Note: Descriptions are shown in the official language in which they were submitted.
CA 02468048 2004-06-07
,0909-173D
1
PROCESS FOR PURIFYING CHEMTCALLY SYNTHESIZED RNA
This is a divisional application of Canadian Patent
Application Ser. No. 2,183,992 filed February 23, 1995.
Field of Invention
The parent application relates to reagents useful
as inhibitors of gene expression relating to diseases such
as inflammatory or autoimmune disorders, chronic myelogenous
leukemia, or respiratory tract illness.
This divisional application relates to a process
for (deprotecting and) purifying chemically synthesized RNA
having one or more chemical modifications.
However, it should be understood that the
expression 'this invention" or the like contained in this
specification encompasses the subject matters of both the
parent and divisional applications.
Summary of the Invention
The invention features novel enzymatic RNA
molecules, or ribozymes, and methods for their use for
inhibiting the expression of disease related genes, e.g.,
ICAM-1, IL-5, relA, TNF-a, p210bcr-abi~ and respiratory
syncytial virus genes. Such ribozymes can be used in a method
for treatment of diseases caused by the expression of these
genes in man and other animals, including other primates.
Ribozymes are RNA molecules having an enzymatic
activity which is able to repeatedly cleave other separate
RNA molecules in a nucleotide base sequence specific manner.
Such enzymatic RNA molecules can be targeted to virtually
any RNA transcript, and efficient cleavage has been achieved
in vitro Kim et al., 84 Proc. Natl. Acad. Sci. USA 8788,
CA 02468048 2004-06-07
,e909-173D
2
1987; Haseloff and Gerlach, 334 Nature 585, 1988; Cech, 260
JAMA 3030, 1988; and Jefferies et al., 17 Nucleic Acids
Research 1371, 1989.
Six basic varieties of naturally-occurring enzymatic
RNAs are known presently. Each can catalyze the hydrolysis of
RNA phosphodiester bonds in traps (and thus can cleave other
RNA molecules) under physiological conditions. Table 1
summarizes some of the characteristics of these ribozymes.
Ribozymes act by first binding to a target RNA.
Such binding occurs through the target RNA binding portion
of a ribozyme which is held in close proximity to an
enzymatic portion of the RNA which acts to cleave the target
RNA. Thus, the ribozyme first recognizes and then binds a
target RNA through complementary base-pairing, and once
bound to the correct site, acts enzymatically to cut the
target RNA. Strategic cleavage of such a target RNA will
destroy its ability to direct synthesis of an encoded
protein. After a ribozyme has bound and cleaved its RNA
target it is released from that RNA to search for another
target and can repeatedly bind and cleave new targets.
The enzymatic nature of a ribozyme is advantageous
over other technologies, such as antisense technology (where a
nucleic acid molecule simply binds to a nucleic acid target to
block its translation) since the effective concentration of
ribozyme necessary to effect a therapeutic treatment is lower
than that of an antisense oligonucleotide. The advantage
reflects the ability of the ribozyme to act enzymatically.
Thus, a single ribozyme molecule is able to cleave many
molecules of target RNA. In addition, the ribozyme is a
highly specific inhibitor, with the specificity of inhibition
depending not only on the base pairing mechanism of binding,
but also on the mechanism by which the molecule inhibits the
CA 02468048 2004-06-07
,n909-173D
2a
expression of the RNA to which it binds. That is, the
inhibition is caused by cleavage of the RNA target and so
specificity is defined as the ratio of the rate of cleavage of
the targeted RNA over the rate of cleavage of non-targeted
RNA. This cleavage mechanism is dependent upon factors
additional to those involved in base pairing. Thus, it is
thought that the specificity of action of a ribozyme is
greater than that of antisense oligonucleotide binding the
same RNA site. With their catalytic activity and increased
site specificity, ribozymes represent more potent and safe
therapeutic molecules than antisense oligonucleotides.
Thus, in a first aspect, this invention relates to
ribozymes, or enzymatic RNA molecules, directed to cleave
RNA species encoding ICAM-1, IL-5, relA, TNF-a, p210bor-abl, or
RSV proteins. Particularly described are the selection and
function of ribozymes capable of cleaving these RNAs and
their use to reduce levels of ICAM-1, IL-5, relA, TNF-a,
p210bcr-abi or RSV proteins in various tissues to treat the
diseases discussed herein. Such ribozymes are also useful
for diagnostic uses.
Indicated here is that these ribozymes are able to
inhibit expression of ICAM-1, IL-5, relA, TNF-a, p210bcr-ably
or RSV genes and that the catalytic activity of the
ribozymes is required for their inhibitory effect. Those of
ordinary skill in the art, will find that it is clear from
the examples described that other ribozymes that cleave
target ICAM-1, IL-5, relA, TNF-a, p210bcr-abi~ or RSV encoding
mRNAs may be readily designed and are within the invention.
These chemically or enzymatically synthesized RNA
molecules contain substrate binding domains that bind to
accessible regions of their target mRNAs. The RNA molecules
also contain domains that catalyze the
CA 02468048 2004-06-07
3
cleavage of RNA. Upon binding, the ribozymes cleave the target encoding
mRNAs, preventing translation and protein accumulation. In the absence
of the expression of the target gene, a therapeutic effect may be observed.
By "gene" is meant to refer to either the protein coding regions of the
cognate mRNA, or any regulatory regions in the RNA which regulate
synthesis of the protein or stability of the mRNA; the term also refers to
those regions of an mRNA which encode the ORF of a cognate polypeptide
product, and the proviral genome.
By "enzymatic RNA molecule" it is meant an RNA molecule which has
complementarity in a substrate binding region to a specified gene target,
and also has an enzymatic activity which is active to specifically cleave
RNA in that target. That is, the enzymatic RNA molecule is able to
intermolecularly cleave RNA and thereby inactivate a target RNA molecule.
This complementarity functions to allow sufficient hybridization of the
enzymatic RNA molecule to the target RNA to allow the cleavage to~ occur.
One hundred percent complementarity is preferred, but complementarity as
low as 50-75% may also be useful in this invention. By "equivalent" RNA to
a virus is meant to include those naturally occurring viral encoded RNA
molecules associated with viral caused diseases in various animals,
including humans, cats, simians, and other primates. These viral or viral-
encoded RNAs have similar structures and equivalent genes to each other.
By "complementarity" it is meant a nucleaic acid that can form
hydrogen bonds) with other RNA sequence by either traditional Watson
Crick or other non-traditional types (for examplke, Hoogsteen type) of base
paired interactions.
In preferred embodiments of this invention, the enzymatic nucleic
acid molecule is formed in a hammerhead or hairpin motif, but may also be
formed in the motif of a hepatitis delta virus, group I intron or RNaseP RNA
(in associateion with an RNA guide sequence) or Neurospora VS RNA.
Examples of such hammerhead motifs are described by Rossi et al., 1992,
Aids Research and Human Refroviruses , 8,183, .of hairpin motifs by
Hampel and Tritz, 1989 Biochemistry. 28, 4929, EP 0360257 and Hampel
et al., 1990, Nucleic Acids Res. 18,299 and an example of the hepatitis
delta virus motif is described by Perotta and Been, 1992 Biochemistry, 31
16 of the RNaseP motif by Guerrier-Takada et al., 1983 ~, 35 849,
CA 02468048 2004-06-07
4
Neurospora VS RNA ribozyme motif is described by Collins (Seville and
Collins, 1990 Cell 61, 685-696; Saville and Collins, 1991 Proc. Natl. Acad.
ci. U A 88, 8826-8830; Collins and Olive, 1993 ~iochemisfrv 32, 2795
2799 Guo and Collins, 1995 EMB ., 14, 368) and of the Group I intron by
Cech et al., U.S. Patent 4,987,071. These specific motifs are not limiting in
the invention and those skilled in the art will recognize that all that is
important in an enzymatic nucleic acid molecule of this invention is that it
has a specific substrate binding site which is complementary to one or
more of the target gene RNA regions, and that it has nucleotide sequences
within or.surrounding that substrate binding site which impart an RNA
cleaving activity to the molecule.
The invention provides a method for producing a class of enzymatic
cleaving agents which exhibit a high degree of specificity for the RNA of a
desired target. The enzymatic nucleic acid molecule is preferably targeted
to a highly conserved sequence region of a target ( i.e., 1 CAM-1, IL-5, reLA,
TNF-a, p210 bcr-abl or RSV proteins encoding mRNA such that specific
treatment of a disease or condition can be provided with either one or
several enzymatic nucleic acids. Such enzymatic nucleic acid molecules
can be delivered exogenously to specific cells as required., Alternatively,
the ribozymes can be expressed from vectors that are delivered to specific
cells. By °vect.ors° is meant any nucleic acid andlor viral-
based technique
used to deliver a desired nucleic acid:
Synthesis of nucleic acids greater than 100 nucleotides in length is
difficult using automated methods, and the therapeutic cost of such
molecules is prohibitive. In this invention small enzymatic nucleic acid
motifs (e.g., of the hammerhead or the hairpin structure) are used for
exogenous delivery. The simple structure of these molecules increases the
ability of the enzymatic nucleic acid to invade targeted regions of the mRNA
structrure. However, these catalytic RNA molecules can also be expressed
within cells from eukaryotic promoters (e.g. Scanion, K.J. et al., 1991, Proc.
Nail. Acad. Sci.. USA, 88, 10591-5; Kashani-Sabet, M., et a1.,1992,
Antis~nse Res. Dev., 2, 3-15; Dropoulic, B., et al., 1992, . Virol, 66, 1432-
41; Weerasinghe, M., et al., 191, Virol 65, 5531-4; Ojwang, J.O., et al.,
1992, Proc. Natl. Acad. Sci.. USA. 89 10802-6; Chen C.J., et al., 1992,
Nucleic Acids Res., 20, 4581-9; Sarver, H., et al., 1990 Science, 247, 1222-
1225). Those skilled in the art would realize that any ribozyme can be
CA 02468048 2004-06-07
J
expressed in eukaryotic cells from the appropriate DNA or RNA vector. The
activity of such ribozymes can be augmented by their release from tl.e
primary transcript by a second ribozyme (Draper et al.; PCT W093123569,
and Sullivan et al., PCT W094102595; Ohkawa, J., et al., 1992, Nucleic
Acids Svmo.
~ 27, 15-6; Taira, K. et al., Nucleic Acids Res.. 19, 5125-30; Ventura, M.,
et al., 1993, Nucleic Acids Res., 21, 3249-55, Chowrira et al., 1994 . Biol
hem 269, 25856 ).
By "inhibit" is meant that the activity or level of ICAM-l,Rel A, IL-5,
TNF-a, p210bcr-abl or RSV encoding mRNA is reduced below that
observed in the absense of the ribozyme, and preferably is below that level
observed in the presence of an inactive RNA molecule able to bind to the
same site on the mRNA, but unable to cleave that RNA.
Such ribozymes are useful for the prevention of the diseases and
conditions discussed above, and any other diseases or conditions that are
related to~ the level of 1CAM-1, IL-5, Rel A, TNF-a, p2lObcr-abl or RSV
protein or activity in a cell or tissue. By "related" is meant that the
inhibition
of ICAM-1, IL-5, Rel A, TNF-a, p210bcr-abl or RSV mRNA translation, and
thus reduction in the level of, ICAM-1, 1L-5, Rel A, TNF-a, p210bcr-abl or
RSV proteins will relieve to some extent the symptoms of the disease or
condition.
Ribozymes are added directly, or can be complexed with cationic
lipids, packaged within liposomes, or otherwise delivered to target cells.
The RNA or RNA complexes can be locally administered to relevant tissues
through the use of a catheter, infusion pump or stent, with or without their
incorporation in biopolymers. In preferred embodiments, the ribozymes
have binding arms which are complementary to the sequences in Tables
2,3,6-9, 11, 13, 15-23, 27, 28, 31, 33, 34, 36 and 37.
Examples of such ribozymes are shown in Tables 4-8, 10, 12, 14-16,
19-22, 24, 26-28, 30, 32, 34 and 36-38. Examples of such _ ribozymes
consist essentially of sequences defined in these Tables. By "consists
essentially of" is meant that the active ribozyme contains a~ enzymatic
center equivalent to those in the examples; and binding arms able to bind
mRNA such that cleavage at' the target site occurs. Other sequences may
be present which do not interfere with such cleavage.
CA 02468048 2004-06-07
s
Those in the art will recognize that, these sequences are
representative only of many more such sequences where the enzymatic
portion of the ribozyme (all but the binding arms) is altered to affect
activity.
For example, stem-loop II sequence of hammerhead ribozymes listed in
the above identified Tables can be altered (substitution, deletion, andlor
insertion) to contain any sequences provided a minimum of two base-
paired stem structure can form. Similarly, stem-loop IV sequence of hairpin
ribozymes listed in the above identified Tables can be altered (substitution,
deletion, and/or insertion) to contain any sequence, provided a minimum of
two base-paired stem structure can form. The sequence listed in the
above identified Tables may be formed of ribonucleotides or other
nucleotides or non-nucleotides. Such ribozymes are equivalent to the
ribozymes described specifically in the Tables. ,
In another aspect of the invention, ribozymes that cleave target
molecules and inhibit ICAM-1, IL-5, Rel A, TNF-a, p210bcr-abl or RSV
gene expression are expressed from transcription units inserted into DNA,
RNA, or viral vectors. Another means of accumulating high concentrations
of a ribozyme(s) within cells is to incorporate the ribozyme-encoding
sequences into a DNA or RNA expression vector. Transcription of the
ribozyme sequences are driven from a promoter for eukaryotic RNA
polymerase I (pol I), RNA polymerase II (pol Il), or RNA polymerase III (pol
III). Transcripts from pol ll or pol III promoters will be expressed at high
levels in all cells; the levels of a given pol II promoter~in a given cell
type
will depend on the nature of .the gene regulatory sequences (enhancers,
silencers, etc.) present nearby. Prokaryotic RNA polymerase promoters are
also used, providing that the prokaryotic RNA polymerase enzyme is
expressed in the appropriate cells (Elroy-Stein and Moss, 1990 Proc. ~Nafl.
Acad. Sci. USA, 87, 6743-7; Gao and Huang 1993 Nucleic Acids Res., 21
2867-72; Lieber et al., 1993 Methods Enzymol., 217, 47-66; Zhou et al.,
1990 Mol. Cell. Biol., 10, 4529-37). Several investigators have
demonstrated that ribozymes expressed from such promoters can function
in mammalian cells (e.g. Kashani-Sabet et ai., 1992 Antisense Res. Dev.;
2, 3-15; Ojwang et al., 1992 Proc. Natl. Acad. Sci. USA, 90, 6340-4;
L'Huiller et al., 1992 EMBO J. 11, 4411-8; Lisziewicz et al., 1993 Proc. Nafl.
Acad. Sci. U.S.A., 90 8000-4). The above ribozyme transcription units can
be incorporated into a variety of vectors for introduction into mammalian
cells, including but not restricted to, plasmid DNA vectors, viral DNA vectors
CA 02468048 2004-06-07
,0909-173D
7
(such as adenovirus or adeno-associated virus vectors), or
viral RNA vectors (such as retroviral or alphavirus
vectors).
As stated above, the subject matter of this
divisional application is a process for (deprotecting and)
purifying chemically synthesized RNA having one or more
chemical modifications.
In a first aspect, purification only is involved.
The process according to a major embodiment of the
first aspect, comprises:
(a) loading the RNA onto an anion exchange high-
performance liquid chromatography (HPLC) column;
(b) eluting the RNA by passing a suitable buffer
through the column to obtain an eluate: and
(c) collecting the eluate from the column and
recovering the RNA from the eluate, under conditions which
allow for purification of the RNA.
The process may further comprise:
(d) recovering the RNA from the desalted eluate
under conditions which allow for purification of the RNA.
In a second aspect, both deprotection and
purification are involved.
In this aspect, the process according to a first
major embodiment, comprises:
(a) loading the RNA onto reverse phase high-
performance liquid chromatography (HPLC) column, wherein the
RNA comprises a 5'-protecting group;
CA 02468048 2004-06-07
,n909-173D
7a
(b) eluting the RNA by passing a suitable buffer
through the reverse phase column;
(c) removing the 5'-protecting group from the RNA
to obtain unprotected RNA;
(d) loading the unprotected RNA onto an anion
exchange high-performance liquid chromatography (HPLC)
column;
(e) eluting the unprotected RNA by passing a
suitable buffer through the anion exchange column to obtain
an eluate; and
(f) collecting the eluate from the anion exchange
column and recovering the RNA from the eluate, under
conditions which allow for purification of the RNA.
In this aspect, the process according to a second
major embodiment, comprises:
(a) contacting the RNA with an alkylamine under
conditions suitable for removing any exocyclic amine
protecting groups or phosphate ester protecting groups;
(b) contacting the RNA with triethylamine-hydrogen
fluoride under conditions suitable to remove any alkylsilyl
protecting groups from the RNA;
(c) loading the RNA onto an anion exchange high-
performance liquid chromatography (HPLC) column;
(d) eluting the RNA by passing a suitable buffer
through the column to obtain an eluate; and
(e) collecting the eluate from the column and
recovering the RNA from the eluate, under conditions which
allow for purification of the RNA.
CA 02468048 2004-06-07
X6909-173D
7b
The process may further comprise:
(f) recovering the RNA from the desalted eluate
under conditions which allow for purification of the RNA.
Other features and advantages of the invention
will be apparent from the following description of the
preferred embodiments thereof, and from the claims.
Description of the Preferred Embodiments
The drawings will first briefly be described.
Drawings:
Figure 1 is a diagrammatic representation of the
hammerhead ribozyme domain known in the art. Stem II can be
>- 2 base-pair long.
Figure 2(a) is a diagrammatic representation of
the hammerhead ribozyme domain known in the art; Figure 2(b)
is a diagrammatic representation of the hammerhead ribozyme
as divided by Uhlenbeck (1987, Nature, 327, 596-600) into a
substrate and enzyme portion; Figure 2(c) is a similar
diagram showing the hammerhead divided by Haseloff and
Gerlach (1988, Nature, 334, 585-591) into two portions; and
Figure 2(d) is a similar diagram showing the hammerhead
divided by Jeffries and Symons (1989, Nucl. Acids. Res., 17,
1371-1371) into two portions.
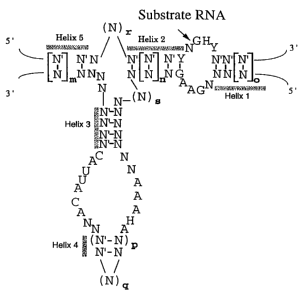
Figure 3 is a diagrammatic representation of the
general structure of a hairpin ribozyme. Helix 2 (H2) is
provided with at least 4 base pairs (i.e., n is 1, 2, 3 or
4) and helix 5 can be optionally provided of length 2 or
more bases (preferably 3-20 bases, i.e., m is from 1-20 or
more). Helix 2 and helix 5 may be covalently linked by one
or more bases (i.e., r is ? 1 base). Helix 1, 4 or 5 may
CA 02468048 2004-06-07
,6909-173D
~C
also be extended by 2 or more base pairs (e. g., 4-20 base
pairs) to stabilize the ribozyme structure, and preferably
is a protein binding site. In each instance, each N and N'
independently is any normal or modified base and each dash
represents a potential base-pairing interaction. These
nucleotides may be modified at the sugar, base or phosphate.
Complete base-pairing is not required in the helices, but is
preferred. Helix 1 and 4 can be of any size (i.e., o and p
is each independently from 0 to any number, e.g., 20) as
long as some base-pairing is maintained. Essential bases
are shown as specific bases in the structure, but those in
the art will recognize that one or more may be
CA 02468048 2004-06-07
8
modified chemically (abasic, base, sugar and/or phosphate modifications)
or replaced with another base without significant effect. Helix 4 can be
formed from two separate molecules, i.e., without a connecting loop. The
connecting loop when present may be a ribonucleotide with or without
modifications to its base, sugar or phosphate. "q" is z 2 bases. The
connecting loop can also be replaced with a non-nucleotide linker
molecule. H refers to bases A, U, or C. Y refers to pyrimidine bases.
" refers to a covalent bond.
Figure 4 is a representation of the general structure of the hepatitis
delta virus ribozyme domain known in the art.
Figure 5 is a representation of the general structure of the self-
cleaving VS RNA ribozyme domain.
Figure 6 is a diagrammatic representation of the genetic map of RSV
strain A2.
Figure 7 is a diagrammatic representation of the solid-phase
synthesis of RNA.
Figure 8 is a diagrammatic representation of exocyclic amino
protecting groups for nucleic acid synthesis.
Figure 9 is a diagrammatic representation of the deprotection of RNA.
Figure 10 is a graphical representation of the cleavage of an RNA
substrate by ribozymes synthesized, deprotected and purified using the
improved methods described herein.
Figure 11 is a schematic representation of a two pot deprotection
protocol. Base deprotection is carried out with aqueous methyl amine at 65
°C for 10 min. The sample is dried in a speed-vac for 2-24 hours
depending on the scale of RNA synthesis. Silyl protecting group at the 2'-
hydroxyl position is removed by treating the sample with 1.4 M anhydrous
HF at 65°C for 1.5 hours.
Figure 12 is a schematic representation of a one pot deprotection of
RNA synthesized using RNA phosphoramidite chemistry. . Anhydrous
methyl amine is used to deprotect bases at 65°C for 15 min. The sample
is
allowed to cool for 10 min before adding TEA~3HF reagent, to the same
CA 02468048 2004-06-07
9
poi, to remove protecting groups at the 2'-hydroxyl position. The .
deprotection is carried out for 1.5 hours.
Figs. 13a - b is a HPLC profile of a 36 nt long ribozyme, targeted to
site B. The RNA is deprotected using either the two pot or the one pot
deprotection protocol. The peaks corresponding to full-length RNA is
indicated. The sequence for site B is CCUGGGCCAGGGAUUA
AUGGAGAUGCCCACU. '
Figure 14 is a graph comparing RNA cleavage activity of ribozymes
deprotected by two pot vs one pot deprotection protocols.
Figure 15 is a schematic representation of an improved method of
synthesizing RNA containing phosphorothioate linkages.
Figure 16 shows RNA cleavage reaction catalyzed by ribozymes
containing phosphorothioate linkages. Hammerhead ribozyme targeted to
site C is synthesized such that 4 nts at the 5' end contain phosphorothioate
linkages. P=O refers to ribozyme without phosphorothioate linkages. P=S
refers to ribozyme with phosphorothioate linkages. The sequence for site C
isUCAUUUUGGCCAUCUC UUCCUUCAGGCGUGG.
Figure 17 is a schematic representation of synthesis of 2'-N-
phtalimido-nucleoside phosphoramidite.
Figure 18 is a diagrammatic representation of a prior art method for
the solid-phase synthesis of RNA using silyl ethers, and the method of this
invention using SEM as a 2'-protecting group.
Figure 19 is a diagrammatic representation of the synthesis of 2'-
SEM-protected nucleosides and phosphoramidites useful for the synthesis
of RNA. B is any nucleotide base as exemplified in the Figure, P is purine
and I is inosine. Standard abbreviations are used throughout this
application, well known to those in the art.
Figure 20 is a diagrammatic representation of a prior art method for
deprotection of RNA using TBDMS protection of the 2'-hydroxyl group.
' Figure 21 is a diagrammatic representation of the deprotection of RNA
having SEM protection of the 2'-hydroxyl group.
CA 02468048 2004-06-07
' 10
Figure 22 is a representation of an HPLC chromatogram of a fully
deprotected 10-mer of uridylic acid.
Figs. 23 - 25 are diagrammatic representations of hammerhead,
hairpin or hepatitis delta virus ribozyme containing self-processing RNA
transcript. Solid arrows indicate self-processing sites. Boxes indicate the
sites of nucleotide substitution. Solid lines are drawn to show the binding
sites of primers used in a primer-extension assay. Lower case letters
indicate vector sequence present in the RNA when transcribed from a
Nindlll-linearized plasmid. (23) HH Cassette, transcript containing the
hammerhead traps-acting ribozyme linked to a 3' cis-acting hammerhead
ribozyme. The structure of the hammerhead ribozyme is based on
phylogenetic and mutational analysis (reviewed by Symons, 1992 su ra).
The traps ribozyme domain extends from nucleotide 1 through 49. After 3'-
end processing, the traps-ribozyme contains 2 non-ribozyme nucleotides
(UC at positions 50 and 51) at its 3' end. The 3' processing ribozyme is
comprised of nucleotides 44 through 96. Roman numerals I, II and III,
indicate the three helices that contribute to the structure of the 3' cis-
acting
hammerhead ribozyme (Hertel et al., 1992 Nucleic Acids Res 20, 3252).
Substitution of G70 and A71 to U and G respectively, inactivates the
hammerhead ribozyme (Ruffner et al., 1990 Biochemistry 29, 10695) and
generates the HH(mutant) construct. (24) HP Cassette, transcript
containing the hammerhead traps-acting ribozyme linked to a 3' cis-acting
hairpin ribozyme. The structure of the hairpin ribozyme is based on
phylogenetic and mutational analysis (Berzal-Herranz et al., 1993 EMEMBO. JJ
12, 2567). The traps-ribozyme domain extends from nucleotide 1 through
49. After 3'-end processing, the traps-ribozyme contains 5 non-ribozyme
nucleotides (UGGCA at positions 50 to 54) at its 3' end. The 3' cis-acting
ribozyme is comprised of nucleotides 50 through 115. The transcript
named HP(GU) was constructed with a potential wobble base pair
between G52 and U77; HP(GC) has a Watson-Crick base pair between
G 52 and C77. A shortened helix 1 (5 base pairs) and a stable tetraloop
(GAAA) at the end of helix 1 was used to connect the substrate with the
catalytic domain of the hairpin ribozyme (Feldstein 8~ Bruening, 1993
Nucleic Acids Res. 21, 1991; Altschuler et al., 1992 su ra). (25) HDV
Cassette, transcript containing the traps-acting hammerhead ribozyme
linked to a 3' cis-acting hepatitis delta virus (HDV) ribozyme. The
secondary structure of the HDV ribozyme is as proposed by Been and
CA 02468048 2004-06-07
11
coworkers (Been et al., 1992 Biochemistry 31, 11843). The trans-ribozyme
domain extends from nucleotides 1 through 48. After 3'-end processing,
the traps-ribozyme contains 2 non-ribozyme nucleotides (AA at positions
49 to 50) at its 3' end. The 3' cis-acting HDV ribozyme is comprised of
nucleotides 50 through 114. Roman numerals I, Il, III 8~ IV, indicate the
location of four helices within the 3' cis-acting HDV ribozyme (Perrota &
Been, 1991 Nature 350, 434). The ~HDV transcript contains a 31
nucleotide deletion in the HDV portion of the transcript (nucleotides 84
through 115 deleted).
Fig. 26 is a schematic representation of a plasmid containing the
insert encoding self-processing cassette. The figure is not drawn to scale.
Fig. 27 demonstrates the efifect of 3' flanking sequences on RNA self-
processing in vitro. H, Plasmid templates linearized with Hindlll restriction
enzyme. Transcripts from H templates contain four non-ribozyme
nucleotides at the 3' end. N, Plasmid templates linearized with Ndel
restriction enzyme. Transcripts from N templates contain 220 non-
ribozyme nucleotides at the 3' end. R, Plasmid templates linearized with
Rcai restriction enzyme. Transcripts from R templates contain 450 non-
ribozyme nucleotides at the 3' end.
Fig. 28 shows the effect of 3' flanking sequences on the trans-
cleavage reaction catalyzed by a hammerhead ribozyme. A 622 nt
internally-labeled RNA (<10 nM) was incubated with ribozyme (1000 nM)
under single turn-over conditions (Herschlag and Cech, 1990 Biochemistry
29, 10159). HH+2, HH+37, and HH+52 are traps-acting ribozymes
produced by transcription from the HH, ~HDV, and HH(mutant) constructs,
respectively, and that contain 2, 37 and 52 extra nucleotides on the 3' end.
The plot of the fraction of uncleaved substrate versus time was fit to a
double exponential curve using the KaleidaGraph graphing program
(Synergy Software, Reading, PA). A double exponential curve fit was used
because the data points did not fall on a single exponential curve,
presumably due to varying conformers of ribozyme and/or substrate RNA.
Fig. 29 shows RNA self-processing in OST7-1 cells. !n vifro lanes
contain full-length, unprocessed transcripts that were added to cellular
lysates prior to RNA extraction. These RNAs were either pre-incubated
with MgCl2 (+) or with DEPC-treated water (-) prior to being hybridized
CA 02468048 2004-06-07
with 5' end-labeled primers. Cellular lanes contain total cellular RNA from
cells transfected with one of the four self-processing constructs. Cellular
RNA are probed for ribozyme expression using a sequence specific primer-
exiension assay. Solid arrows indicate the location of primer extension
bands corresponding to Full-Length RNA and 3' Cleavage Products.
Figs. 30,31 are diagrammatic representations of self-processing
cassettes that will release trans-acting ribozymes with defined, stable stem-
loop structures at the 5' and the 3' end following self-processing. 30,
shows various permutations of a hammerhead self-processing cassette. 31,
shows various permutations of a hairpin self-processing cassette.
Figs. 32a-b Schematic representation of RNA polymerse III promoter
structure. Arrow indicates the transcription start site and the direction of
coding region. A, B and C, refer to consensus A, B and C box promoter
sequences. I, refers to intermediate cis-acting promoter sequence. PSE,
refers to proximal sequence element. DSE, refers to distal sequence
element. ATF, refers to activating transcription factor binding element. ?,
refers to cis-acting sequence element that has not been fully characterized.
EBER, Epstein-Barr-virus-encoded-RNA. TATA is a box well known in the
art.
Figs. 33a-a Sequence of the primary IRNAimet and D3-5 transcripts.
The A and B box are internal promoter regions necessary for pol lil
transcription. Arrows indicate the sites of endogenous tRNA processing.
The D3-5 transcript is a truncated version of tRNA wherein the sequence 3'
of B box has been deleted (Adeniyi-Jones et al., '1984 supra). This
modification renders the d 3-5 RNA resistant to endogenous tRNA
processing.
Figure 34. Schematic representation of RNA structural motifs inserted
into the o3-5 RNA, e3-51HH1- a hammerhead (HHI) ribozyme was cloned
at the 3' region of D3-5 RNA; S3- a stable stem-loop structure was
incorporated at the 3' end of the D3-5/HH1 chimera; S5- stable, stem-loop
structures were incorporated at the 5' and the 3' ends of 03-5/HH1 ribozyme
chimera; S35- sequence at the 3' end of the D3-5/HH1 ribozyme chimera
was altered to enable duplex formation between the 5' end .and a
complementary 3' region of the same RNA; S35PIus- in addition to
structural alterations of S35, sequences were altered to facilitate additional
CA 02468048 2004-06-07
13
duplex formatiow within the non-ribozyme sequence of the e3-5/HHI
chimera.
Figures 35 and 36. Northern analysis to quantitate ribozyme
expression in T cell lines transduced with D3-5 vectors. 35) D3-5/HHI and
its variants were cloned individually into the DC retroviral vector (Sullenger
et al., 1990 supra). Northern analysis of ribozyme chimeras expressed in
MT-2 cells was performed. Total RNA was isolated from cells
(Chomczynski 8 Sacchi, 1987 Analytical Biochemistry 162, 156-159), and
transduced with various constructs described iri Fig. 34. Northern analysis
was carried out using standard protocols (Curt. Protocols Mol. Biol. 1992,
ed. Ausubel et al., Wiley 8 Sons, NY). Nomenclature is same as in Figure
34. This assay measures the level of expression from the type 2 pol 111
promoter. 36) Expression of S35 constructs in MT2 cells. S35 (+ribozyme),
S35 construct containing HHI ribozyme. S35 (-ribozyme), S35 construct
containing no ribozyme.
Figure 37. Ribozyme activity in total RNA extracted from transduced
MT-2 cells. Total RNA was isolated from cells transduced with D3-5
constructs described in Figs. 35 and 36 In a standard ribozyme cleavage
reaction, 5 pg total RNA and trace amounts of 5' terminus-labeled ribozyme
target RNA were denatured separately by heating to 90°C for 2 min in
the
presence of 50 mM Tris-HCI, pH 7.5 and 10 mM MgCl2. RNAs were
renatured by cooling the reaction mixture to 37°C for 10-15 min.
Cleavage
reaction was initiated by mixing the labeled substrate RNA and total
cellular RNA at 37°C. The reaction was allowed to proceed for - 18h,
following which the samples were resolved on a 20 % urea-polyacrylamide
gel. Bands were visualized by autoradiography.
Figures 38 and 39. Ribozyme expression and activity levels in S35-
transduced clonal CEM cell lines. 38) Northern analysis of S35-
transduced clonal CEM cell lines. Standard curve was generated by
spiking known concentrations of in vitro transcribed S~5 RNA into total
cellular RNA isolated from non-transduced CEM cells. Pool, contains RNA
from pooled cells transduced with S35 construct. Pool (-G418 for 3 Mo),
contains RNA from pooled cells that were initially selected for resistance to
6418 and then grown in the absence of 6418 for 3 months. Lanes A
through N contain RNA from individual clones that were generated from the
pooled cells transduced with S35 construct. tRNAimet~ refers to the
CA 02468048 2004-06-07
14
endogenous tRNA. S35, refers to the position of the ribozyme band. M,.
marker lane. 39) Activity levels in S35-transduced clonal CEM cell lines.
RNA isolation and cleavage reactions were as described in Fig.37.
Nomenclature is same as in Figs. 35 and 36 except, S, 5' terminus-labeled
substrate RNA. P, 8 nt 5' terminus-labeled ribozyme-mediated RNA
cleavage product.
Figures 40 and 41 are proposed secondary structures of S35 and
S35 containing a desired RNA (HHI), respectively. The position of HHI
ribozyme is indicated in figure 41. Intramolecular stem refers to the stem
structure formed due to an intramolecular base-paired interaction between
the 3' sequence and the complementary 5' terminus. The length of the
stem ranges from 15-16 base-pairs. Location of the A and the B boxes are.
shown.
Figures 42 and 43 are proposed secondary structures of S35 plus
and S35 plus containing HHI ribozyme.
Figures 44, 45, 46 and 47 are the nucleotide base sequences of S35,
HHIS35, S35 Plus, and HHIS35 Plus respectively.
Figs. 48a-b is a general formula for pol III RNA of this invention.
Figure 49 is a digrammatic representation of 5T construct. In this
construct the desired RNA is located 3' of the intramolecular stem.
Figures 50 and 51 contain proposed secondary structures of 5T
construct alone and 5T contruct containing a desired RNA (HHI ribozyme)
respectively.
Figure 52 is a diagrammatic representation of TRZ-tRNA chimeras.
The site of desired RNA insertion is indicated.
Figure 53 shows the general structure of HHITRZ-A ribozyme chimera.
A hammerhead ribozyme targeted to site I is inserted into the stem II region
of TRZ-tRNA chimera.
Figure 54 shows the general structure of HPITRZ-A riboZyme chimera.
A hairpin ribozyme targeted to site I is cloned into the indicated region of
TRZ-iRNA chimera.
CA 02468048 2004-06-07
Figure 55 shows a comparison of RNA cleavage activity of HHITRZ-A,
HHITRZ-B and a chemically synthesized HHI hammefiead ribozymes.
Figure 56 shows expression of ribozymes in T cell lines that are stably
transduced with viral vectors. M, markers; lane 1, non-transduced CEM
5 cells; lanes 2 and 3, MT2 and CEM cells transduced with retroviral vectors;
lanes 4 and 5, MT2 and CEM cells transduced with AAV vectors.
Figs. 57a-b Schematic diagram of adeno-associated virus and
adenovirues vectors for ribozyme delivery. Both vectors utilize one or more
ribozyme encoding transcription units (RZ) based on RNA polymerase II or
10 RNA polymerase III promoters. A. Diagram of an AAV-based 'vector
containing minimal AAV sequences comprising the inverted terminal
repeats (ITR) at each end of the vector genome, an optional selectable
marker (Neo) driven by an exogenous promoter (Pro), a ribozyme
transcription unit, and sufficient additional sequences (stuffer) to maintain
a
15 vector length suitable for efficient packaging. B. Diagram of ribozyme
expressing adenovirus vectors containing deletions of one or more wild
type adenoviorus coding regions (cross-hatched boxes marked as E1, pIX,
E3, and E4), and insertion of the ribozyme transcription unit at any or
several of those regions of deletions.
Fig. 58 is a graph showing the effect of arm length variation on the
activity of ligated hammerhead (HH) ribozymes. Nomenclature 5/5, 6/6,
7/7, 8/8 and so on refers to the number of base-pairs being formed between
the ribozyme and the target. For example, 5/8 means that the HH ribozyme
forms 5 by on the 5' side and 8 by on the 3' side of the cleavage site for a
total of 13 bp. -DG refers to the free energy of binding calculated for base-
paired interactions between the ribozyme and th.e substrate RNA (Turner
and Sugimoto, 1988 Ann. Rev. Biophys. Chem. 17, 167). RPI A is a HH
ribozyme with 6/6 binding arms.
Figs. 59 and 60 and 61 show cleavage of long substrate (622 nt) by
ligated HH ribozymes.
Fig. 62 is a diagrammatic representation of a hammerhead ribozyme
(HH-H) targeted against a site termed H. Variants of HH-H are also shown
that contain either a 2 base-paired stem II (HH-H1 and HH-H2) or a 3 .base-
paired stem II (HH-H3 and HH-H4).
CA 02468048 2004-06-07
Figs. 63 and 64 show RNA cleavage activity of HH-I and its variants
(see Fig.62). 63) cleavage of matched substrate RNA (15 nt). 64), cleavage
of long substrate RNA (613 nt).
Figs. 65a-b is a schematic representation of a method of this invention
to synthesize a full length hairpin ribozyme. No splint strand is required for
ligation but rather the two fragments hybridize together at helix 4 prior to
ligation. The only prerequisite is that the 3' fragment is phosphorylated at
its 5' end and that the 3' end of the 5' fragment have a hydroxyl group. The
hairpin ribozyme is targeted against site J. H1 and H2 are intermolecular
helices formed between the ribozyme and the substrate. H3 and H4 are
intramolecular helices formed within the hairpin ribozyme motif. Arrow
indicates the cleavage site.
Fig. 66 shows RNA cleavage activity of ligated hairpin ribozymes
targeted against site J.
Figs. 67a-b is a diagrammatic representation of a Site K Hairpin
Ribozyme (HP-K) showing the proposed secondary structure of the hairpin
ribozyme ~substrate complex as described in the art (Berzal-Herranz et al.,
1993 EMBO. J.12, 2567). The ribozyme has been assembled from two
fragments (bimolecular ribozyme; Chowrira and Burke, 1992 Nucleic Acids
Res. 20, 2835); #H1 and H2 represent intermolecular helix formation
between the ribozyme and the substrate. H3 and H4 represent
intramolecular helix formation within the ribozyme (intermolecular helix in
the case of bimolecular ribozyme). Left panel (HP-K1 ) indicates 4 base-
paired helix 2 and the right panel (HP-K2) indicates 6 base-paired helix 2.
Arrow indicates the site of RNA cleavage. All the ribozymes discussed
herein were chemically synthesized by solid phase synthesis using RNA
phosphoramadite chemistry, unless otherwise indicated. Those skilled in
the art will recognize that these ribozymes could also be made
transcriptionally in vitro and in vivo.
Figure 68 is a graph showing RNA cleavage by hairpin ribozymes
targeted to site K. A plot of fraction of the target RNA uncleaved (fraction
uncleaved) as a function of time is shown. HP-K2 (6 by helix 2) cleaves a
422 target RNA to a greater extent than the HP-K1 (4 by helix 2).
CA 02468048 2004-06-07
17
To make internally-labeled substrate RNA for traps-ribozyme
cleavage reactions, a 422 nt region (containing hairpin site A) was
synthesized by PCR using primers that place the T7 RNA promoter
upstream of the amplified sequence. Target RNA was transcribed in a
standard transcription buffer in the presence of [a-32P]CTP (Chowrira ~
Burke, 1991 supra). The reaction mixture was created with' 15 units of
ribonuclease-free DNasel, extracted with phenol followed
chloroform:isoamyl alcohol (25:1), precipitated with isopropanol and
washed with 70% ethanol. The dried pellet was resuspended in 20 p.l
DEPC-treated water and stored at -20°C.
Unlabeled ribozyme (1 pM) and internally labeled 422 nt substrate
RNA (<10 nM) were denatured and renatured separately in a standard
cleavage buffer (containing 50 mM Tris~HCl pH 7.5 and 10 mM MgCl2) by
heating to 90°C for 2 min. and slow cooling to 37°C for 10 min.
The
reaction was initiated by mixing the ribozyme and substrate mixtures and
incubating at 37°C. Aliquots of 5 ~I were taken at regular time
intervals,
quenched by adding an equal volume of 2X formamide gel loading buffer
and frozen on dry ice. The samples were resolved on 5% polyacrylamide
sequencing gel and results were quantitatively analyzed by radioanalytic
imaging of gels with a Phosphorlmager (Molecular Dynamics, Sunnyvale,
CA).
Figs. 69a-b is the Site L Hairpin Ribozyme (HP-L) showing proposed
secondary structure of the hairpin ribozyme~substrate complex. The
ribozyme was assembled from two fragments as described above. The
nomenclature is the same as above.
Figure 70 shows RNA cleavage by hairpin ribozymes targeted to site
L. A. plot of fraction of the target RNA uncleaved (fraction uncleaved) as a
function of time is shown. HP-L2 (6 by helix 2) cleaves a 2 KB target RNA
to a greater extent than the HP-L1 (4 by helix 2). To make internally-
labeled substrate RNA for traps-ribozyme cleavage reactions, a 2 kB region
(containing hairpin site L) was synthesized by PCR using primers.that place
the Z7 RNA promoter upstream of the amplified sequence. The cleavage
reactions were carried out as described above.
CA 02468048 2004-06-07
18
Figs. 71 a-b shows a Site M Hairpin Ribozyme (HP-M) with the
proposed secondary structure of the hairpin ribozyme~substrate complex.
The ribozyme was assembled from two fragments as described above.
Figure 72 is a graph showing RNA cleavage by hairpin ribozymes
targeted to site M. The ribozymes were tested at both 20°C and at
26°C.
To make internally-labeled substrate RNA for traps-ribozyme cleavage
reactions, a 1.9 KB region (containing hairpin site M) was synthesized'by
PCR using primers that place the T7 RNA promoter upstream of the
amplified sequence. Cleavage reactions were carried out as described
above except that 20°C and at 26°C temperatures were used.
Figs. 73a-d shows various structural modifications of the present
invention. A) Hairpin ribozyme lacking helix 5. Nomenclature is same as
described under figure 3. B) Hairpin ribozyme lacking helix 4 and helix 5.
Helix 4 is replaced by a nucleotide loop wherein q is z 2 bases.
Nomenclature is same as described under figure 3. C) Hairpin ribozyme
lacking helix 5. Helix 4 loop is replaced by a linker 103"L", wherein L is a
non-nucleotide linker molecule (Benseler ef al., 1993 J. Am. Chem. Soc.
115, 8483; Jennings et al., WO 94/13688). Nomenclature is same as
described under figure 3. D) Hairpin ribozyme lacking helix 4 and helix 5.
Helix 4 is replaced by non-nucleotide linker molecule "L" (Benseler et al.,
1993 supra; Jennings et aL, supra). Nomenclature is same as described
under figure 3.
Figs. 74a-b shows Hairpin . ribozymes containing nucleotide spacer
region "s" at the indicated location, wherein s is >_ 1 base. Hairpin
ribozymes containing spacer region', can be synthesized as one fragment
or can be assembled from multiple fragments. Nomenclature is same as
described under figure 3.
Figs. 75a-a shows the structures of the 5'-C-alkyl-modified
nucleotides. Rt is as defined above. R is OH, H, O-protecting group, NH, or
any group described by the publications discussed above, and ~ those
described below. B is as defined in the Figure or any other equivalent
nucleotide base. CE is cyanoethyl, DMT is a standard blocking group.
Other abbreviations are standard in the art.
CA 02468048 2004-06-07
Figure 76 is a diagrammatic representation of the synthesis of 5'-G
alkyl-D-allose nucleosides and their phosphoramidites.
Figure 77 is a diagrammatic representation of the synthesis of 5'-C-
alkyl-~-talose nucleosides and their phosphoramidites.
Figure 78 is a diagrammatic representation of hammerhead
ribozymes targeted to site O containing 5'-C-methyl-L-talo modifications at
various positions.
Figure 79 shows RNA cleavage activity of HH-O ribozymes. Fraction
of target RNA uncleaved as a function of time is shown.
Figure 80 is a diagrammatic representation of a position numbered
hammerhead ribozyme (according to Hertel et al. Nucleic Acids Res. 1992,
20, 3252) showing specific substitutions.
Figs. 81 a-j shows the structures of various 2'-alkyl modified
nucleotides which exemplify those of this invention. R groups are alkyl
groups, Z is a protecting group.
Figure 82 is a diagrammatic representation of the synthesis of 2'-G
allyl uridine and cytidine.
Figure 83 is a diagrammatic representation of the synthesis of 2'-C-
methylene and 2'-C-difluoromethylene uridine.
Figure 84 is a diagrammatic representation of the synthesis of 2'-C-
methylene and 2'-C-difluoromethylene cytidine.
Figure 85 is a diagrammatic representation of the synthesis of 2'-C-
methylene and 2'-C-difluoromethylene adenosine.
Figure 86 is a diagrammatic representation of the synthesis of 2'-C-
carboxymethylidine uridine, 2'-C-methoxycarboxymethylidine uridine and
.derivatized amidites thereof. X is CH3 or alkyl as discussed above, or
another substituent.
Figure 87 is a diagrammatic representation of a synthesis of
nucleoside 5'-deoxy-5'-difluoromethyiphosphonates.
CA 02468048 2004-06-07
Figure 88 is a diagrammatic representation of the synthesis of
nucleoside 5'-deoxy-5'-difluoromethylphosphonate 3'-phosphoramidites,
dimers and solid supported dimers.
Figure 89 is a diagrammatic representation of the synthesis of
5 nucleoside 5'-deoxy-5'-difluoromethylene triphosphates.
Figures 90 and 91 are diagrammatic representations of the synthesis
of 3'-deoxy-3'-difluoromethylphosphonates and dimers.
Figure 92 is a schematic representation of synthesizing RNA
phosphoramidite of a nucleotide containing a 2'-hydroxyl group
10 modification of the present invention.
Figs. 93a-b describes a method for deprotection of oligonucleotides
containing a 2'-hydroxyl group modification of the present invention.
Figure 94 is a diagrammatic representation of a hammerhead
ribozyme targeted io site N. Positions of 2'-hydroxyl group substitution is
15 indicated.
Figure 95 shows RNA cleavage activity of ribozymes containing a 2'-
hydroxyl group modification of the present invention. All RNA, represents
hammerhead ribozyme (HHN) with no 2'-hydroxyl group modifications. U7-
ala, represents HHN ribozyme containing 2'-NH-alanine modification at the
20 U7 position. U4/U7-ala, represents HHA containing 2'-NH-alanine
modifications at U4 and U7 positions. U4 lys, represents HHA containing
2'-NH-lysine modification at U4 position. U7 lys, represents HHA containing
2'-NH-lysine modification at U7 position. U4/U7-lys, represents HHN
containing 2'-NH-lysine modification at U4 and U7 positions.
Figures 96 and 97 are schematic representations of synthesizing
(solid-phase synthesis) 3' ends of RNA with modification of the present
invention. B, refers to either a base, modified base or an H.
Figure 98 and 99 are schematic representations of synthesizing
(solid-phase synthesis) 5' ends of RNA with modification of the present
invention. B, refers to either a base, modified base or an H.
Figures 100 and 101 are general schematic representations of the
invention.
CA 02468048 2004-06-07
21
Fig. 102a-d is a schematic representation of a method of the invention.
Fig. 103 is a graph of.the results of the experiment diagrammed in
figure 104.
Figure 104 is a diagrammatic representation of a fusion mRNA used
in the experiment diagrammed in Fig. 102.
Figure 105 is a diagrammatic representation of a method for selection
of useful ribozyrnes of this invention.
Figure 106 generally shows R-loop formation, and an R-loop
complex. In addition, it indicates the location at which ligands can be
provided to target the R-loop complex to cells wing at least three different
procedures, such as ligand receptor interaction, lipid or calcium phosphate
mediated delivery, or electroporation.
Figure 107 shows a method for use of self-processing ribozymes to
generate therapeutic ribozymes of unit length. This method is essentially
i 5 described by Draper et al., PCT WO 93/23509.
Figure 108 shows a method of linking ligands like folate,
carbohydrate or peptides to R-loop forming RNA.
Ribozymes of this invention block to some extent /CAM-1, iL-5, rei A,
TNF-a, p210bcr-abl, or RSV genes expression and can be used to treat
diseases or diagnose such diseases. Ribozymes will be delivered to cells
in culture and to tissues in animal models. Ribozyme cleavage of /CAM-1,
Il- .5, rel A, TNF-a ,p210bcr-abl, or RSV mRNA in these systems may prevent
or alleviate disease symptoms or conditions.
1 Target sites
Targets for useful ribozymes can be determined as disclosed in
Draper et al PCT W093/23509, Sullivan et al., PCT~W094/02595 as welt
as by Draper et' aL, PCT/US94113129.
Rather than repeat the guidance provided in
those documents here, below are provided specific examples of such
30. methods, not limiting to those in the art. Ribozymes to such targets are
designed as described in those applications and synthesized to be tested
in vitro and in vivo, as also described. Such ribozymes can also be
~i
CA 02468048 2004-06-07
22
optimized and delivered as described therein.. .White specific examples to
animal and human RNA ate provided, those in the art will recognize that
the equivalent human RNA targets described can be used as described
below. Thus, the same target may be used, but binding arms suitable for
targeting human RNA sequences are present iri the ribozyme. Such
targets may also be selected as described below.
It must be established that the sites predicted by the computer-based.
RNA folding algorithm correspond to potential cleavage sites.
Hammerhead or hairpin ribozymes are designed that could bind and are
individually analyzed by computer folding (Jae,ger et al., 1989 Proc. Natl.
Acid. Sci., USA; 86 7706-7710) to assess whether the ribozyme
sequences fold into the appropriate secondary structure. Those ribozymes
with unfavorable intramolecular interactions between the binding arms and
the catalytic core are eliminated from consideration. Varying binding arm
lengths can be chosen to optimize activity. Generally, at least 5 bases on
each arm are able to bind to, or otherwise interact with, the target RNA.
mRNA is screened for accessible cleavage sites by the method
described generally in Draper et al., PCT W093123569.
Briefly, DNA oligonucleotides
representing potential hamriierhead or hairpin ribozyme cleavage sites are
synthesized. A polymerise chain reaction is used to generate a substrate
for T7 RNA polymerise transcription from cDNA clones. Labeled RNA
transcripts are synthesized in vitro from DNA templates. The
oligonucleotides and the labeled trascripts are annealed, RNaseH is
added and the mixtures are incubated for the designated times at 37°C.
Reactions are stopped and RNA separated on sequencing polyacrylamide
gels. The percentage of the substrate cleaved is determined by
autoradiographic quantitation using a phosphor imaging system. from
these data, hammerhead or hairpin ribozynme sites are chosen as the
most accessible.
Ribozymes of the hammerhead or hai~pi~ motif are designed to
anneal to various sites in the mRNA message. The binding arms are
complementary to the target site sequences desribed above. The
ribozymes are chemically synthesized. The method of synthesis used
follows the procedure for normal RNA synthesis as described in Usman et
al., 1987 J. Am. Chem. Soc., 109, 7845 and in Scaringe et at., 1990
CA 02468048 2004-06-07
23
Nucleic Acids Res., 18, 5433 and made use of common nucleic acid
protecting and coupling groups, such as dimethoxytrityl at the 5'-end,
phosphoramidites at the 3'-end. The average stepwise coupling yeilds are
>98%. Inactive ribozymes are synthesized by substituting a U for G5 and a
U for A14 (numbering from Hertel et al., 1992 Nucleic Acids Res., 20,
3252). Hairpin ribozymes are synthesized in two parts and annealed to
reconstruct the active ribozyme (Chowrira and Burke, 1992 Nucleic Acids
Res., 20, 2835-2840). Ribozymes are also synthesized from DNA
templates using bacteriophage T7 RNA polymerase (Milligan and
Uhlenbach, 1989, Mefhods Enzymol, 180, 51 ). All ribozymes are modified
extensively to enhance stability by modification with nuclease resistant
groups, for example, 2'-amino, 2'-C-allyl, 2'-flouro, 2'-O-methyl, 2'H (for a
review see Usman and Cedergren, 1992 TIBS 17,34). Ribozymes ace
purified by gel electrophoresis using heneral methods or are purified by
high pressure liquid chromatography and are resuspended in water.
Example 1: ICAM-1
Ribozymes that cleave ICAM-1 mRNA represent a novel therapeutic
approach to inflammatory or autoimmune disorders. ICAM-1 function can
be blocked therapeutically using monoclonal antibodies. Ribozymes have
the advantage of being generally immunologically inert, whereas
significant neutralizing anti-IgG responses can be observed with some
monoclonal antibody treatments.
The following is a brief description of the physiological role of ICAM-1.
The discussion is not meant to be complete and is provided only for
understanding of the invention that follows. This summary is not an
admission that any. of the work described below is prior art to the claimed
invention.
Intercellular adhesion molecule-1 (ICAM-1) is a cell surface protein
whose expression is induced by inflammatory mediators. ICAM-1 is
required for adhesion of leukocytes to endothelial cells and for several
immunological functions including antigen presentation, immunoglobulin
production and cytotoxic cell, activity. Blocking ICAM-1 function prevents
immune cell recognition and activity during transplant rejection and in
animal models of rheumatoid arthritis, asthma and reperfusion injury.
CA 02468048 2004-06-07
24
Cell-cell adhesion plays a pivotal role in inflammatory and immune
responses (Springer et al., 1987 Ann. Rev. ImmunoG 5, 223-252). Cell
adhesion is required for leukocytes to bind to and migrate through vascular
endothelial cells. In addition, cell-cell adhesion is required for antigen
presentation to T cells, for B cell induction by T cells, as well as for the
cytotoxicity activily of T cells, NK cells, monocytes or granulocytes.
Intercellular adhesion molecule-1 (ICAM-1) is a 110 kilodalton member of
the immunoglobulin superfamily that is involved in all of these cell-cell
interactions (Simmons et al., 1988 Nature (London) 331, 624-627).
ICAM-1 is expressed on only a limited number of cells and at low
levels in the absence of stimulation (Dustin et al., 1986 J. lmmunol. 137,
245-254). Upon treatment with a number of inflammatory mediators
(lipopolysaccharide, ~-interferon, tumor necrosis factor-a, or interleukin~1
),
a variety of cell types (endothelial, epithelial, fibroblastic and
hematopoietic
cells) in a variety of tissues express high levels of ICAM-1 on their surface
(Sringer ef. al. supra; Dustin et al., supra; and Rothlein et al., 1988 J.
lmmunol. 141, 1665-1669). Induction occurs via increased transcription of
ICAM-1 mRNA (Simmons et aG, supra). Elevated expression is detectable
after 4 hours and peaks after 16 - 24 hours of induction.
ICAM-1 induction is critical for a number of inflammatory and immune
responses. In vitro, antibodies to ICAM-1 block adhesion of leukocytes to
cytokine-activated endothelial cells (Boyd,1988 Proc. Natl. Acad. Sci. USA
85, 3095-3099; Dustin and Springer, 1988 J. Cell Biol. 107, 321-331).
Thus, ICAM-1 expression may be required for the extravasation of immune
cells to sites of inflammation. Antibodies to ICAM-1 also block T cell
killing,
mixed lymphocyte reactions, and T cell-mediated B cell differentiation,
suggesting that ICAM-1 is required for these cognate cell interactions
(Boyd et aL, supra). The importance of ICAM-1 in antigen presentation is
underscored by the inability of ICAM-1 defective murine B cell mutants to
stimulate antigen-dependent T cell proliferation (Dang et al., 1990 J.
lmmunoG 144, 4082-4091). Conversely, murine L cells require transfection
with human ICAM-1 in addition to HLA-DR in order to present antigen to
human T cells (Altmann et al., 1989 Nature (London) 338, 512-514). In
summary, evidence in vitro indicates that ICAM-1 is required for .cell-cell
interactions critical to inflammatory responses, cellular immune responses,
and humoral antibody responses.
CA 02468048 2004-06-07
By, engineering ribozyme motifis we have designed several ribozymes
directed against /CAM-1 mRNA sequences. These have been synthesized
with modifications that improve their nuclease resistance. These
ribozymes cleave 1CAM-1 target sequences in vitro.
5 The sequence of human, rat and mouse /CAM-1 mRNA can ~ be
screened for accessible sites using a compter folding algorithm. Regions
of the mRNA that did not form secondary folding structures and that contain
potential hammerhead or hairpin ribozyme cleavage sites can be
identified. These sites are shown in Tables 2, 3, and 6-9. (All sequences
10 are 5' to 3' in the tables) While rat, mouse add human sequences can be
screened and ribozymes thereafter designed, the human targeted
sequences are of most utility.
The sequences of the chemically synthesized ribozymes useful in this
study are shown in Tables 4. - 8 and 10. Those in the art will recognize that
15 these sequences are representative only of many more such sequences
where the enzymatic portion of the ribozyme (all but the binding, arms) is
altered to affect activity and rnay be formed of ribonucleotides or other
nucleotides or non-nucleotides. Such ribozymes are equivalent to the
ribozymes described specifically in the Tables.
20 The ribozymes will be tested for function in vivo by exogenous
delivery to human umbilical vein endothelial cells (HUVEC). Ribozymes
will be delivered by incorporation into liposomes, by complexing with
cationic lipids, by microinjectivn, or by expression from DNA or RNA
vectors described above. Cytokine-induced /CAM-1 expression will be
25 monitored by ELISA, by indirect immunofluoresence, andlor by FACS
analysis. /CAM-1 mRNA levels will be assessed by Northern, by RNAse
protection, by primer extension or by quantitative RT-PCR analysis.
Ribozymes that block the induction of /CAM-1 protein and mRNA by more
than 90°/a will be identified.
As disclosed by Sullivan et al.,. PCT W094102595,
ribozymes and/or genes encoding them will be locally
delivered to transplant tissue ex vivo in animal models. Expression of the
ribozyme wilt be monitored by its ability to block ex vivo induction of 1CAM-
1 mRNA and protein. The effect of the anti-/CAM-7 ribozymes on graft
rejection will then be' assessed. Similarly, ribozymes will be introduced
CA 02468048 2004-06-07
26
into joints of mice with collagen-induced arthritis or rabbits with
Streptococcal cell wall-induced arthritis. Liposome delivery, cationic lipid
delivery, or adeno-associated virus vector delivery can be used. One dose
(or a few infrequent doses) of a stable anti-ICAM-1 ribozyme or a gene
construct that constitutively expresses the ribozyme may abrogate
inflammatory and immune responses in these diseases.
Use
ICAM-1 plays a central role in immune cell recognition and function.
Ribozyme inhibition of ICAM-1 expression can reduce transplant rejection
and alleviate symptoms in patients with rheumatoid arthritis, asthma or
other acute and chronic inflammatory disorders. We have engineered
several ribozymes that cleave ICAM-1 mRNA. Ribozymes that efficiently
inhibit ICAM-1 expression in cells can be readily found and their activity
measured with regard to their ability to block transplant rejection and
arthritis symptoms in animal models. These anti-ICAM-1 ribozymes
represent a novel therapeutic for the treatment of immunological or
inflammatory disorders.
The therapeutic utility of reduction of activity of ICAM-1 function is
evident in the following disease targets. The noted references indicate the
role of ICAM-1 and the therapeutic potential of ribozymes described herein.
Thus, these targets can be therapeutically treated with agents that reduce
ICAM-1 expression or function. These diseases and the studies that
support a critical role for ICAM-1 in their pathology are listed below. This
list is not meant to be complete and those in the art will recognize further
conditions and diseases that can be effectively treated using ribozymes of
the present invention.
~ Transplant rejection
ICAM-1 is expressed on venules and capillaries of human cardiac biopsies
with histological evidence of graft rejection (Briscoe et al., 1991
Transplantation
51, 537-539).
Antibody to ICAM-1 blocks renal (Cosimi et al., 1990J.~ Immunol. 144, 4604-
4612) and cardiac (Flavin et al., 1991 Transplanf. Proc. 23, 533-534) graft
rejection in primates.
CA 02468048 2004-06-07
27
A Phase I clinical trial of a monoclonal anti-ICAM-1 antibody showed
significant
reduction in rejection and a significant increase in graft function in human
kidney transplant patients (Haug, et al., 1993Transplantation 55, 766-72).
~ Rheumatoid arthritis
ICAM-1 overexpression is seen on synovial fibroblasts, endothelial cells,
macrophages, and some lymphocytes (Chin et al., 1990 Arthritis Rheum 33,
1776-86; Koch et al., 1991 Lab Invest 64, 313-20).
Soluble ICAM-1 levels correlate with disease severity (Mason et al., 1993
Arthritis Rheum 36, 519-27).
Anti-ICAM antibody inhibits collagen-induced arthritis in mice (Kakimoto et
al.,
1992 Cell lmmunol 142, 326-37).
Anti-ICAM antibody inhibits adjuvant-induced arthritis in rats (ligo et al.,
1991 J
lmmunol 147, 4167-71 ).
~ Myocardial ischemia, stroke, and reperfusion injury
Anti-ICAM-1 antibody blocks adherence of neutrophils to anoxic endothelial
cells (Yoshida et al., 1992 Am J Physiol262, H1891-8).
Anti-ICAM-1 antibody reduces neurological damage in a rabbit model of
cerebral stroke (Bowes et al., 1993 Exp Neurol 119, 215-9).
Anti-ICAM-1 antibody protects against reperfusion injury in a cat model of
myocardial ischemia (Ma et al., 1992Circulation 86, 937-46).
~ Asthma
Antibody to ICAM-1 partially blocks eosinophil adhesion to endothelial cells
and is overexpressed on inflamed airway endothelium and epithelium in vivo
(Wegner et al., 1990 Science 247, 456-9).
In a primate model of asthma, anti-ICAM-1 antibody blocks airway eosinophilia
(Wegneret al., supra) and prevents the resurgence of airway inflammation and
hyper-responsiveness after dexamethosone treatment (Gundel et al., 1992 Clin
Exp Allergy 22, 569-75).
~ Psoriasis
CA 02468048 2004-06-07
28
Surface ICAM-1 and a clipped, soluble version of ICAM-1 is expressed in
psoriatic lesions and expression correlates with inflammation (Kellner et al.,
1991 Br J Dermafol 125, 211-6; Griffiths 1989 J Am Acad Dermafol 20, 617-29;
Schopf et al., 1993 Br J Dermatol 128, 34-7).
Anti-ICAM antibody blocks keratinocyte antigen presentation to T cells
(Nickoloff et al., 1993) lmmunol 150, 2148-59 ).
~ Kawasaki disease
Surface ICAM-1 expression correlates with the disease and is reduced by
effective immunoglobulin treatment (Leung, et al., 1989Lancef 2, 1298-302).
Soluble ICAM levels are elevated in Kawasaki disease patients; particularly
high levels are observed in patients with coronary artery lesions (Furukawa et
al., 1992Arthritis Rheum 35, 672-7; Tsuji, 1992 Arerugi 41, 1507-14).
Circulating LFA-1+ T cells are depleted (presumably due to ICAM-1 mediated
extravasation) in Kawasaki disease patients (Furukawa et al., 1993Scand J
Immunol37, 377-80).
Example 2: IL-5
Ribozymes that cleave IL-5 mRNA represent a novel therapeutic
approach to inflammatory disorders like asthma. The invention features
use of ribozymes to treat chronic asthma, e.g_, by inhibiting the synthesis
of LL-5 in lymphocytes and preventing the recruitment and activation of
eosinophils.
A number of cytokines besides IL-5 may also be involved in the
activation of inflammation in asthmatic patients, including platelet
activating
factor, IL-1, IL-3, IL-4, GM-CSF, TNF-a, gamma interferon, VCAM, ILAM-1,
ELAM-1 and NF-xB. In addition to these molecules, it is appreciated that
any cellular receptors which mediate the activities of the cytokines are also
good targets for intervention in inflammatory diseases. These targets
include, but are not limited to, the IL-1 R and TNF-aR on keratinocytes,
epithelial and endothelial cells in airways. Recent data suggest that certain
neuropeptides may play a role in asthmatic symptoms. These peptides
include substance P, neurokinin A and calcitonin-gene-related peptides.
These target genes may have more general roles in inflammatory
diseases, but are currently assumed to have a role only in asthma.
CA 02468048 2004-06-07
29
Ribozymes of this invention block to some extent IL-5 expression and
can be used to treat disease or diagnose such disease. Ribozyrries will be
delivered to cells in culture and to cells or tissues in animal models of
asthma (Clutterbuck et al., 1989 supra; Garssen et al., 1991 Am. Rev.
Res~ir. Dis. 144, 931-938; Larsen et al., 1992 J. Clin. Invest. 89, 747-752;
Mauser et al., 1993 supra). Ribozyrne cleavage of IL-5 mRNA in these
systems may prevent inflammatory cell function and alleviate disease
symptoms.
The sequence of human and mouse IL-5 mRNA were screened for
accessible sites using a computer folding algorithm. Potential
hammerhead or hairpin ribozyme cleavage sites were identified. These
sites are shown in Tables 11, 13, and 14, 15. (All sequences are 5' to 3' in
the tables.) While mouse and human sequences can be screened and
ribozymes thereafter designed, the human targeted sequences are of most
utility. However, mouse targeted ribozymes are useful to test efficacy of
action of the ribozyme prior to testing in humans. The nucleotide base
position is noted in the Tables as that site to be cleaved by the designated
type of ribozyme. (In Table 12, lower case letters indicate positions that are
not conserved between the Human and the Mouse IL-5 sequences.)
The sequences of the chemically synthesized ribozymes useful in this
study are shown in Tables 12, 14 - 16. Those in the art will recognize that
these sequences are representative only of many more such sequences
where the enzymatic portion of the ribozyme (all but the binding arms) is
altered to affect activity. For example, stem loop II sequence of
hammerhead ribozymes listed in Tables 12 and 14 (5'-GGCCGAAAGGCC-
3') can be altered (substitution, deletion and/or insertion) to contain any
sequence provided, a minimum of two base-paired stem structure can form.
Similarly, stem-loop IV sequence of hairpin ribozymes listed in Tables 15
and 16 (5'-CACGUUGUG-3') can be altered (substitution, deletion and/or
insertion) to contain any sequence provided, a minimum of two base-
paired stem structure can form. The sequences listed in Tables 12; 14. - 16
may be formed of ribonuclectides or other nucleotides or non-nucleotides.
Such ribozymes are equivalent to the ribozymes described specifically in
the Tables.
By engineering ribozyme motifs we have designed several ribozymes
directed against IL-5 mRNA sequences. These ribozymes are synthesized
CA 02468048 2004-06-07
with modifications that improve their nuclease resistance. The ability of
ribozymes to cleave IL-5 target sequences in vitro is evaluated.
The ribozymes will be tested for function in vivo by analyzing IL-5
expression levels. Ribozymes will be delivered to cells by incorporation
5 into liposomes, by complexing with cationic lipids, by microinjection, or by
expression from DNA or RNA vectors. IL-5 expression will be monitored
by biological assays, ELISA, by indirect immunofluoresence, and/or by
FACS analysis. IL-5 mRNA levels will be assessed by Northern analysis,
RNAse protection or primer extension analysis or quantitative RT-PCR.
10 Ribozymes that block the induction of 1L-5 activity and/or IL-5 mRNA by
more than 90°!° will be identified.
Use
Interleukin 5 (IL-5), a cytokine produced by CD4+ T helper cells and
mast cells, was originally termed B cell growth factor I) (reviewed by
15 Takatsu et al., 1988 Immunol. Rev. 102, 107). It stimulates proliferation
of
activated B cells and induces production of IgM and IgA. IL-5 plays a major
role in eosinophil function by promoting differentiation (Clutterbuck et al.,
1989 Blood 73, 1504-12), vascular adhesion (Walsh et al., 1990
Immunoloav 71, 258-65) and in vitro survival of eosinophils (Lopez et al.,
20 1988 J. Exp. Med: 167, 219-24). This cytokine also enhances histamine
release from basophils (Hirai et al., 1990 I. Ex~. Med. 172, 1525-8). The
following summaries of clinical results support the selection of IL-5 as a
primary target for the treatment of asthma:
Several studies have shown a direct correlation between the number
25 of activated T cells and the number of eosinophils from asthmatic patients
vs. normal patients (Oehling et al., 1992 J. Investig. Allergol. Clin.
Immunol.
2, 295-9). Patients with either allergic asthma or intrinsic asthma were
treated with corticosteroids. The bronchoalveolar lavage was monitored for
eosinophils, activated T helper cells and recovery of pulmonary function
30 over a 28 to 30 day period. The number of eosinophils and activated T
helper cells decreased progressively with subsequent improvement in
pulmonary function compared to intrinsic asthma patients with .no
corticosteroid treatment.
Bronchoalveolar lavage cells were screened for production of
cytokines using in situ hybridization for mRNA. In situ hybridization signals
CA 02468048 2004-06-07
31
were detected for 1L-2, IL-3, IL-4, IL-5 and GM-CSF. Upregulation of mRNA
was observed for IL-4, IL-5 and GM-CSF (Robinson et al., 1993 . Aller
Clin. Immunol. 92, 313-24). Another study showed that upregulation of IL-5
transcripts from allergen challenged vs. saline challenged asthmatic
patients (Krishnaswamy et al., 1993 Am. J. Respir. Cell. Mol. Biol. 9., 279-
86).
An 18 patient study was performed to determine a mechanism of
action for corticosteroid improvement of asthma symptoms. Improvement
was monitored by methacholine responsiveness. A correlation was
observed between the methacholine responsiveness, a reduction in the
number of eosinophils, a reduction in the number of cells expressing IL-4
and IL-5 mRNA and an increase in number of cells expressing interferon-
gamma.
Bronchial biopsies from 15 patients were analyzed 24 hours after
allergen challenge (Bentley et al., 1993 Am. J. Respir. Cell. Mol. Biol 8,
35-42). Increased numbers of eosinophils and IL-2 receptor positive cells
were found in the biopsies. No differences in the numbers of total
leukocytes, T lymphocytes, elastase-positive neutrophils, macrophages or
mast cell subtypes were observed. The number of cells expressing IL-5
and GM-CSF mRNA significantly increased.
In another patient study, the eosinophil phenotype was the same for
asthmatic patients and normal individuals. However, eosinophils from
asthmatic patients had greater leukotriene C4 producing capacity and
migration capacity. There were elevated levels of IL-3, IL-5 and GM-CSF in
the circulation of asthmatics but not in normal individuals (Bruijnzeel et
al.,
1992 Schweiz. Med. Wochenschr. 122, 298-301 ).
Efficacy of antibody to IL-5 was assessed in a guinea pig asthma
model. The animals were challenged with ovalbumin and assayed for
eosinophilia and the responsiveness to the bronchioconstriction substance
P. A 30 mg/kg dose of antibody administered i.p. blocked ovalbumin-
induced increased sensitivity to substance P and blocked increases in
bronchoalveolar and lung tissue accumulation of eosinophils (Mauser et
al., 1993 Am. Rev. Respir. Dis. 148, 1623-7). In a separate study guinea
pigs challenged for eight days with ovalbumin were treated with
monoclonal antibody to IL-5. Treatment produced a reduction in the
CA 02468048 2004-06-07
32
number of eosinophils in bronchoalveolar lavage. No reduction was
observed for unchallenged guinea pigs and guinea pigs treated with a
control antibody. Antibody treatment completely inhibited the development
of hyperreactivity to histamine and arecoline after ovalbumin challenge
(van Oosterhout et al., 1993 Vim. Rev, Re~pir. Did 147, 548-52)
Results obtained from human clinical analysis and animal studies
indicate the role of activated T helper cells, cytokines and eosinophils in
asthma. The role of (L-5 in eosinophil development and function makes IL-
5 a good candidate for target selection. The antibody studies neutralized
IL-5 in the circulation thus preventing eosinophilia. Inhibition of the
production of lL-5 will achieve the same goal.
A st h m a - a prominent feature of asthma is the infiltration of
eosinophils and deposition of toxic eosinophil proteins (e.g. major basic
protein, eosinophil-derived neurotoxin) in the lung. A number of T-cell-
derived factors like IL-5 are responsible for the activation and maintainance
of eosinophils (Kay, 1991 . Allergy Clin. Immun. 87, 893). Inhibition of IL-5
expression in the lungs can decrease the activation of eosinophils and will
help alleviate the symptoms of asthma.
Atopy - is characterized by the developement of type I hypersensitive
reactions associated with exposure to certain environmental antigens. One
of the common clinical manifestations of atopy is eosinophilia
(accumulation of abnormally high levels of eosinophils in.~the blood).
Antibodies against IL-5 have been shown to lower the levels of eosinophils
in ri~ice (Cook et al., 1993 in Immunopharmacol. Eosinoohils ed. Smith and
Cook, pp. 193-216, Academic, London, UK)
Parasitic infection-related eosinophilia- infections with
parasites like helminths, can lead to severe eosinophilia (Cook et al., 1993
supra). Animal models for eosinophilia suggest that infection of mice, for
example, can lead to blood, peritoneal and/or tissue eosinophilia, all of
which seem to be lowered to varying degrees by antibodies directed
against IL-5.
Pulmonary infiltration eosinophilia- is characterised by
accumulation of high levels of eosinophils in pulmonary parenchyma
(Gleich, 1990 ~. Allergy Clin. Immunol. 85, 422).
CA 02468048 2004-06-07
33
L-Tryptophan-associated eosinophilia-myalgia syndrome
(EMS)- The EMS disease is closely linked to the consumption of L-
tryptophan, an essential aminoacid used to treat conditions like insomnia
(for review see Varga et al., 1993 J Invest. Dermatol. 100, 97s). Pathologic
and histologic studies have demonstrated high levels of eosinophils and
mononuclear inflammatory cells in patients with EMS. It appears that IL-5
and transforming growth factor play a significant role in the development of
EMS (Varga et al., 1993 supra) by activating eosinophils and other
inflammatory cells.
Thus, ribo~ymes of the present invention that cleave IL-5 mRNA and
thereby IL-5 activity have many potential therapeutic uses, and there are
reasonable modes of delivering the ribozymes in a number of the possible
indications. Development of an effective ribozyme that inhibits IL-5
function is described above; available cellular and activity assays are
numerous, reproducible, and accurate. Animal models for IL-5 function
and for each of the suggested disease targets exist (Cook et al., 1993
su ra) and can be used to optimize activity.
Example 3: NF-xB
Ribozymes that cleave rel A mRNA represent a novel therapeutic
approach to inflammatory or autoimmune disorders. Inflammatory
mediators such as lipopolysaccharide (LPS), interleukin-1 (IL-1) or tumor
necrosis factor-a (TNF-a) act on cells by inducing transcription of a number
of secondary mediators, including other cytokines and adhesion
molecules. In many cases, this gene activation is known to be mediated by
the transcriptional regulator, NF-xB. One subunit of NF-x8, the relA gene
product (termed ReIA or p65) is implicated specifically in the induction of
inflammatory responses. Ribozyme therapy, due to its exquisite specificity,
is particularly well-suited to target intracellular factors that contribute to
disease pathology. Thus, ribozymes that cleave mRNA encoded by rel A or
TNF-a may represent novel therapeutics for the treatment of inflammatory
and autoimmune disorders.
The nuclear DNA-binding activity, NF-xB, was first identified as a
factor that binds and activates the immunoglobulin x light chain enhancer
in B cells. NF-xB now is known to activate transcription of a variety of other
cellular genes (e.g., cytokines, adhesion proteins, oncogenes and viral
CA 02468048 2004-06-07
34
proteins) in response to a variety of stimuli (e.g., phorbol esters, mitogens,
cytokines and oxidative stress). In addition, molecular and biochemical
characterization of NF-xB has shown that the activity is due to a
homodimer or heterodimer of a family of DNA binding subunits. Each
subunit bears a stretch of 300 amino acids that is homologous to the
oncogene, v-rel. The activity first described as NF-xB is a heterodimer of
p49 or p50 with p65. The p49 and p50 subunits of NF-xB (encoded by the
nf-xB2 or nf-xB1 genes, respectively) are generated from the precursors
NF-xB1 (p105) or NF-xB2 (p100). The p65 subunit of NF-xB (now
termed Rel A ) is encoded by the rel A locus.
The roles of each specific transcription-activating complex now are
being elucidated in cells (N.D. Perkins, et al., 1992 Proc. Natl Acad. S
I~SA- 89, 1529-1533). For instance, the heterodimer of NF-xB1 and Rel A
(p50/p65) activates transcription of the promoter for the adhesion molecule,
VCAM-1, while NF-xB2/ReIA heterodimefs (p49/p65) actually inhibit
transcription (H.B. Shu, et al., Mol. Cell. Biol. 13, 6283-6289 (1993)).
Conversely, heterodimers of NF-xB2/ReIA (p49/p65) act with Tat-I to
activate transcription of the HIV genome, while NF-xB1/ReIA (p50/p65)
heterodimers have little effect (J. Liu, N.D. Perkins, R.M. Schmid, G.J.
Nabel, Virol. 1992 66, 3883-3887). Similarly, blocking re! A gene
expression with antisense oligonucleotides specifically blocks embryonic
stem cell adhesion; blocking NF-xB1 gene expression with antisense
oligonucleotides had no effect on cellular adhesion (Narayanan et al.,
1993 Mol. Cell. Biol. 13, 3802-3810). Thus, the promiscuous role initially
sssigned to NF-xB in transcriptional activation (M.J. Lenardo, D. Baltimore,
1989 Cell 58, 227-229) represents the sum of the activities of the rel family
of DNA-binding proteins. This conclusion is supported by recent transgenic
"knock-out" mice of individual members of the rel family. Such "knock-
outs" show few developmental defects, suggesting that essential
transcriptional activation functions can be performed by more than one
member of the rel family.
A number of specific inhibitors of NF-xB function in cells exist,
including treatment with phosphorothioate antisense oliogonucleotide,
treatment with double-stranded NF-xB binding sites, and over expression
of the natural inhibitor MAD-3 (an IxB family member). These agents have
CA 02468048 2004-06-07
been used to show that NF-xB is required for induction of a number of
molecules involved in inflammation, as described below.
~NF-xB is required for phorbol ester-mediated induction of IL-6 (I.
Kitajima, et al., Science 258, 1792-5 (1992)) and IL-8 (Kunsch and Rosen,
5 1993 Mol. Cell. Biol. 13, 6137-46).
~NF-xB is required for induction of the adhesion molecules ICAM-1
(Eck, et al., 1993 Mol. Cell. Biol. 13, 6530-6536), VCAM-1 (Shu et al.,
supra), and E-selectin (Read, et al., 1994 J. Exp. Med. 179, 503-512) on
endothelial cells.
10 ~NF-xB is involved in the induction of the integrin subunit, CD18, and
other adhesive properties of leukocytes (Eck et al., 1993 supra).
The above studies suggest that NF-KB is integrally involved in the
induction of cytokines and adhesion molecules by inflammatory mediators.
Two recent papers point to another connection between NF-xB and
15 inflammation: glucocorticoids may exert their anti-inflammatory effects by
inhibiting NF-xB. The glucocorticoid receptor and p65 both act at NF-xB
binding sites in the ICAM-1 promoter (van de Stolpe, et al., 1994 J. Biol.
Chem. 269, 6185-6192). Glucocorticoid receptor inhibits NF-xB-mediated
induction of IL-6 (Ray and Prefontaine, 1994 Proc. Natl Acad. Sci USA 91,
20 752-756). Conversely, overexpression of p65 inhibits glucocorticoid
induction of the mouse mammary tumor virus promoter. Finally, protein
cross-linking and co-immunoprecipitation experiments demonstrated direct
physical interaction between p65 and the glucocorticoid receptor (Id.).
Ribozymes of this invention block to some extent NF-xB expression
25 and can be used to treat disease or diagnose such disease. Ribozymes
will be delivered to cells in culture and to cells or tissues in animal models
of restenosis, transplant rejection and rheumatoid arthritis. Ribozyme
cleavage of relA mRNA in these systems may prevent inflammatory cell
function and alleviate disease symptoms.
30 The sequence of human and mouse relA mRNA can be screened for
accessible sites using acomputer folding algorithm. Potential
hammerhead or hairpin ribozyme cleavage sites were identified. These
sites are shown in Tables 17; 18 and 21-22. (All sequences are 5' to 3' in
the tables.) While mouse and human sequences can be screened and
CA 02468048 2004-06-07
3s
ribozymes thereafter designed, the human targetted sequences are of most
utility.
The sequences of the chemically synthesized ribozymes useful in this
study are shown in Tables 19 - 22. Those in the art will recognize that
these sequences are representative only of many more such sequences
where the enzymatic portion of the ribozyme (all but the binding arms) is
altered to affect activity and may be formed of ribonucleotides or other
nucleotides or non-nucleotides. Such ribozymes are equivalent to the
ribozymes described specifically in the Tables.
By engineering ribozyme motifs we have designed several ribozymes
directed against rel A mRNA sequences. These ribozymes are synthesized
with modifications that improve their nuclease resistance. The ability of
ribozymes to cleave relA target sequences in vitro is evaluated.
The ribozymes will be tested for function in vivo by analyzing cytokine-
induced VCAM-1, /CAM-1, IL-6 and IL-8 expression levels. Ribozymes will
be delivered to cells by incorporation into liposomes, by complexing with
' cationic lipids, by microinjection, or by expression from DNA and RNA
vectors. Cytokine-induced VCAM-1, /CAM-1, IL-6 and IL-8 expression will
be monitored by ELISA, by indirect immunofluoresence, and/or by FACS
analysis. Rel A mRNA levels will ~ be assessed by Northern analysis,
RNAse protection or primer extension analysis or quantitative RT-PCR.
Activity of NF-x8 will be monitored by gel-retardation assays. Ribozymes
that block the induction of NF-xB activity and/or rel A mRNA by more than
50% will be identified.
RNA ribozymes and/or genes encoding them will be locally delivered
to transplant tissue ex vivo in animal models. Expression of the ribozyme
will be monitored by its ability to block ex vivo induction of VCAM-1, ICAM-
1, IL-6 and II_-8 mRNA and protein. The effect of the anti-rel A ribozymes
on graft rejection will then be assessed. Similarly, ribozymes will be
introduced into joints of mice with collagen-induced arthritis or rabbits with
Streptococcal cell wall-induced arthritis. Liposome delivery, cationic lipid
delivery, or adeno-associated virus vector delivery can be used. One dose
(or a few infrequent doses) of a stable anti-relA ribozyme or a gene
construct that constitutively expresses the ribozyme may abrogate
inflammatory and immune responses in these diseases.
CA 02468048 2004-06-07
- 37
Use
A therapeutic agent that inhibits cytokine gene expression, inhibits
adhesion molecule expression, and mimics the anti-inflammatory. effects of
glucocorticoids (without inducing steroid-responsive genes) is ideal for the
treatment of inflammatory and autoimmune disorders. Disease targets for
such a drug are numerous. Target indications and the delivery options
each entails are summarized below. In all cases, because of the potential
immunosuppressive properties of a ribozyme that cleaves rel A mRNA,
uses are limited to local delivery, acute indications, or ex vivo treatment.
~Rheumatoid arthritis (RA).
Due to the chronic nature of RA, a gene therapy approach is logical.
Delivery of a ribo~yme to inflamed joints is mediated by adenovirus,
retrovirus, or adeno-associated virus vectors. For instance, the appropriate
adenovirus vector can be administered by direct injection into the
synovium: high efficiency of gene transfer and expression for several
months would be expected (B.J. Roessler, E.D. Allen, J.M. Wilson, J.W.
Hartman, B. L. Davidson, J. Clin. Invest. 92, 1085-1092 (1993)). It is
unlikely that the course of the disease could be reversed by the transient,
local administration of . an anti-inflammatory agent. Multiple
administrations may be necessary. Retrovirus and adeno-associated virus
vectors would lead to permanent gene transfer and expression in the joint.
However, permanent expression of a potent anti-inflammatory agent may
lead to local immune deficiency.
~Restenosis.
Expression of NF-xB in the vessel wall of pigs causes a narrowing of
the luminal space due to excessive deposition of extracellular matrix
components. This phenotype is similar to matrix deposition that occurs
subsequent to coronary angioplasty. In addition, NF-xB is required for the
expression of the oncogene c-myb (F.A. La Rosa, J.W. Pierce, G.E.
Soneneshein, Mol. Cell. Biol. 14, 1039-44 (1994)). Thus NF-xB induces
smooth muscle proliferation and the expression of excess matrix
components: both processes are thought to contribute to reocclusion of
vessels after coronary angioplasty.
~Transplantation.
CA 02468048 2004-06-07
38
NF-xB is required for the induction of adhesion molecules (Eck et al.,
supra, K. O'Brien, et al., J. Clin. Invest. 92, 945-951 (1993)) that function
in
immune recognition and inflammatory responses. At least two potential
modes of treatment are possible. In the first, transplanted organs are
treated ex vivo with ribozymes or ribozyme expression vectors. Transient
inhibition of NF-xB in the transplanted endothelium may be sufficient to
prevent transplant-associated vasculitis and may significantly modulate
graft rejection. In the second, donor B cells are treated ex vivo with
ribozymes or ribozyme expression vectors. Recipients would receive the
treatment prior to transplant. Treatment of a recipient with B cells that do
not express T cell co-stimulatory molecules (such as ICAM-1, VCAM-1,
and/or B7 an B7-2) can induce antigen-specific anergy. Tolerance to the
donor's histocompatibility antigens could result; potentially, any donor
could be used for any transplantation procedure.
~Asthma.
Granulocyte macrophage colony stimulating factor (GM-CSF) is
thought to play a major role in recruitment of eosinophils and other
inflammatory cells during the late phase reaction to asthmatic trauma.
Again, blocking the local induction of GM-CSF and other inflammatory
mediators is likely to reduce the persistent inflammation observed in
chronic asthmatics. Aerosol delivery of ribozymes or adenovirus ribozyme
expression vectors is a feasible treatment.
~Gene Therapy.
Immune responses limit the efficacy of many gene transfer
techniques. Cells transfected with retrovirus vectors have short lifetimes in
immune competent individuals. The length of expression of adenovirus
vectors in terminally differentiated cells is longer in neonatal or immune-
compromised animals. Insertion of a small ribozyme expression cassette
that modulates inflammatory and immune responses into existing
adenovirus or retrovirus constructs will greatly enhance their potential.
Thus, ribozymes of the present invention that cleave rel A mRNA and
thereby NF-xB activity have many potential therapeutic uses, and there are
reasonable modes of delivering the ribozymes in a number of the possible
indications. Development of an effective ribozyme that inhibits NF-xB
CA 02468048 2004-06-07
39
function is described above; available cellular and activity assays are
number, reproducible, and accurate. Animal models for NF-KB function
(Kitajima, et al., supra) and for each of the suggested disease targets exist
and can be used to optimize activity.
Example 4: TNF-a
Ribozymes that cleave the specific cites in TNF-a mRNA represent a
novel therapeutic approach to inflammatory or autoimmune disorders.
Tumor necrosis factor-a (TNF-a) is a protein, secreted by activated
leukocytes, that is a potent mediator of inflammatory reactions. Injection of
TNF-a into experimental animals can simulate the symptoms of systemic
and local inflammatory diseases such as septic shock or rheumatoid
arthritis.
TNF-a was initially described as a factor secreted by activated
macrophages which mediates the destruction of solid tumors in mice (Old,
1985 cien a 230, 4225-4231). TNF-a subsequently was found to be
identical to cachectin, an agent responsible for the weight loss and wasting
syndrome associated with tumors and chronic infections (Beutler, et al.,
~ 985 Nature 316, 552-554). The cDNA and the genomic locus for TNF-a
have been cloned and found to be related to TNF-f3 (Shakhov et al., 1990
,J. Exo. Med. 171, 35-47). Both TNF-a and TNF-f3 bind to the same
receptors and have nearly identical biological activities. The two TNF
receptors have been found on most cell types examined (Smith, et al.,
1990 cienc 248, 1019-1023). TNF-a secretion has been detected from
monocytes/macrophages, CD4+ and CD8+ T-cells, B-cells, lymphokine
activated killer cells, neutrophiis, astrocytes, endothelial cells, smooth
muscle cells, as well as various non-hematopoietic tumor cell lines ( for a
review see Turestskaya et al., 1991 in Tumor Necrosis Factor' Structure
Function. and Mechanism of Action B. B. Aggarwal, J. Vilcek, Eds. Marcel
Dekker, Inc., pp. 35-60). TNF-a is regulated transcriptionally and
translationally, and requires proteolytic processing at the plasma
membrane in order io be secreted (Kriegler et al., 1988 Cell 53, 45-53).
Once secreted, the serum half life of TNF-a is approximately 30 minutes.
The tight regulation of TNF-a is important due to the extreme toxicity of this
cytokine. Increasing evidence indicates that overproduction of TNF-a
CA 02468048 2004-06-07
during infections can lead to severe systemic toxicity and death (Tracey &
Cerami, 1992 Am. J. Trod. Med. HVQ. 47, 2-7).
Antisense RNA and Hammerhead ribozymes have been used in an
attempt to lower the expression level of TNF-a by targeting specified
5 cleavage sites (Sioud et al., 1992 J. Mol. Biol. 223; 831; Sioud WO
94/10301; Kisich and co-workers, 1990 abstract (FA B 4, A1860; 1991
slide presentation (J. Leukocyte Biol. sup. 2, 70); December, 1992 poster
presentation at Anti-HIV Therapeutics Conference in SanDiego, CA; and
"Development of anti-TNF-a ribozymes for the control of TNF-a gene
10 expression"- Kisich, Doctoral Dissertation, 1993 University of California,
DavisJ listing various TNFa targeted ribozymes.
Ribozymes of this invention block to some extent TNF-a expression
and can be used to treat disease or diagnose such disease. Ribozymes
will be delivered to cells in culture and to cells or tissues in animal models
15 of septic shock and rheumatoid arthritis. Ribozyme cleavage of TNF-a
mRNA in these systems may prevent inflammatory cell function and
alleviate disease symptoms.
The sequence of human and mouse TNF-a mRNA can be screened
for accessible sites using a computer folding algorithm. Hammerhead or
20 hairpin ribozyme cleavage sites were identified. , These sites are shown in
Tables 23, 25, and 27 - 28. . (All sequences are 5' to 3' in the tables.)
While
mouse and human sequences can be screened and ribozymes thereafiter
designed, the human targeted sequences are of most utility. However,
mouse targeted ribozymes are useful to test efficacy of action of the
25 ribozyme prior to testing in humans. The nucleotide base position is noted
in the Tables as that site to be cleaved by the designated type of ribozyme.
(In Table 24, lower case letters indicate positions that are not conserved
between the human and the mouse TNF-a sequences.)
The sequences of the chemically synthesized ribozymes useful in this
30 study are shown in Tables 24, 26 - 28. Those in the art will recognize that
these sequences are representative only of many more such sequences
where the enzymatic portion of the ribozyme (all but the binding arms) is
altered to affect activity. For example, stem-loop II sequence of
hammerhead ribozymes listed in Tables 24 and 26 (5'-GGCCGAAAGGCC-
35 3') can be altered (substitution, deletion, and/or insertion) to contain
any
CA 02468048 2004-06-07
41
sequences provided a minimum of two base-paired stem structure can
form. Similarly, stem-loop IV sequence of hairpin ribozymes listed in
Tables 27 and 28 (5'-CACGUUGUG-3') can be altered (substitution,
deletion, and/or insertion) to contain any sequence, provided a minimum of
two base-paired stem structure can form. The sequences listed in Tables
24, 26 - 28 may be formed of ribonucleotides or other nucleotides or non-
nucleotides. Such ribozymes are equivalent to the ribozymes described
specifically in the Tables or AAV .
In a preferred embodiment of the invention, a transcription unit
expressing a ribozyme that cleaves TNF-a RNA is inserted into a plasmid
DNA vector or an adenovirus DNA viral vector or AAV or alpha virus or
retroviris vectors. Viral vectors have been used to transfer genes to the
intact vasculature or to joints of live animals (Willard et al., 1992
Circulation, 86, I-473.; Nabel et al., 1990 Science, 249, 1285-1288) and
both vectors lead to transient gene expression. The adenovirus vector is
delivered as recombinant adenoviral particles. DNA may be delivered
alone or complexed with vehicles (as described for RNA above). The DNA,
DNA/vehicle complexes, or the recombinant adenovirus particles are
locally administered to the site of treatment, e.g., through the use of an
injection catheter, scent or infusion pump or are directly added to cells or
tissues ex vivo.
In another preferred embodiment of the invention, a transcription unit
expressing a ribozyme that cleaves TNF-« RNA is inserted into a retrovirus
vector for sustained expression of ribozyme(s).
By engineering ribozyme motifs we have designed several ribozymes
directed against TNF-a mRNA sequences. These ribozymes are
synthesized with modifications that improve their nuclease resistance. The
ability of ribozymes to cleave TNF-a target sequences in vitro is evaluated.
The ribozymes will be tested for function in cells by analyzing
bacterial lipopolysaccharide (LPS)-induced TNF-a expression levels.
Ribozymes will be delivered to cells by incorporation into liposomes, by
complexing with cationic lipids, by microinjection, or by expression from
DNA vectors. TNF-a expression will be monitored by ELISA, by indirect
immunofluoresence, and/or by FACS analysis. TNF-a mRNA levels will be
assessed by Northern analysis, RNAse protection, primer extension
CA 02468048 2004-06-07
42
analysis or quantitative RT-PCR. Ribozymes that block the induction of
TNF-a activity . and/or TNF-a mRNA by more than 90% will be identified.
RNA ribozymes and/or genes encoding them will be locally delivered
to macrophages by intraperitoneal injection. After a period of ribozyme
uptake, the peritoneal macrophages are harvested and induced ex vivo
with LPS. The ribozymes that significantly reduce TNF-a secretion ace
selected. The TNF-a can also be induced after ribozyme treatment with
fixed Streptococcus in the peritoneal cavity instead of ex vivo. In this
fashion the ability of TNF-a ribozymes to block TNF-a secretion in a
localized inflammatory response are evaluated. In addition, we will
determine if the ribozymes can block an ongoing inflammatory response by
delivering the TNF-a ribozymes after induction by the injection of fixed
Streptococcus.
To examine the effect of anti-TNF-a ribozymes on systemic
inflammation, the ribozymes are delivered by intravenous injection. The
ability of the ribozymes to inhibit TNF-a secretion and lethal shock caused
by systemic LPS administration are assessed. Similarly, TNF-a ribozymes
can be introduced into the joints of mice with collagen-induced arthritis.
Either free delivery, liposome delivery, cationic lipid delivery, adeno-
associated virus vector delivery, adenovirus vector delivery, retrovirus
vector delivery or plasmid vector delivery in these animal model
experiments can be used to supply ribozymes. One dose (or a few
infrequent doses) of a stable anti-TNF-a ribozyme or a gene construct that
constitutively expresses the ribozyme may abrogate tissue damage in
these inflammatory diseases.
Macrophage isolation.
To produce responsive macrophages i ml of sterile fluid thioglycollate
broth (Difco, Detroit, MI.) was injected i.p. into 6 week old female
C57b1/6NCR mice 3 days before peritoneal favage. Mice were maintained
as specific pathogen free in autoclaved cages in a laminar flow hood and
given sterilized water to minimize "spontaneousN activation of
macrophage:.. The resulting peritoneal exudate cells (PEC) were obtained
by lavage using Hanks balanced sail solution (HESS) and were plated at
2.5X105/well in 96 well plates (Costar, Cambridge, MA.) with Eagles
minimal essential medium (EMEM) containing 10% heat inactivated fetal
CA 02468048 2004-06-07
43
bovine serum. After adhering for 2 hours the wells were washed to remove
non-adherent cells. The resulting cultures were 97% macrophages as
determined by morphology and staining for non-specific esterase.
Transfection of ribozymes into macrophages:
The ribozymes were diluted to 2X final concentration, mixed with an
equal volume of llnM lipofectamine (Life Technologies, Gaithersburg,
MD.), and vortexed. 100 ml of lipid:ribozyme complex was then added
directly to the cells, followed immediately by 10 mi fetal bovine serum.
Three hours after ribozyme addition 100 ml of 1 mg/ml bacterial
lipopolysaccaride , (LPS) was added to each well to stimulate TNF
production.
Quantitation of TNF-a in mouse macrophages:
Supernatants were sampled at 0, 2, 4, 8, and 24 hours post LPS
stimulation and stored at -70oC. Ouantitation of TNF-a was done by a
specific ELISA. ELISA plates were coated with rabbit anti-mouse TNF-a
serum at 1:1000 dilution (Genzyme) followed by blocking with milk proteins
and incubation with TNF-a containing supernatants. TNF-a was then
detected using a murine TNF-a specific hamster monoclonal antibody
(Genzyme). The ELISA was .developed with goat anti-hamster IgG coupled
to alkaline phosphatase.
Assessment of reagent toxicity:
Following ribozyme/lipid treatment of macrophages and harvesting of
supernatants viability of the cells was assessed by incubation of the cells
with 5 mg/ml of 3-(4,5-dimethylthiazol-2-yl)-2,5-Biphenyl tetrazolium
bromide. (MTT). This compound is reduced by the mitochondrial
dihydrogenases, the activity of which correlates well with cell viability.
After
12 hours the absorbance of reduced MTT is measured at 585 nm.
Uses
The association between TNF-a and bacterial sepsis, rheumatoid
3D arthritis, and autoimmune disease make TNF-a an attractive target for
therapeutic intervention [Tracy & Cerami 1992 supra; Williams et al., 1992
Proc. Natl, Acad. Sci. USA 89, 9784-9788; Jacob, 1992 J. Autoimmun. 5
(Supp. A), 133-143].
CA 02468048 2004-06-07
44 '
Septic Shock
Septic shock is a complication of major surgery, bacterial infection,
and polytrauma characterized by high fever, increased cardiac output,
reduced blood pressure and a neutrophilic infiltrate into the lungs and
other major organs. Current treatment options are limited to antibiotics to
reduce the bacterial load and non-steroidal anti-inflammatories to reduce
fever. Despite these treatments in the best intensive care settings, mortality
from septic shock averages 50%, due primarily to multiple organ failure
and disseminated vascular coagulation. Septic shock, with an incidence of
200,000 cases per year in the United States, is the major cause of death in
intensive care units. In septic shock syndrome, tissue injury or bacterial
products initiate massive immune activation, resulting in the secretion of
pro-inflammatory cytokines which are not normally detected in the serum,
such as TNF-a, interleukin-1 ~i (IL-1 f3), ~-interferon (IFN-Y), interleukin-6
(IL-
6), and interleukin-8 (iL-8). Other non-cytokine mediators such as
leukotriene b4, prostaglandin E2, C3a and C3d also reach high levels (de
Boer et al., 1992 Immunopharmacoloav 24, 135-148).
TNF-a is detected early in the course of septic shock in a large fraction
of patients (de Boer et al., 1992 suflra). In animal models, injection of TNF-
a has been shown to induce shock-Pike symptoms similar to those induced
by LPS injection (Beutler et al:, 1985 Science 229, 869-871); in contrast,
injection of tL-1 C3, IL-6, or IL-8 does not induce shock, Injection of TNF-a
also causes an elevation of IL-1 f3, IL-6, IL-8, PgE2, acute phase proteins,
and TxA2 in the serum of experimental animals (de Boer et al., 1992
su ra). In animal-models the lethal effects of LPS can be blocked by pre-
administration of anti-TNF-a antibodies. The cumulative evidence indicates
that TNF-a is a key player in the pathogenesis of septic shock, and
therefore a good candidate for therapeutic intervention.
Rheumatoid Arthritis
Rheumatoid arthritis (RA) is an autoimmune disease characterized by
chronic inflammation of the joints leading to bone destruction and toss of
joint function. At the cellular level, autoreactive T- lymphocytes and
monocytes are typically present, and the synoviocytes often have altered
morphology and immunostaining patterns. RA joints have been shown to
contain elevated levels of TNF-a, IL-1 a and IL-1 t3, iL-fi, GM-CSF, and TGF-
CA 02468048 2004-06-07
13 (Abney et al., 1991 Imm. Rev. 119, 105-123), some or all of which may
contribute to the pathological course of the disease.
Cells cultured from RA joints spontaneously secrete all of the pro-
inflammatory cytokines detected in vivo. Addition of antisera against TNF-a
5 to these cultures has been shown to reduce IL-ia!(3 production by these
cells to undetectable levels (Abney et al., 1991 a r ). Thus, TNF-a may
directly induce the production of other cytokines in the RA joint. Addition of
the anti-inflammatory cytokine, TGF-f3, has no effect on cytokine secretion
by RA cultures. Immunocytochemical studies of human RA surgical
10 specimens clearly demonstrate the production of TNF-a, IL-ia/f~, and IL-6
from macrophages near the cartilage/pannus junction when the pannus in
invading and overgrowing the cartilage (Chu et al., 1992 Br. ,~
Rheumatofoav 31, 653-661). GM-CSF was shown to be produced mainly
by vascular endothelium in these samples. Both TNF-a and TGF-f3 have
15 been shown to be fibroblast growth factors, and may contribute to the
accumulation of scar tissue in the RA joint. TNF-a has also been shown to
increase osteociast activity and bone resorbtion, and may have a role in
the bone erosion commonly found in the RA joint (Cooper et al., 1992 Clin.
Exp. Immunol. 89, 244-250).
20 Elimination of TNF-a from the rheumatic joint would be predicted to
reduce overall inflammation by reducing induction of MHC class ll, lL-1a!(3,
II-fi, and GM-CSF, and reducing T-cell activation. Osteoclast activity might
also fall, reducing the rate of bone erosion at the joint. Finally,
elimination
of TNF-a would be expected to reduce accumulation of scar tissue within
25 the joint by removal of a fibroblast, growth factor.
