Note: Descriptions are shown in the official language in which they were submitted.
CA 02469307 2004-06-28
79565-77D
1
QESCRIPTION
piaanostio Devices and &nnaratus for the Controlied
Movement of Reaqent^ 1litbout Xembrane^
This is a divisional of Canadian Patent
Application 2,113,198 filed May 20, 1993.
Field of the Invention
This invention relates to devices for conducting
assays, including qualitative, semi-quantitative and
quantitative determinations of one or more analytes in=a
single test format. Unlike assay devices of the prior
art, the inventive assay devices described herein do not
involve the use of bibulous materials, such as pap4rs or
membranes. The inventive devices of the present invention
rely on the use of defined surfaces, including grooved
surfaces, and capillarity alone or in various combinations
to move the test reagents. The inventive devices
described herein provide means for the controlled, timed
movement of reagents within the device and do not require
precise pipetting steps. The concepts and devices of the
present invention are especially useful in the performance
of immunoassays of environmental and industrial fluids,
such as water, and biological fluids and products, such as
urine, blood, serum, plasma, spinal and amniotic fluids
and the like.
Background of the Invention
over the years, numerous simplified test systems have
been designed to rapidly detect the presence of a target
ligand of interest in biological, environmental and indus-
trial fluids. In one of their simplest forms, these assay
systems and devices usually involve the combination of a
test reagent which is capable of reacting with the target
--.--
--- ---
-
ligand to give a visual response and an absorbent paper or
CA 02469307 2004-06-28
79565-77D
2
membrane through which the test reagents flow. Paper
products, glass fibers and nylon are commonly used for the
absorbent materials of the devices. In certain cases, the
portion of the absorbent member containinq the' test
reagents is brought into contact, either physically or
through capillarity, with the sample containinq the target
ligand. The contact may be accomplished in a variety of
ways. Most commonly, an aqueous sample is allowed to
traverse.a porous or absorbent member, such as porous
polyethylene or polypropylene or membranes by capillarity
through the portion of the porous or absorbent member
containing the test reagents. In other cases, the test
reagents are pre-mixed outside the test device and then
added to the absorbent member of the device to ultimately
generate a signal.
Commercially available diagnostic products employ a
concentrating zone methodology. In these products, such
as ICONR (Hybritech Incorporated), TESTPACKM (Abbott.
Laboratories) or ACC[TLEVELR (Syva Corporation),.the device
contains an immunosorbing or capture zone within a porous
member to which a member of a specific binding pair is
immobilized. The surface of the porous member also may bw
treated to contain one or more elements of a siqnal
development system. In these devices, there is a liquid
absorbing zone which serves. to draw liquid through the
immunosorbing zone, to absorb liquid sample and reagents
and to control the rate at which the liquid is drawn
through the immunosorbing zone. The liquid absorbing zone
is either an additional volume of the porous member out-
side of the immunosorbing zone or an absorbent material in
capillary communication with the immunosorbing zone. Many
commercially available devices and assay systems also
involve a wash step in which the immunosorbinq zone is
washed free of non-specifically bound signal generator so
that the presence or amount of target ligand in the sample
can be determined by examining the porous member for a
_ ____________signal_at_the_appropriate_zone.
CA 02469307 2004-06-28
79565-77D
3
The devices described herein do not use bibulous or
porous materials, such as membranes and the like as sub-
strates for the immobilization of reagents or to control
the flow of the reagents throuqh the device. A dis-
advantage of, for example, membranes in diagnostic devices
is that on both microscopic and macroscopic scales the
production of inembranes is not easily reproducible. This
can result in diagnostic devices which have differential
properties of non-specific binding and flow characteris-
tics. The time gates of this invention can, however, be
embedded in membranes or used in devices with membranes.
Membranes are very susceptible to non-specific binding
which can raise the sensitivity limit of the assay. In
the case of immunochromatographic assay formats such as
those described in U.S. Pat. Nos. 4,879,215, 4,945,205 and
4,960,691, the use of inembranes as the diagnostic element
requires, an even flow of reagents through the membrani.
The problem of uneven flow of assay reagents in
immunochromatographic assays has been addressed in U.S.
Patents 4,756,828, 4,757,004 and 4,883,688.
These patents teach that modifying -
the longitudinal edge of thebibulous material controls
the shape of the advancing front. The devices of the
current invention circumvent these membrane associated
problems by the use of defined surfaces, including grooved
surfaces, capillarity, time gates, novel capillary means,
including channels and novel fluid flow control means
alone or in various combinations, all of which are
constructed from non-absorbent materials. In a preferred
mode of this invention, the capillary channel of the
diagnostic element is composed of grooves which are
perpendicular to the flow of the assay reagents. The
manufacture of grooved surfaces can be accomplished by
injection molding and can be sufficiently reproducible to
provide control of the flow of reagentEs through the
----device.
CA 02469307 2004-06-28
79565-77D
4
In addition to the limitations of the assay devices
and systems of the prior art, including the limitations of
usinq absorbsnt membranes as carriers for sample and rea-
gents, assay devices generally involve numerous steps,
including critical pipetting steps which must be performed
by relatively skilled users in laboratory settings.
Accordinqly, there is a need for one step assay devices
and systems, which, in addition to controlling the flow of
reagents in the device, control the timing of the flow of
lo reagents at specific areas in the device. In addition,
there is a need for assay devices which do not require
critical pipetting steps but still perform semi-
quantitative and quantitative determinations. The inven-
tive devices and methods of this invention satisfy these
needs and others by introducing devices which do not
require precise pipettinq% of sample, which do not use
absorbent members, which include novel elements called
time gates for the controlled movement of reagents in the
device and which are capable of providinq quantitative
assays.
Definitions
In interpreting the claims and specification, the
following terms shall have the meanings sst forth below.
Target ligand - The binding partner to one or more
receptors.
Ligand - Binding partner to a ligand receptor.
Liqand Analoque - A chemical derivative of the tarqet
ligand which may be attached either covalently or non-
covalently to other species, for example, to the siqnal
development element. Ligand analoque and target ligand
may be the same and both are capable of bindinq to the
receptor.
Ligand Analogue Conjugate - A conjugate of a ligand
analogue and a signal development element;
CA 02469307 2004-06-28
79565-77D
Signal Development Phase - The phase containing the
materials involving the signal development element to
develop signal, e.g., an enzyme'substrate solution.
Receptor - Chemical or biochemical species capable of
5 reacting with or binding to target ligand, typically an
antibody, a binding fragment, a complementary nucleotide
sequence or a chelate, but which may be a ligand if the
assay is designed to detect a target ligand which is a
receptor. Receptors may also include enzymes or chemical
reagents that specifically react with tha tarqet ligand.
Ligand Receptor Conjugate - A conjugate of a ligand
receptor and a signal development element.
Signal Development Element - The element which dir-
ectly or indirectly causes a visually or instrumentally
detectable signal as a result of the assay process.
Receptors and ligand analogues may be bound, either
covalently or noncovalently to the signal development
element to form a conjuqate. The element of the ligand
analogue conjugate or the receptor conjugate which, in
conjunction with the signal development phase, develops
the detectable signal, e.q., an enzyme.
Reaction Mixture - The mixture of sample suspected of
containing target ligand and the reagents for determining
the presence or amount of target liqand in the sample, for
example, the ligand analogue conjugate or the receptor
conjugate. As used herein the Reaction Mixture may com-
prise a proteinaceous component which may be the target,
a component of the sample or additive (e.g., serum albu-
min, gelatin, milk proteins).
Ligand Complement - A specialized ligand used in
labelling ligand anaa.oque conjugates, receptors, ligand
analogue constructs or signal development elements.
Ligand Complement Receptor - A receptor for ligand
complement.
Ligand Analogue-Ligand Complement Conjugate - A con-
jugate composed of a ligand analogue, a liqand complement
and a siqnal_ development element. _ - . _
CA 02469307 2004-06-28
79565-77D
6
Capture Efficiency - The binding efficiency of the
component or components in the reaction mixture, such as
the ligand analogue conjugate or the receptor conjuqate,
to the capture zone of the diaqnostic element.
Capture Zone - The area on the diagnostic element
which binds at least one component of the reaction mix-
ture, such an the ligand analogue conjugate or the
receptor conjugate.
Capillarity - The state of being capillary or the
exhibition of capillary action. Capillarity can be.
affected by the solid surtace or the liquid surface or
both.
Biosensor - Any electrochemical, optical, electro-
optical or acoustic/mechanical device which is used to
measure the presence or amount of tarqet ligands. For
example, electrochemical biosensors utilize potentiometric
and amperometric measurements, optical biosensors utilize
absorbance, fluorescence, luminescence and evanescent
waves. Acoustic/mechanical biosensors utiliae piezo-
electric crystal resonance, surface acoustic waves, field-
effect transistors, chemical field-effect transistors and
enzyme field-effect transistors. Hiosensors can also
detect chanqes in the physical properties of solutions in
which receptor binding events take place. For example,
biosensors may detect changes in the degree of agglutina-
tion of latex particles upon binding antigen or they may
detect changes in the viscosity of solutions in response
to receptor bindinq events.
Descrivtion of the Drawinas
Fiqure 1 is a partially schematic, top perspective
view of a device in accordance with the present invention.
Figure la is a partially schematic, perspective
exploded view of the device showing the detail in the area
of the sample addition reservoir, the sample-reaction bar-
rier, the reaction chamber, the time gate and the beqin-
- - - ninq- of _ the_ dimgnostic- elsment, _ .._ _ - - - - - -- ,- -- - - - - -
- - - - - - - - - - - - - - - - - - -
CA 02469307 2004-06-28
79565-77D
7
Figure lb is a partially schematic, perspective exploded
view of the device showing the detail in the area of the optional
reagent reservoir, the sample addition reservoir, the sample-
reaction barrier, the reaction chamber, the time gate and the
beginning of the diagnostic element.
Figure ic is a partially schematic, perspective exploded
view of the device showing the detail in the area of the optional
reagent reservoir in fluid contact with the sample addition
reservoir and the reaction chamber.
Figure ld is a partially schematic, perspective cutaway
view of the flow control means.
Figure 2 is a partially schematic, perspective view of a=
second device in accordance with this present invention, which may
be used to add pre-mixed reaction mixtures.
Figures 3A and 3B are partially schematic top views of
the diagnostic element showing some potential placements of
capture zones.
Figure 4 is a partially schematic, perspective view of a
used reagent reservoir.
Figure 5 is a partially schematic view of embodiments of
these devices which are columnar or have curved opposing surfaces.
Figures 6A-6F are top views of time gates.
Figures 7A-7F show typical dimensions for a preferred
time gate.
Figures 8A-8F show top views of sequential time gates.
Summary of the Invention
The assay devices, assay systems and device components
of this invention comprise at least two opposing surfaces disposed
CA 02469307 2008-08-14
71884-88D
8
a capillary distance apart, at least one of which is capable
of immobilizing at least one target ligand or a conjugate in
an amount related to the presence or amount of target ligand
in the sample from a fluid sample in a zone for controlled
fluid movement to, through or away the zone. The inventive
device components may be incorporated into conventional
assay devices with membranes or may be used in the inventive
membrane-less devices herein described and claimed. These
components include flow control elements, measurement
elements, time gates, elements for the elimination of
pipetting steps, and generally, elements for the controlled
flow, timing, delivery, incubation, separation, washing and
other steps of the assay process.
In a broad aspect the invention provides an assay
device for use in detecting or measuring an analyte in a
sample comprising: a housing having a top; a sample port for
receiving the sample, the sample port located on the
housing; a sample addition reservoir for receiving the
sample from the sample port; a reaction chamber for
combining the sample with at least one reagent to form a
reaction mixture, wherein the sample undergoes a physically
detectable change correlated to the amount of the analyte in
the sample; a sample reaction barrier comprising a capillary
which separates the sample addition reservoir from the
reaction chamber, and which capillary facilitates the flow
of the sample from the sample addition reservoir to the
reaction chamber; and a diagnostic element comprising
opposing surfaces spaced a capillary distance apart, which
diagnostic element comprises at least one biosensor that
detects a signal in response to the physically detectable
change and which diagnostic element receives the reaction
mixture from the reaction chamber, and wherein the sample
reaction barrier and the diagnostic element are free of
CA 02469307 2008-11-12
71884-88D
8a
bibulous materials adapted to control the flow of the sample
and the reaction mixture.
