Note: Descriptions are shown in the official language in which they were submitted.
1 11
CA 02461952 2010-08-31
ACID GAS ENRICHMENT PROCESS
FIELD OF THE INVENTION
The present invention relates to treatment of natural gas generally, and
in particular relates to processes for enriching acid gases for sulphur plant
feeds.
BACKGROUND OF THE INVENTION
a) Industry Background
Petroleum reservoirs, whether primarily oil reservoirs or gas reservoirs,
often contain significant quantities of hydrogen sulphide (H2S) and carbon
dioxide
(C02) in addition to hydrocarbons. These contaminants must be removed or at
least
reduced to meet commercial specifications for purity before the natural gas
can be
marketed to consumers. The hydrogen sulphide and carbon dioxide, usually
referred
to as "acid gases", have commercial value as by-products in and of themselves
if, for
example, the hydrogen sulphide is converted to sulphur and the CO2 is used for
miscible flooding of oil reservoirs. Otherwise, the acid gases are considered
to have
no marketable value, and are disposed of either by pumping down a disposal
well or
by flaring.
Commercial specifications for natural gas require that essentially all of
the hydrogen sulphide be removed from the gas, typically to a final
concentration of 4
PPM (parts per million) by volume or less. Carbon dioxide must likewise be
reduced,
but being non-toxic, the tolerance for CO2 is much higher (typically 2% by
volume for
commercial pipeline quality gas).
The extremely stringent specification for H2S content in natural gas has
dictated the type of process that must be used, and virtually all natural gas
being
1
CA 02461952 2010-08-31
"sweetened" today is treated by one of the various alkanolamines that are
available
for this purpose. More than half a century ago, the Girbitol process was
introduced in
which the primary amine, monoethanol amine (popularly known as "MEA"), was
used
as the absorbent. Since then, other amines have become popular, namely
diethanoamine (DEA), and a current favourite, methyidiethanol amine (MDEA),
which
is popular because of its preferential affinity for hydrogen sulphide over
carbon
dioxide. In most cases, generic amine in an aqueous solution is used, although
various processes are available In which chemical additives are used in the
amine
solution to enhance certain characteristics of the absorbent. Amine has gained
widespread acceptance and popularity because it can produce a natural gas
product
that reliably meets the strict requirements for gas purity, especially the
requirements
for hydrogen sulphide, and can do it relatively inexpensively.
Alternative processes for acid gas removal, such as physical absorption
in a solvent or distillation for removal of acid gases, have not been used
extensively,
except possibly for bulk removal followed by cleanup with amine or as a
scavenger
for small volumes. Amine is able to remove acid gas components by reacting
with
them, which in an equilibrium situation can potentially totally remove the
acidic
components from the gas. Acid gases can be removed by other processes based on
chemical reaction, such as the hot carbonate process and various forms of the
iron
oxide process, which can meet the specifications for gas purity. However, for
many
practical reasons these processes have never gained widespread popularity.
Historically, the primary concern of the gas processing industry has
been to produce natural gas that will meet the stringent requirements for gas
purity
2
CA 02461952 2010-08-31
imposed by pipeline and distribution companies who establish the
specifications for
natural gas. There has been much less attention directed toward the by-product
of
the amine process; the acid gas mixture of H2S and CO2 that is co-absorbed in
the
process. Typically, these two gases are not subjected to any separation
process to
recover them as two separate entities, but are sent directly as feed to a
sulphur plant.
Most sulphur plants utilize some version of the Claus process in which one
third of the
H2S is oxidized by combustion to SO2, which then subsequently reacts with the
remaining two thirds of the H2S to produce elemental sulphur and water. The
second
acid gas component, carbon dioxide, is an inert gas and a none-participant in
the
chemical reaction, but because of the thermodynamics of the Claus process,
carbon
dioxide will detrimentally affect the reaction to produce sulphur. The
presence of
carbon dioxide dilutes the reactants - hydrogen sulphide, oxygen, and sulphur
dioxide, retarding the reaction and reducing the percentage conversion to
sulphur.
The dilution effect directly influences the chemical equilibrium of the Claus
process,
fundamentally reducing the attainment of high rates of sulphur conversion. In
cases
where the acid gas feed to the sulphur plant is rich in H2S, the effect of
dilution by
CO2 may not be too serious, but in those cases where the quantity of CO2
exceeds
the quantity of H2S by a factor of five or more, the effect on thermodynamic
equilibrium conversion to sulphur is very significant.
A secondary effect of dilution of H2S by excessive quantities of CO2 is
flame stability in the reaction furnace where H2S is oxidized to SO2. Carbon
dioxide is
an effective fire extinguishing chemical, and when present in excessive
amounts in
the reaction furnace it can inhibit combustion, and in some cases completely
quench
3
CA 02461952 2010-08-31
the flame. The dilution effect of CO2 in the firebox of the furnace will also
reduce
furnace temperature to the extent that complete combustion does not occur.
This
necessitates the addition of natural gas to the acid gas entering the sulphur
plant in
order to improve combustion and maintain flame temperature in the reaction
furnace.
Natural gas in the reaction furnace causes a further complication by
increasing the
undesirable reaction by-products, carbonyl sulphide and carbon disulphide.
These
are the products of reaction between methane and other hydrocarbons, CO2, H2S
and
oxygen, and although they may be present in the furnace effluent in
concentrations of
less than 1%, they effectively bind up a portion of the sulphur which does not
completely hydrolyze back to H2S in the catalyst beds of the sulphur plant,
thus
reducing the overall conversion of H2S to sulphur.
It is apparent that there is a clear need for a process that will increase
the concentration of H2S in the feed gas entering a sulphur plant. Preferably
the
process should improve the conversion of H2S to sulphur, and should also solve
many of the operational problems associated with feed gases that are too lean
in
H2S.
b) Relevant Technology
Advances toward improvement of H2S/CO2 ratios in sulphur plant feed
have generally been based on the selectivity of methyldiethanol amine (MDEA)
for
H2S over CO2 when in contact with sour gas. Tertiary amines such as MDEA and
also
di-isopropyl amine (DIPA) exhibit this preferential affinity for H2S. Other
amines such
as MEA and DEA tend not to exhibit significant preferential affinity, and will
therefore
strongly absorb both H2S and CO2.
4
CA 02461952 2010-08-31
In studying the relative affinities between tertiary amines and the acid
gases hydrogen sulphide and carbon dioxide, two characteristics must be
considered.
One is reaction equilibrium, which is defined as the final concentrations of
reactants
and reaction products after sufficient time has elapsed to attain steady
levels.
Equilibrium in thermodynamic terms occurs when the total free energy of the
mixture
reaches a minimum. The second characteristic to consider 'is reaction
kinetics, which
refers to the rate at which a reaction occurs. While consideration of reaction
equilibrium is important, in the practical application of industrial
chemistry,
consideration of reaction kinetics is equally important since reaction time
will greatly
influence the final distribution of components in a reaction mixture. Such is
the case
with the tertiary amines, and also with DIPA. While the reaction with H2S is
rapid, the
reaction with CO2 is slow. Therefore, although consideration of reaction
equilibrium
alone would suggest that both H2S and CO2 could react almost to completion,
when
the reaction kinetics are considered, only the H2S reaction approaches
completion,
while the CO2 reaction goes only part way. Selective absorption of H2S can
therefore
be improved by limiting contact time. The mechanical design of contacting
equipment,
the operating conditions, and the presence of special chemical promoters can
all
have a bearing on selectivity of tertiary amines for H2S over CO2.
The popular amines MEA, DEA, MDEA, DGA, and DIPA all have in
common a trivalent nitrogen atom to which are attached alcohol radicals
(either
ethanol or propanol). For example, the primary amine, monoethanol amine, has
one
ethanol group and two free hydrogen atoms. The secondary amine, diethanol
amine,
has two ethanol groups (as the name suggests) and one hydrogen atom. DGA has a
CA 02461952 2010-08-31
single ether-ethanol chain with two hydrogens. MEA, DEA, and DGA all react
rapidly
with carbon dioxide, combining with the available proton of the amine molecule
to
form a carbamate radical as follows:
CO2 + AMIN9 -+ [AMINE]' + [AMINE - O]
DIPA, which has two propanol structures and a single hydrogen atom, is not
fully
substituted, and is therefore not a tertiary amine. DIPA does not exhibit the
rapid
reaction with CO2 that is characteristic of the primary and secondary amines,
each of
which have an available proton. Apparently, the proton is not available for
reaction
with C02, so the carbamate reaction does not occur readily with DIPA. Other
amines,
the so called hindered amines use the physical structure of the amine molecule
to
block accessibility of CO2 to the reactive hydrogen. Methyl diethanol amine
(MDEA)
is a tertiary amine which has no proton attached to the nitrogen atom. As the
name
suggests, the three valences of nitrogen are occupied by two ethanol groups
and one
methyl group, so the carbamate reaction, which requires a labile proton,
cannot
occur.
