Note: Descriptions are shown in the official language in which they were submitted.
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
PHARMACEUTICALLY ACCEPTABLE PHOSPHATE-GLYCEROL
CARRYING BODIES
BACKGROUND OF THE INVENTION
Field of the Invention
This invention relates to three-dimensional synthetic and semi-synthetic
compositions having biological activity, and to the uses thereof in the
treatment
and/or prophylaxis of various disorders in mammalian patients. More
particularly it
relates to preparations and uses of synthetic and semi-synthetic bodies, which
after
introduction into the body of a patient, produce beneficial anti-inflammatory,
organ
protective and immune regulatory effects. The invention also relates to
treatments
and compositions for alleviating inflammatory and autoimmune diseases and
their
symptoms.
Background of the Invention
Professional antigen-presenting cells (APCs), including dendritic cells (DCs)
and
macrophages (Mph), actively capture and process antigens (Ags), clear cell
debris,
and remove infectious organisms and dying cells, including the residues from
dying
cells. During this process, APCs can stimulate the production of either
inflammatory Thl pro-inflammatory cytokines (IL-12, IL-1, INF-y, TNF-a, etc.);
or
regulatory Th2/Th3 cytokines (such as IL-10, TGF-(3, IL-4 etc.) dominated
responses; depending on the nature of the antigen (Ag) or phagocytosed
material and
the level of APC maturation/activation.
APCs remove cellular debris, some of which is derived from cell membranes of
the
body, some from bacterial and parasitic infections and commensal organisms,
such
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
as gut bacteria. While some of this cellular debris will initiate a pro-
inflammatory
response, some initiates a protective and anti-inflammatory response.
A normally functioning immune system is capable of distinguishing between the
antigens of foreign invading organisms (non-self) and tissues or debris
derived from
"self," mounting an immune response only against foreign antigens. When a
patient's immune system fails to discriminate between self and non-self,
autoimmune disorders arise.
Summary of the Invention
This invention is directed to the discovery that pharmaceutically acceptable
bodies,
such as liposomes, beads or similar particles, which comprise phosphate-
glycerol
groups, will, upon administration to a mammalian patient, cause an anti-
inflammatory effect and therefore may be used to treat a number of diseases.
These
bodies may further comprise as a minor component an inactive constituent,
and/or
constituent which is active through a different mechanism.
In a preferred embodiment, the invention is directed to a composition of
matter
capable of producing an anti-inflammatory response in vivo in a mammal, said
composition comprising pharmaceutically acceptable bodies of a size from about
20
nanometers (nm) to 500 micrometers (pm), comprising a plurality of phosphate-
glycerol groups or groups convertible to such groups. Preferably, the bodies
are
essentially free of non-lipid pharmaceutically active entities. Preferably the
phosphate-glycerol groups constitute 60% - 100% of the active groups on the
bodies.
Following administration to a mammal, the bodies, through the phosphate-
glycerol
groups, are believed to interact with the immune system. As a result, when so
administered an anti-inflammatory response is elicited.
In another embodiment, this invention is directed to a three-dimensional
synthetic or
semi-synthetic body, otherwise referred to herein as pharmaceutically
acceptable
bodies, having a size ranging from 20 nm to 500 Vim, and having been modified
to
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
comprise, as a major component, at least one anti-inflammatory promoting
ligand
wherein said ligand has phosphate-glycerol groups.
In still another embodiment, this invention is directed to three-dimensional
synthetic
and semi-synthetic bodies, otherwise referred to herein as pharmaceutically
acceptable bodies, having sizes ranging from 20 nm to 500 pm, and having
phosphate-glycerol groups on the surface thereof.
In another aspect, the invention is directed to a method for treating a T-cell
function-
mediated disorder comprising administering to a mammalian patient an effective
amount of pharmaceutically acceptable bodies carrying an effective number of
phosphate-glycerol groups to inhibit and/or reduce the progression of the T-
cell
function-mediated disorder.
This invention is further directed to a method for treating an inflammatory
disorder
comprising administering to a patient an effective amount of pharmaceutically
acceptable bodies carrying an effective number of phosphate-glycerol groups to
inhibit and/or reduce the progression of the inflammatory disorder.
Yet another embodiment of this invention is a method for treating an
endothelial
function disorder comprising administering to a mammalian patient an effective
amount of pharmaceutically acceptable bodies carrying an effective number of
phosphate-glycerol groups to inhibit and/or reduce the progression of the
endothelial
function disorder.
Another embodiment is a method for treating an immune disorder characterized
by
inappropriate cytokine expression comprising administering to a mammalian
patient
an effective amount of pharmaceutically acceptable bodies carrying an
effective
number of phosphate-glycerol groups to inhibit and/or reduce the progression
of the
immune disorder.
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
This invention is further directed to a process for treating or prophylaxis of
a
mammalian cardiac disorder, the presence of or the susceptibility to which is
detectable by observing a prolonged QT-c interval on an electrocardiogram of
the
patient, which process comprises administering to a mammalian patient
suffering
therefrom or susceptible thereto a pharmaceutical composition comprising
pharmaceutically acceptable biocompatible synthetic or semi-synthetic bodies,
otherwise referred to herein as pharmaceutically acceptable bodies, and a
pharmaceutically acceptable carrier, wherein at least a portion of said bodies
have a
size in the range from about 20 nm to 500 Vim, and wherein the surfaces of
said
bodies have been modified to carry, as a major component, at least one anti-
inflammatory promoting group, said group being a phosphate-glycerol.
Another embodiment of the invention is a pharmaceutical composition, in unit-
dosage form, for administration to a mammalian patient, comprising
pharmaceutically acceptable bodies and a pharmaceutically acceptable carrier,
wherein at least a portion of the bodies has a size in the range from about 20
nm to
500 pm, and wherein the surfaces of said bodies comprise phosphate-glycerol
groups or groups convertible to phosphate-glycerol groups, said unit dosage
comprising from about 500 to about 2.5 x 109 bodies.
A further embodiment of this invention is a pharmaceutical composition
comprising
a pharmaceutically acceptable biocompatible synthetic or semi-synthetic bodies
(otherwise referred to herein as pharmaceutically acceptable bodies) and a
pharmaceutically acceptable carrier, wherein at least a portion of said bodies
has a
size from about 20 nm to 500 pm, and wherein the surfaces of said bodies have
been
modified to comprise, as a major component, at least one anti-inflammatory
promoting group, wherein said group is phosphate-glycerol.
A still further embodiment of this invention is a pharmaceutical composition
comprising pharmaceutically acceptable biocompatible synthetic or semi-
synthetic
bodies (otherwise referred to herein as pharmaceutically acceptable bodies)
and a
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
pharmaceutically acceptable carrier, wherein at least a portion of said bodies
has a
from about 20 nm to 500 pm, and comprises cardiolipin.
Optionally, the bodies described above may additionally comprise an inactive
constituent surface group andlor a constituent surface group, which is active
through
another mechanism, e.g. phosphatidylserine. (See, e.g. Fadok et al.,
International
Publication WO 01/66785).
In another embodiment, this invention is directed to lyophilized or freeze-
dried
pharmaceutically acceptable bodies carrying phosphate-glycerol groups or
groups
convertible to phosphate-glycerol groups, and kits comprising lyophilized or
freeze
dried bodies comprising phosphate-glycerol groups, or groups convertible to
phosphate-glycerol groups, and a pharmaceutically acceptable carrier.
In another aspect, this invention is directed to a method for treating a T-
cell
function-mediated disorder comprising administering to a mammalian patient
suffering from or at risk of suffering from a T-cell function mediated
disorder, an
effective amount of a composition comprising pharmaceutically acceptable
bodies
having a size from about 20 nm to about 500 pm, comprising on the surface
thereof
a plurality of phosphate-glycerol groups, or groups convertible to said
phosphate-
glycerol groups, such that upon administration, the progression of the T-cell
function
mediated disorder is inhibited and/or reduced.
Yet another embodiment of this invention is directed to a method for treating
an
endothelial function disorder comprising administering to a mammalian patient
suffering from or at risk of suffering from an endothelial function disorder
an
effective amount of a composition comprising pharmaceutically acceptable
bodies
having a size of from about 20 nm to about 500 pm, comprising on the surface
thereof a plurality of phosphate-glycerol groups, or groups convertible to
said
phosphate-glycerol groups, such that upon administration, the progression of
the
endothelial function disorder is inhibited and/or reduced.
