Note: Descriptions are shown in the official language in which they were submitted.
CA 02454890 2004-O1-21
WO 03/014700 PCT/US02/25034
USE OF ISOTOPES TO PROVIDE INFORMATION TO AN ARTICLE
BACKGROUND OF THE INVENTION
A. Field of Invention
The invention relates to the use of isotopes to provide information to an
article.
B. Description of the Prior Art
As a result of technology advances in reproduction techniques, many articles
have become vulnerable to counterfeiting. This problem is pal-ticularly acute
for items
such as credit cards, compact discs, certificates, passports, identification
cards, designer
fashion accessories a-nd clothing. In addition, an even more serious problem
is the
counterfeiting of currency, bank notes and other financial paper. A related
problem is
the unauthorized use of a financial item, such as a credit card, a registered
security or an
identity document.
Many techniques have been developed for labeling articles to prevent
counterfeiting or fraudulent use. Techniques such as holograms on credit cards
and
magnetic coding on various articles have been in use for some time. These
prior art
techniques have been less than fully effective either because the
counterfeiters have
found ways to duplicate the label, or the apparatus for detecting the label
and verifying
its authenticity has been too expensive or cumbersome to be accepted for
widespread
use.
U.S. Patent Nos. 4,742,340 and 4,862,143 teach the use of a y-ray radiation
technique to detect counterfeit articles by labeling the articles with an
enriched isotope
such as iron-57, tin-119 or europium-151. However, this method requires a y-
ray
source and y-ray detector, which are perceived to be too hazardous for general
use.
It is an object of certain embodiments of the present invention to provide a
labeling composition for associating information with an article, which cannot
be easily
CA 02454890 2004-O1-21
WO 03/014700 PCT/US02/25034
2
duplicated.
It is also an object of certain embodiments of the present invention to
provide a
method to obtain information about an article, which is fast, inexpensive arid
simple,
yet provides a high level of security against fraud, counterfeiting or
duplication.
It is also an object of certain embodiments of the present invention to
provide an
article including a label which can be easily read to provide information
about the
article, yet provides a high level of security against counterfeiting, fraud
or duplication.
These and other obj ects of the invention will be apparent from the surmnary
and
detailed description, which follow.
SUMMARY OF THE 1NVENT10N
In a first aspect, the present invention provides a composition for labeling
an
article that includes at least two isotopes of the same element at a
predetermined
abundance ratio.
In a second aspect, the present invention relates to a method for labeling an
article with at least two isotopes of the same element and detecting the
abundances
and/or abundance ratio of the at least two isotopes of the same element to
obtain
information about the article.
In a third aspect, the present invention relates to an article labeled with at
least
two isotopes of the same element to thereby associate information about the
article with
the article by virtue of the isotope-containing label.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 is a schematic view of an NMR spectrometer that is suitable for
identifying or authenticating articles according to the present invention.
Fig. 2 shows a credit card labeled with an isotopic labeling composition in
accordance with the present invention.
CA 02454890 2004-O1-21
WO 03/014700 PCT/US02/25034
3
Fig. 3 a schematic view of an NQR spectrometer that is suitable for
identifying
or authenticating articles according to the present invention.
Fig. 4 is a signal output result of an NQR spectrometer in detecting chlorine-
35
operating in a marginal oscillator mode according to one embodiment of the
invention.
Fig. 5 is another signal output result in a computer printout format of an NQR
spectrometer in detecting chlorine-35 according to one embodiment of the
invention.
Fig. 6 is a signal output result, which is based on an average of 20 pairs of
sweeps of an NQR spectrometer in detecting chlorine-35 according to one
embodiment
of the invention.
Fig. 7 is a signal output result, which is based on an average of 300 pairs of
sweeps of an NQR spectrometer in detecting chlorine-35 according to one
embodiment
of the invention.
Fig. ~ is a signal output result of an NQR spectrometer in detecting chlorine-
37
according to one embodiment of the invention.
Fig. 9 is a signal output result, which is based on an average of 100 pairs of
sweeps of an NQR spectrometer in detecting chlorine 37 according to one
embodiment
of the invention.
Fig. 10 is a signal output result, which is based on an average of 300 pairs
of
sweeps of an I~TQR spectrometer in detecting chlorine-37 according to one
embodiment
of the invention.
Fig. 11 schematically illustrates the configuration and circuit of an NQR
spectrometer which can be used in detecting abundances and/or abundance ratios
according to the invention.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIIVIENTS
In a fast aspect, the present invention relates to an isotopic labeling
composition
for labeling an article for associating information with the axticle. The
composition
CA 02454890 2004-O1-21
WO 03/014700 PCT/US02/25034
4
contains a predetermined amount of two or more isotopes of at least one
element.
Suitable chemical elements for use in the present invention exist in the form
of
two or more isotopes. Each isotope of a particular element typically exists in
nature in
a particular, known abundance relative to other isotopes of the same element.
This so-
y called natural abundance of each pauticular isotope is a well-defined
constant. Nearly
all materials used to manufacture goods are used in a form that has the same
relative
abundances of the various isotopes as occurs in nature. The natural abundances
of
various isotopes can be found in most physical chemistry handbooks.
