Note: Descriptions are shown in the official language in which they were submitted.
CA 02460526 2004-03-16
WO 03/025581 PCT/SE02/01665
NEW LANDMARKS AND USE THEREOF
TECHNICAL FIELD
This invention relates to a marker compound or a set thereof, suitable for gel-
s electrophoresis, a method for determining of characteristics of said
compound or
set, a method for detection and quantification of a sample in a gel as well as
the use
of said compound and set. Also disclosed is a lcit of said external marker
compound
or said set.
BACKGROUND OF THE INVENTION
Analysing, detecting and ident~ing proteins quantitatively aid qualitatively
in cells
and tissue
In a cell, from e.g. a cell culture or tissue sample, an existing pool of
proteins,
a proteome, exists as a part of biological processes and functions. Knowledge
about
the identity and amount of a protein or a group of proteins at a certain time
point and
also of certain changes over time find applications in clinical, diagnostical
and
analytical situations.
Certain biological processes are defined by changes in morphology and
physiology due to changes in the expression, i.e. the protein level, of
particular
genes. Also, developmental stages of cells can be defined and monitored by
their
global pattern of gene expression and the progressive changes that occur over
time
of particular genes or groups of proteins. Even more, as a response to
treatment of
cells with chemical factors, e.g. drugs, hormones, nutrient factors,
environmental
factors and other growth condition factors, specific proteins of groups of
proteins
can change the/their expression and as such allow for monitoring or
identifying a
response or treatment. Information of this type can be used to e.g. detect,
identify
and classify tumours in terms of malignancy, evaluation of therapies,
adjustment of
therapies as well as diagnosis and prognosis. Also, changes in proteins due to
mutations, cleavage, phosphorylation or glycosylation can be obtained.
Further identification of unknown proteins can be done by e.g. mass-
spectrometry of unidentified sample protein spots on the gel.
Two-dimensional gel electr~opho~esis
Two-dimensional gel electrophoresis is used as a general method for
detecting, identifying, monitoring and quantifying the compositions of complex
biological mixtures. Separation performed in two dimensions enables detection
and
identification of a large number of components that would not be separable and
distinguishable in a linear separation.
CA 02460526 2004-03-16
WO 03/025581 PCT/SE02/01665
2
A frequent way of monitoring the protein expression by 2D gel-
electrophoresis is by analysing a set of multiple gels in one run.
Detection and identifzcatioh of sample spots in a 2D polyacf ylamide gel
By using large gels, the number of proteins to be 'detected and identified can
be several hundred up to about 10 000 in one gel. Each spot is detected by
means of
a signal derived from the individual spot.
In a typical gel size of 24x18 cm, several thousands of sample protein spots
are detected. Normally, about 10% of the total number of sample spots need to
be
selected as landmarks. This further involves a few hundred manually selected
landmarks in each gel. The process of detection, identification and analysis
of the
separate components in a two-dimensional electrophoresis gel is a complex and
difficult task, and often involves tedious and time consuming steps manual
demanding months of experience to perform. Several attempts have been made to
develop means and methods to simplify, standardize and automatize the
procedure
as described in Smilansky et al., 2001, Electrophoresis 22: 1616-1626,
Thompson et
al., 1998, in 20~' Annual International Conference of the IEEE Engineering in
Medicine and Biology Society, Vol. 20: 1060-1063, Wanatabe, et al., 1998, in
20~'
Annual International Conference of the IEEE Engineering in Medicine and
Biology
Society, Vol. 20:804-808.
The manner in which the sample protein spots are distributed across the two-
dimensional gel-electrophoresis depend on the separation parameters used in
the
first and second dimension in the two-dimensional electrophoresis procedure.
Two
parameters commonly used are isoelectric point and molecular size (Gorg et
al.,
2000, Electrophoresis 21:1037-1053).
Various types of signals have been used as post-gel colouring techniques,
such as silver staining as described in Sinha et al., 2001, Proteomics 1:853-
840,
Shevchenko et al., 1996, Anal. Chem. 68:850-858. Also used is e.g., optical
density,
radioactive emission, fluorescence emission, and colorimetric signals (Pawn et
al.,
1999, J Interferone Cytokine Res.19:589-599, Steinberg et al., 2001,
Proteomics,
1:841-855, Herich et al., 2001; Biotechniques, 31:146-149, Kemper at al.,
2001,
Electrophoresis, 22:970-976, Lauber et al., 2001, Electrophoresis 22:919-932,
Berggren et al., 2000, Electrophoresis 21:2509-2521, Steinberg et al., 2000,
Electrophoresis 21:486-496). Detection of the spots is commonly achieved by
the
use of imaging devices that convert these signals into digital data and store
the data
as information on computer storage media.
CA 02460526 2004-03-16
WO 03/025581 PCT/SE02/01665
Landmarks
One of the lcey features in identifying the sample spots is the assignment and
use of reference spots known as "landmarlcs". The landmarks are actual protein
spots that are manually selected by the user, i.e. manual landmarlcing. The
software
used is further processing the manually selected spots to automatically detect
and
identify the same spots in all of the member gels in one matchset.
These user-selected landmarks are relatively few in number compared to the
total number of sample spots in an individual electropherogram. The selection
criteria of the manual landmarks are that the spots should be well-resolved,
that they
are well isolated from other spots, and that they appear in all the gels of
the match-
set.
The number of spots to be assigned as landmarlcs must be large enough so
that all of the remaining protein spots among the various gels will be
successfully
matched by the automatic processing. The function of the landmarlcs is to
serve as
guideposts in the gel-to-gel comparison, thereby aiming at reducing and compen-
sating differences and distortions among the member gels in the matchset to
assure
that there will be a proper correspondence of protein spots among different
gels in
the matchset.
Further, by using known sample proteins manually chosen as landmarks in
the 2-DE one has to consider only proteins that exists in all gels in one
matchset.
Such protein must exist in a relatively large amount to be readily repeatable
and
detectable in all of the gels. Due to this, the spots chosen as protein
landmarlcs are
often large and blob-like. This gives less defined landmarks that are
difficult to
position accurately, i.e. to find the exact centre of the landmark, which is a
source of
inaccuracy, on the analysis process of the sample protein spots.
The process of selecting and marking spots to be used as landmarks is slow
and tedious and is one of the limiting factors in two-dimensional gel
electrophoresis
analysis. Normally, since about 10% of the total number of protein spots needs
to be
selected as protein landmarks to make the image analysis algorithm work
correctly,
several hours needs to be used for each run to pick said landmarks manually.
Also,
in addition to assigning the above described selection criteria for manually
chosen
landmarks, the time involved in making the selections and making the spots
adds to
the cost and time involved in performing the analysis. Even further, the level
of user
involvement raises question regarding reproducibility and reliability.
Dendrimers
Dendritic macromolecules, or dendrimers, are synthetic 3-dimensional
macromolecules that are prepared in a step-wise fashion from simple branched
monomer units, the nature and functionality of which can be easily controlled
and
CA 02460526 2004-03-16
WO 03/025581 PCT/SE02/01665
4
varied. Their unique architecture and monodisperse structure has been shown to
result in numerous previously unknown or significantly improved physical and
chemical characteristics when compared to traditional linear polymers. As a
con-
sequence, dendrimers and dendrimer complexes are now considered to be one of
the
prime nanometer-scale building blocks for construction of nanoscale objects
used
for e.g. drug delivery and detection of various components of a sample using
dendrimers bearing probes and labels as described in W00102861.
