Note: Descriptions are shown in the official language in which they were submitted.
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
3-(IMIDAZOLYL)-2-ALKOXYPROPANOIC ACIDS AS TAFIA INHIBITORS
The present invention relates to a series of novel 3-(imidazolyl)-2-(c~-
aminoalkyloxy)
propanoic acid derivatives that are inhibitors of TAFIa inhibitors and are
useful in the
treatment of disease.
Background
Sophisticated mechanisms have evolved in mammals to repair the body in the
event
of vascular injury and so maintain hemostasis. The injured blood vessel
constricts
to reduce the blood flow to the area, platelets aggregate to reduce the loss
of blood
from the area, and fibrinogen is cleaved to produce fibrin which then
polymerises
and forms a clot. This clot covers the area of vascular damage, preventing
blood
loss. Polymerised fibrin also provides a provisional matrix which enhances the
subsequent repair process. Once the blood vessel has been repaired the clot
dissolves. The process leading to the formation of the clot is the coagulation
cascade, and the process leading to its dissolution is the fibrinolysis
cascade.
Imbalances in the blood coagulation process are thought to be at the origin of
a large
and disparate number of disease conditions, which are linked by an unwanted
build
up of fibrin. The scale of fibrin build up is determined by the delicate
equilibrium
between the two biochemical cascades in the human body. Agents that can
modulate the balance between coagulation and fibrinolysis are therefore
potentially
valuable in the treatment of these disease conditions.
Studies have shown that coagulation and fibrinolysis are linked through the
generation of a-thrombin. a-Thrombin is the final product of the blood
coagulation
cascade and is responsible for the conversion of fibrinogen into fibrin. In
addition to
mediating coagulation, a-thrombin also reduces the rate at which blood clots
are
broken down by the serine protease plasmin. The protein that mediates this
antifibrinolytic effect of a-thrombin is TAFI (Thrombin Activatable
Fibrinolysis
Inhibitor).
TAFI is a 60kDa glycoprotein found in human plasma. It is also known as
procarboxypeptidase B, carboxypeptidase B, plasma carboxypeptidase B,
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
2
carboxypeptidase U and carboxypeptidase R. Following initiation of the
coagulation
cascade it is transformed into an activated form, TAFIa, whereupon it acts
upon the
fibrin matrix of the developing blood clot to prevent its dissolution. TAFI
circulates in
normal plasma at a concentration of about 75nM in an inactive form. Thrombin
converts the inactive zymogen to the active TAFI (TAFIa), a reaction that is
augmented about 1250-fold by thrombomodulin. Once activated, TAFIa cleaves
both C-terminal arginine and lysine residues from the developing fibrin clot.
The
removal of these dibasic amino acids from the surface of the fibrin matrix
attenuates
clot lysis by inhibiting the binding of the key mediators of fibrinolysis:
tissue
plasminogen activator (tPA) and its substrate, plasminogen, which is the
precursor of
plasmin. Both tPA and plasminogen contain a structural motif called a kringle
domain which binds tightly to C-terminal lysine residues. The removal of these
binding sites prevents the formation of a ternary complex between tPA,
plasminogen
and fibrin and this inhibits the conversion of plasminogen to plasmin, thus
protecting
the clot from rapid degradation.
In the presence of a TAFIa inhibitor, TAFIa will not be able to act upon a
developing
fibrin clot as described above to inhibit fibrinolysis of the clot. Thus a
TAFIa inhibitor
should serve to enhance fibrinolysis.
It can be seen that, in pathologies where the normal equilibrium between
coagulation
and fibrinolysis is disturbed in favour of coagulation, there will be a larger
amount of
fibrin present than normal. This makes it more likely that the subjects will
develop
one or more of the conditions in which thrombus build up is implicated. Such
subjects can be expected to benefit from treatment with a pro-fibrinolytic
agent.
McKay et al. (Biochemistry 1978, 17, 401 ) disclose the testing of a number of
compounds as competitive inhibitors of bovine carboxypeptidase B of pancreatic
origin. Inhibition was measured by the inhibitor's efficiency in protecting
the active
centre tyrosine and glutamic acid of bovine carboxypeptidase B from
irreversible
alkylation by bromoacetyl-D-arginine or bromoacetamidobutylguanidine. It is
suggested that such inhibitors could act as bradykinin potentiators. Bovine
enzymes
of pancreatic origin are very different to those found in human plasma, so one
would
not expect inhibitors of one to inhibit the other. Moreover, such inhibitors
are
directed towards a very different utility. Accordingly this disclosure
provides no
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
3
teaching of TAFIa inhibitors or their utility.
Redlitz et al. (J. Clin. Invest. 1995, 96, 2534) teach the involvement of
plasma
carboxypeptidase B (pCPB, or TAFI) in the formation of clots. The lysis of
blood
clots was followed in the absence and presence of pCPB, whereupon it was found
that the presence of pCPB slowed clot lysis. To confirm that pCPB was
responsible
two control reactions were run; one where the lysis experiment was repeated in
the
presence of pCPB and potato carboxypeptidase inhibitor, PCI, and a second
where
the lysis reaction was conducted in the presence of plasma from which pCPB was
removed. In both cases lysis proceeded uninhibited.
Boffa et al. (J. Biol. Chem. 1998, 273, 2127) compare plasma and recombinant
TAFI
and TAFIa with respect to glycosylation, activation, thermal stability and
enzymatic
properties. Inhibition constants for three competitive inhibitors were
determined:
E-aminocaproic acid (~-ACA), 2-guanidinoethylmercaptosuccinic acid (GEMSA) and
potato carboxypeptidase inhibitor (PCI).
There are large numbers of carboxypeptidases (i.e. enzymes that cleave the
C-terminal amino acid from a peptide). They may be classified as acidic,
neutral or
basic, depending on the type of amino acid they cleave. Basic
carboxypeptidases
cleave arginine, lysine and histidine. TAFIa is a member of a specific subset
of the
basic carboxypeptidases. In terms of the present invention, the inhibitors
disclosed
above by Redlitz et al. and Boffa et al. are too weak, non-specific or
otherwise
unsuitable to be considered as suitable TAFIa inhibitors for therapeutic
application.
Further, whilst the role of TAFIa in clot lysis is explained, there is no
suggestion that
TAFIa inhibitors can be used to treat disease.
US-A-5993815 teaches the use of a peptide that binds to the TAFI zymogen,
thereby
inhibiting its activation, to treat those disorders where a C-terminal lysine
or arginine
is cleaved from an intact peptide. Suitable disorders are arthritis, sepsis,
thrombosis, strokes, deep vein thrombosis and myocardial infarctions. The
peptide
used is an antibody or a functionally active fragment. The peptide should be
used in
an amount to promote fibrinolysis in vivo.
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
4
WO00/66550 and WO00/66557 disclose broad classes of compounds useful as
inhibitors of carboxypeptidase U. Inhibitors of carboxypeptidase U are
postulated to
facilitate fibrinolysis and thus the compounds are taught as useful in the
treatment of
thrombotic conditions. There is no data to support this assertion, though
details of a
suitable assay are given.
WO00/66152 discloses formulations containing a carboxypeptidase U inhibitor
and a
thrombin inhibitor. Suitable carboxypeptidase U inhibitors are those of
WO00/66550. The formulations are taught as primarily useful in treating
thrombotic
conditions.
W001/19836 discloses a series of phosphonate esters and analogues thereof as
carboxypeptidase B inhibitors that are suitable for the treatment or
prevention of
thrombotic diseases.
W002/14285 discloses a series of a-imidazolylmethyl-w-aminocarboxylic acids
and
N°'-(w-aminoalkyl)-histidine derivatives that are inhibitors of TAFIa.
The compounds
are considered to be potentially useful in the treatment of a number of
conditions.
The present invention discloses a further class of TAFIa inhibitors.
Description of the Invention
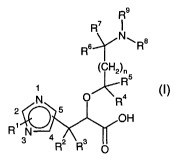
In a first aspect, the present invention provides compounds according to
general
formula (I)
Rs
R'
Rs~NwRa
-~CIrH2~n
Rs
N O Ra
5
OH
R~ N
3 4 R2 Rs
O
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
wherein:
nis0,1,2or3;
R' is selected from
(a) an optionally substituted straight chain or branched chain C~_6 alkyl
group,
5 (b) an optionally substituted straight chain or branched chain C2_6 alkenyl
group,
(c) an optionally substituted straight chain or branched chain C2_6 alkynyl
group,
(d) Aryl,
(e) Aromatic heterocycle,
(f) Heterocycle, and
(g) hydrogen;
where the optional substituents in groups (a), (b) and (c) above are selected
from:
C3_, cycloalkyl, Aryl, Aromatic heterocycle, Heterocycle, OR'°,
NR'°R", S(O)pR'°,
OC(O)R", C02R'°, CONK'°R", S02NR'°R", halo and
NHS02R'°, and where p is 0,
1 or 2;
R2, R3, R4, R6, R' and R9 are each independently selected from hydrogen and
straight chain or branched chain C1_6 alkyl optionally substituted by
OR'° or halo;
R5 and R8 are each independently selected from hydrogen and straight chain or
branched chain C,_s alkyl optionally substituted by OR'° or halo, or
together are a C2_
6 alkylene chain;
R'° and R" are each independently selected from hydrogen and straight
chain or
branched chain C1_6 alkyl;
Aryl is a 6-14 membered aromatic monocyclic or fused polycyclic carbocyclic
group
optionally substituted with one or more groups selected from R'2, halo, OR'3,
NR'3R'4, NR'3C02R'2, C02R'3, NR'3S02R'2, CN, haloalkyl, O(haloalkyl), SR'3,
S(O)R'2, S02R'2, OC(O)R'3, SO2NR'3R'4, C(O)NR'3R'4, C3_~ cycloakyl, O(C3_,
cycloalkyl), R'S and OR'S, where R'2 is straight chain or branched chain C~_6
alkyl,
R'3 and R'4 are each independently selected from hydrogen and straight chain
or
branched chain C,_6 alkyl, and R'S is phenyl optionally substituted by R'2,
OR'3, halo
or haloalkyl;
Aromatic heterocycle is a 5 to 7 membered aromatic ring containing from 1 to 3
heteroatoms, each independently selected from O, S and N, said ring being
optionally substituted with one or more groups selected from OR'3, NR'3R'4,
CO2R'3,
NR'3C02R'2, R'2, halo, CN, haloalkyl, O(haloalkyl), SR'3, S(O)R'2, S02R'2,
OC(O)R'3, NR'3S02R'2, S02NR'3R'4 and C(O)NR'3R'4; and
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
6
Heterocycle is a 3 to 8 membered ring containing from 1 to 3 heteroatoms, each
independently selected from O, S and N, said ring being saturated or partially
saturated, said ring further being optionally substituted with one or more
groups
selected from OR'3, NR'3R'4, C02R'3, NR'3C02R'4, R'2, halo, CN, haloalkyl,
O(haloalkyl), SR'3, S(O)R'2, S02R'2, OC(O)R'3, NR'3S02R'2, S02NR'3R'4 and
C(O)NR'3R'4,
or a tautomer thereof, or a pharmaceutically acceptable salt or solvate of
said
compound or said tautomer.
As used herein:
i) Halo includes fluoro, chloro, bromo and iodo groups.
ii) Haloalkyl includes monohaloalkyl, polyhaloalkyl and perhaloalkyl, such as
2-bromoethyl, 2,2,2-trifluoroethyl, chlorodifluoromethyl and trichloromethyl.
iii) Unless otherwise indicated, alkyl includes straight chain and branched
chain
alkyl.
It will be understood that, in the compounds according to general formula (I),
the R'
group and C(R2)(R3)(amino acid) group may be attached at any atom of the
imidazole ring that is available to form a covalent bond, and that it is not
intended
that the general formula should be interpreted as limiting the R' group to the
G'~ and
N3-positions, nor the C(R2)(R3)(amino acid) group to the Ca- and G's-
positions. It will
further be understood that the two groups cannot both be attached to the same
atom
of the imidazole ring, and that only one of the nitrogen atoms (by convention
designated N') of the imidazole ring is available to form a covalent bond.
Thus the
possible substitution patterns are 1,2-; 1,4-; 1,5-; 2,4- and 2,5-. When the
imidazole
is 2,4- or 2,5-substituted then there is a hydrogen atom attached at the N'-
position.
Certain compounds according to formula (I) may exist in more than one
tautomeric
form. If the imidazole of general formula (I) is substituted at the 2- and 4-
positions
the 2,4-disubstituted imidazole can tautomerise to form the corresponding 2,5-
disubstituted imidazole. Furthermore, where a compound includes an Aromatic
heterocyle that is substituted with a hydroxyl group it may exist as the
'keto'
tautomer. The tautomeric relationship between 2-hydroxypyridine and 2-pyridone
is
a well known example of this phenomenon. All such tautomers of compounds of
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
7
formula (I), including mixtures thereof, are included in the scope of the
present
invention.
The compounds of formula (I) contain one or more asymmetric carbon atoms
(chiral
centers) and can therefore exist in two or more optical stereoisomeric forms
such as
enantiomers, diastereomers and epimers. Where the compounds of formula (I)
contain a carbon-carbon double bond, cis (Z) / trans (E) stereoisomerism may
also
occur. All such individual stereoisomers of the compounds of formula (I) and
mixtures thereof, including racemates, are included in the scope of the
present
invention.
Individual stereoisomers may be separated from mixtures by conventional
techniques such as, for example, by fractional crystallization or by
chromatography
of the mixture of compounds or of a suitable salt or derivative thereof. In
particular,
individual enantiomers of the compounds of formula (I) may be prepared by
resolution, such as by H.P.L.C. of the corresponding racemate using a suitable
chiral
support or by fractional crystallisation of the diastereoisomeric salts formed
by
reaction of the corresponding racemate with a suitable optically active acid
or base,
as appropriate. The individual enantiomers may also be obtained from a
corresponding optically pure intermediate prepared by such a resolution
method.
These general principles are discussed in more detail by J. Jacques and A.
Collet
("Enantiomers, Racemates and Resolutions", Wiley, NY, 1981) and by W. Liu
("Handbook of Chiral Chemicals", D. Ager (ed.), M. Dekker, NY, 1999; chapter
8).
It will be appreciated that the compounds of formula (I) have both acidic and
basic
functional groups. Therefore, in addition to the uncharged form depicted in
the
general formula, they may exist as internal salts (zwitterions). Furthermore,
they
may form pharmaceutically acceptable salts with acids and bases. Such
zwitterions
and salts are included within the scope of the invention.
A pharmaceutically acceptable salt of a compound of the formula (I) may be
readily
prepared by mixing together solutions of a compound of the formula (I) and the
desired acid or base, as appropriate. The salt may precipitate from solution
and be
collected by filtration or may be recovered by evaporation of the solvent.
Salts may
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
8
also be prepared by ion exchange, such as by equilibrating a solution of a
compound
of formula (I) with an appropriate ion exchange resin. Ion exchange may also
be
used to convert one salt form of a compound of formula (I), such as a salt
with an
acid or base that is not pharmaceutically acceptable, to another salt form.
These
methods are generally well known in the art. Suitable acid addition salts are
formed
from acids which form non-toxic salts and examples are the hydrochloride,
hydrobromide, hydroiodide, sulfate, bisulfate, nitrate, phosphate, hydrogen
phosphate, acetate, maleate, fumarate, lactate, tartrate, citrate, gluconate,
succinate,
saccharate, benzoate, methanesulphonate, ethanesulphonate, benzenesulphonate,
p-toluenesulphonate and pamoate salts. Suitable base salts are formed from
bases
which form non-toxic salts and examples are the sodium, potassium, aluminium,
calcium, magnesium, zinc and diethanolamine salts. For a review of
pharmaceutically acceptable salts see Berge et al. (J. Pharm. Sci., 1977, 66,
1 ).
The compounds of formula (I) may form pharmaceutically acceptable solvates
(including hydrates). These solvates are also included in the scope of the
present
invention.
The compounds of formula (I) may exist in one or more crystalline forms. These
polymorphs, including mixtures thereof are also included within the scope of
the
present invention.
The scope of the present invention further includes prodrugs of compounds of
formula (I), i.e. pharmaceutically acceptable derivatives of the compounds in
which
one or more of the functional groups explicitly recited above have been
modified
such that they are converted to the parent compounds in vivo. Suitable
prodrugs
are discussed in Drugs of Today 1983, 19, 499-538 and Annual Reports in
Medicinal
Chemistry 1975, 10, 306-326.
The absolute stereochemistry of the compounds of formula (I) may be as
depicted in
formula (IA) or formula (IB) below. By convention the absolute stereochemisfry
at
the chiral center of (IA) is designated as 'S° and that of (IB) is
'H°. The compounds
of formula (IA) are particularly preferred.
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
9
R9 R9
R~ I R~
Rs~NwRa Rs~NwRa
(CH2)~ (CHZ)n
~R5 ~R5
N O Ra N O Ra
OH ~~ OH
R N ~ R N
R2 Rs O R2 Ra O
(IA) (IB)
Preferred compounds of formula (I) include those where the imidazole is
substituted
at the G'~ or C'4 positions by the C(R2)(R3)(amino acid) group to give
compounds of
formulae (IC) and (ID) respectively. Particularly preferred are those
compounds of
formula (I) where R' is attached at the C'4 position of the imidazole moiety
and the
C(R2)(R3)(amino acid) group is attached at the G'~ position so as to give the
2,4-disubstituted imidazole of formula (IC') or where R' is attached at the N'
position
of the imidazole moiety and the C(R2)(R3)(amino acid) group is attached at the
C4
position so as to give the 1,4-disubstituted imidazole of formula (ID'). Most
preferred are those compounds of formula (I) where R' is attached at the N'
position
of the imidazole moiety and the C(R2)(R3)(amino acid) group is attached at the
C'4
position so as to give the 1,4-disubstituted imidazole of formula (ID').
R9 R9
R~ I R~
Rs~/NwRa Rs~/NwRa
\CH'1)I1 ,CH2)(1
Rs ~ Rs
N O Ra N O Ra
R' N OH R N OH
R2 Rs O R2 R3 O
(IC) (ID)
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
Ra Rs
R~ I R~
Rs~NwRe Rs~NwRe
Ri R~ ~CH2~n
IV ~ Rs
4
OH
H N
Rz Rs ll
O
(IC') (ID')
Preferably n is 0 or 1. More preferably n is 0.
5 Preferably R' is hydrogen, Aryl, or a C~_s alkyl or C2_6 alkenyl group
optionally
substituted by one or more groups selected from C3_~ cycloalkyl, Aryl,
Aromatic
heterocycle, Heterocycle, OR'°, NR'°R", S(O)pR'°,
OC(O)R", C02R'°, CONR'°R",
S02NR'°R", halo and NHS02R'°. More preferably R' is hydrogen,
Aryl, C2_6 alkenyl
or a C1_s alkyl group optionally substituted by one or more groups selected
from C3_~
10 cycloalkyl, Aryl, Aromatic heterocycle, OR'°, C02R'°, halo
and NHS02R'°. Yet more
preferably R' is hydrogen, Aryl or a C1_6 alkyl group optionally substituted
by a group
selected from C3_~ cycloalkyl, Aryl, Aromatic heterocycle, OR'°,
C02R'° and
NHS02R'°. Yet more preferably R' is hydrogen, Aryl or a C1_6 alkyl
group optionally
substituted by a group selected from cyclohexyl and Aryl. Yet more preferably
R' is
hydrogen or C~_3 alkyl. Most preferably R' is hydrogen.
Preferably R2 and R3 are each independently selected from hydrogen and C1_6
alkyl.
More preferably R2 and R3 are hydrogen.
Preferably R4 is hydrogen or C,_s alkyl. More preferably R4 is hydrogen.
Preferably R6, R' and R9 are each independently selected from hydrogen and
C~_6
alkyl. More preferably R6, R' and R9 are each independently selected from
hydrogen and C~_3 alkyl. Yet more preferably R6, R' and R9 are each
independently
selected from hydrogen and methyl. Most preferably R6, R' and R9 are all
hydrogen.
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
11
When R5 and R8 do not constitute a C2_6 alkylene link then R5 is preferably
hydrogen
or C~_6 alkyl, more preferably hydrogen or C~_3 alkyl, yet more preferably
hydrogen or
methyl, and most preferably methyl, and R$ is preferably hydrogen or C~_6
alkyl, more
preferably hydrogen or C,_3 alkyl, yet more preferably hydrogen or methyl, and
most
preferably hydrogen.
When R5 and R8 constitute a C2_6 alkylene link then the link is preferably a
C2_3
alkylene link and more preferably it is a C2 alkylene link.
Preferably R'° and R" are each independently selected from hydrogen
and Ci-s
alkyl. More preferably R'° and R" are each independently selected from
hydrogen
and methyl.
Aryl includes optionally substituted phenyl, naphthyl, anthracenyl and
phenanthrenyl.
Preferably Aryl is phenyl or naphthyl optionally substituted by 1-3 groups
selected
from R'2, halo, OR'3, NR'3R'4, NR'3CO2R'2, C02R'3, NR'3S02R'2, CN, haloalkyl,
O(haloalkyl), SR'3, S(O)R'2, S02R'2, OC(O)R'3, S02NR'3R'4, C(O)NR'3R'4, Csa
cycloakyl, O(C3_~ cycloalkyl), R'S and OR'5. More preferably Aryl is phenyl
optionally
substituted by C~_s alkyl, halo, O(C~_6 alkyl), CF3, C3_~ cycloakyl, O(C3_~
cycloalkyl),
R'S or OR'S and R'S is phenyl optionally substituted by C~_6 alkyl, halo,
O(C1_s alkyl)
or CF3. Yet more preferably Aryl is phenyl optionally substituted by C1_6
alkyl, CF3,
cyclohexyl, O(cyclohexyl), R'S or OR'S and R'5 is phenyl optionally
substituted by C~_6
alkyl, CI, F, O(C~_6 alkyl) or CF3. Most preferably Aryl is phenyl.
Preferably Aromatic heterocycle is a 5 or 6 membered aromatic ring containing
1 or 2
heteroatoms each independently selected from O, S and N, including optionally
substituted furyl, thienyl, pyrrolyl, oxazolyl, isoxazolyl, thiazolyl,
isothiazolyl,
imidazolyl, oxadiazolyl, thiadiazolyl, triazolyl, pyridyl, pyridazinyl,
pyrimidinyl, pyrazinyl
and triazinyl, optionally substituted by 1-3 groups selected from OR'3,
NR'3R'4,
C02R'3, NR'3C02R'2, R'2, halo, CN, haloalkyl, O(haloalkyl), SR'3, S(O)R'2,
S02R'2,
OC(O)R'3, NR'3SO2R'2, S02NR'3R'4, C(O)NR'3R'a. More preferably Aromatic
heterocycle is a 5 or 6 membered aromatic ring containing from 1 to 3
heteroatoms,
each independently selected from O, S and N, optionally substituted by 1-3
groups
selected from OR'3, NR'3R'4, C02R'3, NR'3C02R'2, R'2, halo, CN, haloalkyl,
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
12
O(haloalkyl), SR'3, S(O)R'2, S02R'2, OC(O)R'3, NR'3S02R'2, S02NR'3R'4,
C(O)NR'3R'4. Most preferably Aromatic heterocycle is an un~ubstituted 5 or 6
membered aromatic ring containing 1 or 2 heteroatoms, each independently
selected
from O, S and N.
Preferably, Heterocycle is a 3 to 8 membered ring containing 1 or 2
heteroatoms,
each independently selected from O, S and N, said ring being saturated or
partially
saturated, optionally substituted by 1 to 3 groups selected from OR'3,
NR'3R'4,
C02R'3, NR'3C02R'2, R'2, halo, CN, haloalkyl, O(haloalkyl), SR'3, S(O)R'2,
S02R'2,
OC(O)R'3, NR'3S02R'2, S02NR'3R'4, C(O)NR'3R'4. More preferably, Heterocycle is
a 5 or 6 membered ring containing 1 or 2 heteroatoms, each independently
selected
from O, S and N, said ring being saturated or partially saturated, optionally
substituted by 1 to 3 groups selected from OR'3, NR'3R'4, C02R'3, NR'3C02R'2,
R'2,
halo, CN, haloalkyl, O(haloalkyl), SR'3, S(O)R'2, S02R'2, OC(O)R'3,
NR'3SO2R'2,
S02NR'3R'4, C(O)NR'3R'4. Most preferably, Heterocycle is an unsubstituted 5 or
6
membered ring containing 1 or 2 heteroatoms, each independently selected from
O,
S and N, said ring being saturated or partially saturated, including oxiranyl,
azetidinyl,
tetrahydrofuranyl, thiolanyl, pyrrolidinyl, dioxolanyl, dihydropyranyl,
tetrahydropyranyl,
morpholinyl, piperidinyl and piperazinyl.
Preferred compounds of the present invention are:
(2S)-(-)-2-(2-aminoethoxy)-3-(1-phenyl-1 H imidazol-4-yl)propanoic acid
(Example 6);
(2S)-2-{[(1 f~-2-amino-1-methylethyl]oxy}-3-[1-(2-cyclohexylethyl)-1 H
imidazol-4-yl]-
propanoic acid (Example 15);
(2S)-2-{[(1 f~-2-amino-1-methylethyl)oxy}-3-(1-phenyl-1 H imidazol-4-
yl)propanoic
acid (Example 17);
(25~-2-{[(2S)-2-aminopropyl]oxy}-3-[1-(2-cyclohexylethyl)-1H imidazol-4-
yl]propanoic
acid (Example 34);
(2S)-2-(2-aminoethoxy)-3-(1 H imidazol-4-yl)propanoic acid (Example 50);
(2S)-2-{[(1 ~-2-amino-1-methylethyl]oxy}-3-(1 H imidazol-4-yl)propanoic acid
(Example 51 ); and
(2S)-2-{[(1 R)-2-amino-1-methylethyl]oxy}-3-[1-(2-pyridinyl)-1'-I imidazol-4-
yl]-
propanoic acid (Example 52).
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
13
Particularly preferred is (25~-2-{[(1 F~-2-amino-1-methylethyl]oxy}-3-(1 H
imidazol-4-
yl)propanoic acid (Example 51 ).
The compounds of formula (I) are inhibitors of TAFIa. Inhibition of TAFIa can
be
demonstrated using an assay based on the method of Boffa et al. (J. Biol.
Chem.
1998, 273, 2127) as further described below. The activity of the compounds is
characterized by a calculated K; value. Generally the compounds of the present
invention have a K; value of 1 Op.M or less. Better compounds have a K; value
of
1 ~,M or less, or even 1 OOnM or less. The most potent compounds have a K;
value of
25nM or less.
The compounds of formula (I) are selective for TAFIa over other
carboxypeptidases,
and particularly carboxypeptidase N (CPN). Unwanted inhibition of CPN is
considered to be the most likely cause of undesirable side effects in clinical
use.
Selectivity can be expressed as the ratio of the K; for TAFIa to the K; for
CPN.
Generally the compounds of the present invention have a selectivity ratio of
at least
5. Better compounds have a selectivity ratio of at least 10. The most
selective
compounds have a selectivity ratio of at least 50.
The compounds of formula (I) may be prepared according to the general methods
which are described below and in the Examples and Preparations section. These
methods provide a further aspect of the present invention. Nevertheless, the
skilled
man will appreciate that the compounds of the invention could be made by
methods
other than those herein described, by adaptation of the methods herein
described
and/or adaptation of a plethora of methods known in the art. It is to be
understood
that the synthetic transformation methods specifically mentioned herein may be
carried out in various different sequences in order that the desired
substances can
be efficiently assembled. The skilled chemist will exercise his judgement and
skill
as to the most efficient sequence of reactions for the synthesis of a given
target
substance.
It will be apparent to those skilled in the art that sensitive functional
groups may need
to be protected and deprotected during the synthesis of a substance of the
invention.
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
14
This may be achieved by conventional techniques, for example as described by
T. W.
Greene and P. G. M. Wuts ("Protective Groups in Organic Synthesis", 3R°
edition,
Wiley-Interscience, NY, 1999).
Compounds of formula (I) may be prepared from the corresponding esters of
formula
(II) (wherein P' is a lower alkyl group, a benzyl group or any other carboxyl
protecting
group).
R9 R9
R~ I R~
Rs~NwRs Rs~NwRe
~CH2~n ~CH2~n
O I -Rs ~Rs
N ~Ra ~ N
O~ ~ ~ OH
R N ~ ~ P R N
R2 R3 O R2 Ra O
(II) (I)
P' is preferably a lower alkyl group such as methyl or ethyl, in which case
suitable
conditions for this step include treatment with NaOH in dioxan for 1-3 days.
Compounds of formula (II) may be prepared from the corresponding protected
amines of formula (III) (wherein P2 is a tent butyloxycarbonyl,
benzyloxycarbonyl or
fluorenylmethyloxycarbonyl group, or any other amine protecting group). Where
R9
is H then the preparation involves only a deprotection step. Where R9 is other
than
H then a further step is necessary to introduce R9, such as a reductive
amination
reaction.
P2
R9
R' I R' H R~ (
Rs~NwRs Rs~,NwRa Rs NwRa
~CH2y \CHz)ft lCH2/n
I 'Rs ~Rs ~Rs
N O Ra ~ N O Ra ~ N
O~P, R,
R N ~ N ~ R N ~ ~ P
R2 R3 O R2 R3 O R2 R3 O
(III) (Il, R9 = H) (II)
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
Alternatively, compounds of formula (III) may be converted to the
corresponding
acids (IV) prior to deprotecting the amine to give the compounds of formula
(I).
Pz Pz
R, I
wRs s~NwRs
-IR
(CHz)n
s ~ Rs
N Ra
OH
' ~ P' R'
R N R~Rs
O
(III) (IV)
s
R
R H R'
Rs~N~Re s N~Rs
R
(CHz)Rs (CHz)ns
-.~ ~ a ~ ~ R
N R N O Ra
OH ~ OH
R N R
Rz R3 O N R Rs
O
(I' Rs H) (I)
Compounds of formula (III) may be prepared from imidazoleacetic acid
derivatives of
5 formula (V), wherein X is a leaving group such as a chlorine, bromine or
iodine atom,
or a methanesulphonate or trifluoromethanesulphonate group, by reaction with a
alcohol of formula (VI).
Pz
R' I
i z Rs~NwRe
R II'
N X Rs~N~Re (CHz)ns
R
R~ N O~p' (CHz)~
z ~ a ~) ~R5 N R
R R ~~
O + HO R4 Ri~ OwP,
(V) N
(VI) Rz~R3 O
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
16
Compounds of formula (V) may be prepared from the corresponding hydroxyacid
derivatives of formula (VII) or, where X is Br, by direct halogenation of the
esters of
formula (VIII).
N OH
Ri N OwP~
R2 R3 O N X
(VII)
O
or ~ R' N ~P'
N R2 Ra
0
R~ N OwP,
(V)
R2 R3 O
(VIII)
Compounds of formula (VI), (VII) and (VIII) are known or may be prepared by
methods analogous to those used for the preparation of such known compounds.
Compounds of formula (III) may alternatively be prepared from a-
hydroxyimidazole-
acetic acid derivatives of formula (VII) by reaction with a compound of
formula (IX)
wherein Y is a leaving group such as a chlorine, bromine or iodine atom, or a
methanesulphonate or trifluoromethanesulphonate group.
P2
R'
P2 N w s
R' I Rs~ R
N OH Rs~N~Re
' (CHz)~
R N
R2 R3 ~ + ~Rs
Y Ra Ri
(VII)
(IX)
Compounds of formula (II) where neither of Rs and R9 are hydrogen may also be
prepared from compounds of formula (X). When R3 is hydrogen the transformation
may be accomplished by hydrogenation in the presence of a suitable catalyst.
When R3 is other than hydrogen the transformation may be accomplished using a
reagent such as R3-M, where M is a metal such as lithium or magnesium, in the
presence of a copper(I) salt.
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
17
Rs Rs
R~ I R~
Rs~N~Re R5 NwRa
(CHz)~5 ( Hz)n
R Rs
N Ra ~ N Ra
R~~ ~ O~p' ,
N ~z R R X 3 O P
N
(X) (I I)
Compounds of formula (X) may be prepared from compounds of formula (XI) by
dehydration. The transformation may be accomplished using, for example,
methanesulfonyl chloride and a tertiary amine.
Rs Rs
R~ I R~
RB~N~R$ R5 NwRe
f ( ICHz)~ (CHz)n
R5
R
O~4 a
N R ~ N O R
R N ~ R N ~ ~ P
Rz OH p Rz O
(XI) (X)
Compounds of formula (XI) may be prepared by an aldol-type reaction between
alkoxy-esters of formula (XII) and aldehydes or ketones of formula (X111) in
the
presence of a strong base, such as lithium diisopropylamide.
Rs
R~ ( Rs
R5 NLRB R'
Rs NwRs
N
CH )
( z R5 i ~ O (CHz)"
-I- R N R5
O Ra Rz --~ O
N Ra
(X111) R
~N X ~
O R? OH IOI
(XII)
(XI)
Compounds of formula (X111) are generally known, or may be prepared by methods
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
18
analogous to published methods. Compounds of formula (XII) may be prepared by
reacting the corresponding amino-alcohols R$R9NC(R6)(R')(CH2)"C(R4)(R5)OH with
a
bromoacetate BrCH2C02P' in the presence of a base such as sodium hydride.
Compounds of formula (I) wherein R9 is hydrogen may alternatively be prepared
by
the hydrolysis of lactams of formula (XIV).
R' H
4Rs s~NwRa
R -IR
-(CH2)" s (CH2)~
N p ~R I 'Rs
/\R'
N N O Ra
R1 N ~ ERs
R2 R3 ~ OH
O R' N ~
R2 Rs
(XIV)
(I, R9 = H)
Compounds of formula (XIV) may be prepared from the corresponding unsaturated
compounds of formula (XV). When R3 is hydrogen the transformation may be
accomplished by hydrogenation in the presence of a suitable catalyst. When R3
is
other than hydrogen the transformation may be accomplished using a reagent
such
as R3-M, where M is a metal such as lithium or magnesium, in the presence of a
copper(I) salt.
5
a Rs Ra R
R
~'-(CH2)~ s ~(C''H2)~ s
N O \ _R N O ~R
~R' ~ / _R'
/ N ~ X N~
R R2' \Rs O
R N ~ ~ R N ~ Rs
R O
(XV) (XIV)
Compounds of formula (XV) may be prepared by dehydration of alcohols of
formula
(XVI).
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
19
Ra Rs Ra Rs
~(CH2)~ s ~(CH2)n s
N ~R N O ~R
/\R' /\R'
N ~ ~~ / N
R N R~ H ~ vRs R N ~ ~ vRa
z 2
(XVI) (XV)
Compounds of formula (XVI) may be prepared by reacting an aldehyde or ketone
of
formula (X111) with a lactam of formula (XVII) in the presence of a strong
base, such
as lithium diisopropylamide.
Ra Rs Ra Rs
N ~-(CH2)~ s ~(CHZ)" s
O O R N O R
RW ~ ~
N / _R / _R
R2 ~- N~ a R~~ N~
R N ~ ~ R
(X111) Q R2 OH p
(XVI I) (XVI)
Compounds of formula (XVII) may be prepared from aminoalcohols of formula
(XVIII)
by reaction with chloroacetyl chloride. Compounds of formula (XVIII) are
generally
known, or may be prepared by adaptation of generally known methods.
s
Ra Rs Ra R
CI ~(C'HZ)" s
HO (CH2)~ O ~R
Rs CI / -R'
Nv
HN R I Ra
Ra O O
(XVI I I)
(XVI I)
When R8 is H, the foregoing method can be problematic, particularly at the
step
involving the reaction of compounds of formula (X111) and (XVII). It is
generally
convenient in this case to use a protected aminoalcohol of formula (XVllla),
where P3
is a nitrogen protecting group. A particularly useful embodiment of P3 is the
4-methoxybenzyl group. This may be removed following the elaboration of
intermediate (XIVa) by treatment with ceric ammonium nitrate.
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
R5 R5
5 4 4
Ra R R~(CH2)n s R~-(CH2)~ s
~(CH2)~ ................~ N R ~ N 0 R
HO ~ ~
/ _R' l _R'
Rs
N~ ~ NH
HN R R N ~ ~ P3 R N
\Ps R2 Rs O R2 Rs
O
(XVl l la) (XIVa)
(XIV, R8 = H)
When R' is H it may be necessary or convenient to protect the imidazole as its
trityl
derivative. Accordingly, when R' is H, compounds of formula (XIX), (XX) or
(XXI)
may be elaborated by the foregoing methods to provide compounds of formula
(XXII)
5 which, upon deprotection, give compounds of formula (III).
N OH
O~P,
N
Ph3C R2 R3 0
P2
(XIX) R'
Rs~NwRs
~N
(CH2)~
Ow ~ ~~ ~R5
N 0 a
N ~ ~ P
Ph3C RZ R3 0 R
O~Pi
N
(XX) Ph3C R2 R3 p
/N (XXII)
\O o
N
Ph3C
(XXI)
This route may also be useful for the preparation of certain compounds
according to
formula (I) wherein R' is attached at the N' position of the imidazole ring.
Compounds of formula (III) wherein R' is H may be alkylated or arylated to
give
10 compounds of formula (III) wherein R' is other than H and is attached at
the N'
position.
