Note: Descriptions are shown in the official language in which they were submitted.
CA 02462991 2004-04-01
CROSSLINKED POLYVINYL ALCOHOL FIBER
AND METHOD FOR PRODUCING THE SAME
BACKGROUND OF THE INVENT'ION
Field of the Invention
[01] The present invention relates to crosslinked polyvinyl
alcohol fiber and method for producing the same, and more
particularly, to crosslinked polyvinyl alcohol fiber, in which
PVA resin having a degree of polymerization of more than 1,000
and a degree of saponification of more than 97.0 molo is
dissolved in dimethyl sulfoxide (hereinafter, referred to as
DMSO), the solution is subjected to dry and wet gel spinning
using methanol as a coagulation solution, drawn and thermally
treated, the resulting polyvinyl alcohol drawn yarn with 500-
3,000 deniers is twisted to produce a cabling yarn, the cabling
yarn is plied into a 2-ply or 3-ply yarn to produce a raw cord,
the raw cord is wound on a bobbin for crosslinking and
crosslinked in an aqueous crosslinking solution containing an
aromatic aldehyde compound and an acid catalyst. Moreover, the
present invention relates to a crosslinker-introducing apparatus,
which is used in the above method and can effectively induce the
crosslinking reaction of the wound raw cord.
1
CA 02462991 2004-04-01
Background of the Related Art
[02] A polyvinyl alcohol fiber (hereinafter, referred to as
PVA) shows superior strength and modulus to general purpose
fibers, such as polyamide, polyester, and polyacrylonitrile
fibers, and is very excellent in particularly adhesion, water
dispersibility, alkaline resistance and chemical resistance.
Thus, it is used as materials of various industrial fields.
[03] Recently, PVA is also used as a reinforcement material
for concrete, cement, rubber, plastic and the like, and studied
and developed as a material with high applicability to new fields.
[04] Till now, various methods for producing a high-
strength PVA fiber were proposed.
[05] US Patent No. 4,440,711 discloses a method for
preparing a high-strength PVA fiber using a gel spinning
technique (US patent No. 4,698,194) in which high-molecular
weight polyethylene as a raw material is drawn to high draw ratio
to produce the high-strength fiber. The gel spinning technique
is a general method for producing the high-strength fiber, in
which a polymer compound is mixed with solvent to prepare a
uniform solution, and then, the solution is drawn to high draw
ratio while suitably adjusting phase separation and gelling
occurring in a spinning process.
2
CA 02462991 2004-04-01
[06] Furthermore, a technology on method for producing PVA
fiber having more excellent physical properties using this gel
spinning technique was also known.
[07] Japanese patent laid-open publication No. Heisei 7-
109616 discloses a method for producing a PVA multifilament fiber
with a tensile strength of at least 22 g/denier, an initial
modulus of at least 440 g/denier and a yarn CV of less than 5%,
in which dry and wet spinning processes are performed using a
spinneret with an orifice diameter of 0.1-1 mm and an orifice
length-to-diameter (L/D) ratio of 3-20.
[08] However, although the PVA fiber produced by this
method has excellent mechanical properties, the PVA resin is
dissolved in hot water with a high temperature above 100 C or
has reduced mechanical properties, due to the hydrophilicity of
PVA resin itself. Thus, it has many limitations for use in
applications, such as tire cords which have the biggest market
among the industrial fibers.
[09] Although a very small amount of water is present in
the inside of a tire, excess water can flow into the tire when
the tire gets damaged. Also, when the tire temperature is
increased to 130 C as a result of high-speed running of
automobile, the water is thermally hydrated to cause damages to
the PVA fiber, so that the stability of automobiles is endangered.
3
CA 02462991 2004-04-01
Thus, the PVA fiber according to the prior art could not be used
as a tire reinforcement material without anxiety.
[10] Also, since the high crystallinity of the PVA fiber
results in a reduction in fatigue resistance when it is used for
tire cords, this problem needs to be solved..
[11] To improve hot water resistance and fatigue resistance,
various methods were developed in which PVA. with a high degree of
polymerization is spun, thermally drawn to high draw ratio,
thermally treated, acetalized and crosslink:ed by an acid catalyst.
