Note: Descriptions are shown in the official language in which they were submitted.
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
METHODS AND APPARATUS FOR PERFORMING SUBMICROLITER REACTIONS WITH
NUCLEIC ACIDS OR PROTEINS
STATEMENT REGARDING FEDERALLY SPONSORED RESEARCH
[0001] This invention was made with government support. The government has
certain
rights in the invention
CROSS-REFERENCE TO RELATED APPLICATIONS
[0002] This application claims priority from U.S, Provisional Application
Serial No.
l 0 601355,660, filed February 8, 2002; U,S, Provisional Application Serial
No. 601355,648, filed
February 8, 2002; and U.S. Application Serial No.101262,476, filed September
30, 2002.
FIELD OF THE INVENTION
[0003] This invention is in the field of biotechnology, and relates to methods
and
apparatus for preparing and performing small scale reactions that use nucleic
acid or
protein.
BACKGROUND OF THE INVENTION
[0004] The original goal of the federally-funded Human Genome Project had been
to
complete the sequence of the human genome at ten-fold coverage by the year
2005. With
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 2 -
dramatic acceleration in pace, a partial draft has recently been presented.
Rather than
decreasing the need for rapid, inexpensive DNA sequencing, however, this feat
has spurred
the need for rapid, inexpensive sequencing of nucleic acids. Completion of the
draft human
genome sequence has also spurred a need for methods and apparatus for
analyzing
s directly the complex collection of genome-encoded proteins, collectively
termed the
proteome.
[0005] With respect to DNA sequence needs, there is growing interest in
sequencing the
genomes of non-human organisms, including bacteria, plants and animals.
[0006] More importantly, the burgeoning fields of molecular pathology and
1 o pharmacogenomics will require the resequencing of multiple genes from
individual patients.
Molecular pathology relates to the diagnosis, and often formulation of a
prognosis, for
human diseases by identifying mutations in particular genes. Pharmacogenomics
refers to
understanding how allelic differences that exist in all human populations
affect the
therapeutic response, and susceptibility to side effects, of individuals to
drugs.
15 [0007] As the need to sequence genes from individual patients grows, so
will the demand
for point of care sequencing capability, There will need to be a shift from
large, centralized,
high throughput DNA sequencing facilities that only exist at well-funded
academic research
centers and genomics companies to small, less complicated, middle-throughput
gene
sequencing systems that can be installed in the majority of hospitals and
clinics. This shift
2 o in the market for DNA sequencing technologies will put a premium on
reducing the cost of
reagents and making the sample processing steps as simple and seamless as
possible.
[0008] In the late 1970s, Sanger et al. developed an enzymatic chain
termination method
for DNA sequence analysis that produces a nested set of DNA fragments with a
common
starting point and random terminations at every nucleotide throughout the
sequence. Lloyd
2 s Smith, Lee Hood, and others modified the Sanger method to use four
fluorescent labels in
sequencing reactions enabling single lane separations. This resulted in the
creation of the
first automated DNA sequencers, which used polyacrylamide slab gels for
separations.
More recently, fluorescent energy-transfer dyes have been used to make dye
sets that
enhance signals by 2- to 10-fold and simplify the optical configuration.
3 0 [0009] Automated fluorescent capillary array electrophoresis (CAE) DNA
sequencers
appear to be the consensus technology to replace slab gels. Capillary gel
electrophoresis
speeds up the separation of sequencing products and has the potential to
dramatically
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 3 -
decrease sample volume requirements. The 96-channel capillary electrophoresis
instrument, MegaBACET"", which is commercially available from Amersham
Biosciences,
Inc. (Sunnyvale, CA), uses a laser-induced fluorescence (LIF) confocal
fluorescence
scanner to detect up to an average of about 625 bases per capillary (Phred 20
window) in
90 minute runs with cycle times of two hours. Confocal spatial filtering
results in a higher
signal-to-noise ratio because superfluous reflections and fluorescence from
surrounding
materials are eliminated before signal detection at the photomultiplier tube
(PMT).
Accordingly, sensitivity at the level of subattomoles per sequencing band is
attainable.
Confocal imaging is also particularly important in microchip analysis systems
using capillary
1 o electrophoresis, where the background fluorescence of a glass or plastic
microchip may be
much higher than that of fused silica capillaries. Capillary array
electrophoresis systems will
solve many of the initial throughput needs of the genomic community for DNA
analysis.
However, present methods for low volume sample preparation still present a
significant
barrier to increased throughput and reduced cost.
1 s [0010] While fluorescent DNA sequencers are improving the throughput of
DNA sequence
acquisition, they have also moved the throughput bottleneck from sequence
acquisition
back towards sample preparation. In response, rapid methods for preparing
sequencing
templates and for transposon-facilitated DNA sequencing have been developed,
as have
magnetic bead capture methods that eliminate centrifugation. Thermophilic
Archae DNA
2 o polymerises have been screened and genetically engineered to improve
fidelity, ensure
stability at high temperatures, extend lengths, and alter affinities for
dideoxynucleotides and
fluorescent analogs. These improvements have resulted in lower reagent costs,
simpler
sample preparation, higher data accuracy, and increased read lengths.
[0011] The sequencing community has also developed higher throughput methods
for
2 s preparing DNA templates, polymerise chain reaction (PCR) reactions, and
DNA
sequencing reactions. Sample preparation has been increasingly multiplexed and
automated using 96- and 384-well microtiter, multi-channel pipettors, and
laboratory robotic
workstations. In general, these workstations mimic the manipulations that a
technician
would perform and have minimum working volumes of about a microliter, although
stand-
3 o alone multi-channel pipettors are being used to manipulate smaller
volumes.
[0012] A typical full-scale sample preparation method for DNA shotgun
sequencing on
capillary systems begins by lysing phage plaques or bacterial colonies to
isolate subcloned
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 4 -
DNA. Under some circumstances it may be desirable to PCR-amplify the subcloned
DNA
insert to exponentially increase its concentration in the sample. Next,
exonuclease I (Exol)
and arctic shrimp alkaline phosphatase (SAP) are added to perform an enzymatic
cleanup
reaction to remove primer and excess dNTPs that interfere with cycle
sequencing. Exol is
s used to degrade the single-stranded primers to dNMPs without digesting
double-stranded
products. SAP converts dNTPs to dNPs and reduces the dNTP concentration from
200 NM,
as used for the PCR reaction, to less than 0.1 pM for use with fluorescent
sequencing. The
reaction is performed at 37°C and then heated to 65°C
irreversibly denature the Exol and
SAP.
[0013] Because PCR amplification can produce excess template DNA for cycle
sequencing, the ExoIISAP treated PCR sample can be diluted five-fold before
cycle
sequencing. This reduces the concentration of contaminants into a range that
causes less
interference with capillary electrophoresis analysis. Cycle sequencing
reagents are added,
typically with fluorescently labeled dye primers or terminators and the
reaction is thermal
1 s cycled to drive linear amplification of labeled fragments. Finally, after
cycling, the samples
are post-processed, typically by ethanol precipitation or spin filtration,
resuspended in
formamide, another denaturant, or water, and the sample is electrokinetically
injected into
the capillary electrophoresis system.
[0014] This workflow has resulted in a dramatic improvement in the performance
of the
2 o MegaBACET"" system, and similar work flows currently appear to be the
methods of choice
for other capillary electrophoresis systems as well. Using actual samples from
single
plaques and colonies of human genomic random subclones or Expressed Sequence
Tags
(ESTs), this workflow with linear polyacrylamide as a separation matrix has
improved the
success rate of samples over 200 base pairs from about 60% to 85-90%, and has
improved
2 s the average read length from about 400 to greater than 600 bases.
Furthermore, this
method has proven to be quite robust.
[0015] While the above sample preparation methods have greatly increased
throughout,
the cost of reagents remains a major component of the cost of sequencing.
Capillary
electrophoresis requires only subattomoles of sample, but presently samples
are prepared
3 o in the picomole range. Reducing the reaction volume will therefore reduce
the cost of DNA
sequencing and still provide enough material for analysis. However,
substantial reductions
in reaction volume can only be achieved if satisfactory methods can be
developed for
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
manipulating and reacting samples and reagents. Ideally, such a method would
be
automated and configured to produce multiple samples at one time. Moreover, it
would be
desirable to integrate such a method as a module capable of interfacing with
additional
components, such as capillary electrophoresis and a detector for separation
and analysis.
s [0016] Several devices have been designed to aid in the automation of sample
preparation. For example, U.S. Pat. No. 5,720,923 describes a system in which
small
cycling reactions take place in tubes with diameters as small as 1 mm. The
tubes are
subsequently exposed to thermal cycles produced by thermal blocks to effect
the desired
reaction. Multiple samples may be processed in a single tube by drawing in
small amounts
i o of sample, each of which are separated in the tube by a liquid which will
not combine with
the sample. Fluid moves through the tubes by means of a pump. These features
are
incorporated into a system which automatically cleans the tubes, moves sample
trays
having sample containing wells, and brings the tubes into contact with the
wells in the
sample trays.
1 s (0017] U.S. Pat. No. 5,785,926 discloses a system for transporting small
volumes of
sample. In this system, at least one capillary tube is used to transport small
amounts of
sample. A precision linear actuator connected to a computer controlled motor
acts as a
pneumatic piston to aliquot and dispense liquid using the tube. The sample
amount is
monitored by an optical sensor that detects the presence of liquid within the
capillary
2 o segment. The system includes a fluid station containing liquids to be
deposited and a
positioning device for positioning the transport capillary.
(0018] U.S. Pat. No. 5,897,842 discloses a system for automated sample
preparation
using thermal cycling. In this system a reaction mixture is pumped into a
capillary tube.
One end of the tube is sealed using pressure from an associated pump while the
other end
2 s is sealed by pressing the tube against a barrier. The pump also serves to
move fluid within
the tube. Once the ends are sealed, the tube is exposed to thermal cycles. In
this system a
robotic transfer device moves the tubes between the sample preparation station
where the
pump loads the components of the reaction mixture into the tubes and the
thermal cycling
station.
3 0 (0019] In the systems discussed above, it is necessary to first mix
together a sample,
such as DNA template for sequencing, and reagents, prior to introducing the
mixture into a
reaction chamber. This intermediate mixing step inevitably requires additional
reagent and
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 6 -
sample handling steps that results in wastage. For example, if separate
micropipets are
used to dispense sample and reagent into a mixing chamber, small amounts of
sample and
reagent will be retained in the respective pipets, and reaction mixture will
be retained in the
mixing chamber. In a high throughput system the cost of this wastage and
providing new or
properly cleaned pipets and mixing chambers rapidly mounts. Extent of wastage
is often
exacerbated by the need to dispense relatively large volumes of liquids
containing reaction
components at low concentration as a strategy to compensate for inaccuracies
in
dispensing low volumes of higher concentration liquids. Usually, after the
reaction mixture
is formed, only a small proportion is required for analysis, and the remainder
is discarded.
i o [0020] Thus, there exists a need for means by which a biological sample to
be analyzed
could be introduced into a reaction chamber without the need to first mix the
sample with
the reagents necessary to effect the reaction.
[0021] U.S. Pat. No. 5,846,727 discloses affinity-capture methods wherein
template DNA
is immobilized inside a glass capillary tube that serves as a reaction chamber
for thermal
1 s cycling. The capillary is first prepared by immobilizing biotin molecules
to the inner surface
of the capillary, followed by charging the column with avidin or streptavidin
which binds
tightly the biotin. Template DNA to be sequenced is covalently linked to a
biotin moiety by
PCR, and is then exposed to the avidin inside the capillary. This results in
immobilization of
the template to the capillary wall through a biotin-avidin-biotin linkage.
After unbound
2 o template is washed away, sequencing reagent is added, and the contents of
the capillary
are subjected thermal cycling to activate the sequencing reaction. In this
manner it is
unnecessary to mix template DNA with sequencing reagent prior to loading the
capillary.
[0022] However, the method just described requires that biotin be linked to
the template
DNA by PCR, necessitating setting up and carrying out a reaction even before
the
2 s sequencing reaction. This requisite preliminary step adds to the time and
cost associated
with acquiring the sequence data. Furthermore, the immobilization of the DNA
is effectively
irreversible because the biotin-avidin linkage is so strong it can only be
broken using agents
that denature avidin, a treatment that would also denature any other protein
components in
a reaction. As a result the template DNA must stay bound to the inner surface
of the
3 o capillary. Because the DNA is not free in solution, additional time is
required for reaction
components to diffuse to the walls where they can interact with the DNA.
Furthermore,
when it is desired to recycle the capillary, it is necessary to remove the
template DNA via
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
denaturation of the avidin, washing and then recharging of the avidin in the
capillary, all of
which add to time and reagent costs.
[0023] Thus, there is continued need in the art for methods to introduce
molecules into
reaction chambers without an initial sample-reagent mixing step, without the
need to attach
s an affinity capture moiety to all the molecules in the sample, and wherein
template
immobilization is reversible. In this way reagent costs would be minimized and
processing
speed maximized.
[0024] Capillary array electrophoresis systems and capillary electrophoresis
microchip
analytical systems can detect subattomoles of DNA sequencing reaction
products. This
1 o extraordinary sensitivity comes at the cost of reduced tolerance, compared
to slab gels, for
deviations from the ideal amount of template DNA in the sequencing reactions.
For
example, if there is too little template DNA in the sequencing reaction, there
will be poor
yield of fluorescently labeled primer extension products. This results in weak
signal strength
when the reaction products are scanned by the laser. This prevents the
software that
1 s analyzes the chromatogram from adequately performing spectral separation,
resulting in
shorter than average sequence read lengths; the reaction will have to be
repeated or the
sequence information will be lost.
[0025] Too much template DNA causes problems as well, due to overloading of
the
capillary. While there is adequate yield of fluorescently labeled reaction
product, if the
2 o template is in excess, it competes with sequencing products for entry into
the capillary
during electrokinetic injection. The presence of the large template DNA
molecules can
result in an overall reduction, or sudden drop in capillary current, which can
manifest itself in
a variety of ways. Overloading can cause weak signal strength, late appearance
of
interpretable fluorescence intensity peaks in the chromatogram, and poor
resolution of the
2 s reaction products because the fluorescence emission is broad and diffuse.
All these effects
lead to shorter reads and lower sequencing data quality.
[0026] The problem of overloading is typically solved by either diluting the
sequencing
reaction, or carefully titrating the amount of template DNA introduced into
the sequencing
reaction. While both these solutions are simple in principle, the former
requires repeating
3 o the analysis of the reaction, and the latter is difficult to implement
using conventional means
in a high-throughput system. These means include detecting, and comparing to
standard
concentration curves, the quantity of fluorescent dye that binds DNA in a
sample, or
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
_ g _
measuring the absorbance of ultraviolet light at 260 nm wavelength, which can
be converted
into an absolute measure of DNA concentration. Thus, there is continued need
in the art for
methods to titrate the quantity of template DNA for sequencing reactions to be
analyzed
using high-throughput capillary electrophoresis systems, where minimizing cost
and
maximizing speed are crucial.
[0027] There is an additional need for an automated system that is able to
perform small-
scale thermal cycling reactions in a highly parallel manner. The system should
allow for
rapid preparation of cycling reactions with minimal consumption of reagents.
The
combination of reducing the amount of reagents required for a reaction and
reducing the
1 o time required for a reaction will greatly reduce the overall cost of
preparation of cycling
reactions.
[0028] With respect to proteomics, analysis of the proteome requires
separation,
quantification and identification of large protein collections.
[0029] Typically, such analysis is achieved by a combination of different
techniques, such
as 2-D electrophoresis separation, followed by enzymatic digestion and
identification by
matrix-assisted laser desorptionlionization mass spectrometry (2D PAGE-
MALDIIMS) or by
electrospray ionization mass spectrometry (2D PAGE-ESI/MS). Another common
approach
is LC/LC-MS/MS, i.e., proteins are digested, separated by strong cation
exchange liquid
chromatography and reversed phase liquid chromatography (LC/LC), and then
identified by
2 o tandem mass spectrometry (MSIMS). Current limitations include the
requirement for
extensive sample preparation prior to proteolytic digestion, analyte loss, and
low reaction
efficiencies at low protein concentrations.
[0030] In an alternative, methods and apparatus have been developed that
permit both
partial purification and mass spectal identification using a single
derivatived laser desorption
probe. See, e.g., U.S. Patent Nos. 6,225,047, 6,124,137, 5,719,060. Such
methods,
however, require specialized equipment and familiarity with mass
spectrometers.
[0031] There is, therefore, a continued need in the art for an automated
system that is
able to perform small-scale proteomic reactions in a highly parallel manner.
The system
should allow for rapid preparation of enzymatic reactions with minimal
consumption of
3 o reagents. The combination of reducing the amount of reagents required for
a reaction and
reducing the time required for a reaction will greatly reduce the overall cost
of preparation of
proteomic reactions while a highly parallel system will improve throughput.
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 9 -
SUMMARY OF THE INVENTION
[0032] Accordingly, certain embodiments of the instant invention are set forth
in the
following numbered paragraphs:
[0033] 1. A system for performing small scale reactions, the system
comprising: a
capillary cassette having a substrate and a plurality of capillaries extending
through said
substrate, wherein each of said capillaries has first and second open ends on
opposing
1 o sides of said substrate; a pair of membranes orientated and spaced such
that they may
temporarily seal the opposed ends of said capillaries; a thermal cycler having
an internal
chamber of sufficient capacity to hold said capillary cassette and said
membranes; and an
automated transfer device positioned to contact and move the capillary
cassette to a
location where the ends of the capillary may be sealed by the pair of
membranes and the
1 s capillary cassette with ends sealed by said membranes may be located
within the internal
chamber of the thermal cycler.
(0034] 2. The system of paragraph 1, further comprising a dispenser that
dispenses a fluid
from capillaries of the capillary cassette onto a location on a receiving
substrate, wherein
the automated transfer device may move the capillary cassette in relation to
said dispenser
2 o and receiving substrate such that the fluid contained within the
capillaries of the capillary
cassette are dispensed onto the substrate.
[0035] 3. The system of paragraph 2, wherein the dispenser is a centrifuge.
[0036] 4. The system of paragraph 2, wherein the dispenser is an air
displacement
dispenser.
2 s [0037] 5. The system of paragraph 2, further comprising an analytical
stage positioned
such that the automated transfer device may transfer said capillary cassette
in relation to
said dispenser such that contents within said capillary cassette may be
dispensed onto a
substrate located upon said stage.
[0038] 6. The system of paragraph 5, wherein said substrate is a sample
preparation
3 o microchip and the automated transfer device is disposed to dispense the
capillary cassette
directly into a plurality of sample preparation microchip sample receiving
wells.
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 10 -
[0039] 7. The system of paragraph 5, wherein said substrate is an array of
capillaries and
the automated transfer device is dispersed to disperse the capillary cassette
directly into the
capillaries.
[0040] 8. The system of paragraph 2, wherein said substrate is a multiwell
plate.
[0041] 9. The system of paragraph 1 wherein the capillaries have an interior
volume of 10-
1000 n L.
[0042] 10. The system of paragraph 1, further including a capillary cassette
wash station,
wherein said automated transfer device may transfer a capillary cassette into
contact with
said wash station, said wash station directing a wash solution through the
capillaries of the
1 o capillary cassette when said capillary cassette is placed within said wash
station.
[0043] 11. The system of paragraph 10, wherein said wash station has a wash
solution
tank and an upper wash manifold that may be moved to a position above said
wash solution
tank, wherein a wash fluid may be introduced into said wash solution tank and
drawn by
suction into the wash manifold when the capillary cassette is inserted into
said wash station.
[0044] 12. The system of paragraph 11, wherein said wash station further
includes a
plurality of wash fluid bottles each containing a wash fluid and a selector
valve allowing
selection of a wash fluid from one of said bottles to fill said wash solution
tank.
[0045] 13. The system of paragraph 1, further comprising an electronic control
which may
be programmed to send electronic instructions to components of the system.
2 0 [0046] 14. The system of paragraph 1 wherein said pair of membranes are
affixed to
opposing sides of the internal chamber of the thermal cycling device.
[0047] 15. The system of paragraph 1 further comprising a plurality of
microplate holder
magazines which dispense microplates to a location where said automated
transfer device
may contact and move the microplates.
2 5 [0048] 16. The system of paragraph 1 wherein said membranes are deformable
membranes held with a spring bias to temporarily seal the ends of the
capillaries.
[0049] 17. A system for nanoscale reaction preparation, the system comprising:
a
capillary cassette including a substrate and a plurality of capillaries
extending through said
substrate, each capillary having an internal volume of between 10 nl and about
1 uL,
3 o wherein each of said capillaries has a first and a second open end on
opposing sides of
said substrate, wherein the length of the capillary extending through
substrate on one side
of the substrate is matched to be shorter than the depth of a microplate well;
a multiwell
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 11 -
plate having a plurality of wells into which the capillaries of the capillary
cassette may be
inserted; a dispenser that dispenses fluid contained within the capillaries of
the capillary
cassette into wells of said multiwell plate when said capillary is transported
to the dispenser;
an automated transfer robot having a transfer head to carry articles selected
from the group
comprising capillary cassettes, multiwell plates, and multiwell plates with
capillaries of a
capillary cassette inserted into the wells of the multiwell plates; a pair of
opposing
membrane surfaces, wherein the ends of the capillaries may be temporarily
sealed by
pressing the membranes against said ends; and a thermal cycler having an
internal
chamber of sufficient capacity to hold said capillary cassette and said
membranes when
1 o said membranes are sealing the ends of the capillaries of the capillary
cassette, wherein the
thermal cycler is disposed such that the automated transfer robot may place a
capillary
cassette into an internal chamber within said thermal cycler wherein said
membranes may
seal the end of the capillaries of said capillary cassette within said
internal chamber.
[0050] 18. The system of paragraph 17 wherein said dispenser is an
electrokinetic
1 s injector.
[0051] 19. The system of paragraph 17 wherein said dispenser is a centrifuge.
[0052] 20. The system of paragraph 17 wherein said dispenser is an air
displacement
head.
[0053] 21. The system of paragraph 17 wherein said dispenser is disposed to
dispense
2 0 liquid from the capillaries onto an analytical substrate located on an
analytical stage.
[0054] 22. The system of paragraph 17, further comprising a capillary cassette
wash
station, wherein said automated transfer device may transfer a capillary
cassette into
contact with said wash station, said wash station directing a wash solution
through interiors
of the capillaries of the capillary cassette when said capillary cassette is
placed within said
2 5 wash station.
[0055] 23. The system of paragraph 22, wherein said wash station includes a
lower wash
solution tank and an upper wash manifold, wherein a wash fluid may be
introduced into said
wash solution tank and drawn by suction into the wash manifold when the
capillary cassette
is inserted into said wash station.
3 0 [0056] 24. The system of paragraph 23, wherein said wash station further
includes a
plurality of wash fluid bottles and a selector valve in fluid communication
with said bottles for
selection of a wash fluid to fill said wash solution tank.
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 12 -
[0057] 25. The system of paragraph 17, further comprising an electronic
control, said
control sending electronic instructions to effect programmed operation of said
system.
[0058] 26. A system for preparing nanoscale reactions, the system comprising:
a
substrate having integrally associated elongate submicroliter volume reaction
containers
having two opposing ends; a reaction mixture contained within said reaction
containers; a
pair of membranes disposed to temporarily seal said opposing ends of said
reaction
containers; a thermal cycler having an internal chamber of sufficient
dimension to receive
said substrate with associated elongate reaction chambers sealed by said
membranes.
[0059] 27. The system of paragraph 26, wherein said substrate has capillaries
extending
1 o through said substrate, wherein said capillaries act as the reaction
chambers.
[0060] 28. The system of paragraph 26, wherein said elongate reaction
containers pass
through the thickness of said substrate.
[0061] 29. The system of paragraph 26, wherein said thermal cycler circulates
heated air
through a continuous circuit, wherein said internal chamber is part of said
continuous circuit.
1 s [0062] 30. The system of paragraph 29, wherein said continuous circuit may
be vented by
blocking a section of said internal passageway and venting said heated air
thereby allowing
rapid temperature adjustment of said heated air.
[0063] 31. The system of paragraph 30, wherein said internal chamber contains
said
membranes mounted on opposing surfaces of said internal chamber.
2 0 [0064] 32. The system of paragraph 31, wherein at least one of said
membranes is
mounted within said internal chamber with a spring bias which provides a
sealing force of
said membranes against said ends of said reaction containers.
[0065] 33. The system of paragraph 26, further comprising a means for
dispensing said
reaction containers.
2 s [0066] 34. The system of paragraph 26, further comprising a means for
combining
reagents to form said reaction mixture and a means for filling said reaction
containers with
said reaction mixture.
[0067] 35. The system of paragraph 26, further comprising a wash station which
may hold
and wash said reaction containers.
3 0 [0068] 36. A method to prepare nanoscale thermal cycling reaction
mixtures, the steps
comprising; combining compounds to form a reaction mixture; introducing said
reaction
mixture into a plurality of reaction containers disposed on a substrate, each
reaction
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 13 -
container having an internal volume less than one microliter and having a
first and second
open end; temporarily sealing the ends of reaction containers by pressing a
pair of opposing
membranes against a first and second set of reaction container ends; exposing
the sealed
reaction containers to temperature cycles to effect a reaction in the reaction
mixture; and
dispensing the reaction containers onto a substrate.
[0069] 37. The method of paragraph 36 wherein the steps of combining compounds
to
form a reaction mixture includes the steps: metering an amount of a first
liquid reaction
component by placing one end of a plurality of capillaries of a capillary
cassette into contact
with the first liquid reaction component wherein the capillaries fill by
capillary action;
1 o dispensing the first liquid reaction component onto discrete locations on
a substrate;
metering an amount of a second liquid reaction component by placing one end of
the
capillaries of a capillary cassette into contact with the reaction reagents
wherein the
capillaries fill by capillary action; and dispensing the second liquid
reaction component onto
the discrete locations, thereby combining said first and second liquid
reaction components
to form a reaction mixture.
[0070] 38. The method of paragraph 37 wherein the step of introducing said
reaction
mixture into a plurality of reaction containers is effected by providing a
capillary cassette
and dipping one open end of capillaries of the capillary cassette into contact
with the
reaction mixture so that the capillaries fill by capillary action.
2 0 [0071] 39. The method of paragraph 36 wherein the steps of combining
compounds to
form a reaction mixture includes the steps: immobilizing a biomolecule sample
on an interior
surface of the reaction container; metering an amount of reaction reagents
into the
capillaries of the capillary cassette by placing one end of the capillaries of
a capillary
cassette into contact with the reaction reagents wherein the capillaries fill
by capillary action,
2 s whereby the reaction reagents and the immobilized biomolecule combine to
form the
reaction mixture.
[0072] 40. The method of paragraph 39, wherein the biomolecule is a nucleic
acid.
[0073] 41. The method of paragraph 36 wherein the steps of combining compounds
to
form a reaction mixture include the steps: coating a plurality of surface
locations with a layer
3 0 of desiccated reaction reagents; and adding to each surface location a
nucleic acid sample
in solution of sufficient volume to dissolve the solid layer of reaction
reagents, thereby
forming a reaction mixture.
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 14 -
[0074] 42. The method of paragraph 36 wherein the steps of combining compounds
to
form a reaction mixture include the steps: coating an interior surface of each
capillary in a
capillary cassette with a layer of desiccated reaction reagents; and metering
an amount of
nucleic acid sample in solution into the capillaries of the capillary cassette
by placing one
s end of the capillaries of a capillary cassette into contact with the nucleic
acid sample in
solution, whereby the capillaries fill by capillary action, whereby the
solution allows the layer
of reaction reagents to dissolve, forming the reaction mixture.
[0075] 43. The method of paragraph 36, wherein the step of dispensing the
reaction
containers onto a substrate is effected by: placing the substrate with
associated reaction
1 o containers in a centrifuge; positioning a substrate at a radially distal
end of one open end of
said reaction containers; and applying centrifugal force such that liquid
reaction mixtures
contained within said reaction containers are dispensed onto said substrate.
[0076] 44. The method of paragraph 36, wherein the step of dispensing the
reaction
containers onto a substrate is effected by: displacing the contents of the
reaction containers
1 s onto a substrate using air displacement.
[0077] 45. The method of paragraph 36 wherein the step of temporarily sealing
the ends
of the reaction containers by pressing a pair of opposing membranes against a
first and
second set of reaction container ends is effected by: placing the reaction
containers within
an interior chamber of a thermal cycler, wherein when the reaction containers
are enclosed
2 o within said thermal cycler, deformable membranes on opposing interior
surfaces of said
interior chamber temporarily seal the reaction containers' ends on each end of
the reaction
containers.
[0078] 46. The method of paragraph 36 wherein the step of exposing the sealed
reaction
to temperature cycles to effect a reaction is effected circulating heated air
past the reaction
2 s containers through a conduit which allows rapid venting of air to the
exterior of said conduit
to effect rapid temperature changes during the temperature cycles.
[0079] 47. A thermal cycling device for exposing reaction mixtures to
temperature cycles,
the device comprising: a housing enclosing a continuous interior circuit
passageway, said
housing having a section that may be temporarily opened to allow access to the
interior of
3 o the housing; a blower disposed within said circuit passageway to direct
air flow in one
direction in the internal circuit passageway; a heating element disposed in
said internal
circuit passageway such that air circulating within said passageway passes
through said
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 15 -
heating element; a sample holding compartment having two membranes positioned
in
opposing orientation within said sample holding compartment, wherein said
membranes
may be biased against opposing ends of containers inserted into the sample
holding
compartment; housing air vent which may be opened to rapidly exhaust heated
circulating
air; and a housing air intake for drawing air into said interior circuit
passageway when the
vent exhausts heated circulating air.
[0080] 48. The thermal cycling device of paragraph 47 further comprising a
temperature
monitoring device disposed in the internal passageway proximate to a sample
holding
compartment.
to [0081] 49. The thermal cycling device of paragraph 47 further comprising at
least one air
diffuser disposed in the internal passageway between the blower and the sample
holding
compartment, said diffuser promoting uniform temperature in the air
circulating in the
internal passageway.
[0082] 50. The thermal cycling device of paragraph 47 wherein at least one of
the
membranes within the sample holding compartment is spring biased.
[0083] 51. The thermal cycling device of paragraph 47 further comprising
insulation
affixed to the surfaces of the interior circuit passageway.
[0084] 52. The thermal cycling device of paragraph 47 further comprising an
electronic
control which sends instruction to components of the thermal cycling device.
2 0 [0085] 53. The thermal cycling device of paragraph 47 wherein said vent is
opened by
moving a section of said housing located between said sample holding
compartment and
said air intake such that the internal passageway is at least partially
restricted and an
opening to outside said housing is created.
[0086] 54. The thermal cycling device of paragraph 47 wherein the housing has
a
2 5 sealable opening to admit access to the sample holding compartment.
[0087] 55. A method for performing reactions, the method comprising, a)
introducing
reaction mixtures into a reaction container set, each container in the set
having two
opposing ends and an internal volume between 10 to 1000 nl; b) temporarily
sealing the
ends of the reaction chambers by pressing a deformable membrane against the
opposing
3 o ends of said reaction containers; c) effecting a reaction within said
reaction containers; d)
dispensing reaction mixtures onto discrete locations on a substrate; and e)
combining said
reaction mixtures with at least 1 µl of a liquid reagent mixture.
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 16 -
(0088] 56. The method of paragraph 55, further comprising the step of: f)
reacting the
completed reaction mixture with the liquid reagent mixture.
[0089] 57. The method of paragraph 56, further comprising the step of: g)
combining
reacted mixtures of step f with a reaction reagent set to form a second
reaction mixture set;
h) introducing said second reaction mixture set into a second reaction
container set, each
reaction container having two opposing ends and an internal between 10 and
1000 nl; i)
temporarily sealing the ends of the set of reaction containers by pressing
deformable
membranes against the opposing ends of said reaction containers; j) effecting
a reaction
within said second reaction container set; and k) dispensing reacted mixtures
from said
1 o second reaction container set.
(0090] 58. The method paragraph 57, wherein step f occurs under isothermal
reaction
conditions.
[0091] 59. The method of paragraph 57, wherein the reaction mixture of step a
is a PCR
mixture, the liquid reagent mixture of step a contains exonuclease I and
shrimp alkaline
phospotase, and the second reaction mixture.
[0092] 60. The method of paragraph 57 wherein steps c and j include exposing
the
reaction container sets to temperature cycles.
(0093] 61. The method of paragraph 60 wherein the exposing reaction container
sets to
temperature cycles is effected by a circulating air thermal cycler.
2 0 [0094] 62. The method of paragraph 57 wherein the second reaction
container set is
dispensed onto an analytical substrate.
[0095] 63. The method of paragraph 57 wherein the second reaction container
set is
dispensed into the ends of capillaries in a capillary electrophoresis array.
[0096] 64. The method of paragraph 57 wherein the second reaction container
set is
2 5 dispensed into the wells of a microplate.
[0097] 65. A method of obtaining substantially the same quantity of nucleic
acid from a
first and a second sample, comprising: saturably binding nucleic acid from
said first sample
directly on an inner surface of a first capillary tube by contacting said
inner surface with a
solution comprising a nucleic acid and a chaotropic agent for a time
sufficient for the nucleic
3 o acid to become saturably bound to said inner surface; and saturably
binding nucleic acid
from said second sample directly on an inner surface of a second capillary
tube by
contacting said inner surface with a solution comprising a nucleic acid and a
chaotropic
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 17 -
agent for a time sufficient for the nucleic acid to become saturably bound to
said inner
surface, wherein said inner surfaces of said first and second capillary tubes
are capable of
saturably binding substantially the same quantity of nucleic acid from each of
said first and
second samples, respectively.
s [0098] 66. The method of paragraph 65, wherein the quantity of nucleic acid
saturably
bound to the inner surfaces of said first and second capillary tubes differs
by less than about
10%.
(0099] 67. The method of paragraph 65, wherein said binding steps are effected
substantially contemporaneously.
[0100] 68. The method of paragraph 65, wherein said second capillary tube is
the same
capillary tube as said first capillary tube, and wherein said binding steps
are effected
iteratively.
[0101] 69. The method of paragraph 65 further comprising, prior to said
binding steps, the
step of size-selecting a nucleic acid to be saturably bound.
1 s [0102] 70. The method of paragraph 65 further comprising, after said
binding steps, the
step of using the nucleic acid of either of said first or second capillary
tubes in an enzymatic
reaction.
[0103] 71. The method of paragraph 65 wherein the saturably bound nucleic acid
of either
of said first or second capillary tubes is DNA.
2 0 [0104] 72. The method of paragraph 71 further comprising, after said
binding steps, the
step of using the DNA of either of said first or second capillary tubes in an
enzymatic
reaction.
[0105] 73. The method of paragraph 72 wherein said enzymatic reaction is a DNA
sequencing reaction.
2 s [0106] 74. The method of paragraph 65, wherein either of said first or
second capillary
tubes comprises glass.
[0107] 75. The method of paragraph 65, wherein said capillary tubes are
present in an
array.
[0108] 76. The method of paragraph 75, wherein said array comprises at least 8
capillary
3 o tubes.
[0109] 77. The method of paragraph 75, wherein said array comprises at least
16 capillary
tubes.
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 18 -
[0110] 78. The method of paragraph 75, wherein said array comprises at least
96 capillary
tubes.
