Note: Descriptions are shown in the official language in which they were submitted.
CA 02454204 2003-12-23
IMPLANT FOR USE IN AESTHETIC REGIONS OF THE MOUTH WITH
COLOURED CONTOURED EDGE PORTION
SCOPE OF THE INVENTION
The present invention relates to a dental implant to be used in areas in the
mouth where aesthetics are of a high concern, and more preferably an implant
which
has a bone engaging coated, textured and/or porous portion which has a contour
selected to approximately mirror a predetermined surface contour of bone
tissues at
the site of implant placement, and an upper coronal band portion which has a
colour
selected to complementarily blend with the colouring of the patient's gum
tissue.
BACKGROUND OF THE INVENTION
Conventional implant constructions are generally of a two-part design and
include a body portion which is adapted to be recessed into the patient's jaw,
and a
prosthesis in the form of a ceramic tooth which is adapted for coupling to a
proximal
end of the body. The implantable body which is made from stainless steel,
titanium
or other suitable metals or alloys is configured to be recessed into a
suitable bore
hole formed in the patient's jaw bone at the site of a lost tooth. Typically,
the body
has a threaded interior or is otherwise configured to mechanically receive
thereto an
attachment post which serves as a support for the ceramic tooth.
United States Patent No. 5,344,457 to Pilliar et al., entitled "Porous
Surfaced
Implant", discloses a frustoconical shaped implant which is characterized by a
I
CA 02454204 2003-12-23
porous coated bone engaging lower portion and a smooth non-porous upper bone
attachment region or collar. The implant is press-fitted into a complementary
sized
bore formed in the patient's jaw bone at the site of placement and over time,
bone
tissues grow into and engage the porous coating on the lower portion of the
implant
to firmly anchor it in the place of a natural tooth. The implant which is the
subject
of United States Patent No. 5,344,457 has achieved a significant degree of
success
in the market place, and is presently sold by Innova Corp. of Toronto, Canada,
under the name Endopore~. Endopore~ dental implants are used in the
replacement
of various teeth including, lost molars and bicuspid teeth in the anterior and
posterior regions of the mouth.
Other conventional implant constructions are characterized by the implantable
body being cylindrical in shape and provided with a roughened lower exterior
surface, texture, external thread configuration and/or coating, to facilitate
the
engagement of the implant body with the patient's surrounding bone tissues and
its
anchoring in place.
A difficultly exists with conventional implants in that todate, they have
achieved limited success in replacing incisors and teeth in the frontal-most
regions of
the mouth where high aesthetic demands exist. The abutment-implant interface,
also
termed "microgap", is believed to harbor bacteria and bacterial products
following
exposure to the oral environment. This in turn results in the establishment of
a
"biological width" around the implant (i. e. the distance from the peri-
implant bone
crest to the microgap). The biological width is relatively constant and seems
to be
approximately 2 mm, similar to the biological width present around natural
teeth. It
has been found that following implantation, crestal bone remodeling occurs,
2
CA 02454204 2003-12-23
whereby supporting bone tissues and the overlying bone tissues tend to recede
to the
uppermost peripheral edge of the textured or porous coated bone engaging
portion of
the implant body. A further variable that can play a role in crestal bone
remodeling
is lack of mechanical coupling around any smooth upper collar surface. For
example, it has been demonstrated that the crestal bone resorption around
Endopore~ implants stops at the junction of the smooth collar and the porous
surface. It has been suggested that the lack of mechanical coupling around the
smooth collar surface results in "disuse atrophy" of the crestal bone to the
level of
the junction with the porous surface. This has been demonstrated also with
other
textured implant surfaces.
The receding supporting tissues or crestal bone loss around dental implants
has led to an aesthetic challenge when attempting dental restorations in that
it may
result in exposure of the metal implant body, greatly detracting from the
natural
appearance of the prosthesis. Conventional implants suffer the disadvantage in
that
the alveolar bone which encases the tooth root tends to gradually disappear
along the
portion of the implant where engagement of bone tissue with the implant body
does
not occur. This leads to a corresponding recession of the sulcus and overlying
gum
tissues which gradually results in the exposure of the silver/grey stainless
or
titanium steel body of the implant. In the more aesthetically important
regions of
the mouth, the exposure of the stainless steel portion of the implant body may
be
seen through the patient's gum tissue as a grey tinted band, greatly
detracting from
the natural look of the prosthesis.
This problem is particularly pronounced in the anterior regions of the mouth
and when using two implants positioned adjacent to each other. Loss of inter-
implant
3
........ ....,......_ . ,.,..._ n....~...
...aS.mH.KSrGqt~.t~rs,bx"..u,yp~,p~~p),.~,~gq~.~yy,.a~pyyy.~~,y,a"...,....,.,..
......_.......__...._._..- "q~ p".,..",."........v.._....._ . . ..
