Note: Descriptions are shown in the official language in which they were submitted.
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
1
COMPOUND
FIELD OF INVENTION
The present invention relates to a compound.
In particular the present invention relates to a compound and to a
pharmaceutical
composition comprising the compound. The present invention also relates to the
use of
the compound or composition in therapy applications.
BACKGROUND TO THE INVENTION
Evidence suggests that oestrogens are the major mitogens involved in promoting
the
growth of tumours in endocrine-dependent tissues, such as the breast and
endometrium. Although plasma oestrogen concentrations are similar in women
with or
without breast cancer, breast tumour oestrone and oestradiol levels are
significantly
higher than in normal breast tissue or blood. In situ synthesis of oestrogen
is thought to
make an important contribution to the high levels of oestrogens in tumours and
therefore
inhibitors, in particular specific inhibitors, of oestrogen biosynthesis are
of potential value
for the treatment of endocrine-dependent tumours.
Over the past two decades, there has been considerable interest in the
development of
inhibitors of the aromatase pathway - which converts the androgen precursor
androstenedione to oestrone. However, there is now evidence that the oestrone
sulphatase (El-STS) pathway, i.e. the hydrolysis of oestrone sulphate to
oestrone (E1S
to El), and aromatase (i.e. conversion of androstenedione to oestrone) account
for the
production of oestrogens in breast tumours.
Figures 1 and 2 are schematic diagrams showing some of the enzymes involved in
the
in situ synthesis of oestrone from oestrone sulphate, oestradiol and
androstenedione.
In Figure 2, which schematically shows the origin of oestrogenic steroids in
postmenopausal women, "ER" denotes Oestrogen Receptor, "DHA-S" denotes
Dehydroepiandrosterone-Sulphate, "Adiol" denotes Androstenediol, "E1-STS"
denotes
Oestrone Sulphatase, "DHA-STS" denotes DHA-sulphatase, "Adiol-STS" denotes
Adiol
CA 02464770 2009-11-27
WO 03/045925 PCTIGBO2105214
2
Sulphatase, and "17B-HSD" denotes Oestradiol 17B-hydroxysteroid dehydrogenase.
As can be seen, the main two enzymes that are involved in the peripheral
synthesis of
oestrogens are the aromatase enzyme and the enzyme oestrone suiphatase.
In short, the aromatase enzyme converts androstenedione, which is secreted in
large
amounts by the adrenal cortex, to oestrone. Recent reports have suggested that
some
flavones could inhibit aromatase activity.
Much of the oestrone so formed, however, is converted to oestrone sulphate
(EIS) and
there is now a considerable body of evidence showing that EIS in plasma and
tissue
acts as a reservoir for the formation of oestrone by the action of oestrone
sulphatase.
In this regard, it is now believed that the oestrone suiphatase (E1-STS)
pathway - i.e.
the hydrolysis of oestrone sulphate to oestrone (EIS to E1) is a major source
of
oestrogen in breast tumours. This theory is supported by a modest reduction of
plasma
oestrogen concentration in postmenopausal women with breast cancer treated by
aromatase inhibitors, such as aminoglutethimide and 4-hydroxyandrostenedione
and
also by the fact that plasma El S concentration in these aromatase inhibitor-
treated
patients remains relatively high. The long half-life of EIS in blood (10-12 h)
compared
with the unconjugated oestrogens (20 min) and high levels of steroid
suiphatase activity
in liver and, normal and malignant breast tissues, also lend support to this
theory.
Thus, oestrogen formation in malignant breast and endometrial tissues via the
sulphatase pathway makes a major contribution to the high concentration of
oestrogens
which are present in these tumours. However, inhibition of both the aromatase
and
suiphatase pathways could offer considerable therapeutic benefit.
CA2114630 teaches novel steroid suiphatase inhibitors and pharmaceutical
compositions containing them for use in the treatment of oestrone dependent
tumours,
especially breast cancer. These steroid sulphatase inhibitors are sulphamate
esters,
such as N,N-dimethyl oestrone-3-sulphamate and, preferably, oestrone-3-
sulphamate
(otherwise known as "EMATE"). EMATE has the following structure:
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
3
0
H 0
H \S"0
0
It is known that EMATE is a potent E1-STS inhibitor as it displays more than
99%
inhibition of E1-STS activity in intact MCF-7 cells at 0.1 nM. EMATE also
inhibits the
E1-STS enzyme in a time- and concentration-dependent manner, indicating that
it acts
as an active site-directed inactivator. Although EMATE was originally designed
for the
inhibition of E1-STS, it also inhibits dehydroepiandrosterone sulphatase (DHA-
STS),
which is an enzyme that is believed to have a pivotal role in regulating the
biosynthesis
of the oestrogenic steroid androstenediol. Also, there is now evidence to
suggest that
androstenediol may be of even greater importance as a promoter of breast
tumour
growth. EMATE is also active in vivo as almost complete inhibition of rat
liver E1-STS
(99%) and DHA-STS (99%) activities resulted when it is administered either
orally or
subcutaneously. In addition, EMATE has been shown to have a memory enhancing
effect in rats. Studies in mice have suggested an association between DHA-STS
activity
and the regulation of part of the immune response. It is thought that this may
also occur
in humans. The bridging O-atom of the sulphamate moiety in EMATE is important
for
inhibitory activity. Thus, when the 3-0-atom is replaced by other heteroatoms
as in
oestrone-3-N-sulphamate and oestrone-3-S-sulphamate, these analogues are
weaker
non-time-dependent inactivators.
In addition to oestrone, the other major steroid with oestrogenic properties
which is
produced by postmenopausal women is androstenediol (see Figure 2).
Androstenediol, although an androgen, can bind to the oestrogen receptor (ER)
and can
stimulate the growth of ER positive breast cancer cells and the growth of
carcinogen-
induced mammary tumours in the rat. Importantly, in postmenopausal women 90%
of
the androstenediol produced originates from the androgen
dehydroepiandrosterone
sulphate (DHA-S) which is secreted in large amounts by the adrenal cortex. DHA-
S is
converted to DHA by DHA sulphatase, which may be the same as, or different
from, the
enzyme, oestrone sulphatase, which is responsible for the hydrolysis of E1 S.
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
4
During the last 10-15 years considerable research has also been carried out to
develop
potent aromatase inhibitors, some of which are now marketed. However, in three
recent
reports of postmenopausal women with breast cancer who received aromatase
inhibitor
therapy, plasma EIS concentrations remained between 400-1000 pg/ml.
In summation therefore in situ synthesis of oestrogen is thought to make an
important
contribution to the high levels of oestrogens in tumours and therefore
specific inhibitors
of oestrogen biosynthesis are of potential value for the treatment of
endocrine-
dependent tumours.
Moreover, even though oestrogen formation in malignant breast and endometrial
tissues
via the sulphatase pathway makes a major contribution to the high
concentration of
oestrogens, there are still other enzymatic pathways that contribute to in
vivo synthesis
of oestrogen.
The present invention seeks to provide novel compounds suitable for the
inhibition of
steroid sulphatase activity and aromatase activity.
SUMMARY ASPECTS OF THE PRESENT INVENTION
The present invention is based on the surprising finding that certain
polycyclic
compounds could be used as effective steroid sulphatase inhibitors and/or
aromatase
inhibitors and/or as agents that can influence cell cycling and/or as agents
that can
influence apoptosis.
In one aspect, the present invention is based on the surprising finding that
certain
polycyclic compounds could be used as effective steroid sulphatase inhibitors
and/or
aromatase inhibitors and/or as modulators of cell cycling and/or as modulators
of
apoptosis.
The polycyclic compounds comprise at least a central trivalent atom to which
is attached
either direct or indirectly via a linker at least two or three ring systems.
At least one of
the ring systems comprises a sulphamae group as a further substituent on the
ring
system.
CA 02464770 2010-09-10
The compounds of the present invention may comprise other substituents. These
other substituents may, for example, furthe (increase the activity of the
comopunds of
the present invention and/or increase stability (ex vivo and/or in vivo).
BRIEF DESCRIPTION OF THE FIGURES
Figure 1 is a schematic showing key enzymes involved in steroidogenesis;
Figure 2 is a schematic showing the origin of oestrogenic steroids in
postmenopausal
women;
Figure 3 graphically illustrates the effect of aromatase-sulphatase inhibitors
on
plasma oestradiol; and
Figure 4 graphically illustrates the effect of aromatase-sulphatase
inhibitiors
on sulphatase activity.
DETAILED ASPECTS OF THE PRESENT INVENTION
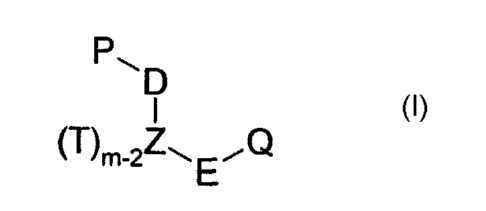
According to one aspect of the present invention, there is provided a compound
of
'Formula I
Pip Formula I
(T) m-2/--I E "U
wherein each T is independently selected from H, hydrocarbyl, -F-R, and a bond
with
one of D, E, P or Q, or together with one of P and Q forms a ring; Z is a
suitable atom
the valency of which is m; D, E and F are each independently of each other an
optional
linker group, wherein when Z is nitrogen E is other than CH2 and C=O; P, Q and
R are
independently of each other a ring system; and at least Q comprises a
sulphamate
group.
According to one aspect of the present invention, there is provided a method
comprising
(a) performing a steroid sulphatase (STS) assay and/or aromatase assay with
one or
more candidate compounds defined herein; (b) determining whether one or more
of said
candidate compounds is/are capable of modulating STS activity and/or aromatase
activity and/or cell cycling and/or cell growth and/or apoptosis; and (c)
selecting one or
more of said candidate compounds that is/are capable of modulating STS
activity and/or
aromatase activity and/or cell cycling and/or cell growth and/or apoptosis.
CA 02464770 2010-09-10
5a
According to one aspect of the present invention, there is provided a method
comprising
(a) performing a steroid suiphatase assay and/or aromatase assay with one or
more
candidate compounds as defined herein; (b) determining whether one or more of
said
candidate compounds is/are capable of inhibiting STS and/or aromatase
activity; and (c)
selecting one or more of said candidate compounds that is/are capable of
inhibiting STS
activity and/or aromatase activity and/or cell cycling and/or cell growth
and/or apoptosis.
In any one of the methods of the present invention, one or more additional
steps may be
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
6
present. For example, the method may also include the step of modifying the
identified
candidate compound (such as by chemical and/or enzymatic techniques) and the
optional additional step of testing that modified compound for STS inhibition
effects
(which may be to see if the effect is greater or different) and/or aromatase
inhibition
effects (which may be to see if the effect is greater or different). By way of
further
example, the method may also include the step of determining the structure
(such as by
use of crystallographic techniques) of the identified candidate compound and
then
performing computer modelling studies - such as to further increase its STS
and/or
aromatase inhibitory action. Thus, the present invention also encompasses a
computer
having a dataset (such as the crystallographic co-ordinates) for said
identified candidate
compound. The present invention also encompasses that identified candidate
compound when presented on a computer screen for the analysis thereof - such
as
enzyme and/or protein binding studies.
According to one aspect of the present invention, there is provided a compound
identified by the method of the present invention.
According to one aspect of the present invention, there is provided a compound
according to the present invention for use in medicine.
According to one aspect of the present invention, there is provided a
pharmaceutical
composition comprising the compound according to the present invention
optionally
admixed with a pharmaceutically acceptable carrier, diluent, excipient or
adjuvant.
According to one aspect of the present invention, there is provided the use of
a
compound according to the present invention in the manufacture of a medicament
for
use in the therapy of a condition or disease associated with STS and/or
aromatase
and/or cell cycling and/or apoptosis and/or cell growth.
According to one aspect of the present invention, there is provided the use of
a
compound according to the present invention in the manufacture of a medicament
for
use in the therapy of a condition or disease associated with adverse STS
levels and/or
adverse aromatase levels and/or cell cycling and/or apoptosis and/or cell
growth.
According to one aspect of the present invention, there is provided the use of
a
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
7
compound according to the present invention in the manufacture of a medicament
for
inhibiting STS activity and/or inhibiting aromatase activity.
According to one aspect of the present invention, there is provided the use of
a
compound according to the present invention in the manufacture of a medicament
for
inhibiting STS activity and inhibiting aromatase activity.
According to one aspect of the present invention, there is provided the use of
a
compound in the manufacture of a medicament for use in the therapy of a
condition or
disease- associated with -aromatase and-optionally associated with STS,
wherein the
compound is of Formula I
Pip Formula I
(T)m-2Z\E"Q
wherein each T is independently selected from H, hydrocarbyl, -F-R, and a bond
with
one of D, E, P or Q, or together with one of P and Q forms a ring; Z is a
suitable atom
the valency of which is m; D, E and F are each independently of each other an
optional
linker group, P, Q and R are independently of each other a ring system; and at
least on
of P, Q and R comprises a sulphamate group.
According to one aspect of the present invention, there is provided the use of
a
compound in the manufacture of a medicament for use in the therapy of a
condition or
disease associated with adverse aromatase levels and optionally associated
with
adverse STS levels, wherein the compound is of Formula I
-P~D- Formula I
I
)m-2/-,, E "Q
wherein each T is independently selected from H, hydrocarbyl, -F-R, and a bond
with
one of D, E, P or Q, or together with one of P and Q forms a ring; Z is a
suitable atom
the valency of which is m; D, E and F are each independently of each other an
optional
linker group, P, Q and R are independently of each other a ring system; and at
least on
of P, Q and R comprises a sulphamate group.
According to one aspect of the present invention, there is provided the use of
a
compound in the manufacture of a medicament for inhibiting aromatase activity
and
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
8
optionally for inhibiting STS activity, wherein the compound is of Formula I
Pip Formula I
I
)m-21-,, E "Q
wherein each T is independently selected from H, hydrocarbyl, -F-R, and a bond
with
one of D, E, P or Q, or together with one of P and Q forms a ring; Z is a
suitable atom
the valency of which is m; D, E and F are each independently of each other an
optional
linker group, P, Q and R are independently of each other a ring system; and at
least on
.of P, Q and R comprises a sulphamate group.
The present invention also encompasses the novel compounds of the present
invention
(such as those presented herein), as well as processes for making same (such
as the
processes presented herein) as well as novel intermediates (such as those
presented
herein) for use in those processes.
For ease of reference, these and further aspects of the present invention are
now
discussed under appropriate section headings. However, the teachings under
each
section are not necessarily limited to each particular section.
SOME ADVANTAGES
One key advantage of the present invention is that the compounds of the
present
invention can act as STS inhibitors.
One key advantage of the present invention is that the compounds of the
present
invention can act as aromatase inhibitors.
One key advantage of the present invention is that the compounds of the
present
invention can act as STS inhibitors and aromatase inhibitors.
Another advantage of the compounds of the present invention is that they may
be potent
in vivo.
Some of the compounds of the present invention may be non-oestrogenic
compounds.
Here, the term "non-oestrogenic" means exhibiting no or substantially no
oestrogenic
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
9
activity. Here, by the term "non-oestrogenic" means exhibiting no or
substantially no
systemic oestrogenic activity, such as that determined by Protocol 4.
Another advantage is that some of the compounds may not be capable of being
metabolised to compounds which display or induce hormonal activity.
Some of the compounds of the present invention are also advantageous in that
they
may be orally active.
Some-of-the-compounds of-the-present--invention-may useful for-the prevention
and/or
treatment of cancer, such as breast cancer, as well as (or in the alternative)
non-
malignant conditions, such as the prevention and/or treatment of inflammatory
conditions - such as conditions associated with any one or more of:
autoimmunity,
including for example, rheumatoid arthritis, type I and II diabetes, systemic
lupus
erythematosus, multiple sclerosis, myasthenia gravis, thyroiditis, vasculitis,
ulcerative
colitis and Crohn's disease, skin disorders e.g. acne, psoriasis and contact
dermatitis;
graft versus host disease; eczema; asthma and organ rejection following
transplantation.
The compounds of the present invention are useful particularly when
pharmaceuticals
may need to be administered from an early age.
Thus, some of the compounds of the present invention are also believed to have
therapeutic uses other than for the treatment of endocrine-dependent cancers,
such as
the treatment of autoimmune diseases.
The compounds of the present invention may also be useful as an inducer of
apoptosis.
The compounds of the present invention may also be useful as a cell growth
inhibitors.
PREFERABLE ASPECTS
Z
As will be clear from Formula I, Z may be any atom which is capable of forming
a bond
or link with the optional linker groups D and/or E or ring systems P and/or Q
and which
is capable of forming a bond or link with the or each T. The valency of Z is
denoted as
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
M.
In one aspect Z is trivalent i.e. m=3.
5 In a preferred aspect Z(T)m_2 is selected from Z(-F-R), ZH and Z-hydrocarbyl
Suitable and preferred trivalent atoms include nitrogen (N), phosphorus (P)
and boron
(B). Preferably Z is Nitrogen.
10 In a preferred aspect Z(T)m_2 is selected from N(-F-R), NH and N-
hydrocarbyl
In one aspect Z is tetravalent i.e. m=4.
In a preferred aspect Z(T)m_2 is selected from ZH(-F-R), Z(-F-R)(-F-R), Z(-F-
R)(hydrocarbyl), ZH2i ZH-hydrocarbyl and Z(hydrocarbyl)(hydrocarbyl)
In a preferred aspect Z(T)m_2 is selected from ZH(-F-R), ZH2 and ZH-
hydrocarbyl
Suitable and preferred tetravalent atoms include carbon (C) and silicon (Si).
Preferably Z
is C. Thus preferably Z(T)m_2 is selected from CH(-F-R), CH2 and CH-
hydrocarbyl
T
Each T of Z(T)m_2 is independently selected from H, hydrocarbyl, a bond with
one of D,
--E, P -or Q-and -F=R, or together, with one-of P and Q forms a ring"
In one aspect each T of Z(T)m_2 is independently selected from H, hydrocarbyl
and -F-R.
When T is a hydrocarbyl group it may be selected from
= Cl-Clo hydrocarbyl,
= C1-C5 hydrocarbyl
= C1-C3 hydrocarbyl.
= hydrocarbon groups
= C,-C,o hydrocarbon
= C1-C5 hydrocarbon
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
11
= C,-C3 hydrocarbon.
= alkyl groups
= CI-Clo alkyl
= C1-C5 alkyl
= C1-C3 alkyl.
The hydrocarbyl/hydrocarbon/alkyl of T may be straight chain or branched
and/or may
be saturated or unsaturated.
to -The hydrocarbyl/hydrocarbon/alkyl of T may be straight or branched
hydrocarbon
groups containing at least one hetero atom in the group.
I Optional Linker - D, E and F
Independently of each other linker groups D, E and F may or may not be
present. If
present D, E or F may be selected from C=O and hydrocarbyl groups.
The term "hydrocarbyl group" as used herein means a group comprising at least
C and
H and may optionally comprise one or more other suitable substituents.
Examples of
such substituents may include halo, alkoxy, nitro, an alkyl group, a cyclic
group etc. In
addition to the possibility of the substituents being a cyclic group, a
combination of
substituents may form a cyclic group. If the hydrocarbyl group comprises more
than one
C then those carbons need not necessarily be linked to each other. For
example, at
least two of the carbons may be linked via a suitable element or group. Thus,
the
hydrocarbyl group may contain hetero atoms. Suitable hetero atoms will be
apparent to
those skilled in the art and include, for instance, sulphur, nitrogen and
oxygen. A non-
limiting example of a hydrocarbyl group is an acyl group.
A typical hydrocarbyl group is a hydrocarbon group. Here the term
"hydrocarbon"
means any one of an alkyl group, an alkenyl group, an alkynyl group, which
groups may
be linear, branched or cyclic, or an aryl group. The term hydrocarbon also
includes
those groups but wherein they have been optionally substituted. If the
hydrocarbon is a
branched structure having substituent(s) thereon, then the substitution may be
on either
the hydrocarbon backbone or on the branch; alternatively the substitutions may
be on
the hydrocarbon backbone and on the branch.
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
12
Preferably D, E and F are independently selected from C1-C10 hydrocarbyl, C1-
C5
hydrocarbyl or C1-C3 hydrocarbyl.
Preferably D, E and F are independently selected from hydrocarbon groups,
preferably
C1-C10 hydrocarbon, C1-C5 hydrocarbon or C1-C3 hydrocarbon.
Preferably D, E and F are independently selected from alkyl groups, C1-C10
alkyl, C1-C5
'Olkyl or C1-C3 alkyl.
The hydrocarbyl/hydrocarbon/alkyl of D, E and F may be straight chain or
branched
and/or may be saturated or unsaturated.
In one preferred aspect D, E and F are independently selected from straight or
branched
hydrocarbon groups containing at least one hetero atom in the group.
In a preferred aspect D, E and F are independently selected from hydrocarbon
groups
and a group of the formula
Y
CH
2)n
wherein n is I to 6 and Y = O, S or CH2.
In a preferred aspect D, E and F are independently selected linear or branched
hydrocarbon -groups-having-a-carbon chain of from 1-to 6 carbon atoms and a
group of
the formula
Y
""K
CH2)n
wherein n is 1 to 6 and Y = 0, S or CH2
In one preferred aspect only one of optional linker groups D, E and F is
present. It will be
understood that by only one it is meant that one of the linkers is present and
the other
optional linker group(s) is/are not present.
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
13
In one preferred aspect E (and preferably D and/or F) is selected from
hydrocarbyl
groups comprising at least two carbons or wherein the total number of carbons
and
hetero atoms is at least two.
In one preferred aspect E (and preferably D and/or F) is selected from
hydrocarbyl
groups containing at least one hetero atom in the group. Preferably the hetero
atom is
selected from sulphur, nitrogen and oxygen.
In one preferred aspect E (and preferably D and/or F) is selected from
straight or
(ranched hydrocarbon groups containing at least one hetero atom in the group.
Preferably the hetero atom is selected from sulphur, nitrogen and oxygen.
In one preferred aspect E (and preferably D and/or F) is selected from
hydrocarbon
groups comprising at least 2 carbons and a group of the formula
Y
A(CH
2>n
wherein n is 1 to 6 and Y = Oxygen, Sulphur or CH2.
In one preferred aspect E (and preferably D and/or F) is selected from linear
or
branched hydrocarbon groups having a carbon chain of from 2 to 6 carbon atoms
and a
group of the formula
Y
A(CH2)n-
wherein n is 1 to 6 and Y = Oxygen, Sulphur or CH2.
In one preferred aspect E (and preferably D and/or F) is selected from
straight or
branched alkyl groups, preferably C1-1o alkyl, more preferably C1_5 alkyl,
containing at
least one hetero atom in the group. Preferably the hetero atom is selected
from sulphur,
nitrogen and oxygen.
In one preferred aspect E (and preferably D and/or F) is selected from
straight chain
alkyl groups, preferably C1_10 alkyl, more preferably C1.5 alkyl, containing
at least one
hetero atom in the group. Preferably the hetero atom is selected from sulphur,
nitrogen
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
14
and oxygen.
When E (or D and/or F) contains a hetero atom, preferably the hetero atom is
attached
to the ring Q (or D or R in the case of D and F).
In a highly preferred aspect the compound of the present invention is of the
formula
P" or P"
I I
(T)m-2Z, 0" (T)m-2Z\
(CH2)1-10 Q (CH2)1-10 Q
In a highly preferred aspect the compound of the present invention is of the
formula
P., D
(T)m-2Z~ --
(CH Q
2)2-10
Rings - P, Q and R
The present compound comprises two or three ring systems each of which is
attached
directly or indirectly via linker D, E or F to Z. For each of P, Q and R the
ring system
need not be a cyclic structure. In this regard, the ring system may be a
linear structure
that may have the ability to conform to a ring like structure when in in vivo.
However in
preferred aspects each ring system is a cyclic structure.
In a preferred aspect, P, Q and R are independently selected from cyclic
groups.
At least one of the cyclic groups P, Q and R may be a heterocyclic group (a
heterocycle) or
a non-heterocyclic group. Suitable hetero atoms of a heterocyclic group
include N, S and
0.
At least one of the cyclic groups P, Q and R may be ring systems comprising
carbon and
optionally one or more hetero atoms.
In a preferred aspect at least one of P, Q and R is, or P, Q and R are
independently
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
selected from a ring system comprising carbon and optionally one, two or three
hetero
atoms. Preferably at least one of P, Q and R is, or P, Q and R are
independently
selected from a ring system comprising carbon and one or more hetero atoms.
5 When hetero atoms are present in a ring system to provide a heterocyclic
group, the
hetero atoms may be present in any amount. In one preferred aspect at least
one of P,
Q and R is, or P, Q and R are independently selected from, a ring system
comprising
carbon and one or more hetero atoms selected from N, S and 0.
10 -When one of P,-Q and R is a heterocyclic group the other of P, Q and R may
or may not
be heterocyclic groups. In a preferred aspect one of P, Q and R is a ring
system
comprising carbon and one or more hetero atoms and the other of P, Q and R are
independently carbocyclic ring systems. It will be understood that by
carbocyclic it is
meant a ring system in which the ring contains only carbon atoms together with
optional
15 substituents on the ring. In this aspect preferably one of P, Q and R is a
ring system
comprising carbon and one or more hetero atoms selected from N, S and 0 and
two of
P, Q and R are independently carbocyclic ring systems.
In one aspect of the invention at least one of P, Q and R, or -P, Q and R are
independently selected a saturated ring structure or an unsaturated ring
structure (such
as an aryl group).
In one aspect of the invention at least one of P, Q and R, or P, Q and R are
independently selected a saturated ring structure such a cycloalkyl group.
Preferably, at least one P, Q and R is an aryl ring.
In one aspect of the invention at least one of P, Q and R, or P, Q and R are
independently selected from substituted or unsubstituted aromatic rings.
In one aspect of the invention at least one of P, Q and R is or comprises a
substituted or
unsubstituted aromatic ring.
In one aspect one of P, Q or R may be a polycyclic group, which need not be a
fused
polycycle. The term "polycyclic" includes fused and non-fused ring structures
including
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
16
combinations thereof. If the ring system of P, Q or R is polycyclic some or
all of the ring
components of the ring system may be fused together or joined via one or more
suitable
spacer groups.
The ring size of any of P, Q and R may be chosen by one skilled in the art to
achieve
compounds having desired activity. Typically P, Q and R are independently ring
systems
comprising from 3 to 10 members, such as ring systems comprising from 5, 6 or
7
members.
Heterocyclic ring systems for use in the present invention include imidazole,
tetrazole,
pyrazole, triazole, such as 1H-1,2,3-triazole, 1H-1,2,4-triazole, 4H-1,2,4-
triazole;
optionally substituted 5- or 6- membered heterocyclic group containing 1 to 3
hetero
atoms each selected from N, 0 and S, optionally substituted aryl (monocyclic
or
polycyclic aromatic), pyridazine, pyrimidine, triazine such as 1,3,5 triazine,
and
1s optionally substituted bicyclic condensed heterocyclic group consisting of
the above
heterocyclic group condensed with benzene.
In a highly preferred aspect at least one of P, Q and R is, or P, Q and R are
independently selected from triazole, in particular 1H-1,2,3-triazole, 1H-
1,2,4-triazole,
4H-1,2,4-triazole
In a highly preferred aspect at least one of P, Q and R is 4H-1,2,4-triazole.
In a highly preferred aspect at least one of P, Q and R is triazole, in
particular 1H-1,2,3-
triazole, 1H-1,2,4-triazole, 4H-1,2,4-triazole and the other of P, Q and R are
substituted
or unsubstituted benzyl rings.
In a highly preferred aspect at least one of P, Q and R is 4H-1,2,4-triazole
and the other
of P, Q and R are substituted or unsubstituted benzyl rings.
In the above aspects the triazole may be linked to X via a C in the triazole
ring or a N in
the triazole ring. In one aspect the triazole is linked to X via a C in the
triazole ring.
In a preferred aspect P is a ring system comprising carbon and one or more
hetero
atoms and Q and R, if present, are independently carbocyclic ring systems.
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
17
In a preferred aspect P is a ring system comprising carbon and one or more
hetero
atoms selected from N, S and 0 and Q and R, if present, are independently
carbocyclic
ring systems.
In a preferred aspect Q and R, if present, are independently carbocyclic ring
systems,
and P is a ring system selected from imidazole, tetrazole, pyrazole, triazole,
such as 1 H-
1,2,3-triazole, 1H-1,2,4-triazole, 4H-1,2,4-triazole; optionally substituted 5-
or 6-
,rtmembered heterocyclic group containing 1 to 3 hetero atoms each selected
from N, 0
---ond -S; optionally---substituted-aryl-(ronocyclic or , polycyclic
aromatic), pyridazine,
pyrimidine, triazine such as 1,3,5 triazine, and optionally substituted
bicyclic condensed
heterocyclic group consisting of the above heterocyclic group condensed with
benzene.
In a preferred aspect Q and R, if present, are independently carbocyclic ring
systems,
and P is a ring system selected from triazoles, in particular 1H-1,2,3-
triazole, 1H-1,2,4-
triazole, 4H-1,2,4-triazole
In a preferred aspect Q and R, if present, are independently carbocyclic ring
systems,
and P is 4H-1,2,4-triazole.
In a preferred aspect P is a ring system comprising carbon and one or more
hetero
atoms and Q and R, if present, are independently optionally substituted benzyl
rings.
In a preferred aspect P is a ring system comprising carbon and one or more
hetero
atoms selected from N, S and 0 and Q and R, if present, are independently
optionally
substituted benzyl rings.
In a preferred aspect Q and R, if present, are independently optionally
substituted
benzyl rings, and P is a ring system selected from imidazole, tetrazole,
pyrazole,
triazole, such as 1H-1,2,3-triazole, 1H-1,2,4-triazole, 4H-1,2,4-triazole;
optionally
substituted 5- or 6- membered heterocyclic group containing 1 to 3 hetero
atoms each
selected from N, 0 and S, optionally substituted aryl (monocyclic or
polycyclic aromatic),
pyridazine, pyrimidine, triazine such as 1,3,5 triazine, and optionally
substituted bicyclic
condensed heterocyclic group consisting of the above heterocyclic group
condensed
with benzene.
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
18
In a preferred aspect Q and R, if present, are independently optionally
substituted
benzyl rings, and P is a ring system selected from triazoles, in particular 1H-
1,2,3-
triazole, 1 H-1,2,4-triazole, 4H-1,2,4-triazole
In a preferred aspect Q and R, if present, are independently optionally
substituted
benzyl rings, and P is 4H-1,2,4-triazole.
The ring systems P, Q and R may be substituted by one or more substituents.
Preferred
--substituents - (other- than- the- required --sulphamate group) -include
hydrocarbyl,
oxyhydrocarbyl, halo and cyano (-C=-N) groups. The ring systems P, Q and R may
also
be substituted by one or more substituents selected from phosphonate groups,
,thiophosphonate groups, sulphonate groups and sulphonamide groups.
The term "oxyhydrocarbyl" group as used herein means a group comprising at
least C, H
and 0 and may optionally comprise one or more other suitable substituents.
Examples
of such substituents may include halo-, alkoxy-, nitro-, an alkyl group, a
cyclic group etc.
In addition to the possibility of the substituents being a cyclic group, a
combination of
substituents may form a cyclic group. If the oxyhydrocarbyl group comprises
more than
one C then those carbons need not necessarily be linked to each other. For
example, at
least two of the carbons may be linked via a suitable element or group. Thus,
the
oxyhydrocarbyl group may contain hetero atoms. Suitable hetero atoms will be
apparent to those skilled in the art and include, for instance, sulphur and
nitrogen.
-In one embodiment of the -present invention, the oxyhydrocarbyl group is a
oxyhydrocarbon group.
Here the term "oxyhydrocarbon" means any one of an alkoxy group, an oxyalkenyl
group, an oxyalkynyl group, which groups may be linear, branched or cyclic, or
an
oxyaryl group. The term oxyhydrocarbon also includes those groups but wherein
they
have been optionally substituted. If the oxyhydrocarbon is a branched
structure having
substituent(s) thereon, then the substitution may be on either the hydrocarbon
backbone
or on the branch; alternatively the substitutions may be on the hydrocarbon
backbone
and on the branch.
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
19
Typically, the oxyhydrocarbyl group is of the formula C,-60 (such as a C1-30).
Preferably Q is substituted with one or more halo atoms. Preferably the halo
atoms are
at a position ortho to the sulphamate group,
Preferably R, particularly when it is a carbocyclic group, is substituted with
a cyano (-
'C-N) group.
Further Preferred Compounds
In one preferred aspect the compound of the present invention is of the
Formula II
Formula II
R7 s/Q
E
wherein each T is independently selected from H, hydrocarbyl, -F-R', and a
bond with
one of E, P or Q, or together with one of P and Q forms a ring; Z is a
suitable atom the
valency of which is m; when Z is Nitrogen E is an optional linker group other
than CH2
and C=O; P, Q, R and R' are independently of each other a ring system; and Q
comprises a sulphamate group.
In one preferred aspect the compound of the present invention is of the
Formula III
P., Formula III
I
R,, F,N~E-Q
wherein D,-E and F are each independently of each other an optional linker
group,
wherein E is other than CH2 and C=O; P, Q and R are independently of each
other a
ring system; and at least Q comprises a sulphamate group.
In one preferred aspect the compound of the present invention is of the
Formula Ilia
P Formula Ilia
R,, F~N,E"Q
wherein E and F are each independently of each other an optional linker group,
wherein
E is other than CH2 and C=O; P, Q and R are independently of each other a ring
system; and at least Q comprises a sulphamate group.
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
In one preferred aspect the compound of the present invention is of the
Formula Illb
P Formula Ilib
I
RN%, E~'Q
wherein E is an optional linker group, wherein E is other than CH2 and C=O; P,
Q and R
are independently of each other a ring system; and at least Q comprises a
sulphamate
group.
5
In one preferred aspect the compound of the present invention is of the
Formula IlIc
P Formula IIIc
I
RN,E"Q
wherein E is a linker group, wherein E is other than CH2 and C=O; P, Q and R
are
independently of each other a ring system; and at least Q comprises a
sulphamate
group.
In one preferred aspect the compound of the present invention is of the
Formula Illd
P Formula Illd
R,N~E~'Q
wherein E is a straight chain or branched hydrocarbon group, preferably a C,-
C1o
hydrocarbon group containing at least two carbons or at least one hetero atom
in the
group; P, Q and R are independently of each other a ring system; and at least
Q
comprises a sulphamate group.
In one preferred aspect the compound of the present invention is of the
Formula IV
Pip Formula IV
IH
R%,
F~C%-% E"Q
wherein D, E and F are each independently of each other an optional linker
group, P, Q
and R are independently of each other a ring system; and at least Q comprises
a
sulphamate group.
In one preferred aspect the compound of the present invention is of the
Formula Na
P Formula Na
IH
R", F"I C%, E"Q
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
21
wherein E and F are each independently of each.other an optional linker group,
P, Q
and R are independently of each other a ring system; and at least Q comprises
a
sulphamate group.
In one preferred aspect the compound of the present invention is of the
Formula IVb
P H Formula IVb
I C~E"Q
wherein E is an optional linker group, P, Q and R are independently of each
other a ring
system; and at least Q comprises a sulphamate group.
In one preferred aspect the compound of the present invention is of the
Formula IVc
P H Formula IVc
RC~Q
wherein P, Q and R are independently of each other a ring system; and at least
Q
.comprises a sulphamate group.
in formulae II, III, Ilia, Illb, IIIc, IIId, IV, IVa, IVb, IVc, preferably one
of P, Q and R is a
ring system comprising carbon and one or more hetero atoms and two of P, Q and
R are
independently selected from carbocyclic ring systems.
In formulae II, III, Ilia, Illb, IIIc, Illd, IV, IVa, IVb, IVc, preferably one
of P, Q and R is a
ring system comprising carbon and one or more hetero atoms selected from
Nitrogen,
Sulphur and Oxygen and two of P, Q and R are independently selected from
carbocyclic
ring systems.
In formulae II, III, Ilia, Illb, Illc, Ilid, IV, IVa, IVb, and IVc, preferably
one of P, Q and R is
4H-1,2,4-triazole and two of P, Q and R are independently selected from
substituted or
unsubstituted benzyl rings.
In one preferred aspect the compound of the present invention is of the
Formula V
PAD Formula V
IH
H~C~% E"Q
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
22
wherein D and E are each independently of each other an optional linker group,
P and Q
are independently of each other a ring system; and at least Q comprises a
sulphamate
group.
In one preferred aspect the compound of the present invention is of the
Formula Va
P Formula Va
H
H.,C"~ E,Q
wherein E is an optional linker group, P and Q are independently of each other
a ring
system; and at least Q comprises a sulphamate group.
In one preferred aspect the compound of the present invention is of the
Formula Vb
P H Formula Vb
HC~Q
wherein P and Q are independently of each other a ring system; and at least Q
comprises a sulphamate group.
In formulae V, Va, and Vb, preferably one of P and Q is a ring system
comprising carbon
and one or more hetero atoms and the other of P and Q is a carbocyclic ring
system.
In formulae V, Va, and Vb, preferably one of P and Q is a ring system
comprising carbon
and one or more hetero atoms selected from Nitrogen, Sulphur and Oxygen and
the
other of P and Q is a carbocyclic ring system.
In formulae V, Va, and Vb, preferably one of P and Q is 4H-1,2,4-triazole and
the other
of P and Q is a substituted or unsubstituted benzyl ring.
In a particularly preferred aspect the compound of the present invention is of
Formula VI
P Formula VI
I
(T)M-2Z,, E~,Q
wherein each T is independently selected from H, hydrocarbyl, a bond with one
of D, E,
P or Q and -R, or together with one of P and Q forms a ring, and wherein m is
the
valency of Z; E is an optional linker group; wherein P, Q and R are each
independently
of each other a ring system; and Q comprises a sulphamate group.
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
23
A highly preferred compound of the present invention is a compound selected
from
compounds of the formula
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
24
//N,
``NN 4-[1,2,4]Triazol-1-ylmethylphenol (LWO02015A, STX269)
HO STX269
N
\ II Sulfamic acid 4-[1,2,4]triazol-1-ylmethylphenyl ester (LWO02017A, STX270)
N
H2NO2S0--) N' STX270
N-N
~Nf 4-[(4-Hydroxylbenzyl)-[1,2,4]triazol-4-ylamino]benzonitrile (LW002030,
STX265)
HO STX265
~N
CN
/N-N
Sulfamic acid 4-{[(4-cyanophenyl)-[1,2,4]triazol-4-ylamino]methyl}phenyl ester
(LW002031, STX258)
H2NO2SON STX258
~CN
N-N
" 4-{[2-(4-Hydroxyphenyl)ethyl]-[1,2,4]triazol-4-yl-amino}benzonitrile
(LW002063, STX290)
HO 0 N / STX290
UCN
N-N
) Sulfamic acid 4-{2-[(4-cyanophenyl)-[1,2,4]triazol-4-ylamino]ethyl}phenyl
ester (LW002066, STX273)
H2NO2SON STX273
~CN
N-N
4-{[2-(4-Hydroxyphenoxy)ethyl]-[1,2,4]triazol-4-yl-amino}benzonitrile
(LW002076, STX291)
N STX291
HO-f**A
OWN
)aCN
N-N
3 Sulfamic acid 4-{2-[(4-cyanophenyl)-[1,2,4]triazol-4-yl-amino]ethoxy}phenyl
ester (LW002077, STX292)
H2NO2SO- N STX292
O-,,,,N ~IaCN
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
/N - `N
`N) STX287 4-{[4-(4-Hydroxyphenoxy)butyl]-[1,2,4]triazol-4-
ylamino}benzonitrile (LWO02067, STX287)
HO~O,~ ^ N
)aCN
N-N
3 Sulfamic acid
H2NO 2 SO-<:~O,~N STX288 4-{4-[(4-cyanophenyl)-[1,2,4]triazol-4-ylamino]buto
v _CN
N-N
Br
J 4-{ [2-(3-Bromo-4-hydroxyphenoxy)ethyl]-[1,2,4]triazol-4 -t~ HO N O STX300 -
ylamino}benzonitrile (STX300)
~ N ~
CN
N-N
Br
Sulfamic acid 2-Bromo-4-{2-[(4-cyanophenyl)-[1,2,4]triazol-4-ylamino]
H2NO2SOON STX301 ethoxy)phenyl ester (STX301)
CN
N-N
1
N STX335 4-[(6-Hydroxy-naphthalen-2-ylmethyl)-[1,2,4]triazol-4-yl-amino]
NN,,::> -benzonitrile (JRL01012, STX 335)
CN
HO N-N
N )
STX336 Sulfamic acid 6-{[(4-cyano-phenyl)-[1,2,4]triazol-4-yl-amino]-methyl}-
N naphthalen-2-yl ester (JRL01014, STX 336)
~CN
H2NO2SO
NN
4-[(3-Hydroxybenzyl)(4-cyanophenyl)amino]-4H-[1,2,4]triazol
HON STX333 e (OBS01022, STX333)
N
N-N
J~)C N. STX334 4-[(3-O-Sulfamoylbenzyl)(4-cyanophenyl)amino]-4H-[1,2,4]triazol
H2NO2SON e (OBS01030, STX334)
N
N-N
HO`s ~N STX355
JJJ~yyyl~ 4-[(3,4-Bis(hydroxybenzyl)(4-cyanophenyl)amino]-4H-[1,2,4]triazol
HO N e (OBS01067, STX355)
N
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
26
N-N
HO N STX362 4-[(3-Hydroxy-4-methoxybenzyl)(4-cyanophenyl)amino]-4H-
[1,2,4]triazole
N (OBS01076, STX362)
0
I~
N
O N=N
H2N S O
O NE STX660 4-[(4-Methoxy-3-O-sulfamoylbenzyl)(4-cyanophenyl)amino]-4H-
[1,2,4]triazole
0 N-()" (OBS01135, STX660)
ON
N-N
9
iO N STX363 4-[(3-Hydroxy-4-methoxybenzyl)(4-cyanophenyl)amino]-4H-
[1,2,4]triazole
HO N (OBS01080, STX363)
N
NN
0 ~~ N STX661 4-[(4-Methoxy-3-O-sulfamoylbenzyl)(4-cyanophenyl)amino]-4H-
[1,2,4]triazole
S (OBS01137, STX661)
N
Br NN
HO N STX405 4-[(3-Bromo-4-hydroxybenzyl)(4-cyanophenyl)amino]-4H-
[1,2,4]triazol
e (OBS01132, STX405)
-0~, N
Br NN
H2NO2SO N STX681 4-[(3-Bromo-4-0-sulfamoylbenzyl)(4-cyanophenyl)amino]-4H-
[1,2,4]triazol
e (OBS01141, STX681)
N
NN
HO I N STX456 4-{[2-(4-Hydroxy-phenylsulfanyl)-ethyl]-[1,2,4]triazol-4-yl-
amino}-benzonitrile
S- N (CAB02137, STX456)
CN
N-N
N
N STX512 4-{[2-(3-Hydroxy-phenylsulfanyl)-ethyl]-[1,2,4]triazol-4-yl-amino}-
benzonitrile
HO'O' S^'N 1 (CAB02149, STX512)
CN
NN
N
N STX596 4-{[3-(4-Hydroxy-phenylsulfanyl)-propyl]-[1,2,4]triazol-4-yl-amino}
S-----""N -benzonitrile (CABO182, STX596)
HOI~ CN
N-N
STX597 Sulfamic acid 4-{3-[(4-cyano-phenyl)-[I,2,4]triazol-4-yl-
S1-1--~N amino]-propylsulfanyl}-phenyl ester (CAB02184, STX597)
H2NO2SO CN
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
27
NN
STX698
N 4-{[5-(4-Hydroxy-phenylsulfanyl)-pentyl]-[1,2,4]triazol-4-yl-amino
1S~^,N -benzonitrile (CAB03014, STX698)
HO CN
NN
N STX699
~ Sulfamic acid 4-{5-[(4-cyano-phenyl)-[1,2,4]triazol-4-yl-amino]
-pentylsulfanyl}-phenyl ester (CAB03025, STX699)
H2N02SO ' CN
NN
,HO
STX625 4-{[10-(4-Hydroxy-phenylsulfanyl)-decyl]-[1,2,4]triazol-4-yl-
S amino}-benzonitrile (CAB03011, STX625)
CN
N-N
H2NO2SO N Sulfamic acid 4-{10-[(4-cyano-phenyl)-[1,2,4]triazol-4-yl-
N STX655 amino]-decylsulfanyl}-phenyl ester (CAB03012, STX655)
S
' CN
Cl N-N
HO
N STX435 4-[(3,5-Dichloro-4-hydroxy-benzyl)-[1,2,4]triazol-4-yl-amino]
CI -benzonitrile (CAB02120, STX435)
CN
N-N
HO
N
STX447 4-[(3-Chloro-4-hydroxy-benzyl)-[1,2,4]triazol-4-yl-amino]-benzonitril
CI I e (CAB02130, STX447)
CN
N-N
H2NO2SO )0, N Sulfamic acid
CI 0STX694 2-chloro-4-f f(4-cyano-phenyl)-f 1,2,4ltriazol-4-yl-aminol-methyl}-
phen N-N CN
a
' 4- 4-Chloro-3-h drox -ben 1 - 1 2 4 triazol-4- l-amino -benzonitril
HO I N STX483 e (CAB02141, STX483) ~) [ ] y ]
' CN
N-N
CI
N Sulfamicacid-2-chloro-5-{ [(4-cyano-phenyl)-[1,2,4]triazol-4-yl-amino
H2NO2SO N STX559 1-methyll-phenyl ester (CAB02176, STX559))
' CN
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
28
OMe N-N
HO N STX600 4-[(3-chloro-4-hydroxy-5-methoxy-benzyl)-[1,2,4]triazol-4-yl-
amino]
CI -benzonitrile (CAB02179, STX600)
~)ICN
OMe N-N
H2NO2SO
N STX601 Sulfamicacid-2-chloro-4-{[(4-cyano-phenyl)-[1,2,4]triazol-4-yl-amino]
CI -methyl}-6-methoxy-phenyl ester (CAB02181, STX601)
I CN
N-N
HO
N 4-[(3-Fluoro-4-hydroxy-benzyl)-[1,2,4]triazol-4-yl-amino]-benzonitril
F N STX696 e (CAB03020, STX696)
CN
N-N
H2NO2SO , N
STX700 Sulfamicacid-4-{[(4-cyano-phenyl)-[1,2,4]triazol-4-yl-amino]-methyl}-2-
F I fluoro-phenyl ester (CAB03021, STX700)
CN
N-N
HO ):X N 4-[(4-Fluoro-3-hydroxy-benzyl)-[1,2,4]triazol-4-yl-amino]-benzonitril
STX488 e (CAB02154, STX488)
CN
N-N
4-[(4-H drox 3 trifluorometh I ben 1)1 2 4]triazol-4- 1 amino
HO , N Y Y- - Y - zY -[, , Y - ]
F C N -benzonitrile (CAB03059, STX781)
STX781
3
CN
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
29
NN NN
N STX541 N
SO N N STX636
CN CN
HO N N Me2NO2SO N N
N STX357 N
I N
STX732
\ O~ 0CN Br \ 0CN
HO Me2NO2SO N N
N N CND
N STX340 I
STX787
I -N N _ OCN
HO I CI \ / CN Me2NO2SO NN
Y
OH MeO \ NC, STX739
N N CN
N \ STX341 Me2NO2SO N N N
HNOSO N
2 2 ` C N _ N STX796
Me2N02S0 \ CN
OSO2NH2 MeO N N
N
N'N MeO N STX747
`N \ CN
Br Br STX414 Me2NO2S0
Br
HO \ OH N N
Br Br N
N Br _ N I STX740
N ~N \ / CN
HN, Me2NO2SO
Br
STX489
c1 c1
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
N-1
N' STX267
HO OH
1-[Bis-(4-Hydroxyphenyl)methyl]-IH-[1,2,4]triazole (LW002020, STX267)
NN
STX268
H2NO2SO OSO2NH2
1-[Bis-(4-sulfamoyloxyphenyl)methyl]-1H-[1,2,4]triazole (LWO02021, STX268)
N
N' STX356
Br Br
HO OH
1-[Bis-(3-bromo-4-hydroxyphenyl)methyl]-1H-[1,2,4]triazole (JRLO1105, STX356)
N
N' STX566
Br Br
H2NO2SO OSO2NH2
1-[Bis-(3-bromo-4-sulfamoyloxyphenyl)methyl]-1H-[1,2,4]triazole (JRLO1109,
STX566)
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
31
N-N N-N
X = H, F, Cl, Br, I, CN or bicyclic
N heterocycles
N
N /N Ar-Y-N = CO, (CH2)n, CO(CH2)n, (CH2)nCO,
Y~ C Y C CS(CH2)n, (CH2)nCS, O(CH2)n,
C S(CH2)n, SO(CH2)n, S02(CH2)n
OSO2NH2 oso2Nx2
N-N N- N
X = H, F, Cl, Br, I, CN or bicyclic heterocycles
On, CO(CH2)n, (CH2)nCO,
N-Y 11 N-Y-Ar = CO, (CH
N-Y \ CS(CH2)n, (CH2)nCS, (CH2)nO,
C )G-C I/ C (CH2)nS, (CH2)nSO, (CH2)nS02
OSO2NH2 OSO2NH2
N-N N -N
N N X=H, F, Cl, Br, I, CN or
bicyclic heterocycles
I I
Y
Y N N Y = (CH2)n, CO(CH2)n, (CH2)nCO,
C CS(CH2)n, (CH2)nCS, SO(CH2)n,
C I C S02(CH2)n, (CH2)nSO, (CH2)nS02,
OS02NH2 OSO2NH2
N-N N-N
A = CH2, 0, S, NH or other
N Ii bicyclic heterocycles
N N
C A / C X = H, F, Cl, Br, I, CN or
N other bicyclic heterocycles
C C
OSO2NH2 OSO2NH2
DASI Patent 1. doe
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
32
X = H, F, Cl, Br, I, CN A = bicyclic ring or bicyclic
heterocycles
N- N N-
N N A N
X \ X X \
A X
--~ OSOzNHz S02NH2 OS02NH2
% N ---------- (1,2,4-triazol-l-yl) --------- <NN
N
/
H2NO2SO X X
OSO NH2 qIQ---
2
S02NH2 2NH2
X O
N-N N-N
// \\ ----------- (1,2,4-triazol-4-yl) ------------
N N
X \ N HN
/
H2NO2SO OSO 2NH2 H2NO 2SO z z
/ OSO NH
X X X X
DASI Patent 2.doc
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
33
Further Aspects
In a further aspect of the present invention we have found that one may
provide a
compound of Formula I
P" Formula I
I
(T )m-2Z,, E"Q
wherein each T is independently selected from H, hydrocarbyl, -F-R, and a bond
with
,one of D, E, P or Q, or together with one of P and Q forms a ring; Z is a
suitable atom
the valency of which is m; D, E and F are each independently of each other an
optional
linker group, P, Q and R are independently of each other a ring system; and at
least on
of P, Q and R comprises a sulphamate group, wherein when Z is N (an optionally
when
Z is other than N) the sulphamate group is of the formula:
(R4)( R5)N-S(O)(O)-O-
wherein R4 and R5 are independently selected from alkyl, cycloalkyl, alkenyl,
acyl and
aryl, or combinations thereof, or together represent alkylene, wherein the or
each alkyl
or cycloalkyl or alkenyl or optionally contain one or more hetero atoms or
groups.
