Note: Descriptions are shown in the official language in which they were submitted.
CA 02456538 2004-02-05
WO 03/018908 PCT/FI02/00684
1
METHOD IN A PAPER OR PULP PROCESS TO CONTROL THE CHEMICAL STATE
OF THE PULP AND CIRCULATION WATER SYSTEM
The present invention relates to a method in a paper or pulp
process to control the chemical state of the pulp and circula-
tion water system, in which said process one or more raw-
material components diluted in liquid, possible fillers, and
one or more additives are mixed to form stock.
At paper mills, various fibrous raw-material components are
stored in storage towers. These are advantageously combined, to
create a wet-formable stock, i.e. a watery sludge of fibres and
other raw materials for manufacturing paper.
The said raw-material components can be, for example,
mechanicals pulps, for instance mechanical pulps from chips and
groundwood pulps (SGW, RMP, TMP, etc.) or chemical pulps, such
as cellulose. Nowadays, a large amount of deinked waste paper
fibre, i.e. so-called DIP pulp (DeInked Pulp) is also used,
along with the reject pulp that arises during production,
sometimes even in large quantities, and which is also taken as
one of the raw-material components in stock preparation, to
utilize it as efficiently as possible.
According to the state of the art, the control of the chemical
state of the pulp and circulation water system, for example,
the control of phase changes, significantly affects the success
of the papermaking process. The chemical state can be used to
affect such things as the runnability properties of the
machine, the paper quality, the dirtying of the machine, the
environmental load, and the operation of the waste-water
treatment plant of the paper or pulp mill. Disequilibrium in
the chemical state can lead to sedimentation, gas formation,
and diminished retention in the stock, for example.
CA 02456538 2004-02-05
WO 03/018908 PCT/FI02/00684
2
According to the state of the art, several variables, such as
pH level, salt content, and temperature are regulated in an
attempt to control the chemical state. This takes place, for
example, by adding chemicals after the raw materials have been
mixed.
If the raw-material components must be held in the storage
towers for a inconveniently long period, for example during a
break in production, changes in their chemical state that are
detrimental to the process may take place. Thus, for example,
bacterial activity may result in the formation of sulphur
compounds, such as hydrogen sulphide, in chemical pulps.
Similar problems also appear with DIP and reject pulps.
The changes in the chemical state of the raw-material compo-
nents cause uncontrollable reactions when the components are
mixed in the mixing tank of the paper machine. For example,
sedimentations or gases that lead to porosity in the paper web
can then arise. Such factors substantially weaken the runn-
ability properties of the paper machine.
According to the state of the art, the pH value, i.e. the
acidity level, presently appears to be an important variable,
for example, in controlling the chemical state of the raw-
material components of the paper process, as changes take place
in the charge level and solubility of the wood material when
the pH value changes. Good pH-value control is an essential
variable in achieving even retention, i.e. the percentage of
solids remaining in the web in the wire section compared to
that in the headbox feed. Further, the pH value affects not
only the retention substances, but also the action of other
additives, the control of the phase changes, the runnability
and dirtying of the machine, and the quality of the paper.
The pH value is controlled with the aid of lye or sulphuric
acid, which can be dosed, for example, into the wire pit,
CA 02456538 2009-05-22
3
either automatically or manually. Careful control movements are
essential, as sudden changes can cause pH shocks or other
disturbances. A sudden change in the pH value will cause the
sedimentation of a dissolved or dispersed substance. Control of
the pH value is slow, because the regulating chemical must be
allowed to enter the fibre and the pH measurement point must be,
located to provide the measurement result after a specific
delay.
On the other hand, controlling the pH value with the said
substances introduces additives, such as sodium and sulphur,
which are detrimental to the process, and which for their part
will cause problems, such as reduced retention;.-in the wire
section.
-
The pH value is usually well controlled in the pulp and
circulation water system of a paper machine. However, attempts
to control phase changes in the paper process have generally
taken place through sensitive control of the pH value, thus
affecting, for instance the general runnability of the machine,
due to the varying charge level.
