Note: Descriptions are shown in the official language in which they were submitted.
CA 02460073 2004-03-09
WO 03/025574 PCT/US02/29327
1
EMBOSSED TEST STRIP SYSTEM
REFERENCE TO RELATED APPLICATIONS
The present application claims the benefit of U.S. Provisional Patent
Application No. 60/323,426 filed September 17, 2001, and the present
application
is a continuation-in-part of U.S. Patent Application Serial No. 09/658,000
filed
September 8, 2000, which is a continuation U.S. Patent Application Serial No.
09/215,686 filed December 18, 1998, now U.S. Patent No. 6,162,639, which
claims the benefit of U.S. Provisional Patent Application Serial No.
60/068,307
filed December 19, 1997, all of which are incorporated by reference in their
entirety.
FIELD OF THE INVENTION
The present invention relates to a system and method for determining the
presence or concentration of analytes or biological agents in a sample of
bodily
fluid using a specific amount of membrane imbibed with dry reagent. In the
most
preferred embodiment the meter and a single use, reagent bearing test strip is
used
to measure the concentration of glucose in a bodily fluid such as whole blood
or
interstitial fluid (ISF)
BACKGROUND OF THE INVENTION
The need for simple methods to determine the chemical and biological
constituents in bodily fluids has increased as point of care testing has
gained in
popularity. The most common application is the self monitoring of blood
glucose
concentrations by patients with diabetes. Diabetic patients frequently
administer
insulin or take other therapeutic actions based on the test results. As
testing is
CA 02460073 2004-03-09
WO 03/025574 PCT/US02/29327
2
generally recommended multiple times daily and may occur in any setting, an
easy
to use, low sample volume test is required. The issues associated with sample
volume are significant to many diabetic patients, especially elderly patients
with
compromised circulatory systems.
In addition to chronic disease monitoring, there are other applications
where simple, low sample size testing at the point of care may be desired. For
example, many practitioners believe that certain medications could be
administered much more effectively, both from a medical outcome and from a
cost
perspective, if the circulating level of such medications could be monitored
during
the course of treatment. Generally, if the level of an analyte or biological
agent is
important enough, the patient needs to go to a clinic or laboratory and submit
to a
venipuncture so a test may be run on an expensive clinical instrument. The
ability
to monitor the patient either in the doctor's office or at home could lead to
improved outcomes. By providing a simple low sample volume test, the
practitioner is given a means of performing a test utilizing a small sample
which in
most cases is easier to obtain from the patient by using a simple finger
stick.
The National Institute of Health conducted a large scale study to evaluate
the benefit of long term tight control of the blood glucose for the diabetic
patient.
The study, known as the DCCT, proved that long term tight control of the blood
glucose levels in patients had a direct relationship to the health of the
patient. One
way for the medical profession to monitor the control of a patient is for the
patient
to use a blood glucose monitoring system. One of the main obstacles to testing
is
the sample size needed to perform the test. As patients age and their
circulation
decreases, the ability to extract an adequate sample of body fluid is
affected. A test
which more efficiently utilizes the bodily fluid would aid in reducing the
problems
associated with larger sample size tests. Current blood glucose monitoring
devices
such as the One Touch systems manufactured by LifeScan, Inc. of Milpitas,
Calif.
require the patient to place between 8 and 12 microliters of blood on the test
strip.
CA 02460073 2004-03-09
WO 03/025574 PCT/US02/29327
3
Many patients apply substantially more blood to the test to minimize the
failure of
the test due to not having enough sample applied to the strip. This unmeasured
sample leads to accuracy problems due to more sample than dried chemistry
present on the test strip. A system which self meters the amount of sample to
a
specific amount of carrier consisting of a matrix which holds a relatively
constant
amount of chemistry and provides a consistent volume for absorbing the sample
to
promote the test reaction would be a significant advancement to the patient
community.
Many diabetics currently use a test method described in U.S. Pat. No.
5,304,468 Phillips et al. This system is comprised of an electronic meter and
a
disposable reagent strip. The meter reads the color change of the strip which
correlates to the concentration of the analyte in the sample applied to the
strip. The
meter is an expensive and complex instrument which uses multiple light sources
or
detectors to isolate the reagent color change from the sample color. The user
must
select the calibration code for the meter to match the calibration code of the
test
strips. In this way, the meter accommodates a wide range of test strip
performance
values.