Background
W00107920 discloses a method for automated landmarking for two-
dimensional gel electrophoresis by the addition of marker proteins to the
sample
proteins. Though, the use of proteins as external landmarks suffers form
several
disadvantages. The production costs are high and the shelf life of the final
protein
product is low compared to other organic molecules.
US 5 139 630 discloses a method for detecting and identifying protein
species in a sample by capillary zone electrophoresis by the addition of at
least two
external marlters, one being an ionic species and one being a neutral charged
species. This method is only applicable for capillary electrophoresis, i.e.
where the
separation is in one dimension, here disclosed for charge densities, and not
applicable for a separation using more than one dimension as in e.g. a two-
dimensional gel electrophoresis.
WO9707398 discloses dendrimer-polypeptide complex and malting thereof.
Said complexes are used for binding assays in biological samples, and not for
the
identification of unknown proteins.
It is thus highly desirable in the light of the aforementioned problems to
develop means and methods for reducing time and costs, as well as increasing
the
reproducibility and reliability of two-dimensional gel electrophoresis used
for
separation, detection, identification and quantification of proteins, which
can also
avoid the problems associated with the prior art means and methods. In this
respect,
the present invention addresses this need and interest.
SUMMARY OF THE INVENTION
In view of the foregoing disadvantages known in the art when using and
analysing two-dimensional gel electrophoresis (2-I7E), the present invention
provides marker compounds, external landmarks, methods and use thereof, for
detection and matching samples in a two-dimensional gel electrophoresis.
An object of the present invention is thus to provide efficient marlter
compounds, external landmarlts, as well as methods and use thereof for a rapid
and
efficient detection and matching of samples in a two-dimensional gel
CA 02460526 2004-03-16
WO 03/025581 PCT/SE02/01665
electrophoresis. Within the object is also considered a way for reducing time
and
costs, as well as increasing the reproducibility and reliability of two-
dimensional gel
electrophoresis used for separation, detection, matching and quantification of
proteins.
5 Thus the present invention provides a marker compound suitable for gel
electrophoresis, wherein the compound is not a protein. This marker compound
may
comprise at least one monomer unit, at least one functional group unit and op-
tionally at least one core unit. The marlcer compound may be characterised by
a pI of
about 1-12, preferably about 3-10, and/or with a molecular size of 5-106 Da,
or 103-
105 Da. The marker molecule may be a dendrimer, comprising at least one mono-
mer, at least one functional group and optionally by at least one core.
The present invention also provides a set of at least two said marker com-
pounds, suitable for gel electrophoresis. Said set form at least two marker
spots , i.e.
external landmarks, in a gel after electrophoresis, forming a grid on the gel
after gel
electrophoresis. The grid may be evenly or unevenly distributed on the gel.
Furthermore, the present invention provides a kit of external landmarks
comprising at least two of the marker compounds according to the invention or
at
least one of the sets according to the invention, and optionally at least one
buffer or
buffer system.
The use of said marker compounds according to the invention or at least one
of the sets according to the invention may be for detection and/or
quantification of a
sample or sample molecule.
Different embodiments may include a use, wherein the detection and/or
quantification of a sample or sample molecule is dependent on the pI and
molecular
size of the marlcer compound(s).
According to the invention, a method for determining and/or verifying the
characteristics of a marker compound according to the invention or a set of
external
landmarks according to the invention is included comprising the steps of a)
preselecting a theoretic positions where a marker compound according to the
invention or a set of external landmarks according to the invention is to
position, b)
designing a marker compound according to the invention or a set of external
landmarlcs according to the invention so as to achieve correct
characteristics, c)
applying the marker compound or the set in b) above onto the gel, d)
separating the
marker compound or the set in c) in a first dimension, e) optionally
separating the
marker compound or set in a second or further dimension, f) collecting
information
about the separation in d) and optionally in e), g) registrating the
information in f) as
digital information, and h) determining and/or verifying the characteristics
after
separation.
The use of said marker molecules and set will give a more reliable and rapid
CA 02460526 2004-03-16
WO 03/025581 PCT/SE02/01665
6
way of performing and analysing gel electrophoresis, such as two-dimensional
gel
electrophoresis. This is partly due to the quality of said markers, forming
even spots
on the gel after electrophoretic separation, not dependent on much manual
input of
the analysis compared to lcnown methods in the art.
SHORT DESCRIPTION OF DRAWINGS
Fig. la shows an example of a two-dimensional gel with protein samples, in
blaclc, and with 25 positions for landmarks. The positions are marked with x.
The
marker spots are positioned evenly over the gel,
Fig. 1b shows the same as in la except that the marker spots are positioned
unevenly over the gel,
Fig. lc shows the same as in la except that nine marker spots are positioned
evenly over the gel,
Fig. 1d shows the same as in lc except that the marker spots are positioned
unevenly over the gel,
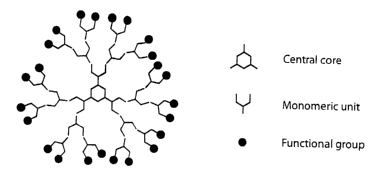
Fig. 2 shows a general formula of a dendrimer with a central core, marked
with a hexagon, several layers of monomeric units, Y-shaped, and an outer
layer of
functional groups shown as blacked filled circles. The central core may be
mono-,
bi-, tri-, or polyfunctional, with one or several layers of monomeric units
which can
be mono-, bi- or polyfunctional and an outer layer of functional groups,
Fig. 3 shows examples of structures that may be used as core units in the
dendrimer according to the invention,
Fig. 4 shows an example of a dendrimer according to the invention using
diaminoethane as the core and 3, 5-diaminobenzoic acid as the monomer and
aspar-
tic acid as the functional group,
Fig. 5 shows examples of structures that may be used as monomers in the
dendrimer according to the invention,
Fig. fi shows examples of structures that may be used as functional groups in
the dendrimer according to the invention,
Fig. 7 shows building blocks to be used in the synthesis of dendrimers in
example l,
Fig. 8 shows the coupling of building block Sa and Sb so as to give a number
of dendrimeric structures with different molecular weights according to
structures
7a or 7b,
Fig. 9 shows the synthesis of the marlcer compound lOc from the dendrimer
DAB-Am-16 (Polypropylenimine hexadecaamine dendrimer, Generation 3.0) shown
as 8a. Boc-Asp(OBzI)-OH is shown as 2b,
Fig. 10 shows the synthesis of the marker compound 11 c from the dendrimer
DAB-Am-64 (Polypropylenimine tetrahexacontaamine dendrimer, Generation 5.0)
CA 02460526 2004-03-16
WO 03/025581 PCT/SE02/01665
7
shown as 8b. Boc-Asp(OBzI)-OH is shown as 2b,
Fig. 11 shows the synthesis of compound 10a by coupling DAB-Am-16
shown as 8a. Phthalic anhydride is shown as 9a,
Fig. 12 shows the synthesis of compound lOb by coupling DAB-Am-16
shown as 8a. Succinic anhydride is shown as 9b,
Fig. 13 shows the synthesis of compound l 1b by coupling DAB-Am-64
shown as 8a. Succinic anhydride is shown as 9b, and
Fig. 14 shows the synthesis of compound 11 a by coupling DAB-Am-64
shown as 8a. Phthalic anhydride is shown as 9a.
