Note: Descriptions are shown in the official language in which they were submitted.
CA 02475066 2004-08-04
WO 03/066679 PCT/AU03/00135
-1-
"Rhamnose Binding Protein"
Field of the Invention
The present invention relates to an isolated rhamnose binding protein (RBP)
that
is over expressed in cancer cells relative to non-cancer cells, The present
invention also relates to methods of diagnosing cancer by detecting RBP levels
and to RBP agonists such as antibodies and methods of treating and diagnosing
cancer using RBP agonists.
Background Art
BEC~ is a mixture of the triglycosides: solasonine and solamargine that has
anti-
cancer activity. Studies on the mode of action of BEC~ indicate that the
glycosides gain entry to cancer cells via a cell surface receptor and that the
in
vitro toxicity of BEC~ to cancer cells is reduced by co-administration of
rhamnose.
The presence of endogenous endocytic ligand receptors (EEL) has been an area
of clinical research for over 2 decades. The first EEL to be identified was
the
asialoglycoprotein receptor on mammalian hepatocytes with specificity for
galactose (Ashwell & Hardford 1982). Since this time other hepatic receptors
have been identified. For example, fucose (Lehrman et al 1986), GaINAc (Kolb-
Bachofen etal 1984), as. well as a number of cell receptors identified by
Cramer
and Gabius (1991). EEL's may be involved in cellular recognition, cell
adhesion
or substrate binding.
To date no one has isolated and/or characterised the cell surface receptor
that is
central to BEC~'s mode of action. The present invention seeks to overcome or
at
least partially alleviate this problem.
Summary of the Invention
The present invention provides an isolated RBP with at Least one of the
following
characteristics:
CA 02475066 2004-08-04
WO 03/066679 PCT/AU03/00135
-2-
(a) a molecular weight of approximately 65-70 kDa and more preferably 66-
69kDa;
(b) a pl of greater than 10 or less than 3;
(c) a dissociation constant of approximately 1.5 x 10-6 when bound to the
rhamnose moiety of solamargine;
(d) adapted to bind to a rhamnose affinity column prepared according to
example 1 and under the conditions set out therein ;
(e) adapted to be eluted from the column in example 1 with a 100mM
rhamnose solution;
(f) insoluble in aqueous solution; and
(g) soluble in highly denaturing buffers containing greater that approximately
2% surfactant.
The ability of the RBP to bind ligands such as rhamnose to a RBP bearing cell,
such as a carcinoma, render it useful in various methods. For example, it has
been found that when the RBP binds a ligand, such as rhamnose, cell adhesion
of
the RBP bearing cells is inhibited. Thus, the present invention also provides
a
method of inhibiting cell adhesion between RBP bearing cells comprising the
step
of contacting the RBP bearing cells with an effective amount of a RBP ligand.
The effective amount may be varied depending on the circumstances and may be
determined by those skilled in the art. However, when the RBP ligand is
rhamnose the effective amount may be approximately 70 picograms/cell.
Upon binding of a ligand to a cell associated RBP of the present invention,
depending on the ligand, the ligand may be internalised in the cell or remain
on
the cell surFace. Whether or not a ligand is internalised after binding to a
cell
associated RBP of the present invention depends on a variety of factors such
as
the molecular weight, charge, structure and/or biological activity of the
ligand.
CA 02475066 2004-08-04
WO 03/066679 PCT/AU03/00135
-3-
Thus, the present invention also provides a method of delivering an agent to a
RBP bearing cell comprising contacting an agent-ligand complex with the RBP
bearing cell.
The agent may be delivered to the cell surface or inside the cell by selecting
an
appropriate ligand-agent complex. For example, by selecting an agent-complex
of a certain molecular weight or structure it is possible to control the
delivery of
the agent to the cell surface or the inside of the cell. In this regard, it
has been
found that if the agent is above a certain threshold weight then it cannot be
efficiently internalised in by the RBP bearing cell and will remain at the
cell
surface.
The ability of RBP to bind ligands and either internalise or retain them on a
cell
surface, means the RBP may be utilised to locate and identify RBP bearing
cells.
Thus, the present invention also provides a method of detecting a RBP bearing
cell comprising the steps of: (i) contacting a cell or tissue sample with an
agent
adapted to selectively bind to RBP and (ii) detecting the RBP bearing cells.
The agent may be varied and includes antibodies and other ligands or agonists
that are adapted to bind to RBP. Furthermore, to ease detection of the RBP
bearing cells the agent may be adapted to be visualised.
Thus, the RBP of the present invention may be used to identify agents that
bind to
the RBP and thus can be used in assays for the RBP, as diagnostics to identify
RBP bearing cells or to target therapeutic agents to cancer cells via the RBP.
The RBP may also form a component of a screening system for antagonists or
agonists of agents that bind to the RBP.
These and additional uses for the reagents described herein will become
apparent to those of ordinary skill in the art upon reading this
specification.
CA 02475066 2004-08-04
WO 03/066679 PCT/AU03/00135
-4-
Brief Description of the~,Figures
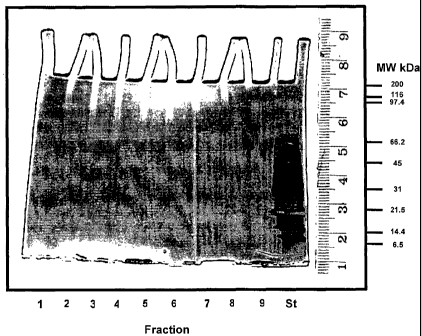
Figure 1: depicts a PAGE gel containing fractions 1-9 eluted from a
biotinylated
rhamnose-ITC affinity column using 100mM free rhamnose and lane 10 contains
standard molecular weight markers;
Figure' 2A: is a fluoro-image of proteins crosslinked to FRITC and analysed on
a
4-20% polyacrylamide gel. Standards (tagged with assorted coloured dyes hence
some visible by fluoro-imaging); Sample: 1) 5~rM FRITC (Batch 7) + 100pM CDI;
2) 5pM FRITC + 500pM CDI; 3) 5pM FRITC + 10mM CDI; 4) 5pM FRITC (Batch
2) + 100J~M CDI; 5) No FRITC + 100pM CDI; 6) No FRITC + 500pM CDI;
Figure 2B: is the total proteins from the gel depicted in Figure 2A stained
with
Coomassie brilliant blue;
Figure 3: is a graph used to calculate the molecular mass of the proteins in
Figure
2A;
Figure 4: is an image of A2058 cells following incubation with 12pM
fluorescein
rhamnose-ITC at 37°C for l5min in HEPES buffered saline containing 2mM
Ca2
and Mg2+;
Figure 5: is a plot of the relationship between dose per cell at LDSO and the
Day 1
cell density for each cell tine;
Figure 6: depicts the data in Figure 5 condensed and fitted to a single
exponential
function;
Figure 7: is a plot of the relationship between dose per cell at LDSO and the
Day 1
cell density for two particular breast cancer lines;
Figure 8: is a comparison of the plots in Figures 6 and 7;
Figure 9: is table containing single point LD50 data from another 11
carcinomas;
Figure 10: is a graphical representation of the data presented in the table in
Figure 9; and
Figure 11: illustrates the protective effects of rhamnose when co-administered
with BEC~ via a graph of % cell (A2058, 600 cells) survival v's concentration
of
BEC~;
Figure 12: illustrates the protective effects of rhamnose when co-administered
with BEC~ via a graph of % cell (A2058, 5000 cells) survival v's concentration
of
BEC~;
CA 02475066 2004-08-04
WO 03/066679 PCT/AU03/00135
-5-
Figure 13 illustrates a fluoro-image of A2058 proteins crosslinked to FR1TC,
solvent extracted and analysed on a 4-20% SDS polyacrylamide gel, From left,
Lane 1: Standards 83, 42.3, 32.2, 18.8kD; Lane 2 blank; Lanes 3-8: replicate
flasks of cells + approx 5pM FR1TC + 100pM carbonyl di-imidazole; and
Figure 14 illustrates immunoprecipitation of FRITC -protein cross-linked
complex
analysed on a 4-20% SDS polyacrylamide gel.