U.S. Pat. No. 4,637,403, to Garcia et al., describes an integrated system
which provides a method by which the patient lances the finger to get a sample
of
blood which is then used by the device to read the quantity of analyte in the
sample. This system uses a complex reflectance system to read the analyte
level in
the sample.
U.S. Pat. No. 5,279,294, to Anderson et al., describes a hand held, shirt
pocket device for quantitative measurement of glucose or analytes in
biological
fluids. The device has a sophisticated electronics system and a sampling
system
integrated into one device to determine the quantity of analyze in a bodily
fluid
sample
CA 02460073 2004-03-09
WO 03/025574 PCT/US02/29327
4
U.S. Pat. No. 5,515,170, to Matzinger et al., describes the difficulties of
keeping a strip holder and optics system clean and the need to present the
test strip
in the proper perspective to the optics.
European Patent Specification 0 351 891 B 1, to Hill et al., describes an
electrochemical system and electrodes which are suitable for the in vitro
determination of blood glucose levels. The system requires the use of
expensive
electrodes and a sophisticated reader to determine blood glucose levels.
U.S. Pat. No. 4,994,167, to Shults et al., describes a measuring device for
determining the presence and amount of a substance in a biological fluid using
electrochemical methods. This system requires a complex instrument and method
for the patient to determine the quantitative result.
U.S. Pat. No. 5,580,794, to Allen et al., describes a single use disposable
measuring device for determining the presence and amount of a substance in a
biological fluid using reflectance methods. This system utilizes optics and
electronics packages which are mated in a single plane.
Single use disposable devices have been designed for the analysis of
analytes in bodily fluids. U.S. Pat. No 3,298,789, to Mast, describes a system
in
which whole blood is applied to a reagent strip. After a precise, user-timed
interval, the blood must be wiped off by the user. An enzyme system reacts
with
the glucose present in the sample to create a color change which is
proportional to
the amount of glucose in the sample. The strip may be read visually by
comparing
to a printed color intensity scale, or in an electronic instrument.
CA 02460073 2004-03-09
WO 03/025574 PCT/US02/29327
U.S. Pat. No. 5,418,142, to Kiser et al., describes a single use device which
does not require blood removal or color matching. The amount of analyte
present
in the sample is read in a semiquantitative fashion.
5 U.S. Pat. No. 5,962,215, to Douglas et al., describes a series of
semiquantitative, single use devices which are used to determine the level of
an
analyte in a biological sample. These devices do not require blood removal or
color matching.
U.S. Pat. No. 5,451,350, to Macho et al., describes a single use system for
the determination of an analyte in a biological sample.
European Patent Application No. EP 0 759 555 A2, to Douglas et al.,
describes a multilayer reagent test strip which measures the concentration of
analyte in a liquid sample that is applied to it.
U.S. Pat. No. 4,994,238, to Daffern et al., describes a multilayer test device
which uses a defined area of absorbent, reagent bearing matrix.
Although many improvements have been made, the cost and complexity of
measuring analyte levels in biological samples remains a significant issue for
patients and for the health care system. The need to deliver a sizable sample
of
bodily fluid to the strips or electrodes in use leads to errors in performance
and
presents problems for the patient. The availability of a low sample volume
which
meters the sample to the test matrix reduces the issues with short sampling or
over
sampling of the test. This is a great advantage to the patient to insure an
accurate
test. A simplified quantitative test system of this invention for the periodic
monitoring of constituents of biological fluids, such as glucose in blood,
would
make testing more accessible to patients and would improve their well-being.
CA 02460073 2004-03-09
WO 03/025574 PCT/US02/29327
6
A system which requires a smaller fluid sample is attractive to many
patients. There has been a trend toward smaller sample sizes, but most devices
still
require about 10 p,L of blood. Many patients have difficulty routinely
applying an
adequate sample to the strips or electrodes. Inadequate sampling can cause
erroneous results or may require that the user discard an expensive test strip
and
repeat the sample application procedure. A system which would require about 3
p,L or less, which is a fraction of the volume required for most blood glucose
tests
and could be more readily obtained by patients, would be advantageous.
An object of the present invention is to provide a method for measuring the
amount of analyte in a sample of biological fluid using a simple, low sample
volume, reagent test strip with a built in metering system.
Another object of this invention is to provide reagent test strips that can
meter the sample into the reaction matrix.
