Note: Descriptions are shown in the official language in which they were submitted.
CA 02470042 2004-06-04
METHOD FOR MANUFAOTURING GALVANNEALED STEEL SHEET
A1~TD GALVANNEALED STEEL SHEET
Related Application
[0001] This application claims priority of JP 2003-307072, filed August 29,
2003, and JP 2003-
307073, filed August 29, 2003.
Field of the Invention
[0002] This invention relates to a method for manufacturing a galvamnealed
steel sheet having
excellent press formability and also excellent chemical conversion treatment
performance and
adhesiveness in a stable manner. It also relates to a galvannealed steel sheet
having excellent
press formability and chemical conversion treatment performance and
adhesiveness.
Background
[0003] Galvannealed steel sheets are used in wide industrial fields such as
automobile bodies
due to the excellent weldability and paintability compared to galvanized steel
sheets. Such
galvannealed steel sheets are used after being subjected to press forming.
However, the
galvannealed steel sheets have disadvantage of poor press formability as
compared to cold-rolled
steel sheets. That is because sliding resistance of the galvannealed steel
sheets during press
forming is larger than that of cold rolled steel sheets. The reasor,~ for such
resistance is due to the
fact that galvannealed steel sheets are difficult to smoothly enter into the
die at a portion where
the sliding resistance between the die and the bead is large. This often
induces fractures.
[0004] In a galvannealed steel sheet, heat treatment is carried out after
subjecting the steel sheet
to zinc-coating and, as a result of an alloying reaction where Fe in the steel
sheet and Zn in the
coating layer are diffused, an Fe-Z.n alloy phase is formed. The Fe-Zn alloy
phase usually
1
CA 02470042 2004-06-04
comprises r phase, 81 phase and ~ phase. When the Fe concentration decreases,
there is a
tendency that hardness and melting point decrease in the order of T phase -~
81 phase ~ ~ phase.
Accordingly, from the point of sliding performance during press forming, it is
effective to form
an alloy phase containing a Large amount of Fe, having high hardness and high
melting point.
This makes it difficult to induce adhesion. The galvannealed steel sheet where
press formability
is important is manufactured in such a manner that the average Fe
concentration in the coating
layer is made a bit high.
[0005] If, however, an alloy phase having a high Fe concentration is formed, a
hard and brittle T
phase appears at the interface between the coating layer and the steel sheet.
This likely induces
what is called "powdering," or a phenomenon of separation of the coating layer
from the
interface during press-forming. As a means to provide both the sliding
performance and the anti-
powdering property, Japanese CJnexamined Patent Publication No. 01/319,661
discloses a
method for forming a hard iron-base alloy layer as a second layer on the
coating layer using
electroplating or the like.
[0006] A widely used method for improving the press formability of zinc-coated
steel sheet is to
apply high viscosity lubricant oil on the steel sheet. That method, however,
generates painting
defects during painting caused by insufficient degreasing, and causes instable
press formability
due to lack of oil during press forming. Accordingly, there has been a strong
demand for
improvement of the press formability of the galvannealed steel sheet itself.
[0007] As the methods for solving the above problems, Japanese lJnexamined
Patent Publication
Nos. 53/060,332 and 02/190,483 disclose methods to improve the weldability or
press
formability by forming an oxide layer consisting mainly of Zn0 on the surface
of the zinc coated
steel sheet using electrolytic treatment, immersion treatment, aplrlication
and oxidation treatment
or heating treatment.
2
CA 02470042 2004-06-04
[0008] Japanese Unexamined Patent Publication No. 04/088,196 discloses a
method to improve
the press formability and chemical conversion treatment performance by forming
an oxide layer
consisting mainly of phosphorus oxide on the surface of the zinc coated steel
sheet by immersing
the zinc coated steel sheet in an aqueous solution of pH 2 to 6 containing 5
to 60 g/1 of sodium
phosphate, by conducting an electrolytic treatment or applying the aqueous
solution on the
surface of the zinc coated steel sheet.
[0009] Japanese Unexamined Patent Publication No. 03/191,093 discloses a
method to improve
the press formability and chemical conversion treatment performance by forming
a nickel oxide
on the surface of zinc-coated steel sheet using electrolytic treatment,
immersion treatment,
application treatment, application and oxidation treatment or heating
treatment.
Summary of the Invention
[0010] We conducted detailed studies on the causes of the failiue to attain
stable and excellent
press formability even when the above-mentioned methods are applied to
galvannealed steel
sheets and found that the reactivity at the surface is poor due to the
presence of Al oxide, and that
the surface irregularity is large. That is, we discovered that when the prior
art methods are
applied to the galvannealed steel sheets, it is difficult to form a desired
layer even when
electrolytic treatment, immersion treatment, application and oxidation
treatment or heating
treatment are carried out because the reactivity of the surface is low. The
thickness of the film
becomes too thin in portions where reactivity is low or, in other words, in
portions where the
amount of aluminum oxide is large. In addition, since the suri~ace
irregularity is large, the die
directly contacts the convex part of the coating layer. At that moment, the
sliding resistance
increases at the convex past of the coating layer having a thin oxide Layer
and the desired effect
for achieving good press formability is not well achieved.
3
CA 02470042 2004-06-04
[0011] A flat part of the surface of the galvannealed steel sheet is present
as a convex part as
compared to the surrounding portion. The main part that actually contacts the
die during gress
forming is such a flat part. Therefore, when the sliding resistance in the
flat part is made small, it
is possible to stably improve press formability. It is effective to prevent
adhesion of the coating
layer on the die to make the sliding resistance at the flat part small. It is
effective to form a hard
and high-melting layer on the surface of the coating layer for such a purpose.
We found that
control of the thickness of the oxide layer on the flat part is effective and
that, when the oxide
layer thickness on the flat part is controlled as such, adhesion of the
coating layer with the die
does not take place but a good sliding performance is available. It has been
also found that, for
the formation of the oxide layer as such, a method for forming an oxide layer
on the surface of
the coating layer by contacting the coating layer to an acid solution is
effective.
