Note: Descriptions are shown in the official language in which they were submitted.
CA 02471910 2004-06-28
WO 03/055861 PCT/EP02/14514
Benzocyclodecane derivatives with antitumor activity
The present invention relates to benzocyclodecane derivatives, to a process
for their
preparation, to pharmaceutical compositions containing them, and to the use of
such
compounds in the prevention, control and treatment of cancer.
In the field of antitumor compounds, a specific class comprises compounds from
natural
sources acting by mitotic arrest through induced tubulin polymerization.
Examples of these
natural product:.-ire p~W taxel, isolated from Taxus Brevifolia, Sarcodictyins
A and B,
isolated in 1987 by Pietra et al. from the Mediterranean stoloniferan coral
Sarcodictyon
to f~oseufn, and the diterpene glycoside eleutherobin, isolated from an
Eleutlaerobia species
of australian soft coral.
Now, there is a strong need for simplified molecules, which nevertheless
maintain the
useful properties referred to above characterizing the natural products.
In J.Chem. Soc. 1967 (7), 565-568 there is described the synthesis of
benzocyclodecenone
derivatives, whithout any suggestion on their pharmacoloigcal activity.
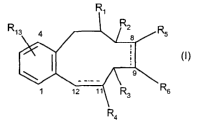
The present invention relates to a new class of antitumor compounds. In
particular, the
present invention provides a compound which is a benzocyclodecane of formula
(I)
R,
~
5
R6
Ra
(
wherein:
----- at positions 8-9 and 11-12 independently represents a single or double
bond,
-Rl represents oxygen (=O), or a residue -OR7, wherein R7 represents hydrogen,
linear or
branched C1-C7 alkanoyl, benzoyl, Cl-Clo alkyl, CZ-Clo alkenyl or a residue of
the formula
O
R8
CA 02471910 2004-06-28
WO 03/055861 PCT/EP02/14514
2
wherein R8 is an optionally substituted aryl or heterocyclyl;
-R2 and -R3 independently represents hydrogen, oxygen atom (=O) or a residue -
OR9,
wherein R9 represents hydrogen, CI-C7 alkanoyl or benzoyl;
when ----- at position 11-12 represents a single bond, then -R4 represents
- oxygen atom (=O),
- methylene (=CHz),
- =CHCOORIn, wherein RIO represents CI-CIO alkyl or optionally substituted
aryl;
=CH(OCH3),
- or a residue of formula -OR9, wherein R~ is as defined above; -CH20RI1
wherein RI l
l0 represents hydrogen or a sugar residue, -CORIZ wherein RIZ represents
hydrogen,
-OH or -ORIO, wherein RIO is as defined above; or
when ----- at position 11-12 represents a double bond, then -R4 represents a
residue of formula
-CH20RI1 or -CORI2 as defined above;
- RS and -R6 are both hydrogen atoms or, when ----- at position 8-9 represents
a single bond,
taken together with the carbon atoms to which they are attached form a
cyclopropane ring;
RI3 represents hydrogen or from one to three substituents selected from CI-C6
alkyl, Cz-C~
alkenyl, optionally substituted phenyl, phenyl CI-C6 alkyl, halogen, hydroxy,
CI-C6 alkoxy,
aryloxy, cyano, vitro, amino, CI-CIO alkylamino, arylamino, CI-C7
allcanoylamino,
aroylamino, hydroxycarbonyl, aminocarbonyl, CI-C6 alkylcarbonyl,
CI-C6 alkylaminosulfonyl and arylaminosulfonyl group;
with the provisos that if RI and R4 are both oxygen atom (=O), then one of R2,
R3, R5, Rb
and RI3 is not hydrogen atom; or a pharmaceutically acceptable salt thereof.
As used herein the teens "CI-CIO alkyl" and "CI-C6 alkyl" refer to a straight
or branched
chain alkyl moiety having respectively from 1 to 10 or from 1 to 6 carbon
atoms, including
for example, methyl, ethyl, propyl, isopropyl, n-butyl, i-butyl, sec-butyl,
tert-butyl, n-
pentyl, isopentyl, n-hexyl, n-heptyl and n-octyl.
The terms "CZ-CIO alkenyl" and "C2-C6 alkenyl" as used herein refer to a
straight or
branched chain alkenyl moiety having respectively from 2 to 10 and from 2 to 6
carbon
atoms and having in addition one double bond of either E or Z stereochemistry
where
applicable. Examples of alkenyl groups include: vinyl, allyl, metallyl,
butenyl and crotyl.
The term "aryl" as used herein refers to a monocyclic or bicyclic aromatic
hydrocarbon group
of 6 to 10 carbon atoms, such as phenyl, naphthyl, indanyl; furthermore,
"aryl" as used herein
CA 02471910 2004-06-28
WO 03/055861 PCT/EP02/14514
may refer to a diphenyl group (-C6Hq.-C6H5). The term "C1-C7 alkanoyl" refers
to acyl
residues such as formyl, acetyl, and pentanoyl groups.
The term "heterocyclyl" as used herein refers to a 3- to 7-membered,
substituted or
unsubstituted, saturated or unsaturated heterocyclyl ring, containing at least
one heteroatom
selected from O, S and N, any ring carbon may be oxidized as a carbonyl, and
wherein said
heterocyclyl ring may be optionally fused to a second 5- or 6-membered,
saturated or
unsaturated heterocyclyl ring, or to a C3 -C7 cycloalkyl ring, or to a benzene
or naphthalene
ring. Examples of heterocyclyl groups are pyrrolyl, pyrrolidinyl, pyrazolyl,
imidazolyl,
triazolyl, tetrazolyl, oxazolyl, isoxazolyl, thiazolyl, isothiazolyl,
thiadiazolyl, thienyl, .
to tetrahydrothienyl, furyl, tetrahydrofuryl, aziridinyl, oxiranyl,
azetidilryl, succinimido, pyridyl,
piperidinyl, pyrazinyl, piperazinyl, pyridazinyl, hexahydropyridazinyl,
pyrimidinyl, pyranyl,
tetrahydropyranyl, benzothienyl, benzothiazolyl, benzoxazolyl,
isobenzofuranyl,
benzofuranyl, benzimidazolyl, indazolyl, chromenyl, indolyl, oxindolyl,
phthalimido, 1-oxo-
2-isoindolyl, quinolyl, isoquinolyl, tetrahydroisoquinolyl, indolizinyl,
isoindolyl, 2-
oxoisoindolyl, 1,2-(methylenedioxy)phenyl, quinuclidinyl, hydantoinyl,
saccarinyl,
cinnolinyl, purinyl, morpholinyl, thiomorpholinyl, dioxanyl, dithianyl and
azepinyl.
Most preferred heterocyclyl groups are N-methyl-imidazolyl, 2-methyl-
thiazolyl, 2-methyl-
oxazolyl and pyridyl group. The term "C3 -C7 cycloalkyl" as used herein refers
to a 3- to
7-membered, substituted or unsubstituted, saturated or unsaturated carbon
ring. Examples of
2o cycloalkyl groups include: cyclopropyl, cyclobutyl, cyclopentyl and
cyclohexyl groups.
Preferably, when ORl1 is a sugar residue, it has the formula
-O
O
~O Ra
O
O Rb
wherein Ra and Rb independently represent hydrogen, a hydroxy protecting
group, or Cl-C7
alkanoyl.
From all of the above, it is clear to the skilled man that any of the groups
or substituents being
defined, for instance, as alkoxy, allcylaminocarbonyl, alkylaminosulphonyl,
CA 02471910 2004-06-28
WO 03/055861 PCT/EP02/14514
4
arylaminosulphonyl and the like, have to be construed from the names of the
groups from
which they originate.