DETAILED DESCRIPTION OF THE INVENTION
Defir~itio~s
As used herein, the term "spot/s" intends to mean a cluster of compounds
migrating identically in a gel electrophoresis thereby ending up at the same
relative
position on the gel. The spot may be formed by sample molecules or compounds,
the spots then herein referred to as "sample spots"; or from marker compounds,
such
spots then herein referred to as "landmarks", "external landmarks", "landmark
spots" or "marker spots".
The term "external" is herein intended to mean not originating, selected or
chosen from the original sample or sample molecules. Thus, "external
landmarks" or
"external marker compounds" is therefore not originating, selected or chosen
from
the original sample or sample molecules.
The term "sample" or "sample molecule(s)" is herein intended to mean a
known or unknown biological sample comprising proteins to be separated in a
gel
electrophoresis for further analysis. "Further analysis" is herein intended to
mean
identification and/or quantification.
The term "identification" is herein intended to mean to find identity with a
previous migration pattern in a gel electrophoresis due to physical, chemical
or
physiochemical characteristics of the sample, or identity with a known
compound.
The term "matching" is herein intended to mean a process of detecting and
matching corresponding spots in different gels. Following this, the term
"sample
matching" is herein intended to mean a process of detecting and matching the
same
sample in different gels.
The term "matchset" is herein intended to mean a group of gels that are to be
compared to each other for purpose of observing changes in individual sample
spots.
The term "natural" is herein intended to mean anything existing in nature, not
being derived from a synthetic process, except for a purification and/or an
enrich-
ment step.
CA 02460526 2004-03-16
WO 03/025581 PCT/SE02/01665
g
The term "non-natural" is herein intended to contrast the term "natural", i.e.
a
synthetic or artificial compound being man-made, not existing in a natural
form.
The term "protein" is herein intended to consist essentially of linear com-
binations of amino acids in peptide linkage, forming polypeptides. A protein
may be
built from more than one polypeptide chain.
The term "monomer" is herein intended to mean one building block or
building molecule. Several monomers may be collected in a "monomer unit"
distributed over several layers.
The term "functional group" is herein intended to mean a molecule or a part
of a molecule or building block, that gives a certain characteristic to said
molecule.
The term "core" is herein intended to mean a centre, focus, the central or
innermost part of the molecule or building block.
External landmarks
As revealed above, the present invention relates to gel-electrophoresis and to
marker compounds to be used, which are more reliable and more rapid than by
using
current means and methods known in the art. Such marker compounds form spots,
or external landmarlcs, after gel electrophoresis to be used for
identification and/or
quantification of a sample or sample molecule(s).
Present ways of detecting and matching proteins on a gel after gel electro-
phoresis include the denotation of sample molecules, e.g. the sample proteins,
in the
gel seen as spots, to marker proteins or marker spots. This will introduce
technical
problems such as less accuracy between different gels and matchsets and is
tedious
for the analyst as well as difficult to repeat.
Accordingly, as revealed above, marker compounds with known
characteristics are therefore added externally according to the invention,
thus
forming external landmarks on the gel after electrophoresis. As external
marker
compounds, they may be added to, but are not selected and chosen from the
sample
molecules, e.g. proteins. The externally added marker compounds may in
different
embodiments be added separately to the preparation of sample molecules, e.g.
proteins, or included separately to the gel before or during the run.
By selecting marker compounds with known and pre-chosen characteristic,
such as, e.g. pI and molecular size values, the external added marlcer
compounds
may position in a desired way over the gel. Such information about
characteristics of
the external marker compounds may be used for further analysis of unknown
samples, such as unknown proteins.
CA 02460526 2004-03-16
WO 03/025581 PCT/SE02/01665
9
The ma~kef° compound
According to the invention, a marker compound suitable for gel electro-
phoresis, wherein the compound is a natural, non-natural compound or a mixture
thereof and wherein the compound is not a protein is used. The marker
compounds
are added externally, and not chosen from the samples, as revealed above.
The marker compound may be a polymer or it may be selected from the
group consisting of glycoconjugates, carbopeptoides, polynucleotides, proteo-
glycanes, fullerenes, carbohydrates and mixtures thereof.
The size and pI of the marlcer compound should be in the range of the sample
molecules to be detected and identified.
In specific embodiments, the marker compound is characterised by a pI of
about 2-12. In still further embodiments, it is characterised by a pI of about
3-10.
Further embodiments include marker compound characterised by a Mw of
about 5-106 Da. In still even further embodiments, the Mw may be of about 103-
105
Da, 5-1000 Da, 5-600 Da, 5-250 Da or an otherwise suitable interval of
molecular
size due to the size of the samples to be separated and detected.
Specific embodiments comprise marker compounds characterised by a pI of
about 1-12 and by a Mw of about 100-106
The marker compound may also be a dendrimer, further described in the
paragraph below.
Dendrime~s as marker compounds
The external marker compound according to the invention may in specific
embodiments of the invention be a dendrimer. Dendrimers are built up from
different structural building blocks that may include branching points to
achieve a
tree like structure characteristic for dendrimers. The different building
blocks are
coupled together to achieve said treelike structure.
The dendrimers offer two important features when used as marker
compound, namely a) the size can be easily modified by adding more layers and
b)
the feature of the dendrimeric compounds can be modified by adding different
functional groups to the layers. Since the individual building blocks may be
composed of repeatable units, the dendrimer molecule may be built up from only
a
few numbers of coupling steps, which is economically and technically
advantageous.
According to the invention, the dendrimer may comprise at least one
monomer, at least one functional group and optionally at least one core as
building
blocks.
The dendrimer may be built up from separate building blocks as shown in
figure 2. Said dendrimers may be built up either by a divergent strategy or by
a
CA 02460526 2004-03-16
WO 03/025581 PCT/SE02/01665
convergent strategy as known in the art of dendrimer synthesis.
In one embodiment of the invention, the dendrimer to be used as external
marlcer compound according to the invention is synthesised according to the
divergent strategy.
5 The dendrimer may be represented by the general formula
(core)"(monomen...o)X (functional group l,.,p~
wherein n is an integer from 0-5 representing number of different co-existing
10 optional cores,
wherein o is an integer from 2-1000 representing number of different monomer
building blocks within the monomer distributed over x layers,
wherein x is an integer from 1-20 representing number of layers, and
wherein p is an integer from 1-20 representing the number of different
functional
groups within one functional group building block.
