Note: Descriptions are shown in the official language in which they were submitted.
Case 1/1268-PCT BOEHRINGER INGELHEIM PHARMA KG
CA 02463146 2004-04-07
79157pct.209
Crystalline sodium salt of telmisartan and the use of same as an
angiotensin antagonist
The invention relates to a crystalline sodium salt of 4'-[2-n-propyl-4-methyl-
6-(1-
methylbenzimidazol-2-yl)benzimidazol-1-ylmethylJbiphenyl-2-carboxylic acid
(INN:
telmisartan), processes for preparing it and the use thereof for preparing a
pharmaceutical composition.
~o
Background to the invention
The compound telmisartan is known from European Patent EP 502 314 B1 and has
the following chemical structure:
Me
M N I ~ N~Me
~ "N
\ O OH
Telmisartan, and the physiologically acceptable salts thereof, have valuable
pharmacological properties. Telmisartan is an angiotensin antagonist,
particularly an
2o angiotensin-II-antagonist which by virtue of its pharmacological properties
may be
used for example to treat hypertension and cardiac insufficiency, to treat
ischaemic
peripheral circulatory disorders, myocardial ischaemia (angina), to prevent
the
progression of cardiac insufficiency after myocardial infarct, to treat
diabetic
neuropathy, glaucoma, gastrointestinal diseases and bladder diseases. Other
25 possible therapeutic applications can be found in EP 502314 B1, the
contents of
which are hereby referred to.
Telmisartan is commercially obtainable under the brand name Micardis~.
Starting
from the free acid of telmisartan, the preparation in the form in which
telmisartan is
3o marketed is produced by a complex spray drying process. Because of the
limited
solubility of the free acid, less complex methods of preparing an alternative
preparation are difficult to achieve.
CA 02463146 2004-04-07
2
The aim of the present invention is to prepare telmisartan in a form which
enables
this active substance to be presented in a less complex form. It has to be
borne in
mind that generally the production of a composition containing a
pharmaceutically
active substance is based on various parameters which are linked to the nature
of
the active ingredient itself. Without being tied thereto, examples of these
parameters
are the stability of effect of the starting material under different
environmental
conditions, the stability during the manufacture of the pharmaceutical
formulation
and the stability in the final compositions of the pharmaceutical preparation.
The
pharmaceutically active substance used to prepare the abovementioned
~o pharmaceutical compositions should be as pure as possible and its stability
on long-
term storage must be guaranteed under various environmental conditions. This
is
absolutely essential, in order to prevent pharmaceutical compositions being
used
which contain, in addition to the active substance proper, breakdown products
thereof. In such a case the content of active substance present in a
preparation
produced therefrom may be less than the specified amount.
Another aspect which is important in the production of solid preparations is
that the
active substance should have the most stable possible crystalline morphology
for the
pharmaceutical quality of a medicinal formulation. If this is not the case,
the
2o morphology of the active substance may change in certain circumstances
under the
conditions of manufacture of the preparation. Such a change may in turn affect
the
reproducibility of the manufacturing process and thus lead to final
formulations which
do not meet the high quality requirements imposed on formulations of
pharmaceutical compositions. To this extent it should generally be borne in
mind that
25 any change to the solid state of a pharmaceutical composition which can
improve its
physical and chemical stability gives a significant advantage over less stable
forms of
the same drug.
The object of the invention is thus to provide a new stable form of
telmisartan which
3o complies with the abovementioned stringent requirements imposed on
pharmaceutically active substances.
~
, CA 02463146 2004-04-07
3
Detailed description of the invention
Surprisingly, it has been found that telmisartan can be obtained in
crystalline form, in
the form of its sodium salt of formula 1
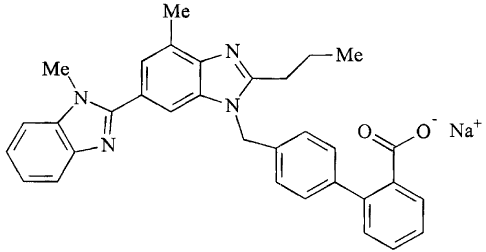
O N a+
1
By a suitable choice of manufacturing conditions, the polymorphic form of the
crystalline sodium salt which meets the requirements mentioned above can be
obtained selectively.