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
Another embodiment of this invention is directed to a method for treating an
immune disorder in a mammalian patient suffering from or at risk of suffering
from
an immune disorder, comprising administering to said mammalian patient an
effective amount of a composition comprising pharmaceutically acceptable
bodies
having a size of from about 20 nm to about 500 pm, comprising on the surface
thereof a plurality of phosphate-glycerol groups, or groups convertible to
said
phosphate-glycerol groups, such that upon administration, the progression of
the
immune disorder is inhibited and/or reduced.
The present invention can also be viewed, from another aspect, as the use of a
receptor on cells of the mammalian immune system, e.g. macrophages, which
specifically bind to the phosphate-glycerol group. The invention embraces
bodies
comprising ligands and groups that will bind to such receptor and consequently
produce an anti-inflammatory response. Accordingly, the present invention can
be
defined as bodies comprising ligands or active groups thereof that compete
with the
binding or uptake of phosphate-glycerol expressing bodies as described herein
by
antigen-presenting cells. ~A person skilled in the art can readily determine
whether a
particular body is one, which will so compete, by conducting simple test
experiments. For example, the bodies can be tested with a readily available
monocytic cell line such as U937 cells. In a first experiment, U937 cells are
incubated with fluorescently labeled PG liposomes alone, and in other
experiments
the U937 cells are incubated in the presence of both fluorescently labeled PG
liposomes and differing amounts of test compound. If the uptake of the
fluorescently
labeled PG liposomes in the other experiments is reduced in comparison with
that of
the first experiment, then the test compound is competing for the specific
receptor
and is a compound within the scope of the present invention.
Brief description of the drawings
FIG. 1 is a bar graph presentation of the results of Example 1 below, murine
contact
hypersensitivity (CHS, acute T-cell mediated inflammatory model) experiments
using liposomes in accordance with a preferred embodiment of the invention, in
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
comparison with other liposomes and controls.
FIG. 2 is a similar graphical presentation, showing the use of liposomes of
various
phosphatidylglycerol (PG) contents, in the murine CHS model, Example 2 below.
FIG. 3 is a similar graphical presentation of the results of Example 3 below
where
different concentrations of 75% PG liposomes were used in the murine CHS
model.
FIG. 4 is a similar graphical presentation of the results of Example 4 below,
where
different concentrations of 100% PG liposomes were used in the murine CHS
model.
FIG. 5 is a similar graphical presentation of the results of Example 5 below,
using
liposomes of different sizes in the CHS model.
FIG. 6 is a similar graphical presentation of the results of Example 6 below,
using a
murine model of delayed type hypersensitivity (DHS, chronic T-cell mediated
inflammatory model).
FIG. 7 is a similar graphical presentation of the results of Example 7 below,
cardiolipin liposomes in a DHS murine model.
FIG. 8 is a similar graphical presentation of the results of Example 8 below,
cardiolipin liposomes in a CHS murine model.
FIG. 9 shows the change in the percentage of excitatory post-synaptic
potential
(EPSP) slope in control and treated mice, which is indicative of the effect on
long
term potentiation (LTP), Example 9.
FIG. 10 displays the data shown in FIG. 9 in the format of a bar chart,
Example 9
below.
7
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
FIG. 11 Shows the difference in the concentration of the anti-inflammatory
cytokine
IL-4 in the hippocampus of control and treated animals, Example 10 below.
FIG. 12 shows the difference in the concentration of the pro-inflammatory
cytokine
IL-1 [3 in a single cell suspension of spleen cells of control and treated
animals,
Example 11 below.
FIG. 13 shows the difference in the concentration of TNF-a in the U937
monocyte
cell line treated with varying concentration of 75% PG liposomes, Example 12
below.
FIG. 14 is a graphical presentation of the results of Example 13 below,
endothelin-1
content in ears of mice treated according to a preferred embodiment of the
invention
versus control.
FIG. 15 is a graphical presentation of the results of Example 14, ICAM-1
positive
cells from HUVEC cultures in the presence and absence of compositions of the
preferred embodiment of the invention.
Description of preferred embodiments
According to the present invention, pharmaceutically acceptable bodies
carrying
phosphate-glycerol groups on their surface are administered to patients.
Without
being limited to any one theory, is believed that these bodies interact with
the
immune system of the patient with accompanying beneficial effects such as
inhibition of pro-inflammatory cytokines in vivo and/or promotion of anti-
inflammatory cytokines. The reacting cells may be immune cells such as
professional or non-professional antigen presenting cells, endothelial cells,
regulatory cells such as NK-T cells and others.
These pharmaceutically acceptable bodies include synthetic and semi-synthetic
8
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
bodies having shapes which are typically but not exclusively spheroidal,
cylindrical,
ellipsoidal, including oblate and prolate spheroidal, serpentine, reniform
etc., and
sizes from about 20 nm to about 500 pm in diameter, preferably measured along
its
longest axis, and comprising phosphate-glycerol groups on the surface thereof.
The pharmaceutically acceptable bodies have phosphate-glycerol groups of
predetermined characteristics on the exterior surface. Without being limited
to any
one theory, it is believed that these groups are capable of interacting with
the
appropriate receptor(s), other than exclusively the PS receptor, on antigen
presenting
cells in vivo. The structure of these groups may be synthetically altered and
include
all, part of or a modified version of the original phosphate-glycerol group.
For
example, the negatively charged oxygen of the phosphate group of the phosphate-
glycerol group may be converted to a phosphate ester group (e.g., L-
OP(O)(OR')(OR"), where L is the remainder of the phosphate-glycerol group, R'
is
-CHzCH(OH)CHZOH and R" is alkyl of from 1 to 4 carbon atoms or hydroxyl
substituted alkyl of from 2 to 4 carbon atoms, and 1 to 3 hydroxyl groups
provided
that R" is more readily hydrolyzed in vivo than the R' group; to a diphosphate
group
including diphosphate esters (e.g., L-OP(O)(OR')OP(O)(OR")2 wherein L and R'
are
as defined above and each R" is independently hydrogen, alkyl of from 1 to 4
carbon atoms, or a hydroxyl substituted alkyl of from 2 to 4 carbon atoms and
1 to 3
hydroxyl groups provided that the second phosphate group [-P(O)(OR")Z] is more
readily hydrolyzed in vivo than the R' group; or to a triphosphate group
including
triphosphate esters (e.g., L-OP(O)(OR')OP(O)(OR")OP(O)(OR")2 wherein L and R'
are defined as above and each R" is independently hydrogen, alkyl of from 1 to
4
carbon atoms, or a hydroxyl substituted alkyl of from 2 to 4 carbon atoms and
1 to 3
hydroxyl groups provided that the second and third phosphate groups are more
readily hydrolyzed in vivo than the R' group; and the like. Such synthetically
altered
phosphate-glycerol groups are capable of expressing phosphate-glycerol in vivo
and,
accordingly, such altered groups are phosphate-glycerol convertible groups.
Phosphatidylglycerol is a known compound. It can be produced, for example, by
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
treating the naturally occurring dimeric form of phosphatidylglycerol,
cardiolipin,
with phospholipase D. It can also be prepared by enzymatic synthesis from
phosphatidylcholine using phospholipase D - see, for example, U. S. Patent
5,188,951 Tremblay, et al. Chemically, it has a phosphate-glycerol group and a
pair
of similar but different C ~ 8-CZO fatty acid chains.
As used herein the term "PG" is intended to cover phospholipids carrying a
phosphate-glycerol group with a wide range of at least one fatty acid chains
provided that the resulting PG entity can participate as a structural
component of a
liposome. Preferably, such PG compounds can be represented by the Formula I:
R-CO-O- ~ HZ
R~-CO-O-CH O
CHZ-O-P-O-CHZCH(OH)CHZOH
O
where R and R' are independently selected from C~ - C24 hydrocarbon chains,
saturated or unsaturated, straight chain or containing a limited amount of
branching
wherein at least one chain has from 10 to 24 carbon atoms. Essentially, the
lipid
chains R and R' form the structural component of the liposomes, rather than
the
active component. Accordingly, these can be varied to include two or one such
lipid
chains, the same or different, provided they fulfill the structural function.
Preferably,
the lipid chains may be from about 10 to about 24 carbon atoms in length,
saturated,
mono-unsaturated or polyunsaturated, straight-chain or with a limited amount
of
branching. Laurate (C 12), myristate (C 14), palmitate (C 16), stearate (C
18),
arachidate (C20), behenate (C22) and lignocerate (C24) are examples of useful
saturated lipid chains for the PG for use in the present invention.
Palmitoleate (C 16),
oleate (C 18) are examples of suitable mono-unsaturated lipid chains.
