Note: Descriptions are shown in the official language in which they were submitted.
CA 02472088 2004-07-08
WO 03/075981 PCT/CA03/00103
1
TITLE OF THE INVENTION
DUAL INLET MIXED-FLOW BLOOD PUMP
FIELD OF THE INVENTION
The present invention relates to a mixed-flow blood pump displaying
characteristics of both radial-flow and axial-flow pumps.
BACKGROUND OF THE INVENTION
The present specification mentions a number of references which are herein
incorporated by reference.
In North America, heart related diseases are stilt the leading cause of death.
Among the causes of heart mortality are congestive heart failure,
cardiomyopathy and cardiogenic shock. The incidence of congestive heart
failure increases dramatically for people over 45 years of age. In addition, a
large part of the population in North America is now entering this age group.
Thus, the people who will need treatment for these types of diseases
comprise a larger segment of the population. Many complications related to
congestive heart failure, including death, could be avoided and many years
added to these persons' lives if proper treatments were available.
The types of treatment available for patients of heart failure depend on the
extent and severity of the illness. Many patients can be cured with rest and
drug therapy but there are still severe cases that require various heart
surgery, including heart transplantation. Actually, the mortality rate for
patients
with cardiomyopathy who receive drug therapy is about 25.% within two years
CA 02472088 2004-07-08
WO 03/075981 PCT/CA03/00103
2
and there still is some form of these diseases that cannot be treated
medically. One of the last options that remain for these patients is heart
transplantation. Unfortunately, according to the procurement agency UNOS
(United Network for Organ Sharing in the United States), the waiting list for
heart transplantation grows at a rate of more than twice the number of heart
donors.
Considering these facts, it appears imperative to offer alternative treatments
to heart transplantation. The treatment should not only add to a recipient's
longevity but also improve his quality of life. In this context, mechanical
circulatory support through Ventricular Assist Devices (VAD) is a worthwhile
alternative given the large deficiency in the number of available organ
donors.
In the 1980's, successful experiments with mechanical hearts and VADs
serving as a bridge to transplantation increased significantly. The
accumulated knowledge in all aspects of patient care, device designs and
related problems led to the use of VADs as permanent implants. Now, it
appears appropriate to address the problem of end stage heart failure with
permanent mechanical heart implants. Among the various mechanical support
devices, axial-flow VADs with a projected life span of five to ten years
provides a very interesting approach. It is estimated that eight thousand
(8,000) patients per year in Canada and seventy-six thousand patients
(76,000) per year in the United States could benefit from VADs.
In 1980, the National Heart, Lung and Blood Institute (NHLBI) of the United
States defined the characteristics for an implantable VAD (Altieri, F.D. and
Watson, J.T., 1987, "Implantable Ventricular Assist Systems", Artif Organs,
Vol. 11, pp. 237-246). These characteristics include medical requirements
including restoration of hemodynamic function (pressure and cardiac index)
avoidance of hemolysis, prevention of clot formation, infection .and bleeding,
CA 02472088 2004-07-08
WO 03/075981 PCT/CA03/00103
3
and minimisation of the anti-coagulation requirement. Further technical
requirements include: small size, control mode, long life span (> 2 years),
low
heating, noise and vibration.
VADs can be used in several circumstances where a patient has poor
hemodynamic functions (low cardiac output, low ejection fraction, low systolic
pressure). Whatever the origin of the cardiac failure, the goal of the VAD is
to
help the heart in its pumping action. The VAD reduces the load on the heart
by enhancing circulation, thus restoring the hemodynamic functions and
providing improved end organ perfusion. Many current VAD devices can
achieve these goals; however they are not optimal, and hemolysis and
thrombus formation are still important problems requiring investigation.
In the 1970's, the first approach to the problem of mechanical support was to
imitate as much as possible the heart physiology. This resulted in the
development of several pulsatile devices, some of these initial designs being
still in use. The first developments were pneumatically driven devices while
a second generation of pumps was electrically actuated. In the 1990's, a new
generation of pumps has emerged which addresses certain problems
associated with previous devices (size and power consumption). These
pumps are non-pulsatile devices divided into two main categories: radial-flow
blood pumps, and axial-flow blood pumps.
In a non-pulsatile VAD, an impeller is enclosed in a housing and continuously
rotates to produce a pumping action. The faster the rotation, the higher the
blood flow. These devices are called non-pulsatile or continuous because
they provide for a constant blood flow. Most axial-flow blood pumps operate
around 10 000 RPM (Rotations Per Minute). However, in in-vivo conditions,
there is a dynamic range (about 1000 RPM around the operating point) over
CA 02472088 2004-07-08
WO 03/075981 PCT/CA03/00103
4
which the output flow is pulsatile. Since the native heart is still
contracting, a
pressure difference between the ventricle (inlet) and the aorta (outlet) is
created. This pressure variation will produce a variation in the pump flow.
The
range of rotational speed over which pulsatile flow occurs is small; at lower
speed back flow is observed (in diastole) and at higher speed the load on the
heart is reduced to zero. In the latter case, no pressure variation occurs
resulting in non-pulsatile flow.
Many advantages are associated with the use of non-pulsatile VADs and they
all have a strong impact on the physiology as well as on the clinical
management. These advantages include:
Size:
Non-pulsatile VADs provide a much smaller volume than pulsatile
VADs, around 25 cc for an axial-flow blood pump, and 100 cc for
a radial-flow blood pump, compared to 150 cc and more for
pulsatile devices. For the sake of comparison a complete axial-flow
VAD is usually smaller than the graft used for pulsatile pump. The
clinical impact is the possibility to use this type of VADs in small
adults as well as in children. Also, the small dimensions allow for
placement of the pump in a more orthotopic position; that is, in the
thorax near the heart instead of the upper abdomen. This
eliminates the use of long cannula passing through the diaphragm.
Furthermore, for axial-flow VADs, the shape and size can be
selected allowing for placement of the VAD in an intra-ventricular
position.
Powe r:
The electrical power required to drive a non-pulsatile VAD is lower
CA 02472088 2004-07-08
WO 03/075981 PCT/CA03/00103
than for pulsatile VADs.