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
21
P2 P2 P2
R' I R' I R~ N
NLRB Rs~N~RB R6~ wRe
~CH2~~5
Rs Rs ~ R
N Ra N O Ra N Ra
Ow , ~O Ow , 'O OwP,
a ~ P N ~ ~ P N X
Ph C R R O H R2 R3 O R~ R' \R3 O
3
(XXII) (III, R' = H) (III, R' at N')
When R' is an alkyl, alkenyl or alkynyl group it may be introduced in an
alkylation
reaction. Suitable conditions for this step include treatment with 1.1 eq of
cesium
carbonate and 1.1 eq of an alkylating agent in N,N-dimethylformamide, or with
sodium hydride and 1.1 eq of an alkylating agent in THF. Suitable alkylating
reagents include R'-CI, R'-Br, R'-I, R'-OS02CH3 and R'-OS02CF3. When R' is
Aryl
or Aromatic heterocycle it may be introduced in an arylation reaction.
Suitable
conditions for this step include treatment with 2eq of Aryl-B(OH)2 or Aromatic
heterocycle-B(OH)2 in the presence of 1.5 eq of copper acetate, 2eq of
pyridine, air
and 4R molecular sieves.
For the compounds of formula (I) wherein the imidazole is 2,4- or 2,5-
disubstituted, it
may also be convenient or necessary to use a protecting group at the N'
position.
The compounds of formula (I) are useful as therapeutic agents. The compounds
will
generally be formulated so as to be amenable to administration to the subject
by the
chosen route. In a further aspect, therefore, the present invention provides
for a
pharmaceutical composition comprising a compound of formula (I) or a
stereoisomer,
tautomer or pharmaceutically acceptable salt, solvate or prodrug thereof and a
pharmaceutically acceptable excipient, diluent or carrier selected with regard
to the
intended route of administration and standard pharmaceutical practice. For
example, the compounds of formula (I) can be administered orally, buccally or
sublingually in the form of tablets, capsules, ovules, elixirs, solutions or
suspensions.
These formulations may contain flavouring or colouring agents, and may be
adapted
for immediate-, delayed-, modified-, sustained-, pulsed- or controlled-release
applications.
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
22
Tablets may contain excipients such as microcrystalline cellulose, lactose,
sodium
citrate, calcium carbonate, dibasic calcium phosphate and glycine,
disintegrants such
as starch (preferably corn, potato or tapioca starch), sodium starch
glycollate,
croscarmellose sodium and certain complex silicates, and granulation binders
such
as polyvinylpyrrolidone, hydroxypropylmethylcellulose (HPMC), hydroxypropyl-
cellulose (HPC), sucrose, gelatin and acacia. Additionally, lubricating agents
such
as magnesium stearate, stearic acid, glyceryl behenate and talc may be
included.
Solid compositions of a similar type may also be employed as fillers in
gelatin
capsules. Preferred excipients in this regard include lactose, starch,
cellulose and
derivatives thereof, milk sugar and high molecular weight polyethylene
glycols.
For solutions, suspensions and elixirs, the compounds of formula (I) may be
combined with various sweetening or flavouring agents, colouring matter or
dyes,
with emulsifying and/or suspending agents, and with diluents such as water,
ethanol,
propylene glycol and glycerin, and combinations thereof.
The compounds of formula (I) may also be administered in the form of a
solution- or
suspension-filled soft or hard gelatin capsule. Such capsules are generally
made of
gelatin, glycerin, water and sorbitol. Hard capsules are distinguished from
soft
capsules by containing less water and thus having a correspondingly stronger
shell.
Additional excipients suitable for use in such capsules include propylene
glycol,
ethanol, water, glycerol and edible oils.
The compounds of formula (I) can also be administered parenterally, for
example,
intravenously, intra-arterially, intraperitoneally, intrathecally,
intraventricularly,
intraurethrally, intrasternally, intracranially, intramuscularly or
subcutaneously. Such
administration may be as a single bolus injection or as a short- or long-
duration
infusion. For such parenteral administration the compounds are preferably
formulated as a sterile solution in water or another suitable solvent or
mixture of
solvents. The solution may contain other substances such as: salts,
particularly
sodium chloride, and sugars, particularly glucose or mannitol, to make the
solution
isotonic with blood; buffering agents such as acetic, citric and phosphoric
acids and
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
23
their sodium salts, such that the pH of the solution is preferably between 3
and 9;
and preservatives. The preparation of suitable parenteral formulations under
sterile
conditions is readily accomplished by standard pharmaceutical techniques well
known to those skilled in the art.
The compounds of formula (I) can also be administered intranasally or by
inhalation
and are conveniently delivered in the form of a dry powder inhaler or an
aerosol
spray presentation from a pressurised container, pump, spray, atomiser or
nebuliser,
with or without the use of a suitable propellant such as
dichlorodifluoromethane,
trichlorofluoromethane, dichlorotetrafluoroethane, a hydrofluoroalkane such as
1,1,1,2-tetrafluoroethane (HFA 134AT"") or 1,1,1,2,3,3,3-heptafluoropropane
(HFA
227EAT""), carbon dioxide or other suitable gas. In the case of a pressurised
aerosol, the dosage unit may be determined by providing a valve to deliver a
metered amount. The pressurised container, pump, spray, atomiser or nebuliser
may contain a solution or suspension of the active compound, e.g. using a
mixture of
ethanol and the propellant as the solvent, which may additionally contain a
lubricant,
e.g. sorbitan trioleate. Capsules and cartridges (made, for example, from
gelatin)
for use in an inhaler or insufflator may be formulated to contain a powder mix
of a
compound of the formula (I) and a suitable powder base such as lactose or
starch.
Alternatively, the compounds of formula (I) can be administered by the vaginal
or
rectal routes in the form of a suppository or pessary, or The compounds of
formula
(I) may also be administered dermally or transdermally, for example, by the
use of a
skin patch.
Alternatively, the compounds of formula (I) can be applied topically in the
form of a
gel, hydrogel, lotion, solution, cream, ointment or dusting powder. Suitable
ointments may contain the active compound suspended or dissolved in, for
example,
a mixture with one or more of the following: mineral oil, liquid petrolatum,
white
petrolatum, propylene glycol, polyoxyethylene polyoxypropylene compound,
emulsifying wax and water. Suitable lotions or creams may contain the active
compound suspended or dissolved in, for example, a mixture of one or more of
the
following: mineral oil, sorbitan monostearate, a polyethylene glycol, liquid
paraffin,
polysorbate 60, cetyl esters wax, cetearyl alcohol, 2-octyldodecanol, benzyl
alcohol
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
24
and water.
Alternatively, the compounds of formula (I) may be administered by the ocular
route.
For ophthalmic use, the compounds can be formulated as micronised suspensions
in
isotonic, pH adjusted, sterile saline, or, preferably, as solutions in
isotonic, pH
adjusted, sterile saline, optionally in combination with a preservative such
as a
benzylalkonium chloride. Alternatively, they may be formulated in an ointment
such
as petrolatum.
The compounds of formula (I) may also be used in combination with a
cyclodextrin.
Cyclodextrins are known to form inclusion and non-inclusion complexes with
drug
molecules. Formation of a drug-cyclodextrin complex may modify the solubility,
dissolution rate, bioavailability and/or stability property of a drug
molecule. Drug-
cyclodextrin complexes are generally useful for most dosage forms and
administration routes. As an alternative to direct complexation with the drug
the
cyclodextrin may be used as an auxiliary additive, e.g. as a carrier, diluent
or
solubiliser. Alpha-, beta- and gamma-cyclodextrins are most commonly used and
suitable examples are described in W091/11172, W094/02518 and W098/55148.
Because the compounds of formula (I) are inhibitors of TAFIa they are useful
as
therapeutic agents in pathologies in which inhibition of TAFIa is beneficial.
In a
further aspect, therefore, the present invention provides for a compound of
formula
(I) or a stereoisomer, tautomer, solvate, pharmaceutically acceptable salt or
prodrug
thereof for use as a medicament. In particular, the present invention provides
for
the use of a compound of formula (I) or a stereoisomer, tautomer, solvate,
pharmaceutically acceptable salt or prodrug thereof in the preparation of a
medicament for the treatment or prevention of a condition selected from
thrombotic
conditions, atherosclerosis, adhesions, dermal scarring, cancer, fibrotic
conditions,
inflammatory diseases and those conditions which benefit from maintaining or
enhancing bradykinin levels in the body. The utility of TAFIa inhibitors for
the
treatment of thrombotic conditions derives from their potential to promote
fibrinolysis
while not interfering with coagulation. In most clinically relevant situations
thrombus
formation is sub-acute, i.e. the thrombus forms slowly. Conventional anti-
thrombotic
agents block the coagulation pathway and so prevent thrombus growth, but as an
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
unavoidable consequence they also block the clotting response to vascular
damage,
which results in an increased incidence of hemorrhaging. By promoting
fibrinolysis,
TAFIa inhibitors accelerate the dissolution of the developing thrombus without
interfering with the clotting response. Accordingly, one preferred embodiment
of the
5 present invention provides for the use of a compound of formula (I) or a
pharmaceutically acceptable salt, solvate or prodrug thereof in the
preparation of a
medicament for the treatment of a thrombotic condition selected from
myocardial
infarction, deep vein thrombosis, stroke, young stroke, cerebral infarction,
cerebral
thrombosis, cerebral embolism, peripheral vascular disease, angina and other
forms
10 of acute coronary syndromes, disseminated intravascular coagulation,
sepsis,
pulmonary embolism, embolic events secondary to cardiac arrhythmias and the
prevention of cardiovascular events following surgical revascularisation or
intervention, or for improving the outcome of organ transplantation by
reducing blood
clotting and so preserving organ function. Cardiovascular events following
15 intervention surgery include conditions such as restenosis or reocclusion
following
interventions such as percutaneous transluminal coronary angioplasty,
grafting, stent
in-placement, coronary bypass surgery or any other forms of surgical
revascularisation or intervention. Disseminated intravascular coagulation
includes
all conditions resulting from intravascular activation of the coagulation
process. This
20 might occur acutely through the release of procoagulant substances (eg.
obstetric
emergencies, snakebite, crush injury malignancy), by abnormal contact of the
blood
(eg. infections, burns, extracorporeal circulation, grafts) or though
generation of
procoagulants in the blood (transfusion reactions, leukemia); or chronically,
(eg.
toxemia, malignant hypertension, severe liver cirrhosis). Deep vein thrombosis
also
25 encompasses what is known as 'economy class syndrome', where clots form in
subjects forced to endure cramped conditions for a period of time, such as
those
sitting in the economy class seats of an aeroplane.
A role for thrombus formation in the pathophysiology of atherosclerosis has
recently
been highlighted by several independent groups. Non-occlusive thrombi not only
restrict blood flow leading to myocardial ischemia and angina pectoris but
also, due
to incomplete endogenous lysis, may be incorporated into the arterial wall as
solidified plaque material enhancing the atherosclerotic process. Long-term
administration of a TAFIa inhibitor promotes the lysis of developing thrombi
and
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
26
therefore provides a safe and efficacious treatment which alleviates the
symptoms of
angina pectoris while impairing the progression of the underlying disease.
Conventional treatment of myocardial ischaemia in clinically stable coronary
artery
disease is predominately designed to reduce cardiac workload and enhance blood
flow. Such approaches clearly reduce myocardial ischaemia thus increasing
quality
of life. However, these strategies have little effect on the pathogenesis of
coronary
atherosclerosis which is a chronic process of continuous remodeling of the
vascular
tree in response to varying degrees of vascular injury. Accordingly, another
preferred embodiment of the present invention provides for the use of
compounds of
formula (I) and pharmaceutically acceptable salts, solvates and prodrugs
thereof in
the preparation of a medicament for the treatment or prevention of
atherosclerosis,
including atherosclerosis as a consequence of peripheral vascular disease,
insulin
resistance and Syndrome X, and further including myocardial ischaemia and
angina
pectoris resulting from atherosclerosis. Atherosclerosis is taken to include
both
primary and secondary coronary artery disease, in which atherosclerosis
restricts the
blood supply to the heart. Primary prevention of coronary artery disease means
preventing the onset of ischemic complications such as myocardial infarction
in
patients with no history of coronary artery disease but who have one or more
risk
factors. Secondary prevention of coronary artery disease means preventing
ischemic
complications in patients with established coronary artery disease, such as
patients
who have had a previous myocardial infarction. Syndrome X is a term often used
to
group together a number of interrelated diseases. The first stage of syndrome
X
consists of insulin resistance, abnormal cholesterol and triglyceride levels,
obesity
and hypertension. Any one of these conditions may be used to diagnose the
start of
Syndrome X. The disease may then progress with one condition leading to the
development of another in the group. For example insulin resistance is
associated
with high lipid levels, hypertension and obesity. The disease then cascades,
with
the development of each additional condition increasing the risk of developing
more
serious diseases. This can progress to the development of diabetes, kidney
disease and heart disease. These diseases may lead to stroke, myocardial
infarction and organ failure. Atherosclerosis is common in patients with
Syndrome
X.
TAFIa inhibitors are also effective in preventing the formation of adhesions
in the
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
27
body. Most surgical procedures and physical traumas result in bleeding into
the
cavities between tissues. The blood which collects at these sites then clots
forming
fibrin-rich thrombi. These thrombi bridge the gaps between adjacent tissues
and act
as foci for the accumulation of inflammatory cells and fibroblasts. Invading
fibroblasts lay down a collagen-rich extracellular matrix which strengthens
the
adhesion of the tissues producing a firm bond which may then restrict
movement.
Adhesions have been characterised according to their location and may result
following any surgery, e.g. abdominal, orthopaedic, neurological,
cardiovascular and
ocular surgery. This inappropriate adhesion of tissues post-surgery or trauma
is a
major issue which can lead to various outcomes, e.g. "aches and pains",
"twinges",
local inflammation, restriction in mobility, pain, intestinal obstruction and
sometimes,
in the most severe cases, death. In the case of gynaecological surgery,
infertility
may result. Additionally clots forming fibrin-rich thrombi are implicated in
dermal
scarring and restenosis. Without being bound by any theory, it is believed
that
adhesion formation may be enhanced when a deficiency in fibrinolysis results
in
enhanced and maintained clot formation. Treatment with a TAFIa inhibitor
around
and/or after surgical intervention may enhance fibrinolysis of the fibrin-rich
thrombi
and hence inhibit thrombus formation, accretion and stabilization, thereby
inhibiting
adhesion formation. A TAFIa inhibitor given either locally as a topical
application or
systemically may be seen to be of benefit in a range of surgical procedures.
In
addition, administration of a TAFIa inhibitor may be used to treat adhesions
resulting
from other forms of non-surgical physical trauma where this has caused
internal
bleeding. Examples of such trauma might include sporting injuries or anything
else
resulting in a tear, cut, bruise or induration of the body. Accordingly,
another
preferred embodiment of the present invention provides for the use of
compounds of
formula (I) and pharmaceutically acceptable salts, solvates and prodrugs
thereof in
the preparation of a medicament for the treatment or prevention of a
medicament for
the treatment or prevention of adhesions or dermal scarring.
TAFIa inhibitors are also effective in inhibiting tumour maturation,
progression and
metastasis. Without being bound by any theory, it is believed that the
hemostatic
system is involved at several levels of cancer pathology, including
neovascularisation, shedding of cells from the primary tumour, invasion of the
blood
supply, adherence to the vessel wall and growth at the metastatic site. It is
thought
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
28
that the efficacy of TAFIa inhibitors stems from an ability to reduce fibrin
deposition
around solid tumours and thereby inhibit the above processes. Accordingly,
another
preferred embodiment of the present invention provides for the use of
compounds of
formula (I) and pharmaceutically acceptable salts, solvates and prodrugs
thereof in
the preparation of a medicament for the treatment or prevention of cancer.
TAFIa inhibitors are efficacious in treatment of any condition in which
fibrosis is a
contributing factor. Suitable fibrotic conditions include cystic fibrosis,
pulmonary
fibrotic diseases such as chronic obstructive pulmonary disease (COPD), adult
respiratory distress syndrome CARDS), fibromuscular dysplasia and fibrotic
lung
disease, and fibrin deposition in the eye during opthalmic surgery.
Accordingly,
another preferred embodiment of the present invention provides for the use of
compounds of formula (I) and pharmaceutically acceptable salts, solvates and
prodrugs thereof in the preparation of a medicament for the treatment or
prevention
of fibrotic disease, and in particular for the treatment or prevention of a
fibrotic
condition selected from cystic fibrosis, pulmonary fibrotic diseases, chronic
obstructive pulmonary disease (COPD), adult respiratory distress syndrome
CARDS),
fibromuscular dysplasia, fibrotic lung disease and fibrin deposition in the
eye during
opthalmic surgery.
TAFIa inhibitors are efficacious in the treatment of inflammation,
inflammatory
diseases such as asthma, arthritis, endometriosis, inflammatory bowel
diseases,
psoriasis and atopic dermatitis and neurodegenerative diseases such as
Alzheimer's
disease and Parkinson's disease. Accordingly, another preferred embodiment of
the present invention provides for the use of compounds of formula (I) and
pharmaceutically acceptable salts, solvates and prodrugs thereof in the
preparation
of a medicament for the treatment or prevention of inflammation, inflammatory
diseases such as asthma, arthritis, endometriosis, inflammatory bowel
diseases,
psoriasis and atopic dermatitis and neurodegenerative diseases such as
Alzheimer's
disease and Parkinson's disease.
TAFIa binds to and breaks down bradykinin (Tan et al., Biochemistry 1995, 34,
5811 ). There are many conditions which are known to benefit from maintaining
or
enhancing levels of bradykinin such as hypertension, angina, heart failure,
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
29
pulmonary hypertension, renal failure and organ failure. Accordingly, another
preferred embodiment of the present invention provides for the use of
compounds of
formula (I) and pharmaceutically acceptable salts, solvates and prodrugs
thereof in
the preparation of a medicament for the treatment or prevention of conditions
which
benefit from maintaining or enhancing levels of bradykinin.
In a further aspect, the present invention provides a method of treating or
preventing
thrombotic conditions, atherosclerosis, adhesions, dermal scarring, cancer,
fibrotic
conditions, inflammatory diseases and those conditions which benefit from
maintaining or enhancing bradykinin levels in the body which comprises
administering a therapeutically effective amount of a compound of formula (I)
or a
stereoisomer, tautomer or pharmaceutically acceptable salt, solvate or prodrug
thereof to a patient in need of such treatment.
One preferred embodiment of the present invention provides for a method of
treating
or preventing thrombosis, particularly myocardial infarction, deep vein
thrombosis,
stroke, young stroke, cerebral infarction, cerebral thrombosis, cerebral
embolism,
peripheral vascular disease, angina and other forms of acute coronary
syndromes,
disseminated intravascular coagulation, sepsis, pulmonary embolism, embolic
events
secondary to cardiac arrhythmias and preventing cardiovascular events
following
intervention surgery which comprises administering a therapeutically effective
amount of a compound of formula (I) or a stereoisomer, tautomer or
pharmaceutically acceptable salt, solvate or prodrug thereof to a patient in
need of
such treatment. Subjects with thrombotic conditions who are suitable for
treatment
by the present invention include those having conditions associated with
hypercoagulability, such as factor V mutation, antithrombin III deficiency,
heparin
cofactor II deficiency, protein C deficiency, protein S deficiency and
polycythemia
vera, and those exhibiting homocystinaemia or homocystinuria.
Another preferred embodiment of the present invention provides for a method of
treating or preventing atherosclerosis which comprises administering a
therapeutically effective amount of a compound of formula (I) or a
stereoisomer,
tautomer or pharmaceutically acceptable salt, solvate or prodrug thereof to a
patient
in need of such treatment.
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
Another preferred embodiment of the present invention provides for a method of
treating or preventing adhesions or dermal scarring which comprises
administering a
therapeutically effective amount of a compound of formula (I) or a
stereoisomer,
5 tautomer or pharmaceutically acceptable salt, solvate or prodrug thereof to
a patient
in need of such treatment.
Another preferred embodiment of the present invention provides for a method of
treating or preventing cancer which comprises administering a therapeutically
10 effective amount of a compound of formula (1) or a stereoisomer, tautomer
or
pharmaceutically acceptable salt, solvate or prodrug thereof to a patient in
need of
such treatment.
Another preferred embodiment of the present invention provides for a method of
15 treating or preventing a fibrotic condition such as cystic fibrosis,
pulmonary fibrotic
diseases, chronic obstructive pulmonary disease (COPD), adult respiratory
distress
syndrome CARDS), fibromuscular dysplasia, fibrotic lung disease and fibrin
deposition in the eye during ophthalmic surgery which comprises administering
a
therapeutically effective amount of a compound of formula (I) or a
stereoisomer,
20 tautomer or pharmaceutically acceptable salt, solvate or prodrug thereof to
a patient
in need of such treatment.
Another preferred embodiment of the present invention provides for a method of
treating or preventing an inflammatory disease such as asthma, arthritis,
25 endometriosis, inflammatory bowel diseases, psoriasis or atopic dermatitis
or a
neurodegenerative disease such as Alzheimer's disease or Parkinson's disease
which comprises administering a therapeutically effective amount of a compound
of
formula (I) or a stereoisomer, tautomer or pharmaceutically acceptable salt,
solvate
or prodrug thereof to a patient in need of such treatment.
Another preferred embodiment of the present invention provides for a method of
treating or preventing conditions which benefit from maintaining or enhancing
levels
of bradykinin which comprises administering a therapeutically effective amount
of a
compound of formula (I) or a stereoisomer, tautomer or pharmaceutically
acceptable
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
31
salt, solvate or prodrug thereof to a patient in need of such treatment.
It is to be appreciated that all references herein to treatment include
curative,
palliative and prophylactic treatment. The amount of compound administered and
the frequency of administration will be determined by the attending physician
taking
into account the characteristics of the patient, such as age, weight and state
of
health, and the degree of inhibition of TAFIa desired. The total daily dose
for a
typical 70kg adult will generally be between 1 mg and 5g, preferably between 1
Omg
and 1 g, more preferably between 50mg and 750mg. The total dose may be given
as a single or divided dose.
The compounds of the present invention may be used alone or in combination
with
other therapeutic agents. When used in combination with another therapeutic
agent
the administration of the two agents may be simultaneous or sequential.
Simultaneous administration includes the administration of a single dosage
form that
comprises both agents and the administration of the two agents in separate
dosage
forms at substantially the same time. Sequential administration includes the
administration of the two agents according to different schedules provided
that there
is an overlap in the periods during which the treatment is provided. Suitable
agents
with which the compounds of formula (I) can be co-administered include
antithrombotics, including antiplatelet agents, anticoagulants and
profibrinolytics.
Suitable antithrombotics include: aspirin, PlavixT"", ticlopidine, warfarin
(CoumadinT""),
unfractionated heparin, hirudin (LepirudinT""), streptokinase, urokinase,
recombinant
tissue plasminogen activator (tPA), dipyridamole, ReoproT"", AggrastatT"", and
IntegrilinT"". The compounds of formula (I) can also be administered together
with
antihypertensive agents and with agents to treat dyslipidaemia such as statins
eg
LipitorT"". Further suitable drug classes for co-administration include Factor
X
inhibitors and antiarrhythmics such as amiodarone or digoxin. Accordingly, in
a
further aspect, the present invention provides for the use of a compound of
formula
(I) or a stereoisomer, tautomer or pharmaceutically acceptable salt, solvate
or
prodrug thereof in combination with an antithrombotic agent for the
preparation of a
medicament for the treatment of thrombosis. In a preferred embodiment the
antithrombotic is an profibrinolytic. In a more preferred embodiment the
antithrombotic is recombinant tissue plasminogen activator (tPA).
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
32
In a further aspect, the present invention provides for a method of treating
or
preventing thrombosis, which comprises administering a therapeutically
effective
amount of a compound of formula (I) or a stereoisomer, tautomer or
pharmaceutically acceptable salt, solvate or prodrug thereof in combination
with an
antithrombotic to a patient in need of such treatment. In a preferred
embodiment
the antithrombotic is a profibrinolytic. In a more preferred embodiment the
antithrombotic is recombinant tissue plasminogen activator (tPA).
In a further aspect, the present invention provides for a kit comprising:
a) a composition comprising a compound of formula (1) or a stereoisomer,
tautomer or pharmaceutically acceptable salt, solvate or prodrug thereof
as disclosed herein and a pharmaceutically acceptable diluent or carrier;
b) a composition comprising an antithrombotic and a pharmaceutically
acceptable diluent or carrier; and
c) a container
The components of this kit may be administered separately, simultaneously or
sequentially.
The present invention also provides for the use a compound of formula (I) or a
stereoisomer, tautomer or pharmaceutically acceptable salt, solvate or prodrug
thereof as a coating on intravascular devices such as indwelling catheters for
dialysis, replacement heart valves or arterial stents; and as a coating on
extra-
corporeal blood circulation devices such as heart, lung and kidney dialysis
machines,
to prevent thrombosis, particularly myocardial infarction, deep vein
thrombosis,
stroke, young stroke, cerebral infarction, cerebral thrombosis, cerebral
embolism,
peripheral vascular disease, angina and other forms of acute coronary
syndromes,
disseminated intravascular coagulation, sepsis, pulmonary embolism, embolic
events
secondary to cardiac arrhythmias and the prevention of cardiovascular events
such
as restenosis following intervention surgery such as percutaneous transluminal
coronary angioplasty, grafting, stent in-placement, coronary bypass surgery or
any
other forms of surgical revascularisation or intervention.
The invention provides for intravascular devices, of which the intravascular
portion is
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
33
coated with a compound of formula (I) or a stereoisomer, tautomer or
pharmaceutically acceptable salt, solvate or prodrug thereof; and extra
corporeal
blood circulation devices such as heart, lung and kidney dialysis machines,
where
the portion coming into contact with the subjects blood is coated with a
compound of
formula (I) or a stereoisomer, tautomer or pharmaceutically acceptable salt,
solvate
or prodrug thereof.
The compounds of the present invention are TAFIa inhibitors, whose utility is
based
upon preventing the reaction between a developing thrombus and TAFIa. It has
been found that the compounds of the present invention are also capable of
binding
to the unactivated TAFI molecule, at the site implicated in the reaction
between
TAFIa and the developing clot. The use of TAFIa inhibitors as described above
in
terms of scope and utility, includes such TAFIa inhibitors which bind to TAFI.
The invention is further illustrated by the following, non-limiting examples.
Melting points were determined on a Gallenkamp melting point apparatus using
glass capillary tubes and are uncorrected. Unless otherwise indicated all
reactions
were carried out under a nitrogen atmosphere, using commercially available
anhydrous solvents. '0.88 Ammonia' refers to commercially-available aqueous
ammonia solution of about 0.88 specific gravity. Thin-layer chromatography was
performed on glass-backed pre-coated Merck silica gel (60 F254) plates, and
silica
gel column chromatography was carried out using 40-63,um silica gel (Merck
silica
gel 60). Ion exchange chromatography was performed using with the specified
ion
exchange resin which had been pre-washed with deionised water. Proton NMR
spectra were measured on a Varian Inova 300, Varian Inova 400, or Varian
Mercury
400 spectrometer in the solvents specified. In the NMR spectra, only non-
exchangeable protons which appeared distinct from the solvent peaks are
reported.
Low resolution mass spectra were recorded on either a Fisons Trio 1000, using
thermospray positive ionisation, or a Finnigan Navigator, using electrospray
positive
or negative ionisation. High resolution mass spectra were recorded on a Bruker
Apex II FT-MS using electrospray positive ionisation. Combustion analyses were
conducted by Exeter Analytical UK. Ltd., Uxbridge, Middlesex. Optical
rotations
were determined at 25°C using a Perkin Elmer 341 polarimeter using the
solvents
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
34
and concentrations specified. Example compounds designated as (+) or (-)
optical
isomers are assigned based on the sign of optical rotation when determined in
a
suitable solvent.
Abbreviations and Definitions
ArbocelT"' Filtration agent, from J. Rettenmaier & Sohne,
Germany
Amberlyst~ 15 Ion exchange resin, available from Aldrich Chemical
Company
atm Pressure in atmospheres (1 atm = 760 Torr = 101.3
kPa)
BiotageT"" Chromatography performed using Flash 75 silica
gel cartridge,
from Biotage, UK
BOC tert Butyloxycarbonyl group
br Broad
c Concentration used for optical rotation measurements
in g per
100 ml (1 mg/ml is c 0.10)
cat Catalytic
d Doublet
dd Doublet of doublets
Degussa~ 101 10 wt% palladium on activated carbon, Degussa type
E101
available from Aldrich Chemical Company
Dowex~ Ion exchange resin, from Aldrich Chemical Company
ee Enantiomeric excess
HRMS High Resolution Mass Spectrocopy (electrospray
ionisation
positive scan)
HyfIoT"" Hyflo supercel~, from Aldrich Chemical Company
liq liquid
LRMS Low Resolution Mass Spectroscopy (electrospray
or thermospray
ionisation positive scan)
LRMS (ES~) Low Resolution Mass Spectroscopy (electrospray
ionisation
negative scan)
m Multiplet
m/z Mass spectrum peak
MCIT"" gel High porous polymer, CHP20P 75-150~m, from Mitsubishi
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
Chemical Corporation
psi Pounds per square inch (1 psi = 6.9 kPa)
q Quartet
Rf Retention factor on TLC
s Singlet
Sep-Pak° Reverse phase C,$ silica gel cartridge, Waters
Corporation
t Triplet
TLC Thin Layer Chromatography
8 Chemical shift
Example 1
(2S')-(-)-2-(2-Aminoethoxy)-3-(1-propel-1 H imidazol-4-yl)propanoic acid
~NH2
H3C~ ~N O
N / OH
I
5 O
A solution of the compound of Preparation 88 (437mg, 1.96mmol) in 6M
hydrochloric
acid (35m1) was heated at reflux for 72 hours, then allowed to cool and
concentrated
under reduced pressure. The residue was dissolved in water (2ml) and the
solution
was purified by column chromatography on Dowex~ 50WX8-200 ion exchange resin
10 using an elution gradient of water:0.88 ammonia (100:0 to 98:2). The
product
containing fractions were combined and evaporated under reduced pressure, and
the product was freeze-dried to afford the title compound, 456mg. 'H-NMR
(CDCI3,
400MHz) S: 0.82 (t, 3H), 1.70 (m, 2H), 2.80 (m, 1 H), 3.01 (m, 3H), 3.44 (m, 1
H), 3.78
(m, 3H), 3.89 (dd, 1 H), 6.70 (s, 1 H), 7.35 (s, 1 H). LRMS: m/z (ES+) 264
[MNa+].
15 Microanalysis found: C, 49.04; H, 8.17; N, 15.51. C"H,9N303;1.6H20 requires
C,
49.04; H, 8.28; N, 15.60%. [a]p = -33.43 (c = 0.193, methanol).
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
36
Examples 2 to 4
The following compounds of general formula
~NHZ
~N
R~N / OH
0
were prepared from the corresponding morpholinone compounds following the
procedure of Example 1.
Yield
Ex R Data
(%)
'H-NMR (D20, 400MHz) 8: 0.76 (t, 3H),
1.10 (m, 2H), 1.60 (m, 2H), 2.75 (dd,
1 H), 2.82 (dd, 1 H), 3.00 (m, 2H), 3.55
37 (m, 2H), 3.62 (t, 2H), 3.90 (dd, 1 H), 6.83
2 H3~ sticky (s, 1 H), 7.48 (s, 1 H). LRMS: m/z (TSP+)
solid 256.2 [MH+]. Microanalysis found: C,
53.59; H, 8.35; N, 15.57.
C~2H2~N303;0.75H20 requires C, 53.62;
H, 8.44; N, 15.63%.
'H-NMR (D20, 300MHz) S: 0.82 (m, 2H),
~/' 48 1.05 (m, 4H), 1.57 (m, 7H), 2.77-2.97 (m,
3 sticky 2H), 3.05 (m, 2H), 3.59 (m, 2H), 3.95 (m,
gum 3H), 6.94 (s, 1 H), 7.59 (s, 1 H). LRMS:
m/z (ES+) 310 [MHO].
'H-NMR (CDCI3, 400MHz) 8: 2.80 (dd,
1 H), 2.99 (m, 5H), 3.42 (m, 1 H), 3.80 (m,
1 H), 3.92 (m, 1 H), 4.02 (t, 2H), 6.64 (s,
1 H), 7.01 (d, 2H), 7.20 (m, 4H). LRMS:
4 ( 61
\ m/z (ES+) 304 [MHO]. Microanalysis
found: C, 58.93; H, 7.17; N, 12.82.
C~6H21 N303;1.27H20 requires C, 58.91;
H, 7.27; N, 12.88%.
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
37
Example 5
(25~-(- -~2-Aminoethoxy)-~ 3-f1-(2-cyclohexylethyl)-1 H imidazol-4-
vllpropanoic acid
NH2
~-N o~
~N / OH
I
O
A solution of the compound of Preparation 133 (72mg, 0.25mmol) in concentrated
hydrochloric acid (5ml) was heated at 110°C for 18 hours, then allowed
to cool and
concentrated under reduced pressure. The residue was dissolved in water and
the
solution was purified by column chromatography on Dowex~ 50WX8-200 ion
exchange resin using an elution gradient of water:0.88 ammonia:methanol
(95:5:0 to
90:5:5). The product was dissolved in water (5ml) and freeze-dried to afford
the title
compound as a sticky gum, 45mg. 'H-NMR (CD30D, 400MHz) 8: 0.99 (m, 2H), 1.21
(m, 4H), 1.61-1.78 (m, 7H), 2.86 (dd, 1 H), 3.02 (m, 3H), 3.58-3.70 (m, 2H),
3.98 (m,
3H), 6.93 (s, 1 H), 7.50 (s, 1 H). LRMS: m/z (ES+) 310 [MH+]. Microanalysis
found:
C, 59.56; H, 8.76; N, 12.91. C16H27N3O3;O.75H2O requires C, 59.51; H, 8.90; N,
13.01 %. [a]o = -23.34 (c = 0.102, methanol).
Example 6
(2S1-(-)-2-(2-Aminoethoxy)-3-(1-phenyl-1 H imidazol-4-yl)propanoic acid
~NH2
~N O
N / OH
O
The title compound was obtained as a fawn solid in 87% yield from the
morpholinone
of Preparation 104, following the procedure of Example 5. 'H-NMR (D20, 400MHz)
S: 2.88 (dd, 1 H), 3.00 (dd, 1 H), 3.10 (t, 2H), 3.62 (t, 2H), 4.02 (m, 1 H),
7.30 (s, 1 H),
7.39 (m, 1 H), 7.45 (m, 4H), 7.98 (s, 1 H). LRMS: m/z (ES+) 298 [MNa~].
Microanalysis found: C, 59.15; H, 6.39; N, 14.71. C~4H1~N303;0.5H20 requires
C,
59.14; H, 6.38; N, 14.78%. [a]o = -16.8 (c = 0.10, methanol).
Example 7
(25~-2-(2-Aminoethoxy)-3-(1-f3,5-bis(trifluoromethyl)phenyll-1 H imidazol-4-
yl)-
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
38
pro~~anoic acid
FaC NH2
~N O~
N / OH
FsC O
The title compound was obtained as a white solid in 45% yield from the
morpholinone of Preparation 106, following the procedure of Example 5. ' H-NMR
(CD30D, 400MHz) 8: 3.00-3.18 (m, 3H), 3.65 (m, 1 H), 3.75 (m, 1 H), 4.04 (m,
2H),
7.58 (s, 1 H), 7.98 (s, 1 H), 8.22 (s, 2H), 8.30 (s, 1 H). LRMS: m/z (ES-) 410
[M-H-].
Microanalysis found: C, 43.95; H, 3.79; N, 9.99. CigH15F6N3~3~1 .25H20
requires C,
44.30; H, 4.07; N, 9.69%.
Example 8
~2RS)-2-f(2-(Methylamino)ethoxyl-3-(1-propel-1H imidazol-4-yl)propanoic acid
H
N
H C~ ~N O~ ~CH3
N / OH
O
The title compound was obtained from the morpholinone of Preparation 51,
following
the procedure of Example 1. 'H-NMR (D20, 400MHz) 8: 0.70 (t, 3H), 1.62 (m,
2H),
2.56 (s, 3H), 2.76 (dd, 1 H), 2.84 (dd, 1 H), 3.05 (m, 2H), 3.53-3.63 (m, 2H),
3.80 (t,
2H), 3.94 (dd, 1 H), 6.84 (s, 1 H), 7.50 (s, 1 H). LRMS: m/z (TSP+) 256.2
[MH+].
Example 9
(2R5')-2-f(2-(Dimethylamino)ethoxyl-3-(1-propel-1 H imidazol-4-yl)propanoic
acid
CH3
N
H C~ ~N ~ \CH3
N / OH
I
A solution of the protected acid of Preparation 142 (200mg, 0.62mmol) in
trifluoroacetic acid (5ml) and dichloromethane (5ml) was stirred at room
temperature
for 18 hours, then concentrated under reduced pressure. The residue was
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
39
dissolved in water and the solution was purified by column chromatography on
Dowex~ 50WX8-200 ion exchange resin using water:0.88 ammonia (96:4) as eluant.
The product containing fractions were concentrated under reduced pressure and
the
residue was dissolved in water and freeze-dried to afford the title compound
as a
sticky gum, 100mg. 'H-NMR (D20, 400MHz) 8: 0.66 (t, 3H), 1.60 (m, 2H), 2.60-
2.78
(m, 7H), 2.82 (dd, 1 H), 3.14 (m, 2H), 3.57 (m, 1 H), 3.61 (m, 1 H), 3.78 (t,
2H), 3.92
(m, 1 H), 6.82 (s, 1 H), 7.48 (s, 1 H). LRMS: m/z (TSP+) 270.2 [MHO].
