Note: Descriptions are shown in the official language in which they were submitted.
CA 02466954 2004-06-03
ANTIBACTERIAL AND ANTIFOULING OXATHIAZINES
AND THEIR OXIDES AS COATING COMPOSITIONS
The present invention concerns a method of controlling bacteria and fouling
organisms
with 3-aryl-5,6-dihydro-1,4,2-oxathiazines and their oxides, a method of
protecting non-
living materials other than wood, and industrial antibacterial and antifouling
compositions. Surfaces of objects -exposed to humid or aqueous environments
are
readily colonized by micro-organisms and occasionally by other, higher life
forms such
as molluscs and crustacea. As these organisms settle on or attach to said
surfaces, the
value of the exposed objects diminishes. The exterior, but possibly also the
interior of
the object may deteriorate, the surface changes (e.g. from smooth, clean and
streamlined
to rough, foul and turbulent), the weight of the object increases by the
deposit of the
organisms and their remnants, and the vicinity of the object may become
obstructed or
encumberecL The function of the object and system involved lowers and the
quality of
the aqueous environment deteriorates. Similar problems beset industrially used
compositions such as coatings, lubricants and the like. All these phenomena
are referred
to as fouling. The oxathiazines of the present method and their oxides are
disclosed in
US-4,569,690 as herbicides, plant fungicides, plant desiccants and defoliants.
The present invention provides a method of controlling bacteria and fouling
organisms;
said method comprising applying to said bacteria or fouling organisms, or to
the locus
thereof, an effective antibacterial or antifouling amount of a compound having
the formula
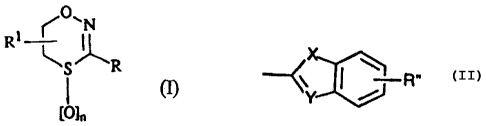
(~O, N
RI
SR. ~ =
[O]:, .
wherein n is 0, 1 or 2; R1 is hydrogen, Ci.4alkyl or benzyl; and R represents
(a) phenyl; phenyl substituted with 1 to 3 substituents independently selected
from
hydroxyl, halo, CZ-12alicyl, C5{cycloalkyl, trihalomethyl, phenyl, C? -
Salkoxy;
C1_5allcylthio, tetrahydropyranyloxy, phenoxy, C1,4allcy1carbonyl,
phenylcarbonyl,
CZ-4alkylsulfinyl, Cl-4alkylsulfonyl, carboxy or its alkali metal salt, Cl-
4alkyloxy-
carbonyl, C4-4allcylaminocarbonyl, phenylaminocarbonyl, tolylaminocarbonyl,
morpholinocarbonyl; amino, nitro, cyano, dioxolanyl or C14alkyloxyiminomethyl;
naphthyl; pyridinyl; thienyl, preferably when n is not 2; furanyl; or thienyl
or furanyl
CA 02466954 2004-06-03
-2-
substituted with one to three substituents independently selected from Cl-
4allcyl,
C1.4alkyloxy, C1..4allcylthio, halo, cyano, formyl, acetyl, benzoyl, nitro, Cl-
4alkyloxy-
carbonyl, phenyl, phenylaminocarbonyl and C1..4alkyloxyiminomethyl; or R
represents a
radical of formula
(b)
wherein X is oxygen or sulfur, Y is nitrogen, CH or C(C1..4ai.kyloxy); and R"
is
hydrogen or Ci.4alkyl.
The present invention in particular provides a method of protecting non-living
materials
other than wood, and the objects made of or covered by said non-living
materials,
against bacteria and/or fouling organisms, said method comprising applying to
the
surface or incorporating into said materials or objects an effective
antibacterial or.
antifouling amount of a compound of formula (1).
In the foregoing definitions halo is generic to fluoro, chloro, brorno and
iodo; CI-49cyl
defines straight and branch chained saturated hydrocarbon radicals having from
I to 4
carbon atoms comprising methyl, ethyl, n-propyl, 1-methyle:thyl, n-butyl, 1,1-
dimethyl-
ethyl,1-niethylpropyl, 2-methylpropyl; CI_5alkyl includes Cl.q.alkyl radicals
as defined
above and. saturated hydrocarlwn radicals having five carbon atoms, e.g. n-
pentyl and
the branched pentyl isomers; Cl-6alkyl includes Ci_galkyl radicals as defined
above and
six carbon containing homologs, e.g. n-hexyl and the branched hexyl isomers.
C1_12allcyl includes C1.6alkyl and saturated hydrocarbon radicals having from
7 to 12
carbon atoms, e.g. heptyl,,octyl, nonyl, decyl, undecyl and their isomers. The
term
alkali metal cation in particular is a sodium or potassium cati.on.