Preferably R4 and R5 are independently selected from alkyl groups, such as C1-
10 alkyl,
C1-5 alkyl, methyl and ethyl. Preferably R4 and R5 are both ethyl.
In a further aspect of the present invention we have found that one may
provide a
compound of Formula I
P" Formula I
(T)m-2Z-l E"Q
wherein each T is independently selected from H, hydrocarbyl, -F-R, and a bond
with
one of D, E, P or Q, or together with one of P and Q forms a ring; Z is a
suitable atom
the valency of which is m; D, E and F are each independently of each other an
optional
linker group, P, Q and R are independently of each other a ring system; and at
least on
of P, Q and R comprises a sulphamate group and wherein when Z is N (an
optionally
when Z is other than N) R is substituted by a halogen.
Sulphamate Group
At least Q one of the compound of the present invention comprises a sulphamate
group.
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
34
For some compounds of the present invention, it is highly preferred that the
compound
comprises at least two or more sulphamate groups.
For some compounds of the present invention, it is highly preferred that the
compound
comprises at least two sulphamate groups, wherein said sulphamate groups are
not on
the same. ring.
For some applications, preferably the compounds have no, or a minimal,
oestrogenic
1o effect.
For some applications, preferably the compounds have an oestrogenic effect.
For some applications, preferably the compounds have a reversible action.
For some applications, preferably the compounds have an irreversible action.
In one embodiment, the compounds of the present invention are useful for the
treatment
of breast cancer.
The present invention also covers novel intermediates that are useful to
prepare the
compounds of the present invention. For example, the present invention covers
novel
alcohol precursors for the compounds. By way of further example, the present
invention
covers bis protected precursors for the compounds. Examples of each of these
precursors are presented herein. The present invention also encompasses a
process
comprising each or both of those precursors for the synthesis of the compounds
of the
present invention.
STEROID SULPHATASE
Steroid sulphatase - which is sometimes referred to as steroid sulphatase or
steryl
sulphatase or "STS" for short - hydrolyses several sulphated steroids, such as
oestrone
sulphate, dehydroepiandrosterone sulphate and cholesterol sulphate. STS has
been
allocated the enzyme number EC 3.1.6.2.
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
STS has been cloned and expressed. For example see Stein et al (J. Biol. Chem.
264:13865-13872 (1989)) and Yen et al (Cell 49:443-454(1987)).
STS is an enzyme that has been implicated in a number of disease conditions.
5
By way of example, workers have found that a total deficiency in STS produces
ichthyosis. According to some workers, STS deficiency is fairly prevalent in
Japan. The
same workers (Sakura et al, J Inherit Metab Dis 1997 Nov;20(6):807-10) have
also
reported that allergic diseases - such as bronchial asthma, allergic rhinitis,
or atopic
10 dermatitis - may be associated with a steroid sulphatase deficiency.
In addition to disease states being brought on through a total lack of STS
activity, an
increased level of STS activity may also bring about disease conditions. By
way of
example, and as indicated above, there is strong evidence to support a role of
STS in
15 breast cancer growth and metastasis.
STS has also been implicated in other disease conditions. By way of example,
Le Roy
et al (Behav Genet 1999 Mar;29(2):131-6) have determined that there may be a
genetic
correlation between steroid sulphatase concentration and initiation of attack
behaviour in
20 mice. The authors conclude that sulphatation of steroids may be the prime
mover of a
complex network, including genes shown to be implicated in aggression by
mutagenesis.
STS INHIBITION
It is believed that some disease conditions associated with STS activity are
due to
conversion of a nonactive, sulphated oestrone to an active, nonsulphated
oestrone. In
disease conditions associated with STS activity, it would be desirable to
inhibit STS
activity.
Here, the term "inhibit" includes reduce and/or eliminate and/or mask and/or
prevent the
detrimental action of STS.
STS INHIBITOR
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
36
In accordance with the present invention, the compound of the present
invention is
capable of acting as an STS inhibitor.
Here, the term "inhibitor" as used herein with respect to the compound of the
present
invention means a compound that can inhibit STS activity - such as reduce
and/or
eliminate and/or mask and/or prevent the detrimental action of STS. The STS
inhibitor
may act as an antagonist.
The ability of compounds to inhibit oestrone sulphatase activity can be
assessed using
either intact JEG3 choriocarcinoma cells or placental microsomes. In addition,
an
animal model may be used. Details on suitable Assay Protocols are presented in
following sections. It is to be noted that other assays could be used to
determine STS
activity and thus STS inhibition. For example, reference may also be made to
the
teachings of WO-A-99/50453.
In one aspect, for some applications, the compound is further characterised by
the
feature that if the sulphamate group were to be substituted by a sulphate
group to form a
sulphate derivative, then the sulphate derivative would be hydrolysable by an
enzyme
having steroid sulphatase (E.C. 3.1.6.2) activity - i.e. when incubated with
steroid
sulphatase EC 3.1.6.2 at pH 7.4 and 37 C.
In one preferred embodiment, if the sulphamate group of the compound were to
be
replaced with a sulphate group to form a sulphate compound then that sulphate
compound would be hydrolysable by an enzyme having steroid sulphatase (E.C.
3;1.6.2) activity and would yield a Km value of less than 200 mmolar,
preferably less
than 150 mmolar, preferably less than 100 mmolar, preferably less than 75
mmolar,
preferably less than 50 mmolar, when incubated with steroid sulphatase EC
3.1.6.2 at
pH 7.4 and 37 C.
In a preferred embodiment, the compound of the present invention is not
hydrolysable
by an enzyme having steroid sulphatase (E.C. 3.1.6.2) activity.
For some applications, preferably the compound of the present invention has at
least
about a 100 fold selectivity to a desired target (e.g. STS and/or aromatase),
preferably
at least about a 150 fold selectivity to the desired target, preferably at
least about a 200
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
37
fold selectivity to the desired target, preferably at least about a 250 fold
selectivity to the
desired target, preferably at least about a 300 fold selectivity to the
desired target,
preferably at least about a 350 fold selectivity to the desired target.
It is to be noted that the compound of the present invention may have other
beneficial
properties in addition to or in the alternative to its ability to inhibit STS
and/or aromatase
activity.
,~ULPHAMATE GROUP
The term "sulphamate" as used herein includes an ester of sulphamic acid, or
an ester
of an N-substituted derivative of sulphamic acid, or a salt thereof.
If R3 is a sulphamate group then the compound of the present invention is
referred to as
a sulphamate compound.
Typically, the sulphamate group has the formula:
(R4)( R5)N-S(O)(O)-O-
wherein preferably R4 and R5 are independently selected from H, alkyl,
cycloalkyl,
alkenyl, acyl and aryl, or combinations thereof, or together represent
alkylene, wherein
the or each alkyl or cycloalkyl or alkenyl or optionally contain one or more
hetero atoms
or groups.
When substituted, the N-substituted compounds of this invention may contain
one or two
N-alkyl, N-alkenyl, N-cycloalkyl or N-aryl substituents, preferably containing
or each
containing a maximum of 10 carbon atoms. When R4 and/or R5 is alkyl, the
preferred
values are those where R4 and R5 are each independently selected from lower
alkyl
groups containing from 1 to 6 carbon atoms, that is to say methyl, ethyl,
propyl etc. R4
and R5 may both be methyl. When R4 and/or R5 is aryl, typical values are
phenyl and
tolyl (PhCH3; o). Where R4 and R5 represent cycloalkyl, typical values are
cyclopropyl,
cyclopentyl, cyclohexyl etc. When joined together R4 and R5 typically
represent an
alkylene group providing a chain of 4 to 6 carbon atoms, optionally
interrupted by one or
more hetero atoms or groups, e.g. to provide a 5 membered heterocycle, e.g.
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
38
morpholino, pyrrolidino or piperidino.
Within the values alkyl, cycloalkyl, alkenyl, acyl and aryl substituted groups
are included
containing as substituents therein one or more groups which do not interfere
with the
sulphatase inhibitory activity of the compound in question. Exemplary non-
interfering
,substituents include hydroxy, amino, halo, alkoxy, alkyl and aryl.
In some embodiments, the sulphamate group may form a ring structure by being
fused
to (or associated with) one or more atoms in or on group X.
In some embodiments, there may be more than one sulphamate group. By way of
example, there may be two sulphamates (i.e. bis-sulphamate compounds).
In some preferred embodiments, at least one of R4 and R5 is H.
In some further preferred embodiments, each of R4 and R5 is H.
PHOSPHONATE GROUP
If the compound of the present invention comprises a phosphonate group then
the
compound of the present invention is referred to as a phosphonate compound.
Typically, the phosphonate group has the formula:
(R6)-P(O)(OH)-O-
wherein preferably R6 is H, alkyl, cycloalkyl, alkenyl, acyl or aryl, or
combinations
thereof, wherein the or each alkyl or cycloalkyl or alkenyl or optionally
contain one or
more hetero atoms or groups.
When substituted, the N-substituted compounds of this invention may contain
one or two
N-alkyl, N-alkenyl, N-cycloalkyl or N-aryl substituents, preferably containing
or each
containing a maximum of 10 carbon atoms. When R6 is alkyl, R6 may be a lower
alkyl
groups containing from 1 to 6 carbon atoms, that is to say methyl, ethyl,
propyl etc. By
way of example, R6 may be methyl. When R6 is aryl, typical values are phenyl
and tolyl
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
39
(PhCH3;o). Where R6 represents cycloalkyl, typical values are cyclopropyl,
cyclopentyl,
cyclohexyl etc. R6 may even comprise an alkylene group providing a chain of 4
to 6
carbon atoms, optionally interrupted by one or more hetero atoms or groups,
e.g. to
provide a 5 membered heterocycle, e.g. morpholino, pyrrolidino or piperidino.
Within the values alkyl, cycloalkyl, alkenyl, acyl and aryl substituted groups
are included
containing as substituents therein one or more groups which do not interfere
with the
suiphatase inhibitory activity of the compound in question. Exemplary non-
interfering
substituents include hydroxy, amino, halo, alkoxy, alkyl and aryl.
In some embodiments, the phosphonate group may form a ring structure by being
fused
to (or associated with) one or more atoms in or on group X.
In some embodiments, there may be more than one phosphonate group. By way of
example, there may be two phosphonates (i.e. bis-phosphonate compounds). If
these
compounds are based on a steroidal nucleus, preferably the second (or at least
one of
the additional) phosphonate group is located at position 17 of the steroidal
nucleus.
These groups need not be the same.
THIOPHOSPHONATE GROUP
If the compound of the present invention comprises a thiophosphonate group
then the
compound of the present invention is referred to as a thiophosphonate
compound.
Typically, the thiophosphonate group has the formula:
(R')-P(S)(OH)-O-
wherein preferably R7 is H, alkyl, cycloalkyl, alkenyl, acyl or aryl, or
combinations
thereof, wherein the or each alkyl or cycloalkyl or alkenyl or optionally
contain one or
more hetero atoms or groups.
When substituted, the N-substituted compounds of this invention may contain
one or two
N-alkyl, N-alkenyl, N-cycloalkyl or N-aryl substituents, preferably containing
or each
containing a maximum of 10 carbon atoms. When R7 is alkyl, R7 may be a lower
alkyl
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
groups containing from 1 to 6 carbon atoms, that is to say methyl, ethyl,
propyl etc. By
way of example, R7 may be methyl. When R7 is aryl, typical values are phenyl
and tolyl
(PhCH3;o). Where R7 represents cycloalkyl, typical values are cyclopropyl,
cyclopentyl,
cyclohexyl etc. R7 may even comprise an alkylene group providing a chain of 4
to 6
5 carbon atoms, optionally interrupted by one or more hetero atoms or groups,
e.g. to
provide a 5 membered heterocycle, e.g. morpholino, pyrrolidino or piperidino.
Within the values alkyl, cycloalkyl, alkenyl, acyl and aryl substituted groups
are included
containing as substituents therein one or more groups which do not interfere
with the
to sulphatase inhibitory activity of the compound in question. Exemplary non-
interfering
substituents include hydroxy, amino, halo, alkoxy, alkyl and aryl.
In some embodiments, the thiophosphonate group may form a ring structure by
being
fused to (or associated with) one or more atoms in or on group X.
In some embodiments, there may be more than one thiophosphonate group. By way
of
example, there may be two thiophosphonates (i.e. bis-thiophosphonate
compounds). If
these compounds are based on a steroidal nucleus, preferably the second (or at
least
one of the additional) thiophosphonate group is located at position 17 of the
steroidal
nucleus. These groups need not be the same.
SULPHONATE GROUP
If the compound of the present invention comprises a sulphonate group then the
compound of the present invention is referred to as a sulphonate compound.
Typically, the sulphonate group has the formula:
(R8)-S(O)(O)-O-
wherein preferably R8 is H, alkyl, cycloalkyl, alkenyl, acyl or aryl, or
combinations
thereof, wherein the or each alkyl or cycloalkyl or alkenyl or optionally
contain one or
more hetero atoms or groups.
When substituted, the N-substituted compounds of this invention may contain
one or two
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
41
N-alkyl, N-alkenyl, N-cycloalkyl or N-aryl substituents, preferably containing
or each
containing a maximum of 10 carbon atoms. When R8 is alkyl, R8 may be a lower
alkyl
groups containing from 1 to 6 carbon atoms, that is to say methyl, ethyl,
propyl etc. By
way of example, R8 may be methyl. When R8 is aryl, typical values are phenyl
and tolyl
(PhCH3io). Where R8 represents cycloalkyl, typical values are cyclopropyl,
cyclopentyl,
cyclohexyl etc. R8 may even comprise an alkylene group providing a chain of 4
to 6
carbon atoms, optionally interrupted by one or more hetero atoms or groups,
e.g. to
provide a 5 membered heterocycle, e.g. morpholino, pyrrolidino or piperidino.
Within the values alkyl, cycloalkyl, alkenyl, acyl and aryl substituted groups
are included
containing as substituents therein one or more groups which do not interfere
with the
sulphatase inhibitory activity of the compound in question. Exemplary non-
interfering
substituents include hydroxy, amino, halo, alkoxy, alkyl and aryl.
In some embodiments, the sulphonate group may form a ring structure by being
fused to
(or associated with) one or more atoms in or on group X.
In some embodiments, there may be more than one sulphonate group. By way of
example, there may be two sulphonates (i.e. bis- sulphonate compounds). If
these
compounds are based on a steroidal nucleus, preferably the second (or at least
one of
the additional) sulphonate group is located at position 17 of the steroidal
nucleus. These
groups need not be the same.
OTHER SUBSTITUENTS
The compound of the present invention may have substituents other than those
of
formula I. By way of example, these other substituents may be one or more of:
one or
more sulphamate group(s), one or more phosphonate group(s), one or more
thiophosphonate group(s), one or more sulphonate group(s), one or more
sulphonamide
group(s), one or more halo groups, one or more 0 groups, one or more hydroxy
groups,
one or more amino groups, one or more sulphur containing group(s), one or more
hydrocarbyl group(s) - such as an oxyhydrocarbyl group.
ASSAY FOR DETERMINING STS ACTIVITY USING CANCER CELLS
(PROTOCOL 1)
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
42
Inhibition of Steroid Sulphatase Activity in JEG3 cells
Steroid sulphatase activity is measured in vitro using intact JEG3
choriocarcinoma cells.
This cell line may be used to study the control of human breast cancer cell
growth. It
possesses significant steroid sulphatase activity (Boivin et al.,J. Med.
Chem., 2000, 43:
4465 - 4478) and is available in from the American Type Culture Collection
(ATCC).
'Cells are maintained in Minimal Essential Medium (MEM) (Flow Laboratories,
Irvine,
Scotland) containing 20 mM HEPES, 5% foetal bovine serum, 2 mM glutamine, non-
essential amino acids and 0.075% sodium bicarbonate. Up to 30 replicate 25 cm2
tissue culture flasks are seeded with approximately 1 x 105 cells/flask using
the above
medium. Cells are grown to 80% confluency and the medium is changed every
third
day.
Intact monolayers of JEG3 cells in triplicate 25 cm2 tissue culture flasks are
washed with
Earle's Balanced Salt Solution (EBSS from ICN Flow, High Wycombe, U.K.) and
incubated for 3-4 hours at 37 C with 5 pmol (7 x 105 dpm) [6,7-3H]oestrone-3-
sulphate
(specific activity 60 Ci/mmol from New England Nuclear, Boston, Mass., U.S.A.)
in
serum-free MEM (2.5 ml) together with oestrone-3-sulphamate (11
concentrations: 0;
1fM; 0.01 pM; 0.1 pM; 1pM; 0.01 nM; 0.1 nM; 1 nM; 0.01mM; 0.1mM; 1 mM). After
incubation each flask is cooled and the medium (1 ml) is pipetted into
separate tubes
containing [14C]oestrone (7 x 103 dpm) (specific activity 97 Ci/mmol from
Amersham
International Radiochemical Centre, Amersham, U.K.). The mixture is shaken
thoroughly for 30 seconds- with toluene (5 ml). Experiments have shown that
>90%
[14C] oestrone and <0.1 % [3H]oestrone-3-sulphate is removed from the aqueous
phase
by this treatment. A portion (2 ml) of the organic phase is removed,
evaporated and the
3H and 14C content of the residue determined by scintillation spectrometry.
The mass of
oestrone-3-sulphate hydrolysed was calculated from the 3H counts obtained
(corrected
for the volumes of the medium and organic phase used, and for recovery of
[14C]
oestrone added) and the specific activity of the substrate. Each batch of
experiments
includes incubations of microsomes prepared from a sulphatase-positive human
placenta (positive control) and flasks without cells (to assess apparent non-
enzymatic
hydrolysis of the substrate). The number of cell nuclei per flask is
determined using a
Coulter Counter after treating the cell monolayers with Zaponin. One flask in
each batch
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
43
is used to assess cell membrane status and viability using the Trypan Blue
exclusion
method (Phillips, H.J. (1973) In: Tissue culture and applications, [eds:
Kruse, D.F. &
Patterson, M.K.]; pp. 406-408; Academic Press, New York).
Results for steroid sulphatase activity are expressed as the mean 1 S.D. of
the total
product (oestrone + oestradiol) formed during the incubation period (3-4
hours)
calculated for 106 cells and, for values showing statistical significance, as
a percentage
reduction (inhibition) over incubations containing no oestrone-3-sulphamate.
Unpaired
Student's t-test was used to test the statistical significance of results.
ASSAY FOR DETERMINING STS ACTIVITY USING PLACENTAL MICROSOMES
(PROTOCOL 2)
Inhibition of Steroid Sulphatase Activity in Placental Microsomes
Sulphatase-positive human placenta from normal term pregnancies are thoroughly
minced with scissors and washed once with cold phosphate buffer (pH 7.4, 50
mM) then
re-suspended in cold phosphate buffer (5 ml/g tissue). Homogenisation is
accomplished
with an Ultra-Turrax homogeniser, using three 10 second bursts separated by 2
minute
cooling periods in ice. Nuclei and cell debris are removed by centrifuging (4
C) at 2000g
for 30 minutes and portions (2 ml) of the supernatant are stored at 20 C. The
protein
concentration of the supernatants is determined by the method of Bradford
(Anal.
Biochem., 72, 248-254 (1976)).
Incubations (1 ml) are carried out using a protein concentration of 100 mg/ml,
substrate
concentration of 20 mM [6,7-3H]oestrone-3-sulphate (specific activity 60
Ci/mmol from
New England Nuclear, Boston, Mass., U.S.A.) and an incubation time of 20
minutes at
37 C. If necessary eight concentrations of compounds are employed: 0 (i.e.
control);
0.05mM; 0.1 mM; 0.2mM; 0.4mM; 0.6mM; 0.8mM; 1.0mM. After incubation each
sample
is cooled and the medium (1 ml) was pipetted into separate tubes containing
[14C]oestrone (7 x 103 dpm) (specific activity 97 Ci/mmol from Amersham
International
Radiochemical Centre, Amersham, U.K.). The mixture is shaken thoroughly for 30
seconds with toluene (5 ml). Experiments have shown that >90% [14C]oestrone
and
<0.1% [3H]oestrone-3-sulphate is removed from the aqueous phase by this
treatment.
A portion (2 ml) of the organic phase was removed, evaporated and the 3H and
14C
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
44
content of the residue determined by scintillation spectrometry. The mass of
oestrone-
3-sulphate hydrolysed is calculated from the 3H counts obtained (corrected for
the
volumes of the medium and organic phase used, and for recovery of
[14C]oestrone
added) and the specific activity of the substrate.
ANIMAL ASSAY MODEL FOR DETERMINING STS ACTIVITY
(PROTOCOL 3)
Inhibition of oestrone sulphatase activity in vivo
The compounds of the present invention may be studied using an animal model,
in
particular in ovariectomised rats. In this model compounds which are
oestrogenic
stimulate uterine growth.
The compound (0.1 mg/Kg/day for five days) is administered orally to rats with
another
group of animals receiving vehicle only (propylene glycol). At the end of the
study
samples of liver tissue were obtained and oestrone sulphatase activity assayed
using
3H oestrone sulphate as the substrate as previously described (see
PCT/GB95/02638).
ANIMAL ASSAY MODEL FOR DETERMINING OESTROGENIC ACTIVITY
(PROTOCOL 4)
The compounds of the present invention may be studied using an animal model,
in
particular in ovariectomised rats. In this model, compounds which are
oestrogenic
stimulate uterine growth.
The compound (0.1 mg/Kg/day for five days) was administered orally to rats
with
another group of animals receiving vehicle only (propylene glycol). At the end
of the
study uteri were obtained and weighed with the results being expressed as
uterine
weight/whole body weight x 100.
Compounds having no significant effect on uterine growth are not oestrogenic.
BIOTECHNOLOGICAL ASSAYS FOR DETERMINING STS ACTIVITY
(PROTOCOL 5)
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
The ability of compounds to inhibit oestrone sulphatase activity can also be
assessed
using amino acid sequences or nucleotide sequences encoding STS, or active
fragments, derivatives, homologues or variants thereof in, for example, high-
through put
5 screens.
Any one or more of appropriate targets - such as an amino acid sequence and/or
nucleotide sequence - may be used for identifying an agent capable of
modulating STS
in any of a variety of drug screening techniques. The target employed in such
a test
10 may be free in solution, affixed to a solid support, borne on a cell
surface, or located
intracellularly. The abolition of target activity or the formation of binding
complexes
between the target and the agent being tested may be measured.
The assay of the present invention may be a screen, whereby a number of agents
are
15 tested. In one aspect, the assay method of the present invention is a high
through put
screen.
Techniques for drug screening may be based on the method described in Geysen,
European Patent Application 84/03564, published on September 13, 1984. In
summary,
20 large numbers of different small peptide test compounds are synthesised on
a solid
substrate, such as plastic pins or some other surface. The peptide test
compounds are
reacted with a suitable target or fragment thereof and washed. Bound entities
are then
detected - such as by appropriately adapting methods well known in the art. A
purified
target can also be coated directly onto plates for use in a drug screening
techniques.
25 Alternatively, non-neutralising antibodies can be used to capture the
peptide and
immobilise it on a solid support.
This invention also contemplates the use of competitive drug screening assays
in which
neutralising antibodies capable of binding a target specifically compete with
a test
30 compound for binding to a target.
Another technique for screening provides for high throughput screening (HTS)
of agents
having suitable binding affinity to the substances and is based upon the
method
described in detail in WO 84/03564.
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
46
It is expected that the assay methods of the present invention will be
suitable for both
small and large-scale screening of test compounds as well as in quantitative
assays.
In one preferred aspect, the present invention relates to a method of
identifying agents
that selectively modulate STS, which compounds have the formula (I).
ASSAY FOR DETERMINING AROMATASE ACTIVITY USING JEG3 CELLS
(PROTOCOL 6)
Aromatase activity is measured in JEG3 choriocarcinoma cells, obtained from
the
ATCC. This cell line possesses significant aromatase activity and is widely
used to
study the control of human aromatase activity (Bhatnager et al., J.Steroid
Biochem.Molec. Biol. 2001, 76: 199 - 202 ). Cells are maintained in Minimal
Essential
Medium (MEM, Flow Laboratories, Irvine, Scotland) containing 20mM HEPES, 10 %
foetal bovine serum, 2mM glutamine, non-essential amino acids and 0.075%
sodium
bicarbonate. Intact monolayers of JEG3 cells (2.5 x 106 cells) in triplicate
25cm2 tissue
culture flasks are washed with Earle's Balanced salt solution (EBSS, from ICN
Flow,
High Wycombe, UK) and incubated with [1 R 3H] androstenedione (2-5nM, 26
Ci/mmol,
New England Nuclear, Boston, MA, USA) for 30min with inhibitors over the range
of
1.Opm-10 M . During the aromatase reaction, 3H2O is liberated which can he
quantified
using a liquid scintillation spectrometer (Beckman-Coulter, High
Wycombe,Bucks. UK).
This 3H2O-release method has been widely used to measure aromatase activity (
Newton et al., J.Steroid Biochem. 1986,24: 1033 -1039 ). The number of cell
nuclei per
flask is determined using a Coulter Counter after treating the cell monolayers
with Z
aponin.
Results for aromatase activity are expressed as the mean 1 S.D. of the
product
formed during the incubation period (30min) calculated for 106 cells and, for
values
showing a statistical significance, as a percentage reduction (inhibition)
over incubations
containing no aromatase inhibitor. Unpaired Student's t test was used to test
the
statistical significance of results. IC50 values were calculated as the
concentration of
inhibitor required to obtain a 50% inhibition of aromatase activity.
ANIMAL ASSAYS FOR DETERMINING AROMATASE ACTIVITY
(PROTOCOL 7)
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
47
(i) Inhibition of PMSG-induced oestrogen synthesis
The ability of compounds to inhibit aromatase activity in vivo was tested
using a
pregnant mare serum gonadotrophin (PMSG)-induced oestrogen synthesis assay.
For
this, female rats (250g) were injected with PMSG (200 IU, s.c.). After 72h
rats were
administered vehicle (propylene glycol) or various doses of test compounds
orally. At 2h
after dosing blood samples were obtained by cardiac puncture (under
anaesthesia).
Plasma oestradiol levels were measured in control groups and groups receiving
drugs.
The efficacy of . aromatase inhibition was determined by measurement of plasma
oestradiol concentrations by radioimmunoassay. This method has been widely
used to
determine the effectiveness of aromatase inhibitors in vivo (Wouters et al.,
J.Steroid
Biochem., 1989, 32 : 781 - 788 ).
(ii) Inhibition of androstenedione stimulated uterine growth in ovariectomised
rats
Female rats (250g) were ovariectomised and used to determine the effectiveness
of
aromatase inhibition on androstenedione stimulated uterine growth.
Administration of
androstenedione (30mg/kg/d) for a 2-week period results in a significant
increase in
uterine growth in ovariectomised animals. This increase in uterine growth is
stimulated
by oestrogen which is derived from the administered androstenedione as a
result of the
action of the aromatase enzyme. By co-administration of compounds with
androstenedione the extent of aromatase inhibition can be determined by
measurements of uterine weights in treated and untreated animals.
REPORTERS
A wide variety of reporters may be used in the assay methods (as well as
screens) of
the present invention with preferred reporters providing conveniently
detectable signals
(e.g. by spectroscopy). By way of example, a reporter gene may encode an
enzyme
which catalyses a reaction which alters light absorption properties.
Other protocols include enzyme-linked immunosorbent assay (ELISA),
radioimmunoassay (RIA) and fluorescent activated cell sorting (FACS). A two-
site,
monoclonal-based immunoassay utilising monoclonal antibodies reactive to two
non-
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
48
interfering epitopes may even be used. These and other assays are described,
among
other places, in Hampton R et at (1990, Serological Methods, A Laboratory
Manual, APS
Press, St Paul MN) and Maddox DE et al (1983, J Exp Med 15 8:121 1).
Examples of reporter molecules include but are not limited to (f3-
galactosidase,
invertase, green fluorescent protein, luciferase, chloramphenicol,
acetyltransferase, (-
glucuronidase, exo-glucanase and glucoamylase. Alternatively, radiolabelled or
fluorescent tag-labelled nucleotides can be incorporated into nascent
transcripts which
are then identified when bound to oligonucleotide probes.
By way of further examples, a number of companies such as Pharmacia Biotech
(Piscataway, NJ), Promega (Madison, WI), and US Biochemical Corp (Cleveland,
OH)
I supply commercial kits and protocols for assay procedures. Suitable reporter
molecules
or labels include those radionuclides, enzymes, fluorescent, chemiluminescent,
or
chromogenic agents as well as substrates, cofactors, inhibitors, magnetic
particles and
the like. Patents teaching the use of such labels include US-A-3817837; US-A-
3850752; US-A-3939350; US-A-3996345; US-A-4277437; US-A-4275149 and US-A-
4366241.
HOST CELLS
The term "host cell" - in relation to the present invention includes any cell
that could
comprise the target for the agent of the present invention.
Thus, a further embodiment of the present invention provides host cells
transformed or
transfected with a polynucleotide that is or expresses the target of the
present invention.
Preferably said polynucleotide is carried in a vector for the replication and
expression of
polynucleotides that are to be the target or are to express the target. The
cells will be
chosen to be compatible with the said vector and may for example be
prokaryotic (for
example bacterial), fungal, yeast or plant cells.
The gram negative bacterium E. coli is widely used as a host for heterologous
gene
expression. However, large amounts of heterologous protein tend to accumulate
inside
the cell. Subsequent purification of the desired protein from the bulk of
E.coli
intracellular proteins can sometimes be difficult.
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
49
In contrast to E.coli, bacteria from the genus Bacillus are very suitable as
heterologous
hosts because of their capability to secrete proteins into the culture medium.
Other
bacteria suitable as hosts are those from the genera Streptomyces and
Pseudomonas.
Depending on the nature of the polynucleotide encoding the polypeptide of the
present
invention, and/or the desirability for further processing of the expressed
protein,
eukaryotic hosts such as yeasts or other fungi may be preferred. In general,
yeast cells
'are preferred over fungal cells because they are easier to manipulate.
However, some
;'proteins are either poorly, secreted--from the yeast cell, or in some cases
are not
processed properly (e.g. hyperglycosylation in yeast). In these instances, a
different
fungal host organism should be selected.
Examples of suitable expression hosts within the scope of the present
invention are
fungi such as Aspergillus species (such as those described in EP-A-0184438 and
EP-A-
0284603) and Trichoderma species; bacteria such as Bacillus species (such as
those
described in EP-A-0134048 and EP-A-0253455), Streptomyces species and
Pseudomonas species; and yeasts such as Kluyveromyces species (such as those
described in EP-A-0096430 and EP-A-0301670) and Saccharomyces species. By way
of example, typical expression hosts may be selected from Aspergillus niger,
Aspergillus
niger var. tubigenis, Aspergillus niger var. awamori, Aspergillus aculeatis,
Aspergillus
nidulans, Aspergillus orvzae, Trichoderma reesei, Bacillus subtilis, Bacillus
licheniformis,
Bacillus amyloliquefaciens, Kluyveromyces lactis and Saccharomyces cerevisiae.
The use of suitable host cells - such as yeast, fungal and plant host cells -
may provide
for post-translational modifications (e.g. myristoylation, glycosylation,
truncation,
lapidation and tyrosine, serine or threonine phosphorylation) as may be needed
to
confer optimal biological activity on recombinant expression products of the
present
invention.
ORGANISM
The term "organism" in relation to the present invention includes any organism
that
could comprise the target according to the present invention and/or products
obtained
therefrom. Examples of organisms may include a fungus, yeast or a plant.
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
The term "transgenic organism" in relation to the present invention includes
any
organism that comprises the target according to the present invention and/or
products
obtained.
5
TRANSFORMATION OF HOST CELLS/HOST ORGANISMS
As indicated earlier, the host organism can be a prokaryotic or a eukaryotic
organism.
Examples of suitable prokaryotic hosts include E. coli and Bacillus subtilis.
Teachings
10 on the transformation of prokaryotic hosts is well documented in the art,
for example see
Sambrook et al (Molecular Cloning: A Laboratory Manual, 2nd edition, 1989,
Cold
Spring Harbor Laboratory Press) and Ausubel et al., Current Protocols in
Molecular
Biology (1995), John Wiley & Sons, Inc.
15 If a prokaryotic host is used then the nucleotide sequence may need to be
suitably
modified before transformation- such as by removal of introns.
In another embodiment the transgenic organism can be a yeast. In this regard,
yeast
have also been widely used as a vehicle for heterologous gene expression. The
20 species Saccharomyces cerevisiae has a long history of industrial use,
including its use
for heterologous gene expression. Expression of heterologous genes in
Saccharomyces cerevisiae has been reviewed by Goodey et al (1987, Yeast
Biotechnology, D R Berry et al, eds, pp 401-429, Allen and Unwin, London) and
by King
et al (1989, Molecular and Cell Biology of Yeasts, E F Walton and G T
Yarronton, eds,
25 pp 107-133, Blackie, Glasgow).
For several reasons Saccharomyces cerevisiae is well suited for heterologous
gene
expression. First, it is non-pathogenic to humans and it is incapable of
producing certain
endotoxins. Second, it has a long history of safe use following centuries of
commercial
30 exploitation for various purposes. This has led to wide public
acceptability. Third, the
extensive commercial use and research devoted to the organism has resulted in
a
wealth of knowledge about the genetics and physiology as well as large-scale
fermentation characteristics of Saccharomyces cerevisiae.
35 A review of the principles of heterologous gene expression in Saccharomyces
cerevisiae
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
51
and secretion of gene products is given by E Hinchcliffe E Kenny (1993, "Yeast
as a
vehicle for the expression of heterologous genes", Yeasts, Vol 5, Anthony H
Rose and
J Stuart Harrison, eds, 2nd edition, Academic Press Ltd.).
Several types of yeast vectors are available, including integrative vectors,
which require
recombination with the host genome for their maintenance, and autonomously
replicating plasmid vectors.
In order to prepare the transgenic Saccharomyces, expression constructs are
prepared
by inserting the nucleotide sequence into a construct designed for expression
in yeast.
Several types of constructs used for heterologous expression have been
developed.
The constructs contain a promoter active in yeast fused to the nucleotide
sequence,
usually a promoter of yeast origin, such as the GAL1 promoter, is used.
Usually a signal
sequence of yeast origin, such as the sequence encoding the SUC2 signal
peptide, is
used. A terminator active in yeast ends the expression system.
For the transformation of yeast several transformation protocols have been
developed.
For example, a transgenic Saccharomyces according to the present invention can
be
prepared by following the teachings of Hinnen et al (1978, Proceedings of the
National
Academy of Sciences of the USA 75, 1929); Beggs, J D (1978, Nature, London,
275,
104); and Ito, H et al (1983, J Bacteriology 153, 163-168).
The transformed yeast cells are selected using various selective markers.
Among the
markers used for transformation are a number of auxotrophic markers such as
LEU2,
HIS4 and TRP1, and dominant antibiotic resistance markers such as
aminoglycoside
antibiotic markers, e.g. G418.
Another host organism is a plant. The basic principle in the construction of
genetically
modified plants is to insert genetic information in the plant genome so as to
obtain a
stable maintenance of the inserted genetic material. Several techniques exist
for
inserting the genetic information, the two main principles being direct
introduction of the
genetic information and introduction of the genetic information by use of a
vector
system. A review of the general techniques may be found in articles by
Potrykus (Annu
Rev Plant Physiol Plant Mol Biol [1991] 42:205-225) and Christou (Agro-Food-
Industry
Hi-Tech March/April 1994 17-27). Further teachings on plant transformation may
be
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
52
found in EP-A-0449375.
Thus, the present invention also provides a method of transforming a host cell
with a
nucleotide sequence that is to be the target or is to express the target. Host
cells
transformed with the nucleotide sequence may be cultured under conditions
suitable for
the expression of the encoded protein. The protein produced by a recombinant
cell may
be displayed on the surface of the cell. If desired, and as will be understood
by those of
skill in the art, expression vectors containing coding sequences can be
designed with
signal sequences which direct secretion of the coding sequences through a
particular
prokaryotic or eukaryotic cell membrane. Other recombinant constructions may
join the
coding sequence to nucleotide sequence encoding a polypeptide domain which
will
facilitate purification of soluble proteins (Kroll DJ et al (1993) DNA Cell
Biol 12:441-53).
VARIANTS/HOMOLOGUES/DERIVATIVES
In addition to the specific amino acid sequences and nucleotide sequences
mentioned
herein, the present invention also encompasses the use of variants, homologue
and
derivatives thereof. Here, the term "homology" can be equated with "identity".
In the present context, an homologous sequence is taken to include an amino
acid
sequence which may be at least 75, 85 or 90% identical, preferably at least 95
or 98%
identical. Although homology can also be considered in terms of similarity
(i.e. amino
acid residues having similar chemical properties/functions), in the context of
the present
invention it is preferred to express homology in terms of sequence identity.
Homology comparisons can be conducted by eye, or more usually, with the aid of
readily available sequence comparison programs. These commercially available
computer programs can calculate % homology between two or more sequences.
% homology may be calculated over contiguous sequences, i.e. one sequence is
aligned with the other sequence and each amino acid in one sequence is
directly
compared with the corresponding amino acid in the other sequence, one residue
at a
time. This is called an "ungapped" alignment. Typically, such ungapped
alignments are
performed only over a relatively short number of residues.
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
53
Although this is a very simple and consistent method, it fails to take into
consideration
that, for example, in an otherwise identical pair of sequences, one insertion
or deletion
will cause the following amino acid residues to be put out of alignment, thus
potentially
resulting in a large reduction in % homology when a global alignment is
performed.
Consequently, most sequence comparison methods are designed to produce optimal
alignments that take into consideration possible insertions and deletions
without
penalising unduly the overall homology score. This is achieved by inserting
"gaps" in
the sequence alignment to try to maximise local homology.
However, these more complex methods assign "gap penalties" to each gap that
occurs
in the alignment so that, for the same number of identical amino acids, a
sequence
alignment with as few gaps as possible - reflecting higher relatedness between
the two
compared sequences - will achieve a higher score than one with many gaps.
"Affine
gap costs" are typically used that charge a relatively high cost for the
existence of a gap
and a smaller penalty for each subsequent residue in the gap. This is the most
commonly used gap scoring system. High gap penalties will of course produce
optimised alignments with fewer gaps. Most alignment programs allow the gap
penalties to be modified. However, it is preferred to use the default values
when using
such software for sequence comparisons. For example when using the GCG
Wisconsin
Bestfit package (see below) the default gap penalty for amino acid sequences
is -12 for
a gap and -4 for each extension.
Calculation of maximum % homology therefore firstly requires the production of
an
optimal alignment, taking into consideration gap penalties. A suitable
computer program
for carrying out such an alignment is the GCG Wisconsin Bestfit package
(University of
Wisconsin, U.S.A.; Devereux et al., 1984, Nucleic Acids Research 12:387).
Examples
of other software than can perform sequence comparisons include, but are not
limited
to, the BLAST package (see Ausubel et al., 1999 ibid - Chapter 18), FASTA
(Atschul et
al., 1990, J. Mol. Biol., 403-410) and the GENEWORKS suite of comparison
tools. Both
BLAST and FASTA are available for offline and online searching (see Ausubel et
al.,
1999 ibid, pages 7-58 to 7-60). However it is preferred to use the GCG Bestfit
program.
A further useful reference is that found in FEMS Microbiol Lett 1999 May
15;174(2):247-
50 (and a published erratum appears in FEMS Microbiol Lett 1999 Aug
1;177(1):187-8).
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
54
Although the final % homology can be measured in terms of identity, the
alignment
process itself is typically not based on an all-or-nothing pair comparison.
Instead, a
scaled similarity score matrix is generally used that assigns scores to each
pairwise
comparison based on chemical similarity or evolutionary distance. An example
of such
a matrix commonly used is the BLOSUM62 matrix - the default matrix for the
BLAST
suite of programs. GCG Wisconsin programs generally use either the public
default
values or a custom symbol comparison table if supplied (see user manual for
further
details). It is preferred to use the public default values for the GCG
package, or in the
base of other software, the default matrix, such as BLOSUM62.
Once the software has produced an optimal alignment, it is possible to
calculate %
homology, preferably % sequence identity. The software typically does this as
part of
the sequence comparison and generates a numerical result.
The sequences may also have deletions, insertions or substitutions of amino
acid
residues which produce a silent change and result in a functionally equivalent
substance. Deliberate amino acid substitutions may be made on the basis of
similarity
in polarity, charge, solubility, hydrophobicity, hydrophilicity, and/or the
amphipathic
nature of the residues as long as the secondary binding activity of the
substance is
retained. For example, negatively charged amino acids include aspartic acid
and
glutamic acid; positively charged amino acids include lysine and arginine; and
amino
acids with uncharged polar head groups having similar hydrophilicity values
include
leucine, isoleucine, valine, glycine, alanine, asparagine, glutamine, serine,
threonine,
phenylalanine, and tyrosine.
Conservative substitutions may be made, for example according to the Table
below.
Amino acids in the same block in the second column and preferably in the same
line in
the third column may be substituted for each other:
ALIPHATIC Non-polar G A P
I L V
Polar - uncharged CST M
NQ
Polar - charged D E
KR
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
AROMATIC H F W Y
EXPRESSION VECTORS
The nucleotide sequence for use as the target or for expressing the target can
be
5 incorporated into a recombinant replicable vector. The vector may be used to
replicate
and express the nucleotide sequence in and/or from a compatible host cell.
Expression
may be controlled using control sequences which include promoters/enhancers
and
other expression regulation signals. Prokaryotic promoters and promoters
functional in
eukaryotic-cells may be --used. Tissue specific or- stimuli specific promoters
may be
10 used. Chimeric promoters may also be used comprising sequence elements from
two
or more different promoters described above.
The protein produced by a host recombinant cell by expression of the
nucleotide
sequence may be secreted or may be contained intracellularly depending on the
15 sequence and/or the vector used. The coding sequences can be designed with
signal
sequences which direct secretion of the substance coding sequences through a
particular prokaryotic or eukaryotic cell membrane.
FUSION PROTEINS
The target amino acid sequence may be produced as a fusion protein, for
example to
aid in extraction and purification. Examples of fusion protein partners
include
glutathione-S-transferase (GST), 6xHis, GAL4 (DNA binding and/or
transcriptional
activation domains) and (3-galactosidase. It may also be convenient to include
a
proteolytic cleavage site between the fusion protein partner and the protein
sequence of
interest to allow removal of fusion protein sequences. Preferably the fusion
protein will
not hinder the activity of the target.
The fusion protein may comprise an antigen or an antigenic determinant fused
to the
substance of the present invention. In this embodiment, the fusion protein may
be a
non-naturally occurring fusion protein comprising a substance which may act as
an
adjuvant in the sense of providing a generalised stimulation of the immune
system. The
antigen or antigenic determinant may be attached to either the amino or
carboxy
terminus of the substance.
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
56
In another embodiment of the invention, the amino acid sequence may be ligated
to a
heterologous sequence to encode a fusion protein. For example, for screening
of
peptide libraries for agents capable of affecting the substance activity, it
may be useful
to encode a chimeric substance expressing a heterologous epitope that is
recognised by
a commercially available antibody.
THERAPY
the compounds of the present invention may be used as therapeutic agents -
i.e. in
therapy applications.
The term "therapy" includes curative effects, alleviation effects, and
prophylactic effects.
The therapy may be on humans or animals, preferably female animals.
PHARMACEUTICAL COMPOSITIONS
In one aspect, the present invention provides a pharmaceutical composition,
which
comprises a compound according to the present invention and optionally a
pharmaceutically acceptable carrier, diluent or excipient (including
combinations
thereof).
The pharmaceutical compositions may be for human or animal usage in human and
veterinary medicine and will typically comprise any one or more of a
pharmaceutically
acceptable diluent, carrier, or excipient. Acceptable carriers or diluents for
therapeutic
use are well known in the pharmaceutical art, and are described, for example,
in
Remington's Pharmaceutical Sciences, Mack Publishing Co. (A. R. Gennaro edit.
1985). The choice of pharmaceutical carrier, excipient or diluent can be
selected with
regard to the intended route of administration and standard pharmaceutical
practice.
The pharmaceutical compositions may comprise as - or in addition to - the
carrier,
excipient or diluent any suitable binder(s), lubricant(s), suspending
agent(s), coating
agent(s), solubilising agent(s).
Preservatives, stabilisers, dyes and even flavouring agents may be provided in
the
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
57
pharmaceutical composition. Examples of preservatives include sodium benzoate,
sorbic acid and esters of p-hydroxybenzoic acid. Antioxidants and suspending
agents
may be also used.
There may be different composition/formulation requirements dependent on the
different
delivery systems. By way of example, the pharmaceutical composition of the
present
invention may be formulated to be delivered using a mini-pump or by a mucosal
route,
for example, as a nasal spray or aerosol for inhalation or ingestable
solution, or
parenterally in which the composition is formulated by an injectable form, for
delivery,
by, for example, an-intravenous, intramuscular-or subcutaneous route.
Alternatively, the
formulation may be designed to be delivered by both routes.
Where the agent is to be delivered mucosally through the gastrointestinal
mucosa, it
should be able to remain stable during transit though the gastrointestinal
tract; for
example, it should be resistant to proteolytic degradation, stable at acid pH
and resistant
to the detergent effects of bile.
Where appropriate, the pharmaceutical compositions can be administered by
inhalation,
in the form of a suppository or pessary, topically in 'the form of a lotion,
solution, cream,
ointment or dusting powder, by use of a skin patch, orally in the form of
tablets
containing excipients such as starch or lactose, or in capsules or ovules
either alone or
in admixture with excipients, or in the form of elixirs, solutions or
suspensions containing
flavouring or colouring agents, or they can be injected parenterally, for
example
intravenously, intramuscularly or subcutaneously. For parenteral
administration, the
compositions. may be best used in the form of a sterile aqueous solution which
may
contain other substances, for example enough salts or monosaccharides to make
the
solution isotonic with blood. For buccal or sublingual administration the
compositions
may be administered in the form of tablets or lozenges which can be formulated
in a
conventional manner.
COMBINATION PHARMACEUTICAL
The compound of the present invention may be used in combination with one or
more
other active agents, such as one or more other pharmaceutically active agents.
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
58
By way of example, the compounds of the present invention may be used in
combination
with other STS inhibitors and/or other inhibitors such as an aromatase
inhibitor (such as
for example, 4-hydroxyandrostenedione (4-OHA)) and/or steroids - such as the
naturally
occurring neurosteroids dehydroepiandrosterone sulfate (DHEAS) and
pregnenolone
sulfate (PS) and/or other structurally similar organic compounds. Examples of
other STS
inhibitors may be found in the above references. By way of example, STS
inhibitors for
use in the present invention include EMATE, and either or both of the 2-ethyl
and 2-
methoxy 17-deoxy compounds that are analogous to compound 5 presented herein.
In addition, or in the alternative, the compound of the present invention may
be used in
combination with a biological response modifier.
The term biological response modifier ("BRM") includes cytokines, immune
modulators,
growth factors, haematopoiesis regulating factors, colony stimulating factors,
chemotactic, haemolytic and thrombolytic factors, cell surface receptors,
ligands,
leukocyte adhesion molecules, monoclonal antibodies, preventative and
therapeutic
vaccines, hormones, extracellular matrix components, fibronectin, etc. For
some
applications, preferably, the biological response modifier is a cytokine.
Examples of
cytokines include: interleukins (IL) - such as IL-1, IL-2, IL-3, IL-4, IL-5,
IL-6, IL-7, IL-8, IL-
9, IL-10, IL-11, IL-12, IL-19; Tumour Necrosis Factor. (TNF) - such as TNF-a;
Interferon
alpha, beta and gamma; TGF-(3. For'some applications, preferably the cytokine
is
tumour necrosis factor (TNF). For some applications, the TNF may be any type
of TNF -
such as TNF-a, TNF-R, including derivatives or mixtures thereof. More
preferably the
cytokine is TNF-a. Teachings on TNF may be found in the art - such as WO-A-
98/08870
and WO-A-98/13348.
ADMINISTRATION
Typically, a physician will determine the actual dosage which will be most
suitable for an
individual subject and it will vary with the age, weight and response of the
particular
patient. The dosages below are exemplary of the average case. There can, of
course,
be individual instances where higher or lower dosage ranges are merited.
The compositions of the present invention may be administered by direct
injection. The
composition may be formulated for parenteral, mucosal, intramuscular,
intravenous,
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
59
subcutaneous, intraocular or transdermal administration. Depending upon the
need, the
agent may be administered at a dose of from 0.01 to 30 mg/kg body weight, such
as
from 0.1 to 10 mg/kg, more preferably from 0.1 to 1 mg/kg body weight.
By way of further example, the agents of the present invention may be
administered in
accordance with a regimen of 1 to 4 times per day, preferably once or twice
per day.
The specific dose level and frequency of dosage for any particular patient may
be varied
and will depend upon a variety of factors including the activity of the
specific compound
employed, the metabolic stability and length of action of that compound, the
age, body
weight, general health, sex, -diet, mode and time of administration, rate of
excretion, drug
combination, the severity of the particular condition, and the host undergoing
therapy.
