Note: Descriptions are shown in the official language in which they were submitted.
CA 02455805 2004-02-23
COMPOUNDS AND METHODS FOR THERAPEUTIC INTERVENTION IN PREVENTING DIABETIC
COMPLICATIONS
This application is a divisional application of co-
pending application 2,279,428 filed February 5, 1998
Pursuant to 35 U.S.C. ~202(c), it is hereby
acknowledged that the U.S. Government has certain
rights in the invention described herein, which was
made in part with funds from the National Institutes
of Health (Grant Nos. DK44050, DK50317 and DK50364).
BACICGROOND OF THE INVENTION
The present invention relates to therapeutic
agents and their use for the treatment of diabetics
and in particular for preventing, reducing or delaying
the onset of diabetic complications and other
disorders of related etiology. More particularly, the
present invention relates to a class of enzyme
inhibitors which inhibit the enzymatic conversion of
fructose lysine (FL) to fructose-lysine-3-phosphate
(FL3P), which is believed to be an important step in
the biochemical mechanism leading to diabetic
complications. This invention also relates to a
method of assessing a diabetic patient°s risk of
experiencing diabetic complications, as well as a
method of determining the efficacy of therapeutic
intervention in preventing, reducing or delaying .the
onset of diabetic complications.
There are four particularly serious
complications of diabetes, namely, diabetic
nephropathy or kidney disease; diabetic retinopathy
which causes blindness due to destruction of the
retina; diabetic neuropathy involving the loss of
peripheral nerve function; and circulatory problems
due to capillary damage. Both retinopathy and
CA 02455805 2004-02-23
F
- 2 -
nephropathy are thought to be subsets of the general
circulatory problems associated with this disease
state. The role of microvascular dysfunction in late
stage diabetes has been recently summarized (Tooke,
Diabetes, 44: 721 (1995)). Throughout this
disclosure, the terms "diabetes-associated pathologic
conditions" and synonymous terms are meant to include
the various well-known retinopathic, neuropathic,
nephropathic, macroangiopathic, as well as other
complications of diabetes.
The similarities between the pathologies
arising from diabetes and those resulting from aging
have been extensively reported. Studies have shown
that many diabetes-associated pathologic conditions
are clinically very similar to the pathologies
normally associated with aging. It has been shown,
far example, that in diabetes arteries and joints
prematurely stiffen, lung elasticity and vital
capacity prematurely decrease. Moreover,
atherosclerosis, myocardial infarction and strokes
occur more frequently in diabetics than in age-matched
non-diabetic individuals. Diabetics are also more
susceptible to infections, and are more likely to have
hypertension, accelerated bane loss, osteoarthritis
and impaired T-cell function at a younger age than
non-diabetics.
The similarities between diabetes-associated
pathologic conditions and aging would appear to
suggest a common mechanistic rationale. A variety of
mechanisms have been proposed as a common biochemical
basis for both diabetes-associated pathologic
conditions and aging. The hypothesis most strongly
supported by data from human subjects is premised on a
non-enzymatic glycosylation mechanism. This
hypothesis states that the aging process and
diabetes-associated pathologic conditions, such as
CA 02455805 2004-02-23
-
those described above, are caused, at least in part,
by protein modification and cross-linking by glucose
and glucose-derived metabolites via the Maillard
reaction (Monnier et al., Proc. Natl. Acad. Sci. USA,
81: 583 (1984) and Dee et al., Biochem. Biophys. Res.
Comm., ~: 888 (1984)). The modified proteins
resulting from such glycosylation reactions are
referred to herein as advanced glycation end product-
modified proteins (AGE-proteins). It is widely ~-
accepted that 3-deoxyglucosone (3~G) is a key
intermediate in the mufti-step reaction sequence
leading to AGE-protein formation. 3DG is a glucose-
derived metabolite that can react with proteins
leading to the cross-linking of both intracellular and
extracellular proteins, such as collagen and basement
membranes.
In the case of diabetic complications, the
reactions that lead to AGE-proteins are thought to be
kinetically accelerated by the chronic hyperglycemia
associated with this disease. Evidence supporting
this mechanism includes data showing that long-lived
proteins such as collagen and lens crystallins from
diabetic subjects contain a significantly greater AGE-
protein content than do those from age-matched normal
controls. Thus, the unusual incidence of cataracts in
diabetics at a relatively early age is explainable by
the increased rate of modification and cross-linking
of lens crystalline. Similarly, the early onset of
joint and arterial stiffening, as well as loss of lung
capacity observed in diabetics is explained by the
increased rate of modification and cross-linking of
collagen, the key structural protein. Because these
proteins are long-lived, the consequences of
modification tend to be cumulative.
Another factor demonstrating cause and
effect relationship between diabetic complications and
CA 02455805 2004-02-23
a
- 4 -
hyperglycemia is hyperglycemic memory. One
particularly striking example of this phenomenon is
the development of severe retinopathy in dogs that
were initially diabetic, then treated to restore
normal blood glucose levels. Although the dog eyes
were histologically normal at the time of the
treatment, over time diabetic retinogathy developed in
these animals in spite of the normalized glucose
concentrations (Engerman et al., Diabetes, 36: 808
(1987)). Thus, the underlying damage to the eyes
irreversibly occurred during the period of early
hyperglycemia, before clinical symptoms were evident.
Diabetic humans and animals have been shown
to have higher than normal concentrations of early and
late sugar modified AGE-proteins. In fact, the
increase in AGE-proteins is greater than the increase
in blood glucose levels. The concentration of AGE-
proteins can be estimated by fluorescence measurement,
as some percentage of sugar molecules rearrange to
produce protein-bound fluorescent molecules.
The pathogenic role of AGE-proteins is not
limited to diabetes. Protein glycation has been
implicated in Alzheimer's disease (Harrington et al.,
Nature, 370: 247 (1994)). Increased protein
fluorescence is also seen with aging. Indeed, some
theories trace the aging process to a combination of
oxidative damage and sugar-induced protein
modification. Thus, a therapy that reduces
AGE-protein formation may also be useful in treating
other etiologically-similar human disease states, and
perhaps slow the aging process.
It has generally been assumed that the
formation of AGE-proteins begins with the reaction of
a protein amino group and a sugar, primarily glucose.
One typical literature citation states "The initial
adduct formed by glycation of e-amino groups of lysine
CA 02455805 2004-02-23
- 5 -
residues is the Amadori compound, fructoselysine....
Glycation is an initial step in a complex series of
reactions, known collectively as the Maillard or
browning reaction, which ultimately leads to the
formation of crosslinked, precipitated, oxidized,
brown and fluorescent proteins°'. K.J. Knecht et al.,
Archives of Biochem. Biophys., 294: 130 (1992).
The formation of AGE-proteins from sugars is
a multi-step process, involving early, reversible
l0 reactions with sugars to produce fructose-lysine
containing proteins. These modified proteins then
continue to react to produce irreversibly modified
AGE-proteins. It is clear that AGE-proteins axe not
identical to proteins containing glycated-lysine
residues, as antibodies raised against AGE-proteins do
not react with fructose-lysine. It is also clear that
AGE-proteins exist as multiple chemical species;
however few have been identified. The chemical
species (e-Amino-(carboxymethyl)lysine has been
identified as one important final AGE-protein
structure in recent studies (Reddy et al., Biochem.,
34: 10872 (1995) and Ikeda et al., Biochemistry, 35:
8075 (1996?). This study failed to chemically
identify another AGE-protein epitope that made up
approximately 50% of the modified sites. A method of
studying the kinetics of AGE-protein formation from
ribose has recently been developed (Khalifah et al.,
Biochemistry, 35: 4645 (1996)). However, this study
suggests that ribose may play an important
physiological role in AGE-protein formation,
supporting the relatively broad definitions of
glycated-lysines and fructose-lysine provided below.
Other references point out the distinction
between proteins containing glycated lysine residues
and AGE proteins, "Equilibrium levels of Schiff-base
and Amadori products are reached in hours and weeks,
CA 02455805 2004-02-23
a
6
respectively. The reversible, equilibrium nature of
early glycosylation products is important, because it
means that the total amount of such products, even on
very long-lived proteins, reaches a steady-state
plateau within a short period of time..... Since these
early glycosylation products do not continue to
accumulate on collagen and other stable tissue
proteins over years in chronic diabetes, it is not
surprising that their concentration does not correlate
with either the presence or the severity of diabetic
retinopathy...Some of the early glycosylation products
on collagen and other long-lived proteins of the
vessel walls do not dissociate, however. Instead,
they undergo a slow, complex series of chemical
rearrangements to form irreversible advanced
glycosylation end products". M. Brownlee et al., New
England Journal of Medicine, 318: 1315 (1988). The
only route for production of these modified proteins
which is described in the scientific literature
involves an initial reaction between proteins and
sugar molecules.
Numerous references point out that the
formation of AGE-proteins occurs thraugh a multi-step
pathway and that 3-deoxyglucosone (3-DG) is a key
intermediate in this pathway. M. Brownlee, Diabetes,
43: 836 (1994); M. Brownlee, Diabetes Care, 15: 1835
(1992); T. Niwa et al., Nephron, 69: 438 (1995); ~1,L.
Dills, Jr., Am. J. Clin. Nutr., 58: 5779 (1993); H.
Yamadat et al., J. Biol. Chem., 269: 20275 (1994); N.
Igaki et al., Clin. Chem., 36: 631 (1990). The
generally accepted pathway for formation of 3DG from
the reaction of sugars and proteins is illustrated in
Figure 1. As can be seen in Figure Z, a sugar
(glucose) molecule initially forms a Schiff base with
a protein-lysine amino group (I). The resulting
Schiff base then rearranges to produce fructose-lysine
CA 02455805 2004-02-23
modified proteins (II). The reactions leading up to
(II) are freely reversible. (II) can rearrange to
produce 3DG and free protein lysine. Subsequent
reaction between 3DG and protein is the first
irreversible step in AGE-protein formation.
Insofar as is known, it has never been
reported that 3DG can be produced by alternative
pathways, or indeed, that the major source of 3-DG is
from an enzyme catalyzed metabolic pathway, rather
than from the uncatalyzed reactions shown in Figure 1.
Diabetic patients have significantly more
3DG in serum than do non-diabetic patients (12.78~2.49
ACM versus 1.94~0.17 ~M). (Toshimitsu Niwa et a1_,
Nephron, 69: 438 (1995)). Nonetheless, this toxic
compound is found in normal healthy individuals.
Thus, it is not surprising that the body has developed
a detoxification pathway for this molecule.. One of
these reactions is catalyzed by aldehyde reductase
which detoxifies 3DG by reducing it to 3-deoxyfructose
(3DF) which is efficiently excreted in urine
(Takahashi et al., Biochem, 34: 1433 (1995)). Another
detoxification,reaction oxidizes 3DG to 3-deoxy-2-
ketogluconic acid (DGA) by oxoaldehyde dehydrogenase
{Fujii et al., Biochem. Biophys. Res. Comm., 210: 852
{1995)).