However, it is difficult to use the PVA fibers as filaments for
industrial purpose. And, when the PVA resin produced by such
methods is used in hot water above 130 C, a problem occurs.
[12] Particularly in the crosslinking technology proposed
in the prior art, a crosslinker is added to a spinning dope
before a drawing process or during an extraction or oil-treating
process.
[13] Korean patent registration Noõ 210727 discloses a
method for producing a polyvinyl alcohol fiber with excellent hot
water resistance, in which a yarn containing an acetal compound
of aliphatic dialdehyde as a crosslinker is prepared, subjected
to dry heat drawing and crosslinked by an acid.
[14] Korean patent laid-open publication No. 96-41438
discloses a method for producing a polyvinyl alcohol fiber with
excellent hot water resistance, in which a yarn containing an
4
CA 02462991 2007-06-04
-5-
ammonium suifate crosslinker is subjected to dry heat drawing and then
crosslinked.
[15] As described above, in the crosslinking technologies proposed till now,
the crosslinker is added to the spinning dope before the drawing process or in
the
extraction or oil-treating process. In such prior crosslink+ng methods, when
subjected
to thermal drawing at a high temperature above 200 C, the crosslinker
contained in
the PVA undrawn yarn cause crosslinking reaction to reduce drawability, or the
crosslinker with low boiling point is volatilized to reduce crosslinking
efficiency. Thus,
the crosslinker hardly has a hot water resistance above 130 c.
[16] Furthermore, the crosslinking treatment methods as described above
have a problem in that, since crosslinking treatment is performed by simply
dipping a
bobbin wound with a undrawn yam into the crosslinker, a portion of the undrawn
yarn
wound inside the bobbin is not impregnated with the crosslinker, and thus,
incompletely crosslinked, or the outer side of the undrawn yarn wound on the
bobbin
is crosslinked at a significantly different level from the inside of the
undrawn yam.
SUMMARY OF THE INVENTION
[17] The present invention provides a crosslinked polyvinyl alcohol fiber, in
which a polyvinyl alcohol drawn yam with 500-3,000 deniers is twisted to form
a
cabling yam, the cabling yam is plied into a 2-ply or 3-ply yam to prepare a
raw cord,
the raw cord wound on a bobbin for crosslinking reaction is crosslinked in an
aqueous
crosslinking solution containing an aromatic aidehyde compound and an acid
catalyst.
[18] Another aspect of the present invention is to provide a
crosslinker-introducing apparatus, which is used in the above inventive method
and
,
CA 02462991 2007-06-04
-6-
alkr+vs the polyvinyl alcohol, fiber to have excellent hot water resistance
and high
strength.
[19] According to another aspect of the present invention, there is provided a
crosslinked raw cord which is produced by a method comprising the steps of:
(A)
spinning polyvinyl alcohol having a degree of polymerization of 1,000-7,000
according
to a dry and wet spinning technique or a wet spinning technique, drawing the
undrawn
yam to high draw ratio, and thermally treating the drawn yam; (8) twisting the
polyvinyl alcohol drawn yam to prepare a cabling yam, and plying the cabling
yam
into a 2-ply or 3-ply yarn to produce a raw cord; and (C) crosslinking the raw
cord by
dipping it into the crosslinker.
1201 According to another aspect of the present invention, there is provided a
crosslinker-introducing apparatus comprising a cylindrical bobbin which has a
hollow
formed therein, a plurality of through-holes formed on the circumferential
surface and
on which a raw cord is wound and a closed container which is charged with the
crosslinker and provided in such a manner that the bobbin for crosslinking is
dipped
in the crosslinker.