Simplicity:
Non-pulsatile VADs are mechanically simpler than pulsatile VADs;
5 they do not require complex structures such as valves, diaphragms,
blood sacs, vents or compliance chambers. Non-pulsatile
continuous VADs are made of a simple motor to which is coupled
an impeller contained in a housing. One important advantage of a
simple mechanical design is its extended durability. Durability as
long as five to ten years (Nose, Y., 1995a, "Can We Develop a
Totally Implantable Rotary Blood Pump? ", Artificial Organs, Vol.
19, pp. 561-562; and Jarvik, R.K., 1995, "System Consideration
Favoring Rotary Artificial Hearts with Blood-Immersed Bearing",
Artificial Organs, Vol. 19, pp. 565-570) could be achieved with
continuous VADs compared with two years for pulsatile VADs
(Pierce, W.S., Sapirstein, J.S. and Pae, W.E., 1996, "Total Artificial
Heart: From Bridge to Transplant to Permanent Use", Ann Thorac
Surg, Vol. 61, pp. 342-346). In principle, this would allow not only
to use a non-pulsatile VAD as a bridge to transplantation but also
as long term mechanical support.
Hemolysis:
Hemolysis, or tearing of the red blood cells, can be estimated in
vitro with a parameter called the Normalised Index of Hemolysis
(NIH).
Infection:
Interestingly, the probability of infection is reduced with continuous
VADs. This is due in large part to the transcutaneous vent of a
CA 02472088 2004-07-08
WO 03/075981 PCT/CA03/00103
6
pulsatile VAD which is an open door for opportunist infections and
therefore requires daily cleaning.
Patient Issues:
Non-pulsatile VADs require less maintenance allowing the patient
a greater autonomy. Also, most patients with a VAD are discharged
from the hospital and returned to a normal life after about a month.
Presently, because of the vent in pulsatile VADs, patients cannot
take a bath or swim since water could enter the motor
compartment. Continuous VADs are less restrictive and allow the
patient to practice more activities.
Radial-flow blood pumps were first used in cardio-pulmonary bypass for heart
surgery. Based on results obtained with the Bio-Medicus pump (Medtronic
Bio-Medicus Inc., Eden Prarie, MN), several groups decided to develop much
smaller radial-flow blood pumps so that they could be totally implantable. In
radial-flow blood pumps, the rotation of the impeller produces a centrifugal
force that drags blood from the inlet port on top to the outlet port at the
bottom. To produce rotation of the impeller, the impeller is coupled to an
electric motor. This coupling is made either (a) magnetically by means of
permanent magnets located under the impeller and on the rotor of the motor
or (b) mechanically by means of a shaft interposed between the impeller and
the motor's rotor. Magnetically coupled devices generally show better
functionality because no seal is required between the motor and the impeller
shaft.
A problem related to radial-flow blood pumps is that although they are much
smaller than pulsatile pumps, they are still too large to be totally implanted
in
a human thorax thus eliminating any intra-ventricular implantation.
CA 02472088 2004-07-08
WO 03/075981 PCT/CA03/00103
7
To overcome the above-mentioned problem related to radial-flow blood
pumps, axial-flow ventricular assist blood pumps were developed. These
axial-flow blood pumps can decrease the hemolysis rate by decreasing the
time of exposure of the blood to friction forces and by reducing the intensity
of these forces. Another interesting advantage is that axial-flow blood pumps
are generally much smaller than radial-flow blood pumps.
The first commercially available axial-flow blood pump was the HemopumpT""
(Medtronic Inc. Minneapolis, MN) used as short term circulatory support.
Based on the good results obtained with this pump, several groups have
initiated the development of totally implantable axial-flow VADs for long term
use.
A few axial-flow VADs are presently under intensive development. Examples
are: the Jarvik 2000T"", by the Texas Heart Institute (Houston, Texas);
MicroMed DeBakeyT"" by MicroMed Technology (also of Houston, Texas); and
HeartMate IIT"", by Thoratec (Pleasanton, California). All of these groups
have
already started in-vivo experiments on animals and humans although they still
perform in-vitro trials. An overview of the operation of these pumps is given
hereinbelow.
Jarvik 2000T""
This axial-flow blood pump comprises two stators, one at the inflow
and one at the outflow. These stators have two functions: they
support the bearing shaft around which the impeller will rotate
(middle part) and they modify the blood flow path. The inflow stator
initiates the rotation of the flow so that the blade tip of the rotor
does not create too much shear stress on the blood cells. The
CA 02472088 2004-07-08
WO 03/075981 PCT/CA03/00103
8
outflow stator straightens the flow so that blood from the pump
enters the blood stream with a generally axial-flow profile.
Permanent magnets are enclosed in the center of the impeller and
two motor windings are located in the casing on each side of the
rotor. This configuration constitutes a DC brushless motor; this is
a simple and durable motor which minimises the number of
mechanical parts. The power cable is connected directly to the DC
brushless motor controller to change motor speed.
To implant the device, the chest is opened by means of a left
thoracotomy and no cardiopulmonary bypass is used. The pump
axial outflow is anastomosed to the aorta with a DacronT"~ graft.
Then a ventriculotomy is made to insert the pump into the ventricle
through a sewing ring attached to the apex.
Micromed DeBakeyT""
The Micromed DeBakeyT"" VAD is very similar to the Jarvik 2000T""
design. Indeed, this concept of VAD is based on a DC brushless
motor with blood-immersed bearings, a central impeller and two
fixed side pieces. The Micromed VAD is described in U.S. patent
No. 5,527,159 (Bozeman, Jr. et al.) issued on June 18, 1996.
Blood enters on the left side and passes through a flow
straightener preventing pre-rotation thereof. Then, the blood
reaches the inducer/impeller; the inducer initiates rotation of blood
before this blood reaches the impeller. However, it should be noted
that the impeller produces the effective pumping action. Finally, a
flow diffuser converts the tangential flow into an axial flow. The
inducer comprises three blades and the impeller is provided with
CA 02472088 2004-07-08
WO 03/075981 PCT/CA03/00103
9
six blades. The three blades of the inducer co-extend with three
associated blades of the impeller. Each blade of the impeller
contains eight cylindrical permanent magnets. Finally, a winding is
placed outside the pump to complete the motor assembly, and the
rotor is supported by a pair of bearings.