Results of studies to date show that the
efficiency of at least one of these enzymes, aldehyde
reductase, is adversely affected in diabetes. When
isolated from normal rat liver, a fraction of this
enzyme is partially giycated on lysines 67, 84 and 140
and has a low catalytic efficiency when compared with
the normal, unmodified enzyme (Takahaski et al.,
Biochem., 34: 1433 (1995)). Since diabetic patients
have higher ratios of glycated proteins than
normoglycemic individuals they are likely to have both
higher levels of 3DG and a reduced ability to detoxify
CA 02455805 2004-02-23
this reactive molecule by reduction to 3DF.
The mechanism of aldehyde reductase has been
studied. These studies determined that this important
detoxification enzyme is inhibited by aldose reductase
inhibitors (ARIs) (Barski et al., Biochem., 34: 112&4
(1995)). ARTs are currently under clinical
investigation for their potential to reduce diabetic
complications. These compounds, as a class, have
shown some effect on short term diabetic
complications. However, they lack clinical effect on
long term diabetic complications and they worsen
kidney function in rats fed a high protein diet. As
will appear hereinbelow, this finding is consistent
with the newly discovered metabolic pathway for lysine
recovery underlying the present invention. A high
protein diet will increase the consumption of
fructose-lysine, which undergoes conversion into 3DG
by the kidney lysine recovery pathway. The
detoxification of the resulting 3DG by reduction to
3DF will be inhibited by ARIs therapy, which
consequently leads to an increase in kidney damage, as
compared to rats not receiving ARIs. This is because
inhibition of the aldose reductase by the ARIs would
reduce availability of aldose reductase for reducing
3DG and 3DF.
The role of 3-DG in contributing to human
disease has been previously investigated as will~be
appreciated from a review of the patents summarized
below.
U.S. Patent 5,4?6,849 to Ulrich et al
describes a method of inhibiting the formation of
AGE-proteins using amino-benzoic acids and
derivatives. These compounds presumably act by
reacting with 3-DG and removing it from the system
before it can react with proteins to begin the
irreversible steps of AGE-protein formation.
CA 02455805 2004-02-23
- 9 -
U.S. Patents 4,798,583 and 5,128,360 to
Cerami et al describes the use of aminoguanidine to
prevent AGE-protein formation and diabetes-induced
arterial wall protein cross-linking. Aminoguanidine
was shown to react with an early glycosylation
product. This early product is 3DG, as defined
herein. These patents do not contemplate the
possibility of inhibiting the formation of 3-DG. They
focus exclusively on complexing this toxic molecule.
U.S. Patent 5,468,777 to France et al
describes methods and agents far preventing the
staining of teeth caused by the non-enzymatic browning
of proteins in the oral cavity. Cysteine and cysteine
derivatives are described as particularly useful in
this application.
U.S. Patent 5,358,960 to Ulrich et al
describe a method for inhibiting AGE-protein formation
using aminosubstituted imidaaoles. These compounds
were shown to react with an early glycosylation
product (3DG).. No mention is made in this patent that
a metabolic source of 3DG may exist. This patent
envisions that 3DG is made exclusively as an
intermediate in the non-enzymatic browning of
proteins.
U.S. Patent 5,334,617 to Ulrich et al
describes amino acids useful as inhibitors of
AGE-protein formation. Lysine and other bifunctional
amino acids are described as particularly useful in
this regard. These amino acids are described as
reacting with the early glycosylation product fxom the
reaction of glucose and proteins. It appears that the
early glycosylation product described in this patent
is 3DG.
U.S. Patent 5,318,982 to Ulrich et al
describes the inhibition of AGE-protein formation
using as the inhibitory agent 1,2,4-triazoles. The
CA 02455805 2004-02-23
- 10 -
inhibitors described in this patent contain
diamino-substituents that are positioned to react with
and complex 3DG. The patent describes these compounds
as reacting with an early glycosylation product t3DG
as defined herein).
U.S. Patent 5,272,165 to Ulrich et al
describes the use of 2-alkylidene-aminoguanidines as
inhibitors of AGE-protein formation. The inhibitars
described in this patent are said to be highly
reactive with 3DG. No mention is made of inhibiting
the metabolic formation of 3DG in this patent.
U.S. Patent 5,262,152 to Ulrich et al
describes the use of amidrazones and derivatives to
inhibit AGE-protein formation. The compounds
described in this patent are cx-effect amines. W.P.
Jencks, 3rd ed., McGraw Hill, New York. Compounds of
this category are known to react with dicarbonyl
compounds, e.g. 3DG.
U.S. Patent 5,258,381 to Ulrich et al
describes the use of 2-substituted-2-imidazolines to
inhibit AGE-protein formation. The compounds
described in this patent contain adjacent amino groups
that can readily react with 3DG.
U.S. Patent 5,243,071 to Ulrich et al
describes the use of 2-alkylidene-aminoguanidies to
inhibit AGE-protein formation. The compounds
described in this patent are highly reactive with-3DG
and function by complexing this reactive, toxic
molecule.
U.S. Patent 5,221,683 to Ulrich et al
describes the use of diaminopyridine compounds to
inhibit AGE-protein formation. The diaminopyridine
compounds described as particularly useful will react
with 3DG to form a stable, six-member ring containing
complex.
U.S. Patent 5,130,337 to Ulrich et al
CA 02455805 2004-02-23
0 1
- 11 -
describes the use of amidrazones and derivatives to
inhibit AGE-protein formation. The inhibitors
described in this patent are a-effect amines which, as
is know in the art, will rapidly react with 3DG and
form stable complexes.
U.S. Patent 5,130,324 to Ulrich et al
describes the use of 2-alkylidene-aminoguanidines to
inhibit AGE-protein formation. The compounds
described in this patent function by reacting with the
early glycosylation product resulting from the
reaction of glucose with proteins (3DG).
U.S. Patent 5,114,943 by Ulrich et al
describes the use of amino-substituted pyrimidines to
inhibit AGE-protein formation. The compounds
described in this patent are said to rapidly react
with and detoxify 3DG.
None of the above-mentioned patents suggest
inhibition of the metabolic formation of 3DG as a
means of therapeutic intervention to prevent diabetic
complications. Indeed, none of these patents even
suggest the involvement of an enzymatic pathway in the
production of 3DG.
U.S. Patent 5,108,930 to Ulrich et al
describes a method for detecting the levels of
aminoguanidine in biological samples. This assay is
described as having potential utility in determining
kidney function by measuring the aminoguanidine _
elimination time. The principal utility intended for
the assay method described in this patent is in the
measurement of tissue levels of aminoguanidine, so
that doses sufficient to inhibit AGE-protein formation
can be maintained in animal and human studies. No
mention is made in this patent of using urine 3DG, 3DF
or DGA ratios to determine diabetics at risk for
complications.
U.S. Patent 5,231,031 to Szwergold et al
CA 02455805 2004-02-23
- 12 -
describes a method for assessing the risk of
diabetic-associated pathologic conditions and
determining the efficacy of therapies for these
complications. This patent describes the measurement
of two phosphorylated compounds in erythrocytes of
diabetic patients. These two Compounds were not
chemically identified in this patent. However,
neither compound is 3DG or 3DF, whose levels are
measured in urine in the diagnostic embodiment of the
present invention.
Methods for monitoring metabolic control in
diabetic patients by measurement of glycosylation end-
products are known. The concentration of glycosylated
hemoglobin is known to reflect mean blood glucose
concentration during the preceding several weeks.
U.S. Patent 4,37~.,37~, issued to A. Cerami et al.,
describes a method for monitoring glucose levels by
quantitation of the degradation products of
glycosylated proteins, more specifically non-
enzymatically glycosylated amino acids and peptides,
in urine. This method purports to utilize the
affinity of alkaline boronic acids for forming
specific complexes with the coplanar cis-diol groups
found in glycosyiation end-products to separate and
quantitate such end-products.
U.S. Patent 4,761,368 issued to A. Cerami
describes the isolation and purification of a
chromophore present in browned polypeptides, e.g.,
bovine serum albumin and poly-L-lysine. The
chromophore, 2- (2-furoyl) -4 (5) -2 (furoyl) -1H-imidazole
(FFI) is a conjugated heterocycle derived from the
condensation of two molecules of glucose with two
lysine-derived amino groups. This patent further
describes the use of FFI in a method for measuring
"aging" (the degree of advanced glycosylation) in a
protein sample wherein the sample "age" is determined
CA 02455805 2004-02-23
4
- 13 -
by measuring the amount of the above-described
chromophore in the sample and then comparing this
measurement to a standard (a protein sample having an
amount of FFI which has been correlated to the "age"
of the sample).
There is a long-standing, unfilled need in
existing treatment regimens of diabetic patients for
effective means to identify those at risk of
developing diabetes-associated pathologic conditions,
to prevent, reduce or delay the onset of such
conditions by therapeutic intervention and to
determine the benefit of such therapeutic
intervention.
SUMMARY OF. THE_ INVENTION
The present invention arose from the
discovery of a metabolic pathway that involves the
enzyme-mediated conversion of FL to FL3P and produces
relatively high concentrations of 3-deoxyglucosone
(3DG) in organs affected by diabetes. Subsequent
research into the biochemical function of this newly
discovered pathway tends to indicate that it has an
important role in the etiology of diabetic kidney
disease. It is also suspected that this pathway
contributes to the development of the various known
diabetes-associated pathologic conditions.
This discovery has found practical
application in the present invention which, in one
aspect, provides a class of compounds which have
enzyme inhibitory activity and are effective to
inhibit the enzymatic conversion of fructose-lysine to
fructose-lysine-3-phosphate. The relevant enzyme
inhibitory activity of the compounds of the present
invention is readily determinable by assay. The assay
method comprises providing an aqueous solution of
fructose-lysine, adenosine triphosphate (ATP), a
CA 02455805 2004-02-23
t
- 14 -
source of fructose-lysine-3-phosphate kinase and a
compound of the present invention in an amount
sufficient to demonstrate inhibitory activity,
subjecting the resulting solution to conditions
promoting the formation of fructose-lysine-3-phosphate
and adenosine diphosphate as products of the
interaction of the above-mentioned kinase, fructose-
lysine and adenosine triphosphate, and measuring the
production of at least one of such products, the
compounds of the present invention reducing the amount
of such products, as compared to an aqueous solution
of the same relative amounts of fructose-lysine,
adenosine triphosphate and source of fructose-lysine-
3-phosphate kinase, without the addition of a compound
~.5 of the present invention. The assay method just
described is also within the scope of the present
invention.
According to another aspect, the present
invention provides a pharmaceutical preparation for
preventing, reducing or delaying the onset of diabetic
complications in a diabetic patient, comprising, as an
active agent, a cornpaund of the invention, as
described above, and a pharmaceutically acceptable
vehicle.