Total protein stain - Lane 1: Standards 206, 124, 83, 42.3, 32.2, 18.8kD;
Both); Lane 2 Protein A pre-clear (-ve); Lane 3 a-FITC antibody precipitation
Detailed Description of the Invention
Rhamnose binding protein~RBP)
The present invention is based on the isolation and identification of a
cellular
receptor of the lectin group that is more abundant on neoplastic (cancer)
cells
than non-cancer cells. The receptor ("RBP") is adapted to bind and internalise
rhamnose and thus represents a valuable diagnostic and therapeutic tool.
The present invention provides an isolated RBP comprising at least one of the
following characteristics:
(a) a molecular weight of appraximately 65-70 kDa and more preferably 66-
69kDa;
(b) a pl of greater than 10 or less than 3;
(c) a dissociation constant of approximately 1.5 x 10-6 when bound to the
rhamnose moiety of solamargine;
(d) adapted to bind to a rhamnose affinity column prepared according to
example 1 and under the conditions set out therein ;
(e) adapted to be eluted from the column in example 1 with a 100mM
rhamnose solution;
CA 02475066 2004-08-04
WO 03/066679 PCT/AU03/00135
-6-
(f) insoluble in aqueous solution; and
(g) soluble in highly denaturing buffers containing greater that approximately
2°l° surFactant.
Throughout the specification, unless the context requires otherwise, the word
"comprise" or variations such as "comprises" or "comprising", will be
understood to
imply the inclusion of a stated integer or group of integers but not the
exclusion of
any other integer or group of integers.
The RBP and other polypeptides of the invention may be in a substantially
isolated form. In this regard, it will be understood that they may be mixed
with
carriers or diluents that will not interFere with their intended purpose and
still be
regarded as substantially isolated. A polypeptide of the invention may also be
in
a substantially purified form, in which case it will generally comprise the
polypeptide in a preparation in which at least 90%, 95°!°, 98%
or 99% of the
protein in the preparation is a polypeptide of the invention.
Assays for Compounds that Bind RBP
The RBP of the present invention may be used in assays to identify compounds
that interact with (e.g., bind to) it.
The compounds which may be screened in accordance with the invention include,
but are not limited to peptides, antibodies and fragments thereof, and other
organic compounds (e.g., peptidomimetics) that bind to the RBP and either
mimic
the activity triggered by the natural ligand - rhamnose (i.e., agonists) or
inhibit the
activity triggered by the natural ligand - rhamnose (i.e., antagonists).
Other compounds that may be screened according to the present invention are
peptides, antibodies or fragments thereof, and other organic compounds that
mimic the extra cellular domain of the RBP (or a portion thereof) and bind to
and
"neutralize" natural ligand such as rhamnose.
CA 02475066 2004-08-04
WO 03/066679 PCT/AU03/00135
-7-
Such compounds may include, but are not limited to, peptides such as, for
example, soluble peptides, including but not limited to members of random
peptide libraries; and combinatorial chemistry-derived molecular library made
of
D- and/or L- configuration amino acids, phosphopeptides including, but not
limited
to, members of random or partially degenerate, directed phosphopeptide
libraries,
antibodies (including, but not limited to, polyclonal, monoclonal, humanized,
anti-
idiotypic, chimeric or single chain antibodies, and FAb, F(ab')2 and FAb
expression library fragments, and epitope-binding fragments thereof), and
small
organic or inorganic molecules.
Computer modelling and searching technologies permit identification of
compounds, or the improvement of already identified compounds, that can
modulate RBP expression or activity. Having identified such a compound or
composition, the active sites or regions are identified. Such active sites
might
typically be ligand binding sites, such as the interaction domains of rhamnose
with
RBP itself. The active site can be identified using methods known in the art
including, for example, from study of complexes of RBP with rhamnose. In this
regard, chemical or X-ray crystallographic methods can be used to find the
active
site by finding where on the factor the complexed ligand is found. Next, the
three
dimensional geometric structure of the active site is determined. This can be
done by known methods, including X-ray crystallography, which can determine a
complete molecular structure. On the other hand, solid or liquid phase NMR can
be used to determine certain intra-molecular distances.
Having determined the structure of the active site, either experimentally, by
modelling, or by a combination, candidate modulating compounds can be
identified by searching databases containing compounds along with information
on their molecular structure. Such a search seeks compounds having structures
that match the determined active site structure and that interact with the
groups
defining the active site. Such a search can be manual, but is preferably
computer
assisted. These compounds found.from this search are potential RBP modulating
compounds.
CA 02475066 2004-08-04
WO 03/066679 PCT/AU03/00135
_$_
Alternatively, these methods can be used to identify improved modulating
compounds from an already known modulating compound or ligand. The
composition of the known compound can be modified and the structural effects
of
modification can be determined using the experimental and computer modelling
methods described above applied to the new composition. The altered structure
is then compared to the active site structure of the compound to determine if
an
improved fit or interaction results. In this manner systematic variations in
composition, such as by varying side groups, can be quickly evaluated to
obtain
modified modulating compounds or ligands of improved specificity or activity.
Further experimental and computer modelling methods useful to identify
modulating compounds based upon identification of the active sites of rhamnose
and RBP will be apparent to those of skill in the art.
Although described above with reference to design and generation of compounds
that could alter binding, one could also screen libraries of known compounds,
including natural products or synthetic chemicals, and biologically active
materials, including proteins, for compounds that are inhibitors or
activators.
Compounds identified via assays such as those described herein may be useful,
for example, in elaborating the biological function of the RBP and for
treating
cancer. -
The compounds capable of binding RBP may also be used to identify and isolate
RBP homologues. In this regard, the compounds may be used to screen various
cell types such as cancer cell types to locate variants of the RBP that could
be
used to design specific therapeuticlagents for treatment of related cancers.
In vitro systems may be designed to identify compounds capable of interacting
with (e.g., binding to) RBP (including, but not limited to, the extra cellular
domain
of RBP). These compounds may be useful, for example, in modulating the
activity of wild type and/or mutant RBP; elaborating the biological function
of the
RBP; screening for compounds that disrupt normal RBP interactions; or may in
themselves disrupt such interactions.
CA 02475066 2004-08-04
WO 03/066679 PCT/AU03/00135
_g_
The principle of the assays used to identify compounds that bind to the RBP
involves preparing a reaction mixture of the RBP and the test compound under
conditions and for a time sufficient to allow the two components to interact
and
bind, thus forming a complex which can be removed and/or detected in the
reaction mixture. The RBP species used can vary depending upon the goal of the
screening assay. For example, where agonists of the natural ligand are sought,
the full length RBP, or a soluble truncated RBP, e.g., in which the
transmembrane
or cellular domain is deleted from the molecule, a peptide corresponding to
the
extracellular domain or a fusion protein comprising the RBP extracellular
domain
fused to a protein or polypeptide that affords advantages in the assay system
(e.g., labelling, isolation of the resulting complex, etc.) can be utilized.