CA 02454204 2003-12-23
bone height (as a result of the normal crestal bone remodeling that is
associated with
each of the implants) results in the absence of a papilla between the two
implants
due to lack of bone support. This creates an aesthetic deformity, often termed
"black triangle", between the two implant crowns. "lack triangles" are
particularly visible when present in the maxillary anterior region and the
patient has
a high lip line. The patient's perception of a successful implant-supported
prosthesis
depends not only on restoring function, but also on restoring normal anatomy
and
aesthetics. The lack of a papilla and the presence of a "black triangle" can
lead to
patients' dissatisfaction with the whole implant treatment, even with patients
having
a low smile line. Heretofore, the dental profession has been forced to come up
with
techniques to deal with "black triangles" . Most commonly, pink acrylic or
porcelain is added to the final restoration to replace the missing papilla.
This
solution is far from ideal since it is impossible to replicate the gingival
tissue with
acrylic or porcelain in terms of texture and colour. Several attempts have
also been
made in establishing surgical procedures that will regenerate the missing
papilla;
however, these procedures are very unpredictable and seldom result in 100
regeneration.
Conventional implants are poorly suited to accommodate for the crestal bone
remodeling which occurs with implants. With conventional implant designs, most
often any bone engaging textured, porous or coated surface extends downwardly
from an uppermost radial edge surface which is located a constant distance
from the
lower apex of the body. Conventional implant designs suffer the disadvantage
that
they fail to account for the fact that with natural incisor teeth, the surface
contour of
healthy supporting bone tissues tends to be higher along the distal and medial
surfaces of the tooth than along the lingual and buccal regions. Heretofore,
the bone
4
CA 02454204 2003-12-23
engaging regions for conventional implants have either been limited by the
lowermost extent of expected bone recovery, weakening the integrity of the
dental
implant attachment, or suffer the disadvantage that the lingual and buccal
portions of
the implantable portion of the implant body may be visible at the patient's
gum line.
SUMMARY OF THE INVENTION
To at least partially overcome the disadvantages of the prior art, the present
invention seeks to provide an implant which includes an implantable body
portion
adapted to be at least partially recessed within a patient's alveolar bone,
and which
has a peripheral surface portion which is configured to stimulate and/or
facilitate the
engagement of bone tissues with the implant.
In a healthy jaw, the root of the tooth is supported by alveolar bone, with
lamellated bone surrounding the root of the tooth where periodontal ligament
fibres
attach. The shape and crestal outline of interdental bone will to a large
extent
depend upon the shape and size of the tooth roots, with the distance from the
crest
of the alveolar bone to the cementoenamel junction of the tooth in a healthy
periodontium being about 2 mm and a healthy sulcus extending about .5 mm. The
present implant construction preferably seeks to stimulate osteoblasts, namely
the
bone forming cells, so as to promote bone ingrowth into and otherwise engage
the
implantable portion of the implant, with the crestal surface of the regrown
bone
tissues substantially mirroring that of a healthy tooth, to firmly anchor the
implant in
place.
s
_.. .,._.___,. ... .. .t'N;".,... ..,.s.....~rv..ywy;
.t."...::;ny;yak.Wp;,,y~;~..~:wtS~2"YrtaC~s.-:;.-....:,a..,.,.~m-
u,.au.:.~.w.....w.,..,........n.n.nw,...>~w" ,..d...~..,......-
...,...~..........
CA 02454204 2003-12-23
Another object of the invention is to provide an improved dental implant body
for use in the anterior regions of the mouth, and which provides bone engaging
regions along one or more of the distal andlor mesial implant surfaces which
are
elongated relative to bone engaging regions on the lingual and/or buccal
surfaces of
the implant body.
Another object of the invention is to provide an implant body configuration
which is configured to stimulate crestal bone tissue remodeling to a normal
pre-
implant height.
Another object of the invention is to provide a dental implant body which has
a bone engaging porous, textured, threaded and/or coated exterior surface,
which is
applied to the peripheral surface of the implant body in a configuration which
reflects the actual or a preselected optimum contour of the crest of the
alveolar bone
and/or lamellated bone tissues at the site of implant placement.
The implantable portion of the implant body could for example include about
all or only part of its periphery, a bone engaging surface which, when the
implant
body is fully seated within the patient's jaw bone, extends from a distal
portion of
the implant body to a remote proximal-most edge. The proximal-most edge has a
contour selected to generally follow a predetermined crestal outline of the
supporting
bone tissue. The bone engaging surface could take a number of possible forms
including without restriction: an externally threaded portion, in which the
proximal-
most thread patterns are configured to generally follow the surface contour of
alveolar and/or lamellated bone; an acid etched, physically abraded or other
roughened or textured peripheral surface of the implant body; a porous coated
6
_._.. , ri a.~ u.. ~ . .~~ ,~,.,~,; ~". ~:,~~~~,.. .~.~~~ _ .,,~ r_. ~
~~..._... a _e , .n., ~ w _ ~_ _ __. _ _____ _. _...
CA 02454204 2003-12-23
surface which, for example, could consist of titanium, metal or ceramic beads
and/or a chemically coated portion. Suitable chemical coatings for use with
the
bone engaging surface would typically comprise bioreactive coatings, including
coatings formed from hydroxyapatite and other compounds suitable for
stimulating
bone tissue growth, and which facilitate the anchoring of the implant body by
bone
tissues following its placement.
