Note: Descriptions are shown in the official language in which they were submitted.
CA 02471583 2004-07-05
WO 03/055381 PCT/CA03/00006
Sample Identification, Chemical Composition Analysis and
Testing of Physical State of the Sample Using Spectra
Obtained At Differ ent Sample Temperatures
The present invention relates to a method of identifying components of a
sample at different temperatures. I\~Iore specifically, this invention
provides a method
of sample identification, chemical composition analysis, and testing the
physical state
of a sample by analyzing spectra obtained from a sample subjected to different
temperatures.
BACKGROUND OF THE IN~~'ENTION
Material objects produce or absorb electromagnetic radiation in certain parts
of the spectrum of electromagnetic radiation. Spectral distribution of the
produced
radiation by an object, or effect of the object on the spectrum of the
radiation
followin~~ interaction with the object, depends on the chemical composition of
the
object, its physical state (for example, but not limited to gas, liquid,
solid), and
existing physical conditions acting on the object (for example but not limited
to,
presstwe, temperature). Under certain conditions spectral characteristics of
the
radiation produced by an object, or changes in the spectrum of radiation
interacting
with the object can be used for identifying chemical elements and compounds in
the
object, or for identifying physical conditions acting on the object. While
spectral
specificity of the. radiation produced by an object, or spectral specificity
of change
introduced to radiation interacting with an object, may be used for
identification or the
analysis of the chenucal composition of an object, the specificity of spectral
changes
under different conditions, temperature in particular, is not recognized and,
as a result,
is not used in practice.
Currently, emission, absorbance and Raman spectroscopy are used to extract
information from a sample using spectral measurements of electromagnetic
radiation
of the sample. Each of these methods, under certain condition can be used for
identification of chemical elements a.nd compounds of a sample, or for the
determination of a relative or absolute, concentration of the elements and
compounds
in the sample.
CA 02471583 2004-07-05
WO 03/055381 PCT/CA03/00006
It is well known that a chemical element generates or absorbs electromagnetic
radiation in a characteristic set of narrow spectral bands distributed in a
specific
fasluon .within the electromagnetic radiation spectrum, including its visible
part, when
the element is in a form of free atoms and heated to sufficiently high
temperature. The
pattern of these. bands can be used for identifying chemical elements present
in the
sample. After suitable calibration, information on relative intensity of
radiation or
absorption levels at these bands can be used for measuring the relative
concentration
of one or more elements in the sample under investigation. Furthermore, after
additional calibration, when volume of sample is normalized, the absolute
concentration of the element in the sample may be determined.
Substances containing molecular compounds also produce specific images of
emission and absorption bands due to transition bet<veen different vibration
modes of
molecules. Since the amount of energy needed to change a vibration mode of
molecule is much lower than that needed to change electron energy level within
the
atom, emission and absorption bands of molecules are usually placed in middle
and
far infrared bands of the spectrum. Detection of such bands is difficult and
emission
spectra of molecules are seldom recorded. However, various indirect detection
methods for measurement of absorption spectra may be used, for example Raman
spectroscopy, which allows detection of transitions bet<veen different
vibration modes
of molecules.
Transition bet<veen vibration modes of molecule which are normally
responsible for long wavelength absorption and emission bands can be excited
by
radiation with shorter wavelengths, thereby producing overtone absorption
bands in
regions of the near infrared, or visible regions of the spectrum, which are
easier to
detect. UnforhuZately, the spectral patterns associated with these bands are.
not as easy
to interpret as those obtained from free atoms, and their use for
identification of
molecules requires further data processing using methods such as PLS (partial
least
squares), PCA (principle components analysis) or neural nerivorks.
Even with application of these advanced data processing methods,
identification and recognition of the molecular composition of a complex
substance is
CA 02471583 2004-07-05
WO 03/055381 PCT/CA03/00006
not an easy task, and there still exists a need for methods that produce well
defined
spectrum-dependent patterns, that are specific for a particular chemical
compound,
and that can be used for identification of chemical compounds
Another limitation of presently used methods of absorption spectroscopy
relates to the manner in which measurements are performed. Spectrum radiation
affected by a light-absorbing sample may be. determined by t<vo factors: the.
spectral
characteristics of radiation used for measurement, and the absorbing
properties of the
tested sample. To ensure that measurement results are independent of the
spectral
characteristics of the used radiation, the result needs to be. suitably
corrected. Such a
correction is possible when spectral characteristics of used radiation are
known.
Since properties of all radiation sources can change with time, sometimes
quite
rapidhj, the spectral characteristics of used radiation needs to be measured
at a time
that is close to the time of sample measurement. Furthermore, it is prefeiTed
that this
measurement is made using the same measurement equipment as that used for the
measurement of radiation affected by the sample. Once these rivo measurements
are
taken, the impact of samples on the radiation can be identified as a ratio of
spectral
distribution of radiation affected by the sample to that of radiation incident
on the
sample. The negative decimal logarithm of this ratio is called the spectral
optical
density of the sample and it can be used for identification of one or more
chemical
compounds. The spectral optical density may also be used, with further
processing, to
determine the relative or absolute concentration of one or more compounds in a
sample. This can be determined with acceptable precision if the sample average
absorbance is relatively small and if the differences in the absorbance in
bands where
radiation is absorbed, and non-absorbed, are large enough to provide a
sufficient
signal to noise ratio. For highly absorbing samples, this condition usually
cannot be
satisfied, and calculation of spectral optical density cannot be performed
with required
precision. To resolve tlus problem additional attenuators, that are not
affected by
radiation, are used for sample illumination, but the use of attenuators
influences the
precision of the measurement. Therefore, there exists a need for a method of
measurement that eliminates the requirement for characterizing radiation that
is used
for testing.