[0012] Thus, in one aspect, the invention relates to a method for
manufacturing a ga.lvannealed
steel sheet comprising: hot dip galvanizing a steel sheet; heating the hot dip
galvanized steel
sheet to alloy the coating layer; temper rolling the galvannealed steel sheet
to form a flat portion
on the coating layer; contacting the temper rolled steel sheet with an acid
solution; allowing the
temper rolled steel sheet to stand for about 1 to about 30 second(s); and
forming an oxide Layer
on the coating layer by washing with water.
[0013] We also found that, although formation of an oxide layer effective for
improving press
formability is possible, there are some cases where close adhesion of the
coating layer to the
oxide layer is poor and, accordingly, that the method is not always excellent
in terms of
adhesiveness. We found that, when the method is used in a. actual
manufacturing line, the
thickness of the oxide layer formed on the surface changes as a result of
changes in
manufacturing conditions such as line speed and coating weight of the acidic
solution and that,
when a thick oxide layer is formed, a uniform chemical conversion layer is not
formed.
4
CA 02470042 2004-06-04
[0014] Thus, in another aspect, this invention provides a method for
manufacturing a
galvannealed steel sheet having excellent sliding performance during press
forming and also
excellent chemical conversion treatment performance and adhesiveness in a
stable manner. It
also provides a galvannealed steel sheet having an excellent sliding
performance during press
forming and excellent chemical conversion treatment performance and
adhesiveness.
[0015] We investigated the influence of elements added to the treating
solution or the acidic
solution and found that, when Fe ion is contained in the above treating
solution, it has no bad
influence on chemical conversion treatment performance and also provides for
excellent
adhesiveness of the coating layer to the oxide layer whereupon it generates an
excellent
adhesiveness.
(0016] Accordingly, in yet another aspect, the invention provides a method for
manufacturing a
galvannealed steel sheet, comprising:
(a) hot dip galvanizing a steel sheet;
(b) heating the hot dip galvanized steel sheet to alloy the coating layer,
thereby
forming a galvannealed steel sheet;
(c) temper rolling the galvannealed steel sheet;
(d) contacting the temper-rolled steel sheet with an acidic solution having pH-
buffering action and containing Fe ion, and then allowing the temper-rolled
steel sheet to stand
for about 1 to about 30 second(s),to form an oxide layer on the surface of the
temper-rolled steel
sheet; and
(e) washing the temper-rolled steel sheet, on which the oxide layer is formed,
with
water.
[0017] It is preferable that the acidic solution contains Fe ion where Fe3+
ion concentration is
about 2 g/ liter or less and the balance is Fe2+ ion.
CA 02470042 2004-06-04
[0018] It is preferred that a "pH-increasing degree" of the acidic solution is
within a range of
about 3 to about 20. The pH-rising degree is defined as the amount (ml) of a 1
mol/liter aqueous
solution of sodium hydroxide necessary for increasing the pH of one liter of
the acidic solution
from 2 to 5.
[0019] It is preferred that the acidic solution contains at least one of
acetate, phthalate, citrate,
succinate, lactate, tartrate, borate and phosphate in an amount of within a
range of about S to
about SO glliter and has a pH within a range of about 1 to about 5.
[0020] It is preferred that the acidic solution contains at least one of
sulfate, nitrate and chloride
of Fe in an amount of within a range of about 0.1 to about 100 g/liter in
terms of Fe ion
cancentration.
[0021] It is preferable that the method far manufacturing a galvannealed steel
sheet further
comprises the step of contacting the temper-rolled steel sheet with an
alkaline solution to activate
the surface, before contacting with the acidic solution step (d).
[0022] It is preferable that the method for manufacturing a galvannealed steel
sheet further
comprises the step of contacting the temper-rolled steel sheet with an
alkaline solution to conduct
a neutralizing treatment for the acidic solution remaining on the surface,
after contacting with the
acidic solution step (d).
[0023] It is preferred that the step of contacting with the acidic solution
comprises contacting the
temper-rolled steel sheet with an acidic solution such that a solution film
formed on the surface
of the steel sheet after contacting to the acidic solution has a caating
weight of about 3 g/m2 or
less.
[0024] Further, this invention provides a galvannealed steel sheet having an
oxide layer with a
thickness of about 10 nrn or more on the surface flattened Bart of the hot dip
galvanized steel
sheet manufactured by the method comprising:
6
CA 02470042 2004-06-04
(a) hot dip galvanizing a steel sheet;
(b) heating the hot dip galvanized steel sheet to alloy the coating layer,
thereby
forming a galvannealed steel sheet;
(c) temper rolling the galvannealed steel sheet;
(d) contacting the temper-rolled steel sheet with an acidic solution having a
pH-
buffering action and containing Fe ion, and then allowing the temper-rolled
steel sheet to stand
for about 1 to about 30 second(s),to form an oxide layer on the surface of the
temper-rolled steel
sheet; and
(e) washing the temper-rolled steel sheet, on which the oxide layer is formed,
with
water.
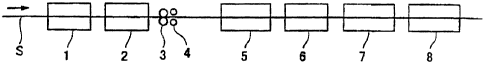
Brief Description of the Drawings
[0025] Fig. 1 shows an essential part of the apparatus for forming an oxide
layer used in
Examples.
[0026] Fig. 2 is a rough front view which shows an apparatus for the
measurement of a frictional
coefficient.
[0027] Fig. 3 is a schematic oblique view which shows the shape and size of a
bead in Fig. 2.
[0028] Fig. 4 is a schematic oblique view which shows the shape and size of a
bead in Fig. 2.
[0029] Fig. 5 is a schematic oblique view which illustrates an assembling
process of a test
material for an adhesion test.
[0030] Fig. 6 is a schematic oblique view which shows the state of a tensile
test in a test material.
[0031] Fig. 7 is a schematic scheme which shows a draw bead tester.
Detailed Description
7
CA 02470042 2004-06-04
[0032] It will be appreciated that the following description is intended to
refer to specific
embodiments of the invention selected for illustration in the drawings and is
not intended to
define or limit the invention, other than in the appended claims.
[0033] In the manufacture of a galvannealed steel sheet, a steep sheet is hot
dip galvanized and
then heated to carry out an alloying treatment. At that time, due to the
difference in the reactivity
of the interface between the steel sheet and the coating layen~ during the
alloying treatment,
irregularities are present on the surface of the galvannealed steel sheet.