The predetermined crestal outline could by way of non-limiting example, be
selected as a crestal outline of the patient's own alveolar and/or lamellated
bone
tissues at the site of implant placement or at the site of one or more of the
patient's
own teeth, or the crestal outline of alveolar and/or lamellated bone tissues
of a
typical healthy jaw, and more preferably the crestal outline at the site of or
proximate to the intended site of implant placement.
The bone engaging portion of the implant body could, for example, consist of
a narrow band between the proximal exposed end of the implant body, and the
distalmost end tip of the implant which is recessed into the patient's jaw.
The bone
engaging portion may further extend partially or completely about the
circumference
of the implant body. More preferably, however, the bone engaging region
extends
from approximately adjacent to the distalmost apex of the implant body to a
proximal edge surface which, following placement of the implant body,
approximately coincides with the crest of the alveolar bone of either the
patient's
missing tooth or a healthy tooth.
In one aspect, the "aesthetic implant" design features of the present
invention
are therefore based on the principles governing peri-implant crestal bone
loss, and
CA 02454204 2003-12-23
aim at maintaining the interproximal bone at a level that is coronal to the
buccal and
lingual bone levels. In one simplified construction, the implant is designed
to be
inserted in a single stage surgery, thereby ensuring adequate biological width
between the microgap and the crest of the bone.
Although not essential, the implant body could be generally frustoconical in
shape and, for example, be provided with a porous coated and/or textured bone
engaging exterior surface which is designed to be "press-fit" in a specific
buccal/lingual and mesial/distal orientation. In such an embodiment, the
invention
is directed to an improved dental implant which is suitable far use in
aesthetic
regions of the mouth, including as replacement for upper incisor teeth, and
may be
developed as a modification of the Endopore~ implant disclosed in United
States
Patent No. 5,344,457. The implantable portion of the implant may optionally be
provided with a smooth upper collar portion which, for example, is provided to
prevent or minimize the accumulation of oral bacteria. The smooth collar
portion
could, in a first embodiment, be provided as a smooth band which extends from
the
proximal edge of the bone engaging surface to a proximal end of the implant
body
which is provided with a substantially constant width, extending from the bone
engaging surface to a contoured implant end surface which also follows the
general
contour of the crestal surface of the alveolar bone. In an alternate
embodiment, the
smooth collar of the implant body could extend from the proximal-most edge of
the
lower bone engaging portion to a generally flat proximal implant body surface.
In another construction, the present invention seeks to provide an improved
cylindrical implant body which is characterized by a bone engaging portion
which,
by way of non-limiting example, could comprised helical threads, ribs and/or a
s
CA 02454204 2003-12-23
roughened implant surface formed by grit blasting and/or acid etching. The
bone
engaging portion most preferably extends from a lowermost distal end of the
implant
to a contoured upper edge which at least generally follows the contour of the
patient's crestal bone or a pre-selected typical contour of healthy bone
tissue at the
site where the implant is to be used.
A further construction of the invention provides an improved implant coating
which is selected to provide enhanced engagement between the bone tissue and
the
implant body, and which for example could comprise a hydroxyapatite or other
dentally active coating used to facilitate the anchoring of the implant ire
situ in a
patient's jaw. The dentally active coating is applied about at least part of
the
circumference of the implant body, and depending upon the intended site of
implant
placement, extends from a distalmost end portion of the implant body to a
proximal-
most edge. The coating is applied so as to be elongated along one or more of
the
lingual, distal mesial and/or buccal sides of the implant body. More
preferably, the
coating is applied such that its proximal edge of the coating generally
mirrors the
typical crestal surface contour of either the patient's own or healthy bone
tissues at
the site at which the implant is to be used.
It is envisioned that the dental implant could also be placed in the patient's
alveolar bone in two stages. During the first stage surgery, the implant body
is
submerged into a complementary size bone formed in the bone to the level of a
proximal end cap or platform used as a temporary cover over the proximal end
of
the implant. Following initial placement, a period of time is provided to
allow bone
tissue regrowth so as to grow into and engage the bone engaging surface and
firmly
anchor the implant body in place. As a next stage the proximal end platform is
9
_. _ _. a , ,. ~ .~ a r ry..~ . M. ~,..~ . s~u n »x, ~:~, .~ ~~, ~~:~~ ~,~ ~ a
. ~,~.." ~ .4_..a. N o_m _ . _ _ ___.
CA 02454204 2003-12-23
removed, and an abutment and suitable prosthesis are then coupled to the
proximal
end of the implant body in a mechanical and/or chemically bonded fit
arrangement.
Accordingly, in one aspect the present invention resides in a dental implant
for use in replacing a missing tooth in a patient's jaw bone comprising,
an implant body adapted to be at least partially recessed into a portion of
said
patient's jaw bone, said implant body extending longitudinally along an axis
from a
distalmost apex to a proximal end portion,
a coloured coronal band portion provided about a peripheral surface of said
implant body adjacent said proximal end portion, said coloured portion having
a
colour which is complementary to a natural gum tissue colour of said patient
so as
not to significantly discolour the gum tissue if seen therethrough,
a bone engaging surface provided about at least a portion of said peripheral
surface of said implant body, and being spaced from said coloured band portion
towards said apex said bone engaging surface selected to promote bone tissue
ingrowth or attachment thereto and extending longitudinally along said
periphery of
said implant body to a proximal edge spaced towards said proximal end portion,
wherein at least a portion of said proximal edge having a contour selected to
generally follow a crestal surface contour of preselected bone tissues.
