Note: Descriptions are shown in the official language in which they were submitted.
CA 02455548 2004-O1-22
-1
ALKALINE MANGANESE DIOXIDE CELL WITH IMPROVED OPEN
CIRCUIT VOLTAGE
FIELD OF THE INVENTION
The invention relates to alkaline manganese dioxide cells, particularly to the
use of an
oxidizing additive to improve the open circuit voltage of such cells without
adversely
affecting the cell impedance.
BACKGROUND OF THE INVENTION
Primary (non-rechargeable) manganese dioxide-based alkaline cells are well
known and include a positive electrode having manganese dioxide (Mn02) as an
active
material, a negative electrode utilizing zinc as the active material, an
aqueous solution of
potassium hydroxide as electrolyte, and a separator betwc;en the positive and
negative
electrode.
One important parameter that is easily measured by the manufacturer and
consumers is the Open Circuit Voltage (OCV) and the Closed Circuit Voltage
(CCV).
2o The OCV/CCV measurement is strongly dependent on the materials used in the
cell.
Generally, primary alkaline cells with an OCV of greater than 1.60V and a CCV
of
greater than 1.50V are considered good quality cells for certain applications.
However, it
is quite desirable to increase the OCV and expand the cell usage in numerous
devices.
According to practical experience the open circuit voltage of cells having
identical
design depend on how the manganese dioxide cathode material was made. Even
products
of the same manufacturer having an identical product number but made at
different times
can produce different OCV values when used in cells. On the other hand, end
users of
cells prefer cells having largely uniform OCV values.
The primary active material to increase this voltage is the positive
electrode, in
3o this case, manganese dioxide. Numerous approaches have been proposed to
increase the
open circuit voltage (OCV) by employing various additives to the
electrochemical cell or
by producing an electrolytic manganese dioxide with greater potentials.
CA 02455548 2004-O1-22
-2-
Magahed in U.S. Patent 4,397,925 issued August 9~, 1983, describes button
cells
having cathodes, which are extended with high potential oxide. The cathode in
this
situation is a monovalent silver oxide (Ag20) extended with divalent silver
oxide (Ag0),
mercury oxide (Hg0), and/or permanganate compound (IT~IVIn04, LiMn04,
BaMn04...
s etc.). These extended cathodes will show high OCV and impedance values in
addition to
two distinct plateaus of discharge. Magahed addresses the undesirable high
voltage and
high impedance levels by including in the electrolyte a reducing agent from
the group of
soluble complex hydrides. The reducing agent signifi<;antly lowers cell OCV
and
impedance that in turn offers a single voltage plateau discharge.
io Dhanji in U.S. Patent 4,555,457 issued November 26, 1985, describes a con-
ventional magnesium-manganese dioxide cell comprising a combined cathode dry
mix
and electrolyte with the addition of 0.1 to 10% by weight of potassium mono-
peroxysulfate, 2KHS05(OXONE~ sold by Dupont) to the electrolyte, which
consists of
magnesium perchlorate Mg(ClO4)z and lithium chromate Li2Cr04 for improving the
open
15 circuit voltage from 1.8 volts to 2.3 volts and capacity of the cell. It is
important to note
that the preferred embodiment of that patent incorporates a cathode and a non-
alkaline
electrolyte mix that has about 50% to 60% wetness, as required for the flow of
current. In
addition, in a comparison with the prior art as shown in Figure 11 of U.S.
Patent
4,555,457, an improvement in the open circuit voltage also increases cell
impedance. This
2o increase is not beneficial for the cell capacity at different discharge
rates.
As various additives, methods, and processes are employed to increase the OCV
of primary electrochemical cells, there is a need for providing a means of
regulating the
OCV/CCV without adversely affecting (increasing) cell impedance.
25 SUMMARY OF THE INVENTION
The main object of the present invention is to provide an alkaline manganese
dioxide-zinc cell incorporating suitable oxidizers as direct additives to the
electrolytic
manganese dioxide (EMD) that enhance the open circuia voltage (OCV) of the
cell
3o without adversely affecting cell impedance.
CA 02455548 2004-O1-22
-3-
A further object of the invention is to enable production of cells with highly
uniform open circuit voltages.