Treatment with an anti-TNF-a antibody reduces joint swelling and the
histological severity of collagen-induced arthritis in mice (Williams et al.,
1992 Proc. Natl. Acad. ci. USA 89, 9784-9788). In addition; a study of RA
patients who have received i.v, infusions of anti-TNF-a monoclonal
30 antibody reports a reduction in the number and severity of inflamed joints
after treatment. The benefit of monoclonal antibody treatment in the long
term may be limited by the expense and immunogenicity of the antibody.
Psoriasis .
Psoriasis is an inflammatory disorder of the skin characterized by
35 keratinocyte hyperproliferation and immune cell infiltrate (Kupper, 1990
,j~,
CA 02468048 2004-06-07
Clin. Invest. 86, 1783-1789). It is a fairly common condition, affecting 1.5-
2.0% of the population. The disorder ranges in severity from mild, with
small flaky patches of skin, to severe, involving inflammation of the entire
epidermis. The cellular infiltrate of psoriasis includes T-lymphocytes,
neutrophils, macrophages, and dermal dendrocytes. The majority of T-
lymphocytes are activated CD4+ cells of the TH-1 phenotype, although
some CD8+ and CD4-/CD8- are also present. B lymphocytes are typically
not found in abundance in psoriatic plaques.
Numerous hypotheses have been offered as to the proximal cause of
psoriasis including auto-antibodies and auto-reactive T-cells,
overproduction of growth factors, and genetic predisposition. Although
there is evidence to support the involvement of each of these factors in
psoriasis, they are neither mutually exclusive nor are any of them
necessary and sufficient for the pathogenesis of psoriasis (Reeves, 1991
~emin. Dermatol. 10, 217).
The role of cytokines in the pathogenesis of psoriasis has been
investigated. Among those cytokines found to be abnormally expressed
were TGF-a , IL-1 a, IL-1 f3, IL-1 ra, ll-6, IL-8, IFN-y, and TNF-a . In
addition
to abnormal cytokine production, elevated expression of ICAM-1, SLAM-1,
and VCAM has been observed (Reeves, 1991 su ra . This cytokine profile
is similar to that of normal wound healing, with the notable exception that
cytokine levels subside upon heating. Keratinocytes themselves have
recently been shown to be capable of secreting EGF, TGF-a, IL-6, and
TNF-a, which could increase proliferation in an autocrine fashion (Oxhoim
et al., 1991 APMI 99, 58-64).
Nickoloff et al., 1993 (J Dermatol Sci. 6, 127-33) have proposed the
following model for the initiation and maintenance of the psoriatic plaque:
Tissue damage induces the wound healing response in the skin.
Keratinocytes secrete IL-1 a, IL-1 t3, IL-6, IL-8, TNF-a. These factors
activate the endothelium of dermal capillaries, recruiting PMNs,
macrophages, and T-cells into the wound site.
Dermal dendrocytes near the dermal/epidermal junction remain
activated when they should return to a quiescent state, and subsequently
secrete cytokines including TNF-a, IL-6, and IL-8. Cytokine expression, in
CA 02468048 2004-06-07
47
turn, maintains the activated state of the endothelium, allowing
extravasation of additional immunocytes, and the activated state of the
keratinocytes which secrete TGF-a and IL-8. Keratinocyte IL-8 recruits
immunocytes from the dermis into the epidermis. During passage through
the dermis, T-cells encounter the activated dermal dendrocytes which
efficiently activate the TH-1 phenotype. The activated T-cells continue to
migrate into the epidermis, where they are stimulated by keratinocyte-
expressed ICAM-1 and MHC class Il. IFN-y secreted by the T-cells
synergizes with the TNF-a from dermal dendrocytes to increase
keratinocyte proliferation and the levels of TGF-a, IL-8, and IL-6 production.
1FN-y also feeds back to the dermal dendrocyte, maintaining the activated
phenotype and the inflammatory cycle.
Elevated serum titres of IL-6 increases synthesis of acute phase
proteins including complement factors by the liver, and antibody production
by plasma cells. Increased complement and antibody levels increases the
probability of autoimmune reactions.
Maintenance of the psoriatic plaque requires continued expression of
all of these processes, but attractive points of therapeutic intervention are
TNF-a expression by the dermal dendrocyte to maintain activated
endothelium and keratinocytes, and 1FN-'y expression by T-cells to maintain
activated dermal dendrocytes.
There are 3 million patients in the United States afflicted with
psoriasis. The available treatments for psoriasis are corticosteroids. The
most widely prescribed are TEMOVATE (clobetasol propionate), LIDEX
(fluocinonide), DIPROLENE (betamethasone propionate), FSORCON
(diflorasone diac~etate) and TRIAMCINOLONE formulated for topical
application. The mechanism of action of corticosteroids is multifactorial.
This is a palliative therapy because the underlying cause of the disease
remains, and upon discontinuation of the treatment the disease returns.
Discontinuation of treatment is often prompted by the appearance of
adverse effects such as atrophy, telangiectasias and purpura.
Corticosteroids are not recommended for prolonged treatments or when
treatment of large and/or inflamed areas is required. Alternative treatments
include retinoids, such as etretinate, which has been .approved for
treatment of severe, refractory psoriasis. Alternative retinoid-based
treatments are in advanced clinical trials. Retinoids act by converting
CA 02468048 2004-06-07
48
keratinocytes to a differentiated state and restoration of normal skin
development. Immunosuppressive drugs such as cyclosporine are also in
the advanced stages of clinical trials. Due to the nonspecific mechanism of
action of corticosteroids, retinoids and immunosuppressives, these
treatments exhibit severe side effects and should not be used for extended
periods of time unless the condition is life-threatening or disabling. There
is a need for a less toxic, effective therapeutic agent in psoriatic patients.
HIV and AIDS
The human immunodeficiency virus (HIV) causes several
fundamental changes in the human immune system from the time of
infection until the development of full-blown acquired immunodeficiency
syndrome (AIDS). These changes include a shift in the ratio of CD4+ to
CD8+ T-cells, sustained elevation of IL-4 levels, episodic elevation of TNF-
a and TNF-fi levels, hypergammaglobulinemia, and lymphoma/leukemia
(Rosenberg & Fauci, 1990 Immun. Todav 11, 176; Weiss 1993 cienc
260, 1273). Many patients experience a unique tumor, Kaposi's sarcoma
and/or unusual opportunistic infections (e.g. Pneumocystis carinii,
cytomegalovirus, herpesviruses, hepatitis viruses, papilloma viruses, and
tuberculosis). The immunological dysfunction of individuals with AIDS
suggests that some of the pathology may be due to cytokine dysregulation.
Levels of serum TNF-a and IL-6 are often found to be elevated in
AIDS patients (Weiss, 1993 supra). In tissue culture, HIV infection of
rnonocytes isolated from healthy individuals stimulates secretion of both
TNF-a and IL-6. This response has been reproduced using purified gp120,
the viral coat protein responsible for binding to CD-4 (Buonaguro et al.,
1992 . Vir I, 66, 7159). It has also been demonstrated that the viral gene
regulator, Tat, can directly induce TNF transcription. The ,ability of HIV to
directly stimulate secretion of TNF-a and IL-6 may be an adaptive
mechanism of the virus. TNF-a has been shown to upregulate transcription
of the LTR of HIV, increasing the number of HIV-specific transcripts in
infected cells. IL-6 enhances HIV production, but at a post-transcriptional
level, apparently increasing the efficiency with which HIV transcripts are
translated into protein. Thus, stimulation of TNF-a secretion by the HIV
virus may promote infection of neighboring CD4+ cells both by enhancing
virus production from latently infected cells and by driving replication of
the
virus in newly infected cells.
CA 02468048 2004-06-07
49
The role of TNF-a in HIV replication has , been well established in
tissue culture models of infection (Sher et al., 1992 Immun. Rev. 127, 183),
suggesting that the mutual induction of HIV replication and TNF-a
replication may create positive feedback in vivo. However, evidence for the
presence of such positive feedback in infected patients is not abundant.
TNF-a levels are found to be elevated in some, but not all patients tested.
Children with AIDS who were given zidovudine had reduced levels of TNF-
a compared to those not given zidovudine (Cremoni et al., 1993 AID 7,
128). This correlation lends support to the hypothesis that reduced viral
replication is physiologically linked to TNF-a levels: Furthermore, recently
it has been shown that the polyclonal B cell activation associated with HIV
infection is due to membrane-bound TNF-a. Thus, levels of secreted TNF-a
may not accurately reflect the contribution of this cytokine to AIDS
pathogenesis.
Chronic elevation of TNF-a has been shown to shown to result in
cachexia (Tracey et al., 1992 Am. J. Trop. Med. Hvo. 47, 2-7), increased
autoimrnune disease (Jacob, 1992 supra), lethargy, and immune
suppression in animal models (Aderka et al., 1992 Isr. J. Med. Sci. 28, 126-
130). The cachexia associated with AIDS may be associated with
chronically elevated TNF-a frequently observed in AIDS patients.
Similarly, TNF-a can stimulate the proliferation of spindle cells isolated
from Kaposi's sarcoma lesions of AIDS patients (Barillari et al., 1992 ~
Immunol 149, 3727).
A therapeutic agent that inhibits cytokine gene expression, inhibits
adhesion molecule expression, and mimics the anti-inflammatory effects of
glucocorticoids (without inducing steroid-responsive genes) is ideal for the
treatment of inflammatory and autoimmune disorders. Disease targets for
such a drug are numerous. Target indications and the delivery options
each entails are summarized below. In all cases, because of the potential
immunosuppressive properties of a ribozyme.that cleaves the specified
sites in TNF-a mRNA, uses are limited to local delivery, acute indications,
or ex vivo treatment.
~Septic shock.
CA 02468048 2004-06-07
50 '
' Exogenous delivery of ribozymes. to macrophages can be achieved
by intraperitoneal or intravenous injections. Ribozymes will be delivered
by incorporation into liposomes or by complexing with cationic lipids.
~Rheumatoid arthritis (RA).
Due to the chronic nature of RA, a gene therapy approach is logical.
Delivery of a ribozyme to inflamed joints is mediated by adenovirus,
retrovirus, or adeno-associated virus vectors. For instance, the appropriate
adenovirus vector can be administered by direct injection into the
synovium: high efficiency of gene transfer and expression for several
months would be expected (B.J. Roessler, E.D. Allen, J.M. Wilson, J.W.
Hartman, B. L. Davidson, J. Clin. Invest. 92, 1085-1092 (1993)). It is
unlikely that the course of the disease could be reversed by the transient,
local administration of an anti-inflammatory agent. Multiple
administrations may be necessary. Retrovirus and adeno-associated virus
vectors would lead to permanent gene transfer and expression in the joint.
However, permanent expression of a potent anti-inflammatory agent may
lead to local immune deficiency.
~Psoriasis
The psoriatic plaque is a particularly good candidate for ribozyme or
vector delivery. The stratum corneum of the plaque is thinned, providing
access to the proliferating keratinocytes. T-cells and dermal dendrocytes
can be efficiently targeted by traps-epidermal diffusion .
Organ culture systems for biopsy specimens of psoriatic and normal
skin are described in current literature (Nickoloff et al., 1993 a ra).
Primary human keratinocytes are easily obtained and will be grown into
epidermal sheets in tissue culture. In addition to these tissue culture
models, the flaky skin mouse develops psoriatic skin in response to UV
light. This model would allow demonstration of animal efficacy for
ribozyme treatments of psoriasis. _
~Gene Therapy.
Immune responses limit the efficacy of many gene transfer
techniques. Cells transfected with retrovirus vectors have short lifetimes in
immune competent individuals. The length of expression of adenovirus
CA 02468048 2004-06-07
51
vectors in terminally differentiated cells is longer in neonatal or immune-
compromised animals. Insertion of a small ribozyme expression cassette
that modulates inflammatory and immune responses into existing
adenovirus or retrovirus constructs will greatly enhance their potential.
Thus, ribozymes of the present invention that cleave TNF-a mRNA
and thereby TNF-a activity have many potential therapeutic uses, and
there are reasonable modes of delivering the ribozymes in a number of the
possible indications. Development of an effective ribozyme that inhibits
TNF-a function is described above; available cellular and activity assays
are number, reproducible, and accurate. Animal models for TNF-a function
and for each of the suggested disease targets exist and can be used to
optimize activity.
Example 5: p210bcr-a I
Chronic myelogenous leukemia exhibits a characteristic disease
course, presenting initially as a chronic granulocytic hyperplasia, and
invariably evolving into an acute leukemia which is caused by the clonal
expansion of a cell with a less differentiated phenotype (i.e., the blast
crisis
stage of the disease). CML is an unstable disease which ultimately
progresses to a terminal stage which resembles acute leukemia. This
lethal disease affects approximately 16,000 patients a year.
Chemotherapeutic agents such as hydroxyurea or busulfan can reduce the
leukemic burden but do not impact the life expectancy of the patient (e.c~,~
approximately 4 years). Consequently, CML patients are candidates for
bone marrow transplantation (BMT) therapy. However, for those' patients
which survive BMT, disease recurrence remains a major obstacle
(Apperley et al., 1988 Br. J. Haematol. 69, 239).
he Philadelphia (Ph) chromosome which results from the
translocation of the abl oncogene from chromosome 9 to the bcr gene on
chromosome 22 is found in greater than 95% of CML patients and in 10-
25% of all cases of acute lymphoblastic leukemia [(ALL); Fourth
International Workshop on Chromosomes in Leukemia 1982, Cancer
Genet. Cytogenet. 11, 316J. In virtually all Ph-positive CMLs and
approximately 50% of the Ph-positive ALLs, the leukemic cells express bcr-
abI fusion mRNAs in which axon 2 (b2-a2 junction) or axon 3 (b3-a2
junction) from the major breakpoint cluster region of the bcr gene is spliced
CA 02468048 2004-06-07
52
to exon 2 of the abl gene. Heisterkamp et al., 1985 Nature 315, 758;
Shtiveiman et al., 1987, 8100 69, 971). In the remaining: cases of Ph-
positive ALL, the first exon of the bcr gene is spliced to exon 2 of the abl
gene (Hooberman et al., 1989 Proc Nat Acad Sci USA 86, 4259;
Heisterkamp et ai., 1988 Nucleic Acids Res. 16, 10069).
The b3-a2 and b2-a2 fusion mRNAs encode 210 kd bcr-abl fusion
proteins which exhibit oncogenic activity (Daley et al., 1990 Science 247,
824; Weisterkamp et al., 1990 Nature 344, 251). The importance of the bcr
abl fusion protein (p210bcr-abl) in the evolution and maintenance of the
leukemic phenotype in human disease has been demonstrated using
antisense oligonucleotide inhibition of p2'lObcr-abl expression. These
inhibitory molecules have been shown to inhibit the in vitro proliferation of
leukemic cells in bone marrow from CML patierits. Szczylik et al., 1991
ci n a 253, 562).
Reddy, U.S. Patent 5,246,921
describes use of ribozymes as therapeutic agents for leukemias,
such as chronic myelogenous leukemia (CML) by targeting the specific
junction region of bcr-abl fusion transcripts. It indicates causing cleavage
by a ribozyme at or near the breakpoint of such a hybrid chromosome,
specifically it includes cleavage at the sequence GUX, where X is A, U or
G. The one example presented is to cleave the sequence 5' AGC AG
AGUU (cleavage site) CAA AAGCCCU-3'.
Scanlon WO 91/18625, WO 91/18624, and WO 91/18913 and
Snyder et al., W093/03141 and W094113793 describe a ribozyme effective
to cleave oncogenic variants of H-ras RNA. This ribozyme is said to inhibit
H-ras expression in response to ex3emal stimuli.
The invention features use of ribozymes to inhibit the development or
expression of a transformed phenotype in man and other animals by
modulating expression of a gene that contributes to the expression of CML.
Cleavage of targeted mRNAs expressed in pre-neoplastic and transformed
cells elicits inhibition of the transformed state.
The invention can be used to treat cancer or pre-neoplastic
conditions. Two preferred administration protocols can be used, either iLn
viv administration to reduce the tumor burden, or ex v- ivo treatment to
CA 02468048 2004-06-07
53
eradicate transformed cells from tissues such as bone marrow prior to
reimplantation.
This invention features an enzymatic RNA molecule (or ribozyme)
which cleaves mRNA associated with development or maintenance of
CML. The mRNA targets are present in the 425 nucleotides surrounding
the fusion sites of the bcr and abl sequences in the b2-a2 and b3-a2
recombinant mRNAs. Other sequences in the 5' portion of the bcr mRNA or
the 3' portion of the abl mRNA may also be targeted for ribozyme cleavage.
Cleavage at any of these sites in the fusion mRNA molecules will result in
inhibition of translation of the fusion protein in treated cells.
The invention provides a class of chemical cleaving agents which
exhibit a high degree of specificity for the mRNA causative of CML. Such
enzymatic RNA molecules can be delivered exogenously or endogenously
to afflicted cells. In the preferred hammerhead motif the small size (less
than 40 nucleotides, preferably between 32 and 36 nucleotides in length)
of the molecule allows the cost of treatment to be reduced.
The smallest ribozyme delivered for any type of treatment reported to
date (by Rossi et al., 1992 supra) is an in vitro transcript having a length
of
142 nucleotides. Synthesis of ribozymes greater than 100 nucleotides in
length is very difficult using automated methods, and the therapeutic cost of
such molecules is prohibitive. Delivery of ribozymes by expression vectors
is primarily feasible using only ex vivo treatments. This limits the utility
of
this approach. In this invention, an alternative approach uses smaller
ribozyme motifs and exogenous delivery. The simple structure of these
molecules also increases the ability of the ribozyme to invade targeted
regions of the mRNA structure. Thus, unlike the situation when the
hammerhead structure is included within longer transcripts, there are no
non-ribozyme flanking sequences to interfere with correct folding of the
ribozyme structure, as well as complementary binding of the ribozyme to
the mRNA target.
The enzymatic RNA molecules of this invention can be used to treat
human CML or precancerous conditions. Affected animals can be treated
at the time of cancer detection or in a prophylactic manner. This timing of
treatment will reduce the number of affected cells and disable cellular
CA 02468048 2004-06-07
54
replication. This is possible because the ribozymes are designed to
disable those structures required for successful cellular proliferation.
Ribozymes of this invention block to some extent p210bcr-abl
expression and can be used to treat disease or diagnose such disease.
Ribozymes will be delivered to cells in culture and to tissues in animal
models of CML. Ribozyme cleavage of bcrlabl mRNA in these systems
may prevent or alleviate disease symptoms or conditions.
The sequence of human bcrlabl mRNA can be screened for
accessible sites using a computer folding algorithm. Regions of the mRNA
that did not form secondary folding structures and that contain potential
hammerhead or hairpin ribozyme cleavage sites can be identified. These
sites are shown in Table 29 (All sequences are 5' to 3' in the tables). The
nucleotide base position is noted in the Tables as that site to be cleaved by
the designated type of ribozyme.
The sequences of the chemically synthesized ribozymes most useful
in this study are shown in Table 30. Those in the art will recognize that
these sequences are representative only of many more such sequences
where the enzymatic portion of the ribozyme (all but the binding arms) is
altered to affect activity. For example, stem-loop II sequence of
hammerhead ribozymes listed in Table 30 (5'-GGCCGAAAGGCC-3') can
be altered (substitution, deletion, and/or insertion) to contain any sequence
provided, a minimum of two base-paired stem structure can form. The
sequences listed in Tables 30 may be formed of ribonucleotides or other
nucleotides or non-nucleotides. Such ribozymes are equivalent to the
ribozymes described specifically in the Tables.
By engineering ribozyme motifs we have designed several ribozymes
directed against bcr-abl mRNA sequences. These have been synthesized
with modifications that improve their nuclease resistance as described
above. These ribozymes cleave bcr-abi target sequences in vitro.
The ribozymes are tested for function in vivo by exogenous delivery to
cells expressing bcr-abl. Ribozymes are delivered by incorporation into
liposomes, by compfexing with cationic lipids, by microinjection, .or by
expression from DNA vectors. Expression of bcr-abl is monitored by
EL1SA, by indirect immunofluoresence, and/or by FACS analysis. Levels of
CA 02468048 2004-06-07
bcr-abI mRNA are assessed by Northern analysis, RNase protection, by
primer extension analysis or by quantitative RT-PCR techniques.
Ribozymes that block the induction of p210bcr-abl) protein and mRNA by
more than 20% are identified.
5 Example 6: RSV
This invention relates to the use of ribozymes as inhibitors of
respiratory syncytial virus (RSV) production, and in particular, the
inhibition
of RSV replication.
RSV is a member of the virus family paramyxoviridae and is classified
10 under the genus Pneumovirus (for a review see Mclntosh and Chanock,
1990 in Virology ed. B.N. Fields, pp. 1045, Raven Press Ltd. NY). The
infectious virus particle is composed of a nucleocapsid enclosed within an
envelope. The nucleocapsid is composed of a linear negative single-
stranded non-segmented RNA associated with repeating subunits of
15 capsid proteins to form a compact structure and thereby protect the RNA
from nuclease degradation. The entire nucleocapsid is enclosed by the
envelope. The size of the virus particle ranges from 150 - 300 nrn in
diameter. The complete life cycle of RSV takes place in the cytoplasm of
infected cells and the nucleocapsid never reaches the nuclear
20 compartment (Hall, 1990 in Principles and Practice of Infectious Diseases
ed. Mandell et al., Churchill Livingstone, NY).
The RSV genome encodes ten viral proteins essential for viral
production. RSV protein products include two structural glycoproteins (G
and F) found in the envelope spikes, two matrix proteins [M and M2 (22K)]
25 found in the inner membrane, three proteins localized in the nucleocapsid
(N, P and L), one protein that is present on the surface of the infected cell
(SH), and two nonstructural proteins [NS1 (1C) and NS2 (1B)] found only
in the infected cell. The mRNAs for the 10 RSV proteins have similar 5'
and 3' ends. UV-inactivation studies suggest that a single promoter is used
30 with multiple transcription initiation sites (Barik et al., 1992 J. Virol.
66,
6813). The order of transcription corresponding to the protein assignment
on the genomic RNA is 1 C, 1 B, N, P, M, SH, G, F, 22K and L genes (Huang
et al., 1985 Virus Res. 2, 157) and transcript abundance corresponds to
the order of gene assignment (for example the 1 C and 1 B mRNAs are
35 much more abundant than the L mRNA. Synthesis of viral message begins
CA 02468048 2004-06-07
58
immediately after RSV infection of cells and reaches a maximum at 14
hours post-infection (Mclntosh and Chanock, supra).
There are two antigenic subgroups of RSV; A and B, which can
circulate simultaneously in the community in varying proportions in different
years (Mclntosh and Chanock, supra). Subgroup A usually predominates.
Within the two subgroups there are numerous strains. By the limited
sequence analysis available it seems that homology at the nucleotide level
is more complete within than between subgroups, although sequence
divergence has been noted within subgroups as well. Antigenic
determinates result primarily from both surface glycoproteins, F and G. For
F, at least half of the neutralization epitopes have been stably maintained
over a period of 30 years. For G however, A and B subgroups may be
related antigenically by as little as a few percent. On the nucleotide level,
however, the majority of the divergence in the coding region of G is found
in the sequence for the extracellular domain (Johnson et al., 1987, Proc.
Natl. Acad. Sci. USA 84, 5625).
Respiratory Syncytial Virus (RSV) is the major cause of lower
respiratory tract illness during infancy and childhood (Hall, supra) and as
such is associated with an estimated 90,000 hospitalizations and 4500
deaths in the United States alone (Update: respiratory syncytial virus
activity ' United States, 1993, Mmwr Morb Mortal Wkly Rep, 42, 971).
Infection with RSV generally outranks all other microbial.agents Leading to
both pneumonia and bronchitis. While primarily affecting children under
two years of age. immunity is not complete and reinfection of older children
and adults, especially hospital care givers (Mclntosh and Chanock, supra),
is not uncommon. Immunocompromised patients are severely affected and
RSV infection is a major complication for patients undergoing bone marrow
transplantation .
Uneventful RSV respiratory disease resembles a common cold and
recovery is in 7 to 12 days. Initial symptoms (rhinorrhea; nasal congestion,
slight fever, etc.) are followed in 1 to 3 days by lower respiratory tract
signs
of infection that include a cough and wheezing. In severe cases, these
mild symptoms quickly progress to tachypnea, cyanosis, and listlessness
and.hospitalization is required. In~infants with underlying cardiac or
respiratory disease, the progression of symptoms is especially rapid and
can lead to respiratory failure by the second or third day of illness. With
CA 02468048 2004-06-07
S7
modem intensive care however, overall mortality is usually less than 5% of
hospitalized patients (Mclntosh and Chanock, supra).
At present, neither an efficient vaccine nor a specific antiviral agent is
available. An immune response to the viral surface glycoproteins can
provide resistance to RSV in a number of experimental animals, and a
subunit vaccine has been shown to be effective for up to 6 months in
children previously hospitalized with an RSV infection (Tristam et al., 1993,
J. Infect. Dis. 167, 191 ). An attenuated bovine RSV vaccine has also been
shown to be effective in calves for a similar length of time (Kubota et al.,
1992 J. Vet. Med. Sci. 54, 957). Previously however, a formalin-inactivated
RSV vaccine was implicated in greater frequency of severe disease in
subsequent natural infections with RSV (Connors et al., 1992 J. Virol. 66,
7444).
The current treatment for RSV infection requiring hospitalization is the
use of aerosolized ribavirin, a guanosine analog [Antiviral Agents and Viral
Diseases of Man, 3rd edition. 1990. (eds. G.J. Galasso, R.J. Whitley, and
T.C. Merigan) Raven Press Ltd., NY.]. Ribavirin therapy is associated with
a decrease in the severity of the symptoms, improved arterial oxygen and a
decrease in the amount of viral shedding at the end of the treatment
period. It is not certain, however, whether ribavirin therapy actually
shortens the patients' hospital stay or diminishes the naed for supportive
therapies (Mclntosh and Chanock, supra). The benefits of ribavirin therapy
are especially clear for high risk infants, those with the most serious
symptoms or for patients with underlying bronchopulmonary or cardiac
disease. Inhibition of the viral polymerise complex is supported as the
main mechanism for inhibition of RSV by ribavirin, since viral but .not
cellular polypeptide synthesis is inhibited by ribavirin in ~ RSV-infected
cells
(Antiviral Agents and Viral Diseases of Man, 3rd edition. 1990. (eds. G.J.
Galasso, R.J. Whitley, and T.C. Merigan) Raven Press Ltd., NY]. Since
ribavirin is at least partially effective against RSV infection when delivered
by aerosolization, it can be assumed that the target cells are at or near the
epithelial surface. In this regard, RSV antigen had not spread any deeper
than the superficial layers of the respiratory epithelium in autopsy studies
of
fatal pneumonia (Mclntosh and Chanock, supra).
Jennings et al., WO 94/13688 indicates that targets for specific types
of ribozymes include respiratory syncytical virus.
CA 02468048 2004-06-07
58
The invention features novel enzymatic RNA molecules, or ribozymes,
and methods for their use for inhibiting production of respiratory syncytial
virus (RSV). Such ribozymes can be used in a method for treatment of
diseases caused by these related viruses in man and other animals. The
invention also features cleavage of the genomic RNA and mRNA of these
viruses by use of ribozymes. In particular, the ribozyme molecules
described are targeted to the NS7 (7C), NS2 (7B) and N viral genes.
These genes are known in the art (for a review see Mclntosh and Chanock,
1990 supra ).
Ribozymes that cleave the specified sites in RSV mRNAs represent a
novel therapeutic approach to respiratory disorders. Applicant indicates
that ribozymes are able to inhibit the activity of RSV and that the catalytic
activity of the ribozymes is required for Their inhibitory effect. Those of
ordinary skill in the art, will find that it is clear from the examples
described
that other ribozymes that cleave these sites in RSV mRNAs encoding 1C,
1 B and N proteins may be readily designed and are within the invention.
Also, those of ordinary skill in the art, will find that it is clear from the
examples described that ribozymes cleaving other mRNAs encoded by
RSV (P, M, SH, G, F, 22K and L) and the genomic RNA may be readily
designed and are within the invention.
In preferred embodiments, the ribozymes have binding arms which
are complementary to the sequences in Tables 31, 33, 35, 37 and 38.
Examples of such ribozymes are shown in Tables 32, 34, 36-38. Examples
of such ribozymes consist essentially of sequences defined in these
Tables. By "consists essentially of" is meant that the active ribozyme
contains an enzymatic center equivalent to those in the examples, and
binding arms able to bind mRNA such that cleavage at the target site
occurs. Other sequences may be present which do not interfere with such
cleavage.
Ribozymes of this invention block to some extent RSV production and
can be used to treat disease or diagnose such disease. Ribozymes will be
delivered to cells in culture and to cells or tissues in animal models of
respiratory disorders. Ribozyme cleavage of RSV encoded mRNAs or the
genomic RNA in these systems may alleviate disease symptoms.
CA 02468048 2004-06-07
59
While all ten RSV encoded proteins (1 C, 1 B, N, P, M, SH, 22K, F, G,
and L) are essential for viral life cycle and are all potential targets for
ribozyme cleavage, certain proteins (mRNAs) are more favorable for
ribozyme targeting than the others. For example RSV encoded proteins 1C,
1 B, SH and 22K are not found in other members of the family
paramyxoviridae and appear to be unique to RSV. In contrast the
ectodomain of the G protein and the signal sequence of the F protein show
significant sequence divergence at the nucleotide level among various
RSV sub-groups (Johnson et aL, 1987 supra).. RSV proteins 1C, 1B and N
are highly conserved among various subtypes at both the nucleotide and
amino acid levels. Also, 1C, 1B and N are the most abundant of all RSV
proteins.
The sequence of human RSV mRNAs encoding 1 C, 1 B and N
proteins are screened for accessible sites using a computer folding
algorithm. Hammerhead or hairpin ribozyme cleavage sites were
identified. These sites are shown in Tables 31, 33, 34, 37 and 38 (All
sequences are 5' to 3' in the tables.) The nucleotide base position is
noted in the Tables as that site to be cleaved by the designated type of
ribozyme.
Ribozymes of the hammerhead or hairpin motif are designed to
anneal to various sites in the mRNA message. The binding arms are
complementary to the target site sequences described above. The
ribozymes are chemically synthesized. The method of synthesis used
follows the procedure for normal RNA synthesis as described in Usman et
al., 1987 J. Am. Chem. Soc., 109, 7845-7854 and in Scaringe et al., 1990
Nucleic Acids Res., 18, 5433-5441 and makes use of common nucleic acid
protecting and coupling groups, such as dimethoxytrityl at the 5'-end, and
Phosphoramidites at the 3'-end. The average stepwise coupling yields
were >98%. Inactive ribozymes were synthesized by substituting a U for
G5 and a U for A14 (numbering from Hertel et al., 1992 Nucleic Acids Res.,
20, 3252). Hairpin ribozymes are synthesized in two parts and annealed to
reconstruct the active ribozyme (Chowrira and Burke, 1992 Nucleic Acids
Res., 20, 2835-2840). Hairpin ribozymes are also synthesized from DNA
templates using bacteriophage T7 RNA polymerase (Milligan and
Uhlenbeck, 1989, Methods Enzymoi. 180, 51 ). All ribozymes are modified
extensively to enhance stability by modification with nuclease . resistant
CA 02468048 2004-06-07
so
groups, for example, 2'-amino, 2'-C-allyl, 2'-flouro, 2'-o-methyl, 2'-H (for a
review see Usman and Cedergren, 1992 TIBS 17, 34). Ribozymes are
purified by gel electrophoresis using general methods or are purified by
high pressure liquid chromatography and are resuspended in water.
The sequences of the chemically synthesized ribozymes useful in this
study are shown in Tables 32, 34, 36, 37 and 38. Those in the art will
recognize that these sequences are representative only of many more such
sequences where the enzymatic portion of the ribozyme (all but the binding
arms) is altered to affect activity. For example, stem-loop II sequence of
hammerhead ribozymes listed in Tables 32 and 34(5'-GGCCGAAAGGCC-
3') can be altered (substitution, deletion, and/or insertion) to contain any
sequences provided a minimum of two base-paired stem structure can
form. Similarly, stem-loop IV sequence of hairpin ribozymes listed in
Tables 37 and 38 (5'-CACGUUGUG-3') can be altered (substitution,
deletion, andlor insertion) to contain any sequence, provided a minimum of
two base-paired stem structure can form. The sequences listed in Tables
32, 34, 36, 37 and 38 may be formed of ribonucleotides or other
nucleotides or non-nucleotides. Such ribozymes are equivalent to the
ribozymes described specifically in the Tables.
By engineering ribozyme motifs we have designed several ribozymes
directed against RSV encoded mRNA sequences. These ribozymes are
synthesized with modifications that improve their nuclease resistance. The
ability of ribozymes to cleave target sequences in vitro is evaluated.
Numerous common cell lines can be infected with RSV for
experimental purposes. These include HeLa, Vero and several primary
epithelial cell lines. A cotton rat anima! model of experimental human RSV
infection is also available, and the bovine RSV is quite homologous to the
human viruses. Rapid clinical diagnosis is through the use of kits designed
for the immunofluorescence staining of RSV-infected cells or an ELISA
assay, both of which are adaptable for experimental study. RSV encoded
mRNA levels will be assessed by Northern analysis, RNAse protection,
primer extension analysis or quantitative RT-PCR. Ribozymes that block
the induction of RSV activity and/or 1 C, 1 B and N protein encoding
mRNAs by more than 90% will be identified.
CA 02468048 2004-06-07
Oatimizin Rq ibozyme Activity
Ribozyme activity can be optimized as described by Draper et al., PCT
W093I23569. The details will not be repeated here, but include altering
the length of the ribozyme binding arms or chemically synthesizing
ribozymes with modifications that prevent their degradation by serum
ribonucleases (see e.g., Eck~tein et aG, International Publication No.
WO 92/07065; Perrault ef aL, 1990 Na_ furs 344, 565; Pieken et al., 1991
cienc 253, 314; Usman and Cedergren, 1992 Trends in Biochem Sci
17, 334; Usman et al., International Publication No. WO 93/15187; and
Rossi et al., International Publication No. WO 91/03162, as well as
Jennings et al., WO 94/13688, which describe various chemical
modifications that can be made to the sugar moieties of enzymatic RNA
molecules, modifications which enhance their efficacy in cells, and .removal
of
stem II bases to shorten RNA synthesis times and reduce chemical
requirements.
Sullivan, et al., PCT W094/02595,
describes the general methods for delivery of enzymatic RNA molecules .
Ribozymes may be administered to cells by a variety of methods known to
those familiar to the art, including, but not restricted to, encapsulation in
liposomes, by iontephoresis, or by incorporation into other vehicles, such
as hydrogels, cyclodextrins, biodegradable nanocapsules, and
bioadhesive microspheres. The RNA/vehicle combination is locally
delivered by direct injection or by use of a catheter, infusion pump or stmt.
Alternative routes of delivery include, but are not limited to, intravenous
injection, intramuscular injection, subcutaneous injection, aerosol
inhalation, oral (tablet or pill form), topical, systemic, ocular,
intraperitoneal
and/or intrathecal delivery. More detailed descriptions of ribozyme delivery
and administration are provided .in Sullivan, et al., supra and Draper, et
al.,
su ra,
Another means of accumulating high concentrations of a ribozyme(s)
within cells is to incorporate the ribozyme-encoding sequences into a DNA
expression vector. Transcription of the ribozyme sequences are driven
from a promoter for eukaryotic,RNA polymerise I (pol I), RNA poiymerase II
(pol ll), or RNA polymerise 111 (pol III). Transcripts from pol II or pol Ill
promoters ,will be expressed at high levels in all cells; the levels of a
given
CA 02468048 2004-06-07
.
pol If promoter in a given cell type will depend on the nature of the gene
regulatory sequences (enhancers, silencers, etc.~ present nearby.
Prokaryotic RNA polymerise promoters are also used, providing that the
prokaryotic RNA polymerise enzyme is expressed in the appropriate cells
(Elroy-Stein and Moss, 1990 proc. Natl. Acid. Sci. U S A, 87, 6743-7; Gao
and Huang 1993 Nucleic Acids Res., 21, 2867-72; Lieber et al., 1993
Methods Enzymol., 217, 47-66; Zhou et al., 1990 Mol. Cell. Biol., 10, 4529-
37). Several investigators have demonstrated that ribozymes expressed
from such promoters can function in mammalian cells (e.g. Kashani-Sabet
et al., 1992 Antisense Res. Dev , 2, 3-15; Ojwang et al., 1992 Proc. Natl.
Acid. Sci. U S A, 89, 10802-6; Chen et al., 1992 Nucleic Acids Res_., 20,
4581-9; Yu et al., 1993 Proc: Natl. Acid. Sci. U S A, 90, 6340-4; L'Huillier
et al., 1992 EM8 . 11, 4411-8; Lisziewicz et al., 1993 Proc. Natl, Acad.
Sci. U. S. A., 90, 8000-4). The above ribozyme transcription units can be
incorporated into a variety of vectors for introduction into mammalian cells,
including but not restricted to, plasmid DNA vectors, viral DNA vectors
(such as adenovirus or adeno-associated virus vectors), or viral RNA
vectors (such as retroviral, or alpha virus vectors).
In a preferred embodiment of the invention, a transcription unit
expressing a ribozyme that cleaves target RNA is inserted into a plasmid
DNA vector, a retrovirus DNA viral vector, an adenovirus DNA viral vector
or an adeno-associated virus vector or alpha virus vector. These and other
vectors have been used to transfer genes to live animals (for a review see
Friedman, 1989 cienc 244, 1275-1281; Roemer and Friedman, 1992
Eur. J. Biochem. 208, 211-225) and leads to transient or stable gene
expression. The vectors are delivered as recombinant viral particles. DNA
may be delivered alone or complexed with vehicles (as described for RNA
above). The DNA, DNAlvehicle complexes, or the recombinant virus
particles are locally administered to the site of treatment, e.g., through the
use of a catheter, stent or infusion pump.
Diagnostic uses
Ribozymes of this invention may be used as diagnostic tools to
examine genetic drift and mutations within diseased cells. The close
relationship between ribozyme activity and the structure of the target RNA
allows the detection of mutations in any region of the molecule which alters
the base-pairing and three-dimensional structure of the target RNA. 8y
CA 02468048 2004-06-07
63
using multiple ribozymes described in this invention, one may map
nucleotide changes which are important to RNA structure and function in
vitro, as well as in cells and tissues. Cleavage of target RNAs with
ribozymes may be used to inhibit gene expression and define the role
(essentially) of specified gene products in the progression of disease. In
this manner, other genetic targets may be defined as important mediators
of the disease. These experiments will lead to better treatment of the
disease progression by affording the possibility of combinational therapies
(e~a., multiple ribozymes targeted to different genes, ribozymes coupled
with known small molecule inhibitors, or intermittent treatment with
combinations of ribozymes andlor other chemical or biological molecules).
Other in vitro uses of ribozymes of this invention are well known in the art,
and include detection of the presence of mRNA associated with ICAM-t,
relA, TNF-a, p210, bcr-abl or RSV related condition. Such RNA is detected
by determining the presence of a cleavage product after treatment with a
ribozyme using standard methodology.
In a specific example, ribozymes which can cleave only wild-type or
mutant forms of the target RNA are used for the assay. The first ribozyme is
used to identify wild-type RNA present in the sample and the second
ribozyme will be used to identify mutant RNA in the sample. As reaction
controls, synthetic substrates of both wild-type and mutant RNA will be
cleaved by both ribozymes to demonstrate the relative ribozyme
efficiencies in the reactions and the absence of cleavage of the "non-
targeted" RNA species. The cleavage products from the synthetic
substrates will also serve to generate size markers for the analysis of wild-
type and mutant RNAs in the sample population. Thus each analysis will
require two ribozymes, two substrates and. one unknown sample which will
be combined into six reactions. The presence of cleavage products will be
determined using an RNAse protection assay so that full-length and
cleavage fragments of each RNA can be analyzed in one lane of a
polyacrylamide gel. It is not absolutely required to quantify the results to
gain insight into the expression of mutant RNAs and putative risk of the
desired phenotypic changes in target cells. The expression of mRNA
whose protein product is implicated in the development of the phenotype
(i.e., 1CAM-1, rel A, TNF~, p210bcr-abl or RSV) is adequate to establish
risk. If probes of comparable specific activity are used for both transcripts,
then a qualitative comparison of RNA levels will be adequate and will
CA 02468048 2004-06-07
64
' decrease the cost of the initial diagnosis. Higher mutant form to wild-type
ratios will be correlated with higher risk whether RNA levels are compared
qualitatively or quantitatively.
ii. Chemical Synthesis Of Ribozyrmes_,
There follows the chemical synthesis, deprotection, and purification of
RNA, enzymatic RNA or modified RNA molecules in greater than milligram
quantities with high biological activity: Applicant has determined that the
synthesis of enzymatically active RNA in high yield and quantity is
dependent upon certain critical steps used during its preparation.
Specifically, it is important that the RNA phosphoramidites are coupled
efficiently in terms of both yield and time, that correct exocyclic amino
protecting groups be used, that the appropriate conditions far the removal
of the exocyclic amino protecting groups and the alkylsilyl protecting
groups on the 2'-hydroxyl are used, and that the correct work-up and
purification procedure of the resulting ribozyme be used.
To obtain a correct synthesis in terms of yield and biological activity of
a large RNA molecule (l.e., about 30 to 40 nucleotide bases), the protection
of the amino functions of the bases requires either amide or substituted
amide protecting groups, which must be, on the one hand, stable enough
to survive the conditions of synthesis, and on the other hand, removable at
the end of the synthesis. These requirements are met by the amide
protecting groups shown in Figure 8, in particular, benzoyl for adenosine,
isobutyryl or benzoyl for cytidine, and isobutyryl for guanosine, which may
be removed at the end of the synthesis by incubating the RNA in NH3lEtOH
(ethanolic ammonia) for 20 h at 65 °C. In the case of the phenoxyacetyl
type protecting groups shown in Figure 8 on guanosine and adenosine
and acetyl protecting groups on cytidine, an incubation in ethanolic
ammonia for 4 h at 65 °C is used to obtain complete removal of these
protecting groups. Removal of the alkylsilyl 2'-hydroxyl protecting groups
can be accomplished using a tetrahydrofuran solution of TBAF at room
temperature for 8-24 h.
The most quantitative procedure for recovering the fully deprotected
RNA molecule is by either ethanol precipitation, or an anion exchange
cartridge desalting, as described in Scaringe et al. Nucleic Acids Res. .
1990, 18, 5433-5341. The purification of the long RNA sequences may be
CA 02468048 2004-06-07
fi5
accomplished by a two-step chromatographic procedure in which the
molecule is first purified on a reverse phase column with either the trity)
group at the 5' position on or off. This purification is accomplished using an
acetonitrile gradient with triethylammonium or bicarbonate salts as the
aqueous phase. In the case of the trityl on purification, the trityl group may
be removed by the addition of an acid and drying of the partially purified
RNA molecule. The final purification is carried out oh an anion exchange
column, using alkali metal perchlorate salt gradients to elute the fully
purified RNA molecule as the appropriate metal salts, e.g. Na+, Li+ etc. A
final de-salting step on a small reverse-phase cartridge completes the
purification procedure. Applicant has found that such a procedure not only
fails to adversely affect activity of a ribozyme, but may improve its activity
to
cleave target RNA molecules.
Applicant has also determined that significant (see Tables 39-41)
improvements in the yield of desired full length product (FLP) can be
obtained by:
1. Using 5-S-alkyltetrazole at a delivered or effective
concentration of 0.25-0.5 M or 0.15-0.35 M for the activation of the RNA (or
analogue) amidite during the coupling step. (By delivered is meant that the
actual amount of chemical in the reaction mix is known. This is possible for
large scale synthesis since the reaction vessel is of size sufficient to allow
such manipulations. The term effective means that available amount of
chemical actually provided to the reaction mixture that is able to react with
the other reagents present in the mixture. Those skilled in the art wilt
recognize the meaning of these terms from the examples provided herein.)
The time for this step is shortened from 10-15 rn, vide supra, to 5-10 m.
Alkyl, as used herein, refers to a saturated aliphatic hydrocarbon, including
straight-chain, branched-chain, and cyclic alkyl groups. Preferably, the
alkyl group~has 1 to 12 carbons. More preferably it is a lower alkyl of from 1
to 7 carbons, more preferably 1 to 4 carbons. The alkyl group may be
substituted or unsubstituted. When substituted the substituted groups) is
preferably, hydroxyl, cyano, alkoxy, =O, =S,, NOp or N(CH3)2, amino, or SH.
The term also includes alkenyl groups which are unsaturated hydrocarbon
groups containing at least one carbon-carbon double bond, including
straight-chain, branched-chain, and cyclic groups. Preferably, the alkenyl
group has 1 to 12 carbons. More preferably it is a lower alkenyl of from 1 to
CA 02468048 2004-06-07
fib
7 carbons, more preferably 1 to 4 carbons. The alkenyl group may be
substituted or unsubstituted. When substituted the substituted groups) is
preferably, hydroxyl, cyano, alkoxy, =0, =S, N02, halogen, N(CH3)2,
amino, or SH. The term "alkyl" also includes alkynyl groups which have an
unsaturated hydrocarbon group containing at least one carbon-carbon
triple bond, including straight-chain, branched-chain, and cyclic groups.
Preferably, the alkynyl group has 1 to 12 carbons. More preferably it~is a
lower alkynyl of from 1 to 7 carbons, more preferably 1 to 4 carbons. The
alkynyl group may be substituted or unsubstituted. When substituted the
substituted groups) is preferably, hydroxyl, cyano, alkoxy, =O, =S, N02 or
N(CH3)2, amino or SH.
Such alkyl groups may also include aryl, alkylaryl, carbocyclic aryl,
heterocyclic aryl, amide and ester groups. An "aryl" group refers to an
aromatic group which has at least one ring having a conjugated n electron
system and includes carbocyclic aryl, heterocyclic aryl and biaryl groups,
all of which may be optionally substituted. The preferred substituent(s) of
aryl groups are halogen, trihalomethyl, hydroxyl, SH, OH, cyano, alkoxy,
alkyl, alkenyl, alkynyl, and amino groups. An "alkylaryl" group refers to an
alkyl group (as described above) covalently joined to an aryl group (as
described above. Carbocyclic aryl groups are groups wherein the ring
atoms on the aromatic ring are all carbon atoms. The carbon atoms are
optionally substituted. Heterocyclic aryl groups are groups having from 1 to
3 heteroatoms as ring atoms in the aromatic ring and the remainder of the
ring atoms are carbon atoms. Suitable heteroatoms include oxygen, sulfur,
and nitrogen, and include furanyl, thienyl, pyridyl, pyrrolyl, N-lower alkyl
pyrrolo, pyrimidyl, pyrazinyl, imidazoiyl and the like, all optionally
substituted. An "amide" refers to an -C(O)-NH-R, where R is either alkyl,
aryl, alkylaryl or hydrogen. An "ester" refers to an -C(O)-OR', where R is
either alkyl, aryl, alkylaryl or hydrogen.
2. Using 5-S-alkyltetrazole at an effective, or final, concentration
of 0.1-0.35 M for the activation of the RNA (or analogue) amidite during the
coupling step. The time for this step is shortened from 10-15 m, vide supra,
to 5-10 m.
3. Using alkylamine (MA, where alkyl is preferably methyl, ethyl,
propy! or butyl) or NH40Hlalkylamine (AMA, with the same preferred alkyl
groups as noted for MA) ~ 65 °C for 10-15 m to remove the exocyclic
CA 02468048 2004-06-07
s~
amino protecting groups (vs 4-20 h C~ 55-65 °C using NH4OH/EtOH or
NH3/EtOH, vide supra). Other alkylamines, e.g. ethylamine, .propyiamine, .
butylamine etc. may also be used.
4. Using anhydrous triethylamine~hydrogen fluoride (aHF~TEA)
Q 65 °C fot 0.5-1.5 h to remove the 2'-hydroxyl alkylsilyl
protecting group
(vs 8 - 24 h using TBAF, vide supra or TEA~3HF for 24 h (Gasparutto et al.
Nucleic Acids Res.1992, 20, 5159-5166). Other alkylamine~HF
complexes may also be used, e.g. trimethylamine or diisopropylethylamine.
5. The use of anion-exctlange resins to purify and/or analyze the
fully deprotected RNA. These resins include, but are not limited to,
quartenary or tertiary amino derivatized stationary phases such as silica or.
polystyrene. Specific examples include, Dionex-NA100~, Mono-Q~, Poros-
Q~.
Thus, the invention features an improved method for the coupling of
RNA phosphorarnidites; for the removal of amide or substituted amide
protecting groups; and for the removal of 2'-hydroxyl alkylsilyl protecting
groups. Such methods enhance the production of RNA or analogs of the
type described above (e.g., with substituted 2'-groups), and allow efficient
synthesis of large amounts of such RNA. Such RNA may also have
enzymatic .activity and be purified without loss of that activity. While
specific
examples .are given herein, 3hose in the art will recognize that equivalent
chemical reactions can be pertormed with the alternative chemicals noted
above, which can be optimized and selected by routine experimentation.
fn another aspect, the invention features an improved method for the
purification or analysis of RNA or en2ymatic RNA molecules (e.g. 28-70
nucleotides in length) by passing said RNA or enzymatic RNA molecule
over an ~HPLC, e.g., reverse phase and/or an anion exchange
chromatography column. The method of purification improves the catalytic
activity of enzymatic RNAs over the gel purification method (see Figure 10).
Draper et al., PCT W093/23569,
disclosed reverse phase HPLC purification. The purification of.long RNA
molecules may be accomplished using anion exchange chromatography,
particularty in conjunction with alkali perchlorate salts. This system may be
used to purify very long RNA~molecules. 1n particular, it is advantageous to
CA 02468048 2004-06-07
68 '
use a Dionex NucleoPak 100' or a Pharmacia Mono Q~ anion exchange
column for the purification of RNA by the anion exchange method. This
anion exchange purification may be used following a reverse-phase
purification or prior to reverse phase purification. This method results in
the
formation of a sodium salt of the ribozyme during the chromatography.
Replacement of the sodium alkali earth salt by other metal salts, e.g.,
lithium, magnesium or calcium perchlorate, yields the corresponding saif of
the RNA molecule during the purification.
In the case of the 2-step purifiication procedure, in which the first step
is a reverse phase purification followed by an anion exchange step, the
reverse phase purification is best accomplished using polymeric, e.g.
polystyrene based, reverse-phase media, using either a 5'-trityl-on or 5'-
trityl-off method. Either molecule may be recovered using this reverse-
phase method, and then, once detritylated, the two fractions may be pooled
and then submitted to an anion exchange purification step as described
above.
The method includes passing the enzymatically active RNA
molecule over a reverse phase HPLC column; the enzymatically active
RNA molecule is produced in a synthetic chemical method and not by an
enzymatic process; and the enzymatic RNA molecule is partially blocked,
and the partially blocked enzymatically active RNA molecule is passed
over a reverse phase HPLC column to separate it from other RNA
molecules.
In more preferred embodiments, the enzymatically active RNA
molecule, after passage over the reverse phase HPLC column, is
deprotected and passed over a second reverse phase HPLC column
(which may be the same as the reverse phase HPIC column), to remove
the enzymatic RNA molecule from other components. In. addition, the
column is a silica or organic polymer-based C4, C8 or C18 column having
a porosity of at least 125 ~, preferably 300 ~, and a particle size of at
least
2 pm, preferably 5 Vim.
Activation
The synthesis of RNA molecules may be accomplished chemically or
enzymatically. In the case of chemical. synthesis the use of letrazole as an
activator of RNA phosphorarnidites is known (Usman et al, J. Am. Chem.
CA 02468048 2004-06-07
~ 69
Soc. 1987, 109, 7845-7854). In this, and subsequent reports, a 0.5 M
solution of tetrazole is allowed to react with the RNA phosphoramidite and
couple with the polymer bound 5'-hydroxyl group for 10 m. Applicant has
determined that using 0.25-0.5 M solutions of 5-S-alkyltetrazoles for only 5
min gives equivalent or bEtter results. The following exemplifies the
procedure.
Example 7: Synthesis of RNA and Ribozymes Using 5-S-Alkyltetrazoles
as Activating Aoent
The method of synthesis used follows the general procedure for RNA
synthesis as described in Usman et al., 1987supra and in Scaringe et al.,
Nucleic Acids Res. 1990, 18, 5433-5441 and makes use of common
nucleic acid protecting and coupling groups, such as dimethoxytrityl at the
5'-end, and phosphoramidites at the 3'-end. The major difference used
was the activating agent, 5-S-ethyl or -methyftetrazole ~ 0.25 M
concentration for 5 min.
All small scale syntheses were conducted on a 394 (ABI) synthesizer
using a modified 2.5 pmol scale protocol with a reduced 5 min coupling
step for alkylsilyl protected RNA and 2.5 m coupling step for 2'-O-
rnethylated RNA. A 6.5-fold excess (162.5 ~L of 0.1 M = 32.5 pmol) of
phosphoramidite and a 40-fold excess of S-ethyl tetrazole (400 ~L of 0.25
M = 100 umol) relative to polymer-bound 5'-hydroxyl was used in each
coupling cycle. Average coupling yields on the 394, determined by
colorimetric quantitation of the trityl fractions, was 97.5-
99°!°. Other
oligonucleotide synthesis reagents for the 394: Detritylation solution was
2% TCA in methylene chloride; capping was performed with 16% N-Methyl
imidazole in THF and 10% acetic anhydridell0% 2,6-lutidine in THF;
oxidation solution was 16.9 mM i2, 49 mM pyridine, 9°!° water in
THF.
Fisher Synthesis Grade acetonitrile was used directly from the reagent
bottle. S-Ethyl tetrazole solution (0.25 M in acetonitrile) was made up from
3.0 the solid obtained from Applied Biosystems. ~ .
All large scale syntheses were conducted on a modified (eight amidite
port capacity) 3902 (ABI) synthesizer using a 25 umol scale protocol with a
5-15 min coupling step for alkylsilyf protected RNA and 7.5 m coupling step
for 2'-Qmethylated RNA. A six-fold excess (1.5 mL of 0.1 M : 150 pmol) of
phosphoramidite and a forty-five-fold excess of S-ethyl tetrazole ~{4.5 mL of
CA 02468048 2004-06-07
7~
0.25 M = 1125 ~mol) relative to polymer-bound 5'-hydroxyl was used in
each coupling cycle. Average coupling yields on the 3902, determined by
colorimetric quantitation of the trityl fractions, was 95.0-96.7%.
Oligonucleotide synthesis reagents for the 3902: Detritylation solution was
2% DCA in methylene chloride; capping was performed with 1fi% N-Methyl
imidazole in THF and 10% acetic anhydride/10% 2,6-lutidine in THF;
oxidation solution was 16.9 mM 12, 49 mM pyridine, 9°I° water in
THF.
Fisher Synthesis Grade acetonitrile was used directly from the reagent
bottle. S-Ethyl tetrazole solution (0.25-0.5 M in acelonitrile) was made up
from the solid obtained from Applied 8iosystems.
Dearotection
The first step of the deprotection of RNA molecules may be
accomplished by removal of the exocyclic amino protecting groups with
either NHQOH/EtOH:3/1 (Usman et al. J. Am. Chem. Soc. 1987, 109, 7845-
7854} or NH3/EtOH (Scaringe et al. Nucleic Acids Res. 1990, 18, 5433-
5341 ) for -20 h ~ 55-65 °C. Applicant has determined that the use of
methylamine or NH40H/methylamine for 10-15 min C~? 55-65 °C gives
equivalent or better results. The following exemplifies the procedure.
Examt~le 8: RNA and Ribozvme Deorotection of Exocyclic Amino
Protecting Groups Using Methylamine (MAl or NHAOH/Methylamine ~,."AMAI
The polymer-bound oligonucleotide, either trityl-on or off, was
suspended in a solution of methylamine (MA) or NH4OH/methylamine
(AMA) C~? 55-65 °C for 5-15 min to remove the exocyclic amino
protecting
groups. The polymer-bound oiigoribonucleotide was transferred from the
synthesis column to a 4 mL glass screw top vial. NH40H and aqueous
methylamine were pre-mixed in equal volumes. 4 mL of the resulting
reagent was added to the vial, equilibrated for 5 m at RT and then heated at
55 or 65 °C for 5-15 min. After cooling to -20 °C, the
supernatant was
removed from the polymer support. The support was washed with 1.0 mL
of EtOH:MeCN:H2O/3:1:1, vortexed and the supernatant was then added to
the first supernatant. The combined supernatants, containing the
oligoribonucleotide, were dried to a white powder. The same procedure
was followed for the aqueous methylamine reagent.
Table 40 is a summary of the results obtained using the improvements
outlined in this application for base deprotection.
CA 02468048 2004-06-07
7
The second step of the deprotection of RNA molecules may be
accomplished by removal of the 2'-hydroxyl alkylsilyl protecting group
using TBAF for 8-24 h (Usman et al. J. Am. Chem. Soc: 1987, 709, 7845-
7854). Applicant has determined that the use of anhydrous TEA~HF in N-
methylpyrrolidine (NMP) for 0.5-1.5 h C~3 55-65 °C gives equivalent or
better
results. The following exemplifies this procedure.
Example 9; RNA and Ribozyme Deorotection of 2'-Hydroxyl Alkvlsilvl
Protectina Groups Using, Anhydrous TEA~HF
To remove the alkylsilyi protecting groups, the ammonia-deprotected
oligoribonucleotide was resuspended in 250 pL of 1.4 M anhydrous HF
solution (1.5 mL N-methylpyrrolidine, 750 uL TEA and 1.0 mL TEA~3HF)
and heated to 65 °C for 1.5 h. 9 mL of 50 mM TEAB was added to quench
the reaction. The resulting solution was loaded onto a Gaiagen 500~ anion
exchange cartridge (Oiagen Inc.) prewashed with 10 mL of 50 mM TEAB.
After washing the cartridge with 10 mL of 50 mM TEAB, the RNA was eluted
with 10 mL of 2 M TEAR and dried down to a white powder.
Table 41 is a summary of the results obtained using the improvements
outlined in this application for alkylsilyl deprotection.
Example 10: HPLC Purification. Anion Exchan
qe column
For a small scale synthesis, the crude material was diluted to 5 mL
with diethylpyrocarbonate treated water. The sample was injected onto
either a Pharmacia Mono Q~ 16/10 or Dionex NucleoPac~ column with
100% buffer A (10 mM NaCl04). A gradient from 180-210 mM NaCl04 at a
rate of 0.85 mMlvoid volume for a Pharmacia Mono Q~ anion-exchange
column or 100-150 mM NaCl04 at a rate of 1.7 mMlvoid volume for a
Dionex NucIeoPac~ anion-exchange column was used to elute the RNA.
Fractions were analyzed by~ a HP-1090 HPLC with a Dionex NucIeoPac~
column. Fractions containing full length product at >_80% by peak area
were pooled.
For a trityi-ofif large scale synthesis, the crude material was desalted
by applying the solution that resulted from quenching of the desilylation
reaction to a 53 mL Pharmacia HiLoa~d 26!10 Q-Sepharose~ Fast Flow
column. The column was thoroughly washed with 10 mM sodium
perchlorate bufifer. The oligonucleotide was eluted from the column with
CA 02468048 2004-06-07
72
300 rnM sodium perchlorate. The eluent was quantitated and an analytical
HPLC was run to determine the percent full length material in the synthesis.
The eluent was diluted four fold in sterile H20 to lower the salt
concentration and applied to a Pharmacia Mono D~ 16!10 column. A
gradient from 10-185 mM sodium perchlorate was run over 4 column
volumes to elute shorter sequences, the full length product was then eluted
in a gradient from 185-214 mM sodium perchlorate in 30 column volumes.
The fractions of interest were analyzed on a HP-1090 HPLC with a Dionex
NucIeoPac~ column. Fractions containing over 85% full length material
were pooled. The pool was applied to a Pharmacia RPC~ column for
desalting.
For a trityl-on large scale synthesis, the crude material was desalted
by applying the solution that resulted from quenching of the desilylation
reaction to a 53 mL Pharmacia HiLoad 26!10 Q-Sepharose~ Fast flow
column. The column was thoroughly washed with 20 mM NH~C03H/10%
CH3CN buffer. The oligonucleotide was eluted from the column with ~1.5 M
NH~C03H/10% acetonitrile. The eluent was quantitated and an analytical
HPLC was run to determine the percent full length material present in the
synthesis. The oligonucleotide was then applied to a Pharmacia Resource
RPC column. A gradient from 20-55°!° B (20 mM NH4C03H125%
CH3CN,
buffer A = 2D mM NH4C03H/10% CH3CN) was run over 35 column
volumes. The fractions of interest were analyzed on a HP-1090 HPLC with
a Dionex NucleoPac~ column. Fractions containing over 60% full length
material were pooled. The pooled fractions were then submitted to manual
detritylation with 80% acetic acid, dried down immediately, resuspended in
sterile H20, dried down and resuspended in H20 again. This material was
analyzed on a HP 1090-HPLC with a Dionex NucIeoPac~ coturnn. The
material was purified by anion exchange chromatography as in the trityl-off
scheme (vide supra).
Example 11 Ribozyme Activity Assav_
Purified 5'-end labeled RNA substrates (15-25-mers) and purified 5'-
end labeled ribozymes (,36-mers) were both heated to 95 °C, quenched
on ice and equilibrated at 37 °C, eparately. RiboZyme stock solutions
were t uM, 200 nM, 40 nM or 8 nM and the final substrate RNA
concentrations were - 1 nM. Total reaction volumes were 50 ~L. The
assay buffer was 50 mM Tris-CI, pH 7.5 and 10 mM MgCl2. Reactions were
CA 02468048 2004-06-07
73
initiated by mixing substrate and ribozyme solutions at t = 0. Aliquots of 5
pL were removed at time points of 1, 5, 15, 30, 60 and 120 m. Each aliquot
was quenched in formamide loading buffer and loaded onto a 15%
denaturing polyacrylamide gel for analysis. Quantitative analyses were
performed using a phosphorimager (Molecular Dynamics).
Example 12: One ,pot deprotection of RNA
Applicant has shown that aqueous methyl amine is an efficient
reagent to deprotect bases in an RNA molecule. However, in a time
consuming step (2-24 hrs), the RNA sample needs to be dried completely
prior to the deprotection of the sugar 2'-hydroxyl groups. Additionally,
deprotection of RNA synthesized on a large scale (e.g., 100 pmol)
becomes challenging since the volume of solid support used is quite large.
In an attempt to minimize the time required for deprotection and to simplify
the process of depratection of RNA synthesized on a large scale, applicant
describes a one pot deprotection protocol (Fig. 12). According to this
protocol, anhydrous methylamine is used in place of aqueous methyl
amine. Base deprotection is carried out at 65 °C for 15 min and the
reaction is allowed to coot for 10 min. Deprotection of 2'-hydroxyl groups is
then carried out in the same container for 90 min in a TEA~3HF reagent.
The reaction is quenched with 16 mM TEAB solution.
Referring to Fia. 13, hammerhead ribozyme targeted to site B is
synthesized using RNA phosphoramadite chemistry and deprotected using
either a two pot or a one pot protocol. Profiles of these ribozymes on an
HPLC column are compared. The figure shows that RNAs deprotected by
either the one pot or the two pot protocols yield similar full-length product
profiles. Applicant has shown that using a one pot deprotection protocol,
time required for RNA deprotection can be reduced considerably without
compromising the quality or the yield of full length RNA.
Referring to F_ ig. 14, hammerhead ribozymes targeted io site B (from
Fi . 13 are tested far their ability to cleave RNA. As shown in the figure 14,
ribozymes that are deprotected using one pot protocol have catalytic
activity comparable to ribozymes that are deprotected using a two pot
protocol.
CA 02468048 2004-06-07
74
Example l2a:lmproved protocol for the synthesis of hosphorothioate
containina RNA and ribozymes using 5-S-Alkyltetrazoles as Activating
A_4ent .
The two sulfurizing reagents that have been used to synthesize
ribophosphorothioates are tetraethylthiuram disulfide (TETD; Vu and
Hirschbein, 1991 Tetrahedron Letter 31, 3005), and 3H-1,2-benzodithiol-3-
one 1,1-dioxide (Beaucage reagent; Vu and Hirschbein, 1991 supra).
TETD requires long sulfurization times (600 seconds for DNA and 3600
seconds for RNA). It has recently been shown that for sulfurization of DNA
oligonucleotides, ~ Beaucage reagent is more efficient than TETD
(Wyrzykiewicz and Ravikumar, 1994 Bioorganic Med. Chem. 4, 1519).
Beaucage reagent has also been used to synthesize phosphorothioate
oligonucleotides containing 2'-deoxy-2'-fluoro modifications wherein the
wait time is 10 min (Kawasaki et al., 1992 J. Med Chem).
The method of synthesis used follows the procedure for RNA
synthesis as described herein and makes use of common nucleic acid
protecting and coupling groups, such as dimethoxytrityl at the 5'-end, and
phosphoramidites at the 3'-end. The sulfurization step for RNA described
in the literature is a 8 second delivery and 10 ruin wait steps (Beaucage
and lyer, 1991 Tetrahedron 49, fi123). These conditions produced about
95% sulfurization as measured by HPLC analysis (Morvan et al., 1990
Tetrahedron Letter 31, 7149). This 5% contaminating oxidation could arise
from the presence of oxygen dissolved in solvents andlor slow release of
traces of iodine adsorbed on the inner surface of delivery lines during
previous synthesis.
A major improvement is the use of an activating agent, 5-S-
ethyitetrazole or 5-S-methyltetrazole at a concentration of 0.25 M for 5 min.
Additionally, for those linkages which are phosporothioate, the iodine
solution is replaced wish a 0.05 M solution of 3H-1,2-benzodithiote-3-one
1,1-dioxide (Beaucage reagent) in acetonitrile. The delivery time for the
sulfurization step is reduced to 5 seconds and the wait time is reduced to
300 seconds.
RNA synthesis is conducted on a 394 . (ABL) synthesizer using a
modified 2.5 pmol scale protocol with a reduced 5 min coupling step for
alkylsilyl protected RNA and 2.5 min coupling step for 2'-O-methylated
RNA. A 6.5-fold excess (162.5 pl of 0.1 M = 32.5 pmol) of phosphoramidite
CA 02468048 2004-06-07
and a 40-fold excess of S--ethyl tetrazole (400 ~L of 0.25 M = 100 ~mol)
relative to polymer-bound 5'-hydroxyl was used in each coupling cycle.
Average coupling yields on the 394 synthesizer, determined by colorimetric
quantitation of the trity) fractions, was 97.5-99%. Other oligonucleotide
5 synthesis reagents for the 394 synthesizer: detritylation solution was 2%
TCA in methylene chloride; capping was performed with 16% N-Methyl
imidazole in THF and 10% acetic anhydride/10% 2,6-futidine iri THF;
oxidation solution was 16.9 mM 12, 49 mM pyridine, 9% water in THF.
Fisher Synthesis Grade acetonitrile was used directly from the reagent
10 bottle. S-Ethyl tetrazole solution (0.25 M in acetonitrile) was made up
from
the solid obtained from Applied Biosystems. Sulfurizing reagent was
obtained from Glen Research.
Average sulfurization efficiency (ASE) is determined using the
formula: ASE = (PSJTotal)1~n-1
15 where, PS = integrated 31 P NMR values of the P=S diester
Total = integration value of all peaks
n = length of oligo
Referring to tables 42 and 43, effects of varying the delivery and the
wait time for sulfurization with Beaucage's reagent is described. These
20 data suggest that 5 second wait time and 300 second delivery time is t'he
condition under which ASE is maximum.
Using the above conditions a 36 mer hammerhead ribozyme is
synthesized which is targeted to site C. The ribozyme is synthesized to
contain phosphorothioate linkages at four positions towards the 5' end.
25 RNA cleavage activity of this ribozyme is shown in Fig. 16 Activity of the
phosphorothioate ribozyme is comparable to the activity of a ribozyme
lacking any phosphorothioate linkages.
Examofe 13' Protocol for the synthesis of 2'-N-phtalimido nucleosidg
~hosphoramidite .
30 The 2'-amino group of a 2'-deoxy-2'-amino nucleoside is normally
protected with N-(9-flourenylmethoxycarbonyl) (Fmoc; Imazawa and
Eckstein, 1979 supra,; Pieken et al., 1991 Science 253,. 314). This
protecting group is not stable in CH3CN solution or even in dry form during
CA 02468048 2004-06-07
76 '
prolonged storage at -20 oC. These problems need to be overcome in
order to achieve large scale synthesis of RNA.
Applicant describes the use of alternative protecting groups for the 2'-
amino group of 2'-deoxy-2'-amino nucleoside. Referring to Figure i7.
phosphoramidite 17 was synthesized starting from 2'-deoxy-2'-
aminonucleoside (12) using transient protection with Markevich reagent
(Markiewicz J. Chem. Res. 1979, S, 24). An intermediate 13 was obtained
in 50% yield, however subsequent introduction of N-phtaloyl (Pht) group by
Nefken's method (Nefkens, 1960 Nature 185, 306), desilylation (15),
dimethoxytrytilation (16) and phosphitylation led to phosphoramidite 17.
Since overall yield of this multi-step procedure was low (20%) applicant
investigated some alternative approaches, concentrating on selective
introduction of N-phtaloyl group without acylation of 5' and 3' hydroxyls.
When 2'-deoxy-2'-amino-nucleoside was reacted with 1.05
equivalents of Nefkens reagent in DMF overnight with subsequent
treatment with Et3N (1 hour) only 10-15°!° of N and 5'(3')-bis-
phtatoyl
derivatives were formed with the major component being N-Pht-derivative
15. The N,0-bis by-products could be selectively and quantitively
converted to N-Pht derivative 15 by treatment of crude reaction mixture
with cat. KCN/MeOH.
A convenient "one-.pot° procedure for the synthesis of key
intermediate 16 involves selective N-phthaloylation with subsequent
dimethoxytrytilation by DMTCIlEt3N and resulting in the preparation of DMT
derivative 16 in 85% overall yield as follows. Standard phosphytilation of
16 produced phosphoramidite 17 in 87% yield. One gram of 2'-amino
nucleoside, for example 2'-amino uridine (US Biochemicals~ part #
77140) was co-evaporated twice from dry dimethyl formamide (Dmf) and
dried in vacuo overnight. 50 mls of Aldrich sure-seal Dmf was added to the
dry 2'-amino uridine via syringe and the mixture was stirred for 10 minutes
to produce a clear solution. 1.0 grams (1.05 eq.) of N-
carbethaxyphthalimide (Nefken's reagent, 98% Jannsen Chimica) was
added and the solution was stirred overnight.. Thin layer chromatography
(TLC) showed 90% conversion to a faster moving products (10% ETOH in
CHC13) and 57 pl of-TEA (0.1 eq.) was added to effect closure of the
phthalimide ring. After 1 hour an additional 855 ~I (1.5 eq.) of TEA was
added followed by the addition of 1.53 grams (1.1 eq.) of DMT-C!
CA 02468048 2004-06-07
77
(Lancaster SynthesisC3~, 98°!°}. The reaction mixture was left
to stir
overnight and quenched with ETOH after TLC showed greater than
90°!°
desired product. Dmf was removed under vacuum and the mixture was
washed with sodium bicarbonate solution (5°!° aq., 500 mls) and
extracted
with ethyl acetate (2x 200 mls). A 25mm x 300mm flash column (75 grams
Merck flash silica) was used for purification. Compound eluted at 80 to
85°l° ethyl acetate in hexanes (yield: 80% , purity:
>95°!° by ~ H N M R).
Phosphoramidites were then prepared using standard protocols described
above.
With phosphoramidite 17 in hand applicant synthesized several
ribozymes with 2'-deoxy-2'-amino modifications. Analysis of the synthesis
demonstrated coupling efficiency in 97-98% range. RNA cleavage activity
of ribozymes containing 2'-deoxy-2'-amino-U modifications at U4 and/or
U7 positions (see Figure 1), wherein the 2'-amino positions were either
protected with Fmoc or Pht, was identical. Additionally, complete
deprotection of 2'-deoxy-2'-amino-Uridine was confirmed by base-
composition analysis. The coupling efficiency of phosphoramidite 17 was
not effected over prolonged storage {1-2 months) at low temperatures.
Protecting 2' Position with a SEM Group
There follows a method using the 2'-(trimethylsilyl)ethoxymethyl
protecting group (SEM) in the synthesis of oligoribonucleotides, and in
particular those enzymatic molecules described above. For the synthesis
of RNA it is important that the 2'-hydroxyl protecting group be stable
throughout the various steps of the synthesis and base deprotection. At the
same time, this group should also be readily removed when desired. To
that end the t-butyldimethylsily) group has been efficacious (Usman,N.;
Ogilvie,K.K.; Jiang,M.-Y.; Cedergren,R.J. J. Am. Chem. Soc. 1987, 709,
7845-7854 and Scaringe,S.A.; Franklyn,C.; Usman,N. Nucl. Acids Res.
1990, 18, 5433-5441). However, long exposure times to tetra-n-
butylammonium fluoride (TBAF) are generally required to fully remove this
protecting group from the 2'-hydroxyl. In addition, the bulky alkyl
substituents can prove to be a hindrance to coupling thereby necessitating
longer coupling times, Finally, it has been shown that the TBDMS group is
base labile and is partially deprotected during treatment with ethanolic
ammonia (Scaringe,S.A.; Franklyn,C.; Usman,N. Nucl. Acids Res. 1990,
CA 02468048 2004-06-07
?8
78, 5433-5441 and Stawinski,J.; Stromberg,R.; Thelin,M.; Westman,E.
Nucleic Acids.Res. 1988, 76, 9285-9298).
The (trimethylsilyl)ethoxymethyl ether (SEM) seems a suitable
substitute. This protecting group is stable to base and alt but the harshest
acidic conditions. Therefore it is stable under the conditions required for
oligonucleotide synthesis. It can be readily introduced and the oxygen
carbon bond makes it unable to migrate. Finally, the SEM group can be
removed with BF3~OEt2 very quickly.
There follows a method for synthesis of RNA by protecting the 2'-
position of a nucleotide during RNA synthesis with a
(trimethylsilyl)ethoxymethyl (SEM) group. The method can involve use of
standard RNA synthesis conditions as discussed below, or any other
equivalent steps. Those in the art are familiar with such steps. The
nucleotide used can be any normal nucleotide or may be substituted in
various positions by methods well known in the art, e.g., as described by
Eckstein et aL, International Publication No. WO 92!07065, Perrault et al.,
Nature 1990, 344, 565-568, Pieken et al., Science 1991, 253, 314-317,
Usman,N.; Cedergren,R.J. Trends in Biochem. Sci. 1992, 17, 334-339,
Usman et al., PCT W093/15187, and Sproat,B. European Patent
Application 92710298.4 .
This invention also features a method for covalently linking a SEM
group to the 2'-position of a nucleotide. The method involves contacting a
nucleoside with an SEM-containing molecule under SEM bonding
conditions. In a preferred embodiment, the conditions are dibutyltin oxide,
tetrabutylammonium fluoride and SEM-CI. Those in the -art, however, will
recognize that other equivalent conditions can also be used.
In another aspect, the invention features a method for removal of an
SEM group from a nucleoside molecule or an oligonucleotide. The method
involves contacting the molecule or oligonucleotide with boron trifluoride
etherate (BF3~OEt2) under SEM removing conditions, e.g., in acetonitrile.
Referring to Fic_ur~ e~18, there is shown the method for solid phase
synthesis of RNA. A 2',5'-protected nucleotide is' contacted with a solid
phase bound nucleotide under RNA synthesis conditions to form a
dinucleotide. The protecting group (R) at the 2'-position in prior art
CA 02468048 2004-06-07
79
methods can be a silyl ether, as shown in the Figure. 1n the method of_ the
present invention, an SEM group is used in place of the silyl ether.
Otherwise RNA synthesis can be performed by standard methodology.
Referring to Figure 19, there is shown the synthesis of 2'-O-SEM
protected nucleosides and phosphoramadites. Briefly, a 5'-protected
nucleoside (1 ) is protected at the 2'- or 3'-position by contacting with a
derivative of SEM under appropriate conditions. Specifically, those
conditions include contacting the nucleoside with dibutyltin oxide and SEM
chloride. The 2 regioisomers are separated by chromatography and the 2'-
protected moiety is converted into a phosphoramidite by standard
procedure. The 3'-protected nucleoside is converted into a succinate
derivative suitable for derivatization of a solid support.
Referring to Fioure 20, a prior art method for deprotection of RNA using silyl
ethers is shown. This contrasts with the method shown in Figure 21 in
which deprotection of RNA containing an SEM group is performed. In step
1, the base protecting groups and cyanoethyl groups are removed by
standard procedure. The SEM group is then removed as shown in the
Figure. The details of the synthesis of phosphoramidites and SEM
protected nucleosides and their use in synthesis of oligonucleotides and
subsequent deprotection of
Examofe 14: Synthesis of 2'-O-((trimethylsilyl}ethoxymethyll-5'-O- Di-
methoxytrityl Uridine (21
Referring to Figure 19, 5'-D-dimethoxytrityl uridine 1 (1.0 g, 1.83
mmol) in CH3CN (18 mL) was added dibutyltin oxide (1.0 g, 4.03 mmol)
and TBAF (1 M, 2.38 mL, 2.38 mmol). The mixture was stirred for 2 h at RT
(about 20-25°C) at which time (trimethylsilyl)ethoxymethyl chloride
(SEM-
CI} (487 pL, 2.75 mmol) was added. The reaction mixture was stirred
overnight and then filtered and evaporated. Flash chromatography (30%
hexanes in ethyl acetate) yielded 347 mg (28.0%} of 2'-hydroxyl protected
nucleoside 2 and 314 mg {25.3%) of 3'-hydroxyl protected nucleoside 3.
Exam le 15: nthesis of 2'- trimeth Isil I ethox m h ridin 4
Nucleoside 2 was detritylated following standard methods, as shown
in Fi r 1
CA 02468048 2004-06-07
Example 16: Synthesis of 2'-D-((trimethylsilyllethoxymethyll-5' 3'-O-Acetyl
Uridi
Nucleoside 4 was acetylated following standard methods, as shown
in Fi ure 1 .
5 Example 17: Synthesis of 5'.3'-D-Acet~rl Uridinel6l
Referring to Figure 19. the fully protected uridine 5 (32 mg, 0.07
mmol) was dissolved in CH3CN (700 pL) and BFg~OEtp (17.5 ~tL, 0.14
mmol) was added. The reaction was stirred 15 m and MeOH was added to
quench the reaction. Flash chromatography (5°l° MeOH in CH2C12)
gave
10 20 mg (88°I°) of SEM deprotected nucleoside 6.
Example 18: Synthesis of 2'-D-((trimethylsily~etho~methy~-3'-Q
Succinyl-5'-O- Dimethox~rtrityl l~ridine~21
Nucleoside 3 was succinylated and coupled to the support following
standard procedures, as shown in Figure 19.
15 Example 19: Synthesis of 2'-O-((trimethylsilyl ethoxymethyl -5~Di-
methoxytrityl Uridine 3'-(2-Cyanoethyl N N-diisopropylphosphoramiditel
i~1
Nucleoside 3 was phosphitylated following standard methods, as
shown in Fi ur 1
20 Example 20: Synthesis of RNA Using 2'-O-SEM Protection
Referring to Figure 18, the method of synthesis used follows the
general procedure for RNA synthesis as described in Usman,N.;
Ogilvie,K.K.; Jiang,M.-Y.; Cedergren,R.J. J. Am. Chem. Soc. 1987, 709,
7845-7854 and in Scaringe,S.A.; Franklyn,C.; Usman,N. Nucl. Acids Res.
25 ~ 1990, 78, 5433-5441. The phosphoramidite 8 was coupled following
standard RNA methods to. provide a 10-mer of uridylic acid. Syntheses
were conducted on a 394 (ABI) synthesizer using a modified 2.5 pmol
scale protocol with a 10 m coupling step. A thirteen-fold excess (325 pL of
0.1 M = 32.5 pmol) of phosphoramidite and a 80-fold excess of tetrazole
30 (400 pL of 0.5 M = 200 ~moi) relative to polymer-bound 5'-hydroxyl was
used in each coupling cycle. Average coupling yields on the 394,
determined by colorimetric quantitation of the trityl fractions, were 98-99%.
Other oligonucleotide synthesis reagents for the 394: Detrityiation solution
was 2% TCA in methylene chloride; capping was performed with 16% N-
CA 02468048 2004-06-07
81
Methyl imidazole in THF and 10% acetic anhydride/10% 2,6-lutidine in
THF; oxidation solution was 15.9 mM 12, 49 mM pyridine, 9% water in THF.
Fisher Synthesis Grade acetonitrile was used directly from the reagent
bottle.
Referring to Figure 21, the homopolymer was base deprotected with
NH3/EtOH at 65 °C. The solution was decanted and the support was
washed twice with a solution of 1:1:1 H20:CH3CN:MeOH. The combined
solutions were dried down and then diluted with CH3CN (1 mL). BF3~OEt2
(2.5 ~L, 30 umol) was added to the solution and aliquots were removed at
ten time points. The results indicate that after 30 min deprotection is
complete, as shown in Figure 22.
111. Vectors Expressing Ribozymes
There follows a method fior expression of a ribozyme in a bacterial or
eucaryotic cell, and for production of large amounts of such a ribozyme. In
general, the invention features a method for preparing multi-copy cassettes
encoding a defined ribozyme structure for production of a ribozyme at a
decreased cost. A vector is produced which encodes a plurality of
ribozymes which are cleaved at their 3' and 5' ends from an RNA transcript
producted from the vector by only one other ribozyme. The system is useful
for scaling up production of a ribozyme, which may be either modified or
unmodified, in situ or in vitro. Such vector systems can be used to express
a desired ribozyme in a specific cell, or can be used in an in vitro system to
allow productiuon of large amounts of a desired riboqyne, The vectors of
this invention allow a higher yield synthesis of a ribozyme in the form of an
RNA transcript which is cleaved ~in situ or in vitro before'or after
transcript
isolation.
Thus, this invention is distinct from the prior art in that a single
ribozyme is used to process the 3' and 5' ends of each therapeutic, trans-
acting or desired ribozyme instead of processing only one end, or only one
ribozyme. This allows smaller vectors to be derived with multiple trans-
acting ribozymes released by only one other ribozyme from the mRNA
transcript. Applicant has also provided methods by which the activity of
. such ribozymes is increased compared to those in the art, by designing
ribozyme-encoding vectors and the corresponding transcript such that
CA 02468048 2004-06-07
82
folding, of the mRNA does not interfere with processing by the releasing
ribozyme.
The stability of the ribozyme produced in this method can be
enhanced by provision of sequences at the termini of the riboZymes as
described by t7raper et al., PCT WO 93!23509.
The method of this invention is advantageous since it provides high
yield synthesis of ribozymes by use of low cost transcription-based
protocols, compared to existing chemical ribozyme synthesis, and can use
isolation techniques currently used to purify chemically synthesized
oligonucleotides. Thus, the method allows synthesis of ribozymes in high
yield at low cost for analytical, diagnostic, or therapeutic applications.
The method i5 also useful for synthesis of ribozymes in vifro for
ribozyme structural studies, enzymatic studies, target RNA accessibility
studies, transcription inhibition studies and nuclease protection studies,
much is described by Draper et al., PCT WO 93/23509.
The method can also be used to produce ribozymes in situ either to
increase the intracellular concentration of a desired therapeutic ribozyme,
or to produce a concatarneric transcript for subsequent in vitro isolation of
unit ,length ribozyme. The desired ~ibozyme can be used to inhibit gene
expression in molecular genetic analyses or in infectious cell systems, and
to test the efificacy of a therapeutic molecule or treat afflicted cells.
Thus, in general, the invention features a vector which includes a
bacterial, viral or eucaryotic promoter within a plasmid, cosmid, phagmid,
virus, viroid, virusoid or phage vector..Other vectors are equally suitable
and include double-stranded, or partially double-stranded DNA, formed by
an amplification method such as the polymerase chain reaction, or double-
stranded, partially double-stranded or single-stranded RNA, formed by sife-
directed homologous recombination into viral or viroid RNA genomes.
Such vectors..need not be circular. Transcriptionally linked to the promoter
region is a first ribozyme-encoding region, and nucleotide sequences
encoding a ribozyme cleavage sequence which is placed on either side of
a region encoding a therapeutic or otherwise desired second ribozyme.
CA 02468048 2004-06-07
83
Suitable restriction endonuclease sites can be provided to ease
construction of this vector in DNA vectors or in requisite DNA vectors of an
RNA expression system. The desired second ribozyme may be any
desired type of ribozyme, such as a hammerhead, hairpin , hepatitis delta
virus (HDV) or other catalytic center, and can include group 1 and group II
introns, as discussed above. The first ribozyme is chosen to cleave the
encoded cleavage sequence, and may also be any desired ribozyme, for
example, a Tetrahymena derived ribozyme, which may, for example,
include an imbedded restriction endonuclease site in the center of a self-
recognition sequence to aid in vector construction. This endonuclease site
is useful for construction of the vector, and subsequent analysis of the
vector.
When the promoter of such a vector is activated an RNA transcript is
produced which includes the first and second ribozyme sequences. The
first ribozyme sequence is able to act, under appropriate conditions, to
cause cleavage at the cleavage sites to release the second ribozyme
sequences. These second ribozyme sequences can then act at their target
RNA sites, or can be isolated for later use or analysis.
Thus, in one aspect the invention features a vector which includes a
first nucleic acid sequence (encoding a first ribozyme having
intramolecular cleaving activity), and a second nucleic acid sequence
(encoding a second ribozyme having intermolecular cleaving enzymatic
activity) flanked by nucleic acid sequences encoding RNA which is cleaved
by the first ribozyme to release the second ribozyme from the RNA
transcript encoded by the vector. The second ribozyme may be flanked by
the first ribozyme either on the 5' side or 3' side. If desired, the first
ribozyme may be encoded on a separate vector and may have
. intermolecular cleaving activity.
As discussed above, the first ribozyme can be chosen to be any self-
cleaving ribozyme, and the second ribozyme may be chosen to be any
desired ribozyme. The flanking sequences are chosen to include
sequences recognized by the first ribozyme. When the vector is caused to
express RNA from these nucleic acid sequences, that RNA has the ability
under appropriate conditions to cleave each of the flanking regions and
thereby release one or more copies of the second ribozyme. If desirEd,
several different second ribozymes can be produced by the same vector, or
CA 02468048 2004-06-07
84 '
- several different vectors can be placed in the same vessel or cell to
produce different ribozymes.
In prefierred embodiments, the vector includes a plurality of the nucleic .
acid sequences encoding the second ribozyme, each flanked by nucleic
acid sequences recognized by the first ribozyme. Most preferably, such a
plurality includes at least six to nine or even between 60 - 100 nucleic acid
sequences. In other preferred embodiments, the vector includes a
promoter which regulates expression of the nucleic acid encoding the
ribozymes from the vector; and the vector' is chosen from a plasmid,
cosmid, phagmid, virus, viroid or phage. In a most preferred embodiment,
the plurality of nucleic acid sequences are identical and are arranged in
sequential order such that each has an identical end nearest to the
promoter. If desired, a poly(A) sequence adjacent to the sequence
encoding the first or second ribozyme may be provided to increase stability
of the RNA produced by the vector; and a restriction endonuclease site
adjacent to the nucleic acid encoding the first ribozyme is provided to atfow
insertion of nucleic acid encoding the second ribozyme during construction
of the vector.
In a second aspect, the invention features a method for formation of a
ribozyme expression vector by providing a vector including nucleic acid
encoding a first ribozyme, as discussed above, and providing a single-
stranded DNA encoding a second ribozyme, as discussed above. The
single-stranded DNA is then allowed to anneal to form a partial duplex
DNA which can be filled in by a treatment with an appropriate enzyme,
such as a DNA polymerise in the presence of dNTPs, to form a duplex
DNA which can then be ligated to the vector. Large vectors resulting from
this method can then be selected to insure that a high copy number of the
single-stranded DNA encoding the second ribozyme is incorporated into
the vector.
In a further aspect, the invention features a method for production of
ribozymes by providing a vector as described above, expressing RNA from
that vector, and allowing cleavage by the first ribozyme to release the
second ribozyme.
In preferred embodiments, three different ribozyme motifs are used as
cis-cleaving ribozymes. The hammerhead, hairpin, and hepatitis delta
CA 02468048 2004-06-07
' ~ 85
virus (HDV) ribozyme motifs consist of small, well-defined sequences that
rapidly self-cleave in vitro (Symons, 1992 Annu. Rev. Biochem. 61, 641).
While structural and functional differences exist among the three ribozyme
motifs, they self-process efficiently in vivo. A11 three ribozyme motifs self-
process to 87-95% completion in the absence of 3' flanking sequences. In
vitro, the self-processing constructs described in this invention are
significantly more active than those reported by Taira et al., 1990 su r ;
and Altschuler et al., 1992 .G_ene 122, 85. The present invention enables
the use of cis-cleaving ribozymes to efficiently truncate RNA molecules at
specific sites in vivo by ensuring lack of secondary structure which
prevents processing.
fsoiation of Therapeutic RiboZ~rme
The preferred method' of isolating therapeutic ribozyme is by a
chromatographic technique. The HPLC purification methods and reverse
HPLC purification methods described by Draper et al., PCT WO 93/23509.
can be used. Alternatively, the
attachment of complementary oligonucleotides to cellulose or other
chromatography columns allows isolation of the therapeutic second
ribozyme, for example, by hybridization to the region between the flanking
arms and the enzymatic~RNA. This hybridization will select against.the
short flanking sequences without the desired enzymatic RNA, and against
the releasing first ribozyme. The hybridization can be accomplished in the
presence of a chaotropic agent to prevent nuclease degradation. The
oligonucleotides on the matrix can be modified to minimize .nuclease
activity, for example, by provision of 2'-O-methyl RNA oligonucleotides.
Such modifications of the oligonucleotide attached to the column matrix will
allow the multiple use of the column with minimal oligo degradation. Many
such modifications a.re known in the art; but a chemically stable non-
reducible modification is preferred. For example, phosphorothioate
modifications can also be used. w
The expressed ribozyme RNA can be isolated from bacterial or
eucaryotic cells by routine procedures such as lysis followed by guanidine
isothiocyanate isolation.
The current known self-cleaving site of Teirahymena can be used in
an alternative vector of this invention. If desired, the full-length
CA 02468048 2004-06-07
as
Tetrahymena sequence may be used, or a shorter sequence may be used.
It is preferred that, in order to decrease the superfluous sequences in the
self-cleaving site at the 5' cleavage end, the hairpin normally present in the
Tetrahymena ribozyme should contain the therapeutic second ribozyme 3'
sequence and its complement. That is, the first releasing ribozyme-
encoding DNA is provided in two portions, separated by DNA encoding the
desired second ribozyme. For example, if the therapeutic second ribozyme
recognition sequence is CGGACGA/CGAGGA, then CGAGGA is provided
in the self-cleaving site loop such that it is in a stem structure recognized
by
the Tetrahymena ribozyme. The loop of the stem may include a restriction
endonuclease site into which the desired second ribozyme-encoding DNA
is placed.
If desired, the vector may be used in a therapeutic protocol by use of
the systems described by Lechner, PCT WO 92/13070,
to allow a timed expression of the
therapeutic second ribozyme, as well as an appropriate shut off of cell or
gene function. Thus, the vector wilt include a promoter which appropriately
expresses enzymatically active RNA only in the presence of an RNA or
another molecule which indicates the presence of an undesired organism
or state. Such enzymatically active RNA wiH then kill or harm the cell in
which it exists, as described by Lechner, id., or act to cause reduced
expression of a desired protein product.
A number of suitable RNA vectors may also be used in this invention.
The vectors include plant viroids, plant viruses which contain single or
double-stranded RNA genomes and animal viruses which .contain RNA
genomes, such as the picornaviruses, myxoviruses, paramyxoviruses,
hepatitis A virus, reovirus and retroviruses. In many instances cited, use of
these 'viral vectors also results in tissue specific delivery of the
ribozymes.
Example 21: Desi4n of self-processing cassettes
In a preferred embodiment, applicant compared the in vitro and in
vivo cis-cleaving activity of threedifferent ribozyme motifs-the
hammerhead, the hairpin and the hepatitis delta virus ribozyme-in order to
assess their potential to process the ends of transcripts in vivo. To make a
direct comparison among the three, however, it is important to design the
ribozyme-containing transcripts to' be as similar as possible. To this end,
CA 02468048 2004-06-07
8T
all the ribozyme cassettes contained the same trans-acting hammerhead
ribozyme followed immediately by one of the three cis-acting ribozymes
(Fioure 23-25). For simplicity, applicant refers to each cassette by an
abbreviation that indicates the downstream cis-cleaving ribozyme only.
Thus HH refers to the cis-cleaving cassette containing a hammerhead
ribozyme, while HP and HDV refer to the cassettes containing hairpin and
hepatitis delta virus cis-cleaving ribozymes, respectively. The general
design of the ribozyme cassettes, as well as specific differences among the
cassettes, are outlined below.
A sequence predicted to form a stable stem-loop structure is included
at the 5' end of all the transcripts. The hairpin stem contains the T7 RNA
polymerise initiation sequence (Milligan & Uhlenbeck, 1989 Methods
Enzymol. 180, 51) and its complement, separated be a stable tetra-loop
(Antao et al., 1991 Nucleic Acids Res. 19, 5901). By incorporating the T7
initiation sequence into a stem-loop structure, applicant hoped to avoid
nonproductive base pairing interactions with either the traps-acting
ribozyme or with the cis-acting ribozyme. The presence of a hairpin at the
end of a transcript may also contribute to the stability of the transcript in
vivo. These are non-limiting examples. Those in the art will recognize that
other embodiments can be readily generated using a variety of promoters,
initiator sequences and stem-loop structure combinations generally known
in the art.
The traps-acting ribozyme used in this study is targeted to a site B
(5'~~~CUGGAGU_C~GACCUUC~-~3'). The 5' binding arm of the ribozyme, 5'-
GAAGGUC-3', and the core of the ribozyme, 5'-
CUGAUGAGGCCGAAAGGCCGAA-3', remain constant in all cases. In
addition, all transcripts also contain a single nucleotide between the 5'
stem-loop and the first nucleotide of the ribozyme. The linker nucleotide
was required to obtain the same activity in vitro that was measured with an
identical ribozyme Packing the 5' hairpin. Because the three cis-cleaving
ribozymes have different requirements at the site of cleavage, slight
differences were unavoidable at the 3' end of the processed transcript. The
junction between the traps- and cis-acting ribozyme is; however, designed
so that there is minimal extraneous sequence left at the 3' end of the trans-
cleaving ribozyme once cis-cleavage occurs. The only differences
between the constructs lie in the 3' binding arm of the riboZyrne, where
CA 02468048 2004-06-07
88
either 6 or 7 nucleotides, 5'-ACUCCA(+!-G)-3', complementary to the target
sequence are present and where, after processing, two to five extra
nucleotides remain.
The cis-cleaving hammerhead ribozyme used in the HH cassette is
based on the design of Grosshans and Cech, 1991 su ra. As shown in
Figure 23, the 3' binding arm of the traps-acting ribozyme is included in the
required base-pairing interactions of the cis-cleaving ribozyme to form stem
I. Two extra nucleotides, UC, were included at the end of the 3' binding
arm to form the self-processing hammerhead ribozyme site (Ruffner et al.,
1990 supra) which remain on the 3' end of the traps-acting ribozyme
following self-processing.
The hairpin ribozyme portion of the HP self-processing construct is
based on the minima! wild-type sequence (Hampel & Tritz, 1989 su ra). A
tetra-loop at the end of helix 1 (3' side of the cleavage site) serves to link
the two portions and thus allows a minimal five nucleotides to remain at the
end of the released traps-acting ribozyme following self-processing. Two
variants of HP were designed: HP(GU) and HP(GC). The HP(GU) was
constructed with a G~U wobble base pair in helix 2 (A52G substitution;
Fioure 4). This slight destabilization of helix 2 was intended to improve
self-processing, activity by promoting product release and preventing the
reverse reaction (Berzal-Herranz et al., 1992 Genes & Dev. 6, 129;
Chowrira et al., 1993 Biochemistry 32, 1088). The HP(GC) cassette was
constructed as a control for strong base-pairing interactions in helix 2
(U~7C and A52G substitution; Figure 24). Another modification to
discourage the reverse ligation reaction of the hairpin ribozyme was to
shorten helix 1 (Fioure 24) by one base pair relative to the wild-type
sequence (Chowrira & 8urke, 1991 Biochemistry 30, 8518).
The HDV ribozyme self-processes efficiently when the nucleotide 5' to
the cleavage site is a pyrimidine, and somewhat less so when adenosine is
in that position. No other sequence requirements have been identified
upstream of the cleavage site, however, we have observed some decrease
in activity when a stem-loop structure was present within 2 nt of the
cleavage site. The HDV self-processing construct (F_ is 25) was designed to
generate the traps-acting hammerhead ribozyme with only two additional
nucleotides at its 3' end after self-processing. The HDV sequence used
here is based on the anti-genomic sequence (Perrota 8~ Been, 1992 su ra)
CA 02468048 2004-06-07
89
but includes the modifications of Been et al., 1992 (~iochemistw 31,
11843) in which cis-cleavage activity of the ribozyme was improved by the
substitution of a shortened helix 4 for a wild-type stem-loop (Fi ur ,
To prepare DNA inserts that encode self-processing ribozyme
cassettes, partially overlapping top- and bottom-strand oligonucleotides
(60-90 nucleotides) were designed to include sequences for the T7
promoter, the trans-acting ribozyme, the cis-cleaving ribozyme and
appropriate restriction sites for use in cloning (see Fiq_ 261, The single-
strand portions of annealed oligonucleotides were converted to doubie-
strands using Sequenase~ (U.S. Biochemicals). Insert DNA was ligated
into EcoR7lHind111-digested pucl8 and transformed into E. coli strain DHSa
using standard protocols (Maniatis et al., 1982 in Molecular Cloning Cold
Spring Harbor Press). The identity of positive clones was confirmed by
sequencing small-scale plasmid preparations.
Larger scale preparations of plasmid DNA for use as in vitro
transcription templates and in transactions were prepared using the
protocol and columns from OIAGEN Inc. (Studio City, CA) except that an
additional ethanol precipitation was included as the final step.
Example 22. RNA Processing in vitr,Q
Transcription reactions containing linear plasmid templates were
carried out essentially as described (Milligan & Uhlenbeck, 1989 Su r ;
Chowrira 8 Burke, 1991 a ra). In order to prepare 5' end-labeled
transcripts, standard transcription reactions were carried out in the
presence of 10-20 pCi [7-32P)GTP, 200 pM each NTP and 0.5 to 1 ~g of
linearized plasmid template. The concentration of MgCl2 was maintained
at 10 mM above the total nucleotide concentration.
To compare the ability of the different ribozyme cassettes to self-
process in vitro, each construct was transcribed and allowed to undergo
self-processing under identical conditions at 37°C. For these
comparisons,
equal amounts of linecrized DNA templates bearing the various ribozyme
cassettes were transcribed in the presence of ~[Y-32p~GTP to generate 5'
end-labeled transcripts. In this manner only the full-length, unprocessed
transcripts and the released trans-ribozymes are visualized by
autoradiography. In all reactions, Mg2+ was included at 10 mM above the
nucleotide concentration so that. cleavage by all the ribozyme cassettes
CA 02468048 2004-06-07
9~ .
would be supported. Transcription templates were linearized at several
positions by digestion with different restriction enzymes so that self-
processing in the presence of increasing lengths of downstream sequence
could be compared (see Fig. 26). The resulting transcripts have either 4-5
non-ribozyme nucleotides at the 3' end (Hind111-digested template), 220
nucleotides (Ndel digested templates) or 454 nucleotides of downstream
sequence (Real digested template). .
As shown in Figure 27, all four ribozyme cassettes are capable of self-
processing and yield RNA products of expected sizes. Two nucleotides
essential for hammerhead ribozyme activity (Ruffner et al., 1990 supra)
have been changed in the HH(mutant) core sequence (see Figure 23) and
so this transcript is unable to undergo self-processing (Fi . 2 ). This is
evidenced by the lack of a released 5' RNA in the HH(mutant), although the
full-length RNAs are present . Comparison of the amounts of released
traps-ribozyme (Fia~27) indicate that there are differences in the ability of
these ribozymes to self-process in vitro, especially with respect to the
presence of downstream sequence. For the two HP constructs, it is clear
that HP(GG) is more efficient than the HP(GU) ribozyme, both in the
presence and in the absence of extra downstream sequence. In addition,
the aclivity of HP(GU) falls off more dramatically when downstream
sequence is present. The stronger G:C base pair likely contributes to the
HP(GC) construct's ability to fold correctly (and/or more quickly) into the
productive structure, even when as much as 216 extra nucleotides are
present downstream. The HH- ribozyme construct is also quite efficient at
self-processing, and slightly better than the HP(GU) construct even when
downstream sequence is present.
Of the three ribozyme motifs, the presence of extra downstream
sequence seems to most affect the efficiency of HDV. When no extra
sequence is present downstream, HDV is quite efficient and self-processes
to approximately the same level as the HH and HP(GC) cassettes.
However, when extra downstream sequence is present, the self-processing
activity seems to decrease almost as dramatically as is seen with the (sub-
optimal) HP(GU) casserie.
CA 02468048 2004-06-07
9t
Example 23' Kinetics of self-orocessina reaction
Hindlll-digested template (250 ng) was used in a standard
transcription reaction mixture containing: 50 mM Tris~HCl pH 8.3; 1 mM
ATP, GTP and UTP; 50 ~M CTP; 40 pCi jcc-32P]CTP; 12 mM MgCl2; 10 mM
DTT. The transcription/self-processing reaction was initiated by the
addition of T7 RNA polymerise (15 U/pl). Aliquots of 5 ~I were taken at
regular time intervals and the reaction was stopped by adding an equal
volume of 2x formamide loading buffer (95% formamide, 15 mM EDTA, 8~
dyes) and freezing on dry ice. The samples were resolved on a
10°!°
pofyacrylamide sequencing gel and results were quantitated by
Phosphorlmager*(Molecuiar Dynamics, Sunnyvale, CA). Ribozyme self=
cleavage rates were determined from non-linear, least-squares fits
(KaleidaGraph, Synergy Software,Reeding, PA) of the data to the equation:
(Fraction Uncleaved Transcript) _ ~ (1-e-~)
where t represents time and k represents the unimolecular rate
constant for cleavage (Long ~ Uhlenbeck, 1994 Proc. Natl. Acid. Sci USA
91, 6977).