In another aspect, the present invention resides in a dental implant for use
in
replacing a missing tooth in a patient's jaw bone comprising,
...........,..~ . . , . y.".. ~I,~,ryeu.,.pj~wa,,~ma, Xg.~q~y;p~p3nv..~fry.~
;.~.~n~l.,.p.,.,.."... ~_.__..._. ..._.,_ . ""..".,.....o .",A..~."~-
._.,._...... ... ~ ~,.___...._,.-._.....-. _.___..-.~,.""u,"~
CA 02454204 2003-12-23
an implant body portion adapted to be recessed into a portion of said
patient's
jaw bone, said implant body extending longitudinally along an axis from a
distalmost
apex to a proximal end portion,
a coloured coronal band portion provided about said implant body portion
immediately adjacent said proximal end portion, said coloured band portion
comprising a gold coloured plating or coating and extending axially between
0.5 to
2.5 mm along said implant body.
a bone engaging surface spaced distally from said coloured band coating
towards said apex and providing a peripheral surface of said implant body,
said bone
engaging surface selected to promote bone tissue ingrowth or attachment
thereto and
extending longitudinally along said periphery of said implant body to a
proximal
edge spaced towards said proximal end portion, wherein the proximal edge of
the
bone engaging surface has a contour selected to generally follow a crestal
surface
contour of a pre-selected jaw bone adjacent said missing tooth.
In a further aspect, the present invention resides in a dental implant for use
in
replacing a natural tooth in a patient's jaw bone comprising,
an implant body extending longitudinally along an axis from a lowermost
apex to an upper end portion and including,
an uppermost coloured coronal band surface adjacent to said upper end
portion and providing a first peripheral surface portion of said implant body,
said coloured band portion comprising a substantially smooth portion having
applied thereto a coating selected from a group consisting of a gold-coloured
CA 02454204 2003-12-23
titanium nitride coating, a yellow gold coating, a yellow gold alloy coating,
a
pink gold coating and a pink gold alloy coating,
a bone engaging surface providing a second peripheral surface portion
of said implant body adapted to be recessed into said patient's jaw bone, said
bone engaging surface selected to promote bone tissue ingrowth or attachment
thereto and extending longitudinally along said periphery of said implant body
substantially from said apex to an upper edge spaced towards said upper end
portion, wherein the upper edge of the bone engaging surface has a contour
selected to generally follow a crestal surface contour of healthy bone tissues
at a site of implant placement, and
a textured peripheral portion intermediate said bone engaging surface
and said coloured band portion, the textured peripheral portion selected from
a laser abraded portion, an acid etched portion, and mechanically abraded
portion, and
an abutment for supporting a prosthesis thereon.
BRIEF DESCRIPTION OF THE DRAWING
Reference may be now had to the accompanying detailed description, together
with the accompanying drawing pages in which:
Figure 1 illustrates an exploded view of a dental implant construction in
accordance with a first embodiment of the invention;
12
CA 02454204 2003-12-23
Figure 2 shows an enlarged schematic side view of the distal surface of the
implant body used in the implant construction of Figure 1;
Figure 3 shows an enlarged plan view of the proximal end of the implant
body shown in Figure 2;
Figure 4 shows a schematic side view of the lingual surface of the implant
body of Figure 2 seated within a patient's jaw bone;
Figure 5 shows a schematic view of the implant construction shown in Figure
1 seated in place within a patient's jaw bone;
Figure 6 shows a schematic side view of a distal/mesial side of an implant
body in accordance with a second embodiment of the invention;
Figure 7 illustrates a schematic view of a distal/mesial side of an implant
body in accordance with a third embodiment of the invention;
Figure 8 illustrates a schematic view of a distal/mesial side of an implant
body construction in accordance with a fourth embodiment of the invention; and
Figure 9 illustrates schematically a side view of a distal/mesial side of an
implant body in accordance with a further embodiment of the invention.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
13
CA 02454204 2003-12-23
Figure 1 illustrates an exploded implant construction 10 used in the
replacement of a lost anterior or maxillary tooth, in accordance with a first
preferred
embodiment of the invention. The implant construction 10 consists of an
implantable titanium or stainless steel body 12, a stainless steel abutment
assembly
14, a stainless steel retaining screw 16, and a ceramic tooth prosthesis 18
which has
a profile and size selected to mimic the patient's natural tooth which is to
be
replaced. As will be described, the implant construction 10 is adapted to be
recessed at the site of a missing anterior or maxillary tooth within the
lamellated and
alveolar bone tissues 20,22 of the patient's jaw 24 (Figure 4) in the place of
the lost
natural tooth.
The abutment assembly 14 serves as the base support for the prosthesis 18
and has an exterior shape selected for fitted engagement within a
complementary
sized recess 30 formed in the bottom of the prosthesis 18. A central bore 32
is
formed through the center of the abutment 14 which is sized to receive therein
the
screw 16 while preventing the screw head 34 from moving therethrough. It is to
be
appreciated that although Figure 1 illustrates a one-part abutment assembly
14, its
individual abutment components and configuration may vary having regard to the
configuration of the prosthesis I8. In the final assembly of the implant
construction
10, the prosthesis I8 is secured in place over the abutment assembly 14 by a
suitable
dental cement.