CA 02460073 2004-03-09
WO 03/025574 PCT/US02/29327
7
SUMMARY OF THE INVENTION
The method of this invention involves the use of single use test strips
capable of reading small sample sizes and determining the amount of an analyte
in
the small sample. The low sample size feature of the strip permits the patient
to
use less invasive systems to acquire a sample than the 21 gauge lancing
devices in
current use. The device is structured with a capillary to meter a specific
quantity of
sample to the test matrix, thereby eliminating a significant source of error
associated with short sampling. The capillary is designed so that, when placed
in
contact with a sample of bodily fluid, it transfers the sample to the test
matrix. If
the sample is insufficient to travel the full length of the capillary, then
the sample
does not reach the test matrix and will not wick into the test matrix, which
prevents
the patient from short sampling the test strip. The user can add additional
sample
to the capillary to complete the test. Once the sample contacts the test
matrix, the
sample will wick into the test matrix until the test matrix is filled, then
stop.
Excess sample remains in the capillary and serves as a signal to the patient
that the
test matrix has the correct amount of sample for the test. This provides many
advantages to the patient including the elimination of wasted strips due to
short
sampling which results in a substantial cost savings for the patient and
reduces the
number of inaccurate tests from marginal samples.
The capillary design also provides another interesting benefit. As blood
travels down through the capillary to the test area, the blood warms the peg,
thus
regulating the temperature of the strip and the test. This is beneficial in
two ways;
the first is that each test is performed under somewhat controlled conditions,
regardless of whether or not the surrounding temperature is warm or cold.
Second,
this effect alleviates the problem of fogging over the test area. This is a
problem
with many blood glucose monitors when testing in cooler ambient conditions.
CA 02460073 2004-03-09
WO 03/025574 PCT/US02/29327
8
The formation of a captivated microtitration zone is described in U.S. Pat.
No. 5,872,713 Douglas et al. When constructed according to this invention, the
microtitration zone can be achieved with a specific volume by following a
simple
series of steps: (a) applying a specific amount of reagent such that it does
not
saturate the matrix and is developed to indicate a specific analyte, (b)
drying the
reagent so that the active ingredients adhere to the substrate of the matrix,
(c)
embossing or compressing the matrix to collapse the matrix surrounding the
reaction zone so that the void volume of the resulting test matrix
microtitration
volume is approximately equal to the sample size desired, (d) installing it
into a
performed pocket which completely surrounds all the circumference of the
pillow
where the capillary is in communication with the top sidelsample side of the
pillow, and (e) sealing the system together. The embossed/collapsed areas have
had their void volume reduced to approximately zero and the test matrix
reaction
zone forms a small bibulous pillow which retains its void volume and has the
desired total volume. This limits the ability of the reagents imbibed into the
embossed matrix to participate in the reaction of the result zone. The test
pad can
be made from various matrix materials which will hold the test reagent in a
dried
form including polyethersulphone (Gelman sciences Supor 200D), polysulphone
(Memtec filtration asymmetric membrane) and nylon (Pall biodyne). The wicking
material which can be selected from various materials, including Pall Accuwick
and Whatman 41, which provide a high enough capillary action to wick and
absorb
the sample from the capillary peg and spread it into and fill the reaction
matrix
microtitration volume.
The applied bodily fluid reacts with the reagents impregnated in the test pad
within the test strip and the resulting color change is read by the optics
system of
the meter adapted to read the strip.
The patient uses the test strip by removing it from the packaging and
placing it into a meter designed to utilize the test strip. The patient turns
the meter
CA 02460073 2004-03-09
WO 03/025574 PCT/US02/29327
9
on or it can be automatically started from the test strip insertion. The
patient uses
either a sampler from the kit or one procured separately to draw a sample of
capillary blood. This sample is applied to the test strip, the meter reads the
sample,
and the meter displays the result after an appropriate time.
One aspect of the present invention concerns a bodily fluid sampling
assembly. The assembly includes a support member that defines an aperture
adapted to receive a bodily fluid sample and a cover member. A test strip is
compressed between the support member and the cover member to form an
embossed pillow within the aperture. The embossed pillow is adapted to absorb
the bodily fluid sample. The test strip has an incision surrounding the
embossed
pillow to minimize leakage of the bodily fluid sample from the embossed
pillow.
Another aspect of the present invention concerns a test strip assembly that
includes a wicking layer for collecting a bodily fluid sample and a support
member.
