Note: Descriptions are shown in the official language in which they were submitted.
CA 02471242 2004-06-16
Glaucoma Treatment Device and Method
BACKGROUND
The invention relates to devices and methods for treating glaucoma.
Glaucoma is the leading cause of irreversible blindness in the world. It is
s estimated that 70 million people worldwide have glaucoma, arid that nearly 7
million are
bilaterally blind from this disease. In the United States, 2.5 - 3 million
people suffer
from glaucoma, and it is the third most common reason for adults to visit a
medical
doctor. Elevated intraocular pressure is the outstanding risk factor for the
development of
glaucoma, arid the main reason for progression of the disease. Accordingly,
treatment of
o glaucoma has been focused on lowering the intraocular pressure in the
affected eye.
Glaucoma treatment has customarily comprised a three-step process. First,
medicines are tried, such as beta-adrenergic antagonists and alpha-adrenergic
agonists.
These have proven only moderately, and inconsistently, effective, and can lead
to many,
sometimes life threatening, side effects, such as respiratory and cardiac side-
effects. If
~5 medical treatment is either not effective or not tolerated, argon laser
trabeculoplasty
(ALT) is usually the next step. ALT success is often limited, and is
ultimately temporary.
The final therapeutic step involves surgery. Trabeculectomy is by far the most
common
type of surgery done for treatment of glaucoma. It was first described by
Cairns in 1969,
slightly modified by Watson 1969-71, and has changed little during the last
three decades.
2o In a trabeculectomy, a hole is made in the eye near the limbus and into the
anterior
chamber, under an overlying scleral flap. The aqueous humor thereby is allowed
to drain
into the subconjunctival space. Subsequent scarnng circumscribes this area of
subconjunctival drainage into a bleb. Sometimes, the scarnng progresses to
completely
scar down the bleb, stopping the flow of aqueous humor, and causing the
surgery to fail.
25 Mitomycin C, an anti-fibroblastic drug, has been used to combat scarring
attendant to
trabeculectomy. While increasing surgical success, however, the use of this
drug has
significantly added to the risks and complications of filtering surgery;
mitomycin C
causes thinning of the conjunctiva and can lead to leaking through the thinned
conjunctiva, and such leaking often leads to hypotony and intraocular
infection.
3o Glaucoma drainage devices (GDD) are an attempt to control the scarring
which so
commonly tends to seal conduits made in tissue. Molteno, in 1969, described
the first of
CA 02471242 2004-06-16
the currently used type of GDD. They consist of a tube and a plate made of
synthetic
biomaterials. The tube is inserted into the anterior chamber and conducts the
aqueous
humor to the plate, which is in the subconjunctival space. The problem
remains,
however, of scarring of the bleb which forms around the plate. About 80 % of
GDDs
appear to be successful for one year, with a 10 % additional failure rate each
year
thereafter. There are significant complications associated with these devices,
both in the
perioperative and postoperative periods, including hypotony, flat anterior
chamber,
suprachoroidal hemorrhage, retinal detachment, a hypertensive phase,
endophthalmitis,
diplopia, corneal decompensation, conjunctiva) melting, and others. One or
more
~o complications have been found to occur in 60-70 % of cases.
SUMMARY
In one aspect, the invention features a device for treating glaucoma in an
afflicted
eye. The device includes a body defining a lumen, the body having first and
second ends,
and external and lumenal surfaces. The body has a length sufficient to provide
fluid
~5 communication (i.e. the flow of aqueous humor) between the anterior chamber
and tear
film of the eye when the device is implanted in the sclera. The device further
includes a
filter membrane capable of providing outflow resistance to aqueous humor
flowing
through the lumen. In addition to the filter for providing outflow resistance,
the device
also includes at least one debris filter positioned at the first end or
between the first end
2o and the filter membrane.
In preferred embodiments, the second end of the device body is adapted to lie,
and
remain, substantially flush with the sclera) surface when the device is
implanted in the
sclera. The device is preferably flared at the second end to aid in providing
an endpoint
for the depth of insertion of the device into the sclera.
25 The body of the device is preferably fabricated from a material selected
from the
group consisting of silicone, acrylic, polyimide, polypropylene, polymethyl
methacrylate,
and polytetrafluoroethylene.