According to the present invention these objectives have been met by an
alkaline
manganese dioxide cell, wherein the positive electrode employs a variety of
suitable
oxidizing additives in an amount of less than 5% relative to the mass of the
manganese
dioxide, whereby the open circuit voltage increases and the cell impedance
stays
essentially the same.
The addition of the oxidizer occurs directly and not through the aqueous
electrolyte as disclosed in the above referenced prior art patents, which
increases the
la chance of cell shortages. The oxidizer can be added as a solid powder or an
aqueous
solution in potassium hydroxide directly to the cathode mass during the
cathode powder
processing.
The present invention increases the voltage potential of the EMD cathode
material
in a simple way and enables manufacturing of cells that precisely meet the
final cell
voltage requirements.
Cells according to the present invention may also have a number of additives
for
purposes of enhancing the conductivity and the structuravl integrity of the
manganese
dioxide positive electrode, or for enhancing hydrogen recombination at the
electrode. For
example, the manganese dioxide electrode may include at least one additive
that
2o comprises: graphite, carbon black, an inorganic binder, an organic binder,
or a
combination thereof.
Where the cells are intended for commercial use, the electrolyte may be a 4N
to
12N aqueous solution of potassium hydroxide, optionally with Zn0 dissolved in
it. A
separator between the zinc electrode and the manganese dioxide electrode and
appropriate
terminal means contacting the zinc electrode and manganese dioxide electrode
are
provided.
In keeping with the present invention, the positive manganese dioxide
electrode
includes an oxidizing additive. Any suitable oxidizing additive may be used.
The
oxidizing additive may comprise permanganate and/or peroxysulfate compounds in
an
3o amount of up to about 5%, preferably up to about 2.5%, by weight of the
manganese
CA 02455548 2004-O1-22
-4-
dioxide. Suitable permanganate and peroxysulfate compounds may comprise
potassium,
barium, calcium, silver, copper, and/or lithium. Preferably, the oxidizing
additive is
potassium permanganate, potassium peroxysulfate, or potassium peroxodisulfate.
Oxidizing additives may be present in an amount of from about 0.05 to
5.0°!°, preferably
about 0.1% to 0.75%, by weight of manganese dioxide.
BRIEF DESCRIPTION OF THE DRAWINGS
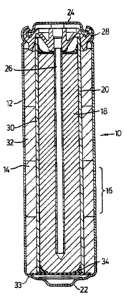
FIG.1 is a cross-sectional view of a cell according to the present invention.
1o
DESCRIPTION OF THE PREFERRED EMBODIMENTS:
As shown in Figure 1, an embodiment of a primary alkaline manganese dioxide-
15 zinc cell 10 comprises the following main units: a steel can 12, optionally
coated with a
conductive coating on the inside of the can, defining a cylindrical inner
space, a
manganese dioxide cathode 14 formed by a plurality of hollow cylindrical
pellets 16
pressed in the can, a zinc anode 18 made of an anode gel and arranged in the
hollow
interior of the cathode 14, and a cylindrical separator ZO separating the
anode 18 from the
20 cathode 14. The ionic conductivity between the anode and the cathode is
provided by the
presence of potassium hydroxide, KOH, electrolyte added into the cell in a
predetermined
quantity.
The can 12 is closed at the bottom, and it has a central circular pip 22
serving as
the positive terminal. The upper end of the can 12 is hermetically sealed by a
cell closure
25 assembly which comprises a negative cap 24 formed by a thin metal sheet, a
current
collector 26 attached to the negative cap 24 and penetrating deeply into the
anode gel to
provide electrical contact with the anode, and a plastic top 28 electrically
insulating the
negative cap 24 from the can 12 and separating gas spaces formed beyond the
cathode
and anode structures, respectively.
30 The material of separator 20 may consist of a single non-woven layer or a
cellophane layer or combinations thereof. Typically the separator material is
a
CA 02455548 2004-O1-22
-5-
conventional ion permeable absorbent membrane comprising non-woven materials
including polyamide, polyvinyl alcohol, rayon or cellulosic (rayon) fibers. A
micro-
porous cellophane film layer may be incorporated to prevent shorting of the
cell due to
the formation of zinc dendrites during discharge.
The separator 20 is wound to form a cylindrical tube of two or three turns,
which
results in multiple layers separating the anode from the cathode, and is
placed into the
hollow cylindrical cathode structure.
