Note: Descriptions are shown in the official language in which they were submitted.
DEMANDES OU BREVETS VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVETS
COMPREND PLUS D'UN TOME.
CECI EST LE TOME 1 DE 3
NOTE: Pour les tomes additionels, veillez contacter le Bureau Canadien des
Brevets.
JUMBO APPLICATIONS / PATENTS
THIS SECTION OF THE APPLICATION / PATENT CONTAINS MORE
THAN ONE VOLUME.
THIS IS VOLUME OF
NOTE: For additional volumes please contact the Canadian Patent Office.
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
BENZIMIDAZOLES AND ANALOGUES AND THEIR USE AS PROTEIN KINASES INHIBITORS
This invention is directed to benzimidazoles of formula (Ix), their
preparation, pharmaceutical
compositions containing these compounds, and their pharmaceutical use in the
treatment of disease
states capable of being modulated by the inhibition of the protein kinases.
Such protein kinases belong
especially to the following group: EGFR, Fak, FLK-1, FGFRl, FGFR2, FGFR3,
FGFR4, FGFR5, flt-1,
IGF-1R, KDR, PDGFR, tie2, VEGFR, ITK and SYK.
Protein kinases are a family of enzymes that participate in the signalling
events which control the
activation, growth and differentiation of cells in response to extracellular
mediators and to changes in
the etivironment. In general, these kinases fall into several groups; those
which preferentially catalyse
the phosphorylation of hydroxy groups of serine and/or threonine residues and
those which
preferentially catalyse the phosphorylation of hydroxy grbups of tyrosine
residues [S.K.Hanks and
T.Hunter, FASEB. J., 1995, 9, pages 576-596]. Such phosphorylations may
greatly modify the
function of the proteins; thus, protein kinases play an important role in
regulating a wide variety of cell
processes including, especially, metabolism, cell proliferation, cell
differentiation or cell survival.
Ainong the various cellular functions in which the activity of a kinase
protein is involved, certain
processes represent attractive targets for treating certain diseases. As an
example, mention may be
made especially of angiogenesis and the control of the cell cycle, in which
kinase proteins can play an
essential role. These processes are essential for the growth of solid tumours
and also for other diseases.
Angiogenesis or the formation of new blood vessels by sprouting from the
preexisting vasculature is of
central importance for embryonic development and organogenesis. Should the
need arise, the vascular
system has the potential to generate a network of new vessels so as to
maintain the correct functioning
of the tissues and organs. Angiogenesis is a complex multistage process which
includes activation,
migration, proliferation and survival of endothelial cells. In adults,
angiogenesis is fairly limited,
appearing mainly only in the processes of repair after an injury or of
vascularization of the
endometrium. (Merenmies et al., Cell Growth & Differentiation, 8, 3-10, 1997).
However, uncontrolled
angiogenesis is found in certain pathologies such as retinopathy, psoriasis,
rheumatoid arthritis,
diabetes, muscle degeneration or cancer (solid tumours) (Folkman, Nature Med.,
1, 27-31, 1995). The
kinase proteins whose involvement it has been possible to demonstrate in the
angiogenesis process
include three members of the family of growth factor receptor tyrosine
kinases: VEGF-R2 (vascular
endothelial growth factor receptor 2, also known as KDR, kinase insert domain
receptor, or FLK-1),
FGF-R (fibroblast growth factor receptor) and TEK (also known as Tie-2).
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-2-
In conjunction with other systems, the Vascular Endothelial Growth Factor
receptors (VEGFRs)
transmit signals involved in the migration, proliferation and survival of
endothelial cells. The family
VEGFR includes VEGFR-1 (Flt-1), VEGFR-2 (KDR) and VEGFR3 (F1t4). The receptor
VEGF-R2,
which is expressed only in the endothelial cells, binds to the angiogenic
growth factor VEGF, and thus
serves as a transduction signal mediator via the activation of its
intracellular kinase domain. Thus, the
direct inhibition of the kinase activity of VEGF-R2 makes it possible to
reduce the phenomenon of
angiogenesis in the presence of exogenous VEGF (Strawn et al., Cancer
Research, 56, 3540-3545,
1996), this process being demonstrated especially with the aid of VEGF-R2
mutants (Millauer et al.,
Cancer Research, 56, 1615-1620, 1996). The VEGF-R2 receptor appears to have no
other function in
adults than that associated with the angiogenic activity of VEGF. Thus, a
selective inhibitor of the
kinase activity of VEGF-R2 should show only little toxicity.
In addition to this central role in the dynamic angiogenic process, recent
results suggest that the
expression of VEGF contributes towards the survival of tumoral cells after
chemotherapy and
radiotherapy, underlining the potential synergism of KDR inhibitors with other
agents (Lee C.G., Heijn
M. et al., (2000), Cancer Research, 60 (19), 5565-70). The KDR inhibitors thus
especially constitute
anti-angiogenic agents and such agents might be used as a first line treatment
against tlie emergence or
regrowth of malignant tumours. The inhibition or regulation of VEGFR-2 (KDR)
thus provides a
powerful new mechanism of action for the treatment of a large number of solid
tumours.
Extensive studies in the field of tumor angiogenesis in the past two decades
have identified a number
of therapeutic targets including kinases, proteases and integrins resulting in
the discovery of many new
anti-angiogenic agents, including KDR inhibitors some of which are currently
under clinical evaluation
(Jekunen, et al Cancer Treatment Rev. 1997 , 23, pages 263-286.).
The present patent application thus relates particularly to novel inhibitors
of the VEGFR-2 (KDR)
receptor that may be used especially for anti-angiogenic treatment in
oncology.
The protein kinases which preferentially catalyse the phosphorylation of
hydroxy groups of serine
and/or threonine residues include for example, protein kinase C isoforms
[A.C.Newton, J. Biol. Chem.,
1995, 270, pages 28495-28498] and a group of cyclin-dependent kinases such as
cdk2 [J.Pines, Trends
in Biochemical Sciences, 1995, 18, pages 195-197]. The protein kinases which
preferentially catalyse
the phosphorylation of hydroxy groups of serine and/or threonine residues
include membrane-spanning
growth factor receptors such as the epidermal growth factor receptor
[S.Iwashita and M.Kobayashi,
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-3-
Cellular Signalling, 1992, 4, pages 123-132], and cytosolic non-receptor
kinases such as p561ck,
p59fYn, ZAP-70 and csk kinases [C.Chan et. al., Ann. Rev. Immunol., 1994,12,
pages 555-592].
Inappropriately high protein kinase activity has been implicated in many
diseases resulting from
abnormal cellular function. This might arise either directly or indirectly,
for example by failure of the
proper control mechanisms for the kinase, related for example to mutation,
over-expression or
inappropriate activation of the enzyme; or by over- or underproduction of
cytokines or growth factors
also participating in the transduction of signals upstream or downstream of
the kinase. In all of these
instances, selective inhibition of the action of the kinase might be expected
to have a beneficial effect.
SYK (Spleen Tyrosine Kinase) is a 72-kDa cytoplasmic protein tyrosine kinase
that is expressed in a
variety of hematopoietic cells and is an essential element in several cascades
that couple antigen
receptors to cellular responses. Thus, SYK plays a pivotal role in signalling
of the high affinity IgE
receptor, FcgRl, in mast cells and in receptor antigen signalling in T and B
lymphocytes. The signal
transduction pathways present in mast, T and B cells have common features. The
ligand binding
domain of the receptor lacks intrinsic tyrosine kinase activity. However, they
interact with transducing
subunits that contain immunoreceptor tyrosine based activation motifs (ITAMs)
[M.Reth, Nature,
1989, 338, pages 383-384]. These motifs are present in both the (3 and y
subunits of the FcERl, in the
~-subunit of the T cell receptor (TCR) and in the IgGa and IgG (3 subunits of
the B cell receptor
(BCR). [N.S.van Oers and A.Weiss, Seminars in Immunology, 1995, 7, pages 227-
236] Upon binding
of antigen and multimerization, the ITAM residues are phosphorylated by
protein tyrosine kinases of
the Src family. SYK belongs to a unique class of tyrosine kinases that have
two tandem Src homology
2 (SH2) domains and a C terminal catalytic domain. These SH2 domains bind with
high affinity to
ITAMs and this SH2 -mediated association of SYK with an activated receptor
stimulates SYK kinase
activity and localises SYK to the plasma membrane.
In SYK deficient mice, mast cell degranulation is inhibited, suggesting that
this is an important target
for the development of mast cell stabilising agents [P.S.Costello, Oncogene,
1996, 13, pages 2595-
2605]. Similar studies have demonstrated a critical role for SYK in BCR and
TCR signalling
[A.M.Cheng, Nature, 1995, 378, pages 303-306, (1995) and D.H.Chu et al.,
Immunological Reviews,
1998, 165, pages 167-180]. SYK also appears to be involved in eosinophil
survival in response to
IL-5 and GM-CSF [S.Yousefi et al., J. Exp. Med., 1996, 183, pages 1407-1414].
Despite the key role
of SYK in mast cell, BCR and T cell signalling, little is known about the
mechanism by which SYK
transmits downstream effectors. Two adaptor proteins, BLNK (B cell Linker
protein, SLP-65) and
SLP-76 have been shown to be substrates of SYK in B cells and mast cells
respectively and'nave been
postulated to interface SYK with downstream effectors [M.Ishiai et al.,
Immunity, 1999, 10, pages 117-
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-4-
125 and L.R.Hendricks-Taylor et al., J.Biol. Chem, 1997, 272, pages 1363-
1367]. In addition SYK
appears to play an important role in the CD40 signalling pathway, which plays
an important role in B
cell proliferation [M.Faris et al., J.Exp. Med., 1994, 179, pages 1923-1931].
SYK is further involved in the activation of platelets stimulated via the low-
affinity IgG receptor (Fc
gamma-RIIA) or stimulated by collagen [F.Yanaga et al., Biochem. J., 1995,
311, (Pt. 2) pages 471-
478].
ITK, is a T cell specific tyrosine kinase of the Tec family that is required
for normal Th2 function.
Asthma is a disease characterised by increased Th2 cytokine production
including IL-4. An inhibitor of
ITK should therefore have an impact on disease progression in asthma through
inhibition of Th2
cytokine production.
We have now found a novel group of benzimidazoles, which have valuable
pharmaceutical properties,
in particular, the ability to inhibit protein kinases, more particularly, the
ability to inhibit the protein
kinase SYK, the protein kinase KDR, the protein kinase tie2 or the protein
kinase ITK.
Thus, in one aspect, the present invention is directed to pharmaceutical
compositions comprising
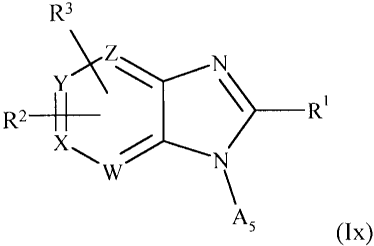
compounds of general formula (Ix):-
Y N
II R
X~
W N
A5
(Ix)
wherein, for the purposes of (Ix):-
X represents C-R2 and W, Y and Z, which may be identical or different,
represent CH or CR3; or
W represents CH, X represents N, Y represents CH or CR3, and Z represents CH
or CR3; or
W represents N, X represents CH or CR2, Y represents CH and CR3, and Z
represents CH or CR3; or
W represents N, X represents CH or CR2, Y represents N, and Z is CH or CR3; or
W represents N, X represents CH or CR2, Y represents CH or CR3, and Z
represents N; or
W represents N, X represents N, Y represents CH or CR3, and Z represents CH or
CR3;
A5 represents H or alkyl;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-5-
Rl represents aryl or heteroaryl, each optionally substituted by one or more
groups selected from
carboxy, cyano, halo, haloalkyl, hydroxy, nitro, R4, -C(=O)R4, -C(=O)NYlY2, -
C(=O)OR4,
-N(R6)C(=O)R4, -N(R6)C(=O)NYIY2, -N(R6)C(=O)OR4, -N(R6)S02R4, -N(R6)SO2NYlY2,
-NYlY2, -OR4, -OCF2H, -OCF3, -OC(=O)R4, -OC(=O)NYlY2, -OS(O)nR4, -S(O)nR4,
-S(O)nNYlY2 and -S(O) nOR4;
R2 and R3 are such that:
R2 and R3, which may be identical or different, represent H, carboxy, cyano,
halo, haloalkyl, hydroxy,
nitro, R4, -C(=O)R4, -C(=O)NYlY2, -C(=O)OR4, -NNYlY2, -N(R6)C(=O)R4, -
N(R6)C(=0)NYlY2,
-N(R6)C(=O)OR4, -N(R6)SO,R4, -N(R6)SO2NYlY2, -OR4, -OCF2H, -OCF3, -OC(=O)R4,
-OC(=0)NYlY2, -S(O)nR4, -S(O)nNYlY2 or -S(O) nOR4; or
R2 represents H, carboxy, cyano, halo, haloalkyl, hydroxy, nitro, R4, -
C(=O)R4, -C(=0)NYlY2,
-C(=O)OR4, -NYlY2, -N(R6)C(=O)R4, -N(R6)C(=O)NYlY2, -N(R6)C(=O)OR4, -
N(R6)SO,,R4,
-N(R6)SO2NY1Y2, -OR4, -OCF2H, -OCF3, -OC(=0)R4, -OC(=0)NYlY2, -S(O)nR4, -
S(O)nNYlY2
or -S(O) nOR4 and R3 represents allcyl, haloalkyl, halogen and OR6; or
R2 and R3 groups on adjacent carbon atoms may form a 5- to 6-membered carbon-
based ring
containing one or more heteroatoms, which may be identical or different,
chosen from 0, N and S, and
which may be optionally substituted by alkyl [examples include those where 0
and R3 form a group
selected from -O-CH2-O-, -O-CH2-CH2-O-; -CH2-O-CH2-, -CH2-N(R14)-CH2-, -CH2-
CH2-CH2-,
-CH2-C(CH3)2-CH2-, -CH2-O-CH2-CH2-, -CH2-N(R14)-CH2-CH2-, -CH2-CH2-CH2-CH2-,
-CH2-C(CH3)2-CH2-CH2-, -CH=CH-CH=CH-, -N=CH-CH=CH-, -CH=N-CH=CH-, -CH=CH-N=CH-
or -CH=CH-CH=N, in which R14 is H or a1ky1)];
R4 is alkyl, alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl or
heteroaryl, each optionally
substituted with one or more substituents selected from alkyl, aryl,
cycloalkyl, heteroaryl,
heterocycloalkyl, halo, hydroxy, hydroxyalkyl, -C(=O)NY3Y4, -C(=0)OR6, -
N(R6)C(=0)NYlY2,
-NYlY2, -OR5 or alkyl substituted by -NY3y4;
R5 is alkyl, alkenyl, aryl, arylalkyl, cycloalkyl, cycloalkylalkyl,
heteroaryl, heteroarylalkyl,
heterocycloalkyl or heterocycloalkylalkyl;
R6 is chosen from the values of R5;
n is zero or an integer 1 or 2;
Yland Y2 are independently hydrogen, alkenyl, aryl, cycloalkyl, heteroaryl,
heterocycloalkyl,
heterocycloalkylalkyl or alkyl optionally substituted by one or more groups
selected from cyano, aryl,
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-6-
heteroaryl, hydroxy, -C(=O)OR6, -C(=O)NY3y4, -N-y3y4 or -OR5, or the group -
NYlY2 may form a
cyclic amine;
Y3 and Y4 are independently hydrogen, alkenyl, alkyl, aryl, arylalkyl,
cycloalkyl, heteroaryl or
heteroarylalkyl; or the group -NY3Y4 may form a cyclic amine;
all the alkyl (or alk, which represents alkyl), alkenyl, cycloalkyl,
heterocycloalkyl, aryl, arylalkyl,
heteroaryl and heteroarylalkyl radicals present in the above radicals
furthermore being optionally
substituted with one or more radicals chosen from halogen atoms and hydroxyl,
cyano, alkyl, alkoxy,
acylamino (NH-COalk), -C(=O)OR6, -C(=O)R6, hydroxyalkyl, carboxyalkyl, S(O)n-
alk, S(O)n-NH2,
S(O)n NH(alk), S(O)ri N(alk)2, CF3, OCF3, NO2, arylalkoxy, aryl, heteroaryl,
aryloxy, aryloxyalkyl,
-C(=O)-NY3Y4 and NY3Y4 radicals, the latter radicals containing alkyl, aryl
and heteroaryl being
themselves optionally substituted with one or more radicals chosen from
halogen atoms and alkyl
radicals, free, salified or esterified carboxyl radicals and acylamino
radicals NH-C(O)R5;
and their corresponding N-oxides, and their prodrugs, and their acid
bioisosteres; and pharmaceutically
acceptable salts and solvates (e.g. hydrates) of such compounds and their N-
oxides and their prodrugs,
and their acid bioisosteres; together with one or more pharmaceutically
acceptable carriers or
excipients.
In another aspect, the invention concerns the compounds of formula (Ix) as
defined above wherein Rl
R9
R 8
~
is a pyrazolyl moiety in which R7 is hydrogen or alkyl, and R8 and R9 are
N~N R~
independently selected from hydrogen, carboxy, cyano, halo, haloalkyl,
hydroxy, nitro, R4, -C(=O)R4,
-C(=O)NYlY2, -C(=O)OR4, -N(R6)C(=O)R4, -N(R6)C(=O)NY1Y2, -N(R6)C(=O)OR4,
-N(R6)SO2R4, -N(R6)SO2NYlY2, -NYlY2, -OR4, -OC(=O)R4, -OC(=O)NYlY2, -S(O)nR4
and
-S(O)2NYlY2; or R8 and R9 together with the carbon atoms to which they are
attached form (i) a 5 to
8 membered carbocyclic ring optionally substituted by one or more carbocyclic
ring substituents; (ii) a
phenyl ring optionally substituted by one or more aryl group substituents;
(iii) a 5 or 6 membered
heteroaromatic ring in which one or more of the ring members is/are nitrogen,
oxygen or sulfur
(examples of such groups include furyl, imidazolyl, isoxazolyl, isothiazolyl,
oxadiazolyl, pyrazinyl,
pyridazinyl, pyrazolyl, pyridyl, pyrimidinyl, pyrrolyl, 1,3,4-thiadiazolyl,
thiazolyl, thienyl and triazolyl
groups) and which is optionally substituted by one or more groups selected
from haloalkyl, hydroxy,
halo, cyano, nitro, R4, -C(=O)NYlY2, -N(R6)C(=O)R4, -N(R6)C(=O)NYlY2, -
N(R6)S02R4,
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-7-
-NYlY2 and -OR5; or (iv) a 5 or 6 membered heterocyclic ring optionally
substituted by alkyl or oxo,
and containing a heteroatom-containing group selected from 0, S, SO2, or NY5
(where Y5 is
hydrogen, R4, -C(=O)R4, -C(=O)NYlY2, -C(=O)OR4 or -S02R4); but excluding the
compounds:
2-(2H-pyrazol-3-yl)-1H-benzoimidazole; 2-(5-methyl-2H-pyrazol-3-yl)-1H-
benzoimidazole; 5-methyl-
6-[2-(2H-pyrazol-3-yl)-3H-benzoimidazol-5-yl]-4,5-dihydro-2H-pyridazin-3-one;
5-methyl-6-[2-(2H-
pyrazol-3 -yl)- 1 H-benzoimidazol-4-yl] -4,5 -dihydro-2H-pyridazin-3 -one; 3,5-
bis(benzimidazol-2-yl)-1H-
pyrazole; 5,6-dimethyl-2-(5-methyl-lH-pyrazol-3-yl)-1H-benzoimidazole; 6-
methyl-2-(5-methyl-lH-
pyrazol-3-yl)-1H-benzoimidazole; 5,6-dichloro-2-(5-methyl-lH-pyrazole-3-yl)-1H-
benzoimidazole; 5-
nitro-2-(5-methyl-lH-pyrazole-3-yl)-1H-benzoimidazole; 2-(5-methyl-lH-pyrazole-
3-yl)-1H-
benzoimidazole-5-carboxylic acid; 2-(5-phenyl-lH-pyrazole-3-yl)-1H-
benzoimidazole; 5,6-dimethyl-2-
(5-phenyl-lH-pyrazole-3-yl)-1H-benzoimidazole; 5-methyl-2-(5-phenyl-lH-
pyrazole-3-yl)-1H-
benzoimidazole; 6-chloro-2-(5-methyl-lH-pyrazole-3-yl)-1H-benzoimidazole; 5-
chloro-2-(5-phenyl-
1H-pyrazole-3-yl)-1H-benzoimidazole; 5,6-dichloro-2-(5-phenyl-lH-pyrazole-3-
yl)-1H-
benzoimidazole; N-[2-(5-isoquinolin-4-yl-lH-indazol-3-yl)-3H-benzoimidazol-5-
yl]-
methanesulfonamide; 3-(1H-benzoimidazol-2-yl)-5-(1H-indazol-4-yl)-1H-indazole,
3-[3-(1H-
benzoimidazol-2-yl)-1H-indazol-5-yl]-2-methoxyphenol; 4-[3-(1H-benzoimidazol-2-
yl)-1H-indazol-5-
yl]isoquinoline; 4-{3-[6-(4-methyl-piperazin-1-yl)-1H-benzoimidazol-2-yl]-1H-
indazol-5-yl}-
isoquinoline; 4-[3-(4-chloro-lH-benzoimidazol-2-yl)-1H-indazol-5-yl]-
isoquinoline; 4-[2-(1H-indazol-
3-yl)-1H-benzoimidazol-5-yl]-phenol; 3-[5-(4-methoxy-phenyl)-1H-benzoimidazol-
2-yl]-1H-indazole;
3-[5-(4-methoxy-phenyl)-1H-benzoimidazol-2-yl]-1H-indazole; 3-[5-(3-methoxy-
phenyl)-1H-
benzoimidazol-2-yl]-1H-indazole; 3-(1H-benzoimidazol-2-yl)-5-phenyl-lH-
indazole; 2-(4-bromo-l-
methyl-lH-pyrazol-3-yl)-1H-benzoimidazole; 2-(5-tert-butyl-lH-pyrazol-3-yl)-1H-
benzoimidazole; 3-
(1H-benzoimidazol-2-yl)-6-(3-methoxy-phenyl)-1H-indazole; 3-(1H-benzoimidazol-
2-yl)-1H-indazole-
6-carboxylic acid; 5-{[3-(1H-benzoimidazol-2-yl)-1H-indazole-6-carbonyl]-
amino}-2-hydroxy-benzoic
acid methyl ester; 5-{[3-(1H-benzoimidazol-2-yl)-1H-indazole-6-carbonyl]-
amino}-f-uran-2-carboxylic
acid methyl ester; 3-(1H-benzoimidazol-2-yl)-1H-indazole-6-carboxylic acid (3-
hydroxy-4-methoxy-
phenyl)-amide; 3-(1H-benzoimidazol-2-yl)-1H-indazole-6-carboxylic acid (5-
hydroxy-lH-pyrazol-3-
yl)-amide; 3-(1H-benzoimidazol-2-yl)-1H-indazole-6-carboxylic acid (1H-pyrazol-
3-yl)-amide; [3-(1H-
benzoimidazol-2-yl)-1H-indazol-6-yl]-[4-(2-hydroxy-ethyl)-piperidin-l-yl]-
methanone; 3-(1H-
benzoimidazol-2-yl)-1H-indazole-6-carboxylic acid (9H-purin-6-yl)-amide; 3-(1H-
benzoimidazol-2-yl)-
1H-indazole-6-carboxylic acid dimethylamide; [3-(1H-benzoimidazol-2-yl)-1H-
indazol-6-yl]-
morpholin-4-yl-methanone; 3-(1H-benzoimidazol-2-yl)-1H-indazole-6-carboxylic
acid pyrazin-2-
ylamide; 3-(1H-benzoimidazol-2-yl)-1H-indazole-6-carboxylic acid
cyclohexylamide; 3-(1H-
benzoimidazol-2-yl)-1H-indazole-6-carboxylic acid (1H-indazol-5-yl)-amide; [3-
(1H-benzoimidazol-2-
yl)-1H-indazol-6-yl]-pyrrolidin-1-yl-methanone; 3-(1H-benzoimidazol-2-yl)-1H-
indazole-6-carboxylic
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-8-
acid (1H-indazol-5-yl)-amide; [3-(1H-benzoiinidazol-2-yl)-1H-indazol-6-yl]-[4-
(furan-2-carbonyl)-
piperazin-1-yl]-methanone; [3-(1H-benzoimidazol-2-yl)-1H-indazol-6-yl]-(4-
methyl-piperazin-1-yl)-
methanone; 1-{4-[3-(1H-benzoimidazol-2-yl)-1H-indazole-6-carbonyl]-piperazin-l-
yl}-ethanone; 3-
(1H-benzoimidazol-2-yl).-1H-indazole-6-carboxylic acid (6-methoxy-pyridin-3-
yl)-amide; 3-(1H-
benzoimidazol-2-yl)-1H-indazole-6-carboxylic acid (3-hydroxy-phenyl)-amide; 3-
(1H-benzoimidazol-
2-yl)-1H-indazole-6-carboxylic acid pyridin-4-ylamide; 3-(1H-benzoimidazol-2-
yl)-1H-indazole-6-
carboxylic acid (2-morpholin-4-yl-ethyl)-amide; 3-(1H-benzoimidazol-2-yl)-1H-
indazole-6-carboxylic
acid (2-hydroxy-ethyl)-methyl-amide; 3-{[3-(1H-benzoimidazol-2-yl)-1H-indazole-
6-carbonyl]-
amino}-butyric acid ethyl ester; 3-(1H-benzoimidazol-2-yl)-1H-indazole-6-
carboxylic acid (3-hydroxy-
propyl)-amide; 3-(1H-benzoimidazol-2-yl)-1H-indazole-6-carboxylic acid
phenylamide; 3-(1H-
benzoimidazol-2-yl)-1H-indazole-6-carboxylic acid pyridin-3-ylamide; 3-(6-
methoxy-lH-
benzoimidazol-2-yl)-1H-indazole-6-carboxylic acid (4-hydroxy-phenyl)-amide; 3-
(1H-benzoimidazol-
2-yl)-6-pyridin-4-yl-lH-indazole; 3-(5-chloro-lH-benzoimidazol-2-yl)-1H-
indazole-6-carboxylic acid
(4-hydroxy-phenyl)-amide; 3-(5,6-dimethoxy-lH-benzoimidazol-2-yl)-1H-indazole-
6-carboxylic acid
(4-hydroxy-phenyl)-amide; 3-(5-fluoro-lH-benzoimidazol-2-yl)-1H-indazole-6-
carboxylic acid (4-
hydroxy-phenyl)-amide; 3-(6-trifluoromethyl-lH-benzoimidazol-2-yl)-1H-indazole-
6-carboxylic acid
(4-hydroxy-phenyl)-amide; 3-(6-tert-butyl-lH-benzoimidazol-2-yl)-1H-indazole-6-
carboxylic acid (4-
hydroxy-phenyl)-amide; 3-(6,7-dimethyl-lH-benzoimidazol-2-yl)-1H-indazole-6-
carboxylic acid (4-
hydroxy-phenyl)-amide; 3-(5,6-dichloro-lH-benzoimidazol-2-yl)-1H-indazole-6-
carboxylic acid (4-
hydroxy-phenyl)-amide; 3-(5,6-difluoro-lH-benzoimidazol-2-yl)-1H-indazole-6-
carboxylic acid (4-
hydroxy-phenyl)-amide; 3-(1H-benzoimidazol-2-yl)-1H-indazole-6-carboxylic acid
(3-fluoro-4-
hydroxy-phenyl)-amide; 3-(1H-benzoimidazol-2-yl)-1H-indazole-6-carboxylic acid
amide; 3-(1H-
benzoimidazol-2-yl)-1H-indazole-6-carboxylic acid (4-hydroxy-2,3-dimethyl-
phenyl)-amide; 3-(1H-
benzoimidazol-2-yl)-1H-indazole-6-carboxylic acid (4-hydroxy-2-methyl-phenyl)-
amide; 3-(1H-
benzoimidazol-2-yl)-1H-indazole-6-carboxylic acid (4-hydroxy-phenyl)-amide; 3-
(1H-benzoimidazol-
2-yl)-1H-indazole-6-carboxylic acid cyclopropylamide; 2-[6-(4-hydroxy-2-
methoxy-phenyl)-1H-
indazol-3-yl]-3H-benzoimidazole-5-sulfonic acid amide; 4-[3-(6-dimethylamino-
lH-benzoimidazol-2-
yl)-1H-indazol-6-yl]-3-methoxy-phenol; 2-[6-(4-hydroxy-2-methoxy-phenyl)-1H-
indazol-3-yl]-3H-
benzoimidazole-5-carboxylic acid methylamide; 3-methoxy-4-{3-[6-(4-methyl-
piperazin-1-yl)-1H-
benzoimidazol-2-yl]-1H-indazol-6-yl}-phenol; 2-[6-(4-hydroxy-2-methoxy-phenyl)-
1H-indazol-3-yl]-
3H-benzoimidazole-5-carboxylic acid (2-morpholin-4-yl-ethyl)-amide; 4-[3-(1H-
imidazo[4,5-c]pyridin-
2-yl)-1H-indazol-6-yl]-3-methoxy-phenol; 3-[3-(1H-benzoimidazol-2-yl)-1H-
indazol-6-yl]-2-methoxy-
phenol; 3-[3-(1H-benzoimidazol-2-yl)-1H-indazol-6-yl]-phenol; 4-[3-(1H-
benzoimidazol-2-yl)-1H-
indazol-6-yl]-3,5-dimethyl-phenol; 4-[3-(1H-benzoimidazol-2-yl)-1H-indazol-6-
yl]-3-phenoxy-phenol;
4-[3-(1H-benzoimidazol-2-yl)-1H-indazol-6-yl]-benzene-1,3-diol; 4-[3-(1H-
benzoimidazol-2-yl)-1H-
indazol-6-yl]-3-methoxy-phenol; 4-[3-(1H-benzoimidazol-2-yl)-1H-indazol-6-yl]-
2-methoxy-phenol;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-9-
N-{3-[3-(1H-benzoimidazol-2-yl)-1H-indazole-6-carbonyl]-phenyl}-benzamide; 6-
[2-(1,5-dimethyl-
1H-pyrazol-3-yl)-3H-benzoimidazol-5-yl]-5-methyl-4,5-dihydro-2H-pyridazin-3-
one; 5-methyl-6-[2-(1-
methyl-1H-pyrazol-3-yl)-3H-benzoimidazol-5-yl]-4,5-dihydro-2H-pyridazin-3-one;
8-(1,5-dimethyl-
1H-pyrazol-3-yl)-7H-purine; 2-(1,5-dimethyl-lH-pyrazol-3-yl)-1H-imidazo[4,5-
b]pyridine and 2-(5-
methyl-lH-pyrazol-3-yl)-1H-imidazo[4,5-b]pyridine.
In the present specification, the term "compounds of the invention", and
equivalent expressions, are
meant to embrace compounds of general formula (Ix) as hereinbefore described,
which expression
includes the prodrugs, the pharmaceutically acceptable salts, and the
solvates, e.g. hydrates, where the
context so permits. Similarly, reference to intermediates, whether or not they
themselves are claimed,
is meant to embrace their salts, and solvates, where the context so permits.
For the sake of clarity,
particular instances when the context so permits are sometimes indicated in
the text, but these instances
are purely illustrative and it is not intended to exclude other instances when
the context so permits.
As used above for compounds of formula (Ix), and throughout the description of
the invention
hereinafter, the following terms unless otherwise indicated, shall be
understood to have the following
meanings:-
"Patient" includes both human and other mammals.
"Acid bioisostere" means a group which has chemical and physical similarities
producing broadly
similar biological properties to a carboxy group (see Lipinski, Annual Reports
in Medicinal Chemistry,
1986,2l,p283 "Bioisosterism In Drug Design"; Yun, Hwahak Sekye, 1993, 33,
pages 576-579
"Application Of Bioisosterism To New Drug Design"; Zhao, Huaxue Tongbao, 1995,
pages 34-38
"Bioisosteric Replacement And Development Of Lead Compounds In Drug Design";
Graham,
Theochem, 1995, 343, pages 105-109 "Theoretical Studies Applied To Drug
Design:ab initio
Electronic Distributions In Bioisosteres"). Examples of suitable acid
bioisosteres include:
-C(=O)-NHOH, -C(=O)-CH2OH, -C(=O)-CH2SH, -C(=O)-NH-CN, sulfo, phosphono,
alkylsulfonylcarbamoyl, tetrazolyl, arylsulfonylcarbamoyl,
heteroarylsulfonylcarbamoyl,
N-methoxycarbamoyl, 3-hydroxy-3-cyclobutene-1,2-dione, 3,5-dioxo-1,2,4-
oxadiazolidinyl or
heterocyclic phenols such as 3-hydroxyisoxazolyl and 3-hydoxy-l-
methylpyrazolyl.
"Acyl" denotes a radical R-C(=O)- in which R represents a radical chosen from
a hydrogen atom,
linear or branched alkyl radicals containing not more than 6 carbon atoms;
optionally substituted
amino; aryl, heteroaryl, cycloalkyl or heterocycloalkyl radicals, for example
phenyl or pyrrolidinyl
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-10-
radicals: the term "acyl" thus especially denotes, for example, formyl
radicals and acetyl, propionyl,
butanoyl, pentanoyl, hexanoyl, benzoyl and pyrrolidinylcarbonyl radicals.
"Acylainino" denotes -C(=O)-NH2, -C(O)-NH(alk) and -C(O)-N(alk)(alk) radicals:
in these radicals,
NH(alk) and N(alk)(alk) have the meanings given hereinafter defined.
"Alkenyl" means an aliphatic hydrocarbon group containing a carbon-carbon
double bond and which
may be straight or branched having about 2 to about 15 carbon atoms in the
chain and containing one
or more double bonds. Preferred alkenyl groups have 2 to about 12 carbon atoms
in the chain; and
more preferably 2 to about 6 carbon atoms (e.g. 2 to 4 carbon atoms) in the
chain. "Branched," as used
herein and throughout the text, means that one or more lower alkyl groups such
as methyl, ethyl or
propyl are attached to a linear chain; here a linear alkenyl chain. "Lower
alkenyl" means about 2 to
about 4 carbon atoms in the chain, which may be straight or branched.
Exemplary alkenyl groups
include ethenyl, propenyl, n-butenyl, i-butenyl, 3-methylbut-2-enyl, n-
pentenyl, heptenyl, octenyl,
cyclohexylbutenyl, decenyl, and 3,7-dimethyl-octa-2,6-dienyl.
"Alkoxy" means an alkyl-O- group in which the alkyl group is as described
herein. Exemplary alkoxy
groups include difluoromethoxy, methoxy, trifluoromethoxy, ethoxy, n-propoxy,
i-propoxy, n-butoxy,
s-butoxy, t-butoxy, pentoxy, hexoxy and heptoxy, and also the linear or
branched positional isomers
thereof.
"Alkoxycarbonyl" means an alkyl-O-CO- group in which the alkyl group is as
described herein.
Exemplary alkoxycarbonyl groups include methoxy- and ethoxycarbonyl.
"Alkyl" means, unless otherwise specified, an aliphatic hydrocarbon group
which may be straight or
branched chain having about 1 to about 15 carbon atoms in the chain,
optionally substituted by one or
more halogen atoms. Particular alkyl groups have from 1 to about 6 carbon
atoms. "Lower alkyl" as a
group or part of a lower alkoxy, lower alkylthio, lower alkylsulfinyl or lower
alkylsulfonyl group
means unless otherwise specified, an aliphatic hydrocarbon group which may be
a straight or branched
chain having 1 to about 4 carbon atoms in the chain. Exemplary alkyl groups
include methyl, ethyl,
n-propyl, i-propyl, n-butyl, isobutyl, s-butyl, t-butyl, n-pentyl, 3-pentyl,
hexyl, isohexyl, heptyl, octyl,
nonyl, decyl and dodecyl, and also the linear or branched positional isomers
thereof. Exemplary alkyl
groups substituted by one or more halogen atoms include trifluoromethyl,
difluoromethyl,
trifluoroethyl and difluoroethyl.
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-11-
"Alkylene" means an aliphatic bivalent radical derived from a straight or
branched alkyl group, in
which the alkyl group is as described herein. Exemplary alkylene radicals
include methylene, ethylene
and trimethylene.
"Alkylenedioxy" means an -O-alkylene-O- group in which alkylene is as defined
above. Exemplary
alkylenedioxy groups include methylenedioxy and ethylenedioxy.
"Alkylsulfinyl" means an alkyl-SO- group in which the alkyl group is as
previously described.
Preferred alkylsulfinyl groups are those in which the alkyl group is C1-
4alkyl.
"Alkylsulfonyl" means an alkyl-S02- group in which the alkyl group is as
previously described.
Preferred alkylsulfonyl groups are those in which the alkyl group is C1-
4alkyl.
"Alkylsulfonylcarbamoyl" means an alkyl-S02-NH-C(=0)- group in which the alkyl
group is as
previously described. Preferred alkylsulfonylcarbamoyl groups are those in
which the alkyl group is
C 1-4alkyl.
"Alkylthio" means an alkyl-S- group in which the alkyl group is as previously
described. Exemplary
alkylthio groups include methylthio, ethylthio, propylthio, isopropylthio,
butylthio, isobutylthio,
sec-butylthio, tert-butylthio, pentylthio, isopentylthio, hexylthio,
isohexylthio and heptylthio, and also
the linear or branched positional isomers thereof. Preferred alkylthio groups
have not more than 4
carbon atoms.
"Alkynyl" means an aliphatic hydrocarbon group containing a carbon-carbon
triple bond and which
group may be a straight or branched chain having about 2 to about 15 carbon
atoms in the chain.
Preferred alkynyl groups have 2 to about 12 carbon atoms in the chain; and
more preferably 2 to about
6 carbon atoms (e.g. 2 to 4 carbon atoms) in the chain. Exemplary alkynyl
groups include ethynyl,
propynyl, n-butynyl, i-butynyl, 3-methylbut-2-ynyl, and n-pentynyl.
"Aroyl" means an aryl-CO- group in which the aryl group is as described
herein. Exemplary aroyl
groups include benzoyl and 1- and 2-naphthoyl.
"Aroylamino" is an aroyl-NH- group wherein aroyl is as previously defined.
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-12-
"Aryl" as a group or part of a group denotes: (i) an optionally substituted
monocyclic or multicyclic
aromatic carbocyclic moiety of about 6 to about 14 carbon atoms, such as
phenyl or naphthyl; or (ii) an
optionally substituted partially saturated multicyclic aromatic carbocyclic
moiety in which a
monocyclic aromatic carbocyclic moiety and a cycloalkyl or cycloalkenyl group
are fused together to
form a cyclic structure, such as a tetrahydronaphthyl, indenyl or indanyl
ring. Except where otherwise
defined, aryl groups may be substituted with one or more aryl group
substituents, which may be the
same or different, where "aryl group substituent" includes, for example, acyl,
acylamino, alkoxy,
alkoxycarbonyl, alkylenedioxy, alkylsulfinyl, alkylsulfonyl, alkylthio, aroyl,
aroylamino, aryl,
arylalkyloxy, arylallcyloxycarbonyl, arylalkylthio, aryloxy, aryloxycarbonyl,
arylsulfinyl, arylsulfonyl,
arylthio, carboxy (or an acid bioisostere), cyano, cycloalkyl, halo,
heteroaroyl, heteroaryl,
heteroarylalkyloxy, heteroaroylamino, heteroaryloxy, heterocycloalkyl,
hydroxy, nitro, trifluoromethyl,
-C(=O)NYlY2, -NY1-C(=O)alkyl, -NY1SO2alkyl, -NYlY2, -SO2NYlY2 or alkyl,
alkenyl or alkynyl
each optionally substituted with aryl, cycloalkyl, heteroaryl, hydroxy, -
C(=0)OR6, -C(=O)NYlY2,
-NYlY2 or -OR5.
"Arylalkyl" means an aryl-alkyl- group in which the aryl and alkyl moieties
are as previously
described. Preferred arylalkyl groups contain a C1-4alkyl moiety. Exemplary
arylalkyl groups
include benzyl, 2-phenethyl and naphthlenemethyl.
"Arylalkyloxy" means an arylalkyl-O- group in which the arylalkyl group is as
previously described.
Exemplary arylalkyloxy groups include benzyloxy and 1- or 2-
naphthalenemethoxy.
"Arylalkyloxycarbonyl" means an arylalkyl-O-CO- group in which the arylalkyl
group is as previously
described. An exemplary arylalkyloxycarbonyl group is benzyloxycarbonyl.
"Arylalkylthio" means an arylalkyl-S- group in which the arylalkyl group is as
previously described.
An exemplary arylalkylthio group is benzylthio.
"Aryloxy" means an aryl-O- group in which the aryl group is as previously
described. Exemplary
aryloxy groups include phenoxy and naphthoxy, each optionally substituted.
"Aryloxycarbonyl" means an aryl-O-C(=O)- group in which the aryl group is as
previously described.
Exemplary aryloxycarbonyl groups include phenoxycarbonyl and
naphthoxycarbonyl.
"Arylsulfinyl" means an aryl-SO- group in which the aryl group is as
previously described.
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-13-
"Arylsulfonyl" means an aryl-S02- group in which the aryl group is as
previously described.
"Arylsulfonylcarbamoyl" means an aryl-S02-NH-C(=O)- group in which the aryl
group is as
previously described.
"Arylthio" means an aryl-S- group in which the aryl group is as previously
described. Exemplary
arylthio groups include phenylthio and naphthylthio.
"Carbocyclic" means a saturated ring system comprising carbon atoms.
"Carbocyclic group substituent" includes, for example, acyl, acylamino,
alkoxy, alkoxycarbonyl,
alkylenedioxy, alkylsulfinyl, alkylsulfonyl, alkylthio, aroyl, aroylamino,
aryl, arylalkyloxy,
arylalkyloxycarbonyl, arylalkylthio, aryloxy, aryloxycarbonyl, arylsulfinyl,
arylsulfonyl, arylthio,
carboxy (or an acid bioisostere), cyano, cycloalkyl, halo, heteroaroyl,
heteroaryl, heteroarylalkyloxy,
heteroaroylamino, heteroaryloxy, heterocycloalkyl, hydroxy, nitro,
trifluoromethyl, -C(=O)NYlY2,
-NYI-C(=O)alkyl, -NY1SO2all.yl, -NYlY2, -SO2NYlY2 or alkyl, alkenyl or alkynyl
each optionally
substituted with aryl, cycloalkyl, heteroaryl, hydroxy, -C(=O)OR6, -
C(=O)NYlY2, -NYlY2 or -OR5.
"Cyclic amine" means a 3 to 8 membered monocyclic cycloalkyl ring system
wherein one of the ring
carbon atoms is replaced by nitrogen and which (i) may also contain a further
heteroatom-containing
group selected from 0, S, SO2, or NY6 (where Y6 is hydrogen, alkyl, aryl,
arylalkyl, -C(=0)R5,
-C(=O)OR5, -C(=O)NYlY2 or -S02R5); and (ii) may be fused to additional aryl
(e.g. phenyl),
heteroaryl (e.g. pyridyl), heterocycloalkyl or cycloalkyl rings to form a
bicyclic or tricyclic ring system.
Exemplary cyclic amines include pyrrolidine, piperidine, morpholine,
piperazine, indoline, pyrindoline,
tetrahydroquinoline and the like groups.
"Cycloalkenyl" means a non-aromatic monocyclic or multicyclic ring system
containing at least one
carbon-carbon double bond and having about 3 to about 10 carbon atoms.
Exemplary monocyclic
cycloalkenyl rings include cyclopentenyl, cyclohexenyl and cycloheptenyl.
"Cycloalkyl" means a saturated monocyclic or bicyclic ring system of about 3
to about 10 carbon
atoms, optionally substituted by oxo. Exemplary monocyclic cycloalkyl rings
include C3-8cycloalkyl
rings such as cyclopropyl, cyclopentyl, cyclohexyl and cycloheptyl.
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-14-
"Cycloalkylalkyl" means a cycloalkyl-alkyl- group in which the cycloalkyl and
alkyl moieties are as
previously described. Exemplary monocyclic cycloalkylalkyl groups include
cyclopropylmethyl,
cyclopentylmethyl, cyclohexylmethyl and cycloheptylmethyl.
"Halo" or "halogen" means fluoro, chloro, bromo, or iodo. Preferred are
fluoro, bromo and chloro.
"Haloalkyl" means an alkyl group having about 1 to about 6 carbon atoms in the
chain and substituted
by one or more halo atoms. Exemplary haloalkyl groups include trifluoromethyl.
"Heteroaroyl" means a heteroaryl-C(=O)- group in which the heteroaryl group is
as described herein.
Exemplary heteroaryl groups include pyridylcarbonyl.
"Heteroaroylamino" means a heteroaroyl-NH- group in which the heteroaryl
moiety is as previously
described.
"Heteroaryl" as a group or part of a group denotes: (i) an optionally
substituted aromatic monocyclic or
multicyclic organic moiety of about 5 to about 10 ring members in which one or
more of the ring
members is/are element(s) other than carbon, for example nitrogen, oxygen or
sulfur (examples of such
groups include benzoimidazolyl, benzothiazolyl, furyl, imidazolyl, indazolyl,
indolyl, indolizinyl,
isoxazolyl, isoquinolinyl, isothiazolyl, oxadiazolyl, pyrazinyl, pyridazinyl,
pyrazolyl, pyridyl,
pyrimidinyl, pyrrolyl, quinazolinyl, quinolinyl, 1,3,4-thiadiazolyl,
thiazolyl, thienyl and triazolyl
groups, optionally substituted by one or more aryl group substituents as
defined above except where
otherwise defined); (ii) an optionally substituted partially saturated
multicyclic heterocarbocyclic
moiety in which a monocyclic heteroaromatic moiety and a cycloalkyl,
cycloalkenyl or
heterocycloalkyl group are fused together to form a cyclic structure (examples
of such groups include
tetrahydro-indazole, tetrahydro-pyrazolopyridine, 5-oxo-1,4,5,6,7,8,9,9a-
octahydro-1,2,4,5a-tetraza-
cyclopenta[a]naphthyl, optionally substituted by one or more "aryl group
substituents" as defined
above, except where otherwise defined). Optional substituents include one or
more "aryl group
substituents" as defined above, except where otherwise defined. When Rl is
heteroaryl this may
particularly represent pyrazolyl, triazolyl, isoxazolyl, isothiazolyl,
thiazolyl, oxazolyl, imidazolyl,
pyrrolyl, furanyl, thiophenyl, phenyl, pyridinyl, oxodihydropyridinyl,
pyrimidinyl, indolyl, indazolyl,
thienopyrazolyl, tetrahydroindazolyl, tetrahydrocyclopentapyrazolyl,
dihydrofuropyrazolyl,
oxodihydropyridazinyl, tetrahydropyrrolopyrazolyl,
oxotetrahydropyrrolopyrazolyl,
tetrahydropyranopyrazolyl, tetahydropyridinopyrazolyl, or
oxodihydropyridinopyrazolyl.
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-15-
"Heteroarylalkyl" means a heteroaryl-alkyl- group in which the heteroaryl and
alkyl moieties afe as
previously described. Preferred heteroarylalkyl groups contain a C1-4alkyl
moiety. Exemplary
heteroarylalkyl groups include pyridylmethyl.
"Heteroarylalkyloxy" means an heteroarylalkyl-O- group in which the
heteroarylalkyl group is as
previously described. Exemplary heteroaryloxy groups include optionally
substituted pyridylmethoxy.
"Heteroaryloxy" means an heteroaryl-O- group in which the heteroaryl group is
as previously
described. Exemplary heteroaryloxy groups include optionally substituted
pyridyloxy.
"Heteroarylsulfonylcarbamoyl" means a heteroaryl-S02-NH-C(=0)- group in which
the heteroaryl
group is as previously described.
"Heterocycloalkyl" means: (i) a cycloalkyl group of about 3 to 10 ring members
which contains one or
more heteroatoms or heteroatom-containing groups selected from 0, S and NY6
and may be optionally
substituted by oxo (examples of such groups include hexahydropyran,
pyrrolidinyl, piperidinyl,
tetrahydropyranyl and octahydro-pyrido[1,2-c]pyrimidin-l-one); (ii) a
partially saturated multicyclic
heterocarbocyclic moiety in which an aryl (or heteroaryl) ring, each
optionally substituted by one or
more "aryl group substituents," and a heterocycloalkyl group are fused
together to form a cyclic
structure (examples of such groups include chromanyl, dihydrobenzofuranyl,
indolinyl and
pyrindolinyl groups).
"Heterocycloalkylalkyl" means a heterocycloalkyl-alkyl- group in which the
heterocycloalkyl and alkyl
moieties are as previously described.
"Hydroxyalkyl" means an alkyl group substituted by one or hydroxy groups.
"NH(alk)" and "N(alk)(alk)" denote an amino radical substituted, respectively,
with one or two alkyl
radicals, such alkyl radicals being linear or branched and chosen from alkyl
radicals as defined above,
preferably containing not more than 4 carbon atoms.
"Prodrug" means a compound which is convertible in vivo by metabolic means
(e.g. by hydrolysis) to a
compound of formula (Ix), including N-oxides thereof. For example an ester of
a compound of
formula (Ix) containing a hydroxy group may be convertible by hydrolysis in
vivo to the parent
molecule. Alternatively, an ester of a compound of formula (Ix) containing a
carboxy group may be
convertible by hydrolysis in vivo to the parent molecule.
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-16-
Suitable esters of compounds of formula (Ix) containing a hydroxy group are,
for example acetates,
citrates, lactates, tartrates, malonates, oxalates, salicylates, propionates,
succinates, fumarates,
maleates, methylene-bis-(3-hydroxynaphthoates, gentisates, isethionates, di-p-
toluoyltartrates,
methanesulfonates, ethanesulfonates, benzenesulfonates, p-toluenesulfonates,
cyclohexylsulfamates
and quinates.
Suitable esters of compounds of formula (Ix) containing a carboxy group are,
for example, those
described by F.J.Leinweber, Drug Metab. Res., 1987, 18, page 379.
An especially useful class of esters of compounds of formula (Ix) containing a
hydroxy group, may be
formed from acid moieties selected from those described by Bundgaard et. al.,
J. Med. Chem., 1989, 32
, pages 2503-2507, and include substituted (aminomethyl)-benzoates, for
example
dialkylamino-methylbenzoates in which the two alkyl groups may be joined
together and/or interrupted
by an oxygen atom or by an optionally substituted nitrogen atom, e.g. an
allcylated nitrogen atom, more
especially (morpholino-methyl)benzoates, e.g. 3- or 4-(morpholinomethyl)-
benzoates, and
(4-alkylpiperazin-1-yl)benzoates, e.g. 3- or 4-(4-alkylpiperazin-1-
yl)benzoates.
Where the compound of the invention of formula (Ix) contains a carboxy group,
or a sufficiently acidic
bioisostere, base addition salts may be formed and are simply a more
convenient form for use; in
practice, use of the salt form inherently amounts to use of the free acid
form. The bases which can be
used to prepare the base addition salts include preferably those which
produce, when combined with
the free acid, pharmaceutically acceptable salts, that is, salts whose cations
are non-toxic to the patient
in pharmaceutical doses of the salts, so that the beneficial inhibitory
effects inherent in the free base
are not vitiated by side effects ascribable to the cations. Pharmaceutically
acceptable salts, including
those derived from alkali and alkaline earth metal salts, within the scope of
the invention include those
derived from the following bases: sodium hydride, sodium hydroxide, potassium
hydroxide, calcium
hydroxide, aluminium hydroxide, lithium hydroxide, magnesium hydroxide, zinc
hydroxide, ammonia,
ethylenediamine, N-methyl-glucamine, lysine, arginine, omithine, choline,
N,N'-dibenzylethylenediamine, chloroprocaine, diethanolamine, procaine, N-
benzylphenethylamine,
diethylamine, piperazine, tris(hydroxymethyl)aminomethane, tetramethylammonium
hydroxide, and
the like.
Some of the compounds of the present invention of formula (Ix) are basic, and
such compounds are
useful in the form of the free base or in the form of a pharmaceutically
acceptable acid addition salt
thereof.
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-17-
Acid addition salts are a more convenient form for use; and in practice, use
of the salt form inherently
amounts to use of the free base form. The acids which can be used to prepare
the acid addition salts
include preferably those which produce, when coinbined with the free base,
pharmaceutically
acceptable salts, that is, salts whose anions are non-toxic to the patient in
pharmaceutical doses of the
salts, so that the beneficial inhibitory effects inherent in the free base are
not vitiated by side effects
ascribable to the anions. Although pharmaceutically acceptable salts of said
basic compounds are
preferred, all acid addition salts are useful as sources of the free base form
even if the particular salt,
per se, is desired only as an intermediate product as, for example, wlien the
salt is formed only for
purposes of purification, and identification, or when it is used as
intermediate in preparing a
pharmaceutically acceptable salt by ion exchange procedures. Pharmaceutically
acceptable salts within
the scope of the invention include those derived from mineral acids and
organic acids, and include
hydrohalides, e.g. hydrochlorides and hydrobronudes, sulfates, phosphates,
nitrates, sulfamates,
acetates, citrates, lactates, tartrates, malonates, oxalates, salicylates,
propionates, succinates, fumarates,
maleates, methylene-bis-b-hydroxynaphthoates, gentisates, isethionates, di-p-
toluoyltartrates,
methane-sulfonates, ethanesulfonates, benzenesulfonates, p-toluenesulfonates,
cyclohexylsulfamates
and quinates.
As well as being useful in themselves as active compounds, salts of compounds
of the invention of
compounds of formula (Ix) are useful for the purposes of purification of the
compounds, for example
by exploitation of the solubility differences between the salts and the parent
compounds, side products
and/or starting materials by techniques well known to those skilled in the
art.
It will be appreciated that compounds of the present invention of formula (Ix)
may contain asymmetric
centres. These asymmetric centres may independently be in either the R or S
configuration. It will be
apparent to those skilled in the art that certain conipounds of the invention
may also exhibit
geometrical isomerism. It is to be understood that the present invention
includes individual
geometrical isomers and stereoisomers and mixtures thereof, including racemic
mixtures, of
compounds of formula (Ix) hereinabove. Such isomers can be separated from
their mixtures, by the
application or adaptation of known methods, for example chromatographic
techniques and
recrystallisation techniques, or they are separately prepared from the
appropriate isomers of their
intermediates. Additionally, tautomers of the compounds of formula (Ix) are
possible, and the present
invention is intended to include all tautomeric forms of the compounds.
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-18-
One subject of the present invention is thus the compounds of formula (I):
Y z N
11 \> Rl
X~, W CN
A5 (I)
in which:
X represents C-R' and W, Y and Z, which may be identical or different,
represent CH or CR3;
R' represents aryl or heteroaryl chosen from pyrazolyl, triazolyl, imidazolyl,
indolyl, indazolyl, thieno-
pyrazolyl, tetrahydroindazolyl, tetrahydrocyclopentapyrazolyl,
dihydrofuropyrazolyl,
oxodihydropyridazinyl, tetrahydropyrrolopyrazolyl,
oxotetrahydropyrrolopyrazolyl,
tetrahydropyranopyrazolyl, tetrahydropyridinopyrazolyl, and
oxodihydropyridinopyrazolyl radicals, all
these radicals being optionally substituted with one or more radicals X', X2
or X3 chosen from H,
halogen, haloalkyl, OH, W, NO2, CN, S(O)nR4, OR¾, NY'Y', COR4, -C(=0)NY'Y', -
C(=O)OR4,
-C(=O)OH, -N(R6)C(=O)R4, -N(R6)SO,R4, -N(R6)C(=O)NY'Y2, -N(R6)C(=O)OR4, -
S(O)nOR¾,
-S(O)õNY'Y', -OC.(=O)NY'Y', -OS(O)õR4, -OC(=O)R4 and optionally substituted
thienyl,
R' and R3 are such that:
either R' and R3, which may be identical or different, represent H, W,
halogen, haloalkyl, OH, NO2,,
CN, OR4, COR 4, S(O)õR4, -C(=O)NYIY', -C(=O)OR4, -C(=O)OH, -NY'Y', -
N(R6)C(=O)R4,
-N(R6)SO,R4, -N(R6)C(=O)NY'Y', -N(R6)C(=0)OR¾, -S(O)õOR4, -S(O)õNY'Y'', -
OC(=0)NY'Y' and
-OC(=O)R`'
or R' represents H, R4, halogen, haloalkyl, OH, NO2), CN, OR¾, COR4, S(O)r,R4,
-C(=O)NY'Y',
-C(=O)OW, -C(=O)OH, -NY'Y2, -N(R6)C(=O)W, -N(W)SO'R4, -N(R6)C(=O)NY'Y2,
-N(R6)C.(=O)OW, -S(O)nOR4, -S(O)õNY'Y2, -OC(=0)NY'Y2 and -OC(=O)R4
and R3 represents alkyl, haloalkyl, halogen and OW
or R' and R3 together form a 5- to 6-membered carbon-based ring containing one
or more hetero atoms,
which may be identical or different, chosen from 0, N and S,
R4 represents alkyl, alkenyl, alkynyl, cycloalkyl, aryl, heteroaryl,
cycloalkylalkyl, heterocycloalkyl,
heteroarylalkyl and arylalkyl, all these radicals being optionally substituted
with one or more radicals
chosen from aryl (optionally substituted), halogen, alkyl, hydroxyalkyl, OH,
ORS, C(=O)NY3Y4,
NY3Y4, alk-NY3Y4 and C(=0)OR6,
RS represents alkyl, alkenyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl,
arylalkyl, cycloalkylalkyl,
heteroarylalkyl and heterocycloalkylalkyl.
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-19-
Y' and Y' are such that: either Y' and Y2, which may be identical or
different, represent H and
optionally substituted alkyl, alkenyl, cycloalkyl, heterocycloalkyl,
heterocycloalkylalkyl, aryl,
arylalkyl, heteroaryl and heteroarylalkyl,
or Y' and Y2 form, together with the nitrogen atom to which they are attached,
a cyclic amino radical,
Y3 and Y4 are such that: either Y3 and Y4, which may be identical or
different, represent hydrogen,
alkenyl, alkyl, aryl, arylalkyl, cycloalkyl, heteroaryl or heteroarylalkyl or
Y3 and Y4 form, together with
the nitrogen atom to which they are attached, an optionally substituted cyclic
amino radical,
A5 represents H or alkyl,
R6 is chosen from the values of R5,
all the alkyl (or alk, which represents alkyl), alkenyl, cycloalkyl,
heterocycloalkyl, aryl, arylalkyl,
heteroaryl and heteroarylalkyl radicals present in the above radicals
furthermore being optionally
substituted with one or more radicals chosen from halogen atoms and hydroxyl,
cyano, alkyl, alkoxy,
acylamino (NH-COalk), -C(=0)OR6 , acyl -C(=O)R6, hydroxyalkyl, carboxyalkyl,
S(O)n alk,
S(O),,-NH2, S(O),,-NH(alk), S(O),,-N(alk)2, CF3, OCF3, NO2, arylalkoxy, aryl,
heteroaryl, aryloxy,
aryloxyalkyl, -C(=0)-NY3Y4 and NY3Y4 radicals,
the latter radicals containing alkyl, aryl and heteroaryl being themselves
optionally substituted with one
or more radicals chosen from halogen atoms and alkyl radicals, free, salified
or esterified carboxyl
radicals and acylamino radicals NH-C(O)R5,
the phenyl radicals furthermore being optionally substituted with a dioxole
radical,
n represents an integer from 0 to 2,
it being understood that when R' represents an indazolyl radical
to give the compounds of formula (F) below:
w
\ N
X ' \N
~ N~ H
H (F)
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-20-
with X representing H, R' or R3 as defined above, then W necessarily
represents H or unsubstituted
alkyl,
the said compounds of formula (I) being in any possible racemic, enantiomeric
or diastereoisomeric
isomer form, and also the addition salts with mineral and organic acids or
with mineral bases.
One subject of the present invention is thus the compoundss of formula (I) as
defined above
corresponding to the formula (Ia):
Ya,Za N
I I ~>--- R,a
Xa.Wa.
A5 (1a)
in which:
Xa represents C-R2a and Wa, Ya and Za, which may be identical or different,
represent CH or CR3a;
Rla represents aryl or heteroaryl chosen from pyrazolyl, triazolyl and
indazolyl radicals, all these
radicals being optionally substituted with one or more radicals X'a, X'a or
X3a chosen from H,
halogen, OH, R4a, OWa, NY'aY'a, S(O)õWa, -C(=O)NY'aY'a, -C(=O)OR4a, -
N(R~a)C(=O)Wa,
-N(R'a)SO-,R4a, -N(R6a)C(=O)NYIaY2a, -N(R6a)C(=O)OR¾a, -OC(=O)NYIaY'a, -
OC(=0)R4a,
-OS(O)õR4 a and thienyl optionally substituted with an alkyl radical,
Wa and R3a are such that:
either Wa and R3a, which may be identical or different, represent H, R4a,
halogen, OH, OR4a,
C(=O)NY'aY2a, -C(=O)OR4a and -C(=O)OH, and R3a represents allcyl, halogen and
OR6a,
or Wa represents H, R4a, halogen, OH, OR4a, C(=O)NY'aY2a, -C(=O)OR4a and -
C(=O)OH, and R3a
represents alkyl, halogen and OR6a,
or Wa and R3a together form an -O-CHZ-O- or -O-CH2-CH2,-O- ring,
Wa represents allcyl, alkenyl, cycloalkyl, aryl, heteroaryl, cycloalkylalkyl,
heterocycloalkyl,
heteroarylalkyl and arylalkyl, all these radicals being optionally substituted
with one or more radicals
chosen from aryl (optionally substituted), halogen, alkyl, hydroxyalkyl, OH,
ORSa, C(=O)NY3aY4a,
NY3aY4a, alk-NY3aY4a and C(=O)OR6a,
RSa represents alkyl, alkenyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl,
arylalkyl, cycloalkylalkyl,
heteroarylalkyl and heterocycloalkylalkyl, all these radicals being optionally
substituted,
Y'a and Y2a are such that: either Y'a and Y2a, which may be identical or
different, represent H, alkyl,
alkoxyalkyl, aryloxyalkyl, arylalkyl, heteroarylalkyl, heterocycloalkylalkyl,
cycloalkyl, aryl and
heteroaryl, all these radicals being optionally substituted, or Yla and Y2a
form, together with the
nitrogen atom to which they are attached, an optionally substituted cyclic
amino radical,
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-21-
Y3a and Y4a are such that: either Y3a and Y¾a, which may be identical or
different, represent hydrogen,
alkyl, aryl, arylalkyl, cycloalkyl, heteroaryl or heteroarylalkyl,
or Y3a and Y¾a form, together with the nitrogen atom to which they are
attached, a cyclic amino
radical,
A5 represents H or alkyl,
all the alkyl, alkenyl, cycloalkyl, heterocycloalkyl, aryl, arylalkyl,
heteroaryl and heteroarylalkyl
radicals present in the above radicals furthermore being optionally
substituted with one or more
radicals chosen from halogen atoms and hydroxyl, cyano, alkyl, alkoxy,
acylamino (NH-C(O)R6a),
-C(=O)OR6a, acyl -C(=O)R6a, hydroxyalkyl, carboxyalkyl, S(O)n-alk, S(O)n NH,,
S(O),,-NH(alk),
S(O),,-N(alk)2, CF3, OCF3, NO7,, arylalkoxy, aryl, heteroaryl, aryloxy,
aryloxyalkyl, -C(=0)-NY3aY4 a
and NY3aY4 a radicals,
the latter radicals containing alkyl, aryl and heteroaryl themselves being
optionally substituted with one
or more radicals chosen from halogen atoms and alkyl radicals, alkoxy
radicals, free, salified or
esterified carboxyl radicals and acylamino radicals NH-C(O)R6 a,
the phenyl radicals furthermore being optionally substituted with a dioxole
radical,
R6a is chosen from the values of R5a,
n represents an integer from 0 to 2,
the said compounds of formula (Ia) being in any possible racemic, enantiomeric
or diastereoisomeric
isomer form, and also the addition salts with mineral and organic acids or
with mineral bases.
One subject of the present invention is thus the compounds of formula (I):
Y~'z N
I I ~>--- Rl
X, W N
A5 (I)
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-22-
in which:
X represents C-R2 and W, Y and Z, which may be identical or different,
represent CH or CR3;
R' represents aryl or heteroaryl chosen from pyrazolyl, triazolyl, imidazolyl,
indolyl, indazolyl, thieno-
pyrazolyl, tetrahydroindazolyl, tetrahydrocyclopentapyrazolyl,
dihydrofuropyrazolyl,
oxodihydropyridazinyl, tetrahydropyrrolopyrazolyl,
oxotetrahydropyrrolopyrazolyl, tetrahydropyrano-
pyrazolyl, tetrahydropyridinopyrazolyl, and oxodihydro-pyridinopyrazolyl
radicals, all these radicals
optionally being substituted with one or more radicals Xl, X2 or X3 chosen
from H, halogen, haloalkyl,
OH, R4, NO2,, CN, S(O)õR4, OR4, NY'Y2, COR4, -C(=O)NY'Y2, -C(=O)OR4, -C(=O)OH,
-
N(R6)C(=O)R4, -N(R6)SO,W, -N(R6)C(=O)NY'Y', -N(R6)C(=O)OR4, -S(O)õOR4, -
S(O)nNY'Y', -
OC(=O)NY'Y', -OS(O) R4, -OC(=O)R4 and optionally substituted thienyl,
W and R-' are such that:
either R' and R3, which may be identical or different, represent H, R4,
halogen, haloalkyl, OH, NO',
CN, OW, COR4, S(O),,W, -C(=O)NY'Y2, -C(=O)OW, -C(=O)OH, -NY'Y2, -N(R6)C(=O)R¾,
-N(R6)SO2R4, -N(R6)C(=0)NY1Y2, -N(R6)C(=O)OR4, -S(O)nOR4, -S(O)nNY1Y2, -
OC(=O)NY'Y'
and -OC(=O)W
~
or R' represents H, R`', halogen, haloalkyl, OH, NOz, CN, OR4, COR¾, S(O)õR~, -
C(=O)NY Y-,
-C(=O)OR¾, -C(=O)OH, -NY'Y2, -N(R6)C(=O)W, -N(R6)SO,R4, -N(R6)C(=O)NY'Y',
-N(R6)C(=O)OR¾, -S(O)"OR4, -S(O),'NYIY', -OC(=O)NY'Y'` and -OC(=O)R4
and R3 represents alkyl, haloalkyl, halogen and OR6
or R2 and R3 together form a 5- to 6-membered carbon-based ring containing one
or more hetero atoms,
which may be identical or different, chosen from 0, N and S,
W represents alkyl, alkenyl, cycloalkyl, aryl, heteroaryl, cycloalkylalkyl,
heterocycloalkyl,
hetero-arylalkyl and arylalkyl, all these radicals being optionally
substituted with one or more radicals
chosen from aryl, OH, OR5, C(=O)NY3Y4, NY3Yd and C(=O)OR6,
Rj represents alkyl, alkenyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl,
arylalkyl, cycloalkylalkyl,
heteroarylallcyl and heterocycloalkylalkyl.
R6 represents H and Cl-C4 alkyl,
n represents an integer from 0 to 2
Y' and Y2 are such that: either Y' and Y2, which may be identical or
different, represent H, alkyl,
alkenyl, cycloalkyl, aryl, arylalkyl, heteroaryl or heteroarylalkyl, all these
radicals being optionally
substituted with one or more radicals chosen from hydroxyl, -C(=O)-NY" Y4, -
C(=O)OR6 and NY3Y4,
or Y' and Y2 form, together with the nitrogen atom to which they are attached,
a cyclic amino radical,
Y3 and Y4 are such that: either Y3 and Y4, which may be identical or
different, represent hydrogen,
alkenyl, alkyl, aryl, arylalkyl, cycloalkyl, heteroaryl or heteroarylalkyl or
Y3 and Y4 form, together with
the nitrogen atom to which they are attached, a cyclic amino radical,
A5 represents H or alkyl,
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-23-
it being understood that when R' represents an indazolyl radical
to give the compounds of formula (F) below:
w
~ ~
X l ,N C N
N H
H (F)
with X representing H, R2 or R3 as defined above, then W necessarily
represents H or unsubstituted
alkyl,
the said compounds of formula (F) being in any possible racemic, enantiomeric
or diastereoisomeric
isomer form, and also the addition salts with mineral and organic acids or
with mineral bases.
It is obvious that, according to the ring represented by R' and its number of
members, R' can comprise
one, two or three substituents represented by X', X2 and X3.
One subject of the present invention is thus the compounds of formula (I) as
defined above
corresponding to the formula (1a):
Ya,Za N
.
II ~>--Ria
Xa.Wa N
A5
(Ia)
in which:
Xa represents C-R'a and Wa, Ya and Za, which may be identical or different,
represent CH or CR3a;
Rla represents aryl or heteroaryl chosen from pyrazolyl, triazolyl or
indazolyl radicals, all these
radicals being optionally substituted with one or more radicals X'a, X2a or
X3a chosen from H,
halogen, OH, R¾a, OR4a, NY'aY2a, S(O)nR4a, -C(=O)NY'aY2a, -C(=O)OR¾a, -
N(R6a)C(=0)R4a,
-N(R6a)SO,R'a, -N(R6a)C(=O)NY'aY'`a, -N(R6a)C(=0)OR4a, -OC(=O)NY'aY2a and -
OC(=O)R4a,
-OS(O)nR4a and thienyl optionally substituted with an alkyl radical,
R''a and R3a are such that:
either RZa and R3a, which may be identical or different, represent H, R4a,
halogen, OH, OR4a,
C(=O)NYIaYZa, -C(=O)OR¾a, -C(=O)OH, and R3a represents alkyl, halogen and
OR6a,
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-24-
or R2a represents H, R¾a, halogen, OH, OWa, C.(=O)NYlaY'a, -C(=O)OR4a, -
C(=O)OH, and R3a
represents alkyl, halogen and OR6,
or R2a and R3a together form an -O-CH2,-O or -O-CH?-CH2-O- ring,
R4a represents alkyl, cycloalkyl, aryl, heteroaryl, heterocycloalkyl,
heteroarylalkyl or arylalkyl, all
these radicals being optionally substituted with one or more radicals chosen
from aryl, OH, ORSa,
C(=O)NY3aY4 a, NY3aY4 a and C(=O)OR6a,
R5a represents alkyl, alkenyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl,
arylalkyl, cycloalkylalkyl,
heteroarylallcyl and heterocycloalkylalkyl,
R6a represents H and C1-C4 alkyl,
n represents an integer from 0 to 2,
Y'a and Y'a are such that: either Y'a and Y2a, which may be identical or
different, represent H, alkyl,
cycloalkyl, aryl and heteroaryl, all these radicals being optionally
substituted with one or more radicals
chosen from hydroxyl, -C(=0)-NY3Y¾, -C(=O)OR6 and NY3Y4, or Y'a and Y2a form,
together with the
nitrogen atom to which they are attached, a cyclic amino radical,
Y3a and Y4 a are such that: either Y3a and Y4a, which may be identical or
different, represent hydrogen,
alkyl, aryl, arylalkyl, cycloalkyl, heteroaryl or heteroarylalkyl, or Y3a and
Y4a form, together with the
nitrogen atom to which they are attached, a cyclic amino radical,
A5 represents H or alkyl,
the said compounds of formula (Ia) being in any possible racemic, enantiomeric
or diastereoisomeric
isomer form, and also the addition salts with mineral and organic acids or
with mineral bases.
One subject of the present invention is thus the compounds of formula (1) as
defined above
corresponding to the formula (IA):
A,
A2 N
I ~>-A
A
3 N
A4 A5 (IA)
in which A represents a saturated heterocyclic radical which is either a 5- or
6-membered
monocyclic radical or a bicyclic radical that is not more than 10-membered,
these members
being such that at least two of them represent a nitrogen atom and the others,
which may be
identical or different, represent a carbon member or a hetero atom member
chosen from 0, N
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-25-
and S, this heterocycle A being optionally substituted with one or more
radicals XA1, XA2 or
XA3 chosen from the values indicated hereinabove for the radicals X~, X2 or
X3,
Al, A, A3 and A4, which may be identical or different, are chosen from a
hydrogen atom, halogen
atoms and hydroxyl, alkyl, alkenyl, alkoxy, nitro, cyano, aryl, heteroaryl and
aryloxy radicals, a
carboxyl radical which is free, salified, esterified with an alkyl radical or
amidated with a radical
NA6A7 such that either A6 and A7, which may be identical or different, are
chosen from a hydrogen
atom and optionally substituted alkyl, alkoxyalkyl, phenoxyalkyl, aryl,
arylalkyl, cycloalkyl,
cycloalkylalkyl, heterocycloalkylalkyl and heteroarylalkyl radicals, or A6 and
A7 form, together with
the nitrogen atom to which they are attached, an optionally substituted 5- or
6-membered cyclic radical,
it being understood that two consecutive radicals among Al, A2, A3 and A4 can
form, with the
benzimidazole radical to which they are attached, a 5- to 6-membered carbon-
based ring
containing one or more hetero atoms, wliich may be identical or different,
chosen from 0, N
and S,
A5 represents a hydrogen atom or an alkyl radical,
R6b represents hydrogen, alkyl, alkenyl, cycloalkyl, phenyl, phenylallcyl and
cycloalkylalkyl,
all the alkyl, alkenyl, aryl, heteroaryl, aryloxy, cycloalkyl and
heterocycloalkyl radicals present in the
above radicals being optionally substituted with one or more radicals chosen
from halogen atoms and
hydroxyl, cyano, alkyl, alkoxy, amino, alkylamino, dialkylamino, phenylamino,
phenylalkylamino,
acylamino (NH-COR), -C(=0)OR6b, acyl -C(=O)R6b, hydroxyalkyl, carboxyalkyl,
phenoxyalkyl,
S(O),,-alk, S(O)õNH,, S(O)õNH(alk), S(O)n N(alk)2, CF3, OCF3, NO-2, CN,
phenyl, itself optionally
substituted with one or more halogen atoms, thienyl, phenoxy, phenylalkoxy, -
C(=0)-NHZ,
-C(=O)-NH(alk) and C(=O)-N(alk)2 radicals,
all the above alkyl, alkenyl, alkoxy and alkylthio radicals being linear or
branched and containing not
more than 4 carbon atoms,
all the phenyl radicals of the above radicals furthermore being optionally
substituted with a dioxole
radical,
n represents an integer from 0 to 2,
the said compounds of formula (IA) being in any possible racemic, enantiomeric
or diastereoisomeric
isomer form, and also the addition salts with mineral and organic acids or
with mineral and organic
bases of the said compounds of formula (IA).
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-26-
A subject of the present invention is thus the compounds of formula (I) as
defined above,
corresponding to the formula (IAa):
A,a
A2a N
I ~>-Aa
A3a N
A4a A5a
(IAa)
in which Aa represents a pyrazolyl, triazolyl or indazolyl radical, this
heterocycle Aa being optionally
substituted with one or more radicals XA', XA' or XA3 chosen from the values
indicated hereinabove
for the radicals X', X' or X3,
Aia, A2a, A3a and A4a, which may be identical or different, are chosen from a
hydrogen atom,
halogen atoms, hydroxyl, alkyl, alkoxy, nitro, cyano, phenyl and phenoxy
radicals, and a
carboxyl radical which is free, salified, esterified with an alkyl radical or
amidated with a
radical NA6 aA7 a such that either A6 a and A7a, which may be identical or
different, are chosen
from a hydrogen atom and alkyl, phenyl, phenylalkyl, cycloalkylalkyl,
cycloalkyl, furylalkyl,
thienylalkyl and pyridylalkyl radicals, or A6a and A7a form, together with the
nitrogen atom to
which they are attached, a pyrrolidinyl, pyrazolidinyl, pyrazolinyl,
piperidyl, morpholino or
piperazinyl radical optionally substituted on the second nitrogen atom with an
alkyl or phenyl
radical, which are themselves optionally substituted,
it being understood that two consecutive radicals from among Ala, A,a, A3a and
A4a may form,
with the benzimidazole radical to which they are attached, an optionally
substituted 5- to 6-
membered carbon-based ring containing one or two oxygen atoms,
A5a represents a hydrogen atom or an alkyl radical,
the phenyl and phenoxy radicals above being optionally substituted with one or
more radicals
chosen from halogen atoms and hydroxyl, cyano, trifluoromethyl,
trifluoromethoxy, alkyl,
alkoxy, amino, alkylamino, dialkylamino, phenylamino, phenylalkylamino, free,
salified or
esterified carboxyl, and dioxole radicals,
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-27-
all the alkyl, alkoxy and alkylthio radicals above being linear or branched
and containing not
more than 6 carbon atoms,
the said compounds of formula (IAa) being in any possible racemic,
enantiomeric or diastereoisomeric
isomer form, and also the addition salts with mineral and organic acids or
with mineral and organic
bases of the said compounds of formula (IAa).
The substituents X', X2 and X3 as defined above are in particular such that
one represents a hydrogen
atom and the other two, which may be identical or different, are chosen from
halogen atoms and OH,
R4a, OR4a, CF3, OCF3, NO7, CN, NY'aY2a, acylamino (NH-COR6b), S(O)õalk,
S(O)õNH,,
S(O),,-NH(alk), S(O),,-N(alk)2, -C(=O)-NH2,, -C(=O)-NH(alk), C(=O)-N(alk)2, -
C(=O)OR4a,
-N(R'b)C(=O)Wa, -N(R6 b)SO,,R4a, -N(R6b)C(=0)NY'aY2a, -N(R~b)C(=O)OR4a, -
OC(=O)NY'aY2a
and thienyl radicals, the thienyl radical being optionally substituted with an
alkyl radical,
R4a, Y'a, Y2a and R6 b having the values defined above and alk representing a
linear or branched allcyl
radical including not more than 6 carbon atoms and optionally substituted as
indicated above.
All the alkylthio radicals are such that the sulfur atom is optionally
oxidized to sulfone or sulfoxide
with one or two oxygen atoms.
Tables I, II and III described below give examples of compounds illustrating
the present invention, with
in particular substituents chosen from the values of Xl, X2 and X3 as defined
above.
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-28-
TABLE I
R
0
RB ""'". N
):D
`; 1 N
N H
H
o):=O o oo
HN x HN ~ HN ~ Q
I DI 7 'N \ N HN v
N N N
~ N H I ~ N ::c[N
N
~
N ~
H H H H H C H HN.
/
-N):=O H ~.N H
HN \ x HN ~ x HN ~CO x
NN :)CrN N rm\'~ N ~N ~ ~
N H .N
N
H H H H
with X represents hydrogen, halogen or alkoxy as defined above.
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-29-
TABLE II
R`
N-R
` N f N
I-'
ti H
H
H N \N N
CI CI
N O / H N~ ~~N O CI
N N CI ~' ' N
N H ~ N H
H NN O H
N` H H
H H H N~
N I\NN N ~~N o I
N~"\N N H
~ N N H
::cc N H H
H H
N O H H N
)QN~NOC F\N I /
)NJNOC ~ H
H N H H
H
H
~ .S~ ~
N N N;S~ N
~~~ .y' NO OO IN ~ N N NH ~v N H )NJNd"b
N
H H H H
HN
N
~ H N O O )XbocQ N~ ~
H H .y~N
H I N H
H
H ~
\ N ~ N; S CI
I ~NO O, CI
N H
H
in which NR'R represents NYIYZ as defined above.
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-30-
TABLE III
R
N N
)(::CN H
H
OH NH2 HaN HO
x x x X
)C\'NN )I'NN ~ N. ~ N N\N
DCCN H
H H H H N
H
NH2
OH N H 2
O O
....-
x x x
)(~c N N.N )00 N N QN)N
N N
H H H H
in which X represents hydrogen, alkynyl or NHCOCHZPh which is optionally
substituted.
The subject of the present invention is thus the compounds of formula (I) as
defined above in which the
substituents of the said compounds of formula (I) have the any of the values
indicated as defined
hereinabove and in which the aryl radicals represent the phenyl and naphthyl
radicals; the heteroaryl
radicals represent the furyl, thienyl, benzothienyl, thianthrenyl, pyridyl,
pyrazolyl, benzimidazolyl,
benzofuran, isobenzof-uran and dihydrobenzofuran radicals; the cycloalkyl
radicals represent a
cyclopropyl, cyclobutyl, cyclopentyl or cyclohexyl radical; the
heterocycloalkyl radicals represent the
hexahydropyran, piperidyl or morpholino radicals; the heterocycloalkylalkyl
radicals represent the
hexahydropyranylalkyl, piperidylallryl and morpholinoalkyl radicals; the
arylalkyl radicals represent
the phenylalkyl, ethylenedioxyphenylalkyl and naphthylalkyl radicals; the
heteroarylalkyl radicals
represent the thienylalkyl, pyridylalkyl, furylalkyl, pyrazolylalkyl,
benzothienylalkyl,
dihydrobenzofuranylalkyl and benzimidazolylalkyl radicals; the aryloxy
radicals represent the phenoxy
and naphthyloxy radicals; the arylalkoxy radicals represent the phenylalkoxy
and naplithylalkoxy
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-31-
radicals; and the aryloxyalkyl radicals represent the phenoxyalkyl radical;
all these radicals being
optionally substituted as indicated hereinabove.