The present invention is based on the concept that by artificially changing
the
relative abmdances of one or more isotopes of a particular chemical element, a
unique
label based on the particular composition can be created for use in labeling
articles with
information about the article.
The relative abundances of isotopes of a chemical element in a particular
material or composition can be altered by several different methods. One
method
involves enriching the material with one or more isotopes. A second method
involves
extracting one or more isotopes from the material to thereby alter the
relative
abundances of the different isotopes contained in the material. The isotopes
may be
used in elemental form, in the form of a chemical compound or mixtures
thereof.
A specific isotope of a composition can be prepared or isolated by using
conventional methods ef extracting isotopes. Conventional methods for
extracting
isotopes include plasma separation processes (PSP), electro-magnetic
separation,
molecular laser isotope separation (MLIS), atomic vapor laser isotope
separation
(AVLIS), gas centrifugation, gas diffusion and distillation. All of these
methods are
well known to persons skilled in the art. The preferred method to prepare the
isotopes
for use in the present invention is PSP due to the quantities of isotopes that
may be
required for this application.
Although these methods of altering isotope ratios axe widely known, each of
CA 02454890 2004-O1-21
WO 03/014700 PCT/US02/25034
them require specialized equipment that is not easily available to
counterfeiters. This
provides a high degree of security against counterfeiting the label. It should
be noted
that chemical processing does not significantly alter isotope ratios.
Any chemical element having two or more isotopes can be employed in the
present invention. The preferred element should display a good sensitivity
towards the
particular detection method employed. For example, when NMR spectroscopy
(hereafter "NMR") is used as the means for detecting the isotopes, the
relaxation times
Tl and T2 of the isotopes, among many other factors, have to be considered in
order to
select a suitable labeling composition. The isotopes to be detected by NMR
should also
have spins equal to or greater than'/2. When "zero-field NMR," also called
nuclear
quadrupole resonance spectroscopy (hereafter "NQR'), is used to detect the
abundances
andlor abundance ratio of the isotopes in the composition, the isotopes being
detected
should have spins greater than 1/2. Methods of detecting isotopes using NQR
are knowm
to a skilled person in the art. See Abragan, The Principles of Nuclear
Magnetism,
Oxford, 1961, pages 16 and 249; Methods of Experimental Physics, Vol. 3,
Molecular
Physics, Dudley Williams ed., Academic Press, 1962, pages 501-524; and Clark,
Pulsed
Nuclear Resonance Apparatus, Review of Scientific Instruments, Vol. 35, No. 3,
March
1964, pages 316-333, all of which are incorporated by reference herein for
their
disclosures of NQR detection methods and apparatus. "Spin" used herein is a
quantum
parameter of the nuclei of an isotope. For a particular isotope, the spin is
generally a
known value which can be found in a handbook.
The isotopes used in the present invention may be incorporated in the
composition of the invention in a metallic form or as an element of one or
more
compounds. Exemplary elements and compounds that can be incorporated in the
composition of the invention are Re, ReBr3, Re02, ReS2, Cu, CuF2,
Cu(N03)2.3H2O,
CuO, Cu20 Cu2S, CuS, Rb, Rb2CO3, RbF, RbN03, Rb~S04, Sb, Sb205, Sb203, SbCl3,
SbF3, Eu, Eu203, EuCl3, EuF3, EuBr3, In, InF3, InCl3, InBr3, C6H4C12 and
C6H4Br2.
CA 02454890 2004-O1-21
WO 03/014700 PCT/US02/25034
6
Alternatively, the isotopes used in the present invention may be employed in
the
form of alloys, powdered metals or eutectic compounds. Such alloys, powdered
metals
or eutectic compounds can include two or more isotopes of a single element
and/or two
or more elements. At least one element in an alloy, powdered metal or eutectic
compound is present in the form of two or more isotopes, although it is also
possible to
employ two or more isotopes of each of two or more different elements,
particularly if it
is desirable to increase the quantity of information contained in the label.
This
embodiment may be employed to provide a more complex signature by combining
several isotopes and elements into a single composition to thereby increase
the number
of isotopic ratios that can be employed to provide information.
When NMR or NQR is used as a preferred detection method, the composition of
the present invention that is used to label an article preferably comprises an
isotope-
containing compound or material that has the following characteristics:
1. The compound is suitable for isotope ratio modification using one of the
above-mentioned methods or other suitable methods;
2. At least one element of the compound preferably has at least two isotopes
that are present in sufficient quantities to provide a detectable signal;
3. The isotopes of the element in the compound should preferably have high
NMR or NQR resonant frequencies;
4. The compound should preferably produce NMR or NQR signals with narrow
line widths;
5. The compound preferably contains a relatively large amount of the element
to be detected;
6. The compound is preferably used' in one form of crystalline structure
instead
of being a mixture of multiple crystalline forms to avoid multiple resonance
frequencies
and avoid unnecessary reduction of signal strength;
7. The compound should preferably be relatively stable (e.g., not prone to
CA 02454890 2004-O1-21
WO 03/014700 PCT/US02/25034
7
oxidation, or hydrolysis); and
8. The compound should preferably be substantially non-toxic to the users of
the labeled article and/or detection equipment.