In another aspect the invention provides use of
the assay device as described herein to detect a target
ligand.
In another aspect, the invention provides an assay
device comprising: a housing having a top; a sample port for
receiving a sample, the sample port located on the housing;
a reagent within the housing, wherein the sample contacts
the reagent, yielding a physically detectable change which
correlates with the amount of a selected analyte in the
sample; and a diagnostic element within the housing which
diagnostic element is in contact with the reagent, the
diagnostic element comprising a biosensor configured and
arranged to detect an electrochemical signal related to the
physically detectable change said electrochemical signal
being mediated by an enzyme conjugated to an antibody or a
binding fragment thereof reactive with the selected analyte
in the sample.
In another aspect, the invention provides a method
for detecting a target ligand using an assay device, the
device comprising a housing that has a sample port, a
reagent, and a diagnostic element in contact with the
reagent, the diagnostic element comprising a biosensor, the
method comprising: depositing a sample containing the target
ligand into the sample port; flowing the sample from the
sample port to the reagent; reacting the sample and the
reagent to create an electrochemically detectable change,
said electrochemically detectable change mediated by an
enzyme conjugated to an antibody or a binding fragment
thereof reactive with the target ligand; and detecting the
electrochemically detectable change by detecting a signal
CA 02469307 2008-11-12
71884-88D
8b
generated in response to the electrochemically detectable
change using the biosensor comprised in the diagnostic
element.
Detailed Description of the Invention
The present invention is directed to diagnostic
testing devices for determining the presence or amount of at
least one target ligand. Figure 1 shows a preferred
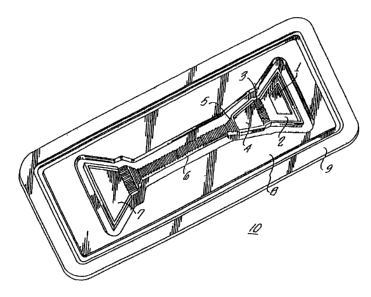
embodiment of a device 10 according to the invention.
Generally, the devices of the invention have thicknesses of
about 2 mm to 15 mm, lengths of about 3 cm to 10 cm and
widths of about 1 cm to 4 cm. The dimensions may be
adjusted depending on the particular purpose of the assay.
One device of this invention, as depicted in Fig. 1,
generally illustrates some features of the inventive devices
and portions of devices herein disclosed and claimed. The
device 10 comprises various elements, a sample addition
zone 1, a sample addition reservoir 2, a sample reaction
barrier 3, a reaction chamber 4, a time gate 5, a diagnostic
element 6, and a used reagent reservoir 7. The devices are
comprised of capillary channels which are formed when a top
member 8 is placed on the bottom member 9 a capillary
distance apart and which move the reagents and sample
throughout the device. The top and bottom members may be
married, the various chambers sealed and the capillaries
formed by a number of techniques, including but not limited
to, gluing, welding by ultrasound, riveting and the like.
The elements of the device can be used in various
combinations with the diagnostic element 6 to achieve a
variety of desired functions. As one skilled in the art
will recognize these elements may be combined to
CA 02469307 2004-06-28
79565-77D
9
perform one-step or multistep assays. The devices 10 may
also be used in the formation, of reaction mixtures for the
assay process. The device 20 in Fig. 2 may be used to add
pre-mixed reaction mixtures for the generation of signal
which relates to the presence or amount of the target
ligand.
An optional reagent chamber 17 may be incorporated
into device 10 or 20 as depicted in Fig. lb and Fig. ic.
The devices 10 and 20 may also be used with an optional
fluid control means 18 as shown in Fig. id.
Features include, but are not limited to: 1) diag-
nostic elements which are not comprised of bibulous mater-
ials, such as membranes, 2) means to control the volume of
sample or reaction.mixture, 3) time gates, 4) novel capil-
lary means, termed fingers herein and 5) novel flow con-
trol means, sometimes referred to as a"gap" herein and
6) used reagent reservoir which prevents backward flow ot
reagents. Those of skill in the art will appreciatec that
these elements are separately novel and nonobvious, and
may be.incorporated into diagnostic devices in various
combinations and may be used with other elements known to
those skilled in the art to achieve novel and nonobvious
diagnostic test devices and heretofore unrealized
benefits.
Each of the elements of devices 10 and 20 will be
described separately, then representative descriptions of
the devices of this invention will follow.
Samnle Addition Zone
Referring to Figs. 1 and 2, the sample addition zone
1 of the devices 10 and 20 is the area where sample is
introduced to the device. The sample addition zone 1 can
be a port of various configurations, that is, round,
oblong, square and the like or the zone can be a trough in
the device.
CA 02469307 2004-06-28
79565-77D
SamQle Addition Reservoir
Referring to Figs. 1 and 2, the sample addition
reservoir 2 is an element of the device which receives the
sample. Referring now to Fig. 1, the volume of the sample
5 addition reservoir 2 should be at least the volume of the
reaction chamber 4 or greater. The sample addition reser-
voir 2 can be a capillary space or it can be an open
trough. In addition, a filter element can be placed in or
on the sample addition reservoir 2 to filter particulates
10 from the sample or to filter blood cells from blood so
that plasma can further travel through the device. In a
preferred embodiment, the volume or capacity of the sample
addition reservoir 2 is 1 to 5 times the volume of the
reaction chamber. 4. In general, one selects a volume or
capacity of this reservoir 2 such that if the excess sam-
ple is used to wash the diagnostic element 6 then enough
volume of sample is needed to thoroughly remove any
unbound reagents from the diagnostic element 6 arising
from the assay process. This reservoir 2 may also contain
certain dried reagents which are used in the assay pro-
cess. For example, a surfactant can be dried in this
reservoir 2- which dissolves when sample is added. The
surfactant in the sample would aid in the movement of the
sample and reaction mixture through the device by lowering
the surface tension of the liquid. The sample addition
reservoir 2 is generally in direct fluid contact with the
sample-reaction barrier 3 (Fig. 1) or the diagnostic ele-
ment 6 (Fig. 2).
S.amDle-Reaction Barrier
As depicted in Fig. 1, the sample-reaction barrier 3
separates the sample in the sample addition reservoir 2
from the reaction mixture in the reaction chamber 4. The
sample-reaction barrier is desired because it provides the
device with the capability of forming a precise reaction
mixture volume. A precise volume of the reaction mixture
is generally necessary for assays in which semi-quantita-
--------------
CA 02469307 2004-06-28
79565-77D
11
tive or quantitative results are desired. Thus, a precise
pipetting step of the sample to the device is not required
because the sample reaction barrier forms a reaction cham-
ber of precise volume into which the sample is capable of
flowing. The sampla reaction barrier 3 is desired because
the reactions which take place in the reaction chamber 4
should preferably be separated from the excess sample in
the sample addition reservoir 2. The sample reaction
barrier 3 comprises a narrow capillary, generally ranging
from about 0.01 mm to 0.2 mm and the surfaces of the
capillary can be smooth or have a single groove or a
series of grooves which are parallel or perpendicular to
the flow of sample. In a preferred embodiment of the
sample reaction barrier 3, now referring to Fig. la,
grooves 12, parallel to the flow of sample, are incor-
porated onto one surface of the device a capillary dis-
tance, for example, 0.02 mm to 0.1 mm, from the other
surface. The volume of sample which fills the sample-
reaction barrier 3 (Fig. la) should be kept to a minimum,
from about 0.014 to 10% of the reaction chamber 4 volume
so that the reagents of the reaction chamber 4 do not
significantly diffuse back into the sample in_the sample
addition reservoir 2. That is, the diffusion of the
reaction mixture back into the excess sample should be
kept to a ninimum so that the chemical or biochemical
reactions occurring in the reaction mixture are not sub-
stantially influenced by the excess sample in the sample
addition reservoir 2. Groove depths can range from about
0.01 mm to 0.5 mm and preferably from about 0.05 mm to
0.2 mm. When more than one groove is used for this ele-
ment, the number of grooves in this element is typically
between 10 and 500 grooves per cm and preferably from
about 20 to 200 grooves per cm. Sample from the sample
addition reservoir 2 flows over the grooves 12 by capil-
lary action and then into the reaction chamber 4. In a
further preferred embodiment, grooves, hereafter termed
_ "finger$!'_ i6,_ -are _ si.tuated _in -the _ wall -of -the- reaction
CA 02469307 2004-06-28
79565-77D
12
chamber 4 in fluid contact with the grooves 12 or capil-
lary space of the sample-reaction barrier 3. These fin-
gers 16 are typically 0.5 mm to 2 mm wide, preferably 1 mm
to 1.5 mm wide and typically 0.1 mm to 1.5 mm in depth,
preferably about 0.2 to 1 mm in depth. The fingers 16 in
the wall of the reaction chamber 4 aid in the capillary
flow of the sample into the reaction chamber 4. That is,
the fingers allow fluid to move from a capillary where the
capillarity is relatively high to a capillary where the
capillarity is lower. Thus, the capillary at the sample-
reaction barrier is generally more narrow and has a
greater capillarity than the capillary or space of the
reaction chamber. This difference in capillarity can
cause the flow of sample or fluid in the device to stop in
the sample-reaction barrier capillary. Presumably, the
fingers break the surface tension of the fluid at the
interface of the two capillaries or spaces and thereby
cause the fluid to move into a capillary or space of lower
capillarity. One can appreciate that the utility of
fingers can be extended to any part of the device where
fluid must flow from high capillarity to low capillarity.
- In practice, this is usually when the direction of fluid
flow is from a narrow capillary (higher capillarity)to a
wider capillary (lower capillarity). The top surface of
the sample reaction barrier may also be used to immobilize
reagents used in the assay process such that the sample
flows over the sample reaction barrier, dissolves the
reagents and moves into the reaction chamber. The move-
ment of the sample and reagents into the reaction chamber
may act as a mixing means.
Reaction Chamber
Referring to Fig. 1, the sample moves into the reac-
tion chamber 4 from the sample-reaction barrier 3. The
reagents of the device 10 are preferably placed in the
reaction chamber 4, for example, as dried'or lyophilized
owders, such that when-the-sam
p - - - - -- - - - - - - - ple- -enters- - the- -reaction - - - - - - - -
CA 02469307 2004-06-28
79565-77D
13
chamber 4 the reagents quickly reconstitute. The volume
oY the reaction chamber 4 is the volums of eample which
defines the reaction mixture. The reaction chamber may be
sealed on 2 sides, for example, by ultrasonic welding of
the top and bottom members. Thus, delivery of the sample
to the device 10 at the sample addition zone 1 does not
require a precise pipetting step to define the volume of
the reaction mixture. Mixing features which mix the reac-
tion mixture can also be incorporated in conjunction with
the reaction chamber element 4, such as those described in
WO 92/21434 published December 10, 1992.
The sample fills the
reaction chamber 4 because of capillary forces and also,
potentially, because of the hydrostatic pressure exertsd
by the sample in the sample addition reservoir Z. The
floor of the reaction chamber 4 may be smooth or comprised
of a grooved surface to aid in the movement of tho sample
into the reaction chamber 4. The volume of the reaction
chamber 4, and thereby the reaction mixture, may be any
volume which accommodates the reagents and which provides
the desired sensitivity of- the assay. The shape of the
reaction chamber 4 should be such that the movement of the
reaction mixture from the reaction chamber 4 is not turbu-
lent and eddies are not formed as a result of the movement
out of the reaction chamber 4. A preferred shape of the
reaction chamber 4 is shown in Fig. 1. The depth of the
reaction chamber 4 should be commensurate with the width
of the chamber to accommodate the desired reaction mixture
volums. The depth of the reaction chamber can range from
about 0.05 mm to 30 mn and preferably from 0.1 ma to
0.6 mm. To accommodate a particular volume of the reac-
tion chamber, the length and width of the reaction chamber
should be adjusted and the depth maintained as narrow as
is practical. The reaction chamber 4 is in direct fluid
contact with the sample-reaction barrier 3 and the diag-
nostic element 6 or time gate 5. In addition, the reac-
CA 02469307 2004-06-28
79565-77D
14
tion chamber 4 may also be in direct fluid contact with an
optional reagent reservoir 17 as shown in Figs. lb and lc.