Microanalysis
found: C, 54.41; H, 8.72; N, 14.58. C,3H23N3O3;H2O requires C, 54.34; H, 8.77;
N,
14.62%.
Example 10
(2RS)-3-(1-Propel-1 H imidazol-4-yl)-2-f(3f~-pyrrolidin-3-~xylpropanoic acid
H3C
Concentrated hydrochloric acid (3ml) was added to a solution of the protected
amino
acid of Preparation 143 (232mg, 0.55mmol) in dioxan (2ml), and the mixture was
stirred at room temperature for 18 hours, then concentrated under reduced
pressure.
The residue was dissolved in water (1 ml) and the solution was purified by
column
chromatography on Dowex~ 50WX8-200 ion exchange resin using an elution
gradient of water:0.88 ammonia (100:0 to 98:2). The product was freeze-dried
to
afford the title compound as a white solid, 67mg. 'H-NMR (D20, 400MHz)
(mixture
of diastereoisomers) 8: 0.72 (m, 3H), 1.63 (m, 2H), 1.80, 1.98, 2.08 (3xm,
2H), 2.65,
2.84 (2xm, 3H), 3.03-3.38 (m, 3H), 3.81 (m, 2H), 3.94 (m, 1 H), 4.14 (m, 1 H),
6.89
(m, 1 H), 7.53 (m, 1 H). LRMS: m/z (ES+) 290 [MNa+].
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
Example 11
(2R5')-2 j2-Aminoethoxy)-3-{1-f2-(4'-eth Ify 1,1'-biphenyll-4-yl)ethvll-1 H
imidazol-4-Y,}-
propanoic acid
~NH2
H3C ~ \ ~ ~ N N
'- / OH
O
5 A mixture of the compound of Preparation 121 (170mg, 0.43mmol) and
concentrated
hydrochloric acid (2ml) was heated at 110°C for 18 hours, then allowed
to cool and
concentrated under reduced pressure. The residue was azeotroped with ethanol,
methanol and dichloromethane, then purified by column chromatography on Dowex~
50WX8-200 ion exchange resin using an elution gradient of water:0.88 ammonia
10 (100:0 to 98:2) to afford the title compound, l5mg. 'H-NMR (CD30D, 400MHz)
8:
1.22 (t, 3H), 2.62 (q, 2H), 2.82 (m, 1 H), 2.98 (m, 3H), 3.02 (t, 2H), 3.50-
3.62 (m, 2H),
3.90 (m, 1 H), 4.18 (t, 2H), 6.86 (s, 1 H), 7.14 (d, 2H), 7.20 (d, 2H), 7.30
(s, 1 H), 7.43
(m, 4H). Microanalysis found: C, 64.05; H, 6.99; N, 9.35. C25H29N303;2.7H20
requires C, 64.28; H, 7.21; N, 8.99%.
Examples 12 to 14
The following compounds of general formula
~NH2
~N
N / OH
O
were prepared from the corresponding morpholinones following the procedure of
Example 11.
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
41
Yield
Ex R Data
(%)
'H-NMR (CD30D, 400MHz) 8: 2.15
(m,
\ ~ 2H), 2.62 (t, 2H), 2.90 (dd,
1 H), 3.02 (m,
35
3H), 3.60 (m, 1 H), 3.66 (m,
1 H), 3.98 (m,
12 \ white 3H
7
40
),
.
(m,
3H), 6.96 (s, 1 H), 7.25 (m,
solid
2H), 7.54 (m, 3H), 7.58 (d, 2H).
LRMS:
m/z (ES-) 392 [M-H-].
H3~ 'H-NMR (CD30D, 400MHz) 8: 2.09
(m,
2H), 2.34 (s, 3H), 2.59 (m, 2H),
2.86 (m,
\ ~ 17 1 H), 3.00 (m, 3H), 3.60 (m,
2H), 3.97 (m,
13 white 3H), 6.96 (s, 1 H), 7.19 (m,
4H), 7.40-7.60
\ foam (m, 5H). LRMS: m/z (ES+) 430
[MNa~].
'H-NMR (CD30D, 400MHz) S: 2.18
(m,
\ 2H), 2.78 (t, 2H), 2.88 (dd,
1 H), 3.02 (m,
21
\ 3H), 3.58-3.72 (m, 2H), 3.97
(m, 3H),
14 yellow
6.95 (s, 1 H), 7.40 (m, 2H),
7.50 (s, 1 H),
solid
7.60 (s, 1 H), 7.78 (m, 3H).
LRMS: m/z
(ES-) 366 [M-H-].
Example 15
~25~-2-~f(1 f~-2-Amino-1-meth Iy ethylloxyf-3-f1-(2-cyclohex IV ethyl)-1 H
imidazol-4-yll-
propanoic acid
CH3
j~NH2
~N O
~N / OH
I
O
The title compound was obtained in 45% yield from the morpholinone of
Preparation
92, following the procedure of Example 11. 'H-NMR (CD30D, 400MHz) 8: 0.94 (m,
4H), 1.18 (m, 4H), 1.59-1.76 (m, 7H), 2.72 (m, 2H), 2.96 (m, 1 H), 3.03 (m, 1
H), 3.26
(m, 1 H), 3.55 (m, 1 H), 3.98 (m, 3H), 6.88 (s, 1 H), 7.48 (s, 1 H). LRMS: m/z
(ES+)
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
42
324 [MNa~]. Microanalysis found: C, 58.97; H, 9.01; N, 11.85. C»H29N303;1.2H20
requires C, 59.18; H, 9.17; N, 12.18%.
Exami~le 16
~2R5~-2-(2-Aminoethoxy)-3-(1-phenyl-1 H imidazol-4-yl)propanoic acid
~NH2
~N
N / OH
O
A solution of the compound of Preparation 99 (70mg, 0.27mmol) in concentrated
hydrochloric acid (2ml) was heated at 110°C for 18 hours, then allowed
to cool and
concentrated under reduced pressure. The residue was azeotroped with water,
then purified by column chromatography on Dowex~ 50WX8-200 ion exchange resin
using water:0.88 ammonia (95:5) as eluant to afford the title compound as a
pale
yellow solid, 50mg. 'H-NMR (CD30D, 400MHz) 8: 2.93-3.14 (m, 3H), 3.24 (m, 1
H),
3.59-3.73 (m, 2H), 4.00 (m, 1 H), 7.35 (m, 2H), 7.45 (m, 4H), 7.98 (s, 1 H).
LRMS:
m/z (ES+) 298 [MNa~]
Example 17
(2S~-2-f f (1 R)-2-Amino-1-methylethylloxy~~-3-(1-phenyl-1 H imidazol-4-
yl)propanoic
acid
CH3
j~NH2
~N O
N / OH
O
The title compound was obtained in 75% yield from the morpholinone of
Preparation
109, following the procedure of Example 16, except that an elution gradient of
methanol:water:0.88 ammonia (20:80:5 to 30:65:5) was used for the ion exchange
chromatography. 'H-NMR (D20, 400MHz) S: 0.90 (d, 3H), 2.80 (m, 2H), 3.00 (m,
2H), 3.59 (m, 1 H), 4.14 (dd, 1 H), 7.26 (s, 1 H), 7.37 (m, 1 H), 7.42 (m,
4H), 7.96 (s,
1 H). LRMS: m/z (ES-) 288 [M-H-]. Microanalysis found: C, 58.76; H, 6.72; N,
13.37. CISH~gN303;H2O requires C, 58.63; H, 6.89; N, 13.67%. [a]p = -83.0 (c =
0.1, methanol).
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
43
Example 18
~25~{f (1 R)-2-Amino-1-methylethylloxy?-3-f 1-(3'.4'-dichlorof 1.1'-biphenyll-
3-yl -1 H
imidazol-4-vl)aropanoic acid
ri
CH3
CI
NH2
/-N o~
/ OH
O
The title compound was obtained as a white foam from the morpholinone of
Preparation 115, following the procedure of Example 17, except that an elution
gradient of water:methano1:0.88 ammonia (75:20:5 to 15:80:5) was used for the
ion
exchange chromatography. 'H-NMR (CD30D, 400MHz) 8: 1.00 (d, 3H), 2.77 (dd,
1 H), 2.92 (m, 2H), 3.15 (dd, 1 H), 3.59 (m, 1 H), 4.10 (m, 1 H), 7.42 (s, 1
H), 7.50-7.627
(m, 5H), 7.78 (s, 1 H), 7.86 (s, 1 H), 8.10 (s, 1 H). LRMS: m/z (ES+) 434, 436
[MH~].
Example 19
(2S~-2-{f (1 R)-2-Amino-1-methylethylloxy}-3-f 1-(4-phenoxyphenyl)-1 H
imidazol-4-yll-
propanoic acid
CH3
j~NH2
~N O
O ~ ' N / OH
0
A solution of the morpholinone of Preparation 111 (130mg, 0.36mmol) in
concentrated hydrochloric acid (l0ml) was heated at 110°-C for 18
hours, then
allowed to cool and concentrated under reduced pressure. The residue was
purified
by column chromatography on Dowex~ 50WX8-200 ion exchange resin using
water:methano1:0.88 ammonia (70:30:5) as eluant. The product was dissolved in
water and freeze-dried to afford the title compound as an off-white powder,
70mg.
'H-NMR (CD30D, 400MHz) 8: 1.00 (d, 3H), 2.78 (dd, 1 H), 2.86-3.00 (m, 2H),
3.17
(dd, 1 H), 3.60 (m, 1 H), 4.15 (dd, 1 H), 7.02 (d, 2H), 7.08 (d, 2H), 7.17 (m,
1 H), 7.30
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
44
(s, 1 H), 7.39 (m, 2H), 7.50 (d, 2H), 7.95 (s, 1 H). LRMS: m/z (ES+) 404
[MNa~].
Microanalysis found: C, 62.70; H, 6.22; N, 10.30. C2,H2sN3O4;1.2H20 requires
C,
62.58; H, 6.35; N, 10.43%. [a]p = -50.9 (c = 0.117, methanol).
Examples 20 to 31
The following compounds of general formula
R
V NH2
~N O ,
N / OH .2HC1
O
were prepared by heating a solution of the corresponding morpholinone (0.2 to
0.4mmol) in concentrated hydrochloric acid (2ml) at reflux for 18 hours,
concentrating
the cooled solution, and azeotroping the residue with ethanol, ethyl acetate
and
dichloromethane.
Ex R Y Data
'H-NMR (CD30D, 400MHz) 8: 1.22
(d,
CH3 6H), 2.90 (m, 1 H), 3.10-3.22
(m, 6H),
H3C 3.78 (m, 2H), 4.36 (m, 1 H),
4.45 (t, 2H),
~ ~ - 7' 18 (d, 2H), 7.24 (d, 2H),
) 7.44 (m, 3H),
-(CH
2
2
7.52 (d, 2H), 8.62 (s, 1 H).
Microanalysis found: C, 56.65;
H, 7.03;
N, 7.92. C25Hs1 NsOa;2HC1;2H20
requires C, 56.60; H, 7.03;
N, 7.92%.
F C~ 'H-NMR (CD30D, 400MHz) 8: 3.18
(m,
5H), 3.78 (m, 3H), 4.15-4.38
(m, 1 H),
21 ~ ~ -(CH2)2-4.44 (m, 2H), 7.21 (m, 3H),
7.50 (m,
4H), 7.63 (m, 1 H), 8.58-8.65
(m, 1 H).
LRMS: m/z (ES+) 432, 434 [MHO].
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
Ex R Y Data
'H-NMR (CD30D, 400MHz) S: 3.10-
3.30 (m, 4H), 3.78 (m, 4H),
4.30 (m,
F3C 1 H), 4.46 (m, 2H), 7.24 (m,
2H), 7.50
(m, 1 H), 7.60 (m, 2H), 7.74
(m, 4H),
22 -(CH2)2-
8.62 (m, 1 H). Microanalysis
found: C,
50.27; H, 5.39; N, 7.34. C23H24F3-
N303;2HCI;1.7H20 requires C,
50.14;
H, 5.38; N, 7.63%.
'H-NMR (CD30D, 400MHz) S: 3.18
(m,
3H), 3.21 (m, 2H), 3.76 (m,
0.5H), 3.80
(m, 2H), 4.20 (m, 0.5H), 4.32
(m, 1 H),
23 -(CH2)r 4.48 (m, 2H), 7.20 (m, 4H),
7.30 (d,
F3C 1 H), 7.50 (m, 2H), 7.60 (m,
1 H), 7.76
(m, 1 H), 8.60 (m, 1 H). HRMS:
m/z
(ES+) 448.1842 [MH+].
'H-NMR (CD30D, 400MHz) 8: 3.18
(m,
2H), 3.20 (m, 2H), 3.74 (m,
1 H), 3.80
(m, 2H), 4.20 (m, 1 H), 4.35
(m, 1 H),
4.48 (t, 2H), 7.21 (m, 2H),
/ \ 7.26-7.40
24 -(CH2)2-(m, 4H), 7.48 (m, 1 H), 7.52
(m, 1 H),
8.59-8.65 (m, 1 H). Microanalysis
Ci
found: C, 50.18; H, 5.15; N,
7.32.
C22H23CI2N3O3;2HCI;H2O requires
C,
50.02; H, 5.11; N, 7.61 %.
H3C 'H-NMR (CD30D, 400MHz) 8: 1.20
(m,
CH3 6H), 2.81 (m, 1 H), 3.17 (m,
6H), 3.70
25 / ~ -(CH2)2-(m' 2H), 3.78 (m, 2H), 4.18
(m, 1 H),
4.30 (m, 1 H), 4.46 (m, 2H),
6.78-7.16
(m, 5H), 7.39 (d, 1 H), 7.44
(m, 2H),
Me0
8.60 (m, 1 H).
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
46
Ex R Y Data
'H-NMR (CD30D, 400MHz) S: 3.17
(m,
4H), 3.78 (m, 3H), 4.18 (m,
1 H), 4.30
(m, 1 H), 4.45 (m, 2H), 7.20
/ (m, 2H),
26 \ -(CH2)2-7.40 (m, 2H), 7.56 (m, 5H),
8.61 (m,
1 H). Microanalysis found: C,
52.64; H,
5.82; N, 7.91. C22H2aCIN303;2HCI;H20
requires C, 52.34; H, 5.59;
N, 8.32%.
'H-NMR (CD30D, 400MHz) 8: 2.24
(d,
6H), 3.07-3.22 (m, 5H), 3.77
(m, 3H),
H3C CH3 4.30 (m, 1 H), 4.42 (m, 2H),
7.23 (d,
1 H), 7.30 (s, 1 H), 7.45 (m,
~ ~ 3H), 8.60
27 -(CH2)r
(2xs, 1 H). Microanalysis found:
C,
57.44; H, 6.74; N, 8.02. C24HpgNg03;-
2HCI;1.3H20 requires C, 57.21;
H,
6.72; N, 8.34%.
'H-NMR (CD30D, 400MHz) 8: 3.12-
3.23 (m, 8H), 3.75 (m, 1 H),
3.80 (m,
2H), 4.32 (m, 1 H), 4.45 (m,
2H), 6.90
(m, 1 H), 6.99 (m, 1 H), 7.10
/ \ (m, 1 H),
28 -(CH2)2-7.19 (m, 4H), 7.50 (s, 1 H),
8.61 (m,
1 H). Microanalysis found: C,
54.72; H,
H3C
6.14; N, 7.96. C23H2sFNsOs;2HCl;-
1.2H20 requires C, 54.59; H,
6.06; N,
8.30%.
'H-NMR (CD30D, 400MHz) S: 1.00
(t,
3H), 2.56 (q, 2H), 3.19 (m,
5H), 3.80
29 -(CH2)2-(m, 2H), 4.19 (m, 1 H), 4.30
(m, 1 H),
H3C 4.46 (m, 2H), 7.04 (d, 1 H),
7.19 (m,
7H), 7.50 (m, 1 H), 8.62 (2xs,
1 H)
CA 02472238 2004-06-30
WO 03/061652 t', : '_<.r';=', :rir:''z9 ''r~'-~~".~ .t, PCT/IB03/00060
R Y pata
.;r;r, ,y. 'fT ,.. a ;x,.'a
. ,.. ... ~':;' :~f,.,; ..
y
D30D; 400MHz) S: 2.23 (m,
H-NMR (C
2H), 2.70 (t, 2H), 3.17 (m,
3H), 3.21 (m,
1 H), 3.80 (t, 2H), 4.22 (m,
2H), 4.30 (m,
1 H), 7.25 (m, -2H), 7.40 (d,
2H), 7.48-
30 -(CH2)3-:
. , ;
~_64 (m, 5H0, 8.6,2 (m, 1 H).
' Microanalysis found: C, 51-.77;
I-I, 5.72;
N; 7.61. C23H26CIN303;2HC1;2H20
-=r:;~~:.;3';~=',.; . . ,' , .
:: . ~ v . : requires C, 51~:46; H;~6.01;
N,'7.83%.
H=NMR (CD30D, 400MHz) S: ~2:23'(m,
'
." ____...w.~ .. ...._..::_.._.-2H);-2:74-(rig; 2H);~31:7-(rri;'4H);
.,.~. ... 3:80 .~-
_ . ~ . . :.. _ .. .. . ,. ~ .___.-_.
., , . _,F..::...: ' . (ioi; 2H)~~:2~;(r'i'i;3H);'~.7~05
. _: .. ..._. (i7it,l;H);~~;.,
.
7.1:8 (rn;. ;2H);; 7..29
(rn~, 2H);, 7.44 (m, ; : ~.,
:
:
~
.
,
.
31 -(CH2)3-,
. . . , , .
: ..
.
: . .
. .
.. .. :
2H), 7.58-7.7.0 (m,; 1:H);
s,,~1 H).,
..
8
.8.82.~(
,
,
,
,
Microanalysis:found: C, .50.15;
'' H;
5:38;
., y .
' , ~~' ': . ....: _,a,- . .: , .. ,
" '' " ''~'~~,.,.
'v . . N .7,.39. ,_C H F N O ~2HCIv2.5H
..-..:- O
i , . 23 25 2 3 , 3s . . . .
~ 2
. . . . , : ,
requires:.C,,,50:46; H, 5.89;
N; 7.68%'.~~.
. , .~~.:~ ' . ... ~.." ...,.", t,;'. . , ,v..~'y:s~W~i°;;; w
- Example.32 :. . , ,
, .~ . , ~;:..., , ~ v ~ ~ ', '. , .-,
- (2R5~=2=(2=AminoethoxyO3-f 1-(3-f 1:,1-.'=biph-enyll-3Tylpropyl)=1 H:
imidazol=4-yll-
T , , , . . . " . . .. . . .
.s. - -. . .... propanoic acid dihydrochloride. -.; , , -- . ; . -.
~ - ~ _ , . . ~.. , , ,
__ .. .. ..k:\ .~ . ~ _ _ .; ;. ,... .,,,,, , 1.... ;I v.,, ;~:-'~;~, :.
°.'~~. ~~::i, ;
, ~ . ~.~ ' r ~,
..-',,,,"
' , ~ . '.. ~/
. 1 ~ _ ' i ~ rt ~~ n e~ 1.- :.9 ~-k't? .:.:r;t -~'...9 .'~. h-'!.~.
..
'.° ' :2~HCI '
.. ...... ~ . , ' .,
i ~-:.w ..'.,.~~t,.;'' ~.k.n;, wj~ ~:.b~H., t.';. 'v, , .
.. 1 ' ~ ' ' , '-E.v. . ' ~.~'~;:~V ~:::~ .i-~~ r~t~'~. ;v' - ' i;.'
, . , . , ,
- .. i ,
jT'he title compound was obtained as
ari"off=viihife~fo~arii~from'aiie'c~iinpou~~nYa'of
__..__________ _ ..._....__... .. .. a_. __._. _. _,..._._..__.
...._..__.___..._____._._. .._...._..__ __ .
Preparation 145, following the procedure of Example 20: '.H-NMR:(CD30D,
400MHz) 8: 2.26 (m, 2H), 2.75 (m, 2H); '~3~ 1~4'~(ii~;~2H); -3:27 {rim; .1
H)3.74: (rn, 1 H),
.;,n,..~, ..~.,,?._~.~ W.,A,t,~E_.,,."itT~i;~~"' ~. z. ~ t ~...~,._rsT .~,,- r-
-.t-w -~ -,'.r'..~ i~-~i:y..A.
''3.78 ;(ri~i, 2H)°4-.22°(m;'3H); 7:18'' (m; '1 H),~
7:25i7'.4'2':(ni;' 6H)'' 7.:45°(s; '~1'-H)", -7:5$ "(d;
~...;>,
'2H), 8:80 (s, 1 H). LRMS: ~rii%zv(ESy3~4: [MFI~]~.''~ Mie't:o'.analysis
found: C, 54.03; H,
,6:73;' N, 8.09. C23H2~N3032HC1;2.4H2:0 requires C;--54:21;;H;
6::68;~:N!=8.24%..
. . . , -. . . .. . . ;' : := 47 '. . . .
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
48
Example 33
(2RS)-2- 2-Aminoethoxy)-3-f1- 3-X1.1'-bia~hen~l-2-ylpropyl)-1 H imidazol-4 yl1
propanoic acid dihvdrochloride
The title~corripound viias obtained as an offwtiite'foam~in 30% yield frorn
tli~e
compound~of Preparation 146, following the procedure of EXamp~le 20. ~'H=NMR
(CD30D, 4OOMHz) 8: 1.96 (m, 2H), 2:60'-(t2'H)~~3.05-3.23 (m, 4H), 3.79 (m,
2H),
S..~: .".,~, ~ t - sr-.~,,~s~~ . ~.~.~ , ' (e,..(.,.~d.l~/a,;y'.~:'.t~~..'~
:_;(',:~:.iv;'i~..!t.S
4.02°(t~; ~2H);~4:4$~(m; 1H), ~.1~4~(d;~1=I-I); 7.19 7:30
(m!'~6H);.'7:36w(m;.3W).;°-8:62w(s;~lH).
Microanalysis found: C, 54.89;'H~; ~6:24;~N~, 8:35:wC23H2~N303;2HCI;2H20
requires C,
54.98; H, 6.62; N, 8.36%:'" ~,
Exarnple 34 ,
(2S)-2-{f (2S)-Aminopropylloxy?-3-f 1-(2-cVclohexylethyl)=1'H=imidazol-4-
yllpropanoic
~
~ ~~ acid dihydrochloride .
t . H~C~~.;t NH2.,.,;; .r"..; x~ , , ,
~ t :: t. _ . . ~ ~~' , . . . . . ; ~i.. . . '.r. .~. ~.~ i~ ~,
_.. :2H~~ . . ;... ,
': ~ . ~, .._ ~ ,~ ' '. ' .. ,.. ''~t r ;w't ~i r.t ; ,. > i:; ; Y,°
.;~
~7:~~:~:a..r'~. ; ''~.,,~.a~ V~....~, , , ;~,;. ~;,~y~~-H..~~t':
'
~.. ._v
f: J~~, ~ i~v;,' ,...:. _t~" ,a",; 'f . ..~.' i: _ ' ~';.Oy.;; . x
J,a..~. , .
v,p: ~heifitle~cor~pound._was'obtained in 64% yield from the morpholinone of
Preparation
95, following the procedure, of Example 20. 'H-NMR (CD30D, 400MHz) S: 0.80-
1.32
(m, 9H), 1.60-1.80 (m, 7H), 3.20 (m, 1.H)3.57 (m, 3,H), 3.80 (m, 1 H), 4.20
(m, 2H),
4.'35 (rri,,,l~H),'.7:48'..(s;~IHj;F8:86.(s;..~,H) ,
..MRMS:y:.,rn/z~(ESI)'324,.22$0~[MHO, : ~v. ~,__ v~
., , . ~ . ~' . . . .
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
..
Example 35 ,
(2R)-2-(f(2R)-Aminopropvlloxy)-3-fi- 2-c cly ohexylethyl)-1 H imidazol-4-
yllaro~~anoic
acid dihydrochloride
. . H3C, NH2
~ .2HCI
/ -N
' . ~ .. ~S' ~ ~'. . . N. / , ~H ,. ,.',;
. . . ~ p ..' , , .,.
,.., ;w: :ar r..
' ;.:,
The title compound was obtainecf'from the morpholinone of Preparation 96,
following
the procedure of Example 20. 'H=NMR (CD30D; 400MHz)'8: 0.80-1.32 (m, 9H),
1.60=1.80 (m, 7H), 3.20 (m, 1 H), 3.57 (m3H),, 3:80 (m, 1 H), 4.20 (m, 2H),
4.35 (m,
1 ~I La~:48' s F~1 H ~-.78:86'y s ~~:1~H ~: : -HRMS:. m/z, ESI. :324:2275'
MH;~ .
..)~_.~ .(...~.~. ...)~. ..... .(. :~.... . ) . . _ - . _. _. . . ..(. .. )
.._ . ...... . .[.. . ...~]..::' ':.,:.,, , .:......;. :.:.:.~~..: _ _
., ., ~. .
Example 3'6~ =°~ ,
(2RS')-2-(2-Aminoethoxy)-3-f1-(2-mettivlahenvl)-1 ,H-imidazol-4-yllpropanoic
acid
. , ~ ~ dihydiochloride' f
... , , '~_. %CH3 . . - . ...-'.. . NH2 .
~N
.2HCI
N / OH ~.
:<"-.r w.':',.. _ "a-a.~. '~. a,~~,..~'. .;~;n.;_ ...
,,, ,..,. :f'y, ~ p . ', r
A. solution ,of,.the.amorpholinone.of; Preparation.1,00
w(1~.OOrng;,;0.:37mmol), in,. , , ,
concentrated:~hy~irachloriGacid.;(2ml).was:'heated!.,at;;1.10.°Cv:fo'r~3
6~hours';_then;cooled
and concentrated under reduced pressure: The; residue was azeotroped with
;3 methanol and dichloromethane to afford.,the,aitle;_compound. 'H-NMR (CD30D,
400MHz)_5;2:1OK(:s:"3H);:3.~19~(m,t2H); 3:26
(m;~l:H),.3:39.(m~,~.1'H)',,3:82~(t,:2H),,~4.42
(m, 1 H), 7.40 (m, 2H)., 7.46 (m, 2H); _7:62_~(s; '1 H.),:9.14 (s, 1 H).'
.~HRMS: m/z..
290.1502 [MHO]. - - w ' w
-_'- ..: , . . .,
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
Example 37
(2~-2-ff(1 S"1-2-Amino-1-methyleth I~loxy,}-3-[1-(2-cyclohexylethyl)-1 H
imidazol-4-yll-
propanoic acid dihydrochloride
NH2
.2HCI
O
5 The t~itle'compound was obtained from the morpholinone of Preparation 91,
following
the procedure of Example 20. 'H-NMR (CD30D, 400MHz) 8: 1.01 (m, 5H), 1.21 (m,
4H), 1.74 (m, 7H), 2.93-3.14 (m, 3H),,.3.25,,(m,,1 H), 3.79 (m, 1 H), 4.21 (t,
2H), 4.38
(m, 1, H), .7.55, (s, 1 H), 8.90 (s,,1 H). _ LRMS:~ m%z,(ES~). 322 [M-H-].,
Microanalysis
v . ;~r_ ..y".~. ' °"5 ':'.i '~ . ,'~. v . , f ,, .! r, f. , ~, 1. t. .
~ .ter5." ~.~',.,
. '.' M '.. .,y ...,;. . ; .~!_ ._...~.~.,k~._...... . ....
found~C,~47.73; 'H, 7.85;~~IV, 9.66. ~~C»H291V303;2HCI~;1.6H20 requires C,
48.02;~H,~
. . _ _f:~p... _.._ . . "'~ _:-_~~a~' ~..°'.... ~:::~~~~::
10 8.11; N, 9.88%.
' y y . . ~ EXample 38 ~ ..
, . . .: .t ' . ; ,....
(2S)-2-df(1 f~-2-Amino-1'-meth I~~iIloxV~-3-d1-(2-(4;4-dimeth
~~ycl~ohexy)ethyll-1 H
imidazol-4-~)propanoic acid dih~rdrochloride
~ ~ '~~~,.ii:li W ."~n'~ ~. .. . . " .. . . CH.3.; ,".' "~..j,av°. ,;~
.rr..
. r . P .~ C.:c... .. ,. . ~~ . : ~'~.: - ~ ~. . _ ',"im 1.'... . ., ~-t.!,.
.,~ ~ , ._.~P...r . .. Il ' Y..rt7~ . , r
.. '' . .;~, :, ~ N::; y:; t , ~~'.,''t'"/ ,~/.NHz.i , . _: ; . :: . ', s ...
L , ,
;-%;. ~ . . y~~ ~~kH'C.~.~ . , . .~',N, :~." ' . . ~ °'Ol:.p: ~;;..:,,
.?~HCI ~'y' E,°'.~ ~'.°r~i
.",-;;
~'t ~,~ s. :- ~y. ,, ~._~_~ :. ...4 '~~~t'~';;si,'-~ : i~ ~7'~;' ;';,.'..,
ri.';~~ ';m;W
'rf'; , :S. 6.a1.; . " _ '~.3 ~~. 5.y., ~a:~.. ., .~ ~i,s~.' : ......_,a. : f,
. .,t..'! .,
15 p .,' 4 :.' ~ ' ! ..~ ~ 3 .r 1.~.~ i ' ~' .n . ~ ~'' c ~ t ' V ,La 4 y
'r'...i
The title compound was 'obtained: in' 83%~yield from'the: morpholinone of
Preparation
', r~ ai, . a r .->? Z.,-.~,y~ ~r.f
93, following the procedure of Example 20. 'H-NMR (CD30D, 400MHz) S: 0.88 (s,
6H), 1.04 (d, 3H), 1,:17-1.34 (m, 5H), ,1. .40 (m, 2H), ~ 1.60 (iii, 2H), 2.97-
3.18 (m, 3H),
' ~ . - ~ ~-wr;r.'.
3.30"(m,,1 H),, 3.81 (m, 1 H), 4.24~ (m;' 2H), ,4.40. (m, 1 H),. 7.58 (s,_-1
H), 8.94;(s, 1_ ,H).:
'tSf~ ~ n ,-~ ~ , t . ~ .~ 7a( ! i ''~,n 'k ~s
..'t_-. :.
20 HRIVIS:~m%z~(ES+)~ 374 2484 [MNa+]: ; ._.. .. ~ ~ _. ... ... .... ._ . .._
._.__ .._.
vP~ ; C' ' , t ' , r u. v.~ .'fty;'
'.., ,... . ' ; ' . . . . , . ~ . ''
,. . j 1 ' 'a,' . , ". n-.~" . ~ ~. ,.. .. . , . , ,
.~' . ~ . ' ~ " 'r;, .
~. . ,, ~a .. . ~ '
~~ .
'f . . . . . ' - . ' . '
,. i j ' -,.,t :~2~ ~ ~'"f ~'t. -' C y'-~~',~'~c~~ - 'f~~ '' ea l,pt:.ea~~ 51.
~ i..mF,.~'~ _,y~,.i .~ ~ ;- t <~. lit ~,-.~.
,.. ~g(~-. .. .. .. . ~ ..l ...5 ... ,...[. , .~. y,. c. . . ~ . .'..,. .~!, .
. ,. . -~ . ! .,t.t ~ .5.~... ., . v.~~t..a...~..~.
.,
. , ... ..
1~'.e ~'~ Y."'.W t' _~ ~L~~:. ' iw f~. v. .. .. i _ ~ H~'~.=~ti.n 'y , '... .
.,~ , ..~'.~._ , ,',
,.., ~t~,~'1A~. ~, .B , . . .... . , '..t ..s . _ . " ,..t. .... , . . ".. A
a.
~t~..~~ ~5 r ft~, ,~ ~ j ~~' n P.", 'L 5 tF~ ~ '," y f. . ry
':5 .' .'..~'', , ~'.'. .. .. o ~:f~ .i~"(~, ~ 4. '.~.1 p,, .'..v; ~i~~:
A.~.'.,, b......f i.. . , n . ~..t L
4_5
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
51
Example 39
L2 -SL2-{fy1 f~-2-Amino-1-methylethylloxy~-3-[~3-cyclohexyl-3-methylbutyl)-1 H
imidazol-4-~lpropanoic acid dihydrochloride
CH3
j~NH2
~N O
H3C N / OH .2HC1
CH3
O
The title compound was obtained from the morpholinone of Preparation 94,
following
the procedure of Example 20. 'H-NMR (CD30D, 400MHz) 8: 0.94 (s, 6H), 0.98-1.30
(m, 10H), 1.62 (m, 1 H), 1.78 (m, 6H), 2.90-3.15 (m, 3H), 3.78 (m, 1 H), 4.20
(m, 2H),
4.38 (m, 1 H), 7.52 (s, 1 H), 8.90 (s, 1 H). LRMS: m/z (ES+) 366 [MH~].
Example 40
2-(2-Aminoethoxy)-3-f 1-(3-phenoxyphenyl)-1 H imidazol-4 ~illpropanoic acid
dihydrochloride
O ~N O~/NH2
% OH .2HCI
v
O
A solution of the morpholinone of Preparation 102 (25mg, 0.07mmol) in
concentrated
hydrochloric acid (5ml) was heated at 110°C for 18 hours, then cooled
and
concentrated under reduced pressure. The residue was dissolved in water and
freeze-dried to afford the title compound as a fawn solid, 35mg. 'H-NMR (D20,
400MHz) 8: 3.04 (m, 3H), 3.18 (dd, 1 H), 3.63 (t, 2H), 4.19 (m, 1 H), 6.98 (d,
2H), 7.06
(m, 3H), 7.19 (d, 1 H), 7.46 (dd, 2H), 7.50 (dd, 1 H), 7.54 (s, 1 H), 8.87 (s,
1 H).
LRMS: m/z (ES+) 368 (MH+].
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
52
Examples 41 to 44
The following compounds of general formula
~NH2
~N
R N / OH .2HC1
O
were prepared from the corresponding morpholinones following the procedure of
Example 40
Ex R Data
'H-NMR (D20, 400MHz) 8: 3.01-3.20
(m, 4H),
3.65 (m, 2H), 4.21 (m, 1 H), 7.10
(m, 2H), 7.25
41 ~ (s, 1 H), 7.32 (m, 3H), 7.54 (m, 3H),
7.61 (m,
1 H), 8.59 (s, 1 H). LRMS: m/z (ES+)
352
[MHO]; 374 [MNa~].
'H-NMR (D20, 400MHz) S: 3.18-3.35
(m, 4H),
3.80 (t, 2H), 4.30 (dd, 1 H), 7.60
(m, 3H), 7.77
(s, 1 H), 7.98 (m, 2H), 8.04 (m, 2H),
9.06 (s,
42 \ 1 H). LRMS: m/z (ES+) 348 [MNa+].
Microanalysis found: C, 49.16; H,
5.60; N,
9.54. C~$H~9N303;2HCI;2.3H20 requires
C,
49.18; H, 5.65; N, 9.58%.
'H-NMR (D20, 400MHz) 8: 3.17-3.35
(m, 4H),
- 3.79 (m, 2H), 4.30 (m, 1 H), 7.40
(m, 2H), 7.52
43 (m, 2H), 7.60 (m, 2H), 7.70 (m, 3H),
9.02 (s,
1 H). LRMS: m/z (ES+) 386 [MH~].
CI
'H-NMR (D20, 400MHz) 8: 3.20-3.35
(m, 4H),
- 3.80 (m, 2H), 4.30 (t, 1 H), 7.42-7.75
(m, 8H),
44 9.05 (s, 1 H). LRMS: m/z (ES+) 420,
422
[MH+].
CI CI
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
53
Example 45
(25~-(-)-2-(2-Aminoethoxy)-3-f1-(4-tert-butylphenyl)-1 H imidazol-4-
yl]propanoic acid
dihydrochloride
NH2
~N o~
H3C ~ \ N / OH .2HCI
H3C
HsC O
The title compound was obtained as a fawn solid from the morpholinone of
Preparation 105, following the procedure of Example 40. 'H-NMR (D20, 400MHz)
8:
1.20 (s, 9H), 3.04-3.35 (m, 4H), 3.78 (m, 2H), 4.38 (m, 1 H), 7.41 (m, 2H),
7.58 (m,
2H), 7.61 (s, 1 H), 8.98 (s, 1 H). LRMS: m/z (ES+) 332 [MH~]. Microanalysis
found:
C, 49.58; H, 6.75; N, 9.84. C~gH25N3O3;2HCI;1.75H20 requires C, 49.58; H,
7.06; N,
9.64%. [a]p = -2.00 (c = 0.30, methanol).
Examales 46 to 48
The following compounds of general formula
CH3
NH2
~N O~
R N / OH .2HCI
I
O
were prepared from the corresponding morpholinones following the procedure of
Example 40
Ex R Data
'H-NMR (D20, 400MHz) 8: 1.00 (d,
3H), 1.16-
1.42 (m, 6H), 1.64 (m, 1 H), 1.78
(m, 3H), 2.59
(m, 1 H), 2.89-3.17 (m, 4H), 3.68
(m, 1 H), 4.30
46 (m~ 1 H), 7.44 (m, 4H), 7.66 (s,
1 H), 8.98 (s,
1 H). LRMS: m/z (ES+) 372 [MH~].
Microanalysis found: C, 52.74; H,
7.27; N,
8.54. C21H29N303;2HC1;2H20 requires
C,
52.50; H, 7.34; N, 8.75%.