HeartMate IIT""
This pump is similar to the Micromed DeBakeyT"~ and the Jarvik
2000T"~ pumps. It is placed next to the heart and is connected
between the apex of the heart and the aorta. It is also a sealed
bearing type pump and, accordingly, requires a purge system. This
system has a second pump which injects 15 ml/day of sterile
solution in the sealed area. A pump without purge system is now
under development. Three animals have been supported for one
month with the HeartMate IIT"" (Konishi, H., Antaki, J.F. et al.,
1996b, "Long-term Animal Survival with an Implantable Axial Flow
Pump as a Left Ventricular Assist Device", Artificial Organs, vol. 20,
pp. 124-127). '
Other axial-flow blood pumps have been proposed. For example, U.S. patent
No. 5,205,721 (Isaacson) issued on April 27, 1993 discloses an axial-flow
blood pump having a hydrodynamically suspended rotor centrally positioned
with respect to the stator. Hydrodynamic bearings are created by two spaces
in which blood must flow to create the hydrodynamic support; this in turn
produces shearing forces applied to the blood. In addition, Isaacson teaches
three types of impeller blades.
U.S. patent No. 5,211,546 granted to Isaacson et al. on May 18, 1993
teaches an axial-flow blood pump which is similar to that of U.S. patent No.
CA 02472088 2004-07-08
WO 03/075981 PCT/CA03/00103
5,205,721. A rotor is suspended radially by hydrodynamic bearings. In certain
embodiments, a radially centered thrust bearing element is provided to
stabilise rotation of the suspended rotor.
5 U.S. patent No. 5,290,227 (Pasque) granted on May 1St, 1994 proposes an
axial-flow blood pump having a rotor assembly described generally as a
hollowed-out cylinder provided with rotor vanes which extend from the inner
surtace of the hollowed cylindrical rotor towards the central rotation axis of
the
rotor. This design generates two pumping zones inside the pump, one of
10 these zones being an outer annulus which is expected to create substantial
shearing of the blood in the outer part of the rotor.
European Patent No. EP 0 060 569 granted to Olson et al. on September
22"d, 1982 teaches a magnetically suspended and rotated impeller which
comprises a bulky valve member which may be included as part of the
impeller. Moreover, this European patent teaches impeller blades which
axially extend outside of a shroud to connect to the valve member.
Pumps with impeller blades attached to a hollow cylindrical shaft, the shaft
rotating around a fixed axle when a magnetic field is applied, reveal a
secondary fluid flow path in the same direction as the primary path. A primary
annular flow path is formed between the hollow shaft and the pump housing,
and a secondary annular flow is formed between the hollow shaft and the
supporting axle. However, these secondary annular paths have minimal flow
rates and are used to insure proper lubrication of bearing faces or the
cleaning of regions which would otherwise collect debris.
CA 02472088 2004-07-08
WO 03/075981 PCT/CA03/00103
11
SUMMARY OF THE INVENTION
The present invention relates to a mixed-flow blood pump presenting features
of both axial-flow and radial-flow pumps. This mixed-flow blood pump
comprises a stationary housing structure and a rotative impeller. The
stationary housing structure defines at least one blood inlet, a blood outlet,
and a blood conduit between these blood inlet and blood outlet, and the
rotative impeller is mounted in the blood conduit. The at least one blood
inlet,
the blood outlet, the blood conduit and the rotative impeller have respective
structures and configurations that operate the mixed-flow blood pump at a
given point of a maximum hydraulic efficiency curve relating a specific pump
rotational speed and a specific pump diameter, that given point being located
within a transition region of the maximum hydraulic efficiency curve between
axial-flow and radial-flow pumps.
Operating a mixed-flow blood pump, which presents the flow characteristics
of an axial-flow blood pump while maintaining the throughput of a radial-flow
blood pump, at the given point of the maximum hydraulic efficiency curve
located within the transition region of the curve between radial-flow and
axial-
flow pumps enables obtention of a pumping efficiency as high as 53%.
The foregoing and other objects, advantages and features of the present
invention will become more apparent upon reading of the following non-
restrictive description of illustrative embodiments thereof, given by way of
example only with reference to the accompanying drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
In the appended drawings:
CA 02472088 2004-07-08
WO 03/075981 PCT/CA03/00103
12
Figure 1 is a cross sectional view of a human heart in which an illustrative
intra-ventricular embodiment of the mixed-flow blood pump according to the
present invention is installed;
Figure 2 is a graph showing, for different types of pumps, a curve relating a
specific pump rotation speed NS with a specific pump diameter DS at the points
where the pump is operating at maximum hydaulic efficiency;
Figure 3 is a side elevational view of the external shape of the illustrative
intra-ventricular embodiment of the mixed-flow blood pump according to the
present invention;
Figure 4a is a side elevational and cross sectional view of the illustrative
intra-
ventricular embodiment of the mixed-flow blood pump according to the
present invention;
Figure 4b is a side elevational and cross sectional view of an illustrative
extra-
ventricular embodiment of the mixed-flow blood pump according to the
present invention;
Figure 5a is an enlarged, partial side cross sectional view showing a first
end
mount of the drive shaft of the mixed-flow blood pump of Figure 4a or 4b;
Figure 5b is an enlarged, partial side cross sectional view showing a second
end mount of the drive shaft of the mixed-flow blood pump of Figure 4a or 4b;
and
Figure 6 is a schematic view of an illustrative embodiment of a VAD system
CA 02472088 2004-07-08
WO 03/075981 PCT/CA03/00103
13
implanted in a human being and comprising the mixed-flow blood pump of
Figure 4a.
DESCRIPTION OF THE ILLUSTRATIVE EMBODIMENT
In the following description, important aspects of the pump design are
addressed. In particular, the pump design takes into consideration anatomical
and physiological considerations combined with mechanical, electrical and
material requirements. Finally, following this discussion, global
characteristics
of a VAD system are presented.
It should first be noted that the mixed-flow blood pump of the present
invention is not restricted to an application to an implantable VAD system.