Linear templates were prepared by digesting the plasmids with Hindlll
so that transcripts will contain only four to five vector-derived nucleotides
at
the 3' end (see Fioure 23-25). By comparison of the unimolecular rate
constant (k) determined for each construct, it is clear that HH is the most
efificient at self-processing (Table 44). The HH transcript self-processes 2-
fold fester than HDV and 3-fold faster than HP(GC) transcripts. Although
the HP(GU.) RNA undergoes self-processing, it is at least 6-fold slower than
the HP(GC) construct. This is consistent with previous observations that
the stability of helix 2 is essential for self-processing and traps-cleavage
activity of the hairpin ribozyme (Hampel et al:, 1990 su ra; Chowrira 8~
Burke, 1991 su ra). The rate of HH self-cleavage during transcription
measured here (1.2 min-1) is similar to the rate measured by Long and
3'D Uhlenbeck 1994 s_u_pra using a HH that has a different stem I and stem
II1.
Self-processing rates during transcription for HP and HDV have not been
previously reported. However, self-processing of the HDV ribozyme-as
measured here during transcription-is significantly slower than when
testedafter isolation from a denaturing gel (Been et al., 1992 su ra . This
decrease likely reflects the.difference in protocol as well as the presence of
5' flanking sequence in the HDV construct used here.
*Trade-mark
CA 02468048 2004-06-07
92 '
Example 24: Effect of downstream seauences on tram-cleavage in viirg
Transcripts containing the traps ribozyme with or without 3' flanking
sequences were assayed for their ability to cleave their target in traps. To
this end, transcripts from three templates were resolved on a preparative
gel and bands corresponding both to processed traps-acting ribozymes
from the HH transcription reaction, and to full-length HH{mutant) and ~HDV
transcripts were isolated. In all three transcripts the traps-acting ribozyme
portion is identical-with the exception of sequences at their 3' ends. The
HH traps-acting ribozyme contains only an additional UC at its 3' end,
while HH(mutant) and aHDV have 52 and 37 nucleotides, respectively, at
their 3' ends. A 622 nucleotide, internally-labeled target RNA was
incubated, under ribozyme excess conditions, along withi the three
ribozyme transcripts in a standard reaction buffer.
To make internally-labeled substrate RNA for traps-ribozyme
cleavage reactions, a 622 nt region (containing hammerhead site P) was
synthesized by PCR using primers that place the T7 RNA promoter
upstream of the amplified sequence. Target RNA was transcribed in a
standard transcription buffer in the presence of [a-32P]CTP (Chowrira 8
Burke, 1991 suflra). The reaction mixture was treated with 15 units of
ribonuclease-free DNasel,, extracted with phenol followed
chloroform:isoamyl alcohol (25:1), precipitated with isopropanol and
washed with 70% ethanol. The dried pellet was resuspended in 20 pi
DEPC-treated water and stored at -20°C.
Unlabeled ribozyme (1pM) and internally labeled 622 nt substrate
RNA (<10 nM) were denatured and renatured separately in a standard
cleavage buffer (containing 50 mM Tris~HCl pH 7.5 and 10 mM MgCl2) by
heating to 90°C for 2 min, and slow cooling to 37°C for 10 min.
The
reaction was initiated by mixing the ribozyme and substrate mixtures and
incubating at 37°C. Aliquots of 5 ul were taken at regular time
intervals,
quenched by adding an equal volume of 2X forinamide gel loading buffer
and frozen on dry ice. The samples were resolved on 5% polyacrylamide
sequencing gel and results were quantitatively analyzed by radioanalytic
imaging of gels with a Phosphorlmager~ (Molecular Dynamics; Sunnyvale,
CA).
The HH traps-acting ribozyme cleaves the target RNA approximately
10-fold faster than the oHDV transcript and greater than 20-fold faster than
CA 02468048 2004-06-07
93
the HH(mutant) transcript (Fi ure 2 ). The additional nucleotides at the
end of HH(mutant) form 7 base-pairs with the 3' target-binding arm of the
traps-acting ribozyme (Fi ure 3). This interaction must be disrupted (at a
cost of 6 kcallmole) to make the traps-acting ribozyme available for binding
the target sequence.. In contrast, the additional nucleotides at the end of
~HDV were not designed to form any strong, alternative base-pairing with
the traps-ribozyme. Nevertheless, the aHDV sequences are predicted to
form multiple structures involving the 3' target-binding arm of the traps
ribozyme that have stabilities ranging from 1-2 kcallmole. Thus, the
observed reductions in activity for the ~HDV and HH(mutant) constructs are
consistent with the predicted folded structures, and it reinforces the view
that the flanking sequences can decrease the catalytic efficiency of a
ribozyme through nonproductive interactions with either the ribozyme or
the substrate or both.
Exam,~le 25: RNA self-processing in vivo
Since three of the constructs (HH, HDV and HP(GC)) self-process
efficiently in solution, the affect of the mammalian cellular milieu on
ribozyme self-processing was next explored by applicant. A transient
expression system was employed to investigate ribozyme activity in vivo. A
mouse cell line (OST7-1) that constitutively expresses T7 RNA polymerase
in the cytoplasm was chosen for this study (Elroy-Stein and Moss, 1990
Proc. Natl. Acad. Sci. USA 87, 6743). In these cells plasmids containing a
ribozyme cassette downstream of the T7 promoter will be transcribed
efficiently in the cytoplasm (Elroy-Stein & Moss, 1990 supra).
Monolayers of a mouse L9 fibroblast cell line (OST7-1; Elroy-Stein
and Moss, 1990 supra) were growr5 in 6-well plates with - 5x105 cells/virell.
Cells were transfected with circular plasmids (5 pglwell) using the calcium
phosphate-DNA precipitation method (Maniatis et al., 1982 su ra). Cells
were lysed (4 hours post-transfection) by the addition of standard lysis
buffer (200 ullwell) containing 4M guanadinium isothiocyanate, ,25 mM
sodium citrate (pH 7.0), 0.5% sarkosyl (Chomczynski and Sacchi, 1987
Anal. Biochem. 162, 156), and 50 mM EDTA pH 8Ø The lysate was
extracted once with water-saturated phenol followed by one extraction -with
chloroform:isoamyl alcohol (25:1 ). Total cellular RNA was precipitated with
an equal volume of isopropanol. The RNA pellet was resuspended in 0.2
CA 02468048 2004-06-07
sa
M ammonium acetate and reprecipitated with ethanol. The pellet was then
washed with 70% ethanol and resuspended in DEPC-treated water.
Purified cellular RNA (3 Irg/reaction) was first denatured in the
presence of a 5' end-labeled DNA primer (100 pmol) by heating to 90°C
for
2 min. in the absence of Mg2+, and then snap-cooling on ice for at least 15
min. This protocol allows for efficient annealing of the primer to its
complement~a~ry RNA sequence. The primer was extended using
Superscript II reverse transcriptase (8 U/~I; BRL) in a buffer containing 50
mM Tris~HCl pH 8.3; 10 mM DTT; 75 mM KCI; 1 mM MgCl2; i mM each
dNTP. The extension reaction was carried out at 42°C for 10 min. The
reaction was terminated by adding an equal volume of 2x formamide gel
loading buffer and freezing on crushed dry ice. The samples were
resolved on a 10°~o polyacrylamide sequencing gel. The primer sequences
are as follows: HH primer, 5'-CTCCAGTTTCGAGCTTT-3 ; HDV primer, 5'-
AAGTAGCCCAGGTCGGACC-3'; HP primer, 5'-
ACCAGGTAATATACCACAAC-3'.
As shown in Figure 29. specific bands corresponding to full-length
precursor RNA and 3' cleavage products were detected from cells
transfected with the self-processing cassettes. All three constructs, in
addition to being transcriptionally active, appear to self-process efficiently
in the cytoplasm of OST7-1 cells. In particular, the HH and HP{GC)
constructs self-process to greater than 95%. The overall extent of self-
processing in OST7-1 cells appears to be strikingly similar to the extent of
self-processing in vitro {Fi ure 2 "In Vitro +MgCl2" vs. "Cellular").
Consistent with the in vitro self-processing results, the HP(GU)
cassette self-processed to approximately 50% in OST7-1 cells. As
expected, transfection with, plasmids containing the HH(mutant) cassette
yielded a primer-extension product corresponding to the full-length RNA
with no detectable cleavage products {Figure 29). The latter result strongly
suggests that the primer extension band corresponding to the 3' cleavage
product is not an artifact of reverse transcription. ~ .
Applicant was concerned with the possibility that RNA self-processing
might occur during cell lysis, RNA isolation and for the primer extension
assay. Two precautions were taken~to exclude this possibility. First, 50 mM
EDTA was included in the lysis buffer. EDTA is a strong chelator of divalent
TY~- ~.~.
CA 02468048 2004-06-07
metal ions such as Mg2+ and Ca2+ that are necessary for ribozyme
activity. Divalent metal ions are therefore unavailable to self-processing
RNAs following cell lysis. A second precaution involved using primers in
the primer-extension assay that were designed to hybridize to essential
5 regions of the processing ribozyme. Binding of these primers . should
prevent the 3' cis-acting ribozymes from folding into the conformation
essential for catalytic activity.
Two experiments were carried out to further eliminate the possibility
that self-processing is occurring either during RNA preparations or during
10 the primer extension analysis. The first experiment involves primer
extension analysis on full-length precursor RNAs that were added to non-
transfected OST7-1 lysates after cell lysis. Thus, only if self-processing is
occurring at some point after lysis would cleavage products be detected.
Full-length precursor RNAs were prepared by transcribing under conditions
15 of low Mg2+ (5 mM) and high NTP concentration (total 12 mM) in an
attempt to eliminate the free Mg2+ required for the self-processing reaction
(Michel et al. 1992 Genes 8 Dev. 6, 1373). The full-length precursor RNAs
were gel-purified, and a known amount was added to fysates of non-
transfected OST7-1 cells. RNA was purified from these lysates and
20 incubated for 1 hr in DEPC-treated water at 37o C prior to the standard
primer extension analysis (Figure 29, in vitro "-MgCf2" control). The
predominant RNA detected in all cases corresponds to the primer
extension product of full-length precursor RNAs. lf, instead, the purified
RNA containing the full-length precursor is incubated in 10 mM MgCl2 prior
25 to.the primer extension analysis, most or all of the RNA detected by primer
extension analysis undergoes cleavage (Figure 29, in vitro "+MgCl2"
control). These results indicate that the standard RNA isolation and primer
extension protocols used here do not provide a favorable environment for
RNA self-processing, even though the RNA in question is inherently able to
30 undergo self-cleavage.
In a second experiment to demonstrate lack of self-processing during
work up, internally-labeled precursor RNAs were prepared and added to
non-transfected OST7-1 lysates as in the previous control. The internally-
labeled precursor RNAs were carried through the RNA purification and
35 primer extension reactions (in the presence of unlabeled primers) and
analyzed to determine the extent of self-processing. 8y this analysis, the
CA 02468048 2004-06-07
ss
vast majority of the added full-length RNA remained intact during the entire
process of RNA isolation and primer extension.
These two control experiments validate the protocols used and .
support applicant's conclusion that the self-processing reactions catalyzed
by HH, HDV and HP(GC} cassettes are occurring in the cytoplasm of
~ST7-1 cells.
Sequences in figures 23 through 25 are meant to be non-limiting
examples. Those in the art will recognize that other embodiments can be
readily generated using techniques generally known in the art.
In addition, those in the art will recognize that Applicant provides
guidance through the above examples as to how to best design vectors of
this invention so that secondary structure of the mRNA allows efficient
cleavage by releasing ribozymes. Thus, the specific constructs are not
limiting in this invention. Such constructs can be readily tested as
described above for such secondary structure, either by computer folding
algorithms or empirically. Such constructs will then allow at least
80°l0
completion of release of ribozymes, which can be readily determined as
described above or by methods known in the art. That is, any such
secondary structure in the RNA does not reduce release of the ribozymes
by more than 20°t°.
IV. Ribozvmes Expressed by RNA Polymerase lil
Applicant has determined that the level of production of a foreign
RNA, using a RNA polymerase III (pol III) based system, can be significantly
enhanced by ensuring that the RNA is produced with the 5' terminus and a
3' region of the RNA molecule base-paired together to form a stable
intramolecular stem structure. This stem structure is formed by hydrogen
bond interactions (either Watson-Crick or non-Watson-Crick) between
nucleotides in the 3' region (at least 8 bases) and complementary
nucleotides in the 5' terminus of the same RNA molecule.
Although the example provided below involves a type 2 pol ill gene
unit, a number of other pol Ill promoter systems can also be used, for
example, tRNA (Hall et al., 1982 Cell29, 3-5), 5S RNA {Nielsen et al.,
7 993, Nucleic Acids Res. 21, 3631-3636), adenovirus VA RNA (Fowlkes
and Shenk, 1980 Cell 22, 405-413), U6 snRNA (Gupta and. Reddy, 1990
CA 02468048 2004-06-07
s7
Nucleic Acids Res. 19, 2073-2075), vault RNA (Kickoefer et al., 1993 J.
Biol. Chem. 268, 7868-7873), telomerase RNA (Romero and Blackburn,
1991 Cell67, 343-353), and others.
The construct described in this invention is able to accumulate RNA to
a significantly higher level than other constructs, even those in which 5'
and 3' ends are involved in hairpin loops. Using such a construct the level
of expression of a foreign RNA can be increased to between 20,000 and
50,000 copies per cell. This makes such constructs, and the vectors
encoding such constructs, excellent for use iri decoy, therapeutic editing
and antisense protocols as well as for ribozyme formation, in addition, the
molecules can be used as agonist or antagonist RNAs (affinity RNAs).
Generally, applicant believes that the intramolecular base-paired
interaction betwean the 5' terminus and the 3' region of the RNA should be
in a double-stranded structure in order to achieve enhanced RNA
accumulation.
Thus, in one preferred embodiment the invention features a pol II1
promoter system (e.g,s, a type 2 system) used to synthesize a chimeric RNA
molecule which includes tRNA sequences and a desired RNA (e~a., a
tRNA-based molecule).
The following exemplifies this invention with a type 2 pol I11 promoter
and a tRNA gene. Specifically to illustrate the broad invention, the RNA
molecule in the following example has an A box and a B box of the type 2
pol III promoter system and has a 5' terminus or region able to base-pair
with at least 8 bases of a complementary 3' end or region of the same RNA
molecule. This is meant to be a specific example. Those in the art will
recognize that this is but one example, and other embodiments can be
readily generated using other pol 111 promoter systems and techniques
generally known in the art.
By "terminus" is meant the terminal bases of an RNA~molecule, ending
in a 3' hydroxyl or 5' phosphate or 5' cap moiety. By "region° is meant
a
stretch of bases 5' or 3' from the terminus that are involved in base-paired
interactions. It need not be adjacent to the end of the RNA. Applicant has
determined that base pairing of at least one end of the RNA molecule with
a region not more than about 50 bases, and preferably only 20 bases, from
CA 02468048 2004-06-07
the other end of the molecule provides a useful molecule able to be
expressed at high levels.
By "3' region" is meant a stretch of bases 3' from the terminus that are
involved in intramolecular bas-paired interaction with complementary
nucleotides in the 5' terminus of the same molecule. The 3' region can be
designed to include the 3' terminus. The 3' region therefore is z 0
nucleotides from the 3' terminus. For example, in the S35 construct
described in the present invention (Fi . 4 the 3' region is one nucleotide
from the 3' terminus. 1n another example, the 3' region is - 43 nt from 3'
terminus. These examples are not meant to be limiting. Those in the art
will recognize that other embodiments can be readily generated using
techniques generally known in the art. Generally, it is preferred to have the
3' region within 100 bases of the 3' terminus.
By "tRNA molecule" is meant a type 2 pol Ill driven RNA molecule that
is generally derived from any recognized tRNA gene. Those in the art will
recognize that DNA encoding such molecules is readily available and can
be modified as desired to alter one or more bases within the DNA encoding
the RNA molecule and/or the promoter system. Generally, but not always,
such molecules include an A box and a B box that consist of sequences
which are well known in the art (and examples of which can be found
throughout the literature). These A and B boxes have a certain consensus
sequence which is essential for a optimal pol III transcription. ,
By "chimeric tRNA molecule" is meant a RNA molecule that includes a
pol III promoter (type 2) region. A chimeric tRNA molecule, for example,
might contain an intramolecular base-paired structure between the 3'
region and complementary 5' terminus of the molecule, and includes a
foreign RNA sequence at any location within the molecule which does not
affect the activity of the type 2 pol III promoter boxes. Thus, such a foreign
RNA may be provided at the 3' end of the B box, or may be provided in
between the A and the B box, with the B box moved ~to an appropriate
location either within the foreign RNA or another location such that it is
effective to provide pol III transcription. In one example, the. RNA molecule
may include a hammerhead ribozyme with the B box of a type 2 pol 111
promoter provided in stem II of the ribozyme. In a second example, the B
box may be provided in stem IV region of a hairpin ribozyme. A specific
example of such RNA molecules .is provided below. Those in the art will
CA 02468048 2004-06-07
~99
recognize that this is but one example, and other embodiments can be
readily generated using techniques generally known in the art.
By "desired RNA" molecule is meant any foreign RNA molecule which
is useful from a therapeutic, diagnostic, or other viewpoint. Such
molecules include antisense RNA molecules, decoy RNA molecules,
enzymatic RNA, therapeutic editing RNA and agonist and antagonist RNA.
By "antisense RNA" is meant a non-enzymatic RNA molecule that
binds to another RNA (target RNA) by means of RNA-RNA interactions and
alters the activity of the target RNA (Eguchi et al., 1991 Annu. Rev.
Biochem. 60, 631-652). By "enzymatic RNA" is meant an RNA molecule
with enzymatic activity (Cech, 1988 J.American. Med. Assoc. 260, 3030-
3035). Enzymatic nucleic acids (ribozymes) act by first binding to a target
RNA. Such binding occurs through the target binding portion of a
enzymatic nucleic acid which is held in close proximity to an enzymatic
portion of the molecule that acts to cleave the target RNA. Thus, the
enzymatic nucleic acid first recognizes and then binds a target RNA
through base-pairing, and once bound to the correct site, acts
enzymatically to cut the target RNA.
By "decoy RNA" is meant an RNA molecule that mimics the natural
binding domain for a ligand. The decoy RNA therefore competes with
natural binding target for the binding of a specific ligand. For example, it
has been shown that over-expression of HIV trans-activation response
(TAR) RNA can act as a "decoy" and efficiently binds H!V tat protein,
thereby preventing it from binding to TAR sequences encoded in the HIV
RNA (Sullenger et al., 1990 Cell 63, 601-608). This is meant to be a
specific example. Those in the art will recognize that this is but one
example, and other embodiments can be readily generated using
techniques generally known in the art.
By "therapeutic editing RNAn is meant an antisense RNA that can bind
to its cellular target (RNA or DNA) and mediate the modification of a
specific base.
By "agonist RNA" is meant an RNA molecule that can bind to protein
receptors with high affinity and cause the stimulation of specific cellular
pathways. .
CA 02468048 2004-06-07
boo '
By "antagonist RNA" is meant an RNA molecule that can bind to
cellular proteins and prevent it from performing its normal biological
function (for example, see Tsai et af., 1992 Proc. Natl. Acad. Sci. USA 89,
8864-8868).
In other aspects, the invention includes vectors encoding ~ RNA
molecules as described above, cells including such vectors, methods for
producing the desired RNA, and use of the vectors and cells to produce this
RNA.
Thus, the invention features a transcribed non-naturally occuring RNA
molecule which includes a desired therapeutic RNA portion and an
intramolecular stem formed by base-pairing interactions between a 3'
region and complementary nucleotides at the 5' terminus in the RNA. The
stem preferably includes at least 8 base pairs, but may have more, for
example, 15 or 16 base pairs.
In preferred embodiments, the 5' terminus of the chimeric tRNA
includes a portion of the precursor molecule of the primary tRNA molecule,
of which >_ 8 nucleotides are involved in base-pairing interaction with the 3'
region; the chimeric tRNA contains A and B boxes; natural sequences 3' of
the B box are deleted, which prevents endogenous RNA processing; the
desired RNA molecule is at the 3' end of the B box; the desired RNA
molecule is between the A and the B box; the desired RNA molecule
includes the B box; the desired RNA molecule is selected from the group
consisting of antisense RNA, decoy RNA, therapeutic editing RNA,
enzymatic RNA, agonist RNA and antagonist RNA; the molecule has an
intramoiecular stem resulting from a base-paired interaction between the 5'
terminus of the RNA and a complementary 3' region within the same RNA,
and includes at least 8 bases; and the 5' terminus is able to base pair with
at least 15 bases of the 3' region.
In most preferred embodiments,, the molecule is transcribed by a RNA
polymerase 111 based promoter system, e.g., a type 2 pol 111 promoter
system; the molecule is a chimeric tRNA, and may have the A and B boxes
of a type 2 pol Ill promoter separated by between 0 and 300 bases; DNA
vector encoding the RNA molecule of claim 51.
CA 02468048 2004-06-07
In other related aspects, the invention features an RNA or DNA vector
encoding the above RNA rnolecule, with the portions of the vector encoding
the RNA functioning as a RNA pol lil promoter; or a cell containing the
vector ; or a method to provide a desired RNA molecule in a cell, by
introducing the molecule into a cell with an RNA molecule as described
above. The cells can be derived from animals, plants or human beings.
In order for RNA-based gene therapy approaches to be effective,
sufficient amounts of the therapeutic RNA must accumulate in the
appropriate intracellular compartment of the treated cells: Accumulation is
a function of both promoter strength of the antiviral gene, and the
intracellular stability of the antiviral RNA. Both RNA polymerise 11 (pot II)
and RNA polymerise III (pol III) based expression systems have been used
to produce therapeutic RNAs in cells (Sarver & Rossi, 1993 AlOS Res. &
Human Retroviruses 9, 483-487; Yu et af., 1993 P.N.A.S.(USA) 90, 6340-
6344). However, pol III based expression cassettes are theoretically more
attractive for use in expressing antiviral RNAs for the following reasons.
Pol II produces messenger RNAs located exclusively in the cytoplasm,
whereas pol III produces functional RNAs found in both the nucleus and the
cytoplasm. Pol II promoters tend to be more tissue restricted, whereas pol
III genes encode tRNAs and other functional RNAs necessary for basic
"housekeeping" functions in all cell types. Therefore, pol III promoters are
likely to be expressed in all tissue types. Finally, pol 111 transcripts from
a
given gene accumulate to much greater levels in cells relative to pol II
genes.
Intracellular accumulation of therapeutic RNAs is also dependent on
the method of gene transfer used. For example, the retroviral vectors
presently used to accomplish stable gene transfer, integrate randomly into
the genome of target cells. This random integration Leads to varied
expression of the transferred gene in individual cells comprising the bulk
treated cell population. Therefore, for maximum effectiveness, the
transferred gene must have the capacity to express therapeutic amounts of
the antiviral RNA in the entire treated cell population, regardless of the
integration site.
CA 02468048 2004-06-07
~~2
Pol III System
The following is just one non-limiting example of the invention. A pol
III based genetic element derived from a human tRNAimet gene and
termed 03-5 (Fi . ; Adeniyi-Jones et al., 1984 supra), has been adapted
to express antiviral RNAs (Sullenger et al., 1990 MoL CeII. Biol. 10, 6512-
6523). This element was inserted into the DC retroviral vector (Sullenger
et al., 1990 Mol. Cell. Biol. 10, 6512-6523) to accomplish stable 'gene
transfer, and used to express antisense RNAs against moloney murine
leukemia virus and anti-HIV decoy RNAs (Sullenger et al., 1990 Mol. Cell.
Biol. 10, 6512-6523; Sullenger et al., 1990 Gell63, 601-608; Sullenger et
al., 1991 J. Virol. 65, 6811-6816; Lee et al., 1992 The New Biologist 4, 66-
74). Clonal lines are expanded from individual cells present in the bulk
population, and therefore express similar amounts of the therapeutic RNA
in all cells. Development of a vector system that generates therapeutic
levels of therapeutic RNA in all treated cells would represent a significant
advancement in RNA based gene therapy modalities.
Applicant examined hammerhead (HHI) ribozyme (RNA with
enzymatic activity) expression in human T cell lines using the ~3-5 vector
system (These constructs are termed u~3-5/HHI"; Fia. 34). On average,
ribo2ymes were found to accumulate to less than 100 copies per cell in the
bulk T cell populations. In an attempt to improve expression levels of the
~3-5 chimera, the applicant made a series of modified D3-5 gene units
containing enhanced promoter elements to increase transcription rates,
and inserted structural elements to improve the intracellular stability of the
ribozyme transcripts (F, ia. 34). One of these modified gene units, termed
S35, nave rise to more than a 100-fold increase in ribozyme accumulation
in bulk T cell populations relative to the original e3-5/HH1 vector system.
Ribozyme accumulation in individual clonal lines from the pooled T cell
populations ranged from 10 to greater than 100 fold more than those
achieved with the original d3-5/HHl version of this vector.
The S35 gene unit may be used to express other therapeutic RNAs
including, but not limited to, ribozymes, antisense, decay, therapeutic
editing, agonist and antagonist RNAs. Application of the S35 gene . unit
would not be limited to antiviral therapies, but also to other diseases, such
as cancer, in which therapeutic RNAs may be effective. The S35 gene unit
may be used in the context of other vector systems besides retroviral
CA 02468048 2004-06-07
103
vectors, including but not limited to, other stable gene transfer systems
such as adeno-associated virus (AAV; Carter, 1992 Curr. Opin. Genet. Dev.
3, 74), as well as transient vector systems such as plasmid delivery and
adenoviral vectors (Berkner, 1988 BioTechniques 6, 616-629).
As described below, the S35 vector encodes a truncated version of a
tRNA wherein the 3' region of the RNA is base-paired to complementary
nucleotides at the 5' terminus, which includes the 5' precursor portion that
is normally processed off during tRNA maturation. Without being bound by
any theory, Applicant believes this feature is important in the level of
expression observed. Thus, those in the art can now design equivalent
RNA molecules with such high expression levels. Below are provided
examples of the methodology by which such vectors and tRNA molecules
can be made.
d3-5 Vectors
The use of a truncated human tRNAimet gene, termed D3-5 (Fia~33;
Adeniyi-Jones et al., 1984 supra), to drive expression of antisense RNAs,
and subsequently decoy RNAs (Sullenger et al., 1990 supra) has recently
been reported. Because tRNA genes utilize internal pol 111 promoters, the
antisense and decoy RNA sequences were expressed as chimeras
contGining tRNAimet sequences. The truncated tRNA genes were placed
into the U3 region of the 3' moloney murine leukemia virus vector LTR
(Sullenger et al., 1990 supra).
Base-Paired Structures
Since the 03-5 vector combination has been successfully used to
express inhibitory levels of both antisense and decoy RNAs, applicant
cloned ribozyme-encoding sequences (termed as °d3-5lHHI") into this
vector to explore its utility for expressing therapeutic ribozymes. However,
low ribozyme accumulation in human T cell lines stably transduced with
this vector was observed {Fi4~35,). To try and improve accumulation of the
~ibozyme, applicant incorporated various RNA structural elements (Fi . 4
into one of the ribozyme chimeras (a3-5/HHI).
Two strategies were used to try and protect the termini of the chimeric
transcripts from exonucleolytic degredation. One strategy involved the
incorporation of stem-loop structures into the termini of the transcript. Two
CA 02468048 2004-06-07
1 oa
such constructs were cloned, S3 which contains a stem-loop structure at
the 3' end, and S5 which contains stem-loop structures at both ends of the
transcript (Fi ure 4). The second strategy involved modification of the 3'
terminal sequences such that the 5' terminus and the 3' end sequences
can form a stable base-paired stem. Two such constructs were made: S35
in which the 3' end was altered to hybridize to the 5' leader and acceptor
stem of the tRNAimet domain, and S35Pfus which was identical to S35 but
included more extensive structure formation within the non-ribozyme
portion of the e3-5 chimeras {Fi ur 4). These stem-loop structures are
also intended to sequester non-ribozyme sequences in structures that will
prevent them from interfering with the catalytic activity of the ribozyme.
These constructs were cloned, producer cell lines were generated, and
stably-transduced, human, MT2 (Harada et"al., 1985_,_sup~a~_and__.CEM. .(tiara
8 Fischinger, 1988 supra) cell lines were established (Curr. Protocols Mol.
Bio!. 1992, ed. Ausubel et al., Wiley 8 Sons, NY). The RNA sequences and
structure of S35 and S35 Plus are provided in Figures 40-47.
Referring to Fioure 4 , there is provided a general structure for a
chimeric RNA molecule of this invention. Each N independently represents
none or a number of bases which may or may not be base paired. The A
and B boxes are optional and can be any known A or B box, or a
consensus sequence as exemplified in the figure. The desired nucleic acid
to be expressed can be any location in the molecule, but preferably is on
those places shown adjacent to or between the A and B boxes (designated
by arrows). Fi ur 4 shows one example of such a structure in which a
desired RNA is provided 3' of the intramolecular stem. A specific example
of such a construct is provided in Figures 50 and 51.
Example 2fi: Cloning of o3-5-Ribozyme Chimera
Oligonucleotides encoding the S35 insert that overlap by at least 15
nucleotides were designed (5' GATCCACTCTGCTGTTCTGTTI'TTGA 3'
and 5' CGCGTCAAAAACAGAACAGCAGAGTG 3'). The oligonucleotides
{10 pM each) were denatured by boiling for.5 min in a buffer containing 40
mM Tris.HCl, pH8Ø The oligonucleotides were allowed to anneal by snap
cooling on ice for 10-15 min.'
The annealed oligonucleotide mixture was converted into a double-
stranded molecule using Sequenase~ enzyme (US Biochemicals) in a
CA 02468048 2004-06-07
105
buffer containing 40 mM Tris.HCl, pH7.5, 20 mM MgCl2, 50 mM NaCI, 0.5
mM each of the four deoxyribonucleotide triphosphates, 10 mM DTT. The
reaction was allowed to proceed at 37°C for 30 min. The reaction was
stopped by heating to 70°C for 15 min.
The double stranded DNA was digested with appropriate restriction
endonucleases (BamHl and Mlul) to generate ends that were suitable for
cloning into the D3-5 vector.
The double-stranded insert DNA was ligated to the o3-5 vector DNA
by incubating at room temperature (about 20°C) for 60 min in a buffer
containing 66 mM Tris.HCl, pH 7.6, 6.6 mM MgCl2, 10 mM DTT, 0.066 ItM
ATP and 0.1 U/~I T4 DNA Ligase (US Biochemicals).
Competent E. colt bacterial strain was transformed with the
recombinant vector DNA by mixing the cells and DNA on ice for 60 min.
The mixture was heat-shocked by heating to 37°C far 1 min. The
reaction
mixture was diluted with LB media and the cells were allowed to recover for
60 min at 37°C. The cells were plated on LB agar plates and incubated
at
37°C for - 18 h.
Plasmid DNA was isolated from an overnight culture of recombinant
clones using standard protocols (Ausubel et al., Curr. Protocols Mol.
Biology 1990, Wiley & Sons, NY).
The identity of the clones were determined by sequencing the plasmid
DNA using the Sequenase~ DNA sequencing kit (US Biochemicals}.
The resulting recombinant D3-5 vector contains the S35 sequence.
The HHI encoding DNA was cloned into this ~3-5-S35. containing vector
using Sacll and BamHl restriction sites.
Example 27: Northern analysis
RNA from the transduced MT2 cells were extracted and the presence
of e3-Slribozyme chimeric transcripts were assayed by Northern analysis
(Curr. Protocols Mol. Biol. 1992, ed. Ausubel et al., Wiiey & Sons, NY).
Northern analysis of RNA extracted from MT2 transductants showed that
D3-5/ribozyme chimeras of appropriate sizes were expressed (Fig. 35.3f,).
In addition, these results demonstrated the relative differences in
accumulation among the different constructs (Figure 35.36). The pattern of
CA 02468048 2004-06-07
expression seen from the D3-5/HHl ribozyme chimera was similar to 12
other ribczymes cloned into the ~3-5 vector (not shown), In MT-2 cell line,
03-5/HHI ribozyme chimeras accumulated, on average, to less than 100
copies per cell.
Addition of a stem-loop onto the 3' end of D3-5/HHI did not lead to
increased D3-5 levels (S3 in F, i-.q 35,36). The S5 construct containing both
5' and 3' stem-loop structures also did not lead to increased ribozyme
4evels (Fig; 35.36).
Interestingly, the S35 construct expression in MT2 cells was about
100-fold more abundant relative to the original D3-5/HHI vector transcripts
(Fia. 35,36}. This may be due to increased stability of the S35 transcript.
Example 28: Cleavage activity
To assay whether ribozymes transcribed in the transduced cells
contained cleavage activity, total RNA extracted from the transduced MT2 T
cells were incubated with a labeled substrate containing the HH1 cleavage
site (Fi ure 7). Ribozyme activity in all but the S35 constructs, was too
low to detect. However, ribozyme activity was detectable in S35-
transduced T cell RNA. Comparison of the activity observed in the S35-
transduced MT2 RNA with that seen with MT2 RNA in which varying
amounts of in vitro transcribed S5 ribozyme chimeras, indicated that
between 1-3 nM of S35 ribozyme was present in S35-transduced MT2
RNA. This level of activity corresponds to an intracellular concentration of
5,000-15,000 ribozyme molecules per cell.
Example 29: Clonal variation
Variation in the ribozyme expression levels among cells making up
the bulk population was determined by generating several clonal cell lines
from the bulk S35 transduced CEM line (Curr. Protocols Mol. 8iol. 1992,
ed. Ausubel et al., Wiley & Sons, NY) and the ribozyme expression and
activity levels in the individual clones were measured (Figure 38 and 39).
All the individual clones were found to express active ribozyme. The
ribozyme activity detected from each clone correlated well with the relative
amounts of ribozyme observed by Northern analysis. Steady state
ribozyme levels among the clones ranged from approximately 1,000
molecules per cell in clone G to 11,000 molecules per cell in clone H (Fig=
CA 02468048 2004-06-07
~$,). The mean accumulation among the clones, calculated by averaging
the ribozyme levels of the clones, exactly equaled the level measured in
the parent bulk population. This suggests that the individual clones are
representative of the variation present in the bulk population.
The fact that all i4 clones were found to express ribozyme indicate
that the percentage of cells in the bulk population expressing ribozyme is
also very high. In addition, the lowest level of expression in the clones was
still more than 10-fold that seen in bulk cells transduced with the original
t~3-5 vector. Therefore, the S35 gene unit should be much more effective
in a gene therapy setting in which bulk cells are removed, transduced and
then reintroduced back into a patient.
Example 3Q: Stability -
Finally, the bulk S35-transduced line, resistant to 6418, was
propogated for a period of 3 months (in the absence of 6418) to determine
if ribozyme expression was stable over extended periods of time. This
situation mimicks that found in the clinic in which bulk cells are transduced
and then reintroduced into the patient and allowed to propogate. There
was a modest 30% reduction of ribozyme expression after 3 months. This
difference probably arose from cells with varying amount of ribozyrne
expression and exhibiting different growth rates in the culture becoming
slightly more prevalent in the culture. However, ribozyme expression is
apparently stable for at least this period of time.
Example 31: Design and construction of TRZ-tRNA Chimera
A transcription unit, termed TRZ, is designed that contains the S35
motif (Fioure ).~ A desired RNA (e.g. ribozyme) can be inserted into the
indicated region of TRZ tRNA chimera. This construct might provide
additional stability to the c+esired RNA. TRZ-A and TRZ-B are non-limiting
examples of the TRZ-tRNA chimera.
Referring to Fig. 53-54, a hammerhead ribozyme targeted to site 1
(HHITRZ-A; Fig. 53) and a hairpin ribozyme, (HPITRZ-A; Fig. 54), also
targeted to site f, is cloned individually into the indicated region of TRZ
tRNA chimera. The resulting ribozyme trancripts retain full RNA cleavage
activity (see for example Fia~55). Applicant has shown that efficient
CA 02468048 2004-06-07
108
expression of these TRZ tRNA chimera can be achieved in mammalian
cells. .
Besides ribezymes, desired RNAs like antisense, therapeutic editing ,
RNAs, decoys, can be readily inserted into the indicated region of TRZ-
tRNA chimera to achieve therapeutic levels of RNA expression in
mammalian cells.
Sequences fisted in Figures 40-47 and 50 - 54 are meant to be non-
limiting examples. Those skilled in the art will recognize that variants
(mutations, insertions and deletions) of the above examples can be readily
generated using techniques known in the art, are within the scope of the
present invention.
Example 32: Ribo_zyme expression in T cell lines
Ribozyme expression in T cell lines stably-transduced with either a
retroviral-based or an Adeno-associated virus (AAV)-based ribozyme
expression vector (Figure 56). The human T cell lines MT2 and CEM were
transduced with either retroviral or AAV vectors encoding a neomycin
slelctable marker and a ribozyme (S35/HHI) expressed from pol III meti
tRNA-driven promoter. Cells stably-transduced with the vectors were
selectivelyt expanded medium containing the neomycin antibiotic
derivative, 6418 (0.7 mg/ml). Ribozyme expression in the stable cell lines
was then alalyzed by Northern analysis. The probe used to detect
ribozyme transcripts also cross-hybridized with human meti tRNA
sequences. Refering to Figure 56, S35/HHI RNA accumulates to significant
levels in MT2 and CEM cells when transduced with either the retrovirus or
the AAV vector.
These are meant to be non-limiting examples, those skilled in the art
will recognize that other vectors such as adenovirus vector (Figure 57),
plasmid DNA vector, alpha virus vectors and the other derivatives there of,
can be readily generated to deliver the desired RNA, using techniques
known in the art and are within the scope of this invention. Additionally, the
transcription units can be expressed individually or in~ multiples using pol
II
and/or pol III promoters.
References cited herein, as well as Draper WO 93/23569, 94/02495,
94/06331, Sullenger WO 93/12657, Thompson W0 93104573, and Suflivan
CA 02468048 2004-06-07
109
WO 94!04609, and 93!11253 describe methods for use of vectors decribed
herein. ~ In particular these
vectors are useful for administration of antisense and decoy RNA
molecules.
Examale 33: Ligated Ribozymes are catalyticallyr active
The ability of ribozymes generated by ligation methods, described in
Draper et al., PCT WO 93123569, to cleave target RNA was tested on either
matched substrate RNA (Fi . 5 or long (622 nt) RNA (Fi~~59,,
f~0 and 61 ~,
Matched substrate RNAs were chemically synthesized using solid-
phase RNA synthesis chemistry (Scaringe et al., 1990 Nucleic Acids Res.
18, 5433-544'1). Substrate RNA was 5' end-labeled using [~y~-32P) ATP and
polynuc~eotide kinase (Curr. Protocols Mol. BQI. 1992, ed. Ausubel et al.,
Wiley 8~ Sons, NY). Ribozyme reactions were carried out under ribozyme
excess conditions (kcat~KM~ Herschlag and Cech, 1990 Biochemistry 29,
10159-10171). Briefly, ribozyme and substrate RNA were denatured and
renatured separately by heating to 90°C and snap cooling on ice for 10
min
in a bufter containing 50 mM Tris. HCI pH 7.5 and 10 mM MgCl2.
Cleavage reaction vuas initiated by mixing the ribozyme with the substrate
at 37°C. Aliquots of 5 pf~were taken at regular intervals of time and
the
reaction was stopped by mixing wish equal volume of formamide gel
loading bufifer (Curr. Protocols Mol. Biol. 1992, ed. Ausube) et al.; Wifey 8~
- Sons, NY). The samples were resolved on 20 % polyacrylamide-urea gel.
Refering to F. ig_58, -DG refers to the free energy of binding calculated for
base-paired interactions between the ribozyme and the substrate RNA
(Turner and Sugimoto, 1988 a ra). , RPI A is a HH ribozyme with 6/6
binding arms. This ribozyme was synthesized chemically either as a one
piece ribozyme or was synthesized in two fragments followed by ligation to
generate a one piece ribozyme. The kcat/KM values for the two ribozymes
were comparable.
A template containing T7 RNA pofyrnerase promoter upstream of 622
nt long target sequence, was PCR amplified from a DNA clone, The target
RNA (containing HH ribozyme cleavage sites B, C and D) was transcribed
from this PCR amplified template using T7 RNA polymerase. The transcript
was internally labeled during transcription by including [a-32p) CTP as one
of the tour ribonucleotide triphosphates. The transcription mixture was
CA 02468048 2004-06-07
110
treated with DNase-1, fiollowing transcription at 37°C for 2 hours, to
digest
away the DNA template used in the transcription. RNA was precipitated
with fsopropanol and the pellet was washed two times with 70°l°
ethanol to
get rid of salt and nucleotides used in the transcription reaction. RNA is
resuspended in DEPC-treated water and stored at 4°C, Ribozyme
cleavage reactions were carried out under ribozyme excess (kcat~KM)
conditions [Herschlag and Cech 1990 su ra . Briefly, 1000 nM ribozyme
and 10 nM internally labeled target RNA were denatured separately by
heating to 90°C for 2 min in the presence of 50 mM Tris.HCl, pH 7.5 and
10
mM MgCl2. The RNAs were renatured by cooling to 37°C for 10-20 min.
Cleavage reaction was initiated by mixing the ribozyme and target RNA at
37°C. Aliquots of 5 lrl were taken at regular intervals of time and the
reaction was quenched by adding equal volume of stop buffer. The
samples were resolved an a sequencing gel.
Examale 34' Hammerhead ribozymes with >_ 2 base-paired stem I1 a_re
catal,~tically a~tivg
To decrease the cost of chemical synthesis of RNA, applicant was
interested in determining whether the length of stem II region of a typical
hammerhead ribozyme (~ 4 by stem ll) can be shortened without
decreasing the catalytic efficiency of the HH ribozyme. The length of stem ll
was systematically shortened by one base-pair at a time, HH ribozymes
with three and twa base-paired stem II were chemically synthesized using
solid-phase RNA phosphoramidite chemistry (Scaringe et al., 1990 su ra .
Matched and long substrate RNAs were synthesized and ribozyme
assays were carried out as described in example 33. Referring to fi4ures ,
~2, 63 and 64, data shows that shortening stem II of a hammerhead
ribozyme does not significantly alter the catalytic efficiency. It is
applicant's
opinion that hammerhead ribozymes with Z 2 base-paired stem II region
are catalytically active.
Examflle 35: Synthesis of catal fcally active hairpin ribozvmes
RNA molecules were chemically synthesized. having the nucleotide
base sequence shown in Fia~65 for both the 5' and 3' fragments. The 3'
fragments are phosphorylated and ligated to the 5' fragment essentially as
described in example 37. As is evident from the Fiau-~- re 65, the 3' and 5'
fragments can hybridize together. at helix 4 and are covalently (inked via
CA 02468048 2004-06-07
GAAA sequence. When this structure hybridizes to a substrate, a
ribozyme~substrate complex structure is formed. White helix 4 is shown as
3 base pairs it may be formed with only 1 or 2 base pairs.
40 nM mixtures of ligated ribozymes were incubated with 1-5 nM 5'
end-labeled matched substrates (chemically synthesized by solid-phase
synthesis using RNA phosphoramidite chemistry) for different times in 50
mM Tris/HCl pH 7.5, 10 mM MgCl2 and shown to cleave the substrate
efficiently (Fia~66).
The target and the ribozyme sequences shown in Fip~. 62 and 65 are
meant to be non-limiting examples. Those in the art will recognize that
other embodiments can be readily generated using other sequences and
techniques generally known in..the .art. ~ --
V , Constructs of Hairpin Ribozymes
There follows an improved traps-cleaving hairpin ribozyme in which a
new helix (i.e., a sequence able to form a double-stranded region with
another single-stranded nucleic acid) is provided in the ribozyme to base~
pair with a 5' region of a separate substrate nucleic acid. This helix is
provided at the 3' end of the ribozyme after helix 3 as shown in i ur In
addition, at least two extra bases may be provided in helix 2 and a portion
of the substrate corresponding to helix 2 may be either directly linked io the
5' portion able to hydrogen bond to the 3' end of the hairpin or may have a
linker of atlEast one base. By traps-cleaving is meant that the ribozyme is
able to act in traps to cleave another RNA molecule which is not covalently
finked to the ribozyme itself. Thus, the ribozyme is not able to act on itself
in an intramolecufar cleavage reaction.
By "base-pair" is meant a nucleic acid that can form hydrogen bonds)
with other RNA sequence by either traditional Watson-Crick or other non-
traditional types (for example Hoogsteen type) of interactions.
The increase in length of helix 2 of a hairpin ribozyme (with or without
helix 5) has several advantages. These include improved stability of the
ribozyme-target complex in vivo , In addition, an increase in the
recognition sequence of the hairpin ribozyme improves the specificity of the
ribozyme. This also makes possible the targeting of potential hairpin
CA 02468048 2004-06-07
7
ribozyme sites that would otherwise be inaccessible due to neighboring
secondary structure.
The increase in length of helix 2 of a hairpin ribozyme (with or without
helix 5) enhances trans-ligation reaction catalyzed by the ribozyme. Trans-
ligation reactions catalyzed by the regular hairpin ribozyme (4 by helix 2) is
very inefficient (Komatsu ef al., 1993 Nucleic Acids Res. 21, 185). This is
attributed to weak base-pairing interactions between substrate RNAs and
the ribozyme. By increasing the length of helix 2 (with or without helix 5)
the rate of ligation (in vitro and in vivo) can be enhanced several fold.
Results of experiments suggest that the length of H2 can be 6 by
without significantly reducing the activity of the hairpin ribozyme. The H2
arm length variation does not appear to be sequence dependent. HP
ribozymes with 6 by H2 have been designed against five different target
RNAs and all five ribczymes efificiently cleaved their cognate target RNA,
Additionally, two of these ribozymes were able to successfully inhibit gene
expression (e.g., TNF-a) in mammalian cells. Results of these experiments
are shown below.
HP ribozymes with 7 and 8 by H2 are also capable of cleaving target
RNA in a sequence-specific manner, however, the rate of the cleavage
reaction is lower than those catalyzed by HP ribozymes with 6 by H2.
Example 3~: 4 and 6 base pair H2
Referring to Figures 67-72, HP ribozymes were synthesized as
described above and tested for activity. Surprisingly, those with 6 base
pairs in H2 were still as active as those with 4 base pairs.
VI. Chemical Modificat~n
Oliaonucleotides with 5'-C-alkyl Group
The introduction of an alkyl group at the 5'-position of a nucleoside or
nucleotide sugar introduces an additional center of chirality into the sugar
moiety. Referring to Fia~75, the general structures of 5'-C-alkylnucieotides
belonging to the D-allose, 2, and L-talose, 3, sugar families are shown.
The family names are derived from the known sugars D-allose and ~-talose
(Ri = CH3 in 2 and 3 in Figure 75). Useful specific D-allose and L-talose
CA 02468048 2004-06-07
' 113
nucleotide derivatives are shown in Figure 76. 29-32 and Figure 77, 58-
61 respectively.
This invention relates to the use of 5'-C-alkylnucleotides in
oligonucleotides, which are particularly useful for enzymatic cleavage of
RNA or single-stranded DNA, and also as antisense oligonucleotides. As
the term is used in this application, 5'-C-alkylnucleotide-containing
enzymatic nucleic acids are catalytic nucleic molecules that contain 5'-C-
alkylnucleotide components replacing, but not limited to, double stranded
stems, single stranded "catalytic core" sequences, single-stranded loops or
single-stranded recognition sequences. These molecules are able to
cleave (preferably, repeatedly cleave) separate RNA or DNA molecules in
a nucleotide base sequence specific manner. Such catalytic nucleic acids
can also act to cleave intramolecularly if that is desired. Such enzymatic
molecules can be targeted to virtually any RNA transcript.
Also within the invention are 5'-C-alkylnucleotides which may be
present in enzymatic nucleic acid o.r even in antisense oligonucleotides.
Such nucleotides are useful since they enhance the stability of the
antisense or enzymatic molecule; and can be used in locations which do
not affect the desired activity of the molecule. That is, while the presence
of
the 5'-C-alkyl group may reduce binding affinity of the oligonucleotide
containing this modification, if that moiety is not in an essential base pair
forming region then the enhanced stability that it provides to the molecule
is advantageous. In addition, while the reduced binding may reduce
enzymatic activity, the enhanced stability may make the loss of activity of
less consequence. Thus, for example, if a 5'-C-alkyl-containing molecule
has 10% the activity of the unmodified molecule, but has 10-fold higher
stability in vivo then it has utility in the present invention. The same
analysis is true for antisense oligonucleotides containing such
modifications. The invention also relates to novel intermediates useful in
, the synthesis of such nucleotides and oligonucleotides (examples of which
are shown in the Figures), and to methods for their synthesis. .
Thus, in one aspect, the invention features 5'-C-alkylnucleosides, that
is a nucleotide base having at the 5'-position on the sugar molecule an
alkyl moiety. In a related aspect, the invention also features 5'-C-
alkylnucleotides, and in preferred embodiments features those where the
nucleotide is not uridine or thymidine. That is; the invention preferably
CA 02468048 2004-06-07
includes all those nucleotides useful for making enzymatic nucleic acids or
antisense molecules that are not described by the art discussed above. In
preferred embodiments, the sugar of the nucleoside or nucleotide is in an
optically pure form, as the talose or allose sugar.
Examples of various alkyl groups useful in this invention are shown in
Fi ure 7 , where each Ry group is any alkyl. These examples are not
limiting in the invention. Specifically, an "alkyl" group refers to a
saturated
aliphatic hydrocarbon, including straight-chain, branched-chain, and cyclic
alkyl groups. Preferably, the alkyl group has 1 to 12 carbons. More
preferably it is a lower alkyl of from 1 to 7 carbons, more preferably 1 to 4
carbons. The alkyl group may be substituted or unsubstituted. When
substituted the substituted groups) is preferably, hydroxyl, cyano, alkoxy,
=O, =S, N02 or N(CH3)2, amino, or SH. The term also iricludes alkenyl
groups which are unsaturated hydrocarbon groups containing at least one
carbon-carbon double bond, including straight-chain, branched-chain, and
cyclic groups. Preferably, the alkenyl group has 1 to 12 carbons. More
preferably it is a lower alkenyl of from 1 to 7 carbons, more preferably 1 to
4
carbons. The alkenyl group may be substituted or unsubstituted. When
substituted the substituted groups) is preferably, hydroxyl, cyano, alkoxy,
=O, =S, NO~, halogen, N(CH3)2, amino, or SH. The term 'alkyl" also
includes alkynyl groups which have an unsaturated hydrocarbon group
containing at least one carbon-carbon triple bond, including straight-chain,
branched-chain, and cyclic groups. Preferably, the alkynyi group has 1 to
12 carbons. More preferably it. is a lower alkynyl of from 1 to 7 carbons,
more preferably 1 to 4 carbons. The alkynyl group may be substituted or
unsubstituted. When substituted the substituted groups) is preferably,
hydroxyl, cyano, alkoxy, =O, =S, N02 or N(CH3)2, amino or SH.
Such alkyl groups may also include aryl, alkylaryl, carbocyclic aryl,
heterocyclic aryl, amide and ester groups. An "aryl" group refers to an
aromatic group which has at least one ring having a conjugated n electron
system and includes carbocyclic aryl,. heterocyclie aryl and biaryl groups,
all of which may be optionally substituted. The preferred substituent(s) of
aryl groups are halogen, trihalomethyl, hydroxyl, SH, OH, cyano, alkoxy,
alkyl, alkenyl, alkynyl, and amino groups. An "alkylaryl" group refers to an
alkyl group (as described above) covalently joined to an aryl group (as
described above. Carbocyclic aryl groups are groups wherein the ring
CA 02468048 2004-06-07
115
atoms on the aromatic ring are all carbon atoms. The carbon atoms are
optionally substituted. Heterocyclic aryl groups are groups having from 1 to
3 heteroatoms as ring atoms in the aromatic ring and the remainder of the
ring atoms are carbon atoms. Suitable heteroatoms include oxygen, sulfur,
and nitrogen, and include furanyl, thienyl, pyridyl, pyrrolyl, N-lower alkyl
pyrrolo, pyrimidyl, pyrazinyl, imidazofyl and the like, all optionally
substituted. An "amide" refers to an -C(O)-NH-R, where R is either alkyl,
aryl, alkylaryl or hydrogen. An "ester" refers to an -C(O)-OR', where R is
either alkyl, aryl, alkylaryl or hydrogen.
In other aspects, also related to those discussed above, the invention
features ofigonucleotides having one or more 5'-C-alkylnucleotides; e.g.
enzymatic nucleic acids having a 5'-C-alkylnucleotide; and a method for
producing an enzymatic nucleic acid molecule having enhanced activity to
cleave an RNA or single-stranded DNA molecule, by forming the enzymatic
molecule with at least one nucleotide having at its 5'-position an alkyl
group. In other related aspects, the invention features 5'-C alkylnucleotide
triphosphates. These triphosphates can be used in standard protocols to
form useful oligonucleotides of this invention.
The 5'-C-alkyl derivatives of this invention provide enhanced stability
to the oligonulceotides containing them. While they may also reduce
absolute activity in an in vitro assay they will provide enhanced overall
activity in vivo. Below are provided assays to determine which such
molecules are useful. Those in the art will recognize that equivalent
assays can be readily devised.
In another aspect, the invention features a method for conversion of a
protected allo sugar to a protected talo sugar. In the method, the protected
allo sugar is contacted with triphenyl phosphine, diethylazodicarboxylate,
and p-nitrobenzoic acid under inversion causing conditions to provide the
protected talo sugar. While one example of such conditions is provided
below, those in the art will recognize other such conditions. Applicant has
found that such conversion allows for ready synthesis of all types of
nucleotide bases as exemplified in the figures. .
While this invention is applicable to all oligonucleotides, applicant has
found that the modified molecules of this invention are particulary useful for
enzymatic RNA molecules. Thus, below is provided examples of such
CA 02468048 2004-06-07
yes -
molecules. Those in the art will recognize that equivalent procedures can
be used to make other molecules without such enzymatic activity.
Specifically, Figure 1 shows base numbering of a hammerhead motif in
which the numbering of various nucleotides in a hammerhead ribozyme is
provided. This is not to be taken as an indication that the Figure is prior
art
to the pending claims, or that the art discussed is prior art to those claims.
Referring to Fi ur 1, the preferred sequence of a hammerhead ribozyme
in a 5'- to 3'-direction of the catalytic core is CUGANGAG[base paired
with]CGAAA. In this invention, the use of 5'-C-alkyl substituted nucleotides
that maintain or enhance the catalytic activity and or nuclease resistance of
the hammerhead ribozyme is described. Substitutions of any nucleotide
with any of the modified nucleotides shown in Fi ur 7 are possible.
' The following are non-limiting examples showing the synthesis of
nucleic acids using 5'-C-alkyl-substituted phosphoramidites and the
syntheses of the arnidites.
Example 37: Synthesis of Hammerhead Ribozymes Containing 5'-GAlkvl-
nucleotides & Other Modified Nucleotides
The method of synthesis would follow the procedure for normal RNA
synthesis as described in Usman,N.; Ogilvie,K.K.; Jiang,M.-Y.;
Cedergren,R.J, J. Am. Chem. Soc.1987, 709, 7845-7854 and in
Scaringe,S,A.; FrankIyn,C.; Usman,N. Nucleic Acids Res. 1990, 78, 5433-
5441 and makes use of common nucleic acid protecting ahd coupling
groups, such as dimethoxytrityi at the 5'-end, and phosphoramidites at the
3'-end (compounds 26-29 and 56-59). These 5'-C-alkyl substituted
2,5 phosphoramidites may be incorporated not only into hammerhead
ribozymes, but also into hairpin, hepatitis delta virus, Group 1 or Group 2
intron catalytic nucleic acids, or into antisense oligonucleotides. They are,
therefore, of general use in any nucleic acid structure.
Examale 38: Methyl-2.3-O-Isooropylidine-6-Deoxy-J3-D-allofuranoside (4_1_
A suspension of ~-rhamnose (100 g, 0.55 mol), CuS04 (120 g) and
conc. H2S0~ (4.0 mL) in 1.0 L of dry acetone was mixed for 24 h at RT,
then filtered. Conc. NH40H (5 mL) was added to the filtrate and the newly
formed precipitate was filtered. The residue was concentrated in vacuo,
coevaporated with pyridine (2 x 300 mL), dissolved in pyridine (500 mL)
and cooled to 0 °C. A solution of p-toluenesufonylchloride (107 g ,
0.56
CA 02468048 2004-06-07
mmol) in dry DCE (500 mL) was added dropwise over 0:5 h. The reaction
mixture was left for 16 h at RT. The reaction was quenched by adding ice-
water (0.5 L) and, after mixing for 0.5 h, was extracted with chloroform (0.75
L). The organic layer was washed with H20 (2 x 500 mL), 10% H2S04 (2 x
300 mL), water (2 x 300 mL), sat. NaHC03 (2 x 300 mL), brine (2 x 300
mL), dried over MgS04 and evaporated to dryness. The residue (115 g)
was dissolved in dry MeOH (1 L) and treated with NaOMe (23.2 g, 0.42
mmol) in MeOH. The reaction mixture was left for 16 h at 20 °C,
neutralized
with dry C02 and evaporated to dryness. The residue was suspended in
chloroform (750 mL), filtered , concentrated to 100 mL and purified by flash
chromatography in CHC13 to yield 45 g (37%) of compound 4.
Example 39: Methvl-2.3-O-Isooropvlidine-5-D-t-Butyldiphenylsilvl-6-
Deoxv~D-Allofuranoside f5~
To solution of methylfurancside 4 (12.5 g 62.2 mmol) and AgN03
(21.25 g, 125.0 mmol) in dry DMF (300 mL) t-butyldiphenylsilyl chloride
(22.2 g , 81 mmol) was added dropwise under Ar over 0.5 h. The reaction
mixture was stirred for 4 h at RT, diluted with CHC13 (200 mL), filtered and
evaporated to dryness (below 40 °C using a high vacuum oil pump). The
residue was dissolved in CH2C12 (300 mL) washed with sat. NaHC03 (2 x
50 mL), brine (2 x 50 mL), dried over MgS04 and evaporated to dryness.
The residue was purified by flash chromatography in CH2C12 to yield 20.0
g (75%) of compound 5.
Example 40: Methvl-5-O-t-Butvldiphenylsilyl-6-Deoxy-Q-D-Allofuranoside
Methylfuranoside 5 (13.5 g, 30.6 mmol) was dissolved in
CF3COOH:dioxane:H20 l 2:1:1 (v/vlv, 200 mL) and stirred at 24 °C
for 45
m. The reaction mixture was cooled to -10 °C, neutralized with cone.
NH40H (140 mL) and extracted with CH2C12 (500 mL). The organic layer
was separated, washed with sat. NaHC03 (2 x 75 mL), brine (2 x 75 mL),
dried over MgS04 and evaporated to dryness. The product 6 was purified
by flash chromatography using a 0-10% MeOH gradient i~ CH2C12. Yield
9.0 g (76%).
CA 02468048 2004-06-07
118 -
Example 41: Methyl-2.3-di-O-Benzoyl-5-D-f-Butyldiphenylsilyl-6-Deoxy_(3-
p-Allofuranoside (7).
Methylfuranoside 6 (7.0 g, 17.5 mmol) was .coevaporated with
pyridine (2 x 100 mL) and dissolved in pyridine (100 mL). Benzoyl chloride
(5.4 g, 38.5 mmol) was added and the reaction mixture was left at RT for 16
h: Dry EtOH (50 mL) was added and the reaction mixture was evaporated
to dryness after 0.5 h. The residue was dissolved in CH2C12 (300 mL),
washed with sat. NaHC03 (2 x 75 mL), brine (2 x 75 mL) dried over MgS04
and evaporated to dryness. The product was purified by flash
chromatography in CH2C12 to yield 9.5 g (89%) of compound T.
Example 42: 1-O-Acetyl-2,3-di-D-benzoyl-5-O-t-Bu~ldiphenylsi~l-6-
Deoxy-J' -D-Allofuranose (8).
Dibenzoate 7 (4.7 g, 7.7 mmol) was dissolved in a mixture of AcOH
(10.0 mL), Ac20 (20.0 mL) and EtOAc (30 mL) and the reaction mixture was
cooled 0 °C. 98% H~S04 (0.15 mL) was then added. The reaction mixture
was kept at 0 °C for 16 h, and then poured into a cold 1:1 mixture of
sat.
NaHC03 and EtOAc (150 mL). After 0.5 h of vigorous stirring the organic
phase was separated, washed with brine (2 x 75 mL), dried over MgS04,
evaporated to dryness and coevaporated with toluene (2 x 50 mL). The
product was purified by flash chromatography using a gradient of 0-5%
MeOH in CH2C12. Yield: 4.0 g (82% as a mixture of oc and p isomers).
Example 43: 1-(2',3'-di-O-Benzovl-5'-O-i-Butyldiphenylsilyl-6'-Deoxy-Q-D-
Allofuranosyl}uracif (91.
Uracil (1.44 g, 11.5 mmol) was suspended in mixture of
hexamethyldisilazane (100 mL) and pyridine (50 mL) and boiled under
reflux until complete dissolution (3 h) occurred, and then for an additional
hour. The. reaction mixture was cooled to RT, evaporated to dryness and
coevaporated with dry toluene (2 x 50 mL). To the residue was added a
solution of acetates 8 (6.36 g, 10.0 mmol) in dry CH3CN (100 mL), followed
by CF,S03SiMe3 (2.8 g, 12.6 mmol). The reaction mixture was kept at 24
°C for 16 h, concentrated to 1/3 of its original volume, diluted with
100 mL
of CH2C12 and extracted with sat. NaHC03 (2 x 50 mL), brine (2 x 50 mL)
dried over MgSO~, and evaporated to dryness. The product 9 was purified
by flash chromatography using a gradient of 0-5% MeOH in CH2C12. Yield:
5.7 g (80%).
CA 02468048 2004-06-07
119
Example 44: IVg-Benzoyl-1-(2' 3'-Di-O-Benzoyl-5'-O-t-But,~rldiphenylsilyl-6'-
Deoxy-D-D-Allofuranosyl)Cytosine (101
IV4-benzoylcytosirie (1.84 g, 8.56 mmol) was suspended in mixture of
hexamethyldisilazane (100 mL) and pyridine (50 mL) and boiled under
reflux until complete dissolution (3 h) occurred, and then for an additional
hour. The reaction mixture was cooled to RT evaporated to dryness and
coevaporated with dry toluene (2 x 50 mL). To the residue was added a
solution of of acetates 8 (3.6 g, 5.6 mmol) in dry CH3CN (100 mL), followed
by CF3S03SiMe3 (4.76 g, 21.4 mmol). The reaction mixture was boiled
under reflux for 5 h, cooled to RT, concentrated to 1/3 of its original
volume,
diluted with CH2C12 (100 mL) and extracted with sat. NaHC03 (2 x 50 mL),
brine (2 x 50 mL) dried over MgS04 and evaporated to dryness.
Purification by flash chromatography using a gradient of 0-5% MeOH in
CH2C12 yielded 1.8 g (55°l°) of compound 10.
Example 45: IVY-Benzoyl-9-(2'.3'-di-O-Benzoyl-5'-O-t-Butyldii~henylsilyl-6'-
Deoxy-~~D-Allofuranosyl)adenine X111.
!V6-benzoyladenine {2.86 g, 11.86 mmol) was suspended in mixture
of hexamethyldisilazane (100 mL) and pyridine (50 mL) and boiled under
reflux until complete dissolution (7 h) occurred, and then for an additional
hour. The reaction mixture was cooled to RT evaporated to dryness and
coevaporated with dry toluene (2 x 50 mL). To the residue was added a
solution of of acetates 8 (3.6 g, 5.6 mmol) in dry CH3CN (100. mL) followed
by CF3SO;,SiMe3 (6.59 g, 29.7 mmol). The reaction mixture was boiled
under reflux for 8 h, cooled to RT, concentrated to 1/3 of its original
volume,
diluted with CH2C12 (100 mL) and extracted with sat. NaHC03 (2 x 50 mL),
brine (2 x 50 mL). dried over MgS04 and evaporated to dryness. The
. product 11 was purified by flash chromatography using a gradient of 0-5%
MeOH in CHZCIp. Yield: 2.7 g (60%).
Example 46: I~-Isobutvrvl-9-l2'.3'-di-O-Benzoyl-5'-O-f-Butyldiphenvlsilvl-
6'-Deoxy-f3-o-Allofuranosyl)duanine,~l2~
!Vz-Isobutyrylguanine (1.47 g , 11.2 mmol) was suspended in mixture
of hexamethyldisilazane (100 mL) and pyridine (50 mL) and boiled under
reflux until complete dissolution (6 h) occurred; and then for an additional
hour. The reaction mixture was cooled to RT evaporated to dryness and
coevaporated with dry toluene (2 x 50 mL). To the residue was added a
CA 02468048 2004-06-07
'f 20
solution of of acetates 8 (3.4 g; 5.3 mmol) in dry CH3CN {100 mL) followed
by CFJSOaSiMe3 (6.22 g, 28.0 mmol). The reaction mixture was boiled
under reflux for 8 h, cooled to RT, concentrated to 1I3 of its original
volume,
diluted with CH2C12 {100 mL) and extracted with sat. NaHC03 (2 x 50 mL),
brine (2 x 50 mL) dried over MgS04 and evaporated to dryness. The
product 12 was purified by flash chromatography using a gradient of 0-2%
MeOH in CH2C12. Yield: 2.1 g (54%).
Example 47: IVY-Benzovl-9-(2'.3'-di-O-benzoyl-6'-Deoxy~~i-D-Allofurano-
~r1)adenine (151.
Nucleoside 11 (1.65 g, 2.0 mmol) was dissolved in THF (50 mL) and
a 1 M solution of TBAF in THF (4 mL) was added. The reaction mixture was
kept at RT for 4 h,, evaporated to dryness and the product purified by flash
chromatography using a gradient of 0-5% MeOH in CH2C12 to yield 1.0 g
(85%) of compound 15.
Example 48: IVY-B_enzoyl-9-(2'.3'-di-D-BenzoVl-5'-O-Dimethoxvtri~ tvl-fi'-
Deox -f3-D-Allofuranosyll-adenine~191.
Nucleoside 15 (0.55 g, 0.92 mmol) was dissolved in dry CH2C12 (50
mL). AgNOa (0.34 g, 2.0 mmol), dimethoxytrityl chloride (0.68 g, 2.0 mmol)
and sym-collidine (0.48 g) were added under Ar. The reaction mixture was
stirred for 2h, diluted with CH2C12 (100 mL), filtered, evaporated to dryness
and coevaporated with toluene (2 x 50 mL). Purification by flash
chromatography using a gradient of 0-5% MeOH in CH2C12 yielded 0.8 g
(97°l°) of compound 19.
Example 49: IVY-Benzovl-9-!-5'-O-Dimethoxytrityl-6'-Deoxy_ -D-Allo-
furanosylladenine (23L
Nucleoside 19 (1.8 g, 2 mmol) was dissolved in dioxane (50 mL),
cooled to 0 °C and 2 M NaOH (50 mL) was added. The reaction mixture
was kept at 0 °C for 45 m, neutralized with Dovi~ex 50 (Pyre form),
filtered
and the resin was washed with MeOH {2 x 50 mL). The filtrate was then
evaporated to dryness. Purification by flash chroillatography using a
gradient of 0-10% MeOH in CH2C12 yielded 1.1 g (80°l°) of 23.
CA 02468048 2004-06-07
121
Examrle 50: fV5-Benzoyl-9-(-5'-O-Dimethoxytrityl-2'-øt butvldimethvlsilyl-
6'-Deoxv~3-~-Allofuranosyl)adenine (271,
Nucleoside 23 (1.2 g, 1.8 mmol) was dissolved. in dry TNF (50 mL).
Pyridine (0.50 g, 8 mmol) and AgN03 (0.4 g, 2.3 mmol) were added. After
the AgN03 dissolved (1.5 h), t-butyldimethylsilyl chloride (0.35 g , 2.3
mmol) was added and the reaction mixture was stirred at RT for 16 h. The
reaction mixture was diluted with CH2C12 (100 mL), filtered into sat.
NaHC03 (50 mL), extracted, the organic layer washed with brine (2 x 50
mL), dried over MgS04 and evaporated to dryness. The product 27 was
purified by flash chromatography using a hexanes:EtOAc / 7:3 gradient.
Yield: 0.7 g (50%).
Example ~1: N~-Benzovl-9-(-5'-O-Dimethoxytrityl-2'-O-t-bu~ldimethylsil~rl-
5'-Deoxy-B-D-Allofuranosvlladenine-3'-(2-Cyanoethyl N N dii~oprogvl-
phosphoramidite) (311.
Standard phosphitylation of 27 according to Scaringe,S.A.;
FrankIyn,C.; Usman,N. Nucleic Acids Res. 1990, 18, 5433-5441 yielded
phosphoramidite 31 in 73% yield.
Example 52. Methy;-5-O-o-Nitrobenzoyl-2.3-D-Isooropylidin~-6-deoxy-Q-u-
Tallofuranoside (51
Methylfuranoside 4 (3.1 g 14.2 mmol) was dissolved in dry dioxane
(200 mL), p-nitrobenzoic acid (10.0 g, 60 mmol) and triphenylphosphine
(15.74 g, 60.0 mmol) were added followed by DEAD (10.45 g, 60.0 mmol).
The reaction mixture was lefit at RT for 16 h, EtOH (5 mL) was added, and
after 0.5 h the reaction mixture was evaporated to dryness. The residue
was dissolved in CH2C12 (300 mL) washed with sat. NaHC03 (2 x 75 mL),
brine (2 x 75 mL) dried over MgS04 and evaporated to dryness.
Purification by flash chromatography using a hexanes:EtOAe / 9:1 gradient
yielded 4.1 g (78%) of compound 33. Subsequent debenzoylation
(NaOMe/MeOH) and silylation (see preparation of 5) fed to l.-
taiofuranoside 34 which was converted to phosphoramidites 58-61 using
the same methodology as described above for the preparation of the
phosphoramidites of the D-allo-isomers 29-32.
The alkyl substituted nucleotides of this invention can be used to form
stable oligonucleotides as discussed above for use in enzymatic cleavage
CA 02468048 2004-06-07
122
or anti~ense situations. Such oligonucleotides can be formed
enzymatically using triphosphate forms by standard procedure.
Administration of such oligonucleotides is by standard procedure. See
Sullivan et al., PCT WO 94J 02595.
The ribozymes and the target RNA containing site 0 were
synthesized, deprotected and purified as described above. RNA cleavage
assay was carried our at 37°C in the presence of 10 mM MgCl2 a s
described above.
Applicant has substituted 5'-C-Me-L-talo nucleotides at positions A6,
A9, A9 + G10, C11.1 and C11.1 + G10, as shown in Figure 78 (HH-O'1 to
HH-05). HH-O '1,2,4 and 5 showed almost wild type activity (Figure 79).
However, HH-03 demonstrated low catalytic activity. Ribozymes HH-01,
2, 3, 4 and 5 are also extremely resistant to degradation by human
serum nucleases.
Ofiaonucleotides with 2'-Deoxy-2'-Alkylnucleotide
This invention uses 2'-deoxy-2'-alkylnucleotides in oligonucleotides,
which are particularly useful for enzymatic cleavage of RNA or single-
stranded DNA, and also as antisense oligonucleotides. As the term is used
in this application, 2'-deoxy-2'-alkylnucleotide-containing enzymatic
nucleic acids are catalytic nucleic molecules that contain 2'-deoxy-2'-
aikylnucleotide components replacing, but not limited to, double slranded
stems, single stranded "catalytic care" sequences, single-stranded loops or
single-stranded recognition sequences. These molecules are able to
cleave (preferably, repeatedly cleave) separate RNA or DNA molecules in
a nucleotide base sequence specific manner. Such catalytic nucleic acids
can also act to cleave intramolecularly if that is desired. Such enzymatic
molecules can be targeted to virtually any RNA transcript.
Also within the invention are 2'-deoxy-2'-alkylnucleotides which may
be present in enzymatic nucleic acid or even in antisense oligonucleotides.
Contrary to the findings of De Mesmaeker et al. applicant has found that
such nucleotides are useful since they enhance. the stability of the
antisense or enzymatic molecule, and can be used in locations which do
not affect the desired activity of the molecule. That is, while the presence
of
the 2'-alkyl group may reduce binding affinity of the oligonucleotide
containing this modification, if that moiety is not in an essential base pair
CA 02468048 2004-06-07
- 123
forming region then the enhanced stability that it provides to the molecule
is advantageous. In addition, while the reduced binding may reduce
enzymatic activity, the enhanced stability may make the loss of activity of
less consequence. Thus, for example, if a 2'-deoxy-2'-alkyl-containing
molecule has 10% the activity of the unmodified molecule, but has 10-fold
higher stability in vivo then it has utility in the present invention. The
same
analysis is true for antisense oligonucleotides containing such
modifications. The invention also relates to novel intermediates useful in
the synthesis of such nucleotides and ofigonucieotides (examples of which
are shown in the Figures), and to methods for their synthesis.
Thus, in one aspect, the invention features 2'-deoxy-2'-
alkylnucleotides, that is a nucleotide base having at the 2'-position on the
sugar molecule an alkyl moiety and in preferred embodiments features
those where the nucleotide is not uridine or thymidine. That is, the
invention preferably includes all those nucleotides useful for making
enzymatic nucleic acids or antisense molecules that are not described by
the art discussed above.
Examples of various alkyl groups useful in this invention are shown in
Figure 81. where each R group is any alkyl. The term "alkyl" does not
include alkoxy groups which have an "-D-alkyl" group, where "alkyl" is
defined as described above, where the O is adjacent the 2'-position of the
sugar molecule.
In other aspects, also related to those discussed above, the invention
features oligonucleotides having one or more 2'-deoxy-2'-alkylnucleotides
(preferably not a 2'-alkyl- uridine or thymidine); e.g. enzymatic nucleic
acids having a 2'-deoxy-2'-alkylnucleotide; and a method for producing an
enzymatic nucleic acid molecule having enhanced activity to cleave an
RNA or single-stranded DNA molecule, by forming the enzymatic molecule
with at least one nucleotide having at its 2'-position. an alkyl group. In
other
related aspects, the invention features 2'-deoxy-2'-alkylnucleotide
triphosphates. These triphosphates can be used in standard protocols to
form useful oligonucleotides of this invention.
The 2'-alkyl derivatives of this invention provide enhanced stability to
the oligonulceotides containing them. While they may also reduce
absolute activity in an in vitro assay they will provide enhanced overall
CA 02468048 2004-06-07
124
activity in vivo. Below are provided assays to determine which such
molecules are useful. Those in the art will recognize that equivalent
assays can be readily devised.
1n another aspect, the invention features hammerhead motifis having
enzymatic activity having ribonucleotides at locations shown in Figure 80 at
5, 6, 8, 12, and 15.1, and having substituted ribonucleotides at other
positions in the core and in the substrate binding arms ifi desired. (The term
"core" refers to positions between bases 3 and 14 in Figure 80, and the
binding arms correspond to the bases from the 3'-end to base 15.1, and
from the 5'-end to base 2). Applicant has found that use of ribonucleotides
at these five locations in the core provide a molecule having suffiicient
enzymatic activity even when modified nucleotides are present at other
sites in the motif. Other such combinations ofi useful ribonucleotides can be
determined as described by Usman ef al. supra.
Figure 80 shows base numbering of a hammerhead motif in which the
numbering ofi various nucleotides in a hammerhead ribozyme is provided.
This is not to be taken as an indication that the Figure is prior art to the
pending claims, or that the art discussed is prior art to those claims.
Referring to Figure 80 the preferred sequence of a hammerhead ribozyme
in a 5'- to 3'-direction of the catalytic core is CUGANGAG[base paired
with)GGAAA. In this invention, the use of 2'-Galkyl substituted nucleotides
that maintain or enhance the catalytic activity and or nuclease resistance ofi
the hammerhead ribozyme is described. Although substitutions of any
nucleotide with any of the modified nucleotides shown in Figure 81 are
possible, and were indeed synthesized, the basic structure composed of
promarily 2'-O-Me nucleotides weth selected substitutions was chosen to
maintain maximal catalytic activity (Yang et al. Biochemistry 1992, 31,
5005-5009 and Paolella et a!. , EMBO J. 1592, 11, 1913-1919) and ease
of synthesis, but is not limiting to this invention.
Ribczymes from Figure 80 and Table 45 were synthesized and
assayed for catalytic activity and nuclease resistance. With .the exception
of entries 8 and 17, all of the modified ribozymes retained at lease 1/10 of
the wild-type catalytic activity. From Table 45, all 2'-modified ribozymes
showed very large and significant increases in stability in human serum
(shown) and in the other fluids described below (Example 55, data not
shown). The order of most agressive nuclease activity was fetal bovine
CA 02468048 2004-06-07
X25
serum, > human serum >human plasma > human synovial fluid. As an
overall measure of the effect of these 2'-substitutions on stability and
activity, a ratio (3 was calculated (Table 45). This f3 value indicated that
all
modified ribozymes tested had significant, >100 - >1700 fold, increases in
overall stability and activity. These increases in fi indicate that the
lifetime
of these modified ribozymes in vivo are significantly increased which
should lead to a more pronounced biological effect.
More general substitutions of the 2'-modified nucleotides from Figure
81 also increased the t1 i2 of the resulting modified ribozymes.
However the catalytic activity of ~ these ribozymes was
decreased > 10-fold.
In Figure 86 _ compound 37 may be used as a general
intermediate to prepare derivatized 2'C-alkyl phosphoramidites,
where X is CH3, or an alkyl, or other group described above.
The following are non-limiting examples showing the synthesis of
nucleic acids using 2'-C-alkyl substituted phosphoramidites, the syntheses
of the amidites, their testing for enzymatic activity and nuclease resistance.
Example 53: Synthesis of Hammerhead Ribozymes Containing 2'-Deoxv-
2'-Alkvlnucieotides & Other 2'-Modified Nucleotides
The method of synthesis used generally follows the procedure for
normal RNA synthesis as described in Usman,N.; Ogilvie,K.K.; Jiang,M.-Y.;
Cedergren,R.J. J. Am. Chem. Soc. 1987, 709, 785-7854 and in
Scaringe,S.A.; Franklyn,C.; Usman,N. Nucleic Acids Res. 1990, 78, 5433-
5441 and makes use of common nucleic acid protecting and coupling
groups, such as dimethoxytrityl at the 5'-end, and phosphoramidites at the
3'-end (compounds 10, 12, 17, 22, 31, 18, 26, 32, 36 and 38). Other
2'-modified phosphoramidites were prepared according to: 3 & 4, Eckstein
ef al. International Publication No. WO 92/07065; and 5 Kois et al.
Nucleosides & Nucleotides 1993, 72, 1093-1109. The .average stepwise
coupling yields were -98%. The 2'-substituted phosphoramidites were
incorporated into hammerhead ribozymes as shown. in Figure 80.
However, these 2'-alkyl substituted phosphoramidites may be incorporated
not only into hammerhead ribozymes, but also into hairpin, hepatitis delta
virus, Group I or Group 11 intron catalytic nucleic acids, or into antisense
CA 02468048 2004-06-07
126
oligonucleotides. They are, therefore, of general use in any nucleic acid
structure.
Example 54: Ribozvme Activity Assav
Purified 5'-end labeled RNA substrates (15-25-mers) and purified 5'-
end labeled ribozymes (-36-mers) were both heated to 95 °C, quenched
on ice and equilibrated at 37 °C, separately. Ribozyme stock solutions
were 1 mM, 200 nM, 40 nM or 8 nM and the final substrate RNA
concentrations were - 1 nM. Total reaction volumes were 50 mL. The
assay buffer was 50 mM Tris-CI, pH 7.5 and 10 mM MgCl2. Reactions were
initiated by mixing substrate and ribozyme solutions at t = 0. Aliquots of 5
mL were removed at time points of 1, 5, 15, 30, 60 and 120 m. Each time
point was quenched in formamide loading buffer and loaded onto a 15%
denaturing polyacryfamide gel for analysis. Quantitative analyses were
performed using a phosphorimager (Molecular Dynamics).
Example 55: Stability Assay
500 pmol of gel-purified 5'-end-labeled ribozyrnes were precipitated
in ethanol and pelleted by centrifugation. Each pellet was resuspended in
mL of appropriate fluid (human serum, human plasma, human synovial
fluid or fetal bovine serum) by vortexing for 20 s at room temperature. The
20 samples were placed into a 37 °C incubator and 2 mL aliquots were
withdrawn after incubation for 0, 15, 30, 45, 60, 120, 240 and 480 m.
Aliquots were added to 20 mL,of a solution containing 95% formamide and
0.5X TBE (50 mM Tris, 50 mM borate, 1 mM EDTA) to quench further
nuclease activity and the samples were frozen until loading onto gels.
Ribozymes were size-fractionated by electrophoresis in 20%
acrylamide/8M urea gels. The amount of intact ribozyme at each time point
was quantified by scanning the bands with a phosphorimager (Molecular
Dynamics) and the half-life of each ribozyme in the fluids was determined
by plotting the percent intact ribozyme vs the time of incubation and
extrapolation from the graph.
Exam I 56: ' '-Q Tetraiso ro 1-disiloxane-1 3-di I - ' Ph nox hi -
carbonyl-Uridine (7)
To a stirred solution of 3',5'-O-(tetraisopropyl-disiloxane-1,3-diyl)-
uridine, 6, (15.1 g, 31 mmol, synthesized according to Nucleic Acid
CA 02468048 2004-06-07
127
Chemistry, ed. Leroy Townsend, 1986 pp. 229-231) and dimethylamino-
pyridine (7.57 g, 62 mmol) a solution of phenylchlorothionoformate (5.15
mL, 37.2 mmol) in 50 mL of acetonitrile was added dropwise and the
reaction stirred for 8 h. TLC (EtOAc:hexanes ! 1:1) showed disappearance
of the starting material. The reaction mixture was evaporated, the residue
dissolved in chloroform, washed with water and brine, the organic layer
was dried over sodium sulfate, filtered and evaporated to dryness. The
residue was purified by flash chromatography on silica gel with
EtOAc:hexanes / 2:1 as eluent to give 16.44 g (85%) of 7.
Example 57: 3'.5'-O-(Tetraisooropvl-disiloxane-1 3-d~~~-2'-C-Ally) -Uridine
To a refluxing, under argon, solution of 3',5'-O-(tetraisopropyl-
disiloxane-1,3-diyi)-2'-D-phencxythiocarbonyl-uridine, 7, (5 g, 8.03 mmol)
and allyltributyltin (12.3 mL, 40.15 mmol) in dry toluene, benzoyl peroxide
(0.5 g) was added portionwise during 1 h. The resulting mixture was
allowed to reflux under argon for an additional 7-8 h. The reaction was
then evaporated and the product 8 purified by flash chromatography on
silica gel with EtOAc:hexanes / 1:3 as eluent. Yield 2.82 g (68.7%).
Example 58: 5'-O-Dimethoxytrityl-2'-C-Allyl-Uridine X91
A solution of 8 (1.25 g, 2.45 mmol) in 10 mL of dry tetrahydrofuran
(THF) was treated with a 1 M solution of tetrabutylammoniumfluoride in
THF (3.7 mL) for 10 m at room temperature. The resulting mixture was
evaporated, the residue was loaded onto a silica gel column, washed with
1 L of chloroform, and the desired deprotected compound was eluted with
chloroform:methanol / 9:1. Appropriate fractions were combined, solvents
removed by evaporation, and the residue was dried by coevaporation with
.dry pyridine. The oily residue was redissolved in dry pyridine,
dimethoxytritylchloride (1.2 eq) was added and the reaction mixture was
left under anhydrous conditions overnight. The reaction was quenched
with methanol (20 mL), evaporated, dissolved in chloroform, washed with
5% aq. sodium bicarbonate and brine. The organic layer was dried over
sodium sulfate and evaporated. The residue was purified by flash
chromatography on silica gel, EtOAc:hexanes / 1:1 as eluent, to give 0.85 g
(57%) of 9 as a white foam.
CA 02468048 2004-06-07
128 '
Example 59: 5'-O-Dirnethoxvtritvl-2'-GAIIyI-Uridine ~C~anoethyl N N-
diiso~ropylphosphoramidite~ ~1 Ol
5'-O-Dimethoxytrityl-2'-C-allyl-uridine (0.64 g, 1.12 mmol) was
dissolved in dry dichloromethane under dry argon. N,N-Diisopropylethyl-
amine (0.39 rnL, 2.24 mmol) was added and the solution was ice-cooled.
2-Cyanoethyl N,N-diisopropylchlorophosphoramidite (0.35 mL, 1.57 mmol)
was added dropwise to the stirred reaction solution and stirring was
continued for 2 h at RT. The reaction mixture was then ice-cooled and
quenched with 12 mL of dry methanol. After stirring for 5 m, the mixture
was concentrated in vacuo (40 °C) and purified by flash chromatography
on silica gel using a gradient of 10-60% EtOAc in hexanes containing 1
°!°
triethylamine mixture as eluent. Yield: 0.78 g (90°1°), white
foam.
Example 60: 3'.5'-D-fTetraisopropyl-disiloxane-1 3-diyl)-2'-C-All~l~_
Acet~~l-Cytidine (111
Triethylamine (6.35 mL, 45.55 mmol) was added dropwise to a stirred
ice-cooled mixture of 1,2,4-triazole (5.66 g, 81.99 mmol) and phosphorous
oxychloride (0.86 mL, 9.11 mmol) in 50 mL of anhydrous acetonitrile. To
the resulting suspension a solution of 3',5'-O-(tetraisopropyl-disiloxane-
1,3-diyl)-2'-C-allyl uridine (2.32 g, 4.55 mmol) in 30 mL of acetonitrile was
added dropwise and the reaction mixture was stirred for 4 h at room
temperature. The reaction was concentrated in vacuo to a minimal volume
(not to dryness). The residue was dissolved in chloroform and washed with
water, saturated aq. sodium bicarbonate and brine. The organic layer was
dried over sodium sulfate and the solvent was removed in vacuo. The
resulting foam was dissolved in 50 mL of 1,4-dioxane and treated with 29%
aq. NH40H overnight at room temperature. TLC (chloroform:methanol
9:1) showed complete conversion of the starting material. The solution was
evaporated, dried by coevaporation with anhydrous pyridine and
acetylated with acetic anhydride (0.52 mL, 5.46 mmol} in pyridine
30. overnight. The reaction mixture was quenched with methanol, evaporated,
the residue was dissolved in chloroform, washed with sodium bicarbonate
and brine. The organic layer was dried over sodium sulfate, evaporated to
dryness and purified by flash chromatography on silica gel (3% MeOH in
chloroform). Yield 2.3 g (90%) as a white foam.
CA 02468048 2004-06-07
129
Example 61: 5'-O-Dimethoxytrityl-2'-GA11y1-N'4-Acetyl-Cytidine
This compound was obtained analogously to the uridine derivative 9
in 55% yield.
Exam,~le 62: 5'-O-Dimethox~rtrityl-2'-C-allyl-N4-Acetyl-Cytidine 3'-12-
Cyan~oeth_yl N,N-diisoprooylohosphoramiditel 1121
2'-O-Dimethoxytrityl-2'-C-allyl-N4-acetyl cytidine (0.8 g, 1.31 mmol)
was dissolved in dry dichloromethane under argon. N,N-Diisopropylethyl-
amine (0.46 mL, 2.62 mmol) was added and the solution was ice-cooled.
2-Cyanoethyl N,N-diisopropylchlorophosphoramidite (0.38 mL, 1.7 mmol)
was added dropwise to a stirred reaction solution and stirring was
continued for 2 h at room temperature. The resction mixture was then ice-
cooled and quenched with 12 mL of dry methanol. After stirring for 5 m, the
mixture was concentrated in vacuo (40 'C) and purified by flash
chromatography on silica gel using chloroform:ethanol / 98:2 with 2%
triethylamine mixture as eluent. Yield: 0.91 g (85°l°), white
foam.
Exam~~le 63: 2'-Deoxy-2'-Methylene lJridine
2'-Deoxy-2'-methylene-3',5'-D-(tetraisopropyldisiloxane-1,3-diyl)- v
uridine 1 a (Hansske,F.; Madej,D.; Robins,M. J. Tetrahedron 1984, 40, 125
and Matsuda,A.; Takenuki,K.; Tanaka,S.; Sasaki,T.; Ueda,T. J. Med. Chem.
1991, 34, 812) (2.2 g, 4.55 mmol ) dissolved in THF (20 mL) was treated
with 1 M TBAF in THF (10 mL) for 20 m and concentrated,in vacuo. The
residue was triturated with petroleum ether and chromatographed on a
silica gel column. 2'-Deoxy-2'-methylene-uridine (1.0 g, 3.3 mmol, 72.5%)
was eluted with 20% MeOH in CH2C12.
Example 64: 5'-O-DMT-2'-Deoxy-2'-Meth~rlene-Uridine (151
2'-Deoxy-2'-methylene-uridine (0.91 g, 3.79 mmol) was dissolved in
pyridine (10 mL) and a solution of DMT-CI in pyridine (10 mL) was added
dropwise over 15 m. The resulting mixture was stirred at RT far .12 h and
MeOH (2 mL) was added to quench the reaction. The mixture was
concentrated in vacuo and the residue taken. up in CH2Cl2 (100 mL) and
washed with sat. NaHCOg, water and brine. The organic extracts' were
dried over MgS04, concentrated in vacuo and purified over a silica gel
column using EtOAc:hexanes as efuant to yield 15 (0.43 g, 0.79 mmol,
22%).
CA 02468048 2004-06-07
130
Example 65: 5'-O-DMT-2'-Deoxy-2'-Methylene-Uridine 3'-(2~yanoethyl
N, N-diisoprowlahosphoramidite,}~1~
1-(2'-Deoxy-2'-methylene-5'-O-dimethoxytrityl-(i-D-ribofuranosyl)
uracil (0.43 g, 0.8 mmol) dissolved in dry CH2C12 (15 ~mL) was placed in a
round-bottom flask under Ar. Diisopropyiethylamine {0.28 mL, 1.6 mmol)
was added, followed by the dropwise addition of 2-cyanoethyl N,N-diiso-
propylchlorophosphoramidite (0.25 mL, 1.12 mmol). The reaction mixture
was stirred 2 h at RT and quenched with ethanol (1 mL}. After 10 m the
mixture evaporated to a syrup in vacuo (40 °C). The product (0.3 g, 0.4
mmoi, 50%) was purified by flash column chromatography over silica gel
using a 25-70% EtOAc gradient in hexanes, containing 1°I°
triethylamine,
as eluant. Rf 0.42 (CH2C12: MeOH / 15:1 )
Example 66: 2'-Deoxv-2'-Difluoromethvlene-3'.5'-O-
lTetrai°oprop,~ldi°ilox~
ane-1.3-d~rl)-Uridinj
2'-Keto-3',5'-D-(tetraisopropyldisiloxane-1,3-diyl)uridine 14 (1.92 g,
12.6 mmol) and triphenylphosphine (2.5 g, 9.25 mmol} were dissolved in
diglyme (20 mL), and heated to a bath temperature of 160 °C. A warm (60
°C) solution of sodium chlorodifluoroacetate in digiyme (50 mL) was
added
(dropwise from an equilibrating dropping funnel) over a period of -1 h. The
resulting mixture was further stirred for 2 h and concentrated in vacuo. The
residue was dissolved in. CH2C12 and chromatographed over silica gel. 2'-
Deoxy-2'-difluoromethylene-3',5'-O-(tetraisopropyldisiloxane-1,3-diyl)-
uridine (3.1 g, 5.9 mmol, 70°!°) eluted with 25°I°
hexanes in EtOAc.
Example 67: 2'-Deoxv-2'-Difluoromethylene-Uridine
2'-Deoxy-2'-methylene-3',5'-O-(tetraisopropyldisiloxane-1,3-diyl)-
uridine (3.1 g, 5.9 mmol) dissolved in THF (20 mL) was treated with 1 M
TBAF in THF (10 mL} for 20 m and concentrated in vacuo. The residue was
triturated with petroleum ether and chromatographed on silica gel column.
2'-Deoxy-2'-difluoromethylene-uridine (1.1 g, 4:0 mmol, 68%) was eluted
with 20% MeOH in CH2C12.
_Examole 68: 5'-O-DMT-2'-Deoxv-2'-Difluoromethvlene-Uridine i16)
2'-Deoxy-2'-difluoromethylene-uridine (1.1 g, 4.0 mmol) was
dissolved in pyridine (10 mL) and a solution of DMT-CI (1.42 g, 4.18 mmol)
in pyridine (10 mL) was added dropwise over 15 m. The resulting mixture
CA 02468048 2004-06-07
131
was stirred at RT for 12 h and MeOH (2 mL) was added to quench the
reaction. The mixture was concentrated in vacuv and the residue taken up
in CH2C12 (100 mL) and washed with sat. NaHC03, water and brine. The
organic extracts were dried over MgS04, concentrated in vacuo and
purified over a silica gel column using 40% EtOAc:hexanes as eluant to
yield 5'-O-DMT-2'-deoxy-2'-difluoromethylene-uridine 16 (1.05 g, 1.8
mmol, 45°!°).
Example 69: 5'-O-DMT-2'-Deoxy-2'-Difluorometh~lene-Uridine 3'~j2-
Cyanoethyl N.N-diisopropyl~hosphoramidite~~l8,~
1-(2'-Deoxy-2'-difluoromethylene-5'-O-dimethoxytrityl-~-D-ribofurano-
syl)-uracil (0.577 g, 1 mmol) dissolved in dry CH2C12 (15 mL) was placed in
a round-bottom flask under Ar. Diisopropylethylamine (0.36 mL, 2-mmol}
was added, followed by the dropwise addition of 2-cyanoethyl N,N-diiso-
propylchlorophosphoramidite (0.44 mL, 1.4 mmol). The reaction mixture
was stirred for 2 h at RT and quenched with ethanol (1 mL). After 10 m the
mixture evaporated to a syrup in vacuo (40 °C). The product (0.404 g,
0.52
mmol, 52%) was purified by flash chromatography over silica gel using 20-
50% EtOAc gradient in hexanes, containing 1 % triethylamine, as eluant. Rt
0.48 (CH2C12: MeOH ! 15:1).
Example 70: 2'-Deoxv-2'-Methvlene-3'.5'-O-(Tetraisoprooyldisiloxane-1
diy,-4-N-Acetxl-Cytidine 20
Triethylamine (4.8 mL, 34 mmol) was added to a solution of POC13
(0.65 mL, 6.8 mmol) and 1,2,4-triazole (2.1 g, 30.6 mmol) in acetonitriie (20
mL) at 0 °C. A solution of 2'-deoxy-2'-methylene-3',5'-O-
(tetraisopropyldi-
siloxane-1,3-diyl) uridine 19 (1.65 g, 3.4 mmol) in acetonitrile (20 mL) was
added dropwise to the above reaction mixture and left to stir at room
temperature for 4 h. The mixture was concentrated in vacuo, dissolved in
CH2C12 (2 x 100 mL) and~washed with 5% NaHC03 (1 x100 mL). The
organic extracts were dried over Na~S04 concentrated in vacua, dissolved
30~ in dioxane (10 mL) and aq. ammonia (20 mL). The mixture was stirred for
12 h and concentrated in vacuo. The residue was azeotroped with
anhydrous pyridine, (2 x 20 mL). Acetic anhydride (3 mL) was added to the
residue dissolved in pyridine, stirred at RT for 4 h and quenched with sat.
NaHC03 (5 mL). The mixture was concentrated in vacuo, dissolved in
CH2C12 (2 x 100 mL) and washed with 5% NaHC03 (1 x 100 mL). The
CA 02468048 2004-06-07
. 132
organic extracts were dried over Na2S04, concentrated in vacuo and the
residue chromatographed over silica gel. 2'-Deoxy-2'-methylene-3',5'-O-
(tetraisopropyldisiloxane-1,3-diyl)-4-N-acetyl-cytidine 20 (1.3 g, 2.5 mmol,
73%) was eluted with 20% EtOAc in hexanes.
Example 71: 1-(2'-Deoxy-2'-Methyrlene-5'-O-Dimethox~~tritvl-8-D-ribo-
furanosYl_)-4-N-Acetyl-Cytosine 21
2'-Deoxy-2'-methylene-3',5'-O-(tetraisopropyldisiloxane-1,3-diyl)-4-N
acetyl-cytidine 20 (1.3 g, 2.5 mmol) dissolved in THF (20 mL) was treated
with 1 M TBAF in THF (3 mL) for 20 m and concentrated in vacuo. The
residue was triturated with petroleum ether and chromatographed on silica
gel column. 2'-Deoxy-2'-methylene-4-N-acetyl-cytidine (0.56 g, 1.99 mmol,
80%) was eluted with 10% MeOH in CH2C12. 2'-Deoxy-2'-methylene-4-N
acetyl-cytidine (0.56 g, 1.99 mmol) wGS dissolved in pyridine (10 mL) and a
solution of DMT-CI (0.81 g, 2.4 mmol) in pyridine (10 mL) was added
dropwise over 15 m. The resulting mixture was stirred at RT for 12.h and
MeOH (2 mL) was added to quench the reaction. The mixture was
concentrated in vacuo and the residue taken up in CH2C12 (100 mL) and
washed with sat. NaHC03 (50 mL), water (50 mL) and brine (50 mL). The
organic extracts were dried over MgS04, concentrated in vacuo and
purified over a silica gel column using EtOAc:hexanes ! 60:40 as eluant to
yield 21 (0.88 g, 1.5 mmol, 75%).
Examale 72: 1-(2'-Deoxv-2'-Methvlene-5'-O-Dimethoxytrityl- -D-ribo-
furanosyll-4-N-Acetyl-Cytosine 3'-f2-Cyanoethyl-N N-diisopropylphosohor-
amidite~~221
1-(2'-Deoxy-2'-methylene-5'-O-dimethoxytrityl-(3-v-ribofuranosyl)-4-N-
acetyl-cytosine 21 (0.88 g, 1.5 mmol) dissolved in dry .CH2C12 (10 mL) was
placed in a round-bottom flask under Ar. Diisopropylethylamine (0.8 mL,
4.5 mmol) was added, followed by the dropwise addition of 2-cyanoethyl
N,N-diisopropylchlorophosphoramidite (0.4 mL, 1.8 mmol). The reaction
mixture was stirred 2 h at room temperature and quenched with ethanol (1
mL). After 10 m the mixture evaporated to ~a syrup in vacuo (40 °C).
The
product 22 (0.82 g, 1.04 mmol, 69%) was purified by flash chromatography
over silica gel using 50-70% EtOAc gradient in hexanes, containing 1
triethylamine, as eluant. Rf 0.38 (CH2C12:MeOH / 20:1).
CA 02468048 2004-06-07
- 133
Example 73' 2'-DeoxX-2'-Difluoromethylene-3' S'-O-(Tetraisopropvl
di°iloxane-1,3-dyl~-4-N-Acetyl-Cytidine (241
Et3N (6.9 mL, 50 mmol) was added to a solution of POC13 (0.94 mL,
mmol) and 1,2,4-triazole (3.1 g, 45 mmol) in acetonitrile (20 mL) at 0
°C.
5 A solution of 2'-deoxy-2'-difluoromethylene-3',5'-O-(tetraisopropyldisilox-
ane-1,3-diyl)uridine 23 ([described in example 14] 2.6 g, 5 mmol) in
acetonitrile (20 mL) was added dropwise to the above reaction mixture and
left to stir at RT for 4 h. The mixture was concentrated in vacua, dissolved
in CH2C12 (2 x 100 mL) and washed with 5% NaHC03 (1 x 100 mL). The
10 organic extracts were dried over Na~S04 concentrated in vacuo, dissolved
in dioxane (20 mL) and aq. ammonia (30 mL). The mixture was stirred for
12 h and concentrated in vacuo. The residue was azeotroped with
anhydrous pyridine (2 x 20 mL). Acetic anhydride (5 mL) was added to the
residue dissolved in pyridine, stirred at RT for 4 h and quenched with sat.
NaHC03 (5mL). The mixture was concentrated in vacuo, dissolved in
CH2C12 (2 x 100 mL) and washed with 5% NaHC03 (1 x 100 mL). The
organic extracts were dried over Na2S04, concentrated in vacuo and the
residue chromatographed over silica gel. 2'-Deoxy-2'-difluoromethylene-
3',5'-O-(tetraisopropyldisiloxane-1,3-diyl)-4-N-acetyl-cytidine 24 (2.2 g, 3.9
mmol, 78%) was eluted with 20% EtOAc in hexanes.
Example 74' 1-f2'-Deexy-2'-Difluoromethylene-5'-O-Dimethoxytrityl~-D-
ribofuranosyl)-4-N-Acetyl-Cytosine 2~
2'-Deoxy-2'-difluoromethylene-3',5'-D-(tetraisopropyldisiloxane-1,3-
diyl)-4-N-acetyl-cytidine 24 (2.2 g, 3.9 mmol) dissolved in THF (20 mL) was
treated with 1 M TBAF in THF (3 mL) for 20 m and concentrated in vacuo.
