Note: Descriptions are shown in the official language in which they were submitted.
CA 02465957 2004-05-17
Z
Catalyst Testing Process and
Apparatus
Background of the Invention
I. Field of the Invention:
The present in~renrion relates to the general field of
catalyst testing. . .
io 22. Problems Preserited by Prior Art
Catalyst testing is conventionally accomplished in
bench scale or larger pilot plants in which the feed is
contacted with a catalyst under reaction conditions,
3$nerally with effluent products being sampled, often With
samples being analyzed and results subjected to data
resolution tecbnigues. Such procedures can take a day or
more. for a single run on a single catalyst_ While such
techniques will have value in fine-tuning the optimum
matrices, pellet shape, etc., the present invention permits
the. scanning of dozens of catalysts in a single set-up,
often in less time than required far a single catalyst to be
evaluated by conventional methods. Further, when practiced
in its preferred robotic embodiments, the invention can
sharply reduce the labor costs per catalyst-screened.
CA 02465957 2004-05-17
1a
Further details with respect to the prior art can be found in the following
references:
(a) "Proceedings of the 6t" International Congress of Catalysis," Vol. 2,
1977,
pages 796-805, Jensen J.V., et al., 'A deactivation reactor for catalyst
screening and
s evaluation,' which relates to a deactivation reactor for catalyst screening
and
evaluation using a multitube converter for simultaneous aging of catalyst
samples
followed by determination of fundamental kinetic parameters in a separate
reactor.
The fundamental kinetic parameters of both fresh and spent catalysts are
determined
by measurement in a conventional recirculation reactor.
io (b) US-A-4 877 584 which relates to temperature programmed spectrometry
wherein particles of a substrate under investigation are attached in a non-
overlapping
manner to a heating filament. Gases from the filament desorbed at increased
temperatures are commonly measured by a quadruple mass spectrometer and ace
not
individually selectively determined.
(c) "International Journal of Hydrogen Energy", Vol. 7, No. 9, 1982, pages 729-
736, Haruta M. et al., 'Catalytic combustion of hydrogen, II. An experimental
investigation of fundamental conditions for burner design,' which relates to
catalytic
combustion of hydrogen. A catalyst body carrying four types of catalysts
tested is
inserted into a chamber and spot temperature and spot compositions of gas
mixtures
20 on the front surface of the catalysts are measured with nine chromel-alumel
thermocouples connected to a digital multi-temperature recorder, and with nine
microprobes for gas sampling connected to a gas chromatograph.
(d) JP-A-08015139 which relates to a gas adsorption/desorption measuring
method of catalyst for automobile. A cell containing a single sample under
constant
2s flow rate of reactant is investigated.
CA 02465957 2004-05-17
2
Scary of ti~.e =nvention
General Statement of the Invention
According to the invention, a multisample holder
(support) e.g. a honeycomb or plate, or a collection of
individual support particles, is treated with
solutions/suspensions of catalyst ingredients to fill.well s
1n plates, or to produce cells, spots or pellets., holding
each of a variety of combinations of the ingredients, is~
Vied, calcined or otherwise treated as necessary to
stabilize the ingredients in the cells, spots or pellets,
then is contacted with a potentially reactive feedstream or
batch e.g_, to catalyze biochemical reactions catalyzed by
proteins, cells,. enzymes; gas oil, hydrogen plus oxygen,
y5 ethylene or other polymerizable moxsomer, propylene plus
oxygen, or CC12E2 and hydrogen. The reaction occurring in
each cell is measured, e.g. by infrared thermography,
spectroscopic, electrochemical, photometric, thermal
conductivity or other method of detection of products or
residual reactants, or by sampling, e.g. by multistreaming
through low volume tubing, from the vicinity of each
combination, followed by analysis e_g. spectral analysis,
chromatography etc, or by observing temperature change irl
the vicinity of the catalyst e.g. by thermographic
9
techniques, to determine the relative efficacy of~ the
catalysts in each combination. Robotic techniques can be
employed in producing the cells, spots, pellets, etc__.
CA 02465957 2004-05-17
2a
The invention also concerns a method for evaluating a library of candidate
catalysts, the method comprising depositing a plurality of candidate catalyst
precursors
on a common support, treating each of the plurality of catalyst precursors in
place on
the support to form the plurality of candidate catalysts on the support,
contacting each
of the plurality of candidate catalysts in place on the support with one or
more reactants
under reaction conditions to catalyze at least one reaction, and determining
the relative
efficacy of the plurality of candidate catalysts by (i) detecting radiation
emitted or
absorbed during the course of the reaction, or (ii) detecting reaction
products or
unreacted reactants.