However, after the
alloying treatment, temper rolling is usually carried out to secure the
quality of the galvannealed
steel sheet and, as a result of contact with the roll during the temper
rolling, the surface of the
coating layer is made smooth whereby the irregularities are relaxed/reduced.
Accordingly, upon
press forming, the force necessary for flattening the convex portions of the
coating layer is
decreased and the sliding performance is improved.
[0034] Since the flattened part of the surface of the galvannealed steel sheet
is a part to which a
die directly contacts during press forming, it is important in attempting to
improve the sliding
performance that a hard and high-melting substance which prevents adhesion to
the die is present.
The presence of an oxide layer on the coating layer is effective for improving
the sliding
performance since the oxide layer prevents adhesion to the die.
[0035] The oxide on the surface layer is abraded and scraped off during press
forming and,
therefore, the presence of a sufficiently thick oxide layer is necessary when
the contacting area of
the die with the material to be processed is large. Although an oxide layer is
formed on the
coating layer by heating during the alloying treafiment, most of the oxide
layer is destroyed by
the contact with the roll upon the temper rolling and a new surface is
exposed. Therefore, it is
necessary to form a thick oxide layer before temper rolling to achieve good
sliding performance.
Even when a thick oxide layer is formed before temper rolling taking the above
into
8
CA 02470042 2004-06-04
consideration, it is not possible to avoid destruction of the oxide Layer
during temper rolling and,
therefore, an oxide layer of the flattened part is unevenly present and a good
sliding performance
is unable to be achieved in a stable manner.
[0036] Because of the above, a good sliding performance is stably achieved
when a treatment to
form a uniform oxide layer on the temper-rolled galvannealed steel sheet,
particularly on the
flattened part of the coating surface, is carried out.
[0037] When the galvannealed steel sheet is contacted with an acidic solution,
then kept for
about 1 to about 30 seconds) under such a state that a liquid film of the
acidic solution is formed
on the surface of the steel sheet and washed with water followed by drying, an
oxide layer is able
to be formed on the coated steel sheet. When the acidic solution has a pH-
buffering action and is
a solution containing Fe ion, an oxide layer having an excellent sliding
performance is able to be
stably formed on the flattened part of the coating layer. Since the oxide
formed as such is very
fine, it does not badly affect the formation of a chemical conversion film
even if it remains
immediately before the chemical conversion treatment. Further, as compared to
an oxide layer
formed by the use of an acidic solution containing no Fe ion, it has been
found to have an
excellent close contact of an oxide layer and also to have adhesiveness.
[0038] Although the mechanism for the formation of the oxide layer as such is
not fully
understood, we believe that it is as follows. When a galvannealed steel sheet
is contacted with
an acidic solution, zinc from the side of the steel sheet dissolves. Since
such a dissolving of zinc
results in a reaction of hydrogen generation at the same time, hydrogen ion
concentration in the
acidic solution decreases as dissolving of zinc proceeds and, a.s a result,
the pH of the acidic
solution increases and an oxide layer mainly comprising Zn is believed to be
formed on the
surface of the galvannealed steel sheet. When an acidic solution having no pH-
buffering action
is used at that time, the pH of the acidic solution immediately rises and
dissolving of zinc which
9
CA 02470042 2004-06-04
is sufficient for the formation of an oxide layer is not achieved whereupon
formation of an oxide
layer which is sufficient for an improvement of a sliding performance is not
formed.
[0039] On the contrary, when an acidic solution having a pH-buffering action
is used, zinc is
dissolved and, even when a hydrogen generation reaction takes place,
dissolving of zinc briskly
proceeds since a rise in pH of the solution is mild and, as a result,
formation of an oxide which is
sufficient for improving a sliding performance takes place. Further, when Fe
ion is contained in
the acidic solution, a reduction reaction of Fe ion takes place and a very
small amount of Fe is
separated on the coated surface and it is presumed that, as a result, an
excessive growth of the
oxide layer mainly comprising Zn is suppressed and a very fine oxide layer is
formed.
[0040] There are two kinds of Fe ion and they are Fe2+ ion a~ad Fe+~ ion.
Although both are
effective for the formation of a fine oxide layer, much sludge is generated in
the solution when
Fe+3 is present and problems in terms of appearance such as formation of
damage caused by
pressing on the surface of the steel sheet occur. Therefore, it is better that
the Fe+3 ion
concentration is as small as possible. However, Fe+2 ion contained in the
acidic solution is
oxidized due to a change with the passage of time whereupon Fe~3 ~S present
and, therefore, it is
substantially impossible to conduct an operation using an acidic solution
containing no Fe+3 ion.
Accordingly, it is important to control the Fe+3 ion concentration in the
acidic solution and, in
view of the goal of avoiding damage caused by pressing, it is effective to
control the Fe+3 ion
concentration to an extent of not more than about 2.0 g/l. With regard to the
means for
controlling the Fe+3 ion as such, a means where a treating solution is renewed
when the Fe+3 ion
concentration becomes more than about 2.0 g/1 and a means where Fe is
dissolved in a solution
to utilize a reduction reaction of Fe+~ ion may be exemplified.
[0041] It is preferred that an acidic solution having a pH-buffering action
has a pH-buffering
action within a range of pH about 2.0 to about 5Ø That is because, when an
acidic acid solution
CA 02470042 2004-06-04
having a pH-buffering action is used with the above pH range, an oxide layer
is stably prepared
upon contact with the acidic solution followed by keeping for a predetermined
time. As a
yardstick for the pH-buffering action as such, an evaluation is able to be
conducted by means of
an increasing degree of pH which is defined as the amount (ml) of a 1.0 mol/1
aqueous solution
of sodium hydroxide required to raise the pH of one liter of the acidic
solution from about 2.0 to
about 5.0 and, when the value is within a range of about 3.0 to about 20.0, an
oxide layer having
a thickness of not less than about 10 nm is able to be stably formed on a
flattened part of the
coating layer. The reason why the range of pH is made about 2.0 to about 5.0
here is that, in a
region where pH is more than about 5.0, zinc oxide is produced and, even when
it is contacted
with an acidic solution and kept for a predetermined time, it is hard to form
an oxide layer
having a thickness of about 10 nm or more and that the pH increasing behavior
when pH is less
than about 2.0 does not substantially affect the easiness of production of the
oxide. When the pH
rising degree is less than about 3.0, the pH increases quickly and dissolving
of zinc which is
sufficient for the formation of an oxide layer is not achieved whereby a
sufficient production of
an oxide Layer is not available. When the value is more than about 211.0,
dissolving of zinc is
promoted and a long time is needed far the production of an oxide layer. In
addition, damage to
the coating layer is significant whereupon it is likely that the role which is
inherent to a rust-
preventing steel sheet is lost as well. The pH increasing degree of an acidic
solution where the
pH is mare than about 2 is evaluated by such a manner that an inorganic acid
which rarely has a
pH-buffering property within a range of pH about 2 to about 5 such as sulfuric
acid is added to
the acidic solution to lower the pH to about 2.
