Note: Descriptions are shown in the official language in which they were submitted.
CA 02475069 2004-08-20
AMINE DERIUATTYES AS PROTEASE INHIBITORS
THE INVENTION
This application relates to compounds and compositions for treating.diseases
associated
with cysteine protease activity, particularly diseases associated with
activity of cathepsins B, K,
LorS.
DESCRIPTION OF THE FIELD
Cysteine proteases represent a class of peptidases characterized by the
presence of a
cysteine residue in the catalytic site of the enzyme. Cysteine proteases are
associated with the
1 Q normal degradation and processing of proteins. The aberrant activity of
cysteine proteases, e.g.
as a result of increased expression or enhanced activation, however, may have
pathological
consequences. in this regard, certain cysteine proteases are associated with a
number of
disease states, including arthritis, muscular dystrophy; inflammation, tumor
invasion,
giomerulonephritis, malaria, periodontal disease, metachromatic Ieukodystrophy
and others. For
i5 example; increased cathepsin B levels and redistribution of the enzyme are
found in tumors;
thus, suggesting a role for the enzyme in tumor invasion and metastasis. in
addition, aberrant
cathepsin B activity is implicated in such disease states as rheumatoid
arthritis, osteo arthritis,
pneumocystis carinii, acute panereatitis, inflammatory airway disease and bone
and joint
disorders.
20 The prominent expression of cathepsin K in osteociasts and osteoclast-
related
multinucleated cells and its high collagenolytic activity suggest that the
enzyme is involved in
ososteoclast-mediated hone resorption and, hence, in bone abnormalities such
as occurs in
osteoporosis. In addition, cathepsin K expression in the lung and its
elasrinolytic activity suggest
that the enzyme plays a role in pulmonary disorders as well.
25 Cathepsin L is implicated in normal lysosomal proteolysis as well as
several disease
states, including, but not limited to, metastasis of melanomas. Cathepsin S is
implicated in
Alzheimer's disease and certain autoimmune disorders, including, but not
limited to juvenile
onset diabetes, multiple sclerosis, pemphigus vulgaris, Graves' disease,
myasthenia gravis,
systemic lupus erythemotasus, rheumatoid arthritis and Hashimoto's
thyroiditis; allergic
-1-
CA 02475069 2004-08-20
disorders, including, but not limited to asthma; and allogeneic invnune
responses, including, but
not limited to; rejection of organ transplants or tissue grafts.
In view of the number of diseases wherein it is recognized that an increase in
cysteine
protease activity contributes to the pathology andlor symptomatology of the
disease, molecules
which are_shown to inhibit the activity of this class of enzymes, in
particular molecules which
are inhibitors of cathepsins B, K, L andlor S, will be useful as therapeutic
agents.
SUMMARY OF THE INVENTION
In one particular embodiment, the present invention relates to protease
inhibitors ~f
Formula I:
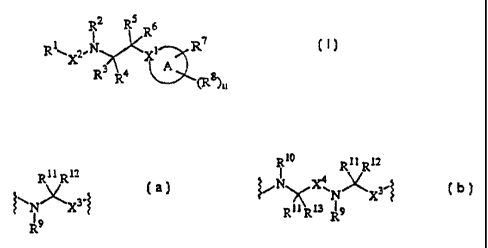
RZ R5 ~
R
RlXZ.N Xt R7
R3 4 A
. R (R8)n
in which:
A comprises a heteromonocyclic ring containing 5 to 6 ring member atoms or a
fused
heteropolycyclic ring system containing S to 14 ring member atoms, wherein
each ring contains
S to 7 ring member atoms, X' is a ring member carbon atom and each ring member
atom other
I5 than X' is a carbon atom or a heteroatom, with the proviso that (i) at
least one ring member
atom is a heteroatom and (ii) when A is a heteromonocycIic radical containing
5 ring member
atoms, no more than two of the ring member atoms comprising A are heteroatoms;
nis0, l,2or3;
X' is =C- or -CH-;
X2 is a bond or a divalent group of Formula (a) or (b):
R11 R12 R20 Ril R12
~\~N~x3-~ ~~N X~~N~,Xg
R9 R11 Rt3
(a) (b)
wherein:
_2_
CA 02475069 2004-08-20
X3 and X4 independently are -C(O)- or -CH2S(0~~~
R9 and R'° independently are hydrogen, (C,.~alkyY or as defined
below;
R" at each occurrence independently is hydrogen or (C,_6)alkyl;
R'Z and R'3 independently are (i) (C~.~)alkyl optionally substituted with
cyano,
halo, vitro, 'NR'4R14~ _~14(~(O)ORl4' _~t4C(O)NR'4R'49 -NR'4C(NR'4)NR'4R'4,
_~RI4~ _~R14~ _C(o)OR14~ _C(O)~I4RI4' _~'(~)2~t4R14~ -~.1(p)(~R14)OR'4,
-OP(O)(OR'4)OR'4, -NR'4C(O)Rts~ _S(O)Rts~ -S(O)aRls~ _C(O)Rls~ _ORIS~ -sR,6~
_S(O)Rts~ _S(O)zRle~ _C(O)Rts~ _C(O)ORts~ _OC(O)Rn~ _yaRn' _~t7C(O)Rte~
_NRt7C(O)ORts~ _C(~)~16R27~ _S(O)~'°Rn~ -NRnC(O)NR'6Rn or
-NR"C(NR")NR'6R", wherein R'4 at each occurrence independently is hydrogen,
(C,_6)alkyl or halo-substituted (CI_3)alkyl, R's is (C,~)alkyl or halo-
substituted
(C,_3)alkyl, halo, (C,~)alkyl or R'6 is (C3_,a)~Ycloalkyl(C~)alkyl,
hetero(C3.~2)cycloalkyl(C~)alkyl, (C~,Z)aryl(Cab)alkyl,
hetero(Cs.,Z)aryl(C~)alkyl,
(C~.,~polycycloaryl(C~)aIkyl ar hetero(Cs_t2)polycycloaryl(C~)alkyl and R" is
1~ hydrogen or (C»)alkyl, and wherein within R'6 said cycloalkyl,
heterocycloalkyl, aryl,
heteroaryl, polycycloaryl or heterpolycycloaryI ring optianally is substituted
by a group
selected from -R's, -XSOR's, -XsSR's, -XsS(O)R's, -XsS(O)ZR'a, -XsC(O)R's,
-XsC(O)OR's, -XsOC(O)R's, _XsNR'$Rt9~ _Xs~lsC(O)Rls9 _XsNRt9C(O)ORts~
_XSC(O)~18RI9~ _X5~(O~2NRt8R19~ _XS~t9C(O)NRtaRl9 or
-XsNR'9C(NR'9)NR'sR'9, wherein Xs is a bond or (C;~,)alkylen~, R's is hydrogen
or
(C,~)alkyl and R'9 is (C3_,2)cycloalkyl(Co~)alkyl,
hetero(C3_=2)cycloalkyl(Ca6)alkyl,
(C~,2)aryl(C~)alkyl, hetero(C~12)aryl(C~)alkyl, (Cø~2)polycycloaryl(C~)alkyl
or
hetero(Cs_t2)polycycloaryl(C~)alkyl, or (ii) a group selected from
(C~,2)cycloalkyl(C~)alkyl, hetero(C3_t2)cycloalkyl(C~)alkyl,
(C~l~aryl(Co.,b)alkyl,
hetero(Cs.l2)aryl(Co-b)alkyl, (C~l~polycycloaryl(C~)alkyl and
hetero(Cs.l~polycycloaryl(C~)alkyl, wherein said cycloalkyl, heterocycloalkyl,
aryl,
heteroaryl, polycycloaryl or heterpolycycloaryl ring optionally is substituted
by a group
selected from -R's, -XsOR's, -XSSR's, -XsS(O)R's, -XsS(O)zR's, -XsC(O)R's,
-XsC(O)ORIS' _XsOC(O)Rte9 _XSNR18RI9~ -Xs~tsC(O)Rts~ _Xs~tvC(O)ORIS~
-XSC(O)NR'sR'9, -XSS(03zNR'sR'9, -XSNR'9C(O)NR'sR'9 or
-X'NR'9C(NR'9)NR'$R'~, wherein Xs, R's and R'9 are as defined above; wherein
within R'Z andlor R'3 any alicyclic or aromatic ring system present may be
substituted
-3-
CA 02475069 2004-08-20
further by I to S radicals independently selected from (C,,~)alkyl,
{C»)alkylidene,
cyano, halo, halo-substituted (C,.,aaikyl, vitro, -XsNR'4Rz4, -
XsNR'4C(O)OR'.4,
_XsNRI4C~O)NR14R14~ _XSN'R14C~Ri4)~14R14~ _XSOR14~ _XSSRi4~ -XsC(O)oRl4'
_XsC(O)y4Rta~ -xsS(O)z~~4R~i~4~~ _Xsp(O)(OR'4)OR'4, -X'OP(O){OR'4)OR'4,
_Xsy4C(O)RIS~ -Xss(O)RIS~ _xsS(O)zRu ~d -XsC(O)RIS~ wherein Xs, R14 and R's
are as defined above; or
R'2 together with R9 andlor R's together with R'° form
trimethylene;
tetramethylene or phenylene-1,2-dimethyiene; optionally substituted with 1 to
3 radicals
independently selected from (C,.~)alkyl, (C,,~alkylidene, cyano, halo; halo-
substituted
70 (C,~,)alkyl, vitro, oxo, -XSNR'4C(O)OR'4, -XsNR'4C(O)NR'4R'$,
_X5~14C~~14}NR'''RI49 -XSOR14~ -XS~R14~ -XsC(O)OR'4, -XsC(O)NR74Rla~
-XsS(O)2NR'°R'4, -XSP(O)(OR'4)OR'4, -XsOP(O)(OR'4}OR'4, -
XsNR'°C(O)R's,.
-XsS(O}R's, -XsS(O)zIt's and -XsC{O)R's, wherein Xs, R'4 and R's are as
defined
above; and
R' is -X6X'R~°, wherein Xg is -C(O)-, -C(O)C(O)- or -S(O)2-, X' is a
bond, -O- or
-NRZ'-, wherein R2' is hydrogen or (CI~)alkyl, and R~ is (i) {C,,~)alkyl
optionally substituted by
cyano, halo, vitro, -NR'°R'4, -NR'aC(O)OR'4, -NR'4C(O)NR'°R14' -
~r4Cy4)NR'4R'4,
_ORt4~ -SR14' -C(O)ORi4' -C(O}NR14R14~ -S(O)INRI4RI4~ -p(O)(OR'4)OR'4,
-OP{O)(OR'4)OR'", -NR'4C(O)R~s~ -S(O)Ras' _S(O)xRls~ -C(o)RIS~ _pltz2, _SR'~, -
S{O)R~,
-S(O)zR22, -C(O)RD, -C(O)ORS, -C(O)NR~R~, -NRzzR~, -NR~C(O)R'~, -NR23C(O)ORu,
-NR23C(O)NRZZR23 Or -NRz3C(NR23)NRZZR~, wherein R''' and R's are as defined
above, R~
is (C3_i_)cycloalkyl(Co~,)alkyl, hetero(C~.,~cycloalkyl(C~)alkyl,
(C~.,2)aryl(C~)alkyl,
hetero(Cs.;z)aryl(C~)alkyl, (C9.,~}bicycloaryl(Co.~)aIkyl or
hetero(C8_~Z)bicycloaryl(C,~..6)alkyl
and R~ at each occurrence independently is hydrogen or (C~~)alkyi, ar
(ii) {C3_iz)cycloalkyl(C~)alkyI, hetero(C~.,~cycloalkyl(C~)alkyl,
(C~.l~aryl(C~,6)alkyI,
hetero(Cs_,~aryl(C~)alkyl, (Cg_~2)bicycloaryl(C~)alkyl or
hetero(C$_a2)bicycloaryl(C~)alkyl or
(iii) (C~)cycloalkyl(Co.~)alkyl, hetero(C~)cycloalkyl(C~)alkyl, phenyl(C~alkyl
or
hetero{Cs_b)aryl(C~alkyl, wherein said cycloalkyl, heterocycloalkyl, phenyl or
heteroaryl is
substituted by -R'~, -XsOR~°, -XsSR'~, -XSS(O)R2°, -X5S(O)ZR~, -
XsC(O}R~', -XsC(O)OR~°,
-X5C(O)NR~'R's, -XSNRz'R=s, -X3NR~C(O)R~'', -XsNRzsC(~)OR24, -XSNR2sC(O)NR~R~
or -XSNR'~C(NR~)NR~°Ru; wherein Xs is as defined above, R~ is
(C3,~)cycloalkyl(C~)alkyl,
hetero(C~)cycloalkyl(C~)alkyl, phenyl(C,~)alkyl or hetero(Cs~aryl(C~)alkyl and
R~ at each
-4-
CA 02475069 2004-08-20
occurrence independently is hydrogen or (C,.~)alkyl; wherein within R' any
alicyclic or aromatic
ring system present may be substituted further by i to 5 radicals
independently selected from
(C,.~)alkyl, {C,~)alkylidene, cyano, halo, halo-substituted (C,~,)alkyl,
nitro, -XsNR'4R'4,
_X3~14C~O)OR14~ -XsNRI4C{o)NR14R14~ _X5~14C~)4)~14R14~ -X5o~14~ -XSSR14~
-XsC(O~~R14~ _XsC(O)NR14R14~ -XSS{Oy'4R14~ -Xsp(O){OR'4)OR'4,
-XSOP(O)(OR'4)OR'4, -XSNR'4C(O)R's, -XsS{0)R's, -XsS(O)2Rls ~d -XsC(0)R's,
wherein
Xs, R'4 and R's are as defined above; or when X2 is a divalent group of
formula (a) or (b) then
R' may also represent hydrogen, carboxy, oxalo or carbamoyl;
R= is hydrogen or (C,.b)aIkyl;
R3 is (i) (C,~)alkyl optionally substituted with cyano, halo, vitro, -SRzb, -
C{O)OR~,
_C(O)NR2sR26, -p(O)(ORzb)OR26, -OP(O)(0Rz6)OR~, -S(0)R27, -S(0)zRzy or -
G(O)Rz',
wherein R~ at each occurrence independently is hydrogen, (C,~)alkyl or halo-
substituted
(C,-3)alkyl and Rr' is (C,~)alkyl or halo-substituted (C,_3)alkyl, or (ii)
(Cs~cycloalkyl{Cz_3)alkyl,
hetero(C3~)cycloalkyl(Cz_3)alkyl, (C6,z)aryl(Cz_3)alkyl or
hetero(C~)aryl(Cz_3)alkyl, wherein
said cycloalkyl, heterocycloalkyl, aryl or heteroaryl optionally is
substituted further with I to 5
radicals independently selected from {C,~alkyl, (C,~)alkylidene, cyano, halo,
halo-substituted
(C1.4)alkYl, vitro, -XSNR'"C(0)OR'4, ..XSNR'''C(0)NR14R14~ -X5~14C~14j~14R14~
.
-xsORt4~ -XsSRl4~ -XsC(O)OR14~ -xsC(O)NR1aR14~ -Xss(O)zNR"R14~ -
Xsp(O)(OR'4)OR'4,
-XSOP(O)(OR'4)OR'4, -XSNR'4C(O)R's, -XsS{0)R's, -XsS(O)zR's and -XSC(O)R's,
wherein
Zu Xs, R'4 a:~ld R's are as defined above, provided that when R3 is
unsubstituted {C,_s)alkyl and R4
is hydrogen or unsubstituted (C,_s)alkyl, then X2 may not represent (i) a bond
when R' is
-C(0)Rz°, -C(O)zRz° or -S(O)zRz° in which R'° is
(C,.~)alkyl, phenyl(C,~)alkyl, phenyl,
(C3.7)cycloalkyi, camphan-10-yl, naphth-1-yl, naphth-2-yl, phenyl substituted
by one or more of
(C,~)alkyl, perfluoro{C,.~)alkyl, {C,~)alkoxy, hydroxy, halo, amido, vitro,
amino,
(C,~,)alkylamino, (C,~)dialkylamino, carboxy or (C,,~)alkoxycarbonyl, or
naphth-I-yI or
naphth-2-yl substituted by one or more of (C,.~)alkyl, perfluoro(C,~)alkyl,
(C,,~)alkoxy, hydroxy,
halo, amido, vitro, amino, carboxy or (C~~)alkoxycarbonyl or (ii) a divalent
group of formula (a)
or (b) in which the moiety R'z is methyl, isopropyl, n-butyl, sec-butyl; tent-
butyl, 1-methylgropyl,
benzyl, naphth-1-ylmethyl, naphth-2-ylmethyl, thien-2-ylmethyl, thien-3-
ylmethyl, or wherein R9
and R'z form ethylene, trimethylene, hydroxy-substituted trimethylene,
tetramethylene or
phenylene-I,2-dimethylene; or
R3 and R4 taken together with the carbon atom to which both R3 and R4 are
attached
-5-
CA 02475069 2004-08-20
form {C~)cycloalkyIene or (Cs.8)heterocycloalkylene, wherein said
cycloalkylene or
hetexocycloalkylene is optionally substituted with 1 to 3 radicals
independently selected from
(C,~)alkyl, (C,~alkylidene, cyano, halo, halo-substituted (C~~)alkyl, vitro, -
XsNR'4C(O)OR'4,
-XsNR'°C(O)NR'4R14~ _xsNRI4C~R14)NR'4R14, -Xs~R14~ -XsSRl4' -
XsC(O)OR14'
-XsC(O)y4R14~ _xsS(O)zNR'4R14~ _Xsp(O)(OR'4)OR'°, -XSOP(O)(OR'4)OR'4,
-XsNR'4C(O)R's, -XsS{O)R's, -XsS{U)zR's and -X5C{O)R's, wherein Xs, R'°
and R's are as
defined above;
R' is hydrogen, (C,.~alkyl or as defined above;
Rs is hydrogen and R6 is hydroxy or Rs and R6 together form oxo;
R' is a group selected from cyano, halo, vitro, -Rte, -X3NRz9Rs°, -
XsI~'Rz~C(G)ORZ9,
-XsNR3oC(O)NR2sR3o, -XsNRsoC(NRso)NR29Rs°, -XSORZ9, -XsSRz9' -
XsC(O)OR~,
-XsC(O)NR29R3°, -XSS(O)2NR29R30~ _xsp(p)(OR~OR~'', -XsOP(O)(ORz9)OR~,
-XsNR3°C(O)R3', -XSS(O)R3', -XsS(O)zR3' and -XSC{O)R3', wherein Xs is
as defined above,
Rz9 is hydrogen or -R3', Rs° at each occurrence is hydrogen or
(C,~)alkyl and R3' is (Cl.~)alkyl,
75 (C3.l~cycloalkyl(C~)alkyl, hetero(C3_,~cycloallyl(C~)alkyl,
(C~lz)aryl(C~)alkyl or
hetero(CS_li)aryl(C°.~)alkyl, wherein within R' any alicyclic or
aromatic ring system present may
be substituted further by 1 to 5 radicals independently selected from
(Cl~)alkyl, {C~~)alkylidene,
cyano, halo, halo-substituted (C,~)alkyl, vitro, -XsNR'4R'4, -XSNR'4C(O)OR'4,
-XS~I4c(O)~14RI4~ _X3~t4C~~14)~14R74~ -XSoRl4~ _x5SR14~ -X5C(~)OR149
-XsC(O)lvTR'4R'4, -Xss(O)zNR14R,1~4,~ _Xsp(O)(OR14)OR'4, -XsOP(U)(OR'4)OR'4,
-XsNR'4C(O)R's, -XsS(O)R's, -XsS(O)zR's and -XSC{O)R's, wherein Xs, R'4 and
R's are as
defined above; and
R$ at each occurrence independently is selected from (C~.s)alkyl,
(Cl,~)alkylidene,
cyano, halo, halo-substituted (C,,~)alkyl, vitro, -XsNR'4R'4, -XsNR'4C(O)OR'4,
-XsNR'4C(O)NR'4R74~ -XsNRI4C(Ny4)NR'4R14~ -XsORl4~ _XsSRl4~ -XsC(~)ORt4~
-XSC{O)N,R14R74~ -Xss(~)2~74RI4~ -Xsp{O)(OR~4)OR'°, -XSOP(U)(OR'4)OR'4,
-Xs~l4C(O)Rls~ -XsS(O)R's, -XsS(O)2R's and -XsC(O)R's, wherein Xs, R'4 and R's
are as
defined above; and the N-oxide derivatives, prodrug derivatives, protected
derivatives, individual
isomers and mixtures of isomers; and the pharmaceutically acceptable salts
thereof.; but
excluding compounds selected from the group consisting of
((S)-1-{ (S)-1-[(S)-1-(1-benzooxazol-~-yl-methanoyl)-3-methyl-butyIcarbamoyl~-
3-methyl-
butylcarbamoyl}-3-methyl-butyl)-carbamic acid benzyl ester, { 1-[1-(1-111-
imidazol-2-yl-
-6-
CA 02475069 2004-08-20
methanoyl)-3-methyl-butylcarbamoyl]-3-methyl-butyl }-carbamic acid tart-butyl
ester,
[(S)-3-methyl-1-((S)-3-methyl-I-{ 1-[1-(2-trimethylsilanyl-ethoxymethyl~lH
imidazol-2-yl]-
methanoyl}-butylcarbamoyl)-butyl]-carbamic acid benzyl ester,
{ (S)-1-[(S)-1-(I-1X imidazol-2-yl-methanoyl)-3-methyl-butylcarbamo~l]-3-
methyl-butyl }-
carbamic acid benzyl ester, ((S)-1-{ (S)-1-[1-(1-benzyl-1H-imidazol-2-yl)-
methanoyl]-3-methyl-
butylcarbamoyl}-3-methyl-butyl)-carbamic acid benzyl ester, {(S)-1-[(Sr1-(1-1H
imidazol-2-yl-
methanoyl)-3-methyl-butylcarbamoyl]-3-methyl-butyl }-carbamic acid tart-butyl
ester,
3-{ [1-(4-chloro-phenyl)-methanoyl]-amino}-4-oxo-4-pyridin-3-yl-butyric acid
ethyl ester,
4-furan-2-yl-4-oxo-3-{ jI-(4-trifluoromethyi-phenyl)-methanoyl]-amino}-butyric
acid ethyl ester,
3-(2-methyl-propanoylamino)-4-oxo-4-thiophen-2-yl-butyric acid ethyl ester, 4-
oxo-
4-thiophen-2-yl-3-[(1 p-tolyl-methanoyl)-amino]-butyric acid ethyl ester, 4-(5-
bromo-
thiophen-2-yl}-3-{ [1-(4-chloro-phenyl)-methanoyl]-amino}-4-oxo-butyric acid
ethyl ester,
3-{[i-(4-chloro-phenyl)-methanoyl]-amino}-4-(5-methyl-thiophen-2-yl)-4~-oxo-
butyric acid ethyl
ester, 4-oxo-4-thiophen-3-yI-3-[(1 p-to)yl-methanoyl)-amino]-butyric acid
ethyl ester,
3-{ [1-(4-methoxy-phenyl)-methanoyl]-amino}-4-oxo-4-thiophen-3-yl-butyric acid
ethyl ester,
3-{ [I-(3,4-dichloro-phenyl)-methanoyl]-amino}-4-oxo-4-thiophen-3-yl-butyric
acid ethyl ester,
4-fluoro-N-[1-(1-thiophen-3-yl-methanoyl)-propyl]-benzamide, 4-{[1-(4-fluoro-
phenyl)-
methanoyl]-amino}-5-oxo-S-thiophen-3-yl-pentanoic acid ethyl ester and 3-{ [I-
(4-fluoro-
phenyl)-methanoyl]-amino}-2-methyl-4-oxo-4-thiophen-3-yl-butyric acid ethyl
ester.
In another particular embodiment, the present invention relates to protease
inhibitors of
Formula I:
R2 RS 6
R
Rry2. N XI R7
R3 4 A
R (R8)n
in which:
A comprises a heteromonocycIic ring containing 5 to 6 ring member atoms or a
fused
heteropolycycIic ring system containing 8 to 14 ring member atoms, wherein
each ring contains
5 to 7 ring member atoms, X' is a ring member carbon atom and each ring member
atom other
than X' is a carbon atom or a heteroatom, with the proviso that (i) at least
one ring member
CA 02475069 2004-08-20
atom is a heteroatom and (ii) when A is a heteromonocyclic radical containing
5 ring member
atoms, no more than two of the ring member atoms comprising A are heteroatoms;
nis0, l,2or3;
X' is =C- or -CH-;
XZ is a bond or a divalent group of Formula (a) or (b):
Rii Rig Rio Rii R12
wN~Xg- ~I~l ~.N~X3~~.
t I
R9 R11 R13 R9
(a) (b)
wherein:
X3 and X4 independently are -C{O)- or -CHZS(O)2-;
R9 and R'° independently are hydrogen, (Cj~)alkyl or as defined
below;
70 R" at each occurrence independently is hydrogen or (Cl-6)alkyl;
R'Z and R'3 independently are (i) (C,~)alkyl optionally substituted with
cyano,
halo, IlltrO, -NRt4R14~ _~14~{O~OR14~ _~14C(O)~14R14~ _~14C~14)~14R149
-OR14' -sRl4~ _C~o)OR14~ -C{~)~14R14' -S{O)2~TR'14R14~ -P(o)~~R14)OR'4,
-OP(O)(OR'4)OR'4, -NR'4C(O)Rls~ _S(O)Rls~ -S(O)ZRIS~ _C(~)Rrs~ _ORIS~ _SR16'
-S(O)R'6, -S(O)2R'6, -C(O)R'6, -C(O)OR'6, -OC(O)R'6, -NR'eRm, -NR"C{O)R16'
_~nC(O)ORIS~ _C(O)ysRa~ _S(O)zNR'6Rt~~ -NR17C(O)NRISRm or
-NR"C(NR")NR'6R", wherein R'4 at each occurrence independently is hydrogen,
(C,.~)alkyI or halo-substituted (C~_3)alkyl, R's {Cl~)aikyl or halo-
.substituted (C~_3)aIkyl,
R'° is (C3_12)cycloaIkyl(C~)alkyl,
hetero{C~,2)cycloalkyl(C~)alkyl,
24 (C~."~aryJ(C~alkyl, hetero(C~I~aryl(C~)alkyl, (C~,~polycycloaryl(C~alkyl or
hetera(C8.12)Polycycloaryl(Co_6)alkyl and R" is hydrogen or (C~~)alkyl, and
wherein
within R'6 said eycloalkyl, heterocycloalkyl, aryl, heteroaryl, polycycloaryl
or '
heterpolycycloaryl ring optionally is substituted by a group selected from -
R'$, -XsOR'$,
_XsSRIS~ _Xss(O)Rn~ -x5f(O)2RI8~ _XsC(O)Rla~ -XsC(~)ORra~ _Xs~C(O)R=$~
_Xs~18R19~ _XsNRr9C(O)Rls9 _Xs~mC{O)~Rls~ -XsC(O)NR18R19~
-XsS(O)zNR'8R'9, _XsNR'9C{O)NR'8R'9 or _XsNR'9C(NR'~NR18R19~ wherein Xs is a
bond or {C,~)alkylene, R'$ is hydrogen or (C,~alkyl and R'9 is
_8-
CA 02475069 2004-08-20
(C~,~cycloalkyl(C~)alkyl, hetero(C3.i~cycloalkyl(C~}alkyl,
(C~,z)aryl(C~)alkyl;
hetero(Cs.,~aryl(C~)alkyl, (C~,i)polycycloaryl(C~)alkyl or
hetero(Cs.,z)polycycloaryl(C~}alkyl, or (ii) a group selected from
(C~.,~cycioalkyl(C°.,6)alkyl, hetero(C~.~~cycloalkyl(C°.6)alkyl,
{C~,~aryl(C~)alkyl,
hetero(Cs.,z)aryl(C~)alkyl' (C~.,~polycycloaryl(C°.~)alkyl and
hetero(C&,~polycycloaryl(Co-6)alkyl, wherein said cycloalkyl,
heterocycloalkyl, aryl,
heteroaryl, polycycloaryl or heterpolycycloaryl ring optionally is substituted
by a group
selected from-R's, -XsOR's, -XsSR'$, -XsS(O)R'$, -XsS(O~R'$, -X5C(O)R'8,
-XsC(O)ORIS~ -XsOC(O)Rls9 -XsysRa°~ -Xsy9C(O)R1s -XsNR'9C(O)OR's,
-XsC(O)y sRl9~ -XsS(O)zNR'sRI9~ _XS~l9C(O)~t8R19 or '
XSNR'9C(NR'9)NR'sR'9, wherein Xs, R's and R'9 are as defined above; wherein
within R'z and/or R'3 any alicyclic or aromatic ring system present may be
substituted
further by 1 to S radicals independently selected from (C,.~)alkyl,
(C,_6)alkylidene,
cyano, halo, halo-substituted (C,~)alkyl, vitro, -XSNR'4R'4, -XsNR'4C(O)OR'4,
-X5~74C(O)NRI4Ri4' -XsNR~4C(ya)p4R14~ -XSOR14~ _XsSRl4~ -XSC(O)OR14~
-XsC(~)NR1aR14~ _XsS(O)zNR'4Rla~ _Xsp(O)(OR'4)OR'4, -XsOP(O)(OR'4)OR'4,
-XsI'TR'4C(O)R's, -XsS(O)R's, -XsS(O)zR's and -XSC(O)R's, wherein Xs, R'4 and
Rls
are as defined above; or
R'z together with R9 and/or R'3 together with R'° form
trimethylene,
tetr~methylene or phenylene-1,2-dimethylene, , optionally substituted with 1
to 3 radicals
independently selected from (C,~)alkyl, (C~.~)alkylidene, cyano, halo, halo-
substituted
(C,~)alkyl, vitro, oxo, -XSNR'~C(O)OR'°, -XSNR'4C(O)NR'°R'4,
-XSNR'4C(NR'4}NR'°R'4, -XSORI4~ _xsSRl4~ _XSC(o)oRl4~ _XsC(O)~14RI4~
-XsS(O)zNR'4R'4, -XsP(O)(OR'4)OR'4, -XSOP(O)(OR'4)OR'4, -XsNR'4C{O)R's, .
-XSS(O)R's, -XSS(O)zR's and -XsC(O)R's, wherein Xs, R'4 and R's are as defined
above; and
R' is -X6X'Rz°, wherein X6 is -C(O)-, -C(O)C(O)- or -S(O)z-, X' is a
bond, -O- or
-NRZ'-, wherein Rz' is hydrogen or (C,.~)alkyI, and Rz° is (i)
(C,.~alkyl optionally substituted by
cyano, halo, vitro, -NR'4R'4, -NR'4C(O)OR'4, -NR'4C(O)NR'4R'4, -
NR'4C(NR'4)NR"R'4,
-OR'4, -SR'4, -C(O)OR'4, -C(O)NRI4R14~ -S(O)zNR'4R14~ _p(O)(OR")OR's,
-OP(O)(OR'4)OR'4, -NR'4C(O)Ris~ _S(O)RIS~ _S(O)zRls~ -C(O)Rls~ _~Rzz~ -SRn~ -
S(~)R~~
-S(O)zRzz~ -C{O)Rzz, -C(O)ORzz, -C(O}NRzzRzs~ -~zzRza~ _~z3C(O)Ru'
_NR~C(O}ORS, .. .
-9-
CA 02475069 2004-08-20
-NRz3C(O)NR'~R~ or -NR23C(NR~)h'tR~R'~, wherein R'4 and R's are as defined
above, R~
is (C~I~cycloalkyl(C~)alkyl, hetero{C~.,z)cycloalkyl(C~)alkyl,
(C~tz)aryl(C~)alkyl,
hetero(C~.~~aryl(C~alkyl, (C~,~bicycloaryl(C~alkyl or
hetero(C:~,z)bicycloaryi(C~)alkyl
and R~ at each occurrence independently is hydrogen or (C,.~)alkyl, or
(ii) (C3_,z)cycloalkyl(C~)alkyl, hetero(C3_l~cycloalkyl(C~jalkyl,
(C~,z)aryl(C~aIkyl,
diphenyl(C~alkyl, hetero{Cs.,2)aryl(C~)alkyl, dihetero(Cs.~)aryl(C~)alkyl,
(C9_,~bicycloaryl(C~6)alkyl or hetero(C$_12)bicycloaryl(C~)alkyl wherein said
cycloalkyl,
heterocycloalkyl, aryl or heteroaryl may be substituted by -Rz4, -XSOR~'; -
XSSR~, -XSS(O)R~,
-XSS(O)zR~~ -XSC(~)R',a, -XSC{O)ORz4~ -XSC(O)~24R25~ _XsNRzaRzs~ -
Xs~zsC(O)Rz4'
-XSNR'~C(O)OR'~, -XSNR'~C(O)NR~'R'~ or -XsNRz3C(NRz3)NRz4R's, wherein Xs is as
defined above, R'~ is (C3_,z)cycloalkyl(Co.,b)alkyl,
hetero(C~,,)cycloalkyl(C~)alkyl,
(C~lz)aryl(C~.6)alkyl, hetero(C~,~aryl(C~)alkyl, (C9_,z)bicycloaryl(Co~)alkyl
or
hetero(C&,z)bicycloaryl(C~)alkyl and Rzs at each occurrence independently is
hydrogen or
(C,~)alkyl; wherein within R: any alicyclic or aromatic ring system present
may be substituted
further by 1 to 5 radicals independently selected from (C,.~)alkyl,
{C,.~)alkylidene, cyano, halo,
halo-substituted (Cl~)alkyl, nitro, -XSNR'4R'4, -XsNR'4C(O)OR'4, -
XSNR'4C(O)IvTR'4R'4,
-Xs~l4C~R14)~l4Ru~ _XSORi4~ _XSSRi4~ -XSC(O)oRl4~ -XsC{O)NRl4R~e~
'X3'S(O)2~TR14RI4' -Xsp(p)(OR'4)OR'°, -XSOP(O)(OR'4)OR'4, -
XSNR'4C(O)R's, -XsS(O)RIS~
-XSS(O)zR's and -XSC(O)R's, wherein Xs, R'4 and R's are as defined above; or
when Xz is a
2d divalent group of formula (a) or (b) then R' may also represent hydrogen,
carboxy, oxalo or
carbamoyl;
Rz is hydrogen or (C,~)alkyl;
R3 is (i) {C,~)alkyl optionally substituted with cyano, halo, vitro, -SR's, -
C(O)OR''',
-C(O)NR'~R~°, -P(O)(ORz°)OR'~, -OP{O)(ORz')ORz°, -S(O)RB,
-S(O)zR~ or -C(O)RD,
wherein Rz4 at each Qccurrence independently is hydrogen, (C,,~)alkyl or halo-
substituted
(C,_3)alkyl and R'~ (C,_6)alkyl or halo-substituted (C,.3)alkyI, or {ii)
(C~)cycIoalkyl(Cz_3)aIkyl,
hetero(C~)cycloalkyl(Cz_3)alkyl, {C~,2)aryl(C2_3)alkyl or
hetero(C~)aryl(Cz_3)alkyl, wherein
said cycloalkyl, heterocycloalkyl, aryl or heteroaryl optionally is
substituted further with 1 to 5
radicals independently selected from (C,,~)alkyl, (C,~)alkylidene, cyano,
halo, halo-substituted
(C,~alkyl, vitro, -XSNR'4C(O)OR'4, -XSNR'4C(U)NR'4R'4, -XSNR'4C(NR'4)NR'4R'4,
-XSOR149 -x3SR14~ -XSC{O)ORI4~ -XS~{O)~14R14' -XSS(O)2~14R74~ -
Xsp{O)(OR'4)OR'4,
-XSOP(O)(OR'4)OR'4, -XsNR'4C{O)R'3, -XSS(O)R's, -XsS{O)zR's and -XSC(O)R's,
wherein
-10-
CA 02475069 2004-08-20
Xs, R'4 and R's are as defined above, provided that when R' is unsubstituted
(C~.s)alkyl and R4
is hydrogen or unsubstituted (C,.s)adkyl, then X2 may not represent (i) a bond
when It' is
-C(O)Rz°, -C(O)2Rz° or -S(O)ZR~° in which R~°
is~(C,.~)alkyl, phenyl(C,~)alkyl, phenyl, (Cs.
~)cycloaIkyl, carnphan-10-yl, naphth-1-yl, naphth-2-yl, phenyl substituted by
one or more of (C,,
,alkyl, perfluoro(C~~alkyl, (C,.,~alkoxy, hydroxy, halo, amido, vitro, amino,
(C,,~alkylamino,
(Cl~dialkylamino, carboxy or (C,~)alkoxycarbonyl, or naphth-1-yl or naphth-2-
yl substituted by
one or more of (Cl.~)alkyl, perfluoro(C~,,)alkyl, (C,~)alkoxy, hydroxy, halo,
amido, vitro, amino,
carboxy or (C,,~)alkoxycarbonyl or (ii) a divalent group of fonmula (a) or (b)
in which the moiety
R'2 is methyl, isopropyl, n-butyl, sec-butyl, tern-butyl, 1-methylpropyl,
benzyl,,naphth-1-ylmethyl,
7 0 naphth-2-ylmethyl, thien-2-ylmethyl, thien-3-ylmethyl, or wherein R9 and'
R'2 form ~ethyleile;
' trirriethylene, hydroxy-substituted trimethylene, teiramethylene or
phenylene-1,2-dimethylene; or
R3 and R4 taken together with the carbon atom to which both R3 and R4 are
attached
form (C3_$)cycloalkylene or (C~)heterocycloalkylene, wherein said
cycloalkylene or
heterocycioalkylene is optionally substituted with 1 to 3 radicals
independently selected from
(C~.~)alkyl, (C~.~)alkylidene, cyano, halo, halo-substituted (C,.~)alkyl,
vitro, -XsNR'4C(O)OR'4,
-Xs~l4C(O)NrR14R14~ -Xsp4Cy4)NR'"R14~ -XsORl4' -XsSRr4~ -XsC(O)OR'4,
-XsC(O)NRI4R14~ -XSS~O)xNR14R14~ -XsP(O)(OR'4)OR'4, -XSOP(O)(OR'4)OR'4,
-XsNR'4C(O)R's, -XsS(O)R's, -XsS(~}ZR's and -XSC(O)R's, wherein Xs, R'4 and
R's are as
defined above;
R3 and R4 taken together with the carbon atom to which both R3 and R4 are
attached
form (C3.8)cycloalkylene or (Cs.B)heterocycloalkylene, wherein said
cycloalkylene or
heterocycloalkylene is optionally substituted with 2 to 3 radicals
independently selected from
(C,.s)aikyl, (C~.~)alkylidene, cyano, halo, halo-substituted (Cl~)alkyl,
vitro, -XsNR'4C(O)OR'4,
-XsNRI4C(O}y4Ri4~ _XsNRIeC(ya)y4R14~ _XsORl4~ -XsSRl4t _XsC(O)ORl4;
-XsC(O)NR'°R'4~ _XsS(p)z~14R14~ -Xsp(O)(OR'4)OR'4, -XsOP(O)(OR'4)OR'4,
-XsNR'4C(O}R's, -XsS(O)R's, -XsS(O)2R's and -XSC(O)R's, wherein X5, R'4 and
R's are as
defined above;
R4 is hydrogen, (C~.~)atkyl or as defined above;
Rs is hydrogen and R° is hydroxy or Rs and R6 together form oxo;
R' is a group selected from cyano, halo, vitro, -Rte, -XsNR29R30,
_X5~3°C(O)OR29,
-X5NR3°C(O)NR29R3°, -XsNR30C(NR30)NR~R3°, -XsOR~'', -
XsSR~'', -XSC(O}ORS,
-XsC(O}NR~gR3°, -X5S(O)_NR29R3°, -X5P(O)(OR3~OR'-9, -
XsOP(O)(ORz9)OR~,
-I 1-
CA 02475069 2004-08-20
_Xs~3oC(O)Rw~ _XsS(O)Rzo9 _Xss(O)zRz°~ 'XsC(O)Rzo and -
C(O)NR4zCHR°3C(O}OR~~
wherein Xs and Rz° are as defined as above, R~ is hydrogen or -
Rz°, wherein Rz° is defined as
above, R3° at each occurrence is hydrogen or (C,~}alkyl, R°z is
hydrogen, (C,~aIkyl or together
with R43 forms trimethylene, tetramethylene or phenylene-1,2-dimethylene,
optionally substituted
with hydroxy or oxo, and R43 is as defined above or is (i) (C,.~}alkyl
optionally substituted with
cyano, halo, vitro, -NR'4R'4, -NR'4C(O}OR''', -NR'4C(O}NR"R'°, -
NR'4C(NR'a)NRI4R74y
_OR~a~ -SRr4~ _C(O)ORre~ _C(O)~14R14~ _S(o)z~J4Rl4~ -p(p)(OR")OR'4,
-OP(O)(OR'4)OR'4, -IvTR'4C(0}Rrs~ _S(O}R~s~ _S(0}'R~s~ -C(O)Rrs' _OR~s~ -SR~s~
_s(O)R~e~
-S(~)zR~a~ _C(0)Rrs~ -C(0)ORre~ _OC(O)Rrs~ _NRr6Rn~ _NRnC(O)Rn~ -y7C(O)OR~6~
-C(O)NR'aRp _S(O)zNR'6R~7~ _~nC(O)NyaRn flr _~rzC7)NR'SR~: or (ii) a group
selected from (C~l~eycloalkyl(C~,.6)alkyl, hetero(C~,z)cycloalkyl(C~)alkyl,
(Cb.,z)aryI(C~)alkyl, hetero(Cs_,~aryl(Ca~alkyl,
{C9.,~polycycloary3(C°.6)alkyl and
hetero(C&l2~olycycloaryl(C°..6)aIkyl, wherein said cycloalkyl,
heterocycloalkyl, aryl, heteroaryl,
polycycloaryl or heterpolycycloaryl ring optionally is substituted by a group
selected fronn -R'g,
't5 -XSOR'8, -XSSR'a, -X5S(O)R's, -XsS(O}2818' _XsC(O)R~s~ -XsC(O)OR'g, -
XsOC(0)R'8,
_XsNRrsRr9~ _Xs~aC(O)R~s~ _Xs~r9C(O)OR~a~ _XsC(O}~I8RI9~ _Xss(O)z~'sRts~
-XsNR'9C(0)NR'BR'9 or -XsNR'gC(NR'9)NR'BR'9, wherein Xs, R'°, R's, R'6,
R", R'e and R'9
are as defined above; wherein within R' any alicyclic or aromatic ring system
present may be
substituted further by 1 to 5 radicals independently selected from
(C~_6)alkyl, (C~,~)alkylidene,
cyano, halo, halo-substituted (C,~)alkyl, vitro, -XSNR'4R''°, -
XsNR'4C(O)OR''',
'X5~74C(O)~74R14~ -Xs~r4C(NRr4)NR'4Rr4~ _XsORm~ _XsSRm~ -XsC(O}OR'e~
_XsC(O}NRr4Rra~ _~SS(Q)2~14R74~ -Xsp(O)(OR'4)OR''°, -
XsOp(O)(ORr"}OR''',
-XsNR'4C(0)R's, -XsS(0}R's, -XsS(0)zR's and -XsC(0}R's, wherein Xs, R'°
and R's are as
defined above; and
R8 at each occurrence independently is selected from (C,~)alkyl, halo-
substituted
(C~.~)alkyl, (Cr.s)alkylidene, cyano, halo, halo-substituted (Ci~,)alkyl,
vitro, -XsNR'°R14~
_Xs~l4C(O)OR14~ _Xsy4C(0 NR'4R'4, -XSNRJ4C(NR14)NR'4R14~ _XSORId' _XssR)4'
_XsC(O) flRr4~ _XSC(O}~14RI4y -XsS(0)zNR"Rr4~ -Xsp(O)(OR'4)OR'4,
-XsOP(O)(OR'4)OR'°, -XSNR'4C(O}R's, -XsS(0)R's, -XsS(0}zR's and -
X5C(O}R's, wherein
Xs is a bond or (C~~)alkylene, R'4 at each occurrence independently is
hydrogen, (C,~)alkyl or
haIo-substituted (C~_3}alkyl and R's (C,.~)alkyl or halo-substituted
(C,_3)alkyl; and the N oxide
derivatives, prodrug derivatives, protected derivatives, individual isomers
and mixtures of
-12-
CA 02475069 2004-08-20
isomers; and the pharmaceutically acceptable salts thereof.
In a further embodiment, the present invention relates to a compound of
formula I:
R2 Rs
i
R .Xar N Xt R~
R~ 4
R (R$)~
in which:
A comprises a benzooxazole or naphthooxazole ring, each substituted by a
group R' and optionally substituted with a group Rg, wherein R~ is hydrogen,
halo,
(CI_4)alkoxy, (C1~)alkoxycarbonyl, vitro or phenyl, R8 at each occurrence
independently is halo, (C1~)alkoxy, (Cl~)alkoxycarbonyl, vitro or
trifluoromethyl;
nis0, l,2or3;
X' is =C-;
XZ is a bond or a divalent group of Formula (a):
Rt r Rt2
~~ N~ X3,
R9
(a)
X3 is -C-(O) or -CH2S(O)2-;
wherein within Formula (a) R9 is hydrogen, Rj 1 is hydrogen or methyl and R12
is
(CI~)alkyl substituted with -SR14, -S(O)Ri4 or -S(O)aRi4, wherein R14 is
(C6_i2)aryl(Co~)alkyl or hetero(CS_12)aryl(Co-6)alkyl; wherein within R12 the
aromatic
ring system present may be substituted further by 1 to 5 radicals
independently
selected from (C1_6)alkyl, (C1_6)alkylidene, cyano, halo, halo-substituted
(Ci_4)alkyl,
vitro, -XSNR1aR14, -XSNR14C(p)OR14,
1~
CA 02475069 2004-08-20
-X5~14~-r(O)~14R14' -X5~14C~14)~14R14' -XSOR14' -XSS,R14' -XS(~(O)OR14,
-XSc(~)~14R14' -x5s(O)2~14R1214~'1~-XSP(O)(~R14)OR14, -XSOP(O)(OR~4)OR14,
-xs~l4C(O)Rls, -XSS(O)R15, -X5S(O)2Rls ~d -XsC(O)Rls, wherein XS is a bond or
(C1_6)alkylene, R14 at each occurrence independently is hydrogen, (Cl_6)alkyl
or
halo-substituted (C1_3)alkyl and R15 is (Cl~)alkyi or halo-substituted
(Cl_3)alkyl;
Rl is -X6X~R2°, wherein X6 is -C(O)- or -S(O)2-, X' is a bond, -O- or -
NR21-,
wherein R21 is hydrogen or (Cl_6)alkyl, and R2° is (i) (C1_6)alkyl
optionally substituted
by -C(O)OR14 or (ii) (C3_12)cycloalkyl(Co_6)alkyl,
hetero(C3_12)cycloalkyl(Co_6)alkyl,
(C~12)aryl(Co_s)alkyl or hetero(CS_12)aryl(Co_6)alkyl or (iii)
(C3~)cycloalkyl(Co_6)alkyl,
hetero(C3_6)cycloalkyl(Co_6)allcyl, phenyl(Co_6)alkyl or
hetero(CS_6)aryl(Co_6)alkyl,
wherein said cycloalkyl, heterocycloalkyl, phenyl or heteroaryl is substituted
by
-XSOR24'-XSC.(O)R24' -XSCr(o)OR24, -x5C(O)NR24R25' -X5~24R25'
-Xs~2sC(O)Rz4~ -~r5~25C((~)oR24, -XS~R25C,(o)~24R,25 ~r .
-XS~25~.~25)~24R25~ wherein XS is a bond or (C1_6)alkylene, R24 is
(C3_6)cycloalkyl(Co~)alkyl, hetero(C3_6)cycloalkyl(Co_~)alkyl,
phenyl(Co_6)alkyl or
hetero(CS_6)aryl(Co-6)alkyl and R25 is hydrogen or (Cl_6)alkyl; wherein within
Rl any
alicyclic or aromatic ring system present may be substituted further by 1 to 5
substituents independently selected from (Cl_6)alkyl, halo, halo-substituted
(Cl~)alkyl,
-OR14 and -C(O)OR14 wherein R14 is as defined above, or when X2 is a divalent
group
of formula (a) then Rl may be, but is not limited to, hydrogen or oxalo;
R2 is hydrogen;
R3 is hydrogen, (C1~)alkyl (optionally substituted with cyano, halo, vitro,
-SR24' -~(O)OR24' -C(o)~24R24' -P(O)(~R24)OR24, -Oh(O)(OR24)OR24, -S(O)R25
-S(O)2R25 or -C(O)R25, wherein R24 at each occurrence independently is
hydrogen,
(C1_6)alkyl or halo-substituted (C1_3)alkyl and R25 is halo, (C1_6)alkyl or
halo-substituted (C1_3)alkyl) or (C6_12)aryl(C2_3)alkyl, wherein said aryl
optionally is
substituted further with 1 to 5 radicals independently selected from
(C1_6)alkyl,
(C1_6)alkylidene, cyano, halo, halo-substituted (C1_4)alkyl, vitro,-
X$NR14C(O)ORl4,
-x5~14C(O)~14R14'
13a
CA 02475069 2004-08-20
-X~14C~14)~14R14' -XsORIa' -XsSRIa' -XsC(~)OR14' -XsC(o)~14R14'
-Xs~SV(tCO)ZN\1R'ila~Ria, -XsP(O)(~RIa)OR~a, -XSOP(O)(ORIa)ORIa, -
XsNRIaC(O)Rts9
-X5S(O)Rls, -XSS(O)2Rls and -XsC(O)Rls, wherein Xs is a bond or (C1~)alkylene
and
R'a and Rls are as defined above, or R3 and Ra or R3 and Ra taken together
with the
carbon atom to which both R3 and R4 are attached form cyclopropylene,
cyclobutylene, cyclopentylene or cyclohexylene;
Ra is hydrogen or as defined above; and
Rs and R6 together form oxo; and the N oxide derivatives, and individual
stereoisomers and mixtures of stereoisomers thereof; and the pharmaceutically
acceptable salts thereof.
In a further embodiment, the present invention relates to a pharmaceutical
composition comprising a compound of formula I or II as defined above, or a N-
oxide
derivative, individual steoizimer, or mixture steoizimer thereof, or a
pharmaceutically
acceptable salt thereof in admixture with one or more suitable excipients.
In a further embodiment, the present invention relates to use of a compound of
formula I as defined above, or an N oxide derivative thereof or an individual
steoizimer or a mixture of steoizimers thereof or a pharmaceutically
acceptable salt
thereof to treat a disease selected from the group consisting of juvenile
onset diabetes,
multiple sclerosis, pemphigus vulgaris, rheumatoid arthritis, Hashimoto's
thyroiditis,
asthma, organ transplant or tissue graft rejections, chronic obstructive
pulmonary
disease, bronchiolitis, excessive airway elastolysis in asthma and bronchitis,
pneumonitis, plaque rupture and atheroma in an animal in need of such
treatment.
In a further embodiment, the present invention relates to use of a compound of
formula II as defined above, or an N oxide derivative thereof or an individual
steoizimer or a mixture of steoizimers thereof or a pharmaceutically
acceptable salt
thereof to treat, a disease selected from the group consisting of juvenile
onset
diabetes, multiple sclerosis, pemphigus vulgaris, rheumatoid arthritis,
Hashimoto's
thyroiditis, asthma, organ transplant or tissue graft rejections, chronic
obstructive
pulmonary disease, bronchiolitis, excessive airway elastolysis in asthma and
bronchitis, pneurnonitis, plaque rupture and atheroma in an animal in need of
such
treatment.
13b
CA 02475069 2004-08-20
In a further embodiment; the present invention relates to use of a compound of
formula I or II as described above or a N oxide derivative thereof; or an
individual
stereoisomer or mixture of stereoisomers thereof; or a pharmaceutically
acceptable
salt thereof, in the preparation of a medicament for use to treat, in an
animal, a disease
selected from the group consisting of juvenile onset diabetes, multiple
sclerosis,
pemphigus vulgaris, rheumatoid arthritis, Hashimoto's thyroiditis, asthma,
organ
transplant or tissue graft rejections, chronic obstructive pulmonary disease,
bronchiolitis, excessive airway elastolysis in asthma and bronchitis,
pneumonitis,
plaque rupture and atheroma.
In a further embodiment, the present invention relates to use of a compound of
formula I or II as described above or a N oxide derivative thereof; or an
individual
stereoisomer or mixture of stereoisomers thereof; or a pharmaceutically
acceptable
salt thereof, in the preparation of a medicament for use to treat, in an
animal, a disease
selected from the group consisting of juvenile onset diabetes, multiple
sclerosis,
pemphigus vulgaris, rheumatoid arthritis, Hashimoto's thyroiditis, asthma,
organ
transplant or tissue graft rejections, chronic obstructive pulmonary disease,
bronchiolitis, excessive airway elastolysis in asthma and bronchitis,
pneumonitis,
plaque rupture and atheroma.