In one preferred embodiment, when NQR is used as the preferred detection
method, the most preferred element for use in the composition of the present
invention
is selected from the group consisting of bromine, chlorine, copper and
antimony.
Compounds of bromine, chlorine, copper and antimony can also be used in th.e
compositions of the present invention. For example, Cu20 (having an NQR
frequency
of 20.337 MHz and a line width of 20 I~Hz), CuO, CuS (having NQR frequencies
of
13.7 and 14.7 MHz, and line widths of 50 KHz), Cu2S, antimony metal powder
(having
NQR frequencies of 21.506 and 19.580 MHz and line widths about 10 KHz at room
temperature), SbZO3 (such as antimony ores Senarmontite or Valentinite),
paradichlorobenzene, and paradibromobenzene can be used in the composition of
the
present invention. These compounds and/or metals can be used alone, with a
carrier, or
encapsulated in a substantially inert material. The carrier used in the
composition may
be any inert material to facilitate the composition being incorporated into an
article to
be labeled. An inert material is a material that does not react with the
compound
containing the isotopes. In addition, the carrier is preferably a material
that does not
contribute to or interfere with the signal of the isotopes being detected and
does not
adversely affect the detection of the isotope abundance and/or abundance
ratios, other
than the normal effect of diluting the isotopes with an inert material. The
carrier may
be homogeneously or heterogeneously mixed with the element or compound, or be
in
contact with the element or compound. Preferably, the carrier used for an NQR
detection method is a solid. For example, a compound containing the desired
isotopes
may be mixed or in contact with an adhesive material to form the composition
of the
present invention to facilitate the incorporation of the composition into an
article being
labeled.
CA 02454890 2004-O1-21
WO 03/014700 PCT/US02/25034
The isotopic label should be incorporated into the object labeled in such a
way
that counterfeiters cannot easily remove the label and insert the removed
label into
other counterfeit objects.
In another embodiment, when an NMR spectrometer is used to detect the
isotope abundances or abundance ratios, the preferred elements for use in the
compositions ofthe present invention include tin, antimony and indium. The
most
preferred elements for use in the compositions of the present invention
include tin and
indium. Tin and indium may offer several advantages when NMR is used as the
preferred detection method. First, isotopes of these elements are difficult to
manufacture (in large enough quantities) except via PSP, which is a process
that can
only be performed at a few locations that have suitable equipment and
experience. This
provides a high degree of security since the isotopes needed for the labeling
composition will be difficult to manufacture or obtain and the high startup
cost of
building and operating a PSP will serve to further deter would be
cowterfeiters.
Further, certain isotopes of these elements provide excellent detection
signals in the
environments in which they are to be employed. This can be used to ensure the
accuracy of the detection method, simplify the detection apparatus and permit
a greater
number of possible isotope combinations for use as labeling compositions.
Another
advantage of tin, and indium is that these isotopes axe non-toxic in the
environment in
which they will be used and at the levels required to provide a suitable
labeling
composition. Furthermore, normal chemical and physical tests cannot be used to
distinguish among certain isotopes of these materials making it even harder to
duplicate
these labeling compositions. The preferred isotopes for use in compositions of
the
present invention for use with NMR detection include tin-115, tin-117, tin-
119, indium-
113, and indium-115.
When NMR is used as the preferred detecting method, the isotopic labeling
composition may also include a suitable carrier. The caxrier used in the
present
CA 02454890 2004-O1-21
WO 03/014700 PCT/US02/25034
9
invention is preferably a material that can disperse or dissolve one or more
isotopes.
The caaTier used in the composition may be any inert material (a material does
not react
with the compound containing the isotopes) to facilitate the composition being
incorporated into an article to be labeled. In addition, the carrier should
not adversely
affect the detection of the isotopes in the composition. The caiTier may be a
solid with
a micro-structural mobility, which dissolves one or more isotopes to provide a
substantially homogenous product. Alternatively, the carrier is a liquid,
which
dissolves one or more of the isotopes to provide a substantially homogeneous
solution.
Suitable liquids include solvents, water and alcohols. It is preferred to use
two isotopes
in a homogeneous mixture since such a mixture is more difficult to
counterfeit.
The solid with a micro-structural mobility may include plastics, rubbers,
oils,
waxes and mixtures thereof. Preferably the solid is natural or synthetic
rubber. Persons
skilled in the art know the concept of micro-structural mobility as applied to
at least
plastics and rubber. Micro-structural mobility is desirable in the labeling
compositions
of the present invention because micro-structural mobility provides NMR
measurements with nasTOwer line widths, which are more consistently indicative
of the
true isotopic abundances in the article than would be NMR measurements of a
simple
solid. This provides a higher degree of accuracy in measurements using the
labeling
compositions of the invention with the asCociated advantages as discussed
above.