One subject of the present invention is, more particularly, the compounds of
formula (I) as defined
above corresponding to the formula (IA):
A,
A2 N
I ~>--- A
A N
3 \
A4 A5
(IA)
in which A represents a saturated heterocyclic radical which is either a 5- or
6-membered monocyclic
radical or a bicyclic radical that is not more than 10-membered, these members
being such that at least
two of them represent a nitrogen atom and the others, which may be identical
or different, represent a
carbon member or a hetero atom member chosen from 0, N and S, this heterocycle
A optionally being
substituted with one or more radicals XA', XA2 or XA3 chosen from halogen
atoms, alkyl, alkoxy or
alkylthio radicals or thienyl radicals optionally substituted with an alkyl
radical,
Al, A2, A3 and A4, which may be identical or different, are chosen from a
hydrogen atom, halogen
atoms and hydroxyl, alkyl, alkoxy, nitro, cyano, phenyl and phenoxy radicals,
a carboxyl radical which
is free, salified, esterified with an alkyl radical or amidated with a radical
NA6A' such that either A6
and A7, which may be identical or different, are chosen from a hydrogen atom
and alkyl, phenyl,
phenylalkyl, cycloalkylalkyl, cycloalkyl and heteroarylalkyl radicals, or A6
and A7 form, together with
the nitrogen atom to which they are attached, a 5- or 6-membered cyclic
radical,
it being understood that two consecutive radicals among Al, A2, A3 and A4 can
form, with the
benzimidazole radical to which they are attached, a 5- to 6-membered carbon-
based ring containing one
or more hetero atoms, which may be identical or different, chosen from 0, N
and S,
A5 represents a hydrogen atom or an alkyl radical,
all the phenyl, phenoxy, cycloalkyl and heteroarylalkyl radicals above being
optionally substituted with
one or more radicals chosen from halogen atoms and hydroxyl, cyano,
trifluoromethyl,
trifluoromethoxy, alkyl, alkoxy, amino, alkylamino, dialkylamino, phenylamino,
phenylalkylamino,
free, salified or esterified carboxyl, and dioxole radicals,
all the alkyl, alkoxy and alkylthio radicals above being linear or branched
and containing not more than
6 carbon atoms,
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-32-
the said compounds of formula (IA) being in any possible racemic, enantiomeric
or diastereoisomeric
isomer form, and also the addition salts with mineral and organic acids or
with mineral and organic
bases of the said compounds of formula (IA).
A subject of the present invention is also, more particularly, the compounds
of formula (I) as defined
above, corresponding to the formula (IAb):
Alb
Azb N
I \
Ab
A3b N
A4b '45b (IAb)
in which Ab represents a pyrazolyl or indazolyl radical optionally substituted
with one or two radicals
chosen from halogen atoms and OH, alkyl, alkynyl, -OR6 b (including alkoxy), -
COR6 b, -O-COR6 b,
-OS(O) R6b, -O(CH,,)R CO-R6 b, phenyl, phenylalkyl, CF3, OCF3, NO2, CN,
NY'bY2b,
-NH-C(=O)NY'bY'b, acylamino (NH-CO-R6b), S(O)õalk, S(O)-NY'bY'b, -C(=O)-
NY'bY2b,
-C(=O)OR6 b, -NH-C(=O)R6 b, -NH-S(O)õR6b, -NH-C(=O)OR6b, -N(R6 b)C(=O)NY'bY'b,
-OC(=O)NY'bY'b and thienyl radicals, all these radicals being optionally
substituted,
with NY'bY'b such that either Y'b and Y2b, which may be identical or
different, are chosen from
hydrogen and optionally substituted alkyl, cycloalkyl, cycloalkylalkyl,
phenyl, naphthyl, phenoxy,
phenylalkyl, phenylalkylthio and naphthylalkyl or Ylb and Y2b form, together
with the nitrogen atom
to which they are attached, a piperidyl, hexahydrofuran, morpholinyl or
morpholinylalkyl radical,
Alb, A,b, A3b and A4b, which may be identical or different, are chosen from a
hydrogen atom,
halogen atoms, hydroxyl, alkyl, alkenyl, -OR6b (including alkoxy), -CO-R~b, -O-
COR6 b,
-OS(O)õR'b, -O(CH2)n CO-R6 b, nitro, cyano, furyl, thienyl, benzothienyl,
naphthyl,
thianthrenyl, phenyl and phenoxy radicals and a carboxyl radical which is
free, salified,
esterified with an alkyl radical or amidated with a radical NA6 bA7b such that
either A6b and
A7b, which may be identical or different, are chosen from hydrogen and alkyl,
alkoxyalkyl,
phenoxyalkyl, phenyl, phenylalkyl, cycloalkylalkyl, cycloalkyl, furylalkyl,
naphthylalkyl,
thienylalkyl, piperidylalkyl, pyridylalkyl, benzothienylalkyl, pyrazolylalkyl,
dihydrobenzofuranylalkyl, hexahydropyranylalkyl, ethylenedioxyphenylalkyl and
benz-
imidazolylalkyl radicals, all these radicals being optionally substituted, or
A6b and A7b form,
together with the nitrogen atom to which they are attached, a pyrrolidinyl,
morpholino or
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-33-
piperazinyl radical, the piperazinyl radical being optionally substituted on
the second nitrogen
atom with an alkyl radical itself optionally substituted,
it being understood that two consecutive radicals among Alb, A?b, A3b and A4b
can form, with
the benzimidazole radical to which they are attached, an optionally
substituted
4,5-ethylenedioxybenzimid-azole radical or an optionally substituted
4,5-methylenedioxybenzimidazole radical,
A5b represents a hydrogen atom,
all the above radicals containing alkyl, alkenyl, phenyl, phenoxy, furyl,
thienyl, piperidyl, pyridyl,
pyrazolyl and benzimidazolyl being optionally substituted with one or more
radicals chosen from
halogen atoms and hydroxyl, cyano, alkyl, alkoxy, amino, alkylamino,
dialkylamino, phenylamino,
phenylalkylamino, acylamino (NH-COR6 b), -C(=O)OR6b, acyl -C(=O)R6b,
hydroxyalkyl,
carboxyalkyl, phenoxyalkyl, S(O)õalk, S(O)õNH,, S(O), NH(alk), S(O)õ-N(alk),,
CF3, OCF3, NO?,
CN, phenyl, itself optionally substituted with one or more halogen atoms,
thienyl, phenoxy,
phenylallcoxy, -C(=O)-NH?, -C(=O)-NH(alk) and C(=O)-N(alk)2 radicals,
with n representing an integer from 0 to 2,
and R6 b representing hydrogen, alkyl, alkenyl, cycloalkyl, phenyl, pyridyl,
thienyl, naphthyl, isoxazole,
adamentyl, quinoline, quinolone, dihydroquinolone, -NH-phenyl, phenylalkyl and
cycloalkylalkyl, all
these radicals being optionally substituted with a morpholino, piperidyl or
phenyl radical itself
optionally substituted with one or more radicals chosen from halogen atoms and
the cyano, CF3, OCF3,
alkyl, phenyl-S(O)n-alk-phenyl, alkoxy, NH,,, NHalk, N(alk)2, SO2INH.2,
SO2Nalk or SO2N(alk)2 radical,
all the alkyl, alkenyl, alkoxy and alkylthio radicals above being linear or
branched and
containing not more than 10 carbon atoms,
all the phenyl radicals of the above radicals furthermore being optionally
substituted with a
dioxole radical,
the said compounds of formula (IAb) being in any possible racemic,
enantiomeric or diastereomeric
isomer form, and also the addition salts with mineral and organic acids or
with mineral and organic
bases of the said compounds of formula (IAb).
One subject of the present invention is thus in particular the compounds of
formula (I) as defined
above corresponding to the formula (IAb) in which Ab represents a pyrazolyl or
indazolyl radical
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-34-
optionally substituted with one or two radicals chosen from halogen atoms and
OH, alkyl, alkynyl,
alkoxy, phenyl, phenylalkyl, CF3, OCF3, NO2, CN, NY'bY2b, -NH-C(=O)NY'bY'b,
acylamino
(NH-CO-R6b), S(O), alk, S(O),,-NY'bY'b, -C(=O)-NYIbY'b, -C(=O)ORbb, -NH-
C(=O)R6b,
-NH-S(O)õR6 b, -NH-C(=O)OR6 b, -N(R~b)C(=O)NY'bY'b, -OC(=O)NY'bY'b and thienyl
radicals
which are optionally substituted,
with NY'bY'b such that either Y'b and Y2b, which may be identical or
different, are chosen from
hydrogen and optionally substituted alkyl, cycloalkyl, cycloalkylalkyl,
phenyl, naphthyl, phenoxy,
phenylalkyl, phenylalkylthio and naphthylalkyl or Ylb and Y2b form, together
with the nitrogen atom
to which they are attached, a piperidyl, hexahydrofuran, morpholinyl or
morpholinylalkyl radical,
Aib, A?b, A3b and A4b, which may be identical or different, are chosen from a
hydrogen atom,
halogen atoms, hydroxyl, alkyl, alkenyl, alkoxy, nitro, cyano, furyl, thienyl,
benzothienyl,
naphthyl, thianthrenyl, phenyl and phenoxy radicals and a carboxyl radical
which is free,
salified, esterified with an alkyl radical or amidated with a radical NA6 bA7
b such that either
A6 b and A'b, which may be identical or different, are chosen from hydrogen
and alkyl,
alkoxyalkyl, phenoxyalkyl, phenyl, phenylalkyl, cycloalkylalkyl, cycloalkyl,
furylalkyl,
naphthylalkyl, thienylalkyl, piperidylalkyl, pyridylalkyl, benzothienylalkyl,
pyrazolylalkyl,
dihydrobenzofuranylalkyl, hexahydropyranylalkyl, ethylenedioxyphenylalkyl and
benzimidazolylalkyl radicals, all these radicals being optionally substituted,
or A6 b and A'b
form, together with the nitrogen atom to which they are attached, a
pyrrolidinyl, morpholino or
piperazinyl radical, the piperazinyl radical being optionally substituted on
the second nitrogen
atom with an alkyl radical itself optionally substituted,
it being understood that two consecutive radicals among Alb, A,b, A3b and A4b
can form, with
the benzimidazole radical to which they are attached, an optionally
substituted
4,5-ethylenedioxybenzimidazole radical or an optionally substituted
4,5-methylenedioxybenzimidazole radical,
A5b represents a hydrogen atom,
all the above radicals containing alkyl, alkenyl, phenyl, phenoxy, furyl,
thienyl, piperidyl, pyridyl,
pyrazolyl and benzimidazolyl being optionally substituted with one or more
radicals chosen from
halogen atoms and hydroxyl, cyano, alkyl, alkoxy, amino, alkylamino,
dialkylamino, phenylamino,
phenylalkylamino, acylamino (NH-COR6b), -C(=O)OR6b, acyl -C(=O)R6b,
hydroxyalkyl,
carboxyalkyl, phenoxyalkyl, S(O),; alk, S(O)õNH2, S(O)õNH(alk), S(O),,-
N(alk)2, CF3, OCF3, NOz,
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-35-
CN, phenyl, itself optionally substituted with one or more halogen atoms,
thienyl, phenoxy,
phenylalkoxy, -C(=O)-NH2, -C(=O)-NH(alk) and C(=0)-N(alk)2 radicals,
with n representing an integer from 0 to 2,
and R~b representing hydrogen, allcyl, alkenyl, cycloalkyl, phenyl,
phenylalkyl and cycloalkylalkyl,
all the alkyl, alkenyl, alkoxy and alkylthio radicals above being linear or
branched and
containing not more than 10 carbon atoms,
all the phenyl radicals of the above radicals furthermore being optionally
substituted with a
dioxole radical,
the said compounds of formula (lAb) being in any possible racemic,
enantiomeric or diastereoisomeric
isomer form, and also the addition salts with mineral and organic acids or
with mineral and organic
bases of the said compounds of formula (IAb).
A subject of the present invention is thus in particular the compounds of
formula (I) as defined above
corresponding to the formula (lAb) in which Ab represents a pyrazolyl radical
substituted with one or
two radicals such that one is chosen from hydrogen, halogen atoms and alkyl,
alkynyl, -COR6 b, phenyl,
phenylalkyl, CF3, NO2, CN, NY'bY'b, -NH-C(=0)NY'bY2b, NH-CO-R6 b, S(O),,-alk,
S(O),,-NY'bY'b,
-C(=O)-NY'bY2b, -C(=O)OR6b, -NH-C(=O)R6b, -NH-S(O)õR6b, -NH-C(=O)OR6b,
-N(R6 b)C(=O)NY'bY'b and thienyl radicals, all these radicals being optionally
substituted,
and the other is chosen from OH, -OR6b, -O-COR6 b, -OS(O),,R6b, -O(CHZ)n CO-
R6b and
-OC(=O)NY'bY'b radicals, all these radicals being optionally substituted,
with NYIbY'b such that Y'b and Y2b, which may be identical or different, are
chosen from hydrogen
and optionally substituted alkyl, cycloalkyl, cycloalkylalkyl, phenyl,
naphthyl, phenoxy, phenylalkyl,
phenylalkylthio and naphthylalkyl or Y'b and Y2b form, together with the
nitrogen atom to which they
are attached, a piperidyl, hexahydrofuran, morpholinyl or morpholinylalkyl
radical,
Alb, A2b, A3b and A4b, which may be identical or different, are such that two
of them represent
hydrogen and the other two, which may be identical or different, are chosen
from a hydrogen
atom, halogen atoms, hydroxyl, alkyl, alkenyl, -OR6 b (including alkoxy), -CO-
R6b, -O-COR6b,
-OS(O)õR6b, -O(CH,)R CO-R6b, nitro, cyano, furyl, thienyl, benzothienyl,
naphthyl,
thianthrenyl, phenyl and phenoxy radicals and a carboxyl radical which is
free, salified,
esterified with an alkyl radical or amidated with a radical NA6 bA7b such that
either A6 b and
A7b, which may be identical or different, are chosen from hydrogen and alkyl,
alkoxyalkyl,
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-36-
phenoxyalkyl, phenyl, phenylalkyl, cycloalkylalkyl, cycloalkyl, furylalkyl,
naphthylalkyl,
thienylalkyl, piperidylalkyl, pyridylalkyl, benzothienylalkyl, pyrazolylalkyl,
dihydrobenzofuranylalkyl, hexahydropyranylalkyl, ethylenedioxyphenylalkyl and
benz-
imidazolylalkyl radicals, all these radicals being optionally substituted, or
A6b and A7b foim,
together with the nitrogen atom to which they are attached, a pyrrolidinyl,
morpholino or
piperazinyl radical, the piperazinyl radical being optionally substituted on
the second nitrogen
atom with an alkyl radical itself optionally substituted,
A5b represents a hydrogen atom,
all the above radicals containing alkyl, alkenyl, phenyl, phenoxy, furyl,
thienyl, piperidyl, pyridyl,
pyrazolyl and benzimidazolyl being optionally substituted with one or more
radicals chosen from
halogen atoms and hydroxyl, cyano, alkyl, alkoxy, amino, alkylamino,
dialkylamino, phenylamino,
phenylalkylamino, acylamino (NH-COR6 b), -C(=O)OR6b, acyl -C(=O)R6b,
hydroxyalkyl,
carboxyalkyl, phenoxyallcyl, S(O), alk, S(O)õNH,, S(O),,-NH(alk), S(O),,-
N(alk),, CF3, OCF3, NO2,
CN, phenyl, itself optionally substituted with one or more halogen atoms,
thienyl, phenoxy,
phenylalkoxy, -C(=O)-NH2, -C(=O)-NH(alk) and C(=O)-N(alk)2 radicals,
with n representing an integer from 0 to 2,
and R6b representing hydrogen, alkyl, alkenyl, cycloalkyl, phenyl, pyridyl,
thienyl, naphthyl, isoxazole,
adamentyl, quinoline, quinolone, dihydroquinolone, -NH-phenyl, phenylalkyl and
cycloalkylalkyl, all
these radicals being optionally substituted with a morpholino, piperidyl or
phenyl radical itself
optionally substituted with one or more radicals chosen from halogen atoms and
the cyano, CF3, OCF3,
alkyl, phenyl-S(O)n-alk-phenyl, alkoxy, NH2, NHalk, N(alk)2, SO2NH2, SOzNalk
or SO,N(alk), radical,
all the alkyl, alkenyl, alkoxy and alkylthio radicals above being linear or
branched and
containing not more than 10 carbon atoms,
all the phenyl radicals of the above radicals furthermore being optionally
substituted with a
dioxole radical,
the said compounds of formula (IAb) being in any possible racemic,
enantiomeric or diastereoisomeric
isomer form, and also the addition salts with mineral and organic acids or
with mineral and organic
bases of the said compounds of forrnula (IAb).
A subject of the present invention is thus in particular the compounds of
formula (I) as defined above
corresponding to the formula (IAb) in which Ab represents a pyrazolyl or
indazolyl radical optionally
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-37-
substituted with one or more radicals chosen from halogen atoms and alkyl,
alkoxy and thienyl
radicals,
Alb, A-,b, A3b and A4b, which may be identical or different, are chosen from a
hydrogen atom;
halogen atoms; radicals of the following types: hydroxyl, alkyl, alkenyl
optionally substituted
with phenyl itself optionally substituted with one or more halogen atoms,
alkoxy, nitro, cyano,
furyl, thienyl optionally substituted with acyl COalk, benzothienyl, naphthyl,
thianthrenyl,
phenyl and phenoxy which are optionally substituted; and a carboxyl radical
which is free,
salified, esterified with an alkyl radical or amidated with a radical NA6 bA7
b such that either
A6 b and A'b, which may be identical or different, are chosen from hydrogen
and radicals of the
following types : alkyl, alkoxyalkyl containing not more than 6 carbon atoms,
phenoxyalkyl
optionally substituted with acylamino NH-C(O)alk, phenyl, optionally
substituted phenylalkyl,
cycloalkylalkyl, cycloalkyl, furylalkyl optionally substituted with one or
more alkyl radicals,
naphthylalkyl, thienylalkyl optionally substituted with alkyl or thienyl,
piperidylalkyl
optionally substituted with a carboxyl radical which is free, salified or
esterified with an alkyl
radical, pyridylalkyl optionally substituted with one or more radicals chosen
from halogen and
CF3, benzothienylallcyl, pyrazolylalkyl optionally substituted with one or
more alkyl radicals,
dihydrobenzofuranylalkyl, hexahydropyranylalkyl, ethylenedioxyphenylalkyl, and
benzimidazolylalkyl optionally substituted with one or more alkyl radicals,
or A6 b and A7b form, together with the nitrogen atom to which they are
attached, a
pyrrolidinyl, morpholino or piperazinyl radical, the piperazinyl radical being
optionally
substituted on the second nitrogen atom with an alkyl radical,
it being understood that two consecutive radicals among Aib, A,,b, A3b and A4b
can form, with
the benzimidazole radical to which they are attached, an optionally
substituted
4,5-ethylenedioxybenzimidazole radical or an optionally substituted
4,5-methylenedioxybenzimidazole radical,
A5a represents a hydrogen atom,
the phenyl, phenoxy and phenylalkyl radicals above being optionally
substituted with one or more
radicals chosen from halogen atoms, hydroxyl, cyano, alkyl, alkoxy, amino,
alkylamino, dialkylamino,
phenylamino, phenylalkylamino and NH-COalk radicals, a carboxyl radical which
is free, salified or
esterified with an alkyl radical, and hydroxyalkyl, carboxyalkyl,
phenoxyalkyl, alkylthio, SO2alk,
SO-2NH2, S02-NH(alk), SO,,-N(alk)z, CF3, OCF3, NOZ, CN, phenyl, itself
optionally substituted with
one or more halogen atoms, thienyl, phenoxy, phenylalkoxy, -C(=O)-NH2, -C(=0)-
NH(alk),
C(=O)-N(alk)2 and C(O)CH3 radicals,
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-38-
all the allryl or alk, alkenyl, alkoxy and alkylthio radicals above being
linear or branched and
containing not more than 4 carbon atoms,
all the phenyl radicals of the above radicals furthermore being optionally
substituted with a dioxole
radical,
the said compounds of formula (IAb) being in any possible racemic,
enantiomeric or diastereoisomeric
isomer form, and also the addition salts with mineral and organic acids or
with mineral and organic
bases of the said compounds of formula (IAb).
A subject of the present invention is thus in particular the compounds of
formula (I) as defined above
corresponding to the formula (IAb) in which Ab, Alb, A~b, A3b, A4b and A5b
have any of the meanings
indicated hereinabove,
and when one of Alb, A2b, A3b and A4b represents a carboxyl radical amidated
with a radical
NA6 bA'b, then either one of A6 b and A'b represents a hydrogen atom or an
alkyl radical and
the other of A6 b and A'b is chosen from the values defined for A6 b and A'b,
or A6 b and A7b
form, together with the nitrogen atom to which they are attached, a 5- or 6-
membered cyclic
radical,
the other substituents of the said compounds of formula (I) having the any of
the values
indicated hereinabove,
the said compounds of formula (IAb) being in any possible racemic,
enantiomeric or diastereoisomeric
isomer form, and also the addition salts with mineral and organic acids or
with mineral and organic
bases of the said compounds of formula (IAb).
A subject of the present invention is thus in particular the compounds of
formula (I) as defined above
in which X, W, Y and Z are such that two or three of them represent CH and the
others are chosen
from the values of CR' or CR3 and, if appropriate, i.e., when two of them
represent CH and CRZ and
CR3 are adjacent to each other, can form a dioxole radical,
R', R3 and the other substituents of the said compounds of formula (I) having
any of the values as
defined hereinabove,
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-39-
the said compounds of formula (I) being in any possible racemic, enantiomeric
or diastereoisomeric
isomer form, and also the addition salts with mineral and organic acids or
with mineral and organic
bases of the said compounds of formula (I).
The present invention thus relates in particular to the compounds of formula
(IA) as defined above in
which Al, & A3 and A4 are such that two or three of them represent a hydrogen
atom and the others
are chosen from the values of Al, A2, A3 and A4 and, if appropriate, i.e.,
when two of them represent a
hydrogen atom and the other two are on adjacent carbons, can form a dioxole
radical,
the other substituents of the compounds of formula (IA) having any of the
values as defined
hereinabove,
the said compounds of formula (IA) being in any possible racemic, enantiomeric
or diastereoisomeric
isomer form, and also the addition salts with mineral and organic acids or
with mineral and organic
bases of the said compounds of formula (IA).
A subject of the present invention is also, more particularly, the compounds
of formula (I) as defined
above, corresponding to the formula (IAa):
Ala
A2a N
I \> Aa
A3a N
A4a A5a (IAa)
in which Aa represents a pyrazolyl, triazolyl or indazolyl radical, this
heterocycle Aa being optionally
substituted with one or more radicals XA1, XA2 or XA3 chosen from halogen
atoms, alkyl, alkoxy or
alkylthio radicals and thienyl radicals optionally substituted with an alkyl
radical,
Ala, A,,a, A3a and A4a, which may be identical or different, are chosen from a
hydrogen atom, halogen
atoms, hydroxyl, alkyl, alkoxy, nitro, cyano, phenyl and phenoxy radicals, and
a carboxyl radical which
is free, salified, esterified with an alkyl radical or amidated with a radical
NA6 aA'a such that either A6 a
and A7 a, which may be identical or different, are chosen from a hydrogen atom
and alkyl, phenyl,
phenylalkyl, cycloalkylalkyl, cycloalkyl, furylalkyl, thienylalkyl and
pyridylalkyl radicals, or A6a and
A7a form, together with the nitrogen atom to which they are attached, a
pyrrolidinyl, pyrazolidinyl,
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-40-
pyrazolinyl, piperidyl, morpholino or piperazinyl radical optionally
substituted on the second nitrogen
atom with an alkyl or phenyl radical, which are themselves optionally
substituted,
it being understood that two consecutive radicals from among Ala, A,,a, A3a
and A4a may form, with
the benzimidazole radical to which they are attached, an optionally
substituted 5- to 6-membered
carbon-based ring containing one or two oxygen atoms,
A5a represents a hydrogen atom or an alkyl radical,
the phenyl and phenoxy radicals above being optionally substituted with one or
more radicals chosen
from halogen atoms and hydroxyl, cyano, trifluoromethyl, trifluoromethoxy,
alkyl, alkoxy, amino,
alkylamino, dialkylamino, phenylamino, phenylalkylamino, free, salified or
esterified carboxyl, and
dioxole radicals,
all the alkyl, alkoxy and alkylthio radicals above being linear or branched
and containing not more than
6 carbon atoms,
the said compounds of formula (IAa) being in any possible racemic,
enantiomeric or diastereoisomeric
isomer form, and also the addition salts with mineral and organic acids or
with mineral and organic
bases of the said compounds of formula (IAa).
One subject of the present invention is, more particularly, the compounds of
formula (I) as defined
above in which R represents a pyrazolyl or indazolyl radical, the other
substituents having the values
indicated above or below.
Among the preferred compounds that are particularly noted are the compounds of
formula (I) in which
Aa represents a pyrazolyl or indazolyl radical optionally substituted as
indicated above and below,
Ala, A?a, A3a and A4a are chosen from the following values:
- Aia represents hydrogen or carboxyl or forms a ring with
the adjacent member A,,a
- A4a represents hydrogen or carboxyl or forms a ring with the adjacent member
A3a
- A2a represents a carboxyl radical that is free, salified, esterified with an
optionally substituted alkyl
radical or an amidated carboxyl as indicated above or below,
- A2a and A3a represent two optionally substituted alkyl radicals,
A5 represents hydrogen.
One subject of the present invention is, even more particularly, the compounds
of formula (I) as
defined above, corresponding to the formula (IAb):
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-41-
Al' b
AP N
~}-- Ab
y\
A3b
A4b A5b (lAb)
in which Ab represents a pyrazolyl or indazolyl radical optionally substituted
with one or more radicals
chosen from halogen atoms and alkyl, alkoxy and thienyl radicals,
Alb, A~b, A3b and A4b, which may be identical or different, are chosen from a
hydrogen atom, halogen
atoms, hydroxyl, alkyl and alkoxy, nitro, cyano, phenyl and phenoxy radicals,
and a carboxyl radical
that is free, salified, esterified with an alkyl radical or amidated with a
radical NA6 bA'b such that
either A6 b and A7 b, which may be identical or different, are chosen from
alkyl, phenyl, phenylalkyl,
cycloalkylalkyl, cycloalkyl and furylalkyl radicals, or A6b and A'b form,
together with the nitrogen
atom to which they are attached, a pyrrolidinyl, morpholino or piperazinyl
radical optionally
substituted on the second nitrogen atom with an alkyl radical,
it being understood that two consecutive radicals from among Alb, A~b, A3b and
A4b may form, with
the benzimidazole radical to which they are attached, an optionally
substituted
4,5-ethylenedioxybenzimidazole radical or 4,5-methylenedioxybenzimidazole
radical,
A5b represents a hydrogen atom,
the phenyl and phenoxy radicals above being optionally substituted with one or
more radicals chosen
from halogen atoms and hydroxyl, cyano, alkyl, alkoxy, amino, alkylamino,
dialkylamino,
phenylamino, phenylalkylamino and free, salified or esterified carboxyl
radicals,
all the alkyl, alkoxy and alkylthio radicals above being linear or branched
and containing not more than
4 carbon atoms,
the said compounds of formula (IAb) being in any possible racemic,
enantiomeric or diastereoisomeric
isomer from, and also the addition salts with mineral and organic acids or
with mineral and organic
bases of the said compounds of formula (IAb).
With reference to formula (Ix) above, the following are particular and
preferred groupings:
Rl may particularly represent optionally substituted heteroaryl. Exemplary
optionally substituted
heteroaryls include dihydrofuropyrazolyl, imidazolyl, indazolyl, indolyl,
isoxazolyl,
oxodihydropyridazinyl, oxodihydropyridinopyrazolyl, oxodihydropyridinyl,
oxotetrahydropyrrolopyrazolyl, pyrazolyl, thiazolyl, thienopyrazolyl,
tetrahydrocyclopentapyrazolyl,
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-42-
tetrahydroindazolyl, tetrahydropyranopyrazolyl, tetahydropyridinopyrazolyl,
tetrahydropyrrolopyrazolyl or triazolyl. Optional substituents include one or
more groups selected
from carboxy, cyano, halo, haloalkyl, hydroxy, nitro, R4, -C(=O)R4, -
C(=O)NYlY2, -C(=O)OR4,
-N(R6)C(=O)R4, -N(R6)C(=O)NYlY2, -N(R6)C(=O)OR4, -N(R6)SO2R4, -N(R6)SO2NYlY2,
-NYlY2, -OR4, -OCF2H, -OCF3, -OC(=O)R4, -OC(=0)NYlY2, -S(O)nR4 and -
S(0)2NYIY2. Rl
R9
Rs
more preferably represents a heteroaryl moiety in which R7, R8 and R9 are as
N--N R7
hereinbefore defined. It will be appreciated that compounds of formula (Ix) in
which Rl represents a
R9
Rs
heteroaryl moiety and R7 is hydrogen can exist in the tautomeric forms
N'N R7
R9 ~
s R
Y N Z Rs
jQNH W H X' W N N--NH
QR 9 R9
Z N Z 10 I and Y ~
W CN --N Xl- W N N' N
H H H
W may particularly represent CH when X is CR2, Y is CH or CR3 an.d Z are CH or
CR3.
W may also particularly represent CH when X is N, Y is CH or CR3 and Z is CH
or CR3.
W may also particularly represent N when X is CH or CR2, Y is CH or CR3 and Z
is CH or CR3.
W may also particularly represent N when X is CH or CR2, Y is CH or CR3 and Z
is N.
It is to be understood that this invention covers all appropriate combinations
of the particular and
preferred groupings referred to herein.
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-43-
A particular group of compounds of the invention are compounds of formula
(Ixa):-
R9
Y C N Rs
~
~
W H N~N R7
(Ixa)
in which W, X, Y, Z and R7 are as hereinbefore defined for compounds of
formula (Ix), and R8 and R9
are independently selected from hydrogen, carboxy, cyano, halo, haloalkyl,
hydroxy, nitro, R4,
-C(=O)R4, -C(=O)NYIY2, -C(=O)OR4, -N(R6)C(=O)R4, -N(R6)C(=O)NYlY2, -
N(R6)C(=O)OR4,
-N(R6)S02R4, -NYlY2, -OR4, -OC(=O)R4, -OC(=O)NYlY2, -S(O)nR4 and -S(O)2NYlY2;
and their
corresponding N-oxides, and their prodrugs, and their acid bioisosteres; and
pharmaceutically
acceptable salts and solvates (e.g. hydrates) of compounds of formula (Ixa)
and their N-oxides and their
prodrugs, and their acid bioisosteres.
Compounds of formula (Ixa) in which W represents CH, X represents CH, Y
represents CH and Z
represents CH or C-CH3 are preferred.
Compounds of formula (Ixa) in which W represents CH, X represents CH, Z
represents CH and Y
represents:
(i) C-Cl-q.allcyl [e.g. C-CH3, C-CH2CH3'C-CH2CH2CH3 or C-CH(CH3)2];
CH3 CH3
(ii) C-aryl [e.g. C / \ , C / \ , C / \
CN CH3O
CH3 , C / \ , C / \ , C / \ Cl ,
F O
-~
C / \ or C / O ,];
(iii) C-CN;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-44-
(iv) C-NO2;
(v) C-halo [e.g. C-Br, C-Cl or C-F];
(vi) C-haloalkyl [e.g. C-CF3];
N
(vii) C-heteroaryl [e.g. C / \N or C
(viii) C-OR4 [e.g. C-OCH3 , C-OCH2CH3 , C-OCHFZ , C-OCF3 , C-O / \
C-O-CH, or C-O-(CH2)2 N --~O
(ix) C-C.(=0)R4 [e.g. C-C(=0) o ];
(x) C-C=O)NYlY2 [e.g. C-C(=O)-NH-CH3 , C-C(=O)-N(CH3)z ,
C-C(=O)-NH-CH,CH3 , C-C(=O)-NH-CH(CH3)2 ,
C-C(=O)-NH-C(CH3),-CH,OH , C-C(=O)-NH-CH,CHZCN ,
C-C(=O)-NH-CH2CH,OCH3 , C-C(=O)-NH-CH2
~
CH3 CH3
C-C(=O)-NH-CH, / \ , C-C(=O)-NH-CHZ / \ ,
C.-C(=O)-NH-CHZ / CH3 C-C(=O)-NH-CHz / \
N-
C-C(=O)-NH-CH2 / \ C-C(=O)-NH-(CH2)2 / \
-N -
C-C(=O)-NH-(CH,)Z N O, C-C(=O)-NH-(CH,)Z N ,
\-j
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-45-
H
N-- \
C-C(=O)-NH-(C,H,)<\ N , C-C(=O)-NH-(CH,)3 N
N/ ~N
O
C-C(=O)-NH-(CH,)3 N or C-C(=0)-NH
(xi) C-C(=O)OR4 [e.g. C-C(=0)OH or C-C(=O)OCH3];
(xii) C-NHC(=0)R4 [e.g. C-NHC(=O)CH3, C-NHC(=0)CH(CH3)2,
C-NH-C(=O) o or C-NH-C(=O)-CH2 \ /]; or
(xiii) C-CH(OH)aryl [e.g. C-CH(OH) \ / ];
(xiv) C-S(O)2NYlY2 [e.g. C-SO; NH-CHz
(xv) C-S(O)nR4 [e.g. C-SO2CH3];
are also preferred.
Compounds of formula (Ixa) in which W represents CH, X represents C-CH3, C-
CH2CH3,
C-CH(CH3)2, C-OCH3, C-OCH2CH3, C-Br or C-Cl, Y represents C-CH3, C-CH2CH3, C-
OCH3,
C-Br, C-Cl, C-F, C / \ or C-C(=O)-NH-CH` / \ and Z represents CH are also
preferred.
Compounds of formula (Ixa) in which W represents CH, X represents CH, Y
represents C-CH3 and Z
represents C-CH3 are also preferred.
Compounds of formula (Ixa) in which W represents CH, X represents CR2 and Y
represents CR3
where R2 and R3 form the group -CH2-O-CH2-, and Z represents CH are also
preferred.
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-46-
Compounds of formula (Ixa) in which W represents CH, X represents CR2 and Y
represents CR3
where R2 and R3 form the group -CH7)-CH2-CH2-, and Z represents CH are also
preferred.
Compounds of formula (Ixa) in which R7 represents hydrogen are preferred.
Compounds of formula (Ixa) in which R8 represents:
(i) hydrogen;
(ii) C1-4alkyl [e.g. CH3, CH2CH3, CH(CH3)2 or CH(CH3)CH2CH3];
(iii) -SR`i [e.g. -S-CH3 , -S-CH2CH3 or -S-CH---a
-S-CHz / \ , -S-CH2 O-OCH 3 ,
N S
-S-CH,-CH, / ~ , -S-CH, / ~ or -S-CH2
~ I
(iv) -NYlY2 [e.g. or
\-j
(v) -OR5 [e.g. -OCH?CH3]
are preferred.
Compounds of formula (Ixa) in which R9 represents:
(i) hydrogen;
(ii) C1-7alkyl [e.g. -CH3, -CH2CH2CH3, -CH(CH3)2 or -CH2-CH2-CH(CH3)2];
(iii) aryl [e.g. phenyl];
(iv) -C(=O)NY'Y2 [e.g. -C(=O)-NH-CH2CH3 , -C(=O)-NH-CHzCH,CH3 ,
-C(=O)-NH-CHzCH(CH3)2 , -C(=O)-NH-CH(CH3), ,
-C(=O)-NH-C(CH3)3 , -C(=O)-NH-C(CH3)2CH2OH ,
-C(=O)-NH-CH2CH,OCH3 , -C(=O)-N(CH3)2 , -C(=O)-N(CH,CH3)Z ,
-C(=O)-NH--a I -C(=O)-NH-CHa or -C(=O)-NH O ];
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-47-
(v) -N(R6)C(=O)R¾, particularly -NHC(=O)R4, in which (a) R4 is alkyl
optionally
substituted by aryl, cycloalkyl, heteroaryl, heterocycloalkyl, NYlY2 or -OR5
[e.g.
-NH-C(=O)-CH3 , -NH-C(=O)-(CH,),CH3 , -NH-C(=O)-CH(CH3), ,
-NH-C(=O)-C(CH3)3 , -NH-C(=O)-CH2CH(CH3)1 ,
-NH-C(=O)-CH(CH3)CH,CH3 , -NH-C(=O)-CH2C(CH3)3 ,
-NH-C(=O)-CH2
-NH-C(=0)-CI z<
~- N
-NH-C(=O)-CH2 N/\ / N , -NH-C(=O)-CHZ N(CH3)2 ,
N
~
-NH-C(=O)-CHTN -NH-C(=O)-CHz N O or
-NH-C(=O)-CHZOCH3 ], (b) R4 is aryl [e.g.
H3C
-NH-C(=O) \ / , -NH-C(=O) or
-NH-C(=O) \ / CH3 ], (c) R4 is cycloalkyl [e.g. -NH-C(=O)---<
or -NH-C(=O) ], (d) R4 is heteroaryl [e.g. -NH-C(=O) 0~/
O'
H;C
O
-NH-C(=0)
iN O
H C ' -NH-C(=0) /~ or
3
-NH-C(=O) C\N ] or (e) heterocycloalkyl [e.g.
-NH-C(=O) O ];
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-48-
(vi) -N(R6)C(=0)NYIY', particularly -NHC(=O)NY'Y' [e.g. -NH-C(=0)-NHCH3 ,
-NH-C(=0)-NHCH,CH3 , -NH-C(=0)-NHCH(CH3)2 '
-NH-C(=O)-NHCH,CH(CH3)Z ' -NH-C(=O)-NHC(CH3); ~
-NH-C(=O)-N(CH3)2 ' -NH-C(=O)-N(CH,CH3); 1
-NH-C(=0)-NH--a > -NH-C(=O)-NH-CH,a
-NH-C(=0)-NH-CH:, / \ , -NH-C(=O)-NH / \ ,
-NH-C(=O)-N , -NH-C(=O)- N N-CH3 or
-NH-C(=0)-N O ];
(vii) -NYlY2 [e.g. -NHZ]; or
(viii) alkyl substituted by -N(R6)C(=O)NYlY2 [e.g. -CH2-NH-C(=O)-CH(CH3)2 or
-CHz NH-C(=0)- O 1;
are preferred.
A preferred group of compounds of the invention are compounds of formula (Ixa)
in which:- W
represents CH; X represents CH; Y represents CH; Z represents CH or C-CH3; R7
represents
hydrogen; R8 represents (i) hydrogen, (ii) C1-4alkyl [e.g. CH3, CH2CH3,
CH(CH3)2 or
CH(CH3)CH2CH3], (iii) -SR4 [e.g. -S-CH3 , -S-CH1CH3 or -S-CH---<
,
-S-CH2/ \ , -S-CH2 / Y-OCH 3 , -S-CH2 CH2 01
N
-S-CHZ ~ ~ or -S-CHZ (iv) -NYlY2 [e.g. -N O ] or (v) -OR5
--
[e.g. -OCH2CH3]; R9 represents (i) hydrogen; (ii) C1-7alkyl [e.g. -CH3, -
CH2CH2CH3, -CH(CH3)2
or -CH2-CH2-CH(CH3)2]; (iii) aryl [e.g. phenyl]; (iv) -C(=O)NY'Y2 [e.g.
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-49-
-C(=O)-NII-CH2CH3 , -C(=O)-NH-CH,CH,CH3 , -C(=O)-NH-CHZCH(CH)2 ,
-C(=O)-NH-CH(C.H3)21 -C(=O)-NH-C(CH3)3 1 -C(=O)-NH-C(CH3)2 CH,OH ,
-C(=O)-NH-CH,CH1OCH , -C(=O)-N(C.H3)Z , -C(=O)-N(CH,CH;), ,
-C(=O)-NH--a -C(=O)-NH-CH,< or --C(=0)-NH O
(v) -N(R6)C(=O)W, particularly -NHC(=O)R4, in which (a) R4 is alkyl optionally
substituted by aryl,
cycloalkyl, heteroaryl, heterocycloalkyl, NYlY2 or -OR5 [e.g. -NH-C(=O)-CH3,
-NH-C(=O)-(CH2)2 CH3 , -NH-C(=O)-CH(CH3), , -NH-C(=O)-C(C.H3)3 ,
-NH-C(=O)-CH2CH(CH3)2 , -NH-C(=O)-CH(CH3)CH,CH3 ,
-NH-C(=O)-CH,C(CH3)3 , -NH-C(=O)-CH2/ \ , -NH-C(=0)-CH2 < ,
~-N
-NH-C(=O)-CH` N/\ / N , -NH-C.(=O)-CH; N(CH3)2,
N
~
-NH-C(=0)-CH2 - N , -NH-C(=0)-CH2 -N 0 or
-NH-C(=O)-CH2OCH3 ], (b) R4 is aryl [e.g.
H3C
-NH-C(=O) \ / , -NH-C(=O) or -NH-C(=O) \ / CH3 ],
(c) R4 is cycloalkyl [e.g. -NH-C(=O)--<j or -NH-C(=O) ], (d) R4 is heteroaryl
H3C
O
-NH-C(=0) N
or
[e.g. -NH-C(=0) N -NH-C(=0) ~O
p HC
-NH-C(=O) C\N ] or (e) heterocycloalkyl [e.g. -NH-C(=O) O ];
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-50-
(vi) -N(R6)C(=O)NYIY', particularly -NHC(=O)NYiY' [e.g. -NH-C(=0)-NHCH3 ,
-NH-C(=O)-NHCH1CH3 , -Nff-C( O)-NHCH(CH3)z ~
~
-NH-C(=O)-NHCH,CH(CH;)1' -NH-C(=O)-NHC(CH3)3 ' -NH-C(=O)-N(CH3)1
-NH-C(=O)-N(CH,CH3)2, -NH-C(=0)-NH-a , -NH-C(=0)-NH-CHZa
-NH-C(=O)-NH-CH2 / \ , -NH-C(=O)-NH / \
-NH-C(=0)-N , -NH-C(=0)-N N-CH; or -NH-C(=0)-N O ],
(vii) -NYlY2 [e.g. -NH2] or (viii) alkyl substituted by -N(R6)C(=O)NYlY2 [e.g.
-CH2-NH-C(=O)-
CH(CH3)2 or -CHNH-C(=O)- Oand their corresponding N-oxides, and their
-
prodrugs, and their acid bioisosteres; and pharmaceutically acceptable salts
and solvates (e.g. hydrates)
of compounds of formula (Ixa) and their N-oxides and their prodrugs, and their
acid bioisosteres.
A further preferred group of compounds of the invention are coinpounds of
formula (Ixa) in which:- W
represents CH; X represents CH; Z represents CH; Y represents (i) C-C1-4alkyl
[e.g. C-CH3,
CH3
C-CH2CH3'C-CH2CH2CH3 or C-CH(CH3)2], (ii) C-aryl [e.g. C / \, C / \,
O
CH3 CN CHib
C / \ , C / \ CH3 , C / \ , C , F O~-I
C Cl, C / \ or C / O ], (iii) C-CN, (iv) C-N02, (v) C-halo
[e.g. C-Br, C-Cl or C-F], (vi) C-haloalkyl [e.g. C-CF3], (vii) C-heteroaryl
[e.g. C / \N or
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-51-
C (viii) C-OR4 [e.g. C-OCH3 , C-OCH2CH3 , C-OCHFZ , C-OCF3,
C-0 C-O-C.Hz / \ or C-O-(CH2); N O], (ix) C-C(=0)R4 [e.g.
-
C-C(=O) \ / ], (x) C-C=O)NYlY2 [e.g. C-C(=O)-NH-CH3 , C-C(=O)-N(CH3)2,
C-C(=O)-NH-CH,CH3 , C-C.(=O)-NH-CH(CH3), , C-C(=O)-NH-C(CH3)2 -C.H2OH ,
C-C(=O)-NH-CHzCHZCN , C-C(=O)-NH-CH2CH2 OCH3 ,
C3
C-C(=O)-NH-CH, / \ , C-C(=O)-NH-CH, / \ ,
CH3
C-C(=O)-NH-CH2 / \ , C-C(=O)-NH-CH2 O-CH 3 ,
C-C(=0)-NH-CHZ C-C(=0)-NH-CH, / \
-N
C-C(=O)-NH-(CHz), / \ , C-C(=O)-NH-(CH,)Z N 05
-
~
C-C(=O)-NH-(CH,)y N , C-C(=O)-NH-(C.HZ)3 NN ,
H O
N--
C-C(=O)-NH-(CHz)z ~ N, C-C(=O)-NH-(CH2)3 N N
N~
or C-C(=O)-NH \ /], (xi) C-C(=O)OR4 [e.g. C-C(=0)OH or C-C(=O)OCH3], (xii)
C-NHC(=O)R4 [e.g. C-NHC(=O)CH3 or C-NHC(=0)CH(CH3)2, C-NH-C(=0) \ f or
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-52-
C-NH-C(=O)-C(=O)-CH, \ / ], (xiii) C-CH(OH)aryl [e.g. C-CH(OH) o ],
(xiv) C-S(O)2NYIY2 [e.g. C-SO; NH-CHZ / \] or (xv) C S(O)nR4 [e.g. C-SO2CH3];
R7
represents hydrogen; R8 represents (i) hydrogen, (ii) C.1-4alkyl [e.g. CH3,
CH2CH3, CH(CH3)2 or
CH(CH3)CH2 )CH3], (iii) -SR4 [e.g. -S-CH3 , -S-CHCH3 or -S-CHz---a
-S-CH -S-CH2/ \ OCH3 , -S-CH` CHZ / \ ,
S
ON
-S-CH
or -S-CHZ ~ I ], (iv) -NYlY2 [e.g. -N O ] or (v) -OR5 [e.g. -OCH2CH3]; R9
represents (i) hydrogen; (ii) Cl-7alkyl [e.g. -CH3, -CH?CH2CH3, -CH(CH3)2
or -CH2-CH2-CH(CH3)2]; (iii) aryl [e.g. phenyl]; (iv) -C(=O)NYIY2 [e.g.
-C(=O)-NH-CH,CH3, -C(=O)-NH-CH,CHiCH, -C(=O)-NH-CH,CH(CH3)2,
-C(=O)-NH-CH(CI~,), , -C.(=O)-NH-C(CH)3 1 -C(=O)-NH-C(CH3)ZCH,OH ,
-C(=O)-NH-CH,CH2OCH3 , -C(=O)-N(CH)21 -C(=O)-N(CH,CH: ), ,
-C(=O)-NH----a I -C(=O)-NH-CHa or -C(=0)-NH O
(v) -N(R6)C(=O)R4, particularly -NHC(=O)R4, in which (a) R4 is alkyl
optionally substituted by aryl,
cycloalkyl, heteroaryl, heterocycloalkyl, NYlY2 or -OR5 [e.g. -NH-C(=0)-CH3 ,
-NH-C(=O)-(CH,),CH3 , -NH-C(=O)-CH(CH3)2 1 -NH-C(=O)-C(CH3)31
-NH-C(=O)-CH2CH(CH3)Z , -NH-C(=O)-CH(CH3)CH2CH3 ,
-NH-C(=O)-CHC(CH3)3 , -NH-C(=O)-CHZ / \ , -NH-C(=O)-CH,a ,
-N
-NH-C(=0)-CH; N/\ ~~N -NH-C(=O)-CHZ N(CH3)2 ,
N
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-53-
-NH-C(=O)-CH,-N -NH-C(=O)-CH; N -\O or
- 0
~
-NH-C(=0)-CHOCH3 ], (b) R4 is aryl [e.g.
H3C
-NH-C(=O) \ / , -NH-C(=O) or -NH-C(=O) \ / CH3 ],
(c) R4 is cycloalkyl [e.g. -NH-C(=O)--a or -NH-C(=O) ], (d) R4 is
II3 C
O
-NH-C(=0) N
heteroaryl[e.g. -NH-C(=O) eFI N , ,
-NH-C(=0)
O' H3 C
or -NH-C(=O) / \N ] or (e) heterocycloalkyl [e.g. -NH-C(=O) O ];
(vi) -N(R6)C(=O)NY'Y', particularly -NHC(=O)NY'Y2 [e.g. -NH-C(=O)-NHCH3 ,
-NH-C(=O)-NHCH2CH3 , -NI-I-C(=0)-NHCH(CH3)2'
-NH-C(=O)-NHCH,CH(CH), ' -NII-C(=O)-NHC(CH3)3' -NH-C(=O)-N(CH3)2'
-NH-C(=O)-N(CH,CH3), , -NH-C(=O)-NH--a , -NH-C(=O)-NH-CH. ,<
-NH-C(=O)-NH-CH2 -NH-C(=O)-NH 0
-NH-C(=O)-N , -NH-C(=O)-N N-CH3 or -NH-C(=O)-N O ],
(vii) -NYlY2 [e.g. -NH2] or (viii) alkyl substituted by -N(R6)C(=O)NYlY2 [e.g.
-CH2-NH-C(=0)-
CH(CH3)2 or -CH2 NH-C(=O)-N O]; and their corresponding N-oxides, and their
prodrugs, and their acid bioisosteres; and pharmaceutically acceptable salts
and solvates (e.g. hydrates)
of compounds of formula (Ixa) and their N-oxides and their prodrugs, and their
acid bioisosteres.
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-54-
A further preferred group of compounds of the invention are compounds of
formula (Ixa) in which:- W
represents CH; X represents C-CH3, C-CH2CH3, C-CH(CH3)2, C-OCH3, C-OCH2CH3, C-
Br or
C-Cl; Y represents C-CH3, C-CH2CH3, C-OCH3, C-Br, C-Cl, C-F, C / \ or
C-C(=O)-NH-CH, / \; Z represents CH; R7 represents hydrogen; R8 represents (i)
hydrogen, (ii) Cl-q.alkyl [e.g. CH3, CH2CH3, CH(CH3)2 or CH(CH3)CH2CH3], (iii)
-SR4 [e.g.
-S-CH3 , -S-CH,CH3 or -S-CH2 < , -S-CH 2 / \ ,
ON
-CH, / \ OCH3 , -S-CH; CHz / \ , -S-CH, or
-S
S
-S-CHz \ I ], (iv) -NYlY2 [e.g. - ] or (v) -OR5 [e.g. -OCH2CH3]; R9
represents (i) hydrogen; (ii) C1-7alkyl [e.g. -CH3, -CH2CH2CH3, -CH(CH3)2 or -
CH2-CH2-
CH(CH3)2]; (iii) aryl [e.g. phenyl]; (iv) -C(=O)NY'Y' [e.g. -C(=O)-NH-CHCH3 ,
-C.(=O)-NH-CH,CHZCH , -C(=O)-NH-CH2CH(CI13)21 -C(=O)-NH-CH(CH3)2,
-C(=O)-NH-C(CH3)3 , -C(=O)-NH-C(CH3),CH,OH , -C(=O)-NH-CHzCHzOCH3 ,
-C(=O)-N(CH3)z , -C(=O)-N(CH2CH3), , -C(=0)-NH-a
-C(=O)-NH-CH,--a or -C(=O)-NH O ]; (v) -N(R6)C(=0)R4, particularly
-NHC(=O)R4, in which (a) R4 is alkyl optionally substituted by aryl,
cycloalkyl, heteroaryl,
heterocycloalkyl, NYlY2 or -OR5 [e.g. -NH-C(=O)-CH3, -NH-C(=O)-(CHz),CH3 ,
-NH-C(=O)-CH(CH3)2, -NH-C(=O)-C(CH3)3, -NH-C(=O)-CH2CH(CH3)2,
-NH-C(=O)-CH(CH3)CH,CH3 , -NH-C(=O)-CH2C(CH3)3 ,
N
-NH-C(=0)-CH -NH-C =O -CH--a , / \
z ~ ( ) z -NH-C(=0)-CHZ N~ ~N i
N
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-55-
-NH-C(=O)-CH2-N(CH3)2 , -NH-C(=O)-CHZ N
-NH-C(=O)-CH2-N \O or -NH-C(=0)-CHZOC.H3 ], (b) R4 is aryl [e.g.
~
H3C
-NH-C(=O) \ / , -NH-C(=O) or -NH-C(=O) O CH3 ],
(c) R4 is cycloalkyl [e.g. -NH-C(=O)--a or -NH-C(=O) ], (d) R4 is
H3C
O
-NH-C(=0)
heteroaryl[e.g. -NH-C(=0) / N , , -NH-C(=0)
O H3c
or -NH-C(=O) C\N ] or (e) heterocycloalkyl [e.g. -NH-C(=O) O ];
(vi) -N(R')C(=O)NYIY', particularly -NHC(=O)NYIY' [e.g. -NH-C(=0)-NHCH3 ,
-NH-C(=O)-NHCH,CH3 , -N-H-C(=O)-NHCH(CH3)z '
-NH-C(=O)-NHCH,C.H(CH3)2' -NH-C(=O)-NHC(CH3)3 ' -NH-C(=O)-N(CH3)2 ~
, -NH-C(=0)-NH--a , -NH-C(=0)-NH-CH,a
-NH-C(=O)-N(CH2CH~)2
-NH-C(=O)-NH-CH2 01 , -NH-C(=O)-NH / \ ,
/D , -NH-C(=0)-N N-CH3 or -NH-C(=O)- O ],
(vii) -NYlY2 [e.g. -NH2] or (viii) alkyl substituted by -N(R6)C(=0)NYlY2 [e.g.
-CH?-NH-C(=O)-
CH(CH3)2 or -CHNH-C(=0)- O]; and their corresponding N-oxides, and their
_
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-56-
prodrugs, and their acid bioisosteres; and pharmaceutically acceptable salts
and solvates (e.g. hydrates)
of compounds of formula (Ixa) and their N-oxides and their prodrugs, and their
acid bioisosteres.
A further preferred group of compounds of the invention are compounds of
formula (Ixa) in which:-W
represents CH; X represents CH; Y represents C-CH3; Z represents C-CH3; R7
represents hydrogen;
R8 represents (i) hydrogen, (ii) CI-4alk-yl [e.g. CH3, CH2CH3, CH(CH3)2 or
CH(CH3)CH2CH3], (iii)
-SR4 [e.g. -S-CH3, -S-CH2CH3 or -S-CH,---a , -S-CH, / \
N
-S-CHZ / ]OCH3 , -S-CH7CH2/ \ , -S-CHZ / ~ or
S
-S-CHZ 0 ], (lv) -NYlY2 [e.g. -N O ] or (v) -OR5 [e.g. -OCH2CH3]; R9
represents (i) hydrogen; (ii) C1-7alkyl [e.g. -CH3, -CH2CH2CH3, -CH(CH3)2 or -
CH2-CH2-
CH(CH3)2]; (iii) aryl [e.g. phenyl]; (iv) -C(=O)NYlY2 [e.g.
-C(=O)-NH-CH,CH , -C(=O)-NH-CH7C.H2CH3 , -C(=O)-NH-CH2CH(CH3)2,
-C(=O)-NH-CH(CH)21 -C(=O)-NH-C(CH3)3 , -C(=O)-NH-C(CH3)2CHzOH ,
-C(=O)-NII-CH,CH,OCH3 , -C(=O)-N(CH)2 , -C(=O)-N(CH,CH)2,
--C(=O)-NH---a ~ C(=O)-NH-CHa or -C(=O)-NH O ];
(v) -N(R6)C(=O)R4, particularly -NHC(=O)R4, in which (a) R4 is alkyl
optionally substituted by aryl,
cycloalkyl, heteroaryl, heterocycloalkyl, NYIY2 or -OR5 [e.g. -NH-C(=0)-CH3 ,
-NH-C(=O)-(CH2)2 CH3 , -NH-C(=O)-CH(CH3)z , -NH-C(=O)-C(CH3)3,
-NH-C(=O)-CHZCH(CH3)2, -NH-C(=O)-CH(CH3)CH2CH3 ,
-NH-C(=O)-CHZC(CH3)3 , -NH-C(=O)-CHZ -NH-C(=O)-CH--a ,
~--N
-NH-C(=O)-CHZ ~~N ~ -NH-C(=O)-CH2 N(CH3)2 ,
N
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-57-
-NH-C(=O)-CH; N -\O or
-NH-C(=O)-CH2 N
0
-NH-C(=0)-CH,OCH3 ], (b) R4 is aryl [e.g.
H3C.
-NH-C(=O) \ / , -NH-C(=O) or -NH-C(=O) \ / CH3 ],
(c) R4 is cycloalkyl [e.g. -NH-C(=O)---a or -NH-C(=O) ], (d) 0 is
I-~JC
O
-NH-C(=O) / f N
heteroaryl[e.g. -NH-C(=O) C~/11 , ,
-NT-I-C(=0)
O' H3C
or -NH-C(=O) C\N ] or (e) heterocycloalkyl [e.g. -NH-C(=O) O ];
(vi) -N(R6)C(=O)NYlY2, particularly -NHC(=O)NYIY2 [e.g. -NH-C(=O)-NHCH3 ,
-NII-C(=O)-NHCH2CH31 -NH-C(=0)-NHCH(CH3)2 '
-NH-C(=O)-NHCHZCH(CH;), ' -NH-C(=O)-NHC(CH3)3' -NH-C(=O)-N(CH3)2'
-NH-C(=O)-N(CH9CH3)2 , -NH-C(=0)-NH- < ? -NH-C(=0)-NH-CH,- a ~
-NH-C(=O)-NH-CH, / \ , -NH-C(=O)-NH / \ ,
-NH-C(=O)-N , -NH-C(=0)-N N-CH3 or -NH-C(=0)-N o
(vii) NYlY2 [e.g. -NH2] or (viii) alkyl substituted by -N(R6)C(=O)NYlY2 [e.g. -
CH7?-NH-C(=O)-
CH(CH3)2 or -CHL NH-C(=0)- O]; and their corresponding N-oxides, and their
prodrugs, and their acid bioisosteres; and pharmaceutically acceptable salts
and solvates (e.g. hydrates)
of compounds of formula (Ixa) and their N-oxides and their prodrugs, and their
acid bioisosteres.
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-58-
A further preferred group of compounds of the invention are compounds of
formula (Ixa) in which:-W
represents CH; X represents CR2 and Y represents CR3 where R2 and R3 form the
group
-C.H2-O-CH2-; Z represents CH; R7 represents hydrogen; R8 represents (i)
hydrogen, (ii) Cl_4alkyl
[e.g. CH3, CH2CH3, CH(CH3)2 or CH(CH3)C.H2CH3], (iii) -SR4 [e.g. -S-CH3 , -S-
CH2CH3
--
or -S-CHa , -S-CH2 / \ , -S-CH2/ \ OCH3 ,
N S
-S-CHZ CH, / \ , -S-CH, / ~ or -S-CH2 (iv) -NYIY2 [e.g.
or (v) -OR5 [e.g. - ~ OCH~CH R9 represents (i) hydrogen; = ii (=~) C_ alkY1
[e.g. -CH3,
3]~ 1 7 3,
-CH2CH2CH3, -CH(CH3)2 or -CH2-CH2-CH(CH3)2]; (iii) aryl [e.g. phenyl]; (iv) -
C(=O)NY'Y' [e.g.
-C(=O)-NH-CH,CH , -C(=O)-NH-CHZCH2CH3 , -C(=O)-NH-CH,CH(CH3), ,
-C(=O)-NH-CH(CH3), , -C(=O)-NH-C(CH3)3, -C(=O)-NH-C(CH3)2CH,OH ,
-C(=O)-NH-CH2CH,OCH3, -C(=O)-N(CH,),, -C(=O)-N(CHCH3), ,
-C(=0)-NH-7a , -C(=0)-NH-CH,--a or -C(=0)-NH O ];
(v) -N(R6)C(=O)R4, particularly -NHC(=O)R4, in which (a) R4 is alkyl
optionally substituted by aryl,
cycloalkyl, heteroaryl, heterocycloalkyl, NYlY2 or -OR5 [e.g. -NH-C(=O)-CH ,
-NH-C(=O)-(CH,)2CH3 , -NH-C(=O)-CH(CH3)2, -NH-C(=O)-C(CH3)3 1
-NH-C.(=O)-CH,CH(CH3)2, -NH-C(=O)-CH(CH3)CH2CH3 ,
-NH-C(=O)-CH2C(CH3)3 , -NH-C(=O)-CH2 01 , -NH-C(=O)-CH,a ,
-N
-NH-C(=0)-CHZ N ~ -NH-C(=O)-CH; N(CH3)Z ,
-NH-C(=0)-CH2 - N -NH-C(=O)-CHz N O or
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-59-
-NH-C(=O)-CH,OCH3 ], (b) R4 is aryl [e.g.
H3C
-NH-C(=O) \ / , -NH-C(=O) or -NH-C(=O) \ / CH ],
(c) R4 is cycloalkyl [e.g. -NH-C(=O)---a or -NH-C(=O) ], (d) R4 is
H3C
O
-NH-C(=0)
~ O
heteroaryl[e.g. -NH-C(=0) / N , -NH-C(=0)
O~ H3C ~
or -NH-C(=O) CN ] or (e) heterocycloalkyl [e.g. -NH-C(=O) O ];
(vi) -N(R6)C(=O)NYlY2, particularly -NHC(=O)NYlY2 [e.g. -NH-C(=O)-NHCH3 ,
-NH-C(=O)-NHCH,CH3 , -NH-C(=O)-NHCH(CH3)2 '
~
-NH-C(=O)-NHCH,CH(CH3), ' -NH-C(=O)-NHC(CH)3 ' -NH-C(=O)-N(CH3)2
-NH-C(=O)-N(CH2CH)21 -NH-C(=O)-NH--a , -NH-C(=O)-NH-CH a ~
-NH-C(=O)-NH-CH2 -NH-C(=O)-NH / \
-NH-C(=O)-N , -NH-C(=O)-N N-CH3 or -NH-C(=0)- ],
(vii) -NYlY2 [e.g. -NH2] or (viii) alkyl substituted by -N(R6)C(=0)NYlY2 [e.g.
-CH2-NH-C(=O)-CH(CH3)2 or -CH NH-C(=0)-N O]; and their corresponding
_
N-oxides, and their prodrugs, and their acid bioisosteres; and
pharmaceutically acceptable salts and
solvates (e.g. hydrates) of compounds of formula (Ixa) and their N-oxides and
their prodrugs, and their
acid bioisosteres.
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-60-
A further preferred group of compounds of the invention are compounds of
formula (Ixa) in which:-W
represents CH; X represents CR2 and Y represents CR3 where R2 and R3 form the
group
-CH2-CH2-CH2- ; Z represents CH; R7 represents hydrogen; R8 represents (i)
hydrogen, (ii) C1-4a1ky1
[e.g. CH3, CH2CH3, CH(CH3)2 or CH(CH3)CH2CH3], (iii) -SR4 [e.g. -S-CH3, -S-
CH2CH3
or -S-CH--a , -S-CH2 / \ , -S-CH2 O-OCH31
N S
-S-CH; CHZ / \ , -S-CH, / ~ or -S-CHZ \ I ], (iv) -NYlY2 [e.g.
-N p] or (v) -OR5 [e.g. -OCH2)CH3]; R9 represents (i) hydrogen; O ii C1_7a1kY1
[e.g. -CH3,
3~
-CH2CH2CH3, -CH(CH3)2 or -CH2-CH,)-CH(CH3)2]; (iii) aryl [e.g. phenyl]; (iv) -
C(=O)NY'Y2 [e.g.
-C(=O)-NH-CH2CH; , -C(=O)-NH-CH2CH7CH3 , -C(=O)-NH-CH2CH(CH)2 ,
-C(=O)-NH-CH(CH3)Z , -C(=O)-NH-C(CH3)3 , -C(=O)-NH-C(CH3),CH,OH ,
-C(=O)-NH-CH,CH,OCH3, -C(=O)-N(CH;), , -C(=O)-N(CH,CH)2 ,
-C.(=O)-NH---< I -C(=O)-NH-CH2 < or -C(=O)-NH O ];
(v) -N(R6)C(=O)R4, particularly -NHC(=O)R4, in which (a) R4 is alkyl
optionally substituted by aryl,
cycloalkyl, heteroaryl, heterocycloalkyl, NYlY2 or -OR5 [e.g. -NH-C(=O)-CH3 ,
-NH-C(=O)-(CHZ)2 CH3 1 -NH-C(=O)-CH(CH3)z ,
-NH-C(=O)-C(CH3)3 1 -NH-C(=O)-CH2CH(CH3)21 -NH-C(=0)-CH(CH3)CH,CH3 ,
-NH-C(=O)-CH2C(CH3)3, -NH-C(=O)-CHz / \ , -NH-C(=O)-CH< ,
-N
-NH-C(=O)-CH7-N/\ //N , -NH-C(=O)-CHZ N(CH3)2 ,
N
-NH-C(=0)-CH2 - N -NH-C(=0)-CH2 -N O or
-NH-C(=O)-CH2OCH3 ], (b) R4 is aryl [e.g.
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-61-
HC
-NH-C(=O) \ / , -NH-C(=O) \ / or -NH-C(=O) \ / CH3 ],
(c) R4 is cycloalkyl [e.g. -NH-C(=O)--< or -NH-C(=O) ], (d) R4 is
H3C
O
-NH-C(=O)
heteroaryl[e.g. -NH-C(=0) / N ,
-NH-C(=0)
O-~ H 3
or -NH-C(=O) CN ] or (e) heterocycloallcyl [e.g. -NH-C(=O) O ];
(vi) -N(R6)C(=O)NYIY2, particularly -NHC(=O)NYlY2 [e.g. -NH-C(=O)-NHCH3 ,
-NH-C(=O)-NHCH,CH 1 -NH-C(=0)-NHCH(CH3)Z ~
-NH-C(=O)-NHCH,CH(CH3), -NH-C(=O)-NHC(CH)3 ' -NH-C(=O)-N(CH3)2'
-NH-C(=O)-N(CH2CH3), , -NH-C(=O)-NH-< -NH-C(=O)-NH-CHa
-NH-C(=O)-NH-CH2 -NH-C(=O)-NH / \
-NH-C(=O)-N , -NH-C(=0)- N-CH3 or -NH-C(=O)-N O
(vii) -NYIY2 [e.g. -NH2] or (viii) alkyl substituted by -N(R6)C(=O)NYIY2 [e.g.
-CH2-NH-C(=O)-CH(CH3)2 or -C.H2 NH-C(=0)- O]; and their corresponding
N-oxides, and their prodrugs, and their acid bioisosteres; and
pharmaceutically acceptable salts and
solvates (e.g. hydrates) of compounds of formula (Ixa) and their N-oxides and
their prodrugs, and their
acid bioisosteres.
Compounds of formula (Ixa) in which R8 is hydrogen or -CH3 and R9 is -CH2-CH2-
CH(CH3)2,
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-62-
-C(=O)-NH-CH2CH3 , -C(=O)-NH-CHZCH,CH3, -C(=O)-NH-CH(CH3), ,
-C(=O)-NH-C(CH3)3 1 -C(=O)-NH-C(CH),CHOH, -C(=O)-NH---a
-C(=O)-NH-CHZCH,OCH , -C(=O)-N(CH)1 , -C(=O)-N(CH,CH3), ,
-C(=O)-NH O , -NH-C(=O)-CH3, -NI-I-C(=O)-(CH,)zCH3 ,
-NH-C(=O)-CH(CH3), , -NH-C(=O)-C(CH3)3 , -NH-C(=O)-CH,C.H(CH3)Z ,
-NH-C(=O)-CH(CH3)CH2CH3, -NH-C(=O)-CH2C(CH3)3 ,
N
-NH-C(=O)-CHZ -NH-C(=0)-CH2- < , -NH-C(=O)-CH2-N/\ N ~
-NH-C(=O)-CH2 -N(CH3)2 , -NH-C(=0)-CH2 -N
-NH-C(=O)-CH, N , -NH-C(=O)-CH,OCH3 , -NH-C(=0) `
\ ~
HC
-NH-C(=O) -NH-C(=O) O CH3 , -NH-C(=O)--<l ,
H~IC
O
-NH-C(=O) , -NH-C(=O) -NH-C(=O)
I,
ON N
H3C
-NH-C(=O) o\N , -NH-C(=0) // I -NH-C(=O)-NHCH3 ,
-NH-C(=O)-NHCH2CH3 , -NH-C(=O)-NHCH(CH3)2 5 -NH-C(=0)-NHC(CH)3 '
-NH-C(=O)-N(CH3)2 1 -NH-C(=O)-N(CH2 CH3)2 , -NH-C(=O)-NH-a
-NH-C(=O)-NH-CHa , -NH-C(=0)-NH-CH2 / \ ,
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-63-
-NH-C(=O)-NH / \ , -NH-C(=O)-N
-NH-C(=O)-N N-CH3 -NH-C(=0)-N O -CH2-NH-C(=0) CH(CH3)) or
-CHZ NH-C(=0)-N j are particularly preferred.
Compounds of formula (Ixa) in which R9 represents hydrogen and R8 represents -
CH(CH3)2,
-S-CH3 ,-S-CH1CH3 or -S-CH--~ are also particularly preferred.
Compounds of formula (Ixa) in which W is CH, X is CH, Y is CH, C-CH,)CH3, C-
CH2CH2CH3,
CN F O~
~
C / , C / \ , C / \ , C , C-CN, C-Br, C-CF3,
N
C / \N , C / ~ , C-OCH3 , C-OCH,CH3 , C-OCHF, , C-OCF3 ,
C-O-CHZ / \ , C-C(=O)-NH-CH3 , C-C(=O)-NH-CH,CH3,
C-C(=O)-NH-CH(CH3)Z , C-C(=O)-NH-C(CH3)2-CH,OH , C-C(=O)-NH-CH2CH2CN ,
C-C(=O)-NH-CHCH2OCH3 , C-C(=0)-NH-CH / \ ,
C3 CH3
C-C(=O)-NH-CH2 C-C(=O)-NH-CH2 / \ ,
C-C(=0)-NH-CH CH3 1 C-C(=O)-NH-(CH,)2 0 1
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-64-
C-C(=O)-NH-(CH,); N --~O , C-C(=O)-NH-(CH,), N
N
C-C(-O)-~-(CH~~~ 11 ~
C-C(=0)-NH-(CH,)3 ~N N--N
C-C(=0)-NH-CH, / \ ? C-C(=O)-NH-CHZ O-N O
C-C(=O)-NH-(CH,)3 N , C-C(=O)-NH \ /, C-C(=O)OCH3, C-C(=O)OH,
C-CH(OH) \ /, C-SO2CH3 or C-SO; NH-CH2 / \ and Z is CH are particularly
preferred.
Compounds of formula (Ixa) in which W is CH, X is C-CH3 or C-CH2CH3, Y is C-
CH3, C-CH2CH3,
C-CH(CH3)2, C-Br, C-Cl, C-F, C / \ or C-C(=O)-NH-CH, / \, and Z is CH
are also particularly preferred.
Compounds of formula (Ixa) in which W is CH, X is C-OCH3, Y is CH, C-CH3, C-
CH2CH3, C-Cl or
C-OCH3 and Z is CH are also particularly preferred.
Compounds of formula (Ixa) in which W is CH, X is C-OCH2CH3, Y is C-F and Z is
CH are also
particularly preferred.
Compounds of formula (Ixa) in which W represents CH, X represents CR2 and Y
represents CR3
where R2 and R3 atoms form the group -CH2-CH2-CH2-, and Z represents CH are
also particularly
preferred.
Compounds of formula (Ixa) in which W represents CH, X represents CR2 and Y
represents CR3
where R2 and R3 form the group -CH2-O-CH?-, and Z represents CH are also
particularly preferred.
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-65-
Compounds of formula (Ixa) in which R8 is hydrogen or -CH3 and R9 is -C(=O)-NH-
CHzCH3 ,
-C.(=O)-NH-CHCH,CH3 , -C(=O)-NH-CH(CH3), , -C(=O)-NH-CH2CH(CH3)2
,
-C(=O)-NH-C(CH3)3 1 -C(=O)-NH-C(CH3),CH,OH , -C(=O)-N(CH,CH), ,
-C(=O)-NH--< I -C(=O)-NH-CHa ~ -C(=O)-NH O ,
-C(=O)-NH-CHCH7OCH3, -NH-C(=O) O , -NH-C(=O)-(CH2),CH3 ,
-NH-C(=O)-CH(C.H3), , -NH-C(=0)-C(CH3)3 , -NH-C(=O)-CH2CH(CH3)2 ,
-NH-C(=O)-CH(CH3)CH,CH3, -NH-C(=O)-CH2C(CH3)3 , -NH-C(=O)-CH,--a ,
N
-NH-C(=0)-CHi N //N , -NH-C(=O)-CH` N O\ /, -NH-C(=O)-CH,OCH3 ,
N -/
-NH-C(=O) \ / , -NH-C(=O)-a , -NH-C(=O) ,
-NH-C(=O) / /N, -NH-C(=0) ~ \N, -NH-C(=0) O
O -
II3 C
O
-NH-C(=0) N , -NH-C(=O)-NHCH3 , -NH-C(=O)-NHCH,CH3 ,
zI3C
-NH-C(=0)-NHCH(CH3)2 . -NH-C(=O)-NHC(CH)3 , -NH-C(=O)-N(CH3)Z ,
-NH-C(=O)-N(CH2CH3)z , -NH-C(=O)-NH-a , -NH-C(=O)-NH-CH< ~
-NH-C(=O)-NH-CH2 / \ , -NH-C(=O)-NH / \ ,
-NH-C(=0)-N -NH-C(=0)- N N-CH3 or -NH-C(=0)- 0 are
especially preferred.
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-66-
Compounds of formula (Ixa) in which W is CH, X is CH, Y is C OCH3, C-OCH2CH3,
C-OCHF2,
d Z is CH are
an
C-CF3, C-C(=O)-NH-CH, / \ or C-C(=O)-NH-CH2 O-N
- especially preferred.
Compounds of formula (Ixa) in which W is CH, X is C-CH3 or C-CH2CH3, Y is C-
CH3 or
C-CH2CH3, C-Cl or C-F and Z is CH are also especially preferred.
Compounds of formula (Ixa) in which W is CH, X is C-OCH3, Y is C-CH3, C-
CH2CH3, C-Cl, C-F or
C-OCH3 and Z is CH are also especially preferred.
Compounds of formula (Ixa) in which W is CH, X is C-OCH2CH3, Y is C-Cl or C-F
and Z is CH are
also especially preferred.
Compounds of formula (Ixa) in which W represents CH, X represents CR2 and Y
represents CR3
where R2 and R3 form the group -CH2-CH2-CH2-, and Z represents CH are also
especially preferred.
Compounds of formula (Ixa) in which W represents CH, X represents CR2 and Y
represents CR3
where R2 and R3 form the group -CH2)-O-CH2-, and Z represents CH are also
especially preferred.
Another particular group of compounds of the invention are compounds of
formula (Ix) wherein Rl is a
R9
R8
heteroaryl moiety in which R8 and R9 together with the carbon atoms to which
they
N--N R7
are attached form an optionally substituted phenyl ring, i.e. compounds of
formula (Ixb):-
5
4 O 6 (R10)P
Y Z N \ 7
1 \
I \
X" W H ~N le
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-67-
(Ixb)
in which W, X, Y, Z and R7 are as hereinbefore defined for compounds of
formula (Ix); RlO is
carboxy, cyano, halo, haloalkyl, hydroxy, nitro, R4, -C(=O)R4, -C(=O)NYlY2, -
C(=0)OR4,
-N(R6)C(=O)R4, -N(R6)C(=O)NYlY2, -N(R6)C(=0)0R4, -N(R6)S02R4, -N(R6)SO2NYlY2,
-NYlY2, -OR4, -OCF2H, -OCF3, -OC(=0)R4, -OC(=0)NYlY2, -S(O)nR4 or -S(O)2NYlY2;
and p
is zero, or an integer 1; and their corresponding N-oxides, and their
prodrugs, and their acid
bioisosteres; and pharmaceutically acceptable salts and solvates (e.g.
hydrates) of compounds of
formula (Ixb) and their N-oxides and their prodrugs, and their acid
bioisosteres.
Compounds of formula (Ixb) in which W represents CH, X represents CH, Y
represents CH and Z
represents CH or C-CH3 are preferred.
Compounds of formula (Ixb) in which W represents CH, X represents CH, Z
represents CH and Y
represents:
(i) C-C1-4alkyl [e.g. C-CH3, C-CH2CH3'C-CH2CH2CH3 or C-CH(CH3)2];
CH3 CH3
(ii) C-aryl [e.g. C / \ , C / \ , C / \ ,
CN CH30
C / \ CH3 C / \ IC / \ ~
F 0`
C Cl,C / \ orC 0 01;
(iii) C-CN;
(iv) C-N02;
(v) C-halo [e.g. C-Br, C-Cl or C-F];
(vi) C-haloalkyl [e.g. C-CF3];
\
(vii) C-heteroaryl [e.g. C / N or C / ~];
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-68-
(viii) C-OR4 [e.g. C-OCH3 , C-OCH,CH3, C-OCHFZ , C-OCF3 , C-O / \ C-O-C.H, / \
or C-O-(CH,); N ~O ];
~
(ix) C-C(=0)R4 [e.g. C-C(=O) o ];
(x) C-C=O)NYlY2 [e.g. C-C,(=O)-NH-CH3, C-C(=O)-N(CH3),
C-C(=O)-NH-CH,CH3 ,
C-C(=O)-NH-CH(CH3)2 , C-C(=O)-NH-C(CH3)2-CHzOH ,
C-C(=O)-NH-CH2CH2CN , C-C(=O)-NH-CH2CH,OCH3 ,
CH3
C-C(=0)-NH-CH2 / \ , C-C(=0)-NH-CH2 CH3
C-C(=O)-NH-CH, / \ , C-C(=O)-NH-CH, CH3 ,
C-
C(=O)-NH-CH, O-N
C-C(=O)-NH-CH, ON-\
C(=O)-NH-(CH2)z 0 , C-C(=O)-rH-(CHz), N o ,
C-
N
C-C(=O)-NH-(CH2)2 N , C-C(=0)-NH-(CH2)--C~ --N ,
N
0
~
C-C(=O)-NH-(CH2)3 N, C-C(=O)-NH-(CH,)3 N or
C-C(=O)-NH \ / ];
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-69-
(xi) C-C(=O)OR4 [e.g. C-C(=O)OH or C-C(=O)OCH3];
(xii) C-NHC(=O)R`l [e.g. C-NHC(=0)CH3, C-NHC(=0)CH(CH3)2,
C-NH-C(=O) \ / or C-NH-C(=O)-CH,
(xiii) C-CH(OH)aryl [e.g. C-CH(OH) \ / ];
(xiv) C-S(O)2NYlY2 [e.g. C-SO; NH-CHi or
(xv) C-S(O)nR4 [e.g. C-SO2CH3];
are also preferred.
Compounds of formula (Ixb) in which W represents CH, X represents C-CH3, C-
CH2C.H3,
C-CH(CH3)2, C-OCH3, C-OCH2CH3, C-Br or C-Cl, Y represents C-CH3, C-CH2CH3, C-
OCH3,
C-Br, C-Cl, C-F, C / \ or C-C(=O)-NH-CH, / \. and Z represents CH are also
preferred.
Compounds of formula (Ixb) in which W represents CH, X represents CH, Y
represents C-CH3 and Z
represents C-CH3 are also preferred.
Compounds of formula (Ixb) in which W represents CH, X represents CR2 and Y
represents CR3
where R'~ and R3 form the group -CH?-O-CH2-, and Z represents CH are also
preferred.
Compounds of formula (Ixb) in which W represents CH, X represents CR2 and Y
represents CR3
where R2 and R3 form the group -CH2-CH2-CH2-, and Z represents CH are also
preferred.
Compounds of formula (Ixb) in which R7 represents hydrogen are preferred.
Compounds of formula (Ixb) in which p is zero or one are preferred.
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-70-
Compounds of formula (Ixb) in which RIO represents:
(i) cyano
(ii) halo [e.g. chloro, fluoro];
(iii) C1-4alkyl [e.g. methyl,
(iv) -OR4 [e.g. -OCH3, -OCH2CH3]; or
(v) -C(=O)NYlY2 [e.g. -C(=O)-NH2, -C(=0)-NHCH(CH3)2, -C(=O)-N(CH3)2
are preferred.