A preferred embodiment of the reaction chamber util-
izes a ramp which extends from the bottom of the reaction
chamber to the surface of the diagnostic element. The
ramp minimizes or prevents mixinq and eddie formation of
the reaction mixture with the sample at the interface of
the reaction chamber and the diagnostic element as the
fluid moves through the device. Thus, the ramp allows a
smooth transition of the fluid out of the reaction chamber
and onto the diaqnostic element. The length of the ramp
should be optimized for each depth of the reaction cham-
ber, but generally, the ramp is at an angle of betwean 25
and 45 degrees relative to the floor of the reaction
chamber.
Time Gate
Referring to Fig. la, the time gate 5 holds the reac-
tion mixture in the reaction chamber 4 for a given period
of time. The concept of the time gate is that a predomi-
nantly aqueous solution cannot pass through a sufficiently
hydrophobic zone until the hydrophobic zone is made sutfi-
ciently hydrophilic. Furthermore, the hydrophobic zone is
made hydrophilic through the binding of a component in the
aqueous solution to the hydrophobic zone. The suffi-
ciently hydrophobic zone is generally in a capillary
space. The drivinq force for fluid movement over or
through the time gate may be either the capillarity of the
space or hydrostatic pressure exerted by the sample or a
combination of both of these forces. The amount of time
which is required to hold the reaction mixture in the
reaction chamber 4 is relative to the assay process such
that the reactions which occur in the reaction chamber 4
as a result of the assay process will reflect the presence
or amount of target ligand in the sample. Thus, the time
gate 5 delays the flow of the reaction mixture onto the
diagnostic element 6. The time gate 5 delays _the _f low of
- - ------- -----------
CA 02469307 2004-06-28
79565-77D
the reaction mixture by the principle that a hydrophilic
liquid, such as an aqueous solution or one which has a
dielectric constant of at least 40, cannot move past a
sufficiently hydrophobic barrier in a capillary channel.
5 In desiqning and building a time gate, one can begin with
a hydrophobic surface, such as are found on native plas-
tics. and elastomers (polyethylene, polypropylene, poly-
styrene, polyacrylates, silicon elastomers and the like)
or silicon chip surfaces or metal surfaces, either smooth,
10 grooved or textured and a capillary is formed by an oppos-
ing surface which can be hydrophobic or hydrophilic in
nature and smooth, grooved or textured. The hydrophobic
surface(s) in the capillary have a microscopic surface
area onto which can bind components which are generally
15 soluble in a predominantly aqueous solution. The hydro-
philic character and the concentration of the component(s)
in the reaction mixture and =the overall surface area -of
the time gate affects the mechanics of the time qate. The
amount of time for which the time gate 5 holds the reac-
tion mixture is related to the rate of binding of a com-
ponent(s) from the reaction mixture to the hydrophobic
barrier. The binding of the component(s) from the reac-
tion mixture changes the hydrophobic barrier to a zone
which is sufficiently hydrophilic over which or throuqh
which the reaction mixture can flow. Creating the suffi-
ciently hydrophilic surface then allows the fluid to flow
as if the time gate had not been in the device. Thus,
fluid flow through the remainder of the device is not
affected once the time gate has been made hydrophilic.
Other devices described which incorporate fluid delay
means, for example, in U.S Patent Nos. 4,426,451 and
4,963,498, require
an external manipulation of the device to start fluid flow
or utilize capillary constrictions to slow fluid flow. In
this latter case, the capillary constriction used to delay
fluid flow will affect the fluid flow through the - - --- - -
remainder of the device.
CA 02469307 2004-06-28
79565-77D
16
In a preferred embodiment, for example, the time gate
can be composed of latex particles 15 (Fig. la, not
drawn to scale), such as polystyrene latexes with diame-
ters of between about 0.01 m and 10 m or hydrophobic
5 polymers, such as polypropylene, polyethylene, polyesters
and the like, which are introduced onto the device in the
appropriate zone where the reaction mixture must travel.
In another preferred embodiment, the time gate can be
created by application of a hydrophobic chemical, such as
an ink or a long chain fatty acid, or a hydrophobic decal
to the desired zone. The hydrophobic chemical or decal is
generally not soluble or is poorly soluble in the reaction
mixture. In yet another preferred embodiment, the time
gate can also be formed by changing a hydrophilic surface
to a hydrophobic surface. For example, hydrophobic sur-
faces made hydrophilic by plasma treatment can be con-
verted back to a hydrophobic surface by the application of
solvents, ultraviolet light or heat and the like. Thiese
treatments can act to change the molecular structure of
the hydrophilic, plasma modified surface back to a
hydrophobic form.
The component(s) in the reaction mixture which bind
to the hydrophobic zone may be various proteins, polypep-
tides, polymers or detergents. A preferred protein is
bovine serum albumin. The time delay provided by the time
gate 5 depends on the concentration of the component(s) in
the reaction mixture, for example, bovine serum albumin,
which binds to the hydrophobic zone, for example, the sur-
face area provided by the latex particles 15. Another
preferred embodiment of the time gate 5 utilizes poly-
electrolytes which are hydrophobic and which become hydro-
philic by exposure to the buffering capacity of the reac-
tion mixture. The time gate 5 would be comprised of, for
example, polyacrylic acid, which in its protonated form it
is hydrophobic. The reaction mixture, if buffered above
the pKa of the polyacrylic acid, would deprotonate the acid
groups and form the hydrophilic salt of the polymer. In
------ ---------
CA 02469307 2004-06-28
79565-77D
17
this case, the time delay is related to the mass of poly-
electrolyte and the pH and the buffering capacity of the
reaction mixture.
The geometry or shape of the time gate can influence
the area of the time gate that the fluid will pass over or
through. That is, the time gate can be designed to direct
the flow of liquid through a specific area of the time
gate. By directing the fluid to flow through a defined
area of the time gate the reproducibility of the time
delay is improved. Figure 6 shows representative geome-
tries of time gates. For example, as shown in figure 6,
time gates a-d, the time gates have.V-shapes incorporated
into their design, and more specifically, the length of
the time gate (defined as the distance the fluid must'
cross over or through in order to pass the time gate) is
less at the tip of the V than in the body of the time
gate. Thus, in a preferred mode, the fluid will cross
over or pass through the time gate where the length is
shortest=thereby directing fluid flow through the time
gate in a consistent manner. In general, the direction-
ality of fluid flow over or through the time gates is
represented by opposing arrows in Figure 6. In a pre-
ferred embodiment, the orientation of the time gates b, c
and d of figure 6 are such that the fluid touches the flat
portion of the time gate first rather than the V shape.
In other words, the preferred direction of flow for the
time gates b, c and d of figure 6 is represented by the up
arrow. In cases where the time gate is simply a line, for
example as seen in figure 6, time gate e and f, the path
of fluid flow over or through the time gate can occur at
any point on the time gate. Thus, the time gates which
have geometries directing the fluid flow over or throuqh
a consistent area of the time gate are preferred. For
example, time gates with lengths ranging from about 1.3 mm
to 0.13 mm achieve delay times of approximately 0.3 min to
5.5 min, respectively, when the distance'between surfaces
-is _ - about __ 0. 018 - mm. - When -the time -gate - is - V,shaped -
- ---- - -- . -the ----- -- -
CA 02469307 2004-06-28
79565-77D
18
length of the time gate at the tip of the V has dimensions
smaller than the lenqth of the time gate at the remaining
portion of the V; that is, the arms of the V should have
a lenqth roughly 2 to 5 times the length of the V tip, as
for example, figure 7, time gate a, illustrates. Figure
7, time gate b, shows that only a small area of the time
gate is crossed over or through at the tip of the V as
compared with the remainder of the time gate. The time
gate should span the width of the capillary or space so
that the entire fluid front comes in contact with the time
gate. If the time gate was not as wide as, for example,
the diagnostic element, then the fluid front would go
around the time gate. Thus, the time gate should "seal"
the fluid in the space durinq the delay period.
Referring to Fig. 1, one skilled in the art can
recognize that each device 10 could incorporate one or
more time gates to achieve the desired function of the
device. Figure 8 shows some examples of the sequential
placement of several time gates of figure 6. For example,
as discussed in the next section, Optional Reagent Cham-
bers, if a sequential addition immunoassay was to be per-
formed by the device then 2 time gates would allow 2
sequential incubation steps to be performed by the device
prior to the movement of the reaction mixture to the diag-
nostic element. In another example, if an incubation of
the reaction mixture on the capture zone or zones of the
diagnostic element(s) 6 was required then a time gate(s)
would be placed immediately behind the capture zone or
zones. This use of the time gate may arise in cases where
poor efficiency of binding of the component in the reac-
tion mixture to the capture zone of the diagnostic element
would prevail.
Another application of the time gate involves the
placement of a time gate on a surface which is not part of
a capillary space. For example, the time gate can be
placed on a hydrophilic surface, which aaone without a
capillary space will allow liquids to move. __This__is_ - - - - - -
CA 02469307 2004-06-28
79565-77D
19
generally the case when a substantial volume of liquid is
placed on a surface and it spreads because of surface
tension and because of the hydrostatic pressure of the
liquid pushing the meniscus outwardly. The time gate then
would function to delay the advance of the fluid front
because the hydrostatic nature of the surface of the time
gate would stop the movement of liquid. As the meniscus
of the advancing liquid touches the time gate, the
component or components in the liquid binds to the time
gate to create a sufficiently hydrophilic surface for a
continued advance of the liquid on the surface.
Yet another embodiment of the time gate involves the
positioning of a time gate prior to a membrane which is
used to capture a conjugate or receptor. In yet another
embodiment of the time gate, the time gate can becomposed
of hydrophobic surfaces in a membrane. In those cases,
the hydrophobic membrane is positioned prior to the por-
tion of membrane which captures the conjugate or receptor
and may be positioned after a reaction chamber or a por-
tion of membrane where reagents of the assay are placed or
embedded. and where the reagents incubate for a defined
period of time. The time gate in the membrane can be
formed by application of raw latex particles in the mem-
brane at an appropriate solids concentration ranging from
about 0.014 to 10%. The size of the latex particles
should be slightly less than the pore size of the membrane
so that the latex becomes imbedded within the membrane.
The density of latex within the membrane at the time gate
should be uniform so that the reaction mixture does not
circumvent the time gate. For example, the latex size
used to create a time gate for a membrane with a pore size
of 1 m can range between 0.05 and 0.2 m. Since the dis-
tribution of pore sizes in membranes varies widely, the
actual size of latex used must be arrived at by experi-
mentation. The hydrophobic nature of the membrane used
for the time gate can also be formed by plasma treatment
or by treatment of the membrane with hydrophobic-chemicals
CA 02469307 2004-06-28
79565-77D
or polymers that adsorb to the membrane. One skilled in
the art can appreciate that the teachings described herein
of the inventive features of the time gate can be utilized
to design time gates in a variety of diagnostic devices
5 which utilize membranes. That is, devices describid, for
example, in U.S. Patents 4,435,504, 4,.727,019, 4,857,453,
4,877,586 and 4,916,056,
can incorporate a time gate, for example, prior
to the membrane or in the membrane which captures the
10 conjugate or receptor.
Optional Reaaent Chambers
Referring to Figi. lb and ic, the optional reagent
chamber 17 is useful for the introduction of reagents into
the assay process. In general, the optional reagent chaa-
15 ber 17 may be in direct fluid contact with the sample
addition reservoir 2 via a sample reaction barrier. 3 or a
port the reaction chamber 4 or the diagnostic element6,
via a sample reaction barrier 3 or a port. For example,
Fig. lb shows the optional reagent chamber 17 in direct
20 fluid contact with the reaction chamber 4. The flow of
the introduced reagent may be controlled by a time gate 5a
and fingers 16 can aid in the movement of reagents into
the reaction chamber 4. Referring now to Fig. ic, for
example, if a sequential addition immunoassay was to be
performed by the device then 2 time gates 5 atid 5a would
and fingers 16 can aid in the movement of reagents into
the reaction chamber 4. Referring now to Fig. lc, for
example, if a sequential addition immunoassay was to be
performed by the device then 2 time gates 5 and 5a would
allow 2 sequential incubation steps to be performed in the
optional reagent chamber 17 and then in the reaction cham-
ber 4 by the device prior to the movement of the reaction
mixture onto the diagnostic element 6. That is, sample
would be applied to the sample addition reservoir 2
through the sample addition zone 1 and the sample flows
- pver the -sample - reaction barrier- 3- and- imto- thar -optional - - - - - -
- - - -
CA 02469307 2004-06-28
79565-77D
21
reagent chamber 17 by the aid 'of fingers 16 where the
first set of reactions would occur. The time gate 5a,
after the appropriate amount of time, would allow the
reagents to flow over the sample reaction barrier 3a and
into the reaction chamber 4 by the aid of fingers 16a
where the next set of reactions would take place. After
the appropriate amount of time, the time gate 5 allows the
flow of reaction mixture onto the diagnostic element 6.
