Note: Descriptions are shown in the official language in which they were submitted.
CA 02468142 2004-05-19
WO 03/050843 PCT/GB02/05628
METHOD OF MASS SPECTROMETRY
With the decoding of the 20-30,000 genes that compose the
human genome, emphasis has switched to the identification of the
translated gene products that comprise the proteome. Mass
spectrometry has firmly established itself as the primary
technique for identifying proteins due to its unparalleled speed,
sensitivity and specificity. Strategies can involve either
analysis of the intact protein or more commonly digestion of the
protein using a specific protease that cleaves at predictable
residues along the peptide backbone. This provides smaller
stretches of peptide sequence which are more amenable to analysis
via mass spectrometry.
The mass spectrometry technique providing the highest degree
of specificity and sensitivity is Electrospray Ionisation ("ESI")
interfaced to a tandem mass spectrometer allowing fragmentation
studies by low energy MS/MS. These experiments involve separation
of the complex digest mixture by microcapillary liquid
chromatography with on-line mass spectral detection using
automated acquisition modes whereby conventional MS and MS/MS
spectra are collected in a data dependant manner. This
information can be used directly to search databases for matching
sequences leading to identification of the parent protein. This
approach has recently allowed the identification of proteins that
are present at low endogenous concentrations. However, often the
limiting factor for identification of the protein is not the
quality of the MS/MS spectrum produced but is the initial
identification of the multiply charged peptide precursor ion in
the MS mode. This is due to the level of background chemical
noise, largely singly charged in nature, which may be produced in
the ion source of the mass spectrometer. Fig. 1 shows a
conventional mass spectrum and shows how doubly charged species
may be obscured in a singly charged background.
It would be desirable to reduce the singly charged chemical
noise thereby allowing the mass spectrometer to specifically
target multiply charged peptide related ions. The ability to be
able to discriminate against singly charged ions in favour of
multiply charged ions would be particularly advantageous for the
study of protein digests.
CA 02468142 2010-08-16
2 -
With an Electrospray Ionisation orthogonal acceleration Time of
flight ("ESI-oaTOF") mass spectrometer it is known to favour the
transmission of multiply charged species in preference to singly
charged species by increasing the discriminator voltage and/or
lowering the gain. The orthogonal acceleration Time of Flight mass
spectrometer counts the arrival of ions using a Time to Digital
Converter ("TDC") which has a discriminator threshold. The voltage
pulse of a single ion must be high enough to trigger the
discriminator and so register the arrival of an ion. The detector
producing the voltage may be an electron multiplier or Microchannel
Plate detector ("MCP"), These detectors are charge sensitive so the
size of signal they produce increases with increasing charge state.
Discrimination in favour of higher charge states may therefore be
accomplished by either increasing the discriminator voltage level of
the TDC and/or by lowering the detector gain or a combination of
both. Fig. 2A shows a conventional mass spectrum obtained with an
orthogonal acceleration Time of Flight mass spectrometer and Fig. 2B
shows a corresponding mass spectrum obtained by lowering the gain of
the ion detector. As can be seen from comparing Figs. 2A and 2B one
of the disadvantages of this technique is that lowering the gain
and/or increasing the discriminator level decreases the detection
efficiency for the desired charge state and hence the sensitivity is
reduced. Furthermore, it is impossible pick out an individual charge
state according to this method. All that can be done is to reduce
the efficiency of detection of lower charge states with respect to
higher charge states.
It is therefore desired to be able to preferentially transmit
multiply charged ions whilst attenuating singly charged ions without
substantially reducing sensitivity.
According to the present invention there is provided a method of
mass spectrometry comprising enhancing the relative proportion or
abundance of multiply charged ions to singly charged ions in a sample
of ions, the method comprising trapping the sample of ions in an AC
or RF ion guide in the presence of a gas at a pressure P for a period
of time T, wherein the product P x T is at least 1 mbar-ms.
Preferably, the product P x T is at least 2, 3, 4, 5, 6, 7, 8,
9, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 110, 120, 130, 140, 150,.
160, 170, 180, 190, 200, 210, 220, 230, 240, 250, 260, 270, 280, 290,
300, 310, 320, 330, 340, 350, 360, 370, 380, 390, 400,
CA 02468142 2004-05-19
WO 03/050843 PCT/GB02/05628
3 -
410, 420, 430, 440, 450, 460, 470, 480, 490, 500, 550, 600, 650,
700, 750, 800, 850, 900, 950 or 1000, 1100, 1200, 1300, 1400,
1500, 1600, 1700, 1800, 1900, 2000, 2500, 3000, 3500, 4000, 4500,
5000, 5500, 6000, 6500, 7000, 7500, 8000, 8500, 9000, 9500 or
10000 mbar-ms.
Preferably, the product P x T is less than 2, 3, 4, 5, 6, 7,
8, 9, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 110, 120, 130, 140,
150, 160, 170, 180, 190, 200, 210, 220, 230, 240, 250, 260, 270,
280, 290, 300, 310, 320, 330, 340, 350, 360, 370, 380, 390, 400,
410, 420, 430, 440, 450, 460, 470, 480, 490, 500, 550, 600, 650,
700, 750, 800, 850, 900, 950 or 1000, 1100, 1200, 1300, 1400,
1500, 1600, 1700, 1800, 1900, 2000, 2500, 3000, 3500, 4000, 4500,
5000, 5500, 6000, 6500, 7000, 7500, 8000, 8500, 9000, 9500 or
10000 mbar-ms.
Preferably, T falls within a range selected from the group
consisting of: (i) 50-100 ps; (ii) 100-150 ps; (iii) 150-200 us;
(iv) 200-250 us; (v) 250-300 us; (vi) 300-350 ps; (vii) 350-400
us; (viii) 400-450 ps; (ix) 450-500 us; (x) 500-550 us; (xi) 550-
600 ps; (xii) 600-650 ps; (xiii) 650-700 ps; (xiv) 700-750 us;
(xv) 750-800 ps; (xvi) 800-850 ps; (xvii) 850-900 ps; (xviii) 900-
950 us; and (xix) 950-1000 ps. Preferably, T falls within a range
selected from the group consisting of: (i) 1-2 ms; (ii) 2-3 ms;
(iii) 3-4 ms; (iv) 4-5 ms; (v) 5-6 ms; (vi) 6-7 ms; (vii) 7-8 ms;
(viii) 8-9 ms; and (ix) 9-10 ms. Preferably, T falls within a
range selected from the group consisting of: (i) 10-15 ms; (ii)
15-20 ms; (iii) 20-25 ms; (iv) 25-30 ms; (v) 30-35 ms; (vi) 35-40
ms; (vii) 40-45 ms; (viii) 45-50 ms; (ix) 50-55 ms; (x) 55-60 ms;
(xi) 60-65 ms; (xii) 65-70 ms; (xiii) 70-75 ms; (xiv) 75-80 ms;
(xv) 80-85 ms; (xvi) 85-90 ms; (xvii) 90-95 ms; and (xviii) 95-100
ms. Preferably, T falls within a range selected from the group
consisting of: (i) 100-110 ms; (ii) 110-120 ms; (iii) 120-130 ms;
(iv) 130-140 ms; (v) 140-150 ms; (vi) 150-160 ms; (vii) 160-170
ms; (viii) 170-180 ms; (ix) 180-190 ms; and (x) 190-200 ms.