[0042 With regard to the acidic solution having a pH-buffering action as such,
it is possible to
use an aqueous solution containing about 5 to about 50 g/1 of at least one
substance selected from
acetate such as sodium acetate (CH3COONa), phthalate such as potassium
hydrogen phthalate
11
CA 02470042 2004-06-04
({KOOC)2C6H4), citrate such as sodium citrate (Na3C6H50~) and potassium
dihydrogen citrate
(KHZC6H507), succinate such as sodium succinate (Na2C4H404), lactate such as
sodium lactate
(NaCH3CHOHCO2), tarirate such as sodium tartrate (NaZC4H40~), borate and
phosphate. When
the above concentration is less than about 5 g/l, an increase ire pH of the
solution takes place
relatively quickly together with dissolving of zinc and, therefore, it is not
possible to form an
oxide layer which is sufficient for improving the sliding performance. When it
is more than
about SO g/l, dissolving of zinc is promoted and the result is believed to be
that not only is a long
time needed for formation of an oxide layer, but also damage of the coating
layer is significant
whereby the role inherent to the rust-preventive steel sheet is lost. When the
pH of the acidic
solution is too low, although dissolving of zinc is promoted, an oxide is
hardly formed and,
therefore, the pH is preferred to be about 1.0 or more. On the other hand,
when the pH is too
high, the reaction rate for dissolving of zinc becomes low and, therefore, it
is preferred that pH of
the acidic solution is about 5.0 or Less. When the pH of the acidic solution
is higher than the
range of about 1.0 to about 5.0, the pH may be adjusted using an inorganic
acid having no pH-
buffering property such as sulfuric acid or an acid solution of the salt used
such as acetic acid,
phthalic acid or citric acid.
[0043] With respect to the Fe ion contained in the acidic solution, it is
preferred that at least one
member selected from sulfate, nitrate and chloride of Fe is added and the
range of Fe ion
concentration is about 0.1 to about I00 g/l. When the Fe ion concentration is
less than about 0.1
g/1, there is a possibility that an oxide is formed by an effect of a pH
buffer only and that control
of the thickness of the oxide layer and making the oxide fine are difficult.
When it is more than
about 100 g/l, there is a possibility that suppression of grov~th of an oxide
layer becomes
excessive and that formation of an oxide necessary for improving the sliding
performance is not
possible. Addition of Fe ion is effective for controlling the thickness of an
oxide layer and
12
CA 02470042 2004-06-04
making the oxide fine but, on the other hand, Fe ion in the solution promotes
the dissolving of a
Zn coating layer and the coating layer becomes fragile and, as a result,
peeling-off of the coating
or the so-called "powdering" is apt to happen by the process upon pressing.
From such a view, it
is preferred that Fe ion is not mare than about IO g/1 and, when application
to a site receiving
severer bending and deformation upon return from the bending is taken into
consideration, it is
preferred to treat with a treating solution where Fe ion is not more than
about 5 gll. The term
"Fe ion concentration" means the sum of Fe2+ ion and Fe+3 ion concentration.
[0044] As such, according to the invention, an oxide layer having an excellent
sliding
performance is stably formed and it is also excellent in chemical conversion
treatment
performance and adhesiveness when the acidic solution used has a pH-buffering
action and also
contains Fe ion and, accordingly, the advantage of the invention is not
deteriorated even if other
metal ions and inorganic compounds are contained in the acidic solution either
as impurities or
intentionally. Especially because Zn ion is an ion which is eluted when the
steel sheet contacts
the acidic solution, an increase in Zn ion is noted in the acidic solution
during the operation, but
the amount of Zn ion concentration as such does not affect the advantage of
the invention at all.
[0045] There is no particular limitation for the method of contacting a
galvannealed steel sheet to
an acidic solution and, although there are methods where a coated steel sheet
is immersed in an
acidic solution, an acidic solution is sprayed on a coated steel sheet, an
acidic solution is applied
to a coated steel sheet via an application roll, etc., it is preferred that a
thin liquid film is finally
present on the surface of the steel sheet. That is because, when the amount of
the acidic solution
present on the surface of the steel sheet is too high, the pH of the solution
does not increase even
when zinc is dissolved, but dissolving of zinc just takes place successively.
Thus, a long time is
needed for the oxide layer to form. In addition, damage of the coating layer
is significant
whereupon it is likely that an inherent role as a rust-preventing steel sheet
is lost as well. From
I3
CA 02470042 2004-06-04
such a viewpoint, it is preferred and effective when the amount of the
solution membrane formed
on the surface of the steel sheet is adjusted to not more than about 3 g/cm2.
Adjustment of the
coating weight of the solution film may be carried out using a s~~ueezing
roll, an air wiper or the
like.
[0046) With regard to the time from contacting with an acidic solution until
washing with water
{retention time until washing with water), about 1 to about 30 seconds) is
used. That is because,
when the time until washing with water is less than about 1 second, the pH of
the solution
increases and an acidic solution is washed out before an oxide layer mainly
comprising Zn is
formed whereby the effect of improving the sliding performance is not achieved
while, even
when it is more than about 30 seconds, there is no change in the amount of the
oxide layer.
[0047] When the contact with an alkaline solution is carried out to conduct an
activating
treatment before formation of an oxide layer by contacting with an acidic
solution as mentioned
above, that is more effective. That is because, although the surface oxide
layer is destructed by
contacting to a roll upon a temper rolling, a part thereof still remains and,
therefore, reactivity of
the surface is not uniform. From such a viewpoint, it is important that the
oxide layer remaining
on the surface layer is removed as much as possible and, when the cantact to
an alkaline solution
as a means therefor is conducted, it is possible to treat relatively easily.