[0111] 79. The method of paragraph 65 wherein said chaotropic agent is
selected from
the group consisting of: urea, sodium perchlorate, potassium perchlorate,
sodium bromide,
s potassium bromide, sodium iodide, potassium iodide, sodium thiocyanate,
potassium
thiocyanate, guanidine thiocyanate, sodium isothiocyanate, potassium
isothiocyanate,
guanidine hydrochloride, guanidine isothiocyanate, lithium chloride, sodium
trichloroacetate,
dimethylsulfoxide, tetra-amine halides, tetraethylamine chloride, and
potassium
trichloroacetate.
[0112] 80. The method of paragraph 65 further comprising the step of removing
the
solution, wherein said removing step occurs after said contacting step.
[0113] 81. The method of paragraph 80 further comprising the step of washing
the inner
surface of either of said first or second capillary tubes, wherein said
washing step occurs
after said removing step.
1 s [0114] 82. The method of paragraph 81 further comprising the step of
drying the inner
surface of either of said first or second capillary tubes, wherein said drying
step occurs after
said washing step.
[0115] 83. A method of performing an enzymatic reaction in a capillary tube
using a
normalized quantity of a nucleic acid, comprising: performing said enzymatic
reaction in a
2 o capillary tube using a normalized quantity of said nucleic acid, said
nucleic acid having been
saturably bound from an excess thereof directly on an inner surface of said
capillary tube by
contacting said inner surface with a solution comprising a nucleic acid and a
chaotropic
agent for a time sufficient for the nucleic acid to have become saturably
bound to said inner
surface; and said excess of nucleic acid having been removed therefrom.
2 s [0116] 84. The method of paragraph 83 further comprising the step of
introducing into said
capillary tube an enzymatic reaction mixture after said excess of nucleic acid
has been
removed therefrom.
[0117] 85. A method of performing an enzymatic reaction in a capillary tube
using a
normalized quantity of a nucleic acid, comprising: introducing an enzymatic
reaction mixture
3 o into a capillary tube having a normalized quantity of a nucleic acid,
wherein said reaction
mixture comprises an oligonucleotide primer, a DNA polymerise, and at least
one
dideoxynucleotide triphosphate (ddNTP), said nucleic acid having been
saturably bound
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 19 -
from an excess thereof directly on an inner surface of said capillary tube by
contacting said
inner surface with a solution comprising nucleic acid and a chaotropic agent
for a time
sufficient for the nucleic acid to have become saturably bound to said inner
surface; and
said excess of nucleic acid having been removed therefrom; and performing said
enzymatic
reaction in said capillary tube using said normalized quantity of nucleic
acid.
[0118] 86. The method of paragraph 85, further comprising subjecting said
enzymatic reaction mixture to at least one thermal cycle.
[0119] 87. The method of paragraph 85, further comprising, after said step of
removing said excess of nucleic acid, the step of washing said inner surface
of said capillary
1 o tube.
[0120] 88. The method of paragraph 87, further comprising, after said step of
washing said inner surface of said capillary tube, the step of drying said
inner surface of
said capillary tube.
[0121] 89. The method of paragraph 85, wherein said enzymatic reaction
1 s mixture is introduced into said capillary tube by capillary action.
[0122] 90. The method of paragraph 85, further comprising, after said step of
performing said enzymatic reaction, the step of expelling the product of said
reaction.
[0123] 91. The method of paragraph 85, further comprising, after said step of
performing said enzymatic reaction, the step of removing unincorporated
dideoxynucleotide
2 o triphosphates (ddNTPs).
[0124] 92. The method of paragraph 91, wherein said unincorporated ddNTPs
are removed by contacting the product of said reaction with gel filtration
media.
[0125] 93. The method of paragraph 85, further comprising, after said step of
performing said enzymatic reaction, the step of inactivating unincorporated
2 s dideoxynucleotide triphosphates (ddNTPs).
[0126] 94. The method of paragraph 93, wherein said unincorporated ddNTPs
are inactivated by treating the product of said reaction with calf intestinal
alkaline
phosphatase (CIAP).
[0127] 95. The method of paragraph 85, wherein the dideoxynucleotide
3 o triphosphates (ddNTPs) included in said enzymatic reaction mixture are
selected from
among the group consisting of: A only; C only; G only; T only; A,C; A,G; A,T;
C,G; C,T; G,T;
A,C,G; A,C,T; A,G,T; C,G,T and A,C,G,T.
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 20 -
[0128] 96. The method of paragraph 85, wherein said dideoxynucleotide
triphosphate (ddNTP) is conjugated to a fluorophore.
[0129] 97. The method of paragraph 96, wherein said fluorophore is base-
specific.
s [0130] 98. The method of paragraph 96, wherein said fluorophore is selected
from among the group consisting of. fluorescein, 5-carboxy-fluorescein, 6-
carboxy-
rhodamine, N,N,N',N'-tetramethyl-5-carboxyrhodamine and 5-carboxy-X-rhodamine,
rhodamine 110, rhodamine-6-G, tetramethyl rhodamine and rhodamine X.
[0131] 99. The method of paragraph 96, wherein said fluorophore is an
1 o energy-transfer fluorophore.
[0132] 100. The method of paragraph 85, wherein said primer is
complementary to a plurality of contiguous nucleotides in said nucleic acid;
and wherein
said primer terminates immediately before a nucleotide present in said nucleic
acid, the
identity of which is desired to be determined.
15 [0133] 101. The method of paragraph 100, wherein said primer is conjugated
to a fluorophore.
[0134] 102. The method of paragraph 101, wherein said fluorophore is selected
from among the group consisting of: fluorescein, 5-carboxy-fluorescein, 6-
carboxy-
rhodamine, N,N,N',N'-tetramethyl-5-carboxyrhodamine and 5-carboxy-X-rhodamine,
2 o rhodamine 110, rhodamine-6-G, tetramethyl rhodamine and rhodamine X.
[0135] 103. The method of paragraph 101, wherein said fluorophore is an
energy-transfer fluorophore.
[0136] 104. The method of paragraph 85, further comprising analyzing a
product of said enzymatic reaction to determine the identity of a ddNTP
incorporated at the
2 5 3'-end of the primer.
[0137] 105. The method of paragraph 104, wherein said step of analyzing a
product of said enzymatic reaction to determine the identity of a base present
in said nucleic
acid is effected using a technique selected from among the group consisting of
gel
electrophoresis, capillary electrophoresis, mass spectroscopy, MALDI mass
spectroscopy,
3 o SELDI mass spectroscopy, fluorescence emission detection, scanning
confocal laser-
induced fluorescence detection, fluorescence polarization (FP) and analytical
microchip
analysis.
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 21 -
[0138] 106. The method of paragraph 104, further comprising inferring the
identity of said ddNTP incorporated at the 3'-end of said primer from the
emission spectrum
of a fluorophore conjugated to said ddNTP.
[0139] 107. The method of paragraph 106, further comprising inferring the
identity of a nucleotide present in said nucleic acid from the identity of
said ddNTP
incorporated at the 3'-end of said primer.
[0140] 108. The method of paragraph 107, further comprising inferring, from
the identity of said nucleotide in said nucleic acid, the identity of a
nucleotide present in a
second nucleic acid.
to [0141] 109. The method of paragraph 107, wherein the identity of said
nucleotide defines a single nucleotide polymorphism (SNP) in said nucleic
acid.
[0142] 110. The method of paragraph 109, wherein said SNP is a
heterozygous SNP.
[0143] 111. The method of paragraph 109, wherein said SNP is a homozygous
is SNP.
[0144] 112. The method of paragraph 109, wherein the identity of said
nucleotide is stored as data in a computer data structure.
[0145] 113. The method of paragraph 112, wherein said computer data
structure is embodied in a computer readable medium.
2 0 [0146] 114. The method of paragraph 85, wherein said DNA polymerise is
thermostable.
[0147] 115. The method of paragraph 85, wherein said DNA polymerise is a
DNA-dependent DNA polymerise.
[0148] 116. The method of paragraph 85, wherein said DNA polymerise is an
2 s RNA-dependent DNA polymerise.
[0149] 117. The method of paragraph 85, wherein said nucleic acid is selected
from among the group consisting of: DNA, double stranded DNA, single stranded
DNA,
DNA produced by polymerise chain reaction, DNA produced by reverse
transcription
reaction, DNA isolated from a eukaryotic cell, DNA isolated from a prokaryotic
cell, DNA
3 o isolated from an archaea cell, DNA isolated from a fungal cell, DNA
isolated from a plant
cell, DNA isolated from a virus, DNA isolated from a bacteriophage, genomic
DNA, plasmid
DNA, episomal DNA, RNA, messenger RNA, double stranded RNA, single stranded
RNA,
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 22 -
RNA isolated from a eukaryotic cell, RNA isolated from a prokaryotic cell, RNA
isolated from
an archaea cell, RNA isolated from a fungal cell, RNA isolated from a plant
cell, RNA
isolated from a virus, genomic RNA, DNA-RNA hybrid, nucleic acid obtained from
frozen
glycerol stocks of bacteria and nucleic acid obtained from bacterial colonies
grown on solid
growth media.
[0150] 118. The method of paragraph 85, wherein said nucleic acid is DNA;
and further comprising the step of preparing said DNA by polymerise chain
reaction (PCR).
[0151] 119 The method of paragraph 118, wherein the template used in said
polymerise chain reaction is genomic DNA.
Z o [0152] 120. The method of paragraph 118, further comprising, after said
step of
preparing said DNA by PCR, the step of removing unincorporated PCR primer
using a
single stranded Dnase.
[0153] 121. The method of paragraph 118, further comprising, after said step
of
preparing said DNA by PCR, the step of removing unincorporated dNTP using a
1 s phosphatase.
[0154] 122. The method of paragraph 118, further comprising, after said step
of
preparing said DNA by PCR, the step of treating said DNA with Exonuclease I
(Exol) and
shrimp alkaline phosphatase (SAP).
[0155] 123. The method of paragraph 85, further comprising, after said steps
of
2 o saturably binding said DNA from an excess thereof directly on an inner
surface of said
capillary tube and removing said excess therefrom, the step of removing
unincorporated
PCR primer and dNTP by washing said inner surface of said capillary.
[0156] 124. The method of paragraph 85, wherein said enzymatic reaction is
performed in a reaction volume of about 10 - 5000 nanoliters.
2 s [0157] 125. The method of paragraph 85, wherein said capillary tube is
present
in a spatially addressable array of capillary tubes.
[0158] 126. The method of paragraph 125, wherein said spatially addressable
array of capillary tubes is an array having a number of capillaries selected
from among the
group consisting of: 2, 4, 8, 12, 16, 24, 32, 48, 64, 96, 128, 192, 288, 384,
480, 576, 672,
3 0 768, 864, 960 and 1536 capillaries.
[0159] 127. A product of an enzymatic reaction using a normalized quantity of
nucleic acid produced by the method of paragraph 85.
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 23 -
[0160] 128. A method of obtaining substantially the same quantity of nucleic
acid from a first and a second sample for use in an enzymatic reaction
effective to detect a
single nucleotide polymorphism (SNP), comprising: saturably binding nucleic
acid from said
first sample directly on an inner surface of a first capillary tube by
contacting said inner
s surface with a first solution comprising a nucleic acid and a chaotropic
agent for a time
sufficient for the nucleic acid to become saturably bound to said inner
surface; and saturably
binding nucleic acid from said second sample directly on an inner surface of a
second
capillary tube by contacting said inner surface with a second solution
comprising a nucleic
acid and a chaotropic agent for a time sufficient for the nucleic acid to
become saturably
1 o bound to said inner surface, wherein said inner surfaces of said first and
second capillary
tubes are capable of saturably binding substantially the same quantity of
nucleic acid from
each of said first and second samples, respectively; and using the nucleic
acid of either or
both of said first or second capillary tubes in an enzymatic reaction
effective to detect a
single nucleotide polymorphism (SNP) present in said nucleic acid.
15 [0161] 129. The method of paragraph 128, wherein said enzymatic reaction is
selected from among the group consisting of: oligonucleotide ligation assay
genotyping
(Ol_A) reaction, minisequencing reaction, TaqManT"" genotyping reaction,
InvaderT"" assay
reaction, dye labeled oligonucleotide ligation reaction, pyrosequencing
reaction, rolling circle
amplification (RCA) reaction and single-base extension (SBE) reaction.
2 0 [0162] 130. The method of paragraph 129, wherein said enzymatic reaction
is
a single-base extension reaction.
[0163] 131. The method of paragraph 128, further comprising analyzing a
product of said enzymatic reaction.
[0164] 132. A product of an enzymatic reaction using a normalized quantity of
2 s a nucleic acid produced by the method of paragraph 128.
[0165] 133. A method of performing an enzymatic reaction in a capillary tube
using a normalized quantity of a nucleic acid effective to detect a single
nucleotide
polymorphism (SNP), comprising: performing said enzymatic reaction in a
capillary tube
using a normalized quantity of said nucleic acid, said nucleic acid having
been saturably
3 o bound from an excess thereof directly on an inner surface of said
capillary tube by
contacting said inner surface with a solution comprising a nucleic acid and a
chaotropic
agent for a time sufficient for the nucleic acid to have become saturably
bound to said inner
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 24 -
surface; and said excess of nucleic acid having been removed therefrom,
wherein said
enzymatic reaction is selected from among the group consisting of:
oligonucleotide ligation
assay genotyping (OLA) reaction, minisequencing reaction, TaqManT"~ genotyping
reaction,
InvaderT"" assay reaction, dye labeled oligonucleotide ligation reaction,
pyrosequencing
s reaction, rolling circle amplification (RCA) reaction and single-base
extension (SBE)
reaction.
[0166] 134. The method of paragraph 133, wherein said enzymatic reaction is
a single-base extension reaction.
[0167] 135. The method of paragraph 133, further comprising analyzing a
1 o product of said enzymatic reaction.
[0168] 136. A product of an enzymatic reaction using a normalized quantity of
a nucleic acid produced by the method of paragraph 133.
[0169] 137. A method of performing an enzymatic reaction in a capillary tube
using a normalized quantity of an enzyme, comprising: performing said
enzymatic reaction
in a capillary tube using a normalized quantity of said enzyme, said enzyme
having been
saturably bound from an excess thereof directly on an inner surface of said
capillary tube by
contacting said inner surface with a solution comprising an enzyme for a time
sufficient for
the enzyme to have become saturably bound to said inner surface; and said
excess of
enzyme having been removed therefrom.
2 0 (0170] 138. A method of performing an enzymatic reaction in a capillary
tube
using a normalized quantity of an enzyme, comprising: performing said
enzymatic reaction
in a capillary tube using a normalized quantity of said enzyme, said enzyme
having been
specifically and saturably bound from an excess thereof on a modified inner
surface of said
capillary tube by contacting said modified inner surface with a solution
comprising an
2 s enzyme for a time sufficient for the enzyme to have become specifically
and saturably
bound to said modified inner surface; and said excess of enzyme having been
removed
therefrom.
[0171] 139. The method of paragraph 138, wherein the modification of said the
inner surface of said capillary is effected by silanization.
3 0 [0172] 140. The method of paragraph 138, wherein said modified inner
surface
of said capillary tube is modified with a functional group.
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 25 -
[0173] 141. The method of paragraph 140, wherein said functional group is
selected from among the group consisting of: an amino group, a pyridyldithio
group, a
disuccinimidyl suberate group, an oxirane group, a streptavidin molecule and a
surface
active hydrogel.
[0174] 142. The method of paragraph 138, wherein said bound enzyme is
coupled covalently to said functional group.
[0175] 143. The method of paragraph 138, wherein said bound enzyme is
coupled noncovalently to said functional group.
[0176] 144. The method of paragraph 138, wherein a plurality of said enzymes
1 o is uniformly oriented on said modified inner surface of said capillary.
[0177] 145. The method of paragraph 138, further comprising the step of
releasing said saturably bound enzymes by the addition of an excess of
thiopyridone.
[0178] 146. The method of paragraph 138, wherein said enzyme is selected
from among the group consisting of: protease, sequence-specific protease,
trypsin,
chymotrypsin, proteinase K, papain, pepsin, endoproteinase, endoproteinase Glu-
C,
endoproteinase Arg-C, endoproteinase Lys-C, endoproteinase Pro-C,
endoproteinase Asp-
N, V8 protease, glycosidase, [i-galactosidase, lipase, oxidise, oxygenase,
glucose oxidise,
cholesterol oxidise, lactate monooxygenase, ligase, DNA ligase, RNA ligase,
methylase,
polymerise, DNA-dependent DNA polymerise, terminal transferase enzyme, RNA-
2 o dependent DNA polymerise, DNA-dependent RNA polymerise, phosphatase,
kinase, DNA
gyrase, topoisomerase, nuclease, exonuclease, S1 exonuclease, mung bean
nuclease,
endonuclease, restriction endonuclease, ribonuclease and urease.
[0179] 147. The method of paragraph 138, further comprising, prior to said
step
of performing said enzymatic reaction, the step of filling said capillary with
a solution
comprising a substrate.
[0180] 148. The method of paragraph 147, wherein said step of filling said
capillary with a solution comprising a substrate is effected by capillary
action.
[0181] 149. The method of paragraph 147, wherein said solution comprises a
volume of about 100 - 2,000 nanoliters.
3 0 [0182] 150. The method of paragraph 138, wherein said enzymatic reaction
is
effected isothermally.
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 26 -
[0183] 151. The method of paragraph 138, wherein said capillary is present in
a spatially addressable array.
[0184] 152. The method of paragraph 138, wherein said enzymatic reaction is
effected in parallel with at least one additional enzymatic reaction.
s [0185] 153. The method of paragraph 138, further comprising the step, after
said step of performing said enzymatic reaction, the step of analyzing a
product of said
enzymatic reaction.
[0186] 154. The method of paragraph 153, wherein said step of analyzing a
product of said enzymatic reaction is effected using a technique selected from
among the
1 o group consisting of: mass spectroscopy, capillary electrophoresis,
fluorescent scanning and
high performance liquid chromatography (HPLC).
[0187] 155. The method of paragraph 153, further comprising the step, before
said step of analyzing a product of said enzymatic reaction, the step of
fluorescently labeling
said product.
15 [0188] 156. A method of performing a protein-based reaction in a capillary
tube
using a normalized quantity of a protein, comprising: performing said protein-
based reaction
in a capillary tube using a normalized quantity of said protein, said protein
having been
saturably bound from an excess thereof on an inner surface of said capillary
tube by
contacting said inner surface with a solution comprising a protein for a time
sufficient for the
2 o protein to have become saturably bound to said inner surface; and said
excess of protein
having been removed therefrom.
[0189] 157. A method of performing a protein-based reaction in a capillary
tube
using a normalized quantity of a protein, comprising: performing said protein-
based reaction
in a capillary tube using a normalized quantity of said protein, said protein
having been
2 s specifically and saturably bound from an excess thereof on a modified
inner surface of said
capillary tube by contacting said modified inner surface with a solution
comprising a protein
for a time sufficient for the protein to have become specifically and
saturably bound to said
modified inner surface; and said excess of protein having been removed
therefrom.
[0190] 158. The method of paragraph 157, wherein said protein is a
3 o noncatalytic protein.
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 27 -
[0191] 159. The method of paragraph 158, wherein said noncatalytic protein is
selected from among the group consisting of: antibody, antigen-binding
fragment of an
antibody, IgG, IgE, IgM, protein G, protein A and streptavidin.
[0192] 160. The method of paragraph 157, wherein said protein-based reaction
s is a molecular binding reaction.
[0193] 161. The method of paragraph 160, wherein the substrate of said
molecular binding reaction is selected from among the group consisting of:
protein, enzyme,
nucleic acid, DNA, RNA, carbohydrate, lipid, and other chemical.
[0194] 162. A method of obtaining substantially the same quantity of protein
1 o from a first and a second sample, comprising: saturably and specifically
binding protein from
said first sample directly on a modified inner surface of a first capillary
tube by contacting
said inner surface with a solution comprising a protein for a time sufficient
for the protein to
become saturably and specifically bound to said modified inner surface; and
saturably and
specifically binding protein from said second sample directly on a modified
inner surface of
15 a second capillary tube by contacting said inner surface with a solution
comprising a protein
for a time sufficient for the protein to become saturably and specifically
bound to said
modified inner surface, wherein said modified inner surfaces of said first and
second
capillary tubes are capable of saturably and specifically binding
substantially the same
quantity of protein from each of said first and second samples, respectively.
BRIEF DESCRIPTION OF THE DRAWINGS
(0195] The above and other objects and advantages of the present invention
will be
apparent upon consideration of the following detailed description taken in
conjunction with
2 5 the accompanying drawings, in which like characters refer to like parts
throughout, and in
which:
[0196] FIG. 1 is a schematic of an integrated system for the preparation of
cycle
sequencing reaction products, which system can advantageously use the methods
of the
present invention;
3 0 [0197] FIG. 2 is a flow chart illustrating the steps in production of
cycling reactions, the
first step of which can advantageously be improved by use of the methods of
the present
invention;
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 28 -
[0198] FIG. 3A is a perspective view of a capillary cassette that is used in a
high
throughput embodiment of the present invention;
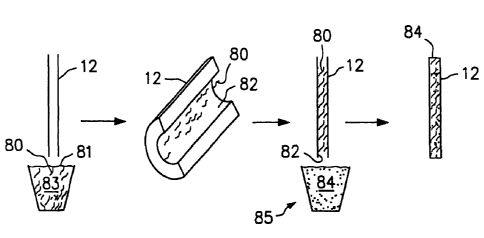
[0199] FIG. 3B is a perspective view of the capillary cassette of FIG. 3A
inserted into a
capillary cassette holder in a system for high throughput application of the
methods of the
s present invention;
[0200] FIG. 3C is a flexible capillary cassette that advantageously can use
the methods of
the present invention;
[0201] FIG. 3D illustrates the capillary cassette of FIG. 3C bent to a curved
orientation
such that the capillary ends are in a curved pattern;
l o [0202] FIG. 3E is a microchip device containing channels, functionally
equivalent to
capillary tubes, for sample preparation, including the direct reversible
immobilization of
nucleic acid, according to the present invention;
[0203] FIG. 4A illustrates a dispense head for dispensing liquid from the
capillary cassette
of FIG. 3, for use in the present invention;
15 [0204] FIG. 4B shows an internal cross section of an air displacement
dispense head of
FIG. 4A;
[0205] FIG. 4C shows the dispense head of FIG. 4A with the dispense head
closed;
[0206] FIG. 5A illustrates a top view of a centrifuge that can be used to
dispense fluid
from the capillary cassette of FIG. 3A;
2 0 [0207] FIG. 5B illustrates a cross-section of a rotor arm of FIG. 5A
holding a swinging
microplate bucket containing a capillary cassette inserted into a microtiter
plate;
[0208] FIG. 6 shows a schematic of an air-based thermal cycling device with
the capillary
cassette and holder shown in FIG. 3B inserted into the temperature cycling
device, for
performing parallel reactions that advantageously can use the template capture
and
2 5 normalization methods of the present invention;
[0209] FIG. 7A shows an internal cross section of an air-based thermal cycler
with
integrated capillary cassette sealing membranes, which can advantageously be
used with
the template capture methods of the present invention;
[0210] FIG. 7B shows a perspective detail of the air-based thermocycler of
FIG. 7A, with
3 o the lid raised to illustrate the chamber into which the capillary cassette
is inserted;
[0211] FIG. 7C shows a cross section of the cassette compartment with the
capillary
cassette inserted into the internal chamber of the thermal cycler of FIG. 7A;
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 29 -
[0212] FIG. 8A is a front view of a capillary cassette wash station useful in
high
throughput performance of the methods of the present invention;
[0213] FIG. 8B is a side view of the capillary cassette wash station of FIG.
8A with the
wash manifold lowered and the wash tank raised;
s [0214] FIG. 8C is a further view of the capillary wash station of FIGS. 8A
and 8B with the
wash manifold raised and the wash tank lowered;
[0215] FIG. 8D is an interior cross-section of the wash manifold;
[0216] FIG. 8E is a schematic plumbing diagram of the wash station;
[0217] FIG. 8F is a top perspective view of the wash tank;
[0218] FIG. 9 shows a histogram of the percent success versus read length
window for
the sequencing analysis of example 1;
[0219] FIG. 10 is an electropherogram of the reaction products of example 2;
[0220] FIG. 11 shows a histogram of the percent success versus read length
window for
the sequencing analysis of example 3;
[0221] FIG. 12A shows a scanned gel image of electrophoretically separated PCR
products prepared at full volume;
[0222] FIG. 12B show a scanned gel image of electrophoretically separated PCR
products prepared at a nanoscale volume (500 nL);
[0223] FIG. 13 is an electropherogram of analysis of sequencing mixtures
prepared by
2 o performing PCR at 500 nL volumes, a cleanup reaction at full volumes,
followed by cycle
sequencing reactions performed at 500 nL;
[0224] FIG. 14 is a graph comparing signal strength of an isothermal reaction
for products
prepared in tubes, capillaries, and capillaries using surface binding;
[0225] FIG. 15 is a flowchart explaining the methodology for preparing
capillary tubes in
2 s which nucleic acid is reversibly directly immobilized;
[0226] FIG. 16 illustrates an embodiment of the method of the present
invention;
[0227] FIG. 17A shows the results of sequencing PCR products mixed with the
reaction
mixture prior to sequencing; FIG. 17B shows the results of first mixing the
PCR template
with sodium thiocyanate, binding the DNA to the inner surface of the
capillary, washing the
3 o DNA with 80% ethanol, followed by sequencing;
[0228] FIG. 18 represents the retained mass of DNA following a template
capture
protocol;
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 30 -
[0229] FIG. 19 shows a plot of read length versus starting DNA mass for
samples
prepared by premixing DNA and sequencing reagents (~ ) compared to samples
prepared
by template capture (~);
(0230] FIG. 20 shows products of PCR reactions after template binding of the
indicated
s starting amount o M13mp18, electrophoresed through a 1.5% agarose gel,
stained with
SYBR Green dye and imaged with a Fluorimager apparatus;
[0231] FIG. 21 represents the relative signal intensity obtained with
increasing template
concentration;
[0232] FIG. 22 represents the relative signal intensity obtained with
increasing template
1 o concentration, showing peak height increasing with increasing template
concentration;
[0233] FIGS. 23A and 23B show a trace that had a Phred 20 score of 561 bases
obtained
by nanoscale direct cycle sequencing from glycerol stocks;
[0234] FIG. 24 are MegaBACET"" traces from four nanoscale single base
extension
reactions, without template capture, demonstrating heterozygosity in trace 2;
1 s [0235] FIG. 25 shows the results of quantitative analysis of nanovolume
PCR products
(FIG. 25B) in comparison with that of full volume PCR products (FIG. 25A);
[0236] FIG. 26 shows the results of full volume SBE reactions and a negative
control;
[0237] FIG. 27 presents the MegaBACET"" traces of (A) a full volume single
base
extension reaction; and (B) a nanovolume single base extension reaction, from
full volume
2 o PCR and ExoIISAP treatment of the PCR product;
[0238] FIG. 28 presents the MegaBACET"" traces of (A) a full volume single
base
extension reaction; and (B) a nanovolume single base extension reaction, from
nanovolume
PCR and template capture of the PCR product;
(0239] FIG. 29 presents the MegaBACET"" traces of (A) a nanovolume single base
2 s extension reaction, from nanovolume PCR and template capture of the PCR
product, but
with no subsequent cleanup of the SBE products; (B) a nanovolume SBE reaction
with
CIAP cleanup and injected into MegaBACET"" with MegaBACET"" loading solution;
(C) a
nanovolume SBE reaction with CIAP cleanup and injected into MegaBACET"" with
deionized
water; and (D) a nanovolume SBE reaction with Sephadex cleanup and injected
into
3 o MegaBACET"" with deionized water;
[0240] FIG. 30 shows the results of a validation experiment comparing full
volume and
nanovolume SBE;
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 31 -
[0241] FIG. 31 illustrates a representative peptide profile of cytochrome C,
after protease
digestion by trypsin in a capillary cassette. The profile was generated by
MegaBACETM
analysis. Trypsin is either in solution (FIG. 31A) or immobilized on magnetic
beads (FIG.
31 B);
s [0242] FIG. 32 presents a representative first run of a peptide profile of
cytochrome C,
after protease digestion by trypsin that is covalently coated onto an internal
surface of a
capillary in a multi-capillary cassette. The internal capillary surface was
modified by either
aminoalkylsilane reagent or streptavidin modification. The profile was
generated by
MegaBACETM analysis;
[0243] FIG. 33 presents a representative second run of a peptide profile of
cytochrome C,
after protease digestion by covalently surface-coated trypsin. The capillary
surface was
modified by either aminoalkylsilane reagent or streptavidin modification. The
profile was
generated by MegaBACETM analysis;
[0244] FIG. 34 presents a representative third run of a peptide profile of
cytochrome C,
1 s after protease digestion by covalently surface-coated trypsin. The
capillary surface was
modified by either aminoalkylsilane reagent or streptavidin. The profile was
generated by
MegaBACETM analysis;
[0245] FIG. 35 presents a representative HPLC profile of cytochrome C, after
protease
digestion by covalently surface-coated trypsin. The capillary surface was
modified by either
2 o aminoalkylsilane reagent or streptavidin modification; and
[0246] FIG. 36 presents the relationship between Asp-N concentration and the
amount of
polypeptides digested, represented here by signal intensity of the Cy3
emission from the
digested peptides.
25 DETAILED DESCRIPTION OF THE INVENTION
[0247] In order that the invention herein described may be fully understood,
the following
detailed description is set forth.
[0248] In the present invention, it was realized that a capillary segment
could be used
3 o both to meter reagents and as a reaction container for performing
temperature cycling
reactions. The length of the capillary and the internal diameter (I.D.) of the
bore of the
capillary tube define the volume of the interior of the capillary tube
segment. Capillaries with
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 32 -
a 50-150 um I.D. are commonly available. The small internal diameter of the
capillary tubes
allows creation of a reaction container having an interior volume less than
one microliter.
With the present invention, capillaries having volumes from 10-500 nanoliters
are adaptable
to the preparation of DNA cycle sequencing reactions or any other reaction.
s [0249] The process carried out by the present automated system is shown in
the flow
chart of FIG. 2. The process begins by the assembly of the reaction mixture,
box 52, by
combination of reagents and a sample nucleic acid. The combined reagents are
then
introduced into the capillaries of a capillary cassette, box 54. The ends of
the capillaries are
next sealed, box 56. The sealed capillary segments are exposed to thermal
cycles, box 58,
1 o which effect the cycling reaction. The capillaries of the capillary
cassette are then dispensed
onto a substrate, box 60. The substrate is then transferred to an analytical
system for
analysis of the reaction mixture, box 62. Details of this process and the
structure of the
apparatus for carrying out this process are detailed herein.
[0250] In reference to FIG. 1, an automated system is shown for assembly of
reaction
15 mixtures, temperature cycling to effect the chemical reaction, and
dispensing the volume of
the completed reaction mixture onto a substrate for subsequent analysis. In
the system an
automated robot 102 may move the length of stage 114 and may rotate such that
automated robot 102 may be moved in relation to other components of the
automated
system. The automated robot 102 may be rotated to allow the transfer head 104
on
2 o automated robot 102 to access objects on all sides adjacent to stage 114.
The assembly of
a reaction mixture would begin by the transfer head 104 picking up a capillary
cassette from
cassette hotel 106.
[0251] Capillary cassette 15 is shown in FIG. 3A. The capillary cassette is
comprised of a
number of capillary tubes 12 extending through a substrate 10. It is preferred
that the
2 5 capillary cassette have at least one row of eight capillary tubes and that
the capillary tubes
have equal spacing. The capillary cassette shown has substrate 10 with 96
capillary tubes
arranged in an 8 by 12 array, with spacing of the tubes matching the spacing
of the wells of
a 96 well microplate. The length of capillary tubes 12 extending from either
side of substrate
is unequal. It is preferred that the shorter end of capillary tube segments 12
be shorter
3 o than the depth of a microplate well. This allows the short end of
capillary tubes 12 to be
inserted into the wells of a microplate while substrate 10 rests on the top of
the microplate.
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 33 -
[0252] The capillary tubes may be made of any material compatible with the
assay and
preparation to be performed, but preferred capillary materials include, but
are not limited to,
glass and silica capillaries, although plastic, metals and other materials may
also be used.
Capillary tubes of various dimensions may be used, such as 75 um ID capillary
tubes or 150
s um I.D.1360 um O.D. capillary tubes.
[0253] The capillary tubes 12 extend through a substrate 10 and preferably are
arranged
in a uniform pattern. The capillary tubes are of equal length and extend
through the
substrate in a substantially parallel orientation such that each of the two
opposing ends of
the capillary tubes 12 are coplanar and the planes defined by the ends of the
capillary tubes
12 are substantially parallel to the substrate 10. The spacing of the
capillary tubes may be
uniform and selected to match the center-to-center spacing of wells on a
microplate. For
example on a standard 96 well microplate the capillary tubes would be arranged
with a 9
mm center to center spacing, on a 384 well microplate the capillary tubes 12
would be
arranged with a 4.5 mm center to center spacing. Higher density capillary
formats,
compatible with 1536 well microplates or plates with even higher well density,
should also
be possible. The capillary tubes 12 are preferably secured within the
substrate such that the
length of capillary tubes 12 extending from one side of the substrate 10 are
shorter than the
length of the capillary tube on the opposite side of substrate 10. The length
of the capillary
tubes 12 on the shorter side of the substrate may be matched to the depth of
wells in a
2 o microplate, such that the length of the shorter side is a shorter length
than the depth of a
well in a microplate. This feature enables the capillary cassette to be
inserted into a
microplate such that the substrate 10 rests against the top lip of the
multiwell plate and the
capillaries on one side of the substrate may extend into the multiwell plate
without touching
the bottom. For example, in a 96 well microplate the capillary tubes may be
disposed on a
2 5 substrate such that the shorter side of the capillary tube extending from
the substrate may
be inserted into wells in a microplate without the capillary touching the
bottom of the well.
This ensures that liquid dispensed into a well is clear of the capillary to
prevent re-entering
the capillary.
[0254] The capillary cassette substrate 10 may be made of a fiberglass board
or other
3 o rigid or semi-flexible material. The capillary tubes 12 may be inserted
through evenly
spaced holes in the substrate and secured with adhesive. In one embodiment,
the length
and width of the substrate are similar to the length and width of a standard
96 well
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 34 -
microplate. This simplifies adapting automated systems designed for
manipulation of
microplates to handle the capillary cassette.
[0255] In some embodiments it may be advantageous to coat the interior of the
capillary
with various surface coatings such as ionic and non-ionic surfactants.
Coatings that may be
s used include bovine serum albumin (BSA), glycerol, polyvinyl alcohol and
Tween 20. The
coatings are introduced into the capillary and dried onto the interior surface
of the capillary
tube. Alternatively, covalent modification of the interior surface with
silanization or Griganard
reaction may be desired. For example, covalent modification of capillary tubes
interior
surfaces that reduce electroendoosmosis may also be useful in reducing charge
surface
1 o effects between a capillary interior surface and components of a reaction
mixture. U.S.
patent application Ser. No. 091324,892, hereby expressly incorporated by
reference for all
purposes herein, discloses the use of acryloyldiethanolamine as a covalent
capillary coating
with advantageous alkaline stability. In addition to this coating, acrylimide
or other known
coatings may also be used to covalently modify capillary interior surfaces.