The reaction between a molecule of MDEA and a molecule of CO2 is
somewhat more complex. When a CO2 molecule is dissolved in an aqueous
solution,
due to its acid nature it hydrolyzes to form carbonic acid (H2CO3). In a
process which
occurs slowly, the carbonic acid then dissociates to form positive hydrogen
ions and
negative bicarbonate ions. The bicarbonate may, to some extent, dissociate
further to
form additional positive hydrogen ions and negative carbonate ions. The MDEA
molecule, being mildly basic in character, will bond loosely with the
available
6
CA 02461952 2010-08-31
hydrogen ions to form a positively charged amine-hydrogen ion that coexists in
solution with negatively charged bicarbonate and carbonate ions as follows:
CO., + H.O -* H3 C03.
H2CO3 -+ H` +HCO;-
HCO, -3 H'' - CO3-
H* +AM NE'-.o (AMIN'E.-HJ"
Since the carbonic acid dissociation step is relatively slow kinetically, the
overall
sequence of steps must also proceed slowly. The overall kinetic acid-base
reaction
between tertiary amines and carbon dioxide must therefore occur quite slowly.
In
contrast, the acid-base reaction of hydrogen sulphide occurs rapidly. In
typical
contacting devices, the H2S reaction rate is at least ten times faster than
the CO2
reaction. These differential rates of reaction help to explain the selectivity
of tertiary
amines for H2S over CO2.
As the reaction between the amine and acid gas proceeds, more of the
available amine molecules become bound to acid gas molecules, leaving fewer
unreacted amine molecules available to react with the acid gas. This lack of
available
reactive amine molecules in the presence of acid gas slows the rate of
reaction.
Solution loading is therefore another factor influencing the selectivity of
tertiary
amines for H2S.
Reaction kinetics, however, is only one factor to consider in analyzing
the absorption of acid gases by amine solutions. As in physical absorption,
acid gas
molecules must migrate to the gas liquid interface under the action of the
concentration gradient that exists in the gas film adjacent to the interface.
The
7
CA 02461952 2010-08-31
molecule must then penetrate the interface and migrate inward until an
unreacted
amine molecule is encountered. As the mass transfer of acid gas molecules from
the
bulk gas phase into the liquid phase occurs by diffusion, the process of
transfer
requires a finite amount of time. Diffusion in this case occurs in two
sequential steps.
First, diffusion through the gas phase occurs near the interfacial boundary at
the gas
diffusion rate and, second, diffusion through the liquid phase occurs near the
liquid
boundary of the interface at the liquid diffusion rate. As a rate determining
factor for
tertiary amines, mass transfer by diffusion must be considered in addition to
chemical
rates of reaction. It has also been observed that selectivity for H2S
increases as
contact pressure decreases.
As previously mentioned, H2S reacts almost instantly with amine, so
mass transfer by diffusion through the gas phase is the rate-limiting step for
hydrogen
sulphide. For carbon dioxide, the dissociation to form hydrogen and
bicarbonate ions
proceeds so slowly that the concentration gradient in the liquid phase that
drives the
mass transfer is impeded. This impedance constitutes an additional resistance
to
absorption of CO2.
For many reactions the temperature at which the reaction takes place
has a profound effect on the rate at which the reaction occurs. This is
particularly
true of the reaction between CO2 and tertiary amines during absorption where a
reduction in temperature of even a few degrees can significantly slow the rate
at
which the CO2 and amine react. The H2S reaction however is not as dramatically
effected by a reduction in reaction temperatures, and this characteristic can
be used
to increase the differential rates of reaction between the two acid gases and
thus
8
CA 02461952 2010-08-31
increase the amines selectivity for H2S. By slowing the rate of the C02
reaction by
reducing the temperature in the absorber the "slipping" of CO2 can be
increased
without significantly affecting the absorption of H2S.
Obviously, one way to reduce the operating temperature of the absorber
is to reduce the temperature of the streams feeding into the absorber. The
reaction
of acid gas with amine is exothermic however, and in some cases considerable
amounts of heat can be generated by the reaction within the absorber itself.
So even
though the feed streams to the absorber are relatively cool, the heat of
reaction will
create a bulge in the temperature profile somewhere in the midsection of the
tower.
One way to mitigate this bulge is to use a more dilute amine solution so that
the
greater mass of the solution will attenuate the temperature rise. A more
direct way to
reduce the temperature bulge is to use inter-stage cooling on a stage near the
peak
of the temperature bulge. This will reduce the contact temperature throughout
the
tower which will in turn slow the C02 reaction and will improve the amine's
selectivity
for H2S.
The extent of reaction between acid gas and amine is the product of the
rate of reaction and the contact time of the reactants. Slowing the rate of
reaction will
improve the amines affinity for H2S over CO2. Reducing the contact time
between the
reactants will have a similar effect. The object should be to choose a type of
contact
device that can absorb H2S to meet the required specification in the briefest
possible
time so as to prevent the C02 reaction from proceeding any further than
necessary.
The choice of an efficient multistage contacting device, whether it be
conventional
trays, random packing, structured packing or equivalent, can also have a
significant
9
CA 02461952 2010-08-31
effect on the selectivity of the amine for H2S.
Practical applications for the selectivity of tertiary amines for H2S over
CO2 have, for the most part, been limited to absorption of acid gases from
natural gas
in a primary absorber. Circulation rate and residence time in the absorber
permit a
portion of the CO2 to remain unabsorbed while H2S is totally removed from the
gas.
Commercial specifications for natural gas require near to total removal of
H2S, but in
most cases up to 2% carbon dioxide in the purified gas is acceptable. The
tertiary
amine, methyldiethanol amine (MDEA), is usually the preferred absorbent. The
practice of partially removing CO2 from the natural gas is referred to as
"slipping" the
CO2.
In the technical record, references to MDEA's preferential affinity for
H2S over CO2 appear as early as 1950, when Frazier and Kohl first noted the
phenomena (see Is Frazier, H. D. and A. L. Kohl, "Selective Absorption of
Hydrogen
Sulfide from Gas Streams", Ind. Eng. Chem., 42, 2258-2292 (1950)). Since then,
the
technical literature has traced the development of design methods for the use
of
MDEA. By the 1980's MDEA had gained widespread use in the gas industry, but
applications were generally restricted to the relatively simple operation of
slipping a
portion of the CO2 in the high pressure absorber while totally absorbing the
H2S. The
formidable challenges of quantitatively predicting the combined chemical
reaction and
mass transfer relationships were not met until recent years, and although
present
methods are adequate, there is still significant room for improvement.
Present methods involve computational procedures to establish both
chemical and mass transfer equilibrium relationships between the amine and the
acid
CA 02461952 2010-08-31
gases. The concentrations of the various chemical species seek to arrive at
final
equilibrium concentrations at which point no further change will occur. It is
the
difference between actual concentrations and equilibrium concentrations that
provides the driving force for change to occur. Because there are various
resistances
to these changes, change does not to occur instantaneously; it occurs at a
definite
rate determined by the nature of the components, and by process conditions.
Rate of
change is proportional to driving force and inversely proportional to
resistance, so if
driving force and resistance can be calculated, the rate of change can also be
calculated. If infinite time were available, equilibrium concentrations would
eventually
be attained. In reality, however, time constraints dictate that only a partial
approach to
equilibrium is attainable. This procedure forms the basis for the design of
processing
equipment to preferentially absorb H2S from gases containing a mixture of both
H2S
and CO2.