This crystalline form of the sodium salt of telmisartan is characterised by a
melting
point of T=245 ~ 5°C (determined by DSC=Differential Scanning
Calorimetry; heating
~s rate:10lVmin).
The present invention therefore relates to crystalline telmisartan sodium salt
characterised by a melting point of T=245 ~ 5°C (determined by DSC).
The above value was obtained using a DSC821 made by Messrs Mettler-Toledo.
The crystalline form of the sodium salt of telmisartan according to the
invention was
examined more closely by spectroscopy. The X-ray powder diagram obtained is
shown in Figure 1.
The following Table 1 summarises the data obtained in this spectroscopic
analysis:
TahlP 1
2 O [] d [A] rel. intensity2 p [] d [d] rel. intensity
[%] [%]
3.54 24.96 7 13.17 6.72 7
4.21 20.95 100 13.68 6.47 7
4.45 19.83 20 14.36 6.16 10
4.98 17.72 54 14.98 5.91 13
5.69 15.52 8 15.51 5.71 14
CA 02463146 2004-04-07
4
6.32 13.97 34 15.70 5.64 12
6.48 13.63 35 16.21 5.46 8
7.12 12.41 12 17.09 5.18 10
7.49 11.80 11 17.48 5.07 9
8.08 10.93 4 18.10 4.90 9
8.49 10.41 6 19.18 4.62 11
8.96 9.86 7 19.43 4.56 13
9.50 9.31 5 19.95 4.45 11
10.19 8.68 5 20.89 4.25 11
10.80 8.18 8 21.29 4.17 10
11.16 7.92 18 22.19 4.00 9
11.88 7.44 7 23.07 3.85 10
12.51 7.07 7 23.76 3.74 9
12.79 6.92 11 24.43 3.64 8
In the above Table the value "2 O [°]" denotes the angle of diffraction
in degrees and
the value "d [A]" denotes the lattice plane spacings determined in A.
According to the findings given in Table 1, the present invention relates to
crystalline
telmisartan sodium salt, characterised in that in the X-ray powder diagram it
has the
characteristic values d= 20.95 A, 17.72 A, 13.97 A and 13.63 A, inter alia.
The X-ray powder diagrams were recorded within the scope of the present
invention
~o using a Bruker D8 Advanced with an SSD (= site-sensitive detector) (CuKa -
radiation, ~, = 1.5418 A, 30 kV, 40 mA).
The present invention also relates to the crystalline sodium salt of
telmisartan
according to the invention in the form of the solvates and hydrates thereof,
~5 preferably in the form of the hydrates, most preferably in the form of the
hemihydrate
thereof.
In another aspect, the present invention relates to a method of producing the
crystalline sodium salt of telmisartan according to the invention. The
starting material
2o used to prepare the crystalline sodium salt of telmisartan according to the
invention
may be the free acid of telmisartan, which may be obtained by methods known in
the
art (e.g. according to EP 502314 A1 ).
CA 02463146 2004-04-07
To prepare the crystalline sodium salt according to the invention the free
acid of
telmisartan is taken up in a suitable solvent, preferably in an organic
aprotic solvent,
most preferably in an organic, aprotic and non-polar solvent. The solvents
used
according to the invention are most preferably toluene, chloroform,
dichloromethane,
s tetrahydrofuran, diethylether, diisopropyfether, methyl-tert. butylether,
acetone,
methylisobutylketone, benzene or acetonitrile, of which toluene, benzene and
methylisobutylketone are particularly preferred. Of outstanding importance
according
to the invention is toluene as solvent.