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
54
Ex R Data
'H-NMR (D20, 400MHz) 8: 0.98 (d,
3H), 2.90
(m, 1 H), 3.05 (m, 2H), 3.24 (m,
1 H), 3.78 (m,
1 H), 4.38 (m, 1 H), 7.02 (d, 2H),
7.15 (m, 3H),
47 7.26 (d, 1 H), 7.38 (m, 2H), 7.48
(m, 1 H), 7.62
(s, 1 H), 8.99 (s, 1 H). LRMS: m/z
(ES+) 382
[MHO]. [a]p = -72.0 (c = 0.05, methanol)
'H-NMR (D20, 400MHz) 8: 1.01 (d,
3H0, 2.98
(m, 1 H), 3.15 (m, 2H), 3.29 (m,
1 H), 3.79 (m,
1 H), 4.37 (m, 1 H), 7.40 (m, 2H),
7.58 (d, 2H),
7.62 (m, 2H), 7.75 (m, 3H), 9.05
(s, 1 H).
48
LRMS: m/z (TSP+) 400.2 [MHO].
Microanalysis found: C, 47.90; H,
5.48; N,
7.90. C2~H22CIN303;2HCI;3H20 requires
C,
47.88; H, 5.74; N, 7.98%.
Example 49
(2S)-2-ff(1 F~-2-Amino-1-methylethylloxy~3-(1-[4~- cyclohexyloxy)phenyll-1 H
imidazol-
4-yl)propanoic acid
CH3
NH2
~N o~
O ~ \ N / OH
O
A solution of the protected amino acid of Preparation 120 in a mixture of 2N
hydrochloric acid (1 ml), water (1 ml) and dioxan (1 ml) was stirred at room
temperature for 2 hours, then concentrated under reduced pressure. The residue
was dissolved in water and purified by column chromatography on Dowex~ 50WX8-
200 ion exchange resin using water:methano1:0.88 ammonia (48:48:4) as eluant.
The product was dissolved in water and freeze-dried to afford the title
compound,
25mg. 'H-NMR (D20, 400MHz) S: 0.94 (d, 3H), 1.35 (m, 4H), 1.55 (m, 3H), 1.72-
2.01 (m, 3H), 2.78-2.97 (m, 2H), 3.10 (m, 2H), 3.60 (m, 1 H), 4.01 (m, 1 H),
4.21 (m,
1 H), 6.92 (d, 2H), 7.01 (s, 1 H), 7.20 (d, 2H), 7.62 (s, 1 H). LRMS: m/z
(ES+) 388
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
[MH~]. Microanalysis found: C, 59.33; H, 7.65; N, 9.80. C21H29N30a;2H20
requires
C, 59.56; H, 7.85; N, 9.92%.
Example 50
5 (2S~-2-(2-AminoethoxY)-3-(1 H imidazol-4-y))proaanoic acid dihydrochloride
~NH2
~N O
HN / OH .2HC1
I
O
A suspension of the morpholinone of Preparation 118 (90mg, 0.24mmol) and
lithium
hydroxide (32mg, 1.33mmol) in a mixture of water (2ml) and tetrahydrofuran (1
ml)
was stirred at room temperature for 18 hours, then concentrated under reduced
10 pressure. The residue was suspended in concentrated hydrochloric acid (3ml)
and
the mixture was heated at 110°C for 18 hours, then cooled and
concentrated under
reduced pressure. The residue was triturated with acetone and the resulting
solid
was collected and dried, then recrystallised from methanol/acetone to afford
the title
compound as a white solid, l2mg. 'H-NMR (CD30D, 400MHz) 8: 3.20 (m, 2H), 3.30
15 (m, 2H), 3.83 (m, 2H), 4.37 (m, 0.5H), 4.41 (m, 0.5H), 7.41 (s, 1 H), 8.81
(s, 1 H).
LRMS: m/z (ES-) 198 [M-H-].
Example 51
~25~-2-~f(1 R)-2-Amino-1-methylethylloxy)-3-(1 H imidazol-4-yl)propanoic acid
CH3
NH2
~N O~
HN / OH
I
20 O
A solution of the morpholinone of Preparation 105 (320mg, 1.64mmol) in
concentrated hydrochloric acid (5ml) was heated at 110°C for 18 hours,
then allowed
to cool and diluted with water (80m1). The resulting solution was purified by
column
chromatography on Dowex~ 50WX8-200 ion exchange resin using an elution
25 gradient of water:0.88 ammonia (100:0 to 95:5). The product was dissolved
in water
and freeze-dried to afford the title compound as a colourless foam, 290mg. 'H-
NMR
(D20, 400MHz) 8: 0.90 (d, 3H), 2.80 (dd, 2H), 2.98 (dd, 2H), 3.57 (m, 1 H),
4.04 (m,
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
56
1 H), 6.84 (s, 1 H), 7.60 (s, 1 H). LRMS: m/z (ES+) 236 [MNa~]. Microanalysis
found:
C, 46.00; H, 7.41; N, 17.81. C9H15N3O4;1.25H20 requires C, 45.85; H, 7.48; N,
17.82%. [a]p = -94.62 (c = 1.72, water).
Alternative synthesis
A mixture of the protected lactone of Preparation 55a (1.58g, 4.42mmol) and
methanesulphonic acid (6.5m1) was heated at 70°C for 2.5 hours. The
cooled
solution was diluted with ether (40m1), the mixture was stirred, and the ether
was
decanted off. This process was repeated twice. Water was added and the mixture
was stirred vigorously then filtered. The filtrate was allowed to stand at
room
temperature for 24 hours then purified by column chromatography on
Dowex°
50WX8-200 ion exchange resin using water:0.88 ammonia (95:5) as eluant, and
the
fractions obtained were evaporated under reduced pressure (with water-bath
temperature below 33°C). The product obtained was dissolved in
concentrated
hydrochloric acid (5ml), and the solution was heated at 100°C for 18
hours, then
cooled and diluted with water (30m1). This solution was purified by column
chromatography on Dowex° 50WX8-200 ion exchange resin using water:0.88
ammonia (95:5) as eluant. The product was stirred in acetonitrile (l0ml) for 1
hour,
filtered and dried in vacuo for 18 hours. Microanalysis found: C, 46.58; H,
7.50; N,
17.98. C9H15N3.1 H20 requires C, 46.75; H, 7.41; N. 18.17%.
Example 52
(25~-2-(f(1 F~-2-Amino-1-methylethylloxy)-3-f 1-(2-pyridinyl)-1 H imidazol-4-
yll-
propanoic acid dihydrochloride
CH3
j~NH2
N ~N O
N / OH .2HC1
A solution of the morpholinone of Preparation 152 (72mg, 0.26mmol) in
concentrated
hydrochloric acid (3ml) was heated at 100°C for 18 hours, then allowed
to cool,
diluted with water, and concentrated under reduced pressure. The residue was
purified by column chromatography on Dowex° 50WX8-200 ion exchange
resin
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
57
using an elution gradient of water:0.88 ammonia (100:0 to 95:5). The product
was
dissolved in 2N hydrochloric acid and the resulting solution was concentrated
under
reduced pressure. The residue was dissolved in water (l0ml) and freeze-dried
to
afford the title compound as a solid, 58mg. 'H-NMR (D20, 400MHz) (mixture of
imidazole regioisomers) 8: 0.79, 0.99 (2xd, 3H), 2.56, 2.92 (2xm, 1 H), 3.02-
3.44 (m,
3H), 3.58, 3.78 (2xm, 1 H), 7.55 (m, 1 H), 7.71 (m, 1 H), 7.96-8.19 (m, 2H),
8.52, 8.61
(2xm, 1 H), 9.03, 9.38 (2xs, 1 H). LRMS: m/z (ES+) 291 [MH+].
Example 53
(2S)-2-ff(1 f~-2-Amino-1-methylethylloxy~~-3~1-propel-1 H imidazol-4-
y~propanoic acid
HsC NHz
N ~ O ~CH3
OH
N a
O
A solution of the compound of Preparation 157 (560mg, 2.36mmol) in
concentrated
hydrochloric acid (l0ml) was stirred at 110°C for 18 hours. The cooled
solution was
concentrated under reduced pressure and the residue was purified by column
chromatography on ion-exchange resin (Dowex~ 50WX8-200) using an elution
gradient of water:0.88 ammonia (100:0 to 95:5) to afford the title compound as
a
foam, 350mg. 'H-NMR (D20, 400MHz) S: 0.66 (t, 3H), 0.81 (d, 3H), 1.60 (m, 2H),
2.60-2.78 (m, 2H), 2.80-2.98 (m, 2H), 3.45 (m, 1 H), 3.78 (t, 2H), 3.98 (dd, 1
H), 6.82
(s, 1 H), 7.43 (s, 1 H). LRMS: m/z (ES+) 278 [MNa~]. Microanalysis found: C,
53.44;
H, 8.13; N, 15.40 C12H2~N303;0.8H20 requires C, 53.44; H, 8.45; N, 15.58%.
[a]o =
-86.93° (c = 0.11, methanol)
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
58
Example 54
(21~-2-(f(1 F~-2-Amino-1-meth I~~illoxy]~-3-LH imidazol-4-~)propanoic acid
NHZ
H
N CHs
OH
N a
O
A mixture of the lactam of Preparation 158 (80mg, 0.50mmol) and 2N
hydrochloric
acid (2ml) was heated at 110°C for 16 hours, then allowed to cool and
diluted with
water (l0ml). This solution was purified by column chromatography on Dowex~
50WX8 resin, using water:0.88 ammonia (95:5) as eluant. The product was
triturated with acetone and the resulting solid was dried in vacuo to afford
the title
compound as a brown solid, 68mg. 'H-NMR (D20, 400MHz) 8: 1.00 (d, 3H), 2.76
(dd, 2H), 2.83 (m, 2H), 3.60 (m, 1 H), 3.86 (m, 1 H), 6.79 (s, 1 H), 7.57 (s,
1 H).
Preparation 1
1-n-Propel-1 H imidazole-4-carboxaldehyde
/N O
IN ~'~~~~~
HC~ H
3
Imidazole-4-carboxaldehyde (30g, 0.31 mol) was added portionwise to a solution
of
sodium hydride (13.9g, 60% dispersion in mineral oil, 0.348mo1) in
tetrahydrofuran
(450m1), and the solution was stirred for 45 minutes. n-Propyl bromide
(31.2m1,
0.344mo1) was then added portionwise, followed by 18-crown-6 (150mg), and the
mixture was heated under reflux for 18 hours. Aqueous ammonium chloride
solution was added to the cooled solution, and the mixture was extracted with
ethyl
acetate (2x) and dichloromethane (2x). The combined organic extracts were
dried
(MgS04), filtered, and concentrated under reduced pressure. The crude product
was purified by column chromatography on silica gel, eluting with ethyl
acetate:
pentane (40:60), to give the title compound, 20.2g, 47% yield. 'H-NMR (DMSO-
ds,
400MHz) 8 : 0.80 (t, 3H), 1.76 (m, 2H), 3.98 (t, 2H), 7.84 (s, 1 H), 8.04 (s,
1 H), 9.70
(s, 1 H). LRMS: m/z (TSP+) 277.3 [2M+H]+
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
59
Preparation 2
1-n-Butyl-1 H imidazole-4-carboxaldehyde
'N O
INr~~~.i,
H3C~~/ H
The title compound was obtained in 28% yield from imidazole-4-carboxaldehyde
and
n-butyl bromide, following a similar procedure to that described in
Preparation 1.
'H-NMR (CDCI3, 300MHz) 8: 0.97 (t, 3H), 1.37 (m, 2H), 1.80 (m, 2H), 4.00 (t,
2H),
7.55 (s, 1 H), 7.62 (s, 1 H), 9.88 (s, 1 H). LRMS: m/z (TSP+) 153.3 [MH~]
Preparation 3
1-(2-Cyclohex Iy ethyl)-1 H imidazole-4-carboxaldeh~
~N
N / O
H
Imidazole-4-carboxaldehyde (4.8g, 50mmol) was added portionwise to a
suspension
of sodium hydride (2.20g, 60% dispersion in mineral oil, 55mmol) in
tetrahydrofuran
(150m1), and the mixture was then stirred at room temperature for 1 hour. 2-
Cyclohexylethyl bromide (8.6m1, 55mmol) was added, and the mixture was heated
under reflux for 18 hours. The cooled mixture was evaporated under reduced
pressure and the residue was partitioned between water (500m1) and
dichloromethane (500m1). The layers were separated, and the organic phase was
dried (MgS04) and evaporated under reduced pressure. The crude product was
purified by column chromatography on silica gel using an elution gradient of
toluene:ethyl acetate (100:0 to 96:4) to afford the title compound, 1.78g. 'H-
NMR
(CDC13, 400MHz) S: 0.98 (m, 2H), 1.20 (m, 4H), 1.68 (m, 7H), 4.00 (t, 2H), 7.4
(s,
1 H), 7.60 (s, 1 H), 9.80 (s, 1 H). LRMS: m/z (TSP+) 207.2 [MHO]
Preparation 4
1-(2-Phenylethyl)-1 H imidazole-4-carboxaldeh~de
~N
\ N / O
i
H
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
Imidazole-4-carboxaldehyde (6.738, 70mmol) was added portionwise to a
suspension of sodium hydride (1.68g, 60% dispersion in mineral oil, 70mmol) in
tetrahydrofuran (280m1), and the mixture was then stirred at room temperature
for 30
minutes. (2-Bromoethyl)benzene (9.56m1, 70mmol) was added, and the mixture
5 was stirred at room temperature for 72 hours. The mixture was evaporated
under
reduced pressure and the residue was partitioned between water (300m1) and
dichloromethane (500m1), and the layers were separated. The organic phase was
dried (MgS04) and evaporated under reduced pressure. The crude product was
pre-adsorbed onto silica gel, and purified by column chromatography on silica
gel
10 using an elution gradient of ethyl acetate:pentane (50:50 to 100:0) to
afford the title
compound, 1.44g. 'H-NMR (CDCI3, 400MHz) 8: 3.16 (t, 2H), 4.23 (t, 2H), 7.02
(d,
2H), 7.28 (m, 3H), 7.36 (s, 1 H), 7.50 (s, 1 H), 9.83 (s, 1 H). LRMS: m/z
(ES+) 223
[MNa~].
15 Preparation 5
1-Trityl-1 H imidazole-4-carboxaldehyde
/ ~N o
N
I H
A solution of trityl chloride (9.5g, 34.3mmol) in N,N-dimethylformamide (50m1)
was
added dropwise to an ice-cooled solution of imidazole-4-carboxaldehyde (3g,
20 31.2mmol) and triethylamine (l7ml, 125mmol) in N,N-dimethylformamide (30m1)
and
the solution was stirred for 2 hours. The reaction was then allowed to warm to
room
temperature, and was stirred for a further 18 hours. Water (200m1) was added,
and
the resulting pink solid was collected and dried, then dissolved in
dichloromethane
(200m1). The resulting solution was washed with water (2x100m1), dried (MgS04)
25 and evaporated under reduced pressure. The product was recrystallised from
ethanol to afford the title compound as a solid, 7.8g. 'H-NMR (CDC13, 400MHz)
8:
7.06 (m, 6H), 7.32 (m, 9H), 7.48 (s, 1 H), 7.58 (s, 1 H), 9.82 (s, 1 H).
Microanalysis
found: C, 81.54; H, 5.37; N, 8.24. C23H~8N20 requires C, 81.63; H, 5.36; N,
8.28%.
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
61
Preparation 6
2-f~4-Methoxybenz~)aminolethanol
\ OwCH3
I~
HO
Acetic acid (ca. 150m1) was added to a solution of p-anisaldehyde (58.2g,
0.42mo1)
and ethanolamine (152m1, 2.52mo1) in methanol (1 L), to achieve a pH of 6.
Sodium
triacetoxyborohydride (100g, 0.47mo1) was added portionwise, and once addition
was complete, the mixture was stirred at room temperature for 72 hours. The
mixture was concentrated under reduced pressure, basified using 1 N sodium
hydroxide solution and extracted with dichloromethane (10x300m1). The combined
extracts were evaporated and the crude product was purified by column
chromatography on silica gel using an elution gradient of
dichloromethane:methanol
(98:2 to 90:10) to afford the title compound, 42g. 'H-NMR (CDCI3, 400MHz) 8:
2.78
(t, 2H), 3.62 (t, 2H), 3.75 (m, 5H), 4.24 (s, 2H), 6.81 (d, 2H), 7.22 (d, 2H).
LRMS:
m/z (ES+) 182 [MHO].
Preparation 7
(2Fih-1-((4-Methoxybenzyl)aminol-2 propanol
Hs \ OwCHs
,,,,. N I /
HO
A solution of (F~-(-)-1-amino-2-propanol (9.OOg, 0.12mo1) in tetrahydrofuran
(40m1)
and acetic acid (5ml) was added dropwise to a solution of p-anisaldehyde
(5.45g,
0.04mo1) in tetrahydrofuran (40m1), and once addition was complete, the
solution
was stirred at room temperature for 2 hours. The solution was cooled in an ice-
bath, and sodium triacetoxyborohydride (9.50g, 0Ø045mo1) was added
portionwise,
and then the mixture was stirred at room temperature for 18 hours. The
reaction
mixture was concentrated under reduced pressure and the residue was
partitioned
between dichloromethane (150m1) and sodium hydroxide solution (150m1, 0.5N).
The
layers were separated and the aqueous phase was saturated with sodium
chloride,
then extracted with further dichloromethane (3x30m1). The combined organic
solutions were dried (MgS04) and evaporated under reduced pressure. The
residue
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
62
was purified by chromatography on silica gel (gradient elution 98:2:0.2 to
97:3:0.3
dichloromethane:methano1:0.88 NH3) to afford an orange oil (4.9g). 'H-NMR
(CDCI3, 400MHz) 8: 1.12 (d, 3H), 2.39 (dd, 1 H), 2.70 (dd, 1 H), 3.62-3.79 (m,
6H),
6.82 (d, 2H), 7.19 (d, 2H). LRMS: m/z (ES+) 196 [MHO].
Preparation 8
i(2S~-1-( 4-Methoxybenzvl)amino]-2-~~roaanol
O
Hs I \ wCHs
N /
HO
A mixture of (S~-(+)-1-amino-2-propanol (9g, 0.12mo1), p-anisaldehyde (5.45g,
0.04mo1), acetic acid (5ml), and sodium triacetoxyborohydride (9.5g, 0.045mo1)
in
methanol (80m1) was stirred at room temperature for 72 hours. The reaction
mixture
was concentrated under reduced pressure and the residue was partitioned
between
dichloromethane (150m1) and sodium hydroxide solution (100m1, 0.5N). The
layers
were separated, and the aqueous phase was extracted with further
dichloromethane
(4x30m1). The combined organic solutions were dried (MgS04) and concentrated
under reduced pressure. The residual yellow oil was purified by column
chromatography on silica gel using an elution gradient of
dichloromethane:methano1:0.88 ammonia (98:2:0.2 to 95:5:0.5) to afford the
title
compound, 6.2g. 'H-NMR (CDCI3, 400MHz) 8: 1.10 (d, 3H), 2.24-2.40 (m, 2H),
2.65
(dd, 1 H), 3.62-3.80 (m, 6H), 6.82 (d, 2H), 7.19 (d, 2H). LRMS: m/z (ES+) 218
[MNa~]
Preparation 9
(21~-2-f (4-Methoxybenzyl~aminol-1-propanol
CH3
N j~OH
H
H3C~0
(F~-(-)-2-Amino-1-propanol (10.36m1, 133mmol) was added dropwise to a solution
of
p-anisaldehyde (5.85g, 42.9mmol) in methanol (90m1), and the solution was
cooled
in an ice-bath. Acetic acid (2.5m1) and sodium triacetoxyborohydride (lO.Og,
47.2mmol) were added and the reaction mixture was allowed to warm to room
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
63
temperature over an hour. The solution was warmed to 40°C and stirred
for a
further 48 hours then concentrated under reduced pressure. The residue was
partitioned between saturated sodium bicarbonate solution (50m1) and
dichloromethane (100m1) and the layers were separated. The aqueous phase was
extracted with further dichloromethane (10x50m1), and the combined organic
solutions were dried (MgS04) and evaporated under reduced pressure. The
residue
was purified twice by column chromatography on silica gel using an elution
gradient
of dichloromethane:methano1:0.88 ammonia (97:3:0.3 to 90:10:1) to afford the
title
compound, 6.Og. 'H-NMR (CDCI3, 400MHz) 8: 1.04 (d, 3H), 2.80 (m, 1H), 322 (dd,
1 H), 3.58 (dd, 1 H), 3.62 (d, 1 H), 3.78 (m, 4H), 6.82 (d, 2H), 7.20 (d, 2H).
LRMS:
m/z (ES+) 196 [MH+]. [a]p = -34.85 (c = 0.137, methanol)
Preparation 10
(25~-2-f (4-Methoxybenzyl)aminol-1-propanol
CH3
/ ~OH
_N
H
H3~~0 \
The title compound was obtained in 67% yield from (S~-(+)-2-amino-1-propanol
and
p-anisaldehyde, following the procedure described in Preparation 9. 'H-NMR
(CDCI3, 400MHz) 8: 1.08 (d, 3H), 2.83 (m, 1 H), 3.30 (dd, 1 H), 3.59 (dd, 1
H), 3.66 (d,
1 H), 3.77 (s, 3H), 3.82 (d, 1 H), 4.08 (bs, 2H), 6.82 (d, 2H), 7.22 (d, 2H).
[a]p =
+39.19 (c = 0.146, methanol)
Preparation 11
4-(4-Methoxybenzyl)-3-morpholinone
O~ \ OwCHs
N I /
O
A solution of sodium hydroxide (7.08g, 0.177mo1) in water (150m1) was added to
a
solution of the amino alcohol of Preparation 6 (32g, 0.177mo1) in
dichloromethane
(250m1), and the mixture was cooled to 0°C. A solution of chloroacetyl
chloride
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
64
(14.3m1, 0.177mo1) in dichloromethane (50m1) was added dropwise over 30
minutes,
and the mixture was stirred at room temperature for 18 hours. The phases were
separated, and the organic layer was washed with sodium hydroxide (2N, 150m1),
2N
hydrochloric acid (150m1), and brine (50m1), then dried (MgS04) and
concentrated
under reduced pressure. The residual oil was dissolved in ethanol (200m1), a
solution of potassium hydroxide (9.93g, 0.177mo1) in ethanol (200m1) was added
and
the mixture was stirred at room temperature for 18 hours. The mixture was
filtered,
the filtrate was evaporated under reduced pressure, and the residue was
triturated
with diethyl ether/pentane to afford the title compound as a white powder,
26g. 'H-
NMR (CDC13, 400MHz) 8: 3.20 (t, 2H), 3.78 (m, 5H), 4.19 (s, 2H), 4.54 (s, 2H),
6.82
(d, 2H), 7.18 (d, 2H). LRMS: m/z (ES+) 244 [MNa~].
Preparation 12
(6~-4-(4-Methoxybenzyl)-6-methyl-3-morpholinone
CH3
O ~ O~CH3
N /
The title compound was obtained in 76% yield from the alcohol of Preparation 7
and
chloroacetyl chloride, following a similar procedure to that described in
Preparation
11, except, the compound was additionally purified by column chromatography on
silica gel using dichloromethane:methano1:0.88 ammonia (98:2:0.2) as eluant.
'H-
NMR (CDC13, 400MHz) 8: 1.18 (d, 3H), 2.99-3.14 (m, 2H), 3.79 (m, 4H), 4.17 (d,
1 H),
4.25 (d, 1 H), 4.38 (d, 1 H), 4.61 (d, 1 H), 6.82 (d, 2H), 7.17 (d, 2H). LRMS:
m/z (ES+)
258 [MNa+]
Preparation 13
(65~-4-(4-Methoxybenzyl)-6-meth~il-3-morpholinone
CH3
O ~ O~CH3
N ~ /
O
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
The title compound was obtained as a yellow oil in 61 % yield from the amino
alcohol
of Preparation 8, following the procedure described in Preparation 11. 'H-NMR
(CDCI3, 400MHz) 8: 1.18 (d, 3H), 2.98-3.10 (m, 2H), 3.78 (m, 4H), 4.18 (d, 1
H), 4.25
(d, 1 H), 4.38 (d, 1 H), 4.60 (d, 1 H), 6.61 (d, 2H), 7.17 (d, 2H). LRMS: m/z
(ES+) 258
5 [MNa~]
Preparation 14
155'1-4-(4-Methoxybenzyl)-5-meth I-~ra~holinone
0~,,~,.CH3 ~ O~CH
1I 3
N /
O
10 A solution of chloroacetyl chloride (2.34m1, 29mmol) in dichloromethane
(25m1) was
added dropwise over 10 minutes to a stirred ice-cooled mixture of the amino
alcohol
of Preparation 10 (5.6g, 28.7mmol) in sodium hydroxide solution (1.16g, 29mmol
in
water (20m1)) and dichloromethane (50m1). The mixture was stirred at room
temperature for 18 hours, and then the layers were separated. The organic
phase
15 was washed with 1 N sodium hydroxide solution (25m1), 2M hydrochloric acid
(20m1)
and brine (20m1), then dried (MgS04) and evaporated under reduced pressure.
The
residue was dissolved in ethanol (40m1) the solution was cooled in an ice-
bath, and a
solution of potassium hydroxide (1.63g, 29mmol) in ethanol (40m1) was added
dropwise over 5 minutes. The mixture was then allowed to warm to room
20 temperature and was stirred for a further 18 hours. The resulting
precipitate was
filtered off, the filtrate was concentrated under reduced pressure, and the
residue
was dissolved in dichloromethane (150m1). This solution was dried (MgS04) and
evaporated under reduced pressure. The crude product was purified by column
chromatography on silica gel using an elution gradient of
25 dichloromethane:methano1:0.88 ammonia (97:3:0.3 to 95:5:0.5) to afford the
title
compound, 4g. 'H-NMR (CDCI3, 400MHz) S: 1.26 (d, 3H), 3.37 (m, 1 H), 3.62 (dd,
1 H), 3.70 (dd, 1 H), 3.80 (s, 3H), 3.90 (d, 1 H), 4.20 (d, 1 H), 4.24 (d, 1
H), 5.33 (d, 1 H),
6.83 (d, 2H), 7.19 (d, 2H). [aJp = -109.66 (c = 0.139, methanol)
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
66
Preparation 15
(5H~-4-~4-Metho benz~, -5-methyl-3-morpholinone
CHs \ OwCHs
N ~ /
O
The title compound was obtained in 49% yield from the alcohol of Preparation 9
following a similar procedure to that described in Preparation 14. 'H-NMR
(CDC13,
400MHz) S: 1.24 (d, 3H), 3.30 (m, 1 H), 3.60 (dd, 1 H), 3.70 (dd, 1 H), 3.78
(s, 3H),
3.85 (d, 1 H), 4.18 (d, 1 H), 4.22 (d, 1 H), 5.28 (d, 1 H), 6.81 (d, 2H), 7.18
(d, 2H).
Preparation 16
4-Methyl-3-morpholinone
N
~CH3
O
A solution of chloroacetyl chloride (3.81 ml, 50mmol) in dichloromethane (1
OOmI) was
added dropwise over 30 minutes to a suspension of 2-(methylamino)ethanol (4m1,
50mmol) and sodium hydroxide (2g, 50mmol) in dichloromethane (50m1) and water
(50m1), and the mixture was stirred at room temperature for 72 hours then
evaporated under reduced pressure. The residue was dissolved in ethanol
(100m1),
potassium hydroxide (2.8g, 50mmol) was added, and the mixture was stirred at
40°C
for 18 hours then filtered, and the filtrate was concentrated under reduced
pressure.
The crude product was purified by column chromatography on silica gel using an
elution gradient of pentane:ethyl acetate (100:0 to 50:50 to 0:100), to afford
the title
compound, 3.42g. 'H-NMR (CDC13, 400MHz) 8: 2.98 (s, 3H), 3.34 (t, 2H), 3.84
(t,
2H), 4.14 (s, 2H). Found: C, 51.55; H, 8.02; N, 12.01. C5H9N02;0.1 H20
requires C,
51.36; H, 7.93; N, 11.98%.
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
67
Preparation 17
tert Butvl f2-(dimethylamino)ethoxylacetate
H3 O
H C O~O~N~CH3
CHs
CH3
N,N-Dimethylethanolamine (5.02m1, 50mmol) was added dropwise over 5 minutes to
an ice-cooled suspension of sodium hydride (2.2g, 60% dispersion in mineral
oil,
55mmol) in tetrahydrofuran (100m1), and the solution was stirred for 30
minutes.
tert Butyl bromoacetate (7.38m1, 50mmol) was added dropwise over 5 minutes,
then
the mixture was allowed to warm to room temperature and stirred for a further
18
hours. The mixture was pre-adsorbed onto silica gel, and purified by column
chromatography on silica gel using an elution gradient of pentane:ethyl
acetate:methanol (50:50:0 to 0:100:0 to 0:80:20) to afford the title compound
as a
yellow oil, 1.46g. 'H-NMR (CDC13, 400MHz) S: 1.50 (s, 9H), 2.30 (s, 6H), 2.59
(t,
2H), 3.64 (t, 2H) 4.00 (s, 2H). LRMS: m/z (ES+) 204 [MHO]
Preparation 18
tert-Butyl (31~-3-(2-tent butoxy-2-oxoethoxy~pyrrolidine-1-carboxylate
O O CH3
CH3
HsC N~ O
HCHs
H3C O~ C s
\\0
Sodium hydride (704mg, 60% in mineral oil, 17.6mmol) was added to an ice-
cooled
solution of tert-butyl (31~-3-hydroxypyrrolidine-1-carboxylate (J. Med. Chem.
1998,
41(25), 4983) (5g, 26.7mmol) in tetrahydrofuran (100m1), and the mixture was
allowed to warm to room temperature and stirred for 20 minutes. tert Butyl
bromoacetate (5.2g, 26.7mmol) was added and the mixture was heated under
reflux
for 18 hours, then cooled and concentrated under reduced pressure. The residue
was partitioned between ethyl acetate and water and the phases separated. The
organic layer was dried (MgS04) and evaporated under reduced pressure. The
crude product was purified by column chromatography on silica gel using an
elution
gradient of ethyl acetate:pentane (0:100 to 20:80) to afford the title
compound,
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
68
1.95g. 'H-NMR (CDC13, 400MHz) 8: 1.42 (s, 9H), 1.44 (s, 9H), 1.85-2.05 (m,
2H),
3.40 (m, 4H), 3.94 (m, 2H), 4.10 (m, 1 H). LRMS: m/z (ES+) 324 [MNa~]
Preparation 19
Ethyl 3-cvclohexvl-3-methylbutanoate
H3C CH3
O O CH3
Trimethylsilyl chloride (1.3m1, 10.2mmol), copper (I) chloride (30mg, 0.3mmol)
and
cyclohexylmagnesium chloride (4.6m1, 2N in diethyl ether, 9.2mmol) were added
slowly to an ice-cooled solution of ethyl 3,3-dimethylacrylate (1 g, 8.5mmol)
in
tetrahydrofuran (l0ml). The solution was stirred for 10 minutes, then allowed
to
warm to room temperature and stirred for an hour. Saturated aqueous ammonium
chloride solution (l0ml) was added and the mixture was partitioned between
water
(l0ml) and diethyl ether (20m1). The layers were separated and the aqueous
phase
was extracted with diethyl ether (2x10m1). The combined organic extracts were
dried (MgS04) and concentrated under reduced pressure. The crude product was
purified by column chromatography on silica gel using ethyl
acetate:pentane(5:95) as
eluant to afford the title compound, 1.2g. 'H-NMR (CDC13, 400MHz) 8: 0.75 (m,
8H),
1.05-1.28 (m, 7H), 1.62 (m, 1 H), 1.78 (m, 4H), 2.20 (s, 2H), 4.10 (q, 2H).
Preparation 20
3-Cyclohexyl-3-methyl-1-butanol
H3C CH3
OH
Lithium borohydride (1.23g, 56.6mmol) was added to a solution of the ester of
Preparation 19 (4g, 18.9mmol) in tetrahydrofuran (30m1), and the mixture was
stirred
at 50°C for 18 hours. Aqueous ammonium chloride solution (l5ml) was
added
carefully to the cooled solution, and the mixture was extracted with ethyl
acetate
(3x30m1). The combined organic extracts were washed with brine, dried (MgS04)
and evaporated under reduced pressure. The crude product was purified by
column
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
69
chromatography on silica gel using ethyl acetate:pentane (20:80) as eluant to
afford
the title compound, 1g. 'H-NMR (CDC13, 400MHz) 8: 0.80 (s, 6H), 0.88-1.18 (m,
6H), 1.50 (t, 2H), 1.60 (m, 1 H), 1.70 (m, 4H), 3.64 (m, 2H).
Preparation 21
(3-Bromo-1.1-dimethylpropvl)cvclohexane
H3C CH3
Br
Triphenylphosphine (1.8g, 7.1 mmol) was added portionwise to an ice-cooled
solution
of the alcohol of Preparation 20 (1 g, 5.9mmol) and carbon tetrabromide (2.9g,
8.8mmol) in dichloromethane (l5ml), and once addition was complete, the
mixture
was stirred at room temperature for 72 hours. The solution was concentrated
under
reduced pressure and the residue was suspended in a mixture of pentane:ethyl
acetate (5:1, by volume). The resulting precipitate was filtered off through a
pad of
silica gel and washed with pentane:ethyl acetate (5:1, by volume, 300m1). The
combined filtrates were concentrated under reduced pressure, and the product
was
purified by column chromatography on silica gel using pentane as eluant to
afford
the title compound, 1.1g. 'H-NMR (CDCI3, 400MHz) 8: 0.80 (2xs, 6H), 0.90-1.20
(m,
6H), 1.62 (m, 3H), 1.75 (m, 2H), 1.81 (m, 2H), 3.36 (m, 2H).
Preaaration 22
4-(2-Bromoethyl)-1.1-dimethvlcvclohexane
H3C
H3C ~/ ~-gr
A mixture of 2-(4,4-dimethylcyclohexyl)ethanol (W099/59971 ) (2g, 12.8mmol),
concentrated sulphuric acid (750w1) and 48% hydrobromic acid (3ml) was stirred
at
90°C for 7 hours. The cooled mixture was then carefully quenched by the
addition
of water (25m1), and the mixture was extracted with dichloromethane (3x30m1).
The
combined organic extracts were washed with 2M sodium carbonate solution and
brine (30m1), then dried (MgS04) and concentrated under reduced pressure. The
residual black gum was purified by distillation to afford the title compound,
330mg.
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
'H-NMR (CDC13, 400MHz) b: 0.82 (2xs, 6H), 1.02-1.20 (m, 4H), 1.35 (m, 3H),
1.50
(m, 2H), 1.78 (m, 2H), 3.40 (t, 2H).