Since H2S proceeds toward equilibrium rapidly, it approaches
equilibrium more closely than CO2, which proceeds slowly. In real absorbers,
equilibrium can be approached, but is never attained. In a multistage
contacting
device such as a trayed tower, if each actual stage had sufficient time to
reach
equilibrium, the stages would be said to be 100% efficient. This hypothetical
scenario
provides a measure of the change that takes place on each actual stage if the
actual
change is expressed as a percentage of the change that would take place if
equilibrium were attained. The actual change taking place on the stage could
then be
calculated from the known 100% efficiency of the stage if equilibrium is
attained. For
example, in a typical trayed MDEA absorber, the tray efficiency for H2S is
11
CA 02461952 2010-08-31
approximately 50%, whereas the tray efficiency for CO2 is typically about one-
tenth as
much, or 5%. If this preferential effect is factored into multiple stages of
contact, the
separation of H2S from CO2 can be significant. In practical situations,
however, it
must be recognized that the final concentration of H2S in the treated gas must
be very
low, while the concentration of C02 is many times higher. The driving force
for
absorption of H2S is low, while the driving force to absorb CO2 is relatively
high in the
top trays of the absorber tower. This means that, in the process of absorbing
essentially all of the H2S, significant quantities of CO2 will inevitably also
be absorbed,
and that the rich MDEA exiting from the bottom of the absorber column will
contain a
large amount of CO2 along with the absorbed H2S.
Over the years various schemes have been proposed to improve the
selectivity of tertiary amines for H2S over CO2, but unless the true
complexity of the
absorption process is recognized, the success of these schemes will be
compromised. For example, many schemes attribute to the tertiary amines a
strong
similarity to physical absorption, in which acid gases are absorbed or
desorbed in
response to changes in pressure or temperature. Physical absorbents generally
follow the principle of Henry's Law, which states that the concentration of a
distributed
component in the liquid phase is proportional to the partial pressure of the
component
in the gas phase. Due to chemical reactions that inevitably occur in the amine
solution, amines do not behave in this manner. When the chemical bond between
the
amine and the acid gas is formed, it is not easily broken. Attempts to desorb
the acid
gases by pressure reduction, gentle heating, or gas stripping will therefore
have only
limited success. The only way to release significant amounts of acid gas from
the
12
CA 02461952 2010-08-31
amine solution is to break the chemical bond by vigorous steaming of the
solution in
the amine regenerator. Some proposed process schemes are based on mild partial
regeneration to create a semi-lean amine solution, which because it is
supposedly
already loaded with C02i will resist further absorption of C02, and absorb H2S
instead. Such schemes have not gained wide acceptance.
SUMMARY OF THE PRESENT INVENTION
According to a first aspect of the invention there is provided a method of
selective absorption of hydrogen sulfide relative to carbon dioxide from a
supply gas
stream comprising:
providing a supply gas stream containing at least a product gas,
hydrogen sulfide and carbon dioxide;
providing an absorbent which absorbs both hydrogen sulfide and carbon
dioxide while exhibiting preferential affinity for hydrogen sulfide relative
to carbon
dioxide;
generating a stream of the absorbent which moves from a lean
condition in countercurrent flow over a series of contact stages to a stream
of the
supply gas so as to contact the absorbent with the supply gas stream so as to
absorb
at least partly the hydrogen sulfide and the carbon dioxide to form a rich
absorbent
while generating a stream of sweetened product gas which contains levels of
hydrogen sulfide and carbon dioxide below a predetermined maximum allowable
value;
passing the rich absorbent through a regeneration process which strips
substantially all of the hydrogen sulfide and carbon dioxide from the rich
absorbent
13
CA 02461952 2010-08-31
returning the absorbent to the lean condition for said stream while generating
a
stream of the hydrogen sulfide and carbon dioxide;
wherein the absorption of the hydrogen sulfide and carbon dioxide by
the absorbent is carried out in two steps in which:
in a first operation the absorbent in lean condition is contacted with the
supply gas stream;
and in a second operation the selectivity for hydrogen sulfide relative to
carbon dioxide is enhanced by contacting the rich absorbent leaving the first
operation with a second gas which has a higher ratio of hydrogen sulfide
relative to
carbon dioxide than the supply gas stream so as to cause the already rich
absorbent
to become even more heavily loaded with hydrogen sulfide and carbon dioxide,
but
because of the high ratio of the second gas, the increased loading is
preferentially in
favor of hydrogen sulfide.
Preferably the source of the second gas is the stream of the hydrogen
sulfide and carbon dioxide from the regenerator, a portion of which is
recycled back to
the second operation, where the stream of the hydrogen sulfide and carbon
dioxide
contains approximately the same ratio as existed in the rich absorbent after
the
second operation. .
Preferably the contact with the second gas in the second operation
occurs counter currently over a series of contact stages.
Preferably the first and second operations take place in the same
countercurrent absorption column which operates throughout at substantially
the
same pressure.
14
CA 02461952 2010-08-31
Preferably lean amine entering at the top of the upper section of the
column comes in contact with sour gas containing both H2S and CO2 which enters
the
column at an intermediate stage in the mid section of the absorber at the
point where
the first operation interfaces with the second operation., wherein the first
operation
occurs in the upper section of the column and the second operation occurs in
the
lower section such that the combined actions of the first and second
absorption
operations will attain an internal balance in which the rich amine leaving the
base of
the column will be enriched in H2S, while the CO2 thus excluded from the rich
amine
solution will exit from the top of the column along with the sweetened product
gas
from which the H2S has been removed.
Preferably there is provided a single feed of the lean absorbent at a top
of the first operation.
Preferably there are provided a plurality of feeds of the lean absorbent
at different positions through the first and second operations.
Preferably the first and second operations take place in at least two
different absorber towers operating at different pressures.
Preferably the first operation takes place substantially wholly in the first
tower which operates at a higher pressure than the second operation in the
second
tower.
Preferably the second absorber tower is arranged to control the amount
of carbon dioxide in the sweetened product gas from the first tower by taking
greater
advantage of the natural preference of the absorbent for hydrogen sulfide to
effect
"slipping" in which a portion of the carbon dioxide is permitted to exit with
the
CA 02461952 2010-08-31
sweetened product gas.
Preferably the method includes contacting the gas in the upper section
of the second tower with a stream of the absorbent in lean condition that is
fed at a
top stage of the second tower such that the lean absorbent, having a very low
residual hydrogen sulfide content, is capable of removing essentially all of
the
hydrogen sulfide from the acid gas stream, producing an overhead carbon
dioxide
vapour that is almost entirely free of hydrogen sulfide.
Preferably the method includes interposing a flash drum in the feed
stream of rich upstream absorbent where the reduced pressure of the flash drum
allows light dissolved vapors to evolve and be removed from the absorbent.
Preferably the first and second operations in the first and second towers
are arranged such that the partially loaded absorbent from the upper stages of
the
second absorber combine with the rich absorbent from the first tower and flow
downward to the lower stages of the second tower and wherein the overall
operation
of the first and of the second towers reaches an internal balance in which the
rich
absorbent leaving the bottom of the second tower is enriched in hydrogen
sulfide,
while the carbon dioxide thus excluded from the absorbent exits from the top
of the
second tower as a water saturated carbon dioxide stream essentially free of
hydrogen
sulfide.
Preferably providing a plurality of lean absorbent feed points on both the
first and second towers in order to optimize selectivity of the amine for
hydrogen
sulfide under varying operating conditions.
Preferably the source of the second gas stream is a portion of the
16
CA 02461952 2010-08-31
hydrogen sulfide enriched acid gas overhead from the absorbent regenerator,
which
is recycled back to the second tower.
Preferably there is provided a first and a second tower wherein the acid
gas product from said regenerator and a portion of the rich amine from the
first
absorber tower are sent to a third absorber tower and rich amine from the base
of the
third absorber tower is sent to a second regenerator which produces an
overhead
acid gas in which the H2S is more concentrated than the acid gas from the
first
regenerator and wherein a portion of the enriched acid gas from the second
regenerator is recycled to the base of the third absorber tower where it is
contacted
with a side stream of rich amine from the first absorber tower, which enters
at a mid
section of the third absorber tower, and by lean amine that enters at a top of
the third
absorber tower, producing an overhead stream of essentially pure C02 and water
vapor from the third absorber tower.
