Note: Descriptions are shown in the official language in which they were submitted.
CA 02453424 2009-12-22
=51096-3
-1-
POLYVINYLIDENE FLUORIDE COMPOSITES AND METHODS FOR
PREPARING SAME
Field Of The Invention
The invention relates generally to electrically conductive polyvinylidene
fluoride composites containing carbon nanotubes, and the methods for preparing
them.
Background Of The Invention
Polyvinylidene Fluoride
Plastics are synthetic polymers which have a wide range of properties that
make them useful for a variety of applications ranging from packaging and
building/construction to transportation; consumer and institutional products;
fu niture
and furnishings; adhesives, inks and coatings and others. In general, plastics
are
valued for their toughness, durability, ease of fabrication into complex
shapes and
their electrical insulation qualities.
One such widely used plastic is polyvinylidene fluoride (-H2C=CF2-),
("PVDF"), which is the homopolymer of 1,1-difluoroethylene, and is available
in
molecular weights between 60,000 and 534,000. This structure, which contains
alternating -CH2- and -CF2- groups along the polymer backbone, gives the PVDF
material polarity that contributes to its unusual chemical and insulation
properties.
PVDF is a semicrystalline engineered thermoplastic whose benefits include
chemical and thermal stability along with melt processibility and selective
solubility.
PVDF offers low permeability to gases and liquids, low flame and smoke
characteristics, abrasion resistance, weathering resistance, as well as
resistance to_
creep and other beneficial characteristics. As a result of its attractive
properties,
PVDF is a common item of commerce and has a wide variety of applications
(e.g.,
cable jacketing, insulation for wires and in chemical tanks and other
equipments).
In addition to forming a homopolymer, PVDF also form copolymers with
other polymer and monomer families, most commonly with the co-monomers
CA 02453424 2004-01-08
WO 03/007314 PCT/US02/21754
-2-
hexafluoropropylene (HFP), chlorotrifluoroethylene (CTFE), and
tetrafluoroethylene
(TFE), as well as terpolymers and olefins. The properties of the copolymers is
strongly dependent on the type and fraction of the co-monomers as well as the
method
of polymerization. For example, HFP makes a homogenous copolymer with PVDF.
On the other hand, the PVDF copolymer phase segregates if the other monomer is
not
fluorinated.
Conductive Plastics
Recently, demand and applications for electrically conductive plastics have
grown. In these uses, one seeks to exploit the unique properties of plastics,
often as
an alternative to metals. For example, electrically conductive polymeric
materials are
desirable for many applications including the dissipation of electrostatic
charge from
electrical parts, electrostatic spray painting and the shielding of electrical
components
to prevent transmission of electromagnetic waves.
Conductivity (i.e., the ability of material to conduct or transmit heat or
electricity) in plastics is typically measured in terms of bulk resistivity
(i.e., volume
resistivity). Bulk resistivity, which is the inverse of conductivity, is
defined as the
electrical resistance per unit length of a substance with uniform cross
section as
measured in olun-cm. Thus, in this manner, the electrical conductivity of a
substance
is determined by measuring the electrical resistance of the substance.
Electrically conductive plastics can be divided into several categories
according to their use. For example, high level of resistivity (i.e., low
level of
conductivity) ranging from approximately 104 to 108 ohm/cm generally confer
protection against electrostatic discharge ("ESD") and is referred to as the
ESD
shielding level of conductivity. This is also the level of conductivity needed
for
electrostatic painting. The next level of resistivity, which ranges from
approximately
104 ohm/cm and lower, protects components contained within such plastic
against
electromagnetic interference ("EMI") as well as prevents the emission of
interfering
radiation, and is referred to as the EMI shielding level of conductivity. In
order for a
plastic article to be used as a conductive element like a current collector or
separator
plate in an electrochemical cell, resistivity less than 102 olun/cm is
required.
CA 02453424 2004-01-08
WO 03/007314 PCT/US02/21754
-3-
The primary method of increasing the electrical conductivity of plastics have
been to fill them with conductive additives such as metallic powders, metallic
fibers,
ionic conductive polymers, intrinsically conductive polymeric powder, e.g.,
polypyrrole, carbon fibers or carbon black. However, each of these approaches
has
some shortcomings. Metallic fiber and powder enhanced plastics have poor
corrosion
resistance and insufficient mechanical strength. Further, their density makes
high
weight loadings necessary. Thus, their use is frequently impractical.
When polyacrylonitrile ("PAN") or pitch-based carbon fiber is added to create
conductive polymers, the high filler content necessary to achieve conductivity
results
in the deterioration of the characteristics specific to the original resin. If
a final
product with a complicated shape is formed by injection molding, uneven filler
distribution and fiber orientation tends to occur due to the relatively large
size of the
fibers, which results in non-uniform electrical conductivity.
Principally because of these factors and cost, carbon black has become the
additive of choice for many applications. The use of carbon black, however,
also has
a number of significant drawbacks. First, the quantities of carbon black
needed to
achieve electrical conductivity in the polymer or plastic are relatively high,
i.e. 10-
60%. These relatively high loadings lead to degradation in the mechanical
properties
of the polymers. Specifically, low temperature impact resistance (i.e., a
measure of
toughness) is often compromised, especially in thermoplastics. Barrier
properties also
suffer. Sloughing of carbon from the surface of the materials is often
experienced.
This is particularly undesirable in many electronic applications. Similarly,
outgassing
during heating may be observed. This adversely affects the surface finish.
Even in
the absence of outgassing, high loadings of carbon black may render the
surface of
conductive plastic parts unsuitable for automotive use.
Taken as a whole, these drawbacks limit carbon black filled conductive
polymers to the low end of the conductivity spectrum. For EMI shielding or
higher
levels of conductivity, the designer generally resorts to metallic fillers
with all their
attendant shortcomings or to metal construction or even machined graphite.
What ultimately limits the amount of carbon black that can be put into plastic
is the ability to form the part for which the plastic is desired for.
Depending on the
plastic, the carbon black, and the specific part for which the plastic is
being made, it
CA 02453424 2009-12-22
51096-3
-4-
becomes impossible to form a plastic article with 20-60 wt % carbon black,
even if
the physical properties are not critical.
Carbon Fibrils
Carbon fibrils have been used in place of carbon black in a number of polymer
applications. Carbon fibrils, `referred to alternatively as nanotubes,
whiskers,
buckytubes, etc., are vermicular carbon deposits having diameters less than
1.0 .t and
usually less than 0.2 p. They exist in a variety of forms and have been
prepared
through the catalytic decomposition of various carbon-containing gases at
metal
surfaces. Such fibers provide significant surface area when incorporated into
a
structure because of their size and shape. They can be made with high purity
and
uniformity.