According to a further aspect of the present
invention, there is provided a method for preventing,
reducing or delaying the onset of diabetic .
complications in a patient at risk of developing same,
which method comprises administering to the patient a
compound of the present invention in an amount
effective to inhibit the enzymatic conversion of
fructose-lysine to fructose-lysine-3-phosphate. This
same method may be used for the prevention or
treatment of other etiologically-similar disease
states, as will be further described hereinbelow.
According to still another aspect, the
CA 02455805 2004-02-23
- 15 -
present invention provides a method for assessing a
diabetic patient's risk of experiencing a diabetes-
associated pathologic condition. This method
comprises administering to the patient a source of
glycated-lysine residues in an amount providing a
predetermined dose of the glycated-lysine residues,
and measuring the ratio of 3-deoxyglucosone to 3-
deoxyfructose in a biological sample obtained from the
patient, with reference to the ratio of 3-
deoxyglucosone to 3-deoxyfructose in a normal subject,
i.e., a non-diabetic subject or one having no clinical
symptoms of diabetes. The higher ratio of 3-
deoxyglucosone to 3-deoxyfructose in the diabetic
patient sample, in comparison to that of the
asymptomatic subject is indicative that the diabetic
patient is at higher risk of experiencing a diabetes-
associated pathologic condition.
The present invention also provides a method
for assessing the efficacy of therapeutic intervention
. 20 in preventing diabetic complications. The method
involves measuring the concentration of 3-
deoxyglucosone, 3-deoxyfructose and fructose-lysine in
biological samples obtained from a diabetic patient,
both before and after initiation of the therapeutic
intervention. The sum of the 3-deoxyglucosone.and 3-
deoxyfructose concentrations are then compared to the
concentration of fructose-lysine in the samples. .A
decrease in the sum of 3-deoxyglucosone and 3-
deoxyfructose concentrations relative to the fructose-
lysine concentration in the biological sample taken
after initiation of therapeutic intervention, as
compared to the same concentrations measured in the
biological sample taken before initiation of the
therapeutic intervention, is indicative of the
efficacy of the therapeutic intervention.
As yet another aspect of the present
CA 02455805 2004-02-23
_16_
invention, there is provided a method for apprising a
diabetic person of the potential of a food product to
contribute to the development of a diabetic-associated
pathologic condition. This method involves measuring the
content of glycated-lysine residues in the food product and
providing this information to diabetic patients, e.g., on
the package of the food product or in a publication intended
for use by diabetics.
Also of interest is a method for inducing a pathological
condition in a test animal, said pathological condition
being selected from the group consisting of diabetic
nephropathy, diabetic neuropathy, diabetic macroangiopathy,
diabetic retinopathy, cardiovascular disease,
neurodegenerative disease, insulin-dependent diabetes,
diabetes-related birth defects and cancer, said method
comprising feeding to said test animal a glycated protein
diet in an amount and for a time sufficient to maintain the
3DG content of a biological fluid of said test animal
relative to the 3DG content of a biological fluid from the
same species of animal fed a diet substantially free of said
glycated protein.
BRIEF DESCRIPTION OF DRAWINGS
FIGURE 1 shows the initial step involved in the multi-
step reaction leading to irreversibly-modified AGE-proteins.
FIGURE 2 illustrates the reactions involved in the
lysine recovery pathway.
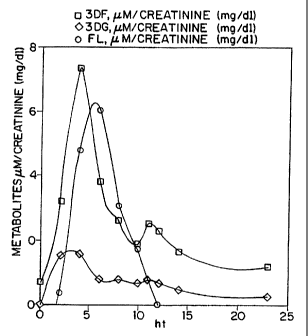
FIGURE 3 is a graphical representation of a urinary
profile showing the variation overtime of 3DF, 3DG and FL
from a single individual fed 2 g. of FL and followed for 24
hours.
CA 02455805 2004-02-23
16a -
FIGURE 4 is a graphical representation of urinary
excretion over time of 3DF from seven volunteers fed 2 g, of
fructoselysine.
FIGURE 5 shows a graphical comparison of 3DF and N-
acetyl-a-glucosaminidase (NAG) between a group of control
animals and an experimental group maintained on a feed
containing 0.3% glycated protein.
FIGURE 6 is a graph showing the linear relationship
between 3DF and 3DG levels in urine of rats fed either a
control diet or one enriched in glycated protein.,
CA 02455805 2004-02-23
_ 17
FIGURES 7A and 78 are graphical
representations of fasting levels of 3DG in the urine
of normals and diabetic patients plotted against the
fasting level of 3DF.
DETAILED DESCRIPTION OF TFiE INVENTION
The following definitions are provided to
facilitate understanding of the present invention, as
described in further detail hereinbelow:
1. Glycated-Lysine Residues - The
expression "glycated lysine residues", as used herein,
refers to the modified lysine residue of a stable
adduct produced by the reaction of a reducing sugar
and a lysine-containing protein.
The majority of protein lysine residues are
located on the surface of proteins as expected for a
positively charged amino acid. Thus, lysine residues
on proteins which come in contact with serum, or other
biological fluids, can freely react with sugar
molecules in solution. This reaction occurs in
multiple stages. The initial stage involves the
formation of a Schiff base between the lysine free
amino group and the sugar keto-group. This initial
product then undergoes the Amadori rearrangement, to
produce a stable ketoamine compound.
This series of reactions can occur with
various sugars. When the Sugar involved is glucose,
the initial Schiff base product will involve imine
formation between the aldehyde moiety on C-1 of the
glucose and the lysine e-amino group. The Amadori
rearrangement will result in formation of lysine
coupled to the C-1 carbon of fructose, 1-deoxy-1-
(E-aminolysine)-fructose, herein referred to as
fructose-lysine or FL.
Similar reactions will occur with other
aldose sugars, for example galactose and ribose
CA 02455805 2004-02-23
- 18 -
(Dills, Am. J. Clin. Nutr., 58: S779 (1993}). For the
purpose of the present invention, the early products
of the reaction of any reducing sugar and the e-amino
residue of protein lysine are.included within the
meaning of glycated-lysine residue, regardless of the
exact structure of the modifying sugar molecule.
Also, the terms glycated-lysine residue,
glycated protein and glycosylated protein or lysine
residue are used interchangeably herein, which is
consistent with current usage in scientific journals
where such expressions are often used interchangeably.
2. Fructose-lysine - The term
"fructose-lysine" (FL) is used herein to signify any
glycated-lysine, whether incorparated in a
protein/peptide or released from a proteinJpeptide by
proteolytic digestion. This term is specifically not
limited to the chemical structure commonly referred to
as fructose-lysine, which is reported to form from the
reaction of protein lysine residues and glucose. As
noted above, lysine amino groups can react with a wide
variety of sugars. Indeed, one report indicates that
glucose is the least reactive sugar out of a group of
sixteen (16) different sugars tested (Bunn et al.,
Science, 213: 222 (1981)). Thus, tagatose-lysine
formed from galactose and lysine, analogously to
glucose is included wherever the term fructose-lysine
is mentioned in this description, as is the
condensation product of all other sugars, whether
naturally-occurring or not. It will be understood
from the description herein that the reaction between
protein-lysine residues and sugars involves multiple
reaction steps. The final steps in this reaction
sequence involve the crosslinking of proteins and the
production of multimeric species, known as AGE-
proteins, some of which are fluorescent. Proteolytic
CA 02455805 2004-02-23
1g _
digestion of such modified proteins does not yield
lysine covalently linked to a sugar molecule. Thus,
these species are not included within the meaning of
"fructose-lysine", as that term is used herein.
3. Fructose-lysine-3-phosphate - This
compound is formed by the enzymatic transfer of a high
energy phosphate group from ATP to FL. The term
fructose-lysine-3-phosphate (FL3P), as used herein, is
meant to include all phosphorylated fructose-lysine
moieties that can be enzymatically formed whether free
or protein-bound.
4. Fructose-lysine-3-phosphate kinase -
This term refers to one or more proteins which can
enzymatically convert FL to FL3P, as defined above,
when additionally supplied with a source of high
energy phosphate.
5. 3-Deoxyglucosone - 3-Deaxyglucosone
(3DG) is the 1,2-dicarbonyl-3-deoxysugar (also known
as 3-deoxyhexulosone) which is formed upon breakdown
of FL3P to yield free lysine and inorganic phosphate.
For purposes of the present description, the term
3-deoxyglucosone is intended to include all possible
dicarbonyl sugars which are formed upon breakdown of
FL3P, having the broad definition of FL3P stated
above.
6. FL3P Lysine Recovery Pathway -
A lysine recovery pathway exists in human kidney and
possibly other tissues, which regenerates unmodified
lysine as a free amino acid or incorporated in a
polypeptide chain. As will be further explained
below, this pathway is an important factor
contributing to the complications of diabetes.
7. AGE-Proteins - The term "AGE-proteins"
(Advanced Glycation End-product modified proteins) has
been used in scientific journals, and is used herein,
to refer to the final product of the reaction between
CA 02455805 2004-02-23
y
- 20 -
sugars and proteins (Brownlee, Diabetes Care, 15: 1835
(1992) and Niwa et al., Nephron, 69: 438 (1995)). It
is clear that the reaction, for example, between
protein lysine residues and glucose does not stop with
the formation of fructose-lysine. FL can undergo
multiple dehydration and rearrangement reactions to
produce non-enzymatic 3DG, which reacts again with
free amino groups, leading to cross-linking and
browning of the protein involved. Indeed, there is
l0 reasonable evidence that 3DG, as defined hereinabove,
is a central intermediate in this modification
reaction.
9. "Glycated Diet°' - As used herein, this
expression refers to any given diet in which a
percentage of normal protein is replaced with glycated
protein. The expression "glycated diet" and "glycated
protein diet" are used interchangeably herein.
At least some, and possibly all, of the
complications of diabetes are due to the covalent
modification of proteins by glucose arid other reactive
sugars. M. Brownlee, Diabetes, 43: 836 (1994). As
noted above, diabetic humans and animals have been
shown to have higher concentrations of sugar modified
proteins than normal. In fact, the increase in
diabetes-associated AGE-proteins is greater than the
increase in blood glucose levels.
Previously, it had been generally accepted
that the origin of 3DG in vivo was from the
decomposition of proteins containing glycated lysine
residues. It had also been commonly believed that
these glycated-lysines could not be used as an amino
acid source. As will appear hereinbelow, this
previous belief was incorrect.
As mentioned above, the present invention
evolved from the discovery of a previously unknown
metabolic pathway which produces 3DG in an enzyme-
CA 02455805 2004-02-23
- 21 -
catalyzed reaction. This enzymatic pathway is capable
of enzymatic inhibition, thereby reducing the
production of toxic 3DG.