A preferred group of compounds of the invention are compounds of formula (Ixb)
in which:- W
represents CH; X represents CH; Y represents CH; Z represents CH or C-CH3; R7
represents
hydrogen; R10 represents (i) cyano, (ii) halo [e.g. chloro, fluoro], (iii) Cl-
q.alkyl [e.g. metliyl], (iv)
-OR4 [e.g. -OCH3 or -OCH2CH3] or (v) -C(=O)NYlY2 [e.g. -C(=O)-NH2, -C(=O)-
NHCH(CH3)2 or
-C(=O)-N(CH3)2]; and their corresponding N-oxides, and their prodrugs, and
their acid bioisosteres;
and pharmaceutically acceptable salts and solvates (e.g. hydrates) of
compounds of formula (Ixb) and
their N-oxides and their prodrugs, and their acid bioisosteres.
A further preferred group of compounds of the invention are compounds of
formula (Ixb) in which:- W
represents CH; X represents CH; Z represents CH; Y represents (i) C-C14alkyl
[e.g. C-CH3,
CH3
C-CH2CH3'C-CH2CH2CH3 or C-CH(CH3)2], (ii) C-aryl [e.g. C / \, C / \
CH3 CN CH30
C / \ , C/ \ CH3,C / \ ,C / \ ,
F O`
C / \ C1, C / \ or C / \ O], (iii) C-CN, (iv) C-NO2, (v) C-halo
[e.g. C-Br, C-Cl or C-F], (vi) C-haloalkyl [e.g. C-CF3], (vii) C-heteroaryl
[e.g. C / \N or
N
C / ~ ], (viii) C-OR4 [e.g. C-OCH3 , C-OCH2C.H3 3 C-OCHF, , C-OCF3 ,
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-71-
C-O / \ , C-O-CH, / \ or C-O-(CH,)-N O ], (ix) C-C(=O)R4 [e.g.
- 2 \-/
C-C(=O) \ / ], (x) C-C=O)NYlY-[e.g. C-C(=O)-NH-CH3, C-C(=O)-N(CH3)2,
C-C(=O)-NH-CH2CH3, C-C(=O)-NH-CH(CH3)2, C-C(=O)-NH-C(CH3)2-CH2OH,
C-C(=O)-NH-CHZCH2CN, C-C(=O)-NH-CH,CH2OCH3 ,
CH3
C-C(=O)-NH-C.H2 / \ , C-C(=O)-NH-CH,
CH3
C.-C(=O)-NH-CHZ / \ , C-C(=O)-NH-CH, O-CH 3 ,
C-C(=O)-NH-CH, ON-\ C-C(=O)-NH-CO-N
C-C(=O)-NH-(CH,), / \ C-C(=O)-NH-(CH2); N o
-
~
C-C(=O)-NH-(CH2); N , C-C(=O)-NH-(CH,)3 NN,
0
N
C-C(=0)-NH-(CHZ)--C~ N, C-C(=0)-NH-(CH2)3 N
N,or C-C(=0)-NH 0 ], (xi) -C(=0)OR4 [e.g. C-C(=0)OH or C-C(=0)OCH3], (xii)
C-NHC(=0)R4 [e.g. C-NHC(=0)CH3 or C-NHC(=0)CH(CH3)2, C-NH-C(=0) \ / or
C-NH-C(=O)-CH2 \ / ], (xiii) C-CH(OH)aryl [e.g. C-CH(OH) \ / ], (xiv)
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-72-
C S(O)2NYIY2 [e.g. C-SO--NH-CH, / \] or (xv) C-S(O)nR4 [e.g. C-SO2CH3]; R7
represents hydrogen; p is zero or one; R10 represents (i) cyano, (ii) halo
[e.g. chloro, fluoro], (iii)
C1-4alkyl [e.g. methyl], (iv) -OR4 [e.g. -OCH3 or -OCH2CH3] or (v) -C(=0)NYIY2
[e.g.
-C(=O)-NH2,-C(=O)-NHCH(CH3)2 or -C(=O)-N(CH3)2]; and their corresponding N-
oxides, and
their prodrugs, and their acid bioisosteres; and pharmaceutically acceptable
salts and solvates (e.g.
hydrates) of compounds of formula (Ixb) and their N-oxides and their prodrugs,
and their acid
bioisosteres.
A further preferred group of compounds of the invention are compounds of
formula (Ixb) in which:- W
represents CH; X represents C-CH3, C-CH2CH3, C-CH(CH3)2, C-OCH3, C-OCH2CH3, C-
Br or
C-Cl; Y represents C-CH3, C-CH2CH3, C-OCH3, C-Br, C-Cl, C-F, C / \ or
C-C(=O)-NH-CH, Z represents CH; R7 represents hydrogen; p is zero or one; RIO
represents (i) cyano, (ii) halo [e.g. chloro, fluoro], (iii) C1-4alkyl [e.g.
methyl], (iv) -OR4 [e.g. -OCH3
or -OCH2CH3] or (v) -C(=O)NYlY2 [e.g. -C(=O)-NH2, -C(=O)-NHCH(CH3)2 or -C(=O)-
N(CH3)2];
and their corresponding N-oxides, and their prodrugs, and their acid
bioisosteres; and pharmaceutically
acceptable salts and solvates (e.g. hydrates) of compounds of formula (Ixb)
and their N-oxides and
their prodrugs, and their acid bioisosteres.
A further preferred group of compounds of the invention are compounds of
formula (Ixb) in which:- W
represents CH; X represents CH; Y represents C-CH3; Z represents C-CH3; R7
represents hydrogen; p
is zero or one; R10 represents (i) cyano, (ii) halo [e.g. chloro, fluoro],
(iii) C1-4alkyl [e.g. methyl], (iv)
-OR4 [e.g. -OCH3 or -OCH2CH3] or (v) -C(=O)NYlY2 [e.g. -C(=0)-NH2, -C(=O)-
NHCH(CH3)2 or
-C(=O)-N(CH3)2]; and their corresponding N-oxides, and their prodrugs, and
their acid bioisosteres;
and pharmaceutically acceptable salts and solvates (e.g. hydrates) of
compounds of formula (Ixb) and
their N-oxides and their prodrugs, and their acid bioisosteres.
A further preferred group of compounds of the invention are compounds of
formula (Ixb) in which:- W
represents CH; X represents CR2 and Y represents CR3 where R2 and R3 form the
group
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-73-
-CH2-O-CH2-; Z represents CH; R7 represents hydrogen; p is zero or one; R10
represents (i) cyano,
(ii) halo [e.g. chloro, fluoro], (iii) Cl-4alkyl [e.g. methyl], (iv) -OR4
[e.g. -OCH3 or -OCH2CH3] or
(v) -C(=O)NYlY2 [e.g. -C.(=O)-NH2, -C.(=O)-NHCH(CH3)2 or -C(=0)-N(CH3)2]; and
their
corresponding N-oxides, and their prodrugs, and their acid bioisosteres; and
pharmaceutically
acceptable salts and solvates (e.g. hydrates) of compounds of formula (Ixb)
and their N-oxides and
their prodrugs, and their acid bioisosteres.
A further preferred group of compounds of the invention are compounds of
formula (Ixb) in which:- W
represents CH; X represents CR2 and Y represents CR3 where R2 and R3 form the
group
-CH2-CH2-CH2- ; Z represents CH; R7 represents hydrogen; p is zero or one; R10
represents (i)
cyano, (ii) halo [e.g. chloro, fluoro], (iii) C1-4alkyl [e.g. methyl], (iv) -
OR4 [e.g. -OCH3 or
-OCH2CH3] or (v) -C(=O)NYlY2 [e.g. -C(=0)-NH2, -C(=0)-NHCH(CH3)2 or -C(=0)-
N(CH3)2];
and their corresponding N-oxides, and their prodrugs, and their acid
bioisosteres; and pharmaceutically
acceptable salts and solvates (e.g. hydrates) of compounds of formula (Ixb)
and their N-oxides and
their prodrugs, and their acid bioisosteres.
Compounds of formula (Ixb) in wllich R7 represents hydrogen and p is zero are
particularly preferred.
Compounds of formula (Ixb) in which R7 represents hydrogen; p is one and R10
represents cyano,
chloro, fluoro, methyl, -OCH3, -OCH2CH3, -C(=O) NH2, -C(=O)-NHCH(CH3)2 or
-C(=0)-N(CH3)2 are also particularly preferred.
Compounds of formula (Ixb) in wllich W is CH, X is CH, Y is CH, C-CH2CH3, C-
CH2CH2CH3,
CN F 0
C O , C C3, C b , C (2 0 , C-CN, C-Br, C-CF3,
N
C / \N , C C-OCH3, C-OCHZCI I3 , C-OCHF, , C-OCF3,
C-O-CHz C-C(=O)-NH-CH3, C-C(=O)-NH-CH,CH3 ,
C-C.(=O)-NH-CH(CH3)2' C-C(=O)-NH-C(CH3)2-CH20H , C-C(=O)-NH-CHZCHZCN ,
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-74-
~
C-C(=O)-NH-CH,CH,OCH3, C-C(=O)-NH-CH2
CH3 CH3
C-C(=O)-NH-CHZ C-C(=O)-NH-CH,
C-C(=O)-NH-CH, O-CH3, C-C(=O)-NH-(CH2)Z
C-C(=O)-NH-(CH2)2 d-\O, C-C(=O)-NH-(CH2)2 N
~
N
~ , C-C(=0)-NH-(CHz)~ N ~
C-C(=0)-NH-(CH2)3 NN N--
C-C(=O)-NH-CHZ C-C(=O)-NH H
-C, O-N - O
C-C(=O)-NH-(CH,)3 N , C-C(=O)-NH \ /, C-C(=O)OCH3 C-C(=O)OH
C-CH(OH) O , C-SO2CH3 or C-SO2 NH-CH2 / \ and Z is CH are particularly
preferred.
Compounds of formula (Ixb) in which W is CH, X is C-CH3 or C-CH2CH3, Y is C-
CH3, C-CH2CH3,
C-CH(CH3)2, C-Br, C-Cl, C-F, C / \, C-C(=O)-NH-CH2 / \, and Z is CH are
also particularly preferred.
Compounds of formula (Ixb) in which W is CH, X is C-OCH3, Y is CH, C-CH3, C-
CH2CH3, C-Cl or
C-OCH3 and Z is CH are also particularly preferred.
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-75-
Compounds of formula (Ixb) in which W is CH, X is C-OCH2CH3, Y is C-F and Z is
CH are also
particularly preferred.
Compounds of formula (Ixb) in which W represents CH, X represents CR2 and Y
represents CR3
where R2 and R3 form the group -CH2-CH2-CH2-, and Z represents CH are also
particularly
preferred.
Compounds of formula (Ixb) in which W represents CH, X represents CR2 and Y
represents CR3
where R2 and R3 form the group -CH2-O-CH2-, and Z represents CH are also
particularly preferred.
Compounds of formula (Ixb) in which R7 represents hydrogen and p is zero are
especially preferred.
Compounds of formula (Ixb) in which R7 represents hydrogen; p is one and RlO
represents -OCH3,
-OCH7)CH3 or -C(=O)-NHCH(CH3)2 attached to position 5 of the indazolyl ring
are also especially
preferred.
Compounds of formula (Ixb) in which W is CH, X is C-CH3 or C-CH2CH3, Y is C-
CH3 or
C-CH-)CH3 and Z is CH are also especially preferred.
Another particular group of compounds of the invention are compounds of
formula (Ix) wherein Rl is a
R9
R8
pyrazolyl moiety in which R8 and R9 together with the carbon atoms to which
they
N--N R7
are attached form an optionally substituted C5-8cycloalkyl ring, i.e.
compounds of formula (Ixc):-
(Ri2)
q
A
Y ~ N
I
W H N'N R 7
(Ixc)
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-76-
in which W, X, Y, Z, X and p are as hereinbefore defined for compounds of
formula (Ix), A is a
C5-8cycloalkyl ring and R12 is acyl, acylamino, alkoxy, alkoxycarbonyl,
alkylenedioxy, alkylsulfinyl,
alkylsulfonyl, alkylthio, aroyl, aroylamino, aryl, arylalkyloxy,
arylalkyloxycarbonyl, arylalkylthio,
aryloxy, aryloxycarbonyl, arylsulfinyl, arylsulfonyl, arylthio, carboxy (or an
acid bioisostere), cyano,
cycloalkyl, halo, heteroaroyl, heteroaryl, heteroarylalkyloxy,
heteroaroylamino, heteroaryloxy,
heterocycloalkyl, hydroxy, nitro, trifluoromethyl, -C(=O)NYlY2, -NYI-
C(=0)alkyl, -NYISO2alkyl, -
NYlY2, -SO2NYlY2 or alkyl, alkenyl or allcynyl each optionally substituted
with aryl, cycloalkyl,
heteroaryl, hydroxy, -C(=O)OR6, -C(=O)NYlY2, -NYlY2 or -OR5; and their
corresponding
N-oxides, and their prodrugs, and their acid bioisosteres; and
pharmaceutically acceptable salts and
solvates (e.g. hydrates) of compounds of formula (Ixc) and their N-oxides and
their prodrugs, and their
acid bioisosteres.
Compounds of formula (Ixc) in which W represents CH, X represents CH, Y
represents CH and Z
represents CH or C-CH3 are preferred.
Compounds of formula (Ixc) in which W represents CH, X represents CH, Z
represents CH and Y
represents:
(i) C-C1-4alkyl [e.g. C-CH3, C-CH2CH3'C-CH2CH2CH3 or C-CH(CH3)2];
CH3 CH3
(ii) C-aryl [e.g. C / \ , C / \ , C / \ ,
CN CH3O
C / \ CH3 , C / \ , C / \ ,
F O'~
C Cl, C / \ or C / \ O ];
(iii) C-CN;
(iv) C-N02;
(v) C-halo [e.g. C-Br, C-Cl or C-F];
(vi) C-haloalkyl [e.g. C-CF3];
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-77-
N
(vii) C-heteroaryl [e.g. C / \N or C
(viii) C-OR4 [e.g. C-OCH3 , C-OCII2CH3 , C-OCHF,, C-OCF~ , C-O / \
C-O-CHZ / \ or C-O-(CHz); N -\O ];
-
(ix) C-C(=0)R4 [e.g. C-C(=0) G ];
(x) C-C=O)NYIY2 [e.g. C-C(=O)-NH-CH3 , C-C(=O)-N(CH3); ~
C-C(=O)-NH-CH,CH3 )
C-C(=O)-NH-CH(CH3), , C-C(=O)-NH-C(CH3)2-CH2 OH ,
C-C(=O)-NH-CH,CH,CN, C-C(=O)-NH-CH,CH,OCH3 '
CH3
C-C(=O)-NH-CHZ / \ , C-C(=O)-NH-CHz / \ ,
CH3
C-C(=O)-NH-CH2 C-C(=O)-NH-CH2 O-CH 3 ,
C-C(=0)-NH-CHZ C-C(=0)-NH-CH2 / \
-N
C-C(=O)-NH-(CHZ)Z C-C(=O)-NH-(CH2)2 N O,
- ~~
N~N
C-C(=0)-NH-(CH,), N , C-G(=0)-NH-(CH,)--C~ /N,
N
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-78-
O
~
C-C(=O)-NH-(CH,)3 N N ~ C-C(=0)-NH-(CH,)3 N or
C-C(=O)-NH 0 ];
(xi) C-C(=O)OR4 [e.g. C-C(=O)OH or C-C(=O)OCH3];
(xii) C-NHC(=0)R4 [e.g. C-NHC(=0)CH3, C-NHC(=O)CH(CH3)2,
C-NH-C(=O) \ / or C-NH-C(=O)-CH, or
(xiii) C-CH(OH)aryl [e.g. C-CH(OH) o
(xiv) C-S(O)2NYlY2 [e.g. C.-SO; NH-CH2 / \ ];
(xv) C-S(O)nR4 [e.g. C-SO2CH3];
are also preferred.
Compounds of formula (Ixc) in which W represents CH, X represents C-CH3, C-
CH2CH3,
C-CH(CH3)2, C-OCH3, C-OCH2CH3, C-Br or C-Cl, Y represents C-CH3, C-CH2CH3, C-
OCH3,
C-Br, C-Cl, C-F, C / \ or C-C(=O)-NH-CH2 Q and Z represents CH are also
preferred.
Compounds of formula (Ixc) in which W represents CH, X represents CH, Y
represents C-CH3 and Z
represents C-CH3 are also preferred.
Compounds of formula (Ixc) in which W represents CH, X represents CR2 and Y
represents CR3
where R2 and R3 form the group -CH2-O-CH2-, and Z represents CH are also
preferred.
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-79-
Compounds of formula (Ixc) in which W represents CH, X represents CR2 and Y
represents CR3
where R2 and R3 form the group -CH7)-CH2-CH7)-, and Z represents CH are also
preferred.
Compounds of formula (Ixc) in which R7 represents hydrogen are preferred.
Compounds of formula (Ixc) in which A represents a cyclopentyl, cyclohexyl and
cycloheptyl,
especially cyclohexyl, ring are preferred.
Compounds of formula (Ixc) in which q is zero are preferred.
A preferred group of compounds of the invention are compounds of formula (Ixc)
in which:- W
represents CH; X represents CH; Y represents CH; Z represents CH or C-CH3; R7
represents
hydrogen; A represents a cyclopentyl, cyclohexyl or cycloheptyl ring; q is
zero; and their
corresponding N-oxides, and their prodrugs, and their acid bioisosteres; and
pharmaceutically
acceptable salts and solvates (e.g. hydrates) of compounds of formula (Lxc)
and their N-oxides and their
prodrugs, and their acid bioisosteres.
A further preferred group of compounds of the invention are compounds of
formula (Ixc) in which:- W
represents CH; X represents CH; Z represents CH; Y represents (i) C-C1-4alkyl
[e.g. C-CH3,
CH3
C-CH2CH3'C-CH2CH2CH3 or C-CH(CH3)2], (ii) C-aryl [e.g. C / \, C / \,
CH3 CN CH3O
C / CH3 1 C / \ , C / \ ,
F O\
C Cl, C / \ or C / 0 ], (iii) C-CN, (iv) C-NO2, (v) C-halo
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-80-
[e.g. C-Br, C-Cl or C-F], (vi) C-haloalkyl [e.g. C-CF3], (vii) C-heteroaryl
[e.g. C / \N or
N
C (viii) C-OR4 [e.g. C-OCH3, C-OCH,CH3 , C-OCHF,, C-OCFj ,
C-O , C-O-CHZ o or C-O-(CH,)2 N - ~O ], (ix) C-C(=O)R4 [e.g.
\-/
C-C(=0) \ / ], (x) C-C=0)NYlY2 [e.g. C'-C(=O)-NH-CH3 , C-C(=O)-N(C.H3)2 ,
C-C(=O)-NH-CH,CH3 , C-C(=O)-NH-CH(CH3), , C-C(=O)-NH-C(CH3)2-CH2OH ,
C-C(=O)-NH-CHZCH,CN, C-C(=O)-NH-CH,CH2OCH3 '
C3
C-C(=O)-NH-CH, C-C(=O)-NH-CHZ / \ ,
CH3
C-C(=O)-NH-CH2 / \ , C-C(=O)-NH-CHZ O-CH 3 ,
C-C(=O)-NH-CH, C-C(=O)-NH-CH2 O-N 10 C-C(=O)-NH-(CH,), / \, C-C(=O)-NH-(CHZ);
N o
~
C-C(=O)-NH-(CH2)2 N , C-C(=O)-NH-(CH,)3 N ,
\5:- N
\
N 0
C.-C(=O)-NH-(CHz);<\ N , C-C(=O)-NH-(CH2)3 N
N~
or C-C.(=O)-NH \ / ], (xi) -C(=O)OR4 [e.g. C-C(=O)OH or C-C(=0)OCH3], (xii)
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-81-
C-NHC(=0)R4 [e.g. C-NHC(=O)CH3 or C-NHC(=0)CH(CH3)2, C-NH-C(=O) \ / or
C-NH-C(=O)-CH, \ / ], (xiii) C-CH(OH)aryl [e.g. C-CH(OH) (xiv)
C-S(O)2NYlY2 [e.g. C-SO; NH-CH2 / \] or (xv) C-S(O)nR4 [e.g. C-SO2CH3]; R7
represents hydrogen; A represents a cyclopentyl, cyclohexyl or cycloheptyl
ring; q is zero; and
their corresponding N-oxides, and their prodrugs, and their acid bioisosteres;
and pharmaceutically
acceptable salts and solvates (e.g. hydrates) of compounds of formula (Ixc)
and their N-oxides and
their prodrugs, and their acid bioisosteres.
A further preferred group of compounds of the invention are compounds of
formula (Ixc) in which:- W
represents CH; X represents C-CH3, C-CH2CH3, C-CH(CH3)2, C-OCH3, C-OCH2CH3, C-
Br or
C-Cl; Y represents C-CH3, C-CH2CH3, C-OCH3, C-Br, C-Cl, C-F, C / \ or
C-C(=O)-NH-CH, Z represents CH; R7 represents hydrogen; A represents a
cyclopentyl, cyclohexyl or cycloheptyl ring; q is zero; and their
corresponding N-oxides, and their
prodrugs, and their acid bioisosteres; and pharmaceutically acceptable salts
and solvates (e.g. hydrates)
of compounds of formula (Ixc) and their N-oxides and their prodrugs, and their
acid bioisosteres.
A further preferred group of compounds of the invention are compounds of
formula (Ixc) in which:-W
represents CH; X represents CH; Y represents C-CH3; Z represents C-CH3; R7
represents hydrogen;
A represents a cyclopentyl, cyclohexyl or cycloheptyl ring; q is zero; and
their corresponding
N-oxides, and their prodrugs, and their acid bioisosteres; and
pharmaceutically acceptable salts and
solvates (e.g. hydrates) of compounds of formula (Ixc) and their N-oxides and
their prodrugs, and their
acid bioisosteres.
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-82-
A further preferred group of compounds of the invention are compounds of
formula (Ixb) in which:- W
represents CH; X represents CR9- and Y represents CR3 where R-9 and R3 form
the group
-CH2-O-CH?-; Z represents CH; R7 represents hydrogen; A represents a
cyclopentyl,
cyclohexyl or cycloheptyl ring; q is zero; and their corresponding N-oxides,
and their prodrugs, and
their acid bioisosteres; and pharmaceutically acceptable salts and solvates
(e.g. hydrates) of compounds
of formula (Ixc) and their N-oxides and their prodrugs, and their acid
bioisosteres.
A further preferred group of compounds of the invention are compounds of
formula (Ixb) in which:- W
represents CH; X represents CR2 and Y represents CR3 where R-9 and R3 form the
group
-CH2-CH2-CH2-; Z represents CH; R7 represents hydrogen; A represents a
cyclopentyl,
cyclohexyl or cycloheptyl ring; q is zero; and their corresponding N-oxides,
and their prodrugs, and
their acid bioisosteres; and pharmaceutically acceptable salts and solvates
(e.g. hydrates) of compounds
of formula (Ixc) and their N-oxides and their prodrugs, and their acid
bioisosteres.
Compounds of formula (Ixc) in which R7 represents hydrogen and p is zero are
particularly preferred.
Compounds of formula (Ixc) in which W is CH, X is C-CH3, Y is C-CH3 and Z is
CH are also
particularly preferred.
Compounds of formula (Ixc) in which A is a cyclopentyl ring are particularly
preferred.
Another particular group of compounds of the invention are compounds of
formula (Ix) wherein RI is a
R9
Rs
pyrazolyl moiety in which R8 and R9 together with the carbon atoms to which
they
N',N R7
are attached form an optionally substituted heterocycloalkyl ring, i.e.
compounds of formula (Ixd):-
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-83-
X1
(R131r
Y ~ N
\ /
\
I \
W H N~N R7
(Ixd)
in which W, X, Y, Z and X are as hereinbefore defined for compounds of formula
(Ix), Xl is 0, S,
S02, or NY5 (where Y5 is hydrogen, R4, -C(=0)R4, -C(=O)NYIY2, -C(=0)OR4 or -
S02R4), r is
zero or an integer one or two and R13 is alkyl or two R13 groups attached to
the same carbon atom
form an oxo group; and their corresponding N-oxides, and their prodrugs, and
their acid bioisosteres;
and pharmaceutically acceptable salts and solvates (e.g. hydrates) of
compounds of formula (Ixd) and
their N-oxides and their prodrugs, and their acid bioisosteres.
Compounds of formula (Ixd) in which W represents CH, X represents CH, Y
represents CH and Z
represents CH or C-CH3 are preferred.
Compounds of formula (Ixd) in which W represents CH, X represents CH, Z
represents CH and Y
represents:
(i) C-C1-4alkyl [e.g. C-CH3, C-CH2CH3'C-CH2CH2CH3 or C-CH(CH3)2];
C3 CH3
(ii) C-aryl [e.g. C / \ , C / \ , C / \ ,
CN CH3O
C / \ CH3 , C / \ , C / \ , C / \ Cl ,
F O`
C / \ or C / O ];
(iii) C-CN;
(iv) C-NO2;
(v) C-halo [e.g. C-Br, C-Cl or C-F];
(vi) C-haloalkyl [e.g. C-CF3];
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-84-
(vii) C-heteroaryl [e.g. C / \N or C
(viii) C-OR4 [e.g. C-OCH3 , C-OCH,CH3 , C-OCHFZ , C-OCF3, C-O / \ I
C-O-CHZ / \ or C-O-(CHZ) N \O ];
~
(ix) C-C(=0)R4 [e.g. C-C(=0) \ / ];
(x) C-C=O)NYlY2 [e.g. C-C(=O)-NH-CH3 , C-C(=O)-N(CH3),,
C-C(=O)-NH-CH2CH3 , C-C(=O)-NH-CH(CH3), ,
C-C(=O)-NH-C(CH3),-CH2OH , C-C(=O)-NH-CHzCH,CN ,
C-C(=O)-NH-CH,CH2OCH3 , C.-C(=O)-NH-CH, / \ ,
CH3 CH3
C-C(=O)-NH-CH~ C-C(=O)-NH-CH2 / \ ,
C-C(=O)-NH-CHZ CH3 , C-C(=O)-NH-CH, / \ ,
N-
C
-C(=O)-NH-(CH,)Z / \ -
C-C(=O)-NH-CH2 O-N
C-C(=O)-NH-(CH2)2 N O, C-C(=O)-NH-(CH2)2 N ,
~
N
C-C(=O)-NH-(CHZ)-C~ ~N
N
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-85-
O
~
C-C(=O)-NH-(CH`)3 NN , C-C(=O)-NH-(CHZ)3 N or
C-C(=O)-NH \ / ];
(xi) C-C(=O)OR4 [e.g. C-C(=O)OH or C-C(=O)OCH3];
(xii) C-NHC(=0)R4 [e.g. C-NHC(=O)CH3, C-NHC(=0)CH(CH3)2,
C-NH-C(=O) o or C-NH-C(=O)-CHz 0 ];
(xiii) C-CH(OH)aryl [e.g. C-CH(OH) \ / ];
(xiv) C-S(O)2NYlY2 [e.g. C.-SOf NH-CHZ or
(xv) C-S(O)nR4 [e.g. C-SO2CH3];
are also preferred.
Compounds of formula (Ixd) in which W represents CH, X represents C-CH3, C-
CH2CH3,
C-CH(CH3)2, C-OCH3, C-OCH2CH3, C-Br or C-Cl, Y represents C-CH3, C-CH2CH3, C-
OCH3,
C-Br, C-Cl, C-F, C / \ or C-C(=O)-NH-CH, / \ and Z represents CH are also
preferred.
Compounds of formula (Ixa) in wliich W represents CH, X represents CH, Y
represents C-CH3 and Z
represents C-CH3 are also preferred.
Compounds of formula (Ixd) in which W represents CH, X represents CR2 and Y
represents CR3
where R2 and R3 form the group -CH2-O-CH2-, and Z represents CH are also
preferred.
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-86-
Compounds of formula (Ixd) in which W represents CH, X represents CR9- and Y
represents CR3
where R2 and R3 form the group -CH2-CH2-CH2-, and Z represents CH are also
preferred.
Compounds of formula (Ixd) in which R7 represents hydrogen are preferred.
Compounds of formula (Ixd) in which Xl is:
(i) 0;
(ii) N-C(=0)R4 [e.g. N-C(=O)CH3, N-C(=0)CH2CH(CH3)2, N-C(=0)CH(CH3)2, or
N-C(=0)C(CH3)3 or N-(C=0)--a ];
(iii) N-C(=0)NYlY2 [e.g. N-C(=0)N(CH3)2, N-C(=0)NCH(CH3)2, N-C(=0)N(CH2CH3)2
N-(C=0)-N , N-(C=0)-N or N-(C=0)- O];
(iv) N-C.(=0)OR4 [e.g. N-C(=0)OCH3 or N-C(=0)OCH2CH3]; or
(v) N-S02R4 [e.g. N-SO2CH3 or N-SO2CH(CH3)21;
are preferred.
Compounds of formula (Ixd) in which r is zero are preferred.
A preferred group of compounds of the invention are compounds of formula (Ixd)
in which:- W
represents CH; X represents CH; Y represents CH; Z represents CH or C-CH3; R7
represents
hydrogen; Xl is (i) 0; (ii) N-C(=0)R4 [e.g. N-C(=0)CH3, N-C(=0)CH2CH(CH3)2, N-
C(=0)CH(CH3)2, or N-C(=0)C(CH3)3 or N-(C=0)--a ]; (iii) N-C(=0)NYlY2 [e.g.
N-C(=0)N(CH3)2, N-C(=0)NCH(CH3)2, N-C(=0)N(CH2CH3)2 N-(C=0)-N ,
N-(C=0)-N or N-(C=0)- O]; (iv) N-C(=0)OR4 [e.g. N-C(=0)OCH3 or
N-C(=0)OCH2CH3]; or (v) N-S02R4 [e.g. N-SO2CH3 or N-SO2CH(CH3)2] and r is
zero; and their
corresponding N-oxides, and their prodrugs, and their acid bioisosteres; and
pharmaceutically
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-87-
acceptable salts and solvates (e.g. hydrates) of compounds of formula (Ixd)
and their N-oxides and
their prodrugs, and their acid bioisosteres.
A further preferred group of compounds of the invention are compounds of
formula (Ixd) in which:- W
represents CH; X represents CH; Z represents CH; Y represents (i) C-C1-4a1ky1
[e.g. C-CH3,
CH3
C.-CH2CH3'C-CH2CH2CH3 or C-CH(CH3)2], (ii) C-aryl [e.g. C
CH3 CN CH3O
C / \ , C CH3 , C / \ , C / \ ,
F O
Cl, C. b or C 6 0 ], (iii) C-CN, (iv) C-NO2, (v) C-halo
[e.g. C-Br, C-Cl or C-F], (vi) C-haloalkyl [e.g. C-CF3], (vii) C-heteroaryl
[e.g. C / \ N or
C (viii) C-OR4 [e.g. C-OC.H3 , C-OCH,Cl~,, C-OCHF2 , C-OCF3 ,
C-0 / \ , C-O-CH2 / \ or C-O-(CH2)N~O ], (ix) C-C(=O)R4 [e.g.
-
C-C(=0) \ / ], (x) C-C=O)NYlY2 [e.g. C-C(=O)-NH-CH3, C-C(=O)-N(CH3); ~
C-C(=O)-NH-CHZCH3 , C-C(=O)-NH-CH(CH3)2, C-C(=O)-NH-C(CH3),-CH2OH ,
C-C(=O)-NH-CH2CH2CN, C-C(=O)-NH-CH2CH2OCH3
CH3
C-C(=O)-NH-CH, / \ , C-C(=O)-NH-CH, / \ ,
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-88-
CH3
C-C(=O)-NH-CH2 / \ , C-C(=O)-NH-CHz / \ CH3 ,
c-c(=o)-NH-cH, ~ ~ C-C(=O)-rrH-CH,
N-
C-C(=O)-NH-(CH,)Z C-C(=O)-NH-(CH,); N\
~
C-C(=0)-NH-(CH2); N O , C-C(=O)-NH-(CH,)3 ~N ,
O
N
C-C(=O)-NH-(CH2)2 N , C-C(=0)-NH-(CH2)3 N
or C-C(=O)-NH (xi) -C(=0)OR4 [e.g. C-C(=0)OH or C-C(=0)OCH3], (xii)
C-NHC(=O)R4 [e.g. C-NHC(=O)CH3 or C-NHC(=0)CH(CH3)2, C-NH-C(=O) \ / or
C-NH-C(=O)-CH2 \ / ], (xiii) C-CH(OH)aryl [e.g. C-CH(OH) \ / ], (xiv)
NH-CH2 ] or (xv) C-S(O)nR4 [e.g. C-S02CH3]; R7
C-S(O)2NYlY2 [e.g. C-SO; / \
represents hydrogen; Xl is (i) 0; (ii) N-C(=0)R4 [e.g. N-C(=0)CH3, N-
C(=0)CH2CH(CH3)2, N-
C(=O)CH(CH3)2, or N-C(=O)C(CH3)3 or N-(C=O)--a ]; (iii) N-C(=0)NYlY2 [e.g.
N-C(=0)N(CH3)2, N-C(=0)NCH(CH3)2, N-C(=O)N(CH2CH3)2 N-(C=0)-N ,
N-(C=0)-N or N-(C=0)-N p]; (iv) N-C(=0)OR4 [e.g. N-C(=0)OCH3 or
\--j
N-C(=0)OCH2CH3]; or (v) N-S02R4 [e.g. N-SO2CH3 or N-SO2CH(CH3)2] and r is
zero; and their
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-89-
corresponding N-oxides, and their prodrugs, and their acid bioisosteres; and
pharmaceutically
acceptable salts and solvates (e.g. hydrates) of compounds of formula (Ixd)
and their N-oxides and
their prodrugs, and their acid bioisosteres.
A further preferred group of compounds of the invention are compounds of
formula (Ixd) in which:- W
represents CH; X represents C-CH3, C-CH2CH3, C-CH(CH3)2, C-OCH3, C-OCH2CH3, C-
Br or
C-Cl; Y represents C-CH3, C-CH2CH3, C-OCH3, C-Br, C-Cl, C-F, C / \ or
C-C(=O)-NH-CH2 Z represents CH; R7 represents hydrogen; XI is (i) 0; (ii)
N-C(=0)R4 [e.g. N-C(=0)CH3, N-C(=0)CH2CH(CH3)2, N-C(=0)CH(CH3)2, or N-
C(=0)C(CH3)3
or N-(C=0)--a ]; (iii) N-C(=0)NYlY2 [e.g. N-C(=0)N(CH3)2, N-C(=0)NCH(CH3)2,
N-C(=0)N(CH2CH3)2 N-(C=O)-N , N-(C=0)-N or N-(C=0)- O];
(iv) N-C(=0)OR4 [e.g. N-C(=0)OCH3 or N-C(=O)OCH2CH3]; or (v) N-S02R4 [e.g. N-
S02CH3 or
N-S02CH(CH3)2] and r is zero; and their corresponding N-oxides, and their
prodrugs, and their acid
bioisosteres; and pharmaceutically acceptable salts and solvates (e.g.
hydrates) of compounds of
formula (Ixd) and their N-oxides and their prodrugs, and their acid
bioisosteres.
A further preferred group of compounds of the invention are compounds of
formula (Ixd) in which:-W
represents CH; X represents CH; Y represents C-CH3; Z represents C-CH3; R7
represents hydrogen;
Xl is (i) 0; (ii) N-C(=0)R4 [e.g. N-C(=0)CH3, N-C(=0)CH2CH(CH3)2, N-
C(=0)CH(CH3)2, or
N-C(=0)C(CH3)3 or N-(C=O)---a ]; (iii) N-C(=0)NYlY2 [e.g. N-C(=0)N(CH3)2, N-
C(=0)NCH(CH3)2, N-C(=O)N(C.H2CH3)2 N-(C=O)-N , N-(C=0)-N or
N-(C=O)-N O]; (iv) N-C(=O)OR`I [e.g. N-C(=0)OCH3 or N-C(=0)OCH2CH3]; or (v)
N-S02R4 [e.g. N-S02CH3 or N-S02CH(CH3)2] and r is zero; and their
corresponding N-oxides, and
their prodrugs, and their acid bioisosteres; and pharmaceutically acceptable
salts and solvates (e.g.
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-90-
hydrates) of compounds of formula (Ixd) and their N-oxides and their prodrugs,
and their acid
bioisosteres.
A further preferred group of compounds of the invention are compounds of
formula (Ixd) in which:-W
represents CH; X represents CR2 and Y represents CR3 where R2 and R3 form the
group
-CH2-O-CH2-; Z represents CH; R7 represents hydrogen; Xl is (i) 0; (ii) N-
C(=0)R4 [e.g. N-
C(=0)CH3, N-C(=0)CH2CH(CH3)2, N-C(=0)CH(CH3)2, or N-C(=0)C(CH3)3 or
N-(C=0)--a ]; (iii) N-C(=0)NYlY2 [e.g. N-C(=0)N(CH3)2, N-C(=0)NCH(C.H3)2,
N-C(=0)N(CH2CH3)2 N-(C=0)-N , N-(C=0)-N or N-(C=0)- O];
(iv) N-C(=0)OR4 [e.g. N-C(=O)OCH3 or N-C(=0)OCH2CH3]; or (v) N-S02R4 [e.g. N-
S02CH3 or
N-SO,)CH(CH3)2] and r is zero; and their corresponding N-oxides, and their
prodrugs, and their acid
bioisosteres; and pharmaceutically acceptable salts and solvates (e.g.
hydrates) of compounds of
formula (Ixd) and their N-oxides and their prodrugs, and their acid
bioisosteres.
A further preferred group of compounds of the invention are compounds of
formula (Ixd) in which:-W
represents CH; X represents CR-9 and Y represents CR3 where 10 and R3 form the
group
-CH2-CH2-CH2)-; Z represents CH; R7 represents hydrogen; Xl is (i) 0; (ii) N-
C(=0)R4 [e.g. N-
C(=0)CH3, N-C(=0)CH2CH(CH3)2, N-C(=0)CH(CH3)2, or N-C(=0)C(CH3)3 or
N-(C=0)--< ]; (iii) N-C(=0)NYIY2 [e.g. N-C(=0)N(CH3)2, N-C(=0)NCH(CH3)2,
N-C(=0)N(CH2CH3)2, N-(C=0)-N , N-(C=0)-N or N-(C=0)- O];
~-~
(iv) N-C(=0)OR4 [e.g. N-C(=O)OCH3 or N-C(=O)OCH2CH3]; or (v) N-S02R4 [e.g. N-
S02CH3 or
N-S02CH(CH3)2] and r is zero; and their corresponding N-oxides, and their
prodrugs, and their acid
bioisosteres; and pharmaceutically acceptable salts and solvates (e.g.
hydrates) of compounds of
formula (Ixd) and their N-oxides and their prodrugs, and their acid
bioisosteres.
Compounds of formula (Ixd) in which Xl is N-C(=O)CH(CH3)2, N-C(=0)CH2CH(CH3)2,
N-C(=0)C(CH3)3; N-(C=O)--< , N-C(=O)N(CH3)2, N-C(=0)NCH(CH3)2,
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-91-
N-C(=O)N(CH2CH3)2, N-(C=O)-N , N-(C=O)-N , N-(C=0)-N O,
N-C(=0)OCH3 or N-C(=O)OCH2CH3and r is zero are particularly preferred.
Compounds of formula (Ixd) in which W is CH, X is CH, Y is CH, C-CH2CH3, C-
CH2CH2CH3,
CN F O
\ \ \ ,
C / , C ~ \ , C / , C / , C-CN, C-Br, C-CF3,
N
C / \ N , C-OCH3 , C-OCH2CH3, C-OCHF,, C-OCF3,
C-O-CH, C,-C(=O)-NH-CH3 , C-C(=O)-NH-CH,CH3 ,
C-C(=O)-NH-CH(CH3), , C-C(=O)-NH-C(CH3),-CHZOH , C-C(=O)-NH-CH,CH,CN ,
C-C(=O)-NH-C.H,CH,OCH3 , C-C(=O)-NH-CHZ 0
,
CH3 CH3
C-C(=O)-NH-CH, C-C(=O)-NH-CH,
C-C(=O)-NH-CH, CH3 1 C.-C(=O)-NH-(CH,)`
C-C(=O)-NH-(CH2)2 N O, C-C(=O)-NH-(CHZ)TN
N~N
C-C(=O)-NH-(CH,)-C~ II ,
G-C(=0)-NH-(CH,)3N \ ~N , N--N
C-C(=O)-NH-CHZ / \ C-C(=O)-NH-CH` / \ `-N
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-92-
O
C-C(=O)-NH-(CH,)3 N , C-C(=O)-NH o , C-C(=O)OCH3 C-C(=O)OH
C-CH(OH) \ /, C-SO2CH3 or C-SOz NH-CH2 / \ and Z is CH are particularly
preferred.
Compounds of formula (Ixd) in which W is CH, X is C-CH3 or C-CH2CH3, Y is C-
CH3, C-CH2CH3,
C-CH(CH3)2, C-Br, C-Cl, C-F, C C-C(=O)-NH-CH, / \, and Z is CH are
also particularly preferred.
Compounds of formula (Ixa) in which W is CH, X is C-OCH3, Y is CH, C-CH3, C-CH-
)CH3, C-Cl or
C-OCH3 and Z is CH are also particularly preferred.
Compounds of formula (Ixd) in which W is CH, X is C-OCH2CH3, Y is C-F and Z is
CH are also
particularly preferred.
Compounds of formula (Ixd) in which W represents CH, X represents CR2 and Y
represents CR3
where R2 and R3 form the group -CH2-CH2-CH2-, and Z represents CH are also
particularly
preferred.
Compounds of formula (Ixd) in which W represents CH, X represents CR2 and Y
represents CR3
where R2 and R3 form the group -CH2-O-CH2-, and Z represents CH are also
particularly preferred.
Compounds of formula (Ixd) in which Xl is N-(C=O)--< , N-C(=0)N(CH3)2,
N-C(=0)NC.H(CH3)2, N-C(=0)N(CH2CH3)2, N-(C=O)-N or N-(C=O)-N and r
is zero are especially preferred.
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-93-
Compounds of formula (Ixd) in which W represents CH, X represents C-CH3, Y
represents C-CH3 or
C-Cl and Z represents CH are especially preferred.
Particular compounds of the invention of formula (Ix) are selected from the
compounds formed by
joining the carbon atom (C*) of one of the benzoimidazole, imidazo[4,5-
b]pyridine,
imidazo[4,5-c]pyridine or imidazo[4,5-b]pyrazine fragments (Al to Al 10) shown
in Table 1 to the
carbon atom (*C) in the heteroaryl moiety of one of the fragments (B 1 to B
168) shown in Table 2.
Particular compounds of the invention of formula (Ixa) are selected from the
compounds formed by
joining the carbon atom (C*) of one of the benzoimidazole, imidazo[4,5-
b]pyridine,
imidazo[4,5-c]pyridine or imidazo[4,5-b]pyrazine fragments (Al to A110) shown
in Table 1 to the
carbon atom (*C) in the pyrazole ring of one of the fragments (B 1 to B48, B74
to B 107, B 124 to B 127,
130 to 142 or 144 to 150) shown in Table 2.
Particular compounds of the invention of formula (Ixb) are also selected from
the compounds formed
by joining the carbon atom (C*) of one of the benzoimidazole, imidazo[4,5-
b]pyridine,
imidazo[4,5-c]pyridine or imidazo[4,5-b]pyrazine fragments (Al to A110) shown
in Table 1 to the
carbon atom (*C) in the five membered ring of one of the fragments (B63 to
B73, B108 to B 114, B128
or B151) shown in Table 2.
Particular compounds of the invention of formula (Ixc) are selected from the
compounds formed by
joining the carbon atom (C*) of one of the benzoimidazole, imidazo[4,5-
b]pyridine,
imidazo[4,5-c]pyridine or imidazo[4,5-b]pyrazine fragments (Al to Al 10) shown
in Table 1 to the
carbon atom (*C) in the five membered ring of one of the fragments (B56, B59
or B129) shown in
Table 2.
Particular compounds of the invention of formula (Ixd) are selected from the
compounds formed by
joining the carbon atom (C*) of one of the benzoimidazole, imidazo[4,5-
b]pyridine,
imidazo[4,5-c]pyridine or imidazo[4,5-b]pyrazine fragments (Al to A110) shown
in Table 1 to the
carbon atom (*C) in the five membered ring of one of the fragments (B 115 to B
123 or B 157) shown in
Table 2.
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-94-
TABLE 1
Al Nv A2 CH3 ai~ Nv
cc* A3 CH; A4 CFs N
\\
i*
N\%*
A5 OH A6 CH3ON\
c*
N C* I/ H
A8 F N\
A7 cI N\\ C
C
A9 CH; \ N\ A10 0
*
C
CH3 C Ns
\ / N
All e.1 N~ A12 CH3 N
v
C* c
CI Cl
A13 tCH_;)3C \ N~ A14 0
1
/ NC HO N~
H I / N/
A15 0 A16 0
C.H3NH N\ CH3(CH2)3NH NC*
C
A17 0 A18 0 s0
I\ ~ I\ N c HzN~s N ~,
A 19 A20 \ 0 N\
C*
O N / N
j
'* H ,-c
C
A21 CH3O I\ N c* A22 0 N / cQc*
N
CH3 H H
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-95-
A23 CH3O\ N A24 (CH;)ZN N\
::jj C* %
CH3O H
A26 N~
A25 (CH3)2N N\ N
%* CN NC
CH; N H
A27 CH3CH2 N\ ~g CH3CH,CHz N\
\C* C
H H
A29 N/\/O N~ A30 F~CH O N\
~* I 1 ;
O F N
N H
H
N A32 B
r N\ .C
~
A31 CF3O1:~,-N
H.
A33 NC \ N A34 OH
l / j * / \ N\
%
0 A36 0
A35
N CH;CH,~ N
CH3O\\C * H I \\C*
0 A38 0
A37
(CH3)zCH,H N ~ HsC~N N~
\ %
CH3 / H
A39 0 A40 0
N
I/ H I/ N C* I
/ H I/ C*
N
H
o A42 0
A41
~\ H I\ H3C
N C* I\ H I\ N C*
N
H H
A43 0 A44 CH3 0
I\ H N C* I\ H I\ N C*
HC / H
s H
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-96-
A45 c A46 c
NN \ N~ CH;O(CH,)-HI N C
H I C*
/ N
H
C
A47 0 o A48 0
N~/\N N \--\N N
H C* H C*
H H
A49 0 A50 0
4 N N~ NC-(CH,),-NH
(\/ H C* ~
N
H
A51 o A52 0
I \ N
H I\ N C* H \C
N
H
A53 0 A54 (cH02CH v Nv
c*
(CH3)ZCHNH N C CH I/ N
3 H
F H
A55 CH3CH, \ N A56 CH3CH, N\
I ~C* C
CI=I3 / CH3CH,
A57 A58 CH3 \ N\
~/c*
N
\ Br C CH3 H
DO,
A59 cH3 A60 /
H3C N\ N
C* \\
j *
H H
A61 A62 H3C H3C \ I\ N C* \ I\ N c
H
A63 CH3 A64 cl
\ I \ N C * \ I \ NC*
/ rj / H
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-97-
A65 p A66 O o N
N C* O I \ C*
F N N
H H
A67 / A68 101-C:Q \ I N~C N
C \C*
OCHs H
A69 N A70
N
\ \ N \c I \ ~C
O \% 'N
H H
A71 Hi C N N A72 (CH3),CH N N
C* C*
~ \\ ~ \\
O O
H
N A74
A73 H
NC* \ I O /
I
H
\ \ NN
/ N
H
A77 N A78 H~C CH 0
\ ~ \ N~ HOCH,H N c*
%*
A79 N N-I 0 A80 0
j~,~
~ \ N C* I D-_- H I~*
I / H3C
A81 HO O A82 0
(CH3)2CHCH,,, N
N \C
\
I N/* I H
A83 0 A84 0
~ I\ N\ C*
O
H H
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-98-
A85 0 o A86 N N-I N 0
N \N " N \ N\
H C* H I C*
H
N /
H
A87 x;c cx, 0 A88 0
N H0 N
HOCH N I \ \\ ~N\N \
x N H3C CH3x / N C*
H H
A89 o A90 0
H3C a S N\ C*
CH3 A91 o A92 0
J I C*
CjC* \ N\ ~N \ N\
N O\/ / 1`j
H H
A93 0 A94 0 0
N
N
H
NC
CH N
;/ N
H;C H
A95 0 A96 0
N \ N~ (CH3),CHNH N~
I j j
OJH3C F
A97 0 A98 o
H * I\ I I\ N C
O \H I
\ NC
s C 3 N N
H 3 C H F H
A99 cF3 N\ A100 0 \ N~
C* C
N
F O H
A101 A102 ~ x
H3N
~
CC*
O rJ ):::c
CN H3C
H
z I \ N *
A103 Ox A104 HOCx
H3C-CH N /C
/
I \ ~C* x3C
/
HOCHz H
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-99-
A105 N N A106 H3C~ I \ N~ (CH3),CH y ):),N
~C*
O / N~C ~
H
A107 N ~ A108 N
\~
C~ C* C
N ~ / N ~ N
N H H
A109 N % N~ A110 N N\
c*
i ~
~N H N H
TABLE 2
B 1 ~ B2 CH,
*CNH
N *C\~ NH
B3 ~CH,CH3 B4 ~CH,OH
*C~~ NH *C'Nz NH
B5 CF3 B6 ~NH,
*C\~ ~NH *C~~ " NH
N N
B7 CD B8 OCHzCH;
*C~~N' NH
~ =
*C\~ " NH
N
B9 ~SCH3 B 10 SCH,CH3
C\IN ~NH *C~~ NH
B11 \ B12 s \
s._/y
s
*C ~ ~NH
*C~NZNH
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-100-
B13 / ~ B 14 s
*C-~ ~NH
N
*C\I oNH
N
B15 B16 CH3~
N
s / ~ N
- *C~ ~NH
F-~
*C\~N/NH
B17 HOCH, B18 HOCH,CH,~
,NH *C~~ NH
*C N
B 19 CH3OCH, B20
*C~ ~NH
~N
*C\~ NI-I
N B
B21 Q
22 0
*C~~ ~NH
*C\~ " NH
N
B23 (CH3)7 B24 NH(CHZ)zOH
O
*C~~ NH *C.\~ NH
N N
C(CH3)2CH,OH B26 N
B25 O o
*C"N' NH O
*C'N~NH
B27 Hz \/}- B28 (CH3)2CHNH~
~\
*C -'N'~ NH *C'~"Ni
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-101-
B29 B30
HN
HN
*C\~ "NH
N *C\~V NH
B31 p CH3 B32
HN
'\ HN
~
*C~~ " NH *C \N/NH
N ~
N- B34 N
B33 p
I-1
*C~ NH *C~~ NH
N N
B36
B35 p o\-
HN p
*C~\N/NH HN \
r--\
*C~~ z NH
N
B37 B38 0 )__NHCH3
0
O HN HN
*C\\ NH
J~ N
*C/\~ NH
B39 B40
~-H 0
HN
HN
*C\~ NH
*C\,N,,NH
B41 B42 p~ ~p
~IN SLCH3
p~}--~
HN C"'N NH
*C\~ ZNH
N
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-102-
B43 cx,cH,o B44 ~
~
*C~~ NH -
0
*C~~NNH
B45 CH3,,SC.H3 B46 CH3C.H2CH2,,SCH3
*CNH *C
B47 ocx3 B48 / \
(CH3)2CI ~S
P
CH;CH,CH,~S
*C\~ ZNH
*C~~ " NH N
N
B49 ~SCH, B50 SCH3
%~
*C\\ C *CNH
B51 SCHzCH3 B52 ~ N_~ ~
*C-- ZNH N
N
B53 H,N B54
*c
*CNH N
O
B55 o B56
*c~ A
~N-H
*C\~ ~NH
B57 o B58 ~ S
HN
*C--N IINH
*C\~ ZNH
N
B59 B60
*C~~ ~NH *C\\ ~NH
N N
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-103-
B61 B62 0
HN
HN
*C~~ ~NH
N *C, NH
"Z N
B63 B64 CH3
- ~ ~
*c ~NH ^
N *C\~ ~NH
B65 cH.3 B66 CF3
*C\~ NH *C\~ NH
N N
B67 CF3 B68 CH3O
*C~N N ~NH *C\~ NoNH
B69 OCH3 B70 F
*C\~ NH *C\~ ~NH
N N
B71 F B72 CN
*C\~ N NH *C\~ NH
B73 CN B74 cH3s~
*C ,NH
\ ~
N
*C~~ ~NH
N
B75 0 B76 0NHcx3
cH
r 3
0 \ N14
NH
~--~ ~
*C\~ ~NH
*C NH
N N
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-104-
B77 B78 CH3
/ \ CH3~N
O O
NH
*C"'N ~NH
*C\~ NH
B79 ~ H3 B80 HOCHz
HN \ CH3
0 HN CH3
*C"' ~NH - 0
N
*C\~ NH
B81 B82
S HN
O
*C~~ NH
*C"' NH
N
B83 cH(cH3)2 B84
*C~~N" NH
*C~~ NH
B85 o\\ B86 0)-CH,CH(CH3),
-CH(CH3), HN ~
*C"' ~NH *C\ NH
N ~N
B87 0\\ B88 :--
NNNH *C~" NH
N
B89 0 B90
0
HN
MN
*C~~
N *C~~ NH
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-105-
B91 B92
HN
HN
kC ~NiNH *C ~~ ~--A
z NH
N
B93 0~ B94 0 0
HN
HN
*C NH
*C-N ZNH
B95 N_ B96 \ -I
~-j HN
HN
*C, NH
*C~~ NH B97 ~ N~N
B98
N
O N.J O
~-j
HN HN
*C~~N~NH *C'-~NH
N
B99 B 100 c o
0 N_J)
O, N O~
HN HN
*C~~ ~NH *C~~ ~NH
N N
B101 B102
o
~\ /
HN
*C~~ NH N *C~~ ~NH
N
B103 B 104 N
o r --~I
- H
HN
*C~~ NH
*C-'~NH
N
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-106-
B 105 N 11 B106 HCH(CH;)z
_~I O
O
*C sNH
*C~~ z NH
N
B107 NH(CH3)1 B108 HO
o CH3
*C~~ ~NH
*C\~ NH
B109 cI B110
F/ \
*C\~ ~NH
*C\~ ~NH
N
B i l l P B112 EtO
cI
*C IINH
*C"' NH
N
B113 NHz B114 N(CH3)2
0 0
*C ~NH *C"'N "INH
N
H3
B115 cR B116 CH3
0
*C-'N *C\~ N ' NH
Q-
IINH
B 117 0=< CH(CH3), B 118 0=< CH2CH(CH3)2
N
p p
*C\~N" NH *C~~NeNH
B 119 0~ N(CH3)2 B120 0~ OCH
N N
*C\-N ~NH *C\X N INH
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-107-
B121 <NHCH(CHI)2 B122
0
N O
N
*C\~ NH
C\~ N
B 123 C(CH3)3 B 124 0
O-( ~-N V
HN
*C~~ NH
*C~~ ,NH
N
NI-IC(CH3)3 B126 0
B 125 )_N(CH.
O
HN
*C~~ NH
*C~~ ~NH
N
B127 B128 NHCH(CI I3),
p~-No O
FrN
*C NH
*C~~ NH
B129 B130
s
*C~~ ~NH *C~~ ~NH
N
B131 Br B132 O j H3
*C\\ N ~NH FfN OH
*C~~N "INH
B133 0 B134 0
\/ ~ ~-aOH
HN H
NH
*C\\N ~NH CN
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-108-
B 135 O HC CH3 B136
~~n H
HN 0
CH3
*C~~ NH H
*C~~N ~NH
B137 \\ B138 H3C
O
y-No
HN/
HN
*C~~ NH
N *C\~ ' NH
N
B139 B140 0 OH
YNO-OH YND
HN ~ CH3
*c NH *C1`j NH
~
B141 0 B142 N(CH3)2
y N~ y
HN C1I3 HNCH3
*CNH *CNH
B143 YNW B 144 NHC(CH3)2CH,OH
O
HN*C~~ NH
*C~~ NH
B145 NHCHzC(CH3)20H B146 NHC(CH3)3
O
*C~~ NH *C--N IINH
B147 H~ B148
N
O
*C~~ z NH O N
N
*C~~ ~NH
N
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-109-
B 149 B 150 Co
NJ
O
O
- *C-- ~NH
N
*C\~ NH
B 151 NHCH2C(CH3)2OH B 152 3
O /\
N~ S
>==<
I
- *C' NI
N
*C~~ NH
B153 - B154 CH3
IIN O
HN O
*C~~ " NH
N
*C "NH
B155 CH; B156 N, o
H3C O ~CH3
-~HN
r
*C~o NH
~N *C~ H
N
B157 N B158 pN
*C NH *C-N ~NH
B159 N
~ B160 0 ~-- ~--N-
H3C - (CH3)zCH
*C\~ eNE *C\~ NH
N N
B 161 --~N(CH3)2 B 162
0
NH
O
NH
*C/
\N ~ \
*C
O \ N
H 0
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-110-
O O
B 163 (CHa)aN B 164 CH3CH,NH
HN
C/
~N H
H 0
O
H(CH,)Z
B165 B166 ~H
0
N
N
O
OC
HN ~\N
O
C\
x
N-H
B167 NHCHzCH(CH;)z B168 O
O *C/
*C N
\N-H
B169
*C 0-1
Particular compounds of the invention of formula (Ix) denoted as the product
of the combination of
group Al to Al 10 in Table 1 with B 1 to B 169 in Table 2 are illustrated
below:
A1-B1; Al-B2; A1-B3; A1-B4; Al-B5; A1-B6;
A1-B7; A1-B8; Al-B9; Al-BlO; A1-B11; A1-B12;
Al-B13; A1-B14; A1-B15; A1-B16; A1-B17; A1-B18;
Al-B19; A1-B20; A1-B21; A1-B22; A1-B23; A1-B24;
Al-B25; A1-B26; A1-B27; Al-B28; Al-B29; Al-B30;
A1-B31; A1-B32; A1-B33; Al-B34; A1-B35; Al-B36;
A1-B37; Al-B38; Al-B39; Al-B40; Al-B41; Al-B42;
A1-B43; Al-B44; A1-B45; A1-B46; Al-B47; A1-B48;
Al-B49; Al-B50; A1-B51; Al-B52; A1-B53; A1-B54;
A1-B55; A1-B56; A1-B57; Al-B58; A1-B59; A1-B60;
A1-B61; Al-B62; Al-B63; A1-B64; Al-B65; A1-B66;
Al-B67; Al-B68; Al-B69; A1-B70; Al-B71; A1-B72;
Al-B73; A1-B74; A1-B75; Al-B76; Al-B77; Al-B78;
Al-B79; A1-B80; Al-B81; A1-B82; A1-B83; Al-B84;
Al-B85; A1-B86; A1-B87; A1-B88; Al-B89; A1-B90;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-111-
Al-B91; Al-B92; Al-B93; A1-B94; Al-B95; Al-B96;
A1-B97; Al-B98; A1-B99; A1-B100; A1-B101; A1-B102;
Al-B103; A1-B104; A1-B105; A1-B106; A1-B107; A1-B108;
A1-B109; Al-B110; A1-B111; Al-B112; Al-B113; Al-B114;
A1-B115; Al-B116; A1-B117; A1-B118; A1-B119; A1-B120;
Al-B121; A1-B122; Al-B123; Al-B124; A1-B125; A1-B126;
Al-B127; Al-B128; Al-B129; Al-B130; A1-B131; A1-B132;
Al-B133; Al-B134; Al-B135; A1-B136; A1-B137; Al-B138;
Al-B139; Al-B140; Al-B141; Al-B142; A1-B143; A1-B144;
A1-B145; Al-B146; Al-B147; A1-B148; Al-B149; Al-B150;
A1-B151; Al-B152; Al-B153; Al-B154; Al-B155; A1-B156;
Al-B157; Al-B158; A1-B159; Al-B160; Al-B161; Al-B162;
Al-B163; Al-B164; A1-B165; A1-B166; A1-B167; Al-B168;
A1-B169; A2-B1; A2-B2; A2-B3; A2-B4; A2-B5;
A2-B6; A2-B7; A2-B8; A2-B9; A2-B10; A2-B11;
A2-B 12; A2-B 13; A2-B 14; A2-B 15; A2-B 16; A2-B 17;
A2-B 18; A2-B 19; A2-B20; A2-B21; A2-B22; A2-B23;
A2-B24; A2-B25; A2-B26; A2-B27; A2-B28; A2-B29;
A2-B30; A2-B31; A2-B32; A2-B33; A2-B34; A2-B35;
A2-B37; A2-B38; A2-B39; A2-B40; A2-B41;
A2-B36;
A2-B42; A2-B43; A2-B44; A2-B45; A2-B46; A2-B47;
A2-B48; A2-B49; A2-B50; A2-B51; A2-B52; A2-B53;
A2-B54; A2-B55; A2-B56; A2-B57; A2-B58; A2-B59;
A2-B60; A2-B61; A2-B62; A2-B63; A2-B64; A2-B65;
A2-B66; A2-B67; A2-B68; A2-B69; A2-B70; A2-B71;
A2-B72; A2-B73; A2-B74; A2-B75; A2-B76; A2-B77;
A2-B78; A2-B79; A2-B80; A2-B81; A2-B82; A2-B83;
A2-B84; A2-B85; A2-B86; A2-B87; A2-B88; A2-B89;
A2-B90; A2-B91; A2-B92; A2-B93; A2-B94; A2-B95;
A2-B96; A2-B97; A2-B98; A2-B99; A2-B100; A2-B101;
A2-B 102; A2-B 103; A2-B 104; A2-B 105; A2-B 106; A2-B 107;
A2-B108; A2-B 109; A2-B 110; A2-B 111; A2-B 112; A2-B 113;
A2-B 114; A2-B 115; A2-B 116; A2-B 117; A2-B 118; A2-B 119;
A2-B 120; A2-B 121; A2-B 122; A2-B 123; A2-B 124; A2-B 125;
A2-B 126; A2-B 127; A2-B 128; A2-B 129; A2-B 130; A2 B 131;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-112-
A2-3132; A2-B 133; A2-B 134; A2-B 135; A2-B 136; A2-B 137;
A2-B138; A2-B 139; A2-B 140; A2-B 141; A2-B 142; A2-B 143;
A2-B 144; A2-B 145; A2-B 146; A2-B 147; A2-B 148; A2-B 149;
A2-B150; A2-B151; A2-B152; A2-B153; A2-B154; A2-B155;
A2-B 156; A2-B 157; A2-B158; A2-B 159; A2-B160; A2-B 161;
A2-B 162; A2-B 163; A2-B 164; A2-B 165; A2-B 166; A2-B 167;
A2-B 168; A2-B 169; A3-B 1; A3-B2; A3-B3; A3-B4;
A3-B5; A3-B6; A3-B7; A3-B8; A3-B9; A3-B 10;
A3-B11; A3-B12; A3-B13; A3-B14; A3-B15; A3-B16;
A3-B 17; A3-B 18; A3-B 19; A3-B20; A3-B21; A3-B22;
A3-B23; A3-B24; A3-B25; A3-B26; A3-B27; A3-B28;
A3-B29; A3-B30; A3-B31; A3-B32; A3-B33; A3-B34;
A3-B35; A3-B36; A3-B37; A3-B38; A3-B39; A3-B40;
A3-B41; A3-B42; A3-B43; A3-B44; A3-B45; A3-B46;
A3-B47; A3-B48; A3-B49; A3-B50; A3-B51; A3-B52;
A3-B53; A3-B54; A3-B55; A3-B56; A3-B57; A3-B58;
A3-B59; A3-B60; A3-B61; A3-B62; A3-B63; A3-B64;
A3-B65; A3-B66; A3-B67; A3-B68; A3-B69; A3-B70;
A3-B71; A3-B72; A3-B73; A3-B74; A3-B75; A3-B76;
A3-B77; A3-B78; A3-B79; A3-B80; A3-B81; A3-B82;
A3-B83; A3-B84; A3-B85; A3-B86; A3-B87; A3-B88;
A3-B89; A3-B90; A3-B91; A3-B92; A3-B93; A3-B94;
A3-B95; A3-B96; A3-B97; A3-B98; A3-B99; A3-B100;
A3-B 101; A3-B 102; A3-B 103; A3-B 104; A3-B 105; A3-B 106;
A3-B107; A3-B108; A3-B109; A3-B110; A3-B111; A3-13112;
A3-B 113; A3-B 114; A3-B 115; A3-B 116; A3-B 117; A3-B 118;
A3-B 119; A3-B 120; A3-B 121; A3-B 122; A3-B 123; A3-B 124;
A3-B 125; A3-B 126; A3-B 127; A3-B 128; A3-B 129; A3-B 130;
A3-B131; A3-B132; A3-B133; A3-B134; A3-B135; A3-B136;
A3-B137; A3-B138; A3-B139; A3-B140; A3-B141; A3-B142;
A3-B143; A3-B 144; A3-B 145; A3-B 146; A3-B 147; A3-B 148;
A3-B 149; A3-B 150; A3-B 151; A3-B 152; A3-B 153; A3-B 154;
A3-B155; A3-B156; A3-B157; A3-B158; A3-B159; A3-B160;
A3-B161; A3-B162; A3-B163; A3-B164; A3-B165; A3-B166;
A3-B167; A3-B168; A3-B169; A4-B1; A4-B2; A4-B3;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-113-
A4-B4; A4-B5; A4-B6; A4-B7; A4-B8; A4-B9;
A4-B 10; A4-B 11; A4-B12; A4-B13; A4-B14; A4-B 15;
A4-B16; A4-B 17; A4-B 18; A4-B 19; A4-B20; A4-B21;
A4-B22; A4-B23; A4-B24; A4-B25; A4-B26; A4-B27;
A4-B28; A4-B29; A4-B30; A4-B31; A4-B32; A4-B33;
A4-B34; A4-B35; A4-B36; A4-B37; A4-B38; A4-B39;
A4-B40; A4-B41; A4-B42; A4-B43; A4-B44; A4-B45;
A4-B46; A4-B47; A4-B48; A4-B49; A4-B50; A4-B51;
A4-B52; A4-B53; A4-B54; A4-B55; A4-B56; A4-B57;
A4-B58; A4-B59; A4-B60; A4-B61; A4-B62; A4-B63;
A4-B64; A4-B65; A4-B66; A4-B67; A4-B68; A4-B69;
A4-B70; A4-B71; A4-B72; A4-B73; A4-B74; A4-B75;
A4-B76; A4-B77; A4-B78; A4-B79; A4-B80; A4-B81;
A4-B82; A4-B83; A4-B84; A4-B85; A4-B86; A4-B87;
A4-B88; A4-B89; A4-B90; A4-B91; A4-B92; A4-B93;
A4-B94; A4-B95; A4-B96; A4-B97; A4-B98; A4-B99;
A4-B 100; A4-B 101; A4-B 102; A4-B 103; A4-B 104; A4-B 105;
A4-B 106; A4-B 107; A4-B 108; A4-B 109; A4-B 110; A4-B 111;
A4-B 112; A4-B 113; A4-B 114; A4-B 115; A4-B 116; A4-B 117;
A4-B 118; A4-B 119; A4-B 120; A4-B 121; A4-B 122; A4-B 123;
A4-B 124; A4-B 125; A4-B 126; A4-B 127; A4-B 128; A4-B129;
A4-B 130; A4-B 131; A4-B 132; A4-B 133; A4-B 134; A4-B 13 5;
A4-B136; A4-B137; A4-B138; A4-B139; A4-B140; A4-B141;
A4-B 142; A4-B 143; A4-B 144; A4-B 145; A4-B 146; A4-B 147;
A4-B 148; A4-B 149; A4-B 150; A4-B 151; A4-B 152; A4-B 153;
A4-B 154; A4-B155; A4-B156; A4-B 157; A4-B158; A4-B 159;
A4-B 160; A4-B 161; A4-B 162; A4-B 163; A4-B 164; A4-B 165;
A4-B 166; A4-B 167; A4-B 168; A4-B 169; A5-B 1; A5-B2;
A5-B3; A5-B4; A5-B5; A5-B6; A5-B7; A5-B8;
A5-B9; A5-B10; A5-B11; A5-B12; A5-B13; A5-B14;
A5-B 15; A5-B 16; A5-B 17; A5-B 18; A5-B 19; A5-B20;
A5-B21; A5-B22; A5-B23; A5-B24; A5-B25; A5-B26;
A5-B27; A5-B28; A5-B29; A5-B30; A5-B31; A5-B32;
A5-B33; A5-B34; A5-B35; A5-B36; A5-B37; A5-B38;
A5-B39; A5-B40; A5-B41; A5-B42; A5-B43; A5-B44;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-114-
A5-B45; A5-B46; A5-B47; A5-B48; A5-B49; A5-B50;
A5-B51; A5-B52; A5-B53; A5-B54; A5-B55; A5-B56;
A5-B57; A5-B58; A5-B59; A5-B60; A5-B61; A5-B62;
A5-B63; A5-B64; A5-B65; A5-B66; A5-B67; A5-B68;
A5-B69; A5-B70; A5-B71; A5-B72; A5-B73; A5-B74;
A5-B75; A5-B76; A5-B77; A5-B78; A5-B79; A5-B80;
A5-B81; A5-B82; A5-B83; A5-B84; A5-B85; A5-B86;
A5-B87; A5-B88; A5-B89; A5-B90; A5-B91; A5-B92;
A5-B93; A5-B94; A5-B95; A5-B96; A5-B97; A5-B98;
A5-B99; A5-B 100; A5-B 101; A5-B 102; A5-B103; A5-B 104;
A5-B 105; A5-B 106; A5-B 107; A5-B 108; A5-B 109; A5-B 110;
A5-B111; A5-B112; A5-B113; A5-B114; A5-B115; A5-B116;
A5-B 117; A5-B 118; A5-B 119; A5-B 120; A5-B 121; A5-B 122;
A5-B123; A5-B124; A5-B125; A5-B126; A5-B127; A5-B128;
A5-B129; A5-B130; A5-B131; A5-B132; A5-B133; A5-B134;
A5-B135; A5-B136; A5-B137; A5-B138; A5-B139; A5-B140;
A5-B 141; A5-B 142; A5-B 143; A5-B 144; A5-B 145; A5-B 146;
A5-B 147; A5-B148; A5-B 149; A5-B 150; A5-B 151; A5-B 152;
A5-B 153; A5-B 154; A5-B155; A5-B 156; A5-B 157; A5-B 15 8;
A5-B 159; A5-B 160; A5-B 161; A5-B 162; A5-B 163; A5-B 164;
A5-B 165; A5-B 166; A5-B 167; A5-B 168; A5-B169; A6-B 1;
A6-B2; A6-B3; A6-B4; A6-B5; A6-B6; A6-B7;
A6-B8; A6-B9; A6-B 10; A6-B 11; A6-B 12; A6-B 13;
A6-B 14; A6-B 15; A6-B 16; A6-B 17; A6-B 18; A6-B 19;
A6-B20; A6-B21; A6-B22; A6-B23; A6-B24; A6-B25;
A6-B26; A6-B27; A6-B28; A6-B29; A6-B30; A6-B31;
A6-B32; A6-B33; A6-B34; A6-B35; A6-B36; A6-B37;
A6-B38; A6-B39; A6-B40; A6-B41; A6-B42; A6-B43;
A6-B44; A6-B45; A6-B46; A6-B47; A6-B48; A6-B49;
A6-B50; A6-B51; A6-B52; A6-B53; A6-B54; A6-B55;
A6-B56; A6-B57; A6-B58; A6-B59; A6-B60; A6-B61;
A6-B62; A6-B63; A6-B64; A6-B65; A6-B66; A6-B67;
A6-B68; A6-B69; A6-B70; A6-B71; A6-B72; A6-B73;
A6-B74; A6-B75; A6-B76; A6-B77; A6-B78; A6-B79;
A6-B80; A6-B81; A6-B82; A6-B83; A6-B84; A6-B85;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-115-
A6-B86; A6-B87; A6-B88; A6-B89; A6-B90; A6-B91;
A6-B92; A6-B93; A6-B94; A6-B95; A6-B96; A6-B97;
A6-B98; A6-B99; A6-B 100; A6-B 101; A6-B 102; A6-B 103;
A6-B 104; A6-B 105; A6-B 106; A6-B 107; A6-B 108; A6-B 109;
A6-B 110; A6-B 111; A6-B 112; A6-B 113; A6-B 114; A6-B 115;
A6-B 116; A6-B 117; A6-B 118; A6-B 119; A6-B 120; A6-B 121;
A6-B 122; A6-B 123; A6-B 124; A6-B 125; A6-B 126; A6-B 127;
A6-B 128; A6-B 129; A6-B 130; A6-B 131; A6-B 132; A6-B 133;
A6-B 134; A6-B 135; A6-B 136; A6-B 137; A6-B 138; A6-B 139;
A6-B 140; A6-B 141; A6-B 142; A6-B 143; A6-B 144; A6-B 145;
A6-B 146; A6-B 147; A6-B 148; A6-B 149; A6-B 150; A6-B 151;
A6-B 152; A6-B 153; A6-B 154; A6-B 155; A6-B 156; A6-B 157;
A6-B 158; A6-B 159; A6-B 160; A6-B 161; A6-B 162; A6-B 163;
A6-B 164; A6-B 165; A6-B 166; A6-B 167; A6-B 168; A6-B 169;
A7-B1; A7-B2; A7-B3; A7-B4; A7-B5; A7-B6;
A7-B7; A7-B8; A7-B9; A7-B10; A7-B11; A7-B12;
A7-B 13; A7-B 14; A7-B 15; A7-B 16; A7-B17; A7-B 18;
A7-B19; A7-B20; A7-B21; A7-B22; A7-B23; A7-B24;
A7-B25; A7-B26; A7-B27; A7-B28; A7-B29; A7-B30;
A7-B31; A7-B32; A7-B33; A7-B34; A7-B35; A7-B36;
A7-B37; A7-B38; A7-B39; A7-B40; A7-B41; A7-B42;
A7-B43; A7-B44; A7-B45; A7-B46; A7-B47; A7-B48;
A7-B49; A7-B50; A7-B51; A7-B52; A7-B53; A7-B54;
A7-B55; A7-B56; A7-B57; A7-B58; A7-B59; A7-B60;
A7-B61; A7-B62; A7-B63; A7-B64; A7-B65; A7-B66;
A7-B67; A7-B68; A7-B69; A7-B70; A7-B71; A7-B72;
A7-B73; A7-B74; A7-B75; A7-B76; A7-B77; A7-B78;
A7-B79; A7-B80; A7-B81; A7-B82; A7-B83; A7-B84;
A7-B85; A7-B86; A7-B87; A7-B88; A7-B89; A7-B90;
A7-B91; A7-B92; A7-B93; A7-B94; A7-B95; A7-B96;
A7-B97; A7-B98; A7-B99; A7-B100; A7-B101; A7-B102;
A7-B 103; A7-B 104; A7-B 105; A7-B 106; A7-B 107; A7-B 108;
A7-B 109; A7-B 110; A7-B 111; A7-B 112; A7-B 113; A7-B 114;
A7-B 115; A7-B 116; A7-B 117; A7-B 118; A7-B 119; A7-B 120;
A7-B 121; A7-B122; A7-B 123; A7-B 124; A7-B 125; A7-B126;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-116-
A7-B 127; A7-B 128; A7-B 129; A7-B130; A7-B 131; A7-B132;
A7-B 133; A7-B 134; A7-B 135; A7-B 136; A7-B 137; A7-B 138;
A7-B139; A7-B 140; A7-B 141; A7-B 142; A7-B 143; A7-B 144;
A7-B 145; A7-B 146; A7-B 147; A7-B 148; A7-B 149; A7-B 150;
A7-B 151; A7-B 152; A7-B 153; A7-B 154; A7-B 155; A7-B156;
A7-B157; A7-B158; A7-B159; A7-B160; A7-B161; A7-B162;
A7-B 163; A7-B 164; A7-B 165; A7-B 166; A7-B167; A7-B 168;
A7-B169; A8-B1; A8-B2; A8-B3; A8-B4; A8-B5;
A8-B6; A8-B7; A8-B8; A8-B9; A8-B10; A8-B11;
A8-B 12; A8-B 13; A8-B 14; A8-B 15; A8-B 16; A8-B 17;
A8-B 18; A8-B 19; A8-B20; A8-B21; A8-B22; A8-B23;
A8-B24; A8-B25; A8-B26; A8-B27; A8-B28; A8-B29;
A8-B30; A8-B31; A8-B32; A8-B33; A8-B34; A8-B35;
A8-B36; A8-B37; A8-B38; A8-B39; A8-B40; A8-B41;
A8-B42; A8-B43; A8-B44; A8-B45; A8-B46; A8-B47;
A8-B48; A8-B49; A8-B50; A8-B51; A8-B52; A8-B53;
A8-B54; A8-B55; A8-B56; A8-B57; A8-B58; A8-B59;
A8-B60; A8-B61; A8-B62; A8-B63; A8-B64; A8-B65;
A8-B66; A8-B67; A8-B68; A8-B69; A8-B70; A8-B71;
A8-B72; A8-B73; A8-B74; A8-B75; A8-B76; A8-B77;
A8-B78; A8-B79; A8-B80; A8-B81; A8-B82; A8-B83;
A8-B84; A8-B85; A8-B86; A8-B87; A8-B88; A8-B89;
A8-B90; A8-B91; A8-B92; A8-B93; A8-B94; A8-B95;
A8-B96; A8-B97; A8-B98; A8-B99; A8-B100; A8-B101;
A8-B 102; A8-B 103; A8-B 104; A8-B 105; A8-B 106; A8-B 107;
A8-B 108; A8-B 109; A8-B 110; A8-B 111; A8-B 112; A8-B 113;
A8-B 114; A8-B 115; A8-B 116; A8-B 117; A8-B 118; A8-B 119;
A8-B 120; A8-B 121; A8-B 122; A8-B 123; A8-B 124; A8-B 125;
A8-B 126; A8-B 127; A8-B 128; A8-B 129; A8-B 130; A8-B 131;
A8-B 132; A8-B133; A8-B 134; A8-B 135; A8-B 136; A8-B 137;
A8-B138; A8-B139; A8-B 140; A8-B 141; A8-B 142; A8-B 143;
A8-B 144; A8-B 145; A8-B 146; A8-B147; A8-B 148; A8-B 149;
A8-B 150; A8-B 151; A8-B 152; A8-B 153; A8-B 154; A8-B 155;
A8-B 156; A8-B 157; A8-B 158; A8-B 159; A8-B 160; A8-B161;
A8-B 162; A8-B 163; A8-B 164; A8-B 165; A8-B 166; A8-B 167;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-117-
A8-B168; A8-B 169; A9-B 1; A9-B2; A9-B3; A9-B4;
A9-B5; A9-B6; A9-B7; A9-B8; A9-B9; A9-B10;
A9-B 11; A9-B 12; A9-B 13; A9-B 14; A9-B 15; A9-B 16;
A9-B 17; A9-B 18; A9-B 19; A9-B20; A9-B21; A9-B22;
A9-B23; A9-B24; A9-B25; A9-B26; A9-B27; A9-B28;
A9-B29; A9-B30; A9-B31; A9-B32; A9-B33; A9-B34;
A9-B35; A9-B36; A9-B37; A9-B38; A9-B39; A9-B40;
A9-B41; A9-B42; A9-B43; A9-B44; A9-B45; A9-B46;
A9-B47; A9-B48; A9-B49; A9-B50; A9-B51; A9-B52;
A9-B53; A9-B54; A9-B55; A9-B56; A9-B57; A9-B58;
A9-B59; A9-B60; A9-B61; A9-B62; A9-B63; A9-B64;
A9-B65; A9-B66; A9-B67; A9-B68; A9-B69; A9-B70;
A9-B71; A9-B72; A9-B73; A9-B74; A9-B75; A9-B76;
A9-B77; A9-B78; A9-B79; A9-B80; A9-B81; A9-B82;
A9-B83; A9-B84; A9-B85; A9-B86; A9-B87; A9-B88;
A9-B89; A9-B90; A9-B91; A9-B92; A9-B93; A9-B94;
A9-B95; A9-B96; A9-B97; A9-B98; A9-B99; A9-B 100;
A9-B 101; A9-B 102; A9-B 103; A9-B 104; A9-B 105; A9-B 106;
A9-B 107; A9-B 108; A9-B 109; A9-B 110; A9-B 111; A9-B 112;
A9-B 113; A9-B 114; A9-B 115; A9-B 116; A9-B 117; A9-B 118;
A9-B 119; A9-B 120; A9-B 121; A9-B 122; A9-B 123; A9-B 124;
A9-B 125; A9-B 126; A9-B 127; A9-B 128; A9-B 129; A9-B130;
A9-B131; A9-B132; A9-B133; A9-B134; A9-B135; A9-B136;
A9-B 137; A9-B 138; A9-B 139; A9-B 140; A9-B 141; A9-B 142;
A9-B 143; A9-B 144; A9-B 145; A9-B 146; A9-B147; A9-B 148;
A9-B 149; A9-B 150; A9-B 151; A9-B152; A9-B153; A9-B 154;
A9-B 155; A9-B156; A9-B 157; A9-B 158; A9-B159; A9-B 160;
A9-B161; A9-B 162; A9-B 163; A9-B 164; A9-B 165; A9-B 166;
A9-B167; A9-B168; A9-B169; A10-Bl; A10-B2; A10-B3;
A10-B4; A10-B5; A10-B6; A10-B7; Al0-B8; A10-B9;
A10-B10; A10-B11; A10-B12; A10-B13; A10-B14; Al -B15;
A10-B16; A10-B17; A10-B18; A10-B19; A10-B20; A10-B21;
A10-B22; A10-B23; A10-B24; A10-B25; A10-B26; A10-B27;
A10-B28; A10-B29; A10-B30; A10-B31; A10-B32; A10-B33;
A10-B34; A10-B35; Al0-B36; A10-B37; A10-B38; A10-B39;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-118-
A10-B40; A10-B41; A10-B42; A10-B43; A10-B44; A10-B45;
A10-B46; A10-B47; A10-B48; A10-B49; A10-B50; A10-B51;
A10-B52; A10-B53; A10-B54; A10-B55; A10-B56; A10-B57;
A10-B58; A10-B59; A10-B60; A10-B61; A10-B62; A10-B63;
A10-B64; A10-B65; A10-B66; A10-B67; A10-B68; A10-B69;
A10-B70; A10-B71; A10-B72; A10-B73; A10-B74; A10-B75;
A10-B76; A10-B77; A10-B78; A10-B79; A10-B80; A10-B81;
A10-B82; A10-B83; A10-B84; A10-B85; A10-B86; A10-B87;
A10-B88; A10-B89; A10-B90; A10-B91; A10-B92; A10-B93;
A10-B94; A10-B95; A10-B96; A10-B97; A10-B98; A10-B99;
A10-B100; A10-B101; A10-B102; A10-B103; A10-B104; A10-B105;
A10-B106; A10-B107; A10-B108; A10-B109; A10-B110; A10-B111;
A10-B112; A10-B113; A10-B114; A10-B115; A10-B116; A10-B117;
A10-B118; A10-B119; A10-B120; A10-B121; A10-B122; A10-B123;
A10-B124; A10-B125; A10-B126; A10-B127; A10-B128; A10-B129;
A10-B130; A10-B131; A10-B132; A10-B133; A10-B134; A10-B135;
A10-B136; A10-B137; A10-B138; A10-B139; A10-B140; A10-B141;
A10-B142; A10-B143; A10-B144; A10-B145; A10-B146; A10-B147;
A10-B148; A10-B149; A10-B150; A10-B151; A10-B152; A10-B153;
A10-B154; A10-B155; A10-B156; A10-B157; A10-B158; A10-B159;
A10-B160; A10-B161; A10-B162; A10-B163; A10-B164; A10-B165;
A10-B166; A10-B167; A10-B168; A10-B169; A1l-Bl; A11-B2;
A11-B3; A11-B4; A11-B5; A11-B6; All-B7; All-B8;
A11-B9; All-B10; All-B11; A11-B12; A11-B13; A11-B14;
A11-B15; A11-B16; A11-B17; A11-B18; A11-B19; A11-B20;
A11-B21; A11-B22; A11-B23; A11-B24; A11-B25; A11-B26;
A11-B27; A11-B28; A11-B29; A11-B30; A11-B31; All-B32;
A11-B33; A11-B34; A11-B35; All-B36; All-B37; All-B38;
A11-B39; All-B40; A11-B41; All-B42; A11-B43; A11-B44;
A11-B45; A11-B46; Al1-B47; All-B48; A11-B49; All-B50;
All-B51; All-B52; A11-B53; A11-B54; A11-B55; All-B56;
A11-B57; A11-B58; A11-B59; A11-B60; All-B61; All-B62;
All-B63; A11-B64; A11-B65; A11-B66; All-B67; All-B68;
A11-B69; A11-B70; All-B71; A11-B72; A11-B73; A11-B74;
A11-B75; A11-B76; A11-B77; A11-B78; All-B79; A11-B80;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-119-
A11-B81; All-B82; All-B83; All-B84; A1l-B85; A11-B86;
All-B87; All-B88; A11-B89; A11-B90; All-B91; A11-B92;
A11-B93; All-B94; All-B95; All-B96; All-B97; All-B98;
A11-B99; All-B100; All-Bl01; A11-B102; A11-B103; All-B104;
A11-B105; All-B106; All-B107; A11-B108; A11-B109; A1l-B110;
A1l-B111; A11-B112; A11-B113; A11-B114; A11-B115; A11-B116;
A11-B117; A11-B118; A11-B119; All-B120; A11-B121; A11-B122;
A1l-B123; A11-B124; A11-B125; All-B126; A11-B127; A11-B128;
All-B129; A11-B130; A11-B131; A11-B132; A11-B133; All-B134;
A11-B135; All-B136; All-B137; All-B138; A11-B139; A11-B140;
A11-B141; All-B142; All-B143; All-B144; All-B145; All-B146;
A11-B147; A11-B148; All-B149; All-B150; A11-B151; All-B152;
A11-B153; A11-B154; A11-B155; A1l-B156; All-B157; A11-B158;
A11-B159; A11-B160; A1l-B161; All-B162; All-B163; All-B164;
A11-B165; All-B166; A11-B167; A11-B168; A11-B169; A12-Bl;
A12-B2; A12-B3; A12-B4; A12-B5; A12-B6; A12-B7;
A12-B8; A12-B9; A12-B10; A12-B11; A12-B12; A12-B13;
A12-B14; A12-B15; A12-B16; A12-B17; A12-B18; A12-B19;