When NMR is used to measure the abundances and/or abundance ratios of the
isotopes in the labeling compositions, carriers allowing micro-structural
mobility may
improve the detectability of the isotopes. The isotopes used in the labeling
compositions of the invention may preferably be dissolved in a liquid such as
a solvent
when the isotopes are in soluble forms such as InCl3. Alternatively, an
isotope is
2~ retained or captured in a clathrate to achieve a good mobility. Clathrates
are molecules
with hollow spaces in which other atoms can be trapped. Therefore, isotopes
trapped in
clathrates may provide narrow easily detectable resonance when they are,
detected using
CA 02454890 2004-O1-21
WO 03/014700 PCT/US02/25034
a detector such as an NMR instrument. Furthermore, an isotope can be retained
or
captured in a bulkyball to achieve a good mobility. A bullcyball is a well-
known,
hollow, giant molecule comprising mainly carbon. For example, one type of
bulkyball
is C6o.
Other methods can also be used to detect isotope abundances and/or abundance
ratios in the labeling composition of the invention. These methods include,
for
example, laser ablatioumagnetic spectroscopy, infrared (hereafter "IR")
spectroscopy,
and microwave spectroscopy using a laser ablation/magnetic spectrometer, an IR
spectrometer and a microwave spectrometer, respectively.
10 There are a variety of methods for associating information to an article
using the
isotopic composition in accordance with the present invention. One method
involves
providing an amount of a particular isotope in the composition, which amount
is
different from the amount of that isotope which would be present in the
composition in
its natural abundance. The enrichment or depletion of one or more of a
plurality of
isotopes of a particular element in a composition to thereby alter the
relative
abundances of the isotopes from their natural abundances can be employed to
provide a
unique composition for providing information about an article. In addition,
bar coding
may be constructed based on patches of at least two different compositions
having
different ratios of isotope abundances.
In a prefeiTed embodiment, the labeling composition contains isotopes that
have
abundances and/or abundance ratios) that are detectably different from the
natural
abundances and/or abundance ratios) of the respective isotopes. "Detectably
different"
abundances and/or abundance ratios means the difference between the first
abundances
and/or abundance ratios of a labeling composition of the invention and the
respective
second abundances and/or abundance ratios being detected is larger than the
experimental error of a particular detection method that is capable of
detecting the
isotope abundances and/or abundance ratios in the labeling composition.
CA 02454890 2004-O1-21
WO 03/014700 PCT/US02/25034
11
In another embodiment of the invention, specific ratios of two or more
isatopes
of a particular element can be employed to provide unique labeling
compositions,
which can be used to provide information about an article. It is also possible
to use
ratios of isotopes of two or more elements to provide additional variations in
the
compositions, which can be used or to provide additional information about the
article
or a higher level of security by making such compositions even more difficult
to
duplicate.
The article to be identified or authenticated is typically an important or
valuable item. For example, the article may be a banknote, currency, a credit
card, a.n
I O identification card, a passport, a ticket, a certificate, etc.
Alternatively, the article may
be anything for which the association of information with the article is
important, for
example, luxury goods, designer goods, currencies, charge cards, debit cards,
identification documents, passports, licenses, negotiable instruments,
tickets, collector's
items, precious metals, jewels, art worlcs, rare goods, ingestible products,
stock
1 S certificates, medical devices and similar products.
The ratio between the amount of the one or more isotopes and the amount of
carrier can vary greatly depending on the nature of the carrier and the
isotopes. Other
factors, such as the cost of the isotope, the nature of the article to be
labeled, the
intended detection technique and the sensitivity of the detection technique,
may affect
20 the selection of the ratio of carrier to isotopes. A person skilled in the
art can determine
the appropriate ratio for a particular application.
To associate certain information with a particular article and/or authenticate
a
particular article, an article in accordance with the present invention may be
labeled
with a labeling composition of the present invention. The article can be
labeled with
25 the isotope composition of the present invention in a variety of different
ways. For
example, a labeling composition can be printed on the surface of the article,
embedded
inside tile article, or dispersed in the article. The labeling composition can
be located
CA 02454890 2004-O1-21
WO 03/014700 PCT/US02/25034
12
throughout the article or located in a selected portion of the article.
Suitable printing processes for labeling an article with the isotopic labeling
composition of the present invention are known to persons skilled in the art.
In this
embodiment, the isotopic labeling composition can be dispersed or dissolved in
a
printing ink or a suitable carrier for printing the composition onto the
surface of an
article. In this manner, the isotopic labeling composition can be included in
the inks
used to print currency, bank notes, and other important documents.
The isotopic labeling composition may also be embedded into an article. This
can be accomplished by mixing the labeling composition with the article,
encapsulating
the labeling composition and impregnating the capsules into the article. Such
capsules
are preferably sufficiently strong to survive normal use and handling of the
article, in
order to prevent the isotopes from being separated from the labeled article or
from
migrating to other locations in the article to thereby upset the distribution
of the
isotopes in the labeling composition.