Other suitable dendrimers to be used as marker compounds according to the
invention are commercially available dendrimers. Though, such dendrimers lack
at
least one functional group according to the invention. Such a functional group
may
then, of course, be coupled onto the commercially available dendrimers using
techniques known to the skilled man in the art. Examples of, but not limited
to,
commercially available dendrimers that may be used according to the invention
are
AstramolTM (DSM Agro, The Netherlands) and Starbust~ (Aldrich)
In specific embodiments of the invention, synthetic amino acid dendrimers .
will be used. In still further specific embodiments, a diamin or a triamin is
used as a
core, diaminobenzoic acid as a monomer and aspartic acid as a functional
group. By
introducing different numbers of diaminobenzoic acid as a monomer a huge range
of molecular masses may be achieved as exemplified in table 2.
Table 2 - Molecular size of dendrimers
made of a diamin as core building block,
diaminobenzoic acid as a monomer (mon)
and aspartic acid as a functional group.
mon Molecular size
0 290
1 788
2 1785
3 3779
4 7767
5 15743
CA 02460526 2004-03-16
WO 03/025581 PCT/SE02/01665
11
6 31694
7 63597
The dend~irvte~ co~~e
According to the invention the external marker compound may comprise a
dendrimer, as exemplified above. The dendrimer may comprise at least one core.
According to specific embodiments, the core may be divalent, trivalent,
tetravalent, or a multivalent core and mixtures thereof. According to the
invention,
the at least one core may be selected from the group consisting of the
formulas in
figure 3 and mixtures thereof.
In a preferred embodiment, the core may be a diamine where n=2 or a
triamine where n=1.
The core may contain further branching units, allowing the treelike structure
characteristic of the dendrimer to form.
An example of a dendrimer using diaminoethane as the core is shown in
figure 4.
The monomer
The dendrimer also comprises at least one monomer. The monomer may
include further branching possibilities to the dendrimer molecule to achieve
the
treelike structure characteristic of the dendrimer. Due to the numbers of
branching
possibilities, the monomer may be monovalent, i.e., to elongate without
branching,
divalent, trivalent, tetravalent, multivalent or mixtures thereof. Examples of
such
monomers with monovalent and divalent branching units are shown in figure 5.
Specific embodiments use amid bondings between the different monomers.
In still a further embodiment, the at least one monomer may be 3,5-
diaminobenzoic acid, as shown in figure 4.
The number of monomers will contribute to the final molecular size of the
dendrimer. According to specific embodiments, using 3,5- diaminobenzoic acid
as
monomer the monomer may be in a number of 1-10.
The fuuctiohal group
The dendrimer may also contain a functional group according to the inven-
tion. The at least one functional group will to the dendrimer ad known charac-
teristics. An example of lcnown characteristics is, e.g. a desired net charge
of the
molecule.
In specific embodiments, at least one functional group may be added to the
CA 02460526 2004-03-16
WO 03/025581 PCT/SE02/01665
12
free ends generated in the coupling step. Due to the addition of the at least
one
functional group, the landmark will according to the invention be able to
position in
the gel if the gel parameters used for separation are selected so as to enable
separa-
tion of the marlcer compound characteristics.
According to different embodiments the at least one functional group may be
selected from the group of functional groups shown in figure 6, and
zwitterions,
anionic or cationic, oligopeptides, alcohols, tiols, carboxy acids, amines;
fluorochromes, such as fluorescamine, isotopes and mixtures thereof .
In a specific embodiment, the fluorescamine used is the commercially
available Fluram~ available from Molecular Probes, U.K.
In still further specific embodiments, the at least one functional group is
selected from the group consisting of an amino acid such as aspartic acid,
glutamic
acid.
According to the invention, the marker compound has known characteristics
affecting its migration in a gel during gel electrophoresis so as to position
the
marker compound in said gel. Such characteristics may, of course, be included
by
the addition of a functional group to the dendrimer. Even further, it may
reflect the
molecular size of the marker compound.
In specific embodiments, the known characteristics may affect the migration
in at least two dimensions in said gel.
In still further embodiments, such known characteristics may be dependent
on pI and molecular size.
The set of external landmarks
According to the invention, above said marker compounds can form a set of
external marlcers suitable for gel electrophoresis, comprising at least two
different
marker compounds described above.
According to the invention, said set may form at least two marker spots, i.e.
external landmarks, in a gel. As such, said two spots will appear in different
areas in
the gel, due to the known characteristics of the marker compounds that form
said
spots due to a separation in at least one dimension.
The number of marker spots in a gel may differ. This is due to the large
number of different types of gels to be used in the gel electrophoresis step.
Another
factor that may affect the number of markers needed are the number of sample
spots
on the gel, which is obvious for the skilled man in the art of gel
electrophoresis and
separation of samples such as e.g., protein samples. Other factors that
affects the
number of marker spots needed are the number of separation dimensions which
are,
according to the invention, at least one, preferably two, but may also be more
than
two, e.g. three. The number of marker spots needed may therefore be, e.g.
about
CA 02460526 2004-03-16
WO 03/025581 PCT/SE02/01665
13
0-50% of the number of sample spots, preferably 1-20%, even more preferably
1-10% of the number of sample spots.
Specific embodiments of the invention may use marker spots, wherein the at
least two marker spots may be from about 2-1000 marker spots per said gel.
In still further embodiments, the at least two marlcer spots may be from about
2-500, such as about 2, 3, 4, 5,6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17,
18, 19, 20,
21, 22, 23, 24, 25; or about 30, 35, 40, 45, 50, 55, 60, 65, 70, 75, 80, 85,
90, 95, 100;
or about 100, 200, 300, 400, 500 marker spots per gel.
Even further embodiments include about 500-1000 marker spots per gel, such
as about 500, 600, 700, 800, 900 or 1000 marker spots per gel.
In a specific embodiment, the number of marker spots needed is such as
about 5-40 marlcer spots. This may apply for a gel, such as a two dimensional
gel
e.g. a polyacrylamid gel, in a size of about 24x18 cm.
Specific embodiments are wherein the at least two marker spots form a grid
in said gel. Still further embodiments are wherein said grid may be evenly or
un-
evenly distributed in the gel, i.e., in different densities over the gel,
according to
chosen preferences. This is to overcome and compensate for differences and/or
dis-
tortions in a gel or group of gels to be analysed and compared. This will
improve
and render the spot matching of the landmarks and the protein samples more
effec-
tive. Examples of such grids are shown in figure 1 a-d. Figure 1 a shows an
example
of a two-dimensional gel with protein samples, in blaclc, and with positions
for
marker spots, i.e. external landmarks. The positions for said marker spots are
marked with an "X" in the gel. In figure 1 a, the spots are distributed evenly
over the
gel. In figure 1b, the same is shown, but the marker spots are distributed
unevenly
over the gel. Figure 1 c and 1 d shows the same as in 1 a and 1 b, but with
nine
marker spots.
Said marker spots contain different number of marlcer compounds per marker
spot. The amount of marker compounds should be, though, as is evident to the
skilled man in the art, enough to allow a clear and concise detection of the
marker
spot on the gel. This is, of course, dependent on how the sample spots are
detected,
equipment and/or gel resolution.