A12-B20; A12-B21; A12-B22; A12-B23; A12-B24; A12-B25;
A12-B26; A12-B27; A12-B28; A12-B29; A12-B30; A12-B31;
A12-B32; A12-B33; A12-B34; A12-B35; A12-B36; A12-B37;
A12-B38; A12-B39; A12-B40; A12-B41; A12-B42; A12-B43;
A12-B44; A12-B45; A12-B46; A12-B47; A12-B48; A12-B49;
A12-B50; A12-B51; A12-B52; A12-B53; A12-B54; A12-B55;
A12-B56; A12-B57; A12-B58; A12-B59; A12-B60; A12-B61;
A12-B62; A12-B63; A12-B64; A12-B65; A12-B66; A12-B67;
A12-B68; A12-B69; A12-B70; A12-B71; A12-B72; A12-B73;
A12-B74; A12-B75; A12-B76; A12-B77; A12-B78; A12-B79;
A12-B80; A12-B81; A12-B82; A12-B83; A12-B84; A12-B85;
A12-B86; A12-B87; A12-B88; A12-B89; A12-B90; A12-B91;
A12-B92; A12-B93; A12-B94; A12-B95; A12-B96; A12-B97;
A12-B98; A12-B99; A12-B100; A12-B101; A12-B102; A12-B103;
A12-B104; A12-B105; A12-B106; A12-B107; A12-B108; A12-B109;
A12-B110; A12-B111; A12-B112; A12-B113; A12-B114; A12-B115;
A12-B116; A12-B117; A12-B118; A12-B119; A12-B120; A12-B121;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-120-
A12-B122; A12-B123; A12-B124; A12-B125; A12-B126; A12-B127;
A12-B128; A12-B129; A12-B130; A12-B131; A12-B132; A12-B133;
A12-B134; A12-B135; A12-B136; A12-B137; A12-B138; A12-B139;
A12-B140; A12-B141; A12-B142; A12-B143; A12-B144; A12-B145;
A12-B146; A12-B147; A12-B148; A12-B149; A12-B150; A12-B151;
A12-B152; A12-B153; A12-B154; A12-B155; A12-B156; A12-B157;
A12-B158; A12-B159; A12-B160; A12-B161; A12-B162; A12-B163;
A12-B164; A12-B165; A12-B166; A12-B167; A12-B168; A12-B169;
A13-B1; A13-B2; A13-B3; A13-B4; A13-B5; A13-B6;
A13-B7; A13-B8; A13-B9; A13-B10; A13-B11; A13-B12;
A13-B13; A13-B14; A13-B15; A13-B16; A13-B17; A13-B18;
A13-B19; A13-B20; A13-B21; A13-B22; A13-B23; A13-B24;
A13-B25; A13-B26; A13-B27; A13-B28; A13-B29; A13-B30;
A13-B31; A13-B32; A13-B33; A13-B34; A13-B35; A13-B36;
A13-B37; A13-B38; A13-B39; A13-B40; A13-B41; A13-B42;
A13-B43; A13-B44; A13-B45; A13-B46; A13-B47; A13-B48;
A13-B49; A13-B50; A13-B51; A13-B52; A13-B53; A13-B54;
A13-B55; A13-B56; A13-B57; A13-B58; A13-B59; A13-B60;
A13-B61; A13-B62; A13-B63; A13-B64; A13-B65; A13-B66;
A13-B67; A13-B68; A13-B69; A13-B70; A13-B71; A13-B72;
A13-B73; A13-B74; A13-B75; A13-B76; A13-B77; A13-B78;
A13-B79; A13-B80; A13-B81; A13-B82; A13-B83; A13-B84;
A13-B85; A13-B86; A13-B87; A13-B88; A13-B89; A'13-B90;
A13-B91; A13-B92; A13-B93; A13-B94; A13-B95; A13-B96;
A13-B97; A13-B98; A13-B99; A13-B100; A13-B101; A13-B102;
A13-B103; A13-B104; A13-B105; A13-B106; A13-B107; A13-B108;
A13-B109; A13-B110; A13-B111; A13-B112; A13-B113; A13-B114;
A13-B115; A13-B116; A13-B117; A13-B118; A13-B119; A13-B120;
A13-B121; A13-B122; A13-B123; A13-B124; A13-B125; A13-B126;
A13-B127; A13-B128; A13-B129; A13-B130; A13-B131; A13-B132;
A13-B133; A13-B134; A13-B135; A13-B136; A13-B137; A13-B138;
A13-B139; A13-B140; A13-B141; A13-B142; A13-B143; A13-B144;
A13-B145; A13-B146; A13-B147; A13-B148; A13-B149; A13-B150;
A13-B 151; A13-B 152; A13-B 153; A13-B 154; A13-B155; A13-B 156;
A13-B157; A13-B158; A13-B159; A13-B160; A13-B161; A13-B162;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-121-
A13-B 163; A13-B 164; A13-B 165; A13-B 166; A13-B 167; A13-B 168;
A13-B169; A14-B1; A14-B2; A14-B3; A14-B4; A14-B5;
A14-B6; A14-B7; A14-B8; A14-B9; A14-B10; A14-B11;
A14-B12; A14-B13; A14-B14; A14-B15; A14-B16; A14-B17;
A14-B18; A14-B19; A14-B20; A14-B21; A14-B22; A14-B23;
A14-B24; A14-B25; A14-B26; A14-B27; A14-B28; A14-B29;
A14-B30; A14-B31; A14-B32; A14-B33; A14-B34; A14-B35;
A14-B36; A14-B37; A14-B38; A14-B39; A14-B40; A14-B41;
A14-B42; A14-B43; A14-B44; A14-B45; A14-B46; A14-B47;
A14-B48; A14-B49; A14-B50; A14-B51; A14-B52; A14-B53;
A14-B54; A14-B55; A14-B56; A14-B57; A14-B58; A14-B59;
A14-B60; A14-B61; A14-B62; A14-B63; A14-B64; A14-B65;
A14-B66; A14-B67; A14-B68; A14-B69; A14-B70; A14-B71;
A14-B72; A14-B73; A14-B74; A14-B75; A14-B76; A14-B77;
A14-B78; A14-B79; A14-B80; A14-B81; A14-B82; A14-B83;
A14-B84; A14-B85; A14-B86; A14-B87; A14-B88; A14-B89;
A14-B90; A14-B91; A14-B92; A14-B93; A14-B94; A14-B95;
A14-B96; A14-B97; A14-B98; A14-B99; A14-B100; A14-B101;
A14-B102; A14-B103; A14-B104; A14-B105; A14-B106; A14-B107;
A14-B108; A14-B109; A14-B110; A14-B111; A14-B112; A14-B113;
A14-B114; A14-B115; A14-B116; A14-B117; A14-B118; A14-B119;
A14-B120; A14-B121; A14-B122; A14-B123; A14-B124; A14-B125;
A14-B126; A14-B127; A14-B128; A14-B129; A14-B130; A14-B131;
A14-B132; A14-B133; A14-B134; A14-B135; A14-B136; A14-B137;
A14-B138; A14-B139; A14-B140; A14-B141; A14-B142; A14-B143;
A14-B144; A14-B145; A14-B146; A14-B147; A14-B148; A14-B149;
A14-B150; A14-B151; A14-B152; A14-B153; A14-B154; A14-B155;
A14-B156; A14-B157; A14-B158; A14-B159; A14-B160; A14-B161;
A14-B162; A14-B163; A14-B164; A14-B165; A14-B166; A14-B167;
A14-B168; A14-B169; A15-B1; A15-B2; A15-B3; A15-B4;
A15-B5; A15-B6; A15-B7; A15-B8; A15-B9; A15-B10;
A15-B11; A15-B12; A15-B13; A15-B14; A15-B15; A15-B16;
A15-B17; A15-B18; A15-B19; A15-B20; A15-B21; A15-B22;
A15-B23; A15-B24; A15-B25; A15-B26; A15-B27; A15-B28;
A15-B29; A15-B30; A15-B31; A15-B32; A15-B33; A15-B34;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-122-
A15-B35; A15-B36; A15-B37; A15-B38; A15-B39; A15-B40;
A15-B41; A15-B42; A15-B43; A15-B44; A15-B45; A15-B46;
A15-B47; A15-B48; A15-B49; A15-B50; A15-B51; A15-B52;
A15-B53; A15-B54; A15-B55; A15-B56; A15-B57; A15-B58;
A15-B59; A15-B60; A15-B61; A15-B62; A15-B63; A15-B64;
A15-B65; A15-B66; A15-B67; A15-B68; A15-B69; A15-B70;
A15-B71; A15-B72; A15-B73; A15-B74; A15-B75; A15-B76;
A15-B77; A15-B78; A15-B79; A15-B80; A15-B81; A15-B82;
A15-B83; A15-B84; A15-B85; A15-B86; A15-B87; A15-B88;
A15-B89; A15-B90; A15-B91; A15-B92; A15-B93; A15-B94;
A15-B95; A15-B96; A15-B97; A15-B98; A15-B99; A15-B100;
A15-B101; A15-B102; A15-B103; A15-B104; A15-B105; A15-B106;
A15-B107; A15-B108; A15-B109; A15-B110; A15-B111; A15-B112;
A15-B113; A15-B114; A15-B115; A15-B116; A15-B117; A15-B118;
A15-B119; A15-B120; A15-B121; A15-B122; A15-B123; A15-B124;
A15-B125; A15-B126; A15-B127; A15-B128; A15-B129; A15-B130;
A15-B131; A15-B132; A15-B133; A15-B134; A15-B135; A15-B136;
A15-B137; A15-B138; A15-B139; A15-B140; A15-B141; A15-B142;
A15-B143; A15-B144; A15-B145; A15-B146; A15-B147; A15-B148;
A15-B149; A15-B150; A15-B151; A15-B152; A15-B153; A15-B154;
A15-B155; A15-B156; A15-B157; A15-B158; A15-B159; A15-B160;
A15-B161; A15-B162; A15-B163; A15-B164; A15-B165; A15-B166;
A15-B167; A15-B168; A15-B169; A16-Bl; A16-B2; A16-B3;
A16-B4; A16-B5; A16-B6; A16-B7; A16-B8; A16-B9;
A16-B10; A16-B11; A16-B12; A16-B13; A16-B14; A16-B15;
A16-B16; A16-B17; A16-B18; A16-B19; A16-B20; A16-B21;
A16-B22; A16-B23; A16-B24; A16-B25; A16-B26; A16-B27;
A16-B28; A16-B29; A16-B30; A16-B31; A16-B32; A16-B33;
A16-B34; A16-B35; A16-B36; A16-B37; A16-B38; A16-B39;
A16-B40; A16-B41; A16-B42; A16-B43; A16-B44; A16-B45;
A16-B46; A16-B47; A16-B48; A16-B49; A16-B50; A16-B51;
A16-B52; A16-B53; A16-B54; A16-B55; A16-B56; A16-B57;
A16-B58; A16-B59; A16-B60; A16-B61; A16-B62; A16-B63;
A16-B64; A16-B65; A16-B66; A16-B67; A16-B68; A16-B69;
A16-B70; A16-B71; A16-B72; A16-B73; A16-B74; A16-B75;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-123-
A16-B76; A16-B77; A16-B78; A16-B79; A16-B80; A16-B81;
A16-B82; A16-B83; A16-B84; A16-B85; A16-B86; A16-B87;
A16-B88; A16-B89; A16-B90; A16-B91; A16-B92; A16-B93;
A16-B94; A16-B95; A16-B96; A16-B97; A16-B98; A16-B99;
A16-B100; A16-B101; A16-B102; A16-B103; A16-B104; A16-B105;
A16-B106; A16-B107; A16-B108; A16-B109; A16-B110; A16-B111;
A16-B112; A16-B113; A16-B114; A16-B115; A16-B116; A16-B117;
A16-B118; A16-B119; A16-B120; A16-B121; A16-B122; A16-B123;
A16-B124; A16-B125; A16-B126; A16-B127; A16-B128; A16-B129;
A16-B130; A16-B131; A16-B132; A16-B133; A16-B134; A16-B135;
A16-B136; A16-B137; A16-B138; A16-B139; A16-B140; A16-B141;
A16-B142; A16-B143; A16-B144; A16-B145; A16-B146; A16-B147;
A16-B148; A16-B149; A16-B150; A16-B151; A16-B152; A16-B153;
A16-B154; A16-B155; A16-B156; A16-B157; A16-B158; A16-B159;
A16-B160; A16-B161; A16-B162; A16-B163; A16-B164; A16-B165;
A16-B166; A16-B167; A16-B168; A16-B169; A17-B1; A17-B2;
A17-B3; A17-B4; A17-B5; A17-B6; A17-B7; A17-B8;
A17-B9; A17-B10; A17-B11; A17-B12; A17-B13; A17-B14;
A17-B15; A17-B16; A17-B17; A17-B18; A17-B19; A17-B20;
A17-B21; A17-B22; A17-B23; A17-B24; A17-B25; A17-B26;
A17-B27; A17-B28; A17-B29; A17-B30; A17-B31; A17-B32;
A17-B33; A17-B34; A17-B35; A17-B36; A17-B37; A17-B38;
A17-B39; A17-B40; A17-B41; A17-B42; A17-B43; A17-B44;
A17-B45; A17-B46; A17-B47; A17-B48; A17-B49; A17-B50;
A17-B51; A17-B52; A17-B53; A17-B54; A17-B55; A17-B56;
A17-B57; A17-B58; A17-B59; A17-B60; A17-B61; A17-B62;
A17-B63; A17-B64; A17-B65; A17-B66; A17-B67; A17-B68;
A17-B69; A17-B70; A17-B71; A17-B72; A17-B73; A17-B74;
A17-B75; A17-B76; A17-B77; A17-B78; A17-B79; A17-B80;
A17-B81; A17-B82; A17-B83; A17-B84; A17-B85; A17-B86;
A17-B87; A17-B88; A17-B89; A17-B90; A17-B91; A17-B92;
A17-B93; A17-B94; A17-B95; A17-B96; A17-B97; A17-B98;
A17-B99; A17-B 100; A17-B 101; A17-B102; A17-B 103; A17-B104;
A17-B105; A17-B106; A17-B107; A17-B108; A17-B109; A17-B110;
A17-B111; A17-B112; A17-B113; A17-B114; A17-B115; A17-B116;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-124-
A17-B117; A17-B118; A17-B119; A17-B120; A17-B121; A17-B122;
A17-B123; A17-B124; A17-B125; A17-B126; A17-B127; A17-B128;
A17-B129; A17-B130; A17-B131; A17-B132; A17-B133; A17-B134;
A17-B135; A17-B136; A17-B137; A17-B138; A17-B139; A17-B140;
A17-B141; A17-B142; A17-B143; A17-B144; A17-B145; A17-B146;
A17-B147; A17-B148; A17-B149; A17-B150; A17-B151; A17-B152;
A17-B153; A17-B154; A17-B155; A17-B156; A17-B157; A17-B158;
A17-B159; A17-B160; A17-B161; A17-B162; A17-B163; A17-B164;
A17-B165; A17-B166; A17-B167; A17-B168; A17-B169; A18-B1;
A18-B2; A18-B3; A18-B4; A18-B5; A18-B6; A18-B7;
A18-B8; A18-B9; A18-B10; A18-B11; A18-B12; A18-B13;
A18-B14; A18-B15; A18-B16; A18-B17; A18-B18; A18-B19;
A18-B20; A18-B21; A18-B22; A18-B23; A18-B24; A18-B25;
A18-B26; A18-B27; A18-B28; A18-B29; A18-B30; A18-B31;
A18-B32; A18-B33; A18-B34; A18-B35; A18-B36; A18-B37;
A18-B38; A18-B39; A18-B40; A18-B41; A18-B42; A18-B43;
A18-B44; A18-B45; A18-B46; A18-B47; A18-B48; A18-B49;
A18-B50; A18-B51; A18-B52; A18-B53; A18-B54; A18-B55;
A18-B56; A18-B57; A18-B58; A18-B59; A18-B60; A18-B61;
A18-B62; A18-B63; A18-B64; A18-B65; A18-B66; A18-B67;
A18-B68; A18-B69; A18-B70; A18-B71; A18-B72; A18-B73;
A18-B74; A18-B75; A18-B76; A18-B77; A18-B78; A18-B79;
A18-B80; A18-B81; A18-B82; A18-B83; A18-B84; A18-B85;
A18-B86; A18-B87; A18-B88; A18-B89; A18-B90; A18-B91;
A18-B92; A18-B93; A18-B94; A18-B95; A18-B96; A18-B97;
A18-B98; A18-B99; A18-B100; A18-B101; A18-B102; A18-B103;
A 18-B 104; A18-B 105; A18-B106; A18-B107; A 18-B 108; A 18-B 109;
A18-B110; A18-B111; A18-B112; A18-B113; A18-B114; A18-B115;
A18-B116; A18-B117; A18-B118; A18-B119; A18-B120; A18-B121;
A18-B122; A18-B123; A18-B124; A18-B125; A18-B126; A18-B127;
A18-B 128; A18-B 129; A18-B130; A18-B 131; A18-B 132; A18-B 133;
A18-B134; A18-B135; A18-B136; A18-B137; A18-B138; A18-B139;
A18-B140; A18-B141; A18-B142; A18-B143; A18-B144; A18-B145;
A18-B146; A18-B147; A18-B148; A18-B149; A18-B150; A18-B151;
A18-B152; A18-B153; A18-B154; A18-B155; A18-B156; A18-B157;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-125-
A18-B158; A18-B159; A18-B160; A18-B161; A18-B162; A18-B163;
A18-B164; A18-B165; A18-B166; A18-B167; A18-B168; A18-B169;
A19-Bl; A19-B2; A19-B3; A19-B4; A19-B5; A19-B6;
A19-B7; A19-B8; A19-B9; A19-B10; A19-B11; A19-B12;
A19-B13; A19-B14; A19-B15; A19-B16; A19-B17; A19-B18;
A19-B19; A19-B20; A19-B21; A19-B22; A19-B23; A19-B24;
A19-B25; A19-B26; A19-B27; A19-B28; A19-B29; A19-B30;
A19-B31; A19-B32; A19-B33; A19-B34; A19-B35; A19-B36;
A19-B37; A19-B38; A19-B39; A19-B40; A19-B41; A19-B42;
A19-B43; A19-B44; A19-B45; A19-B46; A19-B47; A19-B48;
A19-B49; A19-B50; A19-B51; A19-B52; A19-B53; A19-B54;
A19-B55; A19-B56; A19-B57; A19-B58; A19-B59; A19-B60;
A19-B61; A19-B62; A19-B63; A19-B64; A19-B65; A19-B66;
A19-B67; A19-B68; A19-B69; A19-B70; A19-B71; A19-B72;
A19-B73; A19-B74; A19-B75; A19-B76; A19-B77; A19-B78;
A19-B79; A19-B80; A19-B81; A19-B82; A19-B83; A19-B84;
A19-B85; A19-B86; A19-B87; A19-B88; A19-B89; A19-B90;
A19-B91; A19-B92; A19-B93; A19-B94; A19-B95; A19-B96;
A19-B97; A19-B98; A19-B99; A19-B100; A19-B101; A19-B102;
A19-B103; A19-B104; A19-B105; A19-B106; A19-B107; A19-B108;
A19-B109; A19-B110; A19-B111; A19-B112; A19-B113; A19-B114;
A19-B115; A19-B116; A19-B117; A19-B118; A19-B119; A19-B120;
A19-B121; A19-B122; A19-B123; A19-B124; A19-B125; A19-B126;
A19-B127; A19-B128; A19-B129; A19-B130; A19-B131; A19-B132;
A19-B133; A19-B134; A19-B135; A19-B136; A19-B137; A19-B138;
A19-B139; A19-B140; A19-B141; A19-B142; A19-B143; A19-B144;
A19-B145; A19-B146; A19-B147; A19-B148; A19-B149; A19-B150;
A19-B151; A19-B152; A19-B153; A19-B154; A19-B155; A19-B156;
A19-B157; A19-B158; A19-B159; A19-B160; A19-B161; A19-B162;
A19-B163; A19-B164; A19-B165; A19-B166; A19-B167; A19-B168;
A19-B169; A20-B1; A20-B2; A20-B3; A20-B4; A20-B5;
A20-B6; A20-B7; A20-B8; A20-B9; A20-B10; A20-B11;
A20-B12; A20-B 13; A20-B 14; A20-B 15; A20-B 16; A20-B 17;
A20-B18; A20-B19; A20-B20; A20-B21; A20-B22; A20-B23;
A20-B24; A20-B25; A20-B26; A20-B27; A20-B28; A20-B29;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-126-
A20-B30; A20-B31; A20-B32; A20-B33; A20-B34; A20-B35;
A20-B36; A20-B37; A20-B38; A20-B39; A20-B40; A20-B41;
A20-B42; A20-B43; A20-B44; A20-B45; A20-B46; A20-B47;
A20-B48; A20-B49; A20-B50; A20-B51; A20-B52; A20-B53;
A20-B54; A20-B55; A20-B56; A20-B57; A20-B58; A20-B59;
A20-B60; A20-B61; A20-B62; A20-B63; A20-B64; A20-B65;
A20-B66; A20-B67; A20-B68; A20-B69; A20-B70; A20-B71;
A20-B72; A20-B73; A20-B74; A20-B75; A20-B76; A20-B77;
A20-B78; A20-B79; A20-B80; A20-B81; A20-B82; A20-B83;
A20-B84; A20-B85; A20-B86; A20-B87; A20-B88; A20-B89;
A20-B90; A20-B91; A20-B92; A20-B93; A20-B94; A20-B95;
A20-B96; A20-B97; A20-B98; A20-B99; A20-B100; A20-B101;
A20-B 102; A20-B 103; A20-B 104; A20-B 105; A20-B 106; A20-B 107;
A20-B 108; A20-B 109; A20-B 110; A20-B 11 1; A20-B 112; A20-B 113;
A20-B 114; A20-B 115; A20-B 116; A20-B 117; A20-B 118; A20-B 119;
A20-B 120; A20-B 121; A20-B 122; A20-B123; A20-B 124; A20-B125;
A20-B 126; A20-B 127; A20-B 128; A20-B 129; A20-B130; A20-B 13 1;
A20-B 132; A20-B133; A20-B 134; A20-B 135; A20-B 136; A20-B 137;
A20-B 138; A20-B 139; A20-B 140; A20-B 141; A20-B 142; A20-B 143;
A20-B 144; A20-B 145; A20-B 146; A20-B 147; A20-B 148; A20-B 149;
A20-B 150; A20-B 15 1; A20-B 152; A20-B153; A20-B 154; A20-B 155;
A20-B156; A20-B 157; A20-B 158; A20-B159; A20-B 160; A20-B 161;
A20-B 162; A20-B 163; A20-B 164; A20-B 165; A20-B 166; A20-B 167;
A20-B168; A20-B169; A21-B1; A21-B2; A21-B3; A21-B4;
A21-B5; A21-B6; A21-B7; A21-B8; A21-B9; A21-B10;
A21-B11; A21-B12; A21-B13; A21-B14; A21-B15; A21-B16;
A21-B17; A21-B18; A21-B19; A21-B20; A21-B21; A21-B22;
A21-B23; A21-B24; A21-B25; A21-B26; A21-B27; A21-B28;
A21-B29; A21-B30; A21-B31; A21-B32; A21-B33; A21-B34;
A21-B35; A21-B36; A21-B37; A21-B38; A21-B39; A21-B40;
A21-B41; A21-B42; A21-B43; A21-B44; A21-B45; A21-B46;
A21-B47; A21-B48; A21-B49; A21-B50; A21-B51; A21-B52;
A21-B53; A21-B54; A21-B55; A21-B56; A21-B57; A21-B58;
A21-B59; A21-B60; A21-B61; A21-B62; A21-B63; A21-B64;
A21-B65; A21-B66; A21-B67; A21-B68; A21-B69; A21-B70;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-127-
A21-B71; A21-B72; A21-B73; A21-B74; A21-B75; A21-B76;
A21-B77; A21-B78; A21-B79; A21-B80; A21-B81; A21-B82;
A21-B83; A21-B84; A21-B85; A21-B86; A21-B87; A21-B88;
A21-B89; A21-B90; A21-B91; A21-B92; A21-B93; A21-B94;
A21-B95; A21-B96; A21-B97; A21-B98; A21-B99; A21-B100;
A21-B101; A21-B102; A21-B103; A21-B104; A21-B105; A21-B106;
A21-B107; A21-B108; A21-B109; A21-B110; A21-B111; A21-B112;
A21-B113; A21-B114; A21-B115; A21-B116; A21-B117; A21-B118;
A21-B119; A21-B120; A21-B121; A21-B122; A21-B123; A21-B124;
A21-B125; A21-B126; A21-B127; A21-B128; A21-B129; A21-B130;
A21-B131; A21-B132; A21-B133; A21-B134; A21-B135; A21-B136;
A21-B137; A21-B138; A21-B139; A21-B140; A21-B141; A21-B142;
A21-B143; A21-B144; A21-B145; A21-B146; A21-B147; A21-B148;
A21-B149; A21-B150; A21-B151; A21-B152; A21-B153; A21-B154;
A21-B155; A21-B156; A21-B157; A21-B158; A21-B159; A21-B160;
A21-B161; A21-B162; A21-B163; A21-B164; A21-B165; A21-B166;
A21-B 167; A21-B 168; A21-B 169; A22-B 1; A22-B2; A22-B3;
A22-B4; A22-B5; A22-B6; A22-B7; A22-B8; A22-B9;
A22-B 10; A22-B 11; A22-B 12; A22-B 13; A22-B 14; A22-B 15;
A22-B 16; A22-B 17; A22-B 18; A22-B 19; A22-B20; A22-B2 1;
A22-B22; A22-B23; A22-B24; A22-B25; A22-B26; A22-B27;
A22-B28; A22-B29; A22-B30; A22-B31; A22-B32; A22-B33;
A22-B34; A22-B35; A22-B36; A22-B37; A22-B38; A22-B39;
A22-B40; A22-B41; A22-B42; A22-B43; A22-B44; A22-B45;
A22-B46; A22-B47; A22-B48; A22-B49; A22-B50; A22-B51;
A22-B52; A22-B53; A22-B54; A22-B55; A22-B56; A22-B57;
A22-B58; A22-B59; A22-B60; A22-B61; A22-B62; A22-B63;
A22-B64; A22-B65; A22-B66; A22-B67; A22-B68; A22-B69;
A22-B70; A22-B71; A22-B72; A22-B73; A22-B74; A22-B75;
A22-B76; A22-B77; A22-B78; A22-B79; A22-B80; A22-B81;
A22-B82; A22-B83; A22-B84; A22-B85; A22-B86; A22-B87;
A22-B88; A22-B89; A22-B90; A22-B91; A22-B92; A22-B93;
A22-B94; A22-B95; A22-B96; A22-B97; A22-B98; A22-B99;
A22-B 100; A22-B 101; A22-B 102; A22-B 103; A22-B 104; A22-B105;
A22-B 106; A22-B107; A22-B 108; A22-B 109; A22-B 110; A22-B 111;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-128-
A22-B 112; A22-B 113; A22-B 114; A22-B 115; A22-B 116; A22-B 117;
A22-B 118; A22-B 119; A22-B 120; A22-B 121; A22-B 122; A22-B123;
A22-B 124; A22-B 125; A22-B126; A22-B 127; A22-B 128; A22-B 129;
A22-B 130; A22-B 131; A22-B 132; A22-B 133; A22-B 134; A22-B 135;
A22-B136; A22-B 137; A22-B 138; A22-B139; A22-B 140; A22-B 141;
A22-B 142; A22-B 143; A22-B 144; A22-B 145; A22-B 146; A22-B 147;
A22-B148; A22-B 149; A22-B 150; A22-B 151; A22-B 152; A22-B 153;
A22-B 154; A22-B 155; A22-B 156; A22-B 157; A22-B158; A22-B 159;
A22-B 160; A22-B 161; A22-B 162; A22-B 163; A22-B 164; A22-B 165;
A22-B166; A22-B167; A22-B168; A22-B169; A23-B1; A23-B2;
A23-B3; A23-B4; A23-B5; A23-B6; A23-B7; A23-B8;
A23-B9; A23-B10; A23-B11; A23-B12; A23-B13; A23-B14;
A23-B 15; A23-B 16; A23-B 17; A23-B 18; A23-B 19; A23-B20;
A23-B21; A23-B22; A23-B23; A23-B24; A23-B25; A23-B26;
A23-B27; A23-B28; A23-B29; A23-B30; A23-B31; A23-B32;
A23-B33; A23-B34; A23-B35; A23-B36; A23-B37; A23-B38;
A23-B39; A23-B40; A23-B41; A23-B42; A23-B43; A23-B44;
A23-B45; A23-B46; A23-B47; A23-B48; A23-B49; A23-B50;
A23-B51; A23-B52; A23-B53; A23-B54; A23-B55; A23-B56;
A23-B57; A23-B58; A23-B59; A23-B60; A23-B61; A23-B62;
A23-B63; A23-B64; A23-B65; A23-B66; A23-B67; A23-B68;
A23-B69; A23-B70; A23-B71; A23-B72; A23-B73; A23-B74;
A23-B75; A23-B76; A23-B77; A23-B78; A23-B79; A23-B80;
A23-B81; A23-B82; A23-B83; A23-B84; A23-B85; A23-B86;
A23-B87; A23-B88; A23-B89; A23-B90; A23-B91; A23-B92;
A23-B93; A23-B94; A23-B95; A23-B96; A23-B97; A23-B98;
A23-B99; A23-B 100; A23-B 101; A23-B 102; A23-B103; A23-B104;
A23-B 105; A23-B106; A23-B107; A23-B 108; A23-B109; A23-B 110;
A23-B 111; A23-B 112; A23-B 113; A23-B114; A23-B 115; A23-B 116;
A23-B117; A23-B118; A23-B119; A23-B120; A23-B121; A23-B122;
A23-B 123; A23-B 124; A23-B 125; A23-B 126; A23-B 127; A23-B 128;
A23-B 129; A23-B 130; A23-B 131; A23-B 132; A23-B 133; A23-B134;
A23-B135; A23-B136; A23-B137; A23-B138; A23-B139; A23-B140;
A23-B141; A23-B142; A23-B143; A23-B144; A23-B145; A23-B146;
A23-B147; A23-B148; A23-B149; A23-B150; A23-B151; A23-B152;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-129-
A23-B 153; A23-B 154; A23-B155; A23-B 156; A23-B 157; A23-B 158;
A23-B159; A23-B 160; A23-B 161; A23-B 162; A23-B 163; A23-B 164;
A23-B 165; A23-B 166; A23-B 167; A23-B168; A23-B 169; A24-B 1;
A24-B2; A24-B3; A24-B4; A24-B5; A24-B6; A24-B7;
A24-B8; A24-B9; A24-B 10; A24-B 11; A24-B 12; A24-B 13;
A24-B 14; A24-B 15; A24-B 16; A24-B 17; A24-B 18; A24-B 19;
A24-B20; A24-B21; A24-B22; A24-B23; A24-B24; A24-B25;
A24-B26; A24-B27; A24-B28; A24-B29; A24-B30; A24-B31;
A24-B32; A24-B33; A24-B34; A24-B35; A24-B36; A24-B37;
A24-B38; A24-B39; A24-B40; A24-B41; A24-B42; A24-B43;
A24-B44; A24-B45; A24-B46; A24-B47; A24-B48; A24-B49;
A24-B50; A24-B51; A24-B52; A24-B53; A24-B54; A24-B55;
A24-B56; A24-B57; A24-B58; A24-B59; A24-B60; A24-B61;
A24-B62; A24-B63; A24-B64; A24-B65; A24-B66; A24-B67;
A24-B68; A24-B69; A24-B70; A24-B71; A24-B72; A24-B73;
A24-B74; A24-B75; A24-B76; A24-B77; A24-B78; A24-B79;
A24-B80; A24-B81; A24-B82; A24-B83; A24-B84; A24-B85;
A24-B86; A24-B87; A24-B88; A24-B89; A24-B90; A24-B91;
A24-B92; A24-B93; A24-B94; A24-B95; A24-B96; A24-B97;
A24-B98; A24-B99; A24-B 100; A24-B 101; A24-B 102; A24-B 103;
A24-B 104; A24-B 105; A24-B 106; A24-B 107; A24-B 108; A24-B 109;
A24-B 110; A24-B 111; A24-B 112; A24-B 113; A24-B 114; A24-B 115;
A24-B 116; A24-B 117; A24-B 118; A24-B 119; A24-B 120; A24-B 121;
A24-B 122; A24-B 123; A24-B 124; A24-B 125; A24-B 126; A24-B 127;
A24-B128; A24-B 129; A24-B 130; A24-B 13 1; A24-B 132; A24-B 133;
A24-B 134; A24-B 13 5; A24-B 136; A24-B 137; A24-B 13 8; A24-B139;
A24-B 140; A24-B 141; A24-B 142; A24-B 143; A24-B144; A24-B 145;
A24-B 146; A24-B 147; A24-B148; A24-B 149; A24-B 150; A24-B 151;
A24-B 152; A24-B 153; A24-B 154; A24-B 155; A24-B 156; A24-B 157;
A24-B 158; A24-B 159; A24-B 160; A24-B 161; A24-B 162; A24-B 163;
A24-B 164; A24-B 165; A24-B 166; A24-B 167; A24-B 168; A24-B 169;
A25-Bl; A25-B2; A25-B3; A25-B4; A25-B5; A25-B6;
A25-B7; A25-B8; A25-B9; A25-B 10; A25-B 11; A25-B 12;
A25-B13; A25-B14; A25-B15; A25-B16; A25-B17; A25-B18;
A25-B19; A25-B20; A25-B21; A25-B22; A25-B23; A25-B24;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-130-
A25-B25; A25-B26; A25-B27; A25-B28; A25-B29; A25-B30;
A25-B31; A25-B32; A25-B33; A25-B34; A25-B35; A25-B36;
A25-B37; A25-B38; A25-B39; A25-B40; A25-B41; A25-B42;
A25-B43; A25-B44; A25-B45; A25-B46; A25-B47; A25-B48;
A25-B49; A25-B50; A25-B51; A25-B52; A25-B53; A25-B54;
A25-B55; A25-B56; A25-B57; A25-B58; A25-B59; A25-B60;
A25-B61; A25-B62; A25-B63; A25-B64; A25-B65; A25-B66;
A25-B67; A25-B68; A25-B69; A25-B70; A25-B71; A25-B72;
A25-B73; A25-B74; A25-B75; A25-B76; A25-B77; A25-B78;
A25-B79; A25-B80; A25-B81; A25-B82; A25-B83; A25-B84;
A25-B85; A25-B86; A25-B87; A25-B88; A25-B89; A25-B90;
A25-B91; A25-B92; A25-B93; A25-B94; A25-B95; A25-B96;
A25-B97; A25-B98; A25-B99; A25-B 100; A25-B 101; A25-B102;
A25-B 103; A25-B 104; A25-B105; A25-B 106; A25-B 107; A25-B 108;
A25-B109; A25-B110; A25-B111; A25-B112; A25-B113; A25-B114;
A25-B 115; A25-B 116; A25-B 117; A25-B 118; A25-B 119; A25-B 120;
A25-B 121; A25-B 122; A25-B 123; A25-B 124; A25-B 125; A25-B126;
A25-B127; A25-B128; A25-B 129; A25-B 130; A25-B 131; A25-B 132;
A25-B133; A25-B134; A25-B135; A25-B136; A25-B137; A25-B138;
A25-B139; A25-B 140; A25-B 141; A25-B 142; A25-B143; A25-B144;
A25-B145; A25-B 146; A25-B 147; A25-B 148; A25-B 149; A25-B150;
A25-B151; A25-B152; A25-B153; A25-B154; A25-B155; A25-B156;
A25-B157; A25-B158; A25-B159; A25-B 160; A25-B161; A25-B162;
A25-B 163; A25-B 164; A25-B 165; A25-B 166; A25-B 167; A25-B168;
A25-B169; A26-B1; A26-B2; A26-B3; A26-B4; A26-B5;
A26-B6; A26-B7; A26-B8; A26-B9; A26-B10; A26-B11;
A26-B 12; A26-B 13; A26-B 14; A26-B 15; A26-B16; A26-B 17;
A26-B18; A26-B19; A26-B20; A26-B21; A26-B22; A26-B23;
A26-B24; A26-B25; A26-B26; A26-B27; A26-B28; A26-B29;
A26-B30; A26-B31; A26-B32; A26-B33; A26-B34; A26-B35;
A26-B36; A26-B37; A26-B38; A26-B39; A26-B40; A26-B41;
A26-B42; A26-B43; A26-B44; A26-B45; A26-B46; A26-B47;
A26-B48; A26-B49; A26-B50; A26-B51; A26-B52; A26-B53;
A26-B54; A26-B55; A26-B56; A26-B57; A26-B58; A26-B59;
A26-B60; A26-B61; A26-B62; A26-B63; A26-B64; A26-B65;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-131-
A26-B66; A26-B67; A26-B68; A26-B69; A26-B70; A26-B71;
A26-B72; A26-B73; A26-B74; A26-B75; A26-B76; A26-B77;
A26-B78; A26-B79; A26-B80; A26-B81; A26-B82; A26-B83;
A26-B84; A26-B85; A26-B86; A26-B87; A26-B88; A26-B89;
A26-B90; A26-B91; A26-B92; A26=B93; A26-B94; A26-B95;
A26-B96; A26-B97; A26-B98; A26-B99; A26-B100; A26-B101;
A26-B 102; A26-B103; A26-B104; A26-B 105; A26-B 106; A26-B 107;
A26-B 108; A26-B 109; A26-B 110; A26-B 111; A26-B 112; A26-B 113;
A26-B 114; A26-B 115; A26-B116; A26-B 117; A26-B 118; A26-B 119;
A26-B 120; A26-B 121; A26-B122; A26-B 123; A26-B124; A26-B 125;
A26-B 126; A26-B 127; A26-B 128; A26-B 129; A26-B 130; A26-B 13 1;
A26-B 132; A26-B 133; A26-B 134; A26-B135; A26-B 136; A26-B 137;
A26-B 138; A26-B 139; A26-B140; A26-B 141; A26-B 142; A26-B 143;
A26-B 144; A26-B 145; A26-B146; A26-B 147; A26-B 148; A26-B 149;
A26-B 150; A26-B 151; A26-B 152; A26-B 153; A26-B 154; A26-B 155;
A26-B156; A26-B 157; A26-B 158; A26-B 159; A26-B 160; A26-B 161;
A26-B 162; A26-B 163; A26-B 164; A26-B 165; A26-B 166; A26-B167;
A26-B 168; A26-B 169; A27-B 1; A27-B2; A27-B3; A27-B4;
A27-B5; A27-B6; A27-B7; A27-B8; A27-B9; A27-B10;
A27-B 11; A27-B 12; A27-B 13; A27-B 14; A27-B 15; A27-B 16;
A27-B 17; A27-B 18; A27-B 19; A27-B20; A27-B21; A27-B22;
A27-B23; A27-B24; A27-B25; A27-B26; A27-B27; A27-B28;
A27-B29; A27-B30; A27-B31; A27-B32; A27-B33; A27-B34;
A27-B35; A27-B36; A27-B37; A27-B38; A27-B39; A27-B40;
A27-B41; A27-B42; A27-B43; A27-B44; A27-B45; A27-B46;
A27-B47; A27-B48; A27-B49; A27-B50; A27-B51; A27-B52;
A27-B53; A27-B54; A27-B55; A27-B56; A27-B57; A27-B58;
A27-B59; A27-B60; A27-B61; A27-B62; A27-B63; A27-B64;
A27-B65; A27-B66; A27-B67; A27-B68; A27-B69; A27-B70;
A27-B71; A27-B72; A27-B73; A27-B74; A27-B75; A27-B76;
A27-B77; A27-B78; A27-B79; A27-B80; A27-B81; A27-B82;
A27-B83; A27-B84; A27-B85; A27-B86; A27-B87; A27-B88;
A27-B89; A27-B90; A27-B91; A27-B92; A27-B93; A27-B94;
A27-B95; A27-B96; A27-B97; A27-B98; A27-B99; A27-B100;
A27-B 101; A27-B 102; A27-B 103; A27-B 104; A27-B 105; A27-B 106;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-132-
A27-B 107; A27-B 108; A27-B 109; A27-B 110; A27-B 111; A27-B 112;
A27-B 113; A27-B 114; A27-B 115; A27-B 116; A27-B 117; A27-B 118;
A27-B 119; A27-B 120; A27-B 121; A27-B 122; A27-B123; A27-B 124;
A27-B 125; A27-B 126; A27-B 127; A27-B 128; A27-B 129; A27-B130;
A27-B 131; A27-B 132; A27-B133; A27-B 134; A27-B 135; A27-B136;
A27-B 137; A27-B 138; A27-B 139; A27-B 140; A27-B 141; A27-B 142;
A27-B 143; A27-B 144; A27-B 145; A27-B 146; A27-B 147; A27-B 148;
A27-B 149; A27-B 150; A27-B 151; A27-B 152; A27-B 153; A27-B 154;
A27-B155; A27-B156; A27-B157; A27-B158; A27-B159; A27-B160;
A27-B 161; A27-B 162; A27-B 163; A27-B 164; A27-B 165; A27-B 166;
A27-B167; A27-B168; A27-B169; A28-B1; A28-B2; A28-B3;
A28-B4; A28-B5; A28-B6; A28-B7; A28-B8; A28-B9;
A28-B10; A28-B11; A28-B12; A28-B13; A28-B14; A28-B15;
A28-B 16; A28-B 17; A28-B 18; A28-B19; A28-B20; A28-B21;
A28-B22; A28-B23; A28-B24; A28-B25; A28-B26; A28-B27;
A28-B28; A28-B29; A28-B30; A28-B31; A28-B32; A28-B33;
A28-B34; A28-B35; A28-B36; A28-B37; A28-B38; A28-B39;
A28-B40; A28-B41; A28-B42; A28-B43; A28-B44; A28-B45;
A28-B46; A28-B47; A28-B48; A28-B49; A28-B50; A28-B51;
A28-B52; A28-B53; A28-B54; A28-B55; A28-B56; A28-B57;
A28-B58; A28-B59; A28-B60; A28-B61; A28-B62; A28-B63;
A28-B64; A28-B65; A28-B66; A28-B67; A28-B68; A28-B69;
A28-B70; A28-B71; A28-B72; A28-B73; A28-B74; A28-B75;
A28-B76; A28-B77; A28-B78; A28-B79; A28-B80; A28-B81;
A28-B82; A28-B83; A28-B84; A28-B85; A28-B86; A28-B87;
A28-B88; A28-B89; A28-B90; A28-B91; A28-B92; A28-B93;
A28-B94; A28-B95; A28-B96; A28-B97; A28-B98; A28-B99;
A28-B 100; A28-B 101; A28-B 102; A28-B 103; A28-B 104; A28-B 105;
A28-B 106; A28-B 107; A28-B 108; A28-B109; A28-B 110; A28-B 111;
A28-B112; A28-B113; A28-B114; A28-B115; A28-B116; A28-B117;
A28-B 118; A28-B 119; A28-B120; A28-B121; A28-B 122; A28-B123;
A28-B124; A28-B125; A28-B126; A28-B127; A28-B128; A28-B129;
A28-B130; A28-B131; A28-B132; A28-B133; A28-B134; A28-B135;
A28-B136; A28-B137; A28-B138; A28-B139; A28-B140; A28-B141;
A28-B142; A28-B143; A28-B144; A28-B145; A28-B146; A28-B147;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-133-
A28-B148; A28-B149; A28-B150; A28-B151; A28-B152; A28-B153;
A28-B154; A28-B155; A28-B156; A28-B157; A28-B158; A28-B159;
A28-B160; A28-B161; A28-B162; A28-B163; A28-B164; A28-B165;
A28-B 166; A28-B 167; A28-B 168; A28-B 169; A29-B l; A29-B2;
A29-B3; A29-B4; A29-B5; A29-B6; A29-B7; A29-B8;
A29-B9; A29-B 10; A29-B 11; A29-B 12; A29-B 13; A29-B 14;
A29-B 15; A29-B 16; A29-B 17; A29-B 18; A29-B 19; A29-B20;
A29-B21; A29-B22; A29-B23; A29-B24; A29-B25; A29-B26;
A29-B27; A29-B28; A29-B29; A29-B30; A29-B31; A29-B32;
A29-B33; A29-B34; A29-B35; A29-B36; A29-B37; A29-B38;
A29-B39; A29-B40; A29-B41; A29-B42; A29-B43; A29-B44;
A29-B45; A29-B46; A29-B47; A29-B48; A29-B49; A29-B50;
A29-B51; A29-B52; A29-B53; A29-B54; A29-B55; A29-B56;
A29-B57; A29-B58; A29-B59; A29-B60; A29-B61; A29-B62;
A29-B63; A29-B64; A29-B65; A29-B66; A29-B67; A29-B68;
A29-B69; A29-B70; A29-B71; A29-B72; A29-B73; A29-B74;
A29-B75; A29-B76; A29-B77; A29-B78; A29-B79; A29-B80;
A29-B81; A29-B82; A29-B83; A29-B84; A29-B85; A29-B86;
A29-B87; A29-B88; A29-B89; A29-B90; A29-B91; A29-B92;
A29-B93; A29-B94; A29-B95; A29-B96; A29-B97; A29-B98;
A29-B99; A29-B 100; A29-B 101; A29-B 102; A29-B 103; A29-B104;
A29-B 105; A29-B 106; A29-B 107; A29-B 108; A29-B 109; A29-B 110;
A29-B 111; A29-B 112; A29-B 113; A29-B 114; A29-B 115; A29-B 116;
A29-B117; A29-B118; A29-B119; A29-B120; A29-B121; A29-B122;
A29-B 123; A29-B 124; A29-B125; A29-B 126; A29-B 127; A29-B 128;
A29-B129; A29-B130; A29-B 131; A29-B 132; A29-B 133; A29-B 134;
A29-B135; A29-B136; A29-B137; A29-B138; A29-B139; A29-B 140;
A29-B 141; A29-B 142; A29-B 143; A29-B 144; A29-B 145; A29-B 146;
A29-B 147; A29-B 148; A29-B 149; A29-B150; A29-B 151; A29-B 152;
A29-B153; A29-B 154; A29-B 15 5; A29-B 156; A29-B 157; A29-B 15 8;
A29-B159; A29-B 160; A29-B 161; A29-B 162; A29-B 163; A29-B 164;
A29-B165; A29-B 166; A29-B 167; A29-B168; A29-B 169; A30-B 1;
A30-B2; A30-B3; A30-B4; A30-B5; A30-B6; A30-B7;
A30-B8; A30-B9; A30-B10; A30-B11; A30-B12; A30-B13;
A30-B 14; A30-B15; A30-B 16; A30-B17; A30-B18; A30-B19;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-134-
A30-B20; A30-B21; A30-B22; A30-B23; A30-B24; A30-B25;
A30-B26; A30-B27; A30-B28; A30-B29; A30-B30; A30-B31;
A30-B32; A30-B33; A30-B34; A30-B35; A30-B36; A30-B37;
A30-B38; A30-B39; A30-B40; A30-B41; A30-B42; A30-B43;
A30-B44; A30-B45; A30-B46; A30-B47; A30-B48; A30-B49;
A30-B50; A30-B51; A30-B52; A30-B53; A30-B54; A30-B55;
A30-B56; A30-B57; A30-B58; A30-B59; A30-B60; A30-B61;
A30-B62; A30-B63; A30-B64; A30-B65; A30-B66; A30-B67;
A30-B68; A30-B69; A30-B70; A30-B71; A30-B72; A30-B73;
A30-B74; A30-B75; A30-B76; A30-B77; A30-B78; A30-B79;
A30-B80; A30-B81; A30-B82; A30-B83; A30-B84; A30-B85;
A30-B86; A30-B87; A30-B88; A30-B89; A30-B90; A30-B91;
A30-B92; A30-B93; A30-B94; A30-B95; A30-B96; A30-B97;
A30-B98; A30-B99; A30-B 100; A30-B 101; A30-B102; A30-B103;
A30-B 104; A30-B 105; A30-B 106; A30-B 107; A30-B 108; A30-B 109;
A30-B 110; A30-B 111; A30-B 112; A30-B 113; A30-B 114; A30-B 115;
A3 0-B 116; A30-B 117; A30-B 118; A30-B 119; A30-B 120; A30-B 121;
A30-B 122; A30-B 123; A30-B 124; A30-B 125; A30-B 126; A30-B 127;
A30-B 128; A30-B129; A30-B 130; A30-B 131; A30-B 132; A30-B133;
A30-B 134; A30-B 135; A30-B136; A30-B 137; A30-B 138; A30-B 139;
A30-B 140; A30-B 141; A30-B142; A30-B 143; A30-B144; A30-B 145;
A30-B 146; A30-B 147; A30-B 148; A30-B 149; A30-B 150; A30-B 151;
A30-B 152; A30-B 153; A30-B 154; A30-B155; A30-B156; A30-B 157;
A30-B 158; A30-B 159; A30-B 160; A30-B 161; A30-B162; A30-B 163;
A30-B 164; A30-B165; A30-B 166; A30-B 167; A30-B 168; A30-B 169;
A31-B1; A31-B2; A31-B3; A31-B4; A31-B5; A31-B6;
A31-B7; A31-B8; A31-B9; A31-B10; A31-B11; A31-B12;
A31-B13; A31-B14; A31-B15; A31-B16; A31-B17; A31-B18;
A31-B19; A31-B20; A31-B21; A31-B22; A31-B23; A31-B24;
A31-B25; A31-B26; A31-B27; A31-B28; A31-B29; A31-B30;
A31-B31; A31-B32; A31-B33; A31-B34; A31-B35; A31-B36;
A31-B37; A31-B38; A31-B39; A31-B40; A31-B41; A31-B42;
A31-B43; A31-B44; A31-B45; A31-B46; A31-B47; A31-B48;
A31-B49; A31-B50; A31-B51; A31-B52; A31-B53; A31-B54;
A31-B55; A31-B56; A31-B57; A31-B58; A31-B59; A31-B60;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-135-
A31-B61; A31-B62; A31-B63; A31-B64; A31-B65; A31-B66;
A31-B67; A31-B68; A31-B69; A31-B70; A31-B71; A31-B72;
A31-B73; A31-B74; A31-B75; A31-B76; A31-B77; A31-B78;
A31-B79; A31-B80; A31-B81; A31-B82; A31-B83; A31-B84;
A31-B85; A31-B86; A31-B87; A31-B88; A31-B89; A31-B90;
A31-B91; A31-B92; A31-B93; A31-B94; A31-B95; A31-B96;
A31-B97; A31-B98; A31-B99; A31-B100; A31-B101; A31-B102;
A31-B103; A31-B104; A31-B105; A31-B106; A31-B107; A31-B108;
A31-B109; A31-B110; A31-B111; A31-B112; A31-B113; A31-B114;
A31-B115; A31-B116; A31-B117; A31-B118; A31-B119; A31-B120;
A31-B121; A31-B122; A31-B123; A31-B124; A31-B125; A31-B126;
A31-B127; A31-B128; A31-B129; A31-B130; A31-B131; A31-B132;
A31-B133; A31-B134; A31-B135; A31-B136; A31-B137; A31-B138;
A31-B139; A31-B140; A31-B141; A31-B142; A31-B143; A31-B144;
A31-B145; A31-B146; A31-B147; A31-B148; A31-B149; A31-B150;
A31-B151; A31-B152; A31-B153; A31-B154; A31-B155; A31-B156;
A31-B157; A31-B158; A31-B159; A31-B160; A31-B161; A31-B162;
A31-B 163; A31-B164; A31-B 165; A31-B 166; A31-B 167; A31-B 168;
A31-B169; A32-B1; A32-B2; A32-B3; A32-B4; A32-B5;
A32-B6; A32-B7; A32-B8; A32-B9; A32-B10; A32-B11;
A32-B 12; A32-B 13; A32-B 14; A32-B 15; A32-B 16; A32-B 17;
A32-B18; A32-B 19; A32-B20; A32-B21; A32-B22; A32-B23;
A32-B24; A32-B25; A32-B26; A32-B27; A32-B28; A32-B29;
A32-B30; A32-B31; A32-B32; A32-B33; A32-B34; A32-B35;
A32-B36; A32-B37; A32-B38; A32-B39; A32-B40; A32-B41;
A32-B42; A32-B43; A32-B44; A32-B45; A32-B46; A32-B47;
A32-B48; A32-B49; A32-B50; A32-B51; A32-B52; A32-B53;
A32-B54; A32-B55; A32-B56; A32-B57; A32-B58; A32-B59;
A32-B60; A32-B61; A32-B62; A32-B63; A32-B64; A32-B65;
A32-B66; A32-B67; A32-B68; A32-B69; A32-B70; A32-B71;
A32-B72; A32-B73; A32-B74; A32-B75; A32-B76; A32-B77;
A32-B78; A32-B79; A32-B80; A32-B81; A32-B82; A32-B83;
A32-B84; A32-B85; A32-B86; A32-B87; A32-B88; A32-B89;
A32-B90; A32-B91; A32-B92; A32-B93; A32-B94; A32-B95;
A32-B96; A32-B97; A32-B98; A32-B99; A32-B100; A32-B101;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-136-
A32-B102; A32-B 103; A32-B 104; A32-B 105; A32-B106; A32-B 107;
A32-B 108; A32-B 109; A32-B 110; A32-B 111; A32-B 112; A32-B 113;
A32-B 114; A32-B 115; A32-B 116; A32-B117; A32-B 118; A32-B 119;
A32-B 120; A32-B 121; A32-B 122; A32-B123; A32-B 124; A32-B 125;
A32-B 126; A32-B 127; A32-B 128; A32-B 129; A32-B 130; A32-B 131;
A32-B 132; A32-B 133; A32-B 134; A32-B135; A32-B136; A32-B 137;
A32-B 13 8; A32-B 13 9; A32-B 140; A32-B 141; A32-B 142; A32-B 143;
A32-B144; A32-B 145; A32-B 146; A32-B 147; A32-B 148; A32-B 149;
A32-B 150; A32-B 151; A32-B 152; A32-B153; A32-B 154; A32-B 155;
A32-B 156; A32-B 157; A32-B 158; A32-B159; A32-B 160; A32-B 161;
A32-B162; A32-B 163; A32-B 164; A32-B 165; A32-B 166; A32-B 167;
A32-B168; A32-B169; A33-B1; A33-B2; A33-B3; A33-B4;
A33-B5; A33-B6; A33-B7; A33-B8; A33-B9; A33-B10;
A33-B11; A33-B12; A33-B13; A33-B14; A33-B15; A33-B16;
A33-B17; A33-B18; A33-B19; A33-B20; A33-B21; A33-B22;
A33-B23; A33-B24; A33-B25; A33-B26; A33-B27; A33-B28;
A33-B29; A33-B30; A33-B31; A33-B32; A33-B33; A33-B34;
A33-B35; A33-B36; A33-B37; A33-B38; A33-B39; A33-B40;
A33-B41; A33-B42; A33-B43; A33-B44; A33-B45; A33-B46;
A33-B47; A33-B48; A33-B49; A33-B50; A33-B51; A33-B52;
A33-B53; A33-B54; A33-B55; A33-B56; A33-B57; A33-B58;
A33-B59; A33-B60; A33-B61; A33-B62; A33-B63; A33-B64;
A33-B65; A33-B66; A33-B67; A33-B68; A33-B69; A33-B70;
A33-B71; A33-B72; A33-B73; A33-B74; A33-B75; A33-B76;
A33-B77; A33-B78; A33-B79; A33-B80; A33-B81; A33-B82;
A33-B83; A33-B84; A33-B85; A33-B86; A33-B87; A33-B88;
A33-B89; A33-B90; A33-B91; A33-B92; A33-B93; A33-B94;
A33-B95; A33-B96; A33-B97; A33-B98; A33-B99; A33-B100;
A33-B101; A33-B102; A33-B103; A33-B104; A33-B105; A33-B106;
A33-B107; A33-B108; A33-B109; A33-B110; A33-B111; A33-B112;
A33-B 113; A33-B 114; A33-B 115; A33-B 116; A33-B 117; A33-B 118;
A33-B119; A33-B120; A33-B121; A33-B122; A33-B123; A33-B124;
A33-B125; A33-B126; A33-B127; A33-B128; A33-B129; A33-B130;
A33-B131; A33-B 132; A33-B133; A33-B134; A33-B135; A33-B 136;
A33-B137; A33-B138; A33-B139; A33-B140; A33-B141; A33-B142;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-137-
A33-B 143; A33-B 144; A33-B 145; A33-B 146; A33-B 147; A33-B 148;
A33-B149; A33-B 150; A33-B 151; A33-B 152; A33-B 153; A33-B 154;
A33-B155; A33-B156; A33-B157; A33-B158; A33-B159; A33-B160;
A33-B 161; A33-B 162; A33-B 163; A33-B 164; A33-B 165; A33-B 166;
A33-B 167; A33-B 168; A33-B 169; A34-B 1; A34-B2; A34-B3;
A34-B4; A34-B5; A34-B6; A34-B7; A34-B8; A34-B9;
A34-B 10; A34-B 11; A34-B 12; A34-B 13; A34-B 14; A34-B 15;
A34-B 16; A34-B 17; A34-B 18; A34-B 19; A34-B20; A34-B21;
A34-B22; A34-B23; A34-B24; A34-B25; A34-B26; A34-B27;
A34-B28; A34-B29; A34-B30; A34-B31; A34-B32; A34-B33;
A34-B34; A34-B35; A34-B36; A34-B37; A34-B38; A34-B39;
A34-B40; A34-B41; A34-B42; A34-B43; A34-B44; A34-B45;
A34-B46; A34-B47; A34-B48; A34-B49; A34-B50; A34-B51;
A34-B52; A34-B53; A34-B54; A34-B55; A34-B56; A34-B57;
A34-B58; A34-B59; A34-B60; A34-B61; A34-B62; A34-B63;
A34-B64; A34-B65; A34-B66; A34-B67; A34-B68; A34-B69;
A34-B70; A34-B71; A34-B72; A34-B73; A34-B74; A34-B75;
A34-B76; A34-B77; A34-B78; A34-B79; A34-B80; A34-B81;
A34-B82; A34-B83; A34-B84; A34-B85; A34-B86; A34-B87;
A34-B88; A34-B89; A34-B90; A34-B91; A34-B92; A34-B93;
A34-B94; A34-B95; A34-B96; A34-B97; A34-B98; A34-B99;
A34-B 100; A34-B 101; A34-B 102; A34-B 103; A34-B 104; A34-B 105;
A34-B 106; A34-B 107; A34-B 108; A34-B 109; A34-B 110; A34-B 111;
A34-B 112; A34-B 113; A34-B 114; A34-B 115; A34-B 116; A34-B 117;
A34-B 118; A34-B 119; A34-B 120; A34-B 121; A34-B 122; A34-B 123;
A34-B 124; A34-B125; A34-B 126; A34-B 127; A34-B128; A34-B 129;
A34-B 130; A34-B 131; A34-B 132; A34-B 133; A34-B 134; A34-B135;
A34-B 136; A34-B 137; A34-B 13 8; A34-B 13 9; A34-B 140; A34-B 141;
A34-B 142; A34-B 143; A34-B 144; A34-B 145; A34-B 146; A34-B 147;
A34-B 148; A34-B149; A34-B 150; A34-B 151; A34-B 152; A34-B 153;
A34-B 154; A34-B155; A34-B 156; A34-B 157; A34-B 158; A34-B159;
A34-B 160; A34-B 161; A34-B 162; A34-B 163; A34-B 164; A34-B 165;
A34-B 166; A34-B 167; A34-B 168; A34-B 169; A35-B 1; A35-B2;
A35-B3; A35-B4; A35-B5; A35-B6; A35-B7; A35-B8;
A35-B9; A35-B10; A35-B11; A35-B12; A35-B13; A35-B14;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-138-
A35-B15; A35-B 16; A35-B 17; A35-B 18; A35-B 19; A35-B20;
A35-B21; A35-B22; A35-B23; A35-B24; A35-B25; A35-B26;
A35-B27; A35-B28; A35-B29; A35-B30; A35-B31; A35-B32;
A35-B33; A35-B34; A35-B35; A35-B36; A35-B37; A35-B38;
A35-B39; A35-B40; A35-B41; A35-B42; A35-B43; A35-B44;
A35-B45; A35-B46; A35-B47; A35-B48; A35-B49; A35-B50;
A35-B51; A35-B52; A35-B53; A35-B54; A35-B55; A35-B56;
A35-B57; A35-B58; A35-B59; A35-B60; A35-B61; A35-B62;
A35-B63; A35-B64; A35-B65; A35-B66; A35-B67; A35-B68;
A35-B69; A35-B70; A35-B71; A35-B72; A35-B73; A35-B74;
A35-B75; A35-B76; A35-B77; A35-B78; A35-B79; A35-B80;
A35-B81; A35-B82; A35-B83; A35-B84; A35-B85; A35-B86;
A35-B87; A35-B88; A35-B89; A35-B90; A35-B91; A35-B92;
A35-B93; A35-B94; A35-B95; A35-B96; A35-B97; A35-B98;
A35-B99; A35-B100; A35-B101; A35-B102; A35-B103; A35-B104;
A35-B 105; A35-B 106; A35-B 107; A35-B 108; A35-B 109; A35-B 110;
A35-B 111; A35-B 112; A35-B 113; A35-B 114; A35-B 115; A35-B116;
A35-B117; A35-B118; A35-B119; A35-B120; A35-B121; A35-B122;
A35-B123; A35-B124; A35-B125; A35-B126; A35-B127; A35-B128;
A35-B129; A35-B130; A35-B131; A35-B132; A35-B133; A35-B134;
A35-B135; A35-B136; A35-B137; A35-B138; A35-B139; A35-B140;
A35-B141; A35-B142; A35-B 143; A35-B 144; A35-B145; A35-B 146;
A35-B147; A35-B148; A35-B149; A35-B150; A35-B151; A35-B152;
A35-B153; A35-B154; A35-B155; A35-B156; A35-B157; A35-B158;
A35-B159; A35-B160; A35-B161; A35-B162; A35-B163; A35-B164;
A35-B165; A35-B166; A35-B167; A35-B168; A35-B169; A36-Bl;
A36-B2; A36-B3; A36-B4; A36-B5; A36-B6; A36-B7;
A36-B8; A36-B9; A36-B10; A36-B11; A36-B12; A36-B13;
A36-B 14; A36-B15; A36-B 16; A36-B 17; A36-B 18; A36-B 19;
A36-B20; A36-B21; A36-B22; A36-B23; A36-B24; A36-B25;
A36-B26; A36-B27; A36-B28; A36-B29; A36-B30; A36-B31;
A36-B32; A36-B33; A36-B34; A36-B35; A36-B36; A36-B37;
A36-B38; A36-B39; A36-B40; A36-B41; A36-B42; A36-B43;
A36-B44; A36-B45; A36-B46; A36-B47; A36-B48; A36-B49;
A36-B50; A36-B51; A36-B52; A36-B53; A36-B54; A36-B55;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-139-
A36-B56; A36-B57; A36-B58; A36-B59; A36-B60; A36-B61;
A36-B62; A36-B63; A36-B64; A36-B65; A36-B66; A36-B67;
A36-B68; A36-B69; A36-B70; A36-B71; A36-B72; A36-B73;
A36-B74; A36-B75; A36-B76; A36-B77; A36-B78; A36-B79;
A36-B80; A36-B81; A36-B82; A36-B83; A36-B84; A36-B85;
A36-B86; A36-B87; A36-B88; A36-B89; A36-B90; A36-B91;
A36-B92; A36-B93; A36-B94; A36-B95; A36-B96; A36-B97;
A36-B98; A36-B99; A36-B 100; A36-B 101; A36-B 102; A36-B 103;
A36-B104; A36-B105; A36-B106; A36-B107; A36-B108; A36-B109;
A36-B 110; A36-B 111; A36-B 112; A36-B 113; A36-B 114; A36-B 115;
A36-B 116; A36-B 117; A36-B 118; A36-B 119; A36-B120; A36-B 121;
A36-B 122; A36-B 123; A36-B 124; A36-B 125; A36-B126; A36-B 127;
A36-B128; A36-B129; A36-B130; A36-B131; A36-B132; A36-B133;
A36-B134; A36-B135; A36-B136; A36-B137; A36-B138; A36-B139;
A36-B 140; A36-B 141; A36-B 142; A36-B 143; A36-B 144; A36-B 145;
A36-B146; A36-B147; A36-B148; A36-B149; A36-B150; A36-B151;
A36-B152; A36-B153; A36-B154; A36-B155; A36-B156; A36-B157;
A36-B158; A36-B159; A36-B160; A36-B161; A36-B162; A36-B163;
A36-B 164; A36-B165; A36-B 166; A36-B 167; A36-B 168; A36-B 169;
A37-B 1; A37-B2; A37-B3; A37-B4; A37-B5; A37-B6;
A37-B7; A37-B8; A37-B9; A37-B 10; A37-B 11; A37-B 12;
A37-B 13; A37-B 14; A37-B 15; A37-B 16; A37-B 17; A37-B 18;
A37-B 19; A37-B20; A37-B21; A37-B22; A37-B23; A37-B24;
A37-B25; A37-B26; A37-B27; A37-B28; A37-B29; A37-B30;
A37-B31; A37-B32; A37-B33; A37-B34; A37-B35; A37-B36;
A37-B37; A37-B38; A37-B39; A37-B40; A37-B41; A37-B42;
A37-B43; A37-B44; A37-B45; A37-B46; A37-B47; A37-B48;
A37-B49; A37-B50; A37-B51; A37-B52; A37-B53; A37-B54;
A37-B55; A37-B56; A37-B57; A37-B58; A37-B59; A37-B60;
A37-B61; A37-B62; A37-B63; A37-B64; A37-B65; A37-B66;
A37-B67; A37-B68; A37-B69; A37-B70; A37-B71; A37-B72;
A37-B73; A37-B74; A37-B75; A37-B76; A37-B77; A37-B78;
A37-B79; A37-B80; A37-B81; A37-B82; A37-B83; A37-B84;
A37-B85; A37-B86; A37-B87; A37-B88; A37-B89; A37-B90;
A37-B91; A37-B92; A37-B93; A37-B94; A37-B95; A37-B96;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-140-
A37-B97; A37-B98; A37-B99; A37-B100; A37-B101; A37-B102;
A37-B103; A37-B 104; A37-B 105; A37-B 106; A37-B 107; A37-B 108;
A37-B 109; A37-B 110; A37-B 111; A37-B 112; A37-B 113; A37-B 114;
A37-B 115; A37-B 116; A37-B 117; A37-B 118; A37-B 119; A37-B 120;
A37-B 121; A37-B 122; A37-B 123; A37-B 124; A37-B 125; A37-B 126;
A37-B127; A37-B128; A37-B129; A37-B130; A37-B131; A37-B132;
A37-B133; A37-B134; A37-B135; A37-B136; A37-B137; A37-B138;
A37-B 139; A37-B 140; A37-B 141; A37-B 142; A37-B 143; A37-B144;
A37-B 145; A37-B 146; A37-B 147; A37-B 148; A37-B149; A37-B 150;
A37-B 15 1; A37-B 152; A37-B153; A37-B 154; A37-B 155; A37-B156;
A37-B157; A37-B158; A37-B159; A37-B160; A37-B161; A37-B162;
A37-B163; A37-B 164; A37-B 165; A37-B 166; A37-B 167; A37-B 168;
A37-B169; A38-B1; A38-B2; A38-B3; A38-B4; A38-B5;
A38-B6; A38-B7; A38-B8; A38-B9; A38-B10; A38-B11;
A38-B12; A38-B13; A38-B14; A38-B15; A38-B16; A38-B17;
A38-B18; A38-B19; A38-B20; A38-B21; A38-B22; A38-B23;
A38-B24; A38-B25; A38-B26; A38-B27; A38-B28; A38-B29;
A38-B30; A38-B31; A38-B32; A38-B33; A38-B34; A38-B35;
A38-B36; A38-B37; A38-B38; A38-B39; A38-B40; A38-B41;
A38-B42; A38-B43; A38-B44; A38-B45; A38-B46; A38-B47;
A38-B48; A38-B49; A38-B50; A38-B51; A38-B52; A38-B53;
A38-B54; A38-B55; A38-B56; A38-B57; A38-B58; A38-B59;
A38-B60; A38-B61; A38-B62; A38-B63; A38-B64; A38-B65;
A38-B66; A38-B67; A38-B68; A38-B69; A38-B70; A38-B71;
A38-B72; A38-B73; A38-B74; A38-B75; A38-B76; A38-B77;
A38-B78; A38-B79; A38-B80; A38-B81; A38-B82; A38-B83;
A38-B84; A38-B85; A38-B86; A38-B87; A38-B88; A38-B89;
A38-B90; A38-B91; A38-B92; A38-B93; A38-B94; A38-B95;
A38-B96; A38-B97; A38-B98; A38-B99; A38-B100; A38-B101;
A38-B102; A38-B103; A38-B104; A38-B105; A38-B106; A38-B107;
A38-B108; A38-B109; A38-B110; A38-B111; A38-B112; A38-B113;
A38-B114; A38-B115; A38-B116; A38-B117; A38-B118; A38-B119;
A38-B120; A38-B121; A38-B122; A38-B123; A38-B124; A38-B125;
A38-B126; A38-B127; A38-B128; A38-B129; A38-B130; A38-B131;
A38-B132; A38-B133; A38-B134; A38-B135; A38-B136; A38-B137;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-141-
A38-B138; A38-B139; A38-B140; A38-B141; A38-B142; A38-B143;
A38-B144; A38-B145; A38-B146; A38-B147; A38-B148; A38-B149;
A38-B150; A38-B151; A38-B152; A38-B153; A38-B154; A38-B155;
A38-B156; A38-B157; A38-B158; A38-B159; A38-B160; A38-B161;
A38-B162; A38-B163; A38-B164; A38-B165; A38-B166; A38-B167;
A38-B168; A38-B169; A39-Bl; A39-B2; A39-B3; A39-B4;
A39-B5; A39-B6; A39-B7; A39-B8; A39-B9; A39-B10;
A39-B11; A39-B12; A39-B13; A39-B14; A39-B15; A39-B16;
A39-B17; A39-B18; A39-B 19; A39-B20; A39-B21; A39-B22;
A39-B23; A39-B24; A39-B25; A39-B26; A39-B27; A39-B28;
A39-B29; A39-B30; A39-B31; A39-B32; A39-B33; A39-B34;
A39-B35; A39-B36; A39-B37; A39-B38; A39-B39; A39-B40;
A39-B41; A39-B42; A39-B43; A39-B44; A39-B45; A39-B46;
A39-B47; A39-B48; A39-B49; A39-B50; A39-B51; A39-B52;
A39-B53; A39-B54; A39-B55; A39-B56; A39-B57; A39-B58;
A39-B59; A39-B60; A39-B61; A39-B62; A39-B63; A39-B64;
A39-B65; A39-B66; A39-B67; A39-B68; A39-B69; A39-B70;
A39-B71; A39-B72; A39-B73; A39-B74; A39-B75; A39-B76;
A39-B77; A39-B78; A39-B79; A39-B80; A39-B81; A39-B82;
A39-B83; A39-B84; A39-B85; A39-B86; A39-B87; A39-B88;
A39-B89; A39-B90; A39-B91; A39-B92; A39-B93; A39-B94;
A39-B95; A39-B96; A39-B97; A39-B98; A39-B99; A39-B100;
A39-B101; A39-B102; A39-B103; A39-B104; A39-B105; A39-B106;
A39-B107; A39-B 108; A39-B 109; A39-B 110; A39-B 111; A39-B 112;
A39-B113; A39-B114; A39-B115; A39-B116; A39-B117; A39-B118;
A39-B119; A39-B120; A39-B121; A39-B122; A39-B123; A39-B124;
A39-B 125; A39-B 126; A39-B127; A39-B 128; A39-B 129; A39-B130;
A39-B131; A39-B132; A39-B133; A39-B134; A39-B135; A39-B136;
A39-B137; A39-B138; A39-B139; A39-B140; A39-B141; A39-B142;
A39-B143; A39-B144; A39-B145; A39-B146; A39-B147; A39-B148;
A39-B149; A39-B150; A39-B151; A39-B152; A39-B153; A39-B154;
A39-B155; A39-B156; A39-B157; A39-B158; A39-B159; A39-B160;
A39-B161; A39-B162; A39-B163; A39-B164; A39-B165; A39-B166;
A39-B167; A39-B168; A39-B169; A40-B1; A40-B2; A40-B3;
A40-B4; A40-B5; A40-B6; A40-B7; A40-B8; A40-B9;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-142-
A40-B 10; A40-B 11; A40-B 12; A40-B 13; A40-B 14; A40-B 15;
A40-B 16; A40-B 17; A40-B 18; A40-B 19; A40-B20; A40-B21;
A40-B22; A40-B23; A40-B24; A40-B25; A40-B26; A40-B27;
A40-B28; A40-B29; A40-B30; A40-B31; A40-B32; A40-B33;
A40-B34; A40-B35; A40-B36; A40-B37; A40-B38; A40-B39;
A40-B40; A40-B41; A40-B42; A40-B43; A40-B44; A40-B45;
A40-B46; A40-B47; A40-B48; A40-B49; A40-B50; A40-B51;
A40-B52; A40-B53; A40-B54; A40-B55; A40-B56; A40-B57;
A40-B58; A40-B59; A40-B60; A40-B61; A40-B62; A40-B63;
A40-B64; A40-B65; A40-B66; A40-B67; A40-B68; A40-B69;
A40-B70; A40-B71; A40-B72; A40-B73; A40-B74; A40-B75;
A40-B76; A40-B77; A40-B78; A40-B79; A40-B80; A40-B81;
A40-B82; A40-B83; A40-B84; A40-B85; A40-B86; A40-B87;
A40-B88; A40-B89; A40-B90; A40-B91; A40-B92; A40-B93;
A40-B94; A40-B95; A40-B96; A40-B97; A40-B98; A40-B99;
A40-B 100; A40-B 101; A40-B 102; A40-B 103; A40-B 104; A40-B 105;
A40-B 106; A40-B 107; A40-B 108; A40-B 109; A40-B 110; A40-B 111;
A40-B 112; A40-B 113; A40-B 114; A40-B 115; A40-B 116; A40-B 117;
A40-B 118; A40-B 119; A40-B 120; A40-B 121; A40-B 122; A40-B123;
A40-B 124; A40-B 125; A40-B 126; A40-B 127; A40-B 128; A40-B 129;
A40-B130; A40-B131; A40-B132; A40-B133; A40-B134; A40-B135;
A40-B136; A40-B137; A40-B138; A40-B139; A40-B 140; A40-B141;
A40-B 142; A40-B 143; A40-B 144; A40-B 145; A40-B 146; A40-B 147;
A40-B148; A40-B 149; A40-B 150; A40-B 151; A40-B152; A40-B153;
A40-B154; A40-B155; A40-B156; A40-B157; A40-B158; A40-B159;
A40-B 160; A40-B 16 1; A40-B 162; A40-B 163; A40-B 164; A40-B165;
A40-B166; A40-B167; A40-B168; A40-B169; A41-Bl; A41-B2;
A41-B3; A41-B4; A41-B5; A41-B6; A41-B7; A41-B8;
A41-B9; A41-B10; A41-B11; A41-B12; A41-B13; A41-B14;
A41-B15; A41-B16; A41-B17; A41-B18; A41-B19; A41-B20;
A41-B21; A41-B22; A41-B23; A41-B24; A41-B25; A41-B26;
A41-B27; A41-B28; A41-B29; A41-B30; A41-B31; A41-B32;
A41-B33; A41-B34; A41-B35; A41-B36; A41-B37; A41-B38;
A41-B39; A41-B40; A41-B41; A41-B42; A41-B43; A41-B44;
A41-B45; A41-B46; A41-B47; A41-B48; A41-B49; A41-B50;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-143-
A41-B51; A41-B52; A41-B53; A41-B54; A41-B55; A41-B56;
A41-B57; A41-B58; A41-B59; A41-B60; A41-B61; A41-B62;
A41-B63; A41-B64; A41-B65; A41-B66; A41-B67; A41-B68;
A41-B69; A41-B70; A41-B71; A41-B72; A41-B73; A41-B74;
A41-B75; A41-B76; A41-B77; A41-B78; A41-B79; A41-B80;
A41-B81; A41-B82; A41-B83; A41-B84; A41-B85; A41-B86;
A41-B87; A41-B88; A41-B89; A41-B90; A41-B91; A41-B92;
A41-B93; A41-B94; A41-B95; A41-B96; A41-B97; A41-B98;
A41-B99; A41-B100; A41-B101; A41-B102; A41-B103; A41-B104;
A41-B105; A41-B106; A41-B107; A41-B108; A41-B109; A41-B110;
A41-B111; A41-B112; A41-B113; A41-B114; A41-B115; A41-B116;
A41-B117; A41-B118; A41-B119; A41-B120; A41-B121; A41-B122;
A41-B123; A41-B124; A41-B125; A41-B126; A41-B127; A41-B128;
A41-B129; A41-B130; A41-B131; A41-B132; A41-B133; A41-B134;
A41-B135; A41-B136; A41-B137; A41-B138; A41-B139; A41-B140;
A41-B141; A41-B142; A41-B143; A41-B144; A41-B145; A41-B146;
A41-B147; A41-B148; A41-B149; A41-B150; A41-B151; A41-B152;
A41-B153; A41-B154; A41-B155; A41-B156; A41-B157; A41-B158;
A41-B159; A41-B160; A41-B161; A41-B162; A41-B163; A41-B164;
A41-B165; A41-B166; A41-B167; A41-B168; A41-B169; A42-B1;
A42-B2; A42-B3; A42-B4; A42-B5; A42-B6; A42-B7;
A42-B8; A42-B9; A42-B 10; A42-B 11; A42-B 12; A42-B13;
A42-B 14; A42-B 15; A42-B 16; A42-B 17; A42-B 18; A42-B19;
A42-B20; A42-B21; A42-B22; A42-B23; A42-B24; A42-B25;
A42-B26; A42-B27; A42-B28; A42-B29; A42-B30; A42-B31;
A42-B32; A42-B33; A42-B34; A42-B35; A42-B36; A42-B37;
A42-B38; A42-B39; A42-B40; A42-B41; A42-B42; A42-B43;
A42-B44; A42-B45; A42-B46; A42-B47; A42-B48; A42-B49;
A42-B50; A42-B51; A42-B52; A42-B53; A42-B54; A42-B55;
A42-B56; A42-B57; A42-B58; A42-B59; A42-B60; A42-B61;
A42-B62; A42-B63; A42-B64; A42-B65; A42-B66; A42-B67;
A42-B68; A42-B69; A42-B70; A42-B71; A42-B72; A42-B73;
A42-B74; A42-B75; A42-B76; A42-B77; A42-B78; A42-B79;
A42-B80; A42-B81; A42-B82; A42-B83; A42-B84; A42-B85;
A42-B86; A42-B87; A42-B88; A42-B89; A42-B90; A42-B91;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-144-
A42-B92; A42-B93; A42-B94; A42-B95; A42-B96; A42-B97;
A42-B98; A42-B99; A42-B 100; A42-B 101; A42-B 102; A42-B 103;
A42-B 104; A42-B 105; A42-B 106; A42-B 107; A42-B 108; A42-B 109;
A42-B 110; A42-B 111; A42-B 112; A42-B 113; A42-B 114; A42-B 115;
A42-B 116; A42-B 117; A42-B 118; A42-B 119; A42-B 120; A42-B 121;
A42-B 122; A42-B 123; A42-B 124; A42-B 125; A42-B126; A42-B 127;
A42-B128; A42-B 129; A42-B 130; A42-B 131; A42-B 132; A42-B 133;
A42-B 134; A42-B135; A42-B136; A42-B 137; A42-B 138; A42-B139;
A42-B 140; A42-B 141; A42-B 142; A42-B 143; A42-B 144; A42-B 145;
A42-B 146; A42-B 147; A42-B 148; A42-B 149; A42-B 150; A42-B 151;
A42-B 152; A42-B 153; A42-B 154; A42-B 155; A42-B 156; A42-B 157;
A42-B 158; A42-B 159; A42-B 160; A42-B 161; A42-B 162; A42-B 163;
A42-B 164; A42-B 165; A42-B166; A42-B 167; A42-B 168; A42-B 169;
A43-Bl; A43-B2; A43-B3; A43-B4; A43-B5; A43-B6;
A43-B7; A43-B8; A43-B9; A43-B10; A43-B11; A43-B12;
A43-B13; A43-B 14; A43-B 15; A43-B 16; A43-B 17; A43-B 18;
A43-B19; A43-B20; A43-B21; A43-B22; A43-B23; A43-B24;
A43-B25; A43-B26; A43-B27; A43-B28; A43-B29; A43-B30;
A43-B31; A43-B32; A43-B33; A43-B34; A43-B35; A43-B36;
A43-B37; A43-B38; A43-B39; A43-B40; A43-B41; A43-B42;
A43-B43; A43-B44; A43-B45; A43-B46; A43-B47; A43-B48;
A43-B49; A43-B50; A43-B51; A43-B52; A43-B53; A43-B54;
A43-B55; A43-B56; A43-B57; A43-B58; A43-B59; A43-B60;
A43-B61; A43-B62; A43-B63; A43-B64; A43-B65; A43-B66;
A43-B67; A43-B68; A43-B69; A43-B70; A43-B71; A43-B72;
A43-B73; A43-B74; A43-B75; A43-B76; A43-B77; A43-B78;
A43-B79; A43-B80; A43-B81; A43-B82; A43-B83; A43-B84;
A43-B85; A43-B86; A43-B87; A43-B88; A43-B89; A43-B90;
A43-B91; A43-B92; A43-B93; A43-B94; A43-B95; A43-B96;
A43-B97; A43-B98; A43-B99; A43-B 100; A43-B 101; A43-B 102;
A43-B103; A43-B104; A43-B105; A43-B106; A43-B107; A43-B108;
A43-B109; A43-B110; A43-B111; A43-B112; A43-B113; A43-B114;
A43-B 115; A43-B 116; A43-B 117; A43-B 118; A43-B 119; A43-B120;
A43-B 121; A43-B122; A43-B 123; A43-B 124; A43-B 125; A43-B 126;
A43-B127; A43-B128; A43-B129; A43-B130; A43-B131; A43-B132;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-145-
A43-B 133; A43-B 134; A43-B 135; A43-B 136; A43-B 137; A43-B 138;
A43-B 139; A43-B 140; A43-B 141; A43-B 142; A43-B 143; A43-B 144;
A43-B 145; A43-B 146; A43-B 147; A43-B148; A43-B 149; A43-B 150;
A43-B151; A43-B 152; A43-B153; A43-B 154; A43-B 155; A43-B156;
A43-B 157; A43-B 158; A43-B 159; A43-B 160; A43-B 161; A43-B 162;
A43-B 163; A43-B 164; A43-B 165; A43-B 166; A43-B 167; A43-B 168;
A43-B169; A44-B1; A44-B2; A44-B3; A44-B4; A44-B5;
A44-B6; A44-B7; A44-B8; A44-B9; A44-B10; A44-B11;
A44-B 12; A44-B 13; A44-B 14; A44-B 15; A44-B 16; A44-B 17;
A44-B 18; A44-B 19; A44-B20; A44-B21; A44-B22; A44-B23;
A44-B24; A44-B25; A44-B26; A44-B27; A44-B28; A44-B29;
A44-B30; A44-B31; A44-B32; A44-B33; A44-B34; A44-B35;
A44-B36; A44-B37; A44-B38; A44-B39; A44-B40; A44-B41;
A44-B42; A44-B43; A44-B44; A44-B45; A44-B46; A44-B47;
A44-B48; A44-B49; A44-B50; A44-B51; A44-B52; A44-B53;
A44-B54; A44-B55; A44-B56; A44-B57; A44-B58; A44-B59;
A44-B60; A44-B61; A44-B62; A44-B63; A44-B64; A44-B65;
A44-B66; A44-B67; A44-B68; A44-B69; A44-B70; A44-B71;
A44-B72; A44-B73; A44-B74; A44-B75; A44-B76; A44-B77;
A44-B78; A44-B79; A44-B80; A44-B81; A44-B82; A44-B83;
A44-B84; A44-B85; A44-B86; A44-B87; A44-B88; A44-B89;
A44-B90; A44-B91; A44-B92; A44-B93; A44-B94; A44-B95;
A44-B96; A44-B97; A44-B98; A44-B99; A44-B100; A44-B101;
A44-B 102; A44-B 103; A44-B 104; A44-B 105; A44-B 106; A44-B 107;
A44-B 108; A44-B 109; A44-B 110; A44-B 111; A44-B 112; A44-B 113;
A44-B 114; A44-B 115; A44-B 116; A44-B 117; A44-B 118; A44-B 119;
A44-B 120; A44-B 121; A44-B122; A44-B 123; A44-B 124; A44-B 125;
A44-B 126; A44-B 127; A44-B128; A44-B 129; A44-B 130; A44-B 131;
A44-B 132; A44-B133; A44-B 134; A44-B135; A44-B136; A44-B 137;
A44-B 13 8; A44-B 139; A44-B 140; A44-B 141; A44-B 142; A44-B143;
A44-B 144; A44-B 145; A44-B 146; A44-B 147; A44-B 148; A44-B 149;
A44-B150; A44-B 151; A44-B 152; A44-B 153; A44-B 154; A44-B 155;
A44-B 156; A44-B 157; A44-B 158; A44-B159; A44-B 160; A44-B 161;
A44-B 162; A44-B 163; A44-B 164; A44-B 165; A44-B 166; A44-B 167;
A44-B168; A44-B169; A45-Bl; A45-B2; A45-B3; A45-B4;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-146-
A45-B5; A45-B6; A45-B7; A45-B8; A45-B9; A45-B10;
A45-B 11; A45-B 12; A45-B 13; A45-B 14; A45-B 15; A45-B16;
A45-B 17; A45-B18; A45-B 19; A45-B20; A45-B21; A45-B22;
A45-B23; A45-B24; A45-B25; A45-B26; A45-B27; A45-B28;
A45-B29; A45-B30; A45-B31; A45-B32; A45-B33; A45-B34;
A45-B35; A45-B36; A45-B37; A45-B38; A45-B39; A45-B40;
A45-B41; A45-B42; A45-B43; A45-B44; A45-B45; A45-B46;
A45-B47; A45-B48; A45-B49; A45-B50; A45-B51; A45-B52;
A45-B53; A45-B54; A45-B55; A45-B56; A45-B57; A45-B58;
A45-B59; A45-B60; A45-B61; A45-B62; A45-B63; A45-B64;
A45-B65; A45-B66; A45-B67; A45-B68; A45-B69; A45-B70;
A45-B71; A45-B72; A45-B73; A45-B74; A45-B75; A45-B76;
A45-B77; A45-B78; A45-B79; A45-B80; A45-B81; A45-B82;
A45-B83; A45-B84; A45-B85; A45-B86; A45-B87; A45-B88;
A45-B89; A45-B90; A45-B91; A45-B92; A45-B93; A45-B94;
A45-B95; A45-B96; A45-B97; A45-B98; A45-B99; A45-B100;
A45-B 101; A45-B 102; A45-B 103; A45-B104; A45-B105; A45-B 106;