The screening assays can be conducted in a variety of ways. For example, one
method to conduct such an assay involves anchoring the RBP or fusion protein
or
the test substance onto a solid phase and detecting RBP/test compound
complexes anchored on the solid phase at the end of the reaction. In one
embodiment of such a method, the RBP may be anchored onto a solid surface,
and the test compound, which is not anchored, may be labelled, either directly
or
indirectly.
In practice, microtiter plates may conveniently be utilized as the solid
phase. The
anchored component may be immobilized by non-covalent or covalent
attachments. Non-covalent attachment may be accomplished by simply coating
the solid surface with a solution of the RBP or test compound and drying.
Alternatively, an immobilized antibody, such as a monoclonal antibody,
specific
for the protein to be immobilized may be used to anchor the protein to the
solid
surface.
In order to conduct the assay, the nonimmobilized component is added to the
coated surface containing the anchored component. After the reaction is
complete, unreacted components are removed (e.g., by washing) under
conditions such that any complexes formed will remain immobilized on the solid
surface. The detection of complexes anchored on the solid surface can be
accomplished in a number of ways. Where the previously nonimmobilized
CA 02475066 2004-08-04
WO 03/066679 PCT/AU03/00135
-10-
component is pre-labelled, the detection of label immobilized on the surFace
indicates that complexes were formed. Where the previously nonimmobilized
component is not pre-labelled, an indirect label can be used to detect
complexes
anchored on the surface; e.g., using a labelled antibody specific for the
previously
nonimmobilized component (the antibody, in turn, may be directly labelled or
indirectly labelled with a labelled anti-Ig antibody).
Alternatively, a reaction can be conducted in a liquid phase, the reaction
products
separated from unreacted components, and complexes detected; e.g., using an
immobilized antibody specific for RBP or the test compound to anchor any
complexes formed in solution, and a labelled antibody specific for the other
component of the possible complex to detect anchored complexes.
Cell-based assays can also be used to identify compounds that interact with
RBP.
To this end, cell lines that naturally express RBP such as a cancer cell line
selected from the group comprising: HT-29, LS174-T. AGS, 5637, A431, 786-O,
Hs578Bst, CCD 18Lu, HeLa 229, HepG2, JAM, N036, U87-MG, DV145, LNCaP
and A2058, or cell lines (e.g., COS cells, CHO cells, fibroblasts, etc.) that
have
been genetically engineered to express RBP (e.g., by transfection or
transduction
of RBP DNA) can be used. Interaction of the test compound with, for example,
the extracellular domain of RBP expressed by the host cell can be determined
by
comparison or competition with native rhamnose.
Diagnostics
The RBP of the present invention and agonists thereof can be employed for the
diagnostic and prognostic evaluation of cancer. Such methods may, for example,
utilize reagents such as the antibodies described herein. Specifically, such
reagents may be used, for example, to detect an over-abundance of RBP relative
to normal cells.
Thus, the present invention provides a method for detecting cancer in a sample
comprising the steps of: (i) detecting the level of RBP in the sample; and
(ii)
comparing it to the level of RBP in a sample from a non-cancer source.
CA 02475066 2004-08-04
WO 03/066679 PCT/AU03/00135
-11-
The detection method of the present invention may be used to diagnose cancer
in
vitro. Thus, the present invention provides a method of diagnosing cancer in a
patient comprising the steps of: (i) detecting the level of RBP in a sample
from the
patient; and (ii) comparing it to the level of RBP in a sample from a non-
cancer
source.
Alternatively, the detection method may be used to diagnose cancer in vivo. In
this regard, agents that are adapted to bind to RBP can be labelled and
administered to a subject suspected of having cancer and later detected to
perForm the diagnosis. Thus, the present invention also provides a method of
diagnosing cancer in a patient comprising the steps of: (i) detecting the
level
and/or distribution of RBP in the patient; and (ii) analysing the distribution
and/or
levels of RBP to identify differences that are indicative of cancer.
The methods described herein may be performed, for example, by utilizing pre-
packaged diagnostic kits comprising at least one specific RBP antibody reagent
described herein, which may be conveniently used, e.g., in clinical settings,
to
diagnose patients suspected of having cancer.
RBP antibodies and other agonists of RBP may be used as cancer diagnostics
and prognostics, as described herein. Such diagnostic methods may be used to
detect abnormalities in the level of RBP and may be performed in vivo or in
vitro,
such as, for example, on biopsy tissue.
For example, antibodies directed to epitopes of the RBP can be used in vivo to
detect the pattern and level of expression of the RBP in the body. Such
antibodies can be labelled, e.g., with a radio-opaque or other appropriate
compound and injected into a subject in order to visualize binding to the RBP
expressed in the body using methods such as X-rays, CAT-scans, or MRI.
Labelled antibody fragments, e.g., the Fab or single chain antibody comprising
the
smallest portion of the antigen binding region may also be used for this
purpose.
When interpreting the patterns produced according to the diagnostic method,
account must be taken on background signal or "noise" from non-cancer cells
that
also bear the RBP, albeit at lower levels. However, those skilled in the art
are
-10-
component is pre-labelled, t
CA 02475066 2004-08-04
WO 03/066679 PCT/AU03/00135
-12-
readily able to discern noise from actual signal in performing the diagnosis.
Immunoassays or fusion protein detection assays can also be used to diagnose
or type cancer in biopsy or autopsy samples in vitro.
Agonists described herein including antibodies, or fragments of antibodies may
also be used to quantitatively or qualitatively detect the presence of RBP or
conserved variants or peptide fragments thereof. This can be accomplished, for
example, by immunofluorescence techniques employing a fluorescently labelled
antibody coupled with light microscopic, flow cytometric, or fluorimetric
detection.
The agonists such as antibodies (or fragments thereof) of the present
invention
may, additionally, be employed histologically, as in immunofluorescence,
immunoelectron microscopy or non-immuno assays, for in situ detection of RBP
or conserved variants or peptide fragments thereof.
In situ detection may be accomplished by removing a histological specimen from
a patient,, and applying thereto a labelled antibody or fusion protein of the
present
invention. The antibody (or fragment) or fusion protein is preferably applied
by
overlaying the labelled antibody (or fragment) onto a biological sample.
Through
the use of such a procedure, it is possible to determine not only the presence
of
the RBP, or conserved variants or peptide fragments, but also its distribution
in
the examined tissue. Using the present invention, those of ordinary skill will
readily perceive that any of a wide variety of histological methods (such as
staining procedures) can be modified in order to achieve such in situ
detection.
Immunoassays and non-immunoassays for RBP or conserved variants or peptide
fragments thereof will typically comprise incubating a sample, such as a
biological
fluid, a tissue extract, freshly harvested cells, or lysates of cells which
have been
incubated in cell culture, in the presence of a detestably labelled antibody
capable
of identifying RBP or conserved variants or peptide fragments thereof, and
detecting the bound antibody by any of a number of techniques well-known in
the
art.
CA 02475066 2004-08-04
WO 03/066679 PCT/AU03/00135
-13-
The biological sample may be brought in contact with and immobilized onto a
solid phase support or carrier such as nitrocellulose, or other solid support
that is
capable of immobilizing cells, cell particles or soluble proteins. The support
may
then be washed with suitable buffers followed by treatment with the detectably
labelled RBP antibody or other agonist. The solid phase support may then be
washed with the buffer a second time to remove unbound antibody. The amount
of bound label on solid support may then be detected by conventional means.