The support member defines an opening and has a blade extending around the
opening. The blade contacts the wicking layer to minimize flow of the fluid
sample in the wicking layer from the opening.
A further aspect of the present invention concerns a test strip. The test
strip
includes a test matrix to test a bodily fluid sample. A wicking layer is
provided
over the test matrix. The wicking layer has an embossed pillow for absorbing
the
bodily fluid sample. The wicking layer has an incision surrounding the
embossed
pillow to minimize leakage of the bodily fluid sample from the embossed
pillow.
Other forms, embodiments, objects, features, advantages, benefits and
aspects of the present invention shall become apparent from the detailed
drawings
and description contained herein.
CA 02460073 2004-03-09
WO 03/025574 PCT/US02/29327
BRIEF DESCRIPTION OF DRAWINGS
FIG. 1 is an exploded, side elevation view of one embodiment of a test pad
5 matrix and wicking layer prior to being embossed in a die formed by plates.
FIG. 2 is a cross-sectional view of one embodiment of a test pad matrix and
wicking layer during embossing in a die formed by plates.
FIG. 3 is an exploded perspective, cut away view of the test pad matrix,
wicking layer and upper and lower plates of the embossing die.
10 FIG. 4A is an assembled view and 4B is an exploded perspective view of
one embodiment of the strip showing assembly of the handle, test pad, wicking
layer, and capillary.
FIG. 5 is an enlarged, cross-sectional view of a test strip constructed
according to the present invention.
FIG. 6 is an exploded, cross-sectional view of an alternative embodiment of
the test strip in accordance with the present invention.
FIG. 7 is a cross-sectional side view of the alternative embodiment of the
test strip as assembled.
FIG. 8 is an exploded, cross-sectional side view of another alternative
embodiment of a test strip in accordance with the present invention.
FIG. 9 is a cross-sectional side view of the test strip of Figure 8 as
assembled for use.
FIG. 10 is an enlarged, cross-sectional view of a test strip constructed
according to another embodiment of the present invention.
CA 02460073 2004-03-09
WO 03/025574 PCT/US02/29327
11
DESCRIPTION OF SELECTED EMBODIMENTS
For the purposes of promoting an understanding of the principles of the
invention, reference will now be made to the embodiments illustrated in the
drawings and specific language will be used to describe the same. It will
nevertheless be understood that no limitation of the scope of the invention is
thereby intended, such alterations and further modifications in the
illustrated
device, and such further applications of the principles of the invention as
illustrated
therein being contemplated as would normally occur to one skilled in the art
to
which the invention relates. One embodiment of the invention is shown in great
detail, although it will be apparent to those skilled in the art that some of
the
features which are not relevant to the invention may not be shown for the sake
of
clarity.
The present invention provides improvements over existing technology in
use today in several ways. A preferred embodiment of the invention creates a
microtitration zone which permits the accurate testing of a small fluid sample
and
prevents oversampling, while the integrated capillary provides a means to
eliminate
the problems associated with short sampling which frequently occurs in the
current
commercial products. The capillary also provides a means of absorbing the
fluid
sample from a non-fingerstick location. This permits the use of non-
traditional
lancing systems. The small test pad used in this invention reduces the cost of
the
matrix employed and the quantity of expensive reagents needed to conduct an
accurate assay using an oxidase and peroxidase chemistry. With a smaller test
pad,
a smaller sample volume is adequate. It should be noted also that an electrode
based test system could be used with the basic structure and elements of this
invention. A further feature of the capillary is that the capillary acts as a
retaining
chamber where a sample of appropriate volume is initially collected and then
delivered to the test pad. The sample is only delivered to the test pad when a
CA 02460073 2004-03-09
WO 03/025574 PCT/US02/29327
12
sufficient amount of sample has been collected within the capillary.
Furthermore,
the capillary may be constructed to further include a fluid chamber. The fluid
chamber may be disposed adjacent the test media, such that in use, the distal
end of
the capillary is placed in contact with a bodily fluid to be sampled. The
bodily
fluid is received within the capillary through capillary action or other means
such
as a wicking material. As bodily fluid is drawn into the capillary the bodily
fluid
fills the fluid chamber. After the fluid chamber is filled, the sample of
bodily fluid
collected in the fluid chamber may then be deposited upon the test media. A
benefit of using a fluid chamber is that a smaller sized sample may be
utilized to
perform a desired test because the entire amount of bodily fluid needed for
the test
can be accurately delivered to a test site, thereby reducing the overall
amount of
sample needed to perform the test. Furthermore, the use of a fluid chamber to
collect the sample may also lead to fewer failed tests due to inadequate
sample
volume, because the sample will not be delivered to the test media until a
sufficiently sized sample is collected.