A. Assembly of Reaction Mixture
[0256] Returning to FIG. 1, the automated system allows for the combination of
reaction
reagents and sample DNA using the capillary cassette. A capillary cassette
would be taken
2 o by transfer head 104 from the cassette hotel 106 and brought into contact
with the samples
contained in a sample plate at location a. The sample plate is dispensed from
sample plate
hotel 108. The sample would be drawn into the capillary tubes of the capillary
cassette by
capillary action. The internal volume of the capillary tube is defined by the
length of the
capillary tube and its internal diameter. The capillary cassette of FIG. 3A
acts as a fixed
2 5 volume parallel pipettor, allowing a number of capillary tubes to be
filled in parallel. Each
capillary tube segment will meter a discrete amount of liquid that may be
subsequently
dispensed.
[0257] Once one end of each capillary is inserted into the sample containing
well, liquid
will be drawn into the capillary. This small amount of sample may be combined
with other
3 0 liquids to form a reaction mixture. The sensitivity of analytical
instruments such as a
capillary array electrophoresis system and the amplification of reaction
mixture products
enabled by cycling reactions allow for nanoscale reactions and analysis. Very
small-scale
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 35 -
reaction are able to reliably produce reaction mixture products of sufficient
quantity for
analysis on a capillary array electrophoresis system, a capillary
electrophoresis chip, a
mass spectrometer, or other analysis instrumentation. Significantly less
reaction reagents
are required if a nanoscale reaction mixture is enabled.
(0258] The automated system may be used in various ways to prepare reaction
mixtures.
A few of the many such methods for use of the system in production of reaction
mixtures
follow.
Reaction Mixture Preparation Example 1: Metering Reagents with Capillary
Cassette and
1 o Mixing on a Substrate
[0259] One method to prepare the reaction mixture is to use the pipettor to
separately
meter the components of a reaction mixture. For example for a PCR mixture, the
nucleic
acid sample and PCR reagents would be separately metered and dispensed into a
single
container in which the liquids are combined. In using the automated system of
FIG. 1, the
automated robot 102 moves transfer head 104 containing a capillary cassette to
location a
where a sample plate is located. The ends of the capillary tubes of the
capillary cassette are
dipped into the wells. The capillary tubes fill by capillary action, metering
precise amounts of
the samples. The wells of sample plate contain the nucleic acid sample to be
PCR
2 o amplified. The DNA sample should be sufficiently dilute such that 5-20 ng
of DNA is
contained in the 10-10,000 nL volume metered by each capillary tube segment in
the
capillary cassette.
[0260] FIG. 4A shows a 16 channel capillary cassette transferring fluid
samples from a
multiwell plate 36 into a capillary cassette 15. The capillary tube segments
12 on capillary
2 5 cassette 15 are extended into the wells of multiwell plate 36. The wells
of multiwell plate 36
are conical and liquid in the well will flow to the bottom central area of
each well. This allows
a small amount of liquid within the well to be positioned such that a
capillary inserted into
the center of the well and above the bottom of the well will contact the
liquid. The capillary
tube segments of the capillary cassette then fill by capillary action with the
liquid in the
3 o wells. It is preferred that the capillary cassette have. capillary tube
segments which have the
same center to center spacing as the wells of the multiwell plate containing
the DNA
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 36 -
samples. In one embodiment the capillary cassette has the same number of
capillary tube
segments as the number of wells in a multiwell plate holding samples.
[0261] Returning to FIG. 1, after the capillary cassette is dipped into the
nucleic acid
sample containing wells, the filled capillary cassette may be moved by
transfer head 104 to
s a dispensing device location 122. At the dispensing device location 122, the
sample is
dispensed onto a substrate. A clean capillary cassette is then retrieved and
dipped into a
sample plate containing the PCR reagents. As seen earlier, the capillary
cassette meters a
precise amount of liquid defined by the interior volume of the capillary tubes
held in the
capillary cassette. The metered amount of reaction reagents may be the same
volume as
1 o the volume of sample dispensed or it may be different, depending on the
requirements of
the application. At the dispensing device location 122, the reaction reagents
are dispensed
from each capillary tube segment onto locations on the mixing substrate
containing the
nucleic acid sample.
[0262] The present reaction mixture assembly may be used for assembly of
numerous
1 s types of reactions. The same basic method used to assemble the PCR
reaction mixture
may be adapted to assembly of a cycle sequencing mixture, rolling circle
amplification
reaction mixture, enzymatic assays, chemical reactions, or other reaction
mixtures.
[0263] When dispensing the contents into a microplate some care must be taken
to mix
the sample and reaction reagents in a manner which avoids splattering. A
number of
2 o different methods have been envisioned to dispense liquid from the
capillary cassette.
Capillary Cassette Dispensing Example 1: Centrifugal Force
[0264] The first method to dispense the contents of the capillary cassette
while avoiding
2 s splattering uses a centrifuge to dispense the fluid by centrifugal force.
The centrifugal force
is applied evenly to all of the capillaries in the capillary cassette such
that capillaries
independently dispense into the microplate wells. The dispensed liquid is
drawn by
centrifugal force to the bottom of wells in the multiwell plate.
[0265] In FIG. 5A, the centrifuge 42 is shown having a swinging microplate
bucket 43 that
3 o may contain a multiwell plate with an inserted capillary cassette. The
swinging microplate
buckets are held on rotor 41.
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 37 -
(0266] FIG. 5B shows a cross-section of swinging microplate bucket 43. The
capillary
tubes 12 of the capillary cassette are inserted into wells 36a of multiwell
plate 36. The
cassette is inserted such that the portions of the capillary tubes 12
extending from the
substrate 10 are shorter than the depth of the wells 36a. As shown in FIG. 5B,
the capillary
s tube 12 extending from substrate 10 do not reach the bottom of the wells 36a
of multiwell
plate 36. Microplate swinging bucket 43 is comprised of an arm 45 and a
platform 44. An
upper end of arm 45 fits onto latch head 42 on rotor 41. Microplate 36 is
positioned on
platform 44 of microplate swinging bucket 43. When the centrifuge is in
motion, platform 44
rotates on latch head 42 such that the multiwell plate faces the side wall of
the centrifuge
1 o and the centrifugal force on the liquid in the capillary tubes dispenses
the liquid into the
bottom of the wells 36a of the multiwell plate 36. When conical shaped wells
are used, the
centrifugal force will draw the liquids within the well to the well center,
causing the sample to
locate at a more precise location. The liquid will be displaced from the
capillary at fairly low
centrifuge speeds.
i s (0267] In FIG. 1, a low speed centrifuge may optionally be included in the
automated
system at the dispensing device location 122. Automated robot 102 uses
transfer head 104
to pick up a microtiter plate dispensed onto location b by microtiter plate
hotel 110. Transfer
head 104 transfers the microtiter plate to the stage having the low speed
centrifuge. A
capillary cassette is filled with samples or reaction reagents as described
and is transferred
2 0 onto the microtiter plate on the stage of the low speed centrifuge. The
plate and cassette
are then spun in the centrifuge, dispensing the liquid from the capillaries
into the wells of the
microtiter plate. Once the liquid has been dispensed and the centrifuge has
stopped
rotating, the capillary cassette may by removed by the transfer head and
transferred to the
cassette washer 118. The transfer head 104 can then pick up a clean capillary
cassette
2 s from the capillary cassette hotel 106. The clean capillary cassette can be
used to meter a
second liquid reaction component that is similarly dispensed into the
microtiter plate using
the centrifuge. In the automated system the centrifuge includes a sensor
associated with the
rotor used in conjunction with a rotor braking system to stop the rotor in a
position that
transfer head 104 can access. Such a sensor could be magnetic, optical,
mechanical, or
3 o use other known means of sensing rotor position.
Capillary Cassette Dispensing Example 2: Air Displacement
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 38 -
[0268] A second method of dispensing the liquid contained in the capillary
tube segments
of a capillary cassette is through the use of an air displacement device. With
reference to
FIG. 1, a microtiter plate dispensed from microtiter plate hotel is
transferred by transfer head
s 104 to the dispensing device location 122. At this location an air
dispenser, such as the one
pictured in FIG. 4A-C is located. Subsequently a capillary cassette is
retrieved by transfer
head 104, and filled with either sample from a sample multiwell plate or with
reaction
reagents. The capillary cassette is then moved to the dispensing device
location 122 and
brought into contact with air displacement head. The substrate of the
capillary cassette is
1 o placed on a receiving platform on the air displacement head.
Alternatively, the air
displacement head may be joinable to automated transfer robot 102.
[0269] With reference to FIG. 4A, the air displacement head 301 is shown with
a capillary
cassette 15 held on bottom plate 302. The bottom plate 302 is attached to a
manifold
assembly by hinge 318. Capillary cassette substrate 10 is held on foam rubber
pad 304 that
1 s is secured onto bottom plate 302. An array of holes 325 extend through
foam rubber pad
304 and bottom plate 302, which are spaced to allow the capillary tubes 12 to
extend
through foam rubber pad 304 and bottom plate 302 when the capillary cassette
is positioned
on bottom plate 302. The manifold assembly of the air displacement head is
comprised of
an upper housing 306, chamber unit 310 and a set of clamps 314. Clamps 314
secure
2 o membrane 312 to the lower surface of the chamber unit 310. Membrane 312
forms a seal to
the top surface of the capillary cassette 15 when the manifold assembly is
closed over the
cassette. Membrane 312 has holes 316 corresponding to capillary positions in
the cassette
when the capillary cassette 15 is placed on bottom plate 302. When the top
manifold of air
displacement head 301 is closed against bottom plate 302, capillary tubes 12
are positioned
2 s extending through capillary tube receiving holes 316 on membrane 312. When
the air
displacement head 301 is closed it may be secured by latch 322 which mates
with hole 324
to clamp the capillary cassette between the foam rubber pad 304 and membrane
312
resulting in a seal between the top surface of cassette 15 and the membrane
312.
(0270] FIG. 4B illustrates a cross sectional view of displacement head 301.
Upper housing
3 0 306 is constructed of metal, acrylic or other rigid material. Gas input
coupler 303 is disposed
on upper housing 306. When a pressurized gas or vacuum line 305 is attached to
gas input
coupler 303, a vacuum or pressure force may be introduced into upper chamber
307. Held
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 39 -
between upper housing 306 and chamber unit 310 is a gas impervious elastic
membrane
308. The area between elastic membrane 308 and upper housing 306 defines upper
chamber 307. Secured onto clamps 314 is membrane 312. Membrane 312 is pressed
against substrate 10 of a capillary cassette inserted into displacement head
301. Substrate
s 10 is secured within displacement head 301 by bottom plate 302. Rubber pad
304 provides
a deformable surface that exerts uniform force pressing substrate 10 against
membrane
312. Membrane 312 has an array of holes 316 that allow the capillaries 12 of
the capillary
cassette to extend through membrane 312. When a capillary cassette is inserted
into air
displacement head 301, the substrate seals holes 316 enclosing lower chamber
313. When
1 o pressurized gas is introduced into chamber 307 by gas line 305, elastic
membrane 308 will
be pressed into lower chamber 313. Membrane 308 is located between upper
chamber 307
and lower chambers 313. Membrane 308 serves both as seal for the upper end of
chambers 313 and the chamber displacement actuator when pressure is applied to
the
upper chamber 307 through coupler 303. The degree of displacement is dependent
on the
1 s pressure applied and the elasticity of membrane 308. The resulting air
displacement will act
to dispense liquid from capillary tubes 12 that extend through the capillary
cassette 10 and
into the lower chamber 313. By regulating the amount of pressure applied
through line 305,
a consistent displacement force will be applied to each capillary tube. Given
the
submicroliter volume of the capillary tube segments, fluctuations in the
amount of
2 o dispensing pressure should not adversely affect displacement from the
tubes.
[0271] FIG. 4C illustrates the closed air displacement head 301. Upper housing
306 is
pulled toward bottom plate 302 by latch 322 in order to compress membrane 312
against
the top of the capillary cassette substrate thereby forming a seal. Clamps 314
secure
membrane 312 onto chamber unit 310. Air displacement head 301 is mounted on
arm 320.
2 s Arm 320 may extend from automated transfer robot 102 shown in FIG. 1 or be
positioned at
dispense location 122. Pressurized gas may be introduced into upper housing
306 through
gas input couple 303.
[0272] This displacement head provides an individual displacement chamber for
each of
the capillaries dispensed. Although a 16 capillary cassette is depicted, the
displacement
3 o head may be constructed to dispense capillary cassettes having an array of
96 capillaries or
greater capillary densities. The dispensing force applied to each capillary is
sufficiently small
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 40 -
to allow dispensing directly onto a substrate with the sample dispensed at a
discrete
location.
[0273] Air displacement or centrifugal displacement may be used to dispense
liquid from
the capillary tube segments in a capillary cassette. It may also be possible
to dispense liquid
s from the capillary tubes using a bank of syringe pumps, applying pressure
through a gas
permeablelliquid impermeable (hydrophobic) membrane, using electrokinetic
dispensing, or
other known dispensing means. The air displacement head may also be used to
dispense
finished reaction mixtures onto a substrate for subsequent analysis.
1 o Reaction Mixture Assembly Example 2: Dehydrated Reagents
[0274] A second method to assemble the reaction mixture is to have the regents
required
for the reaction stored as a dehydrated coating either on the interior of a
capillary or on a
substrate, such as within a well of a multiwell plate. If the reaction
reagents were
15 dehydrated onto the interior of capillary tube segments in a capillary
cassette, introducing a
sample into the capillary would cause rehydration, mixing and formation of the
reaction
mixture. In a similar manner, if the wells of a microplate were coated with
the dehydrated
reaction reagents, adding a nucleic acid sample into the wells would bring the
reaction
reagents into solution, forming an assay mixture. The sample could be metered
with a
2 o capillary cassette and dispensed from the capillary cassette by one of the
methods set out
above. The sample would bring the dehydrated reaction reagents into solution
and mix with
the sample containing nucleic acid by diffusion. This provides a method to
assemble the
reaction mixture in a very simple manner, potentially without the need to
dispense the
capillary tubes with a centrifuge or air displacement device. This could both
simplify the
2 5 reaction processing system and shorten the reaction assembly time.
[0275] For PCR, a dehydrated reagent mixture is commercially available, sold
as Ready-
to-Go~ (Amersham Pharmacia Biotechnology, Piscataway, N.J.). The stabilized,
dehydrated reagents may be coated onto the interior surface of capillary
segments or the
interior of the wells of a multiwell plate. The Ready-to-Go~ product uses a
carbohydrate
3 o matrix to stabilize the reaction reagents (DNA polymerise, buffer
reagents, dNTPs) in a
desiccated state. Bringing the reagents in the Ready-to-Go~ mixture into
solution with the
liquid nucleic acid sample and primers in solution produces the final reaction
mixture
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 41 -
required for the reaction. The combination of the stabilized Ready-to-Go~
compounds, the
template DNA, primers, and sufficient water produces a final reaction mixture.
It is
contemplated that reagents for chain termination sequencing reactions and
other reactions
could also be stored in a desiccated state.
s [0276] The coating could be applied to surfaces by a number of different
methods
including vapor phase coating, filling a capillary (by capillary action,
pressure filling, etc.)
with the Ready-to-Go~ mixture and emptying the bulk phase (under vacuum,
pressure or
other forces), or dipping a substrate (such as a bead) into the reagents and
subsequently
drying the bead.
to
Reaction Mixture Assembly Example 3: Solid Phase Capture
[0277] A third method of assembly of the reaction mixture is to capture
material from the
sample on the surface of a substrate, such as the interior of a capillary tube
segment. The
is material captured can be nucleic acid, enzymes, other biopolymers, or
chemicals. The
desired material from the sample may be attached onto the surface by a number
of
methods. These include covalent attachment, binding by antibodies, DNA
hybridization,
hydrophobic interactions, electric field, magnetic field, or other chemical or
physical forces.
Once the sample has been attached, the remaining liquid in which the sample
was
2 o suspended may evacuated from the capillary or microchip by chemical
reaction or physical
force. Air displacement or centrifugal dispensing method may be used to empty
the
capillary, as can a vacuum. The sample material would remain on the surface of
the
substrate. In this single step, the sample material may be substantially
purified. The reaction
reagents may then be combined with the sample material, producing the reaction
mixture.
2 s [0278] For nucleic acids, one method to immobilize a nucleic acid sample
is to attach the
nucleic acid directly to a surface. This may be done by non-covalent
modification (such as
surface treatment with NaSCN, DMSO, etc.) or covalent linkage. There are a
number of
different covalent attachment methods for DNA known in the art. For example,
an amino
group can be attached to the deoxyribose base of DNA and incorporated during a
synthetic
3 o reaction, such as during PCR amplification of a DNA plasmid insert. The
glass or silica of a
capillary interior could be silanized and the amino group on the modified DNA
would
covalently bond to the silanized interior of the capillary. Alternatively,
other chemistries are
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 42 -
available to covalently immobilize DNA onto a surface. Once the DNA is bound
to the
surface of a capillary or other substrate, the liquid in which the DNA was
suspended may be
eliminated from the capillary and the capillary may be filled with reaction
reagents.
[0279] An alternative method of attaching a nucleic acid to the interior of
the capillaries of
s a capillary cassette is through affinity chemistry. One common affinity
chemistry procedure
labels a biomolecule with biotin and then binds the biotinylated biomolecules
to avidin or
streptavidin. The avidinlstreptavidin may be used to link the biotinylated
molecules. Nucleic
acid labeled with biotin may be subsequently attached to a surface, such as
the interior of a
capillary tube. This may be accomplished by binding streptavidin to the
interior of the
1 o capillary.
[0280] One example of the use of affinity chemistry for the binding of DNA to
the interior
of a capillary is disclosed in U.S. Pat. No. 5,846,727, hereby expressly
incorporated herein
for all purposes. This reference describes the binding of DNA to the interior
surface of the
capillary tubes. The technique requires primers labeled with biotin that are
combined with
i5 dNTPs, a DNA polymerise, and a reaction buffer. This is combined with
template DNA,
such as plasmids or M13 from a DNA library with sub-cloned DNA inserts, to
form the
reaction mixture. In the present invention a microplate may contain 96 or more
reaction
mixtures, each with a unique template with a subcloned DNA sequence. This
reaction
mixture could be assembled by the method stated in reaction mixture assembly
example 1:
2 o namely the reaction reagents and the template sample could be separately
metered and
dispensed into a 384 well microtiter plate. In a microplate well the liquids
are combined to
form a reaction mixture. The reaction mixture is metered into the capillary
tube segments of
a capillary cassette. The PCR reaction may be effected by temporarily sealing
the ends of
the capillary tube segments and exposing the capillary cassette to thermal
cycles, as
2 s described below. The results of the PCR reaction are exponentially
amplified copies of the
subcloned plasmid DNA insert containing the biotin labeled primer.
(0281] The PCR amplified DNA containing the biotin labeled primer may then be
immobilized on the walls of the capillary tubes of a capillary cassette. The
immobilization
capillary cassette would have capillary tubes with avidin or streptavidin
coated onto the
3 o interior surface of each capillary tube. The chemistry for attachment of
avidinlstreptavidin
may be that disclosed in, for example, L. Amankwa et al., "On-Line Peptide
Mapping by
Capillary Zone Electrophoresis," Anal. Chem., vol. 65, pp. 2693-2697 (1993).
The capillary
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 43 -
is filled with (3-aminopropyl) trimethoxysilane (3-ATPS), incubated for 30
minutes, and air
dried. The dried capillaries in the capillary cassette are next filled with
sulfosuccinimidyl-6-
(biotinamido)hexonate (NHS-LC biotin) which is again incubated followed by air
drying.
Avidin or streptavidin in phosphate buffer at 7.4 pH is added to each
capillary tube. The
s avidin binds to the biotin immobilized on the interior of each capillary.
The double stranded
amplified biotinylated PCR products suspended in a buffer (e.g. Tris-HCI, or
EDTA with
either NaCI or LiCI at 1-3M added for efficaceous binding) are added to the
capillary tube
and incubated for 5-10 min. This results in a capillary wall modified as
follows: capillary wall-
-Si--C.sub,3 H6 --NH--CO-biotin-avidin or streptavidin-amplified
oligonucleotide with
1 o associated biotin primer.
[0282] In this embodiment biotin, rather than avidin or streptavidin, is
covalently attached
first to the capillary wall. This aids in the regeneration of the capillary
cassette for
subsequent binding reactions. After completing the cycle sequencing reaction,
it would be
difficult to remove the amplified biotinylated DNA without also denaturing the
avidin protein.
1 s By having biotin bound to the interior surface of the capillary the
amplified DNA may be
easily removed by filling the capillary with phenol or formamide solution at
65-90 degrees C.
This denatures the avidin protein without removal of the biotin bound to the
interior surface
of the capillary. This mixture is then dispensed. The capillary cassette may
then again be
filled with the avidin containing solution and reused for binding subsequent
biotinylated
2 o amplified template DNA.
[0283] Once the DNA is immobilized on the interior surface of the capillary,
the contents
of the capillary tube may be dispensed in one of the methods described and the
DNA would
remain bound to the surface of the capillary. This removes debris and other
impurities from
the DNA presenting a rapid and effective method of DNA purification. The
capillary may be
2 s rinsed with a buffer for additional purification. The defined area of the
interior surface of the
capillary provides a known amount of binding sites for the DNA attachment.
This provides a
simple method for normalization of DNA concentrations. The normalization of
DNA
concentrations is important in improving the success rate of CAE analysis of
cycle
sequencing reactions. The capillary cassette may then be dipped into wells or
a reagent
3 o reservoir containing the reagents for cycle sequencing. The cycle
sequencing reaction can
be performed by temporarily sealing the ends of the capillary tubes by
pressing each end of
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 44 -
the capillary tubes against a deformable membrane. The capillary cassette may
then be
exposed to thermal cycles that effect the cycle sequencing reaction.
[0284] Prior to filling, the capillary tube segments of the capillary cassette
may be coated
with a variety of compounds. Coating the interior surface of the capillary
tube segments with
s bovine serum albumin (BSA) or polyvinyl alcohol has been shown to improve
performance
of some reactions, such as preparation of chain termination sequencing
reactions.
B. Thermal Cycling
[0285] Once the reaction mixture is introduced into the capillary tube
segments of the
capillary cassette, the ends of the capillaries of the capillary cassette are
sealed and the
capillary cassette is exposed to temperature cycles. The ends of the capillary
cassette
capillaries are sealed by pressing each of the ends of the capillary tubes
against a
deformable membrane. Returning to FIG. 1, once the capillary cassette has been
filled with
1 s the reaction mixture, the ends of the capillaries are sealed and the
capillaries are exposed
to thermal cycles in thermal cycling device 116.
[0286] In one thermal cycling device, shown in FIGS. 7A-7C, the thermal
cycling device
has integrated membranes that seal the ends of the capillaries and exposes the
capillary
cassette to thermal cycles. In this apparatus the means for sealing the ends
of the
2 o capillaries in the capillary cassette is incorporated into the thermal
cycling device.
[0287] With reference to FIG. 7A and 7B, the capillary cassette 15 is held on
lip 280 within
internal passageway 256 between deformable membranes 264a and 264b. As seen in
FIG.
7B, deformable membrane 264a is mounted on upper platform 261. Lid 262 is
secured on
upper platform 261. Platform 261 is attached by pivot 286 to base 265.
Pneumatics 284a,
2 s 284b are attached at an upper end to upper platform 261 at pivot 263.
Screw 282 acts as a
stop for upper platform 261 when upper platform 261 is lowered onto housing
270,
enclosing internal passageway 256. Diffuser 258 promotes temperature uniformly
of air
circulating in internal passageway 256. Thermocouple 260 measures temperature
of the
circulating air. The function of pivot 277 and bottom membrane platform 200 is
described in
3 o conjunction with FIG. 7C.
[0288] FIG. 7C shows a cross section of the capillary cassette holding chamber
with
capillary cassette 15 inserted into the internal passageway 256. The capillary
cassette could
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 45 -
be inserted into this area by automated robot 102 of FIG. 1 after the
capillary tube segments
have been filled with the samples and reaction mixture.
[0289] Capillary cassette 15 is positioned such that substrate 10 rests on
ledge 280.
Capillary cassette is positioned such that the ends of capillary tube segments
12 are
depressed against top deformable membrane 264a and bottom deformable membrane
264b when upper platform 261 is lowered over the capillary cassette and lower
platform 271
is raised. Lid 262 seals against housing 270 when upper platform 261 is
lowered to provide
a flush seal. Screw 282 acts as a stop for upper platform 261 to prevent the
platform from
lowering so far that capillary tube segments are bowed or damaged. Base
platform 266 is
1 o secured to post 273 and secured to housing 270. The lower end of
pneumatics, 284b is
secured at a lower pivot 271 a to lower platform 271. Extending through lower
platform 271
are shoulder screws 268 which extend through housing 270 and stationary
platform 266 and
are secured to lower platform 200. When upper platform 261 is lowered by
pneumatic 284b
lower platform 271 is also raised toward housing 270. When pneumatic cylinders
284b,
284a are retracted, the pneumatic cylinders move to a vertical orientation.
Upper platform
261 is lowered and lower platform 271 is raised slightly in an arc. Lower
platform 271 will
arc upward on pivot 277 to move to a position substantially parallel to upper
platform 261
when pneumatic cylinder 284b is fully retracted. When a capillary cassette 15
is inserted
into internal chamber 258 the ability of platform 200 to "float" on springs
275 prevents
2 o excess pressure from damaging capillary tubes 12 or membranes 264a, 264b.
Platforms
261 and 200 exert 400 pounds per square inch force on capillary tubes 12
providing
sufficient sealing pressure. With upper platform 261 lowered, the capillary
tube segments 12
are sealed at each end by deformable membranes 264a, 264b. Deformable
membranes
264a, 264b may be made of silicon rubber or other deformable material.
2 5 [0290] Returning to FIG. 7A, a motor 250 turns shaft 251 that rotates
squirrel cage blower
253. Blower 253 produces air movement through diffuser 254 to flow into
internal
passageway 256. The blower generates sufficient circulation flow that the air
flowing
through internal passageway 256 circulates at 2,000 feet per minute. Diffuser
254 ensures
that the heat of the air blown by blower 253 is uniform throughout passageway
256. Cone
3 0 255 on diffuser 254 aids in mixing the flowing air, promoting temperature
uniformity through
passageway 256. Diffuser 254 acts to ensure an even flow of air through
passageway 256
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 46 -
in the region of the capillary cassette and reduces non-uniformity from wall
loss effects in
internal passageway 256.
[0291] The internal passageway 256 is defined by outer housing 270. Outer
housing 270
is preferably of rectangular cross section and comprised of sheet metal,
plastic or other
durable materials. The internal surface of outer housing 270 at all locations
except for inlet
278 is lined with thermal foam insulation 272. Insulation 272 prevents excess
heating of
outer housing 270 and helps retain heat and aids temperature uniformity of the
air
circulating through internal passageway 256. After flowing through first
diffuser 254 the air
flows through second diffuser 258. Diffusers 254 and 258 promote uniform air
flow and
1 o temperature uniformity through internal passageway 256. Past first
diffuser 254 internal
passageway 256 transitions to match the dimensions of the capillary cassette.
The heated
air flows past thermocouple 260 that is vertically disposed at the center of
internal
passageway 256 just beyond second diffuser 258. Thermocouple 260 acts to
monitor the
temperature within internal passageway 256. Thermocouple 260 may be a
temperature-
15 monitoring device inserted into a capillary tube section that extends
through outer housing
270 and through foam insulation 272. Alternatively thermocouple 260 may be
selected such
that it accurately reflects the internal temperature of a capillary tube.
[0292] The air circulating through internal passageway 256 passes thermocouple
260 and
flows past the capillary tube segments 12 of capillary cassette 15. The ends
of the capillary
2 o tube segments are sealed at their upper end by deformable membrane 264a
mounted on
upper platform 261 that has been lowered to form an air tight seal with
housing 270. The
lower ends of capillary tube segments 12 are sealed by deformable membrane
264b.
Deformable membrane 264b is mounted on platform 200 that is secured on a
bottom
surface by shoulder screws 268. Shoulder screws 268 extend through housing 270
and
2 s retained by platform 271. Springs 275 located between platform 200 and
platform 271
provide a biasing force while allowing for platform 200 to float such that the
deformable
membrane 264b is biased against the ends of capillaries 12. The function of
double acting
pneumatics in sealing lid 262 and applying force to position platform 271 is
described in
conjunction with FIG. 7C. Lid 262 fits onto housing 270 such that the sheet
metal or other
3 o material comprising the edge of lid 262 fits on top of housing 270.
Membrane 264a is
mounted on upper platform 261 preferably such that membrane 264a extends into
internal
passageway 256 at least far enough that membrane 264a is even with insulation
272. As
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 47 -
the air travels past capillary tube.segments 12, the length of the capillary
tube segments 12
below substrate 10 are rapidly heated and cooled to the temperature of the air
rapidly
moving through internal passageway 256.
[0293] Door 274 controlled by motor 276 is used in conjunction with
thermocouple 260
s and heating element 252 to control the temperature within internal
passageway 256. When
door 274 is closed, the air circulating within internal passageway will not be
exchanged with
outside air. As the air continuously passes over heating element 252 the air
is rapidly
heated until the air comes to the selected temperature. Once thermocouple 260
senses that
the temperature is at a selected temperature, heating element 252 may be kept
at a lower
1 o heat output such that the internal temperature is maintained. If the
temperature needs to be
rapidly dropped, as in during a thermal cycling reaction, door 274 may be
moved to
orientation 274a by motor 276 with the door 274 moved into internal passageway
256,
allowing all air which has passed capillary cassette 15 to be exhausted from
internal
passageway 256 to the outside. It is envisioned that a filter or exhaust duct
could be
1 s mounted about door 274 to prevent compounds in the circulating air from
being exhausted
to the environment. The rapidly circulating air will be quickly exhausted to
outside of the
thermal cycler while ambient air is drawn in through air intake 278. Air drawn
into internal
passageway 256 through intake 278 flows through heater 252. The area through
which the
air moves is restricted by block 259 positioned above heater 252 within
internal chamber
2 0 256. Again the temperature of the air within internal passageway 256 is
monitored by
thermocouple 260 and when the desired temperature drop has occurred, door 274
will be
brought toward housing 270, reducing air volume drawn through air intake 278.
[0294] By connecting heating element 252, thermocouple 260 and door motor 276
to an
electronic control system, such as a computer controller, this thermal cycler
may perform
2 s precise air temperature varying sequences. Additional heat is added when
needed by
heating element 252 and heat is exhausted by opening door 274, with the
temperature
result of either action monitored by thermocouple 260. Exhausting circulating
air through
door 274 allows air within internal passageway to drop in temperature at a
rate greater than
degrees per second.
3 0 [0295] The rapid temperature change combined with the rapid transfer of
heat to or from
the capillaries allows for efficient temperature cycling reactions. For
example in reactions
using a thermostable polymerise, the denaturing of nucleic acid strands and
the annealing
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 48 -
of primer to template strands each may take place in one to five seconds. The
extension of
the primer will require less time to effect since the rapidly circulating air
and the thin walls of
the capillaries rapidly bring the internal volume of the capillaries to the
selected
temperature. The thin walls of the capillaries and the small capillary volume
enable a rapid
s temperature change and heat transfer throughout the internal capillary
volume. This greatly
reduces the time required for each cycle of the reaction, allowing more
efficient use of the
thermal cycler and greater throughput in sample preparation. Presently, a 30
cycle PCR
amplification may be performed in under 30 minutes. It should be possible to
reduce this
time to less than 8 minutes.
1 o Once the thermal cycling reaction is complete, upper platform 261 may be
raised and
capillary cassette 15 removed from internal passageway 256. During the
temperature
cycling process, the liquid within each capillary tube segment will expand
somewhat and
some liquid will leak from the capillary and be carried away by the rapidly
flowing air.
However, such loss is only a few percent of the volume of the capillary tube
segment and
1 s should not present either a contamination problem or cause enough reaction
product loss to
materially affect subsequent analysis. To prevent the small opening of
capillaries 12 from
being contaminated by the small residual of material on deformable membranes
264a and
264b, if desired, disposable materials such as a thin film can be placed over
the deformable
membranes. The disposable materials can be individual sheets or rolls of
material that
2 o advance after each use to prevent the capillary openings from touching a
section of material
previously used. In addition, the portion of capillary tube segments 12
located between
substrate 10 and deformable membrane 264a will receive only poor air flow and
will be less
likely to rapidly reach the denaturation temperature. However since this
length is short, the
failure of this area to as rapidly reach the denaturation temperature should
not adversely
2 s affect the ability of the remaining portion of the capillary from
producing sufficient reaction
product for subsequent analysis.
(0296] An alternative device for sealing the ends of the capillary is a
capillary cassette
holder that seals the ends of capillary tube segments of a capillary cassette.
With reference
to FIG. 3B the capillary cassette holder is comprised of a pair of parallel
deformable
3 o membranes 14a, 14b each secured onto platforms 16a, 16b. The deformable
membranes
may be silicon rubber seals, Teflon, plastics or other resilient, deformable
material. A pair
of parallel posts 9 extend from bottom platform 16a to top support platform 24
where the
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 49 -
posts are secured by internally threaded nut 18. Post 9 passes through
platform 24 and nut
18 is retained on an annular lip of platform 24. Shoulder screws 20 extend
through holes in
support 24 and are secured to top platform 16b. Springs 22 bias the top
platform 16b
against the ends of capillary tube segments 12 while allowing 16b to float.
The substrate 10
s of capillary cassette 15 may be designed to have holes which conform to the
spacing and
dimension of posts 18 such that capillary cassette 15 may be more easily and
securely held
within holder 23.
[0297] Once the ends of the capillary cassette are sealed in holder 23, the
combined
capillary cassette and holder may be exposed to thermal cycles. The holder
seals 16
1 o capillaries. However, a holder may be designed to hold capillary cassettes
having 96
capillaries or higher densities of capillaries. In addition to capillary
cassettes, chips of other
substrates may be used as the reaction containers. FIG. 3E shows a chip
substrate 70
comprised of two bonded substrate layers 72, 74. One layer 72 has grooves 76
extending
the length of the chip. The affixed top substrate 72 encloses a capillary
dimension passage
1 s 76 with opposite open ends. A liquid reaction mixture may be introduced
into the enclosed
passage. The ends of these passages may be sealed by pressing the ends against
a
deformable membrane, as was done with the capillary cassettes. Temperature
cycling may
require longer times because of greater mass material comprising the chip, but
cycling
times should still be more rapid than conventional cycling.
2 0 [0298] For isothermal reactions, such as rolling cycle amplification,
temperature cycling is
not required to effect the reaction. Once an isothermal reaction mixture is
combined and
introduced into a capillary cassette, incubation of the cassette at a reaction
temperature will
allow the reaction to occur. With reference to FIG. 1, the automated transfer
device may
transfer a capillary cassette into incubator 124 where the capillary cassette
is incubated at a
2 s selected temperature. A set of deformable membranes may be used to seal
the ends of the
capillaries during incubation. As was seen in other system components,
incubator 124 may
be used at the same time as other system components.
[0299] In the case of PCR or chain termination sequencing reactions it is
necessary to
expose the reaction mixture to temperature cycles. In FIG. 1 the transfer head
104 moves
3 o the capillary cassette into thermocycler 116. The thermocycling device may
be any device
that can expose the capillary tube segments of the capillary cassette to
temperature cycles.
Thermal cycling devices that use water, electric field, heating blocks, or
other means may
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 50 -
be used. Alternatively, air based thermal cycling devices are rapid and
adaptable to the low
volume cycling of the present invention.