Preparation 23
5 2-fHydroxy~l-propyl-1 H imidazol-4- I)~methyll-4-(4-methoxybenzyl)-3-
morpholinone
O
HaC~ ~N ~ ~ CHs
N / N
OH O
A solution of the compound of Preparation 11 (13g, 58.7mmol) in
tetrahydrofuran
(100m1) was added dropwise to a solution of lithium diisopropylamide (35.3m1,
2M in
tetrahydrofuran/heptane/ethylbenzene, 70.5mmol) at -78°C, and the
solution was
10 stirred at -78°C for 20 minutes. The aldehyde of Preparation 1
(9.75g, 70.5mmol)
was added dropwise and the mixture was allowed to warm to room temperature,
then stirred for 1.5 hours. Ammonium chloride solution (100m1) was added and
the
mixture was diluted with water (100m1) and tetrahydrofuran (300m1). The layers
were separated, the aqueous phase was extracted with tetrahydrofuran (250m1),
and
15 the combined organic solutions were dried (MgS04) and evaporated under
reduced
pressure. The crude product was purified by column chromatography on silica
gel
using an elution gradient of ethyl acetate:dichloromethane:methano1:0.88
ammonia
(100:0:0:0 to 0:95:5:0.5) to afford the title compound, 14g. 'H-NMR (CDC13,
400MHz) (mixture of diastereoisomers) 8: 0.90 (t, 3H), 1.76 (m, 2H), 3.00 (m,
1 H),
20 3.32-3.43 (m, 1 H), 3.61-3.81 (m, 6H), 3.98 (m, 1 H), 4.42-4.58 (m, 3H),
4.75 (m,
0.5H), 5.03 (m, 0.5H), 6.81 (m, 3H), 7.15 (m, 2H), 7.38 (s, 1 H). LRMS: m/z
(ES+)
360.0 [M H+]
Preparation 24
25 2-~Hydroxy(1-propyl-1 H imidazol-4-yl)methyll-4-methyl-3-morpholinone
H3C~ ~N O
~N / N~
CH3
OH O
Lithium diisopropylamide (17.4m1, 2M in heptane/tetrahydrofuran/ethylbenzene,
34.8mmol) was added over 10 minutes to a cooled (-78°C) solution of the
compound
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
71
of Preparation 10 (3.42g, 29mmol) in tetrahydrofuran (100m1), and the
resulting
solution was stirred for 20 minutes. The aldehyde of Preparation 1 (4.81 g,
34.8mmol) was added and the mixture was allowed to warm slowly to room
temperature, then stirred for 18 hours. Aqueous ammonium chloride solution
(20m1)
was added, and the mixture was evaporated under reduced pressure. The residue
was purified by column chromatography on silica gel using an elution gradient
of
pentane:ethyl acetate:methanol:diethylamine (50:50:0:0 to 0:100:0:0 to
0:90:5:5) to
afford the title compound as a yellow gum, 5.47g. 'H-NMR (CDCI3, 400MHz)
(mixture of diastereoisomers) 8: 0.90 (t, 3H), 1.78 (m, 2H), 2.98 (2xs, 3H),
3.10 (m,
1 H), 3.57 (m, 2H), 3.80 (m, 3H), 3.98-4.08 (m, 1 H), 4.39, 4.49, 4.86, 4.98,
5.22 (5xm,
2H), 6.88 (s, 1 H), 7.38 (s, 1 H). LRMS: m/z (TSP+) 254.2 [MH~]
Preparation 25
2-f(1-Butyl-1 H imidazol-4-yl)(hydroxy)methyll-4-(4-methoxybenzyl)-3-
moreholinone
H3C
~N O~ ~ O~CH3
N / N
OH O
A solution of the compound of Preparation 11 (3.63g, 16.4mmol) in
tetrahydrofuran
(40m1) was added dropwise over 5 minutes to a solution of lithium
diisopropylamide
(9.8m1, 2M in tetrahydrofuran/heptane/ethylbenzene, 19.7mmol) at -78°C,
and the
solution was stirred at -78°C for 30 minutes. The aldehyde of
Preparation 2 (3.Og,
19.7mmol) was added dropwise and the mixture was allowed to warm to room
temperature, then stirred for 18 hours. The mixture was partitioned between
ammonium chloride solution and ethyl acetate (300m1). The layers were
separated,
and the organic solution was dried (MgS04) and evaporated under reduced
pressure. The crude product was purified by column chromatography on silica
gel
using an elution gradient of ethyl acetate:methanol: (100:0 to 90:10) to
afford the title
compound, 4.35g. 'H-NMR (CDCI3, 400MHz) (mixture of diastereoisomers) S: 0.96
(t, 3H), 1.35 (m, 2H), 1.78 (m, 2H), 3.03 (m, 1 H), 3.42 (m, 1 H), 3.66-3.80
(m, 5H),
3.87 (m, 2H), 4.00 (m, 1 H), 4.50 (m, 0.5H), 4.57 (m, 2.5H), 4.83 (m, 0.5H),
5.06 (m,
0.5H), 6.90 (m, 3H), 7.20 (m, 2H), 7.41 (s, 1 H). LRMS: m/z (TSP+) 374.0 [MH~]
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
72
Preparation 26
2-ff1-(2-Cyclohexylethyl)-1H imidazol-4- I~ydroxy)methyll-4- 4-methoxybenzyl
morpholinone
~N ~ \ O~CH3
N / N
OH O
The title compound was obtained as a sticky gum in 66% yield from the compound
of
Preparation 11 and the aldehyde of Preparation 3, following a similar
procedure to
that described in Preparation 25. 'H-NMR (CDCI3, 400MHz) (mixture of
diastereoisomers) 8: 0.98 (m, 2H), 1.22 (m, 4H), 1.58-1.78 (m, 7H), 3.03 (m, 1
H),
3.38-3.50 (m, 1 H), 3.70-3.82 (m, 4H), 3.90 (m, 2H), 4.01 (m, 1 H), 4.50 (d,
0.5H),
4.58 (m, 2.5H), 4.87 (m, 0.5H), 5.08 (m, 0.5H), 6.82-6.95 (m, 3H), 7.19 (m,
2H), 7.41
(s, 1 H). LRMS: m/z (TSP+) 428.1 [MH+]
Preparation 27
2-(Hydroxyf 1-(2-phenylethyl)-1 H imidazol-4-yllmethyl)-4-(4-methoxybenzyl)-3-
morpholinone
\ ~'N O~ \ O~CHa
- \-N
/ N
OH O
The title compound was obtained in 54% yield from the compound of Preparation
11
and the aldehyde of Preparation 4, following a similar procedure to that
described in
Preparation 25, except pentane:ethyl acetate:methanol (50:50:0 to 0:100:0 to
0:87:13) was used as the elution gradient. 'H-NMR (CDC13, 400MHz) (mixture of
diastereoisomers) 8: 3.02 (m, 3H), 3.42 (m, 1 H), 3.74 (m, 4H), 3.95-4.05 (m,
1 H),
4.12 (m, 2H), 4.45 (m, 0.5H), 4.58 (m, 2.5H), 4.81 (m, 0.5H), 5.06 (d, 0.5H),
6.84 (m,
3H), 7.07 (d, 2H), 7.19 (m, 2H), 7.27 (m, 4H). LRMS: m/z (ES+) 422 [MHO]
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
73
Preparation 28
2-fHydroxy~l-trig-1 H imidazol-4-vlymethyll-4-(4-methoxybenzyl)-3-morpholinone
0
N O~ ~ ~CH3
/ N /
OH O
A solution of lithium diisopropylamide (88.5m1, 1.5M in cyclohexane, 133mmol)
and
tetrahydrofuran (100m1) was cooled to -78°C. A solution of the compound
of
Preparation 11 (26g, 1 l8mmol) in tetrahydrofuran (100m1) was added dropwise,
and
the solution was stirred for 30 minutes. A solution of the imidazole of
Preparation 5
(39.9g, 118mmol) in tetrahydrofuran (350m1) was added dropwise over 1 hour,
and
once addition was complete, the reaction was allowed to warm slowly to room
temperature with stirring, over 3 hours. Saturated ammonium chloride solution
(200m1) and water (100m1) were added, the phases were separated, and the
aqueous layer was extracted with ethyl acetate (100m1). The combined organic
solutions were dried (MgS04) and evaporated under reduced pressure. The
residual orange oil was dissolved in ethyl acetate/methanol, the solution was
sonicated, and the resulting white precipitate was filtered off, washed with
diethyl
ether and dried, to give the title compound, 21 g. The filtrate was evaporated
under
reduced pressure and the residue was purified by column chromatography on
silica
gel using ethyl acetate:methanol (96:4) as eluant to afford additional
product, 28g.
'H-NMR (CDCI3, 400MHz) (mixture of diastereoisomers) S: 2.98 (m, 1H), 3.35 (m,
1 H), 3.66 (m, 1 H), 3.73 (s, 3H), 3.96 (m, 1 H), 4.40 (d, 1 H), 4.54 (s, 1
H), 4.60 (d, 1 H),
5.21 (m, 1 H), 6.79 (m, 3H), 7.08 (m, 6H), 7.15 (m, 2H), 7.26 (m, 9H), 7.37
(s, 1 H).
LRMS: m/z (TSP+) 560.2 [MHO]
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
morpholinone
74
Preparation 29
(6~-2-~Hydroxy(1-tritvl-1 H imidazol-4-yl methyll-4-(4-methoxybenzyl -6-methyl-
3-
morpholinone
CH3
~N O ~ O~CH3
/\ N
~/ ~ N /
off o
The title compound was obtained in 62% yield from the morpholinone of
Preparation
12 and the imidazole of Preparation 5, following a similar procedure to that
described
in Preparation 28. 'H-NMR (CDCI3, 400MHz) (mixture of diastereoisomers) S:
1.00,
1.06 (2xm, 3H), 2.87-3.04 (m, 2H), 3.76 (m, 3H), 3.80-4.74 (m, 4H), 4.98-5.21
(m,
1 H), 6.78 (m, 3H), 7.05-7.20 (m, 8H), 7.23-7.40 (m, 1 OH). LRMS: m/z (ES+)
574
[MH+]
Preparation 30
(6S~-2-fHydroxy(1-trityl-1 H imidazol-4-yl)methyll-4-(4-methoxybenzyl)-6-
methyl-3-
CH3
/ \ ~N O ~ O~CH3
N ~ N
OH O
\
The title compound was obtained as a yellow foam in 53% yield from the
morpholinone of Preparation 13 and the imidazole of Preparation 5, following a
similar procedure to that described in Preparation 28. 'H-NMR (CDCI3, 400MHz)
(mixture of diastereoisomers) b: 1.00, 1.05 (2xm, 3H), 2.85-3.04 (m, 2H), 3.76
(m,
3H), 3.80-3.96 (m, 1 H), 4.39-4.70 (m, 3H), 4.98-5.20 (m, 1 H), 6.78 (m, 3H),
7.05-
7.20 (m, 8H), 7.25 (m, 9H), 7.39 (m, 1 H). LRMS: m/z (ES+) 574 [MH~]
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
Preparation 31
j55~-2-LHydrox~r(1-tritvl-1 H imidazol-4=,yl)methvll-4-(4-methoxybenzyl)-5-
methyl-3-
morpholinone
~,,~,.CH3 ~ O~CH3
lIN
OH 0
5 The title compound was obtained in 57% yield from the morpholinone of
Preparation
14 and the imidazole of Preparation 5, following a similar procedure to that
described
in Preparation 28. 'H-NMR (CDC13, 400MHz) (mixture of diastereoisomers) 8:
1.03-
1.19 (m, 3H), 2.99-4.83 (m, 8H), 5.02-5.38 (m, 2H), 6.78-6.88 (m, 3H), 7.10-
7.21 (m,
8H), 7.22-7.42 (m, 10H).
Preparation 32
(5F~-2-fHydroxy(1-trityl-1 H imidazol-4-yl)methyll-4-(4-methoxybenzyl)-5-
methyl-3-
~CH3
The title compound was obtained as a yellow foam in 80% yield from the
morpholinone of Preparation 15 and the imidazole of Preparation 5, following a
similar procedure to that described in Preparation 28. 'H-NMR (CDC13, 400MHz)
(mixture of diastereoisomers) b: 1.00-1.16 (m, 3H), 3.16-4.94 (m, 8H), 5.00-
5.37 (m,
2H), 6.72-6.83 (m, 3H), 7.04-7.19 (m, 8H), 7.21-7.40 (m, 1 OH).
morpholinone
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
76
Preparation 33
tent Butyl 2-f2-(dimeth lad)ethoxyl-3-hydrox~3-(1-propyl-1 H imidazol-4-
yl)propanoate
~Hs
N
H3C~ ~N ~ ~CH3
~N / O
i
OH O"CH3
H3C' ICHs
Lithium diisopropylamide (4.3m1, 2M in heptane/tetrahydrofuran/ethylbenzene,
8.6mmol) was added dropwise over 5 minutes to a solution of the amine of
Preparation 17 (1.46g, 7.2mmol) in tetrahydrofuran (20m1) and the solution was
stirred at -78°C for 20 minutes. The aldehyde of Preparation 1 (1.18g,
8.6mmol)
was added and the mixture was stirred for 3 hours then allowed to warm to -
20°C.
Water was added, and the mixture pre-adsorbed onto silica gel. The product was
purified by column chromatography on silica gel using an elution gradient of
ethyl
acetate:methanol:diethylamine (100:0:0 to 96:2:2) to afford the title
compound,
1.36g. 'H-NMR (CDC13, 400MHz) (mixture of diastereoisomers) S: 0.88 (m, 3H),
1.35
(2xs, 9H), 1.75 (m, 2H), 2.22 (s, 6H), 2.42 (m, 1 H), 2.58 (m, 1 H), 3.55 (m,
1 H), 3.80
(m, 2H), 3.90 (m, 1 H), 4.17 (m, 1 H), 4.82, 5.00 (m, 1 H), 6.90 (2xs, 1 H),
7.35 (2xs,
1 H). LRMS: m/z (TSP+) 342.2 [MH~]
Preparation 34
tert-Butyl (3S~-3-f1-tert-butoxycarbonyl-2-hydroxy-2-(1-propel-1 H imidazol-4-
y~-
ethoxv)pvrrolidine-1-carboxylate
O
O CH3
N y-CH3
H3 ~C
H3C~ ~N O
N / O CH3
~CH3
OH O CH3
A solution of the compound of Preparation 18 (5.678, 18.8mmol) in
tetrahydrofuran
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
77
(20m1) was added dropwise to a solution of lithium diisopropylamide (11.3m1,
2M in
heptane/tetrahydrofuran/ethylbenzene, 22.6mmol) at -78°C, and the
solution was
stirred at -78°C for 20 minutes. The aldehyde of Preparation 1 (3.12g,
22.6mmol)
was added portionwise, and the mixture was allowed to warm to room temperature
then stirred for 18 hours. Ammonium chloride solution (50m1) was added
carefully
and the mixture was extracted with tetrahydrofuran (2x200m1). The combined
organic solutions were dried (MgS04) and evaporated under reduced pressure.
The
residual orange oil was purified by column chromatography on silica gel using
an
elution gradient of dichloromethane:methano1:0.88 ammonia (100:0:0: to 90:10:1
) to
afford the title compound, 3.7g. 'H-NMR (CDC13, 400MHz) (mixture of
diastereoisomers) S: 0.92 (t, 3H), 1.42 (s, 18H), 1.79 (m, 2H), 1.94-2.14 (m,
1 H),
2.75-3.50 (m, 5H), 3.84 (m, 2H), 4.03-4.35 (m, 2H), 4.81-5.08 (m, 2H), 6.88
(m, 1 H),
7.39 (s, 1 H). LRMS: m/z (ES+) 440 [MH+]
Pre~oaration 35
(2E~-4-(4-Methoxybenzyl)-2-(~-propyl-1 H imidazol-4-yl)methylidenel-3-
morpholinone
HsC~ ~N O~ \ OwCHs
N / / N
O
Triethylamine (9.19m1, 65.9mmol) was added to a solution of the alcohol of
Preparation 23 (15.8g, 44.Ommol) in dichloromethane (300m1). The solution was
cooled in ice, methanesulphonyl chloride (5.1 ml, 65.9mmol) was added, and the
solution was stirred for 2 hours at room temperature. Additional triethylamine
(3.06m1, 22mmol) was added, and the mixture was stirred at 40°C for 18
hours then
cooled. The mixture was diluted with dichloromethane (1000m1) and washed with
sodium bicarbonate solution (200m1). The aqueous wash was extracted with
dichloromethane (400m1), and the combined organic solutions were dried (MgSOa)
and evaporated under reduced pressure. The residue was purified by column
chromatography on silica gel using an elution gradient of ethyl
acetate:dichloromethane:methano1:0.88 ammonia (100:0:0:0 to 0:95:5:0.5 to
0:90:10:1) to afford the title compound, 8.3g. 'H-NMR (CDCI3, 400MHz) (mixture
of
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
78
geometric isomers) b: 0.90 (t, 3H), 1.78 (m, 2H), 3.39 (t, 2H), 3.77 (s, 3H),
3.83 (t,
2H), 4.15 (t, 2H), 4.61 (s, 2H), 6.81 (d, 2H), 7.00 (s, 1 H), 7.19 (d, 2H),
7.28 (s, 1 H),
7.41 (s, 1 H). LRMS: m/z (ES+) 342 [MHO]
Preaaration 36
f2EZ~~4-Methoxybenzyl)-2-[;1-trityl-1 H imidazol-4-yl)methylidenel-3-
morpholinone
w
O
~N O~ \ ~CH3
N ~ / N /
O
The title compound was obtained in 77% yield as a yellow foam from the alcohol
of
Preparation 28 following the procedure described in Preparation 35. 'H-NMR
(CDCI3, 400MHz) (mixture of geometric isomers) S: 3.34 (t, 2H), 3.78 (s, 3H),
4.00 (t,
2H), 4.59 (s, 2H), 6.80 (d, 2H), 6.98 (s, 1 H), 7.10 (m, 6H), 7.17 (d, 2H),
7.28 (m,
10H), 7.39 (s, 1 H). LRMS: m/z (ES+) 542 [MHO]
Preparation 37
(2EZ.61~-4-(4-Methoxybenzyl)-6-methyl-2-f(1-trityl-1 H imidazol-4-
yl)methylidenel-3-
moraholinone
CH3
~'N \ OwCH3
N
/ / N /
O
The title compound was obtained in 57% yield as a pale yellow foam, from the
alcohol of Preparation 29, following the procedure described in Preparation
35. 'H-
NMR (CDC13, 400MHz) (mixture of geometric isomers) 8: 1.02 (d, 3H), 3.08 (dd,
1 H),
3.20 (dd, 1 H), 3.77 (s, 3H), 4.10 (m, 1 H), 4.50 (d, 1 H), 4.60 (d, 1 H),
6.80 (d, 2H),
6.99 (s, 1 H), 7.14 (m, 8H), 7.28 (m, 10H), 7.38 (s, 1 H). LRMS: m/z (ES+) 556
[MHO]
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
79
Preparation 38
~2EZ.65~-4-(4-Methoxybenzyl)-6-methyl-~1-trityl-1 H imidazol-4-yl)methylidenel-
3-
morpholinone
CH3
/ ~N \ OwCHs
N
/ N I /
0
Methanesulphonyl chloride (911 p,l, 11.78mmol) was added dropwise to an ice-
cooled
solution of the alcohol of Preparation 30 (4.5g, 7.85mmol) in dichloromethane
(40m1)
and triethylamine (1.64m1, 11.78mmol), and the solution was stirred at room
temperature for 1 hour. Additional triethylamine (546w1, 3.93mmol) was added,
and
the mixture was stirred at 40°C for 18 hours. The cooled mixture was
partitioned
between dichloromethane (50m1) and water (50m1) and the layers were separated.
The organic phase was dried (MgS04) and concentrated under reduced pressure.
The residual orange oil was purified by column chromatography on silica gel
using
an elution gradient of dichloromethane:methano1:0.88 ammonia (99:1:0.1 to
98:2:0.2) to afford the title compound as a yellow foam, 2.5g. iH-NMR (CDCI3,
400MHz) (mixture of geometric isomers) b: 1.02 (d, 3H), 3.05 (m, 1 H), 3.20
(m, 1 H),
3.78 (s, 3H), 4.06 (m, 1 H), 4.47-4.63 (m, 2H), 6.80 (d, 2H), 6.98 (s, 1 H),
7.12 (m,
8H), 7.27 (m, 1 OH), 7.38 (s, 1 H). LRMS: m/z (ES+) 556 [MH+]
Preparation 39
(2EZ,5S~-4-(4-Methoxybenzyl)-5-methyl-2-((1-trityl-1 H imidazol-4-
yl)methylidenel-3-
morpholinone
~N O~ ,,,,.CH3 \ O~CH3
N / / 1IN I /
O
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
The title compound was obtained in 28% yield from the alcohol of Preparation
31
following a similar procedure to that described in Preparation 38. 'H-NMR
(CDCI3,
400MHz) (mixture of geometric isomers) 8: 1.22 (d, 3H), 3.38 (m, 1 H), 3.78
(s, 3H),
3.81 (d, 1 H), 3.95 (m, 2H), 5.28 (d, 1 H), 6.80 (d, 2H), 6.95 (s, 1 H), 7.10-
7.19 (m, 9H),
5 7.28 (m, 9H), 7.40 (s, 1 H).
Preparation 40
j2EZ,5R)-~4-MethoxybenzYl)-5-meth~jl;i-trit~rl-1 H imidazol-4-yl)methylidenel-
3-
morpholinone
CHs \ O
~N O~ ~ CH3
N / / N /
O
The title compound was obtained in 27% yield from the alcohol of Preparation
32
following a similar procedure to that described in Preparation 38. 'H-NMR
(CDC13,
400MHz) (mixture of geometric isomers) 8: 1.25 (d, 3H), 3.41 (m, 1 H), 3.78
(s, 3H),
3.83 (dd, 1 H), 3.98 (m, 2H), 5.30 (d, 1 H), 6.82 (d, 2H), 6.98 (s, 1 H), 7.18
(m, 9H),
7.32 (m, 9H), 7.41 (s, 1 H).
Preparation 41
(2E~-2-fj1-Butyl-1 H imidazol-4-yl~methylidenel-4-(4-methoxybenzyl)-3-
morpholinone
HsC O
~N ~ \ ~CHa
N / / N /
O
Triethylamine (1.78m1, 12.8mmol) was added to a solution of the alcohol of
Preparation 25 (4.34g, 11.6mmol) in dichloromethane (50m1). The solution was
cooled in ice and methanesulphonyl chloride (990,1, 12.8mmol) was added. The
solution was stirred for 30 minutes, additional triethylamine (1.78m1,
12.8mmol)
added, and the mixture was stirred at room temperature for 18 hours. The
mixture
was concentrated under reduced pressure and the residue was purified by column
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
81
chromatography on silica gel using an elution gradient of
dichloromethane:ethyl
acetate:methanol (100:0:0 to 0:90:10) to afford the title compound as a sticky
gum,
1.12g. ' H-NMR (CDCI3, 400MHz) (mixture of geometric isomers) S: 0.95 (t, 3H),
1.34 (m, 2H), 1.78 (m, 2H), 3.42 (t, 2H), 3.80 (s, 3H), 3.94 (t, 2H), 4.19 (t,
2H), 4.63
(s, 2H), 6.84 (d, 2H), 7.02 (s, 1 H), 7.22 (d, 2H), 7.32 (s, 1 H), 7.44 (s, 1
H). LRMS:
m/z (TSP+) 356.2 (MH+]
Preparation 42
12E~-2-(f 1-(2-Cyclohexylethyl)-1 H imidazol-4-yllmethylidene)-4-(4-
methoxybenzyl)-
3-morpholinone
~N O~ \ OwCHs
~N / / N
O
The title compound was obtained as a sticky gum in 57% yield from the alcohol
of
Preparation 26 following the procedure described in Preparation 41. 'H-NMR
(CDC13, 400MHz) (mixture of geometric isomers) 8: 0.94 (m, 2H), 1.18 (m, 4H),
1.64
(m, 7H), 3.40 (t, 2H), 3.78 (s, 3H), 3.90 (t, 2H), 4.17 (t, 2H), 4.61 (s, 2H),
6.81 (d,
2H), 6.99 (s, 1 H), 7.20 (d, 2H), 7.25 (s, 1 H), 7.40 (s, 1 H). LRMS: m/z
(TSP+) 410.1
(MH+]
Preparation 43
(2E~-4-(4-Methoxybenzyl)-2-(f 1-(2-phenylethyl)-1 H imidazol-4-
yllmethylidene]~-3-
morpholinone
~'N O~ \ O~CHs
--N//NI
O
Triethylamine (0.98m1, 7.03mmol) was added to a solution of the alcohol of
Preparation 27 (1.41 g, 3.35mmol) in dichloromethane (15ml). The solution was
cooled in ice, methanesulphonyl chloride (311 wl, 4.02mmol) was added, and the
mixture was warmed to 40°C and stirred for 18 hours, then concentrated
under
reduced pressure. The residue was purified by column chromatography on silica
gel
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
82
using an elution gradient of pentane:ethyl acetate:methanol (75:25:0 to
0:100:0 to
0:95:5) to afford the title compound as an orange oil, 449mg. 'H-NMR (CDC13,
400MHz) (mixture of geometric isomers) 8: 3.03 (t, 2H), 3.42 (t, 2H), 3.80 (s,
3H),
4.18 (m, 4H), 4.63 (s, 2H), 6.85 (d, 2H), 7.02 (s, 1 H), 7.06 (d, 2H), 7.25
(m, 7H).
LRMS: m/z (ES+) 404 [MHO]
Preparation 44
tert-But~(2EZ1-2-f2-(dimethylamino ethoxyl-3-(1-propyl-1 H imidazol-4-
propenoate
CH3
N
HaC~ ~N O~/ wCH3
N / / O CH3
~CH3
O CH3
Methanesulphonyl chloride (340w1, 4.4mmol) was added dropwise to an ice-cooled
solution of the alcohol of Preparation 33 (1.36g, 4.Ommol) and triethylamine
(616.1,
4.4mmol) in dichloromethane (20m1). The solution was stirred at room
temperature
for 1 hour, additional triethylamine (616,1, 4.4mmol) was added, and the
solution
was stirred at room temperature for 18 hours. TLC analysis showed starting
material remaining, so the solution was heated to reflux and stirred for a
further 3
hours. The cooled mixture was pre-adsorbed onto silica gel and purified by
column
chromatography on silica gel using an elution gradient of ethyl
acetate:diethylamine:methanol (100:0:0 to 96:2:2) to afford the title
compound,
650mg. 'H-NMR (CDCI3, 400MHz) (mixture of geometric isomers) 8: 0.98 (t, 3H),
1.56 (s, 9H), 1.84 (m, 2H), 2.34 (s, 6H), 2.65 (t, 2H), 3.94 (t, 2H), 4.04 (t,
2H), 7.08
(s, 1 H), 7.44 (s, 1 H), 7.98 (s, 1 H). LRMS: m/z (TSP+) 324.2 [MH+]
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
83
Preparation 45
tert-Butvl (3Sl-3-ff(E~-1-(terf butoxvcarbonyl -2-(1-propyl-1 H imidazol-4-
ethenvlloxy]~ayrrolidine-1-carboxylate
O
O CH3
N ~CH3
H3 /C
H3C~ ~N O
N / / O CH3
~CH3
O CH3
The title compound was obtained as an orange oil in 36% yield from the alcohol
of
Preparation 34 following the procedure described in Preparation 44. 'H-NMR
(CDC13, 400MHz) (mixture of geometric isomers) 8: 0.93 (t, 3H), 1.35-1.56 (m,
18H),
1.80 (m, 2H), 1.98 (m, 1 H), 2.17 (m, 1 H), 3.26-3.66 (m, 4H), 3.86 (m, 2H),
5.15 (m,
1 H), 7.06, 7.15 (2xs, 1 H), 7.35, 7.39 (2xs, 1 H), 7.41 (s, 1 H). LRMS: m/z
(ES+) 422
[MH+]
Preparation 46
(2EZ1-4-Methyl-2-f(1-propyl-1 H imidazol-4-yl)methylidenel-3-morpholinone
H3C~ ~N O
N
/ / N~CH3
O
Triethylamine (3.32m1, 23.8mmol) was added to a solution of the alcohol of
Preparation 24 (5.47g, 21.6mmol) in dichloromethane (80m1), and the solution
was
cooled in ice. A solution of methanesulphonyl chloride (1.84m1, 23.8mmol) in
dichloromethane (3ml) was added over 5 minutes, and the solution was stirred
at
room temperature for 1 hour. The mixture was evaporated under reduced
pressure,
the residue was dissolved in N,N-dimethylformamide (l5ml), triethylamine
(3.32m1,
23.8mmol) was added, and the solution was heated at reflux and stirred for 20
minutes. The cooled solution was concentrated under reduced pressure and the
residue was dissolved in methanol (100m1) then adsorbed onto silica gel and
purified
by column chromatography on silica gel using an elution gradient of ethyl
acetate:methanol:diethylamine (100:0:0 to 95:5:0.5) to afford the title
compound,
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
84
2.5g. 'H-NMR (CDC13, 400MHz) (mixture of geometric isomers) 8: 0.98 (t, 3H),
1.82
(m, 2H), 3.14 (s, 3H), 3.59 (t, 2H), 3.90 (t, 2H), 4.26 (t, 2H), 7.00 (s, 1
H), 7.35 (s, 1 H),
7.45 (s, 1 H). LRMS: m/z (TSP+) 236.2 [MH~]
Preparation 47
(-)-(2S)-4-(4-Methox~rbenz~)-2-f(1-propyl-1 H imidazol-4-vl methyll-3-
morpholinone
O
H3C~ ~N O~ \ ~CH3
N / N /
O
A mixture of the alkene of Preparation 35 (8.3g, 24.3mmol) and 10% Pd/C
(Degussa~ 101 ) (800mg) in ethanol (240m1) was hydrogenated at 1 OOpsi
(690kPa)
and 50°C for 18 hours, then cooled and filtered through Arbocel~. The
filtrate was
evaporated under reduced pressure to give an orange oil. This product was
purified
by column chromatography on a Chiralcel~ OJ column, using hexane: isopropyl
alcohol:diethylamine (70:30:0.5) as eluant, to afford enantiomer 1, 1.65g,
followed by
enantiomer 2, the title compound, 1.54g. 'H-NMR (CDC13, 400MHz) 8: 0.92 (t,
3H),
1.78 (m, 2H), 3.07 (m, 2H), 3.38 (m, 2H), 3.74 (m, 1 H), 3.79 (m, 5H), 3.98
(m, 1 H),
4.52 (m, 3H), 6.72 (s, 1 H), 6.82 (d, 2H), 7.18 (d, 2H), 7.38 (s, 1 H). LRMS:
m/z (ES+)
344 [MHO]. [a]p = -66.10, (c = 0.368, methanol)
Preparation 48
(2RS)-2-f(1-Butyl-lHimidazol-4-yl)methyll-4-(4-methoxybenzyl)-3-morpholinone
HsC O
~N O~ \ ~CH3
N / N /
O
A mixture of the alkene of Preparation 41 (2.5g, 6.96mmol) and 10% Pd/C
(Degussa~ 101 ) (250mg) in ethanol (100m1) was hydrogenated at 50°C and
60 psi
(410kPa) for 18 hours. The cooled mixture was filtered through Arbocel~ and
the
filtrate was evaporated under reduced pressure to afford the title compound as
an
oil, 2.44g. 'H-NMR (CDC13, 400MHz) S: 0.95 (t, 3H), 1.32 (m, 2H), 1.73 (m,
2H),
3.04 (m, 2H), 3.34-3.42 (m, 2H), 3.73 (m, 1 H), 3.78 (s, 3H), 3.82 (t, 2H),
3.98 (m,
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
1 H), 4.45-4.60 (m, 3H), 6.74 (s, 1 H), 6.82 (d, 2H), 7.18 (d, 2H), 7.38 (s, 1
H). LRMS:
m/z (TSP+) 358.2 [MHO]
Preparations 49 to 50
5 The compounds of the following general formula
~N ~ ~ O~CH3
R_N / N
O
were prepared from the appropriate alkene (Preparations 42 and 43), following
a
similar procedure to that described in Preparation 48.
Prep. R Yield Data
No. (%)
'H-NMR (CDCI3, 400MHz) 8: 0.96 (m,
2H),
1.20 (m, 4H), 1.68 (m, 7H), 3.04
75, (m, 2H),
3.40 (m, 2H), 3.74 (m, 1 H), 3.80
49 sticky (s, 3H),
3.86 (s, 3H), 3.98 (m, 1 H), 4.55
(m, 2H),
gum
6.74 (s, 1 H), 6.84 (d, 2H), 7.18
(d, 2H), 7.38
(s, 1 H). LRMS: m/z (TSP+) 412.2
[MHO]
'H-NMR (CDC13, 400MHz) 8: 3.00-3.10
(m,
4H), 3.35 (dd, 1 H), 3.40 (dd, 1
~ ~' 91 H), 3.70 (m,
I 1 H), 3.79 (s, 3H), 3.98 (m, 1 H),
50 oran 4.08 (t, 2H),
/ ge 4.45 (m, 1 H), 4.55 (m, 2H), 6.69
(s, 1 H),
oil
6.68 (d, 2H), 7.06 (d, 2H), 7.18
(d, 2H), 7.25
(m, 4H). LRMS: m/z (ES+) 406 [MHO]
10 1 = purified by column chromatography on silica gel using an elution
gradient of ethyl
acetate:methanol (100:0 to 90:10)
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
86
Preparation 51
(2RS)-4-Methyl-2-f(1-propyl-1 H imidazol-4-~~methvll-3-morpholinone
H3C~ ~N
N / N~
CH3
O
A mixture of the alkene of Preparation 46 (2.5g, 1.06mmol) and 10% Pd/C
(Degussa° 101 ) (250mg) in ethanol (50m1) was hydrogenated at
50°C and 60 psi
(410kPa) for 18 hours. The cooled mixture was filtered through Arbocel~ and
the
filtrate was evaporated under reduced pressure. The residue was purified by
column chromatography on silica gel using an elution gradient of ethyl
acetate:methanol:diethylamine (100:0:0 to 96.5:1.75:1.75) to afford the title
compound as a colourless oil, 2.08g. 'H-NMR (CDCI3, 400MHz) 8: 0.95 (t, 3H),
1.80
(m, 2H), 3.00 (m, 4H), 3.18 (m, 1 H), 3.37 (m, 1 H), 3.57 (m, 1 H), 3.82 (m,
3H), 4.03
(m, 1 H), 4.46 (dd, 1 H), 6.78 (s, 1 H), 7.39 (s, 1 H). LRMS: m/z (ES+) 238
[MH+]
Preparation 52
(2RS)-2-f(1 H Imidazol-4-yl)methvll-4-(4-methoxybenzvl)-3-morpholinone
~N ~ ~ O~CH3
HN / N
O
A mixture of the protected imidazole of Preparation 36 (25g, 46mmol) and Pd/C
Degussa° 101 catalyst (2.5g) in ethanol (500m1) was hydrogenated at
50°C and 60
psi (410kPa) for 18 hours. TLC analysis showed starting material remaining, so
the
mixture was filtered through Arbocel~ and the filtrate was hydrogenated using
fresh
catalyst (2.5g) in ethanol (500m1) at 50°C and 60 psi (410kPa) for a
further 18 hours.
The mixture was filtered through Arbocel~ and the filtrate was evaporated
under
reduced pressure. The product was purified by column chromatography on silica
gel using an elution gradient of dichloromethane:methano1:0.88 ammonia
(98:2:0.2
to 95:5:0.5) to afford the title compound as a white foam, 9g. 'H-NMR (CDC13,
400MHz) 8: 3.01 (m, 1 H), 3.18 (m, 2H), 3.38 (m, 1 H), 3.70 (dd, 1 H), 3.78
(s, 3H),
3.97 (dd, 1 H), 4.32 (m, 1 H), 4.50 (dd, 2H), 6.81 (d, 2H), 6.86 (s, 1 H),
7.02 (d, 2H),
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
87
7.46 (s, 1 H). LRMS: m/z (ES+) 302 [MH+]
Preparation 53
(-)-(2S)-2-f(lHlmidazol-4- I)~~1-4-(4-methoxybenzyl)-3-morpholinone
and
Preparation 54
(+)-(2R)-2-[(1 H Imidazol-4yl)methyll-4-(4-methoxybenzyl)-3-morpholinone
The racemic compound of Preparation 52 was further purified by HPLC using a
Chiralpak~ OD column, and hexane:isopropyl alcohol (80:20) as eluant to afford
the
title compound of preparation 53, >99%ee. 'H-NMR (CDCI3, 400MHz) S: 3.01 (m,
1 H), 3.18 (m, 2H), 3.38 (m, 1 H), 3.70 (dd, 1 H), 3.78 (s, 3H), 3.97 (dd, 1
H), 4.32 (m,
1 H), 4.50 (dd, 2H), 6.81 (d, 2H), 6.86 (s, 1 H), 7.02 (d, 2H), 7.46 (s, 1 H).
LRMS: m/z
(ES+) 324 [MNa~]. [a]p = -529.3 (c = 0.05, methanol).
Further elution gave the title compound of preparation 54, >99%ee. 'H-NMR
(CDCI3, 400MHz) S: 3.01 (m, 1 H), 3.18 (m, 2H), 3.38 (m, 1 H), 3.66-3.79 (m,
4H),
3.97 (dd, 1 H), 4.32 (t, 1 H), 4.50 (dd, 2H), 6.81 (d, 2H), 6.86 (s, 1 H),
7.02 (d, 2H),
7.46 (s, 1 H). LRMS: m/z (ES+) 324 [MNa~]
Preparation 55a
(2S,6R)-2-f(1 H Imidazol-4-girl methyll-4-(4-methoxybenz~l~-6-methyl-3-
morpholinone
and
Preparation 55b
(2R,6F~-2-f(1 H Imidazol-4-yl methyl~4-methoxybenzyl)-6-methyl-3-morpholinone
CH3 CH3
O / OMe N / OMe
I =~ I I
C\ N ~ C, N ~ I
N v ~ \/ N
A mixture of the alkene of Preparation 154 (41 g, 131 mmol) and 10% palladium
on
carbon (Degussa type 101 ) (8g) in ethanol (500m1) was hydrogenated at 50 psi
(345kPa) and 60°C for 24 hours. The mixture was filtered through
Arbocel~, the
filtrate was concentrated under reduced pressure and the residue was
azeotroped
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
88
with dichloromethane. The crude product was purified by column chromatography
on silica gel using a Biotage column, eluting with a solvent gradient of
dichloromethane:methano1:0.88 ammonia (97.5:2.5:0.1 to 90:10:1 ). The higher
running, major product was repurified by column chromatography on silica gel
using
dichloromethane:methano1:0.88 ammonia (95:5:0.25) to afford the title compound
of
preparation 55a as a white foam. 'H-NMR (CDC13, 400MHz) S: 1.19 (d, 3H), 2.97-
3.22 (m, 4H), 3.78 (s, 3H), 3.84 (m, 1 H), 4.32 (t, 1 H), 4.40 (d, 1 H), 4.50
(d, 1 H), 6.80
(d, 2H), 6.86 (s, 1 H), 7.00 (d, 2H), 7.46 (s, 1 H). LRMS: m/z (ES-) 314 [M-H]-
The lower running, minor product was further purified by column chromatography
on
silica gel using ether:methano1:0.88 ammonia (90:10:1 ) as eluant and the
product
was azeotroped with dichloromethane to afford the title compound of
preparation 55b
as a white foam, 220mg. 'H-NMR (CDCI3, 400MHz) 8: 1.19 (d, 3H), 2.97-3.22 (m,
4H), 3.78 (s, 3H), 3.84 (m, 1 H), 4.32 (t, 1 H), 4.40 (d, 1 H), 4.50 (d, 1 H),
6.80 (d, 2H),
6.86 (s, 1 H), 7.00 (d, 2H), 7.46 (s, 1 H).