Although the fluid chamber has been described in use with a capillary, it is
contemplated that other collection devices may be utilized with the fluid
chamber
of the present invention. For example, the fluid chamber may be included
within a
test strip wherein the sample is placed on a portion of the test strip and
transported
to the fluid chamber. The descriptions above should not be considered limiting
and are intended to be exemplary.
The test strip is comprised of a test pad situated in a test pad holder. This
holder provides a means for accurately positioning the test pad with respect
to the
optics system in the meter and for providing a means for blocking ambient
light
from affecting the analysis. The test pad is impregnated with the appropriate
chemistry to permit a colorimetric analysis of the analyte being tested, and
must
therefore provide a stable absorbent substrate. If the system is developed
with an
electrode based system, the function of the test pad holder is to position the
CA 02460073 2004-03-09
WO 03/025574 PCT/US02/29327
13
electrode contacts on the strip with those corresponding to the meter. The
test pad
can be made from various materials which will hold the test reagent in a dried
form, including polyethersulphone (Gelman Sciences Supor 200D), polysulphone
(Memtec filtration asymmetric membrane) and nylon (Pall Biodyne). The wicking
layer can likewise be selected from various materials, including Pall Accuwick
and
Whatman 41, which provide a high enough capillary action to absorb the sample
and spread it to the reaction matrix.
The test strip of this invention provides a support for the test pad and the
capillary peg contacting the test pad. The peg positively seats in the meter
in a
detent and is locked from rotation by a corresponding key in the test strip
which
fits into a slot in the meter test strip holder. The test strip holder is
positioned to
the optics block using pins on the optics block assuring proper alignment of
the test
strip. It also seals the optics area from ambient light and any excess blood
contamination. These features are more fully disclosed in U.S. Pat. No.
5,872,713,
which is incorporated herein by reference.
The signal producing system impregnated in the test pad matrix can be
formed from different indicator systems, such as 3-methyl-2-benzothiazolinone
hydrazone (MBTH) and 8-anilino-1-naphthalenessulfonate(ANS) [U.S. Pat. No.
5,453,360 to Yu], MBTH and 3-dimethylaminobenzoic acid (DMAB) [U.S. Pat.
No. 5,049,487 to Phillips et al.],
3-methyl-2-benzothiazolinone-hydrazone-sulfonate sodium salt (MBTHS) and
-Ethyl-N-(3-sulfopropyl)aniline (ALPS) [U.S. Pat. No. 4,396,714 to Maeda et
al.].
One skilled in the art could devise an alternate indicator system. The oxidase
enzyme system contained in the reagent pad produces hydrogen peroxide which is
used to convert the indicator with the assistance of peroxidase which acts as
the
catalyst.
CA 02460073 2004-03-09
WO 03/025574 PCT/US02/29327
14
In the most preferred embodiment the reagents are impregnated into a
porous membrane by submerging the dry membrane into a reagent dip. Excess
fluid is wiped from the membrane surface and the membrane is gently dried in
an
oven. At this point, subsequent dipping and drying can be conducted. A
preferred
embodiment for a two dip process is:
CA 02460073 2004-03-09
WO 03/025574 PCT/US02/29327
MBTHS & ALPS Formulation
Final
Concentrations
5
A Dip
In Citrate buffer, pH 7 0.1 M
stock A Dip
10 EDTA 0.08
%
mannitol 0.19
Gantrez-S95 0.53%
Klucel 99-EF 20 uM
Crotein-SPA 7.45
%
enzyme reagents
Glucose Oxidase 0.92%
Peroxidase 0.54%
B Dip
In 70% Ethanol
MBTHS 0.66%
ALPS 2.00%
SOS 0.20%
The color formed after applying the bodily fluid to the reagent test pad is
proportional to the amount of analyte in the applied sample. The meter
measures
CA 02460073 2004-03-09
WO 03/025574 PCT/US02/29327
16
the change in reflectance due to the development of the specific color
generated by
the indicator. This is either used as the input to a function which relates
reflectance
to analyte level or to a table which correlates reflectance value to analyze
level.