As illustrated in Figure l, one method of achieving sealing of the separator
bottom
is by means of a hot-melt bead 34, which is used to seal the separator 20 to a
washer 33 in
1o the cell. In another variation the washer is omitted and hot-:melt adhesive
only is used.
Furthermore, bottom seal cups may be employed without the use of a hot-melt
adhesive.
The negative zinc electrode comprises powdered metallic zinc or zinc alloys
and
optionally zinc oxide together with a suitable gelling agent such as
carboxymethyl
cellulose, polyacrylic acid, starches, and their derivatives.
The electrolyte is an aqueous alkaline solution of usually 4N to 12N potassium
hydroxide. The electrolyte may contain additives, such as dissolved zinc oxide
(Zn0), so
as to reduce the gassing of the active zinc within the negative electrode, and
so as to
permit overcharge of the cell without damaging it.
A suitable manganese oxide positive electrode comprises electrolytic or
2o chemically synthesized manganese dioxide containing typically over 90% of
four valent
manganese and minor amounts of lower valence oxides. The oxidizing additives
are
added to the manganese dioxide powder in the dry process of blending the
positive
electrode materials. Depending on the nature of the cell, the positive
electrode may be
molded into pellets and inserted into the can, followed optionally by re-
compaction.
Otherwise, the positive electrode may be extruded directly into the can, or it
may be
rolled or cast as a flat positive electrode for use in flat plate cells,
button or coin cells.
As discussed above, it has been found that the addition of oxidizing additives
to
the cathode material produces a higher open circuit voltage of the cell
without increasing
the cell impedance. The additives comprise permanganate and peroxysulfate
compounds,
3o preferably 0.05% to 5% by weight of manganese dioxide, more preferably 0.1%
to 0.75%
CA 02455548 2004-O1-22
-6
by weight of manganese dioxide. The compounds are present in the cell
preferably in the
form of potassium permanganate or potassium peroxysulfate. The amount of
various
additive compounds will vary depending on the size or format of the cell.
As noted, the present invention is applicable not only to bobbin type cells,
but also
to spirally wound cells, button or coin cells, and to flat plate cells. The
invention is
applicable to both primary and rechargeable alkaline cells.
The following examples will assist those skilled in the art to better
understand the
invention and its principles and advantages. It is intended that these
examples be
illustrative of the invention and not limit the scope thereof.
to
Example 1:
Alkaline AA-size cells were prepared according to the above description of
Figure
1 with variations in the cathode formulation due to the addition of the
appropriate
oxidizing agent. In all cases, the oxidizer replaced part of the active
electrolytic manga-
nese dioxide (EMD) material in a way that the volume of the total cell
materials was
maintained at a constant level.
Each AA-size cell contained 3 cathode pellets weighing approximately 3.3 to
3.5
g. The cathode formulation typical of primary alkaline cell consists about 88
to 92 wt
EMD, 4.5 to 9.0 wt % conductive powder, and the remainder being other
additives such
as binders and electrolyte including the oxidizing agent that are blended and
pressed into
a cathode pellet. As referenced above, the oxidizer agent, in this example,
potassium
permanganate (KMn04) was added by adjusting the amount of EMD accordingly.
Furthermore, in this example, the EMD was supplied by Delta Limited and the
KMn04
addition is expressed in weight % based on EMD in the cathode formulation.
Test cells (50 cells per group) were tested for the basic electrical tests.
These tests
include open circuit voltage (OCV), closed circuit voltage (CCV) carried out
at SOOmA
for 200 msec. load, short circuit current (SCC), and the impedance (IMP).
Table 1 shows the basic electrical tests of the test cells with the addition
of
3o various levels of KMn04 using Delta Ltd. EMD.
CA 02455548 2004-O1-22
_?_
Table 1: Basic Electrical Tests of AA Test Cells with Various Levels of
KMn04 Employing Delta Ltd. EMD
Test KMn04 OCV dVocv CCV dVccv SCC IMP
Cell (mV) (mV) (mV) (mV) (A) (m
)
Group
Control0% 1597 1524 15.7 102
#1 0.25% 1616 17 1542 18 15.2 107
#2 0.6% 1623 26 1551 27 16.5 98
#3 0.9% 1656 59 1571 47 15.2 109
#4 1.2% 1684 85 1614 90 20.3 83
#5 2.4% 1750 153 1619 95 17.4 100
#5b 4.8% 1760 163 1640 11 15.5 110
ti
The results of Table 1 clearly show the increase in the OCV/CCV ratio with an
increase in KMnOa. However, surprisingly, the impedance value, unlike the data
disclosed in the prior art, is not negatively affected by the addition of the
oxidizer.