In preferred embodiments, at least a portion of the external surface of the
body is
coated with a porous cellular ingrowth coating. Preferably, the porous
cellular ingrowth
3o coating is coated on the portion of the device that is in contact with the
sclera when the
CA 02471242 2004-06-16
device is implanted. The remaining surfaces of the device -- the entire
lumenal surface,
the portion of the external surface not in contact with the sclera, and the
filter surfaces of
the device -- are preferably coated with a bio-inert surface coating.
The filter is preferably a microporous/nanoporous filter membrane. In
preferred
embodiments, a filter membrane may be fused to the periphery of the body at
the lumenal
opening at the second end of the body to form a one-piece device for
implantation in the
sclera. The filter has an inflow face, an outflow face and a peripheral edge.
Preferably
the peripheral edge of the filter is contiguous with the body of the device,
and more
preferably is contiguous with the device body at the lumenal opening in the
second end of
t o the body. Alternatively, in other embodiments the device may be a unitary
article in
which a microporous filter membrane is integral with the material used to form
the body
of the device.
In preferred embodiments of the device, the microporous filter membrane may
comprise a silicone) or silicon(e)-based microporous filter membrane, a
microporous
~ 5 polymer network, a fiber network, or microcapsular material.
The debris filter preferably includes an inflow face, and outflow face, and a
peripheral edge contiguous with the body. Preferably, the inflow and outflow
faces of the
debris filter include a bio-inert surface coating. The debris filter
preferably includes a
filter membrane having pores with a diameter between 0.5 and 2 microns. The
filter
2o membrane is preferably a silicone) or silicon(e)-based porous filter
membrane. The
peripheral edge may preferably be bonded to the body at the first end. In
another
embodiment, the device includes a second debris filter positioned at or near
the second
end of the body, external to the microporous filter membrane that provides
desired
outflow resistance to aqueous humor.
z5 Preferably, micropores in the microporous filter membrane have a diameter
less
than or equal to about 0.2 microns.
The devices of the present invention have a length sufficient to provide fluid
communication between the anterior chamber and tear film of the eye when the
device is
implanted in the sclera. Preferably, the devices have a length of at least
about 2.5 mm.
CA 02471242 2004-06-16
The diameter of the lumen of the device is preferably about 0.5 mm or less.
The
diameter of the lumen may increase at the second end of the device in those
embodiments
where the second end of the device is flared.
In preferred embodiments of the devices of the present invention, at least a
portion
of the external surface of the body, preferably corresponding to the portion
of the surface
extending into the anterior chamber, all of the surface of the lumen, and the
filter surfaces
includes a bio-inert surface coating. The bio-inert surface coating may, for
example,
include phosphoryl choline, polyethylene glycol, or polyethylene oxide.
The device preferably may also include at least one barb, preferably extending
~o from the external surface of the device body in the portion of the body in
contact with the
sclera when the device is implanted. The barb or barbs are adapted to engage
with the
sclera to provide stability when the device is implanted.
The device is preferably beveled at its first end to aid in the implantation
process.
In another embodiment, the device includes, at its second end, a lip extending
around at least a portion of the periphery of the second end, the lip having
an external lip
surface continuous with the external surface of the body. A portion of the
external
surface of the lip is adapted to contact the external scleral surface of the
eye when the
device is implanted in the sclera. Preferably, the lip includes a porous
cellular ingrowth
coating on at least a portion of its external surface, and the lip preferably
extends around
2o at least half the circumference of the second end of the device.
In still another embodiment, the second end of the device body includes a head
portion having a cavity in communication with the lumen of the body, wherein
the head
portion has opposing inner and outer faces such that the inner face is in
contact with the
surface of the eye when the device is implanted, and the outer face includes
the filter.
2s The inner surface of the head portion may include a porous cellular
ingrowth coating.
Preferably, the filter is contiguous, at its peripheral edge, with the
peripheral edge of the
outer opposing face of the head portion that defines an opening in the cavity.
In another aspect, the invention features a one piece device for treating
glaucoma
in an afflicted eye. The device includes a body defining a lumen and having
first and
3o second ends. The body is of sufficient length to provide fluid
communication between
the anterior chamber and the tear film of the eye when the device is implanted
in the
CA 02471242 2004-06-16
sclera. The device further includes a filter membrane portion having inflow
and outflow
faces, and a peripheral edge. The filter membrane is capable of providing a
desired
outflow resistance (preferably to achieve a low normal intraocular pressure)
to aqueous
humor flowing through the lumen. The device further includes a debris filter
positioned
at the first end or between the first end and the filter membrane portion. At
least a portion
of the peripheral edge of the filter is contiguous with the body.