Linoleate
(C 18), linolenate (C 18) and arichidonate (C20) are examples of suitable poly-
unsaturated lipid chains for use in PG in the liposomes of the present
invention.
Phospholipids with a single such lipid chain, also useful in the present
invention, are
known as lysophospholipids. The present invention also extends to cover use of
liposomes in which the active component is the dimeric form of PG, namely
cardiolipin but other dimers of Formula I are also suitable. Preferably, such
dimers
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
are not synthetically cross-linked with a synthetic cross-linking agent, such
as
maleimide but rather are cross-linked by removal of a glycerol unit as
described by
Lehniger, Biochemistry, p. 525 (1970) and depicted in the reaction below:
R-CO-O-CH2
R~-CO-O- ~ H O
CH2-O-P-O-CH2CH(OH)CH20H
O-
PG
R-CO-O- ~ H2 ~ H2-O-CO-R
R'-CO-O-CH O O CH-O-CO-R~
H - PO-II-O,CH2CH(OH)CH20-II-O-IH2
-
cardiolipin
HOCH2CH(OH)CH20H
where each R and R' are independently as defined above.
As noted above and again without being limited to any one theory, the PG group
and
its dimer are believed to be a ligand since it is believed that it binds to a
specific site
on a protein or other molecule ("PG receptor") and, accordingly, this molecule
of
phosphatidylglycerol (and its dimeric form) is sometimes referred to herein as
a
"ligand" or a "binding group." Such binding is believed to take place through
the
phosphate-glycerol group -O-P(=O)(OH)-O-CHz-CH(OH)-CHZ-OH, which is
sometimes referred to herein as the "head group," "active group," or "binding
group." In view of the above, reference to "binding," "binding group," or
"ligand"
herein is not to infer any mechanism or mode of action. Nevertheless, it is
believed
that the above phosphate-glycerol groups are presented on the exterior
surfaces of
11
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
the bodies of the present invention for interaction with components of the
patient's
immune system. This interaction, it should be noted, is not the same as the
specific
interaction of apoptotic cells with the phosphatidylserine receptor on antigen
presenting cells.
Examples of "three-dimensional body portions" or pharmaceutically acceptable
bodies" include biocompatible synthetic or semi-synthetic entities such as
liposomes, solid beads, hollow beads, filled beads, particles, granules and
microspheres of biocompatible materials, natural or synthetic, as commonly
used in
the pharmaceutical industry. The beads may be solid or hollow, or filled with
biocompatible material. The term "biocompatible" refers to substances which in
the
amount employed are either non-toxic or have acceptable toxicity profiles such
that
their use in vivo is acceptable. Likewise the term "pharmaceutically
acceptable" as
used in relation to "pharmaceutically acceptable bodies" refers to bodies
comprised
of one or more materials which are pharmaceutically acceptable and suitable
for
delivery in vivo. Such bodies can include liposomes formed of lipids, one of
which
is PG. Alternatively, the pharmaceutically acceptable bodies can be solid
beads,
hollow beads, filled beads, particles, granules and microspheres of
biocompatible
materials, which comprise one or one or more biocompatible materials such as
polyethylene glycol, poly(methylmethacrylate), polyvinylpyrrolidone,
polystyrene
and a wide range of other natural, semi-synthetic and synthetic materials,
with
phosphate-glycerol groups attached thereto.
As noted above, analogues of phosphatidylglycerol with modified active groups,
which also interact with PG receptors on the antigen presenting cells, through
the
same receptor pathway as PG or otherwise resulting in an anti-inflammatory
reaction
in the recipient body are contemplated within the scope of the term
phosphatidylglycerol. This includes, without limitation, compounds in which
one or
more of the hydroxyl groups and/or the phosphate group is derivatized, or in
the
form of a salt. Many such compounds form free hydroxyl groups in vivo, upon or
subsequent to administration and, accordingly, comprise convertible PG groups.
12
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
Preferred compositions of matter are liposomes, which may be composed of a
variety of lipids. Preferably, however, none of the lipids are positively
charged. In
the case of liposomes, phosphatidyl glycerol PG may constitute the major
portion or
the entire portion of the liposome layers) or wall(s), oriented so that the
phosphate-
glycerol group portion thereof is presented exteriorly, to act as the binding
group,
and the lipid chain or chains form the structural wall.
Liposomes, or lipid vesicles, are sealed sacs, in the micron or sub-micron
range, the
walls (monolayer or multilayer) of which comprise suitable amphiphiles. They
normally contain an aqueous medium, although for the present invention the
interior
contents are unimportant, and generally inactive. Accordingly, in a preferred
embodiment, the liposomes, as well as other pharmaceutically acceptable
bodies, are
essentially free of non-lipid pharmaceutically active entities (e.g. <1%) and
more
preferably are free of non-lipid pharmaceutically acceptable entities. Such
liposomes are prepared and treated so that the active groups are presented
exteriorly
on the liposomal body. The PG in the liposomes of the preferred embodiments of
this invention thus serves as both a ligand and a structural component of the
liposome itself.
Thus a preferred embodiment of this invention provides liposomal bodies which
expose or can be treated or induced to expose, on their surfaces, one or more
phosphate-glycerol groups to act as binding groups. Phosphatidylglycerol is a~
preferred PG ligand and such lipids should comprise from 10% - 100% of the
liposome, with the balance being an inactive constituent, e.g.
phosphatidylcholine
PC, or one which acts through a different mechanism, e.g. phosphatidylserine
PS, or
mixtures of such. Inactive co-constituents such as PC are preferred.
At least 10% by weight of such liposome is composed of PG, preferably at least
SO%, more preferably from 60-100% and most preferably from 70-90%, with the
single most preferred embodiment being about 75% by weight of PG.
13
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
Mixtures of PG liposomes with inactive liposomes and/or with liposomes of
phospholipids acting through a different mechanism can also be used, provided
that
the total amount of PG remains above the minimum of about 10% and preferably
above 60% in the total mixture.
As regards to non-liposomal bodies for use in the present invention, these as
noted
to include biocompatible solid or hollow beads of appropriate size. The
biocompatible non-liposomal synthetic or semi-synthetic bodies may be selected
from polyethylene glycol, poly(methylmethacrylate), polyvinylpyrrolidone,
polystyrene and a wide range of other natural, semi-synthetic and synthetic
materials, with phosphate-glycerol groups attached to the surfaces thereof.
Such
materials include biodegradable polymers, such as disclosed by Dunn, et al.
U.S.
Patent 4,938,763, which is hereby incorporated by reference in its entirety.
Biodegradable polymers are disclosed in the art and include, for example,
linear-
chain polymers such as polylactides, polyglycolides, polycaprolactones,
polyanhydrides, polyamides, polyurethanes, polyesteramides, polyorthoesters,
polydioxanones, polyacetals, polyketals, polycarbonates, polyorthocarbonates,
polyphosphazenes, polyhydroxybutyrates, polyhydroxyvalerates, polyalkylene
oxalates, polyalkylene succinates, poly(malic acid), poly(amino acids),
polyvinylpyrrolidone, polyethylene glycol, polyhydroxycellulose, chitin,
chitosan,
and copolymers, terpolymers and combinations thereof. Other biodegradable
polymers include, for example, gelatin, collagen, etc.
Suitable substances for derivatization to attach the phospholipid(s), or
portions
thereof with groups or binding groups, to three-dimensional bodies are
commercially
available e.g. from Polysciences Inc., 400 Valley Road, Warnngton, PA 18976,
or
from Sigma Aldrich Fine Chemicals. Methods for their derivatization are known
in
the art. Specific preferred examples of such methods are disclosed in
International
Patent Application PCT/CA02/01398 Vasogen Ireland Limited, which is
14
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
incorporated herein by reference.
It is contemplated that the patient may be a mammal, including but not limited
to
humans and domestic animals such as cows, horses, pigs, dogs, cats and the
like.
Phospholipids are amphiphilic molecules (i.e. amphiphiles), meaning that the
compound comprises molecules having a polar water-soluble group attached to a
water-insoluble hydrocarbon chain. The amphiphiles serving as the layers of
the
matrix have defined polar and apolar regions. The amphiphiles can include, in
addition to PG in this invention, other, naturally occurring lipids used alone
with the
phospholipid carrying the active group, or in a mixture with another. The
amphiphiles serving as the layers) of the liposomes can be inert, structure-
conferring synthetic compounds such as polyoxyethylene alkylethers,
polyoxyethylene alkylesters and saccharosediesters.