It has been recognized that the addition of carbon fibrils to polymers in
quantities less than that of carbon black can be used to produce conductive
end
products. For example, U.S. Pat. No. 5,445,327, to
Creehan disclosed a process for preparing composites by introducing matrix
material,
such as thermoplastic resins, and one or more fillers, such as carbon fibers
or carbon
fibrils, into a stirred ball mill. Additionally, U.S. Pat. No. 6,403,696,
entitled "Fibril-
Filled Elastomer Compositions,", disclosed composites
comprising carbon fibrils and an elastomeric matrix, and methods of preparing
such.
It has also been recognized that the addition of carbon fibrils to polymers
can
be used to enhance the tensile and flexural characteristics of end products.
See e.g.
Goto et al., U.S. Pat. No. 5,304,326.)
Additionally, prior work by Moy et al., U.S. Pat. No. 5,456,897, and Uehara et
al.,
U.S. Pat. No. 5,643,990 disclosed the production of fibril aggregates
and their usage in creating conductive polymers. Moy et aldisclosed the
production
of a specific type of carbon fibril aggregate, i.e. combed yarn, and alluded
to its use in
composites. Uehara et al. also disclosed the use of fibril aggregates in
polymeric
materials. The fibril aggregates have a preferred diameter range of 100-250
microns.
When these fibril aggregates are added to polymeric compositions and
processed,
conductivity is achieved.
CA 02453424 2009-12-22
51096-3
U.S. Patent No. 5,643,502 to Nahass et al. ,
disclosed that a polymeric composition comprising a polymeric binder and 0.25-
50
weight % carbon fibrils had significantly increased IZOD notched impact
strength
(i.e., greater than about 2 ft-lbs./in) and decreased volume resistivity
(i.e., less than
about lx 101 ohm-cm). Nahass disclosed a long list of polymers (including
polyvinylidene fluoride) into which carbon fibrils may be dispersed to form a
composite. The polymers used by Nahass in the Examples of the `502 patent for
preparing conductive, high toughness polymeric compositions include polyamide,
polycarbonate, acrylonitrile-butadiene-styrene, poly (phenylene ether), and
thermoplastic urethane resins and blends.
While the nanotube-containing polymer composites of the art are useful and
have valuable strength and conductivity properties, many new uses for such
composites require that very high strength and low conductivity be achieved
with low
nanotube loading in the polymer. Accordingly, the art has sought new composite
compositions which achieve these ends.
Summary Of The Invention
The invention relates to a polymer composite which is mechanically strong
and electrically conductive.
The invention relates to a polymer composite which has a higher level of
conductivity than known polymer composites.
The invention relates to polymer composites which achieve extraordinary
levels of conductivity at low levels of nanotube loading.
The invention relates to methods for preparing a polymer composite which is
mechanically strong and electrically conductive.
It has now been discovered that composites containing polyvinylidene fluoride
polymer or copolymer and carbon nanotubes have extraordinary electrical
conductivity. Composites with less than 15/4') by weight of carbon nanotubes
have
been found to have a bulk resistivity many times lower than the bulk
resistivity of
other polymer composites having similar nanotube loading. Composites with as
little
CA 02453424 2009-12-22
51096-3
-6-
as 13% by weight carbon nanotubes have a bulk resistivity similar to that of
pure
carbon nanotube mats.
Composites containing polyvinylidene fluoride polymer or copolymer
and carbon nanotubes may be prepared by dissolving the polylmer in a solvent
to
form a polymer solution and then adding the carbon nanotubes into the
solution.
The solution is mixed using a sonicator or a Waring blender. A precipitating
component is added to precipitate out a composite comprising the polymer and
the nanotubes. The composite is isolated by filtering the solution and drying
the
composite.
Composites containing polyvinylidene fluoride polymer or copolymer
and carbon nanotubes may also be prepared by adding carbon nanotubes to a
polymer emulsion and then mixing the emulsion with a Waring blender. The
water/solvent is then removed by evaporation to obtain the composite
comprising
the polymer and the nanotubes.
In one polymer composite aspect, the invention relates to a polymer
composite comprising: polyvinylidene fluoride; and carbon nanotubes in an
amount from about 0.01 to 30% by weight of said composite, wherein said
nanotubes have a diameter less than about 100 nanometers; wherein said
composite has a bulk resistivity of less than about 10 ohm-cm.
In a further polymer composite aspect, the invention relates to a
polymer composite comprising: a copolymer of vinylidene fluoride, and carbon
nanotubes in an amount from about 0.01 to 30% by weight of said composite,
wherein said nanotubes have a diameter less than about 100 nanometers;
wherein said composite has a bulk resistivity of less than about 10 ohm-cm.
In a still further polymer composite aspect, the invention relates to a
polymer composite comprising: a composition comprising a mixture of at least
two
substances selected from the group consisting of polyvinylidene fluoride,
copolymer of vinylidene fluoride and another monomer, and another polymer; and
carbon nanotubes in an amount from about 0.01 to 30% by weight of said
CA 02453424 2009-12-22
51096-3
-6a-
composite, wherein said nanotubes have a diameter less than
about 100 nanometers.
In one method aspect, the invention relates to a method for
preparing an electrically conductive composite comprising the steps of: (a)
dissolving a polymer selected from the group consisting of polyvinylidene
fluoride
and copolymer of vinylidene fluoride in a solvent to form a solution; (b)
dispersing
nanotubes in said solution; and (c) adding a precipitating component into said
solution to precipitate a composite comprising said polymer and said
nanotubes.
In a further method aspect, the invention relates to a method for
making bipolar plates comprising the steps of: (a) dissolving a polymer
selected
from the group consisting of polyvinylidene fluoride and copolymer of
vinylidene
fluoride in a solvent to form a solution; (b) dispersing said nanotubes in
said
solution; (c) adding a precipitating component into said solution to
precipitate out a
composite comprising said polymer and said nanotubes; (d) isolating said
composite; (e) extruding said composite; and (f) engraving one or more flow
channels on said composite.
In a still further method aspect, the invention relates to a method for
preparing an electrically conductive composite comprising the steps of: (a)
mixing
carbon nanotubes with a polymer emulsion, said emulsion comprising a liquid
and
a polymer selected from the group consisting of polyvinylidene fluoride and
copolymer of vinylidene fluoride; and (b) removing said liquid to form a
composite
comprising said nanotubes and said polymer.