The residue was triturated with petroleum ether and chromatographed on a
silica gel column. 2'-Deoxy-2'-difluoromethylene-4-N-acetyl-cytidine (0.89
g, 2.8 mmol, 72°l°) was eluted with 10% MeOH in CH2C12. 2'-Deoxy-
2'-
difluoromethylene-4-N-acetyl-cytidine (0.89 g, 2.8 mmol) was dissolved in
pyridine (10 mL) and a solution of DMT-CI (1.03 g, 3.1 mmol) in pyridine
(10 mL) was added dropwise over 15 m. The resulting mixture was stirred
at RT for 12 h and MeOH (2 mL) was added to quench the reaction. The
mixture was concentrated in vacuo and the residue taken up in CH2C12
(100 mL) and washed with sat. NaHC03 (50 mL), water (50 mL) and brine
(50 mL). The organic extracts were dried over MgS04, concentrated in
CA 02468048 2004-06-07
134
vacuo and purified over a silica ge! column using EtOAc:hexanes / 60:40
as eluant to yield 25 (1.2 g, 1.9 mmol, 68%).
Example 75: 1-(2'-Deoxy-2'-Difluoromethylene-5'-D-Dim~thoxvtritvl-Q-D
ribofuranosvll-4-N-Acetylcytesine 3'-(2-c anoethyl-N N-diisonrowlflhos
phoramidite,~ j261
1-(2'-Deoxy-2'-difluoromethylene-5'-D-dimethoxytrityl-~i-D-ribofurano-
syl)-4-N-acetyicytosine 25 (0.6 g, 0.97 mmol) dissolved in dry CH2C12 (10
mL) was placed in a round-bottom flask under Ar. Diisopropylethylamine
(0.5 mL, 2.9 mmol) was added, followed by the dropwise addition of 2-
cyanoethyl N,N diisopropylchlorophosphoramidite (0.4 mL, 1.8 mmol). The
reaction mixture was stirred 2 h at RT and quenched with ethanol (1 mL).
After 10 m the mixture was evaporated to a syrup in .vacuo (40
°C). The
product 26, a white foam (0.52 g, 0.63 mmol, 65%) was purified by flash
chromatography over silica gel using 30-70% EtOAc gradient in hexanes,
containing 1 °!° triethylamine, as eluant. Rt 0.48 (CH2CI2:MeOH
l 20:1 ).
Example 76: 2'-Keto-3'.5'-O-(Tetraisoprooyldisiloxane-1 3-diyll-6-N-(4-f
Butylbenzoyl~-Adenosine (,2~~
Acetic anhydride (4.6 mL) was added to a solution of 3',5'-O-(tetraiso-
propyldisiloxane-1,3-diyl)-6-N-(4-t-butylbenzoyl)-adenosine (Brown,J.;
Christodolou, C.; Jones,S.; Modak,A.; Reese,C.; Sibanda,S.; Ubasawa A.
J. Chem .Soc. Perkin Tans. l 1989, 1735) (6.2 g, 9.2 mmol) in DMSO (37
mL) and the resulting mixture was stirred at room temperature for 24 h. The
mixture was concentrated in vacuo. The residue was taken up in EtOAc
and washed with water. The organic layer was dried over MgS04 and
concentrated in vacuo. The residue was purified on a silica gel column to
yield 2'-keto-3',5'-O-(tetraisopropyldisiloxane-1,3-diyl)-6-N-(4-t-butylben-
zoyl)-adenosine 28 (4.8 g, 7.2 mmol, 78%).
Examnfe 77: 2'-Deoxy-2'-methvlene-3'.5'-O-(Tetraisooropvldisiloxane 1 3
_diyll-6-N-(4-t-Butylbenzovl)-Adenosine
Under a pressure of argon, sec-butyllithiurn in hexanes (1.1.2 mL, 14.6
mmol) was added to a suspension of triphenylmethyiphosphonium iodide
(7.07 g,17.5 mmol) in THF (25 mL) cooled at -78 °C. The homogeneous
orange solution was allowed to warm to -30 °C and a solution of 2'-keto-
3',5'-O-(tetraisopropyldisiloxane-1,3-diyl)-6-N-(4-t-butylbenzoyl)-adenosine
CA 02468048 2004-06-07
135
28 (4.87 g, 7.3 mmol} in THF {25 mL) was transferred to this mixture under
argon pressure. After warming to RT, stirring was continued for 24 h. THF
was evaporated and replaced by CHZC12 (250 mL), water was added (20
mL), and the solution was neutralized with a cooled solution of 2% HCI.
The organic layer was washed with H20 (20 mL), 5% aqueous NaHC03
(20 mL), H20 to neutrality, and brine (10 mL). After drying (Na2S04), the
solvent was evaporated in vacuo to give the crude compound, which was
chromatographed on a silica gel column. Elution with light petroleum
ether:EtOAc / 7:3 afforded pure 2'-deoxy-2'-methylene-3',5'-O-(tetraiso-
propyldisiloxane-1,3-diyl)-6-N-(4-i-butylbenzoyl)-adenosine 29 (3.86 g, 5.8
mmol, 79°I°).
Example 78: 2'-Deoxy-2'-Methyfene-6-N-f4-t-Butytbenzoy~-Adenosine
2'-Deoxy-2'-methylene-3',5'-O-(tetrai~opropyldisiloxane-1,3-diyl)-6-N-
(4-t-butylbenzoyl)-adenosine (3.86 g, 5.8 mmol) dissolved in THF (30 mL)
was treated with 1 M TBAF in THF {15 mL) for 20 m and concentrated in
vacuo. The residue was triturated with petroleum ether and
chromatographed on a silica ge! column. 2'-Deoxy-2'-methylene-6-N-(4-t
butylbenzoyl)-adenosine (1.8 g, 4.3 mmof, 74°I°) was eluted with
10°l°
MeOH in CH2C12.
Example 79: 5'-O-DMT-2'-Deoxy-2'-Meth~~fene-6-N-(4-t-6utylbenzoyll-
Adenosine (29)
2'-Deoxy-2'-methylene-6-N-(4-t-butylbenzoyl)-adenosine (0.75 g,
1.77 mmol) was dissolved in pyridine (10 mL) and a solution of DMT-CI
(0.66 g, 1.98 mmol) in pyridine (10 mL) was added dropwise over 15 m.
The resulting mixture was stirred at RT for 12 h and MeOH (2 mL) was
added to quench the reaction. The mixture was concentrated in vacuo and
the residue taken up in CH~C12 (100 mL) and washed with sat. NaHC03,
water and brine. The organic extracts were dried over MgS04,
concentrated in vacuo and purified over a silica gel column using
50°!°
EtOAc:hexanes as an eluant to yield 29 ~{0.81 g, 1.1 mmol, 62%}.
Example 80: 5'-D-DMT-2'-Deoxv-2'-Methvlene-6-N j4-t-8utylbenzoyll-
Adenosine 3'-(2-Cyanoethvl N,N diisopro ylohosphoramiditel 1317
1-(2'-Deoxy-2'-methylene-5'-O-dimethoxytrityl-(3-D-ribofuranosyl)-6-N
(4-f-butylbenzoyl)-adenine 29 dissolved in dry CH2C12 (15 mL) was placed
CA 02468048 2004-06-07
136 '
in a round bottom flask under Ar. Diisopropylethylamine was added,
followed by the dropwise addition ofi 2-cyanoethyl N, N-
diisopropylchlorophosphoramidite. The reaction mixture was stirred 2 h at
RT and quenched with ethanol (1 mL). After 10 m the mixture was
evaporated to a syrup in vacuo (40 °C). The product was purified by
flash
chromatography over silica gel using 30-50% EtOAc gradient in hexanes,
containing 1 % triethylamine, as eluant (0.7 g, 0.76 mrnol, 68%). Rf 0.45
(CH2C12: MeOH / 20:1 )
Example 81: 2'-Deoxy-2'-Difluoromethylene-3'.5'-O-(Tetraisoprooyldi~ifox-
ane-1,3-d~r1)-6-N (4-t-But,~lbenzoyl)-Adenosine
2'-Keto-3',5'-O-(tetraisopropyldisiloxane-1,3-diyl)-6-N-(4-t-butyl-
benzoyl)-adenosine 28 (6.7 g, 10 mmol) and triphenylphosphine (2.9 g, 11
mmol ) were dissolved in diglyme (20 mL), and heated to a bath
temperature of 160 °C. A warm (60 °C} solution of sodium
chlorodifluoroacetate (2.3 g, 15 mmol) in diglyme (50 mL) was added
(dropwise from an equilibrating dropping funnel) over a period ofi ~1 h. The
resulting mixture was further stirred for 2 h and concentrated in vacuo. The
residue was dissolved in GH2Cl2 and chromatographed over silica gel. 2'-
Deoxy-2'-dilluoromethylene-3',5'-O-{tetraisopropyldisiloxane-1,3-diyl}-6-N
(4-i-butylbenzoyl)-adenosine (4.1 g, 6.4 mmol, 64°I°) eluted
with 15%
hexanes in EtOAc.
>=xample 82: 2'-Deoxv-2'-Difluoromethvlene-6-N-~4-t-Butxlbenzovl)-
Adenosine
2'-Deoxy-2'-difluoromethylene-3',5'-O-(tetraisopropyldisiloxane-1,3-
diyl)-6-N-(4-i-butylbenzoyl)-adenosine (4.1 g, 6.4 mmol) dissolved in THF
(20 mL) was treated with 1 M TBAF in THF (10 mL) for 20 m and
concentrated in vacuo. The residue was triturated with petroleum ether
and chromatographed on a silica gel column. 2'-Deoxy-2'-difluoromethyl
ene-6-N-(4-i-butylbenzoyl)-adenosine (2.3 g, 4.9 mmol, 77%) was eluted
with 20% MeOH in CH2C12.
Example 83: 5'-O-DMT-2'-Deoxv-2'-Difluoromethvlene-6-N (4-t-Bu~i-
benzoyll-Adenosine j301
2'-Deoxy-2'-difluoromethylene-6-N-(4-f-butylben2oyl)-adenosine X2.3
g, 4.9 mmol) was dissolved in pyridine (10 mL) and a solution of DMT-CI in
CA 02468048 2004-06-07
137
pyridine (10 mL) was added dropwise over 15 m. The resulting mixture
was stirred at RT for 12 h and MeOH (2 mL) was added to quench the
reaction. The mixture was concentrated in vacuo and the residue taken up
in CH2C12 (100 mL) and washed with sat. NaHC03, water and brine. The
organic extracts were dried over MgS04, concentrated in vacuo and
purified over a silica gel column using 50% EtOAc:hexanes as efuant to
yield 30 (2.6 g, 3.41 mmol, 69%).
Example 84: 5'-O-DMT-2'-Deoxy-2'-Difluoromethvlene-6-NS4-t-Butyt
benzo~~IlAdenosine 3'-(2-C~ranoethyl N N-diisonrop~lphos~horamiditel
32
1-(2'-Deoxy-2'-diffuoromethylene-5'-O-dimethoxytrityl-j3-D-ribofurano-
syl)-6-N-(4-t-butylbenzoyl)-adenine 30 (2.6 g, 3:4 mmol) dissolved in dry
C H 2 C 12 (25 rnL) was placed in a round bottom flask under Ar.
Diisopropyfethylamine (1.2 mL, 6.8 mmol) was added, followed by the
dropwise addition of 2-cyanoethyl N,N-diisopropylchlorophosphoramidite
(1.06 mL, 4.76 mmol). The reaction mixture was stirred 2 h at RT and
quenched with ethanol (1 mL). After 10 m the mixture evaporated to a
syrup in vacuo (40 °C). 32 (2.3 g, 2.4 mmol, 70%} was purified by flash
column chromatography over silica gel using 20-50% EtOAc gradient in
hexanes, containing 1% triethylamine, as eluant. Rf 0.52 (CH2C12: MeOH /
15:1).
Example 85: 2'-Deoxy-2'-Methoxycarbonylmethylidine-,~-O-(Tetraiso-
propyldisiloxane-1.3-diyl)-Uridine (331
Methyl(triphenylphosphoranylidine)acetate (5.4 g,. 16 mmol) was
added to a solution of 2'-keto-3',5'-O-(tetraisopropyl disiloxane-1,3-diyl)-
uridine 14 in CH2C12 under argon. The mixture was left to stir at RT for 30
h. CHZC12 {100 mL) and water were added (20 mL), and the solution was
neutralized with a cooled solution of 2% HCI. The organic layer was
washed with H20 (20 mL), 5% aq. NaHC03 (20 mL), H20 to neutrality, and
brine (10 mL). After drying (Na~S04), the solvent was evaporated in vacuo
to give crude product, that was chromatographed on a silica gel column.
Elution with light petraleurn ether:EtOAc / 7:3 afforded pure 2'-deoxy-2'-
methoxycarbonylmethylidine-3',5'-O-(tetraisopropyldisiloxane-1,3-diyl)-
uridine 33 {5.8 g, 10.8 mmol, 67.5%).
CA 02468048 2004-06-07
138
Example 88: 2'-Deoxy-2'-Methoxvcarbonylmethylidine-Uridine (341
Et3N~3 HF {3 mL) was added to a solution of 2'-deoxy-2'-methoxy-
carboxylmethylidine-3',5'-O-(tetraisopropyldisiloxane-1,3-diyl)-uridine 33
(5 g, 9.3 mmol) dissolved in CH2C12 (20 mL) and Et3N {15 mL). The
resulting mixture was evaporated in vacuo after 1 h and chromatographed
on a silica gel column eluting 2'-deoxy-2'-methoxycarbonylmethylidine-
uridine 34 (2.4 g, 8 mmol, 86%} with THF:CH2C12 / 4:1.
Example 87: 5'-O-DMT-2'-Deoxv-2'-Methoxycarbonylmethylidine-Uridine
2'-Deoxy-2'-methoxycarbonylmethylidine-uridine 34 (1.2 g, 4.02
mmol) was dissolved in pyridine (20 mL). A solution of DMT-CI (1.5 g, 4.42
mmol) in pyridine {10 mL} was added dropwise over 15 m. The resulting
mixture was stirred at RT for 12 h and MeOH (2 mL) was added to quench
the reaction. The mixture was concentrated in vacuo and the residue taken
up in CH2C12 (100 mL} and washed with sat. NaHC03, water and brine.
The organic extracts were dried over MaS04, concentrated in vacuo and
purified over a silica gel column using 2-5% MeOH in CH2C12 as an eluant
to yield 5'-O-DMT-2'-deoxy-2'-methoxycarbonylmethylidine-uridine 35
(2.03 g, 3.46 mmol, 86%).
Example 88: 5'-O-DMT-2'-Deoxv-2'-Methoxycarbonvlmethylidine-Uridine
3'-l2-cvanoethvl-N.N-diisonropv Ir ohosphoramiditel (361
1-(2'-Deoxy-2'-2'-methoxycarbonylmethylidine-5'-O-dimethoxytrityl-(i-
D-ribofuranosyl)-uridine 35 (2.0 g, 3.4 mmol) dissolved in dry CH2Cf2 (10
mL) was placed in a round-bottom flask under Ar. Diisopropylethylamine
(1.2 mL, 6.8 mmol) was added, followed by the drapwise addition of 2-
cyanoethyl N,N-diisopropylchlorophosphoramidite (0.91 mL; 4.08 mmol).
The reaction mixture was stirred 2 h at RT and quenched with ethanol (1
mL). After 10 m the mixture was evaporated to a syrup in vacuo (40 °C).
5'-O-DMT-2'-deoxy-2'-methoxycarbonylmethylidine-uridine 3'-(2-
cyanoethyl-N,N-diisopropylphosphoramidite) 36 (1.8 g, 2.3 mmol, 67%)
was purified by flash column chromatography over silica gel using a 30-
60°!° EtOAc gradient in hexanes, containing 1% triethyiamine, as
eluant. Rf
0.44 (CH2C12:MeOH / 9.5:0.5).
CA 02468048 2004-06-07
139
Examele 89' 2'-Deoxv-2'-Carboxymethylidine-3' S'-O-(Tetraisoprooyldi-
~iloxane-1.3-diyILUridine 37
2'-Deoxy-2'-methoxycarbonylmethylidine-3',5'-O-(tetraisopropyldi
siloxane-1,3-diyl)-uridine 33 (5.0 g, 10.8 mmol) was dissolved in MeOH
(50 mL) and 1 N NaOH solution (50 ml_) was added to the stirred solution
at RT. The mixture was stirred for 2 h and MeOH removed in vacuo. The
pH of the aqueous layer was adjusted to 4.5 with 1 N HCI solution,
extracted with EtOAc (2 x 1.00 mL), washed with brine, dried over MgS04
and concentrated in vacuo to yield the crude acid. 2'-Deoxy-2'-
carboxymethylidine-3',5'-O-(tetraisopropyldisiloxane-1,3-diyl)-uridine 37
(4.2 g, 7.8 mmol, 73%) was purified on a silica gel column using a gradient
of 10-15% MeOH in CHZC12.
The alkyl substituted nucleotides of this invention can be used to form
stable oligonucleotides as discussed above for use in enzymatic cleavage
or antisense situations. Such oligonucleotides can be formed
enzymatically using triphosphate forms by standard procedure.
Administration of such oligonucleotides is by standard procedure. See
Sullivan et al. PCT WO 94!02595.
Oliaonucleotides with 3' and/or 5' Dihalc~phosphonate
This invention synthesis and uses 3' and/or 5' dihalophosphonate-,
e.g., 3' or 5'-CF2-phosphonate-, substituted nucleotides that maintain or
enhance the catalytic activity and/or nuclease resistance of an enzymatic or
antisense molecule.
As the term is used in this application, 5'- and/or 3'-
dihalophosphonate nucleotide containing ribozymes, deoxyribozymes (see
Usman et al., PCT/US94/11649, and
' chimeras, of nucleotides, are catalytic nucleic molecules that contain 5'-
and/or 3'-dihalophosphonate nucleotide components replacing, but not
limited to, double-stranded stems, single-stranded catalytic core
sequences, single-stranded loops or single-stranded recognition
sequences. These molecules are able to cleave (preferably, repeatedly
cleave) separate RNA or DNA molecules in a nucleotide base sequence
specific manner. Such catalytic nucleic acids can also act to cleave
intramolecularly if. that is desired. Such enzymatic molecules can be
targeted to virtually any RNA or DNA transcript. This invention concerns
CA 02468048 2004-06-07
' 140 '
nucleic acids formed of standard nucleotides or modified nucleotides,
which also contain at least one 5'-dihalophosphonate and/or one 3'-
dihalophosphonate group. _
The synthesis of 1-O-Ac-2,3-di-O-8z-D-ribofuranose 5-d-
5+dihalomethylphosphonate in three steps from 1-O-methyl-2,3-O-
isopropylidene-t3-D-ribofuranose 5-deoxy-5-dihalomethylphosphonate is
described (e.g., for the difluoro, in Figure 87). Condensation of this
suitably
derivatized sugar with silylated pyrimidines and purines affords novel
nucleoside 5'-deoxy-5'-dihalomethylphosphonates, These intermediates
may be incorporated into catalytic or antisense nucleic acids by either
chemical {conversion of the nucleoside 5'-deoxy-5'-
dihalomethylphosphonates into suitably protected phosphoramidites 12a
or solid supports 12b, e.g., Figure 88) or enzymatic means (conversion of
the nucleoside 5'-deoxy-5'-dihalomethylphosphonates into their
triphosphates, e.g., 14 Figure 89, for T7 transcription).
Thus, in one aspect the invention features 5' andlor 3'-
dihalonucleotides and nucleic acids containing such 5' and/or 3'-
dihalonucleotides. The general structure of such molecules is shown
below.
0 0
II
(R~O)2pCXp R II
O B 2 O 8 (R30)zPCXz
O B
i I
Rz R~ i Xz R~ CXz R~
_ I
(R30~zP - O (Rs0)zP = O
where R1 is H, 4H, or R, where R is a hydroxyl protecting group, e.g.,
acyl, alkysilyl, er carbonate; each R2 is separately H, OH, or R; each R3 is
separately a phosphate protecting group, e.g,, methyl, ethyl, cyanoethyl, p-
nitrophenyl, or chlorophenyl; each X is separately any halogen; and each B
is any nucleotide base.
The invention in particular features nucleic acid molecules having
such modified nucleotides anti enzymatic activity. In a related aspect the
invention features a method for synthesis of such nucleoside 5'-deoxy-5'-
dihalo and/or 3'-deoxy-3'-dihalophosphonates by condensing a
CA 02468048 2004-06-07
141
dihalophosphonate-containing sugar with a pyrimidine or a purine under
conditions suitable to form a nucleoside 5'-deoxy-5'-dihalophosphonate
and/or a 3'-deoxy-3'-dihalophosphonate.
Phosphonic acids may exhibit important biological properties
because of their similarity 1o phosphates (Engel, Chem. Rev. 1977, 77,
349-367). Blackburn and Kent (J. Chem. Soc., Perkin Trans. 1986, 913-
917) indicate that based on electronic and steric considerations ,_-fluoro
and _,_ difluoromethylphosphonates might mimic phosphate esters better
than the corresponding phosphonates. Analogues of pyro- and
triphosphates 1, where the bridging oxygen atoms are replaced by a
difluoromethylene group, have been employed as substrates in enzymatic
processes (Blackburn et al., Nucleosides & Nucleofides 1985, 4, 165-167;
Blackburn et al., Chem. Scr. 1986, 26, 21-24). 9-(5,5-Difluoro-5-
phosphonopentyl)guanine (2) has been utilized as a multisubstrate
analogue inhibitor of purine nucleoside phosphorylase (Halazy et al., J.
Am. Chem. Soc. 1991, 113, 315-317). Oligonucleotides containing
methylene groups in place of phosphodiester 5'-oxygens are resistant
toward nucleases that cleave phosphodiester linkages between
phosphorus and the 5'-oxygen (Breaker ei al., Biochemistry 1993, 32,
9125-9128), but can still form stable complexes with complementary
sequences. Heinemann et al. (Nucleic Acids Res. 1991, 19, 427-433)
found that a single 3'-methylenephosphonate linkage had a minor
influence on the conformation of a DNA octamer double helix.
CA 02468048 2004-06-07
142
' NH2
O O 0 N
O P x-F O F O
O' O' 0'
OH OH
1
0
N H
I ., N.
N
N NH2
(H0~20PCF2~
2
(ETO}2POCF2Li
3
One common synthetic approach to a,a-difluoro-alkylphosphonates
features the displacement of a leaving group from a suitable reactive
substrate by diethyl (lithiodiffuoromethyl)phosphonate (3) (Obayashi et al.,
Tetrahedron Left. 1982, 23, 2323-2326). However, our attempts to
synthesize nucleoside 5'-deoxy-5'-difluoro-methylphosphonates from 5'-
deoxy-5'-iodonucleosides using 3 were unsuccessful, i.e. starting
compounds were quantitatively recovered. The reaction of nucleoside 5'-
aldehydes with 3, according to the procedure of Martin et al. (Martin et al.,
Tetrahedron Lett. 1992, 33, 1839-1842), led to a complex mixture of
products. Recently, the synthesis of sugar a,a-difluoroalkylphosphonates
from primary sugar triflates using 3 was described (Berkowitz et al., J. Org.
Chem. 1993, 58, 6174-6176). Unfortunately, our experience is that
nucleoside 5'-triflates are too unstable to be used in these syntheses.
The following are non-limiting examples showing the synthesis of
nucleoside 5'-deoxy-5'-difluoromethyl-phosphonates. Those in the art will
recognize that equivalent methods can be readily devised based upon
CA 02468048 2004-06-07
143
these examples. These examples demonstrate that it is possible to
achieve synthesis of 5'-deoxy-5'-difluoro derivatives in good yield and thus
guide those in the art to such equivalent methods. The examples also
indicate utility of such synthesis to provide useful oligonucleotides as
described above.
Those in the art will recognize that useful modified enzymatic nucleic
acids can now be designed, much as described by Draper et al.,
PCT/US94/13129.
Example 90: Synthesis of Nucleoside 5'-Deoxv-5'-
difluorometh~rlpho~honates
Referring to Fia. 87, we synthesized a suitable glycosylating agent
from the known D-ribose a,a-difluoromethylphosphonate (4) (Martin ef al.,
Tetrahedron Lett. 1992, 33, 1839-1842) which served as a key
intermediate for the synthesis of nucleoside 5'-
difluoromethylphosphonates.
Methyl 2,3-O-isopropylidene-~-D-ribofuranose a,a-
difiuoromethylphosphonaie (4) was synthesized from the 5-aldehyde
according to the procedure of Martin ei al. (Tetrahedron Leit. 1992, 33,
1839-1842) (Figure 87). Removal of the isopropylidene group was
accomplished under mild conditions (12-MeOH, reflux, 18 h (Szarek ei al.,
Tetrahedron Lett. 1986, 27, 3827) or Dowex 50 WX8 (H+), MeOH, RT
(about 20-25°C), 3 days) in 72% yield. The anomeric mixture thus
obtained was benzoylated with benzoyl chloridelpyridine to afford the 2,3-
di-O-benzoyl derivative, which was subjected to mild acetolysis conditions
(Walczak et al., Synthesis, 1,993, 790-792) (Ac20, AcOH, H2S04, EtOAc,
0°C. The desired 1-O-acetyl-2,3-di-O-benzoyl-D-ribofuranose
difluoromethylphosphonate (5) was obtained in quantitative yield as an
anomeric mixture. These derivatives were used for selective glycosylation
of silylated uracil and N4-acetylcytosine under Vorbruggen conditions
(Vorbruggen, Nucleoside Analogs. Chemistry, Biology and Medical
Applications, NATO ASI Series A, 26, Plenum Press, New York, London,
1980; pp. 35-69. The use of F3CS020Si(CH3)3 as a glycosylation
catalyst is precluded because it is expected to lead to the undesired 1-
. ethyluracil or 9-ethyladenine byproducts: Podyukova, et al., Tetrahedron
CA 02468048 2004-06-07
144
Lett. 1987, 28, 3623-3626 and references cited therein) (SnCl4 as a
catalyst, boiling acetonitrile) to yield p-nucleosides (62% 6a, 75% 6b).
Glycosylation of silylated N6-benzoyladenine under the same conditions
yielded a mixture of N-9 isomer 6c and N-7 isomer 7 in 34% and 15%
yield, respectively. The above nucleotides were successfully deprotected
using trimethylsilylbromide for the cleavage of the ethyl groups, followed by
treatment with ammonia-methanol to remove the acyl protecting groups.
Nucleoside 5'-deoxy-5'-difluoromethylphosphonates 8 were finally
purified on a DEAE Sephadex A-25 (HC03-) column using a 0.01-0.25 M
TEAR gradient for elution and obtained as their sodium salts (82% 8a; 87%
8b; 82°l° 8c).
Selected analytical data: 31 P-NMR {31 P) and 1 H-NMR (1 H) were
recorded on a Varian Gemini 400. Chemical shifts in ppm refer to H3P04
and TMS, respectively. Solvent was CDC13 unless otherwise noted. 5: 1 H
8 8.07-7.28 (m, Bz), 6.66 (d, J 1,2 4.5, aH1 ), 6.42 {s, ~3H1 ), 5.74 (d, J2,3
4.9,
pH2), 5.67 (dd, J3,2 4.9, J3,4 6.6, ~iH3), 5.63 (dd, J3,2 6.7, J3,4 3.6, aH3),
5.57 {dd, J2,1 4.5, J2,3 6.7, aH2), 4.91 {m, H4), 4.30 (m, CH2CH3), 2.64 (m,
CH2CF2), 2.18 (s, ~iAc), 2.12 (s, aAc), 1.39 {m, CH2CH3). 31 P 8 7.82 (t,
Jp,F 105.2), 7.67 {t, Jp,F 106.5). 6a: 1 H 8 9.11 {s, 1 H, NH), 8.01 (m, 11 H,
Bz, H6), 5.94 (d, J1~,2~ 4.1, 1H, H1'), 5.83 (dd, J5,6 8.1, 1H, H5), 5.79 (dd,
J2,,1 ~ 4.1, J2~,3~ 6.5, 1 H, H2'), 5.71 (dd, Jg~,2~ 6.5, J3~,4~ 6.4, 1 H,
H3'), 4.79
(dd, J4~,3~ 6.4, J4~,F 11.6, 1 H, H4'), 4.31 (m, 4H, CH2CH3), 2.75 (tq, JH,F
19.6, 2H, CH2CF2), 1.40 (m, 6H, CH2CH3). 31P 8 ?.7? (t, Jp,F 104.0). 8c:
31 p (ys DSS) (D20) b 5.71 (t, Jp,F 87.9).
Compound 7 was deacylated with methanolic ammonia yielding the
product that showed ~~max {H20) 271 nm and min 233 nm, confirming that
the site of glycosylation was N-7. .
Example 9l:Svnthesis of Nucleic Acids Containing Modified Nucleotide
~ntaininp Cores
The method of synthesis used follows the procedure for norrria! RNA
synthesis as described in Usman et al., J. Am. Chem. Soc. 1987, 109,
7845-7854 and in Scaringe et aL, Nucleic Acids Res. 1990, y8, 5433-5441
and makes use of common nucleic acid protecting and coupling groups,
such as dimethoxytrityl at the 5'-end, and phosphoramidites at the 3'-end
(Figure 88 and Janda et al., Science 1989, 244:437-440.). These
CA 02468048 2004-06-07
145
nucleoside 5'-deoxy-5'-difluoromethylphosphonates may be incorporated
not only into hammerhead ribozymes, but also into hairpin, hepatitis delta
virus, Group 1 or Group 2 introns, or into antisense oligonucleotides, They
are, therefore, of general use in any nucleic acid structure.
Example 92: Synthesis of Modified Triphosohate
The triphosphate derivatives of the above nucleotides can be formed
as shown in F_ig. 89, according to known procedures. Nucleic Acid Chem.,
Leroy B. Townsend, John Wiley & Sons, New York 1991, pp. 337-340;
Nucleotide Analogs, Karl Heinz Scheit; John Wiley 8 Sons New York 1980,
pp. 211-218.
Equivalent synthetic schemes for 3' dihalophosphonates are shown in
Figures 90 and 91 using art recognized nomenclature. The conditions can
be optimized by standard procedures.
The nucleoside dihalophosphonates described herein are
advantageous as modified nucleotides in any nucleic acid structure, e.g.,
catalytic or antisense, since they are resistant to exo- and endonucleases
that normally degrade unmodified nucleic acids in vivo. They also do not
perturb the normal structure of the nucleic acid in which they are
incorporated thereby maintaining any activity associated with that structure.
These compounds may also be of use as monomers as antiviral and/or
antitumor drugs.
Olic~onucfeotides with Amido or Peptido Modification
This invention replaces 2'-hydroxyl group of a ribonucl.eotide moiety
with a 2'-amido or 2'-peptido moiety. In other embodiments, the 3' and 5'
portions of the sugar of a nucleotide may be substituted, or the phosphate
group may be substituted~with amido or peptido moieties. Generally, such
a nucleotide has the general structure shown in Formula 1 below:
CA 02468048 2004-06-07
146
O
B
0
0
~~ R2
O N"
H R~ R3
p",. p..p
I
O.
F,C~RMULA I
The base (B) is any one of the standard bases or is a modified
nucleotide base known to those in the art, or can be a hydrogen group. In
addition, either R1 or R2 is H or an alkyl, alkene or alkyne group containing
between 2 and 10 carbon atoms, or hydrogen, an amine (primary,
secondary or tertiary, egj, R3NR4 where each R3 and R4 independently is
hydrogen or an alkyl, alkene or alkyne having between 2 and 10 carbon
atoms, or is a residue of an amino acid, i.e., an amide), an alkyl group, or
an amino acid (D or L forms) or peptide containing between 2 and 5 amino
acids. The Zigzag lines represent hydrogen, or a bond to another base or
other chemical moiety known in the art. Preferably, one of R1, R2 and R3 is
an H, and the other is an amino acid or peptide.
Applicant has recognized that RNA can assume a much more
complex structural form than DNA because of the presence of the 2'-
hydroxyl group in RNA. This group is able to provide additional hydrogen
bonding with other .hydrogen donors, acceptors and metal ions within the
RNA molecule. Applicant now provides molecules which have a modified
. amine group at the 2' position, such that significantly more complex
structures. can be farmed by the modified oligonucleotide. Such
modification with a 2'-amido or peptido group leads to expansion and
enrichment of the side-chain hydrogen bonding network. The amide and
peptide moieties are responsible for complex structural formation of the
oligonucleotide and can form strong complexes with other bases, and
interfere with standard base pairing interactions. Such interference will
allow the formation of a complex nucleic acid and protein conglomerate.
CA 02468048 2004-06-07
Oligonucleotides of this invention are significantly more stable than
existing oligonucleotides and can potentially form biologically active
bioconjugates not previously possible for oligonucleotides. They may also
be used for in vitro selection of unique aptamers, that is, randomly
generated oligonucleotides which can be folded into an effective ligand for
a target protein, nucleic acid or polysaccharide.
Thus, in one aspect, the invention features an oligonucleotide
containing the modified base shown in Formula 1, above.
In other aspects, the oligonucleotide may include a 3' or 5' nucleotide
having a 3' or 5' located amino acid or aminoacyl group. Iri all these
aspects, as well as the 2'-modified nucleotide, it will be evident that
various
standard modifications can be made. For example; an "O" may be
replaced with an S, the sugar may lack a base (i.e., abasic) and the
phosphate moiety may be modified to include other substitutions (see
Sproat, supra).
Example 93: General procedure for the preparation of 2'-aminoacwl-2'-
deoxv-2'-aminonucleoside coniuQates.
Referring to Fig~92, to the solution of 2'-deoxy-2'-amino nucleoside (1
mmol) and N-Fmoc L- (or D-) amino acid (1 mmol) in methanol
[dimethylformamide (DMF) and tetrahydrofuran (THF) can also be used], 1-
ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDD) [or 1-
isobutyloxycarbonyl-2-isobutyloxy-1,2-dihydroquinoline (IIDQ)] (2 mmol) is
added and the reaction mixture is stirred at room temperature or up to 50
'C from 3-48 hours. Solvents are removed under reduced pressure and
the residual syrup is chromatographed on the column of silica-gel using 1-
10 % methanol in dichloromethane. Fractions containing the product are
concentrated yielding a white foam with yields ranging from 85 to 95 %.
Structures are confirmed by ~ H NMR spectra of conjugates which show
correct chemical shifts for nucleoside and aminoacyl part of the molecule.
Further proofs of the structures are obtained by cleaving the aminoacyl
protecting groups under appropriate conditions and assigning 1 H NMR
resonances for the fully deprotected conjugate.
Partially protected conjugates described above are converted into
their 5'-O-dimethoxytrityl derivatives and into 3'-phosphoramidites using
standard procedurQS (Oligonucleotide Synthesis: A Practical Approach,
CA 02468048 2004-06-07
~ 148
M.J. Gait ed.; IRL Press, Oxford, 1984). incorporation of these
phosphoramidites into RNA was performed using standard protocols
(Usman et aL, 1987 supra).
A general deprotection protocol for oligonucleotides of the present
invention is described in F-_ia. 93.
The scheme shows synthesis of conjugate of 2'-d-2'-aminouridine.
This is meant to be a non-limiting example, and those skilled in the art will
recognize that, variations to the synthesis protocol can be readily
generated to synthesize other nucelotides (e.g., adenosine, cytidine,
guanosine) and/or abasic moieties.
Example 94: RNA cleavacte by hammerhead ribozymes containing 2'-
aminoacyl modifications.
Hammerhead ribozymes targeted to site N (see Fig. 94) are
synthesized using solid-phase synthesis, as described above. U4 and U7
positions are modified, individually or in combination, with either 2'-NH-
alanine or 2'-NH-lysine.
RNA cleavaoe 2~say m vitro: Substrate RNA is 5' end-labeled using
(.~-32p) ATP and T4 polynucleotide kinase (US Biochemicals). Cleavage
reactions were carried out under ribozyme "excess" conditions. Trace
amount (< 1 nM) of 5' end-labeled substrate and 40 nM unlabeled
ribozyme are denatured and renatured separately by heating to 90°C for
2
min and snap-cooling on ice for 10 -15 min. The ribozyme and substrate
are incubated, separately, at 37°C for 10 min in a buffer containing 50
mM
Tris-HC1 and 10 mM MgCl2. The reaction is initiated by mixing the
ribozyme and substrate solutions and incubating at 37°C. Aliquots of 5
~tt
are taken at regular intervals of time and the reaction is quenched by
mixing with equal volume of 2X formamide stop mix. The samples are
resolved on 20 % denaturing polyacrylamide gels. The results are
quantified and percentage of target RNA cleaved is plotted as a function of
time.
Referring to F_ia~95, hammerhead ribozymes containing 2'-NH-
alanine or 2'-NH-lysine modifications at U4 and U7 positions cleave the
target RNA efficiently.
CA 02468048 2004-06-07
' 149
Sequences listed in Figure 94 and the modifications described . in
Fi ure ~ are meant to be non-limiting examples. Those skilled in the art
wilt recognize that variants (base-substitutions, deletions, insertions,
mutations, chemical modifications) of the ribozyme and RNA containing
other 2'-hydroxyl group modifications, including but not limited to amino
acids, peptides and cholesterol, can be readily generated using techniques
known in the art, and are within the scope of the present invention.
Example 95: Aminoac~rlation of 3'-ends of RNA
!. Referring to F- ig. 96. 3'-OH group of the nucleotide is converted to
succinate as described by Gait, supra. This can be linked with amino-alkyl
solid support (for example: CpG). Zig-zag line indicates linkage of 3'OH
group with the solid support.
11. Preparation of aminoacyl-derivatized solid support
A~ S..ynthesis of O-Dimethox rityl f0-DMT,) amino acid,
Referring to Fig. 97. to a solution of L- (or D-) serine, tyrosine or
threonine (2 mmol) in dry pyridine (15 ml) 4,4'-dimethoxytrityl chloride (3
mmol) is added and the reaction mixture is stirred at RT (about 20-
25°C) for
16 h. Methanol (10 ml) is then added and the solution evaporated under
reduced pressure. The residual syrup was partitioned between 5% aq.
NaHC03 and dichloromethane, organic layer was washed with brine, dried
(NaZSO~) and concentrated in~ vacuo. The residue is purified by flash
silicagel column chromatography using 2-10% methanol in
dichloromethane (containing 0.5 % pyridine). Fractions containing product
are combined and concentrated in vacuo to yield white foam (75-85
°l°
yield).
B~ Preparation of the solid suonort and its derivatization with amino acids
Referring to Fig. 97, the modified solid support (has an OH group
instead of the standard NH2 end group) was prepared according to
Haralambidis et al., Tetrahedron Lett. 1987, 28, 5199, (P denotes
aminopropyl CPG or polystyrene type support). O-DMT or NH-
monomethoxytrityl (NH-MMT amino acid was attached to the above solid
support using standard procedures for derivatization of the solid support
(Gait, 1984, supra) creating a base-labile ester bond between amino acids
CA 02468048 2004-06-07
150
and the support. This support is suitable for the construction of RNAlDNA
chain using suitably protected nucleoside phosphoramidites.
Example 96: Aminoac~rlation of 5'-ends of RNA
1. Referring to Fia. 98, 5'-amino-containing sugar moiety was
synthesized as described (Mag and Engels, 1989 Nucleic Acids f?es. 17,
5973). Aminoacylation of the 5'-end of the monomer was achieved as
described above and RNA phosphoramidite of the 5'-aminoacylated
monomer was prepared as described by Usrraan ef al., 1987 supra. The
phosphoramidite was then incorporated at the 5'-end of the ofigonucleotide
using standard solid-phase synthesis protocols described above.
II. Referring to Fia. 99, aminoacyl groups) is attached to the phosphate
group at the 5'-end of the RNA using standard procedures described
above.
V11. Reversing Genetic Mutations
Modification of existing nucleic acid sequences can be achieved by
homologous recombination. In this process a transfected sequence
recombines with homologous chromosomal sequences and can replace
the endogenous cellular sequence. Boggs, 8 International J. Cell Cloning
80, 1990, describes targeted gene modification. It reviews the use of
homologous DNA recombination to co«ect genetic defects. Banga and
Boyd, 89 Proc. Nafl. Acad. Sci. U.S.A. 1735; 1992, describe a specific
example of in vivo site-directed mutagenesis using a 50 base
oligonucleotide. In this methodology a gene or gene segment is
essentially replaced by the oligonucleotide used.
This invention uses a complementary oligonucleotide to position a
nucleotide base changing activity at a particular site on a gene (RNA or
genomic DNA), such that the nucleotide modifying activity will change (or
revert) a mutation to wild-type, or its equivalent. By reversion or change of
a mutation, we refer to reversion in a broad sense, such as when a
mutation at a second site which leads to functional reversion to a wild type
phenotype. Also, due to the degeneracy of the genetic code, a revenant
may be achieved by changing any one of the three codon positions.
Additionally, creation of a stop codon in a deleterious gene (or transcript)
is
defined here as reversing a mutant phenotype to wild-type. An example of
CA 02468048 2004-06-07
151
this type of reversion is creating a stop colon in a critical HIV proviral
gene
in a human.
Referring to Figures 100 and 101, broadly there are two approaches
to causing a site directed change in order to revert a mutation to wild-type.
In one (Fig. 100) the oligonucleotide is used to target RNA specifically.
RNA is provided with a complementary (Watson-crick) oligonucleotide
sequence to that in the target molecule. In this case the sequence
modifying ofigonucleotide would (analogously to an antisense
oligonucleotide or ribozyme) have to be continuously present to revert the
RNA as it is made by the cell. Such a reversion would be transient and
would potentially require continuous addition of more sequence modifying
oligonucleotide. The transient nature of this approach is an advantage, in _
that treatment could be stopped by simply removing the sequence
modifying oligonucleotide (as with a traditional drug).
A second approach targets DNA (Fi . 1 1) and has the advantage
that changes may be permanently encoded in the target cell's genetic
code. Thus, a single course (or several courses) of treatment may lead to
permanent reversion of the genetic disease. If inadvertent chromosomal
mutations are introduced this may cause cancer, mutate other genes, or
cause genetic ,changes in the germ-line (in patients of reproductive age).
However, if the base changing activity is a specific methylation that may
modulate gene expression it would not necessarily lead to germ-line
transmission. See Lewin, Genesj1983 John Wilely & Sons, Inc. NY pp
493-496.
Complementary base pairing to single-stranded DNA or RNA is one
method of directing an oligonucleotide to a particular site of DNA. This
could occur by a strand displacement mechanism or by targeting DNA
when it is single-stranded (such as during replication, or transcription).
Another method is using triple-strand binding (tripl.ex formation) to double-
stranded DNA, which is an established technique for binding poly-
pyrimidine tracts, and can be extended to recognize all 4 nucleotides. See
Povsic, T., Strobel, S., & Dervan, P. (1992). Sequence-specific double-
strand alkylation and cleavage of DNA mediated by triple-helix formation.
J. Am. Chem. Soc. 114, 5934-5944 (1992). Knorre, D.G., Valentin, V.V.,
Valentina, F.Z., Lebedev, A.V. & Federova, O.S. Design and targeted
reactions of oligonucleotide derivatives 1-366 (CRC Press, Novosibirsk,
CA 02468048 2004-06-07
~ 152
1993) describe conjugation of reactive groups or enzyme to
oligonucleotides and can be used in the methods described herein.
Recently, antisense oligonucleotides have been used to redirect an
incorrect splice into order to obtain correct splicing of a splice mutant
globin
gene in vitro. Dominski Z; Kole R (1993) Restoration of correct splicing in
thalassemia pre-mRNA by antisense oligonucleotides. Proc Natl Acad Sci
U S A 90:8673-7. Analogously, in one preferred embodiment of this
invention a complementary oligomer is used to correct an existiing mutant
RNA, instead of the traditional approach of inhibiting that RNA by
antisense.
In either the RNA or DNA mode, after binding to a particular site on the
RNA or DNA the oligonucleotide will modify the nucleic acid sequence.
This can be accomplished by activating an endogenous enzyme (s, ee
Figure 102), by appropriate positioning of an enzyme (or ribozyme)
conjugated (or activated by the duplex) to the oligonucleotide, or by
appropriate positioning of a chemical mutagen. Specific mutagens, such as
nitrous acid which deaminates C to U, are most useful, but others can also
be used if inactivation of a harmful RNA is desired.
RNA editing is an naturally occurring event in mammalian cells in
which a sequence modifying activity edits a RNA to its proper sequence
post-transcriptionally. Higuchi, M." Single, F., Kohler, M., Sommer; B., and
Seeburg, P. (1993) RNA Editing of AMPA Receptor Subunit GIuR-B: A
base-paired intron-exon structure determines. position and efficiency Cell
75:1361-13?0. The machinery involved in RNA editing can be co-opted by
a suitable oligonucleotide in order to promote chemical modification.
The changes in the base created by the methods of this invention
cause a change in the nucleotide sequence, either directly, or after DNA
repair by normal cellular mechanisms. These changes functionally correct
a genetic defect or introduce a stop codon. Thus, the invention is distinct
from techniques in which an active chemical group (e.g., an alkylatbr) is
attached to an antisense or triple strand oligonucleotide in order to
chemically inactivate the target RNA or DNA.
Thus, this invention crEates an alteration to an existing base in a
nucleic acid molecule so that the base is read in vivo as a different base.
CA 02468048 2004-06-07
153
This includes correcting a sequence instead ofi inactivating a gene but can
also include inactivating a deleterious gene.
Thus, in one aspect, the invention features a method for altering ,j,n_
vivo the nucleotide base sequence of a naturally occurring mutant nucleic
acid molecule. The method includes contacting the nucleic acid molecule
in vivo with an oligonucleotide or peptide nucleic acid or other sequence
specific binding molecules able to form a duplex or triplex molecule with
the nucleic acid molecule. After formation of the duplex or triplex molecule
a base modifying activity chemically or enzymatically alters the targeted
base directly, or after nucleic acid repair in vivo. This results in the
functional alteration of the nucleic acid sequence.
By "alter", as it is used in this context, is meant that one or more
chemical moieties in a targeted base, or bases, is altered so that the mutant
nucleic acid will be functionally different. Thus, this is distinct from prior
methods of correcting defects in DNA, such as homologous recombination,
in which an entire segment of the targeted sequence is replaced with a
segment of DNA from the transfected nucleic acid. This is also distinct from
other methods that use reactive groups to inactivate a RNA or DNA target,
in that this method functionally corrects the sequence of the target, instead
of merely damaging it, by causing it to be read by a polymerase as a
different base from the original base. As noted above, the naturally
occurring enzymes in a cell can be utilized to cause the chemical
alteration, examples of which are provided below.
By "functionally alter" is meant that the ability of the target nucleic acid
to perform its normal function (i.e.., transcription or translation contfol)
is
changed. For example, an RNA molecule may be altered so that it can
cause production of a desired protein, or a DNA molecule can be altered
so that upon DNA repair, the DNA sequence is changed.
By "mutant" it is meant a nucleic acid molecule which is altered in
some way compared to equivalent molecules present in a' normal
individual. Such mutants may be well known in the art, and include,
molecules present in individuals with known genetic deficiencies, such as
muscular dystrophy, or diabetes and the like. It also includes individuals
with diseases or conditions characterized by abnormal expression of a
gene, such as cancer, thalassemia's and sickle cell anemia, and cystic
CA 02468048 2004-06-07
154 '
fibrosis. It allows modulation of lipid metabolism to reduce artery disease,
treatment of integrated AIDS genomes, and AIDs RNA, and Alzeimer's
disease. Thus, this invention concerns alteration of a base in a mutant to
provide a "wild type" phenotype and/or genotype. For deleterious
conditions this involves altering a base to allow expression or prevent
expression as is necassary. When treating an infection, such as HIV, it
concerns inactivation of a gene in the HIV RNA by mutation of the mutant
(i.e., non-human gene) to a wild type (i.e., no production of a non-human
protein). Such modification is performed in traps rather than in cis as in
prior methods.
In preferred embodiments, the oligonucleotide is of a length (at least
12 bases, preferably 17 - 22) sufficient to activate dsRNA deaminase in
viyo to cause conversion of an adenine base to inosine; the
oligonucleotide is an enzymatic nucleic acid molecule that is active to
chemically modify a base (see below); the nucleic acid molecule is DNA or
RNA; the oligonucleotide includes a chemical mutagen, e.g., the mutagen
is nitrous acid; and the oligonucleotide causes deamination of 5-
methylcytosine to thymidine, cytosine to uracil, or adenine to inosine, or
methtylation of cytosine to 5-methylcytosine.
In a most preferred embodiment, the invention features correction of a
mutation, rather than inactivation of a target by causing a mutation.
Using in vitro directed evolution, it is possible to screen for ribozymes
with catalytic activities different than RNA cleavage. Bartel, D. and
Szostak, J. (1993) Isolation of new ribozymes from a large pool of random
sequences. cience 261:1411-1418. Using these methods of in vitro
directed evolution, an enzymatic nucleic acid molecule, or ribozyme that
mutates bases, instead of cleaving the phosphodiester backbone can be
selected. This is a convenient method of obtaining an enzyme with the
appropriate base sequence modifying activities for use in the present
invention.
Sequence modifying activities can change one nucleotide to another
(or modify a nucleotide so that it will be repaired by the cellular machinery
to another nucleotide). Sequence modifying activities could also delete or
add one or more nucleotides to a sequence. A specific embodiment of
adding sequences is described by Sullenger and Cech, PCTlUS94/12976
CA 02468048 2004-06-07
155
in which entire exons with wild-
type sequence are spliced into a mutant transcript. The present invention
features only the addition of a few bases (1 - 3).
Thus,Y in another aspect, the invention features ribozymes or
enzymatic nucleic acid molecules active to change the chemical structure
of an existing base in a separate nucleic acid molecule. Applicant is the
first to determine that such molecules would be useful, and to provide a
description of how such molecules might be isolated.
Molecules used to achieve in situ reversion can be delivered using
the existing means employed for delivering antisense molecules and
ribozymes, including liposomes and cationic lipid complexes. If the in situ
reverting molecule is composed. only of RNA, then expression vectors can
be used in a gene therapy protocol to produce the reverting molecules
endogenously, analogously to antisense or ribozymes expression vectors.
There are several advantages of using such an expression vector, rather
than simply replacing the gene through standard gene therapy. Firstly, this
approach would limit the production of the corrected gene to cells that
already express that gene. Furthermore, the corrected gene would be
properly regulated by its natural transcriptional promoter. Lastly, reversion
can be used when the mutant RNA creates a dominant gain of function
protein (e.g., in sickle cell anemia), where correction of the mutant RNA is
necessary to stop the production of the deleterious mutant protein, and
allow production of the corrected protein.
Endogenous Mammalian RNA Editing System
It was observed in the mid-1980s that the sequence of certain cellular
RNAs were dififerent from the DNA sequence that encodes them. By a
process called RNA editing, cellular RNA are post-transcriptionally
modified to a) create a translation initiation and termination codons, b)
enable tRNA and rRNA to fold into a functional conformation (for a review
see,Bass, B. L. (1993) In The RNA World, R. Gesteland, R. and Atkins, J.
eds. (Cold Spring Harbor,.New York; CSH Lab. Press) pp. 383-418). The
process of RNA editing includes base modification, deletion and insertion
of nucleotides.
Although, the _ RNA editing. process is widespread among lower
eukdryotes, very few Hi~As (four) have been reported to undergo editing in
CA 02468048 2004-06-07
156
mammals (Bass, supra). The predominant mode of RNA editing in
mammalian system is base modification (C ~ U and A -~ G). The
mechanism of RNA editing in the mammalian system is postulated to be
that C--~U conversion is catalyzed by cytidine deaminase. The mechanism
of conversion of A-~G has recently been reported for glutamate receptor B
subunit (gluR-B) in rat PC12 cells (Higuchi, M. et al. (1993) Cell 75, 1361-
1370). According to Higuchi gluR-B mRNA precursor attains a structure
such that intron 11 and exon 11 can form a stable stem-loop structure. This
stem-loop structure is a substrate for a nuclear double strand-specific
adenosine deaminase enzyme. The deamination will result in the
conversion of A--~ I. Reverse transcription followed by double strand
synthesis will result in the incorporation of G in place of A.
In the present invention, the endogenous deaminase activity or other
such activities can be utilised to achieve targeted base modification.
The following are examples of the invention to illustrate different
methods by which in vivo conversion of a base can be achieved. These
are provided only to clarify specific embodiments of the invention and are
not limiting to the invention. Those in the art will recognize that equivalent
methods can be readily devised within the scope of the claims.
Example 97: Exploitin4 celluiar dsRNA dependent Adenine to Inosin,~
converter:
An endogenous activity in most mammalian cells and Xenopus
oocytes converts about 50% of adenines to inosines in double stranded
RNA. (Bass, B. L., 8 Weintraub, H. (1988). An unwinding activity that
covalently modifies it double-stranded RNA substrate. ell, 5;z, 1089-
1098.). This activity can be used to cause an in situ reversion of a
mutation at the RNA level. Referring to Figures 102 and 104, for
demonstration purposes a stop codon is incorporated into the coding
region of dystrophin, which is fused, to the reporter gene luciferase. This
stop codon can be reverted by targeting an antisense RNA which is long
enough to activate the dsRNA deaminase, which converts Adenines to
Inosines. The A to I transition will be read by the ribosome as an A to G
transition in some cases and will thereby functionally revert the stop codon. -
While other A's in this region may be converted to I's and read as G,
converting an A to 1 (G) cannot create a stop codon: The A to I transitions
CA 02468048 2004-06-07
~ 157
in the region surrounding the target mutation will create some point
mutations, however, the function of the dystrophin protein is rarely
inactivated by point mutations.
The reverted mRNA was then translated in a cell lysate and assayed
for luciferase activity. As evidenced by the dramatic increase in luciferase
counts in the graph in figure 103, the A to I transition was read by the
ribosome as an A to G transition and the stop codon has successfully been
reverted with the lysate treated complex. As a control, an irrelevant non-
complementary RNA oligonucleotide was added to the
dystrophinJluciferase mRNA. As expected, in this case no translation
(luciferase activity) is observed because of the stop codon. As an
additional control, the hybrid was not treated with extract, and again no
translation (luciferase activity) is observed (Figure 103).
While other A's in the targeted region may have been converted to I's
and read as G, converting an A to I (G) cannot create a stop codon, so the
ribosome will still read through the region. Dystrophin is not generally
sensitive to point mutations if the open reading frame is maintained, so a
dystrophin protein made from an mRNA reverted by this method should
retain full activity.
The following detail specifics of the methodology: RNA
oligonucleotides were synthesized on a 394 (ABI) synthesizer using
phosphoramidite chemistry. The sequence of the synthetic~complementary
RNA that binds to the mutant dystrophin sequence is as follows (5' to 3'):
CCCGCGGTAGATCTTTCTGGAGGCTTACAGTTTTCTACAAACCTCC
CTTCAAA (Seq. ID No. 1 }
Referring to Fioure 104j fifty-nine base pairs of a human dystrophin
mutant sequence containing a stop codon was fused in frame to the
luciferase coding region using standard cloning technology, into the Hind
111 and Not I sites of pRC-CMV (Invitrogen, San Diego, CA). The AUG of
luciferase was deleted. The sequences of the insert from the Hind III site to
the start of the luciferase coding region is (5' to 3'):
GCCCCTGAGGAGCGATGGAGGCGTTGAAGGGAGGTTTGTGGAAAA
CTGTAAGCCTCCAGAAAGATCTACCGCGG (Seq ID No. 2)
CA 02468048 2004-06-07
. 158
This corresponds to base pairs 3649-3708 of normal dystrophin
(Entrez ID # 311627) with a Sac 11 site at the 3' end. This plasmid was used
as a template for in vitro transcription of mRNA using T7 polymerase with
the manufacturers protocol (Promega, Madison, WI).
Xenopus nuclear extracts were prepared in 0.5X TGKED buffer (0.5X=
25mM Tris (pH 7.9), 12.5°l° glycerol, 25 mM KCI, 0.25mM DTT and
0.05mM
EDTA), by vortexing nuclei and resuspended in a volume of 0.5X TGKED
equal to total cytoplasm volume of the oocytes. Bass, B.L.. 8 Weintraub, H.
Cell 55, 1089-i 098 (1988).
The target mRNA at 500ng/ul was pre-annealed to 1 micromolar
complementary or irrelevant RNA oligonucleotide by heating to 70°C, and
allowing it to slowly cool to 37°C over 30 minutes. Fifty nanograms of
mRNA pre-annealed to the RNA oligonucleotides was added to 7u1 of
nuclear extracts containing 1 mM ATP, 15mM EDTA, 1600un/ml RNasin
and 12.5mM Tris pH 8 to a total volume of l2ul. Bass, B.L. & Weintraub, H.
supra. This mixture, which contains the dsRNA deaminase activity, was
incubated for 30 minutes at 25°C. Next, l.5ul of this mixture was added
to
a rabbit reticulocyte lysate in vitro translation mixture and translated for
two
hours according to the manufacturers protocol (Life Technologies,
Gaithersberg, MD), except that an additional 1.3 mM magnesium acetate
was added to compensate for the EDTA carried through from the nuclear
extract mixture. 1-uciferase assays were performed on l5u) of extract with
the Promega luciferase assay system (Promega, Madison, WI), and
luminescence was detected with a 96 well luminometer, and the results are
displayed in the graph in figure 102.
Example 98: Base changing activities
The chemical synthesis of antisense and triple-strand forming
oligomers conjugated to reactive groups is well studied and characterized
(Knorre, D.G., Valentin, V.V., Vafentina, F.Z., Lebedev, A.V. 8. Federova,
O.S. Design and targeted reactions of oiigonucleoiide derivatives 1-366
(CRC Press, Novosibirsk, 1993) and Povsic,.T., Strobel, S. 8 Dervan, P.
Sequence-specific double-strand alkylation and cleavage of DNA
mediated by triple-helix formation J. Am. Chem. Soc. 114, 5934-5944
(1992). Reactive groups such as alkylators that can modify nucleotide
bases in targeted RNA or DNA have been conjugated to oligonucleotides.
CA 02468048 2004-06-07
~ 159
Additionally enzymes that modify nucleic acids have been conjugated to
oligonucleotides. (Knorre, D.G., Valentin, V.V., Valentina, F.Z., Lebedev,
A.V. 8~ Federova, O.S. Design and targeted reactions of oligonucleotide
derivatives 1-366 (CRC Press, Novosibirsk, 1993). In the past these
conjugated chemical groups or enzymes have been used to inactivate
DNA or RNA that is specifically targeted by antisense or triple-strand
interactions. Below is a list of useful base changing activities that could be
used to change the sequence of DNA or RNA targeted by antisense or
triple strand interactions, in order to achieve in situ reversion of
mutations,
as described herein (see figure 100-104).
1. Deamination of 5-methylcytosine to create thymidine
(performed by the_enzyme ,cytidine deami,nase .(Bass,, .B.L. "in."T.he RNA .._
lNorld (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, 1993).
Also, nitrous acid or related compounds promote oxidative deamination of
C to be read at T(Microbial Genetics, David Freifelder, Jones and Bartiett
Publishers, Inc., Boston,1987, PP.226-230.). Additionally hydroxylamine
or related compounds can transforrii C to be read at T (Microbial Genetics,
David Freifelder, Jones and Bartlett Publishers, inc., Boston,1987, PP.226
230.)
2. Deamination of cytosine to create uracii (performed by the
enzyme cytidine deaminase (Bass, B.L. in The RNA World (Cold Spring
Harbor Laboratory Press, Cold Spring Harbor, 1993) or by chemical
groups similar to nitrous acid that promote oxidative deamination (Microbial
Genetics, David Freifelder, Jones and Bartlett Publishers, Inc.,
Boston,1987, PP.226-230.)
3. Deamination of Adenine to be read like G (Inosine) (as done
by the adenosine deaminase, AMP deaminase or the dsRNA deaminating
activity ( Bass, B.L. in The RNA World (Cold Spring Harbor Laboratory
Press, Cold Spring Harbor, 1993).
4. Methylation of cytosine to 5-methylcytosine
5. Transforming thymidine (or uracil) to 02-methyl thymidine (or
02-methyl uracil), to be read as cytosine by alkynitrosoureas (Xu, and
Swann, Tetrahedron Letters 35:303-306 (1994)).
CA 02468048 2004-06-07
' 160 '
6. Transforming guanine to 6-0-methyl (or other alkyls) to be
read as adenine (Mehta and Ludlum, Biochimica et Biophysica Acta,
521:770-778 (1978) which can be done with the mutagen ethyl methane
sulfonate (EMS) Microbial Genetics, David Freifelder, Jones and Bartlett
Publishers, Inc., Boston,1987, PP.226-230.
7. Amination of uracil to cytosine (as performed by the cellular
enzyme CTP synthetase (Bass, B.L, in The RNA World (Cold Spring Harbor
Laboratory Press, Cold Spring Harbor, 1993).
The following are examples of useful chemical modifications that can
be utilized in the present invention. There are a few preferred
straightforward chemical modifications that can change one base to
another base. Appropriate mutagenic chemicals are placed on the
targetting oligonucleotide, e.g., nitrous acid, or a suitable protein with
such
activity. Such chemicals and proteins can be attatched by standard
procedures. These include molecules which introduce fundamental
chemical changes, that would be useful independent of the particular
technical approach. See Lewin, Genes.1983 John Wilely & Sons, Inc. NY
pp 42-48.
The following matrix shows that the chemical modifications noted can
cause transversion reversions (pyrimidine to pyrimidine, or purine to
purine} in RNA or DNA. The transversions (pyrimidine to purine, or purine
to pyrimidine) are not preferred because these are more difficult chemical
transformations. The footnotes refer to the specific desired chemical
transformations. The bold footnotes refer to the reaction on the opposite
DNA strand. For exsmple, if one desires to change an A to a G, this can be
accomplished at the DNA level by using reaction ~5 to change a T to a C in
the opposing strand. !n this example an ArT' base pair goes to A/C , then
when the DNA is replicated, or mismatch repair occurs this can become
G/C, thus the original A has been converted to a G.
~ ~ ISR matrix
Reverted Base
Mutant base A T(U) C G
CA 02468048 2004-06-07
i61
A Transversion Transversion DNA~.~3~RNA3
T(U) Transversion DNASiRNA7 Transversion
C Transversion RNA2/DNA6 Transversion
G DNA6iRNA6 Transversion Transversion
1 Deamination of 5-methylcytosine to create thymidine.
2 Deamination of cytosine to create uracil.
3 Deamination of Adenine to be read like G (Inosine).
4 Methylation of cytosine to 5-methyfcytosine.
5 Transforming thymidine (or uracil) to 02-methyl thymidine (or
02-methyl uracil), to be read as cytosine (Xu, and Swann, Tetrahedron
Letters 35:303-306 (1994)).
6 Transforming guanine to 6-O-methyl (or other alkyls) to be
read as adenine (Mehta and Ludlum, Biochimica et Biophysica Acta,
521:770-778 (1978)).
7. Amination of uracil to. cytosine. Bass supra, fig. 6c.
In Vitro defection Strateqyr
Referring to Figure 105, there is provided a schematic describing an
approach to selecting for a ribozyme with such base changing activity. An
RNA is designed that folds back on itself (this is similar to approaches
already used to select for RNA ligases, Bartel, D. and Szostak, ,1. (1993)
Isolation of new ribozymes from a large pool of random sequences.
Science 261:1411-1418). A degenerate loop opposing the base to be
modified provides for diversity. After incubating this library of molecules in
a buffer, the RNA is reverse transcribed into DNA (that is, using standard in
vitro evolution protocol. Tuerk and Gold, 249 cience 505, 1990) , .and
then the DNA is selected for having a base change. A restriction enzyme
cleavage and size selection or its equivalent is used to isolate the fraction
of DNAs with the appropriate base change. The cycle could then be
repeated many times.
CA 02468048 2004-06-07
~s2 '
The in vitro selection (evolution) stralegy is similar to approaches
developed by Joyce (Beaudry, A. A. and Joyce, G.F. (1992) Science 257,
635-641; Joyce, G. F. (1992) scientific American 267, 90-97) and Szostak
(Bartel, D, and Szostak, J. (1993) cience 2fi1:1411-1418; Szostak, J. W.
(1993) TIB 17, 89-93). Briefly, a random pool of nucleic acids is
synthesized wherein, each member contains two domains: a) one domain
consists of a region with defined (known) nucleotide sequence; b) the
second domain consists of a region with degenerate (random) sequence.
The known nucleotide sequence domain enables: 1) the nucleic acid to
bind to its target (the region flanking the mutant nucleotide), 2)
complimentary DNA (cDNA) synthesis and PCR amplification of 'molecules
selected for their base modifying activity, 3) introduction of restriction
endonuclease site far the- purpose of cloning.- The degenerate domain can
be created to be completely random (each of the four nucleotides
represented at every position within the random region) or the degeneracy
can be partial (Beaudry, A. A. and Joyce, G.F. (i992) ci nc 257, 635-
641 ). In this invention, the degenerate domain is flanked by regions
containing known sequences (see Figure 105), such that the degenerate
domain is placed across from the mutant base (the base that is targeted for
modification). This random library of nucleic acids is incubated under
conditions that ensure folding of the nucleic acids into conformations that
facilitate the catalysis of base modification (the reaction protocol may also
include certain cofactors like ATP or GTP or an S-adenosyl-methionine (if
methylation is desired) in order to~ make the selection more stringent).
Following incubation, nucleic acids are converted into complimentary DNA
(if the starting pool of nucleic acids is RNA). Nucleic acids with base
modification (at the mutant base position) can be separated from rest of the
population of nucleic acids by using a vGriety of methods. For example, a
restriction endonuclease cleavage site can either be created or abolished
as a result of base modification. If a restriction endonuclease site is
created as a result of base modification, then the library can be digested
with the restriction endonuclease (RE). The fraction of the population that
is cleaved by the RE is the population that has been able to catalyze the
base modification reaction (active pool). A new piece ~ of DNA (containing
oligonucleotide primer binding sites for PCR and RE sites for cloning) is
ligated to the termini of the active pool to facilitate PCR amplification and
subsequent cycles (if necessary) of selection. The final pool of nucleic
acids with the best base modifying activity is cloned in to a plasmid vector
CA 02468048 2004-06-07
' 163
and transformed into bacterial hosts. Recombinant plasmids can then be
isolated from transformed bacteria and the identity of clones can be
determined using DNA sequencing techniques,
Base modifying enzymatic nucleic acids (identified via in vitro
selection) can be used to cause the chemical modification in vivo.
In addition, the ribozyme could be evolved to specifically bind a
protein having an enzymatic base changing acitivity.
Such ribozymes can be used to cause the above chemical
modifications in vivo. The ribozymes or above noted antisense-type
molecules can be administered by methods discussed in the above
referenced art.
VIII. Administration of Nucleic Acids
Applicant has determined that double-stranded nucleic acid lacking
a transcription termination signal can be used for continuous expression of
the encoded RNA. This is achieved by use of an R-loop, i.e., an RNA
molecule non-covalently associated with the double-stranded nucleic acid
and which causes localized denaturation ("bubble" formation) within the
double stranded nucleic acid (Thomas et al., 1976 Proc. Natl. Acad. Sci.
U A 73, 2294). In addition, applicant has determined that that the RNA
20' portion of the R-laop can be used to target the whole R-loop complex to a
desirable intracellular or cellular site, and aid in cellular uptake of the
complex. Further, applicant indicates that expression of enzymatically
active RNA or ribozymes can be significantly enhanced by use of such R
loop complexes.
Thus, in one aspect, the invention features a method for introduction
of enzymatic nucleic acid into a cell or tissue. A complex of a first nucleic
acid encoding the enzymatic nucleic acid and a second nucleic acid
molecule is provided, The second nucleic acid molecule has sufficient
complementarity with the first nucleic acid to be able to form an R-loop
base pair structure under physiological conditions, The R-loop is formed in
a region of the first nucleic acid molecule which promotes expression of
RNA from the first nucleic acid under physiological conditions. The method
further includes contacting the complex with a cell or tissue under
CA 02468048 2004-06-07
' 164
conditions in which the enzymatic nucleic acid is produced within the cell
or tissue.
By "complex" is simply meant that the two nucleic acid molecules
interact by intermolecular bond formation (such as by hydrogen bonding)
between two complementary base-paired sequences. The complex will
generally be stable under physiological condition such that it is able to .
cause initiation of transcription firom the first nucleic acid molecule.
The first and second nucleic acid molecules may be formed from any
desired nucleotide bases, either those naturally occurring (such as
adenine, guanine, thymine and cytosine), or other bases well known in the
art, or may have modifications at the sugar or phosphate moieties to allow
greater stability or greater complex formation to be achieved. In addition,
such molecules may contain non-nucleotides in place of nucleotides.
Such modifications are well known in the ari, see e.g:, Eckstein et al.,
International Publication No. WO 92/07065; Perrault ei aL, 1990 Nature
344, 565; Pieken ei al., 1991 cience, 253, 314; Usman and Cedergren,
1992 Trends in Biochem. Sci. 17, 334; Usman et al., International
Publication No. WO 93/15187; and Rossi ei al., International Publication
No. WO 91/03162, as well as Sproat,B. European Patent Application
927 7 0298.4 which describe various chemical modifications that can be
made to the sugar moieties of enzymatic RNA molecules.
By "sufficient complementarity" is meant that sufficient base pairing
occurs so that the R-loop base pair structure can be formed under the
appropriate conditions to cause transcription of the enzymatic nucleic acid.
These in the art will recognize routine tests by which such sufficient base
pairs can be determined. In general, between about 15 - 80 bases is
sufficient ~in this invention.
By "physiological condition" is meant the condition in the cell or .
tissue to be targeted by the first nucleic acid molecule, although the R-loop
complex may be formed under many other conditions. One example is use
of a standard physiological saline at 37oC, but it is simply desirable in this
invention that the R-loop structure exists to some extent at the site of
action
so that the expression of the desired nucleic acid will be achieved at that
site of action. While it is preferred~that the R-loop structure be stable
under
CA 02468048 2004-06-07
' . 165
those conditions, even a minimal amount of formation of the R-loop
structure to cause expression will be sufficient. Those in the art will
recognize that measurement of such expression is readily achieved,
especially in the absence of any promoter or leader sequence on the first
nucleic acid molecule (Daube and von Hippel, 1992 cien a 258, 1320).
Such expression can thus only be achieved if an R-Poop structure is truly
formed with the second nucleic acid. if a promoter of leader sequence is
provided, then it is preferred that the R-loop be formed at a site distant
from
those regions so that transcription is enhanced.
In a related aspect, the invention features a method for introduction
of ribonucleic acid within a cell or tissue by forming an R-loop base-paired
structure (as described above) with the first nucleic acid molecule lacking
any promoter region or transcription termination signal such that once
expression is initiated it will continue until the first nucleic acid is
degraded.
In another related aspect, the invention features a method in which
the second nucleic acid is provided with a localization factor, such as a
protein, e.g., an antibody, transferin, a nuclear localization peptide, or
folate, or other such compounds well known in the art, which wilt aid in
targeting the R-loop complex to a desired cell or tissue.
In preferred embodiments, the first nucleic acid is a plasmid, e.g.,
one without a promoter or a transcription termination signal ; the second
nucleic acid is of length between about 40-200 bases and is formed of
ribonucleotides at a majority of positions; and the second nucleic is
covalently bonded with a ligand. such as a nucleic acid,, protein, peptide,
lipid, carbohydrate, cellular receptor, nuclear localization factor, . or is
attached to maleimide or a thiol group: the first ~ nucleic acid is an
expression plasmid lacking a promoter able to express a desired gene,
e.g., it is a double-stranded molecule formed with a majority of
deoxyribonucleic acids; the R-loop complex is a RNAlDNA heteroduplex;
no promoter or leader region is provided in the first nucleic acid; and the R-
loop is adapted to prevent nucleosome assembly and is designed to aid
recruitment of cellular transcription machinery. ,
1n other preferred embodiments, the first nucleic acid encodes one or
more enzymatic nucleic acids, e.g., it is formed. with a plufality of
CA 02468048 2004-06-07
intramolecular and intermolecular cleaving enzymatic nucleic acids to
allow release of therapeutic enzymatic nucleic acid in vivo.
In a further related aspect, the invention features a complex of the
above first nucleic acid molecules and second nucleic acid molecules.
R-loop comr'lex
An R-loop complex is designed to provide a non-integrating plasmid
so that, when an RNA polymerase binds to the plasmid, transcription is
continuous until the plasmid is degraded. This is achieved by hybridizing
an RNA molecule, 40 to 200 nucleotides in length, to a DNA expression
plasmid resulting in an R-loop structure (see fi4ure 106). This RNA, when
conjugated with a ligand that binds to a cell surface receptor, triggers
internalization of the plasmid/RNA-ligand complex. Formation of R-loops in
general is described by DeWet, 1987 Methods in Enzymo~,, 145, 235;
Neuwald et al., 1977 J. Virol. 21,1019; and Meyer et al., 1986 J. Ult. Mol,
tr. Re . 96, 187. Thus, those in the art can readily design complexes of
this invention following the teachings of the art.
Promoters placed in retroviral genomes have not always behaved as
planned in that the additional promoter will serve as a stop signal or
reverses the direction of the pofymerase. Applicant was told that crealion
of an R-loop between the promoter and the reporter gene increased the
transfection efficiency. Incubation of an RNA molecule with a double-
stranded DNA molecule, containing a region of complementarity with the
RNA will result in the formation of a stable RNA-DNA hetroduplex and the
DNA strand that has a sequence identical to the RNA will be displaced into
a loop-like structure called the R-loop. This displacement of DNA strand
occurs because an RNA-DNA duplex is more stable compared to a DNA-
DNA duplex. Applicant was also told that an 80 nt long RNA was used to
generate a R-loop structure in a plasmid encoding the f3-galactosidase
gene. The R-loop was initiated either in the promoter region or in the
leader sequence. Plasmids containing ,an R-loop structure were
micr~oinjected into the cytoplasm of CAS cells and the gene expression
was assayed. R-loop formation in the promoter region of the plasmid
inhibited expression of the gene. RNA that hybridized to the leader
sequence between the promoter and the gene, or directly to the first 80
nucleotides of the mRNA increased the expression levels 8-10 fold. The
CA 02468048 2004-06-07
proposed mechanism is that R-loop formation prevents nucleosome
assembly, thus making the DNA more accessible for transcription.
Alternatively, the R-loop may resemble a RNA primer promoting either DNA
replication or transcription (Daube and von Hippel, 1992, su ra .
~ O~ie of the salient features of this invention is to generate R-loops in
expression vectors of choice and introduce them into cells to achieve
enhanced expression from the expression vector. The presence of an R-
loop may aid in the recruitment of cellular transcription machinery. Once
an RNA polymerase binds to the plasmid and initiates transcription, the
process will continue until a termination signal is reached, or the plasmid is
degraded.
This invention will increase the expression of ribozymes inside a
cell. The idea is to construct a plasmid with no transcription termination
signal, such that a transcript-containing multiple ribozyme units can be
generated. In order to liberate unit length ribozymes, self-processing
ribozymes can be cloned downstream of each therapeutic ribozyme (seg
figure 107) as described by Draper supra.
Liaand Tar etina
Another salient feature of this invention is that the RNA used to
generate R-loop structures can be covalently finked to a ligand (nucleic
acid, proteins, peptides, lipids, carbohydrates, eic.). Specific ligands can
be chosen such that the ligand can bind selectively to a desired cell
surface receptor. This ligand-receptor interaction will help internalize a
plasmid containing an R-loop. Thus, RNA is used to attach the ligand to the
DNA such that localization of the gene to certain regions of the cell is
achieved. One of several methods can be used to attach a ligand to RNA.
This includes the incorporation of deoxythymidine containing a 6 carbon
spacer having a terminal primary amine into the RNA (see figure 108). This
amino group can be directly derivatized with the ligand, such as folate (Lee
and Low, 1994 J. Biol. Chem. 269, 3198-3204). The RNA containing a fi
carbon spacer with a terminal amine group is mixed with folate and the
mixture is reacted with activators like 1-(3-Dimethyiaminopropyl)-3-
ethylcarbodiimide hydrochloride (EDC). This reaction should be carried
out in the presence of 1-Hydroxybenzotriazole hydrate (HOST) to prevent
any undesirable side reactions. .
CA 02468048 2004-06-07
The RNA can also be derivatized with a heterobifuctional
crosslinking agent (or linker) like succinimidyl 4-(p-
maleimidopheny!)butyrate (SMPB): The SMPB introduces a maleimide
into the RNA. This maleimide can then react with a thiol moiety either in a
peptide or in a protein. Thiols can also be introduced into proteins or
peptides that Pack naturally occurring thiols using succinylacetylthioacetate.
The amino linker can be attached at the 5' end or 3' end of the RNA. The
RNA can also contain a series of nucleotides that do not hybridize to the
DNA and extend the linker away from the RfVAIDNA complex, thus
increasing the accessibility of the ligand for its receptor and not
interfering
with the hybridization. These techniques can be used to fink peptides such
as nuclear localization signal (NLS) peptides (t-anford et al., 1984 ell 37,
801-813; Kalderon et al., 1984 ell 39, 499-509; Goldfarb et al., 1986
Nature 322, 641-644)and/or proteins like the transferrin (Curiel et al., 1991
Proc. Natl. Acad. Sci. USA 88, .8850-8854; Wagner et al., 1992 Proc. Natl.
Acad. Sci. USA 89, 6099-6103; Giulio et al., 1994 Cell. Signal. 6, 83-90) to
the ends of R-loop forming RNA in order to facilitate the uptake and
localization of the R-loop-DNA complex. To link a protein to the ends of R-
loop forming RNA, an intrinsic thiol can be used to react with the maleimide
or the thiols can be introduced into the protein itself using either
iminothiofate or succinimidyl acetyl thioacetate (SATA; Duncan et al., 1983
Anal. Biochem 132, 68). The SATA requires an additional deprotection
step using 0.5 M hydroxylamine.
In addition liposomes can be used to cause an R-loop complex to be
delivered to an appropriate intracellular cite by techniques well known in
the art. For example, pH-sensitive liposomes (Connor and Huang, 1986
dancer Res, 46, 3431-3435) can be used to facilitate DNA transfection.
Calcium phosphate mediated or electroporation-mediated delivery of
the R-loop complex in to desired cells can also be readily acomplished.
In vitro Selection
In vitro selection strategies can be used to select nucleic acids that a)
can form stable R-loops b) selectively bind to specific cell surface
receptors. These nucleic acids can then be covalently linked to each other.
This will help internalize the R-Poop-containing plasmid efficiently using
receptor-mediated endocytosis. The in vitro selection (evolution) strategy is
CA 02468048 2004-06-07
iss
similar to approaches developed by Joyce (BEaudry and Joyce, 1992
Science 257, 635-641; Joyce, 1992 Scientific American 267, 90-97) and
Szostak (Barrel and Szostak, 1993 cience 261:1411-1418; Szostak,
1993 T I B 17, 89-93). Briefly, a random pool' of nucleic acids is
synthesized wherein each member contains two domains: a) one domain
consists of a region with defined (known) nucleotide sequence; b) the
second domain consists of a region with degenerate (random) sequence.
The known nucleotide sequence domain enables: 1 ) the nucleic acid to
bind to its target (a specific region of the double strand DNA), 2)
complimentary DNA (cDNA) synthesis and PCR amplification of molecules
selected for their affinity to form R-loop and/or their ability to bind to a
specific receptor, 3) introduction of a restriction endonuclease site for the
purpose of cloning. The degenerate domain can .be .created to be
completely random (each of the four nucleotides represented at every
position within the random region) or the degeneracy can be partial
(Beaudry and Joyce, 1992 cience 257, 635-641). In this invention, the
degenerate domain is flanked by regions containing known sequences.
This random library of nucleic acids is incubated under conditions that
ensure equilibrium binding to either double-stranded DNA or cell surface
receptor. Following incubation, nucleic acids are converted into
complementary DNA (if the starting pool of nucleic acids is RNA). Nucleic
acids with desired characteristics can be separated from the rest of the
population of nucleic acids by using a variety of methods (Joyce, 1992
su ra . The desired pool of nucleic acids can then be carried through
subsequent rounds of selection to enrich the population with the most
desired traits. These molecules are then cloned in to appropriate vectors.
Recombinant plasmids can then be isolated from transformed bacteria and
the identity of clones can be determined using DNA sequencing
techniques.
Other embodiments are within the following claims.
CA 02468048 2004-06-07
1
TA
Ch2racteristics of Ribozvmes
Group 1 lntrons
Size: -200 to >1000 nucleotides.
Requires a U in the target sequence immediately 'S' of the cleavage
site.
hinds 4-6 nucleotides at 5' side of cleavage site.
Over 75 known members of this class. Found in Tefrahyme~a
thermophila rRNA, fungal mitcchondria, chloroplasts, phage T4, blue-
green algae, and others.
RNAseP RNA (M1 RNA)
Size: -290 to 400 nucleotides.
RNA portion of a ribonucleoprotein enzyme. Cleaves tRNA precursors
to form mature tRNA.
Roughly 10 known members of this group all are bacterial in origin.
Hammerhead Ribozyme
Size: -13 to 40 nucleotides.
Requires the target sequence UH immediately 5' of the cleavage site.
Binds a variable number nucleotides on both sides of the cleavage
site.
14 known members of this class. Found in a number of plant
pathogens (virusoids} that use RNA as the infectious agent (Figures 1
and 2)
Hairpin Ribozyme
Size: -50 nucleotides.
Requires the target sequence GUC immediately 3' of the cleavage site.
Binds' 4-6 nucleotides at 5' side of the cleavage site and a variable
number to the 3' side of the cleavage site.
Only 3 known member of this class. Found in three plant pathogen
(satellite RNAs of the tobacco ringspot virus, arabis mosaic virus
and chicory yellow mottle virus) which uses RNA as the
infectious agent (Figure 3).
Hepatitis Delta Virus (HDV) Ribozyme
Size: 50 - 60 nucleotides (at present).
Cleavage of target RNAs recently demonstrated.
Sequence requirements not fully determined.
Binding sites and structural requirements not fully determined,
although no sequences 5' of cleavage site are required.
Only 1 known member of this class. Found in human HDV (Figure 4).
Neurospora VS RNA Ribozyme
Size: -144 nucleotides (at present)
CA 02468048 2004-06-07
l~l
Cleav~oe of ;~roet RNAs recently demonstrated.
Sequence requirements not fully determined.
binding sites and structural requirements not fully determined. Only 1
known member of this class. Found in Neurospora VS RNA (Figure 5).
CA 02468048 2004-06-07
172
Table 2
HumGn ICBM HH Taraet sequence
nt. Position Teraet Sequences nt. Position Target Sequences
i C C C C=.GJC ~CC-~ JG 2 b 6 nC C v'GU '~C',;
C~::
~,C'J
23 C'JC~G'~J C C:JCUG:J ?~4 CUC~C'J C C: -C~.CG
26 ~G.."'L'CC',JC UG~'"L:~C'J420 C~CCC ~ C C;C'LJCL'U
Cv'CL~-CTJA CUC'-~G-':GS2s C'JCCC~J C LZ;Cz-CiG
34 L,'G.:~~C'JC F~G.=.GL'UG427 CCC:.'UC'JU G:~:AG~.C
40 UC_'-.GnGUU GC=ACL'C7 4~0 ~G~,ACCU 'J.~CCC'JAC
48 C~-,~,~,CCUC tiGCC'JCG 4~_ C~_CCULi .~C:.C'JACG
~4 UC~GCCU C G~;r:UG: 456 L'U?~CC::LJA CC-C"UGC~
58 cc,;c~,~ ~ UGGL--ccc 4 a ~ cc~accu c ~ccGUG;,
J
64 L:-,UC~J C CCi:C-C<yG.10 L'GCL'G..'"UC CGUCz,C-G
6 CCGC~CU C CUG~JCC 564 CUG~.C~~U C =_C;~CC~
102 UC;: uC...':IJC CUG.: JCG 3 S 2 G~C~U C ? CC~UG::
108 UC~JG..:J C GCi-~~:C 6u7 ~C-C~.~,~,UiJUC'UCG'JG
115 CGC.C-:n~UC LwuC'CC EG8 GCCFAL'U U CLC'GUG.~.
'_,g C~JC'u'GU U CCC~C-::~ 60~ CC~L'L,'U C L;C~JGC;.
120 C'JCL'C-uDC CCAG~C c 1= :-..:~L'L'UC'JC G'.~GCCG~.
14 C~Gr.CJiU C UGUGUCC E = c" GaC~~1J U UCH
6 G'J C,~C
1 S2 UCUGJGU C CCC_~JCJy E'_- 7 ~~L;G',,'UU GCr_r~C~
158 UCCCCCU C nn:AGUC 668 ~C~CCLJ C Ciz.CCCC
l E~ C~,~-u:GU C nUCCUGC 6 7 GCCCCC'J ~ CCrC-w"LTC
168 ra-.GUG~U C C'JGCCCC 684 ACC~C~Ci1 C C~C~CCU
8 5 C-;~GG~: C C ~"L3G E 9 2 C? GACCU U UGL'CCUG
J
209 ~G~.~CCU C WJGUGAC E' ~CCW U GL:~CWJGC
227 CcCT~GU U GLL'GwC E96 CCL'L'UG'JC CIJCiCAG
2 3 :zAGULGU U G:~;~CAUA 7 0 r GC C:~CUC C C
0 C C~C~
237 UGC..~U A GnGrCCC 720 C~C~ACL1 U G'JGGrCC
248 hCCCCGU L'GCCL?~? X23 :-ACWGiJ C AC-CCCCC
2 S GL~ a GC A n.:-.~,~;G;~,7 ? ~ C C C ~ C CL~G.Cz:
3 ~J :,"J
2 6. ~=.C~Gv U Gw~JCCUG ~ 3 6 C-C,C-L7CCU? G=_C~.':UGG
2 67 nG'JL.'~G~JC CUGCCTG 7 6= CCG'JCvJ C UGu'LiCCs
.
293 iu;C-.=JVJA 'v'Gf-.ACUG7E9 G,~-JCUGU U CCCL'GGA
'l9 r.G?.:yGAUr.GCC'CC 77G GJCUGUU C CC'UG:~.C
_' nUGJC-' ~ L'UC~AC 7 8 5 Cv:,CUGU U CCCAGUC
S J
3 3 GJC'''LTT~UU C~u-.nCUG 7 8 6 GC,C"LiGW C CC.=.GUCLJ
7
.38 UGC"J'rsULJC W-.~.:C"JGC'?92 UCCCT~GU C UCC~GG
'_ GC.,~C: C ~Cr C-..~L;7 9 4 C C=~GUCU C GC:.~
:GJ G.~...CC
9
367 r.~:Cr.G~:JA ~,CCUL,' 807 CCG:G.,"U C C=~CCUGG
374 r.:~:CCU U CCUCyCC 833 C~G~G;J U Gr_~CCCC
375 FA~:CCUL' C CUC~CCG c846 CCT~C~G'J C .~CC'UF.UG
378 CCtTJCCU C ACCGJGU 851 G'JGCCU ~,UC1-C.P
nC
CA 02468048 2004-06-07
173
E63 ."-.AC~C~J C CQUCUCG1408 UCCrG-'~U C UUGaG.~_~
866 GCJCCU U C'~.iCC~CC 1410 CAG:~LiC'J U ~~~
867 nCUCCW C UC'v:~CCr~ 1421 GGCACCU A CCUCUGLT
869 UCCL'QCU C GG..:.~G 1425 CCUACCU C UGUCGw
881 AaGGCCO C AGUCAGU 1429 CCUCL'GU C GGGCCAG
685 CCUCAGL1 C AGL~UGA 1444 GAGCACU C AAGGGGA
9~3 GJGCAG'J A AIJACUGG 1455 ~G.~.GGU C ACCCGV.G
936 CAGUAAU A CGG~~ 1482 AUGL'G.."'CT C UCCCCCC
978 'uG~CC?.U C UAC'-~CC~T1484 GUG...~'GCU C CCCCC~.u
S80 ACCyUCU A CAC-W.JUU 1493 CCCCCy~U A UGAGAUU
966 UACAG.=J U UCCC,GCG 1500 AUGAGAU U GUCAUCA
987 ACAGC'W U CC~.NCC1 1503 AC.AL'UGU C AUCAUCA
988 C?G..~.IW C CGG..~CC1506 L'tSGUC?.U C AUCACUG
1005 ACGL'GAU U CQCACGA '_509 UCAUCAU C ACUGUGG
1006 CGi.JGL'U C UGACGAA 1518 CUGUCv--LJ A GCAG.~.CG
1023 CAG~J C UC~AAG 1530 CC~~AGQ C AUAAUG;
1025 G~JCU C AGAAC,GG 1533 GGUCAU A AU ~G~G~,
1066 CCACCCU A GAGCCAA 1551 C1~GGCCU C AGCACG'J
1092 aUG~~D U CCAGCCC 1559 AGCACGU A CCUCUAU
1093 UC-:T.vJU C C~,GCCCA1563 CGUACCU C UALTAACC
1125 CCCi~G.."U C CGG~~UGA1565 UACCUCU ?. UAACCGC
1163 C~"U U CLTCCL1GC 1567 CCUCUAU A ACCGCCA
1164 GCAG.'W C UCCUGCU 1584 GGAACaU C AAGAAAU
116 AG'~UtTCU C CCT~COCU? 5 9 2 AAGAF.AU A CAGACUA
6
172 UCCL7G._~U C UG~.rAACC'_ 5 99 ACAGAC'J A Cp.ACAGG
2 0 CZ CAG'"Q U r'.L~C~.C~.16 51 CACGC CU C CCUGAAC
0
1201 CCyG.~JU A L'ACACAA 1661 UGAACCU A UCCCGG
1203 :~C-w.'VJAU A CACAAGA1663 AACCVAU C CCGG AC
1227 CZ~::~G~~U U CGUGUCC1678 ~~~CU C UUCCUCG
1228 G::ACz~JtJ C GJGUCCU1680 GGCCUCU U CCUCGC~
1233 UUCGJGU C CUGUAUG 1681 GCCUCW C CUC'GGCC
1238 ~JCC~J A UGGCCCC 1684 UCUL'CCU C GGCCUUC
12 64 GACv::aU U GOCC'GGG 1690 ~ UCGGCCU U CCCAUAU
1267 G:~UUGU C CGwAAA 1691 CGGCCL'U C CCAUAW
12 9 AG.F.r.AU U CCCAGCA 16 9 6 UUCCCAU A UUG.~~UGG
4
.295 G.~W C CCAGCAG 1698. CCCAUAU U G.~~JGG~
306 G:..T~C1~CU C CAAUGUG1737 F.AGACAU A UGCCAUG
1321 CC:~GGCZ U ~~G.~AA 1750 UGCAG.~U A CACCUAC
1334 nACCCAU U GCCC'GAG 1756 UACACC'J A CCGG.~CC
1344 CCGAGC'U C AAGUGUC 1787 A ~~~ U U GUCCUCA
13 51 Cr~GtIGU C LRAAGGA 17 9 0 GCAUUGU C CUCAGUC
1353 AGUGUCU A AAGGAUG 1793 UUGUCCU C AGUCAGA
1366 UG:~CACU U UCCCACU 1797 CCUCAGU C AGAUACA
1367 G:vC'~CUU U CCCACLiG1802 ~ G'JCAGAU A G'aACAGC
13 68 G:~CLZ'U C CCACUGC 1812 ACAC~.AU U UG~wGCC
380 UGCCCAU C ~G.~AU 1813 CAGC.AL'U U ~~~
CA
.388 C-CPU C AGL1GACU 1825 CCAUG"~U A CCL~GCAC
1398 UCACUGU C AC"JCGAG 1837 CACACCU A AAACACU
1402 UGUCACU C GACAUCU 1845 AAACACU A GGCCACG
CA 02468048 2004-06-07
174
1256 C=~CC-Ci;U C UG:~UCL'G 3? 89 UAUUL'AU U CnG'vGUC
861 ~'u'C'.'~GiU C L'W.r.GV.iC =~ 96 LT~-'..GUv"~J C L'UUUaUG
1865 G-':UCt.'GU :y GUC?~G.U 2'_98 'r.GLlG~ICp U L~JAUC'JA
186E C'JcwsGL' C ACr.UG~C 2, 9A GUGUCUU U UeAL'cJAG
1877 C=.iJG'eC'u 'r. AGCC'~G 2200 UGUCUL1U U AUCUAG~v
'_ 9 01 C'~.G,CU C AAC.ACAU 2 2 O 1 GZJ~ A ~~
1012 aC:,Ln~,L' U G.T.~L;C-.~-.AU 1205 ULZJAUGU A C~'V'~,?.A
c 2 2 UG:z.= L'cJ U nA,=,CJC~J 2 2 . 0 cvTAG:~."U A =~~LT?,.~C
° 2 3 G'.~ :L'G'~TIJ y ~-~, C",TA 2 2 2 0 L'G? AC:,U A CuTC'JC'J
19 2 8 L'L~~-.; :cJ c U'r~ C UG 2 2 2 4 GG~LTAG""U C UCL'Gv:. C
c30 ~cvC;.v A C-C~..'GAU 2225 L'AG""'UC'.J C UGV,C~JC
1964 G=1~C'-.U A GC~~CAC 22?3 C','C~.CU C ?~;,C:r,G'~
1 ° 83 : ~CuC.,:~U =i C~CL,'~Cv 22 S2 C""vuAG~~[j C CC'-~GUCC
1 o c 6 C.'.~,,;_f.,L' A- - CLJC:nAAC 22 4 8 UCCCAGU C CA L'G'~1C~
2005 UCAi,nCU U G~:,JG.-'CU 2251 UCC'~L1GU C ?,C'yUUC'~
2 013 G'~LTGC C U .~ L JC-:~~UA 2 2 5 9 GUCAG~U U C '-~AGGUC
2 015 UGCC'uTAU U G;"=,L~,LJG 22 60 UCACAUU C AAG"~Z7CA
2020 sL'UGC-cJ A LT~uGAG 2260 UGw-,AC,"--U C ACC.AGv"U
20?° AGAG;,CU U AC~,G 2274 nCCAG.;~1 ?, ~GU
2 0 4 0 CnGAC"uTU A CyG~.G.A 2 2 7 9 G'uU G~GG,
z o s ~ UGC-,: c cU c c=: ~..cac 2 2 82 c-,cuUGU a c~cc~.TG
2 0 61 C C uCCJ,U ~, GAC~,I;G,U 2 2 8 8 LTyCAG"--U U cJAC~.CU
2 0 71 G U cJ GU A GG»CAA 2 2 91 AG~~UUGU A CACUGCA
2 07 6 cur.GC_,;U C a-.F,:~,C~C 2 3 21 ~-.A~,GAU C ' '~' UG""
20-° % CCf:GaCU U CCL'GACG 2338 L'G~~ U ~~G
2 0 9 8 c~cacJU C C'.'Cr CGG 2 ? 3 9 GG::ACUU C Uc~UUG;~
21 1= C-C Cr;Gw~U U G;~...C=,CU 2 3 41 G:yCUUCU C A UL'GG CC
212 8 C'J G.: JG"J C L:.~CUGC 2 3 4 4 L-L7C~U U C
2130 G~~v'cJC'J ?, C'u'G~CCC 2358 CCL7GCC'U U UCCCCAG
214 5 G'u;CCC'L.' U G;.,UG,U~, 2 3 59 CL'GCCW U CCC'Cp.GA
2152 UG AUGAU A Uc~,T'r~UW 2360 UGCCUUU C CCCA~,A
21 5 6 G Lrt,'cJ A L'L JAUUC 2 3 7 6 Gf-~U U UUUCCIAU
21 S 8 Lr L'Gu~U U ,T L'L7CA U 2 3 77 AG'JGF,UU U UUCUAUC
21 5 8 Aucu~UU U A L'L:GULi 2 3 7 8 cJGAUUU U UCUAUCG
216 0 U GL~L~tJIJ A L'UCyUJU 2 3 7 9 L'GAUUUU U ~7CGG
2162 L~.:L'L~unU U CnUL'GGU 2380 GiWt]UZ7 C UAUCGGC
t? 63 A'u'L unL'IJ C I:L'L'L'cJU 2 3 82 L'ULJWCU A UCGw~,CAC
2166 LnL'LiCrU LT L?cJLi:L'U 2384 UL'UCUAU C C-vC~C~
2167 AL~JCr.L'L,'~ U cJ'uT'nLTJU 2399 ~.r°.GCACU A U?yUGGAC
2170 C :'u'uu'GU U ~:ULTuu'AC 2 4 O1 G:.~CLTAU A UGC:.ACUG
2171 AUL'UCJU A UUUUACC 2411 CAC'JGGU A AUGw,~UUC
2173 UUG~1'~iU U Lu7ACG,G 2417 L~t,AUGGLJ U CACAGGU
2174 UGLTurL'U LT LTACCAGC 2418 ~.r.UG,~L7U C ACAC,GU[7
21 7 5 GL~ a r UUL7 U- nC CAG''"U 2 4 2 5 ~ CA CAG~,'U U G:GAGAU
2176 L'L~L'L'UU A CCAGCUA 2426 ACAGv,''UU C AGAGAUU
218:: ACC~G.:.u A L~uuT't,L'LiG 2433 GGAGAU U ACCCAGU
2186 G'G"'~'U U Lr~L'L.'~GAG 2434 AGACAW A CCCAG'UG
AG: .,?~UU U AUUGAGU 2 4 4 8 -C iGGCCU U AUUCCUC
2187 GCTurL"W A UUCnGUG 2449 AGGCCW A UUCCUCC
CA 02468048 2004-06-07
175
2451 C-CCUL1AU CCUCCCLJ 2750 UAUG'JGU A Gr'.CfaP_G~.
U
2452 C~v'U'r~L'UCUCCCUU 2 759 A "G,nG.~U C UCGw."L1CU
C
2455 UAUUCC'J CCUUCCC 2761 AAG.~'JC'J C G.."L3CUG'J
C
2459 CCUCCCU CCCCCCA 2765 UCUC~.T.~U C UG'UCACC
U
2 4 CUCCCUU CCCCCAA 27 69 GCUCUGU C ACCCZICz,
60 C
2 4 GACACCU UGUUAGC 2797 GUGCAAU C AUG.~"LJUC
7 U
9
2 4 ACnCC UU GUUT~GCC 2 8 03 UCAL'Gw,"U U CACUGC_A
8 U
0
2483 CCU'JL;GU AGCCACC 2804 CAUG.~'W C ACUGCAG
U
2 4 CL'UtJGUU G~~CACCCT 2 813 C'JG'AGJ C UUGACCU-
84 A
2492 GCCACCU~ CCC'~CCC 2815 G.;.AGUCU U CACCUUU
C
2504 CC ::C AU CAUUUCU 2821 L'UGACC'J U UUG:~."~J
A
2508 CAUAC=~U UCL'GC;~. 2822 UGACCUL7 U UG:~.JC
U
2 5 A L'ACAUQ CI7GC CAG 2 8 23 G?.CCUUU iJ GCV...~UC~
0 U
9
2510 UACAUUtl UGC.~.AGU 2829 UU ~~~tJ C AI.GUGAU
C
2520 CCAGUGU CACAAUG 2837 AAGUCAU C CUCCCAC
U
2521 CAGvGW C ACAAUGA 2840 UGAUCCU C CC4CCUC
2533 UGACACU AGCG.~~UC 2847 CC:~.CCU C AGCCUCC
C
2 5 C? G~'~G.~"UAUGUCUG 2 8 53 UCAGCC'J C CUGaGLTA
4 C
0
2545 GUCrUGU UG:~CAU 2860 C~J A G~~LJGG:~
C
2568 AC~AU A UGCCCAA 2872 G~C~,.,AU A GG,--L7CAC
2579 CCnAGCU UGCCUUG 2877 AUAGG.."'J C ACAAC~.C
?.