Preparation 56
(2R.6S~-2-f(1 H Imidazol-4-yl)methyll-4-(4-methoxybenzyl)-6-methyl-3-
morpholinone
CH3
~N O ~ O~CH3
HN / N I /
O
The title compound was obtained in 20% yield from the protected alkene of
Preparation 38 following the procedure described in 52. 'H-NMR (CDCI3, 400MHz)
8: 1.19 (d, 3H), 2.99 (m, 2H), 3.17 (m, 2H), 3.78 (s, 3H), 3.83 (m, 1 H), 4.30
(m, 1 H),
4.44 (dd, 2H), 6.80 (d, 2H), 6.85 (s, 1 H), 7.00 (d, 2H), 7.45 (s, 1 H). LRMS:
m/z
(ES+) 316 [MH]+
Preparation 57
(2S.5S~-2-f(1 H Imidazol-4-yl)methyll-4-(4-methoxybenzyl)-5-methyl-3-
morpholinone
,.CH3 O~
~N O~'' ~ CH3
HN / N
O
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
89
The title compound was obtained in 47% yield from the protected alkene of
Preparation 39 following a similar procedure to that described in Preparation
52. ~H-
NMR (CDCI3, 400MHz) (~3:1 mixture of depicted C-2 stereoisomer) 8: 1.15 (d,
3H),
3.22 (m, 3H), 3.48, 3.70-4.00 (2xm, 6H), 4.38 (m, 1 H), 5.18-5.37 (m, 1 H),
6.83 (d,
2H), 6.98 (m, 1 H), 7.18 (d, 2H), 7.62 (s, 1 H). LRMS: m/z (ES-) 314 [M-H-]
Preparation 58
~2R.5F~-2-f(1 H Imidazol-4-yl)methvll-4-(4-methoxvbenz~)-5-methyl-3-
morpholinone
CH3 O~
~N O~ ~ CH3
HN / N /
O
The title compound was obtained in 67% yield from the protected imidazole of
Preparation 40 following a similar procedure to that described in Preparation
52. 'H-
NMR (CDCI3, 400MHz) (~3:1 mixture of depicted C-2 stereoisomer) S: 1.16 (d,
3H),
3.22 (m, 3H), 3.46, 3.70-3.98 (2xm, 6H), 4.35, 4.39 (2xm, 1 H), 5.20, 5.30
(2xd, 1 H),
6.82, 6.94 (2xm, 3H), 7.18 (d, 2H), 7.53 (2xs, 1 H). LRMS: m/z (ES~) 314 [M-H-
]
Preparation 59
(2RS)-(~1-f2-(4-Bromophen~)ethyll-1 H imidazol-4-yl~methyl)-4-(4-methoxybenz~L
morpholinone
Br ~ \ ~N ~ \ O\CH3
'_ N / N
O
Sodium hydride (836mg, 60% dispersion in mineral oil, 20.9mmol) was added
portionwise to an ice-cooled solution of the imidazole of Preparation 52 (5g,
19.9mo1)
in tetrahydrofuran (100m1), and the solution was stirred for 15 minutes. 4-
Bromophenylethyl methanesulphonate (Bioorg. Med. Chem. 1996; 4(5); 645) (6.1
g,
21.9mmol) was then added, and the mixture was stirred at room temperature for
3
days. The reaction was quenched with water (50m1), and the mixture was
extracted
with ethyl acetate (2x100m1). The combined organic extracts were dried (MgS04)
and evaporated under reduced pressure. The crude product was purified by
column
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
chromatography on silica gel using an elution gradient of
dichloromethane:methano1:0.88 ammonia (99:1:0.1 to 95:5:0.5) to afford the
title
compound, 4.4g. 'H-NMR (CDCI3, 400MHz) 8: 2.97 (t, 2H), 3.04 (m, 2H), 3.36 (m,
2H), 3.70 (m, 1 H), 3.78 (s, 3H), 3.97 (m, 1 H), 4.03 (t, 2H), 4.45 (m, 1 H),
4.54 (dd,
5 2H), 6.62 (s, 1 H), 6.82 (d, 2H), 6.90 (d, 2H), 7.18 (d, 2H), 7.20 (s, 1 H),
7.39 (d, 2H).
Preparation 60
(- -(2S)-2-{[1-(2-Cyclohex ly ethyl)-1 H imidazol-4-yllmethyl~-4-(4-
methoxybenzyl;l-3-
morpholinone
O
~N O~ \ ~CH3
~N
/ N /
10 O
A mixture of the compound of Preparation 53 (400mg, 1.32mmol), cesium
carbonate
(472mg, 1.45mmol), and 2-cyclohexylethyl bromide (227,1, 1.45mmol) in N,N-
dimethylformamide (4ml) was stirred at 80°C for 18 hours. The cooled
mixture was
partitioned between ethyl acetate (250m1) and water (100m1), and the layers
were
15 separated. The organic phase washed with water (3x100m1), dried (MgS04) and
evaporated under reduced pressure. The crude product was purified twice by
column chromatography on silica gel using first an elution gradient of
dichloromethane:methano1:0.88 ammonia (100:0:0 to 95:5:0.5), then using an
elution
gradient of ethyl acetate:diethylamine (100:0 to 95:5), to afford the title
compound,
20 as a colourless gum, 191 mg. ' H-NMR (CDC13, 400MHz) 8: 0.90 (m, 2H), 1.04-
1.24
(m, 4H), 1.60 (m, 7H), 3.02 (m, 2H), 3.26-3.40 (m, 2H), 3.70 (m, 1 H), 3.78
(s, 3H),
3.81 (t, 2H), 3.97 (m, 1 H), 4.42-4.58 (m, 3H), 6.68 (s, 1 H), 6.80 (d, 2H),
7.14 (d, 2H),
7.35 (s, 1 H). LRMS: m/z (ES+) 412 [MH+]. [a]o = -50.37, (c = 0.112 in
methanol)
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
91
Preaaration 61
~2R.65~-2-(f 1-(2-C~clohex~eth~)-1 H imidazol-4-yllmethvl}-4-(4-methoxybenzvl
methyl-3-morpholinone
CH3
~N O ~ O~CH3
~N
/ N /
O
A mixture of the compound of Preparation 56 (200mg, 0.635mmol), cesium
carbonate (248mg, 0.762mmol) and 2-cyclohexylethyl bromide (1091, 0.70mmol) in
N,N-dimethylformamide (8ml) was stirred at 70°C for 18 hours. The
cooled mixture
was partitioned between ethyl acetate (l0ml) and water (l0ml), and the layers
were
separated. The organic phase washed with water (2x20m1) and brine (20m1), then
dried (MgS04) and evaporated under reduced pressure. The crude product was
purified by column chromatography on silica gel using an elution gradient of
dichloromethane:methano1:0.88 ammonia (99:1:0.1 to 98:2:0.2) to afford the
title
compound as a colourless gum, 120mg. 'H-NMR (CDCI3, 400MHz) S: 0.90 (m, 2H),
1.18 (m, 7H), 1.62 (m, 7H), 2.94-3.08 (m, 3H), 3.32 (dd, 1 H), 3.78 (s, 3H),
3.80 (m,
3H), 4.43 (m, 3H), 6.66 (s, 1 H), 6.80 (d, 2H), 7.14 (d, 2H), 7.32 (s, 1 H).
LRMS: m/z
(ES+) 426 [MH~j
Preparation 62
(2S.6R)-2-(f 1-(2-Cvclohexvlethyl)-1 H imidazol-4-vllmethvl~-4-(4-
methoxybenzvl)-6-
methyl-3-morpholinone
CH3
~N O ~ O~CH3
N
/ N /
O
The title compound was obtained as a colourless oil in 38% yield from the
imidazole
of Preparation 55 and 2-cyclohexylethyl bromide following the procedure
described
in Preparation 61. 'H-NMR (CDCI3, 400MHz) 8: 0.90 (m, 2H), 1.17 (m, 7H), 1.61
(m,
7H), 2.94-3.08 (m, 3H), 3.32 (dd, 1 H), 3.78 (s, 3H), 3.81 (m, 3H), 4.43 (m,
3H), 6.66
(s, 1 H), 6.80 (d, 2H), 7.12 (d, 2H), 7.32 (s, 1 H). LRMS: m/z (ES+) 448
[MNa~]
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
92
Preparation 63
j2S.5S~-2-(f1-(2-Cvclohex~ethvl)-1 H imidazol-4- Il~methyl}-4-(4-methoxybenz~)-
5-
methyl-3-morpholinone
0~,~,.CH3 ~ O~CH3
N ~ 1I
/ N /
O
The title compound was obtained in 21 % yield from the imidazole of
Preparation 57
following a similar procedure to that described in Preparation 61, except that
ethyl
acetate:methanol:diethylamine (98:1:1) was used as the column eluant. 'H-NMR
(CDCI3, 400MHz) (mixture of C-2 diastereoisomers) 8: 0.98 (m, 2H), 1.20 (m,
7H),
1.68 (m, 7H), 3.05-3.98 (m, 11 H), 4.50 (m, 1 H), 5.22-5.40 (m, 1 H), 6.76,
6.84 (s and
m, 3H), 7.14, 7.20 (2xd, 2H), 7.39 (m, 1 H). LRMS: m/z (ES+) 426 [MHO]
Preparation 64
(2R,5R~{[1-(2-Cyclohexylethyl)-1 H imidazol-4-yllmethyl~-4-(4-methoxybenzyl)-5-
methyl-3-moraholinone
~N O~ 'CH3 ~ O~CH3
~N
/ N /
O
The title compound was obtained in 40% yield from the imidazole of Preparation
58
following a similar procedure to that described in Preparation 61. 'H-NMR
(CDC13,
400MHz) (mixture of C-2 diastereoisomers) 8: 0.90 (m, 2H), 1.18 (m, 7H), 1.63
(m,
7H), 3.01-3.33 (m, 3H), 3.61-3.90 (m, 8H), 4.42 (m, 1 H), 5.22 (m, 1 H), 6.72
(s, 1 H),
6.80 (d, 2H), 7.16 (d, 2H), 7.35 (s, 1 H). LRMS: m/z (ES+) 426 [MHO]
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
93
Preparation 65
~2S.6R)-2-~[~3-Cyclohexyl-3-methylbut~)-1 H imidazol-4-vllmethyl}-~4-
methox~,rben~l)-6-methyl-3-morpholinone
CH3
O
H3C ~N O~ ~ ~CH3
CH3 N / N /
O
A mixture of the bromide of Preparation 21 (420mg, l.8mmol), the imidazole of
Preparation 55 (470mg, 1.5mmol) and cesium carbonate (586mg, l.8mmol) in N,N-
dimethylformamide (5ml) was stirred at 70°C for 18 hours. The cooled
mixture was
concentrated under reduced pressure and partitioned between ethyl acetate
(20m1)
and water (20m1) and the layers were separated. The aqueous phase was
extracted
with ethyl acetate (3x15m1), and the combined organic solutions were washed
with
brine (l5ml), dried (MgS04) and evaporated under reduced pressure. The crude
product was purified by column chromatography using a Biotage° silica
column and
toluene:diethylamine (99:1 to 85:15) as eluant to afford the title compound,
60mg.
'H-NMR (CDCI3, 400MHz) 8: 0.82 (s, 6H), 0.90-1.10 (m, 8H), 1.63 (m, 3H), 1.78
(m,
5H), 2.94-3.06 (m, 3H), 3.30 (dd, 1 H), 3.79 (m, 6H), 4.40-4.55 (m, 3H), 6.68
(s, 1 H),
6.80 (d, 2H), 7.10 (d, 2H), 7.32 (s, 1 H). LRMS: m/z (ES+) 468 [MHO]
Preparation 66
(2S.6f~-2-((1-f2-(4.4-Dimethylcyclohexyl)ethyll-1H imidazol-4-yl?methyl)-4-(4-
methoxybenzy~-6-methyl-3-morpholinone
CH3
H3C ~N O ~ O~CH3
N
H3C / N /
O
The title compound was obtained in 10% yield from the bromide of Preparation
22
and the imidazole of Preparation 55 following a similar procedure to that
described in
Preparation 65. 'H-NMR (CDC13, 400MHz) 8: 0.84 (2xs, 6H), 1.16 (m, 8H), 1.37
(m,
2H), 1.50 (m, 2H), 1.63 (m, 2H), 2.97-3.10 (m, 3H), 3.35 (dd, 1 H), 3.79 (s,
3H), 3.85
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
94
(m, 3H), 4.48 (m, 3H), 6.70 (s, 1 H), 6.82 (d, 2H), 7.16 (d, 2H), 7.35 (s, 1
H). LRMS:
m/z (ES+) 454 [MH+]
Preparation 67
~2RS)-2-((1-f(2E~-3-Bromo-2-propen~-lHimidazol-4-girl]~methy~-4-(4-
methox beryl -3-morpholinone
Br
~N O~ ~ O~CH3
N / N
O
Sodium hydride (133mg, 60% dispersion in mineral oil, 3.32mmol) was added to
an
ice-cooled solution of the morpholinone of Preparation 52 (1 g, 3.32mmol) in
tetrahydrofuran (l0ml). 1,3-Dibromo-1-propene (mixture of cis and traps)
(332p,1,
3.32mmol) was added dropwise over 5 minutes then the mixture was allowed to
warm to room temperature and stirred for 2 hours. The mixture was partitioned
between water (50m1) and ethyl acetate (50m1), and the phases were separated.
The aqueous layer was extracted with ethyl acetate (1 Oml), and the combined
organic solutions were dried (Na2S04) and evaporated under reduced pressure.
The crude product was purified by column chromatography on silica gel using
dichloromethane:methano1:0.88 ammonia (98:2:0.2) as eluant to afford the title
compound as a colourless oil, 1.1g. 'H-NMR (CDCI3, 400MHz) (mixture of
geometric isomers) 8: 3.02 (m, 2H), 3.26-3.40 (m, 2H), 3.68 (m, 1 H), 3.78 (s,
3H),
3.96 (m, 1 H), 4.40 (d, 1 H), 4.42-4.55 (m, 3H), 4.60 (m, 1 H), 6.22 (m, 1 H),
6.70 (2xs,
1 H), 6.80 (m, 3H), 7.15 (m, 2H), 7.38 (2xs, 1 H). LRMS: m/z (ES+) 420, 422
[MH~]
Preparation 68
(2RS)-4-(4-Methox b~ I)-~f(1-phenyl-1 H imidazol-4-yl)methyll-3-morpholinone
~N O~ ~ O~CH3
N / N I /
O
4~ Molecular sieves, copper (II) acetate (452mg, 2.49mmol) and benzeneboronic
acid (405mg, 3.32mmol) were added to a solution of the imidazole of
Preparation 52
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
(500mg, 1.66mmol) and pyridine (269w1, 3.32mmol)in dichloromethane (20m1), and
the solution was stirred at room temperature for 18 hours. Compressed air was
then
bubbled through the solution for 10 hours. The solvent was retained by using a
"cold finger". The solution was then stirred under a nitrogen atmosphere for a
5 further 18 hours. A solution of ethylenediaminetetraacetic acid (800mg) in
saturated
sodium bicarbonate solution (35m1) was added and the mixture was stirred for
20
minutes, then extracted with dichloromethane (70m1). The aqueous phase was
further was extracted with dichloromethane (20m1) and the combined organic
solutions were washed with water (l0ml), dried (MgS04) and concentrated under
10 reduced pressure. The residual black solid was purified by column
chromatography
on silica gel using an elution gradient of dichloromethane:methano1:0.88
ammonia
(98:2:0.2 to 97:3:0.3) to afford the title compound as a pale yellow oil,
175mg. 'H-
NMR (CDC13, 400MHz) 8: 3.02 (m, 1 H), 3.18 (m, 1 H), 3.38 (m, 2H), 3.70-3.79
(m,
4H), 3.99 (m, 1 H), 4.42 (d, 1 H), 4.50 (m, 1 H), 4.60 (d, 1 H), 6.77 (m, 2H),
7.10 (m,
15 3H), 7.32 (m, 3H), 7.41 (m, 2H), 7.78 (bs, 1 H). LRMS: m/z (ES+) 400 [MNa~]
Preparation 69
(2RS)-2-ff 1-(2-methylphenyl)-1 H imidazol-4- Il~methyl)-4-(4-Methox~zylL
morpholinone
CH3
~N ~ ~ ~~CH3
N
/ N /
The title compound was obtained in 16% yield from the imidazole of Preparation
52
and 2-methylbenzeneboronic acid following the procedure described in
Preparation
68. 'H-NMR (CDC13, 400MHz) 8: 2.15 (s, 3H), 3.02 (m, 1 H), 3.18 (m, 1 H), 3.38
(m,
2H), 3.77 (m, 4H), 3.98 (m, 1 H), 4.54 (m, 3H), 6.78 (d, 2H), 6.85 (bs, 1 H),
7.15 (m,
3H), 7.21 (s, 1 H), 7.25 (m, 2H), 7.45 (bs, 1 H). LRMS: m/z (TSP+) 470.2 [MHO]
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
96
Preparation 70
(2RS)-2-ff 1-(3-phenoxyphenvl)-1 H imidazol-4-yllmethyl~-4-(4-Methoxybenzyl~-3-
moraholinone
O
~N ~ ~ CH3
N
/ N /
O
4~ Molecular sieves (50mg), copper (II) acetate (174mg, 0.96mmol) and 3-
phenoxyphenylboronic acid (J. Chem. Soc. 1970; 488) (350mg, l.6mmol) were
added to a solution of the imidazole of Preparation 52 (240mg, 0.8mmol) and
pyridine (130.1, 1.6mmol) in dichloromethane (2ml). Compressed air was then
bubbled through the solution, maintained at 20-25°C using a water bath,
for 7 hours,
and the solvent was retained by using a "cold finger". The solution was then
stirred
under a nitrogen atmosphere for a further 18 hours. The mixture was
partitioned
between dichloromethane (80m1) and a solution of ethylenediaminetetraacetic
acid
tetrasodium salt (1 g) in saturated sodium bicarbonate solution (30m1) and the
phases
were separated. The organic layer was dried (MgS04) and concentrated under
reduced pressure. The residue was purified by column chromatography on silica
gel
using an elution gradient of ethyl acetate:diethylamine (100:0 to 95:5). The
product
was further purified by column chromatography using a Biotage~ silica gel
column,
and an elution gradient of toluene:diethylamine (100:0 to 88:12) to afford the
title
compound as a pale yellow gum, 120mg. 'H-NMR (CDC13, 400MHz) 8: 3.01 (m,
1 H), 3.15 (dd, 1 H), 3.38 (m, 2H), 3.72 (m, 4H), 3.97 (m, 1 H), 4.45 (d, 1
H), 4.50 (m,
1 H), 4.58 (d, 1 H), 6.77 (d, 2H), 6.90 (m, 1 H), 6.97 (m, 1 H), 7.00-7.18 (m,
7H), 7.37
(m, 3H), 7.75 (s, 1 H). LRMS m/z (TSP+) 470.2 [MHO]
Preparations 71 to 73
The following compounds of general structure
~N ~ ~ O~CH3
R~N / N
O
were prepared from the morpholinone of Preparation 52 and the appropriate
boronic
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
97
acids, following a similar procedure to that described in Preparation 70.
Prep R Yield Data
(%)
'H-NMR (CDC13, 400MHz) 8: 3.00 (m,
2H),
\
3.26 (m, 2H), 3.62 (m, 1 H), 3.75
(s, 3H), 3.90
(m, 1 H), 4.38 (m, 1 H), 4.48 (s,
2H), 6.61 (s,
1 8
\ 1 H), 6.78 (d, 2H), 7.02 (m, 2H),
7.14 (d, 2H),
7.22 (m, 4H), 7.30 (m, 1 H), 7.40
(m, 3H).
/
LRMS: m/z (ES+) 454 [MH+]
'H-NMR (CDC13, 400MHz) 8: 3.06 (m,
1 H),
3.22 (m, 1 H), 3.41 (m, 2H), 3.62
(s, 3H), 3.79
19 (m, 1 H), 4.01 (m, 1 H), 4.46 (d,
1 H), 4.59 (m,
72 I / / orange 1 H), 4.62 (d, 1 H), 6.78 (d, 2H),
7.15 (d, 2H),
oil 7.24 (m, 1 H), 7.50 (m, 3H), 7.79
(s, 1 H), 7.84
(dd, 1 H), 7.95 (m, 2H). LRMS: m/z
(ES+) 450
[M Na+]
'H-NMR (CDC13, 400MHz) 8: 3.05 (m,
1H),
gr 3.18 (dd, 1 H), 3.40 (m, 2H), 3.77
\ 20 (m, 4H), 4.00
(m, 1 H), 4.48 (d, 1 H), 4.55 (m,
~ 1 H), 4.62 (d,
73 / colour-
1 H), 6.80 (d, 2H), 7.14 (d, 2H),
7.21-7.37 (m,
less
oil
3H), 7.45 (m, 1 H), 7.54 (s, 1 H),
7.78 (s, 1 H).
LRMS: m/z (TSP+) 457.7 [MHO]
Preparations 74 to 76
The following compounds of general structure
~N 0~ \ OwCHs
R N ~ N
O
were prepared from the morpholinone of Preparation 53 and the appropriate
boronic
acids, following a similar procedure to that described in Preparation 70.
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
98
Prep R Yield Data
(%)
'H-NMR (CDC13, 400MHz) 8: 3.02 (m,
1 H),
3.18 (dd, 1 H), 3.39 (m, 2H), 3.72
(m, 4H), 3.98
(m, 1 H), 4.43 (d, 1 H), 4.52 (m,
1 H), 4.60 (d,
30
4 ~ 1 H), 6.75 (d, 2H), 7.10 (m, 3H),
7.31 (m, 3H),
/ solid
7.42 (dd, 2H), 7.78 (s, 1 H). LRMS:
m/z
(TSP+) 378.1 [MHO]. [a]p = -34.8,
(c = 0.10,
methanol)
'H-NMR (CDC13, 400MHz) 8: 1.37 (s,
9H), 3.04
(m, 1 H), 3.19 (dd, 1 H), 3.40 (m,
2H), 3.76 (m,
~ ~ 9 4H), 4.00 (m, 1 H), 4.46 (d, 1 H),
4.58 (m, 1 H),
oil 4.62 (d, 1 H), 6.78 (d, 2H), 7.14
(m, 3H), 7.26
H3C
CH (d, 2H), 7.44 (d, 2H), 7.77 (s, 1
H). LRMS: m/z
s
H C
(TSP+) 434.2 [MHO]
'H-NMR (CDCI3, 400MHz) 8: 3.10 (m,
1 H),
F3~ \ 3.19 (dd, 1 H), 3.41 (m, 2H), 3.78
(m, 4H), 4.00
12 (m, 1 H), 4.58 (m, 3H), 6.80 (d,
2H), 7.17 (d,
76
( 1 H), 7.80 (s, 2H), 7.83 (d, 2H).
gum 2H , 7.20 s,
CFs
LRMS: m/z (TSP+) 514.0 [MHO]. [a]p
=
-28.96, (c = 0.096, methanol)
Preparations 77 to 80
The following compounds of general structure
CHs
~N O ~ O~CH3
R_N
/ N /
O
5 were prepared from the morpholinone of Preparation 55 and the appropriate
boronic
acids, following a similar procedure to that described in Preparation 70.
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
99
Prep R Yield Data
(%)
'H-NMR (CDC13, 400MHz) 8: 1.18 (d,
3H),
2.98-3.18 (m, 3H), 3.35, 3.38 (2xd,
1 H), 3.70
Br ~ 33 (m, 3H), 3.85 (m, 1 H), 4.41 (d, 1
H), 4.52 (m,
77 I sticky1 H), 4.58 (d, 1 H), 6.77 (d, 2H),
7.05 (m, 4H),
gum 7.20-7.34 (m, 1 H), 7.42 (d, 1 H),
7.47 (s, 1 H),
7.75 (s, 1 H). LRMS: m/z (TSP+) 470.1,
472.0
[MH~J
'H-NMR (CDCI3, 400MHz) 8: 1.19 (d,
3H), 3.00
(dd, 1 H), 3.05-3.22 (m, 2H), 3.40
(dd, 1 H),
3.75 (s, 3H), 3.92 (m, 1 H), 4.42
(d, 1 H), 4.57
78 I 28
/ (m, 1 H), 4.60 (d, 1 H), 6.78 (d,
2H), 7.10 (m,
3H), 7.34 (m, 3H), 7.43 (m, 2H), 7.78
(s, 1 H).
LRMS: m/z (ES+) 392 [MH~]
'H-NMR (CDC13, 400MHz) S: 1.18 (d,
3H), 3.00
(dd, 1 H), 3.04-3.19 (m, 2H), 3.38
(dd, 1 H),
' 3.76 (s, 3H), 3.87 (m, 1 H), 4.42
(d, 1 H), 4.55
79 0 11 (m, 1 H), 4.58 (d, 1 H), 6.78 (d,
2H), 6.96 (m,
2H), 7.02-7.18 (m, 7H), 7.38 (m, 3H),
7.76 (s,
1 H). LRMS: m/z (ES+) 484 [MHO]
'H-NMR (CDCI3, 400MHz) 8: 1.19 (d,
3H), 3.00
(dd, 1 H), 3.07-3.20 (m, 2H), 3.40
(dd, 1 H),
41
3'77 (s, 3H), 3.90 (m, 1 H), 4.42
(d, 1 H), 4.55
80 white
o (m, 1 H), 4.60 (dd, 1 H), 6.78 (d,
~ 2H), 7.01-7.18
I foam
(m, 8H), 7.26 (d, 2H), 7.38 (dd, 2H),
7.72 (s,
1 H). LRMS: m/z (ES+) 484 [MH~]
1 = 3-phenoxyphenylboronic acid (J.Chem.Soc. 1970; 488) was used
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
100
Preparation 81
~2S,6~-2-(f1-(4-Cvclohexy_Iphenyl)-1 H imidazol-4-yllmethyl)-4-(4-methox~zy~-6
metal-3-morpholinone
CH3
~N O ~ O~CH3
~ N
/ N /
O
A mixture of copper (II) acetate (398mg, 2.2mmol), 4-cyclohexylbenzeneboronic
acid
(980mg, 4.8mmol), the imidazole of Preparation 55 (790mg, 2.4mmol) and
pyridine
(390p1, 4.8mmol) in dichloromethane (20m1) was stirred at room temperature
while
compressed air was bubbled through the solution for 8 hours, and the solvent
was
retained by using a "cold finger". The solution was then stirred under a
nitrogen
atmosphere for a further 18 hours. The mixture was partitioned between
dichloromethane (200m1) and water (200m1) containing
ethylenediaminetetraacetic
acid tetrasodium salt (1 g) and aqueous sodium bicarbonate solution (35m1) and
the
layers were separated. The. organic phase was dried (MgS04) and concentrated
under reduced pressure. The residue was purified by column chromatography
using
a Biotage° silica gel column and an elution gradient of
toluene:diethylamine (95:5 to
92:8) to afford the title compound, 140mg. 'H-NMR (CDCI3, 400MHz) S: 1.15-1.47
(m, 9H), 1.72-1.94 (m, 4H), 2.55 (m, 1 H), 2.99 (dd, 1 H), 3.03-3.20 (m, 2H),
3.39 (dd,
1 H), 3.73 (s, 3H), 3.90 (m, 1 H), 4.42 (d, 1 H), 4.56 (m, 2H), 6.78 (d, 2H),
7.02-7.19
(m, 5H), 7.20 (m, 2H), 7.74 (s, 1 H). LRMS: m/z (ES+) 474 [MHO
Preparation 82
4-(Cyclohexyloxy)ahenylboronic acid
O
I/
B(OH)2
A solution of 4-(cyclohexyloxy)phenylbromide (J. Am. Chem. Soc. 1978; 3559) in
tetrahydrofuran (40m1) was degassed then cooled to -78°C. n-Butyl
lithium
(13.47m1, 1.45M in hexanes, 19.5mmol) was added dropwise, and the mixture was
stirred for 30 minutes. Triisopropyl borate (5.01 ml, 26.6mmol) was added
dropwise
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
101
over 10 minutes, and the mixture was allowed to warm slowly, with stirring, to
room
temperature, then poured into sodium hydroxide solution (0.25M, 300m1). This
mixture was stirred for 15 minutes then extracted with diethyl ether
(2x150m1). The
aqueous layer was acidified to pH 1 using concentrated hydrochloric acid and
extracted with dichloromethane (2x150m1). These combined organic extracts were
dried (MgS04) and evaporated under reduced pressure to give the title compound
as
a white solid, 3.1 g. ' H-NMR (CDCI3, 400MHz) S: 0.88-1.62 (m, 7H), 1.80 (m,
2H),
2.00 (m, 2H), 4.39 (m, 1 H), 6.98 (d, 2H), 8.17 (d, 2H).
Preparation 83
~2S,6~-2-(~1-f4-(Cyclohexyloxy)phenyll-1 H imidazol-4-yl)methyl)-4-(4-
methoxybenzyl)-6-methyl-3-morpholinone
CH3
~N O ~ O~CH3
/ v N
O / N /
O
The title compound was obtained in 41 % yield from the imidazole of
Preparation 55
and the boronic acid of Preparation 82 following a similar procedure to that
described
in Preparation 81. 'H-NMR (CDCI3, 400MHz) 8: 1.19 (d, 3H), 1.38 (m, 3H), 1.57
(m,
3H), 1.80 (m, 2H), 1.99 (m, 2H), 2.99 (dd, 1 H), 3.04-3.20 (m, 2H), 3.39 (dd,
1 H), 3.77
(s, 3H), 3.88 (m, 1 H), 4.22 (m, 1 H), 4.42 (d, 1 H), 4.56 (m, 1 H), 4.60 (d,
1 H), 6.79 (d,
2H), 6.96 (d, 2H), 7.02 (s, 1 H), 7.10-7.28 (m, 4H), 7.65 (s, 1 H). LRMS: m/z
(ES+)
490 (MHO]
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
102
Preparation 84
~2RS)2- f 1 ~4'-Chlorof 1.1'-biphenyll-3-yl)-1 H imidazol-4- Il~~ meth I)-
methoxvbenzvl -3-morpholinone
~N ~ \ O~CHs
N / N ~ /
O
A mixture of the bromide of Preparation 73 (200mg, 0.44mmol), 4-chlorophenyl-
boronic acid (207mg, 1.32mmol), lithium chloride (56mg, 1.32mmol), cesium
carbonate (433mg, 1.32mmol) and tetrakis(triphenylphosphine)palladium(0) (51
mg,
0.044mmol) in water (2ml) and tetrahydrofuran (5ml) was stirred at 75°C
for 18
hours. The cooled mixture was partitioned between dichloromethane and 2M
sodium carbonate solution containing 6% v/v 0.88 ammonia. The organic phase
was separated, dried (MgS04) and concentrated under reduced pressure. The
residue was purified by column chromatography on silica gel using an elution
gradient of toluene:diethylamine (95:5 to 93:7) to afford the title compound
as a pale
yellow oil, 150mg. 'H-NMR (CDCI3, 400MHz) 8: 3.05 (m, 1 H), 3.20 (dd, 1 H),
3.42
(m, 2H), 3.72 (s, 3H), 3.78 (m, 1 H), 4.00 (m, 1 H), 4.48 (d, 1 H), 4.58 (dd,
1 H), 4.62 (d,
1 H), 6.78 (d, 2H), 7.14 (d, 2H), 7.20 (s, 1 H), 7.35-7.59 (m, 8H), 7.82 (s, 1
H).
Preparation 85
(2S.6R)-2-df 1-(3'-Chlorof 1,1'-biphenyll-3-yl)-1 H imidazol-4-yllmethyl -) 4-
(4-
methoxybenzvl)-6-methyl-3-morpholinone
CH3
~N O \ O~CH3
N
/ N /
O
The title compound was obtained as a white solid in 63% yield from the bromide
of
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
103
Preparation 77 and 3-chlorobenzeneboronic acid following a similar procedure
to that
described in Preparation 84. 'H-NMR (CDC13, 400MHz) S: 1.20 (d, 3H), 3.00 (dd,
1 H), 3.07-3.21 (m, 2H), 3.41 (dd, 1 H), 3.72 (s, 3H), 3.90 (m, 1 H), 4.44 (d,
1 H), 4.58
(m, 2H), 6.78 (d, 2H), 7.13 (d, 2H), 7.20-7.58 (m, 9H), 7.82 (s, 1 H). LRMS:
m/z
(ES+) 502, 504 [MHO]
Preparation 86
(2RS)-~f 1-(3',4'-Dichloro[1,1'-biphenyll-3-y~-1 H imidazol-4-vllmethyl}-4-~4-
methoxybenzyl)-3-morpholinone
~N O~ \ OwCHs
N
/ N
A mixture of the bromide of Preparation 73 (200mg, 0.44mmol), 3,4-dichloro-
benzeneboronic acid (102mg, 0.53mmol), lithium chloride (56mg, 1.32mmol),
cesium
carbonate (433mg, 1.32mmol) and tetrakis(triphenylphosphine)palladium(0)
(26mg,
0.022mmol) in water (2ml) and tetrahydrofuran (5ml), was stirred at
75°C for 2.5
hours. TLC analysis showed starting material remaining, so additional 3,4-
dichlorobenzeneboronic acid (204mg, 1.06mmol) and tetrakis(triphenylphosphine)-
palladium(0) (26mg, 0.022mmol) were added and the mixture was heated for a
further 18 hours at reflux. The cooled mixture was partitioned between
dichloromethane and 2M sodium carbonate solution containing 6% v/v 0.88
ammonia. The organic phase was separated, dried (MgS04) and concentrated
under reduced pressure. The residue was purified by column chromatography on
silica gel using an elution gradient of toluene:diethylamine (95:5 to 93:7) to
afford the
title compound as a crystalline solid, 165mg. 'H-NMR (CDC13, 400MHz) 8: 3.06
(m,
1 H), 3.20 (dd, 1 H), 3.41 (m, 2H), 3.75 (m, 4H), 4.00 (m, 1 H), 4.48 (d, 1
H), 4.58 (m,
1 H), 4.62 (d, 1 H), 6.58 (2xs, 2H), 7.18 (m, 3H), 7.40 (m, 2H), 7.52 (m, 4H),
7.65 (s,
1 H), 7.82 (s, 1 H). LRMS: m/z (ES+) 522, 524 [MH~]
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
104
Preaaration 87
(2S,6F~-2-ff 1-(3',4'-Dichlorof 1.1'-biphenyll-3 yl)-1 H imidazol-4-
Il~methyl)-4-(4-
methoxybenzyl -6-methyl-3-morpholinone
CH3
~N O ~ O~CH3
/ N /
O
A mixture of the bromide of Preparation 77 (200mg, 0.425mmol), 3,4-dichloro-
benzeneboronic acid (243mg, 1.27mmol), lithium chloride (54mg, 1.27mmol),
cesium
carbonate (416mg, 1.32mmol) and tetrakis(triphenylphosphine)palladium(0)
(23mg,
0.02mmol) in water (2ml) and tetrahydrofuran (5ml) was stirred at 75°C
for 3 hours.
The cooled mixture was partitioned between ethyl acetate (100m1) and water
(50m1).
The organic phase was separated, dried (MgS04) and concentrated under reduced
pressure. The residue was purified by column chromatography on silica gel
using
an elution gradient of pentane:ethyl acetate:methanol (50:50:0 to 0:100:0 to
0:95:5)
to give a white foam. This was further purified by column chromatography using
a
Biotage~ silica gel column, and an elution gradient of ethyl acetate:methanol
(100:0
to 93:7) to afford the title compound as a white foam, 140mg. 'H-NMR (CDCI3,
400MHz) S: 1.20 (d, 3H), 3.00-3.21 (m, 3H), 3.40 (m, 1 H), 3.75 (s, 3H), 3.90
(m, 1 H),
4.42 (m, 1 H), 4.59 (m, 2H), 6.78 (d, 2H), 7.13 (d, 2H), 7.19 (s, 1 H), 7.38
(m, 1 H),
7.40 (m, 1 H), 7.50 (m, 4H), 7.65 (s, 1 H), 7.81 (s, 1 H). LRMS: m/z (ES+)
536, 538
[MHO]
Preparation 88
(2S)-2-f(1-Propel-1 H imidazol-4yl)methyll-3-morpholinone
H3C~ ~N O
N / NH
I
O
Ammonium cerium (IV) nitrate (4.55g, 8.30mmol) was added to a solution of the
compound of Preparation 47 (1.43g, 4.15mmol) in acetonitrile (9ml) and water
(9ml)
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
105
and the mixture was stirred at room temperature for 18 hours then concentrated
under reduced pressure and the residue was dissolved in methanol. This
solution
was purified by column chromatography on silica gel using an elution gradient
of
dichloromethane:methano1:0.88 ammonia (95:5:0.5 to 90:10:1 ) to afford an
orange
oil. This was further purified by column chromatography on Dowex~ 50WX8-200
ion
exchange resin, using an elution gradient of water:0.88 ammonia (100:0 to
98:2) to
afford the title compound, 522mg. 'H-NMR (CDCI3, 400MHz) 8: 0.88 (t, 3H), 1.75
(m, 2H), 3.00 (dd, 1 H), 3.23 (m, 2H), 3.50 (m, 1 H), 3.74 (m, 1 H), 3.79 (t,
2H), 4.00
(m, 1 H), 4.43 (dd, 1 H), 5.94 (bs, 1 H), 6.73 (s, 1 H), 7.36 (s, 1 H). LRMS:
m/z (ES+)
224 [MHO]
Preparation 89
(2RS)-2-f (1-Butyl-1 H imidazol-4-yl)methyll-3-morpholinone
H3C
~N O
N / NH
O
Ammonium cerium (IV) nitrate (5.7g, 10.4mmol) was added to a solution of the
compound of Preparation 48 (1.87g, 5.2mmol) in acetonitrile (50m1) and water
(50m1), and the mixture was stirred at room temperature for 18 hours. The
solvents
were evaporated under reduced pressure and the residue was purified using a
Dowex° 50WX8-200 ion-exchange column and 5% 0.88 ammonia as
eluant. This
product was further purified by column chromatography on silica gel using an
elution
gradient of dichloromethane:methano1:0.88 ammonia (100:0:0 to 90:10:1) to
afford
the title compound, 300mg. 'H-NMR (CDCI3, 400MHz) 8: 0.96 (t, 3H), 1.34 (m,
2H),
1.75 (m, 2H), 3.02 (dd, 1 H), 3.28 (m, 2H), 3.56 (m, 1 H), 3.78 (m, 1 H), 3.82
(t, 2H),
4.02 (m, 1 H), 4.48 (dd, 1 H), 5.98 (bs, 1 H), 6.77 (s, 1 H), 7.38 (s, 1 H).