Preferably, between 0.5 and 5 ml, more preferably between 1 and 3 ml, most
preferably between 1.5 and 2.5 ml of the abovementioned solvent are used per
gram of telmisartan (free acid).
A suitable sodium salt is then added as a base to this solution or suspension.
Suitable sodium salts within the scope of the present invention include sodium
hydroxide, sodium hydride, sodium carbonate, sodium hydrogen carbonate or
sodium alkoxides. By sodium alkoxides are meant the sodium salts which are
formed
with lower alcohols, preferably with alcohols selected from among methanol,
ethanol,
isopropanol, n-propanol, tert-butanol, sec.-butanol, isobutanol, n-butanol and
tert.-
amyfalcohol. Of particular interest according to the invention are sodium
salts
2o selected from among sodium hydroxide, sodium hydride, sodium ethoxide and
sodium methoxide; of these, sodium hydroxide and sodium methoxide are of
particular importance according to the invention. The abovementioned sodium
salts
may be added to the reaction mixture as solids. In the case of sodium
hydroxide this
is preferably added in the form of aqueous solutions, however. It is
particularly
preferable to use concentrated aqueous solutions of sodium hydroxide. For
example,
sodium hydroxide solution may be used in a concentration of about 45 wt.-%.
The amount of sodium salt to be used naturally depends on the amount of free
acid
telmisartan used. According to the invention at least 1 mol of sodium salt has
to be
added per mol of telmisartan. It is also possible according to the invention
to add an
so excess of sodium salt. Preferably, 1-2.5, more preferably 1-2, most
preferably 1-1.5
mol of sodium salt are added per mol of the acid telmisartan used.
If sodium hydroxide is used as the sodium salt and this is added in the form
of an
aqueous solution, according to a preferred embodiment of the process according
to
the invention, it may be helpful in some cases to add a water-miscible organic
s5 solvent. This is preferably selected from among methanol, ethanol,
isopropanol,
acetone, tetrahydrofuran, tert.-butanol, 2-butanol, butanol, glycol,
ethyldiglycol,
1,3-butanediol, 1,4-butanediol, tert.-amylalcohol, acetonitrile, nitromethane,
formamide, dimethylformamide, N-methylpyrrolidinone, dimethylsulphoxide,
dimethylacetamide, nitroethane and methoxy-2-propanol, of which the
CA 02463146 2004-04-07
6
abovementioned alcohols are particularly significant. It is particularly
preferred,
within the scope of the process according to the invention, to use methanol or
ethanol, most preferably ethanol. Preferably, between 50 and 500 ml, more
preferably between 100 and 400 ml, most preferably between 200 and 350 ml of
this
solvent are used per mol of telmisartan used, according to the invention.
Then the reaction mixture may be heated to speed up the progress of the
reaction.
Preferably, the reaction mixture is heated to a temperature of >40°C,
most preferably
to over 60°C, with thorough mixing. The maximum temperature which may
be
selected is naturally determined by the boiling temperature of the solvents
used. If
the preferred solvents as described hereinbefore are used according to the
invention, the mixture is preferably heated to over 70°C. This heating
is generally
carried out for a period of from 15 minutes to 2 hours, preferably between 20
minutes and one hour. Then the solution obtained is filtered and any solid
remaining
in the filter is washed with one or more of the abovementioned solvents.
The filtrate obtained by the process described above is added slowly,
preferably
dropwise, to an organic solvent which is heated to a temperature of
>40°C,
preferably above 60°C, most preferably to boiling point. The solvent
used is
preferably an organic aprotic solvent, more preferably an organic, aprotic and
non-
2o polar solvent. Solvents which may be used according to the invention are,
most
preferably, toluene, chloroform, dichloromethane, tetrahydrofuran,
diethylether,
diisopropylether, methyl-tert. butylether, acetone, methylisobutylketone,
benzene or
acetonitrile, of which toluene, benzene and methylisobutylketone are
particularly
preferred. The solvent toluene is of exceptional importance according to the
25 invention. At the same time as the filtrate is added to the heated solvent,
in a
preferred embodiment of the invention, some of the solvent is distilled off
(optionally
azeotropically). After all the filtrate has been added, more solvent (e.g.
about one to
two thirds of the total amount of solvent added by this stage) may optionally
be
removed by distillation.