Trihalomethyl defines a
methyl group being fully substituted with halo atoms, in particular
trifluoromethyl and
trichloromethyl. CZ -4alkyloxyiminomethyl defines a radical of formula
-CH=N-O-C1.4alkyl=
Particular compounds of formula (1) for use in the method are those wherein n
is 0, .1 or
,2; Rl is hydrogen, C14.alkyl or benzyl; and R represents phenyl; naphthyl;
pyridinyl;
thienyl provided that n is not 2; faranyl optionally substituted with 1 to 3
substituents
independently selected from C?.-4aikyl and Ct-4aikyloxycarbonyl; or phenyl
substituted
with 1 or 2 substituents indepently selected from hydroxyl, halo, CI-12alkyl,
C5-6cyclo-
allcyl, trihalomethyl, phenyl, C2_5alkoxy, C1-5alkylthio,
tetrahydropyranyloxy, phenoxy,
CA 02466954 2006-01-31
-3-
Cl-aalkylcarbonyl, phenylcarbonyl, C1.4alkylsulfinyl, C1.4alkylsulfonyl,
carboxy or its
alkali metal salt, C1-5alkoxycarbonyl, Cl-4alkylaminocarbonyl,
phenylaminocarbonyl,
tolylaminocarbonyl, morpholinocarbonyl, amino, nitro, cyano, or dioxolanyl.
Of interest are those compounds wherein n is 1 and R represents phenyl,
thienyl or
phenyl substituted with one or two substituents selected from halo and
trihalomethyl; or
those wherein n is 2 and R represents phenyl or phenyl substituted with one or
two
substituents selected from halo and trihalomethyl.
Of further interest are the compounds wherein R1 is hydrogen, n is 1 or 2, and
R
represents phenyl, Cl-6alkylphenyl, halophenyl, dihalophenyl, biphenyl, CI-
5alkyl-
oxyphenyl, trihalomethylphenyl, nitrophenyl, phenyl substrituted with
Cl.a,alkyloxy-
carbonyl, C1-6alkylnitrophenyl, unsubstituted furanyl or thienyl, or furanyl
or thienyl
substituted with ethoxycarbonyl, cyano, chlorine or bromine:
Of particular interest are the compounds wherein R1 is hydrogen, n is 1 or 2,
and R
represents 3-fluorophenyl, 4-chiorophenyl, 3,4-dichlorophenyl, 3,5-
dichlorophenyl,
3-trifluoromethylphenyl, 4-trifluoromethylphenyl, 4-methylphenyl, 3-
ethanonephenyl,
3-nitrophenyl, 3-methyl-4-nitrophenyl or 2-thienyl.
Also of further interest are those compounds of formula (I) wherein R1 is
hydrogen, R
is a radical of formula (b) wherein X is sulfur, Y is nitrogen or CH, and R"
is hydrogen.
Preferred compounds are 5,6-dihydro-3-(2-thienyl)-1,4,2-oxathiazine-4-oxide
and
3-(4-chlorophenyl)-5,6-dihydro-1,4,2-oxathiazine-4,4-dioxide.
All compounds of formula (I) can be prepared following the procedures
described in
US-4,569,690.
The compounds of formula (I) are active against gram-positive bacteria and,
more importantly for use in material protection, also against gram-negative
bacteria.
Susceptible bacteria belong to genera such as Aeromonas, Alcaligenes,
Brevibacteriwn,
Cellulomonas, Citrobacter, Corynebacteriurn, Enterobacter, Escherichia,
Klebsiella;
Micrococcus, Proteus, Providencia, Pseudomonas, Shewanella, Acinobacter,
Bacillus,
Serratia, Staphylococcus, Streptococcus and Xanthomonas.
The compounds of formula (I) further are active against fouling organisms. The
term
"fouling organisms" is meant to comprise organisms that grow on or adhere to
various
CA 02466954 2004-06-03
-4-
kinds of surfaces, in particular in humid or aqueous environments such'as,
marine
waters, fresh waters, brackish waters, rain water, and also cooling water,
drainage
water, waste water and sewage. Fouling organisms are Algae such as, for
example,
Microalgae, e.g. Amphora, Achnanthes, Navicula, Amphipora, Melosira,
Cocconeis,
Chlamydomonas, Chlorella, Ulothrix, Anabaena, Phaeodactylum, Porphyridium;
Macroalgae, e.g. Enteromorpha, Cladophora, Ectocarpus, Acrochaetium, Ceramium,
Polysiphonia; Molluscs, e.g. Mytilus, Crustacea, e.g. Artemea, Balanus,
Elminius
Modestus, Verruca, Lepas and Ascidia, Hydrozoa and Bryozoa.
The present invention also provides a method for the preservation of non-
living snaterials
other than wood against fungi that spoil or destroy such materials. Said
method involves
treating such materials with an effective fungicidal amount.of a compound of
formula (I).
The compounds of formula (I) are useful to protect a wide variety of non-
living materials
other than wood, and the objects made thereof or covered thereby. Examples of
non-
living materials and the objects made thereof or covered thereby comprise
adhesives;
sizes; paper and cardboard; pulp; textiles; leather; paints; plastics, e.g.