(0300] A thermal cycling device that uses air as the temperature transfer
medium is
shown in FIG. 6. The reaction mixture is contained in capillary tube segments
that have a
high surface to volume ratio and small material thickness. This allows very
rapid transfer of
heat through the walls of the capillary and throughout the liquid reaction
mixture. An
equilibrium temperature is reached rapidly throughout the liquid in the
capillary. The use of
air as a heat transfer medium enables the rapid vamping of temperature in the
reaction
chamber. Rapid circulation of the air ensures rapid and more uniform heating
or cooling of
1 o the capillary segments and their contents,
[0301] With reference to FIG. 6, the capillary cassette 15 sealed within
holder 8 is inserted
through opening 215 in housing 202 of the air based thermal cycler. The holder
8 is
supported by housing surface 215 of the thermal cycling chamber 210. The
capillary tubes
12 mounted to substrate 10 are exposed to the air of thermal cycling chamber
210 such that
is the air may freely flow around capillary tube segments 12. Thermocouple 216
monitors the
temperature of the air moving past capillary tubes 12.
[0302] In the air based thermal cycling device, paddle 208 driven by motor 206
rapidly
circulates air within chamber 210. The air is rapidly circulated past the
capillaries 12 of
capillary cassette 15. Halogen bulb 220 acts as a heat source to heat the air
within the
2 o thermal cycling chamber 210. To effect a thermal cycling reaction, the
circulating air is held
at a selected temperature for a selected period of time. The thermocouple 216
transmits the
temperature of the capillary tube segment 12 to microprocessor 218. To effect
the needed
temperature changes the microprocessor instructs actuator 222 to open door 226
allowing
air to pass through vent 224. As air passes through vent 224 additional air is
drawn into the
2 5 reaction chamber through air inlet 203 by fan blade 204. Fan blade 204 is
driven by motor
206. The venting of hot air and replacement with cooler ambient temperature
air, combined
with the rapid circulation of air by fan 208, a relatively small thermal
cycling chamber 210
and precise measurement of sample temperatures by thermocouple 216 enables
rapid
temperature vamping. The time required for effecting the thermal cycles is
greatly reduced.
3 o A typical thermal cycling reaction requires different temperatures for
denaturing of nucleic
acid strands, annealing of a primer, and extension of a polymerase. The
denaturing and
annealing steps occur rapidly in a capillary tube where the small internal
volume of liquid will
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 51 -
rapidly come to equilibrium, while the extension of the DNA molecule takes
less than 10
seconds for a 500 base extension. The time required for each thermal cycle of
the three
temperatures (annealing, extension, denaturing) may be reduced to less than 15
seconds
by using the rapid heat transfer of the air based thermal cycling apparatus. A
program of 30
cycles, each cycle exposing the capillary to three temperatures for varying
amounts of time,
theoretically may be effected in less than 8 minutes.
[0303) The use of the capillary cassette in combination with an air based
thermal cycler
allows additional advantages. The capillary cassette holder temporarily seals
the capillary,
allowing rapid and simplified sealing of each capillary tube segment. The
capillary cassette
1 o contains a number of capillary tubes in parallel arrangement, allowing for
more efficient use
of the thermal cycler and allowing greater sample throughput. Once the thermal
cycles are
completed the capillary cassette 15 contained within holder 8 is removed
through opening
215. The capillary cassette 15 is released from the holder and is subsequently
dispensed.
[0304] The thermal cyclers of FIGS. 6 and 7A-C were illustrated as being used
with
capillary cassettes. The same devices are adaptable to other containers with
opposing
ends. For example, a chip-like substrate with a plurality of passageways
extending through
the chip (as seen in FIG. 3E) has, like a capillary cassette, evenly spaced
opposed open
ends. Several chips could be placed into a thermal cycler with the open ends
temporarily
sealed and exposed to thermal cycles. The rapid temperature changes may be a
bit slower
2 o due to increased material thickness. Other containers with opposing open
ends may also be
used with either temperature cycling device.
C. Dispensing Completed Reaction Mixture
2 5 [0305] Following the completion of the thermal cycling or isothermal
reactions, the
prepared reaction mixture is dispensed into a substrate for analysis by an
analytical system.
As noted above, the capillary cassette may be dispensed by air displacement,
centrifugal
force, vacuum or any other displacement method. The substrate into which the
reaction
mixture is displaced may be the wells of a multiwell plate, locations on a
planar substrate, or
3 o wells that lead into an analytical chip. The reaction mixture, though
small, still may produce
enough reaction products that dilution is necessary.
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 52 -
Dispensing Completed Reaction Mixture Example 1: Direct Dilution
[0306] In reference to FIG. 1, following completion of the temperature cycling
process, the
capillary cassette may be removed from air thermal cycler 116 by transfer head
104. The
capillary cassette may be moved by transfer head 104 to be placed in a plate
dispensed
from finished sample hotel 112. The plate, located at position c, may be a
multiwell plate
such as a 384 well microplate. The wells of the plate contain a dilution
liquid, such as
formamide, water, TBE, or other selected buffers. The reaction mixture may be
dispensed
from the capillary tube segments of the capillary cassette by positive
displacement,
1 o centrifugation, or other dispensing means. The reaction may also be
dispensed into a
solution for further chemical or biochemical reaction.
Dispensing Completed Reaction Mixture Example 2: Ethanol Precipitation
[0307) Ethanol precipitation may be effected in a dispensing means similar to
the means
of direct dilution. Transfer head 104 of FIG. 1 would again take the capillary
cassette from
air thermal cycler 116 and place the short ends of the capillaries in a
multiwell plate located
at position c. In this case the wells of the plate would contain an alcohol,
such as 90%
ethanol chilled to 4° C. The reaction mixture would be dispensed from
the capillary
2 o cassette into the ethanol by centrifuge. Air displacement or other
dispensing methods can
also be used. After allowing time for the precipitation, the multiwell plate
can be moved into
the centrifuge by transfer head 102 and a low speed centrifugation performed
to collect the
precipitated nucleic acid in the bottom of the multiwell plate. The alcohol
could then be
removed by aspiration or other means. The precipitated DNA could then be
resuspended in
2 5 formamide, water or other suitable diluent. Once the sample plate is
prepared, by either
direct dilution or ethanol precipitation, the plate may be transferred by
transfer head 104 to
analytical stage 120. Analytical stage 120 may feed the sample plate directly
into an
analytical device, for example a capillary array electrophoresis system, such
as
MegaBACETM produced by Amersham Biosciences, Sunnyvale Calif. Alternatively,
the
3 o analytical stage could direct the product to other systems for further
processing. It is also
possible to dispense the samples onto a substrate for mass spectrometry
analysis,
calorimetric analysis, or other analytical methods.
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 53 -
Dispensing Completed Reaction Mixture Example 3: Dispense Directly into
Analytical
System
[0308] In the previous two examples the samples were dispensed into multiwell
plates.
These plates could then be moved manually or robotically onto a stage for
analysis by an
analytical system. Alternatively the capillary cassette could be dispensed
directly into the
wells of an analytical device, such as an electrophoresis chip. For example a
capillary
cassette having 16 capillaries disposed in the substrate in two parallel rows
of eight
1 o capillaries may dock with 16 wells in an analytical microchip. Such a
microchip would have
an array of analytical lanes in fluid communication with a sample port.
(0309] The capillary cassette may be designed such that the spacing of the
capillaries
matches the spacing of the sample reservoir inlets. For example, the capillary
cassette
illustrated in FIG. 3C includes capillaries 12 extending through flexible
strip 11. Flexible strip
11 may be used alone or in combination with other such strips. The orientation
of the
capillaries in an essentially straight line may be altered by bending strip 11
to form an arc.
FIG. 3D illustrates strip 11 bent to allow capillaries 12 to mate with input
ports that are
disposed on a substrate in a circular pattern. The liquid in capillaries 12
may then be
electrokinetically injected or otherwise dispensed from capillaries 12 into
ports of an
2 o analytical chip if an appropriate electrode array or other dispensing
methods are used. Strip
11 may be positioned in the curved orientation by pressing strip 11 against a
curved form,
such as a curved metal block. This may be done by an automated strip mover
incorporated
into an automated sample preparation system. The capillary cassette could be
dispensed by
air displacement or other dispensing means preferably selected to minimize
splattering and
2 5 bubble formation. Prior to dispensing the prepared reaction mixture into
the wells for
analysis, a small amount of a dilutant could be added to each analytical
microchip well.
When the capillary cassette is dispensed, the diluent will dilute the samples
in the sample
wells. The sub-microliter volume reaction mixtures prepared in the capillary
cassette, such
as a DNA sequencing reaction product mixture, can readily be integrated with
the analytical
3 o microchip for sequencing or other analysis methods.
D. Washing Capillary Cassettes
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 54 -
[0310] Following each use of a capillary cassette, the capillary cassette may
be disposed
of or it may be washed and reused. After the contents of the capillary
cassette have been
dispensed or a capillary cassette has otherwise been used, the capillary
cassette is taken to
cassette washer 118 where the cassette is washed. Following washing, the
cassette is
returned to the cassette hotel 106 where the cassette may be reused.
[0311] With reference to FIG. 8A, capillary cassette washer 410 is comprised
of wash
manifold 412 and wash tank stage 416. Between wash manifold 412 and wash tank
stage
416 is capillary cassette platform 414. Extending from wash tank stage 416 is
leg 419. In
1 o this wash system, a wash liquid is pumped from one or more of containers
452, 454, 456,
458 through respective tubes 1, 2, 3, 4 into respective router inputs 453,
455, 457, 459. The
router directs the selected wash fluid through router outflow 451 through line
451 a into the
wash tank 440. The fluid is drawn from wash tank 440 through capillary tube
segments of a
capillary cassette. The capillary cassette substrate is held between wash
manifold 412 and
wash tank 440 such that if suction is applied to wash manifold 412, wash fluid
will be drawn
through capillary tube segments from wash tank 440. The wash solution is drawn
by
vacuum through wash manifold 412 and into waste receptacle 490.
[0312) FIG. 8E provides a schematic of the working of the wash station.
Nitrogen tank 460
provides a pressure source to direct fluid flow. Opening manual valve 462
allows gas to flow
2 o through regulator 466 and through filter 468. Regulator 466 regulates the
pressure from the
pressure source. Pressure sensor 464 monitors gas pressure from the nitrogen
source and
indicates if gas pressure is below a selected pressure. The pressurized gas
flows through
filter 468 into line 470. Pressurized gas line 470 branches into the top of
sealed wash
bottles 471, 472, 473, and 474. The pressurized nitrogen pumps the wash liquid
within each
wash bottle into respective fluid lines 471a, 472a, 473a and 474a respectively
through an
intake filter 476 on each of said respective fluid lines. Each of the sealed
wash solution
bottles may contain a different wash solution, such as water, alcohol, a
buffer or other wash
solution. Although four wash bottles are illustrated, the system is adaptable
for use with
more or fewer wash fluids. In addition, exchange of wash bottles simply
requires venting
3 o nitrogen pressure on bottles 471, 472, 473, 474 at valve 462, the removal
of the cap from
the selected bottle and replacement of the cap with attached pressure and
fluid lines into a
new or refilled wash fluid bottle. Each of the fluid lines 471 a, 472a, 473a
and 474a terminate
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
_ 55 _
in selector valve 478. According to a preset program, the selector valve
routes one of the
selected fluids from the input line into valve output line 480. The valve
output line then
transports the pressurized liquid into wash tank 440.
[0313] The capillary tubes in the capillary cassette function as a conduit for
transport of
fluid from the wash tank 440 into the wash manifold interior 425. Vacuum
source 496
provides a vacuum force once valve 492 is open. When vacuum valve 498 is open,
a
vacuum force is directed into waste bottle 490 creating negative pressure
within line 490a.
When valve 495 is open, suction will be applied through suction line 490a,
suction line 495a
and suction lines 424x. As suction is applied through suction ports 424 by
suction lines
424a the negative pressure through interior wash manifold 425 will draw liquid
up through
the capillary tube segments extending into wash manifold interior 425. The
liquid will travel
through suction passageways 424, into suction lines 424a, past valve 495,
through suction
lines 495a and 490a and into waste bottle 490.
[0314] FIG. 8D illustrates a view of the wash manifold. The bottom of the wash
manifold
1 s contains holes 426 into which the capillaries are inserted. Wash manifold
interior 425 is
comprised of lanes joined at a first end to suction passageways 424 and at a
second end to
purge passageways 423. When suction is applied through line 424a fluid will be
drawn
through capillaries into the lanes comprising interior 425, through
passageways 424 and
into line 424a. When the purge valve is opened, air will pass through line
423a, through
2 o passageway 423, into interior 425, and into passageway 424, clearing
interior 425 of any
liquid remaining in interior 425.
[0315] Following a wash procedure, wash tank 440 is lowered relative to the
capillary
cassette platform such that the ends of the capillary tube segments are not in
contact with
the liquid in wash tank 440. The liquid within wash tank 440 is drained
through drain 484
2 5 which transmits the fluid into drain line 484a when valve 485 is opened
and suction is
applied through suction line 490a. The fluid within wash tank 440 will then
drain into waste
bottle 490.
[0316] Before each wash solution is introduced into wash tank 440, wash fluid
supply line
480 and the wash tank distribution manifold 480a are purged to empty the line
of any
3 o previous liquid. This is effected by opening one of the valves in selector
valve 478 and
flowing wash fluid through supply line 480 and through bleed lines 482.
Opening valve 487
allows a vacuum force to be transmitted through line 490a through line 488
providing
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 56 -
suction which in conjunction with fluid pressure is used to purge the
distribution manifold
through bleed lines 482. Once wash fluid supply line 480 and distribution
manifold are
purged, valve 487 is closed and the wash tank is raised and filled. The fill
level of wash tank
440 is controlled by the selected wash fluid fill time and wash fluid
pressure. Overflow port
486 acts as a safety drain to drain off fluid overfill. If the fluid level
within wash tank 440 is
too high, liquid will flow from wash tank 440 into overflow port 486 and into
line 486a. When
valve 487 is open, the suction force from line 490a and 488 will draw overflow
liquid from
overflow port 486 into waste bottle 490. Restriction flow valve 441 limits
liquid fluid flow
through lines 482.
[0317] FIG. 8F shows the top perspective of wash fluid tank 440. An input line
introduces
a wash solution into wash fluid distribution manifold 480a. This manifold
supplies wash fluid
ports 481 that fill tank 440. The spacing of wash fluid ports 481 aids in
uniform filling across
the width of tank 440. The fill time and fluid pressure regulate the amount of
fluid filling tank
440. If excess fluid enters tank 440 it will drain from overflow port 486.
[0318] To empty the tank, the tank is lowered by the pneumatics as described,
and drain
484 is opened. The shape of tank 440 directs fluid to drain 484 when the end
of tank 440
containing drain 484 is lowered. This configuration is designed for efficient
filling, emptying
and purging of tank 440 and associated fill lines.
[0319] Again with reference to FIG. 8E, once a wash cycle has been completed,
any liquid
2 o remaining within wash manifold interior 425 may be eliminated by opening
valve 491 while
suction is applied through the manifold. Opening valve 491 causes a pulse of
air to be
drawn in through vent 493. The air is introduced into wash manifold interior
425 through
purge lines 423a and is removed by suction lines 424a. If the manifold is in
contact with
capillaries, the relatively narrow bores of the capillaries in the capillary
cassette provide a
2 5 limited capacity for drawing air through the wash manifold. By opening
valve 491, a much
greater amount of air may be drawn through the manifold through purge lines
423a which
have a much greater capacity for drawing air. This will result in a sudden
rush of air drawn
through the manifold. This acts to clear the wash manifold of any liquid
remaining within the
wash manifold interior 425. Preferably manifold interior 425 is purged before
and after
3 o raising the wash manifold.
[0320] With reference to FIG. 8B, the wash station 410 is shown in side view.
The
capillary cassette platform 414 is mounted on support legs 445. The reservoir
section,
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
_ 57 _
shown in internal cross section has at a back lower end of the reservoir,
drain outlet 484.
Upwardly positioned from the drain outlet at the back wall of the tank is
overflow outlet 486.
Disposed at the front of the reservoir is reservoir bleed outlet 446. Each
outlet is associated
with a respective tube and valve, as described in conjunction with FIG. 8E.
Each tube
carries liquid flowing from an associated outlet when the associated valve is
opened and
vacuum source applied.
[0321] Capillary cassette platform 414 is held in a fixed position by support
legs 445.
Extending downward from the front of capillary cassette platform 414 is hinge
418 with pivot
432. Attached to a lower end of hinge 418 is wash tank stage 416. Extending
from below
1 o wash tank stage 416 is leg 419 that is attached at a lower end by pivot
443 to pneumatic
cylinder 429. At the back end of the stationary capillary cassette platform
414, the wash
manifold is attached at pivot 420. When pneumatic cylinder 429 is extended
from the lower
end, wash tank stage 416 will be lowered in an arc away from stationary
capillary platform.
This occurs when no pressure is applied to 429 and gravity causes the wash
tank stage to
pivot down. When pneumatic cylinder 429 is extended from the upper end by
applied
pressure, wash manifold 412 will be raised in an arc away from capillary
cassette platform
414.
[0322] Disposed above capillary cassette platform 414 is wash manifold 412.
The wash
manifold has a purge passageway 423 disposed at a front end and a suction
passageway
2 0 424 disposed toward the back end. The respective lines carrying air to the
manifold or
removing gas or liquids from the manifold are described in conjunction with
FIG. 8E.
[0323] With reference to FIG. 8C, pneumatic cylinder 429 is shown fully
extended from a
lower connection pivot 443 on leg 419, through hole 333 in capillary cassette
platform 414,
to an upper connection at pivot 428 on wash manifold 412. The extended height
of the wash
2 s manifold is limited by plate 430 that is secured to the top of manifold
412. Plate 430 abuts
pin 422 on capillary cassette platform 414 when the wash manifold is raised to
a selected
level and prevents the wash manifold 412 from being raised beyond this level.
When suction
is applied to wash manifold interior 425 by applying suction through suction
passageway
424, fluid is drawn through capillaries 12 from tank 440.
3 0 [0324] The front end of capillary cassette platform 414 is joined at pivot
432 to hinge 418
and wash tank stage 416 and the back end of capillary cassette platform 414 is
joined at
pivot 420 to wash manifold 412. Extending through capillary cassette platform
414 is cutout
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 58 -
434. The dimensions of cutout 434 are such that capillary cassette 15, when
placed on
capillary cassette platform 414 has associated capillary tube segments 12
extending
through capillary cassette platform 414 while the four edges of capillary
cassette substrate
are retained on the capillary cassette platform 414 on the edge of cutout 434.
Alignment
5 pins may be added to capillary cassette platform 414 to properly position
the capillary
cassette.
[0325] To effect the cassette wash sequence, an electronic controller
implements a
sequence of steps. The electronic controller instructs associated controlled
devices of the
wash station to carry out a programmed wash sequence. The programmed sequence
1 o begins with the capillary cassette being placed on the capillary cassette
stage by the robotic
transfer device. The wash manifold lowers onto the capillary cassette such
that the shorter
end of capillary tube segments extend into the wash manifold and the opposite
end of the
capillary tube segments are within the wash liquid in the wash tank once
filled. The
substrate provides a partial seal between the wash manifold and cassette such
that when
suction is applied to the capillary tube segments by the wash manifold, fluid
will be drawn up
into the wash manifold through the capillary tube segments. The wash solution
supply line is
purged with the first selected solution to clear the previous solution from
the line. As noted
in relation to FIG. 8E, the purge solution is removed through distribution
manifold to drain
484 and bleed lines 482 to wash waste line 488 and 490a then into waste bottle
490. The
2 o wash tank 440 is then raised and filled with the selected wash solution.
[0326] A vacuum is applied to the wash manifold causing the solution in the
wash tank to
be drawn up through all of the capillary tube segments in the capillary
cassette. After the
programmed wash duration, the wash tank is drained and lowered. The vacuum
force is
continued through the wash manifold, drawing air through the capillary tube
segments.
2 5 Once the capillary tube segments are dried, the vacuum line of the wash
manifold is turned
off. The wash solution supply line is purged with the next wash solution and
the steps of
raising and filling the wash tank, drawing the wash solution through the
capillary tube
segments and emptying the wash tank are repeated for each selected solution.
The
specified sequence may repeat these steps for any number of wash solutions.
After the final
3 o wash has been completed and the tank emptied, air is drawn through the
capillaries by
applying a vacuum to the wash manifold, drying the capillary tube segments.
Periodically
the purge valve 491 is opened and air is drawn through vent 493 into purge
lines 423a into
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 59 -
purge inlets 423. This draws a blast of air through wash manifold interior 425
and clears the
wash manifold interior of any remaining liquid, ensuring that any remaining
liquid within the
wash manifold will not wick back into the capillaries. The manifold vacuum is
then shut off
and the manifold is raised, removing the manifold from the capillary cassette.
The manifold
vacuum is again applied and the purge valve 491 is opened and air is drawn
through vent
493.into purge line 423a into purge inlet 423. This ensures that any remaining
liquid is
removed from the wash manifold interior. The vacuum is then shut off. The
washed and
dried capillary cassette may then be moved by the transfer robot to a
capillary cassette
hotel or other location.
System Integration
[0327] The components of the system could be integrated in a combined system
that
allows several elements of the complete system of FIG. 1 to operate at the
same time. For
example, electronic control device 123 may be used to send instructions to the
components
of the integrated system. The electronic control device may be a computer that
sends
electronic signals to various system components to effect a programmed set of
instructions.
Elements of the system could operate simultaneously, increasing system
efficiency. For
example, automated robot 102 could retrieve a capillary cassette from cassette
hotel 106,
2 o place the capillary cassette in a sample plate at stage a. An amount of
sample from the
plate is drawn into the capillary tubes by capillary action. The capillary
cassette could then
be moved and placed on top of a microtiter plate such that the short ends of
the capillary
tube segments are in the wells of the microtiter plate. The robot 102 could
then transfer the
combined microtiter plate/capillary cassette to dispense location 122 for
dispensing. The
2 5 movement of the robot 102, transfer head 104 and dispensing device located
at location
122 are controlled by electronic control device 123.
[0328] At the same time that a reaction mixture is being assembled, the
electronic control
device could also be sending electronic signals to thermocycler 116. The vent
door, heating
element, and thermocouple of thermocycler 116 could be linked to electronic
control device
3 0 123, allowing electronic control device 123 to effect a selected
temperature cycling
procedure by regulating the temperature at which air is cycling within the
thermal cycler.
This precise monitoring allows the temperature cycling procedure to be
effected in a
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 60 -
minimum amount of time. Once the thermal cycling procedure is complete, the
electronic
control device 123 could electronically instruct the thermal cycler to shut
off the
thermocycler fan and heating element and open the lid pneumatically to allow a
capillary
cassette to be removed from the interior of the thermal cycler.
[0329] While automated robot 102 is moving capillary cassettes to assemble a
reaction
mixture and the thermocycler is operating, the cassette washer 118 could also
be cleaning a
capillary cassette. Again the electronic control device 123 could instruct the
cassette washer
118 to perform a wash sequence in which a capillary cassette is cleaned with a
selected
sequence of wash liquids and air-dried.
[0330] Electronic control device 123 enables each element of the system to be
used with
maximum efficiency. A single set of instructions to electronic control device
123 could allow
assembly of the reaction mixture, thermal cycling of the reaction mixture to
effect the
desired reaction, dispensing of the completed reaction mixture onto an
analytical substrate,
movement of the analytical substrate to a stage for processing by an
analytical instrument,
1 s and cleaning of used capillary cassettes.
Submicroliter Template-Normalized Nucleic Acid Reactions
[0331] In a further aspect, the invention provides methods and apparatus for
performing
2 o nucleic acid reactions in reduced volume, and for normalizing the amount
of nucleic acid
template present in such reactions.
[0332] The present invention is based, in part, upon the novel use of the
saturable, yet
reversible, binding of nucleic acids by certain materials to control the mass
of nucleic acid
delivered as template to a subsequent reaction, without a required antecedent
2 s determination of the concentration of nucleic acid in the solution from
which the nucleic acid
is to be captured. In particular embodiments, the internal surface of a
capillary is used to
effect nucleic acid capture, permitting nucleic acid template to be captured
directly in the
chamber in which subsequent reaction is to be performed.
3 o Further Advantages of the Present Invention
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 61 -
(0333] The present invention is described herein with particular reference to
its use for
performing DNA sequencing reactions, especially in the context of a high-
throughput
sample processing system employing capillary electrophoresis, for which the
methods and
apparatus of the present invention are particularly advantageous. However, it
will be clear
s to the skilled artisan, as will be described in more detail below, that this
invention can be
used in the course of performing many types of biochemical and chemical
reactions using
DNA, as well as RNA, as the substrate.
[0334] As disclosed in detail below, the present invention provides methods
for reversibly
immobilizing nucleic acid directly on the inner surface of a reaction chamber,
such as a
1 o glass capillary tube, or the functional equivalent thereof. After
immobilization and other
processing steps, the nucleic acid is ready to be used in a chemical,
biochemical or
enzymatic reaction performed inside the capillary tube. Alternatively, the
nucleic acid can
be eluted and expelled from the capillary so as to dispense a controlled
amount of nucleic
acid for subsequent use.
1 s (0335] For successful analysis of DNA sequencing reactions using highly
sensitive
capillary electrophoresis systems, such as the MegaBACET"" system (Amersham
Biosciences, Sunnyvale, CA), it is important to use consistent, predetermined
amounts of
template DNA in the reactions, so that the amount of template is neither too
low nor too
high. By employing capillary tubes with consistent DNA binding capacity, it is
possible to
2 0 "normalize" the amount of template DNA used across all reactions, thereby
ensuring that all
start with a similar quantity of template. Although normalization can be
accomplished in
other ways, use of capillary tubes results in dramatic savings of time by
reducing the steps
necessary to ensure consistency.
[0336] Although nucleic acid binding is an inherent property of glass
surfaces, it will be
2 5 appreciated that the capture surface can be modified to alter its binding
capacity or binding
selectivity. For example, for capturing non-modified DNA, major binding forces
are
hydrophobic forces, charge-charge (electrostatic) forces, and hydrogen
bonding. Thus, to
capture non-modified DNA, vinyl groups can be added to the capture surface by
reaction in
the solution phase, propyl amine groups can be added by CVD, other amines,
preferably
3 o tertiary amines, can be added by known reactions to maximize the charge-
charge
interaction. In other alternatives, oligo d(T) can be covalently linked to
aminated surface,
increasing capture of poly(A) mRNA. A spacer of the general form Cn can be
added
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 62 -
between the silicon surface and the functional groups. For each of these, the
characteristics andlor binding capacity can be altered by changing the
concentration of the
functional groups.
[0337] An additional advantage of the present invention is that it is useful
for reducing the
s number of processing steps associated with, and the quantity of nucleic acid
and reagents
needed for, carrying out a reaction with nucleic acid, especially in the
context of a high-
throughput sample processing system. For example, for a DNA sequencing
reaction, it is
necessary to combine template DNA with a reaction mixture comprising
sequencing primer,
DNA polymerise, dideoxynucleotides, dNTPs, buffers, salts and water, prior to
performing
1 o thermal cycling that activates the reaction. Typically, this involves
preparing a 20 NI reaction
by aliquoting the reaction mixture into a tube, followed by the addition of
200 ng template
DNA. The pipet tip used to aliquot the DNA is typically discarded to avoid
contamination of
the DNA stock. The components are then mixed, thermal cycled and analyzed.
[0338] According to an embodiment of the present invention, a capillary tube
is filled with
1 s a DNA solution, resulting in the reversible immobilization of 5 ng of the
template inside the
capillary. After several processing steps, the capillary is then filled with
500 nl of reaction
mixture, which causes the template to elute from the inside of the tube into
the mixture. The
capillary is then sealed and thermocycled, with subsequent analysis of the
reaction products
by a high sensitivity capillary electrophoresis system. Because the capillary
serves
2 o simultaneously as a pipettor that is filled by capillary action, and as a
reaction chamber, it is
unnecessary to separately aliquot, with dedicated pipetting systems, either
template DNA
solution, or the reaction mixture. It is only necessary to provide a stock of
each into which
the capillary is dipped to fill it. This saves processing steps and materials
such as
disposable pipettor tips. It also saves reagent that would otherwise be
carried over during
2 s processing steps, and not introduced into a reaction.
[0339] It will also be apparent that a sequencing reaction performed in the
capillary can be
accomplished in only 1110 to 1/40 of the reaction volume, and therefore 1/10
to 1/40 the
cost for reagents. Collectively, these advantages result in reduced
processing, increased
speed, and reduced cost. In the design of high-throughput sample processing
systems,
3 o capillaries, or functional equivalent thereof, can be arranged in
parallel, in ways well known
to those skilled in the art, to increase the number of reactions that can be
processed
simultaneously. The scale of the benefits enjoyed employing the various
embodiments of
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 63 -
the present invention disclosed herein grow in proportion to the number of
samples
processed.
Reversible Direct Immobilization of Nucleic Acid in a Reaction Chamber
[0340] FIG. 15 is a flowchart, and FIG. 16 is a schematic that shows the steps
associated
with embodiments of the instant invention, whereby nucleic acid is reversibly
immobilized to
the inner surface of a reaction chamber, such as a glass capillary tube.
Reaction chambers
prepared in this way can then be used to carry out a sequencing reaction with
nucleic acid,
1 o to effect another type of enzymatic or biochemical reaction with nucleic
acid, or for
dispensing a predetermined quantity of nucleic acid onto a substrate, such as
a microtiter
dish well, or into an analysis instrument, such as a capillary electropheresis
device.
[0341] With reference to FIG. 15, and FIG. 16, in step 1 the nucleic acid
sample is
prepared from a suitable source, after which, in step 2, the nucleic acid 80
is dissolved in a
solution 81 containing chaotropic ions. In step 3, the reaction chamber is
filled with the
nucleic acid-chaotrope solution and incubated, in step 4, for sufficient time
to allow
reversible binding of the nucleic acid 80 to the inner surfaces 82 of the
reaction chamber 12.
In step 5, the nucleic acid-chaotrope solution is removed, followed by
washing, step 6, and
drying, step 7, of the reaction chamber. At this point the reaction chamber is
useable. Part
2 0 12 refers to a capillary tube, or more broadly, a reaction chamber,
including capillary tubes
and structures equivalent in function thereto. Part 80 refers to DNA, or more
broadly,
nucleic acid, including DNA and RNA and derivatives thereof.
[0342] The process begins by obtaining nucleic acid, FIG. 15, step 1, from a
suitable
source. The nucleic acid may be deoxyribonucleic acid (DNA), ribonucleic acid
(RNA) or
2 5 derivatized forms of these molecules. Nucleic acids can be isolated and
purified according
to methods well known in the art (see Current Protocols in Molecular Biology,
John Wiley &
Sons, Inc., 2000, Edited by Fred M. Ausubel et al., ISBN 0-471-50338-X) from a
variety of
living organisms or self-replicating systems that rely on living cells. Cells
can be eukaryotic
cells, including human and non-human mammalian cells, non-mammalian animal
cells,
3 o plant cells and fungal cells. Additionally, eukaryotic cells can be free
living single celled
organisms, such as amoebae or other parasites. Cells can also be prokaryotic
cells
including bacteria and archaebacteria. Nucleic acids can also be obtained from
viruses,
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 64 -
including RNA and DNA viruses, and viruses that infect animal cells, plant
cells, fungal cells,
and bacterial cells. Nucleic acids can also be produced according to chemical
synthetic
methods well known in the art.
[0343] After obtaining template nucleic acid from the appropriate source, the
nucleic acid,
s FIG. 16 80, is resuspended and/or dissolved into a solution containing a
chaotropic agent,
FIG. 15, step 2, and FIG. 16 82. The chaotropic agent is desirably at
sufficiently high
concentration (e.g., about 0.5 M to 8.0 M) to effect the reversible binding of
the nucleic acid,
but not so high as to cause the nucleic acid, or the chaotrope itself to
precipitate out of the
solution under all of the conditions to which the solution is subjected in
carrying out the
1 o invention.
[0344] A chaotropic agent is a substance that affects the partitioning of
molecules from a
nonaqueous to an aqueous phase due to the disruptive effect that the substance
has on the
local structure of water. Chaotropic agents are salts of chaotropic ions, and
are highly
soluble in aqueous solutions. At sufficiently high concentration in aqueous
solutions the
s 5 chaotropic ions provided by such salts cause nucleic acids to lose
secondary or tertiary
structure, and double-stranded nucleic acids to melt (i.e., strand-separate).
It is
hypothesized that chaotropic ions have these effects by disrupting hydrogen-
bond networks
existing in water, causing the denatured form of the nucleic acids to be more
thermodynamically stable as compared to the structure of more highly ordered
structures
2 0 (e.g. the double helix) that exist in a typical aqueous environment.
[0345] As described previously by Vogelstein et al., Proc. Natl. Acad. Sci.
USA 76, 615-
619 (1979) and by Chen and Thomas, Anal. Biochem. 101, 339-341 (1980), in the
presence
of a sufficiently high concentration of chaotropic ions (e.g. about 0.5 M to
about 8.0 M),
nucleic acids will reversibly bind certain substances, such as silica. The
mechanism of
2 s nucleic acid binding to silica may involve chaotropic ion disruption of
the water structure at
the surface of the negatively charged silica, allowing a cation (e.g. Na+ or
K+) mediated salt
bridge to form between it and the negatively charged phosphate backbone of the
nucleic
acid strand. To effect nucleic acid silica binding, a chaotropic agent may be
used singly or
as a mixture of two or more chaotropes. The salt bridge is not a permanent
bond and can
3 o be disrupted when the ionic concentration in the proximity of the bond is
lowered. In this
way, nucleic acid can be eluted from silica or similar material with water or
other suitable
low ionic strength aqueous buffer.
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 65 -
[0346] Chaotropic ions include guanidinium, iodide, perchlorate and
trichloroacetate.
Chaotropic salts include sodium perchlorate, potassium perchlorate, sodium
bromide,
potassium bromide, sodium iodide, potassium iodide, sodium thiocyanate,
potassium
thiocyanate, guanidine thiocyanate, sodium isothiocyanate, potassium
isothiocyanate,
guanidine hydrochloride, guanidine isothiocyanate, lithium chloride, sodium
trichloroacetate,
and potassium trichloroacetate. Other substances with chaotropic properties
include
dimethylsulfoxide (DMSO), urea, and the tetra-amine halides, including
tetraethylamine
chloride.
[0347] After dissolving the nucleic acid in the solution of the chaotrope, the
nucleic acid-
1 o chaotrope solution, FIG. 16 83, is introduced into a reaction chamber,
FIG. 15, step 3, and
FIG. 16 12.
(0348] For the purpose of reducing the cost of reagents used to effect the
sequencing
reaction, the reaction chamber will typically be of very small volume,
desirably from about 1
-1000 nanoliters (nl), more desirably from about 10 - 500 nl, most desirably
from about 100
i 5 - 500 nl.
[0349] In most circumstances, the reaction chamber is configured so that
solutions can be
introduced into it passively, by taking advantage of capillary action.
Capillary action is the
phenomenon by which the elevation of a liquid rises where it is in contact
with a solid, such
as the sides of a tube, and is most marked in capillary tubes, i.e., tubes of
very small
2 o diameter. Capillary action depends on the forces created by surface
tension and by wetting
of the sides of the tube. If the forces of adhesion of the liquid to the solid
(wetting) exceed
the forces of cohesion within the liquid (surface tension), the liquid will
rise up the tube, i.e.,
it will rise above the hydrostatic level. Alternatively, the solution can be
introduced into the
reaction chamber actively, such as by pumping using positive or negative
atmospheric
2 5 pressure.