In the paper and pulp process, the state of the art is also
represented by the measurement of the charge levels of the
liquids, for example, through the definition of the zeta
potential. However, as this is a complicated way to carry out
definition, it is very difficult and cannot be carried out with
complete reliability directly from the production environment.
The present invention is intended to create an new type of
method in a paper or pulp process to control the chemical state
of the pulp and circulation water system. The paper process can
also be held to include board and pulp manufacture.
CA 02456538 2004-02-05
WO 03/018908 PCT/FI02/00684
4
It has been observed that there may be quite natural signifi-
cant differences in the electrochemical states of the raw-
material components, without any particular disturbing factors.
Thus sedimentation or gas formation, for instance, can occur in
the stock. The pilot-stage research has also shown that
differences between the electrochemical states of the raw-
material components have a significant effect on their mutual
miscibility and the formation of bounds between them. The
method according to the invention is characterized by the
regulation in the pulp and circulation water system of the
electrochemical state of at least one raw-material component
and/or the stock. The regulation can be carried out on, for
example, a raw-material component, on part of it, or on a
mixture of two or more raw-material components, before the
stock formation, or, for example, on the stock in the short
circulation, or before it is fed from the headbox to the web-
formation section.
In the method according to the invention no essential changes
are caused in the pH values of the raw-material components
and/or of the stock. This has the effect of substantially
reducing, among other things, the dosing cycle of additives,
caused by the operating manner according to the state of the
art.
In the method according to the invention, when a raw-material
component and/or stock is in the reduction area, its electro-
chemical state is regulated, for example, by equalizing it or
increasing it in relation to the other pulp components,
according to one preferred embodiment by using a suitable
oxidizing or reducing compound or substance, while the electro-
chemical state of a raw-material component in the oxidizing
area is regulated by using a suitable reducing or oxidizing
compound or substance.
CA 02456538 2004-02-05
WO 03/018908 PCT/FI02/00684
According to a second preferred embodiment, the electrochemical
state of a raw-material component or stock can be regulated by
connecting it to a selected potential level by means of an
external source of alternating or direct current. Thus no
5 additives are required. Further, the regulation by means of
chemical can be used in conjunction with regulation using an
external power source. In regulation carried out using an
external power source, the polarity can also be reversed.
According to a third preferred embodiment, in the method
according to the invention the electrochemical state of the
process can be regulated through the order of mixing the raw-
material components. The order can be optimized to suit
different running situations.
The method according to the invention substantially improves
the control of the chemical state, the paper quality, and the
runnability of the machine in general, besides reducing the
environmental load. Further, corrosion in the machine struc-
tures and in the pulp and circulation water systems is signifi-
cantly reduced and the formation of gas diminishes, leading to
a substantial improvement in the reliability of consistency
measurements, among other things.
Further, the method according to the invention can also be used
to advantageously affect the retention of the wire section, the
amount of fines, and possible bacteria growths in the liquid.
This further reduces the need for chemicals. In addition, the
method according to the invention brings further advantages in
improved paper strength and formation and in the control of the
paper-web edges, which significantly affects the effective
production capacity.
The other characteristic features of the method according to
the invention will be apparent from the accompanying Claims
CA 02456538 2004-02-05
WO 03/018908 PCT/FI02/00684
6
while other advantages to be gained are stated in the descrip-
tion section.
In the following, examples of embodiments relating to the
method according to the invention are examined with reference
to the accompanying drawings, in which
Figure 1 shows a schematic example of one embodiment
of the method according to the invention,
Figure 2a shows a schematic example of a second embodi-
ment of the method according to the inven-
tion, and
Figure 2b shows a schematic example of a third embodi-
ment of the method according to the inven-
tion.
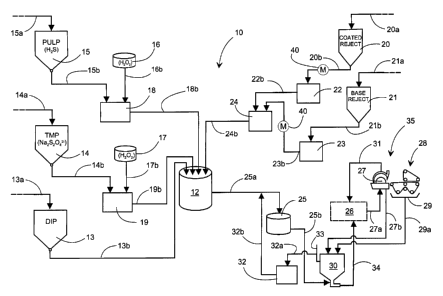
Figure 1 shows a diagram of one preferred embodiment of the
method according to the invention, which depicts very roughly
on a schematic level the pulp and circulation water system 10
of a paper machine 35.