By "solid phase support or carrier" is intended any support capable of binding
an
antigen or an antibody. Well-known supports or carriers include glass,
polystyrene, polypropylene, polyethylene, dextran, nylon, amylases, natural
and
modified celluloses, polyacrylamides, gabbros, and magnetite. The nature of
the
carrier can be either soluble to some extent or insoluble for the purposes of
the
present invention. The 'support material may have virtually any possible
structural
configuration so long as the coupled molecule is capable of binding to an
antigen
or antibody. Thus, the support configuration may be spherical, as in a bead,
or
cylindrical, as in the inside surface of a test tube, or the external surface
of a rod.
Alternatively, the surface may be flat such as a sheet, test strip, etc.
Preferred
supports include polystyrene beads. Those skilled in the art will know many
other
suitable carriers for binding antibody or antigen, or will be able to
ascertain the
same by use of routine experimentation.
With respect to antibodies, one of the ways in which the antibody can be
detectably labelled is by linking the same to an enzyme. This then renders the
antibody suitable for use in an enzyme immunoassay (EIA). The enzyme that is
bound to the antibody will react with an appropriate substrate, preferably a
chromogenic substrate, in such a manner as to produce a chemical moiety that
can be detected, for example, by spectrophotometric, fluorimetric or by visual
means. Enzymes which can be used to detectably label the antibody include, but
are not limited to, malate dehydrogenase, staphylococcal nuclease, delta-5-
steroid isomerase, yeast alcohol ~dehydrogenase, alphaglycerophosphate,
dehydrogenase, triose phosphate isomerase, horseradish peroxidase, alkaline
phosphatase, asparaginase, glucose oxidase, beta-galactosidase, ribonuclease,
urease, catalase, glucose-6-phosphate dehydrogenase, glucoamylase and
CA 02475066 2004-08-04
WO 03/066679 PCT/AU03/00135
-14-
acetylcholinesterase. The detection can be accomplished by calorimetric
methods that employ a chromogenic substrate for the enzyme. Detection may
also be accomplished by visual comparison of the extent of enzymatic reaction
of
a substrate in comparison with similarly prepared standards.
Detection may also be accomplished using any of a variety of other
immunoassays. For example, by radioactively labelling the antibodies, antibody
fragments or other agonists, it is possible to detect RBP through the use of a
radioimmunoassay (RIA). The radioactive isotope can be detected by such
means as the use of a gamma counter or a scintillation counter or by
autoradiography.
It is also possible to label the antibody or other agonist with a fluorescent
compound. When the fluorescently labelled antibody is exposed to light of the
proper wave length, its presence can then be detected due to fluorescence.
Among the most commonly used fluorescent labelling compounds are fluorescein
isothiocyanate, rhodamine, phycoerythrin, phycocyanin, allophycocyanin, o-
phthaldehyde and fluorescamine.
Agonists such as antibodies can also be detectably labelled using fluorescence
emitting metals such as ~52Eu, or others of the lanthanide series. These
metals
can be attached to the antibody using such metal chelating groups such as
diethylenetriaminepentacetic acid (DTPA) or ethylenediaminetetraacetic acid
(EDTA).
The antibody or other agonist can also be detectably labelled by coupling it
to a
chemiluminescent compound. The presence of the chemiluminescent-tagged
antibody is then determined by detecting the presence of luminescence that
arises during the course of a chemical reaction. Examples of particularly
useful
chemiluminescent labelling compounds are luminol, isoluminol, theromatic
acridinium ester, imidazole, acridinium salt and oxalate ester.
Likewise, a bioluminescent compound may be used to label the antibody or other
agonist of the present invention. Bioluminescence is a type of
CA 02475066 2004-08-04
WO 03/066679 PCT/AU03/00135
-15-
chemiluminescence found in biological systems in, which a catalytic protein
increases the efficiency of the chemiluminescent reaction. The presence of a
bioluminescent protein is determined by detecting the presence of
luminescence.
Important bioluminescent compounds for purposes of labelling are luciferin,
luciferase and aequorin.
Methods of Treatment
The ability of the agonists of the present invention bind to RBP and
subsequently
become internalised in the target cell renders them useful for preferentially
delivering agents to cells with a higher load of RBP, such as cancer cells.
For therapeutic purposes, the agents linked to the agonists of the present
invention may be any agent that is adapted to prevent cell growth or division
or
cause cell death such as, Doxorubicin, Daunorubicin, Vincristine, Vimblastine,
Vindesine, Methothrexate, Cytarabine, Etopside, Cisplatin, Carboplatin, 5-
Fluorouracil, Bleomycin, Epirubicin, Cyproterone, Irinotecan etc. When linked
to
such agents the agonists of the present invention may be used to treat cancer
in a
patient.
Thus, the present invention provides a method of treating cancer in a subject
comprising administering a therapeutically, effective amount of a RBP agonist
anticancer conjugate to said subject.
The agonists of the present invention may also be used to treat BEC~ overdose.
In this regard, if BEC~ has been administered to a patient at too high a dose,
then
an agonist of the present invention may be administered to bind to the RBP of
the
present invention and prevent or at least reduce BEC~ binding.
Thus, the present invention also comprises a method of treating BEC~ overdose
in a subject, the method comprising administering an effective amount of an
RBP
agonist to the subject. Agonists for use in this aspect of the invention may
be
varied and include RBP antibodies, rhamnose or some other RBP ligand.
CA 02475066 2004-08-04
WO 03/066679 PCT/AU03/00135
-16-
Compositions/Administration
This invention also contemplates pharmaceutical or veterinary compositions
comprising an agonist of the present invention and a pharmaceutically
acceptable
carrier. Preferably, the compositions will further comprise an agent adapted
to
cause cell death such as a glycoside. Pharmaceutical compositions of
proteineous drugs of this invention are particularly useful for parenteral
administration, i.e., subcutaneously, intramuscularly or intravenously. The
compositions for parenteral administration may comprise a solution of the
compounds of the invention or a cocktail thereof dissolved in an acceptable
carrier, preferably an aqueous carrier, an emulsion or formulated as micelles
in an
appropriate carrier. A variety of aqueous carriers may be employed, e.g.,
water,
buffered water, 0.4% saline, 0.3% glycine, and the like. These solutions are
preferably sterile and generally free of particulate matter. These solutions
may be
sterilized by conventional, well known sterilization techniques. The
compositions
may further contain pharmaceutically acceptable auxiliary substances as
required
to approximate physiological conditions such as pH adjusting and buffering
agents.
The concentration of the compounds of the invention in such pharmaceutical
formulation can very widely, i.e., from less than about 0.1 %, usually at or
at least
about 1 % to as much as 15 or 20% by weight and will be selected primarily
based
on fluid volumes, viscosities, etc., according to the particular mode of
administration selected.
Thus, a pharmaceutical composition of the invention for intramuscular
injection
could be prepared to contain 1 mL sterile buffered water, and 50 mg of a
compound of the invention. Similarly, a pharmaceutical composition of the
invention for intravenous infusion could be made up to contain 250 ml of
sterile
Ringer's solution, and 150 mg of a compound of the invention. Actual methods
for
preparing parenterally administrable compositions are well known or will be
apparent to those skilled in the art and are described in more detail in, for
example, Remington's Pharmaceutical Science, 15th ed., Mack Publishing
Company, Easton, Pa.
CA 02475066 2004-08-04
WO 03/066679 PCT/AU03/00135
-17-
The compounds described herein can be lyophilized for storage and
reconstituted
in a suitable carrier prior to use. This technique has been shown to be
effective
with conventional proteins and art-known lyophilization and reconstitution
techniques can be employed.