Since the mixed-flow blood pump according to the invention overcomes a
number of drawbacks of the current blood pumps, those of ordinary skill in the
art will understand that such a mixed-flow blood pump can be used as part of
an intra-corporal system such as an intra-ventricular VAD, or an extra-
ventricular VAD (for example a VAD located in the abdomen or thorax), or
alternatively as a para-corporal or extra-corporal VAD (for example in a
bridge
to heart transplantation). It shall also be understood that the mixed-flow
blood
pump of the present invention can be used in temporary VADs (for example
a bridge to heart transplant) or permanent VADs:
ANATOMICAL, PHYSIOLOGICAL AND SURGICAL CONSIDERATIONS
As previously discussed, bleeding is an important problem associated with
patients who receive a VAD; in fact 30 % of patients suffer from this problem
(Defraigne, J.O., Limet, R., 1996a, "Les assistances circulatoires: Partie I.
Indications et description des systemes", Rev Med Liege, Vol. 51, pp.295-
CA 02472088 2004-07-08
WO 03/075981 PCT/CA03/00103
14
306). The risk of infection is another quite important problem. These medical
and surgical considerations are met by the illustrative intra-ventricular
embodiment of the mixed-flow blood pump as shown in Figure 1. This position
eliminates the need for inflow and outflow grafts and their anastomoses to
thereby reduce the risk of bleeding and infection. This has also the obvious
advantage of considerably reducing the implantation time. Figure 1 illustrates
the proposed position of the illustrative intra-ventricular embodiment of
mixed-
flow blood pump 2 in the left ventricle 4.
The mixed-flow blood pump 2 has also been designed to fit in small adults
and in teens. Since the physical size and shape of the mixed-flow blood pump
2 are greatly influenced by the desired location of the pump, a good
description of the ventricle anatomy is required. Feigenbaum, Harvey,
"Echocardiography", 5th edition, 1994, Lea & Febiger, Philadelphia, presents
several dimensions of the heart normalised by the BSA (Body Surface Area).
These anatomical dimensions have been statistically determined and are
known to represent 95% of the population. Taking into consideration the
above-identified statistics, a ventricular dimension for humans corresponding
to a BSA of 1.5 m2 was used to design the pump.
It will be understood that the physical size and shape of the mixed-flow blood
pump 2 could also be adapted to meet the anatomical dimensions of
individuals falling outside this 95% of the population. Similarly, the size
and
shape could be adapted to specific and particular individuals and heart
conditions.
For 95% of the population, the internal diameter of the left ventricle 4
ranges
from 37 to 46 mm in diastole and between 22 to 31 mm in systole. This
diameter is determined at the centre of the ventricular length (segment AB in
CA 02472088 2004-07-08
WO 03/075981 PCT/CA03/00103
Figure 1 ). The diameter near the apex at the first third of the ventricular
length
is about 1.5 cm (segment CD of Figure 1 ). The internal length of the
ventricle
from the apex to the aortic valve ranges from 55 to 70 mm. Finally, the other
important parameter is the surface of the aortic valve opening which ranges
5 from 2.5 to 4 cm2.
From a surgical point of view, the favoured insertion procedure is to use the
same approach as with valve replacement. According to this procedure, an
incision is made at the root of the aorta 6 (Figure 1 ) and the mixed-flow
blood
10 pump 2 is inserted though the aortic valve and then into the left ventricle
4.
The mixed-flow blood pump 2 is then pushed until its base reaches the
myocardium at the apex 8. In order to prevent motion thereof, the mixed-flow
blood pump 2 should be fixed. Also in accordance with an illustrative
embodiment, an outflow cannula should pass through the aortic valve to
15 further reduce bleeding.
One of the main roles of the mixed-flow blood pump 2 is to restore a
hemodynamic function in patients with cardiac failure. Depending on the
severity of the failure and the BSA, the pump 2 is susceptible to work at flow
rates between 2 to 6 litres per minute (I/min) against a pressure as high as
120 mmHg and, more commonly, at a flow rate between 3 to 5 I/min against
a pressure of 80 mmHg.
Another important consideration for blood pump design is the hemolysis rate.
Hemolysis is the tearing of red blood cells which empties the content of the
cells in the blood stream resulting in free haemoglobin; the normal level of
plasma free haemoglobin is around 10 mg/dl. A blood pump with a normalised
index of hemolysis (NIH) of 0.005 g/100 I and lower is considered to be almost
athromatic for red blood cells. A NIH of about 0.05 g/100 I could be
tolerated.
CA 02472088 2004-07-08
WO 03/075981 PCT/CA03/00103
16
A NIH situated between 0.005 g/100 I to 0.05 g/100 I can therefore be
envisaged for a VAD. Of course, a NIH as close to 0.005 g/100 I as possible
is desirable.
Platelets are other important blood elements; their activation by high
hydromechanical forces should be avoided in order to prevent clot formation.
MIXED-FLOW FLOW BLOOD PUMP DESIGN: Mechanical aspects
This section of the disclosure is divided into parts A, B and C. Part A
describes the general approach used for the selection of the pump
configuration. Part B describes the external shape and size of an illustrative
embodiment of the mixed-flow blood pump according to the invention, and
part C describes the internal structure of this illustrative embodiment of
mixed-
flow blood pump.
Part A: Selection of a general pump configuration
There are three existing non-pulsatile pump configurations, all turbines, and
having characteristics which make them potential candidates for a cardiac
blood pump: radial-flow, axial-flow and mixed-flow pumps. Given their
relatively small diameter, cylindrical shape and high throughput, axial-flow
pumps display a number of characteristics which make them particularly well
suited for implantation. However, other pump configurations also exhibit
characteristics, different from those of the axial-flow pump, which would also
be useful in a cardiac blood pump.
When designing turbine pumps, dimensionless characteristic values are used
to compare different pump configurations. Dimensionless characteristic values
CA 02472088 2004-07-08
WO 03/075981 PCT/CA03/00103
17
provide useful indications to pump designers of expected performance
regardless of the size of the pump, a comparison which would otherwise
prove difficult given a virtually infinite number of operating parameters that
depend on infinite variations of internal pump geometry. These dimensionless
characteristic values, therefore, can be used to provide an objective starting
point for the selection of a general pump configuration.