CA 02471583 2004-07-05
WO 03/055381 PCT/CA03/00006
It is known that observation of the mesomorphic phases of a substance is a
difficult task because of a lack of sufficiently sensitive methods for
identifying
different phases. It is also known that absorption spectra of a chemical
compound
depends not only on the molecular composition of the compound but also on the
physical state of the sample (gas, liquid, solid), and the mesomorphic phase
of the
substance. Differences in the spectra of substances in different mesomorphic
phases
are very small and they are typically attributed to temperature changes
required for
phase transition of the sample. As a result spectroscopy has not been used for
analysis
of phase state of samples. Therefore there exists a need for a measurement
method
for easy and relatively simple identification of phase transition in the
molecular
substances.
It is an object of the invention to overcome disadvantages of the prior art.
The above object is met by the combinations of features of the main claims,
the sub-claims disclose further advantageous embodiments of the invention.
SUNII~~IARL' OF THE INVENTION
The present invention relates to a method of identifying components of a
sample at different temperatures. More specifically, this invention provides a
method
of sample identification, chemical composition analysis, and testing the
physical state
of a sample by analyzing spectra obtained from a sample subjected to different
temperatures.
The present invention provides a method for identifying a chemical
compound, analyzing a chemical composition or investigating a physical change
of a
sample, the method comprising obtaining spectra of electromagnetic radiation
of the.
sample at t<vo or more than two different temperatures.
The present invention also provides a method (A) for identifying a chemical
compound, analyzing a chemical composition or investigating a physical change
of a
sample comprising:
CA 02471583 2004-07-05
WO 03/055381 PCT/CA03/00006
i) collecting one or more than one spectrum of radiation of the sample at a
first
temperature and averaging the result to obtain a first spectrum, and obtaining
one or
more than one spectrum of radiation of the sample at one or more than one
second
temperature, and averaging the result at each temperature, to obtain one or
more than
one second spectrum;
iii) calculating a difference in absorbance between the first and the one or
more
than one second spectrum; and
iv) using the difference in the absorbance, identifying a chemical compound,
analyzing a chemical composition or investigating a physical changes of the
sample.
The invention also pertains to the method as just defined (IVethod A), wherein
in the step of calculating (step iii)), a negative decimal logarithm of a
ratio of
radiation intensity registered for the sample at the one or more than one
second
temperature to a radiation intensity obtained at the first temperahu~e, is
determined.
This invention embraces method (Method A) as defined above wherein in the
step of calculating (step iii)) comprises
a) determining a mean value of registered spectra for each wavelength measured
for the fist spectrum and the one or more than one second spectrum;
a) calculating the standard deviation for each wavelength; and
b) obtaining a ratio of the mean value to the standard deviation.
The present invention also provides the method as defoined above (Method
A), wherein in the step of collecting (step i)), the temperature of the sample
continuously changes between the first temperature, and the one or more than
one
second temperature. Alternatively, in the step of collecting (step i)), the
temperature
of the sample can be changed in discrete manner bet<veen the first temperature
and the
one or more than one second temperature. 9. Furthermore, in the step of
collecting
(step i)), the first temperature and the one or more than one second
temperature of the
sample can be adjusted outside of, or inside of, a sample compartment. 10. The
first
temperature and the one or more than one second temperature of the sample may
result from radiation that is used for spectral analysis. The first
temperature c.an be
lower than the one or more than one. second temperature, or the first
temperature can
be higher than the one or more than one second temperature.
CA 02471583 2004-07-05
WO 03/055381 PCT/CA03/00006
The present invention also pertains to the method as defined above (Method
A),, .wherein, in the step of calculating (step iii)), a running average, a
standard
deviation, and a relative thermal spectral change are calculated for sets of
measurements, where each set of measurements is collected at the one or more
than
one second temperature, and a thermal dynamic of change in the sample is
determed.
This invention embraces the method defined above (Method A) wherein, in
the step of collecting (step i)), a spectral analyzer is used to obtained the
first
spectrum and the one or more spectrum, the spectral analyzer selected from the
group
consisting of a grating scanning spectrometer, a prism scanning spectrometer,
a
grating and prism spectrometers with detector arrays, a Fourier transform
spectrometer, a filter switching spectrometer, a tuneable filter spectrometer,
a Fabry-
Perot scanning spectrometer, an acousto-optical tuneable filter and an
instrument
allowing for spectrum analysis.
According to the present invention there is provided a method for chemical
composition analysis bf a sample using spectra obtained from a sample at
different
temperatures. In one embodiment, which is provided here by ~vay of an example
only,
the method consists in multiple repetition of the following steps:
- bringing the sample temperature to a certain level,
- placing the sample in the spectrometer,
- illuminating the sample with electromagnetic radiation in required spectral
range,
- collecting of one or more spectra of radiation subjected to interaction with
the
sample at set temperature and repeating the above steps at least one more time
at
different sample temperature.
As is evident to those of shill in the art, the methods defined above can be
perfornled in many different ways, provided that a set of spectra of the
electromagnetic radiation obtained following interaction with the sample
obtained at
t'vo or more than rivo different temperatures is collected. In the case of
samples of
complex molecular composition, it has been observed that the spectral
absorbance of
the sample. depends on te.mperature., therefore spectra obtained at different
temperature have different spectral content. Once spectra of the sample at
different
CA 02471583 2004-07-05
WO 03/055381 PCT/CA03/00006
temperatures are obtained the resulting data can be processed in different
ways as
required.
This invention overcomes existing limitations in the art of spectral
measurement methodology and provides a method for conducting spectral
measurements and data processing resulting in a specific response. The methods
described herein do not require measurements of spectral intensity
distribution of
radiatiori used for illumination of the sample during absorption measurements.
Furthermore, the present invention overcomes existing limitations of in the
recognition of phase transition of a chemical compound, and provides a
spectral
measurement methodology based on analysis of thermal dynamics of absorption
spectra of a compound using the dependence of the thermal dynamic of the
absorption
spectra on temperature range in wl>ich the at~sorbance spectra are collected.
As described in more detail below, changes in spectra of substances caused by
change of temperature have. been observed to be substance specific, and these
spectra
can be also used for identif5ring chemical compounds in a sample, or for
composition
analysis of chemically complex samples.
This smnmarv does not necessarily describe all necessary features of the
invention but that the invention may also reside in a sub-combination of the
described
features.