2 5 U'r.UG.~. GUCCUCU 2 8 9 G:,Cr,AAU i1 UGA
8 CU U 9 UUUU
2 5 GC CU'JGU CUCUUGU 2 9 00 G:=-~F,AW U CAWtJW
8 C
8
2 5 UGGUCC'J UUGUCCU 2 9 04 AULZT~U U LTLJULTULJtT
91 C
2593 GUCCUCU GUCCUGU 2905 UUUGAUU U LWWW
U
2co6 CUCUUGU CUGJWG 2906 UUGAUL'U 'J L'WL'tJUU
C
2601 GUCCUGU UGCAUUU 2907 UCAUUW U UL,~JUt~U
U
2 6 UCC'UGUU GCAUUUC 2 9 08 GAUUUUU U UUtTW~,-U
02 U
2 6 UL'UG:AU UC~CUGG 2 9 09 AUUW W U UULW W
07 U '
2608 UUGC~.UU CACUGGG 2910 LU
U
2609 UGCAUUU AC'UC-GGA 2911 tU
C
2620 C-~,~~U GC_'.~CUAU2912 UUtJLT~7LT~7 U UtILTUWC
U
2 62 UUG~CU A UUGCAGC 2 913 L~tJLILZTW U WUWCzI
5
2628 G.=ACUAU GCT~G~~UC 2914 UUWUUL1.U LZJWCAG
U
2 6 UGC~.G~~U CAGL1UUC 2 915 LU UUUCACA
3 C
5
2 6 CUCG'-.GU UCCL7G:A 2 916 UUUUUUU U UUC~GAG
4 U
0
2641 UCCAGJU C.CLJGCJiG2917 UUUUUUU U UCAGAGA
U
2 642 CCAGJUU CUC~CAGU 2918 WUUUW U CAC~G,~,C
C
2 6 Cz GUGAU :~G~'"UCC 2 919 UUUUUW C A --CAGACG
5 C
3
2 6 UCAGw''U CU ~G.~AG 2931 AC ~~~U C UCGCAAC
59 C
2 6 C CAAG.~~U UUG:~C~G 2 9 3 G:~C,.,~LTCU C GC~.ACAU
8 A 3
9
2 691 AAG.~''tTAUCr:AC-.GP.C2941 C-CA.ACAU U GCCGAGA
U
2700 G.C-::ACU CCUCCCA 2951 CCi.GACU U CCUUUGU
C
2704 ACUCCCU CGAGCUU 2952 " Cr.GACUU C CULZTGUG
C
2711 CCCnG,~U UG~.:AAGG 2955 ACUUCCU U UGC;GUUA
U
2712 CCAG.~L'U GGAAGGG 2956 CWCCUU U GUGJUAG
U
2 7 GAACZ-~''U AUCCGCG 2 9 61 UUUG'JGU U A GUUAAU
21 C
2 724 G.~,-.~''UCAUCC,~~~IJGU2962 WGUGW A GUIJAp,UA
C
2 7 UGUGUGU UGtJGLTAG 2 9 65 UGUUAGU U A AUAAAG
4 A
4
CA 02468048 2004-06-07
2 ~ c~.z~~~,ccr~a r c
6 6
2 c nG'u'Lrcs~,UA .'-.r'sGL.'"f.;L~
6 c
c ~ LT'~"'~ O L'CLWC
7 5
2576 '~,G,,~ U
X577 .'-.:r.G~~,rC;t1C ~GSnC'~JG
2 S C-~ JL'LCUC ~CQGCC
7 9
CA 02468048 2004-06-07
177
Table 3
Mouse ICAM HH Taraet Sequence
nt. Position .t argot Sequence nt. Position Target Sequence
11 CCCacG'J ac ~uUG 367 ~AugG~"'U cF,.3CCcg
C a
23 CaGuGcU C;:CVGCU 374 eF.:.rCCU CCUccCC
a U
? LGcth:CU 6 375 =.~cCCUU CL'ScCCc
C UG..'"ClcCU C
31 C'JCUG~~U CUCczca 3 78 CuacCaU C ACCGL,'GU
c
34 UuCUca U 386 ACCwGU A uU~'uuU
a r.G-c~GLTcG
40 cG.cncU GuAgCC'J 394 CcG.~-~_CU ucG~uC,1
U a
48 ccCACCO F~.'~CUgG 420 yCZC',lU CCCCCcg
C C C
54 UgcGCCU GucAUGG 425 aCCCCU C ccaGC~G
C C
58 CaUccCU UaG~~UCC 427 CacC'JCU aC-:~Gug
a c
64 cAcccCU C~.~a~GC 450 rl,~ACCU ACCCUgC
C AG c
96 C',:cscfiJ CUC~cCC 451 GAF,aCcU uCCrJuuG
C a
-
102 UgCcaGU CUGw~UgG 456 L~IJACCCU aGCcaCl1
t c
08 cuCUGCU - cuG~CcC 4 95 Cl~AcCaU ACCGLTGu
C C
1'_5 uG.~"'~.vCUUGcUCCu .10 UG..~UC-..~UC~.'C-G
C C ;G
119 GcaaUGU zCC'AG:~?,'0'4 CUcF~G.~J uCc~,uCc
c a
~0 CUCUGcU CsS~ccC 592 Gra.~.CaU ACaugGG
C C
146 CAGuCgU c~.,cuUCC E07 AC-:.~J~AU UCUCaL7G
C U
152 UCL'G'JGJ acCCaCu '008 G.:Cla.UU CUCzUGC
C U
158 UCCucuU A~cC 609 C~~UL"U C UCaUGCC
a
165 CAgAr.GU cUuuUGC E' ~ AAUtJtWCU aUGCCGC
a C
168 A?.GcCuU C?G.~CCC 56 ~JGU U UG'lcug
C
185 C~:uG.~cU CG'JGLaG 6~7 f,G..~UCW G ;Gn,gA
C U
209 ccf~C~U CUcOGgC 668 cgacCCU a GGCCaCC
C -
227 CaSAAGU GUUuuGC 677 GsCCuCU A CCAGCcu
U
230 :~.nGG'LiGUuuGCucc 684 LuG.G~"'tJ CgGuCCU
U C
237 LTGuG..~sU GAGnaCu 692 CcG.l~:U cGauCUu
a U
248 rzCCCa uCCVAAA U AGgaCcU c acCCUGC
c 693
253 ccUG.CU AggAaGA E96 CCUcUuU C CUGCCuc
A
263 AcC-.~'LuU uCUaCtIG 709 cGCGSCU C CaCCuCA
c
267 AC-ccGCU C'UGCCUa 720 uACr.ACU uUCAGC~
C U
293 Ar.GcUGU ~ UCnSCUG 723 :~CL'UuU AGCuCCg
a C
3?9 rC-cAGAU c~cAgCC 735 aCCaGaU C CUcCAGa
A
335 cUCUG..~U UgaSAAC 738 LC-C-g~CU GuGaUG.;
a c
337 GUcC4AU CAcACLIG 765 CaGUcGU C cGcUuCC
U
338 aG..~UgUU SAgCL'Ga 769 C- GcCUGU uCCDGcc
a U
59 GuGCAG'J cuCcGCU 770 uL'uUGcU CCUC-:~
C C
i85 ' C-C-cCUGUuCCuGcC 1 =53 . AGLC-cgU c~~GgUG
U c -
786 GcCUGUtJ CCIiGeCU 1366 UeaCAcU a UaCaAC'U
a
792 UcgecGU UC~~,aG 1367 eGC~CcU c CCCACcu
C
794 c,:cGgCU GGAGaCu ~ '! 3 GuACCigU CCACUcu
a 68 a
807 CuCcGaU L.ACCL'GG 1380 UGCCC'~U GGVG
a C
ugg
833 CA~GcU c GAcaCCC 1388 GGaGcU C AGUGgCU
846 CCcuvGU ACCcuUG 1398 UGgCUG'J ACagaAc
C C
Q5 G~c~,CCU 1 1402 ' UGUcc~~U GaCAeCU
c UacCAgC a
CA 02468048 2004-06-07
178
863 ~cCcnCU ~u CcLlC:.'gG 1408- cCGF.GP,U C cgGgaGG
666 G.acCC'J U Cc,:GcCC 1410 GAC,gUC~,7 c GgaaGgg
867 =.uLTCcUU a cCC~-acA 1421 ccCACCL7 A Cl~UuUGU
669 L:C~.:Uc~J C aucG~.G 1425 zCUcCW a aGUaGaG
881 =.uC-;~sU C ~.cCcGJ 1429 uCUC'JaU a GccC~G
685 C'JLJucGU a cac-GJGA 1444 G~ag~ C AaGaG.GA
°33 ct'auAaU c F.L~uC:~C-:~ 1455 G;:aAuGZ7 C ACCaGga
°36 uraL'csU a CUG.~.~Gc 1482 iyguUC~l.~.U a LgC'sCCC
S 78 ~ Uar'.C~cU C ~C'~CU 1484 cUGuUCU a CCI~CauG
880 =.CacUCU =. CAcC'~'W 1493 C'ugt:GcU a UGAGAac
°86 Lr:c~C'J U L'~:CaG~'~ 1500 AUGT,~.~t7 c aUcSUCc
68 7 ~:G'~C"uJ U uCaGC~C 1503 gGAcUzU a AUGyUuc
X88 C'.zC'JLJ a CaC~.:CC .506 WaL'cuU a AUzACcG
1005 ACcaGAU C CUGcaGn 1509, cu.AcCAU C ACcGI3Gu
1006 uGaCrcU C UGccGAA ls? 8-- L:caUG"'"U c cC~GgCG
1023 ueG~~J C UCcG~G 1530 CuauAaU C AUucUGG
1025 G~G;JC'U C cGf,F,G;", 1533 ugGUCAU a gUG:~.;Cc
1 066 CG C.:C'J c at,~auAi, ~ X51 CAuGCCU a AGCA cU
9
.092 rc,:G:-aLT c uCnC-cCC 1«9 AG~,..;CcU c CCcaccU
1093 UGCaccU a ChG~~Ca~, 1563 CuUAugU a UALRACC
1125 CCC~:'U C uUc.~L?G': ?5c'S LTAucUuU A UP.ACCGC
_163 C~aF;C.'J TJ C'UuuUGC 1567 ugUuUAU A ACCGCCA
1164 Ga.=.C~JLJ C L~:urGt"L7 1584 GaAAGF,U C rlgG.AuAU
66 :_C-~JUCL7 a aUG.~"TJCL7 1 592 AgGAuAU A CAacuUA
_172 UCCUG~U a ~F,r,CC lcog F,C~aguU A CAgaAGG
1200 c,:C~Gt~' C C'.,Cv-~C~: 1 E51 CcCaCCV C CCUC,AgC
1201 cC',:C-'~vU a L;c~CAc 1661 caF,ACCU a UCCZ:uuG
12 03 r c.:L ~'~~U a Ct,CG =Gu ~ 6 63 AACCULU C CvuuGAa
1227 G~,:.t-.caU a CL~'~'cC ~ 678 AGGaCCU C agCCUgG
1228 Ga.~.C.C'u'U C uUuUcCU 1680 aGCCaW U CCUCuGg
1233 LuCGJuU C CcGacaG 1681- - GCCaCW C WCuGgC
1238 G'~aCC-G-J A UG..~-uCC~,: 1684 aCWCCU C uGaCUgu
264 G-.a GC-cU c Guc'~~G 1690 cCG~aCU U uCaAUcU
.267 L;r-~'..caG~1 C uC-C-C.cAA 1691 CG:~aCW a CcAUcW
1284 nC=r~F,U a C,:cAGCc 1696 Ue-CCG1U a eaG.;~lGG
-205 C=.ccccU C uC_=.G~TiG 1698 CcaAL'AU a ccUG~,ag
'-_ 06 G=-'-.C:r:CL, C v.:cr.~.UG 1 737 cAC,ACcU c UaCCAgc
.321 c~._C~~U c aG.CaGcA 1750 gGCcG~.~U c CACCUca
'--'34 =.~CCC~.U c uC~~~A.Z 1 7 56 cAacCCU a CCuGCCC
1344 a.,:e=~G~.:~T C crG~GJc 1 78 7 gaGaCAU U GUCCcCA
-'S, ucAaUG'J a UT-~Fc,~uA 1790 G.=.r:WGU a C'UCliaau
1793 U_~~,.'UCCL' C QGc~eGF, 2173 WagagU U UUACCAG
179 7 C~cC?:GL' C F.cFL~.aA 2174 UacagW U UACCAGC
1802 ~cC=.Gr:U c C'uccrGa 2175 ~ acacL'W U ACCAGCU
1812 «.:GcrU c UcaGGCC 2176 cagL'UW A CCAGC~7A
1 613 Cr'.G~L ~' U a c c : ~ G 2183 ACC~GCU A UUUAWG
1 825 CG.cC~cU :~ CCUcJgC 2185 GGCLTpU U UAWGAG
183 7 C~.ucCCU a uACCuCc 21 86' - AGC'JAW U AWGAW
1845 ccAccC'J A GC-CCACc 2187 GCUAUW A UUGAGUa
CA 02468048 2004-06-07
179
~~6 c=~~csU a c~.LCVu 2189 L~Lnr~~L
~ -c~Wacc
1861 AeaUCAU a Ue: ~GLIa2196 ea,e'.~e'JeU
a eWgAUG
1865 cAc~UG'J G~'.:CAg 2198 gcaGcCU
A c G'UAL'G'Ju
1 8 Ca c c~:GU AG~UaAa 2 ~ 9 Gc c UCUO
6 C a UgUuUAu
a
1877 CAUC~cCU AGCagcs 2200 UcJuccU
a c AUC-cAzG
1501 uAA.~,ACU AAG=cAc 2201 ~cUULZI
C A vGUc''.~GC
nuAL'agU GAUcaSU 2205 DGUAUGJ GGCcugA
a C
22 UGaFUGU a uAr.GJUa 22''_0 GcAGaCU
c AgUGgcs
1923 uGr':UGcU AgGZI2Uc 2220 c,:ccCAU ~:L;~CUC'J
c a
1 S28 Iiunc:~GU UuaCCaG 2224 csc~G.~~Li UCcauCC
a a
1930 ncF:G'JuU eCCaGcU 2226 L'cGaUC'J eCvCCgC
a C
1954 G_G:C~U a GuC'CCa 2233 C'JGaC~J c~G::AGg
C
_, =G::.f:uAU C'"~L'ua 2242 uC~.G..~U gCgGaCC
83 A a
c6 aG::.AcAU Cl3G~gcC 2248 auCcaU C C~UccCA
A J
2005 UC-cFrcCU GCgGaCc 2254 UCCAzuU ACAcUgA
a C
20.3 G'~LauuU WGaGJA 2259 aUCAC~U C.~.cG.~~Ug
A U
2015 UGCCcAU c G~~cugG 2260 UCAC=~W acGGiJgc
C
2020 ccUG.'~U UuCaG:~G 2266 ggAAuGU C~~.~G:~a
c a C
2039 cCsGgCU a gCAGAgG 2274 AC~.~GaU Cl:GgaGa
c
2040 C~CAC'cU CuGgAC-g 2279 GaT,ccG'J CUcrCAaG
c c
205 U~Gc~CC'J CAc~ucC 2282 GcUGU a ucaGcUG
7 C
206''_C.~eC:~U acCgUGU 2288 L?:uAeG'J aUggcCU
c U
2 0 CA c.:UGU GC cL'C? 2 2 91 c aGUgGU CuCUGCu
71 A g a
2076 CLnGCCU C AgAL't~',:22321 gAAAG~,U ~.C~aUC-G
C
2097 CaAC~CU U CuUG;uG 2338 UGaGAW c CQgcctrG
208 CzCi~CJU CcccCCG 2339 Gau,CcU GCcULIuG
C a
2115 G:.Cr.G..~U G~:aggaU 2341 GACcUW a caGcCu
c c
2.28 CaGC"JaU LTF.uUGAC2344 Wuc=AU c uuCCAgC
a
21 cCUC'UuU CUGcC.:C 2 3 58 CCcacCU UCaSC~G
? c c
0
2145 C'-~AC~CU cuUG:yLlg2_59 CUGCVW U gaaCAGA
U
2152 UauUe.AU UecAgW 2360 ~eCCUUU C',:uuGAA
a C
2156 uucAL'GU ULJUAWa 237fi eG'u'GgU cUUWga
A z U
2158 cAUC'-.~?~U UAUUaAIJ 2377 gGUGgW c DUCUaag
U
2 i.UG'..~:UU ? 2 3 7 8 a gGgUW UCUAcuG
~ c U AUDaAIJLT c
2160 UGL~UW A WaADL'U 2379 UGcUUW c ucAUaaG
2162 LTAUU~~AU ~WUag 2380 aAcUUUU UgUCGGC
U a
21c3 ?UcL~W a AWaaW 2382 aUUcUCU UuGcCcC
A
2166 acUUCnU U cucUAW 2384 aUcCagU GaCACAA
a
2167 Auct:~:W cU~L'U 2? 99 AAzCACU UcUG~C
U A
2170 uAUWaU U AaBLTLmg 2401 eacCilgU UGagCUG
a
_
2171 i c WGW Ug cDcCC 2 411 uACUG.,~U AgGaUgC
a c
2417 cF.AUC~"U CAuAcG'J 2691 AAuGUcU cGACGcC
a c
2418 FcUGGaU C uCAGGcc 2700 CAaGcCU CCUgCCc
a
2425 CzugG~''U gAGgGuU 2704 ~ gacCuW CCAGCcU
c a
2426 =.uuaaW a F.GAGuW 2711 ~ CCCAGCU UcegcaG
c
2433 uAG~.GuU uaCCAGc 2712 cagGucU C-~AGG:,
U c
2534 ~.Ci.GUW aCCAGcu 2721 G~GG~~U cUcCaaG
a C
244E -C~GCCU U ccUgCcC 2724 GGuaCAU CCuGUGc
a
2499 A~GCCW c cUgCcCC 2744 cGLGaGLI cG'JGcAG
c
CA 02468048 2004-06-07
180
2551 C-CC:"c,:UUC~ TcCCU 2750 'u~r.L;uL'aUaC?_guAcC
2452 C ~c,:W CWcCWc 2?59 cCgcaCU aUCGaUW
L45 C~ac~CU a5 2161 AgGaC..~..1CaCCWGC
CCTJCCCC
24cc CCaCzCLi UC~~Cc 2765 UuUuC-~~rJCUGcCct~
24 60 CaCaCW CCCCCCcg 2? 69 agUC',:G'JCAaaCr',G"
2479 G~.cACCU cUaccAGC 2?97 UGzAAU CAUG,"UcC
z
2480 uC~C'LU UGUcAuCC 2803 UCAL'G"~U cCcacG.~.g
2483 CCaaUGU anGCCT~CC 2804 ggUGGcU CcgU~AG
2484 C'~.'WcW caCCAcuc 28.3 C!:;cCgGU CcUC~.CCc
2492 acCACW CCCC~CC'u 2815 CAGUW ac'~CLW
z
2504 CCCiCcU AC'~L'L'LicU2821 c UG.CCL1 =cLG:
,agg
2508 uAUcCyU ccaLcCCA 2822 cCi,gCcU c~
.
c
2509 uUAgAg UuUaCCAG U L:eCC'JLZ3azC
- - 2823 C Ju
e.:CcCA
2510 L'hgRgW aUaCC~Gc 829 UGGaW auAaUcAU
2
c
2520 C;:uuUGU UCcCF,AUG 2837 AcWGcU aCUuCsga
2'21 CAGca aACccUcA W UG?.gaW CC~gCC'Ug
2840 -
2533 U--CnucC'IJCAGauaUC 2847 C CaAugU CaGCCaCC
2540 C'-.GCaGU CcccUcUG 2853 cCAGCC'J CuUauGUu
2545 CUgcUG'J aUG.~'UCcC 2860 cCcaF..GU AaCL-CuC~,
-
2 5 c,:G~oU cUGuCa.AA 2 8 ~ 2 G::ACCuU caGt
68 caAg
L579 aLr'.r'Sl:U.~.ZiGCCC~.~G28 i7 LUCC'C-w."UaC~'.r'.llC_sC
2585 cucC.~aU 'JG'JuCUW 2899 GcAcuU UcGr',UcUU
c
2588 GCaL~t.,'GUaCUCUaaU 2900 uuAAuW aG:aUL'L'U
2'9 UcG'JuCU C? 2904 cWcAU UcUcUaW
UgcUCW
h
'93 cUuCLJuU UGcuC'JGc 2905 c'JUc~L'U cTJcUeL'Li
g
2_'96 CL'uLu'G'JaCccaaUG 2906 L'L'GF,UcUaLZ.ZaL'Ue
2601 acCcUG'J aUuCgWL' 2907 UCI~aUL'U aUt~~aUUU
L602 LJCCcGGU aCCAUCCC 2908 GAcCC'.uU CL'JL'UgCU
2607 cUc'-gAU aL'acC'LTGG2909 AccWcU UL'L~gcTJcU
2608 caC-:~,cU cCgC'JG~G 2910 UoUaUULI aUUaaLJW
2609 c-GarUg CACcaCuA U aUaUW aUU~aL'UU
U 2911
2620 eC-~cCU caCcC'Jgc 2912 L'UcUUcU cUzzUoL'C
2626 UL'uCcaU cUUc'",~?GC2913 UUL'cUeU acL'ceUG
2628 GC:.Cac UGL:nGCca U UgcL'UW ccaUa
2914 L AG
2635 L?uGC.~J CCcGDccu 2015 aUUUaW a.
aL'L7uAGA
2640 ScCC~GJ UUCC'JGCc 2916 ~'aL'UcgU UUcCcGAG
2641 cCCrGcL cuCaGCAG T aUL?c U
291; W
g cCcCAGa,
2642 CCuG~~TL1 CCUGCcuc 2918 '
L~JcgUL cCcGGAg
2653 T U
uAcL C~.G;-aUcC L LJUcUcaU arC-cGuCG
Gc - 291c
~
2 E e~:~~c-~,7CcUG;~,F,G 2 31 ucGaC- CUCG
59 ~U
" cAAg
2689 C1~.P.uGU cUccGAGG 2933 '
GaC-.. CGe.~.Acg
ZCU r.
2941 GacAC~U UCuCCccA ~
- -
2 51 CC~,ccCU aCCUcUGc
2952 C:~GcagU CCgcLTGUG
2955 ~:gugacv cucJcJc~,
2956 uWCCUU UGaaUcAa
2961 UcUGUGU cAGccAcU
2962 aUGUaW aaUUAAUu
2965 UuUgAaU cAAUAAAG
CA 02468048 2004-06-07
181
266 GcUcC-cU A ccAe:,C-c
2 ~ ?.aUc~r'.U A ~AG1:L'UCT
6
2975 Lr"~aF~lU 'J UzcG'~C
2976 cAcGgUU U CGCuACZJ
2977 AAGC'LJgU a Uglsc~CLG
2979 uCaL'UCU C uAuUG~.C
CA 02468048 2004-06-07
182
?able 4
f-;;urr,en IC: M HH F;ibczyme SequEnces
nt. Potion Ribczyme Sequence
11 C~C-'VJC CTCrC~~.~-".,~.u~G~.CG'1AACUC-.NG
~3 =_C~..r.GGC:."'~L~C~C~~CvCC~.~'nAAGCUCiIG
26 nC~y CLGtCAC-:~CC",.:-.i~GWAC-:riG."'LT
C'"anA
z, c~c~enG ~-e~L-~c~-c~ =~,~,c1-~c~AaG:~c.~.G
3 4 C-'~:CUC'JC' 'GJ,i~GAG.~.-C ~..'AGC~,
C Gi- ',~,~,G~,,C
CG?.?
4 0 .'-.Ci C ~ GUG~G:n : ~,-~.i?iCL'C'"G~
vJGC Cv:. C -C.nA
48 C;.~C-C-L:JC:.'GUGnGC,CC~~C ?C-.~v'UC-v
-CnA
4 C ;l. nC-CC'~; GLiCY.G.:-C AG.w"L~G?,
C :: ~ "C -C~
~E C-Gi:GCCn CuGBU.C-:~:.C"'~'~,GC-CCGAAAGw
.'
G'~.,G
54 C".'C-C'JGGC'~'GIJGAG:~C"'~CuCC:AA:~"C~L'A
6 C-;~.C:AG C'~'G::L'GGvCC~._. AG'JGw.v"
ifiAG~~C~.~-'-.A
102 C'.-~'~~GG~GC''GL~nC-.~_-CC~'=.nAGC,CCACCr~.G:A
-Cr'~A
C-.C~ C C"~'G.'-. UGr.C-:~. AC~GGA
8 C C C".~.AACr,v. CG.~.A
115 C-:.ACA C"~~L'.f:G:.CCC~G:~Cf,GCCCCG
-CAi,
c c, ~c~.cc ~;~ L~~.c-:.z c~.~,G:~: ~c~.
c -c.~A _.c~;Gc
,? c,~~ c c~ cl : L: c;~c-c.~ hA~GaG
o c"c-,~ c a~G:~ c;y-~
14 G:~:G'- C'~-G; : ~ G,G:.-C :yL'GUCGG
6 CA C"'.1:-~aG;~C C
CvaA
, r.r-. r ~ rrw
2 ,'Cr G:~.-aC,.'G L'GAC-:~...~~,..~r,AC.ACAG~
l~s e;:c.~;~ ccUL~~.;~c.:..c;.:_;."-.Ac~CC..~a~rr~_
16~ G,:J-.C-:r;-.:UCC'CrUG~C~~~~C'="..ruai,GuCC:~~'1ACL1WZJG
1 58 GC~-.~-~~AGC..'C:;~L'~CnC-:..C~nUCACW
-aaar',C~~,CCGar
lE5 Cf-.G:ACG C~'C:~-:L'.a-.C.C-CC'',:l,ri,C.;CCGnA~,GCCt7CC
2 09 GL.'G:~C=~GC:G uC::~C-.~.-CCG..:~AGGCCGrIAAC.~~UGCU
227 GCC~=~i~C C'.'CruC~..C-C-CCC~-.:-.CvCC:~AACULiGC,G
230 LT.~uGCCC C'uC::L'GGC-,:.C~,GV,CCAGACL'U
-G:A
2 3 G_-~u C'JCC'~ G C~C?:C-.:.-C hUGC
7 C;~~,GC-C C -CAA CCA
2 4 L1 L r.GGCC"~ GA L'GAC~C
E ~.a..-~C-.n.CC~,~. ACG'-.~Gp
2 _' FTC ' F'L'UC',C:r L ~.C~;.-C :~C-.:.:.~C
3 ~ ~;r..r,C~:,c CG.A
2 6 ' ~ r.:~C-CCL'. GnuG :C-C-C i~CUCCL'U
3 C G.a-AGw C -GA
y. ......
2 6 r CT~ G ~ ~ GC.C C P.G~AACU
~ ~-~-~-:G ~GC,C CC~
203 L.~-=~.-uCiaC.'G~'C.n~~"-CL''~.~vCCG'~AnC~.CCUU
31 C-~'-~.~t C:'Gr :.'~C. :CSC i:UCUUCU
c C-:~C CC=~~sC CC~ni~
1 ? .~ ~ W~'"~:.r~,C'~;:Y L.'~C".~GC~ A C-:ACAU
5 C :~l.,G:A CC.AA
33 .;=_C'-"..:~T~1GC'~~r:~~~G.:-C ~.',f-r.G.:~CCG~.AAU'~:GCAC
7
3 E C~.yG'~'W C'vGh i_'~C~-,Cv: 'u'~.'~GCA
wr''-':~.=~C~.:~C
CGAA
ACLGCCC
30C ~C-'aGJL CL'Gi::~C:rGC-CCL::.,~-.r
''~CGJ,F,
36 rhC-GL~~TtJCt'C:':i_'~C~:C-C-CC~~.GGCCCAAAGC'UGL1L7
~
374 C-~vGhCw C'LC::-.i.'G:;G:~cCCr-'u.."C~CCv~~.AG:mT~JW
373 C i':LGAG C;'CrUGC-s..CCG.~-.~,C~;,CCG:.u~,;AG""LJW
378 ACf~CC~::JW.'Grt.'~'.;.:C-C~CC~-~:G.:~CCG~.ArG;~,i,G"
386 2:GvTC~=.GCUi:.':i.'G,G:~cC~G.GCCCAAACACG,,"U
.94 C~-~'Z:~CuGC'~Cf'-.U -Cs'-.G,:~.CC~-'~~~,G[;CCAG
T-.C, ;CCC"',~,
9 2 n~-LA GC~GCJG~ :CG-':CvC C'.l-.i-~r GC-C,.~''UG
0 :CvC CCZ.A
925 C"vGCC?A C't.'Cr.L' -G':C- F~CvC,G.pG
GCCG.~,CvCCGnn
CA 02468048 2004-06-07
183
427 C-.:a~"UC~CCL~GnU -CnC~~~.CG'r.Ai.G.~r~.CGAA
AG ~~~
SSD G'JAGCV'"U C'JCnDG"rCv~w~C'"'~CG?.A
AG"'"DQCU
S51 CG:.TAC-:~ CUGAU ' ~ CC:WC,CCGP.A
AAG.~"WC
456 G:,~ ~GCG CUCr -UC AC~CCC,AAAC~:~CC'.,~,A
AC~P,
495 CCACCv"Z7 CLiCu':UCv'sC-~v~~C""anAe~'.G~CCGr~A
AC'v"CUG~~
S1D CCCCACG CUG AUGAGGCC -' ~ CGAA
AGCAGC.A
564 UG..~-UCGL1CUGAL"~C~CCG~aAAC:~CC:
AA ACCQC~G
~ 92 C.~.."~TJCv~UCLiCa'rjWr-'.G.~ss.~CGFCCv~A
AUCUCQC
607 CAC~~ CL'GAL'C.ACG..~~"C GAA
AUL'Gw~LJ
608 C~CG~,G CUGAUGAGC~CC " ~~CCsAA
AAUQGGC
609 C:.~~.C~ CUCAVGnGGCC -" 'G:~CC~~
AnAUUGG
6.1 C' ~'~~C NGAL"'.C~~CC~.sAa AG'yArlW
656 C'UL~NCA CL'GAV"~:AC~CCC,AF~GGCC~~A
ACAGCrJC
657 vcnccC cvG;. -C~CGaA AaC~cCU
668 G~.-:~.C CGC~UG ;GGCCGAAAG:~CC~..?.A
AG.~"UGaL1
677 GAG.."LJGG C'UCn~GGCCGAAAGGCC~~?.A
~~ ;,~,C
684 r.G~~~?CUG CUCnUGAGC,CCC~AGGCCGAA
AG.~UC~"U
E92 C~C~. CC1GAVGr'"~CCG:~AGGCCGAA
AG~CUG
E93 G.:~C~~AC NCAUCAGGCCCAAAG:CCCP.A
F~AG~JCU
E96 CQC~."CAG C'tlGA -L~CCGAF~AGGCCCAA
ACT~AG.;
7 09 UGUCZ-.:.-s,C'JGQG.C-GCCGAAhG:~CC~~A
~.GUCGw.~U
720 G~~UGAC CQG.AUGAG~~~CC,AAAGC,CCGAA
AGUUGUG
723 G~w.."CT NGACGAGGCCGAAAGGCCGAA AC~,AGUU
735 CCQc :7AG CC1GALCGF~AGC,CCGAA ACCC~.~,
738 CCACNC CUGAUG?C~CCG~AGCcCGAA AG.~~CCC
7 65 G~.i:zACA CL3G?L'GF~GGCCGF~G.~~CCG?
A ACCT~CGG
7 69 UCCAC~G NG:~.t~F~GG~CCG~GC~CCGAF,
ACAGACC
770 GJCCAGG CUGAL'GnGGCCGAAAGGCCGAA
AACAGAC
785 GF~NC-w NGT~LT~AGGCCGAAAG'-CCG1A
ACAGCCC
786 ACACiiGG CUGAVG'"raGvCCGAAAG.~CCGnP,
AACAGCC
792 CNCCGA NGAU -C:~CCGAAAG:~CCGAA
ACL1C~GA
794 C-GCCUCC NCAUC~G GCCGAAAGC~CC -C
A AGAC~JGC,
&07 CC~.G.~~L;GNCaU -C'rf'. "~''CGnAAGC~CCGAA
ACCUGGG
83. Ci-:.~,':?UCNCAUG~,C'~CCC,AAAGGCCGAA
ACCUCUG
846 CnL~G.~"U NGAUGAGGCC~CaAA ACUGLJGG
851 CWGCCA CUGAUGAG~vCCCT~AtaCvCCGr'~A
ACv~LJGAC
863 CG'.Gr.AG NGAU -C~.GGCC: AAAG:~CCGaA
AGL7CGUtJ
86E CvCC~.~G NGt:UGFaG iCCGT~aTsGC,CCGr'~..'~a
AG~~sr~aGUC
867 UGC-CCGA NGGUGA~CCGAF~CvCCGAA AA
~G~U
869 CWGGCC NG'.UG.~.GGCCGT~AAC~CGAP,
F,GAAGGA
881 ANGACU NGfiUGAGGCCGriAAG:~CCG,AA
AGGCCW
8 8 UCACACU NGALfi~F GGCC'GF AAGGC
S CGAA ACDC'~G
933 CGGJAQ NGAUC=~G GCCGAAAGC-CCGAA
ACUG~.J~C
936 UCCCCAG NGAUGF~GGCCGT~AAG.~CCGnA
AU~CUG
978 AGC'UCUA NGFiUCAG.~~CCGroAAG:~CCG.'.Fs
AUG~~UCA
980 AAF.G~~UG CUGAUGAC~SCCGT~AAGC,CCGr=~
AGF~UGGU
S86 CGCCC-.~-~,CUGAUGACyCCGAF~AGGCCGa.A
AG~.~UGUA
987 GCG.CGG CUGhUGAGGCCGAAAGGCCG~.A
AAGCUGU
988 G:~CGCCG NGAUG.G.~~CC~AG:zCCG.AP,
AAAGw~UG
CA 02468048 2004-06-07
184
1005 UC~~;C~G CL',~G.U';~C-:~CCGAAAGGCCC-.AA
AuC~CG'J
1006 UUCGUCA CUGnL'GAC~.CGAAG:~CCG.~
AAUCACG
1 G23 CL'uC"JGA CJG.nU''~~C -'CnAAGCCCGAA
ACCUC'JG
1025 CCC'JUCU CUG~UCAC~CCGAAAGC~CCGAA
AC-~' CC'JC
1 0 L u'Gv.: CL'GAUGF G~ C'"~GCn. C"~A
6 a JC ~.G:~..':L~'GG
9 Gu~"UGG CL'G:~UCAGuC C~~u~GG CCG?
2 A A C C:. C.AU
.093 UGC~~uG CUG~~C-:~CC~C -CnA AACCCCA
1125 UCAGCAG CUG;yL~nGC~CCGAAAG:~CCGAA
~.CZJC-G:,
1 6 GCAC-GAG CUG:w~,~,GC-C C 'G~A~ CGAA
3 ~.Ci ;1GCG
64 .'->GC~C-:~nC'uG;~LunGG:.CC~i~AGGCC~A
:-~Gw~JGC
115c .:G;~G~G C'UGnL~AG:~CC"~AnAGC-CCGAA
~~=.AGE~J
1 _ c-.~-.~-uGC~c~Gn~-.c-~ccc'~c~cGAa :
~ 2 ~-;~ r-:~
'_ 2 UG'JGJAU CUGAL'G?.G~~C CGAAAC,CCCGAA
0 0 AC-CTJG:~.
12 C UL'c'JGL'ACUGhU"~C~CCGAAAGG~"CC,AA
1 '~AG.."'tJGG
'_203 UCUUGUG CUGr.U-Cr'~G:~CCGFsAACv:.CC?.A
:.,tTF~SGv.,.'J
1227 GC:ACACG CLTGnUCAGGCCGAnAC~CCGAA
AGC"iJCC:.
1228 :~GGACAC CUGAL'GAGGCCGisnAC-GCCGAA
:i-~'VCC
;233 CALTACAG CUGAL'G~'~C:~CCGF~F~AGGCC'C~.A
ACl_CGAA
123 GC GG C CUGAUGhC-GC.CGF~.F~C~~CCGAA
E CA AC~C~?.C
1264 CCW.-:zAC C'.'C~FUGAGGCC~uFFaACvC.CG'ar~
iyUC'CCUC
12 67 UUL'CCCG CUG~-: -UCAG:~CGArr'~G:
CCGAA ACAAUCC
_ 2 U c.~~t c~~,:~t~;-.~.:~ cc~AaGG~ccAA
9 4 ~ G',G A~L~UUCv
12 9 c.~~~ sG,~c Tca.~L~cAG~ cc~G;~,: cca~
s raULroUC
_ ? G~C~UUG C'JG:~UGnC'~:~C C~ C GAA
0 6 AGuCUGC
_32. UuccccC c~~,_AUG.~ccc~.G~.c:~A AclcUCG
_334 cL?cG:Nc c~c,:~t~-.cc..ccG~AG~cc~:~A
At;c-G~,-U
3 4 CA C~CilU CuGnLW,G:~c CGAAAGGCCGAA
4 AC-WJCGG
3 S UC~tr~p CL'Ga:UG.=.G.-C C Cz7=.AAC-:~CCGAA
1 z Ci.CLJL~G
_ 3 C =.UC CUGnUGnG:n C'GAAAGGC C G
5 ? C_ W A:y AGAG'yCU
_ 3 r.GUC-:~nACUG~L'C~CNCCCv~F,CW.C -CAA
6 o AC'u'GCCA
1367 CAC'JG'G CUG~L'C~G:~CC'"~nAAGGCCGAA
AF.,C-LiGCC
13 6 GCAG'UGG CUGAUCAG;~CC'C~GC-C CCAA
8 A.~.AGiJC-C
.380 AUUCCCC CUGL~GGCCGF~r.G:~:.CC~A
AL'C-GGC~
1 3 r.GUCACU C'~~GAL~GJ-.G~,CCG~,F,AG
E 8 CCGAA AL'UCCCC
'_ 3 C'~ C GnGUCLlCu :UC:AGGC C~~F F~GGC
9 8 CGA~, ACAGL1CA
0 r -G':UCilCC'~ GA L'GnG:~C CG'~:.C-GCCGAA
2 A G'~ACA
140E CCCLG;r, C"~'GnL~~GC-CCG~~nG:,CCGAA
AUCUCGA
1510 UG~.CCL1C C'ui~-.U"'~-.C~Crur'u'-.~GGCC
-G'~n AGnUCUC
1 S A C :GnGG CU~UCr'-.GC-C. CGhAr'~GGC
2 ~ C G~ :~G.:-JGCC
S25 CCCG?CA C'~~G:~UG.~~CG~.nAG;~CCC~
AC-~.TAGG
1529 CL'G~CC CL'GAUGG.=-CCG.F.i~AGGCCGAA
ACAG'~,G.;
1444 UCCCCUU CUC.?:UGnGGCC'GAAAGGCC'GAF,
AGL'C,CUC
1555 C~C~-~"U Ct.'C~:UCf:GGCCC~F,C-GCCCAA
ACC'JCCC
S 82 C-C-:.~~-C-~~?CUGAL'CAGv~~.CuAr.AGC,CC~~iaA
.'-'.GCsCAU
S 8 C C C-:~C-GGCUG~ :UG-':C-:~C C G-.hAGGC
S C G:~ A GL.CAC
1493 t..::UCUCACuGnUG:C-CiCGAAAGC~CCG.~u~
ACCG:~GG
50G UGUCAC CUGZ:UG~C-~CCGAAAC~CCGAF.
ALTCLJCAU
'~ 503 UCAUCAU CUGYUC,AGC,CCGAAAG:~CCGAA
ACAAUCU
506 G=.GUGAU C'L'GAUGGGCC "'GGCCGAA AUGACAA
CA 02468048 2004-06-07
185
1509 CCACAGtJ CUC~UCF:G.~cCG?~GGCCC,AA
AL'GnUGa
1518 CC~"IJG:. CUGA~GGCC -C.AAAGC~CCuAA
A,CCAC~.G
530 CCWJLIAU CGGA~C~CC~~AAGGCCGAA ACL'GC~vv
1533 UGCCCAU CUG~AC~CC"~:?AF~GGV.~CG?~A
AUGACUG
' S51 AC~.nJG.~U CUGUG~~C~.~AACIZ "~A aCGCCVG
1 ==9 AL~G.G CUGALIGAGuC ACGUG.~U
1503 GcJUAUA ~~"~'~AF~GGCCG?.A AGc~TAC~v
1565 GWv"L'UA CUGAUGnGGCCGrr~AGGCC"aaA
AGAG"JA
1567 UG:~..w~U CLG:~UGnCw..~C~u~AC,GCC~~A
aUAGaGG
5 a auLVCUU c~a~c;~cccc~caA aUCU~cC
a
1592 UACJCJG c,;G;~L~cG.ccc-,aA,aG;~ccGAa
aUwcfw
_ _= ccJcwG cL-G.ccc~AaG;~,.~cGaA AGL'cLCJ
9 9
16=1 GZTJCi~GS, CLTGALK~C~-.~~CGAA rlG~~
1661 CCCC~GA C~iK~...~C~~AG~CC,?.A AGGUUCA
16 63 CLiCCCGG CDGhUG~."C'"'~r~AA~,CCG?.A
ALTAGGUL1
167 C~.G:~fi.A CL1G'~DGrIG:~C~ '~AAAC"~~CGAA
8 AGGCCCZl
1680 GCCGnGG CLKY.UGP.GGCC~~AAGGCCC,P.A
AGAGGCC
1681 C-:~CCG?G CQGn '' ~ , '"~CGAA AAGAGGC
6 8 G:~CNCC CDGA~C:~C C~~AAG.~CCCAA AG:~?
4 AGE
1690 FLTAUC-:.~, CUCALIGnC.vCCGAAAG~~~.C~"AA
AGG~~CGA
1691 F.F.UAUGG CU -C~LG~GGCC'".~-.F~,GGCC~.~A?.
AAGG.~CG
1 E CCACCAA CGGAI~GGw.'GGCCGAA ~~
9 6
1698 UGCCACC CQGAUG:~C:~~C~CGAA ADAIIGGG
1737 CF~UC~C~CA CBGF~~'C'GAAAGGCCC~.A
AUGUCW
17 5 GUAC.,~"LJG CCuAL""'~.i~.GGCC~~GCCGnA
0 AG."QGCA
1756 G~.-G,:.CGG CUGAUGAG~CC~.~AAGG~CGAA
AGGUGUA
1787 UGnGGAC CUGA " ~ CCG?~P. AUGCCCU
17 9 C.ACtJGF.G CUt~C-GCCGAP. ACTeAUGC
0
17 9 UCUGACU cUGAUG :GG~. -'~'CC~AAGGC
3 Cc~A :~CuACAA
1797 UcJAUCU CUGA'' ~ CGAA ACUGAGG
1802 GC'uGUUG CU~CCGAF~AGGCCGAA AUCUGAC
1812 C-:~cCCCA CUG;AIK~GGCC~~~CGCCGAA
AUGCZ1GL1
1813 UG:nCCC CUGAU'"'w,r~GGCCGAA AAUG'~UG
1825 GL'G:hGG CUGAU'-.Y.C,~~CGAAAC~:~cCGAA
ACCAUG.;
1837 F.GUGUW CUGAL'GnC~CC~CGAA AG~~3GLJG
18 S CGL'C:~:. C CUGaUGAC-G~.~C"~F~GGCCG?.F,
5 I?,GUGUUU
18 5 CfiCAUCA CUGAL'GnC-GC CGAAA~C CGAA
6 AUG~~GUG
1861 G.ACLTACA CUGAUC':AGGCCC'~GG.:CGAA
AUCACAU
18 6 AL'cJGF~C CUGAUG~GGC C~~F.GGCCGAA
5 ACAGAUC
18 6 cJCA UGU CUGAU -'' '' CAA AC'UACAG
8
1877 CUUC-GCLJ C2JGAI~GG~~t'c~AAAGGCCGAA
AGUCAUG
'_901 AUCJCUU CUGAL~CCGF~AAGGCCGAA AGUCUUG
1912 AUCCJyUC CUC,AL7Gr.GGCCG~GGCCGAA
AUCAUGU
1922 F~GACUW CUGF~ -~"CGAF,AGGCCGAA ACAUCCA
c23 Up,C',~CUU CUGAUCAGGC -CCr'~G: CCGAA
AACAUCC
0 2 CAC:~~UA CUGAUGP.C~G C CGw.F,F,G:~CCGAA
8 ACLJUL7AA
1930 AUCACvC CUGAUG.CGCCCF~FaAGGCCCAA
AGACUULJ
1964 cJGC,G GC CUCAUG:.GGCCGT~AAC- GCCGAA
AUGUCUC
1983 CCAGUUG CUGAUG=.G~CCCF.AAGGCC'GA~.
AUGUCCU
CA 02468048 2004-06-07
186
1996 G~l~uCyG ~.,C.~LTG~C1.~C~~Z~C -C :A
aLTLILJCCC
2005 ~.C~.-:=-~C~C'T~LW C-:~.~C~c~:~CC".~
AG.1UUC~
2 013 .~ C... . .
L~ cc,.~A c~c~-e~.:~: ,~c..~,. ,-~,A
ac~,:~G;.
20~ c~.~nrLC c~c~UCAc-c~ c-.~rv.~.c--,~A
s AU~GC~
2020 CUCrr.CA COGL'G1-Cue. ~.f-r1-CCGAA
ACC~~'r.AU
2 03 C UUCuGQ CL'r -G'~~C-:~ C~~.AAC-:~CCC,hA
9 AGUCUGU
2 0 ~ UC~'t1C'JGCUG:yL -uAC-:n. C~G:~~CG'~A
4 0 AAGUCUG
2057 cvC"J'nLiGC'uC~nL'GAC-:~~'~G:.-:.CG,AA
aGwCC~
2061 ~:C:L'GvC C'JG.AC~G-ZCG~A AUG.~-.AG~,
2 071 L'L'G.AUG.~.C'GGaUG~-~Clz C ~'.~AG.=-CCG.?
A ? CAC? L:G
2076 G~G.~'UU C'uGAUGnGC-.:C:~AAG:~CG~.A
AL'G..~UAC
2097 C-'vG=LG CUG~aL~-.G~'"'C".~.AAC1.-.:CGAA
?G'u'GGC-G
2 0 C..W: a CL'C=.~aUC -'C.~a~aG:~ CG~.A
9 8 C.''~G AAG'v'G"vG
2115 AG'JGCCC C'LC~t~CAC-:~C~'..nA.'~CCC~A
AGCUGGc
2128 G-JC=~G'JACUG:,UGAG:~:.C "G.~AGGCCG:,A
ACAGC~.G
2'_30 C-:.-.GUC~GCUGU -CAC-C-'CG~AAC~CCGAA
AGACAC-C.
2145 LT''nIiCAUCCUGF.~CC.-CCGAAAG;~cCGA?~
nG:w~iJGG
2152 '~AUACA CUGAUC,AC~ ~"~.GCC''.y,A
AUCAUCA
2156 GnAL~ri~A CJGr~GnC-.~CCG.~aCCl1~CG~.A
rICALTALC
LISP !'SVL:.MV11C"V~~1~'~llCGr~ A
215 nnU -CAAU CCGnUG,:-s~. C G'~C~.-C CC~.A
9 i~AUACAU
~16o AhA~~;yA c~L~c-c-c~ _..c -C'~. AAAVACa
2162 nG'-.BUG C~~G-'-.L~GC-W CGAr=hGGCCGpA
AUl~a.eiUA
2163 ~C~AAU CUG:~L;CnG:~C'-~CuCGF.~.
AAUAAAU
21 6 ~L'~ACA CUG UG~=~GC,C C'~"..~AG:~
6 C'Cr~P. AU -Cr,AUA
2167 ~f-.n~AC C~L'GAC-:~.= ~:A:~C~.iC -CriA
AAU -C~.U
2170 G~~.f-.r.AUC-LJGALuAC-GCC~~C-:~CCGAA
ACAAAUG
2171 C-' ~.TAAHACUG:~L'GAC-C1 C'~~C~z C.~-~A
AACAAp.U
217 C'u'C-~'=LT'nACUG-',L%GAC,C~ C'".=Ani~~.sC
3 CG'1A AUAACAA
2174 C-GuGw:vIAC'uG-.L~~-'~GC-C~~Ar~C~,.:r~:
r~tRACA
2175 AGCCL'C-..~"UCCJC=~L~'~z~Cu~ AAAUT~AC
217 U ".~C-.."tJG.GCf~'G CGrr-:~~C"~:aC C~.:AA
6 AAnAUAA
2183 CnrUAFu? C"v'G=.L:~-CCCr'~:A~ ACn"L:~C~'-U
2185 C'~C~:LTA CUG;~tJGnG:~CC~C -CAA AL~.GC'~7G
2186 ACJC~.AU CUG:-S'~C,AG-CC~.~AAG.:~CC
-CnA ~-,ALTAGCLJ
2187 CnCt~Cr.A C'JCG~GCCC~CGAA nAnLIAC-C
218 G C :NC C'JCf-',L'~~G:.-C C"'~:AAAC-.=-C.
9 C -CAA A LTF~,UA
2196 Cl:L~nF.nACUG=~L~G:~c~:,i.AC-;~C~-~'A
nCr.CIICA
219 i.''~L=~L'AACUGAL'Cr.C-:~ C'-.~AAGC-C.
8 CG?.A ::CR., ACU
219 CL~1C= C"~ G :L_'G.G:W C:~F~AC-GC
9 L''t, C~.:r.A r.A CACAC
2200 CCLr'r.C_tUCOG,:~'"~ACG:.C"ViaAACi-CCG'~F.
AAAGACA
2 2 G:. C'.TACACUGAUG'r.GG..~'C'-,=~ C -CAA
O 1 AF~.F~,GAC
2 2 L'L~ JAGC COGnUGAGGCC'"v,.at,AG:~C
Q 5 C CGnA ACAUAAA
2210 GJUChULT CL'G=.L'.Gr:C-C-CC~~.G:~CC
-C'nA AGCCLTAC
2 2 .~G :GAC C'~,'GaCCi :C-C-.~. C~v:.Ai~AG.:aC
2 0 C C -GA nUGO[JCA
2220 C-:~C~.G:yC"JGnL;Cr.C.C~-C~AG.:iC~.A
:yC~JAUG
2226 Gr.G:~CCA CUG:-.L~GnG:~CCCAAi:G.:~CC
-C.AA AGACCUA
2233 C-GVCCGU C'JGrL'GAG:.i -C'C.~,C-:ACC
-CnA AGC,CCAG
2242 C-.=AC'OGGCUCnUGGC~CC~~.FJ~.AC:~CC
-GaA ~.C-CiICCG
CA 02468048 2004-06-07
187
2 2 UGaCaUG CC -Cr ~C-:yC C~~nAAG GCCGAA
4 8 aCUG
.254 UG,aUGU CG -C.:~G'r.CvCCGAAAGGCCGAA
ACAUGG?~
2259 G~CCULIG C'~'CAUGnC-:~CCGAAAG~CC~~
AUGUGAC
2260 U --CaCCW CL'G:~UGAG:~CCC~AAGGCCGAA
AAUGL~G?r
2266 ACCLJG.~"fJCLIGhUC,AC~CCC AA,AGGCCGAp.
ACCUQGA
2274 AC-'sACUG CUGhUGAGGCCGAAAGGCCGAA ACCUGGU
2279 CCUG'UAC CL~~GGCC"'~F~3'~AGGCCGAA
AC'~.C
2282 ChACCUG CQCALTu~.G GCC'GAAAG.GCCGr~A
ACnACCiG
2288 apGJF.C CGCaL'CAG~C~~.AAGGCCC~A
ACCUGUA
2291 L'C~GL~G CL'GAUG~.C~CC'G~AGGCCG~
ACAACCLT
2321 CCCAUW CG'GnDGACvCC~~AAGGCCC?.A
AUC'JUUU
2338 Clr'iUC.AGCCW-:.-CC 'C,ruaAGvC.CGnA
aGUCCCA
2339 C:_'-~AUGACG -G;DGAC.GCC~.~nAGGCCG~
AAGUCCC
2341 C~v.,~~"~.UCLICaUGAGGCCCv'-.AAGGCCG?.A
AGAAGUC
2349 GUi3GGCC CUCnLK,nC~~~.CuAAAGGvCG'~A
AUGAGAA
2358 CLlG.v::a C'~G?UGAGGCC".~AA~GrCG~.A
AG:~:.AGG
2359 UCUGC-.~G CUGAUG~GGCCGAAAGGCCCAA AaG:~CAG
2360 UUCUG:~ CL~GF.WanGvC:CC~T~AAGGCCG:~P.
AAAGGCA
2376 Ai~CJ;AA CUGAUGAC- GCCGAAAGGCCGAA
AUCACUC
2377 G. CUGF.LTGAGGCC"'.~F~AAGGCCGAA
aAUCACU
2378 CG~UAGA C'JGAUGAC-~w~CCnr"aAC,~CG'"r.A
AAF.UGAC
2379 CCGAUAG CL1GADGAGGCC'GaTaAGGCCG:~A
AAAAUCA
2 3 GC C~.~UA CUG:~UGAGGCC'GAAAGGC C~~AA
8 0 AAAAAUC
2382 CUGCCGa CUGA~GCCGF.AAGGCCGAA AGAAAAA
2384 UUGJG~_C CL1GF:UGnCGCCGAAAG:T CGAA
AUAGAAA
2399 GLiCC:~UA C'~~Cf~L'G :GC-CCGAAAGG.~.CGr.A
AGL1GCULT
2401 CF~GJCCA CUGAL7GAGuCCGAAAGGCCGAA
AUaGUGC
2411 C.AAGCt.U CUGAI3GAGGC'C~' ~~CGAA ACCFGUC
2417 ACCUGUG CQCABCAGC-CCGAi~AGGCCGAA
ACCAUUA
2918 T~F.CCL'GUC'LTGAUCAGGCCGAAAGGCCGAA
AACCAW
2425 AUCUCUG CGGAUGAGGCCGT~AAGGCCGT,A
ACCUGLG
2426 ' nF.UCQCUCUGrLIGAC acC"~ATa?.GGCC~.~A
AACCUGU
2433 ~.cvG~,~~uc~GnUG~CCGaAA~cCGAA aUCOCVG
2434 c~cUCw c~GnvGacGCCeAp.AGCCCGaA
AAUCUCU
2448 G:-.G~.AU CUG:~UG~CG:~T,AG.3CCGAA
AGGCCUC
2449 CAA CiIGaUGAGGCCGAAAGGCCGAA
AAGGCCU
2451 :. "C~GC, CUG.UCAGGCCGi~nAGGCCG?.A
aUAAGGC
2452 ar.Gu;.G cvcz~UGAG~CGaAAG;~CCG~A
AaUaAGG
2455 C~GG CUG'aUGAGGCCC~A~.AG.~CCGAr~.
AGGAAUA
2459 UGC~,.~w CUCAUGnGvCCGnFaPC~~~.CGnP,
AGGGAGG
2460 UUGG:~c, CUCAUGr.G,~~~~~~CAAAC~~.,~~CGAA
aAG~.,G AG
2479 Gv.~UAACA CUGAUGAGGCCGAAAGGCCGAP.
AG.~~UGUC
2480 GG."UAAC C"~lCf:UGAGGCCGAAAGGCCGAA
AAG,~~UGU
2483 C-.~~UGW.~UCUGAUGAGGCCGAAAGGCCGAA ACAF~GG
2484 AG.~"UGGC CUCAU -Cr.GGCCGAAAGG.~,CGAA
AACAAAG
2492 G:~.~"'UG.G:CUGAUCAG~CG yA,AGG~CCG?.A
AG.~~UGGC
2504 ACF.~iAUG CUGnUCr''~GGCCGAAAG:nCGAA
aUGUGGG
2508 U6GCAGA CUGAUCAGGCCGAAAGGCCGAA AUGUAUG
2509 CUGGCAG CUC:-RUC ;G3CC'~~,AGGvCGAA
F.AUGUAU
CA 02468048 2004-06-07
2 510 AC'JC-:-CACCG uCnC1-CC~~W.-.:C~.:nA
?.~DGLIA
2520 G=,L't:~GJGCL'GrUG:~.'~C~CC:~ 1C~
2 521 LTC_':L~GLICL~C~BG:C-uCCCGAA
2 533 G~CCC-...~UCL'G?L~x-'"t-.:.-: CGAF.r'aGuCCG'AA
ACA
2540 C'-~C~U CUG.~''u :C~CCGF~~.CGAA
ACC"~~L'G
2 S AL'["JCCA CUCr.UGAGC~.. _ A C~I~AC
4 5
2 5 L'UC-:~-:~CUGr:L~G:~:. CC GAA ADL
o E
257 C.'-~t~:~ Cu'GriWGC-CCGAF~.G-.:C~.~?.~1
:~G~JBG;
2 ' n -G'~G-.?C'uG'-.IK.~?.AAGu:.C~.~1A
2 S C ACy:.AVA
2 5 nC'-u.GAG CL' -Cr'.L'~=rt-:~ C~~f~-v.C
8 E GrlA nCA?.Gx~.
2 c r'.C=.:AG.iCU -Cr.C~i~CvW.~"~A =aCWs?
o 1 ~ C.'-~A
G 5 AC~.C-~:~CCL'G~C1-..-CCG,~A ~
r 3
2 5 C~ =. AC~.GCL'C~.u'GnC:~.=CC~,AGS,CCGAP,
9 6 AC'-GAG
2 601 ~.F.AUC~:.ACU -C:~L~AGGCCGAAAC'".":.CGAA
aC~CGAC
2602 GAAnQGC C'J-CALG:~.C-:~CCssAr~G.~aCCGrIA
,~?,f~Ga~
t 607 C''.~L.'.GACLIG:-.L,'~GnC~~CGAAAC.GCCG?A
AUCr:~AA
2608 CCCnCUG C'~JG"C'GAAA~GC1CG~A .~AL~C~,A
2 60 UCC"~' CL1GAL~~CGAAAC'~,aCCGr~.A
GL7 i~iaAI3GCA
2 620 ALTF.GLTG~_C'CC~LIGnG GCC: rnAGGCC:~'.A
AG~'17CCC
2 62 C~JC-C.AA C'CGr.I~nG~CC~C,G.."C iAA
6 AG'~GC~A
2628 Grt-.."UGCC'JG~G:.-CCGAAAC'~y:..CG~.A
:~~C
c 63 -C'".r~.nCUGCL'Gt"'~-C'CGhAAG:~."C"vAA
5 nG.~DGCA
2640 UC-CAGG?~ CuG.W. :C- G.:.C~.z?r'~AGuCCGr'~A
ACUGGAG
2 641 CLTC~-.~.GGCUGUG-t.:~CC'GAA~GC~:CGAA
A~.C'JGGA
2 64'2 AC'LiCCnG CUGnLGiGvC'C'.~'iAAGGCCGAA
AAACQG~v
2 653 G.:=.?,cccUcu'e'.: ~~-'.G;~CCGAAAGC-,:.CGAA
ADCs
:: E CL'(JJCGC~aGC'~'CnL-C'CiaA~aGGCC"aAA
9 .~tCVLTGA
2 6 C. CUC C~JG:~'"'u~,G:~C CGnA~"~GCI.
8 9 C~.A CGr~F, AC C G'LJGG
2 691 GJCCUCC C'JG:,L~'~ :GGCC''CGAA
iiL~~CLlp
2 r UC~unGS, C'vGnL~JsC-GC C "~AGC,C
0 G C~u'~A AC~7CCUC
17 0 A~,G~~LTG:~CC7G .L~~C CGA~AC'wC CGAA
4 AG~~.SCT
271= CCUt,'CG. C',T'~L~:G:~CCC~'.AAC~c
CCAA AGCL~GGG
L ~ CCCL'UCC CLL.nU '~:G~~.-~.~.CGhAACI~.C~.~A
1 L r,AGCLJGG
2721 Cue..-:~U C'JG="'C ''C,~AAGGCC~~AA
,ACCCWC
2 i nCr.C"J-CGCUGL~.GGCC'CAAAC,GCCGAA
2 4 AUCACCC
2 ~ C"w~CACA CUG~-t-.:~CCGl~AGGCCGr~P,
44 ACACF,Cp,
2750 GCLIiGUC NGv~~C-GCC~~-~'AAG~.~~CG?A
ACAGAUA
2759 l:CnC-~~.aAC"uG.i,i',=.:-.C-~C~~.-.AAFt'n."CGAA
AGCQLIG'J
2 r AC'-~.Gr.L',w~CUGnL'G..':CvCCC,~JsAGG:.C
61 'Gw-.A r,GF~GCULT
c 7 G:.:GA CL'::hL~:.r,C~~CG~F~CCGAA
6. CA AG~~~~AGA
L % CCL3C-;~,~''Z;CUCnL~.~-t-GCC~~i~GGCC"'"AA
6 AC?.GaGC
2 r G.A CcT~U CUG. L~J-t.:~CC~: CCGAA
9 7 AL'DGCAC
2803 UC-CAGUG CUGAUG.C:,CC".3Ai~AGC-CCGF~A
ACCAUGA
2 8 CUG.~_r.GUCUG'~UG:~C-;~CCC~.AGGCCGAA
04 AnCCAUG
2813 AG.~JCAA CUG=:uG~C~~nAGGCCGAA ACUGC~aG
2 E''_ iv-aAG.~-.C'JC,t I:~.nG:~C C vFsAAG;~C
5 JC CGiaA AC~~CC~C
2 E21 AG~.CCAA CUCv:UG.rt. ;CCCZr'.AAGGCCGAA
AG~~UCAA
2822 GAGCCCA CUC,FaUCi:C-GCCGT~'~TiGGCCGAA
AAG,nUCF,
2 E2? U -C~~CC CUCAUCr:C-C,CCG~AAAGuCCGAA
A~AG.~"UC
CA 02468048 2004-06-07
189
2E29 AUCACW COCAUCAGGCCr' .-CG?A AG~_CC.AA
2837 G'J ~"-' CL'Gn -~C~AG~C.~~ .'~TJC~CLJU
2 8 4 GAC~~~'GG CL3GnUGAC:~C'J 'G.~iAC,~~CGAA
0 AG~.~:~CA
2897 ~G~~0 CUGnUGAGGCt'GAAAG~~~.C~.~'~a
ACv"'JG~G
~
2E53 UACUCAG CUGADGnGGCC: AAACGCC~..~AA
AG~_-v."C1GA
2 8 6 UCCCAC-C' C9GnL~GGCCGAAAG~.~CGAA
0 ACUGACv
2872 c~C cvcA ~ ,.,~AA~~,~.~-as A Uc-~-CC
2877 GL3GWGLT CUGA ' ~~C~-~aAAG~CC~.~?.ea
AG~_CQAU
2 Q99 AAAAUCA CL7 -CnL'CriG:~c ' ""CG~?~
AUUL'G.~.C
2900 AAAAaUC CU -Cr'~UC:~GC-CC'C~.AG~~~~C~~A
n?.WG~
2904 AAAAAAA CUG:-:L -' ,.~CG~.?.AGC-CC~.-?A
AUG'~?.?aU
2905 A C'JC.aUG'~GGCC~C~.-~ A.~UC'-~A
2 9 0 AA~AA CfJGAUCAGvC ~"CC~1 AAALICAPr
6
290? AAF~AAAA C'JGAUGAGGCCGA~~C~ AAAAUCA
2908 AAAAr~.T~A COCA '' r~CC~.sr'u~. iaF.f.F~.isL'C
2909 ~~AF~A7. CUGT,~,~',G~~~C"C~~.A 'Ar,i,F,AA(1
2910 AAAAAAA CUGF~UGAGGCC~~:FaAAG.~~~~CG?.A
?~Fu,AAe~a
2911 A~ CC~~C~GGCC,G:yA A.~A.AaA
2912 ' '' COGAUGAGGCCC,AAAGGCCG.AA AF~A.Arl~
2913 UGAAAAA COGnLT'v~GC~..~CC,F~AAC~C3CC'".yu~r
.~F~aAAe~.
2914 C~ CUGhi~GC~CCC~ i.~r'.Ae~
2915 UCUGAAA CtIGAL'GFS,~C~CCG~AAAG~CC~.-,'~
'ruJir~AAA
2916 CUCUG~A COCA ~ .
2 917 UCtJC2JGA COGAUGF~C ' ~ CG~A AAAAAAA
2918 GUCUCUG CUGAUCAGGCCCG~
201 o CCUCUCU CuGhUGnGGCCG:~nGC,CCG?.A
~xAt~ra
2931 GUUGCGA COCnUGAGGCCG~?AAGGCCC?.A
ACCCCG'J
2933 AUGUL'GC NG:.UGAG:~CC~~ ACACCCC
2941 UcCTG~.~ COGAUC.AG~GCC -CC:~ ARGJQGC
2951 A ''GJSF~GG CLTC~L1CAG~c~CG~u~. AGOCUGG
2952 G.CAAAG COGAUGnGG~CGAAAGGCCCsAP.
A~JCOG
2 ~ 5 LTF ACACA CUG AUGAG:~CC 'GA~AG~CC~.-~,?.
AG~~'~AGT
2 5 CUAACAC CLiGAUC~.CC~CCCFu':~aG~~~~C~W
6 A 'rnCL~-T~r',G
2 9 61 AUtIAA CU CtICAUCAGuCC 'C~CC~uF.A
ACAC
2 9 62 LTF~UtJAAC cJG :UG.GGC C'CAAAC,C,CC~~
:yACAG~A
2 9 6 CUULJAUU CUGAUGAGGC c~.~AAG,~~C~a?
5 A ACUAACA
266 G..~UUUAU CUGAL'C~:GC.~_CC?.AAGGCC~~.
AACUAAC
2 9 6 AF.AG.''W CUCALJG.G~n C'~3AFv~Gv~CC~.A
9 AUOJyACU
2975 GLIUGp.C~ CUG~:UGAWCGAF~ACG~CGAA
AG~'VOZTP.
2 97 AGWGAG CUG'r.U -C. AGGCCCJ~C~C~-~.
6 ~AG..~OUCT
2977 CAGUUGA CUGF~UG~.GGCCGFhAGGCCG~.
F.AAGCtJU
2979 GC.cAGUU CL3CAUCACvCCG.AF~.AGCCC~.r.A
:~-GnnAGC
CA 02468048 2004-06-07
190
Table ~
Mouse lCAM HH Ribczyme Sequence
nt. Pcsition Ribozyme Sequence
c~.cG~J c~G~~G~~c~T ~ GGccc~A Acc~GC,~
23 ;yC-C.oGAG C'JGnt;~G/=~GV-'C~.-~ia~.C-:w~CC,nr1
ACC_'~.C',~G
25 A G~iC-:.~'~iC'TsnLiGnC~~~.C~' ,v~CCCz?JA
A 'CAACC~
31 UGuG;~G CL'GAL'GAG~C''.~G:~~C".~A
AGCAGaG
34 C~CCCU C'JGA ~ :~CCG~ AUGAGAA
40 ~.c:~.--uACc,~Gr.~ -chG:~c~cc:~AG;
ccr~ A~ucuGc
48 CCAC~CLT C'JGL'C,AGCr.~CGAAp.GuC'CG?.a
AC,~"LJCC.'LJ
s4 CCABC~.C CU -C~JCAC-~CCGA~ AG.aCCCA
s8 C-::AG~~UA CL'G~LT~GC-~~C:,CCGAA AC-GCALTG
64 CUG.~GGG CBGADGAGGCCGnhAGCCCGnA
AGC-~ Gv~UG
9 6 G:~.GC CAG C'UG~~U~~,F.GGCC~CG? A
A G~GAG
.02 CC~:C-.~_r'aC-C'vGALGi~C~",~.CG:~FaFaGGCCC~
ACLJG~Cn,
1 0 C-:~.' CJ? CUGr'.L"".~GGCC ~ A GCAGP.G
8 G
s z~c~::nGC.~cJczU -cAG~ccc~cc,AA Ac~ACC.~
1 , UCCL~G.~'"CTC'JCAL1G~.G~C~.~~AGGCCGa.A
o ACALJOCC
12 GGC-CG'~G CUGALIC,AC~GC CG" '' ~,C~CAC;aG
0
146 C~.G:.G C'JGAUCnGGCCG?.AAGGCCGAA
AC:3ACCiG
l s2 AGL~~U CUi,nLTGAC-:~CCGnFu~G~.CG:aF.
ACACAGA
sa ~~~.~ou c-~GAU~cch;;AG~ccc~ AACAGcA
1 6~ G:~AnAC CUGn2,' -C.:~C-.GCCGAT~AGGCC~~.A
ACUUCUG
168 C-GGGCAG CUCAUGAC-C,CCGAT~AGGCCGaA
AAGGCW
l Es CUGCACG CL'GAUGAG:~CCGAAAGGCCGAr~
ACCCACC
209 GCCAC~:G CLJGT,UGAG~~CCG:~AGGCCGF.A
?.AGUGGC
L27 C-:=.AAAC CLGAUG:~C~-C~ " ~ CG~ AC~JUCtJG
230 C-a:GCAFa CLJGAUGF~GGCCCAAACri,CCCT,A
A~aACUU
237 AGv'UCLiC CUG AUGnC-C,CCGT~AGGCCGAA
AAGCACA
248 U'uLT"r~C~.C~.CUGAUGAGC,cCCAAAGGCCGAA
AUGC,.~~UU
2 53 UCLUCCU C'uGnUGF:C-GCC~T,GGCCCAA
A GC~CAGG
2 63 Cr.C'u .GA CLJCnUC~:GGCCGAA.AG~,CCGAA
AAACCCU
2 67 L~~C.:~CS,GCL;GAUC.;~-C-CC'C,nt"~AGGCCGAA
AG.:.CCCU
293 G:G..~UCA CuGf,UG :GC~CCG~GGCCGAA
ACAGCUU
319 C-GCLJCAG CUGAUG~GGCC"~fi.AAGGCCG~A
AUC'UCCU
335 CULiCUCA CUGAUC.YGGCCG'r,F,F.GGCCGAA
AGCACAG
3 3 C GUGUG CUCAUGAGCCCG:AAAGGCCCAA
7 AUUGGAC
338 L;C=.G'~UC C'JGr.UG:~C-GCCG:~F.G,CCGAA
~,AG~GCU
3 5 r G:. C-.~-,ACC'G.L.'.G C-.:-CCG''yAAGGCC~.~F.A
9 ACUC,CAC
367 CC-C-~:~JUGCL,'~G=.UGr:GC~C'',-~.AGGCCG?.F,
AGCCAW
379 GC.~-:i.GG CL'CnUG~:C- G:.CCi,F..AGG~CCC~.A
AG: CUL1C
375 GC-CMG C'JCALG~.Ci-CCGAAAG:~CCGAA
AAGGCW
378 nCACG.~"tJ CUC.:-.UG-'~GC,CC~~AAG:~CCGAA
AL'GGUAG
3 8 t =.AC -CnACUGAUC:~GGCCCAAAG ;CCGp.A
6 A CACGGU
3 94 ~. -G:UCGA C'JCAUG G.~C C ~~.AAGC,CCCYf,
A G'JCCGG
42 C ~-~.:W C:.'Ci L.'G C-C-C C,~-'~.,~-.i.,CvCCGi.'r.
0 , =.r GUGUG
425 cJ~C-..~c;C-;~C',;C:AUGG;~CCG:-u-.AC-CiCG,=.
AGC~"wuG
CA 02468048 2004-06-07
191
427 CaCL'Gw~U C'vCiLT-_'r.Cv~CC~-~F GW.CGr~
~GlaGC'~TG
45O C-~''zGC.v~UC'sGAUC~'G~~~.Cv~~r'aF~eiGCcC~w'~A
.~Cv~UCCU
4.1 Crai-EGG?.CUG'nL -"G'aC~C~-'~' aG~v:,CGr~A
aG"' uCUC
456 .'-_GJG.~~~UCGGaU -CnGC,CC -'Cru'~aGvC.C~VaA
?.GC-C~JAr1
495 i:CnCGv'v C'~.~JC~?.L"GaGV,CCGn?l1G~v:.C~.a?.a
. e~.i:G.~~UPl',
51p CCCC?CG CUGAUG'~G G: CGAAAGGCC~.~A
?~G:~.GC.~
64 ::YLG,~-.acUGnceacl-ccc~ac~lc~,~aa~
ac~JG~~
?2 CCGaUG'J CL:C?.UCnC:~CGanhCt-:.C~~ae1
ai;C'JUGC
607 Cr:UCCa C'UG'~UGaC-~v~~C~.~n~C~CG'a~
. aL'CiGVsC'J
608 GC?UGAG CUGrVGaGC,CCC-aFaaC~.CGe>~a
.'-sr.LVC"-.,C
sp9 c-;~vca cUGavcacvCc~.-.aaAGGCc~~
anavcG"
011 C,: Czt~U CUGnL'CnC-GC CG~aaaCvC CGr~?r
r1 -C~'ar~AUU
656 C~.GCLJC~ C"uGAUG?.GV,CCGAT~P.GGCCG?.A
isCAGC'JU
657 L'G~C~."UCCUGnUG~GGCC:~AAGGCC~~ AACAGCCT
668 C-GJC-C C2JGAUGG:~CCGAF.AGC~CC -G'~
:C ?Cu."UCG
077 _~'VGG C'UGa.BC,~CCGMAG:,CCG:~,
rG?~GGLiC
684 AC-.::ACCGCUG aUCAC-C,CC -G'~'~GG:.CGF.a
AGL~CIG~,
692 r.~GUCG CUG~L'GnCvCC 'CGAA '.~JCCG
693 G~jG:n,~J CUG~UGr~C- GCC~~F:GGCCGAA
sG~~UCCp
696 CAG~_-:~G CUGALTGaC,GCCG~AAaGGCCGnA
'r~nACAGv
709 L'~.~C,~.~-UGCL'G'.UC'~GG.:.CG'ruiAGG.~.CGaA
AGCCG~.C
7 2 t:C-CZJG'iAC'JG~UG'~G.~r:. C~.~?.G~~~~.
0 CG'~A isG'JUGUa
723 cc~-,~~u c~GAUCac.~C~.'~G~ccGaa :,'~aGw
735 UCUCCAG C'~:CAUC GGCCC?.F~aGC,CCG~.
AUCCiGG'J
~38 cc~ue~e eJGauGG,~.~ccaAAGG,:cGaa
aG;~.ee~
765 G:f-.F~GCGCtJGaUC.~sG~.C.~-~P.GGC
C~~nP, :.CC?.CUG
769 Cw~C~ CUGAUGAGC-CCGA~sF~GGCCGT~P.
AC~GGCC
77p uVCCAGG CUGF~GTGGCiCGnr.FG.~_zCGA.~
~G.~1A?~.
7 E Ci-:~ G:~.CUGAUC:.G:~CGF.~AC=~ CG?.?.
5 ? C=.GG:C
706 ?-Ci-:~GG C'L~CAUG:.GG.:C:~.?.Ci,CCG?.?~
AaCr~GGC
752 CL'UCCGa CUC,T,UCnGGCCGF~.G:~CCG.~A
ACCUCCA
794 ?-CVCUCC CUGAUGf-.GGCC -C~-.e:Ct-v~.CGAA
e'-GCCCaG
807 C':C-..':L1ACLGr.UC:~C-C,CCG.AAGG:.CG~A
AUCC:?~G
g33 G..v"tJG(iCCL3Gi-.UG?.C-W.CGT~nG isCG~Pa
nG.'-'W~7G
a46 c ~ccvJ c,?cAVCac~c;~.~.aG~cG~,a
ac ? ~~
851 GCi,IG~:JAG'JGAUCAc.~C~~AG: CCGAA
r?G.~'"UCL'C
ss3 c~Gr.GG cc~cavcsac~,~.ccaaAGccc~
acvGGcu
ass c-~~G~ cvGnv -c~ccc~GG.ccGAA AGCCUVc
867 UCUCCCv CL"GAUCnGC-s.CG'nr'1F~.G:~CGAA
F.nCGi~AU
g69 CWC-;~.U CVGAUGAG~C.~-.F.A~:G:~CCGAA
AC-:SAGA
8$1 :~CC~JU CUGAUG?Cv.~.C~~nAAG:~CCGAA
~r?GCCAU
885 UC~CCUC CUGAU -CACz-CC -Ca~AG:~CCGAA
FCCp~.GG
933 CC~aGrr~U
CGGT,UG~G.~.zCGA~WG:~CCGAA
AUUAiJA~
936 C~ACC?.G CCiCYIVGaGC-CCGAAAGGCCGr'~.A
AUGAUUA
978 FGJUGUA
CLJGr'iUGACvCCC~GGCCC~A
ACUGUUA
980 ra~GUUG CLGAVGaG ~CCGfiF.AGGCCGAA
AC?.CUGU
9 8 ~ C-.."IJ C'~JGnUC'~.Cv.~, CG.AnC-vCC.~'aF~
6 -G'~'- AGUUGUA
S o C :C-~~'C?CL'GrI7G CW-CC ~r.AF GWCG.a.?
7 ?_ G'~uGJ
.cc v:jC-W,:G ' C'vGAUGCI~CGi ::C~cC~~
i=::C'~'CiG
CA 02468048 2004-06-07
192
1005 UCUCG~G
C',.~~UGAGGCC'"'
~ C-GCCG~u~.
AUCUG.~"U
1006 'u'UCCCCA
NG:~UG:yG:~cCG:v~~CCGAA
.~CUCLTCe~
1023 c,~~,~cce~
c~GnUGhG~ccGhA:~GG~~'GAA
AccuccA
1025 CCCJUCC
C'JGnUG'r.G:~CC'"~aGG.=CGAA
AGACCVC
1066 L'C;rL~LJW
CL'CAL'GAGGCCGAAAGGCCCAA
AGAGUGG
1092 GG.~_CDGA
cJGALGAGGCCGAAAGGCC~.3AA
AUCCAGU
1093 L'UGGCUG
CUCnUCnG~CCCi~AaG:.-~CG?u'~.
AG.~"UCC'~1
11 2 UC'-~AGAA
CUGAL -'GG.-C
C:~AAGC-~
~'".3AA
AGUUGC,G
1163 C-C.'-~AAG
CUGAUGAC.GCCGAAAGGCCGAA
AGCUUC;~
1164 AGCznAA CUG_UGAC~GCCGnaAGGCCG.~.P.
AAGCWC
1166 AGyGC~A CLTGAUC:~GCCC~.-~AGGCC'".~P.
a
1172 G.:JL'UUL7 CUCnUG~-~~CC,AAAGGCCGAA
AAGAGC,A
1200 L'G'JGGnG CUGAUC,AGGCC'"~'-.AAGGCCGnA
AG.:AG?G
12 01 CUC'JUCA CUGAUG~:GGC CG''~r~AGGCCCr"~A
P,AGCAGC
X03 AC:.'~G.~~JGC'JG:yUGAGGCC 'GnAAGGCCG~r.A
AAAAAGU
X27 C-C.ACf~CG CUGAUG.AC~-CC~C~.:AA AUCL'ACC
'228 AC-Cr.AnA CUGr.L'GhGGCC"~GGCCG?.A
?~'UUC
X33 CUCJCCG C'JGnUCnGGLCGi~AG:~CGAA
AAACGAA
1238 AGC_ACCA NGAU -G'~GC ,,C'Gr~AAGGCCGAA
ACAGCAC
12 64 CL'UGCC C"JC,nUGnGGCCGF~AGGCCGAA
ACCCWC
7 L'UCCCC_~ CUGP.UCnGG...~C "' GGCCCAA
ACUCUCA
1294 Ci-cJC.:~G CuCnUGAGGLC~.AGC-CCGAA AUCUCCU
1295 cJG~-'~UGA C',JGAUGr-.GC,CCGAAAGGCCGAA
ACCCC'UC
1306 G_uUuCA C'~~AUGrGGC.CGnAAGGCCG~.P.
AGUCUGC
1321 UCCJCCU CUGnUC,nGC,CC'"~GGCCGAA
AGCCUUC
13 3 UL'LT'r~ cJGAUGAGGC CGnAF~GGCCGAA
4 AUGG""UtJ
1344 CACUCJC CL'GAUGnGGCC~CC~A AG~~UCAU
1351 'r~.cJUA cJG'r.UGAGGCCGnAAGGCCGAA
ACAUUCA
1353 C~C~JUC CUCAUGAGGCCG~AGGCCGAA ACCCACU
13 66 i-.G'Jt'Wi.'ACUGnUGnGGCCG'r~i~T.G:aCCGnA
ACUGWA
1367 ~:C-._'~.'C-;~GCUCAUGJ-.GGCCGr~AGGCCGAA
AGuJGCU
1368 ~GAGJGG c~~UGhGGCCGi~AGGCCGAA ACAGUAC
1380 c: ~cccC cJC.hL.'~G::GGCCGAAAGGCCGAA
AUGGGCA
1388 AGCCAcJ C"~~CnUG~-GCCGAnAGGCCGAA
AGUCUCC
1398 CWCJGU C'LGAUGF.C-:~CCGAAAGGCCGAA
ACAGCCA
1402 :-~GLTJCL1CCUGAUGhGGCCG'nhAGGCCGAA
AAGCACA
1408 CcJCCCC CUCAUG::GGCCGAAAGGCCGAA
AUCUC'GC
1410 CCCJUCC CU FsUGr.C-C-CCC~GGCCGAA
AGACCUC
1421 AG.~.:~~G C"JGAUCr.C~.-CCGAAAGGCCGAA
AGGLJGGG
425 cJC,.~~ncC cJcAUC~GG:.cGAAAGGccGAA
AGG~Gu
1429 Cf:C-C~-;C CUCAUCAGGCCGAAAGGCCGAA AUAGAC~
1444 UCCUCCU CUCAUCAGGCCGAAAGGCCGAr~
AGCCUUC
1455 UCC'JG.~"U CLICAUGr.GGCCGaAAGGCCGAA
AC~UUCC
14 82 GC.-:AGCA C"~JGAUGr~G:~CCG :AAGGCCCAA
pACAACU
1484 CAUG~-~GG CUGAUGnGGCCC'.~AAGwCGAA
AGr.ACAG
14 9 G'vJUC"~JCAcJCAUGAGG.~_ C C,AAAGGL
3 C GAP. A GCA CAG
1500 C-.:=?CCr.UCGCAUGAGGCCCi.~.GGCCGP.A
AUUUCAU
1503 C=?_UCAU CUCnL'GC-:nCGr.~_C-GCCG_~A
AUAGUCC
~ 506 CC~ WAU CLiGAUGhCi-C.C~~_GGCCG ~
At~C.F~UAe~
CA 02468048 2004-06-07
. 193
1509 ACACGGtI CUGAUGr.G~CC -G'J~CGAA AUC-~~UAG
1518 CG.~.CQG.G CUG'~ -UCAGGCCG~AAG~CCGaA
ACC~UGA
1530 CC_~G~nU C'CIC~GaGC-CC~.~CGAA AU~UAG
1533 GGCCCAC CUG?.UGAGGCCG~CCGA~. aUG?~CCA
551 AG."UCr.~U CL'C~~aGC,CC _ ,G~nnGaCC~vr~t~
a ~G.~At~a
1559 AG.~'"Z7GGG NC,aL'G~C-G."C=" '.~n~C~~CGr~.A
.'-~G.~"QG~.~U
1563 G.~JCTAUA G.i~L'C,~.GG..' ~'=" ' ..,.'..~
ACS
1565 GC~JUA CUG~L'Gi.C.GCC~-' v.."CGaa
:~C_~.UA
150'7 UCvCG~;J CL'GAL'GnGC.-:.CC~.C-~vCCG?~.
.~~r AACa
1584 AUAUCC'J CUGAUG:~CCG Ar~G.~CCG?~ AUCUUVC
1592 vAacw,G NcavGf~G~.:C~~aG~~CC,aA av~.UCC~
15 99 CNUCUG CLG?.UG~~CGAF.AGGCC~~? A aACUl7G0
1651 GNCAGG NGnUG~.C-.GCCG~aFanG: CCG?.A
AG,~UGGG
1661 C=.AnG:~?,. CU -CnLGaG.~~~.CG~CZ,CCCAA
:,C,w,~UUUC
1663 UUCAr.~~ CUC~UG?GGCCGA~CvCCGAA AAaG~'"W
1678 CC?.C~CQ CUGAUGaGG:.C'"~GGCCGAA AG"~UCCU
1680 CCACAGG CZJC.nUCi-'sG:~:.C "G.~i.G~CCG?A
AGaGGCQ
1681 GCCACaG CJCia _ ~~C~-' ~CCCsr~?~ 'nnGUGGC
1684 ACAGCCA NGAUGAGG.~CG~r'~GG.~_CGA~.
aGGnAGU
1690 AC~UCG'~ CUGAUG~GG..~C'-'~'.GG..~CG?A
AGDCCGG
1691 ArGAUCG CUCAUG~GGCCG~C~CC~.~AA aAGQCCG
1696 CC_~.CCCC CL;GAUG~C~GCGAT,~~GCCGAa
aUGC,GCA
1698 CUCCAG G CGG~BG:~GG:.C'CA~'u'~C~CCG?.F.
AL1ADCCG
1737 GCUG.~~JA CUGa.U~~AGGCCG?sPI~CG:~
AG.~~UCUC
17 5 UGisGG"JG NG.TaUG.GC-LCGAe'aAC-CsvCCa'~A
0 : _GCCGCC
1756 GC-:~.p.G~, CUGnUG?Cw.=CGAA'r~G~~CCGnr~.
AG~aCWC
1787 U ~'"' C NGAU -C AC.GCCGAAAC-GCCG.~,A
AUC~iTCtJC
1790 AUUAGAG NG:~UC,AGGCCGAAaG~CCC~ aCnP.UGC
1793 UCCAGCC NGAL1G~GGCC -"Cn~G:~.C:~A
AC-:UCCA
17 97 UUVAUw Nc"vcac-;~ccc~~cGAa aNC,~,~c
1802 UCUCCAG CUC,AUG?,GGCC:~G~CCGF,A AUCUGGU
1 8' GGCNGA NGAUG ;G:~,.~CG?.F~.AGC1CG'-~A
2 AUC~AGiJ
1813 UGr"-.G~-,~"t) CL1GP.UC.?.G:~: C"~GC'~CC.~A
i~UGv.~UG
1 E25 C-:.F:C. GG NGnUGp.G:~CCGAAAC- GCCG~?,
P.CCGUGG
1837 GGAC~'"ITA C'uGAUC,nC~CCGA~.ACvCCC,?~
AGGCAUG
1845 G.~"UGGCC CUGAUGGC,CCGnAAGG:.C.~A
ACCCUCG
1856 Ap.GAUCG CUGAUGAGG~CC'GAAAGGCCGAA
AaGTICCG
18 61 UACLIGGA NC?~UGGC-CCGAAAGGCCGAA AUCAUGU
1865 CUGAGGC CU ,CnIJG:~GGCCGAaAGGCC''~A
ACAAG'UG
1868 UUUAUGV NGAL'GrGGCCGAAAG: vC:.ap.
ACCTG.~"UG
1877 AGCUGCU CUGAUGAC-:~CCCAF~AGC-CC~.~
AG~AUG
1901 GUCCCN NGr~UG~sC-GCCCsnF~r:CuCG~,
AGL'UNA
1912 ANGAUC C'UGAUGAGGCCGAF~G.~.-CCG?A
. ACUAUAU
922 UAACUUA CL3GAUGAGC,CCGAnAG GCCG'~A
1 ACAUUCA
1 02,3 GAVACN CUGnUG?.G~T~.CGFar~.GWCC~.A
AGCAUCA
1028 CUG.~~UAA CUGAUGGGCCG.FJ~ACv.~.CG.'~.r'1
ACLICrJAA
1930 AC-NGv~U CUCnL~Ci=.C-CSCC~-fir' "~C-v.-v.CGn?a
~ ~=.C'JCU
1064 LC~"'' .C NG~LiGs?Cz-CC~~-r~~-C1-w.G~l.
:.~~~UC'JC
1583 U?.ACL'UG CUGU -Cr'sC~CGirC~,iC~-~
.'-.u~UCCLT
CA 02468048 2004-06-07
19~
c 9 C~"L?C~G
6 C U~~-~"
UGAG.-C
C C"r~GC-C
C ~.:?~
AUCUCCCT
2005 ~:JCCG~.
CUGAUGnGC-CC~AAC-:~C::AA
AG."UCC~
2013 LT~hCtiC?A
CUCAL'G?~:.iCGAAaC-W
CA?. ihALTACi
2015 c;~cccc
c.;GnUC,~c;~.;.~,-~ac~;.c::na
aUG'..~,~_.~
2a2o c~.-C~.c.~A
c~cA~e~c:~_:~~.~ic:~.a
AACC~Cc
2039 CCUCUGC
CGCsnUC~,AGC,CC~-~"~u~C-.~sCC~.-~~sr1
AG.CnC-v
2040 CCUCC~G CUGAUG1C-C,.=C~G:~C~.l.A
aG.'=iJC'S.G
2 0 C-.~-.AUGvGC'~JC~L'C.A G:zC C.:r.?~GCi
57 C a.?~ AC~GC~.
2 0 aC~C G~J CUGAUGAG:.1 C ~~-r~ 'n~.C-GC
61 C ~,;rfs AUG~"Lr'lG
2071 CUGAG:~C CUCAUGAGGCC~?.GGCC~~ .~C~,aG'u'G
2076 LJAG~."UCUCJCAUGACv:.CC:r~nC~CGl~ AGC~"UAC
2 097 CAUCAAG CUGaUGAC~c C~'~GG.~. C ".-~
AGAGJL'G
2 0 C C~-C.:~;CUGAU GnC~c CCAAAGC~ C u'~,
9 8 AAG'JGL'G
2115 AUCNCC CUCAUGAGuCCC.~.AAGGCC:.-.Ap.
AG~~UGGv
2128 CUGy:UA CL~L'GAGGCC''.~AAG:~CC -CrA
AUAGCUG
2130 Gt.C-C.CnGCUCaUGAG:~CC -C~.r'.C~-:.C
-CAA '.~P.C~GG
2145 G'.UCr.AG CUCAU -G.C-GCC"'=" "' ~CCC=~
AGAGLn;G
2152 AnCLTC"JA CUGAU -CnGGCC'G~-.r~G~vCC''.:r.A
AUUT~'UA
2156 U'rr?L~.'rACQGAL'GnG~CC~-.=C~.:?A ACAUCAA
2158 hULr.AUA Cu'CAUGAC-:~CC~C;~A AUaCAUC
2159 RAUL~'i,AUCUGAU -CAGGCC~-v~AAC~~a.~C~~'a~
Ar3.Ue~.Cr'~.U
2160 AhnUUAA CUGL: -G.C-G.:.C~-~anC-GCCG.A
:t.AUrlC_A
2 ? CL~.u-.HL'UC'uG~=aUG~aG:~ C~~G;sC C
o' GAA A ' " IT?.
2
2103 ~.nLTiJ?~'aUCUGAUG~aGCtCCC~~r'ar~ AnUAC~U
2106 nr.'v'~.GAGCL3GAUG.G:~cCG-'~AaC~CC~..nA
AUGr.ACaU
2167 AnL'U'r.AUCUG?UGAG~~cC -G'~nAGC,:.CC.
iA AAUACAU
2170 CL'AI-.~:UUCUGAU 'C~CC'",~AAC-C-CC~.A
AUT~.AUA
2171 GC-GAG: CL1G=.UGAC-:~CC -'G~ACZ-CC~.:~A
AACAaCU
2173 CUC-.uP.A CLIGhUGAG~CG~J~:C1-C.C~r~
hCUC'L'?.A
2174 C-CJC~"L7aCL~GAUGr'1-GvCC -"CnAAG~cC
-Gsn AAC'JCUA
2175 ~G.=,.'G~"C7CUGL1G-'~GGCCC~AC-GCCGr~
r.I.ACUCU
217 L~ GC"JGG CL~GAUG:~G:~C CC~G:~C C.~~.~.
6 AAAA CUC
21 83 C:.~:LT'~-.F~F~CUGAUGi:C-C-CC -Gn~CvC.CG~?
AGw~UGGLJ
2185 CUC::nLTA CUG AUGAC-:~CCGJ~C-CiCCAA
AUAGCL7G
2186 ACUC'r.AU C'JGnUC~-C~CCCr'~'rrCi-C.C~.-t'.r~
A,r',UAG.~U
2187 L'ACUC~A CUGAUG=.G: CC~'CCG:~ AI.F_UAC-C
21 8 G.:..?F CUGAUGAG:~CCG.i~AGGCCGAA
9 CUC ALTAAP,UA
2196 CALTCr.F.GCUGAUGAGG.=CGrI~AGC~.CC?.A
AGAGUUG
2198 AAC~L~r~A CC'C,AUGAGGCCGAAAC~C -CnA
AGGCUGC
21 00 .t,Lr~ CUGr~UGAGGCCG~_G.=.CC.=.rte
~?C~ ~-.r"~.GAGGC
2200 C'CT..~C-CAUCUG'=.UGAC-CZCCi.AAG GCCG=A
AC.GAAGA
2201 GCCC:ACA C'UGAUGAGGCCG-'~nAG: CC~,,~.A
A"nAACW
2205 UCAGGCC CLTCAUG,:.C-:~CCG~AGC-CCG~'.?
ACAUA~
2210 AGCCACU CUGAUGAGGCCGAAAGGCCG~A AGiJCUCC
2220 AG.CAAC CUGAUGAC-:~CCGAi~C-GCCC?.A~AUGCCAG
2224 G._.AUGGA CUCAUGAC-:~CC~~AC-C-CCGrJ'
ACCUG1G
2226 GCGC~CCU CVCAUGAC-GCCC-'-lrCllC ~~=.
ACAUCC~
2233 CCUCCAG CUC_'_UG.C-C.-:.C.~-_=i_.CZ-CCC=.=_
rC-.~UCiG
22x2 CvJCCGC C'LJC-r'yUG=.GC-:.C.~-r".r_.GC-:.~.~_.
=.GC'LiCC:=
CA 02468048 2004-06-07
195
2248 UCH: UG C'JGAU -CnGGCC~~GGCCGAA
AUG:~.UA
2 2 UCAGUGU CUCAUC-~ CCAAAGGC CG'1~.
54 r'~F~UtIGGA
2259 C.~CCGJG CUGrBG?t-:~CCG:,AAGGCC~.~
aUGUG?~U
22 60 GCACCGLT C~JG:.UGAG:~: CGAAaG~CG~
AAUGUGA
22 66 UCCUCv--U CLC~?.i.'. _C~CG ;AAGG:.C
;:11 ACrUUCC
2274 UCUCC-'~G Ct7G?;U -CAC~C "GnAnGGCCGr~r~
AUCiJG.~"U
2279 CUGGC~C CuGAU -C. AGC,CCG~.AG:~~C~.,nA
ACCCUL'C
2 2 c~G.,~UCA CUGaU~t-.~C C:~1? G:~c.
8 2 CGAa aG~GCUU
2288 ~ AC- GCC_~.UC'JCAU -C.AG.~CCCAT~AGGCC.~A
ACUUAUA
2 2 .'-t-CF CUCAUCAGGC C -CnAaGGC CGnA
91 G".G ACC_~CUG
2321 CCC-~LTGLTCOCA -DC~"-.GCZCGAT~AGG.~.C~.:?.A
AUCUL'UC
2338 CAC''"" CUGnUG~G~.C~"CGF~ AGJCUCA
2339 CAF.AC-:~ CUGAU -C~CG~FGAA AG.~"LTUCC
2341 AG:n.~JGG CUGnUGAC-:~CCGF~PtvCCG?.t~.
AGAG~"UC
2344 GCVG3AA CUGAUG~t~:~CC-CnAAGGGCCAA
AUCGAAA
2358 CUGC~ C'JGAUG.GG:. -CG:~P. AG~~UGGG
2?5c UCVC-DUC CUGAUGnGGCCGAr~.G.:n.C~~
Ar'J~1GCAG
2360 UUCnF~FaG CUG'~DGr'~G:~CGrn~.G~~~.C
-C~'u~ Ar'~GGUU
2376 UCAG.~G CUGAL7GAG:~CCG~u'.e~GGCCG?.~.
ACCACCU
2377 CUCAG~ CUGrL3G~GGCCGF~GGCCGAA AACCACC
2378 CA~~ C~~~CG~AC~CCGa.A AAACCCLJ
2379 CUL7~L'GA CUGAUGAG:~CCGAF~,~GGCC~~
AAAAGCA
2380 G.:CGACA CLGAUGAGGCCGAAAGGCC~~?.A
AAAACUU
23 82 G:~C"~'" CUC~UGnC~CC:~AAG~~CC~~ AGaGhAU
23 $4 UUGUGUC CLGAU -CAC~CCGAFuIG.~CCG?.A
ACUG ?.U
23 og GJCCACA CL1GAUG:~C- GCCG-'~GGCC
GAA AGUGWU
2401 C~~ N~C~~~ AC~GCW
2411 ~GCAUCCU CUGUGAG:~CC -CAC- CC~.~?~
ACCAGUA
2417 ACGU'r~UG CUC,AUGrt-GCCC,AAaGC~CCGAA
ACCAUUC
2418 GGCCUC-~ CUGUGrGG:.C -CAF~P.GGCCGAA
AUCCAGU
2425 AACCCUC CUGAUGAG~C -C~AGG: C~.~A
ACCCAUG
2426 AAACUCU CUGAUG~.G~CCGSAAGGCCG~.A
AAUUAAU
2433 G..'"UG.~"LJACUGAUGI.C-;~CCG~.F.F~GGVCC~,
AACUCUA
2434 AG.'"IJG.~"VCUGi~UGnGGCCG~.FaP.G:~CCGr~~'
AAACUCU
2448 G.;~CAGG CUG.~.UGAG:~CGAAAG GCCGAA
AG~~LTfJC
2449 ''G. ~~G CUGAUCCi:C- GCCGT~AG~~CC:~,A
AAGC,CW
2451 AGGCAC-G CUGAUG~CGAAAGGCCGAA AACAGGC
2 4 GAGGCAG CUCAUG.T~G:~C C -G=.AAC-vCCG?.A
2 AToACAG;
2 4 C ~-~cAGG CUGhUCY.C~C CGAAAG:~ CGAA
5 5 AGG.,.~UUC
2 4 GC~~G CUG'~UGAC1-C C~.~AC11CGAA
5 9 AGLJGUGG
2460 C ~G.~GGG CUCAUG?~G~: CCAAAG.zCGAA
AAGUGUG
2479 G.~UG..~.LTACUCrUGAG3.~.CGr.AAG.~.-CC.~.-,~.a
AG.~"LICUC
2480 G GAUCAC CUCAU -CAGCZCGAT~GGCCG~
ACC,.,'"UGA
2483 G~"UGGCU CUGnUGAGGCCGAAAGGCCGAA ACAUUGG
2484 GACCTGv~U CUGAUGr~GGCC.:AAAGGCCGPJ~
AAAAAAG
2492 AGGUC-Cv CVGAUGF:C-GCCGAAACv~CCGAA
AG,~UGCU
2504 ACA'r~.AG CUC?sUCr.C1-CCG=~C-GCCG..A
AG.,~UGGG
2506 UCz-C:~:UGC~GF-i.'~C~.CizCCA.aC-CzCG.i.
=.UC-:QUA
2509 CUCZ'=tlA.~.CUCrUC=.CW.-CC~=~ ~CZZC.~-_=~
ACUCUFs~.
CA 02468048 2004-06-07
196
c 510 C-.=vC-' C'(.~CnLG.C-:~CCC~_G:~CCGAP.
17F. AACUCUA
2 52 C .UL'GGG CUGAUGAGGC CC~~AGGCCGAA
0 ACAAAAG
2521 vGnGC-._~-UCUG~ -LlCnC~.:~:~G.nnGC~CC"L:?A
:~:L'C,CC~;
2533 CnL''"rsCC'JCUGn -UG'.cGCC"~~.nGGCCG~
AGC=~DCA
2 5 C =~Ci_GC CLOG=~UGt-' CCr_~.C~. CGaA
4 0 G ACT~'Gw~GT
2 5 AG::ACC_T1CL CnUG CvC CG''~anG:zC
4 5 CG~?.A AG~Cu~.C
2568 L'UUGAC~ C'JGUG'.G.~C~~-t~CC".:AA
?CLGC~.C
2579 CAGGCC_~ CLC~UG.?.GG.:CC-.~~C:~A
~CUQAU
2585 ~c~c:-.ac cc~~L~G~Cc -c~..c1-;.ccaA
a-~GCc~c
2 5 hL'L~.G CUG:-.L; Gi.GGCC G~AGC1
8 8 G CGAA AC~-~.DGC
2 5 aG;~G;~. c~~ caUCaGC-cc G~AG~;.cGaA
g 1 ac~ACCA
2593 G~G'~C~ C2.'-GnUC,AG:~CC,~:G:.-CCG~
~.AAG?.r~G
2 5 CAUUGC-G CUG.=.UG:yGGC CGnAaC,GC
9 6 CGAP. ACAAAaG
2 6 A~-.nC~.-~C'UGAUG~GGCC~~.G.GCCG?.A
O1 ?,C~ C'~w"Q
2602 C~-",-.,.-'UGGC'uGnUGnC~C -'Cn~GGCCGr'~a
AG..'RGuA
2607 CC:G..JA CUGhUCl'-_G:~CC~:G.;~CCG'~A
AL'CCGAG
2 608 G~CAC-CG CtiGUGt-:nCG~nCvCCGrZr~.
ACUG..."~JG
2609 L,'CC'~Gu'JCL'GnUGAC-GCCG"~G:~CCGAA
AC~DLJCC
2 6 C-C..':GC-.~~UC"JG UG?t~C CGA:-.r GGC
2 0 CGAA ,~G' JCCU
2 6 G."L~ C-GA?.C'JGAUGa.GC~ CG:~-.r CvC
2 6 C~..~A aUC~P.A
2628 nG:n."UaC C"JCl:UGnGC-CCG~-.nG:~CCG?.A
~GC
2 635 ~.G:;ACCG CUGUGr:G.~ C~~.~=.~G:~CCGAA
AG~'21G~
2 6 G..-CAG.~-~Ct.'~G? L'CriC~.-C C -G'~AnCvCCG?
4 0 A ACAGGCC
2 641 C'~GCt CC7G.nUG.GGCCG~ ~GGs.CGAA
GA AGE
2 6 G~GC-G:G CLTG.L'G GGC C Gr.~.C-GC
4 2 C GAA AA.AC?~GG
2653 G.=AUCC'J C'UG:,UGnGCCC~A:~Cv:.CG?~?.
ACCAGUA
2659 C'JUGCAC C"JG::UGAG.:,CCGW '-.nCv:.CGr'~.A
ACCCWC
2 6 C CUCC-:~.CUC~UG? C-GC C G~CvC CGr.A
8 9 AC~UUAG
2691 G~CUCG C'UG~UCu:C-C.-CCC:~.:CZ-CCGAA
aCACAUU
2 7 G'-C-~:C~ C'CG~UGhGC~CC~C-.;vCGAA
OG AGC~UC
2704 ~_C-:~CUG:~CL:~G-~U -G',G:~CCG~t~CGAA
AGAGGLJC
2711 CUG''"L~Cr'~C'UGnUGAG:~CCG:~.nC-GCCGAA
AC~~UGGG
2712 CCCL'UCC C"~GnUGG.:~CCGn~:G:~CCGAA
AGaCC'UC
2721 C"JUGCAC CUGuGG.:.-CCGnr.~-~C~.CGAA
ACCCUUC
2724 C-:r:C=_CGCLiGAIJGGaCCCr.F~GGCCGAA
AUGUACC
2 7 C'~.TC-C~CGCUGAL?GnC-;~CC''-~ :G.:.~.CGAA
4 4 ACCCACC
2 7 G.: JACUC C"JCAUGnG:~C C -CAAAC-C,C
0 CGAA ALTAAAUA
2759 ACAUCGA CUGnUCr_G:~CCGT~F~GCCGAA
AGUCCGG
2761 C~~:GC~"U CUGAUCr.C-:~CCG~AAG:~CCGAA
AG.~~UCCU
2765 :GCGG:.A CUGAUC~GC,CCG'.yArt-GCCGAP.
P.G~A.F~,
2 7 C C LTGJW CUG=.UCrt-GC CG.i~.r~C-C-C
6 9 CGAA ACAGACU
2797 C~CCAU CUC~UCiGGCCG.rrCI.zCGAP
AUUUCAU
2803 CC-CCUGG CUCAUG.nGC-CCG~~-.AC~CCC~'P.
ACCAUGA
2804 CUGCACG CUGAUGAGGC -CCr~GC~CCGAA
ACCCACC
2 813 G:N~LiG.G CUGr.UG.~G:~CCC~=.nrlG~~~~CGAA
ACCGGr'iG
2 815 =rf:GJUG CUC~UCG? C-C-CCCI=.rr Ci-C
CGAA AG AC'JGU
2821 CCVCCr'1G CUGnUC~?G:.-CCG.=_.r C1-v.CGhe~,
AC~s~UCaG
2 222 _ _ .GUCCGCUG_'=L.'GG:.-CCC-'-: rGC;.CG,'.,p,
rGCi,;CC
_023 JCiuC-C C'UC=L~G_'11CG==t-=CCG?~
r_.r?Civ.