PVC and
polyester, industrial compositions such as cooling media, e.g: cooling
lubricants and
cutting fluids, coating compositions, bath compositions (process liquids),
lubricants and
the like; metals and alloys such as iron and steel; building materials, e.g.
bricks, (paving)
stones, cement and concrete; decorating materials, e.g. plaster, tiles; and
any other
materials that can be contaminated or destroyed by bacteria, fungi or fouling
organisms.
Of particular interest are coating materials conventionally employed for
decorative and
protective purposes. The present method is especially suited for protecting
adherent
coatings, whether clear or colored (i.e. comprising one or more dyes or
pigments), and
whether natural or synthetic, such as, for example, paints - especially
antifouling paint
compositions -, varnishes, lacquers, finishes, whitewash and similar coatings.
The
particular aptness. of the present method of protecting said coating materials
resides in the
fact that the oxathiazines employed in the method not only effectively protect
the coating
material in storage containers, during "in can" conservation (especially
against bacteria)
thus ensuring a long pot (or can) life and good storage stability, but alsq
effectively
protect the coating material and optionally its underlying substrate when it
has been
applied as a film to said substra.te ("film" conservation). The simultaneous
utility of the
oxathiazines for both "in can" and "film" conservation of coatings is of great
practical
value.
As constructions made of or covered by said non-living materials there can be
mentioned
CA 02466954 2006-01-31
-5-
swimming pools, baths, cooling water circulation circuits and industrial baths
in various
installations, e.g. in manufactering plants or in air-conditioning
installations, the function
of which can be impaired by the presence and/or the multiplication of bacteria
and fouling
organisms. Further examples are buildings and parts of buildings such as
floors, outer
and inner walls or ceilings, of places suffering from dampness such as
cellars,
bathrooms, kitchens, washing houses and the lil,ce, and which are hot-beds for
bacteria
and/or fouling organisms. The presence of these organisms not only is
problemat ic from
the viewpoint of hygiene and aesthetics, but also causes economic losses
because said
buildings and/or decorating materials deteriorate more rapidly than desired.
The method is especially suitable to protect underwater objects such as, for
example,
shiphulls, harbour installations, drying docks, sluice-gates, locks, mooring
masts,
buoys, drilling platforms, bridges, pipelines, fishing nets, cables and any
other object in
constant or frequent contact with water, by applying to said.objects an
antifouling
composition, e.g. paint composition, comprising an effective antifouling
amount of a
compound of formula (I).
In this context it should be noted that the present method provides a safer
and
ecologically more acceptable alternative for current methods using antifouling
products
based on heavy metals such as cuprous oxide and the like, or those based on
organometallic derivatives such as organo-tin compounds. The toxicity of the
oxathiazines to mammals is acceptable and as such they are less hazardous for
humans
whether these compounds reach the human body by direct physical contact (e.g.
during.
handling or application) or via the food chain. Their bio-degradability
ensures that they
are less persistent in the environment and that they cause less and shorter
environmental
pollution and stress. The chemical stability of the oxathiazines fiirthermore
implies that
they are compatible with most non-living materials as such and do not need
special
precautions such as the addition of agents for stabilizing the active
ingredient. In
materials that should form films such as lubricants, cutting fluids and
coating materials,
they do not impair the fomlation of uniform films and the practicability. In
particular, in
coating materials they do not impair rapid curing in practical circumstances
such as room
temperature and outdoor conditions, and allow for strong adhesion of the
composition to
the substrate and /or the topcoat.
The method of application is chosen in accordance with the intended objective
and the
prevailing circumstances. For instance, the technique used in the case of
protecting
lubricants, coatings or cutting fluids comprises mixing the active ingredient
in said
lubricants, coatings or fluids either during the manufacturing process or
altematively
CA 02466954 2004-06-03
-6-.
afterwards in the finished product. The active ingredient can be added in neat
form or
dissolved oz suspended in a sufficient amount of diluent. Preferably. the
diluent consists
of one or more of the solvents as they occur in the final composition.
The method also comprises applying compounds of formula (I), optionally in an
appropriate formulation, to non-living materials by any of the techniques
known,in the
art such as, for example, brushing, spraying, atomising, dipping, soaking,
immersing,
scattering and pouring. In some instances, the application may involve
impregnation
techniques using pressure or vacuum systems, thermal systems, injection or
diffusion.
Appropriate formulations are formulated following art-known procedures to
emulsifiable
concentrates, directly sprayable or dilutable solutions, dilute emulsions,
wettable
powders, soluble powders, pastes, dusts, granulates, coating compositions,
e.g. paints -
in particular antifouling paint compositions -, lacquers and the like. For
example, the
active compound can be mixed with an extender, which consists of a liquid,
semi-solid
or solid carrier, and optionally surface-active agents such as emulsifiers
and/oii
dispersing agents. In coating compositions, the active compound may
advantageously.
be incorporated in polymers, copolymers or resins. These can consist of such
monomers as dialkyl (dimethyl) siloxanes, (meth)acrylic acid, (meth)acrylic
acid esters,
vinyl and allyl alcohols and derived esters (e.g. vinyl acetate), maleic acid,
styrene,
vinylchloride, butadiene, acrylamide, acrylonitrile and the liike
copolymerizable
monomers. As resins there may be mentioned alkyd resins, polyurethanes, epoxy
resins, phenolic resins and urea-formaldehyde resins. Further useful additives
in said
compositions comprise water-repelling agents and surface slipping agents that
are
capable of imparting a low surface tension of the coating film formed by the
polymer or
copolymer in the coating compositions.