In another particular embodiment, the present invention relates to a compound
of
Formula II:
R3z
O~ a ~0
SwX~ R2 RS
6
R
Ro N 1 R7
g ~ R3 4 X A
R O ~ ~RE)n
in which:
A comprises a heteromonocyclic ring containing 5 to 6 ring member atoms or a
fused
heteropolycyclic ring system containing 8 to 14 ring member atoms, wherein
each ring contains
5 to 7 ring member atoms, X' is a ring member:,carbon atom and each ring
member atom other
than X' is a carbon atom or a heteroatom, with the proviso that at least one
ring member atom is
a heteroatom;
13c
CA 02475069 2004-08-20
nis0, l,2or3;
X' is =C- or -CH-;
X8 is (C~.~alkylene;
R' is hydrogen, carboxy, axalo, carbamoyl or -XbX'R~°, wherein Xs is
-C(Q~,
-C(O)C(O)- or -S(O)z-, X' is a bond, -O- or -NR~'-, wherein Ra' is hydrogen or
(C,~alkyI, and
R~° is (i) (C;.~)aIkyl optionally substituted by cyano, nalo, vitro,
=IYR'°R'4, -NR'''C(O)OR'a,
-NR~4C(O)~ryaR~e~ _NR~4C(y<~NR'''R~4~ _ORu~ -SR'4, -C(0)OR'4, -C{0)NR"Ra~
-S(0)zNR"R'4, -P(O)(OR'4)OR"; -OP(O)(OR'°)OR", -NROsC(0)R's, -S(O)R's; -
S(O)ZRu, .
-C(0)R's, -ORu, -SRu, -S(0)RZZ, -S(O)zIt~, -C(O)RD, -C(O)OR'~; -C(O)NRnR~, .
1 G -NR~zR~, -NR=3C(O)Rz=, -NR~C{O)OR~,-NR=~C(O)NRZZR~ or -NR~C(NR~)NRuR~,
wherein R" at each occurrence independently is hydrogen, (C~.~)alkyl or halo-
substituted
UC~.3)alicyl, R's is (C,.6)alkyl or halo-substituted (C,.3)alkyl, R~ as
(~_l~'icycloal'r.-Y1(C~;alkyh
hetero(C~.,~cycloalkyl(C~)alkyl, (C~i~aryl(C~)alkyl,
hetero(Cs.,2)aryl(C~)alkyl,
(C9.~~bicycloaryl(C~)alkyl or hetero(Ca.,~bicycloaryl(Ca,.b)alkyl and R'a at
each occurrence
independently is hydrogen or (C,~)alkyl, or (ii) (C3.~~cycloalkyl{Co.,b)alkyl,
13d
CA 02475069 2004-08-20
hetero(Cs.,~cycIoalkyl{C~alkyl, (C~.la)aryl{C~)alkyl, hetero(Cs.~~aryl(Co-
b)alkyl,
(C9_~~bicycloaryl(C~)alkyl or hetero(Cs.,~bicycloaryl(C~)alkyl or
(iii) (C~)cycloalkyl(C~)alkyl, hetero(C~cycloalkyl(C~)alkyl, phenyl(C~alkyI or
hetero(Cs,~)aryl(C~.b)alkyl substituted by -XSOR~, -XsSRu, -XSS(O)R'~, -
XsS(O)aR?'',
-X'C(O)Rz4, -X5C(O}ORz'', -XsC(O)NRz'°Ru, -XSNRz''R~, -XsNR'sC(O)R~,
-XSNR~C(O)OR~', -XSNRzsC(O}NR~'Rzs or -XsNRuC(NRzs}NR~'R'-s, wherein Xs is a
bond
or (C~.~alkylene, Rz° is (C3~)cycloalkyl(C~alkyl,
hetero(C3.~)cycloalkyl(C~)alkyl,
phenyl(C~alkyl or hetero(C~aryl(C~)alkyl and R~ at each occurrence
independently is
hydrogen or (C,~)alkyl; wherein within R' any alicyclic or aromatic ring
system present may be
substituted further by 1 to 5 radicals independently selected from (C,~)alkyl,
(C~~)alkylidene,
cyano, halo, halo-substituted (C~.~)alkyl, vitro, -XsNR'4R'4, -XsNR'4C{O)OR'4,
-XsNRI4C{~)NR14R14~ -XsNRI4C~R14)NR'4R14~ -XsORl4~ -XsSRl4~ -XsC{~}OR,4~
-XSC(o)~14R14~ _XSS(0~2~14R14~ -Xsp{O){ORI4)OR'4, -XSOP(O){OR'4)OR'4,
-XsNR'4C{O)R's, -XsS(O}R's, -XsS(O)zR's and -XSC(O)R's, wherein Xs, R'4 and
R's are as
defined above;
Rz is hydrogen or (C,.~alkyl;
R3 is {i) (CL.6)alkyl optionally substituted with cyano, halo, vitro, -
NR'4R'4,
_~14C{O)ORl4' _NRI4C(O)NR14R14~ -~14'''~~14)~14R14' -~R149 -SR14' -c{o)OR14'
-C{O)~laRl4~ -S(O}zNR'°R14~ -p{O){OR'4}OR'4, -OP(O)(OR'4)OR'4, -
NR'4C(O)Rls~
-S(O)R's, -S(O)zRls~ -C(O)R~s~ -ORIe' -SRIS~ _S{~)Rls~ _S(O)zRls~ _C{~}R16~ -
C~~)OR,6~
_OC(O)Rle~ -yeRn~ _~p{O)Rls~ _N'R17C(O)ORl6r _C(O)NR~~Ray _S(O)z~leRn~
=NR"C{O)NR'6R" or -NR"C(NR")NR'6R", wherein R'4 at each occurrence
independently
is hydrogen, (Cl.~)alkyl or halo-substituted (C~_3)alkyl, R's is (C,.~)alkyl
or halo-substituted
(C,_3)alkyl, R'6 is {C3_,~cycloalkyl{C~)alkyl,
hetero(C~.,z)eycloalkyl(C~)alkyl,
(C~.,z)aryl(Co-b)alkyl, hetero(Cs_lz)aryl(C~)alkyl,
(C9_lz}Polycycloaryl{C~)aIky1 or
hetero(C8.lz)polycycloaryl(Co..b)alkyl and R" is hydrogen or (C,.~)aIkyl, and
wherein within R'6
said cycloalkyl, heterocycloalkyl, aryl, heteroaryl, polycycloaryl or
heterpolycycloaryl ring
optionally is substituted by a group selected from -R'$, -XSOR'8, -XSSR'e, -
XSS(O)R'$,
-Xss(O)zRls~ _XsC{O}Rls~ -XsC{0)ORIS~ -XsdC(O)Rla~ _XsNRIaRo~ -X5~19C{O)Rls~
-XsNR'9C(O)OR'$, -XsC(O)NR'$R'9, -XsS(O)zNR'$R'9, -XsNR'9C{O)NR'8R'9 or
_X5~19C~19)~1R18R19~ wherein Xs is as defined above, R'$ is hydrogen or
(C,~)alkyl and
R'9 is (C3.I~Zl)~c~~ycloalkyI(C~)alkyl, hetero(C3.,~cycloalkyl(C~)alkyl,
(C6_l~aryl(C~)alkyl,
-14-
CA 02475069 2004-08-20
hetero(Cs.,~ary1(Co.b)alkyl, (C~.,~polycycloaryl(C~)alkyl or
hetero(C&,~polycycloaryl(Co-b)alkyl, or (ii) a group selected from
(C3.,2)cycloalkyl(Co-6)alkyl;
hetero(Cs.,~cycIoalkyl(C~)alkyl, (Cb.,Z)aryl(C~.6)aIkyl,
hetero(Cs,,~aryl(C~)alkyl,
4~.,~PaIYcYcloaryl(C~)aIkyl and hetero(C&,~polycycloaryl(C~)aIkyl, wherein
said cycloalkyl;
heterocycloalkyl, aryl, heteroaryI, poIycycloaryl or heterpolycycloaryl ring
optionally is
substituted by a group selected from -R's, -XSOR'8, -XSSR's, -XSS(O)R'8, -
XSS(O)2R's,
_XsC(O}Rls~ _XsC{O}ORIS~ _XsOC{O}Rls~ _XsNRlaRis~ _XspsC(~)R~s' -Xsy
9C(O}OR~e~
_XsC(O)~laRls~ _XSS(O)2~I8RI9~ _~S~t9C(O}~18R19 or _X3~T9C~lS~~18R19~
wherein Xs, R's and R'9 are as defined above; wherein within R''
andlo\rl~Rl'~3 alny alicycIic or
~0 aromatic ring system present may be substituted further by 1 to 5
radicals'independently
selected from (C,~)alkyl, (C~.~)alkyIidene, cyano, halo, halo-substituted
(C,.')alkyl; vitro,
_Xsy4R14~ _XsNRIaC(O)ORIa~ _Xs~~RIaC(O)NR14R14~ _Xs~laC~aa}yaR~a~ _XsORI'~
_XsSRn~ _XsC(O)ORIa~ -XSC(O)~14R14~ _XsS(o~yaRla~ _Xsp(O}(OR'')OR",
-XSOP(O)(OR")OR'°, -XSNR''C(O)R's, -XsS{O}R's, -XsS{O)2Ris and -
XSC(O)R's, wherein
Xs, R" and R's~are as defined above, or
R3 and R' taken together with the carbon atom to which both R3 and R' are
attached
form {C~s)cycloalkylene or (C~s)heterocycloalkylene, wherein said
cycloalkylene or
heterocycloaIkylene is optionally substituted with 1 to 3 radicals
independently selected from
(C,~)alkyl, (C,.~)alkylidene, cyano, halo, halo-substituted (C,~)aIkyl, vitro,
-XSNR"C(O)OK'°,
-XSNR'4C(O}NR'"R14~ _XsNRIaC~RIa)NR"Rv4~ _XsORIe~ _XsSRl4~ _XsC(O)oR~4~
_X3C(O}~14R1<~ _XSS(0)2~14R14' _Xsp(O)(OR'°)OR'°, -
XsOP(O)(OR")OR",
=XSNR'4C(O)R'3, -XSS(O}R's, -XSS{O)2R's and -XSC(O)R's, wherein Xs, R" and R'3
are as
defined above;
R4 is hydrogen, (C,~)aIkyl or as defined above;
Rs is hydrogen and R6 is hydroxy or Rs and R6 together form oxo;
R' is a group selected from cyano, halo, vitro, -RZ9, -XSNR2'Rs°, -
XSNR3oC(O)OR~,
_X3~30C(Q)~29R30~ _XsNRaoC~R3o}~zgRso~ _Xs~R29~ _XSSR29~ _x5C(~)oR29~
_XsC(O}NR29Rr30~ _Xss(O)zNR~R3°~ 'Xsp(O)(OR3°}OR$9, -
XSOP(O){OR29)ORz9,
_X3~30C(O)R3', -XSS(O)R3', -XSS(O)ZR3' and -XSC(O)R3', wherein Xs is as
defined above,
R~ is hydrogen or -R3', R3° at each occurrence is hydrogen or
(C,_6)alkyl and R3' is (C,~)alkyl,
(C3.,2)cycloalkyl(C~)alkyl, hetero(C:3_,Z)cycloalkyl(C~.6)alkyl,
(C~,~aryl(C~)alkyi or
hetero(Cs_,~aryl(C~)alkyl, wherein within R' any alicyclic or aromatic ring
system present may
-1 S-
CA 02475069 2004-08-20
be substituted further by 1 to 5 radicals independently selected from
(C~~)alkyl, (C,.~lkylidene,
cyano, halo, halo-substituted (C~~)alkyl, vitro, -XSNR"R", -XsNR"C(O)OR'4,
-XS~id'",{~)~14R145 -XsyaC(~~4)NR"Ry4~ _XsOR~e~ _XsSR~4~ _XsC(O)OR14~ .
-XsC{O)NR~4R~4~ _'x5s~(~)2NR1dRl4~ _Xsp(O)(OR'4)OR", -XsOP(O)(OR")OR",
-XsNR'4C(O)R's; -XSS(O)R's, -XsS(O)aR's and -XsC(O)R's, wherein Xs, R" and R'f
are as
defined above; and
Rs at each occurrence independently is selected from (C~~)alkyl,
(Cl~alkylidene,
cyano, halo, halo-substituted (C,~)aIkyl, vitro, -XSNR"R", -XsNR"C(O)OR",
-XsNRI4C~o)~14R14~ _X5~14C~I4)~t4R14~ _XSORI4~ _XSSR14' _XSC(O)OR~4'
~,, _XsC(O)NR'4R~'' _XsS(O)ZW4R~4~ _XsP(OXOR'°)OR", -
XSOP(O)(OR"'~OR'°,
s~
-XsNR"C{O)R's, -XsS(O)R's, -XsS(O)2R's and -XsC(O)R's, wherein Xs, R" and R'$
are as
defined above;
R9 is hydrogen or (C~.~)alkyl; and
R3z is (C~_$)alkyl, (C3_~Z)cycloalkyl(C~)alkyl,
hereto(C3.,2)cycloalkyl(C~)alky~,
(C'.,z)aryl(C°.6)all~yl, hetero(C~12)aryl(C~)alkyl;
(C9.,2)polycycloaryl(C~alkyl or
hereto(C8.,2)polycycloaryl(C0.6)alkyl, wherein within R3° any alicyclic
or aromatic ring system
present may be substituted further by 1 to 5 radicals independently selected
from (Cu)alkyl,
(Ca~,)alkylidene, cyano, halo, halo-substituted (C,~)alkyl, vitro, -XsNR'4R",
_~sNR"C(O)OK'",
-X5~14C(O)NR14Ri4~ -Xsy4C~i4)NR'4R'4,. _XsOR", -XsSR", -XsC(O)OR",
-X5C{O)NR'4R'4, _XsS{O)2W4R~4' -Xsp(O)(OR")OR", -XSOP(O)(OR'')OR",
-XsNR'4C(O)R's, -XsS{O)R's, -XsS(O)ZR's and -X$C(O)R's, wherein XS, R" and R's
are as
defined above; and the N oxide derivatives, prodrug derivatives, protected
derivatives, individual
isomers and mixtures of isomers; and tha pharmaceutically acceptable salts
thereof.
In another particular embodiment, the present invention relates to a
pharmaceutical
composition which contains a compound of Formula I or II, or a N oxide
derivative, prodrug
derivative, individual isomer or mixture of isomers, or a pharmaceutically
acceptable salt thereof
in admixture with one or more suitable excipients.
In another particular embodiment, the present invention relates to method of
treating a
disease in an animal in which inhibition of a cysteine protease can prevent;
inhibit or ameliorate
the pathology andlor symptomatology of the disease, which method comprises
administering to
the animal a therapeutically effective amount of compound of Formula I or II
or a N-a~cide
derivative, prodrug derivative, individual isomer or mixture of isomers or a
pharmaceutically
-16-
CA 02475069 2004-08-20
acceptable salt thereof.
In another particular embodiment, the present invention relates to processes
for
preparing compounds of Formula I and II and the N-oxide derivatives, prodrug
derivative,
protected derivatives, individual isomers and mixtures of isomers, and the
pharmaceutically
acceptable salts thereof as set forth in "Detailed Description of the
Invention°'.
In another particular embodiment, the present invention relates to protease
inhibitors of
Formula III:
Ra R5
R6
Rsx2~,.N x1 R~
R3 ~ A
R R8
III
in which:
. A comprises a heteromonocyclic radical containing 5 to 6 annular atoms or a
fused
heteropolycyclic radical containing 8 to 14 annular atoms, wherein each ring
contains 5 to ?
annular atoms, X' is an annular carbon atom and each annular atom other than
X' optionally is a
heteroatom, with the proviso that when A is a heteromonocyclic radical
containing 5 annular
atoms, no more than two of the annular atoms comprising the ring are
heteroatoms;
X' is selected from =C- and -CH-;
Xz is a bond or a divalent group of Formula (a) or (b):
R10
yNe~.X3-~ ~oN.X6.X~N~X~~X3~
R9 R9
(b)
wherein:
X3 and XS independently are -C(O)- or -S{O)2-,
X' is -CI-IR"-, -CHZCHR"- or -CHR"CHZ- and X6 is -CHR'Z-, -CHZCHR'Z-
or -CHR''-CH2- wherein:
R" and R'~ are independently (i) (Cs_6)alkyl or
halo-substituted(C,~)aIkyl optionally substituted with -OR'3, -SR", -S(O)R'3,
-17-
CA 02475069 2004-08-20
-S{~)2Ri3, _C(~)R13, -C{~)~R13, _~13R14, -~14C((~)OR13, -C{O)NR'3R14,
'${~)2~13R14~ _NRIaC(O)~13R14 or -~14C~R14)NR'3R14, wherein R13 is
hydrogen, (C1~)alkyl, (C~.l~cycloalkyl(C~3)alkYl,
hetero(C3.ycYcloaikyl(Co.a)alkyl. (C~.12)aryl(Co-3)alkyl or
hetero(Cs.l~aryl(Co-3)alkyl and R'4 is hydrogen or (C,.~)aIkyl, or
(ii) (C3_y2)cycloalkyl(C°.3)alkyl, hetero(C~12)cyeloalkyl(Ca3)alkyl,
(C6lz)aryl{C°.3)alkyl, hetero(Cs.,z)aryl(Co-3lalkyl,
{C~12)PolYcycloaryl(C°.3)alkYl
or hetero(C&12)polycycloaryl(C°.3)alkyl optionally substituted with -
R's,
-X70R1s, _x~SRls~ _S(O)Rls, _S(O)2R~s~ -C{O)Ru, -C{O)OR's, -X'NR'sRls,
70 -X'NR'bC(O)OR's, -C(O)NR'sR'6: -S{C?)=NR'sRl6, _NRISC{O)NR'sRle or
-NR'bC(NR'6)NR'sR'b, wherein X' is a bond or methylene, R's is
(C3.12)cycloalkyl(C°.3)alkyl, hetera(C3.l~cycloalkyl(Co-3)alkyl,
(C~.lz)aryl(C°.3)alkyl, hetero(Cs_12)aryl(C~3)alkyl,
(C~~2)polycycloaryl(Ca3)alkyl
or hetero(C8.1?)polycycloaryl(C°.3)aIkyl and R'6 is hydrogen or
(C,,°)alkyl, or
(iii) together with R9 or R'° , respectively, when X4 is -CHR"- andlor
X6 is
-CHR'2-, forms trimethylene, tetramethylene or phenylene-1,2-dimethylene,
optionally substituted with hydroxy or oxo; wherein any 1 to 3 annular atoms
of
any aromatic ring with available valences comprising R" andlor R'2 are
optionally independently substituted with halo, vitro, cyano, (C1.~)alkyl,
halo-substituted(C1.~)alkyl, -OR", -C(O}R", -C(0}OR", -C(O)NR"R",
'S(~)2~17Ri?, _X~NR17R1', -X'NR"C(O)OR", -X'NR"C(O)NK"R'a or
-X'NR"C(NR")NR"R", wherein X' is as defned above and each R"
independently is hydrogen or {C1.°)aIkyI; and
R' and R'° are independently hydrogen, (C~.S)alkyl or as defined
above;
R' is hydrogen or -X$X9R'$, wherein X8 is -C(O)- or -S(O)2-, X9 is a bond, -O-
or
-NR'9-, wherein R'9 is hydrogen or (C~.~)alkyl, and R'$ is {i) (Cx~)alkyl or
halo-substituted(C1.~)alkyl optionally substituted with -OR'3, -SR'3, -
S(O)R'3, -S{O)ZR'3,
_C(O)R73~ -C(O)OR13, _~13R14i -~14C(o)~R13, _~(O)~7~13R14, _s{~)2~13R14,
-NR'4C(O)NR'3R'4 or -NR'4C(NR'4)NR13R14~ wherein R'3 and R'4 are as defined
above, or
(ii) (Cs.l~cycloalkyl(C°.6)alkyl, hetero(C~t~cycloalkyl(C~)alkyl,
(C~12)aryl(C~)alkyl,
diphenyl{C~)alkyl, lletero(Cs.l~ary1(C~)alkyl, dihetero(C~)aryl(C~)alkyl,
(C9.,2)polycycloaryl(C~)alkyl or hetero(C&12)polycycloaryl(C~)alkyl optionally
substituted with
-18-
CA 02475069 2004-08-20
-Rls~ _X~OR~s~ -X7SR's, -S(O)R1s' _S(~~)zRis~ _C(O)Ris~ -C(O)ORus _XysRm~
-X7~76C(O)OR15~ ,~(O)NR'sRie~ -S{~)zNR'sR~s~ -yeC{~)~15R16 or
-~16C~16)~75R96~ wherein X', R's and R'6 are as defined above; wherein any 1
to 3
annularlja~tlo~ms of any aromatic ring with available valences comprising R'
optionally
independently are substituted with halo, vitro, cyano, (C,.~)alkyl, halo-
subsdtuted(C,.,~alkyl,
-OR", -C(O)Rn, -C(O)OR», -C(O)NR"R", -S(O)2NR17R1?~ -1781?~
-X'NR"C(O)OK", -X'NR"C(O)NR"R" or -X'NR"C(NR")NR"R", wherein X' aad R"
are as defined above;
Rz is hydrogen or (C,~)alkyl;
R3 is phenyl(Cz_3)alkyl, hetero(Cs_6)aryl(Cz.3)alkyl,
(Cs.~)cycloalkyl(Cz_3)alkyl oa~
hetero(Cs.~)cycloalkyl(Cz_3)alkyl, wherein any 1 to 3 annular atoms of any
aromatic rgng with
available valences comprising R~ optionally independently are substituted with
halo, vitro, cyano,
(C,_6)alkyl, halo-substituted(C,.~)alkyl, -OR", -C(O)R", -C(O)OR", -C{O)NR"R",
-S{O)zNR"Rm~ _X7NRnR17~ -X7~17C{O)ORn~ -X7y7C{O)NRl7Rn oa
-X'NR"C(NR")NR"R", wherein X' and R" are as defined above, and R° is
hydrogen or R3
and R4 are both methyl, ethyl or propyl or together with the carbon atom to
which both R3 and
R4 are attached form cyclopropylene, cyclobutylene or cyclopentylene;
Rs is hydrogen and Rd is hydroxy or Rs and Rs together form oxo;
R' is halo, vitro, -Rz°, -ORz°, -C(O)Rz", -C(O)ORz°, -
S{O)zNRz°Rz', -C{O)NRz°Rz' or
-C{O)NRnCHRz3C(O)ORz° and bonded to any annular carbon atom with a free
valence
comprising A, wherein:
Rz° is hydrogen or R'$, wherein R's is as defined above;
RZ' is hydrogen or (C,~)alkyl;
R'~ is hydrogen, (C,_~aIkyl or together with R~ forms ttimethylene o~
phenylene-1,2-dimethylene, optionally substituted with hydroxy or oxo; and
R~ is as defined above or is (i) (C,.~)alkyl or halo-substituted(C,.~alkyl
optionally substituted with -OR'3, -SR'3, -S(O)R'3, -S(~)zR'3, -C{O)R'3, -
C(O)OR''s,
_NRisR~a~ -~laC{O)~Rn~ _C{O)NR'3R~a~ -S(O)-~y3R14~ -~14C{O)NRt3Rt< or
-NR'°C(NR'°)NR'3R'a, wherein R'3 and R'4 are as defined above,
or
(ii) (C~.,°)cycloalkyl{Co-3)alkyl,
hetero(C~,°)cycloalkyl{C~3)alkyl, (C~,z)aryI(Co-3)alkyl,
hetero(C~sz)aryl(Co-g)alkYl, (C9.,z)PolYcYclaaryl(C0.3)alkyl or
hetero(C~~a)polycycloaryl(C~,3)alkyl optionally substituted with -R's, -
X'OR's, -X'SR's,
-19-
CA 02475069 2004-08-20
-s(o)Rrs~ -g(p)zRis~ -C(p)Ris ~(p)OR's, -X?1VR95R16~ _x~aaC(~)~Ris~
_C(p)NR~sR~a~ _s(p)zNR'sR~a~ _yaC(O)ysR~a or _~laC(NRl6)~ISR16~
wherein X', R's and R'a are as defined above; wherein any 1 to 3 annular atoms
of any
aromatic ring with available valences comprising R~° and/or Rz'
optionally independently
are substituted with halo, vitro, cyano, (C,~)alkyl, halo-
substituted(C,,~)alkyl, -OR's,
-C(O)R", -C(O)OR", -C(~)NR"R", _S(O)iNRnRn. _~'NRnR'?,
-XfiTR"C(O)OR", -X'NR"C(~)NR"R" OT -X71~R17C(~17)~1TR17s wherein X'
and R" are as defined above; and
R8 is hydrogen, halo, hydroxy; formyl, carboxy, carbamoyl, sulfamoyl or
(C~,~)alkyl and
bonded io any annular carbon atom with a free valence comprising A; and tlae N-
oxide
derivatives, prodrug derivatives, protected derivatives, individual isomers
and mixtures of
isomers; and the pharmaceutically acceptable salts thereof.
DETAILED DESCRIPTION OF THE INVENT10N
Definitions:
Unless otherwise stated, the falIowing terms used in the specification and
claims are
defined for the purposes of this Application and have the meanings given this
Section:
"Alicyclic" means a moiety characterized by arrangement of the carbon atoms in
closed
non-aromatic ring structures having properties resembling those of aIiphatics
and may be
saturated or partially unsaturated with two or more double or triple bonds.
"Aliphatic" means a moiety characterized by straight ar branched chain
arrangement of
the constituent carbon atoms and may be saturated or partially unsaturated
with two or more
double or triple bonds.
"Alkenyl" means alkyl, as defined in this Application, provided that the
radical is
comprised of at least one double band. Hence, optionally substituted
(Ci~,)alkenyl as used in
this Application to define R32 includes 2-bromovinyl (-CH~HBr), buts-1,3-
dienyl
(-CHCH-CH~Hz), 2-chioro-I-methylpropenyl (-C(CH3~C1-CH3), 2-chlorovinyl
(-CH~HCI), 4-isopropenyl (-C(CH3~CH~, 1-methylpropenyl (-C(CH3}CH-CH3),
2-methylpropenyl (-CH~(CH3)2), 2-nitrovinyl (-CH~HNO=), propenyl (-CH~H-CH3),
-20-
CA 02475069 2004-08-20
2-trifluoromethylvinyl (-CH:CH-CF3), trifluorovinyl (-CF~F2), vinyl (-CH~H2),
and the like).
"Alkoxy" means the radical -OR, wherein R is alkyl as defined in this
Application,
having the number of carbon atoms indicated (e.g:, (C,~)alkoxy includes the
radicals methoxy,
ethoxy, propoxy, isopropoxy, butoxy, sec-butoXy, isobutoxy, tart-butoxy,
vinyloxy, allyloxy,
1-propenyloxy, isopropenyloxy, I-butenyloxy, 2-butenyloxy, 3-butenyloxy, 2-
methylallyloxy,
ethynyloxy, 1-propynyloxy, 2-propynyloxy, and the like).
"AlkyD" represented by itself means a straight or branched, saturated or
unsaturated,
aliphatic radical having the number of carbon atoms indicated (e.g. (C,~)alkyl
includes methyl,
ethyl, propyl, isopropyl, butyl, see-butyl, isobutyl, ten-butyl, vinyl, allyl,
1-propenyl, isopropenyl,
J I-butenyl, 2-butenyl, 3-butenyl, 2-methylallyl, ethynyl, 1-propynyl, 2-
propynyl, and the like).
Alkyl represented along with another radical (e.g, as in arylalkyl) means a
straight or branched,
saturated or unsaturated aliphatic divalent radical having the number of atoms
indicated or when
no atoms are indicated means a bond (e.g. (Ce,l~laryl(C~.6)alkyl includes
phenyl, benzyl,
phenethyl, I-phenylethyl 3-phenylpropyl, and the Iike).
"Alkylene", unless indicated otherwise, means a straight or branched,
saturated or
unsaturated, aliphatic, divalent radical having the number of carbon atoms
indicated (e.g.
(C~~)alkylene includes methylene (-CH2-), ethylene (-CHiCH2-), trimethylene (-
CH2CHiCH2-),
2-methyltrimethylene (-CH2CH(CH3}CHa-), tetramethylene (-CH2CHzCH2CH2-), 2-
butenylene
(-CH2CH=CHCH2-), 2-methyltetramethylene (-CHZCH(CH3)CH~CHz-), pentamethylene
0 (-CH2CHZCHZCH2CH2-) and the like). Far example: » group of Formula (a),
wherein R" is
hydrogen and R'2 taken together with R' forms optionally substituted
trimethylene is depicted by
the following illustration:
R-
RI
in which R is an optional hydroxy or oxo group and X3 and R' are as defined in
the Summary of
5 the Invention for Formulae I and Il.
"AIkyIidene" means a straight or branched saturated or unsaturaeed, aliphatic,
divalent
radical having the number of carbon atoms indicated (e.g. (C~~)alkylidene
includes methylene
(~HZ), ethylidene (:CHCH3), isopropylidene (:C(CH3)2), propylidene
(:CHCHZCH3),
-21-
CA 02475069 2004-08-20
allylidene (~iCH~H~, and the like).
"Amino" means the radical -NHZ. Unless indicated otherwise, Lhe comgounds of
the
invention containing amino moieties include protected derivatives thereof.
Suitable protecting
groups for amino moieties include acetyl, tert-butoxycarbonyl,
benzyloxycarbonyl, and the like.
"Animal" includes humans, non-human mammals (e.g. dogs, cats, rabbits, cattle,
horses,
sheep, goats, swine, deer, or the like) and non-mammals (e.g. birds, or the
tike).
"Aryl" means a monocyclic or bicyclic ring assembly (fused or linked by a
single bond)
containing the total number of ring carbon atoms indicated, wherein each ring
is comprised of
6 ring carbon atoms and is aromatic or when fused with a second ring forms an
aromatic ring
i 0 assembly. For example,(C~~z)aryl as used in this Application to define R'
includes phenyl,
naphthyl and biphenylyl.
"Aromatic" means a moiety wherein the constituent atoms make up an unsaturated
ring
system, all atoms in the ring system are sp2 hybridized and the total number
of pi electrons is
equal to 4n + 2.
"Carbamoyl" means the radical -C(O)NH2. Unless indicated otherwise, the
compounds
of the invention containing carbamoyl moieties include protected derivatives
thereof. Suitable
protecting groups for carbamoyl moieties include acetyl, tert-butoxycarbonyl,
benzyloxycarbonyl, and the like and both the unprotected and protected
derivatives fall within
the scope of the invention.
"Carboxy" means the radical -C(O)OH. Unless indicated otherwise, the compounds
of
the invention containing carboxy moieties include protected derivatives
thereof. Suitable
protecting groups for carboxy moieties include benzyl, tent-butyl, and the
like. For example; a
compound of Formula I wherein R' contains a carboxy moiety may exist as either
the
unprotected or a protected derivative, e.g. wherein R' is methoxycarbonyl, and
both the
unprotected and protected derivatives fall within the scope of the invention.
.
"CycloaIkyl" means a saturated or partially unsaturated, monocyclic ring,
bicyclic ring
assembly (directly linked by a single bond or fused) or bridged polycyclic
ring assembly
containing the number of ring member carbon atoms indicated, and any
carbocyclic ketone,
thioketone or iniinoketone derivative thereof (e.g. (C3.l~cycIoalkyl includes
cyclopropyl;
cyclobutyi, cyclopentyl, cyclohexyl, cyclohexenyl, 2,5-cyclohexadienyl,
bicyclohexylyl,
cyclopentylcyclohexyl, bicyclo[2.2.2~octyl, adamantan-1-yl,
decahydronaphthalenyl,
oxocyclohexyl, dioxocyclohexyl, thiocyclohexyl, 2-oxobicyclo~2.2.Ijhept-1-yI,
and the like).
-22-
CA 02475069 2004-08-20
"Cycloalkylene" means a saturated or partially unsaturated, monocyclic ring os
bridged
polycyclic ring assembly containing the number of annular carbon atoms
indicated, and any
carbocyclic ketone, thioketone or iminoketone derivative thereof. For example,
the instance
wherein R' and R4 together with the carbon atom to which both R~ and R4 are
attached form
(C~cycloalkylene" includes, but is not limited to, the following:
R2 RS R6 R2 RS R6
~/N ~ ~/N
in which R2, Rs and R6 are as defined in the Summary of the Invention, and any
substituted
derivative thereof.
"Disease" specifically includes any unhealthy condition of an animal or part
thereof and
includes an unhealthy condition which may be caused by, or incident to,
medical or veterinary
therapy applied to that animal, i.e., the "side effects" of such therapy.
"Fused heteropolycyclic ring system" means a saturated, partially saturated or
aromatic
moiety containing two or more rings, wherein at least two ring member atoms of
one ring are
common to a second ring containing the number of ring member atoms indicated
in which at
least one of the ring member atoms is a heteroatom and any carbocyclic ketone,
thioketone,
iminoketone or substituted derivative thereof . For example, the term "a fused
heteropolycyclic
radical containing 8 to 14 ring member atoms" as used in this Application to
define A may
include acridinyl, benzofuryl, benzooxazolyl, benzothiazolyl, carbazolyl,
carbolinyl, chromanyl,
chromenyl, cinnolinyl, indazolyl, indolinyl, indolyl, indoIizinyl,
isobenzofuryl, isochromenyl;
isochromanyl, isoindolinyl, isoquinolyl, naphthyridinyl, perimidinyl,
phenanthridinyl,
phenanthrolinyl, phenazinyI, phenothiazinyl, phenoxathiinyl, phenoxazinyl,
phthalazinyl, pteridinyl,
purinyl, pyrrolizinyl, quinazolinyl, quinolizinyl, quinolyl, quinoxalinyl,
quinuclidinyl, xanthenyl, and
the like.
"Guanidino" means the radical -NHC(NH)NHz. YJnless indicated otherwise, the
compounds of the invention containing guanidino moieties include protected
derivatives thereof.
Suitable protecting groups for aminA moieties include acetyl, rert-
butoxycarbonyl,
benzyloxycarbonyl, and the like and both the unprotected and protected
derivatives fall within
-23-
CA 02475069 2004-08-20
the scope of the invention.
"Halo" means fluoro, chloro, bromo or iodo.
"Halo-substituted alkyl", as a ,group or part of a group, means "alkyl"
substituted by one
or more "halo" atoms, as such terms are defined in this Application. Halo-
substituted alkyl '
includes haloalkyl, dihaloalkyl, trihaloalkyl, perhaloalkyl and the like (e.g.
halo-substituted
(C~.3)alkyl includes chloromethyl, dicloromethyl, difluoromethyl,
trifluromethyl,
2,2,2-trifluoroethyl, pexfluoroethyl, 2,2.,2-trifluoro-i,l-dichloroethyl, and
the like).
"Heteroaryl" means aryl, as defined herein, provided that one or more of the
ring
member carbon atoms indicated, is replaced by heteroatom moiety selected from -
N-, -NR-,
-O- or -S-, wherein R is hydrogen. (C,.~)alkyl or a protecting group, and each
ring contained
therein is comprised of 5 to 6 ring member atoms. For example,
hetero(C~12)aryl as used in this
Application includes benzofuryl, benzooxazolyl, benzothiazolyl,
[2,4']bipyridinylyl, carbazolyl,
carbolinyl, chromenyl, cinnolinyl, furazanyl, furyl, imidazolyl, indazolyl,
indolyl, indolizinyl,
isobenzofuryl, isochromenyl, isoaxazolyl, isoquinolyl, isothiazolyl,
naphthyridinyl, oxazolyl,
perimidinyl, 2-phenylpyridyl, phthalazinyl, pteridinyl, purinyl, pyrazinyl,
pyradazinyl, pyrazolyl,
pyridyl, pyrimidinyl, pyrrolizinyl, pyrrolidinyl, pyrrolyl, pyranyl,
quinazolinyl, quinoIizinyl, quinolyl,
quinoxalinyl, tetrazolyl, thiazolyl, 4-thiazol-4-ylphenyl, thienyl, xanthenyl,
and the like.
"Heteroatom moiety" includes -N:, -NR-, -O-, -S- or -S(O)2-, wherein R is
hydrogen,
(C~.~)alkyl or a protecting group.
"Heterocycloalkyl" means cycloalkyl, as defined herein, provided that one or
more of
the ring member carbon atoms indicated is replaced by heteroatom moiety
selected from -N:,
-NR-, -O- or -S-, wherein R is hydrogen, (C,.~)alkyl or a protecting group,
and any carbocyclic
ketone, thioketone or iminoketone derivative thereof (e.g. the term
hetero(CS_~~cycloalkyl
includes [1,4']bipiperidinylyl, dihydrooxazolyl, morpholinyl, 1-morpholin-4-
ylpiperidinyl,
piperazinyl, piperidyl, pirazolidinyl, pirazolinyl, pyrrolinyl, pyrrolidinyl,
quinuclidinyl, and the Iike).
Suitable protecting groups include tert-butoxycarbonyl, benzyioxycarbonyD,
benzyl,
4-methoxybenzyl, 2-riitrobenzyl, and the dike. For example, a compound of
Formula I wherein
R' is piperidin-4-ylcarbonyl may exist as either the unprotected or a
protected derivative, e.g.
wherein R' is 1-tert-butoxycaxbonylpiperidin-4-ylcarbonyl, and both the
unprotected and
3o protected derivatives fall within the scope of the invention.
"Heterocycloalkylene" means cycloalkylene, as defined in this Application,
provided
that one or more of the ring member carbon atoms indicated, is replaced by
heteroatom moiety
-24-
CA 02475069 2004-08-20
selected from -N;, -NR-, -O-, -S- or -S(O)2-, wherein R is hydrogen or
(C,.~)alkyl. For
example, the instance wherein R3 and Ra together with the carbon atom to which
both R' and
R" are attached form hetero(C~.$)cycloalkylene" includes, but is not limited
to, the following:
R2 R5 R6 ~ r R2 R5 R6
N N
w
N
R
in which R is hydrogen, (C,.~)alkyl or a protecting group and R2 is as defined
in the Summary of
the Invention, and any substituted derivative thereof.
"Heteromonocyclic" means a saturated, partially saturated or aromatic
monocyclic
radical containing the number of ring member atoms indicated in which at Ieast
one of the ring
member atoms is a heteroatom and any carbocyclic ketone, thioketone,
iminoketone or
substituted derivative thereof. For example, the term "a heteromonocyclic
containing 5 to 6 ring
member atoms" as used in this Application to define A may include
dihydrooxazolyl, furazanyl,
furyl, imidazolyl, imidazolidinyl, imidazolinyl, isooxazoIyl, isothiazolyl,
thiazolyl, thienyl,
morpholinyl, oxazolyl, piperazinyl, piperidinyl, pirazolidinyl, pirazolinyl,
pyranyl, pyrazinyl,
pyradazinyl, pyrazolyl, pyridyl, pyrimidinyl, pyrrolidinyl, pyrrolinyl,
pyrrolyl, tetrazolyl, and the like.
75 "Heteropoiycycloaryl" :mans potycycloaryl, as defined herein, except one or
more of
the ring member carbon atoms indicated are replaced by a heteroatom moiety
selected from
-N:, -NR-, -O- or -S-, wherein R is hydrogen, (C,~)alkyl or a protecting
group, and any
carbocyclic ketone, thioketone or iminoketone derivative thereof.. For
example,
hetero(C~l~polycycloaryl includes 1',2'-dihydro-2H-[1,4']bipyridinylyl,
chromanyl, imidazolinyl,
indolinyl, isochromanyl, isoindolinyl, and the Iike.
"Hydroxy" means the radical -OH. Unless indicated otherwise, the compounds of
the
invention containing hydroxy radicals include protected derivatives thereof:
Suitable protecting
groups for hydroxy moieties include benzyl and the like and both the
unprotected and protected
derivatives fall within the scope of the invention:
"Iminoketone derivative" means a derivative containing the moiety -C(NR)-,
wherein R
is hydrogen or (C,,~)alkyl.
-25-
CA 02475069 2004-08-20
"Isomers" mean compounds of Formula I having identical molecular formulae but
differ
in the nature or sequence,of bonding of their atoms or in the arrangement of
their atoms in
space. Isomers that differ in the arrangement of their atoms in space are
termed
"stereoisomers". Stereoisomers that are not mirror images of one another are
termed
"diastereomers" and stereoisomers that are nonsupetimposable mirror images are
termed
"enantiomers" or sometimes "optical isomers". A carbon atom bonded to four
nonidentical
substituents is termed a "chiral center". A compound with one chiral center
has two
enantiomeric forms of opposite chirality is. termed a "racemic mixture". A
compound that has
more than one chiraI center has 2"'' enantiomeric pairs, where n is the number
of chiral centers.
Compoards with more than one chiral center may exist as ether an indiviiiual
diastereomer or as
a mixture of diastereomers, termed a "diastereomeric mixture". When one chiral
center is
present a stereoisomer may be characterized by the absolute configuration of
that chiral; center.
Absolute configuration refers to the arrangement in space of the substituents
attached to the
chiral center. Enantiomers are characterized by the absolute configuration of
their chiral
centers and described by the R- and S-sequencing rules of Cahn, Ingold and
I'relog.
Conventions for stereochemical nomenclature, methods for the determination of
stereochemistry
and the separation of stereoisomers are well known in the art (e.g. see
"Advanced Organic
Chemistry", 3rd edition, March, Jerry, John Wiley & Sons, New Yark, 1985). It
is understood
that the names and illustration used in this Application to describe compounds
of Formula I are
meant to be encompassed all possible stereoisomers arid any mixture, racemic
or otherwise,
thereof.
"Ketone derivative" means a derivative containing the moiety -C(O)-.
"Nitro" means the radical -NOz.
"Optional" or "optionally" means that the subsequently described event or
circumstance
may or may not occur, and that the description includes instances where the
event or
circumstance occurs arid instances in which it does not. For example, the
phrase "(C~.~)alkyl
optionally substituted with cyano, halo, vitro," means that the alkyl group
referred to may or may
not be substituted in order to fall within the scope of the invention.
"Oxalo" means the radical -C(O)C(O)OH.
"N-oxide derivatives" means a derivatives of compound of Formula I in which
nitrogens
are in an oxidized state (i.e., O-N) and which possess the desired
pharmacological activity.
"Oxo" means the radical=O.
-2b-
CA 02475069 2004-08-20
"Pathology" of a disease means the essential nature, causes and development of
the
disease as well as the structural and functional changes that result from the
disease processes:
"Pharmaceutically acceptable" means that which is useful in preparing a
pharmaceutical composition that is generally safe, non-toxic and neither
biologically nor
. otherwise undesirable and includes that which is acceptable for veterinary
use as well as human
pharmaceutical use.
"Pharrrlaceutically acceptable salts" means salts of compounds of Formula I
which are
pharmaceutically acceptable, as defined above, and which possess the desired
pharmacological
activity. Such salts include acid addition salts formed with inorganic acids
such as hydrochloric
t0 acid, hydrobrornic acid, sulfuric acid, nitric acid, pnosphuric acid, and
the like; of with organic
acids such as'acetic acid, propionie acid, hexanoic acid, heptanoic acid,
cyclopentanepropionic
acid, glycolic acid, pyruvic acid, lactic acid; malonic acid, succinic acid,
malic acid, malefic acid;
fumaric acid; tartatic acid, citric acid, benzoic acid; o-(4-
hydroxybenzoyl)benzoic acid, cinnamic
acid, madelic acid, methanesulfonic acid, ethanesulfonic acid, 1,2-
ethanedisulfonic acid,
2-hydroxyethanesulfonic acid, benzenesulfonic acid, p-chlorobenzenesulfonic
acid,
2-naphthalenesulfonic acid, p-toluenesulfonic acid, camphorsulfonic acid,
4-methylbicyclo[2.2.2Joct-2-ene-1-carboxylic acid, glucoheptonic acid,
4,4'-inethylenebis(3-hydroxy-2-ene-1-carboxylic acid), 3-phenylpropionic acid,
trimethylacetic
acid, tertiary butylacetic acid, lauryl sulfuric acid, gluconic acid, glutamic
acid, hydroxynaphthoic
acid, salicylic acid, stearic acid, muconic acid and the like.
Pharmaceutically acceptable salts also include base addition salts which may
be formed
when acidic protons present are capable of reacting with inorganic ox organic
bases.
Acceptable inorganic bases include sodium hydroxide, sodium carbonate,
potassium hydroxide,
ammonium hydroxide, aluminum hydroxide and calcium hydroxide. Acceptable
organic bases
include ethanolamine, diethanolamine, triethanoIamine, tromethamine, N
methylglucatnine and
the like.
"Phenylene-1,2-dimethylene" means the divalent radical -CIi2C6H4CHz-, wherein
the
methylene moieties are attached at the 1- and 2-positions of the phenylene
moiety. For
example, a group of Formula (a) in which R'2 together with R9 forms optionally
substituted
phenylene-1;2-dimethylene is illustrated by the following formula:
-2'7-
CA 02475069 2004-08-20
in which R is an optional hydroxy group and X3 and R' are as defined in the
Summary. of the ,
Invention for Formulae I and Il.
"Polycycloaryl" means a bicyclic ring assembly (directly linked by a single
bond or
fused) containing the number of ring member carbon atoms indicated, wherein at
least ane, bui
not all, of the fused rings comprising the radical is aromatic, and any
carbocyclic ketone,
thioketone or iminoketone derivative thereof (e.g. (Cø~2~olycycloaryl includes
indanyl, indenyl,
1,2,3,4-tetrahydronaphthalenyl, I,2-dihydronaphthalenyl, cyclohexylphenyl,
phenylcyclohexyl,
2,4-dioxo-l,2,3,4-tetrahydronaphthalenyl, and the like):
"Prodrug" means a compound which is convertible in vivo by metabolic means
(e.g.
by hydxolysis) to a compound of Formula ()7. For example an ester of a
compound of
formula (I) containing a hydroxy group may be convertible by hydrolysis in
vivo to the parent
molecule. Alternatively an ester of a compound of Formula (I~ containing a
carboxy group
may be convertible by
i5 hydrolysis in vivo to t!te parent molectlte. Suitable esters of compounds
of Formula (I)
containing a hydroxy group, are for example acetates, citrates, lactates;
tartrates, malonates;
oxalates, salicylates, propionates, succinates, fumarates, maleates,
methylene-bis-b-hydroxynaphthoates, gentisates, isethionates, di p-
toluoyltartrates,
methanesulphonates, ethanesulphonates, benzenesulphonates, p-
tolue~esulphonates,
cyclohexylsulphamates and quinates. Suitable esters of compounds of Formula
(I) containing
a carboxy group, are for example those described by F.J.Leinweber, Drug Metab.
Res.,
1987, 18, page 379. An especially useful class of esters of compounds of
Formula (>)
containing a hydroxy group, may be formed from acid moieties selected from
those described
by Bundgaard et. aL, J. Med. Chem., 1989, 32 , page 2503-2507, and include
substituted
(aminomethyl}-benzoates, for example, dialkylamino-methylbenzoates in which
the two alkyl
-28_
CA 02475069 2004-08-20
groups may be joined together andlor interrupted by an oxygen atom or by an
optionally
substituted nitrogen atom, e.g. an alkylated nitrogen atom, more especially
(morpholino-methyl)benzoates, e.g. 3- or 4-(morpholinomethyl)-benzoates, and
(4-alkylpiperazin-1-yI)benzoates, e.g. 3- or 4-(4-alkylpiperazin-1-
yl)benzoates. A pro~lrug
derivative of a compound of Formula I wherein R3 and R6 together are oxo is
depicted by the
following formula:
x13
r'~ O~
1
R X2,.~h1 Xl R7
R3 4 A
R. ~~ RS
( )n
in which X13 is a bond, straight, saturated ethylene or (-CHZCR"R$ZCPI2-),
wherein R°' and R42
independently are hydrogen, halo or (C~_3)alkyl or taken together form
methylene.
"Protected derivatives" means derivatives of compounds of Formula I in which a
,
reactive site or sites are blocked with protecting groups. Protected
derivatives of compounds of
Formula I are useful in the preparation of compounds of Formula I or in
themselves may be
active cysteine protease inhibitors. For example, the compound of Formula I
which is 2S-
amino-N-(2-benzooxazol-2-yl-2-hydroxy-1S-phenethylethyl)-3-
cyclohexylpropionamide (i.e.,
1 b Compound 55, described in Example 6, infra) may be protected with a
suitable amino protecting
group, e.g. 9H-fluoren-9-ylmethoxycarbonyl, or a suitable hydroxy protecting
group, e.g. reit-
butyldimethylsilanyl, to provide, respectively, 9H-fluoren-9-yimethyl 1S-(2-
benzooxazol-2-y1-
2-hydroxy-1S-phenethylethylcarbamoyl)-2-cyclohexylethylcarbamate (i.e.,
Compound 51,
described in Example 4, infra) and ~S-amino-N [2-benzooxazol-2-yl-
2-(rerr-butyldimethylsiianyloxy)-1S-phenethylethyll-3-cyclohexylpropionamide
(i.e., Compound
S6, described in Example 7, infra). A comprehensive list of suitable
protecting groups can be
found in T.W. Greene, Protecting Groups in Organic Synthesis, John ~7Viley &
Sons, Inc.
1981.
"Ring member", as in fused heteropolycyclic ring system containing 8 to 14
ring
member atoms, means that the atoms referred to are ring members of the fused
heteropolycyclic radical, but not taking into account ring members of any
substituents present.
Thus, for example, a heteropolycyclic radical containing 8 ring member atoms
includes
-29-
CA 02475069 2004-08-20
benzooxaxol-2-yl, benzofur-2-yl, 1H-indol-5-yl, benzothiazol-2-yl, and the
like.
"Sulfamoyl" means the radical -S(O)2Nfi2. Unless indicated otherwise, the
compounds
of the invention containing sulfamoyl radicals include protected derivatives
thereof. Suitable
protecting groups for sulfamoyl radicals include acetyl, rert-butoxycarbonyl,
benzyloxycarbonyl,
and the like and both the unprotected and protected derivatives fall within
the scope of the
invention.
"Therapeutically effective amount" means that amount which, when administered
to an
animal for treating a disease, is sufficient to effect such treatment for the
disease.
"Thioketone derivative" means a derivative containing the moiety -C(S)-.
"Treatment" or "treating" means any administration of a compaurid of the
p~eszrn"
invention and includes:
(1) preventing the disease from occurring in an animal which may be
predisposed to the
disease but does not yet experience or display the pathology or symptomatology
of the disease,
(2) inhibiting the disease in an animal that is experiencing or displaying the
pathology or
symptomatoiogy of the diseased {i.e., arresting further development of the
pathology and/or
symptomatology), or
{3) ameliorating the disease in an animal that is experiencing or displaying
the pathology or
symptomatology of the diseased (i.e., reversing the pathology and/or
symptomatology).
Specific Embodiments or the Invention:
Vdhile the broadest definition of the invention is set forth in the Summary of
the
Invention, certain aspects of the invention are preferred. A preferred aspect
of the invention
are compounds of Formula I in which X' is =C-. In particular, the
heteromonocyclic ring or
fused heteropolycyclic ring system A is selected from 4,5-dihydrooxazol-2-yl,
benzooxazol-2-yl,
benzoihiazol-2-yl and oxazol-2-yl, each substituted by a group R' and
optionally substituted with
a group R8, particularly wherein R' is hydrogen, halo, (C~,~alkoxy,
(Cc~)alkoxycarbonyl, vitro or
phenyl and R$ at each occurrence independently is halo, (C,~,)alkoxy,
(C,.~alkoxycarbonyl, vitro
or trifluoromethyl. The ring system A preferably is benzoxazoI-2-yI
substituted by a group R'
and optionally substituted with a group R8, parricularly wherein R' i's
hydrogen, halo,
(C,~)alkoxy, (C,~)alkoxycarbonyl or vitro and R$ at each occurrence
independently is halo,
(C,.,)alkoxy, {C,.~alkoxycarbonyl, vitro or trifluoromethyl.