CA 02471583 2004-07-05
WO 03/055381 PCT/CA03/00006
BRIEF DESCRIPTION OF THE DRAWINGS
Features of the invention will become more apparent from the following
description in which reference is made to the appended drawings whereil~:
Figure 1 shows a block diagram of a typical spectroscopic system.
Figure ? shows a flow chart of standard measurement process leading to the
production of an absorption spectrum of a sample, and that can be used for
chemical sample identification and analysis.
Figure 3 shows spectra of different samples obtained using a spectrometer and
prior .
art methods. Figure 3A shovJs the spectrum of water. Figure 3B shows the
spectrum of ~0% methanol solution in water. Figure 3C shows spectrum of
methano 1.
Figure ~ shovJs a flow chart of measurement processes leading to the
production of
an absorption difference created by changes in sample temperature.
Figure 5 shows changes of absorbance of a sample obtained while gradually
warming
up a sample from refrigerator (about ~4°C) to room temperature (about
20°C).
Figure SA shows the spectrum of water. Figure SB shows the spectrum of
50% methanol solution in water. Figure 5C shows spectrum of methanol.
Figure 6 shows a scatter plot of measured (predicted) concentrations against
reference data for a mixture of intralipid in water in the presence of t<vo
interfering analytes calculated from a difference of absorption measured at
hvo
different temperatures. Three independent sets of data with correlation
coefficient between all three components below 0.2 were used for model
development, model verification and method validation.
Figure 7 shows a scatter plot of measured concentrations against reference
data for
protein concentration in animal sera calculated from a difference of
absorption
measured at two different temperatures.
Figure 8 shows a scatter plot of measured concentrations against reference
data for
cholesterol concentration in animal sera calculated from a difference of
absorption measured at hvo different temperatures.
8
CA 02471583 2004-07-05
WO 03/055381 PCT/CA03/00006
Figure 9 shows a scatter plot of measured concentrations against reference
data for
bilirubin concentration in animal sera calculated from a difference of
absorption measured at two different temperatures.
Figure 10 shows a scatter plot of measured concentrations against reference
data for
sodium ions concentration in animal sera calculated from a difference of
absorption measured at tlvo different temperatures.
Figure 11 shows a flow chart of a measurement process leading to the
production of a
spectral response representing an average thermal change and the thermal
dymamic of a spectral signal. The obtained value reaches a maximum in
spectral bands where absorbance demonstrates the highest stability (i.e. the
smallest variability).
Figure 12 shows examples of the spectral stability of absorbance spectra of
samples
measured as described herein. The spectra were calculated as a ratio of the
averaged spectral intensity of radiation affected (in this particular case
transnutted) by the sample for a defined period of time, during which
temperature of the sample was gradually increasing, to the standard deviation
of the variability of the spectral intensity of the radiation during the same
time
period. Figure 1?A shows the thermal spectral stability of water. Figure 12B
shows the thermal spectral stability of 50% methanol solution in water. Figure
12C shows the thermal spectral stability of methanol.
Figure 13 shows spectral stability of a water sample over about t<vo minute
time
period as measured using the method described herein, with about a half
minute time beriveen the end of one measurement and beginning next
measurement. Figure 13A shows the relative thermal spectral stability for
water at the beginning of the experiment. Figure 13B shows the relative
thermal spectral stability for water at about ? min. Figure 13C shows the
relative. thermal spectral stability for water at about 4 min. Figure 13D
shows
the relative thermal spectral stability for water at about E min. Figure 13E
shows the relative thermal spectral stability for water at about 8 min.
9
CA 02471583 2004-07-05
WO 03/055381 PCT/CA03/00006
DET:~ILED DESCRIPTION OF THE INVENTION
The present invention relates to a method of sample identification and
chemical composition analysis of a sample at different temperatures, More
specifically, this invention provides a method of sample identification,
chemical
composition analysis, and testing the physical state of a sample by analyzing
spectra
obtained from a sample subjected to different temperatures. This invention
also
pertains to the field of spectral measurements of an object containing a
complex
molecular composition. More specifically, this invention relates, but is not
limited, to
methods of identifying chemical compounds, analyzing chemical compositions,
and
observing physical changes iti a sample absorbuig electromagnetic radiation,
In
particular, the present invention provides methods for identifying a chemical
compound, analyzing the chemical composition of a sample, and testing the
physical
state of a sample using spectra obtained at different temperatures of the
sample.
The following description is of a preferred embodiment by way of example
only and without limitation to the combination of features necessary for
carrying the
invention into effect.
With reference to Figure 1, there is shown a typical spectrometric instrument
which is not to be considered limiting in any manner, comprising:
- a stable source of electromagnetic radiation (101), for example but not
limited to a
lamp;
- a radiation beam forming component (102), for example, but not limited to,
mirrors or lenses, for efficient collection of radiation from the source (101)
and for
forming a radiation beam that can be used for sample ilhunination. Filters
(not
shown) may be used for removal of unwanted radiation as required;
- a shutter (103) to block or open a path of the radiation beam to a sample
(1042);
- a sample compartment (104) equipped with a sample holder (1041) to hold the
sample (1042) in a desired position. The sample compartment, or sample holder
may
be equipped with temperature measuring and controlling elements (1043), for
temperature monitoring and to keep a constant sample. temperature during
m
CA 02471583 2004-07-05
WO 03/055381 PCT/CA03/00006
measurement, or to change the temperature in a controlled fashion by heating
or
cooling;
- radiation collecting optics (105), for example. consisting of lenses or
mirrors to
collect radiation affected by the sample, to form a radiation beam of a
required shape,
and to direct the beam for further analysis;
- a spectrum analyzer (106) that decomposes the delivered radiation beam
received
from the collecting optics (105) into particular spectral components and
directs them
to a radiation detector (107). While any instrument capable to decompose
delivered
radiation into spectral components, and measure the intensity of these
spectral
components can be used as the spectrum analyzer, a non-limiting example of a
spectrum analyzer is a grating spectrometer that angularly disperses radiation
of
different wavelengths in different directions, so that particular spectral
components
are registered with separate detectors, for example, in a form of a detector
array.