LRMS: m/z
(ES+) 238 [MH~]
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
106
Preparation 90
~2RS)-2-ff1-(2-C cly ohexyleth~)-1 H imidazol-4-yl]methyl}-3-morpholinone
~N
N / NH
O
Ammonium cerium (IV) nitrate (1.1 g, 2.Ommol) was added to a solution of the
compound of Preparation 49 (411 mg, 1.Ommol) in acetonitrile (5ml) and water
(5ml),
and the mixture was stirred at room temperature for 18 hours then concentrated
under reduced pressure. The residue was pre-adsorbed onto silica gel and
purified
by column chromatography on silica gel using an elution gradient of ethyl
acetate:dichloromethane:methano1:0.88 ammonia (100:0:0:0 to 75:0:25:0 to
0:90:10:1). The product was further purified by column chromatography on
reverse
phase polystyrene gel, using an elution gradient of water:methanol (100:0 to
0:100)
to afford the title compound, 522mg. 'H-NMR (CD30D, 400MHz) 8: 1.01 (m, 2H),
1:24 (m, 4H), 1.76 (m, 7H), 3.22 (m, 3H), 3.40 (m, 1 H), 3.80 (m, 1 H), 4.02
(m, 1 H),
4.22 (m, 2H), 4.38 (m, 1 H), 7.43 (s, 1 H), 8.85 (s, 1 H). LRMS: m/z (TSP+)
292.2
[MHO]
Preparation 91
(2R,6S~-2-(f 1-(2-Cyclohexylethyl)-1 H imidazol-4-yllmethyl)-6-methyl-3-
mor~~holinone
CH3
~N O
N / NH
v
O
Ammonium cerium (IV) nitrate (387mg, 0.758mmol) was added to a solution of the
compound of Preparation 61 (120mg, 0.283mmol) in acetonitrile (8ml) and water
(5ml) and the mixture was stirred at 40°C for 18 hours then
concentrated under
reduced pressure. The residue was pre-adsorbed onto silica gel and purified by
column chromatography on silica gel using an elution gradient of
dichloromethane:methano1:0.88 ammonia (98:2:0.2 to 95:5:0.5) to afford the
title
compound as a colourless oil, 66mg. 'H-NMR (CDCI3, 400MHz) S: 0.92 (m, 2H),
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
107
1.18 (m, 7H), 1.62 (m, 7H), 2.98 (dd, 1 H), 3.18 (m, 2H), 3.23 (dd, 1 H), 3.83
(m, 3H),
4.41 (m, 1 H), 6.19 (bs, 1 H), 6.70 (s, 1 H), 7.30 (s, 1 H). LRMS: m/z (ES+)
328 [MNa~]
Preparation 92
(2S.6R)-2-ff1-(2-Cyclohexylethyl)-1 H imidazol-4-vllmethyl)-6-methyl-3-
morpholinone
CH3
~N O
~-N
/ NH
O
The title compound was obtained as a pale yellow solid in 63% yield from the
protected morpholinone of Preparation 62 following the procedure described in
Preparation 91. 'H-NMR (CDCI3, 400MHz) 8: 0.92 (m, 2H), 1.18 (m, 7H), 1.62 (m,
7H), 2.98 (dd, 1 H), 3.19 (m, 2H), 3.23 (dd, 1 H), 3.83 (m, 3H), 4.41 (m, 1
H), 6.03 (bs,
1 H), 6.70 (s, 1 H), 7.34 (s, 1 H). LRMS: m/z (ES+) 328 [MNa+)
Preparation 93
(2S.6f~-2-((1-f2-(4.4-Dimethylcyclohexyl)ethyll-1 H imidazol-4-yl)methyl)-6-
methyl-3-
morpholinone
CH3
H3C ~N O
H3C ~-N
/ NH
O
The title compound was obtained in 53% yield from the protected morpholinone
of
Preparation 66 following a similar procedure to that described in Preparation
91,
except that ethyl acetate:methanol:diethylamine (99:0.5:0.5 to 95:2.5:2.5) was
used
as the column eluant. 'H-NMR (CDC13, 400MHz) 8: 0.82 (2xs, 6H), 1.10 (d, 3H),
1.18-1.38 (m, 8H), 1.48 (m, 1 H), 1.63 (m, 2H), 2.98 (m, 1 H), 3.19 (m, 2H),
3.23 (dd,
1 H), 3.84 (m, 3H), 4.41 (m, 1 H), 6.97 (bs, 1 H), 7.21 (s, 1 H), 7.34 (s, 1
H). LRMS:
m/z (ES+) 334 [MH~j
Preparation 94
(2S,6F~-2-(f 1-(3-Cyclohexyl-3-methylbutyl)-1 H imidazol-4-yllmethyl)-6-methyl-
3-
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
108
morbholinone
CH3
H3C ~N O
CH3 ~N / NH
i
O
The title compound was obtained in 52% yield from the protected morpholinone
of
Preparation 65 following a similar procedure to that described in Preparation
91,
except that dichloromethane:methano1:0.88 ammonia (99:1:0.1 to 93:7:0.7) was
used as the column eluant. 'H-NMR (CD30D, 400MHz) 8: 0.86 (s, 6H), 1.00 (m,
2H), 1.03-1.28 (m, 7H), 1.70 (m, 7H), 2.90 (m, 1 H), 3.03 (m, 1 H), 3.18 (m,
2H), 3.82
(m, 1 H), 3.95 (m, 2H), 4.36 (m, 1 H), 5.42 (s, 1 H), 6.86 (s, 1 H), 7.50 (s,
1 H). LRMS:
m/z (ES+) 348 [MH+)
Preaaration 95
(2S,5S)-2-(f1-(2-Cyclohexylethyl)-1 H imidazol-4-yllmethyl~}-5-methyl-3-
morpholinone
~N 0~,,~,.CH3
1IN
/ NH
O
A mixture of the protected morpholinone of Preparation 63 (170mg, 0.4mmol) and
ammonium cerium (IV) nitrate (550mg, 1.Ommol) in water (1 ml) and acetonitrile
(1 ml)
was stirred at 40°C for 18 hours then concentrated under reduced
pressure. The
residue was purified by column chromatography using a Biotage~ silica gel
column
and an elution gradient of ethyl acetate:diethylamine (95:5 to 80:20) to
afford the
title compound, l8mg. 'H-NMR (CDCI3, 400MHz) 8: 0.97 (m, 2H), 1.20 (m, 7H),
1.66 (m, 7H), 3.06 (dd, 1 H), 3.25 (dd, 1 H), 3.58 (m, 1 H), 3.65 (dd, 1 H),
3.83 (m, 3H),
4.43 (dd, 1 H), 6.15 (bs, 1 H), 6.78 (s, 1 H), 7.38 (s, 1 H).
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
109
Preparation 96
(2R,5R)-2-ff 1-(2-C cl~xylethyl)-1 H imidazol-4-yl methy~~-5-meth I-3-
mopholinone
CH3
~N O
~-N
/ NH
O
The title compound was obtained in 34% from the protected morpholinone of
Preparation 64 following the procedure described in Preparation 95. 'H-NMR
(CDCI3, 400MHz) 8: 0.78-0.98 (m, 2H), 1.03-1.22 (m, 7H), 1.62 (m, 7H), 3.02
(m,
1 H), 3.18 (dd, 1 H), 3.55 (m, 1 H), 3.61 (dd, 1 H), 3.77 (dd, 1 H), 3.82 (t,
2H), 4.38 (m,
1 H), 6.24 (bs, 1 H), 6.73 (s, 1 H), 7.37 (s, 1 H).
Preparation 97
(2RS)-2-ff1-(2-Phenylethyl'I-1 H imidazol-4-yllmethyl)-3-morpholinone
~N o~
'--N
/ NH
O
Ammonium cerium (IV) nitrate (883mg, 1.6mmol) was added to a solution of the
compound of Preparation 50 (326mg, 0.81 mmol) in acetonitrile (2.4m1) and
water
(2.4m1) and the mixture was stirred at room temperature for 5 days, then
concentrated under reduced pressure. The residue was dissolved in methanol and
adsorbed onto silica gel, then purified by column chromatography on silica gel
using
an elution gradient of dichloromethane:methano1:0.88 ammonia (100:0:0 to
90:10:1)
to afford the title compound as an orange oil, 97mg. 'H-NMR (CDC13, 400MHz)
8: 3.02 (m, 3H), 3.26 (m, 2H), 3.52 (m, 1 H), 3.77 (m, 1 H), 4.00 (m, 1 H),
4.10 (t, 2H),
4.43 (dd, 1 H), 5.99 (bs, 1 H), 6.70 (s, 1 H), 7.05 (d, 2H), 7.24 (m, 4H).
LRMS: m/z
(ES+) 286 [MHO]
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
110
Preparation 98
(2RS)-2-(~1-f2-(4-Bromophenyl)ethyll-1 H imidazol-4-y~,methyl)-3-mopholinone
Br ~ ~ ~N
N / NH
i
O
A solution of ammonium cerium (IV) nitrate (1.35g, 2.48mmol) in water (5ml)
was
added to a solution of the bromide of Preparation 59 (600mg, 1.24mmol) in
acetonitrile (l0ml) and the mixture was stirred at 40°C for 18 hours.
TLC analysis
showed starting material remaining, so additional ammonium cerium (IV) nitrate
(308mg, 0.56mmol) was added and the mixture was stirred at 40°C for a
further 2
hours. The cooled mixture was concentrated under reduced pressure and the
residue was purified by column chromatography on silica gel using an elution
gradient of dichloromethane:methano1:0.88 ammonia (98:2:0.2 to 95:5:0.5) to
afford
the title compound, 250mg. 'H-NMR (CDCI3, 400MHz) b: 2.98 (t, 2H), 3.03 (m,
1H),
3.25 (m, 2H), 3.52 (m, 1 H), 3.77 (m, 1 H), 4.00 (m, 1 H), 4.05 (t, 2H), 4.42
(m, 1 H),
5.99 (bs, 1 H), 6.65 (s, 1 H), 6.93 (d, 2H), 7.22 (s, 1 H), 7.40 (d, 2H).
Preparations 99 to 101
The following compounds of general structure
~N
N / NH
i
O
were prepared from the corresponding protected morpholinones following a
similar
procedure to that described in Preparation 98.
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
111
Ex R Yield Data
(%)
'H-NMR (CDC13, 400MHz) 8: 3.10 (dd,
1H),
3.25 (m, 1 H), 3.37 (m, 1 H), 3.58
(m, 1 H),
3.78 (m, 1 H), 4.02 (m, 1 H), 4.54
(m, 1 H),
58
/ 5.83 (bs, 1 H), 7.15 (s, 1 H), 7.33
(m, 3H),
7.42 (m, 2H), 7.78 (s, 1 H). LRMS:
m/z (ES+)
280 [MNa~]
'H-NMR (CDCI3, 400MHz) 8: 2.20 (s,
3H),
3.16 (dd, 1 H), 3.30 (m, 1 H), 3.38
(dd, 1 H),
3.58 (m, 1 H), 3.80 (m, 1 H), 4.04
100 ~ 74 (m, 1 H),
/ 4.56 (dd, 1 H), 6.18 (bs, 1 H),
CH3 6.90 (s, 1 H),
7.20-7.35 (m, 4H), 7.50 (s, 1 H).
HRMS: m/z
272.1394 [MHO]
'H-NMR (CD30D, 400MHz) 8: 2.92 (dd,
1 H),
3.05 (m, 1 H), 3.19 (m, 1 H), 3.63
/ (m, 1 H),
101' I \ 3.86 (m, 1 H), 4.22 (m, 1 H), 6.82
(s, 1 H), 7.10
(m, 2H), 7.30 (m, 3H), 7.40 (m,
1 H), 7.50 (m,
4H). LRMS m/z (ES+) 356 [MNa~]
1 = ethyl acetate:methanol:diethylamine (100:0:0 to 90:5:5) used as the column
eluant
Preparation 102
(2RS)-2-~f1-(3-Phenoxyphenyl)-1 H imidazol-4-yllmethyl~-3-morpholinone
O
\ N N O
/ NH
O
A solution of ammonium cerium (IV) nitrate (350mg, 063mmol) in water (2ml) was
added to a solution of the protected morpholine of Preparation (120mg,
0.256mmol)
in acetonitrile (2ml), and the mixture was stirred at 40°C for 18
hours. TLC analysis
showed starting material remaining, so additional ammonium cerium (IV) nitrate
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
112
(500mg, 0.91 mmol) was added, and the mixture was stirred at 40°C for a
further 8
hours. The mixture was evaporated under reduced pressure and the residue was
partitioned between dichloromethane (50m1) and a solution of ethylenediamine-
tetraacetic acid (1 g) in saturated sodium bicarbonate solution (50m1). The
phases
were separated and the organic layer was dried (MgS04) and evaporated under
reduced pressure. The crude product was purified by column chromatography on
silica gel using an elution gradient of ethyl acetate:methanol:diethylamine
(100:0:0 to
96:2:2) to afford the title compound, 25mg. 'H-NMR (CDCI3, 400MHz) 8: 3.05
(dd,
1 H), 3.21-3.18 (m, 2H), 3.57 (m, 1 H), 3.77 (m, 1 H), 4.00 (m, 1 H), 4.48
(dd, 1 H), 6.15
(bs, 1 H), 6.90 (d, 1 H), 6.98 (s, 1 H), 7.02 (m, 3H), 7.14 (m, 2H), 7.37 (m,
3H), 7.74 (s,
1 H). LRMS: m/z (TSP+) 350.0 [MHO]
Preparation 103
j2RS)-2-(f 1-(2-Naphthyl)-1 H imidazol-4-yllmethyl)-3-morpholinone
N O
/ NH
The title compound was obtained as a yellow gum in 56% yield from the
protected
morpholinone of Preparation 72, following a similar procedure to that
described in
Preparation 102, except that dichloromethane:methano1:0.88 ammonia (100:0:0 to
90:10:1) was used as the column eluant. 'H-NMR (CDC13, 400MHz) 8: 3.15 (dd,
1 H), 3.23 (m, 1 H), 3.38 (dd, 1 H), 3.58 (m, 1 H), 3.78 (m, 1 H), 4.02 (m, 1
H), 4.53 (dd,
1 H), 6.01. (bs, 1 H), 7.22 (d, 2H), 7.48 (m, 3H), 7.75-7.94 (m, 4H). LRMS:
m/z (ES+)
308 [MH+]
Preparation 104
(-)-(2S)-2-(f 1-Phenyl-1 H imidazol-4-yllmethyl)-3-morpholinone
~N O
N
/ NH
O
Ammonium cerium (IV) nitrate (1.438, 2.61 mmol) was added to a solution of the
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
113
protected morpholinone of Preparation 74 (330mg, 0.87mmo1) in water (2ml) and
acetonitrile (2ml), and the mixture was stirred at 40°C for 4 hours.
TLC analysis
showed starting material remaining, so additional ammonium cerium (IV) nitrate
(1.43g, 2.61 mmol) was added, and the mixture was stirred at 40°C for a
further 2
hours. The mixture was partitioned between dichloromethane (200m1) and a
solution of ethylenediaminetetraacetic acid (1g) in saturated sodium
bicarbonate
solution (50m1). The phases were separated and the organic layer was dried
(MgS04) and evaporated under reduced pressure. The crude product was purified
by column chromatography on silica gel using an elution gradient of ethyl
acetate:methanol:diethylamine (100:0:0 to 96:2:2). The product was azeotroped
with toluene and dichloromethane to afford the title compound as an oil,
173mg. 'H-
NMR (CDC13, 400MHz) 8: 3.14 (dd, 1 H), 3.28 (m, 1 H), 3.38 (dd, 1 H), 3.58 (m,
1 H),
3.80 (m, 1 H), 4.05 (m, 1 H), 4.56 (dd, 1 H), 5.98 (bs, 1 H), 7.17 (s, 1 H),
7.37 (m, 3H),
7.45 (m, 2H), 7.79 (s, 1 H). LRMS: m/z (TSP+) 258.1 (MHO] [a]p = -70.59, (c =
0.104, methanol)
Preparation 105
(2S)-2-~f1-(4-tent Butylphenyl)-1 H imidazol-4-yllmethyl~-3-morpholinone
HsC -CHs ~ ~ N N O
/ NH
H3C
O
Ammonium cerium (IV) nitrate (297mg, 0.55mmol) was added to a solution of the
protected morpholinone of Preparation 75 (94mg, 0.22mmol) in water (2ml) and
acetonitrile (2ml), and the mixture was stirred at 40°C for 15 hours.
Ethylene-
diaminetetraacetic acid (0.5g) in saturated sodium bicarbonate solution (5ml)
was
added and the mixture was extracted with dichloromethane (2x50m1). The
combined organic extracts were dried (MgS04) and evaporated under reduced
pressure. The crude product was purified by column chromatography on silica
gel
using an elution gradient of ethyl acetate:methanol:diethylamine (98:1:1 to
94:3:3), to
afford the title compound as an oil, 22mg. 'H-NMR (CDCI3, 400MHz) S: 1.37 (s,
9H),
3.12 (dd, 1 H), 3.26 (m, 1 H), 3.38 (dd, 1 H), 3.58 (m, 1 H), 3.79 (m, 1 H),
4.04 (m, 1 H),
4.55 (m, 1 H), 6.22 (bs, 1 H), 7.10 (s, 1 H), 7.25 (d, 2H), 7.42 (d, 2H), 7.77
(s, 1 H).
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
114
LRMS: m/z (TSP+) 314.1 (MH~]
Preparation 106
(-)~2S~~{1-f3,5-Bis(,trifluoromethyl~phenvl]-1 H imidazol-4-yl meths)-3-
morpholinone
F3C
,_N o'1
N
/ NH
FsC O
The title compound was obtained as a solid in 81 % yield from the protected
morpholinone of Preparation 76 following the procedure described in
Preparation
105. 'H-NMR (CDC13, 400MHz) 8: 3.18 (dd, 1 H), 3.30 (m, 1 H), 3.39 (dd, 1 H),
3.60
(m, 1 H), 3.80 (m, 1 H), 4.06 (m, 1 H), 4.55 (m, 1 H), 5.88 (bs, 1 H), 7.20
(s, 1 H), 7.81
(s, 2H), 7.84 (s, 1 H), 7.87 (s, 1 H). LRMS: m/z (TSP+) 394.0 [MHO]. [a]p = -
40.35, (c
= 0.116, methanol)
Preparation 107
(2RS)-2-(f1-(4'-Chlorofl.l'-biphenyll-3-yl)-1 H imidazol-4-yllmethyl)-3-
morpholinone
ci
N O
/ NH
O
The title compound was obtained as a solid in 91 % yield from the protected
morpholinone of Preparation 84 following the procedure described in
Preparation
105. 'H-NMR (CDC13, 400MHz) 8: 3.10 (dd, 1 H), 3.25 (m, 1 H), 3.38 (dd, 1 H),
3.57
(m, 1 H), 3.78 (m, 1 H), 4.02 (m, 1 H), 4.52 (dd, 1 H), 5.96 (bs, 1 H), 7.17
(s, 1 H), 7.37
(m, 3H), 7.42 (m, 1 H), 7.50 (m, 3H), 7.57 (s, 1 H), 7.80 (s, 1 H). LRMS: m/z
(ES-) 366
(M-H-)
Preparation 108
(2RS)-2-(f1-(3'.4'-Dichlorofl.l'-biphenyll-3-yl)-iHimidazol-4~illmethyl~3-
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
115
morpholinone
Ci
C~
N
/ NH
O
The title compound was obtained in 49% yield from the protected morpholinone
of
Preparation 86 following the procedure described in Preparation 105. 'H-NMR
(CDC13, 400MHz) 8: 3.10 (dd, 1 H), 3.25 (m, 1 H), 3.38 (dd, 1 H), 3.57 (m, 1
H), 3.78
(m, 1 H), 4.02 (m, 1 H), 4.52 (dd, 1 H), 5.86 (bs, 1 H), 7.18 (s, 1 H), 7.37
(m, 2H), 7.50
(m, 4H), 7.62 (s, 1 H), 7.80 (s, 1 H). LRMS: m/z (ES+) 402, 404 (MH+)
Preparations 109 to 114
The following compounds of the general structure
CH3
~N O
N / NH
I
O
were prepared from the appropriate protected morpholinones, following a
similar
procedure to that described in Preparation 105.
Prep R Yield Data
(%)
'H-NMR (CDC13, 400MHz) b: 1.22 (d,
3H),
3.08 (dd, 1 H), 3.22 (m, 2H), 3.38
(dd, 1 H),
109 ~ / 50 3.92 (m, 1 H), 4.50 (m, 1 H), 6.62
(bs, 1 H),
7.16 (s, 1 H), 7.37 (m, 3H), 7.42
(m, 2H), 7.78
(s, 1 H).
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
116
Prep R YieldData
(%)
'H-NMR (CDC13, 400MHz) 8: 1.20 (d,
3H),
3.02 (dd, 1 H), 3.20 (m, 2H), 3.34
(dd, 1 H),
3.88 (m, 1 H), 4.44 (m, 1 H), 6.05
(bs, 1 H),
110 \ p 25 6,90 (d, 1 H), 6.98 (s, 1 H), 7.02
(m, 4H), 7.14
(m, 1 H), 7.36 (m, 3H), 7.70 (s,
1 H). LRMS:
m/z (ES+) 364 [ MHO]
'H-NMR (CDC13, 400MHz) b: 1.24 (d,
3H),
3.09 (dd, 1 H), 3.22 (m, 2H), 3.39
(dd, 1 H),
44 3.g5 (m, 1 H), 4.50 (m, 1 H), 5.86
(bs, 1 H),
111 p \ sticky7.03 (m, 4H), 7.18 (m, 2H), 7.36
(m, 4H),
gum 7.70 (s, 1 H). LRMS: m/z (ES+) 364
[MH~]
'H-NMR (CDC13, 400MHz) 8: 1.23 (d,
3H),
3.10 (dd, 1 H), 3.22 (m, 2H), 3.39
(dd, 1 H),
112' 44 394 (m, 1 H), 4.55 (m, 1 H), 5.84
(bs, 1 H),
7.20 (s, 1 H), 7.39 (m, 3H), 7.42-7.60
(m, 5H),
7.80 (s, 1 H).
CI
'H-NMR (CDC13, 400MHz) 8: 1.22 (m,
5H),
1.40 (m, 4H), 1.70-1.90 (m, 4H),
2.55 (m,
40 1 H), 3.09 (dd, 1 H), 3.21 (m, 2H),
3.38 (dd,
113' ~ solid1 H), 3.92 (m, 1 H), 4.50 (m, 1 H),
6.00 (bs,
1 H), 7.10 (s, 1 H), 7.27 (m, 4H),
7.74 (s, 1 H).
LRMS: m/z (ES+) 354 [MHO]
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
117
Prep R Yield Data
(%)
'H-NMR (CDC13, 400MHz) b: 0.88-1.15
(m,
2H), 1.22 (d, 3H), 1.38 (m, 3H),
1.58 (m, 1 H)
1.80 (m, 2H), 1.99 (m, 2H), 3.06
(dd, 1 H),
1142 p ~ 34 3.22 (m, 2H), 3.39 (dd, 1 H), 3.92
(m, 1 H),
4.22 (m, 1 H), 4.50 (m, 1 H), 5.84
(bs, 1 H),
6.97 (d, 2H), 7.05 (s, 1 H), 7.22
(d, 2H), 7.64
(s, 1 H).
1 = Acetonitrile:water (3:1, by volume) was used as the reaction solvent
2 = Acetonitrile:water (2:1, by volume) was used as the reaction solvent
Preparation 115
(2S,6~-2-f f 1-(3',4'-Dichlorof 1,1'-biphenyll-3-yl)-1 H imidazol-4-yllmethyl)-
6-methyl-3-
morpholinone
C~ CI
CH3
~N O
N
/ NH
O
The title compound was obtained as a white foam in 63% yield from the
protected
morpholinone of Preparation 87 following a similar procedure to that described
in
Preparation 105. 'H-NMR (CDCI3, 400MHz) 8: 1.24 (d, 3H), 3.14 (dd, 1 H), 3.23
(m,
2H), 3.40 (dd, 1 H), 3.97 (m, 1 H), 4.56 (dd, 1 H), 5.80 (bs, 1 H), 7.10 (s, 1
H), 7.40 (m,
2H), 7.55 (m, 4H), 7.68 (s, 1 H), 7.81 (s, 1 H). LRMS: m/z (TSP+) 416.1, 420.1
[MH+]
Preparation 116
~2S)-2-(1 H Imidazol-4-ylmethvl)-3-morpholinone
~N O
HN / NH
i
O
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
118
A mixture of the protected morpholinone of Preparation 53 (500mg, 1.66mmol),
and
ammonium cerium (IV) nitrate (2.5g, 4.5mmol) in water (6ml) and acetonitrile
(6ml)
was stirred at 40°C for 18 hours. Potassium carbonate (1.5g) was added
and the
mixture was stirred for 10 minutes then adsorbed onto silica gel. The product
was
isolated by column chromatography on silica gel using ethyl
acetate:methanol:diethylamine (96:2:2 to 80:10:10) and was further purified by
column chromatography on silica gel using dichloromethane:methanol (90:10 to
85:15) to afford the title compound, 240mg. 'H-NMR (CD30D, 400MHz) 8: 3.02-
3.42 (m, 4H), 3.78 (m, 1 H0, 4.00 (m, 1 H); 4.38 (m, 1 H), 6.75 (s, 1 H), 7.78
(s, 1 H).
HRMS: m/z (ES+) 182.0924 [MH~]
Preparation 117
(-)-(2S.6R)-2-(1 H imidazol-4-ylmeth~il)-6-methyl-3-morpholinone
CH3
~N O
HN / NH
O
A mixture of the protected morpholinone of Preparation 55 (1 g, 3.2mmol) and
ammonium cerium (IV) nitrate (5.2g, 9.6mmol) in water (20m1) and acetonitrile
(30m1)
was stirred at 40°C for 18 hours. The solvent was evaporated under
reduced
pressure. The residue was suspended in a mixture of
dichloromethane:methano1:0.88 ammonia (99:1:0.1 by volume) and purified twice
by
column chromatography on silica gel using an elution gradient of
dichloromethane:methano1:0.88 ammonia (90:10:1 ). The resulting oil was
azeotroped with diethyl ether to afford the title compound as a colourless
foam,
380mg. 'H-NMR (CD30D, 400MHz) 8: 1.21 (d, 3H), 3.02 (m, 2H), 3.19 (m, 2H),
3.90 (m, 1 H), 4.36 (m, 1 H), 6.81 (s, 1 H), 7.54 (s, 1 H). LRMS: m/z (ES+)
196 [MHO].
[a]o = -104.56 (c = 0.19, methanol)
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
119
Preparation 118
tert-Butyl (2S)-2-(f1-(tertbutoxvcarbony)-1 H imidazol-4-yllmethylj~-3-oxo-4-
morpholinecarbox I
H3C
H3C~0 ~N ~~ CH
H3C ~N / N O ~H3
O
O O CH3
A solution of the morpholinone of Preparation 116 (70mg, 0.39mmol),
dimethylaminopyridine (3mg) and di-tert-butyl dicarbonate (354mg, 1.62mmol) in
acetonitrile (5ml) was stirred at room temperature for 42 hours. The mixture
was
concentrated under reduced pressure and the residue was purified by column
chromatography on silica gel using an elution gradient of
dichloromethane:methano1:0.88 ammonia (99:1:0.1 to 95:5:0.5) to afford the
title
compound, 96mg. 'H-NMR (CDCI3, 400MHz) S: 1.58 (s, 9H), 1.61 (s, 9H), 3.04
(dd,
1 H), 3.35 (dd, 1 H), 3.78 (m, 3H), 4.05 (m, 1 H), 4.50 (m, 1 H), 7.20 (s, 1
H), 8.00 (s,
1 H). HRMS: m/z (ES+) 382.1972 [MH+]
Preparation 119
tert-Butyl (2S.6~-2-((1-f4-(cyclohexyloxy)phenyll-1 H imidazol-4-yl)methyl)-6-
methyl-
3-oxomorpholine-4-carboxylate
CH3
~N O
CH
O \ N / N O ~H3
O O CH3
4-Dimethylaminopyridine (49mg, 0.4mmol) and di-tert-butyl dicarbonate (174mg,
0.8mmol) were added to a solution of the morpholinone of Preparation 114
(135mg,
0.37mmol) in acetonitrile (5ml), and the mixture was stirred at room
temperature for 5
hours. TLC analysis showed starting material remaining, so additional di-tert-
butyl
Bicarbonate (87mg, 0.4mmol) was added, and the mixture was stirred at room
temperature for a further 18 hours. The reaction mixture was concentrated
under
reduced pressure and the residue was purified by column chromatography on
silica
gel using ethyl acetate as eluant, to give the title compound, 95mg. LRMS: m/z
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
120
(ES+) 470 (MHO
Preparation 120
Lithium (25~-2-({f 1 ~-2-f(tert-butoxycarbon~)aminol-1-meth I~yl}oxy)-3-~(1-[4-
jcyclohexyloxv~phenyll-1 H imidazol-4-yl}propanoate
N O HL~H3
~N O CH ~ CH3
p ~ \ N / O L
O
A mixture of the protected morpholinone of Preparation 119 (87mg, 0.19mmol)
and
lithium hydroxide (24mg, 0.56mmol) in tetrahydrofuran (0.5m1) and water (1 ml)
was
stirred at room temperature for 18 hours. The reaction mixture was evaporated
under reduced pressure to afford the title compound. 'H-NMR (D20, 400MHz)
8: 0.60 (m, 2H), 1.00-1.38 (m, 16H), 1.50 (m, 2H), 1.70 (m, 2H), 2.58 (m, 1
H), 2.80
(m, 2H), 2.94 (m, 1 H), 3.30 (m, 1 H), 3.82 (m, 1 H), 4.00 (m, 1 H), 6.63 (d,
2H), 6.82 (s,
1 H), 7.00 (d, 2H), 7.56 (s, 1 H). LRMS: m/z (ES-) 486 [M-H]-
Preparation 121.
(2RS)-2- f 1-f2-(4'-Ethylf 1,1'-biphenyll-4-yl)ethyll-1 H imidazol-4-
yl)methylL
morpholinone
HsC / ~ ~ ~ ~N O
'-N
/ NH
O
A mixture of the bromo compound of Preparation 98 (250mg, 0.69mmol), 4-
ethylbenzeneboronic acid (154mg, 1.03mmol), tetrakis(triphenylphosphine)-
palladium(0) 78mg, 0.068mmol) and sodium carbonate solution (411 ~I, 2M,
0.823mmol) in water (1 ml) and dioxan (5ml) was heated at 100°C for 3
hours. The
cooled reaction mixture was diluted with water (l0ml), and the mixture was
extracted
with ethyl acetate (3x15m1). The combined organic extracts were dried (MgS04)
and evaporated under reduced pressure. The crude product was purified by
column
chromatography on silica gel using an elution gradient of
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
121
dichloromethane:methano1:0.88 ammonia (99:1:0.1 to 95:5:0.5) to afford the
title
compound, 170mg. 'H-NMR (CDCI3, 400MHz) 8: 1.22 (t, 3H), 2.64 (q, 2H), 3.02
(m,
3H), 3.22 (m, 2H), 3.44 (m, 1 H), 3.72 (m, 1 H), 3.98 (m, 1 H), 4.10 (t, 2H),
4.42 (m,
1 H), 5.90 (bs, 1 H), 6.70 (s, 1 H), 7.08 (d, 2H), 7.22 (m, 3H), 7.44 (m, 4H).
Preparations 122 to 131
~N O
'-N
/ NH
O
Aryl boronic acids (R-B(OH)2), (0.74mmol) were added to solutions of the bromo
compound of Preparation 98 (180mg, 0.49mmol) tetrakis(triphenylphosphine)-
palladium(0) (56mg, 0.051 mmol) and sodium carbonate solution (2951, 2M,
0.593mmol) in water (1 ml) and dioxan (5ml). The reaction mixtures were heated
to
100°C for 4 hours, then allowed to cool. Water (l5ml) was added, and
the mixtures
were extracted with ethyl acetate (3x15m1). The combined organic extracts were
dried (MgS04) and evaporated under reduced pressure. The crude products were
purified by column chromatography on silica gel using an elution gradient of
dichloromethane:methano1:0.88 ammonia (97:3:0.3 to 95:5:0.5) to afford the
desired
products, shown in the following table.
Prep R Yield Data
(%)
'H-NMR (CDC13, 400MHz) 8: 1.28 (d,
6H),
CH3
H3~ 2.95 (m, 1 H), 3.02 (m, 3H), 3.24
(m, 2H),
3.52 (m, 1 H), 3.75 (m, 1 H), 4.00
(m, 1 H),
122 ~ ~ 63
4.12 (t, 2H), 4.44 (m, 1 H), 5.92
(bs, 1 H), 6.75
(bs, 1 H), 7.14 (d, 2H), 7.28 (m,
3H), 7.50 (m,
4H). LRMS: m/z (ES+) 404 [MHO]
'H-NMR (CDC13, 400MHz) b: 3.04 (m,
3H),
3.26 (m, 2H), 3.54 (m, 1 H), 3.76
(m, 1 H),
123 ~ / 78 4.00 (m, 1 H), 4.15 (t, 2H), 4.44
(m, 1 H), 6.06
CI (bs, 1 H), 6.75 (s, 1 H), 7.14 (d,
2H), 7.19 (dd,
1 H), 7.24 (s, 1 H), 7.40 (m, 3H),
7.59 (d, 1 H).
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
122
Prep R Yield Data
(%)
'H-NMR (CDC13, 400MHz) 8: 3.06 (m,
3H),
F C 3.25 (m, 2H), 3.54 (m, 1 H), 3.77
3 (m, 1 H),
4.01 (m, 1 H), 4.15 (t, 2H), 4.44
~ (m, 1 H), 5.83
124 / 61
(bs, 1 H), 6.77 (s, 1 H), 7.17 (d,
2H), 7.24 (s,
1 H), 7.55 (d, 2H), 7.66 (m, 4H).
LRMS: m/z
(ES+) 452 [MNa~]
'H-NMR (CDCI3, 400MHz) 8: 3.05 (m,
3H),
3.26 (m, 2H), 3.55 (m, 1 H), 3.77
(m, 1 H),
4.00 (m, 1 H), 4.17 (t, 2H), 4.45
(m, 1 H), 5.96
CF3
(bs, 1 H), 6.74 (s, 1 H), 7.09 (d,
2H), 7.20-7.34
125 I 66
/ (m, 4H), 7.42 (dd, 1 H), 7.57 (dd,
1 H), 7.75 (d,
1 H). Microanalysis found: C, 62.77;
H, 5.21;
N, 9.46. C23H22N3O2F3:O.5H2O requires
C,
63.01; H, 5.29; N, 9.58%.
'H-NMR (CDC13, 400MHz) 8: 3.02 (m,
3H),
3.22 (m, 2H), 3.50 (m, 1 H), 3.74
(m, 1 H),
( 400 (m, 1 H), 4.14 (t, 2H), 4.42
(dd, 1 H), 5.95
126 / 56
(s, 1 H), 6.74 (s, 1 H), 7.08 (d,
2H), 7.19-7.35
(m, 5H), 7.42 (s, 1 H). LRMS: m/z
(ES+) 430,
432 [MHO]
' H-NMR (CDC13, 400MHz) 8: 1.25
(d, 6H),
2.90 (m, 1 H), 3.05 (m, 3H), 3.22
(m, 2H),
3.44 (m, 1 H), 3.75 (m, 4H), 4.00
(m, 1 H),
127 ~ ( 70 4.15 (t, 2H), 4.44 (m, 1 H), 5.86
(s, 1 H), 6.78
(s, 1 H), 6.92 (d, 1 H), 7.15 (m,
4H), 7.35 (s,
H3C CH3 1 H), 7.44 (d, 2H). LRMS: m/z (ES+)
456
[MNa~]
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
123
Prep R Yield Data
(%)
'H-NMR (CDC13, 400MHz) 8: 3.02 (m,
3H),
3.22 (m, 2H), 3.47 (m, 1 H), 3.70
(m, 1 H),
ci
3.98 (m, 1 H), 4.09 (t, 2H), 4.42
(dd, 1 H), 5.82
128
/ (s, 1 H), 6.74 (s, 1 H), 7.10 (d,
2H), 7.22 (s,
1 H), 7.38 (d, 2H), 7.44 (m, 4H).
HRMS: m/z
396.147 [MHO]
'H-NMR (CDC13, 400MHz) S: 2.35 (2xs,
6H),
H3C ~ 3.06 (m, 3H), 3.30 (m, 2H), 3.55
(m, 1 H),
3.78 (m, 1 H), 4.02 (m, 1 H), 4.17
~ (t, 2H), 4.46
129 / 36
H3C ,(dd, 1 H), 5.83 (bs, 1 H), 6.78
(s, 1 H), 7.15 (d,
2H), 7.21 (d, 1 H), 7.30 (m, 3H),
7.53 (d, 2H).