As shown best in Figure 1, the implantable body 12 is formed with an
internally threaded axial bore 36 which extends downwardly from a proximal
surface 38 of the implant body to which the abutment assembly 14 is mounted
approximately three-quarters along its longitudinal length. The internal
threads of
14
CA 02454204 2003-12-23
the bore 36 are selected for threaded engagement with the retaining screw 16,
to
enable the mechanical coupling of the assembly 14 to the implant body by means
of
the retaining screw 16. As will be described, following the positioning of the
body
12 in a complementary sized bore formed in the patient's jaw 24, the abutment
assembly 14 is coupled to the implant body 12 by inserting the screw through
the
bore 32 and into threaded engagement with the internal treads of the bore 36.
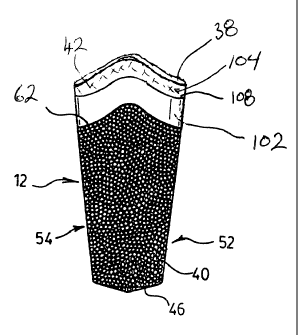
Figure 2 shows best the implant body 12 construction in accordance with the
first embodiment of the invention where the implant body 12 is configured for
use
with a conventional abutment 14 and prosthesis 18, as for example is shown in
Figure 1. Although not essential, the implant body 12 most preferably has a
tapered
frustoconical shape. The body 12 has two principle portions or surfaces,
namely a
distalmost porous coated surface 40 for primary fixation of the implant (i.e.
bone-
engagement) with the patient's bone tissues, as well as a non-porous smooth
upper
or coronal surface 42. The coronal surface 42 is provided to prompt the
maintenance of bone surrounding the surface of the implant 10. As shown best
in
Figures 2 and 3, a hexagonal mount 44 projects upwardly from the proximal end
surface 38 of the implant body 12. The hexagonal mount 44 is centered on the
elongated central axis A-A, (Figure 2) of the implant body. As seen best in
Figure
3, the central bore 36 extends downwardly through the center of the hexagonal
mount 44 in the axial direction. The hexagonal mount 44 more preferably has a
size
and shape selected for fitted placement within a complementary sized recess 46
(Figure 1) which is formed in the bottom of the abutment assembly. Figure 2
further shows best the proximal end surface 38 as comprising a substantially
flat
surface extending radially generally normal to the axis A-A, .
is
. . . . ._. _ ... , . , , . .,,-~~*~. .. a~,: .~,. ~,~,~ ~ .r. . y . ...~~--,
.~..~ .~.. ,.s~~ ~ ..x~,.w~,~, . ~,.~~ ~...,~.._.... . _ _. _ . _
CA 02454204 2003-12-23
The implant body 12 is shown as frustoconically shaped along its entire
length, however, the invention is not so limited and other implant
configurations
which taper only partially along their axial length are also possible.
Preferably, the
implant body 12 tapers inwardly from the proximal end surface 38 to a
distalmost
apex 46, which, as seen best in Figure 4, orients in a downwardmost position
in a
complementary bore 48 formed in the patient's jaw 24 at the site of implant
placement. The implant body 12 tapers toward the distalmost apex 46 at an
angle of
between about 1 and 20 degrees, preferably 2 and 10 degrees, and still more
preferably at approximately 3 to 5 degrees.
Figure 2 shows the distal side face 50 of the implant oriented in a
forwardmost direction, with the mesial side face 51 (Figure 3) having
substantially
the mirror construction, and the lingual and buccal implant side faces
identified by
reference numerals 52 and 54, respectively. As seen best in Figure 2, the non-
porous coronal surface 42 of the implant body 12 adjacent to the end surface
38 is
provided with a smooth polished texture which minimizes the possibility that
bacteria could be trapped thereabouts. Preferably, the smooth coronal surface
42
extends in the axial direction a distance of approximately 2 to 4 mm. The
distalmost
three-quarters of the implant body 12 which functions as a bone engaging
surface is
provided with the porous coated surface 40 formed by spray coating the implant
with titanium beads. The porous surface 40 of the bone-engaging region of the
implant body 12 may be in the form of a coating comprised of discrete titanium
beads or particles adhered to a remainder of the implant body 12 into which
the
patient's bone tissues 20 and/or 22 may grow. Other porous coatings and/or
constructs may also be used including porous coatings formed by mechanical
abrasion, or a roughened portion of the implant. Preferably, the porous
surface 40
16
CA 02454204 2003-12-23
is formed having a porosity of from about 10 to 800 microns, with the porous
coated
surface having a porosity similar to that of the Endopore~ implants. Differing
porosities are, however, also possible.
As shown best in Figures 2 and 4, the porous coated surface 40 is
characterized by two axially elongated and proximally extending regions
60a,60b
which extend on each of the opposing distal and mesial sides SO,Sl of the
implant
body 12. In each of the regions 60a,60b, the porous coated surface 40 extends
an
increased distance from the distal apex 46 of the implant body 12 towards its
proximal end surface 38 relative to the portions of the porous coated surface
40
along the lingual and buccal implant sides S2,S4. More preferably, the regions
60a,60b extend approximately 2 to 4 mm closer to the end surface 38 of the
implant
body 12 than the coated surface 40 at the buccal and lingual surfaces S2,S4.