CA 02453424 2009-12-22
51096-3
- 6b-
Brief Description Of The Drawings
Fig. 1 is a graph plotting composite resistivity as a function of nanotube
loading in a PVDF/HFP composite.
Fig. 2 is a graph plotting composite resistivity as a function of graphite
concentration for PVDF composites with 13% nanotube loading.
Fig. 3 is a logarithmic graph plotting resistivity as a function of nanotube
weight fraction for various polymer composites.
Fig. 4 is a graph plotting composite conductivity as a function of nanotube
loading, in various polymer composites.
Detailed Description Of The Invention
PVDF-Nanotube Composites
It has now been diiscovered that composites containing PVDF polymer or
copolymer and carbon nanotubes have electrical conductivities much higher than
other polymer/carbon nanotube composites known in the art. As used hereafter,
the
term "PVDF composite" refers broadly to any composite containing PVDF or a
copolymer of vinylidene fluoride and another monomer, and carbon nanotubes.
Unlike other polymer composites, PVDF composites with bulk resistivities as
low as
pure carbon nanotubes can be formed. For simplicity, the term percent nanotube
CA 02453424 2004-01-08
WO 03/007314 PCT/US02/21754
-7-
loading (% nanotube loading) will be used to refer to percentage of nanotube
by
weight in the composite.
It has now been discovered that lower loadings of nanotubes in PVDF
composites results in far higher conductivities than similar loadings in other
polymer
composites. For example, a PVDF composite with 5% nanotube loading has a bulk
resistivity of .42 ohm-cm while a poly(paraphenylene sulfide) composite with
5%
nanotube loading has a bulk resistivity of 3.12 ohm-cm.
PVDF composites containing carbon nanotubes in an amount as little as 1% or
less by weight have an exceptionally low bulk resistivity compared to the pure
PVDF
polymer or copolymer, and have exceptionally low resistivity compared to other
polymer composites at similar nanotube loadings. Nanotube loading may be
widely
varied. For example, PVDF composites may be made with nanotube loadings of
broadly from.01-30% desirably from 0.5-20% and preferably from 1-15%. It has
been found that PVDF composites have much lower bulk resistivities compared to
other polymer composites at any given nanotube loading.
It has been further discovered that a PVDF composite with as little as about
13% nanotube loading has a bulk resistivity comparable to a pure nanotube mat,
i.e.,
between.02 ohm-cm to .08 ohm-cm. PVDF composites with about 13% to 20%
nanotube loading all have bulk resistivity values within the range of the
resistivity
values of a pure nanotube mat. PVDF composites can be formed with bulk
resistivity
of less than about 10 ohm-cm or less than about 1 ohm-cm. The bulk resistivity
of
PVDF composite may be adjusted by varying the nanotube loading to meet the
level
of conductivity required for its intended application.
Depending on how the composite is prepared, no further improvements in
conductivity beyond 13-20% nanotube loading in PVDF were observed, the
limiting
resistivity of .02 ohm-cm (i.e., the resistivity of a pure mat of carbon
nanotubes)
having been reached. The lower limit of nanotube loading is set by the limit
of
percolation and will depend on various factors such as the method of composite
formation, the materials used, etc. For example, Table 1 shows that the lower
limit of
nanotube loading under the conditions in Example 1 is well under 1%, but
apparently
above 0.2%.
CA 02453424 2009-12-22
=51096-3
-8-
The monomers which may be used with vinylidene fluoride monomer to form
PVDF copolymers for the composites of the invention include
hexafluoropropylene,
polystyrene, polypropylene, CTFE, TFE, terpolymers or olefins. The copolymers
may be produced broadly from a de minims amount of a monomer other than
vinylidene fluoride to as much as 90% by weight of such monomer. Desirably
copolymers of the invention contain from 1% to 70% by weight of such other
monomer and preferably from 10% to 50% by weight thereof.
The PVDF composites of the invention also include mixtures of PVDF and
other polymers, including those wherein the PVDF and other polymers are
miscible or
immiscible with one another. The PVDF composites of the invention also include
mixtures of PVDF and copolymers formed from vinylidene fluoride and another
monomer, as described above, and mixtures of these mixtures with other
polymers.
Fillers such as graphite may also be used with PVDF copolymer composites.
Carbon Nanotubes
A variety of different carbon nanotubes may be combined with PVDF or
PVDF copolymers to form the composites of the present invention. Preferably,
the
nanotubes used in the invention have a diameter less than 0.1 and preferably
less than
0.05 micron.
United States Patent No. 4,663,230 to Tennent,
describes carbon fibrils that are free of a continuous thermal carbon
overcoat and have multiple ordered graphitic outer layers that are
substantially
parallel to the fibril axis. United States Patent No. 5,171,560 to Tennent et
al.,
describes carbon nanotubes free of thermal overcoat and
having graphitic layers substantially parallel to the fibril axes such that
the projection
of said layers on said fibril axes extends for a distance of at least two
fibril diameters.
As such, these Tennent fibrils may be characterized as having their c-axes,
the axes
which are perpendicular to the tangents of the curved layers of graphite,
substantially
perpendicular to their cylindrical axes. They generally have diameters no
greater than
CA 02453424 2009-12-22
51096-3
-9-
0.1 g and length to diameter ratios of at least 5. Desirably they are
substantially free
of a continuous thermal carbon overcoat, i.e., pyrolytically deposited carbon
resulting
from thermal cracking of the gas feed used to prepare them. These fibrils are
useful
in the present invention. These Tennent inventions provided access to smaller
diameter fibrils having an ordered outer region of catalytically grown
multiple,
substantially continuous layers of ordered carbon atoms having an outside
diameter
between about 3.5 to 70 nm, and a distinct inner core region, each of the
layers and
the core being disposed substantially concentrically about the cylindrical
axis of the
fibrils, said fibrils being substantially free of pyrolytically deposited
thermal carbon.
Fibrillar carbons of less perfect structure, but also without a pyrolytic
carbon outer
layer have also been grown.
Geus, U.S. Patent No. 4,855,091 provides a
procedure for preparation of fishbone fibrils substantially free of a
pyrolytic overcoat.
When the projection of the graphitic layers on the nanotube axis extends for a
distance
of less than two nanotube diameters, the carbon planes of the graphitic
nanotube, in
cross section, take on a herring bone appearance. Hence, the term fishbone
fibrils.
These carbon nanotubes are also useful in the practice of the invention.
The "unbonded" precursor nanotubes may be in the form of discrete
nanotubes, aggregates of nanotubes, or both.