There is no particular
limitation for contacting with the alkaline solution and an effect is achieved
when treated by
means of immersing or spraying. In the case of an alkaline solution, the oxide
layer remained on
the surface layer is able to be removed as much as possible and activate the
surface but, when the
pH is Iow, the reaction is slow and a long time is needed for the treatment
and, therefore, pH of
the alkaline solution is preferably to be not less than about 10. When the pH
is within the above
range, it is possible to use sodium hydroxide or the like regardless of the
type of the solution.
14
CA 02470042 2004-06-04
[0048] When an acidic solution remains on the surface of the steel sheet after
washing with
water and drying, rust is apt to be generated when the steel sheet coil is
preserved for a long
period. From the viewpoint of preventing the generation of the rust as such,
contacting with an
alkaline solution may be conducted either by immersing in the alkaline
solution or by spraying
the alkaline solution thereon after contacting with the acidic solution
whereby the acidic solution
remaining on the surface of the steel sheet may be neutralized. It is
preferred that the pH of the
alkaline solution is not more than about 12 for the prevention of dissolving
of a Zn type oxide
formed on the surface. When the pH is within the above-mentioned range, it is
possible to use
sodium hydroxide, sodium phosphate or the like regardless of the solution
used.
[0049] The oxide layer in the invention is a layer comprising oxide and/or
hydroxide containing
Zn and Fe as essential elements.
[0050] In the manufacture of the galvannealed steel sheet of the invention, A1
is added to a
coating bath and there is no particular limitation for the added element
components which are
other than Al. Thus, besides Al, the advantage of the invention i.s not
deteriorated even when Pb,
Sb, Si, Sn, Mg, Mn, Ni, Ti, Li, Cu or the like are contained therein or added
thereto in addition to
Al.
[0051] Further, the advantage of the invention is not deteriorated even when
S, N, P, B, Cl, Na,
Mn, Ca, Mg, Ba, Sr, Si or the like are incorporated into an oxide layer as a
result of the presence
of impurities in the treating solution used for an oxidizing treatment or the
like.
Example 1
[0052] The invention will now be illustrated in more detail by way of the
following Examples.
[0053] A galvannealed steel sheet was produced by a common method on a cold
rolled steel
sheet having a thickness of 0.8 mm and then a temper rolling was further
carried out. After that,
an oxide layer was formed using a treating device as shown in Fig. 1.
CA 02470042 2004-06-04
[0054] Firstly, after a immersing in an acidic solution of pH 2.0 was carried
out at 50°C in an
acidic solution bath 2, a liquid filin was formed on the surface of the steel
sheet using a
squeezing roll 3. When the pressure of the squeezing roll was changed at that
time, the coating
weight of the liquid film was adjusted. After that, hot water o:F 50°C
was sprayed on the steel
sheet in a washing bath 5, the steel sheet was passed through the neutralizing
bath 6, hot water of
50°C was sprayed on the steel sheet in a washing bath 7 to wash and
drying was conducted in a
drier 8 whereupon an oxide layer was formed on a coated surface.
[0055] With regard to a solution for a immersing treatment in the acidic
solution bath 2, a
solution where 30 g/1 of disodium hydrogen phosphate and 20 g~T of citric acid
were mixed as pH
buffers and a predetermined amount of ferrous sulfate was added for an object
of addition of Fe
ion was used and the pH was adjusted by addition of sulfuric acid. For the
sake of comparison, a
solution which was the same as above except that no pH buffer was used, but
adjustment was
conducted by ferrous sulfate only was used.
[0056] The retention time until the above washing with water is the time until
the start of the
washing in a washing bath 5 when the liquid film amount was adjusted by a
squeezing roll 3 and
an adjustment was conducted by changing the Iine speed and, at the same time,
a thing where the
steel sheet was washed immediately after squeezing using a shower washing
device 4 at the
outlet side of the squeezing roll 3 was partly prepared as well.
[0057] An alkaline solution of pH 10 (an aqueous solution of sodium hydroxide)
was sprayed
during the above treatment in a neutralizing bath 6 so that an acidic solution
remaining on the
surface of the steel sheet was neutralized. Also, before immersing into an
acidic solution,
immersing into an aqueous solution of sodium hydroxide of pH 12 in an
activating bath 1 was
carried out to conduct an activating treatment.
16
CA 02470042 2004-06-04
[0058] After that, the coefficient of friction was measured as a means for
evaluating the press
formability for the steel sheet prepared as above, a peeling-adhesion test was
conducted for
evaluating adaptability of adhesion and evaluating chemical conversion
treatment performance
was carried out. In addition, after the steel sheet was applied with a rust-
preventing oil, it was
allowed to stand outside under such a condition that it was not affected by
external factors such
as dust and, after about six months, generation of rust spots was
investigated. The case where no
rust spot was evaluated as "o" and the case where rust spots were noted was
evaluated as "x".
Measurement of coefficient of friction, peeling-adhesion test and chemical
processing treatment
test were carried out as shown below.
( 1) Test for evaluation of press formability (test by measurement of
frictional coefficient)
[0059] The frictional coefficient of each test material was measured to
evaluate the press
formability as follows.
[0060] Fig. 2 is a rough front view showing the device for the measurement of
frictional
coefficient. As shown in the drawing, a sample 11 for the measurement of
frictional coefficient
collected from the test material was f-wed on a sample stand 12 and the sample
stand 12 was
fixed on the upper side of a slide table 13 which is able to move in a
horizontal direction. On the
lower side of the slide table 13, there is installed a slide table stand 15
which is movable up and
down and has a roller 14 contacting thereto and a first load cell I7 for
measuring the pushed load
N to a sample 11 for measuring the frictional coefficient by a bead 16 when
pushed up is
attached to a slide table stand I5. A second load cell 18 for measuring the
slide resistance F for
moving the slide table 13 in a horizontal direction under a state where the
above pushing force is
applied is attached to one end of the slide table 13. A test was conducted
using 2 liters of Preton
R 352 L which was a washing oiI for pressing manufactured by Sugimura Kagaku
and which
was applied on the surface of the sample 11 as a lubricant.