[21] According to another aspect of the present invention, there is provided a
crosslinked raw oord which is produced by a method comprising the steps of:
(A)
dissolving polyvinyl alcohol having a degree of polymerization of 1,000-7,000
and a
degree of saponification of more than 97.0 mol% in dimethyl sulfoxide,
spinning the
solution according to a dry and wet spinning technique or a wet spinning
technique,
drawing the undrawn yarn to high draw ratio, and thermally treating the drawn
yarn;
(B) twisting the polyvinyl alcohol drawn yam to prepare a cabling yarn, and
plying the
CA 02462991 2007-06-04
-7-
cabling yam into a 2-ply or 3-ply yam to produce a raw cord; and (C)
crosslinking the
raw cord using the crosslinker-introducing apparatus described above in an
aqueous
crosslinking solution containing an aromatic aidehyde compound and an acid
catalyst
while adding aicohol to the aqueous crosslinker solution.
According to another aspect of the invention, there is provided a crosslinked
raw cord which is produced by a method comprising the steps of:
(A) dissolving polyvinyl alcohol having a degree of poiymerization of 1,000-
7,000 and a degree of saponification of more than 97.0 mol% in dimethyl
sulfoxide,
spinning the solution according to a dry and wet spinning technique or a wet
spinning
technique, drawing the undrawn yam, and thermally treating the drawn yarn;
(B) twisting the polyvinyl alcohol drawn yarn to prepare a cabling yam, and
plying the cabling yam into a 2-ply or 3-ply yarn to produce a raw cord; and
(C) crossfinking the raw cord with a crosslinker-introducing apparatus in an
aqueous crosslinking solution containing an aromatic aidehyde compound and an
acid cataiyst while adding alcohol to the aqueous crosslinker solution,
wherein the
crosslinker-introducing apparatus comprise a bobbin having a circumferential
surface
with a plurality of through-holes for winding the raw cord and a hollow
cylindrical
bobbin axis.
[22] Preferably, the alcohol added to the aqueous crosslinking solution in the
step (C) is methanol.
[23] Preferably, the content of the alcohol added to the aqueous
crosslinking, solution in the step (C) is 1-30 wt%.
CA 02462991 2007-06-04
-8-
[24] Preferably, the content of the aromatic aldehyde compound crossiPnked
to the raw cord in the step (C) is 0.1-5.0 wt%,
[25] Preferably, the aromatic aldehyde crosslinked to the raw cord in the
step (C) is terephthakiicarbaxaldehyde (TDA).
[26] Preferably, the acid catalyst is used in the crosslinking of the raw cord
in
the step (C).
[27] Preferably, the acid catalyst used in the step (c) is acetic acid.
[28] In another aspect of the present invention, there is provided a treated
cord for tire cords, which is produced by treating the crosslinked raw cord
described
above with a dipping solution (RFL) and has the following physical properties:
(1) a
breaking load of 20.0-50.0 kgf; (2) a fineness of 1,000-6,000 deniers; (3) hot
water
resistance of at least 130 G, and (4) a fatigue resistance of at least 80%,
[29] The crosslinker which is used in the present invention is preferably an
aidehyde compound capable of crosslinking with the hydroxy group of PVA, and
the
aldehyde compound preferably has two or more aidehyde groups in order to
increase
crossiinking efficiency. The aldehyde compound is more preferably an aromatic
compound which infiltrates only into the non-Crystalline region of the fiber.
CA 02462991 2004-04-01
[30] Examples of this aromatic aldehyde compound include
terephthaldicarboxaldehyde (TDA), isophthaldicarboxaldehyde (IDA)
and naphthaldicarboxaldehyde (NDA) , and a rnixture of two or more
thereof.
[31] As the aromatic aldehyde compound,
terephthaldicarboxaldehyde (TDA) is preferably used in the
present invention.
[32] It is the key technical point of the present invention
that the aromatic aldehyde capable of infiltrating only into the
non-crystalline region of the drawn yarn is used as the
crosslinker. Since the aromatic aldehyde is mainly infiltrated
only into the non-crystalline region of the yarn, the tenacity of
the drawn yarn can be prevented from being reduced due to the
crosslinker.