As
seen best in Figure 2, in this manner the upper edge 62 of the porous coated
surface
40 which is spaced closest to the implant body end 38 rides upwardly into the
collar
42 along both the distal and mesial sides SO,S1 of the implant 10. Most
preferably,
the proximal-most edge 62 of the porous surface 40 is formed so as to follow a
predetermined profile, as for example the profile of the crestal ridge of
typical
healthy bone tissue 20 and/or alveolar tissues 22 at the site of intended
implant use.
It is to be appreciated that providing the proximal edge 62 of the porous
coated portion 40 of the implant body 12 with a profile which generally
follows the
profile of healthy bone tissue advantageously stimulates bone tissues to
engage the
dental implant body 12 in the identical manner that occurs with natural teeth.
As
such, sites of higher bone tissue 20,22 engagement occur along the mesial and
distal
side portions S1,S0 of the implant body 12. It is to be appreciated that the
sites of
m
CA 02454204 2003-12-23
higher bone tissue 20,22 engagement in turn maintain the shape and crestal
outline
of interdental bone and overlying gum tissues 66 (Figure 4) at the optimum
spacing
from the cementoenamel junction of the tooth, eliminating "black triangles"
between
implants 10 and adjacent natural teeth. In addition, the higher sites of bone
tissue
20,22 attachment reduce the likelihood of alveolar bone tissue 22 loss which
may
otherwise result in the exposure of the collar 42 of the implant body 12.
To ensure proper orientation of the implant body 12 seated within the bore
48, the proximal surface 38 of the body 12 may further include visual indicia
68 (see
stamped letters D and M) or other striations, grooves, guides or posts used to
assist
in orienting the implant body 12 following its placement in the patient's jaw
24.
In installation of the implant 10, a frustoconical shaped bore 48 is formed in
the patient's jaw 24 at the site of intended tooth replacement, as for example
is
shown in Figure 5. The bore 48 is formed to a depth selected so that the
cementoenamel junction of the prosthesis 18 and collar 42 locates at a
position
corresponding to that of a natural healthy tooth. The implant body 12 is then
inserted in the bore 48 in a press fit arrangement and the indicia 68 is used
to
facilitate in orienting the implant body 12 so that the distal and mesial
sides 50,51
assume the desired orientation in the patient's jaw bone. Following placement
of the
implant, a temporary cap (not shown) is secured over the hexagonal mount 44
and
proximal end 38 and the implant body 12 is sutured over and allowed to heal
for
several weeks.
After a passage of time which is selected to permit the bone tissue 20,22
ingrowth into the bone engaging porous coated surface 40, so as to
sufficiently
CA 02454204 2003-12-23
anchor the implant body, the temporary cap is removed. The abutment assembly
14
is then secured to the implant body 12 by means of the threaded engagement of
the
screw 16 within the bore 36. The prosthesis 18 is thereafter positioned over
and
affixed over the abutment 14 by a suitable dental cement.
The implant body 12 is constructed such that it may be placed in a press-fit
manner with the raised regions 60a,60b of the porous portions 40 oriented in a
distal/mesial alignment, and the buccal and lingual sides 54,52 of the
positioned
implant body 10 characterized by the porous coated regions of a comparably
shorter
length. In an optimum construction, the implant body 12 is constructed such
that:
~ The proximal edge 62 of the porous coating 40 is curved or follows a contour
whereby the buccal/lingual side portions 54,52 are more apical relative to the
mesialldistal side portions 50,51 by 2 to 4 mm.
~ The implant body 12 is adapted to be "press fitted" in the patient's jaw.
~ The smooth collar 42 of the body 12 is at least 1.5-2 mm wide.
The implant can be offered in the same diameters and lengths as the
Endopore~
implants.
Although Figures 1 to 4 illustrates the implant body 12 as having a bone
engaging porous coated surface 40 which includes elongated regions 60a, 60b
extending towards the proximal end surface 38 and into the collar 42 along the
distal
and mesial sides 50,51 of the positioned implant 10, the invention is not so
limited.
It is to be appreciated that in an alternate configuration, the proximal edge
62 of
bone engaging porous surface 40 of the implant body 12 could include only one
19
CA 02454204 2003-12-23
elongated porous coated region extending into the collar 42 along only one of
the
distal side 50 or mesial side 52 of the implant. In an alternate construction,
the
porous surface 40 could have elongated regions extending proximally along the
lingual and/or buccal sides 52,54 of the implant body 12.
Although Figures 1 to 4 illustrate the implant body 12 as having a generally
flat proximal end surface 38, the invention is not so limited. Reference may
be had
to Figure 6 which shows an alternate implant body construction in which like
reference numerals are used to identify like components. In Figure 6, the
proximal
end 38 of the implant body 12 is contoured so as to generally follow the same
contour as the proximal edge 62 of the bone engaging porous surface 40. It is
to be
appreciated that with the contoured proximal end surface 38, a modified
abutment
assembly is provided which is adapted to substantially mate with the contoured
end
38 in assembly of the implant 10. In the construction shown, the non-porous
coronal surface 42 follows the uppermost edge 62 of the porous coated surface
40 as
a smooth band having a substantially constant width, which most preferably is
selected at about 1.5 to 2.5 mm in width. It is to be appreciated, however,
that a
coronal surface 42 could also be provided which narrows in width at each
raised
region 60a,60b of the porous surface 40.