The function or the table is stored within the meter system for it to produce
and
display a reading of the analyte level. While most meters in use today employ
functions to convert reflectance readings to analyte concentration, this
approach
requires that the function be stable and well understood. The use of a look up
table
permits the storage of specific values for reflectance and their corresponding
analyte levels. The meter uses this table and interpolates between the table
values
to give relatively accurate readings. This is achievable in a system such as
that
described by this invention as the table can quickly be generated for each
reagent
lot produced. The devices of this invention using a read-once calibration
chip, or
being fully disposable, can use a lot- specific look up table to convert
reflectance
reading to analyte levels.
FIG. 1 shows an elevation view of the un-embossed layers, wicking layer 5,
test matrix layer 4, and top layer 1 between the die 17 formed from top plate
16
containing hole 18 and bottom plate 15 containing hole 18A.
FIG. 2 shows an elevation view of the embossed or compressed layers,
wicking layer 5, test matrix layer 4, and top layer 1 between the die 17
formed
from top plate 16 containing hole 18 and bottom plate 15 containing hole 18A.
Hole 18 in die plate 16 forms the microtitration pillow 21 in the wicking
layer 5
and in test matrix layer 4. The areas of the layers surrounding pillow 21 are
compressed to make them essentially impervious to sample liquid flow, thus
forming the microtitration volumetric area around pillow 21. Hole 18A allows
for
the test strip to be placed in an optical meter whereby a color change of the
top
layer and/or matrix layer can be measured.
CA 02460073 2004-03-09
WO 03/025574 PCT/US02/29327
17
FIG. 3 shows an exploded perspective view of the embossed or compressed
layers, wicking 5, test matrix 4, and top layer 1 as formed between the die 17
formed from top plate 16 and bottom plate 15 containing hole 18A.
The assembly of a test strip 20 shown in FIG. 4A is accomplished as shown
in FIG. 4B. In a preferred embodiment bottom or support member 6 which has the
capillary peg 7 and capillary 10 integrally molded therein (e.g., by injection
molding) and constructed so that microtitration pocket 8 has breather holes 9
located within the microtitration pocket 8. Or capillary peg 7 can be formed
as a
separate element and assembled into support member 6 if desired. FIG. 2 shows
the formation of the microtitration pillow 21 in matrix 4 and wicking layer 5.
The
microtitration pillow 21 is formed using die 17 formed from top plate 16 and
bottom plate 15. By using a die to form the pillows the spacing of the pillows
21
can be formed in the matrix 4 and wicking 5 so that they align with the
microtitration pocket 8. When the top layer 1 is assembled on bottom member 6
with test matrix layer 4 and wicking layer 5 properly positioned as shown
between
layers 1 and 6. Test matrix pad 4 is formed from a bibulous matrix which has
been
impregnated with a reagent system comprised of enzymes, indicators and blood
separation agents and the wicking matrix pad 5 provides a means of spreading
the
sample over the test pad 4. Layers or pads 4 and 5 are preferably embossed or
compressed prior to assembly with layers 1 and 6. The holes 22 and 23 formed
in
the top layer 1 and alignment keys 11 and 12 formed in holder 6 provide a
means
of aligning the test strip assembly including pillow 21 and hole 18A to the
microtitration pocket 8. The breather holes 9 provide an escape path for
trapped
air in the assembly pillow 21 when wicking the sample up the capillary 10 and
into
pillow 21. Figure 5 shows an additional preferred feature of the present
invention
where capillary peg 7 and capillary tube 10 are formed with a protruding
collar 25
extending from capillary tube 10 to engage and further compress pillow 21.
This
feature provides a seal between capillary tube 10 and the surface of wicking
layer
5, which better forces the sample flow from capillary tube 10 into the
interior of
CA 02460073 2004-03-09
WO 03/025574 PCT/US02/29327
18
wicking layer 5 to better distribute the sample throughout test matrix layer 4
and
completely fill microtitration volume 8 and to better prevent sample from
flowing
between the surface of wicking layer 5 and the surface of the end of capillary
peg
7.
Figures 6 and 7 illustrate a test strip 200 according to another embodiment
of the present invention. The test strip 200 includes a top layer I, a test
matrix 204
and a wicking Layer 205 of the type as described above. As shown, the test
matrix
204 is sandwiched between the top Layer 201 and the wicking layer 205. Layers
204 and 205 as well as the test matrix 204 are pressed between a bottom plate
(cover member) 15, and a top plate (support member) 216. When pressed
together,
plates 15 and 216 form a die 217 with plates 15 and 216 each defining openings
18A and 218, respectively. In one embodiment, openings 18A and 218 have a
generally cylindrical shape. However, as should be appreciated, openings 18A
and
218 can be shaped differently. Bodily fluid samples are collected through the
opening 218 in the top plate 216, and the opening 18A in the bottom plate 15
allows the sample collected on the test matrix 204 to be analyzed.