Aside from the typical modes of delivery - indicated above - the term
"administered"
also includes delivery by techniques such as lipid mediated transfection,
liposomes,
immunoliposomes, lipofectin, cationic facial amphiphiles (CFAs) and
combinations
thereof. The routes for such delivery mechanisms include but are not limited
to
mucosal, nasal, oral, parenteral, gastrointestinal, topical, or sublingual
routes.
The term "administered" includes but is not limited to delivery by a mucosal
route, for
example, as a nasal spray or aerosol for inhalation or as an ingestable
solution; a
parenteral route where delivery is by an injectable form, such as, for
example, an
intravenous, intramuscular or subcutaneous route.
Thus, for pharmaceutical administration, the STS inhibitors of the present
invention can
be formulated in any suitable manner utilising conventional pharmaceutical
formulating
techniques and pharmaceutical carriers, adjuvants, excipients, diluents etc.
and usually
for parenteral administration. Approximate effective dose rates may be in the
range
from 1 to 1000 mg/day, such as from 10 to 900 mg/day or even from 100 to 800
mg/day
depending on the individual activities of the compounds in question and for a
patient of
average (70Kg) bodyweight. More usual dosage rates for the preferred and more
active
compounds will be in the range 200 to 800 mg/day, more preferably, 200 to 500
mg/day,
most preferably from 200 to 250 mg/day. They may be given in single dose
regimes,
split dose regimes and/or in multiple dose regimes lasting over several days.
For oral
administration they may be formulated in tablets, capsules, solution or
suspension
containing from 100 to 500 mg of compound per unit dose. Alternatively and
preferably
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
the compounds will be formulated for parenteral administration in a suitable
parenterally
administrable carrier and providing single daily dosage rates in the range 200
to 800 mg,
preferably 200 to 500, more preferably 200 to 250 mg. Such effective daily
doses will,
however, vary depending on inherent activity of the active ingredient and on
the
5 bodyweight of the patient, such variations being within the skill and
judgement of the
,physician.
CELL CYCLING
10 The compounds of-the. present invention may be useful in the method of
treatment of a
cell cycling disorder.
,As discussed in "Molecular Cell Biology" 3rd Ed. Lodish et al. pages 177-181
different
eukaryotic cells can grow and divide at quite different rates. Yeast cells,
for example,
15 can divide every 120 min., and the first divisions of fertilised eggs in
the embryonic cells
of sea urchins and insects take only 1530 min. because one large pre-existing
cell is
subdivided. However, most growing plant and animal cells take 10-20 hours to
double
in number, and some duplicate at a much slower rate. Many cells in adults,
such as
nerve cells and striated muscle cells, do not divide at all; others, like the
fibroblasts that
20 assist in healing wounds, grow on demand but are otherwise quiescent.
Still, every eukaryotic cell that divides must be ready to donate equal
genetic material to
two daughter cells. DNA synthesis in eukaryotes does not occur throughout the
cell
division cycle but is restricted to a part of it before cell division.
The relationship between eukaryotic DNA synthesis and cell division has been
thoroughly analysed in cultures of mammalian cells that were all capable of
growth and
division. In contrast to bacteria, it was found, eukaryotic cells spend only a
part of their
time in DNA synthesis, and it is completed hours before cell division
(mitosis). Thus a
gap of time occurs after DNA synthesis and before cell division; another gap
was found
to occur after division and before the next round of DNA synthesis. This
analysis led to
the conclusion that the eukaryotic cell cycle consists of an M (mitotic)
phase, a G, phase
(the first gap), the S (DNA synthesis) phase, a G2 phase (the second gap), and
back to
M. The phases between mitoses (G1, S, and G2) are known collectively as the
interphase.
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
61
Many nondividing cells in tissues (for example, all quiescent fibroblasts)
suspend the
cycle after mitosis and just prior to DNA synthesis; such "resting" cells are
said to have
exited from the cell cycle and to be in the Go state.
It is possible to identify cells when they are in one of the three interphase
stages of the
cell cycle, by using a fluorescence-activated cell sorter (FACS) to measure
their relative
DNA content: a cell that is in G, (before DNA synthesis) has a defined amount
x of DNA;
during S (DNA replication), it has between x and 2x; and when in G2 (or M), it
has 2x of
_,DNA.-_
The stages of mitosis and cytokinesis in an animal cell are as follows
(a) Interphase. The G2 stage of interphase immediately precedes the beginning
of
mitosis. Chromosomal DNA has been replicated and bound to protein during the S
phase, but chromosomes are not yet seen as distinct structures. The nucleolus
is the
only nuclear substructure that is visible under light microscope. In a diploid
cell before
DNA replication there are two morphologic chromosomes of each type, and the
cell is
said to be 2n. In G2, after DNA replication, the cell is 4n. There are four
copies of each
chromosomal DNA. Since the sister chromosomes have not yet separated from each
other, they are called sister chromatids.
b) Early prophase. Centrioles, each with a newly formed daughter centriole,
begin
moving toward opposite poles of the cell; the chromosomes can be seen as long
threads. The nuclear membrane-begins to disaggregate into small vesicles.
(c) Middle and late prophase. Chromosome condensation is completed; each
visible chromosome structure is composed of two chromatids held together at
their
centromeres. Each chromatid contains one of the two newly replicated daughter
DNA
molecules. The microtubular spindle begins to radiate from the regions just
adjacent to
the centrioles, which are moving closer to their poles. Some spindle fibres
reach from
pole to pole; most go to chromatids and attach at kinetochores.
(d) Metaphase. The chromosomes move toward the equator of the cell, where they
become aligned in the equatorial plane. The sister chromatids have not yet
separated.
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
62
(e) Anaphase. The two sister chromatids separate into independent chromosomes.
Each contains a centromere that is linked by a spindle fibre to one pole, to
which it
moves. Thus one copy of each chromosome is donated to each daughter cell.
Simultaneously, the cell elongates, as do the pole-to-pole spindles.
Cytokinesis begins
as the cleavage furrow starts to form.
(f) Telophase. New membranes form around the daughter nuclei; the
chromosomes uncoil and become less distinct, the nucleolus becomes visible
again,
and the nuclear membrane forms around each daughter nucleus. Cytokinesis is
nearly
complete, and the spindle disappears as the microtubules and other fibres
depolymerise. Throughout mitosis the "daughter" centriole at each pole grows
until it is
full-length. At telophase the duplication of each of the original centrioles
is completed,
and new daughter centrioles will be generated during the next interphase.
(g) Interphase. Upon the completion of cytokinesis, the cell enters the G,
phase of
the cell cycle and proceeds again around the cycle.
It will be appreciated that cell cycling is an extremely important cell
process. Deviations
from normal cell cycling can result in a number of medical disorders.
Increased and/or
unrestricted cell cycling may result in cancer. Reduced cell cycling may
result in
degenerative conditions. Use of the compound of the present invention may
provide a
means to treat such disorders and conditions.
Thus, the compound of the present invention may be suitable for use in the
treatment of
cell cycling disorders such as cancers, including hormone dependent and
hormone
independent cancers.
In addition, the compound of the present invention may be suitable for the
treatment of
cancers such as breast cancer, ovarian cancer, endometrial cancer, sarcomas,
melanomas, prostate cancer, pancreatic cancer etc. and other solid tumours.
For some applications, cell cycling is inhibited and/or prevented and/or
arrested,
preferably wherein cell cycling is prevented and/or arrested. In one aspect
cell cycling
may be inhibited and/or prevented and/or arrested in the G2/M phase. In one
aspect cell
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
63
cycling may be irreversibly prevented and/or inhibited and/or arrested,
preferably wherein
cell cycling is irreversibly prevented and/or arrested.
By the term "irreversibly prevented and/or inhibited and/or arrested" it is
meant after
application of a compound of the present invention, on removal of the compound
the
effects of the compound, namely prevention and/or inhibition and/or arrest of
cell cycling,
are still observable. More particularly by the term "irreversibly prevented
and/or inhibited
and/or arrested" it is meant that when assayed in accordance with the cell
cycling assay
protocol presented herein, cells treated with a compound of interest show less
growth after
Stage 2 of the protocol I than control cells. Details on this protocol are
presented below.
Thus, the present invention provides compounds which: cause inhibition of
growth of
oestrogen receptor positive (ER+) and ER negative (ER-) breast cancer cells in
vitro by
preventing and/or inhibiting and/or arresting cell cycling; and/or cause
regression of
nitroso-methyl urea (NMU)-induced mammary tumours in intact animals (i.e. not
ovariectomised), and/or prevent and/or inhibit and/or arrest cell cycling in
cancer cells;
and/or act in vivo by preventing and/or inhibiting and/or arresting cell
cycling and/or act as
a cell cycling agonist.
CELL CYCLING ASSAY
(PROTOCOL 7)
Procedure
Stage 1
MCF-7 breast cancer cells are seeded into multi-well culture plates at a
density of 105
cells/well. Cells were allowed to attach and grown until about 30% confluent
when they
are treated as follows:
Control - no treatment
Compound of Interest (COI) 20 M
Cells are grown for 6 days in growth medium containing the COI with changes of
medium/COI every 3 days. At the end of this period cell numbers were counted
using a
Coulter cell counter.
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
64
Stage 2
After treatment of cells for a 6-day period with the COI cells are re-seeded
at a density
of 104 cells/well. No further treatments are added. Cells are allowed to
continue to grow
for a further 6 days in the presence of growth medium. At the end of this
period cell
numbers are again counted.
CANCER
As indicated, the compounds of the present invention may be useful in the
treatment of
a cell cycling disorder. A particular cell cycling disorder is cancer.
Cancer remains a major cause of mortality in most Western countries. Cancer
therapies
developed so far have included blocking the action or synthesis of hormones to
inhibit
the growth of hormone-dependent tumours. However, more aggressive chemotherapy
is currently employed for the treatment of hormone-independent tumours.
Hence, the development of a pharmaceutical for anti-cancer treatment of
hormone
dependent and/or hormone independent tumours, yet lacking some or all of the
side-
effects associated with chemotherapy, would represent a major therapeutic
advance.
It is known that oestrogens undergo a number of hydroxylation and conjugation
reactions after their synthesis. Until recently it was thought that such
reactions were
part of a metabolic process that ultimately rendered oestrogens water soluble
and
enhanced their elimination from the body. It is now evident that some hydroxy
metabolites (e.g. 2-hydroxy and 16alpha-hydroxy) and conjugates (e.g. oestrone
sulphate, E1 S) are important in determining some of the complex actions that
oestrogens have in the body.
Workers have investigated the formation of 2- and 16-hydroxylated oestrogens
in
relation to conditions that alter the risk of breast cancer. There is now
evidence that
factors which increase 2-hydroxylase activity are associated with a reduced
cancer risk,
while those increasing 16alpha-hydroxylation may enhance the risk of breast
cancer.
Further interest in the biological role of estrogen metabolites has been
stimulated by the
growing body of evidence that 2-methoxyoestradiol is an endogenous metabolite
with
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
anti-mitotic properties. 2-MeOE2 is formed from 2-hydroxy estradiol (2-OHE2)
by
catechol estrogen methyl transferase, an enzyme that is widely distributed
throughout
the body.
5 Workers have shown that in vivo 2-MeOE2 inhibits the growth of tumours
arising from
the subcutaneous injection of Meth A sarcoma, B16 melanoma or MDA-MB-435
estrogen receptor negative (ER-) breast cancer cells. It also inhibits
endothelial cell
proliferation and migration, and in vitro angiogenesis. It was suggested that
the ability of
2-MeOE2 to inhibit tumour growth in vivo may be due to its ability to inhibit
tumour-
10 - induced-angiogenesis rather than-direct inhibition of the proliferation
of tumour cells.
The mechanism by which 2-MeOE2 exerts its potent anti-mitogenic and anti-
angiogenic
effects is still being elucidated. There is evidence that at high
concentrations it can
inhibit microtubule polymerisation and act as a weak inhibitor of colchicine
binding to
15 tubulin. Recently, however, at concentrations that block mitosis, tubulin
filaments in
cells were not found to be depolymerised but to have an identical morphology
to that
seen after taxol treatment. It is possible, therefore, that like taxol, a drug
that is used for
breast and ovarian breast cancer therapy, 2-MeOE2 acts by stabilising
microtubule
dynamics.
While the identification of 2-MeOE2 as a new therapy for cancer represents an
important
advance, the bioavailability of orally administered oestrogens is poor.
Furthermore, they
can undergo extensive metabolism during their first pass through the liver. As
part of a
research programme to develop a steroid sulphatase inhibitor for breast cancer
therapy,
oestrone-3-O-sulphamate (EMATE) was identified as a potent active site-
directed
inhibitor. Unexpectedly, EMATE proved to possess potent oestrogenic properties
with
its oral uterotrophic activity in rats being a 100-times higher than that of
estradiol. Its
enhanced oestrogenicity is thought to result from its absorption by red blood
cells (rbcs)
which protects it from inactivation during its passage through the liver and
which act as a
reservoir for its slow release for a prolonged period of time. A number of A-
ring modified
analogues were synthesised and tested, including 2-methoxyoestrone-3-O-
sulphamate.
While this compound was equipotent with EMATE as a steroid sulphatase
inhibitor, it
was devoid of oestrogenicity.
We believe that the compound of the present invention provides a means for the
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
66
treatment of cancers and, especially, breast cancer.
In addition or in the alternative the compound of the present invention may be
useful in
the blocking the growth of cancers including leukaemias and solid tumours such
as
breast, endometrium, prostate, ovary and pancreatic tumours.
THERAPY CONCERNING OESTROGEN
We believe that some of the compounds of the present invention may be useful
in the
qbntrol of oestrogen levels in the body - in particular in females. Thus, some
of the
compounds may be useful as providing a means of fertility control - such as an
oral
contraceptive tablet, pill, solution or lozenge. Alternatively, the compound
could be in
the form of an implant or as a patch.
Thus, the compounds of the present invention may be useful in treating
hormonal
conditions associated with oestrogen.
In addition or in the alternative the compound of the present invention may be
useful in
treating hormonal conditions in addition to those associated with oestrogen.
Hence, the
compound of the present invention may also be capable of affecting hormonal
activity
and may also be capable of affecting an immune response.
NEURODEGENERATIVE DISEASES
We believe that some of the compounds of the present invention may be useful
in the
treatment of neurodenerative diseases, and similar conditions.
By way of example, it is believed that STS inhibitors may be useful in the
enhancing the
memory function of patients suffering from illnesses such as amnesia, head
injuries,
Alzheimer's disease, epileptic dementia, presenile dementia, post traumatic
dementia,
senile dementia, vascular dementia and post-stroke dementia or individuals
otherwise
seeking memory enhancement.
TH1
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
67
We believe that some of the compounds of the present invention may be useful
in TH1
implications.
By way of example, it is believed that the presence of STS inhibitors within
the
macrophage or other antigen presenting cells may lead to a decreased ability
of
sensitised T cells to mount a TH1 (high IL-2, IFNy low IL-4) response. The
normal
regulatory influence of other steroids such as glucocorticoids would therefore
predominate.
INFLAMATORY CONDITIONS
We believe that some of the compounds of the present invention may be useful
in
treating inflammatory conditions - such as conditions associated with any one
or more
of: autoimmunity, including for example, rheumatoid arthritis, type I and II
diabetes,
systemic lupus erythematosus, multiple sclerosis, myasthenia gravis,
thyroiditis, vasculitis,
ulcerative colitis and Crohn's disease, skin disorders e.g. psoriasis and
contact dermatitis;
graft versus host disease; eczema; asthma and organ rejection following
transplantation.
By way of example, it is believed that STS inhibitors may prevent the normal
physiological effect of DHEA or related steroids on immune and/or inflammatory
responses.
The compounds of the present invention may be useful in the manufacture of a
medicament for revealing an endogenous glucocorticoid-like effect.
OTHER THERAPIES
It is also to be understood that the compound/composition of the present
invention may
have other important medical implications.
For example, the compound or composition of the present invention may be
useful in the
treatment of the disorders listed in WO-A-99/52890 - viz:
In addition, or in the alternative, the compound or composition of the present
invention
may be useful in the treatment of the disorders listed in WO-A-98/05635. For
ease of
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
68
reference, part of that list is now provided: cancer, inflammation or
inflammatory
disease, dermatological disorders, fever, cardiovascular effects, haemorrhage,
coagulation and acute phase response, cachexia, anorexia, acute infection, HIV
infection, shock states, graft-versus-host reactions, autoimmune disease,
reperfusion
injury, meningitis, migraine and aspirin-dependent anti-thrombosis; tumour
growth,
invasion and spread, angiogenesis, metastases, malignant, ascites and
malignant
pleural effusion; cerebral ischaemia, ischaemic heart disease, osteoarthritis,
rheumatoid
arthritis, osteoporosis, asthma, multiple sclerosis, neurodegeneration,
Alzheimer's
disease, atherosclerosis, stroke, vasculitis, Crohn's disease and ulcerative
colitis;
pbriodontitis; gingivitis; psoriasis, atopic -dermatitis, chronic ulcers,
epidermolysis
bullosa; corneal ulceration, retinopathy and surgical wound healing; rhinitis,
allergic
conjunctivitis, eczema, anaphylaxis; restenosis, congestive heart failure,
endometriosis,
atherosclerosis or endosclerosis.
In addition, or in the alternative, the compound or composition of the present
invention
may be useful in the treatment of disorders listed in WO-A-98/07859. For ease
of
reference, part of that list is now provided: cytokine and cell
proliferation/differentiation
activity; immunosuppressant or immunostimulant activity (e.g. for treating
immune
deficiency, including infection with human immune deficiency virus; regulation
of
lymphocyte growth; treating cancer and many autoimmune diseases, and to
prevent
transplant rejection or induce tumour immunity); regulation of haematopoiesis,
e.g.
treatment of myeloid or lymphoid diseases; promoting growth of bone,
cartilage, tendon,
ligament and nerve tissue, e.g. for healing wounds, treatment of burns, ulcers
and
periodontal disease and neurodegeneration; inhibition or activation of
follicle-stimulating
hormone (modulation of fertility); chemotactic/chemokinetic activity (e.g. for
mobilising
specific cell types to sites of injury or infection); haemostatic and
thrombolytic activity
(e.g. for treating haemophilia and stroke); antiinflammatory activity (for
treating e.g.
septic shock or Crohn's disease); as antimicrobials; modulators of e.g.
metabolism or
behaviour; as analgesics; treating specific deficiency disorders; in treatment
of e.g.
psoriasis, in human or veterinary medicine.
In addition, or in the alternative, the composition of the present invention
may be useful
in the treatment of disorders listed in WO-A-98/09985. For ease of reference,
part of
that list is now provided: macrophage inhibitory and/or T cell inhibitory
activity and thus,
anti-inflammatory activity; anti-immune activity, i.e. inhibitory effects
against a cellular
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
69
and/or humoral immune response, including a response not associated with
inflammation; inhibit the ability of macrophages and T cells to adhere to
extracellular
matrix components and fibronectin, as well as up-regulated fas receptor
expression in T
cells; inhibit unwanted immune reaction and inflammation including arthritis,
including
rheumatoid arthritis, inflammation associated with hypersensitivity, allergic
reactions,
asthma, systemic lupus erythematosus, collagen diseases and other autoimmune
diseases, inflammation associated with atherosclerosis, arteriosclerosis,
atherosclerotic
heart disease, reperfusion injury, cardiac arrest, myocardial infarction,
vascular
inflammatory disorders, respiratory distress syndrome or other cardiopulmonary
-djseases, inflammation associated with peptic ulcer, ulcerative colitis and
other diseases
of the gastrointestinal tract, hepatic fibrosis, liver cirrhosis or other
hepatic diseases,
thyroiditis or other glandular diseases, glomerulonephritis or other renal and
urologic
diseases, otitis or other oto-rhino-laryngological diseases, dermatitis or
other dermal
diseases, periodontal diseases or other dental diseases, orchitis or epididimo-
orchitis,
infertility, orchidal trauma or other immune-related testicular diseases,
placental
dysfunction, placental insufficiency, habitual abortion, eclampsia, pre-
eclampsia and
other immune and/or inflammatory-related gynaecological diseases, posterior
uveitis,
intermediate uveitis, anterior uveitis, conjunctivitis, chorioretinitis,
uveoretinitis, optic
neuritis, intraocular inflammation, e.g. retinitis or cystoid macular oedema,
sympathetic
ophthalmia, scleritis, retinitis pigmentosa, immune and inflammatory
components of
degenerative fondus disease, inflammatory components of ocular trauma, ocular
inflammation caused by infection, proliferative vitreo-retinopathies, acute
ischaemic optic
neuropathy, excessive scarring, e.g. following glaucoma filtration operation,
immune
and/or inflammation reaction against ocular implants and other immune and
inflammatory-related ophthalmic diseases, inflammation associated with
autoimmune
diseases or conditions or disorders where, both in the central nervous system
(CNS) or
in any other organ, immune and/or inflammation suppression would be
beneficial,
Parkinson's disease, complication and/or side effects from treatment of
Parkinson's
disease, AIDS-related dementia complex HIV-related encephalopathy, Devic's
disease,
Sydenham chorea, Alzheimer's disease and other degenerative diseases,
conditions or
disorders of the CNS, inflammatory components of stokes, post-polio syndrome,
immune and inflammatory components of psychiatric disorders, myelitis,
encephalitis,
subacute sclerosing pan-encephalitis, encephalomyelitis, acute neuropathy,
subacute
neuropathy, chronic neuropathy, Guillaim-Barre syndrome, Sydenham chora,
myasthenia gravis, pseudo-tumour cerebri, Down's Syndrome, Huntington's
disease,
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
amyotrophic lateral sclerosis, inflammatory components of CNS compression or
CNS
trauma or infections of the CNS, inflammatory components of muscular atrophies
and
dystrophies, and immune and inflammatory related diseases, conditions or
disorders of
the central and peripheral nervous systems, post-traumatic inflammation,
septic shock,
5 infectious diseases, inflammatory complications or side effects of surgery,
bone marrow
transplantation or other transplantation complications and/or side effects,
inflammatory
and/or immune complications and side effects of gene therapy, e.g. due to
infection with
a viral carrier, or inflammation associated with AIDS, to suppress or inhibit
a humoral
and/or cellular immune response, to treat or ameliorate monocyte or leukocyte
io proliferative diseases, e.g. leukaemia, by reducing the amount of monocytes
or
lymphocytes, for the prevention and/or treatment of graft rejection in cases
of
transplantation of natural or artificial cells, tissue and organs such as
cornea, bone
marrow, organs, lenses, pacemakers, natural or artificial skin tissue.
15 COMPOUND PREPARATION
The compounds of the present invention may be prepared by reacting an
appropriate
alcohol with a suitable chloride. By way of example, the sulphamate compounds
of the
present. invention may be prepared by reacting an appropriate alcohol with a
suitable
20 sulfamoyl chloride, of the formula R'R5NSO2CI.
Typical conditions for carrying out the reaction are as follows.
Sodium hydride and a sulfamoyl chloride are added to a stirred solution of the
alcohol in
25 anhydrous dimethyl formamide at 0 C. Subsequently, the reaction is allowed
to warm to
room temperature whereupon stirring is continued for a further 24 hours. The
reaction
mixture is poured onto a cold saturated solution of sodium bicarbonate and the
resulting
aqueous phase is extracted with dichloromethane. The combined organic extracts
are
dried over anhydrous MgSO4. Filtration followed by solvent evaporation in
vacuo and
30 co-evaporated with toluene affords a crude residue which is further
purified by flash
chromatography.
Preferably, the alcohol is derivatised, as appropriate, prior to reaction with
the sulfamoyl
chloride. Where necessary, functional groups in the alcohol may be protected
in known
35 manner and the protecting group or groups removed at the end of the
reaction.
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
71
Preferably, the sulphamate compounds are prepared according to the teachings
of Page
et al (1990 Tetrahedron 46; 2059-2068).
The phosphonate compounds may be prepared by suitably combining the teachings
of
Page et al (1990 Tetrahedron 46; 2059-2068) and PCT/GB92/01586.
The sulphonate compounds may be prepared by suitably adapting the teachings of
Page et al (1990 Tetrahedron 46; 2059-2068) and PCTIGB92/01586.
The thiophosphonate compounds may be prepared by suitably adapting the
teachings of
Page et al (1990 Tetrahedron 46; 2059-2068) and PCT/GB91/00270.
Preferred preparations are also presented in the following text.
SUMMARY
In summation, the present invention provides novel compounds for use as
steroid
sulphatase inhibitors and/or aromatase inhibitors and/or modulators of
apoptosis and/or
modulators of cell cycling and/or cell growth, and pharmaceutical compositions
containing them.
EXAMPLES
The present invention will now be described in further detail by way of
example only with
reference to the accompanying figure in which:-
Figure 1 shows a summary scheme;
Figure 2 shows a summary scheme;
Figure 3 shows a graph; and
Figure 4 shows a graph.
The present invention will now be described only by way of example. However,
it is to
be understood that the examples also present preferred compounds of the
present
invention, as well as preferred routes for making same and useful
intermediates in the
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
72
preparation of same.
Synthetic Routes
Compounds in accordance with the present invention were synthesised in
accordance
with the synthetic routes and schemes.
Compounds of Formula III
-4-([i,24]Triazol-4-ylamino)benzonitrile (LWO02023)
N- N
I ~ 1
N
NH
NC
To a mixture of potassium tert-butoxide (6.7 g, 59.47 mmol) in anhydrous
methyl
sulfoxide (DMSO, 20 mL) was added at 10 -15 C portionwise a solution of 4-
amino-
4H-1,2,4-triazole (5.0 g, 59.47 mmol) in anhydrous DMSO (10 mL). After
stirring the
resulting thick light yellow suspension at room temperature under nitrogen for
60 min,
this was cooled to ice/water temperature and a solution of 4-
fluorobenzonitrile (3.60 g,
29.74 mmol) in anhydrous DMSO (10 mL) was added dropwise over a period of 5
min.
The orange suspension that formed was stirred at room temperature under
nitrogen for 1
h before it was poured into water (500 mL). The pH of the clear yellow mixture
that
formed was brought to neutral by using 5M HCL followed by saturated aqueous
sodium
bicarbonate solution if required. This mixture was allowed to stand at room
temperature
uncovered for 7 days at which yellow crystals were deposited. Upon filtration,
washings
exhaustively with water and air-drying overnight, 4-([1,2,4]triazol-4-
ylamino)benzonitrile (2.08 g, 11.23 mmol, 37.8%) was collected; m.p. 200-204
C; Sx
(400MHz, DMSO-d6) 6.55 (2H, AA'BB'), 7.69 (2H, AA'BB'), 8.85 (2H, s, C3'-H and
C5'-H) and 10.23 (1H, br s, exchanged with D20, NH).
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
73
4-[(4-Benzyloxybenzyl)-[1,2,4]triazol-4-ylamino]benzonitrile (LWO02029)
N--N
N
BnO
~ I \
CN
To a stirred solution of LWO02023 (700 mg, 3.780 mmol) in DMF (10 mL) at
ice/water
temperature was added sodium hydride (60% in mineral oil, 151 mg, 3.780 mmol).
After
stirring at room temperature under an atmosphere of nitrogen for 30 min, 4-
benzyloxybenzyl chloride (968 mg, 4.158 mmol) was added in one portion and the
resulting orange/brown mixture was heated at 80-90 C for 3 h. The yellow
suspension
thus formed at room temperature was diluted with water (200 mL) and the white
precipitate that formed was collected, washed exhaustively with water and air-
dried to
give 4-[(4-benzyloxybenzyl)-[1,2,4]triazol-4-ylamino]benzonitrile (1.35 g,
3.539 mmol,
94%) as white powder; m.p. 206-211 C; Rf 0.37 (neat ethyl acetate), c.f. 0.83
(4-
benzyloxybenzyl chloride); 8H (400MHz, DMSO-d6) 4.98 (2H, s CH2N), 5.06 (2H,
s,
CH2O), 6.77 (2H, AA'BB'), 6.95 (2H, AA'BB'), 7.21 (2H, AA'BB'), 7.30-7.46
(514, m,
Bn), 7.76 (2H, AA'BB') and 8.75 (2H, s, C3'-H and C5'-H); LRMS (FAB+):
763.3[7,
(2M+H)+], 382.2[100, (M+H)+], 313.1[48, (M+H-triazole)+]; (FAB-): 687.3[28,
(M+2NBA)"], 534.2[100, (M+NBA)"]; HRMS (FAB+) 382.16648 C23H2ON50 requires
382.16679.
4-[(4-Hydroxylbenzyl)-[1,2,4]triazol-4-ylamino]benzonitrile (LWO02030, STX265)
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
74
N-N
N STX265
HO N
aCN
To a stirred solution of 4-[(4-benzyloxybenzyl)-[1,2,4]triazol-4-
ylamino]benzonitrile
(705 mg, 1.848 mmol) in distilled THE (120 mL) was added in succession
absolute
ethanol (30 mL) and Pd/C (10%, 40 mg). The black suspensionS was then stirred
under an
atmosphere of hydrogen (balloon) for 3 days. Upon removal by filtration and
washings
of the supported catalyst exhaustively with distilled THF, the filtrate was
evaporated to
give a slight wet light yellow residue (529 mg). The crude was recrystallised
from
DMF/ethyl acetate (1:10, 33 mL) to give 4-[(4-hydroxylbenzyl)-[1,2,4]triazol-4-
ylamino]benzonitrile as yellow crystals (138 mg, 473.7,umol, 25.6%); m.p. 228-
230 C;
Rf 0.24 (neat ethyl acetate), c.f. 0.40 (S.M.); 9f-1(400MHz, DMSO-d6) 4.91
(2H, s CH2N),
6.67 (2H, AA'BB'), 6.77 (2H, AA'BB'), 7.06 (2H, AA'BB'), 7:76 (2H, AA'BB'),
8.71
(2H, s, C3'-H and C5'-H) and 9.49 (111, s, exchanged with D20, OH); LRMS
(FAB+):
583.3[9, (2M+H)+], 445.2[13, (M+H+NBA)+], 292.2[100, (M+H)+], 223.1[50, (M-
triazole)+]; (FAB-): 444.2[36, (M+NBA)"], 184.1 [100, (M-C7H70)-]; HRMS (FAB+)
292.11871 C16H14N50 requires 292.11984.
Sulfamic acid - 4-{[(4-cyanophenyl)-[1,2,4]triazol-4-ylamino]methyl}phenyl
ester
(LWO02031, STX258)
N-N
N STX258
H2NO2SO N
~aCN
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
To a stirred solution of 4-[(4-hydroxylbenzyl)-[1,2,4]triazol-4-
ylamino]benzonitrile (265
mg, 715.4 ,umol) in anhydrous N,N-dimethylacetamide (DMA, 20 mL) was added at
room temperature a solution of sulfamoyl chloride in toluene (ca. 0.68 M, 3.6
mL) and
the resulting mixture stirred under an atmosphere of nitrogen overnight. Ethyl
acetate
5 (100 mL) was added to the reaction mixture and the organic layer that
separated was
washed with brine (100 mL, 4 x 50 mL), dried (MgSO4) and evaporated to give a
light
brown syrup/residue (ca. 400 mg). This crude was fractionated by flash
chromatography
(chloroform/methanol, 7:1 to 3.5:1, gradient) and the third fraction that
isolated gave
sulfamic acid 4-{[(4-cyanophenyl)-[1,2,4]triazol-4-ylamino]methyl}phenyl ester
as a
10 pale beige residue (150 mg, 405.0 ,umol, 57%); m.p. 80-95 C; Rf 0.57
(chloroform/methanol, 5:1), c.f. 0.67 (S.M.); 8x (400MHz, DMSO-d6) 5.10 (2H, s
CH2N), 6.74 (2H, AA'BB'), 7.23 (2H, AA'BB'), 7.42 (2H, AA'BB'), 7.77 (211,
AA'BB'), 8.03 (2H, br s, exchanged with D20, H2NSO2) and 8.85 (2H, s, C3'-H
and
C5'-H); LRMS (FAB+): 371.1 [100, (M+H)+], 302.1 [30, (M-triazole)+]; (FAB-):
15 523.2[30, (M+NBA)"], 369.1 [100, (M-H)], 184.1 [34, (M-Bn-OSO2NH2)-]; HRMS
(FAB+) 371.09116 C16H15N603S requires 371.09264.
2-(4-Benzyloxyphenyl)ethanol (LWO02057)
OH
Bn0
To a stirred solution of 4-hydroxyphenethyl alcohol (3.0 g, 22.16 mmol) in
anhydrous
DMF (50 mL) at ice/water temperature was added sodium hydride (60% in mineral
oil,
886 mg, 22.16 mmol). After stirring at room temperature for 10 min, benzyl
bromide
(3.86 g, 22.16 mmol) was added and the reaction mixture was heated at 50 C
for 30 min.
Upon cooling to room temperature, ethyl acetate (250 mL) was added and the
organic
layer that separated was washed with brine (500 mL, 4 x 100 mL), dried (MgSO4)
and
evaporated to give a white residue (6.05 g). The crude was first dissolved in
hot
isopropanol (10 mL) and hexane (10 mL) was then added dropwise. Upon cooling,
2-(4-
benzyloxyphenyl)ethanol was isolated as soft white crystals (2.45 g, 10.73
mmol). A
second crop of the product (2.04 g, 8.936 mmol, total yield: 89%) was obtained
from the
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
76
residue of the mother liquor upon recrystallisation from hot hexane (ca. 150
mL); Rf 0.71
(neat ethyl acetate), 0.63 (S.M.); bH (400MHz, DMSO-d6) 2.64 (2H, t, J7.2 Hz,
CH CH2OH), 3.54 (2H, in, reduced to t after D20 exchange, CH2CH OH), 4.59 (1H,
t,
J5.2Hz, exchanged with D20, OH), 5.06 (2H, S, CH2O), 6.90 (2H, AA'BB'), 7.11
(2H,
AA'BB') and 7.28-7.46 (5H, in, Bn); LRMS (FAB+): 228.0[94, M+], 91.0[100,
Bn+].
1-Bromo-2-(4-benzyloxyphenyl)ethane (LWO02060)
Bn0
<D-Br
To a stirred solution of 2-(4-benzyloxyphenyl)ethanol (2.02 g, 8.848 mmol) in
anhydrous
distilled THE (30 mL) at ice/water temperature and under an atmosphere of
nitrogen was
added phosphorus tribromide (2.47 g, 8.848 mmol). After stirring at room
temperature
for 30 min, the reaction mixture was evaporated and the pale yellow liquid
thus obtained
was diluted with ethyl acetate (100 mL). The organic layer that separated was
washed
with brine (100 mL, 4 x 50 mL), -dried (MgSO4) and evaporated to give a light
orange
brown syrup (3.21 g) which turned light yellow upon standing overnight. This
crude was
fractionated by flash chromatography (chloroform/ethyl acetate, 1:2 to 1:1,
gradient) and
the first fraction that separated gave 1-bromo-2-(4-benzyloxyphenyl)ethane as
a yellow
solid (990 mg, 3.40 mmol, 38.4%); Rf 0.82 (neat ethyl acetate), 0.68 (S.M.);
SH
(400MHz, DMSO-d6) 3.04 (2H, t, J 7.2 Hz, CH CH2Br), 3.67 (2H, t, J 7.4 Hz,
CH2CH Br), 5.08 (2H, S, CH2O), 6.95 (2H, AA'BB'), 7.20 (2H, AA'BB') and 7.30-
7.48
(5H, in, Bn); LRMS (FAB+): 289.9(9), 73.0(100), (FAB-) 233.9(100).
4-{[2-(4-Benzyloxyphenyl)ethyl]-[1,2,4]triazol-4-yl-amino}benzonitrile
(LWO02061)
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
77
NON
N
BnO
~ I \
CN
To a stirred solution of 4-([1,2,4]triazol-4-ylamino)benzonitrile (549 mg,
2.966 mmol) in
DMF (10 mL) at ice/water temperature was added sodium hydride (60% in mineral
oil,
137 mg, 3.426 mmol). After stirring at room temperature under an atmosphere of
nitrogen for 30 min, 1-bromo-2-(4-benzyloxyphenyl)ethane (950 mg, 3.263 mmol)
was
added in one portion and the resulting dark brown mixture was heated at 70 C
for 3 h.
The reddish brown mixture that formed was diluted with ice/water (200 mL) and
the
precipitate that formed was collected, washed exhaustively with water and air-
dried to
give a light orange yellow residue (1.21 g). This crude was fractionated by
flash
chromatography (dry loading, ethyl acetate as eluant) and the second fraction
that
separated gave 4-{[2-(4-benzyloxyphenyl)ethyl]-[1,2,4]triazol-4-yl-
amino}benzonitrile
as a yellow syrup which solidified upon standing overnight at room temperature
to a light
yellow wax (590 mg, 1.492 mmol, 50.3%); Rf 0.40 (neat ethyl acetate), c.f.
0.85 (1-
bromo-2-(4-benzyloxyphenyl)ethane); 4x (400MHz, DMSO-d6) 2.74 (2H, t, J-7.6
Hz,
CH CH2N), 4.04 (2H, t, J7.4 Hz, CH2CH N), 5.08 (2H, s, CH2O), 6.59 (2H,
AA'BB'),
6.94 (2H, AA'BB'), 7.20 (2H, AA'BB'), 7.28-7.46 (5H, m, Bn), 7.70 (2H, AA'BB')
and
8.83 (2H, s, C3'-H and C5'-H); LRMS (FAB+): 396.1[100, (M+H)+], 369.3[5, (M-
CN)+], 91.0[82, Bn+]; (FAB-): 701.4[25, (M+2NBA)-], 548.3[100, (M+NBA)-],
441.2[35, (M+NBA-OBn)"]; HRMS (FAB+) 396.18192 C24H22N50 requires 396.18244.
4-{ [2-(4-Hydroxyphenyl)ethyl]-[l,2,4]triazol-4-yl-amino}benzonitrile
(LWO02063,
STX290)
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
78
N--N
N STX290
HO \ N
~aCN
To a stirred solution of 4-{[2-(4-benzyloxyphenyl)ethyl]-[1,2,4]triazol-4-yl-
amino}benzonitrile (536 mg, 1.355 mmol) in distilled THE (15 mL) was added in
succession absolute ethanol (30 mL) and Pd/C (10%, 54 mg). The black
suspension was
then stirred under an atmosphere of hydrogen (balloon) for 3 days. Upon
removal by
filtration and washings of the supported catalyst exhaustively with distilled
THF, the
filtrate was evaporated to give a pale yellow syrup which solidified upon
standing at
room temperature to a light yellow wax (192 mg). This crude was fractionated
by flash
chromatography (ethyl acetate) and the second fraction that separated gave 4-
{ [2-(4-
hydroxyphenyl)ethyl]-[1,2,4]triazol-4-yl-amino}benzonitrile as a pale yellow
waxy
residue (115 mg, 376.6 ,umol, 28%); Rf 0.46 (neat ethyl acetate), c.f. 0.59
(S.M.); 4-1
(400MHz, DMSO-d6) 2.70 (2H, t, J 7.4 Hz, CH CH2N), 4.01 (2H, t, J'-7.6 Hz,
CH2N),
6.59 (2H, AA'BB'), 6.69 (2H, AA'BB'), 7.06 (2H, AA'BB'), 7.71 (2H, AA'BB'),
8.78
(2H, s, C3'-H and C5'-H) and 9.29 (1H, s, exchanged with D20, OH); LRMS
(FAB+):
611.2[12, (2M+H)+], 459.1[8, (M+H+NBA)+], 306.0[100, (M+H)+]; (FAB-):
763.5[18,
(2M+NBA)"], 609.4[45, (2M-H)"], 184.0[100, (HOPhCH2CH2)"1; HRMS (FAB+)
306.13477 C17H16N50 requires 306.13549.
Sulfamic acid 4-{2-[(4-cyanophenyl)-[1,2,4]triazol-4-ylamino]ethyl} phenyl
ester
(LWO02066, STX273)
N--N
N STX273
H2NO2SO
CN
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
79
To a stirred solution of 4-{[2-(4-hydroxyphenyl)ethyl]-[1,2,4]triazol-4-yl-
amino}benzonitrile (81 mg, 265.2 ,umol) in anhydrous N,N-dimethylacetamide
(DMA, 5
mL) was added at room temperature a solution of sulfamoyl chloride in toluene
(ca. 0.68
M, 1.2 mL) and the resulting mixture was stirred under an atmosphere of
nitrogen
overnight. Ethyl acetate (50 mL) was then added to the reaction mixture and
the organic
layer that separated was washed with brine (100 mL, 4 x 50 mL), dried (MgS04),
filtered
and evaporated to give a light brown syrup/residue (ca. 100 mg). This crude
was
dissolved in acetone (20 mL) and then concentrated to approximately 3 mL.
Hexane (1.5
to mL) was then added dropwise and upon standing gave sulfamic acid 4-{2-[(4-
cyanophenyl)-[l,2,4]triazol-4-ylamino]ethyl) phenyl ester as white crystals
(52 mg, 135.3
nol, 51%); Rf 0.33 (ethyl acetate), c.f. 0.41 (S.M.); Sji (400MHz, DMSO-d6)
2.84 (2H,
t, J 7 Hz, CH CH2N), 4.09 (2H, t, J-7 Hz, CH2N), 6.63 (2H, AA'BB'), 7.20 (2H,
AA'BB'), 7.38 (2H, AA'BB'), 7.72 (2H, AA'BB'), 7.97 (2H, br s, exchanged with
D20,
OSO2NH2) and 8.87 (2H, s, C3'-H and C5'-H); LRMS (FAB+): 385.0[100, (M+H)+];
(FAB-): 537.2[40, (M+NBA)-], 383.1 [100, (M-H)"]; HRMS (FAB+) 385.10752
C17H17N603S requires 385.10829.
1-Benzyloxy-4-(2-bromoethoxy)benzene (LWO02068)
Br
Bn0
This compound was prepared from 4-(benzyloxy)phenol and 1,2-dibromoethane in
the
same manner as described by Zhou et. Al. (1999) J. Med. Chem. 42: 2993-3000.
4-{[2-(4-Benzyloxyphenoxy)ethyl]-[1,2,4]triazol-4-yl-amino}benzonitrile
(LWO02075)
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
N--N
BnO _c)-o-'-\i N
N ~aCN
To a stirred solution of 4-([1,2,4]triazol-4-ylamino)benzonitrile (1.0 g, 5.40
mmol) in
DMF (15 mL) at ice/water temperature was added sodium hydride (60% in mineral
oil,
5 2'38 mg, 5.94 mmol). After stirring at room temperature under an atmosphere
of nitrogen
for 10 min, 1-benzyloxy-4-(2-bromoethoxy)benzene (1.82 g, 5.94 mmol) was added
in
one portion and the resulting dark brown mixture was heated at 50 C for 18 h.
The
reaction mixture was then passed through a short column of silica and the
filter cake was
washed with ethyl acetate (10 x 20 mL). The combined filtrate was washed with
brine
10 (200 mL, 4 x 50 mL), dried (MgSO4), filtered and evaporated to give a light
yellow
brown residue (2.28 g). This crude was recrystallised from hot ethyl acetate
to give 4-
{[2-(4-benzyloxyphenoxy)ethyl]-[1,2,4]triazol-4-yl-amino}benzonitrile (690 mg,
1.677
mmol) as fluffy creamy crystals. A second crop of the product (915 mg, 2.224
mmol,
total yield, 72%) was obtained from the residue of the mother liquor upon
15 recrystallisation from hot ethyl acetate and hexane; m.p. 160-162 C; Rf
0.45 (neat ethyl
acetate), c.f. 0.89 (1-benzyloxy-4-(2-bromoethoxy)benzene); (400MHz, DMSO-d6)
4.08 (2H, in, CH2), 4.23 (2H, in, CH2), 5.03 (2H, s, CH2O), 6.67 (2H, AA'BB'),
6.82
(2H, AA'BB'), 6.93 (2H, AA'BB'), 7.28-7.46 (5H, in, Bn), 7.74 (2H, AA'BB') and
8.91
(2H, s, C3'-H and C5'-H). Found C 69.7, H 5.16, N 16.7; C24H21N502 requires C
70.06,
20 H 5.14, N 17.02%.
4-{[2-(4-Hydroxyphenoxy)ethyl]-[1,2,4]triazol-4-yl-amino}benzonitrile
(LWO02076,
STX291)
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
81
N-N
t
STX291
HO \ N
~ O I \
CN
To a stirred solution of 4-{[2-(4-benzyloxyphenoxy)ethyl]-[1,2,4]triazol-4-yl-
ainino}benzonitrile (670 mg, 1.628 mmol) in distilled THE (70 mL) was added in
succession absolute ethanol (30 mL) and Pd/C (10%, 67 mg). The black
suspension was
then stirred under an atmosphere of hydrogen (balloon) for 3 days. Upon
removal by
filtration and washings of the supported catalyst exhaustively with distilled
THF, the
filtrate was evaporated to give a light yellow residue (491 mg). This crude
was dissolved
in hot acetone (25 mL) and hexane (15 mL) was then added dropwise. Upon
cooling, 4-
{[2-(4-hydroxyphenoxy)ethyl]-[1,2,4]triazol-4-yl-amino}benzonitrile was
separated as
light green yellow crystals (310 mg, 964.1 umol, 59%); m.p. 184-195 C; Rf
0.31 (neat
ethyl acetate), c.f. 0.45 (S.M.); J1-1 (400MHz, DMSO-d6) 4.03 (2H, t, J 4.6-
5.1 Hz, CH2),
4.21 (2H, t, J 5.1 Hz, CH2), 6.62-6.73 (6H, m, Ar), 7.73 (2H, AA'BB'), '8.89
(2H, s, C3'-
H and C5'-H) and 8.95 (1H, s, exchanged with D20, OH); LRMS (FAB+): 643.2[12,
(2M-H)+], 475.1 [100, (M+H+NBA)+], 322.1 [100, (M+H)+], 253.1 [20, (M-
triazole)+];
(FAB-): 795.1[10, (2M+NBA)], 641.2[30, (2M-H)-], 474.2[90, (M+NBA)"],
320.1[100,
(M-H)]; HRMS (FAB+) 322.12984 C17H16Ns02 requires 322.13040.
Sulfamic acid 4-{2-[(4-cyanophenyl)-[1,2,4]triazol-4-yl-amino]ethoxy}phenyl
ester
(LWO02077, STX292)
N--N
1
NI STX292
H2NO2SO \
CN
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
82
To a stirred solution of 4-{[2-(4-hydroxyphenoxy)ethyl]-[1,2,4]triazol-4-yl-
amino}benzonitrile (212 mg, 659.7 ,umol) in anhydrous N,N-dimethylacetamide
(DMA,
mL) was added at room temperature a solution of sulfamoyl chloride in toluene
(ca.
0.68 M, 2 mL) and the resulting mixture was stirred under an atmosphere of
nitrogen for
5 1.5 h., Ethyl acetate (100 mL) was then added to the reaction mixture and
the organic
layer that separated was washed with brine (100 mL, 4 x 50 mL), dried (MgS04),
filtered
and evaporated to give a white fluffy residue (299 mg). This crude was
fractionated by
flash chromatography (ethyl acetate, then ethyl acetate/acetone 2:1 after the
second
fraction was eluted) and upon evaporation of the third fraction that isolated
gave sulfamic
acid 4-{2-[(4-cyanophenyl)-[1,2,4]triazol-4-yl-amino]ethoxy}phenyl ester as
white fluffy
residue (218 mg, 544.4 umol, 83%); Rf 0.21 (ethyl acetate), c.f. 0.30 (S.M.);
SH
(400MHz, DMSO-d6) 4.14 (2H, in, CH2), 4.28 (2H, in, CH2), 6.68 (2H, AA'BB'),
6.94
(2H, AA'BB'), 7.18 (2H, AA'BB'), 7.75 (2H, AA'BB'), 7.89 (2H, br s, exchanged
with
D20, OSO2NH2) and 8.93 (2H, s, C3'-H and C5'-H); LRMS (FAB+): 401.0[100,
(M+H)+]; (FAB-): 799.1[8,(2M-H)-], 553.2[35, (M+NBA)"], 399.1 [100, (M-H)"];
HRMS
(FAB+) 401.10471 C17H17N604S requires 401.10320.
1-Benzyloxy-4-(4-bromobutoxy)benzene (LW002064)
~ I Br
BnO
To a solution of 4-(benzyloxy)phenol (3.0 g, 15.13 mmol) and 1,4-dibromobutane
(16.34
g, 75.65 mmol) in acetonitrile (25 mL) was added anhydrous potassium carbonate
(5.23
g, 37.83 mmol). The suspension was then refluxed for 18 h. After cooling to
room
temperature, the suspension was filtered through a short column of silica and
the filter
cake washed exhaustively with ethyl acetate. The filtrate was evaporated to
give a
yellow liquid (17.63 g) which upon standing gave a mass of white crystals.
These
crystals were then triturated with hexane and collected by filtration. Upon
drying in the
air, 1-benzyloxy-4-(4-bromobutoxy)benzene was obtained as creamy crystals
(2.87 g,
8.561 mmol, 57%); m.p. 73-75 C; Rf 0.84 (chloroform), c.f. 0.16 (S.M.); 61-
1(400 MHz,
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
83
DMSO-d6) 1.80 (2H, in, CH2), 1.95 (2H, in, CH2), 3.60 (2H, t, J 6.6 Hz,
CH2Br), 3.92
(2H, t, J 6.2 Hz, OCH CH2), 5.03 (2H, s, CH2O), 6.86 (2H, AA'BB'), 6.92 (2H,
AA'BB') and 7.28-7.46 (5H, in, Bn). Found C 60.8, H 5.71; C17H19BrO2 requires
C
60.91, H 5.71%.
4-{ [4-(4-Benzyloxyphenoxy)butyl]-[1,2,4]triazol-4-ylamino}benzonitrile
(LWO02065)
N-N
f / 1
BnO N
O N
CN
To a stirred solution of 4-([1,2,4]triazol-4-ylamino)benzonitrile (1.0 g, 5.40
mmol) in
DMF (15 mL) at ice/water temperature was added sodium hydride (60% in mineral
oil,
238 mg, 5.94 mmol). After stirring at room temperature under an atmosphere of
nitrogen
for 10 min, 1-benzyloxy-4-(4-bromobutoxy)benzene (1.99 g, 5.94 mmol) was added
in
one portion and the resulting dark brown mixture was heated at 50 C for 18 h.