In another aspect, the invention features use of a device as disclosed herein
for
treatment of glaucoma.
In still another aspect, the invention features a method for treating glaucoma
in an
afflicted eye. The method involves the steps of providing a device as
disclosed herein,
and implanting the device in the sclera of the eye such that aqueous humor
flows from the
anterior chamber to the tear film of the eye. In all embodiments of the
method, the
method involves the step of making an incision into the anterior chamber of
the eye prior
to implantation of the device. The method may further involve suturing the
second end of
~5 the device to the sclera following implantation.
The details of one or more embodiments of the invention are set forth in the
accompanying drawings and the description below. Other features, objects, and
advantages of the invention will be apparent from the description and
drawings, and from
the claims.
DESCRIPTION OF DRAWINGS
Figure 1 A is a mid-horizontal cross-sectional view of an eye with one
embodiment of a device illustrative of the present invention implanted and
shown in
longitudinal cross section.
Figure 1 B is an external view of an eye showing the external, intrascleral,
and
intra-anterior chamber portions of the device shown in Fig. lA implanted in an
eye.
Figure 2A is a mid-horizontal cross-sectional view of an eye with another
embodiment of a device illustrative of the present invention implanted and
shown in
longitudinal cross section.
CA 02471242 2004-06-16
Figure ZB is an external view of an eye showing the external, intrascleral,
and
infra-anterior chamber portions of the device shown in Fig. 2A implanted in an
eye.
Figure 3A is a mid-horizontal cross-sectional view of an eye with another
embodiment of a device illustrative of the present invention implanted and
shown in
longitudinal cross section.
Figure 3B is an external view of an eye showing the external, intrascleral,
and
infra-anterior chamber portions of the device shown in Fig. 3A implanted in an
eye.
Like reference symbols in the various drawings indicate like elements.
DETAILED DESCRIPTION
The invention relates to novel devices and methods for treating glaucoma. In
particular, the invention relates to devices wherein a generally tubular body
is provided
which is of sufficient length to allow aqueous humor to flow from the anterior
chamber of
an afflicted eye through a lumen of the tubular body and into the tear film
when the
device is implanted in the sclera. A $lter capable of providing outflow
resistance to
~5 aqueous humor flowing through the lumen is provided in the device. The
device may be
implanted in the sclera of an afflicted eye to treat glaucoma.
The devices of the invention provide numerous advantages. The devices drain
aqueous humor into the tear film, rather than into the subconjuctival space.
No
conjunctiva) bleb is formed, and therefore there is no potential to scar. In
preferred
2o embodiments, a filter portion is fused or bonded to the body to form a one-
piece device
having a simple design and which is easy and safe to insert into an afflicted
eye. The
filter is readily accessible for vacuum or chemical cleaning. Aqueous humor is
expelled
into the tear film, enhancing moisture and lubrication in the eye. Also, in
preferred
embodiments, the filter is comprised of a microporous membrane material. The
25 microporous membrane comprises pores sized to block all bacteria, and pore
number and
length may be calculated to provide aqueous humor outflow that yields
desirable
intraocular pressure. The materials used to make the device may be selected to
provide
bulk biocompatibility by both seeking to match sclera) rigidity, and by
providing the
portion of the device that is in contact with eye tissue with a porous
cellular ingrowth
so surface to promote biointegration. Both the sclera) rigidity compatibility
and the
CA 02471242 2004-06-16
biointegration contribute to the elimination of micromotion of the device. The
biointegration will also eliminate potential dead space around the device,
thus removing
the risk of a tunnel infection into the eye. The surfaces of the device may
also be coated
with other materials, such as polymer coatings or biologically active
molecules, to
promote surface biocompatibility and/or immobilization of the implanted
device.
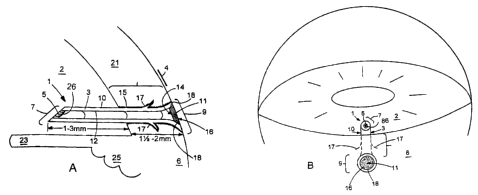
A device illustrative of one embodiment of the present invention is shown in
Figures 1 A and 1 B. As shown in longitudinal cross-section in Fig. 1 A as
implanted in an
eye, the device 1 includes a body 3 defining a lumen 5 and having a first end
7 and a
second end 9. The body has an external surface 10, and a lumenal surface 12. A
filter 11
o is provided at the second end 9 of the device. The filter 11 has an inflow
face 14, and
outflow face 16, and a peripheral edge 18. The device has a length sufficient
to provide
fluid communication between the anterior chamber and tear film of an eye when
the
device is implanted in the sclera. The filter 11 is capable of providing
outflow resistance
to aqueous humor flowing through the lumen S. The device 1 is implanted in the
sclera 6
~ 5 of the eye. Also shown in Figure 1 A are the cornea 21, the iris 23, and
the ciliary body
25.