In situations where the agonist is non-proteineous, it may be administered
alone
or in combination with pharmaceutically acceptable carriers. The proportion of
which is determined by the solubility and chemical nature of the compound,
chosen route of administration and standard pharmaceutical practice. For
example, they may be administered orally in the form of tablets or capsules
containing such excipients as starch, milk sugar, certain types of clay and so
forth. They may be administered sublingually in the form of Troches or
lozenges in
which the active ingredient is mixed with fillers and binders, flavouring
agents and
dyes; and then dehydrated sufficiently to make it suitable for pressing into a
solid
form. They may be administered orally in the form of solutions that may be
injected parenterally, that is, intramuscularly, intravenously or
subcutaneously.
For parenteral administration, they may be used in the form of a sterile
solution
containing other solutes, for example, enough saline or glucose to make the
solution isotonic.
The physician or veterinarian will determine the dosage of the present
therapeutic
agents that will be most suitable and it will vary with the form of
administration and
the particular compound chosen, and' furthermore, it will vary with the
particular
subject under treatment. The physician will generally wish to initiate
treatment
with small dosages substantially less than the optimum dose of the compound
and increase the dosage by small increments until the optimum effect under the
circumstances is reached. It will generally be found that when the composition
is
administered orally, larger quantities of the active agent will be required to
produce the same effect as a smaller quantity given parenterally. The
compounds are useful in the ,same manner as other serotonergic agents and the
dosage level is of the same order of magnitude as is generally employed with
these other therapeutic agents. The therapeutic dosage will generally be from
1
to 1000 milligrams per day and higher although it may be administered in
several
CA 02475066 2004-08-04
WO 03/066679 PCT/AU03/00135
-18-
different dosage units. Tablets containing from 5 to 100 mg. of active agent
are
particularly useful.
Topical administration
The pharmaceutical compositions of the present invention may be adapted for
topical application to a patient.
Various topical delivery systems may be appropriate for administering the
compositions of the present invention depending upon the preferred treatment
regimen. Topical formulations may be produced by dissolving or combining the
agonist of the present invention in an aqueous, or nonaqueous carrier. In
general,
any liquid, cream, .or gel, or similar substance that does not appreciably
react with
the agonist or any other of the active ingredients that may be introduced into
the
composition and which are non-irritating are suitable. Appropriate non-
sprayable
viscous, semi-solid or solid forms can also be employed that include a carrier
compatible with topical application and have a dynamic viscosity preferably
greater than water.
Suitable formulations are well known to those skilled in the art and include,
but
are not limited to, solutions, suspensions, emulsions, creams, gels,
ointments,
powders, liniments, salves, aerosols, transdermal patches, etc, which are, if
desired, sterilized or mixed with auxiliary agents, e.g., preservatives,
stabilizers,
emulsifiers, wetting agents, fragrances, colouring agents, odour controllers,
thickeners such as natural gums etc. Particularly preferred topical
formulations
include ointments, creams or gels.
Ointments generally are prepared using either (1 ) an oleaginous base, i.e.,
one
consisting of fixed oils or hydrocarbons, such as white petroleum or mineral
oil, or
(2) an absorbent base, i.e., one consisting of an anhydrous substance or
substances which can absorb water, for example anhydrous lanolin. Customarily,
following formation of the base, whether oleaginous or absorbent, the active
ingredient is added to an amount affording the desired concentration.
Creams are oil/water emulsions. They consist of an oil phase (internal phase),
30' comprising typically fixed oils, hydrocarbons and the like, waxes,
petroleum,
CA 02475066 2004-08-04
WO 03/066679 PCT/AU03/00135
-19-
mineral oil and the like and an aqueous phase (continuous phase), comprising
water and any water-soluble substances, such as added salts. The two phases
are stabilised by use of an emulsifying agent, for example, a surfiace active
agent,
such as sodium lauryl sulfite; hydrophilic colloids, such as acacia colloidal
clays,
veegum and the like. Upon formation of the emulsion, the agonist is
customarily
added in an amount to achieve the desired concentration.
Gels comprise a base selected from an oleaginous base, water, or an emulsion-
suspension base. To the base is added a gelling agent that forms a matrix in
the
base, increasing its viscosity. Examples of gelling agents are hydroxypropyl
cellulose, acrylic acid polymers and the like. Customarily, the agonist is
added to
the formulation at the desired concentration at a point preceding addition of
the
gelling agent.
The amount of compound incorporated into a topical formulation is not
critical; the
concentration should be within a range sufficient to permit ready application
of the
formulation to the affected tissue area in an amount that will deliver the
desired
amount of agonist to the desired treatment site.
The customary amount of a topical formulation to be applied to an affected
tissue
will depend upon an affected tissue size and concentration of the agonist in
the
formulation.
In therapeutic applications, compositions of the invention are administered to
a
subject afflicted with cancer in an amount sufficient to at least improve the
condition of the patient and preferably cure the patient of cancer.
Single or multiple administrations of the compositions can be carried out with
dose levels and pattern being selected by the treating physician or
veterinarian.
In any event, the composition of the invention should provide a quantity of
the
compounds of the invention sufficient to effectively treat the cancer in the
subject.
Antibodies
CA 02475066 2004-08-04
WO 03/066679 PCT/AU03/00135
-20-
Antibodies that specifically recognize one or more epitopes of RBP, or
epitopes of
conserved variants of RBP, or peptide fragments of the RBP are also
encompassed by the invention. Such antibodies include but are not limited to
polyclonal antibodies, monoclonal antibodies (mAbs), humanized or chimeric
antibodies, single chain antibodies, Fab fragments, F(ab')2 fragments,
fragments produced by a Fab expression library, anti-idiotypic (anti-Id)
antibodies,
and epitope-binding fragments of any of the above.
The antibodies of the invention may be used, for example, in the detection of
the
RBP in a biological sample and may, therefore, be utilized as part of a
diagnostic
or prognostic technique whereby patients may be tested for abnormal amounts of
RBP. Such antibodies may also be utilized in conjunction with, for example,
compound screening schemes, as described herein for evaluating the effect of
test compounds on the ability of RBP to bind its ligand. Additionally, such
antibodies may be used to inhibit RBP activity that may be useful in various
studies on the dynamics of the binding between the RBP and its ligand.
For the production of antibodies, host animals may be immunized by injection
with
the RBP or an immunogenic portion thereof such as one corresponding to a
functional domain of the RBP, e.g. the extracellular domain. Host animals may
include but are not limited to rabbits, mice, and rats, to name but a few.
Various adjuvants may be used to increase the immunological response,
depending on the host species, including but not limited to Freund's (complete
and incomplete), mineral gels such as aluminium hydroxide, surface active
substances such as lysolecithin, pluronic polyols, polyanions, peptides, oil
emulsions, keyhole limpet hemocyanin, dinitrophenol, and potentially useful
human adjuvants such as BCG (bacille Calmette-Guerin) and Corynebacterium
parvum. Polyclonal antibodies are heterogeneous populations of antibody
molecules derived from the sera of the immunized animals.
Monoclonal antibodies may be obtained by any technique that provides for the
production of antibody molecules by continuous cell lines in culture. These
include, but are not limited to, the hybridoma technique of Kohler and
Milstein,
CA 02475066 2004-08-04
WO 03/066679 PCT/AU03/00135
-21 -
(1975, Nature 256:495-497; and U.S. Pat. No. 4,376,110), the human B-cell
hybridoma technique (Kosbor et al., 1983, Immunology Today 4:72; Cole et al.,
1983, Proc. Natl. Acad. Sci. USA 80:2026-2030), and the EBV-hybridoma
technique (Cole et al., 1985, Monoclonal Antibodies And Cancer Therapy, Alan
R.