During the course of a series of studies on
5 diabetic kidneys, examination of 31P NMR spectra from
perchloric acid extracts of kidneys from
streptozotoxin induced diabetic rats revealed an
unusual new peak in the NMR spectrum. Previous
studies by the present inventors had demonstrated the
presence of fructose-3-phosphate in rat lens and human
erythrocytes (A. Petersen et al., Biochem. J., 284:
363-366 (1992); Lal et al., Arch. ~iiochem. Biophys.,
318: 191 (1995); Szwergold et al., Science, 247: 451
(1990) and Lal et al., Investigative Opthalmology and
Visual Science, 36(5): 969 (1995)). Earlier studies
had identified other unusual phosphorylated sugars in
rat lens (Szwergold et al., Diabetes, 44: 810 (1995)
and Kappler et al., Metabolism, 44: 1527 (1995)).
Thus it was reasonable to assume that this newly
identified peak was another phosphorylated sugar.
Further extensive laboratory investigation revealed
that this new compound was not a simple sugar, but
rather fructose-lysine phosphorylated on the 3
position of the fructose component.
This identification was confirmed in two
ways. Authentic fructose-lysine-3-phosphate (FL3P)
was synthesized by the procedure disclosed in Example
2, below, and shown to co-resonate in the 31P NMR
spectrum with the peak in diabetic rat kidneys.
Synthetic fructose-lysine was also injected into
non-diabetic rats. These rats showed a substantial
increase in the levels of FL3P in their kidneys
following this injection.
Two experiments were conducted to
demonstrate that FL3P is derived directly from FL in
an enzyme catalyzed reaction. Fructose-lysine labeled
CA 02455805 2004-02-23
22
with deuterium at the C3 position of the fructose
moiety was synthesized and injected into rats. Three
hours after injection, the kidneys of these rats were
removed and extracted with perchloric acid. NMR
spectroscopy revealed that the FL3P material isolated
from these rats contained the deuterium label at the
C3 position of the fructose moiety. In addition, rat
kidney homogenates demonstrate the ability to produce
FL3P in a reaction requiring both ATP and fructose-
l~rsine. This last-mentioned experiment confirms the
presence of a specific FL3P kinase, as no FL3P is
formed when only fructoselysine and ATP are incubated
together under physiological conditions. Further
experiments which involved the fractionation of kidney
cortex have demonstrated that this kinase activity is
not distributed uniformly in the kidney but is
concentrated in the proximal tubular region, which is
one of the earliest anatomical sites to demonstrate
damage in human and animal diabetic kidneys.
FL3P is not stable in aqueous solution. It
rapidly degrades to form 3DG, lysine and inorganic
phosphate. This reaction also occurs in vivo. It is
not currently know if the degradation of FL3P in vivo
is a spontaneous or enzyme catalyzed reaction. It is
strongly suspected, however, that enzymatic catalysis
is involved, as the production of 3DG from fructose-
lysine occurs very rapidly in intact kidney.
The reaction steps in the FL3P lysine
recovery pathway are presented in Figure 2. In the
first step, fructose-lysine and ATP react to form
fructose-lysine-3-phosphate (FL3P) and ADP in a
reaction catalyzed by FL3P kinase. Phosphorylation
occurs on the 3-position of the fructose moiety,
leading to destabilization of the fructoselysine
molecule. The resulting FL3P then decomposes to form
3-deoxyglucosone (3DG), inorganic phosphate, and
CA 02455805 2004-02-23
.
- 23 -
unmodified, free, reusable lysine, which is available
for utilization in protein synthesis. Aldehyde
reductase detoxifies 3DG by reduction to 3-
deoxyfructose (3DF), which is excreted in urine.
Although Figure 2 illustrates this pathway
using the most prevalent glycated-lysine, fructose-
lysine, it will be readily apparent to those skilled
in the art that a wide variety of similar molecules
can flux through this pathway. Indeed, as will be
explained in further detail below, the substrate
selectivity of the FL3P lysine recovery pathway is
quite broad, warranting the broad definition of the
terms given above.
Additional experiments have shown that the
lysine recovery pathway is faund in a wide variety of
animal species, including sheep, pig, dog, rabbit,
cow, mice and chicken. This pathway is also present
in humans. The ubiquitous presence of the FL3P lysine
recovery pathway can be understood, given that lysine
is an essential amino acid which is present in
relatively low concentrations in most foods. In
addition, an appreciable percentage of the lysine
residues in food will exist in the glycated form and
the proportion of this modified lysine will increase
when the food is cooked. Since these glycated lysine
residues can not be utilized for protein synthesis, a
recovery pathway for lysine is of great utility and
affords a selective advantage to organisms which
possess it.
Diabetes has two effects on the lysine
recovery pathway. Blood proteins contain higher
concentrations of glycated-lysines when isolated from
diabetics than from nan-diabetic individuals. Thus,
diabetics are subject to greater flux through the
lysine recovery pathway than non-diabetics.
Additionally, from preliminary observations on the
CA 02455805 2004-02-23
- 24 -
ratios of 3DG arid 3DF in the urine of diabetics and
normals, diabetics appear to have a reduced ability to
detoxify 3DG that is produced via this pathway. These
two factors combine to produce higher urinary
concentrations of 3DG in diabetics (See Figure 7; also
Lal et al., Arch. Biochem. and Biophys., 342(1): 254-
60 (1997) .
The agents involved in the lysine recovery
pathway have been identified in other tissues besides
kidney, specifically red blood cells, lens, and
peripheral nerve tissues. All of these tissues are
affected by the complications of diabetes. The
location in red blood cells correlates with the
microvascular complications of diabetes, e.g.,
diabetic retinopathy, the kidney location correlates
with diabetic nephropathy, while the location in
peripheral nerve correlates with diabetic peripheral
neuropathy. These agents are also found in pancreas.
Experiments are in progress to determine the presence
of these agents in skin. If found to be present, it
is believed that their deleterious effects may be
ameliorated by a topical treatment using the
inhibitory compounds of the invention in a suitable
vehicle to prevent collagen crosslinking, and thereby
improve skin elasticity.
Experiments have been conducted that tend to
prove that humans produce both 3DG and 3DF from orally
ingested proteins containing glycated-lysine residues.
These experiments, which are described in detail
below, convincingly demonstrate that the lysine
recovery pathway exists in humans. These experiments
also shed light on a puzzling phenomenon, namely, that
some diabetics develop diabetic complications, while
others, even those in poor glycemic control, do not
develop such complications. The reason for this
phenomenon is apparent from the data presented herein.
CA 02455805 2004-02-23
r
c - _
25 -
Diabetics have a differing ability to detoxify 3DG. A
subset of the diabetic population appears to have
relatively higher aldehyde reductase activities than
does the majority of diabetics. Consequently, these
individuals are capable of handling the increased flux
through the lysine recovery pathway by efficiently
detoxifying the higher than normal level of 3DG.
Others with impaired capacity are less able to
detoxify their elevated 3DG levels, and consequently
are at higher risk of developing diabetic
complications.
As will be described in more detail below,
it has been experimentally demonstrated that
stimulation of the lysine recovery pathway can occur
through the use of a glycated protein diet. As was
the case with FL above, elevation of FL3P, 3DG and 3DF
was observed in test animals that were fed the
glycated protein diet.
The enzyme inhibitor compounds of the
invention block the lysine recovery pathway,
preventing formation of toxic 3DG from FL3P.
Described below is a set of extensive
criteria that a suitable enzyme inhibitor should
display for use in the practice of this invention, as
well as certain tests for determining if any putative
inhibitor meets these criteria. Candidate kinase
inhibitors fox use in accordance with this invention
may be natural products isolated from plants or
microorganisms. Alternatively, they may be synthetic
molecules derived from the rational knowledge of the
enzymatic reaction and its mechanism. Inhibitors may
also be synthesized by combinatorial methods.
Combinatorial libraries may be generated from a random
starting point. Furthermore, combinatorial methods
can be utilized to generate a wide variety of
compounds related to previously identified inhibitors
CA 02455805 2004-02-23
a.
~:
- 26 -
of the target FL3P kinase.
Regardless of the source of the putative
inhibitor, compounds that do not meet all of the
criteria listed below are not considered useful
therapeutic agents capable of inhabiting the lysine
recovery pathway and thereby preventing, reducing or
delaying the onset of diabetic complications or
disorders of related etiology.
1. The inhibitor should be a small
molecule and readily taken up by cells. In order to
meet this criteria, the inhibitor must have a
molecular weight of less than 2,000 and more ideally
approximately 1,000 daltons or less.
2. The inhibitor must show competitive,
noncompetitive, irreversible or suicide inhibition of
the FL3P kinase. If the inhibitor is a competitive or
noncompetitive inhibitor, the inhibition constant, Ki,
must be less than about 1 mM. Ideally, it must be
less than 100 ~M and more ideally, about 40 ~,M or
less. If the inhibitor shows suicide or other
irreversible inhibition, this requirement for
inhibition constant is rendered moot.
3. The inhibitor must be both soluble in
aqueous solution and stable in aqueous solution at
physiological pH. The requirement for solubility is
met only if the inhibitor, or a salt of the inhibitar,
is soluble in physiological saline or serum at a
concentration equal to or greater than 10 ~M. This
stability requirement is met only if a solution of
inhibitor dissolved in physiological saline at 37°C
retains greater than 50% of its activity after
incubation for one hour. Ideally, the inhibitor must
retain greater than 50% activity upon incubation far
one day or more.
4. The inhibitor must show acceptable
pharmacokinetics. That is, it must remain at a
1
CA 02455805 2004-02-23
therapeutically effective concentration for at least
one hour following administration of the agent.
Ideally, it should maintain effective concentration
for at least eight hours. More ideally, once per day
S dosing should be all that is necessary in order to
maintain a therapeutic concentration of the inhibitor.
This requirement does not mean that the inhibitor must
be able to establish a therapeutic concentration after
the first dose. i~Tumerous examples of successful
pharmaceuticals exist where medical efficacy is seen
only upon prolonged dosing. The criterion does mean
that, once an efficacious concentration is reached,
this concentration should be able to be maintained for
greater than one hour following the last
administration of medication. A test for therapeutic
efficacy is described herein.
S. The inhibitor must be nan-toxic. This
criteria requires that the inhibitor nat demonstrate
human toxicity when administered at the therapeutic
dose. Ideally, toxicity should not be evident when
the inhibitor is present at blood and/or target tissue
levels of twice that needed for therapeutic effect.
More ideally, there should be no appreciable toxicity
at levels 6 or more times the therapeutic range.
Diabetic complications can only be prevented by long
term inhibitor treatment. Therefore, the requirement
for non-toxicity must include both acute toxicity and
chronic toxicity that may become evident over
extended, long term use. Toxicity of candidate
molecules can be readily assessed using well
established animal studies. Human toxicity is
assessed in stage ane clinical trials.