In another aspect the invention concerns a method for evaluating a library of
candidate catalysts, the method comprising depositing a plurality of candidate
catalyst
precursors on a common support, simultaneously treating each of the plurality
of
catalyst precursors in place on the support to form the plurality of candidate
catalysts
on the support, simultaneously contacting each of the plurality of candidate
catalysts in
place on the support with one or more reactants under reaction conditions to
catalyze at
least one reaction, the reaction conditions including a temperature greater
than 100 °C,
and additionally, or alternatively, a pressure of greater than 1 bar, and
determining the
relative efficacy of the plurality of candidate catalysts by (i) detecting
radiation emitted
or absorbed during the course of the reaction, or (ii) detecting reaction
products or
unreacted reactants.
In yet another embodiment the invention pertains to a method for evaluating a
library of candidate catalysts, the method comprising depositing a plurality
of inorganic
candidate catalyst precursor solutions on a common support, simultaneously
drying
each of the plurality of catalyst precursor solutions in place on the support
to form a
plurality of dried catalyst precursors on the support, simultaneously
calcining each of
the plurality of dried catalyst precursors in place on the support to form a
plurality
CA 02465957 2004-05-17
2b
inorganic catalysts on the support, simultaneously contacting each of the
plurality of
inorganic catalysts in place on the support with one or more reactants under
reaction
conditions to catalyze at least one reaction, the reaction conditions
including a
temperature greater than 100 ~C, and additionally, or alternatively, a
pressure of greater
than 1 bar, and determining the relative efficacy of the plurality of
candidate catalysts
by detecting reaction products or unreacted reactants by spectroscopy or
chromatography.
CA 02465957 2004-05-17
3
$ach of these parameters is discussed below:
Catalysts: Biotechnology catalysts include proteins, cells,
enzymes, etc. Chemical conversion catalysts include most of
the elements of the.periodic table which are solid at the
reaction conditions. Hydrocarbon conversion catalysts
include Bi, Sn, Sb, Ti, Zr, Pt, the rare earths, and many
possible candidates whose potential has not yet been
recognized for the .specific reaction_ Many synergistic
combinations will be useful_ Supported metals and metal
comPl~es are preferred. The chemical catalysts caa be
added to the substrate (support) as elements, as organic or
inorganic compounds which decompose under the temperature of
the stabilizing step, depositing the element or its oxide
onto the substrate, or as stable compounds.
Supports: Supports can be inert clays, zeolites, ceramics,
carbon, plastics, e.g. reactive plastics, stable,
nonreactive metals, or combinations of the foregoing_ Their
shape can be porous honeycomb penetrated by channels,
particles (pellets), or plates onto which patches (spots) of
catalyst candidates are deposited or wells in plates.
Conventional catalyst matrix materials such as.zeolites e.g.
CA 02465957 2004-05-17
4
zeolite USY, kaolin, alumina, etc. are particularly
preferred as they can simulate commercial catalysts.
Preparation: The catalyst candidate precursors can be
deposited onto the supports by any convenient technique,
preferably by pipette or absorbing stamp (like a rubber
stamp), or sil7c screen. In preferred embodiments, the
deposition process will be under robotic control, similar to
that used to load multicell plates in biochemical assays.
MAY of the spots of catalyst will be built up by several
separate depositions e.g. a channel penetrating a honeycomb
can be plugged at one third of its length and the channel
filled With a catalyst solution in its upper third, then the
plug can be moved to the two-thirds point in the channel and
a second catalyst pipetted in, then the plug can be removed
4nd a third catalyst solution added, resulting in a channel
in which reactants contact three catalysts successively as
they flow. through the channel. Catalyst can also be added
by ion exchange, solid deposition, impregnation, or
co~ination of these. The techniques of combinatorial
chemical or biological preparation can preferably be
utilized to prepare an array of candidate catalysts with the
invention. Coprecip~.tates of two or more catalysts can be
slurried, applied to the support, then activated as
,~5 necessary. Catalysts can be silk screened onto a support
plate or inside of a support conduit, and successive
screenings can be used to add different catalyst
combinations to different spots.