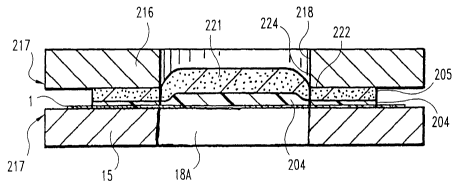
As shown in Figure 7, plate 216 has an interior surface 219 with a blade
member 220 projecting therefrom towards plate 15. In one embodiment, the blade
220 is integrally formed with the top plate 216, and in another embodiment,
the
blade 220 is a separate component attached to plate 216. During assembly, the
top
plate 216 and the bottom plate 115 are compressed to make the portion of the
wicking layer 205 and the test matrix 204 adjacent the aperture 218 virtually
impervious to a bodily fluid sample. Within the opening 218 in plate 216, the
wicking layer 205 and the test matrix 204 are embossed to form a
microtitration
area or pillow 221, which is able to absorb the fluid sample. Moreover, the
blade
220 in one embodiment presses into the wicking material 205 and is
sufficiently
sharp to form an incision or cut 222 at least through part of the wicking
layer 205
in order to minimize the amount of fluid Leakage from the microtitration
pillow
CA 02460073 2004-03-09
WO 03/025574 PCT/US02/29327
19
221. In another embodiment, the blade 220 only compresses the test strip 200
around the microtitration pillow 221 making the periphery of the
micortitration
pillow 221 impervious to fluid so as to minimize fluid leakage from the
microtitration pillow 221. The blade 220 in the illustrated embodiment has a
generally cylindrical shape. However, it should be appreciated that the blade
220
can be shaped differently.
In one embodiment, the blade 220 has a length L that is sized to only cut
the incision 222 through part of the wicking layer 205 so that the
microtitration
pillow 221 of the wicking layer 205 remains attached to the remainder of the
test
strip 200. In another embodiment, the blade 220 is sized to cut the incision
222
completely through the wicking layer 205. The microtitration pillow 221 of the
wicking layer 205 in one form of this embodiment bonded to the test matrix
204,
and in another form, the microtitration pillow 221 of the wicking layer 205 is
retained in opening 18 through frictional engagement. In the illustrated
embodiment, the blade 220 has a closed, continuous shape so that the incision
222
encircles the rnicrotitration pillow 221. Although the incision 222 in the
illustrated
embodiment is continuous to minimize fluid leakage from the microtitration
pillow
221, it should be understood that the incision 222 can be formed in a
discontinuous
manner such that fluid leakage prevention is not severely compronnised. For
example, the blade 220 in another embodiment can include cut out sections that
form retaining webs in the wicking layer 205 such that the formed incision 222
is
discontinuous.
In the illustrated embodiment, the blade 220 has a generally cylindrical
shape to coincide with the shape of opening 218. As depicted in Figure 6,
inner
surface 224 of opening ZI8 is flush with inner surface 226 of the blade. With
the
blade 220 being flush with the opening 218, the incision 222 is formed at the
periphery of the microtitration pillow 221 in order to effectively destroy the
wicking function of the material adjacent the incision 222. This in turn
minimizes
CA 02460073 2004-03-09
WO 03/025574 PCT/US02/29327
leakage of the fluid sample from the microtitration pillow 221. Minimizing
leakage from the microtitration pillow 221 reduces the amount of fluid
required for
the fluid sample. Therefore, as a sample of bodily fluid is placed upon the
microtitration pillow 221, the wicking layer 205 distributes the sample across
the
5 area of the opening 218, and the blade 220 acts to prevent any sample from
flowing
beyond it. Since the blade 220 prevents fluid from passing outside the area of
the
aperture 218, less fluid needs to be collected.
Referring to Figures 8 and 9, a test strip 300 according to another
10 embodiment of the present invention includes top layer 1, test matrix layer
4 and a
wicking layer 305, which is sandwiched between bottom plate 15 and top plate
16.