A45-B 107; A45-B 108; A45-B 109; A45-B 110; A45-B 111; A45-B 112;
A45-B 113; A45-B 114; A45-B 115; A45-B 116; A45-B117; A45-B 118;
A45-B 119; A45-B 120; A45-B 121; A45-B 122; A45-B 123; A45-B124;
A45-B125; A45-B 126; A45-B 127; A45-B 128; A45-B 129; A45-B130;
A45-B 131; A45-B132; A45-B 133; A45-B 134; A45-B 135; A45-B136;
A45-B 137; A45-B 138; A45-B 139; A45-B 140; A45-B 141; A45-B142;
A45-B 143; A45-B 144; A45-B 145; A45-B 146; A45-B 147; A45-B 148;
A45-B149; A45-B150; A45-B151; A45-B152; A45-B153; A45-B154;
A45-B 155; A45-B 156; A45-B 157; A45-B 158; A45-B 159; A45-B160;
A45-B161; A45-B162; A45-B163; A45-B 164; A45-B 165; A45-B 166;
A45-B167; A45-B168; A45-B169; A46-B1; A46-B2; A46-B3;
A46-B4; A46-B5; A46-B6; A46-B7; A46-B8; A46-B9;
A46-B 10; A46-B 11; A46-B12; A46-B 13; A46-B14; A46-B 15;
A46-B 16; A46-B 17; A46-B 18; A46-B 19; A46-B20; A46-B21;
A46-B22; A46-B23; A46-B24; A46-B25; A46-B26; A46-B27;
A46-B28; A46-B29; A46-B30; A46-B31; A46-B32; A46-B33;
A46-B34; A46-B35; A46-B36; A46-B37; A46-B38; A46-B39;
A46-B40; A46-B41; A46-B42; A46-B43; A46-B44; A46-B45;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-147-
A46-B46; A46-B47; A46-B48; A46-B49; A46-B50; A46-B51;
A46-B52; A46-B53; A46-B54; A46-B55; A46-B56; A46-B57;
A46-B58; A46-B59; A46-B60; A46-B61; A46-B62; A46-B63;
A46-B64; A46-B65; A46-B66; A46-B67; A46-B68; A46-B69;
A46-B70; A46-B71; A46-B72; A46-B73; A46-B74; A46-B75;
A46-B76; A46-B77; A46-B78; A46-B79; A46-B80; A46-B81;
A46-B82; A46-B83; A46-B84; A46-B85; A46-B86; A46-B87;
A46-B88; A46-B89; A46-B90; A46-B91; A46-B92; A46-B93;
A46-B94; A46-B95; A46-B96; A46-B97; A46-B98; A46-B99;
A46-B 100; A46-B 101; A46-B 102; A46-B 103; A46-B 104; A46-B 105;
A46-B 106; A46-B 107; A46-B 10S; A46-B 109; A46-B 110; A46-B 111;
A46-B 112; A46-B 113; A46-B 114; A46-B 115; A46-B 116; A46-B 117;
A46-B 118; A46-B 119; A46-B 120; A46-B 121; A46-B 122; A46-B 123;
A46-B 124; A46-B 125; A46-B 12 6; A46-B 127; A46-B 128; A46-B129;
A46-B 130; A46-B 131; A46-B 132; A46-B 133; A46-B 134; A46-B 135;
A46-B136; A46-B137; A46-B138; A46-B139; A46-B140; A46-B141;
A46-B 142; A46-B 143; A46-B 144; A46-B 145; A46-B 146; A46-B 147;
A46-B 148; A46-B 149; A46-B 150; A46-B 151; A46-B 152; A46-B 153;
A46-B 154; A46-B 155; A46-B 156; A46-B 157; A46-B 158; A46-B 159;
A46-B 160; A46-B 161; A46-B 162; A46-B 163; A46-B 164; A46-B 165;
A46-B 166; A46-B 167; A46-B 168; A46-B 169; A47-B 1; A47-B2;
A47-B3; A47-B4; A47-B5; A47-B6; A47-B7; A47-B8;
A47-B9; A47-B 10; A47-B 11; A47-B 12; A47-B 13; A47-B 14;
A47-B 15; A47-B 16; A47-B 17; A47-B 18; A47-B 19; A47-B20;
A47-B21; A47-B22; A47-B23; A47-B24; A47-B25; A47-B26;
A47-B27; A47-B28; A47-B29; A47-B30; A47-B31; A47-B32;
A47-B33; A47-B34; A47-B35; A47-B36; A47-B37; A47-B38;
A47-B39; A47-B40; A47-B41; A47-B42; A47-B43; A47-B44;
A47-B45; A47-B46; A47-B47; A47-B48; A47-B49; A47-B50;
A47-B51; A47-B52; A47-B53; A47-B54; A47-B55; A47-B56;
A47-B57; A47-B58; A47-B59; A47-B60; A47-B61; A47-B62;
A47-B63; A47-B64; A47-B65; A47-B66; A47-B67; A47-B68;
A47-B69; A47-B70; A47-B71; A47-B72; A47-B73; A47-B74;
A47-B75; A47-B76; A47-B77; A47-B78; A47-B79; A47-B80;
A47-B81; A47-B82; A47-B83; A47-B84; A47-B85; A47-B86;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-148-
A47-B87; A47-B88; A47-B89; A47-B90; A47-B91; A47-B92;
A47-B93; A47-B94; A47-B95; A47-B96; A47-B97; A47-B98;
A47-B99; A47-B 100; A47-B 101; A47-B 102; A47-B 103; A47-B 104;
A47-B 105; A47-B 106; A47-B 107; A47-B 108; A47-B 109; A47-B 110;
A47-B 111; A47-B 112; A47-B 113; A47-B 114; A47-B 115; A47-B 116;
A47-B 117; A47-B 118; A47-B 119; A47-B 120; A47-B 121; A47-B 122;
A47-B 123; A47-B 124; A47-B 125; A47-B 126; A47-B 127; A47-B 128;
A47-B 129; A47-B130; A47-B 131; A47-B 132; A47-B 133; A47-B 134;
A47-B 135; A47-B136; A47-B 137; A47-B 138; A47-B 139; A47-B 140;
A47-B 141; A47-B 142; A47-B 143; A47-B 144;, A47-B 145; A47-B 146;
A47-B 147; A47-B 148; A47-B 149; A47-B 150; A47-B 151; A47-B 152;
A47-B 153; A47-B 154; A47-B155; A47-B156; A47-B 157; A47-B158;
A47-B 159; A47-B 160; A47-B 161; A47-B 162; A47-B 163; A47-B 164;
A47-B 165; A47-B 166; A47-B 167; A47-B 168; A47-B 169; A48-B 1;
A48-B2; A48-B3; A48-B4; A48-B5; A48-B6; A48-B7;
A48-B8; A48-B9; A48-B 10; A48-B 11; A48-B12; A48-B 13;
A48-B 14; A48-B 15; A48-B 16; A48-B17; A48-B 18; A48-B 19;
A48-B20; A48-B21; A48-B22; A48-B23; A48-B24; A48-B25;
A48-B26; A48-B27; A48-B28; A48-B29; A48-B30; A48-B31;
A48-B32; A48-B33; A48-B34; A48-B35; A48-B36; A48-B37;
A48-B38; A48-B39; A48-B40; A48-B41; A48-B42; A48-B43;
A48-B44; A48-B45; A48-B46; A48-B47; A48-B48; A48-B49;
A48-B50; A48-B51; A48-B52; A48-B53; A48-B54; A48-B55;
A48-B56; A48-B57; A48-B58; A48-B59; A48-B60; A48-B61;
A48-B62; A48-B63; A48-B64; A48-B65; A48-B66; A48-B67;
A48-B68; A48-B69; A48-B70; A48-B71; A48-B72; A48-B73;
A48-B74; A48-B75; A48-B76; A48-B77; A48-B78; A48-B79;
A48-B80; A48-B81; A48-B82; A48-B83; A48-B84; A48-B85;
A48-B86; A48-B87; A48-B88; A48-B89; A48-B90; A48-B91;
A48-B92; A48-B93; A48-B94; A48-B95; A48-B96; A48-B97;
A48-B98; A48-B99; A48-B100; A48-B101; A48-B102; A48-B103;
A48-B 104; A48-B105; A48-B 106; A48-B 107; A48-B 108; A48-B 109;
A48-B 110; A48-B 111; A48-B 112; A48-B113; A48-B114; A48-B115;
A48-B116; A48-B117; A48-B118; A48-B119; A48-B120; A48-B121;
A48-B 122; A48-B 123; A48-B 124; A48-B 125; A48-B 126; A48-B 127;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-149-
A48-B128; A48-B 129; A48-B 130; A48-B 131; A48-B 132; A48-B 133;
A48-B 134; A48-B135; A48-B 136; A48-B 137; A48-B138; A48-B139;
A48-B140; A48-B 141; A48-B 142; A48-B143; A48-B 144; A48-B 145;
A48-B 146; A48-B 147; A48-B 148; A48-B 149; A48-B 150; A48-B 151;
A48-B152; A48-B153; A48-B154; A48-B155; A48-B156; A48-B 157;
A48-B158; A48-B159; A48-B160; A48-B161; A48-B162; A48-B163;
A48-B164; A48-B165; A48-B 166; A48-B167; A48-B168; A48-B169;
A49-B1; A49-B2; A49-B3; A49-B4; A49-B5; A49-B6;
A49-B7; A49-B8; A49-B9; A49-B10; A49-B11; A49-B12;
A49-B 13; A49-B 14; A49-B 15; A49-B 16; A49-B 17; A49-B 18;
A49-B 19; A49-B20; A49-B21; A49-B22; A49-B23; A49-B24;
A49-B25; A49-B26; A49-B27; A49-B28; A49-B29; A49-B30;
A49-B31; A49-B32; A49-B33; A49-B34; A49-B35; A49-B36;
A49-B37; A49-B38; A49-B39; A49-B40; A49-B41; A49-B42;
A49-B43; A49-B44; A49-B45; A49-B46; A49-B47; A49-B48;
A49-B49; A49-B50; A49-B51; A49-B52; A49-B53; A49-B54;
A49-B55; A49-B56; A49-B57; A49-B58; A49-B59; A49-B60;
A49-B61; A49-B62; A49-B63; A49-B64; A49-B65; A49-B66;
A49-B67; A49-B68; A49-B69; A49-B70; A49-B71; A49-B72;
A49-B73; A49-B74; A49-B75; A49-B76; A49-B77; A49-B78;
A49-B79; A49-B80; A49-B81; A49-B82; A49-B83; A49-B84;
A49-B85; A49-B86; A49-B87; A49-B88; A49-B89; A49-B90;
A49-B91; A49-B92; A49-B93; A49-B94; A49-B95; A49-B96;
A49-B97; A49-B98; A49-B99; A49-B 100; A49-B 101; A49-B 102;
A49-B 103; A49-B 104; A49-B 105; A49-B 106; A49-B 107; A49-B 108;
A49-B 109; A49-B 110; A49-B 111; A49-B 112; A49-B 113; A49-B 114;
A49-B 115; A49-B 116; A49-B 117; A49-B 118; A49-B 119; A49-B 120;
A49-B 121; A49-B 122; A49-B 123; A49-B 124; A49-B 125; A49-B 126;
A49-B 127; A49-B 128; A49-B 129; A49-B 130; A49-B 131; A49-B132;
A49-B 133; A49-B 134; A49-B 135; A49-B 136; A49-B 137; A49-B138;
A49-B 139; A49-B 140; A49-B141; A49-B 142; A49-B 143; A49-B 144;
A49-B 145; A49-B 146; A49-B 147; A49-B 148; A49-B 149; A49-B 150;
A49-B 151; A49-B 152; A49-B 153; A49-B 154; A49-B 155; A49-B 156;
A49-B 157; A49-B158; A49-B 159; A49-B 160; A49-B 161; A49-B162;
A49-B 163; A49-B164; A49-B 165; A49-B 166; A49-B 167; A49-B168;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-150-
A49-B 169; A50-B 1; A50-B2; A50-B3; A50-B4; A50-B5;
A50-B6; A50-B7; A50-B8; A50-B9; A50-B10; A50-B11;
A50-B12; A50-B13; A50-B 14; A50-B 15; A50-B 16; A50-B 17;
A50-B18; A50-B19; A50-B20; A50-B21; A50-B22; A50-B23;
A50-B24; A50-B25; A50-B26; A50-B27; A50-B28; A50-B29;
A50-B30; A50-B31; A50-B32; A50-B33; A50-B34; A50-B35;
A50-B36; A50-B37; A50-B38; A50-B39; A50-B40; A50-B41;
A50-B42; A50-B43; A50-B44; A50-B45; A50-B46; A50-B47;
A50-B48; A50-B49; A50-B50; A50-B51; A50-B52; A50-B53;
A50-B54; A50-B55; A50-B56; A50-B57; A50-B58; A50-B59;
A50-B60; A50-B61; A50-B62; A50-B63; A50-B64; A50-B65;
A50-B66; A50-B67; A50-B68; A50-B69; A50-B70; A50-B71;
A50-B72; A50-B73; A50-B74; A50-B75; A50-B76; A50-B77;
A50-B78; A50-B79; A50-B80; A50-B81; A50-B82; A50-B83;
A50-B84; A50-B85; A50-B86; A50-B87; A50-B88; A50-B89;
A50-B90; A50-B91; A50-B92; A50-B93; A50-B94; A50-B95;
A50-B96; A50-B97; A50-B98; A50-B99; A50-B100; A50-B101;
A50-B 102; A50-B 103; A50-B 104; A50-B 105; A50-B 106; A50-B 107;
A50-B 108; A50-B 109; A50-B 110; A50-B 111; A50-B 112; A50-B 113;
A50-B 114; A50-B 115; A50-B 116; A50-B 117; A50-B118; A50-B 119;
A50-B 120; A50-B121; A50-B 122; A50-B123; A50-B 124; A50-B 125;
A50-B 126; A50-B 127; A50-B 128; A50-B 129; A50-B 130; A50-B 131;
A50-B132; A50-B 133; A50-B 134; A50-B 135; A50-B136; A50-B 137;
A50-B 138; A50-B 139; A50-B 140; A50-B141; A50-B 142; A50-B 143;
A50-B 144; A50-B 145; A50-B146; A50-B 147; A50-B148; A50-B 149;
A50-B 150; A50-B 15 1; A50-B 152; A50-B 153; A50-B 154; A50-B 155;
A50-B 156; A50-B157; A50-B158; A50-B 159; A50-B 160; A50-B161;
A50-B 162; A50-B 163; A50-B 164; A50-B 165; A50-B166; A50-B167;
A50-B168; A50-B169; A51-Bl; A51-B2; A51-B3; A51-B4;
A51-B5; A51-B6; A51-B7; A51-B8; A51-B9; A51-B10;
A51-B11; A51-B12; A51-B13; A51-B14; A51-B15; A51-B16;
A51-B17; A51-B18; A51-B19; A51-B20; A51-B21; A51-B22;
A51-B23; A51-B24; A51-B25; A51-B26; A51-B27; A51-B28;
A51-B29; A51-B30; A51-B31; A51-B32; A51-B33; A51-B34;
A51-B35; A51-B36; A51-B37; A51-B38; A51-B39; A51-B40;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-151-
A51-B41; A51-B42; A51-B43; A51-B44; A51-B45; A51-B46;
A51-B47; A51-B48; A51-B49; A51-B50; A51-B51; A51-B52;
A51-B53; A51-B54; A51-B55; A51-B56; A51-B57; A51-B58;
A51-B59; A51-B60; A51-B61; A51-B62; A51-B63; A51-B64;
A51-B65; A51-B66; A51-B67; A51-B68; A51-B69; A51-B70;
A51-B71; A51-B72; A51-B73; A51-B74; A51-B75; A51-B76;
A51-B77; A51-B78; A51-B79; A51-B80; A51-B81; A51-B82;
A51-B83; A51-B84; A51-B85; A51-B86; A51-B87; A51-B88;
A51-B89; A51-B90; A51-B91; A51-B92; A51-B93; A51-B94;
A51-B95; A51-B96; A51-B97; A51-B98; A51-B99; A51-B100;
A51-B101; A51-B102; A51-B103; A51-B104; A51-B105; A51-B106;
A51-B107; A51-B108; A51-B109; A51-B110; A51-B111; A51-B112;
A51-B113; A51-B114; A51-B115; A51-B116; A51-B117; A51-B118;
A51-B119; A51-B120; A51-B121; A51-B122; A51-B123; A51-B124;
A51-B125; A51-B126; A51-B127; A51-B128; A51-B129; A51-B130;
A51-B131; A51-B132; A51-B133; A51-B134; A51-B135; A51-B136;
A51-B137; A51-B138; A51-B139; A51-B140; A51-B141; A51-B142;
A51-B143; A51-B144; A51-B145; A51-B146; A51-B147; A51-B148;
A51-B149; A51-B150; A51-B151; A51-B152; A51-B153; A51-B154;
A51-B155; A51-B156; A51-B157; A51-B158; A51-B159; A51-B160;
A51-B161; A51-B162; A51-B163; A51-B164; A51-B165; A51-B166;
A51-B167; A51-B168; A51-B169; A52-B1; A52-B2; A52-B3;
A52-B4; A52-B5; A52-B6; A52-B7; A52-B8; A52-B9;
A52-B 10; A52-B 11; A52-B 12; A52-B 13; A52-B 14; A52-B 15;
A52-B 16; A52-B 17; A52-B 18; A52-B 19; A52-B20; A52-B21;
A52-B22; A52-B23; A52-B24; A52-B25; A52-B26; A52-B27;
A52-B28; A52-B29; A52-B30; A52-B31; A52-B32; A52-B33;
A52-B34; A52-B35; A52-B36; A52-B37; A52-B38; A52-B39;
A52-B40; A52-B41; A52-B42; A52-B43; A52-B44; A52-B45;
A52-B46; A52-B47; A52-B48; A52-B49; A52-B50; A52-B51;
A52-B52; A52-B53; A52-B54; A52-B55; A52-B56; A52-B57;
A52-B58; A52-B59; A52-B60; A52-B61; A52-B62; A52-B63;
A52-B64; A52-B65; A52-B66; A52-B67; A52-B68; A52-B69;
A52-B70; A52-B71; A52-B72; A52-B73; A52-B74; A52-B75;
A52-B76; A52-B77; A52-B78; A52-B79; A52-B80; A52-B81;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-152-
A52-B82; A52-B83; A52-B84; A52-B85; A52-B86; A52-B87;
A52-B88; A52-B89; A52-B90; A52-B91; A52-B92; A52-B93;
A52-B94; A52-B95; A52-B96; A52-B97; A52-B98; A52-B99;
A52-B 100; A52-B 101; A52-B102; A52-B 103; A52-B 104; A52-B 105;
A52-B 106; A52-B 107; A52-B 108; A52-B 109; A52-B 110; A52-B 111;
A52-B 112; A52-B 113; A52-B 114; A52-B 115; A52-B116; A52-B 117;
A52-B 118; A52-B 119; A52-B 120; A52-B 121; A52-B 122; A52-B 123;
A52-B 124; A52-B125; A52-B 126; A52-B 127; A52-B 128; A52-B 129;
A52-B 130; A52-B 131; A52-B132; A52-B133; A52-B 134; A52-B 135;
A52-B136; A52-B137; A52-B138; A52-B139; A52-B140; A52-B141;
A52-B 142; A52-B 143; A52-B144; A52-B 145; A52-B 146; A52-B 147;
A52-B 148; A52-B 149; A52-B 150; A52-B 151; A52-B 152; A52-B153;
A52-B 154; A52-B155; A52-B 156; A52-B 157; A52-B 158; A52-B 159;
A52-B 160; A52-B 161; A52-B 162; A52-B 163; A52-B 164; A52-B 165;
A52-B 166; A52-B 167; A52-B 168; A52-B 169; A53-B l; A53-B2;
A53-B3; A53-B4; A53-B5; A53-B6; A53-B7; A53-B8;
A53-B9; A53-B10; A53-B11; A53-B12; A53-B13; A53-B14;
A53-B 15; A53-B 16; A53-B 17; A53-B 18; A53-B19; A53-B20;
A53-B21; A53-B22; A53-B23; A53-B24; A53-B25; A53-B26;
A53-B27; A53-B28; A53-B29; A53-B30; A53-B31; A53-B32;
A53-B33; A53-B34; A53-B35; A53-B36; A53-B37; A53-B38;
A53-B39; A53-B40; A53-B41; A53-B42; A53-B43; A53-B44;
A53-B45; A53-B46; A53-B47; A53-B48; A53-B49; A53-B50;
A53-B51; A53-B52; A53-B53; A53-B54; A53-B55; A53-B56;
A53-B57; A53-B58; A53-B59; A53-B60; A53-B61; A53-B62;
A53-B63; A53-B64; A53-B65; A53-B66; A53-B67; A53-B68;
A53-B69; A53-B70; A53-B71; A53-B72; A53-B73; A53-B74;
A53-B75; A53-B76; A53-B77; A53-B78; A53-B79; A53-B80;
A53-B81; A53-B82; A53-B83; A53-B84; A53-B85; A53-B86;
A53-B87; A53-B88; A53-B89; A53-B90; A53-B91; A53-B92;
A53-B93; A53-B94; A53-B95; A53-B96; A53-B97; A53-B98;
A53-B99; A53-B 100; A53-B 101; A53-B 102; A53-B 103; A53-B 104;
A53-B 105; A53-B 106; A53-B 107; A53-B 108; A53-B 109; A53-B 110;
A53-B111; A53-B112; A53-B113; A53-B114; A53-B115; A53-B116;
A53-B117; A53-B118; A53-B119; A53-B120; A53-B121; A53-B122;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-153-
A53-B 123; A53-B 124; A53-B 125; A53-B 126; A53-B 127; A53-B 128;
A53-B129; A53-B130; A53-B131; A53-B132; A53-B133; A53-B134;
A53-B135; A53-B136; A53-B137; A53-B138; A53-B139; A53-B140;
A53-B141; A53-B142; A53-B143; A53-B144; A53-B145; A53-B146;
A53-B147; A53-B148; A53-B149; A53-B150; A53-B151; A53-B 152;
A53-B153; A53-B154; A53-B155; A53-B156; A53-B157; A53-B158;
A53-B 159; A53-B 160; A53-B 161; A53-B 162; A53-B 163; A53-B 164;
A53-B165; A53-B166; A53-B167; A53-B168; A53-B169; A54-B1;
A54-B2; A54-B3; A54-B4; A54-B5; A54-B6; A54-B7;
A54-B8; A54-B9; A54-B 10; A54-B 11; A54-B 12; A54-B 13;
A54-B 14; A54-B 15; A54-B 16; A54-B 17; A54-B 18; A54-B 19;
A54-B20; A54-B21; A54-B22; A54-B23; A54-B24; A54-B25;
A54-B26; A54-B27; A54-B28; A54-B29; A54-B30; A54-B31;
A54-B32; A54-B33; A54-B34; A54-B35; A54-B36; A54-B37;
A54-B38; A54-B39; A54-B40; A54-B41; A54-B42; A54-B43;
A54-B44; A54-B45; A54-B46; A54-B47; A54-B48; A54-B49;
A54-B50; A54-B51; A54-B52; A54-B53; A54-B54; A54-B55;
A54-B56; A54-B57; A54-B58; A54-B59; A54-B60; A54-B61;
A54-B62; A54-B63; A54-B64; A54-B65; A54-B66; A54-B67;
A54-B68; A54-B69; A54-B70; A54-B71; A54-B72; A54-B73;
A54-B74; A54-B75; A54-B76; A54-B77; A54-B78; A54-B79;
A54-B80; A54-B81; A54-B82; A54-B83; A54-B84; A54-B85;
A54-B86; A54-B87; A54-B88; A54-B89; A54-B90; A54-B91;
A54-B92; A54-B93; A54-B94; A54-B95; A54-B96; A54-B97;
A54-B98; A54-B99; A54-B 100; A54-B 101; A54-B 102; A54-B 103;
A54-B 104; A54-B 105; A54-B 106; A54-B 107; A54-B 108; A54-B 109;
A54-B 110; A54-B 111; A54-B 112; A54-B 113; A54-B 114; A54-B 115;
A54-B 116; A54-B 117; A54-B 118; A54-B 119; A54-B120; A54-B 121;
A54-B 122; A54-B 123; A54-B 124; A54-B 125; A54-B 126; A54-B 127;
A54-B 128; A54-B 129; A54-B130; A54-B 131; A54-B 132; A54-B 133;
A54-B 134; A54-B 135; A54-B 136; A54-B137; A54-B 138; A54-B 139;
A54-B 140; A54-B 141; A54-B 142; A54-B143; A54-B144; A54-B145;
A54-B 146; A54-B 147; A54-B148; A54-B149; A54-B150; A54-B 151;
A54-B 152; A54-B 153; A54-B 154; A54-B 155; A54-B 156; A54-B 157;
A54-B 158; A54-B159; A54-B 160; A54-B161; A54-B 162; A54-B163;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-154-
A54-B 164; A54-B 165; A54-B 166; A54-B 167; A54-B 168; A54-B 169;
A55-B1; A55-B2; A55-B3; A55-B4; A55-B5; A55-B6;
A55-B7; A55-B8; A55-B9; A55-B10; A55-B11; A55-B12;
A55-B 13; A55-B14; A55-B 15; A55-B 16; A55-B 17; A55-B 18;
A55-B19; A55-B20; A55-B21; A55-B22; A55-B23; A55-B24;
A55-B25; A55-B26; A55-B27; A55-B28; A55-B29; A55-B30;
A55-B31; A55-B32; A55-B33; A55-B34; A55-B35; A55-B36;
A55-B37; A55-B38; A55-B39; A55-B40; A55-B41; A55-B42;
A55-B43; A55-B44; A55-B45; A55-B46; A55-B47; A55-B48;
A55-B49; A55-B50; A55-B51; A55-B52; A55-B53; A55-B54;
A55-B55; A55-B56; A55-B57; A55-B58; A55-B59; A55-B60;
A55-B61; A55-B62; A55-B63; A55-B64; A55-B65; A55-B66;
A55-B67; A55-B68; A55-B69; A55-B70; A55-B71; A55-B72;
A55-B73; A55-B74; A55-B75; A55-B76; A55-B77; A55-B78;
A55-B79; A55-B80; A55-B81; A55-B82; A55-B83; A55-B84;
A55-B85; A55-B86; A55-B87; A55-B88; A55-B89; A55-B90;
A55-B91; A55-B92; A55-B93; A55-B94; A55-B95; A55-B96;
A55-B97; A55-B98; A55-B99; A55-B 100; A55-B 101; A55-B 102;
A55-B103; A55-B104; A55-B105; A55-B106; A55-B107; A55-B108;
A55-B109; A55-B110; A55-B111; A55-B112; A55-B113; A55-B114;
A55-B115; A55-B116; A55-B117; A55-B118; A55-B119; A55-B120;
A55-B121; A55-B122; A55-B123; A55-B124; A55-B125; A55-B126;
A55-B127; A55-B128; A55-B129; A55-B130; A55-B131; A55-B132;
A55-B133; A55-B134; A55-B135; A55-B136; A55-B137; A55-B138;
A55-B139; A55-B140; A55-B141; A55-B142; A55-B143; A55-B144;
A55-B145; A55-B146; A55-B147; A55-B148; A55-B149; A55-B150;
A55-B151; A55-B152; A55-B153; A55-B154; A55-B155; A55-B156;
A55-B157; A55-B 158; A55-B159; A55-B160; A55-B 161; A55-B 162;
A55-B163; A55-B164; A55-B165; A55-B166; A55-B167; A55-B168;
A55-B169; A56-B1; A56-B2; A56-B3; A56-B4; A56-B5;
A56-B6; A56-B7; A56-B8; A56-B9; A56-B10; A56-B11;
A56-B 12; A56-B 13; A56-B 14; A56-B 15; A56-B 16; A56-B17;
A56-B18; A56-B19; A56-B20; A56-B21; A56-B22; A56-B23;
A56-B24; A56-B25; A56-B26; A56-B27; A56-B28; A56-B29;
A56-B30; A56-B31; A56-B32; A56-B33; A56-B34; A56-B35;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-155-
A56-B36; A56-B37; A56-B38; A56-B39; A56-B40; A56-B41;
A56-B42; A56-B43; A56-B44; A56-B45; A56-B46; A56-B47;
A56-B48; A56-B49; A56-B50; A56-B51; A56-B52; A56-B53;
A56-B54; A56-B55; A56-B56; A56-B57; A56-B58; A56-B59;
A56-B60; A56-B61; A56-B62; A56-B63; A56-B64; A56-B65;
A56-B66; A56-B67; A56-B68; A56-B69; A56-B70; A56-B71;
A56-B72; A56-B73; A56-B74; A56-B75; A56-B76; A56-B77;
A56-B78; A56-B79; A56-B80; A56-B81; A56-B82; A56-B83;
A56-B84; A56-B85; A56-B86; A56-B87; A56-B88; A56-B89;
A56-B90; A56-B91; A56-B92; A56-B93; A56-B94; A56-B95;
A56-B96; A56-B97; A56-B98; A56-B99; A56-B100; A56-B101;
A56-B102; A56-B103; A56-B104; A56-B105; A56-B106; A56-B107;
A56-B 108; A56-B 109; A56-B 110; A56-B 111; A56-B 112; A56-B 113;
A56-B114; A56-B 115; A56-B 116; A56-B117; A56-B 118; A56-B 119;
A56-B120; A56-B121; A56-B122; A56-B123; A56-B124; A56-B125;
A56-B126; A56-B 127; A56-B 128; A56-B 129; A56-B 130; A56-B 131;
A56-B132; A56-B133; A56-B134; A56-B135; A56-B136; A56-B137;
A56-B138; A56-B139; A56-B140; A56-B141; A56-B142; A56-B143;
A56-B144; A56-B145; A56-B146; A56-B147; A56-B148; A56-B149;
A56-B150; A56-B151; A56-B152; A56-B153; A56-B154; A56-B155;
A56-B156; A56-B157; A56-B158; A56-B159; A56-B160; A56-B161;
A56-B 162; A56-B 163; A56-B164; A56-B 165; A56-B 166; A56-B 167;
A56-B168; A56-B169; A57-B1; A57-B2; A57-B3; A57-B4;
A57-B5; A57-B6; A57-B7; A57-B8; A57-B9; A57-B10;
A57-B 11; A57-B12; A57-B 13; A57-B 14; A57-B 15; A57-B 16;
A57-B17; A57-B 18; A57-B 19; A57-B20; A57-B21; A57-B22;
A57-B23; A57-B24; A57-B25; A57-B26; A57-B27; A57-B28;
A57-B29; A57-B30; A57-B31; A57-B32; A57-B33; A57-B34;
A57-B35; A57-B36; A57-B37; A57-B38; A57-B39; A57-B40;
A57-B41; A57-B42; A57-B43; A57-B44; A57-B45; A57-B46;
A57-B47; A57-B48; A57-B49; A57-B50; A57-B51; A57-B52;
A57-B53; A57-B54; A57-B55; A57-B56; A57-B57; A57-B58;
A57-B59; A57-B60; A57-B61; A57-B62; A57-B63; A57-B64;
A57-B65; A57-B66; A57-B67; A57-B68; A57-B69; A57-B70;
A57-B71; A57-B72; A57-B73; A57-B74; A57-B75; A57-B76;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-156-
A57-B77; A57-B78; A57-B79; A57-B80; A57-B81; A57-B82;
A57-B83; A57-B84; A57-B85; A57-B86; A57-B87; A57-B88;
A57-B89; A57-B90; A57-B91; A57-B92; A57-B93; A57-B94;
A57-B95; A57-B96; A57-B97; A57-B98; A57-B99; A57-B100;
A57-B 101; A57-B 102; A57-B 103; A57-B 104; A57-B 105; A57-B 106;
A57-B107; A57-B 108; A57-B 109; A57-B 110; A57-B 111; A57-B 112;
A57-B 113; A57-B 114; A57-B 115; A57-B 116; A57-B 117; A57-B 118;
A57-B 119; A57-B 120; A57-B 121; A57-B 122; A57-B 123; A57-B 124;
A57-B 125; A57-B 126; A57-B 127; A57-B 128; A57-B 129; A57-B 130;
A57-B131; A57-B 132; A57-B 133; A57-B 134; A57-B 135; A57-B136;
A57-B137; A57-B138; A57-B139; A57-B140; A57-B141; A57-B 142;
A57-B143; A57-B 144; A57-B 145; A57-B 146; A57-B 147; A57-B 148;
A57-B 149; A57-B150; A57-B 151; A57-B 152; A57-B 153; A57-B 154;
A57-B 155; A57-B156; A57-B 157; A57-B 158; A57-B159; A57-B 160;
A57-B161; A57-B 162; A57-B 163; A57-B 164; A57-B165; A57-B166;
A57-B167; A57-B168; A57-B169; A58-Bl; A58-B2; A58-B3;
A58-B4; A58-B5; A58-B6; A58-B7; A58-B8; A58-B9;
A58-B10; A58-B11; A58-B12; A58-B13; A58-B14; A58-B15;
A58-B16; A58-B17; A58-B18; A58-B19; A58-B20; A58-B21;
A58-B22; A58-B23; A58-B24; A58-B25; A58-B26; A58-B27;
A58-B28; A58-B29; A58-B30; A58-B31; A58-B32; A58-B33;
A58-B34; A58-B35; A58-B36; A58-B37; A58-B38; A58-B39;
A58-B40; A58-B41; A58-B42; A58-B43; A58-B44; A58-B45;
A58-B46; A58-B47; A58-B48; A58-B49; A58-B50; A58-B51;
A58-B52; A58-B53; A58-B54; A58-B55; A58-B56; A58-B57;
A58-B58; A58-B59; A58-B60; A58-B61; A58-B62; A58-B63;
A58-B64; A58-B65; A58-B66; A58-B67; A58-B68; A58-B69;
A58-B70; A58-B71; A58-B72; A58-B73; A58-B74; A58-B75;
A58-B76; A58-B77; A58-B78; A58-B79; A58-B80; A58-B81;
A58-B82; A58-B83; A58-B84; A58-B85; A58-B86; A58-B87;
A58-B88; A58-B89; A58-B90; A58-B91; A58-B92; A58-B93;
A58-B94; A58-B95; A58-B96; A58-B97; A58-B98; A58-B99;
A58-B100; A58-B101; A58-B102; A58-B103; A58-B104; A58-B105;
A58-B106; A58-B107; A58-B108; A58-B109; A58-B110; A58-B111;
A58-B112; A58-B113; A58-B114; A58-B115; A58-B116; A58-B117;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-157-
A58-B118; A58-B119; A58-B120; A58-B121; A58-B122; A58-B123;
A58-B 124; A58-B 125; A58-B126; A58-B 127; A58-B 128; A58-B 129;
A58-B130; A58-B131; A58-B132; A58-B133; A58-B134; A58-B135;
A58-B136; A58-B137; A58-B138; A58-B139; A58-B140; A58-B141;
A58-B142; A58-B143; A58-B144; A58-B145; A58-B146; A58-B147;
A58-B148; A58-B149; A58-B150; A58-B151; A58-B152; A58-B153;
A58-B154; A58-B155; A58-B156; A58-B157; A58-B158; A58-B159;
A58-B160; A58-B161; A58-B162; A58-B163; A58-B164; A58-B165;
A58-B166; A58-B167; A58-B168; A58-B169; A59-B1; A59-B2;
A59-B3; A59-B4; A59-B5; A59-B6; A59-B7; A59-B8;
A59-B9; A59-B10; A59-B11; A59-B12; A59-B13; A59-B14;
A59-B 15; A59-B16; A59-B 17; A59-B 18; A59-B 19; A59-B20;
A59-B21; A59-B22; A59-B23; A59-B24; A59-B25; A59-B26;
A59-B27; A59-B28; A59-B29; A59-B30; A59-B31; A59-B32;
A59-B33; A59-B34; A59-B35; A59-B36; A59-B37; A59-B38;
A59-B39; A59-B40; A59-B41; A59-B42; A59-B43; A59-B44;
A59-B45; A59-B46; A59-B47; A59-B48; A59-B49; A59-B50;
A59-B51; A59-B52; A59-B53; A59-B54; A59-B55; A59-B56;
A59-B57; A59-B58; A59-B59; A59-B60; A59-B61; A59-B62;
A59-B63; A59-B64; A59-B65; A59-B66; A59-B67; A59-B68;
A59-B69; A59-B70; A59-B71; A59-B72; A59-B73; A59-B74;
A59-B75; A59-B76; A59-B77; A59-B78; A59-B79; A59-B80;
A59-B81; A59-B82; A59-B83; A59-B84; A59-B85; A59-B86;
A59-B87; A59-B88; A59-B89; A59-B90; A59-B91; A59-B92;
A59-B93; A59-B94; A59-B95; A59-B96; A59-B97; A59-B98;
A59-B99; A59-B100; A59-B101; A59-B102; A59-B103; A59-B104;
A59-B 105; A59-B 106; A59-B107; A59-B 108; A59-B109; A59-B 110;
A59-B111; A59-B112; A59-B113; A59-B114; A59-B115; A59-B116;
A59-B117; A59-B118; A59-B119; A59-B120; A59-B121; A59-B122;
A59-B 123; A59-B124; A59-B125; A59-B 126; A59-B 127; A59-B128;
A59-B 129; A59-B 130; A59-B 131; A59-B132; A59-B 133; A59-B 134;
A59-B 135; A59-B136; A59-B137; A59-B138; A59-B139; A59-B140;
A59-B 141; A59-B 142; A59-B 143; A59-B 144; A59-B 145; A59-B 146;
A59-B147; A59-B148; A59-B149; A59-B150; A59-B151; A59-B152;
A59-B153; A59-B 154; A59-B 155; A59-B156; A59-B 157; A59-B 158;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-158-
A59-B 159; A59-B160; A59-B161; A59-B 162; A59-B 163; A59-B164;
A59-B 165; A59-B 166; A59-B 167; A59-B 168; A59-B169; A60-B l;
A60-B2; A60-B3; A60-B4; A60-B5; A60-B6; A60-B7;
A60-B8; A60-B9; A60-B10; A60-B11; A60-B12; A60-B13;
A60-B 14; A60-B 15; A60-B 16; A60-B 17; A60-B 18; A60-B 19;
A60-B20; A60-B21; A60-B22; A60-B23; A60-B24; A60-B25;
A60-B26; A60-B27; A60-B28; A60-B29; A60-B30; A60-B31;
A60-B32; A60-B33; A60-B34; A60-B35; A60-B36; A60-B37;
A60-B38; A60-B39; A60-B40; A60-B41; A60-B42; A60-B43;
A60-B44; A60-B45; A60-B46; A60-B47; A60-B48; A60-B49;
A60-B50; A60-B51; A60-B52; A60-B53; A60-B54; A60-B55;
A60-B56; A60-B57; A60-B58; A60-B59; A60-B60; A60-B61;
A60-B62; A60-B63; A60-B64; A60-B65; A60-B66; A60-B67;
A60-B68; A60-B69; A60-B70; A60-B71; A60-B72; A60-B73;
A60-B74; A60-B75; A60-B76; A60-B77; A60-B78; A60-B79;
A60-B80; A60-B81; A60-B82; A60-B83; A60-B84; A60-B85;
A60-B86; A60-B87; A60-B88; A60-B89; A60-B90; A60-B91;
A60-B92; A60-B93; A60-B94; A60-B95; A60-B96; A60-B97;
A60-B98; A60-B99; A60-B100; A60-B101; A60-B102; A60-B103;
A60-B 104; A60-B 105; A60-B 106; A60-B 107; A60-B 108; A60-B 109;
A60-B 110; A60-B 111; A60-B 112; A60-B 113; A60-B 114; A60-B 115;
A60-B 116; A60-B 117; A60-B 118; A60-B 119; A60-B 120; A60-B 121;
A60-B 122; A60-B 123; A60-B 124; A60-B 125; A60-B126; A60-B 127;
A60-B 128; A60-B 129; A60-B 130; A60-B 131; A60-B132; A60-B 133;
A60-B 134; A60-B 135; A60-B 136; A60-B 137; A60-B 138; A60-B 139;
A60-B 140; A60-B 141; A60-B 142; A60-B 143; A60-B 144; A60-B 145;
A60-B 146; A60-B 147; A60-B 148; A60-B 149; A60-B 150; A60-B 151;
A60-B 152; A60-B 153; A60-B154; A60-B 155; A60-B 156; A60-B 157;
A60-B 158; A60-B 159; A60-B 160; A60-B 161; A60-B 162; A60-B 163;
A60-B 164; A60-B 165; A60-B 166; A60-B 167; A60-B 168; A60-B 169;
A61-B1; A61-B2; A61-B3; A61-B4; A61-B5; A61-B6;
A61-B7; A61-B8; A61-B9; A61-B10; A61-B11; A61-B12;
A61-B13; A61-B14; A61-B15; A61-B16; A61-B17; A61-B18;
A61-B19; A61-B20; A61-B21; A61-B22; A61-B23; A61-B24;
A61-B25; A61-B26; A61-B27; A61-B28; A61-B29; A61-B30;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-159-
A61-B31; A61-B32; A61-B33; A61-B34; A61-B35; A61-B36;
A61-B37; A61-B38; A61-B39; A61-B40; A61-B41; A61-B42;
A61-B43; A61-B44; A61-B45; A61-B46; A61-B47; A61-B48;
A61-B49; A61-B50; A61-B51; A61-B52; A61-B53; A61-B54;
A61-B55; A61-B56; A61-B57; A61-B58; A61-B59; A61-B60;
A61-B61; A61-B62; A61-B63; A61-B64; A61-B65; A61-B66;
A61-B67; A61-B68; A61-B69; A61-B70; A61-B71; A61-B72;
A61-B73; A61-B74; A61-B75; A61-B76; A61-B77; A61-B78;
A61-B79; A61-B80; A61-B81; A61-B82; A61-B83; A61-B84;
A61-B85; A61-B86; A61-B87; A61-B88; A61-B89; A61-B90;
A61-B91; A61-B92; A61-B93; A61-B94; A61-B95; A61-B96;
A61-B97; A61-B98; A61-B99; A61-B100; A61-B101; A61-B102;
A61-B103; A61-B104; A61-B105; A61-B106; A61-B107; A61-B108;
A61-B109; A61-B110; A61-B111; A61-B112; A61-B113; A61-B114;
A61-B115; A61-B116; A61-B117; A61-B118; A61-B119; A61-B120;
A61-B121; A61-B122; A61-B123; A61-B124; A61-B125; A61-B126;
A61-B127; A61-B128; A61-B129; A61-B130; A61-B131; A61-B132;
A61-B133; A61-B134; A61-B135; A61-B136; A61-B137; A61-B138;
A61-B139; A61-B140; A61-B141; A61-B142; A61-B143; A61-B144;
A61-B145; A61-B146; A61-B147; A61-B148; A61-B149; A61-B150;
A61-B151; A61-B152; A61-B153; A61-B154; A61-B155; A61-B156;
A61-B157; A61-B158; A61-B159; A61-B160; A61-B161; A61-B162;
A61-B163; A61-B164; A61-B165; A61-B166; A61-B167; A61-B168;
A61-B169; A62-B1; A62-B2; A62-B3; A62-B4; A62-B5;
A62-B6; A62-B7; A62-B8; A62-B9; A62-B10; A62-B11;
A62-B 12; A62-B 13; A62-B 14; A62-B 15; A62-B 16; A62-B 17;
A62-B18; A62-B19; A62-B20; A62-B21; A62-B22; A62-B23;
A62-B24; A62-B25; A62-B26; A62-B27; A62-B28; A62-B29;
A62-B30; A62-B31; A62-B32; A62-B33; A62-B34; A62-B35;
A62-B36; A62-B37; A62-B38; A62-B39; A62-B40; A62-B41;
A62-B42; A62-B43; A62-B44; A62-B45; A62-B46; A62-B47;
A62-B48; A62-B49; A62-B50; A62-B51; A62-B52; A62-B53;
A62-B54; A62-B55; A62-B56; A62-B57; A62-B58; A62-B59;
A62-B60; A62-B61; A62-B62; A62-B63; A62-B64; A62-B65;
A62-B66; A62-B67; A62-B68; A62-B69; A62-B70; A62-B71;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-160-
A62-B72; A62-B73; A62-B74; A62-B75; A62-B76; A62-B77;
A62-B78; A62-B79; A62-B80; A62-B81; A62-B82; A62-B83;
A62-B84; A62-B85; A62-B86; A62-B87; A62-B88; A62-B89;
A62-B90; A62-B91; A62-B92; A62-B93; A62-B94; A62-B95;
A62-B96; A62-B97; A62-B98; A62-B99; A62-B100; A62-B101;
A62-B 102; A62-B 103; A62-B 104; A62-B 105; A62-B 106; A62-B 107;
A62-B108; A62-B 109; A62-B 110; A62-B 111; A62-B 112; A62-B 113;
A62-B 114; A62-B 115; A62-B 116; A62-B 117; A62-B 118; A62-B 119;
A62-B 120; A62-B 12 1; A62-B 122; A62-B 123; A62-B 124; A62-B 125;
A62-B 126; A62-B 127; A62-B 128; A62-B 129; A62-B 130; A62-B 13 1;
A62-B 132; A62-B 133; A62-B 134; A62-B 135; A62-B 136; A62-B 137;
A62-B 13 8; A62-B 13 9; A62-B 140; A62-B 141; A62-B 142; A62-B 143;
A62-B 144; A62-B 145; A62-B 146; A62-B 147; A62-B 148; A62-B 149;
A62-B 150; A62-B 15 1; A62-B 152; A62-B 153; A62-B 154; A62-B155;
A62-B 156; A62-B 157; A62-B 158; A62-B 159; A62-B 160; A62-B 161;
A62-B 162; A62-B 163; A62-B 164; A62-B 165; A62-B 166; A62-B 167;
A62-B168; A62-B169; A63-B1; A63-B2; A63-B3; A63-B4;
A63-B5; A63-B6; A63-B7; A63-B8; A63-B9; A63-B10;
A63-B 11; A63-B 12; A63-B 13; A63-B 14; A63-B 15; A63-B 16;
A63-B 17; A63-B 18; A63-B 19; A63-B20; A63-B21; A63-B22;
A63-B23; A63-B24; A63-B25; A63-B26; A63-B27; A63-B28;
A63-B29; A63-B30; A63-B31; A63-B32; A63-B33; A63-B34;
A63-B35; A63-B36; A63-B37; A63-B38; A63-B39; A63-B40;
A63-B41; A63-B42; A63-B43; A63-B44; A63-B45; A63-B46;
A63-B47; A63-B48; A63-B49; A63-B50; A63-B51; A63-B52;
A63-B53; A63-B54; A63-B55; A63-B56; A63-B57; A63-B58;
A63-B59; A63-B60; A63-B61; A63-B62; A63-B63; A63-B64;
A63-B65; A63-B66; A63-B67; A63-B68; A63-B69; A63-B70;
A63-B71; A63-B72; A63-B73; A63-B74; A63-B75; A63-B76;
A63-B77; A63-B78; A63-B79; A63-B80; A63-B81; A63-B82;
A63-B83; A63-B84; A63-B85; A63-B86; A63-B87; A63-B88;
A63-B89; A63-B90; A63-B91; A63-B92; A63-B93; A63-B94;
A63-B95; A63-B96; A63-B97; A63-B98; A63-B99; A63-B100;
A63-B 101; A63-B 102; A63-B103; A63-B 104; A63-B 105; A63-B 106;
A63-B 107; A63-B108; A63-B 109; A63-B 110; A63-B 111; A63-B 112;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-161-
A63-B 113; A63-B 114; A63-B 115; A63-B116; A63-B117; A63-B118;
A63-B 119; A63-B 120; A63-B121; A63-B 122; A63-B123; A63-B 124;
A63-B 125; A63-B 126; A63-B 127; A63-B 128; A63-B 129; A63-B 130;
A63-B 131; A63-B 132; A63-B 133; A63-B 134; A63-B 135; A63-B136;
A63-B 137; A63-B 138; A63-B 139; A63-B 140; A63-B 141; A63-B 142;
A63-B 143; A63-B 144; A63-B 145; A63-B 146; A63-B147; A63-B 148;
A63-B149; A63-B150; A63-B151; A63-B152; A63-B153; A63-B154;
A63-B 155; A63-B156; A63-B 157; A63-B 158; A63-B 159; A63-B 160;
A63-B 161; A63-B 162; A63-B 163; A63-B 164; A63-B 165; A63-B 166;
A63-B 167; A63-B168; A63-B 169; A64-B 1; A64-B2; A64-B3;
A64-B4; A64-B5; A64-B6; A64-B7; A64-B8; A64-B9;
A64-B 10; A64-B 11; A64-B 12; A64-B 13; A64-B 14; A64-B 15;
A64-B 16; A64-B 17; A64-B 18; A64-B 19; A64-B20; A64-B21;
A64-B22; A64-B23; A64-B24; A64-B25; A64-B26; A64-B27;
A64-B28; A64-B29; A64-B30; A64-B31; A64-B32; A64-B33;
A64-B34; A64-B35; A64-B36; A64-B37; A64-B38; A64-B39;
A64-B40; A64-B41; A64-B42; A64-B43; A64-B44; A64-B45;
A64-B46; A64-B47; A64-B48; A64-B49; A64-B50; A64-B51;
A64-B52; A64-B53; A64-B54; A64-B55; A64-B56; A64-B57;
A64-B58; A64-B59; A64-B60; A64-B61; A64-B62; A64-B63;
A64-B64; A64-B65; A64-B66; A64-B67; A64-B68; A64-B69;
A64-B70; A64-B71; A64-B72; A64-B73; A64-B74; A64-B75;
A64-B76; A64-B77; A64-B78; A64-B79; A64-B80; A64-B81;
A64-B82; A64-B83; A64-B84; A64-B85; A64-B86; A64-B87;
A64-B88; A64-B89; A64-B90; A64-B91; A64-B92; A64-B93;
A64-B94; A64-B95; A64-B96; A64-B97; A64-B98; A64-B99;
A64-B 100; A64-B 101; A64-B 102; A64-B 103; A64-B 104; A64-B 105;
A64-B 106; A64-B 107; A64-B 108; A64-B 109; A64-B 110; A64-B 111;
A64-B 112; A64-B 113; A64-B 114; A64-B 115; A64-B 116; A64-B 117;
A64-B 118; A64-B 119; A64-B 120; A64-B121; A64-B122; A64-B 123;
A64-B 124; A64-B 125; A64-B 126; A64-B 127; A64-B 128; A64-B 129;
A64-B 13 0; A64-B 13 1; A64-B 132; A64-B 133; A64-B 134; A64-B 13 5;
A64-B 136; A64-B 137; A64-B 138; A64-B 139; A64-B 140; A64-B141;
A64-B142; A64-B 143; A64-B 144; A64-B 145; A64-B 146; A64-B 147;
A64-B 148; A64-B 149; A64-B150; A64-B 151; A64-B 152; A64-B 153;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-162-
A64-B 154; A64-B 155; A64-B 156; A64-B 157; A64-B 158; A64-B 159;
A64-B 160; A64-B 161; A64-B 162; A64-B 163; A64-B 164; A64-B 165;
A64-B 166; A64-B 167; A64-B 168; A64-B 169; A65-B l; A65-B2;
A65-B3; A65-B4; A65-B5; A65-B6; A65-B7; A65-B8;
A65-B9; A65-B 10; A65-B 11; A65-B 12; A65-B 13; A65-B 14;
A65-B 15; A65-B 16; A65-B 17; A65-B 18; A65-B 19; A65-B20;
A65-B21; A65-B22; A65-B23; A65-B24; A65-B25; A65-B26;
A65-B27; A65-B28; A65-B29; A65-B30; A65-B31; A65-B32;
A65-B33; A65-B34; A65-B35; A65-B36; A65-B37; A65-B38;
A65-B39; A65-B40; A65-B41; A65-B42; A65-B43; A65-B44;
A65-B45; A65-B46; A65-B47; A65-B48; A65-B49; A65-B50;
A65-B51; A65-B52; A65-B53; A65-B54; A65-B55; A65-B56;
A65-B57; A65-B58; A65-B59; A65-B60; A65-B61; A65-B62;
A65-B63; A65-B64; A65-B65; A65-B66; A65-B67; A65-B68;
A65-B69; A65-B70; A65-B71; A65-B72; A65-B73; A65-B74;
A65-B75; A65-B76; A65-B77; A65-B78; A65-B79; A65-B80;
A65-B81; A65-B82; A65-B83; A65-B84; A65-B85; A65-B86;
A65-B87; A65-B88; A65-B89; A65-B90; A65-B91; A65-B92;
A65-B93; A65-B94; A65-B95; A65-B96; A65-B97; A65-B98;
A65-B99; A65-B 100; A65-B 101; A65-B 102; A65-B 103; A65-B 104;
A65-B 105; A65-B 106; A65-B107; A65-B 108; A65-B 109; A65-B 110;
A65-B 111; A65-B 112; A65-B 113; A65-B 114; A65-B 115; A65-B 116;
A65-B 117; A65-B 118; A65-B 119; A65-B120; A65-B 121; A65-B 122;
A65-B 123; A65-B124; A65-B 125; A65-B 126; A65-B127; A65-B 128;
A65-B129; A65-B130; A65-B131; A65-B132; A65-B133; A65-B 134;
A65-B 135; A65-B 136; A65-B 137; A65-B 138; A65-B139; A65-B140;
A65-B 141; A65-B 142; A65-B 143; A65-B 144; A65-B 145; A65-B146;
A65-B147; A65-B148; A65-B149; A65-B150; A65-B151; A65-B152;
A65-B153; A65-B 154; A65-B 155; A65-B156; A65-B 157; A65-B158;
A65-B159; A65-B160; A65-B161; A65-B162; A65-B163; A65-B164;
A65-B 165; A65-B 166; A65-B 167; A65-B 168; A65-B169; A66-B 1;
A66-B2; A66-B3; A66-B4; A66-B5; A66-B6; A66-B7;
A66-B8; A66-B9; A66-B10; A66-B11; A66-B12; A66-B13;
A66-B 14; A66-B 15; A66-B 16; A66-B 17; A66-B18; A66-B19;
A66-B20; A66-B21; A66-B22; A66-B23; A66-B24; A66-B25;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-163-
A66-B26; A66-B27; A66-B28; A66-B29; A66-B30; A66-B31;
A66-B32; A66-B33; A66-B34; A66-B35; A66-B36; A66-B37;
A66-B38; A66-B39; A66-B40; A66-B41; A66-B42; A66-B43;
A66-B44; A66-B45; A66-B46; A66-B47; A66-B48; A66-B49;
A66-B50; A66-B51; A66-B52; A66-B53; A66-B54; A66-B55;
A66-B56; A66-B57; A66-B58; A66-B59; A66-B60; A66-B61;
A66-B62; A66-B63; A66-B64; A66-B65; A66-B66; A66-B67;
A66-B68; A66-B69; A66-B70; A66-B71; A66-B72; A66-B73;
A66-B74; A66-B75; A66-B76; A66-B77; A66-B78; A66-B79;
A66-B80; A66-B81; A66-B82; A66-B83; A66-B84; A66-B85;
A66-B86; A66-B87; A66-B88; A66-B89; A66-B90; A66-B91;
A66-B92; A66-B93; A66-B94; A66-B95; A66-B96; A66-B97;
A66-B98; A66-B99; A66-B 100; A66-B 101; A66-B 102; A66-B 103;
A66-B 104; A66-B 105; A66-B 106; A66-B 107; A66-B 108; A66-B 109;
A66-B 110; A66-B 111; A66-B 112; A66-B 113; A66-B 114; A66-B 115;
A66-B 116; A66-B 117; A66-B118; A66-B 119; A66-B 120; A66-B121;
A66-B 122; A66-B 123; A66-B 124; A66-B 125; A66-B 126; A66-B 127;
A66-B 128; A66-B 129; A66-B 130; A66-B 131; A66-B 132; A66-B 133;
A66-B 134; A66-B 135; A66-B 136; A66-B 137; A66-B 13 8; A66-B 139;
A66-B 140; A66-B 141; A66-B 142; A66-B 143; A66-B 144; A66-B145;
A66-B 146; A66-B 147; A66-B 148; A66-B 149; A66-B 150; A66-B 151;
A66-B 152; A66-B153; A66-B 154; A66-B155; A66-B 156; A66-B 157;
A66-B 15 8; A66-B159; A66-B 160; A66-B 161; A66-B 162; A66-B 163;
A66-B 164; A66-B 165; A66-B 166; A66-B 167; A66-B168; A66-B 169;
A67-B 1; A67-B2; A67-B3; A67-B4; A67-B5; A67-B6;
A67-B7; A67-B8; A67-B9; A67-B10; A67-B11; A67-B12;
A67-B 13; A67-B 14; A67-B 15; A67-B 16; A67-B 17; A67-B 18;
A67-B19; A67-B20; A67-B21; A67-B22; A67-B23; A67-B24;
A67-B25; A67-B26; A67-B27; A67-B28; A67-B29; A67-B30;
A67-B31; A67-B32; A67-B33; A67-B34; A67-B35; A67-B36;
A67-B37; A67-B38; A67-B39; A67-B40; A67-B41; A67-B42;
A67-B43; A67-B44; A67-B45; A67-B46; A67-B47; A67-B48;
A67-B49; A67-B50; A67-B51; A67-B52; A67-B53; A67-B54;
A67-B55; A67-B56; A67-B57; A67-B58; A67-B59; A67-B60;
A67-B61; A67-B62; A67-B63; A67-B64; A67-B65; A67-B66;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-164-
A67-B67; A67-B68; A67-B69; A67-B70; A67-B71; A67-B72;
A67-B73; A67-B74; A67-B75; A67-B76; A67-B77; A67-B78;
A67-B79; A67-B80; A67-B81; A67-B82; A67-B83; A67-B84;
A67-B85; A67-B86; A67-B87; A67-B88; A67-B89; A67-B90;
A67-B91; A67-B92; A67-B93; A67-B94; A67-B95; A67-B96;
A67-B97; A67-B98; A67-B99; A67-B 100; A67-B 101; A67-B 102;
A67-B 103; A67-B104; A67-B 105; A67-B 106; A67-B 107; A67-B108;
A67-B 109; A67-B 110; A67-B 111; A67-B 112; A67-B 113; A67-B 114;
A67-B 115; A67-B 116; A67-B 117; A67-B 118; A67-B 119; A67-B 120;
A67-B 121; A67-B 122; A67-B 123; A67-B 124; A67-B 125; A67-B 126;
A67-B 127; A67-B 128; A67-B 129; A67-B 130; A67-B 131; A67-B132;
A67-B 133; A67-B 134; A67-B 135; A67-B 136; A67-B137; A67-B 138;
A67-B 139; A67-B 140; A67-B 141; A67-B 142; A67-B 143; A67-B 144;
A67-B 145; A67-B 146; A67-B 147; A67-B 148; A67-B 149; A67-B150;
A67-B151; A67-B152; A67-B153; A67-B154; A67-B155; A67-B156;
A67-B157; A67-B 158; A67-B 159; A67-B 160; A67-B 161; A67-B 162;
A67-B 163; A67-B 164; A67-B 165; A67-B 166; A67-B 167; A67-B 168;
A67-B169; A68-B1; A68-B2; A68-B3; A68-B4; A68-B5;
A68-B6; A68-B7; A68-B8; A68-B9; A68-B10; A68-B11;
A68-B 12; A68-B 13; A68-B 14; A68-B 15; A68-B 16; A68-B 17;
A68-B18; A68-B 19; A68-B20; A68-B21; A68-B22; A68-B23;
A68-B24; A68-B25; A68-B26; A68-B27; A68-B28; A68-B29;
A68-B30; A68-B31; A68-B32; A68-B33; A68-B34; A68-B35;
A68-B36; A68-B37; A68-B38; A68-B39; A68-B40; A68-B41;
A68-B42; A68-B43; A68-B44; A68-B45; A68-B46; A68-B47;
A68-B48; A68-B49; A68-B50; A68-B51; A68-B52; A68-B53;
A68-B54; A68-B55; A68-B56; A68-B57; A68-B58; A68-B59;
A68-B60; A68-B61; A68-B62; A68-B63; A68-B64; A68-B65;
A68-B66; A68-B67; A68-B68; A68-B69; A68-B70; A68-B71;
A68-B72; A68-B73; A68-B74; A68-B75; A68-B76; A68-B77;
A68-B78; A68-B79; A68-B80; A68-B81; A68-B82; A68-B83;
A68-B84; A68-B85; A68-B86; A68-B87; A68-B88; A68-B89;
A68-B90; A68-B91; A68-B92; A68-B93; A68-B94; A68-B95;
A68-B96; A68-B97; A68-B98; A68-B99; A68-B100; A68-B101;
A68-B 102; A68-B 103; A68-B 104; A68-B 105; A68-B 106; A68-B 107;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-165-
A68-B 108; A68-B 109; A68-B 110; A68-B 111; A68-B112; A68-B 113;
A68-B 114; A68-B 115; A68-B 116; A68-B 117; A68-B118; A68-B 119;
A68-B 120; A68-B 121; A68-B 122; A68-B 123; A68-B 124; A68-B 125;
A68-B 126; A68-B 127; A68-B 128; A68-B 129; A68-B 130; A68-B 131;
A68-B132; A68-B133; A68-B 134; A68-B 135; A68-B136; A68-B 137;
A68-B 138; A68-B 139; A68-B140; A68-B 141; A68-B142; A68-B 143;
A68-B 144; A68-B 145; A68-B146; A68-B 147; A68-B148; A68-B 149;
A68-B 150; A68-B 151; A68-B 152; A68-B 153; A68-B154; A68-B 155;
A68-B156; A68-B157; A68-B 158; A68-B 159; A68-B 160; A68-B 161;
A68-B 162; A68-B 163; A68-B 164; A68-B165; A68-B166; A68-B 167;
A68-B 168; A68-B 169; A69-B 1; A69-B2; A69-B3; A69-B4;
A69-B5; A69-B6; A69-B7; A69-B8; A69-B9; A69-B10;
A69-B 11; A69-B 12; A69-B 13; A69-B 14; A69-B 15; A69-B 16;
A69-B 17; A69-B 18; A69-B 19; A69-B20; A69-B21; A69-B22;
A69-B23; A69-B24; A69-B25; A69-B26; A69-B27; A69-B28;
A69-B29; A69-B30; A69-B31; A69-B32; A69-B33; A69-B34;
A69-B35; A69-B36; A69-B37; A69-B38; A69-B39; A69-B40;
A69-B41; A69-B42; A69-B43; A69-B44; A69-B45; A69-B46;
A69-B47; A69-B48; A69-B49; A69-B50; A69-B51; A69-B52;
A69-B53; A69-B54; A69-B55; A69-B56; A69-B57; A69-B58;
A69-B59; A69-B60; A69-B61; A69-B62; A69-B63; A69-B64;
A69-B65; A69-B66; A69-B67; A69-B68; A69-B69; A69-B70;
A69-B71; A69-B72; A69-B73; A69-B74; A69-B75; A69-B76;
A69-B77; A69-B78; A69-B79; A69-B80; A69-B81; A69-B82;
A69-B83; A69-B84; A69-B85; A69-B86; A69-B87; A69-B88;
A69-B89; A69-B90; A69-B91; A69-B92; A69-B93; A69-B94;
A69-B95; A69-B96; A69-B97; A69-B98; A69-B99; A69-B 100;
A69-B 101; A69-B 102; A69-B 103; A69-B104; A69-B 105; A69-B 106;
A69-B107; A69-B 108; A69-B 109; A69-B 110; A69-B 111; A69-B 112;
A69-B 113; A69-B 114; A69-B 115; A69-B 116; A69-B 117; A69-B 118;
A69-B 119; A69-B 120; A69-B 121; A69-B 122; A69-B 123; A69-B 124;
A69-B 125; A69-B 126; A69-B 127; A69-B 128; A69-B 129; A69-B130;
A69-B 13 1; A69-B 132; A69-B133; A69-B 134; A69-B 135; A69-B 136;
A69-B 137; A69-B 138; A69-B 139; A69-B 140; A69-B 141; A69-B 142;
A69-B 143; A69-B 144; A69-B 145; A69-B 146; A69-B 147; A69-B 148;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-166-
A69-B 149; A69-B 150; A69-B 151; A69-B 152; A69-B 153; A69-B 154;
A69-B 155; A69-B 156; A69-B 157; A69-B 158; A69-B 159; A69-B 160;
A69-B 161; A69-B 162; A69-B 163; A69-B 164; A69-B 165; A69-B 166;
A69-B 167; A69-B168; A69-B 169; A70-B 1; A70-B2; A70-B3;
A70-B4; A70-B5; A70-B6; A70-B7; A70-B8; A70-B9;
A70-B 10; A70-B 11; A70-B 12; A70-B 13; A70-B 14; A70-B 15;
A70-B 16; A70-B 17; A70-B 18; A70-B 19; A70-B20; A70-B21;
A70-B22; A70-B23; A70-B24; A70-B25; A70-B26; A70-B27;
A70-B28; A70-B29; A70-B30; A70-B31; A70-B32; A70-B33;
A70-B34; A70-B35; A70-B36; A70-B37; A70-B38; A70-B39;
A70-B40; A70-B41; A70-B42; A70-B43; A70-B44; A70-B45;
A70-B46; A70-B47;, A70-B48; A70-B49; A70-B50; A70-B51;
A70-B52; A70-B53; A70-B54; A70-B55; A70-B56; A70-B57;
A70-B58; A70-B59; A70-B60; A70-B61; A70-B62; A70-B63;
A70-B64; A70-B65; A70-B66; A70-B67; A70-B68; A70-B69;
A70-B70; A70-B71; A70-B72; A70-B73; A70-B74; A70-B75;
A70-B76; A70-B77; A70-B78; A70-B79; A70-B80; A70-B81;
A70-B82; A70-B83; A70-B84; A70-B85; A70-B86; A70-B87;
A70-B88; A70-B89; A70-B90; A70-B91; A70-B92; A70-B93;
A70-B94; A70-B95; A70-B96; A70-B97; A70-B98; A70-B99;
A70-B 100; A70-B 101; A70-B 102; A70-B 103; A70-B 104; A70-B 105;
A70-B 106; A70-B 107; A70-B 108; A70-B 109; A70-B 110; A70-B 111;
A70-B 112; A70-B 113; A70-B 114; A70-B 115; A70-B 116; A70-B 117;
A70-B 118; A70-B 119; A70-B120; A70-B 121; A70-B 122; A70-B 123;
A70-B 124; A70-B 125; A70-B126; A70-B 127; A70-B128; A70-B 129;
A70-B130; A70-B131; A70-B132; A70-B133; A70-B134; A70-B135;
A70-B 136; . A70-B137; A70-B138; A70-B 139; A70-B 140; A70-B141;
A70-B 142; A70-B 143; A70-B144; A70-B 145; A70-B146; A70-B 147;
A70-B 148; A70-B149; A70-B150; A70-B 151; A70-B 152; A70-B153;
A70-B 154; A70-B155; A70-B156; A70-B157; A70-B158; A70-B 159;
A70-B 160; A70-B 161; A70-B 162; A70-B 163; A70-B 164; A70-B 165;
A70-B 166; A70-B 167; A70-B168; A70-B 169; A71-B 1; A71-B2;
A71-B3; A71-B4; A71-B5; A71-B6; A71-B7; A71-B8;
A71-B9; A71-B10; A71-B11; A71-B12; A71-B13; A71-B14;
A71-B15; A71-B16; A71-B17; A71-B18; A71-B19; A71-B20;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-167-
A71-B21; A71-B22; A71-B23; A71-B24; A71-B25; A71-B26;
A71-B27; A71-B28; A71-B29; A71-B30; A71-B31; A71-B32;
A71-B33; A71-B34; A71-B35; A71-B36; A71-B37; A71-B38;
A71-B39; A71-B40; A71-B41; A71-B42; A71-B43; A71-B44;
A71-B45; A71-B46; A71-B47; A71-B48; A71-B49; A71-B50;
A71-B51; A71-B52; A71-B53; A71-B54; A71-B55; A71-B56;
A71-B57; A71-B58; A71-B59; A71-B60; A71-B61; A71-B62;
A71-B63; A71-B64; A71-B65; A71-B66; A71-B67; A71-B68;
A71-B69; A71-B70; A71-B71; A71-B72; A71-B73; A71-B74;
A71-B75; A71-B76; A71-B77; A71-B78; A71-B79; A71-B80;
A71-B81; A71-B82; A71-B83; A71-B84; A71-B85; A71-B86;
A71-B87; A71-B88; A71-B89; A71-B90; A71-B91; A71-B92;
A71-B93; A71-B94; A71-B95; A71-B96; A71-B97; A71-B98;
A71-B99; A71-B100; A71-B101; A71-B102; A71-B103; A71-B104;
A71-B105; A71-B106; A71-B107; A71-B108; A71-B109; A71-B110;
A71-B111; A71-B112; A71-B113; A71-B114; A71-B115; A71-B116;
A71-B117; A71-B118; A71-B119; A71-B120; A71-B121; A71-B122;
A71-B123; A71-B124; A71-B125; A71-B126; A71-B127; A71-B128;
A71-B129; A71-B130; A71-B131; A71-B132; A71-B133; A71-B134;
A71-B135; A71-B136; A71-B137; A71-B138; A71-B139; A71-B140;
A71-B141; A71-B142; A71-B143; A71-B144; A71-B145; A71-B146;
A71-B147; A71-B148; A71-B149; A71-B150; A71-B151; A71-B152;
A71-B153; A71-B154; A71-B155; A71-B156; A71-B157; A71-B158;
A71-B159; A71-B160; A71-B161; A71-B162; A71-B163; A71-B164;
A71-B165; A71-B166; A71-B167; A71-B168; A71-B169; A72-B1;
A72-B2; A72-B3; A72-B4; A72-B5; A72-B6; A72-B7;
A72-B8; A72-B9; A72-B 10; A72-B 11; A72-B12; A72-B 13;
A72-B 14; A72-B 15; A72-B 16; A72-B 17; A72-B 18; A72-B 19;
A72-B20; A72-B21; A72-B22; A72-B23; A72-B24; A72-B25;
A72-B26; A72-B27; A72-B28; A72-B29; A72-B30; A72-B31;
A72-B32; A72-B33; A72-B34; A72-B35; A72-B36; A72-B37;
A72-B38; A72-B39; A72-B40; A72-B41; A72-B42; A72-B43;
A72-B44; A72-B45; A72-B46; A72-B47; A72-B48; A72-B49;
A72-B50; A72-B51; A72-B52; A72-B53; A72-B54; A72-B55;
A72-B56; A72-B57; A72-B58; A72-B59; A72-B60; A72-B61;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-168-
A72-B62; A72-B63; A72-B64; A72-B65; A72-B66; A72-B67;
A72-B68; A72-B69; A72-B70; A72-B71; A72-B72; A72-B73;
A72-B74; A72-B75; A72-B76; A72-B77; A72-B78; A72-B79;
A72-B80; A72-B81; A72-B82; A72-B83; A72-B84; A72-B85;
A72-B86; A72-B87; A72-B88; A72-B89; A72-B90; A72-B91;
A72-B92; A72-B93; A72-B94; A72-B95; A72-B96; A72-B97;
A72-B98; A72-B99; A72-B 100; A72-B 101; A72-B 102; A72-B 103;
A72-B 104; A72-B 105; A72-B 106; A72-B 107; A72-B108; A72-B 109;
A72-B 110; A72-B 111; A72-B 112; A72-B 113; A72-B 114; A72-B 115;
A72-B 116; A72-B 117; A72-B 118; A72-B 119; A72-B 120; A72-B 121;
A72-B 122; A72-B 123; A72-B 124; A72-B 125; A72-B 126; A72-B 127;
A72-B128; A72-B 129; A72-B 130; A72-B 131; A72-B 132; A72-B133;
A72-B 134; A72-B 135; A72-B 136; A72-B 137; A72-B 138; A72-B 139;
A72-B 140; A72-B 141; A72-B 142; A72-B 143; A72-B 144; A72-B 145;
A72-B 146; A72-B 147; A72-B 148; A72-B 149; A72-B150; A72-B 151;
A72-B 152; A72-B 153; A72-B 154; A72-B 155; A72-B 156; A72-B157;
A72-B158; A72-B 159; A72-B 160; A72-B 161; A72-B 162; A72-B 163;
A72-B 164; A72-B 165; A72-B 166; A72-B 167; A72-B 168; A72-B 169;
A73-B1; A73-B2; A73-B3; A73-B4; A73-B5; A73-B6;
A73-B7; A73-B8; A73-B9; A73-B10; A73-B11; A73-B12;
A73-B13; A73-B 14; A73-B 15; A73-B 16; A73-B 17; A73-B 18;
A73-B19; A73-B20; A73-B21; A73-B22; A73-B23; A73-B24;
A73-B25; A73-B26; A73-B27; A73-B28; A73-B29; A73-B30;
A73-B31; A73-B32; A73-B33; A73-B34; A73-B35; A73-B36;
A73-B37; A73-B38; A73-B39; A73-B40; A73-B41; A73-B42;
A73-B43; A73-B44; A73-B45; A73-B46; A73-B47; A73-B48;
A73-B49; A73-B50; A73-B51; A73-B52; A73-B53; A73-B54;
A73-B55; A73-B56; A73-B57; A73-B58; A73-B59; A73-B60;
A73-B61; A73-B62; A73-B63; A73-B64; A73-B65; A73-B66;
A73-B67; A73-B68; A73-B69; A73-B70; A73-B71; A73-B72;
A73-B73; A73-B74; A73-B75; A73-B76; A73-B77; A73-B78;
A73-B79; A73-B80; A73-B81; A73-B82; A73-B83; A73-B84;
A73-B85; A73-B86; A73-B87; A73-B88; A73-B89; A73-B90;
A73-B91; A73-B92; A73-B93; A73-B94; A73-B95; A73-B96;
A73-B97; A73-B98; A73-B99; A73-B100; A73-B101; A73-B102;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-169-
A73-B 103; A73-B104; A73-B 105; A73-B 106; A73-B 107; A73-B108;
A73-B 109; A73-B 110; A73-B 111; A73-B 112; A73-B 113; A73-B114;
A73-B 115; A73-B 116; A73-B 117; A73-B118; A73-B 119; A73-B 120;
A73-B 121; A73-B 122; A73-B123; A73-B 124; A73-B125; A73-B 126;
A73-B 127; A73-B 128; A73-B129; A73-B130; A73-B 131; A73-B132;
A73-B 133; A73-B134; A73-B135; A73-B 136; A73-B 137; A73-B 138;
A73-B139; A73-B 140; A73-B141; A73-B 142; A73-B 143; A73-B 144;
A73-B 145; A73-B 146; A73-B 147; A73-B 148; A73-B 149; A73-B 150;
A73-B151; A73-B152; A73-B153; A73-B154; A73-B155; A73-B156;
A73-B157; A73-B158; A73-B159; A73-B160; A73-B161; A73-B162;
A73-B 163; A73-B 164; A73-B 165; A73-B 166; A73-B 167; A73-B 168;
A73-B169; A74-B1; A74-B2; A74-B3; A74-B4; A74-B5;
A74-B6; A74-B7; A74-B8; A74-B9; A74-B 10; A74-B 11;
A74-B 12; A74-B 13; A74-B 14; A74-B 15; A74-B 16; A74-B 17;
A74-B 18; A74-B 19; A74-B20; A74-B21; A74-B22; A74-B23;
A74-B24; A74-B25; A74-B26; A74-B27; A74-B28; A74-B29;
A74-B30; A74-B31; A74-B32; A74-B33; A74-B34; A74-B35;
A74-B36; A74-B37; A74-B38; A74-B39; A74-B40; A74-B41;
A74-B42; A74-B43; A74-B44; A74-B45; A74-B46; A74-B47;
A74-B48; A74-B49; A74-B50; A74-B51; A74-B52; A74-B53;
A74-B54; A74-B55; A74-B56; A74-B57; A74-B58; A74-B59;
A74-B60; A74-B61; A74-B62; A74-B63; A74-B64; A74-B65;
A74-B66; A74-B67; A74-B68; A74-B69; A74-B70; A74-B71;
A74-B72; A74-B73; A74-B74; A74-B75; 'A74-B76; A74-B77;
A74-B78; A74-B79; A74-B80; A74-B81; A74-B82; A74-B83;
A74-B84; A74-B85; A74-B86; A74-B87; A74-B88; A74-B89;
A74-B90; A74-B91; A74-B92; A74-B93; A74-B94; A74-B95;
A74-B96; A74-B97; A74-B98; A74-B99; A74-B100; A74-B101;
A74-B 102; A74-B 103; A74-B 104; A74-B 105; A74-B 106; A74-B 107;
A74-B 108; A74-B109; A74-B 110; A74-B 111; A74-B 112; A74-B 113;
A74-B 114; A74-B 115; A74-B 116; A74-B 117; A74-B 118; A74-B 119;
A74-B 120; A74-B 121; A74-B 122; A74-B 123; A74-B 124; A74-B 125;
A74-B 126; A74-B 127; A74-B 128; A74-B 129; A74-B130; A74-B 131;
A74-B 132; A74-B133; A74-B 134; A74-B135; A74-B 136; A74-B137;
A74-B138; A74-B139; A74-B 140; A74-B 141; A74-B 142; A74-B143;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-170-
A74-B 144; A74-B 145; A74-B 146; A74-B 147; A74-B 148; A74-B 149;
A74-B 150; A74-B 151; A74-B 152; A74-B153; A74-B 154; A74-B 155;
A74-B 156; A74-B 157; A74-B 15 8; A74-B 159; A74-B 160; A74-B 161;
A74-B 162; A74-B 163; A74-B 164; A74-B 165; A74-B 166; A74-B 167;
A74-B 168; A74-B 169; A75-B 1; A75-B2; A75-B3; A75-B4;
A75-B5; A75-B6; A75-B7; A75-B8; A75-B9; A75-B10;
A75-B 11; A75-B 12; A75-B13; A75-B 14; A75-B 15; A75-B16;
A75-B 17; A75-B18; A75-B 19; A75-B20; A75-B21; A75-B22;
A75-B23; A75-B24; A75-B25; A75-B26; A75-B27; A75-B28;
A75-B29; A75-B30; A75-B31; A75-B32; A75-B33; A75-B34;
A75-B35; A75-B36; A75-B37; A75-B38; A75-B39; A75-B40;
A75-B41; A75-B42; A75-B43; A75-B44; A75-B45; A75-B46;
A75-B47; A75-B48; A75-B49; A75-B50; A75-B51; A75-B52;
A75-B53; A75-B54; A75-B55; A75-B56; A75-B57; A75-B58;
A75-B59; A75-B60; A75-B61; A75-B62; A75-B63; A75-B64;
A75-B65; A75-B66; A75-B67; A75-B68; A75-B69; A75-B70;
A75-B71; A75-B72; A75-B73; A75-B74; A75-B75; A75-B76;
A75-B77; A75-B78; A75-B79; A75-B80; A75-B81; A75-B82;
A75-B83; A75-B84; A75-B85; A75-B86; A75-B87; A75-B88;
A75-B89; A75-B90; A75-B91; A75-B92; A75-B93; A75-B94;
A75-B95; A75-B96; A75-B97; A75-B98; A75-B99; A75-B100;
A75-B 101; A75-B 102; A75-B 103; A75-B 104; A75-B 105; A75-B 106;
A75-B 107; A75-B 108; A75-B109; A75-B110; A75-B 111; A75-B 112;
A75-B 113; A75-B 114; A75-B115; A75-B 116; A75-B117; A75-B118;
A75-B119; A75-B120; A75-B121; A75-B122; A75-B123; A75-B124;
A75-B 125; A75-B 126; A75-B 127; A75-B 128; A75-B 129; A75-B 130;
A75-B131; A75-B132; A75-B133; A75-B134; A75-B135; A75-B136;
A75-B137; A75-B138; A75-B139; A75-B140; A75-B141; A75-B142;
A75-B 143; A75-B 144; A75-B 145; A75-B 146; A75-B 147; A75-B 148;
A75-B 149; A75-B 150; A75-B 151; A75-B152; A75-B 153; A75-B 154;
A75-B155; A75-B156; A75-B157; A75-B158; A75-B159; A75-B160;
A75-B 161; A75-B 162; A75-B163; A75-B 164; A75-B 165; A75-B 166;
A75-B167; A75-B168; A75-B169; A76-B1; A76-B2; A76-B3;
A76-B4; A76-B5; A76-B6; A76-B7; A76-B8; A76-B9;
A76-B 10; A76-B 11; A76-B12; A76-B 13; A76-B 14; A76-B 15;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-171-
A76-B 16; A76-B 17; A76-B 18; A76-B 19; A76-B20; A76-B21;
A76-B22; A76-B23; A76-B24; A76-B25; A76-B26; A76-B27;
A76-B28; A76-B29; A76-B30; A76-B31; A76-B32; A76-B33;
A76-B34; A76-B35; A76-B36; A76-B37; A76-B38; A76-B39;
A76-B40; A76-B41; A76-B42; A76-B43; A76-B44; A76-B45;
A76-B46; A76-B47; A76-B48; A76-B49; A76-B50; A76-B51;
A76-B52; A76-B53; A76-B54; A76-B55; A76-B56; A76-B57;
A76-B58; A76-B59; A76-B60; A76-B61; A76-B62; A76-B63;
A76-B64; A76-B65; A76-B66; A76-B67; A76-B68; A76-B69;
A76-B70; A76-B71; A76-B72; A76-B73; A76-B74; A76-B75;
A76-B76; A76-B77; A76-B78; A76-B79; A76-B80; A76-B81;
A76-B82; A76-B83; A76-B84; A76-B85; A76-B86; A76-B87;
A76-B88; A76-B89; A76-B90; A76-B91; A76-B92; A76-B93;
A76-B94; A76-B95; A76-B96; A76-B97; A76-B98; A76-B99;
A76-B 100; A76-B 101; A76-B 102; A76-B 103; A76-B 104; A76-B 105;
A76-B106; A76-B107; A76-B 108; A76-B109; A76-B 110; A76-B 111;
A76-B 112; A76-B 113; A76-B 114; A76-B 115; A76-B 116; A76-B 117;
A76-B 118; A76-B 119; A76-B 120; A76-B 121; A76-B 122; A76-B 123;
A76-B 124; A76-B 125; A76-B126; A76-B 127; A76-B128; A76-B 129;
A76-B 130; A76-B 131; A76-B 132; A76-B 133; A76-B 134; A76-B 135;
A76-B 136; A76-B 137; A76-B 138; A76-B 139; A76-B140; A76-B 141;
A76-B142; A76-B 143; A76-B 144; A76-B 145; A76-B 146; A76-B 147;
A76-B 148; A76-B 149; A76-B 150; A76-B 151; A76-B 152; A76-B 153;
A76-B 154; A76-B 155; A76-B 156; A76-B 157; A76-B 158; A76-B 159;
A76-B 160; A76-B 161; A76-B162; A76-B 163; A76-B 164; A76-B 165;
A76-B 166; A76-B 167; A76-B 168; A76-B169; A77-B 1; A77-B2;
A77-B3; A77-B4; A77-B5; A77-B6; A77-B7; A77-B8;
A77-B9; A77-B 10; A77-B 11; A77-B 12; A77-B 13; A77-B 14;
A77-B 15; A77-B 16; A77-B17; A77-B 18; A77-B 19; A77-B20;
A77-B21; A77-B22; A77-B23; A77-B24; A77-B25; A77-B26;
A77-B27; A77-B28; A77-B29; A77-B30; A77-B31; A77-B32;
A77-B33; A77-B34; A77-B35; A77-B36; A77-B37; A77-B38;
A77-B39; A77-B40; A77-B41; A77-B42; A77-B43; A77-B44;
A77-B45; A77-B46; A77-B47; A77-B48; A77-B49; A77-B50;
A77-B51; A77-B52; A77-B53; A77-B54; A77-B55; A77-B56;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-172-
A77-B57; A77-B58; A77-B59; A77-B60; A77-B61; A77-B62;
A77-B63; A77-B64; A77-B65; A77-B66; A77-B67; A77-B68;
A77-B69; A77-B70; A77-B71; A77-B72; A77-B73; A77-B74;
A77-B75; A77-B76; A77-B77; A77-B78; A77-B79; A77-B80;
A77-B81; A77-B82; A77-B83; A77-B84; A77-B85; A77-B86;
A77-B87; A77-B88; A77-B89; A77-B90; A77-B91; A77-B92;
A77-B93; A77-B94; A77-B95; A77-B96; A77-B97; A77-B98;
A77-B99; A77-B 100; A77-B 101; A77-B 102; A77-B 103; A77-B 104;
A77-B 105; A77-B 106; A77-B 107; A77-B 108; A77-B 109; A77-B 110;
A77-B 111; A77-B 112; A77-B 113; A77-B 114; A77-B 115; A77-B 116;
A77-B 117; A77-B 118; A77-B 119; A77-B 120; A77-B 121; A77-B 122;
A77-B 123; A77-B 124; A77-B 125; A77-B 126; A77-B 127; A77-B128;
A77-B 129; A77-B 130; A77-B 131; A77-B 132; A77-B 133; A77-B 134;
A77-B 135; A77-B 136; A77-B 137; A77-B 138; A77-B 139; A77-B 140;
A77-B 141; A77-B 142; A77-B 143; A77-B 144; A77-B 145; A77-B 146;
A77-B 147; A77-B 148; A77-B 149; A77-B 150; A77-B 151; A77-B 152;
A77-B 153; A77-B 154; A77-B 155; A77-B156; A77-B 157; A77-B 158;
A77-B 159; A77-B 160; A77-B 161; A77-B 162; A77-B 163; A77-B 164;
A77-B165; A77-B 166; A77-B 167; A77-B168; A77-B 169; A78-B 1;
A78-B2; A78-B3; A78-B4; A78-B5; A78-B6; A78-B7;
A78-B8; A78-B9; A78-B10; A78-B11; A78-B12; A78-B13;
A78-B14; A78-B15; A78-B16; A78-B17; A78-B18; A78-B19;
A78-B20; A78-B21; A78-B22; A78-B23; A78-B24; A78-B25;
A78-B26; A78-B27; A78-B28; A78-B29; A78-B30; A78-B31;
A78-B32; A78-B33; A78-B34; A78-B35; A78-B36; A78-B37;
A78-B38; A78-B39; A78-B40; A78-B41; A78-B42; A78-B43;
A78-B44; A78-B45; A78-B46; A78-B47; A78-B48; A78-B49;
A78-B50; A78-B51; A78-B52; A78-B53; A78-B54; A78-B55;
A78-B56; A78-B57; A78-B58; A78-B59; A78-B60; A78-B61;
A78-B62; A78-B63; A78-B64; A78-B65; A78-B66; A78-B67;
A78-B68; A78-B69; A78-B70; A78-B71; A78-B72; A78-B73;
A78-B74; A78-B75; A78-B76; A78-B77; A78-B78; A78-B79;
A78-B80; A78-B81; A78-B82; A78-B83; A78-B84; A78-B85;
A78-B86; A78-B87; A78-B88; A78-B89; A78-B90; A78-B91;
A78-B92; A78-B93; A78-B94; A78-B95; A78-B96; A78-B97;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-173-
A78-B98; A78-B99; A78-B100; A78-B101; A78-B102; A78-B103;
A78-B 104; A78-B 105; A78-B 106; A78-B 107; A78-B 108; A78-B 109;
A78-B 110; A78-B 111; A78-B 112; A78-B 113; A78-B 114; A78-B 115;
A78-B116; A78-B 117; A78-B118; A78-B 119; A78-B120; A78-B121;
A78-B122; A78-B 123; A78-B 124; A78-B125; A78-B 126; A78-B127;