The body 3 of the device is preferably formed of a material selected from the
group consisting of silicone, acrylic, polyimide, polypropylene, polymethyl
methacrylate,
and expanded polytetrafluoroethylene (preferably denucleated and coated with
laminin).
20 'These materials are well known in the art and methods of fabricating
tubular structures
from such materials also are well-known. The material from which the device is
fabricated is selected to provide bulk biocompatibility, as described above.
The bulk
properties of the material may be selected to impart rigidity as close as
possible to that of
the surrounding tissue, e.g. sclera.
25 In accordance with the invention, the device is of sufficient length to
provide fluid
communication between the anterior chamber 2 and tear film 4 when the device
is
implanted in the sclera 6 of an afflicted eye. In general, to provide fluid
communication
between the anterior chamber and tear film, the devices of this invention must
have a
minimum length of about 2 mm. In preferred embodiments, the device has a
length of at
30 least about 2.5 mm. In general, the device may have a length of between
about 2.5 mm
and about 5 mm. The preferred length of at least about 2.5 mm will reduce the
possibility
CA 02471242 2004-06-16
of blockage of the lumenal opening in the anterior chamber by the iris. The
length of the
device within the scleral tract would preferably be greater than the scleral
thickness
because insertion would not be perpendicular to the sclera, but more
tangential to be
parallel to the iris.
As shown in Figure 1, the body 3 of the device defines a generally tubular
lumen
5. In preferred embodiments, the lumen has a diameter less than or equal to
about 0.5
mm. On its external surface 10, the body 3 may preferably include a porous
cellular
ingrowth coating 15 on at least a portion thereof. Preferably, and as shown in
Figure lA,
the portion of the external surface coated with the cellular ingrowth coating
15
o corresponds substantially to the portion of the body in contact with eye
tissue (i.e. sclera)
following scleral implantation. Such porous cellular ingrowth coatings have
been
described with respect to other ophthalmic implants, and have been made of
silicone with
a reported thickness of 0.04 mm. Selected growth factors may be adsorbed on to
this
coating to enhance cellular ingrowth.
~5 The remaining surfaces of the device -- i.e. the entire lumenal surface 12,
the
portion of the external surface 10 not in contact with the sclera, and the
inflow (14) and
outflow (16) faces of the filter -- may further include coatings to enhance
surface
biocompatibility. Such coatings may include bio-inert polymer coatings such as
phosphoryl choline (PC), polyethylene glycol (PEG), and polyethylene oxide
(PEO), and
2o such bio-inert surface coatings may be further modified with biologically
active
molecules such as heparin, spermine, surfactants, proteases or other enzymes,
or other
biocompatible chemicals amendable to surface immobilization. Both PC and PEO
polymer coatings downregulate deleterious biological reactions, primarily by
attracting a
large and stable hydration shell when grafted onto a surface. PEO also is
amendable to
z5 end-group coupling for surface immobilization of biologically active
molecules, which
might include heparin, spermine, surfactants, proteases (eg., papain) or other
enzymes or
chemicals. The addition of such bioactive molecules could advantageously
impart
specific desired functionality, for example, allowing a further increase in
the
hydrophilicity of the surface. Hydrophobic surfaced microporous filters are
known to be
3o much more prone to protein plugging than are microporous filters with
hydrophilic
surfaces.
CA 02471242 2004-06-16
In the portion of the external surface of the body 3 that is in contact with
eye
tissue following implantation, the body may include a barb or barbs 17
designed to
engage with tissue upon implantation and provide stability to the implanted
device. The
barb or barbs 17 may be formed as part of the device body during manufacture
or may be
fused or bonded to the device body by suitable means known in the art. The
device may
also be beveled at its first end 7 to aid in the implantation process.
The devices of the invention include a filter capable of providing outflow
resistance to aqueous humor flowing through the lumen of the device from the
anterior
chamber into the tear film. The filters employed in the devices of this
invention
o preferably are microporous/nanoporous filter membranes.