Fluid Control Means
Referring to Fig. id, the optional fluid control
means 18 is designed to control the flow of the reaction
mixture in the devica. More specifically, the optional
fluid control means 18 causes the volume of the reaction
mixture to flow over the capture zone of the diagnostic
element 6 at a rate which allows for an optimum capture of
reagents onto the capture zone. After the volume of the
reaction mixture flows over the capture zone the rate of
flow of the excess reagents may be increased. The differ-
ential rate of flow of the reagents in the device is
achieved by designing a gap 18 between the surfaces of the
capillary space 19 of the diagnostic element 6. The size
of the gap 18 is larger than the capillary space 19 of the
diagnostic element 6. The gap 18 generally follows the
capture zone or the zone where the rate of flow is
required to be decreased. The gap 18 in the diagnostic
element 6 thus has an associated volume. The volume of
the gap 18 is filled with the reaction mixture by capil-
lary action as it moves through the device. Since the gap
18 after the capture zone is greater than the capillary
space 19 of the diagnostic element 6 a drop in capillary
pressure at the beginning of the gap 18 results in a
decrease in the rate of flow of the reaction mixture into
the gap 18 and therefore a decrease in the rate of flow of
the reaction mixture over the capture zone. Varying the
size of the gap 18 changes the capillarity in the gap and
- - - _thus - the flszw- _of - the- reaction mixture - over - the -oapture - -
- - - - - - - - -
CA 02469307 2004-06-28
79565-77D
22
zone. In the case of immunoassays requiring a wash step
to remove unbound reagents from the diagnostic element 6,
it is generally desired that the rate of flow of the wash
solution over the diagnostic element 6 is faster than the
rate of flow .of the reaction mixture over the diagnostic
element 6 because this decreases the time of the assay.
The shape of the gap can take many forms. As shown in
Fig. id, the gap has square corners, however, the gap can
be shaped as a trapezoid or triangle which would change
the rate of flow of the reaction mixture while flowing
into the gap. One.skilled in the art can also appreciate
that for certain immunoassays a wash step is not required.
The control of the rate of flow of the reagents in
the device can also be used to allow chemical reactions to
take place in one zone of the device before the reagents
move to another area of the device where the extent of
reaction of the reagents is monitored or where further
reaction may take place. For example, several fluid con-
trol means could be incorporated into a device for use in
immunoassays where a sequential addition and incubation of
reagents is necessary. That is, the sample comes in con-
tact with the first reagents and the time for the reaction
of the sample and first reagents is controlled by a first
gap. When the first qap is filled with fluid, the reac-
tion mixture continues to the second reagents at which
time an additional chemical reaction can subsequently take
place. The time required for completion of this second
reaction.can also be controlled by a second gap before
further flow of the reaction mixture along the diagnostic
element. Chemical and biochemical reactions also take
place in the volume of the gap, for example, by immobiliz-
ing reagents in the gap.
Diaanostic Element
Referring to Figs. 1 and 2, the diagnostic element 6
is formed by opposing surfaces which are a capillary dis-
tance apart _ through which the- reastion mixture- -f-l-cww -and - - - - - -
CA 02469307 2004-06-28
79565-77D
23
on which are placed one or more capture zones. The cap-
ture zones are comprised of reagents, such as receptors,
or devices, such as biosensors which bind or react with
one or more components from the reaction mixture. The
binding of the reagents from the reaction mixture to the
capture zones of the diagnostic element 6 is related to
the presence or amount of target ligand in the sample.
One or more receptors or biosensors can be placed on the
diagnostic element 6 to measure the presence or amount of
one or more target ligands. The receptors or biosensors
can be placed in discrete zones on the diagnostic element
6 or they can be distributed homogeneously or heterogene-
ously over the surface. Receptors or other chemical rea-
gents, for example, a receptor against the signal gener-
ator can also be immobilized on the diagnostic element 6
to verify to the user that the reagents of the reaction
mixture are viable and that the reaction mixture passed
through the zones of the receptors'or biosensors. A sin-
gle receptor or biosensor can be placed over the majority
of the diagnostic element 6 such that as the reaction mix-
ture flows through the diagnostic element 6 the components
from the reaction mixture bind to the surface of the diag-
nostic element 6 in a chromatographic fashion. Thus, the
distance which the component of the reaction mixture binds
would be related to the concentration of the tarqet ligand
in the sample. The reagents, such as receptors, are immo-
bilized on the surface of the diagnostic element 6 through
covalent bonds or through adsorbtion. A preferred embodi-
ment is to immobilize receptor coated-latex particles, for
example of diameters ranging from about 0.1 m to 5 m.
In addition, particles termed "nanoparticles" can also be
coated with receptor and the resulting nanoparticles can
be immobilized to the diagnostic element through adsorb-
tion or covalent bonds. Nanoparticles are generally
composed of silica, zirconia, alumina, titania, ceria,
metal sols, and polystyrene and the like and the particle
sizes range _ from about 1 _ nm_ to 100 _nm. The benef it - of
CA 02469307 2004-06-28
79565-77D
24
using nanoparticles is that the surface area ot the pro-
tein coating the nanoparticle as a function of the solids
content is dramatically enhanced relative to larger latex
particles.
The surfaces of the diagnostic element 6 would allow
the receptor coated nanoparticles or latex particles to
bind to the diagnostic element 6. In a preferred embodi-
ment, the receptors bind to the surface of the diagnostic
element through electrostatic, hydrogen bonding and/or
hydrophobic interactions. Electrostatic, hydrogen bonding
and hydrophobic interactions are discussed, for example,
in Biochemistry 2,Q, 3096 (1981) and Biochemistry 21, 7133
(1990). For example, the diagnostic element 6 can be
treated with a plasma to generate carboxylic acid groups
on'the surface. The receptor coated latex particles are
preferably applied to the diagnostic element 6 in a low
salt solution, for example, 1-20 mM, and at a pH whicb, is
below the isoelectric point of the receptor. Thus, the
negative character of the carboxylic acid groups on the
diagnostic element 6 and the positive charge character of
the receptor latex will result in enhanced electrostatic
stabilization of the latex on the diagnostic element 6.
In another preferred embodiment, latex particles or
nanoparticles, which may be coated with receptor or may
compose a time gate, are entrapped on a non-absorbent
surface. The microstructure of the non-absorbent surface
is textured so that the particles are entrapped on the
surface or in the layers of the microstructure, forming
what is generally referred to as a"nanocomposite."
Magnetic fields may also be used to immobilize particles
which are attracted by the magnetic field. These types of
surfaces, generally termed "nanostructured materials" are
described, for example, in Chemical and Engineering News
.U, 18-24 (1992).
In an additional embodiment of the diagnostic ele-
ment, now referring to Fig. 5, the diagnostic element 6 is
- - --- -
- - ---------
- ---- - - a cylincTricar su=face ~ahich may be composed of grooves.
CA 02469307 2004-06-28
79565-77D
When the diagnostic element is! composed. of grooves, the
grooves generally run perpendicular to the flow of the
reaction mixture. A capillary apace is formed around the
diagnostic element by a round= tube which is generally
5 clear; thus, the surface of the diagnostic element and the
opposing surface of the tube are a.capillary distance
apart. The capillary formed allows the flow of the reac-
tion mixture over the round diagnostic element 6. Gener-
ally, the reaction mixture would travel up against gravity
10 or down with gravity through the cylindrical capillary
space. The capture zones of the round diagnostic element
6 can be placed in discrete zones or over the entire
length of the diagnostic element 6. The capture zones may
also circle the diameter of the diagnostic element 6 or
15 may be applied to only a radius of the diagnostic eleaent
6. The reaction mixture may be delivered to the diagnos-
tic element 6 through the tube S. Furthermore, the cylin-
drical volume of the tube 8 may be used as a reaction
chamber 4 and a disc shaped sample reaction barrier 3 with
20 grooves on its perimeter may also be inserted to form tho
reaction chamber 4 and the sample addition rdservoir 2.
From this discussion, now referring to Fig. 1 and 2, one
skilled in the art can also appreciate that the flat
diagnostic element 6 may also be curved such that the
25 curvature is a radius of a circle.=
one skilled in the art can appreciate that various
means can be used for the detection of signal at the
capture zone of the diagnostic element. In the case of
the use of biosensors, such as, for example, a piezo-
electric crystal, the piezoelectric crystal onto which
would be immobilized a receptor, would be the capture zone
and the response generated by binding target ligand would
be generally reflected by an electrical signal. Other
types of detection means include, but are not limited to
visual and instrumental means, such as spectrophotometric
and reflectance methods. The inventive features of the
-- --------
diagnostic element~ -deecribed - herein allows for fmprovad
CA 02469307 2004-06-28
79565-77D
26
capture efficiencies on surfaces over which a reaction
mixture flows and that various means for detection may be
used by one skilled in the art.
The surfaces of the capillaries in the devici are
generally hydrophilic to allow flow of the sample and
reaction mixture through the device. ' In a preferred
embodiment the surface opposing the diagnostic element 6
is hydrophobic such that the reaction mixture repels this
surface. The repulsion of reaction mixture to,the surface
opposing the diagnostic element 6 forces the reaction
mixture, and particularly the protein conjugates, to the
surface where capture occurs, thus improving the capture
efficiency of the components of the reaction mixture to
the capture zone. The hydrophobic surfaces opposing the
diagnostic element cati have a tendency to become hydro-
ph313c as the reaction mixture progresses through the
diagnostic element because various components which may be
present endogenously or exogenously in the sample or reac-
tion mixture, such as, for example,.prote3ns or polymers,
bind to the hydrophobic surface. A preferred hydrophobic
surface opposing the diagnostic element can be composed of
ir
teflon. tt is well known to those skilled in the art that
teflan" surfaces bind proteins poorly. Thus, the teflon
surface opposing the diagnostic element would not become
as hydrophilic as would surfaces composed of, for example,
polystyrene, polyacrylate, polycarbonate and the like,
when the reaction mixture flows through the diagnostic
element.
In another preferred embodiment, the diagnostic ele-
ment 6 is hydrophilic but the areas adjacent to the diag-
nostic element 6 are hydrophobic, such that the reagents
of the assay are= directed through only the hydrophilic
regions of the diagnostic element. One skilled in the art
will recognize that various techniques may be used to
define a hydrophilic diagnostic element or,zone, such as
plasma treatment of hydrophobic surfaces using masks which
- - --- - ---------------
----~-shield the surfaces, except for the diagnostic element,
CA 02469307 2004-06-28
79565-77D
27
from the treatment or by application of hydrophobic adhe-
sives to hydrophilic surfaces to define a diagnostic ele-
ment or by the use of viscous hydrophobic compounds, such
as an oil or a grease. In another preferred embodiment,
the capillary of the diagnostic element can be formed by
ultrasonic welding. The boundaries of the diagnostic
element are dictated by the energy directors which are
used to form'the sonicated weld.
The surfaces of the diagnostic element 6 or of the
other components of the device may be smooth or grooved or
grooved and smooth. Various textured surfaces may also be
employed, alone or in combination with smooth or grooved
surfaces. For example, surfaces composed of posts,
grooves, pyramids and the like, referred to as protru-
sions, or holes, slots, waffled patterns and the like,
referred to as depressions may be utilized. The textured
geometries may be ordered in rows, staggered or totslly
random and different geometries may be combined to yield
the desired surface characteristics. The depressions or
the protrusions of the textured geometries can range from
about - 1 nm to 0. 5 mm and preferably from about 10 nm to
0.3 mm. The distance between the various depressions and
protrusions can range from about 1 nm to 0.5 mm and
preferably from about 2 nm to 0.3 mm.