Any suitable carrier or additive that does not interfere with the
antibacterial nor the .
antifouling activity of the active ingredient can be used in the compositions
of the present
invention. The solid carriers or fillers used e.g. for dusts and powders
include various
inert, porous and pulverous distributing agents of inorganic or organic nature
such as,
for example, the natural mineral fillers, e.g: calcite, talcum, kaolin,
montmorilTonite or
attapulgite,, or fillers of organic nature, e.g. powdered cork, sawdust and
other fine
pulverous materials. In order to improvt their physical properties it can be
advantageous
to add highly dispersed silicic acid or highly dispersed absorbent polymers.
Suitable
granulated absorbent carriers are of the porous type, for example, pumice,
broken brick,
sepiolite or bentonite; and suitable nonsorbent carriers are "imaterials such
as calcite or
CA 02466954 2004-06-03
-7 -
sand. The active ingredient is mixed with these carrier substances, for
example, by
being ground therewith; alternatively, the inert carrier substance is
impregnated with a
solution of the active component in a readily volatile solvent and the solvent
is thereafter
removed by heating or by filtering with suction at reduced pressure. By adding
wetti.ng
and/or dispersing agents, such pulverous preparations can also be made readily
wettable
with water, so that suspensions are obtained upon dilution.
Inert diluents used for the production of liquid preparations should
preferably not be
readily inflammable and should be as far as possible non-toxic to nontarget
animals or
plants and humans in the relevant surrounding. Diluents suitable for this
purpose are,
for example, water or, organic solvents such as, for example, aromatic
hydrocarbons,
e.g. methylbenzene, dimethylbenzene mixtures, substituted naphthalenes;
alcohols and
glycols and their ethers and esters, e.g. ethanol, ethylene glycol, ethylene
glycol
monomethyl or monoethyl ether, ketones e.g. 2-propanone, cyclohexanone and the
like;
strongly polar solvents; e.g. N-methyl-2-pyrrolidone, dimethyl sulfoxide or
dimethylformamide; vegetable oils or epoxidised vegetable oils such as
epoxidised
coconut oil or soybean oil, and mixtures thereof. Solutions can be prepared in
the usual
way, if necessary, with assistance of solution promotors. Other liquid forms
which can
be used consist of emulsions, dispersions or suspensions oi'the active
compound in
water or suitable inert diluents, or also concentrates for preparing such
emulsions,
dispersions or suspensions which can be directly adjusted to the required
concentration.
For this purpose, the active ingredient is, for example, mixed with a
dispersing,
suspending or emulsifying agent. The active component can also be dissolved or
dispersed in a suitable inert solvent and mixed simultaneously or subsequently
with a
dispersing or emulsifying agent. It is also possible to use semi=solid carrier
substances
of cream, ointment, paste or waxlike nature, into which the active ingredient
can be
incorporated, if necessary; with the aid of solution promotors and/or
emulsifiers.
Vaseline, petroleum wax, liquid paraffin, silicone oil and other cream-bases
are examples
of semi-solid carrier substances. Furthermore, it is possible for the active
ingredient to
be used in the form of aerosols. For this purpose the active ingredient is
dissolved or
dispersed in a volatile liquid suitable for use as a propellant; for example,
chlorinated
and/or fluorinated derivatives of methane and ethane and mixtures thereof, or
compressed air. In this way solutions under pressure are obtained which, when
sprayed, yield aerosols that are particularly suitable for controlling or
combatting bacteria
and/or fouling organisms, e.g. in closed chambers and storage rooms. For the
latter
purpose also smoke generators containing the active ingredient can be used.
Besides the compounds of formula (I) and the carrier, appropriate formulations
often
CA 02466954 2004-06-03
-8-
comprise other adjuvants conventionally employed in the art of formulation.