In Figure 1, a microporous filter membrane 11 is shown at the second end 9 of
the
body 3. The microporous filter membrane 11 includes inflow face 14, outflow
face 16,
and is circumscribed by peripheral edge 18. The size of the pores in the
filter-membrane
11 at the exterior surface of the device preferably are approximately 0.2 p,
or smaller.
~5 This is sufficiently small enough to prevent ingress of all known bacteria.
It is also about
the same pore size as has been shown to be present in the capsule formed
around Molteno
implant plates, and through which aqueous humor flows by simple, passive
diffusion.
That capsule is known to act as an "open sieve" for passage of latex
microspheres of 0.2p
and smaller. The filter-membrane of this device would be expected to act as
such an
20 "open sieve," but with a predetermined resistance to outflow to result in a
low to normal
intraocular pressure. The design parameters of microporous membranes suitable
for use
in the present invention may be summarized as follows.
Porous media theory allows the calculation of the resistance of a fluid
through a
porous structure by using the formula: resistance = 8 x fluid viscosity x
length of pore /
z5 number of pores x ~ x pore radius to the fourth power. The viscosity of
aqueous humor is
essentially the same as saline, and the viscosity is stable. The pore radius
could vary only
over a range that would still permit it to act as a barner to bacteria. The
length of the
pores, however, may be varied, and is determined by the thickness of the
filter-
membrane. The number of pores can also be varied to arnve at a desired
resistance.
3o Even though the eye's natural outflow is compromised in glaucoma, it is
rarely zero, and
would in most cases allow for a certain tolerance in the system even after the
present
CA 02471242 2004-06-16
device is in place. In fact, the main natural outflow of the eye, the
conventional or
trabecular meshwork pathway, is intraocular pressure dependent. The trabecular
meshwork pathway serves as a one-way valve, so when the intraocular pressure
is very
low, the trabecular meshwork is compressed with very little outflow, or
backflow,
s allowed through it. When the intraocular pressure increases, to a certain
level, the
outflow can increase also.
In preferred embodiments of the invention, it is desirable to achieve a normal
aqueous humor outflow resistance of about 3.2 mmHg x min / ~,1. In preferred
embodiments, it is desirable to achieve an outflow resistance that produces a
low normal
~o intraocular pressure. For example, if a filter membrane with a diameter of
1.0 mm is
used, that would result in a filter membrane area of 785,000 square ~. If a
pore density of
40% of the filter membrane surface area is used, there would be ten 0.2 ~
pores / square
~. Thus, there would be a total of 7,850,000 pores of 0.2 ~ size. Using a
filter membrane
thickness of 100 ~, the porous membrane theory equation for resistance would
be:
~5 R = 8 x viscosity x pore length / pore number x ~ x pore radius to the
fourth
power
= 8 x 1 x 100 / 7,850,000 x 3.14 x .00001
= 800 / 247
= 3.2, the mean value for outflow resistance of normal, non-glaucomatous, eye.
2o Because episcleral venous pressure would not be a factor in the function of
this
device, as it is in the determination of normal intraocular pressure [eg.,
P(ocular) _
F(inflow) / C(facility of outflow) + P(evp)], the IOP with this device might
be expected to
be below normal. Alternatively, the outflow through the device, rather than
the outflow
resistance, could be adjusted to give the desired intraocular pressure.
25 Microporous filter membranes that have been used with ophthalmic devices or
research include Nuclepore polycarbonate filter membranes, millipore filters,
and
microperforated silicone membranes. However, filter-membrane nanotechnology,
and
specifically microelectromechanical systems (MEMS)-based technology, may be
useful
to fabricate microporous membranes, in accordance with the invention, to be
optimally
3o biocompatible, non-degradable, and immunoisolating. Examples of such
technologies
that are known and characterized in the art include:
to
CA 02471242 2004-06-16
1) Microfabricated silicone) or silicon(e)-based biocapsules, an example of
which would be polycrystalline silicon filter-membranes micrornachined
to present a high density of uniform pores, as small as 0.02.
2) Microporous polymer networks, an example of which would be a
polyurethane network formed by cross-linking a mixture of Iinoleic acid
and a linear poly (etherurethane) with dicumyl peroxide. Microporosity is
introduced by adding salt crystals before cross-linking and leaching it out
afterwards. Pore size in this instance is 0.3-0.7~., with a membrane
thickness of 8~. But, both pore size and membrane thickness can be
varied.