Stabilizing Step: Once the catalysts are in place on the
support, any suitable technique known.to the art can be used
to stabilize, and/or activate the particular catalysts
CA 02465957 2004-05-17
chosen, so they will remain in place during the reaction
step. Calcining, steaming, melting, drying, precipitation
and reaction in place will be particularly preferred.
5 Reactants: The invention has utility with any reaction which
can be enhanced by the presence of a catalyst, including
biological reactions and inorganic and organic chemical
reactions. Chemical reactions include polymerization
reactions, halogenation, oxidation, hydrolysis,
esterification, reduction and any other conventional
reaction which can benefit from a catalyst. Hydrocarbon
conversion reactions, as used in petroleum refining are an
important use of the inventions and include reforming, fluid
catalytic cracking, hydrogenation, hydrocracking,
hydrotreating, hydrodesulfurizing, alkylation and gasoline
sareetening
Sensors: The sensors used to detect catalytic activity in
the candidate catalysts are not narrowly critical but will
preferably be as simple as practical. Chramatographs,
temperature sensors, and spectrometers will be particularly
preferred, especially those adapted to measure temperature
and/or products near each specific catalyst spot e.g. by
multistreaming, multitasking, sampling, fiber optics, or
laser techniques. Thermography, as by an infrared camera
recording the temperature at a number of catalyst sites
simultaneously, is particularly preferred. Other suitable
sensors include electrochemical, fluorescence detectors,
NMR, NIR, F"fNIR, Raman, flame ionization, thermal
conductivity, mass, viscosity and stimulated electron or
X-ray emission Sensors can detect products in a gas or
liquid stream or on the surface of the support. Endothermic
CA 02465957 2004-05-17
6
reactions exhibit reduced temperature at best catalysts.
Some sensors employ an added detection reagent, e.g. ozone
to impart chemiluminesce.
Taggants: Optionally taggants (labels) can be added to
identify particular catalysts, particularly where particles
are employed as the supports for the catalysts. These
taggants can be conventional as discussed in the literature.
Taggants can be chemicals which are stable at reaction
conditions or can be radioactive with distinctive emissions.
The techniques of combinatorial chemistry will beapplicable
with taggants as well as with catalysts chosen to suit the
particular reaction to be enhanced by the catalyst_
Batch or Continuous: While the invention will be preferred
on a flow basis, with reactants flowing by the catalyst
spats under reaction conditions, batch testing e.g. in a
stirred autoclave or agitated containers, can be employed,
particularly in biological reactions.
Temperatures, pressures, space velocities and other reaction
2p conditions: These will be determined by the reactants and
reaction. Elevated pressures can be provided as reaction
conditions by encasing the support in a reaction chamber
with a sapphire or similar window for observation by the
sensing means, or with pressure-tight leads extending
through the reactor walls.
II. Utility of the Inveatioa
The present invention is useful in the testing of catalysts
for biotechnology, for promotion of gas phase and liquid
phase reactions; under batch or, preferably, continuous
flowstream conditions; at elevated, reduced or atmospheric
CA 02465957 2004-05-17
7
pressure; and saves both elapsed time and labor in screening
for improved catalysts to promote a desired reaction.
Brief Description of the Drawings
Figure 1 is a schematic diagram of a preferred
honeycomb support with a robotic pipetting device depositing
different combinations of catalyst ingredients into each of
the channels running through the honeycomb, which is
thereafter calcined to stabilize the catalysts in each
ch~el.
Figure 2 is shows schematically. the honeycomb of Fig.
lbeing contacted by reactants flowing through the channels.
Figures 3a and 3b are alternative schematic diagrams of
one channel of the honeycomb of Fig. 2 with a detector
sensing the products exiting the channel by measuring
absorption in a laser beam directed through the products or
the channel.
Figure 4a shows a channel plugged at its midpoint prior
to receiving a solution of catalyst and Figure 4b shows the
plug moved to the end of the channel, so as to form a
channel having one catalyst in one half its length and
another catalyst in its other half.
Figure 5 shows schematically a sheet of support onto
which 15 spots of different catalyst combinations have been
deposited, as discussed in example 1.
Figure 6a shows an array of particles (pellets) of
support in place in a reactor after having been ion
exchanged with different catalyst combinations on different
pellets (denoted schematically by different markings on the
pellets in the Figure). Figure 6b shows a packed reactor
which is less preferred because upstream pellets see fresh
CA 02465957 2004-05-17
feed, while downstream pellets see partially reacted feed.