These
depend on specific applications and the user's preference. Such adjuvants are,
for
example, organic binding agents (e.g. chemically drying organic binder-forming
polymers such as allcyd resins or physically drying organic binder-forming
solids by
solvent evaporation); insecticides such as, for example, chlorinated
hydrocarbons, e.g.
endosulfan, organophosphates, e.g. chloropyriphos, pyrethroids, e.g.
permethrin and
the like; additional fungicides and bactericides such as alcohols, e.g.
ethanol, 2,3,3-tri-
iodallyl alcohol; aldehydes, e.g. formaldehyde, glutaraldehyde; formaldehyde
releasing
compounds, e.g. 2-bromo-2-nitro-propane-1,3-diol (bronopol), 2-bromo-2-nitro-
propan-l-ol; reaction products of amines and formaldehyde, e.g. triazines, 3,5-
dimethyl-
tetrahydro-1,3,5-2H-thiadiazine-2-thione; reaction products of amides and
formaldehyde, e.g. 1-hydroxymethyl-2-thiono-l:2-dihydzo-benzothiazol-N-
hydroxymethylbenzothiazolinthione; phenols, e.g. 2-phenylphenol,
pentachlorophenol;
organic acids, e.g. propionic acid, benzoic acid, salicylic acid, naphthenic
acid copper '
salts; inorganic acids, e.g. boric acid; amides, e.g. 2,5-dimethyl-N-
cyclohexyi-N-
methoxy-furan-3=carbonarnide; carbamates, e.g. 3-iodopropargyl-N-
butylcarbamate :
(IPBC), methyl-N-benzimidazol-2-ylcarbamate (Carbendazim), methyl-N-(1-
butylcarbamoyl)benzimidazol-2-ylcarbamate (benomyl), zinc
dimethyldithiocarbamate,
zinc ethylenebisdithiocarbam ate, bis-(dimethylthiocarbamoyl)disulphide;
pyridine
derivatives, e.g. 2-mercapto-pyridine-N-oxide, 2-chloro-6-
(trichloromethyl)pyridine,
2,3,5,6-tetrachloro-4-(methylsulphonyl)pyridine, copper 8-hydroxyquinoline, 1-
ethyl-
1,4-dihydro-7-methyl-4-oxo-1,8-naphthyridine-3-carboxylic acid; azoles, e.g.
tebuconazole, propiconazole, azaconazole, imazalil; heterocyclic compounds,
e.g. 2-
methyl-3(2I-,I -isothiazolone, 5-chloro-2-methyl-3(2H)-isothiazolone, 2-n-
octyl-4-
isothiazolin-3-one, 4,5-dichloro-2-(n-octyl)-4-isothiazolin=3-one,1,2-
benzisothiazolin-
3-one, 2-methyl-4,5-trimethylene-4-isothiazolin-3-one, 2-(4-thiazolyl)-
benzimidazole, 2-
mercaptobenzothiazole, 2-(thiocyanomethylthio)benzthiazole; N-haloalkylthio
compounds, e.g. N-trichloromethylthiophthalimide, folpet, N-
trichloromethylthio=4-
cyclohexane-1,2-dicarboximide, captan, N-1,1,2,2-tetrachloroethylthio-4-
cyclohexene-
1,2-dicarboximide, captafol, dichlofluanide, t.olylfluanide; compounds with
activated
halogen groups, e.g. chlorothalonil; surface active agents, e.g. guanidines
and
biguanides; organometallic compounds, e.g. bis(tri-n-butyltin-oxide,
tributyltin esters,
tributyltin naphthenate, tributyltin linoleate, tributyltin benzoate,
tributyltin fluoride;
various compounds, e.g. pimaricine, tridemorph, methylene bisthiocyanate,
tolyl -
sulfone, dicyanobutane; metal salts, e.g. chrome-eopper-ai-senic; active
natural products,
e.g. streptomycin; auxiliary solvents such as ethylglycol acetate and
methoxypropylacetate; processing additives; fixatives 'such as carboxymethyl-
cellulose,
CA 02466954 2004-06-03
-9-
polyvinyl alcohol, paraffin ; plasticizers such as benzoic acid esters and
phthalates, e.g.
dibutyl phthalate, dioctyl phthalate, diuodecyl phthalate; UV-stabilizers- or
stability
enhancers; dyes; color pigments; siccatives such as cobalt octoate, lead
octoate and cobalt
naphthenate; corrosion inhibitors; antisettling agents; anti-skinning agents
such as methyl
ethyl ketoxime; antifoaming agents and the like. Generally, the adjuvants are
not
essential to the practice of the present invention but are included in
particular
formulations, especially in coating formulations to optimize overall
effectiveness and
ease of application. They can be used therein in the conventional amounts.
The antibacterial and/or antifouling compositions which are employed in the
method of .
the invention can contain from 0.001% to 95% of the active ingredient by
weight based
on the total weight of the composition. Ready-to-use compositions such as
paints
preferably contain from 0.5% to 20%, in particular from 1 to 10% by weight of
the
active ingredient. Antifouling paint compositions on the other hand can
contain from 1%
up to 75%, in particular from 20 to 70% of the active ingredient of formula
(I) by weight,
based on the total weight of the dry mass of'said composition (i.e. up to 50%,
in
particular from 5 to 25% of the active ictgredient by weight based on the
total weight of
the antifouling composition). Preferred compositions are composed in
particular of the
following constituents (all percentages are by weight) :
Emulsifiable concentra.tes
active ingredient : 1 to 20%, preferably 5 to 10%
surfactant : 5 to 30%, preferably 10 to 20% ..
liquid carrier : 50 to 94%, preferably 70 to 85%
Dusts
active ingr.edient := 0.1 to 10%, preferably 0.1 to 1%
solid carrier : 99.9 to 90%, preferably 99.9 to 99%
. Suspension concentrates
active ingredient : 5 to 75%; preferably . 10 to 50%
water : 94 to 25%, preferably 88 to 30%
surfactant : 1 to 40%, preferably 2 to 30%
nulates .
active ingredient 0.5 to 30%, preferably 3 to 15%
-solid carrier : 99.5 to 70%, preferably 97 to 85%
The following examples are not intended to limit, but to illustrate the scope
of the
CA 02466954 2004-06-03
-10-
invention.