Non- limiting examples of a spectral analyzer include grating scanning
spectrometer, a prism scanning spectrometer, a grating and prism spectrometers
with
detector arrays, a Fourier transform spectrometer, a filter switching
spectrometer, a
tuneable filter spectrometer, a Fabry-Perot scanning spectrometer, an acousto-
optical
tuneable filter, and any instrument that allows for spectrum analysis of a
sample.
The signal from the radiation detector (107) can be preprocessed or processed
with a suitable electronic circuit (108), converted into a digital form by
means of A/D
converter (109), and directed to computer (110), which can process, store,
display or
send obtained infornnation to an external user (111) for further utilization.
The same
computer (110) can be also used to perform numerous other fimctions, for
example
verification of calibration and perfornnance of the analyzer, and control the
function of
the analyzer for example, but not limited to, opening and closing shutter
(103),
verification of sample (1040 presence in the sample compartment (104), or
measurement and control of sample temperature (10143) and the like.
Spectral absorbance measurement with such the instrument consists of several
steps, as shovrn, but not limited to, in Figure ?. Instrument preparation for
measurement (step 2-1) typically involves verifying, in a trial run, the
functionality of
the instrument and measurement parameters, for example amplification or
integration
11
CA 02471583 2004-07-05
WO 03/055381 PCT/CA03/00006
time, slit width, scanning speed, measurement time, sample temperature or
other
criteria as required. Once working parameters of instrument are deternuned and
set,
one or more than one reference spectral measurements of the radiation beam is
obtained, without a sample present in the optical path, and stored in computer
memory (2-2). However, an attenuator, that replaces the sample, may be present
for
this step (2-2). Immediately before or after reference measurement RL (2-2),
one or
more than one measurement of instrument internal noise RD may be obtained and
stored (2-3) using the. same setting of all system parameters (as for step 2-
2), with
exception that the shutter is in the closed position to block radiation from
the source
to detector. The sample is introduced into the optical path (step ?-4), and
the settings
of the instrument are modified to accommodate instrument performance as
required
by attenuated by the. sample radiation level, and one or more than one light
SL and
dark SD measurements are performed (steps ?-5 and 2-6). The data is stored for
fw-ther processing and analysis.
Usually light measurements (2-5) are conducted either at room temperature or
after the sample reaches a temperature set by a sample temperature controlling
system. Variation in sample temperature during measurement is undesirable
and~is
typically considered as a factor that affects the precision of measurement.
After four
measurements (steps 2-~, ?-~, ?-5 and 2-6; RL, RD, SL and SD, respectively)
are
collected results of the same kind of measurements (if more than one
measurement
was obtained) are averaged (step 2-7; 2-7a - reference light, 2-7b - reference
dark, 2-
7c - sample light and ?-7d - sample dark) and dark signals are subtracted from
the
light signal (step 2-S; ~-Sa - for reference measurement and step 2-Sb - for
sample).
Both measurements, the reference measurement and sample measurement (?-Sa and
2-Sb) are normalized for amplification, or integration times (if different
amplifications
or integration times were applied during these measurements), and the
absorbance is
calculated (step ?-10) as a decimal logarithm of ratio of spectral intensity
of radiation
affected, and not affected,~by the sample. Obtained spectra can be used either
for
identification, or concentration, of a chemical compound (step2-11) with
results
presented, as in step 2-1?, or the results used for measurement of the
concentration of
the compound of interest in the sample (steps 2-21 to 2-27). This includes
collection
of thermal absorbance difference for larger number of samples (?-21),
development of
statistical model (2-'?2), collection a large number of sample data for model
validation
12
CA 02471583 2004-07-05
WO 03/055381 PCT/CA03/00006
(2-23), model validation (?-~4), collection of data for samples to be analyzed
(2-25),
prediction of concentration of compound of interest using developed models (?-
26)
and result presentation (2-27)
Examples of absorption spectra obtained, using the method outlined in Figure
2, of three different substances: water, a s0% solution of methanol in water,
and pure
methanol are shown in Figures 3 A, B and C respectively. While obtained
spectra
show some differences it is clear that they contain only limited number of
specific
features that can be used to make the spectra of these substances easily
distinguishable, especially in comparison to another similar substance.
When properly configured, and the samples to be analyzed obey Beer's law,
the absorbance data can be used for sample identification or, after suitable
calibration,
for analysis of sample composition (steps 2-11 and ~-1?). In many cases,
especially
for light scattering samples such a simple procedure may not be sufficient,
and more
advanced measurement methods are required. These methods include: collecting
data
fi~om larger set of samples (?-211 and development of one or more statistical
models
(steps 2-?2 tot-?4) that represent the relation between measured spectral
values and
the concentrations of analytes of interest in the sample; applying one of many
available mathematical methods for example, but not limited to, Partial Least
Square
fitting (PLS), or Principal Components Analysis (PCA) or Neural Network and
using
the developed method; the model validation, using statistically justified set
of
independent measurements (''-'?4) and predicting in step 2-26 the
concentration of the
analytes for which the mathematical models have already been built, using data
obtained in step ?-25. Results can be presented (2-27).
As will be recognized by one of skill in the art, the order of spectral data
collection steps 2-2 to ?-6 may be varied, and that the required data can be
collected
in any other order. For example, which is not to be considered limiting, a
sample
measurement can be taken before reference and dark measurements. Also the
number
of measurements obtained, the order of data averaging, or the normalization of
the
data can be performed in any order without departure from the spirit of the
method.
13
CA 02471583 2004-07-05
WO 03/055381 PCT/CA03/00006
According to an aspect of the present invention, there is provided a method
for
chemical composition analysis and a method for the analysis of physical state
of a
sample using spectra obtained from a sample ~at different temperatures.
With reference to Figure 4 there is shown a flow chart of a method performed
in accordance with present invention. However it is to be understood that
variations
in tlus method may also be employed.