In the pulp system, the storage towers 15, 14, 13 for the raw-
material components (PULP, TMP, DIP) are shown. Raw materials
are brought to them along lines 15a, 14a, 13a, for example,
from a pulp mill, pulp pretreatment plants, such as disintegra-
tion (bale pulper) and grinding, or other similar places (not
shown). From the storage towers 15, 14, the PULP and TMP pulps
are lead along lines 15b, 14b through possible cleaners,
defibrators, or other operational devices (not shown) to their
dosing tanks 18, 19. The dispersed DIP pulp 13 can also be lead
along a line 13b direct to the mixing tank 12. From the PULP
and TMP dosing tanks 18, 19, the pulp is dosed to lines 18b,
19b, through possible beating stages (not shown), to the mixing
tank 12.
CA 02456538 2004-02-05
WO 03/018908 PCT/FI02/00684
7
On the paper machine 35, the headbox 27 and wire section 28 of
which are shown in Figure 1, even large amounts of reject pulp
can arise at times, the necessary means for collecting and
processing which, such as conveyors and machine pulpers (not
shown), are arranged in connection with the machine 35.
Situations that create reject pulp arise, for example, during
web breaks, and particularly when problematically operating
production is being start up, when reject pulp is formed at the
production speed and over the production width of the machine
Zo 35.
Reject also arises during actual production running, as does,
on a smaller scale, for example, edge strips cut on the machine
35 and edge parts cut from reels during finishing, which
annually represents a considerable amount of highly processed
raw material, obtained from large production inputs. Depending
on the type of machine and the point at which the reject pulp
arises, the reject pulp may be coated or uncoated, i.e. so-
called base reject.
Generally, separate processing is arranged for both types of
reject, including collection lines 20a, 21a for leading the
reject pulp, for example, from machine pulpers (not shown) to
the storage towers 20, 21 for coated and uncoated reject. The
coated reject is led on from the storage tower 20 along line
20b to the reject tank 22 and on along line 22b to the reject
pulp dosing tank 24. The uncoated reject is led along line 21b,
for example, through precipitation (not shown) to the reject
tank 23, and from there along line 23b to the same dosing tank
24 as the coated reject. From there, the reject pulp is dosed
in a suitable ratio over line 24b to the mixing tank 12.
In the mixing tank 12, the raw-material components are mixed
together to form a so-called high consistency pulp, which is
led along line 25a to the machine tank 25. From the machine
tank 25, the high consistency pulp is dosed over the line 25b
CA 02456538 2004-02-05
WO 03/018908 PCT/FI02/00684
8
to the wire pit 30, in which it dilutes to form stock. The
stock is transferred over line 34 to the short circulation 26
of the paper machine 35, which incorporates, for instance,
hydro-cyclone cleaning and deaeration devices, as well as
filters and pumps (not shown).
In the short circulation 26, the stock is diluted, sorted, and
transferred by a head feed pump (not shown) along line 27a to
the headbox 27, which is used to spread the stock evenly to the
subsequent wire section 28. In the wire section 28, the stock
is wet-formed to create a paper web, when most of the water is
removed from it.
In the headbox 27, a bypass circulation 31 is arranged, which
is used to return excess stock that has been led there to the
deaeration (not shown) of the short circulation 26. From the
headbox 27 and the subsequent wire section 28, devices 29 are
used to collect the so-called tail water, which contains both
water and also material not retained in the web (0-water).
The tail water is led by lines 27b, 29a to the wire pit 30,
from which it is reused at various points in the pulp systems
and the short circulation 26. Besides the tail water, chemi-
cally purified water and raw water are generally used in the
processes, all of which together are referred to in the
following as process water. An overflow 33 is arranged in the
wire pit 30, the tail water from which is led by line 32a to
the circulation water tank 32. Water is added from this, for
example, over line 32b to the connecting line 25a between the
mixing tank 12 and the machine tank 25.