[0350] It is simplest and most economical to take advantage of capillary
action to fill the
reaction chamber with the nucleic acid-chaotrope solution, in which case a
capillary tube
serves as the reaction chamber. If the bore of the capillary is of known and
uniform areal
cross section, then the volume of the tube is easily calculated, being
linearly proportional to
3 o its length. Thus, a capillary tube reaction chamber of given total volume
is obtainable by
cutting the tubing to the desired length given by the calculation. In
accordance with the laws
of fluid dynamics however, care must be taken that the density of the solution
is not so
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 66 -
great, its surface tension so low, and the diameter of the tubing
insufficiently small, that the
column of solution cannot overcome gravity, and thereby fails to fill the
tube.
(0351] During filling, one end of the tube is dipped into the nucleic acid-
chaotrope
solution, FIG. 16 83, that is usually provided in volume excess over the total
volume of any
s tube to be filled. In this manner, the tube is filled in one step, reducing
the chance of bubble
formation at the inlet. The opposite end of the capillary must be open, or
otherwise able to
allow air to escape from the filling tube.
[0352] It is not obligatory that the outside of the reaction chamber
approximate the form of
a tall thin cylinder, as it does with a capillary tube. Rather, as will be
apparent to the skilled
1 o artisan, the functional equivalent of a capillary tube can be manufactured
in a variety of
ways. Throughout the specification, the term capillary tube should be
understood to
represent not only that structure commonly referred to as a capillary tube,
but also any
structure that is functionally equivalent thereto. For example, a tunnel,
channel or groove
can be formed that is configured so that fluid can fill it by capillary
action, or by the direct
1 s application of some force, e.g. positive or negative pressure, or
centrifugal force. The
tunnel, channel or groove can be formed mechanically, chemically, thermally,
or by other
means known to the skilled artisan. A channel or tunnel can be formed by
removing
material from a matrix, e.g., using a drill bit, laser, or chemical etching
[0353] As illustrated in FIG. 3E, a groove or channel 78 in the surface of a
substrate 72,
2 o such as a glass slide of any shape and dimension, can be cut with a saw,
or formed by laser
ablation or chemical etching to create a structure called a chip or microchip
70. For
example, grooves in a silicon wafer can be formed by photolithographic
methodologies
known in the art, and grooves in glass slides can be etched using hydrofluoric
acid.
[0354] If a groove or similar depression 78 is formed in the surface of a
substrate 72, it will
2 s usually be advantageous to cover it with a cover 74 to form an enclosed
space. Covering
the groove or depression 78 ensures that there is maximal surface area for the
fluid to
interact with, thereby promoting the capillary action, minimizes the
opportunity for
contaminants to contact the reactants, and creates a vapor barrier to ensure
that during any
elevation in temperature of the reaction, such as during thermal cycling, the
tendency of the
3 o reaction to vaporize is minimized.
[0355] Covers 74, which can be comprised of material identical to, or
different from, that
of the substrate 72 in which the groove is cut, can be applied using a variety
of means
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 67 -
known in the art. For example, the cover 74 can be glued to the substrate
using an epoxy,
cyanoacrylate or other type of glue. The cover can be welded by melting it and
underlying
material until they fuse, through the application of heat or light. The cover
74 can also be
fixed in place mechanically, such as with a clamp, or even magnetically.
[0356] The material of which the reaction chamber is comprised is
advantageously a
material to which template DNA, or other nucleic acid, reversibly and
saturably binds in the
presence of a sufficiently high concentration of chaotropic ions. Frequently,
the reaction
chamber is comprised of glass, especially when configured as capillary tubing.
High quality
glass capillary tubing is readily available in a range of interior dimensions
from a variety of
to manufacturers, including Polymicro Technologies (Phoenix, Arizona, USA).
[0357] If comprised of a fragile, hydrophilic material like glass, it may be
advantageous to
coat the outside of the capillary tubing with a polymer material, such as a
polyimide. A
polyimide coating provides a protective layer that protects the capillary
tubing from
abrasions and breaking by bending. Polyimide also creates a hydrophobic layer
on the
15 outer surface of the capillary which can help prevent the adherence of
aqueous reaction
mixtures when the capillary is filled by dipping it into a reaction mix; this
helps prevent
wastage of reagents. Other potential coatings are acrylates, silicones,
fluoropolymers, and
aluminum.
[0358] Many types of glass may be used including alkali-borosilicate glass,
alumina-
2 o silicate glass, barium flint glass, barium-borate glass, borosilicate
glass, borate glass
comprising 8203, germinate glass comprising Ge02, chalcogenide glass, silicate
glass
comprising Si02, silica glass, fused silica glass, synthetic fused silica
glass, quartz
(crystalline Si02), fused quartz (amorphous Si02), doped synthetic fused
silica (doped with
trace elements such as germanium, fluorine, boron, phosphorous, and titanium),
lanthanum
2 s glass, optical glass, phosphate glass, and soda-lime glass.
[0359] Alternatively, the reaction chamber can be comprised of a metal or
metalloid,
materials that, like glass, can be fashioned into capillaries or wafers.
Suitable pure and
alloyed metals include magnesium, aluminum, titanium, vanadium, chromium,
manganese,
iron, cobalt, nickel, copper, zinc, gallium, zirconium, niobium, molybdenum,
palladium, gold,
3 o silver, cobalt, niobium, indium, rhodium, tin, steel, stainless steel, and
bronze. Suitable pure
and alloyed metalloids include silicon, germanium, arsenic, and gallium
arsenide.
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 68 -
[0360] The reaction chamber can also be comprised of carbon in its multiple
allotropes,
including graphite, diamond, C60 and related allotropes comprising, for
example,
nanotubes, or comprised of organic compounds such as plastic. For these
materials, it may
be necessary to derivatize the carbon or plastic in such a fashion as will
support the
s reversible binding of nucleic acid to the plastic in the presence of
chaotropic ions.
[0361] After the reaction chamber, such as glass capillary, FIG. 16 12, has
been filled with
nucleic acid-chaotrope solution 83, the solution is incubated for such time
and under such
conditions that at least a portion of the DNA in the solution reversibly binds
to the inner
surface, FIG. 16 82, of the chamber or tube, FIG. 15, step 4. In other
embodiments,
1 o irreversible binding can be effected.
[0362] Without wishing to be bound with theory, it is believed, as discussed
above, that if
the inner surface is glass containing Si02 (silica), in the presence of a
sufficiently high
concentration of chaotropic ions the nucleic acid most likely forms salt-
bridge type bonds
with the silica via the phosphate backbone. Usually, binding is allowed to
proceed at about
1 s room temperature (about 24°C), but other temperatures may be chosen
as is deemed
appropriate, so long as the effectiveness of binding is not significantly
hampered, and so
long as neither the DNA nor chaotrope precipitates from the solution.
[0363] After the nucleic acid in the nucleic acid-chaotrope solution has had
the opportunity
to bind to the inner surface 82 of the reaction chamber or tubing, the
solution containing
2 o unbound DNA and the chaotrope is then removed 5, the inner surface is
washed 6 with
washing solution, and then remaining traces of liquid from the wash solution
is removed by
drying 7.
[0364] The greater proportion of nucleic acid-chaotrope solution is removed
from the
chamber by a variety of means including application of positive or negative
air pressure, or
2 s by centrifugation to expel the solution.
[0365] Washing is performed to purify the bound nucleic acid by removing
excess,
unbound nucleic acid, chaotropic agent, and any impurities that may have
contaminated the
nucleic acid. It is important to remove the chaotropic agent because these
ions can severely
interfere with most subsequent chemical and biochemical reactions, even at
very low
3 o concentrations. Washing can be performed in a variety of ways. For
example, a capillary
tube can be filled by capillary action, after which the washing solution is
expelled in similar
manner by which the nucleic acid-chaotrope solution was removed.
Alternatively, a reaction
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 69 -
chamber can be filled and emptied by pumping of the wash solution. Sufficient
volume of
washing solution is used to essentially eliminate the presence of all
contaminants. After
washing, the wash solution is removed from the chamber or tube.
[0366] The composition of the washing solution is chosen so that it does not
remove by
s elution any substantial portion of the nucleic acid that has become bound to
the inner
surface of the chamber or tubing, and is typically a solution of an alcohol
with pure water.
Suitable alcohols include the lower molecular mass alcohols methanol, ethanol
and
isopropanol. The concentration of alcohol is high enough that elution of
nucleic acid
minimized, and is preferably at least 50%, more preferably at least 60%, and
most
1 o preferably at least 70% volume by volume. Typically, ethanol is used at
concentration
greater than about 70% - 80% volume by volume.
[0367] The washing solution can also comprise a salt, preferably in the form
of a buffer,
such as an acetate buffer, or a tris-EDTA buffer (containing, e.g., 10 mM Tris-
HCI and 1 mM
ethylenediamine-tetraacetic acid (EDTA), pH 8.0). The salt can have the effect
of buffering
1 s pH so that the pH is in the range of about 6.5 - 8.5, and also stabilizing
the binding
interaction between DNA and the inner surface of the chamber or tube during
washing.
[0368] It is frequently desirable to remove essentially all traces of the
liquid from any small
volume of the wash solution remaining in the chamber or capillary tubing by
drying.
Although low concentrations of some components of the liquid, such as ethanol,
tend not to
2 o significantly interfere with subsequent biochemical reactions, higher
concentrations can
interfere. Drying can be effected by subjecting the chamber or tube to a high
enough
vacuum so that the liquid vaporizes and is carried away. Alternatively, a dry
gas, such as
air, nitrogen or argon, can be forced at pressure through the chamber or tube
to promote
the evaporation of the liquid. The drying gas can be warmed to further promote
2 5 evaporation.
(0369] After drying, the reaction chamber, now bearing reversibly immobilized
nucleic
acid, can be used immediately to perform a biochemical reaction with the
nucleic acid, or
stored, under appropriate conditions, for future use. Reaction chambers
prepared
according to the steps discussed above can be advantageously used to normalize
the
3 o amount of a nucleic acid to be used in parallel reactions, dispense
predetermined amounts
of DNA or RNA onto a substrate, and to perform nanoscale DNA sequencing
reactions, as
well as many other types of reactions with DNA and RNA. However, as will be
clear to the
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 70 -
skilled artisan, these particular applications should not be seen as limiting
the scope of uses
to which such reaction chambers can be put.
Use of the Present Invention in an Automated System
[0370] Reaction chambers in the form of capillary tubes can be processed as
illustrated in
FIG. 15 and used singly, but it will frequently be advantageous to combine
multiple capillary
tubes in parallel fashion, so as to be able to increase sample throughput,
particularly in an
automated system. For this purpose, capillary tubes can be conveniently
organized into a
1 o capillary cassette; the greater the density of capillary tubes per
cassette, the greater the
potential sample throughput. An apparatus, such as that described in copending
U.S.
Application Serial No. 091577,199, can be used to automate the processing
steps illustrated
in FIG. 1, as well as any subsequent steps associated with carrying out
reactions with the
immobilized nucleic acid, including capillary filling, emptying, washing,
drying, and or
thermal cycling. Used in this way, the cassette becomes an automated, fixed-
volume
parallel pipettor, allowing all the capillary tubes to be filled
simultaneously from the wells of a
sample plate by capillary action.
[0371] Capillary cassette 15 is shown in FIG. 3A. The capillary cassette is
comprised of a
number of capillary tubes extending through a substrate 10. It is preferred
that the capillary
2 o cassette have at least one row of eight capillary tubes and that the
capillary tubes have
equal spacing. The capillary cassette shown has substrate 10 with 96 capillary
tubes
arranged in an 8 by 12 array, with spacing of the tubes matching the spacing
of the wells of
a 96 well microplate.
(0372] The capillary tubes 12 extend through a substrate 10 and preferably are
arranged
2 5 in a uniform pattern. The capillary tubes are of equal length and extend
through the
substrate in a substantially parallel orientation such that each of the two
opposing ends of
the capillary tubes 12 are coplanar and the planes defined by the ends of the
capillary tubes
12 are substantially parallel to the substrate 10. The spacing of the
capillary tubes may be
uniform and selected to match the center-to-center spacing of wells on a
microplate. For
3 o example on a standard 96 well microplate the capillary tubes would be
arranged with a 9
mm center to center spacing, on a 384 well microplate the capillary tubes 12
would be
arranged with a 4.5 mm center to center spacing. Higher density capillary
formats,
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 71 -
compatible with 1536 well microplates or plates with even higher well density,
should also
be possible. The capillary tubes 12 are preferably secured within the
substrate such that
the length of capillary tubes 12 extending from one side of the substrate 10
are shorter than
the length of the capillary tube on the opposite side of substrate 10. The
length of the
capillary tubes 12 on the shorter side of the substrate may be matched to the
depth of wells
in a microplate, such that the length of the shorter side is a shorter length
than the depth of
a well in a microplate. This feature enables the capillary cassette to be
inserted into a
microplate such that the substrate 10 rests against the top lip of the
multiwell plate and the
capillaries on one side of the substrate may extend into the multiwell plate
without touching
1 o the bottom. For example, in a 96 well microplate the capillary tubes may
be disposed on a
substrate such that the shorter side of the capillary tube extending from the
substrate may
be inserted into wells in a microplate without the capillary touching the
bottom of the well.
This ensures that liquid dispensed into a well is clear of the capillary to
prevent re-entering
the capillary.
1 s [0373] The capillary cassette substrate 10 may be made of a fiberglass
board or other
rigid or semi-flexible material. The capillary tubes 12 may be inserted
through evenly
spaced holes in the substrate and secured with adhesive. In one embodiment,
the length
and width of the substrate are similar to the length and width of a standard
96 well
microplate. This simplifies adapting automated systems designed for
manipulation of
2 o microplates to handle the capillary cassette.
Accurate Control and Normalization of the Quantity of Nucleic Acid to be Used
in a
Biochemical Reaction
2 s [0374] When undertaking to carry out a biochemical reaction with nucleic
acid, it is often
crucial for the success of the reaction that the amount of input nucleic acid
be known with
precision. This allows the experimenter to properly calculate the appropriate
ratio of other
reaction components, such as enzymes. For example, as discussed in the
Background
section, if too much template DNA is used in a sequencing reaction to be
analyzed with a
3 o capillary electrophoresis system, poor quality sequencing data often
results. Nucleic acid
concentration in a stock sample is relatively easily determined by measuring
light absorption
at 260 nm, or measuring the amount of dye binding relative to standard curves.
However,
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 72 -
both these approaches use up a portion of the sample and neither approach is
easy to
implement in the context of a high-throughput sample processing system.
Fortunately, the
present invention is useful for precisely controlling the amount of nucleic
acid to be used for
a variety of applications.
s [0375] If during the binding reaction occurring in the reaction chamber, the
nucleic acid-
chaotrope solution is allowed to stay in contact with the inner surface of the
chamber or tube
for sufficient time, and if the nucleic acid is at high enough concentration
in the solution, it is
possible to saturate the available binding sites on the inner surface of the
chamber or
capillary with nucleic acid. This is known as saturable binding. As long as
the amount of
1 o nucleic acid in solution prior to incubation exceeds the binding capacity
of the inner surface
of the chamber, a fixed, maximal quantity of nucleic acid will be immobilized,
regardless of
the amount of nucleic acid initially in the solution. In this way, if the
concentration of nucleic
acid in solution exceeds a minimum, it is not necessary to know the actual
concentration;
the amount of nucleic acid bound will be determined solely by the binding
capacity of the
15 reaction chamber. Accordingly, if the nucleic acid in a capillary tube that
was saturably
bound is eluted into a known volume of liquid, the concentration and amount of
nucleic acid
in the liquid is knowable with a high degree of accuracy.
[0376] Thus, it is possible to use the present invention to obtain, or measure
out,
accurately known, small, consistent quantities of nucleic acid, based on the
binding capacity
2 0 of capillary tubes or other configurations of reaction chamber. For
example, if is desirable to
carry out a reaction using 10 ng of nucleic acid, it is only necessary to
obtain a capillary
tube, or other reaction chamber, with a total of 10 ng of nucleic acid binding
capacity. Then,
the capillary is filled with nucleic acid-chaotrope solution wherein both the
nucleic acid and
chaotrope are at sufficiently high concentration to support saturable binding
in reasonable
2 s time. After the incubation, emptying, washing and drying steps are
complete, the
experimenter is confident that the capillary contains 10 ng of nucleic acid
which can be
eluted for dispensing, or left to reside in the capillary for future use.
[0317] Typically, the binding capacity, or amount of nucleic acid that can be
saturably
bound to the inner surface, is determined empirically. For example, a known
amount of test
3 o nucleic acid is labeled with a radionuclide, such as 35S, ssP or 3zP,
according to methods
known in the art. After labeling, the specific activity of the labeled nucleic
acid is determined
to establish a ratio of disintegrations per minute per mass unit, or
concentration unit of
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 73 -
nucleic acid. The labeled nucleic acid is then dissolved in a solution
containing chaotropic
ions at a predetermined concentration. A standard reaction chamber,
representative of a
general supply, is then tested. For example, a predetermined length of glass
capillary
tubing is cut and filled with the labeled nucleic acid-chaotrope solution.
After sufficient time
for saturable binding to occur, the capillary is emptied and washed. Then, the
amount of
radioactivity retained inside the tube is measured, and, with knowledge of the
specific
activity of labeling, converted to an amount of nucleic acid. This factor can
then be used to
calculate the amount of nucleic acid that will be retained in any length of
capillary tubing cut
from the same lot, so long as similar conditions for binding are used in any
subsequent
1 o experiment.
[0378] An advantage of using the present invention to accurately obtain a
predetermined
quantity of nucleic acid is to normalize quantities of nucleic acid for
subsequent use. This
advantage is especially significant if it is necessary to process many
samples. For example,
in the current state of the art, it is not practical, when preparing different
template DNAs for
~ 5 sequencing, to ensure that the concentration of the templates is the same.
Thus, according
to prior methods it was necessary to normalize the different template DNA
samples, by
separately determining the DNA concentration in each prep, and diluting the
DNA to the
proper concentration for each and every sample. This is especially important
for capillary
electrophoresis because of the sensitivity of that technology to overloading
of the capillaries
2 o with template DNA. The requirement for normalization of the template DNA
added
significant time and cost to obtaining high quality DNA sequence data using
this system, or
required that researchers accept increased failure rates.
[0379] However, the present invention allows very rapid normalization to
minimize
differences in starting template concentration. To normalize the different
templates to a
2 5 predetermined concentration it is only necessary to provide functionally
equivalent capillary
tubes (one for each template) with a known, saturable DNA binding capacity,
and template
DNA-chaotrope solution with sufficiently high concentration of both DNA and
ions that all the
DNA binding sites in the capillary become occupied within a reasonable period
of time.
After emptying and washing, all the capillaries will contain about the same
quantity of
3 o template DNA, and are thus normalized.
[0380] As will be apparent to the skilled artisan, if it is not desirable to
saturate all the
possible nucleic acid binding sites inside a reaction chamber, it is possible
to control the
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 74 -
amount of nucleic acid that is reversibly bound. This is possible because the
kinetics of the
binding reaction depend on a number of variables, including nucleic acid
concentration,
average nucleic acid molecular size, solution pH, chaotropic ion
concentration, the number
of available binding sites on the inner surface of the reaction chamber and
temperature.
Thus, with empirical analysis, it is possible for the skilled artisan to
establish binding
conditions that result in the consistent, predictable, reversible binding of a
predetermined
quantity of nucleic acid that does not saturate all available nucleic acid
binding sites inside a
reaction chamber.
1 o DNA Sequencing for Capillary Electrophoresis
[0381] The advantages of the present invention are beneficially applied to
carrying out
DNA sequencing reactions, particularly for analysis with highly sensitive
capillary
electrophoresis systems such as MegaBACET"". To use the present invention for
DNA
15 sequencing, template DNA must be immobilized in capillary tubes, or the
functional
equivalent thereof. Template DNA is that DNA for which the sequence of
constituent bases
is to be determined. Template DNA can be single stranded, or double stranded,
wherein
two complementary DNA strands are hybridized together, and knowledge of the
sequence
of one strand can be used to infer the sequence of bases in the other strand
according to
2 o the rules of Watson-Crick base pair complementarity.
[0382] Template DNA is typically obtained directly from self-replicating
genetic systems,
grown in a host, into which the DNA fragment to be sequenced was cloned.
Alternatively,
the template can be obtained from any source, e.g., genomic DNA, by amplifying
a
particular DNA sequence using the polymerise chain reaction, or a functionally
equivalent
2 5 linear or exponential amplification process.
[0383] Self-replicating genetic systems include episomal elements, such as
plasmids
containing an origin of replication, or bacteriophage (e.g. lambda or M13),
both of which can
replicate inside bacteria, such as E. coli, after transformation or infection,
respectively.
Plasmids harboring template DNA are obtained by breaking open the bacteria in
which they
3 o have replicated to sufficiently high copy number, and isolating the
plasmid from the
supernatant. Bacteriophage released into bacterial culture supernatant after
lysing the host
bacteria are collected, and the DNA isolated by breaking open the
bacteriophage particles.
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 75 -
It is also possible to grow episomal agents containing mammalian origins of
replication in
mammalian cells, followed by isolation of the DNA according to the Hirt
method.
(0384] Due to the substantial difference in molecular mass between plasmid or
other
episomal DNA, as compared to intact genomic DNA, use of capillary tubes as
reaction
chambers offers a convenient method by which to rapidly purify plasmid DNA
from intact
genomic DNA when both are released after lysing bacteria or other type of
cells. Briefly, a
mixture of plasmid and intact genomic DNA is combined in solution of
chaotropic ions. A
small-bore capillary into which the plasmid is desirably immobilized is dipped
into the
solution. The plasmids, because of their small mass, easily pass into the bore
of the
1 o capillary as it fills, thereby interacting with the glass walls to
establish salt-bridges and
become immobilized. In contrast the intact genomic DNA, being of extremely
large
molecular mass, is excluded from the small bore of the capillary, and is thus
separated by
size exclusion from the plasmids.
[0385] As mentioned, template DNA can also be obtained without the need for
cloning
steps by amplifying a DNA fragment directly from an appropriate source, such
as a virus, a
prokaryotic cell, including bacteria, or eukaryotic cell, including mammals,
other animals, or
plants.
[0386] After the template DNA, FIG. 16 80, is reversibly immobilized directly
to the inner
surface 82 of a glass capillary tube 12, in accordance with the methods of the
present
2 o invention, the capillaries are filled with the sequencing reaction mixture
84 that effects the
DNA sequencing reaction. The reaction is carried out according to techniques
well known
in the art, whereby the products of the DNA sequencing reaction are labeled
with
fluorescent dyes. Well established in the art is the Sanger dideoxynucleotide
chain
termination technique. Briefly, a primer complementary to sequence in the
template DNA
2 5 molecule is permitted to hybridize to the template. Then DNA polymerase
extends the
primer by reading the sequence of bases in the template, by adding dNTPs to
the 3' end of
the growing primer. However, dideoxynucleotide triphosphates that lack the
hydroxyl group
characteristic of the corresponding dNTP prevent the further addition of bases
to the
growing strand. As a result the chain terminates. The pattern of terminated
chains in a
3 o chromatogram permits the experimenter to infer the sequence of bases in
the template.
The terminated reaction products are fluorescently labeled either by
conjugating a
fluorophore to the primer that is extended, or alternatively, by conjugating a
fluorophore to
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 76 -
all the dideoxy terminators that, when incorporated into growing DNA chain,
result in
termination of primer extension.
(0387] In recent years, use of energy transfer, dye-coupled fluorophore
systems,
comprised of a light acceptor dye and fluorescence emitter dye, have improved
the
s performance of laser scanned sequencing systems. Each dideoxy terminator is
labeled with
two dyes. One of these dyes, fluorescein, absorbs light energy from incident
laser light
produced by the laser in the sequencing machine, and transfers the collected
energy via
radiationless energy transfer to an acceptor dye. Each of the four chain
terminators, ddG,
ddA, ddT, and ddC, have a different acceptor dye coupled with the fluorescein
donor. The
1 o acceptor dyes, for example, rhodamine 110, rhodamine-6-G, tetramethyl
rhodamine, and
rhodamine X, then emit light at their characteristic wavelengths. The
fluorescence is
detected by the instrument allowing identification of which nucleotide caused
the termination
event. Use of the energy transfer system results in more efficient excitation
of the acceptor
dyes than direct excitation by the laser, resulting in greater sensitivity. As
an alternative to
15 fluorescently labeling the dideoxy terminators, it is possible to label the
sequencing primer.
If using this system, energy transfer dyes may be used as well by conjugating
to the primer
a donor dye and an acceptor dye. An example of a donor dye to be conjugated to
a primer
is 5-carboxy-fluorescein (FAM), and examples of acceptor dyes to be conjugated
to primers
are rhodamine 110 (R110) for cytosine, 6-carboxyrhodamine (REG) for adenine,
N,N,N',N'-
2 o tetramethyl-5-carboxyrhodamine (TAMRA) for guanine, and 5-carboxy-X-
rhodamine (ROX)
for thymine. The energy transfer dye-coupled fluorophore system is discussed
in greater
detail in issued U.S. Patent Nos. 5,688,648, 5,707,804, 5,728,528, 5,853,992,
5,869,255,
and 6,028,190, all of which are herein incorporated by reference in their
entireties.
(0388] The capillary, FIG. 1612, containing the immobilized template DNA 80 is
filled by
2 5 capillary action by dipping it into a reservoir 85 filled with the
reaction mixture. The reaction
mixture 84 contains all the components at the appropriate concentration to
effect the
sequencing reaction, including water, salts, buffers, primer, DNA polymerase,
dNTPs and
dideoxy terminators. Without wishing to be bound by theory, at present it is
hypothesized
that as the aqueous mixture ascends the capillary, the immobilized DNA likely
rehydrates.
3 o Furthermore, because the ionic strength of the salts in the mixture is
relatively low, the salt-
bridge causing the DNA to be immobilized is disrupted by the water molecules
and the DNA
is eluted from the inner surface of the capillary, and diffuses into the
reaction mixture.
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
_ 77 _
Alternatively or in addition, the DNA desorbs during the thermocycling
reactions. Whatever
the mechanism, physical mixing of the DNA into the mixture is not necessary
for
performance of the reaction.
[0389] Once the capillary is filled, the ends are sealed to prevent
vaporization of the liquid
s contained inside, followed by thermal cycling to activate multiple rounds of
the sequencing
reaction, so as to generate the fluorescently labeled product to be analyzed.
Sealing of the
capillary and thermal cycling may be effected in multiple ways, as will be
apparent to the
skilled artisan. If, as will often be the case, it is desirable to perform
multiple sequencing
reactions in parallel, the experimenter can use a high-throughput apparatus,
such as that
to disclosed in the copending application U.S. Serial No. 09/577,199, which is
hereby
incorporated by reference in its entirety. The disclosed apparatus provides
means both for
sealing multiple capillary tubes arranged into a cassette format, and for
effecting thermal
cycling of the sequencing reaction mixtures contained in the capillaries.
[0390] After the sequencing reaction is completed the reaction products are
expelled from
1 s the capillary tubes, typically in preparation for analysis by capillary
electrophoresis.
[0391] Typically, the reaction product is expelled onto a substrate, or into
some form of
holder for liquid, such as a well of a microtiter dish, from which a capillary
electrophoresis
system may sample the product for analysis. However the skilled artisan will
recognize that
it is possible for the reaction product to be expelled directly from the
reaction capillary into
2 o the electrophoresis capillary. Reaction product may be expelled from the
reaction
capillaries by the application of centrifugal force, electrokinetically, by
the application of
positive or negative air pressure, or by other means known in the art.
[0392] Furthermore, the reaction product can be expelled onto a substrate
adapted for
other types of analytical process, such as a MALDI (matrix-assisted laser
2 s desorption/ionization) or SELDI (surface-enhanced laser
desorption/ionization) substrate for
mass spectrometric analysis.
[0393] During electrophoresis of the fluorescently labeled sequencing reaction
products, a
laser scans a window in the capillaries carrying the products and excites the
fluorophores.
Light emission by the fluorophores is captured and converted into intensity
and light
3 o frequency data that is stored in a computer memory. After scanning and
reading is
complete, the computer assembles a chromatogram representing all the reaction
products
detected by the scanning system. The data in the chromatogram is processed by
computer
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
_ 78 _
software that interprets the chromatogram to infer the sequence of nucleotide
bases in the
starting template DNA. The sequence output is then stored in a computer data
file, either in
random access memory or on a dedicated long term memory device, such as floppy
disk,
ZIP disk, JAZ disk, hard disk, CD-ROM, computer tape, etc. For the convenience
of end
s users of the data, the computer file containing the sequence data can be
stored on a
computer server that can be accessed from remote client computers. When the
file is
transferred it is represented as a data signal associated with a carrier wave
carried through
copper or fiberoptic telephone lines, cable television lines, or by radio
waves.
[0394] Once emptied, the capillary tubes are recycled for immobilization of
new nucleic
1 o acid samples, such as DNA template to be sequenced. Recycling of the tubes
requires
washing to remove detrimental traces of the previous reaction, including
reaction products,
reaction mixture components and the immobilized nucleic acid.
[0395] Typically, the wash solution is an aqueous wash solution of low ionic
strength such
that any remaining immobilized nucleic acid will tend to be eluted and carried
away. Double
i5 distilled water is effective. The wash solution may be heated to increase
the effectiveness
of washes, and the number of washes and/or volume of wash solution per wash
cycle can
be varied as necessary to maximize washing effectiveness. Capillaries can be
filled with
wash solution by capillary action and then emptied using the same methods by
which
reaction product is expelled. If washing is to be effected by electrokinetic
pumping, then the
2 o wash solution must contain some minimum concentration of ions.
Alternatively, a
mechanical pump can be used to drive wash solution through the capillaries.
[0396] The washing can also be accomplished by a mechanical capillary cassette
washer
as disclosed in commonly owned and copending U.S. patent application serial
no.
091577,199, filed May 23, 2000, the disclosure of which is incorporated herein
by reference
2 5 in its entirety.
[0397] The design for a capillary tube washing device designed to wash
multiple
capillaries arranged into a cassette is disclosed in the copending application
U.S. Serial No.
091577,199, herein incorporated by reference in its entirety.
[0398] After the aqueous washes, an alcohol wash, usually comprising a high
3 o concentration of ethanol is used to remove most traces of water and other
components of
the wash solution. The capillaries are then dried, typically by drawing warm
dry air through
them, after which they are ready for storage or reuse.
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
_ 79 _
[0399] For some applications, it is important that essentially no nucleic acid
remain from a
previous reaction in the capillaries. One example is PCR, whereby old residual
template
DNA could be exponentially amplified leading to contamination of a new
reaction. In such
cases, the recycling process can comprise steps effective at destroying traces
of nucleic
s acid. Such means include filling the capillary with a solution containing an
exonuclease and
incubating for such time as is necessary to digest any nucleic acid. Other
means include
chemical degradation of the nucleic acid, such as by washing with highly
acidic or basic
solutions; contact with bleach; irradiating the capillary with ionizing
radiation; or baking to
high temperature. After destroying residual nucleic acids, the capillaries
would typically be
1 o washed using standard solutions.
[0400] One application, though by no means the only one, whereby parallel
processing
using capillaries in cassettes will prove useful is the confirmation of the
sequence of DNA,
often PCR products, for high throughput de novo sequencing, such as for
discovery of
single nucleotide polymorphisms (SNPs). For SNP discovery, the methods and
apparatus
15 of the present invention make possible "deep" sequencing, in which the same
gene or
genetic locus is sequenced from a plurality of individuals, differences in the
sequence
identifying polymorphisms that exist in the sequenced population. Of these,
some SNPs will
be demonstrated to be associated with significant phenotypes, such as
predisposition,
presence, or progressive potential of disease.
2 0 [0401] SNPs are single base changes that occur approximately once every
1000 bases
and are the most common form of genetic variation in humans. If such
polymorphisms
occur in coding sequence or regulatory regions of genes, they can alter the
function of the
gene or gene product, as compared to the wild type sequence. Depending on the
extent to
which gene function is modified, the effect on the organism can minimal, or
result in
2 s deleterious phenotypes, including genetic diseases.
[0402] Analysis of SNPs and their associated phenotypes is useful both in the
search for
genes implicated in defined disease states, as well as the new field of
pharmacogenetics.
[0403] For the purpose of identifying disease genes, SNPs are used as markers
for
genetic linkage analysis to assist in identifying genes responsible for
diseases with a strong
3 o hereditary component. Similarly, SNP analysis has proved useful for
identifying changes in
alleleic variants of genes correlated with important phenotypes, such as
response to drug
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 80 -
compounds or other therapeutic regimes, as well as predisposition to or
progressive
potential of diseases.
[0404] SNP analysis is also useful for customizing drug or other therapeutic
regimes to
individual patients based upon a patient's unique genetic characteristics.
This is concept
s underlies the burgeoning field of pharmacogenetics. For example, a
particular
polymorphism or set of polymorphisms may be correlated with poor
responsiveness to a
particular drug. Further research may then show that the polymorphic changes
reside in a
gene encoding an enzyme responsible for metabolizing the drug, and that the
changes alter
the kinetic rate of the enzyme. As a result, the drug is metabolized more
quickly as
1 o compared to the wild type enzyme.
[0405] Knowledge of the correlation between SNP and enzyme phenotype therefore
presents an opportunity for customizing the care of patients who possess the
SNP. If
physicians could determine, prior to drug administration, which form of an
enzyme a patient
expresses, based on SNP analysis, the patient could, for example, be
prescribed a higher
1 s dose of the drug to compensate for the greater metabolic rate, thereby
obtaining for the
patient an optimal therapeutic effect.
[0406] The approach illustrated above can be generalized to encompass any gene
product that affects a drug or other type of therapeutic regime. In fact, so
long as the
possession, or lack thereof, of particular SNPs can be correlated with a
therapeutic
2 0 outcome, it is not necessary to understand the mechanism by which the
genotypic change,
compared to wild type, results in the altered phenotype. The knowledge of the
correlation
alone can be sufficient to guide physicians in modifying therapeutic regimes
to suit particular
patients.
[0407] SNP analysis therefore, is useful both for identifying genes that
affect therapeutic
2 s regimes in human and non-human patients, and identifying those patients
who will require a
modified therapy compared to the patient population that lacks the SNP marker.
The
usefulness of SNP analysis is not limited to applications related to medical
care alone,
however. Indeed, identification of SNPs in the genes of any organism that can
be
correlated with an interesting phenotype is increasingly useful both for
identifying those
3 o genes responsible for a particular phenotype, as well as those genetic
alterations that cause
the phenotype to be modified. Such knowledge offers an improved understanding
of how
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 81 -
particular gene products function, as well as insights as to how such
functions can be
beneficially modified.
[0408] Typically, SNP analysis is most beneficially undertaken in a high
throughput
manner, for which application of the present invention is particularly well
suited. Depending
s on the information to be obtained, the presence of SNPs in one or a few
genes is analyzed
from a large number of samples from patients, or another type of non-
genetically identical
sources, including non-human sources. This approach is typically, but not
exclusively,
adopted in studies designed to obtain large data sets for correlating
particular SNPs with
particular phenotypes. This approach will often also be adopted by facilities
that analyze
1 o SNPs present in genes of large numbers of human or animal patients, which
information is
to be used for customizing treatment regimes to individual patients.