17
CA 02470042 2004-06-04
[0061] Fig. 3 and Fig. 4 are rough oblique views for showing the shape and the
size of the bead
used. Sliding takes place in such a state that the lower side of the bead 16
is pushed onto the
surface of the sample 11. The shape of the bead I6 as shown in Fig. 3 is that
the width is 10 mm,
the length of the sample in the sliding direction is 12 mm and the Lower part
of both ends in the
sliding direction has a curved surface with a curvature of 4.5 mm R and the
lower side of the
bead to which the sample is pressed has a plane where the width is 10 mm and
the length in the
sliding direction is 3 mm. The shape of the bead 16 as shown in Fig. 4 is that
width was 10 mm,
length of the sample in the sliding direction was 6~ mm and the lower part of
both ends in the
sliding direction has a curved surface with a curvature of 4.5 mm R and the
lower side of the
bead to which the sample is pressed has a plane where the width is 10 mm and
the length in the
sliding direction is 60 mm.
[0062] The test for measuring the frictional coefficient was carried out under
the following two
conditions.
[Condition 1]
[0063] A bead as shown in Fig. 3 was used where a pushing load (N) was set to
400 kgf and a
pulling-out speed for the sample (speed for movement of the slide table 13 in
the horizontal
direction) was set to 100 cm/min.
[Condition 2]
[0064] A bead as shown in Fig. 4 was used where a pushing load (N) was set to
400 kgf and a
pulling-out speed for the sample (speed far movement of the slide table 13 in
the horizontal
direction) was set to 20 cm/min.
[0065] A frictional coefficient p between the test material and the bead was
obtained by ~=FIN.
(2) Adhesiveness test
1S
CA 02470042 2004-06-04
[0066] The following test sample for the following test for adheaiveness was
prepared from each
sample material. Fig. 5 is a rough oblique view illustrating the assembling
process therefor. As
shown in the drawing, two test materials 21 having 25 mm width and 200 mm
length were made
into a test sample for an adhesive test 24 for an adhesive 2?'~ using a spacer
22 of 0.15 mm
between them and baking was carried out at 1 SO°C for 10 minutes. The
test sample 24 prepared
as such was bent in a T-shape as shown in Fig. 6 and pulled at the rate of 200
mm/min using a
tensile tester to conduct a peeling test. Incidentally, an adhesive of a vinyl
chloride resin type for
hemming was used as an adhesive.
[0067] Incidentally, the peeling is generated at the area where the strength
is weakest. For
example, when the close contact of the test material to the adhesive is
sufficient, adhesion failure
inside the adhesive happens. On the other hand, when the close contact of the
test material to the
adhesive is insufficient, peeling occurs at the interface between the test
material and the adhesive.
Thus, adaptability of the adhesive was evaluated according to such a peeling
mode and, in the
case where adhesion failure inside the adhesive was resulted, :it was
evaluated as "o" while, in
case where peeling took place at the interface between the test material and
the adhesive, it was
evaluated as "x".
[0068] In the case of a ga.lvannealed steel sheet, strength of the interface
between plating part
and steel sheet was weak depending upon the Fe% in the coat: particularly in
the coat where T
phase was formed at the interface between plating part and steel sheet and
there were some cases
where peeling was noted at that part. Even in those cases however, close
contact of the test
material to the adhesive was judged to be sufficient and was evaluated as "o".
{3) Test for chemical conversion treatment performance
[0069] Each of the test materials was treated under the conventional condition
using a zinc
phosphate treating solution of a dipping type used for undercoating of
automobiles (PBL 3080
19
CA 02470042 2004-06-04
manufactured by Nippon Parkerizing) and a zinc phosphate coat was formed on
its surface.
Crystalline state of the zinc phosphate coat formed as such was observed under
a scanning
electron microscope (SElvl) and the case where the coat was uniformly formed
was evaluated as
"O" while the case where the coat was not uniform where gaps were noted was
evaluated as "x".
[0070] Result of the test as above is shown in Tahle 1.
(4) Test for powdering-resistant property
(0071] A draw-bead test for each test material was conducted and a peeled
amount of the plating
was measured to evaluate the powdering-resistant property. Fig. 7 is a rough
scheme showing a
draw-bead tester. The weight was measured (Wl (g)) after the coating on the
side of the test
material which did not contact the bead was peeled using hydrochloric acid.
After that, the test
material was set at the sample part of Fig. 7, a triangular bead having an end
angle of O.SR was
pushed with a load of 500 kgf to make the pushing depth 4 mm and then the test
material was
drawn out at a constant speed of 200 mm/min. The drawn-out test material was
subjected to a
compulsory peeling at the area contacting the bead using a tape and the weight
was measured
again (W2 (g)). The difference in the weight obtained as such was divided by
the drawn-out area
to calculate the peeled amount per unit area_
[0072] The following were now apparent from the test result as shown in Table
1.
(1) Since No. 1 and No. 2 were not subjected to a treatment by an acidic
solution,
they do not form an oxide layer which is sufficient for improving a sliding
performance at the
flattened part and a frictional coefficient is high.
(2) Nos. 3 to 5 are comparative examples where a treatment was conducted using
an
acidic solution containing no pH buffer and, as compared with No. 1 and No. 2,
frictional
coe~cient is low but, as compared with the examples of the invention, it is
high and formation
of an oxide layer is insufFcient.
CA 02470042 2004-06-04
(3) Nos. 6 to 8 are comparative examples where a treatment was conducted using
an
acidic solution (aqueous solution of sulfuric acid) having a pH-t>uffering
action but containing no
Fe ion and, although a frictional coefficient is low, an adaptability to
adhesives or a chemical
conversion treatment performance is poor.
(4) Nos. 9 to 14 and Nos. 18 to 20 are the examples of the invention where a
treatment was conducted using an acidic solution (an aqueous solution of
sulfuric acid) having a
gH-buffering action and containing Fe ion whereupon a frictional coefficient
is low and both an
adaptability to adhesives and a chemical conversion treatment performance are
excellent.