Nanotubes aggregate in several stages or degrees. Catalytically grown
nanotubes produced according to U.S. Patent No. 6,031,711
are formed in aggregates substantially all of which will
pass through a 700 micron sieve. About 50% by weight of the aggregates pass
through a 300 micron sieve. The size of as-made aggregates can, of course, be
CA 02453424 2009-12-22
51096-3
-10-
reduced by various means, but such disaggregation becomes increasingly
difficult as
the aggregates get smaller.
Nanotubes may also be prepared as aggregates having various morphologies
(as determined by scanning electron microscopy) in which they are randomly
entangled with each other to form entangled balls of nanotubes resembling bird
nests
("TN'); or as aggregates consisting of bundles of straight to slightly bent or
kinked
carbon nanotubes having substantially the same relative orientation, and
having the
appearance of combed yam ("CY") e.g., the longitudinal axis of each nanotube
(despite individual bends or kinks) extends in the same direction as that of
the
surrounding nanotubes in the bundles; or, as aggregates consisting of straight
to
slightly bent or kinked nanotubes which are loosely entangled with each other
to form
an "open net" ("ON") structure. In open net structures, the extent of nanotube
entanglement is greater than observed in the combed yam aggregates (in which
the
individual nanotubes have substantially the same relative orientation) but
less than
that of bird nest. CY and ON aggregates are more readily dispersed than BN
making
them useful in composite fabrication where uniform properties throughout the
structure are desired.
The morphology of the aggregate is controlled by the choice of catalyst
support. Spherical supports grow nanotubes in all directions leading to the
formation
of bird nest aggregates. Combed yam and open nest aggregates are prepared
using
supports having one or more readily cleavable planar surfaces, e.g., an iron
or iron-
containing metal catalyst particle deposited on a support material having one
or more
readily cleavable surfaces and a surface area of at least 1 square meters per
gram.
Moy et al., U.S. Patent No. 6,143,689 entitled "Improved Methods and
CA 02453424 2010-01-27
51096-3
-11-
Catalysts for the Manufacture of Carbon Fibrils",
describes nanotubes prepared as aggregates having various morphologies.
Further details regarding the formation of carbon nanotube or nanofiber
aggregates may be found in U.S. Patent No. 5,165,909 to Tennent; U.S. Patent
No.
5,456,897 to Moy et al.; U.S. Patent No. 5,707,916 to Snyder et al., and PCT
Application No. US89/00322, filed January 28, 1989
("Carbon Fibrils") WO 89/07163, and MGy et al., U.S. Patent No. 5,456,897 and
PCT Application No. US90105498, ("Fibril Aggregates and Method of Making
Same") WO 91/05089, and U.S. Patent No. 5,500,200 to Mandeville et al. and
U.S. Patent No. 5,965,470 by Bening et at., and U.S. Patent No. 5,456,897 and
U.S. Patent No. 5,569,635 by Moy et al.
Other fibrils of different microscopic and macroscopic morphologies useful in
the present invention include the multiwalled fibrils disclosed in U.S. Patent
Nos.
5,550,200, 5,578,543, 5,589,152, 5,650,370, 5,691,054, 5,707,916, 5,726,116,
and
5,877,110.
Single walled fibrils may also be used in the composites of the invention.
Single walled fibrils and methods for making them are described in U.S. Patent
No.
6,221,330 and WO 00/26138.
Single walled fibrils have characteristics similar to or better than the multi-
walled
fibrils described above, except that they only have a single graphitic outer
layer, the
layer being substantially parallel to the fibril axis.
PVDF composites containing carbon nanotubes with different grades, sizes,
morphologies, or types have different bulk resistivity at a given fibril
loading. For
example, it has been found that combed candy ("CC") nanotubes provide lower
bulk
resistivity than bird nest ("BN") nanotubes in PVDF composites at low nanotube
loading. Without wishing to be bound by any theory, it is believed that CC
nanotubes, which are aggregated in parallel bundles, are easier to disperse in
the
CA 02453424 2004-01-08
WO 03/007314 PCT/US02/21754
-12-
polymer than BN nanotubes, resulting in a more even distribution of fibrils in
the
composite and hence, lower bulk resistivity.
The conductivity levels obtained using PVDF composites formed from PVDF
polymer or copolymers and carbon nanotubes make it possible to use conductive
plastic, with all its property and fabrication advantages, in place of metals
or pure
graphite in a number of applications.
Use of PVDF Composites
PVDF composites of the invention may be used in applications where
exceptional electrical conductivity is important. Examples of such uses
include
current collectors for high power electrochemical capacitors and batteries.
Current
commercial materials used for these purposes have bulk resistivities of
approximately
1 ohm-cm. Other applications include conducting gaskets or EMF shield
coatings. In
these applications, a difference of, for example, .04 ohm-cm in bulk
resistivity will
have a very significant impact on product performance.
Still further uses include bipolar plates for PEM fuel cells as well as
bifunctional (binder and conductivity enhancers) additives to a lithium
battery
cathode. These bipolar plates are formed by preparing a PVDF composite as
disclosed herein and then extruding a PVDF composite sheet with a thickness
of, for
example, 2 mm. A single screw extruder may be used for the sheet extrusion.
Flow
channels may be engraved between two hot plates, one with a mirror pattern of
the
front plate channel and the other with a mirror pattern of the back plate
channel. The
channels may run parallel to each other from one corner to another, with each
channel
separated from the other by 0.5 mm. The channels may have a width and depth of
0.5
mm.
Method of Preparing Composites
PVDF composites may be prepared by a solution method in which PVDF
polymer or copolymer is dissolved in a solvent such as acetone to form a
solution.
Other soluble solvents such as tetrahydrofuran, methyl ethyl ketone, dimethyl
formamide, dimethyl acetamide, tetramethyl urea, dimethyl sulfoxide, trimethyl
phosphate, 2-pyrrolidone, butyrolacetone, isophorone, and carbitor acetate may
be
used.
CA 02453424 2004-01-08
WO 03/007314 PCT/US02/21754
-13-
Nanotubes are dispersed in the solvent by applying energy to the polymer-
nanotube mixture. The energy source can be a mechanical homogenizer,
ultrasonic
sonifier, high speed mixer, Waring blender, or any other mixing means known in
the
art. A precipitating component such as water is added to precipitate or quench
the
solid composite containing the polymer and the nanotubes. The precipitating
component may be any medium which is miscible with the solvent, but in which
the
PVDF polymer or copolymer mixture is insoluble.