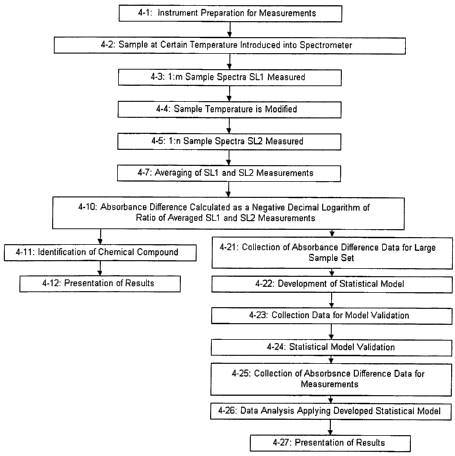
In step 4-1, the instrument is prepared for measurements. This step may
ilzclude spectrum measurement of a radiation beam in the absence of the sample
(as in
step ?-2), but this measurement is not required for use in further steps. Also
step ?-3
and 2-6 (reference and sample dark measurement, respectively) are not required
for
use in this method. Step ~.-1 may be further modified to set the instnunent
parameters
to values that are optimal for light sample collection. After the instrument
is ready, the
sample at the preset temperature is introduced into spectrometer (4-2), where
the
temperature control system either maintains the initial temperature, or it
brings the
sample temperature to a desired level. At this point one or more than one
spectrum
(SL l) of radiation affected by the sample is collected (4-3), after which the
sample
temperature is modified (4-4) using the temperature control system of the
spectrometer (1043, Figure 1) or an external temperature controller (not shown
in
Figure 1), and another one or more than one spectrum (SL?) of radiation
affected by
the sample at the new temperature is collected (4-6). The spectra in each
group are
averaged and difference in absorbance of the sample being at these tvvo
temperatures
is calculated as a negative decimal logarithm or ratio of the measured
spectral
radiation intensity (4-10). Results obtained in this manner can be used in a
similar
fashion (steps 4-11 and 4-12 or 4-? 1 to 4-27) as the sample absorbance
measured in
the process represented in Figure 2.
When active temperature control of a sample is not available or carried out,
alternated methods to conduct measurements can be used. After the instrument
becomes ready to obtain measurements (as a result of step 4-1), the sample at
a certain
temperature, lower or higher than temperature of the instrument, is placed in
the
measurement compartment of the spectrometer (~.-2). For example, which is not
to be
considered limiting in any manner, the sample may be cooled to refrigerator
1~
CA 02471583 2004-07-05
WO 03/055381 PCT/CA03/00006
temperature, for example from about 0°C to about 8°C, and be
introduced into system
in step 4-2 for continuous spectrum and temperature collection (step 4-3)
until the
sample reaches a desired temperature (4-4), for example about 18°C to
about 25°C
(room or any other temperature). The obtained spectra (~-3; in this case steps
4-4 and
4-5 are omitted, since continuous data is collected during the change in
sample
temperature), are. then divided in subgroups containing, for example, but not
limited
to, either equal number of measurements, or measurements corresponding to the
same
temperature change (in tlus case an increase) during which time the selected
group of
measurements were collected. All measurements belonging to a single group are
then
averaged (step 4-7) and a negative decimal logarithm (base 101 of the ratio of
averaged values for t<vo selected groups, obtained in a similar fashion, are
calculated
(4-10). The. results obtained using this method represent differences u1 the
averaged
absorbance, over selected groups of absorbance of the sample, that arise from
the
difference of averaged temperature when the corresponding groups of spectra
were
collected. These measurements may be processed as required (steps 4-11 to ~.-
12, or
steps 4-? 1 to 4-26) as outlined above with respect to Figure 2. Sets of
absorbance
differences obtained using this method, for the first and each consecutive
group, for
the same substances as presented in Figure 3, are shown in Figure 5.
A comparison of spectral absorbance differences (as shown in Figures SA-C)
obtained for large number of different samples for the same temperature
gradient
demonstrates that the difference in absorbance is sample specific as is
evident, for
example, with reference to Figures SA to C, for water, 50°,/o solution
of methanol in
water and pure methanol, respectively. Theoretical analysis also indicates
that these
differences follow Beer's law. Therefore, this analysis can be used for
chemical
composition analysis in a manner similar to the use of absorbance spectra.
An advantage of the approach described herein consists of eliminating a
reference measurement, which in some cases, especially in spectroscopic
systems that
are specific. for measuring light scattering samples, is problematic.
The measurements obtained using the present method may be conducted using
cooled samples, for example which is not to be considered limiting in any
manner, to
CA 02471583 2004-07-05
WO 03/055381 PCT/CA03/00006
refrigerator temperature, and then placing the sample in the compartment of a
spectrometer held at room temperature to permit warming of the sample over
time.
Ten groups of spectral measurements comprising radiation transmitted by a
sample
housed within a radiation scattering container can be collected for each
sample, and
the absorbance difference calculated for selected measurement groups, for
example
the second and ninth groups. The difference bettveen these t<vo groups may
then be
used for further analysis.
The absorbance difference betlveen the any group of measurements may be
used for analysis of the chemical composition of a sample. The difference in
absorbanc.e can be used for analysis of chemical composition of sample in
similar
fasluon as the real absorbance of a sample measured as outlined in Figure 2.
To demonstrate the effectiveness of the method as described herein,
temperature related differences in, absorption have been collected for t<vo
sets of
samples. A preliminary experiment was conducted using a random combination of
intralipid and t<vo interfe.rents solved in water. A second experiment was
conducted
on samples comprising a combination of nine different animal sera containing
physiological analytes in different proportions. In both cases a special care
was taken
to eliminate possibility of correlation between any rivo analytes. In both
cases a lame
number of samples were prepared (over 500 samples in the first experiment and
over
400 samples in the second), as required for chemometry.
Samples vJere cooled and placed into the sample holder at room temperature.
Spectra of radiation affected by the samples during the period of sample
warming,
from refrigerator to room temperature, were continuously collected and
differences in
absorption for the second and ninth groups of spectra calculated as described
above.