Preferably, T falls within a range selected from the group
consisting of: (i) 200-250 ms; (ii) 250-300 ms; (iii) 300-350 ms;
(iv) 350-400 ms; (v) 400-450 ms; (vi) 450-500 ms; (vii) 500-550
ms; (viii) 550-600 ms; (ix) 600-650 ms; (x) 650-700 ms; (xi) 700-
750 ms; (xii) 750-800 ms; (xiii) 800-850 ms; (xiv) 850-900 ms;
(xv) 900-950 ms; and (xvi) 950-1000 ms.
CA 02468142 2004-05-19
WO 03/050843 PCT/GB02/05628
4 -
Preferably, T is at least than: (i) 50 ps; (ii) 60 is (iii)
70 ps; (iv) 80 ps; (v) 90 ps; or (vi) 100 ps. Preferably, T is at
least: (i) 200 ps; (ii) 300 ps (iii) 400 ps; (iv) 500 ps; (v) 600
ps; (vi) 700 ps; (vii) 800 ps; (viii) 900 ps; or (ix) 1000 ps.
Preferably, T is at least: (i) 2 ms; (ii) 3 ms (iii) 4 ms; (iv) 5
ms; (v) 6 ms; (vi) 7 ms; (vii) 8 ms; (viii) 9 ms; or (ix) 10 ms.
Preferably, T is at least: (i) 20 ms; (ii) 30 ms (iii) 40 ms; (iv)
50 ms; (v) 60 ms; (vi) 70 ms; (vii) 80 ms; (viii) 90 ms; or (ix)
100 ms. 'Preferably, T is at least: (1) 100 ms; (ii) 200ms (iii)
300 ms; (iv) 400 ms; (v) 500 ms; (vi) 600 ms; (vii) 700 ms; (viii)
800 ms; or (ix) 900 ms. Preferably, T is at least: (i) 1s; (ii)
2s; (iii) 3s; (iv) 4s; (v) 5s; (vi) 6s; (vii) 8s; (viii) 9s; or
(ix) 10s. Preferably, T is less than: (i) 10s; (ii) 9s; (iii) 8s;
(iv) 7s; (v) 6s; (vi) 5s; (vii) 4s; (viii) 3s; or (ix) 2s.
Preferably, T is less than: (i) 1000 ms; (ii) 900ms (iii) 800
ms; (iv) 700 ms; (v) 600 ms; (vi) 500 ms; (vii) 400 ms; (viii) 300
ms; or (ix) 200 ms. Preferably, T is less than: (i) 100 ms; (ii)
90ms (iii) 80 ms; (iv) 70 ms; (v) 60 ms; (vi) 50 ms; (vii) 40 ms;
(viii) 30 ms; or (ix) 20 ms. Preferably, T is less than: (i) 10
ms; (ii) 9 ms (iii) 8 ms; (iv) 7 ms; (v) 6 ms; (vi) 5 ms; (vii) 4
ms; (viii) 3 ms; or (ix) 2 ms. Preferably, T is less than: (i)
1000 ps; (ii) 900 ps (iii) 800 ps; (iv) 700 ps; (v) 600 ps; (vi)
500 ps; (vii) 400 ps; (viii) 300 ps; or (ix) 200 ps. Preferably,
T is less than: (i) 100 ps; (ii) 90 is (iii) 80 ps; (iv) 70 ps;
(v) 60 ps; or (vi) 50 ps.
Preferably, P falls within a range selected from the group
consisting of: (i) 0.01-0.02 mbar; (ii) 0.02-0.03 mbar; (iii)
0.03-0.04 mbar; (iv) 0.04-0.05 mbar; (v) 0.05-0.06 mbar; (vi)
0.06-0.07 mbar; (vii) 0.07-0.08 mbar; (viii) 0.08-0.09 mbar; and
(ix) 0.09-0.10 mbar. Preferably, P falls within a range selected
from the group consisting of: (i) 0.1-0.2 mbar; (ii) 0.2-0.3 mbar;
(iii) 0.3-0.4 mbar; (iv) 0.4-0.5 mbar; (v) 0.5-0.6 mbar; (vi) 0.6-
0.7 mbar; (vii) 0.7-0.8 mbar; (viii) 0.8-0.9 mbar; and (ix) 0.9-
1.0 mbar. Preferably, P falls within a range selected from the
group consisting of: (i) 1-2 mbar; (ii) 2-3 mbar; (iii) 3-4 mbar;
(iv) 4-5 mbar; (v) 5-6 mbar; (vi) 6-7 mbar; (vii) 7-8 mbar; (viii)
8-9 mbar; and (ix) 9-10 mbar. Preferably, P falls within a range
selected from the group consisting of: (i) 10-20 mbar; (ii) 20-30
mbar; (iii) 30-40 mbar; (iv) 40-50 mbar; (v) 50-60 mbar; (vi) 60-
CA 02468142 2004-05-19
WO 03/050843 PCT/GB02/05628
-
70 mbar; (vii) 70-80 mbar; (viii) 80-90 mbar; and (ix) 90-100
mbar.
Preferably, P is at least: (i) 0.01 mbar; (ii) 0.02 mbar;
(iii) 0.03 mbar; (iv) 0.04 mbar; (v) 0.05 mbar; (vi) 0.06 mbar;
5 (vii) 0.07 mbar; (viii) 0.08 mbar; or (ix) 0.09 mbar. Preferably,
P is at least: (i) 0.1 mbar; (ii) 0.2 mbar; (iii) 0.3 mbar; (iv)
0.4 mbar; (v) 0.5 mbar; (vi) 0.6 mbar; (vii) 0.7 mbar; (viii) 0.8
mbar; or (ix) 0.9 mbar. Preferably, P is at least: (i) 1 mbar;
(ii) 2 mbar; (iii) 3 mbar; (iv) 4 mbar; (v) 5 mbar; (vi) 6 mbar;
(vii) 7 mbar; (viii) 8 mbar; or (ix) 9 mbar. Preferably, P is at
least: (i) 10 mbar; (ii) 20 mbar; (iii) 30 mbar; (iv) 40 mbar; (v)
50 mbar; (vi) 60 mbar; (vii) 70 mbar; (viii) 80 mbar; (ix) 90
mbar; or (x) 100 mbar.
Preferably, P is less than: (i) 100 mbar; (ii) 90 mbar; (iii)
80 mbar; (iv) 70 mbar; (v) 60 mbar; (vi) 50 mbar; (vii) 40 mbar;
(viii) 30 mbar; or (ix) 20 mbar. Preferably, P is less than: (i)
10 mbar; (ii) 9 mbar; (iii) 8 mbar; (iv) 7 mbar; (v) 6 mbar; (vi)
5 mbar; (vii) 4 mbar; (viii) 3 mbar; or (ix) 2 mbar. Preferably,
P is less than: (i) 1 mbar; (ii) 0.9 mbar; (iii) 0.8 mbar; (iv)
0.7 mbar; (v) 0.6 mbar; (vi) 0.5 mbar; (vii) 0.4 mbar; (viii) 0.3
mbar; or (ix) 0.2 mbar. Preferably, P is less than: (i) 0.10
mbar; (ii) 0.09 mbar; (iii) 0.08 mbar; (iv) 0.07 mbar; (v) 0.06
mbar; (vi) 0.05 mbar; (vii) 0.04 mbar; (viii) 0.03 mbar; or (ix)
0.02 mbar.