In addition, prior art incorporates the electrolyte as a vehicle for the
addition of the
oxidizer to the manganese dioxide. Here, in a simple and cost effective way,
the oxidizer
is directly added to the manganese dioxide.
Example 2:
To demonstrate the effect of a different EMD type, alkaline AA-size cells were
prepared as in Example 1 but with EMD supplied by Xiangtan Electrochemical
Company
and three different levels of potassium permanganate. Once again, test cells
were tested
for the basic electrical tests; OCV, CCV, SCC, and IMP.
Table 2 shows the basic electrical tests of the test cells with the addition
of
2o various levels of KMn04 using Xiangtan Electrochemical Company EMD.
CA 02455548 2004-O1-22
_8_
Table 2: Basic Electrical Tests of AA Test Cells with Various Levels of KMn04
Employing Xiangtan Electrochemical Company EMD
Test KMn04 OCV dVocv CCV dVrcv SCC IMP
Cell (mV) (mV) (mV) (mV) (A) (m
Grou )
Control0% 1574 1498 14.8 106
#6 0.25% 1593 19 1517 19 15.7 101
#7 0.5% 1605 31 1529 31 16.2 99
#8 0.75% 1673 99 1596 98 ~9.3 87
~
Once again, Table 2 clearly shows the voltage improvement by applying
permanganate to an alternative EMD supplier, Xiangtan Electrochemical Company.
In
addition, it can be seen as in Table l, that the IMP (cell imp~edance~ is not
negatively
affected by the addition of KMn04 in the practical range for application in
alkaline cells.
However, there is a surprising trend to higher SCC and lowE;r IMP values with
increasing
1 o KMn04 addition.
The OCV maximum according to the International Standard IEC 60086 for
primary alkaline cells is set at 1.65V. Therefore, the maximum practical
amount of
KMn04 additive will depend on this IEC maximum limit value and on the EMD type
used. Tests similar to those made in Examples l and 2 and illustrated in
Tables 1 and 2
can be made when a new type or batch of manganese dioxide material is bought,
and
based on the tested values the amount of the oxidizing additive should be
chosen to
ensure the desirable OCV value.
In all the experimental groups above, permanganate addition has been
extensively
tested and has not shown detrimental effects on other battery performance
parameters
2o such as shelf life, capacity retention and electrolyte
leakagelleaktightness. Tables 3 and 4
show the service time for three selected tests to two voltage levels for cells
made in
experiments 1 and 2.
CA 02455548 2004-O1-22
-S
TABLE 3a: Service Time in Hours for Cells of Experiment 1
KMn04 3.952 3.95 1052 1052 500mA 500mA
to 1.1Vto to to to to 0.8V
0.8V 1.1V 0.9V 1.1V
Control0% 4.05 7.08 14.75 .20.22 1.13 2.95
#1 0.25% 4.53 6.93 15.57 18.87 1.48 2.72
#2 0.60% 4.00 7.50 15.18 19.75 1.15 3.03
#3 0.90% 4.05 7.17 15.05 :20.12 1.20 3.20
#4 1.20% 4.20 7.05 15.08 19.70 1.20 3.12
#5 2.40% 3.63 7.30 15.13 19.60 1.37 3.00
#5b 4.80/a 4.00 6.95 14.68 19.05 1.20 2.85
TABLE 3b: Relative Service Time for Cells of Experiment 1
KMn04 3.95 3.952 1052 1052 500mA 500mA
to 1. to to to to to 0.8V
iV 0.8V 1.1V 0.9V 1.1V
Control0% 100% 100% 100% 100% 100% 100%
#1 0.25% 112% 98% 106% 93% 131% 92%
#2 0.60% 99% 106% 103% 98% 101% 103%
#3 0.90% 100% 101% 102% 100% 106% 108!