The solvent may optionally be removed by filtration or evaporation and dried
to isolate the PVDF composite. The composite may be isolated by drying or
evaporating steps such as heat drying, vacuum drying, freeze-drying, etc.
known in
the art.
PVDF composites may also be prepared by a melt compounding process in
which the PVDF polymer or copolymer is mixed with nanotubes in the mixing head
of a mixer such as a Brabender mixture at high temperatures (i.e., over 200 C)
to melt
and compound the PVDF polymer or copolymer into the carbon nanotubes to form
the composite.
Once the composite has been obtained, it may then be molded as necessary
using compression or injection molding equipment and methods known in the art.
PVDF composites prepared using the solvent solution method have
significantly lower bulk resistivity and thus were better electrical
conductors, than
PVDF composites made using traditional melt compounding methods. Without
wishing to be bound by any theory, it is believed that the solvent solution
method
allows for better intermixing of the PVDF with the carbon nanotubes in the
PVDF
composites, thus resulting in lower bulk resistivity.
It has also been found that at low nanotube loadings, using sonicators or
ultrasonic sonifiers resulted in PVDF composites having lower bulk
resistivities than
PVDF composites made using mechanical mixing means such as Waring blenders.
Without wishing to be bound by any theory, it is also believed that sonicators
or
ultrasonic sonifiers are able to better disperse low levels of nanotubes in
the polymer
than mechanical mixing means, resulting in better distribution of nanotubes
within the
composite and hence, better conductivity.
CA 02453424 2009-12-22
51096-3
-14-
For any industrial processes where use of organic solvents is not preferred,
PVDF composites may be prepared using PVDF emulsions. In this method, carbon
nanotubes are directly mixed with or dispersed in PVDF emulsions (or PVDF
latex, or
any dispersions of PVDF in water) by applying an energy squrce such as a
mechanical
homogenizer, ultrasonic sonifier, high speed mixer, WaringTM blender, or any
other
mixing means known in the art. The water (or liquid) in the mixture is then
removed,
for example, by evaporation or any drying means known in the art to recover
the
PVDF composite.
EXAMPLES
Examples of electrically conductive PVDF composites and methods of
preparing the same are set forth below.
EXAMPLE I:
A PVDF polymer, KynarTM 761, was obtained from Elf Atochem and dissolved in
acetone. HyperionTM CC carbon nanotubes were added and dispersed into the
polymer solution for two to five minutes using a high shear blender (i.e.,
Waring
blender). Water was added to the dispersion to precipitate out the polymer
with the
nanotubes. The material was filtered and the filtrate was dried in a vacuum
oven at
100 C to remove acetone and water, leaving behind the dry nanotube/PVDF
composite. Multiple sheets of the composite with thickness of 0.003-0.01
inches were
made using a compression molder. Bulk resistivity of the thin sheet samples
was
measured using a four probe method. Tensile strength was also measured.
Multiple
batches with different amounts of carbon nanotubes were tested. The results
are
reported in Table 1 below:
Table 1
Tensile'
Batch Nanotubes -PVDF Nanotubes: Thickness Strength Resistivity
# (% inch (si (Ohm-cm.
1 .02 10 0.20 - - 300,000
2 .09 10 0.89 0.0075 7005 73.7404
0.0107 7005 64.5745
0.0035 7005 88.5977
0.0109 7005 87.3533
3 .11 10 1.09 0.0102 8145 22.5572
0.0058 8145 15.7831
CA 02453424 2004-01-08
WO 03/007314 PCT/US02/21754
-15-
Tensile
Batch Nanotubes PVDF Nanotubes Thickness Strength Resistivity
(g) (g) (%) (inch) (psi) (Ohm-cm)
4 .31 10 3.01 0.0090 8072 1.2106
0.0099 8072 1.2189
0.0075 8072 1.3074
0.0067 8072 1.2219
.53 10 5.03 0.0055 6739 0.3924
0.0080 6739 0.6255
0.0100 6739 0.4890
0.0050 6739 0.3912
6 .61 8 7.08 0.0030 6770 0.2934
0.0040 6770 0.2992
0.0030 6770 0.2554
0.0098 6770 0.2819
7 1.5 15 9.09 0.0078 8025 0.2154
0.0100 8025 0.2359
0.0081 8025 0.2470
0.0090 8025 0.2175
8 1.24 10 11.03 0.0115 7144 0.1336
0.0132 7144 0.1177
0.0142 7144 0.1152
0.0130 7144 0.1496
9 1.2 8 13.04 0.0115 7521 0.1012
0.0125 7521 0.0811
0.0108 7521 0.0938
0.0081 7521 0.0769
1.5 6 20.00 0.0120 5318 0.0387
0.0110 5318 0.0443
0.0065 5318 0.0441
0.0075 5318 0.0381
0.0130 5318 0.0419
11 2 6 25.00 0.0097 1918 0.0391
0.0090 1918 0.0371
0.0090 1918 0.0497
0.0120 1918 0.0483
The results of Example I show that a PVDF composite with less than I%
nanotube loading had a significantly lower bulk resistivity than a pure PVDF
polymer. The bulk resistivity of the composites dropped significantly as the
nanotube
5 loading increased to approximately 3%. At approximately 5% nanotube loading,
the
bulk resistivity of the PVDF composite was below 1 ohm-cm. At approximately
13%
nanotube loading, the bulk resistivity approached .08 ohm-cm, which is
comparable to
that of a pure CC nanotube mat. At nanotube loadings higher than 13% (i.e., 13-
25%)
CA 02453424 2004-01-08
WO 03/007314 PCT/US02/21754
-16-
the PVDF composite had bulk resistivities within the ranges of those of pure
CC
nanotube mats.
EXAMPLE II:
Using the procedure of Example I and Kynar 761 PVDF, a comparison was
made of the resistivity of composites prepared with Hyperion CC nanotubes and
with
Hyperion BN nanotubes, respectively. The results are reported in Table 2
below:
Table 2
CC Nanotubes BN Nanotubes
Tensile Tensile
Batch Nanotubes Resistivity Strength Resistivity Strength
(%a) (ohm-cm) (psi) (ohm-cm) (psi),-,
1 1 19.17 8145 12987 -
2 5 0.4242 6739 4.1081 6663
3 7 0.2825 6770 1.6136 7227
4 11 0.1290 7144 0.4664 7201
The results of Example II confirm that at low nanotube loadings, PVDF/CC
nanotube composites have significantly lower bulk resistivity than PVDF/BN
nanotube composites.