Liss, Inc., pp. 77-96). Such antibodies may be of any immunoglobulin class
including IgG, IgM, IgE, IgA, IgD and any subclass thereof. The hybridoma
producing the monoclonals of this invention may be cultivated in vitro or in
vivo.
Production of high titres of monoclonals in vivo makes this the presently
preferred
method of production.
In addition, techniques developed for the production of "chimeric antibodies"
(Morrison et al., 1984, Proc. Natl. Acad. Sci., 81:6851-6855; Neuberger et
al.,
1984, Nature, 312:604-608; Takeda et al., 1985, Nature, 314:452-454) by
splicing
the genes from a mouse antibody molecule of appropriate antigen specificity
together with genes from a human antibody molecule of appropriate biological
activity can be used. A chimeric antibody is a molecule in which different
portions
are derived from difFerent animal species, such as those having a variable
region
derived from a murine monoclonal and a human immunoglobulin constant region.
Alternatively, techniques described for the production of single chain
antibodies
(U.S. Pat. No. 4,946,778; Bird, 1988, Science 242:423-426; Huston et al.,
1988,
Proc. Natl. Acad. Sci. USA 85:5879-5883; and Ward et al., 1989, Nature 334:544-
546) can be adapted to produce single chain antibodies against the RBP. Single
chain antibodies are formed by linking the heavy and light chain fragments of
the
Fv region via an amino acid bridge, resulting in a single chain polypeptide.
Antibody fragments that recognize specific epitopes may be generated by known
techniques. For example, such fragments include but are not limited to: the
F(ab')2 fragments which can be produced by pepsin digestion of the
antibody
molecule and the Fab fragments which can be generated by reducing the
disulfide
bridges of the F(ab')2 fragments. Alternatively, Fab expression libraries
may
be constructed (Huse et al., 1989, Science, 246:1275-1281 ) to allow rapid and
easy identification of monoclonal Fab fragments with the desired specificity.
CA 02475066 2004-08-04
WO 03/066679 PCT/AU03/00135
-22-
Antibodies to the RBP can, in turn, be utilized to generate anti-idiotype
antibodies
that "mimic" the RBP, using techniques well known to those skilled in the art.
(See, e.g., Greenspan & Bona, 1993, FASEB J 7(5): 437-444; and Nissinoff,
1991, J. Immunol. 147(8):2429-2438). For example, antibodies that bind to the
RBP and competitively inhibit the binding of rhamnose to the RBP can be used
to
generate anti-idiotypes that "mimic" the extracellular domain of the RBP and
therefore bind rhamnose.
The present invention will now be described with reference to a number of
examples. The examples are in no way limiting on the preceding description.
Examples
Example 1: Isolation of a rhamnose binding protein using affinity
chromatography
Materials/Methods
1. Labelling of rhamnose probes
Biotin Rhamnose-ITC (BRITC) was formed by dissolving Rhamnose-ITC (Sigma
86881; RMM 297.3) in DMSO, diluting it to 1 mg/ml in 1 OmM sodium bicarbonate
,
pH 9.1 and then adding biotin hydrazide (Sigma, RMM 258.3) at 1:1 or 5:1 molar
ratio and allowing the reaction to proceed at room temperature for 16h.
2. Preparation of rhamnose affinity column
Streptavidin sepharose conjugated columns (Amersham 17-5112-01 ) or free resin
(17-5113-01 ) with a theoretical capacity for biotin labelled rhamnose (BRITC)
of
60pg/ml was used. An excess amount of BRITC was dissolved in phosphate
buffered saline (PBS) and circulated over the pre-equilibrated column at a
flow
rate of 0.2m1/min for 30min. Successful coupling of the BRITC was monitored by
HPLC analysis of the BRITC-PBS solution.
3. Celllysis
CA 02475066 2004-08-04
WO 03/066679 PCT/AU03/00135
-23-
Packed, washed cells were lysed by freeze thawing (-80°C / 4
°C) followed by
brief sonication (30-40 sec at 50% duty pulse using 375W sonicator fitted with
microtip probe). Cells were lysed in the presence of protease inhibitor
cocktail
(Roche 1-836-170) in order to minimise proteolysis.
4. Multiple Surfactant Solution (MSS)
MSS comprises 5 M urea, 2 M thiourea, 0.002 M n-tributyl phosphine, 0.5% pH 3-
Pharmalyte carrier ampholytes (Pharmacia, Uppsala) [only in 2-D
preparations], 2% 3-([3-cholamidopropyl]-dimethylammonio)-1-propanesulfonate
(CHAPS), 2% caprylyl sulfo-betaine, and 0.001 % Orange G dye. Material was
10 also treated with endonuclease EC 3.1.30.2 in order to eliminate
contaminating
DNA.
5. One-dimensional polyacrylamide gel electrophoresis (1 D-PAGE)
Pre-cast Tris-HCI 4-20% polyacrylamide gradient gels (Bio-Rad) were used with
electrode buffer Tris/glycine, pH 8.3. Sample loading solution: Tris pH 6.8,
0.1
SDS, glycerol, dithiothreitol, bromophenol blue marker.
Electrophoresis conditions: 100V for 90min.
6. Protein visualisation in-gel
This was accomplished either by staining with silver, or with Coomassie 8250
in
water/ methanol/acetic acid. Fluorescence was visualised using a Fluoro-imager
(Pharmacia).
7. Affinity chromatography
Whole cell lysis preparations were prepared using MSS on 10$ - 109 A2058
cells.
The solubilised protein was then diluted 1/50 into HEPES buffered saline
containing 140mM NaCI, 2mM MgCl2, and 2mM CaCl2, pH 7.4 (HBS2+) and
passed sequentially over a control column (no rhamnose) and the rhamnose
affinity column. Each column was washed with HBS2+ and eluted with 100mM
CA 02475066 2004-08-04
WO 03/066679 PCT/AU03/00135
-24-
rhamnose. Fractions were analysed using acrylamide mini-gels and 1 D SDS-
PAGE, followed by silver staining.
Results
A band with a molecular weight of approximately 65kD was visualised in the
eluent fractions (Figure 1 ). No bands were visible in eluent from a
corresponding
control column that did not contain BRITC (results not shown).
Example 2: Cross-linking the rhamnose binding protein and its ligand
MaterialslMethods
(a) Labelling of rhamnose probes
Fluorescein Rhamnose-ITC (FRITC) was formed by reacting Rhamnose-ITC with
fluorescein amine (Sigma F1148, RMM 347.3) at a molar ratio of 1:10 in 10mM
sodium bicarbonate pH 9.1.
(b) Cell lysis
As per example 1.
(c) Multiple Surfactant Solution (MSS)
As per example 1.
(d) One-dimensional polyacrylamide gel electrophoresis (1 D-PAGE)
As per example 1.
(e) Protein visualisation in-gel
As per example 1. ,
CA 02475066 2004-08-04
WO 03/066679 PCT/AU03/00135
-25-
(f) Cell probing
A2058 cells were coated at low density in a microscope compatible chamber.
The cells were washed with HBS2+ then incubated with FRITC (top concentration
of DMSO = 2.5%) for 5-15min at 37°C. The FRITC was removed, the cells
washed twice with HBS2+ and examined under a visible light microscope. The
incubation was repeated with 0-100pM fluorescein-amine, and for 25 and 6pM
FRITC using a 103x excess of unlabelled rhamnose.