In a particular embodiment, when an NMR spectrometer is used as the detection
apparatus, the isotopic labeling composition can be impregnated into the
article directly
in the form of solid particles, solid strands or liquid droplets. Preferably,
such an
impregnation process locates the isotopic labeling composition irz a material
having
micro-structural mobility as discussed above. Thus, the isotopes can be
dispersed in
solid pauticles or strands of a material such as rubber, which has micro-
structural
mobility.
Generally, it is preferable to spread the labeling composition over the entire
article if possible or practical to make the counterfeiting even more
difficult. However,
in some situations or out of practicality, it may be sufficient to label only
a small
section of the az-ticle with the labeling composition in order to save costs
and simplify
the labeling and detection processes. The remaining portion of the article
need not be
labeled.
CA 02454890 2004-O1-21
WO 03/014700 PCT/US02/25034
13
If it is desirable to identify the source or origin of the article, a
measurement of
the ratio of the concentrations of two different isotopes can be taken and
cross-
referenced against a key, which indicates the source or origin of the
composition based
on the ratio of the isotopes. If desirable, this can be used as a check in
addition to a
printed indication of source or origin on the article to prevent
counterfeiting of valuable
or rare goods. Other information about the goods can be provided by the
isotopic
labeling composition by, for example, encoding a message in the article, or
encoding a
personal identification.
To provide additional security or to increase the number of possible labeling
compositions, a combination of three or more isotopes can be employed. Using
three
isotopes, it is possible to generate two independently different abundance
ratios that can
be employed for conveying detailed information about the labeled article.
Using four
isotopes, it is possible to generate three independently different ratios that
can be
employed for conveying information about the labeled article. It is also
possible to
combine different combinations of at least two isotopes from at least two
different
elements in one labeling composition to convey even more information.
In an alternative embodiment, a combination of two different isotopes can be
employed using two or more different labels having different ratios of the two
isotopes.
In this manner a type of bar coding effect can be achieved with each bar being
made up
of a different combination of the two different isotopes in a particular ratio
relative to
one another, respectively. This provides a means for conveying large amounts
of
information about the labeled article or for generating a large number of
unique
identifiers using only two different isotopes.
An isotopic label may comprise one or more isotopes, which may be arranged in
a suitable pattern to incorporate information about the article. The label can
be
identified by one or both of its pattern and its isotopic composition. A
suitable pattern
may be achieved by, for example, embedding the labeling composition in the
article in a
CA 02454890 2004-O1-21
WO 03/014700 PCT/US02/25034
14
predetermined pattern or by printing the labeling composition on a surface of
the
article. The printing and embedding processes used for provision of the label
are as
described above. Exemplary patterns include, but are not limited to, bar
codes, graphs,
numbers, symbols and randomized arrays of dots, lines, shapes, etc.
The label can be either visible or invisible to the human eye. The isotope
composition information contained in the label can be detected by the methods
described above using the apparatus described below. The information encoded
in the
pattern of the label can be retrieved by scanning, imaging or other
appropriate methods
using one of the detection methods described above. The retrieved infomnation
can be
processed by a computer to identify the article or authenticate the article
using a
database, well 1V11oWn algorithms, predetermined coding schemes or the like.
The isotope abundances and/or abundance ratios in the composition may be
measured using any suitable apparatus, which is capable of providing
information
regarding the relative abundance of at least two different isotopes of the
same chemical
element present in the composition. In a simple embodiment, if the measured
abundance of at least one isotope matches a predetermined level, this can be
used to
determine specific information about the article or the article can be
authenticated or
identified.
The isotope detection apparatus useful in the present invention typically
employs a non-ionizing radiation source, which emits a radiation which does
not
chemically alter the isotopes being detected, and a detector, which can detect
an output
signal. The output signal results from the radiation interacting with one or
more
isotopes in the isotopic labeling composition. Suitable detection apparatus
include, but
are not limited to, a laser ablation/magnetic spectrometer, an infrared
spectrometer, a
microwave spectrometer, and NQR spectrometer and an NMR spectrometer.
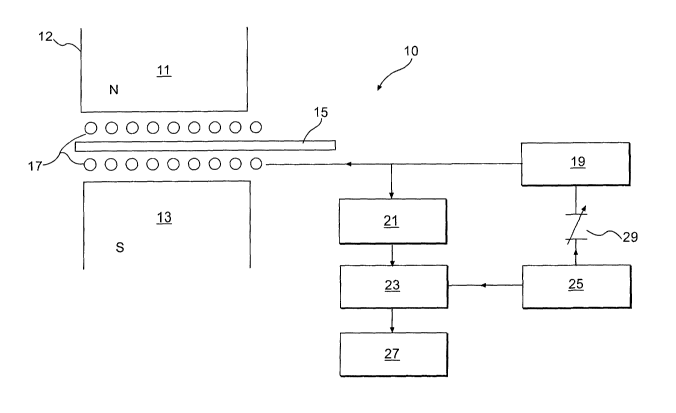
An embodiment of a suitable apparatus for use in determining isotopic
abundances is depicted in the drawing. An NMR spectrometer 10 for suitable for
use as
CA 02454890 2004-O1-21
WO 03/014700 PCT/US02/25034
a detection apparatus in the method of the present invention is illustrated in
Fig 1.