[0409] Alternatively, high throughput SNP analysis may be undertaken on a
large number
of genes obtained from relatively few samples. This approach typically will be
advantageous when a comprehensive analysis of SNPs present in a patient is
desired.
1 s Such information may be necessary to customize treatment regimes in the
context of
diseases with complex multigene etiologies.
[0410] As is known in the art, different methods are useful for detecting SNPs
in genes or
gene fragments of known sequence. Most such techniques rely on indirect
fluorescent
detection of the single base change, as described in greater detail in
"Enabling large-scale
2 o pharmacogenetic studies by high-throughput mutation detection and
genotying
technologies," by M. Shi, published in Clinical Chemistry, 47(2):164-172
(2001), which is
incorporated by reference herein in its entirety. Examples include
oligonucleotide ligation
assay genotyping (OLA); minisequencing; TaqManT"" genotyping; InvaderT""
assay; dye
labeled oligonucleotide ligation; pyrosequencing; and rolling circle
amplification (RCA), as
2 s described further in further detail in "Sniper: a fully automated,
fluorescence platform
incorporating rolling circle amplification for scalable, high-throughput SNP
scoring," by Z.
Clark and J. Pickering, published in Life Science News 6 (2000), Amersham
Pharmacia
Biotech, which is incorporated by reference herein in its entirety.
[0411] An especially useful method of detection of SNPs is single base
extension with
3 o fluorescence detection, also known as single base extention (SBE). SBE, in
part, is based
upon the dideoxyterminator approach to DNA sequencing, described above.
Template
nucleic acid is provided for analysis to determine whether the sequence
contains one or
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 82 -
more SNPs at particular base positions in the sequence. A primer that
specifically
recognizes known sequence immediately 5' of a base to be interrogated in the
template is
then allowed to contact and bind the template via Watson-Crick base pairing.
Thereafter, a
DNA polymerise, which may include a thermostabile version thereof, reads the
template
strand beginning at the base to be interrogated and enzymatically attaches a
complementary dideoxyterminator nucleotide triphosphate (ddNTP), present in
the reaction
mixture, to the 3' hydroxyl group of the primer. Each of the four bases, A, C,
G, T, is
represented among the dideoxyterminators present in the reaction mixture, and
each of the
four bases is labeled with a fluorophore that emits excited photons at a
wavelength that
1 o uniquely identifies which base is present in association with the
particular fluorophore.
Because the dideoxyterminator cannot itself support strand extension by the
DNA
polymerise, extension stops after the addition of the single complementary
labeled
dideoxyterminator. After the extension reaction is complete, the extended
primer is
released, thermally or chemically, from the template and the primer is
analyzed to detect the
fluorophore associated with the dideoxyterminator base attached to primer 3'
end.
Identification of the fluorophore, based on its emission spectrum, permits
unequivocal
identification of the base incorporated by the DNA polymerise during single
base strand
extension, and the base defines the SNP present in the gene at the position
interrogated.
[0412] According to an alternative embodiment, a subset, rather than all four
ddNTPs may
2 o be included in the SBE reaction mixture, according to the needs and
preference of the
skilled artisan. Such ddNTP subsets comprise those listed in the following
table.
A only C, G
C only C, T
G only G, T
T only A, C, G
A, C A, C, T
A,G A,G,T
A,T C,G,T
[0413] Identification of the fluorophore can be accomplished using a variety
of techniques
2 5 according to the knowledge of the skilled artisan. For example, the
products of a single
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 83 -
base extension reaction can be separated from unincorporated dideoxyterminator
nucleotides on a denaturing gel similar to that used for DNA sequencing. After
the SBE
products have been resolved by gel electrophoresis, the fluorophores
associated with the
primers in the gel are excited by light of the appropriate wavelengh and
fluorescence
s emission detected and analyzed according to the knowledge of the skilled
artisan.
Alternatively, unincorporated dideoxynucleotides can be removed prior to
analysis of the
SBE products by gel electrophoresis.
[0414] According to another embodiment, fluorescently labeled dideoxterminator
nucleotides incorporated into SBE extension products are detected using
fluorescence
1 o polarization (FP) according to the knowledge of the skilled artisan. With
this technique,
polarized light is used to stimulate emission from the fluorophores.
Unincorporated
fluorophores are small and therefore emit depolarized light upon fluorescent
excitation,
whereas fluorophores incorporated into the much larger SBE extended primers
emit
polarized light. Preferential detection of polarized fluorescent emission can
therefore be
1 s used to infer the incorporation of particular fluorophores, and therefore
bases, into the
extended primers. Use of FP permits analysis without prior removal of
unincorporated
dideoxyterminators. FP as applied to detection of SNPs is discussed in
additional detail in
U.S. Patent Nos. 6,326,605; 6,310,687; 6,297,018 ; 6,187,267; 6,097,025; and
6,071,748,
each of which is incorporated herein by reference in its entirety.
2 0 [0415] Template can be obtained, according to techniques well known in the
art, from a
variety of sources, including, but not limited to genomic DNA obtained from
eukaroytic cells,
prokaryotic cells, or viruses; episomal DNA, including plasmids; and
messenger, or other
types of RNA. Template can be single stranded DNA or RNA, double stranded DNA
or
RNA, or DNA-RNA hybrids. If template is substantially comprised of RNA, the
DNA
2 s polymerase to be used to extend the primer is a reverse transcriptase
(RT), including
thermostable versions thereof.
[0416] According to one embodiment, the template is a PCR product obtained
from
genomic DNA. In this embodiment, prior to effecting the single base extension
reaction, a
PCR reaction is performed, using methods well known in the art, using primers
that
3 o specifically recognize genomic DNA which serves as the template for PCR.
Thereafter, the
DNA fragment generated by PCR serves as the template for SBE. Amplified
template from
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 84 -
genomic DNA or other nucleic acid can also be obtained by a linear
amplification process,
or an exponential amplification process functionally equivalent to PCR.
[0417] SBE reactions have traditionally been performed in large, so-called
"full volume"
reaction volumes, as described in greater detail in Example 22, below.
According to these
methods, PCR is performed in multiple microliter reaction volumes using
genomic DNA
template to generate the template to be used in subsequent SBE reactions.
Thereafter, the
PCR products are treated with Exol and SAP to degrade single stranded DNA and
excess
dNTPs, respectively. Subsequently, SBE is performed using a portion of the
template
generated by PCR, after which the SBE reaction products are treated with CIAP
and then
1 o analyzed by capillary electrophoresis, e.g., using MegaBACET"~.
[0418] Full volume reactions are performed in volumes of up to about 10, 15,
20, 25, 50,
75, 100 or 200 microliters, and as in volumes as low as about 100, 75, 50, 25,
20, 15, 10, or
5 microliters.
[0419] Although the full volume methods just described have proved
efficacious, they are
1 s also wasteful of reagents and other materials because the mass of SBE
product necessary
to obtain high quality data is very small relative to the actual amount
generated using the full
volume approach. Additionally, the full volume approach demands considerable
time to
effect the various thermal cycling steps in PCR and SBE and to transfer fluid
volumes
between steps.
2 0 [0420] In contrast, application of the methods and apparatus of the
instant invention to
SBE and its antecedent steps can advantageously reduce use of reagents, reduce
the time
necessary to complete the enzymatic reactions and reduce the number of fluid
transfer
steps. An additional advantage is provided by template normalization which
renders it
unnecessary to predetermine, prior to PCR or SBE, the concentration of
template in
2 s whatever solution provides its source.
[0421] As a result, use of the present invention with SBE, or other methods of
SNP
detection, greatly facilitates detection of SNPs in the context of high
throughput methods.
[0422] In part, the advantages of the present invention as applied to SBE and
other
techniques of SNP detection are an effect of performing one or more enzymatic
reactions in
3 o nanoliter volume (also called "nanovolume") reactions using the
capillaries of the instant
invention. In particular, use of nanovolume reactions reduces the quantity of
reagents used,
which translates to saved costs as compared full volume reactions. Nanovolumes
also
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 85 -
reduces the time necessary to proceed from one temperature to another during
thermal
cycling of reactions because the total mass of the reaction mixture is lower,
and the surface
area per unit volume of the reaction is greater when using capillary tubes as
compared to
the reaction tubes used for full volume reactions. Both effects increase the
rate of heat
transfer and thereby reduce the time necessary to perform an entire series of
thermal
cycles. Lastly, as discussed in more detail below, template capture, i.e., the
reversible
binding of template to the internal surface of the capillary in the presence
of a chaotrope,
permits elimination of one or more steps necessary to perform SBE, further
reducing
reagents, costs, and time associated with performing the assay.
l o [0423] As used throughout, nanoliter volume reactions are performed in
volumes of up to
about 25, 50, 100, 250, 500, 750, 1000, 1500, 2000, 2500, 5000, or more
nanoliters, and in
volumes as low as about 2500, 2000, 1500, 1000, 750, 500, 250, 100, 50, 25,
10, or fewer
nanoliters.
[0424] According to an embodiment of the present invention, template dissolved
in
chaotrope solution is withdrawn into a capillary of the present invention by
capillary action,
or other method as described herein, and contacts the inner surface until a
predetermined
approximate mass of such template is caused to bind reversibly thereto. After
binding is
complete excess template in chaotrope is removed and the bound template washed
as
explained elsewhere herein. After a futher optional drying step, SBE reaction
mixture,
2 o containing all ingredients necessary to effect SBE, including buffers,
salts, water, SBE
primer, fluorescently labeled ddNTPs, and DNA polymerase, is drawn into the
capillary by
capillary action, or other method described herein. Subsequently, the
capillary containing
the template and SBE reaction mixture is exposed to thermal cycling as
necessary to effect
SBE.
2 5 [0425] According to an alternative embodiment, SBE template may be
reversibly bound to
the inner surface of a capillary in the presence of chaotrope until an amount
of template, as
determined by the skilled artisan, is bound which is sufficient to yield
detectable SBE
product after conducting the reaction. That is, it is not necessary that a
predetermined
approximate mass of SBE template be reversibly bound inside the capillary for
the
3 o usefulness of the present invention to be realized.
[0426] After the reaction is completed, SBE product is typically expelled from
the capillary,
as described elsewhere herein, for subsequent processing, including removal of
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 86 -
unincorporated ddNTPs, e.g., by treatment with calf intestinal alkaline
phosphatase (CIAP),
according to methods known in the art. As understood by the skilled artisan,
CIAP
treatment removes phosphate groups from ddNTPs, rendering the dephosphorylated
ddNTPs uncharged. As a result, during electrophoresis, e.g., using
MegaBACET"", the
treated ddNTPs are not induced to move by the strong electric field that
causes the charged
SBE products to enter the sieving gel. This approach facilitates separation of
unincorporated ddNTPs from the SBE products.
[0427] CIAP treatment may be effected in full volume reactions, or
alternatively, in
nanovolume reactions. Full volume CIAP treatment is conveniently performed in
the wells
of microtiter plates, e.g., 96, 384, 1536, or higher numbers of wells per
plate. In contrast,
nanovolume CIAP treatment is performed within a capillary of the present
invention after
having mixed the SBE product with CIAP reaction mixture, e.g., within a well
of a microtiter
plate.
[0428] As an alternative to treatment of SBE products with CIAP, excess
unincorporated
ddNTPs may be removed by contacting the SBE reaction products with a gel
filtration media
for sufficient time to separate ddNTPs from SBE products. Complete separation
is not
necessary; the extent of separation which is sufficient is within the
knowledge of the skilled
artisan. Gel filtration media is chosen with properties that ensures that
ddNTPs can enter
the pores of the media whereas SBE products are substantially excluded. In
this manner
2 o ddNTPs are contained in the total volume, whereas SBE product is contained
within the
void volume. Examples of media suitable for use in the present invention
include, but are
not limited to superdex, superose, sephacryl, and sephadex.
[0429] Finally, the SBE products are analyzed to identify the incorporated
bases. As
described elsewhere herein, one such method is capillary electrophoresis using
the
2 5 MegaBACET"" system. Electrophoretic methods coupled with a microfluidic
platform can
also be used to resolve extension products of SBE. Such methods are discussed
in more
detail in IJ.S. Patent Nos. 6,316,201; 6,306,659; 6,306,590; 6,303,343;
6,287,774;
6,274,337; 6,267,858; 6,235,471; 6,235,175; 6,174,675; 6,153,073; 6,107,044;
6,068,752;
6,042,710; 5,976,336; 5,965,410; 5,958,694; and 5,948,227, each of which is
incorporated
3 o herein by reference in its entirety.
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
_ 87 _
[0430] Alternatively, SBE products can be analyzed using mass spectrometric
techniques,
including matrix-assisted laser desorptionlionization (MALDI) or surface-
enhanced laser
desorptionlionization (SELDI).
(0431] According to an alternative embodiment, SBE template can be drawn into
the
s capillary of the instant invention having already been mixed with the SBE
reaction mixture,
in which case template normalization does not occur.
[0432] According to another embodiment of the instant invention, template for
SBE is
prepared by PCR, according to methods well known in the art.
[0433] PCR may be effected in full volume reactions. After PCR is completed,
the
1 o reaction can be treated to remove primers and dNTPs, as described in
further detail below.
Then, according to one embodiment, the PCR products are mixed with chaotrope
and used
to fill a capillary of the instant invention for template normalization of the
SBE template,
followed by the extension reaction, as described above. In an alternative
embodiment, a
portion of the PCR products, after treatment, are added to SBE reaction
mixture and used to
15 fill a capillary of the instant invention for subsequent performance of the
extension reaction,
as described above.
[0434] PCR may also be performed in a capillary of the instant invention using
nanoliter
volume reactions, in which case PCR may be preceded by template normalization
of the
genomic DNA, or other PCR template, to be used in the reaction, similarly as
described
2 o above for SBE template. Alternatively, PCR template may be added to the
PCR reaction
mixture prior to filling the capillary, in which case template normalization
does not occur.
[0435] Following PCR, the reaction product typically is expelled from the
capillary, as
described elsewhere herein, and treated to remove primers and dNTPs, as
described in
further detail below. As in the case of full volume PCR, treated PCR products
may then be
2 s mixed with chaotrope and used for template normalization of the SBE
template, or added to
SBE reaction mixture. Extension reactions are then performed as described
elsewhere
herein.
[0436] As mentioned above, after PCR is completed, PCR product is typically
expelled
from the capillary, as described elsewhere herein, and treated to remove
excess
3 o unincorporated PCR primers and dNTPs by, for example, using a single
stranded Dnase,
e.g., exonuclease I (Exo I), and a phosphatase, e.g., shrimp alkaline
phosphatase (SAP),
respectively, according to methods known in the art. PCR product, as SBE
template, may
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
_ 88 _
then be normalized, or added directly to SBE reaction mixture, and used in SBE
in a
capillary of the present invention, as described above.
[0437] ExoIISAP treatment may be effected in full volume reactions, or
alternatively, in
nanovolume reactions. Full volume ExoI/SAP treatment is conveniently performed
in the
s wells of microtiter plates, which plates may comprise 96, 384, 1536, or
higher numbers of
wells per plate. In contrast, nanovolume ExoI/SAP treatment is performed
within a capillary
of the present invention after having mixed the PCR product with ExoIISAP
reaction
mixture, e.g., within a well of a microtiter plate.
[0438] According to an alternative embodiment, after PCR, whether conducted in
full
to volume or nanovolume reactions, the PCR product treatment step is exluded.
Rather, to
effect removal of excess unincorporated primers and dNTPs, the PCR products
are added
directly to chaotrope, after which the solution is used to fill a capillary of
the instant invention
until such time that a predetermined approximate mass of template, or a mass
of template
sufficient to yield detectable SBE products, is reversibly bound to the inner
surface of the
15 capillary. Thereafter, excess unbound PCR product (i.e., SBE template),
primers, and
dNTPs are removed by washing, as described elsewhere herein. After an optional
drying
step, SBE reaction mixture is then drawn into the capillary for subsequent
performance of
the extension reaction, as described elesewhere herein.
[0439] At each stage of the procedure that uses nanovolume reactions in
capillaries of the
2 o instant invention, a new capillary may be used. Alternatively, the same
capillary from one or
more previous steps may be reused, with, or without first having washed the
interior of the
capillary, or otherwise treat the capillary, to remove or inactivate traces of
reagents,
reactants or products deposited therein from the previous step. Methods of
washing or
treating capillaries of the instant invention are discussed elsewhere herein.
2 s [0440] In a preferred embodiment, a plurality of the capillaries of the
present invention are
provided arranged in a spatially addressible array to facilitate high-
throughput processing of
multiple samples in parallel. Typically, the number and pattern of capillaries
in an array and
the dimensions of an array of capillaries corresponds to the number, pattern
and
dimensions of wells in one or more types of microtiter plates such that an
capillary array and
3 o wells of a plate can be mated, preferably in the context of an automated
or semi-automated
robotic work flow system. Often, but not necessarily, arrays are rectangular,
but may be
circular, triangular,etc. The number of capillaries in an array may include 2,
4, 8, 12, 16, 24,
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 89 .
32, 48, 64, 96, 128, 192, 288, 384, 480, 576, 672, 768, 864, 960, 1536
capillaries, or higher
number of capillaries. Methods of arranging capillaries into arrays of chosen
number,
pattern and dimension are described elsewhere herein, or are otherwise known
to the
skilled artisan. Multiple PCR and SBE reactions can be performed in parallel
using arrays
s of capillaries using an apparatus for performing high-throughput reactions,
such as that
disclosed in the copending application U.S. Serial No. 091577,199, which is
hereby
incorporated by reference in its entirety.
[0441] Another application whereby parallel processing using capillaries in
cassettes will
1 o prove useful is the confirmation of the sequence of DNA, often PCR
products, intended to
be spotted on to a substrate to create a microarray. Such microarrays are
finding increased
use in basic and applied research and are typically comprised of a rectangular
array of
spots of DNA on a glass slide, with a different, known DNA sequence at each
spot. The
experimenter then takes a labeled sample, either RNA or DNA and detects
hybridization
1 s events between the labeled nucleic acid and the DNA spotted to the array.
In this way, the
experimenter can infer the identity andlor partial or complete sequence of the
labeled
nucleic acid.
[0442] To ensure the integrity of the data generated using microarrays, it is
necessary that
the identity of the sequence of the spotted DNA be known with high confidence.
Rearraying
2 o and other sample handling procedures introduce formatting errors that must
be detected.
Furthermore, PCR is often used to generate the DNA to be spotted. As is well
known in the
art, Taq and other thermostable polymerases introduce a certain number of
erroneous base
pairs per thousand as it amplifies the template. If errors have been
introduced, they must
be detected, and the amplified product discarded. Usually, this requires
numerous
2 s processing steps separate from those associated with spotting the PCR
product. However,
use of an embodiment of the present invention greatly increases the efficiency
of sequence
determination and confirmation.
[0443] The DNA sample to be spotted is usually dissolved at a predetermined
concentration in a solution comprising chaotropic ions, for example sodium
thiocyanate.
3 o The DNA is so dissolved because it is to be immobilized to the surface of
the glass
microarray slide in a manner similar to that by which nucleic acid is
immobilized inside
capillary tubes. Typically the different DNA-chaotrope solutions are aliquoted
into wells of
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 90 -
384-well capacity microtiter dishes for storage until ready to be spotted onto
a microarray.
Prior to spotting the dish is picked up by a robot associated with a automated
spotting
system and manipulated into a position whereby the spotting styli or pens can
be dipped
into multiple wells, usually 12, at one time.
s [0444] The present invention can be adapted to sample and sequence the DNA
in
multiple wells of the same 384-well dish used as the DNA source for the
spotting pens. It
will be apparent that it can also be adapted to sample from dishes with more
than 384 wells.
Because the DNA to be sequenced is from the same sample to be spotted,
numerous
processing steps associated with sequencing the DNA from different samples are
obviated.
1 o This results in substantial savings of time and material costs. According
to this embodiment
of the present invention, glass capillaries are arranged into a cassette in
the same pattern
and inter-capillary dimensions as that of the wells in one or more rows or
columns of the
dish. For maximal capacity, a total of 384 capillaries are arranged into a
pattern with
dimensions identical to that of the dish itself. Prior to spotting, the
capillary cassette is filled
15 with DNA-chaotrope solution (usually sodium thiocyanate) according to the
methods of the
present invention. After the DNA samples are immobilized and processed, they
are
sequenced. If any of the templates fails to give the correct sequence, the
operator of the
spotting apparatus knows not to spot that DNA, or if spotted, that data
associated with
hybridization at the corresponding spot is to an unwanted sequence and should
be removed
2 o from the resulting data set.
Alternative Biochemical Reactions With Reversibly Immobilized Nucleic Acids
(0445] The present reaction mixture assembly may be used for assembly of
numerous
2 s types of reactions. The same basic method used to assemble the PCR
reaction mixture
may be adapted to assembly of a cycle sequencing mixture, rolling circle
amplification
reaction mixture, enzymatic assays, chemical reactions, or other reaction
mixtures.
Dispensing a Predetermined Quantity of a Nucleic Acid
(0446] As will be readily apparent, the experimenter is not obligated to carry
out a reaction
with the nucleic acid immobilized inside of a capillary tube. For a variety of
reasons, it may
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 91 -
be preferable to elute the immobilized nucleic acid from the inner surface of
the capillary
and either perform a reaction with it in a different reaction chamber, or to
process the
nucleic acid in some other way outside of the capillary. In such
circumstances, it is possible
to use the capillary as a pipettor to dispense a predetermined approximate
mass of the
s nucleic acid in a fixed volume of liquid, and therefore at a predetermined
approximate
concentration, onto a substrate of the experimenter's choosing. To do so, the
capillary is
filled with elution fluid that elutes essentially all the reversibly
immobilized nucleic acid.
Thereafter, the solution of the elution fluid and nucleic acid is dispensed,
usually onto or into
a substrate. The substrate onto which the reaction mixture is transferred may
be the wells
of a multiwell microtiter plate, locations on a planar substrate, or wells
that lead into an
analytical chip. The reaction may also be dispensed into a solution for
further chemical or
biochemical reaction.
[0447] If multiple capillaries are arranged into a cassette, as described
above, the
cassette becomes a multichannel parallel pipettor, and it becomes possible to
dispense a
large number of normalized nucleic acid samples simultaneously. The dispensing
can be
into microtiter wells, microchips, and other chambers for further reactions.
In addition, the
nucleic acid can be dispensed directly into the reservoirs of a capillary
array electrophoresis
microchip or onto a MALDI or SELDI target, or onto or into a substrate adapted
to be used
in other analytical modalities.
2 0 [0448] Different methods may be used to expel or dispense liquid from
capillary tubes. As
will be appreciated by the skilled artisan, these methods can be employed to
dispense not
just an eluted nucleic acid solution, but also for removing the liquid from a
filled capillary
regardless of purpose, such as to remove reaction product after a reaction, or
to remove
washing solutions.
2 s (0449] One method to dispense the contents of a single capillary tube or
multiple similar
capillaries arranged into a cassette format uses a centrifuge to dispense the
fluid by
centrifugal force. The centrifugal force is applied evenly to all of the
capillaries in the
capillary cassette such that capillaries independently dispense their contents
onto a
substrate situated below the orifice to the capillary from which fluid is
expelled. If the
3 o substrate is a well of a microtiter dish, the dispensed liquid will be
drawn by centrifugal force
to the bottom of the wells. The design for a centrifuge and associated rotor
and buckets to
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 92 -
hold a cassette is disclosed in the copending application U.S. Serial No.
09/577,199, herein
incorporated by reference in its entirety.
(0450] A second method of dispensing the liquid contained in a capillary tube
is through
the use of an air displacement device. The design for an air displacement
device designed
s to dispense the liquid contents of multiple capillaries arranged into a
cassette is disclosed in
the copending application U.S. Serial No. 09/577,199, herein incorporated by
reference in
its entirety.
[0451] Alternatively, the contents of a capillary could be dispensed directly
into a well, or
sample port (FIG. 3E 76) of an analytical device (FIG. 3E 70), such as an
electrophoresis
chip. As shown in FIG. 3E, such an analytical chip would have an array of
analytical lanes
78 in fluid communication with their respective sample inlets or ports 76.
Multiple capillaries
may be arranged into a cassette format such that the spacing of the
capillaries matches the
spacing of the sample inlets 76 in the chip. For example, a capillary cassette
having 16
capillaries in two parallel rows of eight may dock with 16 wells in an
analytical chip.
15 (0452] As an example, the capillary cassette illustrated in FIG. 3C
includes capillaries 12
extending through flexible strip 11. Flexible strip 11 may be used alone or in
combination
with other such strips. The orientation of the capillaries in an essentially
straight line may be
altered by bending strip 11 to form an arc. FIG. 3D illustrates strip 11 bent
to allow
capillaries 12 to mate with input ports that are disposed on a substrate in a
circular pattern.
2 o The liquid in capillaries 12 may then be electrokinetically injected or
otherwise dispensed
from capillaries 12 into ports 76 of an analytical chip 70 if an appropriate
electrode array or
other dispensing methods are used. Strip 11 may be positioned in the curved
orientation by
pressing strip 11 against a curved form, such as a curved metal block. This
may be done
by an automated strip mover incorporated into an automated sample preparation
system.
2 s [0453] The capillary cassette could be dispensed by air displacement or
other dispensing
means preferably selected to minimize splattering and bubble formation. Prior
to dispensing
the prepared reaction mixture into the wells 76 for analysis, a small amount
of a diluent
could be added to each analytical microchip well 76. When the capillary
cassette is
dispensed, the diluent will dilute the samples in the sample wells 76. The
submicroliter
3 o volume reaction mixtures prepared in the capillary cassette, such as a DNA
sequencing
reaction product mixture, can readily be integrated with the analytical chip
for sequencing or
other analysis methods.
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 93 -
[0454] The elution fluid is preferably an aqueous solution of low ionic
strength, more
preferably water or a low ionic strength buffer at about a pH at which the
nucleic acid
material is stable and substantially intact, usually between pH 6.5 and 8.5.
TE Buffer at 1X
concentration (10 mM Tris-HCI, 1 mM ethylenediamine- tetraacetic acid (EDTA),
pH 8.0)
s and distilled or deionized water are particularly preferred elution
solutions for use in the
present invention. The low ionic strength of the preferred forms of the
elution solution
described above will tend to disrupt the salt-bridges established between the
nucleic acid
and the material comprising the inner surface of the capillary, ensuring that
the nucleic acid
is eluted into the solution. Other elution solutions suitable for use in the
methods of this
1 o invention will be readily apparent to one skilled in this art.
[0455] According to the methods of the present invention, nucleic acid binding
to the inner
surface of the glass capillary tube is saturable. Under appropriate
conditions, it is possible
to control, with a high degree of accuracy, the quantity of nucleic acid
immobilized inside
any particular capillary. Thus, if the nucleic acid is eluted into an aqueous
solution and
1 s dispensed, the concentration of the nucleic acid in the solution can be
known, as well as the
total quantity of nucleic acid in any particular volume of that solution. For
example, if a
capillary's binding capacity is 10 ng DNA, and this is eluted into 500 nl of
elution fluid, the
concentration of the solution is 0.02 grams per liter, with the molar
concentration dependent
on the molecular mass of the DNA molecules. If all 500 nl is dispensed, that
droplet
2 o contains 10 ng DNA.
[0456] As will be understood by the skilled artisan, due to small variations
among different
capillary tubes, the amount of nucleic acid that can be immobilized and
eluted, although
highly consistent, is not identical between capillary tubes, or even between
repeated use of
the same tube. For this reason, the predetermined quantity or mass of nucleic
acid eluted
2 s into the elution fluid is an approximate quantity or mass. Preferably, in
this context,
predetermined approximate mass shall mean that between similar capillaries, or
repeated
use of the same capillary, all other conditions being equal, the error between
the mass
expected to be immobilized or dispensed and actually immobilized or dispensed
is not
greater than 10%, more preferably 5%, more preferably 2%, and most preferably
not more
3 o than 1 % error.
[0457] Usually, the dispensing function of the present invention will be
utilized by
immobilizing a saturating quantity of nucleic acid in a particular capillary
and dispensing the
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 94 -
entire volume. Thus, to control the quantity and concentration of dispensed
nucleic acid,
the experimenter will choose a capillary with a predetermined binding capacity
and volume.
However, as discussed above, the experimenter can empirically determine
conditions under
which a predetermined non-saturating quantity of immobilized nucleic acid is
bound.
Accordingly, using these conditions, a non-saturating predetermined quantity
of nucleic acid
can be immobilized and then eluted from a capillary, allowing the experimenter
to dispense
any given amount of nucleic acid at will.
[0458] Under both circumstances, where a capillary has reversibly bound a
predetermined
quantity of nonsaturating, or saturating nucleic acid, if the experimenter,
using methods
1 o familiar to the skilled artisan, controls the amount of nucleic acid-
elution fluid expelled from
the capillary, then knowledge of that volume permits dispensing precise
amounts of nucleic
acid. For example, controlled amounts of the fluid can be expelled by
mechanical pumping,
or electrokinetic pumping.
15 A Highly Parallel Submicroliter System for Enzymatic Reactions
[0459] In another aspect, the invention provides methods and apparatus for
performing
enzymatic reactions - particularly, but not limited to, isothermal reactions -
in small
volumes, particularly submicroliter volumes. The reactions can be performed in
highly
2 o parallel fashion, and can readily be interfaced, in parallel, and without
substantial loss of
reactants to high resolution electrophoresis instrumentation for analysis.
[0460] The enzymes include any that are commonly used in larger-scale assays,
including
proteases, such as trypsin, chymotrypsin, proteinase K, papain, pepsin,
endoproteinase
Glu-C, Arg-C, Lys-C, Pro-C, V8 protease, glycosidases, such as [i-
galactosidase, lipases,
2 5 oxidises and oxygenases, such as glucose oxidise, cholesterol oxidise, and
lactate
monooxygenase, ligases, including DNA and RNA ligases, methylases,
polymerises, such
as DNA-dependent DNA polymerise enzymes, terminal transferase enzymes, RNA-
dependent DNA polymerise enzymes, DNA-dependent RNA polymerise enzymes,
phosphatase enzymes, kinase enzymes, DNA gyrase, topoisomerases, nucleases,
3 o including exonucleases, such as S1, or mung bean nucleases, and
endonucleases, such as
restriction endonucleases, other nuclease enzymes, and ribonuclease enzymes,
and
urease.
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 95 -
[0461] The submicroliter protein reactions are not limited to use of enzymes,
and thus
catalysis of chemical reactions. For example, proteins can be used for their
ability to bind
other molecules, and thus capture them from solution. For example, proteins
can be
antibodies or antigen-binding fragments thereof, such as IgG, IgE, IgM;
protein G and
Protein A; and streptavidin, to name a few.
[0462] Where the protein is an enzyme, the substrates are dictated by the
chosen enzyme
and are, accordingly, as varied as the enzymes, and include nucleic acids,
including DNA
and RNA, carbohydrates, lipids, and other biological and chemical substrates.
[0463] For demonstration purposes herein, submicroliter protease assays using
trypsin
1 o protease - a sequence-specific protease commonly used in the art for mass
spectral
peptide mapping and sequencing - are used herein to demonstrate the usefulness
of such
a system in proteome research and as a drug discovery platform. Submicroliter
protease
assays using endoproteinase Asp-N as the enzymes are presented herein to
demonstrate
the usefulness of such a system in bioassay and drug discovery research. As
would be
understood, other enzymes, indeed other noncatalytic proteins, can be used in
this multiplex
submicroliter reaction system.
Homogeneous assay in small volumes
2 0 [0464] In a first embodiment, capillaries (or channels) having
submicroliter volumes are
used as reaction chambers for small volume enzymatic assays, and can be
usefully be used
in cassettes, or arrays, to conduct such assays in highly parallel fashion
without significant
loss of reagent or reactants before analysis.
[0465] The capillary typically has an internal volume of not more than 5 pL,
often no more
2 5 than about 2 NL, frequently no more than about 1 NL, typically no more
than about 750 nL,
500 nL, 400 nL, and even no more than about 250 nL, 200 nL, even no more than
100 nL.
[0466] For example, Example 26 demonstrates trypsin digestion of cytochrome C
in
homogeneous solution. Mixtures of trypsin and cytochrome C are prepared in
solution at
various trypsin-protein ratios, with the concentration of cytochrome C fixed
at 1 mglmL.
3 o Aliquots of the mixture are drawn into the capillaries of a capillary
cassette by capillary
action, and incubated at 37°C overnight to allow the protease reaction
to complete.
Digestion mixtures are then spun down in parallel to a 96-well microtiter
plate, each of the
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 96 -
wells of which contains fluorescein-5-isothiocyanate (FITC) labeling solution.
After reaction
in the dark, the resulting mixtures are subjected to capillary electrophoresis
(CE) separation
on the MegaBACETM 1000 (Amersham Biosciences, Piscataway, NJ), a high
resolution and
high throughput instrument.
(0467] Example 27 demonstrates homogeneous assay with Asp-N, further
demonstrating
the multiplexing capacity of the current methods. In addition, Example 27
demonstrates that
analysis can be conducted in parallel using scanners.
[0468] Briefly, peptide CyTM5Q-YVADAPVK-Cy3 is reconstituted in assay buffer,
then
mixed with endoproteinase Asp-N of various concentrations. 500nL aliquots of
the mixture
1 o are captured by a capillary cassette system due to capillary action, and
incubated at room
temperature to allow the reaction to complete. Digestion mixtures were then
spun down to
a 384 clear scan plate of which each well contains 10 uL of buffer. The
resulting mixtures
were scanned on TyphoonTM (Amersham Biosciences, Piscataway, NJ) to detect Cy3
emission. The signal intensity of the Cy3 emission increases linearly as the
Asp-N
concentration increases, up to 50 picogram per 500 nL reaction. Beyond that,
Cy3 signal
intensity continues to increases with the Asp-N concentration, up to 180
picogram per 500
nL reaction (FIG. 36).
Bead-immobilized enzyme in a multiplexed capillary reaction
[0469] In a second embodiment, the enzyme is immobilized on a particle, or
bead, so
dimensioned as removably to fit within a capillary or channel, such as those
present in multi-
capillary cassettes, such as that shown in FIG. 3.
[0470] Preferably, the capillary or channel has a small internal volume,
desirably from
2 5 about 1 -1000 nanoliters (nl), more desirably from about 10 - 500 nl, most
desirably from
about 100 - 500 nl; the bead is dimensioned itself to fill no more than about
75%, typically
no more than about 50%, often no more than about 40%, 30%, 20% and even as
little as
10% of the capillary volume. Often, the bead or particle is sufficiently small
as to be
movable solely by entrainment in the reaction volume, and thus to be of such
size as to be
3 o suitable for uptake into the capillary solely by capillary action.
[0471] Beads suitable for surface immobilization of enzymes are known and are
available
commercially from a variety of vendors, such as Dynal, Miltenyi Biotec, and
others.
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 97 -
(0472] Beads can usefully be magnetic or superparamagnetic, and can usefully
be
derivatized to permit the ready attachment of proteins or other moieties
thereto.
[0473] In addition, the beads can usefully include a scintillant, permitting
scintillation
proximity assay (Amersham Biosciences, Inc., Piscataway, NJ). In such assays,
the
s polymer beads contain scintillant that can be stimulated to emit light,
stimulation occurring
only when radiolabelled molecules of interest are bound to the surface of the
bead.
[0474] The enzyme can be immobilized on the external surface of the bead or,
if the bead
is porous and the pores are of sufficient size to permit enzymatic substrate
to diffuse
therewithin, within the bead itself.