The top plate 16, as illustrated, defines aperture or opening 18, and the
bottom
plate 15 defines aperture or opening 18A. In the illustrated embodiment, a
fluid
sample is collected on the test strip 300 through opening 18. Opening 18A
enables
15 a test device to perform a measurement upon the sample collected the test
strip
300, such as a colormetric measurement in which the reflectance of the
collected
sample is measured in order to determine the amount of glucose in the sample.
In one embodiment, the wicking layer 305 and test matrix layer 4 are pre-
20 embossed so as to form a microtitration pillow 321. In another embodiment,
the
microtitration pillow 321 is formed in the test strip 300 in opening 18 when
the test
strip 300 is pressed between the top plate 16 and the bottom plate 15 (Figure
9). In
the illustrated embodiment, the wicking layer 305 has an incision 322 formed
therein before assembly with the other layers of the test strip 300. As shown,
the
incision 322 only partially cuts through the wicking layer 305. It should be
appreciated that in other embodiments the incision 322 can be formed
completely
through the wicking layer 305 and/or the test matrix 4. In one form, the
incision
322 is pre-cut with a blade before assembly. However, as should be understood,
the incision 322 can be fabricated in other manners. As depicted in Figure 9,
the
incision 322 is formed to align with and generally correspond with the shape
of the
CA 02460073 2004-03-09
WO 03/025574 PCT/US02/29327
21
opening 18 in the top plate 16 so that the incision 322 surrounds the
microtitration
pillow 321 formed in the test strip 300.
The incision 322 formed in the wicking layer 305 interrupts fluid flow in
the wicking layer 305 to the area surrounding the microtitration pillow 321.
In use,
a sample of bodily fluid is placed upon the wicking layer 305 whereby the
sample
spreads across the microtitration pillow 321. The incision 322 prevents the
bodily
fluid from flowing past the microtitration pillow 321 defined by the aperture
18.
By not allowing the fluid sample to flow beyond the aperture 18, less fluid is
wasted so that a smaller fluid sample is needed.
Figure 10 illustrates an enlarged view of a bodily fluid sampling assembly
400 incorporating test strip 200, according to another embodiment of the
present
invention. As illustrated, assembly 400 includes a support member 406 that has
capillary peg 7, cover member 15, and test strip 200 sandwiched between the
support member 406 and the cover member 15. The support member 406 defines a
microtitration pocket 8 that fluidly communicates with a capillary tube 10
integrally formed within the capillary peg 7. The capillary tube 10 is used to
draw
a bodily fluid sample into the microtitration pocket 8. In the illustrated
embodiment, the capillary peg 7 is integrally formed with the support member
406.
Nonetheless, it should be understood that the capillary peg 7 can be formed as
a
separate component and attached to the support member 406. In Figure 10, the
support member 406 has a protruding collar 25 that extends from the capillary
tube
10 in the microtitration pocket 8 in order to compress the test strip 200. The
support member 406 further includes a blade member 220 that surrounds and is
aligned with the periphery of the microtitration pocket 8. The cover member 15
defines a sensor aperture 18A that is aligned with the microtitration pocket
8.
As described above, the test strip 200 includes top layer 1, test matrix 204
and wicking layer 205. When the test strip 200 is pressed between the support
CA 02460073 2004-03-09
WO 03/025574 PCT/US02/29327
22
member 406 and the cover member 15, a microtitration pillow 221 in the test
strip
200 is formed within the microtitration pocket 8. The protruding collar 25
engages
and compresses the microtitration pillow 221 to improve the seal between the
capillary tube 10 and the wicking layer 205. This configuration improves
distribution of the bodily fluid sample within the test matrix 204. Further,
in the
illustrated embodiment, the blade 220 forms an incision 222 in the test strip
200
around the microtitration pillow 221. As discussed above, this incision 222
reduces the amount of bodily fluid required for a sample because less of the
fluid is
wasted by leaking from the microtitration pillow 221. In another embodiment,
the
blade 220 does not form the incision 222 in the test strip 200. Instead, the
blade
220 compresses the periphery of the microtitration pillow 221 in order to make
the
periphery of the pillow 221 substantially impervious to fluid so as to
minimize the
amount of fluid required for a sample.
While the invention has been illustrated and described in detail in the
drawings and foregoing description, the same is to be considered as
illustrative and
not restrictive in character, it being understood that only the preferred
embodiment
has been shown and described and that all changes and modifications that come
within the spirit of the invention are desired to be protected.