A78-B128; A78-B 129; A78-B 130; A78-B 131; A78-B 132; A78-B 133;
A78-B134; A78-B135; A78-B136; A78-B137; A78-B138; A78-B139;
A78-B 140; A78-B141; A78-B142; A78-B 143; A78-B 144; A78-B 145;
A78-B 146; A78-B 147; A78-B 148; A78-B 149; A78-B150; A78-B 151;
A78-B 152; A78-B 153; A78-B154; A78-B 155; A78-B156; A78-B 157;
A78-B 158; A78-B 159; A78-B 160; A78-B 161; A78-B 162; A78-B 163;
A78-B 164; A78-B 165; A78-B 166; A78-B 167; A78-B 168; A78-B 169;
A79-Bl; A79-B2; A79-B3; A79-B4; A79-B5; A79-B6;
A79-B7; A79-B8; A79-B9; A79-B 10; A79-B 11; A79-B 12;
A79-B 13; A79-B 14; A79-B 15; A79-B 16; A79-B 17; A79-B 18;
A79-B19; A79-B20; A79-B21; A79-B22; A79-B23; A79-B24;
A79-B25; A79-B26; A79-B27; A79-B28; A79-B29; A79-B30;
A79-B31; A79-B32; A79-B33; A79-B34; A79-B35; A79-B36;
A79-B37; A79-B38; A79-B39; A79-B40; A79-B41; A79-B42;
A79-B43; A79-B44; A79-B45; A79-B46; A79-B47; A79-B48;
A79-B49; A79-B50; A79-B51; A79-B52; A79-B53; A79-B54;
A79-B55; A79-B56; A79-B57; A79-B58; A79-B59; A79-B60;
A79-B61; A79-B62; A79-B63; A79-B64; A79-B65; A79-B66;
A79-B67; A79-B68; A79-B69; A79-B70; A79-B71; A79-B72;
A79-B73; A79-B74; A79-B75; A79-B76; A79-B77; A79-B78;
A79-B79; A79-B80; A79-B81; A79-B82; A79-B83; A79-B84;
A79-B85; A79-B86; A79-B87; A79-B88; A79-B89; A79-B90;
A79-B91; A79-B92; A79-B93; A79-B94; A79-B95; A79-B96;
A79-B97; A79-B98; A79-B99; A79-B100; A79-B101; A79-B102;
A79-B 103; A79-B104; A79-B105; A79-B106; A79-B 107; A79-B 108;
A79-B 109; A79-B 110; A79-B 111; A79-B 112; A79-B 113; A79-B 114;
A79-B 115; A79-B 116; A79-B 117; A79-B 118; A79-B 119; A79-B 120;
A79-B 121; A79-B 122; A79-B 123; A79-B 124; A79-B 125; A79-B 126;
A79-B 127; A79-B 128; A79-B 129; A79-B 130; A79-B 131; A79-B 132;
A79-B133; A79-B134; A79-B135; A79-B136; A79-B137; A79-B138;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-174-
A79-B 139; A79-B 140; A79-B 141; A79-B 142; A79-B 143; A79-B 144;
A79-B 145; A79-B 146; A79-B 147; A79-B 148; A79-B 149; A79-B150;
A79-B 151; A79-B 152; A79-B 153; A79-B 154; A79-B 155; A79-B 156;
A79-B 157; A79-B 158; A79-B 159; A79-B 160; A79-B 161; A79-B 162;
A79-B 163; A79-B 164; A79-B 165; A79-B 166; A79-B 167; A79-B 168;
A79-B169; A80-B1; A80-B2; A80-B3; A80-B4; A80-B5;
A80-B6; A80-B7; A80-B8; A80-B9; A80-B10; A80-B11;
A80-B 12; A80-B 13; A80-B 14; A80-B 15; A80-B 16; A80-B 17;
A80-B 18; A80-B 19; A80-B20; A80-B21; A80-B22; A80-B23;
A80-B24; A80-B25; A80-B26; A80-B27; A80-B28; A80-B29;
A80-B30; A80-B31; A80-B32; A80-B33; A80-B34; A80-B35;
A80-B36; A80-B37; A80-B38; A80-B39; A80-B40; A80-B41;
A80-B42; A80-B43; A80-B44; A80-B45; A80-B46; A80-B47;
A80-B48; A80-B49; A80-B50; A80-B51; A80-B52; A80-B53;
A80-B54; A80-B55; A80-B56; A80-B57; A80-B58; A80-B59;
A80-B60; A80-B61; A80-B62; A80-B63; A80-B64; A80-B65;
A80-B66; A80-B67; A80-B68; A80-B69; A80-B70; A80-B71;
A80-B72; A80-B73; A80-B74; A80-B75; A80-B76; A80-B77;
A80-B78; A80-B79; A80-B80; A80-B81; A80-B82; A80-B83;
A80-B84; A80-B85; A80-B86; A80-B87; A80-B88; A80-B89;
A80-B90; A80-B91; A80-B92; A80-B93; A80-B94; A80-B95;
A80-B96; A80-B97; A80-B98; A80-B99; A80-B 100; A80-B 101;
A80-B 102; A80-B 103; A80-B 104; A80-B 105; A80-B 106; A80-B 107;
A80-B 108; A80-B 109; A80-B 110; A80-B 11 l; A80-B 112; A80-B 113;
A80-B 114; A80-B 115; A80-B 116; A80-B 117; A80-B 118; A80-B 119;
A80-B 120; A80-B 121; A80-B 122; A80-B 123; A80-B 124; A80-B 125;
A80-B 126; A80-B 127; A80-B128; A80-B 129; A80-B130; A80-B 13 1;
A80-B 132; A80-B 133; A80-B 134; A80-B 135; A80-B 136; A80-B 137;
A80-B 138; A80-B139; A80-B 140; A80-B 141; A80-B 142; A80-B 143;
A80-B 144; A80-B 145; A80-B 146; A80-B 147; A80-B 148; A80-B 149;
A80-B 150; A80-B 151; A80-B152; A80-B 153; A80-B 154; A80-B155;
A80-B156; A80-B 157; A80-B158; A80-B 159; A80-B 160; A80-B 161;
A80-B 162; A80-B 163; A80-B 164; A80-B 165; A80-B 166; A80-B 167;
A80-B 168; A80-B 169; A81-B 1; A81-B2; A81-B3; A81-B4;
A81-B5; A81-B6; A81-B7; A81-B8; A81-B9; A81-B10;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-175-
A81-B11; A81-B12; A81-B13; A81-B14; A81-B15; A81-B16;
A81-B17; A81-B18; A81-B19; A81-B20; A81-B21; A81-B22;
A81-B23; A81-B24; A81-B25; A81-B26; A81-B27; A81-B28;
A81-B29; A81-B30; A81-B31; A81-B32; A81-B33; A81-B34;
A81-B35; A81-B36; A81-B37; A81-B38; A81-B39; A81-B40;
A81-B41; A81-B42; A81-B43; A81-B44; A81-B45; A81-B46;
A81-B47; A81-B48; A81-B49; A81-B50; A81-B51; A81-B52;
A81-B53; A81-B54; A81-B55; A81-B56; A81-B57; A81-B58;
A81-B59; A81-B60; A81-B61; A81-B62; A81-B63; A81-B64;
A81-B65; A81-B66; A81-B67; A81-B68; A81-B69; A81-B70;
A81-B71; A81-B72; A81-B73; A81-B74; A81-B75; A81-B76;
A81-B77; A81-B78; A81-B79; A81-B80; A81-B81; A81-B82;
A81-B83; A81-B84; A81-B85; A81-B86; A81-B87; A81-B88;
A81-B89; A81-B90; A81-B91; A81-B92; A81-B93; A81-B94;
A81-B95; A81-B96; A81-B97; A81-B98; A81-B99; A81-B100;
A81-B101; A81-B102; A81-B103; A81-B104; A81-B105; A81-B106;
A81-B107; A81-B108; A81-B109; A81-B110; A81-B111; A81-B112;
A81-B113; A81-B114; A81-B115; A81-B116; A81-B117; A81-B118;
A81-B119; A81-B120; A81-B121; A81-B122; A81-B123; A81-B124;
A81-B125; A81-B126; A81-B127; A81-B128; A81-B129; A81-B130;
A81-B131; A81-B132; A81-B133; A81-B134; A81-B135; A81-B136;
A81-B 137; A81-B138; A81-B 139; A81-B 140; A81-B 141; A81-B 142;
A81-B143; A81-B144; A81-B145; A81-B146; A81-B147; A81-B148;
A81-B149; A81-B150; A81-B151; A81-B152; A81-B153; A81-B154;
A81-B155; A81-B156; A81-B157; A81-B158; A81-B159; A81-B160;
A81-B161; A81-B162; A81-B163; A81-B164; A81-B165; A81-B166;
A81-B167; A81-B168; A81-B169; A82-B1; A82-B2; A82-B3;
A82-B4; A82-B5; A82-B6; A82-B7; A82-B8; A82-B9;
A82-B 10; A82-B 11; A82-B12; A82-B 13; A82-B 14; A82-B15;
A82-B 16; A82-B 17; A82-B18; A82-B 19; A82-B20; A82-B21;
A82-B22; A82-B23; A82-B24; A82-B25; A82-B26; A82-B27;
A82-B28; A82-B29; A82-B30; A82-B31; A82-B32; A82-B33;
A82-B34; A82-B35; A82-B36; A82-B37; A82-B38; A82-B39;
A82-B40; A82-B41; A82-B42; A82-B43; A82-B44; A82-B45;
A82-B46; A82-B47; A82-B48; A82-B49; A82-B50; A82-B51;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-176-
A82-B52; A82-B53; A82-B54; A82-B55; A82-B56; A82-B57;
A82-B58; A82-B59; A82-B60; A82-B61; A82-B62; A82-B63;
A82-B64; A82-B65; A82-B66; A82-B67; A82-B68; A82-B69;
A82-B70; A82-B71; A82-B72; A82-B73; A82-B74; A82-B75;
A82-B76; A82-B77; A82-B78; A82-B79; A82-B80; A82-B81;
A82-B82; A82-B83; A82-B84; A82-B85; A82-B86; A82-B87;
A82-B88; A82-B89; A82-B90; A82-B91; A82-B92; A82-B93;
A82-B94; A82-B95; A82-B96; A82-B97; A82-B98; A82-B99;
A82-B 100; A82-B 101; A82-B 102; A82-B 103; A82-B 104; A82-B 105;
A82-B 106; A82-B 107; A82-B 108; A82-B 109; A82-B 110; A82-B 111;
A82-B 112; A82-B 113; A82-B 114; A82-B 115; A82-B 116; A82-B 117;
A82-B 118; A82-B 119; A82-B 120; A82-B 121; A82-B 122; A82-B 123;
A82-B124; A82-B 125; A82-B 126; A82-B 127; A82-B 128; A82-B129;
A82-B 130; A82-B 13 1; A82-B 132; A82-B 133; A82-B 134; A82-B 135;
A82-B 136; A82-B 137; A82-B 13 8; A82-B139; A82-B 140; A82-B 141;
A82-B 142; A82-B 143; A82-B 144; A82-B 145; A82-B 146; A82-B 147;
A82-B 148; A82-B149; A82-B 150; A82-B 151; A82-B 152; A82-B 153;
A82-B 154; A82-B 155; A82-B 156; A82-B 157; A82-B 158; A82-B 159;
A82-B 160; A82-B 161; A82-B 162; A82-B 163; A82-B 164; A82-B 165;
A82-B 166; A82-B 167; A82-B 168; A82-B 169; A83-B 1; A83-B2;
A83-B3; A83-B4; A83-B5; A83-B6; A83-B7; A83-B8;
A83-B9; A83-B 10; A83-B 11; A83-B 12; A83-B 13; A83-B 14;
A83-B 15; A83-B 16; A83-B 17; A83-B 18; A83-B19; A83-B20;
A83-B21; A83-B22; A83-B23; A83-B24; A83-B25; A83-B26;
A83-B27; A83-B28; A83-B29; A83-B30; A83-B31; A83-B32;
A83-B33; A83-B34; A83-B35; A83-B36; A83-B37; A83-B38;
A83-B39; A83-B40; A83-B41; A83-B42; A83-B43; A83-B44;
A83-B45; A83-B46; A83-B47; A83-B48; A83-B49; A83-B50;
A83-B51; A83-B52; A83-B53; A83-B54; A83-B55; A83-B56;
A83-B57; A83-B58; A83-B59; A83-B60; A83-B61; A83-B62;
A83-B63; A83-B64; A83-B65; A83-B66; A83-B67; A83-B68;
A83-B69; A83-B70; A83-B71; A83-B72; A83-B73; A83-B74;
A83-B75; A83-B76; A83-B77; A83-B78; A83-B79; A83-B80;
A83-B81; A83-B82; A83-B83; A83-B84; A83-B85; A83-B86;
A83-B87; A83-B88; A83-B89; A83-B90; A83-B91; A83-B92;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-177-
A83-B93; A83-B94; A83-B95; A83-B96; A83-B97; A83-B98;
A83-B99; A83-B100; A83-B101; A83-B102; A83-B103; A83-B104;
A83-B 105; A83-B 106; A83-B 107; A83-B 108; A83-B 109; A83-B 110;
A83-B 111; A83-B 112; A83-B 113; A83-B 114; A83-B115; A83-B 116;
A83-B 117; A83-B 118; A83-B 119; A83-B 120; A83-B 121; A83-B 122;
A83-B 123; A83-B 124; A83-B 125; A83-B 126; A83-B 127; A83-B 128;
A83-B 129; A83-B 130; A83-B 131; A83-B 132; A83-B 133; A83-B 134;
A83-B135; A83-B136; A83-B137; A83-B138; A83-B139; A83-B140;
A83-B141; A83-B 142; A83-B 143; A83-B 144; A83-B145; A83-B 146;
A83-B 147; A83-B148; A83-B 149; A83-B 150; A83-B 151; A83-B 152;
A83-B153; A83-B154; A83-B155; A83-B156; A83-B157; A83-B158;
A83-B 159; A83-B 160; A83-B161; A83-B 162; A83-B 163; A83-B 164;
A83-B 165; A83-B 166; A83-B167; A83-B 168; A83-B169; A84-B 1;
A84-B2; A84-B3; A84-B4; A84-B5; A84-B6; A84-B7;
A84-B8; A84-B9; A84-B 10; A84-B 11; A84-B 12; A84-B 13;
A84-B 14; A84-B 15; A84-B 16; A84-B 17; A84-B 18; A84-B 19;
A84-B20; A84-B21; A84-B22; A84-B23; A84-B24; A84-B25;
A84-B26; A84-B27; A84-B28; A84-B29; A84-B30; A84-B31;
A84-B32; A84-B33; A84-B34; A84-B35; A84-B36; A84-B37;
A84-B38; A84-B39; A84-B40; A84-B41; A84-B42; A84-B43;
A84-B44; A84-B45; A84-B46; A84-B47; A84-B48; A84-B49;
A84-B50; A84-B51; A84-B52; A84-B53; A84-B54; A84-B55;
A84-B56; A84-B57; A84-B58; A84-B59; A84-B60; A84-B61;
A84-B62; A84-B63; A84-B64; A84-B65; A84-B66; A84-B67;
A84-B68; A84-B69; A84-B70; A84-B71; A84-B72; A84-B73;
A84-B74; A84-B75; A84-B76; A84-B77; A84-B78; A84-B79;
A84-B80; A84-B81; A84-B82; A84-B83; A84-B84; A84-B85;
A84-B86; A84-B87; A84-B88; A84-B89; A84-B90; A84-B91;
A84-B92; A84-B93; A84-B94; A84-B95; A84-B96; A84-B97;
A84-B98; A84-B99; A84-B100; A84-B101; A84-B102; A84-B103;
A84-B 104; A84-B 105; A84-B 106; A84-B 107; A84-B 108; A84-B 109;
A84-B 110; A84-B 111; A84-B 112; A84-B 113; A84-B 114; A84-B 115;
A84-B 116; A84-B 117; A84-B 118; A84-B 119; A84-B 120; A84-B 121;
A84-B 122; A84-B 123; A84-B 124; A84-B 125; A84-B 126; A84-B 127;
A84-B 128; A84-B 129; A84-B 130; A84-B 13 1; A84-B132; A84-B 133;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-178-
A84-B 134; A84-B 13 5; A84-B 136; A84-B 137; A84-B 13 8; A84-B 139;
A84-B 140; A84-B 141; A84-B 142; A84-B 143; A84-B 144; A84-B 145;
A84-B 146; A84-B 147; A84-B 148; A84-B149; A84-B 150; A84-B 151;
A84-B 152; A84-B 153; A84-B 154; A84-B 155; A84-B 156; A84-B157;
A84-B 158; A84-B 159; A84-B 160; A84-B 161; A84-B 162; A84-B 163;
A84-B 164; A84-B 165; A84-B 166; A84-B 167; A84-B 168; A84-B 169;
A85-B 1; A85-B2; A85-B3; A85-B4; A85-B5; A85-B6;
A85-B7; A85-B8; A85-B9; A85-B10; A85-B11; A85-B12;
A85-B 13; A85-B14; A85-B 15; A85-B 16; A85-B 17; A85-B 18;
A85-B19; A85-B20; A85-B21; A85-B22; A85-B23; A85-B24;
A85-B25; A85-B26; A85-B27; A85-B28; A85-B29; A85-B30;
A85-B31; A85-B32; A85-B33; A85-B34; A85-B35; A85-B36;
A85-B37; A85-B38; A85-B39; A85-B40; A85-B41; A85-B42;
A85-B43; A85-B44; A85-B45; A85-B46; A85-B47; A85-B48;
A85-B49; A85-B50; A85-B51; A85-B52; A85-B53; A85-B54;
A85-B55; A85-B56; A85-B57; A85-B58; A85-B59; A85-B60;
A85-B61; A85-B62; A85-B63; A85-B64; A85-B65; A85-B66;
A85-B67; A85-B68; A85-B69; A85-B70; A85-B71; A85-B72;
A85-B73; A85-B74; A85-B75; A85-B76; A85-B77; A85-B78;
A85-B79; A85-B80; A85-B81; A85-B82; A85-B83; A85-B84;
A85-B85; A85-B86; A85-B87; A85-B88; A85-B89; A85-B90;
A85-B91; A85-B92; A85-B93; A85-B94; A85-B95; A85-B96;
A85-B97; A85-B98; A85-B99; A85-B 100; A85-B 101; A85-B 102;
A85-B 103; A85-B 104; A85-B105; A85-B 106; A85-B 107; A85-B 108;
A85-B109; A85-B 110; A85-B 111; A85-B 112; A85-B 113; A85-B 114;
A85-B 115; A85-B 116; A85-B 117; A85-B 118; A85-B 119; A85-B 120;
A85-B121; A85-B122; A85-B123; A85-B124; A85-B125; A85-B126;
A85-B 127; A85-B 128; A85-B 129; A85-B 130; A85-B131; A85-B 132;
A85-B133; A85-B134; A85-B135; A85-B136; A85-B137; A85-B138;
A85-B139; A85-B140; A85-B141; A85-B142; A85-B143; A85-B144;
A85-B 145; A85-B 146; A85-B 147; A85-B 148; A85-B 149; A85-B 150;
A85-B 151; A85-B 152; A85-B 153; A85-B 154; A85-B 155; A85-B 156;
A85-B157; A85-B158; A85-B159; A85-B160; A85-B161; A85-B162;
A85-B163; A85-B164; A85-B165; A85-B166; A85-B167; A85-B168;
A85-B169; A86-B1; A86-B2; A86-B3; A86-B4; A86-B5;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-179-
A86-B6; A86-B7; A86-B8; A86-B9; A86-B10; A86-B11;
A86-B 12; A86-B 13; A86-B 14; A86-B 15; A86-B 16; A86-B 17;
A86-B 18; A86-B 19; A86-B20; A86-B21; A86-B22; A86-B23;
A86-B24; A86-B25; A86-B26; A86-B27; A86-B28; A86-B29;
A86-B30; A86-B31; A86-B32; A86-B33; A86-B34; A86-B35;
A86-B36; A86-B37; A86-B38; A86-B39; A86-B40; A86-B41;
A86-B42; A86-B43; A86-B44; A86-B45; A86-B46; A86-B47;
A86-B48; A86-B49; A86-B50; A86-B51; A86-B52; A86-B53;
A86-B54; A86-B55; A86-B56; A86-B57; A86-B58; A86-B59;
A86-B60; A86-B61; A86-B62; A86-B63; A86-B64; A86-B65;
A86-B66; A86-B67; A86-B68; A86-B69; A86-B70; A86-B71;
A86-B72; A86-B73; A86-B74; A86-B75; A86-B76; A86-B77;
A86-B78; A86-B79; A86-B80; A86-B81; A86-B82; A86-B83;
A86-B84; A86-B85; A86-B86; A86-B87; A86-B88; A86-B89;
A86-B90; A86-B91; A86-B92; A86-B93; A86-B94; A86-B95;
A86-B96; A86-B97; A86-B98; A86-B99; A86-B100; A86-B101;
A86-B102; A86-B 103; A86-B 104; A86-B 105; A86-B 106; A86-B 107;
A86-B108; A86-B 109; A86-B 110; A86-B 111; A86-B 112; A86-B 113;
A86-B 114; A86-B 115; A86-B 116; A86-B 117; A86-B 118; A86-B 119;
A86-B 120; A86-B 121; A86-B 122; A86-B 123; A86-B 124; A86-B 125;
A86-B 126; A86-B127; A86-B128; A86-B 129; A86-B 130; A86-B 131;
A86-B132; A86-B 133; A86-B 134; A86-B135; A86-B136; A86-B137;
A86-B138; A86-B139; A86-B140; A86-B141; A86-B142; A86-B143;
A86-B 144; A86-B 145; A86-B 146; A86-B 147; A86-B148; A86-B149;
A86-B 150; A86-B 151; A86-B 152; A86-B153; A86-B 154; A86-B155;
A86-B156; A86-B157; A86-B158; A86-B159; A86-B160; A86-B161;
A86-B162; A86-B 163; A86-B164; A86-B 165; A86-B 166; A86-B167;
A86-B 168; A86-B 169; A87-B 1; A87-B2; A87-B3; A87-B4;
A87-B5; A87-B6; A87-B7; A87-B8; A87-B9; A87-B10;
A87-B 11; A87-B 12; A87-B 13; A87-B 14; A87-B15; A87-B16;
A87-B 17; A87-B 18; A87-B 19; A87-B20; A87-B21; A87-B22;
A87-B23; A87-B24; A87-B25; A87-B26; A87-B27; A87-B28;
A87-B29; A87-B30; A87-B31; A87-B32; A87-B33; A87-B34;
A87-B35; A87-B36; A87-B37; A87-B38; A87-B39; A87-B40;
A87-B41; A87-B42; A87-B43; A87-B44; A87-B45; A87-B46;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-180-
A87-B47; A87-B48; A87-B49; A87-B50; A87-B51; A87-B52;
A87-B53; A87-B54; A87-B55; A87-B56; A87-B57; A87-B58;
A87-B59; A87-B60; A87-B61; A87-B62; A87-B63; A87-B64;
A87-B65; A87-B66; A87-B67; A87-B68; A87-B69; A87-B70;
A87-B71; A87-B72; A87-B73; A87-B74; A87-B75; A87-B76;
A87-B77; A87-B78; A87-B79; A87-B80; A87-B81; A87-B82;
A87-B83; A87-B84; A87-B85; A87-B86; A87-B87; A87-B88;
A87-B89; A87-B90; A87-B91; A87-B92; A87-B93; A87-B94;
A87-B95; A87-B96; A87-B97; A87-B98; A87-B99; A87-B 100;
A87-B 101; A87-B 102; A87-B 103; A87-B 104; A87-B 105; A87-B 106;
A87-B 107; A87-B108; A87-B 109; A87-B 110; A87-B 111; A87-B 112;
A87-B 113; A87-B 114; A87-B 115; A87-B 116; A87-B 117; A87-B 118;
A87-B 119; A87-B 120; A87-B 121; A87-B 122; A87-B 123; A87-B 124;
A87-B125; A87-B 126; A87-B 127; A87-B 128; A87-B 129; A87-B 130;
A87-B 13 1; A87-B 132; A87-B 133; A87-B 134; A87-B 135; A87-B 136;
A87-B 137; A87-B 13 8; A87-B 139; A87-B 140; A87-B 141; A87-B 142;
A87-B 143; A87-B 144; A87-B 145; A87-B 146; A87-B 147; A87-B148;
A87-B 149; A87-B 150; A87-B 151; A87-B 152; A87-B 153; A87-B 154;
A87-B155; A87-B 156; A87-B 157; A87-B 158; A87-B 159; A87-B 160;
A87-B161; A87-B 162; A87-B 163; A87-B 164; A87-B 165; A87-B 166;
A87-B 167; A87-B168; A87-B 169; A88-B 1; A88-B2; A88-B3;
A88-B4; A88-B5; A88-B6; A88-B7; A88-B8; A88-B9;
A88-B 10; A88-B 11; A88-B 12; A88-B 13; A88-B14; A88-B15;
A88-B16; A88-B17; A88-B18; A88-B19; A88-B20; A88-B21;
A88-B22; A88-B23; A88-B24; A88-B25; A88-B26; A88-B27;
A88-B28; A88-B29; A88-B30; A88-B31; A88-B32; A88-B33;
A88-B34; A88-B35; A88-B36; A88-B37; A88-B38; A88-B39;
A88-B40; A88-B41; A88-B42; A88-B43; A88-B44; A88-B45;
A88-B46; A88-B47; A88-B48; A88-B49; A88-B50; A88-B51;
A88-B52; A88-B53; A88-B54; A88-B55; A88-B56; A88-B57;
A88-B58; A88-B59; A88-B60; A88-B61; A88-B62; A88-B63;
A88-B64; A88-B65; A88-B66; A88-B67; A88-B68; A88-B69;
A88-B70; A88-B71; A88-B72; A88-B73; A88-B74; A88-B75;
A88-B76; A88-B77; A88-B78; A88-B79; A88-B80; A88-B81;
A88-B82; A88-B83; A88-B84; A88-B85; A88-B86; A88-B87;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-181-
A88-B88; A88-B89; A88-B90; A88-B91; A88-B92; A88-B93;
A88-B94; A88-B95; A88-B96; A88-B97; A88-B98; A88-B99;
A88-B 100; A88-B 101; A88-B 102; A88-B103; A88-B 104; A88-B 105;
A88-B 106; A88-B 107; A88-B 108; A88-B 109; A88-B 110; A88-B 111;
A88-B 112; A88-B 113; A88-B 114; A88-B 115; A88-B 116; A88-B117;
A88-B118; A88-B 119; A88-B 120; A88-B 121; A88-B122; A88-B123;
A88-B 124; A88-B 125; A88-B 126; A88-B 127; A88-B128; A88-B 129;
A88-B130; A88-B131; A88-B132; A88-B133; A88-B134; A88-B135;
A88-B 136; A88-B137; A88-B 138; A88-B 139; A88-B140; A88-B141;
A88-B 142; A88-B 143; A88-B 144; A88-B 145; A88-B 146; A88-B 147;
A88-B 148; A88-B 149; A88-B 150; A88-B 151; A88-B 152; A88-B153;
A88-B154; A88-B155; A88-B156; A88-B157; A88-B158; A88-B159;
A88-B 160; A88-B 161; A88-B 162; A88-B 163; A88-B 164; A88-B165;
A88-B 166; A88-B 167; A88-B 168; A88-B 169; A89-B 1; A89-B2;
A89-B3; A89-B4; A89-B5; A89-B6; A89-B7; A89-B8;
A89-B9; A89-B 10; A89-B 11; A89-B 12; A89-B 13; A89-B 14;
A89-B 15; A89-B 16; A89-B 17; A89-B 18; A89-B19; A89-B20;
A89-B21; A89-B22; A89-B23; A89-B24; A89-B25; A89-B26;
A89-B27; A89-B28; A89-B29; A89-B30; A89-B31; A89-B32;
A89-B33; A89-B34; A89-B35; A89-B36; A89-B37; A89-B38;
A89-B39; A89-B40; A89-B41; A89-B42; A89-B43; A89-B44;
A89-B45; A89-B46; A89-B47; A89-B48; A89-B49; A89-B50;
A89-B51; A89-B52; A89-B53; A89-B54; A89-B55; A89-B56;
A89-B57; A89-B58; A89-B59; A89-B60; A89-B61; A89-B62;
A89-B63; A89-B64; A89-B65; A89-B66; A89-B67; A89-B68;
A89-B69; A89-B70; A89-B71; A89-B72; A89-B73; A89-B74;
A89-B75; A89-B76; A89-B77; A89-B78; A89-B79; A89-B80;
A89-B81; A89-B82; A89-B83; A89-B84; A89-B85; A89-B86;
A89-B87; A89-B88; A89-B89; A89-B90; A89-B91; A89-B92;
A89-B93; A89-B94; A89-B95; A89-B96; A89-B97; A89-B98;
A89-B99; A89-B 100; A89-B 101; A89-B 102; A89-B 103; A89-B 104;
A89-B 105; A89-B 106; A89-B 107; A89-B 108; A89-B109; A89-B 110;
A89-B111; A89-B112; A89-B113; A89-B114; A89-B115; A89-B116;
A89-B 117; A89-B 118; A89-B 119; A89-B 120; A89-B 121; A89-B 122;
A89-B 123; A89-B124; A89-B 125; A89-B 126; A89-B127; A89-B128;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-182-
A89-B 129; A89-B 130; A89-B 131; A89-B 132; A89-B 133; A89-B 134;
A89-B 135; A89-B 136; A89-B 137; A89-B138; A89-B 139; A89-B 140;
A89-B 141; A89-B 142; A89-B 143; A89-B 144; A89-B 145; A89-B 146;
A89-B 147; A89-B 148; A89-B 149; A89-B 150; A89-B 15 1; A89-B 152;
A89-B 153; A89-B 154; A89-B 155; A89-B 156; A89-B157; A89-B 158;
A89-B 159; A89-B 160; A89-B 161; A89-B 162; A89-B 163; A89-B 164;
A89-B 165; A89-B 166; A89-B 167; A89-B168; A89-B 169; A90-B 1;
A90-B2; A90-B3; A90-B4; A90-B5; A90-B6; A90-B7;
A90-B8; A90-B9; A90-B 10; A90-B 11; A90-B 12; A90-B 13;
A90-B 14; A90-B 15; A90-B 16; A90-B 17; A90-B 18; A90-B 19;
A90-B20; A90-B21; A90-B22; A90-B23; A90-B24; A90-B25;
A90-B26; A90-B27; A90-B28; A90-B29; A90-B30; A90-B31;
A90-B32; A90-B33; A90-B34; A90-B35; A90-B36; A90-B37;
A90-B38; A90-B39; A90-B40; A90-B41; A90-B42; A90-B43;
A90-B44; A90-B45; A90-B46; A90-B47; A90-B48; A90-B49;
A90-B50; A90-B51; A90-B52; A90-B53; A90-B54; A90-B55;
A90-B56; A90-B57; A90-B58; A90-B59; A90-B60; A90-B61;
A90-B62; A90-B63; A90-B64; A90-B65; A90-B66; A90-B67;
A90-B68; A90-B69; A90-B70; A90-B71; A90-B72; A90-B73;
A90-B74; A90-B75; A90-B76; A90-B77; A90-B78; A90-B79;
A90-B80; A90-B81; A90-B82; A90-B83; A90-B84; A90-B85;
A90-B86; A90-B87; A90-B88; A90-B89; A90-B90; A90-B91;
A90-B92; A90-B93; A90-B94; A90-B95; A90-B96; A90-B97;
A90-B98; A90-B99; A90-B 100; A90-B 101; A90-B 102; A90-B 103;
A90-B 104; A90-B 105; A90-B 106; A90-B 107; A90-B108; A90-B 109;
A90-B110; A90-B 111; A90-B 112; A90-B 113; A90-B 114; A90-B 115;
A90-B 116; A90-B 117; A90-B 118; A90-B 119; A90-B 120; A90-B 121;
A90-B 122; A90-B 123; A90-B 124; A90-B 125; A90-B 126; A90-B 127;
A90-B128; A90-B129; A90-B 130; A90-B 131; A90-B 132; A90-B 133;
A90-B 134; A90-B 135; A90-B 136; A90-B 137; A90-B 138; A90-B 139;
A90-B 140; A90-B 141; A90-B 142; A90-B 143; A90-B 144; A90-B 145;
A90-B 146; A90-B 147; A90-B 148; A90-B 149; A90-B150; A90-B 151;
A90-B 152; A90-B 153; A90-B 154; A90-B 155; A90-B156; A90-B157;
A90-B 158; A90-B 159; A90-B 160; A90-B 161; A90-B 162; A90-B 163;
A90-B 164; A90-B 165; A90-B 166; A90-B 167; A90-B 168; A90-B 169;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-183-
A91-B1; A91-B2; A91-B3; A91-B4; A91-B5; A91-B6;
A91-B7; A91-B8; A91-B9; A91-B10; A91-B11; A91-B12;
A91-B13; A91-B14; A91-B15; A91-B16; A91-B17; A91-B18;
A91-B19; A91-B20; A91-B21; A91-B22; A91-B23; A91-B24;
A91-B25; A91-B26; A91-B27; A91-B28; A91-B29; A91-B30;
A91-B31; A91-B32; A91-B33; A91-B34; A91-B35; A91-B36;
A91-B37; A91-B38; A91-B39; A91-B40; A91-B41; A91-B42;
A91-B43; A91-B44; A91-B45; A91-B46; A91-B47; A91-B48;
A91-B49; A91-B50; A91-B51; A91-B52; A91-B53; A91-B54;
A91-B55; A91-B56; A91-B57; A91-B58; A91-B59; A91-B60;
A91-B61; A91-B62; A91-B63; A91-B64; A91-B65; A91-B66;
A91-B67; A91-B68; A91-B69; A91-B70; A91-B71; A91-B72;
A91-B73; A91-B74; A91-B75; A91-B76; A91-B77; A91-B78;
A91-B79; A91-B80; A91-B81; A91-B82; A91-B83; A91-B84;
A91-B85; A91-B86; A91-B87; A91-B88; A91-B89; A91-B90;
A91-B91; A91-B92; A91-B93; A91-B94; A91-B95; A91-B96;
A91-B97; A91-B98; A91-B99; A91-B100; A91-B101; A91-B102;
A91-B 103; A91-B 104; A91-B 105; A91-B 106; A91-B 107; A91-B 108;
A91-B109; A91-B110; A91-B111; A91-B112; A91-B113; A91-B114;
A91-B115; A91-B116; A91-B117; A91-B118; A91-B119; A91-B120;
A91-B121; A91-B122; A91-B123; A91-B124; A91-B125; A91-B126;
A91-B127; A91-B128; A91-B129; A91-B130; A91-B131; A91-B132;
A91-B133; A91-B134; A91-B135; A91-B136; A91-B137; A91-B138;
A91-B139; A91-B140; A91-B141; A91-B142; A91-B143; A91-B144;
A91-B145; A91-B146; A91-B147; A91-B148; A91-B149; A91-B150;
A91-B151; A91-B152; A91-B153; A91-B154; A91-B155; A91-B156;
A91-B157; A91-B158; A91-B159; A91-B160; A91-B161; A91-B162;
A91-B163; A91-B164; A91-B165; A91-B166; A91-B167; A91-B168;
A91-B169; A92-B1; A92-B2; A92-B3; A92-B4; A92-B5;
A92-B6; A92-B7; A92-B8; A92-B9; A92-B10; A92-B11;
A92-B 12; A92-B 13; A92-B 14; A92-B 15; A92-B 16; A92-B17;
A92-B 18; A92-B 19; A92-B20; A92-B21; A92-B22; A92-B23;
A92-B24; A92-B25; A92-B26; A92-B27; A92-B28; A92-B29;
A92-B30; A92-B31; A92-B32; A92-B33; A92-B34; A92-B35;
A92-B36; A92-B37; A92-B38; A92-B39; A92-B40; A92-B41;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-184-
A92-B42; A92-B43; A92-B44; A92-B45; A92-B46; A92-B47;
A92-B48; A92-B49; A92-B50; A92-B51; A92-B52; A92-B53;
A92-B54; A92-B55; A92-B56; A92-B57; A92-B58; A92-B59;
A92-B60; A92-B61; A92-B62; A92-B63; A92-B64; A92-B65;
A92-B66; A92-B67; A92-B68; A92-B69; A92-B70; A92-B71;
A92-B72; A92-B73; A92-B74; A92-B75; A92-B76; A92-B77;
A92-B78; A92-B79; A92-B80; A92-B81; A92-B82; A92-B83;
A92-B84; A92-B85; A92-B86; A92-B87; A92-B88; A92-B89;
A92-B90; A92-B91; A92-B92; A92-B93; A92-B94; A92-B95;
A92-B96; A92-B97; A92-B98; A92-B99; A92-B100; A92-B101;
A92-B 102; A92-B 103; A92-B 104; A92-B 105; A92-B 106; A92-B 107;
A92-B108; A92-B 109; A92-B 110; A92-B 111; A92-B 112; A92-B 113;
A92-B 114; A92-B 115; A92-B 116; A92-B 117; A92-B 118; A92-B 119;
A92-B 120; A92-B 121; A92-B 122; A92-B 123; A92-B 124; A92-B 125;
A92-B 126; A92-B 127; A92-B 128; A92-B 129; A92-B 130; A92-B 131;
A92-B 132; A92-B133; A92-B 134; A92-B135; A92-B 136; A92-B 137;
A92-B 13 8; A92-B 13 9; A92-B 140; A92-B 141; A92-B 142; A92-B 143;
A92-B 144; A92-B 145; A92-B 146; A92-B 147; A92-B 148; A92-B 149;
A92-B 150; A92-B 151; A92-B 152; A92-B 153; A92-B 154; A92-B 155;
A92-B 156; A92-B 157; A92-B 158; A92-B 159; A92-B 160; A92-B 161;
A92-B 162; A92-B 163; A92-B 164; A92-B165; A92-B 166; A92-B 167;
A92-B168; A92-B 169; A93-B 1; A93-B2; A93-B3; A93-B4;
A93-B5; A93-B6; A93-B7; A93-B8; A93-B9; A93-B10;
A93-B 11; A93-B12; A93-B 13; A93-B 14; A93-B15; A93-B 16;
A93-B 17; A93-B 18; A93-B 19; A93-B20; A93-B21; A93-B22;
A93-B23; A93-B24; A93-B25; A93-B26; A93-B27; A93-B28;
A93-B29; A93-B30; A93-B31; A93-B32; A93-B33; A93-B34;
A93-B35; A93-B36; A93-B37; A93-B38; A93-B39; A93-B40;
A93-B41; A93-B42; A93-B43; A93-B44; A93-B45; A93-B46;
A93-B47; A93-B48; A93-B49; A93-B50; A93-B51; A93-B52;
A93-B53; A93-B54; A93-B55; A93-B56; A93-B57; A93-B58;
A93-B59; A93-B60; A93-B61; A93-B62; A93-B63; A93-B64;
A93-B65; A93-B66; A93-B67; A93-B68; A93-B69; A93-B70;
A93-B71; A93-B72; A93-B73; A93-B74; A93-B75; A93-B76;
A93-B77; A93-B78; A93-B79; A93-B80; A93-B81; A93-B82;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-185-
A93-B83; A93-B84; A93-B85; A93-B86; A93-B87; A93-B88;
A93-B89; A93-B90; A93-B91; A93-B92; A93-B93; A93-B94;
A93-B95; A93-B96; A93-B97; A93-B98; A93-B99; A93-B100;
A93-B 101; A93-B102; A93-B 103; A93-B 104; A93-B 105; A93-B 106;
A93-B 107; A93-B 108; A93-B 109; A93-B 110; A93-B 111; A93-B 112;
A93-B 113; A93-B114; A93-B 115; A93-B 116; A93-B117; A93-B 118;
A93-B119; A93-B120; A93-B121; A93-B122; A93-B123; A93-B124;
A93-B 125; A93-B 126; A93-B 127; A93-B 128; A93-B129; A93-B 130;
A93-B 131; A93-B132; A93-B 133; A93-B134; A93-B135; A93-B 136;
A93-B137; A93-B138; A93-B139; A93-B140; A93-B141; A93-B142;
A93-B 143; A93-B 144; A93-B 145; A93-B 146; A93-B 147; A93-B 148;
A93-B 149; A93-B 150; A93-B 151; A93-B 152; A93-B 153; A93-B 154;
A93-B 155; A93-B 156; A93-B 157; A93-B158; A93-B159; A93-B 160;
A93-B 161; A93-B 162; A93-B 163; A93-B 164; A93-B 165; A93-B 166;
A93-B167; A93-B 168; A93-B169; A94-B l; A94-B2; A94-B3;
A94-B4; A94-B5; A94-B6; A94-B7; A94-B8; A94-B9;
A94-B 10; A94-B 11; A94-B 12; A94-B 13; A94-B 14; A94-B 15 ;
A94-B 16; A94-B 17; A94-B 18; A94-B 19; A94-B20; A94-B21;
A94-B22; A94-B23; A94-B24; A94-B25; A94-B26; A94-B27;
A94-B28; A94-B29; A94-B30; A94-B31; A94-B32; A94-B33;
A94-B34; A94-B35; A94-B36; A94-B37; A94-B38; A94-B39;
A94-B40; A94-B41; A94-B42; A94-B43; A94-B44; A94-B45;
A94-B46; A94-B47; A94-B48; A94-B49; A94-B50; A94-B51;
A94-B52; A94-B53; A94-B54; A94-B55; A94-B56; A94-B57;
A94-B58; A94-B59; A94-B60; A94-B61; A94-B62; A94-B63;
A94-B64; A94-B65; A94-B66; A94-B67; A94-B68; A94-B69;
A94-B70; A94-B71; A94-B72; A94-B73; A94-B74; A94-B75;
A94-B76; A94-B77; A94-B78; A94-B79; A94-B80; A94-B81;
A94-B82; A94-B83; A94-B84; A94-B85; A94-B86; A94-B87;
A94-B88; A94-B89; A94-B90; A94-B91; A94-B92; A94-B93;
A94-B94; A94-B95; A94-B96; A94-B97; A94-B98; A94-B99;
A94-B 100; A94-B 101; A94-B 102; A94-B103; A94-B 104; A94-B 105;
A94-B 106; A94-B 107; A94-B 108; A94-B 109; A94-B 110; A94-B 111;
A94-B 112; A94-B 113; A94-B 114; A94-B 115; A94-B 116; A94-B 117;
A94-B 118; A94-B 119; A94-B 120; A94-B 121; A94-B 122; A94-B 123;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-186-
A94-B 124; A94-B 125; A94-B 126; A94-B 127; A94-B 128; A94-B 129;
A94-B130; A94-B 131; A94-B 132; A94-B 133; A94-B 134; A94-B 135;
A94-B 13 6; A94-B 137; A94-B 13 8; A94-B 13 9; A94-B 140; A94-B 141;
A94-B 142; A94-B 143; A94-B 144; A94-B 145; A94-B 146; A94-B 147;
A94-B 148; A94-B 149; A94-B 150; A94-B 15 1; A94-B 152; A94-B153;
A94-B 154; A94-B 155; A94-B156; A94-B 157; A94-B 158; A94-B 159;
A94-B 160; A94-B 161; A94-B 162; A94-B 163; A94-B 164; A94-B 165;
A94-B 166; A94-B 167; A94-B168; A94-B169; A95-B 1; A95-B2;
A95-B3; A95-B4; A95-B5; A95-B6; A95-B7; A95-B8;
A95-B9; A95-B 10; A95-B 11; A95-B 12; A95-B 13; A95-B14;
A95-B15; A95-B 16; A95-B 17; A95-B 18; A95-B 19; A95-B20;
A95-B21; A95-B22; A95-B23; A95-B24; A95-B25; A95-B26;
A95-B27; A95-B28; A95-B29; A95-B30; A95-B31; A95-B32;
A95-B33; A95-B34; A95-B35; A95-B36; A95-B37; A95-B38;
A95-B39; A95-B40; A95-B41; A95-B42; A95-B43; A95-B44;
A95-B45; A95-B46; A95-B47; A95-B48; A95-B49; A95-B50;
A95-B51; A95-B52; A95-B53; A95-B54; A95-B55; A95-B56;
A95-B57; A95-B58; A95-B59; A95-B60; A95-B61; A95-B62;
A95-B63; A95-B64; A95-B65; A95-B66; A95-B67; A95-B68;
A95-B69; A95-B70; A95-B71; A95-B72; A95-B73; A95-B74;
A95-B75; A95-B76; A95-B77; A95-B78; A95-B79; A95-B80;
A95-B81; A95-B82; A95-B83; A95-B84; A95-B85; A95-B86;
A95-B87; A95-B88; A95-B89; A95-B90; A95-B91; A95-B92;
A95-B93; A95-B94; A95-B95; A95-B96; A95-B97; A95-B98;
A95-B99; A95-B 100; A95-B 101; A95-B 102; A95-B103; A95-B 104;
A95-B 105; A95-B106; A95-B107; A95-B108; A95-B 109; A95-B 110;
A95-B 111; A95-B112; A95-B113; A95-B114; A95-B 115; A95-B 116;
A95-B 117; A95-B 118; A95-B 119; A95-B 120; A95-B 121; A95-B122;
A95-B123; A95-B124; A95-B125; A95-B126; A95-B127; A95-B128;
A95-B 129; A95-B130; A95-B 131; A95-B 132; A95-B 133; A95-B 134;
A95-B135; A95-B136; A95-B137; A95-B138; A95-B139; A95-B140;
A95-B141; A95-B 142; A95-B 143; A95-B144; A95-B 145; A95-B 146;
A95-B147; A95-B148; A95-B149; A95-B150; A95-B151; A95-B152;
A95-B153; A95-B 154; A95-B155; A95-B 156; A95-B157; A95-B158;
A95-B159; A95-B160; A95-B161; A95-B162; A95-B163; A95-B164;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-187-
A95-B 165; A95-B 166; A95-B 167; A95-B 168; A95-B 169; A96-B 1;
A96-B2; A96-B3; A96-B4; A96-B5; A96-B6; A96-B7;
A96-B8; A96-B9; A96-B 10; A96-B 11; A96-B 12; A96-B 13;
A96-B 14; A96-B 15; A96-B 16; A96-B 17; A96-B 18; A96-B 19;
A96-B20; A96-B21; A96-B22; A96-B23; A96-B24; A96-B25;
A96-B26; A96-B27; A96-B28; A96-B29; A96-B30; A96-B31;
A96-B32; A96-B33; A96-B34; A96-B35; A96-B36; A96-B37;
A96-B38; A96-B39; A96-B40; A96-B41; A96-B42; A96-B43;
A96-B44; A96-B45; A96-B46; A96-B47; A96-B48; A96-B49;
A96-B50; A96-B51; A96-B52; A96-B53; A96-B54; A96-B55;
A96-B56; A96-B57; A96-B58; A96-B59; A96-B60; A96-B61;
A96-B62; A96-B63; A96-B64; A96-B65; A96-B66; A96-B67;
A96-B68; A96-B69; A96-B70; A96-B71; A96-B72; A96-B73;
A96-B74; A96-B75; A96-B76; A96-B77; A96-B78; A96-B79;
A96-B80; A96-B81; A96-B82; A96-B83; A96-B84; A96-B85;
A96-B86; A96-B87; A96-B88; A96-B89; A96-B90; A96-B91;
A96-B92; A96-B93; A96-B94; A96-B95; A96-B96; A96-B97;
A96-B98; A96-B99; A96-B 100; A96-B 101; A96-B 102; A96-B 103;
A96-B 104; A96-B 105; A96-B 106; A96-B 107; A96-B 108; A96-B 109;
A96-B 110; A96-B 11 1; A96-B 112; A96-B 113; A96-B 114; A96-B 115;
A96-B 116; A96-B 117; A96-B 118; A96-B 119; A96-B 120; A96-B 121;
A96-B 122; A96-B 123; A96-B 124; A96-B125; A96-B 126; A96-B 127;
A96-B 128; A96-B 129; A96-B 130; A96-B 131; A96-B 132; A96-B 133;
A96-B 134; A96-B 135; A96-B 136; A96-B137; A96-B 138; A96-B 139;
A96-B 140; A96-B141; A96-B 142; A96-B 143; A96-B 144; A96-B 145;
A96-B 146; A96-B 147; A96-B 148; A96-B 149; A96-B150; A96-B 151;
A96-B 152; A96-B 153; A96-B 154; A96-B 155; A96-B 156; A96-B 157;
A96-B158; A96-B159; A96-B160; A96-B 161; A96-B 162; A96-B 163;
A96-B164; A96-B 165; A96-B 166; A96-B 167; A96-B 168; A96-B 169;
A97-B l; A97-B2; A97-B3; A97-B4; A97-B5; A97-B6;
A97-B7; A97-B8; A97-B9; A97-B10; A97-B11; A97-B12;
A97-B 13; A97-B 14; A97-B 15; A97-B 16; A97-B17; A97-B 18;
A97-B19; A97-B20; A97-B21; A97-B22; A97-B23; A97-B24;
A97-B25; A97-B26; A97-B27; A97-B28; A97-B29; A97-B30;
A97-B31; A97-B32; A97-B33; A97-B34; A97-B35; A97-B36;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-188-
A97-B37; A97-B38; A97-B39; A97-B40; A97-B41; A97-B42;
A97-B43; A97-B44; A97-B45; A97-B46; A97-B47; A97-B48;
A97-B49; A97-B50; A97-B51; A97-B52; A97-B53; A97-B54;
A97-B55; A97-B56; A97-B57; A97-B58; A97-B59; A97-B60;
A97-B61; A97-B62; A97-B63; A97-B64; A97-B65; A97-B66;
A97-B67; A97-B68; A97-B69; A97-B70; A97-B71; A97-B72;
A97-B73; A97-B74; A97-B75; A97-B76; A97-B77; A97-B78;
A97-B79; A97-B80; A97-B81; A97-B82; A97-B83; A97-B84;
A97-B85; A97-B86; A97-B87; A97-B88; A97-B89; A97-B90;
A97-B91; A97-B92; A97-B93; A97-B94; A97-B95; A97-B96;
A97-B97; A97-B98; A97-B99; A97-B 100; A97-B 101; A97-B 102;
A97-B 103; A97-B 104; A97-B 105; A97-B 106; A97-B 107; A97-B 108;
A97-B 109; A97-B 110; A97-B 111; A97-B 112; A97-B 113; A97-B 114;
A97-B 115; A97-B 116; A97-B 117; A97-B 118; A97-B 119; A97-B 120;
A97-B 121; A97-B 122; A97-B 123; A97-B 124; A97-B 125; A97-B 126;
A97-B 127; A97-B 128; A97-B 129; A97-B130; A97-B 131; A97-B 132;
A97-B 133; A97-B 134; A97-B 135; A97-B 136; A97-B 137; A97-B 138;
A97-B 139; A97-B 140; A97-B141; A97-B 142; A97-B 143; A97-B 144;
A97-B 145; A97-B 146; A97-B 147; A97-B 148; A97-B 149; A97-B 150;
A97-B 151; A97-B 152; A97-B153; A97-B 154; A97-B 155; A97-B156;
A97-B 157; A97-B158; A97-B 159; A97-B 160; A97-B 161; A97-B 162;
A97-B 163; A97-B 164; A97-B 165; A97-B 166; A97-B 167; A97-B 168;
A97-B169; A98-B1; A98-B2; A98-B3; A98-B4; A98-B5;
A98-B6; A98-B7; A98-B8; A98-B9; A98-B10; A98-B11;
A98-B 12; A98-B 13; A98-B 14; A98-B 15; A98-B 16; A98-B17;
A98-B18; A98-B19; A98-B20; A98-B21; A98-B22; A98-B23;
A98-B24; A98-B25; A98-B26; A98-B27; A98-B28; A98-B29;
A98-B30; A98-B31; A98-B32; A98-B33; A98-B34; A98-B35;
A98-B36; A98-B37; A98-B38; A98-B39; A98-B40; A98-B41;
A98-B42; A98-B43; A98-B44; A98-B45; A98-B46; A98-B47;
A98-B48; A98-B49; A98-B50; A98-B51; A98-B52; A98-B53;
A98-B54; A98-B55; A98-B56; A98-B57; A98-B58; A98-B59;
A98-B60; A98-B61; A98-B62; A98-B63; A98-B64; A98-B65;
A98-B66; A98-B67; A98-B68; A98-B69; A98-B70; A98-B71;
A98-B72; A98-B73; A98-B74; A98-B75; A98-B76; A98-B77;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-189-
A98-B78; A98-B79; A98-B80; A98-B81; A98-B82; A98-B83;
A98-B84; A98-B85; A98-B86; A98-B87; A98-B88; A98-B89;
A98-B90; A98-B91; A98-B92; A98-B93; A98-B94; A98-B95;
A98-B96; A98-B97; A98-B98; A98-B99; A98-B100; A98-B101;
A98-B 102; A98-B 103; A98-B 104; A98-B 105; A98-B 106; A98-B107;
A98-B108; A98-B109; A98-B110; A98-B111; A98-B112; A98-B113;
A98-B114; A98-B 115; A98-B 116; A98-B117; A98-B118; A98-B 119;
A98-B 120; A98-B 121; A98-B 122; A98-B 123; A98-B124; A98-B125;
A98-B 126; A98-B 127; A98-B 128; A98-B 129; A98-B 130; A98-B 131;
A98-B132; A98-B133; A98-B 134; A98-B 135; A98-B136; A98-B 137;
A98-B138; A98-B139; A98-B140; A98-B141; A98-B142; A98-B143;
A98-B 144; A98-B145; A98-B 146; A98-B147; A98-B 148; A98-B 149;
A98-B150; A98-B 151; A98-B 152; A98-B153; A98-B154; A98-B 155;
A98-B156; A98-B157; A98-B158; A98-B159; A98-B160; A98-B161;
A98-B 162; A98-B 163; A98-B 164; A98-B165; A98-B166; A98-B 167;
A98-B168; A98-B169; A99-B1; A99-B2; A99-B3; A99-B4;
A99-B5; A99-B6; A99-B7; A99-B8; A99-B9; A99-B10;
A99-B 11; A99-B 12; A99-B 13; A99-B 14; A99-B 15; A99-B 16;
A99-B17; A99-B18; A99-B19; A99-B20; A99-B21; A99-B22;
A99-B23; A99-B24; A99-B25; A99-B26; A99-B27; A99-B28;
A99-B29; A99-B30; A99-B31; A99-B32; A99-B33; A99-B34;
A99-B35; A99-B36; A99-B37; A99-B38; A99-B39; A99-B40;
A99-B41; A99-B42; A99-B43; A99-B44; A99-B45; A99-B46;
A99-B47; A99-B48; A99-B49; A99-B50; A99-B51; A99-B52;
A99-B53; A99-B54; A99-B55; A99-B56; A99-B57; A99-B58;
A99-B59; A99-B60; A99-B61; A99-B62; A99-B63; A99-B64;
A99-B65; A99-B66; A99-B67; A99-B68; A99-B69; A99-B70;
A99-B71; A99-B72; A99-B73; A99-B74; A99-B75; A99-B76;
A99-B77; A99-B78; A99-B79; A99-B80; A99-B81; A99-B82;
A99-B83; A99-B84; A99-B85; A99-B86; A99-B87; A99-B88;
A99-B89; A99-B90; A99-B91; A99-B92; A99-B93; A99-B94;
A99-B95; A99-B96; A99-B97; A99-B98; A99-B99; A99-B 100;
A99-B 101; A99-B 102; A99-B 103; A99-B 104; A99-B 105; A99-B 106;
A99-B 107; A99-B108; A99-B109; A99-B 110; A99-B 111; A99-B 112;
A99-B 113; A99-B 114; A99-B 115; A99-B 116; A99-B 117; A99-B 118;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-190-
A99-B 119; A99-B 120; A99-B 121; A99-B 122; A99-B 123; A99-B 124;
A99-B 125; A99-B 126; A99-B 127; A99-B 128; A99-B 129; A99-B130;
A99-B 131; A99-B 132; A99-B 133; A99-B 134; A99-B 135; A99-B 136;
A99-B 137; A99-B 13 8; A99-B139; A99-B 140; A99-B 141; A99-B 142;
A99-B 143; A99-B 144; A99-B 145; A99-B 146; A99-B 147; A99-B 148;
A99-B149; A99-B 150; A99-B 15 1; A99-B 152; A99-B 153; A99-B 154;
A99-B 155; A99-B 156; A99-B 157; A99-B 158; A99-B 159; A99-B 160;
A99-B 161; A99-B 162; A99-B 163; A99-B 164; A99-B 165; A99-B 166;
A99-B167; A99-B168; A99-B169; A100-B1; A100-B2; A100-B3;
A100-B4; A100-B5; A100-B6; A100-B7; A100-B8; A100-B9;
A100-B10; A100-B11; A100-B12; A100-B13; A100-B14; A100-B15;
A100-B16; A100-B17; A100-B18; A100-B19; A100-B20; A100-B21;
A100-B22; A100-B23; A100-B24; A100-B25; A100-B26; A100-B27;
A100-B28; A100-B29; A100-B30; A100-B31; A100-B32; A100-B33;
A100-B34; A100-B35; A100-B36; A100-B37; A100-B38; A100-B39;
A100-B40; A100-B41; A100-B42; A100-B43; A100-B44; A100-B45;
A100-B46; A100-B47; A100-B48; A100-B49; A100-B50; A100-B51;
A100-B52; A100-B53; A100-B54; A100-B55; A100-B56; A100-B57;
A100-B58; A100-B59; A100-B60; A100-B61; A100-B62; A100-B63;
A100-B64; A100-B65; A100-B66; A100-B67; A100-B68; A100-B69;
A100-B70; A100-B71; A100-B72; A100-B73; A100-B74; A100-B75;
A100-B76; A100-B77; A100-B78; A100-B79; A100-B80; A100-B81;
A100-B82; A100-B83; A100-B84; A100-B85; A100-B86; A100-B87;
A100-B88; A100-B89; A100-B90; A100-B91; A100-B92; A100-B93;
A100-B94; A100-B95; A100-B96; A100-B97; A100-B98; A100-B99;
A100-B100; A100-B101; A100-B102; A100-B103; A100-B104; A100-B105;
A100-B106; A100-B107; A100-B108; A100-B109; A100-B110; A100-Blll;
A100-B 112; A100-B 113; A100-B 114; A100-B 115; A100-B 116; A100-B117;
A100-B118; A100-B119; A100-B120; A100-B121; A100-B122; A100-B123;
A100-B124; A100-B125; A100-B126; A100-B127; A100-B128; A100-B129;
A100-B130; A100-B131; A100-B132; A100-B133; A100-B134; A100-B135;
A100-B136; A100-B137; A100-B138; A100-B139; A100-B140; A100-B141;
A100-B142; A100-B143; A100-B144; A100-B145; A100-B146; A100-B147;
A100-B148; A100-B149; A100-B150; A100-B151; A100-B152; A100-B153;
A100-B154; A100-B155; A100-B156; A100-B157; A100-B158; A100-B159;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-191-
A100-B160; A100-B161; A100-B162; A100-B163; A100-B164; A100-B165;
A100-B166; A100-B167; A100-B168; A100-B169; A101-B1; A101-B2;
A101-B3; A101-B4; A101-B5; A101-B6; A101-B7; A101-B8;
A101-B9; A101-B10; A101-B11; A101-B12; A101-B13; A101-B14;
A101-B15; A101-B16; A101-B17; A101-B18; A101-B19; A101-B20;
A101-B21; A101-B22; A101-B23; A101-B24; A101-B25; A101-B26;
A101-B27; A101-B28; A101-B29; A101-B30; A101-B31; A101-B32;
A101-B33; A101-B34; A101-B35; A101-B36; A101-B37; A101-B38;
A101-B39; A101-B40; A101-B41; A101-B42; A101-B43; A101-B44;
A101-B45; A101-B46; A101-B47; A101-B48; A101-B49; A101-B50;
A101-B51; A101-B52; A101-B53; A101-B54; A101-B55; A101-B56;
A101-B57; A101-B58; A101-B59; A101-B60; A101-B61; A101-B62;
A101-B63; A101-B64; A101-B65; A101-B66; A101-B67; A101-B68;
A101-B69; A101-B70; A101-B71; A101-B72; A101-B73; A101-B74;
A101-B75; A101-B76; A101-B77; A101-B78; A101-B79; A101-B80;
A101-B81; A101-B82; A101-B83; A101-B84; A101-B85; A101-B86;
A1 1-B87; A101-B88; A101-B89; A101-B90; A101-B91; A101-B92;
A101-B93; A101-B94; A101-B95; A101-B96; A101-B97; A101-B98;
A101-B99; A101-B100; A101-Bl01; A101-B102; A101-B103; A101-B104;
A101-B105; A101-B106; A101-B107; A101-B108; A101-B109; A101-B110;
A101-B11l; A101-B112; A101-B113; A101-B114; A101-B115; A101-B116;
A101-B117; A101-B118; A101-B119; A101-B120; A101-B121; A101-B122;
A101-B123; A101-B124; A101-B125; A101-B126; A101-B127; A101-B128;
A101-B129; A101-B130; A101-B131; A101-B132; A101-B133; A101-B134;
A101-B135; A101-B136; A101-B137; A101-B138; A101-B139; A101-B140;
A101-B141; A101-B142; A101-B143; A101-B144; A101-B145; A101-B146;
A101-B147; A101-B148; A101-B149; A101-B150; A101-B151; A101-B152;
A101-B153; A101-B154; A101-B155; A101-B156; A101-B157; A101-B158;
A101-B159; A101-B160; A101-B161; A101-B162; A101-B163; A101-B164;
A101-B165; A101-B166; A101-B167; A101-B168; A101-B169; A102-B1;
A102-B2; A102-B3; A102-B4; A102-B5; A102-B6; A102-B7;
A102-B8; A102-B9; A102-B10; A102-Bll; A102-B12; A102-B13;
A102-B14; A102-B15; A102-B16; A102-B17; A102-B18; A102-B19;
A102-B20; A102-B21; A102-B22; A102-B23; A102-B24; A102-B25;
A102-B26; A102-B27; A102-B28; A102-B29; A102-B30; A102-B31;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-192-
A102-B32; A102-B33; A102-B34; A102-B35; A102-B36; A102-B37;
A102-B38; A102-B39; A102-B40; A102-B41; A102-B42; A102-B43;
A102-B44; A102-B45; A102-B46; A102-B47; A102-B48; A102-B49;
A102-B50; A102-B51; A102-B52; A102-B53; A102-B54; A102-B55;
A102-B56; A102-B57; A102-B58; A102-B59; A102-B60; A102-B61;
A102-B62; A102-B63; A102-B64; A102-B65; A102-B66; A102-B67;
A102-B68; A102-B69; A102-B70; A102-B71; A102-B72; A102-B73;
A102-B74; A102-B75; A102-B76; A102-B77; A102-B78; A102-B79;
A102-B80; A102-B81; A102-B82; A102-B83; A102-B84; A102-B85;
A102-B86; A102-B87; A102-B88; A102-B89; A102-B90; A102-B91;
A102-B92; A102-B93; A102-B94; A102-B95; A102-B96; A102-B97;
A102-B98; A102-B99; A102-B100; A102-B101; A102-B102; A102-B103;
A102-B104; A102-B105; A102-B106; A102-B107; A102-B108; A102-B109;
A102-B110; A102-B111; A102-B112; A102-B113; A102-B114; A102-B115;
A102-B116; A102-B117; A102-B118; A102-B119; A102-B120; A102-B121;
A102-B122; A102-B123; A102-B124; A102-B125; A102-B126; A102-B127;
A102-B128; A102-B129; A102-B130; A102-B131; A102-B132; A102-B133;
A102-B134; A102-B135; A102-B136; A102-B137; A102-B138; A102-B139;
A102-B140; A102-B141; A102-B142; A102-B143; A102-B144; A102-B145;
A102-B146; A102-B147; A102-B148; A102-B149; A102-B150; A102-B151;
A102-B152; A102-B153; A102-B154; A102-B155; A102-B156; A102-B157;
A102-B158; A102-B159; A102-B160; A102-B161; A102-B162; A102-B163;
A102-B164; A102-B165; A102-B166; A102-B167; A102-B168; A102-B169;
A103-B1; A103-B2; A103-B3; A103-B4; A103-B5; A103-B6;
A103-B7; A103-B8; A103-B9; A103-B10; A103-B11; A103-B12;
A103-B13; A103-B14; A103-B15; A103-B16; A103-B17; A103-B18;
A103-B19; A103-B20; A103-B21; A103-B22; A103-B23; A103-B24;
A103-B25; A103-B26; A103-B27; A103-B28; A103-B29; A103-B30;
A103-B31; A103-B32; A103-B33; A103-B34; A103-B35; A103-B36;
A103-B37; A103-B38; A103-B39; A103-B40; A103-B41; A103-B42;
A103-B43; A103-B44; A103-B45; A103-B46; A103-B47; A103-B48;
A103-B49; A103-B50; A103-B51; A103-B52; A103-B53; A103-B54;
A103-B55; A103-B56; A103-B57; A103-B58; A103-B59; A103-B60;
A103-B61; A103-B62; A103-B63; A103-B64; A103-B65; A103-B66;
A103-B67; A103-B68; A103-B69; A103-B70; A103-B71; A103-B72;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-193-
A103-B73; A103-B74; A103-B75; A103-B76; A103-B77; A103-B78;
A103-B79; A103-B80; A103-B81; A103-B82; A103-B83; A103-B84;
A103-B85; A103-B86; A103-B87; A103-B88; A103-B89; A103-B90;
A103-B91; A103-B92; A103-B93; A103-B94; A103-B95; A103-B96;
A103-B97; A103-B98; A103-B99; A103-B100; A103-B101; A103-B102;
A103-B103; A103-B104; A103-B105; A103-B106; A103-B107; A103-B108;
A103-B109; A103-B110; A103-B111; A103-B112; A103-B113; A103-B114;
A103-B115; A103-B116; A103-B117; A103-B118; A103-B119; A103-B120;
A103-B121; A103-B122; A103-B123; A103-B124; A103-B125; A103-B126;
A103-B127; A103-B128; A103-B129; A103-B130; A103-B131; A103-B132;
A103-B133; A103-B134; A103-B135; A103-B136; A103-B137; A103-B138;
A103-B139; A103-B140; A103-B141; A103-B142; A103-B143; A103-B144;
A103-B145; A103-B146; A103-B147; A103-B148; A103-B149; A103-B150;
A103-B151; A103-B152; A103-B153; A103-B154; A103-B155; A103-B156;
A103-B157; A103-B158; A103-B159; A103-B160; A103-B161; A103-B162;
A103-B163; A103-B164; A103-B165; A103-B166; A103-B167; A103-B168;
A103-B169; A104-B1; A104-B2; A104-B3; A104-B4; A104-B5;
A104-B6; A104-B7; A104-B8; A104-B9; A104-B10; A104-B11;
A104-B12; A104-B13; A104-B14; A104-B15; A104-B16; A104-B17;
A104-B18; A104-B19; A104-B20; A104-B21; A104-B22; A104-B23;
A104-B24; A104-B25; A104-B26; A104-B27; A104-B28; A104-B29;
A104-B30; A104-B31; A104-B32; A104-B33; A104-B34; A104-B35;
A104-B36; A104-B37; A104-B38; A104-B39; A104-B40; A104-B41;
A104-B42; A104-B43; A104-B44; A104-B45; A104-B46; A104-B47;
A104-B48; A104-B49; A104-B50; A104-B51; A104-B52; A104-B53;
A104-B54; A104-B55; A104-B56; A104-B57; A104-B58; A104-B59;
A104-B60; A104-B61; A104-B62; A104-B63; A104-B64; A104-B65;
A104-B66; A104-B67; A104-B68; A104-B69; A104-B70; A104-B71;
A104-B72; A104-B73; A104-B74; A104-B75; A104-B76; A104-B77;
A104-B78; A104-B79; A104-B80; A104-B81; A104-B82; A104-B83;
A104-B84; A104-B85; A104-B86; A104-B87; A104-B88; A104-B89;
A104-B90; A104-B91; A104-B92; A104-B93; A104-B94; A104-B95;
A104-B96; A104-B97; A104-B98; A104-B99; A104-B100; A104-B101;
A104-B102; A104-B103; A104-B104; A104-B105; A104-B106; A104-B107;
A104-B108; A104-B109; A104-B110; A104-B111; A104-B112; A104-B113;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-194-
A104-B114; A104-B115; A104-B116; A104-B117; A104-B118; A104-B119;
A104-B120; A104-B121; A104-B122; A104-B123; A104-B124; A104-B125;
A104-B126; A104-B127; A104-B128; A104-B129; A104-B130; A104-B131;
A104-B132; A104-B133; A104-B134; A104-B135; A104-B136; A104-B137;
A104-B138; A104-B139; A104-B140; A104-B141; A104-B142; A104-B143;
A104-B144; A104-B145; A104-B146; A104-B147; A104-B148; A104-B149;
A104-B150; A104-B151; A104-B152; A104-B153; A104-B154; A104-B155;
A104-B156; A104-B157; A104-B158; A104-B159; A104-B160; A104-B161;
A104-B162; A104-B163; A104-B164; A104-B165; A104-B166; A104-B167;
A104-B168; A104-B169; A105-B1; A105-B2; A105-B3; A105-B4;
A105-B5; A105-B6; A105-B7; A105-B8; A105-B9; A105-B10;
A105-B11; A105-B12; A105-B13; A105-B14; A105-B15; A105-B16;
A105-B17; A105-B18; A105-B19; A105-B20; A105-B21; A105-B22;
A105-B23; A105-B24; A105-B25; A105-B26; A105-B27; A105-B28;
A105-B29; A105-B30; A105-B31; A105-B32; A105-B33; A105-B34;
A105-B35; A105-B36; A105-B37; A105-B38; A105-B39; A105-B40;
A105-B41; A105-B42; A105-B43; A105-B44; A105-B45; A105-B46;
A105-B47; A105-B48; A105-B49; A105-B50; A105-B51; A105-B52;
A105-B53; A105-B54; A105-B55; A105-B56; A105-B57; A105-B58;
A105-B59; A105-B60; A105-B61; A105-B62; A105-B63; A105-B64;
A105-B65; A105-B66; A105-B67; A105-B68; A105-B69; A105-B70;
A105-B71; A105-B72; A105-B73; A105-B74; A105-B75; A105-B76;
A105-B77; A105-B78; A105-B79; A105-B80; A105-B81; A105-B82;
A105-B83; A105-B84; A105-B85; A105-B86; A105-B87; A105-B88;
A105-B89; A105-B90; A105-B91; A105-B92; A105-B93; A105-B94;
A105-B95; A105-B96; A105-B97; A105-B98; A105-B99; A105-B100;
A105-B101; A105-B102; A105-B103; A105-B104; A105-B105; A105-B106;
A105-B107; A105-B108; A105-B109; A105-B110; A105-B111; A105-B112;
A105-B113; A105-B114; A105-B115; A105-B116; A105-B117; A105-B118;
A105-B119; A105-B120; A105-B121; A105-B122; A105-B123; A105-B124;
A105-B125; A105-B126; A105-B127; A105-B128; A105-B129; A105-B130;
A105-B131; A105-B132; A105-B133; A105-B134; A105-B135; A105-B136;
A105-B137; A105-B138; A105-B139; A105-B140; A105-B141; A105-B142;
A105-B143; A105-B144; A105-B145; A105-B146; A105-B147; A105-B148;
A105-B149; A105-B150; A105-B151; A105-B152; A105-B153; A105-B154;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-195-
A105-B155; A105-B156; A105-B157; A105-B158; A105-B159; A105-B160;
A105-B161; A105-B162; A105-B163; A105-B164; A105-B165; A105-B166;
A105-B167; A105-B168; A105-B169; A106-B1; A106-B2; A106-B3;
A106-B4; A106-B5; A106-B6; A106-B7; A106-B8; A106-B9;
A106-B10; A106-B11; A106-B12; A106-B13; A106-B14; A106-B15;
A106-B16; A106-B17; A106-B18; A106-B19; A106-B20; A106-B21;
A106-B22; A106-B23; A106-B24; A106-B25; A106-B26; A106-B27;
A106-B28; A106-B29; A106-B30; A106-B31; A106-B32; A106-B33;
A106-B34; A106-B35; A106-B36; A106-B37; A106-B38; A106-B39;
A106-B40; A106-B41; A106-B42; A106-B43; A106-B44; A106-B45;
A106-B46; A106-B47; A106-B48; A106-B49; A106-B50; A106-B51;
A106-B52; A106-B53; A106-B54; A106-B55; A106-B56; A106-B57;
A106-B58; A106-B59; A106-B60; A106-B61; A106-B62; A106-B63;
A106-B64; A106-B65; A106-B66; A106-B67; A106-B68; A106-B69;
A106-B70; A106-B71; A106-B72; A106-B73; A106-B74; A106-B75;
A106-B76; A106-B77; A106-B78; A106-B79; A106-B80; A106-B81;
A106-B82; A106-B83; A106-B84; A106-B85; A106-B86; A106-B87;
A106-B88; A106-B89; A106-B90; A106-B91; A106-B92; A106-B93;
A106-B94; A106-B95; A106-B96; A106-B97; A106-B98; A106-B99;
A106-B100; A106-B101; A106-B102; A106-B103; A106-B104; A106-B105;
A106-B106; A106-B107; A106-B108; A106-B109; A106-B110; A106-B111;
A106-B112; A106-B113; A106-B114; A106-B115; A106-B116; A106-B117;
A106-B118; A106-B119; A106-B120; A106-B121; A106-B122; A106-B123;
A106-B124; A106-B125; A106-B126; A106-B127; A106-B128; A106-B129;
A106-B130; A106-B131; A106-B132; A106-B133; A106-B134; A106-B135;
A106-B136; A106-B137; A106-B138; A106-B139; A106-B140; A106-B141;
A106-B 142; A106-B 143; A106-B144; A106-B145; A 106-B 146; A106-B 147;
A106-B148; A106-B149; A106-B150; A106-B151; A106-B152; A106-B153;
A106-B154; A106-B155; A106-B156; A106-B157; A106-B158; A106-B159;
A106-B160; A106-B161; A106-B162; A106-B163; A106-B164; A106-B165;
A106-B166; A106-B167; A106-B168; A106-B169; A107-B1; A107-B2;
A107-B3; A107-B4; A107-B5; A107-B6; A107-B7; A107-B8;
A107-B9; A107-B10; A107-B11; A107-B12; A107-B13; A107-B14;
A107-B15; A107-B16; A107-B17; A107-B18; A107-B19; A107-B20;
A107-B21; A107-B22; A107-B23; A107-B24; A107-B25; A107-B26;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-196-
A107-B27; A107-B28; A107-B29; A107-B30; A107-B31; A107-B32;
A107-B33; A107-B34; A107-B35; A107-B36; A107-B37; A107-B38;
A107-B39; A107-B40; A107-B41; A107-B42; A107-B43; A107-B44;
A107-B45; A107-B46; A107-B47; A107-B48; A107-B49; A107-B50;
A107-B51; A107-B52; A107-B53; A107-B54; A107-B55; A107-B56;
A107-B57; A107-B58; A107-B59; A107-B60; A107-B61; A107-B62;
A107-B63; A107-B64; A107-B65; A107-B66; A107-B67; A107-B68;
A107-B69; A107-B70; A107-B71; A107-B72; A107-B73; A107-B74;
A107-B75; A107-B76; A107-B77; A107-B78; A107-B79; A107-B80;
A107-B81; A107-B82; A107-B83; A107-B84; A107-B85; A107-B86;
A107-B87; A107-B88; A107-B89; A107-B90; A107-B91; A107-B92;
A107-B93; A107-B94; A107-B95; A107-B96; A107-B97; A107-B98;
A107-B99; A107-B100; A107-B101; A107-B102; A107-B103; A107-B104;
A107-B105; A107-B106; A107-B107; A107-B108; A107-B109; A107-B110;
A107-B111; A107-B112; A107-B113; A107-B114; A107-B115; A107-B116;
A107-B117; A107-B118; A107-B119; A107-B120; A107-B121; A107-B122;
A107-B123; A107-B124; A107-B125; A107-B126; A107-B127; A107-B128;
A107-B129; A107-B130; A107-B131; A107-B132; A107-B133; A107-B134;
A107-B135; A107-B136; A107-B137; A107-B138; A107-B139; A107-B140;
A107-B141; A107-B142; A107-B143; A107-B144; A107-B145; A107-B146;
A107-B147; A107-B148; A107-B149; A107-B150; A107-B151; A107-B152;
A107-B153; A107-B154; A107-B155; A107-B156; A107-B157; A107-B158;
A107-B159; A107-B160; A107-B161; A107-B162; A107-B163; A107-B164;
A107-B165; A107-B166; A107-B167; A107-B168; A107-B169; A108-B1;
A108-B2; A108-B3; A108-B4; A108-B5; A108-B6; A108-B7;
A108-B8; A108-B9; A108-B10; A108-B11; A108-B12; A108-B13;
A108-B14; A108-B15; A108-B16; A108-B17; A108-B18; A108-B19;
A108-B20; A108-B21; A108-B22; A108-B23; A108-B24; A108-B25;
A108-B26; A108-B27; A108-B28; A108-B29; A108-B30; A108-B31;
A108-B32; A108-B33; A108-B34; A108-B35; A108-B36; A108-B37;
A108-B38; A108-B39; A108-B40; A108-B41; A108-B42; A108-B43;
A108-B44; A108-B45; A108-B46; A108-B47; A108-B48; A108-B49;
A108-B50; A108-B51; A108-B52; A108-B53; A108-B54; A108-B55;
A108-B56; A108-B57; A108-B58; A108-B59; A108-B60; A108-B61;
A108-B62; A108-B63; A108-B64; A108-B65; A108-B66; A108-B67;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-197-
A108-B68; A108-B69; A108-B70; A108-B71; A108-B72; A108-B73;
A108-B74; A108-B75; A108-B76; A108-B77; A108-B78; A108-B79;
A108-B80; A108-B81; A108-B82; A108-B83; A108-B84; A108-B85;
A108-B86; A108-B87; A108-B88; A108-B89; A108-B90; A108-B91;
A108-B92; A108-B93; A108-B94; A108-B95; A108-B96; A108-B97;
A108-B98; A108-B99; A108-B100; A108-B101; A108-B102; A108-B103;
A108-B104; A108-B105; A108-B106; A108-B107; A108-B108; A108-B109;
A108-B110; A108-B111; A108-B112; A108-B113; A108-B114; A108-B115;
A108-B116; A108-B117; A108-B118; A108-B119; A108-B120; A108-B121;
A108-B122; A108-B123; A108-B124; A108-B125; A108-B126; A108-B127;
A108-B128; A108-B129; A108-B130; A108-B131; A108-B132; A108-B133;
A108-B134; A108-B135; A108-B136; A108-B137; A108-B138; A108-B139;
A108-B140; A108-B141; A108-B142; A108-B143; A108-B144; A108-B145;
A108-B146; A108-B147; A108-B148; A108-B149; A108-B150; A108-B151;
A108-B152; A108-B153; A108-B154; A108-B155; A108-B156; A108-B157;
A108-B158; A108-B159; A108-B160; A108-B161; A108-B162; A108-B163;
A108-B164; A108-B165; A108-B166; A108-B167; A108-B168; A108-B169;
A109-B1; A109-B2; A109-B3; A109-B4; A109-B5; A109-B6;
A109-B7; A109-B8; A109-B9; A109-B10; A109-B11; A109-B12;
A109-B13; A109-B14; A109-B15; A109-B16; A109-B17; A109-B18;
A109-B19; A109-B20; A109-B21; A109-B22; A109-B23; A109-B24;
A109-B25; A109-B26; A109-B27; A109-B28; A109-B29; A109-B30;
A109-B31; A109-B32; A109-B33; A109-B34; A109-B35; A109-B36;
A109-B37; A109-B38; A109-B39; A109-B40; A109-B41; A109-B42;
A109-B43; A109-B44; A109-B45; A109-B46; A109-B47; A109-B48;
A109-B49; A109-B50; A109-B51; A109-B52; A109-B53; A109-B54;
A109-B55; A109-B56; A109-B57; A109-B58; A109-B59; A109-B60;
A109-B61; A109-B62; A109-B63; A109-B64; A109-B65; A109-B66;
A109-B67; A109-B68; A109-B69; A109-B70; A109-B71; A109-B72;
A109-B73; A109-B74; A109-B75; A109-B76; A109-B77; A109-B78;
A109-B79; A109-B80; A109-B81; A109-B82; A109-B83; A109-B84;
A109-B85; A109-B86; A109-B87; A109-B88; A109-B89; A109-B90;
A109-B91; A109-B92; A109-B93; A109-B94; A109-B95; A109-B96;
A109-B97; A109-B98; A109-B99; A109-B100; A109-B101; A109-B102;
Al09-B103; A109-B104; A109-B105; A109-B106; A109-B107; A109-B108;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-198-
A109-B109; A109-B110; A109-B111; A109-B112; A109-B113; A109-B114;
A109-B 115; A109-B 116; A109-B 117; A109-B 118; A109-B119; A109-B 120;
A109-B121; A109-B122; A109-B123; A109-B124; A109-B125; A109-B126;
A109-B127; A109-B128; A109-B129; A109-B130; A109-B131; A109-B132;
A109-B133; A109-B134; A109-B135; A109-B136; A109-B137; A109-B138;
A109-B139; A109-B140; A109-B141; A109-B142; A109-B143; A109-B144;
A109-B145; A109-B146; A109-B147; A109-B148; A109-B149; A109-B150;
A109-B151; A109-B152; A109-B153; A109-B154; A109-B155; A109-B156;
A109-B157; A109-B158; A109-B159; A109-B160; A109-B161; A109-B162;
A109-B163; A109-B164; A109-B165; A109-B166; A109-B167; A109-B168;
A109-B169; AllO-B1; A110-B2; A110-B3; A110-B4; A110-B5;
A110-B6; A110-B7; A110-B8; A110-B9; A110-BlO; A110-B11;
A110-B12; A110-B13; A110-B14; A110-B15; A110-B16; A110-B17;
A110-B18; A110-B19; A110-B20; A110-B21; A110-B22; A110-B23;
A110-B24; A110-B25; A110-B26; A110-B27; A110-B28; A110-B29;
A110-B30; Al10-B31; A110-B32; A110-B33; A110-B34; A110-B35;
A110-B36; A110-B37; A110-B38; A110-B39; A110-B40; A110-B41;
A110-B42; A110-B43; A110-B44; A110-B45; A110-B46; A110-B47;
A110-B48; A110-B49; A110-B50; A110-B51; A110-B52; A110-B53;
Al10-B54; A110-B55; A110-B56; A110-B57; A110-B58; A110-B59;
A110-B60; A110-B61; A110-B62; A110-B63; A110-B64; A110-B65;
A110-B66; A110-B67; A110-B68; A110-B69; A110-B70; A110-B71;
A110-B72; A110-B73; A110-B74; A110-B75; A110-B76; A110-B77;
A110-B78; A110-B79; A110-B80; A110-B81; A110-B82; A110-B83;
A110-B84; A110-B85; A110-B86; A110-B87; A110-B88; A110-B89;
A110-B90; A110-B91; A110-B92; A110-B93; A110-B94; A110-B95;
A110-B96; A110-B97; A110-B98; A110-B99; A110-B100; A110-B101;
A110-B102; A110-B103; A110-B104; A110-B105; A110-B106; A110-B107;
A110-B108; A110-B109; A110-B110; A110-B111; A110-B112; A110-B113;
A110-B114; A110-B115; A110-B116; A110-B117; A110-B118; A110-B119;
A110-B120; A110-B121; A110-B122; A110-B123; A110-B124; A110-B125;
A110-B126; A110-B127; A110-B128; A110-B129; A110-B130; A110-B131;
A110-B132; A110-B133; A110-B134; Al10-B135; A110-B136; A110-B137;
A110-B138; A110-B139; A110-B140; A110-B141; A110-B142; A110-B143;
A110-B144; A110-B145; A110-B146; A110-B147; A110-B148; A110-B149;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-199-
A110-B150; A110-B151; A110-B152; A110-B153; A110-B154; A110-B155;
A110-B156; A110-B157; A110-B158; A110-B159; A110-B160; A110-B161;
A110-B162; A110-B163; A110-B164; A110-B165; A110-B166; A110-B167;
A110-B168; A110-B169.