Substituents which may be present in the aryl or heterocyclyl groups in any of
the above
definitions of Rl-R13 include the following:
- halo (i.e., fluoro, bromo, chloro or iodo);
- hydroxy;
- nitro;
- azido;
- mercapto (i.e., -SH), and acetyl or phenylacetyl esters thereof (i.e., -
SCOCH3 and -
1o SCOCHZC6H5);
- amino (i.e., -NH2 or -NHRI or -NRIRII, wherein RI and RII, which are the
same or different,
are straight or branched C~-C6 alkyl, phenyh biphenyl (i.e., -C6H4-C6H5), or
benzyl groups,
optionally substituted by hydroxy, methoxy, methyl, amino, methylamino,
dimethylamino,
chloro or fluoro; or RI and RII taken together with the nitrogen atom to which
they are
attached form a heterocyclic riilg such as morpholino, pyrrolidino,
piperidino, pyperazino or
N-methylpyperazino;
- guanidino, i.e., -NHC(--NH)NH2;
- formyl (i.e. -CHO);
- cyano;
- carboxy (i.e. -COOH), or esters thereof (i.e., -COORI), or amides thereof
(i.e., -CONH2, -
CONHRI or -CONHRIRII), wherein RI and RII are as defined above, and including
morpholino-amides, pynolidino-amides, and carboxymethylamides -CONHCHZCOOH;
- sulfo (i.e., -S03H);
- acyl, i.e., -C(O)RI, wherein RI is as defined above, including
monofluoroacetyl,
difluoroacetyl, trifluoroacetyl;
- carbamoyloxy (i.e., -OCONHZ) and N-methylcarbamoyloxy;
- acyloxy, i.e., -OC(O)RI wherein RI is as defined above, or formyloxy;
- acylamino, i.e., -NHC(O)RI, or -NHC(O)ORI , wherein RI is as defined above
or is a
group -(CH2)tCOOH where t is l, 2 or 3;
- ureido, i.e., -NH(CO)NHZ , -NH(CO)NHRI, -NH(CO)NRIRII, wherein RI and RII
are as
defined above, including -NH(CO)-(4-morpholino), -NH(CO)-(1-pyrrolidino), -
NH(CO)-(1-
piperazino), -NH(CO)-(4-methyl-1-piperazino);
CA 02471910 2004-06-28
WO 03/055861 PCT/EP02/14514
- sulfonamido, i.e., -NHS02RI wherein RI is as defined above;
- a group -(CHZ)tCOOH, and esters and amides thereof, i.e., -(CH2)tCOORI and -
(CH2)tCONH2 , -(CHZ)tCONHRI, -(CHZ)tCONRIRII, wherein t, RI and RII are as
defined
above;
5 - a group -NH(S02)NHa , -NH(SO2)NHRI, -NH(SO2)NRjRII, wherein RI and RII are
as
defined above, including -NH(S02)-(4-morpholino), -NH(S02)-(1-pyrrolidino), -
NH(S02)-
(1-piperazino), -NH(SOZ)-(4-methyl-1-piperazino);
- a group -OC(O)ORI, wherein RI is as defined above;
- a group -ORI, wherein RI is as defined above, including -OCHZCOOH;
to - a group -SRI, wherein RI is as defined above, including -SCHZCOOH;
- a group -S(O)RI, wherein RI is as defined above;
- a group -S(OZ )RI, wherein RI is as defined above;
- a group -SOZNHZ , -SO2NHRI, or - SO2NRIRII, wherein RI and RII are as
defined above;
- CI -C6 alkyl or C2 -C6 allcenyl;
- C3 -C7 cycloalkyl;
- substituted methyl selected from chloromethyl, fluoromethyl, difluoromethyl,
trifluoromethyl, aminomethyl, N,N-dimethylaminomethyl, azidomethyl,
cyanomethyl,
carboxymethyl, sulfomethyl, carbamoylmethyl, carbamoyloxymethyl,
hydroxyrnethyl,
methoxycarbonylmethyl, ethoxycarbonyhnethyl, tert-butoxycarbonylmethyl and
guanidinomethyl.
When present, carboxy, hydroxy, mercapto and amino groups may be either free
or in
a protected form. Protected forms of said groups are any of those generally
known in the art.
Preferably, carboxy groups are protected as esters thereof, in particular
methyl, ethyl, tert-
butyl, benzyl, and 4-nitrobenzyl esters. Preferably, hydroxy groups are
protected as silyl-
ethers, ethers or esters thereof, in particular trimethyl silyl, tert-
butyldiphenyl silyl, triethyl
silyl, triisopropyl silyl or tert-butyldimethylsilyl ethers, methoxyrnethyl
ethers,
tetrahydropyranyl ethers, benzyl ethers, acetates or benzoates. Preferably,
mercapto groups
are protected as tluoethers or thioesters, in particular tert-butyl
thioethers, thioacetates or
thiobenzoates. Preferably, amino groups are protected as carbamates, e.g. tert-
butoxycarbonyl
3o derivatives, or as amides, e.g. acetamides and benzamides.
As stated above, the present invention provides the salts of those compounds
of formula (~
that have salt-forming groups, especially the salts of the compounds having a
carboxylic
CA 02471910 2004-06-28
WO 03/055861 PCT/EP02/14514
6
group or the salts of the compounds having a basic group, especially an amino.
The salts are
especially physiologically tolerable salts, for example all~ali metal and
alkaline earth metal
salts (e.g. sodium, potassium, lithium, calcium and magnesium salts), ammonium
salts and
salts with an appropriate organic amine or amino acid (e.g. arginine, procaine
salts), and the
addition salts formed with suitable inorganic acids (e.g. hydrochlorides,
hydrobromides,
sulfates, phosphates) or carboxylic and sulfonic organic acids (e.g. acetates,
trifluoroacetates,
citrates, succinates, malonates, lactates, tartxates, fumarates, maleates,
methanesulfonates,
p-toluenesulfonates).
Furthermore, hydrates, solvates of compounds of formula (I), and
physiologically
to hydrolysable derivatives (i.e., prodrugs) of compounds of formula (I) are
included within the
scope of the present invention.
It is to be noted that the Rl, R2, R3 , R4, RS and R6 substituents may be
above or under
the plane, so that the present invention encompasses all the possible stereo
isomers (e.g.
diastereoisomers, epimers, geometrical isomers) of the compounds of formula
(I), as well as
their racemic or optically active mixtures related to these substituents.
In the preferred configuration Rl, which is the substituent at ring position
6, is under the
plane:
R~
R1
Rs
Ra
2o In a preferred compound of the present invention, the benzocyclodecane has
the following
formula (IA.):
CA 02471910 2004-06-28
WO 03/055861 PCT/EP02/14514
7
R~
Ra
(
wherein:
----- at positions 8-9 and 11-12 independently represents a single or double
bond,
R7 represents a residue of the formula
O
R$
wherein R8 is N-methyl imidazolyl, phenyl, methyl-thiazolyl, methyl-oxazolyl
or pyridyl
group;
one of -R2 and -R3 represents hydrogen and the other one is hydrogen or oxygen
(=O),
hydroxy or acetoxy group;
when ----- at position 11-12 represents a single bond, then -R4 represents
oxygen (=O),
- methylene (=CHZ), =CHCOORia, wherein Rlorepresents methyl or ethyl,
=CH(OCH3), -CHO, hydroxy, acetoxy, or -CHZORlI wherein Rl I represents
hydrogen or a
sugar residue having the formula
-O
O
~ORa
O
ORb
wherein Ra and Rb independently represent hydrogen, a hydroxy protecting
group, or Cl-C7
alkanoyl,
or
CA 02471910 2004-06-28
WO 03/055861 PCT/EP02/14514
8
when ----- at position 11-12 represents a double bond, then -R4 represents a
residue of formula
-C02CzH5; and
- RS and -R6 are both hydrogen atoms or, when ----- at position 8-9 represents
a single bond,
taken together with the carbon atoms to which they are attached form a
cyclopropane ring;
R13 represents hydrogen atom, two methyl groups at positions 1 and 4, one
methyl group at
position 4 and one isopropyl group at position 1.