CA 02468048 2004-06-07
197
2E29 ~UGr.U~rA
cUGaUG~:G~cc~AGC,cCGaA
AcuCCAG
2837 UCACAG CL'C~UGAGGCCCAAAGGCCG?.A
ACCACCU
2840 C=GC-CAG C'LiCF~UCAG~CCGnF.AGG CCGrI.A
AG'JCtJCA
2847 G~JC-GC'IJCUGAUC~GGCCG?.AF~GGCCGaA
ACAUUGG
2853 ~C~L~r CUGAUGAG~CCG~GGCC:~A AC~~UG~.
A
2860 UC_ACnGU CUG:~UGnGG CCG~GGCCC~A ACtJLSGGC
2872 CUUGw'U C'JGr~UGAGC,CC~'~n~AG~~CCGaA
?.e'~Gva~UCC
2877 GJGAUGG CUGAUGAGC~CC~~FCGAA AG..~G::aA
2 8 Tx.G:~UCG CUC~UG.GGCCG~GGCC'".z?A
9 AAGQCCG
2 9 F~~ACUC CUGAUGT.GGCCGAAAGGCCGAA
00 AAAOUAA
2904 F.F.UAGAG CUGAUG .GC~CC~.~Fa~AG~."CCr~
AUG:u~GC1
2905 CAAUAGA CUGF.UGACGCC~' ' ~ C~.~
AAUGAAIG
2 06 LT'nPUp.AACUCnUGr~.GGCC".~AAGvCCGAA
ACAUC'aA
2 9 F~UUAA CUCriUGnGGCC 'G~C G?.A AT~ALTACA
07
2908 :sC~~AA CUGnUGAGGCCGAAAGGCCG?.A
AAGCUUC
2909 AGG:AA CUGrUGAGGCCGAAAGGCC-CAA
AG?AGCQ
2 910 F.w:UtJF~ACUCnUGAG~vCCGAAFaGGC CGr'aA
AAFaUACA
2911 ~:~.UtlAA CUGAUG?G: CC~AAGGCCGaA AAAUACA
2912 GCAUUA CUGAUGAG: CCGAAAGGCCG?.A
AGF.ACAA
2913 UCl.CCAG CUGF.UGr.GGCCGAAFGGCCG?~A
AGAGAAA
2914 CUUF:UGA CUCr'~.L1GAGGCC~~.AAG~~~.~.~CGAr~
?~'ar'~AGCA
2915 UCU''t,F..AUCUG?UGrGGCC 'GraAAG~~~.CGara
AAUAAAU
2916 CUCCC~A CUGAUGnGGCC".~G~.CGAA ACGAAUA
2917 UCUCCGG CUGAUGhGGCCGaAAGG.~.CC,AA
AACGAAU
2918 NCUCCG CUGAUGAGGCCGAAAGGCCGAA AF~ACGAA
2919 CGaCCCU CUGAUGAC-GCCG~AAAGGCCG?.A
AUGF~GaA
2931 CL'UCCGA CuGF~UGAGGCCG:~AAGC~CCGAA
ACCUCCA
2933 CCCUUCC CUGr.UGAGG.~_CGAAAC-~CC,~1
AGACCUC
2 9 UC~:~,C CUC,AUGAGGCCGAAAG;~CCCAA
41 AUGUCUC
2951 GC'~GnG~v CUGAUGr.GG.~.CGr~AG:oCCGAA
AGCGUGG
2952 CzC~GCG CUGAUCAGG.~.CGAAAGu'CGAA
ACUG.~UG
2955 UC.~.CACA CUCAUGAGGCCGAAAG:~CCG?.A
AGUCACU
295c UUGAUUC CUGAUGAGGCCGAF.AGC,CCGAA
AACuAAA
2961 AGJGu',"U CUC,AUGAGGCCGF,RAGGvC~s?.A
ACACAGA
2 9 AF:UUF CUGAUGAGGC CG?.AF~GCCGAA
62 AU AAUACAU
2965 CUUUAUU CUGAUGF:GGCCGAAAG:~CC~~?,A
AUUCAAA
2966 CCUCUGC CUGAUGAGGCCGAAAGGCCGAA AGCCAGC
2969 F~CW CUGAUGnGC,CCGAAAGGCCGAA
AUUGAU~3
2975 GCUG.~'"tTACUGAUGrGGCCGAAAGGCCGAA AACUCUA
2976 nCUFGAG C'UCAUGAGGCCCr~G.~CCGr'1A
AACCCLJC
2977 CAGCUCA CUGAUG~GGCCG~GGCCGAA ACAGCUtJ
2979 GC~UA CUGAUGAGGCC
-C~AGGCCGT~
AGnAUGA
CA 02468048 2004-06-07
198
v ~ c~ ~ ~ ~ a ~ ~ a v U ~ a
U U U
W J v ~ W
Z- U U U U a U C7 ~ ~ ~ ~ a ~ ~ U t~ ~
r
C.~ ~ ~ ~ ~ ~ ~ ~ U
C~ ~ ~ ~ ~ U ~ ~t ~ U U ~ ~ U
a ~c a a ~
UC:.'~J~JU~~~UU
U U U U U U U U U U U U U U U U U
~C ~ ~C .2 .G d d d ~C 4 W2 d ft it ~G ~t
a j ~ ~ a
U L U V ~ 'uJ ~ ~ U U U ~ ~ ~ ~ L
V ,~ ~ ~ ,~ a a a a a a a a a a a a a
o _ .~ .~ o 0 0 0 0 0 ~ a o 5 o a a
O
4) ~ U U U U U U V U U U U U U
U ~ ~ U
0
5~~~~~~5~~~~
a ~ U U U
,uc c ~ ~ ~ ~ ~ a a a a a a a a a a
c~ ~ :~ c~ a U c~ ~ ~ ~ a c~ ~ a ~ o
~C ~ U U
y_ U U .~_. U J U ~ ~ a ~ ~ ~ a
Q7 .~ C7 C~ G~ C~ C.7 C7 C9 C9 C9 C7 C~ C7
>,= a ~c a a a a a ~a d a
N ~ C7 L L O C7 C7 C9 C7 C7 C~
o a ~c a a a a a a a a a a a a a ~c a
~~~c~~~~~a~~~~~
U ~ ~ ~ C? C~ U' ~ C?
~a~a~~~va~a~~'~aa
T
Q
U O r, ,., ..~ e, m c ~n N ~o c
CD ~ ...r ~ C vC r ~ t~t1 D ~N O t~ N C\ O f1 N 1~'1 r
'-i ~-i .-i ~-i N N N N N N
'= a
CA 02468048 2004-06-07
199
au~c~~~
».
a dc~c~~'~c~aaac~~~~'~~c
c~c~c7Wc7c7~c~c7c7c7c7t7c7c7c'7c7
o~ U U U U V
cn O C~~~~ ~ c.~c~
~~~~~~~~~~~~~~~t'~~
N=
O
a c~ a ~ a v
c a
a
a d a ~ a ~a a a ~ t~ ~
V , .~ a~ uwn o er ~ o
I~ .fir yp C' N ~ 00 G~ a ~ rWD ~ ~ N U1 Y~'1 t11 CD
m ~D tf1 ~ '-1 er O ~ rl C~ N N H'1 t~1 tt7 CD A
ri N N r~1 a W CO C1 C1 r,.~ rl r.l ri e~i rl ri
O
a
CA 02468048 2004-06-07
200
U a ~ U ~ ~ ~ ~
.. D U
.a ~~~~~~~c~c~~c~~~c~v~~~'~
U rt ~ ~ ~ ~ U
U ~t a
C~
C
47
v v v v v v ... ... .. ,.. .. .. .r ... ,.. _ _ _ _
c j, Uc~~c~c~c~c~~~~a~~c~c~c~c~c~3c~c~J
0
a a a a a a a a a ~x a a a a ~ ~x a a a
,c_
p ~~ ~ C7
a~xa~xaaaaaaaa~~s~xa~a~
a a U c~ c,~,9 ~ c~ c
a ~(~~~~U~
c~ c~ c~ a ~5 c~ ~ a
'o..
= C
N t~ t~ N CD L~ N r'1 C1
_ !l1 01 t~1 10 l0 Q1 t!1 ~ O Ilt CC 111 CD e~-1 O f'~1
~ N e'N7 c ~D O~ D Ot ~ !~f M c''1 QJ D O r1 tf1
ri ~-1 ~-~I ei v-~1 e-I N N N
c ~o
CA 02468048 2004-06-07
201
Table 9: Rat ICAlI~I HH.Ribozyme Target Sequence
at. H8 TaxQet eequeace at. 3H Target Sequence
Position Positioa
GaUCCAAU U C~CACQGa394 GuT~~'G..."U~ ' CAG
U
23 ~~ C ~.'~A 420 ~G,f,CCCCU C;.AG..~G:.A
C
26 G~ACDG~~U C UUCCUCUO425 C~JC~~ U C;,GC~CC
31 CCUC~ C CUG.~"tJCCQ427 L'CCTGUU :,AAAACCA
U
34 cuGr~GCV c AcavAtmC45o AACaacw avc~G..~
c
40 ~~"'Q A C:AAGC'CCC 451 Gw"G~rC~J CCCC~~
C
48 ~CCU C G:~CCL~ 456 CL'C~~~L'U L'C~C~C~,~,
C
54 CCCCG~~CU C CCUGAGCC495. GCC~CC~U
C
58 CCGUGC'CV U UAiGCTJCCC510 GCGCCTGC.v C
C
64 CAAUGG~."U U CAACCCL,U564 ~ U C~~C
96 CCUCLJG~~I7 C CL3G.~~UCCU592 GC~U C CC
1 p2 CUCC~~U C CQG.~~UCGC607 CAGCC~U DCL'CAUC-C
U
~ 08 G GAC'JG."TJ U GC~~608 AGCC~UU C'CCaLG~~LT
'J
~ 5 UCCtmCCV U U~GWCCCA609 C~.C'-,AUOQDC~DGCL~
C
9 cr.CACVCJ c cccAACUC~ 611 caavUUCV aUC-~~WC~
c
0 GWGUCAU C CCCGGGCC E56 GuC'~CUGV Ci~,AL'G
U
46 CCT~GaCCV U G::~ACVCC657 UCACL~'JU ~~ --CAAL'G'J
C
152 ACCCG ECU C CACCDCAA668 G:~ACUGC'J L"CCCLiCW
C
158 AWQCDUU C ACGAG~ 67? GC.ACCCCLT C:?~~.G:~
C
365 UGnACAGU A CWCCCCC 684 AG~.'~.G.."QCC~CUUU
C
168 G~AGCCUtJ C CQGC<vCGE92 CCAGACCU CZA?CUCC
D
185 GG~'"JGGAU C ~ E93 C'GGaCLW GDCVOCC
C
209 CAGCCCtv A ADCUGACCE96 GCCUGL~U C
C
227 GnCCAAGU A J~~AA 709 G~C~.L'L?p CCCCUCAC
A
230 CAAGCflGU U GUGC,C,AGG72'0 CLC'J U a '
237 CUr~AAGCU C C,ACaCCCC723 CAACUL~TQ AG.."'L7~CCC~
C
248 GGCCCCCU A CC'UVAGGA735 CUCCIJG3U CuCvJCGv
C
253 CACUGCCO C AGUGGAGG738 UC'~GCCIT Czv"~CGG;,
C
2 63 GAGCCAAU U UCVCADGC7 65 p,L~GtJC,CVp -~W
U
2 57 -G.~GCCULT C C'UGCCUCG7 69 UCUUGL;GU CC
U
293 G~GCUC~l U C~AGC'U~C,A770 CUUGJGW CC's-::AAG
C
319 CGuAGGAU C ACAAACGA785 AG~CC'UGC7 DCCDGCCU
U
3 3 5 ACUGQGCV U tIGAGAACO7 8 6 G:~CCiK~7U CCCzGCCVC
U
337 t7CUGCLJAU A UG.~~UCCtIC792 C~JCCUG.~~UCVG,-UCGC
C
3 3 8 AAGCUCUU C AAGCL~GAG7 94 UCCUGCCU LG.,Gw.--pC
C
3 5 9 CACGCAGU C CUCGGC 8 07 G..~v?CAGr~aUL~~C~TC~,
UV A
3 67 CAAtTGGCV U . CAACCCGU8 3 3 CCUGG:r.~-UC~ CACUA
U
374 WACCCCU C ACCCACCU 846 CUCACAGU nLiu~.UUG
U
. 3 75 AG~AGCCV v ccvGCCVC851 GcvCACCV vAc-~GCU
U
378 ACCCACCU C ACAGG""'Lm863 CAAVGG CU C~.~srCCGU
U
3 8 6 CGC'UGUGO U UUG~u~Gtv8 6 6 CCAUG~~UU CL-CL,CaC,A
C
CA 02468048 2004-06-07
202
867 GAC~''~CCU C CC,~~C"~,1421 G~CpU C CCCCAGGC
E69 CL7C'DUCCU C U~~".zAAG1425 ACC",~CU CUCVGGV.~U
C
E81 AAUG:~'W C :zACCCGtrG1429 ~,A ~~~
885 G~CCza-~.GU A ACL'G'v'G?.A1444 ~-sGAAGC,~~L7~AG
C
933 UG'JGi~,ULl C GWCCCAG1455 GGGAGUAU ACCAG~~
C
936 G=AGAGnU U UUGUGUC~, 1482 AC.~ACU CCC'CCAGG
U
978 UUGAGAAU C ~ACW 1484 ACTu'G~~UCUC~JCUUG~
U
980 GACAAUCt7 A CAACJp~'(,1493 C~G:~,~,--UCr;~.GAC27A
7 U
9 8 C'JACnAC.'U U UL1CAG'~CCL~ 00 C'J'.JG AUG.,~UCAA
6 AAAU U
987 UACAACvU U UCAG~'VC~ 1503 Gr,A~UGU C~'AACC~C
U
9 8 aC'~AC~UU U C iGCUCCC15 06 UG;";UC,AU ,~
8 A
1005 CLJC~U C GUC,: CGGC 1.509 GCC.~.CC~,UAC'JGUGUA
C
1 006 GJGG.AGZT A UCACCAGG 1518 G'JCCUGGU ~~~U
C
1023 CCGGAG,~U C BCAGAAGG 1530 ACCUG,GU ALmAUUG'J
C
1025 G~.GGUCQ C ~~ 1533 ~~U U GCGuGC~
1 0 CCCD~CCQp U GUUC'CCAAL 51 C~G;~.,~UCUUGC UCC~~1
6 C
6
1092 ~~~U C UCAC~CA.G?~ ~~59 AGU C CC~L~TA
1093 A~~~";AAU C CAG'~CCCp1563 UCCLmCCU UGULICCCA
U
1125 CCCCAACU C U x.565 ULmCACCQ UUACCGCC
A
1163 ACC:ACG."U U CWUI3GC[T'_567 AC~CCpAU ACC'GCCAG
U
1164 C'"ACGCUU C UDC L 84 AG ;AAGAU A
C
G~,UALDr,
166 ACGC"~Cff U LrCGCOCL1G1 592 G~G
CA~U A CA:~C
,172 CWQUGCU C UG..~GG..' 1599 UA~pU A
~U
'_200 AUCCAABp C p.CACL~AA 1E51 CCCCGCCLT
C
CCDGAG;
12 WG~.~W C UCCAC~G;, 16 61 CLU C
O ~
1
GCCCOG.,
12 G~"tlDCU C GEC ; 6 63 U
03
GAACAGAU AAUC",ACA
1227 L~TGC;AC~7 C CALJG'JGCQ C
1678 C,AGAAUCU GGCCUG~~G
2 8 G~~G:~:~ C
~W C ~
U~
. 16 8 GG:~.."t~CUCAG;G"'"LJC
' , 0 C
233 J
"'
CUCC
'
"
_ ~G, 1681 GGCCLTGUU CC'UGCCUC
iJ C C U
t3Gu
UCGC
1238 EAU A UG.~"UCC~1C 1684 CtJG~~~
A
~pCUC
64 GGAAAGAU C ALIF.C'~.,~U1 E 9
0
CCCCACCU CAIJACAW
12 GZJC~C'~ U CAAi" A
67 AAtlG
.z 1 E 91 C CGC,ACUU CGAUCUUC
12 C~.GaC,Am7 U UGUGL~G U
9
4
1 E 9 CUC CUGGU C'OCr,~UCGC
12 AGAGG:r,~p C UCAGCAGA6 C
9
1 E 9 UCAGAUAU CCUGC,AGA
13 A 8 A
0 GCA
6
~ 17 3 GADCACAU CACCn,"UGC
' GAC~7 C L~CA~ 7 U
-321 AACAGAGU C UG.:~.AAA 1 750 GUCCAUUW CACCUAUU
A
13 GUAUUCGLT U Cr'CF.GAGC17 5 CCUCUGCU ~
3 6 C C
4
UG~
1344 UCG,~~UG~~U C AG.~'"i.JADCC1787 UCCU
GAGAACW G~GCCUGGG
C
13 UCAG~"C'U A AGAC~:~F,CL717 9 GACACL1GU
51 0 C
CCCAACUC
1?53 ~ C AACAAUGG 1793
AUG~JCCU ACCUGGAC
13 AC C
6 :
6
~ 17 97 UCCCUGpU AAApp
~GDACp U CCCCC~G U CCA
1367 G~~uACQU C CCCCJ~GC 1802 GCUCAGAU ,
A '
UAC
13 G~UG...~UGa C CCG~GCC1812 AA CUGGA
68
CAO;AGU UGG.:,CApp,
1380 C'UGCCUAU C G; C
=AUC~
~, 1813 GCC~;CUU GUGAUCGU
388 LTG C
; :~AGACU A ACUGGAUG 1E25 GCCACCAU ACUGUGUA
' C
98 CUGG~~UGp C ACAGGACA 1837 ACCCACCU
, C ACAGGGUp,
402 CffGZfiCUU U O;AGAA~ 1845 A
GAC,C,ACU GGAGGG(,,C
1408 UUCGUGAU C C~JGGCGUC 1E56 C
CCCCUAAU UGACCUGC
1 410 C:~P,CL7~1U C CAGUG C
GAC 18 61 CAUGUGCU UAUC-GUCC
A
CA 02468048 2004-06-07
203
1865 UAUCCCyJ A -Cr'~CJ~G 2198 -CAaL'G'JCU C C~.~G~
C~,
1868 UCACG~G'J C :.L~ITA~J2199 ~CUCUU A C~DGCCJiG
1877 ACAGL~CU U CCCCCAGV 2200 GG.,~UACUU C CCCC~G,~.C
1901 W~s~U C 'M'C~.~'LTACr'12201 GCr~sCZILiCU C CACACv~vTC
1 g -Cr'.ACA~U C A?.UG:~C~22 05 UUUGGJG'J C AG~~CACGG
~
1922 aUGU'r~?.GU U .~UL'GCC'LTA2210 LT~C,ACU A aCCGC,ALiG
1923 LT~CG."'J C :~CCWUpG 2220 G?.G~rICCCT C G:~CWGC"
1 a2$ CiJC~ -G'~U ?i ~?ACCCK1G::?,2224 e~.C.~L~nG~.U U
CC'.r~ICCUIJ
1930 L'G.;~G?~C'J A AC'.;G.:~UG2226 CUC~,aCCIT C AGCiC~
1964 -1,~G~~i~? U GUG'JC~.GV2233 UCAL'G~"LZ7 C ~1.?~C?~1~C'J
1983 -C~:?.C~J C C-C-:.C'"'G:~G2242 ?~C~.C~C-.."U C
UC~1U~1
o c L'C,~.C-.."tJ C UUC~~J22 4 8 CL'CCGG.3U C CL'G
6 ~-UCC-v
.
2005 =~G~G'J U AUUG~'"CiTA2254 AUCCAAUU C ?~C?.CQG~A
2013 C".~~~'G.~J A UC~.N::~.L'G2259 G?.UC~CAU U CAC~,vGC
2015 C~CCO~U C ~~G~.:~L1G.~~J2260 AUC~UU C ACGC,UG,-~U
2020 UAWC:~,U A CCCVG~C 2266 AUCAG~~U A ~AGiJCI
2039 C'G ;?.G.:.AU C ACA.AACGA2274 G?.G:_~.G~"U U AAA
2040 C~CGaL'C'J C CUGC.~G.,~U2279 G:~GaU C AUAC'
2057 CGG~"L~CWJ C CAr.UC~C:LT2282 ACAG'v'Oa.U U CAULTi'-~C'aU
2061 G.~,GVCC:U U U~.C'~CCL?~2288 GCCCUC~J C CUC~.~ALG
2 071 AUACVGG'J A GCCUCAGu 22 91 G~,UAU A G~C~C
2076 L~~J C AGC-CCi~ . 2321 ~C~AAGaU C AL1A~CGG"~U
209? C~..:~.CUC'J U GUL'G1UGU2338 L'C'G~~~ULT C UCC'~CAGG
2 09 C'UGAC.: J C CUGG~J 2 3 3 G:u~UACL'U C CCC
8 9 ".~GC-C
2115 LZiCCG?1ZT A GCw~UCCUG2341 G:,-:~CCUG'J C Gw,"L'G..~(1C.~
2128 r.C-JG.."GGU A CCAUGAUC2344 CCGC'LTCGU A G,?,C'CDC:GC
2130 GCCUGUGZJ C CUC-CCCiC'J2358 CCCL'Cz.C'U C CGCCCACa,
2145 CC~ACL~C'J U G"vL'. 2359 CCAUCCAU C C'C~aCAG~
-C~sLIGU
2152 LAG-'~,G~U C UAGACULT23 60 CWGLCW C CCUG"A,~,G
215 UCAG~tJ A L"CUAUaGA 2 3 7 C,~ACUGCLJ C UUCCUC
6 6 GV
2158 L'GrUG~~U U U1~IJ~AL'U2377 G?,C'ULTCCU U CVCUAWA
215 G'! UGJ'riUU U AL'~aAWC2 3 7 GCiiGAUUU C UUUC'~CCA
9 8
2160 AUGUAUUU A UU3~.UUCA 2379 CUG~"UC'W C C'LTCULiGCG
2162 AC.~UUCCU A C~'INGUU 2 3 80 UGAUQL;CU U UCACGnG'J
2163 L~LTULJAW A AUGCAGAG 23 82 AWUCOUU C ACG.GUCA
216 UG'~UGUAU U Ut ULD~AUU2 3 84 UAUCCC-.~"L7 A GACACAAG
6
216 G~.UGURLZJ U .~UL'''.~AUUC2 3 9 UAn~.UACU A UGL'GC,?,CG
i 9
2170 GUAUUUAU U AAUUCAGA 2401 UGUG..~U"nU A UGGUCCUC
2171 C~G'JCIAW U AUGG:,GUp,2411 CAAUULJCU C AUGCWCA
2173 LGUG."LJAU A UG.~"UC'CUC2417 AUCJ;GGaU A UAC'~p
2174 UCUC'OAUU A CCCCUGCU 2 418 UCAUGCW C AC~GF,ACU
2175 nUWCUUU C :~CC~C~CA 2425 WAUUAAU U CAG~UUC
217 GAAAUGG' U CG~ACCAC 2 42 6 CC ~~"'CT U
6
218 UG.CAGJU a UUtTaUUC,A2 4 3 UCAG .G'JU C L'G?~G',G'u'U
3 3
2165 ACnCUtTAU U U?.L'LCGG'J2434 C ~- -~-'U C ACArACGA
218 Cr.CUCIAW U nL'L -"C~CLTA,2 4 4 UGAAC'-aG~'J p,
6 8 [~CCCCC
2187 AG'JUAUUU A UCiG?.~,C_ 2449 C,'rAGCCUU C CUGCCUCG
2189 UUAUUtJAU U GAGLTACCC2451 Cz,CCUG'JU U CCGC-CCUC
2196 CLC,?.CnGLJ U AUUL~r~UUG2552 GCC'UGL'L'U C CUGCCUC'J
CA 02468048 2004-06-07
204
2455 AG;UUC'CLJ A C~ 2761 C~C G
AUCWCC
2459 C'CUC,~CU C CUCC:AC~.2765 "
'
CL
2460 CC~CCW U GWCC~'AA 2 7 69 WUC~
U C UG:WCCU
WCUCUaU U ACCCCUGC
2479 U~CnCCO ,~ WACC~~C
279? CGUGAAAU U AUG.;JC?,A
2480 GUC"~CCvU U GUGAUCCC
2803 CUCAUG..."U U C~~C
2483 AC~~7 U CCC'-
ALJG'J
~ 2804 UCAL"G..~UU C AC'~GAACp
2 4 CCL1UU
84
GUU C CC~C 2 813 GCUCCC'~U C CLGACCCU
2492 GACCACCU C CCCACCDA 2815 C~~,7UU C GALTC?~CC
2504 ACCLg,C~1 A CAUUCCUA 2821 CCUGaCCU C
CLGuAGW
2508 AG.I~C~U U CC~CCUU 2822
UAC~T,CW U UC~~uCC
2509 C~UAG,UU C C
TACC~-U
" 2823 C~.AC'~JULJU C AG~~TCCCA
2510 G';CC:~L~GU A G
C~L~J
, 2829 L'CGG,'GCU C AG.;JAL,7CC
2520 ACCGL7L'W U CCCf
AUGL1
, 2837 CACAC~.;~~U A CUZ,CCCCC
2521 CC'JUf
'GUU C C
2533 . 2840 G~~,CCCCU C CCAG'GC~
CAAI1GUC
,
ACAGGyLI7 U p,CCC~CA 2847 UUACCC'CL7 C
ACCCACCU
2540 UCG.,~~"C~.~p C AG,,,~UAUCC2853
WCGaUCQ U CC~~CC~1G
2545 AG~
-
GCp C CG~~(. 2860 CU CCCL'GGAA
2562 UpU
'
C 2872 C
~GAGAUp U Ur;WUCAG C
G
,
2579 CCUGC~ U UGCCCUG.; 2877 ,
~~ C
U
G.3AGUCU C CCC
2 5 CUGCiIC'GU A GACCUCQC2 g c
85 g
GCU C CGC~C~
2588 UGCC'JCCU C CG~CAGCC 2900 GGC
L1GACU U CCWCUCLT
2 5 CUCWCCp C UUGC'-SAG 2 9 04
91
GAACUG~.~p C UUCCUCW
2593 tlCtTCL~',UQ A CCCCL~CLJ
2905 G:~~~CU U CCWC'UCU
2 5 CUCCUG"'Z7 C CUG
9 6 ~LfiGC
, 2 9 0 G'JUCAUG'J A UUUAUUAA
2601 UGGG'~'LmU A UG 6
~UCCUC
~ 2907 CL'i~~L7CW C CUCWGCG
2 6 GUCCUG~1 C GCS
02
2 9 0 UG~,UG~U U ~UVAAUu
2607 GJ ~c~.,~~,~ A BCACC~GG8
.
2909 CAACUGC~7 C WCCUCUU
2608 CL7CL~,C~U C CC'G'UG~
GA
" 2910 ACWCC'W C UCUAUUAC
2 609 IaGuAGACU A ACQGGAUG
2911 WCCWCU C UAUUACCC
2620 DCAGAL~1 C UGAC~
G~7
, AWQAUW A
2626 2912 UUAAWCA
CQCUCAGp A GUGCUGCp
2913 UGLIG~W C GWCCCAG
2628 LmCAACUt7 U U~~
2914 GUAUUUAU U AAWCAGA
2 63 UC1~CT~AU C C~
,AU~JCAC 2 915 UAUUUAW A AUUCAGAG
2 64 G~"UCJaC~,~Q A UCCAUCCA.
0
2916 C~CUUCCU C WGCGAAG
2641 CCCCACCO A CAL~CAUU
2917 CWCCUCU U G~.GAAGAC
2 642 GCCUGLW C CUGCCUCU
2918 AUWCUW C ACG~Cp,
2 6 CCACAC,"~U C AGC
5 3 GUGC
'CT
, 2 919 UUUUGUGU C AGC C~CUG
2 659 .
AGAACG"~U C C'tlC.CAF.
GC
. 2931 GAUG.,~ilGU C CCGCLTGCC
2 6 AC'JF~GG.,~p C C~
8 9 p~
; 2 9 3 Lfi.GAGL7CU C CG~GCACC
2691 CU 3
UCAGC.'~Cp A pC~CU
2941 CAGL7ACW C CCCCAG;C
2700 AC~CU U CCCCCAGG
2951 ACCAUGCU U CCUCUGI~C
2704 GACCACCCT C CCCAC'WA
2952 CCGGp,CUU U CGAUCWC
2711 CCCL1AC'CZ7 U ' ~.~C~0
~U
,~,~
, 2 UGH-WCCU C UGACAUGG
2 712 ", 55
CCUACCUL1 -
A G
~
UG 2 9 5 CUUUCC'LTCT U GAAL7Cpp,U
2721 W,~, 6
C~-~,C~U C AUACGGGU
2961 L'UL1LG'JGU C AGCGa.CGG
2724 AAGAUGa.U A CG:riv"JUG
2962 UGVGUAW C GWCCCAG
2744 Guu~UGGAU C CC;~G
G 2965 CUWGAAU C AAUAAAGU
2 7 GUCCC'C1GLT U UAA
5 0 AAACC
2 9 6 UGGAAGCU C L1UCAAGCU
2 7 GACGAACU A UCGAGUGG 6
5 9
2 9 69 C~F~,UCAAU A AA GUtWA
CA 02468048 2004-06-07
205
2975 L ~' ~U C L'QC~G..~p
2976 L~.L~DG~~U C CGCACCUG
2977 -C~AGCtICU U CAAGCL~?,
CA 02468048 2004-06-07
206
Table 10: Rat ICA11I HH Ribozyme Sequences
nt. R.at EH
Ribozyme
SeQuence
Pos:.t~on
,1 UG~l~uG'~~GCUCu-UGnG:~cC~'.~GC~.C"wA?,
ALZG::AL'C
23 LnGaG:JyGCUG~~JG:~G~CC~-.~?.GG..'~'",~
AAG'.:C~G~.
i6 ~~AA CUGAL',C'~'"'~"-'--~=....~
31 nG~~C,~"-~GCUGAUG~'"C'-An~GvCC.~-~'~'r
a.G~C?.C~G~u
34 GL~AL~UCUCUC~L'GF.G:~ ~ _. .,.,"~.A
: LG.,.~'J GCAG
40 ""G~.~CVt7GCLJGhUGA ~ ~. '' "~C"~?.A
ACCJUGAG
48 CCC~C~,CCCUG~L'GhG:~CCG?.AF~G~CC~.:Aa
AG.w'UCLC
54 G~CZ3CA~',CJGnLTG'nG~~ .~CG'nr~~LGi,:aCCC,~A
s ACvC
58 "Gw~?~C(1ACLiGAUGnGGCC:~AAGv:.C'~.,a~,
. ~. ..
64 AC''.N.~~OUGC'U"'~FJCG~'~AGGCC'~~1 AGCCADL1G
96 AG: ACCAGCUGAI1G~~.C'GAAFC~CCG~A aG.~_~GG
102 G.."GaCCAGCLT~C~~AAGG:.CGhA ACC'~GGAG
108 hG~.~L7CCCC~G:~UGhG~CC~.~AAGGCC~,~A
A ~ ,JCC
115 UG~Ch CL~t,F,IJGAG:"C'".~AAGG.'"C'".-~
AC-.~~~
119 '~ .. ' ~ .r.AAC~G."CGAA ACAG'~'GUC
12 0 G:~~CCGG;~~' :."".~r ~r A~rG'~.G~AC
146 G:3AG'JUCCC'CTts~iUGkG:~CC'C'CGP~ AG~~?C'UGG
152 L'LJC~G~"QGC'JCAL'GAG:~.C~-"~..w~aG:~CCG~-.:e
AGCC:r,..~-U
158 UCACLTCGVCiJG~~IJGAG:~CC 'C~GG~~.C'~,,=.,?~
~D
163 ....~... CUC~nDGA.C.,GC~'~aF~AC~G:.C"a.A
G ?C"~'C'v'OC'~
1 68 C~~G:T:.AGCT7C~UGnGGCC -C iAAGC-:.C'"~,
A~,G;"~WC
185 CCUGCACG CUGhUG~~CG:~AGGCC'~,.sAA
ADCCACCC
2 09 G~~JG.C~UCLT~UCZF~G:~ 'C"'CC'GAA AG~~CQG
227 UUCACAGU CUGALTG~GC~C'."~T,AGGCC~~A
ACQGG~3C
230 CCQCCCAC CUGA ~ . ~ C:Aa aCAG~,"~GG
237 G:~GGQGUCCUCJ~UGAC~CC"'C~u?A AGC'GpCAG
248 UCC't.TAAGGCL~UGAG~.~'C~FGG CC~~A .
-..... C
253 CCUCCACU C'vG~~UG:~GGC ~l~GGCC~.s:~.
: "-~G.~AGZJG
2 63 G:~U -C. CL'G~:L'GF~G~CC~AnC~C~~ ;A
FGA AUUC:~"UC
267 CGAGwAG C"JGF.UG:~~G:~'~'~~CGAa ~~'WC
293 UCAG~'"ULIGCL'GaUG;yC-:~CCC -G iA A
319 UC.'GUUUGUCUGn " ''~C~CGA.A AUCCUCCG
3 3 5 AGLJCIC"JCACLTGI,UGAG~ C",CC'GAA AG:
AC~GLT
337 G~.ACCA CL~GAUGi~GCCC~~AAGGCC~A AL~.Gt'~.
3 3 8 CtJCAGC'WCUGAUC,AGG.'"C"G~GCCGAA AAG
;GCW
359 AAGCCGAG C'~TGAUGhG~CCG:~'CG~A ACUG."GUG
367 ACG:.-'JUGCUGAUGAGGCCG"~AGGCCCAA i.GCC~,huG
374 i.Gu~L1G:~JC'UG~UGi~C,GCCCi-'v'zF~GGCCGaA
?.G:~:r,"'LTAp,
375 GnGGCAGG _
CUG.~-UGAG:~C -Cr'u'~isGGCCG~A
AGu'~UIjCU
378 UACCCUGU CUC~UG~CG~.isGGCCG~.A AC-GLJC~o.a~U
386 AG'~UCCAACUGAUC~GGCCG:,i~.P..GGCC'"..-.AA
?.C~,C,e Gr~G
CA 02468048 2004-06-07
207
394 Cu~UUC~G C~IGnGG~.~CGiu~G:n."CGr~
AG:~CCAC
420 UGCG~."ffGG.'' ' ~ C'GAF~GGCC'~A AC,:~,~7GC
425 G CGG' ' "~CGAAAGGCCGAA AGCC~~GG
427 UCv~DDDUU CQGAUGr'~G~CCC~v~CCG?.A
AAGyGC~~'
450 U ' .AAA AGuLJIJCOt7
451 GCCUGwG CL'GAUGF~C,GCCG.~'1~F'~~CGaA
AAGJACCC
456 UG.~"'u"'C-ACACDGAUGAGGCCGAAAG~..'~GaA
AAGCCGAG
495 UAC~CAGLJ CQGnUGAG~C AUGuv'GC~
.
5.0 UUCC~~1CG CU'~~CG~.F~AGC~CCGr'~A
7~~C~CAC
564 ~-~'~ ' ~"CGa~~~ ACAUUUUC
592 DCCCUG..~-CC~ -'G~G:~CGAAAG:~CC"aAr~r
ALQ,CQCCC
607 C~L'G~ CLT~GAGGCC~'~AG~CCGAA ADG'GG..~UC
608 :~~ '' ~ ~"CGAA AAULJG~.V
609 ~AU~ CUG%~G~7C'0=~y~AAGGCCGAA
AAAUt~GC
6.1 UC,FJaGC=.UCLK~UGAGGCCG'nA~~G~~CGAA
A~AUL~G
65o CnDDC~G C~UGAG:~CCGAF~AC~:~CC~A
AG:GUGAC
657 ACAUUCW ~
668 AnGAGC,AA C'CtC~AUGAGGCCGAAAGCCCGAA
AC~AGLTGC
677 DG.~G.."DGGCL1GAUG."~CCGAF,T~GGCCGAA
AGGG.,"UGC
684 AAF~GUCCG CDGhUGnC~naCC'GAAAG~GCCGAA
AGCUGCCU
692 G:~GDQCC CUC~AUGAG:~.~C~ ~ AG"~DCL7G
693 G:y~ACAUC C'CGAUGAGGCt'GF~AAG~.~CGAA
AAAGUCCG
~
696 ~ ~
.~AAAGGCCGAA AAACaGGC
709 ~'~ CDGF~GAG CCCGF,F,AGGCC~.~?.A
AAAUGCVG
720 GAG'"DGAA CL'GAU"~G;~CC~ AGUUGL1AG
723 U ''G~GCLJCflL~AG~~CGAAAC~.~CGAA ~,AAAGWG
i35 C,~~G.CCAGC'LT(:~ADGAGGCC~uAAAGGCCG~
ACCAGGAG
i38 UCCACCCC C'L~CGAAAGG...'"CGAA p,GuCAGGA
i 65 AGJUCUCA ~GCCGAAAG~~CGAFa A,G;~CAGL7
769 DUCCAGGG ~ ~ AC7~.C~AGA
770 CDDCCAGG ~ . > >CCGAA p,ACACAAG
?85 "A~ ~ rICAGGCCU
7 8 G,?.G:,CAGG' ~ ~ ~CGAA Ap~,GGCC
6
792 G~'".~ACCAG~ ~ CGAA ACCAGGAG
794 -C~"LTOCA CUGAUGAGCCC~CG~A AGGCAGGA
807 UCCAG.~UA CL1GAUGAG~CC'GAAAGC~CCGAA
AUCUGAGC
833 UAGCJCUCC CUGAU"~,AC~GCCGAAAGGCCGA,A
ACCCCAGG
84 CAAUAAAU COGF~UG:~GC~CC~~CGAA hc.'UGUCAG
6
851 AGCQGCUA CUGAUG~GGCCGAF~AGGCC"~A
AG,~~UGAGC
863 A~ CUG~sIJGAGGCCGT~F~AG~~CGAA
AGCCABLJG
866 UGLJCAGAG C2JGAUGAGGCC~.~F~AGGCCGAA
1?~AGCAIJGG
667 LTAG~'UGGGC'C~C~aIJGAGGC~CGAA p,G"~L7~C
869 CWCGCAA " GT~AAGG.~CGAA AG~~AGp,G
881 CACG:N"W CUGAIJGAGGCCGA~'~AGGCCGAA
AAGCCAUU
885 UL1CACAGU CUGAUGAGGCCGAAA~GGCCGAA
ACWG,~UC
933 ~~ SAC CUGAUCACa~vCCGA.~iP GGCCGRA
AAiJACACA
936 UCACACAA CUGAUGAGGCCGAAAOiCCCGAA
AUCUCUGC
978 AAGDtJGUA CUGAUGAG~CC ~ AUQCQCAA
980 ~F~WG CUGAUCACvCCGAAA,GGCCGAA
AGAUUCUC
CA 02468048 2004-06-07
208
0 8 GnG~"GG~P. CCG~DCAG~ _ -"Gy~F~C'~-CC".~1
6 A.GUi.'GUzIG
087 GnGC'vCA Cu'~-C.CGr'~A ~J~G'JL7GL7A
988 G;u~G.."GG CLJG~DC-~GGC~~'GA:~AGGC'C~.:~A
~,.~AG'JUGLT
'! GGGGCCAC CIJGAUG~GG.~CG~~GG.~.CG~1
005 AnG'~CG~
1006 CCDG~' uG.~CLTGAUG~1G.~ '~C~r~GvWCna
ACCCC~...r~C
1023 cc~ovc,JCa cvc~cG~~C~,.~a
1025 CC'~'C',JGCOCL~~AUGGG'C " 'G~C"~1 AGaCC?CC
1066 C CUGALG~CG~GCZC~.~ .~.?1~,~1GG
102 LCVG~~cGa C~-~~aG~cc~~cGaa AcCCCCcv
1 OS3 :~G~:~"CG CL7G?. -UCr.GC~ ~ ~-.C-.~??r
AL~C
;, aL-cnac~ cCGat~ -~~ ~a:~a~:~CCC~aa
~5 aQUG~;
~ , ~t.." , CLJGADGAG~CC~.-~.~ ~"GLCGC1
53 . G
1 , . .." . ~~ 'CG~ a~G.."C~LC'C
64
11 G. -G~~C~ CtICACTGAG:~CC.a~G~. ~r'A
66 AG?~
1._172aC~CC',~C.'ACLTC~.LIGnG~.'',CC'G~A~G:~CG?.?~
s~C~aArIAG
12 ~CAG'~ ~UG~-'"~"CG~A AAL7L7GG~,LJ
00
Ol CCQGUG~ CL;~C~ A ?~J~CCCAA
1203 GACCt ~iG CQGaUGAG:~CCG~i.~'~C,C,CCGIa
AG'.AGCCC
X27 aGC_yCr.UG CUGAU -CnG.~cCC~.>~CG~?~
AL~CCC?,A
:~ r.C~~UCAC CQGAUGr GuC C G~C.GC C~~
2 ~G.: C C~.~C
8
1233 G~.nCC:~G CUGAUGAGGCCGC~.AA ?~~G
1238 G~G:~.C: C'CtG~UGAG~~'AGG:.CG~?~ ~?~
~.
1264 ACCC'"..rT'1UCC~CG:~aGGCC"'.~ AUCLJCDCC
.267 CnL'UC~uUG CDGFatJGaC~CCG?AAGG:.C~.-~.
?.CaGv'G?.G
1294 CL'G~GYC~ CUGAUGC~~~.CGiv~~C,~~~.C~.z?..~
A?.DC'L'CLTs
125 UC~-~~ CtIGaUG~C~C ' 't-GCCGAA AL~CCUCQ
_306 G;~L'G'u;'.AC'C~CC~-.:SAC-GCC".Aa, ~C~."U
1321 L'UtlCCCC~ CU~"C"'aT~AT~GCC~~A AL'UCDG~
334 G~JCQGw CLTG'r~DGe~G: C ~
1344 GC:~.L~.CCTJCDGApC~G~CCG'~F.AG~.~C'G?A
AG~~CCGA
~?5, AGvICCGCQ CDG?.L1G?C~:CCG.'~FGGC'CG~1
~CUGA
13 C CaUL'G'.JUCUGAUGAG ' ~ C?A AGCt?CCI~
3
13 C C~JC~F~UGAC,GCCGP~,F~AGGCC'v:aA
6 AGUACCCt7
o
1367 GC'C~JG,~:~:7CUGAUGAG.~~CC~''~,nG;CCGaA
A?.GLTACCC
6 2 G,G;r.GC CUC,~UGAG: CCGF~F~.:C~,CCG~,
G:~ ACACC~UC
3 8 ACG~UCCC C'JGADGAGGC ,CC.aAAG.;C CG~A
0 ABC-:SAG
'_388 Cf:UCCAGL7 C~UGT,Gu'CGFi~.?t-CSCCGI~a
AG"uCUCCA
13 L'GJCCOGLJ CUGABGAGGCC GT~1AGGCCGaa,
S ACAGCCAG
8
1 sot c~,c.TUCUC cv~UCAGCCC~3~G,.~G~A AAC~C~CAG
408 GaCGCCAC C'OGFUGAGGCC'G~AAGG.~CG~A
?~CACGAA
1410 GJCCACQC CZTtsF~UGAGn~CCGCCGAA AL~'aQJUCG
1421 GCCLG~_-w CUG~ABGA.C.GCC'GAAAGGCCGAA
"~CCC
142= :~CiG~GAG CUGAUGAC~:.CGAA:~GvCCG?A
~.G~'G:~1
1529 CC'~GG:~ CUGAUGaGGC 'CC' ' ~,CCCirl
AC.i~F,GUAU
14x4 ct;cczccu CuGavchG,;,~.CC~aA~C;,tcc~
aG,~.CVtscv
S. UCCCLiGa~U CUGnUC~G'~CCv~.Ar'.T~.rCCGna
~ AL~GUCCC
1482 COLT~. . C'UG.ADGAGGCC~CGA?, ~.G~ACCCU
1 C G~AP.CAGG CUGAUGAGG.: CG~ARtvCCG~.A
8 A C? C-Ca GU
4
1493 LTaGUCVCC CUGAUC,~GCiCGAF~C~.-CCC?
CCCC=.GG
CA 02468048 2004-06-07
209
1500 BUC~.CCAU
CUGAUC,r.G~~~CC~A
ADQ<TCACG
1503 GJGG'UUGG -CU'CAI1GAGGCC~.~~CC'".~.~A
Ac~WIJtJC
1506 CCAAG~AU CUGAUG~GGCC'GP.A,AGGCCGAA
AUGACCCA
.509 L~G~CAGU CUGnUG?.G:~CC 'G~1GGCCGaA
.3.LT.~uT~ aC
1518 1~ C'JG~aDGaC'~c,CCGAelAGGCCGAA
ACCJ~G~.~AC
1530 ACAAUUAQ CUCF.U~GaC~ -'~AA~C~a~CCGAA
ACCCAG.a"iJ
533 AAGCCCGC CUGaUGF~GGCC'C~A~GGCCG.~A
.3.DGnUCAC',
1551 DACG~ C'C~C~~?.AAG:,CCG~ AGwCCaC
1559 ~CAGG CUG:~UGAG~~CC,AAAG~~CGaA
ACDtJGCCA
1563 ~~ CLlGr'1UGAC,~,C.Cu '~G~~~~.C~~'
aG~AG aA
1565 c-:~c~~,~ c~GavGaG;~C~,~aG~zCG~A ~GG~~aA
1567 CL'G:~CG.~~UC'UGAUGAGGCCG~.AAGGCCGA1
A~RGGUGLT
1584 UAL~UCCU CUGAUCAC,GCCGAAF~~ ADCUUCCtJ
1592 ~CWG CUGAUGAG~C'GF~AGGCCGaA AL~UCCC7G
1599 Gv."C'(JCC'U~2CC1GALIGaGC,C ' ~ CG~a h~ACWGCTA
1651 GG,.~QCAGGC'CtC:AUGAGG~. ..
661 A~G~"~GC ~GCCG~F.AAG:,CCG?~A CAG
1663 UC;JCCAULTCUGAL~GAGGCC~ ~ ADCVGUUC
16?8 CC'C~C CUCAUGAGGC ' ~ CG?.A ~JVCUC
1680 GACCOGLJG CVGF~DGAGGCCG~' '~J~~CCGAa
-AG~r~.GCCC
16 81 'G~GGCAGG CUGAUC~C~."CG~AAC~CC"~AA
AAC~G~CC
1684 G?~C,AG.~"iJCCUGAUGAGGC~'"AAAGG.."CC,AA
AC~
1690 ~.BGUAUG C'CK~"CC,AAAGGCCGAA AG.~
~~~
1691 G~GnUCG CL~j'C ' ~ AAA AAGtICCGG
~ 696 G~~~~.CCAGCUGAUGF~GGCCGFaAFaG s: CGr~A
AC~...FaGGAG
1698 UCUCCT,GG CDG?.UGAGGCCG~AAAG:~CCGAA
AUAUCUGA
1737 C-:ACCGUG CUCAtTGAGG:.C:GAI',AGGCCGaA
ADGUGAIJC
1750 AALI~G.~"'UGCU~C ' ~~CGAA AAAUGGaC
17 5 AG: ACCAG GAAP.GGCCGAA ~ AG~AGAGG
6
1787 C'CCAG:,CCCL7GA ~ ~~CCAp, AG~JVCpC
1790 GAGBOGGG C'C~CJ~1GAGGCC'G~AAAGG:.CGAA
ACAGL1GUC
1793 GWCAG.~"'UOZn~DGAGGCC'"~AAAGGCCGAA
AG:,ACCAU
1797 UG.~"UDDW C'C~CGAF~F.GGCCGAA p~~
1802 W~"VA CUGF~UCAGGCC'G~F.AGGCCGAA
1?~fiUGAGC
1812 UWCCCCA NGAUGT,GGCC'GJ~GGCCGAR ACUCUGUU
1813 AC~~CAC CUGAUGF~CCGATLAGGCCGAA AAGCCCGC
1825 ~ NG~UG~GGCCC~AGGCCGAA AL'G.~~UGGC
1837 Z7ACCCUGU CUGAL1GAGGCCG~GGCCGAA A:G.,"LJGGG~T
1845 GCCCCUCC CUGAUGAGGCCGAAAGGCCGAA AGUCCUCU
1856 GCAG~'"UCACUG1~UGAGGCCGAAAGGCCGAA ADUAG:~GG
18 61 G:~CCAL~ C'I1G~1GAG~C ~ "AA AG:ACAUG
1865 CUUGLJGDC CUGAUGAC~CGAAAGGCCGAA ACCGGAUA
1868 A~UAV CUGAUGAGGC -C~GAA ACUCGUGA
1877 CCUG~~~ CUGAUGAGGCCGAAFlGCCGAA AGUACUGU
1901 UGUACC'OU CUGAUCF~GGCCG~1AAGGCCGAA
AGUUUUAG
1912 UGUCCAW CUGAUGAGGCCC:~'~AAGGCCGAA
AUCUGUtlC
1922 UAGGG~AU CUGAUGAGGCCG~AAGGCCGAA~ ACVCTACAU
1923 ~ C~1GAUGAGGCC'GAAAGGCCCAA
AGCGUCCA
1928 UCCAG~"C~ CUG:~UGAGGCCCAAAGWC~~A AUCUGAt~C~
CA 02468048 2004-06-07
21~
930 CAUCCnGU CGG~G~' C~~l~ CC".~A AGUCDCCA
964 GCL~ -Cl:CACC'C1C'~AL' .~CCG?A, AAAIJCUCU
'G.,G~~~CG:~A:,G
1 gg3 CC~'F~GGCCC'CTC~ ~ AG~70COC
o g ~L CL -' .. -'CGAau~l.~..~WCCA
6
2005 ~~~U ~~~~ C~A AC~CAB
2 013 C=~L'CCCGACU '~"C.2aAaGGCCGap,
A
2 015 :~CCi~UCCCCOC,~~~ CCGAA AUAGGCAG
2 02 G'.1AC,AGCV" ~ ACr3CAAUA
0
2 03 U~ ~-~'O C~ ~~1CCUCCG
9
2040 ACCUCCnG CG' ' aG.~~UC~
2 0 ? Ci CnDLIGCC -G'~C~G~ ~ a~CCAG
7
2 0 iT~G~..'~ACGGADGAC'r.~AG~.~CCGaA ALIGuaCGC
61
2 071 CCUGAGGC CGG~~tK~GGC ~ ACF~.GL~U
2076 Ut~G~"~ C'Cttj~UC,?.GG..~~ ~,G;,~~C~C~
2097 ACAUCAAC Cv'GAI7GA~~CCi?.AAf;GCCGaA Al
2 09 .~CCUCCAG AG~CAG~,
8
2115 G'~.CCC AC~CGGP,p,
2 3.2 c~U ' ~ C~occCG~AAAG uCCGAA ACAGCACU
8
2'_ FLAG C UGJ.UC'~GC~CCC,AAAGGCCGAA AAAC'AGGC
3 0
214 nG~UCAAC ' '
5
2152 :~:~GG'GGUA' AU~7C'QCAA
2'_ CC~A CQ~ ~ AA~3C~ICA
5 6
215 ~ ' ' ~ :~~CC~A atTACAi~,
8
2 ~ cr,AUUAAU aC
9
21 5 L'Gn~LTL~AC'JC~UG'~G:~
0. CGGCCGAA ?.AP.UACAU
2162 "~ C~UGT~GGC ' AC~ALIaT
'
2 _ cJCLGnAU CU _' ,. ~~CGAP. AALg,AAI7p,
6 3
216 RF~UtRF~C~CI?GAOGAGu.. a~CAUCA
6
21 67 c~.ULIAAU CL7 ' ~ AA C, GCCGA,A
RAI~C~C
2170 BCUGi.AW CUGA ' ~ AA AUAAAL~
2171 UACUCAAU ' '
2173 C,:,GGACCACQGn ' ~ p
2174 ACCT: G C'OGnBGAGGCC'GP.AAG~~'CGAA 1~
2175 UGACQCGU ' ~
217 GDG~JUGG '' p,Cppp~7pC
6
21 8 UChAUAAA CUGF.LTG:~C App,
3
2 ? ACQCAADA CUGAL1GAGG.~CGAA'nC,GC:CGAA
8 5 ALRACDGLI
2 ~ c~cJc~AU cJC-s~c~c~CC AA~AACC~
8 6
2187 GL7ACUCAA CGGAITGAGGCCGAF.AGGCCGAA AAAI174ACp
2 ? G:~.~"~C CtTGADGAGGCCGAAAGG.~CGAA
2 9 tTC AUA.AAL~p~
2 ?9 cAFsUAAAtJCvGAUGT.GuCCGF~AAGGCCGAA ACQGUCAG
6
219 UGACCLTCG CUGALrC,AGGCC p~ZJ~JC
8
219 CL~UG CUC~UGAGGCCGAAA.G~GCCGap.
9 AAGAGpCU
2200 G~.~CLG~,NGC'UGU 'C.;GC~CGAF.AGGCCGAp, ~,A~CCC
2 2 G.a C
O1 C'JGUG CUG:oUGAG~CCGAAAGGCCGAA
AGF,~CCC
2205 C:.G'JGGCUCL7CAIi - r~ "~ ~ Ararnnna
C
2210 CAUCCi.Gi1CQGAUGAGG.~.~ CCGpp~ p,Q7CUCCA
2 2 CCChGGCC CUGAUG,AGGCCC p G"~TJpCUC
2 0
2224 ~.G''JAG.~,CtJCI,UCAG:r.CGAAAC~cCGAA AUGUAUGL1
CA 02468048 2004-06-07
211
2226 UC~?G~:.CQCQC~sUG~G~M ~C~a~A ~TCCaG
2233 A~C~ W~~-~~-CC~ 's~~'GCADGA
2242 ACUACDG.A C'C~u~!'~CCCG~ A~~UGCGn
22 4 G~GACG~G CVGAUCA~CGA.~AG~: C~.~1A aCG'~G~,G
8
2254 WCF~1G~7 C'JGADG '' _ ~~CGAA AaDL'G~~1U
2259 GCACCGUG CUGnUGAG~~~~.CGAAAG~CCGr~A AI;Gv'G~sDC
2260 , AG=AC~'GLJCDGnL'GaG~,.'"C'~"r~.AACa~~CC'".a~.A
nauG.JG~U
2260 AACUL7GLTACUGAUG'~G~vCCG~AAG~CC~~e1 AZCCL'GaU
2274 UACABG'.JQCI;~~C"'~AAG~CCGAa ?~CC'JG.~(~
2279 aCCCGUAU C'UG~~QG~GC..~C~~l~.:CG~A ALCL'LTCCC
2282 ACUCAA~A C'GC~L'G~G~C ~'" ~~C~.y~r1 :~t~CUGU
2288 CAWG~~nG CUGAUGAGGCC'G~AAGw"CGaA nC~ ~~~
2291 Gi~C~I~G CL1G?S3~C~.~AAG~CCGAA AL~UCG,~
2321 accccuAV cuC,~UC~~C~,~aAAG~C~~~aA ~tc.~~cvC;.
2338 CC'C1GUG~~aACL'GnL1 ~ ~.aAAe~"~._~C"sAA naGCCCA?a
2339 ~ '"' ~G~C~ ~ :~?~GiTACCC
2341 UGAC~CC ~ ~ ~,G~G~CCC
2344 G~GAC~JC CUGADG~~'C'G~AAG~.~CGAA iC".~GC~G
2358 UC-~JC~::AGC~JG~UGhCuCGAAAG ;CCG?.A x .., ....
2359 WCUGtTGG CUG~rUG~~C~~AAAGG~~C~.~AA ALG.?,UGC,
.
2360 CWCCAG~ CUG~DGF~.."C"'.:~AAG~CCG?~ aa~lCArlG
.
2376 F,AGAG~~P.ACL1CAL1GAGGCCGAAAC,~CV"a?~A ' ~,~.GUUC
2377 UAAUAGAG ~ aAA ?t~~GUC
2378 UCGLTGAAA CUG:AIJG~G~CC'G~AG~CCCaA ~flL'CAG~.
2379 C". =~GrIGCUG:~DGaGCCCGCGaA ?.?~ACCAG
2 3 ACLJCGUGA CZR~ AC'.,AAUCA
8 0
2382 UGACUC3L1 CUG;~~IJ -G'~GGCC~CGAA ~.AGaAaU
2384 CflUGUGUC CDCAUG~G~CCGAAAG~CCGA,A AGr~".~~UA
2399 CGL7CCACA CU~~C~.~AAG~CCGAA AGJAWUA
2 a GAGGACCA cUGa.UGAiGGCC'~;~AAAG~~CCGAA AtIPtt~
o1
2 41 a cvc,~UC~cccaAA~ccc~A a -cs,AA~
~
2417 AACUUGUA CLJGa~DGAGGCCGaAAG;CC'GAA AL2CUGAU
2 418 AGWCUGL7 CVGAflGAG~C~CGAA ~UG?~
2425 GAACUCUG CQGAL1G~~G.~.,~CCG~ A.AG~CCGAA ALZTAAUAA
2 42 UAGUCUCC CUGF.UGAGGCCGA~aAG~CCGP,A ACCCCAG;
6
2 43 AACQGUCA CUGAUGAG~ CCGT.AAG~CCGAA AAG'i;COC,A
3
2434 UCGUUIIGU CUGAUGAGGCCGnAAG~CCGAA AUCCCiCCG
2448 ~ ~ CUGAUGT~GGCCCAF~AGGCCGAA ACuG'JtTCA
2449 CGAG~cAiG CCJG~AUGAGGC'C.'CaAAAG~CCGAA A1~G~CZJUC
2451 GAGG:AC~G CUt;~.BGAGGCCGAAAG~,CCG?.F, AACJ~G.~C
~
2452 a~r~c cvc,AUCA~~ccan~c~CC~.~ A,~.ca~C
2455 AACAAAGG CUGAUGAG~.~'C~G~AAG~.~CGrlA ~'~,L'GLJ
2459 UGtiGG~G CL1CAUGnC~,C.CG~TaAC~CCGAA ACv~~Gv~r.~,
2460 UVC~AAC CUGAUG~G~CCGr~G;CC sAA ArtyJAG"
.
2479 CvCG.~~JAACLGAUG?~C~CCG:~.GC-CCGAA AG,WGIJAA
2480 GwAUCAC CUCAUC~G~...~CG~C-GVCG~~ ACW.CGAC
2483 ACA ~~ CUGAUGAGGCCGAAp.C,GCCGAA ACrnAG~~(J
2484 GACAUUCG CUGAUGAG~CC'G~AAAG:~CGAA AAG,A~,C~~-.
2092 UAC-v~L'C,~~',C'UGnUC,r'~ ~C,~~C -Cr~r~'.AC-GCCG'PA
ACvv'Gw,~UC
CA 02468048 2004-06-07
212
2 504 '= '' c~'-~n~'.CGaF. F~JG~C~~U
2508 :u C'C~CGAF.AGGCCGAP. AUGt~DGLT
2509 'VAG C~~C AAUGi~UG
2510 AAUAGw'G CDG~~GCCGAAAGC,~~C"u AA
AAADCGaC
2520 AG ~ ACAAAGGU
2 521 GAC~UQCG CU'GAI~GGCCGAAAGGCCGAA
l~,ACAAAGG
2 .3 vcaw:~.~--uc,.-~a~.G~CC~~a~AGG,.~ccaa
3 a~cocv
2 5 G:~UF.CC'C C~CAGCACCGA
4
0
2545 A~CCG C2~C~"C"~ AGC'L1GGCU
2 5 CLTGaCAC~ tzJG?~GGv."Ct'y~AAGGCC:
6 ".~P.A aAUCUC~tJG
8
25:9 C ' ~''~ CU'C~~UGAGGCCG~?~AAGGCC~~AA
ArUG~
2585 GAG~.~''L1CC'DGaDGA~G.~CGaA ACG.AGCAG
2 5 GG.~oGOGG C2tC~AIJGAGG~~C~.~AAAG~."C'GaA
8 l~
8
2 591 CUL1C~~A ' ~ ~,C~
2 5 AL~.GGuG AADO~GAGA
9
3
2 5 G~.CCAG C'C~C~AiJGAGG..~CGAAAGGCCGaA
9 AC~.~GGAG
6
2 601 G.A~~~ACCA
2 602 ACAnCG~~ CDGAI1GAGGCCGAAAG~CCGaA
h~GGAC
2 607 CCVG.~~UGA CQGF~UGAG~~:.C'GAAAGGCCGAA
ACUCCG''~C
2608 UCCCACGG cJGnDGAGGCCGAAAGGCCGAA
A~.AAG
2609 G~UC'CF~GL1AGL1CDCCA
2620 AF~UGQCA ~ ~ CG~1 AACQC(7GA
2 62 F.GCAGCAC cJGaD GCCGAA ACQGaGAG
6
2 62 G ' ~~C'GAA AAG~G~
8
2 63 CUGAAtJDG CDGF~JG' ~ ~,~p,
2640 UGuA~A C~ ACCUGAGC
2641 AhTJG~IJG CUGAUCAG~"~CG.~.A AG.~,
~~
2642 A~ ~ .A AAACAGC,C
2 653 AC~~.ACCCO " ~ A~G
2 659 GCLTOC~C.AGCDGADGaGG(.'C~~AAAGt~CCG:~A
ACCCUUCtJ
2689 AGC'~G
2 691 AGOCCCCQ ~ A~~,C~
2700 CCUGG.~~G ~C~J
2704 L''"G.~~~ CUGnUGAC~ AG~~UGGUC
2711 ACCWCCL1 ' ~v~A~ AGE ....
2712 ChCCOUCC CUGnDGAG~.~'t.'GAAAGuCCGAA
AAG.,,~UAGG
2721 ACCC~'OAU CUGAL'GAGJCCGP.AAGG..~CGAA
AUCUUUCC
2724 CAAACCCG CUGAUC~GGCCGAAAG~CCGAA
AUGAUCW
2744 CCUGC'iCG ~ ~ AUCCACCC
2?50 G~1UWUA ~
2759 CCACUCGA CU'GADGAGGCC'G;AAAGuCCGAA
A~pC~C
2761 G~AGADC CLJGT~IJGAGC,CCGAAAGGCCC,AA
F.AAGUCCG
2765 F~G~CC".~ ~ ~ A
2769 c,C~c~:~,~-acUCa~GaC~ccG~,cccccaa AUAGACAA
2797 UUGACCAU CUGAUGAGCCCGAAAGGCCGaF,
AtRJUCACG
2803 GJUCOGUG CUGALT,AG~C~,p, ~U~
2 8 AGWC't7GQ CDCAUGAGGC ~ p ~~J~,
04
2 813 AG.~_~JCAG CL7GF~ ~ C,GCCGAp, AI:~GGAGC
2 815 GGAAGAUC CUGAUC.nG GCC'GAAAGGCCGAA
~~AGUCCG
CA 02468048 2004-06-07
213
2 821 AC:.'UCCAGCQC~.BG~~GGC..~ 'G~,~G~C".~,A
, AG~"UCAGG
2 g22 ~G -,.~~UG?~NUL'GAC~,.~C~'.~Pt~CC~.~,A
A~LTC
2823 U ~.' C~~- '~C:G '~CCCAA AAAAG?L'G
2 829 C:~Q'ACCU CGG~.UGAGGCC~"w~GG.."".~~'il
r~GCACCGA
2837 ~,.~'= C~-'~-~'~"~A ACCNGuG
2840 UG:.C~"LJC,~vC'u'GAUGAG:~C.. -.. ..
2847 AG.wGC-:~J~-:~_CG'r.~lsCw~CC~?.A
2 E CTJAGL'CCNC'u'G-'iDGAGGC.. - . ...
53
2 8 UUC~.~GGG CL~"CG.~CCG?~a ACAC.=.aGa
60
t 872 L~:~1C.~.CCC~'G~GAGCZC~-"~Cv.,."CGr~
ACraC"yCCC
~ 8'77 C~G.'.GG.~C'CG~L'C,nG~...C -\ \G~GGCC,..~
AC,?,CJCCA
2859 AAAGZ7CCG cJC,AL'G~ '~CG'~GGCCGAA Ar~'JGC~T
2 9 ~G ~~-"CG 'ArJ~ AGJCAG:.C
0 0
2 9 A~AC-:~A C'uG~LC~G~~C~~GG.-~C~~ '"'
04 JCC
2 9 ~~ CQ~B~"C~~CCzAa nGUG~.C.CC
05
2 0 C~t~,F~AAAC'C~BGAG~"CGA ' ~ CG?~ ACADU1AC
6
2 9 CG:3,AGAG C'CG~JGAGG.- ~ A,F~
07
2908 'IlA C'GGAUG~CCGAAi~G~."CGA~i
Ai~C~aUCA
c 9 ~~ C~C~~C: " ~~CC=~ AG~UC
09
2 910 GL~I~G~ ~ . \ . ..CC,~r1 '~,AG:~AG'J
2 911 GC.~"'CAAUAC'L'GAIJGAG: C'C~C"~AA 1~~AG~AA
2 912 U~~A~ NCAU~G~ = ' ~ ~ A~~C~J
29.3 COCv:AAC CGGAZJG: GC,CCG " ..
~ a Uc~G~vQ cvcavcxC;~C,. ' r.~cr-.~ap,
l 4 Atm~at~C
~~15 ~~~~ ~J'C7~\. ' ' 'VC~
2 S NUCGCAA N -CAUGAGGC ' . ' .~.CG
15
2 S 6v'CWCG~. NG~DGF.C~CCGr':r'al:.GGCC".~AA
17 ~ -~CAC,~~ACa
2518 L'G-'~CUCGUCQGADG~GC~C'G~LAAGG:.CGAA
AAAC',AAAD
2919 CAGL7GG~U CDC~DGAGG...~'C.'GA?.~'.GGCCC7~,A
ACACAAA~A
2 931 GG:AGCG C~CGaAAG~..~CGAA ACACCALTC
;
2 S33 G.~".JGC'L~GGC'CfC~3~DCsAGuCC~' . CGAa
AGrIG~CCA
2 9 G~CCUGwG CG ' ~C 'CG ~~C C'GAA AAG~CUG
< 1
2956 GUCAGAGG CU _' ~CCGT~,7~;~C,GCCC~AA
AGCAUCGC
252 -C~.CAUCG C~.T-C~'GA~C~C~.AA AAGUCCGG
2955 CCAUGUCA NG?~UGAGGCCGAT~AC~CC~uAA
AGGI'~AC~
2956 r.UtJG~ULJCNCG~A~GGCCC~ F,AGGP.P.P~.
2 S Cr.GLTC. NCsUGAGG'.CGAAAC~CCGA?. ACAC~IAaA
61 GCU
2 62 CLTC NGADGAG;CC.'C,? A>;Gw~tGAA
2 9 ACUUUAULJ NG~UGA~GCCGF,AAGGCC~GAA ADQCAAJ~G
6 5
2966 r'~.G..~UUGr'~r1NGnUGAGGCCGAAAG~..~CGAA ACa~~WC~.
2969 ZTAAAACDU CL3GAUGAGGCCGAAAG:~CC'GAA
AWGAWC
2 9 AG..'ZTtJGaACUGAiJGAGGCC'G~',,AAGGCC'CAA
7 5 AC~~WCCA
2 97 CnC~"UGAG NGUGAGGCC -CAA7~GGCCGAA ACCAUAT1A
6
2 977 VGGCUUG CL7Cr~UG?.GG~~CGAAAGGCC~~?A
n -GGCU(TC
CA 02468048 2004-06-07
214
Table 11: Human LIrS HH Target Sequence
at . HH Ta.r~et SeQveace at . BB Target SeQuence
Poaitioa Position
8 AUG~CU U UCUUDGC 245 AAGAAAU UUUC_3GG
C
9 UG:aCUU U CUU~,CC 247 GAAAUCTJ 7C~C
W
G~~CL'ULT C UDUGCCA 2 4 8 A~UCUU U CAC-G:~~
~aCWUCU U UGCCAAA 249 AAUCUW C U
13 CUWCUU U GCCJ1AAG 257 AG:~U A G~CAC
36 AG~ACGU U UCAGAt'~C 273 G:zAGAGU AAACL7G'J
C
37 C~ACGUU U CAGAGCC 291 ,~U ,~, ~,T~
3 8 AACC~DU C AGAGCG'~ 3 05 AAAG? CLJ U~1~A
F,
5 6 G:~.L'G.."'U U 'CL1GCAW3 07 AGACi7AU C?,AAAp,C
U
57 GAUG..''W C UGC.AWtJ308 GAS C ~A~
63 UCUG~~U U UGAGUUU 316 AAF,AACU GUCC'Wa
U
64 ~ ' W U GAGWUG 319 AACUUW C QUA
69 TJUL7GnGU U UGCUAGC 322 UUGL1C'CU AALTAAAG
U
70 UUGAGiTLJ U GL1~AGCQ323 UGUCCW A F,U'r~
.
7 4 GUUUGCU A GCUCCJlr'G3 2 6 CCUUAAU AY~G?
,~ p~,U
7 8 G~"'~.GCIJ C UUGGAGC3 3 4 AAGAAAU CAUUGAC
A
80 UAG.."UCU U G.~AG~~UG338 AA~U U G~CGGCC
91 G~~UGCCU A CGUGLU1U 380 G~GAGQ ,~, i.ACCAAU
97 u"P.CGUGU A UGCCAUC 388 AACC.AAU CCUAGAC
U
104 AUGCCAU C CCCACAG 389 ACC?AW C 0'UAGACU
116 C~AU U CCCACAA 392 AAWCCU A G,CL,TACC
117 AC~AW C CCACAAG 397 CAW A C
13 0 AGUGCAU U GGUGAAA 4 09 CAAC,AGp L1CWGGL1
U
145 GnGACCU U GGCACBG 410 AA~;A~ U ~
155 CAC'UG~.'"U U UCUACUC4I1 AGAGUW C L~JG..~~UGU
156 ACUGCW U CQACUCA 413 AG~1WCU GGJGVAA
U
157 CUGCUUU C LU~CUCAU 419 UUG,3GU ?.UCAAC.3.
A
159 G:.~1UBL:U A CUCAUCG437 AGOG: AU ALTAGAAA
A
162 UUCLRCU C AUCGAAC 440 G:~UAAU G?.AAG'JU
A
16~ UACJCAU C GAACUCU 447 A~,W U ~~~A
171 UCG :ACU C UGCLK;AU 454 UGAGACU AF,~,-.U
A
179 LT~t7C~D A GCCAAUG 462 F:,~~C'CGGUUGUUGCA
U
192 UC,kGACU C UGAGGAxT S 63 ACUG~~Up CL~G~
U
200 L;~G.~~U U CWGUUC 466 G"~~7W U
201 GAG:~.W C CUGWCC 479 CAAAGAU L'UG"AGG
U
206 WCCUGU U CCUGUAC 480 AAAGF,W UG:=AGG~
U
2 07 UCCUGJU C CUGUAC~. 4 81 F~'aGAUUU Gv:,r~_C,~.,G
U
212 WCCUGU A CAT.1AA.AA 497 AGCAGa,U G'tJACUGC
U
216 UGUACAU A AAAAUCA 498 GGAG~W U UP.CUGCA
222 ~~AAU C ACCAACU 499 GACAUW U ,~~n~
CA 02468048 2004-06-07
215
500 ACJ~WOQ A CQG~GO 684 ~GpUU U UCWAUU
531 ~~ C A~UU 685 ACUQC10U U C'CLTAUfJO
538 C~~ U AAU~tJC 686 COUUQUU C UUAUpDA
539 aGGCCQQ A A~ 688 UUUQUC'U U AUWAAC
542 C~LTAAU U UOCAA~r 689 UQtJOCUU A L10C7AACU
543 CUUAADU U UCAAI~D 691 L1UCUCP.U U UAACUpA
544 UUAAUOU U Cp.A~ 692 UCUUAW U r,~p,
545 L~AUWO C AA~UAA 693 ~ CQ~1UU A ACWAAC
549 UWC~AU A L~ADC10A 697 UUtIAaCU U AACAUUC
551 UC~AI1AD A 698 UUAACOU A AC.~1L1L7CU
554 AUAUaaU U UAAC00C 703 UQAACAU O CJGUAAA
555 L'AUAADU U AACQQCA 704 ~1AC~ C UGt~lAp~
556 ?~.AUUU A AC~G 708 AUUC'UC;LT A AAAUC~JC
560 UtT~~ACU U CAGAGGG 715 AAAADGU C UGUpA,AC
61 ~707sA~ C AGAGuGA 719 UGUCDGU U Ap,CU~
573 ~AAGU A AAIDSDDU 720 GGC~U A ACUUAAU
577 AGUAAAU A UQUCAGG 724 GD~ACU U AA~C~,
579 ~VAU U UCJ~'~CA 725 UD~,ACUU A A
580 AAA~DO U ~ 728 ACUOAAU A
581 FAUAUUC1 C AGGCAI~ 731 A U~g,UGA
588 C~.GGCAU A C~CAC 733 AUAGUAU U UAW
597 LiG~C~ U UGCCAGA 734 ,~U U AWAAAU
598 C~CACDO U GCCAGAA 735 AG~UUU A UGAAAL7G
~
611 AF~G~U A ~DQCUw 745 AA.ABGGU U AA~,AUU
6? 6 AITAAAAU 0 C~AAA 746 AAUG.~~W A AGAPUUQ
617 L~AAADQ C UCAAAAD 752 UAAGAAO U DG~A
619 AAAUC'CU U AAAAQAU 753 AAGAAUU U C,~AAQ
620 AADLJCUU A AAA1~DA 757 ALmt~ A AAUOAGU
625 UD~AU A UAUOUCA 761 G.,~CU~Ap,U U AGLmUpU
627 AAF.F~J A UCOt~GA 7 62 GUAAAUU A GUAIJ~
629 A~UAU U UCAGA$l 765 AAUUP.GU A OQQp,UpU
63 0 AUAUADQ U C~.C'~A~1U 7 67 WAGUA1U U UAUQUAA
631 UAUAUW C AGA~C. 768 ~UU U AUUt~IAU
636 DDCJ~GAU A UCAGAAU 769 AGUADUU A UU~,AW
6 3 8 C ;GAUAO C AGAADCA 771 ~UU~p U U
644 UCAGAAU C AUCGAAG 7?2 AUQ~ U AAU~UA
647 GAAUCAD U GAAC'UAD 773 UpUAppO A A
653 UUGAAGU A UDUDCCU 778 U AUGUUGU
655 G:.AGUAO U UUCC~CC 779 UAAUGGU A OGUUGUG
656 AAGUAW U UCCQCCJ~ 783 GUGUiUGU U GU~UW
657 AGaP~DW U CCOGCAG 788 GUp~U U C~UAA
658 GLD~UUUU C CQCC~GG 789 UU~,~UU C
661 UC1C~CCI7 C CAG~GCAA 791 C~lpCU A p,,,
672 GCAAAAU U GAUAImC 794 U~7~ A ~A~AA
676 AADUGAU A UACOOW 805 G~AAAAU A GACAACU
578 UUCAUAU A CUUUUW
581 :~~JACU U L1UWC'Op
...
682 UAUACW U UWCWA
CA 02468048 2004-06-07
21 fi
Table 12: F3uma.n IIrS HH Ribozpme Sequences
nt. EB Ribozyme SeQueace
position
8 G:.~J.AGr~C'uT~LnAC~CC~~.nr~GG.."~.~~..~
AGvCC3U
9 G~.~AG C'L"GAI~GwC'.AAaGG..~C~.~?.
:AC~r'GC?,
o U ~G~:~A cL~L-GnG~~.:~a~.GVC~-"aa
Aaac-~c1
12 UL'L'G:~ CL'C,AUG~C:~.AG:~C"'.:AA
.~G~G'J
13 CUUUCvC CUCUG :GG;. C C:Ai~IGC-v.
~.:?.A ' -' '~",
36 G..'"UCUGaCL1G~UC~G.:~"C".~?.AGCZC~~.A
AC.~.JUCLT
37 GGCgCt7G C'LJG:~~v:.C~,~.~?.A~C".~,A
.'-.e~.C~GC
3 8 UG:~~UCU C~UC~ C'Cr':~A A?,ACGLJD
56 AAL7G.CAG CGG ;~~C~..,Ar~AG;~CC~.-.Aa
AGC~I7CC
57 AFAUGCA CaGAI~~G~.~C"~?~AGuCCGrA
AAG~UC
63 AAACDCA C'UGALIG~GuCC'-'..~G~C'C?.A
AUC.:AC~
04 G'~T,AC(1CCUGACGJ~.G:~CCC~AG~C~,~A
AAL'GC?.G
69 GGVAGCA CUGnL3~G ;CC~'..~,AGGCCC.A?,
ACL1CAAA
70 nG,~UAGC CUC~,~.C'''~CGAA AACQCaA
74 C~AC~GC CL'G-'~L1GAGGCCGr?.?.Cr""~C~,:AA
e~~?~.aC
78 G..'"UCCAACZJG~-.~,G:~CC~-'~,?.~C~.-~
AGCQAC-C
80 CAGCQCC C'DGnt~,.~C~~.:~~.a AGa,G,_'-tTA
91 ALTACACG CUG:~UGnGuCC~.~C~~C~??~
?L~~,~cAGC
97 GAUGGC~ CUG;,UG~C~_'~.~C~.~,a AG~CGLa
104 C'JG'JGG.;,CUGnUC,~.G:~:.CG:Y~.. -'.?.
A ~~~U
116 UUGUG:r, CUGr.U~~~GGCC -'C~:,C;.~~,A
AUUUCUG
117 CUQCUGG CUG?.UG~,G.~~cCC~p,G,CC~~,Ap,
p,AUWCU
13 0 DLJQCACC ' ~.nAC~CCG?.r~ AUG.:ACU
145 CAGL'GCC COGi~LT~C-C,CC::~AG:,CCG?.a
AG.~7C'JC
155 -ChC~.RG~ CL1G' '" -~CG~?.AGGCCC~A
AGCAGL~
156 UGAG~AG CGG::~C~CC'G;n:~G;,CC~.3?.?.
157 A~"UA CDG:-.L'G ;~C~C~ '
159 C~"f,UGAG CQG:~CCCG~A AGAA?,GC
152 GJtJCGAU CVGL"'.J~C~CC -G'~?G:~CC
-C. AA AG'JAC.?~A
16 5 ~'UIJC CL'C; :~C~ C'=' ',l.~CC?
A AUCAGLJA
171 AUC.~.C~CACUGAL~GGCC'=' '.~hAC~CGA_~
AGUUCGA
179 CAUUGGC CG"~,UG~GGCCG~AC,GCC~,,_,?,P,
AUG"~GCA
192 AUCCUCA CUGA ' ~ .~ -~,AC',,C-CCG3A
AGUCUC~,
200 GAACAGG CL~C -C ;A,AGGCCG?.A AUCCQCA
201 GG?.ACAG CUGAL~AG:~CC~AAGGCC~ AAUCCUC
206 GUAC:.G~G CUG~UGAG~C -G'~AGC-CC;?.1
J~C
2 07 UGJACAG C'~1G::UGF~C-C,CC~-~GC,CCG?.?
AAG~C"A
212 L'ULZ"'rAUGCClG:yUGACvCCG~GGCC -G'A
AC3G.~~~,
216 UGAUUW CUGf,UGAGGCCGnAaG;CC, ?.?,
AUC~LTACA
222 AGJUGGL7 CUGaUGnC.:,cC ~C.~GC,CCG?.?.
AUWIJ~,
245 CCUGhAA CLiG~UCnG~ _ - ' ~~C~.s~
AUUCTCUV
CA 02468048 2004-06-07
21?
247 vcCcJGA c~vGAGuCcc~~cGaA AG~-wC
248 WCCCUG CUGA 'tlCAGw' CGAAAG:~CCGAA
AAG?.UW
249 :~UCICCCU ,CU'G~GAC~.,"C"~AAG.GCCGaFa
AAaGAW
257 GJGUGCC CQG~CGAAF~;CCGAA AWCCCU
273 ACAG~fJ CUGALSGaGGCCG~AAGGCCGaA ACUCL1CC
291 DC~CAG ~~C~ ACCCCCD
3 05 UQUOGAA CUGALT~C~G~".~AAAGGCCGAA AGDCL1L70
307 GJUUUUG CUGAUGAGGCCG~AAnGGCCG~1A AUAGUCU
308 AGJUUUU CUGr~.L7G?.GGCC~CGAA nAUAGVC
316 SAC C~'GCC~~P.AG~CCGAA AGWUW
319 UAW ~~CGaA ACRAGL7fT
322 C'LJtIUAUd CDG:~?.GG..~C"'~AC~vCCGaA
AGGaCAA
323 UCU~U CUC~L~GG.-"CGAAAG~CG~A AAGC~CA
326 ADWCW CDGAUG('': CCGAP.A~~CGAA AtILTAAGa
334 GUGAADG C~L~AGGCCGaAAGGCCGAA AUULJCIJU
3 3 G~ CGQC CT~'"CG~~A?.GuCCGAA AUG~W
8
3 80 ADt3Gu"W CflGA~AG: ..~~~vGAP~ ACQCOCC
388 GUCCT.GG CDCJ~.T3GAGGCCGAAAC~CCGAA
AUUC,GtJQ
3 89 AC~CL~G CDGAL1G ~CC,AA AADUG~
3 92 GGTAGflC ~AA~CCG?~A AGGAAW
3 97 CUGADG~.-'"CGAAA~CCGAA AGUC'~G
4 09 ACG"-.F.GA Cfl~C~TiDGAGG..~C - . ACUCULTG
410 CACCAAG CDGA ~ ~ AACUCULT
411 ACACCAA C2~C~AGGCCGAAAGGCCGAA AAACfJCU
413 DUACACC CUGADGAGGCCGAAAGGCCGAA P.GAAACO
419 UGJtICAU " ~ C'GAAA~CCG?~A ACACCAA
937 WUCQAU CUGnVGAGGCC~CG?~A AUCCACU
440 AACW~JC CUGAL~AGGCCG AAAG,~~CCCAA
AULD~WC
4 47 ~CUC CU~CGAAAG GCCGAA ACUUUCLT
454 ACCT,GW CUGAUC'~GGCCGAAAGGCCGAA AGUCUCA
4 62 DG:~ACA CDGAL1GAGGC ~ ACCAGt7U
4 63 CC3GCAAC C'CfGO~UGAGGCCGAAAGGCCGAA
AACCAGU
4 66 DGGC'UGC C~IC~ADGAGGC~CG?~A ACAAACC
479 CCUCC~A C'C~COCCGAAAGGCCGAA ADCUUL1G
480 UCNCCA COGAUGAGGv.~CGAAAGGCCGAA AAUCVf~
4 81 C'DCCQCC CDGATJGAGGCC'GAAAGGCCGAA
AAAUCt7U
497 G:~GLTAA CUGA ~ " "~CGAA ADGTJCCCT
498 UG~.~. C'L1GADGAGGCCG~AAAGG~CCGAA
AAUGtJCC
499 CQG:AGU CUGAUGAGGCCG~.AAG.~~CCGAA
AF.AtIGOC
500 A,C(JGCAG CUGAL1GAGGCC'G~AAAGG~CCGAA
AAAAI7GU
531 AAGGCCU ~ ACL7C'~JW
538 GAAAAL1Q C'UGADGAGGCC~~AAACGCC'GAA
AGGCCUG
539 DC-~T~AAAU CUGAL7GAG~CCGAAAGGCCGAA
AAGGCCLT
542 VAUQGAA CL7GF.L;~GF.GGCCG~'~AAGGCCGAA
AWAAGG
543 AUAWCA CLIGAiIGAGGCCGAAAG GCCGAA AAWAAG
544 LTAU~:WG CUGAL'GAG~~CCGAA~.G.;CCGAA
AAAUL1AA
545 UL~t~W CUGAUGAGGCC '"G.~AGC,CCGAA
AAAAUUA
549 LT~aeaAUt~a CtJGAtIGAGGCC'GfiAAGGCCGAA
AUUGAAA
551 GUtRAAU CUGAUGAGGCCGAAAGGCCGAA AVAL1UGA
CA 02468048 2004-06-07
218
554 c~GUVA cJe~e~c-~,..~CC,~~G~cCCaA
h~~AU
Uc~:~ACJU c-cGnUGr~.~c;~..~cr~GGCC~aa
AAU~A
550' CU~'Q C~U"~~CG~~AGGCCGAA AA.ADt~,U
6 CCCUCL7G CUGAIC'GAAAGGCCG'r.A AG'CLTAAA
0
561 UCCCUCU CUGAL~G GcC'G~AAAGGCCGArI
AFB.
5 i3 F~ CUOuCC~AAA;C~CCG?.P. ACWQCC
5 i7 CCUGr'~AA CQG?.Z~~F.AGGCCCAP. AUUL'ACLJ
579 UGLCUCA CUGAUGAGC-CCGAA.~vCCG'~A
AUAUUUP,
5 8 AUGC C _' ' . CGAAGC,CC~ A~LJL'U
0 UG
581 UAUGCCQ G~AGGCCG~A AnAIJALJU
58s cJ~cAG cJ~~a.~;~CCCAAACG~~ A~G;.CCC
~~7 Uc~G:~.~ cUCaCC~,aawCccaA AGcGUC~
5 9 ULJCUGG CUGnUGnGC~CCGAAAC,GCC~.~A
8 C .aGUGCC
611 AGAAUW C'UGAL'GAGGCC~' AUG._"CW
616 UUUL:~.AG CCGnUGAGGCCG~AAGuCCC-~A
AD'LJL~U
617 r~UWLTAA CUGAUGAC~GCCGAAAG:sCCGrIF.
AAUUiICTA
619 ADAUUUU CUGALT~C~CCGAAAGGCCGAP,
p,GAAUpLT
620 L~AI~UW C'DGAi7GAG.GCC'GAAAC~CCGaA
AAGAAUL7
625 UG~FF~tJA CffC' ~ ~ C~ Abp,
627 UCUG~A CUGAUGAGGCC'' ~ AL~UQpp
629 L~UCLiCA CUGAUGnC-GCCGAAAG~,:.CC~
A~tJAUU
630 aLTAUCUG C~ 'UC,AGGCC'Gn.AAGGCCCzAA
AAIJADAU
631 GnBAUCLT C~DGAG:~C~"AF,AC~CCC,AA
AAADAUA
63 6 :yUUCQCA CUGAUGAG~~'CC~.F~AGGCCGaA
AUCUGAA
638 UCABQCU CUGaUGAGGCCG~AAG~,CCCAA
AUAUCUG
644 CULTCnAU CUG~CG~~1AGGCC""?,A AUUCUGA
647 y.UACL'UC CLGAUG~aC~,CCGAAF.C'~.~~~.CGAP.
AUGF,UL)C
653 A CUGnUGAL'~CCGAAAG:~CCG~.
ACULJC~F~
555 G;,~.G:~A CUGALTC~G.:~CCGA~AGGCCC~A
AUACUUC
656 UGGAG~A CUGLT~GGCCGAAAGGCCGAA AALTACUU
657 CUGAUGlsGGCCC,AAAGGCC'v,AA
p,AAUAW
658 CCUGGAG CL3GFiD~AGC,CCGAA ~A.~C
661 UL~CCL7G CLIGnUGFaCri,CLI;sAAp.GC~CCGAA
p~,~-.AAA
672 GUALIAUC CUGA '~ GCCGaA AUUUUGC
676 ~ CUGADGAGGCC'C~AAA~CCGAA
AUGAW
678 AAF~:~AAG CUGAUGAG.~,CCGAAAGGCCGAA
AUAUCAA
681 ~A CUGAL7GAGGCCGAAAGGCCGAA
AGUAUAU
682 U''.~AGAAACDGACCT,.F.A(',:~CCGAA
.AAG~LTA
683 F~(~AGr~i~CUGAUGAGCr;.C'C~~GGCCC~AA
AAAGUAU
684 Fv~LRAGA CUGALTGAC-GCCGFap.AGuCCG?.A
AAAAGUA
685 ~AUAAG CUG~L~F.GGCC'C;;4AAGGCCGpp,
~
686 LRAAITAA CUGAUGAGGCCGAA,~~CCGAA
AAAAAAG
688 GUL'A~AU CUGhUGhGGC'CGAAAGGCC~A
~p,~A
6 8 .=sGZJUAAACUGAUGAGGCCC~GGCCGAA p~P.C,F,AAp,
9
691 L1T~CUUA CUG:~UGAGGCCCAAAGGCCGAA
ALTAAGAA
692 L1~GUU CUGnUGAGGCCGAAAG~,CCG_3,,A
t,pt~.AGA
693 GULTAAGU CUGAUG:~GCCGAAAGGCC:~P.A
AAAUAAG
697 --G:AUGUU CUGJ~UGF,GG~CCGA,AAGGCCG~.A
AGU~,p~
698 ACAAL'GU CUCAUGAGC-CCGAAAGGCCGF~A
AAGUUA.A
CA 02468048 2004-06-07
219
703 L'C'JACAG CL1GAUGnGGCCGT,~.AGGCCGAA
AUGvUAA
704 'uG~.C~. CUG~.LRzr':C~:,CC~v'r.FuaC,
sCCG~A AADGO(~,
708 ' ' C'" ,~ . ...~,. ..
715 CCiGr3.UGAGuCC~~aAAAGuCCGAA ACAD~D
7.9 ~ CU~~CC~.aFAF~CC~.~AA ACAGACA
720 AUL~AGQ CUGAC~~CGAA AACAGAC
724 UAC~UQ CL'G~C~~AAAG',..~C~~1.A AGCUAAC
725 A~CUAU CUGADGAGGCC'G~AAACC'CGAA AAGQtmA
728 CDGaI2;AGGCC~ ' ~ AUQAAGO
731 UCz~C~CCGaA ACUAUUA
733 L'WG.UA C~i~AG~' ~aAAGG.~C~.~A AID~L7AU
7; 4 ALZVC~.U c.,-~a~cCC~,~AxAGV,.~,~ AAUAC,~
7.5 CAL'UCCA CL'GAD"aAGGCC~.~AAG~CC~AA
aAA~
7s~ A.~CVU cucca~"AA ACCAVw
746 'nAAWCQ CC~Ci~~GAG~~aCCG~P,AAGaC:CGr'~A
AAC"~~
752 UUQAC~A CL'GAUGT,GV...~CGAAAG~,.~C~AA
AWCOiJA
753 AUOt~CC Csr"CCAA~~CCGAA AADUC00
757 ACCAAUQ C~ADGAGG~'"CGAAAGG.."CGAA ACCAAAU
761 A~ C~U~G~"C~A AUDQACC
762 C CZ~~~"CGAAA~~CGAA.AAUOUAC
765 ?~~P. CLT,~,GGCCGAAAG~.~CGAA ACL1AAL1lJ
7 67 L'OAAAISA C'C~C~KAG~CCC~AA~CC~.~AA
A~CC~,A
762 AL1C~U CUG~'~GAG~C~c:CGAAF.GVCCGAA
AAUACUA
769 G~SAA t'DG-'~DGAGGCCGAAA~' C'GAA AAAL~C,D
771 AAC~ C'~,'tGCCGAAAf '"~.~CCG?~A AVAAAUA
772 U1~CAUU C(JGADGaGC'~CCC ~,F,~.AAU
773 ~TAAC~U CL'c~C'"AAA,GGC'CGAp. AAAUAAA
778 ACAACAU C'C1C:AD~GGCC'G~~7~AG~~CCG?~A
ACADCmA
779 CACJ~C'~ CUCA~GGCCGA~''~AGGCC GAA AACADUA
783 AGACAC CCC,P.DGAGGCCGAAAG3CCGAA ACAUAAC
788 ~G " ACAC~AC
.
789 ~ ~...'~CCAA p.ACACAA
~ C~
791 CL~UUDAU CC1GAUGAGG~~CG~J~i~CCGAA AC,AACAC
794 UC70~T CQGAL~GGC'CGAAAC~CCGAA AUU7~GAF,
805 .~C~UGUC CGGABGAGG._~CG~AAAGGCCCAA
AULI~7QpG
CA 02468048 2004-06-07
- 220
Table 13: louse Ilro HH Ribozyme Taraei Sequence
at. HB Ta.rQetSeQuence at. 3H Ta:Qet Sequence
Position Position
8 c''~'l~CtlUCUUCZG..'~253 AG~,> ;gcJ GaC.~.uAC
C A
11 uCr~LTc~J UG,~ SAA 259 vzSAG~.U C'JGaagA
U a
C'~'UcCtJUGCugAAG 269 GaAGI~U C AAPC'JG'J
U
36 G~cacU CAGAGuC 269 GaAGaaU c aAaCugU
U
36 GaAcAcU cAgAG'Jc 269 CAAgaAU c ~.c:7gU
a
37 AAgac;JU AG~C~. 287 a "G.~.,~U CGC~GGp,
C A
43 QcaGaGU AUGAgaA 301 AAAugCI1 WC~~AA
c A
58 G,=ALG..~UCDGCAcO 301 AAAugCL7 uOCCaaA
U a
59 GaUGCUU UGCAcUO 303 3UGC1~AU CCaAaAc
C a
59 gADGcUU uGcAcUO 303 AucCUAU U Cc~AAC
c
66 CUG:.AcU CAGUgOu 304 , ug-CCTAW cAPAACc
U C
82 UcAc,~cU aGcUGUG 315 ~CcUGU C aULTF~UA
c
91 GcUcL7GU uggGCCA 318 cL'GJCaU ?.hL~AG
c . U
112 ucG?.gAU CCCAugA 3 ~ L'G'CiCaUU ALA
U a
113 SG;~cAUCJ CCAugAG 322 CaUL~ ~U AAGAAAU
C a
141 G:~ACCU GaCACaG 330 =.nGAAAU CALZGC
U A
141 GAcACcU GaCAcAg 334 ~UACAU U GACcGCC
U
-
158 cUCc~ C AcC~C 334 ~~.UaCaU CACcgCC
a
167 cCGAgCU L'Gut7GAc384 AgcCAgU U CCUcGau
C
196 UGAGGcU CCQC~JcC 385 SSCASUU C CUgGAuU
U
197 GAGGcUU CUGUcCC ? 93 C'JeGAuU CCQG:~A
C A
197 gAC~U c CUGuCcC 405 C~,AGaGU cCUL7G,--U
U
202 L'UCCtti~JCCUacuC 406 :,r.~GAGW
c c
202 UUCC'GGL1 CcUAcuc 409 ~~L~ilcCU G,~JGUgA
c U
206 UG'JCccU cuCaUAA. 481 LtczCAAU UAAgUUA
a a
212 UACUCAU aAAaUCa 4 82 c.~cAAW U Ar.SUUaA
a
212 Uac~CAU AAAAUCA 483 :,cAAUW ~, AgUUaP.a
A
218 UazxzaU aCcAG~.~U483 =.cF.~.VuU aGL'IJAaa
c a
218 ~AAU C ACCJygCC1495 '.~UUgU c AAc~gAU
-
218 uAAAnAU acC~aCL1 :53 G.."'UGuuU CaUuUAU
c c
232 uaUGCAU CvaGAAA 557 UuUcCAU U UauaDUU
U
241 cAGAAAU UUUCAGG 564 UUauAuU a aUgUCCU
C
241 gAcAa.AU UUucAGG 564 ULauaUU a AugUcCLT
c
241 cacA.'-,?,UUWCAGG 565 uaUAUL'U ucUCCuG
c a
241 gAcr~AU UUUCAGg 565 L?UrluUt~ USUCcUg
c a
243 g~>;ucU UCAGSGg 569 UUuAUGU c cUGUaGU
U
243 -G'~,AUCU UCAC~G~g 569 LL'L?~UCU cUGUagU
U c
24.4 AAAUCUU C1~GGGgc 613 AFAGuGU a uaaCCUU
U
245 AAUCUUU AGGG9cU 614 AAgUGuU a a_aCcUW
C
CA 02468048 2004-06-07
221
620 UUAACcU a uUuG~U 1407 cC AgUW ,~ CJcCAGg
793 caAGgCIT a UGuGcALT 1407 c~~ a ~C
816 CUGagW n UACQCcc 1410 gUWaCi7 C CAG ;aAp,
818 G~guUAU a cUCCcuC 1434 AUgCUpO U aUuUaAU
825 ACUcCcU c CccC'QCA 1434 aUgcUuD U AUUIJAAu
825 aCDccCL1 c CcCcUCa 1434 aUgcsW a AuUUAAU
839 AuCc,~cU U cGU~ 1435 L
~ a UuUaAW
840 uCcucW c GWGC~IU 1435
ugcUUW a uOt~aW
863 c~St~D U cC 1438 '
UuUUAL
U U AAuUcug
864 AAgQAW c C~G:~g 1438 uUUUAW U AAUucU
g
864 :~Gi~UD c caggCug 1439 ULZJAUW ~, AUucUgU
913 gAaCUCU U G.~~cCaG 1443 L'WaAuU c UGuaAGa
917 UcLiuggU c CAGAuGG 1447 AWCUGL? A AgAUGUu
957 WagcAU c COUUcUc 1458 ugWcaU a UV'r~UUUp,
960 GCAuccU a UcUc~A 1458 ugUUcn,V A uUAUVUA
960 GcaUcCU a uCUCcDa 1460 ~~ a A~ug
962 AUctvuO c UCcUaGC 1461 UcAUAuU A UDL~UGP,
975 gcccCQU a AgAI~gA 1463 ~,~~Q U ~~
g
987 aGaUGAU A cuuF,AUG 1475 AuGgAW
c aGUAAgU
990 UGAuACU a AAugacU 1479
AWcaGU A AgL'tJAsU
1000 UCACVCU c UugCuGA 1
483 as ~~UW
1027 CcgaGCU U cCUgCUC 1483 GLTAAgp U AaUAUW
z
1034 UCCUGcU C CUaUcuA 1484 GUAAgW A aUADWA
1037 UgcLTCcU A UcUAACIT 1487 agUC~AU a W
' A
UA
1039 cOccuAU c LRACDUC 1487 u
u
~.~aU A ~Wa
1039 cUCcL~U c UAACppc 1489 UU'~.,AUaU U uAuVAc~
1041 CcUAUcU A ACWcAa 1489 W~U a ~Wa~
1051 UUcAAuU U AAuAccC 1489 UUAaUAU U U'r,WacA
1148 uCAcUW a cUuaUGU 1490 L~~UeW a ?alt~cpc
1213 GCUoGaU a UUGGAaa 1490 UAzUAW U AUuAcAc
-
213 ocUG.;AU a uUgGAAA 1490 UAaUAW U AUUacAc
1214 c~aGT,Dp U UG.;~an, 1491 AAUAUUp
a uuaCAcg
1215 ugGAUUU U GCAaa.p,G 1491 AAITAUuU a UuAcAcg
1234 gGuAC~U c UccuUGC 1491 AaL~ A U~cG
1236 GACAUcU c cuUGCAG 1491 AaUAUW A U'ilacAcG
1275 ugC~,CCU U AcWcUC 1494 AUuUAW
a CAc LmU
1276 gG ;;.CW A cUUcUCc 1502 g
cACGtIaU A UaauAUu
1280 CUUAcW c UCcgUgU 1502
cAcgUAU a VA.AUaW
1298 UgAACUU a AGAaGc~
, 1507 AUAUAaU a WcUaaU
310
g cAAAGL1 a aAuACcp,15 09 ALTAAuAU U CUaAuAA
1310 GC~AgU a aAUAcca 1509 aUaaUaU U CUAAVAA
,?10 GcaAAgU a AAL~ccA 1510 UAAuAW C UaAuAAa
__50 AAAGCAU A AAAUggU 1510 UAAuAW C UaauAAA
'
_358 AAAUG.,~V U ggCAugU 1510 tT~:AuAuU
c UaaUAAA
370 UgUuaW C AC-gUAUC 1510
UaaUaW C UAAUAAA
1375 WCAGgU A UCAGggU ~ 1512
aUaWCU A AUAAAgC
1377 CAGc~UAU C AGggUCA 1515
WCUAAU A ,~pg~gA
1383 UCAGggU C AcUGgAG
1405 cccCAgU U DACIJcCA
CA 02468048 2004-06-07
222
m
c~
c~
m
v
c
m
N
O
N .G
~i
O R'"
wi
y
x
c
..
c:
...
