Note: Descriptions are shown in the official language in which they were submitted.
CA 02458566 2004-02-13
-2-
Title: SUPPORTED BIOFILM APPARATUS AND PROCESS
Field of the invention
[0001] This invention relates to a gas transfer apparatus and process,
for example to support a biofilm in a liquid, as in a water or wastewater
treatment process or apparatus.
Background of the invention
[0002] Currently, most wastewater treatment plants use an activated
sludge process, based on biological oxidation of organic contaminants in a
suspended growth medium. Oxygen is supplied from air using bubble type
aerators. Efficiency of these systems is poor resulting in very high energy
use. Tank size is large since oxygen demand loadings are low. The result is
high capital and operating cost.
[0003] A second type of established biological oxidation process uses
biofilms grown on a solid media. For example, the wastewater may be
circulated to the top of the reactor and trickles down. Air is supplied at the
bottom. The rate of oxygen transfer is limited by the biofilm surface area,
and
the operating cost is high because of wastewater pumping requirements.
[0004] Recently, development work has been done on a membrane
supported bioreactor concept. For example, US Patent Nos. 4,181,604 and
4,746,435 describe a process for treating wastewater by supplying oxygen
from one side of a gas-permeable membrane to micro-organisms growing on
the other side of the membrane. Hollow fibers with porous walls were used as
the membrane. In US Patent No. 5,116,506, a gas permeable membrane
divides a reactor vessel into a liquid compartment and a gas compartment. A
biofilm is grown on the gas permeable membrane on the liquid side of the
membrane. Oxygen and alternate gases pass through the membrane to the
bacteria growing on the liquid side of the membrane.
Summary of the invention
[0005] It is an object of this invention to improve on the prior art. It is
another object of this invention to provide methods and apparatus suitable for
CA 02458566 2004-02-13
-3-
treating water, for example industrial and municipal wastewater, using
membrane supported bioreactor technology. It is another object of this
invention to provide a hollow fibre gas transfer membrane and module which
is, for example, suitable for supporting a biofilm. These aspects and others
are met by the invention described and claimed herein. The following
summary will introduce the reader to various aspects of the invention but is
not intended to define the invention which may reside in a combination or sub-
combination of various elements or steps found in the following summary or
other parts of this document.
[0006] In one aspect, the invention provides a membrane and module
with a reasonably high gas transfer rate and adequate surface area, for
oxygen transfer, biofilm support or both, to allow a membrane supported
biofilm reactor to provide an operating cost advantage over other processes
used in the art. The membrane and module may have an oxygen transfer
efficiency (OTE) of over 50°10 or in the range of 50°lo to
70°to or more. The
module may be made of non-porous or dense walled hollow fibre membranes
to provide a large surface area while avoiding the tendency of porous fibers
to
wet over time which results in a drastic drop in their oxygen transfer rates.
[0007] In another aspect, the invention provides a very fine dense
hollow fibre made from poly methylpentene (PMP), which has a high
selectivity and diffusion coefficient for oxygen. In particular, PMP has a gas
permeability of about 70,000 cc~mm/m2~24hr~Bar in dense wall, non-wetting
form. While this is significantly less than silicone, which has an extremely
high
gas permeability, PMP may be melt spun into a hollow fibre. The fiber can
have an outside diameter of 500 microns or less or 100 microns or less. Use
of such a small diameter fibre helps reduce module cost as textile fine fibre
technology can be used to create modules. A very large surface area can be
provided to achieve high OTE. The non-porous wall avoids wetting problems
as described above.
[0008] In another aspect, the invention provides a fabric with a very
large number of hollow fibres, for example of PMP, providing sufficient
surface
CA 02458566 2004-02-13
-4-
area so that oxygen transfer does not become a limiting factor in controlling
biological kinetics. The fabric may be made, for example, with the hollow
fibres, optionally collected into units, woven as weft and an inert fibre as
warp
to minimize the damage to the transfer fibre while weaving. Other methods of
preparing a fabric may also be used. The fabric provides strength to the fine
fibre to permit biofilm growth on its surface with minimal fibre breakage.
[0009] In another aspect, the invention provides a module built from
fabric sheets with very high packing density to permit good substrate
velocities across the surface without recirculation of large volume of liquid.
The modules enable a supply of oxygen containing gas, such as air, to be
supplied to the lumens of the hollow fibres without exposing the lumens to the
wastewater. Long fibre elements, for example between 1 and 3 metres or
between 1.5 and 2.5 metres are used and potted in the module header to
provide a low cost configuration.
[0010] In another aspect, a biofilm is grown on a fabric made from a
gas permeable hollow fibre, for example PMP dense wall hollow fibre.
Oxygen bearing gas is introduced into the lumen of the fibre. Aerobic
reactions take place near the surface of the fibre, where the highest levels
of
oxygen exists. These reactions include conversion of organic carbon
compounds to carbon dioxide and water, and ammonia to nitrates. The
surface of the biofilm is maintained under anoxic conditions such that
conversion of nitrates to nitrogen can take place. The result is simultaneous
reduction of organic carbon, ammonia and total nitrogen.
[0011] In another aspect, the invention uses oxygen enrichment as a
means of dealing with peak flows. Need for such oxygen enrichment may be
determined by on-line COD monitors, or set according to time of day for, for
example, municipal applications where diurnal flow and strength variations are
well known.
[0012] In another aspect, the invention uses the module and bioreactor
design to conduct other biological reactions on the surface of the fabric. An
CA 02458566 2004-02-13
-5-
example is biological reduction of compounds such as nitrates in water using
hydrogen gas supplied to the lumen of the hollow fibre.
[0013] In another aspect, the invention uses either air or enriched air to
supply oxygen. Selection of enriched air and level of oxygen present in such
air may be determined by the wastewater strength.
[0014] In another aspect, the invention may be used to digest primary
and/or secondary sludge.
[0015] In another aspect, the fibres may have a small outside diameter,
such as 100 ~,m or less, and substantial hollow area, for example 30% or
more or 40% or more, so as to have a thin wall. The fibres can be woven,
knitted, stitched or otherwise made into a fabric. The use of fine hollow
fibres
allows the thickness of the fibre wall to be low, for example 20 ~,m or less,
which is several times less than what would be required to make a film
handleable. The fine fibres may themselves be difficult to handle on their
own,
but may be combined into units such as threads or tows for handling which
may include forming textile sheets. The fabric, having a large number of
hollow fibres, provides sufficient surface area for oxygen transfer capability
such that air can be used as a feed gas without limiting the growth of the
biofilm or other biological kinetics and with acceptable pressure loss due to
air
flow through the module.
[0016] In another aspect, plug flow or multistage continuous stirred or
batch tank reactors may be used to conduct biological reactions at the highest
possible substrate concentrations for a given feed. This maximizes mass
transfer of organic carbon compounds and ammonia in the biofilm, eliminating
these processes as potential limitations to reaction rates. In multi-stage
reactors, module designs with lower surface areas for oxygen transfer to
biofilm surface area ratios may be used in downstream stages. The total
surface area for oxygen transfer, for example per unit of tank volume or flow
rate of feed, may increase or decrease in the downstream reactor since the
lower ratio may result from an increase in biofilm surface area rather than a
decrease in surface area for oxygen transfer.
CA 02458566 2004-02-13
-6-
[0017] In another aspect, the invention provides a membrane
supported batch biofilm reactor (MSBBR). The reactor includes one nr more
membrane modules which are fed an oxygen containing gas and support a
biofilm layer. The modules are located inside of a tank that is cyclically
filled
and drained to provide a batch treatment process. In an embodiment, the
modules are made of a hollow fibre fabric and are used to reduce the COD,
ammonia, total nitrogen and suspended solids in an industrial wastewater to
concentrations suitable for discharge into a municipal sewer system or for
direct discharge to a receiving stream. In another embodiment, the modules
are used to reduce COD, ammonia, total nitrogen and suspended solids in a
municipal wastewater stream for direct discharge to a receiving stream. In
another embodiment, the modules are used to reduce COD, ammonia, total
nitrogen and suspended solids in a septic tank to reduce the size of the
septic
field or to use simpler, lower cost disposal techniques or for direct
discharge
to a receiving stream.
[0018] In another aspect, the invention provides one or more methods
of controlling the growth or thickness of a biofilm layer growing on the
modules. Some methods) involve applying one or more substances to the
biofilm from the tank side while the tank is drained of feed. These substances
may include gases, such as ozone or chlorine, or liquid such as heated water
or basic or acidic solutions. During the application of the control substance,
conditions in the bio~lm may be cycled from aerobic to anaerobic by turning
the supply of oxygen to the inside of the module on and off. The biofilm may
also be starved prior to the application of the control substance by removing
the feed water, replacing the feed water with clean water or replacing the
feed
water with feed at a loading of 0.1 kg COD per kg MLSS per day or less. After
the application of the control substance, mechanical biofilm control methods
may also be used on the weakened biofilm.
[0019] In another aspect, this invention uses scouring air provided on
the outsides of the fibres as a means of controlling the biofilm thickness to
an
optimum level. Air may be used as a means of controlling the biofilm
CA 02458566 2004-02-13
- _
thickness to a desired level. Treatment with acid, alkali, oxidant, or enzyme,
or anaerobic treatment may be used periodically prior to air scouring to
weaken the biofilm and to improve the efficacy of air in completely or
partially
removing the biofilm. Other methods of biofilm control include in-situ
digestion, periodic ozonation followed by digestion, periodic alkali or acid
treatment followed by digestion, periodic enzyme treatment followed by
digestion, and use of a higher life form, such as worms, to digest the biofilm
periodically. To speed up the biological digestion reactions, the air supplied
to
inside of the module may be preheated to raise the temperature of the
bioreactor.
[0020] In another aspect, the invention provides a tow of hollow fibers,
for example with an outside diameter (OD) of 500 microns or less or 100
microns or less. To facilitate building modules with minimal reduction in the
effective surface area of the fibres, the fibres are processed or used as tows
over a significant portion, for example one half or more, of their length.