In a preferred mode as shown in Figs. 1 and 2, one
surface of the diagnostic element 6 is grooved and the
grooves are perpendicular to the flow of the reaction mix-
ture and the opposing surface is smooth. In another
embodiment, one surface of the diagnostic element 6 is
grooved at the capture zone and the areas adjacent to the
capture zone are smooth. The opposing surface of the
diagnostic element 6 may be smooth or may be grooved, for
example, the grooves of each surface intermesh. The posi-
tioning of the qrooves of the diagnostic element perpendi-
cular to the flow of the reaction mixture is beneficial in
that the flow of the reaction mixture through the diagnos-
- - - -
-- t1c- slement~ -6 -occurs - in- an- orqaifized manrfer with- -
- a dis-
._.... .,.._...,-. . - _ _
..........~.~_
CA 02469307 2004-06-28
79565-77D
28
tinct, straight front dictated by the grooves in the
capillary space. In addition, when one surface is in
close proximity, for example 1 m to 100 m, to the peaks
of the grooves then the capture efficiency of the compo-
nents from the reaction mixture can be enhanced. The
enhancement of capture efficiency at the capture zones in
grooved diagnostic elements as compared to smooth surface
elements may be related to the movement of the reaction
mixture in the capillary space; that is, in the case of
the grooved surface the reaction mixture is forced to move
over the peak of the groove and into the trough of the
next groove. Thus, a finer grooved surface, that is, more
grooves per cm, would provide a better capture efficiency
than a coarser grooved surface. The-reaction mixture is
thus driven closer to the surface of the grooved diaqnos-
tic element than it would be if both surfaces were smooth.
Also, the close proximity of the surfaces decreases the
volume of the bulk reaction mixture above the grooved
surface of the diagnostic element and therefore decreases
the diffusion distance of the components which bind to the
diagnostic element. The proximity of the surfaces of the
-diagnostic element should minimize the volume of reaction
mixture in the diagnostic element at the capture zone
without blocking the capillary flow through the element.
The capture of, for example, the complex of target ligand:
Ligand receptor conjugate at the capture zone can approach
100% efficiency if the proximity of the surfaces is opti-
mized. The capture of nearly all of the ligand receptor
conjugate which is bound by target ligand is most desired
because a greater sensitivity of the assay as a function
of sample volume can be achieved. Other advantages of
improved capture efficiency are that less reagents are
used because the sample volume is decreased, the assay
device can be miniaturized because of the smaller sample
volume and the reproducibility of the assay result will be
improved because changes in the rate 6f flow of the
CA 02469307 2004-06-28
79565-77D
29
reaction mixture through the capture zones will have less
or no effect on the capture of the labelled conjugates.
The capillary space can be defined by a variety of
ways, for example, machining the surfaces to the appropri-
ate tolerances or using shims between the surfaces. In a
preferred embodiment, ultrasonic welding of the surfaces
defines the capillary. In this case, the capillary space
is defined by the energy directors and the distance
between the surfaces is a function of the size of the
energy director, the welding energy, the time of energy
application and the pressure applied durinq welding. The
surfaces of the diagnostic element can be parallel or non-
parallel. In the latter case, the flow rate of the rea-
gents through the diagnostic element will not be unifori
throughout the length. A preferred embodiment is to
maintain the surfaces of the diagnostic element approxi-
mately parallel. The surfaces of the diagnostic element
can be made from materials, such as plastics which are
capable of being milled or injection molded, for example,
polystyrene, polycarbonate, polyacrylate and the like or
from surfaces of copper, silver and gold films upon which
are adsorbed various long chain alkanethiols as described
in J.Am.Chem.Soc. 1992, 2,g,, 1990-1995 and the references
therein. In this latter example, the thiol groups which
are oriented outward can be used to covalently immobilise
proteins, receptors or various molecules or biomolecules
whi-ch have attached maleimide or alkyl halide groups and
which are used to bind components from the reaction mix-
ture for determining the presence or amount of the target
ligand.
Referring to Figs. 3a and 3b, the zones of immobili-
zation of one or more receptdrs ot the placement of bio-
sensors at the capture zone 31 dn the diagnostic element
6 can take many forms. For example, if the target ligand
is very low in concentration in the sample,then one would
desire that all of the reaction mixture pass over the zone
---- - - -
of i=obiiizeit -rece-ptor -o= biosensor to obtain the best
CA 02469307 2004-06-28
79565-77D
signal from the given volume of reaction mixture. In this
case, the placement of the reagents or biosensprs on the
diagnostic element 6 at the capture zones 31 could, for
example, resemble that shown in Fig. 3a. If the target
5 ligand in the sample is high in concentration and the
sensitivity of the-analytical method is not an issue then
the placement of the receptors or biosensors at the cap-
ture zones 31 could, for example, resemble that in Fig.
3b. one skilled in the art can appreciate that the place-
10 ment of receptors or biosensors on the diagnostic element
is a function'of the sensitivity requirements of the ana-
lytical method.
onr or more diagnostic elements can.comprise a
device. The reaction mixture may be applied to a device.
15 with multiple diagnostic elements. In addition, the
sample may be applied to the device and then separated
into different reaction chambers, each with separate,
diagnostic elements. The capture zone can be varidus
geometrical symbols or letters to denote a code when the
20 sample is positive or negative for the target ligand. One
skilled in the art will recognize the useful combinations
of the elements of this invention.
The diagnostic element can also be configured to
perform a semi-quantitative or quantitative assay, as for
25 example, is described in Clinical Chemistry (1993) U,
619-624, herein referred to by reference only: This
format utilizes a competitive binding of antigen and
antigen label along a solid phase membrane. The improve-
ment is that the use of the diagnostic element described
30 herein for the above cited method would require a smaller
sample volume and improved binding efficiency to the solid
phase surface.
Diaanostic Elements other than Canil aries
The inventive teachings described herein of the
adsorbtion of proteins, particularly receptors to plastic
surfaces, _ can be_ utilizea for.adsorbtion _ot receptors to_
CA 02469307 2004-06-28
79565-77D
31
many plastic surfaces which are not a part of a capillary.
Nanoparticles and latex particles coated with receptors
can also be applied to surfaces-of many types of immuno-
assay devices, as for example, to "dipsticka." Dipsticks
are generally used as a solid phase onto which are bound,
as a result of the assay process, for example, the ligand
receptor conjugate. Dipsticks generally incorporate mem-
branes; however, a disadvantage in the use of membranes in
dipsticks is the difficulty in washing the unbound ligand
receptor from the membrane. Thus, an improvement in the
use of dipsticks is to immobilize receptor coated latex or
nanoparticles 'directly onto a plastic surface of the dip-
stick. 'The removal of unbound ligand conjugate from the
plastic surface is thus more efficient than removal from
a membrane.
Used Reaaent Reservoir
Referring to Figs. 1 and 2, the used reagen't reser-
voir 7 receives the reaction mixture, other reagents and
excess sample from the diagnostic element 6. The volume
of the used reagent reservoir 7 is at least the volume of
the sample and extra reagents which are added to or are in
the device. The used reagent reservoir 7 can take many
forms using an absorbent, such as a bibulous material of
nitrocellulose, porous polyethylene or polypropylene and
the like or the used reagent reservoir can be comprised of
a series of capillary grooves. In the case of grooves in
the used reagent reservoir 7, the capillary grooves can be
designed to have different capillary pressures to pull the
reagents through the device or to allow the reagents to be
received without a capillary pull and prevent the reagents
from flowing backwards throuqh the device. The size and
quantity of the grooved capillaries determine the volume
and capillarity of the used reagent reservoir 7. In a
preferred embodiment, as shown in Fig. 4, the fingers 52
at the end of the diagnostic element 6,are in fluid con-
tact with a capillary space 55 and the-capillary space 55
CA 02469307 2004-06-28
79565-77D
32
is in fluid contact with a grooved or textured capillary
space 56. The depth of the grooves or textured surface
can be, for example, about 0.1 mm to 0.6 mm, preferably
about 0.3 mm to 0.5 mm and the density can range from
about 5 to 75 grooves per cm and preferably about 10 to
50 grooves per cm. Referring to Fig. 4, the reagents of
the device move to the fingers 52 at the end of the diag-
nostic element 51 and into the capillary channel 55. The
reagents either partially or completely fill the capillary
space 55 and then come in contact with the grooved or tex-
tured surface 56. The width of the capillary space 55 is
generally about 1 mm to 3 mm and the depth is generally
about 0.1 mm to 2 mm. The length of the capillary space
55 should be sufficient to be in fluid contact with the
grooved or textured surface 56. The grooved or textured
surface 56 partially or completely pulls the reagents from
the capillary channel 55'depending on the rate of delivery
of the reagents into the capillary space 55 from the diag-
nostic element 51. When the flow of reagents is complete
in the device, the grooved or textured surface 56 has
greater capillarity than the capillary channel 55 and the
reagents are removed from the capillary channel 55 by the
grooved or textured surface 56. In addition, the reverse
flow of the reagents from the grooved or textured surface
is not preferred because.the capillarity in the grooved or
textured surface 56 holds the reagents and prevents their
backward flow. One skilled in the art can recognize from
these inventive features that the arrangement of grooves
or a used reagent reservoir within the device can be
adapted to a variety of desired objectives.
The Description of the One-Step Assay Device
The elements of the device which have been described
individually can be assembled in various ways to achieve
the desired function. The term "one-step" implies that
one manual action is required to achieve the assay result,
for example, adding sample to the device is one step. In
CA 02469307 2004-06-28
79565-77D
33
the case of the device performing a one-step assay which
involves both a timed incubation of reagents and a wash
step, the wash solution is excess sample and the assay
device is built with the elements in fluid communication
using the sample addition reservoir, the sample-reaction
barrier, the reaction chamber, the time gate, the diag-
nostic element and the used reagent reservoir as depicted
in Fig. 1. The devices are generally about 3 cm to 10 cm
in length, 1 cm to 4 cm in width and about 2 mm to 15 mm
thick. Typically, a top member with smooth surfaces is
placed onto a bottom member which has a surface onto which
are built the elements stated above. The relationship of
the elements are as depicted in Fig. 1. The reagents
required for performing the assay are immobilized or
placed in the respective elements. The surfaces are
brought together, a capillary distance apart, and in doing
so, the regions of the sample addition reservoir, the
sample reaction barrier, the reaction chamber, the time
gate, the diagnostic element, the gap and the used reagent
reservoir are all formed and are capable of functioning
together. Also, the surfaces are brought together such
that the opposing surfaces touch to form and seal the
sample addition reservoir, the reaction chamber, and the
used reagent reservoir.
When performing a qualitative, non-competitive assay
on one or more target ligands, the signal producing rea-
gents, which could include, for example, a receptor
specific for the target ligand adsorbed to a colloidal
metal, such as a gold or selenium sol, are placed on the
sample reaction barrier or in the reaction chamber in
dried or lyophilized form. Another receptor for each
target ligand is immobilized onto the surface of the
diagnostic element at the capture zone. The time gate is
positioned generally on the diagnostic element between the
reaction chamber and the capture zones by the placement
of, for example, a surfactant-free polystyrene suspension
onto the device in an amount which dictates the desired
--------- ---
CA 02469307 2004-06-28
79565-77D
34
incubation time. The incubation time is usually the
amount of time for the reactions to come to substantial
equilibrium binding. The assay is then performed by
addition of sample to the sample addition reservoir of the
device. The-sample moves over the sample-reaction bar-
rier, into the reaction chamber by the aid of the fingers
and dissolves the reagents in the reaction chamber to form
the reaction mixture. The reaction mixture incubates for
the amount of time dictated by the time gate. The excess
sample remaining in the sample addition reservoir and
reaction mixture in the reaction chamber are in fluid com-
munication but are not in substantial chemical communica-
tion because of the sample-reaction barrier. Thus, the
reaction chamber defines the volume of the reaction mix-
ture. The reaction mixture then moves past the'time gate
and onto the diagnostic element and over the capture
zones. The complex of receptor conjugate and target
ligand formed in the reaction mixture binds to the
respective receptor at the capture zone as the reaction
mixture flows over the capture zones. The reaction mix-
ture may also flow over a positive control zone, which can
be for example, an immobilized receptor to the signal
development element. As the reaction mixture flows
through the diagnostic element and into the used reagent
reservoir by the aid of the fingers, the excess sample
flows behind the reaction mixture and generally does not
substantially mix with the reaction mixture. The excess
sample moves onto the diagnostic element and removes the
receptor conjugate which did not bind to the capture zone.