Magnet 12 creates a magnetic field between its north pole 11 and south pole
13. One or
more excitation coils 17 are located in the magnetic field. Article 15 is
placed in the
spectrometer 10 as shown. Oscillator 19 sends a pulse to excitation coil 17,
which then
5 excites the nuclei of the isotopes of the labeling composition in article 15
to their
excited states. After the pulse, the nuclei return to a lower energy state and
emit a
signal, which is detected by excitation coil 17 and sent to a detector 21. The
output
from detector 21 is then sent to synchronous detector 23. This process of
excitation and
relaxation of the nuclei is controlled by a sweep generator 25, which controls
r 0 synchronous detector 23 and also controls oscillator 19 via a frequency
varying device
29. Synchronous detector 23 measures the isotope abundances in the labeling
composition and optionally determines if the measured result is consistent
with a
predetermined labeling composition for a genuine article. The result of the
determination is sent to a processor 27 to provide information about the
article. In
15 order to optimize the NMR spectrometer 10, a number of factors, such as Tl
and T2 of
the isotopes, number of isotope nuclei per unit volume, magnetic field
strength and
uniformity, the drive electronics and its pulse sequence and amplitude, the
data
acquisition time and processing, the coil configuration and sample handling
arrangement, the robustness or error rate of the accept-reject decision
process, and cost
of production machines, etc, have to be considered. The NMR spectrometer is
advantageous in measuring certain isotope abundances and/or abundance
ratio(s),
because it provides a favorable signal-to-noise ratio, especially when the
labeling
composition is in liquid form. If the labeling composition is a solid at room
temperature, the labeling composition can be heated to an elevated temperature
to
enhance microstructural mobility and thereby improve the signal-to-noise
ratio. Most
commercial NMR spectrometers include their own heating and cooling systems for
this
purpose. Alternatively, an NMR spectrometer with a solid-state probe can be
used to
CA 02454890 2004-O1-21
WO 03/014700 PCT/US02/25034
16
detect isotope concentrations in solid labeling compositions. The solid-state
probe
typically involves using a magic angle spinning apparatus during the taking of
the
measurement.
An article 15 labeled in accordance with the present invention is shown in
Fig.
2. Section 31 of labeled article 15 preferably contains the isotopic labeling
composition. Section 33 of labeled at-ticle 15 need not contain any isotope
composition
or it may contain a reference composition. The article 15 may contain a
reference
composition, which makes up section 33 of labeled article 15 that can be used
to
establish the baseline for a particular measurement apparatus. Preferably, a
reference
composition including the isotopes in the naturally occurring abundance ratio
is
employed. The article 15 may also contain information on a magnetic strip 35
or in any
other suitable form.
Another embodiment of a suitable apparatus for use in determining isotopic
abundances is depicted in Fig. 3. An NQR spectrometer 40 operating in a pulse
mode
suitable to be used as a detection apparatus in the method of the present
invention is
illustrated in Fig 3. One or more resonating coils 42 are located near article
15 to be
tested. Article 15 is placed in the spectrometer 40 as shown. Oscillator 52
sends a
signal and/or pulse to resonating coil 42, which then excites the nuclei of
the isotopes
of the labeling composition in article 15 and causes the nuclei to resonate.
After the
signal and/or pulse, the nuclei continue to resonate, which is detected by
resonating coil
42 and sent to a detector 44. The output from detector 44 is then sent to
synchronous
detector 46. This process of excitation and resonation of the nuclei is
controlled by a
sweep generator 50, which controls synchronous detector 46 and also controls
oscillator
52 via a frequency varying device 54. Synchronous detector 46 measures the
isotope
abunda_nces and/or abundance ratios) in the labeling composition and
optionally
determines if the measured result is consistent with a predetermined labeling
composition for a genuine article. The result of the determination is sent to
a processor
CA 02454890 2004-O1-21
WO 03/014700 PCT/US02/25034
17
48 to provide information about the article. In order to optimize the NQR
spectrometer
40, a number of factors, such as T1 and T2 of the isotopes, number of isotope
nuclei per
unit volume and uniformity, the drive electronics and its pulse sequence and
amplitude,
the data acquisition time and processing, the coil configuration and sample
handling
aiTangement, the robustness or error rate of the accept-reject decision
process, and cost
of production machines, etc, have to be considered.
In this embodiment, the NQR spectrometer 40 is operated in a pulse mode to
detect the abundances and/or abundance ratios) of the isotopes used in the
isotope
labeling composition of the present invention. The resonating coil 42 applies
a strong
pulse at the resonant frequency of the isotope nuclei for a short time
interval. Then as
soon as the pulse ends, a sensitive detector 44 is turned on to observe the
decay of the
resonance of the nuclei through the resonating coil 42. This pulse method has
many
elaborations and is capable of measuring many of the nuclei properties in
addition to
the simple detection of isotope abundances and/or abundance ratio(s). In
addition, the
pulse method allows parameters to be adjusted for maximum sensitivity when the
nuclear properties are known. The pulse and receive sequence typically takes
less than
a millisecond. To achieve high sensitivity it is usual to average the results
of many
pulse sequences, thus averaging out the noise and allowing the signal to build
up.