Modules may be made directly from the tows without first making a fabric. The
tows may also be made into open fabrics to facilitate potting, for example
along the edges of the fabric, while leaving significant portions of the
fibres as
tows, for example a portion between the edges of the fabric. The modules
made from tows may be potted at both ends, or potted at one end only with
the other end left unpotted with fibre ends open to permit exhaust gas to
escape. A single header module may have lower cost than a double header
module. A single header module may be inserted in a vertical configuration
with the header at the bottom and the fibres floating upwards. Such a module
may be aerated from outside the module to remove accumulations of trash
and solids. Feed may also be screened, for example through a 0.5 mm
screen, to reduce trash in the feed before it enters the reactor. Where the
tow
module is used in a downstream stage of a multi-stage reactor, the upstream
stage may also reduce the amount of trash fed to the tow module reactor.
[0021] In another aspect, reactors for treating wastewaters of different
strength are provided with modules having different ratios of surface area for
CA 02458566 2004-02-13
- -
gas transfer to surface area of the attached biofilm. The surface area for gas
transfer is the area of the outer surface of the module that is in contact
with
the supported biofilm. The surface area of the biofilm is the area of the
outer
surface of the biofilm that contacts the wastewater. Is some cases, the
surface area of the biofilm depends on the thickness of the biofilm which, for
calculations or for comparing modules, may be the actual thickness or time
average of thicknesses of a biofilm in a rector or a nominal or design
thickness or average thickness, for example 250 microns. A reactor for
treating wastewater with a COD of over 1000 mg/L may have a module with a
surface area for gas transfer to surface area of attached biofilm ratio of
more
than 1, more than 1.6, or between 1.6 and 10. A reactor for treating
wastewater with a COD of less than 1000 mg/L may have a module with a
surface area for gas transfer to surface area of attached biofilm ratio of
less
than 2.5 or between 0.2 and 2.5. A reactor for treating wastewater with a COD
of less than 300 mg/L may have a module with a surface area for gas transfer
to surface area of attached biofilm ratio less than 1 or between 0.1 and 10.
In
a mufti-stage process, two or more reactors may be connected in series with
the outlet of an upstream reactor connected to the inlet of a downstream
reactor. The COD of the wastewater to be treated decreases through each
reactor and the surface area for gas transfer to surface area of attached
biofilm ratio for modules in a downstream reactor is less than for modules in
an upstream reactor.
(0022) Other aspects of the invention are described in the claims or in
the following drawings or description.
Brief description of the drawings
(0023) Embodiments of the invention will be described below with
reference to the following figures.
(0024] Figure 1 is a picture of a group of hollow fibres.
(0025] Figure 1 a is a cross-section of a hollow fiber.
CA 02458566 2004-02-13
_g_
[0026] Figure 1 b shows a group of hollow fibers and inert fibers
collected into a unit.
[0027] Figures 2a through 2d and 2e show slot arrangements and a
spinneret for melt spinning fibers.
[0028] Figures 3a and 3b show a plan view and cross-section of a
woven fabric respectively.
[0029] Figure 3c shows steps in weaving a fabric.
[0030] Figures 3d shows a warp knitted fabric.
[0031] Figure 4a shows a sheet of hollow fibres with a central portion of
the sheet having the fibres in tows. Figure 4b shows details of a part of the
sheet of Figure 4a.
[0032] Figure 5 is a cross-section of a loose tow module.
[0033] Figure 6 shows a top view of a module having sheets of fibres.
[0034] Figure 7 is a partial section, in elevation view, of the module of
Figure 6.
[0035] Figure 8 is a cross-section of another part of the module of
Figure 6 in plan view.
[0036] Figure 9 is an elevation view of a module according to Figures 6
and 7.
[0037] Figures 10a, 10b and 10c are elevation, plan and partial section
views of another module having sheets of fibres.
[0038] Figures 11 and 12 are plan and elevation views of a tank having
cassettes of modules of sheets of hollow fibres.
[0039] Figure 13 is a drawing of the details of a tensioning mechanism
in the apparatus of Figures 11 and 12.
[0040] Figure 14 is an elevation view of the mechanism of Figure 13.
[0041] Figures 15 and 16 are schematic elevation drawings of reactors.
CA 02458566 2004-02-13
-10-
[0042] Figures 17 and 18 are schematic drawings of other reactors.
[0043] Figure 19a is a bench scale batch reactor using a tow module.
[0044] Figure 19b is a photograph of a biofilm on a tow of fibres
growing in the reactor of Figure 19a taken through a microscope.
[0045] Figure 20 is a schematic elevation drawing of a septic tank
modified to use a supported biofilm module.
[0046] Figures 21 to 31 are results of tests conducted with various
sample modules or reactors.
Descriution of Embodiments
1.0 Module Elements
1.1 Fiber
[0047] Figures 1 and 1 a show a Poly (4-methylpentene-1 ) (PMP) fiber
10 that is hollow inside but non-porous with dense walls. In a group of fibers
10, the fibers 10 may have various diameters, and may be fine fibers having
outside diameters of less than 500 microns or less than 100 microns, for
example, between 30 and 100 microns, or between 50 and 60 microns. The
hollow fibres 10 shown are non-porous, or dense walled, and water does not
flow through the fiber walls by advective flow. However, oxygen or other
gases may permeate or travel through the fiber walls, for example by
molecular diffusion or dissolution-diffusion.
[0048] The hollow fiber 10 can be prepared by melt spinning,
alternately called melt extrusion. In melt spinning a polymer granulate, for
example of PMP, is fed to the hopper of an extruder. The polymer granulate
is heated and melted in the extruder and continuously extruded to a spinning
head under a pressure of several tens of bars. The spinning head consists of
a heated in-line filter and spinneret. The spinneret is essentially a steel
plate
with thin arc shaped slots in circular arrangements. Examples of suitable slot
arrangements for the formation of a hollow fiber are shown in Figures 2a to
2d. As shown in Figure 2e, the spinneret may have multiple groups of slots
so that many fibers, 8 in the spinneret shown, can be extruded
CA 02458566 2004-02-13
-11 -
simultaneously. The molten polymer is extruded through the spinneret,
leaves the slots and closes into a hollow fiber in a cooling zone. The gaps
caused by the segment dividers allow air into the fibre to prevent collapse
before the fibre sections fuse to form the annulus. In the cooling zone, the
polymer fiber form is solidified and cooled by a controlled cross flow of air
and
the end is collected on a take up winder. Suitable fibers 10 may also be
formed by other melt spinning methods. For example, in pipe in hole spinning
the polymer is melted and drawn through an annular spinneret while passing
a gas into the lumen of the extruded fibers through another hole in the
spinneret to prevent fiber collapse. Methods other than melt spinning may
also be used.
[0049] Referring to Figure 1a, in the illustrated embodiment, a melt
spinning method is used to make fibres 10 with an outside diameter 12 of 100
~,m or less. The hollow area (ar area of the lumen 14) of the fibre may be
more than 10% or more than 30% or 40% of the cross-sectional area of the
fibre. The hollow area is typically less than 60% or 50% of the cross-
sectional
area of the fiber. For example, a polymethyl pentene fibre may be made
having an outside diameter 12 of between about 50 to 60 hum and an inside
diameter 16 of 30 ~,m or more, resulting in a wall thickness 18 of 10 ~.m or
less and a gas permeability of over 30,000 cc~mm/m2~24hr~Bar or more.
[0050] In the embodiment illustrated in Figure 1, the textile PMP fibre
10 has about a 45 micron outside diameter 12 and about a 15 to 30 micron
inside diameter 16. The fibre 10 was melt extruded using MX-001 or MX-002
PMP, produced by Mitsui Petrochemical of Japan and sold under the name
TPX, as the raw polymer through a segmented spinneret as described above.
This fiber 10 is used in the embodiments and examples described in this
document, although other fibers 10 may also be used.
1.2 Fiber Aaareaates je.a. Tows
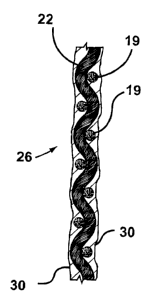
[0051] Referring to Figure 1 b, the hollow fibers 10 may be combined
into fiber units 19 for handling. The fibre units 19 may be individual fibres
10,
tows 20, for example, of 1 to 200 or 16 to 96 fibres 10 each, either twisted
or
CA 02458566 2004-02-13
-12-
untwisted (Figure 1 b), threads, yarns, tubular, flat or cordage braids, or
other
units 19 for handling. Tows 20 are made by re-winding fibers from multiple
take up spools in combination on to a second spool. Stronger inert fibres 22,
such as yarns of PE or PP, may be included in a tow 20 or other unit 19. The
fibres 10 may be curled for use in the units 19. Curled fibres 10 can be made
by winding them onto a bobbin at varying tensions.
1.3 Sheet Structures
[0052] The fibers 10 and/or fiber units 19 can be provided in the form of
sheets 26. In Figures 3a and 3b, the fibres 10 are woven as fibre units 19
into
a basic two-dimensional structure or fabric sheet 26. In the embodiment
illustrated, the units 19 run across the sheet, meaning perpendicular to the
direction in which the sheet 26 advances out of a loom. Inert fibres 22 run
along the length of the sheet 26 to provide support to the fibre units 19.
Figure 3c illustrates steps involved in a weaving process. The fiber units 19
are carried on a shuttle through 2 groups of inert fibers 22 that are
alternately
raised or lowered after each pass of the shuttle. Other weaving or fabric
making methods may also be used. Unit 19 type, unit 19 bundle size, spacing
between units 19 and percent of fibre in each direction can all be tailored to
meet the mechanical or biochemical requirements of each unique application.
[0053] In more detail, the fibre units 19 provide a support surtace for
the growth of a biofilm 30. The number of hollow fibre units 19, and the
number of fibres 10 per unit 19, may be adjusted to provide a desired surface
area for 02 transfer compared to surface area of biofilm 30 or to the planar
surface area of the fabric sheet 26. The planar surface area of the sheet 26
is
simply the sheet length multiplied by its width, multiplied by two (since the
sheet has two sides). The surtace area of the biofilm 30 is the total area of
the biofilm 30 exposed to the liquid in the reactor, which may be generally
the
same as the planar area of the sheet 26 for a substantially two dimensional
sheet configuration.