[0415] In one set of experiments further described in the Examples, below,
trypsin
immobilized on the surface of magnetic beads are used. Introduction of small
magnetic
beads eliminates the need for separating the enzyme from the reaction mixture
prior to
analysis, minimizes contamination by the proteolytic enzyme, and provides high
binding
surface area per unit volume for optimal accessibility of target molecules.
Beads are
prepared by incubating streptavidin-coated magnetic beads M280 (Dynal, Oslo,
Norway)
with biotin-conjugated trypsin (Sigma, St. Louis, MO). These trypsin
immobilized magnetic
beads were then mixed with cytochrome C for tryptic digestion using the
capillary cassette.
After incubation in an oven at 37°C overnight, the digestion mixtures
are separated from
beads, and labeled by FITC fluorescent dye. The resulting protein fragments
are analyzed
2 o by MegaBACETM 1000 (Amersham Biosciences, Piscataway, NJ).
[0476] Capillary electrophoresis separation of tryptic digestion products of
cytochrome C
on the MegaBACET"" shows that the peptide profiles obtained from the two
approaches are
consistent and reproducible. A representative electropherogram of cytochrome C
digestion
from a capillary cassette reaction is illustrated in FIG. 31 B.
2 s (0477] The nanoscale enzymatic reaction systems offer unique advantages
over the full
volume reaction systems. The small reaction volume (nanoliter range) greatly
reduces the
quantity of samples (picomole range) and reagents required, as well as the
sample
preparation time. It also offers enhanced reaction sensitivity. The concurrent
multiplex
format makes it possible to integrate it in a fully automated system for high
throughput
3 o analysis and identification of biomolecules, as well as using it in a
manual format. All these
can be translated into shortened running time per assay, and reduced
consumption of
reagents and samples, resulting in substantial cost savings.
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
_ 98 _
Immobilization of Protein in a Reaction Chamber for Enzymatic Reaction
[0478] In a third embodiment, the enzyme is immobilized to an interior surface
of the
s reaction chamber, usefully a channel or capillary having submicroliter
volume.
[0479] Nonspecific immobilization of enzyme can be achieved by simple
adsorption onto a
relatively hydrophobic solid phase. The passive adsorption of the enzyme is
through its
exposed hydrophobic sites. Such a process, however, is not completely general,
and the
optimal conditions for binding often have to be found by trial and error.
Enzymes bound to
i o the solid phase via multiple amino acid groups risk deformation of the
active site and hence
reduced reactivity.
[0480] Accordingly, there is typically a need to modify the attachment surface
with specific
functional groups to tether enzyme molecules.
(0481] In one approach, silanization with aminoalkylsilane reagents gives a
surface that is
15 functionalized with amino groups to which a wide variety of affinity
ligands can subsequently
be attached.
(0482] Thus, capillaries of capillary cassettes such as those shown in FIG. 3
and
described above, or other kinds of reaction chambers having small volumes, can
be treated,
e.g., by 3-aminopropyltriethoxy silane, followed by N-succinimidyl 3-(2-
pyridyldithio)
2 o propionatel. The pyridyldithio functional group provides a convenient way
to bind proteins,
such as enzymes, through specific -S-S- and -SH exchange reactions.
Furthermore, if
needed the immobilized enzyme can be released by adding an excess amount of
thiopyridone, regenerating the derivatized surface for tethering fresh trypsin
to ensure high
enzyme reactivity.
2 s [0483] Another surface immobilization approach is based on a specific
streptavidin-biotin
reaction. Streptavidin modification enables the surface to bind biotinylated
enzymes. In this
approach, capillary cassettes can be derivatized, e.g., with 3-
aminopropyltriethoxy silane,
and then reacted with a bifunctional linker, such as disuccinimidyl suberate,
which in turn
tethers streptavidin; the streptavidin thereafter can bind any biotinylated
enzyme to the
3 o reaction chamber (typically capillary) interior surface. If the enzyme is
biotinylated at a
unique site - e.g., by enzymatic biotinylation of a biotin-binding site
engineered into the
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 99 -
enzyme - the high affinity, high specificity streptavidin and biotin
interaction results in
uniformly oriented enzymes on the inner surfaces of the capillary.
[0484] These protein immobilization techniques offer high surface reactivity
and
minimized nonspecific binding. In addition, as further described in the
Examples, below, we
s have found that proteins immobilized by such approaches remain bound, and
functional,
even after completing a reaction; the capillary can thus be used serially for
a plurality of
reactions without requiring recharging with enzyme.
(0485] Other surface immobilization approaches can also be utilized. Reaction
with y-
glycidoxylpropylsilane introduces oxirane groups to the solid surfaces that
allow coupling
1 o enzyme at lysine sites. This modification is expected to provide more
hydrophilic surfaces to
reduce unspecific protein uptake. Conjugating enzyme with surface-active
hydrogels offers
yet another convenient means to produce enzyme immobilized surfaces (Caldwell,
Carlsson
and Li, US patent no. 5,516,703). An advantage of this approach is that it
provides protein-
compatible environments and reusable surfaces.
1 s [0486] To evaluate the reactivity of the enzymes immobilized by the above
protocols,
protein digestion reactions were conducted by contacting a model protein,
cytochrome C, to
trypsin-coated capillaries of a capillary cassette. The reactions were carried
out at 37°C
overnight. Protein fragments were then labeled with fluorescein isothiocyanate
(FITC), and
analyzed using a MegaBACETM 1000 machine. Capillary cassettes coated with
trypsin
2 o through nonspecific immobilization were used as the control.
[0487] For a given immobilized-trypsin cassette, three protein digestion
reactions were
performed over a period of two weeks. Fresh cytochrome C solutions were
applied in each
digestion reaction, and immobilized-trypsin capillary cassettes were stored in
0.15 M
phosphate buffered saline at 4°C between runs. Capillary
electrophoresis separation
2 s obtained on MegaBACETM demonstrated that all capillaries of these treated
capillary
cassettes have the same peptide maps across the three runs. Representative
electropherograms of runt, runt and run3 are shown in FIG. 32, 33 and 34,
respectively.
On the contrary, the control capillary cassette showed some protein digestion
only in the
first run, but no protein digest in the second or the third run. As a result,
such adsorbed
3 o enzymes do not have efficient capacity to carry out repeated digestion
reactions. A high
performance liquid chromatography (HPLC) was utilized to further characterize
these
protein digests. A representative HPLC chromatogram is shown in FIG. 35. The
peptide
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 100 -
profile of the protein digests obtained on covalently-coated capillary
cassettes are in
agreement with literature results (Neue et al., HPLC Columns: Theory,
Technology, and
Practice, VCH Publishing, 1997).
EXAMPLES
[0488] The following examples illustrate uses of the methods and apparatus of
the
present invention, and are representative of the many different types of
biochemical or
enzymatic reactions that can be effected with the disclosed methods. These
reactions
1 o include 1 ) dye-primer DNA sequencing; 2) dye-terminator DNA sequencing;
3) PCR
amplification; 4) PCR amplification, enzymatic purification, and DNA
sequencing; and 5)
enzymatic reactions. The following examples are offered by way of illustration
and not by
way of limitation.
EXAMPLE 1
Dye-primer DNA Sequencing Analyzed by Capillary Electrophoresis
[0489] Dye-primer sequencing reactions were performed within a capillary
cassette
comprised of 96 uncoated 2.8 cm long, 150 Nm I.D., 360 Nm O.D. fused-silica
capillaries.
2 o Dye-primer sequencing reactions were performed by amplifying template DNA
with
emission-specific primers corresponding to ddT, ddA, ddC, and ddG terminated
reactions.
The amplification of template was performed as single reactions in each
capillary and
pooled into a common well for post-reaction processing and analysis.
[0490] The color-specific primers were based on the M13-40 FWD primer (5'-FAM-
2 5 GTTTTCCCAGT*CACGACG-3'), with 5-carboxyfluorescein (FAM) as the donor dye,
and a
termination-specific fluor attached to the indicated thymine (T*) as the
acceptor dye. The
thymine was labeled with FAM for ddC-terminated reactions (C-FAM), 6-
carboxyrhodamine
for ddA reactions (A-REG), N,N,N',N'-tetramethyl-5-carboxyrhodamine for ddG
reactions
(G-TMR), and 5-carboxy-X-rhodamine for ddT reactions (T-ROX). A master mix for
100
3 o dye-primer sequencing reactions was prepared by combining 65 NL reaction
buffer (220 mM
Tris-HCI, pH 9.5, 33.2 mM MgCl2), 100 uL dye-primer solution (either 1 uM T-
ROX, 1 NM G-
TMR, 0.5 NM A-REG, or 0.5 NM C-FAM), 100 pL of the corresponding deoxy- and
dideoxynucleotide mix (0.94 mM dATP, dCTP, dTTP, 7-deaza-dGTP, with 3.1 pM
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 101 -
dideoxynucleotide), 10 NL of enzyme (32 units/NL ThermoSequenase), and 225 NL
filtered
demonized water. This solution was aliquoted into a 96-well reagent plate
prior to mixing
with template DNA. The general mixing scheme required the use of two capillary
cassettes
and a 384-well "mix plate." The first capillary cassette (transfer cassette)
was dipped in a
s solution of template DNA (20 ngINL M13mp18), and then inverted onto the top
of a 384-well
"mix plate" with the short ends of the capillaries inserted into the wells.
The inverted
transfer cassette and mix plate were placed inside a bench top centrifuge. A
balance plate
was added to balance the rotor and the centrifuge brought to 3,000 x g for 5
seconds. The
centrifugation uniformly dispensed the contents of the transfer cassette into
individual wells
of the 384-well plate. After the centrifuge step, the transfer cassette was
transferred to the
capillary cassette washer 410 for cleaning, and the mix plate was used for a
subsequent
centrifuge step for reagent addition.
[0491] To add reagents, a second capillary cassette (the reaction cassette),
was dipped
into the wells containing sequencing reagents (prepared as described in the
preceding
1 s paragraph) and inverted over the same wells of the same 384-well plate.
The reaction
cassette and mix plate were placed in the centrifuge, spun at 3,000 x g for 5
seconds, and
removed from the centrifuge. At this point each well contained 500 nL of
template DNA and
500 nL of sequencing reagents to form the final reaction mixture. The second
capillary
cassette (used to add reagents) was then dipped into the 1 NL mixture
contained in the mix
2 o plate, filling the capillaries of the reaction cassette with 500 nL.
[0492] The capillary cassette was inserted into the internal chamber of an air-
based
thermal cycler, as described herein in FIG. 7A-C, where the ends of the
capillary segments
are sealed by depressing the ends of the capillaries against deformable
membranes 264a
and 264b. After 30 cycles of 95°C for 2 seconds, 55°C for 2
second, and 72°C for 60
2 5 seconds, the thermal cycler was opened, removing the ends of the
capillaries from contact
with the deformable membranes. The capillary cassette was removed and placed
on top of
a 96-well "pooling plate" with the short ends of the capillaries inserted into
the wells. The
capillary cassette and mix plate were placed into a centrifuge, with a balance
plate. The
reaction products were dispensed by centrifugal force (2500 x g) into a
microtiter plate
3 o containing 40 pL of 80% isopropyl alcohol. After an initial reaction, the
capillaries were
washed as described herein. After the four dye-primer reactions had been
performed in
four individual capillary cassettes and the four sets products pooled into the
wells of the 96
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 102 -
well pooling microtiter plate, the samples were subsequently centrifuged at
3000 x g for 30
minutes. The alcohol was decanted by a gentle inverted spin, and the samples
were
resuspended in 5 NL of ddH20 for electrokinetic injection and analysis by
MegaBACET""
capillary array electrophoresis.
s [0493] Analysis of the DNA sequencing fragments was performed with
MegaBACET"", a
96-capillary array electrophoresis instrument (Amersham Biosciences,
Sunnyvale, CA)
using scanning confocal laser-induced fluorescence detection. Separations were
performed
in 62 cm long, 75 Nm I.D., 200 pm O.D. fused-silica capillaries with a working
separation
distance of 40 cm. Electroosmotic flow was reduced by Grignard coupling of a
vinyl group
1 o to the capillary surface and acrylamide polymerization. The capillaries
were filled with a
fresh solution of 3% linear polyacrylamide (LPA) (MegaBACET"" Long Read
Matrix,
Amersham Life Sciences, Piscataway, NJ) which was pumped through the
capillaries under
high pressure from the anode chamber to individual wells of a 96-well buffer
plate contained
in the cathode chamber. Each well was filled with 100 NL of Tris-TAPS running
buffer (30
15 mM Tris, 100 mM TAPS, 1 mM EDTA, pH 8.0). The matrix was equilibrated for
20 minutes
followed by pre-electrophoresis for 5 minutes at 180 V/cm. Prior to sample
injection, the
cathode capillary ends and electrodes were rinsed with double distilled water
(ddH20) to
remove residual LPA prior to sample injection.
(0494] DNA sequencing samples were electrokinetically injected at constant
voltage from
2 o a 96-well microtiter plate according to the specified conditions; one
preferred injection
condition for 500 nL samples is 40 seconds of injection at an applied voltage
of 2kV. After
injection, the capillary ends were rinsed with water, the buffer plate was
placed in the
cathode chamber, and the electrophoresis run was commenced. Separations were
typically
for 120 minutes at 8 kV. Computer controlled automation of the instrument and
data
2 s collection was performed using LabBench software (Amersham Biosciences,
Sunnyvale,
CA). Specific injection and run conditions were tailored to the reaction
mixture to be
analyzed.
[0495] The reproducibility of the described method for sub-microliter dye-
primer cycle
sequencing is shown in Figure 9. This histogram shows the percent of samples
in different
3 o read length bins and shows that the method is highly reproducible. Over 80
percent of the
sequenced DNA inserts had read lengths over 600 bases. Overall, this plate of
96 samples
yielded 55,000 high quality "Phred 20" bases, with an average read length of
605 bases.
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 103 -
EXAMPLE 2
Dye-primer DNA Sequencing Analyzed by a Capillary Electrophoresis Microchip
[0496) In another analysis example, dye-primer reactions performed in the same
capillary
cassette were analyzed by direct injection into a 16 channel microfabricated
"chip-based"
analyzer described in detail in S. Liu, H. Ren, Q. Gao, D.J. Roach, R.T. Loder
Jr., T.M.
Armstrong, Q. Mao, I. Blaga, D.L. Barker, and S.B. Jovanovich, Proc. Natl.
Acad, Sci. USA,
5-00. The 16-channel chip is formed by bonding two glass wafers, the top wafer
has 50 um
1 o deep by 100 um wide channels etched into it by standard microfabrication
methods. The
pattern etched has a combination of two 8-channel groups, each with a common
anode
reservoir Sixteen cathode reservoirs were evenly spaced at 4.5-mm intervals in
a line, as
were sixteen sample and sixteen waste reservoirs. The reservoirs were formed
by the
drilled access holes through the top etched wafer. Sixteen 250-Nm long twin-T
injectors
1 s were formed by the offset of channels from the sample and waste reservoirs
joining the
main separation channel. The distance between adjacent channels (center-to-
center) was
600 Nm in the detection region. The two alignment holes were used to align the
chip to the
detector.
[0497) In this example, a dye-primer reaction terminated by ddT was performed
as
2 o described and dispensed into the sample wells of a microchip containing
1.5 pL of ddH20,
Sample injection was performed by applying voltages of 50 and 10 volts
respectively to the
waste and cathode reservoirs, typically for 60 s, while the sample and anode
reservoirs
were grounded. Separations were carried out immediately after sample injection
by
applying 2,000 volts to the anode reservoir, 140 volts to sample and waste
reservoirs, while
25 grounding the cathode reservoir. The corresponding separation field
strength was ca. 227
V/cm. The laser-induced fluorescence was collected, digitized, and processed
into the
electropherogram shown in Figure 10. The electropherogram demonstrates
microchip
analysis of the reactions performed in the described capillary cassette
system.
3 o EXAMPLE 3
Dye-terminator Cycle Sequencing with Alcohol Precipitation Purification
(0498) Dye-terminator cycle sequencing was demonstrated using the capillary
cassette
system and alcohol precipitation for cleanup prior to capillary array
electrophoresis. In this
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 104 -
example, the sequencing reaction mix was prepared by mixing 400 uL of
sequencing
reagents (Dynamic ET terminator kit, Amersham Pharmacia Biotech, Part 81600)
with
100 pL of 5 pmoIIpL of M13-28 FWD primer (5'-TGT AAA ACG ACG GCC AGT-3'). The
reaction mix was distributed in 5 pL aliquots to a 96-well "reagent" plate.
Mixing of template
DNA and sequencing reagents was performed in the same series of steps
described in
Example 1, using a transfer cassette was used to transfer 500 nL of DNA
samples and a
reaction cassette to transfer 500 nL of sequencing reagents from the reagent
plate to the
wells of the mix plate. This same reaction cassette was then filled by
capillary action with
the templatelreagent mixture.
l o [0499] The capillary cassette was transferred to the air-based thermal
cycler where the
capillaries were sealed between the deformable membranes within the thermal
cycler.
Thermal cycling was achieved with 30 cycles of 95°C for 2 seconds,
55°C for 2 seconds,
and 60°C for 60 seconds, After the thermal cycling, the cassette was
removed from the
cycling chamber and the contents of the capillaries dispensed by centrifugal
force (3000 x g)
1 s into a 96-well plate containing 40 pL of 80% ethanol. The samples were
centrifuged at 3000
x g for 30 minutes. The alcohol was decanted by a gentle inverted spin, and
the samples
were resuspended in 5 NL of ddHzO for electrokinetic injection and analysis by
MegaBACET"" capillary array electrophoresis. The cleanup of dye-terminator
reactions by
alcohol precipitation, the reproducibility of the technique, and the
application to "real-world"
2 o templates is represented as a histogram of percent success versus read
length in Figure 11.
Figure 11 demonstrates excellent read lengths and success rates with M13
subclone inserts
prepared from a subclone library of a mouse bacterial artificial chromosome.
EXAMPLE 4
2 s Dye-terminator Cycle Sequencing with Size-exclusion Purification
[0500] In another example, dye-terminator reactions were performed in 500 nL
capillaries
as described in Example 3, and the reaction products dispensed into 15 NL of
ddH20 by
centrifugal force. The 15 NL samples were transferred to a filter plate
containing 45 NL of
3 o hydrated Sephadex G-50. The samples were centrifuged through the Sephadex
matrix at
910 x g for 5 minutes and the fluent collected in a clean 96-well injection
plate. The
samples were electrokinetically injected without further dehydration or
processing into
MegaBACET"". For 16 samples, an average read length of 650 bases was obtained
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 105 -
demonstrating the compatibility of sub-microliter dye-terminator sequencing
with size-
exclusion purification.
EXAMPLE 5
s Pcr Amplification of Plasmid Insert DNA
[0501] The present technology uses the disclosed system for the PCR
amplification of
insert DNA (e.g. subclone inserts from a DNA library). The PCR reaction
mixture was
prepared by mixing 5 pL of 10 NM of M13 -40 FWD primer (5' GTT TTC CCA GTC ACG
AC
3') and 5 NL of 10 uM -40 REV primer (5' GGA TAA CAA TTT CAC ACA GG 3') with
25 uL
of 10x GeneAmp buffer, 15 pL of 25 mM MgCl2, 5 NL of AmpIiTaq Gold, 2.5 NL of
1 mg/mL
bovine serum albumin (BSA), and 67.5 NL of ddH20. This mix was aliquoted in
equal
volumes to sixteen 0.20 mL tubes.
[0502] The reaction was initiated by mixing template DNA with the PCR cocktail
using the
two-capillary cassette and mix-plate method described. The transfer cassette
was dipped
into the glycerol stock solutions of a subclone library and dispensed by
centrifugal force into
the wells of a 384-well plate. A second "reaction" cassette was used to
transfer 500 nL of
PCR cocktail to the same wells by centrifugal force, The capillaries of the
reaction cassette
were subsequently dipped into the combined mixture of template DNA and PCR
reagents,
2 o filling the capillaries by capillary action. Amplification was effected by
placing the capillaries
into the cycling chamber and thermally cycling with an activation step of
95°C for 12 minutes
followed by 30 cycles of 64°C for 4.5 minutes and 95°C for 5
seconds.
[0503] The PCR products were analyzed by agarose gel electrophoresis and
compared
with the same subclones amplified by full volume (25 NL) reactions performed
in 0.20 mL
2 5 tubes. Nanoscale capillary cassette samples were dispensed into 4.5 NL of
ddHzO by
centrifugal force. Equivalent volume aliquots of full volume reactions were
transferred
manually using a low volume pipettor. To each 5 uL sample, 1 NL of 6x loading
dye was
added and the sample quantitatively transferred to the wells of an agarose
gel. Agarose gel
electrophoresis was performed using a 0.7% agarose gel with 1 X Tris-acetate-
EDTA buffer,
3 o pH 8Ø Samples were separated for 40 minutes at 15 V/cm, stained with
Sybr Green II
(Molecular Probes, Eugene, OR), and imaged using a two-dimensional
fluorescence
scanner (Fluorlmager, Amersham Biosciences, Sunnyvale, CA). The scanned gel
image is
shown in Figures 12A and 12B. It can be seen that samples prepared at full
volume (Figure
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 106 -
12A) and 500 nL volume (Figure 12B) have the same molecular weight
distribution. This
example demonstrates nanoscale sample preparation can be used for PCR
reactions and
that the products can be analyzed by traditional macro-scale analysis methods
such as
agarose gel electrophoresis.
EXAMPLE 6
PCR Amplification and Cycle-Sequencing
[0504] A preferred mode of preparing cycle sequencing samples using the
present
1 o invention is to prepare nanoscale PCR samples in the capillary cassette
and related
instrumentation, perform macroscale ExoIISAP reactions, and then perform the
cycle
sequencing in the capillary cassette and related instrumentation. Nanoscale
PCR template
preparation for DNA sequencing was demonstrated by performing PCR
amplification from
glycerol stock subclones. Glycerol stock subclones were PCR amplified in the
capillary
cassette and related hardware as described in Example 5. After PCR
amplification, the
contents of the capillaries were dispensed by centrifugation into the wells of
a 96-well plate
containing 4.5 NL of 7.5 mU of shrimp alkaline phosphatase (SAP) and 37.5 mU
of
exonuclease I (Exol). The PCR products and ExoI/SAP solution were allowed to
incubate
at 37°C for 5 minutes to digest the unincorporated primers and to
dephosphorylate the
2 o unincorporated nucleotides. After an initial incubation, the enzymes were
deactivated by
heating the solution to 72°C for 15 minutes.
[0505] The ExoI/SAP treated PCR products were aliquoted to a fresh 384-well
mix plate
with a transfer capillary cassette and centrifugal dispensing. An equal
aliquot of dye-
terminator sequencing reagents were added to the 500 nL of purified PCR
products using
2 s another capillary cassette, the reaction cassette, and centrifugal
dispensing. The capillaries
of the reaction cassette were then filled by dipping the capillary cassette
into the 1 pL
reaction mixture. The template was amplified according to Example 3, dispensed
into 40 NL
of 80% ethanol and purified as described. Analysis of the sequencing reactions
was
performed by MegaBACET"' using electrokinetic injection. Portions of six base
called
3 o sequencing electropherograms from subclone templates prepared by nanoscale
PCR
amplification from glycerol stock solutions and by nanoscale cycle sequencing
are shown in
Figure 13. By performing PCR in a capillary cassette and subsequently
transferring the
reaction mixture to a microplate, the present system allows a simplified
transition from
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 107 -
nanoscale (less than 1 NL volumes) to greater than nanoscale reaction volumes.
The
present system also allows a simplified transition from macroscale (more than
1 NL
volumes) to nanoscale reaction volumes, as shown by utilizing the Exo I/SAP
reactions for
cycle sequencing in the capillary cassette.
s
EXAMPLE 7
Isothermal Enzyme Performed in
Sub-microliter Capillary Cassette
[0506] The use of the described system for performing enzyme reactions was
demonstrated with a fluorogenic enzymatic assay of ~i-galactosidase hydrolysis
of ~3-D-~i -
galactosidase to the fluorophore resorufin. The ~i-galactosidase catalyzed
hydrolysis of
resorufin-(i-D-galactosidase (RBG) was performed within the capillaries of a
96-capillary
cassette and in control full volume reactions in which ~i-Gal hydrolyzes RBG.
1 s [0507] A stock solution of 35NM RBG was prepared in 5 mL of buffer (100 mM
Tris-HCL,
mM KC1, and 2 mM MgClz) to 5 mg of RBG, vorfexing vigorously, and filtering
the
solution through a 0.40 micron filter and then adding an equal volume of
buffer. A dilution
curve of RBG was then prepared from the stock solution. To each 10 pL of RBG
solution
prepared in 0.20 mL tubes, 200 ug of ~3-galactosidase was added and after
briefly mixing,
2 o filled into a capillary cassette by capillary action. The cassette was
placed in air cycler and
after 2 minutes at 37°C, the capillary cassette was removed and the
contents centrifuged
out of the capillaries into a 384-well scan plate containing 5 NL of 1 M
sodium carbonate.
The wells of the scan plate were subsequently filled with 50 NL of ddH20. In
parallel, the
0.2 mL tubes were incubated at 37°C for 2 minutes and the ful volume
reactions stopped by
2 s adding 1 M sodium carbonate. A control aliquot from the enzyme reactions
performed in
the 0.20 mL tubes was added to the scan plate.
[0508] Solid-phase capture of the ~i-galactosidase was also demonstrated with
this
system by simply filling the cassette with a 20 NgImL solution of ~i-
galactosidase to bind to
the capillary surface followed by removing the excess liquid and drying the
cassette using
3 o the described cassette wash-manifold. After (i-galactosidase binding the
capillaries were
filled with RBG solution by capillary action. The reaction was performed for 2
minutes at
37°C and analyzed by dispensing into 1 M sodium carbonate, and diluting
with water in the
scan plate.
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 108 -
[0509] Once all three sets of reactions (full volume, capillary cassette, and
capillary
cassette with solid phase capture) had been added to the scan plate, the plate
was read by
a fluorescent plate reader (Typhoon, Amersham Biosciences, Sunnyvale, CA). The
results
of the standard curve performed in 0.2 mL tubes (tube rxn), a reaction
performed in the
capillary cassette without solid phase capture (capillary reaction), and in
the capillary
cassette with solid phase capture (capillary with binding reaction) are
summaries in Figure
14. Figure 14 shows the expected signal versus substrate concentration for the
tube
reactions, and data points of signal for the pre-mixed enzyme reaction
performed in the
capillary cassette, and for the capillary-binding (3-galactosidase assay.
[0510] This example serves to illustrate the compatibility of the described
system for
performing a range of general enzyme activity and inhibition assays. In
addition, it
demonstrates that solid phase capture can be applied to proteins and enzymes
as well as
DNA. Finally, it shows the described system can be applied to isothermal
reactions.
EXAMPLE 8
Template purification
[0511] This example demonstrates the effectiveness with which the methods of
the instant
invention can be used to purify template DNA of contaminants that interfere
with sequencing
2 o reactions and acquisition of high quality sequence data.
[0512] Template capture cleanup of PCR products as DNA sequencing template
using
direct reversible binding to the inner surface of a fused-silica capillary
tube. A 500 nl
volume sequence reaction, using the ET dye-terminator cycle sequencing method
was
carried out in a 150 Nm inner diameter capillary tube and analyzed on
MegaBACET"" using a
2 s 2kV, 30s injection. Fig. 17A shows the results of sequencing PCR products
mixed with the
reaction mixture prior to sequencing. Fig. 17B shows the results of first
mixing the PCR
template with sodium thiocyanate, binding the DNA to the inner surface of the
capillary,
washing the DNA with 80% ethanol, followed by sequencing.
3 o EXAMPLE 9
Template Normalization Effect for M13, Plasmid,
and PCR Product DNA
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 109 -
[0513] This example, as do several of those following, demonstrates the
usefulness and
effectiveness of the methods of the present invention for normalizing the
quantity of nucleic
acid directly and reversibly immobilized inside capillary tubes.
[0514] FIG. 18 represents the retained mass of DNA following a template
capture
s protocol. The amount of DNA bound remains constant above 40 ng starting
template for
M13 (~), plasmid (~), and PCR product (~).
[0515] Template DNA was prepared by a restriction digest of M13mp18 and PUC19
DNA
to form linear single and linear double stranded DNA respectively. These
templates, along
with a 800 by PCR product (standard amplification conditions) were end labeled
with 32P
1 o using [y-32P]ATP and T4 polynucleotide kinase. The labeled DNA was seeded
into
unlabeled template of the same type and a calibration curve was generated for
the seeded
DNA solution. Template binding was performed by mixing stock DNA with 10 M
sodium
thiocyanate and loading into 500 nl fused-silica capillaries. After 10 minute
incubation and
80% ethanol washing, the capillaries were placed in scintillation fluid and
quantified. Fig. 18
1 s shows definitive normalization for three sources of template DNA.
EXAMPLE 10
Template Capture Normalization Effect on Read Length
2 0 [0516] FIG. 19 shows a plot of read length versus starting DNA mass for
samples
prepared by premixing DNA and sequencing reagents (D) compared to samples
prepared by
template capture (0). The normalization effect is highlighted by a nearly
constant read
length obtained for the template capture samples, whereas for premixed
samples, template
overloading and reduction in read length occurs above 20 ng starting DNA.
2 s [0517] Template binding was performed by mixing stock M13mp18 DNA with 10
M
sodium thiocyanate and loading into 500 nL fused-silica capillaries. After 10
minute
incubation and 80% ethanol washing, the capillaries were placed filled with ET
terminator
premixed with M13-40FWD sequencing primer. Premixed reagents were prepared in
a 10
pl volume and loaded into clean sample preparation capillaries. The air-based
cycle
3 o sequencing was performed as previously described followed by ethanol
precipitation and
MegaBACET"" analysis at 2kV, 30 second injection, 8kV, 120 minute run time.
EXAMPLE 11
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 110 -
Template Capture Polymerase Chain Reaction with Normalization
[0518] PCR reactions were performed after template binding of indicated
starting amount
of M13mp18. Standard PCR amplification reactions with M13-100 FWD and M13-400
REV
s primers were performed in 500 nl capillary cassette with 10 s at
95°C, 10 s at 55°C, and 120
s at 72°C. Reaction products were dispensed by centrifuge into loading
buffer, and
transferred to a 1.5% agarose gel. The products were stained with SYBR Green
dye and
imaged with a Fluorimager apparatus, as shown in FIG. 20.
1 o EXAMPLE 12
Template Capture Normalization Effect on Peak Height and Migration Time and
Peak
Height and Migration Time for Pre-mixed Samples
[0519] Template capture normalization effect on peak height and migration
time. FIG. 21
1 s represents the relative signal intensity obtained with increasing template
concentration
represented by the intensity of peak 79, peak 308, and peak 604 (ddT-
terminated peaks
early, middle, and late in the electrophoresis chromatogram). The peak
intensity increases
to 40 ng/Nl and levels off, confirming by peak height the normalization effect
and saturation
level of the template capture technique. The migration time of the first peak
is relatively
2 o constant across template concentrations,
[0520] Peak height and migration time for pre-mixed samples. FIG. 22 shows
peak height
increasing with increasing template concentration, reaching a maximum due to
overloading
of the sequencing sample. An excess of template DNA inhibited the
electrokinetic injection,
reducing the current in the sample run, consequently increasing the migration
time of the
2 s sample through the capillary.
EXAMPLE 13
Nanoscale Direct Cycle Sequencing
from Glycerol Stocks of Clone
(0521] Sample preparation for DNA sequencing could be simplified if some of
the many
steps involved in preparing sequencing samples from cloned DNA in bacterial
cells could be
eliminated. Typically for capillary electrophoresis analysis, bacterial cells
are grown and
lysed, PCR amplification is performed, followed by ExoIISAP cleanup and then
cycle
3 s sequencing. The instant invention provides a method to simplify the
workflow by cycle
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 111 -
sequencing directly from glycerol stocks of clones. Equal volumes of glycerol
stock and 10
M NaSCN were pulled into a 96 channel 500 nl capillary cassette. A five minute
binding
was performed at 60°C in the air cycler disclosed in the copending
application U.S. Serial
No. 09/577,199, herein incorporated by reference in its entirety. The
capillary cassette was
washed with an 80% ethanol rinse and dried with flowing nitrogen in the
capillary cassette
washer disclosed in the copending application U.S. Serial No. 09/577,199. The
cassette
was then filled by capillary action with a 1:4:5 mixture of primer, ET
terminator premix and
water and cycled in the air cycler. The cycling protocol was for ET
terminators as described
in Example 1, above. The samples were ethanol precipitated by being dispensed
by
1 o centrifugation (3220 g for 30 minutes at 4°C) into a microtiter
plate containing 80% ethanol.
After decanting and 30 seconds of inverted spinning at 50 g to remove ethanol,
the samples
were resuspended in 5 ul water. The samples were then injected into
MegaBACET"" with a
2 kV, 30 second injection followed by a 8 kV, 140 minute separation. The data
were
analyzed with Sequence Analyzer software (Amersham Biosciences) and then
processed to
determine Phred 20 base calling scores. FIGS. 23 A and B show a trace obtained
by this
method that had a Phred 20 score of 561 bases. This example demonstrates the
application of the instant invention to direct sequencing from frozen glycerol
stocks of
bacteria. It will be apparent to the skilled artisan that this method can be
applied to the
sequencing of bacterial colonies grown on agar plates, or similar solid growth
media,
2 o regardless whether the plates are fresh or desiccated.
EXAMPLE 14
Genotyping with Nanoscale Single
Base Extensions of Nucleic Acids
[0522] The instant invention can be applied to perform nanoscale genotyping
reactions.
[0523] Single-base extension (SBE) reactions were performed in the 96 channel
capillary
cassette. The single base extension analysis consists of the single base
extension of a
DNA primer that terminates immediately before the base to be interrogated. PCR
reactions
3 0 of 25 ul were prepared containing 5 nglul of genomic human DNA, 1 NM of
forward and
reverse primers, buffer, MgCl2 and AmpIiTaq Gold. The PCR cycling was
96°C for 12 min,
cycles of 94°C for 20 sec, 60°C for 20 sec, and 72°C for
30 sec, followed by 72°C for 2
min. An Exo IISAP cleanup was performed by adding 9 units of SAP and 45 units
of Exo I
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 112 -
to the 25 pl of PCR products. The reaction was incubated at 37°C for 45
min and then the
ExoI/SAP enzymes denatured by heating to 95°C for 15 min.
(0524] For full volume control reactions, 9 pl of SBE premix containing
fluorescently
labeled dideoxyterminators, a DNA polymerise, buffer solution and 1 pl of 2 NM
primer was
s added to 10 NI of the ExoIISAP treated PCR products. For reactions in the
500 nl capillary
cassette, samples were loaded by capillary action.
[0525] The single base extension reactions were performed by 25 cycles of
96°C for 10
sec, 50°C for 5 sec, 60°C for 30 sec. The thermal cycling was
carried out in either MJ
Research tetrads (a type of thermal cycling machine) for the full volume
controls, or for the
1 o capillary cassette samples, in the air cycler disclosed in the co-pending
application U.S.
Serial No. 091577,199, herein incorporated by reference in its entirety. The
samples were
dispensed into water and injected into MegaBACET"" for analysis.