Preferably, P is selected from the group consisting of: (i) >
0.01 mbar; (ii) > 0.05 mbar; (iii) > 0.1 mbar; (iv) > 0.2 mbar;
(v) > 0.5 mbar; (vi) > 1 mbar; (vii) > 2 mbar; (viii) > 5 mbar;
and (ix) > 10 mbar.
The sample of ions preferably comprises at least some ions
having similar or substantially the same mass to charge ratios but
different charge states. The at least some ions may have similar
or substantially the same mass to charge ratios preferably wherein
the mass to charge ratios differ by less than: (i) 20 mass to
charge units; (ii) 15 mass to charge units; (iii) 10 mass to
charge units; (iv) 5 mass to charge units; (v) 4 mass to charge
units; (vi) 3 mass to charge units; (vii) 2 mass to charge units;
and (viii) 1 mass to charge unit, wherein 1 mass to charge unit
equals 1 dalton per unit of electronic charge.
CA 02468142 2010-08-16
6 -
The sample of ions may comprise a plurality of ionised
molecules, the molecules comprising a plurality of different
biopolymers, proteins, peptides, polypeptides, oligionucleotides,
oligionucleosides, amino acids, carbohydrates, sugars, lipids,
fatty acids, vitamins, hormones, portions or fragments of DNA,
portions or fragments of cDNA, portions or fragments of RNA,
portions or fragments of mRNA, portions or fragments of tRNA,
polyclonal antibodies, monoclonal antibodies, ribonucleases,
enzymes, metabolites, polysaccharides, phosphorolated peptides,
phosphorolated proteins, glycopeptides, glycoproteins or steroids.
According to another embodiment of the present invention,
there is provided a method of mass spectrometry comprising:
providing a sample of singly charged ions and doubly charged
ions having similar mass to charge ratios;
20
30
CA 02468142 2010-08-16
7 -
onwardly transmitting doubly charged ions whilst at least
partially relatively attenuating singly charged ions; and
mass analysing the doubly charged ions.
Preferably, the AC or RF ion guide comprises electrodes and
the AC or RF ion guide has a central longitudinal axis, and
wherein the combination of pressure and trapping time is such that
singly charged ions are forced radially outwards from the central
longitudinal axis whereas multiply charged ions are caused to
forced towards the central longitudinal axis.
The singly charged ions are preferably substantially ejected
from or lost from the AC or RF ion guide, whereas at least some
preferably a majority of the multiply charged ions are
substantially retained within the AC or RF ion guide.
Preferably, one or more of the following groups of ions are
substantially ejected from or lost from the AC or RF ion guide:
(i) ions having 2 charges; (ii) ions having 3 charges; (iii) ions
having 4 charges; (iv) ions having 5 charges; (v) ions having 6
charges; (vi) ions having 7 charges; (vii) ions having 8 charges;
30
CA 02468142 2010-08-16
8 -
(viii) ions having 9 charges; (ix) ions having 10 charges; (x)
ions having 11 charges; (xi) ions having 12 charges; (xii) ions
having 13 charges; (xiii) ions having 14 charges; (xiv) ions
having 15 charges; (xv) ions having 16 charges; (xvi) ions having
17 charges; (xvii) ions having 18 charges; (xviii) ions having 19
charges; (xix) ions having 20 charges; (xx) ions having 21
charges; (xxi) ions having 22 charges; and (xxii) ions having more
than 22 charges.
Preferably, one or more of the following groups of ions are
substantially retained with the AC or RF ion guide: (i) ions
having 2 charges; (ii) ions having 3 charges; (iii) ions having 4
charges; (iv) ions having 5 charges; (v) ions having 6 charges;
(vi) ions having 7 charges; (vii) ions having 8 charges; (viii)
ions having 9 charges; (ix) ions having 10 charges; (x) ions
having 11 charges; (xi) ions having 12 charges; (xii) ions having
13 charges; (xiii) ions having 14 charges; (xiv) ions having 15
charges; (xv) ions having 16 charges; (xvi) ions having 17
charges; (xvii) ions having 18 charges; (xviii) ions having 19
charges; (xix) ions having 20 charges; (xx) ions having 21
charges; (xxi) ions having 22 charges; and (xxii) ions having more
than 22 charges.
According to another embodiment of the present invention,
unwanted singly charged background ions are removed from a mixture
of singly charged background ions and multiply charged analyte
ions, the method comprising:
transmitting the mixture of ions to the AC or RF ion guide;
trapping the ions within the AC or RF ion guide maintained at
the pressure P;
setting the period of time T during which the ions are
trapped within the AC or RF ion guide at a value such that at
least 50%, 60%, 70%, 80%, 90% or more than 90% of said singly
charged ions will be substantially ejected from or lost from the
AC or RF ion guide whereas at least 50%, 60%, 70%, 80%, 90% or
more than 90% of said multiply charged ions will be substantially
maintained within the AC or RF ion guide.
According to another embodiment of the present invention,
there is provided a method of removing or attenuating singly
and/or
CA 02468142 2010-08-16
9 -
doubly charged ions from a mixture of at least singly, doubly and
triply charged ions.
According to another aspect of the present invention, there
is provided a mass spectrometer comprising an ion source; a vacuum
chamber housing an AC or RF ion guide maintained in use at a
pressure P; an electrode, wherein in a first mode of operation a
potential applied to the electrode causes a sample of ions to be
substantially trapped within the AC or RF ion guide and wherein in
a second mode of operation the potential applied to the electrode
allows ions to be released from the AC or RF ion guide; a further
vacuum chamber housing a mass analyser; and control means arranged
to enhance the relative proportion or abundance of multiply
charged ions to singly charged ions in the sample of ions by
controlling a period of time T that ions are trapped within the AC
or RF ion guide by controlling the potential applied to the
electrode such that the product P x T is at least 1 mbar-ms.
The mass spectrometer preferably further comprises an ion
source for generating mainly molecular or pseudo-molecular ions.
The ion source may comprise an atmospheric pressure
ionization source such as an ion source selected from the group
comprising: (i) an Electrospray ionisation ("ESI") ion source;
(ii) an Atmospheric Pressure Chemical Ionisation ("APCI") ion
source; (iii) an Atmospheric Pressure Photo Ionisation ("APPI")
ion source; (iv) an atmospheric pressure Matrix Assisted Laser
Desorption Ionisation ("MALDI") ion source; and (v) an Inductively
Coupled Plasma ("ICP") ion source. Alternatively, the ion source
may comprise a non-atmospheric pressure ionization source such as
an ion source selected from the group consisting of: (i) a Fast
Atom Bombardment ("FAB") ion source; (ii) a Liquid Secondary Ions
Mass Spectrometry ("LSIMS") ion source; (iii) a Matrix Assisted
Laser Desorption Ionisation ("MALDI") ion source; (iv) a matrix
Assisted Laser Desorption ("MALDI") ion source in combination with
a collision cell for collisionally cooling ions; (v) a Laser
Desorption Ionisation ("LDI") ion source; (vi) an Electron Impact
("EI") ion source; and (vii) a Chemical Ionisation ("CI") ion
source.
Preferably, the AC or. RF ion guide comprises a multipole rod
set e.g. a quadrupole rod set, a hexapole rod set, an octopole rod
set or a rod set having ten or more rods.