X~ particularly represents a bond or a divalent~group of Formula (a);
particularly,
-30-
CA 02475069 2004-08-20
wherein within Formula (a) X3 is -C{Or; R9 represents hydrogen, R" represents
hydrogen or
methyl, typically hydrogen, and R'Z particularly represents (i) {C,.~)aikyl
substituted with -SR'°,
-S(O)R'° or -S(O~R''', wherein R'° is (C~.i~aryl(C~,)alkyl or
hetero(Cs.,2)aryl(C~alkyl or
(ii) (C~l~cycloalkyl(C~alkyl or (C~.,2)aryl(C~)alkyl; wherein within R'2 any
alicyclic or
aromatic ring system present may be substituted further by 1 to 5 radicals
independently
selected from (C,~)alkyl, (C,,~)alkylidene, cyano~ halo, halo-substituted
(C,~alkyl, nitro,
_XsNR~aRta~ _X5~14C(~)ORi4~ _X5~14C(O)~14R14~ -XSNRI4C~Rt4)NR'4R14~ _X5~R14~
_XsSRio~ _XsC(O)pR~a~ _XsC(O)NR~aR~4' _XsS(O)a~nRae~ -Xsp(C)(ORra)OR",
-XSOP(O)(OR'°)OR'4, -XsNR'°C(O)R'$, -XsS(O)R's, -XsS(O)ZR's and -
XsC(O)R's, wherein
Xs is a bond or (C,.~)alkylene, R'4 at each occurrence independently is
hyi3rogen; {C,.~)alkyl or
halo-substituted {Cy-3)alkyl and R's is .(C,.~)alkyl or halo-substituted
(C,_s)alkyl.
Further preferred, within Formula (a), R'~ particularly represents a group
having the
following formula:
in which q is 0, 1, 2, 4 or 5 and R33 at each occurrence independently is
selected from a group
consisting of (C,~)alkyl, cyano, halo, halo-substituted (C,,~)alkyl, nitro, -
XsNR"R'4, -XSOR",
_xsSRve~ -XSC(O)~14R14y -XsC(O)OR~e~ _XsS(O)Ris~ _Xss(O)zR~s and -XSC(O)R'S,
wherein
Xs is a bond or {C,.~)alkylene, R'4 at each occurrence independently is
hydrogen, (C,-3)alkyl or
halasubstituted (C,-3)alkyl and R's is (C,_3)alkyl or halo-substituted
(C,_3)alkyl; more particularly
in which q is 0, I or 2 and R33 at each occurrence independently is selected
from a group
consisting of (C,~)alkyl, cyano, halo, halo-substituted (C,~,)alkyl, vitro,'-
OR'4, -SR" and
-C(O)OR'4, wherein R'a independently is hydrogen, (C,_3)alkyl or halo-
substituted (C,_3)alkyl;
more particularly in which R33 at each occurrence independently is selected
from a group
consisting of {C,.~)aIkyI, bromo, carboxy, chloro, cyano, difluoromethoxy,
fluoro, iodo, methoxy,
vitro, trifluoromethoxy, trifluoromethyl and trifluorosulfanyl
Further preferred, within Formula (a), R'2 particularly represents
benzylsulfonylmethyl,
2-chlorobenzylsulfonyhwethyl, ~-cyanobenzylsulfonylmethyl,
-31-
CA 02475069 2004-08-20
2-difluoromethoxybenzylsulfonylmethyl, 3,5-dimethylisooxazol-~-
ylmethylsulfonylmethyl,
2-methoxybenzylsuifonylmethyl, 4-methylpyrid-2-ylmethyIsulfonylmethyl,
2-nitrobenzylsulfonyhnethyl, pyrid-2-ylmethylsulfonyImethyl, o-
tolylmethylsulfonylmethyl or
2-trifluoromethylbenzylsulfonylmethyl.
R' particularly represents -X6X'Rz°, wherein X6 is -C(O~ or -S(O)z-,
X'.is a bond, -O-
or -NRZ'-, wherein R2' is hydrogen ar (Cl~)alkyl, and R~° is (i)
(C,~)alkyl optionally substituted
by -C(O)OR'° or (ii) (C~~~}cycloalkyl(C~)alkyl,
hetero(C3_,~cycloalkyl(C~)alkyl,
(C~,Z)aryl(C~)alkyl or hetero(Cs.l2)aryl(Co..b)alkyl or (iii)
(C3,~cycloalkyl(C;~)alkyl,
hetero(C~,.~)cycloaIkyl(C~.6)alkyl, phenyl(C~)alkyl or
hetero(C~)aryl(C~)alkyl, wherein said
1U cycloalkyl, heterocycloalkyl, phenyl or heteroaryl ring is substituted by -
X50R~°; XSC(O)R~', .
-XSC(O)OR~, -X5C(O)NRZ'R~, -XSNR~'R~, -X5NR25C(O)R~', -XsNR'~C(O)OR~, '
-XsNR'~C(O)NR~'R~ or -XSNRZSC(NR'~)NRZ°R'~, wherein XS is a bond or
(C,~)alkylene, R?~
is (C3.~)cycloalkyl(C~}alkyl, hetero(C~)cycloalkyl(C~)alkyl; phenyl(C~)alkyl
or
hetero(C~)aryl(Co.,6)alkyl and R~ is hydrogen or (C~~)alkyl; wherein within R'
any alicyclic or
aromatic ring system present may be substituted further by 1 to 5 substituents
independently
selected from (C~~alkyl, halo, halo-substituted (C~~)alkyI, -OR'4 and -
C(O)OR'° wherein R'4
is hydrogen or (C»)alkyl, or when X'- is a divalent group of formula (a} then
R' may be, but is
not limited to, hydrogen or oxalo.
R' preferably is a group selected from acetyl, azetidin-3-ylcarbonyI,
benzyloxycarbonyl,
2d 1-benzyloxycarbonylpiperidin-4-ylcarbonyl, benzylsuifonyl,
bicyclo[2.2.2]hept-2-ylcarbonyl,
bicyclo[2.2.1]hept-2-ylcarbonyl, tart-butoxycarbonyl, carboxyace3yl, 2-
carboxyprogionyl,
3-carboxypropionyl, 2-cyclohexylacetyl, 4-cyclohexyibutyryl, 2-
cyclohexylethylsulfonyl,
cyclohexylmethoxycarbonyl, 3-cyclohexylpropionyl, 2-cyclopentylethylsulfonyl,
3-cyclopentylpropionyl, di(2-methoxyethyl)carbamoyl, dimethylcarbamoyl,
6-hydroxypyrid-3-ylcarbonyl,1H-imidazol-4-ylcarbonyl, iriethoxycarbonyl,
methylsnlfonyl,
4-methylvaleryl, morpholin-4-ylcarbonyl, 2-rnorpholin-4-ylethylcarbonyl,
naphth-1-ylacetyl,
naphth-1-ylmethylcarbonyl, oxalo, 3-phenylpropionyl, piperazim-I-ylcarbonyl,
piperidin-4-ylcarbonyl, pyrazin-2-ylcarbonyl, pyrid-3-yIcarbonyl, pyrid-4-
ylcarbonyl,
pyrid-3-ylaminocarbonyi, tetrahydropyran-4-ylcarbonyl and tetrahydropyran-4-
yloxycarbonyl,
R' especially represents morpholin-4-ylcarbonyl, methoxycarbonyl,
methylsulfonyl;
piperidin-4-ylcarbonyl, pyrazin-2-ylcarbonyl pyrid-3-ylcarbonyl, pyrid-4-
yIcarbonyl,
tetrahydropyran-4-ylcarbonyl or tetrahydropyran-4-yloxycarbonyl.
-32-
CA 02475069 2004-08-20
RZ typically is hydrogen.
R3 particularly represents hydrogen, (C,~alkyl (optionally substituted with
cyano, halo,
vitro, -SRS, -C(O)ORS, -C(O)NR'~Rz6, -P(O)(OR~)OR~, -OP(O)(OR~)OR~, -S(O)RB',
-S(O)2R~' or -C(O)RZ', wherein R~ at each occurrence independently is
hydrogen, (C,~)alkyl,
or halo-substituted (C~.3)alkyl and R~' is {C,.~alkyl or halo-substituted
(C,.3)alkyl) or
(C~.12)aryl(C~_3)aIkyl, wherein said aryl optionally is substituted further
with 1 to 5 radicals
independently selected from (C~,~)alkyl, (C,.~)alkylidene, cyano, halo, halo-
substituted
{G,~)alkYl, intro, -XsNR"C(0)OR'", -XsNR'°C(~)NR~aRt4, -
XsNRI4C(NRI4)~tbRl4~
-xsOR~a~ -XsSR~a~ -XsC(O)OR~4~ -XsC(O)y aR~a~ -XsS(O)2y aR~a~ -
Xsp(O)(OR'~)OR'4,
-XSOP(O)(OR")OR'°, -XsNR'4C(0)R's, -XsS(0)R's, -XsS(0)zR's and
~XsC{0)Rt', wherein
Xs is a bond or (C~~)alkylene, R'4 at each occurrence independently is
hydrogen, {C,,~)alkyl or
halo-substituted {C1.3)alkyl and R's is (C~.~)alkyl or halo-substituted {C,-
3)alkyl, or R3 and R4
taken together with the carbon atom to which both R3 and R4 are attached form
(C~)cycloalkylene. In particular, R3 may be selected from hydrogen,
(C,.~)alkyl (e.g. methyl;
ethyl, n-propyl, n-butyl), phenyl(C2.3)alkyl (e.g. pheneihyl) or
(C,~)alkylsulfanyl(C~)alkyl (e.g.
2-methylsulfonylethyl) or R3 and R4 taken together with the carbon atom to
which both R3 and
R4 are attached form (C3.~)cycloalkylene (e.g. cyclobutylene or
cyclohexylene). R3 preferably
is (Ct~)alkyl.
R° particularly represents hydrogen or R3 and R° taken together
with the carbon atom
to which both R3 and R" are attached form (C~)cycloalkylene (e.g:
cyclohutylene or
cyclohexylene).
Rs and R6 preferably together form oxo.
Compounds of Formula II are preferred in which:
nis0;
1 X' is =C- and the ring system A is selected from 4,5-dihydrooxazol-2-yl,
benzooxazol-2-yl, benzothiazol-2-yl and oxazol-2-yl, each substituted by a
group R' and
optionally substituted with a group RB, particularly wherein R' is hydrogen,
halo, {C,~)alkoxy,
(C~.~)aIkoxycarbonyl, vitro or phenyl and R8 at each occurrence independently
is (Ct,~)alkoxy,
(C~,~)alkoxycarbonyl, vitro or trifluoromethyl.
X8 methylene or ethylene;
R', R3 and R4 are as defined above;
Rs and R6 together form oxo;
-33-
CA 02475069 2004-08-20
R9 is hydrogen; and
R'2 is -X9R3°, wherein X9 is methylene when X8 is methylene and X9 is a
bond when Xg
is ethylene, Rs° is -CR3sCHR36 or -CR~NR38; wherein R3s and R~ together
with the atoms to
which R3s and R~ are attached form (Cz.,e)alkenyl, (Cs.~2)cycloalkenyl,
hetero(Cs.,~cycloalkenyl, (C,~12)aryl, hetero(C6~~aryl, (C~.1~}bieycloaryl or
hetero(C&,~bicycloaryl and Rs' and R3$ together with the atoms to which Rs'
and R38 are
attached form hetero(Cs.,~cycloalkenyl, hetero(C~1Z)aryl or
hetero(CB.,z)bicycloaryl, wherein
within R3° said cycloalkenyl, heterocycloalkenyl, aryl, heteroaryl,
bicyeloaryl or heterobicycloaryl
may be substituted further by 1 to 5 radicals independently selected from
(CL~)alkyl,
(C~.~)alkylidene, cyano, halo, halo-substituted (C,~)alkyl, nitrb, -
XsNR'''R'°, -XsNR'°C(O)OR'°,
-XsNR~aC(O)NRtaR~a~ -x5~TR14C~14)~74RI4~ -XS~R14~ -XSSR14' -XsC(~)pRl4'
-XsC(O)y4R~4~ _XsS(O)zWaRl~~a~~~-~Xsp(~)(OR'°)OR'°, -
XSOP(O)(OR'°)OR'4,
-XsNR'°C(O)R's, -XsS(O)R's, -XsS(O~R's and XsC(O)R's, wherein Xs is a
bond or
(Cl~)alkylene, R'4 at each occurrence independently is hydrogen, (C,~)aIkyl or
halo-substituted
(C1.3)alkyI and R's is (C,.~alkyl or halo-substituted (C,:3)alkyl.
Rs° particularly represents (C~t2)aryl or hetero(C~12)aryl, each
optionally substituted by
1 to 5 radicals selected from a group consisting of (C~~)alkyl, cyano, halo,
halo-substituted
(Ci.4)alkyl, vitro, -XsNR'°R'4~ -XsOR'°, -XsSR'4, -
XsC(O)NR'4Ra4' -XsC(O)ORa4,
-XsS(O)R's, -XsS(O)ZR's and -X5C(O)R's, wherein Xs is a bond or (C,_~alkylene,
R'° at each
occurrence independently is hydrogen, (C~ 3)aIkyI of halo-substituted
(C,.3)alkyl and R's is
(C~_3)aIky1 or halo-substituted (C~.3)alkyl. Rs° more preferably
represents biphenyl, isooxazolyl,
naphthyl, phenyl, pyridyi or thienyi, each optionally substituted by 1 to 5
radicals selected from a
group consisting of (C~~)alkyl, cyano, halo, halo-substituted (C,-
°)alkyl, vitro, -XSNR'°R'4,
-XsOR,4~ -XsSRu' -XSC(O)~14R14s _XsC(p,OR~4' -XsS(O)Ris4 -XsS(O)2Ris ~d
-XsC(O)R's, wherein.X4 is a bond or (C,~)alkylene; R'° at each
occurrence independently is
hydrogen, (C,_3)alkyl or halo-substituted (C~ 3)alkyI and R's is (C'_3)alkyl
or halo-substituted
(Cy.3)alkyl: Rs° more preferably represents biphenyl-2-yl, 2,4-
bistrifluoromethylphenyl,
2,5-bistrifluoromethylphenyl, 4-tert-butylphenyl, 2-bromophenyl, 3-
bromophenyl, 4-bromophenyl,
2-bromo-5-fluorophenyl, 3-chloro-2-fluorophenyl, 2-chlorophenyI, 3-
chlorophenyl,
4-chlorophenyl, S-chlorothien-2-yl, 2-chloro-5-trifluoromethyl, 2-cyanophenyl,
3-cyanophenyl;
4-cyanophenyl, 1,5-dichlorophenyl, 2,6-dichlorophenyl, 3,4-dichlorophenyl, 2,3-
difluorophenyl,
2,4-difluorophenyl, 3,4-difluorophenyl, 2-difluoromethoxyphenyi, 3-
difluoromethoxyphenyl,
-34-
CA 02475069 2004-08-20
4-difluorornethoxyphenyl, 2,5-difluorophenyl, 2,6-difluorophenyl, 3,5-
dimethylisooicaxol-4-yl,
3,5-dimethylphenyl, 2-fluoro-6-nitroptienyI, 2-fluorophenyl, 4-fluorophenyl, 2-
fluoro-
3-trifluoromethylphenyl, 2-fluoro-4-trifluoromethylphenyl, 2-fluora-5-
trifluoromethylphenyl,
2-fluoro-6-trifluoromethylphenyi, 4-fluoro-2-trifluoromethylphenyl, 4-fluoro-
'
3-trifluoromethylphenyl, 2-iodophenyl, 3-iodophenyl, 4-iodophenyl, 2-
methoxyphenyl,
4-methoxyphenyl, 2-methylphenyi, 3-methylphenyl, 4-methytphenyl, 6-methylpyrid-
2-yI,
3-methyl-2-fluorophenyl, naphth-2-yl, 2-niirophenyl, 3-nitrophenyl, 4-
nitrophenyl,
2,3,4,5,6-pentafluorophenyI, phenyl, prop-2-en-1-yl, pyrid-2-yl, pyrid-3-yl,
pyrid-4-yl, thien-3-yl,
o-tolyl, 2-trifluoromethoxyphenyl, 3-trifluoromethoxyphenyl, 4-
trifluoromethoxyphenyl,
. 3-trifluoromethylphenyl, 4-trifluoromethylphenyl, 2-
trifluaromethylsulfariyIphenyl,
3-trifluoromethylsulfanylphenyl, 4-trifluoromethylsulfanyIphenyl, 2,3,4-
trifluorophenyl, °
2,3,5-trifluorophenyl, 2,4,6-trifluarophenyl, 2,4,5-trifluorophenyl or 2,3,6-
trifluorophenyl.
A preferred group of compounds of Formula II are those in which -XgS(O)zR3z
represents a group having the following formula:
r~~ (R33)9
S(O)2
NVW
in which Q is 0, 1, 2, 4 or 5 and R33 ai each occurrence independently is
selected from a group
consisting of (C~.a)alkyl, cyano, halo, halo-substituted (C~.a)alkyl, vitro, -
XsNR'aR'a, -XSOR'4, .
-XsSR~a~ -XsC(O)yaR~a~ _xsC(O)ORn~ -Xss(O)R~s' _XsS(p)zRis and -X'C(O)R's,
wherein .
Xs is a bond or (C,_~alkylene, R'4 at each occurrence independently is
hydrogen, (Ct_3)alkyl or
halo-substituted (C~_~alkyl and R's is (C~_3)alkyl or halo-substituted
(C,°3)alkyl; more particularly "
in which q is 0, 1 or 2 and R33 at each occurrence independently is selected
from a group
consisting of (Ca~alkyl, cyano, halo, halo-substituted (CI,~)alkyl, nitra, -
OR'a; -SR'a and
-C(O)OR'a, wherein R'a at each occurrence independently is hydrogen,
(C~_3)alkyl or
halo-substituted (C,_3)alkyl; more particularly in which R33 at each
occurrence independently is
selected from a group consisting of (CI,~)alkyl, bromo, carboxy, chloro,
cyano, difluoromethoxy,
fluaro, iodo, methoxy, vitro, trifluoromethoxy, trifluoromethyl and
trifluorosulfanyl.
In particular, -XaS(O)2R3z represents benzylsulfonyImethyl, 2-
chlorobenzylsulfonyhnethyl,
-35-
CA 02475069 2004-08-20
2-cyanobenzylsulfonylmethyl, 2-difluoromethoxybenzylsulfonylmethyl,
3,5-dimethylisooxazol-4-ylmethylsulfonylmethyl, 2-methoxybenzylsulfonylmethyi,
6-methylpyrid-2-yhnethylsulfonyhnethyl, 2-nitrobenzylsulfonylmethyl,
pyrid-2-yhnethylsulfonylmethyl, o-tolylmethylsulfonylmethyl or
2-trifluoromethylbenzylsulfonylmethyl.
Reference to the preferred embodiments set forth above is meant to include all
combinations of particular and preferred groups:
Further preferred are compounds of Formula I selected from a group consisting
of:
2S-acetylamino-N (1S-benzooxazol-2-ylcarbonyl}-3-phenylprapyl)-
~t3 3-cyclohexylpropionamide; and
N [ 1 S-( 1 S-benzooxazol-2-ylcarbonyl-3-phenylpropylcarbamoyl)-
2-cyclohexylethylisonicotinamide; and the N oxide derivatives, prodrug
derivatives, protected
derivatives, individual isomers and mixtures of isomers; and the
pharmaceutically acceptable
salts thereof.
Further preferred are compounds of Formula I selected from a group consisting
of:
N [1R-{1S-benzooxazol-2-ylcarbonylbutylcarbamoyl)-2-
benzylsulfonylethyl]motpholine-
4-carboxamide;
methyl
1R-( 1 S-benzooxazol-2-ylcarbonylbutyl carbamayl)-2-
benzylsulfonylethylcarbamate;
N (1S-benzooxazol-2-ylcarbonylbutyl)-
2R-methylsulfonylamino-3-benzylsulfonylpropionamide;
N (1S-benzooxazol-2-ylcarbonylbutylcarbamoyl)-2R-(3,~-dimethylureido)'
3-(2-methoxybenzylsuifonyl)propionamide;
N [1R-(1S-benzooxazol-2-ylcarbonylbutylcarbamoyl)-
2-(2-difluoromethaxybenzylsulfonyI)ethyl]morpboline-4-carboxamide;
N [1R-(1S-benzooxazol-2-yicarbonylbutylcarbamoyl)_
2-(2-methoxybenzylsulfonyl)ethyl]marphoIine-4-carboxamide;
N-[ 1 R-( 1 S-benzooxazal-2-ylcarbonylpentylcarbamoyl)-
2-benzylsulfonylethyl]morpholine-4-carboxamide;
N [1R-(1S-benzooxazol-2-yicarbonylpentylcarbamoyl)-
2-{2-chlorobenzylsulfonyl)ethyl]rnorpholine-4-carboxamide;
1R-(1 S-benzooxazol-2-ylcarbonylpentylcarbamoyl)-
-36-
CA 02475069 2004-08-20
2-(2-difluoromethoxybenzylsulfonyl)ethylcarbamate;
N [1R-(1S-benzooxazol-2-ylcarbonylpentylcarbamoyl}-
2-{2-difluoromethoxybenzylsulfonyi)ethyllmorpholine-4-carboxyamide;
N-[ IR-( 1 S-benzooxazol-2-ylcarbonylpentylcarbamoyl~
2-(3,5-dimethylisoxazol.-4-ylmethylsulfonylethyl]isonicotinamide;
IV [ 1 R-(1 S-benzooxazol-2-ylcarbonylpentylcarbamoyl}-
2-(2-nitrobenzylsulfonyl)ethyl]morpholine-4-carboxamide;
N [1R-(1S-benzooxazol-2-ylcarbonylpentylcarbamoyIr
2-pyridin-2-yimethylsulfonylethyl]morpholine-4-carboxamide;
N [1R-(1S-benzooxazol-2-yIcarbonylpentylcarbamoyl}-
2-o-tolylmethylsulfonylethyIJmorpholine-4-carboxamide;
N [1R-(1S-benzooxazol-2-ylcarbonylpentylcarbamoyl)-
2-(2-trifluoromethylbenzylsulfonyl)zthyl]morpholine-4-carboxamide;
N [IR-(1S-benzooxazol-2-ylcarbonyl-3-phenylpropylcarbamoyl)-
2-benzylsulfonylethyl]nicotinamide;
N [1R-(IS-benzooxazol-2-ylcarbonyl-3-phenylpropylcarbamoyl~
2-benzylsulfonylethyl]pyrazine-2-carboxamide;
N [1R-(1S-benzooxazol-2-ylcarbonyl-3-phenylpropylcarbamoyl)-
2-(2-chlorobenzylsulfonyl)ethyl Jmorpholine-4-carboxamide;
N [1R-(iS-benzooxazol-2-ylcarbonyl-3-phenylpropylcarbamoyl}-
2-(2-cyanobenzylsulfonyl)ethyl]isonicotinamide;
N [1R-(1S-benzooxazol-2-ylcarbonyl-3-methylsulfonylpropylcarbamoyl}-
2-(2-difluoromethoxybenzylsulfonyl}ethyl]morpholine-4-carboxamide;
N [1R-(1S-benzooxazol-2-ylcarbonyipentylcarbamoyI}-
2-(2-difluoromethoxybenzylsulfonyI)ethyl3isonicotinamide;
N [1R-(1S-benzooxazol-2-ylcarbonyl}-3-phenylpropylcarbamoyl)-
2-benzylsulfonyletliyllmorpholine-4-carboxamide;
N-[1R-(1S-benzooxazol-2-ylcarbonyl-3-phenylpropylcaxbamoyl)-
2-{6-methyipyrid-2-ylmethylsulfonyl)ethyl]isonicotinamide;
N-j1R-(IS-benzooxazol-2-ylcarbonyl-3-phenylpropylcarbamoyl)-
2-(2-nitrobenzylsulfonyl)ethyljmorpholine-.4-carboxamide;
N [1R-(1S-benzooxazol-2-ylcarbonyl-3-phenylpropylcarbamoyl)-
-37-
CA 02475069 2004-08-20
2-pyrid-2-ylmethylsulfonylethyl]morpholine-4-carboxamide;
N [1R-{IS-benzooxazol-2-ylcarbonyl-3-phenylpropylcarbamoyl)-
2-oaolylmethyIsulfonylethyl]morpholine-4-carboxamide;
N [1R-(1S-benzooxazol-2-ylcarbonyl-3-phenylpropylcarbamoyl~.
2-(2-trifluoiomethylbenzylsulfonyl)ethyl)tetrahydropyran-4-carboxamide;
tetrahydropyran-4-yl IR-(I S-benzooxazol-2-ylcarbonyl-3-phenylpropylcarbamoyl)-
2-benzylsulfonylethylcarbamate; and
N [1R-(IS-benzooxazol-2-ylcarbonyl-
3-phenyipropylcarbamoyl}-2-(2-cyanobenzylsulfonyl}ethylJpiperidine-4-
carboxamide; and the
N-oxide derivatives, prodrug derivatives, protecaed derivatives,
individual~isomers and mixtures
of isomers; and the pharmaceutically acceptable salts thereof.
A preferred aspect of the invention are compounds of Formula I in which X' is
=C-. In
particular, the heteromonocyclic ring or fused heteropolycyclic ring system A
is selected from
thien-2-yl, oxazol-2-yl, 4,5-dihydrooxazol-Z-yl, fur-2-yl, IH-indol-5-yl,
pyrid-2-yl, pyrid-3-yl,
75 thiazol-2-yl, 1-methyl-1H-imidazol-2-yl, I-benzyl-1H-imidazol-2-yi,
benzooxazol-2-yl,
benzofur-2-yl, benzothiazol-2-yl, 1H-benzoimidazol-2-yl, I,1-dioxo-1H-I7~6-
benzo[b]thien-2-yl,
quinol-3-yl, (1,3]dioxolan-2-yI, naphtho[2,3-d]oxazol-2-yl, naphtho(I,2-
d]oxazol-2-yl and
naphtho(2,1-~oxazol-2-yl, each substituted by a group R' and optionally
substituted with a group
R8, particularly wherein R' is halo, vitro, -R~', -ORZ9, -C(O)R~°, -
C(C?)ORs9,.-S{O)2NRZ9R~°,
-C(O)NR~'R3° or -C(O)NHCHR43C(O)OR29, wherein R~° is
(C,.~)alkyD,
{C3.12)cYcloalkyl(C~)alkyl, hetero(C3_~~cycloalkyl(C~)alkyl,
(C~.12)aryl(C~)alkYl,
diphenyl(C~)alkyl, hetero{C~.,~aryl(C~)alkyl or
hetero(C$:,~polycycloaryl(C~)alkyl and R29
is hydrogen or -R~°, wherein R2° is defined as above, wherein
said heterocycloalkyl may be
substituted with (C~.,~aryl(C0.3)alkyl, R~° at each occurrence is
hydrogen or (CI~)alkyl and R'o
is (C,.~)alkyl, and Rg at each occurrence independently is hydrogen,
(Ca.~)alkyl or
halo-substituted {C»)alkyl; wherein within R' any alicyclic or aromatic ring
system present may
be substituted further by I to 5 radicals independently selected from
(C1~)alkyl, (C,.b)alkylidene,
cyano, halo, halo-substituted (C,.~)alkyl, vitro, -XbNR'4R'4, -XbNR'4C(O}OR'4,
_xs~taC(O)NR~aRl4~ -X6~14C~14)~14Ri4~ -X6OR74' -XbSRl4~ -X6C(o)OR14~
-XbC(U)NR'°R14~ -X6~(p)2NR"R14~ -Xbp(O)(oR'4)~R'4, -XbUP{O)(OR'4)OR'4,
-X6NR'4C(O)R's, -XbS(O)R's, -X6S(O),R's and -XbC(O)R's, wherein X6 is a bond
or
-3 8-
CA 02475069 2004-08-20
(C,~)alkylene, R'° at each occurrence independently is hydrogen,
(C'.~)alkyl or halo-substituted
(C,.3)alkyl and R" (C,.~)alkyl or halo-substituted (C,.3)alkyl. .
The ring system A preferably is oxazol-2-yl, 4,5-dihydrooxazol-2-y1,
benzooxazol-2-yl,
naphthoj2,3-d]oxazol-2-yl, naphtho[1,2-dJoxazol-2-yl or naphtho[2,1-djoxazol-2-
yl; each '
substituted by a group R' and optionally substituted with a group R8,
particularly wherein R' is
halo, -R29, -C(O)R~°, -C(O)OR29, -C(O)NR~'R3° or -
S(O)2NR~''R~°, wherein R~° is (C,.~)alkyl,
(C3.,~cycloalkyl(C~)alkyl, (C~.,2)aryI(C~alkyl, hetero{C5_l~aryi(C~alkyl or
hetero(Cg.i2)polycycloaryI(Co..b)alkyl.
The ring system A more preferably is oxazol-2-yl, 4,5-dihydrooxazol-2-yl,
benzooxazol-2-yl or naphtho[1,2-dJoxazol-2-yl, each substituted by a group R'
and optionally
substituted with a group Rg, particularly wherein R' is adamantan-I-
ylmethylcarbamoyl, benzyl,
benzytcarbamoyl; benzyl(methyl)carbamoyl, I-benzyloxycarbonyl-3-
methylhutylcarbamoyl,
4-benzylpiperidin-1-carbonyl, ten-butyl, chloro, 2,3-dihydroindol-I-
ylcarbonyl, 3,4-dihydro-
IH-isoquinol-2-ylcarbonyl, 3,4-dihydro-IH-quinol-1-ylcarbonyl,
diphenylmethylcarbamoyl,
fur-2-yimethylcarbamoyl, hydrogen, 2-(1H-indol-3-yl)ethylcarbamoyl, methoxy,
methoxycarbonyl, methyl, 3-methylbutylcarbamoyI, methylcarbamoyl, I-
methylethylcarbamoyl,
naphth-I-ylmethylcarbonyl, vitro, phenyl, phenylcarbamoyl, 2-
phenylcyclopropylcarbamoyl,
1-phenylethylcarbamoyl, sulfamoyl, trifluoromethyl, phenethylcarbamoyl,
3-phenylpropylcarbamoyl, piperid-1-ylcarbonyl, pyrid-2-ylmethylcarbamoyl,
pyrid-3-ylmethyIcarbamoyl, pyrid-4-ylmethylcarbamoyl or pyrrolidin-I-
ylcaibonyl and R$ is
methyl.
XZ particularly represents a bond or a divalent group of Formula (a), wherein
within
Formula (a) X3 is -C(0)-, R'represents hydrogen, R" represents hydrogen or
methyl, typically
'hydrogen, and R'z particularly represents (C,~)alkyl, preferably isobutyl,
sec-butyl or isopropyl.
R' particularly represents hydrogen or -X8X9R~°, wherein X$ is -C(O)-
or -S(O)2-; X9 is
a bond or -0- and RZ° is (Cl.~)alkyl, (C3.12)cyclaalkyl(Co.,b)alkyl,
hetero(C3_l~cycloalkyl(Co-b)alkyl, (C~.l~aryl(C~)alkyl or
hetero{CS.~~aryl(Cp,6,)alkyl; wherein
within R' any alicyclic or aromatic ring system present may be substituted
further by I to S
radicals independently selected from (Cl.~)alkyl, -C(O)OR'°, -X6NR'4R'4
and
-X6NR"C{O)OR'", wherein X6 is a bond or (C,.~)alkylene, R" at each occurrence
independently is hydrogen, {Cj.~)alkyl or halo-substituted (C, 3)alkyl and R's
(C,.~)alkyl or
halo-substituted (C~_3)alkyl.
-39-
CA 02475069 2004-08-20
R' particularly represents acetyl, benzoyl, benzyloxycarbonyl, benzylsulfonyl,
bicyclo[2.2.2]hept-2-ylcaxbonyl,: tent-butoxycarbonyl, rent-butyryl,
4-tent-butoxycarbonylpiperazin-I-ylcarbonyl, I-tart-butoxycarbonyipiperidin-4-
ylcarbonyl,
2-cyclohexylacetyl, 4-cyclohexylbutyryl, 2-cyclohexylethyIsulfonyl, 3-
cyclohexylpropionyl,
2-cyclopentylethylsulfonyl, hydrogen, 4-methylpiperazin-I-ylcarbonyl,
methylsulfonyl,
4-methylvaleryl, 3-rnorpholin-4-ylpropionyl, naphth-2-ylmethyl, 3-
phenylpropionyl,
piperazin-1-ylcarbonyl, piperidin-4-ylcarbonyl or pyrid-3-ylcarbonyl, wherein
within Rl any
alicycIie or aromatic ring system present may be substituted further by I to 3
radicals
independently selected from 3-aminomethyl and 3-tart-
butoxycarbonylaminomethyl.
R2 particularly represents hydrogen.
R3 preferably represents (C~~)alkyl or (C~.,o)aryl{C,,3)alkyl, more preferably
phenethyl,
or R3 and R° taken together with the carbon atom to which both R3 and
R4 are attached form
(C3~)cycloalkylene, more preferably cyclopropylene.
R4 preferably represents hydrogen or (C~.~)alkyl, preferably hydrogen or
methyl or R3
and R4 or R3 and R° taken together with the carbon atom to which both
R3 and R° are attached
form (C~)cycloalkylene, more preferably cyclopropylene.
Rs and R6 preferably together form oxo.
Reference to the preferred embodiments set forth above is meant to include all
combinations of particular and preferred groups.
Pharmacology and Utility:
The compounds of the invention are cysteine protease inhibitors, in particular
the
compounds of the invention inhibit the activity of cathepsins B, L, K and/or S
and, as such, are
useful for treating diseases in which cathepsin B, L, K andlor S activity
contributes to the
pathology andlor symptomatology of the disease. For example, the compounds of
the invention
2S are useful in treating tumor invasion and metastasis, in particular as anti-
angiogenic agents,
rheumatoid arthritis, osteo arthritis, pneumoeystis carinii, acute
pancreatitis, inflammatory airway
disease and bone and joint disorders. Furthermore, the compounds of the
invention are useful in
treating bone resorption disorders, e.g. osteoporosis.
The compounds of the invention are inhibitors of cathepsin S and, as such, are
useful for
80 treating diseases in which cathepsin S activity contributes to the
pathology andlor
symptomatology of the disease. For example, the compounds of the invention are
useful in
~40-
CA 02475069 2004-08-20
treating autoimmune disorders, including, but not limited to, juvenile onset
diabetes; multiple
sclerosis, pemphigus vulgaris, Graves' disease, myasthenia gravis, systemic
lupus
erythemotasus, rheumatoid arthritis and Hashimoto's thyroiditis; allergic
disorders, including, but
not limited to, asthma; and allogeneic immune responses, including, but not
limited to, organ
transplants or tissue grafts.
Cathepsin S also is implicated in disorders involving excessive elastolysis,
such as
chronic obstructive pulmonary disease (e.g. emphysema), bronchidlitis,
excessive airway
elastolysis in asthma and bronchitis, pneumonities and cardiovascular disease
such as plaque
rupture and atheroma. Cathepsin S is implicated in fibril formation and,
therefore, inhibitors of
cathepsins S are of use in treatment of systemic amyloidosis.
The cysteine protease inhibitory activities of the compounds of the invention
can be
determined by methods known to those of ordinary skill in the art. Suitable in
visro assays for
measuring protease activity and the inhibition thereof by test compounds are
known. Typically,
the assay measures protease induced hydrolysis of a peptide based substrate.
_ Furthermore, the compounds of the invention are useful as intermediates in
the
preparation of other compounds of Formula I. For example, compounds of Formula
I in which
Rs is hydroxy can be used to prepare compounds of Formula I in which Rs and R6
taken
together form axo.
Nomenclature:
The compounds of Formula I and the intermediates and starting materials used
in their
preparation are named in accordance with ICJ1PAC rules of nomenclature in
which the
characteristic groups have decreasing priority for citation as the principle
group as follows:
acids, esters, amides, etc.. Alternatively, the compounds are named by AutoNom
4.0 (Beilstein
information Systems, Inc.). For example, a compound of Formula I in which A is
bert2ooxazol-2-y1; Xz is a group of Formula (a), wherein R9 is hydrogen and
Rr2 is
cyclohexylmethyl; R' is acetyl; RZ is hydrogen; R~ is phenethyl; R° is
hydrogen; and RS and R6
together form oxa; that is, a compound having the following structure:
-4I -
CA 02475069 2004-08-20
is named 2S-acetylamino-l1~ (1-benzooxazol-2-ylcarbonyl-3-phenylpropyl~.
3-cyclohexylpropionamide; and a compound of Formula I in which A is
benzooxazol-2-yl; X'- is a
group of Formula (a), wherein R9 is hydrogen and R~Z is benzyIsuifonylmethyl;
R' is
morpholin-4-ylcarbonyl; Rz is hydrogen; R3 is phenethyl; R4 is hydrogen; Rs is
hydrogen; and R6
is hydroxy; that is, a compound having the following structure:
is named N [1S-(2-benzooxazol-2-yl-2-hydroxy-1S-phenethylethylcarbamoyl)-
2-benzylsuifonylethyl]-morpholine-4-carboxamide or morpholine-4-carboxylic
acid
i0 {{R)-1-[(S~-i-(1-benzooxazol-2-yl-1-hydroxy-methyl)-3-phenyl-
propylcarbamoyl]-
2-phenylmethanesulfonyl-ethyl }-amide; and a compound of Formula I in which A
is
benzooxazol-2-yl; XZ is a group of Formula (a), wherein R9~is hydrogen and R'2
is
cyclohexymethyl; R' is carboxyacetyl; Rz is hydrogen; R3 is phenethyl; R4 is
hydrogen; and RS
and R6 together form oxo; that is, a compound having the following structure:
-42-
CA 02475069 2004-08-20
is named N-[1S-(IS-benzooxazol-2-ylcarbonyl-3-phenylpropylcarbamoyl)-
2-cyclohexylethyl]malonamic acid orN-{(S~-1-[(S~-1-(1-benzooxazol-2-yl-
znethanoyl)-3-phenyl-
propylcarbarnoylJ-2-cyclohexyl-ethyl )-malonamic acid; and a compound of
Formula I in which
A is benzooxazol-2-yl; Xz is a group of Formula (a); wherein R9 is hydrogen
and R'~ is
2-nitrobenzylsulfonylmethyl; R' is moxpholin-2-ylcarbonyl; RZ is hydrogen; R3
is phenethyl; R° is
hydrogen; and R' and R6 together form oxo; that is, a compound having the
following structure:
O
II
is named N-[1R-(1S-benzooxazol-2-ylcarbonyl-3-phenylpropylcarbamoyl)-
2-(2-nitrobenzylsulfonyl)ethylJmorpholine-4-carboxamide or morphoIine-4-
carboxylic acitt
[(R)-I-[(S~-I-(1-benzooxazol-2-yI-methanoyl)-3-phenyl-propylcarbamoyl)-2-(2-
nitro-
-43-
CA 02475069 2004-08-20
phenylmethanesulfonyl)-ethyl]-amide; and a compound of Formula I in which A is
benzooxazol-2-yI; XZ is a group of Formula (a), wherein R9 is hydrogen and R'2
is
benzylsulfonylmethyl; R' is tetrahydropyran-4-yloxycarbonyl; Rz is hydrogen;
R~ is phenethyl;
R4 ZS hydrogen; and RS and R6 together form oxo; that is, a compound having
the following
structure:
is named tetrahydropyran-4-yl 1R-(IS=benzooxazol-2-ylcarbonyl-3-
phenylpropylcarbamoyl)-
2-benzylsulfonylethylcarbamate or l (R)-1-[(S}-1-(1-benzooxazol-2-yl-
methanoyl)-3-phenyl-
propylcarbamoyl]-2-phenylmethanesulfonyl-ethyl j-carbamic acid tetrahydro-
pyran-4-yl ester,
A compound of Formula I in which A is pyrid-2-yl; XZ is a group of Formula
(a),
wherein R9 is hydrogen and R" is 2-methylpropyl; R' is benzyloxycarbonyl; R2,
Rd and Rs each
are hydrogen; R3 is phenethyl; and R6 is hydroxy; that is, a compound having
tlhe, following
structure:
is named benzyl 1S-(1S-pyrid-2-ylcarbonyl-3-phenylpropylcarbamoyl)-3-
methylbutylcarbamate
,.dq._
CA 02475069 2004-08-20
or { (S)-1-[(S)-1-(I-hydroxy-1-pyridin-2-yI-methyl)-3-phenyl-gropylcarbamoyl]-
3-methyl-butyl }-
carbamic acid benzyl ester; and a compound of Formula I in which A is thiazol-
2-yl; Xz is a
group of .Formula (a), wherein R9 is hydrogen and R" is 2-methylpropyl; R' is
4-methylpiperazin-1-ylcarbonyl; R2 and R'' each are hydrogen; R3 is phenethyl;
and Rs and R6
together form oxo; that is, a compound having the following structure:
0
~N~
/N J
is named N [3-methyl-1S-(3-phenyl-I-thiazol-2-ylcarbonylpropylcarbamoyl)butyl]-
4-methylpiperazine-1-carboxamide or 4-methyl-piperazine-1-carboxylic acid or {
(S)-3-methyl-
1-[(S)-3-phenyl-1-(i-thiazol-2-yl-methanoyl)-propylcarbamoyl)-butyl}-amide;
and a compound
of Formula I in which A is 4,5-tetrahydro-4-methoxycarbonyloxazol-2-yl; X~ is
a group of
Formula (a), wherein R9 is hydrogen and R" is 2-methylpropyl; R' is
benzyloxycarbonyI; RZ and
R4 each are hydrogen; R3 is pijenethyl; aru ;5 and R5 together fornn oxo; that
is, a compound
having the following structure:
0II 0 0
N\ ~ /~\~
\ OJ~H .~~ J vOr
0 O
I
75 is named methyl 2S-(2S-benzyloxycarbonylamino-4-methylvalerylamino)-4-
ghenylbutyryl-
4,5-dihydrooxazole-4-carboxylate or 2-[(S)-2-((S)-2-benzyloxycarbonylamino-4-
methyl-
-45-
CA 02475069 2004-08-20
pentanoylamino)-4-phenyl-butanoylJ-4,5-dihydro-oxazole-4-carboxylic acid
methyl ester.
Certain compounds. of Formula I exist in tautomeric equilibrium. Compounds of
Formula I which exist as tautomers are named, illustrated or otherwise
described in this
application as one possible tautomer. However, it is to be understood that the
all possible
tautomers, are meant to be encompassed by such names, illustrations and
descriptions.
Certain compounds of Formulae I and II exist in tautomeric equilibrium.
Compounds of
Formulae I and II which exist as tautomers are named, illustrated or otherwise
described in this
application as one possible tautomer. However, it is to be understood that the
all possible
tautomers are meant to be encompassed by such names, illustrations and
descriptions.
Administration and Pharmaceutical Compositions:
In general, compounds of Formula I will be administered in therapeutically
effective
amounts via any of the usual and acceptable modes known in the art, either
singly or in
combination with another therapeutic agent. A therapeutically effective amount
may vary
widely depending on the severity of the disease, the age and relative health
of the subject, the
potency of the compound used and other factors. For example, therapeutically
effective
amounts of a compound of Formula I may range from O.I micrograms per kilogram
body weight
(~,vglkg) per day to IO milligram per kilogram body weight (mglkg) per day,
typically 1 iug~kg/day
to I mg/kglday. Therefore, a therapeutically effective amount for a 80 kg
human patient may
range from 10 ~cg/day to 100 mglday, typically 0.1 mg/day to 10 mglday. In
general, one of
ordinary skill in the art, acting in reliance upon personal knowledge and the
disclosure of this
Application, will be able to ascertain a therapeutically effective amount of a
compound of
Formula i for treating a given disease.
The compounds of Formula I can be administered as pharmaceutical compositions
by
one of the following routes: oral, systemic (e.g., transdermal, intranasal or
by suppository) or
parenteral (e.g., intramuscular, intravenous or subcutaneous). Compositions
can take the form
of tablets, pills, capsules, semisolids, powders, sustained release
formulations, solutions,
suspensions, elixirs, aerosols, or any other appropriate composition and are
comprised of, in
general, a compound of Formula I in combination with at least one
pharmaceutically acceptable
excipient. Acceptable excipients are non-toxic, aid administration, and do not
adversely affect
the therapeutic benefit of the active ingredient. Such excipient may be any
solid, liquid,
semisolid or, in the case of an aerosol composition, gaseous excipient that is
generally available
-4b-
CA 02475069 2004-08-20
to one of skill in the art.
Solid pharmaceutical excipients include starch, cellulose, talc, glucose,
lactose, sucrose,
gelatin, malt, rice, flour, chalk, silica gel, magnesium stearate, sodium
stearate, glycerol
monostearate, sodium chloride, dried skim milk, and the tike. Liquid and
semisolid excipients
may be selected from water, ethanol, glycerol, propylene glycol and various
oils, including those
of petroleum, animal, vegetable or synthetic origin (e.g., peanut oil, soybean
oil, mineral oil,
sesame oil, or the like). Preferred liquid carriers, particularly for
injectable solutions, include
water, saline; aqueous dextrose and glycols.
The amount of a compound of Formula I in the composition may vary widely
depending
upon the type of formulation, size of a unit dosage, kind of excipients and
other factors known to
' those of skill in the art of pharmaceutical sciences. In general, a
composition of a~compound of
Formula I for treating a given disease will comprise from 0.01 %w to 10%w,
preferably 0.3%w
to 1 %w, of active ingredient with the remainder being the excipient or
excipients. Preferably the
pharmaceutical composition is administered in a single unit dosage form for
continuous
treatment or in a single unit dosage form ad libitum when relief of symptoms
is specifically
required. _ ... .~. _..
The compounds of Formula I can be administered alone or in combination with
other
compounds of Formula I or in combination with one or more other active
ingredient(s). For
example, the compounds of Formula I can be administered in combination with a
therapeutically
active amount of a bisphosphonic acid or acid ester derivative or any
pharmaceutically
acceptable salt thereof. Suitable bisphosphonic acids and acid ester
derivatives include
compounds corresponding to the following formula:
i (~)(OR43)OR43
R44 X 11,C X45
P(O)(OR43)OR43
wherein X" is a bond or (C~_,)alkylene, each R°3 independently is
hydrogen or (C~.3o)alkyl, R'°
and R43 are selected independently from a group consisting of hydrogen, halo,
optionally
substituted (C,.ao)alkyl, (C3.3o)cyclaalkyl, hetero(C~.3o)cycloalkyt,
optionally substituted
(Cb,o)aryl, hetero(C~.~o)aryl, -NR°6R'~, -OR°~, -SR's, wherein
each R~ independently is
hydrogen, (C,_io)alkyl, (C3_~o)cycloalkyl, optionally substituted (C~.~o)aryl,
provided that both R"
and R4s are not selected from hydrogen or hydroxy when X" is a bond; or R~ and
R4s taken
-47-
CA 02475069 2004-08-20
together form {C~.9)alkylene; wherein (C3_,~cycIoalkyl includes adarnantyl and
the like,
hetero(C~,okycloalkyl includes pyrrolidinyl and the like, (C~,o)aryl includes
phenyl and
naphthyl, and hetero(Cø,o)aryl includes quinolyl, isoquinolyl, pyridyl, furyl,
imidazolyl,
imidazopyridyl and the like.
Instances wherein R°~ andlor Ra5 are substituted (Cl_~~alkyl may
include, but are not
limited to, {C,_~)alkyl substituted by hetero(C~.,o)cycloallryl, (C~.,o)aryl,
hetero(C~,o)aryl,
-~47R4T' -pRa~ and -SR°', wherein each R'r' is independently hydrogen
or {C,_,a)alkyl;
wherein hetero(C~.lo)cycIoalkyl includes pyrrolidinyl and the Like, (C~,o)aryl
includes phenyl and
naphthyl, and hetero(C~.,o)aryI includes quinolyl, isoquinolyl, pyridyl,
furyl, imidazolyl,
imidazopyridyl and the like. Suitable optionally substituted aryl groups
include, but are not limited
to, halo-substituted phenyl.
A non-limiting class of bisphosphonic acids and acid ester derivatives thereof
suitable
for administration in combination with compounds of Formula I include those in
which R°" is
selected from the group consisting of hydrogen, hydroxy or halo, and R'S is
selected from the
group consisting of optionally substituted (C,_~)alkyl, halo and -SR"~,
wherein R''~ is (C,_,o)alkyl
or phenyl.
A non-limiting subclass of bisphosphonic acids and acid ester derivatives
thereof
suitable for administration in combination with compounds of Formula I include
those in which
R~° is selected from the group consisting of hydrogen, hydroxy and
chloro and R43 is selected
from the group consisting of optionally substituted (C,_3o)alkyl, chloro and
chlorophenylthio.
A non-limiting example of a bisphosphonic acid suitable for administration in
combination with compounds of Formula I include that in which X" is a bond,
each R43 is
hydrogen, R"° is hydroxy and R45 is 3-aminopropyl, namely 4-amino-l-
hydroxybutylidene-
I,1-bisphosphonic acid (aka alendronic acid), or the monosodium trihydrate
salt thereof, namely
4-amino-1-hydroxybutylidene-l,I-bisphosphonate monosodium trihydrate (aka
alendronate
monosodium trihydrate), described in LJ.S. Patents 4,922,00?, to Kieczykowski
et al., issued May
I, 1990; 5,019,651, to Kieczykowski et al., issued May 28, 1991; 5,510,517, to
Dauer et al.,
issued April 23, I996; 5,648,491, to Dauer et al., issued July 15, 1997, all
of which patents are
incorporated by reference herein in their entirety. .
Further non-limiting examples of bisphosphonic acids suitable for
administration in
combination with compounds of Formula I include the following: .
cycloheptylaminomethylene-1,I-bisphosphonic acid (aka cimadronic acid),
described in
-48-
CA 02475069 2004-08-20
U.S. Patent 4,970,335, to Isomura et al., issued November 13, 1990;
1,1-dichloromethylene-1,1-diphosphonic acid (aka clodronic acid) and the
disodium salt
thereof, namely clodronate disodium, described in Belgium Patent 672,205
{1966) and J. Org.
Chem 32, 4111 (1967);
1-hydroxy-3-pyrrolidin-1-ylpropylidene-1,1-bisphosphonic acid (aka EB-1053);
1-hydroxyethyIidene-1,1-diphosphanic acid (aka etidronic acid);
1-hydroxy-3-(N-methyl-N pentylamino~ropylidene-1,1-bisphosphonic acid (aka
ibandronic acid), described in U.S. Patent No. 4,927,814, issued May 22, 1990;
6-amino-l-hydroxyhexylidene-1,1-bisphosphonic acid (aka neridronic acid);
3-(dimethylamino)-1-hydroxypropyIidene-1,1-bisphosphonic acid {aka olpadronic
acid);
3-amino-1-hydroxypropylidene-1,I-bisphosphonic acid (aka pamidronic acid);
2-pyrid-2-ylethylidene-1,1-bisphosphonic acid {aka piridronic acid), described
in U.S.
Patent No. 4,761,406;
1-hydroxy-2-pyrid-3-ylethylidene-1,1-bisphosphonic acid (aka risedronic acid);
t5 4-chloraphenylthiomethylenebisphosphonic acid (aka tiludronic acid),
described in U.S.
Patent 4,876,248, to Breliere et al., October 24, 1989; and
I-hydroxy-2-(IH-imidazol-I-yl)ethylidene-1,1-bisphosphonic acid (aka
zoledronic acid);
all of which patents and other documents referred to above are incorporated by
reference
herein in their entirety.
A non-limiting subclass of bisphosphonic acids suitable for administration in
combination
with compounds of Formula I include those selected from the group consisting
of alendronic
acid, cimadronic acid, clodronic acid, tiiudronic acid, etidronic acid,
ibandronic acid, risedronic
acid, piridronic acid, pamidronic acid, xolendronic acid, pharmaceutically
acceptable salts
thereof, and mixtures thereof. A further example of a bisphosphonic acid
suitable for
administration in combination with compounds of Formula I is alendronic acid
or a
pharmaceutically acceptable salt thereof, and mixtures thereof. A further non-
limiting example
is alendronate. monosodium trihydrate.
Compounds of Formula i can be administered in combination with a
therapeutically
active amount of an estrogen receptor agonist. Non-limiting examples of
estrogen receptor
agonists suitable for administration in combination with the compounds of
Formula I include
naturally occurring estrogens such as estradiol, estrone and estroil, or
synthetic estrogen
receptor agonists such as
-49-
CA 02475069 2004-08-20
[6-hydroxy-2-(4-hydroxyphenyl)benzo[b] thien-3-yl] [4-(2-piperidin-1-
ylethoxy~henyl]methanone
(aka raloxifene) and (2-{4-(1,2-diphenylbut-1-enyl~henoxy]ethyl )dimethylamine
(aka
tamoxifen). A non-limiting subclass of estrogen receptor agonists suitable for
administration in
combination with the compounds of Formula I include estrogen receptor partial
agonists (i.e.,
estrogen receptor agonists with mixed agonisdantagonist properties), sometimes
referred to as
estrogen receptor modulators. Estrogen receptor partial agonists can exert
tissue-selective
estrogen agonist effects. Tamoxifen, for example, selectively exerts an
estrogen agonist effect
on the bone, in humans. Additional suitable estrogen receptor partial agonists
are described in
Tissue-Selective Actions Of Estrogen Analogs, Bone Col. 17, No. 4, October
1995, 18IS-190S.