In another embodiment, the NQR spectrometer 40 can be operated in a marginal
oscillator mode that measures the nuclei quadrupole resonance of isotopes to
detect the
isotope abundances or abundance ratios) of the composition of the present
invention.
When NQR spectrometer 40 is used as a marginal oscillator, the resonating coil
42
emits a signal with a varied frequency (a signal with a sweeping frequency)
instead of a
pulse. When the frequency of the signal is swept past the NQR resoi:ant
frequency of
the isotopes, the signal causes the nuclei of the isotopes to resonate and the
NQR
resonance of the isotopes absorbs some power from the oscillator 52 and hence
decreases the amplitude of the oscillation to result in an output signal.
CA 02454890 2004-O1-21
WO 03/014700 PCT/US02/25034
18
In a third embodiment, the NQR spectrometer 40 may also be operated in a
superregenerative detector mode. In this embodiment, the circuit in oscillator
52 is so
arranged that the oscillation signal generated by oscillator 52 starts and
stops many
times each second, e.g., 50,000 times per second. The circuit of the
oscillator is
arranged so that the oscillator 52 is turned "ON" only by changing the gain to
where it
is sufficieizt to oscillate. No starting impulse is supplied to the oscillator
so the
oscillations actually grow from the thermal agitation noise in the circuit
components. If
there is a signal present during this starting interval stronger than the
thermal noise,
then the oscillations grow quicker and result in an output signal. When used
for NQR
detection, the nuclei are resonantly excited during the oscillating period and
when the
oscillations turn off the nuclei continue to resonate and hence induce a
signal during the
next start up interval causing quicker growth and an output signal.
The circuits for the NQR spectrometer 40 to be operated in the marginal
oscillator and the superregenerative detector modes may be similar and often
small
changes can be made that change the mode of operation from one to the other.
In
addition, when the NQR spectrometer 40 is operated in the marginal oscillator
and the
superregenerative detector modes, not all of the components shown in Figure 3
are
necessary. An exemplary configuration and circuitry for another NQR
spectrometer 60
operating in a marginal oscillator mode that can be used to detect isotope
abundances
andlor abundance ratios is illustrated in Figure 11. NQR spectrometer 60 is
used in
detecting abundances and/or abundance ratios of chlorine-35 and chlorine-37 in
the
following example. In Figure 11, a testing sample 62 is placed within an
excitation coil
64. The frequency of excitation coil 62 is adjusted by a main frequency tuning
device
66. NQR spectrometer 60 further includes a sweep voltage input 68, a feedback
circuit
84, a phase adjustment circuit 82, an RF monitor 78 and an audio output 80.
When the
NQR spectrometer 60 in Figure 11 is operated in a superregenerative detector
mode, a
small change is made to allow an external oscillator at 10 KHZ to turn the RF
CA 02454890 2004-O1-21
WO 03/014700 PCT/US02/25034
19
oscillations on and off.
Generally, to determine if a particular a~.-ticle is authentic or genuine, or
to
determine the inforrr~ation associating with the particular article, the
article is subjected
to isotope abundance and/or abundance ratio measurement using one of the
methods
described above. If the isotope abundances and/or isotope abundance ratios
fall within
a predetermined range or are equal to a predetermined value, then the article
is
considered to be authentic or genuine. Otherwise, the article may be
considered
counterfeit or non-authentic.
The invention will be further illustrated by the following non-limiting
example.
EXAMPLE
Detection of Isotopes Using an NQR Spectrometer
An NQR spectrometer was built according to the configuration and circuitry
illustrated in Fig. 11. For this example, the NQR spectrometer was operated in
a
marginal oscillator mode. However, this NQR spectrometer can be operated in a
superregenerative mode with a minor adjustment. The material to be detected in
this
example was paradichlorobenzene (C6H4C12). In the experiment, 150 grams of
paradichlorobenzene in a bottle was kept at the center of a 12 inch cubic box
of the
NQR spectrometer. The bottle was surrounded by the main resonating coil of the
spectrometer. The interior of the box was lined with copper foil to shield out
any
potential radio frequency interference. In addition, the spectrometer further
included a
magnetic field generating coil that generated a magnetic field of about 10
gauss at the
location of the paradichlorobenzene being detected when a current passing
through the
magnetic field coil was turned on. Generally, the current supply to this
magnetic field
generating coil was turned on and off by a 6 Hz square wave. When the current
was on,
the 10 gauss magnetic field squashed out the resonance so that the resonating
coil
CA 02454890 2004-O1-21
WO 03/014700 PCT/US02/25034
would not be able to detect the NQR. Accordingly, in one embodiment, the net
output
signal of the NQR spectrometer was, therefore, the difference between an
output signal
detected by the resonating coil with the current supply to the magnetic field
generating
coil turned off (therefore, zero field NQR active), and an output signal
detected by the
5 resonating coil with the current supply turned on (therefore, no NQR
detected). In one
case, the measurement of the output signal of the spectrometer could be
carried out with
the 6Hz square wave current to the magnetic field generating coil being used.