CA 02468142 2010-08-16
-
Alternatively, the AC or RF ion guide may comprise a
plurality of electrodes having apertures through which the ions
are transmitted. For example, the AC or RF ion guide may comprise
an ion tunnel having a plurality of electrodes each having
5 substantially the same size aperture or an ion funnel having a
plurality of electrodes wherein the size of the apertures becomes
progressively smaller or larger.
According to another embodiment the AC or RF ion guide may
comprise a double helix arrangement of electrodes.
10 According to a yet further embodiment the AC or RF ion guide
may comprise a plurality of plates stacked adjacent to each other.
The mass spectrometer preferably comprises a mass analyzer
such as a Time of Flight mass analyzer, a quadrupole mass
analyzer, a 2D or 3D ion trap, a Fourier Transform mass
spectrometer or a Fourier Transform Ion Cyclotron Resonance mass
spectrometer.
The mass spectrometer preferably further comprises a further
AC or RF ion guide arranged in a further vacuum chamber. A
quadrupole mass filter and/or a collision cell may be arranged in
a yet further vacuum chamber intermediate the vacuum chamber(s)
housing the AC or RF ion guide(s) and the vacuum chamber housing
the mass analyzer. The ion source may comprise an atmospheric
pressure ion source and the mass analyzer may comprise a Time of
Flight mass analyzer.
Preferably, the further AC or RF ion guide comprises: (i) a
rnulti.pole rod set; (ii) an ion funnel comprising a plurality of
electrodes having apertures therein through which ions are
transmitted, wherein the diameter of the apertures becomes
progressively smaller or larger; (iii) an ion tunnel comprising a
plurality of electrodes having apertures therein through which
ions are transmitted, wherein the diameter of the apertures
remains substantially constant; (iv) a double helix arrangement of
electrodes; and (v) a stack of plates wherein adjacent electrodes
are connected to opposite phases of an AC or RF supply.
According to another embodiment of the present invention,
there is provided a mass spectrometer comprising:
an ion source;
CA 02468142 2010-08-16
- 11 -
a first AC or RF ion guide disposed in an upstream ion guide
vacuum chamber, the first AC or RF ion guide being maintained at a
pressure P1;
a second AC or RF ion guide disposed in a downstream ion
guide vacuum chamber, the second AC or RF ion guide being
maintained at a pressure P2; and
a mass analyser disposed in a further vacuum chamber, the
further vacuum chamber being disposed downstream of the upstream
ion guide vacuum chamber and the downstream ion guide vacuum
chamber;
wherein, in use, ions are arranged to be trapped in the first
AC or RF ion guide for a time Ti and/or ions are arranged to be
trapped in the second AC or RF ion guide for a time T2 wherein P1
x Ti is at least 1 mbar-ms and/or P2 X T2 is at least 1 mbar-ms.
Preferably, the AC or RF ion guide and/or the further AC or
RF ion guide comprises: (i) a multipole rod set; (ii) an ion
funnel comprising a plurality of electrodes having apertures
therein through which ions are transmitted, wherein the diameter
of the apertures becomes progressively smaller or larger; (iii) an
ion tunnel comprising a plurality of electrodes having apertures
therein through which ions are transmitted, wherein the diameter
of the apertures remains substantially constant; (iv) a double
helix arrangement of electrodes; and (v) a stack of plates wherein
adjacent electrodes are connected to opposite phases of an AC or
RF supply.
According to another embodiment of the present invention,
there is provided a method of mass spectrometry comprising:
operating an AC or RF device in a first mode wherein the AC
or RF device acts as an ion guide to substantially transmit ions
received at an entrance to the device through to an exit of the
device; and
operating the AC or RF device in a second mode wherein the AC
or RF device acts as an ion trap to substantially trap ions within
the device and to substantially prevent the ions from exiting the
device, wherein in the second mode the AC or RF device is
maintained at a pressure P and ions are trapped within the AC or
RF device for a period of time T, wherein the product P x T is at
least 1 mbar-ms.
CA 02468142 2010-08-16
12 -
Preferably, the period of time T is a continuous or
substantially continuous period of time. Alternatively, the
period of time T is an accumulative period of time.
According to the preferred embodiment ions having a chosen
charge state are selected from a mixture of ions having differing
charge states by trapping the ions in an RF device for a period of
time and in the presence of a buffer gas at a particular pressure.
Ions generated from an Electrospray Ionisation source, for
example, typically contain a mixture of charge states. These ions
are usually generated at atmospheric pressure and admitted to the
mass spectrometer through means of a pumping aperture that forms
part of a differentially pumped vacuum system. In normal
20
30
CA 02468142 2004-05-19
WO 03/050843 PCT/GB02/05628
- 13 -
operation these ions continually stream through an RF device into
regions of lower pressure by means of further differentially
pumped regions which lead in turn to a mass analyser housed in an
analyser vacuum chamber. The resulting mass spectrum therefore
contains ions of all the charge states generated in the ionisation
region of the instrument.
If an electrode is placed at the exit of the RF device then
ions can be trapped by raising the potential of this gate
electrode higher than the body or reference DC potential of the AC
or RF device. During this trapping phase ions are preferably
still able to enter the device at the upstream end through the
differential pumping aperture and hence ions can build up in
concentration. If the electrode voltage is reduced then the
accumulated ions will be released. By adjusting the pressure in
the trapping device it is possible to vary the ratio of singly to
multiply charged species.
Various embodiments of the present invention will now be
described, by way of example only, and with reference to the
accompanying drawings in which:
Fig. 1 shows how doubly charged ions may be obscured amongst
a background of singly charged ions in a typical mass spectrum;
Fig. 2A shows a conventional mass spectrum and Fig. 2B shows
a corresponding mass spectrum obtained by lowering the detector
gain;
Fig. 3A shows a schematic drawing of a collisional trapping
charge state selector device according to the preferred embodiment
and Fig. 3B shows a timing diagram for the voltage applied to an
electrode adjacent the exit of the AC or RF device;
Fig. 4A shows a mass spectrum of ions obtained by guiding
ions through the AC or RF device without trapping the ions when
the AC or RF device was maintained at a pressure of 1.4 mbar, Fig.