Two of these dimensionless characteristic values are the specific rotation
.speed NS of the pump and the specific pump diameter DS. They are defined
as follows:
Qaz
H3/4 (1 )
D . Haa
D.s - Qaz (2)
where i~ is the rotation speed the pump in rad/s, Q is the flow rate in
m3/second, H is the head (i.e. the gain in pressure) of the pump and D the
diameter of the pump, both in meters. NS remains the same regardless of the
size of the pump and therefore provides an accurate measure of the
performance of a given pump design. DS relates the pump diameter to the
pump head H and flow rate Q.
Referring to Figure 2, known are curves which relate the specific speed NS
with the specific diameter D$ at the points where the pump is operating at
maximum hydraulic efficiency. In this regard, hydraulic efficiency is
expressed
as the percentage of the power input to the pump which is converted to
energy of movement of the fluid within the pump. From the curve of Figure 2
CA 02472088 2004-07-08
WO 03/075981 PCT/CA03/00103
18
and equation (1) above, it follows that optimally efficient pumps having a
higher specific speed also have a smaller size.
As referred to above, there are three (3) principal categories of non-
pulsatile
pumps characterised by the direction of flow of fluid through the puriip
relative
to the axis of rotation: axial-flow, radial-flow and mixed-flow pumps.
In axial-flow pumps the direction of fluid flow is parallel to the axis of
rotation.
The pressure differential, or head, is produced by a change in the amount of
tangential movement. Characteristics associated with axial-flow pumps
include high flow rate Q and small head H. This results in high specific
speeds
NS.
In radial-flow pumps a large portion of the throughput, either on the outlet
or
the inlet, is radial, i.e. perpendicular to the axis of rotation. This change
in
direction causes an increase in pressure. Contrary to an axial-flow pump, a
radial-flow pump is characterised by relatively large head H and smaller flow
rate Q, resulting in lower specific speeds NS.
Located between radial-flow pumps and axial-flow pumps are mixed-flow
pumps where the direction of flow at the output or input is composed of both
radial-flow and axial-flow components. As would be expected, the specific
speed of mixed-flow pumps is located between the specific speed of axial-flow
and radial-flow pumps.
In order to determine an optimised choice for a pump, it is necessary to
evaluate the specific speed NS in light of the characteristics in terms of
head
H and flow rate Q projected for the pump. As discussed above, the pump will
typically be operated with a flow rate of 5 I/min and a head of approximately
CA 02472088 2004-07-08
WO 03/075981 PCT/CA03/00103
19
100 mmHg. Additionally, current motor technology provides small yet efficient
motors operating at a speed of 11,000 RPM. This gives a specific speed of
1.62.
Referring again to Figure 2, an indication is given to the ranges of NS and DS
within which a given pump configuration will provide efficient operation. The
specific speed NS of 1.62 falls within a transition region of the curve
between
axial-flow and radial-flow pumps. In this transition region, a mixed-flow pump
topology would yield a higher efficiency than purely radial-flow or axial-flow
pumps. Additionally, the specific diameter DS is around 2 which, by applying
equation (2) above, yields a characteristic diameter of 9.83 X 10-3 for the
pump, i.e. a very small pump.
Part B: External design requirements
The external design (shape and size) of the mixed-flow blood pump 2 (Figure
1 ) depends on the anatomic dimensions of the left ventricle 4. Figure 3
illustrates the external shape of the mixed-flow blood pump 2 and the critical
geometric parameters thereof.
Pump Inlets
Figure 1 shows that the illustrative embodiment of the mixed-flow blood pump
2 rests at the bottom of the left ventricle 4, in the region of the apex 8 of
the
heart 30.
Referring to Figure 3, in order to prevent the inner walls of the left
ventricle 4
from completely obstructing blood intake, two axially spaced apart inlets 12
and 14 are provided. Additionally, a first end of the pump presents the
CA 02472088 2004-07-08
WO 03/075981 PCT/CA03/00103
generaly configuration of a hemisphere 16. The diameter of the hemisphere
16 is set to approximately 20 mm, which is smaller than the segment CD (see
Figure 1) and suitable to limit the level of pressure on the walls of the left
ventricle 4 near the apex 8.
5
The inlet 12 is found at the first end of the pump 2 between the hemisphere
16 and a first end of an axial, cylindrical member 18. As illustrated in
Figure
4, the cylindrical member 18 contains the stator windings 80. A first series
of
narrow, axially extending supports 20 spread out evenly around the axis of
10 the pump 2 connects the hemisphere 16 to the cylindrical member 18.
The second inlet 14 is formed between the second end of the cylindrical
member 18 and an impeller housing 22. A second series of narrow, axially
extending supports 24 spread out evenly around the axis of the pump 2
15 interconnects the cylindrical member 18 to the impeller housing 22. As can
be
seen, the second inlet 14 is axially spaced apart from the first inlet 12. The
separation between the inlets 12 and 14 reduces the effect occlusion of one
of the pump inlets may have on normal operation of the pump 2.
20 Oufiflow Cannula
Referring back to Figures 1 and 3, at the second end of the mixed-flow blood
pump 2, the outflow diameter 26 (see Figure 3) is reduced so as to reduce as
much as possible the obstruction caused by an outflow cannula 28 to the
operation of the aortic valve (not shown); since the function of the mixed-
flow
blood pump 2 is to assist blood circulation, blood flow contribution from the
natural contraction of the heart 30 should be maintained. In an illustrative
embodiment, the area of the outflow cannula 28, corresponding to diameter
26, is 1.3 cmZ.
CA 02472088 2004-07-08
WO 03/075981 PCT/CA03/00103
21
As illustrated, the outflow cannula 28 is, in the illustrative embodiment,
integral with the impeller housing 22.