CA 02455805 2004-02-23
28
Included among the compounds useful in the
practice of this invention are those of the formula:
CHZ - X - R
Y
( (I)
z - c - H
I
io Rl
wherein X is -NR'- or -O-, R' being selected
from the group consisting of H, and linear or branched
15 chain alkyl group (C1-C4) and an unsubstituted or
substituted aryl group (C6-Clo) or aralkyl group (C.,-
Clo); R is a substituent selected from the group
consisting of H, an amino acid residue, a
polyaminoacid residue, a peptide chain, a linear or
20 branched chain aliphatic group (C1-Ce), which is
unsubstituted or substituted with at least one
nitrogen- or oxygen-containing substituent, a linear
or branched chain aliphatic group (C1-Ce), which
is unsubstituted or substituted with at least one
25 nitrogen- or oxygen-containing substituent and
interrupted by at least one -O-, -NH-, or -NR"-
moiety, R" being linear or branched chain alkyl group
(C1-C6) and an unsubstituted or substituted aryl group
(C6-C1Q) or aralkyl group (G,-C,o) , with the proviso that
30 when X represents -NR'-, R and R', together with the
nitrogen atom to which they are attached, may also
represent a substituted or unsubstituted heterocyclic
ring having from S to 7 ring atoms, with at least one
of nitrogen and oxygen being the only heteroatoms in
35 said ring, said aryl group (C6-Clo) or aralkyl group
(C.,-Cla) and said heterocyclic ring substituents being
selected from the group consisting of H, alkyl
(C1-C6) , halogen, CF3, CN, NOz and -O-alkyl (C1-C6) ;
R, is a polyol moiety having 1 to 4 linear carbon
CA 02455805 2004-02-23
Schiff-base intermediate to an amine, thereby
producing an inhibitor having an alcohol moiety (i.e.,
Y=-CH(-OH)-). The reactive moiety of an amino acid
reactant, when used, may be the amine group on the
alpha-carbon, or the amine group or hydroxyl group on
the acid side chain. Suitable amino acids encompass
the essential amino acids. Specific examples include
without limitation, glycine, alanine, valine, leucine,
isoleucine, serine, threonine, methionine, aspartic
1o acid, phenylalanine, tyrosine, histidine and
tryptophan. Other suitable reactants are from the
broader class of aminocarboxylic acid, for example,
pyroglutamic acid, beta-alanine, gamma-arninobutyric
acid, epsilon-amino caproic acid and the like. N-acyl
derivatives of the above-mentioned amino acids, such
as formyl lysine, may also be used if desired.
Other appropriate reactants include, without
limitation, unsubstituted or substituted aryl (C6-C10)
compounds, wherein the substituent may be alkyl (C1-
C3), alkoxy, carboxy, vitro or halogen groups,
unsubstituted or substituted alkanes, wherein the
substituent may be at least one alkoxy group; or
unsubstituted or substituted nitrogen-containing
heterocyclic compounds, wherein the substituents may
be alkyl (C1-C3) , aryl (C6-Clo) . alkoxy, carboxy, vitro
or halogen groups. Illustrative examples of the last-
mentioned group of reactants include m-methyl-, p-
methyl-, m-methoxy-, o-methoxy- and m-nitro-
aminobenzenes, o- and p-aminobenzoic acids; n-
propylamine, n-butylamine, 3-methoxypropylamine;
morpholine and piperdine.
Representative inhibitor compounds having
the above formula are set forth in the attached Table
A. Examples of known compounds that may be used as
inhibitors in practicing this invention include,
without limitation, meglumine, sorbitol lysine and
CA 02455805 2004-02-23
- 31 -
mannitol lysine.
The inhibitor compounds described herein can
form pharmaceutically acceptable salts with various
inorganic or organic acids or bases. Suitable bases
include, e.g., alkali metal salts, alkaline earth
metal salts, ammonium, substituted ammonium and other
amine salts. Suitable acids include, e.g.,
hydrochloric acid, hydrobromic acid and
methanesulfonic acid.
The pharmaceutically acceptable salts of the
compounds of formula I can be prepared following
procedures which axe familiar to those skilled in the
art.
The ability of a compound to inhibit the
FL3P kinase can be determined using a wide variety of
kinase activity assays. One useful assay involves
incubating the potential inhibitor with
fructose-lysine and ATP in the presence of kidney
homogenate or other enzyme source. A solution of the
assay components is prepared, which typically contains
1 millimole or less of the inhibitor compound of this
invention, an amount of fructose lysine (FL) in the
range of 1-10 millimoles, an amount of ATP in the
range of 0.1-10 millimoles and an amount of the enzyme
- source which is sufficient to convert FL to fructose
lysine-3-phosphate. The incubation should be
conducted within a pH range of 4.5 to 9.5 and ideally
at neutral or near neutral pH. The incubation should
be carried out at a temperature that is compatible
with enzyme activity, between 4~ and 40°C. Ideally,
the incubation is carried out at physiological
temperature. After incubation, the reaction is
stopped by acid precipitation of the protein and the
production of FL3P measured by 31P-NMR spectroscopy.
FL3P production will be reduced or eliminated in
samples containing an inhibitor compound when compared
CA 02455805 2004-02-23
32 -
to control samples that are free of inhibitor.
Other assays have utility for the rapid
determination of enzyme inhibition. One such assay
involves the use of fructose-lysine and y-labelled 32p
or 33P-ATP. Since FL3P does not bind to Dow-1 but ATP
and most other phosphates do, it is possible to
separate the product FL3P from the remaining reaction
mixture by passing the assay solution through a column
of Dow-1 resin after a predetermined reaction time,
typically 10 minutes. The resultant solution is added
to a container of scintillation liquid, e.g., Ecoscint
A, and counted to determine the amount of
radioactivity produced.
As it is difficult to obtain large
quantities of human tissue, it is preferable to use a
recombinant version of the kinase that is cloned into
an expression system, such as E. Coli. The cloned
kinase can be readily obtained from the "shotgun"
cloning of tissue specific cDNA libraries. Such
libraries are readily available from commercial
sources. For example they may be obtained from
Clontech, Paio Alto, CA. The shotgun cloning
envisioned may be performed using the lambda cloning
system commercially available from Stratagem, located
in San Diego, California. This cloning kit contains
detailed instructions for its use.
The pharmaceutical preparations of the .
present invention comprise one or mare of the
compounds described above, as the active ingredient,
in combination with a pharmaceutically acceptable
carrier medium or auxiliary agent.
These ingredients may be prepared in various
forms for administration, including both liquids and
solids. Thus, the preparation may be in the form of
tablets, caplets, pills or dragees, or can be filled
in suitable containers, such as capsules, or, in the
CA 02455805 2004-02-23
- 33 -
case of suspensions, filled into bottles. As used
herein, °'pharmaceutically acceptable carrier medium"
includes any and all solvents, diluents, or other
liquid vehicle, dispersion or suspension aids, surface
active agents, isotonic agents, thickening or
emulsifying agents, preservatives, solid binders,
lubricants and the like, as suited to the particular
dosage form desired. Representative examples of
suitable carrier media include gelatine, lactose,
1o starch, magnesium stearate, talc, vegetable and animal
fats and oils, gum, polyalkylene glycol, or the like.
Reminaton's Pharmaceutical Sciences, Fifteenth
Edition, E.W. Martin (Mack Publishing Co., Easton, PA
1975) discloses various carriers used in formulating
pharmaceutical compositions and known techniques for
the preparation thereof. Except insofar as any
conventional carrier medium is incompatible with the
enzyme inhibitors of the invention, such as by
producing any undesirable biological effect or
otherwise interacting in a deleterious manner with any
other components) of the pharmaceutical preparation,
its use is contemplated to be within the scope of this
invention.
In the pharmaceutical preparations of the
invention, the active agents) may be present in an
amount of at least 5% and generally not more than 98%
by weight, based on the total weight of the
preparation, including carrier medium and/or auxiliary
agent(s), if any. Preferably, the proportion of
active agent varies between 65% - 95% by weight of the
composition.
Preferred supplementary active agents are
compounds that bind to 3DG in vivo. This class of
compounds includes, without limitation,
aminoguanidine, amino benzoic acid and derivatives
thereof, cysteine and derivatives thereof, amino-
CA 02455805 2004-02-23
T
- 34 -
substituted imidazoles, 1,2-disubstituted
benzimidazoles, substituted 1,2,4-triazoles,
diaminopyridine and derivatives thereof, amina-
substituted pyrimidines, aminoalcohols, diamines and
the like. Anti-hypertensive drugs, including
particularly the angiotensin-converting enzyme (ACE?
inhibitors, may also be included as supplementary
active agents in the pharmaceutical preparations of
this invention.
Io Auxiliary agents, such as compounds that
will protect the active compound from acid destruction
in the stomach or facilitate the absorption of the
active compound into the bloodstream can also be
incorporated into the pharmaceutical preparation, if
necessary or desirable. Such auxiliary agents may
include, for example, complexing agents such as borate
or other salts which partially offset the acid
conditions in the stomach, and the like. Absorption
can be increased by delivering the active compound as
the salt of a fatty acid (in those cases where the
active compound contains one or more basic functional
groups).
The compounds of the invention, along with
any supplementary active ingredients? may be
administered, using any amount and any route of
administration effective for inhibiting the FL3P
lysine recovery pathway. Thus, the expression
°'therapeutically effective amount", as used herein,
refers to a nontoxic but sufficient amount of the
enzyme inhibitor to provide the desired therapy to
counteract diabetic complications or to inhibit the
metabolic production of 3DG for other medical reasons,
such as reducing the effects of aging or other human
disease states where AGE-Protein formation has a
causative role. The exact amount required may vary,
depending on the species, age, and general condition
CA 02455805 2004-02-23
a" a
- 35 -
of the patient, the nature of the complications, the
particular enzyme inhibitor and its mode of
administration, and the like.
The compounds of the invention are
preferably formulated in dosage form far ease of
administration and uniformity of dosage. Dosage unit
form as used herein refers to a physically discrete
unit of enzyme inhibitor appropriate for the patient
to be treated. Each dosage should contain the
quantity of active material calculated to produce the
desired therapeutic effect either as such, or in
association with the selected pharmaceutical carrier
medium. Typically, the compounds of the invention
will be administered in dosage units containing from
about 1 mg to about 2,500 mg of the compound, by
weight of the preparation, with a range of about 5 mg
to about 250 mg being preferred.
The compounds of the invention may be
administered orally, parenterally, such as by
intramuscular injection, intraperitoneal injection,
intravenous infusion or the like, depending on the
nature of the diabetic complication being treated.
The compounds of the invention may be administered
orally or parenterally at dosage levels of about 0.7
~g to about 20 mg and preferably from about 30 ~.g to
about 3.5 mg/kg, of patient body weight per day, one
or more times a day, to obtain the desired therapeutic
effect .