4B shows a mass spectrum of ions obtained by guiding ions through
the AC or RF device without trapping the ions when the AC or RF
device was maintained at a pressure of 2.7 mbar, Fig. 4C shows a
mass spectrum obtained wherein ions were trapped at a pressure of
1.4 mbar for 60 ms, and Fig. 4D shows a mass spectrum obtained
according to the preferred embodiment wherein ions were trapped
within the AC or RF device at a pressure of 2.7 mbar for 60 ms;
CA 02468142 2004-05-19
WO 03/050843 PCT/GB02/05628
- 14 -
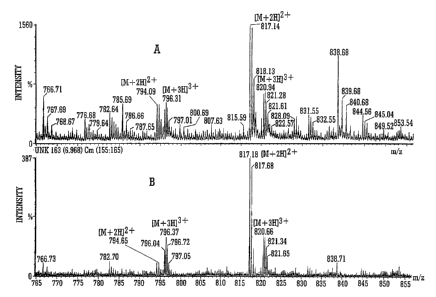
Fig. 5A is an expansion of Fig. 4B and Fig. 5B is an
expansion of Fig. 5D;
Fig. 6A shows a plot of trapping time against pressure for
which the ratio of the intensity of doubly charged ions from
Gramacidin-S (m/z 571) to that of singly charged ions from Leucine
Enkephalin (m/z 556) was doubled over that for no trapping and
Fig. 6B shows a plot of trapping time against pressure for which
the ratio of the intensity of triply charged ions from Renin
Substrate (m/z 586) to that of singly charged ions from Leucine
Enkephalin (m/z 556) was doubled over that for no trapping;
Fig. 7 shows the effect of storage or trapping time on the
intensity of doubly charged Gramacidin-S (m/z 571) ions and singly
charged Leucine Enkephalin (m/z 556) ions at 1.64 mbar;
Fig. 8 shows the effect of storage or trapping time on the
intensity of triply charged Renin Substrate (m/z 586) ions and
singly charged Leucine Enkephalin (m/z 556) ions at 1.64 mbar;
Fig. 9 shows the ratio of intensities of: (i) doubly charged
Gramacidin-S ions (m/z 571) to singly charged Leucine Enkephalin
(m/z 556) ions; and (ii) triply charged Renin Substrate (m/z 586)
ions to singly charge Leucine Enkephalin (m/z 556) ions, as a
function of storage or trapping time at 1.64 mbar;
Fig. 10 shows the effect of storage or trapping time on the
intensity of doubly charged Gramacidin-S (m/z 571) ions and singly
charged Leucine Enkephalin (m/z 556) ions at 1.95 mbar;
Fig. 11 shows the effect of storage or trapping time on the
intensity of triply charged Renin Substrate (m/z 586) ions and
singly charged Leucine Enkephalin (m/z 556) ions at 1.95 mbar;
Fig. 12 shows the ratio of intensities of: (i) doubly charged
Gramacidin-S ions (m/z 571) to singly charged Leucine Enkephalin
(m/z 556) ions; and (ii) triply charged Renin Substrate (m/z 586)
ions to singly charge Leucine Enkephalin (m/z 556) ions, as a
function of storage or trapping time at 1.95 mbar;
Fig. 13 shows the effect of storage or trapping time on the
intensity of doubly charged Gramacidin-S (m/z 571) ions and singly
charged Leucine Enkephalin (m/z 556) ions at 2.23 mbar;
Fig. 14 shows the effect of storage or trapping time on the
intensity of triply charged Renin Substrate (m/z 586) ions and
singly charged Leucine Enkephalin (m/z 556) ions at 2.23 mbar;
CA 02468142 2004-05-19
WO 03/050843 PCT/GB02/05628
- 15 --
Fig. 15 shows the ratio of intensities of: (i) doubly charged
Gramacidin-S ions (m/z 571) to singly charged Leucine Enkephalin
(m/z 556) ions; and (ii) triply charged Renin Substrate (m/z 586)
ions to singly charge Leucine Enkephalin (m/z 556) ions, as a
function of storage or trapping time at 2.23 mbar;
Fig. 16 shows the effect of storage or trapping time on the
intensity of doubly charged Gramacidin-S (m/z 571) ions and singly
charged Leucine Enkephalin (m/z 556) ions at 2.51 mbar;
Fig. 17 shows the effect of storage or trapping time on the
intensity of triply charged Renin Substrate (m/z 586) ions and
singly charged Leucine Enkephalin (m/z 556) ions at 2.51 mbar;
Fig. 18 shows the ratio of intensities of: (i) doubly charged
Gramacidin-S ions (m/z 571) to singly charged Leucine Enkephalin
(m/z 556) ions; and (ii) triply charged Renin Substrate (m/z 586)
ions to singly charge Leucine Enkephalin (m/z 556) ions, as a
function of storage or trapping time at 2.51 mbar;
Fig. 19 shows the effect of storage or trapping time on the
intensity of doubly charged Gramacidin-S (m/z 571) ions and singly
charged Leucine Enkephalin (m/z 556) ions at 2.86 mbar;
Fig. 20 shows the effect of storage or trapping time on the
intensity of triply charged Renin Substrate (m/z 586) ions and
singly charged Leucine Enkephalin (m/z 556) ions at 2.86 mbar; and
Fig. 21 shows the ratio of intensities of: (i) doubly charged
Gramacidin-S ions (m/z 571) to singly charged Leucine Enkephalin
(m/z 556) ions; and (ii) triply charged Renin Substrate (m/z 586)
ions to singly charge Leucine Enkephalin (m/z 556) ions, as a
function of storage or trapping time at 2.86 mbar.
A preferred AC or RF ion guide/ion trap 5 will now be
described in relation to Fig. 3A. Ions from an ion source 1 enter
an upstream vacuum chamber 2 which may have an optional RF ion
guide 3 arranged therein. However, such an ion guide 3 is not
essential and may be omitted. The upstream vacuum chamber 2 is
pumped by a pump. Ions pass through a differential pumping
aperture 9 into an intermediate vacuum chamber 4. Another RF ion
guide 5 is preferably provided in the intermediate vacuum chamber
4 and according to one embodiment this AC or RF ion guide 5 may be
operated in one mode of operation as an ion trap. Ions may, for
example, be trapped in the guide 5 by raising the potential of a
differential pumping aperture 6 which separates the intermediate
CA 02468142 2004-05-19
WO 03/050843 PCT/GB02/05628
- 16 -
vacuum chamber from a downstream vacuum chamber 7 preferably
housing another RF ion guide 8. The electric field resulting from
the voltage applied to the differential pumping aperture 6
preferably extends into the downstream region of the intermediate
ion guide 5 and hence has the effect of preventing ions from
exiting the ion guide 5. A voltage may or may not be applied to
an electrode adjacent an upstream end of the intermediate AC or RF
ion guide 5. However, since the differential pumping aperture 9
is preferably maintained at a higher DC potential than the
reference DC potential of the intermediate AC or RF ion guide 5
then ions are effectively prevented from exiting the AC or RF ion
guide 5 via the entrance. Ions entering the AC or RF ion guide 5
quickly become thermalised i.e. lose their kinetic energy and when
a trapping voltage is removed the ions preferably exit the
intermediate AC or RF ion guide 5 by the repulsive space-charge
effect of further ions entering the ion guide 5. other
embodiments are also contemplated especially in relation to ion
tunnel ion guides wherein an axial voltage gradient is used to
encourage ions to travel through and/or leave the ion guide(s).
When the potential applied to the differential pumping
aperture 6 is lowered, ions may exit the ion guide 5 and pass
through the differential pumping aperture 6 into the downstream
vacuum chamber 7 which preferably houses a downstream AC or RF ion
guide 8. Ions are preferably guided through the downstream vacuum
chamber 7 by the ion guide 8 and may then pass through a further
differential pumping aperture 10 into an analyser vacuum chamber
(not shown) housing a mass analyser (not shown).
A timing diagram of the voltage applied to the differential
pumping aperture 6 or more generally to an exit electrode of the
AC or RF ion trap 5 is shown in Fig. 3B. When the differential
pumping aperture 6 or the exit to the ion trap 4 is at a voltage
Vtrap then ions are unable to exit the AC or RF ion trap 5 and
hence accumulate in the device 5. When the voltage applied to the
exit differential pumping aperture 6 or the exit electrode of the
AC or RF ion trap 5 falls to Vextract then ions are allowed to the
exit the ion trap 5 and pass to the next stages and subsequently
to the ion detector (not shown).