I3e.scriptiosi of the Preferred E~nbod~.mertt3
Example I.
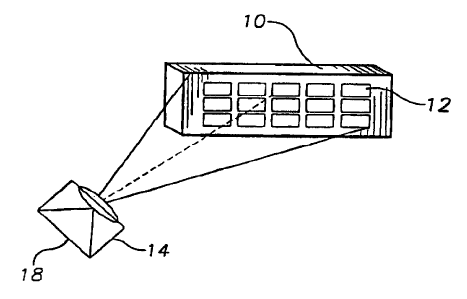
Referring to Figt:re 5, a sheet of. alpha alumina 10 is
wash coated with particles of porous gamma-aluaiina by
standard methods. Solutions of oxalate salts of 12
different transition metal elements are prepared in the
wells of a 24 well aLlcrotiter dish made of polystyrene. A
~u
B~=c~i~2000 roi~otic automatedliquid banal i.ng system
is used to prepare dilutions and mixtures fratn the original
stoc7ts, again in the. wells of microtiter style plates . Tha
robot a.s used to deposit 20 microliter aliquots of each of _
the resulting solutioxis at. defined positions (spots) 3.2 on
t~ surface of the alumina support 10, which is . then dried,
calcined.aad inserted into a reactor capable of,teraperature
control at temperatures from 100 to 350 degrees. centigrade_
After reduction, a potentially reactive mixture of oxygen
sad hydrogen is fed to the reactor. An Ages infra-red
sensitive camera 14 is l~sed to observe the alumina support
through infra-red-transparent sapphire windows via a
polished metal mirror_ The camera is set so that the lower
end of its dynamic range corresponds to a teatperature of
about 40 degrees C below the feed temperature and the
maxim~n signal is associated with a temperature about 200
degrees higher. Compositions catalyzing the reaction are
revealed by the localized temperature increases (decreases
for endothermic reactions) around spots 12 of that
composition, as shown on photograph 18.
CA 02465957 2004-05-17
9
Example la_
Catalysts are alternatively identified by conducting
the reaction in the presence of strong ultraviolet and/or
visible light illumination, with infra-red thermography
being conducted imomediately after the illumination is turned
off, or through the use of a short pass filter on tie
illumination source to eliminate,contaminating infra-red
radiation.
to Example 2..
Referyng to Figure 2, a porous alumina monolith 2 O
.'
(Corning) having square or circular cross-section channels
extending in a regular array through its entire thiclo?ess is
treated in each ~~a~re1 with a solutions of catalyst
15 Precursors of differing compostions, with each composition
being segregated in its own channel. After drying,
calcination, etc., the activated monolith is placed in
contact with a flowing potentially reactive mixture at an
elevated temperature, and observed in the infra-red using an
20 AJema~ m~de1 camera. The enthalpy of reaction produces
localized temperature differences in the vicinity of
composition exha.biting catalytic activity and these are
obsezved as temperature variations near the exits of the
channels_
a s Examp 1 a 3 .
Referring to Figures 2 and 3, a porous ceramic monolith 20 of
the type described in Example 2, bearing various catalyst
compositions in its channels is installed in a reactor (not
shown) in such a way the entire length of each channel can
3D be observed through sapphire windows at the ends of the
CA 02465957 2004-05-17
ZD
reactor. A broad-spectrum thern~al infra-red source is
installed at one end of the reactor, giving an areal
infra-red energy flux density. An~'Agema IR-sensitive camera
is positioned in such a way as to observe the infra- red
source directly through a significant fraction of the pores _
An interferometriclor other filter is installed on one side
of the reactor between the camera and the infra-red source
such that the light reaching the camera from the source is
lp substantially limited to wavelengths between 4 and 4.5
micro~ns_ Observation of absorbency at this wavelength range
is used to compare candidate catalyst compositions on the
basis of their production of carbon dioxide, an undesired
side product of the 'intended reaction. Catalyst
compositions chosen for loaf carbon dioxide formation (in
combination with high overall conversion activity as
measured by infra-red absorbance of the desired .product or
by infra-red thermography) are found to have high
selectivity for the desired product over the carbon dioxide
20 side product.
Example 4.