[33] The most important characteristic of the present
invention is a crosslinking process. In general crosslinking,
there is used a method wherein the crosslinker i_s dissolved in an
organic solvent in an extraction process in order to infiltrate
the crosslinker into the inside of the fiber. However, this
crosslinker within the undrawn fiber causes a reduction in
drawability in a thermal drawing step at high temperature above
200 C, so that the drawn yarn does not have sufficient hot water
resistance and fatigue resistance. The crosslinker used in the
9
CA 02462991 2004-04-01
extraction process makes organic solvent recovery difficult and
thus an entire process difficult.
[34] For this reason, in the present invention, in order to
increase crosslinking efficiency and to prevent fiber damage, the
twisted PVA raw cord is crosslinked after it is infiltrated with
the crosslinker. This gives a high-strenqth PVA fiber having a
hot water resistance above 130 C and a fatigue resistance of at
least 80%.
[35] A key technical point in the present invention is that
the raw cord is crosslinked in a crosslinking solution containing
an aromatic aldehyde compound and an acid catalyst while adding
alcohol to the aqueous crosslinking solution. The addition of
alcohol to the crosslinking solution allows significant
prevention of reduction in tenacity.
[36] Hereinafter, the producing method of the PVA fiber
will be described in detail.
[37] PVA has a degree of polymerization of about 1,000-
7,000, and preferably 1,500-4,000. At a degree of polymerization
lower than 1,000, it is difficult to form it into fibers, and at
a degree of polymerization higher than 7,000, it has so high
viscosity to reduce spinning processability. Since the high-
strength PVA fibers which are mostly used in the industrial
material field need to have hot water resistance, PVA with a
saponification degree of more than 97.0 mol% is used. As the
CA 02462991 2004-04-01
organic solvent, ethylene glycol, glycerin, and DMSO may be used,
but DMSO is suitable for its highest solubility for PVA. This
DMSO is preferably purified to a water content of less than
several tens ppm before use.
[38] The concentration of the PVA dope is adjusted such
that its viscosity is preferably in a rarige of 50-4,000 poise,
and more preferably 500-3,000 poise in order to obtain excellent
physical properties. At a viscosity below 50 poise, it is
difficult to form the PVA dope into a fiber, and at a viscosity
above 4,000 poise, fiber spinnability is reduced.
[39] A coagulation bath has a temperature of -30 to 30 C
for possible spinning, and preferably -10 to 10 C for the
formation of uniform gel. If the temperature of the coagulation
bath is below -30 C, PVA spinning dope may be frozen. If the
temperature of the coagulation bath is higher than 30 C, gel
formation becomes impossible so that spinnability will be reduced.
[40] A method for producing a PVA fiber is performed by a
dry spinning technique, a wet spinning technique, and a dry and
wet spinning technique, but in a method for producing a high-
strength PVA fiber where a drawing process with high draw ratio
is required, the dry and wet spinning technique is preferred.
For the production of a PVA filament, the air-gap in the dry and
wet spinning technique may be 5-200 mm, but for thermal drawing
to high draw ratio, a narrow air-gap of 5-50 mm is preferred. At
11
CA 02462991 2004-04-01
an air-gap below 5 mm, workability will be reduced. On the other
hand, at an air-gap above 200 mm, crystallinity is greater than
gelling to make the thermal drawing at high draw ratio impossible,
and also the fusion between fibers on a nozzle section occurs to
reduce productivity.
[41] In the producing method of the high-strength PVA fiber,
the drawing process is very important for high strength and
improved hot water resistance. Examples of a heating manner in
the drawing process include a hot air heating manner and a roller
heating manner. In the roller heating manner, a filament is in
contact with the roller surface such that the fiber surface is
liable to be damaged. Thus, the hot air heating manner is more
effective for the production of the high-strength PVA fiber. The
heating temperature may be 140-250 C, and preferably 160-230 C.
At a heating temperature below 140 C, molecular chains will not
sufficiently move to make the thermal drawing at high draw ratio
impossible, and above 250 C, PVA is liable to be decomposed to
cause a reduction in physical properties.
[42] Furthermore, in the PVA fiber used as a tire cord
among industrial materials, high strength and fatigue resistance
are required. To meet such requirements, a PVA drawn yarn is
twisted to produce a raw cord. In a general process for twisting
synthetic fibers, an increase in twist number will result in a
reduction in tenacity but an increase in fatigue resistance.