Orally active enzyme inhibitors are
particularly preferred, provided the oral dose is
capable of generating blood and/or target tissue
levels of the inhibitor that are therapeutically
active. Those skilled in the art can readily measure
the levels of a small molecule inhibitor in
deproteinized samples of blood, kidney and other
target tissues. The concentration of inhibitor in
CA 02455805 2004-02-23
3
- 36 -
these samples can be compared with the predetermined
inhibitory constant. Tissue levels that are far below
the inhibitory constant suggest a lack of therapeutic
activity. In the case of irreversible inhibitors,
this lack can be confirmed or refuted by assay of the
FL3P kinase levels in the respective tissue. In all
cases, therapeutic activity can be assessed by feeding
the human or animal subject a food rich in glycated
lysine residues or fructose-lysine and~measuring the
amount of 3DG and 3DF in their urine, both before and
after feeding. Subjects that have therapeutically
active inhibitor in their systems will experience
decreased secretion of both 3DG and 3DF and increased
urinary secretion of fructose-lysine when compared to
25 levels secreted by these same subjects prior to
inhibitor therapy as will be described in further
detail hereinbelow.
The compounds of the invention will
typically be administered once per day or up to 4-5
~ 20 times per day, depending upon the exact inhibitor
chosen. While a dosing schedule of once-a-day is
preferred, diabetic patients are accustomed to paying
close attention to their disease state, and so will
readily accept more frequent dosing schedules if
25 required, so as to deliver the above-mentioned daily
dosage. However, the exact regimen for administration
of the compounds and compositions described herein
will necessarily be dependent on the needs of the
individual patient being treated, the type of
30 treatment administered arid the judgment of the
attending physician. As used herein, the term
"patient" includes both humans and animals.
The inhibitor compounds described herein are
useful in counteracting diabetic complications,
35 especially diabetic nephropathy which affects greater
than forty percent of diabetics and is the primary
CA 02455805 2004-02-23
- 37 -
cause of end stage renal disease requiring dialysis
and transplantation. In addition, these inhibitors
may be used for the prevention or treatment of other
pathological conditions attributable to the formation
of AGE-proteins, such as hypertension, stroke,
neurodegenerative disorders, e.g., senile demential of
the Alzheimers type, circulatory disease,
atherosclerosis, osteoarthritis, cataracts and the
general debilitating effects of aging.
Preliminary experiments have shown that
serious adverse health effects result from stimulation
of the lysine recovery pathway through long-term
consumption of glycated proteins. As was the case
with FL, elevation of FL3P, 3DG and 3DF was observed
in test animals that were fed a glycated protein diet.
See Table B. After eight months of such a diet clear
evidence of kidney pathology, resembling that found in
diabetic kidneys, was seen in the animals on the
glycated protein diet, as described further in Example
10, below. Transient elevation of 3DG and 3DF levels
were also observed in the urine of human volunteers
who ate a small amount of the glycated protein.
TABLE B
% Glycated FL3P cone. 3DG/3DF cones
Protein (aM-in Kidney) (~cM-in plasma)
0 97 1.4/0.05
1 295 -
2.5 605 -
5 937
10 1066 3.6/0.12
20 1259 5.2/0.14
30 1267 6.2/0.28
CA 02455805 2004-02-23
r x
38
Since stimulation of the newly discovered
lysine recovery pathway leads to substantial increases
in systemic 3DG levels, an investigation was carried
out to determine whether a glycated diet would cause
significant effects on pregnancy. The results
obtained so far suggest there is a very strong effect
due to this pathway, as will appear in the examples
that follow.
Furthermore, it is well known that in
susceptible strains of rats and mice the diets on
which the animals are maintained in early life
(following weaning), can have a marked effect on the
incidence of type 1 diabetes, with the incidence
ranging from 10% to 90%. Considerable effort has been
put into investigating this phenomenon over the last
10 years. See, for example, Diabetes, 46(4): 589-98
(1997) and Diabetes Metab. Rev., 12(4): 341-59 (1996),
and references cited therein. An investigation has
been undertaken by certain of the present inventors
with respect to two diets which are at the extremes
for induction of diabetes. AIN-93 (Dyets, Inc.)
causes the least incidence of diabetes and produces
the lowest ratio of urinary 3DF/creatinine (1.0) yet
observed. Purina 500 induces the highest incidence of
diabetes and produces a 2.5 fold increase in the
3DF/creatinine ratio. Since FL3P, 3DG and 3DF were
observed in the pancreas of rats, it is likely that
fructoselysine kinase and the metabolites of this
metabolic pathway are involved in the development of
Type l diabetes. Animals which are susceptible to
this type of diabetes (a useful model of insulin
dependent or Type I diabetes in humans) have an
abnormal immune system which makes them sensitive to
an unknown antigen which develops in the j3-cells of
the pancreas, resulting in an autoimmune attack by the
animal's own immune system on its (3-cells, this
CA 02455805 2004-02-23
- 39 -
results in their subsequent destruction, thereby
depriving the animal of the ability to make insulin.
It is well known that 3DG reacting with proteins can
make new antigenic sites. Thus, the source of the
antigenic properties of the various diets appears to
be the 3DG created by the decomposition of
fructoselysine-3-phosphate in the pancreas.
Also, because 3DG is known to interact with
amines generally, it may be able to interact with DNA
and show mutagenic and carcinogenic potential, as well
as crosslink proteins.
The discovery of the FL3P lysine recovery
pathway makes it practical, for the first time, to
differentiate the diabetic population and to determine
which subset of the population is likely to develop to
diabetic comglications. This determination can be
conveniently carried out on a biological fluid of the
test subject, such as urine, blood fractions
(particularly plasma or serum), lymph fluid,
interstitial fluid or the like.
After an overnight fast, a human subject is
fed a food source containing a relatively high
concentration of glycated-lysine residues. By way of
example, this food can be in the form of a
casein/sugar "cookie", such as described in Example
5, below, or some other suitable source of glycated-
lysines or synthetic fructose-lysine. When proteins
containing glycated-lysine residues are utilized, the
content of glycated-lysine should be preferably
between 0.02 and 10% of total protein amino acid, or
more preferably between about 0.2 and 0.4%. The total
amount of glycated-lysine residues in the oral dose
should be about 0.3 grams. Preferably, a urine sample
is collected before consumption of the glycated-lysine
source, then at one, three and five hours, or such
other appropriate times as may be warranted by the
CA 02455805 2004-02-23
individual clinical situation.
The 3DG and 3DF levels in these urine
samples are measured and the ratios of these
metabolites calculated. The particular methodology
utilized in this measurement is not essential to the
practice of this invention. The GC method described
in Example 5, below, may be utilized, if desired.
Alternatively, colorimetric or immunological assay
methods can be used, as will be apparent to those
skilled in the art.
It is clear that the major risk factor faced
by diabetics is glycemic control, as was clearly
demonstrated by the recently completed Diabetes
Control and Complications Trial. However, the
35 incidence of diabetic complications cannot be
explained solely by blood sugar levels; a fairly wide
scatter is seen when the incidence of diabetic
complications is compared to historical blood sugar
levels.
One method for determining that subset of
the diabetic population which is most at risk for
developing diabetic complications is a particularly
significant aspect of the present invention. This
method involves the measurement of FL, 3DG and 3DF
levels before and, optimally, after ingesting a source
of glycated lysine.
For example, normal subjects have a fasted
3DG to 3DF ratio in urine of about .025, whereas
diabetics have higher ratios, which may be up to five
fold higher, or more. This is borne out by the data
in Figure 7, which shows that normoglycemics have a
3DG/3DF ratio of 0.025 (1/39.77) with quite tight
scatter around this value, whereas diabetics have a
more than 2 fold higher average ratio (average 0.069)
with much more scatter around the average.
As demonstrated herein, diabetics have
CA 02455805 2004-02-23
- 41 -
increased production of 3DG. Therefore, resistance to
diabetic complications requires highly efficient
removal of this toxic metabolite. The ratio of 3DG to
3DF, calculated by the method described herein, allows
one to assess the efficiency of the 3DG detoxification
pathways. Those individuals with low ratio will be
generally resistant to developing diabetic
complications. Individuals with higher ratios,
including ratios contained within the normal range,
are more at risk, while individuals with elevated
ratios above the normal range are particularly at risk
far developing these complications.
Recent measurements of fructoselysine (FL)
in the plasma and urine of four different rat strains
have demonstrated considerable variability in the
manner in which their respective kidneys process FL in
blood. In two of the four strains (Long Evans, Brown
Norway) virtually all of the FL filtered by the kidney
appeared in the urine based upon ratios of this
. 20 compound and its metabolites with creatinine. With
the other two strains (Sprague Dawley, Fischer) 10-200
of the FL in the plasma appeared in the urine, based
on comparisons with creatinine filtration. These
measurements strongly suggest a major variability in
FL processing in the mammalian kidney. Given what is
known about the functional equivalence of rodent and
human kidneys, it is reasonable to assume a similar
variation in FL processing will exist among humans.
Since FL is the primary input to the fructoselysine
recovery pathway, the entire pathway is likely to be
substantially stimulated in those humans in whom a
large amount of FL is absorbed from the ultrafiltrate,
leading to the high local levels of 3-deoxyglucosone
(3DG) in the kidney, as well as systemically
throughout the body. This observation may serve as
the basis of a diagnostic test in which the comparison
CA 02455805 2004-02-23
_ y
- 42
of a sample of plasma or serum contemporaneously
obtained with a urine sample would determine the flux
of FL into the kidney, and the fraction of that flux
which appears in the urine. Those individuals in whom
this ratio is substantially lower than one (1) would
then be at risk for developing a variety of kidney
pathologies including, but not limited to, diabetic
nephropathy, kidney failure in old age and kidney
carcinoma.
Therapeutic efficacy of the kinase
inhibitors of the invention can be easily and safely
determined using a test of the lysine recovery
pathway. The test protocol is identical to the one
presented immediately above, with the exception that
urinary fructoselysine levels are measured in addition
to urinary 3DG and 3DF levels. Tt is useful to
conduct this test both before and after initiating
FZ3P kinase inhibitor therapy. The urine levels of
3DG and 3DF are summed at each time point and compared
to the levels of fructose-lysine measured in the same
sample.
The peak levels of 3DG and 3DF found in
urine following ingestion of food rich in
glycated-lysine residues are derived from the activity
of the lysine recovery pathway. The ratio of the
concentration of these metabolites to unreacted
fructose-lysine (which is a normal component of human
urine) reflects the activity of this pathway.
Inhibition of the lysine recovery pathway will cause a
3o decrease in the amount of 3DG and 3DF excreted, and an
increase in the excreted levels of fructose-lysine.
Thus, therapeutic efficacy of a kinase inhibitor can
be quantitated by measuring the decrease of the (3DG +
3DF)/fructose-lysine ratio following initiation of
therapy. It is noteworthy that urine volume or
metabolite concentrations are not a factor in
CA 02455805 2004-02-23
- 43 -
interpreting this assay, as only a ratio of
metabolites is considered.
It will be appreciated from the foregoing
disclosure that orally digested food containing high
concentrations of glycated-lysine residues will lead
to the production of kidney and serum 3DG. It is
reasonable to caution individuals at risk for kidney
disease, for example diabetics, to avoid food with
these high concentrations. Concentrations of
glycated-lysine residues can be measured using a wide
variety of methods. One such measurement method is
described in Example 4, below. However, any suitable
measurement methodology that accurately determines the
levels of glycated-lysine residues can be substituted
in place of the assay method exemplified below.