The
cooled reaction mixture was then passed through a short column of silica and
the filter
cake was washed with ethyl acetate (10 x 20 mL). The combined filtrate was
washed
with brine (200 mL, 4 x 50 mL), dried (MgSO4), filtered and evaporated to give
a yellow
orange residue (2.50 g). This crude was dissolved in hot ethyl acetate (10 mL)
and
hexane (2 mL) was then added dropwise. Upon cooling to room temperature, 4-{[4-
(4-
benzyloxyphenoxy)butyl]-[1,2,4]triazol-4-ylamino}benzonitrile was obtained as
creamy/pale yellow crystals (1.42 g, 3.231 mmol). A second crop of the product
(363
mg, 825.9 umol, total yield, 75%) was obtained from the residue of the mother
liquor
upon recrystallisation from hot ethyl acetate; m.p. 124.5-126.5 C; Rf 0.45
(neat ethyl
acetate), c.f. 0.88 (1-benzyloxy-4-(4-bromobutoxy)benzene); JH (400MHz, DMSO-
d6)
1.58 (2H, quasi quintet, CH2), 1.78 (2H, quasi quintet, CH2), 3.90 (4H, quasi
q,
OCH CH2CH2CH N), 5.03 (2H, s, CH2O), 6.65 (2H, AA'BB'), 6.84 (2H, AA'BB'),
6.92
(2H, AA'BB'), 7.28-7.45 (5H, in, Bn), 7.72 (2H, AA'BB') and 8.98 (2H, s, C3'-H
and
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
84
C5'-H). Found C 71.05, H 5.74, N 15.8; C26H25N502 requires C 71.05, H 5.73, N
15.93%.
4-{[4-(4-Hydroxyphenoxy)butyl]-[1,2,4]triazol-4-ylamino}benzonitrile
(LWO02067,
STX287)
NON
STX287
. N
HO \
N
~
CN
To a stirred solution of 4-{[4-(4-benzyloxyphenoxy)butyl]-[1,2,4]triazol-4-
ylamino}benzonitrile (800 mg, 1.820 mmol) in. distilled THE (10 mL) was added
in
succession absolute ethanol (30 mL) and Pd/C (10%, 80 mg). The black
suspension was
then stirred under.an atmosphere of hydrogen (balloon) for 2 days. Upon
removal by
filtration and washings of the supported catalyst exhaustively with distilled
THF, the
filtrate was evaporated to give a light yellow frothy residue/syrup (481 mg).
This crude
was fractionated by flash chromatography (ethyl acetate to acetone, gradient)
and the
second fraction that separated upon evaporation gave a soft pale yellow
residue (323 mg)
which was further purified by recrystallisation from acetone/hexane to give 4-
{[4-(4-
hydroxyphenoxy)butyl]-[1,2,4]triazol-4-ylamino}benzonitrile as fine pale
yellow crystals
(280 mg, 801.4 umol, 44%); m.p. 156-159 C; Rf 0.42 (neat ethyl acetate), c.f.
0.51
(S.M.); Sx (400MHz, DMSO-d6) 1.58 (2H, m), 1.76 (2H, m), 3.87 (4H, in,
OCH CH2CH2CH N), 6.61-6.75 (6H, in, Ar), 7.73 (2H, AA'BB'), 8.91 (1H, s,
exchanged with D20, OH) and 8.99 (2H, s, C3'-H and C5'-H). Found C 65.4, H
5.54, N
19.6; C19H19N502 requires C 65.32, H 5.48, N 20.04%.
Sulfamic acid 4-{4-[(4-cyanophenyl)-[1,2,4]triazol-4-ylamino]butoxy}phenyl
ester
(LWO02069, STX288)
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
N-N
H2NO2SO N STX288
` p N
CN
To a stirred solution of 4-{[4-(4-hydroxyphenoxy)butyl]-[1,2,4]triazol-4-
ylamino}benzonitrile (210 mg, 601 pmol) in anhydrous N,N-dimethylacetamide
(DMA,
5 5' mL) was added at room temperature a solution of sulfamoyl chloride in
toluene (ca.
0.68 M, 1.8 mL) and the resulting mixture was stirred under an atmosphere of
nitrogen
for 1.5 h. Ethyl acetate (100 mL) was then added to the reaction mixture and
the organic
layer that separated was washed with brine (50 mL, 4 x 20 mL), dried (MgS04),
filtered
and evaporated to give a creamy residue (291 mg) which was purified by
recrystallisation
10 from acetone/hexane to give sulfamic acid 4-{4-[(4-cyanophenyl)-
[1,2,4]triazol-4-
ylamino]butoxy}phenyl ester as pale yellow crystals (218 mg, 508.8 ,umol,
85%); m.p.
164-172 C; Rf 0.43 (ethyl acetate), c.f. 0.48 (S.M.); SH (400MHz, DMSO-d6)
1.60 (2H,
in, CH2), 1.82 (2H, in, CH2), 3.90 (2H, t, J 7.4 Hz, CH2), 3.98 (2H, t, J 6.2
Hz, CH2),
6.66 (2H, AA'BB'), 6.97 (2H, AA'BB'), 7.18 (2H, AA'BB'), 7.73 (2H, AA'BB'),
8.91
15 (2H, br s, exchanged with D20, OSO2NH2) and 8.99 (2H, s, C3'-H and C5'-H);
LRMS
(FAB+): 429.0[100, (M+H)+]; (FAB-): 427.1 [100, (M-H)"]; HRMS (FAB+) 429.13567
C19H21N6O4S requires 429.13450.
3-Bromo-4-hydroxybenzaldehyde (LWO02081)
Br
HO
CHO
To a stirred solution of 4-hydroxybenzaldehyde *8.0 g, 64.20 mmol) in
chloroform (400
mL) at 40 C was added portionwise a solution of bromine (3.3 mL) in
chloroform (10
mL). The resulting reddish brown mixture was stirred at 40 C for 2 h, cooled
and
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
86
evaporated to give a purple residue which was dissolved in ethyl acetate (200
mL). The
organic layer that separated was washed with brine (4 x 100 mL), dried
(MgS04), filtered
and evaporated to give a light pink/brown residue (12.95 g). The crude was
purified by
recrystallisation from hot toluene twice to give 3-bromo-4-hydroxybenzaldehyde
as light
orange/brown crystals (8.88 g, 44.17 mmol, 69%); m.p. 115-128 C; Rf 0.42
(chloroform/ethyl acetate, 4:1), c.f. 0.36 (S.M.); 8H (400MHz, DMSO-d6) 7.11
(1H, d, J
8'.2 Hz, C5-H), 7.76 (1H, dd, J 1.95 and 8.2 Hz, C6-H), 8.04 (1H, d, J2 Hz, C2-
H), 9.78
(1H, s, CHO) and 11.53 (1H, s, exchanged with D20, OH). This product was used
for
the next reaction without further purification.
4-Benzyloxy-3-bromobenzaldehyde (LWO02082)
I
Br
BnO
CHO
To a stirred solution of 3-bromo-4-hydroxybenzaldehyde (8.0 g, 39.80 mmol) in
anhydrous DMF (50 mL) at ice/water temperature was added sodium hydride (60%
in
mineral oil, 1.67 g, 41.79 mmol). After stirring at room temperature for 10
min, benzyl
bromide (7.64 g, 43.78 mmol) was added and the reaction mixture was heated at
80 C
for 2 h. Upon cooling to room temperature, ethyl acetate (300 mL) was added
and the
organic layer that separated was washed with brine (500 mL, 4 x 50 mL), dried
(MgSO4),
filtered and evaporated to give a light beige residue (13.09 g). The crude was
purified by
recrystallisation from isopropanol/hexane to give 4-benzyloxy-3-
bromobenzaldehyde as
fine light yellow crystals (9.52 g, 32.70 mmol, 82%); m.p. 95-96.5 C [Lit.'
(ethanol),
m.p. 95 C]; Rf 0.75 (ethyl acetate/hexane, 1:1), 0.52 (S.M.); SH (400MHz,
DMSO-d6)
5.35 (2H, s, CH2O), 7.33-7.53 (6H, in, Bn and C5-H), 7.93 (1H, dd, J2 and 8.4
Hz, C6-
H), 8.13 (1H, d, J 1.9 Hz, C2-H) and 9.87 (1H, s, CHO).
1 Buu-Hoi et. Al. (1953) J. Org. Chem. 18: 121-125.
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
87
4-Benzyloxy-3-bromophenol (LWO02085)
Br
BnO / \
OH
To a stirred solution of 4-benzyloxy-3-bromobenzaldehyde (4.5 g, 15.46 mmol)
in
chloroform (30 mL) at room temperature was added m-chloroperoxybenzoic acid
(57-
86%, 5.62 g) and the resulting suspension was stirred for 4 h. Ethyl acetate
(200 mL)
was then added and the organic layer that separated was washed with saturated
aqueous
sodium bicarbonate (1 x 100 mL, 3 x 50 mL) followed by brine (2 x 50 mL),
dried
(MgSO4), filtered and evaporated to give a clear brown oil which upon standing
at room
temperature overnight gave some light yellow deposits (6.05 g). To this crude
in
methanol (45 mL) at room temperature was added 1 M NaOH (aq) (30 mL). After
stirring for 2 h, the resulting brown mixture was acidified with 5 M HCl
followed by
dilution with ethyl acetate (200 mL). The organic layer that separated was
washed with
brine (100 mL, 4 x 50 mL), dried (MgSO4), filtered and evaporated to give a
brown oil
(5.33 g). Upon fractionation by flash chromatography (ethyl acetate/hexane,
1:2 to 1:1
gradient), the fourth fraction that isolated gave 4-benzyloxy-3-bromophenol as
a light
golden yellow oil which solidified upon standing at room temperature to form a
light
brown wax (3.92 g, 14.04 mmol, 91%); Rf 0.48 (ethyl acetate/hexane, 1:2), 0.61
(4-
benzyloxy-3-bromobenzaldehyde); 8H (400MHz, DMSO-d6) 5.07 (2H, s, CH2O), 6.72
(1H, dd, J 2.7 and 8.9 Hz, C6-H), 6.98 (1H, d, J 3.1 Hz, C2-H), 7.02 (1H, d, J
8.9 Hz,
C5-H), 7.28-7.50 (5H, in, Bn) and 9.39 (1H, br s, exchanged with D20, OH);
LRMS
(FAB+): 278.0[35, (79M)+], 91.0[100, Bn+]; (FAB-): 433.1 [83, (81M+NBA)-],
276.9[84,
(79M-H)"], 187.9 [100, (81M-H-Bn)"]; HRMS (FAB+) 277.99390 C13H1179BrO2
requires
277.99424, (FAB+) 279.99213 C13H1181BrO2 requires 279.99219. The product was
not
further purified before use.
2-(4-Benzyloxy-3-bromophenoxy)ethanol (LWO02086)
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
88
Br 0
I OH
BnO
To a solution of 4-benzyloxy-3-bromophenol (2.0 g, 7.165 mmol) in anhydrous
DMF (10
mL) at room temperature was added anhydrous potassium carbonate (1.04 g)
followed by
2-bromoethanol (990 mg, 7.523 mmol). The resulting suspension was stirred
under an
atmosphere of nitrogen at 80 C overnight. After cooling, ethyl acetate (100
mL) was
added and the organic layer that separated was washed with brine (150 mL, 4 x
50 mL),
dried (MgS04), filtered and evaporated to give a brown syrup (2.51 g).. This
crude was
fractionated by flash chromatography (chloroform/ethyl acetate, 6:1 to 2:1
gradient) and
the third fraction that separated upon evaporation gave 2-(4-benzyloxy-3-
bromophenoxy)ethanol (1.58 g, 4.889 mmol, 68%); Rf 0.49 (chloroform/ethyl
acetate,
4:1), c.f. 0.68 (S.M.); Sx (400 MHz, DMSO-d6) 3.67 (2H, --q, J 5Hz, CH OH),
3.94 (2H,
t, J 5.1 Hz, CH CH2OH), 4.86 (1H, t, J 5.5 Hz, exchanged with D20, OH), 5.13
(2H, s,
CH2O), 6.92 (1H, dd, J3.1 and 8.9 Hz, C6-H), 7.12 (1H, d, J9 Hz, C5-H), 7.19
(1H, d, J
3.1 Hz, C2-H) and 7.30-7.50 (5H, m, Bn); LRMS (FAB+): 322.0[45, (79M)+],
91.0[100,
Bn+]; (FAB-): 475.2[24, (79M+NBA)"], 323.1 ['100, (81M-H)"], 231.9(100) ; HRMS
(FAB+) 324.01846 C15H1581BrO3 requires 324.01841.
2-Bromo-l-(4-benzyloxy-3-bromophenoxy)ethane (LWO02087)
Br / O\\~
Br
Bn0
To a solution of 2-(4-benzyloxy-3-bromophenoxy)ethanol (1.44 g, 4.456 mmol) in
anhydrous dichloromethane (15 mL) at ice/water temperature was added carbon
tetrabromide (1.88 g, 5.570 mmol) followed by triphenylphosphine (1.77 g,
6.684 mmol)
portionwise over a period of 5 min. After stirring under an atmosphere of
nitrogen at
ice/water temperature for 15 min, the reaction mixture was evaporated to give
a pale
orange syrup (5.22 g). This crude was fractionated by flash chromatography
(chloroform/ethyl acetate, 4:1) and the first fraction that separated upon
evaporation gave
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
89
2-bromo-l-(4-benzyloxy-3-bromophenoxy)ethane as a pale yellow liquid (1.81 g,
4.688
mmol); Rf 0.79 (chloroform/ethyl acetate, 4:1), c.f. 0.32 (S.M.); 4x (400 MHz,
DMSO-
d6) 3.76 (2H, t, J 5.5 Hz, CH Br), 4.28 (2H, - t, J 4-5 Hz, OCH CH2), 5.14
(2H, s,
CH2O), 6.96 (1H, dd, J3.1 and 8.9 Hz, C6-H), 7.13 (1H, d, J8.9 Hz, C5-H), 7.24
(1H, d,
J2.7 Hz, C2-H) and 7.30-7.50 (5H, m, Bn); LRMS (FAB+): 385.9[36, M+],
91.0[100,
Bn+]; (FAB-): 385.0[29, (M-H)], 231.9(100) ; HRMS (FAB+) 385.93362
C 15H 14O279Br81 Br requires 385.93401.
4- { [2-(4-Benzyloxy-3 -bromophenoxy) ethyl] - [ 1,2,4] triazol-4-ylamino }
benzonitrile
(LWO02088)
N- N
Br 1
J
/ N
Bn0 \
N
CN
To a stirred solution of 4-([1,2,4]triazol-4-ylamino)benzonitrile (741 mg,
4.001 mmol) in
anhydrous DMF (10 mL) at ice/water temperature was added sodium hydride (60%
in
mineral oil, 176 mg, 4.401 mmol). After stirring at room temperature under an
atmosphere of nitrogen for 10 min, a solution of 2-bromo-l-(4-benzyloxy-3-
bromophenoxy)ethane (1.70 g, 4.401 mmol) in DMF (5 mL) was added. The
resulting
mixture was heated at 60 C for 2 h, then cooled and diluted with ethyl
acetate (500 mL).
The organic layer that separated was washed with brine (200 mL, 4 x 100 mL),
dried
(MgSO4), filtered and evaporated to give a light brown residue (2.04 g). This
crude was
purified by recrystallisation from hot ethyl acetate and upon cooling gave 4-
{[2-(4-
benzyloxy-3-bromophenoxy)ethyl]-[1,2,4]triazol-4-ylamino}benzonitrile as
fluffy pale
beige powder (1.20 g, 2.447 mmol, 61%); m.p. 163-166 C; Rf 0.30 (ethyl
acetate), c.f.
0.75 [2-bromo-l-(4-benzyloxy-3-bromophenoxy)ethane]; 9-1 (400MHz, DMSO-d6)
4.11
(2H, t, J 4-5 Hz, CH2N), 4.23 (2H, t, J 4-5 Hz, CH2O), 5.13 (2H, s, CH2O),
6.66 (2H,
AA'BB'), 6.86 (1H, dd, J 2.7 and 8.9 Hz, C6'-H), 7.12 (1H, d, J 8.9 Hz, C5'-
H), 7.16
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
(1H, d, J 2.7 Hz, C2'-H), 7.30-7.48 (5H, in, Bn), 7.74 (2H, AA'BB') and 8.94
(2H, s,
C3"-H and C5"-H). Found C 58.6, H 4.17, N 14.38; C24H2OBrN5O2 requires C
58.79, H
4.11, N 14.28%.
5 4-{ [2-(3-Bromo-4-hydroxyphenoxy)ethyl]-[1,2,4]triazol-4-
ylamino}benzonitrile
(STX300) and 4-{[2-(4-Hydroxy-phenoxy)ethyl]-[1,2,4]triazol-4-
ylamino}benzonitrile
(STX291) (LWO02089)
N N N-N
Br
HO N N
O~I HO \
~ O~ N
STX300 CN STX291 CN
10 To a stirred solution of 4-{[2-(4-benzyloxy-3-bromophenoxy)ethyl]-
[1,2,4]triazol-4-
ylamino}benzonitrile (902 mg, 1.839 mmol) in distilled THE (45 mL) was added
in
succession absolute ethanol (45 mL) and Pd/C (10%, 90 mg). The black
suspension was
then stirred under an atmosphere of hydrogen (balloon) for 2 days. Upon
removal by
filtration and washings of the supported catalyst exhaustively with distilled
THF, the
15 filtrate was evaporated to give a beige residue (860 mg). This crude in DMF
(10 mL)
was fractionated by flash chromatography (ethyl acetate/acetone, 4:1 to
acetone,
gradient) and the third fraction that separated upon evaporation gave a wet
creamy
residue which was triturated with ether (50 mL). The precipitate that formed
was
filtered, washed exhaustively with water and air-dried overnight to give
LWO02089 as
20 off-white powder (453 mg); Rf 0.40 (ethyl acetate), c.f. 0.50 (S.M.); 41
(400MHz,
DMSO-d6) 4.03 (- 0.4H, t, J4.6-5.1 Hz, CH2N of STX291), 4.06 (1.6H, t, J4.6-
5.1 Hz,
CH2N of STX300), 4.21 (2H, t, J4.6-5.1 Hz, OCH CH2N of STX291 and STX300),
6.65
(-j 2H, AA'BB' of STX291 and STX300), 6.71 (-j 0.4H, AA'BB' of STX291), 6.74 (-
0.82, dd, J 2.8-3.1 and 8.8 Hz, C6'-H of STX300), 6.85 (- 0.8H, d, J 8.9 Hz,
C5'-H of
25 STX300), 7.04 (0.8H, d, J 3.1 Hz, C2'-H of STX300), 7.74 (2H, AA'BB' of
STX291
and STX300), 8.91 (-. 0.4H, s, C3"-H and C5"-H of STX291), 8.93 (- 1.6H, s,
C3"-H
and C5"-H of STX300), 8.98 (- 0.2H, br s, exchanged with D20, OH of STX291)
and
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
91
9.74 (- 0.8H, br s, exchanged with D20, OH of STX300) This implies that the
product
contains approx. 20% ofSTX291; LRMS (FAB+): 553.0[18, (79M+H+NBA)+], 475.1[12,
(STX29l+H+NBA)+], 400.0[100, (79M+H)+], 322.1 [85, (STX291+H)+], 252.1 [50,
(79M-
79Br-triazole)+]; (FAB-): 552.0[60, (79M+NBA)"], 475.3[32, (STX291+H+NBA)"],
398.1 [100, (79M-H)"]; HRMS (FAB+) 400.04045 C17H1579BrN5O2 requires
400.04091,
(FAB+) 322.12936 C17H16N502 requires 322.13040.
Sulfamic acid 2-bromo-4-{2-[(4-cyanophenyl)-[1,2,4]triazol-4-
ylamino]ethoxy}phenyl
ester (STX301) and Sulfamic acid 4-{2-[(4-cyanophenyl)-[1,2,4]triazol-4-
ylamino]ethoxy}phenyl ester (STX292) (LWO02090)
N-N
Br
N STX301
H2NO2SO \
CN
C
H2NO2SO N STX292
N ~aCN
To a stirred solution of LWO02089 (289 mg, 699.5 ,umol) in anhydrous N,N-
dimethylacetamide (DMA, 10 mL) was added at room temperature a solution of
sulfamoyl chloride in toluene (ca. 0.59 M, 4.7 mL) and the resulting mixture
was stirred
under an atmosphere of nitrogen overnight. Ethyl acetate (100 mL) was then
added to
the reaction mixture and the organic layer that separated was washed with
brine (100 mL,
4 x 50 mL), dried (MgSO4), filtered and evaporated to give a white fluffy
residue (347
mg). This crude in ethyl acetate was filtered through a short column of silica
and the
combined filtrate upon evaporation gave LWO02090 as a white fluffy residue
(310 mg,
646.8 pmol, 92.5%); Rf 0.29 (ethyl acetate), c.f. 0.35 (S.M.); 8H (400MHz,
DMSO-d6)
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
92
4.14 (- 0.4H, t, J 4.6-5.1 Hz, CH2N of STX292), 4.17 (-. 1.6H, t, J 4.7 Hz,
CH2N of
STX301), 4.27 (2H, t, J 3.9-4.6 Hz, OCH CH2N of STX292 and STX301), 6.68 (2H,
AA'BB' of STX292 and STX301), 6.91-6.99 (- 1.2H, mixture of AA'BB' and dd of
STX292 and C6'-H of STX301), 7.18 (-. 0.4H, AA'BB' of STX292), 7.25 (0.8H, d,
J3.1
Hz, C2'-H of STX301), 7.37 (' 0.8H, d, J 8.9 Hz, C5'-H of STX301), 7.75 (2H,
AA'BB'
of STX292 and STX301), 7.91 (- 0.4H, br s, exchanged with D20, SO2NH2 of
STX292),
8.16 (- 1.6H, br s, exchanged with D20, SO2NH2 of STX301), 8.94 (- 0.4H, s,
C3"-H
and C5"-H of STX292) and 8.99 (- 1.6H, s, C3"-H and C5"-H of STX301) This
implies that the product contains approx. 20% of STX292; LRMS (FAB+):
481.0[100,
(81M+H)+], 401.1 [73, (STX292+H)+], (FAB-): 631.1 [35, (79M+NBA)"], 477.1
[100, (79M-
H)J, 399.1 [64, (STX292-H)]; HRMS (FAB+) 479.01430 C17H1579BrN6O4S requires
479.01371, (FAB+) 401.10349 C17H17N604S requires 401.10320.
6-Benzyloxy-naphthalene-2-carboxylic acid benzyl ester (JRL01001)
COOBn
/ I \
BnO
To a stirred suspension of NaH (60%, 1.37 g, 34.3 mmol) in DMF (40 mL) at 0 C
under
nitrogen was added 6-hydroxy-2-naphthoic acid (3.0 g, 15.6 mmol). The
resulting brown
mixture was stirred for 30 min before benzyl bromide (5.99 g, 34.3 mmol) was
added.
After stirring overnight, the reaction mixture was poured into water and the
aqueous
layer was extracted with ethyl acetate (3 x 100 mL). The combined organic
extracts was
washed with brine (4 x 100 mL), dried (Na2SO4) and evaporated to give the
crude
product which upon fractionation with flash chromatography (hexane/ethyl
acetate, 10:1)
gave JRLO1001 as a white solid (4.3 g, 75 %); Rf (hexane/ethyl acetate, 10:1)
0.42; 9x
(400MHz, CDC13) 5.19 (2H, s), 5.41 (2H, s), 7.20-7.60 (12H, m), 7.74 (1H, d, J
8.6 Hz),
7.85 (1H, d, J 9.0 Hz), 8.06 (1H, dd, J 1.9 and 8.6Hz) and 8.57 (1H, s); 6c
(100 MHz,
CDC13) 67.1 (t), 70.5 (t), 107.2 (d), 120.2 (d), 125.5 (s), 126.3 (d), 127.2
(d), 127.5 (d),
128.2 (s), 128.4 (d), 128.5 (d), 128.6 (d), 128.9 (d), 131.2 (d), 136.5 (s),
136.7 (s), 137.4
(s), 158.6 (s), 166.8 (s).
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
93
(6-Benzyloxy-naphthalen-2-yl)-methanol (JRL01003)
/ I \ OH
BnO
To a stirred suspension of LiAlH4 (240 mg, 6.0 mmol) in THE (100 mL) at room
temperature under nitrogen was added a solution of JRLO1001 (1.99 g, 5.4 mmol)
in
THF. After 2 h of stirring at which time no starting material was detected by
TLC, the
reaction mixture was evaporated. The residue obtained was treated with ethyl
acetate
(100 mL) and the organic layer resulted was washed with dilute ammonium
chloride
solution (50 mL) and brine (3 x 50 mL), dried (Na2SO4) and evaporated. The
crude
product thus obtained was recrystallised from hot ethanol to give JRL01003 as
white
solid (1.16 g, 81 %); m.p. 138.0 -138.5 C; Rf 0.26 (hexane/ethyl acetate,
2:1); cj
(400 MHz, CDC13) 1.82 (1H, t, J 5.9 Hz), 4.79 (2H, d, J 5.9 Hz), 5.16 (2H, s)
and 7.20-
7.80 (11H, m); (100 MHz, CDC13) 65.8 (t), 70.3 (t), 107.3 (d), 119.5 (d),
125.7 (d),
126.0 (d), 127.4 (d), 127.7 (d), 128.2 (d), 128.8 (d), 129.1 (s), 129.6 (d),
134.2 (s), 136.2
(s), 137.0 (s), 157.0 (s). Found: C 81.77, H 6.11; C1SH16O2 requires C 81.79,
H 6.10%.
2-Benzyloxy-6-bromomethyl-naphthalene (JRL01006)
/ I \ Br
BnO
To a stirred solution of JRL01003 (1.29 g, 4.9 mmol) in dry CH2C12 (60 mL) at
0 C
under nitrogen was added PBr3 (1.33 g, 4.9 mmol). A white suspension was
formed
initially but it turned into a pale yellow mixture subsequently. After
stirring the reaction
mixture for 2 h at 0 C and at room temperature for 1 h, it was poured onto
ice/water.
The organic layer was separated and the aqueous layer extracted with
dichloromethane (3
x 50 mL). The combined organic extracts was dried (Na2S04) and evaporated to
give
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
94
JRL01006 as a white solid (1.51 g, 94 %); Rf 0.28 (hexane/ethyl acetate,
10:1); Sx (400
MHz, CDC13) 4.65 (2H, s), 5.17 (2H, s), 7.10-7.30 (2H, m), 7.30-7.50 (6H, m)
and 7.68-
7.78 (3H, m); LRMS (FAB+) 327.9 [25, (M+H)+], 247 (45), 91.0 [100, (Bn+)].
4-[(6-Benzyloxy-naphthalen-2-ylmethyl)-[1,2,4]triazol-4-yl-amino]-benzonitrile
(JRLO1010)
N-N
N
BnO
To a stirred solution of NaH (60%, 144 mg, 3.6 mmol) in DMF (10 mL) at 0 C
under
nitrogen was added 4-([1,2,4]triazol-4-ylamino)benzonitrile (LWO02023, 667 mg,
3.6
mmol) in DMF (10 mL). A white suspension/mixture was resulted initially but it
turned
orange subsequently. After stirring at 40-50 C for 1 h under nitrogen, the
reaction
mixture was cooled to room temperature and JRL01006 (1.21 g, 3.7 mmol) was
added.
The solution was stirred at room temperature overnight under nitrogen and then
diluted
with dichloromethane (100 mL). The organic layer was washed with brine (4 x 50
mL),
dried (Na2SO4) and evaporated to give JRLO1010 as a pale yellow solid (994 mg,
64 %);
Rf0.42 (ethyl acetate); 9x (400 MHz, CDC13) 5.00 (2H, s), 5.20 (2H, s), 6.72
(2H,
AA'BB'), 7.20-7.80 (13H, m) and 8.10 (2H, s); LRMS (FAB+) 432.1 [70, (M+H)+],
363.1 [50, (M-triazole)+], 247.1 (35), 91.0 [100, (Bn)+].
4-[(6-Hydroxy-naphthalen-2-ylmethyl)-[1,2,4]triazol-4-yl-amino]-benzonitrile
(JRL01012, STX 335)
CA 02464770 2009-11-27
WO 031045925 PCT/GB02/05214
N`~N
3
N STX335
N
I \
~ 1 / ~ CN
HO
To a stirred solution of JRLO1010 (906 mg, 2.1 mmol) in THF/MeOH (1 : 1, 180
mL)
was added Pd-C (10 %, 250 mg). After stirring the suspension under an
atmosphere of
5 hydrogen (balloon) overnight, it was filtered through Celite and the
filtrate evaporated to
give a grey crude product. Upon trituration in hot ethyl acetate, the pale
grey solid that
'resulted was filtered and dried to give JRL01012 (STX335, 444 mg, 62 %); m.p.
281 -
284 C; Rf 0.26 (ethyl acetate); 4 (40OMHz, DMSO-d6) 5.18 (2H, s), 6.80 (2H,
AA'BB'),
7.07 (2H, m), 7.34 (1 H, d, J 8.2 Hz), 7.60-7.70 (3H, m), 7.76 (2H AA'BB'),
8.80 (2H, s)
10 and 9.79 (1H, s); LRMS (FAB) 342.1 [100, (M+H)+3, 274.1 [37, (M+H-
triazole)1, 158.0
(25); HRMS (FAB+) 342.13594, C20H16N50 requires 342.13549.
Sulfamic acid 6-{[(4-cyano-phenyl)-[1,2,4]triazol-4-yi-amino]-methyl}-
naphthalen-
2-yl ester (JRL01014, STX 336)
N-N
N STX336
~ CN
H2NO2SO
To a stirred solution of JRL01012 (181 mg, 530 pmol) in DMA (2 mL) under
nitrogen
was added sulfamoyl chloride (1.2 mmol). After stirring overnight at room
temperature,
the reaction mixture was diluted with ethyl acetate (30 mL). The organic layer
was then
washed with brine (4 x 30 mL), dried (Na2SO4) and evaporated to give JRL01014
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
96
(STX336, 189 mg, 85%) as a white solid. An amount of 70 mg of this solid was
recrystallised from acetone/hexane to obtain white crystals (40 mg); m.p. 125 -
127 C;
Rf 0.23 (ethyl acetate); SH (400MHz, DMSO-d6) 5.23 (2H, s), 6.80 (2H, AA'BB'),
7.42
(1H, dd, J 2.3 and 9.0 Hz), 7.55 (1H, br dd, J 8.6 Hz), ; 7.74-7.82 (3H, m),
7.85 (1H, s),
7.94 (2H, m), 8.04 (2H, br s, H2NSO2O) and 8.84 (2H, s); LRMS (FAB+) 421.1
[100,
(M+H)+], 352.0 [58, (M-triazole)+], 341.1 (10), 236.0 (25), 158.0 (10); HRMS
(FAB+)
421.10766, C20H17N603S requires 421.10829.
?-Benzyoxybenzyl bromide (OBS01018)
\ I O Br
Using the procedure reported by K. Thakkar et al., J. Med. Chem., 1993, 36
(20), 2950.
Phosphorus tribromide (1.96 mL, 20.6 mmol) was added to a solution of 3-
benzyloxybenzyl alcohol (4.29 g, 20 mmol) in anhydrous dichloromethane (90 mL)
at 0
C. The mixture was stirred at 0 C for 2 h and then at room temperature for 1
h. The
reaction was poured onto ice/water (400 mL) and allowed to warm to room
temperature.
The aqueous solution was extracted with Et2O (5 x 100 mL) and the combined
ethereal
solution dried (MgS04). Concentration in vacuo gave a light yellow oil which
crystallised on standing to give OBS01018 as colourless needles (4.93 g, 89
%). TLC
[Si02, EtOAc-n-hexane (1:1)] Rf = 0.9; m.p. 55 - 56 C [Lit. (Petroleum
ether): 55 C];
'H-NMR (400 MHz, CDC13) 4.44 (2H, s), 5.05 (2H, s); 6.78 (1H, d, J= 2), 6.93
(1H, m),
7.22 (1H, t, J= 8), 7.40 (5H, m).
4-[(3-Benzyloxybenzyl)(4-cyanophenyl)amino]-4H-[1,2,4]triazole (OBS01019,
STX675)
N-N
N STX675
O
N
To a suspension of NaH (60 % dispersion in oil, 0.22 g, 5.4 mmol) in anhydrous
DMF
(20 mL) at room temperature was added a solution of 4-[(4-cyanophenyl)amino]-
4H-
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
97
[1,2,4]triazole (see LWO02023) (1.0 g, 5.4 mmol) in anhydrous DMF (4 mL) and
the
mixture stirred under a positive flow of dry nitrogen for 1 h. The orange-
yellow
suspension was then treated with a solution of OBS01018 (1.57 g, 5.66 mmol) in
anhydrous DMF (5 mL) and the mixture was stirred at room temperature
overnight. The
mixture was transferred to a separating funnel and diluted with EtOAc (100
mL). The
organic layer was washed with water (4 x 100 mL), brine (100 mL), and dried
(MgS04).
Concentration in vacuo gave a residue which was recystallised from i-PrOH to
give
OBS01019 as a white solid (0.58 g, 28 %). TLC [Si02, EtOAc-n-hexane (1:1)] Rf=
0.15
(blue fluorescence at 254 nm); 1H-NMR (400 MHz, CDC13) 4.85 (2H, s), 5.03 (2H,
s);
6.62 (2H, AA'BB'), 6.77 (1H, d, J= 7.8), 6.79 (1H, d, J= 2.4), 6.96 (1H, dd,
J= 7.8,
2.4), 7.26 (1H, t, J= 7.8), 7.34 - 7.37 (5H, m), 7.57 (2H, AA'BB'), 8.04 (2H,
s); LC-MS:
tR = 6.81 min, M+H = 382 (Waters 2790 Alliance HPLC / ZQ MicroMass
spectrometer
with PDA detector using APCI, gradient elution : 5 : 95 MeCN / H2O - 95 : 5
MeCN /
H2O over 10 mins then 95 : 5 MeCN / H2O - 5 : 95 MeCN / H2O using Waters
"Symmetry" C18 (packing : 3.5 ,um), 100 mm column); HPLC (Waters
717+Autosampler with PDA detector, using Waters "Symmetry" C18 (packing : 3.5
,um), 4.6 x 150 mm column, 90: 10 MeOH / H2O) tR = 2.27 min (98 % purity).
4-[(3-Hydroxybenzyl)(4-cyanophenyl)amino]-4H-[1,2,4]triazole (OBS01022,
STX333)
N-N
N STX333
HO /I
N I \
N
To a solution of OBS01019 (0.4 g, 1.05 mmol) in THF-MeOH (1:1) (20 mL) was
added
a slurry of Pd-C (10%, 0.24 g, 2.26 mmol) in THE (2 mL) and the suspension
stirred
under an atmosphere of hydrogen (balloon) for 21 h. The suspension was
filtered
through Celite and the combined filtrates concentrated in vacuo to give a
brown residue
which solidified on standing. Recrystallisation from EtOH gave OBS01022
(STX333) as
a white solid (0.27 g, 88 %). TLC [Si02, EtOAc-n-hexane (1:1)] Rf= 0.6; 1H-NMR
(400
MHz, d6-DMSO) 4.97 (2H, s), 6.68 (2H, d, AA'BB'), 6.72 - 6.75 (3H, m), 7.11
(1H, m),
7.76 (2H, AA'BB'), 8.77 (2H, s), 9.49 (1H, bs). MS (FAB+) = 292 (M+H, 100 %),
223
(M+H-triazole, 42); Acc. MS for C16H13N50 (Required, 292.1192; Found,
292.1198);
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
98
HPLC (Waters 717+ Autosampler with PDA detector, using Waters "Symmetry" C18
(packing : 3.5 ,um), 4.6 x 150 mm column, 70:30 MeOH / H2O) tR = 2.22 min (92
%
purity)-
4-[(3-O-Sulfamoylbenzyl)(4-cyanophenyl)amino]-4H-[1,2,4]triazole (OBS01030,
STX334)
N-N
N STX334
H2NO2SO/ )
N I \
N
To an ice-cooled solution of OBS01022 (100 mg, 340 , anol) in DMA (2 mL) was
added
sulfamoyl chloride (0.59 M solution toluene, 1.2 mL, 0.69 mmol) in DMA (2 mL)
and
the mixture stirred under nitrogen overnight. The mixture was diluted with
EtOAc (30
mL) and washed with brine (3 x 100 mL). The organic solution was dried
(Na2S04) and
concentrated in vacuo to give a colourless residue which solidified on
standing.
Recrystallisation from acetone-petroleum ether (40 - 60) gave OBS01030
(STX334) as a
colourless solid (0.06 g, 47 %). TLC [Si02, EtOAc-n-hexane (1:1)] Rf = 0.55;
'H-NMR
(400 MHz, d6-DMSO) 5.11 (2H, s), 6.74 (2H, d, J= 8.6), 7.20 - 7.27 (3H, m),
7.40 (1H,
t, J = 7.8), 7.77 (2H, d, J = 8.6), 7.98 (2H, br s), 8.81 (2H, s). MS (FAB+) =
371 (M+H,
100 %), 302 (M+H-triazole, 28); Acc. MS for C16H15N603S (Required, 371.0946;
Found,
371.0926); HPLC (Waters 717+ Autosampler with PDA detector, using Waters
"Symmetry" C18 (packing : 3.5 ,um), 4.6 x 150 mm column, 70:30 MeOH / H2O) tR
=
2.13 min (99 % purity).
3,4-Bis(benzyloxy)benzaldehyde (OBS01058)
/
O
Using the procedure reported by A. F. Barrero et al., Tetrahedron, 1998, 54,
5635.
To a suspension of 3,4-dihydroxybenzaldehyde (6.9 g, 50 mmol) and potassium
carbonate (14.5 g) in acetone (150 mL) was added benzyl bromide (11.96 mL, 101
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
99
mmol) and the mixture heated at reflux for 15 h. The reaction was cooled and
the solvent
removed in vacuo to give a light brown residue. The residue was redissolved in
Et2O
(200 mL), washed with water (3 x 200 mL) and dried (Na2SO4). Concetration in
vacuo
gave a pale yellow solid which was recrystallised from EtOH to give OBS01058
as a
white powder (13.84 g, 87 %). TLC [Si02, EtOAc-n-hexane (1:1)] Rf= 0.7; m.p.
93 - 94
C [Lit. (EtOH): 93 - 94 C]; 'H-NMR (400 MHz, d6-DMSO) 5.22 (2H, s), 5.28 (2H,
s),
7.29 (1H, d, J= 8.4), 7.30 - 7.48 (10H, m), 7.51 (1H, d, J= 2), 7.55 (1H, dd,
J= 2, 8.4),
.9.81 (1H, s).
3,4-Bis(benzyloxy)benzyl alcohol (OBS01060)
O
I OH
Qo
Reference: L. Lisowski et al., Bioorg. Med. Chem. Lett., 2001, 11(16), 2205
To a solution of OBS01058 (3.18 g, 10 mmol) in anhydrous THE (50 mL) was added
sodium borohydride (378 mg, 10 mmol) and the mixture stirred at room
temperature for
4 h. The reaction was quenched with cautious addition of water (CARE N),
filtered
through Celite and concentrated in vacuo to give a yellow oil. The oil was
recrystallised
from MeOH to give OBS01060 as colourless needles (2.97 g, 93 %). TLC [Si02,
EtOAc-n-hexane (1:1)] Rf = 0.45; m.p. 65 - 67 C [Lit. (MeOH): 65 - 66 C]; 'H-
NMR
(400 MHz, CDC13) 4.58 (2H, d, J = 5), 5.16 (2H, s), 5.17 (2H, s), 6.86 (1H,
dd, J = 2,
8.2), 6.91 (1H, d, J= 8.2), 7.00 (1H, d, J= 2), 7.29 - 7.39 and 7.43 - 7.47
(10H, m), OH
signal too broad to be observed.
3,4-Bis(benzyloxy)benzyl bromide (OB S 01061)
\ I O Br
O
Reference: K. Thakkar et al., J. Med. Chem.., 1993, 36(20), 2950.
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
100
Phosphorus tribromide (0.98 mL, 10.3 mmol) was added to a solution of OBS01060
(3.20 g, 10 mmol) in anhydrous DCM (45 mL) at 0 C. The mixture was stirred at
0 C
for 2 h and then at room temperature for 1 h. The reaction was poured onto ice
water
(200 mL) and allowed to warm to room temperature. The aqueous solution was
extracted
with Et20 (5 x 50 mL) and the combined ethereal solution dried (MgSO4).
Concentration in vacuo gave a colourless oil which solidified on standing to
give
OBS01061 as a white solid (3.10 g, 78 %). TLC [Si02, EtOAc-n-hexane (1:1)] Rf
=
0.89; m.p. 72 - 75 C [Lit: 72 - 75 C]; 1H-NMR (400 MHz, CDC13) 4.53 (2H, s),
5.15
(2H, s), 5.17 (2H, s), 6.83 (1H, dd, J= 2, 8.2), 6.90 (1H, d, J= 8.2), 6.99
(1H, d, J= 2),
7.52 (1OH, m).
4-[(3,4-Bis(benzyloxy)benzyl)(4-cyanophenyl)amino]-4H-[ 1,2,4]triazole
(OBS01066,
STX676)
I N -N
N~ STX676
O
N
To a suspension of NaH (60 % dispersion in oil, 0.22 g, 5.4 mmol) in anhydrous
DMF
(20 mL) at room temperature was added a solution of 4-[(4-cyanophenyl)amino]-
4H-
[1,2,4]triazole (1.0 g, 5.4 mmol) in anhydrous DMF (4 mL) and the mixture
stirred under
nitrogen for 1 h. The orange-yellow suspension was then treated with a
solution of
OBS01061 (2.07 g, 5.67 mmol) in anhydrous DMF (5 mL) and the mixture stirred
at
room temperature overnight. The mixture was transferred to a separating funnel
and
diluted with EtOAc (100 mL). The organic layer was washed with water (4 x 100
mL),
brine (100 mL), and dried (MgS04). Concentration in vacuo gave a residue which
was
recystallised from EtOH to give OBS01066 (STX676) as an off-white solid (1.66
g, 63
%). TLC [Si02, EtOAc-n-hexane (1:1)] Rf = 0.1 (blue fluorescence at 254 nm);
1H-
NMR (400 MHz, d6-DMSO) 4.92 (2H, s), 5.06 (2H, s), 5.07 (2H, s), 6.72 - 6.76
(3H, m);
6.94 (1H, d, J = 8.2), 7.01 (1H, d, J = 2.2), 7.28 - 7.42 (10H, m), 7.74 (2H,
AA'BB'),
8.65 (2H, s). MS (FAB+) 488 (M+H, 45 %), 419 (M+H-triazole, 21), 91 (Bn,
100%);
Ace MS for C30H26N502 (Required, 488.2096; Found, 488.2087); LC-MS (Waters
2790
Alliance HPLC / ZQ MicroMass spectrometer with PDA detector using APCI), tR
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
101
(gradient elution : 5:95 MeCN / H2O - 95:5 MeCN / H2O over 10 mins then 95:5
MeCN
/ H2O - 5:95 MeCN / H2O using Waters "Symmetry" C18 (packing : 3.5 ,um), 100
mm
column) = 7.68 min (M+H = 488); HPLC (Waters 717+ Autosampler with PDA
detector,
using Waters "Symmetry" C18 (packing : 3.5 ,um), 4.6 x 150 mm column, 90:10
MeOH /
H2O) tR = 2.43 min (98 % purity).
4-[(3,4-Bis(hydroxybenzyl)(4-cyanophenyl)amino]-4H-[1,2,4]triazole (OBS01067,
STX355)
N -N
HO / N STX355
HO N I \
N
To a solution of OBS01066 (0.98 g, 2.01 mmol) in THF-MeOH (1:1) (20 mL) was
added
a slurry of Pd-C (10%, 0.10 g) in THE (2 mL) and the mixture stirred under an
atmosphere of hydrogen (balloon) for 24 h. The suspension was filtered through
Celite
and the combined filtrates concentrated in vacuo to give a brown residue.
Recrystallisation from McOH gave OBS01067 (STX355) as a white solid (0.14 g,
21 %).
TLC [Si02, EtOAc-n-hexane (1:1)] Rf = 0.6; 1H-NMR (400 MHz, d6-DMSO) 4.84 (2H,
s), 6.50 (1H, d, J = 8.2), 6.62 (2H, m), 6.75 (2H, AA'BB'), 7.75 (2H, AA'BB'),
8.68
(2H, s); LC-MS (Waters 2790 Alliance HPLC / ZQ MicroMass spectrometer with PDA
detector using APCI), tR (gradient elution : 5:95 MeCN / H2O - 95:5 MeCN / H2O
over
10 mins then 95:5 MeCN / H2O - 5:95 MeCN / H2O using Waters "Symmetry" C18
(packing : 3.5 pm), 100 mm column) = 7.68 min (M+H = 488); HPLC (Waters 717+
Autosampler with PDA detector, using Waters "Symmetry" C 18 (packing : 3.5
,um), 4.6
x 150 mm column, 70:30 MeOH / H2O) tR = 3.02 min (99 % purity).
3-Methoxy-4-benzyloxybenzaldehyde (OBS01056)
,0 I \ `-~O
O
Using the procedure reported by A. I. Meyers et al., Heterocycles, 1989, 295.
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
102
To a solution of vanillin (7.7 g, 50.67 mmol) in EtOH (40 mL) was added
potassium
carbonate (7.9 g, 57.35 mmol) and benzyl bromide (6.0 mL, 50.67 mmol) and the
mixture stirred at room temperature overnight. The reaction was filtered
through Celite
and the filtrates concentrated in vacuo. The residue was redissolved in DCM
(250 mL),
washed with aqueous NaOH (5 % w/v, 2 x 100 mL) and the organic layer dried
(Na2S04). Concentration in vacuo and recrystallisation of the residue obtained
from
EtOH gave OBS01056 as a white powder (11.16 g, 91 %). TLC [SiO2, EtOAc-n-
hexane
(1:1)] Rf = 0.8; m.p. 63 - 64 C [Lit. (MeOH): 63 - 64 C]; 'H-NMR (400 MHz,
CDC13)
3.95 (3H, s), 5.25 (2H, s), 6.99 (1H, m), 7.33 - 7.45 (7H, m), 9.84 (1H, s)
3-Methoxy-4-benzyloxybenzyl alcohol (OBS01063)
0 I \\ O
Using the procedure reported by A. van Oeveran et al., J Org. Chem., 1994, 59
(20),
5999.
To a solution of OBS01056 (5.0 g, 20.64 mmol) in anhydrous DCM (25 mL) was
added
a suspension of sodium borohydride (0.97 g, 25.59 mmol) in MeOH (12 mL) and
the
mixture stirred at room temperature for 18 h. The reaction was poured into
water (50
mL) (CARE !!) and extracted with DCM (3 x 50 mL) and dried (MgSO4).
Concentration
in vacuo gave a white residue. Recrystallisation from Et20-petroleum ether
gave
OBS01063 as colorless needles (4.91 g, 97 %). TLC [Si02, EtOAc-n-hexane (1:1)]
Rf =
0.49; m.p. 72 - 74 C [Lit. (Et2O - Pet. ether): 72 - 73 C]; 'H-NMR (400 MHz,
d6-
DMSO) 3.74 (3H, s), 4.39 (2H, d, J= 5.7), 5.05 (2H, s), 5.08 (1H, t, J= 5.7,
OH), 6.84
(1H,dd,J=2, 8.2), 6.91 (1H,d,J=8.2),7.01 (1H, d, J= 2),7.31 -7.46(5H, m).
3 -Methoxy-4-benzyloxybenzyl bromide (OB S 01070)
i0 I \\ D~Br
0
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
103
Adapting the procedure reported by A. van Oeveran et al., J Org. Chem., 1994,
59 (20),
5999.
Phosphorus tribromide (0.98 mL, 10.3 mmol) was added to a solution of OBS01063
(2.44 g, 10 mmol) in anhydrous DCM (20 mL) at 0 T. The mixture was stirred at
0 C
for 2 h and then at room temperature for 1 h. The reaction was poured onto ice
water
(400 mL) and allowed to warm to room temperature. The aqueous solution was
extracted
,with DCM (5 x 100 mL) and the combined organic extracts dried (MgS04).
Concentration in vacuo gave an off-white residue which was recrystallised from
n-
hexane to give OBS01070 as colourless needles (2.52 g, 82 %). TLC [Si02, EtOAc-
n-
hexane (1:1)] Rf = 0.92; m.p. 73 - 74 C [Lit. (Pet. ether): 73 C]; 1H-NMR
(400 MHz,
CDC13) 3.91 (3H, s), 4.49 (2H, s), 5.16 (2H, s), 6.81 (1H, d, J = 8.2), 6.88
(1H, dd, J =
8.2, 2), 6.94 (1H, d, J= 2), 7.28 - 7.44 (5H, m).