3) Fiber networks with a porous structure, an example of which would be an
acrylonitrile membrane (AN 69).
4) Microcapsules based on the use of oligomers which participate in
polyelectrolyte complexion reactions.
The application of these technologies to medicine has heretofore been most
prominently related to pancreas cell transplantation.
In Figure 1, the microporous filter membrane 11 is attached at its periphery
18 to
the body 3 at the second end 9 of the body. The lumenal opening at the second
end is
2o thus closed by the microporous filter membrane. As shown in Figure lA, and
in
preferred embodiments of the invention, the filter 11 is bonded, fused or
otherwise
attached to the body at the second end of the device, most preferably at the
edge of the
second end defining the lumenal opening, such that the filter is substantially
flush with
the second end of the body. Although preferred, such placement of the filter
is not
required, the filter could be placed elsewhere, for example in a slightly
recessed or
protruding position, or at any position along the lumen of the body. In an
alternative
embodiment of the device of this invention, the filter may be formed of the
material used
to fabricate the device body and be integral with it. In this alternative
embodiment,
manufacture of the device could occur as a one-step fabrication process to
fabricate the
3o tubular body which would be closed at one end (corresponding to the second
end of the
ultimate device) with body material of a desired thickness. A microporous
filter
n
CA 02471242 2004-06-16
membrane can then be fabricated at the closed end by creating a desired number
of pores
of appropriate diameter, by perforation or other suitable means. This device
could then
be implanted in the sclera in accordance with the present invention.
As shown in Figure lA, the fixation of the filter membrane, by fusion,
bonding, or
other means of attachment, results in a one-piece device that may be implanted
as such in
the sclera of an afflicted eye. 'The shape of the filter membrane may
preferably be either
round or oval.
As also shown in Figure 1, the body 3 of the device flares at the second end
9, and
the filter and second end 9 of the device are situated substantially flush
with the external
o scleral surface 21. The flaring of the body at its second end 9 aids in the
flush mounting
of the device in the eye by providing an endpoint of insertion as the device
is pushed into
the sclera during surgery. The device 1 is also beveled at its first end 7 to
assist in
implantation. In this embodiment, the diameter of the filter membrane thus
exceeds the
diameter of the lumen in the portion of the body that is not flared. The
degree to which
~5 the body flares and the resultant diameter of the microporous filter
membrane may be
adjusted to optimize the functional properties of the filter membrane. With
the second
end of the device, including the filter, in communication with the tear film,
the filter is
readily accessible for cleaning, using methods involving vacuum, chemical,
micro
backflushing, magnetic pulsing, or ultrasonic disruptive processes.
2o Figure 1 B depicts a device, as shown in Figure 1 A, implanted in an eye
with like
numbers signifying like features. The view shown in an external view of an eye
showing
the external, intrascleral, and infra-anterior chamber portions of the device
shown in
Figure lA implanted in the eye. A frontal view of the second end 9 and filter
11 (with
outflow face 16 and peripheral edge 18 visible) is shown, and the device
extends through
25 the sclera 6 and into the anterior chamber 2. The flaring of the second end
9 of the device
within the sclera is shown, and the second end is substantially flush with the
scleral
surface.
Figures 2A and 2B show another embodiment of the device of the present
invention, with like numbers signifying like features. The views of the device
3o embodiment shown in Figures 2A and 2B are similar to those shown in Figures
lA and
IB. The features of the devices shown in Figures lA/1B and 2A/2B are similar
in all
12
CA 02471242 2004-06-16
respects except where noted. A device 41 is shown, having a body 43, a lumen
45, a first
end 47, and a second end 49. Also shown are filter S 1, porous cellular
ingrowth coating
55, stabilization barbs 57, and a bevel at the first end 47. As with other
embodiments, the
device 41 is of sufficient length to allow fluid communication between the
anterior
chamber 42 and tear film 44 of an eye through the lumen 45 when implanted in
the sclera
46.
In the embodiment shown in Figures 2A and 2B, the device comprises a head
portion 61 which is not substantially flush with, but rather extends
externally to the
scleral surface. The body 43 of the device is adapted to form a lip 63 at the
second end
0 49 of the device. The lip 63 extends around at least a portion of the filter
51 of the device
(shown as extending for roughly'/ of the circumference of the head portion
61). The lip
63 has an external lip surface 65 that is continuous with the external surface
50 of the
body. The lip 63 serves to stabilize the device against the scleral surface,
and the external
lip surface 65 may be provided with porous cellular ingrowth coating 55 (as
shown in
~5 Figure 2A) to further stabilize the device in the eye. The lip 63 further
provides an
endpoint of insertion when the device is implanted.