Methods of preparing liposomes of the appropriate size are known in the art
and do
not form part of this invention. Reference may be made to various textbooks
and
literature articles on the subject, for example, the review article "Liposomes
as
Pharmaceutical Dosage Forms", by Yechezkel Barenholz and Daan J. A.
Chrommelin, and literature cited therein, for example New, R. C. "Liposomes: A
Practical Approach", IRL Press at Oxford University Press (1990).
The diameter of the liposomes, as well as the other pharmaceutically
acceptable
bodies, of the preferred embodiment of this invention is from about 20 nm to
about
500 pm, more preferably from about 20 nm to about 1000 nm, more preferably
from
about 50 nm to about 500 nm, and most preferably from about 80 nm to about 120
nm (preferably measured along its longest axis). In one embodiment, the
diameter
of the liposome is from 60nm to SOO~m.
The pharmaceutically acceptable bodies may be suspended in a pharmaceutically
acceptable carrier, such as physiological sterile saline, sterile water,
pyrogen-free
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
water, isotonic saline, and phosphate buffer solutions (e.g. sterile aqueous
solutions
comprising phosphate buffer), as well as other non-toxic compatible substances
used
in pharmaceutical formulations, such as, for example, adjuvants, buffers,
preservatives, and the like. Preferably, the pharmaceutically acceptable
bodies are
constituted into a liquid suspension in a sterile biocompatible liquid such as
buffered
saline and administered to the patient by any appropriate route which exposes
it to
one or more components of the immune system, such as intra-arterially,
intravenously or most preferably intramuscularly or subcutaneously.
It is contemplated that the pharmaceutically acceptable bodies may be freeze-
dried
or lyophilized so that they may be later resuspended for administration. This
invention is also directed to a kit of part comprising lyophilized or freeze-
dried
binding group-carrying bodies and a pharmaceutically acceptable Garner, such
as
physiological sterile saline, sterile water, pyrogen-free water, isotonic
saline, and
phosphate buffer solutions (e.g. sterile aqueous solutions comprising
phosphate
buffer), as well as other non-toxic compatible substances used in
pharmaceutical
formulations, such as, for example, adjuvants, buffers, preservatives, and the
like.
Protectants for freeze drying, as known in the_ art, for example lactose or
sucrose,
may also be included.
A preferred manner of administering the pharmaceutically acceptable bodies to
the
patient is a course of injections, administered daily, several times per week,
weekly
or monthly to the patient, over a period ranging from a week to several
months. The
frequency and duration of the course of the administration is likely to vary
from
patient to patient, and according to the condition being treated, its
severity, and
whether the treatment is intended as prophylactic, therapeutic or curative.
Its design
and optimization is well within the skill of the attending physician.
Intramuscular
injection, especially via the gluteal muscle, is most preferred. One
particular
injection schedule, in at least some of the indications of the invention, is
an
injection, via the gluteal muscle, of an appropriate amount of bodies on day
1, a
further injection on day 2, a further injection on day 14, and then "booster"
16
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
injections at monthly intervals, if appropriate.
It is postulated that, in many embodiments of the present invention,
pharmaceutically acceptable bodies comprising the PG groups as binding groups
on
their surface are acting as modifiers of the patient's immune system, in a
manner
similar to that of a vaccine. Accordingly they are used in quantities and by
administration methods to provide a sufficient localized concentration of the
bodies
at the site of introduction. Quantities of such bodies appropriate for immune
system
modification may not be directly correlated with body size of a recipient and
can,
therefore, be clearly distinguished from drug dosages, which are designed to
provide
therapeutic levels of active substances in a patient's bloodstream and
tissues. Drug
dosages are accordingly likely to be much larger than immune system modifying
dosages.
The correlation between weights of liposomes and numbers of liposomes is
derivable from the knowledge, accepted by persons skilled in the art of
liposomal
formulations, that a 100 nm diameter bilayer vesicle has 81,230 lipid
molecules per
vesicle, distributed approximately 50:50 between the layers (see Richard
Harrigan -
1992 University of British Columbia PhD Thesis "Transmembrane pH gradients in
liposomes (microform): drug-vesicle interactions and proton flux", published
by
National Library of Canada, Ottawa, Canada (1993); University Microfilms order
no. UMI00406756; Canadiana no. 942042220, ISBN 0315796936). From this one
can calculate, for example, that a dose of 5 x 10g vesicles, of the order of
the dose
used in the specific in vivo examples below, is equivalent to 4.06 x 10'3
lipid
molecules. Using Avogadro's number for the number of molecules of lipid in a
gram molecule (mole), 6.023 x 1023, one determines that this represents 6.74 x
10-~ ~
moles which, at a molecular weight of 729 for PG is approximately 3.83 x 10-
ggm,
or 38.3 ng of PG for such dosage.
The quantities of the pharmaceutically acceptable bodies to be administered
will
vary depending on the nature of the mammalian disorder it is intended to treat
and
17
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
on the identity and characteristics of the patient. Preferably, the effective
amount of
pharmaceutically acceptable bodies is non-toxic to the patient, and is not so
large as
to overwhelm the immune system. When using infra-arterial, intravenous,
subcutaneous or intramuscular administration of a sterile aqueous suspension
of
pharmaceutically acceptable bodies, it is preferred to administer, for each
dose, from
about 0.1-SO ml of liquid, containing an amount of bodies generally equivalent
to
10% - 1000% of the number of leukocytes normally found in an equivalent volume
of whole blood. Preferably, the number of bodies administered per delivery to
a
human patient is in the range from about 500 to about 2.5 x 109 (<250 ng of
bodies,
in the case of liposomes, pro-rated for density differences for other
embodiments of
bodies), more preferably from about 1,000 to about 1,500,000,000, even more
preferably 10,000 to about 50,000,000, and most preferably from about 200,000
to
about 2,000,000.
Since the pharmaceutically acceptable bodies are acting, in the process of the
invention, as immune system modifiers, in the nature of a vaccine, the number
of
such bodies administered to an injection site for each administration maybe a
more
meaningful quantitation than the number or weight of bodies per unit of
patient body
weight. For the same reason, it is now contemplated that effective amounts or
numbers of bodies for small animal use may not directly translate into
effective
amounts for larger mammals (i.e. greater than 5 kg) on a weight ratio basis.
The present invention is indicated for use in prophylaxis and/or treatment of
a wide
variety of mammalian disorders where T-cell function, inflammation,
endothelial
dysfunction and inappropriate cytokine expression are involved. A patient
having or
suspected of having such a disorder may be selected for treatment. "Treatment"
refers to a reduction of symptoms, such as, but not limited to, a decrease in
the
severity or number of symptoms of the particular disease or a limit on the
further
progression of symptoms.
With respect to T-cell function (T-cell mediated) disorders, these disorders
include
18
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
any and all disorders mediated at least in party by T-cells and include for
example,
ulcers, wounds, and autoimmune disorders including, but not limited to
diabetes,
scleroderma, psoriasis and rheumatoid arthritis.
The invention is indicated for use with inflammatory allergic reactions, organ
and
cell transplantation reaction disorders, and microbial infections giving rise
to
inflammatory reactions. It is also indicated for use in prophylaxis against
oxidative
stress and/or ischemia reperfusion injury, ingestion of poisons, exposure to
toxic
chemicals, radiation damage, and exposure to airborne and water-borne irritant
substances, etc., which cause damaging inflammation. It is also indicated for
inflammatory, allergic and T-cell-mediated disorders of internal organs such
as
kidney, liver, heart, etc.
With respect to disorders involving inappropriate cytokine expression for
which the
present invention is indicated, these include any and all disorders involving
inappropriate cytokine expression and include, for example, neurodegenerative
diseases. Neurodegenerative diseases, including Down's syndrome, Alzheimer's
disease and Parkinson's disease, are associated with increased levels of
certain
cytokines, including interleukin-1~3 (IL-1(3) (see Griffin WST et al. (1989);
Mogi M.
et al. (1996)). It has also been shown that IL-1~3 inhibits long-term
potentiation in
the hippocampus (Murray, C. A, et al. (1998)). Long-term potentiation in the
hippocampus is a form of synaptic plasticity and is generally considered to be
an
appropriate model for memory and learning (Bliss, T.V.P. et al. (1993)). Thus,
inappropriate cytokine expression in the brain is currently believed to be
involved in
the development and progression of neurodegenerative diseases and
neuroinflammatory disorders.