When sufficient excess sample washes the diagnostic ele-
ment, the signal at the capture zones can be interpreted
visually or instrumentally. Referring to Fig. id, in a
preferred mode of the above description, the reaction mix-
ture moves onto the diagnostic element 6, over the capture
zone or zones and then the reaction mixture proceeds into
a capillary gap 18. The capillary gap 1$ generally has
less capillarity than that of the diagnostic element 6.
CA 02469307 2004-06-28
79565-77D
The capillary space 19 of the diaqnostic element 6 is
generally smaller than the capillary space of the gap 18.
The volume of the capillary qap 18 qenerally approximates
the volume of the reaction mixture such that the capillary
5 gap 18 fills -slowly with the reaction mixture and once
filled, the capillarity of the remaining portion of the
diaqnostic element 6 or used reaqent reservoir is qreater
than the capillarity of the gap 18 resulting in an
increased rate of flow to wash the diagnostic element 6.
10 As one skilled in the art can appreciate, the gap 18 can
be formed in the top member 8 or in the bottom member 9 or
a combination of both members 8 and 9.
In the case of the device performing a one-step assay
which does not involve a timed incubation step but does
15 involve a wash step in which the wash solution'is excess
sample, the assay device is built with the elements in
fluid communication usinq the sample addition reservo~r,
the sample-reaction barrier, the reaction chamber, the
diagnostic element and the used reagent reservoir. The
20 assay reagents are used as described above for the non-
competitive qualitative assay. The assay device without
the time gate allows the reaction mixture to flow onto the
diagnostic element without an extended incubation time.
The capillary flow of the reaction mixture and the excess
25 sample are as described above.
The optional reagent chamber is incorporated into the
device in the case of the device performing a one-step
assay with the introduction of an additional assay reagent
into or after the reaction mixture or the introduction of
30 a wash solution which flows behind the reaction mixture
through the device. The optional reagent chamber may be
in fluid contact with any element of the device and is
generally in fluid contact with the reaction chamber.
When in fluid contact with, for example, the reaction
35 chamber, the optional reagent chamber and the reaction
chamber may be separated by a time gate. Various reagents
may _be _dried -or _lyophilized -in -the -optional-r-eagent-cham- - - - - - - -
- - - - -
CA 02469307 2004-06-28
79565-77D
36
ber, such as detergents for a washing step or reagents
which are sequentially provided to the diagnostic element
after the reaction mixture.
in the case of performing one-step, non-competitive,
quantitative assays the reagents as described above for
the non-competitive, qualitative assay may apply. The
device is comprised of the elements, sample addition
reservoir, sample-addition barrier, reaction chamber, time
gate, diagnostic element and used reagent reservoir. In
this case, the capture zone of the diagnostic element is
generally the entire diagnostic element.' That is, the
capture. zone is a length of the diagnostic element onto
which the receptor conjughte binds. The receptor conju-
gate binds along the length of the captura zone in pro-
portion to the amount of target ligand in the sample. The
device of the present invention is preferred for this
quantitative assay because of the high efficiency of cap-
ture of the reagents, for example, the binding of a com-
plex of target ligand and receptor conjugate to an immo-
bilized receptor to the target ligand on the capture zone,
and because the movement of the reaction mixture over the-
diagnostic element proceeds with a sharp front. The
receptors on the capture zone sequentially become satur-
ated with the complex of target ligand and receptor con-
jugate as the reaction mixture moves over the leng" of
the capture zone. The length of the diagnostic element
containing bound conjugate then determines the concentra-
tion of the target ligand. Those skilled in the art will
recognize the format of this type of immunoassay as a
quantitative immunochromatographic assay as discussed in
U.S. Pat. Nos 4,883,688 and 4,945,205.
In the case of the device performing a one-step,
qualitative, competitive assay which involves both a timed
incubation of reagents and a wash step and the wash solu-
tion is excess sample, the assay device is built with the
--- - --- -
elements in fluid communication using the sample addition
CA 02469307 2004-06-28
79565-77D
37
reservoir, the sample-reaction barrier, tha reaction cham-
ber, the time gate, the diagnostic element and the used
reagent reservoir. when performing a qualitative competi-
tive assay on one or more target ligands, the conjugate is
composed of, for example, a ligand analogue coupled to
signal development element, such as a gold or selenium
sol. The conjugate and receptor for each target ligand
are piaced in the reaction chamber in dried or lyophilized
form, for example, in amounts which are taught by U.S.
Pat. Nos. 5,028,535 and 5,089,391, =
Another receptor for each target ligand is
immobilized onto the surface of the diagnostic element at
the capture zone. The time gate is positioned generally
on the diagnostic element between the reaction chamber and
the capture zones as described previously. The incubation
time.is usually the amount of time for the reactions to
come to substantial equilibrium binding. The assay is
then performed by addition of sample to the deviCe. The
sample moves over the sample-reaction barrier and into the
reaction chamber, dissolves the reagents to form the reac-
tion mixture and incubates for the time dictated by ths
time gate. The excess sample and reaction mixture are in
fluid communication but not in substantial chemical commu-
nication because of the sample-reaction barrier. The
reaction mixture then moves onto the diagnostic element
and over the capture zones. The ligand analogua conjugate
binds to the respective receptor or receptors at the cap-
ture zone or zones. As the reaction mixture flows over
the diagnostic element and into the used reagent reser-
voir, the excess sample flows behind the reaction mixture
and generally does not substantially mix with the reaction
mixture. The excess sample moves onto the diagnostic ele-
ment and removes conjugates which do not bind to the cap-
ture zone or zones. When sufficient excess sample washes
the diagnostic element the.results at the capture zones
can be interpreted visually or instrumentally. In a pre-
---ferred mode -cf - the- above- invention,- t-he -reactton mixture
CA 02469307 2004-06-28
79565-77D
38
moves onto the diagnostic element, over the capture zone
or zones and then the reaction mixture proceeds into a
capillary gap. The capillary gap has less capillarity
than that of the diagnostic element. The volume of the
capillary gap generally approximates the volume of the
reaction mixture such that the capillary gap fills slowly
with the reaction mixture and once filled, the capillarity
of the remaining portion of the diagnostic element or used
reagent reservoir is greater resulting in an increased
rate of flow of excess sample to wash the diagnostic
element.
In another aspect of the one-step, competitive assay,
the reaction mixture is composed of ligand analogue-ligand
complement conjugate to each target ligand and receptors
adsorbed to latex particles with diameters of, for exan-
ple, 0.1 m to 5 m to each target ligand, in appropriate
amounts, for example, as taught by U.S. Pat. Nos.
5,028,535 and 5,089,391. The ligand complement on the
conjugate can be any chemical or biochemical which does
not bind to the receptors for the target ligands. The
assay is begun by addition of sample to the device.
Sample fills the reaction chamber and is incubated for a
time which allows the reagents to come to substantial
equilibrium binding. The reaction mixture flows over the
time gate and onto or into a filter element to prevent
ligand analogue-ligand complement conjugates which have
bound to their respective receptor latexes from passing
onto the diagnostic element. Typical filter elements can
be composed of nitrocellulose, cellulose, nylon, and
porous polypropylene and polyethylene and the like. Thus,
only the ligand analogue-ligand complements conjugate
which were not bound by the receptor latex will pass onto
the diagnostic element. The receptor to the ligand com-
plement of the conjugate is immobilized on the diagnostic
element at the capture zone and binds the conjugate. A
wash step may not be required because t'he filter removes
-- the_conjugate bound to latex; _hDwever, the excess- sample------ --
CA 02469307 2004-06-28
79565-77D
39
or a wash solution from the optional reagent chamber may
be used to wash the diagnostic element.
In the case of a one-step quantitative, competitive
assay, the receptor to the ligand analogue conjugate or
the ligand complement of the conjugate is immobilized onto
the diagnostic element as described previously for the
one-step quantitative, non-competitive assay. Thus, the
concentration of the target ligand in the sample is visual-
ized by the distance of migration on the diagnostic ele-
io ment of the conjugate. In another mode, a quantitative
assay could be performed by the binding of the labelled
conjugate, for example, the ligand analogue-ligand com-
plement conjugate, to sequential, discrete capture zones
of receptor on the diagnostic element. The quantitative
result is achieved by the depletion of the conjugate as
the reaction mixture flows through the capture zones of
the diagnostic element.
The Device as a Diaonostic Element
The diagnostic element of the device can be utilized
with-a sample addition means to perform a separation step
of bound and unbound conjugates. An example of this type
of device which has a sample addition means, a diagnostic
element and a used reagent reservoir is depicted in Fig 2.
For example, in the case of a non-competitive assay, at
least one receptor conjugate is incubated with sample
which is suspected of containing at least one target
ligand in a suitable vessel and this reaction mixture is
applied to the sample addition zone of the device. The
reaction mixture then flows onto the diagnostic element
and over the capture zone of, for example, immobilized
receptor to the target ligand. When target ligand is
present in the sample, the target ligand-receptor conju-
gate complex binds to the receptor on the capture zone.
If the signal development element is an enzyme, then
either a substrate for the enzyme which produces a visual
color or a wash solution followed by a substrate is next
----------- --
CA 02469307 2004-06-28
79565-77D
added to the device. Excess reagents flow to the used
reagent reservoir. The presence or amount of each target
ligand in the sample is then determined either visually or
instrumentally.
5 In the case of a competitive immunoassay, for.example
as taught by U.S. Pat. Nos. 5,028,535 and 5,089,391,
the diagnostic element
may be used to separate bound and unbound ligand analogue
conjugates such that. the unbound ligand analogue conju-
10 gates bind to the receptors of the diagnostic element in
proportion to the presence or amount of target'ligand in
the sample.
one skilled in the art can appreciate that all for-
mats of immunoassays or gene probe assays which require a
15 separation step of free and bound conjugates or the
separation of free of bound reagents which subsequentlf
leads to the ability to detect a signal can utilize the
inventive features of the diagnostic element. One skilled
in the art can also recognize that the inventive elements
20 of this invention, namely, the fingers., the sample reac-
tion barrier, the reaction chamber, the time gate, the
diagnostic element, the fluid control means- and the used
reagent reservoir can be used separately or in various
combinations and in conjunction with other devices not
25 described here. For example, the sample reaction barrier
with fingers and the reaction chamber can be used in con-
junction with devices incorporating porous members, such
as membranes to deliver precise volumes of reagents to the
porous member. The time gate can also be incorporated
30 into the aforementioned devices or the time gate may be
used alone in conjunction with devices incorporating por-
ous members. The fluid control means can also be used in
devices incorporating porous members to control the rate
of flow of reagents through the porous member.
CA 02469307 2004-06-28
79565-77D
41
Exoerimental Procedures
Examflle 1
PreDaration of anti-BhCG Antibodi--Colloidal Gold Coniuaate
Colloidal gold with an average diameter of 45 nm was
prepared according to the method of Prens, Nature, Physi-
cal Sciences, 241, 20 (1973). The colloidal gold conju-
gate was prepared by first adding 5.6 ml of 0.1 M potas-
sium phosphate, pH 7.58, dropwise with rapid stirring to
50 ml of colloidal gold. Anti B-subunit monoclonal anti-
body to hCG (Applied Biotech, San Diego,.CA; 1 ml of 4.79
mg/mi in phosphate buffered saline, 0.024 sodium azide, pH
7) was added in a bolus to the colloidal gold with rapid
stirring. After complete mixing the stirring was stopped
and the solution was incubated at room temperature for
1 h. Polyethylene glycol (average molecular weight-
20,000) was added (0.58 ml) as a lt solution to the col-
loidal gold solution and the solution was mixed. The
colloidal gold solution was subjected to centrifugation at
27,000 g-and 5 C for 20 min. The supernatant was removed
and each pellet was washed twice by resuspension and cen-
trifugation with 35 ml of 10 mM potassium phosphate, 2 mM
potassium borate, 0.01% polyethylene glycol (average mole-
cular weight=20,000), pH 7. After the final centrifuga-
tion, the pellet was resuspended in 0.5 ml of the wash
buffsr. The gold conjugate was diluted for the assay of
hCG into a buffered solution containing 10 mg/ml bovine
serum albumin at pH 8.