4-[(3-Benzyloxy-4-methoxybenzyl)(4-cyanophenyl)amino]-4H-[1,2,4]triazole
(OBS01071, STX677)
N -N
/ \
N ~ STX677
N
O
N
To a suspension of NaH (60 % dispersion in oil, 0.22 g, 5.4 mmol) in anhydrous
DMF
(20 mL) at room temperature was added a solution of 4-[(4-cyanophenyl)amino]-
4H-
[1,2,4]triazole (1.0 g, 5.68 mmol) in anhydrous DMF (4 mL) and the mixture
stirred
under nitrogen for 1 h. The orange-yellow suspension was then treated with a
solution of
OBSO1071 (1.66 g, 5.4 mmol) in anhydrous DMF (5 mL) and the mixture stirred at
room
temperature overnight. The mixture was transferred to a separating funnel and
diluted
with EtOAc (200 mL). The organic layer was washed with water (4 x 200 mL),
brine
(200 mL), and dried (MgS04). Concentration in vacuo gave a residue which was
recystallised from i-PrOH to give OBSO1071 (STX677) as an off-white powder
(769 mg,
%). TLC [Si02, EtOAc-n-hexane (1:1)] Rf = 0.15 (blue fluorescence at 254 nm);
'H-
NMR (400 MHz, d6-DMSO) 3.80 (3H, s), 4.79 (2H, s), 5.12 (2H, s), 6.64 (1H, d,
J= 2),
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
104
6.66 (1H, dd, J= 8.2, 2), 6.70 (2H, AA'BB'), 6.81 (1H, d, J= 8.2), 7.29 - 7.42
(5H, m),
7.59 (2H, AA'BB'), 8.06 (2H, s); MS (FAB+) = 412 (M+H, 100 %), 343 (40), 275
(20),
227 (20); Acc MS for C24H22N502 (Required, 412.1779; Found, 412.1773); LC-MS
(Waters 2790 Alliance HPLC / ZQ MicroMass spectrometer with PDA detector using
APCI), tR (gradient elution : 5:95 MeCN / H2O - 95:5 MeCN / H2O over 10 mins
then
95:5 MeCN / H2O - 5:95 MeCN / H2O using Waters "Symmetry" C18 (packing : 3.5
,um), 100 mm column) = 6.61 min (M+H = 412); HPLC (Waters 717+ Autosampler
with
,PDA detector, using Waters "Symmetry" C18 (packing : 3.5 ,um), 4.6 x 150 mm
column,
X0:10 MeOH / H2O) tR = 2.19 min (95 % purity).
4-[(3-Hydroxy-4-methoxybenzyl)(4-cyanophenyl)amino]-4H-[1,2,4]triazole
(OBS01076,
STX362)
N-N
HO ;J9 STX362
N \
N
To a solution of OBS01071 (411 mg, 999,umol) in THF-MeOH (1:1) (20 mL) was
added
a slurry of Pd-C (10%. 42 mg) in THE (2 mL) and the mixture stirred under an
atmosphere of hydrogen (balloon) for 48 h. The mixture was filtered through
Celite and
the combined filtrates concentrated in vacuo to give a brown residue which
solidified on
standing. Recrystallisation from i-PrOH gave OBS01076 (STX362) as a colourless
powder (232 mg, 72 %). TLC [Si02, EtOAc-n-hexane (1:1)] Rf = 0.52; 1H-NMR (400
MHz, d6-DMSO) 3.71 (3H, s), 4.90 (2H, s), 6.61 (1H, dd, J = 7.8, 2), 6.65 (1H,
d, J =
7.8), 6.79 (2H, AA'BB'), 6.80 (1H, d, J= 2), 7.76 (2H, AA'BB'), 8.72 (2H, s),
9.06 (1H,
br s, OH); MS (FAB+) = 322 (M+H, 100 %), 253 (64); Acc MS for C17H16N502
(Required, 322.1303; Found, 322.1304); HPLC (Waters 717+ Autosampler with PDA
detector, using Waters "Symmetry" C18 (packing : 3.5 ,um), 4.6 x 150 mm
column,
80:20 MeOH / H2O) tR = 2.59 min (95 % purity).
4-[(4-Methoxy-3-O-sulfamoylbenzyl)(4-cyanophenyl)amino]-4H-[ 1,2,4]triazole
(OBS01135, STX660)
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
105
O N -N
H2N-O O N STX660
\O \ I N \
N
To an ice-cooled solution of OBS01076 (100 mg, 310 ,umol) in DMA (10 mL) was
added
sulfamoyl chloride (0.69 M solution toluene, 2.71 mL - the toluene was removed
in
vacuo (not allowing the temperature of the water bath to exceed 30 C) prior
to addition,
8.1 mmol) and the mixture stirred under nitrogen overnight. The mixture was
diluted
with EtOAc (100 mL) and washed with water (3 x 100 mL) and brine (100 mL). The
organic solution was dried (MgSO4) and concentrated in vacuo to give OBS01135
(STX660) as a white residue which was precipitated from EtOAc solution by
addition of
'n-hexane (40 mg, 32 %). TLC [Si02, EtOAc-n-hexane (1:1)] Rf = 0.52; 'H-NMR
(400
MHz, d6-DMSO) 3.77 (3H, s), 5.06 (2H, s), 6.76 (2H, AA'BB'), 6.89 and 6.91
(1H, dd, J
= 8.2, 2.3), 7.08 (1H, d, J= 2.3), 7.23 (1H, d, J= 8.2), 7.78 (2H, AA'BB'),
7.95 (2H, s),
8.87 (2H, s); LC-MS (Waters 2790 Alliance HPLC / ZQ MicroMass spectrometer
with
PDA detector using APCI), tR (gradient elution : 5:95 MeCN / H2O - 95:5 MeCN /
H2O
over 10 min then 95:5 MeCN / H2O - 5:95 MeCN / H2O using Waters "Symmetry" C18
(packing : 3.5 pm), 100 mm column) = 4.29 min (M+H = 401); HPLC (Waters 717 +
Autosampler with PDA detector, using Waters "Symmetry" C18 (packing : 3.5 pm),
4.6
x 150 mm column, 80:20 MeOH / H2O) tR = 3.95 min (88 % purity).
4-Methoxy-3-benzyloxybenzaldehyde (OBS01054)
/
I
\ O "~O
0
\ I /
Using the procedure reported by A. I. Meyers et al., Heterocycles, 1989, 295.
To a suspension of isovanillin (7.7 g, 50.67 mmol) in water (50 mL) was added
potassium hydroxide (3.4 g, 60 mmol) and the mixture stirred for 0.25 h. The
now
homogenous solution was then treated with benzyl bromide (6.0 mL, 50.67 mmol)
and
the mixture heated at reflux for 5 h. The reaction was diluted with DCM (200
mL),
washed with water (2 x 100 mL) and brine (100 mL) and dried (Na2SO4).
Concentration
in vacuo and recrystallisation of the yellow residue obtained from EtOH gave
OBSO1054
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
106
as a colourless powder (11.28 g, 92 %). TLC [Si02, EtOAc-n-hexane (1:1)] Rf =
0.8;
m.p. 62 - 63 C [Lit. (EtOH): 62 C]; 'H-NMR (400 MHz, d6-DMSO) 3.88 (3H, s),
5.17
(2H, s), 7.21 (1H, d, J= 8.2), 7.33 - 7.49 (6H, m), 7.58 (1H, d, J= 8.2), 9.83
(1H, s).
4-Methoxy-3-benzyloxybenzyl alcohol (OBS01062)
off
Using the procedure reported by A. I. Meyers et al., Heterocycles, 1989, 295.
To a solution of OBS01054 (5.0 g, 20.64 mmol) in EtOH (60 mL) was added sodium
borohydride (1.23 g, 32.40 mmol) and the mixture stirred at room temperature
for 18 h.
The reaction was heated at relux for. 1 h, cooled and decanted into aqueous
ammonium
chloride (50 % w/v, 250 mL) (CARE ! !). The aqueous solution was extracted
with Et20
(3 x 100 mL), washed with brine (100 mL) and dried (Na2S04). Concentration in
vacuo
gave a white residue. Recrystallisation from EtOAc-petroleum ether gave
OBS01062 as
colorless needles (4.91 g, 97 %). TLC [Si02, EtOAc-n-hexane (1: 1)] Rf= 0.48;
m.p. 73
- 74 C [Lit. (EtOAc-Pet. ether): 73 C]; 'H-NMR (400 MHz, d6-DMSO) 3.74 (3H,
s),
4.39 (2H, d, J = 5.7), 5.05 (2H, s), 5.08 (1H, t, J = 5.7, OH), 6.84 (1H, dd,
J = 2, 8.2),
6.91 (1H, d, J= 8.2),7.01 (1H, d, J= 2),7.33 - 7.49 (5H, m).
4-Methoxy-3 -benzyloxybenzyl bromide (OB S 01068)
Br
I
0
Using the procedure reported by A. I. Meyers et al., Heterocycles, 1989, 295.
Phosphorus tribromide (0.98 mL, 10.3 mmol) was added to a solution of OBS01062
(2.44 g, 10 mmol) in anhydrous THE (20 mL) at 0 C. The mixture was stirred at
0 C
for 2 h and then at room temperature for 1 h. Concentration in vacuo gave an
off-white
residue which was recrystallised from DCM-n-hexane to give OBS01068 as a white
powder (2.95 g, 96 %). TLC [Si02, EtOAc-n-hexane (1:1)] Rf = 0.9; m.p. 86 - 88
C
[Lit. (DCM-hexane): 86 - 87 C];'H-NMR (400 MHz, d6-DMSO) 3.74 (3H, s), 4.39
(2H,
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
107
s), 5.05 (2H, s), 6.83 (1H, dd, J= 2, 8.2), 6.90 (1H, d, J- 8.2), 7.01 (1H, d,
J= 2), 7.30 -
7.47 (5H, m).
4- [(3 -B enzyloxy-4-methoxybenzyl)(4-cyanophenyl)amino] -4H- [ 1,2,4]triazole
(OBS01069)
N-N
0 N
O \ I N \
N
To a suspension of NaH (60 % dispersion in oil, 220 mg, 5.4 mmol) in anhydrous
DMF
x(20 mL) at room temperature was added a solution of 4-[(4-cyanophenyl)amino]-
4H-
[1,2,4]triazole (1.0 g, 5.68 mmol) in anhydrous DMF (4 mL) and the mixture
stirred
under nitrogen for 1 h. The orange-yellow suspension was then treated with a
solution of
OBS01068 (1.66 g, 5.4 mmol) in anhydrous DMF (5 mL) and the mixture stirred at
room
temperature overnight. The mixture was transferred to a separating funnel and
diluted
with EtOAc (200 mL). The organic layer was washed with water (4 x 200 mL),
brine
(200 mL), and dried (MgS04). Concentration in vacuo gave a residue which was
recystallised from EtOH to give OBS01069 as an off-white powder (970 mg, 44
%).
TLC [Si02, EtOAc-n-hexane (1:1)] Rf = 0.12 (blue fluorescence at 254 nm); 'H-
NMR
(400 MHz, CDC13) 3.89 (3H, s), 4.72 (2H, s), 5.12 (2H, s), 6.59 (2H, AA'BB'),
6.62 (1H,
d, J= 2), 6.64 (1H, dd, J= 7.8, 2), 6.79 (1H, d, J= 7.8), 7.28 - 7.34 (5H, m),
7.57 (2H,
AA'BB'), 7.74 (2H, s); MS (FAB+) 412 (M+H, 100 %), 343 (51), 227 (72); Acc MS
for
C24H22N502 (Required, 412.1776; Found, 412.1774).
4-[(3-Hydroxy-4-methoxybenzyl)(4-cyanophenyl)amino]-4H-[1,2,4]triazole
(OBS01080,
STX363)
N -N
~0 STX363
HO NI \
~\N
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
108
To a solution of OBSO1069 (411 mg, 999 ,umol) in THF-MeOH (1:1) (20 mL) was
added
a slurry of Pd-C (10%, 42 mg) in THE (2 mL) and the suspension stirred under
an
atmosphere of hydrogen (balloon) for 48 h. The suspension was filtered through
Celite
and the combined filtrates concentrated in vacuo to give a brown residue.
Recrystallisation from i-PrOH gave OBS01080 (STX363) as a white powder (164
mg, 51
%). TLC [Si02, EtOAc-n-hexane (1:1)] Rf= 0.52; 1H-NMR (400 MHz, d6-DMSO) 3.71
(3H, s); 4.88 (2H, s), 6.62 (1H, dd, J = 8.2, 1.95), 6.68 (1H, d, J = 1.95),
6.74 (2H,
AA'BB'), 6.80 (1H, d, J= 8.2), 7.74 (2H, AA'BB'), 8.70 (2H, s), 9.00 (1H, s,
OH); MS
(FAB+) 322 (M+H, 100 %), 253 (61); Acc MS for C17H16N502 (Required, 322.1304;
to Found, 322.1304); HPLC (Waters 717+ Autosampler with PDA detector, using
Waters
"Symmetry" C18 (packing : 3.5,um), 4.6 x 150 mm column, 90:10 MeOH / H2O) tR =
2.03 min (99 % purity).
4-[(4-Methoxy-3-O-sulfamoylbenzyl)(4-cyanophenyl)amino] -4H-[ 1,2,4]triazole
(OBS01137, STX661)
N-N
I-O XIN STX661
H2NO2SO N
N
To an ice-cooled solution of OBS01080 (100 mg, 310 pmol) in DMA (10 mL) was
added
sulfamoyl chloride (0.69 M solution toluene, 2.71 mL - the toluene was removed
in
vacuo (not allowing the temperature of the water bath to exceed 30 C) prior
to addition,
8.1 mmol) and the mixture stirred under nitrogen overnight. The mixture was
diluted
with EtOAc (100 mL) and washed with water (3 x 100 mL) and brine (100 mL). The
organic layer was dried (MgSO4) and concentrated in vacuo to give OBS01137
(STX661) as a colourless residue which was precipitated from EtOAc solution by
addition of n-hexane (40 mg, 32 %). TLC [SiO2, EtOAc-n-hexane (1:1)] Rf =
0.53; 1H-
NMR (400 MHz, d6-DMSO) 3.77 (3H, s); 5.00 (2H, s), 6.79 (2H, AA'BB'), 7.06
(1H, d,
J = 8.6), 7.12 and 7.14 (1H, dd, J = 8.2, 2.3), 7.27 (1H, d, J = 2), 7.77 (2H,
AA'BB'),
7.94 (2H, s), 8.75 (2H, s); LC-MS (Waters 2790 Alliance HPLC / ZQ MicroMass
spectrometer with PDA detector using APCI), tR (gradient elution : 5:95 MeCN /
H2O -
95:5 MeCN / H2O over 10 mins then 95:5 MeCN / H2O - 5:95 MeCN / H2O using
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
109
Waters "Symmetry" C18 (packing : 3.5 ,um), 100 mm column) = 4.70 min (M+H =
401);
HPLC (Waters 717+ Autosampler with PDA detector, using Waters "Symmetry" C18
(packing : 3.5 ,um), 4.6 x 150 mm column, 80:20 MeOH / H2O) tR = 2.04 min (88
%
purity)-
3-Bromo-4-hydroxybenzaldehyde (OBS01057)
HO
Br
Adapting the procedure reported by S. Kelly et al., Hely. Chim. Acta., 1989,
72, 594.
To a ice-cooled solution of 4-hydroxybenzaldehyde (30.0 g, 245.67 mmol) in
glacial
rAcOH (120 mL) was added bromine (12.6 mL, 257.61 mmol). The mixture was
stirred
at room temperature overnight, diluted with water (600 mL) and extracted with
DCM (3
x 120 mL). The combined organic fractions were washed with water (600 mL),
dilute
sodium hydrogen carbonate solution (2 x 600 mL) and brine (600 mL). The
organic
layer was dried (Na2SO4) and concentrated in vacuo to give a brown residue.
Recrystallisation from toluene gave OBSO 1057 as a pink-brown crystalline
solid (26.3 g,
53 %). TLC [Si02, EtOAc-n-hexane (1:1)] Rf = 0.6; m.p. 124 - 126 C [Lit.
(CHC13):
125 - 126 C]; 'H-NMR (400 MHz, CDC13) 6.31 (1H, s, OH), 7.15 (1H, d, J= 8.8),
7.77
(1H, dd, J= 8.8, 2), 8.04 (1H, d, J= 2), 9.83 (1H, s, CHO).
3 -Bromo-4-benzoyloxybenzaldehyde (OB S 01072)
O I ~O
O /
Br
ej
To a solution of OBS01057 (8.0 g, 40.0 mmol) in EtOAc (100 mL) was added NEt3
(5.58
mL, 40.0 mmol) and the mixture stirred at room temperature for 0.5 h. Benzoyl
chloride
(4.64 mL, 40.0 mmol) was then added and the reaction stirred at room
temperature for 5
h. The precipitated NEt3.HC1 was filtered off and the organic solution dried
(Na2S04).
Concentration in vacuo gave a grey residue. Recrystallisation from EtOAc-
petroleum
ether gave OBS01072 as a yellow solid (10.9 g, 89 %). TLC [Si02, EtOAc-n-
hexane
(1:1)] Rf= 0.82; 1H-NMR (400 MHz, CDC13) 7.50 (1H, d, J= 8.2), 7.54, 7.56 and
7.58
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
110
(2H, tt, J = 7.4, 1.4), 7.68, 7.70 and 7.71 (1R, tt, J = 7.4, 1.4), 7.91 and
7.93 (1H, dd, J =
8.2, 1.8), 8.20 (1H, d, J= 1.8), 8.24 and 8.27 (2H, dt, J= 8.2, 1.4), 9.98
(1H, s, CHO).
3-Bromo-4-benzoyloxybenzyl alcohol (OBS01074)
O I ~OH
e O 5 .Br
To a solution of OBS01072 (6.10 g, 19.99 mmol) in anhydrous THE (25 mL) was
added
sodium borohydride (1.13 g, 29.99 mmol) and the mixture stirred at room
temperature
for 4 h. The reaction was quenched with cautious addition of water (CARE ! !),
filtered
through Celite and dried (MgSO4). Concentration in vacuo gave a yellowish oil
which
1 crystallised on standing. Recrystallisation from Et2O gave 3-bromo-4-
benzoyloxybenzyl
alcohol as colorless needles (5.89 g, 96 %). TLC [Si02, EtOAc-n-hexane (1:1)]
Rf= 0.5;
1H-NMR (400 MHz, CDC13) = 4.71 (2H, s), 7.26 (1H, d, J = 8.2), 7.36 (1H, dd, J
=
8.2, 1.95), 7.51, 7.53 and 7.55 (2H, tt, J= 8.2, 1.6), 7.64-7.68 (2H, m), 8.24
(1H, t, J=
1.95), 8.26 (1H, t, J= 1.95).
3-Bromo-4-benzoyloxybenzyl bromide (OBS01089)
Br
O 0,1
e Br
Phosphorus tribromide (0.98 mL, 10.3 mmol) was added to a solution of OBS01074
(3.07 g, 10.0 mmol) in anhydrous dichloromethane (45 mL) at 0 C. The mixture
was
stirred at 0 C for 2 h and then at room temperature for 1 h. The reaction was
poured
onto ice water (400 mL) and allowed to warm to room temperature. The aqueous
solution was extracted with diethyl ether (5 x 100 mL) and the combined
ethereal
solution dried (MgS04). Concentration in vacuo gave a yellow oil. Purification
by
gravity column chromatography [Si02, EtOAc-petroleum ether (1:7)] give
OBS01089 as
colourless needles (3.22 g, 87 %). TLC [Si02, EtOAc-n-hexane (1:1)] Rf = 0.87;
1H-
NMR (400 MHz, CDC13) 4.47 (2H, s), 7.27 (1 H, d, J = 8.6), 7.41 (1 H, dd, J =
8.6, 2.3),
7.52, 7.54 and 7.56 (2H, if, J= 8.2, 1.56), 7.66, 7.67 and 7.69 (1H, if, J=
8.2, 1.56), 7.70
(1H, d, J= 2.3), 8.24 (1H, t, J=1.2), 8.26 (1H, t, J= 1.2).
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
111
4-[(4-Benzoyloxy-3-bromobenzyl)(4-cyanophenyl)amino]-4H-[ 1,2,4]triazole
(OBSO1131)
Br N-N
\ I
0,1:t, N
-Y I
0
N
To a suspension of NaH (60 % dispersion in oil, 220 mg, 5.4 mmol) in anhydrous
DMF
(20 mL) at room temperature was added a solution of 4-[(4-cyanophenyl)amino]-
4H-
[1,2,4]triazole (1.0 g, 5.68 mmol) in anhydrous DMF (4 mL) and the mixture
stirred
under nitrogen for 1 h. The orange-yellow suspension was then treated with a
solution of
OBSO 1089 (2.00 g, 5.4 mmol) in anhydrous DMF (5 mL) and the mixture stirred
at room
temperature overnight. The mixture was transferred to a separating funnel and
diluted
with EtOAc (200 mL). The organic layer was washed with water (4 x 200 mL),
brine
(200 mL), and dried (MgSO4). Concentration in vacuo gave a residue which was
recrystallised from i-PrOH to give OBSO1131 as a colourless solid (1.99 g, 78
%). TLC
[Si02, EtOAc (100 %)] Rf = 0.5 (blue fluorescence at 254 nm); 'H-NMR (400 MHz,
d6-
DMSO) 5.14 (2H, s), 6.75 (1H, t, J = 2.7), 6.78 (1H, t, J = 2.7), 7.44 (2H, d,
J = 8.2),
7.61 - 7.66 (2H, m), 7.76 - 7.80 (4H, m), 8.14 (1H, t, J= 2.7), 8.16 (1 H, t,
J= 2.7), 8.92
(2H, s); MS (FAB+) 474 (M, 100 %), 405 (38); Acc MS for C23H16N5O2Br
(Required,
474.0569; Found, 474.0566).
4-[(3-Bromo-4-hydroxybenzyl)(4-cyanophenyl)amino]-4H-[1,2,4]triazole
(OBS01132,
STX405)
Br N-N
HO STX405
\ I N \
N
To a suspension of OBS01131 (2.0 g, 4.22 mmol) in MeOH (10 mL) was added
potassium hydroxide (1.42 g, 25.3 mmol) and the mixture stirred at room
temperature for
2 h. The solvents were removed in vacuo and he pH of the alkaline slurry was
adjusted
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
112
to - pH 6 - 7 by treatment with saturated sodium hydrogen carbonate solution.
The
colourless precipitate was filtered off, washed with the minimum of cold water
and
boiled in i-PrOH to give OBS01091 (STX405) as a colourless powder (1.20 g, 77
%).
TLC [Si02, EtOAc-n-hexane (1:1)] Rf= 0.43; 1H-NMR (400 MHz, d6-DMSO) 4.93 (2H,
s), 6.77 (2H, AA'BB'), 6.85 (1H, d, J= 8.2), 7.06 (1H, dd, J= 8.2, 1.95), 7.41
(1H, d, J
= 1.95), 7.76 (2H, AA'BB'), 8.76 (2H, s); 10.35 (1H, br s, OH); MS (FAB+) 370
(M,
100 %), 301 (55), 260 (67), 242 (64); Acc MS for C16H12N5OBr (Required,
370.0314;
Found, 370.0304); HPLC (Waters 717+ Autosampler with PDA detector, using
Waters
"Symmetry" C18 (packing : 3.5 ,um), 4.6 x 150 mm column, 94:6 MeOH / H2O) tR =
2.73 min (96 % purity).
4-[(3-Bromo-4-O-sulfamoylbenzyl)(4-cyanophenyl)amino] -4H-[ 1,2,4]triazole
(OBS01141, STX681)
Br N-N
H2NO2SO N STX681
\ I N \
N
To an ice-cooled solution of OBS01132 (500 mg, 1.35 mmol) in DMA (10 mL) was
added sulfamoyl chloride (0.69 M solution toluene, 11.7 mL - the toluene was
removed
in vacuo (not allowing the temperature of the water bath to exceed 30 C)
prior to
addition, 8.1 mmol) and the mixture stirred under nitrogen overnight. The
mixture was
diluted with EtOAc (100 mL) and washed with water (3 x 100 mL) and brine (100
mL).
The organic layer was dried (MgSO4) and concentrated in vacuo to give a
colourless
residue which was precipitated from EtOAc solution by addition of n-hexane
(463 mg).
The white solid (200 mg) was then purified by gradient elution gravity column
chromatography [SiO2, EtOAc-n-hexane (1:4) - EtOAc (100 %)] to give OBS01141
(STX681) as a white solid (107 mg, 44 % after chromatography). TLC [Si02,
EtOAc-n-
hexane (1:1)] Rf = 0.42; 1H-NMR (400 MHz, d6-DMSO) 5.17 (2H, s), 6.80 (2H,
AA'BB'), 7.50 (2H, s), 7.78 (1H, s), 7.84 (2H, AA'BB'), 8.38 (2H, s), 8.96
(2H, s); MS
(FAB+) 412 (M+H, 100 %), 343 (40), 275 (20), 227 (20); Acc MS for C24H22N5O2
(Required, 412.1779; Found, 412.1773); LC-MS (Waters 2790 Alliance HPLC / ZQ
MicroMass spectrometer with PDA detectors using APCI), tR (gradient elution :
5:95
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
113
MeCN / H2O - 95:5 MeCN / H2O over 10 mins then 95:5 MeCN / H2O - 5:95 MeCN /
H2O using Waters "Symmetry" C18 (packing : 3.5 ,um), 100 mm column) = 5.52 min
(M+H = 448); HPLC (Waters 717+ Autosampler with PDA detector, using Waters
"Symmetry" C18 (packing : 3.5 pm), 4.6 x 150 mm column, 90:10 MeOH / H2O) tR =
1.89 min (99 % purity). HPLC cannot differentiate OBS01091 (STX 405) and
OBS01141 (STX 681). LC-MS / 1H-NMR indicates that sample of OBS01141 (STX
681) [M+H] = 450 contains ca. 6.66 % of starting material OBS01091 (STX 405)
[M+H]
371.
4-[(2-Bromo-ethyl)-[1,2,4]triazol-4-yl-amino]-benzonitrile (CAB03031)
N-N
N
i
Br,-,,_,N
GN
Sodium hydride (60%, 240 mg, 6.0 mmol) was added to a solution of 4-
([1,2,4]triazol-4-
ylamino)-benzonitrile (926 mg, 5.0 mmol) in DMSO (25 mL) at r.t.. The mixture
was
stirred for 1 hour at this temperature and 1,2-dibromethane (5 mL) was added.
The
reaction mixture was stirred overnight and ethyl acetate (100 mL) was added.
The
mixture was transferred into a separation funnel and extracted with water
(twice 100 mL)
and brine (20 mL). The organic layer was dried over sodium sulphate and
concentrated
under reduced pressure (water bath temperature < 30 C). The resulting orange
oil was
mixed with diethyl ether (100 mL) and filtered through a layer of silica (ca.
5 cm). The
silica was washed with more diethyl ether (ca. 100 mL) to remove the excess of
1,2-
dibromoethane; the crude product was washed from the silica with acetone (120
mL).
The acetone solution was concentrated under reduced pressure and the residue
was
purified by column-chromatography (eluent: ethyl acetate) to give the title
compound as
a white solid. Yield: 628 mg (43%). 'H-NMR (400 MHz, d6-DMSO) 8 = 3.61 (t, J =
6.2
Hz, 2H), 4.30 (t, J = 6.4 Hz, 2H), 6.64 (d, J = 9.0 Hz), 7.74 (d, 9.0 Hz, 2H),
8.97 (s, 2H).
LRMS (FAB+):292.0 (100, [M+H]+).
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
114
4- { [2-(4-Hydroxy-phenylsulfanyl)-ethyl]-[ 1,2,4]triazol-4-yl-amino } -
benzonitrile
(CAB02137, STX456)
N-N
HO N STX456
N )aCN
A mixture of 4-[(2-bromo-ethyl)-[1,2,4]triazol-4-yl-amino]-benzonitrile
(CAB03031,
146 mg, 0.50 mmol), 4-hydroxythiophenol (126 mg, 1.0 mmol) and potassium
carbonate
(138 mg, 1.0 mmol) in DMF (10 mL) was stirred overnight at room temperature.
The
mixture was transferred into a separation funnel and ethyl acetate (50 mL) and
water (50
mL) were added. The organic layer was separated, washed with brine (30 mL),
dried over
sodium sulphate and concentrated under reduced pressure. The residue was
crystallised
from methanol. Yield: 116 mg (69%) colourless crystals. 1H-NMR (400 MHz, d6-
DMSO) 6 = 2.99 (t, J = 7.0 Hz, 2H), 3.93 (t, J = 7.0 Hz, 2H), 6.48 (d, = 9.0
Hz, 2H),
6.73 (d, J = 8.6 Hz, 2H), 7.21 (d, J = 8.6 Hz, 2H), 7.68 (d, J = 9.0 Hz, 2H),
8.89 (s, 2H),
9.66 (s, 1H, -OH). 13C-NMR (100 MHz, d6-DMSO) S = 30.62, 53.07, 102.92,
113.36,
116.86, 119.59, 122.71, 134.07, 134.49, 144.07, 151.17, 157.83. LRMS (FAB+):
338.2
(100, [M+H]+). Found: C 60.6, H 4.57, N 20.6%; C17H15N50S (337.4) requires C
60.52,
H 4.48, N 20.76%.
4-{[2-(3-Hydroxy-phenylsulfanyl)-ethyl]-[1,2,4]triazol-4-yl-amino}-
benzonitrile
(CAB02149, STX512)
N-N
STX512
HO \ _,N
CN
A mixture of 4-[(2-bromo-ethyl)-[1,2,4]triazol-4-yl-amino]-benzonitrile
(CAB02148=CABO3031, 146 mg, 0.50 mmol), 3-(tert-butyl-dimethylsiloxy)-
thiophenol
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
115
(240 mg, 1.0 mmol) and potassium carbonate (276 mg, 2.0 mmol) in DMF (10 mL)
was
stirred for 48 hours at room temperature. The mixture was transferred into a
separation
funnel and ethyl acetate (50 mL) and water (50 mL) were added. The organic
layer was
separated, washed with brine (30 mL), dried over sodium sulphate and
concentrated
under reduced pressure. The residue was crystallised from methanol. Yield: 93
mg (55%)
colourless crystals. 1H-NMR (400 MHz, d6-DMSO) S = 3.14 (t, J = 7.0 Hz, 2H),
4.02 (t,
J = 7.0 Hz, 2H), 6.53 (d, J = 9.0 Hz, 2H), 6.62 (ddd, J = 7.8, 2.0, 0.8 Hz,
1H), 6.70-6.72
('m, 2H), 7.10 (dd, J = 7.8, 7.8, 1H), 7.70 (d, J = 9.0 Hz, 2H), 8.94 (s, 2H),
9.60 (s, 1H, -
PH). 13C-NMR (100 MHz, d6-DMSO) S = 29.53, 52.46, 102.38, 112.86, 113.57,
115.36,
119.00, 130.11, 133.94, 135.82, 143.50, 150.71, 157.90, 169.59. LRMS (FAB+):
338.2
(100, [M+H]+). Found: C 60.6, H 4.53, N 20.8%; C17H15N50S (337.4) requires C
60.52,
H 4.48, N 20.76%.
4-[(3-Bromo-propyl)-[1,2,4]triazol-4-yl-amino]-benzonitrile (CAB02180, STX595)
N-N
STX595
Br~,,-~. N
CN
Sodium hydride (60%, 240 mg, 6.0 mmol) was added to a solution of 4-
([1,2,4]triazol-4-
ylamino)-benzonitrile (926 mg, 5.0 mmol) in DMSO (25 mL) at r.t.. The mixture
was
stirred for 1 hour at this temperature and 1,3-dibromopropane (5 mL) was
added. The
reaction mixture was stirred overnight and ethyl acetate (100 mL) was added.
The
mixture was transferred into a separation funnel and extracted with water
(twice 100 mL)
and brine (20 mL). The organic layer was dried over sodium sulphate and
concentrated
under reduced pressure (water bath temperature < 30 C). The resulting orange
oil was
mixed with diethyl ether (100 mL) and filtered through a layer of silica (ca.
5 cm). The
silica was washed with more diethyl ether (ca. 100 mL) to remove the excess of
1,3 -
dibromopropane, the crude product was washed from the silica with acetone (120
mL).
The acetone solution was concentrated under reduced pressure and the residue
was
purified by column-chromatography (eluent: ethyl acetate) to give the title
compound as
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
116
a white solid. Yield: 792 mg (52%). 'H-NMR (400 MHz, d6-DMSO) 6 = 2.01 (tt, J
= 7.0,
6.6 Hz, 2H), 3.64 (t, J = 6.6 Hz, 2H), 3.95 (t, J = 7.0 Hz, 2H), 6.62 (d, J =
9.0 Hz, 2H),
7.74 (d, J = 9.0 Hz, 2H), 9.02 (s, 2H). 13C-NMR (100 MHz, d6-DMSO) S = 30.38,
32.13,
52.72, 103.17, 113.83, 119.74, 134.63, 144.14, 151.65. LRMS (FAB+): 306.0
(100,
[M+H]+).
4- { [3-(4-Hydroxy-phenylsulfanyl)-propyl]-[ 1,2,4]triazol-4-yl-amino } -
benzonitrile
(bABO182, STX596)
N STX596
HO CN
A mixture of 4-[(2-bromo-propyl)-[1,2,4]triazol-4-yl-amino]-benzonitrile
(CAB02180,
146 mg, 0.50 mmol), 4-hydroxy-thiophenol (240 mg, 1.0 mmol) and potassium
carbonate
(276 mg, 2.0 mmol) in DMF (10 mL) was stirred for 48 hours at room
temperature. The
mixture was transferred into a separation funnel and ethyl acetate (50 mL) and
water (50
mL) were added. The organic layer was separated, washed with brine (30 mL),
dried over
sodium sulphate and concentrated under reduced pressure. The residue was
purified by
column chromatography (eluent: ethyl acetate, Rf: 0.31) to give an yellow oil,
which was
crystallised from methanol. Yield: 211 mg (60%). 'H-NMR (400 MHz, d6-DMSO) 6 =
1.64 (tt, J = 7.0, 7.0 Hz, 2H), 2.89 ( t, J = 7.0 Hz, 2H), 3.92 (t, J = 7.0
Hz, 2H), 6.57 ( d, J
= 9.0 Hz, 2H), 6.72 (d, J = 9.0 Hz, 2H), 7.20 (d, J = 9.0 Hz, 2H), 7.71 (d, J
= 9.0 Hz, 2H),
8.96 (s,2H), 9.59 (s, 1H, -OH). LRMS (FAB+): 352.1 (100, [M+H]+).
Sulfamic acid 4-{3-[(4-cyano-phenyl)-[1,2,4]triazol-4-yl-amino]-
propylsulfanyl}-
phenyl ester (CAB02184, STX597)
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
117
N-N
STX597
S-,,iN
H2NO2SO CN
Sulphamoyl chloride solution in toluene (3 mL, 0.7 M, 2.1 mmol) was
concentrated
under reduced pressure (30 C water bath temperature) to ca. 0.5 mL volume. The
residue
was cooled to 0 C (ice bath) and N,N-dimethylacetamide (5 mL) was added. 4-{[3-
(4-
hydroxy-phenylsulfanyl)-propyl]-[1,2,4]triazol-4-yl-amino}-benzonitrile
(CAB02182,
140 mg, 0.40 mmol) was added to the colourless solution and the mixture was
stirred for
18 hours at room temperature. Ethyl acetate (50 mL) and water (50 mL) were
added to
the solution, the organic layer was separated, washed with water (2 x 30 mL)
and brine
(20 mL), dried over sodium sulphate and concentrated under reduced pressure.
The
residue was dissolved in a small amount of acetone and precipitated by
addition of
hexane. The precipitate was filtered off and dried under high vacuum. Yield:
139 mg
(81%) light yellow powder. 'H-NMR (400 MHz, d6-DMSO) 6 = 1.74 (tt, J = 7.0,
7.0 Hz,
2H), 3.97 (t, J = 7.0 Hz, 2H), 6.62 (d, J = 9.0 Hz, 2H), 7.22 (d, J = 8.6 Hz,
2H), 7.43 (d, J
= 8.6 Hz, 2H), 7.74 (d, J = 9.0 Hz, 2H), 8.03 (s, 2H, -NH2), 9.00 (s, 2H).
LRMS (FAB+):
431.1 (100, [M+H]+).
4-[(4-Bromo-butyl)-[1,2,4]triazol-4-yl-amino]-benzonitrile (CAB03001,STX602)
N-N
STX602
BrN
I:LCN
Sodium hydride (60%, 240 mg, 6.0 mmol) was added to a solution of 4-
([1,2,4]triazol-4-
ylamino)-benzonitrile (926 mg, 5.0 mmol) in DMSO (25 mL) at r.t.. The mixture
was
stirred for 1 hour at this temperature and 1,4-dibromobutane (5 mL, ca 42
mmol) was
added. The reaction mixture was stirred overnight and ethyl acetate (100 mL)
was added.
The mixture was transferred into a separation funnel and extracted with water
(twice 100
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
118
mL) and brine (20 mL). The organic layer was dried over sodium sulphate and
concentrated under reduced pressure (water bath temperature < 30 C). The
resulting
orange oil was mixed with diethyl ether (100 mL) and filtered through a layer
of silica
(ca. 5 cm). The silica was washed with more diethyl ether (ca. 100 mL) to
remove the
excess of 1,4-dibromobutane; the crude product was washed from the silica with
acetone
(120 mL). The acetone solution was concentrated under reduced pressure and the
residue
was purified by column-chromatography (eluent: ethyl acetate) to give the
title
dompound as a white solid. Yield: 984 mg (61%). 1H-NMR (400 MHz, d6-DMSO) S =
1,.53-1.60 (m, 2H), 1.87-1.94 (m, 2H), 3.56 (t, J = 6.6 Hz, 2H), 3.87 (t, J =
7.4 Hz, 2H),
6.64 (d, J = 9.0 Hz, 2H), 7.73 (d, J = 9.0 Hz, 2H), 8.99 (s, 2H). LRMS (FAB+):
320.0
(100, [M+H]+)
4-{ [4-(4-Hydroxy-phenylsulfanyl)-butyl]-[ 1,2,4]triazol-4-yl-amino } -
benzonitrile
(CAB03007)
//N/-N\\
HO / (N~
N
CN
Potassium carbonate (500 mg) was added to a solution of 4-[(2-bromo-butyl)-
[1,2,4]triazol-4-yl-amino]-benzonitrile (CAB03001, 384 mg, 1.2 mmol) and 4-
hydroxy-
thiophenol (227 mg, 1.8 mmol) in DMF (20 mL). The mixture was stirred for 18
hours at
r.t., diluted with ethyl acetate (100 mL) and extracted with water (two times
30 mL) and
brine (30 mL). The. organic layer was dried over sodium sulphate and
concentrated under
reduced pressure. The residue was purified by column chromatography (eluent:
ethyl
acetate, Rf: 0.30). The resulting oil was dissolved in a small amount of ethyl
acetate and
the product was precipitated by addition of hexane. Yield: 289 mg (66%). 1H-
NMR (400
MHz, d6-DMSO) 6 = 1.48-1.64 (m, 4H) 2.81 (t, J = 6.6 Hz, 2H), 3.81 (t, J = 6.8
Hz, 2H),
6.62 (d, J = 9.0 Hz, 2H), 6.72 (d, J = 8.6 Hz, 2H), 7.19 (d, J = 9.0 Hz, 2H),
7.72 (d, J =
9.0, 2H), 8.94 (s, 2H), 9.56 (s, 1H, -OH). LRMS (FAB+): 366.1 (100, [M+H]).
4-[(5-Bromo-pentyl)-[1,2,4]triazol-4-yl-amino]-benzonitrile (CAB03005)
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
119
N-N
N
Br N ~
CN
Sodium hydride (60%, 240 mg, 6.0 mmol) was added to a solution of 4-
([1,2,4]triazol-4-
ylamino)-benzonitrile (926 mg, 5.0 mmol) in DMSO (25 mL) at r.t.. The mixture
was
stirred for 1 hour at this temperature and 1,5 -dibromopentane (5 mL) was
added. The
reaction mixture was stirred overnight and ethyl acetate (100 mL) was added.
The
mixture was transferred into a separation funnel and extracted with water
(twice 100 mL)
and brine (20 mL). The organic layer was dried over sodium sulphate and
concentrated
under reduced pressure (water bath temperature < 30 C). The resulting orange
oil was
mixed with diethyl ether (100 mL) and filtered through a layer of silica (ca.
5 cm). The
silica was washed with more diethyl ether (ca. 100 mL) to remove the excess of
1,5-
dibromopentane. The crude product was washed from the silica with acetone (120
mL).
The acetone solution was concentrated under reduced pressure and the residue
was
purified by column-chromatography (eluent: ethyl acetate) to give the title
compound as
a white solid. Yield: 1.01 g (60%). 1H-NMR (400 MHz, d6-DMSO) 6 = 1.40-1.52
(m,
2H), 1.75-1.85 (m, 2H), 3.52 (t, J = 6.6 Hz, 2H), 3.78-3.89 (m, 2H), 6.64 (d,
J = 9.0 Hz,
2H), 7.72 (d, J = 9.0 Hz, 2H), 8.97 (s, 2H). LRMS (FAB+): 334.1 (100, [M+H]+).
4-{[5-(4-Hydroxy-phenylsulfanyl)-pentyl]-[1,2,4]triazol-4-yl-amino}-
benzonitrile
(CAB03014, STX698)
N-N
N STX698
\\ S N
HO CN
A mixture of 4-[(2-Bromo-pentyl)-[1,2,4]triazol-4-yl-amino]-benzonitrile
(CAB03005,
500 mg, 1.5 mmol), 4-hydroxy-thiophenol (378 mg, 3.0 mmol) and potassium
carbonate
(414 mg, 3.0 mmol) in DMF (10 mL) was stirred for 12 hours at room
temperature. The
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
120
mixture was transferred into a separation funnel and ethyl acetate (50 mL) and
water (50
mL) were added. The organic layer was separated, washed with brine (30 mL),
dried over
sodium sulphate and concentrated under reduced pressure. The residue was
purified by
column chromatography (eluent: ethyl acetate, Rf: 0.43). The resulting yellow
oil was
dissolved in ethyl acetate and precipitated by addition of hexane to give an
light yellow
powder. Yield: 338 mg (59%). 'H-NMR (400 MHz, d6-DMSO) S = 1.40-1.54 (m, 6H),
2.77 (t, J = 6.8 Hz, 2H), 3.79 (t, J = 6.6 Hz, 2H), 6.61 (d, J = 9.0 Hz, 2H),
6.72 (d, J = 9.0
Rz, 2H), 7.20 (d, J = 9.0 Hz, 2H), 7.72 (d, J = 9.0 Hz, 2H), 8.96 (s, 2H),
9.55 (s, 1H. -
QH). 13C-NMR (100 MHz, d6-DMSO) 8 = 25.66, 26.67, 29.35, 35.56, 53.86, 103.06,
113.89, 116.80, 119.84, 124.54, 133.39, 134.47, 144.09, 151.89, 157.26. LRMS
(FAB+):
380.0 (100, [M+H]+). HRMS (FAB+) 380.15355 C20H22N5OS requires 380.154507.
Sulfamic acid 4-{5-[(4-cyano-phenyl)-[1,2,4]triazol-4-yl-amino]-
pentylsulfanyl}-
phenyl ester (CAB03025, STX699)
N-N
STX699
S N
H2NO2SO 1::~CN
Sulphamoyl chloride solution in toluene (3 mL, 0.7 M, 2.1 mmol) was
concentrated
under reduced pressure (30 C water bath temperature) to ca. 0.5 mL volume. The
residue
was cooled to 0 C (ice bath) and NN-dimethylacetamide (5 mL) was added. 4-{[3-
(4-
hydroxy-phenylsulfanyl)-pentyl]-[1,2,4]triazol-4-yl-amino}-benzonitrile
(CAB03014, 95
mg, 0.25 mmol) was added to the colourless solution and the mixture was
stirred for 18
hours at room temperature. Ethyl acetate (50 mL) and water (50 mL) were added
to the
solution, the organic layer was separated, washed with water (two times 30 mL)
and
brine (20 mL), dried over sodium sulphate and concentrated under reduced
pressure. The
residue was dissolved in a small amount of acetone and precipitated by
addition of
hexane. The precipitate was filtered off and dried under high vacuum. Yield:
103 mg
(90%) light yellow powder. 'H-NMR (270 MHz, d6-DMSO) S = 1.35-1.63 (m, 6H),
2.95
(t, J = 7.0 Hz, 2H), 3.75-3.85 (m, 2H), 6.62 (d, J = 9.0 Hz, 2H), 7.20 (d, J =
9.0 Hz, 2H),
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
121
7.37 (d, J = 9.0 Hz, 2H), 7.71 (d, J = 9.0 Hz, 2H), 7.99 (s, 2H, -NH2), 8.96
(s, 2H). LRMS
(FAB+): 459.1 (100, [M+H]-').
4-[(10-Bromo-decyl)-[1,2,4]triazol-4-yl-amino]-benzonitrile (CAB03008)
N-N
N
Br N
CN
Sodium hydride (60%, 240 mg, 6.0 mmol) was added to a solution of 4-
([1,2,4]triazol-4-
ylamino)-benzonitrile (926 mg, 5.0 mmol) in DMSO (25 mL) at r.t.. The mixture
was
stirred for 1 hour at this temperature and 1,10-dibromodecane (5 mL) was
added. The
reaction mixture was stirred overnight and ethyl acetate (100 mL) was added.
The
mixture was transferred into a separation funnel and extracted with water
(twice 100 mL)
and brine (20 mL). The organic layer was dried over sodium sulphate and
concentrated
under reduced pressure (water bath temperature < 30 C). The resulting orange
oil was
mixed with diethyl ether (100 mL) and filtered through a layer of silica (ca.
5 cm). The
silica was washed with more diethyl ether (ca. 100 mL) to remove the excess of
1,10-
dibromodecane. The crude product was washed from the silica with acetone (120
mL).
The acetone solution was concentrated under reduced pressure and the residue
was
purified by column-chromatography (eluent: ethyl acetate) to give the title
compound as
a pale yellow solid.-Yield: 1.32-g-(65-%)-1H-NMR-(400-MHz,-d6=DMSO) 6 = 1.16-
1.40
(m, 14H), 1.76 (tt, J = 7.0, 7.0 Hz, 2H), 3.50 (t, J = 6.6 Hz, 2H), 3.79 (t, J
= 7.0 Hz, 2H),
6.61 (d, J = 9.0 Hz, 2H), 7.70 (d, J = 9.0 Hz, 2H), 8.95 (s, 2H).
4- { [ 10-(4-Hydroxy-phenylsulfanyl)-decyl] - [ 1,2,4]triazol-4-yl-amino } -
benzonitrile
(CAB03011, STX625)
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
122
N-N
HO N STX625
N T:LCN
A mixture of 4-[(2-Bromo-decyl)-[1,2,4]triazol-4-yl-amino]-benzonitrile
(CAB03008,
607 mg, 1.5 mmol), 4-hydroxy-thiophenol (378 mg, 3.0 mmol) and potassium
carbonate
(414 mg, 3.0 mmol) in DMF (10 mL) was stirred for 12 hours at room
temperature. The
mixture was transferred into a separation funnel and ethyl acetate (50 mL) and
water (50
mL) were added. The organic layer was separated, washed with brine (30 mL),
dried over
sodium sulphate and concentrated under reduced pressure. The residue was
purified by
column chromatography (eluent: ethyl acetate, Rf: 0.71). The resulting yellow
solid was
dissolved in acetone and precipitated by addition of hexane to give a light
yellow
powder. Yield: 401 mg (59%). 'H-NMR (400 MHz, d6-DMSO) S = 1.16-1.52 (m, 16H),
2.76 (t, J = 7.2 Hz, 2H), 3.80 (t, J = 7.2 Hz, 2H), 6.62 (d, J = 9.0 Hz, 2H),
6.72 (d, J = 8.6
Hz, 2H), 7.19 (d, J = 8.6 Hz, 2H), 7.72 (d, J = 9.0 Hz, 2H), 8.97 (s, 2H),
9.53 (s, 1H, -
OH). LRMS (FAB+): 450.2 (100, [M+H]+). HRMS (FAB+) 450.23208 C25H32N50S
requires 450.232758.
Sulfamic acid 4-{ 10-[(4-cyano-phenyl)-[1,2,4]triazol-4-yl-amino]-
decylsulfanyl}-phenyl
ester (CAB03012, STX655)
_ -N-N
H2NO2SO ( N STX655
N 1:::~CN
Sulphamoyl chloride solution in toluene (3 mL, 0.7 M, 2.1 mmol) was
concentrated
under reduced pressure (30 C water bath temperature) to ca. 0.5 mL volume. The
residue
was cooled to 0 C (ice bath) and N,N-dimethylacetamide (5 mL) was added. 4-{[3-
(4-
hydroxy-phenylsulfanyl)-decyl]-[1,2,4]triazol-4-yl-amino}-benzonitrile
(CAB03011, 100
mg, 0.22 mmol) was added to the colourless solution and the mixture was
stirred for 18
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
123
hours at room temperature. Ethyl acetate (50 mL) and water (50 mL) were added
to the
solution, the organic layer was separated, washed with water (two times 30 mL)
and
brine (20 mL), dried over sodium sulphate and concentrated under reduced
pressure. The
residue was dissolved in a small amount of acetone and precipitated by
addition of
diethyl ether and hexane. The precipitate was filtered off and dried under
high vacuum.
Yield: 104 mg (88%) light yellow powder. 1H-NMR (400 MHz, d6-DMSO) S = 1.20-
1.41 (m, 14H), 1.56 (tt, J= 7.0 Hz, 2H), 2.96 (t, J = 7.0 Hz, 2H), 3.80 (t, J
= 7.0 Hz, 2H),
61.62 (d, J = 9.0 Hz, 2H), 7.21 (d, J = 9.0 Hz, 2H), 7.37 (d, J = 9.0 Hz, 2H),
7.71 (d, J =
9.0 Hz, 2H), 7.99 (s, 2H, -NH2), 8.95 (s,2H). LRMS (FAB+): 529.2 (100,
[M+H]+).
HRMS (FAB+) 529.20425 C25H33N603S2 requires 529.205558.
4-Benzyloxy-3,5-dichloro-benzoic acid methyl ester (CAB02115)
CI CO2Me
CI
A mixture of 3,5-dichloro-4-hydroxy benzoic acid methyl ester (5.525 g, 25
mmol),
benzyl bromide (5.13 g, 30 mmol) and potassium carbonate (6.91 g, 50 mmol) in
N,N-
dimethylformamide (100 mL) was stirred overnight at room temperature. The
reaction
mixture was poured into crushed ice (ca. 300 g) and the product was extracted
with ethyl
acetate (2 x 100 mL). The combined organic layers were dried over sodium
sulphate and
concentrated under reduced pressure. The solid residue was recrystallised from
ethyl
acetate and hexane. Yield: 7.47 g (96%) fine white needles. 'H-NMR (400 MHz,
CDC13)
8 = 3.93 (s, 3H, -OCH3), 5.12 (s, 2H, -CH2Ph), 7.35-7.44 (m, 3H), 7.54-7.58
(m, 2H),
8.01 (s, 2H). 13C-NMR (100 MHz, CDC13) S = 52.66, 75.12, 127.18, 128.41,
128.48,
129.79, 130.13, 135.57, 154.57, 164.39.