The present invention also provides a process for preparing a compound of the
invention as
defined above, which process comprises:
cyclizing a compound of formula II
R~
ORd
II
wherein R~ represents hydrogen, a hydroxy protecting group, C1-C7 allcanoyl or
benzoyl or,
taken together with Re, forms an acetonide ring; Ra represents hydrogen, a
hydroxy protecting
group, CI-C~ alkanoyl, or benzoyl, or , taken together with Rf, forms an
acetonide ring; Re
represents hydrogen atom and Rf represents hydrogen atom or a free or
protected hydroxy
group, or is linked to the adj acent ORd substituent as defined above; Rf
represents hydrogen
atom and Re represents hydrogen atom or a free or protected hydroxy group or
is linked to the
adjacent ORS substituent as defined above;
and, if desired, converting the resultant compound of formula I',
R
R6
Ra
I,
CA 02471910 2004-06-28
WO 03/055861 PCT/EP02/14514
wherein Rl is ORS, RZ is Re, R3 is Rf, R4 is ORd, in which R~, Rd, Re and Rf
are as defined
above and R5 and R6 are hydrogen atoms, into another different compound of
formula I as
defined above; and/or if desired, converting a compound of formula I' or I
into a
pharmaceutically acceptable salt therof; and/or, if desired convertiilg a
pharmaceutically
acceptable salt of a compound of formula I or I' into the corresponding free
compound.
Preferably, the hydroxy protecting groups are silyl or methoxymethyl group; R~
represents a
C1-C6 alkanoyl group, more preferably an acetyl group, or a silyl protecting
group, more
preferably a t-butyldiphenylsilyl group. The cyclization to give the compound
of formula I'
as single Z isomer can be performed through the Ring Closing Metathesis (RCM)
reaction. In
to particular, the RCM reaction is carried out in the presence of an
appropriate catalyst, more
preferably aNolan and Grubb's catalyst, described for example
in,LAm.Chem.Soc.,1999,
121, 2674 and in Org. Lett.,1999, 1, 953.
CY3 Cy3
CI CI ,,
, (
(
, ,
, .
~ ~
~ ~
CI CI
~ ~
Ph Ph
Mst-NON-Mst Mst-NON-Mst
U U
RCM Catalyst A RCM Catalyst B
1s [Mst=C6H2-2,4,6-(CH3)3]
The conversion of a compound of formula I' or I into another different final
compound of
formula I may be carried out in several ways, depending on the meanings of the
substituents
and the presence of the unsaturated bonds in the ring. Such conversions follow
conventional
procedures known in the art.
2o For example, a compound of formula I wherein -Rl represents a residue of
the formula
O
_O Ra
wherein R$ is as defined above, can be obtained by condensing a corresponding
compound of
the formula I or I' wherein -Rl represents hydroxy group with a the
appropriate derivative of
formula III
O
III
25 H-O R$
CA 02471910 2004-06-28
WO 03/055861 PCT/EP02/14514
wherein R8 is as above defined. These compounds of formula III are known or
can be
prepared according to known procedures.
Therefore, it is a further object of the present invention a process for
obtaining a compound of
5 formula I"'
O
~R
O
Re
Ri3 ~ w
Rf
OH
I"'
wherein Re, Rf, R13 and R8 are as defined above, which process comprises
deprotecting a
compound of formula I":
R~
ORd
10 wherein R~, Re, R~ , Rf and R13 are as defined above, condensing the
resultant compound of
formula f
R~
ORd
wherein Re, Ra , Rf and R13 are as defined above, with a compound of formula
III or an
activated form thereof
CA 02471910 2004-06-28
WO 03/055861 PCT/EP02/14514
11
O
III
H-O Rs
wherein R$ is as above defined, optionally in presence of a condensing agent;
and, if
necessary, deprotecting the resultant compound of formula I°.
O ~ R
a
ORd
IV
wherein Re, Rd , Rf , R8 and R13 are as defined above, and Rd represents a
hydroxy protecting
group, Cl-C6 all~anoyl, or benzoyl, or , talcen together with Rf, forms an
acetonide ring; to
give the desired compound of formula I"' as above defined.
As a more specific example, the process for preparing a compound of formula I
wherein -Rl
represents a residue of the formula
O
_ . _O /
N-CHs
N ~/
is depicted in the scheme 1 below:
Scheme 1
CA 02471910 2004-06-28
WO 03/055861 PCT/EP02/14514
12
O'I
DCC /
ORc OH DMAP O~~~N-CH3
deprotection ~ DCM N~/
I/ I ~ I/ I
ORd ORd -N~COOH I /
~N
ORd
I~a
deprotection N-CH3
OH
la
The reaction with (E)-N-methylurocanic acid can be carried out in
dichloromethane
(DCM) in presence of dicyclohexylcarbodiimmide (DCC) and 4-
dimethylaminopyridine
(DMAP). The deprotection steps can be basic hydrolysis in case R~ and/or Rd
are acetyl
groups.
A compound of formula I wherein R2, R3 or -R4 represents an oxygen atom =O can
be
obtained from a corresponding compound of formula I or I' as defined above
wherein -R2,
-R3 or -R4 represents a hydroxy group by means of oxidation, for example with
Dess-Martin
to periodinane, pyridinium dichromate (PDC) or pyridinium chlorochromate (PCC)
or under
Swern oxidation conditions (dimethylsulfoxide/oxalyl chloride), provided that
the other
hydroxy groups in the molecule, if any, are protected. A compound of formula I
wherein -R4
represents an oxygen atom =O can be conveniently converted into a
corresponding compound
of formula I wherein -R4 represents methylene (=CH2), =CHCOORto wherein Rto is
as
defined above, or =CH(OCH3) by reaction with a suitable Wittig reagent, such
as for
example, respectively, Ph3P=CH2, Ph3P=CHCOORto, wherein Rto is as defined
above and
Ph3P=CH(OCH3). A compound of formula I wherein -R4 represents =CH(OCH3) can be
then
converted by acidic hydrolysis into a corresponding compound of formula I
wherein -R4
represents -CHO, which in turn may be either reacted with a reducing agent to
give a
2o compound of formula I wherein -R4 represents -CHZOH, or oxidised with a
suitable reagent
such as NaC102 to give a compound of formula I wherein -R4 represents -COOH. A
compound of formula I wherein -R4 represents an oxygen atom =O can also be
converted into
a compound of formula I wherein -R4 represents a-COORto group wherein Rto is
as defined
CA 02471910 2004-06-28
WO 03/055861 PCT/EP02/14514
13
above and the bond at position 11-12 is double by treatment with triflic
anhydride in the
presence of a base followed by reaction of the resultant enol-triflate with CO
and Rlo-OH
wherein Rlo is as defined above in the presence of Palladium catalyst and a
base such as
triethylamine according to known procedures as those described in
J.Claem.Soc.PeYkin Ty°a~s.
I, 1991 (5), 969-979. Such compounds of formula I wherein -R4 represents a -
COOH group
and the bond at position 11-12 is double can be converted by selective
reduction into the
corresponding 11-12 unsaturated compounds of formula I wherein -R4 represents
a -CH20H
group, for example by treatment with C1COOEt/NaBH4,
A compound of formula I' or I wherein the bond at position 8-9 is double may
be converted
to into the corresponding compound of formula I with a single bond at the 8-9
position and
wherein RS and R6 are hydrogen atoms by hydrogenation, such as by treatment
with HZ and a
suitable catalyst like a Palladium on charcoal catalyst according to the
methods known in
the art; or into the corresponding compounds of formula I with a single bond
at the 8-9
position wherein RS and R6 taken together with the carbon atoms to which they
are attached,
form a cyclopropane ring by treatment with a suitable reactant such as a zinc
carbenoid
(J.ATn.CIZem.Soc. 2001, 123, 8139-8140).
A compound of formula I may be converted into a pharmaceutically acceptable
salt thereof
using conventional techniques. Suitable salts include those mentioned above.