#4 1.20% 104% 100% 102% '97% 106% 106%
#5 2.40% 90% 103% 103% 97l0 121% 102%
#5b 4.80% 99% 98% 100% 94% 106% 97%
TABLE 4a: Service Time in Hours for Cells of Experiment 2
KMn04 3.952 3.952 10~ 102 500mA 500mA
Controlto l.iV to to to to to 0.8V
0% 3.63 0.8V 1.1V 0.9V l,iV 2.72
6.97 13.78 19.87 0.88
#6 0.25% 3.83 6.98 13.50 1'9.62 1.25 2.75
#7 0.50% 3.75 7.05 13.68 1'9.78 1.22 2.63
#8 0.75% 3.70 7.02 13,53 19.681 1:27' 2.75
CA 02455548 2004-O1-22
- l~ -
TABLE 4b: Relative Service Time for Cells of Experiment 2
KMn04 3.952 3.952 i0S2 1052 500mA 500mA
to 1.1Vto to to to 1.1Vto
0.8V 1.1V 0.!~V 0.8V
Control0% 100% 100% 100% 100% 100% 100%
#6 0.25% 106% 100% 98% 99% 142% 101%
#7 0.50% 103% 101% 99% 100% 138% 97%
#8 0.75% 102% 101% 98% 99% 143% 101%
As can be seen from tables 3 and 4, the service time is not negatively
affected by the
addition of KMn04. In fact, especially at the higher rate of :i00 mA, the
performance to
the higher voltage level of 1.1 V is significantly improved over no KMn04
addition, up to
43% improvement were measured depending on EMD grade and level of addition.
Example 3:
to To demonstrate the effect of other oxidizer additives, potassium
peroxodisulfate,
KZS208, was evaluated in alkaline AA-size cells, which were prepared as in
Example 2
with EMD supplied by Xiangtan Electrochemical Company and two different levels
of
potassium peroxodisulfate (K2S20$). The separator used in this experiment was
different,
therefore, control cells that had no K2S208 addition were prepared as well for
comparison. Once again, test cells were tested for the basic electrical tests;
OCV, CCV,
SCC, and IMP.
Table 5 shows the basic electrical tests of the test cells with the addition
of
various levels of KZS208 using Xiangtan Electrochemical Company EMD.
CA 02455548 2004-O1-22
- 11 -
Table 5: Basic Electrical Tests of AA Test Cells with Various Levels of
KZS208,
Employing Xiangtan Electrochemical Company EMD
Test KZS20$ OCV dVocv CCV dVccv SCC IMP
Cell (mV) (mV) (mV) (mV) (A) (m
Group )
Control0% 1.580 1.504 18.7 84
#9 0.75% 1.584 4 1.504 0 17.8 89
#10 1% 1.589 9 1.511 7 17.9 89
Once again, Tables 5 shows the voltage improvement by applying persulfate to
EMD supplied by Xiangtan Electrochemical Company. In addition, it can be seen
as in
Tables 1 and 2, that the IMP is not negatively affected by the addition of
KZSZOB
However, when compared to Experiment 2, at the same level of additive
addition,
potassium permanganate is a more powerful oxidizing agent and would be
preferred.
Tables 6a and 6b show the service time for three selected to sts to two
voltage levels for
to cells made in experiments 3.
TABLE 6a: Service Time in Hours for Cells of Experiment 3
KzSzOg 3.9SZ 3.9~ 102 1052 500mA 500mA
to to to to
1.1V 0.8V 1.1V 0.9V to to 0.8V
1.1V
Control0% 3.94 7.61 14.22 20.61 1.31 2.88
#9 0.75% 4.00 7.35 13.88 20.31 1.25 2.71
#10 1% 3.69 7.05 14.12 20.38 1.26 2.65
~ ~
TABLE 6b: Relative Service Time for Cells ~of Experiment 3
KzSzOg 3.952 3.952 1052 LOS2 500mA 500mA
to to to to to to 0.8V
1.1V 0.8V 1.1V 0.9V 1.1V
Control0% 100% 100% 100% 100% 100% 100%
#9 0.75% 101% 97% 98% 99% 96% 94%
#10 1% ~ 94% 93% 99% 99% 97% - 92%
~ ~ ~ ~
CA 02455548 2004-O1-22
-12
As can be seen from tables 6a and 6b, the service time is not significantly
affected by the
addition of KZS208.
__ j_.~._.._._T__n_..-.~.~._.._.~