[0054] The surface area for OZ transfer is the total area of the hollow
fibres 10 in the sheet exposed to the biofilm. This is approximately equal to
CA 02458566 2004-02-13
-13-
the product of the effective diameter and length of the fibre 10, multiplied
by
the number of the fibres 10 in the sheet 26. The effective diameter for
diffusion is a logarithmic average of the diameters of the fibre to account
for
the effect of the wall thickness. The inert fibres 22 crossing the hollow
fibres
10 in the sheet 26, and contact between fibres 10, may interfere with oxygen
transfer in some embodiments, for example a tightly woven fabric, but the
interference is generally small and is ignored in surface area for oxygen
transfer calculations.
[0055] Although the surface area of the biofilm 30 is generally the same
as the planar area of the sheet, it may be slightly larger for very rough or
open
fabrics or fabrics having more dispersed fiber units 19. Varying fabric
roughnesses may also be used to affect the thickness of the biofilm 30 or how
readily the biofilm 30 can be reduced or controlled. High ratios of OZ
transfer
surface area to biofilm area (SA OZ/SA biofilm) may be obtained, in the range
of, for example, 6 to 10 or more. However, for treating feed water with a high
concentration of COD, for example, 300 mg/L CODs or more, lower SA 02/SA
biofilm ratios, for example, between 1.6 and 10 are sufficient, and may be
preferred to reduce module cost. An SA 021SA biofilm ratio in the range of
about 2 to 8, or about 4 to 6, can provide satisfactory results in many
treatment applications.
[0056] The surface area of the biofilm 30 can also be larger than the
planar area of the sheet 26 by providing a loose arrangement of fibres 10 and
controlling the thickness of the biofilm 30 to a sufficiently thin layer so
that the
biofilm 30 on adjacent parallel fibres does not form a continuous layer. A
sheet 26 with a rough or textured surface, the height of the surtace
undulations being in the range of the desired biofilm thickness, may also be
desirable since it may facilitate biofilm control. Desired biofilm thickness
may
be 200 to 1,000 microns.
[0057] Provided that oxygen transfer through the module 40 does not
limit reactions in the biofilm 30, the rate of COD reduction in the wastewater
is
roughly proportional to the concentration of COD in the wastewater.
CA 02458566 2004-02-13
-14-
However, for oxygen transfer to not be a limiting factor, more oxygen is
required to flow through the module 40 to support a biomass of the same
surface area as wastewater COD concentrations increase. More oxygen can
be provided by increasing the size or speed of operation of a blower.
However, large head losses, for example 10 psi or more, may result due to
resistance to oxygen flow through the fibre lumens 14. Head loss may be
kept below 10 psi, or in the range of 6 to 9 psi, by choosing a fabric type
and
number of fibres that produces sufficient total lumen area for a given biofilm
outer surface area.
[0058] Also, the inventors have observed that biofilms growing in
wastewater with high concentrations of COD, for example 1000 mglL CODs
or more or 2000 mg/L CODs or more, are more resilient and tend to grow to
undesirable thickness of a few mm or more, faster than biofilms growing in
wastewater with lower COD concentrations. Thus, biofilms growing in high
COD wastewater require more strenuous biofilm control methods which in
turn make a stronger fabric desirable.
[0059] The various issues discussed above make it preferable for
fabrics to be used in high COD wastewater that have more fibres, and
optionally more surface roughness, for the same overall planar area of a
sheet or outer surface area of supported biofilm than for fabrics used to
treat
lower COD wastewater. This can be achieved by choice of method used to
create the fabric and choice of thread or fabric unit count or tightness of
the
fabric. Multi-stage reactors may also be used. In a multi-stage reactor, an
upstream reactor treats the feed at its highest COD concentration and is
fitted
with modules having dense fabrics with large numbers of fibres. A
downstream reactor receives partially treated wastewater with a lower COD
and is fitted with modules having a less dense fabric with fewer fibres for
the
same sheet or biofilm outer surface area. The less dense fabric is more
economical since it has less fibres and may have a higher area of biofilm for
a
sheet of the same planar surface area.
CA 02458566 2004-02-13
-15-
[0060] The fabric sheets 26 may also be made by other methods such
as braiding, stitching or knitting, such as warp knitting. Warp knitting is
desirable, for example, when small units 19 or tows or even individual strands
of fine fiber 10 are used. The fabric sheets 26 may be patterned, as in
pattern
knitting, if desired, to provide areas with fewer fibers or holes to enhance
flow
through the sheets 26.
[0061] In warp knitting, the fabric sheet 26, as shown in Figure 3d,
contains interlaced loops of 'knitted stitches'. The column of stitches being
formed on one needle make a fringe. The fringes in the length direction
('warp') of the fabric can be made by relatively inexpensive, commodity yarns,
e.g. PET, PP, etc., as the inert fibres 22. The inert fibres 22 can withstand
the
stress and strain of processing and use. The fabric sheet 26 is generally
strong and stiff in the warp (length) direction and elastic in the weft
(cross)
direction. The weft is a perpendicular yarn system, which is laid across the
fringes and fixed by stitches (loops) of the warp fibres 22. The weft doesn't
take part in the fabric (loop) formation, therefore the weft fibre units 19
can be
processed very tenderly, being subjected to less stress and strain than the
warp. Accordingly, preparing the sheet 26 with units 19 as the weft can
minimize risk of damage to the fibres 10 during manufacturing the sheet 26.
The weft is usually a parallel layer or band of yarns being moved crosswise to
the fringes (warp) during knitting. The fabric sheet width can be about to 2-3
m.
[0062] In the embodiment of Figures 4a and 4b, the sheets 26 are
constructed of an open fabric made by weaving tows 20 through the shuttle of
a loom and crossing the tows 20 with an inert fibre 22 only along the edges of
the fabric 26. The fabric shown is approximately 1.3 m wide, that is it has
active fibres 10 of about 1.3 m long, and has inert fibers 22 woven
perpendicularly to the tows 20 in a strip of about 2 cm along the edges. As
shown in Figure 4b, the fibers 10 in each tow 20 disperse beyond the strips so
that the tows 20 remain unrestrained and partially open between the strips.
The resulting roll of 1.3 m wide fabric is cut into sections of about 20-200
cm,
CA 02458566 2004-02-13
-16-
or 30-60 cm, width to make individual sheets 26. In Figure 4b, the number of
fibers 10 in each tow 20 is small for clarity but the tows 20 may each have,
for
example, between 1 and 200, for example 16, 48 or 96 fibers 10.
1.4 Modules
1.4.1 Loose Tow Module
[0063] In accordance with the present invention, multiple fiber units 19,
including fibers 10, tows 20 or sheets 26, can be grouped together to form
membrane modules 40. Figure 5 shows a module 40, which may be called a
tow or loose tow module, with fibres 10 arranged and potted in tows 20 of
fibres. The tows 20 are made of a loose collection of a plurality of fibres
10,
for example between 1 and 200 or 16 to 96 fibres 10. The fibres 10 may be
lightly twisted together or left untwisted. The fibres 10 may be curled,
crimped
or undulating to provide three dimensional structure to the each potted row.
Curling may be achieved by re-winding the fibres 10 onto a bobbin while
varying the tension on the fibres. The individual fibres 10 remain separable
from each other in the tow 20. Such a tow 20, when coated with a thin biofilm,
for example of less than 1 mm thickness, may provide a ratio of gas transfer
area through the fibre walls to biofilm outer surface area
(SAoXy9en/SAbiofilm) of
less than 2.5, less than 1 or between 0.1 or 0.2 and 1. Inert fibres 22 may be
added to the tow to strengthen it if required. Each tow 20 is potted into a
plug
of resin 32 so that its ends 34 are open at one face of the resin 32. The plug
of resin 32 is glued into a plastic header enclosure 35 having a port 36 which
forms a header 44 connecting the port 36 to the open ends 34 of the fibers 10
through a cavity 37. There are two headers 44, one associated with each end
of the fibres 10, although modules 40 with only an inlet header 44 may also be
made. With two headers 44, air or other gases may be input into one header
44, flow through the fibres 10 and exhaust from the second header 44. Tows
are potted in a resin 32, such as polyurethane, and the potted ends are cut to
expose the fibre lumen. Alternately, a fugitive potting material may be used
to
block off fibre ends, as described in U.S. Patent No. 6,592,759, or other
potting methods may be used. In Figure 5, the number of tows 20 and the
CA 02458566 2004-02-13
-17-
number of fibers 10 per tow 20 are both small for clarity in the drawing and
may be much larger in practice.
1.4.2 Sheet Module
[0064] A module 40 can also be constructed of a bundle or stack of
sheets 26. The sheets 26 may have perpendicular inert fibers present across
the entire width of sheet 26 as in Figure 3a or only across a portion of the
width of the sheet 26, for example at the ends as in Figure 4. Raw material
for the sheets 26 may be rolled onto a fabric roll. For example, where the
sheets 26 are prepared by weaving, the material is rolled on to a take up roll
at the end of a loom as material is produced. The fiber units 19 may extend
across the roll while the inert fibers spiral around the roll. With the fibers
oriented in this way, individual sheets 26 may be cut from the roll by rolling
out
a length of material from the roll and cutting it off with a hot knife or heat
cutter. The heat cutter melts through the fiber units 19 and inert fibers and
bonds them together to protect the fabric edge from disintegrating or fraying.
Since the heat cutter melts a strip of fibers on either side of the cut line,
for
example a strip about 5 mm wide, the fibers remaining on the roll are
similarly
melted together to produce a stable edge. After a sheet 26 has been cut from
the roll, the other two ends of the sheet, meaning the edges of the sheets 26
at right angles to the heat cut edges, are cut to open the lumens of the fiber
units 19. To minimize distortion or collapse of the ends of the fibers 10
under
the cutting pressure, the area to be cut is first reinforced, for example by
impregrating it with polyurethane to provide a reinforcing coating around the
fibers 10 or fiber units. The cut across the fiber units 19 is then made with
a
sharp cutter, for example a razor edge cutter. The cutter is preferably kept
very sharp, for example by changing blades regularly, to minimize distortions
of the ends of the fibers 10. Other cutting machines or toots used in the
garment and textile industries may also be used.