Thus, for example, in the above list the compound denoted as A9-B9 is the
product of the combination
of group A9 in Table 1 and B9 in Table 2, namely
H3 C SCH3
,~ N
I / \ NH
3C H N
, Example 230(a) hereinafter described.
Particular compounds of the invention of formula (Ix) for the inhibition of
SYK are:
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acid benzylamide;
2-(1H-indazol-3-yl)-1H-benzimidazole=5-carboxylic acid N-methylamide;
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acid N-ethylamide;
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acid N-isopropylamide;
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acid N-phenylamide;
2-(1 H-indazol-3-yl)-1 H-benzimidazole-5-carboxylic acid N-phenethylamide;
5,6-dimethyl-2-(5-methylsulfanyl-lH-pyrazol-3-yl)-1H-benzoimidazole;
6-chloro-5-methyl-2-(5-methylsulfanyl-1 H-pyrazol-3-yl)-1 H-benzoimidazole;
6-chloro-2-(5-ethylsulfanyl-1 H-pyrazol-3-yl)-5-methyl-lH-benzoimidazole;
2-(5 -methylsulfanyl- 1 H-pyrazol-3-yl)-5-trifluoromethyl-1 H-benzoimidazole;
2-(5-cyclopropylmethylsulfanyl-1 H-pyrazol-3-yl)-5,6-dimethyl-1 H-
benzoimidazole;
2-(5-ethylsulfanyl-lH-pyrazol-3-yl)-5,6-dimethyl-lH-benzoimidazole;
5,6-dimethyl-2-[5-(pyridin-3-ylmethylsulfanyl)-1H-pyrazol-3-yl]-1H-
benzoimidazole;
5-fluoro-2-[5-methylsulfanyl)-1 H-pyrazol-3-yl]-1H-benzoimidazole;
5,6-dimethyl-2-(5-phenethylsulfanyl-1 H-pyrazol-3-yl)-1H-benzoimidazole;
4-methyl-2-(5-methylsulfanyl-1 H-pyrazol-3 -yl)-1 H-b enzoimidazole;
5,6-dimethyl-2-(5-benzylsulfanyl-lH-pyrazol-3-yl)-1H-benzoimidazole;
6-chloro-5 -methyl-2-(5 -morpholin-4-yl-1 H-pyrazol-3 -yl)-1 H-b
enzoimidazole;
5,6-dimethyl-2-[5-(thiophen-2-ylmethylsulfanyl)-1H-pyrazol-3-yl]-1 H-
benzoimidazole;
2-(5-ethylsulfanyl-lH-pyrazol-3-yl)-5-methoxy-lH-benzoimidazole hydrochloride;
5-methyl-2-(5-methylsulfanyl-4-propyl-1 H-pyrazol-3-yl)-1H-benzoimidazole;
2-(5-(4-methoxy-benzylsulfanyl)-4-propyl-lH-pyrazol-3-yl)- 5-methyl-lH-
benzoimidazole;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-200-
2-(5-benzylsulfanyl-4-isopropyl-1 H-pyrazol-3-yl)-5-methyl-lH-benzoimidazole;
2-(5-methylsulfanyl-4-methyl-lH-pyrazol-3-yl)-5-methoxy-1 H-benzoimidazole;
2 -(5-methylsulfanyl-4-methyl-1 H-pyrazol-3-yl)-5-methyl-1 H-benzoimidazole;
3-(5-chloro-1 H-benzoimidazol-2-yl)-1 H-pyrazol-4-ylamine;
3-(5,6-dichloro-lH-benzoimidazol-2-yl)-1H-pyrazol-4-ylamine;
3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1 H-pyrazol-4-ylamine;
3-(5-ethyl-6-methyl-lH-benzoimidazol-2-yl)-1 H-pyrazol-4-ylamine;
3-(6-chloro-5-methoxy-1 H-benzoimidazol-2-yl)-1 H-pyrazol-4-ylamine;
3-(5-methoxy-1 H-benzoimidazol-2-yl)-1H-pyrazol-4-ylamine;
3-(5-ethoxy-1H-benzoimidazol-2-yl)-1H-pyrazol-4-ylamine;
3-(5 -fluoro-6-methyl-1 H-benzoiinidazol-2-yl)-1 H-pyrazol-4-ylamine;
3-(5-trifluoromethoxy-1 H-benzoimidazol-2-yl)-1 H-pyrazol-4-ylamine;
3-(5-trifluoromethyl-1 H-benzoimidazol-2-yl)-1 H-pyrazol-4-ylamine;
2-(4-amino-lH-pyrazol-3-yl)-1H-benzoimidazole-5-carboxylic acid methyl ester;
3-(1 H-benzoimidazol-2-yl)-1 H-indazole;
3-(5-methoxy-1 H-benzoimidazol-2-yl)-1 H-indazole;
[2-(indazol-3-yl)-1 H-benzoimidazol-5-yl]-phenyl-methanone;
2-(1 H-indazol-3-yl)-3H-benzoimidazol-4-ol;
2-phenyl-1 H-imidazol[4,5-b]pyrazine;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-indazole;
2-(1 H-indazol-3 -yl)-3 H-imidazo [4, 5 -c]pyridine;
2-(1 H-indazole-3-yl)-3H-imidazo[4,5-b]pyridine;
2-(1 H-pyrazol-3y1)-1 H-benzoimidazole;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-5-methoxy-1 H-indazole;
3-(5-ethyl-6-methyl-lH-benzoimidazol-2-yl)-5-methoxy-lH-indazole;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-5-fluoro-1 H-indazole;
3 -(5, 6-dimethyl-1 H-benzoimidazol-2-yl)-6-fluoro-1 H-indazole;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-5-methyl-lH-indazole;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-6-methoxy-lH-indazole;
5,6-dimethyl-2-(4-phenyl-lH-pyrazol-3-yl)-1H-benzoimidazole;
3-(5 -ethyl-1 H-b enzo imi dazo l-2 -yl)-1 H-indazo le;
3-( 5-ethyl-6-methyl-1 H-b enzo imi dazo l-2-yl) -1 H-indazo le;
3-(5-isopropyl-6-methyl-1 H-benzoimidazol-2-yl)-1H-indazole;
3-(5-bromo-6-methyl-lH-benzoimidazol-2-yl)-1 H-indazole;
3-(5-bromo-lH-benzoimidazol-2-yl)-1H-indazole;
3-(5 -(3 -cyano)phenyl-1 H-b enzoimidazol-2-yl)-1 H-indazo le;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-201-
3 -(5 -(pyrid-3 -yl)-1 H-b enzoimidazol-2-yl)-1 H-indazole;
3-(6-methyl-5-phenyl-1 H-benzoimidazol-2-yl)-1 H-indazole;
3-(5-phenyl-1 H-benzoimidazol-2-yl)-1 H-indazole;
3 -(5 -(2-fluoro)phenyl-1 H-b enzoimidazol-2-yl)-1 H-indazole;
3-(5-(5,6-methylenedioxy)phenyl-lH-benzoimidazol-2-yl)-1H-indazole;
3-(5-(2-methoxy)phenyl-1 H-benzoimidazol-2-yl)-1 H-indazole;
3 -(5-(4-chloro)phenyl-1 H-b enzoimidazol-2-yl)-1 H-indazole;
3-(5-(4-methyl)phenyl-1 H-benzoimidazol-2-yl)-1H-indazole;
3-(5-benzyloxy-1 H-benzoimidazol-2-yl)-1H-indazole;
3-(5,6-methylenedioxy-1 H-benzoimidazol-2-yl)-1 H-indazole;
3-(5, 6-dixnethoxy-1 H-benzoimidazol-2-yl)-1 H-indazole;
3-(5,6-diethyl-1 H-benzoimidazol-2-yl)-1 H-indazole;
3-(4, 5-dimethyl-1 H-benzoimidazol-2-yl)-1 H-indazole;
2-(1 H-indazol-3-yl)-1 H-benzoimidazole-5-carbonitrile;
3-(5-methoxycarbonyl-lH-benzoimidazol-2-yl)-1H-indazole;
3-(5, 6-dimethyl-1 IH-benzoimidazol-2-yl)-5-ethoxy- H-indazole;
3-(5,6-dimethyl-lH-benzoiinidazol-2-yl)-pyrazole-4-carboxylic acid ethyl
ester;
2-(4-isopropylcarbamoyl-lH-pyrazol-3-yl)-1H-benzoimidazole-5-carboxylic acid
methyl ester;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-5-methyl-pyrazole-4-carboxylic acid
ethyl ester;
3-(1,5,6,7-tetrahydro-1,3-diaza-s-indacen-2-yl)-1H-pyrazole-4-carboxylic acid
cyclopropylamide;
3-(5-methoxy-6-methyl-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid
isopropylamide;
3-[5-(2-morpholin-4-yl-ethoxy)-1 H-benzoimidazol-2-yl]-1 H-indazole;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid (2-
methoxy-ethyl)-amide;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid
propylamide;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid
(tetrahydro-pyran-4-yl)-amide;
3-(5-ethyl-6-methyl-1 H-benzoimidazol-2-yl)-1 H-indazole-5-carbonitrile;
3-(5-difluoromethoxy-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid
isopropylamide;
3-(5-difluoromethoxy-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid
cyclopropylamide;
3-(6-ethyl-5-methoxy-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid
isopropylamide;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-indazole-5-carbonitrile;
2-( 5-methyl-1 H-pyrazol-3 -yl)-1 H-b enzoimidazol e;
2-(5 -ethoxy-1 H-pyrazol-3 -yl)-1 H-benzoimidazole;
2-(5-methylsulfanyl-isoxazol-3-yl)-1 H-benzoimidazole;
5-chloro-2-(4-nitro-1 H-pyrazol-3-yl)-1H-benzoimidazole;
5,6-dichloro-2-(4-nitro-lH-pyrazol-3-yl)-1H-benzoimidazole;
(benzoimidazol-2-yl)-5-methylthio-3-pyrazole;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-202-
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-4,5,6,7-tetrahydro-1 H-indazole;
2-(5-isopropyl-1 H-pyrazol-3-yl)-5,6-dimethyl-1 H-benzoimidazole;
2-(5-ethyl-1 H-pyrazol-3-yl)-5,6-dimethyl-1 H-benzoimidazole;
5,6-dimethyl-2-(1,4,5,6-tetrahydro-cyclopentapyrazol-3-yl)-1 H-benzoimidazole;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-4-fluoro-lH-indazole;
4-chloro-3 -(5, 6-dimethyl-1 H-benzoimidazol-2-yl)-1 H-indazole;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-5-chloro-1 H-indazole;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-indazol-5-ol;
3-(5-n-propyl-1 H-benzoimidazo 1-2-yl)-1 H-indazole;
2-(1H-indazol-3-yl)-lH-benzoimidazole-5-sulfonic acid benzylamide;
3 -(5 -methane sulfonyl-1 H-benzoimidazol-2-yl)-1 H-indazole;
[2-(indazol-3-yl)-1 H-benzoimidazol-5-yl]-phenyl-methanol;
[2-(indazol-3-yl)-1H-benzoimidazol-5-yl]-carboxylic acid;
[2-(indazol-3-yl)-1H-benzoimidazol-5-yl]-carboxylic acid, methylamide;
[2-(indazol-3-yl)-1H-benzoimidazol-5-yl]-carboxylic acid, dimethylamide;
[2-(indazol-3-yl)-1H-benzoimidazol-5-yl]-carboxylic acid, isopropylamide;
IH-benzoimidazol-5-yl]-carboxylic acid, benzylamide;
[2-(indazol-3-yl)-1 H-benzoimidazol-5-yl]-carboxylic acid, benzamide;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid
isopropylamide;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid (2-
hydroxy-1,1-dimethyl-
ethyl)-amide;
2-(4-isopropylcarbamoyl-IH-pyrazol-3-yl)-1H-benzoimidazole-5-carboxylic acid
(pyridin-3-ylmethyl)-
amide;
3-(5,6-dimethyl-IH-benzoimidazol-2-yl)-5-methyl-lH-pyrazole-4-carboxylic acid
cyclopropylamide;
2-(4-isopropylcarbamoyl-lH-pyrazol-3-yl)-1H-benzoimidazole-5-carboxylic acid
phenylmethyl-amide;
2-(4-isopropylcarbamoyl-lH-pyrazol-3-yl)-1H-benzoimidazole-5-carboxylic acid
(pyridin-2-ylmethyl)-
amide;
2-(1 H-indazol-3-yl)-1 H-benzoimidazole-5-carboxylic acid (pyridin-3-ylmethyl)-
amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 3-methyl-benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 4-methyl-benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid [3-(2-oxo-pyrrolidin-1-
yl)-propyl]-amide;
2-(1H-indazol-3-yl)-IH-benzoimidazole-5-carboxylic acid (2-morpholin-4-yl-
ethyl)-amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (2-methoxy-ethyl)-
amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (2-cyano-ethyl)-amide;
2-(IH-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (2-hydroxy-1,1-
dimethyl-ethyl)-amide;
2-(1H-Indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (3-imidazol-1-yl-
propyl)-amide;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-203-
3-(5, 6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid isobutyl-
amide;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid
isopropylamide;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid
cyclopropylmethyl-amide;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-5-methyl-lH-pyrazole-4-carboxylic acid
tert-butylamide;
3-(5,6-dimethyl-lH-benzoiinidazol-2-yl)-1H-indazole-5-carboxylic acid
dimethylamide;
2-(4-isobutyrylamino-lH-pyrazol-3-yl)-1H-benzoimidazole-5-carboxylic acid
benzylamide;
[2-(indazol-3-yl)-1H-benzoimidazol-5-yl]-carboxylic acid;
3-(5,6-dimethyl-lH-benzoimidazol-5-yl)-pyrazole-4-carboxylic acid;
2-(4-isopropylcarbamoyl-1 H-pyrazol-3-yl)-1 H-benzoimidazole-5-carboxylic
acid;
3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-5-methyl-pyrazole-4-carboxylic acid;
N-[3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1 H-pyrazol-4-yl]-isobutyramide ;
N-[3-(5, 6-dimethyl-1 H-benzoimidazol-2-yl)-1 H-pyrazol-4-yl]-3-methyl-
butyramide;
N-[3-(5,6-dimethyl-lH-benzoiinidazol-2-yl)-1 H-pyrazol-4-yl]-2-phenyl-
acetamide;
cyclopropanecarboxylic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-
4-yl]-amide;
methoxyacetic acid [3-(5,6-dimethyl-IH-benzoimidazol-2-yl)-IH-pyrazol-4-yl]-
amide;
cyclopentanecarboxylic acid [3-(5,6-dimethyl-IH-benzoimidazol-2-yl)-IH-pyrazol-
4-yl]-amide;
trimethylacetic acid [3-(5,6-dimethyl-IH-benzoimidazol-2-yl)-IH-pyrazol-4-yl]-
amide;
tert-butylacetic acid [3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1 H-pyrazol-4-
yl]-amide;
butanoic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-amide;
isoxazole-5-carboxylic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-
4-yl]-amide;
S(+)-2-methylbutanoic acid [3-(5,6-dimethyl-IH-benzoimidazol-2-yl)-IH-pyrazol-
4-yl]-amide;
cyclopropanecarboxylic acid [3-(5-ethyl-6-methyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide;
piperidine-l-carboxylic acid[3-(6-chloro-5-methoxy-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide;
3-[3-(6-chloro-5-methoxy-lH-benzoimidazol-2-yl)-1 H-pyrazol-4-yl]-1,1-
dimethylurea;
cyclopropanecarboxylic acid [3-(5-methoxy-lH-benzoimidazol-2-yl)-1H-pyrazol-4-
yl]-amide;
cyclopropanecarboxylic acid [3-(5-ethoxy-lH-benzoimidazol-2-yl)-1H-pyrazol-4-
yl]-amide;
cyclopropanecarboxylic acid [3-(5-fluoro-6-methyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide;
cyclopropanecarboxylic acid [3-(5-trifluoromethoxy-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide;
cyclopropanecarboxylic acid [3-(5-trifluoromethyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide;
N-[3-(5-trifluoromethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-isobutyramide;
cyclopropanecarboxylic acid [3-(5-chloro-6-methyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide;
3,5-dimethyl-isoxazole-4-carboxylic acid [3-(5,6-dimethyl-IH-benzoimidazol-2-
yl)-IH-pyrazol-4-yl]-
amide;
N-[3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1 H-pyrazol-4-yl]-acetamide;
furan-3-carboxylic acid [3-(5-chloro-6-methyl-lH-benzoimidazol-2-y1)-1H-
pyrazol-4-yl]-amide;
N-[3 -(5, 6-dimethyl-1 H-benzoimidazol-2-yl)-1 H-pyrazol-4-yl] -4-methyl-
benzamide;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-204-
5, 6-dimethyl-2-(4-nitro-1 H-pyrazo 1-3 -yl)-1 H-benzoimidazole;
5-ethyl-6-methyl-2-(4-nitro-1 H-pyrazol-3-yl)-1 H-benzoimidazole;
6-chloro-5-methoxy-2-(4-nitro-1 H-pyrazol-3-yl)-1 H-benzoimidazole;
5-fluoro-6-methyl-2-(4-nitro-1 H-pyrazol-3-yl)-1 H-benzoimidazole;
2-(4-nitro-lH-pyrazol-3-yl)-5-trifluoromethoxy-lH-benzoimidazole;
2-(4-nitro-1 H-pyrazol-3-yl)-5-trifluoromethyl-lH-benzoimidazole;
5 -chloro-6-methyl-2-(4-nitro-1 H-pyrazol-3 -yl)-1 H-benzoimidazole;
2-(4-nitro-lH-pyrazol-3-yl)-1H-benzoimidazole-5-carboxylic acid methyl ester;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridine-5-carboxylic acid
isopropylamide;
cyclopropyl-[3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-
pyrazolo[4,3-c]pyridin-5-yl]-
methanone;
isopropyl-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-
pyrazolo[4,3-c]pyridin-5-yl]-
methanone;
1-[3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridin-5-yl]-2,2-
dimethyl-propan-l-one;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridine-5-carboxylic acid
methyl ester;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-4,5,6,7-tetrahydro-1 H-pyrazolo[4,3-
c]pyridine;
3-(5-chloro-6-methyl-lH-benzoimidazol-2-yl)-4,5,6,7-tetrahydro-lH-pyrazolo[4,3-
c]pyridine;
3-[5-(2-morpholin-4-yl-ethoxy)-1 H-benzoimidazol-2-yl]-4,5,6,7-tetrahydro-1 H-
pyrazolo [4,3-c]pyridine;
3-(5-trifluoromethyl-1 H-benzoimidazol-2-yl)-4,5,6,7-tetrahydro-1 H-
pyrazolo[4,3-c]pyridine;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridine-5-carboxylic acid
tert-butyl ester;
5-methoxy-2-(4-nitro-lH-pyrazol-3-yl)-1H-benzoimidazole;
5-ethoxy-2-(4-nitro-1 H-pyrazol-3-yl)-1 H-benzoimidazole;
3-(5-chloro-6-methyl-1 H-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridine-5-carboxylic
acid tert-butyl ester;
3-[5-(2-morpholin-4-yl-ethoxy)-1H-benzoimidazol-2-yl]-1,4,6,7-tetrahydro-
pyrazolo[4,3-c]pyridine-5-
carboxylic acid tert-butyl ester;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrano[4,3-
c]pyrazole;
3-(5-trifluoromethyl-1 H-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridine-5-carboxylic
acid tert-butyl ester;
N-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-2-morpholin-4-yl-
acetamide;
2-dimethylamino-N-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1 H-pyrazol-4-yl]-
acetamide;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-205-
N-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]- 2-(1H-1,2,3,4-
tetraazol-1-yl)-acetamide;
N-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1 H-pyrazol-4-yl]-isonicotinamide;
2-cyclopropyl-N-[3 -( 5, 6-dimethyl-1 H-b enzoimidazol-2 -yl)-1 H-pyrazol-4-
yl] -acetamide;
1-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1 H-pyrazol-4-yl]-3-methyl-urea;
1-[3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-3-isopropyl-urea;
1-[3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1 H-pyrazol-4-yl] -3-phenyl-urea;
1-benzyl-3-[3-(5, 6-dimethyl-1 H-benzoimidazol-2-yl)-1 H-pyrazol-4-yl]-urea;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridine-5-carboxylic acid
isopropylamide;
cyclopropanecarboxylic acid[3-(5-ethoxy-6-ethyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]amide;
3-(1,5,6,7-tetrahydro-1,3-diaza-s-indacen-2-yl)-1 H-pyrazol-4-ylamine;
4-methylpiperazine-l-carboxylic acid [3-(1,5,6,7-tetrahydro-1,3-diaza-s-
indacen-2-yl)-1H-pyrazol-4-
yl]amide;
l,1-dimethyl-3-[3-(1,5,6,7-tetrahydro-s-indacen-2-yl)-1H-pyrazol-4-yl] urea;
cyclopropanecarboxylic acid [3-(6-ethoxy-5-fluoro-lH-benzimidazol-2-yl)-1H-
pyrazol-4-yl]amide;
tetrahydropyran-4-carboxylic acid [3-(6-ethoxy-5-fluoro-lH-benzimidazol-2-yl)-
1H-pyrazole-4-
yl] amide;
morpholine-4-carboxylic acid[3-(6-ethoxy-5-fluoro-lH-benzimidazol-2-yl)-1H-
pyrazol-4-yl]amide;
piperidine-4-carboxylic acid[3-(6-ethoxy-5-fluoro-lH-benzimidazol-2-yl)-1H-
pyrazol-4-yl]amide;
3-[6-ethoxy-5-fluoro-lH-benzimidazol-2-yl)-1H-pyrazol-4-yl]-1,1-diethylurea;
5-methoxy-2-(4-nitro-1 H-pyrazol-3-yl)-1 H-benzoimidazole;
morpholine-4-carboxylic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-ylmethyl]-
amide;
3-[3-(5-difluoromethoxy-1 H-benzoimidazol-2-yl)-1 H-pyrazol-4-yl]-1,1-diethyl-
urea;
piperidine-l-carboxylic acid [3-(5-difluoromethoxy-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide;
cyclopropanecarboxylic acid [3-(6-chloro-5-methoxy-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide;
cyclopropanecarboxylic acid [3-(1,5,6,7-tetrahydro-1,3-diaza-s-indacen-2-yl)-
1H-pyrazol-4-yl]amide;
morpholine-4-carboxylic acid[3-(1,5,6,7-tetrahydro-1,3-diaza-s-indacen-2-yl)-
1H-pyrazol-4-yl]-amide;
piperidine-l-carboxylic acid [3-(5-methoxy-lH-benzoimidazol-2-yl)-1H-pyrazol-4-
yl]-amide;
3-[3-(5-methoxy-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-1,1-dimethyl-urea;
piperidine-l-carboxylic acid [3-(5-ethyl-6-methyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide;
3-[3-(5-fluoro-6-methyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-1,1-dimethyl-
urea;
morpholine-4-carboxylic acid [3-(5-trifluoromethyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridine-5-carboxylic acid
diethylamide;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-206-
[3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridin-5-yl]-pyrrolidin-l-
yl-methanone;
[3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridin-5-yl]-piperidin-l-
yl-methanone;
[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridin-5-yl]-morpholin-4-
yl-methanone;
3-(5-chloro-6-methyl-1 H-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridine-5-carboxylic
acid diethylamide;
morpholine-4-carboxylic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide;
piperidine-l-carboxylic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide;
3-[5-(2-morpholin-4-yl-ethoxy)-1 H-benzoimidazol-2-yl]-1,4,6,7-tetrahydro-
pyrazolo[4,3-c]pyridine-5-
carboxylic acid diethylamide;
3-(5-trifluoromethyl-1 H-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridine-5-carboxylic
acid diethylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid [2-(2H-tetrazol-5-yl)-
ethyl]-amide;
1-cyc lopropyl-3 -[3 -( 5-ethyl-6-methyl-1 H-benzoimidazol-2-yl)-1 H-pyrazol-4-
yl] -urea;
1-[3-(5-ethyl-6-methyl-1 H-benzoimidazol-2-yl)-1 H-pyrazol-4-yl]-3-methyl-
urea;
4-methyl-piperazine-l-carboxylic acid [3-(5-ethyl-6-methyl-lH-benzoimidazol-2-
yl)-1H-pyrazol-4-yl]-
amide;
piperidine-l-carboxylic acid [3-(5-fluoro-6-methyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide;
1-[3-(5-fluoro-6-methyl-1 H-benzoimidazol-2-yl)-1 H-pyrazol-4-yl]-3-methyl-
urea;
morpholine-4-carboxylic acid [3-(5-fluoro-6-methyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide;
4-methyl-piperazine-l-carboxylic acid [3-(5-fluoro-6-methyl-lH-benzoimidazol-2-
yl)-1H-pyrazol-4-
yl]-amide;
1-methyl-3-[3-(5-trifluoromethyl-1 H-benzoimidazol-2-yl)-1 H-pyrazol-4-yl]-
urea;
1-[3-(5 -chloro-6-methyl-1 H-benzoimidazol-2-yl)-1 H-pyrazol-4-yl]-3-methyl-
urea;
4-methyl-piperazine-l-carboxylic acid [3-(5-chloro-6-methyl-lH-benzoimidazol-2-
yl)-1H-pyrazol-4-
yl]-amide;
1-tert-butyl-3-[3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-urea;
1-[3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-3-ethyl-urea;
4-methyl-piperazine-l-carboxylic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-
1H-pyrazol-4-yl]-
amide;
1-cyclopropyl-3-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-urea;
3-[3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-1,1-diethyl-urea;
1-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1 H-pyrazol-4-yl]-3-isobutyl-urea;
1-cyclopropylmethyl-3-[3 -(5, 6-dimethyl-1 H-benzoimidazol-2-yl)-1 H-pyrazol-4-
yl] -urea;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-207-
3-(5-chloro-6-methyl-1 H-benzoimidazol-2-yl)-1 H-pyrazol-4-ylamine;
3-(5-ethyl-6-methyl-lH-benzoimidazol-2-yl)-1H-indazole-5-carboxylic acid amide
dihydrochloride;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-indazole-5-carboxylic acid;
2-(4-isobutyrylamino-lH-pyrazol-3-yl)-1H-benzoimidazole-5-carboxylic acid;
3-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-1,1-dimethyl-urea;
3-(5-nitro-1 H-benzoimidazol-2-yl)-1H-indazole;
2-(1H-Indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (2-piperidin-1-yl-
ethyl)-amide;
2-(1H-Indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (pyridin-2-ylmethyl)-
amide;
2-(1H-Indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid [3-(4-methyl-piperazin-
1-yl)-propyl]-amide;
N-[2-(1H-Indazol-3-yl)-1H-benzoimidazol-5-yl]-isobutyramide;
N-[3-(5,6-Dimethyl-1 H-benzoimidazol-2-yl)-1 H-pyrazol-4-yl]-2-piperidin-1-yl-
acetamide;
2-(1 H-indazol-3-yl)-3H-benzoimidazol-5-amine;
piperidine-l-carboxylic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide;
and the corresponding N-oxides, and their prodrugs; and pharmaceutically
acceptable salts and solvates
(e.g. hydrates) of such compounds and their N-oxides and prodrugs.
Preferred compounds of formula (Ixa) of the invention for the inhibition of
SYK are:-
2-(1 H-indazol-3-yl)-1 H-benzimidazole-5-carboxylic acid benzylamide;
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acid N-methylamide;
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acid N-ethylamide;
2-(1 H-indazol-3-yl)-1 H-benzimidazole-5-carboxylic acid N-isopropylamide;
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acid N-phenylamide;
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acid N-phenethylamide;
5,6-dimethyl-2-(5-methylsulfanyl-lH-pyrazol-3-yl)-1 H-benzoimidazole;
6-chloro-5-methyl-2-(5-methylsulfanyl-lH-pyrazol-3-yl)-1H-benzoimidazole;
6-chloro-2-(5-ethylsulfanyl-1 H-pyrazol-3-yl)-5-methyl-1 H-benzoimidazole;
2-(5-methylsulfanyl-lH-pyrazol-3-yl)-5-trifluoromethyl-1 H-benzoimidazole;
2-(5-cyclopropylmethylsulfanyl-1 H-pyrazol-3-yl)-5, 6-dimethyl-1 H-
benzoimidazole;
2-(5-ethylsulfanyl-1 H-pyrazol-3-yl)-5,6-dimethyl-1 H-benzoimidazole;
5,6-dimethyl-2-[5-(pyridin-3-ylmethylsulfanyl)-1H-pyrazol-3-yl]-1H-
benzoimidazole;
5-fluoro-2-[ 5-methylsulfanyl)-1 H-pyrazol-3 -yl] -1 H-b enzo imidazo le;
5, 6-dimethyl-2-(5-phenethylsulfanyl-1 H-pyrazol-3 -yl)-1 H-b enzoimidazole;
4-methyl-2-(5 -methylsulfanyl-1 H-pyrazol-3 -yl)-1 H-b enzoimidazole;
5,6-dimethyl-2-(5-benzylsulfanyl-1 H-pyrazol-3-yl)-1H-benzoimidazole;
5,6-dimethyl-2-[5-(thiophen-2-ylmethylsulfanyl)-1H-pyrazol-3-yl]-1H-
benzoimidazole;
2-(5-ethylsulfanyl-lH-pyrazol-3-yl)-5-methoxy-lH-benzoimidazole hydrochloride;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-208-
5-methyl-2-(5-methylsulfanyl-4-propyl-1 H-pyrazol-3-yl)-1 H-benzoimidazole;
2-(5-(4-methoxy-benzylsulfanyl)-4-propyl-lH-pyrazol-3-yl)- 5-methyl-lH-
benzoimidazole;
2-(5-benzylsulfanyl-4-isopropyl-1 H-pyrazol-3-yl)-5-methyl-1 H-benzoimidazole;
2-(5-methylsulfanyl-4-methyl-1 H-pyrazol-3-yl)-5-methoxy-1 H-benzoimidazole;
2-(5-methylsulfanyl-4-methyl-lH-pyrazol-3-yl)-5-methyl-lH-benzoimidazole;
3 -(5 -chloro-1 H-benzoimidazol-2-yl)-1 H-pyrazol-4-ylamine;
3-(5, 6-dichloro-1 H-benzoimidazol-2-yl)-1 H-pyrazo 1-4-ylamine;
5,6-dimethyl-2-(4-phenyl-1 H-pyrazol-3-yl)-1 H-benzoimidazole;
3-(1,5,6,7-tetrahydro-1,3-diaza-s-indacen-2-yl)-1H-pyrazole-4-carboxylic acid
cyclopropylamide;
3-(5-methoxy-6-methyl-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid
isopropylamide;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid (2-
methoxy-ethyl)-amide;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid
propylamide;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid
(tetrahydro-pyran-4-yl)-amide;
3-(5-difluoromethoxy-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid
isopropylamide;
3-(5-difluoromethoxy-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid
cyclopropylamide;
3-(6-ethyl-5-methoxy-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid
isopropylamide;
2-(5-ethoxy-1 H-pyrazol-3-yl)-1 H-benzoimidazole;
(benzoimidazol-2-yl)-5-methylthio-3 -pyrazole;
2-(5-isopropyl-1 H-pyrazol-3-yl)-5,6-dimethyl-lH-benzoimidazole;
2-(5-ethyl-lH-pyrazol-3-yl)-5,6-dimethyl-lH-benzoimidazole;
3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1 H-pyrazole-4-carboxylic acid
isopropylamide;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid (2-
hydroxy-1,1-dimethyl-
ethyl)-amide;
2-(4-isopropylcarbamoyl-lH-pyrazol-3-yl)-1H-benzoimidazole-5-carboxylic acid
(pyridin-3-ylmethyl)-
amide;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-5-methyl-lH-pyrazole-4-carboxylic acid
cyclopropylamide;
2-(4-isopropylcarbamoyl-lH-pyrazol-3-yl)-1H-benzoimidazole-5-carboxylic acid
phenylmethyl-amide;
3-(5, 6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid isobutyl-
amide;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid
isopropylamide;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid
cyclopropylmethyl-amide;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-5-methyl-lH-pyrazole-4-carboxylic acid
tert-butylamide;
2-(4-isobutyrylamino-lH-pyrazol-3-yl)-1H-benzoimidazole-5-carboxylic acid
benzylamide;
N-[3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-isobutyramide;
N-[3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-3-methyl-
butyramide;
N-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-2-phenyl-acetamide;
cyclopropanecarboxylic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-
4-yl]-amide;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-209-
methoxyacetic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-
amide;
cyclopentanecarboxylic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-
4-yl]-amide;
trimethylacetic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-
amide;
tef-t-butylacetic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-
yl]-amide;
butanoic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-amide;
isoxazole-5-carboxylic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-
4-yl]-amide;
S(+)-2-methylbutanoic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-
4-yl]-amide;
cyclopropanecarboxylic acid [3-(5-ethyl-6-methyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide;
piperidine-1-carboxylic acid[3-(6-chloro-5-methoxy-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide;
3-[3-(6-chloro-5-methoxy-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-1,1-
dimethylurea;
cyclopropanecarboxylic acid [3-(5-methoxy-lH-benzoimidazol-2-yl)-1H-pyrazol-4-
yl]-amide;
cyclopropanecarboxylic acid [3-(5-ethoxy-lH-benzoimidazol-2-yl)-1H-pyrazol-4-
yl]-amide;
cyclopropanecarboxylic acid [3-(5-fluoro-6-methyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide;
cyclopropanecarboxylic acid [3-(5-trifluoromethoxy-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide;
cyclopropanecarboxylic acid [3-(5-trifluoromethyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide;
N-[3-(5-trifluoromethyl-1 H-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-
isobutyramide;
cyclopropanecarboxylic acid [3-(5-chloro-6-methyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide;
3,5-dimethyl-isoxazole-4-carboxylic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-
yl)-1H-pyrazol-4-yl]-
amide;
N-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-acetamide;
furan-3-carboxylic acid [3-(5-chloro-6-methyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide;
N-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1 H-pyrazol-4-yl]-4-methyl-
benzamide;
N-[3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1 H-pyrazol-4-yl]-2-morpholin-4-yl-
acetamide;
2-dimethylamino-N-[3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1 H-pyrazol-4-yl]-
acetamide;
N-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]- 2-(1H-1,2,3,4-
tetraazol-1-yl)-acetamide;
N-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1 H-pyrazol-4-yl]-isonicotinamide;
2-cyclopropyl-N-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1 H-pyrazol-4-yl]-
acetamide;
1-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-3-methyl-urea;
1-[3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-3-isopropyl-urea;
1-[3-(5, 6-dimethyl-1 H-benzoimidazol-2-yl)-1 H-pyrazol-4-yl]-3-phenyl-urea;
1-benzyl-3-[3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-urea;
cyclopropanecarboxylic acid[3-(5-ethoxy-6-ethyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]amide;
4-methylpiperazine-l-carboxylic acid [3-(1,5,6,7-tetrahydro-1,3-diaza-s-
indacen-2-yl)-1H-pyrazol-4-
yl] amide;
1,1-dimethyl-3-[3-(1,5,6,7-tetrahydro-s-indacen-2-yl)-1 H-pyrazol-4-yl]urea;
cyclopropanecarboxylic acid [3-(6-ethoxy-5-fluoro-lH-benzimidazol-2-yl)-1H-
pyrazol-4-yl]amide;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-210-
tetrahydropyran-4-carboxylic acid [3-(6-ethoxy-5-fluoro-1 H-benzimidazol-2-yl)-
1 H-pyrazole-4-
yl]amide;
morpholine-4-carboxylic acid[3-(6-ethoxy-5-fluoro-lH-benzimidazol-2-yl)-1H-
pyrazol-4-yl]amide;
piperidine-4-carboxylic acid[3-(6-ethoxy-5-fluoro-lH-benzimidazol-2-yl)-1H-
pyrazol-4-yl]amide;
3-[6-ethoxy-5-fluoro-lH-benzimidazol-2-yl)-1H-pyrazol-4-yl]-1,1-diethylurea;
morpholine-4-carboxylic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-ylmethyl]-
amide;
3-[3-(5-difluoromethoxy-1 H-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-1,1-diethyl-
urea;
piperidine-l-carboxylic acid [3-(5-difluoromethoxy-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide;
cyclopropanecarboxylic acid [3-(6-chloro-5-methoxy-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide;
cyclopropanecarboxylic acid [3-(1,5,6,7-tetrahydro-1,3-diaza-s-indacen-2-yl)-
1H-pyrazol-4-yl]amide;
morpholine-4-carboxylic acid[3-(1,5,6,7-tetrahydro-1,3-diaza-s-indacen-2-yl)-
1H-pyrazol-4-yl]-amide;
piperidine-l-carboxylic acid [3-(5-methoxy-lH-benzoimidazol-2-yl)-1H-pyrazol-4-
yl]-amide;
3-[ 3-(5 -methoxy-1 H-b enzoimidazol-2 -yl)-1 H-pyrazo 1-4-y1]-1,1-dimethyl-
urea;
piperidine-l-carboxylic acid [3-(5-ethyl-6-methyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide;
3-[3-(5-fluoro-6-methyl-1 H-benzoimidazol-2-yl)-1 H-pyrazol-4-yl]-1,1-dimethyl-
urea;
morpholine-4-carboxylic acid [3-(5-trifluoromethyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide;
morpholine-4-carboxylic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide;
piperidine-l-carboxylic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide;
1-cyclopropyl-3-[3-(5-ethyl-6-methyl-1 H-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-
urea;
1-[3-(5-ethyl-6-methyl-1 H-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-3-methyl-urea;
4-methyl-piperazine-l-carboxylic acid [3-(5-ethyl-6-methyl-lH-benzoimidazol-2-
yl)-1H-pyrazol-4-yl]-
amide;
piperidine-l-carboxylic acid [3-(5-fluoro-6-methyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide;
1-[3-(5-fluoro-6-methyl-1 H-benzoimidazol-2-yl)-1 H-pyrazol-4-yl]-3-methyl-
urea;
morpholine-4-carboxylic acid [3-(5-fluoro-6-methyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide;
4-methyl-piperazine-l-carboxylic acid [3-(5-fluoro-6-methyl-lH-benzoimidazol-2-
yl)-1H-pyrazol-4-
yl]-amide;
1-methyl-3-[3-(5-trifluoromethyl-1 H-benzoimidazol-2-yl)-1 H-pyrazol-4-yl]-
urea;
1-[3-(5-chloro-6-methyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-3-methyl-urea;
4-methyl-piperazine-l-carboxylic acid [3-(5-chloro-6-methyl-lH-benzoimidazol-2-
yl)-1H-pyrazol-4-
yl]-amide;
1-tert-butyl-3-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-urea;
1-[ 3-(5, 6-dimethyl-1 H-b enzo imi dazo l-2-yl)-1 H-pyrazol-4-yl] -3 -ethyl-
ure a;
4-methyl-piperazine-l-carboxylic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-
1H-pyrazol-4-yl]-
amide;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-211-
1-cyclopropyl-3-[3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-
urea;
3-[3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1 H-pyrazol-4-yl]-1,1-diethyl-urea;
1-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1 H-pyrazol-4-yl]-3-isobutyl-urea;
1-cyclopropylmethyl-3-[3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1 H-pyrazol-4-
yl]-urea;
3-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-1,1-dimethyl-urea;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (2-piperidin-1-yl-
ethyl)-amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (pyridin-2-ylmethyl)-
amide;
N-[2-(1 H-indazol-3 -yl)-1 H-b enzoimidazol-5 -yl] -isobutyramide;
N-[3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1 H-pyrazol-4-yl]-2-piperidin-1-yl-
acetamide;
2-(1H-indazol-3-yl)-IH-benzimidazole-5-carboxylic acid N-morpholinoamide;
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acid N-(N'-
methylpiperazino)amide;
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acid N-pyrrolidinoamide;
2-(1 H-indazol-3-yl)-1 H-benzimidazole-5-carboxylic acid N-(isobutyl)amide;
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acid N-
(cyclohexylmethyl)amide;
2-(1 H-indazol-3-yl)-1 H-benzimidazole-5-carboxylic acid N-(2-furfuryl)amide;
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acid N-benzyl-N-methylamide;
methyl2-(1H-indazol-3-yl)-3H-benzimidazole-5- carboxylate;
5,6-dimethyl-2-(1 H-indazol-3-yl)-1H-benzimidazole;
2-(1H-indazol-3-yl)-3H-benzimidazole-4-carboxylic acid;
2-(5-ethoxy-2H-pyrazol-3-yl)-1 H-benzimidazole-4-carboxylic acid;
5, 6-dimethyl-2-(5-methyl-2H-pyrazol-3-yl)-1 H-benzimidazole;
5,6-dimethyl-2-(5-thiophen-2-yl-2H-pyrazol-3-yl)-1H-benzimidazole;
2-(4-bromo-2H-pyrazol-3-yl)-5, 6-dimethyl-lH-benzimidazole;
2-(5-ethyl-2H-pyrazol-3-yl)-5, 6-dimethyl-1 H-b enzimidazole;
2-(5-ethyl-2H-pyrazol-3-yl)-4, 5-ethylenedioxy-1 H-benzimidazole;
2-(5-ethyl-2H-pyrazol-3-yl)-5-methoxy-1 H-benzimidazole;
2-(5-ethyl-2H-pyrazol-3-yl)-4-hydroxy-1 H-benzimidazole
2-(5-ethyl-2H-pyrazol-3-yl)-5-bromo-1 H-benzimidazole;
and the corresponding N-oxides, and their prodrugs; and pharmaceutically
acceptable salts and solvates
(e.g. hydrates) of such compounds and their N-oxides and prodrugs.
Particularly preferred compounds of formula (Ixa) of the invention for the
inhibition of SYK are:-
2-(1H-indazol-3-yl)-IH-benzimidazole-5-carboxylic acid benzylamide, Example 1;
2-(1H-indazol-3-yl)-IH-benzimidazole-5-carboxylic acid N-methylamide, Example
2;
2-(1H-indazol-3-yl)-IH-benzimidazole-5-carboxylic acid N-ethylamide, Example
3;
2-(1H-indazol-3-yl)-IH-benzimidazole-5-carboxylic acid N-isopropylamide,
Example 4;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-212-
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acid N-phenylamide, Example
5;
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acidN-phenethylamide,
Example 6
5,6-dimethyl-2-(5-methylsulfanyl-lH-pyrazol-3-yl)-1H-benzoimidazole, (compound
denoted as
A9-B9), Example 230(a);
6-chloro-2-(5-methylsulfanyl-lH-pyrazol-3-yl)-5-methyl-lH-benzoimidazole,
(compound denoted as
A12-B9), Example 230(b);
6-chloro-2-(5-ethylsulfanyl-lH-pyrazol-3-yl)-5-methyl-lH-benzoimidazole,
(compound denoted as
A12-B10), Example 230(c);
2-(5-methylsulfanyl-lH-pyrazol-3-yl)-5-trifluoromethyl-lH-benzoimidazole,
(compound denoted as
A4-B9), Example 230(d);
2-(5-cyclopropylmethylsulfanyl-lH-pyrazol-3-yl)-5,6-dimethyl-lH-
benzoimidazole, (compound
denoted as A9-B11), Example 230(e);
2-(5-ethylsulfanyl-lH-pyrazol-3-yl)-5,6-dimethyl-lH-benzoimidazole, (compound
denoted as A9-B10),
Example 230(f);
3-(1,5,6,7-tetrahydro-1,3-diaza-s-indacen-2-yl)-1H-pyrazole-4-carboxylic acid
cyclopropylamide,
Example 235(ah);
3-(5-methoxy-6-methyl-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid
isopropylamide,
Example 235(ai);
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid (2-
methoxy-ethyl)-amide,
Example 235(ak);
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid
propylamide, Example
235(al);
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid
(tetrahydro-pyran-4-yl)-amide,
Example 235(am);
3-(5-difluoromethoxy-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid
isopropylamide,
Example 235(ao);
3-(5-difluoromethoxy-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid
cyclopropylamide,
Example 235(ap);
3-(6-ethyl-5-methoxy-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid
isopropylamide,
Example 235(aq);
2-(5-isopropyl-lH-pyrazol-3-yl)-5,6-dimethyl-lH-benzoimidazole, (compound
denoted as A9-B83),
Example 241(b);
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid
isopropylamide, (compound
denoted as A9-B106), Example 246(g);
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid (2-
hydroxy-1,1-dimethyl-
ethyl)-amide, (compound denoted as A9-B25), Example 246(h);
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-213-
2-(4-isopropylcarbamoyl-lH-pyrazol-3-yl)-1H-benzoimidazole-5-carboxylic acid
(pyridin-3-ylmethyl)-
amide, (compound denoted as A40-B 106), Example 246(i);
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-5-methyl-lH-pyrazole-4-carboxylic acid
cyclopropylamide,
(compound denoted as A9-B 105), Example 246(j);
2-(4-isopropylcarbamoyl-lH-pyrazol-3-yl)-1H-benzoimidazole-5-carboxylic acid
phenylmethyl-amide,
(compound denoted as A17-B106), Example 246(k);
3-(5, 6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid isobutyl-
amide, Example
246(v);
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid
isopropylamide, Example
246(w);
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid
cyclopropylmethyl-amide,
Example 246(x);
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-5-methyl-lH-pyrazole-4-carboxylic acid
tert-butylamide,
Example 246(y);
2-(4-isobutyrylamino-lH-pyrazol-3-yl)-1H-benzoimidazole-5-carboxylic acid
benzylamide, Example
246(aa);
N-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-isobutyramide,
(compound denoted as
A9-B85), Example 248(a);
N-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-3-methyl-
butyramide, (compound
denoted as A9-B86), Example 248(b);
N-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-2-phenyl-acetamide,
(compound denoted
as A9-B36), Example 248(c);
cyclopropanecarboxylic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-
4-yl]-amide,
(compound denoted as A9-B89), Example 248(d);
methoxyacetic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-
amide, (compound
denoted as A9-B94), Example 248(e);
cyclopentanecarboxylic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-
4-yl]-amide,
(compound denoted as A9-B87), Example 248(f);
trimethylacetic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-
amide, (compound
denoted as A9-B88), Example 248(g);
tert-butylacetic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-
amide, (compound
denoted as A9-B90), Example 248(h);
butanoic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-amide,
(compound denoted
as A9-B91), Example 248(i);
isoxazole-5-carboxylic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-
4-yl]-amide,
(compound denoted as A9-B96), Example 248(j);
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-214-
S(+)-2-methylbutanoic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-
4-yl]-amide,
(compound denoted as A9-B93), Example 248(k);
cyclopropanecarboxylic acid [3-(5-ethyl-6-methyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide,
(compound denoted as A55-B89), Example 248(1);
piperidine-l-carboxylic acid[3-(6-chloro-5-methoxy-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide,
Example 248(m);
3-[3-(6-chloro-5-methoxy-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-1,1-
dimethylurea, Example
248(n);
cyclopropanecarboxylic acid [3-(5-methoxy-lH-benzoimidazol-2-yl)-1H-pyrazol-4-
yl]-amide, Example
248(o);
cyclopropanecarboxylic acid [3-(5-ethoxy-lH-benzoimidazol-2-yl)-1H-pyrazol-4-
yl]-amide, Example
248(p);
cyclopropanecarboxylic acid [3-(5-fluoro-6-methyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide,
Example 248(q);
cyclopropanecarboxylic acid [3-(S-trifluoromethoxy-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide,
Example 248(r);
cyclopropanecarboxylic acid [3-(5-trifluoromethyl-1H-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide,
Example 248(s);
N-[3-(5-trifluoromethyl-1 H-benzoimidazol-2-yl)-1 H-pyrazol-4-y1]-
isobutyramide, Example 248(t);
cyclopropanecarboxylic acid [3-(S-chloro-6-methyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide,
Example 248(u);
3,5-dimethyl-isoxazole-4-carboxylic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-
yl)-1H-pyrazol-4-yl]-
amide, Exainple 248(v);
N-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-acetamide, Example
248(w);
furan-3-carboxylic acid [3-(S-chloro-6-methyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide,
Example 248(x);
N-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-4-methyl-benzamide,
Example 248(y);
N-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-2-morpholin-4-yl-
acetamide, (compound
denoted as A9-B99), Example 253;
N-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]- 2-(1H-1,2,3,4-
tetraazol-1-yl)-acetamide,
(compound denoted as A9-B97), Example 254(a);
N-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-isonicotinamide;
Example 254(b);
2-cyclopropyl-N-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-
acetamide; Example
254(c);
1-[3-(5,6-dimethyl-lH-benzoimidazol-2-y1)-1H-pyrazol-4-yl]-3-methyl-urea,
(compound denoted as
A9-B38), Example 255(a);
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-215-
1-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-3-isopropyl-urea,
(compound denoted as
A9-B103), Example 255(b);
1-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-3-phenyl-urea,
(compound denoted as
A9-B40), Example 255(c);
1-benzyl-3-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-urea,
(compound denoted as
A9-B39), Example 255(d);
cyclopropanecarboxylic acid[3-(5-ethoxy-6-ethyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]amide,
Example 256(a);
4-methylpiperazine-l-carboxylic acid [3-(1,5,6,7-tetrahydro-1,3-diaza-s-
indacen-2-yl)-1H-pyrazol-4-
yl]amide, Example 256(c);
1,1-dimethyl-3-[3-(1,5,6,7-tetrahydro-s-indacen-2-yl)-1H-pyrazol-4-yl]urea,
Example 256(d);
cyclopropanecarboxylic acid [3-(6-ethoxy-5-fluoro-lH-benzimidazol-2-yl)-1H-
pyrazol-4-yl]amide,
Example 257(a);
tetrahydropyran-4-carboxylic acid [3-(6-ethoxy-5-fluoro-lH-benzimidazol-2-yl)-
1H-pyrazole-4-
yl]amide, Example 257(b);
morpholine-4-carboxylic acid[3-(6-ethoxy-5-fluoro-lH-benzimidazol-2-yl)-1H-
pyrazol-4-yl]amide,
Example 257(c);
piperidine-4-carboxylic acid[3-(6-ethoxy-5-fluoro-lH-benzimidazol-2-yl)-1H-
pyrazol-4-yl]amide,
Example 257(d);
3-[6-ethoxy-5-fluoro-lH-benzimidazol-2-yl)-1H-pyrazol-4-yl]-1,1-diethylurea,
Example 257(e);
morpholine-4-carboxylic acid [3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1 H-
pyrazol-4-ylmethyl]-amide,
Example 257(g);
3-[3-(5-difluoroinethoxy-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-1,1-diethyl-
urea, Example 257(h);
piperidine-l-carboxylic acid [3-(5-difluoromethoxy-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide,
Example 257(i);
cyclopropanecarboxylic acid [3-(6-chloro-5-methoxy-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide,
Example 258(a);
cyclopropanecarboxylic acid [3-(1,5,6,7-tetrahydro-1,3-diaza-s-indacen-2-yl)-
1H-pyrazol-4-yl]amide,
Example 258(b);
morpholine-4-carboxylic acid[3-(1,5,6,7-tetrahydro-1,3-diaza-s-indacen-2-yl)-
1H-pyrazol-4-yl]-amide,
Example 258(c);
piperidine-l-carboxylic acid [3-(5-methoxy-lH-benzoimidazol-2-yl)-1H-pyrazol-4-
yl]-amide, Example
258(d);
3-[3-(5-methoxy-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-1,1-dimethyl-urea,
Example 258(e);
piperidine-l-carboxylic acid [3-(5-ethyl-6-methyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide,
Example 258(f);
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-216-
3-[3-(5-fluoro-6-methyl-lH-benzoimidazol-2-yl)-IH-pyrazol-4-yl]-1,1-dimethyl-
urea, Example 258(g);
morpholine-4-carboxylic acid [3-(5-trifluoromethyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide,
Example 258(h);
morpholine-4-carboxylic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide,
Example 258(n);
piperidine-l-carboxylic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide,
Example 258(o);
1-cyclopropyl-3-[3-(5-ethyl-6-methyl-lH-benzoimidazol-2-yl)-IH-pyrazol-4-yl]-
urea, Example 260(a);
1-[3-(5-ethyl-6-methyl-lH-benzoimidazol-2-yl)-IH-pyrazol-4-yl]-3-methyl-urea,
Example 260(b);
4-methyl-piperazine-l-carboxylic acid [3-(5-ethyl-6-methyl-lH-benzoimidazol-2-
yl)-1H-pyrazol-4-yl]-
amide, Example 260(c);
piperidine-l-carboxylic acid [3-(5-fluoro-6-methyl-lH-benzoimidazol-2-yl)-IH-
pyrazol-4-yl]-amide,
Example 260(d);
1-[3-(5-fluoro-6-methyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-3-methyl-urea,
Example 260(e);
morpholine-4-carboxylic acid [3-(5-fluoro-6-methyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide,
Example 260(f);
4-methyl-piperazine-l-carboxylic acid [3-(5-fluoro-6-methyl-lH-benzoimidazol-2-
yl)-1H-pyrazol-4-
yl]-amide, Example 260(g);
1-methyl-3-[3-(5-trifluoromethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-urea,
Example 260(h);
1-[3-(5-chloro-6-methyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-3-methyl-urea,
Example 260(i);
4-methyl-piperazine-l-carboxylic acid [3-(5-chloro-6-methyl-lH-benzoimidazol-2-
yl)-1H-pyrazol-4-
yl]-amide, Example 260(j);
1-tert-butyl-3-[3-(5,6-dimethyl-IH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-urea,
Example 260(k);
1-[3-(5,6-dimethyl-IH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-3-ethyl-urea,
Example 260(1);
4-methyl-piperazine-l-carboxylic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-
1H-pyrazol-4-yl]-
amide, Example 260(m);
1-cyclopropyl-3-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-IH-pyrazol-4-yl]-urea,
Example 260(n);
3-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-1,1-diethyl-urea,
Example 260(o);
1-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-IH-pyrazol-4-yl]-3-isobutyl-urea,
Example 260(p);
1-cyclopropylmethyl-3-[3-(5,6-dimethyl-IH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-
urea, Example
260(q);
3-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-IH-pyrazol-4-yl]-1,1-dimethyl-urea,
Example 258(r);
2-(IH-Indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (2-piperidin-1-yl-
ethyl)-amide, Example
246(ab);
2-(1H-Indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (pyridin-2-ylmethyl)-
amide, Example
246(ac);
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-217-
N-[3-(5,6-Dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-2-piperidin-1-yl-
acetamide, Example
253(c);
and the corresponding N-oxides, and their prodrugs; and pharmaceutically
acceptable salts and solvates
(e.g. hydrates) of such compounds and their N-oxides and prodrugs.