(5) Nos. 15 to 17 are the examples of the invention where an alkaline
treatment was
conducted in an activating bath before the treatment with an acidic solution
is performed under
the same conditions as in Nos. 12 to 14 and there was achieved the effect that
the frictional
coefficient is far lower as compared with the examples where the retention
time until washing
with water is same. In addition, as a result of the use of a neutralizing
bath, no rust spot was
generated and a steel sheet coil forming an oxide layer was pre served for a
long period before its
use whereby an ability of preventing the generation of rust is excellent.
(6) There is a tendency that the peeled amount of the plating in the draw-bead
test in
each test material is small when treated with a treating solution where Fe
concentration is not
more than S glliter and a powdering-resistant property is excellent as well.
21
CA 02470042 2004-06-04
.--~N m d-ew0 t~-o0
s<sCofoCtos4o-fst~'N cn~Yv7~ t~~oa
I
W W W WW W W W ~;,a?a~a~a~a;: a;?a~? a~
~
L~Ci~OrLL~~ ~ ~ GO.OrOr
~ ~~ ~ ~ ~~ ~ ~
c W c e c
C C C a
~ W W
U U U UU U U U WW W W WW W :xWW
7
o I
~ ~ ~
~ O O x xx x x x xx x x xx O O Ox x x
rn
I
U
~
O I
~~
W
E
~
L I
.67
'
~
~
ir O O O OO O O x OO O O OO O O OO O O
U
''rs
Q.
o
~,
o..",
~ O OO OO O O OO O O~OO O O
d O O x x x
d
-o
o
:oc3TSN
y O N ~ONh:et~ 00vDO cnoochaoN -!h;vWO
~
p"
L
N
W
P. .-i.-;.-.i.-;~ ,-.yj.-.i.-i,-i.-i.-it~jO ~ .-a,-.WC ooC
d
p
C~
t~
N
G
O
p O ~ t'aC -~N ~OOh-~hcn~Da ~t~ Wit'N N
~
U t!7V'1~ O.-na o0a ao000a 00k'WO ~Dv0a o0a
4:O N N N NN ...a,-,...,..,.-a.:..r,-,'.r'r.r..-,'.r'-.~.-a.r
~ U o 0 0 00 0 0 0 00 0 0 00 0 0 00 0 0
0
U
c
o
o .
o m.voNo a N vor.~ ~ --~Qa ~nr wc~nM M
U C t~oo~n~n~ncncnr~cncncncncnN N N Ncncnc~
o ....-.r.-a.-,.....-r,-,r,~.-..-r.-t.--i.-.~.r,-.~,-,.r..r.--~
w U .-. o d o ocao 0 0 0o cico00 0 0 ocio 0
w
tip
o
,.
~N
f
~zra ~ ~ y ~j ~ ~ ~ ~~ ~ ~ ~~ 0 0 0
~
0
y
x
~Qw i i i ri i i i ii i i ii o 0 oi i i
~
.~~.C~ OC O O 0 0 00 0 0 0 0
V
C O OC ~ O O ~O O ~ OO ~ O O~ O O
~ ~ ~
H~3'a3
..
...
N_
~.,
c'~.. o oO o 0 0 0o a o 00 o a o0 0 0
b
r-~ ~ ~ C'')MM Cf1~ CT1MC'7M f%Cn('ntf7M f%Cf1tr7M
LH M
v
~,
x
o M
O ~ o r r o0 00
o
~
U ,
O
~
p 1 O
~
G in b~
~ O 4
w
.- .~ W n o ~n
.~ U
~
~
a~ a
c
~?
~
",n
H o w
o a
~~
b
0
~
v ~ ~ C~
~ ~
a. o'
,
':~
~
U
d
r:
p O
-~ en ~
N ~t N
uW M
d-
v7
y0
t~
x
a
C
I
-r
r
-~
r
-r
~
-r
-
.
O
t~
oo
a
.
.
.
.
,
.
m.
,
.
-a
N
zz
CA 02470042 2004-06-04
EXAMPLE 2
[0073] The invention will now be illustrated in more detail by way of the
following Examples.
[0074] A galvannealed steel sheet was produced by a common method on a cold
rolled steel
sheet having a thickness of 0.8 mm and then a temper rolling was further
carried out. After that,
an oxide layer was formed using a treating device having a constitution as
shown in Fig. 1.
[0075] Firstly, after immersion in an acidic solution of pH 2.0 was carried
out at 50°C in an
acidic solution bath 2, a liquid film was formed on the surface of the steel
sheet using a
squeezing roll 3. When the pressure of the squeezing roll was changed at that
time, the coating
weight of the liquid film was adjusted. After that, hot water of 50°C
was sprayed on the steel
sheet in a washing bath 5, the plate was passed through the neutralizing bath
6, hot water of 50°C
was sprayed on the steel sheet in a washing bath 7 to wash and drying was
conducted in a drier 8
whereupon an oxide layer was formed on a coated surface.
[0076] With regard to a solution for all immersion treatment in the acidic
solution bath 2, a
solution where 30 g/1 of disodium hydrogen phosphate and 20 g,ll of citric
acid were mixed as pH
buffers and a predetermined amount of ferrous sulfate was added far the
purpose of adding Fe
ion was used and the pH was adjusted by addition of sulfuric acid. For the
sake of comparison, a
solution which was the same as above except that no pH buffer was used, but
adjustment was
conducted by ferrous sulfate only was used: In addition, to check the
influence of Fe+3 ion,
solutions to which ferric sulfate was added were partially used.
[0077] The retention time until the above washing with water is the dune until
the start of the
washing in a washing bath 5 when the liquid film amount was adjusted by a
squeezing roll 3 and
a.n adjustment was conducted by changing the line speed and, at the same time,
the steel sheet
was washed immediately after squeezing using a shower washing device 4 at the
outlet side of
the squeezing roll 3.
23
CA 02470042 2004-06-04
[0078] Also, an alkaline solution of pH 10 (an aqueous solution of sodium
hydroxide) was
sprayed during the above treatment in a neutralizing bath 6 so that an acidic
solution remaining
on the surface of the steel sheet was neutralized. Before immersing into an
acidic solution,
dipging into an aqueous solution of sodium hydroxide of pH 12 in an activating
bath 1 was
carried out to conduct an activating treatment.