[0065] The end or ends of single or multiple sheets 26 can be potted
into a header to provide one or more ports 36 in communication with the
lumens of the fibers 10. To pot one or more sheets 26, sheets 26 are cut from
CA 02458566 2004-02-13
-18-
a roll as described above. A plastic spacer strip is attached, for example
with
glue or adhesive transfer tape, on one or both sides of the sheet 26, at the
end of the sheet 26 parallel to but offset from the razor cut line across the
fiber units 19. For potting multiple sheets 26, the sheets 26 with spacer
strips
attached are laid on top of each other and attached together, for example by
glue or adhesive transfer tape, between adjacent spacing strips or between
the spacing strip of one sheet 26 and a second sheet 26. The strips space
adjacent sheets 26 but also form a barrier between a potting material to be
applied later and the cavity of the header containing the ends of the fibers
10.
The ends of the sheet 26 or stack of sheets 26 is fitted into an elongated
header cavity that may be made, for example, by injection molding. Spacing
and sealing to the header walls is maintained with a self-adhesive closed cell
neoprene gasket strip attached to each of the long header walls. Any
openings in the header cavity left by the spacer strips may be covered with
hot melt glue. Final sealing of the header is achieved by pouring a layer of
potting material, for example a two-component polyurethane compound, over
the spacer strips. The layer may be about 45 mm thick and extend between
the insides of walls of the header. If there are multiple sheets, care is
taken to
force or ensure flow of the potting material, as completely and evenly as
practicable, between the sheets 26. After the potting material hardens, a seal
is formed between the outsides of the fibers 10 and the walls of the header
but the ends of the fibers 10 remain in communication with a cavity within the
header.
[0066] Figures 6 to 9 show a module 40 in which a set of parallel
sheets 26 are potted with gaps 42 between them in a header 44. Two headers
44 may be used as shown when a bleed of exhaust air is desired. One header
44 may also be used with exhaust bled through opposed open ends of the
fibres 10 or with the other ends of the fibres 10 closed for dead end
operation.
The gap 42 may be between 2mm and 10 mm thick, or between 3 mm and 15
mm. The chosen gap 42 may depend on the water to be treated or the choice
of method to control biofilm thickness. For example, a module 40 of tensioned
sheets 26 may have a gap 42 of 6 mm when used with air scouring to control
CA 02458566 2004-02-13
-19-
biofilm 30 thickness. Tension may be provided by mounting the headers 44 to
a rigid structure, which may include parts of a tank, with one or both headers
44 movable relative to the structure. Alternately, the headers 44 may be
attached to part of a frame held at an adjustable distance apart. The sheets
of
fabric 26 are potted and separated in the headers 44 by various potting
materials 46 such as one or more of polyurethane, hot melt glue, adhesive
strips, plastic spacing strips or epoxy. The spacing between adjacent sheets
26, or gaps 42, provides space for scouring air and substrate flow through the
module 40. A large sheet of the fabric 26 may also be rolled or folded to
produce a module 40 rather than using separate sheets. The length of the
module 40 is a compromise between OTE and pressure drop and may range
from 1 m to 5 m or between 1 m and 3 m.
[0067] Referring to Figure 8, to make the module 40 a sheet 26 of
fibres 10 is laid onto strips 50 (one on each end) of adhesive located to
crass
the ends of the fibres 10. Additional strips 50 of adhesive and spacing strips
52 are placed over the sheet 26, followed by additional strips 50 of adhesive
and an additional sheet of fabric 26. These steps are repeated as appropriate
for the number of sheets 26 desired. The resulting assembly is then sealed
into the header enclosures 35 of a pair of opposed headers 44 such that the
lumens 14 of the fibres 10 are in communication with ports 36 in the headers
44 through cavities 37. The ends of the fibres 10 are cut before potting to
open them, for example as described above. Additional glue or potting resin
41 may optionally be poured into the header enclosure 35 to further seal the
fibers 10 to the header enclosure 35. Alternately, sheets 26 may be
separately glued to spacing strips at their edges and inserted into a header
cavity and additional glue or potting resin 41 placed around this assembly to
seal it to the header enclosure 35. Further alternately, the first assembly
method described above may be used.
[0068] Figure 9 shows a picture of a module 40 assembled as generally
described above. The headers 44 are about 2 meters apart. Additional
spacers 33 are used mid way between the headers to better preserve the
CA 02458566 2004-02-13
-20-
sheet 26 separation. A thin steel rod 45 is attached to the edges of the
fabric
sheet 26 in the right half of the module to address the folding which can be
seen in the left half of the module. The module 40 has a ratio of SA
oxygen/SA biofilm of about 5.
[0069] Another embodiment of a module 40 can be seen in Figures 10a
to 10c. The module 40 has a single sheet 26 with hollow fibre units 19 and
inert fibres 22. The hollow fibre units 19 extend between headers 44 at either
end of the sheet 26. The width 62 of the headers 44 is such that stacking
multiple modules 40 adjacent each other with the headers 44 of adjacent
modules 40 abutting each other provides the desired spacing between the
adjacent sheets 26. The header enclosures 35 of this module 40 are clear
allowing the cavity 37 to be seen. To pot the sheet 26, the header enclosure
35, which is a folded over plastic strip, is forced open and a sheet 26 is
inserted. The header enclosure 35 springs closed on the sheet 26. Tubes
which function as ports 36 are inserted into the ends of the header
enclosures. Potting resin 31 is laid along the joint between the sheet 26 and
the header enclosure 35, between the ports 36 and the header enclosure 35
and all other openings to seal the cavity 37.
[0070] Referring again to Figure 4, another module, which may be
called a tow or tow sheet module, can be made of open sheets 26 of tows 20
cut along the woven edges to open the ends of the fibres 10 and potted with a
0 to 10 mm space between them into one or a pair of opposed headers.
Depending on the potting method used, which may include potting methods
described above, the fibres 10 may be cut open either before or after they are
inserted into the potting resin. 1 to 100 or 8-20 sheets may be potted into a
pair of headers to produce a module. Modules made in this way using the
fibers of Figure 1 had SAoxygen/SAb;orim ratios of between 1:2.5 (0.4) and 1
/11
(0.1 ) with a biofilm thickness of 250 microns.
1.5 Cassettes/Reactors
[0071] In general, a plurality of modules can be grouped together to
form a cassette, and one or more modules or one or more cassettes can be
CA 02458566 2004-02-13
-21 -
placed in a tank as part of a reactor. Referring to Figures 11 and 12, the
modules 40 of a cassette 110 are mounted in a tank 112 of a pilot reactor for
treating 1 cubic meter per day of industrial wastewater having a COD of over
1,000 mg/L, typically 7,000 mg/L. The feed is treated by either batch or
continuous process to reduce its COD concentration to 300 mg/L as required
for discharge into the municipal sewer that it outlets to. The tank 112 has a
fill
volume of 1.8 m3. Fifteen modules 40 are provided in the tank 112, each
module 114 containing six sheets 26 of 3.6 m2 surface area of a woven fabric
of PMP fibers units 19, woven as tows 20. The fibres 10 are 1.8 m long and
extend between an inlet header 116 and outlet header 122 of the modules 40.
Total number of PMP tows per sheet is 1968, and fibres per sheet are 94464,
there being 48 fibers per tow and a two packing of 50 threads per inch in the
sheet 26. Also, polyester yarn is woven perpendicular to the PMP fibre, and
the total number of yarns per module is 1912. Air pressure drop in the fibre
lumen is in the range of 5 to 10 psi. Total biofilm area per module is 17 m2,
and oxygen transfer area is about 5.1 times the biofilm area.
(0072) The modules in the embodiment illustrated are mounted in such
a way that the tension of the sheets 26 extending between the headers 116,
122 can be adjusted. The cassette provides a rigid structure 150, which can
include elements of the tank 112 or elements of a cassette sub-frame,
adjacent the modules 40, and one or both of the headers 116, 122 are
movable relative to the rigid structure 150.
[0073) In the embodiment illustrated, the rigid structure 150 comprises
a pair of side plates 152 that extend along the distal side surfaces of the
outermost modules 40 of the stack of modules 40. As best seen in Figures 13
and 14, the modules 40 are attached to the side plate 152 by means of a
mounting bracket 154 extending transversely between the side plates 152 at
either end of the modules 40. The mounting brackets 154 are provided with
grooves 156 shaped to receive T-shaped tongues 158 extending from
surfaces of the headers 116, 122, opposite the sheets 26. The module 40
can be secured to the mounting brackets 154 by sliding the tongues 158 of
CA 02458566 2004-02-13
- 22 -
the headers 116 and 122 into the grooves 156 of the brackets 154. The
mounting brackets 154 can be secured to the side plate 152 by, for example,
a bolt 160 passing through an aperture 162 engaging the plate 152 and a
threaded hole 164 in an edge surface of the bracket 154.
[0074] The aperture 162 can be slot-shaped, so that the bracket 154
with the attached header 116, 122 can be shifted horizontally to increase or
decrease the tension of the sheets 26. An eccentrically mounted cam
member 166 can be provided between the head of the bolt 160 and the plate
152, with an outer diameter surface in engagement with an abutment surface
168 fixed to the plate 152. Rotating the cam member 166 can force the
opposed brackets 154 further apart or allow them to draw closer together,
thereby adjusting the tension of the sheets 26 in the modules 40.
[0075] The tension adjustment mechanism can be provided on only
one end or on both ends of the modules 40, and can be modified to provide
individual tension adjustment for each module 40 or for sub-groups of
modules 40. Other mounting methods may also be used to allow modules 40
to be removed or tensioned.
[0076] In another embodiment of the invention, the elements or
modules are stacked in a vertical configuration. Flow of scouring air from
outside the modules or of water in the tank may be from top to bottom or
bottom to top. This minimizes the capital required for scouring air and the
operating cost of air.
2.0 Operation/Aoplications
[0077] The fiber units 19 having one or more fibers 10 can be used as
membranes to support biofilm in a reactor. In general, gas containing oxygen
filows into at least one of the headers 44 of a module 40. The module 40 may
be operated in a dead end mode, with no outlet other than through the fibres.