Preferably a first effect followed by a second effect which by using
multiple stages of absorption and regeneration is able to produce an acid gas
that is
progressively richer in H2S wherein the staging process is continued to a
third effect
or more by adding additional stages of absorption and regeneration and wherein
each additional stage of absorption receives acid gas and rich amine from
preceding
effects and produces a stream of CO2 and rich amine which when regenerated
produces an enriched acid gas, a portion of which is recycled back to its
absorber.
Preferably the lean amine for the first absorber tower is drawn from a
stage in the regeneration process above a reboiler while the lean amine for
the
second absorber tower is drawn from the bottom of the regenerator proces in
order to
17
CA 02461952 2010-08-31
meet the differing tolerances of the first and second absorber towers for
residual acid
gas in the lean amine.
Preferably it is desired to maximize recovery of CO2 as a by-product by
minimizing slipping CO2 in a first absorber tower by adjusting operating
conditions,
solution concentration, circulation rate, type of contacting device and stages
of
contact so as to increase CO2 absorption in the first absorber tower thus
enabling
increased CO2 production in subsequent absorber towers.
Preferably side coolers are used on absorbers to cool the process and
to reduce the rate of the CO2 reaction with amine.
According to a second aspect of the invention there is provided a
method of selective absorption of hydrogen sulfide relative to carbon dioxide
from a
supply gas stream comprising:
providing a supply gas stream containing at least a product gas,
hydrogen sulfide and carbon dioxide;
providing an absorbent which absorbs both hydrogen sulfide and carbon
dioxide while exhibiting preferential affinity for hydrogen sulfide relative
to carbon
dioxide;
generating a stream of the absorbent which moves from a lean
condition in countercurrent flow over a series of contact stages to a stream
of the
supply gas so as to contact the absorbent with the supply gas stream so as to
absorb
at least partly the hydrogen sulfide and the carbon dioxide to form a rich
absorbent
while generating a stream of sweetened product gas which contains levels of
hydrogen sulfide and carbon dioxide below a predetermined maximum allowable
is
CA 02461952 2010-08-31
value;
passing the rich absorbent through a regeneration process which strips
substantially all of the hydrogen sulfide and carbon dioxide from the rich
absorbent
returning the absorbent to the lean condition for said stream while generating
a
stream of the hydrogen sulfide and carbon dioxide;
wherein the absorption of the hydrogen sulfide and carbon dioxide by
the absorbent is carried out in two steps in which:
in a first operation the absorbent in lean condition is contacted
with the supply gas stream;
and in a second operation the selectivity for hydrogen sulfide
relative to carbon dioxide is enhanced by contacting the rich absorbent
leaving the
first operation with a second gas which has a higher ratio of hydrogen sulfide
relative
to carbon dioxide than the supply gas stream so as to cause the already rich
absorbent to become even more heavily loaded with hydrogen sulfide and carbon
dioxide, but because of the high ratio of the second gas, the increased
loading is
preferentially in favor of hydrogen sulfide;
wherein the first and second operations take place in first and second
different absorber towers where the second absorber tower operates at a
pressure
lower than the first and lower than the regeneration process and wherein there
is
provided a liquid pump for pressurizing the absorbent from the second absorber
tower
for supply to the regeneration process.
The process described herein recognizes that co-absorption of CO2 and
H2S by tertiary amines is essentially unidirectional and that, short of
vigorous
19
CA 02461952 2010-08-31
regeneration of the rich solution by steaming, desorption of acid gas from
rich solution
is not significant. Absorption responds to partial pressures, solution
loading, and
temperature. However, because the chemical bond formed during absorption
cannot
be easily broken, in practical situations desorption will not respond to these
measures.
The present process is most applicable to situations where the C02/H2S
ratio in the natural gas (indicated by reference numeral 10 in figs. 4 through
8) that
feeds into the plant is relatively high. In this scenario, the rich amine
solution exiting
the high pressure absorber would also have a relatively high ratio of CO2 to
H2S, even
if CO2 slipping was maximized. In addition, because regeneration strips
essentially all
of the acid gas from the solution, the regenerator overhead vapour in a
conventional
MDEA plant would also have a high CO2 to H2S ratio. This process described
herein
proposes to improve this ratio by recycling an acid gas slip stream, which is
rich in
H2S, to contact the rich amine prior to regeneration where, because of the
higher
partial pressure of H2S in the recycled acid gas, further absorption of H2S
into the rich
solution can occur. The source of the H2S enriched acid gas is the overhead
vapour
from the regenerator. If a sufficient portion of this overhead vapour is
recycled, the
rich amine solution will be enriched in H2S and, since the regeneration
process strips
essentially all acid gas from the rich solution, the regenerator overhead
vapour will
also be H2S-enriched. A portion of this enriched overhead vapour is recycled
back to
enrich the amine solution, and the entire system will come to a new dynamic
equilibrium based on these new conditions, resulting in regenerator overhead
vapours
having a significantly higher proportion of H2S over CO2.
CA 02461952 2010-08-31
In summary, the following process concepts form the basis of the
process described herein.
(1) Tertiary amines exhibit a preferential affinity for H2S over C02
primarily because of differing rates of absorption. Therefore, when H2S and
C02 are
coabsorbed from gases, the relative proportion of H2S to CO2 in the amine will
be
higher than the corresponding proportion in the gas phase. This is because in
the
actual processing equipment H2S is absorbed more rapidly than C02.
(2) Absorption of acid gas by amine involves physical absorption
plus chemical reaction. Absorption occurs readily, but desorption to separate
the acid
gas from the amine is much more difficult because the reaction that bonds the
acid
gas chemically to the amine is not easily reversed except by intense steaming
at
elevated temperature. Mass transfer of acid gas is therefore essentially
unidirectional
throughout most of the process except for the regeneration where the chemical
bond
that links acid gas to amine is broken by steaming the rich solution. After
regeneration
the amine is totally stripped of all acid gas except for very minor residual
amounts.
(3) Rich tertiary amine in contact with sour gas will be loaded with
both H2S and C02 in proportions dictated by the ratio of H2S to C02 in the gas
phase,
by the contact time and by the conditions of contact. While the rich solution
does not
readily give up its acid gas short of vigorous regeneration, it is possible to
more fully
load the rich solution with H2S when the solution is in contact with a gas
which is
enriched with H2S at the proper operating conditions.
(4) If the tertiary amine is initially contacted with gas that is relatively
lean in H2S but rich in C02, the 1-12S will be totally absorbed, but a portion
of the C02
21
CA 02461952 2010-08-31
will remain unabsorbed and will not be removed from the gas. This is referred
to as
"slipping" a portion of the CO2. If the rich amine from the first contact is
then
contacted with the second gas that is richer in H2S than the first gas, then
the rich
amine is capable of absorbing additional H2S from the second gas, provided
that
concentrations and operating conditions are favourable.
However, the rich amine which contacts the second gas is not capable
of totally removing the H2S from the second gas because it is already
partially loaded
with H2S. Equilibrium conditions between the rich amine and the second gas
will
permit only partial absorption of the H2S, but will not permit total removal.
Thus, while
slipping CO2 from the second gas, a portion of the H2S will also be
unavoidably
slipped while in contact with the rich amine. In order to pick up the slipped
H2S from
the second gas, the second gas must be contacted with lean amine which is
sufficient
to absorb the H2S but will continue to allow the CO2 to slip. The second gas,
after
being contacted by both rich and lean amine streams, will consist of
substantially pure
CO2 and water vapour with only a trace of H2S remaining.
(5) Based on the principles described in (4) above, it is possible to
extend the enrichment method by devising a multistage enrichment system
wherein
the acid gas is progressively enriched in stages by contacting rich amine with
recycled acid gases that are progressively richer in H2S in a series of
absorbers and
regenerators.
(6) It is possible to realize some reduction in process heat required
for regeneration of the rich amine solution by tailoring the acid gas
residuals
contained in the lean solution to suit the requirements of the individual
absorbers.
22
CA 02461952 2010-08-31
Absorbers with an extreme intolerance for acid gas residuals would be drawn
from
the bottom of the regeneration column where it would be exposed to the most
intense
degree of steaming. Absorbers with a greater tolerance for acid gas residuals
could
draw their lean amine from an intermediate stage in the column where the
degree of
regeneration heat is less. Overall, the two lean streams require less process
heat
than producing a single lean stream with very low residuals.