[0526] FIG. 24 demonstrates that the capillary-based reactions could correctly
identify
single nucleotide polymorphisms. Traces 1, 3, and 4 were obtained from samples
15 homozygous at the interrogated base. Trace 2 was obtained from a sample
heterozygous
at the interrogated base and demonstrates that allelic polymorphism can be
detected using
nanoscale reactions. Signal is essentially the same as that obtained with the
full volume
reactions.
(0527] The entire process, from PCR to SBE, was accomplished using the
capillary
2 o cassette.
[0528] Template capture in the capillary, as described in this application, is
used in an
improved version of this nanoscale single base extension reaction and provides
even better
results.
[0529] It will also be apparent to the skilled artisan that single base
extension of
2 s messenger RNA using reverse transcriptase and fluorescently-labeled
ribonucleotides
permits genotyping using mRNA as an alternative to genomic DNA.
EXAMPLE 15
Nanoscale Genotyping with Amplified
3 o Fragment Length Polymorphism
(0530] The methods of the instant invention can be used to perform AFLPs
(amplified
fragment length polymorphism) in nanoliter volumes. To perform AFLP reactions,
genomic
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 113 -
DNA is digested with pairs of restriction enzymes. The fragments are either
ligated to a
linker and amplified to amplify fragments of a certain length, in a certain
orientation, as
determined by the two restriction enzymes used, or alternatively, amplified by
PCR directly
using degenerate primers. The amplified fragments are analyzed by capillary
electrophoresis. The AFLP analysis method is used to generate a
"representation" of a
genome, also called an amplicon, with variable fragments as well as constant
ones. The
amplicon is used to assess the diversity of populations of organisms or to
make genome
maps in organisms where little sequence and marker information is available.
to EXAMPLE 16
Nanoscale Genotyping with Direct Display Analysis
[0531] The methods of the present invention can be used to perform direct
display
analysis in nanoliter volumes. To perform direct display analysis reactions,
complementary
1 s DNA is digested with pairs of restriction enzymes. The fragments are
either ligated to a
linker and amplified to amplify fragments of a certain length, in a certain
orientation
depending on the two restriction enzymes used, or alternatively, amplified by
PCR directly
using degenerate primers. The amplified fragments are analyzed by capillary
electrophoresis. The direct display analysis method is used to generate a
"representation"
2 0 of a transcriptosome, with variable fragments as well as constant ones.
Direct display
analysis is used to assess the quantitative change in the level of expression
between
organisms, or differences due to environmental or physiological effects.
EXAMPLE 17
25 Nanoscale Genotyping by Microsatellite Analysis
[0532] The methods of the present invention can be used to perform genotyping
by
microsatellite analysis in nanoliter volumes. To perform genotyping by
microsatellite
analysis reactions, genomic DNA is PCR amplified with marker panels such as PE
Applied
3 o Biosystems Linkage Mapping Sets. For example, 96 human samples are
analyzed with
respect to panels of 12 genotypes in about 30 minutes using a four-color
analysis. Three of
the colors are used with four primer sets, while the fourth color provides
internal size
standards.
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 114 -
[0533] PCR set-up and thermocycling is performed as recommended by the
manufacturer
of the primer panel.
[0534] An example of a polymerise chain reaction mixture is as follows:
In ra edient Volume
10X Gold Buffer 1.50 NI
MgCl2 (25 mM) 1.50 NI
dNTPs Mix (2.5 mM) 1.50 NI
Primer mix 1.00 NI
1 o AmpIiTaq Gold 0.12 pl
Sterile distilled water 1.38 ul
7.00 NI
DNA (5na/ul) 8.0o ul
15.0 NI per well
[0535] The primer mix contains both forward and reverse primers, each at a
final
concentration of 5NM.
[0536] An example of a thermal cycler program is as follows:
Temp Time C cy le No.
95C 12 mins 1 cycle
94C 15 sec
55C 15 sec
2 5 72C 30 sec 10 cycles
89C 15 sec
55C 15 sec
72C 30 sec 20 cycles
72C 10 mins 1 cycle
Pooling.
[0537) Sealed PCR sample trays are stored at -20°C.
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 115 -
[0538] Initially, 1 NI of each PCR product is pooled, after which the final
volume is brought
up to about 15 to 20N1 with water. Then, samples are dialyzed. Dialysis is
done in 0.1 X TE
for 15 minutes, after which the pooled PCR samples are loaded into the
MegaBACET"".
Loading.
[0539] Samples are prepared for loading into the MegaBACET"" as follows:
In r4 edient Volume
Desalted PCR pools 2.00 ul
1 o ET400-R Size Standard 0.25 ul
Formamide loadin sod 2.75 ul
Total loading volume 5.00 ul
EXAMPLE 18
Nanoscale Enzymatic Reactions with Nucleic Acids
[0540] The present invention is advantageously applied to performing nanoscale
enzymatic reactions with nucleic acids in nanoliter volumes, The nucleic acids
are
immobilized in a reaction chamber, such as a glass capillary, prepared
according to the
2 o methods of the instant invention. The capillaries are filled with reaction
mixtures that
comprise one or more of different enzymes, such as a restriction enzyme.
[0541] A typical restriction enzyme digest is performed in a total volume of
20 NL that
includes 0.2 to 1.5 ug of substrate DNA and a 2-10 fold excess of restriction
enzyme over
DNA. Reaction buffer, enzyme, water, and DNA are mixed in a reaction tube and
incubated
at 37°C for 1 to 4 hours. According to the instant invention template
DNA is bound to the
inner surface of a capillary tube. Then, a premix of restriction enzyme (e.g.
Hind III) in a 1 x
KGB buffer (100 mM potassium glutamate, 25 mM Tris-acetate, pH 7.5, 10 mM
magnesium
sulfate, 50 Ng/ml bovine serum albumin, and 1 mM (3-mercaptoethanol) is drawn
into the
capillary by capillary action. The reaction is incubated at 37°C for an
allotted time, after
3 o which the contents are dispensed in gel-loading buffer for agarose gel
sizing, or into a
solution containing 10 mM EDTA,
[0542] Other reactions comprising different enzymes are also possible. These
enzymes
include, but are not limited to methylation enzymes, DNA-dependent DNA
polymerise
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 116 -
enzymes, terminal transferase enzymes, RNA-dependent DNA polymerise enzymes,
DNA-
dependent RNA polymerise enzymes, phosphatase enzymes, kinase enzymes,
exonuclease enzymes, such as S1, or mung bean nucleases, other nuclease
enzymes,
ribonuclease enzymes, or DNA or RNA ligase enzymes. For most of these
reactions,
s control over the ratio of nucleic acid to enzyme is crucial to the success
of the reaction
process.
[0543] Use of the present application beneficially reduces the error
associated with
concentration dependent enzymatic reactions with nucleic acids, as well as
reducing the
consumption of valuable enzymes. Furthermore, through washing, use of the
methods of
1 o the present invention is effective for eliminating residual ions, such as
ammonium acetate,
EDTA, and lithium chloride, and other contaminants, such as polysaccharides
that interfere
with enzymatic activity.
EXAMPLE 19
15 Direct Sequencing from a Microarray Spotting Plate
[0544] To ensure the integrity of the data generated using microarrays, it is
necessary that
the identity of the sequence of the spotted DNA be known with high confidence.
PCR is
often used to generate the DNA to be spotted, and as is well known in the art,
Taq and
2 o related thermostable polymerises introduces a certain number of erroneous
base pairs per
thousand as it amplifies the template. If errors have been introduced they
must be
detected, and the amplified product or data therefrom discarded. Usually, this
requires
numerous processing steps separate from those associated with spotting the PCR
product.
However, use of an embodiment of the present invention greatly increases the
efficiency of
2 5 sequence confirmation.
[0545] Confirmation of the sequence of a series of microarray spotting samples
was
achieved, using the methods of the present invention, as follows.
[0546] Microarray spotting samples were prepared from PCR products, average of
500
bp, from human genomic DNA template. The products were purified using standard
3 o guanidinium hydrochloride glass-filter plate processing and mixed with an
equal volume of
M sodium thiocyanate. Samples were arrayed in a microtiter plate ("spotting
plate") for
subsequent spotting onto the microarray slide.
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 117 -
[0547] To confirm the PCR product sequence and positional arrangement on the
microarray hybridization slide, sequencing reactions were performed by dipping
the ends of
a 96-capillary cassette into the spotting plate and binding the DNA to the
inside surface of
the capillary. After a wash step with 80% ethanol, the capillaries were filled
with sequencing
s mix containing buffer, polymerase, dye-labeled dideoxynucleotides, and
sequencing primer
at 1 x concentration. After thermal cycling (30 cycles at 95 °C for 5
s, 55 °C for 5 s, and 60
°C for 60 s), the sequencing reactions were purified by ethanol
precipitation and analyzed
by MegaBACET"".
[0548] In this example, 60 samples yielded confirmatory sequence, with an
average read
to length of 335 bases (450 by maximum). By directly sequencing from the same
preparation
and source as was spotted on the array, we resolved ambiguities in position or
identity of
the PCR product.
EXAMPLE 20
15 Direct Sequencing of PCR Products Without Preliminary Removal of PCR
Nucleotides and
Primers
[0549] The methods of this invention have been used to simplify the
purification of PCR
products prior to sequencing. Typically, an enzymatic purification of the PCR
product using
2 o exonuclease I (Exol) and arctic shrimp alkaline phosphatase (SAP) to
remove primer and
excess dNTPs is required prior to cycle sequencing. Because template binding
is size
dependent, however, the unincorporated primers and remaining nucleotides can
instead be
removed from the template by differential binding of the template to the
capillary, followed
by removal of nucleotides and primer by washing. This approach obviates
enzymatic
2 s cleanup of the PCR product and greatly simplifies the overall workflow.
[0550] As a demonstration, 96 PCR products of M13 DNA containing a mouse
subclone
insert were directly sequenced without enzymatic purification after PCR
amplification.
[0551] The PCR amplification reactions were performed using M13 templates
containing a
subclone insert (ca. 2000 bp) of mouse genomic DNA. The M13 templates had
previously
3 o been prepared by polyethylene glycol precipitation and detergent solvation
(Thermomax),
diluted 200 fold and rearrayed into a 96-well microtiter plate.
[0552] A 2 pL aliquot of this solution was transferred to a PCR amplification
mix prepared
with 2.5 NL 10X GeneAmp buffer, 0.2 NL of 25 mM each dNTPs, 0.5 uL of 10 NM
M13 -
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 118 -
40FWD (GTT TTC CCA GTC ACG AC), 0.5 NL of 10 NM M13 -40REV primer (GGA TAA
CAA TTT CAC ACA GG), 1.5 NL of 25 mM magnesium chloride, 0.5 pL of 5 U/NL
AmpIiTaq
polymerase, and 17.3 NL water. After mixing and sealing the plate, the
reactions were
thermally cycled at 95 ° C for 10 s, 55 ° C for 10 s, and 72
°C for 2 minutes for thirty cycles.
s After PCR amplification, a 5 pL aliquot was removed and mixed with 5 NL of
10 M sodium
thiocyanate in a separate 96-well plate.
[0553] The capillaries of a 96-capillary cassette were dipped into the
chaotrope-PCR
product mixture, thus filling the cassette. After a 5 minute incubation at 60
°C, the residual
chaotrope, unbound buffer components and DNA were removed with an 80% ethanol
wash
1 o applied by pulling the ethanol through the capillaries under vacuum. After
drying the inside
surface with a 1 minute flow of air, the capillaries were dipped into a
sequencing mixture
containing a 1 x solution of ET terminator reaction mix and forward sequencing
primer, M13
-21 FWD (TGT AAA ACG ACG GCC AGT).
[0554] Cycle sequencing was performed by sealing the ends of the capillaries
in the air-
15 thermal cycle. The reaction was cycled 30 times at 95 °C for 5 s, 55
° C for 5 s, and 60 °C
for 60 s. The cycle-sequencing products were dispensed into a microtiter plate
containing
40 NL of 80% ethanol using centrifugal force. After a 30 minute centrifugation
at 3000 x g,
the alcohol was decanted, the pelleted DNA resuspended in 5 NL of ddH20, and
the
samples were analyzed by MegaBACET"'.
2 0 [0555] For these 96 samples, an average read length of 550 bases was
achieved with
83% pass rate and a sum of 44000 bases. This procedure has been repeated for
over 5000
samples with demonstration of improvements over full-volume and enzymatically
purified
reactions.
2 5 EXAMPLE 21
Comparison of Nanovolume and Full Volume PCR
[0556] PCR premixture is prepared by mixing template specific primer pairs
with 10x
GeneAmp buffer, MgCl2, AmpIiTaq Gold, bovine serum albumin (BSA), dNTPs and
double-
3 o distilled water. Fifteen microliters of the premix is then aliquoted into
24 wells of microtiter
plate. To each well containing PCR premix, 10 ul of genomic DNA (5 nglul) is
added as
template for the reaction. Each of 23 wells receives genomic DNA isolated from
a different
individual, and one well receives no template as a negative control. For
nanovolume PCR,
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 119 -
the capillaries of a reaction cassette are filled by capillary action with
about 500 nl of
reaction mixture by dipping the ends of the capillaries into wells of the
microtiter plate. The
capillary cassette is then placed into the thermal air cycler, disclosed in co-
pending
application U.S. Serial No. 09/577,199, herein incorporated by reference in
its entirety, and
s the capillary ends are sealed. Amplification is then effected by air driven
thermal cycling
using the following program: 30 cycles of 93°C for 10 sec; 60°C
for 10 sec, and 72°C for 60
sec. For full volume PCR, the remaining PCR reaction mixture is transferred to
0.2 ml PCR
tubes and amplification effected by thermal cycling using the following
program: 35 cycles of
94°C for 20 sec; 60°C for 20 sec; 72°C for 30 sec, and
one cycle of 72°C for 2 min.
[0557] After PCR is completed, the contents of the capillaries are expelled
into 7.5 ul 1x
loading dye by centrifugal force. An equivalent volume from each full volume
PCR reaction
is manually transferred using a low volume pipettor into the same amount of
loading dye.
PCR products are then loaded into the wells of a 1.5% agarose gel and
subjected to
electrophoresis for 40 minutes at 15 V/cm in 1 X Tris-acetate-EDTA buffer at
pH 8Ø After
1 s electrophoresis is completed, the gel is stained with the DNA dye Sybr
Green II (Molecular
Probes, Eugene, OR), and is imaged using a two-dimensional fluorescence
scanner
(Fluorlmager, Amersham Biosciences, Sunnyvale, CA).
[0558] Fluorescence signal intensity from each band of PCR product is
converted to DNA
mass and displayed graphically. The results of this experiment are shown in
FIG. 25, which
2 o demonstrates that PCR of genomic DNA in nanovolume reactions in capillary
cassettes
(FIG. 25A) yields a comparable amount of product as full volume PCR reactions
(FIG. 25B).
EXAMPLE 22
Comparison of Nanovolume and Full Volume SBE
[0559] Full volume and nanovolume SBE reactions are performed using PCR
products
generated from genomic DNA, which serve as the template for SBE. PCR is
performed in
full volume reactions as described in Example 21. After PCR is completed,
excess PCR
primers are digested with Exo I and unincorporated dNTPs are inactivated by
treatment with
3 o SAP. To the 25 ul PCR volume is added and mixed 14 ul of ExoI/SAP solution
(consisting
of 9 ul of SAP at 1.0 U/ul and 5 ul of Exo I at 10 U/ul), after which the
mixture is incubated at
37°C for 45 min to effect the reactions, followed by 95°C for 15
min to heat inactivate the
enzymes.
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 120 -
[0560] For full volume SBE, to 10 NI of the ExoI/SAP treated PCR products are
added 9 NI
of SBE premix, and 1 NI of 2 NM SBE primer solution (primer NCBI 422 or primer
NCBI 425)
in the wells of a microtiter plate. SBE premix is similar to PCR premix,
except that primer
pairs are excluded and dNTPs are replaced with fluorescently labeled
dideoxyterminators.
After the ingredients are mixed, the reaction mixture is transferred to 0.2 ul
tubes and SBE
is performed by thermal cycling, as for PCR, using the following program: 25
cycles of 96°C
for 10 sec; 50°C for 5 sec; 60°C for 30 sec.
[0561] After full volume SBE is completed, unincorporated ddNTPs are
dephosphorylated
by CIAP treatment. Ten microliters of each SBE reaction product is transferred
to the well
of a microtiter plate and mixed with 25 ul of CIAP solution, containing 0.1
U/ul of CIAP and
1x CIAP buffer. The mixture is then incubated at 37°C for 60 min to
effect the reaction and
then at 72°C for 15 min to heat inactivate the CIAP enzyme.
[0562] Five microliters of each CIAP-treated full volume SBE reaction is then
mixed with 5
uL of MegaBACET"" loading solution, denatured at 95°C for 1 minute, and
analyzed using
1 s MegaBACET"" (Injection: 6 kV for 15 sec. Run: 6kV for 60 min).
[0563] Results from four samples analyzed by full volume SBE are shown in FIG.
26.
FIG. 26A and FIG. 26C show heterozygous nucleotide polymorphisms at the
interrogated
base, whereas FIG. 26B shows a homozygous polymorphism. FIG. 26D shows that a
negative control, which contained no DNA, produced no single nucleotide
signal.
2 0 [0564] For nanovolume SBE, capillary tubes are dipped into the same SBE
primer-premix
solution reaction mixture prepared for full volume SBE, and filled by
capillary action with
about 500 nanoliters of the mixture. Thereafter, the capillary cassette is
transferred to the
air thermal cycler apparatus and SBE is performed, as for PCR, using the
following
program: 30 cycles of 95°C for 5 sec; 55°C for 5 sec;
60°C for 30 sec.
2 s [0565] After nanovolume SBE is completed, reaction products are expelled
from the
capillary tubes by centrifugation into the wells of a microtiter dish
containing 20 ul of the
CIAP solution described above. The reaction products are treated with CIAP by
incubation
at 37°C for 60 min to effect the reaction and then at 72°C for
15 min to heat inactivate the
CIAP enzyme.
3 0 [0566] Five microliters of each CIAP-treated nanovolume SBE reaction is
then mixed with
5 uL of water, and analyzed using MegaBACET"" (Injection: 2 kV for 45 sec.
Run: 6kV for
60 min).
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 121 -
[0567] FIG. 27 shows the results of an experiment comparing full volume (FIG.
27A) and
nanovolume SBE (FIG. 27B) of the same heterozygous sample. The results
demonstrate
that nanovolume SBE produces similar quality data as full volume SBE.
[0568] Using both full volume and nanovolume SBE, 23 different samples were
analyzed
s using two distinct primers (NCBI 422 and NCBI 425) with 100 % accuracy of
detection of the
polymorphic nucleotide.
EXAMPLE 23
Nanovolume SBE Coupled With Template Capture
[0569] Nanovolume PCR is performed similarly as described in Example 21,
except that 5
ul of genomic DNA template are mixed with 7.5 ul PCR premix in the wells of a
microtiter
plate and then drawn into the capillary tubes by capillary action. After the
reaction is
completed, PCR product is expelled from the capillaries by centrifugation into
the wells of a
microtiter plate containing 500 nanoliters of 9.7M sodium thiocyanate (NaSCN).
After
mixing, about 500 nanoliters of the solution is drawn into new capillaries by
capillary action,
and incubated at 60°C for 5 min to allow the SBE product to bind to the
inner surface of the
capillaries. Thereafter, the solution is expelled by centrifugation, the
capillaries washed with
80% ethano1120% double distilled water and dried with flowing nitrogen.
Treatment of the
2 o nanovolume PCR product with ExoIISAP is not performed.
[0570] Nanovolume SBE and CIAP treatment of the SBE products is then performed
as
described in Example 22, followed by analysis of the products using
MegaBACET"", also as
described.
[0571] FIG. 28 shows the results of an experiment comparing full volume PCR
treated
2 s with ExoIISAP, followed by full volume SBE (FIG. 28A) and nanovolume PCR
with template
capture, followed by nanovolume SBE (FIG. 28B) of the same heterozygous
sample. The
results demonstrate that nanovolume SBE coupled with template capture produces
similar
quality data as full volume SBE coupled with ExoIISAP treatment of the PCR
product that
serves as SBE template.
EXAMPLE 24
Comparison of SBE Product Cleanup Methods
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 122 -
[0572] Nanovolume SBE is performed as described in Example 23 and different
methods
of treating the SBE products to remove or inactivate unincorporated ddNTPs
prior to
analysis using MegaBACET"" are compared for efficacy. Injection into
MegaBACET"" is
performed at 2kV for 45 sec, and running of samples is performed at 6kV for 60
min. As
s shown in FIG. 29A, if ddNTPs are not removed or inactivated prior to
injection they produce
a strong signal. FIG. 29B and FIG. 29C demonstrate the effectiveness of CIAP
treatment in
preventing the ddNTPs from entering the MegaBACET"' gel bed. Denaturation of
SBE
products in deionized water at 95°C for 1 minute prior to injection
results in about 4 fold
greater signal intensity (FIG. 29C) as compared to denaturing the products in
MegaBACET""
to loading solution (FIG, 29B).
[0573] Most effective in removing ddNTPs and increasing signal intensity
however, is
purifying the SBE products using Sephadex (FIG. 29D) which results in a
further 2 fold
increase in signal intensity. Sephadex aliquoted into the wells of a
microtiter plate is
prewashed four times with 150 ul deionized water. Between washes, sephadex is
pelleted
15 in the well by centrifugation at 910 g for 5 min. Nanovolume SBE reactions
are expelled by
centrifugation into 20 ul of water, after which the diluted reactions are
transferred to wells of
the microtiter plate containing the sephadex. After incubation for time
sufficient for ddNTPs
to enter the pores of the sephadex, the sephadex is pelleted by
centrifugation. A sample
from each well is then injected directly into MegaBACET"~.
EXAMPLE 25
Validation of Nanovolume SBE
[0574] Nanovolume SBE is performed as described in Example 23 using 23
unrelated
2 5 human genomic DNA samples, and 12 no-DNA negative controls. Different base
positions
are interrogated using 12 primers. SBE product is purified with sephadex as
described in
Example 24. Full volume SBE using the same samples and primers is performed as
described in Example 22.
[0575] FIG. 30 compares the results of nanovolume and full volume SBE and
shows the
3 o results for 9 of the primers. Average accuracy of nanovolume SBE (98%) is
comparable to
that of full volume SBE (99%).
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 123 -
[0576] The following examples demonstrate the usefulness and effectiveness of
the
methods of the present invention for performing a range of general enzyme
activity and
inhibition assays. In addition, they demonstrate that solid phase
immobilization can be
applied to proteins and enzymes as well as DNA. Finally, they show the
described system
s can be applied to isothermal reactions. A multiplex capillary system used in
the following
examples contains 16, 96 or 384 capillaries.
EXAMPLE 26
In Solution Protein Digestion using Trypsin
to
[0577] The use of the described multiplex system for processing submicroliter
protein-
containing solutions was demonstrated with a trypsin digest of cytochrome C.
The digestion
was performed in homogeneous solutions within the capillaries of a capillary
cassette.
[0578] A mixture of trypsin (Sigma, St. Louis, MO) and cytochrome C (Sigma,
St. Louis,
is MO) were prepared with a tris-HCI buffer (10 mM, pH 8) at a trypsin-protein
ratio of 1:10,
1:20, 1:50 and 1:100, and protein concentrations were kept at 1 mglmL. 500 nL
aliquots of
the mixture were loaded into the capillary cassettes by capillary action, and
incubated at
37°C overnight. Digestion mixtures were then spun down (by
centrifugation at 2700 G for 1
minute) to a 96-well Robbins plate of which each well contains fluorescein-5-
isothiocyanate
2 0 (FITC) (Molecular Probes, Eugene, OR), 1 mglmL in dimethyl sulfoxide. The
plates were
kept in dark at room temperature for 4 hours. The resulting mixtures were then
diluted 20 to
2000 times with tris-HCI buffer, and subjected to capillary electrophoresis
(CE) separation
on MegaBACETM 1000 (Amersham Biosciences, Piscataway, NJ) using the MegaBACE
LPA buffer and the long read matrix. Samples were injected at 1 KV for 5 sec,
and
2 s separated at 9 KV for 50 min.
[0579] The above experimental conditions were run at least 16 times. A
representative
electropherogram of protein digest is illustrated in FIG. 31A. The obtained
peptide profiles of
digested cytochrome C are consistent and reproducible between each run and
comparable
to data obtained with full volume reactions.
EXAMPLE 27
Protease Assay using Endoproteinase Asp-N
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 124 -
[0580] Endoproteinase Asp-N digestion of polypeptides is illustrated here as
an additional
Example. It further demonstrates the use of the described multiplex system for
processing
submicroliter enzymatic reactions. An enzyme-product relationship for
endoproteinase Asp-
N digestion was established, as well as an optimal enzyme concentration.
s (0581] Peptide CyTM5Q-YVADAPVK-Cy3 (Amersham Biosciences, Piscataway, NJ)
was
used as the reaction substrate. When the peptide is intact, CySQ efficiently
quenches Cy3
and the excitation at Cy3 wavelength results in only a residual background
signal. Once the
peptide is cleaved (Asp-N cleaves the peptide at N-terminal side of aspartic
acid residue),
the dyes are no longer in close proximity, and excitation at Cy3 wavelength
results in Cy3
1 o emission. The signal intensity of Cy3 emission is in linear proportion to
the amount of
peptide being cleaved.
[0582) The endoproteinase Asp-N reaction was performed in homogeneous
solutions.
Five micrograms of the peptide was reconstituted with 20 uL dimethyl
sulfoxide, then mixed
with 980 uL of assay buffer (50 mM Tris, pH 8.0, + 0.005% Tween 2OTM). The
1 s endoproteinase Asp-N (Amersham Biosciences, Piscataway, NJ) was
reconstituted with
500 uL glass distilled water, with a final concentration of 4 ug/mL. A series
of dilutions were
performed on the enzyme so that the final amount of Asp-N in a 500 nL reaction
was
between 5 and 180 picograms. Six concentrations of Asp-N were spun mixed with
a 1:20
dilution of the peptide substrate in a 384 well microtiter plate. 500 nL
aliquots of the mixture
2 o were loaded into a 384 capillary cassette system by capillary action, and
incubated at room
temperature for 10 minutes to allow reaction to complete. Digestion mixtures
were then
spun down into a 384-well clear plate (Nalge Nunc International, Rochester,
NY) of which
each well contains 10 uL assay buffer. The plate was read by a fluorescent
plate reader
(TyphoonTM, Amersham Biosciences, Piscataway, NJ) at 532 nm and 650V with Cy3
555
2 5 BP 20 emission filter.
[0583] The reaction at each Asp-N concentration was repeated 24 times in
parallel. FIG.
36 summarizes the result of these reactions. Signal intensity of Cy3 emission
increases
linearly as the Asp-N concentration increases, up to ~50 picogram Asp-N per
500 nL
reaction. Beyond that, Cy3 signal intensity continues to increase - but at a
slower pace -
3 o with the Asp-N concentration, up to 180 picogram per 500 nL reaction. The
optimal amount
of Asp-N in a 500 nL reaction volume is ~ 50 picogram. These results
demonstrate the use
of nanoprep for assaying potential drug targets in a high throughput nanoscale
reaction and
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 125 -
determining response curves. The application of nanoprep to high throughput
drug
screening will minimize the consumption of targets, compound libraries and
natural product
libraries.
EXAMPLE 28
Protein Digestion with Trypsin Immobilized Beads
(0584] The use of the described multiplex system for processing submicroliter
protein-
containing solutions was demonstrated with a trypsin digest of cytochrome C.
The digestion
1 o was performed with enzyme immobilized on magnetic beads within the
capillaries of a
capillary cassette. Introduction of small magnetic beads offers an efficient
separation tool,
and provides high binding surface area per unit volume for optimal
accessibility of target
molecules. As would readily be understood by those of skill in the art, beads
can also be
non-magnetic or scintillation proximity assay (Amersham Biosciences,
Piscataway, NJ), or
15 have other surface properties.
[0585] Trypsin immobilized magnetic beads were prepared by incubating
Streptavidin
coated magnetic beads M280 (Dynal, Oslo, Norway) with biotin conjugated
trypsin (Sigma,
St. Louis, MO) in tris-HCI buffer at a bead-trypsin ratio of 10:1
(weightlweight). After 24
hours incubation under constant end-over-end shaking at room temperature,
beads were
2 o cleaned on a Dynal MPC-96 magnet device by washing off unbound enzymes
with tris
buffer. These trypsin immobilized magnetic beads were then mixed with
cytochrome C
(Sigma, St. Louis, MO) at a bead-protein ratio of 10:1 (weightlweight). 500 nL
aliquots of the
mixture were then transferred to the capillary cassette by dipping the
cassette into the bead
solution with filling by capillary action. After incubation in an oven at
37°C overnight,
2 5 digestion mixtures were then spun down (by centrifugation at 2700 G for 1
minute) to a 96-
well Bobbins plate of which each well contains 1 mg/mL FITC labeling
solutions. The
mixtures were separated from beads by magnetic force, and the resulting
supernatants
which were free from beads were transferred to another plate. After reaction
in the dark for
4 hours, the labeled protein fragments (peptides) were analyzed by MegaBACET"~
1000
3 0 (Amersham Biosciences, Piscataway, NJ) as described in Example 26.
(0586] The above experimental conditions were run at least 16 times. Capillary
electrophoresis characterization showed that the peptide profiles of digested
cytochrome C
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 126 -
obtained from this approach and the approach described in Example 26 are
consistent and
reproducible. A representative electropherogram of protein digest is
illustrated in FIG. 31 B.
[0587] Enzyme immobilized magnetic beads can also be applied to biochemical
reactions
where the substrate involves DNA or RNA molecules, proteins, glycoproteins,
lipids,
s peptides, or other biomolecules in a capillary cassette format.
EXAMPLE 29
Protein Digestion with Surface Modified
Capillary Cassettes
[0588] Heterogeneous reactions using immobilized-enzyme reactors can eliminate
the
need for separating enzymes from the reaction mixtures, and minimize the
contamination of
the digest by the proteolytic enzymes. The current Example demonstrates that
enzymes
covalently bound to the inner surface of the capillary cassettes retain their
activities and
such a system can be used in enzymatic assays. As would be apparent to those
skilled in
the art, immobilized enzymes can also be used in many other reactions
including sandwich
assays, conversion of substrates to products, bioassays, or other reactions.
[0589] Silanization with aminoalkylsilane reagents gives an amino group
functionalized
surface to which a wide variety of affinity ligands can be subsequently
attached. In this
2 o method, capillary cassettes (or other kind of reaction chambers) were
treated by 3-
aminopropyltriethoxy silane, followed by N-succinimidyl 3-(2-pyridyldithio)
propionatel. The
pyridyldithio functional group provides a convenient way to bind trypsin
through specific -S-
S- and -SH exchange reactions, and, if needed, the immobilized enzymes can be
released
by adding an excess amount of thiopyridone (Carlsson, J.; Drevin H.; Axen, R.
Biochem. J.
2 5 1978, 173, 723). Thus, the same capillary surface can be regenerated for
tethering fresh
trypsin to ensure high enzyme reactivity.
[0590] Another surface immobilization approach is based on a specific
streptavidin-biotin
reaction. Streptavidin modification enables the surface to bind biotinylated
enzymes. In this
approach, capillary cassettes were derivatized with 3-aminopropyltriethoxy
silane, and then
3 o reacted with a bifunctional linker, disuccinimidyl suberate, for tethering
streptavidin that
allows biotinylated trypsin to be thereafter linked to the capillary surfaces.
The high
specificity of streptavidin and biotin interaction was utilized to give
uniformly oriented
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 127 -
enzymes on the inner surfaces of the capillary (Wilchek, M.; Bayer, E.A.
Methods in
Enzymology, 1990, 184)
[0591] These two enzyme immobilization techniques are aimed to offer high
surface
reactivity and minimized nonspecific binding. As will be understood by the
skilled artisan,
other surface immobilization approaches can also be utilized. For example,
reaction with y-
glycidoxylpropylsilane introduces oxirane groups to the solid surfaces that
allow coupling
enzyme at lysine sites. This modification is expected to provide more
hydrophilic surfaces to
reduce unspecific protein uptake. Conjugating enzyme with surface-active
hydrogels offers
a convenient means to produce enzyme immobilized surfaces (Caldwell, Carlsson
and Li,
1 o US Patent US5516703, 1996). Advantage of this approach is to provide
protein compatible
environment and reusable surfaces.
[0592] Protein digestion reactions were conducted by directly introducing
cytochrome C (1
mg/mL) to the streptavidin-biotin immobilized-trypsin capillary microreactors
by capillary
action, followed by incubation at 37 °C overnight. Protein fragments
were then spun out,
labeled with fluorescein-5-isothiocyanate (FITC), and the labeled protein
digests
subsequently subjected to MegaBACETM analysis, all as described in Example 26.
Two
untreated capillary cassettes coated with trypsin (by simple adsorption) were
used as the
controls.
[0593] For a given immobilized-trypsin capillary cassette, three protein
digestion reactions
2 o were performed during a period of two weeks using fresh cytochrome C
solutions. The first
reaction was performed on day 0, the second on day 7 and the third reaction on
day 14.
The immobilized-trypsin capillary cassettes were stored in 0.15 M phosphate
buffered saline
at 4°C between runs. Capillary electrophoresis separation obtained on
MegaBACETM
demonstrates that all capillaries of the treated cassettes have the same
peptide maps in the
three runs, as shown in the representative electropherograms of runt (day 0),
runt (day 7)
and run3 (day 14) (FIG. 32, 33 and 34), respectively. On the contrary, the
control capillary
cassette showed some protein digestion only in the first run, but no protein
digestion in the
second or the third run. This is as expected, since nonspecific binding via
physical
adsorption probably reduces enzyme activity, and the binding is not as stable
as covalent
3 o binding. As a result, such immobilized enzymes do not have sufficient
capacity to carry out
repeated digestion reactions. This example demonstrates that enzymes may be
coupled to
CA 02474429 2004-07-26
WO 03/066667 PCT/US03/03986
- 128 -
the surface of a high throughput nanoscale reactor and used to perform
repeated enzymatic
reactions, e.g., a proteolytic digestion, as described hereabove.
(0594] In addition to CE technique described above, high performance liquid
chromatography (HPLC) was utilized to further characterize these protein
digests.
s Experiments were performed on a AKTAexplorer chromatography system 10 with a
fraction
collector Frac-950 and an autosampler A900 (Amersham Biosciences, Piscataway,
NJ). A
tryptic digest sample of cytochrome C prepared on the streptavidin-biotin
immobilized
trypsin capillary cassette was injected into a reversed phase column SOURCE
5RPC ST
4.6/150 (Amersham Biosciences, Piscataway, NJ), and eluted out by a gradient
(eluent A:
0.05% trifluoroacetic acid in 2% acetonitrile; eluent B: 0.05% trifluoroacetic
acid in 80%
acetonitrile). A representative HPLC chromatogram is shown in FIG. 35. The
profile of
cytochrome C digests obtained on capillary cassettes is identical to
literature results (Neue,
U.D.; Zoubair, M.; Fallah EI, HPLC Columns: Theory, Technology, and Practice,
VCH
Publishing, 1997).
[0595] All patents, patent publications, and other published references
mentioned herein
are hereby incorporated by reference in their entireties as if each had been
individually and
specifically incorporated by reference herein. While preferred illustrative
embodiments of
the present invention are described, one skilled in the art will appreciate
that the present
2 o invention can be practiced by other than the described embodiments, which
are presented
for purposes of illustration only and not by way of limitation. The present
invention is limited
only by the paragraphs that follow.