The addition of fillers and additives, diluted to suitable
proportions (not shown), takes place, for example, in connec-
tion with the wire pit 30.
CA 02456538 2004-02-05
WO 03/018908 PCT/FI02/00684
9
It should be noted that the above description of the pulp and
circulation water systems of a paper machine 25 is given by way
of a rough example, so that it lacks, for example, inessential
operating devices from the point of view of the invention, such
as defibrators, pulpers, grinders, precipitators, sorters, and
other intermediate storage. In addition, implementations of the
systems may also differ from each other, due to the paper
grades being manufactured and the machine concepts.
The method according to the invention can be advantageously
applied not only to the pulp and circulation water systems 10
shown in the embodiment example, but also, for example, to a
board machine and the pulp manufacturing process.
In the method according to the invention, the electrochemical
state of at least one raw-material component PROCESS WATER,
TMP, PULP, REJECT, DIP, and/or the stock formed from them is
controlled by regulation. According to one preferred embodi-
ment, an attempt is made to equalize the electrochemical states
of the raw-material components, before mixing them together.
Thus, in addition to the aforementioned pulps, the process
waters and generally the substances (for example, fillers and
additives) mixed into the liquids in the processes described
can also be regarded as raw-material components.
The electrochemical state of a raw-material component PROCESS
WATER, TMP, PULP, REJECT, DIP can be expressed, for example, as
the level of its electrical potential. In the method according
to the invention, the regulation of the electrochemical state
of a raw-material component PROCESS WATER, TMP, PULP, REJECT,
DIP involves according to a first embodiment either raising or
lowering the level of its potential, in such a way that the
electrochemical states of the raw-material components fed into
the mixing tank 12 are essentially more equalized than before
their regulation, without substantially altering the pH value
of the components, due to a rise or drop in the level of their
CA 02456538 2004-02-05
WO 03/018908 PCT/FI02/00684
electrochemical potential. According to a second preferred
embodiment, the difference between the electrochemical states
of the raw-material components can also be increased, to
eliminate detrimental reactions and to increase advantageous
5 reactions.
If the raw-material component PROCESS WATER, TMP, PULP, REJECT,
DIP is in the oxidizing range before it is led to the mixing
tank, according to a first embodiment a reducing agent (for
10 example, sulphur dioxide, SO2) is added to it, to reduce the
value of the electrical potential of the raw-material component
to the chosen level, or else an oxidizing agent (for example,
hydrogen peroxide H202) is added, to raise the value of the
electrical potential to a chosen level that is advantageous to
the mixing to be carried out with the other pulp components.
Correspondingly, if the chemical state of the raw-material
component is in the reducing range, the substance added to it
is an oxidizing agent (for example, H202), which raises the
electrical potential to the chosen level. If it is desired to
lower the potential, a reducing agent, for example SOZ, is
added. In the method, it is essential for the said reducing or
oxidizing agents to be added not to affect the pH value of the
raw-material components.
Figure 1 shows an example, in which the bleaching of a mechani-
cal pulp TMP 14, carried out by using reducing dithionite
(Na2S2O92-) , significantly lowers the potential of the raw-
material component, leading to unfavourable reactions in the
process. According to a preferred embodiment, hydrogen peroxide
(H202), for example, which has a known reducing effect, can be
added from the tank 17. The addition is made through the line
17b to the dosing tank 19, essentially before the mixing tank
12, and thus before the formation of the stock. The addition of
the hydrogen peroxide 17 raises the electrochemical state of
the TMP pulp in question to a level at which it does not react
CA 02456538 2004-02-05
WO 03/018908 PCT/FI02/00684
11
when mixed with the other raw-material components PULP, REJECT,
thus preventing disturbances that diminish the runnability of
the machine 35, for example.
Correspondingly, extremely detrimental hydrogen sulphide (H2S),
which strongly lowers the raw-material components into the
reducing zone, can arise in chemical pulp PULP or especially in
reject pulp. This can be oxidized by adding, for example,
hydrogen peroxide (H202) from a tank 16 to the dosing tank 18
through a line 16b, essentially before the mixing tank 12.