(7) Recognizing that it is primarily the differential rates of reaction
between the acid gases H2S and CO2 with tertiary amine that creates the
preferential
affinity of amines for H2S, methods to control the rates of reaction and the
extent of
reaction will improve selectivity for H2S. Reducing the temperature of
absorption and
reducing the contact time of the acid gas with the amine will both enhance
selectivity
for H2S.
The above described principles recognize the physical and chemical
nature that is inherent in tertiary amines. By employing these principles in
combination it is possible to devise a process that will greatly enrich the
H2S
concentration of the acid gas feed to a sulphur plant. It should also produce
a
secondary benefit of producing a side stream of essentially pure CO2 which may
also
have commercial value.
BRIEF DESCRIPTION OF THE DRAWING FIGURES
Embodiments of the invention will now be described, by way of example
only, with reference to the accompanying drawings, wherein:
Figure 9 shows a typical prior art amine process employing a primary
absorber and regenerator;
23
CA 02461952 2010-08-31
Figure 2 shows a simple acid gas recycle process according to one
embodiment of the present invention;
Figure 3 shows a "single effect" acid gas enrichment process according
to another embodiment of the present invention in which the second absorber
operates at a pressure intermediate between the first absorber and the
regenerator;
and,
= Figure 4 shows a "single effect" acid gas enrichment process according
to another embodiment of the present invention in which the second absorber
operates at a pressure lower than the pressure of the regenerator; and,
Figure 5 shows a "single effect" process with a lean/superlean system
according to a further embodiment of the present invention; and,
Figure 6 shows a "double effect" acid gas enrichment process according
to yet another embodiment of the present invention.
DESCRIPTION OF THE PREFERRED EMBODIMENT
In one embodiment of the present invention shown in figure 2 the
process recycles concentrated acid gas vapours back to the base of the high
pressure amine absorber below the entry point of the sour feed gas for the
purpose of
increasing the concentration of H2S in the amine solution exiting the base of
the
column. The introduction of additional H2S into the absorber column, however,
means
that conditions in the upper section of the column must be altered in order to
maintain
H2S specifications of the product gas while slipping additional CO2. The
process
scheme of Figure 2 requires a high pressure compressor to recycle the acid
gas.
In a second more practical embodiment of the present invention shown
24
CA 02461952 2010-08-31
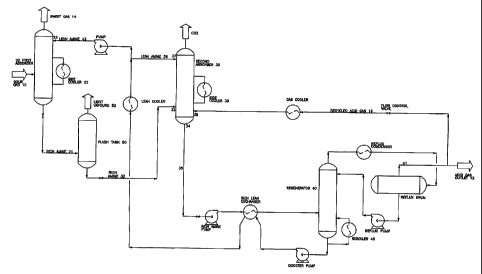
in Figure 3, the process, sometimes referred to herein as a "single effect
process",
uses a second absorber column (indicated by reference numeral 30) which
operates
at a pressure that is intermediate to that of the main high pressure absorber
tower 20,
also referred to herein as the "first absorber", and the pressure of the amine
regenerator 40. Acid gas 16 that is enriched in H2S is fed into the base of
the second
absorber column at 38 where it comes in contact is with rich amine 32 from the
high
pressure absorber 20 (which enters the second column 30 at 32) in counter-
current
flow over a series of contact stages in the second absorber 30. While the
amine
solution picks up additional H2S from the recycled acid gas the CO2 loading
does not
change dramatically, resulting in a rich stream 35 exiting from the base of
the second
column at 34 that has been enriched in H2S. Vapours rising above the feed
tray,
where rich amine from the high pressure absorber enters the second absorber at
33,
will be in contact with a solution that is already significantly loaded with
acid gas. As a
consequence, these vapours will contain both H2S and CO2. In order to achieve
a
useful separation in the second column 30, it is necessary to eliminate
essentially all
of the H2S from the overhead vapour so that it can either be disposed of, or
marketed
as an essentially pure C02 stream 31. This can be accomplished by introducing
a
lean stream of tertiary amine at 36 directly from the amine regenerator 40
onto the
top tray of the second absorber. Because of its low residual concentration of
acid gas,
the lean stream will reduce the H28 in the overhead gas essentially to zero in
the
second absorber, leaving an overhead of essentially pure CO2 and water vapour,
plus
remaining non-condensable hydrocarbons which were dissolved in the rich amine
solution from the high pressure absorber 20.
CA 02461952 2010-08-31
The second column 30 has three feeds. Lean amine enters at the top of
the column at 37, while rich amine from the high pressure absorber 20 enters
at the
midsection of the column at 32, while recycled acid gas enters at the column's
base
at 38. The overhead vapour stream 31 - one of two streams leaving the second
absorber consists mostly of CO2 and water. The bottom liquid stream at 34
consists
of rich amine enriched with H2S. The lean amine stream 36 entering at the top
of the
column contacts the rising acid gas vapours counter-currently through several
stages
of contact where it preferentially absorbs H2S, and allows a substantial
portion of the
CO2 to slip past unabsorbed until it exits at the top stream 31 of the second
column.
At the rich amine feed stage at the mid-section 32 of the absorber, the lean
stream
36, now partially loaded with acid gas, after entering the column flows from
the upper
section of the column and blends with the incoming rich amine feed. As the
combined
amine stream flows downward through the lower section of the second absorber,
it
preferentially absorbs H2S from the acid gases before exiting from the base 34
of the
column.
In another aspect of the present process a flash tank 50 may be added
to the system by locating it between the high pressure absorber 20 and the
second
absorber 30. The tank's purpose is to flash off non-condensable vapours,
namely
principally methane and ethane, which are picked up in small quantities in the
high
pressure absorber where the amine acts as a physical solvent for hydrocarbons.
These hydrocarbons are largely flashed off in the flash tank, along with minor
amounts of H2S and CO2. This flash vapour, exiting at 52, can also be
sweetened and
used as plant fuel. The purpose of the flash tank is to remove non-condensable
26
CA 02461952 2010-08-31
vapours that would otherwise appear in the overhead vapour 31 from the second
absorber 30, contaminating the CO2.
Another aspect of the process may employ side coolers 22 and 39 in
certain cases where heat build-up in the absorbers detrimentally affects the
selective
absorption of H2S over C02-
The process described herein is based on recycling a portion of the
overhead acid gas stream from the regenerator 40 for the purpose of improving
the
ratio of H2S to CO2 in the acid gas stream 12 going to the sulphur plant.
Carbon
dioxide, which is an undesirable contaminant in the sulphur plant, is excluded
at two
points in the process. First, the CO2 is only partially absorbed in the high
pressure
absorber 20, allowing a portion of the CO2 to slip and remain in the residue
gas,
namely the "sweet gas" 14. Second, CO2 is slipped by the amine in the second
absorber 30, where it is removed overhead at 31 as essentially pure CO2
saturated
with water, When the overall plant material balance for CO2 is calculated, the
concentration of H2S in the overhead stream 41 from the regenerator 40 should
be
greatly increased, significantly improving its quality as a sulphur plant feed
and
improving the conversion of H2S to sulphur in the sulphur plant.
Lean tertiary amine that leaves the regenerator 40 is split into two
streams, namely a first stream 43 which flows to the top of the high pressure
absorber 20, and a second stream 36 which flows to the top of the second
absorber
30. The first stream 43 is sufficient to produce a sweet natural gas product,
and the
second stream 36 is used to sweeten recycled acid gas for the purpose of
improving
H2S concentration in the feed 12 to the sulphur plant. This internal recycle
system
27
CA 02461952 2010-08-31
consisting of recycled enriched acid gas 16 requires additional lean amine 36,
additional heat to regenerate the additional amine in reboiler 45, and
additional
pumping and acid gas compression at 46 to recycle the internal streams. With
this
approach, additional process costs will be incurred in improving the H2S/C02
ratio of
the acid gas 12 leaving the plant, but these costs are reasonable and
practical for
most systems. However, with very lean streams, the acid gas ratio in the rich
amine
21 from the high pressure absorber 20 will become increasingly unfavourable,
and a
greater and greater portion of the overhead regenerator vapour 41 must be
recycled
in order to gain a significant improvement in the concentration of H2S in the
acid gas
stream 12 leaving the plant. In this case, the recycle stream 16 and the lean
amine
stream 36 going to the second absorber 30 become the dominant elements in the
plant, resulting in progressively higher process costs for reabsorbing and
regenerating
recycled streams.