LRMS: m/z (ES+) 391 [MH*J
'H-NMR (CDC13, 400MHz) 8: 2.20 (s,
3H),
3.02 (m, 3H), 3.22 (m, 2H), 3.50
(m, 1 H),
3.75 (m, 1 H), 4.00 (m, 1 H), 4.10
(m, 2H),
130 / 62
H3c 4,42 (m, 1 H), 5.81 (s, 1 H), 6.72
(m, 2H),
7.02-7.25 (m, 7H). LRMS: m/z (ES+)
395
[MHO]
'H-NMR (CDC13, 400MHz) 8: 1.04 (t,
3H),
2.55 (q, 2H), 3.01 (m, 3H), 3.22
(m, 2H), 3.48
CH3 (m, 1 H), 3.72 (m, 1 H), 3.98 (m,
1 H), 4.10 (t,
2H), 4.42 (dd, 1 H), 6.10 (s, 1
H), 6.74 (s, 1 H),
131 / 52
( 7.06 (d, 2H), 7.10 (d, 1 H), 7.19
(m, 3H), 7.25
(m, 3H). Microanalysis found: C,
70.93; H,
7.15; N, 10.39. C24H2~N302;H20 requires
C,
70.74; H, 7.17; N, 10.31 %.
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
124
Pre~~aration 132
(2RS -2-((1-[(2EZ~-3-Bromo-2-proaenyll-1 H imidazol-4-yl meths)-3-morpholinone
Br
~N N
/ NH
O
A mixture of ammonium cerium (IV) nitrate (2.6g, 4.75mmol) and the compound of
Preparation 67 (1 g, 2.38mmol) in acetonitrile (1 Oml) and water (5ml) was
stirred at
40°C for 18 hours. TLC analysis showed starting material remaining, so
additional
ammonium cerium (IV) nitrate (650mg, 1.l9mmol) was added and the mixture was
stirred for a further 3 hours at 40°C. The mixture was concentrated
under reduced
pressure and azeotroped with methanol. The crude product was pre-adsorbed onto
silica gel and purified twice by column chromatography on silica gel using an
elution
gradient of dichloromethane:methano1:0.88 ammonia (99:1:0.1 to 95:5:0.5) to
afford
the title compound, 370mg. 'H-NMR (CDCI3,400MHz) (mixture of geometric
isomers) 8: 3.00 (m, 1 H), 3.23 (m, 2H), 3.50 (m, 1 H), 3.74 (m, 1 H), 4.00
(m, 1 H),
4.42 (m, 2H), 4.62 (d, 1 H), 5.98 (bs, 1 H), 6.26 (m, 1.5H), 6.42 (d, 0.5H),
6.75 (2xs,
1 H), 7.40 (2xs, 1 H). LRMS: m/z (ES+) 300, 302 [MHO]
Preparation 133
(-~2S)-2-((1-(2-Cyclohexylethyl)-1 H imidazol-4-yllmethyl)-3-morpholinone
~N O
~N
/ NH
O
Ammonium cerium (IV) nitrate (482mg, 0.88mmol) and water (1 ml) were added to
a
solution of the compound of Preparation 60 (181 mg, 0.44mmol) in acetonitrile
(1 ml)
and the mixture was stirred at 40°C for 18 hours. TLC analysis showed
starting
material remaining, so additional ammonium cerium (IV) nitrate was added
(250mg,
0.46mmol) and the mixture was stirred at 40°C for a further 5 hours.
The mixture
was partitioned between dichloromethane (75m1) and a solution of
ethylenediamine-
tetraacetic acid (1 g) in aqueous sodium bicarbonate (30m1), and the phases
were
separated. The organic layer was dried (MgS04) and evaporated under reduced
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
125
pressure. The crude product was purified by column chromatography on silica
gel
using an elution gradient of dichloromethane:methano1:0.88 ammonia (100:0:0 to
94:6:0.6) to afford the title compound as a sticky gum, 80mg. 'H-NMR (CDCI3,
400MHz) 8: 0.97 (m, 2H), 1.20 (m, 4H), 1.63 (m, 7H), 3.02 (dd, 1 H), 3.26 (m,
2H),
3.56 (m, 1 H), 3.78 (m, 1 H), 3.86 (t, 2H), 4.02 (m, 1 H), 4.45 (m, 1 H), 5.83
(bs, 1 H),
6.76 (s, 1 H), 7.38 (s, 1 H). LRMS: mlz (TSP+) 292.1 [MHO]. [a]o = -60.01, (c
= 0.05,
methanol)
Preparation 134
~2RS)-2-(f1-f(2E~-3-f1.1'-Biphenyll-4-yl-2-propenyll-lHimidazol-4-~,)meth~L
morpholinone
O
\ ~N NH
N,
0
A mixture of the bromo compound of Preparation 132 (185mg, 0.62mmol), 4-
biphenylboronic acid (183mg, 0.925mmol),
tetrakis(triphenylphosphine)palladium(0)
(72mg, 0.062mmol) and sodium carbonate (78mg, 0.74mmol) in water (3ml) and
dioxan (6ml) was heated at 100°C for 3 hours, then cooled and
partitioned between
water (20m1) and ethyl acetate (20m1). The layers were separated and the
aqueous
phase was extracted with ethyl acetate (l0ml). The combined organic extracts
were
dried (MgS04) and evaporated under reduced pressure. The crude product was
purified by column chromatography on silica gel using an elution gradient of
dichloromethane:methano1:0.88 ammonia (99:1:0.1 to 98:2:0.2) to afford the
title
compound as a white foam, 100mg. 'H-NMR (CDCI3, 400MHz) (mixture of
geometric isomers) b: 3.00 (m, 1 H), 3.22 (m, 2H), 3.54 (m, 1 H), 3.75 (m, 1
H), 4.00
(m, 1 H), 4.44 (m, 1 H), 4.62 (m, 1 H), 4.78 (m, 1 H), 5.80, 6.26 (2xm, 1 H),
5.93 (bs,
1 H), 6.54, 6.66-6.80 (2xm, 2H), 7.23-7.60 (m, 1 OH). LRMS: m/z (ES+) 374
[MH~]
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
126
Preparations 135 to 137
The following compounds of general structure
o
-N ~ H
R
O
were prepared from the bromide of Preparation 132 and the appropriate boronic
acid, following similar procedures to that described in Preparation 134.
Prep R YieldData
(%)
'H-NMR (CDC13, 400MHz) (mixture of
geometric isomers) 8: 3.00 (m, 1
H), 3.20-
36 3.35 (m, 2H), 3.50 (m, 1 H), 3.62
(m, 1 H),
135 I ~ v ~' white3.98 (m, 1 H), 4.42 (m, 1 H), 4.62
(m, 1 H),
foam 4.78 (m, 1 H), 5.75 (bs, 1 H), 5.82,
6.30 (2xm,
1 H), 6.57, 6.78 (2xm, 2H), 7.18-7.58
(m,
10H). LRMS: m/z (ES+) 374 [MH+]
'H-NMR (CDC13, 400MHz) (mixture of
geometric isomers) b: 2.98 (dd, 1
H), 3.22 (m,
41 2H), 3.50 (m, 1 H), 3.74 (m, 1 H),
4.00 (m,
136' ~ \ ~ white1 H), 4.42 (m, 1 H), 4.54 (m, 2H),
5.66, 6.18
foam (2xm, 1 H), 5.82 (bs, 1 H), 6.50-6.72
(m, 2H),
7.22-7.42 (m, 10H). LRMS: m/z (ESA
396
[MNa~]
'H-NMR (CDC13, 400MHz) (mixture of
geometric isomers) S: 3.00 (m, 1
H), 3.20-
\ \ 51 3.35 (m, 2H), 3.50 (m, 1 H), 3.75
(m, 1 H),
137 ~ / / white4.00 (m, 1 H), 4.44 (m, 1 H), 4.64
(m, 1 H),
foam 4.80 (m, 1 H), 5.78 (bs, 1 H), 5.84,
6.38 (2xm,
1 H), 6.62-6.85 (m, 2H), 7.30-7.82
(m, 8H).
LRMS: m/z (ES+) 348 (MHO]
1 = isolated without column chromatography
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
127
Preparation 138
(2RS)-2-( 1-f(2E~-3-(4-Bromophenvl)-2-propenyll-lHimidazol-4-yl)methyl)-3-
morpholinone
Br
O
\ ~N NH
N ,
0
The title compound was obtained in 42% yield from the compound of Preparation
67
and 4-bromobenzeneboronic acid following the procedure described in
Preparation
134. 'H-NMR (CDCI3, 400MHz) (mixture of geometric isomers) S: 3.00 (m, 1 H),
3.25
(m, 2H), 3.54 (m, 1 H), 3.76 (m, 1 H), 4.00 (m, 1 H), 4.43 (m, 1 H), 4.59-4.79
(m, 2H),
5.80, 6.22 (2xm, 2H), 6.40, 6.62 (2xm, 1 H), 6.72, 6.78 (2xs, 1 H), 7.06 (d, 1
H), 7.19
(d, 1 H), 7.38-7.58 (m, 3H). LRMS: m/z (TSP+) 376.1, 378.1 [MHO]
Preparation 139
~2RS)-2-(~(1-f(2EZ)-3-(4'-Methyl~l ,1'-biphen I~yl)-2-propenyll-1 H imidazol-4-
yl~methyl)-3-morpholinone
H3C
O
-N ~ H
O
A mixture of the bromo compound of Preparation 138 (132mg, 0.35mmol), 4-
methylbenzeneboronic acid (72mg, 0.53mmol), tetrakis(triphenylphosphine)-
palladium(0) (50mg, 0.04mmol) and sodium carbonate (270w1, 2M, 0.53mmol) in
dioxan (6ml) was heated at 100°C for 1.5 hours. The cooled reaction
mixture was
partitioned between water (20m1) and ethyl acetate (20m1) and the layers were
separated. The aqueous phase was extracted with ethyl acetate (10m1) and the
combined organic extracts were dried (MgS04) and evaporated under reduced
pressure. The residual yellow oil was purified by column chromatography on
silica
gel using an elution gradient of dichloromethane:methano1:0.88 ammonia
(99:1:0.1
to 98:2:0.2) to afford the title compound as a white foam, 77mg. 'H-NMR
(CDCI3,
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
128
400MHz) (mixture of geometric isomers) 8: 2.38 (2xs, 3H), 3.00 (m, 1 H), 3.25
(m,
2H), 3.54 (m, 1 H), 3.75 (m, 1 H), 4.00 (m, 1 H), 4.43 (m, 1 H), 4.62 (m, 1
H), 4.78 (m,
1 H), 5.78, 6.28 (2xm, 2H), 6.55, 6.68-6.80 (2xm, 2H), 7.22 (m, 3H), 7.38-7.63
(m,
6H). LRMS: m/z (ES+) 388 [MH~]
Preparation 140
(2RS)-2-((1-[(2E~-3-(4'-Chlorofl.l'-biphenyll-4-yl~-2-propenyll-1 H imidazol-4-
methyl)-3-morpholinone
CI
O
\ ~N NH
N,
0
A mixture of the bromo compound of Preparation 138 (100mg, 0.27mmol), 4-
chlorobenzeneboronic acid (63mg, 0.4mmol), tetrakis(triphenylphosphine)-
palladium(0) (31 mg, 0.027mmol) and sodium carbonate solution (400p1, 2M,
0.79mmol) in ethanol (1 ml) and toluene (4ml) was heated at 100°C for 3
hours. TLC
analysis showed starting material remaining, so dioxan (3ml), additional 4-
chlorobenzeneboronic acid (21 mg, 0.13mmol) and tetrakis(triphenylphosphine)-
palladium(0) (l5mg, 0.013mmol) were added, and the mixture was stirred at
100°C
for a further 6 hours. The cooled reaction mixture was partitioned between
water
(l0ml) and ethyl acetate (20m1) and the layers were separated. The aqueous
phase
was extracted with ethyl acetate (l0ml) and the combined organic extracts were
dried (MgS04) and evaporated under reduced pressure. The crude product was
purified by column chromatography on silica gel using
dichloromethane:methano1:0.88 ammonia (98:2:0.2) as eluant to afford the title
compound as a white solid, 60mg. 'H-NMR (CDC13, 400MHz) (mixture of geometric
isomers) 8: 3.02 (m, 1 H), 3.27 (m, 2H), 3.57 (m, 1 H), 3.78 (m, 1 H), 4.02 (m
1 H), 4.47
(m, 1 H), 4.65, 4.79 (2xd, 2H), 5.82, 6.32 (m, 1 H), 6.18 (bs, 1 H), 6.57,
6.82 (2xm, 2H),
7.30 (d, 1 H), 7.39-7.79 (m, 8H). LRMS: m/z (ES+) 430 [MNa~]
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
129
Preparation 141
(2RS)-2-(d1-f(2E~2'.5'-Difluoro[1.1'-bi~henyll-4-yIZ-2-proaenyll-1H imidazol-4-
vl)methyl)-3-morpholinone
F
F
i
O
-N ~ H
N,
0
The title compound was obtained from the compound of Preparation 138 and 2,5-
difluorobenzeneboronic acid following the procedure described in Preparation
140.
' H-NMR (CDC13, 400MHz) (mixture of geometric isomers) 8: 3.02 (m, 1 H), 3.28
(m,
2H), 3.57 (m, 1 H), 3.78 (m, 1 H), 4.00 (m, 1 H), 4.45 (m, 1 H), 4.63 (d, 1
H), 4.79 (d,
1 H), 5.82, 6.36 (m, 1 H), 6.14 (bs, 1 H), 6.58, 6.79 (2xm, 2H), 7.00 (m, 1
H), 7.14 (m,
2H), 7.32 (d, 1 H), 7.40-7.70 (m, 4H). LRMS: m/z (ES+) 432 [MNa+]
Preparation 142
tent Butyl (2RS~-2-f2-(dimethylamino)ethoxyl-3-(1-propel-1 H imidazol-4-
yl)propanoate
H3C~ CH3
CH3
CH3
A mixture of the alkene of Preparation 44 (650mg, 2.01 mmol) and 10% palladium
on
charcoal (Degussa~ 101 ) (60mg) in ethanol (20m1) was hydrogenated at
50°C and
60 psi (410kPa) for 18 hours. The cooled mixture was filtered through Arbocel~
and
the filtrate was evaporated under reduced pressure. The residue was purified
by
column chromatography on silica gel using an elution gradient of ethyl
acetate:diethylamine:methanol (100:0:0 to 97:1.5:1.5) to afford the title
compound,
502mg. 'H-NMR (CDC13, 400MHz) 8: 0.92 (t, 3H), 1.42 (s, 9H), 1.77 (m, 2H),
2.21
(s, 6H), 2.48 (t, 2H), 2.90-3.03 (m, 2H), 3.42 (m, 1 H), 3.70 (m, 1 H), 3.80
(t, 2H), 4.08
(m, 1 H), 6.79 (s, 1 H), 7.37 (s, 1 H). LRMS: m/z (TSP+) 326.2 [MHO]
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
130
Preparation 143
tert-But ly. (3 -3-((1 RS)-2-tert-butoxy-2-oxo-1-f(1-propel-1 H imidazol-4-
yl)methyllethoxy~pyrrolidinecarboxylate
O
O CH3
N y--CH3
H3 ~C
H3C~ ~N O
~N / O CH3
a ~ ~CH3
O CH3
A mixture of the alkene of Preparation 45 (1.198, 2.83mmol) and
Degussa° 101
catalyst (120mg) in ethanol (l2ml) was hydrogenated at 50°C and 60 psi
(410kPa)
for 18 hours. TLC analysis showed starting material remaining, so additional
catalyst (120mg) was added and the mixture was hydrogenated at 50°C and
60 psi
(410kPa) for a further 48 hours. The mixture was filtered through Arbocel~,
the
catalyst was washed with ethanol, and the combined filtrates were evaporated
under
reduced pressure. The residual oil was purified by column chromatography on
silica
gel using ethyl acetate as the eluant to afford the title compound as a
colourless oil,
227mg. 'H-NMR (CDCI3, 400MHz) 8: 0.92 (t, 3H), 1.42 (m, 18H), 1.79 (m, 2H),
2.00-2.30 (m, 2H), 2.80-2.95 (m, 1 H), 3.00-3.48 (m, 5H), 3.84 (t, 2H), 4.05-
4.20 (m,
2H), 6.75 (m, 1 H), 7.50 (m, 1 H). LRMS: m/z (ES+) 424 [MH+]
Preparation 144
(2RS)-2-(f 1-(3-f 1,1'-Biphenvll-4-ylpropyl)-1 H imidazol-4- 11~ methyl_)-3-
morpholinone
O
\ ~N NH
N
O
A mixture of the alkene of Preparation 134 (100mg, 0.268mmol) and Degussa~ 101
catalyst (l5mg) in ethanol (l2ml) was hydrogenated at 50°C and 60 psi
(410kPa) for
6 hours. TLC analysis showed starting material remaining, so additional
Degussa~
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
131
101 catalyst (20mg) was added and the mixture was hydrogenated for a further
18
hours. The reaction mixture was filtered through Arbocel~, the catalyst was
washed
with ethanol, and the combined filtrates were evaporated under reduced
pressure.
The residue was purified by column chromatography on silica gel using an
elution
gradient of dichloromethane:methano1:0.88 ammonia (99:1:0.1 to 98:2:0.2) to
afford
the title compound as a colourless oil, 70mg. 'H-NMR (CDCI3, 400MHz) 8: 2.10
(m,
2H), 2.61 (t, 2H), 3.02 (dd, 1 H), 3.23 (m, 2H), 3.49 (m, 1 H), 3.74 (m, 1 H),
3.86 (t,
2H), 4.00 (m, 1 H), 4.42 (m, 1 H), 6.20 (bs, 1 H), 7.20 (d, 2H), 7.29 (m, 1
H), 7.39 (m,
4H), 7.50 (d, 2H), 7.54 (d, 2H). LRMS: m/z (ES+) 398 [MNa~]
Preparations 145 to 150
The following compounds of general structure
O
-N ~ H
R
O
were prepared from the appropriate alkenes, following similar procedures to
that
described in Preparation 144.
Prep R Yield Data
(%)
'H-NMR (CDC13, 400MHz) 8: 2.17 (m, 2H),
2.68 (t, 2H), 3.02 (m, 1 H), 3.24 (m, 2H), 3.50
145' 70 (m, 1 H), 3.74 (m, 1 H), 3.90 (t, 2H), 4.00 (m,
/ 1 H), 4.43 (m, 1 H), 6.50 (bs, 1 H), 6.78 (s, 1 H),
\ ~ 7.16 (d, 1 H), 7.32-7.48 (m, 7H), 7.58 (d, 2H).
LRMS: m/z (ES+) 377 [MH~]
'H-NMR (CDC13, 400MHz) 8: 1.82 (m, 2H),
2.58 (t, 2H), 2.97 (dd, 1 H), 3.22 (m, 2H), 3.50
146 \ \ 71 (m, 1 H), 3.60-3.77 (m, 3H), 3.99 (m, 1 H),
4.40 (m, 1 H), 5.82 (bs, 1 H), 6.55 (s, 1 H),
7.20 (m, 7H), 7.38 (m, 3H). LRMS: m/z
(ES+) 398 [MHO]
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
132
Prep R Yield Data
(%)
'H-NMR (CDC13, 400MHz) S: 2.10 (m,
2H),
2.38 (s, 3H), 2.61 (t, 2H), 3.02
(dd, 1 H), 3.23
(m, 2H), 3.50 (m, 1 H), 3.75 (m,
1 H), 3.83 (t,
147 65 2H), 4.00 (m, 1 H), 4.42 (m, 1 H),
5.82 (bs,
1 H), 6.77 (s, 1 H), 7.19 (m, 4H),
7.38 (s, 1 H),
7.45 (m, 4H). LRMS: m/z (ES+) 412
[MNa~]
' H-NMR (CDC13, 400MHz) 8: 2.10 (m,
2H),
2.60 (t, 2H), 3.02 (dd, 1 H), 3.25
(m, 2H), 3.50
(m, 1 H), 3.74 (m, 1 H), 3.85 (t,
2H), 4.00 (m,
148 57 1 H), 4.42 (m, 1 H), 6.05 (bs, 1
H), 6.77 (s, 1 H),
7.19 (d, 2H), 7.38 (m, 3H), 7.45
(m, 4H).
LRMS: m/z (ES+) 432 [MNa~]
'H-NMR (CDCI3, 400MHz) 8: 2.08 (m,
2H),
2.60 (t, 2H), 3.02 (dd, 1 H), 3.23
(m, 2H), 3.50
F (m, 1 H), 3.74 (m, 1 H), 3.85 (t,
2H), 4.00 (m,
149 76 1 H), 4.42 (m, 1 H), 6.00 (bs, 1
H), 6.75 (s, 1 H),
6.96 (m, 1 H), 7.05 (m, 2H), 7.20
(d, 2H), 7.38
(s, 1 H), 7.42 (d, 2H). LRMS: m/z
(ES+) 434
F
[MNa~]
'H-NMR (CDCI3, 400MHz) 8: 2.20 (m,
2H),
2.78 (t, 2H), 3.06 (dd, 1 H), 3.30
(m, 2H), 3.54
(m, 1 H), 3.75 (m, 1 H), 3.90 (t,
2H), 4.03 (m,
150 ~ 70 1 H), 4.48 (m, 1 H), 5.89 (bs, 1
H), 6.78 (s, 1 H),
7.28 (d, 1 H), 7.39 (s, 1 H), 7.45
(m, 2H), 7.59
(s, 1 H), 7.80 (m, 3H). LRMS: m/z
(ES+) 372
[MNa~]
1 = isolated without column chromatography
Preparation 151
(2S.6~-4-(4-MethoxybenzLrl)-6-methyl-2-(f 1-(2-pyridinyl)-1 H imidazol-4-
yllmethyl)-3-
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
133
morpholinone
CH3
N ~N O ~ O~CH3
~ N _~
/ N
O
A mixture of the imidazole of Preparation 55 (566mg, 1.8mmol), copper (I)
oxide
(20mg, 0.14mmol) and potassium carbonate (372mg, 2.7mmol) in 2-bromopyridine
(1 ml) was heated at 100°C for 18 hours. The cooled mixture was
purified by
Biotage~ column chromatography on silica gel using an elution gradient of
toluene:diethylamine (93:7 to 86:14) to afford the title compound as a foam,
482mg.
'H-NMR (CDCI3, 400MHz) (5:1 mixture of regioisomers) 8: 0.94, 1.20 (2xd, 3H),
2.83-
3.23 (m, 4H), 3.63, 3.78 (2xs, 3H), 3.97 (m, 1 H), 4.16, 4.22 (2xd, 1 H), 4.50-
4.82 (m,
2H), 6.64, 6.82 (2xd, 2H), 7.00, 7.18 (2xd, 2H), 7.35, 7.44 (2xm, 1 H), 7.59
(m, 2H),
7.96 (m, 1 H), 8.40-8.57 (m, 2H). HRMS: m/z (ES+) 393.1926 [MHO]
Preparation 152
(2S,6f~-6-Methyl-2-(f 1-(2-pyridinyl)-1 H imidazol-4-yllmethy~-3-morpholinone
CH3
N ~N O
N
NH
O
A mixture of the protected morpholinone of Preparation 151 (454mg, 1.l6mmol)
and
ammonium cerium (IV) nitrate (1.585g, 2.9mmol) in water (8ml) and acetonitrile
(l6ml) was heated at 40°C for 6 hours. The cooled mixture was diluted
with
methanol (100m1), and the solution was adsorbed onto silica gel. The product
was
isolated by column chromatography on silica gel using
dichloromethane:methano1:0.88 ammonia (95:5:1 ) as eluant and further purified
by
Biotage° column chromatography on silica gel using toluene:diethylamine
(92:8) then
dichloromethane:methano1:0.88 ammonia (95:5:1 ) as eluant to afford the title
compound, 204mg. 'H-NMR (CD30D, 400MHz) (7:1 mixture of regioisomers)
S: 1.01, 1.21 (2xd, 3H), 2.92-336 (m, 4H), 3.78, 3.93 (2xm, 1 H), 4.27, 4.46
(2xm, 1 H),
7.37, 7.45 (2xm, 1 H), 7.58-7.70 (m, 2H), 7.96, 8.00 (2xm, 1 H), 8.40-8.18 (m,
2H).
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
134
Preparation 153
(6R~-2~Hydrox~r(1-propel-1 H imidazol-4-yl meth I~-4-(4-methox by enz~)-6-
methyl-3-
morpholinone
CH3
.H3C~N/~N O / O~CH3
i N \
OH O
A solution of the compound of Preparation 12 (6.81 g, 29.Ommol) in
tetrahydrofuran
was added dropwise to a solution of lithium diisopropylamide (23.2m1, 1.5M in
cyclohexanes, 34.8mmol) at -78°C, and the solution was stirred for a
further 20
minutes at -78°C. A solution of the aldehyde of Preparation 1 (4g,
29.Ommol) in
tetrahydrofuran (80m1 total volume) was then added dropwise, and the mixture
was
allowed to warm slowly to room temperature. Saturated ammonium chloride
solution (50m1) was added, followed by water (100m1), and the mixture was
extracted
with ethyl acetate. The combined organic extracts were dried (MgSOa) and
concentrated under reduced pressure. The residual orange oil was purified by
column chromatography on silica gel using an elution gradient of ethyl
acetate:methanol (98:2 to 95:5) to afford the title compound as an orange oil,
5.71 g.
'H-NMR (CDCI3, 400MHz) (mixture of diastereoisomers) 8: 0.92 (m, 3H), 1.14 (m,
3H), 1.58 (m, 2H), 2.96-3.18 (m, 2H), 3.78-4.00 (m, 6H), 4.22-4.76 (m, 3H),
5.06-
5.30 (m, 1 H), 6.81-6.95 (m, 3H), 7.18 (m, 2H), 7.42 (d, 1 H). LRMS: m/z (ES+)
374
[MHO]
Preparation 154
(2EZ,6f~-4-(4-Methoxybenzyl)-6-methyl-2-f(1 H imidazol-4-~)methylidenel-3-
morpholinone
CH3
H
N O / O~CH3
/ N \
N -''h/ ~ ~/
O
A mixture of the compound of Preparation 37 (91 g, 164mmol) and water (90m1)
in
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
135
glacial acetic acid (900m1) was heated at 40°C for 1 hour. The cooled
mixture was
concentrated under reduced pressure, diluted with water (400m1) and the
resulting
precipitate filtered off. The filtrate was washed with ether (2x400m1), then
neutralised using sodium bicarbonate and extracted with ethyl acetate
(1000m1).
This organic solution washed with water, dried (Na2S04) and evaporated under
reduced pressure to afford the title compound as a gum, 46.4g. 'H-NMR (CDC13,
400MHz) S: 1.41 (d, 3H), 3.24 (dd, 1 H), 3.38 (dd, 1 H), 3.80 (s, 3H), 4.34
(m, 1 H),
4.58 (d, 1 H), 4.68 (d, 1 H), 6.84 (d, 2H), 6.97 (s, 1 H), 7.20 (d, 2H), 7.30
(s, 1 H).
Preparation 155
(2EZ,6F~-4-(4-Methoxybenzvl)-6-methyl-2-f(1-propel-1H imidazol-4-~
methylidenel-
3-morpholinone
CH3
HsC~N~N O / O~CHs
i / N \
O
Triethylamine (3m1, 21.75mmol) was added dropwise to a solution of the alcohol
of
Preparation 153 (5.41 g, 14.50mmol) in dichloromethane (60m1), and the
solution was
cooled to 0°C. Methanesulphonyl chloride (1.68m1, 21.75mmol) was added,
and the
mixture was allowed to warm to room temperature, then stirred for a further 2
hours.
Additional triethylamine (2m1, 14.50mmol) was added, and the mixture was
warmed
to 35°C, then stirred for 18 hours. The solution was washed with water
(100m1),
sodium bicarbonate solution (100m1) and brine (50m1), then dried (MgS04) and
concentrated under reduced pressure. The residue was purified by column
chromatography on silica gel using an elution gradient of
dichloromethane:methano1:0.88 ammonia (99:1:0.1 to 98:2:0.2) to afford one
isomer
of the title compound as an orange oil, 1.8g, and the second isomer, 260mg. 1
H-
NMR (CDC13, 400MHz, major isomer) 8: 0.96 (t, 3H), 1.38 (d, 3H), 1.80 (m, 2H),
3.20
(dd, 1 H), 3.32 (dd, 1 H), 3.78 (s, 3H), 3.86 (t, 2H), 4.25 (m, 1 H), 4.57 (d,
1 H), 4.65 (d,
1 H), 6.84 (d, 2H), 7.02 (s, 1 H), 7.20 (d, 2H), 7.35 (s, 1 H), 7.46 (s, 1 H).
LRMS: m/z
(ES+) 356 [MH~]. Microanalysis found: C, 63.99; H, 6.88; N, 11.00
C2oH25N303;H20
requires C, 64.32; H, 7.29; N, 11.25%.
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
136
Preparation 156
(2S,6R)-4-(4-Methoxybenzy~-6-meth~j(1-propel-1 H imidazol-4-yl)meth I
morpholinone
CH3
HsC~N~N O / O~CHs
N \
O
A mixture of the alkene of Preparation 155 (1.8g, 5.07mmol) and 10% palladium
on
charcoal (Degussa type 101 ) (200mg) in ethanol (50m1) was hydrogenated at 60
psi
(410kPa) and 50°C for 18 hours. TLC analysis showed starting material
remaining.
The mixture was filtered, the filtrate was evaporated under reduced pressure,
and
the residue was re-dissolved in ethanol (50m1). 10% Palladium on charcoal
(Degussa type 101 ) (200mg) was added and the mixture was hydrogenated at
60psi
(410kPa) and 50°C for 18 hours, then filtered. The filtrate was
evaporated under
reduced pressure and the residue was purified by column chromatography on
silica
gel using dichloromethane:methano1:0.88 ammonia (98:2:0.2) to afford the title
compound as a colourless oil, 1.35g. 'H-NMR (CDC13, 400MHz) S: 0.92 (t, 3H),
1.19
(d, 3H), 1.78 (m, 2H), 2.98-3.16 (m, 3H), 3.58 (dd, 1 H), 3.82 (m, 6H), 4.50
(m, 3H),
6.75 (s, 1 H), 6.82 (d, 2H), 7.18 (d, 2H), 7.58 (s, 1 H). LRMS: m/z (ES+) 358
[MHO].
Microanalysis found: C, 62.12; H, 7.58; N, 10.89 C2oH2~N303;1.5H20 requires C,
62.48; H, 7.86; N, 10.93%.
Preparation 157
1;2S,6f~-6-Methyl-2-f(1-propel-1 H imidazol-4-yl)meth 11-y 3mo~~holinone
CH3
HaC~N/~N O
NH
O
A solution of the compound of Preparation 156 (1.2g, 3.36mmol) in methane-
sulphonic acid (5ml) was stirred at 70°C for 2 hours. The cooled
mixture was
washed with ether (2x20m1) by decantation. Water (20m1) was added and the
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
137
mixture was basified using 0.88 ammonia, then washed with ethyl acetate
(20m1).
The aqueous phase was evaporated under reduced pressure, the residue was
suspended in acetonitrile, and this mixture was heated to 50°C. The
acetonitrile
solution was separated by decantation and evaporated under reduced pressure to
give an oil. This was purified by column chromatography on silica gel using an
elution gradient of dichloromethane:methano1:0.88 ammonia (98:2:0.2 to
96:4:0.4) to
afford the title compound as a colourless oil, 560mg. 'H-NMR (CDCI3, 400MHz)
8:
0.94 (t, 3H), 1.22 (d, 3H), 1.59 (m, 2H), 3.04 (dd, 1 H), 3.18-3.37 (m, 3H),
3.85 (m,
3H), 4.42 (m, 1 H), 6.50 (s, 1 H), 6.79 (s, 1 H), 7.68 (s, 1 H). LRMS: m/z
(ES+) 238
[MH~]
Preparation 158
(2R.6R)-2-f(1 H Imidazol-4-yl)methyll-6-methyl-3-morpholinone
CH3
H
N
NH
N a
O
Ammonium cerium (IV) nitrate (1.1 g, 2mmol) was added to a solution of the
protected lactam of Preparation 55b (200mg, 0.63mmol) in water (4ml) and
acetonitrile (4ml) and the mixture was stirred at room temperature for 3
hours. The
solution was diluted with acetonitrile (5ml) and 0.88 ammonia (4ml), and the
mixture
was filtered through Arbocel~, washing through with a solution of
acetonitrile:water
(50:50, l0ml). The filtrate was concentrated under reduced pressure and the
aqueous residue was washed with ether, then evaporated under reduced pressure.
The crude product was purified by column chromatography on silica gel using an
elution gradient of dichloromethane:methano1:0.88 ammonia (95:5:0.25 to
92:8:0.4)
to afford the title compound as a foam, 88mg. 'H-NMR (D20, 400MHz) 8: 1.20 (d,
3H), 3.02-3.30 (m, 6H), 4.01 (m, 1 H), 4.40 (dd, 1 H), 6.86 (s, 1 H), 7.58 (s,
1 H).
The compounds of the present invention may be tested using the following
assay,
which is based on that disclosed in Boffa et aL, J. Biol. Chem. 1998, 273,
2127. The
compounds are incubated with activated TAFI and a standard substrate for
TAFIa,
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
138
the rate of hydrolysis of the substrate is determined and compared to the rate
of
hydrolysis in the absence of the compounds, and the amount of inhibition
expressed
in terms of K~.
Assay for TAFIa inhibition.
i) TAFI activation
Human TAFI (recombinant or purified) was activated by incubating 20w1 of stock
solution (360~g/ml) with 101 of human thrombin (10NIH units/ml), 10.1 of
rabbit
thrombomodulin (30wg/ml), 6p,1 calcium chloride (50mM) in 501 of 20mM HEPES (N-
[2-hydroxyethyl]piperazine-N-[2-ethanesulfonic acid]) buffer containing 150mM
sodium chloride and 0.01% TWEEN 80 (polyoxyethylene-sorbitan monooleate) pH
7.6 for 20 minutes at 22~C. At the end of the incubation period, thrombin was
neutralised by the addition of lOp.L of PPACK (D-Phe-Pro-Arg chloromethyl
ketone)
(100nM). The resulting TAFIa solution was stored on ice for 5 minutes and
finally
diluted with 1751 of HEPES buffer.
ii) K; Determination (TAFIa)
Calculated K;
A number of different dilutions of the test compound in water were made up. To
20w1 of each dilution was added 1501 of HEPES buffer and l0p.l of TAFIa, which
was then pre-incubated for 15 minutes at 24°C. To each dilution was
then added
20p.1 furylacryloyl-alanyl-lysine (FAAL) at a standard concentration.
Substrate
turnover was measured by reading the absorbance of the reaction mixture at
330nm
every 15 seconds for 30 minutes. The reaction was performed at 24°-C
and samples
were mixed for 3 seconds prior to each absorbance reading.
A graph of % inhibition against test compound concentration was then plotted;
from
which was calculated the IC5o value. The K; value was then calculated using
the
Cheng-Prusoff equation.
Two controls, positive and negative, were used to check the accuracy of the
results
in each case. For the first control, the assay was performed as above, but
with 201
of water rather than a dilution of the test compound. This showed minimal
inhibition.
CA 02472238 2004-06-30
WO 03/061652 PCT/IB03/00060
139
For the second control, the assay was performed as above, but with an
effective
amount of a non specific carboxypeptidase inhibitor rather than a dilution of
the test
compound. This showed maximal inhibition. When the two controls did not
demonstrate minimal and maximal inhibition respectively then the results were
discounted and the test compound was reanalysed.
Using the above assay the compounds of the Examples were found to be potent
and
selective inhibitors of TAFIa. All the compounds tested had a K; value less
than
20~.M. The specific K; values of certain compounds are detailed below:
Compound of Example: K; (TAFIa)
4 10 nM
5 10 nM
40 14 nM
49 9 nM
51 26 nM
The selectivity of the compounds of the present invention for TAFIa over CPN
was
determined by calculating the K; of the compounds of the present invention for
CPN,
then comparing it to the K; for TAFIa. The K; was calculated using the assay
for the
calculation of TAFIa K;, but substituting 101 of human CPN for l0p,l of TAFIa.
Those compounds of the present invention tested exhibited a strong selectivity
for
TAFIa over CPN of the order of >50:1. The specific K; values and calculated
selectivities of certain compounds are detailed below:
Compound of Example: K; (CPN) Selectivity
5 > 10 ~M > 1000
51 >10 wM >380