(4-Benzyloxy-3,5-dichloro-phenyl)-methanol (CAB02117)
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
124
CI OH
0
CI
A solution of 4-benzyloxy-3,5-dichloro-benzoic acid methyl ester (CAB02115,
7.20 g,
23.14 mmol) in THE (20 mL) was added slowly with a syringe to a suspension of
lithium
aluminium hydride (1.50 g, 39.5 mmol) in THE (40 mL). The reaction mixture was
stirred for 30 minutes at room temperature and then carefully quenched by
addition of 2N
sodium hydroxide solution in water. After 20 minutes stirring the colour of
the mixture
turned from grey to white. The white precipitate was filtered off, the
filtrate was dried
over sodium sulphate and concentrated under reduced pressure. The residue was
Irecrystallised from dichloromethane/hexane. Yield: 5.83 g (89%) white powder.
1H-
NMR (400 MHz, CDC13) 8 = 1.85 (t, J = 5.9 Hz, 1H, -OH), 4.63 (d, J = 5.9 Hz,
2H), 5.04
(s, 2H), 7.33 (s, 2H), 7.35-7.44 (m, 3H), 7.55-7.59 (m, 2H). 13C-NMR (100 MHz,
CDC13)
8 = 64.05, 75.03, 127.36, 128.70, 128.75, 129.91, 136.39, 138.64, 150.32.
2-Benzyloxy-5-bromomethyl-1,3-dichloro-benzene (CAB02118)
CI Br
Qo
CI
Phosphorous tribromide (2 mL) was added to a solution of (4-benzyloxy-3,5-
dichloro-
phenyl)-methanol (CAB02117, 5.50 g, 19.4 mmol) in dichloromethane (80 mL) at 0
C.
The mixture was stirred at this temperature for 2 h, diluted with diethyl
ether (150 mL),
transferred into a separation funnel and washed with water (2 x 50 mL) and
.brine (50
mL). The organic layer was dried over magnesium sulphate and concentrated
under
reduced pressure. The residue was recrystallised from dichloromethane/hexane.
1H-NMR
(400 MHz, CDC13) 8 = 4.39 (s, 2H, -CH2Br), 5.05 (s, 2H, -CH2Ph), 7.37 (s, 2H),
7.38-
7.44 (m, 3H), 7.54-7.58 (m, 2H). 13C-NMR (100 MHz, CDC13) 8 = 31.48, 75.39,
128.73,
129.68, 129.70, 129.72, 130.06, 135.46, 136.27, 151.23.
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
125
4-[(4-Benzyloxy-3,5-dichloro-benzyl)-[1,2,4]triazol-4-yl-amino]-benzonitrile
(CAB02119, STX434)
CI N-N STX434
BnO ~Nl
CI N
CN
Sodium hydride (60%, 200 mg, 5.0 mmol) was added to a solution of 4-
([1,2,4]triazol-4-
ylamino)-benzonitrile (926 mg, 5.0 mmol) in DMF (20 mL) at r.t.. The mixture
was
stirred for 1 h at this temperature. and 2-benzyloxy-5-bromomethyl-1,3-
dichloro-benzene
(CAB02118, 1.73 g, 5.0 mmol) was added. The reaction mixture was stirred
overnight
and ethyl acetate (75 mL) was added. The mixture was transferred into a
separation
funnel and washed with water (2 x 100 mL) and brine (20 mL). The organic layer
was
dried over sodium sulphate and concentrated under reduced pressure. The
residue was
suspended in 2-propanol (20 mL) and heated to reflux for 5 minutes. The white
solid was
filtered off after cooling to room temperature and dried under high vacuum.
Yield: 1.76 g
(78%).
'H-NMR (400 MHz, d6-DMSO) S = 5.00 (s, 2H), 5.07 (s, 2H), 6.74 (d, J = 9.0 Hz,
2H),
7.36-7.44 (m, 3H), 7.47-7.50 (m, 2H), 7.50 (s, 2H), 7.77 (d, J = 9.0 Hz, 2H),
8.92 (s, 2H).
13C-NMR (100 MHz, CDC13) S = 55.94, 74.59, 102.96, 113.61, 118.80, 128.21,
128.27,
128.63, 128.81, 133.29, 133.75, 135.69, 143.13, 149.62, 150.88. LRMS (FAB+):
450.1
(100, [M+H]+).
4-[(3,5-Dichloro-4-hydroxy-benzyl)-[1,2,4]triazol-4-yl-amino]-benzonitrile
(CAB02120,
STX435)
CI N-N\\\\ STX435
HO / (I N
CI N )aCN
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
126
Palladium on charcoal (100 mg, 10% Pd) was added to a solution of 4-[(4-
benzyloxy-3,5-
dichloro-benzyl)-[1,2,4]triazol-4-yl-amino]-benzonitrile (CAB02119, 1.13 g,
2.50 mmol)
in McOH/THF/EtOAc (30 mL /30 mL / 40 mL). The mixture was stirred under
hydrogen
atmosphere (balloon) for 18 h at room temperature. The reaction mixture was
filtered
through celite and the clear colourless filtrate was concentrated under
reduced pressure.
The residue was suspended in 2-propanol (20 mL) and heated to reflux for 5
minutes.
The white solid was filtered off after cooling to room temperature and dried
under high
vacuum. Yield: 306 mg (34%). 1H-NMR (400 MHz, d6-DMSO) S = 4.97 (s, 2H, -
CH2Ar), 6.76 (d, J = 9.0 Hz, 2H), 7.31 (s, 2H), 7.77 (d, J = 9.0 Hz, 2H), 8.85
(s, 2H),
l0 10.29 (s, 1H, -OH). 13C-NMR (100 MHz, CDC13) b = 56.57, 103.76, 114.55,
119.69,
122.89, 128.14, 129.46, 134.58, 144.03, 149.46, 151.85. LRMS (FAB+): 360.0
(100,
[M+H]+).
4-Benzyloxy-3-chloro-benzoic acid methyl ester (CAB02121)
CI CO2Me
O
A mixture of 3-chloro-4-hydroxy benzoic acid methyl ester (4.665 g, 25 mmol),
benzyl
bromide (5.13 g, 30 mmol) and potassium carbonate (6.91 g, 50 mmol) in N,N-
dimethylformamide (50 mL) was stirred overnight at room temperature. The
reaction
mixture was poured onto crushed ice (ca. 300 g) and the product was extracted
with ethyl
acetate (2 x 100 mL). The combined organic layers were dried over sodium
sulphate and
concentrated under reduced pressure. The residue was dissolved in
dichloromethane and
precipitated by addition of hexane. Yield: 6.47 g (94%) white powder. 'H-NMR
(400
MHz, CDC13) 8 = 3.90 (s, 3H, -OCH3), 5.23 (s, 2H, -CH2Ar), 6.99 (d, J = 9.0
Hz, 1H),
7.31-7.48 (m, 5H), 7.89 (dd, J = 9.0, 2.3 Hz, 1H), 8.08 (d, J = 2.3 Hz, 1H).
(4-Benzyloxy-3-chloro-phenyl)-methanol (CAB02127)
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
1Z/
CI
OH
)cr
Qo
A solution of 4-benzyloxy-3-chloro-benzoic acid methyl ester (CAB02121, 4.15
g, 15.0
mmol) in THE (30 mL) was added slowly with a syringe to a suspension of
lithium
aluminium hydride (1.0 g, 26.3 mmol) in THE (30 mL). The reaction mixture was
stirred
,for 30 minutes at room temperature and then carefully quenched by addition of
2N
sodium hydroxide solution in water. After 20 minutes stirring the colour of
the mixture
turned from grey to white. The white precipitate was filtered off, the
filtrate was dried
over sodium sulphate and concentrated under reduced pressure to give an
colourless oil,
which was used without further purification. Yield: 3.61 g (97%). 'H-NMR (400
MHz,
CDC13) 8 = 2.12 (s, 1H, -OH), 4.56 (s, 2H), 5.16 (s, 2H), 6.93 (d, J = 8.6 Hz,
1H), 7.14
(dd, J = 8.6, 2.3 Hz, 1H), 7.31-7.48 (m, 6H).
1-Benzyloxy-4-bromomethyl-2-chloro-benzene (CAB02128)
CI Br
r
Qo
Phosphorous tribromide (2 mL) was added to a solution of (4-benzyloxy-3-chloro-
phenyl)-methanol (CAB02127, 3.40 g, 13.7 mmol) in dichloromethane (50 mL) at 0
C.
The mixture was stirred at this temperature for 1 h, diluted with diethyl
ether (100 mL),
transferred into a separation funnel and washed with water (50 mL) and brine
(50 mL).
The organic layer was dried over magnesium sulphate and concentrated under
reduced
pressure to give a white, analytical pure solid. Yield: 4.27 g (100%).
'H-NMR (400 MHz, CDC13) S = 4.44 (s, 2H, -CH2Br), 5.17 (s, 2H), 6.92 (d, J =
8.6 Hz,
1H), 7.21 (dd, J = 8.6, 2.3 Hz, 1H), 7.31-7.48 (m, 6 H).
4-[(4-Benzyloxy-3-chloro-benzyl)-[1,2,4]triazol-4-yl-amino]-benzonitrile
(CAB02129,
//N/-N STX446)
BnO `N STX446
CI N
CN
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
128
Sodium hydride (60%, 200 mg, 5.0 mmol) was added to a solution of 4-
([1,2,4]triazol-4-
ylamino)-benzonitrile (926 mg, 5.0 mmol) in DMF (20 mL) at r.t.. The mixture
was
stirred for 1 hour at this temperature and 1-benzyloxy-4-bromomethyl-2-chloro-
benzene
(CAB02128, 1.56 g, 5.0 mmol) was added. The reaction mixture was stirred
overnight
and ethyl acetate (75 mL) was added. The mixture was transferred into a
separation
funnel and washed with water (two times 100 mL) and brine (20 mL). The organic
layer
was dried over, sodium sulphate and concentrated under reduced pressure. The
residue
was suspended in 2-propanol (20 mL) and heated to reflux for 5 minutes. The
white solid
was filtered off after cooling to room temperature and dried under high
vacuum. Yield:
1.38 g (66%). 'H-NMR (400 MHz, d6-DMSO) 6 = 4.99 (s, 2H), 5.17 (s, 2H), 6.76
(d, J =
9.0 Hz, 2H), 7.16 (d, J - 8.6 Hz, 1H), 7.20 (dd, J = 8.6, 2.2 Hz, I H), 7.32-
7.47 (m, 6H),
7.76 (d, J = 9.0 Hz, 2H), 8.81 (s, 2H). LRMS (FAB+): 416.1 (100, [M+H]+).
4-[(3-Chloro-4-hydroxy-benzyl)-[1, 2, 4]triazol-4-yl-amino]-benzonitrile
(CAB02130,
STX447)
N-N
HO STX447
N
CI N
CN
Palladium on charcoal (50 mg, 10% Pd) was added to a solution of 4-[(4-
benzyloxy-3-
chloro-benzyl)-[1,2,4]triazol-4-yl-amino]-benzonitrile (CAB02129, 1.04 g, 2.50
mmol)
in McOH/THF/EtOAc (25 mL / 25 mL / 25 mL). The mixture was stirred under
hydrogen atmosphere (balloon) for 18 hours at room temperature. The reaction
mixture
was filtered through celite and the clear colourless filtrate was concentrated
under
reduced pressure. The residue was suspended in 2-propanol (20 mL) and heated
to reflux
for 5 minutes. The white solid was filtered off after cooling to room
temperature and
dried under high vacuum. Yield: 484 mg (59%). 1H-NMR (400 MHz, d6-DMSO) S =
4.93 (s, 2H), 6.76 (d, J = 9.0 Hz, 2H), 6.86 (d, J = 8.6 Hz, 1H), 7.02 (dd, J
= 8.2, 2.0 Hz,
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
129
1H), 7.27 (d, J = 2.0 Hz, I H), 7.76 (d, J = 9.0 Hz, 2H), 8.77 (s, 2H), 10.29
(s, 1H, -OH).
13C-NMR (100 MHz, d6-DMSO) S = 56.87, 103.55, 114.48, 117.28, 119.73, 120.25,
126.72, 129.19, 130.91, 134.56, 144.02, 152.03, 153.50. LRMS (FAB+): 325.0
(100,
[M+H]+).
Sulfamic acid 2-chloro-4-{[(4-cyano-phenyl)-[1,2,4]triazol-4-yl-amino]-methyl}-
phenyl ester (CAB03015, STX694)
N-N
H2NO2SO N > STX694
CI \ I N \
Sulphamoyl chloride solution in toluene (3 mL, 0.7 M, 2.1 mmol) was
concentrated
under reduced pressure (30 C water bath temperature) to ca. 0.5 mL volume. The
residue
was cooled to 0 C (ice bath) and N,N-dimethylacetamide (5 mL) was added. 4-[(3-
Chloro-4-hydroxy-benzyl)-[1,2,4]triazol-4-yl-amino]-benzonitrile (CAB02130,
163 mg,
0.50 mmol) was added to the colourless solution and the mixture was stirred
for 18 h at
room temperature. Ethyl acetate (50 mL) and water (30 mL) were added to the
solution,
the organic layer was separated, washed with water (2 x 30 mL) and brine (20
mL), dried
over sodium sulphate and concentrated under reduced pressure. The residue was
dissolved in a small amount of acetone and precipitated by addition of hexane.
The
precipitate was filtered off and dried under high vacuum. Yield: 59 mg (29%)
white
powder. 'H-NMR (400 MHz, d6-DMSO) 5 = 5.11 (s, 2H), 6.72 (d, J = 9.0 Hz, 2H),
7.38
(dd, J = 8.2, 2.0 Hz, 1 H), 7.44 (d, J = 8.2 Hz, 1 H), 7.59 (d, J = 2.0 Hz, 1
H), 7.77 (d, J =
9.0 Hz, 2H), 8.31 (s, 2H, -NH2), 8.91 (s, 2H). LRMS (FAB+): 405.0 (100,
[M+H]+).
HRMS (FAB+) 405.05349 C16H14N6O3SC1 requires 405.053663
Benzoic acid 2-chloro-5-methyl-phenyl ester (CAB02124)
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
130
OCI
fO CH3
Benzoyl chloride (3.51 g, 25 mmol) was added dropwise to a solution of 2-
chloro-5-
methylphenol (3.92 g, 27.5 mmol) and triethylamine (5 mL) in dichloromethane
(100
mL), The mixture was stirred for 18 hours at room temperature and concentrated
under
,ieduced pressure. Diethylether (200 mL) and water (100 mL) were added. The
organic
layer was separated and extracted with 2N NaOH (2 x 100 mL) and brine (50 mL),
dried
over sodium sulphate and concentrated under reduced pressure. The resulting
colourless
oil solidified in the freezer. Yield: 5.82 g (94%). 1H-NMR (400 MHz, CDC13) 8
= 2.38
(s, 3H, -CH3), 7.05 (d, J = 8.2 Hz, 1H), 7.11 (s, 1H), 7.36 (d, J = 8.2 Hz,
1H), 7.51-7.56
(m, 2H), 7.64-7.7.70 (m, 1H), 8.22-8.28 (m, 2H). 13C-NMR (100 MHz, CDC13) 8 =
21.41, 123.97, 124.58, 128.07, 128.85, 129.15, 130.07, 130.55, 134.01, 138.38,
146.98,
164.51.
Benzoic acid 5-bromomethyl-2-chloro-phenyl ester (CAB02138)
OCI
eo CH2Br
A mixture of benzoic acid 2-chloro-5-methyl-phenyl ester (CAB02124, 2.47 g,
10.0
mmol), N-bromo-succinimide (1.96 g, 11.0 mmol) and dibenzoyl peroxide (10 mg)
in
carbon tetrachloride (25 mL) was heated to reflux for 1 hour (TLC-control).
After
cooling to room temperature water (50 mL) and diethyl ether (100 mL) were
added. The
organic layer was separated, washed with brine (20 mL), dried over sodium
sulphate and
concentrated under reduced pressure. The residue was purified by column
chromatography (EtOAc:hexane, 1:40, Rf: 0.21). Yield: 2.012 g (62%) colourless
oil,
which solidified after a few hours (contains ca. 10% benzoic acid 5-
dibromomethyl-2-
chloro-phenyl ester).
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
131
'H-NMR (400 MHz, CDC13) S = 4.48 (s, 2H, -CH2Br), 7.27 (dd, J = 8.2, 2.0 Hz,
1H),
7.36 (d, J = 2.0 Hz, 1H), 7.46 (d, J = 8.2 Hz, 1H), 7.50-7.58 (m, 2H), 7.64-
7.71 (m, 1H),
8.22-8.28 (m, 2H). LRMS (FAB+): 325.0 (81, [M+H]+), 327.0 (100, [M+H]+).
Benzoic acid 2-chloro-5-{[(4-cyano-phenyl)-[1,2,4]triazol-4-yi-amino]methyl}-
,phenyl ester (CAB02139)
//N/-N\\
GCI / ~N
p I N
C10 Sodium hydride (60%, 200 mg, 5.0 mmol) was added to a solution of 4-
([1,2,4]triazol-4-
ylamino)-benzonitrile (926 mg, 5.0 mmol) in DMF (20 mL) at r.t.. The mixture
was
stirred for 1 hour at this temperature and benzoic acid 5-bromomethyl-2-chloro-
phenyl
ester (CAB02138, 1.63 g, 5.0 mmol) was added. The reaction mixture was stirred
overnight and ethyl acetate (75 mL) was added. The mixture was transferred
into a
separation funnel and washed with water (2 x 50 mL) and brine (20 mL). The
organic
layer was dried over sodium sulphate and concentrated under reduced pressure.
The
residue was purified by column chromatography (EtOAc, Rf:0.31) to give a white
solid.
Yield: 1.773 g (82%). 1H-NMR (400 MHz, CDC13) b = 4.93 (s, 2H), 6.66 (d, 9.0
Hz,
2H), 7.06 (dd, J = 8.2, 2.0 Hz, 1 H), 7.27 (d, J = 2.0 Hz, 1H), 7.45 (d, J =
8.2 Hz, 1H),
7.47-7.58 (m, 4H), 7.60-7.69 (m, 1H), 8.16-8.21 (m, 2H), 8.24 (s, 2H). LRMS
(FAB+):
430.1 (100, [M+H]+).
4-[(4-Chloro-3-hydroxy-benzyl)-[1, 2, 4]triazol-4-yl-amino]-benzonitrile
(CAB02141,
STX483)
N-N STX483
CI )::
HQ N
CN
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
132
A solution of benzoic acid 2-chloro-5-{[(4-cyano-phenyl)-[1,2,4]triazol-4-yl-
amino]methyl}-phenyl ester (CAB02139, 1.13 g, 2.63 mmol) and sodium methoxide
(500.mg) in methanol (20 mL) and water (5 mL) was heated to reflux for 30
minutes.
After cooling to room temperature most of the solvent was removed under
reduced
pressure and concentrated sodium bicarbonate solution (20 mL) and ethyl
acetate (50
mL) were added. The organic layer was separated, dried over sodium sulphate
and the
solvent was removed under reduced pressure. The resulting white powder was
refluxed in
ethyl acetate (10. mL, product did not dissolve completely). After cooling to
room
temperature the product was filtered off and dried under high vacuum. Yield:
412 mg
(48%) white powder. 1H-NMR (400 MHz, d6-DMSO) S = 4.97 (s, 2H), 6.71-6.75 (m,
3H), 6.84 (d, J = 2.0 Hz, 1H), 7.25 (d, J = 8.2 Hz, 1H), 7.75 (d, J = 9.0 Hz,
2H), 8.76 (s,
2H), 10.21 (s, 1H, -OH). LRMS (FAB+): 326.1 (100, [M+H]+).
Sulfamic acid 2-chIoro-5-{[(4-cyano-phenyl)-[1,2,4]triazol-4-yl-amino]-methyl}-
phenyl ester (CAB02176, STX559)
N-N
CI > STX559
N
H2NO2SO N
CN
Sulphamoyl chloride solution in toluene (3 mL, 0.7 M, 2.1 mmol) was
concentrated
under reduced pressure (30 C water bath temperature) to ca. 0.5 mL volume. The
residue
was cooled to 0 C (ice bath) and N,N-dimethylacetamide (5 mL) was added. 4-[(4-
Chloro-3-hydroxy-benzyl)-[l,2,4]triazol-4-yl-amino]-benzonitrile (CAB02141,
200 mg,
0.614 mmol) was added to the colourless solution and the mixture was stirred
for 18
hours at room temperature. Ethyl acetate (50 mL) and water (30 mL) were added
to the
solution, the organic layer was separated, washed with water (2 x 30 mL) and
brine (20
mL), dried over sodium sulphate and concentrated under reduced pressure. The
residue
was dissolved in a small amount of acetone and precipitated by addition of
diethyl ether
and hexane. The precipitate was filtered off and dried under high vacuum.
Yield: 136 mg
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
133
(55%) white powder. 1H-NMR (400 MHz, d6-DMSO) 8 = 5.11 (s, 2H), 6.75 (d, J =
9.0
Hz, 2H), 7.25 (dd, J = 8.2, 2.0 Hz, 1H), 7.46 (d, J = 2.0 Hz, 1H), 7.54 (d, J
= 8.2 Hz, 1H),
7.77 (d, J = 9.0 Hz, 2H), 8.32 (s, 2H, -NH2), 8.82 (s, 2H). LRMS (FAB+): 87.0
(100),
404.9 (40, [M+H]+). HRMS (FAB+): 405.05338 C16H14N603SC1 requires 405.053663.
4-Benzyloxy-3-chloro-5-methoxy-benzoic acid benzyl ester (CAB02162)
CI CO2Bn
We
A mixture of 5-chloro-vanillic acid (4.05 g, 20 mmol), benzyl bromide (8.55 g,
50 mmol)
and potassium carbonate (6.90 g, 50 mmol) in DMF (60 mL) was stirred at room
temperature for 18 hours. The mixture was transferred into a separation funnel
and ethyl
acetate (100 mL) and water (100 mL) were added, the organic layer was
separated,
washed with water (2 x 50 mL) and brine (50 mL), dried over sodium sulphate
and
concentrated under reduced pressure. The residue was crystallised from
dichloromethane/hexane. Yield: 7.34 g (96%) colourless needles. 'H-NMR (400
MHz,
CDC13) 8 = 3.92 (s, 3H, -OCH3), 5.13 (s, 2H), 5.36 (s, 2H), 7.31-7.51 (m,
IOH), 7.53 (d, J
= 2.0 Hz, 1H), 7.73 (d, J = 2.0 Hz, 1H). LRMS (FAB+): 91.0 (100), 382.1 (15,
[M+H]+).
(4-Benzyloxy-3-chloro-5-methoxy-phenyl)-methanol (CAB02170)
CI OH
We
A solution of 4-benzyloxy-3-chloro-5-methoxy-benzoic acid benzyl ester
(CAB02162,
3.83 g, 10.0 mmol) in THE (20 mL) was added dropwise with a syringe to a
suspension
of lithium aluminium hydride (500 mg, 13.15 mmol) in THE (30 mL). The mixture
was
stirred at room temperature for 30 minutes and 2N NaOH (5 mL) was added. The
mixture was stirred for another hour, the aluminium salts were filtered off,
the filtrate
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
134
was dried over sodium sulphate and concentrated under reduced pressure. The
resulting
oil was heated to 120 C under high vacuum in a kugelrohr-destillation-
apparatus to
remove benzyl alcohol for 10 h. The product was obtained as a light yellow oil
and was
used without any further purification. Yield: 2.70 g (97%). 'H-NMR (400 MHz,
CDC13)
6 = 2.09 (2, 1H, -OH), 3.86 (s, 3H, -OCH3), 4.56 (s, 2H), 5.02 (s, 2H), 6.83
(d, J = 2.0
Hz, 1H), 6.95 (d, J = 2.0 Hz, 1H), 7.31-7.41 (m, 3H), 7.52-7.55 (m, 2H).
2-Benzyloxy- l -chloro-5-chloromethyl-3-methoxy-benzene (CAB02174)
CI CI
1 I We
Thionyl chloride (2 mL) was added to solution of (4-benzyloxy-3-chloro-5-
methoxy-
phenyl)-methanol (CAB02170, 2.703 g, 9.7 mmol) in dichloromethane (10 mL). The
solution was stirred for 1 h at room temperature, then diethyl ether (50 mL)
and water (20
mL) were added. The organic layer was separated, washed with cone. sodium
bicarbonate solution (10 mL), dried over sodium sulphate and concentrated
under
reduced pressure. The residue was dissolved in dichloromethane (5 mL) and
precipitated
by addition of hexane (ca. 50 mL). Yield: 2.730 g (95%) white powder. 'H-NMR
(400
MHz, CDC13) 6 = 3.89 (s, 3H, -OCH3), 4.52 (s,2H), 5.07 (s, 2H), 6.87 (d, J =
2.0 Hz,
1H), 7.03 (d, J = 2.0 Hz,-1H); 7.33-7.43 (m, 3H), 7.53-7.58 (m, 2H). LRMS
(FAB+):
91.0 (100), 296.0 (17, [M+H]).
4-[(4-Benzyloxy-3-chloro-5-methoxy-benzyl)-[1,2,4]triazol-4-yl-amino]-
benzonitrile (CAB02177, STX599)
We N-N
STX599
BnO N
CI N IaCN
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
135
Sodium hydride (60%, 200 mg, 5.0 mmol) was added to a solution of 4-
([1,2,4]triazol-4-
ylamino)-benzonitrile (926 mg, 5.0 mmol) in DMF (20 mL) at r.t.. The mixture
was
stirred for 1 h at this temperature and 2-benzyloxy-l-chloro-5-chloromethyl-3-
methoxy-
benzene (CAB02174, 1.49 g, 5.0 mmol) was added. The reaction mixture was
stirred
overnight and ethyl acetate (100 mL) and water (50 mL) were added. The organic
layer
was separated and washed with water (2 x 50 mL) and brine (30 mL). The organic
layer
was dried over sodium sulphate and concentrated under reduced pressure. The
residue
,was suspended in 2-propanol (20 mL) and heated to reflux for 5 minutes. The
white solid
was filtered off after cooling to room temperature and dried under high
vacuum. Yield:
1.76 g (79%).
1H-NMR (400 MHz, d6-DMSO) 8 = 3.78 (s, 3H, -OCH3), 4.80 (s, 2H), 5.05 (s, 2H),
6.52
(d, J = 2.0 Hz, 1H), 6.67 (d, J = 9.0 Hz, 2H), 6.89 (d, J = 2.0 Hz, 1H), 7.32-
7.40 (m, 3H),
7.46-7.50 (m, 2H), 7.61 (d, J = 9.0, 2H), 8.75 (s, 2H). LRMS (FAB+): 91.0
(100), 446.0
(65, [M+H]+). HRMS (FAB+): 446.13840 C24H21N502C1 requires 446.138378.
4-[(3-chloro-4-hydroxy-5-methoxy-benzyl)-[I ,2,4]triazol-4-yi-amino]-
benzonitrile
(CAB02179, STX600)
We N-N STX600
HO `NY
CI N
CN
Palladium on charcoal (100 mg, 5% Pd) was added to a solution of 4-[(4-
benzyloxy-3-
chloro-5-methoxy-benzyl)-[1,2,4]triazol-4-yl-amino]-benzonitrile (CAB02177,
1.34 g,
3.0 mmol) in ethanol (60 mL) and THE (40 mL). The mixture was stirred under
hydrogen atmosphere (balloon) for 24 h (TLC monitored), then filtered through
celite
and concentrated under reduced pressure. The residue was dried under high
vacuum.
Yield: 1.06 g (99%), white powder. 'H-NMR (400 MHz, d6-DMSO) 8 = 3.77 (s, 3H, -
OCH3), 4.92 (s, 2H), 6.79 (d, J = 9.0 Hz, 2H), 6.81 (d, J = 1.8 Hz, 1H), 6.85
(d, J = 1.8
Hz, 1H), 7.77 (d, J = 9.0 Hz, 2H), 8.79 (s, 2H), 9.49 (s, 1H, -OH). LRMS
(FAB+): 356.0
(100, [M+H]+). HRMS (FAB+): 356.09234 C17H15N5O2C1 requires 356.091428.
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
136
Sulfamic acid 2-chloro-4-{[(4-cyano-phenyl)-[1,2,4]triazol-4-yl-amino]-methyl}-
6-
methoxy-phenyl ester (CAB02181, STX601)
OMe /N-N
H2NO2SO ~N STX601
C1 N I \
CN
Sulphamoyl chloride solution in toluene (3 mL, 0.7 M, 2.1 mmol) was
concentrated
under reduced pressure (30 C water bath temperature) to ca. 0.5. mL volume.
The residue
was cooled to 0 C (ice bath) and N,N-dimethylacetamide (5 mL) was added. 4-
[(3-
Chloro-4-hydroxy-5-methoxy-benzyl)-[1,2,4]triazol-4-yl-amino]-benzonitrile
(CAB02179, 212 mg, 0.596 mmol) was added to the colourless solution and the
mixture
was stirred for 18 hours at room temperature. Ethyl acetate (50 mL) and water
(30 mL)
were added to the solution, the organic layer was separated, washed with water
(2 x 30
mL) and brine (20 mL), dried over sodium sulphate and concentrated under
reduced
pressure. The residue was dissolved in a small amount of acetone and
precipitated by
addition of hexane. The precipitate was filtered off and dried under high
vacuum. Yield:
219 mg (84%) white powder. 'H-NMR (400 MHz, d6-DMSO) S = 3.78 (s, 3H, -OCH3),
6.74 (d, J = 9.0 Hz, 2H), 7.04 (d, J = 2.0 Hz, 1H), 7.07 (d, J = 2.0 Hz, I H),
7.77 (d, J =
9.0 Hz, 2H), 7.98 (s, 2H, -NH2), 8.91 (s, 2H). LRMS (FAB+): 435.0 (100,
[M+H]+).
HRMS (FAB+): 435.06476 C17H16N604SC1 requires 435.064228.
4-Benzyloxy-3-fluoro-benzaldehyde (CAB03016)
F CHO
O
A mixture of 3-fluoro-4-hydroxy benzaldyde (4.90 g, 35.0 mmol), benzyl bromide
(6.84
g, 40.0 mmol, 4.80 mL) and potassium carbonate (9.66 g, 70.0 mmol) in DMF (50
mL)
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
137
was strirred for 18 h at room temperature. The reaction mixture was
transferred into a
separation funnel and ethyl acetate (100 mL) and water (50 mL) were added. The
organic
layer was separated, washed with water (2 x 50 mL) and brine (30 mL), dried
over
sodium sulphate and concentrated under reduced pressure. The white solid
residue was
recrystallised from dichloromethane/hexane. Yield: 7.65 g (95%). 1H-NMR (400
MHz,
CDC13) 8 = 5.24 (s, 2H), 7.12 (dd, J = 8.2, 8.2 Hz, 1H), 7.34-7.48 (m, 5H),
7.59-7.66 (m,
2H), 9.85 (d, J = 2.0 Hz, 1H, -CHO). LRMS (FAB+): 91 (100), 231.1 (100,
[M+H]+).
(4-Benzyloxy-3-fluoro-phenyl)-methanol (CABO3017)
F OH
Qo
Sodium borohydride (500 mg, 13.2 mmol) was added to a solution of 4-benzyloxy-
3-
fluoro-benzaldehyde (CAB03016, 7.32 g, 31.8 mmol) in ethanol (40 mL) and THE
(40
mL) at 0 C. The clear and colourless solution was allowed to warm to room
temperature
and stirred for 12 h at this temperature. Ethyl acetate (150 mL) and water (50
mL) were
added to the solution, the organic layer was separated, washed with water (2 x
50 mL)
and brine (50 mL), dried over sodium sulphate and concentrated under reduced
pressure.
The white solid residue was dissolved in dichloromethane and precipitated by
addition of
hexane. The white powder was filtered off and dried under high vacuum. Yield:
7.16 g
(97 %). 'H-NMR (400 MHz, CDC13) 8 = 1.71 (s, 1H, -OH), 4.61 (s, 2H, -CH2OH),
5.15
(s, 2H), 6.97 (dd, J = 8.6, 8.6 Hz, 1 H), 7.02 (dd, J = 8.6, 1.9 Hz, 1 H),
7.13 (dd, J = 11.7,
1.9 Hz, 1H), 7.30-7.46 (m, 5H). LRMS (FAB+): 91 (100), 215.1 (40), 232.1 (100,
[M]}).
1-Benzyloxy-4-chloromethyl-2-fluoro-benzene (CAB03018)
CI
0
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
138
Thionyl chloride (5 mL) was added to solution of (4-benzyloxy-3-fluoro-phenyl)-
methanol (CAB03017, 6.80 g, 29.28 mmol) in dichloromethane (50 mL). The
solution
was stirred for 1 h at room temperature and concentrated under reduced
pressure. Then
diethyl ether (100 mL) and water (20 mL) were added. The organic layer was
separated,
washed with conc. sodium bicarbonate solution (10 mL), dried over sodium
sulphate. and
concentrated under reduced pressure. The residue was dissolved in
dichloromethane (5
mL) and precipitated by addition of hexane (ca. 50 mL). The precipitate was
filtered off
and dried under high vacuum. Yield: 7.01 g (95%) white powder. 'H-NMR (400
MHz,
CDC13) 5 = 4.52 (s, 2H, - CH2C1), 5.15 (s, 2H), 6.96 (dd, J = 8.2, 8.2 Hz,
1H), 7.04-7.06
(m, 1H), 7.15 (dd, J = 11.7, 2.4 Hz, I H), 7.31-7.45 (m.5H). LRMS (FAB+): 91
(100),
215.0 (10), 250.0 (16, [M]+).
I
4-[(4-Benzyloxy-3-fluoro-benzyl)-[1,2,4]triazol-4-yl-amino]-benzonitrile
(CAB03019,
STX695)
N-N
BnO > STX695
N
F I N 1::~CN
Sodium hydride (60%, 400 mg, 10.0 mmol) was added to a solution of 4-
([1,2,4]triazol-
4-ylamino)-benzonitrile (1.852 g, 10.0 mmol) in DMF (50 mL) at room
temperature. The
mixture was stirred for 1 h at this temperature and 1-benzyloxy-4-chloromethyl-
2-fluoro-
benzene (CAB03018, 2.51 g, 10.0 mmol) was added. The reaction mixture was
stirred
overnight and ethyl acetate (200 mL) and water (50 mL) were added. The mixture
was
transferred into a separation funnel and washed with water (2 x 50 mL) and
brine (30
mL). The organic layer was dried over sodium sulphate and concentrated under
reduced
pressure. The residue was suspended in 2-propanol (40 mL) and heated to reflux
for 5
minutes. The white solid was filtered off after cooling to room temperature
and dried
under high vacuum. Yield: 3.12 g (78%). 'H-NMR (400 MHz, d6-DMSO) 8 = 4.97 (s,
2H), 5.12 (s, 2H), 6.73 (d, J = 9.0 Hz, 2H), 7.01 (dd, J = 8.2, 1.2 Hz, 1 H),
7.16 (dd, J =
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
139
8.6, 8.6 Hz 1H), 7.21 (dd, J = 8.6, 2.4 Hz, 1H), 7.30-7.44 (m, 5H), 7.75 (d, J
= 9.0 Hz,
2H), 8.80 (s, 2H). LRMS (FAB+): 400.1 (100, [M+H]+).
HRMS (FAB+): 400.15800 C23H19N50F requires 400.157364.
4-[(3-Fiuoro-4-hydroxy-benzyl)-[1, 2, 4]triazol-4-yl-amino]-benzonitrile
(CAB03020,
STX696)
N-N
HO STX696
~` I N
FN
~aCN
4-[(4-Benzyloxy-3-fluoro-benzyl)-[1,2,4]triazol-4-yl-amino]-benzonitrile
(CAB03019,
2.83 g, 7.09 mmol) was dissolved in a mixture of ethanol (50 mL), THE (50 mL)
and
ethyl acetate (50 mL) by heating and palladium on charcoal (150 mg, 5% Pd) was
added.
The mixture was stirred under hydrogen-atmosphere (balloon) for 18 h, filtered
through a
3 cm layer of celite and concentrated under reduced pressure. The residue was
suspended
in 2-propanol (30 mL), the mixture was heated to reflux for 5 minutes. After
cooling
room temperature the white precipitate was filtered off and dried under high
vacuum.
Yield: 2.13 g (97%). 'H-NMR (400 MHz, d6-DMSO) 6 = 4.93 (s, 2H), 6.75 (d, J =
9.0
Hz, 2H), 6.82-6.89 (m, 2H), 7.07-7.12 (m, 1H), 7.76 (d, J = 9.0 Hz, 2H), 8.77
(s, 2H),
9.95 (s, I H, -OH).
Sulfamic acid 4-{[(4-cyano-phenyl)-[1,2,4]triazol-4-yl-amino]-methyl}-2-fluoro-
phenyl ester (CAB03021, STX700)
N-N STX700
H2NO2SO / N
FN
)aCN
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
140
Sulphamoyl chloride solution in toluene (3 mL, 0.7 M, 2.1 mmol) was
concentrated
under reduced pressure (30 C water bath temperature) to ca. 0.5 mL volume.
The residue
was cooled to 0 C (ice bath) and N,N-dimethylacetamide (5 mL) was added. 4-
[(3-
Fluoro-4-hydroxy-benzyl)-[1,2,4]triazol-4-yl-amino]-benzonitrile (CAB03020,
220 mg,
0.71 mmol) was added to the colourless solution and the mixture was stirred
for 4 h at
room temperature. Ethyl acetate (50 mL) and water (30 mL) were added to the
solution,
the organic layer was separated, washed with water (2 x 25 mL) and brine (25
mL), dried
over sodium sulphate and concentrated under reduced pressure. The residue was
dissolved in acetone (5 mL) and precipitated by addition of diethyl ether. The
precipitate
was filtered off and dried under high vacuum. Yield: 228 mg (83%) white
powder. 1H-
NMR (400 MHz, d6-DMSO) S = 5.11 (s, 2H), 6.71 (d, J = 9.0 Hz, 2H), 7.23 (dd,
8.2, 1.2
Hz, I H), 7.39 (dd, J = 8.2, 8.2 Hz, I H), 7.43 (dd, J = 11.1, 2.1 Hz, 1H),
7.77 (d, J = 9.0
Hz, 2H), 8.28 (s, 2H, -NH2), 8.92 (s, 2H). LRMS (FAB+): 389.1 (100, [M+H]+).
HRMS
(FAB+): 389.08298 C16H14N603SF requires 389.083214.
Benzoic acid 2-fluoro-5-methyl-phenyl ester (CAB02145)
O F
eo CH3
Benzoyl chloride (4.22 g, 30 mmol) was added dropwise with a syringe to a
solution of
2-fluoro-5-methylphenol (3.784 g, 30 mmol) and triethylamine (5 mL) in
dichloromethane (50 mL), The mixture was stirred for 18 h at room temperature
and
concentrated under reduced pressure. Diethyl ether (200 mL) and water (100 mL)
were
added, the organic layer was separated and extracted with 2N NaOH (2 x 30 mL)
and
brine (20 mL), dried over sodium sulphate and concentrated under reduced
pressure to
give a white solid. Yield: 6.601 g (96%). 'H-NMR (400 MHz, CDC13) b = 2.36 (s,
3H, -
CH3), 7.01-7.12 (m, 3H), 7.49-7.55 (m, 2H), 7.63-7.68 (m, 1H), 8.19-8.23 (m,
2H).
LRMS (FAB+): 231.1 (100, [M+H]+)
Benzoic acid 5-bromomethyl-2-fluoro-phenyl ester (CAB02146)
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
141
0 F
eo CH2Br
'A mixture of benzoic acid 2-fluoro-5-methyl-phenyl ester (CAB02145, 2.47 g,
10.0
mmol), N-bromo-succinimide (1.96 g, 11.0 mmol) and dibenzoy lperoxide (10 mg)
in
carbon tetrachloride (25 mL) was heated to reflux for 2 h (TLC monitored).
After cooling
to room temperature, water (50 mL) and diethyl ether (100 mL) were added. The
organic
layer was separated, washed with brine (20 mL), dried over sodium sulphate and
concentrated under reduced pressure. The residue was purified by column
chromatography (EtOAc:hexane, 1:25, Rf: 0.28). Yield: 1.80 g (58%), white
solid. 'H-
NMR (400 MHz, CDC13) S = 4.48 (s, 2H, -CH2Br), 7.18 (dd, J = 9.8, 8.6 Hz, I
H), 7.28
(ddd, J = 8.6, 4.3, 2.3 Hz, 1H), 7.34 (dd, J = 7.0, 2.3 Hz, 1H), 7.50-7.55 (m,
2H), 7.64-
7.69 (m, 1H), 8.18-8.23 (m, 2H). LRMS (FAB+): 229.1 (95), 309.0 (100, [M+H]+).
Found: C 54.1, H 3.22%; C14H10BrFO2 (309.13) requires C 54.39, H 3.26%.
Benzoic acid 5-{[(4-cyano-phenyl)-[1,2,4]triazol-4-yl-amino]methyl}-2-fluoro-
phenyl ester (CAB02147)
N-N
O F N
0 N
CN
Sodium hydride (60%, 200 mg, 5.0 mmol) was added to a solution of 4-
([1,2,4]triazol-4-
ylamino)-benzonitrile (926 mg, 5.0 mmol) in DMF (20 mL) at r.t.. The mixture
was
stirred for 1 h at this temperature and benzoic acid 5-bromomethyl-2-fluoro-
phenyl ester
(CAB02146, 1.55 g, 5.0 mmol) was added. The reaction mixture was stirred
overnight
and ethyl acetate (75 mL) and water (50 mL) were added. The mixture was
transferred
into a separation funnel and washed with water (2 x 50 mL) and brine (20 mL).
The
organic layer was dried over sodium sulphate and concentrated under reduced
pressure.
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
142
The residue was purified by column chromatography (EtOAc, Rf 0.32) to give a
white
solid. Yield: 1.16 g (56%). 1H-NMR (400 MHz, CDC13) S = 4.91 (s, 2H), 6.67 (d,
J = 9.0
Hz, 2H), 7.07 (ddd, J = 8.2, 4.3, 2.0 Hz, I H), 7.18 (dd, J = 9.4, 8.6 Hz,
1H), 7.24 (dd, J =
7.0, 2.3 Hz, 1H), 7.49-7.56 (m, 2H), 7.58 (d, J = 9.0 Hz, 2H), 7.62-7.70 (m,
1H), 8.15-
8.20 (m, 2H), 8.21 (s, 2H). LRMS (FAB+): 414.2 (100, [M+H]+).
4-[(4-Fluoro-3-hydroxy-benzyl)-[1, 2, 4]triazol-4-yl-amino]-benzonitrile
(CAB02154,
STX488)
N-N
F > STX488
~
HO \ N
CN
A solution of sodium hydroxide (250 mg, 6.25 mmol) in water (5 mL) was added
to a
solution of benzoic acid 5-{[(4-cyano-phenyl)-[1,2,4]triazol-4-yl-
amino]methyl}-2-
fluoro-phenyl ester (CAB02147, 958 mg, 2.32 mmol) in methanol (10 mL). The
solution
was heated to reflux for 5 minutes and concentrated under reduced pressure.
Water (10
mL) was added and the milky suspension was neutralised (pH 7-8) with 2N
hydrochloric
acid. The white precipitate was filtered off, washed with a small amount of
water (5 mL)
and dried under high vacuum. Yield: 476 mg (66%). 'H-NMR (400 MHz, d6-DMSO) 8
= 4.95 (s, 2H), 6.70 (ddd, J = 11.4, 8.4, 2.4 Hz, 1 H), 6.75 (d, J = 9.0 Hz,
2H), 6.84 (dd, J
= 8.4, 2.4 Hz, 1 H), 7.06 (dd, J = 11.3, 8.4 Hz, 1 H), 7.76 (d, J = 9.0 Hz,
2H), 8.75 (s, 2H),
9.90 (s, 1H, -OH). LRMS (FAB+): 310.1 (100, [M+H]).
4-Benzyloxy-3-trifluoromethyl-benzoic acid (CAB03046)
0
F3C OH
O
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
143
Sodium hydride (60%, 1.80 g, 45 mmol) was added to a solution of 4-fluoro-3-
trifluoromethyl benzoic acid (4.162 g, 20 mmol) and benzyl. alcohol (3.25 g,
30 mmol) in
DMSO (50 mL). The mixture was stirred overnight at room temperature, poured
into
water (50 mL) and acidified with concentrated hydrochloric acid. The white
precipitate
was filtered off, dissolved in ethyl acetate (ca. 50 mL), dried over sodium
sulphate and
concentrated under reduced pressure. The residue was recrystallised from ethyl
acetate/hexane. Yield: 4.252 g (72%). 'H-NMR (400 MHz, CDC13) S = 5.37 (s,
2H),
7.32-7.48 (m, 6H), 8.12 (d, J = 2.0 Hz, 1H), 8.18 (dd, J = 8.6, 2.0 Hz, 1H),
13.16 (br s,
1H, -COOH). LRMS (FAB+): 91.1 (100), 297.1(18, [M+H]+).
(4-Benzyloxy-3-trifluoromethyl-phenyl)-methanol -(CAB03047)
F3C / OH
\ O
4-Benzyloxy-3-trifluoromethyl-benzoic acid (CAB03046, 3.555 g, 12 mmol) in THE
(20
mL) was added dropwise to a suspension of lithium aluminium hydride (1.0 g,
26.3
mmol) in THE (20 mL). The mixture was stirred for another 30 minutes at room
temperature and then quenched by addition of 2N NaOH (5 mL). The white
preciptate
was filtered off and washed with dichloromethane (100 mL), the filtrate was
dried over
sodium sulphate and concentrated under reduced pressure. The resulting oil was
crystallised from diethyl ether/hexane. Yield: 3.31 g (98%). 1H-NMR (400 MHz,
CDC13)
6 = 1.72 (t, J = 5.9 Hz, 1H, -OH), 4.66 (d, J = 5.9 Hz, 2H, -CH2OH), 5.21 (s,
2H, -
CH2Ph), 7.02 (d, J = 8.2 Hz, 1H), 7.30-7.48 (m, 6H), 7.61 (d, J = 2.3 Hz, 1H).
LRMS
(FAB+): 91.1 (100), 265.2 (45), 282.2 (40, [M+H]+).
I -Benzyloxy-4-chloromethyl-2-trifluoromethyl-benzene (CAB03050)
F3C CI
Qo
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
144
Thionyl chloride (2.0 mL) was added to solution of (4-benzyloxy-3-
trifluoromethyl-
phenyl)-methanol (CAB03047, 3.10 g, 11.0 mmol) in dichloromethane (20 mL). The
solution was stirred for 1 h at room temperature and concentrated under
reduced
pressure. Then diethyl ether (100 mL) and water (20 mL) were added. The
organic layer
was separated, washed with conc. sodium bicarbonate solution (10 mL), dried
over
sodium sulphate and concentrated under reduced pressure. The resulting oil
solidified
After a few minutes and was dried under high vacuum. Yield: 3.25 g (98%). 1H-
NMR
(400 MHz, CDC13) 6 = 4.57 (s, 2H, -CH2C1), 5.22 (s, 2H, -OCH2Ph), 7.02 (d, J =
8.5 Hz,
1H), 7.31-7.52 (m, 6H), 7.63 (d, J = 2.0 Hz, 1H). LRMS (FAB+): 91.1 (100),
265.2 (8),
300.1 (10, [M]+).
4-[(4-Benzyloxy-3-trifluoromethyl-benzyl)-[1,2,4]triazol-4-yl-amino]-
benzonitrile
(CAB03054, STX719)
N-N
BnO > N STX719
F3C N
CN
Sodium hydride (60%, 200 mg, 5.0 mmol) was added to a solution of 4-
([1,2,4]triazol-4-
ylamino)-benzonitrile (926 mg, 5.0 mmol) in DMF (50 mL) at room temperature.
The
mixture was stirred for 1 h at this temperature and 1-benzyloxy-4-chloromethyl-
2-
trifluoromethyl-benzene (CAB03050, 1.50 g, 5.0 mmol) was added. The reaction
mixture
was stirred overnight and ethyl acetate (100 mL) and water (30 mL) were added.
The
mixture was transferred into a separation funnel and washed with water (2 x 30
mL) and
brine (20 mL). The organic layer was dried over sodium sulphate and
concentrated under
reduced pressure. The residue was suspended in 2-propanol (40 mL) and heated
to reflux
for 5 minutes. The white solid was filtered off after cooling to room
temperature and
dried under high vacuum. Yield: 1.87 g (83%). 1H-NMR (400 MHz, d6-DMSO) 8 =
5.05
(s, 2H), 5.23 (s, 2H), 6.78 (d, J = 9.0 Hz, 2H), 7.26 (d, J = 8.6 Hz, 1H),
7.30-7.44 (m,
5H), 7.51 (dd, J = 8.6, 2.0 Hz, 1H), 7.56 (d, J = 2.0 Hz, 1H), 7.77 (d, J =
9.0 Hz, 2H),
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
145
8.79 (s, 2H). LRMS (FAB+): 450.2 (100, [M+H]+). HRMS (FAB+): 450.15404
C24H19N50F3 requires 450.154170
4-[(4-Hydroxy-3-trifluoromethyl-benzyl)-[1,2,4]triazol-4-yl-amino]-
benzonitrile
(CAB03059, STX781)
//'N-N\\\\ STX781
HO `N
F3C N
CN
Palladium on charcoal (100 mg, 10% Pd) was added to a solution of 4-[(4-
benzyloxy-3-
trifluoromethyl-benzyl)-[1,2,4]triazol-4-yl-amino]-benzonitrile (CAB03054,
1.75 g, 3.89
mmol) in EtOH/THF/MeCN (50 ml, / 50 mL / 30 mL). The mixture was stirred under
hydrogen atmosphere (balloon) for 18 h at room temperature. The reaction
mixture was
filtered through celite and the clear colourless filtrate was concentrated
under reduced
pressure. The residue was suspended in 2-propanol (20 mL) and heated to reflux
for 5
minutes. The white solid was filtered off after cooling to room temperature
and dried
under high vacuum. Yield: 1.31 g (94%). 'H-NMR (400 MHz, d6-DMSO) S = 4.98 (s,
2H), 6.78 (d, J = 9.0 Hz, 2H), 6.91 (d, J = 8.6 Hz, I H), 7.31 (dd, J = 8.6,
2.0 Hz, I H),
7.39 (d, J = 2.0 Hz, 1H), 7.76 (d, J = 9.0 Hz, 2H), 8.74 (s, 2H), 10.66 (s,
1H, -OH).