Examples of assay methods specifically contemplated
include but are not limited to colorimetric and
immulogical methods.
Regardless of the method of measurement
employed, it is within the scope of the present
invention to determine the content of glycated-lysine
residues in prepared foods and to apprise individuals
at risk for developing kidney dysfunction of these
determinations, so that such individuals may refrain
from ingesting foods high in glycated-lysine content.
The following examples are provided to
describe the invention in further detail. These
examples are provided for illustrative purposes only,
and should in no way be construed as limiting the
invention. All temperatures given in the examples are
in degrees centigrade unless otherwise indicated.
CA 02455805 2004-02-23
1
- 44 -
EXAMPLE 1
ISOLATION AND IDENTIFICATION OF FL3P: A 31p
NMR analysis of a perchloric acid extract of diabetic
rat kidneys showed a new sugar monophosphate resonance
at 6.24 ppm which is not observed in non-kidney tissue
and is present at greatly reduced levels in non-
diabetic kidney. The compound responsible for the
observed resonance was isolated by chromatography of
the extract on a microcrytalline cellulose column
using 1-butanol-acetic acid-water (5:2:3) as eluent.
The structure was determined by proton 2D COSY to be
fructose-lysine 3-phosphate. This was later confirmed
by injecting animals with FL, prepared as previously
described (Finot and Mauson, Helv. Chim. Acta, 52:
1488 (1969)), and showing direct phosphorylation to
FL3P. Using FL specifically deuterated in position-3
confirmed the position of the phosphate at carbon-3.
This was performed by analyzing the 31P NMR spectra
both coupled and decoupled. The normal P-O-C-H
coupling produces a doublet in FL3P with a J value of
10.3 Hz, whereas P-O-C-D has no coupling and produces
a singlet both coupled and decoupled, as was found for
3-deuterated FL3P. A unique property of FL3P is that
when treated with sodium borohydride it is converted
into two new resonances at 5.85 and 5.95 ppm, which
correspond to marznitol and sorbitol-lysine 3-
phosphates.
EXAhIPLE 2
SYNTHESIS OF FL3P: 1 mmol of dibenzyl-
glucose 3-phosphate and 0.25 mmol of a-carbobenzoxy-
lysine was refluxed in SO ml of MeOH for 3 hours. The
solution was diluted with 100 ml water and
chromatographed on a Dow-SO column (2.5 x 20 cm) in
the pyridinium form and eluted first with water (200
ml) and then with 60o ml buffer (0.1M pyridine and
CA 02455805 2004-02-23
.
a _ 45 _
0.3M acetic acid). The target compound eluted at the
end of the water wash and the beginning of the buffer
wash. Removal of the cbz arid benzyl blocking groups
with S% Pd/C at 20 psi of hydrogen gave FL3P in 6%
yield.
EXAMPLE 3
ENZYMATIC PRODUCTION OF FL3P FROM FL AND ATP
AND ASSAY FOR SCREENING INHIBITORS: Initially 31P NMR
was used to demonstrate kinase activity in the kidney
cortex. A 3 g. sample of fresh pig kidney cortex was
homogenized in 9 ml. of 50 mM Tris~HCl containing 150
mM KC1, 5 mM DTT, 15 mM MgCl2, pH 7.5. This was
centrifuged at 10,000 g for 30 minutes, and then the
supernate centrifuged at 100,000 g for 60 minutes.
Ammonium sulfate was added to 60o saturation. After 1
hour at 4o the precipitate was collected by
centrifugation and dissolved in 5 rnl. of original
buffer. A 2 ml aliquot of this solutian was incubated
with 10 mM ATP and 10 mM of FL (prepared as in Example
1, above) for 2 hours at 370. The reaction was
quenched with 300 uL of perchloric acid, centrifuged
to remove protein, and desalted on a column of
SephadeX G 10 (5 x 10 cm?. '1P NMR analysis of the
reaction mixture detected formation of FL3P.
Based on the proof of kinase activity thus
obtained, a radioactive assay was developed. This
assay was designed to take advantage of the lack of
binding to Dow 1 anion exchange resin by FL3P. This
characteristic of FL3P was discovered during efforts
to isolate it. Since most phosphates bind to this
resin, it was suspected that the bulk of all compounds
that react with ATP as well as any excess ATP would be
bound, leaving FL3P in solution. The first step was
to determine the amount of resin required to remove
the ATP in the assay. This was accomplished by
*Trade-mark
CA 02455805 2004-02-23
- 46 -
pipetting the mixture into a suspension of 200 mg, of
Dow-1 in 0.9 ml HZO, vortexing and centrifuging to pack
the resin. From this 0.8 m1. of supernate was
pipetted onto 200 mg. of fresh dry resin, vortexed arid
centrifuged. A 0.5 ml volume of supernate was
pipetted into 10 ml of Ecoscint A and counted.
Residual counts were 85 cpm. This procedure was used
for the assay. The precipitate from 60% ammonium
sulfate precipitation of the crude cortex homogenate
was redissolved in the homogenate buffer at 4o. The
assay contains 10 mM y33P-ATP (40,000 fpm), 10 mM FL,
150 mM KCl, 15 mM MgCIZ, 5 mM~DTT in 0.1 ml of 50 mM
Tris~HCl, pH 7.5. The relationship between rates of
FL3P production and enzyme concentration was
determined using triplicate determinations with 1,2
and 4 mg of protein for 30 minutes at 370. Blanks run
concurrently without FL were subtracted and the data
recorded. The observed activity corresponds to an
approximate FL3P synthesis rate of 20 nmols/hr./mg.
protein.
EXAMPLE 4
INHIBITION OF THE FORMATION OF 3-DEOXYGLUCOSONE
BY MEGLUMINE AND VARIOUS POLYOLLYSINES
a. Geaexal polyollysine syntJ~esis.
The sugar (11 mmoles), a-carbobenzoxy-lysine
(1o mmols) and NaBH3CN (15 mmoles) were dissolved in 50
ml of MeOH-H20 (3:2) and stirred at 25a for 18 hours.
The solution was treated with an excess of Dow-SO (H)
ion exchange resin to decompose excess NaBH3CN. This
mixture (liquid plus resin) was transferred onto a
Dow-50 iH) column (2.5 x 15 cm) and washed well with
water to remove excess sugar and boric acid. The
carbobenzoxy-polyollysine was eluted with 5% NH4
OH. The residue obtained upon evaporation was
dissolved in water-methanol (9:1) and reduced with
CA 02455805 2004-02-23
s ~.
hydrogen gas (20 psi) using a 10% palladium on
charcoal catalyst. Filtration and evaporation yields
the polyollysine.
b. Experimental protocol for reduction of
urinary and plasma 3-deoxyglucosone by sorbitollysine,
mannitollysine and galactitollysine.
Urine was collected from six rats for three
hours. A plasma sample was also obtained. The
animals were then given 10 ~mols of either
sorbitollysine, mannitollysine, or galactitollysine by
intraperitoneal injection. Urine was collected for
another three hours, and a plasma sample obtained at
the end of the three hours.
3-deoxyglucosone was measured ~.n these
samples, as described in Example 5, below, and
variable volumes were normalized to creatinine.. The
average reduction of urinary 3-deoxyglucosone was 50%
by sorbitollysine, 35% by mannitollysine and 35% by
galactitollysine. Plasma 3-deoxyglucosone was reduced
40% by sorbitollysine, 58% by mannitolysine and 50% by
galactitollysine.
c. Use of meglumine to reduce urinary 3-
deoxyglucosone.
Three rats were treated as in b),
immediately above, except meglumine (100 ~cmols) was
injected intraperitoneally instead of the above-
mentioned lysine derivatives. Three hours after the
injection the average 3-deoxyglucosone concentrations
in the urine were decreased 42%.
EXAMPLE 5
ELEVATION OF URINARY FL, 3DG AND 3DF IN
HUMANS FOLLOWING INGESTION OF GLYCATED PROTEIN:
a. Preparation of glycated protein
containing food product: 260 g. of casein, 120 g. of
glucose and 720 ml. of water were mixed.to give a
CA 02455805 2004-02-23
48 -
homogeneous mixture. This mixture was transferred to
a metal plate and cooked at 65o for 68 hours. The
resulting cake was then pulverized to a course powder.
This powder contained 60% protein as
determined by the ~Cjeldahl procedure.
b. Measurement of glycated lysine content:
1 g of the powder prepared as in step a., above, was
hydrolyzed by refluxing with 6N HCl for 20 hours. The
resulting solution was adjusted to pH 1.8 with NaOH
solution and diluted to 100 ml. The fructoselysine
content was measured on an amino acid.analyzer as
furosine, the product obtained from acid hydrolysis of
fructoselysine. In this way, it was determined that
the cake contained 5.5% (w/w) fructoselysine.
c. Experimental protocol: Volunteers
spent two days on a fructoselysine-free diet and then
consumed 22.5 g of the food product prepared as
described herein, thus effectively receiving a 2 g.
dose of fructoselysine. Urine was collected at 2 hour
intervals for 14 hours and a final collection was made
at 24 hours.
d. Measurement of FL, 3DG and 3DF in
urine: FL was measured by HFLC with a Waters 996
diode Array using a Waters C18 Free Amino Acid column
at 46o and a gradient elution system of acetonitrile-
methyl alcohol-water (45:15:40) into acetonitrile-
sodium acetate-water (6:2:92) at 1 ml./min.
Quantitation employed an internal standard of
meglumine.
3DF was measured by HPLC after deionization
of the sample. Analyses were performed on a DioneX
DX-500 HFLC system employing a PA1 column (Dionex) and
eluting with 32 mM sodium hydroxide at 1 ml./min.
Quantitation was performed from standard curves
obtained daily with synthetic 3DF.
3DG was measured by GC-MS after deionization
*Trade-mark
CA 02455805 2004-02-23
av
- 49
of the sample. 3DG was derivatized with a l0-fold
excess of diaminonaphthalene in PBS. Ethyl acetate
extraction gave a salt free fraction which was
co~verted to the trimethyl silyl ethers with Tri-Sil~
(Pierce). Analysis was performed on a Hewlett-Packardx
5890 selected ion monitoring GC-MS system. GC was
performed on a fused silica capillary column (D8-5,25
mx.25 mm? using the following temperature program:
injector port 2500, initial column temperature 1500
which is held for 1 minute, then increased to 2900 at
15~/minute and held for 15 minutes. Quantitation of
3DG employed selected ion monitoring using an internal
standard of U-13C-3DG,
The graph shown in Figure 3 represents
production of FL, 3DF and 3DG in the urine of one
volunteer after consuming the glycated protein. The
rapid appearance of all three metabolites is clearly
evident. Both 3DF and 3DG show a slight elevation
even after twenty-four hours.