Although Figures 1 to 5 illustrate the implant 10 as having a tapered implant
body 12, the invention is not so limited. It is to be appreciated that the
present
invention is also operable with an implant body 12 which, for example, could
be
parallel sided or for that matter which could include a combination of
parallel sided
and tapered portions, without departing from the spirit and scope of the
present
invention.
Zo
___ ___._~ ~~~~~ ,~ .,.~.r~"~-~.~.~: ..~~"~~h.~".,...~.~..~n_~______
_....__~~._.___ .._ _.__. _______________ ._.
CA 02454204 2003-12-23
Reference is made to Figure 7 which illustrates a further embodiment of the
invention wherein like reference numerals are used to identify like
components. In
Figure 7, the dental implant body 12 is cylindrical in shape. The body 12
includes a
distalmost bone engaging portion 140 which is provided with external helical
threads
80 as the means by which bone engagement and anchorage of the implant body 12
is
achieved. As with the porous coating 40 of the implant body 12 shown in Figure
I ,
the helical threads 80 of the implant body 12 of Figure S extend from a
distalmost
apex 40 upwardly to a proximal-most edge 62. The external threads 80 extend
axially in the proximal direction further along the distal side (SO) and
mesial side
(not shown) of the implant body 12 than the regions of the external threads in
the
lingual and buccal S2,S4 side portions. As with the raised porous region
60a,60b,
the elongated threaded region 60a shown in Figure S thus extends closer to the
proximal end surface 38 of the implant body and further proximally into the
smooth
collar 42 than the threads 80 of the implant body I2 along its lingual and
buccal
sides 52,54.
In Figure 7, the implant body 12 is similarly provided with a hexagonal or
other polygonally shaped mount 44. In addition to serving as a mount for the
abutment assembly 14, the mount 44 advantageously may be used to assist in
theadedly inserting the implant body 12 into the patient's jaw bone. If
desired, the
external hexagonal mount SO on the implant body 12 could be provided with
grooves, slots or other visual indicia which facilitate the positioning of the
implant
body 12 so that the elongated threaded regions 60 are in alignment with the
mesial
and distal surfaces of the patient's immediately adjacent teeth.
21
CA 02454204 2003-12-23
Figure 8 illustrates a further embodiment of the invention in which like
reference numerals are used to identify like components. In Figure 8, the
implant
body 12 includes a conventional externally threaded bone engaging portion 140.
In
the embodiment as shown, the implant body 12 further includes an enlarged
flared
non-porous upper portion 142. A threaded bore (not shown) is formed in the
proximal end surface 38 of the implant body 12 and is adapted for the
mechanical
coupling of an abutment assembly and prosthesis (not shown) thereto in a
conventional manner. To provide an enhanced bone engaging surface, a
bioreactive
coating 144 is provided over the externally threaded portion 140 and part of
upper
portion 142 of the implant body 12. The coating 144 extends further towards
the
proximal surface 38 along the lingual side 52 and buccal side 54 of the
implant body
as regions 160a,160b. It is to be appreciated, however, that the coating 144
could
equally extend further along the mesial and/or distal sides of the implant in
the
similar manner as the porous coated surface 40 shown in Figure 1. The
bioreactive
coating 144 which is selected to facilitate the engagement of bone tissues
with the
implant and suitable coatings include without restriction, hydroxyapatite
coatings
and the like. As with the embodiments shown in Figures 1 to 5, the proximal-
most
edge 162 of the bioreactive coating 144 has a contour which is selected to
follow a
predetermined contour, as for example, which follows the profile of the
crestal
surface of either a healthy jaw bone or the patient's own jaw bone.
Figure 9 illustrates another embodiment of the invention in which like
reference numerals are used to identify like components. In Figure 9, the
implant
body 12 tapers from the distal apex end 46 of the implant body to the proximal
end
38. As with the implant construction shown in Figure 6, the proximal end 38 is
provided with a contoured prof le, which generally follows the same contour as
the
22
CA 02454204 2003-12-23
proximal edge 62 of the bone engaging porous coated surface 40, and the
crestal
surface contour.
As with the implant shown in Figures 1 to 4, the porous surface 40 most
preferably is formed by a coating of discreet titanium of ceramic beads or
particles
which are adhered to the remainder of the implant body. The porous coating is
selected to form a porosity of from between about 10 to 800 microns. Other
manner
of forming the porous coating including mechanical abrasion and/or roughening
are
however also envisioned as possible.
The implant body 12 of Figure 9 includes a textured transition zone 104
which is provided between the porous coated portion 40 of the implant body 12,
and
the smooth coronal surface 42. The transition zone 102 is characterized by a
roughened textured surface which, for example, is preferably formed by laser
abradement, acid etching and/or other mechanical abradement.