A collection of catalyst precursor compositions is
produced by automated liquid handling device, and a catalyst
support particle is contacted with each composition. After
25 further treatment to stabilize and activate the catalyst
precursors, catalyst pellets are arrayed on a surface,
exposed to a potentially reactive environment and their
activity detsT~~red by infrared thermography.
Examp 1 a 5 .
CA 02465957 2004-05-17
11
Solutions of combinations of catalyst precursors are
prepared in a variety of separate vessels. Each composition
also contains a small. quantity of a labeling material (e. g.,
stable isotopes of the element carbon or sulfur in varying
ratios). Catalyst support particles are contacted with
catalyst precursor preparations, and activated. Pellets are
then contacted one at a time With a potentially reactive
mixture (for examples, by eTutriation into an enclosed
volume) and their activity measured (by thermography, by
spectroscopic measurP~nt of products, or sampling of the
surrounding vapor or liquid phase). Particles showing
activity are collected and individually analyzed for their
content of the labeling material so as to determine the
composition giving the desired catalytic activity_
Exantp 1 a 6 .
Fxample 2 is repeated except that only a portion of the
pore length is coated with a catalyst candidate so as to
allow for observation of unmodified monolith pore wall as a
control reference standard foroptical una.fOrmity.
Example 7.
The emissivity of the support monolith pores of the
support of Example 2 is mapped at a wavelength of interest
by holding the monolith at the intended experimental
temperature in the absence of reactants. riigitally stored
maps of the emissivity are used to normalize the infra-red
energy flux measured under experimental conditions, to
improve the accuracy with which local temperatures can be
estimated.
CA 02465957 2004-05-17
12
Example 8.
A surface of high, substantially uniform emissivity is
located at the end of the monolith of Example 2, away from
the camera, in close radiative heat transfer/contact with
the monolith channel material. The temperature of the
portion of the surface closest to the open end of each
channel is observed. In this case, it is necessary that
gas be admitted into the channels past the uniform radiative
surface, either by means of pores or by means of a small
offset between the radiative surface and the monolith.
Exa.mp 1 a 9 .
Alternatively, spots of catalysts can be deposited on
the inner surface of a reactor a . g . a tube formed of the
support material, and temperature of the corresponding spots
on the outside of the reactor can be measured to determine
by conduction whether the respective catalyst has increased
or decreased in temperature under the reaction.
Example 10.
The process of Example 1 is repeated except that the
reactants are in the liquid phase and a liquid phase assay
is used to detect the activity of individual catalyst
candidates.
Example 11.
The experiment of Example 4 is repeated except that the
metal loading is directly measured by dissolving the
pellet and directly analyzing the metal loading.
Example 12.
A sheet,of alpha alumina is wash coated with particles
CA 02465957 2004-05-17
13
of porous gamma-alurnina by standard methods. Solutions of
oxalate salts of 12 different transition metal elements are
prepared in the raells of a 24 well micro titer dish made of '
polystyrene. A BeclonanBiomek2000 automated liquid
handling system is used to prepare dilutions and mixtures of
the original stocks, again in the wells of microtiter styl a
plates . The Biomelc robot is used to deposit 40 m3.croliter
aliquots of each of the resulting solutions at defined
positions on the surface of the alumina support, which is
then dried, calciaed and inserted into a reactor controll ad
at a temperature of 200 degrees centigrade. A gaseous
mixture of hydrogen ( 9 7 . S % ) and oxygen ( 2 . 5 % ) is fed at a
temperature of 200 degrees centigrade. An infra-red
s~sitive camera is used to observe the alumina.support
through infra-red-transparent sapphire windows.. The camera
is set so that its lower range corresponds to the feed
temperature and the max~mum signal is associated with a
temperature degrees 20 degrees higher. Compositions
catalyzing the reaction are revealed by the localized
temperature~increases around spots of that composition_
Example i3.
A porous alumina monolith having square pores extending
in a regular array through its entire thicIaness at a density
of 25 per square inch is washcoated with alumina particles.
The channels are then partially filled with solutions of
differing compositions, each containing one or more metal
oxalate or nitrate salts, with each composition being
segregated in its own channel or set of channels. After
drying and activation in the presence of hyrogen gas, the
CA 02465957 2004-05-17
14
activated monolith is placed into a sapphire-window-equipped
reactor in which it can be observed in the infrared using an
IR-sensitive camera. The camera is positioned in such a way
as to observe the walls of the support. The relative
emissivity of the support at each pixel a.s determined by
imaging the monolith in the IR while holding the reactor and
monolith at each of several constant temperatures while
flowing nitrogen gas through the reactor.