According to an embodiment the AC or RF ion guide/ion trap 5
is maintained in the intermediate vacuum chamber 4 at a pressure
CA 02468142 2004-05-19
WO 03/050843 PCT/GB02/05628
- 17 -
in the range 1-3 mbar. However, according to other embodiments
the upstream AC or RF ion guide 3 and/or the downstream AC or RF
ion guide 8 may also be used to trap ions therein.
By varying or appropriately setting (i) the pressure in the
trapping region, (ii) the cycle time Tm, (iii) the release width W
and (iv) the voltages Vtrap and Vextract it is possible to maximise
the trapping efficiency and to maximise or optimise the
discrimination between singly and multiply charged species.
By way of illustration Figs. 4A and 4B show the mass spectra
obtained when the AC or RF device 5 is operated as an ion guide
substantially without trapping ions therein (e.g. the voltage
applied to the exit of the AC or RF device 5 is maintained at
Vextract- Fig. 4A shows the mass spectrum obtained when the AC or RF
device 5 was maintained at a pressure of 1.4 mbar and Fig. 4B
shows the mass spectrum obtained when the AC or RF device 5 was
maintained at a pressure of 2.7 mbar. In both cases the AC or RF
device 5 acted as an ion guide without trapping ions.
All the experimental results presented in the present
application were obtained using an AC or RF device which comprised
an ion tunnel. An ion tunnel comprises a plurality of electrodes
having preferably circular apertures through which ions are
transmitted in use. The ion tunnel may therefore be considered to
comprise a plurality of stacked rings. According to an embodiment
the ion tunnel comprises two interleaved combed arrangements of
electrodes. Adjacent electrodes in the ion tunnel device are
supplied with opposite phases of an AC or RF voltage supply. The
voltage supply is preferably sinusoidal but other embodiments are
contemplated wherein, for example, a square wave or other non-
sinusoidal waveform may be applied to the device. The ion tunnel
device preferably comprises 10-20, 20-30, 30-40, 40-50, 50-60, 60-
70, 70-80, 80-90, 90-100 or more than 100 electrodes. Preferably,
the vast majority of the electrodes have substantially similar
size apertures in contrast to an ion funnel. According to an
embodiment at least 75%, 80%, 85%, 90%, 95% or 99% of the
electrodes forming the ion tunnel have substantially the same size
and/or area internal apertures.
However, the present invention is not limited to using an ion
tunnel ion guide and other AC or RF devices are intended to fall
within the scope of the present invention.
CA 02468142 2004-05-19
WO 03/050843 PCT/GB02/05628
18 -
An equimolar mixture of Leucine-Enkephalin (which exhibits a
singly charged peak at m/z 556) and Gramacidin-S (which exhibits a
doubly charged peak at m/z 571) was infused into the mass
spectrometer. The slight difference in intensities between the
two species is largely attributable to differing ionisation
efficiencies and is normal in Electrospray mass spectrometry.
Figs. 4C and 4D show mass spectra obtained when the RF device
5 was operated as an ion trap. Ions were trapped within the ion
trap 2 for 60 ms in both cases. Fig. 4C shows the mass spectrum
obtained when the ions were trapped for 60 ms in the ion trap 5 at
a pressure of 1.4 mbar. As is apparent, Fig. 4C is substantially
similar to the mass spectra shown in Figs. 4A and 4B.
Fig. 4D illustrates an embodiment of the present invention
and shows the mass spectrum which resulted from mass analysing the
ions which emerged from the ion trap 5 when the ion trap 5 was
maintained at a pressure of 2.7 mbar and ions were trapped within
the ion trap 5 for 60 ms.
The mass spectra shown in Figs. 4A, 4B and 4C are
qualitatively similar and show that the ratio of the intensity of
the doubly charged mass peaks at m/z 571 to the ratio of the
intensity of the singly charged mass peaks at m/z 556 remained
substantially constant. However, when the ions were trapped at
2.7 mbar for 60 ms then as clearly shown in Fig. 4D singly charged
ions were significantly attenuated whilst the'doubly charged
Gramacidin-S ions at m/z 571 were substantially unattenuated.
Fig. 5A corresponds with the data shown in Fig. 4B and shows
the mass spectrum for ions in the mass to charge ratio range 290-
580 (as opposed to ions having mass to charge ratios in the range
556-573 as shown in Fig. 4B). Similarly, Fig. 5B corresponds with
the data shown in Fig. 4D and shows the mass spectrum for ions in
the mass to charge ratio range 290-580 (as opposed to ions having
mass to charge ratios in the range 556-573 as shown in Fig. 4D).
As can be clearly seen from Figs. 5A and 5B singly charged
ions present in the sample were rejected from the ion trap 5 when
the mixture of ions was trapped at 2.7 mbar for 60 ms whereas
doubly charged ions were substantially unattenuated. The peak at
mass to charge 297.6 is doubly charged and is substantially
unattenuated.
CA 02468142 2004-05-19
WO 03/050843 PCT/GB02/05628
- 19 -
The reasons for the discrimination against singly charged
ions in favour of multiply charged ions will now be discussed
below. In particular, the distribution of ions within
inhomogeneous RF Fields will now be considered.
Through consideration of the average force acting on an ion
in the inhomogeneous RF fields it can be shown that the time
average of the alternating force is finite and is directed towards
the region of weaker field independent of the sign of the ionic
charge. This quadratic potential 0 can be expressed as:
O.5Eo(Ax2 +6y2+yz2)
The corresponding electric field E may be expressed as:
E=E.(.~x+oy+yz)
For a quadrupole rod set A = -o and y = 0, and for a
quadrupole ion trap A. = a= and y = -2cr. For both the quadrupole
rod set and the quadrupole ion trap the field is uncoupled in the
three directions. Hence, the secular motion is simple harmonic
along any given co-ordinate axis.
Evaluation of the kinetic energy along any given co-ordinate
axis, averaged over one period, allows the constant W to be
determined, where W is a constant of the secular motion
corresponding to the total energy in the system with time-
independent conservative forces. That is:
W = kinetic energy + pseudo-potential energy
= 1/2 M. v2 + eW
The maximum kinetic energy in the micro-motion of the ion is
equivalent to the pseudo-potential energy eW. For a quadrupole
ion trap the value of the corresponding effective, or equivalent,
potential W is given by:
ZeE2
`I'=-
4nico2
CA 02468142 2004-05-19
WO 03/050843 PCT/GB02/05628
- 20 -
where m is the mass of the ion, z is the charge of the ion, e is
the charge of an electron and w is the angular frequency of the RF
supply.
Through consideration of the pseudo-potential energy eW for
multipole rod sets it can also be shown that the effective
potential W(R) as a function of the radial distance R is given by:
T(R) N Z zeV o (R ) ZcN-z,
4mCO2Ro !R0
where VO is the peak RF voltage applied to the rods, R0 in the
inscribed radius of the rods, R is the radial distance from the
centre and 2N is the number of rods.