According to specific embodiments of the invention, the at least two marker
compounds forming at least one marker spot may position in at least one
dimension
in a gel, such as a polyacrylamide gel, at pre-chosen positions. As used
herein, the
word pre-chosen is intended to mean pre-chosen due to empirical information,
such
as experimental data, or from theoretical information, such as chemical and/or
physical data characterising the at least one marker compound used. The at
least two
marker compounds may be designed to be dependent upon e.g. pI and molecular
size for their positioning. Of course the marker compounds may be designed in
CA 02460526 2004-03-16
WO 03/025581 PCT/SE02/01665
14
different ways, as obvious to the skilled man in the art, to depend upon other
characteristics for separation according to other dimensions than pI and
molecular
size.
The set of external landmarks may in specific embodiments comprise at least
two marker compounds forming at least two spots, wherein the at least two
marker
compounds are characterized by a pI of about 1-12, such as about 3-10.
In still further embodiments, the at least two marker compounds may be
characterised by a Mw of about 100-106, such as about 103-105.
Specific embodiments may be where the at least two marker compounds may
be characterised by a pI of about 1-12 and a Mw of about 100-106.
In still further embodiments of the invention, the set of external landmarks
form a grid on said gel due to the fact that the characteristics of the marker
forming
the spots are pre-chosen compounds.
Cha~~acter~istics of a de~d~ime~ according to the invention
Examples of dimensions used for separation of sample proteins and external
marker compounds are separations based on e.g. the molecular size and pI of
the
marker molecule and/or sample protein.
As described above, the separation of marker compound and/or the samples
are performed in at least one dimension. This means that the separation,
according-
1y, is dependent on such characteristics of said compound and/or sample.
In the specific embodiments of the invention separation is dependent on the
pI of the external landmark. To achieve this for the marker compound, e.g. a
dendrimer, a functional group is added as described above, till the landmarlc
will
theoretically and/or empirically position at the desired pre-chosen spot in
the gel.
The second dimension may be a separation due to the molecular size of the
landmarlc. This is done simply by adding at least one building block of the
monomer, which may be branched. Different sizes of the dendrimer marker
compound are then simply achieved by adding a different numbers of said
monomer
to the optional core structure.
According to specific embodiments, the free ends may protrude from the
dendrimer structure or reside in the more interior part of the branching
treelike
structure. Further examples of cores, monomers, and functional groups to be
used in
different embodiments are shown in figure 3-6.
A kit of external lavcdma~~ks
The present invention also includes a lcit of external marker compounds,
comprising at least two of the marker compounds according to the invention, or
at
least one of the sets of external marlcers according to the invention, and
optionally at
CA 02460526 2004-03-16
WO 03/025581 PCT/SE02/01665
least one buffer or buffer system.
The lcit may, in different embodiments, comprise at least two marker
compounds according to the invention, or the set according to the invention
wherein
said set or compound is to be dissolved upon usage or is pre-dissolved in a
solution.
5 Thus, the at least two marlcer compounds according to the invention, or the
set according to the invention may specific embodiments in the kit be supplied
in a
dry format, such as a powder form format, in a pellet format, a granula
format, or a
flakes format.
In further embodiments, the at least two marker compounds according to the
10 invention, or the set according to the invention may in the kit be supplied
in a pre-
dissolved format such as in a solution, e.g. in a suitable buffer, an aqueous
solution,
or in an organic solvent.
Still further embodiments may comprise a kit wherein at least one applicator
strip suitable for gel electrophoresis is included. Examples of, but not
limited to,
15 suitable applicator strips comprises agarose and/or polyacrylamide strips,
such as
Immobiline Dry Strips obtainable from Amersham Pharmacia Biotech, e.g. the 3-4
polyacrylamide gel 3-10 NL pI strips. Other suitable strips that may be
included
are given in table 3. The examples in table 3 are only to be considered as
examples
and not in any way limit the scope of the invention.
Table 3 - Suitable applicator strips that may be included
in the kit.
Company Type of strip
Amersham Pharmacia Immobiline Dry StripTM,
Biotech
Biorad Ready Strip IPG-stripTM,
Tube Gel IEF 2-D
systemTM
Even further embodiments may contain a pre-casted gel suitable for gel
electrophoresis. The choice of gel to use is obvious for the skilled man in
the art and
may differ depending on the particular application. It may be a gel suitable
for two
dimensional gel electrophoresis, such as an agarose gel, a polyacrylamid gel
or a
mixture thereof in a suitable concentration or an otherwise known in the art
suitable
gel composition in a suitable concentration.
Still further embodiments may comprise enough of said external marker
compound or set according to the invention to be used in one or multiple gel
runs.
CA 02460526 2004-03-16
WO 03/025581 PCT/SE02/01665
16
Even still further embodiments may include a manual describing the method,
a certificate for the kit, constituents and guidelines for the usage of the
kit as well as
analysis procedure. Said manual may also be supplied on a compact disc, CD, on
a
server reachable for the user or a regular disc floppy and may also be
interactive for
the user.
Use of exte~~nal landmarks
The use of external landmarks, or marlcer compounds, according to the
invention, or a set thereof according to the invention, comprises using marker
compounds in gel electrophoresis where the marlcer compounds may, due to its
chemical, physical or physiochemical characteristics, migrate, and optionally
separate if more than one type of marker is used, in said gel together with
sample
molecules. Marker compounds positioning at the same position in the gel will
form
spots, i.e. marker spots that are readily detectable for further analysis. The
migration
and separation of said marker compounds is done in at least one dimension,
such as
in two dimensions where the two dimensions may be dependent on the pI and
molecular size of the marker molecule.
According to the invention, the use of said marker compounds according to
the invention or at least one of the sets according to the invention may be
for
detection and/or quantification of a sample or sample molecule.
Different embodiments may include a use, wherein the detection and/or
quantification of a sample or sample molecule is dependent on the pI and
molecular
size of the marker compound(s).
A method fog dete~mini~tg andlo~ ve~~ifyihg characteristics of marked
compounds
The invention provides a marker compound with specific and pre-chosen
chemical, physical or physiochemical characteristics. The characteristics may
be a
specific and pre-chosen pI or molecular size, thereby affecting the migration
characteristics of the marker compound during a gel electrophoresis. To
determine
and/or verify the characteristics of such compounds, a method is provided.
According to the invention, a method for determining and/or verifying the
characteristics of a marker compound according to the invention or a set of
external
landmarks according to the invention is included comprising the steps of
a) pre-selecting a theoretic positions where a marker compound
according to the invention or a set of external landmarks according to the
invention
is to position,
b) designing a marker compound according to the invention or a set of
external landmarks according to the invention so as to achieve correct
characteristics,
CA 02460526 2004-03-16
WO 03/025581 PCT/SE02/01665
17
c) applying the marker compound or the set in b) above onto the gel,
d) separating the marlcer compound or the set in c) in a first dimension,
e) optionally separating the marker compound or set in a second or
further dimension,
f) collecting information about the separation in d) and optionally in e),
g) registrating the information in f) as digital information, and
h) determining and/or verifying the characteristics after separation.
In further embodiments, the first dimension may be pI or molecular size.
In further embodiments, the first dimension is pI and the second dimension is
molecular size.
In further embodiments, the first dimension is molecular size and the second
dimension is pI.