Thus, the invention is indicated for the treatment and prophylaxis of a wide
variety
of mammalian neurodegenerative and other neurological disorders, including
Downs
syndrome, Alzheimer's disease, Parkinson's disease, senile dementia,
depression,
Huntingdon's disease, peripheral neuropathies, Guillain Barr syndrome, spinal
cord
19
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
diseases, neuropathic joint diseases, chronic inflammatory demyelinating
disease,
neuropathies including mononeuropathy, polyneuropathy, symmetrical distal
sensory neuropathy, neuromuscular junction disorders, myasthenias and
amyotrophic lateral sclerosis (ALS). Treatment and prophylaxis of these
neurodegenerative diseases represents a particularly preferred embodiment of
the
invention, with treatment of Alzheimer's disease, Parkinson's disease and ALS
particularly preferred.
Regarding disorders involving endothelial dysfunction, the present invention
is
indicated for the treatment and prophylaxis of a wide variety of such
mammalian
disorders including, any and all disorders mediated at least in part by
endothelial
dysfunction and include, for example, cardiovascular diseases, such as
atherosclerosis, peripheral arterial or arterial occlusive disease, congestive
heart
failure, cerebrovascular disease (stroke), myocardial infarction, angina,
hypertension, etc., vasospastic disorders such as Raynaud's disease, cardiac
syndrome X, migraine etc., and the damage resulting from ischemia (ischemic
injury
or ischemia-reperfusion injury). In summary, it can be substantially any
disorder the
pathology of which involves an inappropriately functioning endothelium.
Further indications for the compositions and processes of the present
invention
include the treatment of patients to accelerate their rate of wound healing
and ulcer
healing, and treatment of patients prior to surgical operations, to accelerate
their rate
of recovery from surgery including their rate of healing of surgical wounds
and
incisions.
In regard to "cardiac disorders," the present invention is indicated for the
treatment
and prophylaxis of a wide variety of such mammalian disorders including, any
and
all disorders relating to the heart and include, for example, ventricular
arrhythmias
(ventricular tachycardia or fibrillation) and sudden death from heart disease.
Susceptibility of patients to cardiac disorders such as arrhythmias and sudden
cardiac death is often indicated by prolonged QT-c intervals in the heart beat
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
rhythm. Administration of compositions according to the preferred embodiments
of
the invention is believed to reduce QT-c intervals in mammalian patients,
indicative
of reduced susceptibility of to arrhythmia and sudden cardiac death.
The invention is further described, for illustrative purposes, in the
following non-
limiting examples.
EXAMPLES
In the examples below, the following abbreviations have the following
meanings. If
an abbreviation is not defined, it has it generally acceptable meaning.
~g - microgram
pL - microliter
~m - micrometer
pM - micromolar
CHS contact hypersensitivity
-
cm - centimeter
DMSO dimethylsulfoxide
-
DNFB 2,4-dinitrofluorobenzene
-
DHS delayed-type hypersensitivity
-
EtOH ethanol
-
g - gram
hrs hours
-
Hz - hertz
IM - intramuscular
IP - intraperitoneal
kg - kilogram
LPS lipopolysaccharide
-
LTP long-term potentiation
-
mg - milligram
min minutes
-
21
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
ml - milliliter
mM - millimolar
ms - millisecond
ng - nanogram
nm - manometer
nM - nanomolar
PBS - phosphate-buffered saline
PCR - polymerase chain reaction
POPS - 1-Palmitoyl-2-Oleoyl-sn-Glycero-3-[phospho-L-serine],
referred to
herein as PS
POPG - 1-Palmitoyl-2-Oleoyl-sn-Glycero-3-[phospho-rac-(1-glycerol)]],
referred to herein as PG
POPC - 1-Palmitoyl-2-Oleoyl-sn-Glycero-3-phosphocholine,
referred to
herein as PC
RPM - revolutions per minute
S - second
Unless otherwise stated, the precise form of the lipids used in the
experiments was
POPS, POPG and POPC as set out above.
Example 1
Liposomes of 100 ~ 20 nm in average diameter were prepared according to
standard
methods known in the art and had the following compositions:
Group A - 100% PS
Group B - 100% PG
Group C - control, no liposomes.
A stock suspension of each liposome composition containing 4.8 x 10'4
liposomes
per ml was diluted with PBS to give an injection suspension containing 6 x 106
particles per ml. The liposomal suspensions were injected into female BALB/c
mice
(Jackson Laboratories) aged 6-8 weeks and weighing 19-23 g, to determine the
22
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
effect on ear swelling in the murine contact hypersensitivity (CHS) model. The
CHS model tests for Thl-mediated inflammatory reactions.
The animals were assigned to one of 3 groups, with 5 animals in each group.
Groups A and B received approximately 3 x 105 of the above-identified
liposomes
(i.e., 100% PC and 100% PG, respectively), in a volume of approximately 50
~.1.
Group C was a control group, receiving no liposomes.
Protocol
The following experiments were performed:
TABLEI
Lipo- Day
7
GroupsomesDay 1 Day Day Day Day Day 6 (24
2 3 4 5
hours)
100% Injected Injected
A Ear
PS then InjectedInjectedInjectedInjectedthen
m easured
sensitized challenged
Injected Injected
100% Ear
B then InjectedInjectedInjectedInjectedthen
PG measured
sensitized challenged
On Days 1-6, mice of Groups A and B were injected with the respective
liposomes
preparations. Approximately 300,000 liposomes were injected in 50 ~1 volume
via
intramuscular (IM) injection, for a total administration over the test period
of about
1,800,000 liposomes. Mice of the control group (Group C) received no
liposomes,
23
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
but were sensitized, challenged and tested in the same way as Groups A and B,
as
described below.
Sensitization
On Day l, following liposome injection for that day, mice were anaesthetized
with
0.2 ml 5 mg/ml sodium pentobarbital via IP injection. The abdominal skin of
the
mouse was sprayed with 70% EtOH and a scalpel blade was used to remove about a
one-inch diameter patch of hair from the abdomen. The shaved area was then
painted with 25 ~,1 of 0.5% 2,4-dinitrofluorobenzene (DNFB) in 4:1 acetone:
olive
oil using a pipette tip.
Challenge
Following liposome injection on day 6, mice were challenged with DNFB by
painting 10 ~,l of 0.2% DNFB on the dorsal surface of the right ear with a
pipette tip
and by painting 10 ~.1 of vehicle on the left ear with a pipette tip.
Rac»ltc
On Day 7, 24 hours after challenge, each animal was anaesthetized with
Halothane,
and ear thickness was measured using a Peacock spring-loaded micrometer. Data
was expressed as the difference between the treated right ear thickness and
the
thickness of the vehicle-treated left ear. The experiments were repeated three
times,
on similar animals. Increase in ear swelling was used as a measure of CHS
response. The significance of the data was determined by the two-tailed
student's t-
test. A P value of <0.05 was considered significant.
The results are presented in FIG. 1, a bar graph showing the mean values from
the
three experiments of ear swelling, reported in Vim.
FIG. 1 shows that a significant reduction in ear swelling was achieved by
injection
of liposomes according to the present invention. The reduction achieved with
100%
24
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
PG liposomes is substantially greater than that from 100% PS liposomes.
Example 2
Liposomes of 100 ~ 20 nm in average diameter were prepared according to
standard
methods known in the art and had the following compositions:
Group A - 100% PG
Group B - 75% PG, 25% PC
Group C - 50% PG, 50% PC
Group D - 25% PG, 75% PC
Group E - PBS only
Group F - no injection
A stock suspension of each liposome containing 4:8 x 10'4 liposomes per ml was
diluted to give an injection suspension containing 12 x 106 liposomes per ml.
The
liposomal suspensions were used to inject into mice to determine the effect on
ear
swelling in the murine CHS model, a biological system useful for assaying Thl-
mediated inflammatory reactions. For these experiments, female BALB/c mice
(Jackson Laboratories) aged 6-8 weeks and weighing 19-23 g were used.
The animals were assigned to one of 6 groups (Groups A-F, above) with 10
animals
in each group. Control groups were also included that received no injections
(Group
F) or injections of PBS with no liposomes (Group E). Animals in Groups A-D
were
injected with SO~tI of the above-identified liposome suspensions, each
containing
about 6 x 105 liposomes.
Protocol
The test involves sensitization (Sens) with a potentially inflammation-causing
substance, injection of liposomes (Inj) in test animals or PBS in controls and
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
challenge (Chal) with the potentially inflammation-causing substance following
measurement (Meas) to determine whether the injection of liposomes are
effective
against the development of inflammation by the challenge.