The graph shown in Figure 4 represents the
formation of 3DF in each of the members of a seven
person test~group. A similar pattern was seen in all
cases. As appears in Figure 4, 3DF excretion peaks
about 4 hours after the FL bolus and a slight
elevation of 3DF is noticeable even 24 h after the
bolus.
EXAMPLE 6
FEEDTNG EXPERTMENT: N-acetyl-/3-
glucosaminidase (NAGase) is an enzyme excreted into
the uxine in elevated concentration in diabetics. It
is thought to be an early marker of tubular damage,
but the pathogenesis of increased NAGase in urine is
not well understood. The increased urinary output of
NAGase in diabetics has been proposed to be due to
activation of lysosomes in proximal tubules induced by
*Trade-mark
CA 02455805 2004-02-23
v
- 50 -
diabetes with an increased output into the urine
rather than destruction of cells.
The results obtained in this example show
that in all comparisons 3DF and NAGase levels are
S elevated in the experimental group relative to the
control. Thus, animals fed glycated protein excrete
excess NAGase into their urine, similar to results
obtained with diabetics. There is an approximate 50a
increase in NAGase output compared with control
animals. These animals also have a five-fold increase
in urine 3DF compared with controls. ~ZJrinary 3DF
correlates extremely well with 3DG, as can be seen in
Figures 5 and 6. Both compounds appear to be removed
from the plasma at the glomerular filtration rate,
with no reabsorption.
EXAMPLE 7
SDS GEL GF KIDNEY PROTEINS: Two rats were
injected daily with 5 ~mols. of either FL or mannitol
fused as a control) for 5 days. The animals were
sacrificed and the kidneys removed-and dissected into
the cortex and medulla. Tissues were homogenized in 5
volumes of 50 mM Tris~HCl containing 150 mM KCl, 15 mM
MgCl2 and 5 mM DTT, pH 7.5. Cellular debris was
removed by centrifugation at 10,000 g for 15 minutes,
and the supernate was then centrifuged at 150,000 g
for 70 minutes. The salable proteins were analyzed by
SDS PAGE on 12% polyacrylamide gels as well as on 4-15
and 10-20o gradient gels. In all cases, lower
molecular weight bands were missing or visually
reduced from the kidney extract of the animal injected
with FL when compared with the animal injected with
mannitol.
CA 02455805 2004-02-23
- 51 -
EXAMPLE 8
SYNTHESIS OF 3-O-METHYLFRUCTOSE LYSINE:
A suspension of 19.4 g (0.1 mol) of
anhydrous 3-O-methyl glucose and 1 g of sodium
bisulfate in 30 ml of methanol and 15 ml of glycerol
was refluxed for 30 minutes, followed by the addition
of 0.035 mol of a-carbobenzoxy-lysine arid 4 ml of
acetic acid. This solution was refluxed for 3 hours.
The solution was treated with 1 volume of water and
chromatographed on a Dowex-50 column (4x50 cm) in the
pyridinium form, and eluted first with water and then
with pyridinium acetate. Fractions containing the
pure material were combined and evaporated. The
resulting material was dissolved in 50 ml of water-
methanol (9:1) and reduced with hydrogen gas (20 psi)
using a loo palladium on charcoal catalyst.
Filtration and evaporation gave 3-O-methyl-
fructoselysine.
Other specific compounds having the
structure of formula (I), above, may be made e.g. by
glycation of a selected nitrogen- or oxygen-containing
starting material, which maybe an amino acid,
polyaminoacid, peptide or the like, with a glycating
agent, such as fructose, which may be chemically
modified, if desired, according to procedures well
know to those skilled in the art.
EXAMPLE 9
ADDITIONAL ASSAY FOR FL3P ICINASE ACTIVITY:
a. Preparation of Stock Solutions:
An assay buffer solution was prepared which
was 100 mM HEPES pH 8.0, 10 mM ATP, 2 mM MgCl~, 5 mM
DTT, 0.5 mM PMSF. A fructosyl-spermine stock solution
was prepared which was 2 mM fructosyl-spermine Hcl. A
spermine control solution was prepared which was 2 mM
spermine Hcl.
CA 02455805 2004-02-23
- 52 -
b. Synthesis of Fructosyl-spermine:
Synthesis of fructosyl-spermine was performed by an
adaptation of a known procedure (J. Hodge and B.
Fisher, Methods Carbohydr. Chem., 2: 99-107 (1963)).
A mixture of spermine (500 mg), glucose (500 mg) and
sodium pyrosulfite (80 mg) was prepared in a molar
ratio of 8:4:1 (spermine:glucose:pyrosulfite) in 50 ml
of methanol-water (1:1) and refluxed for 12 hours.
The product was diluted to 200 ml with water and
loaded onto a DOW-50 column (5x90 cm). The unreacted
glucose was removed by 2 column volumes of water and
the product and unreacted spermine were removed with
0.1 M NH40H. Pooled peak fractions of the product were
lyophilized and concentration of fructosyl-spermine
was determined by measuring the integral of the C-2
fructosyl peak in a quantitative ~3C NMR spectrum of
the product (NMR data collected with a 45o pulse, a 10
second relaxation delay and without NOE decoupling).
c. Assay of Kinase for Purification:
An incubation mixture was prepared including
10 ~C1 of the enzyme preparation, 10 ~.1 of assay
buffer, 1.0 ~.Ci of 33P ATP, 10 ~.1 of fructosyl-spermine
stock solution and 70 ~,1 of water and incubated at
37°C for 1 hour. At the end of the incubation 90 ~,1
(2x451) of the sample is spotted onto two 2.5 cm
diameter cellulose phosphate disks (Whatman P-81) and
allowed to dry. The disks were washed extensively
with water. After drying, the disks were placed in
scintillation vials and counted.
Each enzyme fraction was assayed in
duplicate with an appropriate spermine control.
CA 02455805 2004-02-23
- 53 -
EXAMPLE 10
KIDNEY PATHOLOGY 08SER~lED IN TEST
ANIMALS ON GLYCATED PROTEIN DIET
Three rats were maintained on a glycated
protein diet (20a total protein; 3% glycated) for 8
.months and compared to 9 rats of the same age
maintained on a control diet. The primary finding was
a substantial increase in damaged glomeruli in the
animals on the glycated diet. Typical lesions
1.0 observed in these animals were segmental sclerosis of
the glomerular tuft with adhesion to Bowman's capsule,
tubular metaplasia of the parietal epithelium and
intestitial fibrosis. All three of the animals on the
glycated protein diet, and only one of the animals on
the control diet showed more than 13o damaged
glomeruli. The probablity of this happening by chance
is less than 20, In addition to the pathology
observed in the glomeruli, a number of hylinated casts
within tubules were observed. Mare of these were
~ 20 found in animals on the glycated diet, although these
were not quantitated. Increased levels of NAGase were
also observed in the animals on the glycated diet.
From the results of this experiment, the
glycated diet appeared to cause the test animals to
develop a series of histological lesions similar to
those seen in the diabetic kidney.
EXAMPLE 11
EFFECTS OF GLYCATED DIETS ON PREGNANCY
In a preliminary experiment, 5 mice pairs
were placed on a glycated diet (18o total protein; 3%
glycated) and bred six times over a period of 7
months. The resulting six pregnancies produced the
following live pups; 17, 23, 13, 0, 3 and 0. In view
of this sharp drop in live pups after the third
CA 02455805 2004-02-23
c
.,
- 54 -
breeding, two cohorts of ten pairs each were put on
either a glycated diet (13o total protein; 3%
glycated) or a control diet (13% total protein; o0
glycated). Thus far, the two groups of pups have
been bred four times obtaining similar results in both
groups. The first pregnancy produced 49/20
(glycated/control) pups; the second, 18/41; the third
37/27; and the fourth 20/33. The fifth pregnancy is
currently underway. The mice pairs have been tested
for hyperglycemia. The blood glucose levels are 120
and 1.12 mg/dl in the experimental and control groups,
respectively.
Preliminary measurements of the 3DF levels
in the mice urine indicate, as expected, a substantial
elevation (approximately 5-10 fold) of the systemic
3DF when on the glycated diet described herein.
EXAMPLE 12
CARCINOGENIC EFFECTS OF FRUCTOSELYSINE PATHWAY
To investigate the carcinogenic potential of
metabolites formed in the fructoselysine pathway,
experiments have been conducted on a strain of rats
with a high susceptibility to kidney carcinomas. Four
rats were put on a glycated protein diet and three
rats on a control diet. After ten weeks on the diet,
the animals were sacrificed and their kidneys
examined. In all four animals on the diet, kidne~r
carcinomas of size greater than lmm were found,
whereas no lesions this large were found in the
control animals. The probability of this happening by
chance is less than 2%. The data show that the
elevated 3DG levels caused by the excess
fructoselysine coming from the glycated protein in the
animals diet found in the kidney tubular cells (known
to be the cell of origin of most kidney carcinomas)
can interact with the cellular DNA leading to a
CA 02455805 2004-02-23
- 55
variety of mutogenic and ultimately carcinogenic
events. The possibility exists that this process is
important in the development of human cancers in the
kidney and elsewhere.
While certain embodiments of the present
invention have been described and/or exemplified
above, various other embodiments will be apparent to
those skilled in the art from the foregoing
disclosure. The present invention is, therefore, not
limited to the particular embodiments described and/or
exemplified, but is capable of consideration variation
and modification without departure from the scope of
the appended claims.
CA 02455805 2004-02-23
- 56 -
M
x
U
i
U
, o x
x
0
-U - O ~ o O
- , b
-
x
x
x x
x x
x x x o o x x
o~~ U ~ '
-U U ~ -U- U -U- U U
- - -
W rt
N O
U U
H (~I - - U- ro ,. a
d
---t
x
x
m z b
_r,
w
o a
o a~
.a
z
o ~, "~
~ J-) ~ M r-i ~
rr r-I
~ ~ r-i
O
1~ O ~
U O O -r-I
f'3 p rt3 Q1
~
U r~ ~ ~ ~ ~ O
M
M fJ)
CA 02455805 2004-02-23
- 57 -
P 1
0 0
0 1 1
a o -~n-o w x
x
0
0 1
-u- vp o -u-.
,~
x
x
0 0 0 0 0 x x x o
--.U-U -U-U-U ~ -U-~..-U.-U
..C~ m 1 U .-. N
cv
p rv o N N-~ U
- Z
U U
,r,
-V-~ .~ -
'
'
'
~
O
O
. . ~ m
p p x
U
N
la
N
r-I ri
s
N O
U
-
rd
~1
~
4-t O
1
i m
L
ct p
..
~
b ~' O
U
1
a3
(U U N
'r1 ~ f-i
p p ~ ~ ~ ~
_ t/~ (a ~
O
'
r O
L1 l. -I ?" U
U ~ U t (d Gl ~.i r
1
1 ~ ~ M
~ b ~ s 1 1
-1 ss ch u1 N ..Q U