Especially preferred compounds of formula (Ixa), denoted as the product of the
combination of group
Al in Table 1 and Bl in Table 2, of the invention for the inhibition of SYK
are:-
3-(1,5,6,7-tetrahydro-1,3-diaza-s-indacen-2-yl)-1H-pyrazole-4-carboxylic acid
cyclopropylamide,
Example 235(ah);
3-(5-methoxy-6-methyl-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid
isopropylamide,
Example 235(ai);
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid (2-
methoxy-ethyl)-amide,
Example 235(ak);
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid
propylamide, Example
235(al);
3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1 H-pyrazole-4-carboxylic acid
(tetrahydro-pyran-4-yl)-amide,
Example 235(am);
3-(5-difluoromethoxy-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid
isopropylamide,
Example 235(ao);
3-(5-difluoromethoxy-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid
cyclopropylamide,
Example 235(ap);
3-(6-ethyl-5-methoxy-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid
isopropylamide,
Example 235(aq);
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid
isopropylamide, (compound
denoted as A9-B106), Example 246(g);
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid (2-
hydroxy-l,l-dimethyl-
ethyl)-amide, (compound denoted as A9-B25), Example 246(h);
2-(4-isopropylcarbamoyl-lH-pyrazol-3-yl)-1H-benzoimidazole-5-carboxylic acid
(pyridin-3-ylmethyl)-
amide, (compound denoted as A40-B106), Exainple 246(i);
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-5-methyl-lH-pyrazole-4-carboxylic acid
cyclopropylamide,
(compound denoted as A9-B 105), Example 246(j);
2-(4-isopropylcarbamoyl-lH-pyrazol-3-yl)-1H-benzoimidazole-5-carboxylic acid
phenylmethyl-amide,
(compound denoted as A17-B106), Example 246(k);
3-(5, 6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid isobutyl-
amide, Example
246(v);
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-218-
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid
isopropylamide, Example
246(w);
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid
cyclopropylmethyl-amide,
Example 246(x);
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-5-methyl-lH-pyrazole-4-carboxylic acid
tert-butylamide,
Example 246(y);
2-(4-isobutyrylamino-lH-pyrazol-3-yl)-1H-benzoimidazole-5-carboxylic acid
benzylamide, Example
246(aa);
N-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-isobutyramide,
(compound denoted as
A9-B85), Example 248(a);
N-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-3-methyl-
butyramide, (compound
denoted as A9-B86), Example 248(b);
cyclopropanecarboxylic acid [3-(5,6-dimethyl-lH-benzoiinidazol-2-yl)-1H-
pyrazol-4-yl]-amide,
(compound denoted as A9-B89), Example 248(d);
methoxyacetic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-
amide, (compound
denoted as A9-B94), Example 248(e);
cyclopentanecarboxylic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-
4-yl]-amide,
(compound denoted as A9-B87), Example 248(f);
trimethylacetic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-
amide, (compound
denoted as A9-B88), Example 248(g);
tert-butylacetic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-
amide, (compound
denoted as A9-B90), Example 248(h);
butanoic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-amide,
(compound denoted
as A9-B91), Example 248(i);
isoxazole-5-carboxylic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-
4-yl]-amide,
(compound denoted as A9-B96), Example 248(j);
S(+)-2-methylbutanoic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-
4-yl]-amide,
(compound denoted as A9-B93), Example 248(k);
cyclopropanecarboxylic acid [3-(5-ethyl-6-methyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide,
(compound denoted as A55-B89), Example 248(1);
piperidine-l-carboxylic acid[3-(6-chloro-5-methoxy-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide,
Example 248(m);
3-[3-(6-chloro-5-methoxy-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-1,1-
dimethylurea, Example
248(n);
cyclopropanecarboxylic acid [3-(5-methoxy-lH-benzoimidazol-2-yl)-1H-pyrazol-4-
yl]-amide, Example
248(o);
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-219-
cyclopropanecarboxylic acid [3-(5-ethoxy-lH-benzoimidazol-2-yl)-1H-pyrazol-4-
yl]-amide, Example
248(p);
cyclopropanecarboxylic acid [3-(5-fluoro-6-methyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide,
Example 248(q);
cyclopropanecarboxylic acid [3-(5-trifluoromethyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide,
Example 248(s);
N-[3-(5-trifluoromethyl-1 H-benzoimidazol-2-yl)-1 H-pyrazol-4-yl]-
isobutyramide, Example 248(t);
cyclopropanecarboxylic acid [3-(5-chloro-6-methyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide,
Example 248(u);
3,5-dimethyl-isoxazole-4-carboxylic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-
yl)-1H-pyrazol-4-yl]-
amide, Example 248(v);
furan-3-carboxylic acid [3-(5-chloro-6-methyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide,
Example 248(x);
N-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-2-morpholin-4-yl-
acetamide, (compound
denoted as A9-B99), Example 253;
N-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]- 2-(1H-1,2,3,4-
tetraazol-1-yl)-acetamide,
(compound denoted as A9-B97), Example 254(a);
N-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-isonicotinamide;
Example 254(b);
2-cyclopropyl-N-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-
acetamide; Example
254(c);
1-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-3-methyl-urea,
(compound denoted as
A9-B38), Example 255(a);
1-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-3-isopropyl-urea,
(compound denoted as
A9-B103), Example 255(b);
1-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-3-phenyl-urea,
(compound denoted as
A9-B40), Example 255(c);
1-benzyl-3-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-urea,
(compound denoted as
A9-B39), Example 255(d);
cyclopropanecarboxylic acid[3-(5-ethoxy-6-ethyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]amide,
Example 256(a);
4-methylpiperazine-l-carboxylic acid [3-(1,5,6,7-tetrahydro-1,3-diaza-s-
indacen-2-yl)-1H-pyrazol-4-
yl]amide, Example 256(c);
1,1-dimethyl-3-[3-(1,5,6,7-tetrahydro-s-indacen-2-yl)-1H-pyrazol-4-yl]urea,
Example 256(d);
cyclopropanecarboxylic acid [3-(6-ethoxy-5-fluoro-lH-benzimidazol-2-yl)-1H-
pyrazol-4-yl]amide,
Example 257(a);
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-220-
tetrahydropyran-4-carboxylic acid [3-(6-ethoxy-5-fluoro-lH-benzimidazol-2-yl)-
1H-pyrazole-4-
yl]amide, Example 257(b);
morpholine-4-carboxylic acid[3-(6-ethoxy-5-fluoro-lH-benzimidazol-2-yl)-1H-
pyrazol-4-yl]amide,
Example 257(c);
piperidine-4-carboxylic acid[3-(6-ethoxy-5-fluoro-lH-benzimidazol-2-yl)-1H-
pyrazol-4-yl]amide,
Example 257(d);
3-[6-ethoxy-5-fluoro-lH-benzimidazol-2-yl)-1H-pyrazol-4-yl]-1,1-diethylurea,
Example 257(e);
3-[3-(5-difluoromethoxy-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-1,1-diethyl-
urea, Example 257(h);
piperidine-l-carboxylic acid [3-(5-difluoromethoxy-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide,
Example 257(i);
cyclopropanecarboxylic acid [3-(6-chloro-5-methoxy-lH-benzoimidazol-2-yl)-1 H-
pyrazol-4-yl]-amide,
Example 258(a);
cyclopropanecarboxylic acid [3-(1,5,6,7-tetrahydro-1,3-diaza-s-indacen-2-yl)-
1H-pyrazol-4-yl]amide,
Example 258(b);
morpholine-4-carboxylic acid[3-(1,5,6,7-tetrahydro-1,3-diaza-s-indacen-2-yl)-
1H-pyrazol-4-yl]-amide,
Example 258(c);
piperidine-l-carboxylic acid [3-(5-methoxy-lH-benzoimidazol-2-yl)-1H-pyrazol-4-
yl]-amide, Example
258(d);
3-[3-(5-methoxy-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-1,1-dimethyl-urea,
Example 258(e);
piperidine-l-carboxylic acid [3-(5-ethyl-6-methyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide,
Example 258(f);
3-[3-(5-fluoro-6-methyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-1,1-dimethyl-
urea, Example 258(g);
morpholine-4-carboxylic acid [3-(5-trifluoromethyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide,
Example 258(h);
morpholine-4-carboxylic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide,
Example 258(n);
piperidine-l-carboxylic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide,
Example 258(o);
1-cyclopropyl-3-[3-(5-ethyl-6-methyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-
urea, Example 260(a);
1-[3-(5-ethyl-6-methyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-3-methyl-urea,
Example 260(b);
4-methyl-piperazine-l-carboxylic acid [3-(5-ethyl-6-methyl-lH-benzoimidazol-2-
yl)-1H-pyrazol-4-yl]-
amide, Example 260(c);
piperidine-l-carboxylic acid [3-(5-fluoro-6-methyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide,
Example 260(d);
1-[3-(5-fluoro-6-methyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-3-methyl-urea,
Example 260(e);
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-221-
morpholine-4-carboxylic acid [3-(5-fluoro-6-methyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide,
Example 260(f);
1-methyl-3-[3-(5-trifluoromethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-urea,
Example 260(h);
1-[3-(5-chloro-6-inethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-3-methyl-
urea, Example 260(i);
4-methyl-piperazine-l-carboxylic acid [3-(5-chloro-6-methyl-lH-benzoimidazol-2-
yl)-1H-pyrazol-4-
yl]-amide, Example 260(j);
1-tert-butyl-3-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-urea,
Example 260(k);
1-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-3-ethyl-urea,
Example 260(1);
4-methyl-piperazine-l-carboxylic acid [3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-
1H-pyrazol-4-yl]-
amide, Example 260(m);
1-cyclopropyl-3-[3-(5,6-dimethyl-lH-benzoiinidazol-2-yl)-1H-pyrazol-4-yl]-
urea, Example 260(n);
3-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-1,1-diethyl-urea,
Example 260(o);
1-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-3-isobutyl-urea,
Example 260(p);
1-cyclopropylmethyl-3-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-
urea, Example
260(q); 3-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-1,1-
dimethyl-urea, (compound denoted as
A9-B 142), Example 25 8(r);
and the corresponding N-oxides, and their prodrugs; and pharmaceutically
acceptable salts and solvates
(e.g. hydrates) of such compounds and their N-oxides and prodrugs.
More especially preferred compounds of formula (Ixa) of the invention for the
inhibition of SYK are:-
3-(5-methoxy-6-methyl-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid
isopropylamide,
Example 235(ai);
3-(1,5,6,7-tetrahydro-1,3-diaza-s-indacen-2-yl)-1H-pyrazole-4-carboxylic acid
cyclopropylamide,
Example 235(ah);
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid
(tetrahydro-pyran-4-yl)-amide,
Example 235(am);
3-(5, 6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid isobutyl-
amide, Example
246(v);
cyclopropanecarboxylic acid[3-(5-ethoxy-6-ethyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]amide,
Example 256(a);
1,1-dimethyl-3-[3-(1,5,6,7-tetrahydro-s-indacen-2-yl)-1H-pyrazol-4-yl]urea,
Example 256(d);
piperidine-4-carboxylic acid[3-(6-ethoxy-5-fluoro-lH-benzimidazol-2-yl)-1H-
pyrazol-4-yl]amide,
Example 257(d);
3-[6-ethoxy-5-fluoro-lH-benzimidazol-2-yl)-1H-pyrazol-4-yl]-1,1-diethylurea,
Example 257(e);
3-[3-(5-difluoromethoxy-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-1,1-diethyl-
urea, Example 257(h);
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-222-
piperidine-l-carboxylic acid [3-(5-difluoromethoxy-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide,
Example 257(i);
cyclopropanecarboxylic acid [3-(1,5,6,7-tetrahydro-1,3-diaza-s-indacen-2-yl)-
1H-pyrazol-4-yl]amide,
Example 258(b);
piperidine-l-carboxylic acid [3-(5-methoxy-lH-benzoimidazol-2-yl)-IH-pyrazol-4-
yl]-amide, Example
258(d);
piperidine-l-carboxylic acid [3-(5-ethyl-6-methyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide,
Example 258(f);
piperidine-l-carboxylic acid [3-(5,6-dimethyl-IH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide,
Example 258(o);
1-cyclopropyl-3-[3-(5-ethyl-6-methyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-
urea, Example 260(a);
piperidine-l-carboxylic acid [3-(5-fluoro-6-methyl-lH-benzoimidazol-2-yl)-1H-
pyrazol-4-yl]-amide,
Example 260(d);
1-tert-butyl-3-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-urea,
Example 260(k);
1-cyclopropyl-3-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-urea,
Example 260(n);
3-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-IH-pyrazol-4-yl]-1,1-diethyl-urea,
Example 260(o);
1-cyclopropylmethyl-3-[3-(5,6-dimethyl-IH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-
urea, Example
260(q);
3-[3-(5,6-dimethyl-lH-benzoiinidazol-2-yl)-1H-pyrazol-4-yl]-1,1-dimethyl-urea,
Example 258(r);
and the corresponding N-oxides, and their prodrugs; and pharmaceutically
acceptable salts and solvates
(e.g. hydrates) of such compounds and their N-oxides and prodrugs.
Preferred compounds of formula (Ixb) of the invention for the inhibition of
SYK are:-
3-(1 H-benzoimidazol-2-yl)-1H-indazole;
3-(5-methoxy-lH-benzoimidazol-2-yl)-1H-indazole;
[2-(indazol-3-yl)-1 H-benzoimidazol-5-yl] -phenyl-methanone;
2-(1 H-indazol-3-yl)-3H-benzoimidazol-4-ol;
3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1H-indazole;
2-(1H-indazol-3-yl)-3H-imidazo[4,5-c]pyridine;
2-(1H-indazole-3-yl)-3H-imidazo[4,5-b]pyridine;
3 -(5, 6-dimethyl-1 H-benzoimidazol-2-yl)-5 -methoxy-1 H-indazole;
3-(5, 6-dimethyl-1 H-b enzoimidazol-2-yl)-5-fluoro-1 H-indazole;
3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-6-fluoro-lH-indazole;
3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-5-methyl-1 H-indazole;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-6-methoxy-lH-indazole;
3 -(5 -ethyl-1 H-benzoimidazol-2-yl)-1 H-indazole;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-223-
3-(5-ethyl-6-methyl-1 H-benzoimidazol-2-yl)-1 H-indazole;
3-(5-isopropyl-6-methyl-lH-benzoimidazol-2-yl)-1 H-indazole;
3-(5-bromo-6-methyl-1 H-benzoimidazol-2-yl)-1H-indazole;
3-(5-bromo-1 H-benzoimidazol-2-yl)-1 H-indazole;
3-(5-(3-cyano)phenyl-1 H-benzoimidazol-2-yl)-1 H-indazole;
3-(5-(pyrid-3-yl)-1 H-benzoimidazol-2-yl)-1 H-indazole;
3-(6-methyl-5-phenyl-1 H-benzoimidazol-2-yl)-1H-indazole;
3-(5-phenyl-1 H-benzoimidazol-2-yl)-1 H-indazole;
3-(5-(2-fluoro)phenyl- 1 H-benzoimidazol-2-yl)-1 H-indazole;
3-(5-(5,6-methylenedioxy)phenyl-1H-benzoimidazol-2-yl)-1H-indazole;
3-(5-(2-methoxy)phenyl-1 H-benzoimidazol-2-yl)-1 H-indazole;
3-(5-(4-chloro)phenyl- 1 H-benzoimidazol-2-yl)-1 H-indazole;
3-(5-(4-methyl)phenyl-1 H-benzoimidazol-2-yl)-1 H-indazole;
3-(5 -benzyloxy- 1 H-benzoimidazol-2-yl)- 1 H-indazole;
3-(5,6-methylenedioxy-1H-benzoimidazol-2-yl)-1H-indazole;
3-(5, 6-dimethoxy-1 H-b enzo imidazol-2-yl)-1 H-indazole;
3-(5,6-diethyl-1 H-benzoimidazol-2 -yl)-1 H-indazole;
2-(1 H-indazol-3-yl)-1 H-benzoimidazole-5-carbonitrile;
3-(5-methoxycarbonyl- 1 H-benzoimidazol-2-yl)-1 H-indazole;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-5-ethoxy-lH-indazole;
3-[5-(2-morpholin-4-yl-ethoxy)-1 H-benzoimidazol-2-yl] -1 H-indazole;
3-(5-ethyl-6-methyl-1 H-benzoimidazol-2-yl)-1 H-indazole-5-carbonitrile;
3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1 H-indazole-5-carbonitrile;
3-(5,6-dimethyl- 1 H-benzoimidazol-2-yl)-4-fluoro-1 H-indazole;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-5-chloro-lH-indazole;
3-(5-n-propyl-1 H-benzoimidazol-2-yl)-1 H-indazole;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-sulfonic acid benzylamide;
3-(5-methanesulfonyl-1 H-benzoimidazol-2-yl)-1 H-indazole;
[2-(indazol-3 -yl)-1 H-benzoimidazol-5-yl] -phenyl-methanol;
[2-(indazol-3-yl)-1H-benzoimidazol-5-yl]-carboxylic acid;
[2-(indazol-3-yl)-1H-benzoimidazol-5-yl]-carboxylic acid, methylamide;
[2-(indazol-3-yl)-1H-benzoimidazol-5-yl]-carboxylic acid, dimethylamide;
[2-(indazol-3-yl)-1H-benzoimidazol-5-yl]-carboxylic acid, isopropylamide;
[2-(indazol-3-yl)-1H-benzoimidazol-5-yl]-carboxylic acid, benzylamide;
[2-(indazol-3-yl)-1H-benzoimidazol-5-yl]-carboxylic acid, benzamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (pyridin-3-ylmethyl)-
amide;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-224-
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 3-methyl-benzylamide;
2-(1H-indazol-3-yl)-lH-benzoimidazole-5-carboxylic acid 4-methyl-benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid [3-(2-oxo-pyrrolidin-1-
yl)-propyl]-amide;
2-(1 H-indazol-3-yl)-1 H-benzoimidazole-5-carboxylic acid (2-morpholin-4-yl-
ethyl)-amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (2-methoxy-ethyl)-
amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (2-cyano-ethyl)-amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (2-hydroxy-l,l-
dimethyl-ethyl)-amide;
2-(lH-Indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (3-imidazol-1-yl-
propyl)-amide;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-indazole-5-carboxylic acid
dimethylamide;
[2-(indazol-3-yl)-1 H-benzoimidazol-5-yl]-carboxylic acid;
3-(5-ethyl-6-methyl-lH-benzoimidazol-2-yl)-1H-indazole-5-carboxylic acid amide
dihydrochloride;
and the corresponding N-oxides, and their prodrugs; and pharmaceutically
acceptable salts and solvates
(e.g. hydrates) of such compounds and their N-oxides and prodrugs.
Particularly preferred compounds of formula (Ixb), denoted as the product of
the combination of group
Al in Table 1 and B 1 in Table 2, of the invention for the inhibition of SYK
are:-
3-(1H-benzoimidazol-2-yl)-1H-indazole, (compound denoted as A1-B63), Example
234(a);
3-(5-methoxy-lH-benzoimidazol-2-yl)-1H-indazole, (compound denoted as A6-B63),
Example 234(b);
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-indazole, (compound denoted as A9-
B63), Example
234(f);
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-5-methoxy-lH-indazole, (compound
denoted as A9-B68),
Example 235(b);
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-5-fluoro-lH-indazole, (compound denoted
as A9-B70),
Example 235(d);
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-6-fluoro-lH-indazole, (compound denoted
as A9-B71),
Example 235(e);
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-5-methyl-lH-indazole, (compound denoted
as A9-B64),
Example 235(f);
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-6-methoxy-lH-indazole, (compound
denoted as A9-B69),
Example 235(g);
3-(5-ethyl-1H-benzoimidazol-2-yl)-1H-indazole, (compound denoted as A27-B63),
Example 235(i);
3-(5-ethyl-6-methyl-lH-benzoimidazol-2-yl)-1H-indazole, (compound denoted as
A55-B63), Example
235(j);
3-(5-isopropyl-6-methyl-lH-benzoimidazol-2-yl)-1H-indazole, (compound denoted
as A54-B63),
Example 235(k);
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-225-
3-(5-bromo-6-methyl-lH-benzoimidazol-2-yl)-1H-indazole, (compound denoted as
A58-B63), Example
235(1);
3-(5-bromo-lH-benzoimidazol-2-yl)-1H-indazole, (compound denoted as A32-B63),
Example 235(m);
3-(5-(3-cyano)phenyl-lH-benzoimidazol-2-yl)-1H-indazole, (compound denoted as
A68-B63),
Example 235(n);
3-(5-(pyrid-3-yl)-1H-benzoimidazol-2-yl)-1H-indazole, (compound denoted as A69-
B63), Example
235(o);
3-(6-methyl-5-phenyl-lH-benzoimidazol-2-yl)-1H-indazole, (compound denoted as
A57-B63),
Example 235(p);
3-(5-phenyl-lH-benzoimidazol-2-yl)-1H-indazole, (compound denoted as A60-B63),
Example 235(q);
3-(5-(2-fluoro)phenyl-lH-benzoimidazol-2-yl)-1H-indazole, (compound denoted as
A65-B63),
Example 235(r);
3-(5-(3,4 -methylenedioxy)phenyl-lH-benzoimidazol-2-yl)-1H-indazole, (compound
denoted as
A66-B63), Example 235(s);
3-(5-benzyloxy-lH-benzoimidazol-2-yl)-1H-indazole, (compound denoted as A74-
B63), Example
235(w);
3-(5,6-methylenedioxy-lH-benzoimidazol-2-yl)-1H-indazole, (compound denoted as
A22-B63),
Example 235(x);
3-(5,6-dimethoxy-lH-benzoimidazol-2-yl)-1H-indazole, (compound denoted as A23-
B63), Example
235(y);
3-(5,6-diethyl-lH-benzoimidazol-2-yl)-1H-indazole, (compound denoted as A56-
B63), Example
235(z);
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carbonitrile, (compound denoted as A33-
B63), Example
235(ab);
3-(5-methoxycarbonyl-lH-benzoimidazol-2-yl)-1H-indazole, (compound denoted as
A35-B63),
Example 235(ac);
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-5-ethoxy-lH-indazole, (compound denoted
as A9-B63),
(compound denoted as A9-B 112), Example 235(ad);
3-[5-(2-morpholin-4-yl-ethoxy)-1H-benzoimidazol-2-yl]-1H-indazole, Example
235(aj);
3-(5-ethyl-6-methyl-lH-benzoimidazol-2-yl)-1H-indazole-5-carbonitrile, Example
235(an);
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-indazole-5-carbonitrile, Example
235(ar);
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-4-fluoro-lH-indazole, (compound denoted
as A9-B110),
Example 242(a);
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-5-chloro-lH-indazole, (compound denoted
as A9-B109),
Example 242(c);
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-226-
3-(5-n-propyl-lH-benzoimidazol-2-yl)-1H-indazole, (compound denoted as A28-
B63), Example
244(a);
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-sulfonic acid benzylamide,
Example244(b);
3-(5-methanesulfonyl-lH-benzoimidazol-2-yl)-1H-indazole; Example 244(c)
[2-(indazol-3-yl)-1H-benzoimidazol-5-yl]-phenyl-methanol, (compound denoted as
A34-B63),
Example 245;
[2-(indazol-3-yl)-1H-benzoimidazol-5-yl]-carboxylic acid, ethylamide,
(compound denoted as
A36-B63), Example 246(a);
[2-(indazol-3-yl)-1H-benzoimidazol-5-yl]-carboxylic acid, methylamide,
(compound denoted as
A15-B63), Example 246(b);
[2-(indazol-3-yl)-1H-benzoimidazol-5-yl]-carboxylic acid, isopropylamide,
(compound denoted as
A16-B63), Example 246(d);
[2-(indazol-3-yl)-1H-benzoimidazol-5-yl]-carboxylic acid, benzylamide,
(compound denoted as
A17-B63), Example 246(e);
[2-(indazol-3-yl)-1H-benzoimidazol-5-y1]-carboxylic acid, benzamide, (compound
denoted as
A52-B63), Example 246(f);
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (pyridin-3-ylmethyl)-
amide, Exainple
246(m);
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 3-methyl-benzylamide,
Example 246(n);
2-(1H-indazol-3-yl)-lH-benzoimidazole-5-carboxylic acid 4-methyl-benzylamide,
Example 246(o);
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid [3-(2-oxo-pyrrolidin-1-
yl)-propyl]-amide,
Example 246(p);
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (2-morpholin-4-yl-
ethyl)-amide, Example
246(q);
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (2-methoxy-ethyl)-
amide, Example 246(r);
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (2-cyano-ethyl)-amide,
Example 246(s);
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (2-hydroxy-1,1-
dimethyl-ethyl)-amide,
Example 246(t);
2-(1H-Indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (3-imidazol-1-yl-
propyl)-amide, Example
246(u),
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1H-indazole-5-carboxylic acid
dimethylamide, Example
246(x);
[2-(indazol-3-yl)-1H-benzoimidazol-5-yl]-carboxylic acid, (compound denoted as
A14-B63), Example
247(a);
3-(5-ethyl-6-methyl-lH-benzoimidazol-2-yl)-1H-indazole-5-carboxylic acid amide
dihydrochloride,
Example 262;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-227-
and the corresponding N-oxides, and their prodrugs; and pharmaceutically
acceptable salts and solvates
(e.g. hydrates) of such compounds and their N-oxides and prodrugs.
Especially preferred compounds of formula (Ixb) of the invention for the
inhibition of SYK are:-
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-5-methoxy-lH-indazole, (compound
denoted as A9-B68),
Example 235(b);
3-(5-ethyl-6-methyl-lH-benzoimidazol-2-yl)-1H-indazole, (compound denoted as
A55-B63), Example
235(j);
3-(5,6-diethyl-lH-benzoimidazol-2-yl)-1H-indazole, (compound denoted as A56-
B63), Example
235(z);
3-(5,6-diinethyl-lH-benzoimidazol-2-yl)-1H-indazole-5-carboxylic acid
dimethylamide, Example
246(x);
and the corresponding N-oxides, and their prodrugs; and pharmaceutically
acceptable salts and solvates
(e.g. hydrates) of such compounds and their N-oxides and prodrugs.
Preferred compounds of formula (Ixc) of the invention for the inhibition of
SYK are:-
3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-4, 5, 6,7-tetrahydro-1 H-indazole;
5,6-dimethyl-2-(1,4,5,6-tetrahydro-cyclopentapyrazol-3-yl)-1 H-benzoimidazole;
3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1,4,5,6,7,8-hexahydro-
cycloheptapyrazole;
and the corresponding N-oxides, and their prodrugs; and pharmaceutically
acceptable salts and solvates
(e.g. hydrates) of such compounds and their N-oxides and prodrugs.
Particularly preferred compounds of formula (Ixc), denoted as the product of
the combination of group
Al in Table 1 and B 1 in Table 2, of the invention for the inhibition of SYK
are:-
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-4,5,6,7-tetrahydro-lH-indazole,
(compound denoted as
A9-B59), Example 241(a);
5,6-dimethyl-2-(1,4,5,6-tetrahydro-cyclopentapyrazol-3-yl)-1H-benzoimidazole,
(compound denoted as
A9-B56), Example 241(d);
and the corresponding N-oxides, and their prodrugs; and pharmaceutically
acceptable salts and solvates
(e.g. hydrates) of such compounds and their N-oxides and prodrugs.
Preferred compounds of formula (Ixd) of the invention for the inhibition of
SYK are:-
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridine-5-carboxylic acid
isopropylamide;
cyclopropyl-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-
pyrazolo[4,3-c]pyridin-5-yl]-
methanone;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-228-
isopropyl-[3-(5, 6-dimethyl-1 H-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-
pyrazolo[4, 3-c]pyridin-5-yl]-
methanone;
1-[3-(5, 6-dimethyl-1 H-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridin-5-yl] -ethanone;
1-[3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridin-5-yl]-2-methyl-
propan-l-one;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridine-5-carboxylic acid
methyl ester;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridine-5-carboxylic acid
dimethylamide;
1-[3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridin-5-yl]-3-methyl-
butan-l-one;
1-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridin-5-yl]-2,2-
dimethyl-propan-l-one;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridine-5-carboxylic acid
methyl ester;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridine-5-carboxylic acid
isopropylamide;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridine-5-carboxylic acid
diethylamide;
[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridin-5-yl]-pyrrolidin-l-
yl-methanone;
[3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridin-5-yl]-piperidin-l-
yl-methanone;
[3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridin-5-yl]-morpholin-4-
yl-methanone;
3 -(5-chloro-6-methyl-1 H-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridine-5-carboxylic
acid dietllylamide;
3-[5-(2-morpholin-4-yl-ethoxy)-1 H-benzoimidazol-2-y1]-1,4,6,7-tetrahydro-
pyrazolo[4,3-c]pyridine-5-
carboxylic acid diethylamide;
3-(5-trifluoromethyl-lH-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridine-5-carboxylic
acid diethylainide;
1-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridin-5-yl]-2,2-
dimethyl-propan-1-one;
3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-5-(propane-2-sulfonyl)-4,5,6,7-
tetrahydro-lH-pyrazolo[4,3-
c]pyridine;
3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrano[4,3-
c]pyrazole;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-229-
and the corresponding N-oxides, and their prodrugs; and pharmaceutically
acceptable salts and solvates
(e.g. hydrates) of such compounds and their N-oxides and prodrugs.
Particularly preferred compounds of formula (Ixd), denoted as the product of
the combination of group
Al in Table 1 and B 1 in Table 2, of the invention for the inhibition of SYK
are:-
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridine-5-carboxylic acid
isopropylamide, (compound denoted as A9-B121), Example 250(a);
cyclopropyl-[3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-
pyrazolo[4,3-c]pyridin-5-yl]-
methanone, (compound denoted as A9-B 122);
isopropyl-[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-
pyrazolo[4,3-c]pyridin-5-yl]-
methanone;
1-[3-(5, 6-dimethyl-1 H-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridin-5-yl]-2,2-
dimethyl-p rop an-l-one;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridine-5-carboxylic acid
methyl ester;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridine-5-carboxylic acid
isopropylamide; 26(e)
prepared 3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-
pyrazolo[4,3-c]pyridine-5-
carboxylic acid diethylamide;
[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridin-5-yl]-pyrrolidin-l-
yl-methanone;
[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridin-5-yl]-piperidin-l-
yl-methanone;
[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridin-5-yl]-morpholin-4-
yl-methanone;
3-(5-chloro-6-methyl-1 H-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridine-5-carboxylic
acid diethylamide;
3-[5-(2-morpholin-4-yl-ethoxy)-1 H-benzoimidazol-2-yl]-1,4,6,7-tetrahydro-
pyrazolo [4,3-c]pyridine-5-
carboxylic acid diethylamide;
3-(5-trifluoromethyl-lH-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridine-5-carboxylic
acid diethylamide;
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridine-5-carboxylic acid
dimethylamide, (compound denoted as A9-B 119);
1-[3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1,4, 6,7-tetrahydro-pyrazolo [4,3-
c]pyridin-5-yl] -2-methyl-
propan-l-one, (compound denoted as A9-B117);
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-230-
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridine-5-carboxylic acid
methyl ester, (compound denoted as A9-B 120);
1-[3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridin-5-yl]-3-methyl-
butan-l-one, (compound denoted as A9-B118);
1-[3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridin-5-yl]-2,2-
dimethyl-propan-l-one, (compound denoted as A9-B123);
and the corresponding N-oxides, and their prodrugs; and pharmaceutically
acceptable salts and solvates
(e.g. hydrates) of such compounds and their N-oxides and prodrugs.
Especially preferred compounds of formula (Ixd), denoted as the product of the
combination of group
Al in Table 1 and B1 in Table 2, of the invention for the inhibition of SYK
are:-
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridine-5-carboxylic acid
isopropylamide, (compound denoted as A9-B121), Example 250(a);
cyclopropyl-[3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1,4, 6,7-tetrahydro-
pyrazolo [4,3-c]pyridin-5-yl]-
methanone, (compound denoted as A9-B 122); Example 250(b);
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridine-5-carboxylic acid
isopropylamide, Example 255(e);
prepared 3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-
pyrazolo[4,3-c]pyridine-5-
carboxylic acid diethylamide, Example 258(i);
[3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridin-5-yl]-pyrrolidin-l-
yl-methanone, Example 258(j);
[3-(5,6-dimethyl-1 H-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3 -
c]pyridin-5-yl]-piperidin-l-
yl-methanone, Example 258(k);
3-(5-chloro-6-methyl-1 H-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo [4,3-
c]pyridine-5-carboxylic
acid diethylamide, Example 258(m);
3-(5,6-dimethyl-lH-benzoimidazol-2-yl)-1,4,6,7-tetrahydro-pyrazolo[4,3-
c]pyridine-5-carboxylic acid
dimethylamide, (compound denoted as A9-B 119);
and the corresponding N-oxides, and their prodrugs; and pharmaceutically
acceptable salts and solvates
(e.g. hydrates) of such compounds and their N-oxides and prodrugs.
Particular compounds of formula (Ix) of the invention for the inhibition of
KDR are:-
2-(1 H-indazol-3-yl)-1 H-benzimidazole-5-carboxylic acid benzylamide;
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acid N-methylamide;
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acid N-ethylamide;
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acid N-isopropylamide;
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acid N-phenylamide;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-231-
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acid N-phenethylamide;
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acid N-morpholinoamide;
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acid N-(N'-
methylpiperazino)amide;
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acid N-pyrrolidinoamide;
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acid N-(isobutyl)amide;
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acid N-
(cyclohexylmethyl)amide;
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acid N-(2-furfuryl)amide;
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acid N-benzyl-N-methylamide;
methyl2-(1H-indazol-3-yl)-3H-benzimidazole-5- carboxylate;
5,6-dimethyl-2-(1H-indazol-3-yl)-1H-benzimidazole;
5-methoxy-2-(1 H-indazol-3-yl)-1H-benzimidazole;
2-(1H-indazol-3-yl)-3H-benzimidazole-4-carboxylic acid;
5-bromo 2-(1H-indazol-3-yl)-3H-benzimidazole;
2-(5-ethoxy-2H-pyrazol-3-yl)-1H-benzimidazole-4-carboxylic acid;
5,6-dimethyl-2-(5-methyl-2H-pyrazol-3-yl)-1H-benzimidazole;
5,6-dimethyl-2-(5-thiophen-2-yl-2H-pyrazol-3-yl)-1 H-benzimidazole;
2-(4-bromo-2H-pyrazol-3-yl)-5,6-dimethyl-1 H-benzimidazole;
2-(5-ethyl-2H-pyrazol-3-yl)-5,6-dimethyl-1 H-benzimidazole;
2-(5-ethyl-2H-pyrazol-3-yl)-4,5-ethylenedioxy-1 H-benzimidazole;
2-(5-ethyl-2H-pyrazol-3-yl)-5-methoxy-1 H-benzimidazole;
2-(5-ethyl-2H-pyrazol-3-yl)-4-hydroxy-1 H-benzimidazole
2-(5-ethyl-2H-pyrazol-3-yl)-5-bromo-1 H-benzimidazole;
2-(1 H-indazol-3-yl)-1 H-benzoimidazole-5-carboxylic acid 2,4-dichloro-
benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (3-ethoxy-propyl)-
amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 4-bromo-benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 4-methanesulfonyl-
benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (naphthalen-1-
ylmethyl)-amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 4-trifluoromethyl-
benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (thiophen-2-ylmethyl)-
amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 4-dimethylamino-
benzylamide;
4-( { [2-(1 H-indazol-3-y1)-1H-benzoimidazole-5-carbonyl]-amino}-methyl)-
piperidine-l-carboxylic
acid tert-butyl ester;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 4-nitro-benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (pyridin-3-ylmethyl)-
amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 3-bromo-benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 3-methoxy-benzylamide;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-232-
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (benzo[1,3]dioxol-5-
ylmethyl)-amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (benzo[b]thiophen-3-
ylmethyl)-amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (1,3-dimethyl-lH-
pyrazol-4-ylmethyl)-
amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 2-trifluoromethoxy-
benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 2-methyl-benzylamide;
2-(1 H-indazol-3-yl)-1 H-benzoimidazole-5-carboxylic acid (3-methyl-thiophen-2-
ylmethyl)-amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 2-trifluoromethyl-
benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 4-phenoxy-benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 3-trifluoromethoxy-
benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (3-isopropoxy-propyl)-
amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (1-methyl-lH-pyrazol-4-
ylmethyl)-amide;
2-(1 H-indazol-3-yl)-1 H-benzoimidazole-5-carboxylic acid 4-isopropyl-
benzylamide;
2-(1 H-indazol-3-yl)-1 H-benzoimidazole-5-carboxylic acid (2,5-dimethyl-furan-
3-ylmethyl)-amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (benzo[b]thiophen-2-
ylmethyl)-amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid [3-(3-acetylamino-
phenoxy)-propyl]-amide;
2-(1 H-indazol-3-yl)-1 H-benzoimidazole-5-carboxylic acid (6-chloro-pyridin-3-
ylmethyl)-amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid ([2,2']bithiophenyl-5-
ylmethyl)-amide;
2-(1 H-indazol-3-yl)-1 H-benzoimidazole-5-carboxylic acid (2,3-dihydro-
benzofuran-5-ylmethyl)-
amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 4-cyano-benzylamide;
2-(1 H-indazol-3-yl)-1 H-benzoimidazole-5-carboxylic acid (5-chloro-
benzo[b]thiophen-3-ylmethyl)-
amide;
2-(1 H-indazol-3-yl)-1 H-benzoimidazole-5-carboxylic acid 3-trifluoromethyl-
benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 2-methylsulfanyl-
benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (benzo[b]thiophen-3-
ylmethyl)-amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (tetrahydro-pyran-4-
ylmethyl)-amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (2,3-dihydro-
benzo[1,4]dioxin-2-ylmethyl)-
amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (furan-3-ylmethyl)-
amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 2-nitro-benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (thiophen-3-ylmethyl)-
amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 3,5-dimethyl-
benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (1-methyl-lH-
benzoimidazol-2-ylmethyl)-
amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 3-methyl-benzylamide;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-233-
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 3-chloro-benzylamide;
2-(1 H-indazol-3-yl)-3H-benzoimidazole-4-carboxylic acid 4-sulfamoyl-
benzylamide;
2-(1 H-indazol-3-yl)-3H-benzoimidazole-4-carboxylic acid (3-ethoxy-propyl)-
amide;
2-(1H-indazol-3-yl)-3H-benzoimidazole-4-carboxylic acid 4-bromo-benzylamide;
2-(1H-indazol-3-yl)-3H-benzoimidazole-4-carboxylic acid (naphthalen-1-
ylmethyl)-amide;
2-(1 H-indazol-3-yl)-3H-benzoimidazole-4-carboxylic acid (thiophen-2-ylmethyl)-
amide;
2-(1H-indazol-3-yl)-3H-benzoimidazole-4-carboxylic acid 4-dimethylamino-
benzylamide;
2-(1H-indazol-3-yl)-3H-benzoimidazole-4-carboxylic acid 4-nitro-benzylamide;
2-(1H-indazol-3-yl)-3H-benzoimidazole-4-carboxylic acid (pyridin-3-ylmethyl)-
amide;
2-(1H-indazol-3-yl)-3H-benzoimidazole-4-carboxylic acid 3-bromo-benzylamide;
2-(1H-indazol-3-yl)-3H-benzoimidazole-4-carboxylic acid 3-methoxy-benzylamide;
2-(1H-indazol-3-yl)-3H-benzoimidazole-4-carboxylic acid (benzo[b]thiophen-3-
ylmethyl)-amide;
2-(1H-indazol-3-yl)-3H-benzoimidazole-4-carboxylic acid 4-phenoxy-benzylamide;
2-(1H-indazol-3-yl)-3H-benzoimidazole-4-carboxylic acid 3-trifluoromethoxy-
benzylamide;
2-(1H-indazol-3-yl)-3H-benzoimidazole-4-carboxylic acid (6-chloro-pyridin-3-
ylmethyl)-amide;
2-(1H-indazol-3-yl)-3H-benzoimidazole-4-carboxylic acid (2,3-dihydro-
benzofuran-5-ylmethyl)-
amide;
2-(1H-indazol-3-yl)-3H-benzoimidazole-4-carboxylic acid 3-trifluoromethyl-
benzylamide;
2-(1H-indazol-3-yl)-3H-benzoimidazole-4-carboxylic acid 2-methylsulfanyl-
benzylamide;
2-(1H-indazol-3-yl)-3H-benzoimidazole-4-carboxylic acid (furan-3-ylmethyl)-
amide;
2-(1H-indazol-3-yl)-3H-benzoimidazole-4-carboxylic acid 2-nitro-benzylamide;
2-(1H-indazol-3-yl)-3H-benzoimidazole-4-carboxylic acid 3,5-dimethyl-
benzylamide;
2-(1H-indazol-3-yl)-3H-benzoimidazole-4-carboxylic acid 3-chloro-benzylamide;
2-(1 H-indazol-3-yl)-3H-benzoimidazole-4-carboxylic acid phenylamide;
2-(1H-indazol-3-yl)-3H-benzoimidazole-4-carboxylic acid benzylamide;
2-(1H-indazol-3-yl)-3H-benzoimidazole-4-carboxylic acid phenethyl-amide;
3-(6-phenyl-1 H-benzoimidazol-2-yl)-2H-indazole;
3-[6-(2,4-dichloro-phenyl)-1 H-benzoimidazol-2-yl]-2H-indazole;
3-(6-naphthalen-1-yl-1 H-benzoimidazol-2-yl)-2H-indazole;
3-[6-(4-fluoro-phenyl)-1H-benzoimidazol-2-yl]-2H-indazole;
3-[6-(4-chloro-phenyl)-1 H-benzoimidazol-2-yl]-2H-indazole;
3-[6-(4-methoxy-phenyl)-1H-benzoimidazol-2-yl]-2H-indazole;
3-[6-(3-chloro-4-fluoro-phenyl)-1 H-benzoimidazol-2-yl] -2H-indazole;
3-[6-(3,5-dichloro-phenyl)-1 H-benzoimidazol-2-yl]-2H-indazole;
3-(6-thianthren-l-yl-lH-benzoimidazol-2-yl)-2H-indazole;
3-(6-biphenyl-4-yl-l H-benzoimidazol-2-yl)-2H-indazole;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-234-
3 -(6-p-tolyl-1 H-benzoimidazol-2-yl)-2 H-indazole;
3-(6-m-tolyl-1 H-benzoimidazol-2-yl)-2H-indazole;
3-(6-o-tolyl-1 H-benzoimidazol-2-yl)-2H-indazole;
3-(6-thiophen-3-yl-1 H-benzoimidazol-2-yl)-2H-indazole;
3-[6-(3-trifluoromethyl-phenyl)-1 H-benzoimidazol-2-yl]-2H-indazole;
3 -[6-(4-trifluoromethyl-phenyl)-1 H-benzoimidazol-2-yl] -2H-indazole;
3-[6-(3-chloro-phenyl)-1 H-benzoimidazol-2-yl] -2H-indazole;
3-[6-(3 -methoxy-phenyl)- 1 H-benzoimidazol-2-yl]-2H-indazole;
3-[6-(3,5-dimethyl-phenyl)-1 H-benzoimidazol-2-yl]-2H-indazole;
3-[6-(3,4-dimethyl-phenyl)-1H-benzoimidazol-2-yl]-2H-indazole;
3-(6-benzo[ 1,3] dioxol-5-yl-lH-benzoimidazol-2-yl)-2H-indazole;
3-[6-(4-tert-butyl-phenyl)-1 H-benzoimidazol-2-yl]-2H-indazole;
3 -(6-hex-l-enyl-1 H-benzoimidazol-2-yl)-2H-indazole;
3-[6-(3,4-dimethoxy-phenyl)-1 H-benzoimidazol-2-yl]-2H-indazole;
3-[2-(2H-indazol-3-yl)-3H-benzoimidazol-5-yl] -phenol;
4-[2-(2H-indazol-3-yl)-3H-benzoimidazol-5-yl] -phenol;
3-[6-(3,4-dichloro-phenyl)-1 H-benzoimidazol-2-yl]-2H-indazole;
3-[6-(4-trifluoromethoxy-phenyl)-1 H-benzoimidazol-2-yl]-2H-indazole;
1- {4-[2-(2H-indazol-3-yl)-3H-benzoimidazol-5-yl]-phenyl } -ethanone;
3-(6-benzo[b]thiophen-2-yl-lH-benzoimidazol-2-yl)-2H-indazole;
3-[6-(3,4,5-trimethoxy-phenyl)-1 H-benzoimidazol-2-yl]-2H-indazole;
1- { 5-[2-(2H-indazol-3-yl)-3H-benzoimidazol-5-yl]-thiophen-2-yl } -ethanone;
1-{3-[2-(2H-indazol-3-yl)-3H-benzoimidazol-5-yl]-phenyl} -ethanone;
3-[6-(4-benzyloxy-phenyl)-1 H-benzoimidazol-2-yl]-2H-indazole;
3-[6-(2-fluoro-biphenyl-4-yl)-1H-benzoimidazol-2-yl]-2H-indazole;
3 -(6-b enzo [b] thiophen-3 -yl-1 H-benzoimidazol-2-yl)-2H-indazole;
{3-[2-(2H-indazol-3-yl)-3H-benzoimidazol-5-yl]-phenyl } -methanol;
3-[6-(4-ethylsulfanyl-phenyl)-1 H-benzoimidazol-2-yl]-2H-indazole;
3-[6-(2,4-difluoro-phenyl)-1 H-b enzoimidazol-2-yl] -2H-indazole;
3-[6-(3-trifluoromethoxy-phenyl)-1H-benzoimidazol-2-yl]-2H-indazole;
3-[6-(4-fluoro-2-methyl-phenyl)-1 H-benzoimidazol-2-yl] -2H-indazole;
3-{6-[2-(4-fluoro-phenyl)-vinyl]-1 H-benzoimidazol-2-yl} -2H-indazole;
3-{6-[2-(4-chloro-phenyl)-vinyl]-1 H-benzoimidazol-2-yl} -2H-indazole;
3-{4-[2-(2H-indazol-3-yl)-3H-benzoimidazol-5-yl]-phenyl}-propionic acid;
{4-[2-(2H-indazol-3-yl)-3H-benzoimidazol-5-yl]-phenyl}-methanol;
3 -(6-furan-2-yl-1 H-benzoimidazol-2-yl)-2H-indazole;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-235-
3-[6-(3-benzyloxy-phenyl)-1 H-benzoimidazol-2-yl]-2H-indazole;
3-[6-(4-isopropyl-phenyl)-1 H-benzoimidazol-2-yl]-2H-indazole;
3 -[6-(4-methane sulfonyl-phenyl)-1 H-benzoimidazol-2-yl] -2H-indazole;
2-(1 H-indazol-3-yl)-1 H-benzoimidazole-5-carboxylic acid (tetrahydro-pyran-4-
ylmethyl)-amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 4-acetylamino-
benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid methylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid isopropylamide;
[2-(1 H-indazol-3-yl)-1 H-benzoimidazol-5-yl]-morpholin-4-yl-methanone;
[2-(1 H-indazol-3-yl)-1 H-benzoimidazol-5-yl]-(4-methyl-piperazin-l-yl)-
methanone;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid benzyl-methyl-amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 3-nitro-benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 2-fluoro-benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 2,4-difluoro-
benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 2,6-difluoro-
benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 4-bromo-2-fluoro-
benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 4-chloro-2-fluoro-
benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 4-bromo-2-fluoro-
benzylamide;
2-(1 H-indazol-3-yl)-1 H-benzoimidazole-5-carboxylic acid 3,4-difluoro-
benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 3,4,5-trifluoro-
benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (4'-chloro-biphenyl-4-
ylmethyl)-amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (3',5'-dichloro-
biphenyl-4-ylmethyl)-amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (4'-fluoro-biphenyl-4-
ylmethyl)-amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 2-fluoro-benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 2,6-difluoro-3-methyl-
benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 2,4-dichloro-
benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 4-chloro-benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 4-chloro-2-methyl-
benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 4-fluoro-benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (2'-chloro-biphenyl-4-
ylmethyl)-amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (6-trifluoromethyl-
pyridin-3-ylmethyl)-
ainide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (5-pyridin-2-yl-
thiophen-2-ylmethyl)-
amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (3-imidazol-1-yl-
propyl)-amide;
4-[2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carbonyl]-piperazine-l-carboxylic
acid tert-butyl ester;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (2,6-difluoro-4-chloro-
benzyl)amide;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-236-
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (2,4-dichloro-6-fluoro-
benzyl)amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (3-fluoro-4-chloro-
benzyl)amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (2-fluoro-4-chloro-6-
methyl-benzyl)amide;
2-(1 H-indazol-3-yl)-1 H-benzoimidazole-5-carboxylic acid (6-methoxy-pyridin-3-
ylmethyl)-amide;
2-[5-(benzyloxy)-2H-pyrazol-3-yl]-1H-benzoimidazole;
2-[ 5-(3 -phenyl-allyloxy)2H-pyrazol-3-yl] -1 H-benzoimidazole;
2-[5-(2-methyl-allyloxy)2H-pyrazol-3-yl]-1H-benzoimidazole;
2-[5-(3,7-dimethyl-octa-2, 6-dienyloxy)-2H-pyrazol-3-yl]-1H-benzoimidazole;
2-[5-(3-bromo-benzyloxy)-2 H-pyrazol-3-yl]-1 H-benzoimidazole;
3-[5-(1H-benzoimidazol-2-yl)-1H-pyrazol-3-yloxymethyl]-benzonitrile;
2-[5-(4-trifluoromethyl-benzyloxy)-2H-pyrazol-3-yl]-1 H-benzoimidazole;
2-[ 5 -(3,4-dichloro-b enzyloxy)-2H-pyrazol-3-yl]-1 H-benzoimidazole;
2-[5-pentafluorophenylmethoxy)-2H-pyrazol-3-yl]-1 H-benzoimidazole;
2-[5-(4-tert-butyl-benzyloxy)-2H-pyrazol-3-yl]-1 H-benzoimidazole;
2-[5-(2-benzenesulfonylmethyl-benzyloxy)-2H-pyrazol-3-yl]-1H-benzoimidazole;
4-[5 -(1 H-benzoimidazol-2-yl)-1 H-pyrazol-3-yloxymethyl] -benzonitrile;
2-[5-(biphenyl-4-ylmethoxy)-2H-pyrazol-3-yl]-1 H-benzoimidazole;
2,3-dichioro-benzenesulfonic acid 5-(1 H-benzoimidazol-2-yl)-1H-pyrazol-3-yl
ester;
2-[5-(2-morpholin-4-yl-ethoxy)-2 H-pyrazol-3-yl]-1H-benzoiniidazole;
2-[5-(2-piperidin-1-yl-ethoxy)-2H-pyrazol-3-yl]-1H-benzoimidazole;
2-[5-(3-methoxy-benzyloxy)-2H-pyrazol-3-yl]-1H-benzoimidazole;
2-[5-(1H-benzoimidazol-2-yl)-1H-pyrazol-3-yloxy]-1 -p-tolyl-ethanone;
1-[5-(1H-benzoimidazol-2-yl)-1H-pyrazol-3-yloxy]-3,3,4,4,4-pentafluoro-butan-2-
one;
2-[5-(1 H-benzoimidazol-2-yl)-1 H-pyrazol-3 -yloxy] -1-biphenyl-4-yl-ethanone;
1-[5-(1H-benzoimidazol-2-yl)-1H-pyrazol-3-yloxy]-butan-2-one;
2-[5-(1H-benzoimidazol-2-yl)-1 H-pyrazol-3-yloxy]-1-(4-dimethylamino-phenyl)-
ethanone;
2-[5-(1 H-b enzo imidazol-2-yl)-1 H-pyrazol-3-yloxy]-1-(3 -phenyl-isoxazol-5-
yl)-ethanone;
2-[5-(1H-benzoimidazol-2-yl)-1H-pyrazol-3-yloxy]-N-phenyl-acetamide;
1-[5-(1H-benzoimidazol-2-yl)-1 H-pyrazol-3-yloxy]-3,3-dimethyl-butan-2-one;
1-adamantan-1-yI-2-[5-(1H-benzoimidazol-2-yl)-1H-pyrazol-3-yloxy]-ethanone;
2-[5-(1H-benzoimidazol-2-yl)-1H-pyrazol-3-yloxy]-1-naphthalen-2-yl-ethanone;
4-{2-[5-(1H-benzoimidazol-2-yl)-1H-pyrazol-3-yloxy]-acetyl} -benzonitrile;
6- {2-[5-(IH-benzoimidazol-2-yl)-1H-pyrazol-3-yloxy]-acetyl}-3,4-dihydro-lH-
quinolin-2-one;
2-[5-(1 H-benzoimidazol-2-yl)-1 H-pyrazol-3-yloxy]-1-(4-trifluoromethoxy-
phenyl)-ethanone;
5-{2-[5-(1H-benzoimidazol-2-yl)-1H-pyrazol-3-yloxy]-acetyl}-2-chloro-
benzenesulfonamide;
2-[5-(1H-benzoimidazol-2-yl)-1H-pyrazol-3-yloxy]-1-(4-methoxy-phenyl)-
ethanone;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-237-
2-[5-(1H-benzoimidazol-2-yl)-1H-pyrazol-3-yloxy]-1 -cyclopropyl-ethanone;
isonicotinic acid 5-(1H-benzoimidazol-2-yl)-1H-pyrazol-3-yl ester;
2,2-dimethyl-propionic acid 5-(1H-benzoimidazol-2-yl)-1H-pyrazol-3-yl ester;
benzyloxy-acetic acid 5-(1H-benzoimidazol-2-yl)-1H-pyrazol-3-yl ester;
benzoic acid 5-(1H-benzoimidazol-2-yl)-1H-pyrazol-3-yl ester;
4-methoxy-benzoic acid 5-(1H-benzoimidazol-2-yI)-1H-pyrazol-3-yl ester;
phenyl-acetic acid 5-(1H-benzoimidazol-2-yl)-1H-pyrazol-3-yl ester;
2,3,4,5,6-Pentafluoro-benzoic acid 5-(1H-benzoimidazol-2-yl)-1H-pyrazol-3-yl
ester;
cyclopropanecarboxylic acid 5-(1H-benzoimidazol-2-yl)-1H-pyrazol-3-yl ester;
2,2,3,3,4,4,4-heptafluoro-butyric acid 5-(1H-benzoimidazol-2-yl)-1H-pyrazol-3-
yl ester;
cyclopentanecarboxylic acid 5-(1H-benzoimidazol-2-yl)-1H-pyrazol-3-yl ester;
3-phenyl-propionic acid 5-(1H-benzoimidazol-2-yI)-1H-pyrazol-3-yl ester;
biphenyl-4-carboxylic acid 5-(1H benzoimidazol-2-yl)-1H-pyrazol-3-yl ester;
3,5-bis-trifluoromethyl-benzoic acid 5-(1H-benzoimidazol-2-yl)-1H-pyrazol-3-yl
ester;
4-trifluoromethyl-benzoic acid 5-(1H-benzoimidazol-2-yl)-1H-pyrazol-3-yl
ester;
thiophene-2-carboxylic acid 5-(1H-benzoimidazol-2-yl)-1H-pyrazol-3-yl ester;
and the corresponding N-oxides, and their prodrugs; and pharmaceutically
acceptable salts and solvates
(e.g. hydrates) of such compounds and their N-oxides and prodrugs.
Preferred compounds of formula (Ixa), denoted as the product of the
combination of group Al in Table
1 and B 1 in Table 2, of the invention for the inhibition of KDR are:-
2-(5-ethyl-2H-pyrazol-3-yl)-5,6-dimethyl-lH-benzimidazole, (compound denoted
as A9-B3);
2-(5-methyl-2H-pyrazol-3-yl)-5,6-dimethyl-lH-benzimidazole (compound denoted
as A9-B2);
and the corresponding N-oxides, and their prodrugs; and pharmaceutically
accelftable salts and solvates
(e.g. hydrates) of such compounds and their N-oxides and prodrugs.
Preferred compounds of formula (Ixb), denoted as the product of the
combination of group Al in Table
1 and B 1 in Table 2, of the invention for the inhibition of KDR are:-
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acid benzylamide, (compound
denoted as
A17-B63);
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acid N-methylamide,
(compound denoted as
A15-B63);
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acid N-ethylamide, (compound
denoted as
A36-B63);
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acid N-isopropylamide,
(compound denoted as
A37-B63);
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-238-
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acid N-phenylamide,
(compound denoted as
A52-B63);
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acid N-phenethylamide,
(compound denoted as
A51-B63);
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acid N-morpholinoamide,
(compound denoted as
A92-B63);
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acid N-(N'-
methylpiperazino)amide, (compound
denoted as A93-B63);
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acid N-pyrrolidinoamide,
(compouiid denoted as
A91-B63);
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acid N-(isobutyl)amide,
(compound denoted as
A82-B63);
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acid N-
(cyclohexylmethyl)amide, (compound
denoted as A83-B63);
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acid N-(2-furfuryl)amide,
(compound denoted as
A84-B63);
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acid N-benzyl-N-methylamide,
(compound
denoted as A90-B63);
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 2,4-dichloro-
benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (3-ethoxy-propyl)-
amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 4-bromo-benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 4-methanesulfonyl-
benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (naphthalen-1-
ylmethyl)-amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 4-trifluoromethyl-
benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (thiophen-2-ylmethyl)-
amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 4-dimethylamino-
benzylamide;
4-( { [2-(1H-indazol-3-yl)-1 H-benzoimidazole-5-carbonyl]-amino} -methyl)-
piperidine- 1 -carboxylic
acid tert-butyl ester;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 4-nitro-benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (pyridin-3-ylmethyl)-
amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 3-bromo-benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 3-methoxy-benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (benzo[1,3]dioxol-5-
ylmethyl)-amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (benzo[b]thiophen-3-
ylmethyl)-amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (1,3-dimethyl-lH-
pyrazol-4-ylmethyl)-
amide;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-239-
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 2-trifluoromethoxy-
benzylamide;
2-(1 H-indazol-3-yl)-1 H-benzoimidazole-5-carboxylic acid 2-methyl-
benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (3-methyl-thiophen-2-
ylmethyl)-amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 2-trifluoromethyl-
benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 4-phenoxy-benzylamide;
2-(IH-indazol-3-yl)-IH-benzoimidazole-5-carboxylic acid 3-trifluoromethoxy-
benzylamide;
2-(IH-indazol-3-yl)-IH-benzoimidazole-5-carboxylic acid (3-isopropoxy-propyl)-
amide;
2-(IH-indazol-3-yl)-IH-benzoimidazole-5-carboxylic acid (1-methyl-lH-pyrazol-4-
ylmethyl)-amide;
2-(1 H-indazol-3-yl)-1 H-benzoimidazole-5-carboxylic acid 4-isopropyl-
benzylamide;
2-(IH-indazol-3-yl)-IH-benzoimidazole-5-carboxylic acid (2,5-dimethyl-furan-3-
ylmethyl)-amide;
2-(IH-indazol-3-yl)-IH-benzoimidazole-5-carboxylic acid (benzo[b]thiophen-2-
ylmethyl)-amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid [3-(3-acetylamino-
phenoxy)-propyl]-amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (6-chloro-pyridin-3-
ylmethyl)-amide;
2-(IH-indazol-3-yl)-IH-benzoimidazole-5-carboxylic acid ([2,2']bithiophenyl-5-
ylmethyl)-amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (2,3-dihydro-
benzofuran-5-ylmethyl)-
amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 4-cyano-benzylamide;
2-(IH-indazol-3-yl)-IH-benzoimidazole-5-carboxylic acid 2-methylsulfanyl-
benzylamide;
2-(IH-indazol-3-yl)-IH-benzoimidazole-5-carboxylic acid (benzo[b]thiophen-3-
ylmethyl)-amide;
2-(IH-indazol-3-yl)-IH-benzoimidazole-5-carboxylic acid (tetrahydro-pyran-4-
ylmethyl)-amide;
2-(IH-indazol-3-yl)-IH-benzoimidazole-5-carboxylic acid (2,3-dihydro-
benzo[1,4]dioxin-2-ylmethyl)-
amide;
2-(IH-indazol-3-yl)-IH-benzoimidazole-5-carboxylic acid (furan-3-ylmethyl)-
amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 2-nitro-benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (thiophen-3-ylmethyl)-
amide;
2-(1 H-indazol-3-yl)-1 H-benzoimidazole-5-carboxylic acid 3,5-dimethyl-
benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (1-methyl-lH-
benzoimidazol-2-ylmethyl)-
amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 3-methyl-benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 3-chloro-benzylamide;
2-(1H-indazol-3-yl)-3H-benzoimidazole-4-carboxylic acid 4-sulfamoyl-
benzylamide;
2-(1H-indazol-3-yl)-3H-benzoimidazole-4-carboxylic acid (pyridin-3-ylmethyl)-
amide;
2-(1 H-indazol-3-yl)-3H-benzoimidazole-4-carboxylic acid 3-methoxy-
benzylamide;
2-(1 H-indazol-3-yl)-3H-benzoimidazole-4-carboxylic acid 2-methylsulfanyl-
benzylamide;
2-(1H-indazol-3-yl)-3H-benzoimidazole-4-carboxylic acid (furan-3-ylmethyl)-
amide;
2-(1H-indazol-3-yl)-3H-benzoimidazole-4-carboxylic acid 2-nitro-benzylamide;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-240-
2-(1H-indazol-3-yl)-3H-benzoimidazole-4-carboxylic acid 3,5-dimethyl-
benzylamide;
2-(1H-indazol-3-yl)-3H-benzoimidazole-4-carboxylic acid phenylamide;
3-[6-(4-fluoro-phenyl)-1 H-benzoimidazol-2-yl]-2H-indazole;
3-[6-(4-methoxy-phenyl)-1 H-benzoimidazol-2-yl]-2H-indazole;
3-[6-(3-chloro-4-fluoro-phenyl)-1H-benzoimidazol-2-yl]-2H-indazole;
3-(6-m-tolyl-1 H-benzoimidazol-2-yl)-2H-indazole;
3-(6-o-tolyl-1 H-b enzoimidazol-2-yl)-2H-indazole;
3-(6-thiophen-3-yl-1 H-benzoimidazol-2-yl)-2H-indazole;
3-[6-(3-chloro-phenyl)-1 H-benzoimidazol-2-yl]-2H-indazole;
3-[6-(3-methoxy-phenyl)-1H-benzoimidazol-2-yl]-2H-indazole;
3-[6-(3,5-dimethyl-phenyl)-1 H-benzoimidazol-2-yl]-2H-indazole;
3-(6-benzo[ 1,3]dioxol-5-yl-1 H-benzoimidazol-2-yl)-2H-indazole;
3-(6-hex-l-enyl-1 H-benzoimidazol-2-yl)-2H-indazole;
3-[6-(3,4-dimethoxy-phenyl)-1 H-benzoimidazol-2-yl]-2H-indazole;
3-[2-(2H-indazol-3-yl)-3H-benzoimidazol-5-yl]-phenol;
4-[2-(2H-indazol-3-yl)-3H-benzoiinidazol-5-yl]-phenol;
3-[6-(3,4,5-trimethoxy-phenyl)-1 H-benzoimidazol-2-yl]-2H-indazole;
1-{5-[2-(2H-indazol-3-yl)-3H-benzoimidazol-5-yl]-thiophen-2-yl} -ethanone;
{3-[2-(2H-indazol-3-yl)-3H-benzoimidazol-5-yl]-phenyl} -methanol;
3-[6-(2,4-difluoro-phenyl)-1 H-benzoimidazol-2-yl]-2H-indazole;
3-[6-(4-fluoro-2-methyl-phenyl)-1 H-benzoimidazol-2-yl]-2H-indazole;
{4-[2-(2H-indazol-3-yl)-3H-benzoimidazol-5-yl]-phenyl } -methanol;
3-(6-furan-2-yl-1 H-benzoimidazol-2-yl)-2H-indazole;
3-[6-(4-isopropyl-phenyl)-1 H-benzoimidazol-2-yl]-2H-indazole;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (tetrahydro-pyran-4-
ylmethyl)-amide;
2-(1 H-indazol-3-yl)-1 H-benzoimidazole-5-carboxylic acid 4-acetylamino-
benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid methylamide;
2-(1 H-indazol-3-yl)-1 H-benzoimidazole-5-carboxylic acid isopropylamide;
[2-(1H-indazol-3-yl)-1H-benzoimidazol-5-yl]-morpholin-4-yl-methanone;
[2-(1H-indazol-3-yl)-1H-benzoimidazol-5-yl]-(4-methyl-piperazin-1-yl)-
methanone;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid benzyl-methyl-amide;
2-(lH-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 3-nitro-benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 2-fluoro-benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 2,4-difluoro-
benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 2,6-difluoro-
benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 4-bromo-2-fluoro-
benzylamide;
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-241-
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 4-chloro-2-fluoro-
benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 4-bromo-2-fluoro-
benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 3,4-difluoro-
benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 3,4,5-trifluoro-
benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 2,6-difluoro-3-methyl-
benzylamide;
2-(1 H-indazol-3-yl)-1 H-benzoimidazole-5-carboxylic acid 2,4-dichloro-
benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 4-chloro-benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 4-chloro-2-methyl-
benzylamide;
2-(1 H-indazol-3-yl)-1 H-benzoimidazole-5-carboxylic acid 4-fluoro-
benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (2'-chloro-biphenyl-4-
ylmethyl)-amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (6-trifluoromethyl-
pyridin-3-ylmethyl)-
amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (5-pyridin-2-yl-
thiophen-2-ylmethyl)-amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (3-imidazol-l-yl-
propyl)-amide;
4-[2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carbonyl]-piperazine-l-carboxylic
acid tert-butyl ester;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (2,6-difluoro-4-chloro-
benzyl)amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (2,4-dichloro-6-fluoro-
benzyl)amide;
2-(1 H-indazol-3-yl)-1 H-benzoimidazole-5-carboxylic acid (3-fluoro-4-chloro-
benzyl)amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (2-fluoro-4-chloro-6-
methyl-benzyl)amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (6-methoxy-pyridin-3-
ylmethyl)-amide;
2-[5-(benzyloxy)-2H-pyrazol-3-yl]-1 H-benzoimidazole;
2-[5 -(3-phenyl-allyloxy)2H-pyrazol-3-yl]-1 H-benzoimidazole;
2-[5-(3,7-dimethyl-octa-2, 6-dienyloxy)-2H-pyrazol-3-yl]-1H-benzoimidazole;
2-[5 -(3-bromo-benzyloxy)-2H-pyrazol-3-yl]-1 H-benzoimidazole;
2-[5-(3,4-dichloro-benzyloxy)-2H-pyrazol-3-yl]-1H-benzoimidazole;
2-[5-(2-benzenesulfonylmethyl-benzyloxy)-2H-pyrazol-3-yl]-1H-benzoimidazole;
2-[5-(biphenyl-4-ylmethoxy)-2H-pyrazol-3-yl]-1H-benzoimidazole;
2-[5-(3-methoxy-benzyloxy)-2H-pyrazol-3 -yl]-1H-benzoimidazole;
isonicotinic acid 5-(1H-benzoimidazol-2-yl)-1H-pyrazol-3-yl ester;
benzoic acid 5-(1H-benzoimidazol-2-yl)-1H-pyrazol-3-yl ester;
3-phenyl-propionic acid 5-(1H-benzoimidazol-2-yn-lH-pyrazol-3-yl ester;
methyl2-(1 H-indazol-3-yl)-3H-benzimidazole-5- carboxylate;
5 -methoxy-2-(1 H-indazol-3 -yl)-1 H-benzimidazole;
5-bromo 2-(1H-indazol-3-yl)-3H-benzimidazole, (compound denoted as A32-B63);
and the corresponding N-oxides, and their prodrugs; and pharmaceutically
acceptable salts and solvates
(e.g. hydrates) of such compounds and their N-oxides and prodrugs.
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-242-
Particularly preferred compounds of formula (Ixb) of the invention for the
inhibition of KDR are:-
2-(1 H-indazol-3-yl)- 1 H-benzimidazole-5-carboxylic acid N-
(cyclohexylmethyl)amide;
2-(1H-indazol-3-yl)-1H-benzimidazole-5-carboxylic acid N-(2-furfuryl)amide;
2-(1H-indazol-3-yl)-lH-benzoimidazole-5-carboxylic acid 2,4-dichloro-
benzylamide;
2-(1 H-indazol-3-yl)-1 H-benzoimidazole-5-carboxylic acid 4-bromo-benzylamide;
2-(1H-indazol-3-yl)-lH-benzoimidazole-5-carboxylic acid 4-methanesulfonyl-
benzylamide;
2-(1 H-indazol-3-yl)-1 H-benzoimidazole-5-carboxylic acid 4-nitro-benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 2-methyl-benzylamide;
2-(lH-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (6-chloro-pyridin-3-
ylmethyl)-amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (2,3-dihydro-
benzofuran-5-ylmethyl)-amide;
2-(1H-Indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 2-methylsulfanyl-
benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (benzo[b]thiophen-3-
ylmethyl)-amide; 2-
(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 3-methyl-benzylamide;
2-(lH-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 3-chloro-benzylamide;
2-(1H-indazol-3-yl)-3H-benzoiinidazole-4-carboxylic acid 2-methylsulfanyl-
benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 4-bromo-2-fluoro-
benzylamide;
2-(1 H-indazol-3-yl)-1 H-benzoimidazole-5-carboxylic acid 2,4-dichloro-
benzylamide;
2-(1 H-indazol-3-yl)-1 H-benzoimidazole-5-carboxylic acid 4-chloro-
benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid 4-chloro-2-methyl-
benzylamide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (2,6-difluoro-4-chloro-
benzyl)amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (2,4-dichloro-6-fluoro-
benzyl)amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (3-fluoro-4-chloro-
benzyl)amide;
2-(1 H-indazol-3-yl)-1 H-benzoimidazole-5-carboxylic acid (2-fluoro-4-chloro-6-
methyl-benzyl)amide;
2-(1H-indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (6-methoxy-pyridin-3-
ylmethyl)-amide;
and the corresponding N-oxides, and their prodrugs; and pharmaceutically
acceptable salts and solvates
(e.g. hydrates) of such compounds and their N-oxides and prodrugs.
Particular compounds of formula (Ix) of the invention for the inhibition of
ITK are:-
2-(IH-Indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (2-piperidin-1-yl-
ethyl)-amide, Example
246(ab);
2-(1H-Indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid (pyridin-2-ylmethyl)-
amide, Example
246(ac);
2-(1H-Indazol-3-yl)-1H-benzoimidazole-5-carboxylic acid [3-(4-methyl-piperazin-
1-yl)-propyl]-amide,
Example 246(ad);
N-[2-(1H-Indazol-3-yl)-1H-benzoimidazol-5-yl]-isobutyramide, Example 246(ae)
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-243-
N-[3-(5,6-Dimethyl-lH-benzoimidazol-2-yl)-1H-pyrazol-4-yl]-2-piperidin-1-yl-
acetamide, Example
253(c)
and the corresponding N-oxides, and their prodrugs; and pharmaceutically
acceptable salts and solvates
(e.g. hydrates) of such compounds and their N-oxides and prodrugs.
The compounds of formula (Ix) of the invention exhibit useful pharmacological
activity and
accordingly are incorporated into pharmaceutical compositions and used in the
treatment of patients
suffering from certain medical disorders. The present invention thus provides,
according to a further
aspect, compounds of formula (Ix) of the invention and compositions containing
compounds of
formula (Ix) of the invention for use in therapy.
Compounds of formula (Ix) within the scope of the present invention block
kinase catalytic activity
according to tests described in the literature and in vitro procedures
described hereinafter, and which
tests results are believed to correlate to pharmacological activity in humans
and other mammals. Thus,
in a further embodiment, the present invention provides compounds of forinula
(Ix) of the invention
and compositions containing compounds of formula (Ix) of the invention for use
in the treatment of a
patient suffering from, or subject to, conditions which can be ameliorated by
the administration of
protein kinase inhibitors (e.g. Syk, KDR, tie2 or ITK). For example, compounds
of formula (Ix) of the
present invention are useful in the treatment of inflammatory diseases, for
example asthma: atopic
dermatitis, inflammatory dermatoses (e.g. psoriasis, dematitis herpetiformis,
eczema, necrotizing and
cutaneous vasculitis, bullous disease, acute and chronic urticaria,); allergic
rhinitis and allergic
conjunctivitis; joint inflainmation, including arthritis, rheumatoid arthritis
and other arthritic conditions
such as rheumatoid spondylitis, gouty arthritis, traumatic arthritis, rubella
arthritis, psoriatic arthritis
and osteoarthritis. The compounds of formula (Ix) are also useful in the
treatment of Chronic
Obstructive Pulmonary Disease (COPD), adult respiratory distress syndrome,
silicosis, pulmonary
sarcoidosis, acute synovitis, autoimmune diabetes, autoimmune
encephalomyelitis, collitis,
atherosclerosis, peripheral vascular disease, cardiovascular disease,
cutaneous and systemic
anaphylaxis, endotoxemia, sepsis, septic shock, endotoxic shock, gram negative
sepsis, diabetes,
multiple sclerosis, restenosis, myocarditis, B cell lymphomas, systemic lupus
erythematosus, viral
infections, bacterial infections, parasitic infections, graft v host disease
and other transplant associated
rejection events, reperfusion injury, Crohn's disease and ulcerative colitis,
cancers and tumours (such
as colorectal, prostate, breast, thyroid, colon and lung cancers),
atherosclerosis, degenerative muscle
diseases, obesity, conjestive heart failure, Parkinson's, depression,
schizophrenia, stroke, head trauma,
spinal cord injury, Alzheimer's, neuropathic pain syndrome, amyotrophic
lateral sclerosis, cachexia,
osteoporosis, fibrotic diseases of the viscera, and inflammatory bowel
disease.
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-244-
The products of the present patent application as SYK inhibitors may be used
for the treatment of
diseases chosen from the following: asthma, allergic rhinitis, atopic
dermatitis, allergic conjunctivitis,
chronic obstructive pulmonary disease, adult respiratory distress syndrome,
silicosis, pulmonary
sarcoidosis, rheumatoid arthritis, osteoarthritis, rheumatoid spondylitis,
gouty arthritis, traumatic
arthritis, rubella arthritis, psoriatic arthritis, acute and chronic
urticaria, cutaneous and systemic
anaphylaxis, endotoxemia, sepsis, septic shock, endotoxic shock, gram negative
sepsis, diabetes,
multiple sclerosis, systemic lupus erythromatosis, viral infections, bacterial
infections, parasitic
infections, graft vs. host disease, organ transplant rejection, reperfusion
injury, Crohn's disease and
ulcerative colitis.
The products of the present patent application as KDR inhibitors may be used
especially for the
treatment or prevention of diseases chosen from the following group: cancers,
especially breast, colon,
lung and prostate cancer, atherosclerosis, degenerative muscle diseases,
obesity, conjestive heart
failure, Parkinson's, depression, schizophrenia, stroke, head trauma, spinal
cord injury, Alzheimer's,
neuropathic pain syndrome, amyotrophic lateral sclerosis, cachexia,
osteoporosis and fibrotic diseases
of the viscera.
A special embodiment of the therapeutic methods of the present invention is
the treating of asthma.
Another special embodiment of the therapeutic methods of the present invention
is the treating of
psoriasis.
Another special embodiment of the therapeutic methods of the present invention
is the treating of joint
inflammation.
Another special embodiment of the therapeutic methods of the present invention
is the treating of
inflammatory bowel disease.
Another special embodiment of the therapeutic methods of the present invention
is the treating of
cancers and tumours.
According to a further feature of the invention there is provided a method for
the treatment of a human
or animal patient suffering from, or subject to, conditions which can be
ameliorated by the
administration of a protein kinase inhibitor (e.g. . Syk, KDR, tie2 or ITK)
for example conditions as
hereinbefore described, which comprises the administration to the patient of
an effective amount of
compound of the invention or a composition containing a compound of the
invention. "Effective
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-245-
amount" is meant to describe an amount of compound of the present invention
effective in inhibiting
the catalytic activity a protein kinase, such as. Syk, KDR, tie2 or ITK, and
thus producing the desired
therapeutic effect.
References herein to treatment should be understood to include prophylactic
therapy as well as
treatment of established conditions.
The present invention also includes within its scope pharmaceutical
compositions comprising at least
one of the compounds of formula (Ix) of the invention, as defined above, or a
pharmaceutically
acceptable salt or a prodrug, in association, where appropriate, with a
pharmaceutically acceptable
carrier or excipient.
Pharmaceutical compositions of the present invention for the treatment of KDR
or tie2 associated
disease states can also, where appropriate, contain active principles of other
antimitotic medicinal
prbducts such as, in particular, those based on taxol, cis-platin, DNA-
intercalating agents and the like.
Compounds of formula (Ix) of the invention may be administered by any suitable
means. In practice
compounds of formula (Ix) of the present invention may generally be
administered parenterally, locally
by topical application to the skin and mucous membranes, rectally, orally, by
inhalation, or by
intravenous or intramuscular injection, especially by the oral route.
Compositions according to the invention may be prepared according to the
customary methods, using
one or more pharmaceutically acceptable adjuvants or excipients. The adjuvants
comprise, inter alia,
diluents, sterile aqueous media and the various non-toxic organic solvents.
The compositions may be
presented in the form of tablets, pills, granules, powders, aqueous solutions
or suspensions, injectable
solutions, elixirs or syrups, and can contain one or more agents chosen from
the group comprising
sweeteners, flavourings, colourings, or stabilisers in order to obtain
pharmaceutically acceptable
preparations. The choice of vehicle and the content of active substance in the
vehicle are generally
determined in accordance with the solubility and chemical properties of the
active compound, the
particular mode of administration and the provisions to be observed in
pharmaceutical practice. For
example, excipients such as lactose, sodium citrate, calcium carbonate,
dicalcium phosphate and
disintegrating agents such as starch, alginic acids and certain complex
silicates combined with
lubricants such as magnesium stearate, sodium lauryl sulfate and talc may be
used for preparing tablets.
To prepare a capsule, it is advantageous to use lactose and high molecular
weight polyethylene glycols.
When aqueous suspensions are used they can contain emulsifying agents or
agents which facilitate
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-246-
suspension. Diluents such as sucrose, ethanol, polyethylene glycol, propylene
glycol, glycerol and
chloroform or mixtures thereof may also be used.
For parenteral administration, emulsions, suspensions or solutions of the
products according to the
invention in vegetable oil, for example sesame oil, groundnut oil or olive
oil, or aqueous-organic
solutions such as water and propylene glycol, injectable organic esters such
as ethyl oleate, as well as
sterile aqueous solutions of the pharmaceutically acceptable salts, are used.
The solutions of the salts
of the products according to the invention are especially useful for
administration by intramuscular or
subcutaneous injection. The aqueous solutions, also comprising solutions of
the salts in pure distilled
water, may be used for intravenous administration with the proviso that their
pH is suitably adjusted,
that they are judiciously buffered and rendered isotonic with a sufficient
quantity of glucose or sodium
chloride and that they are sterilised by heating, irradiation or
microfiltration.
For topical administration, gels (water or alcohol based), creams or ointments
containing compounds of
formula (Ix) of the invention may be used. Compounds of formula (Ix) of the
invention may also be
incorporated in a gel or matrix base for application in a patch, which would
allow a controlled release
of compound through the transdermal barrier.
For administration by inhalation compounds of formula (Ix) of the invention
may be dissolved or
suspended in a suitable carrier for use in a nebuliser or a suspension or
solution aerosol, or may be
absorbed or adsorbed onto a suitable solid carrier for use in a dry powder
inhaler.
Solid compositions for rectal administration include suppositories formulated
in accordance with
known methods and containing at least one compound of the invention.
The percentage of active ingredient in the compositions of the invention may
be varied, it being
necessary that it should constitute a proportion such that a suitable dosage
shall be obtained.
Obviously, several unit dosage forms may be administered at about the same
time. The dose employed
will be determined by the physician, and depends upon the desired therapeutic
effect, the route of
administration and the duration of the treatment, and the condition of the
patient. In the adult, the
doses are generally from about 0.001 to about 50, preferably about 0.001 to
about 5, mg/kg body
weight per day by inllalation, from about 0.01 to about 100, preferably 0.1 to
70, more especially 0.5 to
10, mg/kg body weight per day by oral administration, and from about 0.001 to
about 10, preferably
0.01 to 1, mg/kg body weight per day by intravenous administration. In each
particular case, the doses
will be determined in accordance with the factors distinctive to the subject
to be treated, such as age,
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-247-
weight, general state of health and other characteristics which can influence
the efficacy of the
medicinal product.
The compounds of formula (Ix) according to the invention may be administered
as frequently as
necessary in order to obtain the desired therapeutic effect. Some patients may
respond rapidly to a
higher or lower dose and may find much weaker maintenance doses adequate. For
other patients, it
may be necessary to have long-term treatments at the rate of 1 to 4 doses per
day, in accordance with
the physiological requirements of each particular patient. Generally, the
active product may be
administered orally 1 to 4 times per day. Of course, for some patients, it
will be necessary to prescribe
not more than one or two doses per day.
Compounds of formula (Ix) of the invention may be prepared by the application
or adaptation of
known methods, by which is meant methods used heretofore or described in the
literature, for example
those described by R.C.Larock in Comprehensive Organic Transformations, VCH
publishers, 1989.
In the reactions described hereinafter it may be necessary to protect reactive
functional groups, for
example hydroxy, amino, imino, thio or carboxy groups, where these are desired
in the final product, to
avoid their unwanted participation in the reactions. Conventional protecting
groups may be used in
accordance with standard practice, for examples see T.W. Greene and P.G.M.Wuts
in "Protective
Groups in Organic Chemistry" John Wiley and Sons, 1991.
Compounds of formula (Ix) wherein W, X, Y, Z and R1 are as hereinbefore
defined for compounds of
formula (Ix) and A5 is H, may be prepared by reaction of compounds of formula
(IIx):-
Y~ ~ NH,
X\ ,
(IIx)
W NHZ
in which W, X, Y and Z are as hereinbefore defined for compounds of formula
(Ix), with acids of
formula (IIIx):-
Rl COZH (IIIx)
in which Rl is as hereinbefore defined for compounds of formula (Ix), at a
temperature at about 160 C.
Alternatively the reaction may (i) be carried in the presence of hydrochloric
acid at about reflux
CA 02465247 2004-04-26
WO 03/035065 PCT/GB02/04763
-248-
temperature, or polyphosphoric acid at a temperature at about 160 C or (ii) be
carried out in a
microwave oven.
Compounds of formula (Ix) wherein W, X, Y, Z and Rl are as hereinbefore
defined and A5 is H, may
=
be prepared by reaction of compounds of formula (IIx) in which W, X, Y and Z
are as hereinbefore
defined for compounds of formula (Ix), with aldehydes of formula (IVx):-
Rl CHO (IVx)
in which RI is as hereinbefore defined for compounds of formula (Ix), in the
presence of in inert
solvent, such as dimethylformamide or nitrobenzene, and at a temperature up to
about 145 C.
Alternatively the reaction may (i) be carried in the presence of sodium
bisulfite at a temperature at
about reflux temperature or (ii) be carried out in a microwave oven at a
temperature up to about 200 C.
Compounds of formula (Ix) wherein W, X, Y, Z and RI are as hereinbefore
defined for compounds of
formula (Ix) and A5 is H, may be prepared by cyclisation of compounds of
formula (Vx):-
I-lZ NH,
11 ~
X~w/ "kRi (Vx)
wherein W, X, Y, Z and R1 are as hereinbefore defined for compounds of formula
(Ix). The
cyclisation may be carried out by heating in the presence of an acid catalyst,
such as acetic acid, and at
a temperature up to about 120 C.
Compounds of formula (Ixa) wherein W, X, Y and Z are as hereinbefore defined
for compounds of
R9
R8
formula (Ix) and Rl is in which R9 is as hereinbefore defined for compounds of
N--N R 7
formula (Ix), R7is hydrogen and R8 is SR4, i.e. compounds of formula (Ixaa),
may be prepared as
shown in scheme 1.
DEMANDES OU BREVETS VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVETS
COMPREND PLUS D'UN TOME.
CECI EST LE TOME DE
NOTE: Pour les tomes additionels, veillez contacter le Bureau Canadien des
Brevets.
JUMBO APPLICATIONS / PATENTS
THIS SECTION OF THE APPLICATION / PATENT CONTAINS MORE
THAN ONE VOLUME.
THIS IS VOLUME 1 OF 3
NOTE: For additional volumes please contact the Canadian Patent Office.