12
CA 02462991 2004-04-01
Thus, selecting suitable twist number according to the purpose of
use is very important. For example, a tire cord used for carcass
of a tire with 1500 d/2p is twisted to 300-500 TPM (turns per
meter) before use.
[43] To enhance hot water resistance and fatigue resistance,
the twisted PVA raw cord is crosslinked by the addition of a
crosslinker.
[44] To infiltrate the crosslinker only into the non-
crystalline region of the PVA fiber drawn to high draw ratio, the
aromatic aldehyde is used as the crosslinker as described above.
[45] The aromatic aldehyde compound, which is used in the
present invention, is preferably terephthaldicarboxaldehyde (TDA).
The crosslinking compound is used at the amount of 0.1-5 wt%
relative to a fiber, and preferably 0.5-2.0 wt%. If it is used
at the amount of less than 0.1 wt%, an insufficient heat water
resistance below 130 C will be caused, and if it is used at the
amount of more than 5.0 wt%, a great reduction in tenacity will
be caused to make the use of the high-tenacity tire cord
difficult.
[46] To react the crosslinking compound with the OH group
of PVA, an acid catalyst is required in an aqueous crosslinking
solution. Although acids, such as sulfuric acid or acetic acid,
may be used as the acid catalyst, the acetic acid is preferable
in view of reaction rate adjustment and stability. The acid
13
CA 02462991 2004-04-01
catalyst is preferably used at the amount of 5-30 wt% relative to
the aqueous crosslinking solution. If the acid catalyst is used
at less than 5 wt%, crosslinking reaction will progress too
slowly, and if it is used at more than 30 wt%, it will be
difficult to remove the acid catalyst in a water-washing process
after reaction.
[47] It is a key technical point in the present invention
that crosslinking is performed with the addition of alcohol to
the aqueous crosslinking solution containing the aromatic
aldehyde compound and the acid catalyst. The addition of alcohol
to the crosslinking solution allows significant prevention of a
reduction in tenacity after crosslinking.
[48] Examples of preferred alcohols, which are added to the
aqueous crosslinking solution in the present invention, include
methanol, ethanol, propanol and butanol. Methanol is more
preferred. The alcohol is added at the amount of 1-30 wt%
relative to the aqueous crosslinking solution. At less than 1
wt%, a great reduction in tenacity will be caused during
crosslinking to make the use for the high-tenacity tire cord
difficult, and at more than 30 wt%, a cost disadvantage will be
caused and also crosslinking will progress at a too slow rate.
[49] Another key technical point in the present invention
is that a polyvinyl alcohol drawn yarn is plied into a 2-ply or
3-ply yarn to produce a raw cord wound on a bobbin for
14
CA 02462991 2004-04-01
crosslinking, and then, the raw cord wound on the bobbin for
crosslinking is crosslinked by dipping it into the crosslinking
solution.
[50] To infiltrate the crosslinking compound into the non-
crystalline region of a PVA fiber having high crystallinity, a
method is used in which the reaction solution is heated to 50 C
to increase the activity of the crosslinking compound, and a
reactor is pressurized before use. Also, crosslinking time
varies depending on the crosslinking compound and conditions, but
is preferably longer than 30 minutes. However, if the
crosslinking is performed for too long time, a great reduction in
tenacity will be caused.
[51] The crosslinked PVA raw cord is washed and dried. To
improve the adhesion to rubber, the dried raw cord is dipped in a
RFL solution, dried and thermally treated. Concretely speaking,
the dipping process is achieved by impregriating the fiber surface
with a resin solution called resorcinol-formaline-latex (RFL),
and this dipping process is performed in order to improve the
problem of low adhesion to rubber of the tire cord fiber.
[52] The dipping solution, which is used for the adhesion
between the PVA raw cord and rubber in the present invention, can
be prepared by, for example, the following method. The following
preparation example is given to more fully understand the present
invention and is not intended to limit the present invention.