Alternately, the module may be operated in a cross flow manner with gas
entering through one header 44, flowing through the fibers 10, then exiting
from the other header 44. The oxygen content and flow rate of the gas may
be set to produce an oxygen transfer that provides aerobic conditions near the
CA 02458566 2004-02-13
-23-
outer surface of the fibers 10, where the level of oxygen is highest. Aerobic
reactions occur in this area, including conversion of organic compounds to
carbon dioxide and water, and ammonia to nitrates. The biofilm may be
maintained under anoxic conditions on its outer surface or near the substrate
being treated and conversion of nitrogen to nitrates can take place. In this
way, multiple and simultaneous reactions, including carbon based organics,
ammonia and total nitrogen reduction, may be performed in the biofilm.
[0078] An example reactor 80 is shown in Figure 15. Figure 15
provides a near plug flow. The reactor 80 has a tank 82, a feed inlet 84 to
the
tank 82, an effluent outlet 86 from the tank 82, a flow path 88 between the
feed inlet 84 and the effluent outlet 86, and a plurality of fiber units 19 in
the
form of modules 40 in the tank 82. Each module 40 can have one or more
sheets 26 extending from one or more headers 44. The plurality of modules
40 can be provided as part of one or more cassettes 110.
[0079] The sheets 26 and modules 40 are sized to fit the tank 82 and
fill a substantial part of its volume. The sheets 26 may be custom made to
provide efficient use of the available space in the tank 82. The sheets 26 are
preferably arranged in the tank 82 in a number of rows, one such row being
shown in Figure 15. The sheets 26 may range from 0.25 to 2 mm in thickness
and adjacent sheets 26 are placed in the tank 82 side by side at a distance of
2 to 15 mm to allow for biofilm growth and wastewater flow between adjacent
sheets 26.
[0080] The tank 82 is longer than it is deep and may have a
generally horizontal flow path 88 with minimal mixing. This is achieved by
leaving some space near the ends (ie. near the inlet 84 and outlet 86) of the
tank 82 for vertical movement of water and leaving minimal free space at the
top, bottom and sides of the tank 82. A baffle 90 may also be placed upstream
of the effluent outlet 86 to force the flow path 88 to go under it. A sludge
outlet
92 is provided to remove excess sludge.
[0081] The flow path 88 is generally straight over a substantial
portion of the tank 82 between the feed inlet 84 and effluent outlet 86. Each
CA 02458566 2004-02-13
-24-
module 40 is held in the tank 82 by its headers 44 attached to a frame (not
shown for clarity) which restrains each module 40 in positions in the reactor
80 whereby the sheets 26 of each module 40 are generally parallel to the flow
path 88. Preferably, a plurality of sheets 26 are spaced in series along the
flow path 88 so that the reactor 80 will more nearly have plug flow
characteristics. Wastewater to be treated may be partially recycled from the
effluent outlet 86 to the feed inlet 84. Such a recycle can increase the rate
of
gas transfer by increasing the velocity of wastewater along the flow path 88,
but it is preferred if the recycle ratio is small so as to not provide more
nearly
mixed flow characteristics in the reactor 80.
[0082] Oxygen containing gas is provided to each module 40 through
its inlet conduit 216 connected to an inlet manifold 94 located above the
water
to be treated. With the inlet manifold 94 located above the water, a leak in
any
module 40 will not admit water into the manifold nor any other module 40. Gas
leaves each module 40 through its outlet conduit 218 which is connected to
an exhaust manifold 95. Although it is not strictly necessary to collect the
gases leaving each module 40, it does provide some advantages. For
example, the gas in the exhaust manifold 95 may have become rich in volatile
organic compounds which may create odour or health problems within a
building containing the reactor 80. These gases are preferably treated further
or at least vented outside of the building.
[0083] Oxygen diffuses or permeates through the fibers 10. The
amount of oxygen so diffused or permeated may be such that an aerobic
biofilm is cultured adjacent the sheets 26, an anoxic biofilm is cultivated
adjacent the aerobic biofilm and the wastewater to be treated is maintained in
an anaerobic state. Such a biofilm provides for simultaneous nitrification and
denitrification. A source of agitation 98 is operated from time to time to
agitate
the sheets 26 to release accumulated biofilm. A suitable source of agitation
is
a series of coarse bubble aerators which do not provide sufficient oxygen to
the water to be treated to make it non-anaerobic.
CA 02458566 2004-02-13
-25-
[0084] Figure 16 shows a second reactor 80 having a tank 82, a feed
inlet 84, an effluent outlet 86, a flow path 88 and a plurality of modules 40.
Frames (not shown) hold each module 40 in a position whereby the sheets 26
of each module 40 are generally parallel to the flow path 88.
[0085] The sheets 26 are sized to fit the tank 82 and fill a substantial
amount of its volume. The sheets 26 may be custom made to provide efi'icient
use of the available space in the tank 182. The sheets 26 may range from
0.25 to 2 mm in thickness and are placed side by side at a distance of 2 to 15
mm to allow for biofilm growth and wastewater flow between adjacent sheets
26.
[0086] The tank 82 is deeper than it is long to encourage a straight and
generally vertical flow path 88 over a substantial portion of the tank 82 with
minimal mixing. This is done by leaving minimal space near the ends and
sides of the tank 82 but a substantial amount of space near the top and
bottom of the tank 82. Water to be treated may be partially recycled from the
effluent outlet 86 to the feed inlet 84 but it is preferred that the recycle
rate be
small if a recycle is used.
[0087] Oxygen containing gas is provided to each module 40 through
its inlet conduit 216 connected to a manifold 94. The manifold 94 may
alternately be located above the water to be treated so that a leak in any
module 40 will not admit water into the manifold 94 nor any other module 40.
Outlet conduits 218 are connected to an outlet manifold 95 which may
alternately be located above the surface of the water to be treated.
[0088] Alternatively, gas flow through the module 40 is produced by
applying a suction to the outlet conduits 218. The inlet conduits 216 are
placed in fluid communication with the atmosphere. By this method, the rate
of gas diffusion across the membrane is slightly reduced, but the exhaust from
the blower may be connected to further apparatus for processing the exhaust
gases.
CA 02458566 2004-02-13
-26-
[0089] Oxygen diffuses or permeates through the membranes 120
preferably such that an aerobic biofilm is cultured adjacent the sheets 26, an
anoxic biofilm is cultivated adjacent the aerobic biofilm and the wastewater
to
be treated is maintained in an anaerobic state. A source of agitation 98 is
operated from time to time to agitate the sheets 26 to release accumulated
biofilm. A suitable source of agitation is a series of mechanical mixers.
[0090] Referring to Figure 17, a reactor 100 has a tank 112 with one or
more membrane supported biofilm module cassettes 110 installed inside of it.
The cassettes may have one or more modules 40, as described above. The
module 40 may also be a tow module, a module of planar elements, or other
types of modules using a membrane to support a biofilm. Each module 40
has a gas inlet header 116 fed with air, or another oxygen containing gas,
through a blower 118. Gas passes from the inlet header 116 to the inside (or
lumens 14) of one or more fibers 10. The walls of the fibers 10 serve as gas
transfer membranes 120. A portion of the gas passes through the
membranes 120 while another portion, and possibly some gasses taken up
from the tank 112, flow to an outlet header 122 of the modules 40 and to an
exhaust 124. The gases leaving the exhaust 124 may be post-treated or
discharged to the atmosphere.
[0091] Feed water enters the reactor 100 through a feed valve 126 and
feed pump 128. The feed is filled to a feed fill level 130 above the modules
40.
After a batch of feed has been treated, a drain valve 131 is opened to drain
the tank 112 of treated water. The treated water may flow to a municipal
sewer, to the environment, discharge directly to a receiving stream, or to
another stage of a MSBBR (membrane supported biofilm batch reactor) or to
another sort of reactor for further processing.
[0092] A biofilm 132 grows on the outside of the membranes 120. To
control the thickness of the biofilm 132, one or more aerators 134 are
provided below the modules 140 and connected to a scouring air blower 136
through an aeration valve 138. The scouring air blower 136 can be operated
to provide bubbles when the tank 112 is full of water. The bubbles rise
CA 02458566 2004-02-13
-27-
through the module 140 and physically remove some of the biofilm 132 from
the membranes 120. The aerators 134 are also attached to a gas supply 140
through a gas supply valve 142. The gas supply 140 may contain a
pressurized gas or a gas generator and pump or other device for supplying a
gas when the tank 112 is empty. The reactor 100 also has a liquid pump 144
operable to fill the tank 112 with a liquid other than feed water. The liquid
pump 144 may be connected to a reservoir holding the liquid or to a source of
clean water passing through a modifier, such as a chemical injection device or
heater. The tank 112 is generally open to the atmosphere and contains liquid
at generally ambient pressure but has a lid 146 which may be closed from
time to time to provide an enclosed space.
(0093] The main treatment process in the reactor 100 involves the
batch application of feed to the biofilm 132. The tank 112 is filled with feed
to
the feed level 130 using the feed pump 128. The feed pump 128 is connected
to the feed supply through an equalization reservoir 148 to permit batch
operation from a non-batch feed. The feed remains in the tank 112 for a
period of time, for example between 12 and 96 hours, while it is treated by
the
biofilm 32. During treatment, the lid 46 may remain open, but the water in the
tank 112 is generally anoxic or anaerobic. However, oxygen, typically as a
component of air, is supplied to the biofilm 132 through the membrane 120 by
the blower 118 creating an aerobic region on the biofilm 132. From time to
time during the treatment period, a recirculaton valve 149 may be opened and
feed pump 128 operated to mix the feed water in the tank 112.
[0094] After the biofilm 132 has digested the feed to the desired
degree, the drain valve 131 is opened to drain the tank 112. The draining may
occur in two steps. In the first step, the solids slurry present in the bottom
of
the tank is drained to remove settled solids which are then transferred to a
sludge management system. In the second step, the clear decanted liquid is
then drained to a second stage treatment or disinfection system, or
discharged to a sewer, or discharged to a receiving stream.
CA 02458566 2004-02-13
-28-
(0095] The oxygen bearing gas supply may be continued throughout
the filling operations to continue digestion of the material adsorbed on the
biofilm, and to ensure that treatment starts immediately as soon as a portion
of the biofilm is immersed in the wastewater. Similarly aeration may continue
throughout the draining operation to continue treatment as long as a portion
of
the biofilm is immersed and to digest organics adsorbed in the biofilm for a
short period of time even while not immersed, so as to maximize the time of
treatment of each batch.