0
N
. 0
GL
CA 02468048 2004-06-07
223
U
C~
V
d m
r
C m
v N
O
a a
arc
._
0
,e~ o
~w~~i ~B'c-ll~~e~-I~N~'p'7~~~~O~f
~l
~
~ m r
r-i
r1
ri
v-1
W
CA 02468048 2004-06-07
224
~~~~~&FE~~~~~~~~~~~
~ a a
~
B
o
~~~ ~ ~
~~~~a5s ~
~ ~
~~~
~
~~
~g
F
gg
~
g
g
~
g
q
~
kk
'2YZkYi'e~Y'2Y~'Z'dk'2'2Y'd'd
'
~'
~
,
,
e5~F9~'G~R~'sR~~w2aa.:
ro
E~
CA 02468048 2004-06-07
225
Table 17
Mouse re! A HH Target sequence
nt. Position HH Target Sequence nt. Position HH Target Sequence
1g AAUGG~.~U a caCaGgA 467 cCAG~."LT_ c cur~.uUCg
22 aGCOCcU a cG'J~GUG 469 ~ 3aGCc.~4LT a AGcCr4Gv
26 CcUCcaU a GcGgACa 473 UuOgAGU C ?~GauCAy
~3 CAuCt7G0 U uCCCCGC 481 ?~ ~z~U C C.'~G1CCA
94 AuCIIGt3Q a CCCC~1 503 AaCCCCU v? uCAcGC?CT
100 UuCCCCU C AUCUGuC 502 ?.ACC....,, a C.~Utfi
103 CCCOCAU C UUuCCcs 508 ULC~rcGLT U CCL'~.UAG
105 C'JCAUCU U uCCcuCA 509 uC.~cGUQ C CUAUAGA
106 OCAUCW a CCcuC'-~G 5~? c~LCC'J A UACAgGA
129 C.~'1iU C UGGgCCIi 5:.4 UC,~CCOAtT A G
138 C,GgCCI~U A L'G7GV?l 534 fz~CU A uG~UG
148 UGGAC~AtJ C AUcGAtC 556 L'G..~GcCQ C UG."Of3CC
151 AGAUCAU c GAaCAGC 561 CrCOGCU U CCAGGUG
180 ,ADGCGaO U CCGC~Au 562 UCUG4"W C C~~GGA
181 UGCGaW C CGC'UAuA 585 aPr.,C~~a,U a AGcCAGc
vUCCG,.~U a uAAavG,~. ~~e G~Ccccu c cLCCUGa
204 G GG..~G~.~U C aGC'G:~613 CcCCUG? C CUcsCaC
217 G~~i~liAU a CCuC-GCG 610' CL~GUCCQ c uCaCAUC
239 C~GAU A CC~CAA 617 SucCCUU C CUC~gC~'
262 CCACCAU C ~UG1 62J CCULCC'fJ C ~rC'Caug
268 UC~AGAU C ~.A~ 623 UCCUgeU e: CC.AL'CfJe
276 ~AL'C~CIJ A CACAGGA 628 ALiCCgAU a UL'~JGAuA
301 VuCGaAU C UC ,. ~ 630 CCSAUuU U i.'GAuAP.c
303 CGaAI3CU C CCUGGQC 631 C~AUuL'LT U Cr~uAAcC
310 CCCOGV~LT C ACCAAGG 638 UGgCcAU a GvGI~uCC
323 GGcCCC(J C CUCcuga 661 CCG?1~C:LJ C A~UCU
326 uCCaCCD C ACC~~GCC 607 UCaAGAU C L'GCCGaG
335 CCGGCCU C AuCCaCA 687 CGgAACU C UG~gAGC
349 AuGAaC~T U GUS~A - 700 G.~UGCCL7 C G.~~v'G.7GG
3 52 AGaUcaU c Ga.A~cAGc 715 AUCAGAU C WCIiUgC
375 CAUG~~J. a C'UAZJG~"~G717 GAC?wcC1 U cwgctTG
376 AUGGucU C UccGgaG 718 AGUCUU C uUSCUGU
378 G ~CL7aCD A UGAGG..'U 721 UucUCCLJ c CauUGcG
391 CDGAcCU C UG.~CCaG 751 AaGACAU U G?~C~~UGU
409 GCaGuAU C CAuAGcU 759 GAG.,~~UGU A UL'UCACG
416 CCgCJ~Gt7 a UCCAuAQ 7 61 G~GUAU U UCAC~wG
417 CAuAGcU U CCAGAA~C 7 62 GUGUAI7Q O CACGGG?,
418 AuAGcUU C CAG?~ACC 763 UG'JAVW C AC~~~
433 LT"~,gAU C CAGUGUG 792 ~ CGAGGC~T C CZ'UUUCu
795 G~.,.~UCCU U UUCuCAA 3167 GAUGAGU U UuCCcCC
796 G~"'JCCt7U U UCVCA1G 1168 ?.UGAC,ZJLT U v:CCcCCA
797 CUCCUW U CuCAAGC 1169 L'C~GLJW a CCcCCAU
798 UCCUUW C uCAAGC~T ,' ~ - - AUGcUGU U aCCaUCa
3182
829 UG: CCAU U GUGLJUCC 1183 UGcL)GUp a CCaUCaG
CA 02468048 2004-06-07
226
834 AUUGiIGU U CCGGAL1~ 1184 G
;
. CUcCUGa
835 L'UG~L1LT C C'~,~,C~C1187 ccccU C
GVccCIiU CUcaGCc
845 G c
~CCU C CgUACGC 1188 UUaCCaU C aG;"C~G
849 CCUCCgU A CGCcG?,C 1198 G:,gA~U a A~~Ga
872 cCnG:~~tT C CUGUuCG 1209 CAGcCCU
a caCCUUc
8R3 UuCGaGU C UCCAUGC 1215
cuGGCCU U aGCaCCG
885 C~",zGUCLJ C CAUGCAG
1229 GG1~CC'CLT CCvcAGc
-9D5 GC:NCCU U CliGAuCG a
1237 CCCAgcU C CUG~.CCC
906 '
CG:~ 1250 CCAGcCU C CAGgL~C
~ C uGAuCGc
919 GcG:~GC'Q C AC~GC 12 68 CCCaGCQ C
CuGCCcc
936 AUC~gU U CC~C 1279 ~
CC~UG" cL~uCc,1
937 UGLAcUU C CnC~ Q c
l
. 1281 gOGGgcU C AGCUgcG
942 1
-
UUCCAGU A CuDGC~
A
,,. 1286 AUgAGuU a UccCCC_8
053 GCCl~c~lU c CAc~
GA
u 1309 Cud a CgA~C~
962 AGAuGAU C GcCACCG
1315 cCCCAGU a CUAaCCC
965 CagUacU a cCCaG?
c
. 1318 CAGUuCU A aCCCCgG
973 ACCG;~U U GAaGAGA ~
1331 gGG~CW C CcCAG~C
986 GAr~ACcU a cAAGa 13
u
g 34
6 ClzuUuCLJ AaGCUGa
C
AGGACcU A DGAC~liCC 13 89
A~ C
1005 9~GCC
~W U 14 ~
U UGAUGcU
1006 AGACCUO C AAGAGuA
1414 UGCJ,GUp ~U~UG
1015 AGAG~AU C AtKAACp U
, 1437 ~W U
1028 F
G 1441 CCUUGW U
,AGACU C CUW~
1031 -CAGUCCU U BCAauGG 1467
~a~ O
1 032 AG'UCCUU U CAeu~A 14 68 C~CAGAC
'
9aG ACAGACC
1033 GUCCUW C AauGGAC UGUU C
1482 CUGGCAU C uGUgGAC
1058 CCGGCC'U C CAaCcCG
1486 CuUCgGU a GggAACU
1064 UaCACCU a GAucCAa
1494 GaC~IA~ C aGAGUUU
~U U ~C
1082 ? 500 UCaGAGU U UG'~GCAG
UGUGCCU a CCCGaAa
1083 1501 CaGAGUU U CAGCAGC
aaGCCUU C CCGa
A;Gi1
. 1502 aGAGUUU C AGCAGCU
1092 CGzAaCU C AaCpt
-CU
, 1625
9GuGCAU c C
1097 CUCAaCU U CUGUCCC CUGUGu
1566 ADG: A(v CCCUGpa
1098 UCAaCUU C UGUCCCC A
1577 LA UA~~
1102 CUUCUGU C CCCAAGC
1.579 AaGCUAU A ACUCGCC
1125 CAGCCCU A caCCUtlc
1583 UAL~p,W C ~CUgGU
1127 GCCaUAU a gCCUL~
C
r 1588 CUC17CCU GaGAggG
1131 cAUCCCU c agCacCA A
1622 CCCAGCU C CUGCcCC
1132 AcaCCUU c cCzgCAU
1628 UCCZ.1GCU CggUa~
1133 UCCaUcU c CagCVUC a
1648 ~~ a CC~UG
~U a AgCgCgc
1660 cUGaCCU C ugccCAG
cCaQCAU C CCUcAGC
1663 cuCUgCU U cCAGGuG
1153 GCACCAU C AACUuUG
1664 uCUgtW c CAGGuGA
1158 AUCAACU a U'GAUGAG
1665 ' CUCgcUU cG:,AGgU
1680 GAAGACU U CUCCUCC a
1681 AAGACUU C UCCUCCA
1683 C~CUUCU C CUCCAUU
1686 UUCUCCU C CAUUGCG
'_690 CCUCCAU U GCGGACA
CA 02468048 2004-06-07
227
1704 Avc;~cU U Cu~sc..w
l7os vc,;~acw C vc~~,.-~C
1707 GACiIUCU C uG~'vCQu
1721 uuUGAGU C AG~UC4G
172 GUCAC,AU C AG~~UCCU
6
17 31 AUC''~G..~LT C COAAGGt~
17 3 AG..'ZJCCa a AGv"vGcU
4
1754 CaGIigCU C CCaAGaG
CA 02468048 2004-06-07
228
T able 18
Human rel A HH Target Sequences
nt. Pcsition HH Target Sequence nt. Position HH Target Sequence
19 AAUGG.~U GUC'UGL~.4 67 GC~C~'v A
C QC~GUCA
22 G~~UCGQ C UGH 4 69 AGGC'GAU AGUG1GC
C
26 CGJCOGU A GUGCACG 473 u'~UCaGJ
C AGC"'.~~U
93 C'~ACLJGV CCCCCL1C 481 AG..~GC~U CAG?.C'~
U C
94 AnCUGW C CCCCUC~1 501 ?.~1CCC:.v CC~Lr'U
J
100 UCCCCCLT AUCUUCC 502 ACCC'C'uv CAAGUGC
C C
103 CCCUCAU C WC'CCGG 508 CCCA?G'-~7 CCLTAL~G
J
105 CUCnIICU CC..~A 509 CC'~Gvv C CUALTACA
U
106 UCAUCW C CC".~C~G 5.2 AGUL'C~J LaG~Ga
?~
129 CAC,GCCU UGGCCCC 514 QGCCGAU A
C
13 GGCCCCD A rJGLIGVAG534 G.~.~C~J CGP.CCL'G
8 A
148 UGGAGALJ AULJGAGC 556 T;GCG:~J UGC-JUCC
C C
51 AGADCAU O GAGCAGC 561 C'JCVG'~U C~,.~.G.w~~G
v?
18o A~G~~ccU ccccQAC 562 UcUCCtv c caGGacA
U
181 DGCG~"W C CGC'~CA 585 G~.CC~,,~U AGE
C
186 UUCCG.~U C?~GUGC 598 G.:~C'CCU CG:.~GC
A C
204 GC~"CCtT CGCGGGC 613 C 1CLGLT CQL7CCUC
C C
217 GCAGCAU C CCAC~CG 616 CLGG'Cw U C~C~.UC
239 CBCAGAU A CC?.CCAA 617 UGVJCCL'U C'JC.'~1UCC
C
262 CCACCAU C AAGAUCA ~ 620 CCOUCCU C AUCCC~U
268 UCAAGAU C AAL7G~GC'U623 UC~,TCAU CC~UC'JU
C
276 AF.UG.~~ CACAGGA 628 ALCCCAU C L'tJL'C?.C~
A
3Ol UGCGCAU C UCCCQGG 630 CCCAUCU U UGA~?U
3 03 C'',~CAt7CU CCUGGUC 631 CC =UCL'U C~.C~AUC
C U
310 CCCL'G~'"U ACCAAG 638 UC~CT,AU G'JGCCCC
C ; C
323 G.ACCCLT CUCACCG 661 CC"AGCV C AAGaUCU
C
3 2 CCCZTCCLJ ACCGGCC o' 67 UCaAGAU C UGCCG?.G
6 C
335 CC".~CCU ACCCCCA 687 CG~ALV C L'GGC~~C
C
3 4 ACGnGCU U GUAGC'~A.A7 0 0 C-~~Z7G.
9 CU C
352 AGCUUGU A GGAAAGG 775 2,UC?.GAU WCCUAC
C
375 G~DGG.."U CUAUGAG 717 C?G~,UCU CC'LTACL'G
U U
376 AUG:~..~W UAUGAGG 718 ~UCUU C CU'r,CL'GU
C
378 Gu<WCLT A UGAGGCU 721 UCL'C7CCU CUGZ7GL~
A
3 91 CUG~GCV C UGC'CC'GG? 51 r.G~CAU U G:~GGUG'J
409 G'''"JGCAU CACAGUU 759 GnG.;JGU L'WCACG
C A
416 CCACAGU U UCG~GAA 7 61 G.,~LTG~U U~~,
Q
417 CACAGW U CCAC~AAC 7 62 G'JGUAW U C~CGGCA
418 ACAGQUU C CAGAACC 763 UGUAUW C ACC-:~G?C
433 UGG:~AU C C:T~TGUG 792 CC CL'UWCG
7 9 GGCUCCU U UUCGCAA 1167 ~ C? LG? L'CCC1~1CC
GLi U
796 G.:JCCW U UCGCAAG 1168 AUG?GW U CC~~,CC?
797 CUCCUUU U CG:~AGC 1169 UG.G'JUU CCACCAU
C
798 UCCUUUU C ~ 1182 AUCv'vG'J UCUVC1C'J
U
829 UG:~CCAU GUGUUCC 1183 UG.a~v'GUU CCUUCL'G
U U
834 AUUGUGU U CCG:zACC 1184 G"~Z,-C;~ ~-U~,.C~
C
CA 02468048 2004-06-07
229
835 UUGUGJU C CGGACCC 1187 G'JUUCCU U CUG~~C~
845 --CACCCCU C CCUAC~vC 1188 UUUCCUL1 C UG~G
849 CC'UCCCU A CG:AGAC 1198 GGC~U C AGCCAGG
872 GCAGG.."U C C~1GCG 1209 CAGGCCU C G~CC1UG
883 UGCGUGU C UCCAUGC 1215 UCG~CU U GGCCCCG
ses c~uc,~cu c cz~G:~ 1229 cGCCCCV c cccAACU
905 GCGGCCU U CCGACCG 1237 CCG'-.AGU C CUGCCCC
906 CG:~"CW C CGACCG a 1250 CC~CGCU C C?.GCCCC
919 GGG~"Q C AGLJGAGC X68 CCCUG..~'J C CAG.~,CAU
936 aUG:~IU U CCAGUAC 12?9 CC~' U A UCAGC(JC
937 UG~AUU C CAGUACC 1281 AL'G;JAU C AG~.~UCL.'G
942 UUCCAGU A C~CA 1286 AUC~ICn.~LT C UGC.-CCCA
9=_3 GCCAC"s?.U A CAG~CGar1309 CCCCUGU C CCAGL7CC
962 ACACGAU C GUCACCG 1315 UCCCAC~7 C CUAG.~_CC
965 CG?.UCG~? C ACCG ;AU 1318 CAGJCCQ r1 GCCCCAG
973 ACCGGAU U GaG GAGA 1331 AG:~..~CCU C CLTCAG
9 8 GAAACGU A AAAGGAC 13 3 4 CCCUCCLT C AGG C'tlGU
6
996 AC~ACAU A UGAGACC 1389 ACG~~UGp C AGAGGCC
1005 CAGACCQ U CAAt'~AGC 1413 CT ~ , T U UGaUGAU
10 nG~CC UU C AAGAGCA 1414 L1G~W p G~UG
06
1015 A -CAGC.3.U C AUGAAGA1437 ~~~~W U ~~~"C
1028 -GAAGAGCT C CUOUCAG 1441 CCUUGv.~U U ~C,~CAACA
1031 CAGUCCU U UCAG~"G; 1467 GCVGUGZ7 U CACAGAC
1032 AGUCCUtJ U Cr'~GGA 1468 CQGUGUU C ACAGACC
1033 G'JCCUUU C AG~."CGAC 1482 CUGGCaLT C CGUC~"~,C
1058 CCGGCCU C CACCDCG 1486 CAUCCGU C GACAACU
'! UCCACCU C GACGCAU 1494 . GACAACU C C~~,GU<Jt7
064
1072 GAC~~~U U GGUGi7GC 1500 UCCGAGU U UCAGCAG
1082 UGL'GCCU U CCCGCAG 1501 CCGAGUU U G~G~~GC
1083 G'v'GCCULI C CC'GCAGC1502 CGAGUW C AG~?,G.~U
1092 CG:J~~CV C AGCL'QC'Q 1525 AGGCCP,U A CCQG'.IG
1097 CUCAGCLJ U CUGUCCC 1566 AUGGAGV A CCCZ7GAG
1098 UCAC~C'L7U C UGUCCCC 1577 DGAGGCC A L~.ACL7CG
1102 cuUCVrw c ccc~A~c 1579 AcccvAU A Acuc~"cc
,125 CAGCCCU A UCCCUUU 1583 UAUAACU C GCC'UAGU
1127 G~.CCUAU C CCDUUAC 1588 CUCGCCU A GUGACAG
1131 UAUCCCU U UACGUCA 1622 CCCAG.~U C CUG~.~UCC
1132 AUCCCW U ACGUCAU 1628 UCCUGCU C CAC'UGGG
1133 UCCCWU A CGUCAUC 1648 ~~~V C CCCAAJG
1137 UQCTACGU C AUCCCQG 1660 AUG: CCU C CWUCAG
114 ACGUCAU C CCUGAGC 16 63 GCCUCCU U UC~.G
0 ;AG
1153 GCACCAU C AACC~DG 1664 CCUCCULT U CAGuAGA
1158 AUCAACU A UGAI1GAG 1665 CUCCUW C AGGAGAU
1 680 C~.ACACU U CUCCL1CC
1681 AAGACW C UCCUCCA
1683 C~-'~CWCU C CUCCAUU
1686 UUCUCCU C CAUUGCG
1690 CCUCCAU U GCG~ACA
1704 AUGGACU U CUCAGCC
CA 02468048 2004-06-07
230
1705 L'G:~CUQ C UCAC-CCC
17 GACWCL7 C AC.C,'CCVG
07
1721 GCQGAGU C ~CAG
172 GUCAG~IT C nG~."flCCL7
6
1731 ADCAGv."Q C CU~'~GGG
1734 AC'aCUCCU A r~Gw:~~LT
17 CL1G..~CC~ C CCCAGAG
54
CA 02468048 2004-06-07
231
Table 19
Mouse rel A HH Ribozyme Seauences
nt. HH Ribozyme Sequence
Sequence
1,9 UCCDGUG CffGAUGAGCZC"~AAGVCCG?,A AGCCAW
22 CACCACG C'UGAUGAGGCC'GAAAGGCCGAA aGGAG.~U
26 UGUCCGC C'JGAUGAGGCCGAAAGGCCGAA AUG~~GG
.
93 GAG~GA CLrcaAUGAGGCC:~.~AGGCCGaA aG'~GALJC
94 C~GAG~CUA~AGGCCGAA aACAGAU
100 caAACAV cvG~UC~c; :.C:~AAC~cccaa a
~~~
1 p3 aG:~AA CUGAUGaGGCC~.~AAiAGGCCGAA AVGAGGG
105 LIG.~.GC~GA CUGAUG?.G: CCGAAAGSaCC".~AA
aGAUG'aG
106 CUGAGGG CL1GAL~' G~"CGA'rIAC~~,CC~.oAFa
~.AGAUG?~
129 AGGCCCA CUGADGA~CG CCG.~'~AAGGCCGAA
AAGCCL~G
138 CUCCAC~1 CUGAtJGAGGC.. , ~~CG~A AAGGCCC
148 GWC"uAU CUGAVGr'aGGCCG~AGGCCGAe~a AUCUCCA
.51 GCUGUOC CUGAWAG~CGAAAGGCCGAA AVGAUCLT
180 A~GG CDGAUGAGGCC'GAAAGGCCGAA aVCGCAU
181 TJAtTAGCG CUGAUGAC-GCCGAAAGGCCGAA AAUCGCA
186 GCAVUQA CUGAUGAG:CC~.~AAAGGCCGAA AGC~GAA
204 G.~CCGCIJ CUGAUGAGGCCG AGCGCCC
217 CGCCAGG CUGADGAGGCCGAAAGGCCGAA AUACQGC
239 U~~GG CL~CG~UGAGGCC'G~AGGCCGAA AVCUGUG
262 UGAUCW CDGAUGAGGCCGAAAGGCCGAA AVGGUGG
268 P.GCCAUU CUGAUGAGG.~_CGAAA,C~CCGAA
AUCWGA
276 UCCL~GUG CLJG~UGAGGCCGAAAGGCCGAA AGCCAW
301 CCAGwA CQCALTAGGCCGAAAGGCCCAA AUC1CGAA
3 03 c~a.CCAGG CUGAUGAGGCCGAAAC~,CCGAA AGAWCG
310 CCWG~'"IJ CQGAU ~CCGAA ACCAG~G
323 U '' ~ CUGAUGAGGCCGAAAGGCCGAA A ~~
C
326 G GCCG.~~U CUGAVC~GGCCG?.Ap.C,GCCGAA
AGC~JGC",le,
335 VGL1G:~AV CUGAVGAGGCCG~?,~IAGGCCGAA
AGGCCGG
349 UCCCCAC CUGAL1GAGGC'C:cs~,F.GGCCGAA
AGWCAU
352 GVUGUUC CUGALTGAGGCCGAAAGGCCGaA AUGAUCU
375 cucavAG cucAUCAGCCCGaAACGCCCAA AGCCAVc
376 CUCCGGA CL1GAUGAGGCCG~F~AAGC~CCGAA
AGACCAU
378 AGCCQCA CUGAUGAGGCCGAAAGGCCGAA AGUAGCC
391 CUG~~: A CUGAiTGAGGCCGAAAGGCCGAA AG.~"UCAG
409 AGCQAUG CUGAUGAGGCCG~AAAGGv,~CGAA AUACUGC
416 CL'AUG~A CUGAU GGCCGAA ACUGCG~v
417 GUUCUGG CUGAUGAGG~CG~AAC~GCCGAA AGCUAi~G
418 GGUQCUG CUGAUGAGGCCGAAAGGCCGAA AP.Gw~UAU
433 CACACL7G CUGAUGAG ;CCGAAACGCCGAA AUCCCCA
467 CG?ACAG CUGAUGAC~CCCAAAGGCCGAA AGCCUGG
469 GCUG:~CfJ CUGAUGAG: CCGAAAGGCCGAA AUGGCW
473 CUGAUCU CUGAUGAGGCCCAAAGGCCGAA ACUCAAA
481 UG.~"UCUG CUGAUGAG: CCCAAAGGCCGAA AUtTCGCIJ
CA 02468048 2004-06-07
232
O ~C ~ ~A cu~cAUe;.~:~C C G'~G~;.
1 C Gr,A A~:~,~~uU
502 CAAC~G CL"'~UGAGGCCG~AAGGCCGaA
p~
508 CCAUAGG CUCAUC~AGGCC"'.~?.F'~AG~.~CGAA
ACG'L1GAA
509 DC~G CDC-~UGAG.G.."C.'GAAAGG.."CC,AA
AAC
512 L~CCUCLA CuGL~.GC~.:CGAFaAGGCCC_AA
AC-GAACG
514 G."QCCUC CuGAI?GAG :~~C'=' ' A ADACuAA
534 CAAGJCA CUGAL'GAGGCCG?.AAGGCCGaA
AGUCCCC
556 G,3AAGA CLG~UGAGGCCG~CGA~. AC,:~CGC~.
561 CACCUGG CUC~UCzAC~CCC'GAA AC,~~C~G
562 UCACCBG CL'GAUGAGGCCG~AAG~CCG1A
AAG:AGa
5 8 G.~UGG~."U Cu'GAt3G? G:~C CGAAAGGC
5 CG~.A AUGC,C UL~
598 UCAG~G CUC'.:AIKnGG:.C~.~.aAGC-CCC~AA
'r,GC,GGCC
6.3 GL -TC~C~.GC.,nC-'~UGAGGCCG?.AAG~CGAA
AG~GGC~
616 GAUGJGA C'CJ(y~JGAGGCCGAA.AGGCCG~.A
AGGACAG
617 G:~C'JGAG C ~ -.~A?.GS,AAGG~C
620 GaUGGCQ CVGAUGnCGCC~-~' AAGGCCGAA
AG:y4AGG
623 GAGAUGG CQGAUGAGGCC'G~AA.AG: CCCP.F.
A
628 UAUCAAA CUGAUGAGGCC'GAAAGGCCGAA
AUCG:~U
630 GUQAUCA CU~CGAAAGGCCG?.A AAAUCGG
631 G.~"L7LTAUCCL~CCAAAGGCCGJ~A AAAAUCG
638 ~C".,~~ACACCt7GAUGAGGCC~~AACw,CCG?~A
AL'GGCCA
661 AGAUCUU CQG~UC,T~G:~cCGAnAGGCCG~.A
AGCUCGG
667 C'u~CC~C~ C'LT~UG.~'aG:~cCCAAAGGC.CGAA
AUCUUGA
687 GL"C1CCC~ CLTGr',UGAGG,:C~~AA~~,CCGAA
AGULICCG
700 CCCCACC C'BGAUC~GGC~CGp~ AGC.GAGC
715 GJ~iGAA CUCiAUCAGGCC"~AAAC,C,CCG~sA
AUCUC_AU
717 ChGCF.AG CUGAUGAGGCCGAAAGuCC'G?A
AGAUCUC
718 AC.~G:~A CUGAUGAGGCC''-~GGCC:'~A
AAGAUCU
721 C"~"':,AUG CLTGAUC,nGGCC 'G~nAGGCCG'~1A
AGCZnGAA
751 ACACCUC Ct7G'r.L'G?GGCCGAAAGGCCGaA
AUGUCUU
759 CGLJGAAA CLJG:~.UG~~GUCC ' ACACCVC
761 CCCGUGA CCC,A. ' ~ C'C~AAC,GCCGAA
ALTF,C~CC
7 62 UCCCGUG CUCy~I7C;AGG~CGAAAGGCCG~
AAUACAC
7 63 GUCCCGU CUGAIIGnGGCCGaAACC,CCC,AA
AAAUACA
7 9 ~.C~AF~AG CUGAUGAGGC C'C~F AAGGC CGAA
2 AGC CUCG
7 9 L'UGAG~sA CUGAUGAC~CGaAAGC,C CCAA
5 AGuAGCC
7 9 CUUGAGA CUG:yUG:~GvC CC~i~',r'~G:~:.
6 CGAA AaCrinGC
797 C-CUL7C~G COGAUGF~GC-.CC'CAAAC~CCGAA
AAACuAG
7 9 AG~~UUGA C'u'GAUGAG~,CC'f'~' AAG~CCGr'~'s.
8 ~iFaAAGC;A
829 C-,~AACAC CUCAUGAGGCCG.AA~.GuCCGAa.
AUG.GCCA
834 AGUCCGG CGC,AU ~ ~ CCAA ACACAAU
835 GnGJCCG CDGnUGAGGCCG~AAAGGCCGAA
AACACp.A
845 GCGUACG CLIG~UGAGGCCGAAACGCCGaA
AGGAGUC
849 GUCGC~CG CUGhUGAGGCCC~T,AG;,,CCC?J~
ACGGAGG
872 C''L:~,ACAGCL1GAUGr,GGCCG~.AAGGCCCAA
AGCCUGG
883 GCAUGaA CUGAUGAGC,CC 'G~AGC,CCG?.A
ACUCCAh
8 8 CUGCAUG CL1GAUC,F.GGCC~C.~-_AA AGACUCG
5
905 CCAUCAG CUC,AUGAGGCCGAAAG:~CCG?.A
AGGCCGC
906 GCC,AUCA CUC,AUGAGGCCGAAAGC,CCCAA
AAGGCCG
CA 02468048 2004-06-07
233
of g GCUCAtV
' ~ C~~F~GG.~,CC~A
AC',CZTCGC
c 3 GDACDGG CU ~ ' . '"'C'~~A AC<ICCAZJ
6
g3 ~ AG'~ C'~'.IJGAGC,CCGAAAGGCCC~A
AACDCCA
942 D .~ ~~ ~
g53 DCAUGDG COGAUGAGGCCGAAA~ A~AGGC
9 62 CGG'JG~~ CUC~JG~GGCC~' ~ AUCADCU
9 65 GUCUGGC C ~ AGL7P..CUG
9?3 DC'OCUQC CLTG ;UG~GGv."CG~AAGGCCG~
AUCCGCR
.
S 8 ACQCODG C _tGADGAG~~CG~GG.."~A
6 AG.~"DCL1C
006 GuJCLJC~. CCJ'G;F~I7GAG:~CC~.~AG~~rGAA
AC~CCU
1005 aCUCQGG G:~AGGCCGAA AGuLICUC
1006 Li~CUC'LJQCGGADG~ ~ "AP.
3 015 UCGiJC.~U CG'G~'~DGP.GGCC".~AAG~"CGaA
AUACDCU
1028 L'L'G~AG CUGAL1GAGGCC'GAAAGGCCGaA
ACUCIJUC
1031 CCAUUGA CaG:~GAGC,CCGAAAGGCC''"AA
AG.~~ACL?C
1032 DC~~G ~ ' ~CCGAA AA~ACD
1033 GDCCADU CWAUGTaGGC'C~AGCr.~CGAA
AAAGGAC
1058 CW:~LIG ~ ~ AC,GCCG6
1064 ~'C~ '" AC,~
1072 G~J~C~.GC CtJGADGAGGCCGAF~AG~.~CGAA
ADACGCC'
'! 082 DULiCG~ CGGAUGF~.SGCC~~AAGGCC~"AA
?~~CACA
1 083 ACWCGG cJGT~UGAC;GCC'"~AAG GCCC,AA
AAGGCt7p
1092 ~~ AG~CG
1097 GG~~CAG ~C'CGAAAG~~C'GAA AGODGAG
1098 ~G~C3~ CL3Cp.DGr':GG~C" AAGUDGA
1102 Gt"U~7Gw C.'UGAUGA ~ ~~C;GAA ACAGAAG
1125 GAAGCDG CUGADGAGGCC~.~GGCCGAA AGGGCVG
1127 c~c cvG~~~Cc,ccGAA Atmz~GGC
31 ~Gu~UC~~U c ~ '~:,G ~CCGF~r ~~
32 AVGC'OC~ CVGAOGAGGCC~CGAA h
1133 GAAG..'ZJGAGADGGFa
37 G'~cc~"CV cvc~:UGAGGe~cGr~A ~,~,
1140 GCVGAGG C~.~CCGAAAGGw~CGAA ADGCLA',G
1153 CFJ~GW CUC~F~K~AG~~CCGAAAG:~CCGAF.
ADG~JGC
1 '_ CuCAIICA CUGAUGP.G~~C~GAA AGQI1GAZ7
8
116? C~~"~,Ga,ACVGF, ~ GAA AC(JCADC
,168 L .-.. CL-GA . ~ CCAA AF CQCAU
1169 AU ~GwGG CUGA ~ CCCGAA AAACDCA
182 UGsUG.~~V CG'C,p, " CGAA ACAGCAU
,183 CDCAUGG CUGADGAGGCt~GAJi~F~ AACJ~GCA
1184 UCAG~AG CGGAU ~ ~ CGAA AGGGGCC
11 87 G:~CtJGAG CUGAUGAGGC,'C'Cl~AGGCCGAA
AAGGGAC
6188 CUGCCCO C'CGGC~CCGAA AI7GG~1,A
?198 UCAGACU C'C~CGAAAGC~CCGAA AACUCCC
..2 GA?.GGUG CG ~ GGCCGAA A~GGCVG
09
215 CG~'UGCU CLiGF.VGAGGCCGAAAGGCCGAA
AGGCCAG
'!229 C,CUGAGG CGCAUGaG~CCGAAAGGCCGAA
AG~GACC
_237 GGC-GVAG CUGAL'GAGGCCGAAAGGCCGAA
AGCL?GC,G
1250 GAGCCUG CGG'~.UGAGGCCCT,AAGGCCGAA
AGGCUGG
CA 02468048 2004-06-07
234
1268 ~,.".' CC~CG?.A AG.~UGG
1279 AG~GG CDGAUC,AG:~ "CC~G:~..~CGAA
ACCAUGG
1281 C~~0 CL~C-:~~C~~ AGCCG?~C
1286 -... CL~t,..CG.'~AAGG..,CG?.A
AACUG'~U
.3 09 AGF~CUCG CI:~~t'CAA?~C-:~C CGAA AC~G:y?
G
13J.5 GG.~~L~G CQGhUG~GGCC'GAF~?.GGCCG~
ACL3G~.~,
1318 CCG''~~ C~7GAUGAC:~CCG..ranAG~~~G'~
,"'O AC,~CUG
1?31 GACL~ CVC~UGACv:.C~~G.ZCG~A AGGaCCC
1334 UG'..GCT.1LJC(3Gp.DC~IG:~C~.~G.~.-CCGAA
,~.G
1389 G~"LNCC CQG?.UGaG:r.:."'~ AG~GGGU
1413 AG~J~.UCr"sCUGaUC;AG:r.~~~' CvC~~G-~r~a
'r~CuGC?.IG
1414 ' "' C CuGi C'='W C"~?,A ?.r~CL.'GC?r
1437 GCG.:~GC C~JG~CG?.GGC;.~'.~AG~"'CG~A
~.G~.CC;.'
14 41 UGUUG:. CL'G.'.. - ~ ~C GAA ~~,~,GG
C
1467 GUCUGOG COG"CG~.ACWCG~ ACACQCC
1468 CC~7CUQJ CUG~LJG~C~.~CCGnaAC~CCG?~A
AACAC'LJC
1482 GL1CCACA CUGAUGAG~"'CG~AGGCCG?.A
ADGCC?~G
1486 AGUOCC'C C~~7~GAGGCCC~nAAGGCCGAA
~,~C:CGa~G
1494 AAACQC'IT COGaUGAG~~~CG~AGwCGaA AGUUGL7C
15 00 CUGCUC~A CL1GABG~GGC CGAA.ACuC C
G~ ACUCDGa,
1501 G.."'G'GCUGCflGT~L7GAGGCC~~G~CG?~.
AACUCGG
1502 AGCCLJGCU CUGA~G'~G:~CC~-~'J~aG.~C'~G~
.~r'...aCt7CU
1525 ACAC~.GG CL~~C~.~A.AGC-CCG~A AL'~CC
1566 UUG'zG~vG CUGATrGAC~CGnAAGaGC".-~?.
ACUCC~iU
1577 CG~7UA Ci:~~C~~GGCCG~:A AC-CULJGa
1579 G~~GAGU CL~C~UGnG:~CCCAAACz-LC.~_-?~
AUAG~~UU
1583 ~CCAGGC CL'G:~DG AG:~CCGr~G.C-v."CGaA
AGvILJALm
1588 CCC~CUC CUGT,~G:~..~CG~u~AG:~CC~~:?.A
~G?,G
1622 ~C.G CUG~L'GAGGCC"'C~.~A ?.Gw.~UGGG
1628 CCUACCG CG'GFaUGaC:,C " "~C"'.~.
AGCAGGA
1648 CAUUGGG C~QC'~ '~'CC~AAG~C~.~A AGCCCCG
1660 CQG~ CDG:~UC,AGGCC'G~,~~G.~-CC~.~y?,
aGGUCp.G
1663 CACCUGG CUGAUGAGGCC'G~AGGCCGAA AGG1GAG
1664 UCACCUG CUGhUGAG:~ _~~CGAP. p~G:~G?,
1665 ACC'JCCG CffGaC~C~GC,CCC:?.~'~AC,~:~CCG?.A
AAGCGAG
1680 Cr;AC~G cJG.=.UGAG~CC~CGaA AGUCUUC
16 B "UG.~G:~ C'CJGAUGAG~C~~~AC~C.1 CGAA
1 AAGUCUU
16 83 Ar.UG;s? C'JGAI:CCu'lA AC~~7C
G
1686 CG,IaAUG C~1GAUCv'~C,:~ -C'GFanG:,CCG~.A
AC~?C~,
1690 L?GDCC:yC CLJG~UGnG.~~CC~~ACi-~~CGAA
AUG:~GG
17 04 AGCAG? G CLTGAUGAGG~CCG~GGCCGAA AGL7CCAU
17 05 G~...~GA CLTGr,U ' 1 Ap,~C
1707 AAGAGCA CLTGr~ZfGAC~CC'rlr.?.G.a~CGAA
AGA~GVC
1721 CLJG~UCU CC~UGAG ;CCGAAAG: CCG.Ar?
ACUG~.AA
1726 F~GaG~."U COGAUGAG:~C " ""CC~A AUCUG~:C
1731 ACCULTAG CUGAUGAGGCC~-GCCG ;A AGC~TGAU
1734 AG~~CCLT CUGAUGAGGCC ' ' ~ CCAA AG.3AGCU
1754 CUCZ1UG~a CUG?.UGnGGCCGr'aFaAGGCCGr.'~.
'~C,CACUG
CA 02468048 2004-06-07
235
Table 20
Human rel A HH Ribozyme Sequences
nt. Position HH Ribozyme Sequences
1g UacAGA,c cvG~GAGG,.~r-,~AaccccaA AGCCAVv
22 CACOACA C'CGnUGAG~~.C".~AACvCCGAA
AC~.~GCC
26 C~JGCAC C'OGa~rGaGGCCG~.AAGGCCG~r
AG~t;?~CG
~3 GA ~G~ CUGnUGAGGCCCAAAGGCCGAA AGaGL'ZJC
~
94 UGr ,.,. CflGAVGaGGCCGF~1GGCCGaA AACAGOIT
100 "C -~U C'~GAC,GC'C"~AGGCCGAA AC-C.vCGA
103 cc ~c~.,~~ c~GavG:~Gcc~cc~a, AvcaGw
105 'LG'.~C~ v ' " ~ C""VAA AGr'IUGrIG
106 CUGCCG a CUGr~UG~.G~.CGr~AAGGCCGr'1A
AAGr'~.UGA
i 29 GGGGCCA ~ _~ ....CGA?, AGGCC'CG
138 CUCCACA CC7Ga.UC'l.~C~..~'C.'GAAAGGCCGAA
~~~ C
148 GCDG'-,AU CI7GnL'GAGGCCGAAAGuCCG?~A
AVCUCC?~
151 G..~GGCDC ' " " ADGAUCQ
180 GUAGC"'~G C'C~CJ~ffGAGGCCGAAAGGCCGAA
AC~CGCAU
181 C~AGCG CL7GnUGhG~CCG~AAG;GCGAA AAGCGC?a
186 G:ACWG CU~C~~GCCGAAAGGCC~.~A AG.~~uGAA
204 GCCCGCG C'C1GAUG~GGCCGAaAGGCCGAA AG~_GCCC
217 CGCCOG:, CtJ~CGA~J?~~CCGAA AUGCUGC
239 UUG.~~tiGG CL7GA~GGCtGAAAGGCC".~r1
AUCCTGUG
2 62 VGAUCW CL3GAt;~AAAGGCCGAFa AUGGUGG
268 AGCCAUQ CUGAUGAC~.GCCGAAAGGCCGAA AUCUUGA
'
276 UCCLTGLTG CUGADGAC~CCGAF~AG~~CCGaA
AGCCAW
301 CCnGGGA CL3GALIGAGGCCGF3~GwCGAA ?~UGCCC~,
303 C?CCAGG CDG~.L~AGGCCG~AAAC,GCCGAP,
AC~.UGCG
310 CCUUG~"Q CUGABGAGGC ~ ACCACGG
323 CG~"OCAG CUGFsU - ~ CGAA AG:~.,~LTCC
326 GGCCG.~"U CUGAUG~C' CGAA ~' G
335 U ~c~GGGU C9GA ~ ~ CGAA AG ;CCGV
349 UQCCLTlIC CC1GAUGACuCCty~AGGC'CGAA
AGCUCGU
352 CCL1WCC CUGAC~AAAGGCCGA?, ACAAG~~V
375 CUCAUAG CUGAUGAG.~~CCGAAAGGCC'GaA
AGCCAUC
376 CCC1CAUA CUGF.L1GAGGCC'GAAAG:,CCGAA
AAGCCAU
378 AG..~CUCA CfJGAUGF.CGCCGAAAG GC'CGAA
AGAAGCC
391 CCG~GCA CUGAUGAG~CCGAAAG~CCGAA AGCUCAG
4 09 AACUGUG C tTGAUGAG GCCCaAAAGGCCGr~A
AUGCAGC
416 UOCDGGA C'C~GADGAGGCCGAAAGGCCGAA AC'~1GQGG
417 GWCUG~v CUGA AGGCCGAA AACpGUG
418 GGWCUG C'LJGF.UGAG~~CC'~AAAGGCCGAA
AAACVGU
433 CACACUG CiJGAUGAGGCCGAFAC~GCCG?~ AUUCCCA
467 UGaCUGA CUGAL'GA ~ CCGF~A AGCCUGC
469 GCUGACU CUGAUGT..C- GCCCAAAGGCCG~.A
AVAGCCU
473 AUGCGCU CUGAUGAC,GC'C:Cs?.A.AGGCCGAA
ACUGAUP
481 UG~"tJCUG CUGAUGAGGCC~CGAA AUGCGCU
501 AACUtIGG CVGAIJG~GGCC"~AAAGGCCGAA
AGGGv,~UU
CA 02468048 2004-06-07
X36 '
502 G-nC~UG
CUG:~UG~C-:~CCC~-C-:_CC,~r?
aAC~,'U
508 C'cUC~UGnCNCCC;~A.'~G:~CG:
A ACJL7G:~A
509 Ueur~L~IG
CZGAU"uAG:~.C"'CCGAR
AAC'OUGG
512 UccUC'~
c~~~c~~aa.~ccG~A
Ac;GAACtT
51s G,.~UCJUC
c~.lcaccnaAC~ccc~A
A~G~,
534 Chc-.~~LicG
cJGn -
'' ~CccAA
AGUCCCC
556 G:~F~C.~.
C'L~vnLIGF~C:~.CG~AC""CCG?A
AC,CCGCA
561 CACCJG:a CUG ALTGAGGCC"~GG.."CG?~A
aGG'~GAG
562 UCACCUG CCG~UG~G~..~CG?..'~AGGvCGAA
aaGCAGA
585 CCL'GCCQ CtiC~UG~CCGAAAGGCCG.~.A
~"aC
598 ~i Ca~CC~~C~
613 GAC~G CL7Cr.UCaC~C"'~n~C'~."CGAP.
?.C~GGCG
6.6 GnUGAGG CUC~.U~G:tCCGAF~AC-~~CCAA
:'~
617 c: .~UC~.GcL-ca~e~G: cc~raaAGG:.cGaa,
a~ccac~
620 AL'GCUAU CuCAL'C~GG~~'C'G?~.AGG._~CGAA
AGGAAG3
623 '~UGG CUGn ~ -ZT~.G:T."CGAP.AGGCCr'.~A
AUGAC',:,Fa
628 UGUCAAA CUG:~.UGAC~~'CGAAAGGCCG?.A
AL~.;VAU
630 AL'UGJC.A CDG~.BGAGG'~ AGAIR'~.;G
631 GAC~JC'JC CUGAL~GyGG~~C'".~AAF '~
AaGAUGG
638 C-Gc.-GCyCC'C -CADG.F~G:~.CG~r.AAC~GCCGiaA
~aDCGLICA
661 :-c~UCUU CuGnUGACvCCGinA~GGCCG'Aa.
GCLTCGG
667 C'uCC,C-:~.CLIGAUG<aGG._'GAAAGGCCC~'~.P.
AUC'LJ~
6 a 7 GC'L7GC CUGi~LT~.GGC C'G7"LAAGGCCG?.A
CA AGUI7UCG
700 CCCCACC CUG:,L'C;~G:~cC'CAAAGG."CGAA
AG:~CAGC
715 G-~?~G: C~ ' ~ .~DCDCALT
AA
717 C=.GJAGG CLG~U -C~GGCC~~GGCCGAA AGA~C
71 a :~C.~GL~G CUG:sUGAGc.-~~CC'~Fv,Fac~"CGaA
AAGADC(1
721 C?CGC~G CVGAUGAGGCCG~CGAA ?.GCAAGP,
751 Y.C~Cf~C CUCr.L'GI~GGCC'C~FF~GGs.CGnA
AUGUCCU
7 5 9 C GUG~AA C'~'GAUGr~"C ' ~ ACACC'UC
761 CCCGLICA C'UGAIJCAGGCC~.~AAAGGCCGAA
?UAC~1CC
762 UCCCGL'G -C~C~CGAA ?,,F.UACAC
7 6 3 GU C C ' '" ~F.P~G~"CGAA AAAL7ACA
CGV
792 C''.~G C~ZK~~C.:~CCC,AF.AGGCCC~AP.
F,GCCL3CG
795 L2GCGAA CL~C'GAAAGGCCC~.A AGuAGCC
7 9 6 c,,~~Gc cJC,~cc;.~G:~ccGA~~GC,.~ccAA
GA AACua~C
/97 G~J'LG'"G CUGhUC~G,~CVAAAC~CCGAA F
7 9 8 r G~~L'UG.~_C~UG''~G~.;~CGAA AAAAGGA
829 C~nc~.C CUGAUCAGGCCGAAAGGCCGAA AUGGCCA
834 C-.~JCC'G.GCUGAUC:.:~CGCCGF~AAGGCCGAA
AC.ACAAU
n35 G:NJCC'G CLGAUC~-CCGAF.~'~GGCCGAA
AACACAA
845 G~.GJAGG CL'G:~L~AC~GCCw,FLAF.C~CCGAA
~~ ,
a49 GUCUGCG CUGAUGAGGC'CGAAAGGCCGAA
A,GGGAL,G
a72 cc-cacAC cvc,~UC~G:,cccaa~GGcccaa.
AG;.cvcC
sa3 c,:~UC-:~acuc;..UCaGCcccaaacGCcc~.
AcACCC~
ass c~G~UG cL~:.,L-cr.GVC.cG~G~ccAA
AcAC~cG
905 C 1JCG.;~ CVGnUGr.GGCCGF~AAG:~CGr~?.
AGGCCGC
906 CCCv"UCG CUGr~.UGAGGVCG'~FaAGGCCGAA
AAGGCCG
919 G~~UCACU CUCaUGAGGCCCAAP1GCCGAA AGCLJCCC
CA 02468048 2004-06-07
237
936 cJACUGu
CuGaUGaGucc~~C~~
aUUCC_aU
937 G.~"'~.RCUGCUG~UGAG~C~~AC~CG~ iaL'CTCC.3
042 UG~u~GG CCiGAC~~CvCC~,.~?a ACCG~s?~
'
~
053 UCG'JCUG CC~JGr',G:,w~CC'~F~C, sCCC.?aF~
ABCCGC-C
062 C"w"~?GaC :.UGnUGAC Gc~~~F~AC"~s.~.CG'~.A
aLiCGUCLT
065 aUCCCVU ' .-C~' .CC~a aCCaUCG
973 UCtJCCQC C9GAUGAwCC~.~Al'~AC,~C~a?.~1
AUCCG.~~-J
986 GJCCUW CUG~UGaG~CGAAAG.;CC~v:?~,
?.CGiJULJC
6 G,~~cvcA CvGa~c~GCa~a~ac~cccaa~ aL-~oC~J
005 Gv.'"UCWG CDG~LJGhG: CC~' ~CC~,.-.aA
:,G,.;JC'JC
1006 UGv.~uCCTf3COGADGaG~~CGAAAGGVCG~A :~T~G.,uCJ
1015 UCWCaU CuGAUG~~C~,iaP.3'~.~cGra
aL'G..~UCV
1028 ccc:~aG cJC-~UG;~cC~ac~~ -tea ~c~c~rvc
1031 CCGCUGA CUG:~UGaGGCC'G~AAG aCC"._~.A
AC-.~-~C'~?C
1032 UCCGCUG CUGAUG~GG: C~~aAAG~CGaA
AaC,GaCV
1033 GUCCGv.~U CUGaUGAGGCC~- ~ ~G?1r1 'r1?.?G
GaC
1 058 C~~GGJG CUGnLIGaG~~~~~~GnAAC- vCCG~A
r~~~CG;
1064 AUGCGUC CUGAUG;GGCCGAAaGCICG~ .~G.,~L'GGr1
1072 GG~CAGC CQGaUGAGGC~CG?~ AUG.~GUC
1082 CC1GCGGG CL1GAUGhG~CG~FsAGGCCG?A
aG~,~,Ca
1083 Cn~UGCGG CU -C'r.IlGnC- G: CGr'1A'nCZ,CC.~-~'.A
'.~aC~~sCrlC
1092 AC, ~AGC'UCUGAUGAG~CGAAAGGCCG1~ :~G..~UG'G
1097 "G.~ACAG CUG~UG~C:~CCGAFJ;GGCC~.:AA
?.G..~L'G ;G
1098 G:~GaCA CvGA ' ~ CC~ :~aGw'ZC~
1102 G. ~~ CUC,~UCAGGCCG' : ~ CG?.A
?,C_~GaAG
1125 AF.AC,vGa CQGAUGr'~.GGCCGr " C~.-'fir
~~"L'G
1 ~'1 WJA~ CUGF~UGAGu'CGAAAGGCC~~ AL2.G~~
1131 L'GACGQA CUGAUGnG.~~CCG~AAGGCC"aA
~G~GaUp,
1132 AUGACGU CUGAI1GAGGCC~..nAF~GGCCG?~a
:,?, ~G~.~-~IJ
1133 GAt'~GACG CflGAUGaGGCCCGAA ".~AG:,GA
1137 CAGGGaU CUGaUGAG GCC ' ~~CGAFa ACC,'Vr~1
1140 CCZJCAGG CtyGAUG?~GGCCG~P~~AGGCC".~AA
aUCACGU
1153 Cr.T~GULJ C1TGAUGAGG..~'C'GAAAG~~~~CGaA
i~L'Cw~UGv
1158 CUCAUCA CDGAUGAGC~C -CAAT~GCZCCAA
aG'JUGAU
1167 G.~''LlG::sACLTGAUGAG.~~.:.CG'~F.AGGCC~.:.aA
AC'CJG'~UC
1168 UG.~"UG:~ CUGAL1GF~G:,CC'G~AF.F.GGCCG?~
; ;,r~,CUCALi
1169 AUGGUGG CUGAUGT~C~CCCa~AaGGCCG?~r
iz~.ACL1CA
1182 FGJ~F:GGA CUC~UG~GGCCGT~AAGGCCC?~A
ACACCAV
1183 GG~nGG CUGasUGAGGCCG'~aAGGCCGAA
?.AG~CCs~
184 CCAC:iAG C'UGaUG~G.GCCGAAAGGCCGAA
AF.aC aCC
1187 UGCCCAG CQGAUGAGGCCGAAAGC~CC~.~A
AG:~P,AC
1188 CUGCCCA C~UGAGG:.~CG?~, aAGGAAA
1198 CCUGG'"Q C'CGaLIGAGGCCGAAAGGCCGAA
AUCUGCC
1209 CFaAGG.~C CL1GAUGr'~C~,.~~CGr'~AAGGCC~.anA
ACvCCUG
1215 CC~vGGCC CUGF~UGAC,GCCGr'1AAC,CaCC~.,:r~'r_
~C~~,~~CGa
1229 ACWG:r; CUGAUGhGG.~.C~CGAA ~'~~~C
1237 ~G.~c.~G CDCAUCnGGC -CC~AAGGCCG~
ACZJUGGv,
1250 G~.~~UG CUGUGAG~:~CGAAAGGCC -C~:,
:~G.~CUG~
1268 AUGGCUG C'QGAUGnGGCCCia~'~AG;CCGn?a
GC,C,
CA 02468048 2004-06-07
238
X79 -G'~G..~GGAC~UGAC~~'C'CAAAG~CGAA ACCAIJGG
1281 CAGaG..~0 C~~~'C.'C~F~AG~CGaA ADACC.AIT
1286 CG~~CA CL7GABG:~CCC~AAC~CCG?~A
h~GC~1
13 09 G,~-.ACGGGCQCADGnG~.~CGAAAGC~:C'CAA
ACAG:~ ;G
1315 G~~G'PG CQC,~.~DGAC,GCCGAAAGGCCGAA
ACtJGGG?r
1318 CL7G:~Gw CL1GA0 ~ ~ AGGACUG
13 31 GCCUt's CQGF Z~l~sAG~J
:G
~~34 AGyGCCU C'~T1G~~r:C~AAG.GCCG?.A
.ACG1G:~G
13 89 GGCCQCO C~LGF.GG~ '" .~A AC~GCG'J
1413 ABCAI7CA ~ .AAA ACQG~G
1414 CADCADC ' ~ " ~ AAC"LJGC~.
1437 G~~C~.AGC CQGADGAGG~~t"GAAAGG~CGA?.
aGGCCCC
144' UG~?L'GCC C~GnG~."CCaAAG:~"CGr~r
1 AC-Ci~ACv
14 67 GJCLTCL~G CL1C~.QGAGvCCGaAAG.~~~.
C~.ar'1A ACACAGC
1468 G.~'"tlCGC~CUGADGAGG~~C''~?.T~.AGG
CCC~ ~ACAC.J~G
1482 GUCGACG '' ~ " AUGCCAG
1486 AGLIUGUC C~AUG~~~tlt~AAAG~."CGAA
ACGGAI~G
1494 AAACQCG ~sAC~~'"'CXy9AAGCCCGAA
AGL70GUC
1500 CDG'."QGA CDG AiIG~GCCGAAAGGCCGAA
ACUCG~~,
1501 GCUGCOG '' '' ~ AACUCGG
1502 AGC"UG~."UCLJGUG~GG~t~rGAAAG~:~CC~.~A
AAACUCG
1525 CC~CAGG Cl7Gn3JGAGV~CC'GF.AAG:yCCGaA
AUGCCCLT
1566 CUCAG:~G C0'GAUGAGGCC'GAP.AGC,CCGaA
ACUCCAD
1577 CGA~ CO~U'GAGGw~~..~LGAA AGCCQC~r
1579 GC.~"CAGO CDC,ADGAG~~C''.~AAAGGCCGaA
ALmGCCU
1583 ACL7AGGC CUGADGF~GG~~C.'C~AAGGCC~.~,
~'.G~AITA
1586 CUGUC~.C CI?GACGF~nAGGCCGAA h~GCGAG
1 622 G:zaGCAG CC1GAUGAG~.~CGAAAGGCCGAA
AGCLJGGG
1628 CCCAGUG CQGAtTGAGGC'CG~.AACGCCGr~a
AGCAC,GA
1648 CA~GGG C'OGAI1GAC~C~"CGAF, AGCCCCG
16 6 CLTGAAAG CUGADGAGGCCGAAAGGCC'GAA
0 AGGCCALJ
16 63 CDCCC1C,A CDGnUG:, ~ GAA r3~CC~GC,C
1664 UCUCCUG G~ AA~,G
16 65 AUCQCCU CDGAL1GAGGCCC~AAAGGCCGAA
AA~'~GGAG
1680 GGF~CuAG COGAQGF,GuCCGAAAGGCC'~.A
AGUCtIOC
16 81 ~AG:~A CQGAUGAO;C.C ~'G~AF,AGGCCCAFr
AAGDCUO
1683 AAUGGAG CLIGr.UGAGGCCGT,AAGGCCGAA
AGAAGLTC
1686 CGCAAUG CVGAUGAGGCCGaF~AAGGCCGAp,
~:GGAGAA
1690 UGUCCGC CDGADGAG~:.CGAAAGuCCGaA
ADGGAGG
1704 ~ CUGAUC~C,GCC'G~AAGGCCCAA
AGUCCAU
1705 ~G.~ CCVGACUGALC'CO~AAGG~CCGAA AAGUCCA
17 07 CAC~CU CUGAL7GAGGCCGAAAGGCCGAp.
A,GAAG~
1?21 CUGAUCU CLTGF, ~ ~ CGAA ACUCAGC
17 2 AGGAG."U C'UGAUGAGGCC'G;F~JaAGGCCGAA
6 AUCUGAC
? 731 CCCLTtIAG C'cJGAVGhC-GCCGAAAGGCCGAA
AGw'ZTGAU
1734 ACCCCCU CUGAU-CAGuCCGAAAGGCCCAA
,~.C-;AGCV
754 CLTCUG:,G CUGAVG.AC~CC'G:A3',AGC,CCGAA
AG ;C,C~G
CA 02468048 2004-06-07
239
U ~ ~ ~ ~ U U
a ~ a
U ~ ~ ~ ~ ~ ~ ~ ~ ~ U
~C W C V ~C ~ V
d a Ac a a a a .s a ~t .s a ~t ~t a
U U U U U U U V
.C 'C sC a d it iC ~C a sC iC iC iC ~C iC
a 5' ~ ~ ~ ~ '~ '5 5~ ~5 '5 a ~ a ~
c~5c~uc~c~u5555c~c~c~5
a a d a a a a d a a a a a ,~ a
U U U
~c'~c'~t"~c'~~c~c~c~~c~taJc~c'~c~
~~5~5~~~~~~~5~~
0 ac'~~ c~c~
a~-n c~c~c~~~c~~c~c~~c~c~c~c~t~
ACC UUC~c~c~~c~c~c~ c~
m c ~ a a ~ a a ~ ~ ~ a ~ a a ~c a
,.
U U U C~ C9 5 ~ C~ ~ C'J
~ CU7 C~7 ~
0
~c~'~~' ~'~U5
'a a c~ U
z
ec
-o
N a :~ ~ ~ l~ tf7
C o tn ~ ~ o ~ O
N ~ ~'~ f~1
v .-t
tn
l~~
w
f--~
c
CA 02468048 2004-06-07
240
C9 ' U_
a
S~aU
C7 ~ ~ C~ ~ U ~ ~ ~ ~ ~ ~ U U
U ~ U U U U U U V ~
~c~'~c~c~c~c~~'~c~
(j ~ U C9 ~ V
st ~ ~ a ~ ~ C9
~ a a ~ ~
U ~ U U U U U U ~ U ~ ~ ~ U U U ~ U
V ~ a a U U
a~~c~~~t~ c~3
~~~~~~~~~~~~~~c~~~~c~
cna~ c~c~~5c~5u5c~5555c~55c~c~~
C a a a a a a a a a a a a ~ a ~ a ~ ~ a
c9.~ ~~.U~~. ~C~j,7~~ ~ ~~ UUU
~ U C~
N a a a a a a a a a a a a a a a a a a a
U U U V U
U
~~~c~~5u~~'~a~ ~J~a'~~a ~a
c~ c~ c~
Z
_.
N ~ -O o W .-i u1 .-i o~ e~ o~ er rt a~
N v) c- r-~ r, vo r-, vo c ,-~ o o vo co ~-, c o o N r~ u~
r-1 N fh P'1 ~G ~ CO D "'~ N r"f f'~1 C' aT C7 O ri N f''1
.-~ r-i ra .-i ri r-1 N N N N
O
CA 02468048 2004-06-07
291
Table ~3: Huma.n TI3F-cc HH Ribozyme Target Sequence
nt. ~ Target Sequenc. at. 8H Target Sequence
Position Position
28 C,:~CACv"V U C9C~CC
29 C~?G.~'W C UCiJLVCC~321 G'JC~1U C AL'CL'UCU
31 AG.~JL'C~J C ~DCCL1CU324 AGr~UC.''~U C L'C;CCr'C.~,?r
33 CWCtICCI U CCUCUCA 326 AUCAUCp U W
34 UQC;~2W C C'UCQGAC 327 UC_~,UCL'U C UC~.~,CC
37 L'CGUCCQ C UCACAUA 329 AUCL'UCU C G:~CCCC
39 UUCCUCV C ACAUACU 352 ~:G~."CUGO A GCC--,~,UG
44 CQCACAU A CUGACCC 3 61 CCC.T~UGU U GL7AGCAA
58 CACG:,CQ C CaCCCVC 3 64 aUGL~GU A C
65 CCACCCU C UCUCCCC 374 ?~CCCU C :~AGC'fJGA
67 ACCCQCU C UCCCCaG 391 C~G~.~p C C.~C
69 CCL2'UW C CCC~A 421 ALGCCCU C CpG~CCA
106 GC_~UGAU C C ~GwACG449
136 AG:~w~LJ C C~ ' 468 GUGC CAU C .~~G~G:,~
- '
165 CAG~:~."U C C.~C~s 480 G"CCUGU ,~ CCT3C'-RUC
177 CG.~''UGw."Q U G'JUCCUC4 84 UGQACCV C AUCZJAC'(T
180 UGCUGGV U CCUCAG~. 487 ACCUCAU C UACUCCC
181 G..~L'LGJU C CUC?.t',CC489 CGCa.UCU A CUCCCAG
18'4 UGWCCQ C AG~.COCU 492 AL'CC~CU C CCAC~C
190 UCAGCCU C UUCUCCU 499 CCCT,G""U C CQCUtJCA
192 AGCCUCU U CUCCWC 502 ;,G"~Z,CW C W~~
193 GCCQCW C UCCWCC 504 GLiCCUCU U CAnCGGC
1 c5 ~ C 505 UCCUCW C F.AG~GCC
-
198 UUCUCCU U CCL'GhUC 525 UGCCCCU C CACCCAU
199 L'CVCCW C CQGADCG 538 AUGUGCU C CUCACCC
205 UCCUCAU C ~ 541 UGC'QCCU C ACCCACA
225 CCACG."U C v?UC'UGCC553 ACACCAU C AGCCGCA
228 ACGCt7CU U CU"v..~CUG562 ~ GCCG:AU C GCCGUCU
229 CGCUC'UL7 C UGCCQGC568 UCGC'CGU C UCCUACC
243 CL1G~PCU U UCGAGUG 570 GC'CGVCU C CUACCAG
244 UGC~CW U GGAGLIGrI 573 GUCUCCU A CCAGACC
253 --CAGLJGAU C GGCCCCC586 CCAAG"~U C AACCUCC
273 G:,AGAGU C CCCCAGG 592 UCp~W C Cp
286 G~ACCU C UCUCQAp, 595 ACCUCCU C UCUC-CCA
2 88 G'~CCUCTJ C UCVAAUC597 C~,'CCUCU C UGCCAUC
290 CCUCUCU C UAAUCAG 604 CCGCCAU C F.AC.AGCC
292 UCUCUCU A AUCAGCC 657 CCCUCu,.U A UGAGCCC
295 CUCUAAU C AGCCCUC 667 AGCCCAU C U''...UCL1GG
302 ~:~C-CCCU C UGGCCCA669 CCCAUCU A UCL, ~~p~
CA 02468048 2004-06-07
242
671 C~UCUAU C UG.:,-~.GG 960 UC-.~.~UU C ~ LG
682 GJ C DUCCT.~GC 1001 AACC_AC'J ,AAGAAUUC
684 G:~~UCU U CCAG.."L1G 1007 L~'r~G~,AUU CanACUG
685 G:~.~"'JCJL7 C CAGC'uGG1008 ~,GAAW C ,
? AC.. " C AGCG...~UG 1 021 G:~C-GCC'JC CMG
09 AACt1
721 cv-r~A~ C AAUCC~C ~ a29 cAe.AACU c a ~~
725 CABCAAU C G:~CCCGA 1040 GGC,:,CCU A C?,C~,~UGLT
7 CCCGACU A UCL1C".~AC 1 04 6 LTACAGCU U UG?~UC.~.C
3
737 CC~ACVAU C UC'"~:ACL~J1047 ACaG~"UU U CAUCCCU
739 ACUAUCLJ C GACWUG 1051 CL'LZ1GAU C CCUGAC_~.
7 CUC""uACLT U UGL"C"..=AG? 0 6 C'JGACAU C L'G~?.AL'C
4 0
4
745 UCC,AC.W U G~~CGAGU 1067 C'JC-::AAUC U ~
C
7 GCCG? G'J C UG:~:~.AG10 8 5 G:~GC C(? U L-C.,G-,JUCU
53
7 C~,CAG.~~U C L1ACUULTG2 0 8 GAGCCUU U G.;
63 6 JUCUG
765 CAG.~~UCU A CUUUGGG 1090 CUUZJC'",~UU CL'GGCCA
7 GUCQACU U UG~~v~AUC 1091 WUCv'W C UGC,CCaG
68
7 UCCIACZJU U G;~AUGA L? 3 CAGGACU U C
69
775 WG;~:~U C AUOG'~CC 1124 AACACCV C aC~
778 GGADCAU U GCCC~GU ~~29 CUCACCU A GAAA~
8 CCAACAU C CAACCUU 113 5 ~CAAAU U CAGACAA
O1
808 CCAACCU U CCCAAAC 1151 UC-:=ACCU U rGCICUU
809 CAACCUU C CCAAACG ~ ~ 52 G~~CC',7U ,~,GGCCUUC
820 AACGCC'0 C CCCDGCC 1.158 UAG~,CC'(7U CCUC~,-W
833 CCCCAAU C CC~.D~ ~? 59 AGGCCW C CUCUCUC
837 AAUCC'CU O UAIJQACC 1162 CCL'~CC'J C UCUCCAG
E38 ADCCCQLJ U AU~CCC ? 1 64 L'UCCVCV C L;CCAGAU
839 UCCC'UUU A ULTFrCCCC 1166 CCVCUCU C CAC;aUG'J
841 CCUUL1P.U U ACCCCCt7 1174 C:ACAUG'J U UCCA
GA
C
842 CUULIAUU A CCCCCUC 1175 . aG?~L'GUU U C~yGACU
849 ACCCC'CU C C'QOCAGA 1176 G~:,UGUUU C CAGACUU
852 CCCUCCU U CF.GACAC 1183 CCACACU U CCUUGp~
E53 CCUCCULT C AGACACC 1184 C
863 ACAC'CCU C AACCVCL7 1187 ~ ACVOCCU U G~CAC
869 UCAACCU C WC'DGGC 2208 CACiCCU C CCCAUGG
8?1 AACCUCU U CUG,~,,~UC 1224 C-:.C~.G..~~7C CC'UCL~1U
872 ACCUCW C BGG.'~Ca X28 GCtICCCU C UAUUUAU
878 UCL'C;~Cfl C AAAAAGA X30 LCCC'QCU A UUUAUGZT
8 AGAC~AAU U G~,:~.~U 12 3 2 CCUCUAU U L'AUGUUU
9
0
898 G.~~~"" ;CQ U AGC~CG 1233 CLCVAUU U AUGUUUG
899 GGG; ~~UU A G:~,~JCGG1234 UCUABUU A UGUWGC
9 tJ C CGAACCC 12 3 8 UUUAL'GU U UG:.ACUU
04
917 C'C's U AGAACUU X39 ~,U~ U CMG
918 CAAGCW A G,AACUUt7 ~ 45 UUGG'~CU U G'JCAUUA
9 LTAGAACU U UAAGCAA ~ 51 ~7UG'LG U i LWr~W
2 V
4
9 AGAACUU U AAC-CAAC 12 52 L~GUG~L'U A L'L'tThUUa
2
5
926 GAACL7UU A ~.GCAaCA 1254 UCAUUAU U L~.L'tJAUU
945 CACCACU U CGA~',ACC 1255 C;AL'UAL'UU rJUAUUU
9 ACCACW C GAAACCV 12 5 6 i WAUW A L'UAUUUA
4
6
9 CUC~AU U CAGGAAU ~ 5 8 UAU~, U U ;~~,,~W
5
9
CA 02468048 2004-06-07
243
12 ~ A UUL~LW 14 4 0 uG7L'UL'U U 'LUAU
9
61 ~U U tTAUL'@.U 14 41 G,'OUUUU A ?,A~UAUU
X62 ~'L'~ U ADaLmL"Q 1446 "U A DG~UCL~
12 ~UW A L'~I7~1 14 4 8 "LUAU U p,G'CuC,~,U
63
L~U U UAUtIF,Dp 1449 .~L~W 1U A~ UC~UU
12 AULTD~,L1U U ?.UiRDLIU14 51 :yLT~.L'UAU C BG.~.IJL~A
6
o
67 UO~.D~ A UUAU~ 14 5 6 AUCL'G?~T U U
X69 LRUtIt~.D U AU~J~CT 1457 UcJC.~.vQ a ?.~,wct;C
27 a~ A vuuA~v 14 61 a~-~~J a ~-~cr..,aa
o
X72 UUAUL~D D i~DtJGaU 1464 :~GLJ~,'GU C L~?.CAA
1273 UAU~sUO U ADGLTAUU ? 466 GL'L'GL'CV r7 ,~C'-,,RUC,
X74 ?UUAUCIJ .~ UULRL~Z,T1479 CG.."L~G1U U LT~.,T~,C
i27 I~U~.U U UAULZTAC 14 8 0 Ci JG',L'U U ~..~,CC
6
X77 AU~W U AUOL1ACA 1494 Cr.?CLiGU C i~CLiC~UU
~7 L~RWU A ULJiIAC'aG 14 9 8 L~C~C'J C ~"'(~
8
8 0 LTAL'QiIAO U UACAGaU 15 Ol C~.C UCAU U G~"GGaG,~,
1281 f~~UU U ACAGaUG 1512 C~G,~C~J C L'G..~~CCC
X82 UCD7~DL~ A C~G~ 1517 CUCflGCU C CCCAG~;,
1294 UG?.~~ A U~F.UQO 1528 ~~ U ~,
1296 AAUG~U U UAUQCGG 1533 GJL'GLtGU C UGt~,UC
1.297 :~UGJ'r~J U AUUL'G~, S37 vwcLTcJ a avcC.;~CC
x..298UG'~O A UWG:~:~.1 1540 CLGiIaAU C C-C-:.WsIC
1300 TRUQLJAU U 1546 UCH.-CCU A CUALZ1C~
1301 ADU~DC U GG~C~C ~ ?549 GCCL1ACU A WCAGL,'G
1315 CCCv:~,"C A UC ~~ 1551 CUACL1AU U C'~GvG:aC
317 G~:~.~"L7AU C ~~''~ 15 52 VaCCAW C ?.GL't-:~.G
3 3 CC~L'GU A G:~GCDG 15 6 6 GaGaAAU A ~C-.~
4 ~JC,~
3 4 GC"CGC ~ J U C~GCQCAG157 2 L7~.rl.G~--U U
5 GC'L-Qp W
3 5 CUL1G~.."Q C AC~AL'G 157 6 G.~~-vUG~~U U aG~.AAG
0
359 GACAI3GD U LZJCC~~G 1577 GJUG~~,7L1 a ~C,~r,p,Gp,
1360 ACAUGUU U UCCC~GA
1361 CAUGDOU U CC'G~1?~
13 ~W C CGtIGAAA
62
1386 GAAC~AU A GC~p
1 ? F~C~ U CCCJIDaJ
93
1394 GC,~'"t.iC~LJ C CCAU
.GTA
.401 CCCAL'GU A G:.CCCCV
1414 CUGw'"CQ C UGUGCCV
.422 LGIJGC'Cp U CWtniGa
1423 6JGCCW C LIUiJLJGAU
1425 GCCt~UCU U WGAUL~
1426 . CCUt7CW U UGAUI~1U
1427 CWCZJ~1Q U C,aUUALTG
1431 UQUUG~U U AUGUUUU .
.432 UWCAW A UGUUUW
14 AUUAUGV .U L~r~.
3
6
.437 UUAUGUU U L;~JtJAAAA
1438 UAUGL'L~J U CTtTAAAAU
CA 02468048 2004-06-07
244
Table 24: Human TNF-cc Hammerhead Ribozyme Sequences
nt. 3H Ribozyme SeQueaoe
Position
28 G~.G?.G CUG~UG?.GGCC~CG?~A ACCLIGw"'C
29 nGC~.ACA CUG yUCaGC~CCGAAr.GGCCGAA
AACCUC'aC
31 __-C~G;:~ACL'GyL'G~,GGCCG~F~GGCCG~A
AGaAGCG
33 UGAGAG3 C'LT"~~ -' GGCCGA~AGGCC
GAA .~~GAG~AC
34 GUG.nGAG CC;C,?UG.GGCCGAAAG:~CCGAA
~AGaGAA
37 ~JGA CL'GAL~'~GGCCGAA1G:~CC~~A
AC,G AAGA
39 AGwTA~IGQ CUGA -UCnGGCCGAAAGGCC~.~A
AGAG~~,v~A
44 GG~"DCAG CGGAUGAGGCC'GAAP.GuCCGAA
AUC~G
58 GnGC,uzrG CUG~!~IJGaC~CCGAAAGG."C'C~A
AGC'CC~G
65 C~~~' A -CGC~CCGAF.F.GGCCGAA AG:~.~~UG~
67 G~G:~:1~ CUGABG~"CGAAAGGCCGAA AGAGS,G~
69 UC: ~GC~G CUGAU~~.G~G~C~CCGAA AC,F,GAGG
106 C~JCCCG CL'~~UGAGGCCGAAF.C'~:.C.~-~A
AUG:UGC
13 6 UC~JL7GC~,CL'GAUGaCCGCCGAAnGGCCGAA
AGE-"GCCLT
165 CC"~CCUG CUGn "~GG.~CC~Ar~fC~CCGAA
AGCCCL7G
177 C~G~AC CDGAiIGaG.~CC"'~~C'.~CCGaA
AG'ACCG
1 ao cc~lc~ caG:~.UGAG;,cc~CcGAA ACAAGCa
181 G:~~G CUGAL~,GGCCGAAnGC,~."CGaA
AACAAGC
184 rG.~.G~."UCL'G:~UGAG~C ~ '' -- CGAA
A "G.nACA
190 nGC~GAA CUC~UGACG~.~CGAAnC~CCGAA
AG:~~OGA
192 G~G~'~G CUGAUGAG;CC~.~AAAG.~CCCAA
A~GAGGCU
193 G~G::P. CUGA ~ ~CCGAAAGGCCG?.A
AACAGGC
195 G~-GAAG CUGACGAAi~GG: CGAA AGAAGAG
198 GAI1CAC.G CUGnLTGAG~CCGAAi~G~CCCAA
AGGAGAA
199 C-.~UCAG CUGAUGT~C,GCC~~A:~~GGCCGAA
AAGGAGA
2 OS CUGC CAC CBGI-.LIGGC,C CGF~aAGGC
CCAA AUCAGGA
226 C-:~CAG'1AC'UCAUGAG: CCGAAAG~~CCGAA
AGCGQGG
228 Cry C'JGT,UGAC-:~CCGAAAG.~CCGAA
AGAG~GQ
229 G~.C~ CUGAUC~.G:~CCGr"aF,AGGCCCArI
F.AGAGCG
243 GEC-~1CCA CL~G~LfiAG~C -G'~C.GCCG~.
AGUGCAG
244 UC:-.CUCC C'uGAL~Gr-''.G~~CCGF.~~aAG~CCGAA
AAL~'UGCA
253 C-;~:.-;~cCCQGhUC~C-:,CCGAAAC-GCCGAA
AUCACOC
273 CC~G C 1~C~CG~.A ACL1CWC
286 U'JACy,GA CUGA -L1CAGC,CCGAAAGC,cCGAA
AGGUCCC
288 CnUUAGA C'.1GT,UC'J,G;CCCr'vSAC,vCCG~~,
AGAGa"UC
290 CLTG-'-.UCTACUGF~UGAG:~CC "~G,~CCG?~
AC~GF,GG
292 Cz-,.~UGAUC'UGUG~C.~CCuAAAGGCC -CA.3.
rIG.G~GA
295 -CnGC-C~LJCUGA -UCi.GC,cCG~GC,CCG
aA AUUAGAG
302 UGC~C-CCA CUGAUG-'~GGCC'GAAAGGCCGAA
AGGGCUG
CA 02468048 2004-06-07
245
321 ' -nCnAG?~U CU -CAUGAC~CCGAAAGGCC~.~'ie1
AUCDGaC
324 UCGAC v~ CLiC~UGAC~: ~'~G:~CG1~ P.DC,A
326 c,~%c,~-.~.~ cve~~=.c-;~ccGaaAG~ccc~A
A~:~aUraU
327 G.~"UOCGe'~ CQGA~CC~~rCG'~A AACAUGe'~
329 C-uw"WC CUGr'1C.~."'.:~ACWavC~.,a?~
U
352 C:~IJ ~G:~:,C CL1GAUGAGG..~CGAAAGGCC:~1
AC~W."U
3 61 UQG.."LTAC C~L'GaGw"C'"~s~AAGG."C"~A
AC?LlGGv~
364 G.~~UUL1GC CGG?aLKa~G:sCC~alaA~~.CCv1'~
ACAAC?iU
374 UC~~UU CnGaC~.~AGtIC~.~ AG.~JL~J
3 g G~~C.~CDG C'~AG~..~C".~AAGGCCGAa ?.C-..'"UGCC
~
423 L'C~CCAG CT.1G~AaAG~CCG~ A ~G. ~~.~LT
449 ~~G.."L'G,.'~ CL~i~CvCC~.~?~AG'~.C.~.-~~1
AUCZTCC~C
4 6 G:. C CL'CU CUG~.L'G?.G~~CCAAAG~C
8 ~""~ AUC~C
4 so c~UC~acG c~c.~~aGuccG~AG'-cccy,A c
484 i~GUAG?~U CDG?.L~a~lGw"CGC~.~a?I a
4 87 CZ-::AG9A CUGAUG?~G':-v.'"CG?~~.~.CG~
ABGAGGQ
4 a9 cu~:~~G cvcAt~GAGC,CC~~a~AGucc~..-.a.a
Aca~AG
492 C~CCUGG CGGAUGAGGCCGAAA~C.~~ AGi~U
4 og UCAAC.AG C'.lGaDGAGGC'C~,.~?.AAG~~~~C",~rl
ACC~GC-:~
502 CC'UL'Cr-'~A C'CGAI1GAGGCC"~' At~CCGr~r1
saGCACCfJ
504 C-CCCLJUG C'CG?.UGr'~G~~CCGaAACw~"'C"~.r'1
AGAG~AC
505 CvCCCW CLJG?~LCGAAAGu..~CG?.A ~
525 r'~L7C~.~"UG CL1GCCGAr~A~CCG?~ .~G~~~vC.~
538 G:~~7GAG Cff~GAA,A~CC.~-~A AC,C~CAU
541 U~Gw'"t1 CL1GAL'GAGGCC"~AFa~CC".~~.1
AGGaGCA
553 UGC"u'"D CUGnUGAGGL"CGFCC~~ AUGv~Cs~J
562 ?.GACGGC CUCrL'C?d> >CCGAru~GGCCGAA
AUG."GCC
568 C~JAG.~-A CGC=AUGAG~CC' -Cue' AAG.~~~~C~w~.
ACGw~,w~G~a
570 C:.G~JAG CU~CGAAAGGCC~.~r aG:~GGC
573 C-.~CL1GG CVCAUGAC.vCCGAA,AGGCCG?.a
1?~GGACv~.C
8 C~F~G.~"'UtJ CUGADGP.GGCCGAAAG~C"~ps~.
6 ACCWG:
592 CnGAGAG CUGACCGAAAGuCC~~l~a AG~~flL7GA
595 il~C~:~GAGA ~'GP.C~G:~CCGAAA~CC~~~1
AGGAG.~~7
7 _. ,... ~CGAAAGGCC~.~A AGAGGAG
604 G:~~UCW CQ -G'.UGAC,GCCGAA.~GC,CC~~A
ADG~~AG
657 G:~"UCA CUGAUGaG~C'C'GAAAGGCCGaA ACCAGG
667 CC~VA CUGAUGA~G:~CCGaAAGGCCGP..'~
ALJC,GCCLT
669 UCCCAGA C'v'GAL1GACC,CCGAAAGW."C'GAA
AGAL1G~~,
671 ccvcccA cuc~UC~~c~~AAAC~-y:.ccaa AvAGAUG
ss2 G'rv~caA cvc~r~AC,~caccaA AccccUc
684 CAGCLJGG CUGAL7GAC~CCGAAAGGCCGAA AGACCCC
685 CC~~UG ~ GCCGAA AAGACCC
709 CP.GCGw"0 CLIGw~IJGAGGCCGAAAGGCCG?~A
AGUCGGU
721 ~ccc,~tro ccr<~c~aAAC~ccaa A~cvcAG
725 UC'.~:~CC C('G~.UGAC,GCCGAAAGGCCG?~
AUL1GAUC
735 6JCGAGA CUG-'~UGAGGCCC.-'-~GGCCCAA
?.GUCGGG
737 :~F.GiICGA CLGAL'G~CGG~ACvCCGAF, AUAGUCG
739 C.~G'JC CUG~.UGF.GGCCCJ~J~AG~CCCv'~a
~?~LTAGU
744 CVCC~1 CGGAUGAGGCCGT~fi~rGGCCGP.?~
AGUCGIAG
CA 02468048 2004-06-07
246
745 nCuCG:~C CCGr..Ur~GGCC~'w~rra' "G~w~C~.~ar1
'~,.p.GJCG?.
;53 CLi~CCCA CG 'G~'G~~~C~'~'"G~u~~C~~r~
ACLCGSaC
763 C CUGnDGGC~CC~CC -C~ .~CCCGCC
65 CC~~G CL~F~UGAGGCCGF~r.Ci-~~C~.~1A
AGACCJG
r 68 GnUCCCzI C~JGADC'~A~GGCCG~. AG'JAGAC
769 UG?.IJCCC CDGAI~~GGCCr~F~AGGC.~_G~
' ,,
775 C~~~~' CUGr'a~aF~CCGnr'~~ AUCC.~r'~A
aU
778 C C~~~ ~UCC
801 "~"WG CtTG:~ ~ '"CC~.~ AL~WCG
808 GWDGC~C"s C.'I7C~CG?.nACvCC".,?.?i
~Cv~JLJG~
8 09 C~~JQGGG '' ~ "~,CC~,y~ ~,?.G.~"UL'G
E20 G:.-~.G:u CUCAC~i~T~C~.,CC"'.~?. ?~G~GJU
833 AL'AAAGG CLT3A ""CG?~. ?rL'C~ GGG
837 C-;~JAAUA CUGAL~GGC C'GCC?~r1 AC-C~UCJ
838 G.:~"DAAD CQGF~I~F~GGCCGAAAGGC~ ?.~.G.~~
G?,U
839 G:~;~"CTAAC'CfC~LIGAG.GCCC,~F.F.G:~...~C'".-~
. ..
841 :.G~"U CUO;ADGAGGCCG~AGGCC~.~ ~RAAGG
842 CAC C~I~AGuCC~~AAAC',GCCGAA
paUAAAG
849 UCL1GF~AG " C"".~1 ?~GC~
852 G1GL7CGG CUGADGAC~CCGAT~F~GGCCGAa.
~?~C~..nG~G,
853 C~,JGUCR CDC~L~C-:~CC 'GAn~~,CCG?~r~r
n?G::AGv
863 F.GF~JU CL1GAUG~C-GCCCA~C:~CCG~.?.
a~~UGLT
869 G~.~'".J~AACUG:~ ~ ~ =~C-~3G~
"071 C~C~C'"'.J~GCGGAI7""uAGGCCGAFaF~C~,CCCsrl~.
A,G?.G.~'W
872 UGAGCC:A CtIGADGAGGCCCAFu~GGCC"..:A?,
a
878 UCUL'WU CUGAI~GGCCGARAG:,CC~~.a.
.~C.~""~AGA
890 FGCCCCC CUCriU~~CCGF,AAC,:~CCGa?r
?~'v'tJCZTCU
898 C~:ACCCU CtIGALIGAGGCCG~G.;CCG:~
aGCCCCC
899 CCGaCCC CU~"C ' ~~C~~~?a iaAC~CCC
904 G:N"DOCC CUGADGF~CGAF~~,CCGaa ACCL"L~1P,
917 A~Ci1 CUGAL~~GGCCGAAAG~CG?~ aGC~JC7GG
916 FJ~AGDQC CL AGG:.C~.~rl ~.~G~~WG
X24 t~~~ cuGA~r~G;~-~~c:~~cccaA a~wcvA
S25 GWCCW CDGAL~,A~GG.."CG~AC~.CGaA
AAG<JDCL7
926 CIt~JDG~~UCZ1C~~G~CCC,T~AAGGCCGaA
A~F~
945 G~TUCG CZtGAIIGAGGCCGF~ACvCCGaA
AGUCr,~t,'G
946 AGu~DWC CUGAC~" ' " CG~1 F.i.CZGGtT
59 nULJCCUG CUO~CGF.A~vCCGAA rIUCCCAG
960 CAUUCCD CUGAUGAC~CCGr'~AAC- GCCG:~'
iar~LiCCCA
1001 GAF:UUCLT CL1GAL7GAC~CCGCGAA ?GLJG~JL7
1007 CAG(JUUG C'CfG~~I~GF.GGCC'GAAA~CCG:~A
aWCWA
1 008 CCAGZ1UU CUG~~I~sA.AAGw~CGAA Aa ,L~JCL1U
021 nG~COG CUG ' ~ CG ~AAG:~CCGAA n~CCCC
1029 CCCCAGL1 C'DGAIJG~.C~CC'GiT~AAGvCCGr~.e1
AGL'LJCUG
2040 G CUGAUGAGGCCGAAAC~CG~A ~.G.-CCCC
1046 G:~ s~aUC.~CUGALTGAGGCCGAAAGvCCGr~.
:sC-~"UGUA
1047 " '"~ UC CtIGAUGAGGCCGAAAGG:.CG~A
AAG~.~LGU
1051 UGUCAGG CUGALSCAGGCCGAAAGGCC~~.A
AUCAA.AG
1060 CAVtICCA CUGAUGAGGCCG:~.AGCZCG?.A
Ai,'GUCpl
CA 02468048 2004-06-07
247
1067 GLCDCCA CD -CAL"'~C~- , i-.~,CG?.A
AWCCAG
1085 e'aG'~ACCACDGr~UC~C~,CC~u~GCZ~CG'Aa
aG~vC't?CC
1086 CAG'~ACC CUG3LT~C~C -CA~GG..~CCz?.a,
AAG~.,~UC
1090 U~~ ~~ ~~
~ 091 C~JG~..C.ACOGr~IGF~aCCGaAAG~ICC~1A
AACC'~Ae~a
1113 UCUtICUC CUGnL - ~~CG~nAG~CG?.ar
AGUCCUG
12 4 OCLAG.~"U C'0~"C".:ArIAGGCCGAA AG~7CCW
1~9 CAAUQUC CUG~DGF~G:~ :~1AAGGCC~.~A
AG~TGaG
1135 UU~7GLIC CDG~GGCCG:1AAGG..~CG?J~
~ aUUtnCUA
1151 :~.G:~CIJ CL~C~CGAa AG.~"UCCA
11,52 G~AGuCC CUG~~GGCC~C~~ A~G.~~UCC
1158 A,G'~C~GG C'JG'~G?.~"C'',J~AAG~C'".-~
~C'LTA
1 1 -G~n~' ~Ge~ ' ~.~"~W.: ark u~~~.CU
S~ -rya
62 CC~'~~-.'r.C~TGA -UCH' ~"aCCGCCe~A
-Cue' AG ar'aAG~~
1164 AUCD~"C~A C~JGGGCCGAAAC~CCG~A AG?.CGAA
1166 ~ ACAUCL'GCL3G~C~.~AAC,:~C -CAr1
AG~GG
1174 WCUG.~-~~ CL'Gr'1I~GGCCC,aAAG.;CCG.~
ACAUCC1G
175 ~CDGV ~' CCGAA AACAUCCT
11? AAf~COG CZrc~AGGCCCAAAG~~CGAA AAACA~7C
6
1183 C'CC~.C' CQC,?.DGaGGCC 'CuA.A ~;~CCG?.A
.~., AGDCUGa
1184 UCUCr'ar~aG' ~CCGnA~'~~CG.~A AAGUCUG
1187 GCGiJCUC C'JG~CG~?.~A, AC,AAGL?
X08 CC~UGGG CU ' ~ CGAAAC.GCC~.~A,
""
224 :~L~G~G C~UGAGGCCG:~r~AGGCCGAA
AGCI7C~GGC
122 ~I~AALTA CLTGAC~CG?.A AC~AC,C
8
'_230 AC.~AA CI,n~~C~CG:,a ?~
1232 AAAG~UA C'C~C'C~AGGCCCaP, AUAGAGG
X33 C-T,AACAU CT~C-CGCCC~A AAUAGAG
234 C:~ACA CZtC:~C~CG~1 AAAUAGA
1238 AF~G~'G:A C~TG~DGAGGCC'"~AAAGGCCG,?.a,
ACAL7AAA
1239 CAAGnGC CUCnL7GAC-GCCGAAAGGCCCaA
AACA~A
12 4 UAADCAC C'Cit~AGGC~CG?..~ AGt~F,
1251 F~UAAP.U CO'C~GGw~C"V?.F~AGGCCGrIFa
AUG'~CAA
'_252 LIP.nDAAA CUGF.~AGC,CCGr'~AAGGCCG'aA
AAUCACA
X54 r'.~At7A C'JGA~vCC"CG?.A AUAAUCA
X55 AAAUAAU CU " '' ~~'C.'C~AA~CCGAA
AALJAAUC
2 5 L'~AUA CUGF.L7GAGG~CCG'r.AACGCCGA?~
6 A " ' U
i 258 A~UP.FaAU CUGJ.I7GFaG.~~~C~.:~.AAC~CCCAA
AUAp.AUA
2 5 ~UAAA CUGAL'GAGGCC'G~AAGGCCCAA
9 AAtmP.AU
12 61 AUAFAAUA Ci7GAi;~C~~i~GCCGA~'~CCGAA
AUAAUAA
12 62 AAU~AAU CQG3.L7GTaGGCCG~AAAG~~a~CCGAA
An~AUA
1263 ~AU~r AA CQG:~UG~GGCC'GAAAiGGCCGAA
AAA~AU
1265 QUA CLTGAt7GAGGCCGAAAGGCCCAA
AUAAALJA
12 6 A:~F.UAAU CUGADGAGGCCG?.~AG~CCGAA
6 AALTAAAU
267 L~UAA CUGAL1GP.G~CC~CCAA AAAUAAA
12 69 .~L~.AAU C'tJGAIJG~G~CG~'~AC, ;~~CGA?a
AUAAAt~r
1270 r'~AUAAA C'UGAUGAGG.~.C 'C~AaGGCCG~.A
nAUAAAU
1272 AUAAAUA CUG~UGAG~CG~.r"~AGGCCGAA
AUAALTAA
1273 AALg.AAU C'DGAiIGAC.G CC ' ~~CGA?,
AF,UAAUA
CA 02468048 2004-06-07
298
.274 :~A CUG~UG~CZC~-Y.AGGCCC-~ A~ALTA~rJ
12 7 G'.RAnUA C~-.~~. CGa~'aaG;sC C -Cnr~
6 nUAr'~e~LTA
1277 L'G,TAF~U CGCAUGAG~C~~aGGCCG?~?~ aai~AAU
27a c~c~A cvcaac=J~cGaA~aGCCCGA.~
A,~az~.aA
1280 AUCQGIJA CUGaD~.GGCCC~GGCCas AUAAF~UA
81 C_'~TJCUGUCUGACC~~~A'r~C~CCG~ ?~L~Ae~aU
1282 UG.VCUG CLTG?.UGAGGCCG?~hi~GGCCG~1
arZAUAAA
1294 ~LTA~A CG'GAL'GAG:~cC~CG~ ~.G~.UUC~
1296 CC'~LTA CUG?.UG?~-:ACC~.~.GGCCG?a
AJAC~UU
1297 CCC~U CUG:~UGAG:~CCGaAaGGCC~.~?,
~L~C~U
1298 UCCChAa CGGaL'GAG:~::CGA~G.:zC~.:
~?. ~AL~CA
1300 U ., ~:A C~'c~.L'G:~c-:~Cc~.~cvcccaA
avap.AVa
13 O GuC'JCCC CTTGaL'Ci? C-~C C G3~C~sC
1 C". :~.rl ABU
1315 CCCaG~~r CUGAL'GAG~C -'G~C,GCC~~
aCCCCG:
1317 CCCCCaG CUGAL'GnC-Gv.~CG?.r~~.GGCCG?.A
AUACCCC
1334 CAG,.'VCC CLiG~.UGAG:~..~CGAA~.CvCCC_~.?,
aCAtJ~;
1345 v~- CCC ' ~ C'GAA.AG:~"CG~P. ~.G:~.G~
13 5 C~G)CQ CQG~a~CGaAAG:~~CGAA aG.."CAA~G
0
13 s c~GGaA a~aL~aCr~,,.~CGaa ac~uc
9
1360 OCr~CG.~~ C'UC,AUCAC~ sCC~~ArIGGCCG~,
r~.CAUG'LJ
1361 L''vC'~.C:~CGCaU " ~~CGr'u~r'r:,CCG~a
i~.ACAUG
13 62 L'L'C~C~CGCUCAUGAC~.C -CGr~. A~.~.At~AU
1386 AACJ~GCC CGCAL7GAC~CC~:aCC"'~ ALltr~WC
1393 AGy.UG:~G CLIGnBGAGC-s.C~~GGC.~CGnr~,
AC~GCCU
1394 UACaUGG CUGaDGnGGC ~ AACAGCC
1402 >G~:~C CQGAL'GAGGCCG~C-:~CCG'..a
aGUGG.;
1414 CUGAL'G~C- GCCGA~:~CvCCG:~a
AG:~c CAG
1422 UC_'-.?.SAGCUGALCG~AG:~CC -CA.~ :~G.~cACA
1423 :,LG's~Ap CUGA~C,GCCCne~r'~CvCCG?s~.
'-~nG~~.C
1425 UA~UC'J~A CQGADG~~C~.~:~AC~CCGaA AC~AGGC
1426 :,L~UCA CZJGat.r ' ~~C'C~F.AC,:~.~CG~
:,aC'~aGG
1427 CAUAAUC CUG~UGAGC-CC -CJ~.A.=.CvCCGAa
AA~.CAAG
1431 'A.~.isCAUCUGnL~GaC,.~....~CGAaAG.:~~C'.,~
i :~UCAAA.A
1432 A~.~.ACa CUC,~,CCCa~,ac.~,~.cG,>~
aAVCaAp,
1436 Dt7~,~',~ACUGaLR3AGGCCGA~.1G"CCGA~,
AC3iLIAAU
1437 U'JUL1AA.ACUGa " ~ CG''nr'u~.GGCCGA?,
aACAUAA
14 3 AL'atJtTAP.CLJGAUG.F~C-G.. -CCi,~,F,CT,C
8 C -C:~?. ~.nACALTA
1439 t?~W~, CUGaI3GaG;~:.CGr~AAGGCCGa.A
AFa~.ACAU
1440 hL~LUUn CL-GAL7GaGGCC ' 'G~.,r,GGCC~~.
?.AAp.ACa
1441 :~.LTALW CUGaUGnC~GCCC~liAaGGCCC:Y,
'.~.~F.AAp,C
14 4 CAG~sUAA CUGaL'GAGvCC'GAAAC,C,C CGaa,
6 AL1UUUAA
14 4 T~UCAGAU CUGA ~ C'GAA AUAIJUW
8
1449 AaUCAGA CQGAUGA~' ~ CGAA AaUAUUU
1451 Ut'..T,AUCACUGAL1GAGGCCGAAAG:~CCGAa,
AUAAUAU
1456 i~C~aCUU Ct3GAUGACvCCGAi,aC~,CCGr~A
.'-.UCJ~GaU
1457 GAG~CU CUG.~L'GyGGCCCaF~C1-CCG?~,
~AUCAGA
1461 L'L'LTFaG~CCCiCyaUGAG.;CCC=~.T.GGCCCPA
i_CWAAU
1464 L'UGU-uZTACL'GaTJGC-v.CCG'~AaCvCCGr~?',
~?CAp,CU[7
14 66 CJ~L'UGUU CUGAUGAGGCCC-'-AAA G'C-CCGP.A
: ~GaCAAC
CA 02468048 2004-06-07
299
1479 G?CACCA
C'GGAUGnC-GCCG~r~C-G..~CGAA
AUCAGCA
1480 G.~"'JCACCCGGAI7GaGGCC~CGAA AAUCAGC
1494 AAUG~ CQGADG~C"'~AAG:~~CGAA AG'sG'1UG
1498 CAGCAAU CffG:~ ' ~ ~ ~~CG?~A AGUGACA
1501 CCUCAGC CL7GI3GnGGCCGAARGGCCGAA
AUGAGUG
~~Gv~CA CUGnUGr~G.~~~~CGAAAG~~a~~CGAA
AGGC'CG'C
1517 CCCQGw CUGADGAG:~CGT,AAG~~C~~AA
AG~GAG
1528 CAGACAC CUC,nI7GA,G.:CCGr~~AAG.~C'CGAA
ACUCCCO
1533 GADUACA CUGADG?.GG.C'GAAA~CCGIPa
AC~CAAC
1537 GGCCGAU CLIGAtIGaG:~CGAAAGGCC~.~A
AC.