CA 02462991 2004-04-01
[53] Resorcinol of 29.4 wt% 45.6 weight part
Pure water 255.5 weight part
Formalin of 37% 20 weight part
Sodium Hydroxide of 10 wt% 3.8 weight part
[54] The solution prepared as described above is reacted at
25 C for 5 hours with stirring, and then added with the
following components.
[55] VP-Latex of 40 wt% 300 weight part
Pure water 129 weight part
Ammonia water of 28% 23.8 weight part
[56] The solution containing the above components is aged
at 25 C for 20 hours, and maintained at a solid concentration of
19.05%.
[57] In order to prevent the RFL solution from infiltrating
deeply into the inside of the fiber during the RFL dipping
process, the raw cord is stretched to a stretch ratio of 0.5-3%,
and a dip pick up (DPU) of the RFL is 3.0-9.0 wt%. At a stretch
ratio of less than 0.5%, DPU will exceed 9 wt% so that the RFL
solution will be infiltrated deeply into the inside of the staple
fiber to reduce fatigue resistance. At a stretch ratio of more
than 3%, excessive tension will be applied to the raw cord and
thus will cause damages to the raw cord. Thermal treatment
should be performed at a temperature of 170-230 C, and
preferably 200-220 C where the movement of PVA molecules is the
16
CA 02462991 2004-04-01
best. By minimizing the tension applied to the fiber to allow
for the greatest possible movement of the PVA molecules to
maximize a heat treatment effect, the production of a treated
high-tenacity PVA cord becomes possible. In a heat treatment
process conducted after dipping the raw cord in the RFL solution,
it is important that the dipped cord is maintained at a stretch
ratio of 0 to -5%. If the stretch ratio in the heat treatment
process is above 0%, when the dipped cord is used in a tire cord
requiring high fatigue resistance, a cord cutting or separation
phenomenon will occur which is due to low fatigue resistance
below 60% resulting from the low elongation of the dipped cord.
On the other hand, at a stretch ratio below -5%, molecular
recrystallization in a vertical direction to the fiber axis will
occur due to excessive molecular movement to cause a reduction in
tenacity. If the crosslinker is present within the fiber not
having been washed in the water-washing process after the
crosslinking process, it acts as an impurity in a product where
the PVA fiber was used. Thus, heat treatment is performed above
200 C such that the remaining crosslinker can be reacted or
volatilized to further improve crosslinking efficiency.
[58] Hereinafter, the bobbin for crosslinking used in the
crosslinking as described above, and the use state thereof, will
be described in brief.
17
CA 02462991 2004-04-01
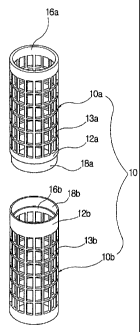
[591 FIG. 1 is a perspective diagram showing the bobbin for
crosslinking according to the present invention, and FIG. 2 is a
use state diagram showing the use state of the bobbin for
crosslinking according to the present invention.
[60] In the crosslinking as described above, a bobbin for
crosslinking 10 is provided. The bobbin for crosslinking 10
comprises a first bobbin l0a forming one portion of the
crosslinking bobbin 10, and a second bobbin lOb, which is
detachably coupled to the first bobbin l0a and forms the other
portion of the crosslinking bobbin 10. In the first and second
bobbins l0a and lOb, hollows 16a and 16b are formed, respectively,
a plurality of through-holes 13a and 13b are formed in the
circumferential portion of the first and second bobbins so that
cylindrical bobbin axes 12a and 12b on which a PVA raw cord is
wound up are provided. In the inner end of the first and second
bobbins l0a and lOb, a coupling protrusion 18a and acoupling
groove 18b are formed which correspond to each other such that
the second bobbin 10b is coupled to the first bobbin l0a.
[61] Herein, the first bobbin wheel 14a coupled to the
first bobbin l0a serves to close the hollow 16a of the bobbin for
crosslinking 10, and the second bobbin wheel 14b coupled to the
second bobbin lOb serves to be connected with a crosslinker-
feeding pipeline 30 in order to supply the crosslinker 2 into the
inside of the PVA raw cord 1 wound on a bobbin for crosslinking
18
CA 02462991 2004-04-01
through a hollow 16a and 16b formed in a bobbin for
crosslinking 10 by pressurizing or depressurizing with supplying
apparatus by which crosslinker 2 is supplied in a specified
pressure.