Oxidizing can also be carried out simply by using pure air. The
electrochemical state of the pulp component PULP will then rise
to a favourable level, thus preventing detrimental reactions
when it is mixed with the other components TMP, REJECT, DIP.
It should be noted that the electrochemical states of the raw-
material components may differ also very naturally, without any
specific factor. The differences in level in the electrochemi-
cal states of the raw-material components caused by the
aforementioned dithionite and hydrogen sulphide are only
individual factors and in no way restrict the scope of protec-
tion of the method according to the invention.
Thus, the electrical potentials of the raw-material components
PROCESS WATER, TMP, PULP, REJECT, DIP are regulated before they
are fed into the mixing tank 12, or the electrochemical state
of the stock formed from the raw-material components is
regulated to an optimal level, thus preventing detrimental
chemical reactions, which cause, for example, sedimentation or
air bubbles in the stock. Differences in the electrochemical
states of the raw-material components or mixtures formed from
them are permitted, in order to find the optimal level,
depending, for example, on the running situation/manner, or on
the other properties of the raw-material components.
CA 02456538 2004-02-05
WO 03/018908 PCT/FI02/00684
12
An attempt is made to make any possible chemical sedimentation
take place, for example, only in the wire section 28 of the
paper machine 35 and to create optimal retention, so that it
will not create problems, for example, in the mixing tank 12 or
in the headbox 27. Instead of continuous regulation, regulation
can also take place, to suit the situation, as a shock effect
in pulses, and not continuously. Using a shock effect, violent
changes will be created in the electrochemical state, so that
a live bacterial strain will not thrive, as it cannot withstand
violent changes in its living conditions. Regulation can take
place also according to the process equipment, or by selecting
an optimal liquid for each process.
Further, the electrochemical state of the stock formed from the
raw-material components can be regulated after the formation of
the stock, however, essentially before it is fed out of the
headbox 27 of the paper machine 35 to the wire section 28.
In the method according to the invention, the minimum require-
ment for determining the electrochemical state is only a single
measurement of the electrical potential, in which the electri-
cal potential of the liquid is measured in relation to a chosen
reference electrode. If the potential distribution is uneven,
the number of measurement locations can be increased to
determine the average potential level. Examples of the said
electrode are Fe, Pt, Rst, Cu, Au, Ag. In pilot-stage tests, it
has been observed that the raw-material components' potential
levels can vary between, for example, - 800 mV, PULP and +350
mV, REJECT.
According to a second preferred embodiment, the correction of
an electrochemical disequilibrium can be achieved in place of,
or possibly along with the chemical additions described above,
by means of an external alternating or direct current source
40.
CA 02456538 2004-02-05
WO 03/018908 PCT/FI02/00684
13
In order to correct the chemical state with the aid of an
external current source 40, one or several electrodes 40
connected to an external power supply are arranged, for
example, in the dosing tanks 18, 19, 22, 23, 24, or before or
after them. This embodiment is most advantageous, for example,
for implementing regulation that takes place in pulses and/or
by reversing polarity.
Further, according to a third preferred embodiment, the order
of mixing the raw-material components PULP, TMP, DIP, REJECT
can be used to equalize or increase their electrochemical
states. For example, the two raw-material components with the
lowest potential levels are mixed first, and then these are
mixed with the component with the next lowest level, and so on.
All of the types of regulation described above can be used
singly or in combinations. Further, in regulation it is
possible to use, in addition to or besides the above types, gas
and/or salts, and/or a magnetic field, and in general all
methods of regulation based on electromagnetic radiation. In
addition, lasers and ultrasound can be used in regulation.
Figures 2a and 2b show further additional application examples
of preferred embodiments of the method according to the
invention. In both, the differences between the electrochemical
states of the raw-material components, or of the divided parts
of individual raw-material components are increased.