A. Biological Examples
Example 1: Efficacy against Bacteria and Yeasts.
Test solutions were prepared by dissolving the compourids shown in Tables 1-4
in
50% ethanol and further diluting with, sterile distilled water. These test
solutions were
pipetted into Petri dishes and mixed with warm tryptose agar to reach an
active
ingredient concentration of 100 ppm. After cooling, the medium was inoculated
with
the following yeasts or bacteria :
Debaryomyces hansenii (yeast) (A)
Pseudomonas alcaligenes (gram neg) (B)
Bacillus cereus mycoides (gram pos) (C)
Pseudomonas aeruginosa (gram neg) (D)
Flavobacterium sp. (gram neg) (E)
Streptomyces albus (gram pos) (F)
Enterobacter aerogenes (gram neg) (G)
Escherichia c"oli (gram neg) (H)
After sufficient growth of the untreated cultures, the compounds were
evaluated using
the following rating system :
0 = growth equal to control.
1 = inhibition of growth by the compound.
2= no growth under the influence of the compound.
The scores measured for antibacterial and antifouling efficacy of the
compounds of this
invention are listed in Tables 1-4. The sign '- signifies the; compound was
not tested.
Table 1 ~s R
[ala
Compounds Bacteria
Co. no. n R A B C D E F G H
1 1 4-chlorophenyl 2 -1 2 0 2 2 0 2
2 2 2,4-dichlorophenyl 2 0 2 0 2 0 0 0
3 1 3-nitro hen- 1 2 2 2. 1 2 2 1 2
CA 02466954 2004-06-03
-11-
Com ounds Bacteria
4 1 3,4-dichlorophenyl 2 1 2 0 2 2 0 2
2 2-methylphenyl 2 0 2 0 2 2 0 1.
6 1 3-fluorophenyl 2 1 2 0 2 2 0 2
7 1 2-furanyl 2 1 2 0 2 0 0 0
8 1 2-thienyl 2 2 2 1 2 2 2 2
9 1 3-methoxyphenyl 0 0 0 0 0 0 0 0
1 4-methylphenyl 2 1 2 1 2 2 0 2
11 2 4-methylphenyl 2 1 1 1 2 1 0 0
12 2 2-furanyl 0 0 0 0 0 0 0 0
13 1 3-trifluoromethyl 2 0 2 0 2 2 0 2
phenyl
14 1 4-ethanonephenyl 2 1 2 1 2 2 0 2
1 2,6-dichlorophenyl 1 0 1 0 0 0 0 0
16 2 2,6-dichlorophenyl 2 0 2 0 0 0 0 0
17 2 phenyl 0 0. 0 0 0 0 0 0
18 2 4-chlorophenyl - 1 2 0 2 2 0 0
19 2 3,5-dichlorophenyl - 0 2 0 2 2 0 0
1 4-butoxyphenyl 0 0 0 0 0 0 0 0
21 1 3,5-dichlorophenyl - 2 2 0 2 2 0 0
22 1 4-benzoic acid - 1 0 0 2 2 0 0
ethyl ester
23 2 3-chlorophenyl 0 . 0 0 0 0 0 0 0
24 2 4-trifluoromethyl - 0 2 0 2 2 2 0
phenyl
1 4-trifluoromethyl - 1 2 0 2 2 0 0
phenyl
26 . 1 3-benzoic acid, - 0 2 0 2 2. 0 0
methyl ester
27 2 3-bromophenyl - 1 2 0 2 2 0 2
28 1 4-ethoxyphenyl - 0 0 0 2 2 0 0
CA 02466954 2004-06-03
-12-
Table 2
R~ 'N
f
S X R4
[0'ln
R R3
Co. No. Rl R2 R3 R4 X n M.P. ( C)
29 H H H CO2CH3 0 0 85-89
30 H H H CO2CH3 0 2 126-129
31 H H H CO2CH3 0 1 118-119
32 H CH3 H H S 0 oil
33 - H CH3 H H S 1 73-75
34 H H H H S 2 99-101
35 H H H Br S 0 82-83
36 H H H Br S 1 113414
37 H CH3 H H S 2 60-62
38 H H H Br S 2 118-119
39 H H C02CH3 CH3 0 0 87-88
40 H H Br H S 0 74-75
41 H H Br . H S 1 169-173
42 H H Br H . . S 2 126-127
43 H H C02CH3 CH3 0 2 156-157