0 Certain 3-[4-(2-phenylindol-1-ylmethyl)phenyl]acrylamides, described iri
U.S: Patent 5,383,910
to Miller et al., November 16, 1999; 'benzothiphene compounds, described in
U.S. Patent
5,985,897 to Meuhl et al., November 16, 1999; naphthyl compounds, described in
U.S. Patent
5,952,350 to Cullinan et al., September I4, 1999; substituted benzothiophene
compounds,
described in U.S. Patent 5,962,475 to Schmid et, al., October 4, 1999, are
suitable estxogen
receptor partial agonists for administration with the compounds of Formula I;
all of which
patents and other documents referred to above are incorporated by reference
herein in their
entirety.
More particularly a pharmaceutical composition of this invention may comprise
a
therapeutically effect amount of a compound of Formula I in combination with
one or more
active ingredients) selected from the group consisting of (i) a
therapeutically effect amount of a
bisphosphonic acid or acid ester thereof or a pharmaceutically acceptable salt
thereof and (ii) a
therapeutically effect amount of an estrogen receptor agonist or a
pharmaceutically acceptable
salt thereof; and one or more pharmaceutically acceptable excipient(s). Non-
limiting examples
of such bisphosphonic acids include 1,1-dichloromethylene-i,l-diphosphonic
acid, 1-hydroxy-
3-pyrrolidin-1-yIpropylidene-1,1-bisphosphonic acid, 1-hydroxyethylidene-1,1-
diphosphonic acid,
1-hydroxy-3=(N-methyl-N-pentylamino)propylidene-1,1-bisphosphonic acid, 6-
amino-
1-hydroxyhexylidene-1,1-bisphosphonic acid, 3-(dimethylamino)-1-
hydroxypropylidene-
1,1-bisphosphonic acid, 3-amino-1-hydroxypropylidene-1,1-bisphosphonic acid,
2-pyrid-2-ylethylidene-1,1-bisphosphonic acid,1-hydroxy-2-pyrid-3-ylethylidene-
1,1-
bisphosphonic acid, 4-chlorophenylttxiomethylenebisphosphonic acid and 1-
hydroxy-
2-(1H-imidazoI-1-yl)ethylidene-1,1-bisphosphonic acid or acid ester thereof or
a
pharmaceutically acceptable salt thereof; particularly 1,1-dichloromethylene-
1,1-diphosphonic
-50-
CA 02475069 2004-08-20
acid or a pharmaceutically acceptable salt thereof and preferably 1,1-
dichloromethylene-
l,l-diphosphonate monosodium trihydrate.
Chemistry:
Processes for Making Compounds of Formula I:
Compounds of Formula I in which Rs and R6 together form oxo can be prepared by
proceeding as in the following Scheme i:
Scheme 1
Li\Xl R~
A
~(Rg)n
2
R2 O
R1 xz.N N.O~
R3 Ra l
3
R2 O
1
R ~XZ. N x? R~
R3 R4
(R8;~;
I(a)
in which n, A, X', Xz, R', R2, R3, R4, R~ and R$ are as defined in the Summary
of the Invention
for Formulae I and II.
Compounds of Formula I in which Rs and R6 together form oxo (Formula I(a)) can
be
prepared by reacting an organometallic compound of Formula 2 with a compound
of Formula 3.
The reaction is carried out in a suitable solvent (e.g. tetrahydrofuran
('I~iF~, ether, or the like) at
-80 to -?0° C, preferably at about -78 ° C, and requires 30
minutes to an hour to complete. The
organometallic compound of Formula 2 is generated by treating a corresponding
organo
compound, or a brominated derivative thereof, with n-butyllithium or tert-
butyllithium in a
suitable solvent (e.g. THF, ether, or the like) at -80 to -?0° C,
preferably at about -?8 ° C, for
approximately 30 minutes to an hour.
-51-
CA 02475069 2004-08-20
Compounds of Formula I in which the ring comprised by X' is a
4,5-tetrahydrooxazol-2-yl or oxazol-2-yl or moiety, Rs is hydrogen and R6 is
hydroxy can be
prepared by proceeding as in the following Scheme 2:
Scheme 2
R2 OH
Rvx~'N ~~'
R3 R4 jrlrH
4
I~o~, R'
H2N_:,:Rs
s(a)
R2 OH
Rvx2.N O ~R~
P
R3 R4
R
I(b)
in which X2, R', Rz, R3, R°, R' and R8 are as defined in the Summary of
the invention for
Formulae I and II.
compounds of Formula I can be prepared by reacting a compound Formula 4 wi:>~:
a~
compound of the Formula s(a). The reaction is carried out in a suitable
solvent (e.g.
70 chloroform, ethanol, or the Iike) at reflux temperatures and reguires 3 to
24 hours to complete.
In a similar fashion, using analogous reaction conditions to those described
in Scheme r,
compounds of Formula I in which A is a heteropolycyclic radical wherein X' is
a ring member
atom of an oxazole ring, RS is hydrogen and R6 is hydroxy can be prepared by
reacting a
compound of Formula 4 with a compound of Formula s(b):
HO
R~
H ~J
~-~'(~8)n
in which n is 0, 1, 2 or 3 and B is a heteromonocyclic radical containing 5 to
6 ring member
-s2-
CA 02475069 2004-08-20
atoms or a fused heteropolycyclic radical containing $ to l l ring member
atoms, wherein each "'
ring contains 5 to 7 ring member atoms and each ring member atom is a carbon
atom or a
heteroatom, and R' and R$ is as defined in the Summary of the invention for
Foranulae I and II.
Compounds of Formula I can be prepared by proceeding as in the following
Scheme 3:
Scheme 3
R2 OH
XZ R7
R~ R4 A
(R~iry
1. RIXZOY
2. optionally deprotecting
R2 OH
Rl~Xz~N X1 R7
R3 4 A
R (R$)n
I(c)
in which Y is hydrogen or an activating group (e.g. 2,5-dioxopyrrolidin-1-yl
(NBS), or the like)
and n, A, X', X2, R', R2, R3, R', R' and R$ are as defined in the Su..rriary
of the Inv~naon for
Formulae I and II.
1 Q Compounds of Formula 1 can be prepared by reacting a compound of Formula
6, or a
protected derivative thereof, with a compound of the formula R'XiOY, or a
protected derivative
thereof, and then optionally deprotecting. The reaction is carried out in
the.presence of a
suitable base (e.g. triethylamine, diisopropylethylamine, or the Iike) and in
a suitable solvent
(e.g. acetonirrile, N,N dimethylformamide (DMF), dichioromethane, or any
suitable combination
thereof, or the like) at 10 to 30°C, preferably at about 25 °C,
and requires 24 to 30 hours to
complete. When Y is hydrogen a suitable coupling agent
(e.g. benzotriazole-1-yloxytrispyrrolidinophosphonium hexafluorophosphate
(PyBOP~),
I-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (FDG), O-
benzotriazol-Z-yl-
N,N,N',N'-tetramethyluronium hexafluorophosphate (HBTU),
-53-
CA 02475069 2004-08-20
O-(7-azabenzotriazol-1-yl)-1,1,3;3-tetramethyluronium hexafluorophosphate
(HAT(n,
1,3-dicyclohexylcarbodiimide (DCC), or the like) and base (e.g. N,N
diisopropylethylamine,
triethylamine, or the like) is required and the reaction requires 2 to 3 hours
to complete.
Deprotection can be effected by any means which removes the protecting group
and gives the
desired product in reasonable yield. A detailed description of the techniques
applicable to the
creation of protecting groups and their removal can be found in T.W. Greene,
Prmtecring
Groups in Organic Synthesis, John Wiley & Sons, Inc. 1981. Detailed
descriptions of the
preparation of a compound of Formula I in accordance with Scheme 3 are set
forth in Examples
8, 9, IO and 12, infra.
Compounds of Formula I can be prepared by proce~.uing as in the following
Scheme 4:
Scheme 4
R2 OH
~2~N~ xI R7
R3 4 A
R ~8)n
7
R3 OOH
RZ OH
R X2,~N xI R7
39
R3 R4 ~~ 8
I(c) (R )n.
in which R3' is -X7X8Rz° and n, X', X2, X', X~, R', RZ, R~, R4, R~, R$
and R~° are as defined in
the Summary of the Invention for Formulae I and II.
Additional Processes for Preparing Compounds of Formula I:
-54-
CA 02475069 2004-08-20
Compounds of Formula i in which A is optionally substituted oxazol-2-yl can be
prepared by oxidizing a corresponding compound of Formula I in which A is
4,5-dihydrooxazol-2-yl. The reduction is carried out in the presence of base
(e.g.
I,8-diazabicyclo[5.4.0]under-7-ene (DBU), I,5-diazabicyclo[3.4.0]non-5-ene
(DBN), or the
like) in a suitable solvent (e.g. dichloromethane, or the like) at 20 to 25
° C and requires 6 to I2
hours to complete.
Compounds of Formula I in which R' is -C(O)OH can be prepared from a
corresponding compound of Formula I in which R' is methoxycarbonyl. The
conversion can be
effected by treating the methyl ester with sodium hydroxide in a suitable
solvent (e.g, ethanol, or
the like) at 20 to 25 ° C and requires 6 to 12 hours to complete.
Compounds of Formula I in which R' is -C(O)NR~'R~° or -
C(O)NR"ZCHRe3C(O)OR=9,
can be prepared by reacting a corresponding compound of Formula I in which R'
is -C(O)OH
with a compound of the formula NHR~°RZ' or NHR42CHR43C(O)OR29,
respectively. The
reaction is carried out in the presence of a suitable coupling agent (PyBOP~,
EDC, HBTU,
DCC, or the like) and base (e.g, N,N diisopropylethyIamine, triethylarnine, or
the like) in a
suitable solvent (e.g., DMF, or the like) at 20 to 25° C and requires 2
to 4 hours to complete.
Compounds of Formula I fn R' is -X6X'R~°can be prepared by reacting a
compound of
Formula I in which R' is hydrogen with a compound of the formula
R~°X'X60H. The reaction
is carried out by procedures analogous to those described above for carrying
out Reaction
Scheme 3.
Compounds of Formula I in which Rs and R6 together form oxo can be prepared by
oxidizing a compound of Formula I in which R5 is hydrogen and R6 is hydroxy.
The oxidation
can be carried out with a suitable oxidizing agent (e.g. Dess-Martin
periodinate, or the like) in a
suitable solvent (e.g, dichloromethane, or the like) at 15 to 25° C and
requires 10 to 20 hours to
complete.
Compounds of Formula I in which R'Z contains a sulfonyl moiety can be prepared
by
oxidizing a corresponding compound of Formula I containing a sulfanyl moiety.
The oxidation is
carried aut with a suitable oxidizing agent (e.g. potassium peroxymonosulfate
(OXONE~, or the
Like) in a suitable solvent (e.g. methanol, water, or the like, or any
suitable combination thereof
at ambient temperature and requires 16 to 24 hours to complete.
A compound of Formula I in which A is 1,1-dioxo-1H-1~.6-benzo[b)thien-2-yl can
be
prepared by oxidizing a corresponding compound of Formula I in which A is
benzo[b)thien-2-yl.
-55-
CA 02475069 2004-08-20
Proceeding in this fashion benzyl 1-tl-11.I-dioxo-1H-l~,b-benzotblthien-2-
ylcarbonvl~
3-nhenxluronvlcarbamoyll-3-meth ly burylca~bamate (Compound 209) was prepared.
'H NMR
(CDCI~: 8 0.83 - 0.95 (m, 6H), 81.35 - 1.52 (m, 1H), 81.61 - 1.69 (m, 2H), 8
2.07 ~ 2.20 (m,
1H), S 2.36 - 2.71 {m, 3H), 8 4.57 (m, 1H), 8 4.76 {m,1H), 8 4.98 - 5.26 (m,
3H), 8 5.35 (bs,
1H), 8 7.06 - 7.62 (m, 14H);
A compound of Formula I can be prepared as a pharmaceutically acceptable acid
addition salt by reacting the free base farm of the compound with a
pharmaceutically acceptable
inorganic or organic acid. Alternatively, a pharmaceutically acceptable base
addition salt of a
compound of Formula I can be prepared by reacting the free acid form of the
compound with a
r0 pharmaceutically acceptable inorganic ar organic base. Inorganic and
organic acids acid bases
' suitable for the preparation of the pharmaceutically acceptable salts of
compounds of Formula I
are set forth in the definitions section of this application. Alternatively,
the salt farms of the
compounds of Formula I can be prepared using salts of the starting materials
or intermediates.
The free acid or free base forms of the compounds of Formula I can be prepaied
from
~ 5 the corresponding base addition salt or acid addition salt form. For
example, a compound of
Formula I in an acid addition salt form can be converted to the corresponding
free base by
treating with a suitable base (e.g. ammonium hydroxide solution, sodium
hydroxide, or the like).
A compound of Formula I in a base addition salt form can be converted to the
corresponding
free acid by treating with a suitable acid {e.g. hydrochloric acid, etc).
20 The N oxides of compounds of Formula I can be prepared by methods known to
those
of ordinary skill in the art. For example, N oxides can be prepared by
treating an unoxidized
form of the compound of Formula I with an oxidizing agent (e.g.
trifluoroperacetic acid,
permaleic acid, perbenzoic acid, peracetic acid, meta-chloroperoxybenzoic
acid, or the Like) in a
suitable inert organic solvent (e.g. a halogenated hydrocarbon such as
dichloromethane) at
25 approximately 0°C. Alternatively, the N oxides of the compounds of
Formula I can be prepared
from the N oxide of an appropriate starting material.
Compounds of Formula I in unoxidized form can be prepared from N oxides of
compounds of Formula I by treating with a reducing agent (e.g. sulfur, sulfur
dioxide, triphenyl
phosphine, lithium borohydride, sodium borohydride, phosphorus trichloride,
tribromide, or the
30 Like) in an suitable inert organic solvent (e.g. acetonitrile, ethanol,
aqueous dioxane, or the like)
at 0 to 80°C.
Prodrug derivatives of the compounds of Formula I can be prepared by methods
known
-56-
CA 02475069 2004-08-20
to those of ordinary skill in the art (e.g. for-further details see Sauinier
et al.(1994), Bioorganic
and Medicinal Chemistry Levers. 4:1985). For example, appropriate prodrugs can
be
prepared by reacting a non-derivatized compound of Formula I with a suitable
carbamylating
agent (e.g. 1,1-acyloxyalkylcarbonochloridate, para-nitrophenyl carbonate, or
the like).
Protected derivatives of the compounds of Formula I can be made by means known
to
those of ordinary skill in the art. A detailed description of the techniques
applicable to the
creation of protecting groups and their removal can be found in T.W. Greene,
Protecting
Groups in Organic Synthesis, John Wiley & Sons, Inc. 1981.
Compounds of Formula I can be prepared as their individual stereoisomers by
reacting a
racemic mixture of the compound with an optically active resolving agent to
form a pair of
diastereoisomeric compounds, separating the diastereomers and recovering the
optically pure
enantiomer. While resolution of enantiomers can be carned out using covalent
diasteromeric
derivatives of compounds of Formula I, dissociable complexes are preferred
(e.g. crystalline
diastereoisomeric salts). Diastereomers have distinct physical properties
(e.g. melting points,
boiling points, solubilities, reactivity, and the like) and can be readily
separated by taking
advantage of these dissimilarities. The diastereomers can be separated by
chromatography or,
preferably, by separation/resolution techniques based upon differences in
solubility. The
optically pure enantiomer is then recovered, along with the resolving agent,
by any practical
means that would not result in racemization. A more detailed description of
the techniques
applicable to the resolution of stereoisomers of compounds from their racemic
mixture can be
found in Jean Jacques Andre Collet, Samuel H: Wilen, Enantiomers, Racemates
and
Resolutions, Honh Wiley & Sons, Inc. {1981).
In summary, an aspect of the invention is a process for preparing a compound
of
Formula I, which process comprises:
(A) reacting an organometallic compound of Formula 2:
~\~1 R7
(R8)n
2
with a compound of Formula 3:
-57-
CA 02475069 2004-08-20
~ ~ x2: N N.o~
R3 R4 I
wherein n, A; X', Xz, R', RZ, R3, R4, R' and R$ are as defined in the Summary
of the Invention
for Formulae'I and It, to give a compound of Formula I in which Rs and R6
together form oxo;
or
(B) reacting a compound of Formula 4:
1E~2 OH
RI' 2.N p~
X
3
4
with a compound of Formula 5(a) or 5(b):
o sR~ H R7
H2N ~ B
H N--::~ s s
(R )n CR )n
5 (a) 5 (b)
wherein the dashed Iine represents an optional bond and B is a monocycIic
radical containing 5
to 6 ring member atoms or a fused polycyclic radical containing 8 to I I ring
member atoms,
wherein each ring contains 5 to 7 ring member atoms and each ring member atom
is a carbon
atom or a heteroatom and n, R', R2, R', R°, R' and R8 are as defined in
the Summary of the
Invention for Formulae I and II, to give a compound of Formula I in which the
ring corhprised by
X' is a 4,5-tetrahydrooxazol-2-yI or oxazol-2-yl or moiety, respectively, R5
is hydrogen and R6 is
~ 5 hydroxy or
(C) reacting a compound of Formula 6:
-58-
CA 02475069 2004-08-20
R2 H
Hhi
X1 R?
R3 R4 A s
CR )n
6
with a compound of the formula R'XzOY, wherein Y is hydrogen or an activating
group and n,
A, X', X2, R', R2, R3, R4, R' and R$ are as defined in the Summary of the
Invention for
Formulae I and II, to give a compound of Formula I in which Rs is hydrogen and
R6 is hydroxy;
or
(D) reacting a compound of Formula ?:
R2 H
H X2..-N XI R7
R3 4 A
R ~Rg)n
7
or a protected derivative thereof, with R390H, wherein R39 is -X'XBR~°
and n, A, X', Xx, X', X8,
R~, R3, R°, R', R$ and R2° are as defined in the Summary of the
Invention for Formulae I and II,
and deprotecting if necessary to give a compound of Formula I in which R' is -
X'X$R~°,
(E) optionally oxidizing a compound of Formula I in which Rs is hydrogen and
R6 is hydroxy
to give a compound of Formula I in which Rs and R6 together form oxo;
(F) optionally oxidizing a compound of Formula I in which A is optionally
substituted
4,5-dihydroxyoxazol-2-yl to give a compound of Formula I in which A is
optionally substituted
oxazol-2-yl;
(G) optionally converting a compound of Formula I in which R' is -C(O)OK to a
compound
of Formula I in which R' is methoxycarbonyl;
(I~ optionally converting a compound of Formula I into a pharmaceutically
acceptable salt,
()] optionally converting a salt form of a compound of Formula I to non-salt
form;
(7) optionally converting an unoxidized form of a compound of Formula I into a
pharmaceutically acceptable N oxide;
(I~ optionally converting an N oxide form of a compound of Formula I its
unoxidized foam;
-59-
CA 02475069 2004-08-20
(L) optionally converting a non-derivatized compound of Formula I into a
pharmaceutically
prodrug derivative; and
(M) optionally converting a prodrug derivative of a compound of Formula I to
its
non-derivatized form.
Processes for Preparing Intezmediates:
Compounds of Formula 3 can be prepared by reacting a compound of the Formula
8:
RZ O
HIV N.Oe
R3
8
with a compound of the formula R'XxOY, in which Y is hydrogen or an activating
group (NBS,
or the like). The reaction is carried out under conditions analogous to those
set for Reaction
~ 0 Scheme 3.
Compounds Formula 8 can be prepared by reacting a corresponding amino
protected
carboxylic acid with N,O-dimethylhydroxylamine hydrochloride and then
deprotecting. The
reaction with the amine is carned out in the presence of a suitable coupling
agent (PyBOP~,
EDC, HBTU, DCC, or the like) and base {e.g. N,N-diisopropylethylamine,
triethylamine, or the
like) in a suitable solvent {e.g. dichloromethane, DNlF, or the like) at 20 to
30° C, preferably at
about 25 ° C, and requires 2 to 4 hours to complete (e.g. see Reference
i, infra.). Deprotecdon
can be effected by any means which removes the protecting group and gives the
desired
product in reasonable yield (e.g. see Example 2, infra.): A detailed
description of the preparation
of a compound of Formula 8 is set forth in References Z and 6, infra.
Compounds of Formula 4 can be prepared by reacting a nitrite of Formula 9:
R2 OH
R1v 2~N
x 'CN
R3 R4
9
with ethanol. The reaction is carried out by adding the nitrite to a mixture
comprising a catalytic
-60-
CA 02475069 2004-08-20
amount of dry hydrogen chloride in a suitable solvent (e.g. chloroform,
ethanol, or the like) and
then allowing the reaction to proceed at 0 to 25 ° C for 4 to 6 hours.
Dry hydrogen chloride is
conveniently generated by combining a slightly excessive amount of ethanol
with acetyl chloride
prior to adding the imidate to the reaction mixture. Alternatively, the
hydrogen chloride is
introduced to the reaction medium as a gas.
Compounds of Formula 6 cah be prepared by methods known to those of ordinary
skill
in the art. For example, compounds of Formula b in which A is optionally
substituted
benzooxazol-2-yl can be prepared by reacting a compound of Formula I0:
R? OH
I
R,~~~N OEt
R3 R4
10 in which R''° is a protecting group, with 2-aminophenol and
deprotecting. The reaction with tlhe
phenol is carried out in the presence of a suitable base (e.g.
diisopropylethylamine, triethylamine,
or the Like) and in a suitable solvent (e.g. chloroform, or the like) at
reflux temperatures to 25 ° C
and requires 10 to I2 hours to complete. Deprotection can be effected by any
means which
removes the protecting group and gives the desired product in reasonable
yield. A detailed
description of the preparation of a compound of Formula 6 is set forth in
Reference , infra.
Compounds of Formula 7 can be prepared by condensing a compound of Formula 6
with a compound of the formula R'°X20Y, wherein R~° is a
protecting group, and then
deprotecting. The condensation is carried out in the presence of a suitable
base
(e.g. triethylamine, diisopropylethylamine, or the like) and in a suitable
solvent (e.g. acetonitriie,
DMF, dichloromethane, or any suitable combination thereof, or the like) at IO
to 34°C,
preferably at about 25 °C, and requires 24 to 30 hours to complete.
When Y is hydrogen a
suitable coupling agent (e.g. PyBOP~, EDC, HBTU, HATU, DCC, or the like) and
base
(e.g. N,N-diisopropylethylamine, triethylamine, or the like) is required and
the reaction requires 2
to 3 hours to complete. Deprotecrion can be effected by any means which
removes the
protecting group and gives the desired product in reasonable yield.
Examples:
-6I-
CA 02475069 2004-08-20
The following abbreviations used in this Application area defined as follows:
PyBOP~ = benzotriazole-1-yloxytrispyrroiidinophosphonium hexafluorophosphate;
THF = tetrahydrofuran;
OXONE~ = potassium peroxymonosulfate;
a EDC =1-(3-dimethylaminopropyl}-3-ethylcarbodiimide hydrochloride;
DMF = N,N dimethylformamide;
HATU = O-(7-azabenzotriazol-1-yt)-1,1,3,3-tetramethyluronium
hexafluarophosphate;
HOBT = I-hydroxybenzotriazole hydrate.
REFERENCE 1
Benz~l S ~N-methoxy-N-methvlcarbamovl)-3-nhenylnropylcarbamate
A solution of 2-benzyloxycarbonylamino-4-phenylbutyric acid (5.05 g, I6.1
mmol) in
dichloromethane (70 mL} was cooled to 0°C arid treated with
diisopropylethylamine (2.82 mL,
I6.2 mrnol) added dropwise and then PyBOP~ (8.53 g, 16.4 mmol) added in one
portion. The
mixture was stirred for 5 minutes and then treated with N,O-
dimethylhydroxylamine
hydrochloride (I.73 g, 1?.71 mmol) added in one portion. The mixture was
neutralized with
diisopropylethylamine (4.6 mL, 26.44 mmol) added dropwise, stirred for 2 hours
at room
temperature and then diluted with dichloromethane (70 mL). The dilution was
washed
sequentially with IN aqueous hydrochloric acid (3x 40 mL), saturated sodium
bicarbonate (3x
40 mL) and brine (40 mL} and then concentrated. The product was purified from
the residue
by column chromatography eluting with 2:3 ethyl acetate/hexane to provide benz
I S- N methoxv-N-methylcarbarno,Lrl,~ 3-ghenvlpropv)carbamate (5.48 g, 15.4
mmol) as an oil.
MS(PCI) mlz = 357 (M +1).
Proceeding as in Reference 1 provided tart-butyl IS-(N methoxv-N
methvlcarbamoyll-
3 ~hen~lnropylcarbamate; 'H NMR (CDCl3): $ 1.35 (s, 9~, 8 I .64 - I .72 (m,
2H), 8 2.40 - 2.54
(m, 1H), b 2.60 - 2.77 (m, 1H), s 3.00 (s, 3H) 3.52 (s, 3H), S 4.23 (m, 1H), b
7.10 - 7.3'7 (m,
SH).
-62-
CA 02475069 2004-08-20
ItE"FERENCE 2
3- 2- anobenz lsulfan 1 -2R- 'd-4- lcarbon lamino ro ionic acid
A mixture of isonicotinic acid (3 g), N hydroxysuccinimide (2.79 g) and
N,N dicyclohexylcarbodiimide (5.52 g) was stirred in THF (200 mL) for 16
hours. The solid
was filtered off and the solvent evaporated under reduced pressure. The
residue was triturated
with ethyl acetate and more solid filtered off: The filtrates were
concentrated under reduced
pressure gave 2,5-dioxogyrrolidin-1-yl isonicotinate (5.27 g). MS: 22I [NIH]'.
A solution of L-cysteine (6 g) in ethanol (57 mL) was treated sequentially
with aqueous
2N sodium hydroxide solution (30 mL) and 2-bromomethylbenzonitrile (9.71 g).
The reaction
mixture was stirred 2 hours at room temperature then neutralized by addition
of concentrated
hydrochloric acid. A resulting solid was collected by filtration and wash
sequentially with water,
ethanol and diethylether to provide 2R-amino-3-(2-
cyanobenzylsulfanyl)propionic acid as a white
solid. MS: 237 [MH]''. MS: 235 [M]'.
A solution of 2R-amino-3-(2-cyanobenzylsulfanyl)propionic acid {590 mg) in --
1~ dichloromethane was treated with 2,5-dioxopyrrolidin-1-yl isonicotinate
(1.41 g) and -.
diisopropylethyamine (0.435 mL). The reaction mixture was stirred for 6 hours
and then
concentrated. The residue was treated with water and a resulting insoluble
solid was filtered
off. The aqueous filtrate was extracted twice with ethyl acetate and the
combined extracts
were dried over magnesium sulfate and then concentrated to provide 3-(2-
cvanobenzyisulfa~ll-
2R pvrid-3-carbonylaminopropionic acid (340 mg) as a gum. MS: 342 [MITj*.
HPLC:RT=.
10.63 minutes.
REFERENCE 3
3-Benz Isulfan~J-2R-tetrahydropyran-4-yloxycarbonylaminopro~ionic acid
A solution of tetrahydropyran-4-of (200 mg) in acetonitrile (5 mL) was treated
with
bis(2,5-dioxocyclopentyI) carbonate (0.753 g) and triethylamine (0.$I mL). The
reaction
mixture was stirred for 4 hours at room temperature and then concentrated. The
residue was '
dissolved in ethyl acetate and the solution was washed with a.saturated sodium
bicarbonate
solution, dried over magnesium sulfate and then concentrated to provide 2,5-
dioxo-pyrrolidin-1-yl
tetrahydropyran-4-yl carbonate.
-63-
CA 02475069 2004-08-20
A solution of 2R-amino-3-benzylsulfanylpropionic acid (1 g) and triethylamine
(0.8 mL)
in dichloromethane (40 mL) was treated with 2,5-dioxo-pyrrolidin-1-yl
tetrahydro-pyran-4-yI
carbonate (1.15 g). The mixture was stirred for 16 hours at room temperature
and then
concentrated. The residue was dissolved in ethyl acetate and the solution was
washed
sequentially with hydrochloric acid and brine, dried over magnesium sulfate
and then
concentrated. The residue was subjected to flash column chromatography on
silica eluting with
a mixture of ethyl acetate and pentane (1:1, v/v) to provide 3-benzylsulfanvl-
2R-
tetrahydronyran-4-ylox caY rbonylamino~~ropionic acid (800 mg) as an oil.
REFERENCE 4
3-Benzyisulfanyl-2R-monphoIin-4.-vlcarbonvlaminopropionic acid
A solution of 3-benzylsulfanyl-2R-aminopropionic acid hydrochloride (25 g,
0.118 mol) in
2N sodium hydroxide (59 mL, 0.118 mol) was cooled in an ice bath and then
treated
sirrmultaneousIy with morpholine-4-carbonyl chloride (I3.8 mL, 0.118 mol) and
1N sodium
hydroxide (I I8 mL, 0.118 mol). The mixture was stirred at 0°C for 30
minutes and then
filtered. The filtrate was acidified with SN hydrochloric acid and extracted
with ethyl acetate
(Sx 100 mL). The combined extracts were dried (MgSO,), filtered and
concentrated to provide
3-benzvlsulfanyl-2R-morphoIin-4-ylcarbonylaminopropionic acid (19.65 g, 60.6
mmol) as a white
solid.
REFERENCE S
3-Benzylsulfonyl-ZR-morpholin-4-ylcarbonylaminopro~ionic acid
A solution of 3-benzylsulfanyl-2R-morpholin-4-ylcarbonylaminopropionic acid
(17.58 g,
54.2 mmol), provided as in Reference 4, in methanol (550 mL) was treated with
a solution of
OXONE~ (50 g, 8I.4 mL) in water (550 mL). The mixture was stirred at room
temperature for
2 hours and then concentrated to dryness. The residue was taken up into water
(90 mL) and
ethyl acetate (600 mL}. The mixture was stirred vigorously and the aqueous
layer was
separated and extracted with ethyl acetate (2x 100 mL). The combined ethyl
acetate layers
vi~ere dried (MgS04) and concentrated. The residue was triturated with diethyl
ether and the
solid material was collected by filtration to provide
CA 02475069 2004-08-20
3-benzvlsulfonvl-2R-momholin-4-ylcarbon3rIaniinonro~ionic acido
REFERENCE 6
2-Amino-ld methoxy-N-metl~l-4-.phenylbutyramide
trifluoroacetic acid salt
A solution of tert-butyl 1-(N methoxy-N-methylcarbamoyl)-3-
phenylpropylcarbamate
(9.32 g, 29 mmol}, provided as~in Reference 1, in dichloromethane (100 mL) was
cooled to 0° C
and then treated with anisole (5 naL, 46.5 mmol} and trifluoroacetic acid (50
mL, 296 mmoi).
The mixture was stirred for 30 minutes, while allowing it to warm to room
temperature, and then
concentrated. The residue was dissolved in toluene (10(1 mL) and the solution
was
concentrated. The residue was again dissolved in toluene (100 mL) and
concentrated to provide
2-amino-N methoxv-N methyl-4-phenylbntyramide trifluoroacetic acid salt (9.74
g 29 mmoI) as
a crude product. MS(PCI} mlz = 223 (M +1).
REFERENCE 7
Ethyl 3S-benzyloxycarbonv)amino-2-hvdroxy 5 phen~pentanimidate
i5 A suspension comprised of lithium aluminum hydride (0.885 g, 23.3 mmol) in
anhydrous
diethyl ether was cooled to -4S ° C under nitrogen and then treated
with a solution of benzyl
1S-(N methoxy-N-methylcarbamoyl}-3-phenylpropylcarbamate (5.53 g, 15.53 mmol),
provided
as in Reference 1, in ether {75 mL) and THF (25 mL) added dropwise over a
period of
30 minutes such that the temperature of the mixture was maintained at -40 to -
45 ° C. The
mixture was allowed to warm to 5 ° C and then recooled to -35 °
C. A saturated solution of
sodium bicarbonate,(? mL, 0.5 M) was added dropwise and the mixture was
allowed to warm to
0° C. The mixture was allowed to warm to room temperature and stirred
far 1 hour to provide
a precipitate. The precipitate was collected by filtration and washed with
ether (I00 mL): The
filtrate and washings were combined and washed sequentially with ice cold 1N
hydrochloric
acid (2x SO mL), saturated sodium bicarbonate {2 x 50 mL) and brine (50 mL),
dried (Na~SO~
and concentrated in vacuo to provide benzyl 1S-formyl-3-phenylpropylcarbamate
(4.01 g,
13.5 mmol) as a colorless oil. MS (PCI) nnJz = 298 (M + I).
A solution of benzyl 1S-formyl-3-phenylpropylcarbamate (4.557 g, 15.3 mmol) in
-65-
CA 02475069 2004-08-20
anhydrous dichloromethane (50 mir) was stirred while sequentially treated with
2-hydroxy-
2-methylgroionitriIe {4.25 mL, 46.2 mmol) and triethylamine (1.28 mL, 9.20
mmol). The
mixture was stirred for 4 hours at room temperature and concentrated in varua.
The residue
was dissolved in ether (100 mL) and the solution was washed sequentially with
water (S x 20
mL) and brine (20 mL), dried (MgSO,~ and concentrated to provide benzyi
2-cyano-2-liydroxy-1S-phenethylethylcarbamate (4.957 g, 15.3 mmol) as a yellow
oil. 'H NMR
(CDC13) 81:75 - 2.01 (m, 2H), b 2.08 - 2.24 {m, III, b 2.51 - 2.80 (m, 2H), 8
3.70 - 4.02 (m,
IH), 8 5.07,, & 5.33 (m, 3H?, E ?.10 - 7.47 (m, 10I~.
A mixture of chloroform (30 mL) and anhydrous ethanol (30 mL, 510 rnmol) was
cooled
.10 to 0° C and then treated with acetyl .chloride 132.6 mL, 459 mmol)
added dropwise over a
period of 30 ininutes. The mixture was cooled by adding a solution of crude
benzyl 2-cyano-
2-hydroxy-1S-phenethylethylcarbamate (4.957 g, 15.3 mmol) in chloroform (30
mL). The
mixture was fitted for 2 hours at 0°C and then 6 hours at room
temperature and concentrated
in vacuo to,provide ethyl 3S-benzyloxvcarbonylamino-2-hydroxy-S-
phenylpentanimidate
(6.212 g 15.3 rnmol) as a crude yellow oil. MS (PCn mlz = 371 (M + I).
REFERENCE 8
2S-Amino-4-vhenvl-1 ~4S=phen 1~-4.5-dihydrooxazol-2-yllbutan-1-of
(a) A mixture comprised of ethyl 3S-benzyloxycarbonylamino-2-hydroxy-
S-phenylpentanimidate (0.78 g, 1.92 mmol), provided as in Reference 7,
diisopropylethylamine
(0.218 pL, 1.26 mmol) and ZS-amino-2-phenylethanol (0.260 g, 1.9 mmol) in
chloroform (2S mL)
was heated at reflux for 3 hours and then was stirred for approximately i2
hours, while allowing
to cool to room temperature. The mixture was concentrated and the residue was
dissolved in
ethyl acetate (50 mL). The solution was washed sequentially with O.SN sodium
hydroxide
(40 mL) and brine (40 mL), dried (MgSC)~) and then concentrated. Product was
purified from
the residue by flash chromatography eluting with 1:3 hexanes/ethyl acetate to
provide benzyl
2-hydroxy-2-(4,5-dihydro-.4S-phenyloxazol-2-yl}-1S-phenyethylethylcarbamate
(0.475 g,
1.1 mmol) as an oily mixture of diastereomers. MS (PCl7 miz = 445 (M +1).
(C27H~Nz04).
(b) A solution comprised of benzyI 2-hydroxy-2-(4,5-dihydro-4S-phenyloxazol-2-
yl}-
1S-phenyethylethylcarbamate (100 mg, 0.22 mmol) in methanol (10 mL) was placed
under a
nitrogen atmosphere and stirred while Pearhaan's catalyst (20 mg) was added.
The mixture
-66-
CA 02475069 2004-08-20
was stirred vigorously under a hydrogen atmosphere until the reaction was
complete and then
filtered. The filter was washed with methanol (2 x 25 mL). The cambined
filtrates were
concentrated to provided 2S-amino-4-phenyl-1-l4S-phenyl-4 5-dih~drooxazol-2-
vl)butan-1-of
(51 mg, 0.16 mmol) as a clear oil. MS (PC>') mlz = 311(M +1). (C,~-IuNZO~.
Proceeding as in Reference 8 provided methyl 2-(2S-benzyloxycarbonylamino-
1-hydroxy-4-phenylbutyl)-4,5-dihydroaxazole-4-carboxylate.
REFERENCE 9
2S-Amino-1-oxazol-2-yl_4-phe~lbutan=1-of
trifluoroacetic acid salt
90 A solution comprised of oxazole (0.25 g, 3.62 mmol) in T~' (20 mL) was
treated with
borane tetrahydrofuran complex (3.62 mL, 3.62 mmol).under nitrogen and the
mixture was
stirred for 30 minutes and then cooled to -78 °C. A solution comprised
of sec-butyl lithium (2.78 v
mL, 3.62 mmol) in cyclohexane was added dropwise and the mixture was stirred
for 30 minutes.
A solution comprised of ten-butyl (S~l-formyl-3-phenylpropylcarbamate (0.476
g, I.81 mm~y
75 in T.I~ (25 mL} was added and the mixture was stirred and allowed to warm
while the reaction
proceeded to completion. The mixture then was cooled to -78°C, quenched
by slowly adding
5% acetic acid in ethanol (20 mL), allowed to warm to ambient temperature and
stirred for 18
hours. The mixture was concentrated to dryness and the residue was extracted
with ether
(2x25 mL}. The combined extracts were washed with brine, dried (MgSO~ and
concentrated
20 to dryness to provide tert-butyl 2-hydroxy-2-oxazol-2-y1-1S-
phenethylethylcarbamate (0.12S g,
0.376 mmol) as a yellow oil. MS (PCn m!z = 333 (M + 1).
A mixture comprised of tent-butyl 2-hydroxy-2-oxazol-2-yl-IS-
phenethylethylcarban~
(0.125 g, 0.376 mmol), anisole (0.2 mL} and trifluoroacetic acid (0.6 mL) in
dichlorornethane
(20 mL) was stirred at room temperature for 2 hours and then concentrated to
provide
25 2S-amino-1-oxazol-2-yi-4-yhenylbutan-1-of trifluoroacetic acid salt ( 0.08
g, 0.229 mmol) asa
yellow oil. MS (PCI) m/z = 233 (M + 1}.
-67-
CA 02475069 2004-08-20
REFERENCE I U
Methyl 2-l2S-amino-1-h~rdroxv-4-QhenvlbutYl)oxazole-4-carboxylate
A solution comprised of methyl 2-(2S-benzyloxycarbonylamino-l-hydroxy-
4-phenylbutyl)-4,5-dihydrooxazole-4-carboxylate (0.100 g, 0.235 mmol),
provided as in
Reference 10, in dichloromethane (3 mL) was cooled to 0° C and then
treated with DBU
(39 mL, 0.26 mmol) and bromotrichloromethane (26 mL, 0.26 mmol). The mixture
was stirred
for 6 hours at 0° C, washed with ammonium, chloride (10 mL) and
concentrated. The residue
was dried (MgSOd) to provide methyl 2-(2S-benzyloxycarbonylamino-I-hydroxy-
4-phenylbutyl)oxazoIe-4-carboxylate. MS(PCn mlz = 425 (M ~I).
' ' Deprotecting provided methyl 2-l2S-amino-1-hydrox~
4-,~henylbutyl)oxazole-.4-carboxylate.
REFERENCE 1 I
2-Benzooxazol-2-yl-2-pert-butyl-dimethvl-silanyloxy)-1S-phenethylethylamine
A solution of 2S-amino-l-benzooxazol-2-yl-4-phenylbutan-I-of (600 mg),
provided as in
Referencel2, in dichlaromethane (IS mL) was cooled to 0°C and then
treated with 2,6-lutidine
(0.57 mL) followed by tert-butyldimethylsilyl trifluoromethanesulfonate (1.08
mL). The solution
was stirred for 3 hours and then additional dichloromethane was added (SO mL).
The mixture
was washed sequentially with a saturated sodium bi-carbonate solution (50 mL)
and brine
(50 mL x2), dried over magnesium sulphate and concentrated under reduced
pressure to provide
2-benzooxazol-2:y1-2-ftert-butyl-dimethyl-silanyloxy)-IS-phenethylethyIamine
as an orange oil.
REFERENCE 12
2S-Amino-1-benzooxazol-2 y1-4-phenylbutan-1-oI
A solution of (S)-2-tern-butoxycarbonylamino-4-phenylbutyric acid (S00 g, 179
mmol),
EDC (37.8 g, 197 mmol), H4BT (4l.lg, 269 mmol) and N,O-dimethylhydroxylamine
hydrochloride (19.2 g, 197 mmol) in , dichIoromethane (S00 mL) was cooled in
an ice bath and
then treated with a solution of triethylamine (27.5 mL, 197 mmol) in
dichloromethane (1S0 mL).
The ice bath was removed and the reaction mixture was stir at room temperature
for
-68-
CA 02475069 2004-08-20
approximately I2 hours. The mixture was concentrated by rotary evaporation and
the residue
was treated with ethyl acetate (450 mL), water (300 mL) and saturated sodium
bicarbonate until
all solids .were dissolved. The ethyl acetate layer was separated and washed
sequentially with
saturated sodium bicarbonate {100 mL), water (100 mL), IN hydrochloric acid
(100 mL), water
(i00 mL) and brine (50 mL). The solution was dried over anhydrous magnesium
sulfate and
concentrated to provide ten-butyl (S)-1-(N methoxy-N methylcarbamoyl~
3-phenylpropyicarbamate (53.41 g, 93°lo yield) as a clear, colorless
oil.
The tert-butyl (S)-1-(N methoxy-N methyIcarbamoyl}-3-phenylpropylcarbamate
provided above was divided into three portions (5.0 g 15.5 mmol; 4.88 g , 15.1
mmol; and 4.54 g,
14.1 mmol). Each portion was azeotroped with toluene by rotary evaporation and
dried under
reduced pressure to remove residual ethyl acetate and water. Each portion of
the ester was
taken up into anhydrous diethyl ether (75 mL) and the mixtures were cooled in
an ice bath under
nitrogen. Each of the mixtures were treated with lithium aluminum hydride (1M
in diethyl
ether, 23.3 mL, 22.7 mL, and 21.1 mL, respectively) added by syringe and the
mixtures were
i5 stirred at 0°C for 90 minutes. The mixtures were treated with ethyl
acetate (5 mL), stirred~for
ZS minutes, further treated with saturated KIi2P04 (5 mL), 1N hydrochloric
acid {I mL) and
then additional 1N hydrochloric acid until the solid mass dissolved. The
resulting solutions were
combined and extracted with ethyl acetate (3x 200 mL). The extracts were dried
over
anhydrous magnesium sulfate and concentrated. The residue was dried under
reduced pressure
to provide tent-butyl (S)-I-formyl-3-phenylpropylcarbamate 111.61 g, 99%
yield).
A solution of tert-butyl (S)-1-formyl-3-phenylpropylcarbamate (1I.15 g, 42.3
mmol) in
dichloromethane (25 mL) was cooled in an ice bath under nitrogen and then
treated sequentially
with acetone cyanohydrin (10.8 mL, I 19 mmol) and triethylamine (3.5 mL, 25.4
mmol). The
reaction was stirred for approximately 12 hours at room temperature and then
concentrated by
rotary evaporation. The residue was dissolved in 1:1 hexanes:ethyl acetate
(250 mL) and the
solution was washed sequentially with water (3x I00 mL) and brine (50 mL),
dried over
anhydrous magnesium sulfate and concentrated. Product was purified from the
residue by silica
gel chromatography using 2:1 hexanes:ethyl acetate eluent to provide ten-butyl
2-cyano-Z-hydroxy-1S-phenethylethylcarbamate (12.05 g, 98% yield).
A mixture of chloroform (12.8 mL) and absolute ethanol (9 mL, 153 mmol), under
a
nitrogen stream with an attached Firestone valve bubbler, was cooled in an ice
bath and then
treated with acetyl chloride (9.2 mL, I29 minol) added by syringe. The mixture
was allowed to
-69-
CA 02475069 2004-08-20
stand for 5 minutes and then a solution of tent-butyl
2-cyano-2-hydroxy-1S-phenethylethylcarbamate (2.34 g, 8 mmol) in chloroform
(19.2 mL) was
added. The nitrogen inlet was removed and the mixture was stirred and slowly
warm to room
temperature over approximately I2 hours. The mixture then was concentrated by
rotary
evaporation and the residue was treated with absolute ethanol (40 mL)and o-
aminophenol
(873 mg, 8 mrnol). The mixture was heated at 95°C under nitrogen for 5
hours and then stirred
at room temperature for approximately I2 hours. The mixture was treated with
diethyl ether
(150 mL) and the resulting solution was washed repeatedly with 1N KOH until
the aqueous
wash layer was colorless. The organic phase was separated, dried over
anhydrous magnesium
sulfate and concentrated. The residue was recrystallized from hot hexane and a
minimum
amount of ethyl acetate to give a tan powder (335 mg). The mother liquor was
combined with
the mixed fractions from a similarly performed reaction nan and purified by
silica gel
chromatography using 5% methanol in dichlorornethane to provide
2S-amino-I-benzooxazoI-2-yl-4.-nhenylbutan-1-of (L2? g, 52% average yield) as
an orange
semi-solid mass.
Proceeding as in Reference 22 provided the following compounds:
2-amino-1-benzooxazol-2-y1-ethanol;
2.-amino-I-benzooxazol-2-yI-2-methyl-propan-1-oI;
~,Sl-2-amino-I-benzooxazol-2-vI-hexan-1-ol;
I-ll-amino-cy.,clopropyl~-1-benzooxazol-2-yl-methanol;
(SZ2-amino-I-benzooxazol-2-yl-propan-1-ol;
~S)-2-amino-I-benzooxazol-2-girl-4-methanesulfonvl-butan-I-ol;
sS)-2-amino-i-benzooxazol-2 yl-pentan-I-oI;
~S~2-amino-7-benzooxazol-2yI-butan-I-ol; and
2-Amino-1-benzooxazol-2-yl-3-methoxy.propan-1-ol;'H NMR (CDC13): 7.70 (m, 1H),
?.53 (m, 1H), 7.34 (rn, 2H), 4.88-5.0 (rn, IH), 3.60 (m; iH), 3.53 (m, 3H),
3.3? (s,1H), 3.30 (s,
1H); .
-70-
CA 02475069 2004-08-20
XAMPLE 1 _.
N- 1R- 2-Benzaoxazol-2- 1-2-h drox -1 - he eth Ieth tcarbamo 1 -2-be z lsulf n
leth
rnorpholine-4-carboxamide
(Compound 1)
A mixture of 2S-amino-1-benzooxazol-2-y1-4-phenylbutan-I-of (2.2 g, ?.8
mrnol),
provided as in Reference 12, 2-mozpholfn-4-ylcarbonylamino-3-
benzylsulfonylpropionic acid _ ,
(2.78 g, 7.8 mmol), EDC (1.64 g, 8.57 mmol), 1-hydroxybenzotriazole hydrate
(1.58 g;
11.7 mmoi) and N methylmorphoIine (2.4 mL, I7.1 mmoI) in dichloromethane was
stirred for I
hour. The mixture was treated with additional amounts of EDC (0.1 eq) and
I-hydroxybenzotriazole hydrate (O.I eq) and stirred for 30 minutes. The
mixture was treated
with an additional amount of EDC (0.1 eq) and stirred for 15 minutes: The
mixture was treated
with an additional amount of EDC .(0.1 eq) and stirred for 30 minutes. The
mixture was
concentrated and the residue was taken up into ethyl acetate. The mixture was
washed
sequentially with 1N hydrochloric acid (3x 50 mL), saturated sodium
bicarbonate solution (2x
50 mL) and brine (50 mL), dried (MgS04) and concentrated to provide
N 1R-(2-benzooxazol-2-yl-2-hydroxy-IS-ph nethylethvlcarbamoyl)-
2-benzylsulfon~leth-yllmorrpholine-4-carboxamide (4 g, 6.44 mmol); tH NMR
(CDC13): 7.6$ (m,
1H), 7.52 (m, 1H), 7.10-7.45 (m, I2H), 6.0-6.25 (m, 1H), 4.95-5.1 (m, 1H),
4.52-4.80 (m, 1H),
4.i5-4.5 (m, 3H), 3.I-3.75 (m, lOH), 2.69 (m, 2H), 2.06 (m, IH), 1.80 (m, 1H);
IVlS: m/e 621.0;
EXAMPLE 2
-71-
CA 02475069 2004-08-20
ZS-Acet lamino-N 2-oxazol-2- 1-2-h drox -IS- heneth Ieth 1 -3-c clohex 1 ro
ionamide
(Compound 2)
A mixture comprised of 2-acetylamino-3-cyclohexylpropionic acid {0.45 g; 0.211
mmol),
PyBOP~ (0.1 I g, 0.21 mmol) and diisopropylethylamine (0.037 g, 0.211 mmol) in
DMF (10 mL)
was stirred for 15 minutes at room temperature and a solution comprised of 2S-
amino-
1-oxazol-2-yl-4.-phenylbutan-I-of trifluoroacetic acid salt, provided as in
Reference 9, in DMF
and neutralized with diisopropylethylamine was added. Additional
diisopropylethylamine
(0.037 g, 0.211 mmol) was added and the mixture was stirred for 2 hours at
room temperature
and then poured into 100 mL of ice cold water. The aqueous phase was extracted
with ethyl
acetate (3 x 25 mL) and the combined organic layers were washed sequentially
with 1 N
hydrochloric acid {2 x 2S mL), water (2 x 25 mL) and brine (2 x 25 mL), drricd
(MgSO~j and
concentrated. JProduct was purified fr~am the residue by flash chromatography
eluting with i:3
hexanes/ethyl acetate to provide 2S-acetylamino-N l2-oxazol-2-vl-
~-hydroxv-1S_phenethylethvl'i-3-cyclohexylpropionamide (0.036 g, 0.084 mmol)
as an oil. MS
(ESI) m/z = 428 (M + i ); 'H-NMR {300 MHz, CD3OD): 8 0.80 (m, 2H), S L 12 (m,
4H),
8 1.40(m, 2H), 8 1.65 (m, 6H), s 1.80 (m, 1H), 8 2.00 (m, 4H), 8 2.70 (m, 1H),
S 2.80 (m, 1H),
8 4.44 (m,1H), b 4.51 (m, iH), 8 7.1 I -:7.47 (m, 6H), b 7.99 {s, 1H),
{C~H33~3~~~
Proceeding as in Example 2 provided the following compounds of Formula I:
3-c clohex 1-N- 2-h drox -2- 5- hen loxazol-2- 1 -1S- heneth leth 1 ro
ionamide
(Compound 3); MS (ESI) m/z = 448 (M + I ); 'H-NMR (300 MHz, CDC13): 8 0.89 (m,
ZH),
-?2-
CA 02475069 2004-08-20
81.20 (m, 4H), b 1.45 (m,1H), E 1.65 (m, 6H), b 1.80 (m, iH}, 8 2.09 (m, 4H),
b 2.73 (t, J = 4 ~-
Hz, 2H), S 4.S I (m, 1 H), 8 4:96 (m, 2H), 8 6.00 {m, i H), 8 7.11 - 7.47 {m,
9H}, 8 7.60 (m, 2H);
{~H3~3~3)~
2S-acetxlamino-N-f 2-hvdroxy-I S-pheneth)!J-2-l5-phenvloxazoI-2-vI)ethyll-
3-cyclohexvlvropionamide (Compound 4); MS (ESI) m/z = 505 (M + 1);'H-NMR (300
MHz,
CDC13): b 0:80 (rn, 2H), 81.12 (m, 4H), $1.40 (m, 2H), b 1.65 (m, 6H), 81.80
(m, 1H), 8 2.00
(m, SH), 8 2.70 (m, 2H), 8 4.5I (m, IH), 8 4.96 (m, 2H), S 6.I9 (m, 1H), 8
6.98 (m, 1H},
8 7.11 - 7.47 {m, 9H), 87.62 (m, 2H), {C3pH38N3~4)9 ~d
N-( I S-benzothiazol-2-vlcarbonvl-3-phenylpropyl)-3-cyclohexylpropionamide
{Compound 5);'H NMR: b 0.83 (m, 2H}, 8 1.20 (m, SH), 8 1.48 (q, 2H, J = 9 Hz),
81.67 (m,
4H), 8 2.20 (m, 3H), 8 2.48 (m, 1H), 8 2.75 (m, 2H), 8 5.95 (m, 1H), 8 6.35
(d, 1H, J = 9 Hz),
8 ?.2S (m, 5H), 8 7.57 (m, 2H), 8 7.93 (d, 1H, J = 9 Hz), 8 8.18 (d, 1H, J = 9
Hz); ES-MS mlz
435 (MH+}; and
2S-acetylamino-N (1S-benzothiazol-2-ylcarbonyl_3-phenylpropyl)-
3-cyclohexyl_vropionamide (Compound 6);'H NMR: 8 0:87 (m, 8H), 8 1.22 (m, 6H),
8 1.92 (m,
IH), 8 2.12 (m, 1H), 8 2.48 (m, iH), b 2.78 (m, 2H), b 3.87 (d, IH, J = 7 Hz),
b 5.62 (m, 1H),
b ?.20 (m, 6H), 8 7.53 (m, 2H), & 7.98 (d, 1H, J = 7 Iiz), S 8.18 (d, 1H, J =
7 Hz); ES-MS m/z
492 (MH+).