Figure 4 shows a variation of the second embodiment referred to as the
"single effect" process in which the second absorber tower (indicated by
numeral 30)
operates at a pressure that is lower than that of the regenerator. A pump is
required
to boost the rich amine stream 35 from the low pressure of the second absorber
to
the higher pressure of the regenerator, indicated by numeral 40. The recycled
acid
gas stream 16 flows to the second absorber and enters the tower at 38 and a
flow
control valve serves to reduce the pressure of the recycled acid gas from
regenerator
pressure to the lower pressure of the second absorber. With the exception of
the
changes described above, the numbering system and the process description of
Figure 3 also applies to Figure 4.
28
CA 02461952 2010-08-31
Lean/Super Lean Amine Systems
It has been stated that in order to remove virtually all of the H2S from a
sour gas stream 10 while allowing a portion of the CO2 to slip through an
absorber,
the lean amine solution must be stripped in a regenerator to a very low
residual H2S
content. An H2S content of 0.0015 mole percent is a typical H2S residual for
lean 50%
(weight) MDEA. If residual H2S rises much above this level, the H2S content in
the
gas exifing the top of the absorber will exceed acceptable limits. It has been
found
that the high pressure absorber 20 is much more tolerant of residual H2S than
the low
pressure secondary absorber 30, even though the specification for HZS in the
gas
from the high pressure absorber is much tighter than the specification for the
low
pressure absorber. The high pressure absorber can tolerate more residual HZS
because it has a much higher partial pressure driving force to cause HZS to
diffuse
through the gas film at the liquid interface and into the body of the amine
liquid. The
low pressure absorber must function with a much lower HZS partial pressure in
the
gas phase at the top of the column with the result that even modest amounts of
residual H2S in the lean amine inevitably create such resistance to diffusion
that final
traces of H2S will not be absorbed and significant amounts of H2S will break
through
with the gas exiting from the top of the second absorber.
In order to meet the strict requirements for low residual H2S in the lean
amine entering the second absorber 30 (which operates at a lower pressure than
the
first absorber 20), it is necessary to create a super lean amine by expending
extra
heat energy in the regenerator. The first absorber requires low residual H2S,
but
because of its higher operating pressure, can tolerate residuals which are
typically
29
CA 02461952 2010-08-31
about five to ten times higher than those required for the low pressure
absorber.
Moderate steam stripping in the regenerator 40 is adequate to produce lean
amine for
the high pressure absorber, but for the low pressure second absorber intense
steam
stripping is necessary to produce a super lean tertiary amine having the
required
extremely low residual H2S content. In a simple system, the single bottom
product
leaving the amine regenerator has been stripped of H2S to the level necessary
to
meet the needs of the low pressure absorber, even though the high pressure
absorber can tolerate a much higher level of H2S residual in the lean
solution.
The different requirements for lean amine purity for the two absorbers
suggest an alternate arrangement for regenerating the amine solution. Instead
of
drawing all of the lean amine from the base of the regenerator still column,
the lean
amine for the high pressure absorber can be drawn from an intermediate tray
approximately five stages above the reboiler 45 located at the base of the
column as
shown in figure 5. The portion of lean amine drawn from the intermediate tray
will
have residual H2S low enough to meet the needs of the high pressure absorber,
while
the balance of the amine remaining in the regenerator still column will
continue to
downflow over the trays in the lower section of the column where it is subject
to the
intense steaming necessary to regenerate a super lean solution suitable for
the low
pressure absorber. The two draw-off points in the still column serve to reduce
the
overall process heat necessary to regenerate the solution. Instead of
expending the
energy required to regenerate the total amine solution to the standard of
purity
required by the low pressure absorber, a lesser amount of energy is expended
to
regenerate a conventional lean amine for the high pressure absorber, plus a
super
CA 02461952 2010-08-31
lean stream for the low pressure absorber. This lean/super lean system is a
relatively
simple enhancement to the process that will improve overall energy efficiency.
The flow scheme for the lean/super lean system is illustrated in fig. 7.
in this third embodiment of the invention there are two amine streams exiting
from the
regenerator, namely a lean stream and a super lean stream. The lean stream 44
is
drawn from an intermediate stage in the regenerator 40 that is several stages
above
the reboiler 45 but is below the feed stream 35 which comes from the second
absorber 30. After leaving the regenerator, the lean stream 44 is cooled by
flow
through the rich/lean exchanger and the lean cooler after which it enters the
first
absorber as stream 43.
The super lean stream 42 exits from the bottom of the regenerator 40 in
a customary manner and is pumped through the rich/super lean exchanger and the
super lean cooler after which it enters the second absorber 30 as stream 36.
It is obvious that the process scheme described in Figure 4 in which the
second absorber operates at a pressure lower than the pressure of the
regenerator
could also apply to the lean-super lean process.
Also the use of side coolers on absorbers 22 and 39 may be beneficial
in some cases in improving the process and in influencing the purity required
for the
lean solutions.
Extremely Lean Acid Gas
For extremely lean streams where, for example, the molar ratio of H2S
to CO2 in the rich amine stream from the high pressure absorber is 1% or less,
yet
another, or fourth, embodiment of the invention shown in Figure 6 should be
31
CA 02461952 2010-08-31
considered. Low molar ratios exist where, for example, H2S in the natural gas
is
0.03%, while CO2 is 5%. In spite of slipping CO2 in the high pressure
absorber, there
will be a very strong predominance of CO2 in the rich amine with a typical
H2S/CO2
ratio of 1 % or less. A 1 % H2S/CO2 ratio as feed to a Claus sulphur plant
following a
conventional amine plant would be literally impossible to operate. Using the
second
embodiment of the invention (i.e. the single effect system) as described
above, the
H2S/CO2 ratio in the acid gas could be increased by a factor of about 5, or
from 1 % to
5%.
In applying the second embodiment of the invention to a system that is
very low in H2S, the acid gas recycled to the second absorber is still a
comparatively
lean gas, even though the H2S has been concentrated by, for example, a factor
of
five. As the proportion of acid gas recycled is increased, the gain in
concentration of
H2S appears to approach a limit beyond which the amount of process energy
expended becomes impractical. In this case, H2S/CO2 ratio can only be improved
by
employing the fourth embodiment of the invention, namely a double effect
system
shown in Figure 6.
Whereas the second embodiment of the invention may be referred to as
a "single effect system", the fourth embodiment of the system may be referred
to as a
"double effect system", which involves coupling together two stages of low
pressure
absorption and regeneration. Components of the system in Figure 6 which are
the
same or similar to those shown in Figure 3 are identified with the same
reference
numerals, except with the addition of a prefix "1". The double effect system
consists
of all the basic component parts of the single effect system, including the
high
32
CA 02461952 2010-08-31
pressure absorber 120, the optional flash tank 150, the second absorber 130,
the
regenerator 140, a compressor 146 to recycle acid gas, and a means of pumping
lean amine to the two absorbers. The double effect system adds to the basic
system
a third absorber tower 160, a second regenerator 170, an additional lean amine
pump
180, and an acid gas compressor 190.
The double effect system attaches directly to the acid gas outlet 112
from the single effect system. The acid gas from the first regenerator enters
near the
base 161 of the third absorber 160, along with H2S enriched acid gas 175
entering at
162 at the base of the column recycled from the overhead 171 of the second
regenerator 170. Lean amine from the second regenerator is divided into two
streams: one stream 173 flows to the top of the third absorber at 163; and a
second
stream 174, which combines with lean amine from the first regenerator 140, and
flows
to the top of the first absorber at 122 and the second absorber at 137.
In the double effect system, greater concentrations of H2S are achieved
by rejecting a stream of essentially pure CO2 and water overhead from the
third
absorber at 164. This C02, which is rejected from the process, may be combined
with
C02 from the second absorber 130. The second effect should improve upon the
first
effect's H2S/CO2 ratio by approximately a factor of three. The overall
Improvement in
the ratio is therefore the product of the improvement in the first and second
effects,
which in the example cited is the product of 5 and 3. (if acid gas from the
first effect
has the H2S/CO2 ratio improved by a factor of 5, the overall ratio improvement
leaving
the second effect will be 15.) Thus, a H2S/CO2 ratio of only 1% should be
improved to
15% in stream 172 by the use of a double effect system. A ratio of 15%, while
still a
33
CA 02461952 2010-08-31
relatively lean acid gas, is a practical concentration of H2S for feed to a
Claus sulphur
plant. Individual cases will obviously vary, with final concentrations
depending an
initial concentrations, and the degree of recycling employed in the process.