As can be better seen from Figure 3, a blood diffuser 32 is formed at the free
end of the cannula 28, integrally therewith. The function of the diffuser 32
is
to reduce the shear stress on blood cells. Without diffuser 32, the velocity
of
blood ejected from the pump 2 is higher than the velocity of blood ejected
from the heart 30. The difference in velocity between these two blood flows
would result in shear stress proportional to this difference. Since the
velocity
is inversely proportional to the cross-sectional area, a solution for reducing
the
relative velocity of the blood flows from the pump 2 and from the heart 30 is
(a) to increase the area of the orifice 34 of the cannula 28 to reduce the
velocity of the flow of blood from the pump 2, and (b) to decrease the area
occupied by the blood flow from the heart to increase the velocity of the
latter
blood flow. This is exactly the role of the diffuser 32. Of course, parameters
such as the angle of opening and the length of the diffuser 32 can be adjusted
at will to fit the mechanical characteristics of the pump 2 in view of
minimising
the shear stress on the blood cells.
Housing
The diameter of the mixed-flow blood pump 2 is a compromise between
pumping requirements and minimal interference with heart contraction. In an
illustrative embodiment, the maximum allowable diameter 36 is about 22 mm
which is the diameter of the left ventricle 4 in systole. This dimension is
reasonable since people with heart failure generally have dilated ventricles.
The maximum length 38 of the mixed-flow blood pump 2, as illustrated in
CA 02472088 2004-07-08
WO 03/075981 PCT/CA03/00103
22
Figure 2, is set in regard of the average distance between the apex 8 and the
aortic valve 40 of the heart 30. In an illustrative embodiment, the length 38
of
the mixed-flow blood pump 2 is about 55 mm. As shown in Figure 1, a
reduction of the pump diameter (see 40) toward the outflow increases the
aortic valve clearance in order to minimise interference with the aortic valve
function.
Since in this illustrative embodiment the mixed-flow blood pump 2 will be
completely located inside the left ventricle 4, blood will circulate around
the
pump 2. As a consequence, all external surfaces of the pump 2 should be as
smooth as possible and avoid as much as possible abrupt deviations to
thereby minimise vortices, turbulence and recirculation zones which may be
at the origin of clot formation. To overcome this problem, the pump 2 and
other components may be machined from surgical quality titanium.
Fixation Mechanism
At the first end of the pump 2, a fixation mechanism 42 is provided. As an
example, fixation mechanism 42 comprises:
- an elongated needle member 44 extending from the hemisphere 16, this
needle member 44 being driven from the inside of the left ventricle 4
through the myocardium and the epicardium at the apex 8 of the heart 30;
and
- a fixation disk 46 fastened to the free end of the needle member 44 on the
outside of the heart 30 to firmly fix the mixed-flow blood pump 2 within the
left ventricle 4.
CA 02472088 2004-07-08
WO 03/075981 PCT/CA03/00103
23
Of course, it is within the scope of the present invention to employ any other
type of fixation mechanism.
Electrical Supply
The required electrical supply for the operation of the motor (to be described
herein below) is made through a wire that could, for example, extend from the
mixed-flow blood pump 2 along the needle member 44 to reach a controller
and an energy source (both to be further described hereinafter).
Part C: Internal design characteristics
In the design of the internal components, some of the characteristics of axial-
flow pumps have been retained and combined with some of the
characteristics of radial-flow pumps to form the mixed-flow blood pump 2.
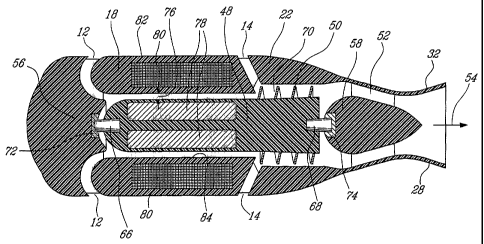
Referring to Figure 4a, the mixed-flow blood pump 2 comprises a stationary
housing structure comprising an inflow bushing mount 56 which defines the
hemisphere 16, the cylindrical member 18, the impeller housing 22, a
stationary outflow stator 52, an outflow bushing mount 58, the outflow cannula
28 and the blood diffuser 32. Blood pump 2 further comprises a rotative
impeller 50 with an impeller shaft 48 and an impeller blade 70.
Current wet motor axial-flow blood pumps use permanent magnets inserted
either in the central hub or in the impeller blades. Both methods require
important compromises. Insertion of the permanent magnets in the central
hub requires a large hub to locate the permanent magnets close to the motor
windings for obvious electromagnetic coupling reasons. In contrast, a small
hub has the advantage of increasing the pumped volume of blood. Insertion
CA 02472088 2004-07-08
WO 03/075981 PCT/CA03/00103
24
of the permanent magnets in the impeller blades yields a compromise since
the geometry of the blades must be curved for pumping efficiency. As a
consequence, some of the embedded magnets move away from the windings
as the blades) curve(s).
In the approach proposed by the illustrative embodiment of the present
invention, the mixed-flow blood pump 2 presents an enclosed-impeller mixed-
flow configuration. In the illustrative intra-ventricular embodiment of this
configuration as illustrated in Figure 4a, the impeller blade 70 is a
spiralling
auger-type impeller blade having a constant height and being rigidly attached
to the outer surtace of the impeller drive shaft 48 and enclosed in the
impeller
housing 22. Obviously, the impeller blade 70 fits snugly withing the impeller
housing 22. Of course, the shape (curvature and angulation) of the impeller
blade 70 should be optimally designed in relation to pumping pertormance
and other hydrodynamic considerations. In particular, the influence of the
blade angulation on the level of shearing stresses, turbulence and cavitation
responsible for red blood cell damage and increase of hemolysis rate must be
carefully taken into consideration.
The end portion of the impeller shaft 48 bearing the impeller blade 70 is
slightly tapered in a direction opposite to the direction of blood flow. This
contributes to create the mixed-flow operation of the pump 2. More
specifically, this slight taper imparts to the blood flow both axial and
radial
components.
Still referring to Figure 4a, at both ends of the impeller drive shaft 48 end
pivots 66 and 68 protrude. The function of the end pivots 66 and 68 is to
support the impeller drive shaft 48 at each end. The end pivots 66 and 68 are
respectively inserted into the respective bushing mounts 56 and 58. Bushing
CA 02472088 2004-07-08
WO 03/075981 PCT/CA03/00103
72 is mounted on the inner face of the inflow bushing mount 56. In the same
manner, bushing 74 is mounted on the adjacent face of the outflow bushing
mount 58. The bearings formed by the bushing and pivot assemblies 66;72
and 68;74 are the only mechanical parts subject to wear. Therefore, these
5 parts are expected to be mostly responsible for the life span of the mixed-
flow
blood pump 2.