N-i 1 S-f 1 S-phenethvl-2-benzaoxazol-2-yl-1-oxoethylcarbamaylZ
2-naphth-2-ylethyllpi~eridine-4-carboxamide (Compound 7),'H NMR (DMSO-db}: 8
i.32 - 1.76
(m, 4H), 81.90 - 2.09 (m, 2H), 8 2.22. - 2.60 (m, 2H), 8 2.65 - 3.26 (rn, 6H),
8 4.?2 - 4.86 (m,
1H), b 5.26 (m,1H), 8 7.06 - 7.31 (m, SH), 8 7.45 (m, 4H), 8 7.55 (dt, J =
1.26, 7.84 Hz, 1H),
8 7.65 (dt, J = 1.18; 8.00 Hz, 1H), 8 7.72 - ?.88 (m, 3H), 8 7.90 (d, J = 8.06
Hz, 1H}, 8 7.99 (d,
J ~ 7.86 Hz, 1H), 8 8.I4 (bs, IH), 8 8.24 (d, J = 8.04 Hz, IH), 8 8.46 (bs,
1H), 8 8.94 (d,
J = 6.43 Hz; 1H);
2S-acetylamino-N (1S-benzooxazol-2 ylcarbonyl-3-nheny_lpropyl)-
3-cyclohexy_Ipropionamide (Compound 8); MS (ESI) m1z = 476 (M + 1);'H-NMR (300
MHz,
CDCl3): 8 0.85 (m, 2H), 8 I.26 (m, 4H}, 8 I.47 (m, 2H), S i.b4 {m, 6H), 8 1.99
(s, 3H}, 8 2.I5
(m, 2H), 8 2.41 (in, iH), 8 2.72 (t, J = 6Hz, 2H), b 4.59 (q, J = 4Hz, 1H), 8
5.65 (q, J = 2Hz,
1H), 8 6.26 (d, J = 6 Hz, 1H), 8 7.10 - 7.26 (m, 6H), 8 7.41 - 7.65 (m, 3H), 8
7.86 (d, J = 6Hz
IH), (CZBH33N3o4}~
rerr-butyl 1 S-( 1 S-benzooxazol-2-ylcarbonyl-3-phenylt~ropylcarbamoxl)-
-73-
CA 02475069 2004-08-20
2-cyclohexvlethylcarbamate (Compound 9);
N f 1-fbenzooxazol-2-ylcarbonyl)-3-phenylpropyll-3-c clohexylpropionamide
(Compound IO);
3-cyclohex~-N f3S=phenyl-1 ~S-phenyloxazoI-2-ylcarbonyl_)pronyl~ropionamide
(Compound 11 ); MS (ESl7 mlz = 445 (M + 1 ); 'H-NMR (300 MHz, CDCI3): 8 0.89
(m, ZH),
8 1.20 (m, 4H), 81.55 (m, 2H), 81.68 (m, 6H), ~ 2.12 (m, 1H), S 2.27 (t, J =
4Hz, 2H), 8 2.48
(m, 1H), S 2.76 (m, 2H), S 5.70 {m, 1H), S 6.35 (d, J = 4 Hz, IH), S 7.19 -
7.30 (m, SH), 8 7.48
(m, 3H), 8 7.57 (s, 1H), 8 7.79 (d, J = 4Hz, 2H), (C2gH32N2o3)~
2S-acet5rlamino-N-f 1S-(5-phenyloxazol-2-ylcarbonvl)-3-phenylprop
3-cyciohexylpropionamide (Compound 12); MS (ESA m/z = 502 (N! + 1);''H-NMR
(300 MHz,
CDCl3): 8 0.80 (m, 2H), 8 1.12 (m, 4H), b 1.50 (m, 1H), $ 1.65 (m, 6H), 8 1.80
(m, 1H), 8 2.05
(s, 3H), S 2.12 (m, 1H), S 2.48 (m, 1H), b 2.70 (t, J = 6Hz, 2H), b 4.52 (q, J
= 2Hz, 1H); 8 5.60
(q, J = 2Hz, iH), S 5.9$ (d, J = 6 Hz, 1H), 8 6.92 (d, J = 6Hz,1H), 8 ?.19 -
7.30 {m, SH), 8 7.48
(m, 3H),. b 7.57 {s,. IH), S 7.79 (d, J = 4Hz, 2H), (C3pH~3N3~4}~
. benzvl 1 S-lbenzooxazol-2-ylcarbonvlmethyicarbamoyl)-3-methylbutvlcarbamate
(Compound 13);
benzyl I S-(5-phenylbenzooxazol-2-vlcarbonvlmethylcarbamoyl)-3-
meth~butylcarbamate
(Compound 14);
2S-ace lamino-N- IS-oxazol-2- lcarbon 1-3- hen 1 ro 1 -3-c clohex I r ionamide
(Compound 15); MS (ESA mlz = 426 (M + 1); 'H-NMR (300 MHz, CDCl3): e~ 0.85 (m,
2H),
8 1.20 (m, 4H), b 1.50 {m, 2H), 81.65 (m, 6H), S 2.05 (s, 3H), 8 2.48 (m, 1H),
& 2.70 (t,
J = 6Hz, 2H), 8 4.52 (q, J = 2Hz, 1H), 8 5.60 (q, J = 2Hz, 1H), F 5.93 (d, J =
~ Hz, IH), 8 6.89
(d, 3 = 6Hz, 1H), 8 7.19 - 7.38 (m, 5H), 8 7.47 (s, 1H), b 7.79 (s, 1H),
(C~H3,N304);
benzyl 1S-benzooxazol-2-vlcarbonyl-3-phenylprop,~rlcarbamate {Compound 16);
2-acetylamino-N (1S-benzooxazol-2-ylcarbonvl-3-phenylpropvl)-3-
,phenvlnropionamide
(Compound 17);
N-~1S-benzooxazol-2 ylcarbonyl-3-oheny~propyl)benzvlsulfonamide (Compound
18);'H
NMR (CDC13): 7.88 (d, J=6.2Hz, 1H), 7.67 (d, J=6.2Hz, 1H), 7.60 (t, J=6.2Hz,
1H), 7.51 (t,
J=6.2Hz, 1H), 7.35 (d, J=6.2Hz, 2H), 7.08-7.29 (m, 7H), 6.96 (t, J=6.2Hz, 1H),
5.52 (d, JK=9.4
Hz, 1H), 4.90 (td, J=9.4, 3.IHz, 1H), 4.31 (dd, J=10.9, 10.9Hz, 2H), 2.80 (m,
IH), 2.27 (m, 1H),
2.04 (m, 1H}; MS: m/e=4.35.0;
N ~1S-benzooxazol-2-ylcarbonyl-3-phenvlpropvl)-2-cyclohexylethanesuIfonamide
-74-
CA 02475069 2004-08-20
(Compound 19); 'H NMR (CDC13): 7.94 (d, J=6.3Hz,1H}, 7.70 (d, J=6.3Hz, IH),
7.62 (t,
J=6.3Hz, 1H), 7.52 (t, J=6.3Hz, IH), 7.17-7.34 (m, SH), 5.42 (d, J~9.5Hz, 1H),
5.17-5.25 (m,'
IH}, 2.79-3.09 (m, 4H), 2.38-2.55 (m, 1H), 2.08-2.21 (m, 1H), 1.52-1.79 (m,
7H), 1.08-1.34. (m,
4H), .77-I.O1 (m, ZH); MS m/e=455.3;
N (1-benzooxazol-2- lcarbonyl-3-nhenylpropyl)-3-cvclopentYlpropionamide
(Compound 20);
N (IS-benzooxazoI-2-ylcarbonyl-3-phenylprogyl)-2-cvclohex~Iacetamide
(Compound 21};
N-(1S-benzooxazoI-2-ylcarbonyl-3 phenylprop~rl)-2-bicyclof2.2.lyhept-2-
ylacetamide
0 {Compound 22};
N-(1S-benzooxazol-2-ylcarbonyl-3-nhenylprog I)-4-met>~Ipentanamide (Compound
23) ;
N l1S-benzooxazol-2-vlcarbonvl-3-phenvlprop~-2-naphthaIen-1- l~tamide
{Compound 24);'H NMR (CDC13): 7.96 (m, 1H), 7.84 (m, 2H), 7.82 (m, IH); 7.42-
7.75 (nn,
6H), 7.14 {m, 4H), 6.86 (m, 2H), 6.25 (m,1H}, 5.64 (m, 2H), 4.08 (m, 1H), 2.45
(m, ZH),2.42
i5 (m, 1H), 1.90 (m, 1H); .
N (1-benzooxazol-2-ylcarbonyl-3-nhenylpropyl)-3-phenvloropionamide (Compound
25);
'H NMR (CDC13): 7.90 (d,J=B.OHz, 1H), 7.65 (d,J=8.OHz,1H), 7.59 (m, 1H}, 7.56
(m, 1T~,
7.05-?.35 (m, 11H), 6.20 (d,1=7.OHz, 1H), 5.76 (m, 1H), 2.97 (m, 2H), 2.5-2.7
(m, 4H), 2.4 (m,
1H), 2.1 (m, 1H);
?0 methyl2-(2-l3S-cvclohexvlpropionvlaminoy-4-nhenvlbutvrvll-
4.5-dihvdrooxazole-4S-carboxvlate {Compound 26); MS {ESI) m!z = 429 (M + 1);'H-
NMR
(300 MHz, CDC13): 8 0.89 (m, 2H), $ 1.22 (m, 4H), 81.51 (m, 1H), S 1.65 (m,
6H), 8 2.05 (m,
IH), S 2.20 (t, J = 4 Hz, 2H), 8 2.46 (m, 1H), b 2.73 (m, 2H), b 3.80 (s, 3H),
8 4.55 {m, 1H),
'~ 4.60 (m, 1H), 8 5.00 (m, 1H), 8 5.45 (m,1H), 8 6.15 (m, 1H), 8 7.13 - 7.35
(m, 5H),
(~H32N2~5}~
methyl 2-f2-(3S-cvclohexvlpronionylaminol-4-phenylbutvrvlioxazole-4-carbox ly
ate
(Compound 27); MS (ESI) mlz = 427 (M + 1);'H-NMR (300 MHz, CDC13): 8 0.89 (m,
2H),
8 L22 (m, 4H), 8 1.49 (m, 1H), S 1.65 (m, 6H), & 2.20 (m, 3H), S 2.46 (rn,
1H), b 2.74 (m, 2H},
8 3.99 (s, 3H), 8 5.62 (m, 1H), b 6.20 {d, J = 4Hz, 1H), S 7.15 - 7.35 (m,
5H), S 8.40 (s, IH),
30 (C~,H~IV20s);
benzyl I S-(I S-benzooxazol-2-y-lcarbon~)-
3-phenvlpropvlcarbamo l~phthalen-2-ylet~lcarbamate {Compound 28);
-75-
CA 02475069 2004-08-20
2-acetvlarnino-,N~- 1S-benzooxazol-2-ylcarbonvl-3-phenvlnrovvll- .
3-(2-fluorophenYl)propionamide (Compound 29);
2S-acetvlamino-N (1S-benzooxazol-2-ylcarbonvl-3-phenvlpropyl)-2-methyl- .
3-nhen~nropionamide {Compound 30);
tert-butyl 1SS,IS benzooxazol-2-vlcarbonyl-3-phenylprop~lcarbarnovl)-
3-nhenylprowlcarbamate (Compound 31);
- 1-benzooxazol-2- lcarbon 1 -3- hen 1 r 1 lohex Iy but~ramide
(Compound 32);'H NMR (CDC13): 7.94 (d, J=7.9Hz, 1H), 7.68 (d, 7.9Hz, 1H), ?.58
(t,J=7.9Hz, 1H), 7.50 (t, J=7.9Hz, IH}, ?.10-7.32 (m, SH), 6.27 (d, J=lI.8Hz,
1H), 5.76-5.89 (m,
1H), 2.74-2.89 (ni, 2H), 2.42-2.61 (m, iH), 2.11-2.32 (m, 3H); 1.53-1.79 (m,
9H), 1.05-1.32 (m,
4H), 0.79-L0 (m, 2H); MS: mle=433;
methyl 2-f 2S-(3-cyclohexylpropionvlamino)-4-phen~t~r~ll-
4y5-dihydrooxazol-4S-ylcarboxvlate (Compound 33); MS (ESI) mlz = 429 (M + 1);
'H-NMR
(300 MHz, CDCl3): 8 0.89 (m, 2H), 8 1.22 (m, 4H), & 1.51 {m, 1H), S 1.65 (m,
6H), S 2.05 (m,
i5 1H), 8 2.20 (t, J = 4 Hz, 2H), 8 2.46 (m, 1H), fi 2.73 (m, 2H), 8 3.80 {s,
3H), 8 4.58 (m, 2H),
8 5.00 (m, 1H), S 5.45 (m, IH), 8 6.15 (m, 1H), 8 7.13 - 7.35 (m, SH),
(C~H3ZN=OS);
3-c clohex 1-N 1- 5-methox benzooxazol-2- lcarbon 1 -3- hen 1 ro 1 ro ionamide
(Compound 34); MS (ESn mlz = 449 (M + 1 ); 'H-NMR (300 MHz, CDC13): S 0.95 (m,
2H),
8 1.22 {m,~4H), 81.51 (m, 2H), b 1.65 {m, 6H), 8 2.15 (m, 1H), ~ 2.20 (t, 3 =
4 Hz, 2H), b 2.50
(m, 1 H), 8 2.7? (q, J = 2 Hz, 2H), S 3.92 (s, 3H), S 5.78 (m, 1H), 8 6.37 (m,
1H), 8 7.13 - 7.35
(m, SH), 8 7.53 (d, J = 6 Hz,1H), (C27H32N2~~~
2-acet~rl-N (1S-benzooxazol-2-vlcarbonvl-
3-phenvlpro-p-yl)-1.2.3.4-tetrahydroisoquinoline-3S-carboxamide (Compound 35);
2S-acet~lamino-N-(I S-benzooxazol-2-vlcarbonyl-3-gheny~pron~!1)
3-(2-chlorophenyl)propionamide (Compound 36);
3-c clohex 1-N 1 S- 6-methox benzooxazol-2- lcarbon 1 -3- hen 1 ro 1 ro
ionamide
(Compound 37); MS (ESI) mlz = 449 (M + 1 ); 'H-NMR (300 MHz, CDCI3): S 0.95
(m, 2H),
81.22 (m, 4H), 81.51 (m, 2H), 81.65 (m, 6H), 8 2.15 (m, 1H), b 2.20 (t, J = 4
Hz, 2H), b 2.50
(m, 1H), 8 2.77 (q, J = 2 Hz, 2H), 8 3.95 (s, 3H), 8 5.78 (m; 1H), & 6.37 (d,
J = b Hz, 1H},
8 7.10 - 7.35 (m, SH), 8 7.77 (d, J = 6 Hz, 1H}, (CZ,H3zNaO,a;.
3-cyclohexyl-N-11S-l5-trifluoromethYlbenzooxazol-2-ylcarbonvl)-
3-phenylprog~_lpropionamide (Compound 38); MS (ESI) m/z = 487 (M + 1); 'H-NMR
(300
-76-
CA 02475069 2004-08-20
MHz, CDC13): 8 0.95 (m, 2H), 8 I.22 (m, 4H), fi i.51 (m, 1H), b 1.65 (m, 6H),
8 2.20 (m, 3H),
8 2.S 1 (m, IH), 8 2.80 (g, J = 2 Hz, 2H), 8 5.76 (m, 1H), S 6.22 (d; J = 6
Hz, iH), 8 ?.15 - 7.35
(m, SH), 8 7.77 (m, 2H), S 8.25(s, IH), (Cnf~29F3N2~3)~
2-acetylamino-Nll-benzooxazol-2-ylcarbonyl-3-phenylpropyl)-
3-l2-trifluorometh~~henyI,Zpro,~,~onamide (Compound 39);
- 1=benzooxazol-2- lcarbon I-3- hen 1 ro I 3-mo holin-4- 1 ro iona 'd
{compound 40);;'H NMR (CDC13): 7.90 {m, 1H), 7.76 (m, IH), 7.06-7.36 (m, 7H),
4.00 (m,
1 H), 3. i 2 (m; 4H), 2.50-3.5 (m, ZH), 2.0-2.S (m, 2H), 1.83 (m, 4H); MS: mle-
42 L9;
3-cvclohexvl-N I1S-(S-nitrobenzooxazol-2ylcarbon ly 1~.3-"phenyl-
propvll~ropionamidg
(Compound 41 ); MS (ESn m/z = 464 (M + 1 ); 'H-NMR (300 MHz, CDC13): 8 0.95
(m, 2H),
8 1.22 (m, 4H), S I .S 1 (m, 1H), 8 I .65 (m, 6H), 8 2.20 {m, 3H), 8 2.S 1 (m,
IH), b 2.80 (m, 2H),
8 5.67 (m, 1H), b 6.17 (d, J = 6 Hz, 1H), 8 7.09 - 7.35 (m, SH), 8 7.?7 (d, J
= 6Hz, 1H), s 8so
(a, J = 6 Hz, ll~, s 8.77 {s, ll~, (C~.H2gN3os);
meth 12- 2S- 3-c clohex l ro ion iamino -4- hen )but 1 benzooxazole-6-carbox
late
(Compound 42); MS (ESI) mlz : 477 (M + 1); 'H-NMR (300 MHz, CDC13): is 0.95
(m, 2H), -
S I.22 (m, 4H), 8 l.Sl (m, 1H), ~ 1.65 (m, 6H), 8 2.23 (m, 3H), s 2.50.(m,
1H), & 2.77 (m, 2H), -
8 4.00 (s, 3H), 8 S.?8 (m, 1H), b 6.27 (d, J = 6 Hz, IH), 7.15 - 7.35 (m, SH),
8 7.98 (d, J = 6 Hz,
1H), 8 8.22 (d, J = 6Hz, 1H ), b 8.39 (s, 1H), (C28H32N2Os):
N 1S- S-chlorobenzooxazol-2- lcarbon l -3- hen I ro l -3-c clohex I ro
ionamide
(Compound 43); MS (ESI} m/z = 4S3 (M + I);'H-NMR (300 MHz, CDC13); S 0.95 (m,
2H),
S 1.22 (m, 4H), b 1.53 (m, 2H), & 1.65 (m, SH), $ 2.20 (m, 3H), 8 2.50 (m,
1H), b 2.77 (m, 2H),
8 5.74 (m, I H), b 6.20 (d, J = 6 Hz, 1 H); 8 7.09 - 7.35 (m, SH), 8 7.60 (m,
2H), & 7.90 {s, 1 H ),
(C2~29c~2~3)~
benzyl 1S-(1S-benzooxazal-2-ylcarbonyl-3 phenvlpropytsulfamo-ylmeth~~-
3-met>~lbutylcarbamate (Compound 44);'H NMR (CDC13): 7.92 (d, J=?.7Hz, IH),
7.64 (m,
1H), 7.57 {m,1H), 7.50 {m, 1H), 7.21-7.34 (m, lOH), 6.30 (d,j=9.ZHz,1H), 5.34
(m; IH), 5.11
(m, 1H}, 4.91 (dJ=9.6Hz, 1H), 4.51 (m, 1H), 3.1I (m, 2H), 2.89 (m,~2H), 2.50
(m, 1H), 2.20 (m,
1H), I.70 (m,1H), I.S (m, IH), 1.23-1.46 (m, 1H), 0.;92 (t,J=7.4Hz, H); MS:
m/e=5?8.1;
N lIS-f1S-Sbenzooxazol-2-ylcarbon l~phen~prop~sulfamoylmethyll-
3-methvIbutvl~acetamide (Compound 45);'H NMR (CDC13): ?.89 (d,J=7.7Hz, 1H),
7.62 (m,
1H), 7.SS (m, IH), 7.49 {m, 1H), 7.18-'7.30 (m, SIi}, 6.7 (d, J=8.9Hz, 1H),
5.61 (d, J=9.4Hz, 1H),
5.34 {m, 1H), 4.86 (m, 1H), 3.06 (m, 2H), 2.90 (t, J=7.7Hz,. 2H), 2.24 (m,
1H), 2.22 (m, 1H),
_77_
CA 02475069 2004-08-20
2.04 (s, 3H), 1.66 (m, 1H), 1.48 (m, 1H), 1.38 (m,1H), 0.91 (t, I=6.2Hz, 6H);
MS: mle=486.1;
benzyl iRSIS-benzooxazol-2-vlcarbonyl-3-phenylpropylsulfamovlmethyl>
3-methvlbutylcarbamate (Compound 46)'H NMR (CDC13): ?.9 (m, 1H), ?.GO (m, 1H),
7.58
(m, iH), 7.S (m, 1H), 7.75-7.4 (m, lOH), 5.85 (m, 1H), 5.0-S.4 (m, 3H), 4.2
(m, 1H), 3.15-3.35
(m, 2H), 2.65-2.85 (m, 2H), 2.45 (m, 1H), 2.i5 (m, 1H), 1.9 (m, IH), 1.4-1.7
(m, 3H), 0.9 (m,
6H); MS: m/e=578.1; and
N ~I~I-benzooxazol-2-vlcarbonyl-3-phen~propylsulfamoylmethvl>-
3-methvlbutyllacetamide (Compound 47)'H NMR (CDC13): 7.9 (m, IH), 7.65
(rn,1H), ?.61
(m, 1H), 7.60 (m, 1H), 7.18-7.30 (m, SH), 6.0 (m, 1H), 5.85 (m, iH), 5.28 (m,
1H), 4.50 (m,
iH), 3.20 (m, 1H), 2.85 (m, 1H), 2.70 (m, iH), 1.8-2.2 (m, 2H), 1 4S (S, 3H),
1.35-1.70 (m, 2H),
0.9 (m, 6H); MS: m/e=486Ø
EXAMPLE 3
tert-Butvl 1R-(2-benzooxazol-2 yl-
2-h drox -1S- heneth leth lcarbamo I -2- 2-c anobenz lsulfan l th Icarbamate
(Compound 48)
il3
~fl
/ \
w
A solution of 2R-ten-butoxycarbonylamino-3-(2-cyanobenzylsulfanyl~ropionic
acid
(336 mg), 2S-amino-1-benzooxazol-2-yl-4.-phenylbutan-1-of (282mg), 1-(3-
dimethylaminopropyl)-
3-ethylcarbodiimide hydrochloride (211 mg) and i-hydroxybenzotriazole (I97 mg)
in
2~ dichloromethane (20 mL) was treated with N methylmorpholine (2.2 mL). The
reaction mixture
was stirred O.S hour and then concentrated by evaporation. The residue was
dissol~red in ethyl
_?8_
CA 02475069 2004-08-20
acetate (40 mL) and the solution was washed sequentially with water (20 mL),
1N hydrochloric
acid (30 mL), a saturated sodium bicarbonate solution (30 mL) and then bryne
(30mL), dried
over magnesium sulfate and concentrated by evaporation. The residue was
subjected to flash
column chromatography on silica eluting with diethyl ether to provide tert-bu
1
IR-l2-benzooxazol-2-yl-2-hvdrox -~1S-~henethylethvlcarbamovl)-
2-(2-cyanobenzylsulfanyl)ethylc_arbamate as an off white solid. MS: 60I [MH]+.
Proceeding as in Example 3 provided ten-butyl 1R-(2-benzooxazol-2-vl-2-
hvdroxy_
1 ~henethvleth~lcarbamoyl~ 2-benzylsulfanvlethylcarbamate (Compound 49), MS:
S76 jh2FiJ''.
EXAMPLE 4
0 N~1R-(2-Benzooxazol-2- I-~2-hvdroxy-1S-phenethylethylcarbamovl~-
2~,2-cyanobenzylsulfan ly lethyllisonicotinamide
(Compound SO)
O
\ w
A solution of 3-(2-cyanobenzylsulfanyI)-2R-(pyrid..4-ylcarbonyl)aminopropionic
acid
(42S mg), provided as in Reference 2, 2S-amino-1-benzooxazoI-2-yl-4-
phenylbutan-1-of
(3Sb mg) and HAT'U (3S6 mg) in dimethylformamide (40 mL) was treated wilts
diisopropylamine (0.239 mL). The reaction mixture was stirred for 16 hours at
room
temperature then concentrated by evaporation. The residue was dissolved in
ethyl acetate and
the solution was washed with saturated sodium bicarbonate solution, dried over
magnesium
_79_
CA 02475069 2004-08-20
sulfate and then concentrated by evaporarion. The residue was subjected to
flash column
chromatography on silica eluting with ethyl acetate to provide N f 1R-l2-
benzooxazol-2;y1-
2-h drox -1S- heneth leth lcarbamo l -2- 2-c anobenz lsul an 1 th 1 isonicoti
ami
(216 mg) as a gum. MS: 646 [MH]*. HPLC: Rz= 13.20 minutes.
Proceeding as in Example 4 provided 9H-fluoren-9-vlmethyl IS-(Z-benzooxazol-2-
yl_-
2-hydroxv-1S,-,gheneth Iy ethylcarbamoyl~-2-cvclohexYlethylcarbamate (Compound
51);
9H-fluoren- - Imeth 1 1S- 2-benzooxazol-2- 1-2- to -bu Idimeth Isilan lox
1S-phenethvlethvlcarbamoyll-2-cyclohexyleth lY carbamate {Compound 52), MS:
772 [MHJ+.
EXAMPLE 5
2R-Amino-N- 2-benzooxazol-2- 1-2-h drox -IS- heneth Ieth I -3- 2-c anoben
Isulfan l -
propionamide hydrochloride
{Compound 53)
A solution tern-butyl IR-(2-benzooxazal-2-yl-2-hydroxy-1S-
phenethylethylcarbamoyI)-
2-(2-cyanobenzylsulfanyl)ethylcarbarnate (145 mg), provided as in Example 3,
in dioxane
(20 mL) was treated with hydrogen chloride, bubbling the gas through the
solution for 30
minutes. The reaction mixture was concentrated by evaporation and the residue
was triturated
with diethyl ether to provide 2R-amino-N (2-benzooxazol-2-vl-2-hydroxy 1S-
phenethylethyl)-3-
~cyanobenzylsulfanyl)proQionamide hydrochloride (I17 mg) as a an off-white
solid. MS: 537
-80-
rvc
CA 02475069 2004-08-20
Proceeding as in Example 5 provided 2R-amino-IV J2-benzooxazol-2-vl-2-~dro~y-
IS- heneth leth I -3-benz lsulfon l ro ionamide h drochloride {Compound 54},
MS: 508
IMH~+.
EXAMPLE 6
2S-Amino-N 2-benzooxazol-2- 1-2-h drox -IS- heneth leth 1 -3-c clohex 1 o
ianami
(Compound 55)
A solution of 9FI-fluoren-9-yImethyl 1S-(2-benzooxazol-2-yl-2-hydroxy-
14 1S-phenethylethylcarbamoyl)-2-cyclohexylethylcarbamate (165 mg), provided
as in Example 4,
in dichloromethane (30 mL) was treated with tris(~-aminoethyl)amine bound to
polysterene
beads (4.48 g). The mixture was stirred at room temperature for 48 hours and
then filtered.
The resin was washed four times with dichloromethane (20 mL) and the combined
filtrates
were concentrated under reduced pressure to provide 2S-amino-N ~2-benzooxazol-
2 ~ rLl-
I5 2-hydroxv-IS-nhenethyleth Iy )-3-cvclohexylpropionamide {14? mg) as a
colourless oil.
-81-
CA 02475069 2004-08-20
EXAMPLE 7
2 -Amino-1V- 2-benzooxazol-2- 1-2- ten-bu ldimeth IsiIan lox -1 - heneth Ieth
1=
3-cycl~hexylQropionamide
(Compound SG), a protected compound of Formula I
A.solution of 9H-fluoren-9-ylmethyl 1S-[2-benzooxazol-2-yl-
2-(tent-butyldimethylsilanyloxy)-1S-phenethylethylcarbamoyl]-2-
cyclohexylethylcarbamate
(1.48 g), provided as in Example 4, in dichloromethane (50 mL) was treated
with Iris-(2-
aminoethyl)amine (14.4 mL). The reaction mixture was stirred for 75 minutes
and then
t0 additional dichloromethane was added (50 mL). The mixture was washed
sequentially with
brine (50 mL x4) and a pH 5.3 buffer (SO mL x3), dried over magnesium sulphate
and
concentrated to provide 2S-amino-N f2-benzooxazol-2-vl-2-ltert-
butyldimethylsilanyloxv)-
1S-phenethYlethyIl-3-cyclohe~lpro,~ionamide as an orange oil.
-82_
CA 02475069 2004-08-20
EXAMPLE 8
tert-Butvl 4-t 1 R-(2-benzooxazol-2-vI-2-hydroxv-I S-phenethylethvlcarbamovl)~
2-(2-clan obenzyl sulfan~l)ethvlcarbamovlpiperi dine-1-carboxvlate
(Compound 57)
A solution of 2R-amino-N-{2-benzooxazol-2-yl-2-hydroxy-1S-phenethylethyl)
3-(2-cyanobenzylsulfanyl)propionamide hydrochloride (170 mg), provided as in
Example 5, in
dimethylformamide (7 mL) was treated with 1-ten-butoxycarbonylpiperidine-4.-
carboxylic acid
tetrafluorophenyl ester tert-butyl ester on resin {excess), prepared according
to the procedure
described in International Patent Application No. W099167228, and
triethylamine (0.053mL).
The suspension was agitated for 16 hours, then filtered, and the filtrate was
washed with
dimethylaforrnamide and then concentrated by evaporation. The residue was
subjected to flash
column chromatography on silica eluting with ethyl acetate to give tert-butyl
_4-t 1 R-(2-benzooxazol-2-vl-2-hydroxy-1 S-phenethylethylcarbamovll-
2-!2-c anobenzylsulfanvl)ethylcarbamoy,Ipiperidine-I-carboxvlate (95mg) as a
gum.
Ms: 71 z ~x~+.
Proceeding as in Example $ provided benzvl 4-lIS-l2-benzooxazol-2-yl-2-l~droxy-
1_S-nhenethylethvlcarbamoyl)-2-cyclohexylethylcarbamoyllpiperidine-I-
carboxylate
(Compound 58), MS: 681 jM]'.
-83-
CA 02475069 2004-08-20
EXAMPLE 9
NjIR-(2-Benzooxazol-2-yl-2-hydroxy-IS-pheneth ly ethylcarbamovl)-2-
benzylsulfonvlethyll-
tetrah"ydropvran-4-carboxamide
(Compound 59)
A mixture of 2R-amino-N (2-benzooxazoI-2-yI-2-hydroxy-IS-phenethylethyl~-
3-benzylsulfonylpropionamide hydrochloride {0.3 g), prepared as in Example 5,
tetrahydropyran-
4-carboxylic acid (0.072 g), I-(3-dimethylaminopropyl)-3-ethylcarbodiimide
hydrochloride
(0:116 g) and 1-hydroxybenzotriazole (0.112 g) in dichloromethane (20 mL) was
treated with
4-N methylmorphoIine (O.IZ mL). After stirring at room temperature for 4 hours
the reaction
mixture was ieft to stand 16 hours and then concentrated by evaporation. The
residue was
treated with dichloromethane (50 mL) and the mixture was washed sequentially
with 1N
hydrochloric acid solution {5 mL), saturated sodium bicarbonate solution {5
mL) and brine
(S mL), dried over magnesium sulfate and then concentrated by evaporation. The
residue was
subjected to flash column chromatography on silica eluting with ethylacetate
to provide N 1R-
~2-benzoox azol-2-vI-2-hydroxy- I S-ph en ethyl_ethylc arbam oYl2 2-benz~rl
sulfonvl etl~ll-
tetrahydropyran-4-carboxamide {66 mg) as a cream solid. MS: 618 [MH]'.
Prcxeeding as in Example 9 ;provided the following compounds of Formula I:
N flR~2-benzooxazol-2-yl-2-hvdrox -L1S-phenethyleth~tlcarbam~l)-
2-benzylsulfonvlethvll-nicotinamide (Compound 60), MS: 613 jMHJ'";
-84-
CA 02475069 2004-08-20
N IR- 2-benzooxazol-2- 1-2-h drox -1S- heneth leth lcarb mo 1 -2-ben lsulfon 1-
ethyliplirazine=2~arboxamide (Compound 61), MS: 614 [N>Hj*;
dal.C=f~_hPnTnnxa~nf-?_-vi-2-hvdrc~xv-1 S-nhenethviethvlcarhgmnvil-
~..c:vclnhPxvl..
ethylcarbarriov~]piperidine-1.-carboxylate (Compound 62); and
1V- S- 2~benzooxazol-2- l-2-h drox -1S- heneth Jeth lcarbamo 1 -2-c clohex
leth 1 -
iso~icotinamide (Compound 63).
EXAMPLE 10
tent-Butvl 4-f 1 R-l2-benzooxazol-2-v1-2-hvdrox~ 1 S-phenethylethvlcarbamoyll-
2-f3-methvlpYrid-2-ylmethvlsulfonyl)ethvlcarbamovllpiperidine-1-carboxvlate
(Compound 64)
A solution of 2R-anvno-N (2-benzooxazol-2-yl-2-hydroxy-ls-phenethylethyl~
3-(3-methylpyrid-2-ylmethylsulfonyl)propionamide (178 mg), IiATU (137 mg) and
1-ten-butoxycarbonylpiperidine-4-carboxylic acid (69 mg) in dimethylformamide
(10 mL) was
treated with N,N-diisopropylethylamine (O.I74 mL). The reaction mixture was
stirred for 9
hours and then concentrated by evaporation. The residue was dissolved in ethyl
acetate and the
solution was washed with saturated sodium bicarbonate solution, dried over
magnesium sulfate
and then concentrated by evaporation. The residue was subjected to flash
column
chromatography on silica eluting with ethyl acetate to provide te_rt-butyl 4-f
1R-(2-benzooxazol-2-
yl-2-l~droxy_1S-phenethylethylcarbamoyll-
2-l3-meth~pvrid-2-ylmethvlsulfon l~,e_th,~~carbamovllpiperidine-I-carboxylate
(81 mg). MS: 734
-85-
CA 02475069 2004-08-20
Proceeding as in Example 10 provided the following compounds of Formula I:
tetrah dro ran-4- I 1R- 2-benzooxazol-2- 1-2-h drox -1S- heneth leth lcarbamo
2-benzvlsulfanyI_ethYlcarbamate (Compound 65);
N LI S-f2-benzooxazol-2-y1-2-hvdroxy=1 S-phenethylethylcarbamoyj,~
2-cvclohexvlethylltetrahydronyran-4-carboxamide (Compound 66), MS: 548 [M]*;
and
N- 1S- 2-ben ooxazol-2- 1-2-h drox -1S- heneth leth lcarbamo 1 -2-c c1 hex
Ieth
6-hvdroxynicotinamide (Compound fr7).
EXAMPLE 1 I
lV 2-Benzooxazol-2- I-2- tert-but Idimeth isiian lox -1S- heneth leth 1 -3-c
clohe 1-
~-f3-nyrid-3-vlureido)propionamide
(Compound 68), a protected compound of Formula I
O
w
N
~Si~
O
x
N p
-s
0 1~ a
r~
A solution of 2S-amino-N-[2-benzooxazol-2-yl-2-{tert-butyldimethyIsilanyloxy~
1S-phenethylethyl]-3-cyclohexylpropionamide (200.1 mg), provided as in Example
7, in
dichloromethane (10 mL) was treated with 3-pyridyI isocyanate (48 mg). The
mixture was
stirred at room temperature for I6 hours and the solvent evaporated under
reduced presssure.
The residue was subjected to flash column chromatography on silica eluting
with a mixture of
pentane and ethylacetate (2:1, v/v) to provide N (2-benzooxazol-2-yl-2-(tent-
bu ldirneth Isilan lox -1S- heneth leth l -3-c clohex 1-2S- 3- 'd-3- lureido
ro ionamide
(I72 mg) as a colorless oil.
-86-
CA 02475069 2004-08-20
EXAMPLE I2
N 1S- 2-Benzooxazol-2- 1- - tart-bu ldimeth lsilan lox -1S- heneth lath
Icarbamo 1 - .
2-cycl ohexyl ethyl 1 morphoIine-4.-carboxamide
(Compound 69), a protected compound of Formula I
A solution of 2S-amino-N-[2-benzooxazoI-2-yl-2-(ten-butyldimethylsilanyloxyr
1S-phenethylethylJ-3-cyciohexylpropionamide (200 mg), provided as in Example
7, in
dichIoromethane (8 mL) was treated with 4-morpholinecarbonyl chloride (0.084
mL) and
triethylamine (0.112 mL). The solution was stirred at room temperature for 2U
hours. The
i 0 solvent was evaporated under reduced pressure and the residue was purified
by flash
chromatography on silica eluting with a mixture of pentane and ethylacetate
(2:I, v/v) to
provide l~ i 1S-f2-benzooxazol-2-xI-2-pert-butyldirneth~rlsilanyloxW-
1S-phenethylethvlcarbamoyll-2-cyclohexvlethvl lmorpholine-4-carboxamide (143
mg) as a white
solid. MH~ 663.
_87_
CA 02475069 2004-08-20
EXAMPLE 13
ten-butyl 4-T 1R-l2-benzooxazol-2-yl-2-h~rdroxv-ZS-phenet~ylethylcarbamoyll-
2-r_2-c~anobenzvlsulfonvl)ethylcarbamoylpiperidine-1-carboxylate
(Compound 70)
A solution of tent-butyl 4-[1R-(2-benzooxazol-2-yl-2-hydroxy-1S-
phenethylethylcarbamoyl]-2-(2-cyanobenzylsulfanyl)ethylcarbamoylpiperidine-1-
carboxylate
(95 mg), provided as in Example 8, in methanol (8 mL) was treated with a
solution of OXONE~
(246 mg) in water (8 mL). After stirring at room temperature for 10 hours the
methanol was
distilled under reduced pressure and the remaining aqueous phase was extracted
four times with
ethyl acetate (20mL). The combined extracts were dried over magnesium sulfate
and then
concentrated by evaporation. The residue was subjected to flash column
chromatography on
silica eluting with ethyl acetate to give the tent-butyl 4-t1R-l2-benzooxazol-
2-yI-2-hydroxy;1S
heneth Ieth )carbamo I -2- 2-c anobenz lsuIfon 1 eth Icarbamo 1 i eridine-1-
carbox at
t5 (35 mg) as a gum. MS: 74.4 [MH]+.
Proceeding as in Example 13 provided N I1R-l2-benzooxazol-2yI-2-hvdroxy
1S-phenethylethvlcarbamoyl)-2-l2-cyanobenzvlsulfonyl)ethxllisonicotinamide
(Compound 71),
HPLC: RT= I2.89 minutes.
-88_
CA 02475069 2004-08-20
~~Al~'L.E 14
tent-Bu 1 1R- 2-benzooxazol-2- 1-2-h drox -1S- a eth leth lcarb o 1
2-benzyl~ulfony,~ethvlcarbamate
(Compound 72)
A solution of tern-butyl 1R-(2-benzooxazol-2-yl-2-hydroxy-IS-
phenethylethylcarbamoyl)-2-benzylsulfanylethylcarbamate (3.62. g), provided as
in Example 3, in
dichloromethane (174 mL) was treated with meta-chloroperbenzoic acid (6.9 g).
After stirring
at room temperature for 5 hours the reaction mixture was diluted with
dichloromethane
i0 (100 mL), washed sequentially with a saturated sodium bicarbonate solution
(100 mL) and brine
(100 mL), dried over magnesium sulfate and then concentrated by evaporation.
The residue was
subjected to flash column chromatography on silica eluting with a mixture of
pentane and
ethylecetate (1:1, v/v) to provide tent-butyl 1R-(2-benzooxazol-2-vl-2-hydroxv-
1S-phenetl~lethylcarbamoyl~2-benzvlsulfonvlethylcarbamate (0.95 g) as a yellow
solid. MS:
608 jMH]''.
Proceeding as in Example 14 provided N 11R-(2-benzooxazol-2y1-2-hydrox,~r-
1S-phenethylethvlcarbar,~oyl)-2-nyrid-3-ylmethvlsulfonyleth~rllpyrazine-2-
carboxamide
(Compound 73).
-89-
CA 02475069 2004-08-20
XANIPLE 15
~V~2-Benzooxazol-2-yl-2-~"~,icy-1S~phenet~ylethyl~3-cyclohexyl
2S-f3-pvrid-3_ylur~~dolprogionamide (Compound ?4)
A solution of N [2-benzooxazol-2-yl-2-(tart-butyldimethylsilanyloxy)-1S-
phenethylethyl]-
3-cyclohexyl-2S-(3-pyrid-3-ylureido)propionamide (1?2 mg) in tetrahydrofuran
(5 mL), provided
as in Example 11, under an inert atmosphere at room temperatwre was treated
with a.soiution of
tetrabutylamrnoniumfluoride in 1M tetrahydrofuran (0.4 mL): After stirring at
room
temperature for 90 minutes, the solvent was distilled under reduced pressure.
The residue was
subjected to flash column chromatography on silica eluting with a mixture of
ethylacetate and
pentane (5:1, vlv) to provide .,lV l2-benzooxazol-2-yI-2-hvdroxv-1S-
pheneth~ethvll-3-cyclohex3rl-
25~3-pyrid-3-Ylureido ropionamide (108 mg) as a white solid.
Proceeding as in Example 13 provided N ~1S-(2-benzooxazol-2-vl-2-hydroxy_
1S-phenethylethylcarbamovl)-2-cyclohex l~ethvllmotpholine-4-carboxamidg
(Compound?5).
-90-
CA 02475069 2004-08-20
EXAMPLE 16 ~.
ten-Bu 14- 1R- 1S-benzooxazo -2- lcarbon 1-3- hen 1 r lcarbamo 1 - - 2-c an
benzylsulfonvl)ethylcarbamov_l,]pigeridine-1-carboxylate
(Compound 76)
A solution tert-butyl 4-[IR-(2-benzooxazol-2-yl-2-hydroxy-1S-
phenethylethylcarbamoyI]-2-(2-cyanobenzylsulfonyl)ethylcarbamoylpiperidine-I -
carboxylate
(35 mg, prepared as in Example 13, in dichloromethane (10 mL) was treated with
Dess-Martin
reagent (60 mg). The reaction mixture was stirred at room temperature for 5
hours, then
washed with sodium thiosulfate in saturated sodium bi-carbonate solution,
dried over magnesium
sulfate and then concentrated by evaporation. The residue was subjected to
flash column
chromatography on silica eluting with a mixture of ethyl acetate and pentane
(1:I, vlv) to give
rert-butyl 4;f 1R-(1S-benzooxazol-2-vIcaibonvl-3-phenyipropylcarbam~ll-
2-f2-eyanobenzylsulfonvl~ethvlearbarnoyIlp~peridine-1-carboxylate (26 mg) as a
gum. MS: 742
[MH]'~-.
Proceeding as in Example 16 provided the following compounds of Formula I:
N f1R-fIS-benzooxazol-2-ylcarbonvl)-3-phen5rlpropylcarbamoyl)-
2-benzylsulfonylethvlltetrahydrouvran-4-carboxamide (Compound ??), m.p. I78-
I80°C, MS:
618 [MH]'';
N f iR-f 1S-benzooxazol-2-~lcarbonyl-3-phenylpropylcarbamoyl)-
2-benzYlsulfonyleth~lnicotinamide (Compound ?8), m.p. 193-195°C, MS:
611 [MH]'';
~V_ -T1R-lIS-benzooxazoI-2-ylcarbonvl-3-phenvlpropvlcarbamo
2-benzylsulfon~rlethvllpyrazine-2-carboxamide (Compound ?9), m.p. 194-
196°C. MS: 6I2
_91_
CA 02475069 2004-08-20
~+.
t
tent-butyl 4-f 1 S-(,1 S-benzooxazol-2-vlcarbonyl,-3-phenylpro,.p~rlcarbamovl)-
2-cyclohexyi_ethylcarbamoyl]piperidine-1-carboxylate (Compound 80);
tert-butyl 4-f 1S-(1-benzooxazol-2-vlcarbonyl-3-phen~propYlcarbamoyl)-
2-l6-meth~nvrid-2~rlmethylsulfonyl)ethylcarbamoyllpiQeridine-1-carboxvlate
(Compound 81),
MS: 732 [MH]+, HPLC: RT =15.I8 minutes;
N-f 1R-l1S-benzooxazol-2 ylcarbonvl-3-phenYlprogvlcarbamoyl)-
2-f2-cvanobenzvlsulfon~)ethyllisonicotinamide (Compound 82), m.p. 2Q4-
206°C, MS: 636
[h,~~.
tetrah dro an-4- 1 1R- IS-benzooxazol-2- lcarbon vl-3- hen 1 io lcarbamo 1 -
2-benzylsulfon~rlethvlcarbamate (Compound 83), m.p. 93°C (with
decomposition), MS: 634
[~7'
benzyl 4-f 1 S-f 1 S-benzooxazol-2-ylcarbonvl-3-phenylpropvlcarbamovl)
2-cyclohexylethYlcarbamoyllpiperidine-1-carboxvlate (Compound 84), MS: 677
[M]';
N f1S-benzooxazol-2ylcarbonYl_-3-phenylpropy,~-Z 3-cvclohexvl-
2S-f3p~rid-3 Xlureido ropionamide (Compound 85), MS: 554 [M]';
N-f 1SS1S-bentooxazol-2-vlcarbonvl-3-phenvIprop~IcarbamoyI)
2-cyclohexylethYllrnorpholine-4-carboxamide (Compound 86), MS: 547 [MHJ'";
N_J' 1 S~1 S-benzoox azol-2-ylcarbon~pheny~r~vlcarbamovl~
2-cyclohexylethyl_isonicotinamide (Compound 87), MS: 53? [M]';
N f1S-l1S-benzooxazol-2-vlcarbonvI-3-phenvlprop~rbamoyl)-
2-cyclohexyleth~,lltetrahvdrop~-4-carboxamide (Compound 88), MS: 546 [M]+; and
N f1S-(1S-benzooxazol-2-vlcarbonvl-3-vhenvlpropvlcarbamoyt)-2-cyclohexvlethvll-
6-hydrox~nicorinamide (Compound 89); MS: SSS [M]'.
-92-
CA 02475069 2004-08-20
EXAMPLE I7 ..
N j1R-F1S-Benzooxazol-2-ylcarbonvI)-3-phenvlpropylcarbamoyl)-
2-benzvlsulfonyleth~lmorpholine-4-carboxamide
(Compound 90)
O
N ~H
O
A mixture of N [1S-(2-benzooxazol-2-yl-2-hydroxy-1S-phenethylethylcarbamoyl)-
2-benzylsulfonylethyl]morpholine-4-carboxamide (7.2 g, 11.6 mmol), prepared as
in Example 1;
and Dess-Martin periodinane (9.87 g, 23.3 mmol) in dichloromethane (57 mL) was
stirred at room temperature for 1 hour and then diluted with a solution of
0.26 M sodium
thiosulfate in saturated sodium bicarbonate. The dilution was extracted with
ethyl acetate and
the extract was filtered. The filtrate was concentrated to provide
N [1S-(1S-benzooxazol-2-ylcarbonyl)-3-phenylpropylcarbamoyl)-
2-benzylsulfonylethyl]morpholine-4-carboxamide (2.33 g) as an orangeltan oil.
The solids
collected from the filtration were taken up into dichloromethane (700 rnL) and
the mixture was
washed sequentially with water and saturated sodium bicarbonate solution,
dried and
- concentrated to provide N '( 1 R-t I S-benzoox azol-2-ylcarbonvt)-3-
phenylpro_pvlcarbamovl)-
2-benzylsulfonylethyllznor~phoIine-4-carboxamide (4.2 g) as a white powder. 'H
NMR (DMSO-
d6) 8.024 (d, J=6.68Hz, 1H), 7.9787 (d, J=7.92Hz, ll~, 7.8857 (d, J=8.16Hz,
1H), 7.6471 (td,
J=8.41, 0.99 Hz, 1H), 7.5455 (td, J=8.16, 1.24Hz, 1H), 7.3806 (s, SH), 7.2479
(m, 5H), 7..1210
(d, J=4.53Hz), 1H, 5.2578 (m, 1H), 4.7395 (m, 1>~, 4.509 (s, 2H), 3.5342 (m,
4H), 3.4082 (m,
2H), 3.30 (m, 4H (+water)), 2.6963 (m, 2H), 2.2768 (m, 1H), 2.0497 (m, 1H). MS
(M''1) 619.2.
-93-
CA 02475069 2004-08-20
Proceeding as in ExampIel7 provided the following compounds of Formula I:
N fIR-(2-benzooxazol-2-vl-1.1-dimethyl-2-oxoethylcarbamoyl~
2-benzvlsulfonvlet~llmorpholine-4-carboxamide (Compound 91);'H NMR: (1~MS0)
9.26 (s,
IH), 7.79 (d, J=.8Hz,1H), ?.73 (d, J=BHz,1H), 7.56 (t, J=BHz, 1H), 7.47 (t;
J=8Hz, IH), 7.36-
7.25 (m, SH), 6.70 (d, J=BHz,1H}, 4.67 (m,1H), 4.39 (d, J=I4Hz,1H), 4.32 (d,
J=I4Hz, 1H),
3.49-3.00 (rn, lOH), 1.56 (s, 3H), 1.51 (s, 3H); MS: (M++1) 543;
N tIR ~,IS-benzooxazol-2-ylcarbonylpentylcarbamovl?-
2-(3.5-dimethylisoxazol-4-ylmethylsulfonyllethvllmorpholine-4-carboxamide
(Compound 92);'H
NMR: {DMSO) 8.66 (d, J=6.6Hz, 1H), 7.99 (d, J=BHz, 1H), 7.88 (d, J=8Hz, IJ~,
7.62 (t,
- J=8Hz, 1H), 7.52 (t, J=8Hz, 1H), 7.02 (d, J=7.7Hz,1H), 5.24 (m, 1H), 4.?6
(m, IH), 4.39 (d,
J=l4Hz, IH), 4.27 (d, J=l4Hz,1H), 3.63-3.20 (m, lOH), 2.33 (s, 3H), 2.15 (s,
3H}, L94 (m, IH),
1.69 (m, 1H), 1.40-1.22 (m, 4H), 0.$4 (t, J=6.7Hz, 3H); MS: (M*+1) 590; and
N-f 1R-(1S-benzooxazol-2-ylcarbonvlpentylcarbamoyll~
2-(3.5-dimethvlisoxazol-4.-ylrnethvlsulfonylethyliisonicotinamide (Compound
~3);'H NMR:
(DMSO) 9.23 (d, J=8Hz, 1H), 8.87 (d, J=7Hz, 1H), 8.71 (m, 2H), 7.98 (d, J=8Hz,
1H), 7.87 (d,
J=8Hz, 1H), 7.70 (m, 2H), 7.62 (t, J=BHz, IH), 7:51 (t, J=8Hz, IH), 5.28 (m,
IH), 5.10 (m, 1H),
4.44 (d, J=l4Hz,1H), 4.37 (d, J=l4Ha, 1H), 3.80-3.52 (m, 2H), 2.33 (s, 3H),
2.14 (s, 3H), 1.95
(m, 1H}, 2.69 (m, 1H}, 1.40-1.22 (m, 4H}, 0:82 (t, J=6.7Hz, 3H); MS: (M''+I)
s82.