The concentrated solution thus obtained is cooled, preferably to ambient
temperature, whereupon the telmisartan sodium salt crystallises out. After
crystallisation is complete the crystals are separated off, optionally washed
with the
organic solvent mentioned above and finally dried.
In another embodiment of the invention the crystalline telmisartan sodium salt
according to the invention may be obtained starting from the acid addition
salts of
formula 2
CA 02463146 2004-04-07
7
Me
M N I ~ N~Me
/~/\
~ 'N x H-X
O OH
2
wherein H-X denotes an acid selected from among hydrochloric acid, hydrobromic
acid, toluenesulphonic acid or methanesulphonic acid. Of the abovementioned
acid
addition salts of formula 2 the salt wherein H-X denotes hydrochloric acid is
of
particular significance. This acid addition salt is also referred to
hereinafter as
telmisartan hydrochloride.
The compounds of formula 2 may be obtained for example from tert.-butyl 4~-[[2-
n-
propyl-4-methyl-6-( 1-methylbenzim idazol-2-yl)-benzim idazol-1-yl]-m ethyl]-
biphenyl-
~0 2-carboxylate (=tert.-butyl ester of telmisartan) known from the prior art
by
saponification in acetic acid in the presence of the acid H-X.
In order to prepare the crystalline telmisartan sodium salt of formula 1
according to
the invention starting from the acid addition salts of formula 2 the following
procedure
95 may be used, according to the invention.
The compound of formula Z is taken up in a suitable solvent and combined with
a
suitable sodium salt.
The solvent may be water and/or a suitable alcohol, such as methanol, ethanol
or
2o isopropanol mixed with an aprotic organic solvent selected from among
toluene,
chloroform, dichloromethane, tetrahydrofuran, diethylether, diisopropylether,
methyl-
tert. butylether, acetone, methylisobutylketone, benzene and acetonitrile. It
is
particularly preferred to use, as the solvent, water mixed with ethanol or
isopropanol
mixed with an aprotic organic solvent selected from among toluene, benzene and
25 methylisobutylketone, most preferably toluene. A mixture of water,
isopropanol and
toluene has proved particularly suitable for this step of the synthesis.
The amount of solvent or solvent mixture used depends on the amount of acid
addition salt 2 used. Preferably, about 0.3 - 3.5 L, preferably about 1 - 2.5
L, more
3o preferably about 1.5 - 2 L of the abovementioned solvent or solvent mixture
are
used per mol of compound 2 used. If the solvent used is the preferred solvent
mixture according to the invention which contains an alcohol as the third
solvent
component in addition to water and an aprotic organic solvent, the ratios by
volume
CA 02463146 2004-04-07
8
of water to aprotic organic solvent according to the invention are preferably
in a
range from 1:5 to 1:50 and the ratio of water to alcohol used is in a range
from 2:1 to
1:40. Preferably, in a solvent mixture of this kind, the ratios of water to
aprotic
organic solvent are in the range from 1:10 to 1:30, preferably in the range
from 1:15
to 1:25 and the ratio of water to alcohol used is in a range from 1:1 to 1:20,
preferably in the range from 1:5 to 1:15.
Preferably, the solvent or solvent mixture mentioned above contains about 10
to 100
ml of water, preferably about 30 to 80 ml of water, most preferably about 40
to 70 ml
of water, per mol of 2. Preferably the solvent or solvent mixture used also
contains
about 100 to 1000 ml of alcohol, preferably about 300 to 800 ml alcohol, most
preferably about 400 to 700 ml alcohol, per mol of 2. Finally, the solvent or
solvent
mixture used preferably contains as the third component of the solvent, about
200 to
2000 ml of the abovementioned aprotic organic solvent, preferably about 600 to
1600 ml, most preferably about 800 to 1400 ml of the abovementioned aprotic
organic solvent, per mol of 2.