Still referring to Figure 4a, the cylindrical gap 76 separating the impeller
shaft
48 and the inner surface of the cylindrical member 18 should be sufficiently
10 thick to produce sufficient blood flow in order to increase washout and
prevent
clot formation. On the other hand, too large a gap 76 may either reduce the
pump efficiency (by reducing the electromagnetic coupling) or result in higher
hemolysis. Just a word to mention that the section of the impeller shaft 48
within the cylindrical member 18 is cylindrical whereby the gap 76 has a
15 constant thickness.
In the illustrative embodiment of Figures 1 and 4a, the volume of blood
pumped through the second inlet 14 is typically 4 liters/minute. This is
higher
than the volume of blood pumped through the first inlet 12 and the cylindrical
20 gap 76 which is typically 1 liter/minute. A number of benefits is
associated
with the higher volume blood pumped through the second inlet 14. For
example, installation of the mixed-flow blood pump 2 in the left ventricle 4
of
a patient with the cannula 28 extending through the aortic valve generally
interferes with proper operation of the aortic valve. Optimally, the aortic
valve
25 should continue to function normally; however, in some cases, it has been
observed that the aortic valve ceases to function further until it remains
closed
around the cannula 28. Typically, blood would have the tendency to collect in
the region close to the aortic valve and the cannula 28 which might lead to
thrombus formation and other adverse effects. The increased volume of blood
CA 02472088 2004-07-08
WO 03/075981 PCT/CA03/00103
26
pumped through the second inlet 14 has the effect of creating turbulence in
the region within the ventricle 4 bordered by the aortic valve and the cannula
28, thus providing improved washout of this region and thereby reducing the
effects of the malfunctioning aortic valve.
On the one hand, the volume of blood pumped through the second inlet 14
contributes to the radial-flow operation of the mixed-flow blood pump 2. On
the other hand, the volume of blood pumped through the first inlet 12 and the
cylindrical gap 76 contributes to the axial-flow operation of the mixed-flow
blood pump 2.
The stationary outflow stator 52 comprises a plurality of blades shaped and
disposed around the outflow bushing mount 58 to transform the rotational
motion of the flow about the longitudinal axis 54 into a translational motion.
Therefore, the stationary outflow stator 52 constitutes a blood flow
straightener.
As previously mentioned, the pump design should minimise shearing stress
in order to minimise hemolysis. In that context, reduction of the rotational
speed would obviously contribute to reduce hemolysis. However, reduction
of the rotational speed while pumping the same volume of blood requires an
increase of the volume of blood contained in the rotor zone of the pump 2.
The volume of blood contained in the rotor zone can be increased by either
increasing the diameter of the rotor zone, or alternatively minimising the
volume of the central hub of the pump rotor.
Design of the internal surfaces of the pump is also important for minimising
shearing stresses in order to minimise hemolysis. Figure 5a shows the
configuration of the region between the inflow bushing mount 56 and the
CA 02472088 2004-07-08
WO 03/075981 PCT/CA03/00103
27
impeller drive shaft 48. Figure 5b shows the configuration of the region
between the impeller drive shaft 48 and the outflow bushing mount 58.
Referring to Figure 5a, the end surtace 60 of the impeller drive shaft 48 is
convex and generally hemispheric while the confronting surface 62 of the
bushing mount 56 is concave and generally hemispheric. Forming the
surfaces in this manner reduces the shearing stress placed on the blood thus
minimising hemolysis (see flow 61 ).
The outflow bushing mount 58 is formed with a similar convex, generally
hemispheric end surface to reduce shearing stress at the outlet (see Figure
5b, in particular flow 65). The confronting end of the impeller shaft 48 is
flat
and perpendicular to the axis 54.
Additionally, referring to both Figures 5a and 5b, end pivots 66 and 68 by
which the respective ends of the impeller shaft 48 are mounted to their
respective bushing mounts 56, 58 are slightly tapered in a direction opposite
to blood flow. This helps prevent the creation of eddies and the collection of
debris in proximity to the end pivots. Normally, a taper of the order of five
degrees (5°) is adequate.
In this manner, pivot 66 has a smaller diameter free end received within the
bushing 72. In the same manner, the pivot 68 has a larger diameter free end
received in the bushing 74.
Figure 4b illustrates an alternative, illustrative extra-ventricular
embodiment
of mixed-flow blood pump 100. Pump 100 is adapted for use externally of the
heart as a ventricle bypass/assist. In this embodiment the pump 100 would
typically be implanted above the diaphragm in the thorax and would be
CA 02472088 2004-07-08
WO 03/075981 PCT/CA03/00103
28
connected to the circulation system using standard vascular grafts, a first
graft
102 being attached to the inflow end of the pump and a second graft 104
being attached to the outflow end of the pump 100.
Similar to the mixed-flow blood pump 2, the alternative illustrative
embodiment
100 as illustrated in Figure 4b comprises a stationary housing structure
including an inflow bushing mount 122, a cylindrical member 108, an impeller
housing 106, a stationary outflow stator 114, an outflow bushing mount 124,
an outflow cannula 150 and a blood diffuser 151. Blood pump 100 further
comprises a rotative impeller 112 with an impeller shaft 110 and an impeller
blade 116.
The impeller blade 116 is a spiralling auger-type impeller blade rigidly
connected to the impeller drive shaft 110 and enclosed in the impeller housing
106.
A series of longitudinal ridges 118, typically five (5) evenly spaced around
the
pump axis 119, support the cylindrical member 108 within the impeller
housing 106 thereby forming a series of longitudinal flow passages such as
120 between the cylindrical member 108 and the impeller housing 106.
The longitudinal ridges 118 extend to meet and hold rigid the inflow bushing
mount 122 with respect to the impeller housing 106 and the cylindrical
member 108. Similarly, the outflow bushing mount 124 is supported within the
impeller housing 106 through the stationary outflow stator 114.