[62] In the crosslinking step, the bobbin for crosslinking
10 on which the PVA raw cord 1 was wound is provided in such a
manner that it is dipped in a crosslinker 2 contained in a closed
container 40 charged with the crosslinker 2. The crosslinker 2
is pressurized or depressurized to a specified pressure and
supplied through a crosslinker-feeding pipeline 30. The supplied
crosslinker is moved from inside to outside of the wound PVA raw
cord 1 through the through-holes 13a and 13b formed in the
respective bobbin axes 12a and 12b or moved from the outside to
inside of the PVA raw cord 1, so that the inside and outside of
the PVA raw cord 1 wound on the crosslinking bobbin 10 can be
uniformly crosslinked.
BRIEF DESCRIPTION OF THE DRAWINGS
[63] FIG. 1 is a perspective diagram showing a bobbin for
crosslinking according to the present invention.
[64] FIG. 2 is a use state diagram showing a crosslinker-
introducing system using the bobbin tor crosslinking according to
the present invention.
19
CA 02462991 2004-04-01
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENT
[65] Hereinafter, the present invention is described in
detail with referring to the following examples, but it is to be
understood that the examples is solely for the purpose of
illustration and do not limit the scope of the present invention.
In the following examples, the estimating method and the
measuring method as following is used.
[66] (a) tenacity(kgf) of PVA cord
[67] The tenacity of the filament is measured using low
speed elongation tester, and the filament is tested after being
dried at 107 C for 2 hours. The filament is twisted by
8OTPM(80turns/meter) and the length of the filament is 250mm and
the elongation speed is 300m/min.
[68] (b) hot water resistance(WTb, C)
[69] A twisted raw cord with 3,000 deniers is selected, cut
into a 4-cm size, and then applied with a load of 3g/ply. The
cord is dipped in water contained in a glass container for
pressurization, and the temperature at which the fiber is broken
is measured while elevating the temperature at a rate of 2
C/minute.
[70] (c) fatigue resistance
[71] Samples were subjected a fatigue test using a Goodrich
disc fatigue tester which is conventionally used for the fatigue
test of tire cords. Then, they were measured for residual
CA 02462991 2004-04-01
tenacity, and fatigue resistances were compared. The fatigue
test was conducted under the following conditions: 120 C; 2,500
rpm, and 10% and 18% compression. After the fatigue test, the
samples were submerged in tetrachloroethy].ene solution to swell
rubber, and then, a cord was separated from the rubber and
measured for residual tenacity. This residual tenacity was
measured after drying at 107 C for 2 hours using a conventional
tensile strength tester by the above-described measurement method
(a).
[72] Example 1
[73] PVA was used in a powder form with a degree of
saponification of 99.9 mol% and a degree of polymerization of
2,000, and methyl alcohol and DMSO were used in a purified
solvent mixture form with a water content of less than l00 ppm.
To prepare the solvent mixture, DMSO and methyl were mixed such
that the content of methyl alcohol content in the solvent was 50
by volume. PVA was dissolved in the solvent mixture such that it
was 22 wt% relative to a PVA spinning dope. Next, the PVA
solution was produced into a PVA fiber by a dry and wet spinning
technique, using gel spinning. In this spinning process, a
circular nozzle with a nozzle hole number of 500, a nozzle hole
diameter of 0.5 mm and a L/D ratio of 5 was used. Also, air-gap
was 50 mm, and methanol was used as a solvent in a coagulation
bath. At this time, the coagulation bath was maintained at a
21
CA 02462991 2004-04-01
solvent/methanol mixing ratio of 20/80 and a temperature of 0 C.