(g) Cell Surface Receptor Cross-Linking
A2058 cells prepared in 40m1 culture flasks were incubated with FRITC (5 or
10pM) as described above and washed once with HBS2+. Carbonyl ~di-imidazole
(Aldrich 115533) was dissolved at 1 M in DMSO immediately prior to use. This
stock solution was then diluted in HBS2+ or DMSO to 100pM-10mM and added to
the cells at room temperature. After 15min the cross-linker was removed and
the
flasks stored on ice. Cells were then removed from the flasks by scraping and
taken up into MSS lysis buffer. Protein fractions were subjected to SDS-PAGE
and the gels visualised as set out above.
(h) Two Dimensional Electrophoresis
Cross linked cells prepared according to method 7 above, using 5pM FRITC,
were lysed into 350p1 of MSS by cell scraping. This material was prepared for
iso-electric focusing by loading onto an 18cm Immobiline DryStrip, pH 3-10
[Amersham], the strip equilibrated and electrophoresed for approx. 150kVhr
(Voltage gradient: 200V, 12hr; 250V, 1.5hr; 500V, 2hr; 1000V, 2.5hr; 8000V,
19hr).
The strip was then run in the second dimension using 1-D PAGE according to
method 4 above, except a 10% polyacrylamide gel was used. The gel was
analysed using a fluoroimager and silver stained.
Results
CA 02475066 2004-08-04
WO 03/066679 PCT/AU03/00135
-26-
The electrophoretic profile of the protein components from the cross linking
experiments are set out in Figure 2A and 2B. Figure 2A depicts the proteins
cross-linked to fluorescein that were visualised by fluorescence scanning and
Figure 2B depicts the total protein stained with Coomassie brilliant blue.
The total protein stain indicates that there are approximately equal amounts
of
protein loaded in each lane. Following FRITC incubation two fluorescently
labelled proteins are detectable that are not present in the CDI only lanes.
Calibration of the gel using the molecular mass markers (Figure 3) gives
masses
of 22kD and 68kD for these proteins. However, these masses include one or
more FRITC molecules and consequently the mass of the receptor is
approximately 67kD.
The results from the two dimensional electrophoresis suggest the fluorescein
tagged protein is running with a pl of approximately 6 - 7, and the molecular
mass
is consistent with prior results.
Example 3: Staining of cells with a fluorescein tagged rhamnose probe (FRITC)
Materials/Methods
A fluorescein tagged rhamnose probe (FRITC) was prepared as previously
described and used to stain A2058 cells.
FRITC at concentrations from 3-25pM was incubated with A2058 cells for 15
minutes at 37°C.
Results
The cells following incubation were found to fluoresce confirming the presence
of
a rhamnose binding protein on the cells. An image of the cells is depicted in
Figure 4 and closer inspection of the stained cells indicates an increased
concentration of staining in the cell nucleus, suggesting the rhamnose probe
is
also taken inside the nuclear membrane. It was found that the staining could
be
CA 02475066 2004-08-04
WO 03/066679 PCT/AU03/00135
-27-
inhibited by co-incubation of the FRITC and cells with free rhamnose at 10mM
concentration.
Example 4 - Effect of Cell Density on Measured LDSO Values
Materials/Methods
Given that the evaluation of the cytotoxicities for different cell lines
needed to be
conducted using different sized cell populations, it was considered prudent to
determine the effect, if any, of cell number on the measured value of LDSO.
Five
cell lines, HT-29, LS174-T, 5637, A431 and MCF-7 were evaluated at four
different seeding cell densities. Hs578T and CCD 18Lu were evaluated at three
seeding densities and Hs578Bst, both early and late passage cells, were
evaluated at two seeding densities.
In order that the full range of cell densities be evaluated with at least one
cell line,
a 3-day version of the cytotoxicity was developed. The cell lines involved
were
recalibtrated for the 3-day format. Multiwell plates were seeded on day 1.
Cells
were treated with BEC~ on day 2 and MTT was added twenty four hours later.
Results
Plotting the relationship between dose per cell at LDSO and the Day 1 cell
density
for each cell line, Figure 5, reveals that the behaviours of the epidermoid
adenocarcinoma A431, the colorectal adenocarcinoma HT-29 and the normal
infant lung fibroblast line, CCD 18Lu, are identical. Similarly, the plots for
the
colon adenocarcinoma LS174-T and the bladder carcinoma 5637 are almost
coincident.
In fact, data from CCD 18Lu, A431, HT-29, LS174T and 5637 can be combined
and fitted to a single exponential function,
Dose per seeded cell at LDSO = Intercept X a k x Seeding density + Limit
as shown in Figure 6.
CA 02475066 2004-08-04
WO 03/066679 PCT/AU03/00135
-28-
This implies that, for these five cell lines the processes of BEC~ uptake,
including
receptor affinity, hydrolytic processing to produce the lysogenic ligand
complex,
as well as the pathway to cell death, are quantitatively identical.
Similarly, data from the two breast cancer lines, Hs578T, an infiltrating
ductal
carcinoma and MCF-7, a metastasis from a breast adenocarcinoma, appear to
behave similarly to each other but differently from the other lines. These
combined data sets can also be fitted to an exponential function, see Figure
7.
Comparing the two fitted functions, two distinct regions are discernable
(Figure 8).
The functions are virtually coincident at cell densities of 1,500 cells per
well and
below, conditions under which receptor affinity can be expected to be the
major
determinant of cytotoxicity (region 1 of figure 8). This suggests that only a
single
type of receptor is involved in virtually all cell lines included in this
study. We
estimate that the dissociation constant for this receptor is likely to be of
the order
of1.5x10-6M.
However, the functions diverge at cell densities greater than 1,500 cells per
well
(region 2 of figure 8). The difference between the limit values, representing
the
minimum dose per cell to kill 50% of the susceptible population, is obvious.
For
LS174-T and 5637 the fitted value of this minimum dose is 300 pg BEC~/cell (71
pg solamargine/cell) while for the breast cancer lines this minimum dose is
some
three-fold higher at 580 pg BEC~/cell (137 pg solamargine/cell). Such a
difference could arise from either a significantly lower number of receptors
per cell
or slower intracellular processing to produce the isolated ligand complex.
Note from Figure 5 that the behaviour of early passage normal breast
fibroblasts
differs from late passage cells of the same line. Within the limitations of
the
restricted data sets these non-tumour cells appear to become more vulnerable
to
BEC~ as they approach senescence.
Example 5 - Single Point Data for Other Cell Lines
CA 02475066 2004-08-04
WO 03/066679 PCT/AU03/00135
-29-
Materials/methods
A range of other cell lines were assessed in a similar manner to the
assessments
carried out in Example 4.
Results
Figure 10 shows that the single data points for the other cell lines evaluated
are
plotted, with the exception of MIA PaCa-2, the breast cancer lines and the
early
passage normal breast fibroblasts, all fall on the same exponential curve of
Figure
6.
Example 6 - Co-administration of BEC~ and Rhamnose
Materials/methods
A2058 cells at two different cell densities were treated with BEC~ +/-
rhamnose
and cell survival was monitored after 4 days. The treatments comprised: (i)
BEC~ only for 4 days (ii) BEC~ only for 5 minutes (iii) BEC~ and 5mM rhamnose
for 4 days and (iv) BEC~ and 5 mM rhamnose for 5 minutes.