In different embodiments the method in a-h above includes different ways of
applying said marlcer compound or set. E.g., the application of the marker
compound or set in step c) above may include applying the marker compound or
set
in the form of application strips or mixing and applying the marker compound
or set
together with the test samples or applying the set at the time of casting of
the gel.
Examples of, but not limited to, suitable application strips are Immobiline
Dry Strips~ made of 3-4 % polyacrylamid gel, such as the 3-10 NL pI strips
obtainable from Amersham Pharmacia Biotech.
The separation in step e) above may be in further dimensions. Different
embodiments may be wherein the further dimensions are 3, 4 or even more
dimensions. A specific embodiment is wherein the separation in step e) above
is a
separation in a second dimension.
The separation of the set may be dependent upon different characteristics of
the marker compounds. As such, different embodiments of the method includes a
method as described above, wherein the separation is in at least two
dimensions and
wherein the two dimensions are dependent on pI and molecular size of the
marker
compound.
Information about the position of each separated marker compound needs to
be collected and stored. Accordingly, embodiments of the invention are wherein
the
collecting information about the position in step f) above, is done by using
any of
the determination processes selected from the group consisting of visual
light, UV,
IR, multispectral imaging, isotope labelling, colouring techniques, e.g.
silver
staining, Comassie staining; fluorescence e.g. DIDGE (R) StainingTM
(Amersham),
fluorochromes such as fluorescamine, and mixtures thereof.
CA 02460526 2004-03-16
WO 03/025581 PCT/SE02/01665
18
A method fog detectiv~g and/or quantification of sample molecules and
extet~hal
landmarks
A method according to the invention for detection and/or quantification of a
sample and/or external landmarlc in a gel comprises the steps of
a) adding the sample to the gel
b) adding the at least two marlcer compounds according to the invention
or the at least one set according to the invention with known identity and
known
characteristics on the gel,
c) separating said sample and/or marker compound in b) above to form,
with said at least two marker compounds or set of external landmarks added, an
array of spots of the sample proteins and at least two of the marker molecules
or at
least one of the sets, respectively,
d) collecting information about the positions of the array of spots in at
least one image and optionally superimposing the images,
. e) registrating the information in d) above as digital data, and
f) analysing and/or correcting and optionally changing the image or
images to detect, quantify and optionally verify the sample. The verification
may
include, if possible, an identification of said sample molecule.
The method may in specific embodiments be a method wherein the adding in
step a) and/or b) above includes adding the sample and/or the at least two of
the
marker molecules or the at least one set in application strips, or mixing and
applying
the at least one set or the at least two marker molecules together with the
test
samples or applying the at least one set or the at least two marker molecules
at the
dine of casting the gel.
Still further, the method may in the separation in step c) above be performed
in at least two dimensions and the array formed in step c) above may then be
an
array in at least two dimensions. In even further embodiments, the said
separation in
two dimensions may be two-dimensional gel-electrophoresis, wherein the gel-
electrophoresis may be polyacrylamide gel-electrophoresis, and wherein the two
dimension may be dependent on pI and molecular size.
The samples further needs to be correlated to the marker molecules so as to
achieve information about the samples. The method according to the invention
may
in different embodiments include correlating the sample to the at least two
marker
molecules or the at least one set with known positions and characteristics,
and
optionally correcting for background noise and distortions, and assigning the
sample
at least one characteristic in the analysing in step f) above.
Further, the known characteristics of said at least two marker compounds or
the at least one set may be dependent on their molecular sizes, and step f)
above may
further comprise assigning a molecular size to at least one sample based on
the
CA 02460526 2004-03-16
WO 03/025581 PCT/SE02/01665
19
molecular size of said set of external landmarks.
Even more further, the lcnown characteristics of said at least two marlcer
molecules or the at least one set are dependent on their pI, and step f) in
the method
above further comprises assigning a pI to at least one sample based on the pI
of said
set of external landmarks.
Also, embodiments include a method wherein the known characteristics of
said set of external landmarks may be dependent on their molecular sizes and
their
pI, and step f) in the method above may further comprise assigning a molecular
size
and a pI to at least one sample based on the molecular size and the pI of said
set of
external landmarks.
Detection of artificial external landmarks
By using external landmarks according to the invention, one may detect the
landmarks in an alternative way compared to the sample molecules. Examples of
different ways of detecting the external landmarks according to the invention
is by
using any of the determination processes selected from the group consisting of
visual light, UV, IR, multispectral imaging, isotope labelling, colouring
techniques,
e.g. silver staining, Comassie staining; fluorescence. e.g. DIDGE (R)
StainingTM
(Amersham) fluorochromes such as fluorescamine and mixtures thereof.
The detection of landmark spots may be done by using:
1. The shapes of the landmark spots, both in two and three dimensions.
2. The patterns of the landmark spots in the gel.
3. A combination of both shapes and patterns of the landmark spots in the gel.
4. Positions of the landmark spots in the gel.
5. A combination of both shapes and positions of the landmark spots in the
gel.
6. A combination of both positions and patternls of the landmark spots in the
gel.
A combination of shapes, patterns and positions of the landmark spots in the
gel.
Said alternative ways of detecting the external landmark will give a more
accurate positioning due to less interference with the protein sample spots.
In specific embodiments of the invention the amount of external landmarks
may be chosen in such a way that the landmark spots positioned are evenly
formed
for all the spots. The spots may also be chosen in preferred embodiments to
allow
for quantitative analysis of the protein sample spots since known and exact
amounts
of the external landmarks may be chosen. Evenly formed landmarks will give a
higher accuracy and reproducibility in the analysis process due to a
simplified
process when choosing the centre of the landmark spot compared to when using
proteins that may form large irregular blobs in the gel. Such regularly shaped
CA 02460526 2004-03-16
WO 03/025581 PCT/SE02/01665
external landmarks according to the invention, that may be detected in a
different
way than the sample proteins, may be added in a small amount due to high
reproducibility and reliability.
The external landmarks according to the invention will reduce the input of
5 manual work due to the readily and accurate detection of said landmarlcs.
The
detection of the landmarlcs may be done fully automatically by in the art
known
techniques for spot detection. An image produced by e.g. scanning of the
landmarks
may further be superimposed onto the image of the sample proteins before
further
processing and analysis of the electrophoresed sample proteins.
Separating the external land~na~ks according to the ihvehtion
A way of separating the external landmarks according to the invention may
be done as described below in this paragraph. In this specific embodiment,
external
dendrimer marker compounds are separated in two dimensions in a polyacrylamide
gel. The experimental procedure is performed over 4 days. One or more
dimension
may be used in other embodiments and the separation may be performed over one
or
several days.
A rehydration of the samples, i.e. both the external marker compound and the
sample molecules, is done on day 1. The samples may be dissolved in 125-500
~,l
solubilisation solution such as of 2M thiourea, 7M urea, 4% CHAPS, 0,3% DTT
and 0,5% IPG buffert. The samples may then be pulled into the strip by
diffusion
overnight in a tray.
On day 2, the separation in the first dimension may take place. The strips may
be aligned in a first dimension separation system such as a Multiphore
(supplied by
e.g. Amersham Pharinacia Biotech). Different embodiments may, of course use
similar equipment as is known by the skilled man in the art of isoelectric
focusing,
such as an IPphore (supplied by e.g. Amersham Pharmacia Biotech) or similar.