The arrangements described herein are based upon a theory of
selective absorption of hydrogen sulphide over carbon dioxide from gas based
on
countercurrent contact between gas and tertiary or other amines which exhibit
preferential affinity for H2S over C02 primarily because of differential rates
of
absorption of the two gases. The process of enhanced selective absorption is
accomplished by performing the absorption in two steps. The first operation is
to
contact lean amine with sour gas which contains both H2S and C02. The object
of
the first operation is to produce an overhead gas that meets an arbitrary
standard for
content of H2S and C02. The second operation is to enhance the selectivity for
H2S
by contacting the rich amine leaving the first operation with a second gas
which is a
highly concentrated acid gas having a higher H2S/CO2 ratio than the first acid
gas.
The contact with the second gas occurs counter currently over a series of
contact
stages which causes the already rich amine to become even more heavily loaded
with acid gas, but because of the high H2S/C02 ratio of the second gas, the
increased
loading will be preferentially in favor of H2S. The rich amine leaving the
second
operation will thus have a much higher proportion of H2S relative to C02 than
rich
amine from the first operation, and when regenerated will therefore produce a
regenerator overhead gas that is likewise enriched in H2S. The regeneration
process
strips essentially all of the acid gas from the rich amine, producing a
regenerated lean
solution having only minor amounts of residual H2S and C02. The regenerator
34
CA 02461952 2010-08-31
overhead vapour contains the acid gases that have been stripped from the rich
amine
and will therefore have approximately the same H2S/CO2 ratio as existed in the
rich
amine. The source of the highly concentrated acid gas that contacts the rich
amine in
the second operation is the overhead vapour from the regenerator, a portion of
which
is recycled back to the lower stages of the second operation.
The process uses absorption to concentrate the amine solution
preferentially with H2S. Acid gases are bound to the amine by chemical
reaction and
this bond is not easily broken short of regeneration. The process
preferentially loads
the rich amine with H2S by pairing up as many as possible of fast reacting H2S
molecules with amine molecules as quickly as possible, thus depriving the slow
CO2
molecules of active reaction sites and thus excluding them from the rich
solution.
A version of the selective absorption process is provided in which the
first and second operations take place in the same countercurrent absorption
column
which operates throughout at essentially the same pressure. In the first
operation as
described above, lean amine entering at the top of the upper section of the
column
comes in contact with sour gas containing both H2S and CO2 which enters the
column
at an intermediate stage in the mid section of the absorber at the point where
the first
operation interfaces with the second operation. The first operation occurs in
the
upper section of the column and the second operation occurs in the lower
section.
Rich amine from the first operation flows downward into the stages of the
second
operation where it comes in contact with a highly concentrated acid gas having
a
higher H2S/CO2 ratio than the first gas which causes it to be much more
heavily
loaded with acid gas. But because of the high H2S/CO2 ratio of the second gas,
the
CA 02461952 2010-08-31
rich solution becomes preferentially loaded with H2S, resulting in a much
higher
H2S/CO2 ratio in the rich amine from the second operation than in the rich
amine from
the first operation. The none-absorbed acid gas components from the second
operation flow upward into the stages of the first operation where they blend
with the
incoming first sour gas, contacting the down flowing amine and giving up their
acid
gas components as the gas flows upward to the top stage of the column. Gas
exiting
the top of the column will meet the required specifications for H2S content.
CO2 in the
overhead gas will be the none absorbed CO2 that was not picked up by the rich
amine exiting the bottom of the column. The combined actions of the first and
second absorption operations will attain an internal balance in which the rich
amine
leaving the base of the column will be enriched in H2S, while the CO2 thus
excluded
from the rich amine solution will exit from the top of the column along with
the
sweetened product gas from which the H2S has been removed. A single lean amine
feed at the top of the first operation will in most cases be adequate, but in
some
circumstances multiple lean amine feeds may be advantageous.
The source of the highly concentrated acid gas which constitutes the
bottom feed for the second operation is normally a portion of the regenerator
overhead vapour which is compressed and recycled back to the base of the
absorber
column.
A version of the selective absorption process is disclosed herein in
which the first and second operations take place in two different absorber
towers
operating at different pressures. The first operation, which typically
operates at a
higher pressure than the second operation, sweetens incoming sour feed gas to
meet
36
CA 02461952 2010-08-31
product specifications for H2S. CO2 is also removed and by appropriate design
methods it is possible, within limits, to control the amount of CO2 removed.
The
second absorber tower provides an additional degree of freedom in controlling
the
amount of CO2 in the sweet gas product from the first tower by taking greater
advantage of the amine's natural preference for H2S. The process of partially
removing the CO2 is referred to "slipping" in which a portion of the CO2 is
permitted to
exit with the sweetened product gas. Lean amine enters the first absorber,
picks up
H2S and a portion of the CO2 and exits from the base of the column as rich
amine.
The rich amine then flows to the second absorber, which typically
operates at a lower pressure than the first absorber, entering on an
intermediate
stage between the top and bottom of the column. A highly concentrated acid gas
stream with an enhanced H2S/CO2 ratio enters at the bottom stage of the second
absorber where it flows upward in contact with downflowing amine solution. A
portion
of the amine is the rich amine from the first operation, which, although
already
partially loaded with acid gas will become much more heavily loaded because of
the
concentrated acid gas entering at the base of the column. Because the acid gas
has
an elevated H2S/CO2 ratio, the rich amine leaving the base of the second
absorber
will be preferentially loaded with H2S.
Rich amine from the first operation is capable of bulk absorption of acid
gas, but because the amine is already partially loaded with acid gas, it is
not capable
of quantitatively absorbing the H2S from the gas. Vapours leaving the
intermediate
feed stage will be mostly CO2 but will also contain significant amounts of
H2S, which
must be removed. Since it is desirable to produce an overhead vapour from the
37
CA 02461952 2010-08-31
second absorber that is essentially free of H2S, it is necessary to contact
the gas in
the upper section of the second absorber with a lean stream of amine that
enters on
the top stage. The lean amine, having a very low residual H2S content is
capable of
removing essentially all of the H2S from the acid gas stream, producing an
overhead
CO2 vapour that is almost entirely free of H2S.
Light vapours such as methane and ethane, which may have been
dissolved in the rich amine from the first operation, will come out of
solution at the
reduced pressure of the second operation and may contaminate the overhead
stream
of CO2. If these light vapours are objectionable in the CO2 it is possible to
exclude
most of them by interposing a flash drum in the rich amine feed stream
upstream of
the second column where the reduced pressure of the flash drum will allow
tight
dissolved vapours to evolve and be removed from the rich amine. The flash
vapours
will also contain minor amounts of H2S and CO2.
At the rich amine feed stage of the second column the partially loaded
amine from the upper stages of the absorber combine with the rich amine from
the
first column and flow downward to the lower stages of the second tower. The
overall
operation of the first and of the second absorbers will reach an internal
balance in
which the rich amine leaving the base of the column will be enriched in H2S,
while the
CO2 thus excluded from the amine will exit from the top of the second absorber
as a
water saturated CO2 stream essentially free of H2S.
Multiple lean amine feed points may be necessary on both the first and
second absorbers in order to optimize selectivity of the amine for H2S under
varying
operating conditions.
38
CA 02461952 2010-08-31
The source of the concentrated acid gas feed for the second absorber is
a portion of the I-12S enriched acid gas overhead from the amine regenerator,
which is
compressed and recycled back to the base of the second absorber.