(0096] Referring now to Figure 18, a reactor 400 is shown having
similar features as the reactor 100, but without the gas supply 140, gas
supply
valve 142, or liquid pump 144.
(0097] In a batch process, the concentration of the wastewater
decreases towards the end of each processing period. Demand for oxygen
supplied to the biofilm also decreases and so the gas supply to the modules
may be reduced. Modules using fibres at least partially in the form of tows
allow a very high surface area for oxygen transfer and biofilm growth. Tow
modules are particularly useful in treating wastewater having a low COD, for
example 1,000 mg/L or less, 500 mglL or less or 300 mglL or less, because
they provide large surface areas. Pressure loss through the fine fibre lumens
is not limiting with the amount of air supply required to deliver oxygen to a
biofilm treating low COD wastewater. Although they may be useful for treating
other wastewaters as well, tow modules can be used where the initial feed
has a low COD or as a second or third stage behind other treatment
processes or apparatus that reduce the COD concentration of stronger
feedwaters. With municipal wastewater or other feeds, for example feeds
having a COD of 1,000 mgJL or more, a two stage apparatus may be used. In
a first stage, membrane supported biofilm modules in the form of a fabric
sheet are used as in Figure 9. The outlet from a reactor containing these
modules is fed to a reactor containing tow modules with sheets as in Figure 4
which provides second stage treatment. The inventors have observed that
rapid reduction in COD from a high COD wastewater limits the denitrification
CA 02458566 2004-02-13
-29-
produced from a membrane supported biofilm reactor. With a two stage
process, the first stage may be optimized for COD removal. The feed to the
second stage has a reduced COD and the second stage may be optimized to
support nitrifying microorganisms, for example of the species nitrobacter and
nitrosomas, over carbon degrading microorganisms to provide improved
ammonia oxidation in the second stage.
[0098] In general, when considering COD, soluble COD is used since
soluble COD is most easily digested by a biofilm 30 and is easily measured.
However, particularly for modules 40 with loose tows 20 over some or all of
their area, some particles of insoluble COD are trapped in the biofilm. Over
time, these particles are broken down into soluble COD and digested.
Accordingly, total, or total biodegradable, COD also may be a relevant
parameter in some embodiments.
(0099] For feeds having a CODs of 1000 mg/L or more, a module 40
may have an SApXYGEN ISAs~oFUM of 1 or more, for example between 1 and
10. Modules 40 having sheets 26 woven across the entire length of the fibers
10, in a dense weave with a high number of fibers for very high loadings, for
example, are useful. For feeds having a CODs of 1000 mg/L or less, a
module 40 may have an SApxYGEN /SAe~oFUM of between 0.2 and 2.5.
Modules 40 having sheets woven across the entire length of the fibers but
with a less dense weave, or sheets 26 with a central open tow 20 area, for
example, are useful. For feeds having a CODs of 300 mg/L or less, a module
40 may have an SApXYGEN /SAB~oFUnn of 1 or less, for example between 1 and
10. Modules 40 with sheets 26 have a central open tow 20 area, or modules
40 of loose tows 20, for example, are useful.
(00100] Figure 19a shows a bench scale reactor having a module 40
made by potting 100 tows 20, each of 96 fibres 10 as shown in Figure 1, into
an opposed pair of headers 44. The module 40 was used to treat a feed water
in a batch process. In the process, the module 40 was located in a tank 112
filled to 4 L of synthetic wastewater. The tank was drained and filled with
fresh
feed every 1 to 7 days. Air was applied to the module at 10 mL/min. A biofilm
CA 02458566 2004-02-13
-30-
30 of stable thickness grew on the module 40 for a period of over 6 months.
The biofilm 30 was essentially endogenous, its rate of growth generally equal
to its rate of decay, except that a small part of the biofilm 30 broke off and
was
discharged with some of the tank drains. A section of a tow 20 is shown in
Figure 19b. Individual fibers 10 are covered in biofilm 30. In some places,
the biofilm 30 around a small group of fibers 10 may merge together for a
portion of the length of the fibers 10. The thickness of the biofilm 30 shown
is
about 250 microns.
[00101] Referring now to Figure 20, another reactor is shown as
suitable, for example, for a septic tank, septic tank retrofit or shipboard
treatment plant. The particular reactor shown is a septic tank retrofit using
a
standard septic tank 410 with an inlet 412 and an outlet 414 on opposite
sides. The tank 410 has two stages including a primary chamber 416 and a
secondary chamber 418. A dividing wall 420 has a submerged orifice 422
that allows flow between the chambers 416, 418. One or more modules 424
are placed in the secondary chamber 418. Air is supplied to the bottom
headers of the modules 424 through inlet tubes 426. Exhaust air is vented
from the upper headers of the modules 424 through exhaust tubes 428.
Scouring air is periodically applied to a sparger 430 located under or near
the
bottom of the modules 424 through scouring air tube 432. The modules 424
each have 1 to 100 or 8-20 sheets as in Figure 4 potted into a pair of headers
to produce a module 424. For example, a septic tank for a single household
may have one 8 to 10 sheet module 424 fed with a 1/4 hp air blower and
creating a pressure drop of about 1 to 7 psi, or about 3 psi. With a typical
household feed, a generally endogenous biofilm grows on the individual fibre
19 and tow 20 surfaces. Biological treatment in the biofilm results in a
reduction in the suspended solids and chemical oxygen demand of the
effluent, allowing the septic tile field to be reduced in size or eliminated.
[00102] In another embodiment of the invention, a number of bioreactors
are installed in series to provide flow patterns approaching plug flow. This
results in higher reaction rates and better utilization of oxygen.
CA 02458566 2004-02-13
-31 -
[00103] In another embodiment of the invention, different oxygen levels
are used in different stages of the bioreactor by oxygen spiking to meet
different levels of oxygen demand and to achieve high bioreactor loadings.
Different oxygen levels may also be used at different times in a single
reactor
or stage of a reactor. To increase the oxygen level, the pressure of the gas
fed to the lumens of the fibers or the oxygen content of the feed gas can be
increased. Similarly, to decrease the oxygen level, the feed gas pressure or
oxygen content can be decreased. Higher oxygen levels may be used in
upstream stages of multi-stage reactors or in highly loaded reactors. Oxygen
levels may also be increased periodically or from time to time to correspond
to
periods of time when the loading on a reactor is temporarily increased, for
example to respond to seasonal or daily variations in wastewater strength or
quantity.
3.0 Biofilm Control
[00104] In a membrane supported biofilm reactor, it can be
advantageous to control the thickness of the biofilm on the membranes. For
example, in the reactor 100 (Figure 17), although the tank 112 is drained
periodically, most of the biofilm 132 remains on the membranes 120,
particularly where the feed has a high COD, for example over 300 mg/L.
Excess thickness of the biofilm 132, for example 2 mm thick or more, provides
minimal, if any, increase in digestion rate, over a thinner layer, for example
of
1 mm thick or less. However, keeping the biofilm 132 thin allows the sheets
26 of the modules 40 to be placed closer together, providing more surface
area per module volume. This increase in surface area generally more than
offsets any minor increase in digestion that may, or may not, be achieved with
a thicker biofilm 132.
[00105] Accordingly, means are provided to prevent the biofilm 32 from
becoming unnecessarily thick. The following methods may be performed
individually or in various combinations. The frequency of treatment varies
with
the growth rate of the biofilm 132. For example, a biofilm 132 may grow by 10
microns a day and the module 40 may be made to tolerate a biofilm of
CA 02458566 2004-02-13
-32-
between 0.2 mm and 0.8 mm. Biofilm control procedures may then be
required every 5 to 10 days. Alternately, the period between biofilm control
procedures may be linked to the amount of COD that the biofilm has digested
since the last control procedure, which is in turn related to the time and
biofilm
thickness increase since the last control procedure. For example, control
procedures may be performed when the biofilm has digested about 20 to 200
grams of CODs per square meter of biofilm area since the last control
procedure. When control or thickness reducing procedures are performed at
this frequently, a stable biofilm layer is maintained over extended periods of
time even though each control period does not have a drastic effect on biofilm
thickness. Control procedures may be applied to the entire biofilm at once or
to a portion of the biofilm at a time.
3.1 Mechanical Methods of Biofilm Control
[00106] Some methods for controlling the thickness of the biofilm 132 on
the membranes 120 involve mechanically removing part of the biofilm 132. In
one such method, still referring to Figure 17, one or more aerators 134 are
provided below the modules 114 and connected to a blower 136 through an
aeration valve 138. With the tank 112 full of liquid, blower 136 is operated
to
create bubbles from aerator 134 below the modules 40. The bubbles
mechanically scour the biofilm 132 and also create a flow of water through the
modules 40 that physically removes some of the biofilm 132. A high velocity of
scouring air of 2-8 feet/second or an air application rate of 5 to 20, for
example about 10, cubic meters per hour per square meter of module
footprint for intervals of 1 to 10 minutes may be used. This may be done, for
example once every day to once every week. Also, air may be used to
periodically mix the contents of the bioreactor.
[00107] Other mechanical methods include spraying the modules 40
with water while the tank 112 is empty and physically removing biofilm 132
such as with a comb, wire or brush. The removed biofilm 132 falls to the floor
of the tank 112 and may be flushed out through drain 131 for further
processing as for waste sludge. These mechanical methods may be
CA 02458566 2004-02-13
-33-
performed less frequently than other methods and, when performed, may be
performed after another method has weakened the biofilm 132.
[00108] Mechanical methods for controlling the biofilm are enhanced by
providing the sheet 26 with a rough or textured surface, the height of the
surface undulations being in the range of the desired biofilm thickness.
Desired biofilm thickness may be 200 to 1,000 microns.
3.2 Chemical Methods
[00109] In another embodiment, ozone gas, introduced in the fibre
lumen is used to oxidize a part of the biofilm to make it digestible. Oxygen
is
then provided to the lumens to permit the biofilm to digest the oxidized
organics, thereby reducing the total amounts of solids generated and to
control the biofilm thickness. The oxygen may be provided as a separate step
or as part of the regular step of digesting wastewater. The reactor may be
treated in this way one module or section at a time.