Suitable sodium salts which may be used for reacting 2 to 1 include sodium
hydroxide, sodium hydride, sodium carbonate, sodium hydrogen carbonate or
sodium alkoxides. By sodium alkoxides are meant the sodium salts which are
formed
2o with lower alcohols, preferably with alcohols selected from among methanol,
ethanol, isopropanol, n-propanol, tert-butanol, sec.-butanol, isobutanol, n-
butanol
and tert.-amylalcohol. Of particular interest according to the invention are
sodium
salts selected from among sodium hydroxide, sodium hydride, sodium ethoxide
and
sodium methoxide, while the sodium alkoxides sodium ethoxide and sodium
25 methoxide, particularly sodium methoxide are of particular importance
according to
the invention for this reaction step. The abovementioned sodium salts may be
added to the reaction mixture as solids. In the case of sodium methoxide
however it
is preferable to add it in the form of a methanolic solution. Methanolic
solutions of
sodium methoxide which contain it in a concentration of at least 10%, most
3o preferably about 20-40 % (w/w) , are particularly preferred. For example,
the
methanolic sodium methoxide solution used may have a concentration of about 30
wt. %.
The amount of sodium salt to be used is naturally dependent on the amount of
free
acid telmisartan used. According to the invention, at least 2 mol of sodium
salt have
35 to be added per mol of telmisartan acid addition salt of formula 2 used.
According to
the invention it is also possible to add an excess of sodium salt.
CA 02463146 2004-04-07
9
It may be useful in some cases to add activated charcoal to the abovementioned
reaction mixture. For example, it may be added in an amount of about 5 - 50 g
per
mol of 2 used, preferably in an amount of about 10 - 40 g per mol of 2 used.
After the sodium salt and optionally the activated charcoal has been added the
reaction mixture obtained is heated to a temperature of about 50-100°C,
preferably
about 60-90°C, most preferably about 70-80°C for a period of
about 10 minutes to 2
hours, preferably for about 20-45 minutes. In the course of this heating, some
of the
solvent, preferably about 10-50%, most preferably about 20-40% of the total
~o quantity of solvent may be distilled off.
The remaining suspension is then filtered, the filter residue is optionally
washed with
one of the abovementioned aprotic organic solvents, preferably with the
aprotic
organic solvent which is also used in the reaction.
~5 The filtrate obtained is then diluted with a solvent or mixture of
solvents. It is
preferable to use a mixture of water and the abovementioned aprotic organic
solvent for this. Preferably, at this point, about 10 to 100 ml of water,
preferably
about 30 to 80 ml of water, most preferably about 40 to 70 ml of water are
used per
mol of the compound 2 originally used. At this point, 250 to 3000 ml,
preferably about
20 800 to 2000 ml, most preferably about 1200 to 1800 ml of aprotic organic
solvent are
used per mole of the compound 2 originally used.
After dilution, the mixture obtained is refluxed. Then about 1-2 L, preferably
about
1200 to 1800 ml of solvent are distilled off per mole of the compound 2
originally
25 used. After the solvent has been distilled off the telmisartan-sodium salt
1 according
to the invention crystallises out. The crystals obtained are isolated,
optionally
washed with one of the abovementioned aprotic organic solvents and finally
dried.
In another aspect the present invention relates to crystalline telmisartan-
sodium salt,
30 optionally in the form of the solvates or hydrates thereof, preferably in
the form of
the hydrates thereof, most preferably in the form of the hemihydrate, which
may be
obtained by the methods described above.