_9ø
CA 02475069 2004-08-20
EXAMPLE 18
N jl R-l I S-Benzooxazol-2-v~carbon~rl- ,:
- hen 1 ro Icarbamo 1 -2- 2-c anobenz lsulfon I th i ridine-4-carboxamide
{Compound 94)
A solution of tert-butyl 4-[1R-{1S-benzooxazol-2-ylcarbonyl-3-
phenylpropylcarbamoyl)-
2-{2-cyanobenzylsulfonyl)ethylcarbamoyl]piperidine-1-carboxylate (26 mg),
provided as in
Example 16, in ethyl acetate (10 rnI,) was treated with hydrogen chloride,
bubbling the gas
through the solution for 3 minutes. A white solid formed which was filtered
and dried under
reduced pressure to provide N-f 1R-(1S-benzooxazol-2-vlcarbony-I-3-phenylprop
Icarbamoxl)-
2-f2-c~,nobenzylsulfonXl~,yllp~eridine-4-carboxamide (19 mg) as a solid, m.p.
=155-157°C.
MS: 678 [M13J+.
Proceeding as in Example 18 provided N f IS-(1S-benzooxazol-2- lcarbony~
3-phenylpropylcarbamoyl)-2-cvclohexylethvllpiperidine-4-carbaxamide
hydrochloride
(Compound 95), MS: 634 [MH]+; and
N f1R-(1S-benzooxazoI-2-ylcarbonyl-3-phenylpropy)carbamo
2-(6-methylnyrid-2-ylmethylsulfonyl?ethyllpiperidine-4-carboxamide {Compound
96), MS: 632
[MH]'', HPLC: Rr = 12.05 minutes.
-95-
CA 02475069 2004-08-20
EXAMPLE 19
N t 1S-Benzooxazol-2-vlcarbonylbutyl)-2R-methylsulfonvlamino-3-
benzvlsulfonvlQropionamide'
(Compound 159)
Qt ~~
~'S~N
H
A solution of (R)-2-(2-methylsulfonylacetyIamino)-3-benzylsulfonylpropionic
acid (212
mg, 0.66 mmol), (S7-2-amino-l-benzooxazol-2-ylpentan-I-of (150 mg, 0.66 mmol),
EDCI
(L65 mg, 0.858 mmol) and HOBT (1 I0 mg, 0.726 xnmol) in methylene chloride (3
mL) was
stirred at room temperature for 2 hours, sequentially washed with hydrochloric
acid, sodium
bicarbonate solution and brine and then concentrated. The residue was
dissolved in
dichloromethane and the solution was treated with Dess-Martin reagent (340 mg,
0.8 mmoI) for
1 hour. The mixture was stirred with a sodium thiosulfate/sodiurn bicarbonate
solution and the
mixture was extracted with ethyl acetate. The extract was washed sequential)
with dilute
f~ydrochloric acid, sodium bicarbonate and brine, dried (MgSO~ and then
concentrated to
provide N f1S-benzooxazol-2-vlcarbonvlbutvl)-
2_R-methylsulfonylamino-3-benzy)sulfonylpropionamide (49 mg, 0.09 mmol). 'H
NMR (DMSO):
9.0 (d,J = 7Hz, IH), 8.0 (d,J = BHz,1H), 7.90 (d,J = 9Hz, IH), 7.66 (t,J =
8Hz, IH), 7.55 (t,J
=9Hz; IH), 7.39 (s, SH), 5.32 (m, 1H), 4.55 (m, 3H), 3.35 (m, 3H), 2.95 (s,
3H), 1.94 (m, IH),
1.71 (m, 1H),1.45(m, 2H), 0.92 (t, J=8Hz, 3H); MS: mle=522.03.
-96-
CA 02475069 2004-08-20
EXAMPLE 20
Meth 1 1R- 1S-benzooXazol-2- lcarbon lbu lcarbamo 1 2-Benz lsulfon leth
lcarbamate
(Compound 158)
A solution of (R)-2-(2-methoxycarbonyIamino)-3-benzyIsulfonylpropionic acid
(200 mg,
0.66 mmoI), (S')-2-amino-I-benzooxazol-2-ylpentan-1-of (150 mg, 0.66 mmol),
EDCI (165 mg,
0.558 mmoI) and HOBT (110 mg, 0.726 mmol) in methylene chloride (3 mL) was
stirred at
room temperature for 2 hours, sequentially washed with hydrochloric acid,
sodium bicarbonate
solution and brine and then concentrated. The residue was treated with Dens-
Martin reagent
(340 mg, 0.8 nuuol) in dichlr~romethane (4 mL) for 1 hour. The mixture was
stirred with a
sodium thiosulfate/sodium bicarbonate solution and the mixture was extracted
with ethyl acetate.
The extract was washed sequentially with dilute hydrochloric acid, sodium
bicarbonate and
brine, dried (MgSO,) and then concentrated. The residue was heated with ethyl
acetate and
then treated with tent-butyloxymethyl. The mixture was let stand for
approximately 12 hours
and then cooled in an ice bath. Resulting solids were collected by filtration
and washed with
cold ethyl acetate to provide meth I
1R-f1S-benzooxazoI-2-vlcarbon~rlbutylcarbamovl)-2-benzvlsulfonylethylcarbamate
(133 mg, 0.26
mmol).'H NMR {DMSO): 8.77 (d,J =7Hz, 1H), 8.01 (d,J = 9Hz,.lH), 7.90 (dyJ =
9Hz,1H), 7.6
(m, 2H), 7.55 (t,T=9Hz, 1H), 7.39 (s, SH), 5.3 (m, 1H), 4.68 (m, 1H), 4.48 (s,
2H), 3.55 (s, 3H);
3.52-3.4 (m, 1H), 3.3 (m, IH), 1.92 (m, 1H), 1.73 (m, 1H), 1.42 (m, 2H), 0.91
(t, J=SHz, 3H);
MS: m/e=502.05.
-97-
CA 02475069 2004-08-20
EXAMPLE 2I
l1~-f 1 R-f 1 S-Benzooxazol-2-~arbonvJbutvlcarbamoyl)-2-
benzvlsulfonylethyllmorpholine
.4-carboxamide
I JN~H II m~
O O
A solution of (R)-2-(2-morpholin-4-ylcarbonylamino)-3-benzylsulfonylpropionic
acid
(356 mg, 1 mmol}, EDCI (240 mg, mmoi) and HOBT (178 mg, mmol) in methylene
chloride (8
mL) was (S)-2-amino-1-benzooxazol-2-ylpentan-I-of (220 mg, mmol). The mixture
was stirred
at room temperature for 1.5 hours and then treated with addtional F..DCI (80
mg). The mixture
was stirred for an additional 0.5 hours and then poured into cold, dilute
hydrochloric acid. The
mixture. was extracted with ethyl acetate (2x) and the extract washed
sequentially with aqeous
sodium bicarbonate and brine, dried (MgS04) and concentrated. The residue was
dissolved in
methylene chloride (8 mL) and the solution was treated with Dess-Martin
reagent (544 mg) .
The mixture was stirred for 1.5 hours and then stirred a sodium
thiosuifate/sodium bicarbonate
t5 solution for 15 minutes. The mixture was extracted with ethyl acetate (2x)
and the extract was
washed with brine, dried (MgS04) and then concentrated. The residue was
triturated with ethyl
acetate and then hexanes. The mixture cooled in an ice bath and resulting
solids were collected
and dried to provide N fIR-l1S-benzooxazol-2-ylcarbon l~tvlcarbamovll-
2-benzvlsulfonvlethyllmor-~holine-4-carboxarnide (408 mg, 73°lo yield).
1H NMR 300m1'iz: 8.65
(d,J=7.1H3, 1H), 8.0i (d, J=8.8H3, 1H), 7.91 (d, J=9.1H3, 1H), 7.65 (t,
J=8.2H3, 1H}, 7.55 (t,
J=9.1H3,1H), 7.38 (s, SH}, 7.05 (d, J=9.4H3; 1H), 5.29 (m, 1H), 4.73 (m, 1H),
4.48 (s, 2H), 3.53
(m, 4H), 3.4-3.2 (m, 6H}, 1.94 (m, 1H), I.73 (m, 1H), 1.42 (m, 2H), 0.91 (t,
J=8H3, 3H);
MS=557.21 M+=556.20.
-98-
(compound 158)
CA 02475069 2004-08-20
Proceeding by methods analogous to those described in this Application
provided the
following compounds of Formula I:
2S-acetylamino-lV~,2-benzooxazol-2-yl-1 S-butyl-2-hydroxvethyl)-
3-c_vclQhexylp;opionamide (Compound 97);'H NMR (CDCl3): 7.6? (d, J=8.OHz, 1H),
7.53 (d,
J=6.OHz, 1H), 7.34 (m, 2H), 6.64 (d, J=8.lHz, IH), 5.99 (d, 3=8.lHz, 1H), 5.03
(m, 1H), 4.39
(m, 2H), 2.02-0.70 (m, 22Hz); MS ESI: MH'' 430;
2S-acetylamino-N (IS-benzooxazol-2-vlcarbonvlpentvll-3-cvclohexylpr~ionamide
(Compound 98);'H NMR (CDCl3): 7.93 (d, J=7.SHz,1H), 7.67 (d, J=8.lHz, 1H),
7.54 (t,
J=7.2Hz, 1H), 7.46 (t, J=7.8Hz, 1H), 6.78 (d, J=7.2Hz, 1H), 5.91 (d, J=8.4Hz,
1H), 5.63 {m, 1H),
4:59 (m, 1H), 2.09-0.85 (m, 24Hz); MS ESI: MH+ 428;
tent-buqrl 1S-f 1-benzooxazol-2-ylcarbonyl'~-3 phenylpropylcarbamoylO-
2-cyclQhexylethyllcarbamate (Compound 99);
2S-acetylamino-N ll-benzooxazol~2-ylcarbonyl~
3-nhen~rlvropyl~ 3-cyclohex~propionamide (Compound I00);
i5 2S-ace lam'no-N- 1-benzooxazol-2- Icarbon Ic clobu I 3-c clohex ro ionamid
(Compound 101);
2S-acetylamino-N (IR-benzooxazoI-2-ylcarbonvl-3-phenvlnrouyl~-
3-cyclohexyIpropionamide (Compound L02);
2S-acetylamino-N-(2-benzooxazol-2-vl-2-hydroxy-lR:phen~ethylethy~l
3-c~clohexylpropionamide (Compound 103);
N-fIS-(IS-benzooxazol-2- Ic~n~-3-pheny3prowlcarbamoyll-
2-c~rclohexylethyllsuccinamic acid (Compound 104);'H NMR {CDC13): 7.87 (m,1H),
?.62 (m,
IH), 7.52 (m, 1H), 7.43 (m, 1H), 7.15 (m, bH), b.89 (m, 1H), 5.62 (an, 1H),
4.5b (m,1H), 2.75
(m, 2H), 2.70 (m, IH), 2.48 (m, 2H), 2.16 (m,1H), 1.6 (m, 7H), 0.7-1.4 (m,
7H); MS: role 534;
N f 1S-(2-benzooxazgl-2-y1~2-hydro~-
IS-phenethvlethylcarbamoyl)-2-c-yclohexylethyl~succinamic acid (Compound
105);'H NMR
(CDC13): 12.04 (s, 1H), 7.89 (m, 1H), 7.80 (m, 1H), 7.65 (m, 2H), 7.36 (m,
2H), 7.13-7.29 (m,
4H), 6.08-b.23 (m, 1H), 4.62-4.93 (m, IH), 4.15 (m, IH), 2.64 (m, iH), 2.50
(m, 1H), 2.34 (m,
6H), 1.78 (m, 1H), 1.45-1.68 (m, 4H), I.37 (m, 1H), 0.95-1.3 (m, 3H), 0.87 (m,
2H); MS:
role=535.8;
-99-
CA 02475069 2004-08-20
hvI]oxalamic acid (Compound 106);'H NMR (CDC13): 6.6-7.9 (m,lOH), 3.6 (m,
1H), 4.5 (nz;_ IH), 2.72 (m, 1H), 2.45 {rn, 1H), 0.8-2.1 {rn, 15H); MS: m/e
506.2;
3X-irnidazoIe=4-carboxamide (Compound 107);'H NMR (CI)Cl3): 8.1 (m,lH), 7.3-
7.15 (m, 3H),
6.95-7.2 (m,, 8H), 5.62 (m, TH), 4.74 {m, 1H), 2.77 (m, 2H), 2.38 (m, 1H),
2.25 (m, IH), 0.8-1.9
(m, 13H); IviS: mle 528.2;
2-c'r~clohexyJethyl_'j~3H-imidazole-4-carboxamide {Compound 108);'H NMR
(CDCI3): 7.0-7.6
(rn; I2H), 5.05 {m, IH), 4.S (m, 1H), 2.75 (m, 2H), 0.6-2.2 (m, ISH); MS: mle
529.6;
(Compound 109), m.p. = 70-85°C, MH'' 542;
2-cvclohex~ethyllmalonamic acid {Compound I10);'H NMR (CI3Cl3): 6.8-7.9 (m,
9H), 5.63
(m, 1H);:4.56 (m, 1H), 2.6-2.8 (m, 4H), 2.0-2.4 (m, 2H), 0.7-2:0 {m, 13H); MS:
m/e 520.4;
_N:~1R-(1S=benzooxazol-2-vlcarbonvl-3-~henvlpropylcarbamoyl)-
2-o-tolylmethylsulfonylethyllmor_pholine-4-carboxamide (Compound I11);'H NMR
300mHz
(DIvISO-db) PPM, 8.841 (d, J=6.2Hz, IH), 7.942 (d, I=5.2Hz, 1H), 7.860 (d, J=
8.4Hz, 1H),
7.618 (t, J=8.lHz, IH), 7.516 (t, J=8.IHz,1H), 7.16 (m, lOH), 5.22 (m, 1H),
4.78 {m, 1H), 4.516
(s, ZH), 3.S67 (m, 2H), 3.500 (m, 6H), 3.3 (s, 3H), 2.75 {m, 1H), 2.65 (m,
1H), 2.44 (m, 1H),
2.26 (m, 2H), 2.01 (m, 1H); MS: M+=633.4 M'=631.4;
N f1R-(1S-benzooxazol-2-Ylcarbon~l-3-,phenvlpropylcarbamo,~)1-
2-(2-nitrobenzylsulfonyl)ethyllinorpholine-4-carboxamide (Compound 112);'H
IVMR 300mHz
(DMSO-db) PPM, 8.840 {d, J=7.OHz, 1H), 8.025 (d, J=8.OHz, 1H), 7.950 (d,
J=8.4Hz, IH),
7.858 {d, J=7.7Hz, 1H), 7.730 (d, J-8.8Hz, IH), 7.646 (i; J=8.4Hz,1H), 7.515
(t, J=7.7Hz, 1H),
5.223 (m, 1H), 5.004 (s, 2H), 4.694 (m, IH), 3.561 (m, 2H), 3.SI0 (m, 6H),
2.756 (m, IH), 2.652
(m, IH), 2.429 (m, 2H), 2.243 (m, IH), I.983 {m, IH); MS: M''=664.2 M-=662.4;
N fIR-lIS-benzooxazol-2-vlcarbon~-3-phenytpropylcarbamovl)-
2-I2-chlorobenzXlsuIfonvl)ethyllmorpholine-4-carboxamide (Compound 1 i3); 'H
NMR 300mHz
(DMSO-db) PPM, 8.851 (d, J=6.2Hz, IH), 7.953 (d, J=8.8Hz, 1H), 7.855 (d,
J=8.4Hz, 1H),
7.627 (t, J=6.6Hz, lI~, 7.498 (m, 3H), 7.365 (m, 2H), 7.211 (m, 6H), 5.220 (m,
1H), 4.774 (m,
1H), 4.659( m, 2H), 3.578 (m, 2H), 3.499 (m, 6H), 2.752 (m, 1H), 2.648 (m,
1H), 2.472 (m, 2H),
-100-
CA 02475069 2004-08-20
2.243 (m, 1H), 1.992 (m, IH); MS: M+=653.2;
N-f 1R-(1S-benzooxazol-2-ylcarbonylventvlcarbamoyl)..
2-benzylsulfon I~yllmorpholine-4-carboxamide (Compound 114); NMR 300n~iz {DMSO-
d~,
8:64 {d, J-7.4H3, 1H), 8.01 (d, J=8.8H3, IH), 7.91 (d, J=9.1H3, 1H), 7.68 (t,
J=6H3, 1H), 7.55 (t,
J=8.2H3,1H), 7.38 (s, SH), 7.05 (d, J=9.6H, 1H), 5.26 (m, 1H), 4.'72 (m, 1H),
4.49 (s, 2H), 3.55
(m, 4H), 3.5-3.2(m, 6H), 1.96 {m,1H), 1.76 (m, 1H), 1.38 (m, 4H), 0.87 (t,
J=7.4H3, 3H); MS:
571.24 M'=570.20;
N-f 1 R-( I S-benzooxazol-2-ylcarbon~~lpentylcarbamoyl)-
2-o-to~lmethylsulfon ley thvllmorpholine-4-carboxamide (Compound II5); NMR
300mHz
(DMSO-db), 8.70 {d, J=6.9H3, 1H), 8.01(d, J=9.1H3, 1H), '7.91 (d, J=8.8H3,
1H), 7.67 (i, J=8H3,
1H), 7.55 (t, J=8.SH, 3H), 7.3-7.I (m, 4H], 7.05 (d, J=9.6H~ H), 5.26 (m, 1H),
4.80 (m, 1H),
4.53 {s, 2H), 3.58 (m, 4H), 3.33 (m, 6H), 2.33 (s, 3H), 1.96 (m, IH), 1.72 (m,
1H), I.35 (m, 4H);
0.87 (t, J=7.7H3); MS=585.30, M*=584.23;
~V f IR-f IS-benzooxazol-2~rlcarbonyl~entvlcarbamovl~
2-(2-nitrobenzylsulfonyl~ethvllmorpholine-4.-carboxamide (Compound 116); NMR
300m.Hz
(DMSO-d~), 8.70 (d, J=7.2H3, 1H), 8.1-7.5 (m, HH), 7.05 (d, J=9.3H3, 1H), 5.26
(rn, 1H), 5:01
(s, 2H), 4.70 (m, IH), 3.57 (m, 5H), 3.30 (m, SH), 1.96 (m, IH), I.72 (m, IH),
I.34 (m, 4H),
0.87 {t, J=7.7H3, 3H); MS: 616.09 M*=615.20;
N fIR-(1S-benzooxazol-2-vJcarbonylpentylcarbamovl)-
2dJ 2-(2-chlorobenzylsulfonyl)ethyllmorgholine-4-carbQxamide (Compound 117);
NMR 300mHz
(DMSO-da), 8.71 (d; J=7.IH3, 1H), 8.1-73 (m, 8H), ?.06 (d,J=9.6H3, 1H), 5.26
(m, IH), 4.79 (m,
1H), 4.72 (d, J=I5H3, 1H), 4.65 (d, J=ISH3, 1H), 3.56 (m, 4H), 3.30 (m, 6H),
1.96 {m, 1H), I.73
{m, 1H), I.35 (m, 4H), 0.87 (t, J=7.7H3, 3H); MS: 605.24 M+=605.10;
N f1R-(2-benzooxazol-2-vl-1.~-dimethyl-2-oxoethylcarbamo"yl~
2_-o-tolvlmeth ls~ ulfonylethyl morpholine-4-carboxamide (Compound I 18); MS:
{M*+1) 557;
N-f IR-(2-benzooxazol-2-yl-1.1-dimethyl~2-oxoethylcarbamovll-
2-l2-chlorobenzylsulfonyl)ethxlimorpholine-4.-carboxamide (Compound 119); MS:
(M*+1) 578;
N f 1R-(2-benzooxazol-2-yl'l,l-dimethyl-2-oxoethvlcarbamoyl)-
2-(2-nitrobenz Isulfonyl)ethyllmorpholine-4-carboxamide (Compound I20);'H NMR:
{DMSO)
9.34 (s, 1H), 8.02 (d, J=7.7Hz, 1H), 7.82-?.45 (m, 7H), 6.74 (d, J=8.8Hz, iH),
4.87 (m, 2H), 4.64
(m, IH), 3.44-3.1I (m, lOH), I.56 (s, 3H),1.50 (s, 3H); MS: (M'+1) 588;
N~ IR-(IS-benzooxazol-2-ylcarbonyl-3,-,phenvlpropylcarbamolrl~
-101-
CA 02475069 2004-08-20
~,p 'rid-2-ylmethvlsulfonylethyllpiperidine-4-carboxamide (Compound 121);
MS:m/e +I=616.2;
N-f 1R-(1S-benzooxazol-2- lcarbonvIpent"~lcarbamovl)-
2-pyrid-2 ylmethvlsulfonvlethyllmorpholine-4-carboxamide (Compound 122); 'H
NMR : 8.62
{d; 6.9 Hz, 1 H), 8.55 (d, 3.2 Hz, 1H), 8.00 (d, 7.0 Hz, 1H), 7.86 (m, 2H),
7.65 (t. 6:2 Hz, lI~,
7.48-7.58 (m, 2H), 7.40 {m, 1H), 7.06-7..25 (m, 3I~, 5.28 (m, 1H), 4.74 (m,
1H), 4.67 (d, 1.1 Hz,
2H), 3.53 (m, 4H), 3.3 I (m, 4H), 1.99 (m, I H); 1. 75 (m, I H), 1.32 (m, 4H),
0.87 (t, 6.7 Hz, 3H);
MS: M+1= 571.8;
N I1R-(IR-benzooxazol-2-yJcarbonvl-3-phe~Ipropylcarbamovl)-
2-benzvlsulfonylethylimorpholine-4-carboxamide (Compound 123);
'f0 N f 1R-(1-benzooxazol-2-ylcar'~c::ylcvclobutylcarbam~l)-
2-benzyIsuIfon~rlethylimorpholine-4-carboxamide (Compound 124), MH* 555;
benzvl 1 S-(2-benzooxazol-2-vl-2-hvdroxvethvlcarbamoyl)-3-methylbutvlcarbamaie
(Compound 125);
2S-ace - lamino-
N (2-benzooxazol-2-y1-1 S-methyl-2-oxoethyl)-3-cyclohexvlnropionamide
{Compound 126); 'H
NMR (CDCl3): 7.92 (d, J=8.4Hz, IH), 7.73-7.67 (m, 1H), 7.60-7.48 (m, 2H), 5.94
(d, J=8.7Hz,
1H), 6.65 (m,1H), 2.03 (d, J=7:2Hz, 2I-1], I.64 {m, 6H), 1.56-0.92 (m, IOHz);
MS ESI: MH*
386;
ten-butyl IR-(I-benzooxazol-2-ylcarbonylcYclobutylearbamoyl~
2-benzylsulfan~rlethvlearbamate (Compound 127);
N f 1R-(1S-benzooxazol-2-ylcarbonvl-3-methylsulfonJrlprop lcarba~royl)
2-benzylsulfonvleth~~IimorphoIine-4-carboxamide (Compound 128);'H NMR (CDCI~:
7.89 (d,
J=7.4Hz, 1H)', 7.65 (m, 1H), 7.57 {m, 1H), 7.48 (m, 1H), 7.4 (m, 5H), 6.0 (m,
1H), 5.7 (m, 1H),
4.93 (m, 1H), 4.33 (m, 3H), 3.70 (m, SH), 3.25-3.4 (m, 7H), 2.93 (m, 3H), 2.8
(m, 1H), 2.35 (m,
IH); MS: m/e 653.2;
- N 1I-(1S-benzooxazol-2-ylcarbon~pentylcarbamoyl)-
3-phenarlsulfanylpropyllmoroholine-4-carboxamide (Compound 129);'H NMR
(DIviSO): 8.52
(d,J = 8Hz, 1H), 8.98 (d,J = 8Hz, 1H), 8.88 (d,J = 9Hz, 1H), 7.64 (t,J =
8Hz,1H), 7.53 (t,J =
9Hz, 1H), 7.30 (m, 4H), 7.19 (m, 1H), 5.25 (m, lI-1?, 4.35 (m, 1H); 3.51 (m,
4H), 3.26 (m, 4H),
2.94 (t, J=8Hz, 2H), 2.9 (m, 3H), 1.7 (m, IH), 1.31 {m, 4H), 0.86 (t,J=BHz,
3H), 6.53 (d,J=9Hz,
1H); MS: mle=539.24;
N-1 IR-(1 S-benzooxazoI-2-vlcarbony_I-pentvlcarbamo,~l)
-102-
CA 02475069 2004-08-20
2-(2-trifJuolromethvlbenzylsulfonyl)ethxllmorpholine-4-carboxamide (Compound
131);'H NMR:
(DMSO) 8.78 (d, J=8Hz, 1H), 8.06-7.50 (m, 8H), 7.04 (d, J=8Hz, 1H), 5.27 (m,
1H), 4.82-4.64
(m, 3H), 3.65-3.25 (m, l0H),1.96 (m, 1H), 1.7I (m, 1H), 1.4I-1.22 (m, 4H), ),
0.84 (t, J=7Hz;
3H). MS: (M++1) 639;
~V-i 1 R-f i S-benzooxazol-2- Iy cax_b_onyl-3-phenyl-progvlcarbamovl)-
2-pyrid-2;~rlmethylsulfonvlethylimor~holine-4-carboxamide (Compound I3Z);'H
NMR (DMSO):
8.78 (d,J=?.2Hz, 1H), 8.56 (d,J=5.4Hz, 1H), 7.98 (d;J=8.4Hz, 1H), 7.85 (m,
2H), 7.64
{t,J=12.1Hz,1H), 7.52 (m, 2H), 7.38 (xn, 1H), 7.10-7.34 (m, 8H), 5.25 (m, 1H),
4.70 (m, 3H);
3.55-3.70 (m, 4H), 3.35 (s, 4H), 2.80 (m, 2H), 2.25 (m, 1H), 2:0 (m, 1H); MS:
mle {+I) = 620.0;
N fIR-(IS-benzooxazol-2-ylcarbonvl-3-methylsulfon~vropylcarbamovl)-
2-pyrid-2-vlmeth Is~ylethy~morpholine-4-carboxamide (Compound I33); '~i NMR
(DMSO): 8.83 (d,J=7.6Hz, 1H), 8.55 (d,J=4.OHz, 1H), 7.97 (d,J=7.6Hz, 1H), 7.88
(m, 3H), 7.64
(t,J=7.2Hz, 1H), 7.39-7.54 (m, 4H), 7.15 (d, J=7.6Hz, IH), 5.36 (m, 1H), 4.70
(m, 3H); 3.56 (m,
6H), 3.24 (m, 4H), 2.40 (m, 1H), 2.I5 {m, IH), 2.99 (s, 3fi); MS: mle (+I) =
622.2;
2-f2~1-benzooxazol-2-ylcarbonvlpentylcarbarnovl)-
2-morpholin-4-ylcarbonylamino)ethanesulfonylmethyl~,pyridine l=oxide (Compound
134);'H
NMR (DMSO): 8.?5 (d, 3=6.SHz, 1H), 8.38 (m, 2H), 7.96 (d, J=?.7Hz, IH), 7.89
(d,J=7.7Hz,
IH), 7.48-7.69 (m, 6H), 7.05 (d, J=6.8Hz, 1H), 5.22 (m, 1H), 4.95 (d, J=2.?Hz,
2H), 5.85 (m,
1H), 5.53 (rn, 4H), 3.30 (s, 4H), 1.95 (m, 1H), 1.70 (m, 1H), 1.30 (m, 4H),
0.88 (t, J=5.4Hz, 3H);
MS: MW = 587.65 M+1 = 588.2;
N ~1R-(IS-benzooxazol-2 y-Jcarbonylbutylcarbamoyll-
2-(2-difluoromethoxybenzylsulfonYl ethyllmorpholine-4-carboxamide (Compound
135); NMR
300mHz (DMSO-db), 8.70 (d, J=7.IH3, 1H); 8.01 {d, J=8.8H3, 1H), 7.91,(d,
J=9.1H3, 1H), 7.65
(t, J=8H3, 1H), 7.55 (t, J=8.2H), 7.I I {t, J=8.2H), ?.4-6.8 (m, SH), 5.28 (m,
1H), 4.76 (m, 1H),
4.5 (s, 2H), 3.SS (m, 4H), 3.3 (m, 6H), 1.93 (m, 1H), I.71 (m, 1H), 1.42 (m,
2H), 0.91 (t, J=8H3,
3H); MS: 623.38 M+=622.19;
N f3-ghen ls~ulfonyl~
1- 1S-benzooxazol-2- lcarbon 1 en lcarbamo I ro 1 rno holine-4-carboxamide
{Compound I36);'H NMR (DMSO): 8.5 (m, 2H), 8.00 (d,J = 9Hz, 1H), 7.9-7.5 (m,
8H), 6.54
(t,J = 9Hz, IH), 4.28 (m, IH), 3.49 (m, 4H), 3.24 (m, 6H), 1.90 (m, 3H), 1.65
(m, 1H), 1.31 (m, w
4H), 0.85 (t,J=7Hz, 3H); MS: m/e =571.39;
N IIR-(1S-benzooxazol-2-~rlcarbonylpentylcarbamoyl)-.
-103-
CA 02475069 2004-08-20
2-~2-difluoromethoxvbenz Is~ulfo_nyI)ethYllmo~holine-4-carboxyamide (Compound
137);'H
NMR: {DMSO) 8.66 (d, J=6.6Hz, 1H), 7.99 (d, J=8Hz,1H),.7.87 (d, J=8Hz,1H),
?.67-6.83 (m,
8H), 5.25 (tn, 1H), 4.73 (m, 1H), 4.54 (s, 2H), 3.60-3.23 (m, lOH), 1.93'(m,
1H), 1.68 (m, 1H),
1.40-I.22 (m, 4H), 0.84 (t, J=6.7Hz, 3H); MS: (M++1) 637;
~V t1R-~1S-benzooxazol-2-vlcarbonylpentylcarbamoyll-
2-(2-difluoromethoxybenzvlsulfonvl)ethvllisonicotinamide (Compound 138); IH
NMR: (DMSU)
9.22 (d, J=8Hz, 1H), 8.87 (d, J=6Hz, iH), 8.70 (m, 2H), 7.97-7.i9 (m, 10H),
7.08 (t,.J~74Hz,
IH), 5.30-5.09 (m, 2H); 4.58 (s, 2H), 3.73-3.59 (m, 2H), 1.94 (m, 1H), 1.71
(m, IH), 1.41-1.22
(m, 4H), ), 0.82 (t, J=6.7Hz, 3H); MS: {M++1) 629;
N-11R-(IS-benzooxazol-2-~~lcarbonylbutYlcarbamoyl)-
2-pyrid-2=ylmethylsulfonyl)ethylimorpboline-4-.carboxamide (Compound 139);'H
NMR
(DMSO): 8.6 (m, 2H), 8.05 (d,J=S.lHz, 1H), 7.85 (m, 2H), 7.3-7.8 (m, 4H), 7.2
(m, 3H), 5.32
{m, 1H), 4.72 (m, IH), 4.65 (d,J=3.lHz, 2H), 3.21-3.75 (m, 8H),1.90 (m, 1H);
1.75 (m; 1H),
1.45 (m, 2H), 0.90 (t,J=4.SHz, 3Ii~; MS:m/e +1=558.2;MS: m/e {+I) = 558.2;
i5 2-f 2R-(1 S-benzooxazol-2-ylcarbonylbutvlcarbamovl)-
2-morpholin-4-ylcarbon~inoethylsulfonylmethYl~p~ridine 1-oxide (Compound
140);'H NMR
(DMSO): 8.57 (m, 3H), 7.97 (m, 1H), 7.63-7.82 (m, 3H), 7.35-7.45 (m, 4H), 6.93
{rn, 1H), 4.50-
4.95 (m, 2H), 4.18 (m, ZH), 3.10-3.80 (m, 8H), 1.10-1.70 (m, 4H), 0.82
(t,J=5.4Hz, 3H); MS:m/e
(+1 ) =574.2;
1 R-l I S-benzooxazol-2-ylcarbanylpentylcarbamoyl)-
2-l2-difluoromethoxvbenzvlsulfon~)eth>rlcarbamate (Compound 141 ); MS: (M'+1 )
582;
N fIR-(1S-benzooxazol-2-vlcarbonvlventvlcarbamovl)-2-benzylsul
onylethy~Lsuccinamic
acid (Compound 142);'H NMR: {DMSO) 12.09 (s, 1H), 8.63 {d, J=6Hz, IH), 8.51
(d, J=8Hz,
1H), 7.98 (d, J=BHz, 1H), 7.87 (d, J=BHz, 1H), 7.62 (t, J=8Hz, IH), 7.52 (t,
J=8Hz, 1H), 7.38-
7.30 (m, SH), 5.25 (m, IH), 4.84 (m, 1H), 4.46 {s, 2H), 3.53-3.21 (m, 2H),
5.28-5.25 (m, 4H),
I.93 (m, 1H), 1.68 (m, 1H), I.40-1.22 (m, 4H), 0.84 (t, J=6.2Hz, 3H); MS:
(M'+I) 558;
2R-f3,3-bis(2-methoxyethyl)ureidol-N-(I S-benzooxazol-2-ylcarbonvlpentyl)-
3-benzylsulfonyl~ropionamide (Compound 143);'H NMR: (DMSO) 8.50 (d, J=6.6Hz,
1H), 7.98
(d, J=8Hz, 1H), ?.88 (d, J=8Hz, 1H), '7.62 (t, J=8Hz, 1H), 7.52 (t, J=8Hz,
1H), 7.38-7.30 (m,
SH), 6.82 (d, J=8Hz, 1H), 5.26 (m, 1H), 4.70 (m, IH), 4.46 (s, 2H), 3.52-3.22
(m, lOH), 3.31 (s,
6H), 1.94 (m, IH), 1.69 (m, IH), I.40-1.22 (m, 4H), 0.85 (t, J=6.6Hz, 3H); MS:
(M'+1) 617;
_N-11R-(1S-benzooxazol-2-ylcarbonyI-3-pheny~rogylcarbamovl)-
-104-
CA 02475069 2004-08-20
2-l6-methv~vrid-2-ylmethylsulfon~rl)ethyIlisonicotinamide (Compound 144);'H
NMR (DMSO): -
8069 (t,J =6Hz, 1H), 8.55 (d,J =9Hz, IH), 7.91 (m, 2H), 7.51 (m, 3H), 4.51 (m,
IH), 4.I I (d,J
6Hz, 2H),1..5 (m, 15H); MS: m/e=328:05;
N f1R-lIS-benzooxazol-2-vlcarbonvl-3-phenyprogylcarbamoyl,~
2-benz lsulfonylethyl~succinamic acid (Compound 145); MS (ESI] MH+ 478:2;
N fIR-(IS-benzooxazol-2-ylcarbonyli3-phenylpropylcarbamo~)-
252-trifluoromethylbenzylsulfonyl)ethylltetrah ~1_dropyran-4-carboxamide
(Compound I46);
N f 1R-(IS-benzooxazol-2 ylcaTbonvl-3-pheny3propylcarbamoyl~
2-thien-3-vlmethylsulfonylethyllisonicotinamide (Compound 147);
N-fIR-l1S-benzooxazol-2-ylcarbonyl-3phenvIprotiylcarbamovl)g~
2-(6-meth~rlpyrid-2-vlmethylsulfonyl~ethvlltetrahydrovvran-4-carboxamide
(Compound 14$);
N f 1R-(1-benzooxazol-2-ylcaibonylcyclobutylcarbamoyl)-
2-(2-trifluorometh lbenzylsulfonyl~ethyl3tetrahydropyran-4-carboxamide
(Compound 149);
N f 1R-l1S-benzooxazol-2-ylcarbonvl-3-phen~rlpropylcarbamovll-
2-gvrid_3-~vlsulfonylethvIlpyrazine-2-carboxamide (Compound I50);
N-f I-(I-benzooxazol-2 ylcarbonvl-3-Qhenylpropylcarbamoyl)-
2-thien-3-ylmethylsulfon Iy ethyllpiperidine-4-carboxamide (Compound 151);
N f I S-(I S-benzooxazol-2-vlcarbonyl-3 phenvlpropylcarbamovl)-
2-thien-3 ylrnethvlsuIfonylethyllazetidine-3-carboxamide (Compound 152);
N-fIR-f1S-benzooxazol-2-ylcarbon~)but~arbamoxl)-
2-pvrid-3- I~methvlsulfonylethylimorpholine-4-carboxamide (Compound 153);'H
NMR
(DMSO): 8.66 (d, J=6.7Hz, 1H), 8.56 (m, 3H), 8.01 (d, J=7.9Hz, 1H), 7.90
(d,J=8.lHz, 1H),
?.79 (m, 1H), ?.65 (t, 3=7.lHz, 1H), 7.55 (t, J=7.lHz, 1H), 7.43 (dd, J-
4.9,7.9Hz, 2H), 6.g3 (d,
J=8.40Hz,1H), 5.30 (m, J=lHz, IH), 4.76 (m, IH), 4.57 (d, J=3.?Hz, 2H), 3.24-
3.?0 (m; 8H),
1.91 (m, IH), 1.73 (m, IH), 1.40 (m, 2H), 0.82 (t, J=5.4Hz; 3H); MS:mle (+1) =
555.8;
N-f l R-(1 S-benzooxazol-2 ylcarbonyl-3-phenv_lpro~, vlcarbamo~I)-
2-benzylsulfo~lethyllninerazine-I-carboxamide (Compound 154);
N-f 1 R-( 1 S-benzooxazol-2"vlcarbonyl-3-meth~lsulfonvlpro~ylcarbamovl)-
2 ~2-difluoromethoxvbenzylsulfonyl~,eth-yllmorpholine-4-carboxamide (Compound
155);'H NMR -
(CDCL~, 300MHz) 7.8944 (d, J=7.92Hz, 1H), 7.67 (m, 1H), 7.58 (m, 1H), 4.49 (m,
2H), 7.415
(m, IH), 7.24 (m, 3H), 6.5811 (t, J=?3.24Hz, IH), 5.7633 (m; IH), 4.9199 (m,
IH), 4.4871 (dd,
J=13.61, 23.75Hz, 2H), 3.7101 (m, 4H), 3.4189 (m, 4H), 3.27 (m, 2H), 2.9289
(s, 3H), 2.77 (m,
-105-
CA 02475069 2004-08-20
1H), 2.37 (rid;' lI~; MS: (M*) 687.3 (M') 685.6;
1V [1R-(IS-benzooxazoI-2-ylcarbonyl-3-methvlesulfonvlnronvlcarbamovll-
2-(2-methoxybenz Isy ulfonvi)ethvllmorpholine-4-carboxamide (Compound 156);'H
NMR
(CDCI3): ?.89 (m, 1H), 7.45-7.8 (m, 3H), 7.35 (m, ZH), 6.9-7.05 (m, 2H), 5.83-
5.9 (m,1H),
5.62-5.8 (m, .1H), 4.82 (m, IH), 4.40 (m, 2H), 3.89 (s, 3H), 3.70 (m, 5H),
3.25-3.42 (m, 7H),
2.95 (s, 3H);-2.75 (m, 1H), 2.35 (m, IH); MS: m/e 651.4;
N f1R-(1S-benzooxazol-2-ylcarbonylpentYlcarbamoyI)-
2-benzvlsulfonylethvllpiperazine-1-carboxamide {Compound I57);'H hTMR: (DMSO)
9.20-
9.11 (m, 2H); 8.73 (m, IH), 7.98 (d, J=BHz, 1H), 7.88 (d, J=8Hz, 1H), ?.63 (t,
J=BHz, 1H), 7.52
(t, J=8Hz, IH); 7.39-7.30 (m, SH), 5.24 (m, IH), 4.74 (m, IH), 4.50 (s, 2H),
3.62-3.30 (m, 6H),
3.05-2.95 (m, 4H), L94 {m,1H), 1.69 (m, 1H), 1.40-1.22 (m, 4H), 0.84 (t,
7=6.6Hz, 3H); MS:
(M'+1 ) 570;
N (1S-benzooxazole-2-vlcarbonyl-3-methvlsulfonylpronvl)-2R-methvlsulfonvlamino-
3-bent Iy sulfonylpropionamide (Compound 160);'H NMR (DMSO-d6) 7.9498 (m, 2H),
7.657?
(m, IH), 7.5556 (m, 1H); 7.3870 (m, SH), 5.4016 (m, 1H), 4.5444 (m, 3H), 3.32
(m, 2T~, 2.9?84
(s, IH), 2.9326 (s, 1H), 2.49 (m, 1H), 2.20 (m, 1H); MS: (M+) 586.0, (M-)
584.0;
methyl 1 R-( 1 S-benzooxazol-2-ylcarbonyi-3-3-phenvlpropyl carbam oyI)-
2-pyrid-2-vlmethvlsulfon-yleth~rlcarbamate (Compound 161); MS: m/e (+1) =
564.6;
meth l 1R- 1S-benzooxazol-2- lcarbon 1-3-meth lsulfon i ro lcarbamo 1
2-benzylsulfon,~ethylcarbamate {Compound 162); 'H NMR (DMSO): 9.03 (d,
J=7.ZHz,1H),
7.97 (d, J-7.9Hz, IH), 7,90 (d, J=8.2Hz, IH), 7.65 (td, J=7.2, l.2Hz, 1H),
?.55 (t, J=7.9Hz, 1H),
7.37 (m; SH), 5.32 (m, IH), 4.65 (m, 1H), 4.50 (m, 2H), 3.53 (m, IH), 3.49 (s,
3H), 3.33 (s, 2H),
3.24 (m, IH), 2.98 (s, 3H), 2.41 (tn, 1H), 2.18 (m, 1H); MS: m/e 653.2;
N-(IS-benzooxazoI-2-vlcarbonylpentyl)-ZR-f3.3-di(2-methoxyethvl)ureidol-
3 pvrid-2~imeth5rlsulfonyloropionamide {Compound 163); MS:m/e +1= 615.6;
N f 1R-(1S-benzooxazol-2-vlcarbonylbutylcarbamovl~
2-(2-methoxybenzvlsuifon l~ethyllmorgholine-4-carboxamide (Compound 164);'H
1~1MR
(DMSO): 8.66 (d,J =6Hz, 1H), 8.03 (d,J = 9Hz, IH), 7.93 (d,J = 9Hz, 1H), 7.68
(t,3 = BHz, 1H),
7.58 (t,J =9Hz, fH), 7.36 (m, 2H), 7.0 (m, 3H), 5.29 (m, 1H), 4.77 (m, 1H),
4.54 (d,J = l4Hz,
IH), 4.43 (d,J =I4Hz, 1H), 3.84 (s, 3H), 3.5-3.3 (m, lOH), 1.95 (m; 1H), 1.74
(m, 1H), 1.46 (m,
2H), 0.93 (t, J=8Hz, 3H); MS: mle=587.31;
N (1S-benzooxazol-2-vlcarbonvlbutvlcarbamoyl~-2R-(3 3-dimeth Iureido>".
-106-
CA 02475069 2004-08-20
3-(2-methoxvbenz Is~nyl)propionamide (Compound lb5); NMIt 300mHz (DiVISC)-d6),
8.63 y
(d, J=6.9H3,1H), 8.03 {d, J=8.8H~; 1H), 7.92 (d, J=9.1, IH), ?:70 (t, J=8:8H3,
1H), 7.38 (t,
J-8.ZH3, IH), ?.37 (m, 2H), 7.08 (d, J=9.IH3, IH), 6.98 (t, J=8.2H3, IH), 6.71
(d, J=9.iH3, 1H);
5.27 (m, 1H), 4.?? (m, 1H), 4.55 (d, J=IS.IH3, 1H), 4.43 (d, J=15.IH3, 1H),
3.79 (s, 3H), 3.47
{d, J=6.9H3, 2H), 2.83 (s, 6H), I.93 (m, 1H), L?5 (xn, IH), I.43 (m, 2H), 0.93
(t, J=8H3, 3H);
N l1S-benzooxazol-2-ylcarbonylbutyl)-2-meth~rlsulfonvlamino-
3S2-methoxvbenzylsulfonvl)propionamide (Compound 166);'H NMR (DMSO): 9.0 (d,J
= 6Hz,
IH), 8.01 (d,J = 8Hz, 1H), 7.9i (d,J = 8Hz,1H), 7.67 (t,J = ?Hz,1H), 7.57 (t,J
= g~, 1H),
7.36 (t, J=BHz, 2H), 7.07 (d, J=8Hz, 1H), 6.97 (dt, J=2,7Hz, 1H), 7.85 (m,
1H); 5.33 (m, 4H), 4.5
0 (m, 3H), 3.8 (s, 3H), 3.35 (m, 2H), 2.92 (s, 3H), 1.93 (m, I H), 1.72 (m>
IH~, 1.44 (m, 2H), 0.9 i
' (t, J=7Hz, 3H); MS: rnle=552.19; .
3-cvclohexvl-N f2-hvdroxv-2-(5-nitrobenzooxazol-2-vly-
ISphenethylethyllpro~ionamide
(Compound 167); MS (ESI) miz = 466 {M + I);'H-NMR (300 MHz, CDC13): b 0.95 (m,
2H),
81.22 (m, 4H), $ I.S1 (m, 2H), 8 I.65 (m, 6H), S 2.15 (m, 2H), 8 2.65 (m, 2H),
8 4.15 (in, 1H),
8 4.50 (m,1H), b 5.08 (m; 1H), b 5.80 (d, J = 6 Hz, IH), b 6.09 (m, IH), 8
7.00 - 7.35 (m, SH),
8 7.60 (m, IH), S 8.40 (m, 1H), 8 B.SS (rxi, iH), (C~H3,N30s);
methyl 2-f 2S-(3-cyclohexvlprogi onylamino)-1-hvdroxv-
4-phenylbut~llbenzooxazole-6-carboxvlate (Compound 168); MS (ESI) m/z = 478 {M
+ 1);
'H-NMR (300 MHz, CDCl3): 8 0.84 (m, 2H), 81.22 (m, 4H), 8 1.51 (m, 7H), 81.90
(m,1H),
?0 8 2,11 (m; 2H), 8 2.65 (m, 2H), 8 3 .95 (s, 3H), 8 4. i 9 (m, 1 H), 8 4.50
(m,1 H), 8 5.09 (s, 1H),
8 6.09 (m, IH), 8 6.49 (m, 1H), b 7.01 - 7.35 (m, SH), & 7.65 (m, 1H), 8 8.01
(m, 1H), 58.17 (m,
IH)9 (CzaH3aNzos)~
N-f2-(5-chlorobenzooxazol-2-yl)-
2-hydroxv-lSphenethylethyll-3-cyclohex.,y_lpro~ionamide (Compound 169); MS
(ESI) mlz ~ 455
(M +.1 ); 'H-NMR (300 MHz, CDCI~: s 0.84 (m, 2H), 8 1.12 (m, 4H), 81.20 (m;
2H), 8 1.51
(m, 6H), b 2.00 (m, 3H), 8 2.65 (m, 2H), b 4.21 (m, IH), 8 4.50 (m,1H), 8 5.02
(s, ll~; 8 6.44
(m, 1H), 8 7.01 - 7.47 (m, 7H), b 7.65 (s,1H), (C~6H31cIN2Og);
benzyl 1 S-(2-benzooxazol-2-yl-2-hydroxv-i S-nhenethylethylsulfamoylmethvl)-
3-methylbutylcarbamate {Compound i70);'H NMR (CDCi3): 7.71 (m,1H), 7.52 m,
1H); 7.20-
7.40 (m, 12H), 5.9 (m, O.SH), 5.6 (m, O.SH), 4.80-5.20 (m, SH),,4.1-4.3 (m,
2H), 2.7-2.9 {m,
4H), 1.7-2.0 {m, 2H), 0.90 (m, 3H), 0:79 (m, 3H), 3.30 (m, 1H);
N 1S- 2-benzooxazol-2- 1-2=h drox - S- heneth let Isulfamo (meth 1
-107-
CA 02475069 2004-08-20
3-methyibutvllacetamide (Compound 171 );
benzyl 1S(2-benzooxazoI-2 y,I-2-h,Ydroxy-
lSphenethylethvlsulfamoylmethyl)-3-methvlbutvlcarbamate (Compound 172); 'HNMR
(DMS4): 7.71 (m, 1H), 7.5 (m, 1H), 7.0-7.4 (m, 12H), 4.9-6.2 (m, 6H), 4.0-4:35
(m, 2H), 3.75
(m, 1H), 3.20-3.60 {m, 2H), 2.5-3.0 (m, 2H), 1.15-2.15 (m, 3H), O.b-1.05 (m;
6H); MS: m!e
580.1;
N fIR-(2-benzooxazol-2-y1-2-hvdroxv-1S-phenethylethylsulfamovimeth~
-methylbutvllacetamide (Compound 173);
2S-acetvlamino-N (2-benzooxazol-2-yl-2-hydrox -~Qheneth ly ethyl~
3-cvclohex~mropionamide (Compound 174);
tent-but~S (2-benzooxazol-2-yI-2-hydroxy-IS-phenethyleth~ll-
2-cvclohexyleth~arbamate (Compound 175);
2-acet~lamino-N 2-benzooxazol-2- ~Ll-1,1-dimeth.,yl-2-oxoethyl)-
3-cyclohexylvronionamide (Compound 176);
benzvl 1S-f2-t5-phenylbenzooxazol-2- l~-hydroxyethylcarbamovll-
3-methylbutylcarbamate (Compound 177);
jV-(2-benzooxazol-2-y1-2-hydroxy-1S-phenethylethyl)-3-c clopentylpropionamide
(Compound 178);'H NMR {CDC13): 7.72 (m, 1H), 7.53 (m, 1H), 7.08-7.19 (m, 8H),
5.98 (m;
1H), 5.05 {m, 2H), 4.51 (m, 1H), 2.6-2.8 (m, 4H), 2.17-2.29 (m, IH), 1.95-2.15
(m, 2H),1.8-
1.95 (m; 1H), 1.68-1.78 (m, IH), I.3-I.7 (m, 6H), 1.0-1.12 (m, 1H), 0.85-1.0
{m, 1H);
N (2-benzoo~cazol-2 yl-2-hydroxy-
1S-pheny_ethvlethvl!~-2-bicycloj2.2.Ilhept-2-~lacetamide (Compound 179);
N-(2-benzooxazol-2-vI-2-hvdroxy-1S-pheneth ~Iy ethyl)-2-naphthalen-1-
ylacetamide
{Compound 180);
N (2-benzooxazol-2-v1-2-hvdroxv-1S-pheneth ly ethyl)-3-phenylpropionamide
(Compound 181);'H NMR (CDC13): 7.69 (m, IH), 7.53 (m, 1H), 7.37 (m, ZH), 7.03-
7.35 {m,
lOH), 5.9 (m,1H), 4.98 {m, 1H), 4.40-4..55 (m, 1H), 3.0 (m, 1H), 2.80 (t,
J=?.7Hz; 2H), 2.55 (m,
2H), 2.38 (t, J=7.SHz, 2H);
methyl 2-f2S-(3-cyclohexvlpro~~ionvlamino)-1-hydroxy-4 ~henylbut~ll-
4.5-dih drooxazole-4S-carhoxylate (Compound 182); MS (ESI) rnlz = 431 {M -~
1);'H-NMR
(300 MHz, CDCl3): 8 0.89 (m, 2H), 8 1.20 {m, 4H), 81.48 (m, 2H), 8 1.65 (m,
6H), 8 2.00 (m,
2H), S 2.15 {m, 2H), 8 2.73 (t, J = 4 Hz, 2H), 8 3.76 {s, 3H), 8 4.30 - 4.65
(m, SH); 8 6.00 (d,
-108-
CA 02475069 2004-08-20
J = 6H2, 1H), b 7.13 - 7.35 (m, SH), (C~H~N20~;
methyl 2-f 2S-!3-cvclohexylp~opi onvlamino~- I -hydroxy.