EXAMPLE III:
Example I was repeated, except that an ultrasound sonicator or a homogenizer
was used instead of a Waring blender to disperse the nanotubes in the polymer
solution. The results are reported below in Table 3:
Table 3
Batch Nanotubes Thickness Dispersion Resistivity
# (%0) (inch) Method (ohm-cm)
1 1.05 0.0058 Sonicator 11.7122
2 3.03 0.0080 Sonicator 0.6144
3 13.04 0.0130 Sonicator 0.0924
0.0110 Sonicator 0.0959
4 13.04 0.0100 Homogenizer 0.1168
0.0110 Homogenizer 0.1028
5 20.00 0.0090 Homogenizer 0.0381
0.0090 Homogenizer 0.0509
These results revealed that PVDF composites with low carbon nanotube
loadings made with a sonicator generally had lower bulk resistivities than
composites
CA 02453424 2004-01-08
WO 03/007314 PCT/US02/21754
-17-
which were made with a Waring blender. PVDF composites made with a
homogenizer had similar bulk resistivity values to those made with a Waring
blender.
EXAMPLE IV:
Example I was repeated, except that the nanotubes were heat treated under
hydrogen, argon, or air before they were dispersed into the polymer solution.
Heat
treatment of the nanotubes was carried out by heating the nanotubes under a
flowing
gas at the following conditions: hydrogen - 600 C for 30 minutes; argon - 1000
C for
30 minutes. Air oxidation was carried out by heating the nanotubes in an oven
in air
at 450 C for 2 hours. The results are reported below in Table 4:
Table 4
Original Nanotubes
Fibril from Ai- Treated Air Oxidized
_ Exam le-1 H2 Treated Nanotubes Narnotubes Nanotubes
R Tensile Tensile R Tensile R Tensile
(ohm- Strength R Strength (ohm- Strength (ohm- Strength
cm) (psi) (ohm-cm) (psi) cm) (psi) cm) (psi)
1.09 19.1701 8145 11.6651 7456 167.0608 7404 609.9 6995
5.03 0.4242 6739 0.4751 6578 0.7111 6648 2.6740 7474
20.00 0.0422 5318 0.0514 5456 0.0783 7855 0.1942 7051
25.00 0.0457 1919 0.0327 2721 - - - -
Generally, heat treatment of nanotubes under hydrogen or argon did not
improve the conductivity of the PVDF composites as compared to composites
formed
from nontreated nanotubes. PVDF composites formed from air oxidized nanotubes
showed significantly poorer conductivity (i.e., higher bulk resistivity) but
higher
tensile strength (at 5-20% fibril loading) compared to composites formed from
nontreated nanotubes.
EXAMPLE V:
Polyvinylidene fluoride-hexafluoropropylene (i.e., PVDF/HFP) copolymer
was obtained from Solvay Advanced Polymers (21508) and the procedure of
Example
I was repeated with the PVDF/HFP copolymer instead of the pure PVDF Kynar 761
polymer. The results are reproduced in Table 5:
CA 02453424 2004-01-08
WO 03/007314 PCT/US02/21754
-18-
Table 5
Nano- PVDF/ Nano- Tensile
Batch tubes HFP tubes Thickness Voltage Current Resistivity Strength
# (g) (g) (% o) (inch) (v) (amp) (ohm-cm) (psi)
1 0.024 20 0.12
2 0.12 20 0.60 0.0095 0.4250 0.001 46.4563 2793
0.0095 0.2750 0.001 30.0599
0.0075 0.4150 0.001 35.8130
0.0080 0.6000 0.001 55.2298
0.0085 0.5200 0.001 50.8574
0.0110 0.3500 0.001 44.2989
3 0.22 20 1.09 0.0055 0.1025 0.001 6.4866 3296
0.0060 0.0700 0.001 4.8326
0.0090 0.0700 0.001 7.2489
4 0.64 20 3.10 0.0090 0.0075 0.001 0.7767 3343
0.0022 0.0380 0.001 0.9619
0.0110 0.0065 0.001 0.8227
1.08 20 5.12 0.0102 0.0048 0.001 0.5633 3206
0.0082 0.0052 0.001 0.4906
0.0050 0.0110 0.001 0.6328
0.0060 0.0095 0.001 0.6559
0.0080 0.0067 0.001 0.6149
0.0100 0.0055 0.001 0.6328
6 2 20 9.09 0.0060 0.0036 0.001 0.2458 3695
0.0060 0.0034 0.001 0.2347
0.0035 0.0072 0.001 0.2908
0.0095 0.0024 0.001 0.2580
0.0060 0.0035 0.001 0.2444
7 3 20 13.04 0.0100 0.0012 0.001 0.1323
0.0045 0.0017 0.001 0.0880
0.0055 0.0022 0.001 0.1392
0.0032 0.0025 0.001 0.0920
0.0080 0.0014 0.001 0.1289
8 1.52 10.1 13.08 0.0060 0.0015 0.001 0.1015 3165
0.0095 0.0008 0.001 0.0874
0.0100 0.0009 0.001 0.1070
0.0050 0.0021 0.001 0.1208
9 1.54 10.04 13.30 0.0055 0.0154 0.010 0.0975
0.0060 0.0124 0.010 0.0856
0.0045 0.0186 0.010 0.0963
0.0050 0.0125 0.010 0.0721
0.0050 0.0187 0.010 0.1076
0.0060 0.0125 0.010 0.0863
1.95 10 16.32 0.0060 0.0012 0.001 0.0828
11 2.54 10.18 19.97 0.0065 0.0177 0.010 0.1322
0.0080 0.0120 0.010 0.1105
12 2.6 10.04 20.57 0.0035 0.0026 0.001 0.1047
The PVDF/HFP copolymer has lower crystallinity than PVDF polymer. The
5 results of Example V showed that as little as 0.6% nanotube loading resulted
in a bulk
resistivity as low as 30 ohm-cm. The bulk resistivity of the PVDF/HFP
composite
continued to drop as the nanotube loading was increased to approximately 13%.
The
CA 02453424 2004-01-08
WO 03/007314 PCT/US02/21754
-19-
bulk resistivity dropped below 1 ohm-cm at 3.1% nanotube loading and the
lowest
reported bulk resistivity observed was 0.072 ohm-cm at 13.3% nanotube loading,
which is within the range of bulk resistivity for a pure CC nanotube mat.