A version of the selective absorption process is disclosed herein in
which enhanced enrichment in H2S is made possible through multiple stages of
enrichment. The single effect system described above is inherently limited in
its
ability to improve the H2S/CO2 ratio in the acid gas above that attainable in
the
conventional amine process to an approximate factor of five. The conventional
system would have approximately the same H2S/CO2 ratio as the rich amine from
the
first operation. Attempts at improving the H2S/CO2 ratio beyond this level
using the
process of claim 3 will require a dramatic increase in the volume of acid gas
recycled
with a corresponding very large increase in energy consumption. So for cases,
which
have an extremely low H2S/CO2 ratio, a multiple effect acid gas enrichment
system
should be considered. For example, if a double effect system were used in
which the
H2S/CO2 ratio could be improved by a factor of 5 for each effect, the overall
improvement would be the product of the factors for each effect, which in this
case
would be a 25 fold improvement in the H2SICO2 ratio.
In a multiple effect system, the regenerator effluent acid gas from the
first effect does not go to a sulphur plant. Instead it goes to a third
absorber tower
where it is contacted by a stream of rich amine split off from the rich amine
feed to
the second absorber. The rich amine enters on an intermediate stage of the
added
absorber and a lean stream of amine enters at the top stage. A stream of
essentially
pure CO2 which Is saturated with water is discharged overhead from the third
column
39
CA 02461952 2010-08-31
and the rich amine from the base of the column flows to a second regenerator
which
produces an overhead acid gas which is considerably richer in H2S than the
acid gas
from the preceding regenerator. A portion of the concentrated acid gas from
the
second regenerator is then compressed and) recycled back to the base of the
third
absorber where it is processed for removal of H2S. The regenerated lean amine
from
the added regenerator is combined with other regenerated lean amine in the
system
and is circulated to the top stages of the absorber columns.
The double effect system retains all elements of the single effect system
and adds an additional absorber tower and an additional regenerator along with
all
the necessary additional exchangers, pumps, and piping.
The double effect system could be extended to a triple effect system or
a quadruple effect system by adding additional stages of absorption and
regeneration.
A version of the process is disclosed herein wherein the differing needs
for lean solution purity in terms of residual H2S and CO2 for each individual
absorber
are addressed by using two different lean amine streams from a single
regeneration
still column. Lean amine following regeneration will contain residual amounts
for both
H2S and C02, which can detract from the performance of the absorbers. Each
individual absorber has a different level of tolerance for residuals in the
lean solution.
With the process of claim 18 the residuals must be reduced to meet the most
severe
needs of either of the two absorbers. Reducing residual H2S and CO2 to very
low
levels requires a high expenditure of energy in the reboiler.
Typically the first absorber tower with its higher operating pressure is
CA 02461952 2010-08-31
more tolerant of residuals than is the lower pressure second tower, which
requires
very low residuals. By allowing the severest needs of one tower to dictate the
purity
of the lean amine for both towers, additional regeneration energy is expended
unnecessarily. A normal lean amine would typically serve the needs of the
first tower,
while a super-lean solution would be required for the low pressure second
absorber.
In the amine regeneration still column the level of residual H2S and CO2
varies at different levels in the column. The lowest residuals are at the base
of the
column where the amine is subjected to the most intense steaming from the
reboiler.
Further up the still column, several stages above the reboiler, the amine's
exposure to
steam and temperature Is lower, and the level of residuals in the solution is
correspondingly higher. It is therefore possible to draw two lean streams of
differing
compositions from the regenerator. One stream with normal levels of residual
H2S
and CO2 would be drawn from a stage above the reboiler. The second super lean
stream would be drawn from the level of the reboiler. These two individual
regenerated lean amine streams would be pumped separately to their own
respective
individual absorbers; the lean stream normally to the first absorber and the
super lean
stream normally to the second absorber. By dividing the lean amine into these
separate streams will result in a reduction in reboiler duty of typically ten
to fifteen
percent will result.
A process can be provided that integrates existing pieces of
conventional plant equipment into a new process that employs any of the
principles
described above. An existing conventional amine plant for example could be
upgraded to be equal to or equivalent to any of the processes described in the
claims
41
CA 02461952 2010-08-31
above by installing additional equipment. It is anticipated that a major
application of
the new technology will be to modify existing gas plants as necessary to
improve
performance by meeting the requirements of the new process. Existing equipment
will be used to the maximum extent possible to minimize the cost of the
upgrade.
The final plant configuration will include the reuse of existing equipment
plus
whatever additional equipment is necessary to meet the demands of the new
process.
A version of the processes described above can be proviced in which
one of the principal purposes of the process described herein is to maximize
recovery
of CO2 for commercial sale. In this case the first operation, which is
conducted in the
first absorber, rather than allowing a portion of the CO2 to be deliberately
"slipped"
into the overhead gas, is designed to recover a major portion of the CO2 in
the rich
amine leaving the first absorber. This differs from the arrangement described
herein
in which CO2 is recovered but merely as a by-product, which may or may not
have
commercial value. In one arrangement described herein the object is to
maximize
recovery of CO2 from the inlet gas for the purpose of producing a marketable
CO2
product.
Having absorbed a major portion of the available CO2 in the rich amine
exiting the first absorber, the next step normally would be to flash off the
light gases,
principally methane and ethane that may have been absorbed in the first
operation. If
it is desired to eliminate a significant portion of these light ends from the
rich amine a
flash tank should be used, employing the optimum combination of preheat and
reduced pressure to attain the desired separation. A small quantity of CO2
will
42
CA 02461952 2010-08-31
unfortunately be lost in the overhead from the flash tank. Light gases, if not
flashed
off, will appear as contaminants in the CO2 product stream. The flash tank is
an
option, depending on the amount of light gas that can be tolerated in the CO2
product.
The rich amine, whether degassed or not, then proceeds to the second
absorber, or in the case of a double effect unit, to the second and third
absorbers.
The rich amine is then contacted with the recycled enriched acid gas and lean
amine
to produce an overhead product that is essentially pure water saturated CO2.
The
rich amine leaving the base of the column is then regenerated to produce lean
amine
plus an enriched acid gas overhead stream, a portion of which is recycled back
to the
base of the column. The balance of this acid gas flows to other processing
units,
typically a sulphur plant.
The CO2 overhead product is typically 98% (MOL) carbon dioxide. The
product will also be contaminated by light hydrocarbon gases and a fraction of
a
percent of hydrogen sulphide. There may also be traces of organic sulphur,
amine or
other contaminants that may have been present in the feed stream.
Commercially marketable carbon dioxide can currently be grouped into
the four following grades. The standards to be met do not necessarily meet any
universally recognized set of specifications, so the quality of this product
is often
determined on a case by case basis, as agreed between buyer and seller:
Injection grade CO2 contains a minimum of 98% (MOL) CO2 and is used
for miscible flooding of oil reservoirs. Specifications for this product are
loosely
defined, and normally the only processing required is dehydration of the
overhead
CO2 from the second (or third) absorber.
43
CA 02461952 2010-08-31
Frac grade CO2 is 98.0% pure and is used for the fracturing reservoir
rock in petroleum reservoirs, which together with a propping agent, is used to
improve
permeability adjacent to the weilbore. Specifications are essentially the same
as for
injection grade CO2 except for a standard for H2S and organic sulphur content.
Beverage grade CO2 must be a minimum of 99.90% (MOL) pure CO2 and has strict
PPM limits for all impurities plus a standard for odor, color and taste when
dissolved
in water. Like all standards for potable products the standards for purity and
processing methods are.extremely high.
Food grade CO2 must be a minimum of 99.8%(MOL) pure CO2 and In
most respects is similar to beverage grade CO2 except that tolerance for
impurities is
in many cases lower.
The CO2 product described above could be a feed stock for various
standard downstream processes to produce commercial grades of CO2.
The above description is intended in an illustrative rather than a
restrictive sense, and variations to the specific configurations described may
be
apparent to skilled persons in adapting the present process described herein
to other
specific applications. Such variations are intended to form part of the
present
invention insofar as they are within the spirit and scope of the claims below.
For
instance, it will be appreciated that the present process may be extended to a
third
effect or more, increasing the concentration of H2S at each stage. For each
succeeding effect, the feed Into the low pressure absorber would be the acid
gas
produced by the preceding effect.
It is obvious that the process scheme described in Figure 4 in which the
44
CA 02461952 2010-08-31
absorber receiving the recycled acid gas operates at a pressure lower than
that of the
regenerator could also apply to the double effect process. In a multiple
effect system
some or all of the low pressure absorbers may operate at a pressure lower than
that
of their respective regenerators.