4-phenvlbut~rlloxazole-4.-carboxvlate (Compound 183);
N !2-benzooxazol-2-yl-2-hydroxY-1S-phenethvl>_4-cyclohexvlbu mide
(Compound 184);'H NMR (CDCl~: 7.62-7.73 (m,1H), 7.46-7.59 (m, 1H), 7.05-7.43
(rn, 2H),
6.22-6.38 (m, 1H), 5.11 (s, 1H), 4.50-4.69 (m, 1H), 2.58-2.82 (m, 2H), 2.14-
2.24 (m, 1H), 2.0,
2.I4 (m, 1H), i.54-I.76 (m, 6H), 1.31-1.50 (m, 1H), 0.94-1.31 (m, 7H), 0.63-
0.93 (m, 2H); MS:
m/e=435.1;
methyl 2-f 2S-!3-cyclohexylpropionylaminol-1-hydroxy-4-phen~butyll-
4.5-dihvdrooxazole-4R-carboxvlate (Compound 18~); MS (LSI) mfz = 431' (M +
1);'H-NMR
(300 MHz, CDCl3): 8 0.89 (rn, 2H), 81.20 (m, 4H), S 1.48 (m, ZH), 81.65 (m,
6H), 8 2.00 (m,
2H), 8 2.15 (m, 2H), b 2.?3 (t, J = 4 Hz, 2H), 8 3.76 (s, 3H), S 4.35 - 4.72
(m, SH), b 5.75 (m,
1H), 8 7.13 - 7.35 (m, SH), (C~,H~N20s);
3-c cly ohexx-N f2-hydroxy-2-!5-trifluoromethvlbenzooxazol-2-yl)-
1S-phenethylethvllpropionamide (Compound 186); MS (ESI~ mlz = 489 (M + 1);'H-
NMR (300
MHz, CDC13): 8 0.77 (m, 2H), b 1.22 (~, 4H), s 1.51 (m, 2H), 8 1.60 (m, 6H), 8
2.15 (m, 4H),
8 2.70 (m, 2H), 8 4.51 (m, 1H), 8 5.11 (s, 1H), 8 6.10 (d, J = 6 Hz, 1H), 8
7.00 - 7.35 (m, SH),
8 7.56 (s, 2H), b 7.99 (s, 1H), (CnH3tF3Na03);
2S-acetylamino-N-!2-benzooxazol-2-vl-2-hvdroxy-1 S-phenethylethyl~
3-!2-trifluorometh~phenvl~pro~ionarnide (Compound 187);
methyl_1-~I-benzooxazol-2-y ~,carbonyl-3-phenylpropvlcarbamovl)-
2-cvclohex~ethvlcarbamate (Compound 188);'H NMR (CDCl~: ?.89 (d,J=7.4Hz, 1H),
7.62
(M, 1H0, 7.54 (m, 1H), ?.46 (m, 1H), 7.13-7.30 (m, IH), 6.87 (d, J=7.9Hz, IH),
5.68 (m, 1H),
5.04 (d, J=9.6Hz, 1H), 4.24 (m, 1H), 3.66 (s, 3H), 2.75 (S,J=8.3Hz, 2H), 2.45
(m, 1H), 2.19 (m,
1H), 2.00 (M, 1H), 1.52-1.80 (m=SH), 1.44 (m,1H), I.12-1.27 (m, 4H), 0.89 (m,
2H); MS:
mle=492.04;
N !1-benzooxazol-2-ylcarbonvl-3 ~henypropyl)-3-cvclohexyl-
2-meth isulfonvlaminopropionamide (Compound 189);'H NMR (CDCI3): 7.87 (m, 1H),
7.62
(m, 1H), 7.55 (m; IH), 7.46 (m, 1H), 7.13-7.28 (m, SH), 6.79 (d, J=7.9Hz, 1H),
5.71 (m,1H),
4.92 (m, 1H), 4.00 (m, 1H), 2.95 (2, 3H), 2.75 (m, 2H), 2.48 (m, IH), 2.21 (m,
1H); 1.78 (m,
1H), 1.61 (m, SH), 1.45 (m, 1H), 1.16 (m, 4H), 0.89 (m, 2H);
cvclohexylmethyll-benzooxazoI-2-ylcarbonvl-3-phenylpropylcarbamate -
- I09-
CA 02475069 2004-08-20
(Compound 190);'H NMR (CDC13): 7.88 (m,1H), 7.62 (m,1H), 7.52 (m, 1H), 7.49
(m, IH),
7.13-7.23 (m, 5H), 5.57 (m, 1H), 3.89 (d, J=6.5Hz, 2H), 2.79 (m, 2H), 2.42 (m,
1H), 2.I2 (m,
1H), 1.50-1.73 (m, 6H),1.24 (m, 6H), 0.89 (m, 2H); MS: mle=421.0;
benz~~-benzooxazol-2-ylcarbonyl-3-phenxlpropylsulfamo i~yl3-
2-methylbutylcarbamate (Compound 191);'H NMR (CDC13): 7.88 (d.J=7.7Hz, 1H),
7.62 (m,
1H), 7:55 (m, IH), 7.4? (m, IH), 7.33 (m, 5H), 7.19 (m, SH), 6.35 (d, J=7.7Hz,
iH), 5.45 (m,
1H), 5.13 (s, 2H), 5.0 (m, 1H), 4.43 (m, 1H),.3.06 (m, 1H), 2.87 (m, IH), 2.45
(m, 1H), 2.I5 (m,
1H), 1.41 (m, 1H), 1.07 (m, 1H), 0.88 (m, 6H); MS: mle=5.78.1;
N-f 1R-(iS-benzooxazol-2-ylcarbonyl-_ 3-phenyl~~ropylcarbamovl)-
2-(6-methyl-nvrid-2-ylmethvlsulfonvl iethvllthionhene-3-carboxamide (Compound
192);
N j1R i1S-benzooxazol-2-ylcarbonyl~-
3- hen I ro lcarbamo 1 -2- 2-meth 1 'd-3- lmeth Isulfon 1 th 1 nicotinamide
(Compound 193);
N-f 1R-(1S-benzooxazol-2- l~onyl-3-phenvIpropylcarbamoyl)-
2-(2-cvanobenzylsulfon~lZethyllazetidine-3-carboxamide (Compound 194);
ten-butyl 1R-(1-benzooxazol-2-vlcarbonylcyclobutylcarbamovl)-
2-(2-difluQromethoxybenzvlsulfonyl)ethylcarbamate (Compound 195);
ten-butyl 1 R-( 1 S-benzooxazol-2-ylcarbonyI-3-phenylpropylcarbamovl)-
2~4-trifluoromethylpvrid-3-vImeth 11L sulfonyl)ethvlcarbamate (Compound 196);
N f 1R-f 1-benzooxazoI-2-ylcarbon~cyclobutylcarbamovl?-
2 ~2-difluoromethoxvbenz lsy ulfonyl)eth~morpholine-4-carboxamide (Compound
I97);
N jIR-l1S-benzooxazol-2-~rlcarbonylpentvlcarbamovll-
2-wrid-3 ~rimethylsulfonvlethvllisonicodnamide (Compound 198);
methyl 1R-(1S-benzooxazol-2-vlearbonylbutvlcarbamovl)-
2-~2-methoxybenz-ylsuIfon-yllethylcarbamate (Compound 199);
N j1R-t1S-benzooxazol-2-ylcarbonylpronylcarbamoyl~
2-benzvJsulfonyl_ethvlimor~,pholine-4-carboxamide (Compound 200); NMR 300mHz
(DIVISO-d~,
8.65 (d, J=?.1H3, 1H), 8.01 (d, J=8.2H3, IH), 7.9I (d, J=8.8H3, 1H), 7.66 (t,
J=8H3, 1H), 7.55 (t.,
J=7.7H3, iH), 7.38 (s, 5H), 7.05 (d, J=9.4H3, 1H), 5.21 (m, 1H), 4.75 (m, 1H),
4.49 (s, 2H), 3.53
(m, 4H), 3.45 (m, 21~, 3.32 (m, 4I-~, 2.02 (m, lI~, 1.77 (m, 1H), 0.96 (t,
J=8H3, 3H);
M=543.24 M'=542.61;
N SIR-benzooxazol-2-vlcarbonvlpropvll-2-(3.3-dimethylurei do)-
-110-
CA 02475069 2004-08-20
3-(2-methox~nzylsulfonvl)propionamide (Compound 201); NMR 30dmHz (DMSO-d6),
8.61
(d, J=7.4H3, 1H), 8.01 (d, J=8.SH3, IH), 7.90 (d, J=7.1H3, 1H), 7.GS {t,
J=8H3, IH), 7.55 (t,
I=8H3, 1H), 7.33 (m, 2H), 7.OS (d, J=8.8H3, 1H), 6.96 (t, J=8.2H3, IH), 6.70
(d, J=9.1I33, 1H),
5.20 (m, 1H), 4.53 (d, J=1S.4H3, 1H), 4.41 (d, J=15.4H3, 1H), 3.77 (s, 3H),
3.45 {d, J=7.1H3,
ZH), 2.81 (s, 6H), 2.0 (m, IH), 1.7 ( m, 1H), 0.96 (t, J=8H3, 3H); MS=651.33
M°=650.59;
methyl 1R-(1S-benzooxazol-2-ylcarbonylpropylcarbamoyl)-
2:L2-methoxybenzylsulfonylethyl)carbamate (Compound 202);
N (1-benzooxazol-2-ylcarbonylpentyl)-2R-f3.3-bis(2-methoxvethvl)ureidoL
3-wrid-3-ylmethylsulfony)propionamide (Compound 203);
~1S-benzooxazol-2-ylcarbonvlpentyl)-2R-f3.3-bis(2-methoxyetlivl)ureidol-
33.5-dimethylisoxazol-4- lmethylsulfonyl)propionamide (Compound 204);
N-(1S-benzooxazol-2-vlcarbonvlnropvl)-3-(3,S-dimethylisoxazoI-4-ylmeth
Isulfonyl)-
2R-methylsulfonylaminopropionamide (Compound 20S); 'H NMR: (DMSO) 9.04 (d,
J=6.6Hz,
1H), 8.00-7.87 (m, 3H), 7.63 (t, J=BHz, 1H), 7.53 (t, J=8Hz, 1H), S.2S (m,
IH), 4.61-4.36 (m,
3H), 3.56-3.31 (m, ZH), 2.91 (s, 3H), 2.36 (s, 3H), 2.17 (s, 3H), 2.02 (m,
1H), 1.74 (m, 1H), 0.96
(t, J=7Hz, 3H); MS: (M++1) 527;
metl~l 1R-ll S-benzooxazol-2-vlcarbonylpropylcarbamoyl)-
2-(3,S-dimeth~rlisoxazol-4-ylmethvlsulfonyl)ethylcarbamate (Compound 206);'H
NMR:
(DMSO) 8.?8 {d, J=S.8Hz, 1H), 7:99 (d, J=BHz, 1H), 7.87 (d, J=8Hz, 1H), 7.69
(d, J-8.SHz,
1H), 7.62 (t, J=8Hz, 1H), 7.52 (t, J=8Hz, 1H), 5.20 (m, IH), 4.68 (m, 1H),
4.39 (d, J=l4Hz, 1H),
4.29 {d, J=l4Hz, 1H), 3.52 (s, 3H), 3.60-3.28 (m, 2H), 2.37 (s, 3H), 2.15 (s,
3H), 2.02 (m, IH),
1.74 (m, 1H), 0.95 (t, J=?Hz, 3H); MS: (M''+1) 507;
N f 1R-(1-benzooxazol-2=ylcarbonYlpentylcarbamoxl)-
2.pyrid-2-ylmethylsulfonylethyllisonicotinamide (Compound 207); NMR 1H: 9.15-
9.30 (m, lI~,
8.4-8.9 (m, 4 H), 7.32-8.OS (m, 9H), 5.28 (m, 1H), 5.10 (m, 1H), 4.75 (m, 2H),
3.75 (m,1H),
3.62 (m, 1H),1.95 (m, 1H), 1.75 (m, 1H), 1.OS-1.45 (m, 4H), 0.87 (m, 3H); MS:
M+1= 564.0;
and
4-f 1R-(1S-benzooxazol-2-ylcarbonylnenty)carbamoyl~
2-p 'md-2=vlmethylsulfonvlethvlcarbamoyllnvridine I-oxide (Compound 208).
REFERENCE I3
Benzyl 1S-IN methoxy-N methylcarbamoyl)-3-phe~lnropylcarbamate
-111-
CA 02475069 2004-08-20
A solution of 2-benzyloxycarbonylamino-4-phenylbutyric acid (S.OS g, 16.1
mmol) in
methylene chloride (70 mL) was cooled to 0°C and treated with
diisopropylethylamine
(2.82 mL, 16.2 mmol) added dropwise and then PyBOP~ (8.53 g,16.4 mmol) added
in one
portion. The mixture was stirred for 5 minutes and then treated with
N,O-dimethylhydroxylamine hydrochloride (1.73 8,17.71 mmol) was added in one
portion. The
mixture was neutralized with diisopropylethylamine (4.6 mL, 26.44 mmol) added
dropwise,
stirred for 2 hours at room temperature and then diluted with methylene
chloride (70 mL). The
dilution, was washed with 1N aqueous hydrochloric acid (3x 40 mL), saturated
sodium
bicarbonate (3x 40 mL) and brine (40 mL) and rlnen concentrated. 'The product
was purified
from the residue by column chromatography eluting with 2:3 ethyl
acetate/hexane to provide
benzyl 1 S-fN-methoxy-N methylcarbarnoyl)-3-phenylpropylcarbamate (5.48 g,
15.4 mmol) as an
oil. MS(PC.1] mfz = 357 (M +1).
Proceeding as in Reference 13 provided tent-butyl 1S-tN-methoxy-
N methylcarbamoyl)-3-phenylpropylcarbamate; 'H NMR (CDC13): b 1.35 (s, 9F~,
81.64 -1.72
(m, 2F~, 8 2.40 - 2.54 (m, lI~, 8 2.60 ~~ 2.77 (m, 11T), S 3.00 (s, 3I~ 3.52
(s, 3I~, 8 4.23 (m,
llE~, S 7.10 - 7.37 (m, SH).
REFERENCE 14
2S-Amino-N methoxy-N methyl-4-phenylbu amide
trifluoroacetic acid salt
A solution of tert-butyl 1-(N-methoxy-N-methylcarbamoyl)-3-
phenylpropylcarbamate .
(9.32 g, 29 mmol), provided as in Reference 13, in methylene chloride (100 mL)
was cooled to
0° C and then treated with anisole (5 mL, 46.5 mmol) and
trifluoroacetic acid (50 mL, 296
mmol). The mixture was stirred for 30 minutes, while allowing it to warm to
room temperature,
.and then concentrated. The residue was dissolved in toluene (100 mL) and the
solution was
concentrated. The residue was again dissolved in toluene (100 ml) and
concentrated to provide
2S-amino-N methox~-N meth-4-phenylbutyramide trifluoroacetic acid salt (9.74 g
29 mmol) as
a crude product. MS(PCn mlz = 223 (M +1).
-112-
CA 02475069 2004-08-20
REFERENCE 1 S
Benzyl 1-f 1-lN-methoxy-N methvlcarbamovl)-3S-phenylnropylcarbamoyli
3-meth~lbutvlcarbamate
A solution comprised of 2S-amino N methoxy-N methyl-4-phenylbutyramide
trifluoroacetic acid salt (9.74 g, 29 mmol), provided as in Reference 2, in
D1VIF (?5 mL) was
cooled to 0° C and then neutralized with diisopropylethylamine added
dropwise. A solution
comprised of 2,5-dioxopynrolidin-I-yl 2-benzyloxycarbonylamino~-methylvalerate
(I0.50 g,
29 mmol) in D1VlF (?5 m>~..} and an additional amount of diisopropylethylamine
(10.10 mL, 58
mmol) were added to the cooled butyramide solution. The mixture was stirred
for 2 hours,
while allowing it to warm to room temperature, and then poured into ice water
(300 mL). 'fee
mixture was let stand for I hour to provide a white precipitate. ~e
precipitate was collected by
fltration and dried (P205} under vacuum to provide benzvl I-f 1-fN methoxy-
N methvTcarbamoylZ3-phenylpropylcarbamovli-3-methylbutvlcarbamate (12.24 g,
26.1 mmol).
'H NMR (CDCI3}: 8 0.91 (d, J = 5.88 Hz, 6H), 81.45 -1.55 (m, 1H), 81.45 -1.S5
(m, 2I~,
8 1.77 - 2.00 (m, 1H), 8 2. i I - 2.22 (m, 1H}, 8 2.70 (m, 2H), b 3.20 (s, 3H)
3.60 ('s , 3H) 4.25
(m, lH), 8 5.00 (m, 1H), 8 5.15 (s, 2I~, 8 6.6 (d, J = 8.15 Hz, 1H}, 8 7.15 -
7.45 (m, 1 OIL.
REFERENCE I6
Ethyl_3S-benzXloxycarbo~lamino-2-hydroxy-5-phe~lpentanimidate
A suspension comprised of lithium aluminum hydride (0.885 g, 23.3 mmol) in
anhydrous
diethyl ether was cooled to -45 ° C under nitrogen and then treated
with a solution of benzyl
IS-(N methoxy-N-methylcarbamoyl)-3-phenylpropylcarbamate (S.S3 g, 15.53 mmol),
provided
as in Reference I3, in ether (7S mL) and THF (25 mL) was added dropwise over a
period of
minutes such that the temperature of the mixture was maintained at -40 to -
45° C: The
mixture was allowed to warm to 5° C and then recooded to -35° C.
A saturated solution of
25 sodium bicarbaonate (7 mL, 0.5 IvI) was added dropwise and the mixture was
allowed to warm
to 0° C. The mixture was allowed to warm to room temperature and
stirred for I hour to
provide a precipitate. The precipitate was collected by filtration and washed
with ether
(100 mL}. The filtrate and washings were combined and washed with ice cold 1N
hydrochloric
acid {2x 50 tnL), saturated sodium bicarbonate (2 x 50 mL) and brine (50 mL},
dried (Na2S04)
-113-
CA 02475069 2004-08-20
and concentrated in vacuo to provide benzyl IS-formyl-3-phenylpropylcarbamate
(4.01 g,
I3.5 mmol) as a colorless oil. MS (PCI) m/z = 298 (1VI + 1).
A solution of benzyl 1S-formyl-3-phenylpropylcarbamate (4.557 g, 15.3 mmol) in
anhydrous methylene chloride {SO mL) was stirred while sequentially treating
with 2-hydroxy-
2-methylpropionitrile (4.25 mL, 46.2 mmol) and triethylamine (I.28 ml, 9.20
mmol). The mixture
was stirred for 4 hours at room temperature and concentrated in vacuo. The
residue was
dissolved in ether (100 mL) and the solution was washed with water (5 x 20 mL)
and brine (20
mL), dried (MgS04) and concentrated to provide benzyl
2-cyano-2-hydroxy-1S-phenethylethylcarbamate (4.957 g, 15.3 mmol) as a yellow
oil. 'H NMR
90 (CDCl3): s 1.75 - 2.01 (m, 2I-i), b 2.08 - 2.24 (m, 1H), 8 2.51 - 2.80 (m,
2I~, S 3.70 - 4.02 (m,
II-~, 8 5.07, 8 5.33 (m, 3I~, 8 7.10 - 7.47 (m, 10I~.
A comprised of chloroform (30 mL) and anhydrous ethanol (30 mL, 510 mmol) was
cooled to 0° C and then treated with acetyl chloride (32.6 mL, 459
mmol) added dropwise over ,
a period of 30 minutes. The mixture was cooled with solution of crude benzyl 2-
cyano-
2-hydroxy-1-phenethylethylcarbamate {4.957 g, .15.3 mmol) in chloroform (30
mL). 'Ihe
mixture was stirred for 2 hours at 0°C and then 6 hours at room
temperature and concentrated
in vacuo to provide ethyl 3S-benzyloxvcarbonylamino-2-hydrox~-5-
phenYlpentanimidate
(6.212 g 15.3 mmol) as a crude yellow oil. MS (PCn m/z = 371 {M + 1).
REFERENCE 17
2S-Amino-4-phenyl-1-(4S-phenyl-4.5-dihydrooxazol-2-vl)butan-1-of
A mixture comprised of ethyl 3S-benzyloxycarbonylaxnino-2-hydroxy-
5-phenylpentanimidate (0.78 g, 1.92 mmol), provided as in F~eference I6,
diisopropylethylamine
(0.218 ~tL, 1.26 mmol) and 2S-amino-2-phenylethanol (0.260 g, 1.9 mmol) in
chloroform (25 mL)
was heated at reflux for 3 hours and then was stirred for approximately 12
hours, while allowing
to cool to room temperature. The mixture was concentrated and the residue was
dissolved in
ethyl acetate (30 rnl). The solution was washed with O.SN sodium hydroxide (40
mL) and brine
(40 mL), dried (MgS04) and then concentrated. Product was purified from the
residue by flash
chromatography eluting with 1:3 hexaneslethyl acetate to provide benzyI 2-
hydroxy-
2-(4,5-dihydro-4S-phenyloxazol-2-yI)-1S-phenyethylethylcarbamate (0.475 g, 1.1
mmol) as an
oily mixture of diastereomers. MS (PCI) m/z = 445 (M +1). (CZ~H~Nz04).
-114-
CA 02475069 2004-08-20
A solution comprised of benzyl 2-hydroXy-2-(4,5-dihydro-4S-phenyloxazol-2-yI~
1S-phenyethylethylcarbamate (100 mg, 0.22 mmol) in methanol (10 raL) was
placed under a
nitrogen atmosphere and stirred while Pearlman's catalyst (20 mg) was added.
The mixture
was stirred vigorously under a hydrogen atmosphere until the reaction was
complete and then
filtered. The filter was washed with methanol (2 x 25 mL). The combined
filtrates were
concentrated to provided 2S-amino-4-nhenyl-1-f4.5-dihvdro-4S phenvloxazol-
2=yt)butan-1-of
(51 mg, 0.16 mmol) as a clear oil. Ma (PCI) m/z = 311 (M +1). (C~gH~NzO~.
REFERENCE 18
2S-Amino-1-oxazol-2-yl-4-phenylbutan-1-oI
A solution comprised of oxazole (0.25 g, 3.62 mmol) in THF (20 mL) was treated
with
borane tetrahydrofuran complex (3.62 mL, 3.62 mmol) under nitrogen and the
mixture was
stirred for 30 minutes and then cooled to -78°C. .A solution comprised
of sec-butyl lithium (2.78
ml, 3.62 mmol) in cyclohexane was added dropwise and the mixture was stirred
for 30 minutes.
A solution comprised of tert-butyl (,f~~~1-formyl-3-phenylpropylcarbamate
(0.476 g; 1.8I mmol)
in THF (25 mL) was added and the mixture was stirred and allowed to. warm
while the reaction
proceeded to completion. The mixture then was cooled to -78°C, quenched
by slowly adding
5% acetic acid in ethanol (20 mL), allowed to warm to ambient temperature and
stirred for 18
hours. The mixture was concentrated to dryness and the residue was extracted
with ether
(2x25 mL). The combined extracts were washed with brine, dried (MgS04) and
concentrated
to dryness to provide tert-butyl 2-hydroxy-2-oxazol-2-yl-1S-
phenethylethylcarbamate (0.125 g,
0.376 mmol) as a yellow oil.
MS (PCn m/z = 333 (M + 1).
A mixture comprised of tent-butyl 2-hydroxy-2-oxazol-2-yl-1S-
phenethylethylcarbamate
(0.125 g, 0.376 mmol), anisole (0.2 mL) and trifluoroacetic acid (0.6 mL) in
methylene chloride
(20 mL) was stirred at room temperature for 2 hours and then concentrated to
provide
2S amino-1-oxazol-2-yl-4-Q,henylbutan-1-of trifluoroacetic acid salt ( 0.08 g,
0.229 mmol) as a
yellow oil. MS (PC>] zn/z = 233 (M + 1 ).
-115-
CA 02475069 2004-08-20
REFERENCE 19
Methyl 2-(2S-amino-1-hydroxy-4-phenylbut~xazole-4-carboxvlate
A solution comprised of methyl 2-(2S-benzyloxycarbonylamino-1-hydroxy-
4-phenylbutyl)-4,5-dihydrooxazole-4-carboxylate (0.100 g, 0.235 mmol) in
methylene chloride
(3 mL) was cooled to 0° C and then treated with DBU (39 mL, 0.26 mmol)
and
bromotrichloromethane (26 ml, 0.26 mmol). The mixture was stirred for 6 hours
at 0° C,
washed with ammonium chloride (10 mL) and. concentrated. The residue was dried
(MgSO~ to
provide methyl 2-(2S-benzyloxycarbonylamino-1-hydroxy-4-phenylbutyl)oxazole-4-
carboxylate.
MS(PCl7 m/z = 425 (M +1). '
Deprotecting provided methyl 2-(2S-amino-1-h~x~r-
4-nhenylbut-,yl)oxazole-4-carbox lLate.
EXAMPLE 19
Benzyl 1S f2-(4:S-dihydrooxazol-2-yl)-2-hydroxy-1S-phenethylethylcarbamoyll-
3-methvlbutvlcarbamate (Compound 210)
'I N
\ O~H
O
A mixture comprised of ethyl 3-(2-benzyloxycarbonylamino-4-methylvalerylamino~
2-hydroxy-5-phenylpentanimidate (0.327 g, 0.63 mmol), diisopropylethylamine
(0.218 mL,
1.26 mmol) and ethanolamine (38.4 mg, 0.63 mmol) in chloroform (20 mL) was
heated (reflex
temperature) for 3 hours and then stirred at room temperature for
approximately 12 hours. The
mixture was concentrated and the residue was dissolvcd in ethyl acetate (50
mL). The solution
was washed with 0.5 M sodium hydroxide (40 mL) and brine (40 mL), dried
(MgSO4) and
-116-
CA 02475069 2004-08-20
concentrated in vacuo. Product was purified from the residue by flash
chromatography eluting
with 3:1 ethyl acetate/hexanes to provide benzyl 1S-f2-(4,5-dihydrooxazol-2-
vl~-2-hydroxy-
1S-phenethvleth~rlcarbamoyll-3-methvlbutylcarbamate (38 mg, 0.079 mmol) as a
white solid.
MS (PCI] m/z = 482 (M +1). y71333~3~3)'
Proceeding as in Example 19 provided benzyl IS-12-(IX benzoimidazol-2-vI)-
2-hvdrox -~henyethylethylcarbamo~-3-meth l~txlcarbamate (Compound 211);
EXAMPLE 20
Benzyl 1S-12-(4.5-dihydro-4S-phenvloxazol-2-yl)-2-hvdroxy-1S-
phenethyleth~rlcarbamoyl7- .
3-rnethylbutylcarbamate
(Compound 212)
O
\ 0
A solution comprised of 2S-amino-4-phenyl-1-(4S-phenyl-
4,5-dihydrooxazol-2-yl)butan-1-of (51 mg, 0.165 mmol), provided as in Example
18, in DMF:(2
mL) was cooled to 0° C and a second solution comprised of 2,5-
diaxopyrroIidin-1-yl
2S-benzyloxycarbonylamino-4-methylvalerate {0.063 g, 0.174 mmol) and
diisopropylethylamine
(30.3 p,L, 0.174 mmoI) in DMF (3 mL) was added. The mixture was stirred for 2
hours, while
allowing to warm to room temperature, and then concentrated. Product was
purified from the
residue by column chromatography eluting with ethyl 1:1 acetate/hexane to
provide benzyl
1S-!2-(4,5-dihvdro-4S-phen~oxazol-2-yl)-2-h~roxy-1S-phenethylethylcarbamo~rll-
3-methylbut~lcarbamate (34 mg, 0.061 mmol) as a clear oil. MS (PC17 m/z =
558(M +1).
{C33H39"3o3)'
-117-
CA 02475069 2004-08-20
Proceeding as in Example 20 provided the following compounds of Formula I:
ben zyl 1 S-f 2-benzooxazoI-2-yl-2-hvdroxy- I S-phenethylethylcarbam ~1);
3-methylbu-t,~lcarbamate (Compound 213); MS (ESn mIz = 530 (M + I);'H-NMIt
(300 MFiz,
CDCl3,): b 0.65 - 0.7 {dd, 6H), 8 0.98 (d, J = 6 Hz 2H), b I.IO - L55 (m, 3H),
S 1.65 - I.85 (m,
1H), 2.08 (m, IH), b 2.70 (m, 2H), 8 3.99 - 4.I3 (m, IH); b 4.50(~a, IH), 8
4.90 - 5.2I (m, 3H),
8 6.40 - 6.70 {dd,1H), 8 7.05 - 7.35 (m, IOH), 8 7.47 (d, J = 4 Hz, 2H), ~
7.5I (d, J = 2 Hz, 2H),
~~fH3~3~~~
benzvl 1-f 2-(4.S-dihvdro-S-x~henvloxazol-2-vI)-2-hydroxv-1-
ahenethvlethylcarbamovll-
3-methylbutylcarbamate (Compound 2I4);
90 benzvll-f2-(4,5-dihvdro-4S-methyl-SS-phenvloxazol-2-y1?-2-hydroxy-
1 phenvethvlcarbamovll-3-methylbutvlcarbamate {Compound 215);
benzyl 3-methyl-1-(2-hvdroxy-2-naphthof2,3-dloxazol-2 y1-
1-nhenethyleth~Icarbamo~,Lbutyicarbamate (Compound ZI6); MS (ESn m/z = 580 (M
+ 1);
'H-NMR (300 MHz, CDCI~: S 0.65 - 0.95 (m, 6H), S 1.25 (m, 3H), b 1.54 (m, 3H),
8 2.20 (m,
i5 1H), fi 2.82 (t, J = 4 Hz, 2H), 8 4.00 - 4.20 (m, 1H), 8 4.35 - 4.55 (m,
1H), 8 4.90 - 5.09 (m, 3H),
8 6.60 (m, 1H), b 7.23 (m, lOH), s 7.56 (m, 2H), b 7.96 (m, 3H), S 8.18 (s,
IH), (C~H3zN3O3);
benzvl IS-(2-benzooxazol-2-yl-2-hydroxv-1S-phenethylethvlcarbamovl)-
2-methYnro~Ylcarbamate (Comgound 217);
benzvl 1S-(2-benzooxazol-2-Y1~2~,hydroxy-1S-phenethylethylcarbamoyl)-
20 3-methylbutvlcarbamate (Compound 2I8);
benzvl 1S-f2-l4.S-dihydro-4.4-dimethyloxazol-2.;yI~-2-h droxv-
1S phenethvlethylcarbamoyll-3-methyIbutylcarbamate (Compound 2l9), MS(PCI) m/z
~ SIO
(M -rl); 'H NMR (CDCI~: 8 0.8 - 0.99 (d, J = 6 Hz, 6H) , 1.11 - I.35 (m, 6H),
S I.4 -1.78 (m,
' 3H), b 1.82 - 2.01 (m, 2H), 8 2.55 - 2.72 (m, 2H), 8 3.95 (m, 1H), 8 4.0 -
4:25 (m, 3H), 8 4.30
25 (s,1H), b 5.10 (s, 2H), 8 5.35 (s, IH), s 6.58 (m, lI3) 7.1 - 7.37 (m,
lOH); (C29H3sN3O~;
~nethyl 2-f2-(2-benzylox~carbonylamino-4-meth Iy valerylamino)-1-hydroxy-
4-phenylbutyll-4.5-dihydrooxazola-4-carboxyIate (Compound 220), MS(PC>7 m/z =
540 (M +1);
'H NMR (CDCI~: cS 0.8 - 0.99 (d, J = 6 Hz, 6H) ,1.25 (m, 1H), F 1.47 (m, IH)
I.65 (m, ZH),
8 1.99 (m, 2H), 8 2.15 (s, 1H), 8 2.65 (t, J = 4Hz, 2H), 8 3.70 (s, 3H) 4.18
(m, II-~,
30 8 4.25 - 4.50 (m, 3H), 8 4.51 - 4.64 (m, 2H), 8 5.17 (m, 2H), 8 5.35 (d, J
= SHz, IH) 6.65 (d,
J = 6Hz, IH), b 7:17 - 7.45( m, 10H); (C~H37N30~);
-118-
CA 02475069 2004-08-20
methyl 2-f2-f2,2-dimethvlpropionylamino)-4-phenylbut~rylloxazole-4-carboxylate
(Compound 221); MS (ES17 m/z = 373 (M + 1); 'H-NMR (300 MHz, CDCI~: S 1.25 (s,
9H),
& 2.20 (m, 1H), 8 2.46 (m,1H), 8 2.77 (t, J = 4 Hz, 2H), b 3.99 (s, 3H), 5
5.55 (m, 1H); 8 6,41
(d, J = 4 Hz,1H), S 7.20.- ?.38 (m, SH), 8 8.41 (s, 1H), (C~T~IV20s);
tert-butyl 4-f IS-f2-lS-ten-but~benzooxazol-2-yl)-2-h~droxy!-
IS-phenethylethylcarbamoyll-3-methylbutylcarbarnoyllpiperidine-I-carbox l~
(Compound 222);
tert-butyl 4-f 1S-f2-hydroxv-IS-phenethyl-
2- 5-sulfamo Ibenzooxazol-2- 1 eth Icarbamo 1 -3-meth lbu lcarbamo I i eridine-
70 I-carboxylate (Compound 223);
tert-bu 14- 1S- 2-h drox -2-na htho 1 2- oxazol-2- 1-1S- heneth leth Icarbamo
1 -
3-methylbutylcarbamoyl~piperidine-1-carbox~late (Compound 224);
tent-bu 14- 1S- 2-h drox -2-na htho 2.1- oxazol-2- 1-1S- heneth leth lcarbamo
3-meth,~rlbutylcarbamoyllpiperidine-1-carboxvlate (Compound 225);
i5 tent-butyl 4-f 1S-f2-hydroxy-IS-phenethyl-
2-l5-nhenvlbenzooxazol-2-vI)ethvlcarbamovll-3-methvlbutvlcarbamovl)piperidine-
I-carboxvlate
(Compound 226);
tent-butvl4-fIS-(2-benzooxazol-2-vI)-2-hydroxy-IS- hp enethylethyIcarbamovl>~
2methylbutylcarbamoyl"Ipiperidine-I-carbox~ lr_ ate (Compound 227); MS (ESn
mlz = 607 (M +
20 1); 'H-NMR (300 MHz, CDCI3): 8 0.50 - 0.6I (m, 1H), b 0.75 - 0.98 (m, 6H),
8 1.22 (m, 1H),
8 1.41 (s, 9H), b 1.81 - I.85 (m, IH), 8 1:99 - 2.06 (m, 1H), S 2.70 (m, 2H);
4.24 (d, J = 2 Hz
2H), S 4.50 - 4.70 (m, 1H), 8 4.99 - 5.14 (m, 2H), 8 6.96 - 7.81 (m, 15H),
(C34H,~N,,O6);
tert-butyl 3-f 1 S-(2-benzooxazol-2-vI)-2-hydroxy-1 S-
pheneth~rlethvlcarbamoyll-
2-methylbut~carbamoyljb_enzylcarbamate (Compound 228);
25 te_n-butyl 4-f 1S-(2-benzooxazol-2-yl)-2-hey-IS-phanethylethvlcarbamoyl~
2-cyclohexylethylcarbamoyllpiperidine-1-carboxy_late (Compound 229);
benzyl 3-methyl-1 S= j2-hydroxy-1 S-~henethvl-
2-__(5 phenyloxazol-2-yl ethylcarbamoyllbuylcarbamate (Compound 230); MS (ESl7
m/z = 556
(M + I);'H-NMR (300 MHz, CDCt3): 8 0.75 - 0.95 (m, 6H), S 1.25 - 1.80 (m, 5H),
b 2.00 (m,
30 ZH), 8 2.67 (m, 2H), S 4.15 (m, IH), 8 4.55(m, 1H), $ 4.85 - 5.20 (m, 2H),
S 5.50 (m, 1H),
b 6.80 (d, 3 = 6Hz, 1H), 8 7.12 - 7.48 (m, 14H), 8 7. 62 (d, J = 2 Hz, 2H),
(C33H37N3C5)~
pyrid-3-yl 3-methyl-1S-[2-hydroxy-1S-phenethyl-
-119-
CA 02475069 2004-08-20
2-(5-phenyloxazol-2-yl)ethylcarbamoyl]butylcartiamate (Compound 231); MS (ESn
m/z = 527
(M + 1);'H-NMR (300 MHz, CDCl3): 8 0.75 - 0.95 (m, 6H), b 1.45 -1.75 (m, 5H),
b 2.00 (m,
2H), 8 2.67 (m, 2H), S 4.40 - 5.10 (m, 3H), b 5.60(s, 1H), 8 7.00 - 7.47 (m,
10H), 8 7.62 (m,
2H), b 8.15 (m, 1H), 8 8.65 ( m, IH), 8 9.15 (m, IH), (C3,H~N404); and
benzyllS-[2-hydroxy-1S-phenethyl-
2-(5-phenyloxazol-2-yl)ethylsulfamoylmethyl]-2R-methyIbutylcarbamate (Compound
232); MS
(ESn mlz = 606 (M + 1 ); 'H-NMR (300 MHz, CDC13): 8 0.75 - 0.95 (m, 6H), S
1.30 -1.50 (m,
5H), 8 1.98 (m, 2H), 8 2.77 (m, 3H), b 3.55 (m, 2H), 8 4.09 (m, 1H), 8 4.90 -
5.10 (m, 3H),
S 5.60 (m, 1H), 8 7.02 - 7.47 (m, 14H), 8 7.62 (m, 2H), (C33H39N306s)~
t 0 EXAMPLE 21
Benz~rl 3-methyl-1S-(IS_pyrid-2-vJcarbonvl-3-
phenylpropylcarbam~l)but~~lcarbamate
(Compound 233j
0
H
p
p
A solution comprised of 2-bromopyridine (0.291 mL, 3.06 nunoI) in dry 1~ (2
mL)
was cooled to -78° C and then a solution of n-butyllithium (L6 ml,,
2.?2 mmol) in pentane was
added dropwise over 2 minutes. The mixture was stirred at -78° C for 10
minutes and then a
solution of benzyl 1-[1-(N methoxy-N methylcarbamoyl)-3-phenylpropylcarbamoyl]-
3-methylbutylcarbamate (0.3 g, 0.64 mmol) in T.~iF' (2 mL) was added slowly.
The mixture was
_ stirred, while allowing to slowly warm to room temperature, and then poured
into a solution
comprising acetic acid (0.163 mL) in diethyl ether (50 mL). The organic phase
was washed
with brine (40 mL), dried (MgSO~ and concentrated in vacuo. Product was purred
from the
'residue by flash chromatography on silica gel eluting with 1:2 ethyl
acetate/hexanes to provide
-120-
CA 02475069 2004-08-20
benzyl 3-methyl-1-(1-pyrid-2-ylcarbonyl-3-phenylpropylcarhamoyl)butylcarbamate
(82 mg,
0.17 mmol) as a white solid. MS (ESl) mlz = 488 (M + 1); 'H NMR (CDCI~I: 8 0.8
-1.05 (d,
3 = 4 Hz, 6H) , 1.5 (m, IH), 81.6 - L78 (t, 2H), $1.99 - 2.20 (m,1H), 8 2.6 -
2.9 (m,1H),
8 2.55 - 2.85 (m, 2H), 8 4.25 (m, 1H), 8 5.17 (s, 2H), S 5.25 (m, ITS, 8 6.00
(m,1H),
8 6.85 - 6.95 (d, J = lOHz, 1H), 8 7.1 - 7.4 (m, lOH) 7.50( t, J = 4Hz, 1H), s
7.85 (t, J = 6Hz,
IH) 8.01 (d, J = 8 hz, 1H), 8 8.69 (m, 1H). Anal (C~~I~N3O,).
Proceeding as in Example 21 provided the following compounds of Formula I:
bent 1 I- I- 'd-3- Icarbon 1 -3- hen 1 ro vlcarbamo 1 -3-meth lbu lcarbamate
(Compound 234), MS(PCI) m/z = 488 (M +1); 'H NMR (CDCI~: 8 0.8 - LOS (d, J = 4
Hz, 6H)
, 1.5 (m, IH), B 1.6 - 1.78 (t, 2H), 8 I .80 - 2.01 (m, 2H), 8 2.25 (m, IH)
2.6 - 2.9 (t, J =3 Hz,
1H), 8 2.55 - 2.85 (m, 2H), 8 4.30 (m, 1H), 8 5.17 (s, 2H), 8 5.35 (d, J =
6Hz, 1H), S 5.55 {m,
1H), 8 7.02 (d, J = 8Hz, IH), 8 7.1- 7.4 (m, l OH) 8.05( d, J =5 Hz, IH), 8
8.78 (d, J = 4Hz, 1H),
8 9.10 (s,1H); (C~H33N3O~; and
benzvl I-~i-fquinol-3-ylcarbonyl)-3-phenylpropylcarbamovll-3-
methylbutylcarbamate
(Compound 235), MS(PCn m/z = 538 (M +1 ); 'H NMR (CDC13): b 0.8 -1.05 (d, J =
4 Hz, 6H)
1.5 (m, 1H), b 1.6 - 1.78 (m, 2H), S L99 - 2.20 {m,1H), 8 2.6 - 2.9 (m, 1H), 8
2.55 - 2.85 (m,
ZH), S 4.35 (m, 1H), S 5.17 - 5.25 (m, 3H), 8 5.70 (m, IH), S 6.75 - 6.85 (d,
J = lOHz, 1H),
8 7.20 - 7.45 (m, IOH), c5 7.65 (t, J = 6Hz, IH), 8 7.77 - 7.90 (m, 2H), 8
8.22 (d, J = 7, 1H),
$ 8.46 (s, 1H), b 9.4 (s, 1H); (C~H~N30,~.
-121-
CA 02475069 2004-08-20
EXAMPLE 22
BenzY 1-~I-(IH-indol-5-ylcarbonvl)-3-phenylpronvlcarbamoyll-3-meth ~Ibut-
ylcarbamate
(Compound 236)
A mixture comprised of potassium hydride (0.29 g, 2.56 mmol; 67% in mineral
oil) in
anhydrous ether (5 mL) was cooled to 0° C and then a solution comprised
of 5-bromo-lH-indole
(0.5 g, 2.56 mmol) in anhydrous ether (5 mL) was added. The mixture was
stirred for
minutes and then cooled to -?8 ° C under nitrogen. A solution comprised
of ten-butyllithium
(3 mL in pentane, 5.08 mmoI) in anhydrous ether (S mL) was cooled to -
?8° C and added to the
10 indole mixture over 2 minutes. The mixture was stirred for 10 minutes and
then a solution
comprised of benzyl 1-[1-(N methoxy-N-methylcarbamoyl)-3-
phenylpropylcarbamoyl]-
3-methyIbutylcarbamate (0.3 g, 0.64 mM) in ether (IO mL) was added. The
mixture was
allowed to warm to room temperature and then poured into a cold solution at
0° C of phosphoric
acid (25 mL, 1 M in water). The aqueous layer was separated and extracted with
ethyl acetate
15 (25 mL). The organic layers were combined and washed with saturated sodium
bicarbonate
(25 mL), dried {MgSO~ and concentrated. The product was purred from the
residue by flash
chromatograpby on silica gel eluting with I:2 ethyl acetatelhexanes to provide
benzyl
I-[I-(IH-indol-2-ylcarbonyl)-3-phenylpropylcarbamayl]-3-methylbutylcarbamate
(1 I2 mg,
0.21 mmol) as a white solid. MS (ESn mlz = 526(M + 1); 'H NMR (CDCI3): 8 0.8 -
1.05 (d,
J = 4 Hz, 6H) , 1.5 (s, IH), S 1.5 - I.?8 (m, 3H), 8 2.00 (m, 1H), 8 2.4 (m,
lI~, $ 2.65 (m, 2H),
8 4.35 (m; 1H), S 5.17 (s, 2H), 8 5.25 (d,J = 6 Hz 1H), S 5.75 (m, 1H), 8 6.55
{s, 1H) 7.05 (d,
J = 4Hz, IH), 5 7.1 - 7.45 (m, lOH) 7.?( d, J = 4Hz, 1H), 8 8.I5 (d, J = 4Hz,
1H) 8.78 {m, 1H).
(~~35~3~4)~
-122-
CA 02475069 2004-08-20
EXAMPLE 23
benzYl 1-f 1-tbenzofur-2-vlcarbonvl)-3 phen~propvlcarbamoyll-3-
methylbutylcarbamate
(Compound 23'7)
A solution comprised of benzofuran {0.302 g, 2.56 mmol) in anhydrous ether (S
mL)
was cooled to -1S ° C under a nitrogen atmosphere and then a solution
of n-buryllithium (1.6 mL
in hexanes) was added dropwise over 2 minutes. The mixture was stirred for 1
how and then a
solution comprised of benzyl 1-[1-(N rnethoxy-N methylcarbamoyl)-3-
phenyIpropylcarbamoyIJ-
3-methylbutylcarbamate,(0.3 g, 0.64 mmol) in diethyl ether was added. The
mixture was stirred
at -1S ° C until the reaction was complete. The mixture was quenched
with a solution of acetic
acid (0.153 mL) in diethyl ether (50 mL). The organic phase was washed with
brine (40.mL),
dried (MgS04) and concentrated in vacuo. The product was purified from the
residue by flash
chromatography eluting with 2:3 ethyl acetatelhexanes to provide Benz 1
I-f 1-(benzofur-2-ylcarbonyl)-3-phen~propylcarbamoyll-3-methvibutylcarbamate
(?0 mg,
0.13 mmol) as a white solid. 'H NMR (CDC13): 8 0.8 - 0.99 (d, J = 4 Hz, 6H) ,
1.5 (m, 1H),
8 I.6 - 1.72 (m, 2H), 8 1.99 - 2.18 (m, IH), 8 2.22 - 2.41 (m, 1H), ~ 2.6 -
2.75 (m, 2H), 8 4.21
(m, 1H), 8 S.Ol (m, 1H), 8 5.17 (s, 2H), 8 5.50 (m, 1H), 8 6.75 - 6.81 (d, J =
7 Hz, 1H),
8 7.I0 - 7.37 (m, I IH) 7.4 - 7.59( m, , 3H), 8 7.64 (d, J = 7 Hz, 1H).
(C32H~N20s).
Proceeding as in Example 23 provided the following compounds of Formula I:
benzvl 1-f 1-lbenzothiazol-2-ylcarbon Iy )-3=phenylpronvlcarbamovl7-
3-methYlbutvlcarbamate (Compound 238),'H NMR (CDC13): 8 0.91 (d, J = 5.88 Hz,
6H),
81.39 - LS4 (m,1H), fi 1.60 -1.72 (m, 2H), S 2.11 - 2.25 (m, 1H), S 2.40 -
2.54 (m, 1H), b 2.?2
-123-
CA 02475069 2004-08-20
{m, 2H), S 4.21 (m, 1H), 8 5.10 (s, 3H), 8 5.84 (m, 1H), S 6.87 (d, J = 8.15
Hz, IH),
8 ?.10 - ?.40 (m, l OH), 8 7.54 (dt, J = 1.62, 8.1U Hz, 1H), 8 ?.58 (dt, J =
1.46, 7.80 Hz, 1H),
S 7.97 (dd, J = 1.80, 8.15 Hz, l I~, 8 8.17 (dd, J = 1.66, 7.67 Hz,1H);
benzyl 3-methyl-1S-(3-phenyl-1 S-thiazol-2-
vlcarbonvlpropylcarbamovl)butylcarbamate
(Compound 239);
N f3-methyl_-IS-(3-phenyl-1S-thiazol-2-ylcarbonylpropylcarbamo 1)y butvf~
4.meth~nip~-1-carboxamide (Compound 240);
tent-butyl 4-f3-methyl-1S-f3-phenyi-
IS-thiazol-2-vlcarbonvlpropvlcarbamo l~r )bu_tylcarbamoyllpiperazine-1-
carboxylate
(Compound 241 );
benz~3-methyl-1S (3-phenyl-IS-thien-2-
ylcarbonylpropylcarbamoyl)butylcarbaniate
(Compound 242);
benzyl IS-f1S-(1-methyl-1X imidazol-2-ylcarbonyl-3-phenyl_propylcarbamoyll-
3-methylbutvlcarbamate (Compound 243);
benzyl IS (1S-thiazol-2-ylcarbonyI-3-phenylnropylcarbamoyl)-2-
methylprogylcarbamate
(Compound 244);
N f3-methyl-1S-(3-Then"Yl-1S-thiazol-2-
ylcarbonylpr_opylcarbamovl~butvllninerazine-
I-carboxamide (Compound 245);
benzyl 1 S-f 1 S-(4-methylthiazol-2-vlcarbonyl)-3-phenvlpropylcarbamoyll-
3-methylbut,~lcarbamate (Compound 246);
benzyl 1 S-f 1 S-furYl-2-ylcarbonyl-3-phenvlpropylcarbamoyl)-3-
methylbutylcarbamate
{Compound Z47), 'H IVMR {CDCI3): s 0.9I (d, J = 6.I8 Hz, 6I-~, S L42 - 1.?0
(m, 3I~,
81.98 - 2.13 (m, 1H), S 2.19 - 2.37 (m, 1H), 8 2.69 (t, J = 7.60 Hz, 2H), 8
4.22 (m, 1H), 8 5.10
(d, J = 7.76 Hz, 1H), S 5.12 (s, 2H), S 5.54 {m, 1H), 8 6.76 (d, J = 8,15 Hz,
1H), 8 7.16 - ?.36
(m, lOH), 8 7.39 (dt, J = 1.82, 7.86 Hz, 1H), 8 7.47 (dt, J =1.63, 7.79 Hz,
1H), 8 7.69 (s, 1H),
S 7.80 (d, J = 7.15 Hz, I H), 8 7.85 (d; J = 8. I 8 Hz, 1 H);
benzyl 1S-f 1S-(I-benzyl-IH imidazol-2-ylcarbonyl-3-nhe~lpropylcarbamovll-
3-meth3rlbutylcarbamate (Compound 248);
benzvl 3-phenyl-1-(4,5-dihydro~-4S-p,~enyloxazol-2-vlcarbonvl)propyllcarbamate
(Compound 249);
benzvl 3-phenyl-1-(4,5-dihydro-5-nhenvloxazol-2-ylcarbonylpropvllcarbamate
(Compound 250);
-I24-
CA 02475069 2004-08-20
benzvl f 1-f4.5-dihydro-4S-methyl-5S-nhenvloxazol-2-vlcarbonvl)-
3-phenyinropvllcarbamate (Compound 251); and
ethyl 2-12-f2-benzyloxycarbon~ylamino-4-methvlvalerylaminol-
4-phenvlbutyr~rl~thiazole-4-carboxvlate (Compound 252}.
EXAMPLE 24
Methyl 2-f2-(2-benzyloxycarbon~amino-4-methvlvalerylamino)-1-hvdrox"y
4-phenylbutylloxazole-4-carboxylate
(Compound 2.53)
A solution comprised of methyl 2-[2-(2-benzyloxycarbonylamino-4-
methylvalerylamino)-
I-hydroxy-4-phenylbutyl]-4,5-dihydrooxazole-4-carboxylate (0.036 g, 0.067
mmol) in methylene
chloride (3 mL) was cooled to 0° C and then DBU {11.2 mg, 72.7 ~Cmo1)
and
bromotrichloromethane {14.6 mg, 73.7 umol) were added. The mixture was stirred
for 6 hours
at room temperature and concentrated. The residue was dissolved in ethyl
acetate (20 mL) and
the solution was dried (MgSO,) and concentrated. The product was purified from
the residue
by flash chromatography eluting with 1:3 hexaneslethyl acetate to provide meth
2-'~2_-(2-benzylox carbonvlamino-4-ri~ethvlvalerylamino)-I-h~droxy-4-
Qhenvlbutylloxazole-
4-carboxylate (12 mg, 0.022 mmol) as a white solid. MS(PCn m/z = 538 (M +1)'H
NMR
(CDCI~: 8 0.8 -1.05 (d, J = 4 Hz, 6H), 81.55 -1.?0 (m, 3H), 8 2.00 (m, lI~, 8
2.40 (m, III,
~ 2.69 (m, 2I~, 8 3.99 (m, 3H) 4.45 (m, 1H), fi 5.17 (s, 2H), 8 5.78 (m, IH),
& 7.01 (d, J = 4Hz
IH), 8 7.I4 - 7.47 (m, IOH) 7.72( d, J = 4Hz, IH), ~ 8.40 {s, 1H).
(CZ~H~SN30,).
-125-
CA 02475069 2004-08-20
EXAMPLE 25
2-T2-l2-BenzVloxycarbonvlamino-4-methvlvalervlamino)-1-
hvdrox~phenylbutvlloxazole-
4-carboxylic acid
(Compound 254)
A mixture comprised of methyl 2-[2-(2-benzyloxycarbonyiamino-4-
methylvalerylamino)- ,
1-hydroxy-4-phenyIbutyl]oxazole-4-carboxylate (2.I6 g, 4.02 mmol), provided as
in Example 18,
and sodium hydroxide (0.815 mL, 1.63 M in water) in methanol (10 mL) was
stirred for
approximately 12 hours at room temperature, acidified with 1 M hydt~ochloric
acid and
concentrated. The residue was dissolved in ethyl acetate (50 mL) and the
solution dried
(MgSO~. The product was recrystallized from methanol and ether to provide
2-[2-(2-benzyloxycarbonylamino-4-methylvalerylamino~ 1-hydroxy-4-
phenylbutyl]oxazole-
4-carboxylic acid (1.77 g, 3.38 mmol) as an off white solid.