IO The reactor is then fed with a gas mixture of 2.5
mole g oxygen in hydrogen. The reactor and feed
temperatures are originally set to 40 degrees centigrade,
and are gradually increased while the catalyst-bearing
monolith is repeatedly imaged in the IR. The temperature in
15 each cell may be judged by observing the cell at a position
adjacent to the end of the catalyst-precursor-coated section
of the channel, or by normalizing the observed IR energy
emission by the emissivity calculated from the images taken
under nonreaetive conditions. The compositions in the cells
20 showing the earliest temperature increase above the reactor
temperature are useful as hydrogen oxidation catalysts.
Examp 1 a 14 .
A porous alumina monolith having square channels in a
regular array extending through its entire 10 centimeter
25 thickness at a density of 25 per square inch is washcoated
with alumina particles. The channels are then partially
filled with solutions of differing compositions, each
containing one or more metal salts and in some cases also
candidate modifiers such as barium, cesium or potassium
30 compounds, each composition being segregated in its own
CA 02465957 2004-05-17
channel or set of channels.
After drying and reduction in the presence of
hydrogen gas, the activated monolith is placed into a
5 reactor in which it can be observed through a sapphire
window using an IR-sensitive camera. This first window is
positioned 0.5 centimeter from the surface of the monolith.
The camera is positioned in such a Way as to look through
the window, through the channels of the support and through
10 a second sapphire Window toward a source of IR radiation.
The reactor is then fed with methane gas, mixed with
oxygen and argon, in such a way that the gas flows through
the channels of the monolith toward the camera. An optical
filter which selectively passes IR radiation at 4_3 microns,
15 a wavelength which is strongly absorbed by carbon dioxide,
is inserted between the IR source and the camera. The
effective concentration of carbon dioxide in each channel is
inferred from the IR intensity at 4.3 microns seen in that
channel. The reading at 4.3 microns for each pixel a.s
divided by the reading taken through a filter selective for
an IR wavelength which is near 4.3 microns, but which is not
absorbed strongly by carbon dioxide, methane or water, to
compensate for potential optical artifacts_
Compositions giving high concentrations of carbon
dioxide after long exposures to operating conditions are
useful in catalytic oxidation of methane.
Example 15.
Solutions of combinations of catalyst precursors are
prepared in a variety of separate vessels. Each composition
also contains a smallquantity of a labeling material (e. g.,
CA 02465957 2004-05-17
Z6
stable isotopes .of the element sulfur in varying ratios
unique to each composition) . Catalyst support particles are
contacted with the preparations of catalyst precursors
compositions, and activated. Pellets are then contacted one
at a time with a potentially reactive mixture (for examples,
by elutriation into an enclosed volume) and their activity
measured (by thermography, by spectroscopic measurement of
products, or sampling of the surround3_ng vapor or liquid
Phase). Particles showing activity are collected and
individually analyzed for their content of the labeling
material so as to dete~~re the composition giving the
desired catalytic activity.
Example l6.
A Teflon block having square channels in a regular
array extending through its satire thickness at.a density of
9 per square iach is prepared in such a way that a shallow
well exists at the bottom of each chanael_ Each well is
r~h--arged with a differeat polymer preparation bearing
sulfonic acid groups on its surface, and a porous retaining
mesh installed to keep t~xe polymer samples in place.
The catalyst-charged monolith is placed into a
reactor in which it can be observed through a window
positioned 0_5 centimeter from the surface of the block. A
Z5 camera is positioned in such a way as to look via through
the sapphire window, through the charnels of the support and
through a second window, toward a source of polarized light.
A polarizer is installed between the block and the camera.
A sucrose solution is fed to the reactor in such a
way as to flow through the channels of the block. The angle
CA 02465957 2004-05-17
of rotation of polarized light in passing through the liquid
in each channel is measured by rotating the polarizes to
various angles, and observingthe variation in brightness of
the light passing through each channel. The candidate
catalysts found in channels giving the greatest change in
the angle of rotation are useful as catalysts of sucrose
hydrolysis_
Exampla 17.