A compound of the formula II rnay be prepared as described in any one of the
following
2o schemes, in which R~ , Rd, Re , Rf and R13 have the meanings above defined:
Scheme 2
R~3 \ / Rl~ \ O
1 2 O
R ORc R OR~
13 ~ / 1 ~ /
\ ~ ~ \
ORa ORd
II, R~=Ra H II
CA 02471910 2004-06-28
WO 03/055861 PCT/EP02/14514
14
Compound 1 where Ri3 represents hydrogen atom is known and can be prepared
according
to known procedures (Tety~ahed~on Lett. (2000), 41(5), 729-731). Compound 1
can also
be obtained by the copper mediated reaction of a vinyl organometallic reagent,
such as
vinyl magnesium bromide, with the appropriate 1,2 dibromomethyl-phenyl
derivative (see
for example J. Ag~ic. Food Cl2em. 45, 1422, I997). Compound 1 can be
conveniently
transformed into compound 2 by oxidation, for example by treatment with an
inorganic or
organic peracid, such as meta-chloroperbenzoic acid, and then compound 2 can
be
converted into the compound II, wherein R~ and Rd are both hydrogen atoms, by
the
addition of a vinyl organometallic reagent, such as vinyl magnesium bromide.
The
to resultant compound II is then protected to yield the desired compound of
formula II
wherein R~ and Rd are hydroxy protecting groups as defined above. By the above
process,
fox example, there are obtained compounds of formula II wherein R~ and Rd are
both acetyl
groups and Re and Rfare hydrogen atoms. It is a further object of the present
invention an
intermediate compound of formula IT
OR~
Rl \\ . ~.
ORd
15- B
wherein R~ and Rd are hydrogen atoms or hydroxy protecting groups, and Rl3 has
the
meanings above defined.
Scheme 3
CA 02471910 2004-06-28
WO 03/055861 PCT/EP02/14514
O oP ORS
R~s H (A>ze~ (4) Rts OP
g OPT 5 U~'~ 6 OH
R~ R
--
n U
II>Rd-N II
Compound 3 wherein R13 represents hydrogen atom and P1 represents acetyl group
is
known, other compounds 3 can be analogously prepared as described in the
literature
(Tet~°alaedrora 1988, 44, 7027). To the properly protected compound 3,
wherein P1
5 represents a hydroxy protecting group such as an acetyl or a silyl
protecting group, is
added the appropriate allylic boronate of formula (4) wherein P represents a
hydroxy
protecting group and A represents a suitable organic residue. These compounds
of formula
(4) are known or can be prepared according to known procedures.
_ Depending on the Z or E stereochemistry of the starting allylic boronate (4)
in scheme 3;
to both syn and anti allylic derivatives 6 can be obtained. Alternatively, a
Compound 3 can be
submitted to Brown's stereoselective allylation reaction, (J. Or~g. Claetn.
1982, 47, 5065).
In this case the desired stereochemistry of the two oxygenated vicinal
substituents can be
controlled in the resultant compound of formula 5 just by choosing the
suitable absolute
stereochemistry of an alpha-pinene-derived allylic reagent (4), wherein A
represents 1-Ipc
15 from (-)-alpha-pinene or d-Ipc from (+)-alpha-pinene.
All possible stereoisomers can be synthesized as a mixture and obtained as
single
stereoisomers also by chromatographic separation. In particular enantiomers
can be
obtained by chiral chromatographic separation (by using for example chiral
solid support).
Compound 5 is protected (introduction of R.~ group) and then deprotected
(removal of PI)
2o to yield Compound 6, that is then oxidized to give the aldehyde derivative
7, for example
under Swern oxidation conditions (dimethylsulfoxide/oxalyl chloride) or with
PCC.
CA 02471910 2004-06-28
WO 03/055861 PCT/EP02/14514
16
Addition to the Compound 7 of an allylic organometallic species (for example
allyl
magnesium bromide) affords the compound II (Rd=H), that is suitably protected
to be
converted into another compound II. By the above process, for example, there
are obtained
compounds of formula II wherein R~, Rd and Re are hydroxy protecting groups
and Rfis
hydrogen atom.
BIOLOGICAL TESTS
Microtubule assembly and disassembly assay.
Pig brain tubulin was prepared by two cycles of assembly and disassembly and
it was
stored in liquid nitrogen in Microtubule Assembly Buffer (MAB: 0.1 M MES, 2.5
mM
to EGTA, 0.5 mM MgS04, 0.1 mM EDTA, 0.1 mM DTT pH 6.4). Assembly was monitored
by the method of Gaskin et al.(Gaskin F, Cantor CR, Shelanski M L, 1974,:
Turbidimetric
studies of the in vitro assembly and disassembly of porcine neurotubules. J.
Mol. Biol. 89:
737-758). The cuvette (1 cm path) containing 0.5 mglml tubulin and 1 mM GTP
was
shifted to 37 °C and continuous turbidity measurements were made at 340
nm on a
spectrophotometer equipped with an automatic recorder and a thermostatically
regulated
sample chamber. After 30 min CaCl2 (5 mM) was added and disassembly was
monitored
for 10 min as decreased turbidity. Scalar doses of test compounds were
monitored at
regular intervals of 15 min.
Data were expressed as percentage of reassembly induced by the tested
compounds and the
dose effecting tubulin assembly by 90% at 37 °C (ED~o) was calculated
on this curve.
The compound Ira prepared in Example 9 showed an ED~o of 10 microM.
Cytotoxicity
A2780 cells (2000/well) were seeded in mufti-well plates (96 wells) in the
presence of 200
q,l of the complete medium RPMI 1640 + 10% FCS. After 24 h, the cells were
treated with
the compounds: the compounds' solution (200 x) was prepared in DMSO 100% and 1
p.l/well was added. 5 scalar concentrations for each compound were tested in
four
replicates. The cells were incubated at 37°C, 5% COZ for 72 h.
Colorimetric assay (SRB: sulforhodamine B): cell cultures were fixed with
trichloroacetic
acid, stained with 0.4% SRB dissolved in 1 % acetic acid. Unbound dye was
removed by
four washes with 1 % acetic acid and protein-bound dye was extracted with l
OmM Tris
base for determination of optical density in a 96-well microtiter plate
reader. ICso and IC9o
CA 02471910 2004-06-28
WO 03/055861 PCT/EP02/14514
17
(concentration inhibiting cell proliferation by 50 or 90 %) were determined by
data
analysis in the Microsoft Excel 97 program.
Effect on cell cycle progression
Human colon carcinoma HCTl 16 cells were seeded in culture flasks and treated
24 h after
incubation at 37°C. At the end of the treatment (24 or 48 or 72 hours),
cells were counted
and resuspended in propidium iodide (PI) staining solution (0.1 % sodium
cifirate; 0.1
nonidet P40, 6.5 ~.glml Rnasi A, 50 ~,g/ml PI). After incubation in the dark
at room
temperature for at least 30 minutes, samples were then analyzed for cell cycle
on FacScan
(Becton Dickinson) flow cytometer.
to Compounds~of formula I of the invention show enhanced antitumor activity
and acceptable
toxicity. They are useful as antitumour agents in the prevention, treatment
and/or control of
cancer, for instance in the treatment of leulcemia and solid tumors, such as
colon, colo-
rectal, ovarian, mammary, prostate, lung, kidney and also melanoma tumors. A
human can
be treated by a method comprising administering thereto a therapeutically
effective amount
i5 of a compound of the invention. The invention therefore provides a method
of treating a
patient in need of an antitumour agent, which method comprises the
administration thereto
of a compound as defined above. The condition of the human patient can thus be
improved. The invention also provides the use of a compound of the invention
as defined
above in the manufacture of a medicament for use as an antitumour agent.