The transitional band 102 is provided as a contoured band extending in the
direction of axis A-A1 between about .25 to 3 mm, preferably between about 0.5
and
2.5 mm, and more preferably from about .75 to about 1.5 mm. In a simplified
construction, the transitional band 102 has a substantially constant width and
mimics
the path of the crestal surface contour. It is to be appreciated, however,
that the
band 102 could be provided so as to narrow or increase in width at each of the
raised and/or lower regions on the porous surface 40 without departing in
scope
from the present invention.
z3
CA 02454204 2003-12-23
The coronal surface 42 is formed as non-porous coloured substantially smooth
coated or plated band 104. The coloured coronal band 104 preferably extends
from
the proximal end 38 of the implant body 12 to a lower edge 108 spaced distally
towards the apex end 46. The coloured coronal band 104 has a width in the
direction of axis A-A1 of between about O.S and 2.S mm, and more preferably
about
.7S to 1.2S mm. Although not essential, the lower edge 108 of the band 104
preferably generally follows the contour of the proximal edge 62 surface of
the
porous surface 40.
It has been found that with conventional silver stainless steel and titanium
implants, when a patient experiences partial recession of supporting gum
tissue, the
silver/grey colour of conventional titanium or stainless steel implants shows
through
the translucent gum tissues, tinting the gum tissues a grayish colour. The
silver/grey colouring presents an unnatural and aesthetically displeasing
appearance
to the patient. To overcome these disadvantages, the coloured band 104 is
provided
with colour which is more complementary to the patient's gum tissue colour,
and
which is selected so that when shown through translucent gum tissue, the band
colour does not significantly tint the gum tissue from its natural colour.
More
preferably, the coloured band 104 is provided with a yellow gold or pink gold
colouring. In such a construction, the coloured coronal band portion is formed
by
applying a gold coloured titanium nitride plating or coating, a, yellow gold
or gold
alloy coating or plating, or a pink gold or gold alloy plating or coating to
the implant
body 12 to form the smooth band 104. Other alloys used to create suitable
coloured
platings and coatings will also now become apparent.
24
,~.r ~ .~~"~,~~ _.,~~~,,,." z:,~~,"",~,.~~s"~ ~ ~..,~ ~._. ... ....._ _
_....... .. ~q.~.. ,.".~. . q.. _.. .....
CA 02454204 2003-12-23
Although Figure 9 illustrates the coloured coronal band of the implant body
12 as extending to a contoured lower edge 108, the invention is not so
limited. It is
to be appreciated that the coloured band could be provided along the non-
contoured
proximal edge 38 of each of the implant bodies shown in Figures 1, 5, 7 or 8
and
which extends the lower edge which is either parallel to the contoured edge 38
or
the proximal edge 62 of the bone engaging portion.
While it may be preferred that the implant body 12 include two opposed
elongated coated regions 160a,160b on both the distal/mesial sides 50,51 of
the
implant body 12, the invention is not so limited. If desired, the body 12
could be
modified to include only a single elongated coated region 160a where for
example,
the implant 10 is to be placed in a position interposed between a natural
tooth and a
second implant. In this construction, any proximally extending or elongated
portion
of the coating 144 would be located adjacent to the second implant alone.
Alternately, it is envisioned that the implant having two or more non-opposed
discrete elongated coated regions 160a,160b could be provided for specific
orientation, where the loss of supporting bone height may otherwise occur.
Although Figures 1 to 4 illustrate the implant body 12 as including a
hexagonal mount or projection 44 sized for complementary insertion within the
bore
46 of the abutment assembly 14, the invention is not so limited. Other forms
and
configurations of mounts may also be used and will now become apparent. In a
less
preferred embodiment, the mount may be omitted in its entirety or alternately,
a
complementary form recess could be provided within the implant body 12. The
hexagonal projection 44, however, is believed to represent a preferred
construction
in that it acts to correctly seat the abutment 14, preventing any rotational
movement
_. . _ . _ _ _ ~-~.~.~-..~,,,~ -~...~.~.~.~... w,.~_~ _.____ ...
CA 02454204 2003-12-23
thereof once secured against the implant body surface 38. The hexagonal
projection
44 most preferably is of a standard size so as to provide compatibility with a
variety
of prosthetic systems from a variety of different manufacturers.
Although Figure 1 illustrates the abutment assembly 14 as comprising a
single piece, the invention is not so limited. It is to be appreciated that
the present
invention is suitable for use with a variety of abutment configurations
including,
without restriction, other single, two-piece and multiple-piece abutments.
Although the preferred embodiments of the invention disclose and describe
the bone engaging regions of the implant body 12 as comprising a porous coated
surface, an externally threaded surface or a biochemically coated surface, the
invention is not so limited. It is to be appreciated that other bone engaging
surfaces
could also be used with the present invention, including, without restriction,
the use
of other textured or roughened surfaces which are provided with a contoured
profile
selected to follow the crestal surface of a patient's or other preselected jaw
bone
tissues.
Although the preferred embodiment of the invention describes and illustrates
various preferred aspects of the invention, the invention is not so limited.
Many
modifications and variations will now occur to persons skilled in the art. For
a
definition of the invention, reference may be had to the appended claims.
26