Example 2
PreDaration of anti-ahCG Antibody Latex
Surfactant-free polystyrene particles (Interfacial
Dynamics Corp., Portland, OR; 0.106 ml of 9.4% solids, 0.4
m) was added while vortexing to anti a-subunit hCG mono-
clonal antibody (Applied Biotech, San Diego, CA; 0.89 ml
of 6.3 mq/ml in 0.1 M 2-(N-morpholino) ethane sulfonic
acid, (MES), pH 5.5) and the suspension'was incubated at
room temperature f or 15 min . - -The suspension -waa subjected - - - - - - - -
-
---- -- - - --- - ---- - - -- --------------
CA 02469307 2004-06-28
79565-77D
42
to centrifugation to pellet the latex particles. The pel-
let was washed three times by centrifugation and resuspen-
sion of the pellet with 10 mM MES, 0.1 mg/ml trehalose, pH
5.5. The final pellet was resuspended in the wash buffer
at a solids concentration of 1t.
Example 3
IMegaration of Goat Anti-Mouse Latex
Surfactant-free polystyrene particles (Interfacial
Dynamics Corp., Portland, OR; 0.11 ml of 9.4% solids, 0.6
m) were added while vortexing to goat IgG antibody
against mouse IgG (Jackson ImmunoResearch Laboratories,
Inc.; 0.t9 ml of 0.34 mg/ml in 0.1 M MES, pH 5) and the
suspension was incubated at 450C for 2 h. The suspension
was subjected to centrifugation to pellet the latex par-
ticles. The pellet was washed three times by centrifuga-
tion and resuspension of the pellet with 10 mM MES, 0.2
mg/ml trehalose, pH 5.5. The final pellet was resuspended
in the wash buffer at a solids concentration of 1%.
Examole 4
Pretiaration of the One-Sten Device for a Oualitative hCG
Assav
A one-step device made of plastic was built having an
80 to loo l sample addition reservoir, a 20 /il reaction
chamber and a 40 l used reagent reservoir. This device
is designed for applying samples of about 20 1 to 100 l,
but the reaction chamber is fixed at 20 l. In cases
where a larger reaction mixture volume is required for the
desired assay, then the reaction chamber would be
increased to that volume and the sample addition resarvoir
would be about 2 to 4 times the volume of the reaction
chamber volume. The devices were plasma treated to graft
functional groups which create a hydrophilic surface.
Those skilled in the art will recognize that the plasma
treatment of plastic is performed in a coiltrolled atmo-
sphere - of - a- spetif ic gas in a, high -f-r-eguency -f ie-1-d.- - The
CA 02469307 2004-06-28
79565-77D
43
gas ior.izes, generating.free radicals which react with the
surface. The sample addition reservoir was shaped as a
trapezoid with dimensions of 14 mm and 7 mm for the paral-
lel sides and 7 mm for the other sides with a depth of
0.49 mm. The sample addition reservoir was adjacent to
the sample reaction barrier. The sample-reaction barrier
was 1.5 mm long and 7 mm wide including grooves running
parallel to the flow of the sample at a density of 50
grooves per cm and a depth of 0.1 mm. In the case of
sample volumes larger than 20 to 80 l, the width of the
reaction barrier and thereby the reaction chamber could be
increased to accommodate the desired flow rate but the
groove size or density could remain as indicated. The
fingers in the walls of the reaction chamber and the used
reagent reservoir were 1 mm wide and 0.4 mm deep with 7
fingers in each wall of the reaction chamber and the used
reagent reservoir. The reaction chamber volume was 20 l.
The reaction chamber was shaped as a trapezoid with dimen-
sions of 7 mm and 3.5 mm for the parallel sides and 7.1 mm
for the other sides with depths of 0.56 mm for 20 l reac-
tion chambers. The diagnostic element was about 2.5 cm
long, 2 min wide and 1 mm from the base of the device
including grooves running perpendicular to the flow of
reaction mixture at a density of 100 grooves per cm and a
depth of 0.05 mm. In the case of a time gate on the
diagnostic element, the time gate was positioned on the
diagnostic element immediately adjacent to the reaction
chamber. The width of the diagnostic element could be
increased to increase the flow of the reaction mixture to
the desired rate past the capture zones. The ant3-chCG
antibody latex (1 l) and the goat anti-mouse latex (1 l)
were applied to the diagnostic element of the devices
approximately 1.5 cm apart. The anti-BhCG antibody col-
loidal gold conjugate (10 l) was pipetted into the trough
of the reaction chamber. The devices were placed under
vacuum for about 15 min. to dry the reagents. The used
r e a g e n t r e s e r v - o i r had t h e _ shape.- of a _trapezoid w i t h
rlimen = - - - - - - - -
CA 02469307 2004-06-28
79565-77D
44
sions of 7 mm and 15 mm for the parallel sides and 8 mm
for the other sides with a depth of 0.5 mm. Referring to
Fig. 4, in a preferred (best mode) embodiment of the used
reagent reservoir, the reaction mixture moved to a capil-
lary space 55 (1.25 mm long, 27.5 mm wide and 0.48 mm
deep) from the diagnostic element 6, aided by fingers 52
(1 mm wide and 0.4 mm deep with 7 fingers), and then into
a grooved capillary structure (13.6 mm long, 25.4 mm wide,
0.61 mm deep with a density of 16 grooves per cm). The
outer walls and the top surface of the walls of the sample
addition reservoir and the reaction chamber had applied a
thin coating of silicon grease to prevent the leakage of
the reagents from the reservoir and chamber of the assem-
bled device. The capillary spaces in the devices were
then formed by placing a clear plastic polycarbonate sheet
on top of the device. The plastic shest was held to the
opposing surface with binder clips. The clear plastic
sheet had a sample port above the sample addition reser-
voir for the introduction of sample.
Example 5
oualitative one-Step Assay for hCG
The devices described in Example 4 were used for the
qualitative one-step assay for hCG. The assay times for
the devices without the time gates were about 5 to 10 min.
A urine solution (60 i) containing 0, 50, 200 and 500 mIU
hCG/ml was added to the sample reservoir of the devices.
The sample moved into the reaction chamber, dissolved the
colloidal gold conjugate and the reaction mixture moved
onto the diagnostic element over the anti-hCG latex and
goat anti-mouse IgG latex capture zones. The reaction
mixture moved into the used reagent reservoir and the
excess sample washed the diagnostic element. The color
density of the capture zones for hCG was measured instru-
mentally using a Minolta Chroma Meter CR 241 at 540 nm.
A red color was visible for samples containing hCG and not
visible for the sample without hCG at the_ capture_ zones
CA 02469307 2004-06-28
79565-77D
for hCG. The eE" values for the 0, 50, 200 and 500 mIU/ml
were 0, 7.78, 12.95 and 20.96, respectively, and for the
positive control (goat anti-mouse IgG) zones a distinctive
red bar was observed with a eE''of about 35.
5 ale 6
Oualitative One-Ste2 Assav for hCG Usina a Time Gate
Devices as described in Example 4 were prepared with
the addition of the time gate. The time gate was formed
on the diagnostic element which is in contact with the
10 reaction mixture in the reaction chamber. The time gate
was prepared by adding 1 l of 2% solids of surfactant-
free, sulfated latex, 1.0 pm, (Interfacial Dynanics Corp.,
Portland, OR). The other reagent latexes and gold conju-
gate were also added to the devices and dried as described
15 in Example 5. Clear plastic sheets were placed on the
devices and sample (about 60 l) containing 0, 50, 200,and
500 mIU hCG/ml was added to the devices. The sample moved
into the reaction chamber, dissolved the colloidal gold
conjugate and the reaction mixture remained in the reac-
20 tion* chamber for about 8 to 10 min, whereas in devices
without time gates the reaction mixture remained in the
reaction chamber for 5 sec to 15 sec. The proteinaceous
components of the reaction mixture, which may be present
in the sample and which was added as a component of the
25 reaction mixture, namely, bovine serum albumin, bound to
the latex particles of the time gate and changed the
hydrophobicsurface of the time gate into a hydrophilic
surface. Other proteins, such as gelatin, serum albumins,
immunoglobulins, enzymes and the like and polypeptides and
30 hydrophilic polymers will also function to bind to the
hydrophobic zone. The gradual transformation of the hydro-
phobic surface to a hydrophilic surface, which resulted
through binding of the proteinaceous components of the
reaction mixture to the latex particles allowed the reac-
35 tion mixture to flow over the area of the time gate. In
control- experiment.s- 3n- which protein, - namely- bovine- serua - -
CA 02469307 2004-06-28
79565-77D
46
albumin, was not added to the reaction mixture, flow of
the reaction mixture over the time gate and onto the diag-
nostic element did not occur during the time (5 h) of the
experiment. This control experiment showed that the urine
sample alone did not contain sufficient protein or compo-
nents which bind to the applied latex of the time gate to
allow a change in the hydrophobic character of the time
gate. In the event that the components in the sample
should only be used to cause the transformation of the
hydrophobic time gate to a hydrophilic one for the reac-
tion mixture to flow, then one would be required to lower
the mass and total surface area of the latex applied to
the time gate to an extent which would allow flow of the
reaction mixture over the time gate in an appropriate
amount of time. The reaction mixture then moved onto the
diagnostic element over the anti-hCG latex and goat anti-
mouse IgG latex capture zones. The reaction mixture moved
into the used reagent reservoir and the excess saipple
washed the diagnostic element. The color density of the
capture zones for hCG was measured instrumentally using a
Minolta Chroma Meter CR 241. A red color was visible for
samples containing hCG and not visible for the sample
without hCG at the capture zones for hCG. The eE" values
for the 0, 50, 200 and 500 mIU/ml were 0, 6.51, 13.14 and
18.19, respectively. A red color bar was visible at the
goat anti-mouse IgG capture zones of each device.
Example 7
Oualitative One-Step Assay for hCG Usinc a Flow Control
Means
Devices as described in Example 4 were prepared with
the addition of the optional flow control means. The
optional flow control means or "gap" was placed behind the
capture zone for hCG gold conjugate on the diagnostic ele-
ment. The gap between the two surfaces was 0.38 man, the
length of the gap was 13.2 mm and the width of the gap on
the top member was 9 mm; however, the effective width_of________
CA 02469307 2004-06-28
79565-77D
47
the gap was the width of the diagnostic, element (2 mm).
This gap volume above the diagnostic element was about 10
l which was, in this case, half the volume of the reac-
tion chamber. The anti-hCG 'and the goat anti-mouse
latexes and gold conjugate were added to the device and
dried as described in Example S. Clear plastic sheets of
polycarbonate having a gap in one surface were placed on
the devices with the gap facing the diagnostic element.
sample (about 60 l) containing 0 and 200 alU hCG/ml was
added to the devices. The sample moved into the reaction
chamber, dissolved the colloidal gold conjugate and the
reaction mixture then moved onto the diagnostic element
over the anti-hCG latex. The reaction mixture then
entered the gap. which was immediately behind the capture
zone of anti-hCG latex. The flow rate over the capture
zone slowed while the reaction mixture moved over the
capture zone and filled the gap. The time for the 10 l
reaction mixture to fill the gap was about 12 min to 16
min, whereas with devices without the optional flow con-
trol means, the times were about 1 min to 3 min. for the
reaction mixture to pass over the capture zone. idhen the
reaction mixture filled the gap, the reaction mixture then
moved into the narrow capillary of the diagnostic element
and over the goat anti-mouse capture zone. The reaction
mixture moved into the used reagent reservoir and the
excess sample washed the diagnostic element. The color
density of the capture zones for hCG was measured instru-
mentally using a Minolta Chroma Meter CR 241. A red color
was visible for samples containing hCG and not visible for
the sample without hCG at the capture zones for hCG. The
AE* values for the 0 and 200 mIU/ml were 0 and 16.12. The
6E* value of the-hCG capture zone for the device without
the flow control means for the 200 miU/mi sample was
16.32. A red color bar was visible at the goat anti-mouse
IgG capture zones of each device.