Results
Figures 11 and 12 indicate that rhamnose competition with BEC~ uptake is more
readily observed at the higher cell density (5000 cells). Under these
conditions,
where the amount of BEC~ available to each cell is a major factor determining
LDSO, the presence of rhamnose at the relatively high concentration of 5 mM
affects the amount of BEC~ taken up by the cells in both 5 minutes and 4 days
from solutions in specific concentration ranges. Data in Figure 12 suggests
that
the rhamnose protective effect is more significant in the pulsed treatment
experiment.
Example 7 - Isolation of RBP
Materials/Methods
CA 02475066 2004-08-04
WO 03/066679 PCT/AU03/00135
-30-
1. Preparation of FRITC
The following reaction mixture was used:
Rhamnose 5mg/ml in 500p1 DMSO
Isothiocyanate [Sigma
86881 ]
Fluorescein [Sigma 50mg in 500p1
F 1148]
100mM sodium 120p1
hydrogen carbonate
MiIIiQ water 80p1
Incubate overnight at room temperature and store at -20°C. Purify FRITC
by RP-
HPLC:
Column: C18 reverse phase
Solvent A: water + 0.06% tri-fluoro acetic acid (TFA)
Solvent B: 80% acetonitrile (ACN)/ water + 0.06% TFA
Analytical gradient 98-50% (A) over 15min. Flow 1 ml/min. Wavelength
255nm or 220nm.
Preparative gradient 98% (A) for 2.5min., 98-50% (A) over 20min. Flow
2ml/min. Wavelength 255nm or 220nm.
2. Cell Surface Receptor Cross-Linking
A2058 cells were grown to 80-90% confluency in 25m1 culture flasks and then
washed with 2x HEPES buffered saline containing 140mM NaCI, 2mM MgCl2, and
2mM CaCl2, pH 7.4 (HBS2+). FRITC was dissolved in a minimal volume of DMSO
(5-10p1) and added to 1ml HBS2+ to,give a concentration of 2-10pM FRITC. The
FRITC solution was added to the cells and the flask incubated for 15min at
37°C.
The FRITC was removed, the cells washed once with HBS2+, and freshly
prepared carbonyl di-imidazole (100pM in 1 ml DMSO) was added immediately at
room temperature. After a minimum of 15min the cross-linker was removed and
the flasks stored on ice.
3. Protein extraction following Receptor Cross-linking
CA 02475066 2004-08-04
WO 03/066679 PCT/AU03/00135
-31 -
All the DMSO is aspirated from the cells and 300-400p1 of MSS (section 10) is
added to the flask. The MSS is spread over the entire surface area of the
flask
and the cells then scraped using a cell scraper/harvester. The cells in MSS
are
allowed to incubate for 15min at room temperature to remove as much of the
protein as possible.
Cell extracts are removed from the flask and added to a fresh tube. The
protein is
precipitated by adding 1 ml of methanol (or acetone) and storing the sample
over
night at -80°C. To remove viscous material (e.g. DNA, lipid etc.) the
tube was
centrifuged for 30min at 13000rpm and 4°C. The methanol was removed and
the
sample resuspended in 300p1 of MSS. To this 1.2 ml of hexane was added and
the sample again centrifuged for 30min at 13000rpm and 4°C. The top
layer was
discarded and any white particulate matter on the surface of the aqueous layer
was also removed. Samples were then analysed by SDS-PAGE.
4. Immunoprecipitation of FRITC cross-linked proteins
100p1 of protein extract (section 1 above) was diluted to 10m1 with 50mM Tris-
CI
pH7 and 0.05% Tween 20. In order to pre-clear non-specific binding material
50p1
of protein A sepharose [Amersham] was added and incubated overnight at
4°C on
a rotating wheel. After incubation, the sample was centrifuged at 2000rpm for
5min to pellet the sepharose. The supernatant was added to 10p1 of anti-FITC
antibody [Sigma], 50p1 of protein A sepharose and incubated overnight at
4°C on
a rotating wheel. After incubation the sepharose was washed 2x1 ml with 10mM
Tris pH 7 and the whole sample (matrix included) was run on 4-20% SDS-PAGE.
The gel was then assessed for FRITC labelled proteins by fluorescent
detection.
5: 1 D PAGE was carried out as in Example 1.
6. Protein visualisation in-gel
To detect fluorescently labelled proteins gels were scanned using a Fluoro-
imager
(Pharmacia): fluorescein excitation wavelength 494nm, emission wavelength
520nm.
CA 02475066 2004-08-04
WO 03/066679 PCT/AU03/00135
-32-
Visual staining was accomplished either with Coomassie 6250 in
water/methanol/acetic acid or silver staining (PI in-house mass spectrometry
compatible protocol; under optimised conditions this is approximately 10-fold
less
sensitive than previously used methods).
Results
1. Preparation of FRITC (Fluorescein Rhamnose Iso-thiocyanate)
Large quantities of fluorescein labelled rhamnose probe (FRITC) were purified
by
HPLC and their viability confirmed by mass spectrometry. The probe appears
stable indefinitely if stored dry at -20°C.
2. Cell Surface Receptor Cross-Linking
Cross linking of FRITC to the surface of A2058 cells was performed
successfully
in 25m1 culture flasks. Large scale FRITC cross-linking (75m1 flasks) using
identical concentrations of reagent was unsuccessful. This suggests the
reaction
is readily influenced by micro-changes in the cell environment. Our
observations
also indicated that an advantageous side effect of using carbonyl di-imidazole
cross linker was that the cells became adhered to the flask surface during the
procedure and hence were easier to wash.
A cross-linked FRITC protein complex was consistently visible on SDS-PAGE
gels by fluorescent imaging (Figure 13).
3. Protein extraction following Receptor Cross-linking
Our procedures have focused on maximising the yield of the FRITC-receptor
complex, and subsequently isolating the complex from unwanted contaminants.
The extraction procedures have involved different protein precipitation
methods
and subsequent solubilisation steps. The A2058 Receptor-FRITC complex
appears fully soluble in multiple surfactant solution (SPRL21111 ), and
partially
soluble in a range of non-ionic detergents (2% Tween 20; 2% Triton X-100, 2%
CA 02475066 2004-08-04
WO 03/066679 PCT/AU03/00135
-33-
CHAPS), however no single non-ionic detergent has been identified that fully
solubilises the complex.
Further, the fluorescent complex appears to be associated with the cell
debris/DNA that is precipitated during the initial methanol precipitation. To
overcome this problem (of contamination and viscosity) we have developed a two-
stage clean-up using methanol, followed by hexane.
4. Immunoprecipitation of FRITC cross-linked proteins
The protein-FRITC complex was diluted into a low detergent, low salt buffer
and
incubated with an antibody directed against fluorescein. Any complexes formed
were absorbed onto protein A, precipitated and analysed by SDS-PAGE.
The experiments produced a feint protein band at approx 70kD that was
detectable by Coomassie blue staining Figure 14).
Further modifications and adaptations not specifically disclosed herein that
are
apparent to those skilled in the art upon reading this specification are
encompassed within the scope of this invention.
CA 02475066 2004-08-04
WO 03/066679 PCT/AU03/00135
-34-
References
1. Ashwell, G and Harford, J. (1982) "Carbohydrate specific receptors of the
Liver". Ann Rev. Biochem, 51, 531 - 554.
2. Lehrman, MA. et al (1986) "The binding of fucose containing glycoproteins
by
hepatic lectins". J. Biol. Chem., 261 (16) 7412 - 7418.
3. Kolb - Bachofen, V, et al (1984) "Gal/NAC/Gal specific rat liver lectins
their
role in cellular recognition". Biol. Cell, 51, 219 - 226.
4. Cramer, F. and Gabius, HJ. (1991) US Patent 5,225,542.