The results were divided in three sets:
- the first set was used for statistical model development of the relation
beriveen absorbance difference and concentration of the analytes of the
interest;
- the second set was used for model validation; and
- the last set was used for verification of the method described herein.
16
CA 02471583 2004-07-05
WO 03/055381 PCT/CA03/00006
The. results of this analysis are presented in set of scatter plots show in
Figures
6 to 10, The abscise on each graph represents the independently measured value
of
concentration of a compound of iliterest, while the ordinate shows the value
predicted
by a developed model from the spectral information obtained in the measurement
process as described above.
Figure 6 shows a scatter plot of intralipid concentrations in water in
presence
of two non-correlated interferents as predicted by a model, against the
concentration
of intralipid used for sample preparation. This result indicates a very good
predictability of intralipid concentration (all measurements line up along the
line with
slope 1.(45° line), and an acceptable measurement precision, as almost
all
measurements are contained within +/- 20% error range.
A further analysis with animal sera was undertaken and results for selected
anal~~tes are presented in Figures 7 to 10, Figure 7 shows a scatter plot for
protein
concentration in animal sera, while Figure 8, 9 and 10 present results for
cholesterol,
bilirubin and sodium ions. While not all analytes can be measured with the
same
precision, since not all analytes in natural concentrations provide. a signal
that is
sufficient for spectroscopic measurement, the results demonstrate a very good
level of
predictability - in all cases results line up along line with slope 1.
These results demonstrate that the method described herein, comprising the
measurement of spectral differences in sample absorbance caused by temperature
differences in a sample, provides information suitable for the. measurement of
the
concentration of chemical components in the sample, This method is also
suitable for
determine the measurement of the concentration of chemical components within a
complex matrix, such as animal sera, without the need for spectral
characterization of
radiation used for measurement. As in other methods measurement, errors depend
on
concentration of the component under consideration and the strength of the.
signal
provided, as it is can be seen with reference to Figures 6-10.
The data sets shown in Figures 6 to 10 demonstrate that measurement of the
concentration of a chemical compound within a sample within simple or complex
m
CA 02471583 2004-07-05
WO 03/055381 PCT/CA03/00006
matrices, such as animal sera, may be obtained using differences in absorbance
of the
samples obtained at different temperatures.
Different information may be derived when the spectral distribution of
radiation intensity obtained during a continuous change in the temperature of
the
sample. is averaged and divided by the. standard deviation of these variations
at each
spectral measurement point. If the instnunent noise is measured in similar way
in the
absence of the sample is low, this ratio, called dynamic range of instrument,
shows a
high value. For non-absorbing samples this ratio would be preserved. For the
samples uniformly absorbing radiation across the whole spectrum, the ratio cm
be
scaled down if the same instrument settings are used for spectral intensity
distribution,
or for determining the radiation affected by the sample, as those used for
unaffected
radiation. However, as described herein, it was noted that in the presence of
the
sample, whose temperature varied during measurement, the response is different
from
that as expected. Furthermore, in portions of spectra where sample absorbance
is
small, and where no changes in dynamic range were expected, a large
variability in
dynamic range was observed.
Further study demonstrates that the temperature-affected dynamic range is
sample specific and strongly depends on a mean value and the temperature.
variability
range during time when the to be averaged spectra of affected by the sample
radiation,
are collected. This method can provide information on the position of
isosbestic
points in a sample spectrum.
Analysis of water demonstrates that its spectrum depends on temperature and
that there also exist points were the absorbance spectnun remains virtually
constant,
which by extension are referred to as thermal isosbestic points. These points
were
observed as intersections of rivo or more water absorption spectra taken at
different
temperatures. To verify the uniqueness of the method described herein, tests
were
performed using different chenucal compounds of organic and non-organic
origin. It
has been observed that each sample produces a different, specific pattern that
is
dependent on mean temperature, and the temperature variability range as shown
in
Figures 12 A-C, and Figures 13 A-E.
18
CA 02471583 2004-07-05
WO 03/055381 PCT/CA03/00006
As opposed to isosbestic points, the method proposed in the present invention
allows for a quantitative characterization of the thermal behavior of
absorbing
properties of a sample. The proposed ratio shows high values in portions of
the
spectra where absorbance of the sample is not affected by the sample and the
absorbance is stable, and low values where the impact of temperature is large,
and
where stability of the sample absorbance is low, Therefore, these measurements
represent the thermal dynamic of the signal across the spectrum that results
from
changes in the temperatures of the sample, The thermal d~mamic of the signal
should
reach a maximum at thermal isosbestic points of the sample.
With reference to Figure 1 l, there is sho~~m a flow chart of measurements
that
are performed in accordance. with present invention to collect data for
determination
of a thermal dynamic. signal of the radiation that is affected by the sample..
In the first
step the instrument is prepared for measurement (11-1). This process may
include
measurements needed to evaluate instrument performance, but these measurements
are usually not used in the process of characterization the thermal dynamic
signal.
Step 11-1 can be used to set the instrument parameters to values that are
optimal for
collection of radiation affected by the sample. After working conditions of
the
instrument are determined and set, the spectra of radiation affected by the
sample are
collected as the sample temperature is changed in a controlled manner. As
outlined
with reference to the analogous step in Figure 4, this step can be performed
in many
ways depending on requirements, and on available equipment, For example, which
is
not to be considered limiting, the sample is cooled to refrigerator
temperature, from
about 0°C to about 8°C, and it is introduced into the sample
holder (step 11-2) and the
continuous spectrum is collected (11-3) until the sample reaches a desired
temperature, for example but not limited to room temperature. The process of
changing the temperature may take different amounts of time, depending of
thermal
properties of sample holder, or if a sample temperature controller used. In
the latter
case, the dynanuc of the process can be adjusted to increase the detail of
thermal
d5mamic to be resolved. Generally speaking, the slower the process, the more
details
can be revealed. However, it is required that the measuring instrument is
stable
enough during the time period for the collection and averaging of each group
of
spectra. Due to the high sensitivity to the temperature and temperature
~rariations, it is
19
CA 02471583 2004-07-05
WO 03/055381 PCT/CA03/00006
preferred that such spectra have to be collected under controlled thermal
conditions.