2o The dosage range adopted will depend on the route of administration and on
the age,
weight and condition of the patient being treated. The compound of formula (~
is typically
administered by parenteral route, for example intramuscularly, intravenously
or by bolus
infusion. A suitable dose range is from 1 to 1000 mg of equivalent per m2 body
surface
area of active drug, for instance from 10 to 500 mg/m2.
25 The compounds of formula (I) may be formulated into a pharmaceutical
composition
together with a pharmaceutically carrier or diluent. The invention therefore
further
provides a pharmaceutical composition which comprises a pharmaceutically
acceptable
diluent or carrier and, as an active ingredient, a compound as defined above.
The
pharmaceutical compositions of the invention are prepared by conventional
methods and
3o are administered in a pharmaceutically acceptable form. For example, the
solid oral forms
may contain, together with the active compound, diluents, e.g. lactose,
dextrose,
saccharose, sucrose, cellulose, corn starch or potato starch; lubricants, e.g.
silica, talc,
CA 02471910 2004-06-28
WO 03/055861 PCT/EP02/14514
18
stearic, magnesium or calcium stearate, and/or polyethylene glycols; binding
agents, e.g.
starches, arabic gum, gelatine, methylcellulose, carboxyrnethylcellulose or
polyvinyl
pyrrolidone; disaggregating agents, e.g. a starch, alginic, alginates or
sodium starch
glycolate; effervescing mixtures; dyestuffs; sweeteners; wetting agents such
as lecithin,
polysorbates, laurylsulphates; and, in general, non-toxic and
pharmacologically inactive
substances used in pharmaceutical formulations. Said pharmaceutical
preparations may be
manufactured in known manner, for example, by means of mixing, granulating,
tabletting,
sugar-coating, or film-coating processes.
The liquid dispersions for oral administration may be e.g. syrups, emulsions
and
suspensions.
The syrups may contain as carrier, for example, saccharose or saccharose with
glycerine
and/or mannitol and/or sorbitol.
The suspensions and the emulsions may contain as carrier, for example, a
natural gum,
agar, sodium alginate, pectin, methylcellulose, carboxymethylcellulose, or
polyvinyl
alcohol.
The suspension or solutions for intramuscular injections may contain, together
with the
active compound, a pharmaceutically acceptable carrier, e.g. sterile water,
olive oil, ethyl
oleate, glycols, e.g. propylene glycol, and, if desired, a suitable amount of
lidocaine
hydrochloride. The solutions for intravenous injections or infusions may
contain as carrier,
2o for example, sterile water or preferably they may be in the form of
sterile, aqueous,
isotonic saline solutions or they may contain as a carrier propylene glycol.
The suppositories may contain together with the active compound a
pharmaceutically
acceptable carrier, e.g. cocoa butter, polyethylene glycol, a polyoxyethylene
sorbitan fatty
ester surfactant or lecithin.
Typically the pharmaceutical compositions are formulated for parenteral
administration,
for example by dissolution in water for injection or physiological saline.
The following examples illustrate the invention without limiting it.
Example 1: 1,2-Diallyl-benzene.
CA 02471910 2004-06-28
WO 03/055861 PCT/EP02/14514
19
A 1.0 M tetrahydrofurane (THF) solution of vinyl bromide (300 ml, 0.30 mol)
was added
to a flask containing magnesium turnings (7.01 g, 0.29 mol) in freshly dried
THF (100 ml)
under an atmosphere of nitrogen. The mixture was heated under reflux until all
the
magnesium disappeared (2 hours) and copper (I) iodide (28.5 g, 0.15 mol) was
added drop-
s wise to the resulting slurry keeping the temperature below -30 °C. A
solution of b'-
dibrornoxylene (13.75 g, 0.052 mol) in dry THF (100 ml) was then slowly
dropped into the
green slurry at -60 °C. The resulting mixture was stirred at -60
°C for 1 hour and at 0 °C
for a further 3 hours. When TLC analysis showed no starting material left, the
mixture was
quenched with a saturated solution of ammonium chloride (100 ml) and extracted
with
1o diethyl ether (3 x 100 ml). The etheral layer was dried over Na2S04,
filtered and
evaporated (no heating, the product is volatile). Flash chromatography
(silicagel, hexane)
afforded the title compound 1,2-diallyl-benzene in 77% yield (6.3 g); ~H (300
MHz,
CDC13) 7.18 (4H, m, Ph), 5.90-6.05 (2H, iim, 2 x CH=), 4.97-5.10 (4H, m, 2 x
CHZ=), 2.40
(4H, m, 2 x CHI,). '
i5 Example 2: 2-f2-(oxiran-2-ylmethyl)benzylloxirane.
O
w
O
To a_ solution. of 1,2-diallyl-benzene __prepared in Example 1(0.55. g, 3.48
mmol) -in- dr-y- -
DCM (50 ml) was added fn-chloroperoxybenzoic acid (3.15 g, 9.10 mmol). The
mixture
was stirred at RT under an atmosphere of nitrogen for 16 hours. A saturated
solution of
2o NaHC03 (50 ml) was added arid the mixture was stirred for a further 15
minutes. The
organic layer was then dried over Na2S04, filtered and evaporated. The residue
was
purified by flash chromatography (silicagel, 10% ethyl acetate in hexane) to
give 2-[2-
(oxiran-2-ylmethyl)benzyl]oxirane in 82% yield (0.54 g); 8H (300 MHz, CDC13)
7.20-7.30
(4H, m, Ph), 3.12-3.22 (2H, m, 2 x CH), 2.94 (4H, m, 2 x CHZ), 2.80 (2H, m, 2
x CHaHb),
25 2.53 (2H, m, 2 x CHaHb); rnlz 208.3 (M+NH~+, 100%), 191.3 (M+H+, 10%).
Example 3: 1-[2'-(2"-Hydroxy-pent-4"-enyl~phenyll-pent-4-en-2-ol.
OH
v
OH
CA 02471910 2004-06-28
WO 03/055861 PCT/EP02/14514
A 1.0 M THF solution of vinyl bromide (220 ml, 0.22 mol) was added to
magnesium
turnings (5.0 g, 0.21 mol) in freshly dried THF (80 ml) under an atmosphere of
nitrogen.
The mixture was heated under reflux until all the magnesium disappeared.
Copper (I)
iodide (19.58 g, 0.10 mol) in dry THF (50 ml) was added drop-wise to the vinyl
5 magnesium bromide at -50 °C and the greenish slurry was stirred for
10 minutes. 2-[2-
(oxiran-2-ylmethyl)benzyl]oxirane prepared in Example 2 (3.9 g, 0.02 mol) in
dry THF (50
ml) was added drop-wise to the slurry keeping the temperature below -65
°C, stirred 1
hour at this temperature and at 0 °C until all starting material
disappeared by TLC analysis.
The mixture was then quenched with a saturated solution of ammonium chloride
and
1o extracted with diethyl ether (3 x 75 ml). The etheral layer was filtered
through a 5-cm pad
of silicagel, dried over Na2S04, filtered and evaporated to dryness. The
residue was
purified by flash chromatography (silicagel, 10% ethyl acetate in hexane) to
furnish 1-[2'-
(2"-hydroxy-pent-4"-enyl)-phenyl]-pent-4-en-2-of in 85% yield (4.3 g) as a
yellowish
powder; 8H (300 MHz, CDC13) 7.21 (4H, m, Ph), 5.90 (2H, m, 2 x CH=), 5.18 (4H,
m, 2 x
15 CH2), 3.89 (2H, q, J 6 Hz, CH), 2.83 (4H, d; J 6 Hz, CH2), 2.37 (4H, m, 2 x
CHZ), 2.23
(2H, bs, 2 x OH); mlz 305.3 (M+CH3C00-, 100%), 264.3 (M+NHø+, 100%), 247.3
(M+H+, 60%).
Example 4: Acetic acid 1-[2' ~2"-acetoxy_pent-4"-enyl)-benz~]-but-3-enyl
ester.
OAc / _ _ . _ .