Furthermore, it is known that the pseudo-potential energy eW
for a stacked ring set is proportional to the exponential function
of radial displacement R. The effective potential W(R,Z) as a
function of the radial distance R and the axial position Z is
given by:
I12 R C[Io21R1 sine Z
_ zeVa Z0 Z0 Zo Z0
'Y(R,Z) 4nza 2Za
I02Ro
Z0
where R0 is the inscribed radius of the rings, nZo is the ring
centre to ring centre separation in the axial direction, I1 is a
first order modified Bessel function of the first kind and 10 is a
zeroth order modified Bessel function of the first kind.
Through consideration of the effect of ion-molecule
collisions in the quadrupole field (F. G. Major and H. G. Dehmelt,
Phys. Rev., 1968, 170, 91) it has been shown that when ions of
mass m undergo purely elastic collisions within an RF field with
relatively cold gas molecules of mass m0 where m >> m0r the
collisions will result in viscous drag which lowers the mean
kinetic energy of the ions as a function of time. The authors go
on to state that the ion micro-motion is not interrupted by the
collisions, but only slightly modified in phase and amplitude,
while any secular motion is damped out exponentially.
CA 02468142 2004-05-19
WO 03/050843 PCT/GB02/05628
- 21 -
The experimental results presented in the present application
show that there is an abundance of doubly charged ions relative to
that of singly charged ions following the accumulation of ions in
a 2D stacked ring ion guide at a pressures of 2.7 mbar (2 torr)
for a trapping period of 60 ms. The data shows enhancement of
ions with higher charge states (z values) but with the same m/z
values as the product of pressure and storage time is increased.
As already discussed, the effective potential W(R) as a
function of the radial distance R for a multipole rod set is given
by:
2(N-2)
`P(R) N2zeVa
-C4mco R0)t,R0
Hence by differentiation of the effective potential with respect
to R the effective radial field r(R) as a function of the radial
distance R is given by:
N2 (N -1)ze Vn (2N-3)
T(R) _ - -
2m w2Ro2 Ro
Therefore, the radial force F(R) as a function of the radial
distance R on ions with mass m and charge z is equal to zer(R).
Hence:
[N2(N_1)z2e2v021 R (2.N-3)
F(R) 2mrv2Ro2 Ro
It will be seen that the radial force F(R) towards the centre
is proportional to z2/m. Similarly, the effective potential
W(R,Z) as a function of the radial distance R and axial position Z
for a ring stack set is given by:
112 R [cos211+ rI02 R sine
zeVt Zo Zo Zo Zo
l'(R,Z)_ ~4mcvtZo
1102 (P20
CA 02468142 2004-05-19
WO 03/050843 PCT/GB02/05628
22 -
A similar treatment shows that the radial force F(R) towards
the centre is again proportional to z2/m. Hence, the radial force
is greater for ions of the same mass m with higher charge states z
i.e. ions of the same substance with lower m/z values.
However, it will be seen that the radial force F(R) towards
the centre is also proportional to z/(m/z). Hence, the radial
force is also greater for ions with the same m/z value but with
higher values of the charge state z as has been observed.
As a consequence of this, ions with the same values of mass
to charge ratio (m/z) but with higher charge states (z) will
experience a greater force directed towards the centre where the
field is weakest. In an environment where ions are free to move,
but frequently in collision with lighter gas molecules, ions that
experience the greater radial force will eventually migrate and
occupy the central space. Ions that experience a smaller radial
force will eventually be squeezed out to occupy larger radial
positions. This arranging of ions according to the force acting
upon them will only take place in situations where the ions lose
their secular motion through collisional damping, and where
adequate time has been allowed for the whole population of ions to
reach a steady state.
This process by which ions arrange themselves into layers or
bands is similar to that which takes place when DNA segments are
centrifuged in a caesium chloride density gradient solution to
separate out the DNA satellites. In the centrifuge the DNA
molecules separate into a number of bands - the main band and
three additional bands (satellites). The different satellite
bands have different densities depending on whether they are AT-
rich or CG-rich segments. This separation of DNA into bands is
the result of the different centrifugal forces acting on the
different classes of DNA molecules. In a similar manner, ions
with the same m/z value, but different z values, will experience
different effective radial forces as a result of the effective
pseudo-potential well generated by the inhomogeneous RF fields,
and will consequentially separate into different bands. Ions with
the lower z values will occupy larger radial positions. Hence,
these ions are more likely to be lost through collisions with the
CA 02468142 2004-05-19
WO 03/050843 PCT/GB02/05628
- 23 -
rods or rings of the ion guide, or not be transmitted through any
small orifice arranged along the axis of the ion guide after its
exit.
As has already been explained, a method for enhancing the
signal from doubly, triply or more highly charged ions from that
of background singly charged ions is particularly advantageous for
the study of protein digests. The peptides from protein digests,
when ionised by electrospray, often yield an abundance of doubly
charged, triply or more highly charged ions. The method, as
described above, of first storing ions at elevated pressures in an
ion guide or ion trap employing inhomogeneous RF fields provides a
means of enhancing the relative abundance of multiply charged ions
to that of singly charged ions having the same m/z values. This
method can therefore be employed before mass analysis so as to
enhance the relative abundance of multiply charged ions to that of
singly charged ions at equivalent m/z values within the mass
spectrum. The relative enhancement of doubly charged ion
abundance to that of singly charged ion abundance becomes very
pronounced at pressures above 1.4 mbar (1 torr) for storage times
of the order of 60 ms. Hence, the enhancement of doubly charged
ion abundance to that of singly charged ion abundance becomes very
pronounced when the product of pressure and storage or transit
time is greater than 8.4 x 10-2 mbar-seconds (6 x 10-2 torr-
seconds).
Figs. 6A and 6B show the results of further investigations
into the relationship between trapping time and pressure. Fig. 6A
shows a plot of trapping time (ms) against pressure (mbar) for
which the ratio of the intensity of doubly charged ions from
Gramacidin-S (m/z 571) to that of the singly charged ions from
Leucine Enkephalin (m/z 556) is doubled over that for no trapping.
The particular data points are:
Pressure (P) Trapping time (T) P x T
1.95 mbar 89 ms 173.55 mbar-ms
2.23 mbar 60 ms 133.80 mbar-ms
2.51 mbar 42.5 ms 106.68 mbar-ms
2.86 mbar 21 ms 60.06 mbar-ms
CA 02468142 2004-05-19
WO 03/050843 PCT/GB02/05628
- 24 -
Pressure and trapping time appear to be exponentially
related. An empirically derived relationship for the results
shown in Fig. 6A is:
-P
T=1450e0.70
where T is the trapping time in ms and P is the pressure in mbar.
Fig. 6B shows a plot of trapping time (ms) against pressure
(mbar) for which the ratio of the intensity of the triply charged
ions from Renin Substrate (m/z 586) to that of the singly charged
ions from Leucine Enkephalin (m/z 556) is doubled over that for no
trapping. The particular data points are:
Pressure (P) Trapping time (T) P x T
1.64 mbar 89 ms 145.96 mbar-ms
1.95 mbar 50 ms 97.50 mbar-ms
2.23 mbar 32 ms 71.36 mbar-ms
2.51 mbar 21 ms 52.71 mbar-ms
2.86 mbar 7 ms 20.02 mbar-ms
Pressure and trapping time again appear to be exponentially
related. An empirically derived relationship for the results
shown in Fig. 6B is:
-P
T=1750e01
where T is the trapping time in ms and P is the pressure in mbar.
These results show that by modestly increasing the pressure
the required trapping time can be drastically reduced.