[00110] In another method, a control substance is applied to the tank
side of the biofilm 132. For example, after the tank 112 is drained, clean
water
heated to, for example, 35-55 C, may be pumped into the tank 112 by the
liquid pump 144. The heated water is kept in the tank 112 for a period of time
(contact period), for example 3-5 hours, sufficient to kill a fraction of the
biofilm 132 and dissolve some of the organics that form the biofilm matrix.
The biofilm is also starved to some extent since feed has been removed.
Oxygen may continue to be applied to the lumens or may be turned off. Air
scouring may also be provided during this period to enhance biofilm removal,
although it may be more economical to carry out this operation without air
scouring, particularly if the blower 136 and aerator 134 can then be
eliminated
from the reactor 100 entirely. The biofilm 132 is also starved to some extent.
After the contact period, the water is drained through drain valve 131. In an
industrial treatment system, the discharge water will have some COD but the
duration of the contact period can be chosen such that the discharge is still
suitable for discharge to a municipal sewer since most of the killed organisms
will remain in the biofilm 32. During a later part of the contact period, the
CA 02458566 2004-02-13
-34-
living inner part of the biofilm 32 will biodegrade the killed organisms. The
effect of the heated water, or unheated water, may be enhanced with the
addition of chemicals such as acids, for example with a pH between 1 and 6
or between 3 and 3, bases, for example with a pH between 8 and 13 or
between 9 and 11, or enzymes. The chemicals and their concentration and
contact time are chosen to partially dissolve or weaken some organics that
are structural component of the biofilm but to kill only a fraction of the
microorganisms while leaving the majority behind in an active biofilm for
rapid
restart of the reactor.
[00111] In another method, a gaseous control substance is applied to
the tank side of the biofilm 132. The gas is applied from gas supply 140 while
the tank 112 is drained at the end of a batch cycle. Lid 146 is closed so that
the gas remains in the tank 112. The gas may be of various types, for
example an acid such as chlorine. Alternately, ozone may be used. The
primary purpose of the ozone is to break up the cell walls of the
microorganisms in the biofilm 132 to make it more biodegradable. The amount
of ozone applied would not be sufficient to oxidize more than about 5% of the
biofilm directly and to kill only a fraction of the microorganisms present in
the
biofilm. However, refractory organic material is converted to organic material
which is later reduced by biological oxidation when the tank is refilled. The
ozone is generated in a gas phase (air or oxygen} and is easily dispersed in
an empty tank 112. The ozone is kept in the tank 112 for a period of time
allowing it to be absorbed by the biofilm 132. Redox conditions can be
controlled in the tank 112 while it is drained to promote sludge reduction.
Alternating aerobic and anaerobic conditions can be established in the biofilm
132 by turning the feed to the inlet header 116 on and off while the tank 112
is
fitted with ozone to enhance the effects of the ozone. Killed and partially
oxidized organisms remain in the biofilm 132 and are later digested in situ
such that excess biomass need not be removed from the tank 112 for further
treatment. Denitrification may also be improved because the carbonlnttrogen
(ClN) ratio increases. Ozone may also be used in this method with
CA 02458566 2004-02-13
-35-
membranes 120 that are sensitive to ozone since the membranes 120 are
protected by the biofilm 32.
3.3 Biological Methods
[00112] In another method, worms or other animals or higher life forms
are used in an isolated section of the reactor to digest excess biofilm to
reduce bio-solids generation. The worms etc. are grown in a separate
bioreactor. When desired, the worms etc. are applied to the biofilm by filling
the tank with a liquid suspension or brine containing the worms etc.
[00113] Another method of biofilm control is endogenous respiration. By
this method, the feed loading applied to the biofilm 132 is kept such that the
rates of decay of the biofilm 132 equals its rate of growth. In practice, the
rate
of growth may exceed the rate of decay by a small amount in a batch process
because some of the biofilm 132 may detach and leave the tank 12 when it is
drained. However, endogenous respiration occurs practically only at low
loading rates and so is more appropriate for feeds with low COD
concentrations, for example 1000 mg/L CODs or less or 300 mglL CODs or
less.
[00114] Another method is periodic starvation. 1n this method, the feed is
kept in the tank 112 for an extended period of time such that the COD
concentration drops to below what it is at the end of a typical batch process.
The biofilm 132 is not nourished and decays rapidly until the start of the
next
batch cycle. The biofilm can also be starve by removing the feed and filling
the tank with clean, for example tap or potable, water, or by loading the
reactor at less than 0.1 kg CODs per kg MLSS per day.
[00115] In another method, the supply of gas to the inlet header 116 of
the module 40 is turned on and off cyclically for a period of time. The
varying
supply of oxygen shocks the biofilm 132 and causes increased decay.
Aerobic and anaerobic areas in the biofilm expand and contract while
consuming, or being consumed by, the other. Alternately, gases such as
CA 02458566 2004-02-13
-36-
ozone or chlorine, may be added to the inlet header 116 to enhance the
shock.
[00116] With chemical or biological biofilm control, closer spacing
between the sheets 26, for example 3-4 mm, may be used since hydraulic
flow through the modules 40 is not required as with air scouring, agitation or
other physical methods of biofilm removal. Chemical or biological methods
are also useful where sheets 26 or fibers 10 or units 19 are not arranged so
that a flow of scouring air will not reach all parts of the biolfim. Chemical
or
biological biofilm control methods may also be useful with open sheets 26 or
modules with unsupported or loose fibers 10, fiber units 19 or tows 20 that
would be damaged by air scouring, agitation or physical methods.
Alternately, one or more chemical methods, one or more mechanical methods
or one or more biological methods may be combined.
Examples:
Example 1 ~ C_hemical oxvaen demand (COD) reduction in a membrane
suuported bioreactor
[00117] A bench scale bioreactor was made using a module generally as
presented in Figures 6-9 except that only a single sheet of the fibres was
used. The length of the sheet was 0.57 m and height 0.45 m, providing a total
biofilm area of approximately 0.5 m2 assuming a with both sides of sheet
available for biofilm growth. The ratio of surface area for gas transfer to
surface area of attached biofilm was between about 5 and 6. Inlet air flow
was 25 ml/min at a pressure of 34.5 kPa. Reactor volume was 30L.
Synthetic wastewater with a COD level of 1000 mgll was introduced in a batch
manner periodically. The synthetic wastewater consisted of 1.0 glL of soluble
peptone and 0.03 glL of sodium hydrogen phosphate dissolved in tap water.
A series of batch reactions were conducted to determine the rate of reaction
and oxygen transfer efficiency. Figure 21 presents the results of three batch
periods: a three day period form day 2 to day 5, a three day period from day 6
to day 9 and a one day period from day 9 to day 10. It can be seen that 80-
90°lo reduction of COD was obtained in each of the three-day batch
periods.
CA 02458566 2004-02-13
-37-
A COD reduction of about 40% Was achieved in the one-day batch period
suggesting that the rate of COD reduction is higher while the concentration of
wastewater is higher and that the COD reduction rate levels off as the COD
concentration in the batch decreases with time. Oxygen transfer efficiency
during these series of tests ranged from 50 to 70%, as measured by the exit
concentration of air.
Example 2: Bench Test with Synthetic Wastewater
[00118] A bench scale bioreactor was designed using a single sheet
module as described for Example 1. Synthetic wastewater with a COD level
of 1000 mg/I, as described in Example 1, was introduced and treated by the
biofilm on the module. Rates of COD removal and oxygen transfer and the
thickness of the biofilm were calculated or measured and recorded. For about
the first 21 days, the reactor (which has a 30 L fill volume) drained and re-
filled with feed after variable batch periods to keep the CODs in the tank
generally between 500 and 1000 mg/L. At day 8 and day 16, in addition to
emptying the tank and re-filling it with new feed, the module was
powerwashed with a water sprayer to remove biofilm. From about day 21 to
day 30, the biofilm was subjected to starvation (i.e. the tank was filled with
tap, i.e. clean or drinkable, water while oxygen supply continued to the
module) and air scouring treatments. On about day 30, the tank was emptied
and re-filled with feed. From then on, the tank was emptied and re-filled with
wastewater daily but no biofilm control steps were taken, to allow the biofilm
to grow in thickness and observe the effect and rate of such growth. The
results of the test are presented in Figure 21. It can be observed that the
COD removal rate varied between about 19 to 38 grams per square metre per
day without being proportional to the biofilm thickness. The oxygen transfer
varied between about 10 to 15% grams per square metre per day, also over a
relatively wide range of biofilm thickness, namely, from about 0.5mm to over
2.3mm, at which thickness the measurement device reached its maximum
thickness.
Example 3: Pilot Study with Industrial Wastewater
CA 02458566 2004-02-13
-38-
[00119] A small pilot study was conducted using four modules generally
as shown in Figures 6 to 9. Each module has 6 sheets of fibers and a total
planar surface area, or area of biofilm, of about 3.6m2, and a ratio of
surface
area for gas transfer to surface area of attached biofilm of between about 5
and 6. The modules were installed in a 300 litre tank. The reactor was
initially
operated with peptone (about 2000 mg/I) and then peptone added to
wastewater in a declining ration to accelerate the initial growth of biofilm
on
the sheets but then acclimatize the biofilm to the wastewater. After
acclimatizing the biofilm, batch operations were conducted, filling the tank
with
industrial wastewater. The wastewater was drawn from multiple sources in
ratios chosen to create an feed COD of about 3000 mg/I. "Pure" oxygen was
supplied to the modules at a feed pressure of about 5 psi. As shown in Figure
23, bulk CODs concentration dropped to less than 1000 mg/I in about 2 to 3
days. It was also noted that COD removal rates declined with bulk CODs
concentration in the wastewater, and with time, during each batch.
[00'120] COD removal rates were calculated at different periods of time
during the batches corresponding to different concentrations of CODs in the
tank. Batches having initial CODs of 5000 mg/I and 7000 mgll were also
tested to observe the effect of higher initial COD concentrations on COD
removal rate. The results are presented in Figure 24. As indicated in Figure
24, removal rate was generally higher at higher loadings except that, in the
reactor tested, very high loadings did not always produce very high removal
rates suggesting that one or more of air feed pressure, surface area for air
transfer to biofilm surface area or total module area were less than optimum
for very high loadings.