Figures 3A and 3B depict still another embodiment illustrative of the device
of the
invention, with like numbers signifying like features. The view of the device
embodiment shown in Figures 3A and 3B are similar to those shown in Figures lA
and
20 1 B. The features of the devices shown in Figures 1 A/1 B and 3A/3B are
similar in all
respects except where noted. A device 71 is shown, having a body 73, a lumen
75, a first
end 77, and a second end 79. Also shown are filter 81, porous cellular
ingrowth coating
85, stabilization barbs 87, and a bevel at the first end 77. The device is of
sufficient
length to allow fluid communication between the anterior chamber 72 and the
tear film 74
25 when the device is implanted in the sclera 76.
In the embodiment shown in Figures 3A and 3B, the device comprises, at its
second end 79, a disc-shaped head portion which is not flush with, but rather
extends
externally to the scleral surface. The body 73 of the device is adapted to
form the disc
portion, which includes a cavity 94 (Fig. 3A), which is in communication with
the lumen
30 75. The disc-shaped head portion has opposing inner and outer faces 93 and
95,
respectively. The inner face 93 (continuous with the external surface 80 of
the body) is in
13
CA 02471242 2004-06-16
contact with the external surface of the sclera 76, and the outer face 95 as
shown in Figure
3A includes the filter 8I. The inner face 93 may be coated with porous
cellular ingrowth
coating 85. In preferred embodiments, a peripheral edge 98 of the filter 81 is
contiguous
with the periphery of the body 73 at the opening to the cavity 94, such that
the filter 81
forms part of the outer face 95 of the disc-shaped head portion.
Another embodiment of the Glaucoma Treatment Device includes an additional
debris filter, or debris filters, within the lumen of the body, to keep debris
from the filter
membrane that is fabricated to provide the desired outflow resistance.
Preferably, a debris
filter is positioned at or near the first end 7 of the body of the device,
within the anterior
o chamber of the eye. The debris filter contains larger pores than the
resistance-providing
microporous filter membrane, for example in the range of 1 ~. in diameter.
While any
porous filter will necessarily provide some resistance to flow through it, the
debris filters)
is fabricated to provide the least possible resistance. The primary function
of the debris
filter is to keep debris from reaching the microporous filter membrane, which
is the outflow
t5 resistance determining element. Porous media flow theory teaches that
resistance is
inversely proportional to the pore radius to the fourth power, so a much
larger pored filter
would provide little resistance to aqueous humor outflow. Number and length of
pores can
also be varied to eliminate most resistance.
While the microporous filter membrane of the device that provides outflow
2o resistance would have modifications, especially related to its surface
chemistry, to prevent
adherence of proteins or cells, limiting its exposure to potentially plugging
debris may also
be important. An additional debris filter placed at or near the first end of
the device body
can block most blood and pigment cells and cell fragments that might be
included in the
aqueous humor outflow. The surface of the debris filter preferably is
accessible for laser
25 photodisruption of accumulated debris, as is used to eliminate debris that
occasionally
collects on the surface of intraocular lens. Because this additional filter
would preferably
be covering the inner, beveled, end of the lumen, its surface area would be
increased, and it
would be facing anteriorly. The larger surface area allows for some plugging
before any
significant resistance develops to outflow; and an anterior orientation would
make laser
3o access easier.
14
CA 02471242 2004-06-16
In addition to placing such a filter at the inner end of the body of the
device, a
similar debris-collecting filter can be positioned at or near the second end 9
of the body,
with the resistance-providing filter membrane internal to it at some position
within the
lumen.
Referring to the figures, a debris filter is shown as 26 in Figs. 1 a and lb,
as 66 in
Figs. 2a and 2b, and 99 in Figs. 3a and 3b.
'The additional, larger pored debris filter(s), designed to keep debris from
the filter
membrane, can be fabricated using various micromachining techniques, including
microelectromechanical systems (MEMS) - based technology, as with the filter
membrane.
~ o Alternatively, soft lithography or focused ion beam (FIB) technologies may
be employed.
Laser perforations could also be used to create the pores. Potential materials
for fabrication
of the debris filter include silicon or silicone, polytetrafluoroethylene,
polypropylene,
polymethyl methacrylate, acrylic, polyurethane and polyimide.