44 H . HCU2CH3 CH3 0 1 . 147-148
45 ' H H CH3 CO2CH3 S 1 150-152
46 H HCH3 CO2CH3 S 2 125-126
47 H H H CH3 S 0 62-63
.48 H H H CH3 S 1109-111
49 H H H CH3 S 2 101-102
50 CH3 H H H S 1 114-115
51 CH3 H H H S 2 147-148
52 CH3 H H H S '0 70-72
53 H H. H CO2CH2CH3 S 0 68-69
54 H H H C02CH2CH3 S 1 109-110
55 H H H C02CH2CH3 S 2- 123-124
56 H H H _ CN S 0 136-137
57 H H H CN S 1 160-162
CA 02466954 2004-06-03
-13-
Co. No. R1 R2 R3 R4 X n M.P. ( C)
58 H H H CN S 2 153-155
59 . H H H Cl S 0 74-77
60 H .H H C1 S 1 102
61 . H H H CI S 2 113-114
62 H H H CHO S 0 48-49
63 H H H NO2 S. 0 162-163
64 H H H NO2 S 1 186-188
65 H H H NO2 S 2 160-161
66 H H H CH=NOCH3 S 2 168-170
67 H -H H . C6H5 S 0 100-103
68' H H H C6H5 S 1 144-147
69 H H H . C6H5 S 2 95-98
70 H H NO2 C6H5 S 0 140-145
71 H H CH3 Br S 0 oil
72 H H CH3 Br S 1 100-104
73 H H Br CH3 S 0 64-67.
74 H H COOH CH3 0 0
75 H H CONHC6H5 CH3 0 0
76 H H CONHC6H5 CH3 0 0
77 H H CONHC6H5 CH3 O 0
Table 2a
~-N R2
~
S
[0]n S
Co. No. R2 n M.P. ( C)
78 H 2 102-104 79 H 1 106-107
CA 02466954 2004-06-03
-14-
Table 3. . ..
O, N
S X
[O Y
l
a
R7
Co. No. R" X Y n M.P.( C)
80 H 0 N 2 255
81 H 0 N 1 190-191
82 H 0 N 0 143-144
83 H S N 2 226-227
84- H S N 0 150-151
85 H S N 1 192-195
86 H S CH 0 132-134
87 H S CH 1 140-142
88 H S CH 2 150-154
89 CH3 0 N 1 209-210
90 CH3 0 N 2 215-216
91 H S GOCH(CH )2 1 ozl
Table 4
oeN
Rlo
Co1õ R
9
R$
Co. No. R8 R9 R10 M,.P.( C
92 H F H 2 136-138
93 . H F H 1 132-133
94 F F H 2 106-108
95 F F H 1 128-130
96 F F . H. 0 63-65
97 CF3 H CF3 1 110-113
98 CF3 H CF3 0 44-48
99 CF3 H CF3 2 76-78
100 F H F 0 103-104
- -- - --------
CA 02466954 2004-06-03
-15-
Co. No. R8 R9 R 10 n M.P. ( .C)
101 F H F 2 108-110
102 F H F 1 138-139
103 CO2CH(CH3)2 Cl H 0 oil
104 CO2CH(CH3)2 Cl H 1 127-129
105 CO2CH(CH3)2 Cl n 2 82-83
106 H CH=NOCH3 H 1 85-87
107 H ICH=NOCH31 H 2 104-106
Exainple 2: Efficacy against Fresh-water algae
9 ml of Bold's algal broth containing an appropriate concentration of test
compound, was
inoculated in a 5.5 cm diameter plastic Petri dish with 1 ml of a two week old
stock
culture of one of three species of green algae (Chlamydomonas dysosmos,
Chlorella
vulgaris or Ulothrix ca,~'ervicola) or the blue-green bacterium Anabaena
cylindrica. The
dishes were incubated in a climate room under a photosynthetically active
radiation level
of 40 mole.m-2.sec-1 at 20 C during the day (16 h) and 18 C at night (8 h).
Evaluation
was performed after 14 days by visually estimating the percentage- of algal
growth as
compared to controls. Results are expressed as minimum test concentration (in
ppm)
giving 90% mortality.
Com. ounds Concentration (ppm)
Co. nQ. n R Chiamydomonas Chlotella Ulothrix Anabaena
dysosmos vulgaris confervicola cylindrica
4 1 3,4-dichlorophenyl 2.5 10 2.5 2.5
8 1 2-thienyl 2.5 5 1 2.5.