The
proteins may further be focused for 5-40h at 3500-8000 V.
On day 3, the 2"d dimension separation may be performed. The strips formed
day 2 may be equilibrated in equilibration solution such as 30% glycerol, 2%
SDS,
6M urea and SOmM Tris/HCL, supplemented with 65mM DTT, and secondly 259
mM iodocetamine to reduce and alkylate the sample. According to knowledge
within the art, samples may be reduced, alkylated and separated optimally for
the
specific samples and markers added to the separation. The strips may be
applicated
onto the polyacrylamide gel and run in the 2°d dimension in a running
buffer
comprising 0,1 % SDS, 24 mM Tris-base and 0,2 M glycin, or similarly. The
separation is performed for a certain number of hours to achieve a proper
separation
e.g., for about 20 h at 100V or similarly as found suitable by the skilled man
in the
art.
CA 02460526 2004-03-16
WO 03/025581 PCT/SE02/01665
21
On day 4, staining and scanning of the samples and the external marlcers may
be performed. Different methods may be used here, as described in the
paragraphs
above. One way of staining and detecting the samples and the markers is silver
staining, as is a well-lcnown procedure in the art. For silver staining, the
gel is fixed
in e.g., 50% ethanol, 5% HAc, 45% water, milliQ quality, for about 1h. Further
on,
the gel is washed in e.g., 50% ethanol and subsequently in water for about 30
minutes each. A silver staining may be done according to methods known in the
art,
or in a modified way, as is obvious for the skilled man in the art. One
example of a
modified silver staining to be performed for developing the gel may be wherein
the
gel is sensitized in 0.02 % sodium thiosulfate and stained with 0,1 % silver
nitrate.
Further, the gel may be developed in 0.04% formaldehyde, 2% sodiumcarbonate
till
the samples are visualised enough for detection. The staining process may be
stopped by adding approximately 500 ml 5% acetic acid, or in another way, as
is
obvious to the skilled man in the art.
The stained gels, both containing separated landmarks and sample molecules,
may be scanned in a scanner with dual detection possibility. With dual
detection
possibility, it is intended to mean that the scanner has the possibility to
detect
signals from different staining techniques, such as silver staining,
fluorescent
staining, radioactivity, or any other staining method used.
The landmarks are detected in a separate image after a scanning step,
enabling detection of the landmarks only. Without changing the position of the
gel,
the parameters of the scanning apparatus are changed, so as to enable a
separate
scanning and detection of the separated sample molecules, i.e. the proteins.
The two gel images, one with the separated landmarks and one with the
separated sample proteins, now collected in digitalized form, are subsequently
used
for the image analysis of the gel.
The gel may also be scanned once, detecting the sample molecules and the
proteins in one image. This may be convenient when the same detection
methodology is used for the sample molecules and the landmarks.
EXAMPLES
Example 1
This example describes without limiting the invention the synthesis of
dendrimers for use as marker compounds and external landmarks in 2D-gel
electrophoresis.
CA 02460526 2004-03-16
WO 03/025581 PCT/SE02/01665
22
Objective
The objective of this example is to synthesize dendrimers of different
molecular sizes and pI. The sizes chosen are approximately 4000 and 16000 and
the
pI approximately 3-4 and 6-8.
Material
N-~i-t-Boc-~i-alanine (Boc-~i-Ala-OH), N-a-t-Boc-L-aspartic acid ~i-benzyl
ester (Boc-Asp(OBzI)-OH), N,N'-dicyclohexylcarbodiimide (DCC), and N-
Hydroxybenzotriazole (HOBt) were purchased from Novabiochem.
N,N-Dimetylformamide (DMF), dichloromethane (DCM), heptane,
diisopropyl ethylamine (DIPEA), metanol (MeOH), trifluoroacetic aid (TFA),
anisole, sodium hydroxide (NaOH), acetonitrile (MeCN), 3,5-diamino-benzoic
acid,
1,2-diaminoethane, and Citric acid were purchased from Merck.
Polypropylenimine hexadecaamine dendrimer, Generation 3.0 (DAB-Am-16)
and Polypropylenimine tetrahexacontaamine dendrimer, Generation 5.0 (DAB-Am-
64) were purchased from Aldrich.
9,10-bis(aminomethyl)anthracene (6a in figure 8) was synthesized according
to Gunnlaugsson et al. (Org. Lett., 2002, 4, 2449-2452) incorporated herein by
reference.
Experimental procedure
Synthesis of de~cdrime~ structures based on 3, 5-diamino-benzoic acid
This section describes the synthesis of compounds Sa and Sb in figure 7 and
the use of them as building block for marker compounds based on dendrimeric
structures.
The two building blocks were synthesized by coupling commercially
available amino acids to 3,5-diamino-benzoic acid as shown in figure 7. The
building blocks were then activated as OBt-esters by treatment with DCC and
HOBt
to give Sa and Sb in figure 7.
The dendrimeric structures were then synthesized by coupling of building
block Sa in figure 7 to a bisamine, as shown in 6a or 6b in figure 8. The
protective
groups were removed and the coupling repeated.
Finally, building block Sb in figure 7 was coupled so as to give the protected
dendrimer in figure 8. Deprotection gave a number of dendrimeric structures
with
different molecular weights according to structures 7a or 7b in figure 8.
CA 02460526 2004-03-16
WO 03/025581 PCT/SE02/01665
23
P~epat~atioh of building blocks
Boc-,Q-Ala-OBt (2a)
Boc-~i-Ala-OH la in figure7, (1.0 g) was dissolved in DMF (20 mL) and
HOBt (0.84 g) was added.
DCC (1.44 g) was added after 10 minutes. The mixture was stirred over night
and then slowly added to ice cold water (250 mL).
The precipitate was collected and dried in vacuum and then dissolved in
DCM (25 mL).
The slurry was then added to ice cold heptane (150 mL), stirred for two hours
and then filtered to give compound 2a in figure 7 ( 1.13 g, 70% yield).
Boc Asp(OBzI)-OBt (2b)
Boc-Asp(OBzI)-OH 1b in figure 7 was treated as in the synthesis of 2a in
figure 7 to give Boc-Asp(OBzI)-OBt shown as 2b in figure 7.
3, 5-di-(Boc-/3-Ala)-Benzoic acid (4a)
3,5-diamino-benzoic acid shown as 3 in figure 7 (0.12 g) was dissolved in
DMF (2 mL) and added to a slurry of 2a in figure 7 (0.58 mg) in DMF (5 mL).
DIPEA (1.3 mL) was added to the mixture. The mixture was stirred at
40°C
over night and then slowly added to ice cold water ( 100 mL) and acidified
with
citric acid ( 1.5 g). The precipitate was filtered off and dried in vacuum to
give 4a in
figure 7 in quantitative yield.
3, 5-di-(Boc-/.3-Ala)-Benzoic acid OBt ester (5a)
Compound 4a in figure 7 (0.51 g) was dissolved in DMF (5 mL) and HOBt
(0.16 g) and DCC (0.28 g) were added. The mixture was stirred over night,
filtered
and then slowly added to ice cold water (60 ml). The precipitate was filtered
off and
dried in vacuum to give Sa in figure 7 (75%).