One of skill in the art appreciates that numerous ways of changing the
temperature
can be realized during or bet'veen time periods when consecutive groups of
spectra
are collected.
The obtained spectra (11-5) are divided in subgroups containing, for example,
but not limited to, groups comprising an equal number of measurements, or
groups
corresponding to the same temperature increase during the time when the
selected
group of measurement were collected. All measurements belonging to a single
group
are averaged (11-7) to obtain a mean value for each wavelength.
Simultaneously, the
standard deviation of variabilit5r within the group at each wavelength is
calculated
(11-8), and for each wavelength, the ratio of the mean to the standard
deviation, is
calculated (11-?0). Alternatively, instead of continuous temperature change,
stepwise
change can be applied (steps 11-~ to 11-10 in the right column in chart of
Figure 11)
and the ratio of the mean to the standard deviation can be calculated This
data can be
presented in a form of a set of graphs (one for each group of measurements),
that
represent the thermal dynamic of the changes (spectral dependence of these
changes
will be referred here as the thermodynamic signal) in a spectrum of radiation
affected
by the sample at a certain mean temperature, and whose temperatwe. changed
during
measurement within the range determined by measurement system. Comparison of
the results obtained for different groups, and registered at different
temperatures of
samples shows the variability of the thermodynamic signal as function of
sample
composition, mean temperature and temperature range, over which the spectra
were
averaged. Because of high sample specificity, the result can be used for
identification
of chemical compounds (11-~1) or used for analysis of spectral thermodynamic
(1-
31). The result of the analysis may be presented (11-22 and 11-32) or
transferred for
further use.
Exemplary results of chemical compound, solutions or mixtures, were
obtained for water, 50% solution of methanol in water and pure methanol, as
shown in
Figures 1 ~A, B and C.
These results demonstrate that by using the method described above, spectral
thermodynamic signals that depend on mean temperature of the sample during
211
CA 02471583 2004-07-05
WO 03/055381 PCT/CA03/00006
measurement, and that depend on the range of temperature change during
collection
of data, may be obtained that are specific. for given substance. The graphs
(Figures
1?A-C) further demonstrate that the thermal spectra contain more pronounced
features that vary significantly between samples than absorption spectra shown
in
Figures 3A-C. The graphs shown in Figure 12 A-C exhibit richer structure than
the
absorption spectra of Figures 3A-C, including ,very strong features in
spectral ranges
where absorption of the sample is relatively weak (e.g. the 600-900 nm range).
Therefore. results obtained using the method outlined above, and with
reference to
Figures 4 and 11 are more suitable for identification of chemical compounds
than
methods associated with obtaining a standard absorption spectrum.
As graphs in Figure 12A-C show, the average spectral thernial dynamic of the
signal affected by the sample radiation (or thernlodynamic signal) strongly
depends
on temperature and the temperature variability range used for averaging. These
results may therefore be used to identify temperature points where properties
of the
substances change dramatically for some reason. Without wishing to be bound by
theory, the observed changes at temperature. points may occur, for example, as
a result
of a mesomorphic phase transition, or other physical change in the sample, for
example a transition from monomer to dimmer and the reverse, or thermal
related
transitions beriveen different internal structures of solids and liquids.
These
temperature points where thennod5mamic signal undergoes dramatic changes may
be
a good indicator of changes in internal structure of the sample, for example.
water, or
bonds bet<veen water molecules and walls of the vial containing the water
('Drost-
Hansen' temperature, for example). Therefore the spectrum analyzing method
described herein may be a powerful tool for testing physical chances in a
substance.
As one of skill in the art understands, the temperature dependent data can be
collected in many different ways for example, but not limited to, by
measurement of
light affected by the sample at t'vo or more than t,vo distinctive different
temperatures, for example one at a low temperature, and the other, or
remaining
measurements, at any elevated temperature, for example but not limited to from
about
0°K to a temperature accessible with present day technology without
damage to
sample or used measuriilg apparatus. Selection of a temperature range to b,e
used will
21
CA 02471583 2004-07-05
WO 03/055381 PCT/CA03/00006
depend upon the sample and the physical state of the sample being analyzed. In
this
regard, spectra may be obtained spanning any desired temperature range.
Measurements can be also taken in reversed order with a first measurement
obtained
at a higher temperature, and a second and all other consecutive measurements
obtained at lower temperatures. Also the transition from one temperature to
another,
or for averaging measurements from low to high or from high to low, can be.
performed either continuously or in discrete steps with constant or variable
temperature change from one temperature setting to another.
The method of the present invention may be used on any sample for which a
chemical composition analysis or analysis of physical state may be desired.
For
example, but not wishing to be limiting the sample may comprise a body part of
a
subject, for example, but not limited to a finger, toe, earlobe, arm or back,
samples of
various natural or synthetic products, self contained or in a container,
samples or
portions of various products and materials. Alternatively the sample may
comprise a
solid, semi-solid, fluid, vapor or gas obtained from any source, for example,
but not
limited to blood, urine, mucus, sweat, lymph, excrement, secretions and the
like, dairy
products, any form of food or drinks, a chemical product for example but not
limited
to plastics, products of semiconductor industry, products of petrochemical
industry,
wood and paper industry products, neutral and hazardous products in loose or
contained fi.~rm, clear and contaminated water, clear or contaminated air,
gases or
vapors and so on. Samples that are not part of living creature are considered
iu vitro
samples, while parts of life forms such as humans, animal, plants, bacteria
and all
other living creatures, which have to be tested without damage to
functionality, are
considered in oivo samples.