The stationary outflow stator 114 comprises a plurality of blades shaped and
disposed around the outflow bushing mount 124 to transform the rotational
motion of the blood flow about the longitudinal axis 119 into a translational
CA 02472088 2004-07-08
WO 03/075981 PCT/CA03/00103
29
motion. Therefore, the stationary outflow stator 114 constitutes a flow
straightener.
At both ends of the impeller shaft 110 end pivots 126 and 128 protrude. The
end pivots 126 and 128 are respectively inserted into respective bushings 130
and 132 to support the impeller drive shaft 110 within the cylindrical conduit
108 while at the same time allowing the impeller shaft 110 to rotate freely.
Bushing 130 is mounted on the inflow bushing mount 122. In the same
manner, bushing 132 is mounted on the outflow bushing mount 124.
In addition to the series of longitudinal flow passages 120, an annular flow
passage 134 is formed between the inner surface 136 of the cylindrical
member 108 and the outer surface 138 of the impeller shaft 110.
Flow of blood through the mixed-flow pump 100 is indicated by the arrows
152-154.
The other characteristics of the mixed-flow pump 2 according to the
illustrative
embodiment of Figure 4a also apply to the illustrative embodiment 100 of
Figure 4b.
Electrical Aspects
In the illustrative embodiment of Figure 4a, the mixed-flow blood pump 2 is
actuated by means of a brushless DC (direct current) motor. This brushless
configuration presents the advantage of minimal wear. Two other interesting
characteristics of brushless DC motors are high rotational speed and high
torque.
CA 02472088 2004-07-08
WO 03/075981 PCT/CA03/00103
In the mixed-flow blood pump 2, the brushless DC motor includes elongated
axial permanent magnets such as 78 inserted in the impeller shaft 48 and
stator windings 80 embedded or housed in the cylindrical member 18.
5 As discussed previously, the cylindrical gap 76 between the outer surface of
the impeller shaft 48 and the inner surface of the cylindrical member 18 must
be sufficiently thick to produce sufficient blood flow in order to increase
washout and prevent clot formation. However, increasing the thickness of the
gap 76 decreases the efficiency of the magnetic coupling between the
10 permanent magnets 78 and the stator windings 80. This requires an increase
in current through the stator windings 80 to compensate for the decreased
efficiency and to maintain the same characteristics in terms of impeller blade
speed and blood volume throughput. Of course, increase in current leads to
an increase in thermal loss from the stator windings 80; this thermal loss
15 increases as the square of the current through the stator windings 80. As
the
temperature of the surface of the stator windings must remain at or below
40°C, the gap 76 must be sufficiently small to provide efficient
magnetic
coupling between the permanent magnets 78 and the stator windings 80.
20 Thermal performance is also improved given the proximate position of the
stator windings 80 to the external surface 82 of the cylindrical member 18.
Blood flow over the external surface 82 efficiently cools the stator windings
80. The flow of blood within the gap 76 between the impeller shaft 48 and the
inner surface 84 of the cylindrical member 18 also contributes in efficiently
25 cooling the stator windings 80.
The alternative illustrative embodiment 100 of the mixed-flow blood pump as
illustrated in Figure 4b maintains the essential electrical characteristics of
the
illustrative embodiment of Figure 4a with the exception that, referring to
Figure
CA 02472088 2004-07-08
WO 03/075981 PCT/CA03/00103
31
4b, the design of the pump 100 overcomes the thermal limitations by allowing
for a second blood flow passage along the series of longitudinal flow
passages 120 between the impeller housing 106 and the cylindrical member
108.
Axial spacing between the impeller blade and the permanent magnets along
the impeller shaft enables separate design of the DC motor and the impeller
to obtain simultaneously both efficient coupling between the permanent
magnets and the stator windings and sufficient pumping volume.
Selection of the Materials
The choice of materials for an implantable device is crucial and several
properties of the available materials should be considered: strength,
durability,
hardness, elasticity, wear resistance, surface finish and biocompatibility.
Biocompatibility is very important to minimise irritation, rejection and
thrombogenesis. The interaction between the surface of the material and the
biological tissues is very complex. In several cases, treatment of the surface
with human proteins, certain drugs like heparin or other biocompatible
material may considerably increase the biocompatibility and minimise
thrombus formation.
VAD SYSTEM
Figure 6 schematically illustrates an embodiment of implantable VAD system
including an axial-flow blood pump 2. The VAD system is composed of four
main parts:
- the axial-flow blood pump 2 implanted in the left ventricle 4 of the patient
CA 02472088 2004-07-08
WO 03/075981 PCT/CA03/00103
32
86;
- an internal controller 88;
- two energy sources, namely an internal rechargeable battery 90 and an
external rechargeable battery 92; and
- a Transcutaneous Energy and Information Transmission (TEIT) system
94.
VAD and TEIT Systems are well known in the art and will not be further
discussed in the present specification.
To conclude, ventricular assist devices (VADs) are now being used world-
wide and their utilisation is becoming more and more accepted as a solution
to treat end stage heart failure. It is generally accepted that VADs extend
life
of patients while improving quality of life of these patients. A poll, made
with
patients who received VADs, concerning their quality of life revealed that
these patients would have preferred a heart transplant but prefer their
situation than having to be on dialyses.
It is also now being accepted that VAD is becoming a cost effective solution
considering the fact that patients are discharged from the hospitals more
rapidly and may return to normal life occupations. In the United States,
several insurance companies are now reimbursing the implantation of VADs.
Finally, the mixed-flow blood pump 2 according to the invention provides an
excellent bridge to heart transplant and aims at long term implant. The new
proposed mixed-flow blood pump 2 should answer most of the remaining
CA 02472088 2004-07-08
WO 03/075981 PCT/CA03/00103
33
problems and limitations of the prior axial-flow blood pumps, especially those
related to hemolysis and bleeding.
Although the present invention has been described hereinabove by way of
illustrative embodiments thereof, these embodiments can be modified at will;
within the scope of the appended claims, without departing from the spirit and
nature of the present invention.