The following experiments were performed:
GroupLipo- Day Day Day Day Day Day Day
1 6
somes 2 3 4 5 7
A 100% Sens Inj Inj Inj Inj Chal Meas
PG & &
Inj Inj
B 75% PG Sens Inj Inj Inj Inj Chal Meas
& &
Inj Inj
C 50% PG Sens Inj Inj Inj Inj Chal Meas
& &
Inj Inj
D 25% PG Sens Inj Inj Inj Inj Chal Meas
& &
Inj Inj
E None Sens Inj Inj Inj Inj Chal Meas
& &
(PBS Inj Inj
only)
F none Sens Chal Meas
On days 1-6 the mice were injected with the respective liposomes as indicated
above. Liposomes were injected in SOpI volume via IM injection, i.e., 600,000
liposomes per injection, for a total administration over the test period of
3,600,000
liposomes. Mice of the control group received no liposomes but were
sensitized,
challenged and tested in the same way as the other groups of mice, as
described
below.
Sensitization (Sens)
On Day 1, following liposome injection for that day, mice were anaesthetized
with
0.2 ml 5 mg/ml sodium phenobarbital via IP injection. The abdominal skin of
the
26
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
mouse was sprayed with 70% EtOH and a blade was used to remove about a one
inch diameter of hair from the abdomen. The bare area was painted with 25 ~1
of
0.5% 2,4-dinitrofluorobenzene (DNFB) in 4:1 acetone: olive oil using a pipette
tip.
Challenge (Chal)
On Day 6, following liposomes injection for that day, mice were challenged
(Chat)
with DNFB as follows: 10 pl of 0.2% DNFB was painted on the dorsal surface of
the right ear with a pipette tip and 10 pl of vehicle was painted on the left
ear with a
pipette tip.
Results
On Day 7, 24 hours after challenge, each animal was anaesthetized with
Halothane,
and ear thickness was measured (Meas) using a Peacock spring-loaded
micrometer.
Increase in ear swelling was used as a measure of CHS response. Data was
expressed as the difference in the treated right ear thickness minus the
thickness of
the vehicle treated left ear. The significance between the two groups is
determined
by a two-tailed student's t-test. A P value of <0.05 is considered
significant.
The results are presented graphically in FIG. 2, a bar graph showing ear
swelling in
pm. The mean value from the respective experiments was used in compiling the
graph.
FIG. 2 shows that a significant reduction in ear swelling with both 100 and
75% PG
is achieved, showing that both these concentrations protect against the
development
of inflammation resulting from contact with the allergenic substance, DNFB.
The
50% and the 25% PG liposomes also showed reductions as compared with both
controls, but the differences did not reach statistical significance in this
experiment.
27
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
Example 3
Liposomes of 100 ~ 20 nm in average diameter were prepared according to
standard
methods known in the art and were composed of 75% PG, 25% PC. A stock
suspension containing 4.8 x 10'4liposomes per ml was used as before and
diluted in
PBS to give an injection suspension containing the following concentrations of
liposomes:
GroupLiposomes Concentration Liposomes Animals
per in
(liposomes per injection Group
mL)
A 75% PG, 12 x 10" 6 x 10"' 10
25%
PC
B 75% PG, 12 x l0y 6 x lOx 10
25%
PC
C 75% PG, 12 x 10 6 x 10' 16
25%
PC
D 75% PG, 12 x 10' 6 x 10 16
25%
PC
E 75% PG, 12 x 10 6 x 10' 16
25%
PC
F none (PBS 16
only)
BALB-c mice were divided into six groups (Groups A-F) including a control
group
receiving no liposomes but injected with 50 ~,L of PBS (Group F). Mice were
sensitized on the flank, injected with their selected liposomal dose,
intramuscularly
to the right leg muscle, on the same day as, but after, sensitisation (day I)
and on
days 2, 3, 4, and 5. On day 6 they were both injected and challenged on the
ear as
described in Example 1. The thickness of the ear was measured as described 24
hours after the challenge.
28
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
The results (FIG. 3) show a significant difference between the control group
(Group
F) and Group C (12 x 10g liposomes per ml) and between the control group and
Group D (12 x 10' liposomes per ml) and between the control group and Group E
(12 x 10~ liposomes per ml). There was little difference between the control
group
and Groups A or B (12 x 10~ I and 12 x 10~ liposomes per ml, respectively),
suggesting that there is an optimum range of liposome concentrations above
which
the beneficial effects may be reduced. In other experiments, a decrease in
effect was
also be observed as the concentration of the liposomes was decreased below 12
x
104 liposomes per ml.
Example 4
Liposomes of formulation 100% PG and 100120 nm in average size were prepared
according to standard methods. Four groups (Groups A-D) of 10 mice were
sensitised, injected and challenged in accordance with the procedure and
schedule
described in Example 3, with the following numbers of 100% PG liposomes
delivered in a 50 pl suspension.
Group A - 6 x 10'
Group B - 6 x 106
Group C - 6 x 1 O5
Group D - 6 x 104
The results, along with the PBS control from Example 4, are presented in
similar bar
graph form in FIG. 4. A significant reduction in ear swelling, as compared
with the
control group is to be noted for each of the test groups, but with little
difference
between the various groups.
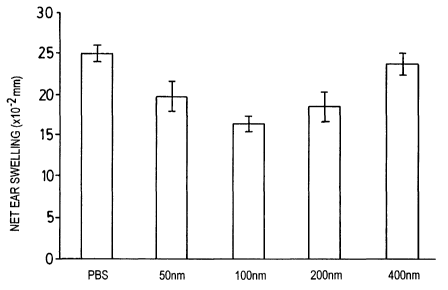
Examule 5
Liposomes of composition 75% PG, 25% PC and of 50, 100, 200, of 400 nm in
average diameter were prepared by standard methods. They were tested in the
murine CHS model, as in Examples 3 and 4, using 6 x 105 liposomes in 50 pl
suspensions for each injection, and a sensitisation-injection-challenge
schedule and
29
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
procedure as in The groups were
Example 3. as follows:
Group A - 50 nm liposomes
Group B - 100 nm liposomes
Group C - 200 nm liposomes
Group D - 400 nm liposomes
Group E - no liposomes
The results are presented in FIG. 5. The result from Group D, using the 400 nm
diameter liposomes, is not significantly different from the control group
(Group E),
indicating a probable size range criticality in this model.
Example 6
A stock suspension of 75% PG liposomes of 100~20 nm in average diameter
containing 4.8 X 104 liposomes per ml was diluted to give an injection
suspension
containing 6 x 105 liposomes per ml. The liposomal suspensions were used to
inject
into mice, to determine the effect on ear swelling in the murine DHS model. As
in
Example 1, female BALB/c mice (Jackson Laboratories) aged 6-8 weeks and
weighing 19-23 g were used.
The animals were assigned to one of 3 groups with 10 animals in each group. A
control group (Group C) received only PBS injections. Animals of Groups A and
B
were injected with 50 ~1 of a suspension containing 6 x 105 liposomes.
D....4......1
On days 13-18 the mice were injected with the 75% PG liposomes as indicated
below. Liposomes were injected in 50 pl volume via IM injection, i.e., 600,000
liposomes per injection, for a total administration over the test period of
3,600,000
liposomes. Sensitization and challenge took place as described in Example 2.
DAY TREATMENT
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
1 Sensitized
6 Challenged
7 Measured
12 Challenged
13 Measured & Injected
14 Injected
15 Injected
16 Measured & Injected
17 Injected
18 Injected & Challenged
19 Measured
Results
The results are presented graphically in accompanying FIG. 6 and show that 75%
PG is effective in the DHS model on day 16, 24 hours after the third injection
following the second challenge.
Example 7
Liposomes of composed of 100% cardiolipin (CL) and 10020 nm in average
diameter were prepared, by standard methods. These were used at a dosage of 6
x
105 liposomes per 50 ~1 per injection in the murine DHS model described in
Example 6. Data obtained from animals injected with CL liposomes (Group A; 10
animals) was compared to data obtained from animals receiving only PBS (Group
B; 10 animals). The sensitisation, injection and challenge procedures were as
described in Example 2. The ear thickness measurement results, taken on day
19, 24
hrs after the 6'h injection, are presented in FIG. 7. The results showed a
significant
reduction in ear swelling within the CL-injected test (Group A).
31
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
Example 8
Liposomes of 100 nm in average diameter, and comprising either 100%
cardiolipin
or 75% cardiolipin and 25% PC, were prepared by standard methods. Three groups
(Groups A-C) of 10 mice were sensitised on day 1. A control group received
injections of PBS on days 1, 2 and 6 (Group C). The other two groups received
injections, of 6 x 105 100% cardiolipin liposomes (Group A) or of 6 x 105 75%
cardiolipin liposomes (Group B), liposomes in 50 ~1 per injection according to
the
same schedule. The mice were challenged on day 7, and the ear thickness
measured,
as described in the previous examples.