LRMS (FAB+): 360.2 (100, [M+H]+).
3-Benzyloxy-phenol (JRL01015)
BnO a OH
To a stirred solution of resorcinol (7.05 g, 63.4 mmol) in DMF (100 mL) at 0
C under
nitrogen was added NaH (60%, 2.54 g, 63.4 mmol). After stirring for 30 min,
benzyl
bromide (7.72 mL, 63.4 mmol) was added and the resulting mixture was stirred
for 4 h at
room temperature. The reaction mixture was diluted with ethyl acetate (300 mL)
and the
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
146
organic layer separated washed with brine (300 mL, 4 x 100 mL), dried
(Na2SO4),
filtered and evaporated to give the crude product which was fractionated by
flash
chromatography (hexane/EtOAc 3:1) to give JRLO1015 as a pale yellow solid
(4.06 g, 32
%); Rf 0.50 (Hexane/EtOAc 3:1); 1H (400 MHz CDC13) 4.97 (1H, s OH), 5.01 (2H,
s,
CH2), 6.42 (1 H, dd, J 2.3 and 8.0 Hz), 6.47 (1H, t, J 2.3 Hz), 6.56 (1 H, dd,
J 2.3 and 8.0
Hz), 7.12 (1 H, t, J 8.0 Hz) and 7.28-7.46 (5H, m).
5-Benzyloxy-2-nitro-phenol (JRL01017A)
NO2
BnO OH
I
To a stirred solution of JRL01015 (4.0 g, 20.0 mmol) in AcOH (40 mL) at 5-10
C was
added HNO3 (69%, 2.74 g, 30.0 mmol) in AcOH (1.80 g, 30.0 mmol). After
stirring for 4
h at room temperature, the reaction mixture was diluted EtOAc (150 mL) and the
organic
layer separated washed with brine (200 mL, 4 x 100 mL), dried (Na2SO4),
filtered and
evaporated to give the crude product which was fractionated by a flash
chromatography
(hexane/EtOAc 3:1) to give JRL01017 as a yellow solid (1.88 g, 38.5 %); Rf
0.65
(hexane/EtOAc 3:1); 1H (400 MHz, CDC13) 5.13 (2H, s, CH2), 6.59 (1H, dd, J 2.4
and
9.0 Hz), 6.62 (1H, d, J2.4 Hz), 7.34-7.45 (5H, m), 8.04 (1H, d, J9.0 Hz) and
11.00 (1H,
s, OH).
2-Amino-5-benzyloxy-phenol (JRL01022)
NH2
BnO OH
To a stirred solution of JRL01017 (500 mg, 2.04 mmol) in EtOH/H20 (1:1, 50 mL)
was
added sodium hydrosulfite (Na2S2O4, - 85%, 1.67 g, 8.16 mmol) and the yellow
suspension resulted was heated at 75 C. After 1 h at this temperature, the
reaction
mixture had become colorless and it was then cooled to room temperature. The
reaction
mixture was diluted with EtOAc (100 mL) and the organic layer separated washed
with
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
147
brine (4 x 50 mL), dried (Na2SO4), filtered and evaporated to give the crude
product
which was purified by a flash chromatography (hexane/EtOAc 3:1) to give JRLO
1022 as
a dark pink solid (418 mg, 95 %); Rf 0.50 (hexane/EtOAc, 3:1); 1H (400 MHz,
DMSO-
d6) - 4.0 (2H, v br s), 4.90 (2H, s), 6.24 (1H, dd, J 2.7 and 8.4 Hz), 6.37
(1H, d, J 2.7
Hz), 6.49 (1H, d, J 8.4 Hz), 7.28-7.46 (5H, m) and - 11.0 (1H, v br s).
6-Benzyloxy-2-bromomethyl-benzooxazole (JRL01026)
N
Br
BnO O
To a stirred mixture of JRL01022 (773 mg, 3.60 mmol) in
trimethylsilylpolyphosphate
(PPSE)/1,2-dichlorobenzene (1:5, 60 mL) under nitrogen was added bromoacetic
acid
(400 mg, 2.88 mmol) and the resulting purple mixture was heated at 150 C for
1 h.
After cooling to room temperature, the reaction mixture was diluted with EtOAc
(100
mL) and the organic layer separated washed with brine (4 x 50 mL), dried
(Na2SO4),
filtered and evaporated to an oil which was fractionated by flash
chromatography
(hexane/EtOAc 5:1) to give JRLO1026 as a white solid (369 mg, 40 %); Rf 0.28
(hexane/EtOAc 5:1); 1H (400 MHz, CDC13) 4.55 (2H, s), 5.10 (2H, s), 7.03 (1H,
dd, J2.2
and 8.6 Hz), 7.10 (1H, d, J2.2 Hz), 7.30-7.46 (5H, m) and 7.59 (1H, d, J8.6
Hz).
4-[(6-Benzyloxy-benzooxazol-2-ylmethyl)-[1, 2, 4]triazol-4-yl-amino]-
benzonitrile
(JRL01029)
N' N
N N,N CN
Bn0 O
To a stirred solution of NaH (60%, 12 mg, 300 ,umol) in DMF (10 mL) at 0 C
under
nitrogen was added 4-[(1,2,4)triazol-4-amino]benzonitrile (55 mg, 300 ,umol)
in DMF
(10 mL). After stirring at 40-50 C under nitrogen for 1 h, the orange
reaction mixture
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
148
was cooled to room temperature and JRL01026 (100 mg, 310 umol) was added. The
resulting mixture was stirred overnight at room temperature under nitrogen.
After
diluting the reaction mixture with CH2C12 (50 mL), the organic layer was
washed with
brine (100 mL, 3 x 50 mL), dried (Na2SO4), filtered and evaporated to give a
crude
product which was fractionated by flash chromatography (EtOAc) to give
JRLO1029 as a
white solid (80 mg, 63 %); Rf 0.24 (EtOAc); 'H (400 MHz, CDC13) *5.11 (2H, s),
5.19
(2H, s), 6.59 (2H AA'BB' ; 7.05 (1H, dd, J2.2 and 8.8Hz), 7.11 (1H, d, J2.2
Hz), 7.30-
7.60 (8H, m) and 8.65 (2H, s).
i o 4-[(6-Hydroxy-benzooxazol-2-ylmethyl)-[1, 2, 4]triazol-4-yl-amino]-
benzonitrile
(JRL01035, STX 357)
N~
N N \ / CN
HO O
To a stirred solution of JRL01029 (235 mg, 5.56 mmol) in THF/MeOH 1:1 (60 mL)
was
added Pd-C 10 % (65 mg) and the resulting suspension was stirred under an
atmosphere
of H2 (balloon) overnight. After filtration on celite, the filtrate collected
was evaporated
to give a grey solid which was purified by trituration in hot EtOAc to produce
JRL01035
as a white solid (128 mg, 69 %); Rf 0.38 (Acetone/EtOAc, 1:2); 'H (400 MHz,
DMSO-d,
400 MHz) 5.47 (2H, s), 6.70 (2H, d, J9 Hz), 6.80 (1H, dd, J2.1 and 8.6 Hz),
7.02 (1H,
d, J2.1 Hz), 7.48 (1 H, d, J 8.6 Hz), 7.74 (2H, d, J9 Hz), 8.95 (2H, s) and
9.86 (1 H, br s).
4-Benzyloxy-phenol (JRL01016)
OH
Bn0
To a stirred solution of hydroquinone (7.00 g, 63.4 mmol) in DMF (100 mL) at 0
C
under nitrogen was added NaH (60%, 2.54 g, 63.4 mmol). After stirring for 30
min,
benzyl bromide (7.72 mL, 63.4 mmol) was added and the resulting mixture was
stirred
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
149
for 4 h at room temperature. The reaction mixture was diluted with ethyl
acetate (300
mL) and the organic layer separated was washed with brine (300 mL, 4 x 100
mL), dried
(Na2SO4), filtered and evaporated to give the crude product which was
fractionated by
flash chromatography (hexane/EtOAc 3:1) to give JRLO1016 as a white solid
(3.11 g, 25
%); Rf 0.35 (Hexane/EtOAc 3:1); 'H (400 MHz CDC13) 4.61 (1H, s OH), 5.01 (2H,
s,
CH2), 6.74 (2H, AA'BB'), 6.85 (2H, AA'BB'), and 7.28-7.44 (5H, m).
4-Benzyloxy-2-nitro-phenol (JRL01023)
OH
BnO Noe
To a stirred solution of JRLO1016 (500 mg, 2.50 mmol) in ethylene glycol
dimethyl ether
(10 mL) at - 50 C under nitrogen was added in one portion tetraborofluorate
nitronium in
sulpholane (0.5 M, 5.1 mL, 2.55 mmol). After 1 h of stirring at - 50 C, the
reaction
mixture at room temperature was filtered through a short silica column. The
eluate
collected was evaporated to give the crude product which was fractionated by
flash
chromatography (hexane/EtOAc 10:1). The first fractionation gave some pure
JRL01023
as a yellow solid. The mixture of fractions retrieved from the first column
was
fractionated again to give more JRL01023 (total amount isolated: 186 mg,
combined
yield: 30 %); Rf 0.33 (hexane/EtOAc, 10:1); 1H (400 MHz, CDC13) 5.05 (2H, s),
7.09
(1H, d, J 9.0 Hz), 7.28 (1H, dd, J 3.0 and 9.0 Hz), 7.32-7.44 (5H, m), 7.59
(1H, d, J 3.0
Hz) and 10.34 (1 H, s, OH).
2-Amino-4-benzyloxy-phenol (JRL01028)
OH
BnO NH2
To a stirred solution of JRL01023 (1.33 g, 5.44 mmol) in EtOH/H2O (1:1, 150
mL) was
added sodium hydrosulfite (Na2S2O4, - 85%, 4.45 g, 21.74 mmol) and the yellow
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
150
suspension resulted was heated at 75 C. After 1 h at this temperature, the
reaction
mixture had become colorless and it was then cooled to room temperature. The
reaction
mixture was diluted with EtOAc (150 mL) and the organic layer separated washed
with
brine (4 x 100 mL), dried (Na2SO4), filtered and evaporated to give the crude
product
which was purified by a flash chromatography (hexane/EtOAc 2:1) to give
JRL01028 as
a brown solid (700 mg, 62 %); Rf 0.20 (hexane/EtOAc, 2:1); 1H (400 MHz, DMSO-
d6)
4.54 (2H, s), 4.90 (2H, s), 6.02 (1H, dd, J 2.8 and 8.6 Hz), 6.28 (1H, d, J
2.8 Hz), 6.52
(1H, d, J8.6 Hz), 7.25-7.45 (5H, m) and 8.50 (1H, s).
5-Benzyloxy-2-bromomethyl-benzooxazole (JRL01030)
BnO 10~ ~Br
To a stirred mixture of JRL01028 (663 mg, 3.08 mmol) in
trimethylsilylpolyphosphate
(PPSE)/1,2-dichlorobenzene (1:5, 60 mL) under nitrogen was added bromoacetic
acid
(333 mg, 2.40 minol) and the resulting purple mixture was heated at 150 C for
1 h.
After cooling to room temperature, the reaction mixture was diluted with EtOAc
(100
mL) and the organic layer separated washed with brine (4 x 50 mL), dried
(Na2SO4),
filtered and evaporated to an oil which was fractionated by flash
chromatography
(hexane/EtOAc 7:1) to give JRLO1026 as a red solid (228 mg, 30 %); Rf 0.21
(hexane/EtOAc 7:1); 1H (400 MHz, CDC13) 4.56 (2H, s), 5.10 (2H, s), 7.06 (1H,
dd, J2.4
and 8.8 Hz), 7.25 (1H, d, J2.4 Hz) and 7.30-7.46 (6H, m).
2-Methyl-benzothiazol-6-ol (JRL01040)
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
151
HO\ I e~
S
BBr3 (1M in CH2C12, 7.7 mL, 7.7 mmol) was slowly added to a stirred solution
of 6-
methoxybenzothiazol (950 mg, 5.14 mmol) in dichloromethane (30 mL) at 0 C
under
nitrogen and the reaction mixture became a dark brown suspension. After
stirring at room
temperature overnight, the reaction was quenched with ice/brine and diluted
with EtOAc
(100 mL). The organic layer that separated was washed with brine (4 x 50 mL),
dried
(Na2SO4), filtered and evaporated to give light brown residue which was
fractionated by
flash chromatography (hexane/EtOAc 3:1) to give JRLO1040 as a white powder
(600 mg,
71 %); Rf 0.16 (hexane/EtOAc 3:1); 1H (400 MHz, acetone-d) 2.72 (3H, s), 6.99
(1H, dd,
J2.4and8.6Hz),7.35(1H,d,J2.4Hz),7.70(1H,d,J8.6Hz)and8.70(1H,brs).
6-Benzyloxy-2-methyl-benzothiazole (JRL01053)
N
I
BnO S
To a stirred solution of JRL01040 (1.47 g, 8.93 mmol) in DMF (30 mL) at 0 C
under
nitrogen was added NaH (60%, 393 mg, 9.83 mmol). After stirring for 30 min,
benzyl
bromide (1.2 mL, 9.83 mmol) was added and the resulting mixture was stirred
for 4 h at
room temperature. The reaction mixture was diluted with ethyl acetate (100 mL)
and the
organic layer separated washed with brine (100 mL, 4 x 50 mL), dried (Na2SO4),
filtered
and evaporated to give the crude product which was fractionated by flash
chromatography (hexane/EtOAc 6:1) to give JRL01053 as a pale yellow solid
(2.20 g, 96
%); Rf 0.15 (Hexane/EtOAc 6:1); 1H (400 MHz CDC13) 2.75 (3H, s), 5.06 (2H, s),
7.10
(1H, dd, J2.2 and 8.8 Hz) 7.28-7.45 (6H, m) and 7.82 (1H, d, J9 Hz).
6-Benzyloxy-2-bromomethyl-benzothiazole (JRL01071)
Br
BnO S
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
152
To a stirred solution of JRL01053 (2.1 g, 8.22 mmol) in CC14 (50 mL) was added
NBS
(1.55 g, 8.64 mmol) and dibenzoyl peroxide (32 mg). The pale yellow suspension
was
refluxed for 2 h, cooled to room temperatue and filtered. The filtrate was
diluted with
EtOAc (100 mL), washed with NaOH (5%, 1 x 100 mL) and then brine (3 x 50 mL)
dried (Na2SO4), filtered and evaporated to give the crude product which was
fractionated
by flash chromatography (Hexane/EtOAc 7:1) to give JRL01071 as a white solid
(1.05 g,
39 %); m.p. 93 - 96 C; Rf 0.23 (hexane/EtOAc 7:1); 1H (400 MHz, CDC13) 4.76
(2H, s),
5'.10 (2H, s), 7.15 (1H, dd, J 2.7 and 9.0 Hz), 7.30-7.46 (6H, m) and 7.89
(1H, d, J 9.0
1o Hz); LRMS (FAB+) 333.9 [55, (M+H)+], 255.0 [17, (M+H-79Br)+], 91.0 {100,
Bn+];
HRMS (FAB+) 333.98873, C15H13BrNOS requires 333.99012.
4-[(6-Benzyloxy-benzothiazol-2-ylmethyl)-[1, 2, 4]triazol-4-yl-aminoJ-
benzonitrile
(JRL01074)
NN
\I- 7
N NN CN
BnO S
To a stirred mixture of NaH (60%, 57 mg, 1.42 mol) in DMF (5 mL) at 0 C under
nitrogen was added 4-[(1,2,4)triazol-4-amino]benzonitrile (264 mg, 1.42 mol)
in DMF (5
mL). After stirring at 40-50 C under nitrogen for 1 h, the orange reaction
mixture was
cooled to room temperature and JRL01071 (500 mg, 1.50 mol) was added. The
resulting
mixture was stirred overnight at room temperature under nitrogen. After
diluting the
reaction mixture with CH2C12 (50 mL), the organic layer was washed with brine
(100 mL,
3 x 50 mL), dried (Na2SO4), filtered and evaporated to give a crude product
which was
fractionated by flash chromatography (EtOAc) to give JRL01071 as a pale yellow
solid
(320 mg, 51 %); Rf 0.26 (EtOAc); 1H (400 MHz, CDC13) 5.12 (2H, s), 5.30 (2H,
s),
6.60 (2H AA'BB'), 7.18 (1H, dd, J 2.6 and 9.0 Hz), 7.30-7.50 (6H, m), 7.54 (2H
AA'BB'), 7.85 (lH, d, J9.0 Hz) and 8.57 (2H, s).
5-Benzyloxy-2-methyl-benzothiazole (JRL01052)
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
153
Bn0 10~ N
To a stirred solution of 2-Methylbenzothiazol-5-ol (4.0 g, 23.48 mmol) in DMF
(40 mL)
at 0 C under nitrogen was added NaH (60%, 1.03 g, 25.75 mmol). After stirring
for 30
min, benzyl bromide (3.2 mL, 25.83 mmol) was added and the resulting mixture
was
stirred for 4 h at room temperature. The reaction mixture was diluted with
ethyl acetate
(300 mL) and the organic layer separated washed with brine (300 mL, 4 x 100
mL), dried
(Na2SO4), filtered and evaporated to give the crude product which was
fractionated by
flash chromatography (hexane/EtOAc 6:1) to give JRLO1052 as a pale yellow
solid (5.63
1g, 94 %); Rf 0.15 (Hexane/EtOAc 6:1); 1H (400 MHz CDC13) 2.80 (3H, s), 5.14
(2H, s),
7.00-7.70 (8H, m).
5-Benzyloxy-2-bromomethyl-benzothiazole (JRL01064)
BnO
~Br
To a stirred solution of JRLO 1052 (3.6 g, 14.10 mmol) in CC14 (100 mL) was
added NBS
(2.66 g, 14.80 mmol) and dibenzoyl peroxide (55 mg). The pale yellow
suspension was
refluxed for 2 h, cooled to room temperatue and filtered. The filtrate was
diluted with
EtOAc (200 mL), washed with NaOH (5%, 1 x 200 mL) and then brine (3 x 100 mL)
dried (Na2SO4), filtered and evaporated to give the crude product which was
fractionated
by flash chromatography (hexane/EtOAc 7:1) to give JRL01064 as a white solid
with
(1.81 g, 38 %); m.p. 87 - 89 C; Rf0.25 (hexane/EtOAc 7:1); 1H (400 MHz,
CDC13) 4.77
(2H, s), 5.13 (2H, s), 7.14 (1H, dd, J 2.5 and 8.6 Hz), 7.30-7.48 (5H, m),
7.55 (1H, d, J
2.5 Hz) and 7.71 (1H, d, J 8.6 Hz); LRMS (FAB+) 333.9 [50, (M+H)+], 255.0 [17,
(M+H-79Br)+], 91.0 1100, Bn+]; HRMS (FAB+) 333.98946, C15H,3BrNOS requires
333.99012.
4-[(5-Benzyloxy-benzothiazol-2-ylmethyl)-[1, 2, 4]triazol-4-yl-amino]-
benzonitrile
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
154
(JRL01078)
N' N
BnO N ,
N N CN
)as
A suspension of JRL01064 (500 mg, 1.5 mmol), 4-[(1,2,4)triazol-4-
amino]benzonitrile
(277 mg, 1.5 mmol) and anhydrous potassium carbonate (207 mg, 1.5 mmol) in
cetonitrile (10 mL) was stirred at room temperature under nitrogen overnight.
The
resulting reaction mixture was diluted with EtOAc (50 mL) and the organic
layer was
washed with brine (4x 50 mL), dried (Na2SO4), filtered and evaporated to give
the crude
product which was fractionated by flash chromatography (EtOAc/acetone. 4:1) to
give
JRLO1078 as a yellow solid (98 mg, 15 %); Rf 0.24 (EtOAc); 'H (400 MHz, CDC13)
5.12 (2H, s), 5.33 (2H, s), 6.58 (2H AA'BB' ; 7.13 (1H, dd, J 2.6 and 9.0 Hz),
7.30-7.48
(5H, m), 7.50-7.56 (3H, m), 7.71 (1H, d, J9.0 Hz) and 8.61 (2H, s).
4-(3-Hydroxy-propylsulfanyl)-phenol (CAB02029)
S,~/OOH
HO
4-Hydroxythiophenol (6.31 g, 50 mmol) was dissolved in ethanol (100 mL) and
potassium tert.-butoxide (6.72 g, 60 mmol) was added. The mixture was stirred
stirred
until a clear, yellow solution was obtained. Then 3-chloro-l-propanol (4.20
mL, 50
mmol) was added with a syringe. The reaction mixture was stirred overnight at
room
temperature, the potassium chloride precipitate was filtered off and the
filtrate was
concentrated under reduced pressure. The residue was dissolved in ethyl
acetate (150
mL) and the organic layer was extracted with water (2 x 100 mL) and brine (100
mL),
dried over sodium sulphate and concentrated under reduced pressure. The
resulting oil
was dissolved in dichloromethane (50 mL) and hexane (100 mL) and left standing
open
overnight. The solid product was filtered off and dried under high vacuum.
Yield: 5.34 g
(58%) pale yellow solid.
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
155
1H-NMR (400 MHz, CD3OD) 5 = 1.71-1.77 (m, 2H), 2.82-2.86 (m, 2H), 3.60-3.64
(m,
2H), 6.73 (d, J = 8.6 Hz, 2H), 7.24 (d, J = 8.6 Hz, 2H).
3-(4-Benzyloxy-phenylsulfanyl)-propan-1-ol (CAB02032)
ScC0
4-(3-Hydroxy-propylsulfanyl)-phenol (CAB02029, 3.686 g, 20 mmol) was dissolved
in
ethanol (50 mL) and potassium tert-Butoxide (2.80 g, 25 mmol) and benzyl
bromide (3.0
UL, ca. 25 mmol) were added. The mixture was stirred overnight at room
temperature,
the precipitated potassium bromide was filtered off and the filtrate was
concentrated
under reduced pressure. The resulting yellow solid was dissolved in ethyl
acetate (100
mL), the solution was washed with water (100 mL) and brine (100 mL), dried
over
sodium sulphate and concentrated under reduced pressure. The residue was
dissolved in
dichloromethane (ca. 10 mL) and the product was precipitated by addition of
hexane (ca.
200 mL). The crystalline product was collected and dried under high vacuum.
Yield:
4.481 g (82%) colourless, small plates. 1H-NMR (400 MHz, CDC13) 5 = 1.45 (br
s, 1H, -
OH), 1.82-1.87 (m, 2H), 2.84 (t, J = 7.4 Hz, 2H), 3.74-3.78 (m, 2H), 5.05 (s,
2H, -
OCH2Ph), 6.90-6.93 (m, 2H), 7.31-7.44 (m, 7H).
1-Bromo-3-(4-benzyloxy-phenylsulfanyl)-propane (CABO2037)
S~~Br
Triphenylphosphine (7.90 g, 30.0 mmol) was added to a solution of 3-(4-
benzyloxy-
phenylsulfanyl)-propan-l-ol (CAB02032, 4.12 g, 15.0 mmol) and carbon
tetrabromide
(4.98 g, 18.0 mmol) in dichloromethane (120 mL) at 0 C (ice/water bath). The
reaction
mixture was allowed to warm up to room temperature and was stirred for another
hour.
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
156
The solution was transferred into a separation funnel and conc. NaHCO3-
solution (50
mL) was added. The organic layer was separated, dried over sodium sulphate ad
concentrated under reduced pressure. The residue was dissolved in ethyl
acetate (25 mL)
and hexane (100 mL) was added with stirring. The precipitated
triphenylphosphine oxide
was filtered off and washed with more EtOAc/hexane-mixture (1:4, 100 mL). The
organic solutions were concentrated and the residue was purified by column
chromatography (ethyl acetate/hexane, 1:10, Rf: 0.48). Yield: 4.96 g (98%)
colourless
dil, which becomes a solid after a couple of days. 1H-NMR (400 MHz, CDC13) 8 =
2.07
- (tt, J = 7.0, 7.0 Hz, 2H), 2.95 (t,-J = -7.0 Hz, 2H), 3.50 -(t, J = 7.0 Hz,
2H), 5.04 (s, 2H, -
1o OCH2Ph), 6.92 (d, J = 9.0 Hz, 2H), 7.27-7.44 (m, 7H). 13C-NMR (100.5 MHz,
CDC13) 8
= 32.30, 32.50, 34.41, 70.44, 115.84, 126.15, 127.72, 128.31, 128.86, 133.65,
136.89,
158.43.
4- { [3-(4-Benzyloxy-phenylsulfanyl)-propyl]-[ 1,2,4]triazol-4-yl-amino } -
benzonitrile
(CAB02038)
N///-N
N
~~ S IaCN
\% Bn0 Sodium hydride (60%, 200 mg, 5.0 mmol) was added to a solution of 4-
([1,2,4]triazol-4-
ylamino)-benzonitrile (926 mg, 5.0 mmol) in DMF (10 mL) at 0 C. The mixture
was
stirred for 30 min at 50 C, cooled to room temperature and 1-bromo-3-(4-
benzyloxy-
phenylsulfanyl)propane (CAB02037, 1.686 g, 5.0 mmol) was added. The reaction
mixture was stirred for 15 h and ethyl acetate (100 mL) was added. The mixture
was
transferred into a separation funnel and extracted with water (2 x 50 mL) and
brine (20
mL). The organic layer was dried over sodium sulphate and concentrated under
reduced
pressure. The residue was purified by column-chromatography (eluent: ethyl
acetate, Rf:
0.41). Yield: 1.724 g (78%) colourless oil. The oil was crystallised from a
small amount
of methanol. Yield: 1.517 g (68%). 'H-NMR (400 MHz, CDC13) 8 = 1.84 (tt, J =
6.4, 6.4
Hz, 2H), 2.90 (t, J = 6.4 Hz, 2H), 3.90 (t, J = 6.4 Hz, 2H), 5.06 (s, 2H, -
OCH2Ph), 6.52
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
157
(d, J = 9.0 Hz, 2H), 6.93 (d, J = 9.0 Hz, 2H), 7.31 (d, J = 9.0 Hz, 2H), 7.33-
7.44 (m, 5H),
7.52 (d, J = 9.0 Hz, 2H), 8.26 (s, 2H). LRMS (FAB+): 442.2 (100, [M+H])
4- { [3-(4-Benzyloxy-benzenesulfonyl)-propyl]-[1,2,4]triazol-4-yl-amino}-
benzonitrile
(CAB02168)
/N/-N
02 N
\\ S N I \\
\% \%~
Bn0 CN
m-Chloroperbenzoic acid (259 mg, 1.50 mmol) was added to a solution of 4-{[3-
(4-
Benzyloxy phenylsulfanyl) propylJ-[1,2,4]triazol-4 yl-amino}-benzonitrile
(CAB02038,
221 mg, 0.50 mmol) in dichloromethane (10 mL) at room temperature. The mixture
was
stirred for 1 h, then ethyl acetate (50 mL) and concentrated NaHCO3-solution
(20 mL)
were added. The mixture was transferred into a separation funnel, the organic
layer was
separated, washed with brine (20 mL), dried over sodium sulphate and
concentrated
under reduced pressure. The residue was purified by column chromatography
(ethyl
acetate, Rf: 0.22). Yield: 186 mg (78%) pale yellow foam. 'H-NMR (400 MHz,
CDC13) S
= 2.08 (tt, J = 7.0, 7.0 Hz, 2H), 3.15 (t, J = 7.0 Hz, 2H), 4.04 (t, J = 7.0
Hz, 2H), 5.16 (s,
2H, -OCH2Ph), 6.61 (d, J = 9.0 Hz, 2H), 7.11 (d, J = 9.0 Hz, 2H), 7.34-7.44
(m, 5H), 7.58
(d, J = 9.0 Hz, 2H), 7.81 (d, J = 9.0 Hz, 2H), 8.30 (s, 2H). LRMS (FAB+):
474.1 (100,
[M+H]+).
HRMS (FAB+) 474.16010 C25H24N503S requires 474.159987
4- { [3-(4-Hydroxy-benzenesulfonyl)-propyl]- [ 1,2,4]triazol-4-yl-amino } -
benzonitrile
(CAB02169, STX541)
N-N
02 N
S N STX541
HO J' CN
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
158
Palladium on charcoal (50 mg, 10% Pd) was added to a solution of 4-{[3-(4-
Benzyloxy-
benzenesulfonyl) propylJ-[1,2,4]triazol-4 yl-amino}-benzonitrile (CAB02168,
118 mg,
0.25 mmol) in THE (10 mL) and ethanol (10 mL). The mixture was stirred under
hydrogen atmosphere (balloon) for 18 h at room temperature. The reaction
mixture was
filtered through celite and the clear colourless filtrate was concentrated
under reduced
pressure. The residue was crystallised from acetone/water. Yield: 68 mg (71%)
colourless crystals. 1H-NMR (400 MHz, d6-DMSO) S = 1.68-1.76 (m, 2H), 3.38 (t,
J =
7.6 Hz, 2H), 3.92 (t, J = 7.2 Hz, 2H), 6.57 (d, J = 9.0 Hz, 2H), 6.95 (d, J =
9.0 Hz, 2H),
7.68 (d, J = 9.0 Hz, 2H), 7.73 (d, J = 9.0 Hz, 2H), 8.97 (s, 2H), 10.64 (s,
1H, -OH).
1o LRMS (FAB+): 384.0 (100, [M+H]+). HRMS (FAB+): 384.11248 C18H18N503S
requires
384.11304.
Bis-(4,4'-benzyloxy)phenyl-chloromethane (CAB02062)
CI
BnO / OBn
Thionylchloride (3.0 mL) was added to bis-(4-benzyloxy-phenyl)-methanol (1.982
g, 5.0
mmol). The resulting pink solution was stirred at room temperature until the
production
of sulphur dioxide and hydrogen chloride ceased (ca. 1.5 h). The excess of
thionyl
chloride was removed under reduced pressure, the crude product was used
without any
further purification. Yield: 2.075 g (100%). 1H-NMR (400 MHz, CDC13) 5 = 5.06
(s, 4H,
2 x -OCH2Ph), 6.11 (s, 1H, Ar2CHC1), 6.94 (d, J = 8.6 Hz, 4H), 7.30-7.46 (m,
14H).
4- { [Bis-(4-benzyloxy-phenyl)-methyl] - [ 1,2,4] triazol-4-yl-amino } -
benzonitrile
(CAB02068)
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
159
N- \N
BnO NNV
N
CN
OBn
Sodium hydride (60%, 200 mg, 5.0 mmol) was added to a solution of 4-
([1,2,4]triazol-4-
ylamino)-benzonitrile (926 mg, 5.0 mmol) in DMF (10 mL) at 0 C. The mixture
was
stirred for 1 h at room temperature and Bis-(4, 4'-benzyloxy)phenyl-
chloromethane
(CAB02062, 2.075 g, 5.0 mmol) was added. The reaction mixture was stirred for
15 h
and ethyl acetate (100 mL) was added. The mixture was transferred into a
separation
funnel and extracted with water (2 x 50 mL) and brine (20 mL). The organic
layer was
dried over sodium sulphate and concentrated under reduced pressure. The
residue was
purified by column-chromatography (ethyl acetate, Rf: 0.41). Yield: 1.319 g
(47%) white
solid. 'H-NMR (400 MHz, CDC13) 8 = 5.02 (s, 4H, 2 x -OCH2Ph), 6.28 (s, 1H),
6.54 (d,
J = 9.0 Hz, 2H), 6.90 (d, J = 8.6 Hz, 4H), 7.06 (d, J = 8.6 Hz, 4H), 7.30-7.44
(m, 1OH),
7.51 (d, J = 9.0 Hz, 2H), 7.88 (s, 2H). 13C-NMR (100.5 MHz, CDC13) 'S = 69.89,
70.44,
104.68, 113.48, 115.63, 118.93, 127.76, 128.39, 128.86, 129.31, 129.76,
134.19, 136.56,
143.92, 150.39, 159.19. LRMS (FAB+): 564.2 (100, [M+H]+).
4- { [Bis-(4-hydroxy-phenyl)-methyl]-[ 1,2,4]triazol-4-yl-amino } -
benzonitrile
(CAB02070, STX340)
N-N
HO N STX340
N 1::~CN
OH
4-{[Bis-(4-benzyloxy phenyl)-methyl]-[1, 2, 4]triazol-4 yl-amino}-benzonitrile
(CAB02068, 564 mg, 1.0 mmol) was dissolved in ethanol (50 mL) and palladium on
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
160
charcoal (50 mg, 10% Pd) was added. The mixture was stirred under hydrogen
atmosphere (balloon) for 48 h (TLC monitored) until all starting material was
consumed.
The Pd/C was filtered off (celite) and the solution was concentrated under
reduced
pressure. A yellow solid was obtained, which was dissolved in ethyl acetate
(10 mL)
upon heating. After cooling to room temperature the white precipitate was
filtered off
,and dried under high vacuum. Yield: 312 mg (81%). 'H-NMR (400 MHz, d6-DMSO) 8
= 5.74 (s, 2H, 2 x -OH), 6.28 (s, 1H), 6.53 (d, J = 9.0 Hz, 2H), 6.61 (d, J =
8.6 Hz, 4H),
7.10 (d, J = 8.6 Hz, 4H), 7.65 (d, J = 9.0 Hz, 2H), 8.83 (s, 2H). LRMS (FAB+):
199.1
(100), 384.1 (50, [M+H]+). LRMS (FAB-): 184.1 (100), 382.1 (41, [M-H]").
4- { [Bis-(4-sulphamoyloxy-phenyl)-methyl]-[ 1,2,4]triazol-4-yl-amino}-
benzonitrile
I(CAB02075)
N-N
H2NO2SO
N
i STX341
\ N \
CN
OSO2NH2
Sulphamoyl chloride solution in toluene (5 mL, 0.7 M, 3.5 mmol) was
concentrated
under reduced pressure (30 C water bath temperature) to ca. 1 mL volume. The
residue
was cooled -to 0 C (ice bath) and N,N-dimethylacetamide (5 mL) was added. 4-
([Bis-(4-
hydroxyphenyl)-methyl]-[1,2,4]triazol-4 yl-amino}-benzonitrile (CAB02070, 250
mg,
0.65 mmol) was added to the colourless solution and the mixture was stirred
for 18 h at
room temperature. Ethyl acetate (50 mL) and water (30 mL) were added to the
solution,
the organic layer was separated, washed with water (2 x 30 mL) and brine (20
mL), dried
over sodium sulphate and concentrated under reduced pressure to give a white
solid. The
solid was dissolved in ethyl acetate and precipitated by addition of hexane.
The white
powder was filtered off and dried under high vacuum. Yield: 299 mg (85%).
1H-NMR (400 MHz, d6-DMSO) 8 = 6.67 (s, 1H), 6.67 (d, J = 8.6 Hz, 2H), 7.17 (d,
J =
8.6 Hz, 4H), 7.47 (d, J = 8.6 Hz, 4H), 7.70 (d, J = 8.6 Hz, 2H), 8.02 (s, 4H,
2 x NH2),
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
161
8.77 (s, 2H). LRMS (FAB+): 542.1 (50, [M+H]). LRMS (FAB-): 184.1 (100), 540.0
(85, [M-H]").
Dimethylsulfamic acid 4-formyl-phenyl ester (JRL01114) (We followed the
synthetic method described in the 1957 German patent no. 1 016 256)
CHO
\
Me2NO2SO
A stirred solution of 4-hydroxybenzaldehyde (1.0 g, 8.02 mmol) in N,N-
dimethylcyclohexylamine (7 mL) was heated to 90-95 C and at this temperature
C1S02NMe2 (0.87 mL, 8.02 mL) was added dropwise. The reaction mixture was then
heated at 90-95 C for 3 h. After cooling to room temperatue, EtOAc (100 mL)
was
added and the organic layer was washed with 1M hydrochloric acid (2 x 100 mL)
and
then brine (3 x 50 mL), dried (Na2SO4), filtered and evaporated to give a
yellow oil
which solidified to a yellow wax upon storage in the refrigerator (1.82 ,g, 99
%); 'H (270
MHz, CDC13) 3.08 (6H, s), 7.46 (2H AA'BB'), 7.94 (2H AA'BB') and 10.0 (1H, s).
Dimethyl-sulfamic acid 4-hydroxymethyl-phenyl ester (JRL01115)
/ I OH
Me2NO2SO \
To a stirred solution of JRLO 1114 (1.81 g, 7.90 mmol) in THE (50 mL) was
added at 0
C NaBH4 (305 mg, 7.90 mmol). After 2 h of stirring, the initial yellow mixture
became a
white suspension and the reaction mixture was then poured into ice/water (-
100 mL).
The aqueous layer was extracted with chloroform (4 x 50 mL). The combined
organic
extracts was dried (Na2SO4), filtered and evaporated to give a yellow oil (1.0
g) which
was purified by flash chromatography (ethyl acetate) to give JRLO1115 as a
clear pale
yellow oil (820 mg, 45%); 1H (400 MHz, CDC13) 1.76 (-lH, br s, exchanged with
D20),
2.98 (6H, s), 4.70 (2H, s), 7.27 (2H AA'BB') and 7.39 (2H AA'BB'); LRMS (FAB+)
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
162
385.1 [7, (M+H+NBA)+], 231.0 (50, M), 214.0 [100, (M+H-H2O)+], 202.0 (10);
HRMS
(FAB+) 231.05557, C9H13NO4S requires 231.05653.
Dimethyl-sulfamic acid 4-chloromethyl-phenyl ester (LW002144)
Me2NO2SO (Orc To a solution of JRL01115 (725 mg, 3.135 mmol) in
dichloromethane (10 mL) at
ice/water temperature was added thionyl chloride (0.35 mL, 4.703 mmol). After
stirring
at room temperature for 1 h, the volatiles were removed from the reaction
mixture and
the oil resulted co-evaporated 3 times with chloroform (3 x 30 mL) to give to
LWO02144
as a yellow oil (767 mg, 98%); Rf 0.52 (EtOAc/hexane, 1:1), c.f. 0.19
(JRL01115); 1H
(400 MHz, CDC13) 2.99 (6H, s, NMe2), 4.58 (2H, s), 7.28 (2H AA'BB') and 7.42
(2H,
AA'BB'); LRMS (FAB+) 403.0 [18, (M+H+NBA)+], 391.2 (21), 249.9 [100, (M+H)+],
214.0 [45, (M+H-Cl)+], 113.0 (17); HRMS (FAB+) 249.02239, C9H12C1NO3S requires
249.02264. LWO02144 was very pure and hence was used without further
purification.
Dimethyl-sulfamic acid 4-([(4-cyano-phenyl) [1,2,4]triazol--4-yl-amino]-
methyl)-
phenyl ester (LWO02145, STX636)
N
N
N
i I N STX636
Me2NO2SO
CN
To a stirred solution of 4-[(1,2,4)triazol-4-amino]benzonitrile (538 mg, 2.903
mol) in
anhydrous DMF (10 mL) at ice/water temperature was added MaH (60%, 128 mg,
3.193
mmol). The pale orange brown mixture that formed was stirred under nitrogen at
50 C
for 10 min. After cooling to room temperature, LWO02144 (725 mg, 2.903 mmol)
in
DMF (total 5 mL) was added to the reaction mixture. The resulting orange/brown
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
163
suspension was stirred and heated at 50-60 C for 4 h. The cooled reaction
mixture was
diluted with ethyl acetate (100 mL) and the organic layer that separated
washed with
brine (100 mL, 4 x 50 mL), dried (MgSO4), filtered and evaporated to give an
orange/brown syrup (1.15 g). This crude product was fractionated on silica by
flash
chromatography (neat ethyl acetate initially followed by neat acetone after
the first
fraction has been collected) and the second fraction that collected upon
evaporation gave
LWO02145 as a clear bright yellow syrup which solidified upon standing at room
,tiemperature overnight to give a light yellow wax (803 mg, 69%); m.p.
crystals broke up
.nd scattered intensively at around 150 C and beyond, crystals melted at 195-
202 C; Rf
0.36 (EtOAc), c.f. 0.13 (LWO02144); 1H (400 MHz, CDC13) 2.98 (6H, s, NMe2),
4.90
(2H, s), 6.68 (2H AA'BB'), 7.27 (4H, m), 7.60 (2H, AA'BB') and 8.13 (2H, s,
triazole-
H); LRMS (FAB+) 399.2 [100, (M+H)+], 330.1 [43, (M-triazole)+]; HRMS (FAB+)
399.12458, C18H,9N603S requires 399.12394. Found: C 53.9, H 4.62, N 22.7%;
C18H18N603S requires C 54.26, H 4.55, N 21.09%
3-Bromo-4-(N,N-dimethylsulfamoyl)benzaldehyde (OBS02001)
Br
N SO
O
To a solution of OBS01057 (6.0 g, 30 mmol) in N, N-dimethylcyclohexylamine (30
mL)
at 80 - 90 C, was added N, N-dimethylsulfamoyl chloride (3.79 mL, 35.27
mmol). The
mixture was stirred at this temperature for 4 h, transferred to a separating
funnel and
diluted with EtOAc (100 mL). The organic layer was washed with water (2 x 200
mL),
6M HCl (aq.) (200 mL), brine (2 x 200 mL), dried (Na2S04) and filtered.
Concentration
in vacuo of the filtrates gave an orange oil which solidified on standing. The
product was
stirred in n-hexane, filtered and air-dried to give OBS02001 as a pale yellow
solid (8.21
g, 89 %). TLC [Si02, EtOAc-n-hexane (1:1)] Rf = 0.9; 'H-NMR (270 MHz, CDC13) _
3.09 (6H, s), 7.68 (1H, d, J= 8.4), 7.84 (1H, dd, J= 1.8, 8.4), 8.12 (1H, d,
J= 1.8), 9.93
(1H, s).
3-Bromo-4-(N,N-dimethylsulfamoyl)benzyl alcohol (OBS02002)
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
164
Br OH
N S-O
/ II
O
To a solution of OBS02001 (6.0 g, 19.47 mmol) in anhydrous THE (50 mL) was
added
sodium borohydride (0.81 g, 21.42 mmol). The mixture was stirred at room
temperature
for 4 h, quenched with water (CARE!!) and filtered through a Celite pad. The
filtrate
was concentrated in vacuo and re-dissolved in DCM (200 mL). The organic layer
was
washed with brine (2 x 200 mL), dried (Na2SO4) and filtered. Concentration in
vacuo of
tie filtrates gave OBS02002 as a pale yellow oil (5.54 g, 92 %). TLC [Si02,
EtOAc-n-
hexane (1:1)] Rf = 0.47; 'H-NMR (270 MHz, CDC13) = 2.97 (6H, s), 3.14 (1H, bs,
OH),
4.51 (2H, s), 7.19 (1H, dd, J= 1.5, 8.1),7.37 (1H, d, J=8.4),7.52(1H,d,J=1.8).
3-Bromo-4-(N,N-dimethylsulfamoyl)benzyl chloride (OBS02003)
Br C1
N-S-O
O
To a solution of OBS02002 (5.0 g, 16.12 mmol) in anhydrous DCM (50 mL) was
added
thionyl chloride (1.76 mL, 24.18 mmol). The mixture was stirred at room
temperature
for 2 h and the volatiles removed in vacuo. The residue was re-dissolved and
co-
evaporated three times with DCM (3 x 20 mL) to give a yellow oil which
solidified on
standing. The solid was stirred in n-hexane, filtered and air-dried to give
OBS02003 as
an off-white solid (5.01 g, 95 %). TLC [SiO2, EtOAc-n-hexane (1:1)] Rf = 0.79;
'H-
NMR (270 MHz, CDC13) = 3.05 (6H, s), 4.51 (2H, s), 7.32 (1H, dd, J = 2.2,
8.4), 7.48
(1H, d, J= 8.4), 7.62 (1H, d, J= 2.2).
Dimethylsulfamic acid 2-bromo-4-{ [(4-cyano-phenyl)-[1,2,4]triazol-4-
ylamino]methyl}phenyl ester (OBS02005, STX732)
eBr N' N_,
N S-O / I STX732
O
CN
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
165
To a suspension of NaH (60 % dispersion in oil, 0.44 g, 11.36 mmol) in
anhydrous DMF
(30 mL) at room temperature was added a solution of 4-[(4-cyanophenyl)amino]-
4H-
[1,2,4]triazole (2.0 g, 10.8 mmol) in anhydrous DMF (10 mL) and the mixture
stirred
under nitrogen for 1 h. The orange-yellow suspension was then treated with a
solution of
OBS02003 (3.73 g, 11.36 mmol) in anhydrous DMF (5 mL) and the mixture stirred
at 80
- 90 C overnight. The mixture was transferred to a separating funnel and
diluted with
EtOAc (200 mL). The organic layer was washed with water (4 x 200 mL), brine
(200
mL), dried (MgSO4) and filtered. Concentration in vacuo of the combined
filtrates gave
a residue which was recystallised from i-PrOH to give OBS02005 as a colourless
solid
(3.34 g, 65 %). TLC [Si02, EtOAc (100 %)] Rf = 0.32 (blue fluorescence at 254
nm);
Anal. Calcd. for C18H17N6SO3Br: C, 45.3; H, 17.6; N, 3.6 %; Found: C. 45.4; H,
17.5; N,
3.6 %; 'H-NMR (400 MHz, d6-DMSO) = 2.98 (6H, s), 5.11 (2H, s), 6.75 (2H,
AA'BB'),
7.43 (2H, s), 7.74 (1H, s), 7.78 (2H, AA'BB'), 8.88 (2H, s); 13C-NMR (400 MHz,
d6-
DMSO) = 39.4 (2 x CH3), 56.9 (CH2), 103.8, 114.5 (2 x CH), 116.1, 119.7, 123.9
(CH),
129.9 (CH), 134.3 (CH), 134.6 (2 x CH), 136.0, 144.0 (2 x CH), 146.9, 151.7;
MS
(FAB+) = 477 (100 %), 410 (30), 274 (22), 113 (32); Acc. MS for C18H17N6SO3Br
(Required, 477.03445; Found, 477.03282); LC-MS (Waters 2790 Alliance HPLC / ZQ
MicroMass spectrometer with PDA detector using APCI), tR (gradient elution :
5:95
MeCN / H2O - 95:5 MeCN / H2O over 10 min then 95:5 MeCN / H2O - 5:95 MeCN /
H2O using Waters "Symmetry" C18 (packing : 3.5 ,um), 100 mm column) = 6.24 min
(M+H = 478.19); HPLC (Waters 717+ Autosampler with PDA detector, using Waters
"Symmetry" C 18 (packing : 3.5 ,um), 4.6 x 150 mm column, 90:10 MeOH / H2O) tR
=
1.98 min (99.8 % purity).
3,5-Dibromo-4-(NN-dimethylsulfamoyl)benzaldehyde (OBS02013)
Br ~ ~O
o
N-S-O
O Br
To a solution of 3,5-dibromo-4-hydroxybenzaldehyde (5.0 g, 17.86 mmol) in N,N-
dimethylcyclohexylamine (30 mL) at 80 - 90 C, was added N,N-dimethylsulfamoyl
chloride (30 mL). The mixture was stirred at this temperature for 4 h,
transferred to a
separating funnel and diluted with EtOAc (100 mL). The organic layer was
washed with
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
166
water (2 x 200 mL), 6M HC1 (aq.) (200 mL), brine (2 x 200 mL), dried (Na2SO4)
and
filtered. Concentration in vacuo of the combined filtrates gave a dark amber
oil which
solidified on standing. The product was stirred in n-hexane, filtered and air-
dried to give
OBS02013 as a pale cream solid (6.09 g, 88 %). TLC [Si02, EtOAc-n-hexane
(1:1)] Rf=
0.73; 1H-NMR (270 MHz, CDC13) = 3.14 (6H, s), 8.06 (2H, s), 9.88 (1H, s).
3,5-Dibromo-4-(N,N-dimethylsulfamoyl)benzyl alcohol (OBS02015)
DT OH
11-S-O
O Br
To a solution of OBS02013 (5.5 g, 14.21 mmol) in anhydrous THE (50 mL) was
added
sodium borohydride (0.59 g, 15.63 mmol). The mixture was stirred at room
temperature
for 4 h, quenched with water (CARE! !) and filtered through a Celite pad. The
filtrate
was concentrated in vacuo and re-dissolved in DCM (200 mL). The organic layer
was
washed with brine (2 x 200 mL), dried (Na2S04) and filtered. Concentration in
vacuo of
the combined filtrates gave OBS02015 as a pale yellow oil (5.21 g, 94 %). TLC
[Si02,
EtOAc-n-hexane (1:1)] Rf = 0.55; 'H-NMR (270 MHz, CDC13) = 1.97 (1H, bs, OH),
3.11
(6H, s), 4.63 (2H, s), 7.55 (2H, t, J= 1.5).
3,5-Dibromo-4-(N,N-dimethylsulfamoyl)benzyl chloride (OBS02018)
Br C1
N-S-O
ii
0 Br
To a solution of OBS02015 (3.93 g, 10.10 mmol) in anhydrous DCM (50 mL) was
added
thionyl chloride (1.11 mL, 15.15 mmol). The mixture was stirred at room
temperature
for 2 h and the volatiles removed in vacuo. The residue was re-dissolved and
co-
evaporated three times with DCM (3 x 20 mL) to give OBS02018 as a brown oil
which
solidified on standing (3.93 g, 96 %). TLC [SiO2, EtOAc-n-hexane (1:1)] Rf =
0.83; 'H-
NMR (270 MHz, CDC13) = 3.11 (6H, s), 4.46 (2H, s), 7.59 (2H, s).