Catalysts for photooxidation of hexane are identified
by conducting the reaction in the apparatus of Example 16 in
the presence of strong ultraviolet andJor visible light
illumination, With infra-red thermography being conducted
immediately after the illumination is turned off, or through
the use of a short pass filter on the illumination source to
eliminate contam3.nating infrared radiation.
Example 18.
Samples of cyanogen bromide-activated cross linked
agarose beads are exposed to solutions of alcohol oxidase at
varied pH's, salt concentrations, and enzyme concentrations.
After coupling of the enzyme, residual active groups are
quenched with ethanolamine, the beads are washed, and each
sample placed in a separate well of a multiwell plate. The
plate is exposed to a flowing air stream containing ethanol
vapor and observed with an Amber infrared-sensitive camera.
The samples showing the greatest temperature increase are
selected as highly active immobilized alcohol oxidase
catalysts.
Example 19.
Samples of cyanogen bromide activated cross linked
CA 02465957 2004-05-17
18
agarose beads are exposed to solutions of anti-alcohol
oxidase antibodies at varied pH~s, salt concentrations, and
antibody concentratipns.
After coupling of the enzyme, residual active groups are
quenchedwith ethanolamine_ The beads are washed, exposed to
a solution of alcohol oxidase, washed again, and each sample
placed in a separate well of a multiwell plate. The plate
is exposed to a flowing air stream containing ethanol vapor
1p and observed with an Amber infrared-sensitive camera. The
samples showing the greatest temperature increase are
selected as highly active immobilized alcohol oxidase
catalysts.
Example 20.
A cerama.e monolith having channels arranged in
perpendicular row/column format passing through its entire
thic3tness is washcoated with porous alumina particles and
all the channels in each column are treated with the same
catalyst precursors, which are activated. A potentially
reactive stream is flowed through the channels of the
monolith, and a multiwavelength beam of radiation is passed
over the surface of the monolith, parallel to each column,
to a detector situated at the end of the colu~. The
composition of the stream leaving the pores in that column
is estimated by processing the detector output,
including Fourier transformation and/or weighted
sumanation/differencing of the intensities at different
wavelengths.
Example 21.
Pellets bearing catalytically-active groups capable of
CA 02465957 2004-05-17
19
catalyzing the conversion of both the D-and L-stereoisomers
of a reactant are treated with a variety of substances
potentially capable of preferentially suppressing
(temporarily or permanently) the conversion of the
L-stereoisomer of that compound by that catalyst. The
pellets are distributed among the wells of a multiwellplate
and exposed to a mixture of the isomers of the compound to
be modified. Pellets treated with the suppressor giving the
9r'eatest reduction in the activity for conversion of the
L-isomer are useful in stereoselective modification of the
D-isomer.
Example 22.
A ceramic monolith having channels arranged in
perpendicular row/column format passing through its entire
thickness is washcoated with porous alumina particles and
the channels treated with catalyst precursors, which are
activated: A potentially reactive stream is flowed through
the channels of the monolith. A manifold consisting of an
ar'r'ay of tubes, each smaller than the dimensions of an
individual channel, is used to introduce a stream containing
ozone into the stream flowing through each channel, near its
outlet. Reaction of the introduced ozone with the desired
product liberates light, which is detected by a camera
directed at the monolith. The catalyst composition giving
the strongest light output is a useful catalyst for
conversion of the reactants to the ozone-reactive desired
product.
Example 23.
A ceramic monolith having channels arranged in
CA 02465957 2004-05-17
perpendicular row/column format passing through its entire
thickness is washcoated with porous alumina particles and
the channels treated with catalyst precursors, which are
activated and then exposed to a potential-deactivating
substance. A potentially-reactive stream isflowed through
the channels of the monolith. A manifold consisting of an
array of tubes, each smaller than the dimensions of an
individual channel, is used to sample the stream flowing
xp within each channel. Samples from each channel in turn are
introduced into a gas chromatograph-mass spectrometer
COmblnat7.On through an arrangement of switching valves, and
catalyst compositions giving the highest yield of desired
products are useful in conversion of that reactive stream.
Modificatio~a.s
Specific compositions, methods, or embodiments
discussed are intended to be only illustrative of the
invention disclosed by this specification. variations on
these compositions, methods, or embodiments are readily
2o apparent to a person of shill in the art based upon the
teachings of this specification and are therefore intended
to be included as part of the inventions disclosed herein.