However,
no improvement in bulk resistivity was observed for PVDF/HFP composites with
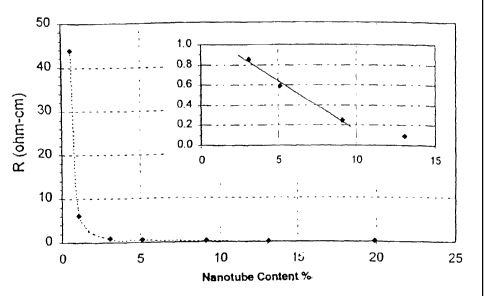
more than 13.3% nanotube loading. Fig. 1 illustrates the steepness of the drop
in bulk
resistivity up to 3% nanotube loading and then the rather linear decrease in
bulk
resistivity above 3 % nanotube loading. An inset plot within the graph of Fig.
1 was
provided to better display this linear decrease in resistivity between 3 and
13%
nanotube loading.
PVDF/HFP composites with nanotube loadings up to approximately 3%
appeared to have lower bulk resistivities than those of PVDF composites with
the
same nanotube loadings. Thus, at low nanotube loading, the conductivity of a
PVDF
composite may be improved by using a PVDF/HFP copolymer, or a lower grade
PVDF with less crystallinity, instead of a pure PVDF polymer. However, it was
also
observed that the tensile strength of this PVDF/HFP composite is lower and
thus the
selection of the copolymer composite or the polymer composite will depend on
the
properties required in the final application.
Conversely, the bulk resistivity of the PVDF composite is lower than that of
the PVDF/HFP composite at higher nanotube loading (i.e., -20% or greater). It
was
further observed that PVDF/HFP composites with over 16% nanotube loading had
rough surfaces and holes, and were difficult to mold since they broke easily.
EXAMPLE VI:
A different grade of PVDF/HFP copolymer (Kynar 2801) was obtained from
Elf Atochem and the procedure of Example I was repeated. Kynar 2801 is also
known as Kynar-Flex. The results are reproduced in Table 6:
Table 6
Kynar-
Batch Nanotubes Flex Nanotubes ` Voltage Current Thickness Resistivity
# O O (%) (v) (amps) (inch) (ohm-cm)
1 0.62 20 3.01% 0.0128 0.001 0.0075 1.1046
0.0075 0.001 0.0105 0.9061
2 1.06 20 5.03% 0.0093 0.001 0.0050 0.5350
0.0064 0.001 0.0075 0.5549
3 1.5 15 9.09% 0.0035 0.001 0.0075 0.3020
0.0019 0.001 0.0104 0.2274
CA 02453424 2004-01-08
WO 03/007314 PCT/US02/21754
-20-
Kynar-
Batch Nanotubes Flex Nanotubes Voltage Current Thickness Resistivity
O (g) (%) (y) (amps) (inch) (ohvn-cm)
0.0021 0.001 0.0090 0.2175
0.0010 0.001 0.0200 0.2186
4 1.51 10 13.12% 0.0105 0.010 0.0100 0.1208
0.0245 0.010 0.0040 0.1128
0.0115 0.010 0.0110 0.1456
1.54 10.14 13.18% 0.0033 0.001 0.0040 0.1519
0.0025 0.001 0.0050 0.1438
0.0125 0.010 0.0100 0.1438
6 1.5 6 20.00% 0.0006 0.001 0.0080 0.0506
0.0058 0.010 0.0078 0.0521
0.0004 0.001 0.0120 0.0525
0.0006 0.001 0.0080 0.0552
Unlike the PVDF/HFP composite of Example V, the Kynar 2801 copolymer
composite exhibited lower bulk resistivity above 13% nanotube loading. At 20%
nanotube loading, the Kynar 2801 composite had bulk resistivity values of
about .05
5 ohm-cm, which is within the range of a pure CC nanotube mat.
EXAMPLE VII:
PVDF composites were prepared by melt compounding PVDF (Kynar 761)
and Hyperion CC nanotubes and/or graphite (Lonza KS-75) in the mixing head of
a
Brabender mixer at 100 RPM for approximately five minutes at the temperature
specified. Each of the mixtures were prepared by sequential addition of the
compounds in the following order: PVDF, nanotubes, then graphite, unless the
mixtures were premixed as indicated by an asterisk (*). Once compounded, flat
sheets were prepared by pressing small pieces of the composite between thin,
chromed plates at approximately 240 C with the thin plates being cooled to
room
temperature in one to two minutes. Resistivity was measured with a linear four
probe
head. The results are reported below in Table 7:
Table 7
Batch PVDF Nanotubes Graphite Nanotubes Temp Resistivity
# (g) () () (%) ( C) (ohm-cm)
1 40 10 - 20 210 0.148*
2 42.5 7.5 - 15 215 0.171
3 51 9 - 15 230 0.117
- 245 0.126
4 49.5 10.5 - 17.5 240 0.105
5 48 12 - 20 245 0.092
6 51 9 - 12.5 250 0.156
7 58.2 1.8 - 3 240 12.1
CA 02453424 2004-01-08
WO 03/007314 PCT/US02/21754
-21-
Batch PVDF Nanotubes Graphite Nanotubes Temp Resistivity
# O () O (%) ( C) (ohm-cm)
- 1.93*
8 45.5 9 14 13.1 240 0.064
9 44.8 8.2 9 13.2 240 0.075
60 10 5 13.3 240 0.109
11 58 10 7.5 13.2 240 0.091
12 56 10 10 13.1 240 0.077
0.055*
13 52 10 12.5 13.4 240 0.068
14 63.9 0 11.4 0 240 1000*
56 0 21.25 0 240 300*
The results show that very conductive composites can be formed by melt
compounding. Premixed materials appear to yield composites with lower bulk
resistivity than composites formed by sequential addition.
5 As shown in Batches 8-12, it was discovered that increasing the graphite
concentration in PVDF composites at a given nanotube loading increases the
conductivity of the composite. Fig. 2, which plots the resistivity for Batch
Nos. 8-12,
illustrates the decrease in bulk resistivity as a function of the increase in
graphite
concentration in the PVDF composite with 13% nanotube loading.
10 EXAMPLE VIII:
Resistivity tests were performed on several polymer composites at several
nanotube loadings. The composites were made using the solution procedure of
Example I or the melt compounding procedure of Example VII. The following
polymers were used:
15 PVDF-Sol (PVDF/nanotube composite made from solution);
Kynar-Flex (PVDF/HFP nanotube composite made from solution);
PVDF-Melt (PVDF/nanotube composite made by melt compounding);
PPS (poly(paraphenylene sulfide)/nanotube compound made by melt
compounding);
EVA (poly(co-ethylene-vinyl acetate)/nanotube compound made by melt
compounding);
PS (polystyrene/nanotube compound made by melt compounding); and
PE (polyethylene/nanotube compound made by melt compounding).