Tl2e
output signal of the spectrometer was monitored by an oscilloscope comlected
to the
audio output 80 shov~m in figure 11.
10 The measurement results with and without use of the 6 Hz square wave
current
are shown in Figure 4. The results were a contorted lissajou plot with the
horizontal
sinusoidally sweeping back and forth at 60 Hz. The vertical axis of the plot
represented
the output from the spectrometer. The same 60 Hz voltage that drove the
horizontal
axis on the oscilloscope also swept the frequency of the spectrometer back and
forth.
15 Accordingly, the horizontal axis of the plot also represented the
spectrometer
frequency. Near the center of the plot in Fig. 4, where the trace was double,
was the
NQR output signal as the NQR spectrometer frequency operating in a marginal
oscillator mode passed through the nuclear quadrupole resonance of the
chlorine-35
isotope: The smoother trace of the plot was the NQR output signal detected by
the
2,0 resonating signal when the 6Hz magnetic field produced by the magnetic
field
generating coil controlled by the 6 Hz square wave current was turned on to
eliminate
the NQR. The zig-zag trace of the plot was the NQR output signal detected by
the
resonating coil when the 6 Hz magnetic field was turned off. The isotope
abundance of
a particular isotope could be correlated with the strength of the NQR output
signal.
One can also infer from Figure 4 that a detection time in milliseconds to
detect
abundances and/or abundance ratio of isotopes, is realistic.
Figure 5 is a computer printout of the chlorine-35 resonance observed by the
CA 02454890 2004-O1-21
WO 03/014700 PCT/US02/25034
21
NQR spectrometer operating in a marginal oscillator mode. This figure was
produced
by connecting the output of the NQR spectrometer to an A/D input of a
computer. The
horizontal axis of the plot in Figure 5 again represented a frequency sweep of
the NQR
spectrometer. In this particular figure, the frequency swept up for the first
half, and
down for the second half of the plot trace in Figure 5. The time for a full
sweep was
1/60th of a second. Actually the sweeps were done in pairs, one with no
mag~.zetic field
and the other with the 10 gauss magnetic field. The vertical axis of Figure 5
represented the difference between the two sweeps and thus represented the
difference
between the two traces of the plot shown in Figure 4, the difference being the
true NQR
output signal without extraneous instrumentation artifacts.
Figure 6 is similar to Figure 5 except that 20 pairs of sweeps have been
carried
out by the NQR spectrometer and the plot in Figure 6 was the average of these
20 pairs.
The noise of the averaged output was clearly reduced. In addition, a second
run in
which the NQR spectrometer frequency was offset by exactly 1 KHz was also
plotted in
Figure 6. This offset of 1 I~Hz was comparable with the NQR output signal
width and
showed that the NQR output signal line width of this material was about 1
I~HHz.
Figure 7 is similar to Figures 5 and 6 except that 300 pairs of sweeps have
been
averaged in the plot of Figure 7 and the NQR spectrometer parameters have been
slightly changed to maximize the visibility of a phenomena referred to in the
literature
as "wiggles." Here one observed that as the NQR spectrometer frequency moved
from
left to right, that the NQR was excited and then as the NQR spectrometer
frequency
moved past the NQR frequency of chlorine-35, the NQR spectrometer oscillator
beats
with the decaying resonance of the chlorine-35 nuclei, going in and out of
phase for
several cycles. From the plot of Figure 7, one could see that the NQR output
signal,
when the sweep was rapid, departed from the simple loading model (when the
sweep
was slow) shown in Figures 4-6.
Figures 8-10 are the corresponding figures to Figures 5-7 for measuring the
CA 02454890 2004-O1-21
WO 03/014700 PCT/US02/25034
22
NQR output signals of the other chlorine isotope, chlorine-37. Generally, the
signals
from chlorine-37 were about six times smaller than the signals from chlorine-
35 for this
particular sample, whose isotope abundance ratio was equal to the natural
abundance
ratio, because the natural abundance of chlorine-37 is about one third of
chlorine-35. In
addition the NQR frequency of chlorine-37 is lower and the magnetic moment of
the
nucleus of chlorine-37 is also smaller in comparison with those of chlorine-
35.
Accordingly, the signal for chlorine-37 in Figure 8 was weaker than the
corresponding
signal of chlorine-35 in Figure 5.
From this example, it is apparent that if the same instrumentation were
applied
to a sample in which the ratio of chlorine-37 to chlorine-35 had been enriched
by a
factor of six relative to the natural abundance ratio, the two isotopes would
then give
comparable output signals and the enriched ratio material would be readily
distinguished from the material containing the chlorine isotopes in their
natural
abundance.
It will be apparent to a skilled person that ceutain changes may be made in
carrying out the above method and in the compositions and articles set forth
without
departing from the spirit and scope of the invention, it is intended that all
matter
contained in the above description and shomz in the accompanying drawing shall
be
interpreted as illustrative and not in a limiting sense. The scope of the
invention is to
be determined from the claims appended hereto.