CA 02469307 2004-06-28
79565-77D
48
Examcle S
Preuaration of the Diacmostic Element for Multi-steR
Bssavs
A device was built -comprising a sample addition
reservoir and a diagnostic element. The devices were
plasma treated to graft functional groups which create a
hydrophilic surface. The sample addition reservoir had
dimensions of.12 mm long, 6 mm wide and 0.05 mm deep. The
diagnostic element was about 5.5 cm long, 1.3 mm wide and
1 mm from the base of the device and included grooves.
running perpendicular to the flow of reaction mixture at
a density of 100 grooves per cm and a depth of 0.05 mm.
In the case of qualitative assays, the antibody latex
(i l) was applied to the diagnostic element, covering the
entire width and 1 cm length of the diagnostic element.
In the case of an immunochromatographic assay, the anti-
body latex (6 l) was applied to the entire width and
length of the diagnostic element. The devices were placed
under vacuum for about 1 h: to dry the reagents. The capil-
lary spaces in the device were then formed by placing a
clear plastic polystyrene sheet on top of the device. The
plastic sheet was held to.the opposinq surface with binder
clips.
Example 9
Assay for hCG Usina the Diaanostic Element
The diagnostic element described in Example 8 was
used for the assay of hCG. Urine samples (20 l) contain-
ing 0, 50, 200 and 500 mIU/ml hCG were added to tubes
containing anti-BhCG antibody colloidal gold conjugate
(2 l). The tubes were vortexed and the reaction mixtures
were incubated for 5 min at room temperature. The reac-
tion mixtures (20 l) were applied in 10 l aliquots to
the sample addition reservoir of the device. The reaction
mixture flowed onto the diagnostic element from the sample
reservoir and over the capture zone. An absorbent at the
end of the capture zone removed the used reagent from the_
-------- - ------
CA 02469307 2004-06-28
79565-77D
49
diagnostic element. The color density of the capture
zones for hCG was measured instrumentally using a Minolta
Chroma Meter CR 241. A red color-was visible for samples
containing hCG and not visible for the sample without hCG
at the capture zones for hCG. The AE* values for the 0,
50, 250 and 500 mIU/ml were 0.00, 1.24, 3.16 and 5.56,
respectively.
Examyle 10
Svnthesis of ineta-Nitrovhencvclidine
To an ice cooled solution of phencyclidine hydro-
chloride (5g, 1.8 X 10-2mol) in concentrated sulfuric acid
(9m1) was added dropwise, and with stirring, fuming nitric
acid (2ml). The reaction mixture was stirred in an ice-
water bath for 1 hour and then poured onto crushed ice/
water. The mixture was made basic with lON sodium hydrox-
ide (50m1) to pH12 and extracted with diethyl ether (2 X
100m1). The combined organic layers were washed with
water (2 X 100m1), dried over anhydrous magnesium sulfate,
filtered,and evaporated under vacuum. The residue was
treated with methyl alcohol (20m1) and heated on a hot
water bath (80 C) until solute dissolved. The flask was
covered with aluminum foil (product is light sensitive)
and the solution was allowed to stir at room temperature
overnight when a yellow solid precipitated. The solid was
collected by filtration and dried under vacuum to afford
3.Og (58%) of m-nitrophencyclidine as fine yellow crystals
which were protected from light: mp 81-826C.
Example 11
Synthesis of ineta-Aminophencvclidine
To a stirrinq solution of m-nitrophencyclidine (3.Og,
10.4 X 10'3mo1) in methyl alcohol (150m1) was added, under
a flow of argon, 10% palladium-carbon (0.5g) followed by
ammonium formate (4.og, 6.3 X 10-2mol). The reaction mix-
ture was stirred at room temperature for 2 hours after
which time the catalyst was removed by filtration and the
------------- ------
CA 02469307 2004-06-28
79565-77D
solvent was evaporated under vacuum. The residue was
treated with iN potassium hydroxide solution (30m1) and
extracted with diethyl ether (2 X 50m1). The combined
orqanic extracts were washed with water (50m1), dried over
5 anhydrous magnesium sulfate, filtered and evaporated under
vacuum. The residue was dissolved in hexane (20m1) and
the solution was stirred at room temperature overnight
when a white solid precipitated. The solid was collected
by filtration and dried under vacuum to afford 1.4g (52%)
10 of m-aminophencyclidine: mp 121-122 C.
Examiple 12
Synthesis of Acetylthiovropionic Acid
To a stirred solution of 3-mercaptoproprionic acid
(7 ml, 0.08 moles) and imidazole (5.4 g, 0.08 moles) in
15 tetrahydrofuran (TIiF, 700 ml) was added dropwise over 15
minutes, under argon, a solution of 1-acetyl imidazole
(9.6 g, 0.087 moles) in THF (100 ml). The solution was
allowed to stir a further 3 hours at room temperature
after which time the THF was removed in vacuo. The resi-
20 due was treated with ice-cold water (18 ml) and the
resulting solution acidified with ice-cold concentrated
HC1 (14.5 ml) to pH 1.5-2. The mixture was extracted with
water (2X50 ml), dried over magnesium sulfate and evapor-
ated. The residual crude yellow oily solid product (10.5
25 g) was recrystallized from chloroform-hexane to afford 4.8
g(418 yield) acetylthiopropionic acid as a white solid
with a melting point of 44-45 C.
Example 13
Synthesis of ineta-Acetylthio~ropionamide Phencvclidine
30 To a stirring solution of m-aminophencyclidine (1.4g,
5.4 X 10-3mol) and acetylthiopropionic acid (0.87q, 5.8 X
10'3mo1) in anhydrous tetrahydrofuran (7m1) was added
dicyclohexylcarbodiimide (1.19g, 5.8 X 10-3mo1). The flask
was purged with argon and the solution, stirred at room
35 temperature for 2 hours. The mixture was filtered from
CA 02469307 2004-06-28
79565-77D
51
insoluble dicyclohexylurea and evaporated under vacuum.
The residual solid was recrystallized from chloroform/
hexane to afford 1.5g (71%) of -m-acetylthiopropionamide
phencyclidine as a white crystalline solid: mp152-4 C.
Examtile 14
Svnthesis of ineta-3-Merca2toprQ2rionamide Phencvclidina
meta-Acetylthiopropionamide phencyclidins (0.01q,
2.57x10-5 mol) was dissolved in 1.29 ml 0.12M potassium
carbonate in 80% methanol/20% water (v/v). The solution
sat at room temperature for 5 min and then 0.2 ml 0.5 N
potassium phosphate, pH 7, was immediately added and the
solution was adjusted to pH 7-7.5 with hydrochloric acid
(1 N). The title compound in solution was used as is to
react with BSA-SMCC.
Example 15
Preoaration of Phencvclidine Analoove Attached to Bovine
Serum Albumin (BSA-PCP)
Bovine serum albumin (BSA, 3.5 ml of 20 mq/ml) was
reacted with succinimidyl 4-(N-maleimidomethyl)-cyclo-
hexane-1-carboxylate (SNCC, Pierce Chemical Co.) by adding
a solution of 6.7 mg SMCC in 0.3 ml acetonitrile and stir-
ring the solution at room temperature for 1 h while main-
taining the pH between 7 and.7.5 with iN potassium hydrox-
ide. The protein was separated from unreacted compounds
by qel filtration chromatography in 0.1 lr! potassium phos-
phate, 0.02 M potassium borate, 0.15 M sodium chloride, pH
7Ø The meta-3-mercaptoproprionamide phencyclidine (0.2
ml of 13 mM) was added to the BSA-maleimide (2 ml at 8.2
mq/ml) and the solution was stirred at room temperature
for 4 h. The solution was then dialyzed 3 times against
1000 ml of 10 mM MES, pH 5.5. Recover 1.8 ml BSA-PCP at
8 mq/ml.
CA 02469307 2004-06-28
79565-77D
52
ExaIl13Zle 16,
Prevaration of Phencvclidine Analogye Colloidal Gold
Coniugate
A solution (4.7 ml) containing BSA (22 mg) and BSA-
PCP (5.6 mg) in 10 mM MES, pH 5.5 was added in a bolus to
colloidal gold (105 ml) in 10 mM MES, pH 5.5 with rapid
stirring. After complete mixing the stirring was stopped
and the solution was incubated at room temperature for
1 h. The colloidal gold conjuqate was subjected to dia-
filtration against 50 mM potassium phosphate, 10 mM potas-
sium borate, pH 7, using a tangential flow device (Sartor-
ius Easy Flow, molecular weight cutoff was 100,000) to
remove BSA and BSA-PCP which was not bound to colloidal
gold. The gold conjugate was diluted for the assay of PCP
into a buffered solution containing 10 mg/ml bovine serum
albumin at pH 7.5.
Examole 17
Prenaration of anti-Phencvclidine Antibody Latex
Surfactant-free polystyrene particles (Interfacial
Dynamics Corp., Portland, OR; 0.074 ml of 9.44 solids, 0.4
m) was added while vortexing to anti-phencyclidine mono-
clonal antibody (0.926 ml of 5.86 mq/ml in 0.1 lri MES, pH
5) and the suspension was incubated at 450C for 2 h. The
suspension was subjected to centrifugation to pellet the
latex particles. The pellet was washed three times by
centrifugation and resuspension of the pellet with 10 mM
MES, 0.1 mg/ml trehalose, pH 5.5. The final pellet was
resuspended in the wash buffer at a solids concentration
of 18.
------- ---------
CA 02469307 2004-06-28
79565-77D
53
Examtile 18
Preflaration of Latex-Immobilized Affinity-Purified Goat
laG Antibody Against the Fc Fraament of Mouse IqG (Goat
anti-mouse Fc latex)
Affinity-purified goat anti-mouse (Fc (Immunosearch)
and polystyrene latex particles (sulfated, 1.07 pm)
(Interfacial Dynamics) were incubated separately at 45 C
for one hour., the antibody solution being buffered with
0.1 M 2-(N-morpholino) ethane sulfonic acid at pH S.S.
While vortexing the antibody solution, the suspension of
latex particles was added to the antibody solution such
that the final concentration of antibody was 0.3 mg/ml and
the solution contained 18 latex solids. The suspension
was incubated for 2 hours at 45 C prior to centrifugation'
of the suspension to pellet the latex particles. The
latex pellet was resuspended in 1%.bovine serum albumin in
phosphate-buffered-saline (PBS) and incubated for one hour
at room temperature. Following centrifugation to pellet
the latex, the pellet was washed three times by resuspen-
sion in PBS and centrifugation. The final pellet was
resuspended in PBS containing 0.1% sodium azide at pH 7.0
at a latex concentration of 1% solids. -
Examole 19
Assay for Phencyclidine Using the Diaanostic Element
The diagnostic element,described in Example 8 was
used for the assay of phencyclidine (PCP). Urine samples
(133 l)containing 0, 100, 200 and 300 ng/ml PCP were
added to tubes containing a lyophilized buffer formulation
(containing 10 mM potassium phosphate, 150.mM sodium chior-
ide and 10 mg/al BSA, pH 8) and phencyclidine analogue
colloidal gold conjugate (4 Nl) was added and the solution
was vortexed. Anti-PCP antibody (2.8 Nl of 0.1 mg/ml) was
added to each tube and the solutions were vortexed and
incubated at room temperature for 5 min. Goat anti-mouse
Fc latex (50 ml of a 1$ suspension) ,was added to the
tubes, the tubes were vortexed and incubated at room
CA 02469307 2004-06-28
79565-77D
54
temperature for 10 min. The solutions were then filtered
to remove the complex of the PCP analogue gold conjugate:
anti-PCP antibody:goat anti-mouse latex from the reaction
mixture usinq a Gelman Acrodisce 3 syringe filter (0.45
Etm). The filtrates of the reaction mixtures (20 /11) were
applied to the diagnostic elements described in example 8.
The reaction mixture flowed onto the diagnostic element
from the sample reservoir and over the capture zone. An
absorbent tissue placed 1 cm after the capture zone
removed the used reagent from the diagnostic element. The
color density of the capture zones was measured instru-
mentally using a Minolta Chroma Meter CR 241. The OE*
values for the 0, 100, 200 and 300 ng/mi samples were
0.69, 9.28, 14.04 and 21.6, respectively.
Although the foregoing invention has been described
in some detail by way of illustration and example, it will
be obvious that certain changes or modifications may be
practiced within the scope of the appended claims.= As
used herein, references to "preferred" embodiments refer
to best modes for practicing the invention.