The results of Example VIII are shown below in Table 8:
CA 02453424 2004-01-08
WO 03/007314 PCT/US02/21754
-22-
Table 8
Nanotube Nanotube
(weight (volume Resistivity
-fraction) fraction) Polymer (ohm-cm)
0.02 0.01 PPS 19.00
0.03 0.03 Kynar-Flex 1.10
0.91
0.04 0.03 PPS 211.00
0.05 0.03 PPS 3.12
0.05 Kynar-Flex 0.55
0.54
0.05 PVDF-Sol 0.42
0.07 0.06 PVDF-Sol 0.28
0.05 PPS 1.97
0.09 0.08 Kynar-Flex 0.30
0.23
0.22
0.22
PVDF-Sol 0.23
0.11 0.10 PVDF-Sol 0.13
0.13 0.11 PVDF-Melt 0.16
0.12 Kynar-Flex 0.15
0.15
0.14
0.14
0.12
0.11
0.12 PVDF-Sol 0.09
0.15 0.11 PPS 0.25
0.25
0.14 PVDF-Melt 0.14
0.13
0.12
0.18 0.16 PVDF-Melt 0.10
0.20 0.09 EVA 0.41
0.18 PVDF-Melt 0.09
0.18 Kynar-Flex 0.06
0.05
0.05
0.05
0.18 PVDF-Sol 0.04
0.09 PE 0.65
0.25 0.13 PS 0.20
0.23 PVDF-Sol 0.05
0.26 0.12 PE 0.34
0.12 EVA 0.24
CA 02453424 2004-01-08
WO 03/007314 PCT/US02/21754
-23-
Nanotube Nanotube
(weight (volume Resistivity
fraction) fraction) Polymer (ohm-cm)
0.27 0.15 EVA 0.25
0.13 EVA 0.23
0.14 PS 0.11
0.28 0.13 PE 0.29
0.29 0.14 PE 0.47
0.30 0.16 PS 0.15
0.14 EVA 0.13
0.33 0.16 EVA 0.20
The nanotube weight fraction was calculated by dividing the nanotube weight
by the composite weight. The nanotube volume fraction was calculated by
dividing
the volume of the nanotubes by the volume of the composite. These volumes were
calculated by dividing each of the nanotube and polymer weights by their
respective
densities (the volume of the composite is the sum of the nanotube and polymer
volumes).
The results of Example VIII showed that the resistivities of the PVDF and
PVDF/HFP composites are orders of magnitude lower than the resistivities of
even
the best conductive polymers at any given nanotube loading level. For example,
at
5% nanotube loading, the PVDF and PVDF/HFP composites had bulk resistivity
values ranging from .42 to .55 ohm-cm, while the bulk resistivity of the PPS
composite was 3.12 ohm-cm. At 20% nanotube loading, The PVDF and PVDF/HFP
composites had bulk resistivity values between .04-.09 ohm-cm, which is within
the
range of a pure CC nanotube mat. No other polymer composite at 20% nanotube
loading or at any higher nanotube loading level had a bulk resistivity value
within the
range of that of a pure CC nanotube mat. These differences are significant for
applications where high electrical conductivity is crucial.
Fig. 3 sets forth a logarithmic plot of nanotube weight vs. resistivity. A
line is
drawn which unequivocally distinguishes the resistivity of the PVDF composites
from
all other polymer composites, illustrating clearly that PVDF composites are
superior
to other polymer composites in electrical conductivity.
Fig. 4 sets forth a plot of nanotube weight vs. conductivity. As Fig. 4
confirms, PVDF composites have clearly superior conductivity than other
polymer
composites known in the art. Additionally, Fig. 4 further shows that, unlike
other
CA 02453424 2004-01-08
WO 03/007314 PCT/US02/21754
-24-
polymer composites known in the art, conductivity for PVDF composites increase
exponentially as the nanotube loading is increased to approximately 20%. PVDF
composites also obtained the conductivity of a pure CC nanotube mat (i.e.,
12.5-
50/ohm-cm). Composites made from other polymers were unable to reach the
conductivity range of a pure CC nanotube mat, even as the nanotube loading was
increased beyond 30%.
EXAMPLE IX:
Multilayered structure comprising a first layer of a PVDF composite, a second
layer of a thermoplastic or thermoplastic blend/composite, and an optional
third
adhesive layer between the first and second layers. The second layer can be a
nylon-6
(6,6, 11, or 12), a nylon-clay composite known for excellent barrier and high
heat
distortion properties, or a nylon blend. The adhesive layer can be a PVDF-
nylon
blend with relatively lower viscosity. This layered structure can be
fabricated in a
sheet form, or into a container of any shape and size, or into a tubing/pipe.
The inner
layer for the container and tubing forms is preferably a PVDF composite layer.
Since
the PVDF polymer is known for its resistance to heat and hydrocarbons, and the
nylon
material, in particular, nylon-clay composite is known for its high heat
distortion
temperature, excellent barrier properties and good mechanical properties,
containers
and tubing formed from this multilayered structure can be used for safe
storage and
transport wide-range of hydrocarbons.
A multilayered tubing may be prepared using the following materials: PVDF
composite of Kynar 761 and 13% loading nanotube, clay-nylon-6 composite and a
PVDF (Kynar 741)-Nylon-6(30%) blend. The preparation of a three-layer tubing
was
carried out in a coextrusion system equipped with three extruders. The inner
diameter
of the tubing and thickness of each layer are: inner diameter: 20 mm;
thickness of
inner PVDF composite layer: 0.6 mm; thickness of adhesive PVDF-nylon blend
layer:
0.2 min; thickness of outer clay-nylon composite layer: 1 mm.
EXAMPLE X
A PVDF emulsion, Kynar 720, was obtained from Elf Atochem. Hyperion
CC carbon nanotubes were added and dispersed into the Kynar 720 emulsion using
a
high shear blender (i.e., Waring blender). The water/liquid present in this
mixture
was removed by evaporation and thin specimens of the PVDF composite with
CA 02453424 2004-01-08
WO 03/007314 PCT/US02/21754
-25-
dimensions of approximately 0.5' x 3" x. 0.01' were prepared by hot press. The
resistivities were measured and reported below:
Nanotubes Resistivity
(%) (Ohm-cm)
3 3.11
13 0.14
20 0.063
The results of Example X confirm that PVDF composites with low bulk
resistivity
may be prepared using PVDF emulsions. At 20% nanotube loading, the PVDF
composite prepared using this method had a bulk resistivity within the range
of a pure
CC nantube mat.