In an aspect of the present invention, the sample may be an in vivo hmnan or
animal sample, for example, but not limited to, a finger of a subject placed
in an
apparatus capable of changing temperature and analyzing the chemical
composition of
a sample by taking the. spectrum of radiation affected by the sample. An
example
such an apparatus is disclosed in US 5,361,755 (which is incorporated herein
by
reference). In an alternate aspect of an embodiment of the present invention,
the
sample may be provided i~z vitro, for example, but not wishing to be limiting
in any
manner by placing blood or other body fluid taken from a subject into a
cuvette or
22
CA 02471583 2004-07-05
WO 03/055381 PCT/CA03/00006
other holder for analysis. Further, if the sample is an ira vitro sample, then
it may be
subjected to processing prior to chemical composition analysis. Any processing
step
known in the art may be employed. Examples of processing steps may include,
but are
not limited to purification, centrifugation, separating contaminating or other
chemical
constituents prior to analysis, diluting, concentrating, reacting components
in the
sample with endogenous or exogenously added reagents or any combination
thereof.
The method of the present invention may be employed to analyze any
chemical component within a sample. Examples of chemical components may
include, but are not limited to one or more proteins, sugars, lipids, and the
like or any
other component of organic or inorganic origin. Any physiological or
nonphysiological chemical component in a biological or nonbiological sample
may be
analyzed by the method of the present invention.
The spectra which are used in the method of the present invention to analyze a
chemical component in a sample or their physical state are obtained by
exposing the
sample to a suitable source of electromagnetic radiation, for example, but not
limited
to that is the infra-red, near infi~a-red, visible, and ultraviolet wavelength
ranges.
Preferably the source employs infrared or near infrared wavelengths.
As described above, the spectra of one or more than one compound of interest
changes with a change in temperature. Furthermore, in samples comprising rivo
or
more compounds, the spectra of the sample changes with changes in temperature,
in a
manner that is distinct from the changes in the spectra of each compound
separately.
Therefore, these changes may be used to identify and characterize compounds of
interest within a sample.
According to an aspect of the present invention, the method comprises
generating a first spectrum of a sample at a first temperature, and obtaining
one or
more second spectra of the sample, at one or more second temperatures.
Therefore, at
least rivo or more spectra may be obtained from a sample. In the. simplest
method,
only t'WO spectra are obtained. Preferably, the first and second spectra are
generated
over a plurality of identical one or more wavelengths. Also contemplated by
the
method of the present invention, multiple spectra may be generated, and
optionally
23
CA 02471583 2004-07-05
WO 03/055381 PCT/CA03/00006
averaged at the first temperature, second temperature, or both. Further, the
present
invention contemplates generating spectra at more than two temperatures and
using
the information to determine the presence and concentration of a chemical
component
of interest within a sample and evaluating a thermal dynamic of the sample,
thereby
obtaining information on its physical state.
The first temperature and second temperature of the first and second spectra
respectively, are different. Any temperature may be used in the present
invention. For
example, but not wishing to be limiting, the first temperature may be
different from
the second temperature, for example but not limited to, by about 0.1°C
to about
500°C, or from about 1°C to about 100°C. In an aspect of
the present invention, both
the first and second temperatures are within the range of about 0°C to
about 50°C, for
example but not limited to about ?0°C to about 40°C. However,
temperatures outside
this range are also contemplated and can be used for different samples,
especially of
non-organic. origin. As would be evident to someone of skill in the art,
preferably the
temperatures are selected so that no damage occurs to the sample, for example,
but
nut limited to denaturation of proteins, precipitation of chemical
constituents, or
destruction of the chemical component of interest in the sample, or causing
discomfort to an individual or living creature.
The temperature of the sample may be adjusted by any means knovm in the
art. For example., but not wishing to be limiting in any manner, the.
temperature of
sample held within a c.uvette or other holder may be adjusted by heating or
cooling a
chamber which holds the sample. In an alternate aspect of an embodiment, which
is
not meant to be limiting in any manner, an ira vivo sample such as a finger or
the like
may be heated or cooled by the receptor device used to hold the sample, or by
the
beam source, for example., but not limited to an infrared beam including that
employed for testing of sample iii an infra-red portion of the spectrum. The
beam
may be the same or different from the electromagnetic radiation source used
for the
analysis of the sample, furthermore, microwave radiation can be used for
sample
heating.
In an aspect of the present invention, the first spectrum obtained for the
sample. at a first temperature is compared to the second spectrum at a second
2~
CA 02471583 2004-07-05
WO 03/055381 PCT/CA03/00006
temperature and the presence and amount of a chemical component of interest
within
the sample is determined. The comparison may involve computing the difference
between the spectra or a point in the spectra. A calibration algorithm may be
employed in the comparison, for example, but not limited to adjust for
interfering or
other components in a sample.
Alternatively, a certain number of spectra are registered at least at t<vo
different temperatures, the results averaged and standard deviation of mean
value
calculated. A ratio of the mean value to the standard deviation can be
calculated
(relative thermal spectral change ratiol, and used for chemical analysis, or
for
investigation of structural changes in a sample that is related to temperature
change.
The ratio can be further processed in any knovrn way, for example but not
limited to
smoothed normalized or normalized to a dynamic range of the instrument, as
required.
As the spectra in samples comprising t,vo or more compounds changes with
temperature. in a manner that is distinct from the changes in the spectra of
each
compound separately, and indicative of the compounds in the sample, then
differences
or the relative spectral thermal change betVVeen spectra obtained from the.
same
sample at different or varying temperatures may be used to identify and
characterize
compounds of interest within an sample, or to observe structural changes of
the
sample. Such a method eliminates need for reference measurements of spectral
content of radiation used for analysis and enables measurement, or increases
the.
accuracy of the measurement, of a compound of interest. Further the method of
the
present invention does not require the use of a reference measurement, since
by
obtaining repeated spectra from the same sample, interfering factors are
corrected for.
The above description is not intended to limit the claimed invention in any
manner, Furthermore, the. discussed combination of features might not be
absolutely
necessary for the inventive solution.
All references are herein incorporated by reference.
The present invention has been described with regard to preferred
embodiments. However, it will be ob~rious to persons skilled in the art that a
number
2s
CA 02471583 2004-07-05
WO 03/055381 PCT/CA03/00006
of variations and modifications can be made without departing from the scope
of the
invention as described herein.
26