Because of the central significance of the compounds of formula 2 as valuable
ss starting materials for the direct synthesis of the telmisartan-sodium salt
1 according
to the invention, in another aspect the present invention relates to compounds
of
formula 2 per se
CA 02463146 2004-04-07
Me
Me
N I /
/ \ ~ '~' ~H x H-X
2
wherein H-X denotes an acid selected from among hydrochloric acid,
hydrobromic acid, toluenesulphonic acid or methanesulphonic acid. The compound
of formula 2 wherein H-X denotes hydrogen chloride, the telmisartan
hydrochloride,
s is particularly preferred.
Most preferably, the present invention further relates to the abovementioned
compounds of formula 2 in crystalline form.
~o Moreover, in view of the pharmaceutical activity of the crystalline
telmisartan sodium
salt according to the invention, the present invention relates to the use
thereof as a
pharmaceutical composition.
In another aspect, in view of the pharmaceutical activity of the crystalline
telmisartan
sodium salt according to the invention, the present invention relates to the
use
thereof for preparing a pharmaceutical composition, particularly for preparing
a
pharmaceutical composition for the prevention or treatment of diseases wherein
the
administration of therapeutically effective doses of one or more angiotensin-
II-
antagonists may provide a therapeutic benefit. Preferably, the present
invention
2o relates to the use of crystalline telmisartan-sodium salt for preparing a
pharmaceutical composition for the prevention or treatment of diseases
selected
from among hypertension, cardiac insufficiency, ischaemic peripheral
circulatory
disorders, myocardial ischaemia (angina), the progression of cardiac
insufficiency
after myocardial infarct, diabetic neuropathy, glaucoma, gastrointestinal
diseases
25 and bladder diseases, the prevention or treatment of hypertension being
particularly
preferred.
Accordingly, in another aspect, the present invention is directed to
pharmaceutical
formulations characterised in that they contain crystalline telmisartan-sodium
salt.
The example of synthesis that follows serves to illustrate a method of
preparing
crystalline telmisartan-sodium salt carried out by way of example. It is
intended
~
CA 02463146 2004-04-07
11
solely as a possible procedure provided by way of example, without restricting
the
invention to its contents.
Synthesis Example 1: Preparation of crystalline telmisartan-sodium salt
starting
from telmisartan:
The starting material used to prepare crystalline telmisartan-sodium salt
according to
the invention may be the free acid, which may be obtained by methods known
from
the prior art (e.g. according to EP 502314 A1 ).
154.4 g of telmisartan are placed in 308.8 ml of toluene in a suitable
reaction vessel.
The suspension is combined with 27.8 g of 44.68% sodium hydroxide solution and
84.9 ml of ethanol and heated to 78°C for about 30 min, then the
mixture is filtered.
If desired, if large amounts of solid are left in the filter, this may be
washed with a
mixture of 61.8 ml of toluene and 15.3 ml of ethanol.
~5 463.2 ml of toluene are placed in another reaction vessel and refluxed. The
filtrate
obtained by the process described above is slowly added dropwise thereto at
boiling
temperature and simultaneously distilled off azeotropically. After it has all
been
added the solution which may have been obtained from washing the filter is
also
added and again distilled off azeotropically. The mixture is distilled at up
to 103°C
2o and the suspension is allowed to cool to ambient temperature. The crystals
are
suction filtered, washed with 154.4 ml of toluene and dried at 60°C in
the circulating
air drier.
Yield: 154.6 g (96%) of colourless crystals;
C33HZ9N402Na x 0,5H20 calc.: C 72.51 H 5.72 N 10.25
found: C 72.57 H 5.69 N 10.21
Synthesis Example 2: Preparation of crystalline teimisartan-sodium salt
starting
so from telmisartan hydrochloride:
A) Preparation of telmisartan-hydrochloride:
411 g of tert.-butyl 4'-[[2-n-propyl-4-methyl-6-(1-methylbenzimidazol-2-yl)-
benzimidazol-1-yl]-methyl]-biphenyl-2-carboxylate are suspended in 822 ml of
glacial
s5 acetic acid and combined with 213 g of concentrated aqueous hydrochloric
acid
(37%). The mixture is refluxed and about 640 ml of solvent are distilled off.