3,5-di-(Boc-Asp(OBzl))-Benzoic acid OBt estey~ (5b)
3,5-diamino-benzoic acid was treated with 2b in figure 7 as in the synthesis
of Sa described above.
General procedure fo~~ dendrimer synthesis
a) Coupling
The diamine 6a or 6b in figure 8 (0.18 mmol) was dissolved in DMF (1.5
mL) and DIPEA (0.5 mL). A solution of the activated acid Sa in figure 8 and 1
(3.5
eq) in DMF (0.5 mL) was added and the mixture was stirred over night and then
slowly added to ice cold water ( 100 mL). The precipitate was filtered off,
dried in
CA 02460526 2004-03-16
WO 03/025581 PCT/SE02/01665
24
vacuum and then purified (Si02, DCM:MeOH 9:1, and Sephadex LH-20,
DCM:MeOH 1:1).
b) Deprotectiov~ of Boc-groups
The Boc groups were removed by addition of a TFA-solution (1 ml,
TFA:DCM:anisole 80:15:5). The mixture was stirred for 2 hours and then
concentrated twice with toluene.
c) Dep~otection of Benzyleste~s:
The benzylesters were removed by treatment with aqeous NaOH ( 1 M, 0.5
mL) at 0°C for 15 min and then neutralized using ice cold acetic acid.
The mixture
was then lyophilized.
d) Procedure to synthesise lzighe~ getze~ations of de~cd~~imeric
stf°uctures
The procedures (a-b) were repeated to get higher generations, i.e. increase in
molecular size, of dendrimeric structures. Finally compound Sb in figure 8 was
coupled and deprotected using procedures b and c above. The final deprotected
dendrimer was purified using reverse phase HPLC (MeCN: 0.1 % aq TFA).
Results
The results of the synthesis are dendrimeric structures shown as 7a and 7b in
figure 8.
Example 2
This example describes without limiting the invention the synthesis of marker
compounds from commercially available dendrimers for use as external
landmarlcs
external landmarks in 2D-gel electrophoresis.
Objective
The objective of this example is to synthesize six different marker
compounds with different pI (approximately 3-4 and 6-8) and molecular sizes
(approximately 4000 and 16000). They are all synthesized from the two
commercially available dendrimers DAB-Am 16 or DAB-Am-64. The dendrimers
were condensed with three different acids to give six different landmarks as
shown
in figure 9-14 as 10a, 10b, 10c, l la, l 1b, and l lc.
Material
DAB-Am 16 (Polypropylenimine hexadecaamine dendrimer, Generation 3.0)
or DAB-Am-64 (Polypropylenimine tetrahexacontaamine dendrimer, Generation
5.0) are commercially available from Aldrich.
CA 02460526 2004-03-16
WO 03/025581 PCT/SE02/01665
Expe~ime~tal p~ocedvcre
Synthesis of IOc a~cd 11 c
The synthesis of compound l Oc and 11 c are shown in figures 9 and 10.
The dendrimers DAB-Am-16, 8a in figure 9, or DAB-Am-64, 8b in figure
5 10, (30 mg) were dissolved in a solution of activated Boc-Asp(OBzI)-OH shown
as
2b in figure 9 ( 160 mg, 1.8 eq/NH2) in DMF (4 mL).
DIPEA (0.8 mL) was added and the mixtures were stirred over night and then
slowly added to ice cold water (100 mL). The precipitate was dried in vacuum
and
then deprotected using procedures b and c in Experiment 1 above, and then
purified
10 using reverse phase HPLC (MeCN: 0.1% aq TFA).
Synthesis of IOa, IOb, I l a, a~td l l b
The synthesis of compound 10a, 10b, l la, and 11b are shown in Figures 11
14. Another set of dendrimers was synthesized by coupling DAB-Am-16 shown as
15 8a in figure 11-14, or DAB-Am-64 shown as 8b in figure 13 with succinic 9b
in
figure 13 or phthalic anhydride shown as 9a in figure 14 using the following
procedure:
The dendrimer (38 mg) was dissolved in DMF (2 mL) and DIPEA (0.5 mL)
and the anhydride shown as 9a or 9b in figure 11-14 (2 eq/NH2) were added. The
;,
20 mixture was stirred over night and then concentrated and dried in vacuum.
The
residue was purified using reverse phase HPLC (MeCN: 0.1 % aq TFA).
Results
The results of the synthesis are dendrimeric structures shown as 10a, l Ob,
25 l la, and l 1b in figure 11-14.
Example 3
This example describes without limiting the invention a two-dimensional gel
electrophoresis with the addition of external dendrimer marker compounds.
Objective
The objective of this example is to position an external dendrimer marker in
two dimensions in a polyacrylamid gel.
Material
Dendrimers synthesized in example 1 are used. The dendrimers G2 and G3
are shown in figure 8 as 7a where n=6 for G2 and n=14 for G3.
CA 02460526 2004-03-16
WO 03/025581 PCT/SE02/01665
26
Experimental procedure
Day 1- Rehydration Sample molecules and the external dendrimer
markers are dissolved in 125-500 ~,l of 2M
thiourea, 7M urea, 4% CHAPS, 0,3% DTT
and 0,5% IPG buffert. The samples are pulled
into the strip by diffusion overnight in a tray.
Day 2 - 1St dimension The strips are put onto the gel and aligned in a
Multiphore. The proteins are focused for lOh
~ at 3500 V.
Day 3 - 2"d dimension Equilibrate the strips from day 2 in 30%
glycerol, 2% SDS, 6M urea and SOmM
Tris/HCL, supplemented with 65mM DTT,
and secondly 259 mM iodocetamine to reduce
and alkylated the sample.
Apply the strips to the gel and run the 2"a
dimension in a running buffer0,1 % SDS, 24
mM Tris-base and 0,2 M glycin for 20h at 100
V.
Day 4 - Staining and scanning
Fix the gel in 50% ethanol, 5% acetic acid,
45% water, milliQ quality, for 1h. Wash in
50% ethanol and subsequently in water for 30
minutes each. A modified silver staining is
performed to develop the gel, wherein the gel
'is sensitized in 0.02 % sodium thiosulfate and
stained with 0,1 % silver nitrate. The gel is
developed in 0.04% formaldehyde, 2%
sodium carbonate till the samples are
visualised enough for detection. The staining
process is stopped by adding approximately
500 ml 5% acetic acid.
The stained gels, both containing separated
landmarks and sample molecules, are scanned
in a scanner with dual detection possibility.
The landmarks are detected in a separate
image after a scanning step, enabling
CA 02460526 2004-03-16
WO 03/025581 PCT/SE02/01665
27
detection of the landmarks only. With~unn
changing the position of the gel, the
parameters are changed, so as to enabLoe oa
separate scanning and detection of th.e
separated sample molecules, i.e. the pr~o~d~f~eiins.
The two gel images, one with the sep~a.~a~ttesd
landmarks and one with the separated sa~immple
proteins, now collected in digitalized fbn:~ctn,
are subsequently used for the image an~a~lyt~sis
of the gel.