OAc
2o A solution of 1-[2'-(2"-hydroxy-pent-4"-enyl)-phenyl]-pent-4-en-2-of
prepared in
Example 3 (0.57 g, 2.32 mmol), acetic anhydride (1 ml), pyridine (0.5 ml), 4-
dimethylaminopyridine (2 mg) in dichloromethane (DCM, 20 ml) was stirred at
room
temperature (RT) for 4 hours, washed with a saturated solution of NaHC03 (20
ml), water
(20 ml), dried over NaZS04, filtered and concentrated. The residue was
purified by flash
chromatography (silicagel, 10% ethyl acetate in hexane) to furnish the desired
acetic acid
1-[2'-(2"-acetoxy-pent-4"-enyl)-benzyl]-but-3-enyl ester in 89% yield (0.68
g); ~H (300
MHz, CDCl3) 7.13 (4H, s, Ph), 5.78 (2H, m, 2 x CH=), 5.10 (4H, m, 2 x CHZ=),
3.73 (2H,
m, 2 x CH), 2.92 (4H, d, J 7 Hz, 2 x CH2), 2.35 (4H, m, 2 x CH2).
CA 02471910 2004-06-28
WO 03/055861 PCT/EP02/14514
21
Example 5: Acetic acid 11-acetoxy-5,6,7,101112-hexahydro-benzocyclodec-8-en-6-
ester.
OAc
OAc
To a solution of acetic acid 1-[2'-(2"-acetoxy-pent-4"-enyl)-benzyl]-but-3-
enyl ester
prepared in Example 4 (0.68 g, 2.06 mmol) in dry DCM (200 ml) was added Grubbs
II
catalyst B (35.4 mg, 2 mol%). The flask was flushed with nitrogen and the pink
solution
was stirred at RT under an atmosphere of nitrogen for 2 hours. After stirring
at ambient air
until the solution turned brown (decomposed catalyst), the solvent was
evaporated. The
residue was purified by flash chromatography (silicagel, 10% ethyl acette in
hexane) to
furnished the cis-cyclized product acetic acid 11-acetoxy-5,6,7,10,11,12-
hexahydro-
benzocyclodec-8-en-6-yl ester in 74% yield (0.46 g); 8H (300 MHz, CDC13) 7.29
(2H, m,
Ph), 7.19 (2H, m, Ph), 5.78 (2H, m, 2 x CH=), 5.30 (2H, m, CH), 3.04 (2H, t, J
12 Hz, 2 x
CHaHb), 2.75 (2H, dd, J 5, 12 Hz, 2 x CHaHb), 2.04-2.18 (1 OH; m, 2 x CH2, 2 x
CH3); mlz
(EI) 302 (M+, 10%), 242 [(M-CH3COOH)+, 25], 182 [(M-2 x CH3COOH)+, 55], 43
(CH3C0+, 100); X-ray.
Example 6: Acetic acid 11-h day-5 6 7 10 11 12-hexahydro-benzocyclodec-8-en-6-
ester.
OH
OAc
To a stirred solution of acetic acid 11-acetoxy-5,6,7,10,11,12-hexahydro-
benzocyclodec-8-
2o en-6-yl ester prepared in Example 5 (30.6 mg, 0.101 mmol) in dry methanol
(5 ml) was
added potassium carbonate (13.2 mg, 0.096 mmol). After 30 minutes, the
solution was
quenched with water (10 ml), acidified with 1N HCl and extracted with DCM (2 x
10 ml).
The organic layer was dried over Na2S04, filtered and evaporated. Flash
chromatography
(silicagel, 10% ethyl acetate in hexane) afforded acetic acid 11-hydroxy-
5,6,7,10,11,12-
hexahydro-benzocyclodec-8-en-6-yl ester as a white powder in 84% yield (22
mg); 8H (300
MHz, CDCl3) 7.10-7.30 (4H, m, Ph), 5.77-5.99 (2H, m, 2 x CH=), 5.23 (1H, m,
CH), 4.22
CA 02471910 2004-06-28
WO 03/055861 PCT/EP02/14514
22
(1H, rn, CH), 2.95-3.17 (2H, m, CH2), 2.88 (2H, m, CH2), 2.19 (3H, s, CH3),
2.02-2.18
(4H, m, 2 x CHZ); fnlz 319.3 [(M+CH3C00-, 100%), 278.3 [(M+NH4)+, 100%].
Example 7: Acetic acid 11-oxo-5 6 7 10 11 12-hexahydro-benzocyclodecen-6-yl
ester.
O
\
OAc
To a solution of acetic acid 11-acetoxy-5,6,7,10,11,12-hexahydro-benzocyclodec-
8-en-6-yl
ester prepared in Example 5 (110.2 mg, 0.365 mmol) in dry methanol (10 ml) was
added
potassium carbonate (49.7 mg, 0.360 mmol). The solution was stirred at RT for
30
minutes, quenched with water (10 ml) and extracted with DCM (2 x 20 ml). The
organic
layer was dried over NaZSO4, filtered and evaporated to give crude acetic acid
11-hydroxy-
l0 5,6,7,10,11,12-hexahydro-benzocyclodec-8-en-6-yl ester. The crude acetic
acid 11-
hydroxy-5,6,7,10,11,12-hexahydro-benzocyclodec-8-en-6-yl ester was redissolved
in DCM
(10 ml) and pyridinium chlorochromate (78.2 mg, 0.363 mmol) was added. The
mixture
was stirred at RT for 2 hours, filtered and evaporated. The residue was
purified by flash
chromatography (silicagel, 5% ethyl acetate in hexane) to afford the tiltle
compound in
46% yield (43 mg); 8H (300 MHz, CDC13) 7.12-7.42 (4H, m, Ph), 5.80 (1H, m,
CH=), 5.63
(1H, m, CH=), 5.38 (1H, m, CH-O), 3.99 (1H, d, J 7 Hz, CHaHb), 3.48 (1H, d, J
7 Hz,
CHaHb), 3.00 (2H, m, CHZ), 2.08 (3H, s, CH3), 2.00-2.22 (4H, m, 2 x CH2); fnlz
276.4
[(M+NH4)+, 100%].
Example 8: 11-Hydroxy-7 10 11 12-tetrahydro-SH-benzocyclodecen-6-one.
OH
O
To a solution of acetic acid 11-oxo-5,6,7,10,11,12-hexahydro-benzocyclodecen-6-
yl ester
prepared in Example 7 (36 mg, 0.140 mmol) in dry methanol (5 ml) was added
potassium
carbonate (30 mg). The mixture was stirred at RT for 1 hour, quenched with
water (10 ml)
and extracted with DCM (2 x 15 ml). The organic layer was dried over Na2S04,
filtered,
evaporated and purified by flash chromatography (silicagel, 30% ethyl acetate
in hexane)
to furnish the title compound in 83% yield (25 mg); 8H (300 MHz, CDCl3) 7.14-
7.34 (4H,
m, Ph), 5.88 (1H, q, J 8 Hz, CH=), 5.62 (1H, q, J 8 Hz, CH=), 4.35 (1H, m, CH-
O), 3.94
CA 02471910 2004-06-28
WO 03/055861 PCT/EP02/14514
23
(1H, d, J 8 Hz, CHaHb), 3.54 (1H, d, J 8 Hz, CHaHb), 2.82 -3.07 (4H, m, 2 x
CH2), 2.15
(2H, m, CH2), 1.87 (1H, bs, OH); m/z 275.3 [(M+CH3C00)-, 100%], 234.3
[(M+NH4)+,
100%].
Example 9: 11-(Acetyloxy)-5,6,7,10,11,12-hexahydrobenzo[a][10]annulen-6-yl
(2E)-3-(1-
methyl-1H-imidazol-4-yl)prop-2-enoate.