The
residue remaining is slowly combined with about 620 ml of water at 50-
60°C. To this
mixture are added 20 g of activated charcoal (e.g. Norit SX 2 Ultra) and the
resulting
mixture is stirred for about 10 min at constant temperature. After filtering,
the residue
CA 02463146 2004-04-07
12
is washed three times with 25 ml of glacial acetic acid and about 620 ml of
water.
The filtrate obtained is again heated to about 50-60°C and about 2 L of
water are
added. After stirring for about 12 hours at about 23°C the crystals
formed are suction
filtered and washed twice with about 500 ml of water, once with about 900 ml
of
acetone and then dried at about 60°C.
Yield: 367 g (92.5%), colourless crystals, melting point: = 278°C
Bl Preparation of crystalline telmisartan sodium salt from telmisartan
hydrochloride
~0 55.1 g of telmisartan hydrochloride are taken up in 110.2 ml of toluene,
5.5 ml of
water, 55.1 ml of isopropanol and this mixture is combined with 36.9 g of
sodium
methoxide (30% in methanol) and 2.75 g of activated charcoal (e.g. Sorit SX 2
Ultra).
The mixture is then heated to about 75°C, and about 50 ml of solvent
mixture are
distilled off at constant temperature over about 30 min. The suspension
obtained is
~5 filtered and the residue is washed with about 20 ml of toluene. The
filtrate is
combined with about 5 ml of water and about 150 ml of toluene. The mixture
obtained is refluxed. During this time about 150 ml of solvent mixture are
azeotropically distilled off (at up to 102°C). The mixture is left to
crystallise for one
hour at 100°C. The crystals are suction filtered, washed with about 50
ml of toluene
2o and dried at about 60°C.
Yield: 53.6 g (99%), colourless crystals ;
C33HZ9N402Na~0.5H20 calc.: C 72.51 H 5.72 N 10.25
found: C 72.44 H 5.68 N 10.20
To prepare a pharmaceutical composition containing the active substance,
particularly an orally administered pharmaceutical composition, most
preferably a
tablet, procedures known in the art may be used.
3o Suitable tablets may be obtained, for example, by mixing the active
substances)
with known excipients, for example inert diluents such as calcium carbonate,
calcium
phosphate or lactose, disintegrants such as maize starch or alginic acid,
binders
such as starch or gelatine, lubricants such as magnesium stearate or talc
and/or
agents for delaying release, such as carboxymethyl cellulose, cellulose
acetate
phthalate, or polyvinyl acetate. The tablets may also comprise several layers.
The following are some examples of pharmaceutical preparations which may be
used according to the invention. They are intended purely as illustrations by
way of
example without restricting the subject matter of the invention thereto.
CA 02463146 2004-04-07
13
Tablet 1:
Ingredients: mg
I elmisartan-sodium salt hemihydrate 1.00
Mannitol 121.50
Maize starch 79.85
Highly dispersed silicon dioxide, anhydrous 2.30
Polyvidon K25 2.35
~o Magnesium stearate 3.00
Total 210.00
Tablet 2:
~5 Ingredients: mg
Telmisartan-sodium salt hemihydrate 0.5
Mannitol 122.0
Maize starch. dried 61.8
2o Maize starch 18.0
Highly dispersed silicon dioxide, 2.4
anhydrous
Polyvidon K25 2.3
Magnesium stearate 3.0
Total 210.0
Tablet 3:
Ingredients: mg
Telmisartan-sodium salt hemihydrate 0.25
Mannitol 61.00
Maize starch 39.90
Highly dispersed silicon dioxide, anhydrous 1.20
Polyvidon K25 1.15
Magnesium stearate 1.5
Total 105.00