FIG. 8 shows the mean measurements in each group. Both groups receiving CL
liposomes showed a statistically significant suppression of CHS compared to
the
control group
Example 9
To study the cellular and molecular mechanisms underlying cognitive function,
the
Long-Term Potentiation (LTP) animal model is used. LTP is a form of synaptic
plasticity that occurs in the hippocampal formation, which has been proposed
as a
biological substrate for learning and memory (Bliss et al. Nature 361:31-39
(1990)).
LTP in rats is monitored electrophysiologically by methods well known to those
in
the art. The animals are then sacrificed to investigate biochemical changes in
hippocampal tissues. Comparing the results of electrophysiological data with
biochemical hippocampal changes is useful for determining how the cellular
events
that underlie LTP may be altered in animals suffering from diseases or
disorders
associated with neuroinflammation such as aging, stress, Alzheimer's disease,
and
bacterial infection.
Systemic administration of lipopolysaccharide (LPS), a cell-wall component of
Gram-negative bacteria, provokes an activation of the immune system by
inducing
an increase in pro-inflammatory cytokines such as IL-1(3. As noted above, one
32
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
example of a neuronal deficit induced by LPS and IL-1(3 is the impairment of
LTP in
the hippocampus. An indicator of LTP is the mean slope of the population
excitatory post-synaptic potential (epsp). Upon tetanic stimulation, the epsp
slope
(%) increases sharply indicating increased synaptic activity. LPS-induced
inhibition
of LTP reduces the increase in slope, and/or causes the epsp slope to revert
more
rapidly to base line, indicating that the increased synaptic activity is short-
lived.
Accordingly measurements of the epsp slope (%) at timed intervals after
tetanic
stimulation can be used to reflect memory and the loss thereof following an
inflammatory stimulus as well as inflammation in the hippocampus of the brain.
Liposomes of 100~20 nm in average diameter were prepared as according to
standard methods known in the art and were composed of 75% PG and 25% PC. A
stock suspension of the liposomes containing about 2.9 x 104 liposomes per ml
was
diluted with PBS to give an injection suspension containing about 1.2 x 10~
liposomes per ml. This was then used to inject into rats, to determine the
effect on
LPS-induced impairment of LTP. For these experiments, male Wistar rats
(BioResources Unit, Trinity College, Dublin), weighing approximately 300 g,
were
used.
The animals were assigned to one of four groups, 8 animals in each group to be
treated as follows:
Group A - saline + control
Group B - saline + PG
Group C - LPS + control
Group D - LPS + PG
150 pl of each above-identified preparation was injected via IM injection on
days 1,
13, and 14. Groups B and D received a total of 5,400,000 liposomes (1,800,000
liposomes per injection). The LTP procedure and tissue~preparation procedure
were
carried out on day 0.
33
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
LTP Procedure
Rats were anaesthetized by IP injection of urethane (1.5 g/kg). Rats received
either
LPS (100 ~g/kg) or saline intraperitoneally. Three hours later a bipolar
stimulating
electrode and a unipolar recording electrode were placed in the perforant path
and in
the dorsal cell body region of the dentate gyros respectively. Test shocks of
0.033
Hz were given and responses recorded for 10 min before and 45 min after high
frequency stimulation (3 trains of stimuli delivered at 30 s intervals, 250 Hz
for 200
ms).
Rats were killed by decapitation. The hippocampus, the tetanized and
untetanized
dentate gyri, the cortex and entorhinal cortex were dissected on ice,
sectioned and
frozen in 1 ml of Krebs solution (composition of Krebs in mM: NaCI 136, KCl
2.54,
KHzPOa 1.18, MgS04.7H20 1.18, NaHC03 16, glucose 10, CaClz 1.13) containing
10% DMSO.
Results
The results are shown in FIG. 9. The graph shows the difference in the
excitatory
post-synaptic potential (epsp) recorded in cell bodies of the granule cells.
The data
presented are means of seven to eight observations in each treatment group and
are
expressed as mean percentage change in epsp slope every 30 s normalized with
respect to the mean value in the 5 minutes immediately prior to tetanic
stimulation.
FIG. 9 shows that the LPS-induced inhibition of LTP in perforant path-granule
cell
synapses was overcome by pre-treatment with the PG liposomes. The filled
triangles represent Group A (saline + control), the open triangles represent
Group B
(saline + PG), the filled squares represent Group C (LPS + control) and the
open
squares represent Group D (LPS + PG).
FIG. 10 shows that analysis of the mean values 40-45 minutes post tetanic
stimulation indicate that the population epsp slope was decreased in the
control-LPS
group (open bars) and that the PG liposomes (hashed bars) significantly
reversed
this effect (*p<0.01). As an index of memory and learning functionality, the
34
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
improvement in sustainability of LTP demonstrated in this Example indicates
suitability of the treatment for dementias e.g. Alzheimer's disease and memory
impairment.
Example 10
IL-4 is one of a number of cytokines secreted by the Th2 subclass of
lymphocytes
and is known for its anti-inflammatory effects. FIG. 11 shows that the IL-4
concentration in the hippocampus was significantly increased in the LPS group
that
had been pre-treated with the PG liposomes (*p<0.05). Open bars represent
control
group (Group E) and hashed bars represent the PG treated group (Group F). IL-4
was measured by ELISA and expressed as PG of IL-4 per mg of total protein.
This upregulation of the anti-inflammatory cytokine IL-4 in the brain is
indicative of
the use of the process and composition of preferred embodiments of the present
invention in treating a wide range of neuroinflammatory disorders, including
Parkinson's disease, ALS, chronic inflammatory demyelinating disease CIPD and
Guillain Barr syndrome.
Example 11
IL-1 (3 is one of a number of cytokines secreted by the Thl subclass of
lymphocytes
and is known for its proinflammatory effects. Spleens from animals treated as
described in Example 9, groups C and D thereof, were extracted and spleen
cells
collected. They were prepared as follows:
FIG. 12 shows that the IL-1 (3 concentration in spleen cells was significantly
reduced
in the LPS group that had been pre-treated with the PG liposomes (*p<0.05). IL-
1 (3
was measured by ELISA and expressed as picagrams of IL-1 (3 per mg of total
protein. This indicates a systemic inflammatory effect of the process and
compositions of preferred embodiments of the present invention.
Example 12
U937 is a monocytic leukemia cell line that can be differentiated into
macrophages
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
by administration of phorbol esters. Treatment with lipopolysaccharide (LPS),
a
component of the cell wall of Gram-negative bacteria, stimulates an
inflammatory
response in U937 cells, with the upregulation of expression of a number of
inflammatory molecules including TNFoc. This model provides an experimental
system for the assessment of anti-inflammatory therapies. The macrophages can
be
grown in culture medium in the presence of a suspected anti-inflammatory
composition, and the expression of TNFa, can be measured.
Liposomes of 100 ~ 20 nm in average diameter were prepared according to
standard
methods known in the art and had a composition of 75% phosphatidylglycerol
(PG),
25% phosphatidylcholine (PC). The stock concentration of liposome was about 40
mM lipid and was diluted to the following final concentrations in the assay:
100 pM phosphatidylglycerol (PG)
40~M PG
~M PG
4.0 ~M PG
1 pM PG
The U937 cells were cultured by growing in RPMI medium (GIBCO BRL)
supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin
and grown at 37°C in an atmosphere containing 5% CO2. 5 x 105 cells
were seeded
into wells of 6-well plates and caused to differentiated into macrophages by
treatment with 150 nM phorbol myristate acetate (PMA) for 2-3 days. The cell
medium was then replaced with complete medium after the U937 cells had
differentiated into macrophages. The cells were then incubated for an
additional 24
hrs to minimize pleotropic effects due to PMA treatment.
The cells were then incubated with either:
Group A Phosphate buffered saline (PBS) - as a negative control,
Group B 10 ng/ml LPS - as a positive control,
Group C 10 ng/ml LPS + 100 ~M PG,
36
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
Group 10 ng/ml LPS + 40 pM
D PG,
Group 10 ng/ml LPS + 10 pM
E PG,
Group 10 ng/ml LPS + 4.0 ~M
F PG, or
Group 10 ng/ml LPS + 1 pM PG.