18 2 4-chlorophenyl 2.5 10 5 2.5
19 2 3,5-dichlorophenyl 5 -10 2.5 2.5
21 1 3,5-dichiorophenyl 1 5 1 2.5
25 1 4-trifluoromethyl 2.5 10 2.5 5
hen. l
Ex=le 3: Efficacy against Marine algae
9 ml of Provasoli A8P-2 (Artificial Seawater-2) medium containing an
appropriate
concentration of test compound, was inoculated in a 5.5 cm diameter plastic
Petn dish
with 1 ml of a two weeks old stock culture of the diatom Phaeodactylurn
tricornutwn or
the unicellar red alga Porphyridiurn sp. The dishes were incubated in a
clirnate room
CA 02466954 2004-06-03
-16-
under a photosynthetically active radiation level of 40 mole.m-2.sec-1 at 20
C during
the day (16 h) and 18 C at night (8 h). Evaluation was performed after 14 days
by
visually estimating the percentage of algal growth as compared to controls.
Compounds 1, 2, 3, 4, 5, 14, 15, 16, 18, 19, 21, 25, 27 caused 90% mortality
at
concentration levels equal to or lower than 1 ppm.
Example 4: Efficacy against Artemia
Into 1 ml of artificial sea-water containing different amounts of the test
compound,
approximately 15 Artemia Instar II larvae were added. After 24 hours of static
incubation with continuous illumination the test was evaluated.
Compounds 2, 4, 5; 11, 13, 18, 19 caused 100% mortality at concentration
levels equal
to or lower than 10 ppm.
B. Composition examples (all percentages.are by weight)
Example 5: Composition examples for solid compounds of formula (I)
a) Emulsifiable concentrates : emulsions of any concentration could be
obtained from
these concentrates by dilution with water.
a) b)
compound of formula (1) 10% 1%
octylphenol polyethylene glycol ether
(4-5 moles of ethylene oxide) 3% 3%
calcium dodecylbenzenesulfonate 3% 3%
castor oil polyglycol ether
(36 moles of ethylene oxide) 4% 4%
cyclohexanone 30% 10%
diniethylbenzene mixture 50% 79%
b) Dusts were obtained by mixing the active ingredient with the carriers, and
grind.ing
the mixture in a suitable mill. a) b)
compound of formula (1) 0.1% 1%
talcum 99.9% -
kaolin - 99%
c) Suspension concentrates from which suspensions of any desired concentration
could
, be obtained by dilution with water were obtained by intimately mixing finely
ground .
active ingredient with the adjuvants. a) b)
compound of formula (I) 40% 5%
ethylene glycol 10% 10%
nonylphenol polyethylene glycol ether
CA 02466954 2004-06-03
= i
-17-
(15 moles of ethylene oxide) 6% 1%
sodium lignosulfate 10% 5%
carboxymethylcellulose 1% 1%
37% aqueous formaldehyde solution 0.2% 0.2%
silicone oil in the form of a 75%
aqueous emulsion 0.8% 0.8%
water 32% 77%
Ex le 6 Composition examples for liquid active ingredients of formula (I)
a) Emulsifiable concentrates : emulsions of any concentration could be
obtained from
these concentrates by dilution.with water.
a) b) . c)
compound of formula (I) 20% 40% 50%
calcium dodecylbenzenesulfonate 5% 8% 5.8%
castor oil polyethylene glycoi ether
(36 moles of ethylene oxide) 5% - -
tributylphenol polyethylene glycol ether
(30 moles of ethylene oxide) .12% 4.2%
cyclohexanone - 1.5% 20%
dimethylbenzene mixture 70% 25% 20%
b) Solutions suitable for application in the form of microdrops.
a) b) c) d)
compounds of fornlula (I) 80% 10% 5% 95 k
ethylene glycol monoethyl ether 20% - - -
polyethylene glycol (1VIG 400) - 70% - -
~T-methyl-2-pyrrolidone - 20% - -
epoxidised coconut oil - - 1% 5%
petroleum distillate (boiling range
160-190- C) - . - 94%
c) Granulates : prepared by dissolving the active ingredient in.
dichloromethane,
spraying the solution onto the carrier, and subsequently evaporating the
solvent.
a) b)
compound of formula (I) 5% 10%
kaolin 94% -
highly dispersed silicic acid 1%
attapulgim . - . 90%
CA 02466954 2004-06-03
-18-
d) Dusts : were obtained by intimately mixing the carriers with the active
ingredient.
a) b)
compound of formula (I) 2% 5%
highly dispersed silicic acid 1% 5%
talcum 97% -
kaolin - 90%
Example 7: Composition examples for paints
a) Compound of formula (I) 6.0 %
Titanium dioxide 17.1 %
Whiting 9.7%
Talc 13.2%
Calgon , 0.1 %
Hydroxyethylcellulose (3% solution in water) 12.5%
Co-solvent 1.2 %
Minor additives 0.4 %
Water 27.7%
Vinyl acetatefversatate copolymer emulsion 12.1 %
b) Compound of formula (I) 1.0 %
Titanium dioxide 26.4 %
Soya alkyd resin 44.0%
White spirit . 27.5 %
Cobalt octoate (8%) 0.3 %
Lead octoate (33%). 0.7 %
Methyl ethyl ketoxime 0.1 %