Dimethylsulfamic acid 2,6-dibromo-4-f [(4-cyano-phenyl)-[1,2,4]triazol-4-
ylamino]methyl}phenyl ester (OBS02019, STX740)
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
167
N
Br N,,,,N
STX740
N-S-O
O Br
CN
To a suspension of NaH (60 % dispersion in oil, 0.22 g, 5.67 mmol) in
anhydrous DMF
(20 mL) at room temperature was added a solution of 4-[(4-cyanophenyl)amino]-
4H-
[1,2,4]triazole (1.0 g, 5.4 mmol) in anhydrous DMF (5 mL) and the mixture
stirred under
nitrogen for 1 h. The orange-yellow suspension was then treated with a
solution of
OBS02018 (2.31 g, 5.67 mmol) in anhydrous DMF (5 mL) and the mixture stirred
at 80
- 90 C overnight. The mixture was transferred to a separating funnel and
diluted with
EtOAc (200 mL). The organic layer was washed with water (4 x 200 mL), brine
(200
mL), and dried (MgS04). Concentration in vacuo gave a residue which was
recystallised
from i-PrOH to give OBS02019 as a white solid (2.06 g, 69 %). TLC [Si02, EtOAc
(100
%)] Rf = 0.53 (blue fluorescence at 254 nm); Anal. Calcd. for C18H16N6SO3Br2:
C, 53.3;
H, 4.7; N, 19.6 %; Found: C. 53.1; H, 4.7; N, 19.3 %; 1H-NMR (270 MHz, d6-
DMSO) =
3.02 (6H, s), 5.09 (2H, s), 6.81 (2H, AA'BB'), 7.73 (2H, s), 7.76 (2H,
AA'BB'), 8.93
(2H, s); 13C-NMR (400 MHz, d6-DMSO) = 39.3 (2 x CH3), 56.7 (CH2), 103.9, 114.5
(2 x
CH), 118.7, 119.7, 133.6 (2 x CH), 134.6 (2 x CH), 137.6, 144.0, 145.1 (2 x
CH), 151.7;
MS (FAB) = 557 (M+H, 100 %), 488 (20), 113 (28); Ace. MS for C18Hl6N6SO3Br2
(Required, 556.9442; Found, 556.9429); LC-MS (Waters 2790 Alliance HPLC / ZQ
MicroMass spectrometer with PDA detector using APCI), tR (gradient elution :
5:95
MeCN / H2O - 95:5 MeCN / H2O over 10 mins then 95:5 MeCN / H2O - 5:95 MeCN /
H2O using Waters "Symmetry" C18 (packing : 3.5 ,um), 100 mm column) = 6.46 min
(M+2H = 558.17); HPLC (Waters 717+ Autosampler with PDA detector, using Waters
"Symmetry" C18 (packing : 3.5 ,um), 4.6 x 150 mm column, 90:10 MeOH / H2O) tR
=
2.01 min (98.7 % purity).
3-Bromo-4-(NN-dimethylsulfamoyl)-5-methoxybenzaldehyde (OBS02022)
Br O
\ 11
N-S-O
0 /O
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
168
To a solution of 5-bromovanillin (3.0 g, 12.98 mmol) in N,N-
dimethylcyclohexylamine
(30 mL) at 80 - 90 C, was added N,N-dimethylsulfamoyl chloride (1.64 mL,
15.26
mmol). The mixture was stirred at this temperature for 4 h, transferred to a
separating
funnel and diluted with EtOAc (100 mL). The organic layer was washed with
water (2 x
200 mL), 6M HCl (aq.) (200 mL), brine (2 x 200 mL), dried (Na2SO4) and
filtered.
Concentration in vacuo of the combined filtrates gave a golden-brown oil which
solidified on standing. The product was stirred in n-hexane, filtered and air-
dried to give
OBS02022 as a cream solid (3.22 g, 73 %). TLC [Si02, EtOAc-n-hexane (1:1)] Rf
=
0.88; 'H-NMR (270 MHz, CDC13) = 3.07 (6H, s), 3.96 (3H, s), 7.43 (1H, d, J=
1.8), 7.68
(1H, d, J= 1.8), 9.87 (1H, s).
3-Bromo-4-(N,N-dimethylsulfamoyl)-5-methoxybenzyl alcohol (OBS02023)
")qr OH
N-S-O
O ,O
To a solution of OBS02022 (3.0 g, 8.87 mmol) in anhydrous THE (50 mL) was
added
sodium borohydride (0.37 g, 9.76 mmol). The mixture was stirred at room
temperature
for 4 h, quenched with water (CARE!!) and filtered through a Celite pad. The
filtrate
was concentrated in vacuo and re-dissolved in DCM (200 mL). The organic layer
was
washed with brine (2 x 200 mL), dried (Na2SO4) and filtered. Concentration in
vacuo of
the combined filtrates gave OBS02023 as a pale yellow oil (1.71 g, 57 %). TLC
[Si02,
EtOAc-n-hexane (1:1)] Rf= 0.68; 'H-NMR (270 MHz, CDC13) = 1.94 (1H, bs, OH),
3.07
(6H, s), 3.91 (3H, s), 4.65 (2H, s), 6.96 (1H, d, J= 2), 7.17 (1H, d, J= 2).
3-Brom6-4-(N,N-dimethylsulfamoyl)-5-methoxybenzyl chloride (OBS02026)
Br C1
N-S-O
O O~
To a solution of OBS02023 (1.56 g, 4.59 mmol) in anhydrous DCM (50 mL) was
added
thionyl chloride (0.5 mL, 6.88 mmol). The mixture was stirred at room
temperature for 2
h and the volatiles removed in vacuo. The residue was re-dissolved and co-
evaporated
three times with DCM (3 x 20 mL) to give OBS02026 as a brown oil (0.79 g, 48
%).
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
169
TLC [Si02, EtOAc-n-hexane (1:1)] Rf = 0.89; 1H-NMR (270 MHz, CDC13) = 3.00
(6H,
s), 3.85 (3H, s), 4.46 (2H, s), 6.92 (1H, d, J= 1.8), 7.16 (1H, d, J= 1.8).
Dimethylsulfamic acid 2-bromo-4-{[(4-cyano-phenyl)-[1,2,4]triazol-4-
ylamino]methyl}-
6-methoxyphenyl ester (OBS02028, STX747)
N
Br NON
-s-0
o o~
CN
To a suspension of NaH (60 % dispersion in oil, 0.07 g, 1.73 mmol) in
anhydrous DMF
(20 mL) at room temperature was added a solution of 4-[(4-cyanophenyl)amino]-
4H-
[1,2,4]triazole (0.31 g, 1.65 mmol) in anhydrous DMF (5 mL) and the mixture
stirred
under nitrogen for 1 h. The orange-yellow suspension was then treated with a
solution of
OBS02026 (0.62 g, 1.73 mmol) in anhydrous DMF (5 mL) and the mixture stirred
at 80
- 90 C overnight. The mixture was transferred to a separating funnel and
diluted with
EtOAc (200 mL). The organic layer was washed with water (4 x 200 mL), brine
(200
mL), dried (MgSO4) and filtered. Concentration in vacuo of the combined
filtrates gave
a residue which was recystallised from i-PrOH to give OBS02028 as an off-white
solid
(0.50 g, 59 %). TLC [Si02, EtOAc (100 %)] Rf = 0.63 (blue fluorescence at 254
rim);
Anal. Calcd. for C19H19N6SO4Br: C, 45.0; H, 3.8; N, 16.6 %; Found: C. 44.9; H,
3.8; N,
15.8 %; 'H-NMR (400 MHz, d6-DMSO) = 2.93 (6H, s), 3.86 (3H, s), 5.07 (2H, s),
6.77
(2H, AA'BB'), 7.11 (1H, d, J= 1.6), 7.24 (1H, d, J= 1.6), 7.79 (2H, AA'BB'),
8.9 (2H,
s); 13C-NMR (400 MHz, d6-DMSO) = 39.0 (2 x CH3), 57.31 (CH2), 57.31 (CH3),
103.9,
113.3 (CH), 114.5 (CH), 118.3, 119.7, 125.1 (CH), 134.6 (CH), 136.3, 137.3,
144.0
(CH), 152.0, 153.4; MS (FAB+) = 509 (M+2H, 100 %), 440 (31), 215 (19), 113
(19);
Acc. MS for C19H19N6SO4Br (Required, 507.04323; Found, 507.04501); LC-MS
(Waters
2790 Alliance HPLC / ZQ MicroMass spectrometer with PDA detector using APCI),
tR
(gradient elution : 5:95 MeCN / H2O - 95:5 MeCN / H2O over 10 mins then 95:5
MeCN
/ H2O - 5:95 MeCN / H2O using Waters "Symmetry" C18 (packing : 3.5 ,um), 100
mm
column) = 6.16 min (M+H = 508.29); HPLC (Waters 717+ Autosampler with PDA
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
170
detector, using Waters "Symmetry" C18 (packing : 3.5 ,um), 4.6 x 150 mm
column,
90:10 MeOH / H2O) tR = 1.96 min (97.5 % purity).
3-Chloro-4-(N,N-dimethylsulfamoyl)benzaldehyde (OBS02043)
Cl -Z O
N-SO
/. O
To a solution of 3-chloro-4-hydroxybenzaldehyde (6.0 g, 38.32 mmol) in N,N-
dimethylcyclohexylamine (30 mL) at 80 - 90 C, was added N,N-dimethylsulfamoyl
chloride (4.84 mL, 45.05 mmol). The mixture was stirred at this temperature
for 4 h,
transferred to a separating funnel and diluted with EtOAc (100 mL). The
organic layer
was washed with water (2 x 200 mL), 6M HCl (aq.) (200 mL), brine (2 x 200 mL),
dried
(Na2SO4) and filtered. Concentration in vacuo of the combined filtrates gave a
brown oil
which solidified on standing. The product was stirred in n-hexane, filtered
and air-dried
to give OBS02043 as a beige solid (5.36 g, 53 %). TLC [Si02, EtOAc-n-hexane
(1:1)] Rf
= 0.92; 1H-NMR (270 MHz, CDC13) = 3.06 (6H, s), 7.67 (1H, d, J= 8.4), 7.79
(1H, dd, J
= 2.2, 8.4), 7.95 (1H, d, J= 1.8), 9.93 (1H, s).
3-Chloro-4-(N,N-dimethylsulfamoyl)benzyl alcohol (OBS02046)
Cl OH
0 N-S-O
u
O
To a solution of OBS02043 (5.0 g, 18.96 mmol) in anhydrous THE (50 mL) was
added
sodium borohydride (0.79 g, 20.86 mmol). The mixture was stirred at room
temperature
for 4 h, quenched with water (CARE!!) and filtered through a Celite pad. The
filtrate
was concentrated in vacuo and re-dissolved in DCM (200 mL). The organic layer
was
washed with brine (2 x 200 mL), dried (Na2S04) and filtered. Concentration in
vacuo of
the combined filtrates gave OBS02046 as a golden-brown oil (3.81 g, 76 %). TLC
[Si02,
EtOAc-n-hexane (1:1)] Rf= 0.64; 'H-NMR (270 MHz, CDC13) = 2.04 (1H, bs, OH),
3.05
(6H, s), 4.66 (2H, s), 7.25 (1 H, dd, J = 2.2, 8.4), 7.46 (1 H, d, J = 2.2),
7.47 (1 H, d, J =
8.1).
3-Chloro-4-(N,N-dimethylsulfamoyl)benzyl chloride (OBS02052)
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
171
C1 C1
0
N S-0
O
To a solution of OBS02046 (3.02 g, 11.37 mmol) in anhydrous DCM (50 mL) was
added
thionyl chloride (1.24 mL, 17.05 mmol). The mixture was stirred at room
temperature
for 2 h and the volatiles removed in vacuo. The residue was re-dissolved and
co-
evaporated three times with DCM (3 x 20 mL) to give OBS02052 as an amber oil
(2.68
g, 83 %). TLC [Si02, EtOAc-n-hexane (1:1)] Rf = 0.91; 1H-NMR (270 MHz, CDCl3)
=
3;:04 (6H, s), 4.52 (2H, s), 7.28 (1H, dd, J= 2.2, 8.4), 7.47 (1H, d, J= 8.5),
7.47 (1H, d, J
= 2.2).
Dimethylsulfamic acid 2-chloro-4-{[(4-cyano-phenyl)-[1,2,4]triazol-4-
ylamino]methyl}phenyl ester (OBS02054, STX787)
CI NNs N
0 ~ I I / STX787
N_S-0
O
CN
To a suspension of NaH (60 % dispersion in oil, 0.34 g, 8.95 mmol) in
anhydrous DMF
(20 mL) at room temperature was added a solution of 4-[(4-cyanophenyl)amino]-
4H-
[1,2,4]triazole (1.66 g, 8.95 mmol) in anhydrous DMF (5 mL) and the mixture
stirred
under nitrogen for 1 h. The orange-yellow suspension was then treated with a
solution of
OBS02052 (2.67 g, 9.4 mmol) in anhydrous DMF (5 mL) and the mixture stirred at
80 -
90 C overnight. The mixture was transferred to a separating funnel and
diluted with
EtOAc (200 mL). The organic layer was washed with water (4 x 200 mL), brine
(200
mL), dried (MgSO4) and filtered. Concentration in vacuo of the combined
filtrates gave
a residue which was recystallised from i-PrOH to give OBS02054 as a pale cream
solid
(2.03 g, 52 %). TLC [Si02, EtOAc (100 %)] Rf = 0.53 (blue fluorescence at 254
nm);
Anal. Calcd. for C18H17N6SO3C1: C, 49.9; H, 4.0; N, 19.4 %; Found: C, 49.7; H,
4.0; N,
19.2 %; 1H-NMR (270 MHz, CDC13) = 3.01 (6H, s), 4.87 (2H, s), 6.62 (2H,
AA'BB'),
7.15 (1H, dd, J = 2.2, 8.4), 7.32 (1H, d, J = 2.2), 7.46 (1H, d, J = 8.4),
7.55 (2H,
AA'BB'), 8.18 (2H, s); 13C-NMR (400 MHz, d6-DMSO) = 38.3 (2 x CH3), 56.0
(CH2),
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
172
102.9, 113.5 (2 x CH), 118.7, 123.4 (CH), 125.8, 128.3 (CH), 130.3 (CH), 133.7
(2 x
CH), 134.9, 143.0 (2 x CH), 144.7, 150.9; MS (FAB) = 433 (M+H, 100 %), 364
(37);
Acc. MS for C18H17N6S03C1(Required M+H, 433.08476; Found M+H, 433.08496); LC-
MS (Waters 2790 Alliance HPLC / ZQ MicroMass spectrometer with PDA detector
using APCI), tR (gradient elution : 5:95 MeCN / H2O - 95:5 MeCN / H2O over 10
min
then 95:5 MeCN / H2O - 5:95 MeCN / H2O using Waters "Symmetry" C18 (packing :
3.5 ,um), 100 mm column) = 6.18 min (M+H = 434.18); HPLC (Waters 717+
Autosampler with PDA detector, using Waters "Symmetry" C18 (packing : 3.5
,um), 4.6
x 150 mm column, 90:10 MeOH / H2O) tR = 1.98 min (99.9 % purity).
4-(N,N-Dimethylsulfamoyl)-3-methoxybenzaldehyde (OBS02011)
O 'o
N-S-O
~ n
O
To a solution of vanillin (4.56 g, 30 mmol) in N,N-dimethylcyclohexylamine (30
mL) at
80 - 90 C, was added N,N-dimethylsulfamoyl chloride (3.79 mL, 35.27 mmol).
The
mixture was stirred at this temperature for 4 h, transferred to a separating
funnel and
diluted with EtOAc (100 mL). The organic layer was washed with water (2 x 200
mL),
6M HCl (aq.) (200 mL), brine (2 x 200 mL), dried (Na2SO4) and filtered.
Concentration
in vacuo of the combined filtrates gave a golden-yellow oil which solidified
on standing.
The product was stirred in n-hexane, filtered and air-dried to give OBS02011
as yellow
plates (6.92 g, 89 %). TLC [Si02, EtOAc-n-hexane (1:1)] Rf = 0.65; 'H-NMR (270
MHz, CDC13) = 2.98 (6H, s), 3.93 (3H, s), 7.44 (1H, d, J = 1.6), 7.48 (1H, dd,
J = 1.6,
8.1), 7.53 (1H, d, J= 8.1), 9.92 (1H, s).
4-(N,N-Dimethylsulfamoyl)-3-methoxybenzyl alcohol (0BS02014)
I
~ OH
0 1
N-S-O
/ O
To a solution of OBS02011 (5.5 g, 21.21 mmol) in anhydrous THE (50 mL) was
added
sodium borohydride (0.88 g, 23.33 mmol). The mixture was stirred at room
temperature
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
173
for 4 h, quenched with water (CARE!!) and filtered through a Celite pad. The
filtrate
was concentrated in vacuo and re-dissolved in DCM (200 mL). The organic layer
was
washed with brine (2 x 200 mL), dried (Na2SO4) and filtered. Concentration in
vacuo of
the combined filtrates gave OBS02014 as a pale yellow oil (5.12 g, 92 %). TLC
[Si02,
EtOAc-n-hexane (1:1)] Rf= 0.18; 1H-NMR (270 MHz, CDC13) = 2.44 (1H, bs, OH),
2.92
(6H, s), 3.84 (3H, s), 4.60 (2H, s), 6.85 (1H, dd, J= 2.2, 8.4), 6.97 (1H, d,
J= 1.8), 7.25
(1H, d, J= 8.1).
4. (N,N-Dimethylsulfamoyl)-3-methoxybenzyl chloride (OBS02016)
O C1
N S0
/ O
To a solution of OBS02014 (2.76 g, 10.55 mmol) in anhydrous DCM (50 mL) was
added
thionyl chloride (1.15 mL, 15.82 mmol). The mixture was stirred at room
temperature
for 2 h and the volatiles removed in vacuo. The residue was re-dissolved and
co-
evaporated three times with DCM (3 x 20 mL) to give OBS02016 as a brown oil
(2.91 g,
99 %). TLC [SiO2, EtOAc-n-hexane (1:1)] Rf = 0.84; 1H-NMR (270,MHz, CDC13) _
2.96 (6H, s), 3.90 (3H, s), 4.55 (2H, s), 6.95 (1H, dd, J= 1.8, 8.0), 7.01
(1H, d, J= 1.8),
7.32 (1H, d, J= 8.0).
Dimethylsulfamic acid 4-{[(4-cyano-phenyl)-[1,2,4]triazol-4-ylamino]methyl}-2-
methoxyphenyl ester (OBS02017, STX739)
O N.Ni N
0
I / STX739
N-
/ S-O
0
CN
To a suspension of NaH (60 % dispersion in oil, 0.22 g, 5.67 mmol) in
anhydrous DMF
(20 mL) at room temperature was added a solution of 4-[(4-cyanophenyl)amino]-
4H-
[1,2,4]triazole (1.0 g, 5.4 mmol) in anhydrous DMF (5 mL) and the mixture
stirred under
nitrogen for 1 h. The orange-yellow suspension was then treated with a
solution of
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
174
OBS02016 (1.59 g, 5.67 mmol) in anhydrous DMF (5 mL) and the mixture stirred
at 80
- 90 C overnight. The mixture was transferred to a separating funnel and
diluted with
EtOAc (200 mL). The organic layer was washed with water (4 x 200 mL), brine
(200
mL), dried (MgSO4) and filtered. Concentration in vacuo of the combined
filtrates gave
a residue which was recystallised from i-PrOH to give OBS02017 as a cream
solid (1.07
46 %). TLC [SiO2, EtOAc (100 %)] Rf = 0.26 (blue fluorescence at 254 nm);
Anal.
Calcd. for C19H2ON6SO4: C, 38.9; H, 2.9; N, 15.1 %; Found: C, 38.9; H, 3.0; N,
14.8 %;
1H-NMR (400 MHz, d6-DMSO) = 2.83 (6H, s), 3.84 (3H, s), 5.07 (2H, s), 6.80
(2H,
AA'BB'), 6.90 (1H, dd, J = 2, 8), 7.12 (1H, d, J = 2), 7.23 (1H, d, J = 8),
7.79 (2H,
1o AA'BB'), 8.83 (2H, s); 13C-NMR (400 MHz, d6-DMSO) = 39.1 (2 x CH3), 56.8
(CH3),
57.6 (CH2), 103.7, 114.1 (2 x CH), 114.5 (CH), 119.7, 121.5 (CH), 124.1 (CH),
134.6 (2
pc CH), 135.2, 138.8, 144.0 (2 x CH), 151.8, 152.0; MS (FAB+) = 429 (M+H, 100
%),
360 (36), 321 (6), 244 (15), 113 (5); Acc. MS for C19H2ON6SO4 (Required,
429.1341;
Found, 429.1345); LC-MS (Waters 2790 Alliance HPLC / ZQ MicroMass spectrometer
with PDA detector using APCI), tR (gradient elution : 5:95 MeCN / H2O - 95:5
MeCN /
H2O over 10 min then 95:5 MeCN / H2O - 5:95 MeCN / H2O using Waters "Symmetry"
C18 (packing : 3.5 ,um), 100 mm column) = 5.83 min (M+2H = 430.27); HPLC
(Waters
717+ Autosampler with PDA detector, using Waters "Symmetry" C18 (packing : 3.5
pm), 4.6 x 150 mm column, 90:10 MeOH / H2O) tR = 1.94 min (99.5 % purity).
3-(NN-Dimethylsulfamoyl)-4-methoxybenzaldehyde (OBS02049)
11
Jc
% -S -O
O O
To a solution of isovanillin (4.56 g, 30 mmol) in NN-dimethylcyclohexylamine
(30 mL)
at 80 - 90 C, was added N,N-dimethylsulfamoyl chloride (3.79 g, 35.27 mmol).
The
mixture was stirred at this temperature for 4 h, transferred to a separating
funnel and
diluted with EtOAc (100 mL). The organic layer was washed with water (2 x 200
mL),
6M HC1(aq.) (200 mL), brine (2 x 200 mL), dried (Na2SO4) and filtered.
Concentration
in vacuo of the combined filtrates gave a brown oil which solidified on
standing. The
product was stirred in n-hexane, filtered and air-dried to give OBS02049 as a
pale yellow
solid (6.91 g, 89 %). TLC [SiO2, EtOAc-n-hexane (1:1)] Rf= 0.57; 1H-NMR (270
MHz,
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
175
CDC13) = 2.98 (6H, s), 3.95 (3H, s), 7.07 (1H, d, J = 8.4), 7.77 (1H, dd, J =
1.8, 8.4),
7.83 (1H, d, J= 2.2), 9.84 (1H, s).
3-(N,N-Dimethylsulfamoyl)-4-methoxybenzyl alcohol (OBS02053)
O
n
N-S-O
Jc
Q OH
3"o a solution of OBS02049 (5.5 g, 21.21 mmol) in anhydrous THE (50 mL) was
added
sodium borohydride (0.88 g, 23.33 mmol). The mixture was stirred at room
temperature
for 4 h, quenched with water (CARE!!) and filtered through a Celite pad. The
filtrate
was concentrated in vacuo and re-dissolved in DCM (200 mL). The organic layer
was
washed with brine (2 x 200 mL), dried (Na2S04) and filtered. Concentration in
vacuo of
the combined filtrates gave OBS02053 as a golden-yellow oil (3.26 g, 59 %).
TLC
[Si02, EtOAc-n-hexane (1:1)] Rf = 0.36; 1H-NMR (270 MHz, CDC13) = 1.73 (1H,
bs,
OH), 2.97 (6H, s), 3.88 (3H, s), 4.62 (2H, s), 6.95 (1H, d, J= 8.4), 7.23,(1H,
dd, J= 2.2,
8.4), 7.36 (1H, d, J= 2.2).
3-(N,N-Dimethylsulfamoyl)-4-methoxybenzyl chloride (OBS02058)
O
\ n
N-S-O
O C1
To a solution of OBS02053 (2.33 g, 8.89 mmol) in anhydrous DCM (50 mL) was
added
thionyl chloride (0.97 mL, 13.34 mmol). The mixture was stirred at room
temperature
for 2 h and the volatiles removed in vacuo. The residue was re-dissolved and
co-
evaporated three times with DCM (3 x 20 mL) to give OBS02058 as a light-
sensitive
brown oil (2.07 g, 83 %); 'H-NMR (270 MHz, CDC13) = 2.98 (6H, s), 3.89 (3H,
s), 4.54
(2H, s), 6.94 (1H, d, J= 8.4), 7.25 (1H, dd, J= 2.2, 8.4), 7.40 (1H, d, J=
2.2).
Dimethylsulfamic acid 5-{[(4-cyano-phenyl)-[1,2,4]triazol-4-ylamino]methyl}-2-
methoxyphenyl ester (OBS02060, STX796)
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
176
e~N-S-O NN
O I / STX796
-0
CN
To a suspension of NaH (60 % dispersion in oil, 0.28 g, 6.91 mmol) in
anhydrous DMF
(20 mL) at room temperature was added a solution of 4-[(4-cyanophenyl)amino]-
4H-
[1,2,4]triazole (1.28 g, 6.91 mmol) in anhydrous DMF (5 mL) and the mixture
stirred
under nitrogen for 1 h. The orange-yellow suspension was then treated with a
solution of
OBS02058 (2.03 g, 7.26 mmol) in anhydrous DMF (5 mL) and the mixture stirred
at 80
- 90 C overnight. The mixture was transferred to a separating funnel and
diluted with
EtOAc (200 mL). The organic layer was washed with water (4 x 200 mL), brine
(200
mL), dried (MgSO4) and filtered. Concentration in vacuo of the combined
filtrates gave
a residue which was recystallised from EtOAc-n-hexane to give OBS02060 as a
pale
cream solid (2.24 g, 76 %); 1H-NMR (270 MHz, CHC13) = 2.88 (6H, s), 3.82 (3H,
s),
4.79 (2H, s), 6.66 (2H, AA'BB'), 6.83 (1H, d, J= 8.4), 6.94 (1H, dd, J= 2.2,
8.4), 7.30
(1H, d, J= 1.8), 7.53 (2H, AA'BB'), 8.12 (2H, s); 13C-NMR (270 MHz, CHC13) =
38.7
(2 x CH3), 56.1 (CH3), 57.2 (CH2), 105.1, 112.9 (CH), 113.6 (2 x CH), 118.5,
123.9
(CH), 125.7 (CH), 127.6 (CH), 133.9 (2 x CH), 139.4, 142.6 (2 x CH), 150.4,
151.8; MS
(FAB+) = 429 (M+H, 100 %), 360 (50), 321 (6), 244 (43); Ace. MS for
Cj9H2ON6SO4
(Required, 429.1343; Found, 429.1345); LC-MS (Waters 2790 Alliance HPLC / ZQ
MicroMass spectrometer with PDA detector using APCI), tR (gradient elution :
5:95
MeCN / .H20 - 95:5 MeCN / H2O over 10 min then 95:5 MeCN / H2O - 5:95 MeCN /
H2O using Waters "Symmetry" C18 (packing : 3.5 ,um), 100 mm column) = 5.80 min
(M+2H = 430.27); HPLC (Waters 717+ Autosampler with PDA detector, using Waters
"Symmetry" C18 (packing : 3.5 ,um), 4.6 x 150 mm column, 90:10 MeOH / H2O) tR
=
1.96 min (99.8 % purity).
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
177
Synthesis of STX258, 265, 273, 287, 288, 290, 291, 292
N-N
N
BnO X-Br + HN
aCN
NaH/DMF
N-N
~N~
BnO XI--,'---a CN
Pd/C, H2
N-N X
N CH2 STX265
CH2CH2 STX290
HO X~ O(CH2)4 STX287
O(CH2)2 STX291
CN
H2SO2NH2 / DMA
'N-N
X
N
CH2 STX258
CH2CH2 STX273
H2NO2SO X~ I O(CH2)4 STX288
CN O(CH2)2 STX292
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
178
Synthesis of STX300 and STX301
N-N
.{- HN
BnO Br I \
:b-o/,\/ N
CN
LWO02087 1) NaH/DMF
4 2) 60 0C
NON
Br N
BnO
~ CN
Pd/C, H2, r.t.
N-N IINII-N
Br + N N
I \
HO IN ~aCN HO / \ O~\/
~ CN
STX300 80% STX291 20%
H2NSO2CI / DMA
N -N 1
N + N
fN
H2NO2SO :L~-O/ IN \CN H2NO2SO-a / ~O~
~ ~ CN
STX301 80% STX292 20%
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
179
Compounds of Formula IV
Bis-(4-Benzyloxyphenyl)methanone (LWO02007A)
O
/ I I \
BnO OBn
To a solution of 4,4'-dihydroxybenzophenone (5.0 g, 23.34 mmol) in anhydrous
DMF
(150 mL) at 0 C was added sodium hydride (60% in mineral oil, 2.1 g, 51.35
mmol), in
two portions. After stirring for 20 min at which no more evolution of hydrogen
was
observed, benzyl bromide (8.96 g, 51.35 mmol) was added. The resulting yellow
suspension was then stirred under an atmosphere of nitrogen at 100 C for 1 h.
Upon
cooling to room temperature, water (500 mL) was added to the suspension and
the
precipitate that formed was filtered and washed exhaustively with water. After
air-drying
overnight at room temperature, the white solid (10.1 g) that collected was
recrystallised
from hot toluene to give LWO02007A as white flaky plate crystals (8.92 g,
22.61 mmol,
96.9%); m.p. 188-190 C.
Bis-(4-Benzyloxyphenyl)methanol (LWO02018)
OH
BnO OBn
To a solution of LWO02007A (3.50 g, 8.873 mmol) in anhydrous THE (250 mL) at 0
C
was added a suspension of lithium aluminium hydride (95%, 425 mg, 10.65 mmol)
in
anhydrous THE (20 mL). After stirring for 30 min at room temperature, the grey
suspension/mixture was concentrated and ethyl acetate (200 mL) was added the
wet
residue that obtained. The organic layer was washed with 1M HCl (200 mL), then
brine
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
180
(4 x 100 mL), dried (MgSO4), filtered and evaporated to give LWO02018 as a
white/pale
yellow residue (3.56 g); m.p. 113-120 C; 5H (400MHz, DMSO-d6) 5.06 (4H, s,
OCH2),
5.58 (1H, d, J4.3 Hz, CH), 5.67 (1H, d, J4.3 Hz, exchanged with D20, OH), 6.93
(4H,
AA'BB'), 7.23 (4H, AA'BB') and 7.38 (1OH, in, Bn).
1-[Bis-(4-Benzyloxyphenyl)methyl]-lH-[1,2,4]triazole (LWO02019)
11
N /N
BnO OBn
1o A mixture of LWO02018 (3.26 g, 8.222 mmol) and 1H-1,2,4-triazole (695 mg,
9.866
mmol) in toluene (350 mL) in the presence of p-toluenesulphonic acid (650 mg)
was
heated under Dean Stark conditions overnight. After cooling to room
temperature and
evaporation of solvent, the light yellow residue that obtained was dissolved
in ethyl
acetate (300 mL). The organic layer was washed with 1M NaOH (2 x 100 mL), then
brine (3 x 50 mL), dried (MgSO4), filtered and evaporated to give a light
yellow/brown
residue (3.36 g). This crude was dissolved in hot ethyl acetate (30 mL) and
hexane (15
mL) was added portionwise. Upon cooling to room temperature, LWO02019A was
obtained as yellow crystals (2.50 g, 5.586 mmol, 68%); m.p. 134-137 C; Sx
(400MHz,
DMSO-d6) 5.09 (4H, s, 2 x OCH2), 6.94 (1H, s, CH), 7.01 (4H, AA'BB'), 7.14
(4H,
AA'BB'), 7.29-7.47 (10H, in, Bn), 8.03 (-1H, s, C3'-H) and 8.53 (1H, s, C5'-
H); LRMS
(FAB+) 447.3[17, M+], 379.3[100, (M-triazole)+], 288,2[8, (M-triazole-Bn)+],
91.1[75,
Bn+]; LRMS (FAB-): no peak was observed; HRMS (FAB+) 447.19588 C29H25N302
requires 447.19468. Found: C 77.6, H 5.63, N 9.26; C29H25N302 requires C
77.83, H
5.63, N 9.37.
1-[Bis-(4-Hydroxyphenyl)methyl]-1H-[1,2,4]triazole (LWO02020, STX267)
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
181
11
N STX267
/ I I \
HO OH
To a solution of LWO02019 (1.50 g, 3.356 mmol) in distilled THE (50 mL) was
added
methanol (30 mL) and Pd/C (10%, 75 mg). The black suspension was stirred at
room
temperature under an atmosphere of hydrogen (balloon) over weekend. After
removal by
filtration and exhaustive washings of the supported catalyst with distilled
THF, the
filtrate was evaporated to give a frothy light yellow residue (839 mg, 3.319
mmol,
93.5%). This crude was dissolved in hot THE (15 mL) and hexane (10 mL) was
added
portionwise. Upon cooling, LWO02020A was obtained as white crystals (483 mg);
m.p.
l0 230 C; 8H (400MHz, DMSO-d6) 6.73 (4H, AA'BB'), 6.79 (1H, s, CH), 6.99 (4H,
AA'BB'), 8.01 (-1H, s, C3'-H), 8.45 (1H, s, C5'-H) and 9.52 (-2H, s, exchanged
with
D20, 2 x OH); &2 (100.4 MHz, DMSO-d6) 65.2 (d, CH), 115.3 (d, Ar), 129.3 (d,
Ar),
129.9 (s, Ar), 144.0 (d, C5'), 151.8 (d, C3'), 157.1 (s, Ar-OH). Found: C
67.2, H 5.08, N
15.4; C15H13N302 requires C 67.4, H 4.90, N 15.72.
1-[Bis-(4-sulfamoyloxyphenyl)methyl]-1H-[1,2,4]triazole (LWO02021, STX268)
11
/N
NN
STX268
/ I I \
H2NO2SO OSO2NH2
To a solution of LWO02020 (257 mg, 1.336 mmol) in NN-dimethylacetamide (20 mL)
at room temperature under an atmosphere of nitrogen was added sulfamoyl
chloride in
toluene (ca. 0.68 M, 7.8 mL). After stirring the reaction mixture overnight,
it was diluted
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
182
with ethyl acetate (100 mL). The organic layer that separated was washed with
brine
(100 mL, 4 x 50 mL), dried (MgSO4), filtered and evaporated to give a light
brown
syrup/residue (612 mg). This crude was purified by flash chromatography (ethyl
acetate)
and the second fraction that collected gave LWO02021A as white residue (310
mg, 728.7
,umol, 54.5%); m.p. 70-85 C; 9x (400MHz, DMSO-d6) 7.19 (1H, s, CH), 7.30 (4H,
AA'BB'), 7.36 (4H, AA'BB'), 8.03 (-4H, br s, exchanged with D20, H2NSO2), 8.10
(~-1H, s, C3'-H) and 8.63 (1H, s, C5'-H); LRMS (FAB+): 851.2[6, (2M+H)+],
579.2[10,
(M+H+NBA)+], 426.2[60, (M+H)+], 357.2[l 00, (M-triazole)+]; (FAB-): 849.1 [16,
(2M-
H)], 578.1[35, (M+NBA)-], 424.1[100, (M-H)], 345.2[25, (M-H2NS02)-]; HRMS
(FAB+) 426.05452 C15H16N506S2 requires 426.05420.
1-[Bis-(3-bromo-4-hydroxyphenyl)methyl]-1H-[1,2,4]triazole (JRL01105, STX356)
N STX356
Br Br
\ I I /
HO OH
To a stirred solution of 1-[Bis-(4-hydroxyphenyl)methyl]-1H-[1,2,4]triazole
(STX267,
500 mg, 1.87 mmol) in CH2C12/MeOH 1:1 (40 ml) at-78'C under nitrogen, a
solution of
benzyltrimethylammonium tribromide (1.49 g, 3.74 mmol) in CH2Cl2/MeOH 1:1 (10
ml)
was added dropwise over 45 min. The orange mixture was kept at 0 C for 7 h and
then at
room temperature overnight, at which time the solution had become colorless.
The
reaction mixture was evaporated and the residue that obtained was dissolved in
a mixture
water (100 mL) and EtOAc (100 mL). The aqueous layer was separated and was
further
extracted with EtOAc (2 x 50 mL). The organic extracts were combined and
washed
with brine, dried (Na2S04) and evaporated. The crude product was fractionated
by flash
chromatography (hexane/ethyl acetate, 1:3) and the second fraction that
collected gave
JRL01105 (STX356) (485 mg) as white solid at about 97% purity; Sll (400MHz,
DMSO-
d6) 6.90 (1H, s, CH), 6.93 (2H, d, J 8.6 Hz), 7.03 (2H, dd, J 2.1 and 8.6 Hz),
7.30 (2H, d,
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
183
J2.1 Hz), 8.06 (1H, s), 8.56 (1H, s) and 10.45 (2H, br s, exchanged with D20,
2 x OH).
The main impurity was the mono-brominated derivative of STX356. A small
quantity of
this fraction was further purified by semi-preparative HPLC (Waters PrepLC RP
18, 25 x
mm, flow rate: 10 mL/min, mobile phase: McOH/H2O, 60:40). The fraction with a
5 retention time of 5.5 min was collected and upon evaporation gave a white
solid; m.p.
198-205 C (dec.). Found: C 42.2, H 2.65, N 9.79; C15H11Br2N3O2 requires C
42.38, H
2.61, N 9.89.
1tiT[Bis-(3-bromo-4-sulfamoyloxyphenyl)methyl]-1H-[1,2,4]triazole (JRLO 1109,
10 STX566)
11
N STX566
Br / I \ Br
\ I /
H2NO2SO OS02NH2
To a solution of JRLO1105 (175 mg, 412 ,umol) in N,N-dimethylacetamide (15 mL)
at
room temperature under an atmosphere of nitrogen was added sulfamoyl chloride
(4.4
eq.). After stirring the reaction mixture overnight, it was diluted with ethyl
acetate (30
mL) and the resulting mixture was washed with brine (50 mL, 4 x 20 mL), dried
(MgSO4), filtered and evaporated to give JRLO1109 as a pale yellow residue
(210 mg);
m.p. 98 -102 C; 6(270MHz, DMSO-d6) 7.21 (1H, s, CH), 7.38 (2H, dd, J2.1 and
8.4
Hz), 7.55 (2H, d, J 8.4 Hz), 7.66 (2H, d, J 2.1 Hz), 8.15 (1 H, s), 8.32 (4H,
br s) and 8.69
(1H, s); LRMS (FAB+): 584.0[10, (M+H)+], 515.0(10), 391.0[100, (M-2H2NSO2O)+];
HRMS (FAB+): 583.87219 C15H14Br2N506S2 requires 583.87318.
1-[Bis-(3,5-dibromo-4-sulfamoyloxyphenyl)methyl]-1H-[1,2,4]triazole
(LWO02128A,
STX414)
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
184
N N
Br Br STX414
I I
HO OH
Br Br
1-[Bis-(4-hydroxyphenyl)methyl]-1H-[1,2,4]triazole (450 mg, 1.684 mmol) was
dissolved in hot acetic acid (100 mL). Upon cooling to ice/water temperature,
potassium
acetate (3.3 g, 33.67 mmol) was added to the yellow mixture followed by a
solution of
bromine in acetic acid dropwise (1.1 g/10 mL acetic acid, 7.5 mL, 5.061 mmol)
over a
period of 30 min. After stirring for another 30 min, water (20 mL) was added
to the pale
yellow gel/solid that resulted and the whole mixture was evaporated to give a
wet pale
yellow/beige residue. This crude was diluted with ethyl acetate (150 mL) and
the blue
green organic layer was washed with brine (4 x 100 mL), dried (MgSO4),
filtered and
evaporated to give a slightly wet yellow residue (1.2 g). Upon standing in the
round-
bottomed flask unstoppered at room temperature overnight, a yellow/brown
residue (850
mg) was obtained which upon trituration with acetone (10 mL) gave yellow
deposits.
After filtration and washing with more acetone, the pale yellow powder
collected was
air-dried to give LWO02128A (205 mg, 21%); m.p. 223-235 C (dec.); 1H (400
MHz,
CDC13) 6.97 (1H, s, CH), 7.40 (4H, s, Ar), 8.11 (1H, s, triazole-H), 8.64 (1H,
s, triazole-
H) and 10.23 (2H, br s, exchanged with D20, 2 x OH); LRMS (FAB+) 584.0 [48,
(M+H)+], 513.0 [68, (M-triazole)+], 427.4 (95), 260.1 (88), 193.2 [100, ((M-
4Br -
triazole)+]; LRMS (FAB-) 579.8 (100, M-), 455.1 (40), 276.1 (70), 195.1 (60);
HRMS
(FAB-) 579.73119, C15H879Br381BrN3O2 requires 579.73295.
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
185
Synthesis of STX267 and STX268
0 0
1) NaH/DMF
I \ I \ 2) BnBr
HO OH BnO OBn
LiA1H4/THF
OH
C\N
N
H
BnO OBn
Toluene/p-TSA/reflux
N
STX267 N Pd/C, H2 N
HO OH BnO OBn
H2NSO2C1 / DMA
C'\.
STX268 N
H2NO2SO OSO2NH2
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
186
Compounds of Formula V
4-Benzyloxybenzyl chloride (LWO02011)
CI
Bn0/ \
To a solution of 4-benzyloxybenzyl alcohol (5.0 g, 22.64 mmol) in
dichloromethane (100
mL) at ice/water temperature was added dropwise thionyl chloride (2.5 mL,
33.96
mmol). The resulting clear pink/red solution was stirred at 0 C for another
40 min
before being evaporated to give a light green/yellow residue. After co-
evaporation of the
crude with dichloromethane three times, a creamy residue (5.77 g) was obtained
which
was dissolved in hot toluene (3 mL) and treated portionwise with hexane (60
mL). Upon
standing at room temperature, LWO02011A was obtained as white crystals (2.89
g,
12.42 mmol). A second crop (LWO02011B, 1.41 g, 6.06 mmol, total yield: 81.6%)
of
the product was obtained from the residue of the mother liquor of the first
crop after it
has been recrystallised from hot hexane (20 mL); m.p. 74-80 C; 8H (400MHz,
DMSO-
d6) 4.72 (2H, s, CH2C1), 5.11 (2H, s, OCH2), 7.01 (2H, in, Ar) and 7.39 (7H,
in, Ar).
1-(4-Benzyloxybenzyl)-1H-[1,2,4]triazole (A) and 4-(4-Benzyloxybenzyl)-4H-
[1,2,4]triazole (B) (LWO02013)
~N ~N\
/ + !1
N N N
BnO / \ (A) BnO / \ (B)
A suspension of (1H)-1,2,4-triazole (890 mg, 12.89 mmol), 4-benzyloxybenzyl
chloride
(2.0 g, 8.594 mmol), anhydrous potassium carbonate (1.19 g) in N,N-
dimethylformamide
(20 mL) was stirred at 90-95 C for 4 h under an atmosphere of nitrogen. After
cooling
to room temperature, the reaction mixture was diluted with ethyl acetate (100
mL) and
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
187
washed with 1M sodium hydroxide (150 mL) and then brine (4 x 50 mL). The
organic
layer was then dried (MgSO4), filtered and evaporated to give a white residue
(2.16 g).
This crude was dissolved in hot ethyl acetate (15 mL) and hexane was added
portionwise
to the resulting solution. Upon cooling, LWO02013 was obtained as white
crystals (1.34
g, 5.051 mmol). 1H NMR has suggested LWO02013 contains a 1 : 1 mixture of the
above isomers (A and B).
4;-[1,2,4]Triazol-1-ylmethylphenol (LWO02015A, STX269)
N
NiN
HO \ /
STX269
To a solution of LWO02013 (1.25 g, 4.711 mmol) in distilled THE (50 mL) was
added
absolute ethanol (10 mL) and Pd/C (10%, 70 mg). The resulting black suspension
was
stirred at room temperature for 72 h under an atmosphere of hydrogen
(balloon). After
removal by filtration and washings of the supported catalyst exhaustively with
distilled
THF, the filtrate was evaporated to give a white residue (580 mg) which was
recrystallised from acetone/hexane to give LWO02015A as off white crystals
(298 mg,
1.701 mmol). A second crop (LWO02015B, 77 mg, 439.5 umol, total yield: 45.4%)
of
the product was obtained from the residue of the mother liquor when it was
recrystallised
in the same manner; m.p. 145-148 C [Lit.' 143-146 C (chloroform/Pet ether)];
9H
(400MHz, DMSO-d6) 5.26 (2H, s, CH2), 6.73 (2H, in, Ar), 7.13 (2H, m, Ar), 7.94
(1H, s,
C3'-H), 8.58 (1H, s, C5'-H) and 9.50 (1H, s, exchanged with D20, OH); LRMS
(FAB+):
351.2[10, (2M+H)+], 176.2[100, (M+H)+], 107.1[47, (M-Triazole)+], (FAB-):
481.3[30,
(M+2NBA)"], 349.2[27, (2M-H)-], 328.2[l 00, (M+NBA)-], 221.2(23), 174.2[l 00,
(M-H)-
];
HRMS (FAB+) 176.08183 C9H10N30 requires 176.08239. Found: C 61.6, H 5.11, N
23.6; C9H9N30 requires C 61.70, H 5.18, N 23.99.
1 Abdreubu et. Al. (1989) Farmaco 44(9) 831-842.
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
188
Sulfamic acid 4-[1,2,4]triazol-1-ylmethylphenyl ester (LWO02017A, STX270)
N
NON
STX270
H2NO2SO
5i
To a solution of LWO02015A (150 mg, 856.2 pmol) in distilled THE (10 mL) at 0
C
was added sodium hydride (60% in mineral oil, 36 mg, 899,umol). After stirring
for 10
min at which no more evolution of hydrogen was observed, sulfamoyl chloride in
toluene
(ca. 0.68M, 2.5 mL) was added under an atmosphere of nitrogen and the
resulting thin
white suspension was stirred at room temperature for 3 h. The reaction mixture
was then
diluted with ethyl acetate (70 mL) and washed with brine (100 mL, 3 x 50 mL).
The
organic layer that separated was dried (MgSO4), filtered and evaporated to
give a white
residue (144 mg) which was recrystallised from acetone/hexane to give
LWO02017A as
white crystals (71 mg, 279.2 ,umol, 32.6%); vm (KBr) 3352, 3127, 2880, 2646,
1509,
1369, 1183, 1158 cm 1; 9x (400MHz, DMSO-d6) 5.44 (2H, s, CH2), 7.27 (2H,
AA'BB'),
7.38 (2H, AA'BB'), 8.0 (-2.7H, m, reduced to one proton, singlet, after D20
exchange,
C3'-H and H2NSO2) and 8.68 (1H, s, C5'-H); Found: C 42.6, H 4.01, N 21.7;
C9H10N403S requires C 42.51, H 3.96, N 22.03.
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
189
Synthesis of STX269 and STX270
CI
Bn0 +
N
H
K2C03/DMF
N N N
N-N - N
IBnO \ / + BnO \ /
Pd/C, H2
N
N-N STX269
HO \ /
H2NSO2Cl / THE
N
H2NO2SO N-N STX270
\ /
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
190
BIOLOGICAL DATA
Compounds were tested for aromatase and steroid sulphatase inhibition in
accordance
with the above Protocols. Each compound in accordance with the present
invention is
found to inhibit steroid sulphatase and aromatase.
The following in vitro data were recorded.
',compound Structure AROMATASE SULPHATASE
ICso nM ICso nM
+H340F1 H340F1 (59% inhibition 10 M
at 10 M)
ICso > 10 M
OS02NH2
H342F1 H342F1 (No inhibition 90
seen)
H2NSO2
OS02NH2
STX258 N 100 227
NJ
NHZ
STX265 <1 N .N 23 n.d.
NJ
N
HO I / / \
\N
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
191
Compound Structure AROMATASE SULPHATASE
ICso (nM) IC5o (nM)
STX 268 N- 3044 >10000
I (31%at10
N M)
HZN\ ~j I \ I \ \\
0 HZ
STX269 N- 41% inhibition n.d.
I/ \\ at 10 M
N /N
HO /
STX270 N- 62% inhibition 14% inhibition at
/\ N at101M 10 M in
placental
microsomes
o
O
H2N-l 0
STX273 69% inhibition at n.d.
0.1 M
/NHZ
\
STX 287 N- N 4.4 n.d.
HO
STX 288 KJ"~,N 31 >10 M
N
O' \ ^ /
V \iN
NH2 \ / I
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
192
Compound Structure AROMATASE SULPHATASE
ICSo (nM) IC5o (nM)
STX 290 <N--N 1.3 n.d.
N-il
I
J1 N
STX 291 N-N 26 n.d.
{ HO N/\\
STX 292 jj ; 767 >10 M
HzN~ \\
O \ N I \
STX 300 "-N 1.6 n.d.
Br l/ ~I1
a N
N
N
STX 301 i 119 n.d.
0~ N
O & I O^/N
STX 310 0 (42% inhibition n.d.
OH at 10 M)
/ I \
ICso> 10 M
0
STX 566 ()N 4.9 476
N
Br \ Br
z
HzN HN
/S\0 I / 01-11
0/ \\
CA 02464770 2004-04-26
WO 03/045925 PCT/GB02/05214
193
Compound Structure AROMATASE SULPHATASE
IC5o (nM) IC5o (nM)
STX 597 N-\ 0.51 >10000
N
S v v
H2N
o N
STX 636 N ~-N 9 >10000
1- (6.6% at 10
M)
N
/ \C
-N
N
STX 681 /N 0.82 39
N _1I
N Br
O
MO-S-NH2
N
STX 694 "-" 2.3 20
0
H2N~
0
CI \ I \
N
STX 699 /j-N 0.73. 1000
FIaN \ S
O
STX 700 N,-" 12 40
0
0
H2N~
F
CA 02464770 2009-11-27
WO 03/045925 PCT/GB02/05214
194
Compound Structure AROMATASE SULPHATASE
Moo nM [Cook M
STX 732 N 19 >10000
JIN (12%at10
&M)
N
Br
0
In vivo data were recorded using the above described aromatase and STS animals
assays. The relevant compounds were administered and for each animal both
aromatase and STS activities were determined. The data are shown In Figures 3
and 4.
Various modifications and variations of the present invention will be apparent
to
those skilled in the art without departing from the scope and spirit of the
invention.
Although the invention has been described in connection with specific
preferred
embodiments, it should be understood that the invention as claimed should not
be
unduly limited to such specific embodiments. Indeed, various modifications of
the
described modes for carrying out the invention which are obvious to those
skilled
in chemistry, biology or related fields are intended to be within the scope of
the
following claims.