For example, statistically-designed experiments, and
automated, iterative experimental process methods can be
employed to obtain further reductions in. time for testing.
Attachrnent/arraying of preformed catalytic elements
(especially precipitates, also single molecules and
complexes such as metallocenes) onto a support, preferably
by precipitating or deposition is useful in many cases.
3p Detection can involve addition of some reagent to the
CA 02465957 2004-05-17
2I
stream leaving each candidate, the reagent allowing
detection of a catalyst product through staining or reaction
to give a detectable~product, light, etc.
The supports can comprise arrays with special
arrangements for uniform flow distribution, e.g., a header
of multiple delivery tubes inserted into each channel in a
blocx.
The detection means can comprise electrochemical means,
or a gamma camera for metals accumulation measurement,
imaging elemental analysis by neutron activation and imaging
by film or storage plate of emitted radioactivity,
temperature measurement by acoustic pyrometry, bolometry,
electrochemical detection, conductivity detection, liquid
Phase assay, preferably dissolving the support pellet and
directly analyzing. the metal loading; measuring refractive
index in the liquid phase; observing the IR emissions of
product gases directly, without the usual source and using
instead the radiation hot gases emit at characteristic
wavelengths_
Other modifications can include testing for
selectivity after deliberately poisoning some sites,
especially in chiral catalysis, etc.
The formulations can be supported in the form of spots
or Layers on the surface of a support containing wells or
channels or channels extending across the entire extent of
the support. The support can comprise a form of carbon,
zeolite and/or plastic. The plastic can comprise a
reactant. The support can hold a form of catalyst made by
coprecipitation, or aluminum, or particles.
At least one of the formulations can preferably
CA 02465957 2004-05-17
zz
comprise a material selected from the group consisting of
transition metals, platinum, iron, rhodium, manganese,
metallocenes, zinc, copper, potassium chloride, calcsum,
zinc, molybdenum, silver, tungsten, cobalt and mixtures of
the foregoing.
The label can comprise different isotopes or different
mixtures of isotopes. The reaction conditions can comprise a
pressure greater than one bar absolute pressure and the
l0 contact can be at a temperature greater than 100 degrees
centigrade.
The method can comprise detection of temperature
changes in the vicinity of a respective formulation due to
reaction endotherm or exotherm. The method can comprise
z5 treatment With a reducing agent. The contacting step can be
carried out in the presence of compounds which modify the
distribution of the metal within the porous support. The
candidate catalyst fornnilations can be contacted in the form
of spots or layers on the surface of a support containing a
20 washcoat supported by an underlayer.
The stabilizing step can be carried out with a
temperature gradient or other means whereby certain
candidate catalyst formulations are exposed to different
temperatures. The stabilizing can comprise calcining,
steaming, drying, reaction, ion exchange and/or
precipitation. The detection of temperature changes due to
reaction can employ a correction for emissivity variations
associated with differences in chemical composition.
The array of formulations to be tested can comprise
30 preformed metallocenes or other catalytic complexes fixed to
a support.
CA 02465957 2004-05-17
23
The infrared radiation can be detected through the use
of nondispersive infrared spectroscopy, or
infrared-sensitive photographic film. The detector means can
comprise means for physically scanning over an array_of
candidate formulations.
Observations at multiple wavelengths can be processed
by mathematical manipulation e.g. transformation, weighted
summation and/or subtraction, etc. Reaction activity,
reactants, or products can be detected through the use of an
added reaction which signals the presence of reaction or
particular compounds or classes of compounds.
Chemiluminescence can be used as an indicator of reaction
activity, or particular compounds or classes of compounds. A
s~stantially collimated radiation source can be employed in
product detection/imaging. Multi-tube sampling can be used
to lead into a mass spectrometer, chromatograph, or optical
monitor. To simulate aging, etc_, the formulations can
exposed to a deleterious agent which reduces the activity of
2p at least one formulation by at least 10%, and then
optionally exposed to steam, heat, H2, air, liquid water or
other different substances) or conditions) which increase
the activity of at least one member of the collection by at
least 10% over its previously-reduced activity whereby
re3enerability, reactivatability, decolcing, or other
catalyst property is measured. The deleterious agent can
comprise elevated temperature, V, Pb, Ni, As, Sb, Sn, Hg,
Fe, S or other metals, H2S, chlorine, oxygen, Cl, and/or
carbon monoxide.