After passing through an extraction tank, the PVA fiber must be
free of the DMSO solvent. If the solvent remains in the filament,
it is discolored in a thermal drawing process at high temperature
to act as a main cause of deteriorating the physical properties
of the final filament. In the thermal drawing process, two-step
hot air heating was used in which the hot air heating temperature
was 200 C at the first step and 220 C at the second step, and
the draw ratio was adjusted such that total draw ratio was 13.5.
As a result, a high-strength PVA fiber with a strength of 13.0
g/d and an elongation of 7.0% was produced. This drawn yarn was
twisted to plying and cabling number of 300 TPM to produce a raw
cord yarn having a tenacity of 34 kgf. The raw cord wound on a
bobbin for crosslinking was crosslinked by dipping it in
terephthaldicarboxaldehyde (TDA) as aromatic aldehyde through a
crosslinker-introducing apparatus capable of effectively inducing
crosslinking. In the crosslinking reaction, 2 wt% of
terephthaldicarboxaldehyde (TDA) and 10 wt% of acetic acid were
dissolved in water to prepare an aqueous crosslinking solution,
and 10 wt% of methanol was added to the aqueous crosslinking
solution, and then, the raw cord wound on the bobbin for
crosslinking was crosslinked by dipping it in the aqueous
crosslinking solution at 70 C for one hour and washed with water.
The crosslinked raw cord with tenacity of 33.6 kgf was
22
CA 02462991 2004-04-01
impregnated with a RFL solution to produce a treated PVA cord.
The treated PVA cord was measured for its physical properties and
the results are summarized in Table 1 below.
[74] Examples 2 and 3
[75] The ratio between terephthaldicarboxaldehyde, acetic
acid and methanol was adjusted to a ratio given in Table 1, and
the resulting raw cord was crosslinked and then measured for its
physical properties, including tenacity and fatigue resistance.
[76] Comparative Examples 1 and 2
[77] Comparative Example 1 is a non-crosslinked case and
the results are given in Table 1, and Comparative Example 2 is a
case where methanol was not used in the aqueous crosslinking
solution. The results for Comparative Example 1 and 2 are given
in Table 1.
[78] Comparative Examples 3 and 4
[79] In Comparative Example 3, crosslinking was performed
for 6 hours and the results are given in Table 1. In Comparative
Example 4, crosslinking was performed at 30 C and the results
are given in Table 1.
23
CA 02462991 2004-04-01
[80] Table 1
Ex. 1 Ex. 2 Ex. 3 Comp. Comp. Comp. Comp.
Ex. 1 Ex. 2 Ex. 3 Ex. 4
Concentration of TDA 2 2 2 - 5 2 2
(wt %/aqueous solution)
Concentration of Acetic acid 10 10 15 - 10 10 10
(wt %/aqueous solution)
Concentration of Methanol 10 5 10 - - 10 10
(wt %/aqueous solution)
Reaction temp. (,C) 70 70 70 - 70 70 30
Reaction time(min) 60 60 60 - 60 360 60
Tensile strength of 13.5 13.5 13.5 13.5 1.3.5 13.5 13.5
drawn yarn ( g / d )
Strength of raw cord 34 34 34 34 34 34 34
(k g f) Strength of treated 33.6 32.8 32.1 - 21.2 28.2 33.4
raw cord ( ic g f)
Strength of dipped cord 37.9 37.2 36.5 38 26.4 31.5 38.2
(k g f)
Fatigue resistance (%) 99 95 97 62 98 98 68
Hot water resistance(C) 172 167 172 107 170 171 108
[81] As described above, the present invention provides the
crosslinked raw cord which is produced by the method comprising
the steps of: twisting a polyvinyl alcohol drawn yarn with 500-
3,000 deniers to prepare a cabling yarn; plying the cabling yarn
into a 2-ply or 3-ply yarn to prepare a raw cord; winding the raw
cord on a bobbin for crosslinking; and crosslinking the raw cord
wound on the bobbin for crosslinking, in an aqueous crosslinking
solution containing an aromatic aldehyde compound and an acid
catalyst, while adding alcohol to the aqueous crosslinking
solution. The crosslinked raw cord has excellent hot water
resistance, and thus, can be suitably used for tire cords.
24