1540 GVAGGCC CLT~C'G~AAAGGCCGAA AUUACAG
1546 ~UAG C'~.DG~'"~'C~AAA~CCGAA
AGG.."C".~
1549
1551 GCCACLG CUGAUGAGGCCGAAAGGCCGAA
AIU1GQAG
~ S5Z CGCCACV C'UGADGAGG:.CGAhAG GCCGAA
AAA
5ss c~accw cvGAVGnc~cGa~AAGuccGaA
Avwcac
1572 CCUAAGC Cn~~GGCCC ~ ACCUO'QA
576 ~ ~G~~G"CG~A AC~ACC
1577 UCUOOCC CTtC~AG:~CCC,AAAG:~CCGaA
AAC~AC
CA 02468048 2004-06-07
Z5~
Table 2b: blouse T.NF-a HH Tasget sequences
nt . HH Ta.rQet SeQvencent . E8 Target Seqveaea
Positioa Foaitioa
60' VcG~AU a GcucCcA 324 Gg~U C GCIiCCCC
101 G~G~"U U CUgBcCC 347 GaGAagU a cCCAaaU
1 O1 c~:~GgU u. CuGUccC 3 64 CCVCcCU C Uc~IDCAG
02 C1-~CvJU C US~UcCCO3 6v LC:CCICU c ADCAGuu
102 cCaGgUU c ugUCCCO 360' UcCC'GCU C auCAGuU
106 GUUC'UgU c CC'UuUCA3 69 CUCUc~U C AC~.~:uCUa
110 UgUcCCU a UCALI~cA 376 CAGuuCU a UC-GCCCA
111 gUCcCW a CaCDCAC 390 AgACCCLT C AcaCQc~
111 gLCCCuU a C.AGIiC~c396 ucaCAcU 'C AGaDCaIT
~ ~ 2 UcC'C'QuU C ANcA~CO401 cUCAfAU C AUC'WCp
116 LJuUCACU C :~cUGgcc404 AC,AUG'1U C UUCUCaA
137 G~CaCAU C uCCcUCc 406 ;,UCADCV U CUCaAAa
139 caCAuCU C CCUCcAg 406 AVcADcU U cUcaAA~,
177 GCAUG;~U C CGcGACG 407 UCAUCUU C UCalAau
207 AC~CU C CCCcAsA 409 AUCZ7tJCU C aAaauuC
228 G:~.:~C'sU C CAGF~ 409 Au~uCO c AaAAUUC
228 G~.'1iU c C~.GzacU 409 aUcUUcU c AAAauOc
236 cac-~cu c c~.-~;~ 432 acccoGU A cc~,cc
23 6 C~GaAC(T c cAGgcGg
249 G..~-ugCCU a UgUCUcA
249 G.~"~GCCU a UGucUCa444 AcGUcGU A GCAAACC
501 AcGCCCL1 C COCA
2 61 UC,AGCCV C UUCUCaU 5.60 gGgUOGU a CCZJuguC
2 61 UG~CCLJ C UL"C'L7cau5 6 0 GGguUGU A ~gpC
263 AGCC'UCZJ O CUC~1L~C564 Dc.~GCp a gUCOACU
2 63 AgCCVCU U CUcruUC 56? ACCUugU C UACLTCCC
264 GCCUCUU C UCaUUCC 569 CUugUCU A CUCCCAG
2 64 gCCLICUJLJ C UcauUCc572 gUCUACU C CCAGGLJu
266 CUCUtICt1 C aUUC:.UG572 GUCUaCU c CCAGguu
269 UUC'UCaU U CCUGcDu 572 GuCOacU C CCAgGUu
270 UCUCaUU C C"JGcUuG 579 CCCAG.~~U a CUC'WCA
276 UCCUC-cU a GJC:.~:~G580 CC~IGguU c uCWcAa
297 CG~C''~CLT C UUCL~C580 CCaGGuU c UCuUcaa
299 ACGC'JCU U CUCI:CDa582 ~1,C~Up~ C egg
300 CGCQCUU C L'Gl~CUaC582 AG.,~UuCU C WCAAGG
304 CUuC'UgU c uAcUGaa 584 GUuCUCU U CAAGGGa
306 UcUGUcU a cUgAAcU 585 UuC'UCUU C AAGGGaC
314 CUGaACU U cG~eGUG 608 CcCGaClJ a CgugCUC
315 UGaACUU c GGgGUGA 615 aCgUGcU C CUCAcCC
315 uGaaCUtJ c GGGguGa 615 AcGUGCV C CUCACCC
324 eGCUGaU c GgUC~CcC 618 UGCUCCU C ACCCACA
CA 02468048 2004-06-07
251
63O ACACCgU C AG'~ =au 94O GuCLTACU z cUCAGaG
630 A~CCAU C AgCCcaU 943 'ur'~.C'UccU C AGaGcCc
638 aS~6AU a uG~~JaUc 972 UC'Ja,aCU a AgA?.AGg
643 aUUt7GcU a uCLIG~uP.972 ucUa~CV a AGAa.AgG
645 UuG~~~aU C UCatTACC 973 CQaACuU A G?~AgSG
647 GCuaUCV C aLTACCAG 984 ~GgAU U auGC,cuc
663 agAAa~ C AACCQCC ~ 984 AGC~gaU U atlC~IJc '
669 UCAACCU C CUCUCL'G 985 G:,:~auU a LC~cUCa
669 Uc~AccO c cDcL~JG 997 UcaGac~U c C3Acscu
672 ACCUCCU C UCL'GCCg 1010 CuguGCU c AGASCW
674 C'JCCOCU C UGCCgUC 1017 c_CAgC'J U Uc~.aC~
681 cUC-CCgO C AaSaGcC .018 aC?~5C'L't? U c~CAJ~C
681 CQG~.~CgU C AAG~GCC 1019 GAS~CLyJ c ?~C3s?G1a
6g1 CUGcCgU C aaGAScC _073 USC:~.~.CLT c ucyUgCA
734 CC~"U A ACC lOg6 A~.GgAcU C ?.?AugCy
734 CccUG.~"U a ugaGCCc ? 106 aCGwcU U ucc~.~AU
744 AGCCCAU a L~cCDG:a '? 07 UG:.-:~cUU a ccGAAUu
746 CCCAUsU A cCUG~r "08 GGgCIaW c cGaaUUC
759 GAgG~U C uuCCAGc "'_5 Cc~-..AauU C ?~CUGGaG
759 GAGGaGU C ULJCCAG~. ~?.33 CGAAugU C CAL~iCcU
761 GGaGUCU U CCAGCUG 1164 cacLT,gU c AgGJUGc
762 GaGUCW C CAG.."UGG 1180 UcTJgUcU c agaAUG?,
7 8 ACC~ACU C AGCG~~OG 12 03 zz.G?s:CU c AGC-CCUtJ
6
798 CUGAGgU C AAUCuG.~ 1210 cAG:CLJ U C=L7acCU
~
802 C-gU~B C uG.~CCaA 12? 1 AGC-~.' ~UQ C CUacCUu
8u CCCzAgU A cuUaGaC 1214 C~'LCCU a c~JuCAG
816 AgUA~U a GACUWG 1218 CcvACcU a CaCACCu
821 uUaGACU U UGCgGAG 1218 CC.:aCCU U C~CnCcu
e22 UaGACW U GCgGAGU '?18 cC.:F.CcU a cngACCU
E30 GCgGAGU C c "G.~AG 1218 CCUacCU a C~G?.ccU
840 ~'"U C G 1219 CuaCCUU C ACACcuu
842 CA~'~ A ~a ~?1 g C~Ac~UI1 c agACcUU
842 CAGgucU a CWucG<1 1226 CagACC'J U uCCAgAC
842 cagGuCU a CQUOgG?. 1226 CAG~ccU U UC~AGAC
845 ~C~U U UGGagUC ?227 agAC~,~ a CCASACu
846 UCUACUU U GGagUCA X27 AGAccW U CC?.C_BCLJ
852 UUG: agU C AUUGCuC '_228 GAccWU C C'~G~.CUc
e55 GagUCAU U GCuCLJGU 1238 gACL'CuU c cC'~'~G,AGG
8g7 AUCCaUU c ucLRCCC 1.262 CAG.."'CIaU C CuC~caG
891 AuucuCU a CCCaGC'C X83 CCCCccU C uaL'LJUAU
905 CCcCaCU C UgaCCCC X83 cCcCCCLJ C UAUUUAU
905 cCCCacU c UgACCCC '1285 cCCCUCL7 A L~7UAUaU
905 CcCCACU c uGAccCC 1287 CcsCVAU a UauAuUU
914 CAcCCcU U uacUCUG 1287 C~JC'UFaU U iTAUaUW
915 ACCCCuU.u acUCuGA 1288 CUC'JAUU U ?UaUUUG
919 CUUUAcU c ugaCCcC 12 89 UCLA u'L'U A LTaL'L1GGC
g28 GACCcCU a UaUugUC 1293 UULT',.,UaU U LC-~:CUU
g2g gAcCCCU U UAUUguC 1293 uWaUsU a LTGcAcUu
g32 CCUUUAU U cuCuaCU X94 L'tJ'T.~tiaL'U U GCACUUa
CA 02468048 2004-06-07
252
100 WG~ACQ U aUuAUUu 1462 aCCuUGU GCCsCCU
a
1303 CAcuUaU a AuUuAW 1470 GccuCcU UUWGcU
C
1304 acDuAUD A U~sUQ'A 14?2 cuCcUCU uZJGcUUA
U
1306 UuAUt~U U UADUAW 14?3 uCcUCW U UGcTJLTAU
1307 uA~I7U U AULTADUU 24 i4 CcUCUW U GcUUAUG
1307 UaWaW U AuuADuO 1478 UUUUGcU AL'GLJULIa
U
1308 AU~UULJ A UU~~UOA 1479 UUUGcW a UGuuuAa
1310 UauUuAU U AUUUF.LZ7 1479 UUUGc'"1U UGUUUaa
A
1310 UA~U U AUUL~DU 1484 UUAUGW U a.aaAcAA
1310 UAUDDAU U AUUUAW 1498 AAAuauU AUCL7aAc
U
1311 AUUUAW A UUUAUUU 1511 Ac c cA~aU GUCUuAA
i1
1311 :~aoaW A ~~ ~ 514 c~WCw c vuAAuAA
131? AuuDAUU A UuUauW 1516 aWGUCU a AAuF.AcG
1313 L1DAUQAU U UAWUAU 1529 CgcugAU UGG~GAC
a
1313 WADL~U U UALTUAU 1529 cG..~UGaU UG~3GaC
U
1313 u~U a UauUDAu 1530 gCUGAW a gGUgacC
14 U AUUtIAUU 15 3 0 GC'UC,AW G"~UGaCC
U
1314 U AWQADQ 1563 UgaAcCU UGcOCCC
c
1315 AUUAUW A 1563 ugaaCCU UGCUCCC
C
1317 BAUD~U U ZIAUUAW ~ 568 CUCUGCU CCCAcG
C
1318 AUULIFsW U AUUAI1W 1589 UGaCUCU AUuGcCC
A
1319 UUiRUW A UQAUUUA ~s92 CUGUAAU GcCCUAC
a
1326 AL'QADW A UGUAUL1U 161? GAG:~AU AAGaUcG
A
1328 IRUWAU U BAUWgC ~ 1623 UAAAGaU G~~UUAaa
c
1329 AUUUAW U AULIUgCu 1633 UUAazaU a.aAA,aCC
a
1330 UUUABW A UWetl:u 25 AcGSaCU gCCagGA
- a
1 3
3 2 LTr~UQLmU U UgCuuAU
13 AUUUAITU U gCl:uAUG
3
3
1337 auUUG."U U AuGAAuG
1 3 uQUGCL7Q A uGAAuGI~
3
8
13 L:C~.AIJGU A UUUAUW
4
6
13 AAUC~U ~ L?AUUUGG
4
8
13 P.UGUAW U AWL7GGa
4
9
1350 UG~DOU A DWG~GaA
13 uAUuLTAIJ a DGGaAGG
2
1352 UAU~U U UGGaAGg
1353 AUUUAW U GGaAGgC
13 G;~:~.~~UgU C CUGGaGG
69
1398 gCUguCQ U cAGACAg
1398 G,.'"UG~CU U cagaCAG
1412 GACAUGU U WCuGUG
1413 AcAUGW U UCuG~A
1414 CADGUOLT U CuGL3GAA
1415 AUGUUW C uGLJGAAA
1415 AUpU(nT c UgugAaA
1438 gaG~."UGU c CCCAccU
1451 CUGGCCU C UcUaCCU
1453 ggCWCU C UaCCuUG
CA 02468048 2004-06-07
253
Table 26: 1'Iouse T'~F-a Hammerhead Ribozyme Sequences
Mouse ~ Ribczyme.SequeaCa
Pos'_t'_oa
2~ LCCL'GGC CG -C~:~.C~ '~C~~.,~~CC'r~1
aGUCC';."J
60 C'IJGAI~C~CCG~.rIaGGCCGAA
A~7UQCCA
GC~1CAG CLT?~L'GC~:a...' ~",r~.~'.aG.~~~CG?.rl
~CCu'G.."'r'
G.~sr~G~GCDGraLiG.~G..1V -"C~?~Gu~."C:s?~e1
ACCDGv."'C
.C2 ~ C'JG1L'G~C~..":.'~.~.aAGGCCGaA
A1CCGGC
i 02 AGGC,AC~ CUGAtIG?.GC,~~~CGau1 A1CCUGC
CC~a:~ CUGUG.~'~C~'CG1~1 '~G;C,?~rC
'.0 ~G~GJCA CL'G?iLTy,GGCCCv~r~C,~,"CCG?.A
AGu:~C?r
GCG~,GOG CGG?.UG:~GGC ' ~ .~IGGGaC
GL~.r'-'~aJGCL~~.DC'v'~C,:sC -'CGn~G~.~CG?.A
Ar~~ S~?~C
..2 '~v -~GLJCVC~GnG:~CC _CAaAGGCCGAA
AnAGGG~
GC,CCJ,GQCL7Cs?.U -C~'~C".J~l",hGGCC"~
AGL'GAAa
~~7 G:~C~A C'JGnL~~C-v,CCG~A~GGCCG~
ACGCGGC
35 CL'GG-'~GGCv?G?.L -'G'~~~vCC~u~r~G:aCC".ylA
AGnDC~L'G
/ 7 CGJCh. CUGn ~ ~ C~"AA AUC~.
V
207 ~~,w CL'GAL'C,?~GG..~'-'~:~GGCCGaA
AGJGCCU
228 ~JG CCGI.~G:~. ~ w ' AAG.~CCC
226 :~GJUCUG G:~.~CGAA AnGCCCC
236 CC''w,:.CUGCUC,:.~-CC~CGAA AG'v'UCUG
236 CCC-CCRG CLiG~L'C,aG:~~CC?v~GGCCGr~/~,
AGUUCUG
249 LG~G~G'~ CL'G:~L'G~GCICGInnG~.~CGna
i~C~' CC
L4S ~GAG.~CA CL'G~.L'GaG.~C 'GMAC~CCGA?,
AC,.~.~CC
2 51 AL'C'M CL1G:~T~AGGC 'C'G~AGG.~CC,AA
AG~'ZJG?~
26. :~L CQGALG?.C:~.,."C".~:~?.C~CCGAA
AC,G..'vG?~
253 -C~F,BC,AGC~G:aL~~GC-CCG~AaGGVC~.~A
AGaGGw~U
253 G:~.T~QC~GCUG:~L1G:~GGCC~.~J~AGGCCGAA
AG:,GG~~U
26C G:~tGA C'CGa~ICG~AC,C,cCG.~ AAG.~GVC
264 G:~ADGA CL'G?.iTGAGC1~ "Grv~C,.GCCGAA
AAGr~GGC
26c C.AG:~.AUCUGnL'GnGW.~~'v"~~CCC~AA
ACvaAGr'~C',
269 CZ1G:~L~C-.;AAAGGCCGaA
AUG?.GAA
270 CJ~AG.:.:~GCUC~L~GGCC 'C,~.G~CCGAA
AFB -CC:~
276 CL'GCC.~CCL'GAL~C"~:,CC~~AAAG:~CCGA1
AGCAC,GA
2S7 G CQGAL3GaGGCC " ~ AGCGUGG
2S9 ~ CGG:.CG~AA~CCGAA AG sGCGG
300 GUF~C~CA CL'GGG~CCG'aAAC,GCCGAA
~'~JaC~,CG
3C4 UL'C~'~GIJACL'Gr~1~CG/~r1'~CCG?u~
ACAGAACs
306 AGv'L7G,GCGT~L1GAG:.-:.CGA.A?.GC,~.~CG1A
e~C~GP
31C C?CCCCG CUG.ALT~GCiCGAr~GGCCG?~A
AGUUC~.~G
~s5 LCACCCC CUG.~LIGr~'C11CG~'a 'hr~v'CG?~P.
Ar'~Gu'CrCr1
CA 02468048 2004-06-07
254
LC;ycCCC c~~-~r-CCap, a:~uC~
324 G~.;~CC C',1G:..UG~G:~:.C".~,G:~cC~aaA
WG~CCC
3 2 C~CC C'UC'~F~C',~GG.."C"~:~Fu,Gu..
4 ~ GaA .'~'C~CCC
347 nL'L1L'G:~C"'u~-.~~~CGF,r'J~G~~ ".AAA
AL'~CQC
3 64 C"'-'W- Cuu~D"~,nC,G..~C",1 '".~A
?~GGG~C,C,
3 60 '.~r'sC'vG%~sIJCL'G:-.L'GnGG..";:Gi-u~.'~GGC~..:~.r1
aG~,C~
3 6 tu~L'LJ"~UC':~G:~~CG:,F~',Gu.."CC,AA
6
3 69 ~CmCU C UGF.UCy,G:~CCC~:~AGG:.C"'~A
?.RGaGaG
3 7 UG:~ C?. C~~C": ,~A AG:~AC~G
6
U~~V~ ~U~~~~1 W
3 0 FCC C~ . ....C..
6
401 U CL'GAUG~:..~.~AF~CC~. aI7C~G?l
4 04 UL~~-' C'JG~G:,..~.~:v.i,C~~.
'.GnF. ~.:~A aL~J
406 L'LTtn~G CUrGAL'~G:~CCG~1G~~C~..:?A
aGaDG~U
406 UtILJL~G CUCGAAAGC~C"~A aG:,UG~LT
407 nUUWGa C~'C".~:~F.nC~._"CGr~.
F~
409 G~I~t7W CL'GF.DG;~G~~CCGaF~~C'.~A
.~'.aAGaO
4 09 GF AUU~ CUG%~I,~"'CG%,AAGGCC".~A
AGaAG?~D
409 G:~L'L7DV CUG:~T~"C~aAAWCL"'.~A AG
aAGAU
,32 CGUG:~ C'U'~:~G:,CC~~el~uuCC'C~A
"O
Y44 WJL~GC CVG~'~..~A aCGaC~J
01 UCNC.r,G CUG~UGG.~..C~J~C"~CCGAA
aC~CGLl
360 Gr.C'~ CU"C~VAF~GG..' ~GAA AC~CCC
9 6 G:zCJ~.F.GGC~UG~G:~ "CG~ ~ACCC
0
364 C CDG;,UG;~G:~"C;~,:~.:~G:~CGr.A
AG.~u~C.~
6 C ~~' ' CL'G~L~~r'aGw C '.~.r~G:~C
7 G~ ~'GaA r~C~IC,~.~,J
3 6 C'JG.-uAG COGAUG:~C~'~C C~~F~C'~"~
9 ',.~A F~G~C~IaG
.72 F.F.CCL'GGCL~ :L~C:~F,F.GGCC".~ AG'~rlC
7 2 ;~,:~c'UGGc,JG~UG~G;~.:."~=.aFu,.~crcaa
AGCAGAC
5 72 ~C'CL'G.G CZ~~CGA:~AGC'".C~.~A T~C
579 LTG:~AG?~GC~G,.~1JG:~G:~CC~CG?p,
ACLU
a o L2-~;~;~a cvc~v~ "aA apccvG
9 8 L~AGA CUG1.U~AG:~C'G~~GC~CC""ap,
0 ~lCCipGG
c 2 CC'JQGnA CLT~L~nC~ CGr'~aF~,GC,CC
GAA ACnACCU
~82 CCUUC'~A CL'Gni~C,GCCC,a'CuAA AGnACCU
304 UCCC'JQG CUt:ABGAG:~."CG~AC,GCCC,?.F.
AG:,GAAC
525 GuCCCW C'.fi~nL'GF.Gu;.~.~ni,GGCCG~A
F~FaGnGAA
608 G~G~CG CUG~L'C~G:~CCC~.F.AG:~:.C~.~,F.
AGJCGw
6? G'-,,~~.G CUG~U"'~'~G~CCC~F.C'NCCG~.
S AGC~CGL7
6.5 GG...TG CL'G:~L~GnGGCCGAF,F,G:~.:C~~A
:G AG.:.ACG'CI
618 L'G~JGG.~"UCUGF.LIGnC~.CGT,F.T,G;,CCG?,A
AG:~~GCA
630 F~UCG~CLJ CVGF.L'G:~Gu'C".~:.u,AGGCCG
AA ACC~.~"'UGU
630 F.UCG:,CV CUC'y~IaGAGC~CCGAhF.GC,CCG?.A
ACGGLIGtJ
E 3 G~IJAGCA CUGF~~C".~F.~AC'~CC'~A
6 AUCG CLJ
643 L"nUG:yGr1CUC,AL'GAG:~CCGi'aAesG:rCCGrIA
AGCAAAU~
6S5 G.~uT?~UG~.C'~'G:yUGAG:~:.C~.~.=.AG:~CCG,?.F,
ALTAC~A
04 CUG.~''IJAUCUGr.UGAG~CCGnMG~CC:~.A
7 AGAUr.GC
CA 02468048 2004-06-07
255
663 ~~"W CZ1G~.UG~.G.:,~'"C " ~,C~:~1
aCLJ01'CU
659 C~CvaGaG CLGaUG:~G:~C 'CG~G~CCCalr1
elGw'~'~?~
663 'C'~rcCGF~F~r:G~CC~~.1 aC~""G~
672 C"~~~~~CC~.rI AG~:~G.,"U
674 G,:~C"~ C'L~GAL~G~CC~~AiC.:~.."AiGAG:~1G
681 G:~JC'Ut7 C~CC~AAG~.."C~',arrel
aC~w:rG
681 GG~"LJC'D'U COC~UGACHCC'GAAAG~.."~A
AC~.~G
sal c;~wc~oo cvc%~ca~.~~aa~CC~;a ac~"',~
734 G:~.."CCa C~C~~G~CCC~ aCCi',Gw
734 COCA CUGa ~ ' "~CG~1 aCC.~.C~
744 CC~CG~ CVGAI~C, a~"'C;GA~AG'~..'C"~11
aU~."'U
746 ~~ "~
759 G~c,JC:~L'G~C~~'CG~i~aG~~CG?~A aC'~~~
7.9 GC'L3G~.A ' , ....C..~ ~..'C
7 61 CAG."fJGv CUG.'i ~ ~CCGr~ AGAL V
CC
7 62 CC~~JG .
7 8 C:~G~~''.~~U CUG:~au ' '" ~ "CGrla
6 ~J
GCa~itJQ CUG:a~a~'aWC'C~'v~.AG~CCG~A
aCC'CGG
a oz v~-~;~cA c~vcawCC~~.aIG.~CCCaA az~"~cc
a, wcv~ac Cuc~v~rcc-c"~~ac~..~ .~ce~;~
z
816 CTu~.GLJC C~CG~ ~AG:,.:.CG~1 a~C'J
s21 c.."CCc-C a~ Cv~,UGaw,.~c"~:,~-"cca~
acvc-~"~.a
s22 acucccc c~,cc~aa~:~;.CCaa a
830 C~~CCG CL~GAU"~.G~CCG~F~AC~C~,~CGa~
aCUCCG'
840 CnAAGiTA C~a'~~G:,CCGaAA~~.~.CC~e1
.'~CCL~."C
842 BCG~AAG CUG~DGACG~.~CG~G:,CC~.~?s.1
AGaCCTG
842 UC~'..,F.AG CUGIsDC',r~G~CCG~aAAG~.,."CG~?,
AG?.CCr:.G
842 UC~~ CUCu~LIGi~GSaCCGAT,?.G:~.CG~1'~
aGACC'uC
845 ChCUCCA CLIGF~t~GGC~C~A aL~~C
84 UG?.CGCC C~uGnl~a'nGw~,~~CCv'JaAG:,C.CGAA
6 .'.~,GTi~ra
852 ~v cv;~cawCCCc~~ acvcc ;a
ass ~:~cc cvc~rc;~~a~cc~a :~czc
se7 c;~,.u~cA c"~,n~r~cc~r-.~ '
891 G~.."DG:~, C17GF~iIGT~G~CCGAAAG:~CCG~r1
AGaG?~1J
905 G~:~.~'RC?~ C'D~OGAG~CC'GAA?~G~CCC~A
905 G~:N~UCA C'CiC~"CGAAhG~CC".~AA ?.G~,
905 G:~.~~UCA CIIC,FaDG:.GCCCGT,AAG:~CC~~Ar1
....."
914 CnGnGUA CZJGAL7C'a:aGGCC'GAAAG~CCG?~A
aC~u'C
915 UCAGP.GU CUG~UGAGGCCG?.AAG~CC~.~.?~
' ~,.
919 G:~:~"UG'1 CUGAUC~?.C;~CC'GAA.AG~CCGAA
aGLg,AAG
S28 GAG.AUA C'J~UGAGs~.~CGT~T,aG~CCC~r1
:~.~. vC
9 2 ~C~~ CUC,~UG~~ ;CCG?.F~ACuCCGAA
8 :~G:~C
932 ~C~3'.C CDGFaUGAC,G: C.'Cv':AALHCCGAA
AI~AAG s
940 CUCOGAG CUGAUG~CGCCGAAAG~CCGAA C
943 ~ GG:~.."'QCU CUGADG' "~ ..,.C:~AA
A;~AC~
972 CCUWCU CUGA ' ~ AAA~..~CGaA AGQ<TAGr,
972 CC~CU CU~UGaG~C~CCGF.F~G~CCC~A :~G:,
973 CCCUUUC CUGAIIGFaG:,CC~C:~CCGrIF,
:,aGLJtTA~G
984 GAGCC.~U CUG'ri~;GGCCGAAAG~CCGaA
aUCCC'''J
CA 02468048 2004-06-07
256
c84 G'~.,C'"~nD
CU~~nC"CG1A
raUCCCCO
9 8 L'G~GC. CA C~G~'~G~.,~.~.r~J,C~'",1"'.~A
'r~rDCCCC
og7 .-~G~GCDG Cv~ "CG~CG.~.A .aC~JLZGrI
1010 ;~-acv c~.v~-~~c~~cc~,a
~
1 Ol? L'~GCQGA C'I7G~L~G'.~GGCC~~F.A~~CC ""?~A
AG~'~CUG
1018 ~'"'uG~CG CilG~.UG:~G~.~C".~F~G:,CCG
;a. ~~UC'J
1019
1073 UG;aBG1 CQG:~CIGAGGCCG'rJ,AG:~"C'.~F'
?~G~.CC~
1096 CC~~ CDG'.~ ~CGFu~AG:~.:,.~,~?.P.
aGDC'CU<7
1106 ~L'CC~~ CiJG~~ G~'"C"~.~GOaCL"Gar1
~1G."CGaU
t 107 :-,:,DpCGG CD~ C'CvCC".yl~, .~G:.C"..A
1 ~ G~a :UDC CL1CCCa~"CGr'1A~'~.'"C"'.i?.A
Q$ .,C
111 ~'CC,'y~ r'a GV.~.~C~~ar'~!~~av.C.~..v~
~~.oV
1133 G CVC~UG~.G~~~P.AGG.."C"..-.~
1164 G:A~CCQ C'~LW CC~"C"~A lCCaCLIC
1180 CCT~UQCV C~G:~L'CAGGCC'w'.~1 :aC~CaGn
1203 1,:,G~"CU C~ C~.'1A AG~CW
1210 C~ -~C~.~AA aC,:~.,."C9G
1211 i~F~G.~~QAG GGCCG~AAG'~..~CGaA
CUG~'~ .~.C,:~CCU
1214 C'~A~G:~ C~~ GGCCGAAA~ AG~~AGG
18 F~C~CLiG CUG:ai~s~',GC~CCG?..'~
AG'~1GG
1218
1218 :,G.~"QC'GG ' "CGAA AC'~:~GG
CU '
1218 ~G.~"~?CL1G sCaC,CC:~AAG s~.'"C"u
CL3GF.L7GF iA 'wa~
1219 ~~ CLIGnL~ G~.' C'~Fv~CCG ;A
~AG:..~G
1219 ~AG.~"'CCO CL~GA. "?.A A?~Cu"UAG
'
1226 Gv7CDG:~?. CUGFL~.G:~CCG~AG~CCG?A
aG~CG'G
1226 GUCL3GG?. CUG.=.L7G;G~~~~CGAAA~CC~.~nA
AG.,u"'CT~G
1227 nGOCL3G~a ~ ~CCG~1A ~C~DCO<
1227 '~G'vC'JGG CUGAVG:,GGCCGA:,AGGCCGa?~
~1G,~.1CU
X28 G~CUG CL'Ga ~ CCG~Fa A?.AG ,CUC
1238 CCtJC:~G:~ C~GAi7G'' ~"C:G?.a AAG:,GC~C
12 62 CL~JG? G '" A~G.~C~G
12 83 ALB,AAUA C'CfC~;C,:aCCCa~AAGW CGaA
"""
12 83 r~Ai~TlA CDGA ' ~ CCGAA AG:~G
12 8 F~~A NG~BG ~C~GCCGA~J,GGCCGAA
5 AC,T,G~~a
.2 87 QA CUG~.UG F~LNCC'C~J~.AAG:~C"~AA
F~GG
'_287 iuaAU'nffA CLIGF~t~,GCC'G:fi~FaaGGCCG?~A
~AGG
12 8 Cry' raL'AU ~nGGCCG:v'~AGGC CGr'ir~r
8 CUCALIC FsFaI~iGaG
1289 G~F~ CUGFL~ '.~GGCCGAAAGGCCG1A
AAALmGA
'_293 :~.GUC~.~.A "~C~~.~ F~?,~~
CDGZ
1293 ~~A C'CGAL7G AGGC FJ~AA
12 9 UF.F~GQGC AAI~A
4
1300 '~L.~r~U :~AAAGGCCGAA AC~CAe'~
?303 'FnI~P" .U C'CGnUGr~.G~CCGnAA~CCGAA
FatTP.AGUG
1304 L~AA CUGAL:IG p~CGCCGAAAG:~~CGaA
AADAAGQ
13 0 ~F L~nAUA CLTGAUGAGG: CC;aF,FaGGCCGaA
6 AITF,F.ITAF,
1307 a,ML'T~FU CvCALIGnGGCC~~.~n~a?.G:,CC~L.u1
r~',UhALTP,
'_307 ~Lr.~U C'JG~UGAG:~CC
' ' GGCCG~?,
r~UAAt~,
CA 02468048 2004-06-07
257
13 08 U''r'.r~~' L?~A CL7GALu:~G~CC~~GGCCGr'1A
naatTAaU
_310 :EAU c,~c~,.~cG~~:~.~C~.:.aa a~.Aazu~
.310 inLRF~.U CUG?.~aGaCC"vaF,rl~~GGCC~va:v~a
.310 : '~~ "~A A~A,r~Vi4
13, :~F. CLTG~~UC~.~~CG: P.AG~CG?.A
, aAI~.AAU
;,A;~rr.~a c;,c~L-~;.~;~;.C~~,a~CC~aA
'~~t
=a :~AAU~ c~~c~ - ;.~ccu aaz~~
313 i~i~Al~. CL1G:~L'G:,GaU~~~G:~.~C~~'
:,Ue~?.~1
'.?13 :~LRAF~~ CuGaDGAGGCCGA<.?G:~CC'',i:~?,
?.~
13.3 AUAAAUA CUG~,LJGaGG.," u:,.~GGCCC?~
~L'AA ~.
13 i1 nAUAAaU C',JG~UG:~GaCC "GnrvaG~CGr'1?r
i,.lUP.AI~
:~IR~.U CLT~i~Cv.."UG?J,~Cv~~CGr~
1r'.'t~~w~
s~15 Luv.UFa~A CQGnL -GSC,v~.CC,n?.~C~-vCGl1
.lrz~lLIF?.D
i~.7 :antJAAUA CUG~L7GhGw~GGCCGaA e~U'na?~.1
~ O '~~ ~G~~~~ f1
i? 19 UAAAVA~ CL1G?.~C~.~AAC~CCG?.A
1325 :-.F.:.~ CUG:~UG~G:~CC~.anA.',G~aUCG?ar1
?~A13~IT
?..3 G:=-~ CL~C 'G.~AAG:~~CG?~?r .~L~J4~
2 8
.329 AGCAAAU C~L~'~GG.. .AAA A..UAAAU
330 f.AG~A CUCT,L~GnC,vCCCG?.r1 .~iv~~
1332 ~c,TC~~.CC~A at~ADA
3 3 CAUAAGC CL7GALT.AC~:~c ~~G:~nC~CC".~?~
3 :~IRAAO
1337 C~CGU CQGAL'GnGC~CC"'~nnACvCCGnA
r~C~~r'~AU
~~38 :,CnDOCA CQGAL'GaC~CCGAnnG~"C"~?~1
:~GC1AA
,346 F.F~~A.A CDG:~L1G~;C,:~C~~GGCC~.~,r1
.1C~
3 4 C~CQGn~F G:~CCGAAF~"C".~?.A ~t'~.CAW
8
1349 L1C~~U C'LJGAL"~G:,CCr'~F.F,AG:,~~CG~
AhU~U
1350 WCCAAP. CW,?.G~G~CG~u~AG:~CCG?.A
:~.~P~1C~
1352 CCUUCCA CUGaUGAGGCC'".~AG:,~.~CG~
1UAA1UA
_2 CCLTL?CCA CUG:~UG~G:~C ~.'w'~~',G:~CC~.~:.u1
:~U'AT.aLm
1==3 G:~WCC C'~JG~LK~G~CC~.~A:~AGGCCCAA
?~U
1309 CCQCCAG CUGAUG:,WCC'G~J~A ~.~CC~~
.'~C~.CCCC
'_398 CLZ;LJCUG COGAUC",AGGCCCG1A ;GACaGC
1395 Ct7G'JCUG CL~CG'~CCG:~r1 A.Cr~CAGC
1s12 G'~CFaGAA CL7Ga.L~,hGGCCGAAAC,:,-~.~.a~A
s 1 v~~ca c.~;..vG;~;~CC~,~a~,c.~cc-~"aa
~ :,acaUw
1414 UDC~.CAG CL7GF.LTCCC~CCC,?~T~~CC,A?.
aAAC,~IUG
1 s1~ L'WCACA CUGhLIGAG.~:.C'GAAAGGCC;~1P.
:v,.'~ACAU
's'_5 L'UC1CACA C'JGaLIGnC~.CGAiu~GwCCv'~'a
r'.r'aAaCAU
1438 :~G.w'G;N CL1GAUGAC,:~.CGnFai~Cw~cCG?.A
r1C?G~"'QC
14=1 ~~UAGA CuG:~L7GnG.;CCGnAACvCCCaA?.
~CrIC,
133 CAr.G.~"'~i CUGAL~CCC~.GGCCGAA ACC
1 s ~AC~.cc c.~t~Ac,G'~r-.~~AAC~cc:~a
s s ~~c.~
14 62 nGGAC'~: C CDGAL~~ ~ .~:,A ACAAG~~U
1470 ACu.F.T,Fa CUGnLIGAGGCC'C~,AAGGCCGAA
?~G~~GGC
1 C72 Ll~' ~~AA CUGAUGAG~,CCGaAAGGCCGnA
isGnGGAG.
1 s ALTAAGCa CL'GAUGr'.CGCCGAAnG~~.:C~.-~'1A
73 A
1 s Cr.UAAGC CUG:~L~Cz,C C~~AAAG:~:.
i 4 C~.A ' 'EGG
1C i U'nn~C~U CL'C,?.UGACv.~.CG.' '~'.G:~CCGnA
2 ~G:.'~AaA
CA 02468048 2004-06-07
258
1079 ~sCA CvJG~L'GaGC..~C~AaAG:T.CGaA AAGC~A
1479 t7L~s:~CA CLTG:~LICnG:~CCG~',~a~G~..' ~"'.~?.A AAGCF.~1A
1484 UUv~7CU~1 C'JGAUC~G:~CC'C~AA~CGAA AACAIJAA
1999 GutRGAQ CQGF~LT~.y~.C~~CC~ ~ .. AAUA~J
1511 vCRAGAC CCGA~nG~~.~C~uAAAG:aCCG?~a AUK
1514 L~'u~DL?aACUGA~G:~ ~ ....CC~?rA ACAALTGlG
1516 Cw'L~.QU CBGALGT~G:~CC~aAAAI~.~CG.~A AGaCAAD
529 G~CC1 CLJG~'..~ ~"CGaA ALTC~GC:G
1529 UCCA C'JC~UG:~C~C~~AG'"VCCGaA AUC~;CG
153 G.~G~CC CGG:~~~~CG:~AG;~~C~..~A AADCAGC
0
1530 G:,uCICC CL'GArGTvGvCCGAAAGG..~CG?~1 AAUCAGC
1563 G:~GCA CUGAUG?C~.~~~C~.a~AAC~aL'C"?~1 AC,~JUCA
1503 G~:.~.G;..~CL'G'CG?~.AG:~.C'~1A AG.~"'CUCA
1 ssa cc~;~ ccc,~,~;~ccca~a~:.c~~ arc
1589 C,~:~C~U CUG:~UC~C;:~CC'G~C~"'C".,~ ACAGUCA
'_ .~ G:~. C~CGr.AAG~CCG?~A AUUACaG
g
2
1 617 C"~:,UCUB CVCADGAG~CL'G~~AAG~~CC~A AWC7CQC
1623 UCOAAGC AUC~,
1633 G:~WOU ~ AUDU~1?r
CA 02468048 2004-06-07
259
V ~ ~ ~ ~ C9 iC ~ 4~ yC n!
C9 a ~ C~
b
m
c~
c
v ~n ~c~c~c~c~c~c~c~c~c~c~c~c~c~~c~c~c~c~c~c~c~.c~c~c~c~c~v'u
5~~~~~~~5~~~5~5~~5~~g~555~~~5~5
0
c a ~~c~c~c~c~c~c~~c~~~~c~c~~c~~~c~
':. ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ U ~ ~ V V
:_
r c~ ~ a c~ a
r
wr
~"i C
. "~ ,n .,, o a ~r vo r e~ a r~, ao W vo e~ .e vo o ~ N m m N o e' 0 0
y y ~~" ~ O O 1'~1 P'1 ll1 01 r'1 ~ O 1f1 r~l tf1 ~O !~ D O N N~1 N ~ ~-1 ,~.~
N~ N N P1 f'1
~ w'1 ri N N N N N f~1 e'~1 a aT IC1 1~1 ttf tf1 ~ f~ !~ !~ m ,."p! ~i v-1 ..;
,.., ,.q rr
CA 02468048 2004-06-07
261
~~~~~~s~~~~~~~~~~~~~~~~~~~
0
0
m
~r
c m
d
cn m
d
N 0
C
a a
n
~, ~~~~~~~~~~~~~~ _
H
:,
~ y'1 ~O N '~-I tlf O t'~1 ~ ~ 1G G1 ~ f~1 ~ ~ ~ G~'1 ~ G~'1 ~ ~ t~11 l'~'~, N
t0'1 ~ P~~
N N e0~1 ~N'1 f~~'1 t0'1 r1 c ~ W f1 ~D v0 W O ~ ~ Q ~ ~ C1
O
CA 02468048 2004-06-07
263
Table 29: Human bcrlabl HH Targei Sequence
Sequence E8 TatQet Sequence
LD No.
ZO ~LL~ pIJA ?L
?1 C CLU C?C.GJ
A~ ~Z
b3-a2a2
s7tz~_ct~ on
T T
C~r~~.n
~~~a~x uvc ~c-~n~
CA 02468048 2004-06-07
264
Table 30: Hun bcr-nbl HH Ribozpme Sequences
Sequence ~ R:.bcryme Se~ence
ID No.
26 G:~"L10C'DUCCLJ C'JGnDG:~ ~'.~:,G~..a;?. T,~JGG~J~"0~
27 aC':,~ ~.:,,G c~~~"'.~c;~..~ -..:,a. a~:~;~,.~cc~C
2 8 LT'nCuG:~. CG~"'J C'~ G: L'GnG:~. ~ ~? AFaC,.:,..4.~r. ~nG:aO,CWCW
29 C~~.~a~~C',~Ar~ r1'~CUCffC,~WA
3 0 nC~~CGCGG C'~C"~'"C~:-.~a~'~Gr.~"C"~1 '
31 C'"~L'LJG:~CGC'U C'LT"~=~L1G~C--:n.C~G.~.."CC-.~?. ?F~GGG..'ZJUUQG
CA 02468048 2004-06-07
265
Table 31: RSV (1B) HH Target Sequence
at. HH Tarpet Sequenceat. fib Target Sequence
positioa positioa
"EAU A AaUC:.aU 276 "'~,2.U A CQG~,UA
14 AALRAAU C A:~WC~,,2 83 ACL,'G~U ,~ C?.AC
aCA
18 ~UC.AAU U C:,O~~~.~A295 U A L'GC~:.~,C'J
ADCn:~~LJ C AGCCnAC303 DG:,G~C~T U UCC~,~
54 C.'-~ADGAU A aLmCACC304 C.:~:~C.W U CCLUAL~
57 UC,AU'.~U A C~CCAG13OS GC_ACUW C CC:,hL'GC
77 UC~U C ACJ~GACA 309 UUUC'JCU A LJGC,;
~1U
94 A~.CC~ U 6JCACW 317 L'GCCArIU A UUG~UC1
97 CCuQLIC~iT C AC~~"AG319 C~.:~I1AU U G~L'G"~7
101 ~ U G:~GACCA 320 C:~:~1UU C :,UG~?.UC
,1 p AA 323 UAUQC?,U C
1,13 CCA~ A AG1DCAC 327 CAUCAAU C aDG?,L'GG
~ 8 AImACAU C ACDAACC 337 G,L'G",~U U
122 CAUCACD A 1CC~GAG 338 :~L'Gw"W C ULTAGaAU
13 4 GAGAGAU C ADAACAC 3 4 0 GCWJt'CU U AG:~L'iGC
137 AC'~L'CAU A nC~UC'~341 Cv"~CCW A GnAUG~..A
14 8 CHAD U UA~C _ 3 5 ~GGG~,U U G:~T~UG~
0
149 AG.AAUD U 356 DCGC"~IJ U AAGC,C~A
150 C.~.hADW A L?A~CUQ357 DGC~.:~L'LJ A AGCCVAC
152 A ~.C~A 363 t~G;.Cp p, CAp.AC.;~
154 ~U A CGUG:.UA 372 ~nG~.U A CUCCC1L7
157 AIJAIJACL7 U G~AU 375 G:.AL~CV C CChU,~I7
161 ACCIDGaU A AF~DCAL7G380 C'JCCG~U A AUAUACA
165 Cy,~AU C ADGAF.L1G383 CGUAiaU A ~IC~IGtT
17 6 U A G~JG:~GAA 3 8 5 AUA:,DF,U A CAAGLTAU
1 B 8 C~AAA~CU U G:~GAAF,3 91 L'.~C~GU A UGaUCGC
2 08 GCC3~AD U UACADUC 3 9 6 GJAL'G?,U C UC~.AUCC
209 CCAC~ U AGAUC'CC 398 AUGaUCLI C AAUCCAU
210 CACAUW A CAUBCCQ 402 UCUCnAU C CFaImAAU
214 OUDACAU U CCBG.~'UC406 :,At7CCAU A Ap,WU~
215 L~JAC~.W C 410 C_~L?~AAU U UC~.ACAC
221 "LJ C AACLIF.I7G 411 ALTn~,:,W U CAACACA
226 C,aCAACU A BGn:~F~UG4?.2 UFmUULT C pp,~~
239 L7~AACQ A ULTACACA421 AC.~C.'.AU A UUCACAC
241 AF~C~U U ACACAAA 423 AG.AUAU U CACACAA
242 AACQAUU A C3~C~AG 424 C:~F.BAL'~J C ACACAAU
251 ACJJJyC~ A ~' ~ 432 ACAG,AU C LZp.AAACA
261 AA~~GU A AAtZA~A 434 AC:,?.UCU A AAACAaC
2 65 ACUAAAU A AAA 4 4 6 AF,~,~7 C U
2 67 ~F.TJAU A AA:~alsA~14 4 8 C:,r~ CGCV A UGCAL'r~.A
274 AAAAAAU A LIACL7GAP,454 U'nL'C~,U A AC'~",UAC
CA 02468048 2004-06-07
266
4 c~~,AC~ A ~A
s
a
9 tTnACL~U A ~~
60
4 CUAIJACQ C CAt~lC
63
4 ~U A GJC..aGa
67
470 CC G~GAUG~
4 t G~aFv~U U :~I1AG~1AA
89
490 GAAAAUU a ~G~,AU
492 :LV;UffAU A GRAI7UU
495 UL1F~ A ADQL~AA
CA 02468048 2004-06-07
267
Tsble 32: RSV (1B) HH Ribozyme Sequence
at. 88 Ribozyms Saguenc~
Poait3on
iaUDGAW CUGAGG.'aGG~.~C"~AG~'GAA ADULTx_"C
14 C'JG~?.W CL~CGAAaG:~..~CGaA
AL'U~W
18 UQG:r.~u~G C'9G1~CCAAAGGC"CGAA
AUL'GAW
1g GQCK~~0 CUGCCG?~A. AG~"CGlr1 AAWG1U
54 G~U. CUG~I~aAGGC'C~~1'~G sCC~.aaA
AUGWG
57 u~G.~"CG CUG~ -LT~G:~'"~
'~AAG~.'~A AWAUGA
77 UGJC0G0 CL1GALG~~C w AUC?.UCA
,
94 A~G%~ ~L~ ~1 ACG.~~UCU
9? C'0 'C.~e~GR CL'GADGAGGCAC'~AC'"..G
1Ol UG.~"QCL'C ~CG~A AGGCCGAA AGUGaCA
110 ADGLTDAU CDG~LJGF,GGCCGAAAGG.."C'G~A
AiJG.~"UGV
1? GL'GAUCa'U C~L~GC,~.~CGaAA~;CCGr~A AD~I1GG
3
118 G.~"UQAGU COGAI"C~~~ aA ABU
122 C'OCCflGGU COGr'~ ~"CGaA A~.1GAUG
134 G~AU C~CC~'~A~CCG~
AUGJCUC
137 UGUQ3GQ C'DG:~BG.~~GGCC'GAAAG: ~"'C"~AA
AUGaL7GJ
148 GL~UA ~an~I~ IGGCCGAP. AWUGC1G
149 ABU ~ .'~ADG~a
T
150 nAL~LU1 COGAL7GAGGCCGaAAG~CC"~rlA
AAAL7C10Ci
152 Q~ ' ' " ALU~AI7U
154 L~L1C'4AG C~UGAGaCCGrl3'~AG.~~.~C~ AL~r~IAA
157 AUUOAUC CUGF~ ~ .~CCGrI~r AG'L1A
?I7A~J
161 CAD"'~FaLJQ CVGF.UGnC,~,~.CG?aFaA~.~vGaA
AIJCAAC~T
165 caWC~u cvc;~uc~CC~AAAG;~~cGaA
A~mvC
17s Wcvcac cvc~~-.~, cG~-r~ A~
~c:A~ ~
AVv
.. ,
.
188 UUUG'~DC CVGAZ~AGG.- ACL7QWC
208 GnFaUGffA '" ADGflGw
2 09 " AADG'JGG
210 A~~AUG CL?GAUG:,6G.,.AAADGUG
214 G~CAGG " " AUG'JAAA
215 ~~G CUG~ GwCGAA AAL'GLTAA
221 CAUF.GQtJ CUGnLK~G:~CC'G~AA~CG?.?a ACCAG
art
226 CAWUC~ CQGALTGAGGCCGAAAAGJUG~IC
239 L~AA CUG~UG~GCCG?~T~AGGCCGAA
AGUUUCA
2 41 ~ " ~ CGaA ALU1GLTUU
242 WWGL~G CVGT,IIGTaG:~CCGnA~~s~~C'CGAA
AA~GW
251 UGCUOCC G~GAL1G~G AG~~CCGaA ACUUUGU
GCCGAA
261 ~ ULRIIAUU.CUGAUG~CCGAAAGGCCGAA AGUGCUU
265 Wtn7UUA CUGADGaGGCC ~ _ ADUUAGU
267 QAUUUUO C~G~ ~GaA AUAL1WA
274 WG'~ CUG1U"~GGGCGAA AGGCCGAA AUUUUUU
276 UAUCCAG CUG~UGAGG.~.CGAA?1~CGAA
AU'fsUVW
CA 02468048 2004-06-07
268
2 83 -uLT~ ~ ~ ~ '"C"~,74A A(JC~'1~l
2 9 ~ C?. C~~GG.~C""'uMAGC-~~C
G.~a nDLJU~T
3 03 ~C~.'~' .~'.,~ G~C".~AA aGUGC:Ca
304 G.UAC~ ' " G,,lr'~Fs ~C'CGAA AAC,'L~'a~,."C
3 05 G: ~.I~.GG C'C's~'C'GAA~CCGa.A
' ~ C
3 09 ~UG~CA C'L~'G~ . ,
.
317 UC~~.A C~J".~"C~AA G:~.."~AA AUOG"~1
319 ~WG:~ITG CUG:~DGT~G:~CC3aAA~"C~.~1r1
AI~L~',
.2O G.~D~JG.~D CJC,~LtC~'~G~..."C~.~.,A~~CG?~A
~1ADAD~.~
323 CAL.K~UUQ ~,G...~C "~ '.a~A AIT,~
327 CC~UUU CDG?.DGAG~~.,~"CGaAr~~G'~?a ADCGrIDG
337 UUC~G CCGADG~CCG1~.~ G'~.~C~~lA
ACS
3 3 ~DL7CL1AA CL'G~~~ C~.~A
8
3 4 NCO " . ~A: ~Gw~C".-~A
0 ~G~,CCC
3 41 UG~.~UC CGGAUG~.. AAGaACC
.
350 LAADG..,.,C az7GCADQ
3 5 '"W CQG:~G?.G~"CGAAA ~.~CG~ ADG~"G1A
6
5 7 ~G~U C'UGa ' .~~CC".~.1A
AAL1GCCA
3 63 CC,C~UffG AGG'pCO~
372 :~L'GG:~.G CUGr.DG ~.."C~A A1~0
~
375 CQG ~CGA<1 Ac~ADGC
3 8O L~U C'UGaIKa~'~a.~'Cl~hAAG~CCGr.A GAG
3 83 F~CUU~ CDGnGC'~75C',:,w"CGAAA~aCC"~A ADS
3 85 nL~.CQUG CUG:.BGAG.~""C:GnAAGG..'"C".~iA
~aUADC~
z of C-~UCA ' w ACQD'~I~
3 9 G,:~~UOG?, ' ~ ~ AtJ~UAC
6
3 9 .~L'G~ADQ CQG~GAGG'"CG~AAG'~CC'".~AA
8 .~DCAD
402 :~UUL~.UG CZJG.UG~GG.."CG'~'~.A ADUGAGA
406 UG~nAW CUG.'. ' ~ ~"CGAA A~AUp
410 G~G~ CGGAaGAG: CC CGAA ADUD~,L'G
4?'_ UG,~1GG C'UGaL~AG~"Ct=.AP.AGCaCCGAA AAIJtIUAU
sit ~ro c~:~~cAG;o~c,~A AGu,.-~ccaa
A~o~,
t21 G~AA CL1GA ~ ADD
423 UQ~lGat"a C~G~DC,AG~~~CG7~Lf~ALr~CCGaA AImOUC'Os
42 F U~GO '~
4
4 3 UGJUC1~ ' App
2
434 G~7GUW CoC~DGnGGCC ~ aGADDW
4 4 :-.2~L~. CCJGAL1C~J~GGCCGAF~"CGAA AGQLIGpQ
6
4 4 LCDGF.DGAC',:,CC AC,~
8
454 Gff~L~GU CL~UGaGG. ,Ap, Ap~At~
458 UGH, CL1GALIG~AG~CCGAAA~CCGaA ApL~:pG
4 60 L~t~c~G A p~p~
4 63 G~aC'~tJG CUGhBGF,CCCGT~;i4I~~,CCGAA
F~P,UAG
4 67 UCQG~C C'LTGr',L1GAC~GA,~,~~
470 C~,..F~DCUG C'UGAt~G~"CCrCGAA ACBADGG
4 8 L'L:AC~D CCIGnL~a
9 ~'
490 AUCACUA Cl?GAL7C'~G~~CGhAF~:".~CCA~,
F~AU~JpC
492 AAATjDAC CUGr~.U~ AG;~CCGnA AL~aUUQ
499 L'LZJ?,AF,U CUG:,L1G~GGCCGAAAG.~,CCG?l.~
AC'UP.LIAp~
CA 02468048 2004-06-07
269
Table 33 : RSV (1C) HH taiget Sequence
at. Tn.=yet Se~sence at Target SeQueace
Positioa Fositioa
l o U A ac~L~ ~ 6=_ ~ a ar~c~
36 UFaaGnAD U UGr~.LTr~IG169 UQ~C,~ A AC'".n:uL'Q
17 AaGr.:~U U G?~ _ 5 ~U U
n ;~coGav A Ac~c:a 17 s aac:~.--t~-Q tt
~",~.-,~~
25 GaUhAGO A CGsCuGA 181 ULJt,C-C,.,.~J
A ,s~,GJ
31 U ?.AAUP '_92 C~GVT,~,U A Cf,LaC~1
32 ACCA~ A :~.UQ~ 196 GAUAG,U A C'~UCaA
36 C~~,F,AQ U ~COCC 201 ?,tJ~C~V C A
37 UDAAAIJQ U AACOCCC206 AU~,~U ~
38. L~AAUQU A r~CQCCCU216 AUC,~U U ~-G
42 UUUAACL1 C CC~.~"'U221 AU~,~ U U
46 ACUCCCIT U G.~~ 222 Ups 'U ~~UG
50 CCUQGGJ U AGaG~aLJG23i UCaG'LT,J U i1L1L7P.G',e~
51 CLJCG~'ZJU A GAGT.LTG.;2.2 G~,'G;,'p A U~
67 CT~,AU U CADflG~G 234 .~~U U AC~AGCTA
68 aGCA~L'tJ C AL'f3G~GJ~ 235 y,W ,~ C~
71 AAUUCAU U GAG 241 ~p A
76 ALZ7GAGU' A UGAL~AA247 ~,~,U ,~ U~~.y
81 6,TAUGa,U A :.i~Aplm249 G"'Gp,U~,U U U~-C
87 L~AGLJ U AGDDAC 250 U~,~W U ~"
88 AAAAG'vJU A G~LTQAC~,256 UG~~ ,~ ,~V,~~
92 GL~GAU U ACAAaAU 259 CCCL~,U :, avAavAU
93 L~GaDD A 'C.~,AAW 2 62 ~,~,~,U A p
100 ACAF~AAU U UGLtOUGA2 65 U A UUGUAGp
1 Ol CF,AAAUQ U GU9~C 2 67 ;,Up"~U Q G.~
104 AAUDUC~ U UG:~CAAD270 aUaD~,'J ?, GNU
?05 AUUUGUU U GaCAAUG 273 UUG~G~J A AAADCCA
12 0 AUGr.AG'J A GCAUC1GU2 7 8 G'JAA~.AU C CAAUL~1C
125 GUAG~U U GUQAAAA 283 AUCCAAU U UCAC~AC
8 G.=:~WGJ U ' ' 2 84 UCCArIL'U U CACAACA
~9 CAzJUGUtJ A AA3~k~A285 CCAAUW C AG'~A~G~A
335 ~'~AAAU A ACAUG.'U:00 LGCG,G'J A CZ'Je~G'~A
143 ACAUGCU A LIAC'UGAU3 03 C~CU .A CT.p.AAilG
145 AUG."L~U A CDGAUAA316 UG~~G"~u U AaAtTAUG
151 LTACUGAZJ A ~aF.Ut~,AU317 ~,~ A ~~~
155 GAUAAAU U AAUACAU 319 AG.~~OUAI7 A UAL
15 6 A~W A AUACAUV 3 21 GJUAUAU A Uc~;~"AAA
15 9 AAUUAAV A CAUULTAA3 3 8 A 'UC~:,AU U ;,:,CaCAL7
163 ~ ?.AUACAU U UAACUAA339 L'G~~AW A ACACAW.
164 AUACAUIT U nACUAAC3 4 6 i.ACACr,U U Ct'Z1CUCA
CA 02468048 2004-06-07
350 C~UD~p C UCnACCCI
3 52 UU~~DCp C AACC~A
.58 UC:,rICCQ A ADG~C~
3 64 L~,AD~ C
3 66 AUG.~"~ A CVAG?LT
3 69 GQC'UT,CQ A W
379 t~ACAAD U G~AAD
387 U AAAUQCQ
3 8 ' ' A AT,UOD~
8
392 AWAF~AU Q C'UCCaAA
3 93 L'DAAF,Dp C UC'CAAApr
3 95 :~,AUOC~ C G~.AAAA~.
405 AAAAACQ A AG3GADU
412 J~GLT~.D U CnACAAD
413 AGUGADL7 C AACAAL~G
427 GACCAAU U
428 ACC~IADp A U
430 CAADI~ A L~A~
436 ~AAD C AADUADC
440 AADCAAD U ADCL7C',?,A
441 AIJC3,F~IJQ p,
443 CAAZTUAQ C UCv,A~
449 UCUGAAU U ACL7~A
4 5 C~GF,ADp A C~~.;AD
0
453 AADt'~CU ~ G;~7pp~
458 C~ p~,~pp
459 UGGG~Dp O GADC~L7F,
4 63 AUQt7GAQ C pL~ADCC
4 s U~cv tr AA~ccau
s
4 6 L3GADC~ A ADL~A~
6
469 C CAIIAAAD
973 AAIJCCAD A AFB
477 c~,I~AAD Q ADAAI70~
478 ALg,AAUp A
480 AAAIJtmD A ADLJAALp,
483 UDA~,A~ U AAffADCA
484 ~AI7L7 A AAA
487 AADL~AD A UCAACQA
4 89 UUAAI~U C AACL~GC
494 AIICA.ACU A "Cu,AADC
501 AGCAAAU C AADGICA
07 t~ C A~AA~
511 L~1CACQ A ACACCAD
519 ACACCAU D AGUL~AD
520 ~p A ~7~,A~
523 ~ ~ A~,~
524 ABL~,G~ A l~t~AA
CA 02468048 2004-06-07
271
Table 34: RSV (1C) $H Ribozyme Sequence
8H Ribc~ym~ Se~ueac~
~oaitioa
1 'r~n:~~Ct7 C~~aAGCCWnAA~G~..~C~'.y,A
Q a~DGCG~"C
.6 C~Fai3C.~ COGAL~:~~CG,'h?~AG:~"'~.1
~
1'7 ?.C'JLRIJC C?G:~DG~IAGa.."'CGr~
~1ADGC~Q
c. L'G.~IaCZ1 CG'G?.Lr~.'~.G:,C ~~C:,C~.~?,
?S~C~.~aD
25 Q'~G: CLT~Li~t-:~..~CGAaF.~1"C~~
1LZ.~t7C
31 tT'.~F~?.LW CGGat~?~~C~.~.AAC~"'C'..:a.1
.~G,'C~
32 L'IT.iu\W CL'GAL~G-',C,~."C~"C.~sarl
3 G;~CCtm CGGL~G:~.,.~CG~AC~'~CG~1
6
37 G;~~GUU " ~ r~rlD~tlAe1
38 :T CffG'r.~'C~G~."CGr~I ~1AU~7C~a
42 :~CC~AG: C~C'~~~C~ ~lA
4 L'CL~CC CCC,A~~C'Ga~~1 ACT
6
50 C,DC'CCZ7 CZG~G:~G:~c~G~.."C~.~.1
aCG'~AG~
~l CG~UCUC CVC~GC1C"~ ~,aG
CL'G~:,TIG CQGW ~~CG.~G.~'~ .1L'Ll'..~CG
68 nC~-iA~ -CflGni~:aG:,~"CC,~AG~.."CC,~r
rl AAL'D~.~~7
71 C.'-~CGC ~' '~~"'CC~AG".~..~C~~?.r1
F~ADQ
6 WQABC~ C9~ ~ ~ ~ . . -.~ ~..~'1~AD
81 L~:.C'CW CCG?I~G:~G:~"CGAV~CCGM
~L~C
87 6JiaAL2TJ CLJGaI7Cv:G~WG:sw"'C"aTv's
aCZJDGC1A
88 L'GJAALlC CQGADG~G~CC"CGaA .~UU
g2 nLTv'OCGLJ CLG:~~C?.C:~C~~ e~.UC~1AC
03 ~.~1UI~'UG CUGT~~"C'".~'~AG:r.~CGA~
aADC'~1A
oo vc~wcJi cvr~;~:~:~a:~aAA~~ccr~.~A
~
o muc~AAC cvc~~c~c~aa~,.-,c~aA A~nw~
104 n ,~C?~ C~CG?.AAGC,w~C~.a:a?, ACaAAW
l ~C ' AA~A~
05
?ZO nC'-~?.BGC CQGFa~r'aG.~~'AG~"CC~A
aCLiJGQ
125 L~AC CDGAL'G~~'C:G~:~CCG :A ?.LT~'~AC
?2 LnUL7LIW CUG?.L7G?.G.~a~~t ''~~C~.~:~A
8 AC?.r'~C
129 L'L~DUOQ CU~~C".~AAG:~CG:~1 aACAAUG
13 F.G:.ADGU CDGF.LT~G~CCGAF~AGGCC.GAA
143 nL'C'~ Ctl~. -UCn'C,~~CCGaF~AG~.."CGAFa
?~G~"'~IJfa~
145 UL7AtiCAG C~1C,T~ ' ~ C'GJ'~AA nVAGC'rIUU
131 '~L~F~u'O COGA ?aQCAfa'C~l
~ .'~.~QtT CUG?.D~' ~
5
r~
5 :~UG~AU ~-~CGAA AADU~rO
6
139 LAG CflL~~C'CAAA~CCGAA nD~AUO
183 UC~G~JL~ CC7C,?~DGAG~CC~~AG~CCG.'~A
aL7GJAxJi1
1 GG~.GIJU CtJGAUGF~~C'~AAG:~CCGAA
~ :~.DC~,U
4
03 CGJIJ?.GU CLiG~.LKar':GCZCG a~aF.C,GCCG.~,
?t. :L'C~Ja
CA 02468048 2004-06-07
272
169 A~-'~ ~~~~'~~~ AGC~.~
175 ~C~ C'CG~.~' ~".yJ~G:~CC"'.~.AA
~"~7LTA
176 CVCAG-~C C~.~G:~CC"~,C'T;yCCGAA
aAGC'G7D
181 ACL1GCL~J C'GG~L~G~~C"~euaG;~~C.~,,AA
~.G~"~?u~
192 DRUG COC~.'~.GG..~CCGAA ~CQG
1 g U~UL1G CG~~,G~ C~,-? A AUG<IALC
6
201 UCAABOU C~'"CGAAAC'~~.~.."CGAA AVOU~U
.
206 G~~CAWC CCTGA ' GV.~CGaA ABWGaU
216 GAACAC CVGhDGF,G:aC ~ ulAC"'~,~CCG~A
AUG~~C'~U
221 :~D""~ CUGADGnGaCCGAAAG~CGA<1 AC~G'-,P.U
222
231 UL7G~AU ~ '~'CG~nF.G,CCCa?~A .~G~UG~
232 C~G'~A C~A~.-"C~~AAG~~C~.~A AaC~LJC-C
234 ''L~CIJCTGtJ C'JCA~GGCCCGAA AUAAC?.U
235 CZ.mCCUG CUGAL ~ ~CGaA AALmACa
241 AUAUCAC CbGAL3G~.GGCCGAAAGGCCGaA
ACOL'GUA
247 G;~.AAA CVGaL~CGAAAGGCCGaA ?.LG'~C',7A
249 BAGwCA COGAC'"~1A~.C~CCGAA
250 DL~GGGC ~ A~.QCA
256 ~ADL~t1 C'CK~"C'GA7?,3~f~C'CGAA
AG~Ar~
2 5 A~nUC~U ~~...~C'~AAAG;~CCGaA AL1L~G~
9
2 62 AC ;ALmU CUGr~'~C1G<".GG~."CC~?~AAG~CC~.~,
aUCTAUUr~.
2 65 ACUACAA ~GF~~CCG.~A ADCIAUG~a
2 67 UC~CL~C ' " - AAC'~.~CCGAA A~DCmU
270 AUtJL7CAC C'L~"CGCGAA AG~AL~U
.
273 ~CGaA ACL~CA?r
U~%~ CHUG ~
278 GAFsAUUG CLCC'GAAAGGCC'".~A AU~C
283 GAGA CflGhUGAGuCCC~Ae,GGCCGaA AWG:~1U
2 84 wG CLTGF.DC'snG'vC s~.AUtJG~
285 ~'~1UGU CDGAL7GhGu'CGAA~aG~CGAA
AAaLTGGG
300 UWG'~.G C'CTC~AGC~CCG:~AGGCCC~A
ACL'C,:~C?~
303 CJ~JUG C " ~ uGGCCGAA AGVALUG
316 G',L~U CUGA . CGAA ACCUCCA
31? CCA~'~L~ COGF,OGAG~C'G74AAGGCCGAA
AACCUCC
319 CCC'".~.~ C'O~I7GacHCCGA3i,~IG~CCCuIA
ALmACCCT
321 UtmCCCA C~GAiIG:~"CGAAAGGCCGAA aI7AL~C
338 AB~.JGUtJ CQGALKAG~~'C:c:~,AG;,CCGaA
ADUCCAU
339 AAL1GUGU CUGAI~FSG:~~CGAAAG~GCCGAA
AAIJUCC~
3 4 BGAGAGC CUGAUGnGG~~'C'GAAAG:~. C~.~A
6 ALT~GQL7
350 AG"~GA CL1GAUGAGG~."'C~"~AC~CC"..AA
AG~~A~JG
3 52 UCAGu''TJU CDGAL~C'~G:~CCGAA~'~GGCCGAA
ACAGCAA
358 AGACCAU Ct7GAL~t~:~C~GCCGAAGGCCG~.A
AG,rJQGa
364 UC~GOA CQGF~GGCCGAAAC~:~CCGAA ACCAL1QA
366 CAUCUAG NGAL~G:~~'C:GAAAGGCCGaA
AC~ACCAU
369 UG'JCAUC C'CGnI7GAG~CC'GAAA~CGAA
p~p,C
379 AUUUCAC C'UGAUG'~~,GCC''"AA AUUGUCA
3 s7 A~:"~uuo cQC~uc~.G~cccvw.GuccG:~
avwc~c
3sa G:~Atnr cvcAUGaGvCcGaAACc,ccG.~
AavwC~,
392 QCIUG:zAG CDGAUGAGG:.C~.~Af~AGGCCG~
AUWAAU
CA 02468048 2004-06-07
273
393 UOWG,~~A Cu'CC:GTaAAG~~~GA~ AAUOQAA
Up~pG ~~CC~1',AAG.'~~~~~.A AG:~u~.D0t7
405 AAUCACD CL7"~~~C'GAAA~CCGaA AGJOO~t7
412 AU~G ~~C~A~.~C".~A ADi~CO~T
413 ~ ~'"~ AAUCACD
427 UflCnIRU COGF ' ' "C'GaA ADL7C,GUC
428 ~ CDGF~GG~ ~ .~AA AADOGGD
430 UGaDOCA CRGF.L~GG.. .~A ADAADDG
436 GraUFaAULI CUGAi~G~~CGAAA~."vGaA AOQCa'
Im
440 U ~~'"'~~ ALnTvIW
443 A~A~ ~~C~ AADtKAI7
443 LTAFaUOU C'GC~G~' ~aAA~"C'".~1A ~1D~
449
450 C~U'"~A AADOCAG
453 G'~AAUCC C'LtC~GAC~C'GAAA~.."CGAA A6.1AAW
458 AAGAUCA CRGA~AC.GCCGAAAGG~GAA AUC~IAG
459 I~1GAUC CGGAL~G~..~C"'WvlA s~.,C'C'.~A
AAUC''.Ae1
4 63 G~ADLmA CLTGAL7GAG~,COGAAA~.."CGAA
AI7CAAaL7
465 ACC~~~AA~C~A AGaDCAA
4 66 ~aDGGaU C~GF.~G:~CCC~aAG.~~~CaA AAGaDCA
4 69 %.DQOT~I~ -CUGUGA~CGAAAG~.""'C".~r1
A~AGA
473 ~'~~:~C ~ A AUG~raL7CT
477 UAAL'tg,U CoG<,'UG~G~~'CCaAhGG..TGaA
ADUC~
478 UUAAULm CUGaDG?,GG.."CGaAA~CCGAA AADQC~4D
480 Ln'~UDAAU CUCCGAAAGGCC~A AUAADOl7
4 83 UGT.~.UtJ CD'Gr.BG.'1G~;~CCG?~A:~"CCAA
AUtmi~,
484 UOGAImU C'.~.~AG~CG?~AAGGCCG1D, Ap.DOAUA
487 L~Ga CQGACGAAA~.."CGaA :,UC~ApO
4 89 G~."OAGW CUGr.L~'~~C~~AAF~CC".~AA ADADQAA
494 G:,D~7~ CUGAi7G~C,C~~'~GAAAGG."CGAF,
AG~T(3GAIJ
501 DG?~CALU CL'GaLK~raC~~~~~C.'Gi~AAG~CCC~A
AUWGCp
507 UGUQ7iGU CCTGA~GGCC'GAAAG~CtGAA ACAT;70GA
511 A~ ~ ~ CGAA AGJGAC~
519 AUOAAC~T CC.'G~,UGAGGCCC,AAW."CGAA
AL~L~
320 WiDDAAC CnGaDGAG.~~.~CGAAA~C.'~Fa AAD~,~pG
523 UtU~DU C~LCC~CGAA AC"~,ADG
324 UUUAI~J CL~G~sUGAG~,.~DGnAAG~sCCGr~A
AAC~T.~AU
CA 02468048 2004-06-07
274
Table 33: RSV (I~ HH Target Sequence
nt . &8 Ta.r Qet 9equeaceat. H8 Tappet SeCuenca
Pcsition Positioa
9 '-CPU A C'~AAGAII 217 G,~'?AUGU U ,~U?,UC~~
21 GLTC.:,.."ZJ C 218 G'v~ A
UGAGCa?.
23 ~ JCJ Q AG:aAaG 2 2 0 AUC,'OL~J A L'GC".~G
24 G:,~'~ .~ GC,'~AaGLT229 G'.~,I~ C v..~
.
32 G''~CL7 C : '~nG~GA231 GnU~CU A Cz'~~~,
37 ~~C~GU U C-~:AL~?.U235 LTCU'.,~G.~"U U
'
45 G~GAQ .~ C?.CLJC~r236 CQAG;JU A G;~"~"~,
0 AUACACU C AACAAAG 2 5 4 .~Cr,C~,.1U ,~
60 C-J~GaU C AACUQCU 2 60 UAA?~1?JJ A C','G'-~G
65 AU 'C.~D U CUGDCAU2 53 AAA~,Cp C
66 UG~ALVU C UGDCADC 277 G~'~U ,'~, LCD
70 C~UC~GU C AUCG'uC-C279 G.~~U C A~pA
73 cJGUCAU C CAC~CAAA2 84 ~ AUCADGLT ,~,
A
82 ~G-;~,AU A CACC~L'C299 r.LT::.=,f,'U ,~
G~AA
89 ACAC;.?U C C.'-.AC~.~305 UAGALGLJ ,'~, ACC
108 AG~G~.U a G~TAUUGA315 AACAG~Q C C;rG~
111 GU A UC,~',~ . 318 AC~.L'C~ C A:~.C~1C~.U
113 AIR .~r U U Gr'~~L'UC3 2 6 naG~G~,U U ~..~.LT~?,
117 LV,BUGAU A CpC~ 32? AG?GWJLJ A AUG
1.2 0 L~LRCLT C CI,RAUUA3 4 6 ?.UGAAa,U U GG~t~,G
123 ZIAC'JCCLJ .~, 347 L'GnA~.UU U -C
AUZ?AUGA ' v T
12 6 UC CL~U U ADG:,UG'J? 5 5 G:~AG~1GV U A?
C.~UUG
12 7 ccz~u a 3 5 s :~,-a a ac~.L~Gc
14 6 :~ACKCAU C Aria 3 61 UUAACAU U
150 G~UCJ-.AU A .~GZ~~IJG370 G:J,AG'~p U 1"~,G~CU
154 ~AAGD U AUG~C 371 G~,AG'~W A p,Ca,~,-,G
155 A UGUC~vCA 383 C~JG~,r~U U C~UC.~
'! 6 6 C~F~BGU U AU~ADC 3 8 4 L'G:Js~.UU C AAAUCAA
16'l G~.~BGJU A ULIF,AIfiA3 89 UL'C'.~.AAU C AAC?,UCG
16 9 A ~ ~ U U AAI1CAC~3 9 5 UCA?.C~U U GaG~G
17 0 UGau.~W A AUCACAG 4 O 1 UL'G?L? U A GnAUCUA
17 3 L~UUAAU C AC:~F~AG4 0 6 ALTAGAAB C L~CA
A~
18 6 :~G:,DG~"G A AUCAUAA4 0 8 AG:~.UC'J A ' '
' UC
1 8 9 v~~~U C AUA~W 415 AG~U C CQAC~.AA
192 L~UG~U A AAUUCAC 418 AAAUCCU A G~AA,p.AA
19 6 CA~r '~U U Cf~CLIG4 31 AAAUGCU A AA.AGAAA
;G
197 AL;AF~AUtJ C ACUG~~.~LJ449 GAGAG,"'U A C,CL'CCaG
205 ACCIG:~.~~IJ U 453 G"~L1AG.~p C
AAITAGGQ
2 0 6 C~JG;N'~T A AUF 4 6 0 C ~U A C-~G"~,U
G.,'ZTA
209 G:w~~~.T'n:~U A 4?2 C~L"G~CU C UCCL'Ga.U
G.~~JAIJGU
213 AAU'nGa.-U A UGUL'AUA4 7 4 UG.CZTCU C CUG,ULTG
CA 02468048 2004-06-07
275
4 8 ~U. U GUGC.:~1 E 9 6 UUUDG.3U A UAGCAC.~
0
4 91 ~GAQ A Ai7?~UL~D E 9 8 UQG.~~JAU ?~ GCAG~AU
494 UG~AD A U~ 706 G~~G'~U C WCCACC
496 A~IJ U AL~Im 708 ACAADCQ U CQACC~G
497 ~~W A UG~.IIAG 709 CAAUC'OU C UACC.~
501 A ~GC 711 AUCULJCU A CG~GaGV,
503 ~~U A GNU 726 UGGCAGU A G~Gpr
511 "G~C~ U AGJAaUA 731 G~G?.V'U U GAAGG
art
'S~ G'1GCAUl7 A G~F.:,L~A740 ~~ U U WUGC3~G
515 C~ A AL~ACa~1 741 AG~~UO U UUGCAGa
518 A ACLU.A.~O 742 G:rC~DL'U U L~uG~1
522 AnBAACO A AADUAGC 743 G:a~U~TUU U G~GaarU
52 AC~AU U A 7 51 GC.~U U G'JCUAUG
6
527 C~:,DU A GGC~G 754 G:~WGU U UAUGA?rU
544 G%~AG~ C ~'~ 755 GaUUGDU U AUGAAUG
549 AUC~ C UOACAGC 756 AUUGaUO A UGAAUGC
551 C~JCQ U AC<.GCCG 766 AAL'GCCU A UG.~~JG~A
552 A G~GQ 787 C~GFsL~ U ACG:~GG
563 CC~U U AG:~,GAG 788 UGat~LJU A CGG'LJG
a6
564 CVs A G~1CAGC 800 G~GU C UCU1GCAA
573 "Q A A~ 802 G~.GUCD U AGC.~AAA
576 AG.."DA~aU A ALG'UCCU803 GhGQCW A GC~.AAaU
581 A~AUW C CaJ,F~T,AA 811 GCAAAAU C AGJUAAA
584 ADGDC'CD A A~A~G 815 aAUC~.GiJ U AAAAAUA
603 G~AACGa U AC~AGG 816 AtICAGW A aAAA~U
604 AAAC.;UU A CAC ~ 822 LJAAAA.rIU A U~JC~
613 AAAGGCU U ACUACCC 824 AAAAL?~U U AUGUUAG
614 AT~COQ A C~CCCA 825 AAAUAUU A UG~Ga
617 GCOUACU A CCCAAGG E29 AUUAUGU U AGCACAU
629 AC~GAU A ~CAACA 830 ULD~UGW A GGACAL'G
640 AAC~G.."'Q U CQAL~,AA840 ACr.UGCU A GuGUG.~~,
641 AG~GC~ C D~.BGAA6 866 AACAAGU U GWGaGa
643 AG~."~C9 A G 869 aAC~GLT U GAG""UnLT
652 G:~AG~ U UGJ~AAA 875 UQG~~U U UAUGAAU
E53 A~ U G'~.:~AC 876 UGAGV~UU U AL'GAAVA
663 AAAACAD C CCCiCDO 877 Ga,C~'~JLJLT A
UGaAVAU
670 CCCCACL7 U ~r~GAU 883 UFsUGaAU A UGCCCAA
671 CC~.:,C~ U AUAG~G 895 CJ,?.AAAU U G:>GLJGa~U
672 ~C.'L70U A UAGhDC~ 913 G:~1C:,~.U U CUACCAU
674 ACUDLIAU A GABGUW 914 CAGGhUU C UACCAUA
680 ~D"' ALT U ~70C 916 G:~:.UUCU A CCA~VA
681 AGAUGW U UUG'WCA 921 CUACCAU A UAUUGAA
682 GAUGDW U DGaUCAU 923 ACCAUAU A UUGAACA
683 AUGUQW U GWCJ,W 925 CAVAUAU U GAACAAC
686 UUQUDGU U CAUWDG 943 AAAG~J,U C AULmULTA
687 UUUamU C ADUCT~ 946 GCAUCAU U AUL~1UCQ
E90 LIGLnJCAU U UUG~D 947 CAUCAUU A UUAUCW
691 GLIffCAUU U UG.~''i~UA949 UCAUVAU U AUCUUE1G
692 WC~.~JU U G~'~nDAG 950 Cr.UUAW A UCUWG?r
CA 02468048 2004-06-07
276
52 UDAUL~SU C UL'UGACO
954 :~UI~,iJCV U
UGaLZJCA
055 ~3'aDCULJ U GAL"DC1~
960 WUGACU C AF~DQDCC
964 ACffCAAU U UCC~C
965 CUCAAUU U CC9C:~1CQ
966 C Cep
969 ~BOQCCU C AC~CGC
973 C~21G~CQ U CUCCAGO
974 C'JC~.CL'U C
UC'C~GL'G
976 UCDUCIJ C CAA
9 e3 c~~ a caA~~aG
986 GLG~GU A Ut~,G,CA
988 C~AC~U U AG:~CAAU
989 A GGCAADG
1007 CQG:,~."'CQ A
CC.~1LJA~,
ol mmc~ a auGGVa~c
102 "G~G~L;LT A CAGAG;G'U
4
.032 C:~~ A CAL~CGAG
1044 G~G~ C CQ
'_ 050 UC :~GaU C
:052 :~T~~ A ~GAL'G
.054 G-'~C~',U A UGa,UGC~a
1072 aACr,CAU A ~~
085 :~.ACAACp C AAAGAAA
1103 ~U U AAC~1CA
.104 BGUGAUU A AL~ACAG
.108 ?.ULmACn A CAGE
i'_15 AC?.GvJGU A CGAGAC'p
.? 18 GJGJACC A GaGZIpGa,
'-123 U C-~AGCA
1 '-3 1,AGAACL1 A GAGGCLm
9
1146 "U A
1 14 AG~C'UAQ C AA.ACADC
8
1_55 CAAACAU C AGCD~,
160 AUCA.GCp U AaDCCAp,
11 61 L1CAC,C~ A Aat:CAp,A
4 t'ZTUAF,U C CAhAAGA
C
7 3 AAP FLU A F.UC~DGU
'_'_ ALIG:yUGU A GAGCUL1I7
81
=a7 -v U UGac~
8 g vp
-- U G:~GOLIAA
--g3 U A.AUAAAA
'-'--94WGAG~p A ADAAAAA
CA 02468048 2004-06-07
277
Table 36: RSV (I~ HH Ribozpme Sequence
at. EH Ribozyme Sequence
positioa
g AL'CUOUCa CVGA~C~CC~.1 ADUG'GCC
21 UCG..~JAA CL'C,AiI~GGCC~"'C".~r1
AG..'"C~aL'C
23 C~TppG,,"U CDC~A~C~'~G:~: C".~ AG?~GCCA
24 ACIItJL'GC CJG?JD~GGCCGAAAC~."C"'~?.ar
M
32 UGaCVII CUC~UG'aG aCCG aAAGVCC:~r1
AC'JUUGC
37 AUCAUUC CUG~GAG: ~."C'G1~~SCC".~1?l
ACGt'GaC
45 UQGAGUG C'CIt~JGAG~.~CG~AGGCCG1~
AUG1UG~C
50 CUtIiIGLIU CUGA ' "'~ :,GJG'JAU
60 aG:.AG'UU CUGaUC,~GGCC~~:~AAGGCC~.~1
ALCUUL7G
65 AUGACAG CDGP.UGDGS,'CCGC".-. AA AG70GAU
66 GC~7GALJGAG ;CCG?~AAGG.~C'.~e1 ~'~
70 Gt'OGGp.U " .. -.~A aCAG?.?~G
73 UWGCUG CUGAUGAG a~"CCCG~ A~GACAG
82 C~UG~"'~7G CZJG~GGCC~.CCG~1 ALUG'G.'V
89 UCCGiIUG CQGAUGAG~~ "~CGAA ~~T,U
~
108 UG'~AUAC CUG?U
~ ~ ~ CGnA AQCUCCO
111 GUAUCAA CUGAUGAGGCV"~A:~AG:~CC".~
a ACUAUC'J
1?3 C~AUC CUGAUGAG~CUGAAAGr.~CGaA ADACDAU
117 UUAGGAG CUGADGAGGCCGaAAG:~."CGaA
AUC1ADA
120 UAAUUAG CW:.L7GT~C,GCCGnAACs:~C'".~1
AGUAUCA
123 UCAUAAU CQGAUGAGGCCG.~AACsav."C~,?.A
AGG1G"UA
12 AGUCAZJ CUGA "~CGAA .'~rL~
6
127 CACADCA CUC,?aL~G.ovC'CGr'~r~' ~'1r~
146 ACOUALJQ CQGAUG~ ' ALGA
.50 CAUAACO CDGADGAGG~~C~C~.~A ADWFaLTG
154 G~"CACAU CUGr.UGAGGCCG ~ ., ACUCTAUU
'~5 DG'' CACA CZIG~DGA~GGC ~"CG?.A AAC~UAU
166 GAUUAAD CCICiA~GCCGTa~arlC~~~.CGaA
AG'aDGv.~C
1 s~ Uc~umA cvcAC~,~ ~ AACaUCc
169 U~JGAW CUGAUG:~G:~CCGA?~CG1A ADAACAU
~
170 CQG'JGAU CCiGAUGnG~C'G~aAAGGCC".yaA
?i.~T~?aCA
173 CWCUGU CiJ~GADGAG;~C'C:GAT~AGG~~C:GnA
AD07iAUA
186 Ut~UGAU CUGAL7GAGGCCG AAC,GCCGaA
AC,~DCU
189 AAUUUAU CUGAUG CGAP. AU~.GC~1
192 GUGAAUU CUGF~I1GAGGCCGAAAG sCCG~
ALG?.WA
196 CC".~,GUG CtJGAL7GAGGCCG.'~F~AG:,cC~a?.A
AIJWAUG
197 ACCCAGU CUGT~I~aGGCC:GaAACGCC:GaPa
AAL'OQAU
205 ACr'JAW CUGAT1GAGGC~CGaA ACCCAGU
206 UACCUAU CUGALJGaGGCCGAAAGGCC~.~,A
AnCCCAG
209 AGUACC CUGAUGAGG..~~AC~.~.C~~ AL'UAACC
~
213 UAUAACA ~ CUGhL~;C;~CGT~'~GGCCC~,
ACCUAUU
CA 02468048 2004-06-07
278
217 C~~ ' ~ '~~ ACF.uACC
218 UC"~ cJC~ G: w "~""C GAA AAGUAC
22o c~cc~a ~x~: C~AAGwCc~A ago
229 cn,~,cc~A c
' ~~cGt.Ar~cccaA
Ac ~ucc~C
231 CCL1FACC CUG'r~GG.~'~AnG:~CG?.A AC,'~.G'iUC
235 CC~C~1 C~B" ~-~C~~C~.3AA AC~CA
236 CL'CUQCC CUGr'.~AG:~C'""..~P.AC~.."CGAA
AACC'~1G
2 54 G~L'~U CUGZ.UGhGGCCG~3!~~CNCC GA,A
AB~Gp
2 6 C'9C'CG.'~ AGGCC"vAA,ALvCt GAA
0 C ~Z~ AUWQC~Fr
263 ' v C' ~ ' AGUAUW
277 LTAC~DGA COGAL~G~s~~AACCCGAA AUCCCGC
279 ~CkU CL1GF~U GAGGC.:.~~AAGuCCGaA
284 ULiC~'~ CL1GAI~GG..~~"CGAA ACAL1GA'U
299 ABC CLTG:~ ~C~~CGaA AL'VC".~U
305 ~ CL~ GGCCGaAA~C'~A ACABCLm
315 UDC CGG~ GC,~,~C'C~AAA~CCGAA
ADG~JU
318 F~DC~JC C~IGA~AG~.Cu%~AG:~-"CC~A
AL'GA~V
326 UBCC3~D0 G~A~ AG~~CGAAAGw'tGAA A~~
327 C'~R ~"~AAC"~"CGaA AADGaCQ
3 4 ~ " AUDC~4D
6
347 ACAC~C CLJC~ GGCCGAAA~CCGAA AAULNCA
355 ~O CUGAD GAGGCC'GAAAG:~CCGAA
AC~CQtaC
356 CG~J CL7G:~ AGGCCGT,AA~CC".~AA
AACACC)t1
~
3 61 G~."'.1BG,."C ~ AUC"~A
370 AGCY CDG.',I ~~,GGC~~CGAA AGCWGC
371 CAC~QG'J ~ AAGC'L7C1G
3 83 BG~G "CG?,A AU~C.1~
384 QL~AU00 AAUC70C~1
3 89 C: ~.UG~ CDC~UGAG~
AD(JUG7~pr
395 CQAUCUC CIJGT~DG:,G.~~CCGAAAGu.."CGAA
AUGaL7GA
401 UACnBOC C~G:,UG3~GGCCGAAAGGCCGAA
AUCDCAA
406 L'QQUCOA CC~ AG~~'~AAAG~,CCGAA
ADnCQAD
4 08 GAUQLJOC COGT~""~'CGAA AGT,DUC"0
415 UU~G COGZ.L ~C~;t~CCGAAA~~CGAA
ALTppOC~
418 U~ COGAD G?~GGCCC',aAT~C~A
AC~AVC7U
431 ~ AGCADOp
449 CL~C ACCCJCQC
453 AC,C~CC
460 AUGC'CUG CDG;,~ 'AA~CCGaA ADUC'OGG
472 AUC~ C~L 3GnGGw~CGAAAGC",CCGAA
A~
474 G,:SUCAG ~ A~
480 AUCCCAC C'L1G~~I1GAGGCCt'~7~.~~lA~CCGAp,
AUCAGGA
491 ~.tIF~BAU C~D ~G~F,GC~CCGAAAG~~CGAA
ADCADCC
4 9 LmCADAA CL~' ~AC~C ~ ADUAL7CA
4
4 9 UAIB~CAg AQAU~
6
497 C~ffACA Ct~ 7GAGGC'0.'C~F.AA~CCGAA
AF.L~UtJA
501 G~.'Z1G.."~ ~AGG..~C'C~.AAGGCCGAA
CL1G:~L ACALmAx1'
503 nUC~' .~GC
CD~VGAG~:CGa,AAGGCCGAA
ALmCF,.LTA
511 LT"nLTJ'i,CLJ 3GAGGiC:CG.~AAG~~CGAA
CUGAL AUGC'UGC
CA 02468048 2004-06-07
279
512 ~t~C CIJGADG' ~ ,, _ , . ...C,..~A
AADG.."L1G
53 ~~ CUGr~~CGiirIAWCC'". ',r~,1 ACG~1r1L7G
518 ADOCAGV C~~ w;AAG~CCGaA ADC~CC~
522 G.~J~W ~ . ....~CGaA
526 ~~0 CC~"CJAAAG~CC~.sAA ADI~GfJ '
527 Cv~~a~ c~C~'~:~cCCaA AF.ov~
544 'UCCA CO~GAL7C~G~CC'~AA~CGAA AOCDGCC
549 G.~'D~A . CQ~v'r~~"v,:~G~..~C"'.~AAG~CCGAA
ACCAGaLI
551 C~~:COGO C ~CG~1 AGACG'~G
_552 AC~.~;~~OG CG~..~~AAG~..'~CGaA AT,GACCA
63 C;1CL1CC0 . .. A~CA~CG~
564 G~"OC~CC CUGaCCG%~'~J~I. ~CCGaW
?~AUCACG
573 aC:~DL~U COG:~~CC~~G:~."CG~1A AG~'UCL:C
57 .aG:~Ca~ C'~aDC~G~.."C'" ~ AD~GCZJ
6
581 TJtnJUflA~ COGAT~G~~C"~ArJaG:~CC".~A
ACliLIt~D
584 C:JJOflW CCG?WG~CC~C'".~A AG~~AG~U
603 C~ C~C~A~CCG?~A ACCVOLIC
604 G."N09G CDG~L~?~AAO~."CGAA AACGi7G0
613 ~ ' AG~~CZJCtT
614 ~'~ ' AAGCCW
617 CC~OG:~G C~D"~AG~CCCAAA~CCG?~1 AGLmAGC
629 ~~CCC~~C~ A~CC'J
640 WCALnG CC1~UG " ' CGAA AG~.'"I7~Cr'LT
641 CC~Lm C~G:~GGC'CG~AA~~'CG~A AAGCO~
643 CACWCA CG~~G~CCGAAAG~~CG?~A AGaAGCU
652 UUQWCA C'"'CG?,h~~C~CCGaA ACJ~CDCC
653 GQUL1WC CGGF~L~ a:,CA~
6 63 iIAGO~ CL'G%~~C~..GGCCa,.~CGAA A'CC~7U~7tJ
670 AL7Ct~.tm CQG:~DGAGGCCG.~r'..'~CGAA
AGLA~:~G s
671 C~C"CFU C~GaG~CC~-' .~CC,Ar1 AAGLIC,
;~;
672 A~~ CL~CCGaAAGr.CGAA AAAGiIG
674 F~F.ACAUC CC~ ~ CGAA AtTAAAGL7
680 G~ACAAA Cue. " CGAA ACAUCQp,
681 CG:~AC~Fa CUG'~r 'nG~CCGAAAG:yCCGAA
AACADCU
682 ACA CflC,F~L~GA ~ "~C~.aAA AAACAUC
.
683 AAI~AC C
" ~ CGAA AAAACAQ
6 8 CP.AAJ~ItG ACAAAAA
6
687 C~~AU ' ~ a~CGAA AACAAAA
6 9 ALmC'CAA CDG:.DG?~G~CC'C~.AACuCCG.~.F,
0 ADGAACA
691 UF~JACCA CUGr'.L~t'GAAAG~CCG?.A
AA~GAAC
692 CLRDACC C' ~ ~ AAAL7GaA
696 L'GLJG~"tD~ CDGALJCv.GC.CCG;AAG~CCGAA
ACCAAAA
698 AL1L1GUGC CUGAL1GAG~CCGAAFaGGCCGaA
AITACCAA
706 G.~"UF.GAA CUGF~ ' ""C~C'GaA Am~C
708 ~ CUC-~"L~G CDG:,IJGAGGCC'GAAA~G~CCGAA
ACADLJGG
7 09 UCL1G~~JA C'DGF.LJ~C'~:~CCGAAAG:~CGAA
AAGAIJG'C;
711 CC~CUGG CLT.J. ' ~ C~CGAA AGAAG?.Q
726 LG'~ACZ7C.Ci3GA~GAG~CCGAAAwCCGAA
AC~GCCA
731 vcccvuc cvcavcacNccc"~,~c;~CCr,:~
AcUCCn~C
CA 02468048 2004-06-07
280
7 4 C~v.=riAAC~C'~'aG:~C CC~lGw'"C
0 -GiA AUCCC~U
741 ~JG:~A C'~Cv,L~AG~CC""C".~1 hAIICCC:J
742 CC~G:~ CGGntIGnC'~CC"~,~',A~xGaA
.~AADCCC
743 :~BCC'JGCC'"'wAL~aGvCCC'~inr?duCCG?~
AAAAUCC
7 51 G~AC CCGi~IR'~~CG~C,~~CG~A
ADC~GC
754 ~WCAUA C'JGnI~G:~CCG~A:,GGCCGAA
ACAAUCC
7 55 G.DQG~U ' AACF.ADC
7 5 C.~.~BL'G~CL'G;~L~A~.."'CG~.ArI~"C~.~A
6 AAACAaU
766 i~:~:CC~ CLTC,~L~C,:,~ GaA~G~."CGA?r
AC,~Up
787 CC~CC"~.JCUGAL~C,hG;~~C".,Ar~C~..-.?~A
AG~L'CAC
7 8 CC G~CCG ' ' aAC~~C?,
8
8 00 ,p, C J - . .
8 02 L'~.."Q CL'~~"Ci'~A:,C,"v.'"CG:~A
AGACL3CC
803 :~L~GC CL'G<1UG:~GGCC~~CG1~ AAGAC':C
e11 Lc~AC~ c~G.:.tx~G~CCG.~aa.~,.~ccaa
A~ov~;.
~uw c~G~. ' a
816 AL~DUW CCC'G~AA~CC~~ ;?, AaC~lU
c22 ~ Ci7~"'CGAA.A~~'C'GaA AUppp0~1
824 C~CAU ,~,BpUp
825 CC~ACP. CLlc"CGAa,F~t~LCGAA AA~Q
E 2 ADGUCCQ CL~~GG..~C~~..~;1 ACA~AU
9
8 3 CADGL1CC C'CGht~G:~CCG?.~~AG~CCGr'L~
0 AACAUAA
0 4 DC,C.~CACCL ~a"C ' ' AG~aUG'J
0
8 6 '~CT~AC C'~ -G.BGAGGC'CGAAAC'~..."CGAA
6 AC~LT
869 AAACCOC CC~C~"CGAAAGGCCG?~ AC~ACW
875 :~UDCF~tIAgyp,
876 BAUOCAU C~ AACCCG
877 AUAL1UCA C'JG~,G:,CC".,Fu~."CGA?,
A:,ACCVC
8 8 LUGw:A ~ - p~p~,
3
c 9 AC",r=.CCCC'JG:~ ~ ~C'"AA AUI7pDUG
5
913 :~'G:~,~GC~?GhL~CG~,G:~..~CG~aA
AQCCQGC
914 ~L7~~ C~UC"~AGGCCGAAA~.~CGAA
916 G CCGA2~,Cr,CC",,,AF,AGu.."~A
AGAp
921 BQGJ~A ' ,w.~CGA1 AUG~"~G
923 UC~CAA
9 2 GuLIC;JOCCDG? 'CGAA ,'~~L~T,
5
943 U~AU ~-~pUp
9 4 AC~AU CQGF.LIGnGGCCGAA~,G~;~CCGaA
6 AaGALTGC
947 AAGA~A CUGAL~F~G:".."CG.~T,A~CCGP.A
~AL1G
9 4 CAA~GF~IJCLJG:-.IJC~G;~~G?..'~
9 A~AI3GF,
o c LT w~.,~,~C'CC"C'CGAAAGGV."CGAA
0 AADAAUG
952 A~ CDG~I7GAGS,~C~~' AnGGCCGap.
ALRAL~A
5 4 L'GAGi7C~C~JG:~L7G~Cr,CC'~'~,AG',CCGAA
AGA~AU
55 L'L'GAGUCC'C1C~AC~CCGAAA~~CCGA?~
AnGAUAA
960 GC~AAW CL'GA w ' CC.CGAA ~GaCAAp,
9 64 ~ CUGAI~."CGAAAGGCCGAa,
AUL~7
965 nGCGi~GG CDGF.UGF~c:~CCCGAAF~CCGAA
AALJG'GAG
966 ~JC~,G CUG;~L'GAGG,.~r'C~A~,T,G:,CCGA?,
,~Ap.L~3Gp~
969 C,~GnAGU CUG:~L'Gr~C,GCCGAF~AGGCCG?.A
?.C,G~AU
CA 02468048 2004-06-07
281
573 ACOG:v~ CUGhLTv,AGGCCGAA~~G:,CC".~.
~.G""'v~GG
974 C~C~~s?,r'~AGv~:C~~ ae~uara' G
976 UACACUG CQGAB'"~~C'CAAF~.."C".~
:~'
083 C.'~'UAC CLIGn,I~AAr'~CC,1A aG~
086 UC,.~.CLTAA CUC,nL~s'nC,.~~CC~aA'n?.G:~C~u~A
r~C?~
988 rUDGCCU CUGi.L'C~~GG.'W~'~r~G:~:.:f~a
a~..CLUsC
c8g CAUO'GCC CQGAI~~CGAFaAG~~~ ar.DACUA
1007 C CUGaL~a~.GVaCCGr~?.AWCCv~a aC,~vW'~1G
1 pi CLICCCfIU C~"C"va."~e1 aL'GCCLJ~s
3
1024 ACCZJCLTG CL7GAUGAGG.."C":AAnG.IC~~
aCLCr'CC
1032 CUC"w;JG CflG,AI~G:~CC"'~SG~~:.CGaA
1044 AGnUCW CCIGnI~~z~CC~aA a~.s~.~CCsn?a
nUrL.'CCrC
1050 UCaUAUA CUGAL'GaGGCC~~?.~Z ".v'v'~
:~':C:rL:GA
10_'2 C~UC.~UA CUGAL~G:,CCGAP.AGG..~A
?.G~rCW
1054 UC.:AIJCA COGAL1G~.G~.CCyIr~C~,:.CGAA
aI~C
1072 UUG~ CUG:,QG:~GGCCGaAaC~cCG:,a aL'CZCW
1085 UG'UCULJU CUC~~'C~AAG:,CCG,?~a aGvL'GL'U
" 03 UC~G7D C'C~t~aI~AGG~~CC~A1C,:,~~CG?.~
?LT~G1C
1104 cvG~~u ~ A~~
pa c,c~ ~ ~:.a aG~a~r
11 ? AL~1L~~G CUGAW' "~C~.y,a aC?.~CGGZT
cc~ACUC cuca~~;T.~ ~aAa~:~c~a~ ac-aACac
1..23 UG'-vGUC cncc~ap.A~c~ ~
1 ~ L'FaC,CCUC CUGnI:~C~CCCGAAAG:~...~'CGnFa
39 r1C",vCTC'~
,146 UC-JIJUC'~A CUGhUGAGGCCGaAAGGCC"~a
aG~.'WUCQ
'! 14 GaUGUUU CL~GA~.G:~..' CC'~CC, a
8 .~~GCCfJ
v~,a.GC-u c~,~;~uc~;~;~c~ - . -
ar~wG
" 60 UL~:~,W CUGALT:.:~G:~.:CGAAF.G~CC''.:A?.
.~,G.."UC,1U
1 1 UUtJGGP.U CUG:~L'~,GGCCGaAF~G;~CCG?.A
61 :.aC~."L7G?a .
1154 UCUUUUG CUGnUG~CCGr'~.r'~AG'vCC~~ar'aa
aUL~G.
1.73 AG.UCAU CU"~I3GAGGCC~~CG?~?~ aLCUUW
11 E1 F.AF.G~'"QC ' ~"C.sAaF.G:~CC"'.~:~
aC?~L'GD
1187 t~CDG1 ~ ~1 aG.'Z.'CUA
11 a UUFaACUC CQGa;~CGr'J~AG,~~C~. .~.e~
8 ar~,GC~C'J
1 ~ UUUUAUU CU~~CGA7~,F~G:~CCGaa aC
9 3
1 ? UUWUAU CUGAL~GwCGnaAC~CCG~ AACJC?.a
94
CA 02468048 2004-06-07
282
~
m ~ 5
m ~ ~
~I
y
~
~ ~
5
'
5~5
m
N
a
O iC
r.
N $ I
~ c~
.r
w
a
~~5
0
y y o .-i c"
~..i r a, r
.a ~ ~ a
G 0
~., w
CA 02468048 2004-06-07
283
m
c's ~
~555~
m
v
c
m
v v
m
c ~ a 5~~5
~
~
o ~~ 55~5
O ~ U
U
~
U
L~l~ C ~ ~
~
<
C~
A
t WC
~ ~
C9
C9
O .
rC G
iC yC
~
~ 5
.. C~ C9
G ~
< C~
C
G
G
G
G
W U
C iC
C9
z
_
G ~~~
ao O
. yo wo
d " O m
y o '
u~
e~
o,
~
y r ,
w wo
o,
~
G O
H
CA 02468048 2004-06-07
284
Table 39: Large-Scale Synthesis
Sequence Activator Amidite Time' % Full
[ AddedlFinal][AddedlFinal] Length
(min) (min) Product
A9T T [0.50!0.33][0.1/0.02] '15 m 85
A9T S (0.2510.17][0.1/0.02] 15 m 89
(GGU)3GGT T [0.5010.33][0.110.02] ~ 5 m 78
(GGU)3GGT S [0.2510.17][0.1!0.02] 15 m 81
C9T T [0.5010.33][0.1!0.02] 15 m 90
C9T S [0.2510.17][0.110.02] 15 m 97
U9T T [0.50/0.33][0.1/0.02] 15 m 80
U9T S [0.25/0.17][0.1/0.02] 1 ~ m 85
A (36-mer) T [0.50/0.33][0.110.02] 15/15m 21
A (36-mer) S [0.25/0.17][0.1/0.02] 15I~5 m 25
A (36-mer) S [0.5010.241[0.1!0.03] 15l'l5 m 25
A (36-mer) S [0.50/0.18]j0.1l0.05] 15115 m 38
A (36-mer) S [0.5010.18][0.110.05] 10J5 m 42
'Where two coupling times ndicated A coupling
sre i the first
refers to
RN
and the second to 2'-O-methylcoupling. 5-S-Ethyltetrazole,
S = T =
tetra.zole activator. cA UCU GAU AGG CCG
A is 5' -ucu c GAG GCC
GAA
AAA Auc ccu -3' where lewerecase represents
2'-O-methylnucleetides.
CA 02468048 2004-06-07
285
Table 40: Base Deprotection
Sequence Deprotection Time T C ~ ~ Full
Reagent (min) Length
Product
i8u(GGU)4 NH,~OHIEiOH 16 h 55 62.5
MA 10 m 65 62.7
AMA 10 m 65 74.8
MA 10 m 55 75.0
AMA 10 m 55 772
iPrP(GGU)4NH4OH/EtOH 4 h 65 44.8
MA 10 m 65 65.9
AMA 10 m 65 59.8
MA 10 m 55 61.3
AMA . 10 m 55 60.1
.
C9U NH40H/EtOH 4 h 65 , 75.2
MA 10 m 65 79.1
AMA 10 m 65 77.1
MA 10 m 55 79.8
AMA 10 m 55 75.5
A (36-mer)NH40H/EiOH 4 h 65 22.7
MA 10 m 65 28.9
.. .
CA 02468048 2004-06-07
286
Table 41: 2'-O-Alkylsilyl Deprotection
Sequence Deprotection Time T % ~Futl
C
Reagent (min) Length
Product
AgT TBAF 24 h 20 84.5
1.4 M HF 0.5 65 81.0
h
(GGU)4 TBAF 24 h 20 60.9
1.4MHF 0.5h 65 67.8
TBAF 24 h 20 86.2
1.4 M HF 0.5 65 86.1
h
U ~ p TBAF 24 h 20 84.8
1.4 M HF 0.5 65 84.5
h
B (36-mer) iBAF 24 h 20 25.2
1.4MHF 1.5h 65 30.6
A (36-mer) THAF 24 h 20 29.7
1.4 M HF 1.5 65 30.4
h
B is 5'- UCU AG GCC
CCA UCU GAU GAA
G AGG
CCG
AAA
AUC
CCU
-3'.
CA 02468048 2004-06-07
' 287
'N .
.
N
~ CD r1 C C
'fJ CIA L'~ GV C C
CV C~ r~
r~ 1~
~ o~
!~3 o ~ O imp
O ~ ~
~
N 1! 1~
rl r-c
IWl !V
~1 , '~ d~ e'" to CD cC
r
~~
~ O ~ p O O
d
v~ ~n m v~
L'~ , Zp
to
k
A N N
N ri r1
h.s O ~ :~ : ;~ ~ .c
r-' ~. ~. ~" ;,
...
cV ~ -~r o c~ co o,
~a ~ N
N N ~ N
r"~.i~ C'~~'~~ ~ L' L'
~ 7
n
U~
CA 02468048 2004-06-07
Zsa
N
.N
N. ,
' N ,-.
0
C ~ 00 CO
V
v~ m
tJ N O
M N M O
yS M
k
N ~ N ,-t
N
'N
p' ~ ~ ~ b ca
~ ~
Ltd ~ ~ o ~ o
'~ >
O L'7 .d,
""r L7 d'
b~ K
1; SG
N r-t N
a~ e~ a~
.
r., : N N CV
L'
w
.
r
, M ~ p
~
_ c''7e'~ m e'~ M
L.
H
CA 02468048 2004-06-07
289
Table ~4. Kinetics of Self=Processing In Intro
SE:lf Processing Constructs k {~ 1)
1.16 ~ 0.08
~y ~ o.5s = o.i5
o.ss = o.os
Hp(G~ i 0.054 = O.G03
k represents the uaimolecular rate constant for n'bozpme self cleavage
determined from a non-linear, least-squares fit (KaleidaGraph, Synergy
Software, Reeling, PA) to the equatioa:
(fraction Uncleaved Transcript) _ ~ (1-e'~
The equation describes the extent of r~ozyme processing in the presense of
ongoing transcription (Long & Uhlenbeck, 1°94 Proc. Natl. Acad. ~ci'
LTSA 91,
697 as a function of time {t) and the unimolecular rate constant for cleavage
(k). Each value of k represents the average (j range) of values determined
from two experiments.
CA 02468048 2004-06-07
290
Table 45
entry Modification t (m) ty~ (m)
p = tS/tA
., ActivityStability X '18
(tA) (tS)
1 U4 & U7 = U 1 0.1 1
2 U4 & U7 = 2'-G~-Me-U4 260 650
3 U4 = 2'=CHrU 6.5 120 180
4 U7 = 2'=CHZ-U 8 280 350
U4 & U7 = 2'=CH2-U 9.5 120 130
6 U4 = 2'=CF~-U 5 320 640
7 U7 = 2'=CFz-U 4 220 550
8 U4 8 U7 = 2'=CF2-U 20 320 160
9 U4 = 2'-F-U 4 320 800
U7 ~ 2'-F-U 8 400 500
11 U4 8~ U7 = 2'-F-U 4 300 750
12 U4 = 2'-C-Afly!-U 3 >500 >1700
13 U7 - 2'-C-Aily!-U 3 220 730
14 U4 ~ U7 = 2'-C-Ally!-U3 120 400
U4 = 2'-araF-U 5 > 500 > 1000
16 U7 ~ 2'-araF-U 4 350 875
17 U4 $ U7 = 2'-araF-U15 500 330
18 U4 = 2'-NH2-U 10 500 500
19 U7 = 2'-NH2-U 5 500 7 000
U4 8 U7 = 2'-NH2-U 2 300 1500
21 U4 = dU 6 100 170
22 U4 8. U7 = dU 4 ' 240 600