G
The cells were incubated as described above at 37°C in 5% COz. After 18
hrs, the
supernatants from each treatment were collected and assayed for TNF-a using a
standard Quantikine Enzyme-Linked Immunosorbent Assay (ELISA) kit (R&D
systems, Minneapolis, USA).
Results
FIG. 13 shows the amount of secreted TNF-a in PG per ml. The results
demonstrates that U937-differentiated macrophage cells express very low levels
of
TNF-a under normal conditions. However, once exposed to LPS, they secrete
large
amounts of TNF-a into the, surrounding medium, which is indicative of cellular
stress occurring. Incubation of the cells with PG liposomes inhibits the
secretion of
TNF-a in a dose-dependent manner, with the highest concentration of 100~M
resulting in a 98% decrease, and even the lowest concentration of 1 pM causing
a
58% decrease in TNF-a expression.
Examule 13
To determine the effect of the PG liposomes of the preferred embodiment of the
present invention on endothelial function, the endothelin-1 (ET-1) content in
the ears
of mice which had been subjected to the CHS studies as described in Example 3
was
determined. Endothelin-1 is a potent vasoconstrictive agent, has inotropic and
mitogenic actions, modulates salt and water homeostasis and plays an important
role
in the maintenance of vascular tone and blood pressure. Various lines of
evidence
indicate that endogenous ET-1 may contribute to the pathophysiology of
conditions
associated with sustained vasoconstriction, such as heart failure. In heart
failure,
elevated levels of circulating ET-1 and big-ET-1 are observed (Giannessi D,
De1 Ry
37
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
S, Vitale RL. "The role of endothelins and their receptors in heart failure."
Pharmacol Res 2001 Feb 43:2 111-26). Thus ET-1 is a marker of endothelial
function and increased production of ET-1 in tissue is indicative of impaired
endothelial function.
In order to determine ET-1 expression, mouse ears (right challenged ear) were
harvested 24 hrs after challenge in CHS experiments. Ears were obtained from
mice injected intramuscularly with PBS for 6 days (Group A) and mice injected
intramuscularly with 75% PG/25% PC liposomes (600,000 liposomes/injection;
Group B). Ears were stored in RNAlater at -20°C until RNA extraction.
RNA was
extracted and cDNA was generated using reverse transcriptase (RT) along with
ET-
1-specific primers, as an internal control, PCR was also performed using 3-
actin-
specific primers. PCR products were resolved on a 1.5% agarose gel and the DNA
bands were quantitated by densitometry analysis. The ratio of ET-1/(3-actin
was
calculated.
38
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
PCR Preparation:
PCR Mix (ET-1) PCR Mix ((3-Actin)
5pl PCR Buffer (lOx)Spl PCR Buffer (10x)
1.5p1 MgCl2 (50mM) 1.5p1 MgCl2 (50mM)
1 pl dNTP ( 1 OmM) 1 ~1 dNTP ( 1 OmM)
0.5p1 Primer 1 (25uM)1 pl Primer 1 (lOuM)
0.5 pl Primer 2 (25uM)1 pl Primer 2 ( 1
OuM)
0.25w1 TAQ 0.251 TAQ
2.5p1 cDNA 2.5p1 cDNA
38p1 Water 37.75p1 Water
501 Total 50p1 Total
39
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
Primers : (as previously described - see for example Yang, L; Husain, M; and
Stewart. D.J., "Conditional cardiac overexpression of endothelin-1 in
transgenic
mice", FASEB J 15(S):Al 138 - Al 138 Part 2, MAR8 2001.
ET-1(r) 5'-CAG CAC TTC TTG TCT TTT TGG-3'
ET-1(f) 5'-CCA AGG AGC TCC AGA AAC AG-3'
(3-Actin(F) 5'-GTG GGC CGC TCT AGG CAC CAA-3'
(3-Actin(r) 5'-CTC TTT GAT GTC ACG CAC GAT TTC-3'
PCR Settings:
94°C - 5minutes
94°C - 30s
60°C - 30s 30 cycles
72°C - 60s
72°C - 10 minutes
4°C - Soak
After 6-daily injections of the 75% PG liposomes, the level of ET-1 was
decreased
by 36% relative to control mice receiving PBS during the same injection
regimen.
The results are shown graphically on FIG. 14. This decrease indicates a
beneficial
effect resulting from the injection of the liposomes of the preferred
embodiment of
the invention on endothelial function in a mammalian patient, through Thl
mediated
inflammation reduction.
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
Example 14
Intercellular adhesion molecule-1 (ICAM-1) is a cell surface molecule
expressed by
several cell types, including leukocytes and endothelial cells. It is involved
in the
adhesion of monocytes to endothelial cells and plays a role in inflammatory
processes and in the T-cell mediated host defense system. ICAM-1 expression
probably contributes to the clinical manifestations of a variety of diseases,
predominantly by interfering with normal immune function. Among these are
malignancies (e.g., melanoma and lymphomas), many inflammatory disorders
(e.g.,
asthma and autoimmune disorders), atherosclerosis, ischemia, certain
neurological
disorders, and allogeneic organ transplantation (Van de Stolpe A, van der Saag
PT,
"Intercellular adhesion molecule-1" J. Mol. Med. (1996) 74:1 13-33).
Human umbilical vein endothelial cells (HUVECs) are a primary cell line of
endothelial cells that are isolated from umbilical vein cords as follows.
T75 flasks were prepared by coating with 0.2% gelatin (5-7 ml/flask) for a
minimum
of 15/20minutes or overnight. The excess was then removed. The cord was
sprayed with 70% ethanol prior to procedure and any placenta still remaining
attached to the cord was cut away. The cord was then cut to an approximate
length
of 5-6 inches. The cord has two arteries which are thick walled and one vein
that is
bigger and thin walled. The vein was located and the serrated edge of a
stopper
placed into it. Approximately 20cm of string was then used to tie the cord
onto the
stopper.
The cord was then washed through with phosphate buffered saline (PBS) a number
of times until the PBS ran clear. Following this 15-20m1s of Collagenase
solution
was placed into the cord; it was wrapped in tinfoil and incubated for
l5minutes at
37°C. After incubation the tied end of the cord was cut and the
collagenase drained
into a SOmI tube. Collagenase was then passed through the cord again, the cord
was
massaged to loosen the endothelial cells and then PBS was passed through the
cord
41
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
and collected into the same tube containing the collagenase solution. This was
then
centrifuged at 1600RPMs, the supernatant removed and the pellet resuspended in
10-12 mls of M199 complete medium. Finally the medium containing the cells was
added to the gelatinized flasks.
Liposomes of 100 ~ 20 nm in average diameter were prepared according to
standard
methods known in the art and had a composition of 75% phosphatidylglycerol
(PG),
25% phosphatidylcholine (PC). The stock concentration of liposome was 40 mM
lipid and was diluted to 100 pM in the assay.
HUVECs split into a number of tissue culture flasks, allowed to adhere to the
surface of the flask and then treated as follows:
Group A - PBS - as a negative control,
Group B - 500 ng/ml LPS - as a positive control,
Group C - 500 ng/ml LPS + 100 pM PG
Group D - S00 ng/ml LPS + 100 pM PC
The cells were incubated at 37°C, 5% CO2. After 18 hrs, the
supernatants from each
treatment were collected and assayed for ET-1 using a standard ELISA kit
(obtained
from Assay Designs) and the cells harvested for analyzing ICAM-1 as follows.
The cells were first washed with PBS and then incubated with a cell
dissociation
buffer at 37°C for 25-30 min. The cells were then washed by
centrifugation and
incubated with an anti-CD54 (ICAM-1) antibody for 30 minutes. A secondary FITC
antibody was then added and incubated with the cells as before. Finally they
were
resuspended in 1 ml of PBS and analyzed for fluorescence on a flow cytometer.
Results
The results are presented on FIG. 15, a graphical presentation of the
percentage of
cells staining positive for ICAM-1 in the respective cultures. It is to be
noted that
the numbers of cells staining positive in the PG liposome-containing culture
is
42
CA 02471740 2004-06-25
WO 03/061667 PCT/CA03/00065
reduced to negative control level, and is much lower than the positive control
level.
Example 15
Microglial cells (brain macrophages) were cultured, and their output of TNF-a,
an
inflammatory cytokine, was measured. The cells were stimulated with the
immunoglobulin (IgG) of patients suffering from ALS, and the TNF-a output
increased about 800-fold as a result. When the same cells were grown in the
presence of both the ALS IgG and PG liposomes, output of TNF-a decreased by
about 75%, indicating the potential of the preferred embodiments of the
present
invention in the treatment of ALS. The results are presented in FIG. 16.
43