[00121] The same reactor was used for a series of trials conducted
under continuous operation. In the trials, HRT and inlet CODs were varied.
The feed gas was "pure" oxygen at a feed pressure of 5 psi. For each trial,
the average inlet CODs, outlet CODs and removal rate, organized by HRT of
the trial, are presented in Figure 25. COD removal rates generally decreased
as HRT increased or as inlet CODs decreased.
CA 02458566 2004-02-13
-39-
[00122] The effectiveness of biofilm control procedures were also
verified in the reactor during the batch trials mentioned above. Gentle
aeration of about 1 scfm/module for 15 seconds every hour was applied,
primarily for mixing, and more aggressive air scouring of about 4 scfmlmodule
for 2-3 minutes every 2-3 days was applied primarily to remove bio~lm. The
biofilm thickness was successfully maintained in a range from about 0.2 mm
to less than 0.8 mm regardless of the average bulk CODs in the reactor,
which varied from about 300 mg/L to about 5,500 mg/L.
Example 4: Pilot Study with Municiaal Wastewater
[00123] Another pilot study was conducted using two modules as
described in Example 3, each having a surface area of about 3.6m2, installed
in an 85 litre tank. Air was supplied to the modules at a feed pressure of
about 5 psi. Peptone was added initially to the sewage to accelerate the
initial
growth of biofilm on the sheets as described for example 3. Batch operations
were conducted, filling the tank with municipal wastewater, screened through
a 3mm screen, having an initial CODs of averaging about 100 to 200 mg/I, but
occasionally up to 700 mg/L. At the ends of the batches, CODs concentration
had generally dropped to less than 30 mg/I and COD removal rate had also
generally dropped to less than 1 g/m2/d. The levels of CODs and CODt with
respect to time within a sample period in a batch are presented in Figure 26.
[00124] A study was also conducted with a continuous process, with
difFerent trials performed over a total period of about 60 days. In the
trials,
HRT varied from 24 hours to 3 hours and inlet CODs from 100 mg/I to 200
mg/l. Average removal rates tended to be lower with lower loading rates.
[00125] Nitrification and denitrification kinetics were also measured in
the continuous process study. The results of 4 trials are presented in the
following table.
Table 1: Nitrification and Denitrification in Continuous Operation
HRT Inlet Inlet Outlet Outlet Outlet
(hr) CODs NH3-N CODs NH3-N N03-N
m /L m !L m /L m /L m /L
CA 02458566 2004-02-13
-40-
11.5 165 18.2 29 3.5 3.4
7.8 117 19.6 25 5.4 4.4
4.4 105 17.7 35.9 5.6 4.3
3.1 84 18.7 37.6 11.6 1.3
[00126] Biofilm control was also tested in the municipal wastewater
study. Biofilm thickness averaging 0.2 mm was observed with air scouring,
but thicker biofilm appeared to collect between some individual sheets
indicating that these areas were not receiving full scouring air.
Example 5: Bench Scale Study with a Tow Module with Wastewater
[00127] A module similar to the one shown in Figure 5, having 100 PMP
fibre tows, each tow having 96 fibres of dense walled PMP, was tested. The
total surface area of the fibres in the module was 0.54 m2. In the module,
each tow was individually potted into an upper and lower header. The module
was fed with a supply of air at a rate of 10 mUmin to the bottom header and
exhausted out of the top header. The module was suspended, with the top
header held in a clamp at the water surface and the bottom header weighed
down, in a container filled to a volume of 4 L. The module was operated in a
batch mode using a synthetic wastewater of 1000 mg/L CODs and also
wastewater from a septic tank. At the start of each batch processing period,
the container was filled with wastewater. Air was supplied to the module to
support a biofilm growing on the fibres for processing periods ranging from
between about 1 to 7 days white wastewater was neither added to nor
withdrawn from the tank. Shorter batch periods were generally used with
wastewater having lower concentrations of COD. At the end of the
processing period, the tank was drained. New wastewater was added to start
the next processing period. At various times, the module was removed to non-
destructively measure the thickness of the biofilm on them and measurements
of the COD in the wastewater were taken.
[00128] The thickness measurements from the tests using synthetic
wastewater are recorded in Figure 27 which shows the thickness of the bio~lm
CA 02458566 2004-02-13
-41 -
on the fibres over the period of 180 days of operation. There was initially no
biofilm but after about 20 or 40 days a biofilm had developed having a
thickness that generally ranged between about 100 and 300 pm. For most of
the test run, no additional methods were used to control the biofilm thickness
and yet the biofilm thickness remained generally stable and acceptable. Small
portions of biofilm were observed to be shed from the module during at least
some of the tank draining operations, and biofilm control was otherwise
provided by endogenous growth of the biofilm. However, for a period of
approximately 15 days, the module was operated in a starvation mode. In this
mode, the tank was filled with tap water and air feed was continued. The
biofilm was reduced in thickness from about 250 Nm to about 100 Nm during
the starvation period indicating that the starvation period was effective at
reducing the thickness of the biofilm.
[00129 Figures 28 and 29 show the removal rate of COD in tests using
the synthetic wastewater. Figure 28 shows removal rate as a function of time
and Figure 29 shows removal rate as a function of COD concentration.
Referring first to Figure 28, each vertical line within the figure indicates
the
start of a new batch processing period. Accordingly, at the times indicated by
the vertical lines, new wastewater having a COD of 1,000 mg/L was added to
the tank. As the batch progresses, the wastewater is treated and accordingly
its COD concentration reduces. As shown in Figure 28, the COD removal rate
tended to drop with time in each batch processing period suggesting that the
removal rate is related to the COD concentration in the wastewater. Further,
the removal rate in the batch between day 154 and day 159 approached zero
indicating that further processing time would have marginal value. In Figure
29, the COD removal rate is plotted directly against the average COD
concentration in the wastewater. As indicated in Figure 29, the relationship
between COD removal rate and COD concentration in the wastewater is
nearly linear with the removal rate being generally proportional to the COD
concentration.
CA 02458566 2004-02-13
- 42 -
[00130] For the tests using septic tank wastewater, the wastewater was
taken from the second chamber of a septic tank. For one trial, the
characteristics of the wastewater were as follows:
Total Chemical Oxygen Demand (CODs): 377 mglL
Soluble COD (CODS): 199 mg/L
Ammonia Nitrogen (AN): 55.1 mg/L
Total Suspended Solids (TSS): 70 mg/L
The module was operated in a batch mode with batch processing periods of
approximately 24 hours to simulate actual reaction conditions in a septic
tank.
Air was supplied during these periods at the rate given above to provide
oxygen to the biofilm. After one processing period of 22 hours and 35
minutes in duration, a sample of the treated wastewater was analyzed and
results were as follows:
CODs: 140 mg/L
CODS: 73 mg/L
AN: 24.7 mg/L
TSS: 1 mg/L
A significant improvement in effluent quality was achieved. In particular, a
huge reduction in TSS was achieved. By visual observation, a large portion of
the TSS removed was in the form of colloidal matter.
[00131] Figure 30 records the results from another trial using septic tank
wastewater. The reactor was operated for a two-day batch period with
concentration of CODt, CODs TSS and ammonia nitrogen measured at the
beginning, middle and end of the batch period. For comparison purposes,
another sample of wastewater taken from the same septic tank on the same
day was placed in a 500 mL graduated cylinder and monitored as a control.
After two days of operation, reduction of Total COD (CODt) in the reactor
approached 75 mg/L, with a removal in excess of 70°f°. TSS
dropped from 34
mg/L to almost no appreciable TSS after two days of treatment. Ammonia
CA 02458566 2004-02-13
-43-
was also reduced during this period. During the same period, the control had
a less than 40% reduction in COD and an increase in TSS. The batch
process and reactor effectively treated the septic tank wastewater by
removing COD but also removing suspended solids, in part because of the
quiescent nature of the process.
Example 6 - Chemical Biofilm Control:
[00132] A biofilm control study was done using the single sheet reactor
described in Example 1 with a very thick biofilm on it. At the start of the
test,
the tank was drained and 30 L of sodium hydroxide solution in deionized
water at a pH of 9.43 and a temperature of 40 C was added to the reactor.
After a first 4 hours of soak, air scouring at 2 scfm was started and was
continued for more than 18 hours while sodium hydroxide solution remained
in the tank. Air supply to the lumens remained on. The biofilm thickness was
reduced slightly (4.6 mm to 4.3 mm) over the first four hour period. After the
18 hours of soaking and air scouring, the thickness of the biofim was reduced
further to 3.2 mm.
[00133] In another biofilm control study, 6 single sheet modules as
shown in Figure 10a and 10b Were used. Each sheet was about 27 cm long
by 20 cm wide and had an available surface area of about 0.11 square
meters. The sheets were woven with the hollow fibers running lengthwise and
open at both ends. The ratio of air transfer area to biofilm area was about 6
to 1. The modules were placed in a 20 L {working volume) reactor operated
in batch mode at room temperature with batch periods of about 3 days. The
reactor was fed with synthetic sewage at concentrations from 2000 to 8000
mg/L CODs. Air was fed to the lumens of the modules at about 2 psi with a
flow rate of about 20 mL/min to an inlet header of each sheet. At intervals of
from 3 to 7 days, between batches, the modules were soaked for 4 hours in a
solution of NaOH in hot water with a pH of 10 at 50 C. Air supply to the
lumens remained on. After the 4 hours, the reactor was re-filled with feed. No
air scouring was provided during the soak periods or during the batch periods.
Figure 31 shows the biofilm thickness over time which was maintained
CA 02458566 2004-02-13
- 44 -
between 0.2 and 0.8 mm and averaged about 550 microns over a 140 day
period. Calculated results from the batches during that period indicate that
during the interval between cleanings the biofilm removed from fib to 120
grams of CODs per square meter.
[00134a Many modifications and variations of the present invention are
possible within the teachings of the invention and the invention may be
practiced other than as described above. The scope of the invention is
defined by the following claims.