Fig. 7 shows the effect of storage or trapping time on the
intensity of doubly charged Gramacidin-S (m/z 571) ions and singly
charged Leucine Enkephalin (m/z 556) ions at 1.64 mbar, Fig. 8
shows the effect of storage or trapping time on the intensity of
triply charged Renin Substrate (m/z 586) ions and singly charged
Leucine Enkephalin (m/z 556) ions at 1.64 mbar and Fig. 9 shows
the ratio of intensities of: (i) doubly charged Gramacidin-S ions
(m/z 571) to singly charged Leucine Enkephalin (m/z 556) ions; and
(ii) triply charged Renin Substrate (m/z 586) ions to singly
CA 02468142 2004-05-19
WO 03/050843 PCT/GB02/05628
- 25 -
charge Leucine Enkephalin (m/z 556) ions, as a function of storage
or trapping time at 1.64 mbar.
Fig. 10 shows the effect of storage or trapping time on the
intensity of doubly charged Gramacidin-S (m/z 571) ions and singly
charged Leucine Enkephalin (m/z 556) ions at 1.95 mbar, Fig. 11
shows the effect of storage or trapping time on the intensity of
triply charged Renin Substrate (m/z 586) ions and singly charged
Leucine Enkephalin (m/z 556) ions at 1.95 mbar and Fig. 12 shows
the ratio of intensities of: (i) doubly charged Gramacidin-S ions
(m/z 571) to singly charged Leucine Enkephalin (m/z 556) ions; and
(ii) triply charged Renin Substrate (m/z 586) ions to singly
charge Leucine Enkephalin (m/z 556) ions, as a function of storage
or trapping time at 1.95 mbar.
Fig. 13 shows the effect of storage or trapping time on the
intensity of doubly charged Gramacidin-S (m/z 571) ions and singly
charged Leucine Enkephalin (m/z 556) ions at 2.23 mbar, Fig. 14
shows the effect of storage or trapping time on the intensity of
triply charged Renin Substrate (m/z 586) ions and singly charged
Leucine Enkephalin (m/z 556) ions at 2.23 mbar and Fig. 15 shows
the ratio of intensities of: (i) doubly charged Gramacidin-S ions
(m/z 571) to singly charged Leucine Enkephalin (m/z 556) ions; and
(ii) triply charged Renin Substrate (m/z 586) ions to singly
charge Leucine Enkephalin (m/z 556) ions, as a function of storage
or trapping time at 2.23 mbar.
Fig. 16 shows the effect of storage or trapping time on the
intensity of doubly charged Gramacidin-S (m/z 571) ions and singly
charged Leucine Enkephalin (m/z 556) ions at 2.51 mbar, Fig. 17
shows the effect of storage or trapping time on the intensity of
triply charged Renin Substrate (m/z 586) ions and singly charged
Leucine Enkephalin (m/z 556) ions at 2.51 mbar and Fig. 18 shows
the ratio of intensities of: (i) doubly charged Gramacidin-S ions
(m/z 571) to singly charged Leucine Enkephalin (m/z 556) ions; and
(ii) triply charged Renin Substrate (m/z 586) ions to singly
charge Leucine Enkephalin (m/z 556) ions, as a function of storage
or trapping time at 2.51 mbar.
Fig. 19 shows the effect of storage or trapping time on the
intensity of doubly charged Gramacidin-S (m/z 571) ions and singly
charged Leucine Enkephalin (m/z 556) ions at 2.86 mbar, Fig. 20
shows the effect of storage or trapping time on the intensity of
CA 02468142 2004-05-19
WO 03/050843 PCT/GB02/05628
- 26 -
triply charged Renin Substrate (m/z 586) ions and singly charged
Leucine Enkephalin (m/z 556) ions at 2.86 mbar and Fig. 21 shows
the ratio of intensities of: (i) doubly charged Gramacidin-S ions
(m/z 571) to singly charged Leucine Enkephalin (m/z 556) ions; and
(ii) triply charged Renin Substrate (m/z 586) ions to singly
charge Leucine Enkephalin (m/z 556) ions, as a function of storage
or trapping time at 2.86 mbar.
It will be seen that in some instances the ion signal can
first increase before eventually decreasing as the trapping time
is increased. This effect can be observed to a greater or lesser
extent in Figs, 7, 10, 13, 14, 17, 19 and 20. It is thought that
the increase in signal intensity is due to ions beginning to
migrate towards the centre of the pseudo-potential well as a
result of frequent collisions with the lighter gas molecules.
This ion migration is likely to be the precursor to the process in
which ions with higher values of z2/m eventually displace ions
which have lower values of z2/m and occupy the central space.
Ions that accumulate in the central region are more likely to be
transmitted through the exit of the ion guide and to the ion
detection system. Hence, ions that initially collapse into the
centre of the pseudo-potential well may be expected to show a
corresponding increase in signal intensity. In fact, by careful
selection of pressure and trapping time, it is possible to enhance
the ratio of the intensity of the multiply charged ions with
respect to that of singly charged ions with similar m/z values and
simultaneously increase the absolute intensity of the multiply
charged ions.
If a mass spectrometer is being switched between two modes of
operation or is being switched from transmitting ions of one m/z
value to those of a different m/z value there will be a period of
time for which the mass spectrometer will not be able to receive
and transmit ions. In this period of time ions may advantageously
be trapped in the AC or RF ion guide/ion trap and then released
when the mass spectrometer is ready to accept these ions thereby
gaining the advantage of the extra sensitivity that is observed
when ions are trapped according to the preferred embodiment
described above.
The preferred embodiment also looks particularly useful for
preferentially transmitting ions having a large number of charges.
CA 02468142 2004-05-19
WO 03/050843 PCT/GB02/05628
- 27 -
For example, horse heart myoglobin has a molecular mass of
16951.48 and ions may in some conditions have 8 or 9 charges or in
other conditions the ions may have between 10-28 charges.
Experimental data suggests that with highly charged ions
preferentially transmission of multiply charged ions in favour of
lower or singly charged ions occurs down to pressures P and
trapping times T wherein the product P x T is 1 mbar-ms.
Experimental data suggests that at or above the product of P x T
equalling 1 mbar-ms the beneficial effect of the selective
enhancement of multiply charged ions is observed.
Although the preferred embodiment above has been described
mainly in relation to preferentially transmitting doubly or triply
charged ions as opposed to singly charged ions, the enhancement of
highly charged ions to those of e.g. singly charged ions also
becomes pronounced at lower products of pressure and storage or
transit time.
The preferred embodiment can be used for removing background
ions from a mixture of ions, wherein the mixture of ions comprises
a plurality of different biopolymers, proteins, peptides,
polypeptides, oligionucleotides, oligionucleosides, amino acids,
carbohydrates, sugars, lipids, fatty acids, vitamins, hormones,
portions or fragments of DNA, portions or fragments of cDNA,
portions or fragments of RNA, portions or fragments of mRNA,
portions or fragments of tRNA, polyclonal antibodies, monoclonal
antibodies, ribonucleases, enzymes, metabolites, polysaccharides,
phosphorolated peptides, phosphorolated proteins, glycopeptides,
glycoproteins or steroids.
Although the present invention has been described with
reference to preferred embodiments and other arrangements, it will
be understood by those skilled in the art that various changes in
form and detail may be made without departing from the scope of
the invention as set forth in the accompanying claims.