As with the filter membrane, the debris filters) is preferably bonded to the
body
~5 within the lumen. The bond needs to provide a robust, permanent, and
totally hermetic
seal. Examples of suitable bonding methodologies are fusion, wafer, covalent,
or anodic
bonding; or the use of various biocompatible adhesives, including silicone
elastomer,
epoxy, cyanoacrylate, or polyurethane.
As with the rest of the device exposed to aqueous humor, the debris filters)
2o preferably has surface modifications to make it as bioinert as possible.
Surface coating
using self assembled monolayers of biomolecules may be used; examples include
phosphoryl choline, polyethylene oxide, or polyethylene glycol. These can
provide a very
hydrophilic surface, thereby decreasing/eliminating protein and cellular
adhesion.
The method for installing this device is simple and consumes little time.
25 Sometime before installation, topical antibiotic and non-steroidal anti-
inflammatory drops
(NSAID) should be applied to the operative eye. These will be continued for
one week
postoperatively four times a day. The NSAID helps stabilize the blood-aqueous
barrier.
All embodiments of the device illustrated herein may be inserted under topical
anesthesia, possibly supplemented subconjunctivally. In general, the devices
of the
30 invention may be inserted into the sclera using routine operative
procedures. The location
of insertion for all embodiments is in the sclera at about the posterior
surgical limbus.
CA 02471242 2004-06-16
The device could be inserted at any site around the limbus, but would
preferably be
inserted at the far temporal limbus.
The insertion procedure is begun by excising a small amount of conjunctiva at
the
site of the anticipated insertion, exposing the underlying sclera. Any
bleeding is then
cauterized. For embodiments of the device as shown in Figure 2 and Figure 3, a
superficial layer of sclera may be excised beneath the anticipated position of
the exterior
portion of the device. This will allow these embodiments to be more flush with
the
surrounding external scleral surface, as occurs easily with the embodiment of
Figure 1.
Then, approximately 1-2 mm posterior to the limbus, at the site of the now
exposed sclera, a diamond blade is used to make a stab incision into the
anterior chamber,
while held roughly parallel to the iris. This blade is of a size predetermined
to make an
opening into the anterior chamber sized appropriately for the introduction of
the device.
This stab incision is made gently, but relatively quickly, assiduously
avoiding any and all
intraocular structures. Such an uneventful paracentesis has been found not to
disrupt the
blood-aqueous barrier in most cases. In any event, any disruption of this
barrier is usually
of less than 24 hours duration without continued insult. In the embodiment of
the device
shown in Figure 1, the paracentesis could be customized to the flared external
shape of
the device by using a diamond blade, or trochar, sized to the device, and
fitted with a
depth guard. This would insure accurate and predictable depth of insertion so
the exterior
2o surface of the device would lie flush with the external scleral surface.
The device is next picked up and held with a non-toothed forceps. The lips of
the
stab incision wound may be gaped with a fine, toothed forceps. The pointed tip
of the
tube element would then be gently pushed through the scleral tract of the stab
incision
and into the anterior chamber, with the tube lying above and parallel to the
iris, with the
bevel up [ie., anteriorly]. Alternately, a dedicated instrument could be used
to facilitate
placement of the device. This instrument would consist of a hollow tube within
which the
device could be placed, and guided into the paracentesis wound. The instrument
would
have a mechanism then to extrude the device into its proper position. The
flare in the
embodiment of Figure 1, the external lip in the embodiment of Figure 2, and
the disc
so portion in the embodiment of Figure 3, provide for a definite endpoint to
the depth of
insertion. The embodiments of the device having a beveled first end, the bevel
is oriented
16
CA 02471242 2004-06-16
anteriorly so as to minimize the potential for blockage of the lumenal opening
by the iris.
The scleral barbs) then stabilizes the device until the biointegration with
the sclera is
complete. This biointegration is a function of its porous cellular ingrowth
surface, likely
enhanced by adsorbed growth factors. In the embodiment of Figure 3, a 10-0
nylon
suture on a broad spatula needle may be used to suture the disc portion into
the sclera,
providing additional stability to the device until the biointegration is
complete. This
suture may then be easily removed. In the embodiments of Figures 1 and 2, a
suture
could also be used to add additional temporary stability.
After insertion of the device, an ocular shield should be placed over the eye.
o Other embodiments of the invention are within the scope of the appended
claims.
m