O
O
~/N_CHs
OAc
Acetic acid 11-hydroxy-5,6,7,10,11,12-hexahydro-benzocyclodec-8-en-6-yl ester
prepared
in Example 6 (22 mg, 0.085 mmol) and 3-(1'-Methyl-1'H-imidazol-4'-yl)-acrylic
acid
prepared as described in J. Am. Chem. Soc., Vol. 121, No. 28, p.6563-6579,
1999 (65 mg)
were stirred in DCM (10 ml) in the presence of DCC (106 mg) and 4-
dimethylaminopyridine (106 mg) at RT under an atmosphere of nitrogen for 2
days. The
mixture was quenched with a saturated solution of ammonium chloride (10 ml),
dried over
Na2S04, filtered and evaporated. The residue was purified by HPLC to afford
>98% pure
title compound (0.80 mg); 8H (300 MHz, CDCl3) 7.60 (1H, d, J 15 Hz, CH=), 7.46
(1H, bs,
CH=), 7.15-7.30 (4H, m, Ph), 7.09 (1H, bs, CH=), 6.60 (1H, d, J 15 Hz, CH=),
5.80 (2H,
m, 2 x CH), 5.42 (1H, m, CH-O), 5.35 (1H, m, CH-O), 3.23 (3H, s, CH3), 3.12
(2H, td, J 1,
7 Hz, CHZ), 2.81 (2H, m, CHZ), 2.18 (3H, s, CH3), 2.00-2.15 (4H, m, 2 x CH2);
nalz 395.3
[(M+H)+, 100%].
Unequivocal assignment of cis stereochemistry of the double bond has been
determined
2o through X-Ray crystal structure.
Example 10: 11-Hydroxy-5,6,7,10,11,12-hexahydrobenzo[a][10]annulen-6-yl (2E)-3-
(1-
methyl-1H-imidazol-4-yl)prop-2-enoate (Ia).
O
O
~ N_CHs
N=/
OH
CA 02471910 2004-06-28
WO 03/055861 PCT/EP02/14514
24
11-(Acetyloxy)-5,6,7,10,11,12-hexahydrobenzo[a][10]annulen-6-yl (2E)-3-(1-
methyl-1H-
imidazol-4-yl)prop-2-enoate prepared in Example 9, was treated with potassium
carbonate
as described in example 6, to give the title compound.
Operating as described in the previous examples, the following compounds are
prepared:
Ib) 11-(Acetyloxy)-5,6,7,10 11 12-hexahydrobenzoL].[10]annulen-6-yl (2E)-3-
phenylprop-
2-enoate.
O
O
w
O
O
Molecular Weight =390.48
Exact Mass =390
Molecular Formula =C25H26O4
Molecular Composition =C 76.90% H 6.71 % O 16.39%
to Ic) 11-(Acetyloxy)-5,6,7,10,11,12-hexahydrobenzo[a][10]annulen-6-yl (2E)-3-
(2-methyl-
1,3-thiazol-4-)prop-2-enoate ~ .
O ~ / S
O N
O
O
Molecular Weight =411.52
Exact Mass =411
Molecular Formula =C23H25N04S
Molecular Composition =C 67.13% H 6.12% N 3.40% O 15.55% S 7.79%
CA 02471910 2004-06-28
WO 03/055861 PCT/EP02/14514
Id) I l-(Acetyloxy)-5,6 7,10 11 I2-hexahydrobenzoL][10]~annulen-6-~(2E)-3-(2-
methyl-
1 3-oxazol-4-y~pro~-2-enoate.
O / / O
O N
O
O
5
Molecular Weight =395.46
Exact Mass =395
Molecular Formula =C23H25N05
Molecular Composition =C 69.86% H 6.37% N 3.54% O 20.23%
Ie) 11-(Acetyloxy)-5,6,7,10,11,12-hexahydrobenzoL][10]annulen-6-~(2E)-
3=pyridin-2-
~prop-2-enoate.
O ~
N
O
O
O
Molecular Weight =391.47
Exact Mass =391
Molecular Formula =C24H25N04
Molecular Composition =C 73.64% H 6.44% N 3.58% O 16.35%
to I~ 11-(Acetyloxy)-7-hydroxy-5,6 7 10 11 I2-hexahydrobenzo[a1f lOlannulen-6-
yI (2E)-3-
(1-methyl-1H-imidazol-4-yl)~ro~-2-enoate.
\ //
N
O
Molecular Weight =410.47
Exact Mass =410
Molecular Formula =C23H26N2O5
Molecular Composition =C 67.30% H 6.38% N 6.82% O 19.49%
CA 02471910 2004-06-28
WO 03/055861 PCT/EP02/14514
26
Ig) 11-(Acetyloxy)-7-oxo-5,6,7101112-hexahydrobenzo[a][l0~lannulen-6-yl (2E)-3-
~l-
methyl-1H-imidazol-4-yl)pro~-2-enoate.
\ //
N
Molecular Weight =408.46
Exact Mass =408
Molecular Formula =C23H24N205
Molecular Composition =C 67.63% H 5.92% N 6.86% O 19.59%
Ih) 711-Bis(acetyloxy)-5,6,7,101112-hexahydrobenzo[a][1~'annulen-6-yl (2E)-3-
(1-
methyl-1 H-imidazol-4-yl)prop-2-enoate.
O
\
N
Molecular Weight =452.51
Exact Mass =452
Molecular Formula =C25H28N2O6
Molecular Composition =C 66.36% H 6.24% N 6.19% O 21.21
CA 02471910 2004-06-28
WO 03/055861 PCT/EP02/14514
27
Ii) 11-(Acetyloxy)-5,6,7,8,9,I0,11,12-octahydrobenzo[a][lOlannulen-6-yl (2E)-3-
(1-
methyl-1H-imidazol-4-)prop-2-enoate.
0
N
Molecular Weight =396.49
Exact Mass =396
Molecular Formula =C23H28N2O4
Molecular Composition =C 69.68% H 7.12% N 7.07% O 16.14%
Il) 10-(Acetyloxy)-la 2 3 4 9 10 11 11a-octahydro-1H-benzoLlcyclopropaLf~f
10]annulen-
3 -~(2E)-3-( 1-methyl-1 H-imidazol-4-yl)prop-2-eno ate.
0
N
Molecular Weight =408.50
Exact Mass =408
Molecular Formula =C24H28N2O4
Molecular Composition =C 70.57% H 6.91 % N 6.86% O 15.67%
Im) 11-(acetyloxy)-10-hydroxy-5,6,7,10,11,12-hexahydrobenzoLlf 10]!annulen-6-
~~
l0 3-(1-methyl-1H-imidazol-4-yl)prop-2-enoate.
CA 02471910 2004-06-28
WO 03/055861 PCT/EP02/14514
28
O
N O
O f
OH
O
Molecular Weight =410.47
Exact Mass =410
Molecular Formula =C23H26N2O5
Molecular Composition =C 67.30% H 6.38% N 6.82% O 19.49%
In) 10 11-Bis~acetyloxy)-5 6 7 10 11 12-hexahydrobenzo[al[lOlannulen-6-yl (2E)-
3-(1-
methyl-1H-imidazol-4-yl)prop-2-enoate.
0
N
Molecular Weight =452.51
Exact Mass =452
Molecular Formula =C25H28N206
Molecular Composition =C 66.36% H 6.24% N 6.19% O 21.21%
To) 11-(Ace~loxy~l0-oxo-5 6,7 10 11 12-hexahydrobenzofa][lOlannulen-6-yl (2E)-
3-(1-
methyl-1H-imidazol-4-yl)pro~-2-enoate.
\ o
N O
O II
O
O
Molecular Weight =408.46
Exact Mass =408
Molecular Formula =C23H24N205
Molecular Composition =C 67.63% H 5.92% N 6.86% O 19.59%
CA 02471910 2004-06-28
WO 03/055861 PCT/EP02/14514
29
Ip) 1-1-Hydroxy-5 6 7 10 11 12-hexahydrobenzoLlf 10]annulen-6-yl (2E)-3-(1-
methyl 1H
imidazol-4-)prop-2-enoate.