In the embodiment example shown in Figure 2a, the electrochemi-
cal state of a raw-material component TMP used in the manufac-
ture of newsprint is regulated in such a way as to reduce, or
even in certain cases to omit entirely, the most cost-intensive
amount of pulp required in the manufacture of the paper grade
in question. The regulation of the electrochemical state takes
place by leading at least part of the TMP pulp to the manipula-
tion M 40 of the electrochemical state, after which both the
CA 02456538 2009-05-22
14
manipulated raw-material component TMPM and the unmanipulated
raw-material component TMP are mixed together. The difference
in potential created between the components creates
advantageous reactions, thus allowing the amount of pulp to be
reduced.
Figure 2b shows a second embodiment example, in which the
difference in the electrochemical state of the TMP pulp
relative to the REJECT pulp is increased M 40 prior to them
being combined. Correspondingly in this case, the increase in
the difference between the electrochemical states of the raw-
material components TMP, REJECT allows the PULP component to be
reduced, and, in the best case, for it to be omitted.
In addition to the measurement of the electrical potential, the
electrochemical state of the raw-material components can be
measured using other forms of electrochemical measurement, such
as the measurement of electrochemical noise, current
measurement, measurement of the linear polarization resistance,
and frequency measurement. In the measurement of
electrochemical noise (EN, Electrochemical Noise), the
potential or current noise, i.e. the fluctuation at a low
frequency and amplitude, is measured between two identical
electrodes. In the measurement of linear polarization
resistance (LPR) the speed of the oxidizing reactions taking
place on the surface of a sensor is measured. The measurement
reacts to such things as changes in the conductivity and
temperature of an electrolyte, i.e. diluting liquid, and the
concentrations of the oxidizing components.
In the method according to the invention, the chemical state
can also be controlled by means of electro-flocculation. In
this embodiment, two or more electrodes of different models
(for example, plates, rods, spheres), between which the
direct/alternating current voltage ratio and/or the polarity is
altered, can be arranged in parallel and/or in series. Further,
CA 02456538 2004-02-05
WO 03/018908 PCT/FI02/00684
the regulation parameters are affected by the electrode
materials used. The diluted raw-material component of the
process water, or the stock is arranged to circulate through
electrodes arranged in parallel in a bank of electrodes.
5
According to a first embodiment exploiting electro-floccula-
tion, the elements in the raw-material components and/or in the
stock can be charged electrically, so that the electrochemical
conditions of the raw-material component and/or the stock can
10 be regulated to be advantageous to the process.
According to a second electro-flocculation embodiment, disturb-
ing substances can be collected from the raw-material compo-
nents and/or the stock onto electrodes, to prevent them from
15 disturbing the process.
According to a third electro-flocculation embodiment, the
materials of the electrodes can be advantageously selected so
that additives, which charge the fibres and/or particles in a
raw-material component and/or the stock, thus advantageously
affecting the electrochemical state of the process, can be
released from them.
The method according to the invention can also be used to
advantageously affect the retention in the wire section,
because conditions favourable to retention can also be created
by regulating the electrochemical state of the stock, thus
reducing the need to use retention agents. Further, the
formation and strength of the paper improve and the bacterial
activity in the process water diminishes. The runnability and
level of cleanliness of the machine 35 also improve.
Yet another application of the method according to the inven-
tion is the improvement of the properties of the edge parts of
the paper web. According to the state of the art, the strength
of the edge parts of the paper web is weaker than that of the
CA 02456538 2004-02-05
WO 03/018908 PCT/FI02/00684
16
web on average. In part, this is due to the fact that the
temperature distribution of the drying cylinders (not shown) of
the dryer section of the paper machine 35 is uneven, so that
the ends of the cylinders are hotter than the central part.
When applying the method according to the invention to the
problem, process water, the electrochemical state of which has
been regulated according to the method of the invention in an
advantageously direction in terms of the desired effect, is
added to the edge parts of the headbox 25 of the paper machine
35. This also improves the properties of the edge parts of the
paper web, thus increasing the effective width of the web.
It should be understood that the above description and the
related figures are only intended to illustrate the method
according to the present invention. Thus, the invention is in
no way restricted to the embodiments described above or defined
in the Claims, instead, many different variations and adapta-
tions of the invention, which are possible within the scope of
the inventive idea defined in the accompanying Claims, will be
obvious to one versed in the art.