[0079] After that, the coefficient of friction was measured as a means for
evaluating the press
formability for the steel sheet prepared as above, a peeling-adhesion test was
conducted for
evaluating adaptability of adhesion and evaluating chemical conversion
treatment erformance
and powdering-resistant property was carried out. In addition, after the steel
sheet was applied
with a rust-preventing oil, it was allowed to stand outside under such a
condition that it was not
affected by external factors such as dust and, after about six months,
generation of rust spots was
investigated. The case where no rust spot was evaluated as "~~" and the case
where rust spots
were noted was evaluated as "X".
[0080] The results of the test obtained by the above are shown in Table 2.
[0081] The following became apparent from the test result as shown in Table 2.
(1) Since No. l and No. 2 were not subjected to a. treatment by an acidic
solution,
they do not form an oxide layer which is sufficient for improving sliding
performance at the
flattened part and the frictional coefficient is high.
(2) Nos. 3 to 5 are comparative examples where a treatment was conducted using
an
acidic solution containing no pH buffer and, as compared with No. 1 and No. 2,
the frictional
coefficient is Iow but, as compared with the examples of the invention, it is
high and formation
of an oxide layer is insufficient.
(3) Nos. 6 to 8 are comparative examples where a treatment was conducted using
an
acidic solution (aqueous solution of sulfuric acid) having a pH-buffering
action, but containing
24
CA 02470042 2004-06-04
no Fe ion and, although the frictional coefficient is low, an adaptability to
adhesives or a
chemical conversion treatment performance is poor.
(4) Nos. 9 to 14 and Nos. 24 to 26 are the examples of the invention where a
treatment was conducted using an acidic solution (an aqueous solution of
sulfuric acid) having a
pH-buffering action and containing Fe ion whereupon the frictional coefficient
is low and both
the adaptability to adhesives and a chemical conversion treatment performance
are excellent.
(5) Nos. 15 to 17 are the examples of the invention where an alkaline
treatment was
conducted in an activating bath before the treatment with an acidic solution
is done under the
same condition as in Nos. 12 to 14 and there was achieved the effect that the
frictional
coefficient is far lower as compared with the examples where the retention
time until washing
with water is the same. In addition, as a result of the use of a neutralizing
bath, no rust spots
were generated and even when a steel sheet coil forming an oxide layer was
preserved for a long
period before its use, an ability of preventing the generation of rust is
excellent.
(6) Nos. 18 to 23 are the examples where Fe+3 ion concentration is changed by
addition of ferric sulfate. All of them show lowering of the frictional
coefficient and both
adaptability of adhesives and chemical conversion treatment performance are
excellent but, in
Nos. 18 to 20, Fe+3 ion concentration is within a range of the invention
whereby generation of
damage caused by pressing is not noted at all while, in comparative examples
of Nos. 21 to 23,
Fe+3 ion concentration is out of the range of the invention whereby damage
caused by pressing is
resulted.
(7) There is a tendency that the peeled amount of the plating in the draw-bead
test in
each test material is small when treated with a treating solution where the
total Fe concentration
is not mare than 5 g/liter and a powdering-resistant property is excellent as
well.
CA 02470042 2004-06-04
....NmepnvOt~as O,_,~
0~
KkKK Kk K~f" NM ~ ~~~ ~ ~K K
~ K
L0tr7GIW f9W W!~m aa~ e~aad da~dd Gz1 v d
GLo.m G o.Go,O,do.pp W c.Go.
~. G.
L1
6 8EEE 6E EEm ~m w mma msmm E ~ mm
E E E
0.~' UUUU UU UUm mn m C7WGCOW~WwU UU WK0.1C1
WkW
c~
en
m
c
"
o
G5 OOOO OO OOO OC O OOO OOOO x O CO
a C x x
n,
'~
o I
~fn OOxx xx xxx xxx x xOO Oxxxx xx x xx
d
C
y
N
E
O
~
U OOOO CO OxO OO O OOO OOOO O O OO
v O O O
~
y
m
..
>
~
u
~a
as o000 o o ooo o oloo ooo00 00 0 00
x xx
=a
sm
a
n
m
y f~N.a.-nOvN .-~00~OP7M N OO~0tnV1mv0 tT N NV'f
d N V1 tn
y~
G., ~ ~~pn ~~NNN M a0f~OC
7 F1 m
C
O
O ~O.O m~!~n y~ N v0.-mt~a0OvV7 N Om
V7n O ~a Q~ h~ ~pI~ V't ~ PO'
n ~ ~
~ ~...~. ~ ~.,., .~.... ~ .~...
O NNNN N r. .. .. ..,, .. . -,a
p ~ .~
U o0oc oc co0 0o o 000 0occ c o 00
c o c
U
~'
b
r
,
E O~-'~ON O~ OW~ I~ oo~oom OC~00m m O.~h
b r~~ M MN O~ m mm Ma0 O
m m
V .~..m~ ~....N ..~ n .. H ~ . N ~
C .w.r r .n rA . r. ~ r
O ~
w
U co0o co 0oc o0 0 000 0000 0 0 oci
0 0 0
0
W
~
~
3
~z~ mll ll m lm lo0 0lll yl I II
C
R
y
H
V
R
yam IIII II III II I Io0 oIII ly I II
I I
~3
'
' o o oo oo o o o o oo o oo
a o
, , ,, ,, , , , , , , ,,
Ey II.o o. ooR oo o oRo o.00 , . 00
~ ~. . W -~. . v'-. ~ p 0
3 ~ i 0
n. mm -m .m ~ m,. mn.-~m - ~n.-,m
n v m
w
o,o,o,o o,o, o,oo,0 0000 0 0 00
c 0 0
r-a IImM mm MMP7Mm m mmtnf~it~7m!~1 M M MTu
v~ M en m
x
~,
a
d
e~7 ~O V7 VO .r m
w O .r
H
A o e~ r~ ao m
o co co
x
a
o boo
m
fir. v;
U
a '
oo
o m ~n p
ao
cz. ~n o vy
U r,
,y
a ::
.
6
c
n ?"' 2~a
0 ~C
~
'
N o $
m ~
'
m
~
eo
p H
x C
C
p
b
r
p
,_,m A
a s
c~
~
U
:::
~'1O O -yNm ~W t~ O.O Nmet~Wo
,"JZ .., M ~Of'~ Oa ~ O oo ..N NNNN NN
N ~ oo .r .~
ut ~.r
26