\ o
N O
OH
Molecular Weight =352.44
Exact Mass =352
Molecular Formula =C21 H24N2O3
Molecular Composition =C 71.57% H 6.86% N 7.95% O 13.62%
s Iq) 11-Oxo-s,6,7,101112-hexahydrobenzo[a]'[lOlannulen-6-~(2E)-3-(1-methyllH
imidazol-4-yl)prop-2-enoate.
\ o
N O
O
Molecular Weight =350.42
Exact Mass =350
-- - - Molecular Formula =C21 H22N2O3
Molecular Composition =C 71.98% H 6.33% N 7.99% O 13.70%
Ir) 11-Methylene-5,6 7 10 11 12-hexahydrobenzo[a]I[l~annulen-6-~(2E)-3-(1
methyl
i 0 1H-imidazol-4-)prop-2-enoate.
0
N O
i
Molecular Weight =348.45
Exact Mass =348
Molecular Formula =C22H24N202
Molecular Composition =C 75.83% H 6.94% N 8.04% O 9.18%
Is) 11-(2-Methoxy-2-oxoethylidene)-5 6 7 10 11 12-hexahydrobenzo[a][10]annulen
6 yl
(2E)-3-( 1-methyl-1 H-imidazol-4-)prop-2-eno ate
CA 02471910 2004-06-28
WO 03/055861 PCT/EP02/14514
\ O
N O
' O
,O
Molecular Weight =406.49
Exact Mass =406
Molecular Forri~ula =C24H26N204
Molecular Composition =C 70.92% H 6.45% N 6.89% O 15.74%
It) 11-(Methoxymethylene)-5 6 7 10 11 12-hexahydrobenzo[a]j10]annulen 6 y~2E)
3~1
methyl-1H-imidazol-4-yl)prop-2-enoate
5
O
N O
O
Molecular Weight =378.48
_ Exact Mass =378 _ _ _ _
Molecular Formula =C23H26N2O3
Molecular Composition =C 72.99% H 6.92% N 7.40% O 12.68%
Iu) 11-(2-Ethoxy-2-oxoethylidene)-5 6 7 10 11 12-hexahydrobenzo[a~[1 ~annulen
6 yl
(2E)-3-(1-methyl-1H-imidazol-4-yl)prop-2-enoate
CA 02471910 2004-06-28
WO 03/055861 PCT/EP02/14514
31
\ O
N
Molecular Weight =420.51
Exact Mass =420
Molecular Formula =C25H28N2O4
Molecular Composition =C 71.41 % H 6.71 % N 6.66% O 15.22%
Iv) l l-Formyl-5,6 7 10 11 12-hexahydrobenzo[a]_[10]annulen-6-yl ~2EL1-methyl
1H
imidazol-4-yl~rop-2-enoate.
0
N O
O H
Molecular Weight =364.45
Exact Mass =364
Molecular Formula =C22H24N2O3
Molecular Composition =C 72.51 % H 6.64% N 7.69% O 13.17%
_5 Iw) __ 1_1-(Hydroxymethyl)-5 6 7 10 11 12-hexahydrobenzo[a]'[10]annulen-6-
yl-- (2E) 3- (1
methyl-1H-imidazol-4-yl)prop-2-enoate
0
N O
HO
Molecular Weight =366.46
Exact Mass =366
Molecular Formula =C22H26N2O3
Molecular Composition =C 72.11 % H 7.15% N 7.64% O 13.10%
Iy)11-f ~(2-O-acetylpentoF~yl)oxy~!methyll-5 6 7 10 11 12-hexahydrobenzo
to fal~lOlannulen-6-yl (2E)-3-(1-methyl-1H-imidazol-4-yl~pro~-2-enoate
CA 02471910 2004-06-28
WO 03/055861 PCT/EP02/14514
32
O
N O
O
~00~
OH
O
OH
Molecular Weight =540,62
Exact Mass =540
Molecular Formula =C29H36N2O8
Molecular Composition =C 64.43% H 6.71 % N 5.18% O 23.68%
Iz) Ethyl 11-ff(2E)-3-(1-methyl-1H-irnidazol-4-y~ ro -2-enoylloxK~-5 6 7 10 11
12-
hexahydrobenzo[a1 10] annulene-6-carboxylate
0
N O
0 0
Molecular Weight =408.50
Exact Mass =408
-- -- Molecular Formula -=C24H28N204 _ _ _
Molecular Composition =C 70.57% H 6.91% N 6.86% O 15.67%
Iaa) Ethyl 7-hydroxy-11-f f(ZE)-3-(1-methyl-1H-imidazol-4-yl)~rop-2-en~lloxy)-
7,10,11,12-tetrahydrobenzo [aj jl 0] annulene-6-carboxylate ' -
0
N O
w
i
O OH
O
Molecular Weight =422.49
Exact Mass =422
Molecular Formula =C24H26N2O5
Molecular Composition =C 68.23% H 6.20% N 6.63% O 18.93%
CA 02471910 2004-06-28
WO 03/055861 PCT/EP02/14514
33
Ibb) 11-(Acetyloxy.-1-isopro~yl-4-methyl-5,6,7,10,11,12-
hexahydrobenzo[al~lOlannulen-
6-yl (2E)-3-(1-methyl-1H-imidazol-4-yl)prop-2-enoate.
\ o
N O
O' /O
Molecular Weight =450.58
Exact Mass =450
Molecular Formula =C27H34N2O4
Molecular Composition =C 71.97% H 7.61 % N 6.22% O 14.20%
Icc) 7,11-Bis acetyloxy)-1-isopropyl-4-methyl-5,6,7,10,11,12-hexahydrobenzo
[al('10]annulen-6-yl (2E~1-methyl-1H-imidazol-4-yl)prop-2-enoate.
0
w ~N \ /
Molecular Weight =508.62
Exact Mass =508
Molecular Formula =C29H36N2O6
Molecular Composition =C 68.48% H 7.13% N 5.51 % O 18.87%
Idd) 10,11-Bis(acetyloxy)-1-isopro~yl-4-methyl-5,6,7,10,11,12-hexahydrobenzo
Ll[lOlannulen-6-yl (2E)-3-(1-methyl-1H-imidazol-4-yl)prop-2-enoate.
to
\ o
N
O
Molecular Weight =508.62
Exact Mass =508
Molecular Formula =C29H36N2O6
Molecular Composition =C 68.48% H 7.13% N 5.51 % O 18.87%
CA 02471910 2004-06-28
WO 03/055861 PCT/EP02/14514
34
Iee) 11-(Acetyloxy)-1 4-dimethyl-5 6 7 10 11,12-hexahydrobenzo[al[,-lOlannulen-
6-yl
(2E)-3 -( 1-meth-1 H-imidazol-4-yl)prop-2-enoate.
0
N O
O O
Molecular Weight =422.53
Exact Mass =422
Molecular Formula =C25H30N204
Molecular Composition =C 71.07% H 7.16% N 6.63% O 15.15%
Iff) 7,11-bis(acetyloxy)-1,4-dimethyl-5,6,7,10,11,12-
hexahydrobenzo[a][10]annulen-6-yl
(2E)-3-(1-methyl-1H-imidazol-4-yl)prop-2-enoate
0
wN \ O
~N \ O O
O O
Molecular Weight =480.57
Exact Mass =480
Molecular Formula =C27H32N2O6
Molecular Composition =C 67.48% H 6.71 % N 5.83% O 19.98%
Igg) 1011-Bis(acetyloxy)-14-dimethyl-5,6,7,10,11,12-
hexahydrobenzo[a][10]annulen-6-
_~2E~ 3-(1-methyl-1H-imidazol-4-yl)prop-2-enoate.
CA 02471910 2004-06-28
WO 03/055861 PCT/EP02/14514
O
N
Molecular Weight =480.57
Exact Mass =480
Molecular Formula =C27H32N2O6
Molecular Composition =C 67.48% H 6.71 % N 5.83% O 19.98%