-126-
CA 02475069 2004-08-20
EXAMPLE 26
Benz~l, 3-methyl-112-h~drox~-1-ghenethyrl-
2-(4-vhenvlcarbamovloxazol-2- I~ethylcarbamoyllbutylcarbamate
(Compound 255)
A solution comprised of 2-[2-(2-benzyloxycarbonylarnino-4-meihylvalerylamino~
1-hydroxy-4-phenylbutylJoxazole-4-carboxylic acid (0.05 g, 0.096 mmol),
provided as in
Example 7, in DMF (5 mL) was stirred while PyBOP~ (0.05 g, 0.096 mmoI) and
aniline (9 mg,
0.096 mmoI) were added. The mixture was stirred for an additional 2 minutes
and
t0 diisopropylethylamine (12.4 mg, 0.096 mmol) was added. The mixture was
stirred for 2 hours at
room temperature, poured into cold water 0° C ai and extracted with
ethyl acetate 14 x 30mL).
The extracts were combined, dried (MgSO~ and then concentrated. 'The product
was purified
from the residue by flash chromatography eluting with 1:2 hexaneslethyl
acetate to provide
benzyl 3-methyl-1-j2-hydroxy-1-phenethyl-
2-(4-phenylcarbamoyloxazol-2-yl)ethylcarbamoyl]butylcarbamate (30 mg, 0.05
mmol) as a white
solid. MS (ESI) ) m/z = 599 (M + 1);'H NMR (CDCl3): 8 0.8 - I.05 (d, J = 4 Hz,
6H) ,1.35
(m, IH), b 1.55 (m, 1H), 8 2.00 - 2.15 (m, 2H), 8 2.62 (m, 2H}, 8 2.80 (m,
2H), ~ 3.65 (m, 2H),
8 4.11 (m, 1H), S 4.30 (m, 1H), 8 4.45 (m, 1H), 8 4.95 (s, 1H) 5.17 (s, 2I~, 8
5.2 (d, J =4Hz,
1H), 8 6.70 (d, J = SHz 1H), S 7.1 - 7.45 (m,15H) 7.7( d, J = 4Hz, 1H), 8 8.19
(s, 1H), b 8.99
(s, IH). (C~H3gN40~.
Proceeding as in Example 26 provided the following compounds of Formula I:
-127-
CA 02475069 2004-08-20
benzyl l-f2-!4-benzvlcarbamov6oxazol-2-v)1-2-hvdroxv-1-
nhenethYlethvlcarbamovll-
3-methvIbutylcarbamate (Compound 256), MS (ESn ) m/z = 613 (M + 1);'H NMR
(CDCI~):
b 0.8 -1.05 (d, J = 4 Hz, 6H), 81.25 - I .7S (m, 3H), 8 2.00 - 2.20 (m, 2H), 8
2.69 (m, 2H),
8 3.85 (m,,1H), S 3.95 (m, 1H), 8 4.25 (m, 1H), 8 4.60 (m, 2H), S 4.$0 (s,
IH), 8 S.I7 (s, 2H), S
5.59 (xn, IH), S 6.59 (d, J = 4Hz 1H), 3 7.05 - ?.47 (m, 15H), 8 8.20 (s,
IH);, (C35H,QN,~06); and
benzyl 3-methyl-1-f2-hydroxy-I pheneehyl-
2-!4-ghenvethvlcarbamoyloxazol-2-vI)eth lcy arbamoyllbutylcarbamag (Compound
257), MS
(ESI] ) mlz = 627 (M + 1);'H NMR (CDCl3): 8 0.8 -1.05 (d, J = 4 Hz, 6H), 81.25
- 1.75 (m,
4H), S 2.00 (m, 2H), b 2.59 (m, 2H) 2.88 (m, 2H), $ 3.65 (m, 2H), S 4.02 (m,
1H),. 8 4.25
(m,lH), 8 4.80 (s, 1H), 8 S.I7 (s, 2H), 8 6.59 (d, J = 4 Hz, 1H), 8 7.00 -
?:42 (m, 15H), S 8.20
($, 1~~ (~6H42N4~6)~
EXAMPLE 27
benzyI 1-fI-!4 5-dihvdro-4S-ghenvloxazol-2-vlcarbonyll-3-
phenylpropy)carbamoyl_1-
3-met~lbutylcarbamate
(Compound 258)
0
~~o~
i
A solution comprised of benzyl 1S-[2-(4,5-dihydro-4S-phenyioxazol-2-yl)-2-
hydroxy-
1S-phenethylethylcarbamoyl]-3-methylbutylcarbamate (0.038 g, 0.0?8 mmol),
provided as.in
Example 14, and Dess-Martin Periodinane (0.031 g, 0.072 mmol) in methylene
chloride (5 mL)
was stirred while a mixture of 0.001:1 methylene chloride/water (2 mL) was
slowly added. The
mixture was stirred until the reaction was complete and then concentrated. The
residue was
dissolved in ethyl acetate (50 mL) and the solurion was washed with saturated
sodium
-128-
CA 02475069 2004-08-20
bicarbonate (40 mL), sodium thiosulfate (40 mL, 109'o wdwt), water (40 mL) and
brine (40 mL),
dried (MgSO~ and then concentrated. Product was purified from the residue by
flash
chromatography eluting with-3:1 ethyl acetateJhexanes to provide benzyl I-[1-
(4,5-dihydro-.
4S-phenyloxazol-2-ylcarbonyl)-3-phenyIpropylcarbamoylJ-3-methylbutylcarbamate
(0.0148,
37.5%) as a white solid. MS (PCI] m/z = 556 (M +I)'H NMR (CDC13): S 4.8 -1.05
(d, J = 6
Hz, 6H), 81.4 -1.78 (m, 3H), b 1.87 - 2.12 (m, IH), 8 2.40 (m, 1H), ~ 2.65(t,
J = 4Hz, 2H),
b 4.25 (t, J = 3Hz, 2H), 8 4.75 (t, J = 4 Hz, IH), 8 S.I O (s, ZH), 8 5.40(d J
= 3Hz , 1H), 8 5.50 (t,
J = 4 Hz, IH), 8 6.97 (d, J = 3Hz, 1H) 7.I - 7.49( m, 15H). (C33H37N3o5)'
Proceeding as in Example 27 provided the following compounds of Formula I:
70 benzyllS-(IS-benzooxazol-2-ylcarbonvl-3-phenylpropylcarbamoyl)-
3-methylbutvlcarbamate (Compound 259);
benzyl 1S-f1S-(4.5-dihvdrooxazol-2-ylcarbonvh-3 phenylpropylcarbamoyll-
3-meth~lbutvlcarbamate (Compound 260), MS (PCI) miz = 480 (M +1)'H NM;It
(CDC13):
s 0.8 - 1.05 (d, J = 6 Hz, 6H), 81.4 - 1.78 (m; 3H), 8 1.82 - 2.01 (m, 2H), 8
2.65 (t, J = 5 Hz
2H), 8 2.99 (t, J = 4Hz, IH), 8 3.75 (d, J = 3Hz,lH), b 4.10 - 4.35 (m, 3H), s
4.50 (m,1H),
S 5.17 (s, 3H), S 6.85 (s, IH), 8 7.I - 7.49( m, IOH), (C27H33Na0~;
N 13-methyl-IS-f3-phenyl-
1S-benzooxazol-2-ylcarbonylpropylcarbamo ly )butyllgiperidine-4-carboxamide
(Compound 261),
'H NMR (DMSO-db): b 0.83 (d, J = 6.9I Hz, 6H), fi 1.34 - 1.87 (m, 7H), 8 1.92 -
2.07 (m, IH),
8 2.20 - 2.33 (m, IH), 8 2.41 - 2.54 (m, 1H), 8 2.62 - 2.92 (m, 4H), 8 3.26
(bd, J = 12.12 2H),
8 4.39 (m, 1H), S 5.18 (m, 1H), b 7.16 - 7.33 (m, SH), 8 7.54 (t, J = 7.64 Hz,
1H), 8 7.64 (t, ?.82
Hz, 1H), 8 7.87 (d, J = 8.40 Hz, IH), 8 7.96 (d, J = 7.67 Hz, 1H), 8 8.07 (d,
J = 8.15 Hz,1H),
8 8.29 (hs, IH), 8.8.60 (bs, IH), S 8.76 (d, J = 6.45 Hz, IH);
benzyl 1-lI-f4.~5-dihydro-5-phenyloxazol-2-ylcarbonyl~
3-phenyl_propylcarbamoy113-rnethylbutylcarbamate (Compound 262);
benzyl 1-1I-f4.5-dihvdro-5S-phell-4S-methvloxazol-2-ylcarbonyl)-
3-phenylpropylcarbamoyll-3-methJrIbutylcarbamate (Compound 263);
benzyl_ IS-(1S-phenethyl-2-benzimidazol-2-yl-1-oxoethylcarbamovll-
3-methylbutylcarbamate (Compound 264),'H IVNflZ (CDCl3): 8 0.82 - 0.96 (m, 6~,
8 1.44 - I.75 (m, 3H), S 2.17 - 2.32 (m, 1H), $ 2.43 - 2.56 (m, 1H), S 2.61 -
2.80 (m, 2H), 8 4.55
-129-
CA 02475069 2004-08-20
(m, 1H), & S.I3 (m, 2H), 8 5.35 (d, J = 8.6? Hz, 1H), S 5.70 - 5.88 (m, IH), S
7.00 - 7.42 (m,
14H), 6 7.50 - ?.83 (m, 2H);
benzvl I-f I-(naphthof2,3-dloxazol-2-ylcarbonyl)-3-phenylpron l~carbamoyll-
3-methylbutvlcarbamate (Compound 265);
b methvl2-f2-f2-be,~z,~rloxycarbonylamino-4-methylvalervlaminol-4-
phenvlbutvrvll-
4_,,5-dihvdrooxazole-4-carboxvlate (Compound 266), MS(PCn m/z = 538 (M +1);'H
NMIt
(CDCI~: 8 0.8 - 0.99 (d; J = 6 Hz, 6H) ,1.25 (m, 1H), 8 1.47 (m, 1H} 1.65 (m,
3H), S L99 (ra,
1H), b 2.35 (m, 1H), s 2.65 (m, 2H), 8 3.70 (m, 3H) 4.18 (m, 2H), s 4.55 (m,
1H), 8 5.17 (s,
2H), 8 5.35 (m, IH) 6.75 (m, 1H), 8 7.1? - 7.45( m, lOH}, (C2c,H35N307)'
~ benz I IS- 1S- 4 5-dih dro-4 4-dimeth loxazoi ~- Icarbon I 3- lien 1 ro
lcarbamo 1
3-methYlbutylcarbamate (Compound 267), .MS(PCI} mlz = 508 (Nl +1);'H NMR
(CDCIs}:
S 0.8 - 0.99 (d, J = 6 Hz, 6H), 81.36 (s, 6H), 81.5 (m, iH), 8 1.65 {rn, 2H)
1.82 - 2.01 (m, lIi),
S 2.35 (m, 1H}, 8 2.6 (t, J = 6 Hz, 2H), b 4.05 (s, 2I~, 8 4.25 (m, 2H), 8
5.I0 (s, 2I~, ~ 5.4 (m,
IH), 8 6.75 (d J = BHz, 1H) 7.1- 7.38( m, lOH); (Cz9H~N3~s);
15- benzyllS-(1S-benzooxazol-2-ylcarbonyl-3-phen~propylcarbamovl)-
2-methylpropYcarbamate (Compound 268),'H NMR (CDCl3): b 0.90 (d, J = 6.91 Hz,
3H),
8 0.97 (d, J = 6.94 Hz, 3H), 8 2.06 - 2.25 (m, 2H), b 2.38 - 2.55 (m, 1H), s
2.74 {m, 2H), 8 4.03
(dd, J =1.73, 6.45 Hz, 1H), 8 5.10 (s, 2H), b 5.29 (d, J = 8.67 Hz, 1H), $
S.'73 (m, iH); 8 6.6G (d,
3 = 7.42 Hz, 1H), $ 7.09 - 7.40 (m, l OH), 8 7.4b (dt, J = 1.62, 8.10 Hz, 1H),
8 7.55 (dt, J = L83,
7.76 Hz,1H), 8 7.64 (d, J = 8.06 Hz, IH), 8 7.89 (d, J = 7.46 Hz, IH);
benz~l 1 S-( 1 S-benzooxazol-2~1_carbon~ 1-3-phen,yluronvlcurbamo~I~-
2-meth~butycarbamate (Compound 269),'H NMR (CDCI3): 8 0.88 (t, J = 7.43 Hz,
3H), $ 0.9i
(d, J = 6.67 Hz, 3H), 8 1.04 -1.21 (m, 1H), S I.40 -1.55 (m, 1H), b I.78 -
1.93 (m, IH),
b 2.10 - 2.24 (m, 1H), 8 2.40 - 2.54 {m, IH), 8 2.74 (t, J = 7.60 Hz, 2H), 8
4.06 (t, J = 6.21 Hz,
1H), 8 5.09 (s, 2H), 8 5.29 (d, J = 8.67 Hz, IH), 8 5.72 (m, 1H), 8 6.66 (d, J
= 8.00 Hz, IH),
8 ?.09 - 7.39 (m,10H), 8 7.46 (dt, J = 1.68, 7.80 Hz, 1H), S 7.55 (dt, J =
1.44, 7.56 Hz, IH),
8 7.63 (d, J = 8.04 Hz, 1H), 8 7.89 (d, J = 7.82 Hz,1H);
benzyl 1S-~'IS-(S-chlorobenzooxazol-2-ylcarbonyt)-3-phen,~lgropylcarbamoyll-
3-methylbut,~carbamate (Compound 270),'H NMR {CDCI3): 8 0.90 (m, 6H), 8 1.39 -
1.53 (m,
IH), S 1.59 - I.70 (m, 2H), S 2.07 - 2..21 (m, 1H), S 2.37 - 2.52 (m, 1H), S
2.73 (t, J = 7.91 Hz,
2H), 8 4.20 (m, 1H), 8 5.06 (d, J = 7.91 Hz, 1H), S 5.10 (s, 2H), 8 5.64 (m,
1H), b 6.77 (d,
3 = ?.6? Hz, IH), 8 7.09 - 7.37 (m, I OH), 8 7.53 (dq, J = 1.86, 8.91 Hz, 2H),
& 7.89 {d, J =1.73
-I30-
CA 02475069 2004-08-20
Hz, IH);
N ( 3-methyl-1 S-f 3-phenyl-
1 - 5-chlorobenzooxazol-2- lcarbon 1 ro learbamo 1 but I i eridine-4-
carboxamide
(Compound 271);
N f2-cyclohex ly~-1,S-l3 phenyl-
IS-benzooxazol-2-y~Icarbonylpropylcarbamo ly-,)ethyllpiperidine-4-carboxamide
(Compound 272);
MS (EST) mlz = 54S (M + 1); 'H-NMR (300 MHz, CDCI~, CD30D): 8 0.85 (m, 2H),
8 1.02 - 1.58 (m, 4H), 8 1.40 - 1.71 (m, 7H), S 1.75 - 2.21 (m, SH), $ 2.38
(m, 1H), S 2.51 (m,
1H), 8 2.69 (t, J = 4 Hz, 2H), 8 3.32 {m, 2H), 8 4.39_ (q, J = 6 Hz IH), 8
5.53 (q, J =.3 Hz 1H),
S 7.11 - 7.21 (m, SH), 8 7.24 (s, 1H); 6 7.38 - 7.61 (m, 3H), 8 7.73 (d, J =
6'Hz, 1H), 8 7.82(d,
J = 6 Hz, 1H), (C3zHaoNdC4);
methyl 2-(2-benzyloxvcarbonylamino-4-meth ly valervlamino)-4-phen~ loxazole-
4-carboxvlate (Compound 273);
Benz 1 1- 1- 4- hen lcarbamo loxazol-2- lcarbon 1 -3- hen 1 ro Icarbamo 1 -
3-methylbutvlcarbamate (Compound 274), MS (ESI) ) m/z = 597 (M + 1);'H NMR
(CDGI3):
8 0.8 - 1.05 (d, J = 4 Hz, 6H), b I.55 {m, IH), 81.70 (s, 2H), a 2.00 - 2.20
(m, lI~, 8 2.40 (m,
1H), b 2.69 (m, 2H); b 2,97 (t, J = 4 Hz, 2H), 8 3.70(q, J = 3 Hz, 2H) 4.25
{m, 1H), b 5.17 (s,
2H), $ 5.59 (m, 1H), 8 6.99 (d, J = 4Hz 1H), 8 7.14 - 7.47 (m, ISH) 7.72( d,.J
= 4Hz, 1H),
8-8.47 (s, 1H), 8 8.65 (s, IH), (C34H3~4~~~
benzvl 1-f 1-(4-benzylcarbamoyloxazol-2-~~Icarbonyl)-3,=phenylpr~ylcarbamoyIl-
3-meth Iy butlr)carbamate (Compound 275), MS (ESn ) mlz = 611 (M + 1);'H NMR
(CDC13):
$ 0.8 - 1.05 (d, J = 4 Hz, 6H), S 1.45 -1.70 (m, 4H), b 2.00 - 2.20 (m, 1H), 8
2.40 (m, 1H),
8 2.69 (m, 2H), 8 4.25 (m, IH), 6 4.67 (t, J = 3 Hz, 2H), S 5.17 (m, 3H), S
5.59 {m, 1H), 8 6.85
{d, J = 4Hz IH), s 7.10 - 7.47 (m, 15H), 8 8.47 (s, 1H), (C3sH38N40s);
ten-bu 14- 1S- 1S- 5-tent-bu lbenzooxazol-2- lcarbon 1 -3- hen 1 ro lcarbamo
3-methylbutvlcarbamovl lpineridine-1-earboxylate (Compound 2?6),'H NMR
(CDCl3):
8 0.86 - 0.97 (m, 6H), 8 1.34 - L85 (m, 7H), b 1.38 (s, 9H), $ 1.43 (s, 9H), 8
2.09 - 2.30 (m,
2H), S 2.37 - 2.52 (m, IH), 8 2.72 (m, 4H), 8 4.I 1 (bd, J =12.85, 2H), 8 4.49
(m, 1H), 8 5.66 (m,
1H); S 5.97 (d, J = 7.91 Hz, 1H), 8 6.89 (d, J = ?.67 Hz, 1H), S 7.11- 7.2?
(m, SH); S 7.50 - 7.64
(m, 2H), 6 7.86 (d, J = 1.56 Hz, 1H);
tent-butyl 4-- j i S-f 1 S-l5-sulfamov)benzooxazol-2-ylcarbonyl)-3-
phenv)propvicarbamoyll-
3-methylbutvlcarbamovl~piperidine-1-carboxylate (Compound 277),'H NMR
(CDCI3):.
-131-
CA 02475069 2004-08-20
8 0.85 - 0:96 (m, 6H), 81.37 -1.82 (m, 7H), S 1.42 (s, 9H), 8 2.08 - 2.46 (m,
3H), 8 2.71 (m,
4H), 8 4.02 (bs, 2H), 8 4.56 (m, 1H), ~ 5.38 (bs, IH), 8 5.78 (bs, 2H), 8 6.38
(d,~J = 8.42 Hz,
1H), b 7.07 - 7.25 (m, SH), 8 7.70 {dd, J = 3.48, 8.64 Hz,1H), 8 8.08 (dd, J
=1.73, 8.67 Hz,1H),
8 8.41 ~ (dd, J = 1.49, 3.96 Hz, 1 H);
S N (3-methyl-1S-f3-phen~-
155-tent butvlbenzooxazol-2-ylcarbonyl_lrn~onylcarbamovllbutvl lniveridine-4-
carboxamide
(Compound 278), 'H NMR (DMSO-cte): 8 0.82 {t, J = 6.18 Hz, 6H), b 1.36 (s,
9H), b 1.33 - 1.88
(m, 7H), 81.91 - 2.06 (m, 1H), b 2.19 - 2:34 (m, 1H), 8 2.42 - 2.54 (m, 1H), 8
2.61 - 2.92 (m,
4H), fi 3.27 (bd, J = 12.02 2H), 8 4.39 (m, IH), 8 5.19 (m, 1H), b 7.15 - 7.33
(m~ SH), 8 7.74 (dcl,
J = 1.97, 7.9I Hz, 2H), 8 7.90 (d, J = L83 Hz, iH), 8 8.0? (d, J = 8.15 Hz,
~lH), 8 8.27 (bs, 1H),
8 8.56 {bs, 1H), 8 8.72 (d, J = 6.43 Hz, 1H);
N !3-methyl-1S-L3-phen~=
,~S-(5-sulfamoylbenzooxazol-2 ylcarbonyl)propylcarbamo~Lbutyl lpiperidine-4-
carboxamide
(Compound 279),'H NMR (DMSO-db): 8 0.80 - 0.88 (m, 6H), 8 1.31 - 1.86 (m, ?H),
8 1.92 - 2.05 (m, IH), 8 2.22 - 2.33 (m, 1H), 8 2.41- 2.52 (m, 1H), 8 2.63 -
2.89 (m, 4H), 8 3.26
(bd, J =11.88 ZH), 8 4.40 (m, 1H), 8 5.i3 {m,1H), $ 7.16 - 7.31 (m, 5H), 8
7.57 (s, 2H), 8 8.05
(m, 3H), 8 8.25 (bs, 1H), 8 8.32 (s, 1H), 8 8.55 (bs, IH), 8 8.82 (d, J = 6.18
Hz, 1H), 8 8.88 (d,
J = 6.84 Hz, 1H);
tart-butyl 4-f 1S-(1S-naphthof 1.2-dloxazol-2-ylcarbony,I-3-
phenylpropylcarbamoyl)-
3-methvlbutylcarbamov_l]piperidine-1-carbox~ (Compound 280),'H NMR (CDCI~:
8 0.87 - 0:95 (m, 6H), 81.39 - 1.85 (m, 7H), 8 1.44 (s, 9H), 8 2.I3 - 2.32 (m,
2H), 8 2.45 - 2.60
(m, 1H), 8 2.65 - 2.8i {m, 4H), fi 4.12 (m, 2H), 8 4.53 (m, 1H), 8 5.79 (m,
1H), $ 6.00 (d,
J = 7.94 Hz, IH), 8 6.90 (d, J = 7.67 Hz, IH), 8 7.12 - 7.26 (m, SH), 8 7.56 -
7.8U (m, 3H),
S 7.93 - 8.00 (m, 2H), b 8.52 (dd, J =1.97, 8.00 Hz, 1H);
tart-bu 14- I - 1S-na htho 2 1- oxazol-2- lcarbon 1-3- hen 1 ro lcarba o
3-methylbut~carbamo~jpiperidine-I-carboxylate (Compound 281),'H NMR (CDC13):
8 0.88 - 0.97 (m, 6H), 81.38 -1.86 (m; ?H), 81.43 {s, 9H), s 2.15 - 2.31.(m,
2H), 8 2.43 - 2.57
(m, 1H), 8 2.67 - 2.?9 (m, 4H), 8 4.11 (m, 2H), b 4.52 (m, 1H), 8 5.73 (m,
1H), 8 5.96 (d,
J = 7.94 Hz,1H), 8 6.90 {d, J = 7.91 Hz, IH), 8 7.12 - 7.26 (m, 5H), 8 7.66
(m, 2H), 8 7.85 (s,
IH), 8 7.99 (dd, J =1.85, 7.80 Hz, 1H), 8 8.33 (dd, J = 1.97, 7.94 Hz, 1H);
tart-bu 1ty 4-~ 1S-f 1S-f5-phenvlbenzooxazol-2-ylcarbonyl)-3-
phenylpro~rlcarbamo
3-methylbut~carbamo~~pigeridine-I-carboxylate (Compound 282); MS (ESl~ m/z =
681 (M +
-132-
CA 02475069 2004-08-20
I);'H-NMR (300 MHz, CDC13): s 0.85 - 0.98 (m, 6H), 8 1.43 (s, 9H), b 1.60 -
1.85 (m, SH),
8 2.14 - 2.30 (m, ZH), ~S 2.56 (m, 1H); ~ 2.75 (m, 4H), 8 4.12 (m, 2H), 8 4.52
(m, 1H), 8 5.69
(m,1H), S 5.92 (d, J = 6 Hz, 1H), 8 6.85 (d, J = 6 Hz, ,1H), S 7.13 - ?.26 (m,
7H), S 7.36 - 7.80
(m, 7H), 8 8:05 (s, 1H), (C,,~H~N406);
N 13-methyl-1S-f3-phenyl-
iS- na htho 1 2- oxazol-2- lcarbon 1 ro Icarbamo 1 but 1 i ridine-4-carboxamid
(Compound 283),'H NMR (DMSQ-d~: 8 0.81 (m, 6H), 81.35 - 1.86 (m, 7H), 8 1.96 -
2.11 (m,
1H), $ 2.26 - 2.53 (m, 2H), 8 2.64 - 2.9I (m, 4H), 8 3.26 (bd, J = 11.63 2H),
8 4.42 {m, 1H),
8 5.27 {m, 1H), 8 7.19 - 7.36 (m, SH), 8 7.70 (t, i =_ '~.~1 Hz, 1H), 8 7.83
(t, J = 7.43 Hz, 1H),
8 8.OI (d, J = 8.91 Hz, 1H), 8 8.08 (m, 1H), S 8.18 (d, J = 8:91 Hz, 2H), S
8.27 (bs, 1H), 8 8.39
(d, J = 7.91 Hz, 1H), S 8.56 (bs, 1H), 8 8.75 (d, J = 6.45 Hz, 1H);
N 13-methyl-1S,_[3-phenvl-
1S-fnaphthof2,I-dlbenzooxazol-2-yl_carbonyl)propylcarbamovllbut ~~ltpiperidine-
4-carboxamide
(Compound 284),.'H NMR (DMSO-d6): 8 0.81 (t, J = 6.43 Hz, 6H), 81.34 - I.8?
(m, 7H),
81.97 - 2.12 (m, 1H), b 2.24 - 2.38 (m, 1H), S 2.42 - 2.53 (m, IH), b 2.66 -
2.93 (m, 4H), 8 3.26
(6d, J =10.12 2H), S 4.41 (m,1H), S 5.26 (m, 1H), 8 7.16 - 7.34 (m, SH), b
7.77 (m, 2H), b 7.97
(d, J = 8.91 Hz, IH), 8 8.05 (d, J = 8.86 Hz, 1H), b 8.07 (d, J = 8.64 Hz,
1H), S 8.19 (d, J = 7.91
Hz, IH), 8 8.26 (bs, IH), 8 8.28 (d, J = 7.67 Hz, 1H), 8 8.56 (bs, 1H), 8 8.78
(d, J = 6.43 Hz,
1H),
N 13-methyl-1-[3-phenvl-
I-f5phen~benzooxazol-2~lcarbony~,propvlcarbamo 1L, lbutvl?piperidine-4-
carboxamide
(Compound 285);
benz~ I-II-(4-nhenyeth~!Icarbamoytoxazol-2-ylcarbonyl)-3-
phenvlprapylcarbamoyll-
3-methylbut~carbamate (Compound 286), MS (ESn ) m/z = 625 (M + 1); 'H NMR
(CDCI~:
8 0.8 - 1.05 (d, J = 4 Hz, 6H), 81.50 (m,1H), 8 i.65 (m, 3H), 8 2.00 - 2.20
(m, IH), 8 2.35 (m,
IH), S 2.60 {m, 2H), 8 2.99 (t, J = 4Hz, 2H), S 3.67(9, J = 3 Hz, 2H),4.19 (m,
1H), S 5.17 (s,
2H), ~ 5.59 (m, IH), 8 6.85 - 6.98 (gin, 2H); 8 7.10 - 7.4? (m, 15H), 8 8.43
(s, IH);
(''36'i40"4o6h
benzyl 1-hl -t4-~3-phenxpropxlcarbamoylloxazol-2-vlcarbonyll-
3-nhenylprop~rlcarbamoyl I-3-methXlbutylcarbamate (Compound 287); M5 (ESI) m/z
= 639 (M +
1); 'H-NMR (300 MHz, CDCl3): 8 0.95 (d, J = 6 Hz, 6H), 8 1.50 (m, IH), S 1.65
(m, 3H),
S 2.00 (m, 4H), 8 2.35 (m, 1H), 8 2.67 (m, 4H), 8 3.49 (m, 2H), S 4.20 (m,
1H), 8 5.09 (s, 2H);
-133-
CA 02475069 2004-08-20
8 5.50 (m, 1H), 8 6.85 {m, 1H), 8 7.23(m, 15H), ~ 8.35 (s, IH), b (C3,H42N40~;
ten-butyl 4-f 1 S-(I S-benzooxazol-2-vIcarbonyI-3-phenvlpropylcarbamoyl)-
2-methylbutylcarbamoyl'~piperidine-I-carboxylate (Compound 28$);
tent-butyl 3-f 1S-(1S-benzoaxazol-2-vlcarbonyl-3-phen~propvlcarbam~l)-
2-methvlbutylcarbamoy~llbenzylcarbamate (Compound 289);
N--,L-methyl-1 S-(3-phenyl-
IS-benzooxazol-2;ylcarbonyl_prop I~bamo l,~.tyllpiperidine-4-carboxamide
(Compound 290);
N f2-methyl-1S-(3_,phen-y1-IS-benzooxazol-2-ylcarbonylprop
r~Icarbamo~rl)butvll-
3-aminomethylbenzamide (Compound 291); t
'10 benzy_1 1-11-L-(2-indol-3 ylethyicarbarnoyi)oxazol-2-vlcarbonyll-'
3-nhen~nro~,vlcarbamoyl ~,-3-meth l~r butylcarbarnate (Compound 292); MS (ES)n
m/z = 664 (M +
I); 'H-NMR (300 MHz, CDC13): b 0.94 (d, J = 6 Hz, 6H), b 1.40 -1.70 (m, 6H), 8
2.00 (m,
IH), 8 2.25{m, 1H), b 2.67 (m, 2H), 8 3.09 {m, 2H), b 3.52 - 3.85 (m; 2H), 8
4.20 (na, 1H),
8 5.09 (s, 2H), 8 5.50 (m, IH), $ 6.80 (d, J = 6 Hz, IH), S 6.99 - ?.41(m,
14H), & ?.65 (d, J = 6
Hz, 1H), & 8.35 (s,1H), 8 8.39 (s, IH), (C38H4,N306);
benzyl 1-f 1-(4-meth~carbamoyloxazol-2-ylcarbon l~phenylpropylcarbamoyl~
3-methvlbutlr)carbamate (Compound 293); MS (ESI] m/z = 535 (M + 1); 'H-NMR
(300 MHz,
CDC13): S 0.95 (d, J = 6 Hz, 6H), 8 1.33 - 1.70 (m, 5H), 8 2.00 (m,1H), E 2.28
(m, 1H), S 2.67
(m, 2H), 8 2.99 (d, J = 2 Hz,.3H), $ 4.15 (m, 1H), 8 5.09 (m, 2H), ~ 5.50 (m,
1H), 8 6.88 (m,
IH}, 8 ?.09 -?.38 (m, lOH), 8 8.35 (s, IH), (C2~3~N406);
benzyl 2-I 2-f 2-(2-berizyloxycarbonylamino-4-rnethylvaler-yiamino)-
4-phen l~t~rylloxazol-2=ylcarbo~larninolvalerate (Compound 294);
benzyl 1S-d 1S-f4-(4-benzyIpiperidin-1 ylcarbonyl)oxazol-2 ylcarbonyll-
3-phenylprop~rbamoyl l-3-met~lbu~lcarbamate (Compound 295); MS l~~ m/z = 679
(M +
1);'H-NMR (300 MHz, CDC13): s 0.92 (m, 6H), b I.25 (m, IH), S I.48 (q, J = 4
Hz, 1H),
a 1.52 -1.85 (m, 6H), 8 2.09(m, IH), f 2.36 (m, 1H), 8 2.53 - 2.?? (m, 3H), b
3.03 (t, J = 8 Hz,
4H), 8 4.19 (m,1H), S 4.65 (m, 1H), 8 5.02 - 5.13 (m, 3H), 8 5.53 (m,1H), 8
6.68 (d, J -- 6 Hz,
1H), 8 7.08 - 7.39 (m, 15H), 8 8.28 (s, 1H), (C,oH,~N,Cb);
benzyl 1S-f1S-(4-fur-2 ylmethYlcarbamoyloxazol-2-ylcarbonyl~
3-phenylpropylcarbamoyll-3-methyIbutvlcarbamate (Compound 296); MS (ESl') m/z
= 601 (M +
1); 'H-NMR (300 MHz, CDCI~: 8 0.98 (d; J = 6 Hz 6H), 8 1.58 (q, J = 6 Hz IH),
1.62 (m,
4H), 8 2.00 (m, 1 H), b 2.27 (m, I H), 8 2.76 (m, 2H), S 4.20 (m, 1 H), 8 4.70
(d, J - 4 Hz, 2H),
-I34-
CA 02475069 2004-08-20
8 4.98 - 5.18 {m, 2H), 8 5.56 (m,1H), S 6.82 (m, iH), 8 ?.OS - 7.42 (m,13H), 8
8.32 (d, J = 4
Hz, 1H)~ (C~Har~4o~)~
benzyl 3-methyl-1S-f1S-(4-pyrid-2-vlmethylcarbamovloxazol-2 ylcarbonyll-
3-nhenvInronvlcarbamoyllbutylcarbamate (Compound 297); MS (ESI) m/z = 612 {M +
I);
'H-NMR (300 MHz, CDCl3): 8 0.98 (d, J = 6 Hz 6H), 8 1.4 - 2.I5 (m, SH), 8 2.32
(m,1H~,
8 2.71 (m, 2H), 8 4.21 (m, IH), S 4.75 (d, J = 2 Hz, 2T-i), S 5.09 (m, 2H), 8
5.15 - 5.5 (m, IH),
S 7.10 - 7.38 (m, I3H), S 7.7 (t, J = 4 Hz, IH), 8 7.95 (m, 1H), S 8.32 (d; J
= 4 Hz, IH), 8 8.59
(s~ iH), (~3~Ns~s);
benzyl 3-methyl-1S-f 1S-f4-pyrid-3-ylmethylcarbamoyloxazol-2- Icarbonyl~
3-phenylpropylcarbam~llbutylcarbamate (Compound 298); MS (hSI) m/z = 612 (M +
1);
'H-NMR (300 MHz, CDC13); 8 0.98 (d, J = 6 Hz 6H), 8 1.5 (q, J= 4 Hz, 1H),
81.65 (m, 2H),
8 1.95 (m, 3H), 2.25 b (m, 1H), S 2.68 (m, 2H), 8 4.19 (m, iH), b 4.72 (d, J =
2 Hz, ZJ;3), 8 5.09
(s, 2H), 8 5.41 (m, IH), 8 6.90 (t, J= 2, Hz, iH), b 7.05 - 7.35 (m, l OH), 8
7.46 (m, 1H), ~ 7.72
(d, 3= 6 Hz, 1H), 8 8.31 (d, J = 4 Hz, iH), 8 8.62 (d, J = 4 Hz 1H), 8 8.73
(s, 1H),
(C3oHsiNsDt~:
benzvl 3-methyl-1 S-f 1 S-(4-pyri d-4-ylmethylcarbamoyloxazol-2-ylcarbonyl)-
3-~henylpropylcarbamoyI~butyIcarbamate (Compound 299); MS (ESI) mJz = 6I2 (M +
1);
'H-NMR (300 MHz, CDCI~: b 0.98 (d, J = 6 Hz, 6H), 81.5 (q, J = 4 Hz, IH), 1.65
(m, 2H),
1.95 (m, 3H), 2.25 (m, IH), 2.68 (m, 2H), 4.I9 (m, IH), 4.72 (t, J = 2 Hz,
ZH), S.I1 (d, J= 4
Hz, 2H); 5.43 (m, 1H), 6.92 {d, J= 6 Hz, Ice, 7.05 - 7.35 (m, I 1H), ?.46
(m,1H), 8.33 (d, J =
4 Hz, 1H), 8.58 (m, 2H), (C3aH37Ns~6):
benzvl 1S-11 S-f4-(2-chlorobenzylcarbamoyl)oxazol-2-,~Icarbon,~l-
3-phenylpropylcarbamoyl 1-3-methylbutylcarbamate (Compound 300); MS (ESn mlz =
646 (M +
1);'H-NMR (300 MHz, CDCl3): S 0.98 (d, J = 6 Hz, 6H), 8 1.5 (q, J = 4 Hz, 1H),
81.62 (m,
4H), 1.95 b (m, 1H), 8 2.30 (m, 1H), 8 2.65 (m, 2H), 8 4.19 (m, 1H), 8 4.70
(d, J = 2 Hz, 2H),
8 5.09 (m, ZH), 8 5.47 (m, 2H), 8 6.82 (m, IH) 8 7.05 - 7.45 (m, 14H), 8 8.33
(d, J = 4 Hz, 1H),
(~37~4~~~
benzyl 1 S-11 S-f4-(3-chlorobenzylcarbamoyI)oxazol-2-ylcarbon~l-
3-phenylpropylcarbamoyl l-3-meth l~butYlcarbamate (Compound 301); MS (ESI) m/z
= 646
(M + I);'H-NMR (300 MHz, CDC13): 8 0.98 (d, ~ J = 6 Hz, 6H), 8 1.5 (q, J = 4
Hz, IH), 8 1.62
(m, 4H), S 2.00 (m, 1H), 8 2.25 (m, 1H), S 2.65 (m, 2H), S 4.20 (m, 1H), 8
4.68 (d, J = 2 Hz,
2H), b 5.09 (gin, 2H), 8 5.43 (m, 1 H), 8 8.85 (d, J = 6 Hz, 1 H), 8 7.05 -
7.45 (m,14H), b 8.33
-135-
CA 02475069 2004-08-20
(d, J = 4 Hz,1H)~ (C3sHs~~NaD6)'.
benzvi 1S-( 1S-f4-(4-chlorobenzylcarbamoyl)oxazol-2=ylcarbon
3-nhenvlnro~ylcarbamo~l l-3-methylbut~carbamate (Compound 302); MS (ESl) m/z =
646 (M +
1 ); 'H-NMR (300 MHz, CDCI~: S 0.98 (d, J = 6 Hz, 6H), 8 L5 (q, J = 4 Hz, 1H),
S 1.62 (m,
4H), s 2.00 (m, IFij, 8 2.25 (rn, 1H), ~ 2.65 (m, 2H), $ 4.20 (m, IH), S 4.68
(d, J = 2 Hz, 2H),
8 5.09 (m, 2H), 8 5.43 (m, 1H), b 6.85 (m, 1H), S 7.05 - 7.45 (m, i4H), 8 8.33
(d, J = 4 Hz, 1H),
(~sHs~C~sD~;
benzyI 3-methyl-IS-( IS-f4-(ZS-nhenylcyclonron-1S-vlcarbarnoylloxazol-2-
ylca~bonyll-
3-vhenvlprow)carbamoyl~ 3-methvlbutylcarbamatg (Compound 303); MS (ESl) m/z =
637 (M +
1 ); ~'H-NMR (300 MHz, CDC13): b 0.92 (d; J = 6 Hz, 6H), 81.46 -1.78 (rri,
6H), b 2.00 (m,
3H), 3 2.31 (m, III, S 2.67 (m, ZH), ~ 2.99 - 3.22 (m, 1H), 8 4.20 (rn, IH),
.8 5:04 .(d, J = 6 Hz,
IH), S 5.11 (s, 2H), 8 5.54 (m, 1H), S 6.87 (m, IH), S 7.08 - 7.47 (m, 1~SH),
S 8.30 (d, J = 2 Hz;
l~s (C374O~~
benzvl 3-methyl-1S-f 1S-(4-dinhenvlmethylmethvlcarbamovloxazol-2-ylCarbonvll-
3-phenvlpropylcarbamoyll-3-methylbutytcarbamate (Compound 304); MS (ESn m/z ~
68? (M +
1); 'H-NMR (300 MHz, CDC13): 8 0.98 (d, J = 6 Hz, 6H), 81.48 (q, J = 4 Hz,
1H), 81.62 (m,
2H), S 2.00 (m,1H), b 2.30 (m, 1H), 8 2.67 (m, 2H), b 4.18 (m, 1H), 8 5.09 (m,
3H), 8 5.43 (m,
1H), b 6.42 (d , J = 6 Hz, 1H), 8 6.80 (d, J = 6Hz, 1H), 8 7.02 - 7.72 (m,
20H), 8 7.79 (d, J = 6
Hz, IH), E 8.33 (d, J = 4 Hz, IH), (CaaH4zNa~s)~
benzyl 1 S-f 1 S-(4-adamantan-1 ~rlmethylcarbamoyioxazol-2-vicarbonyll-
3-phenvlpropylcarbamoyll-3-methvibutylcarbamate (Compound 305); MS (ES>] m/z =
670 (M +
1 ); 'H-NMR (300 MHz, CDCI3): 8 0.92 (m, 8H), 8 L I B - 1.78 (m, 16H), S 2.00
(m, 1H), 8 2.31
(m, 1H), S 2.67 (m, 2H), 8 2.99 - 3.09 (m, 2H), 8 4.21 (m, 1H), 8 5.11 (m,
3H), 8 S.SI_ (m, lI~;
S 6.87 (m, 1H), 8 7.02 (m, 1H), 8 7.08 - 7.47 (m, lOH), S 8.31 (d, J = 2 Hz,
IH), (CN40,~;
benzyl 1-f I-f4-(1-methylethylcarbamoyl)oxazol-2-ylcarbonyI1-
3-phenylpropylcarbamoyl}-3-meth"ylb~lcarbamate (Compound 306);
benzvl 1-i 1-f4-(IS phenvlethvlcarbamoyl oxazol-2-ylcarbonyll-
3-phenvlpron lc~bamoyl )-3-methylbutylcarbamate (Compound 307); MS (ESn m/z =
625 tM +
I); 'H-NMR (300 MHz, CDCI3): 8 0.92 (d, J = 6 Hz, 6H), S 1.54 - I.65 (m, 7H),
8 2.00 (m,
IH), 8 2.25 (m, IH), S 2.65 (m, 2H), 6 4.15 (m, 1H), 8 4.99 (d, J = 2 Hz, 1H),
i; 5.09 (s, 2H),
S 5.32 (m, 1H), 8 5.43 (m, 1H), 8 6.79 (d, J = 6 Hz, 1H), 8 7.05 - 7.45 (m,
15H), 8 8.3I (s, 11~,
(~4~4~6~i
-I36-
CA 02475069 2004-08-20
benzyl 1-I I-f4-!1R-phenvlethvIcarbamoyIZoxazol-2-yl_carbon-,yll-
3;phenylpropylcarbamoyl )-3-methylbut~carbamate (Compound 308); MS (ESI) mlz =
625 (M +
1); 'H-NMR (300 MHz, CDCI~: 8 0.92 (d, J = 6 Hz, 6H), S 1.45 -1.68 (m, 7H), 8
2.00 (m,
1H), 8 2.25 (m, IH), 8 2.65 (m, ZH), f 4.15 (m, 1H), 8 4.99 (d, J = 2 Hz, 1H),
8 5.09 (s, ZH),
S 5.32 (m;1H), 8 5.43 (m,1H), S 6.79 (d, J = 6 Hz; S 1H); 8 ?.O5 - 7.45 (m,
15H), 8 8.31 (s,
1H), (C~H,,aNdO~;
benzvl 1-I 1-f4-tN benzyl-N methylcarbamoyl)oxazol-2~ylcarbonyll-
3-phenylprop,~lcarbamovl 1-3-met~lbutylcarbamate (Compound 309); MS (ESI) mlz
= 625 (M +
1 ); 'H-NMR .(300 MHz, CDCl3): 8 0.9G (d; J = 6 Hz, 6H), S L27 -1.68 (m, 4H),
8 2.00 (m,
1H), S 2.25 (m, 1H), 8 2.65 (m, ZH), i; 3.I0 (s, IH), 8 4.19 (m, 1H), S 4.71
~(s , 2H), S 5.09 (s,
2H), 8 5.22 (m, 1H), b 5.43 (m, IH), 6 6.99 (d, J = 6 Hz, 1H), S 7.OS - 7.45
(m, 1SH), 8 7:60 (m,
IH), 8 8.31 (s, IH), (C36H40N4~~~
benzyl I-f I-(4-pyrrolidin-I-ylcarbonvloxazol-2-vlcarbonvl)-3
phenyprop~rlcarbamovll
3-methylbut5rlcarbamate (Compound 310); MS (ESI) mlz = S7S (M + I); 'H-NMR
(300 MHz,
CDC13): 8 0.93 (d, 1= 6 Hz, 6H), 51.45 -1.73 (m, 3H), b 1.85 - 2.I2 (m, SH), 8
2.34 (m, 1H),
S 2.64 (m, 2H), b 3.62 {t, J = 4 Hz, 2H), 8 3.82 (m, 2H), b 4:21 (m, 1H), 4.99
- 5.11 (m, 2H),
8 S.SS (m, 1H), 8 5.43 (m, 1H), $ 6.79 (m, IH), 8 7.OS - 7.45 (m, lOH), 8 8.31
(d, J = 2Hz, 1H),
(~~38N4~6)i
benzvl I-f 1-(4-vineridin-I-vlcarbonvloxazol-2- lcy arbonyl)-3-
phenvlpropylcarbamoyll-
3-meth l~tylcarbamate (Compound 311); MS BSI) ~z = 589 ~ + 1); 'H-NMR (300
MHz,
CDCI3); 8 0.90 (d, J = 6 Hz, 6H), 8 x.25 (m, 2H), S 1.49 -1.66 (m, 6H), 8 2.12
(m, 1H), b 2:34
(m, IH), S 2.64 (m, 2H), 8 3.65 (m, 2H), 8 3.85 (m, 2H), 8 4.17 (m, 1H), 8
4.99 - 5.11 (m, 3H);
8 S.SS (m, 1H), b 6.67 (m, 1H), 8 7.08 - 7.39 (m, I1H), S 8.27 (s, IH),
(C33H,,oN4O~;
benzvl I-f I-f4-f2.3-dihydroindol-1-vlcarbonvl)oxazol-2-ylcarbon,~ll
3-nhenvlnropyl_carbamoyl ~,3-methylbutvlcarbamate (Compound 312);
benzvl l-I 1-f4-t3.4-dihvdro-1H-isoauinol-2-vlcarbon~~oxazol-2-ylcarbonvll-
3-phenvlprogylcarbamoyl )-3-meth~b-utvlcarbamate (Compound 313); MS (ESI} mlz
= 637 (M +
1); 'H-NMR (300 MHz, CDCl3): 8 0.90 (d, J = 6 Hz, 6Hj, b 1.25 (m, 2H); 81.45 ~
1.79 (m,
4H), b 2.11 (m, IH), 8 2.40 (m, IH), S 2.68 (m, ZH), 8 2.95 {t, J = 4 Hz, 2H),
8 3.96 (t, J = 4
Hz,1H), 8 4.15 (m, 2H), S 4.86 (d, J = 6 Hz, 1H), S 4.99 - S.I 1 (m, 3H), 8
5.59 (m,1H), 86.70
(m, 1H), a 7.OS - 7.45 (m, I2H), 8 8.35 (s, 1H), (C37H~406)s
benzvt 1-1I-f4-(3.4-dihvdro-2H-quinol-I-yl-carbonyl)oxazol-2-ylcarbon ~~11-
-137-
(d, J = 4 Hz,1H)~ (C3sHs~~NaD6)'.
CA 02475069 2004-08-20
3-nhenLrlnropylcarbamovI 1-3-meth l~tylcarbamate (Compound 314); MS BSI) ~z =
637 ~ +
1); 'H-NMR {300 MHz, CDCI3): 6 0.90 (d, J = 6 Hz, 6H), 8 1.25 (m, 2H), 81.40 -
1.69 (m,
3H), 8 2.05 (m, 2H), 8 2.52 (t, J = 6 Hz, 2H), 8 2.82 (t, J = 4 Hz, 2H), 8
3.80 - 4.21 (m, 4H),
8 4.86 (d, J = 6 Hz, 1H), 8 5.09 (s, 2H), 8 5.21 (m, 1H), 8 6.62 (m, lI~, b
6.85 - 7.31 (m, 11I~,
8 7.SI (m,1H), 8 7.67 (m, 1H), 8 8.31 (s, IH), (C37HaoN4~6)i
benzvl 1-f 1-(4-naphth-1-ylmethylcarbamoyloxazol-2:ylcarbon,~,>~.
~nhen ly nronyl_carbamoyll-3-methvlbutylcarbamate (Compound 3I5); MS (ESI) m/z
= 661 (M +
1); 'H-NMR (300 MHz, CDC13): 8 0.90 (d, J = 6 Hz, 6H), 8 I.25 (m, 2H), 81.54
(m, 3H),
8 2.05 (m, iH), 8 2.59 (t, J = 6 Hz, 1H), 8 2.82 (t, J = 4 Hz, 2H), 8 4.12 (m,
1H~ S 4.90 - 5.09
(m, 4H), 8 5.34 (m, 1H), 8 6.71 (m, 1H), 8 6.95 - 7.12 (m, 3H), 8 7.27 (m,
lOH), 8 7.51 (m, 2H),
8 7.88 {t, J = 6 Hz, 1H), 8 8.06 (d, J = 6 Hz, 1H), b 8.35 (s, IH),
(C3gH,,~N40,~;
tent-butyl 4-f 1S-(1S-benzooxazol-2-vlcarbonvl)-3-phenrr_lprogylcarbamoyl)-
2-cyclohexylethylcarbamoyllpi~erid~ne-1-carboxylate (Compound 316);
1 S-11S-f4-(3,4-dihydro-2H-auinol-1-ylcarbonvl)oxazol-2-ylcarbonyll-
ethvlcarbamoyl 1-
3_-methylbutylcarbamate (Compound 317);
benzvl 3-meth-1 S-f 1 S-(5-ghenyloxazol-2-ylcarbon,Yll-
3-phenylprop~carbamoyllbutylcarbamate (Compound 318); MS (ES)] mlz = 554 (M +
1);
'H-NMR (300 MHz, CDCI3): 8 0.97 (d, J = 4 Hz, 6H), 8 1.50 (t, T = 4 Hz, 1H), 8
1.65 - 1.82
(m, 3H), b 2.20 (m, 1H), 8 2.48 (m, 1H), 8 2.75 (t, J = 4 Hz, 2H), 8 4.2? (m,
1H), 8 5.09 (s, 2H),
8 5.65 (m,1H), b 6.85 (d, J = 6Hz, 1H), 8 7.12 - 7.G2 (m, 14H), 8 7. 77 (d, J
= 2 Hz, 2H),
{~3H35N3o3)i
pvrid-3-y1 3-methyl-1 S-11 S-f S-phenyloxazol-2-vlcarbonvl)-
3-phenylpro~vlcarbamoyllbutylcarbamate (Compound 319); MS (FS~ m/z = 525 (M +
1);
'H-NMR (300 MHz, CDCI3): S 0.80 - 1.05 (m, 6H), 8 1.27 (m, 3H), 8 1.72 (m,
3H), 8 2.I5 (m,
IH), 8 2.46 (m, 1H), 8 2.77 (t, J = 4 Hz, ZH), 8 4.75 (m, 1H), 8 5.65 (m, IH);
8 6.95 (d, J = 4Hz,
1H), 8 7.02 (d, J = 4Hz, 1H), 8 7.09 - 7.35 (m, SH), 8 7.37 - 7.62 (m, 3H), 8
7.80 (d, J = 4 Hz,
1H), 8 8.15 (d, J = 6Hz,1H), 8 8.75 ( m, 1H), 8 9.09 (s, 1H), (C3,H~N40~;
benzyl 1S-f 1S-(5-phe~rloxazol-2-ylcarbon~
3-phenylpronYlsulfamo_ytmethyll-2R-methylbut~rlcarbamate (Compound 320); MS
(ESl] m/z =
604 (M + 1);'H-NMR (300 MHz, CDC13): i; 0.95 (m, 6TH, 8 1.25 (m,1H), $ L49 (m,
lHj,
81.65 (m, 1H), 8 2.15 (m, 1H), 8 2.48 (m, iH), 8 2.85 (m, 2H), 8 3.12 (m, 2H),
8 4.46 (m, 1H),
8 4.99 (d, J = 8Hz, 1H), 8 S.I2 (m, 3H), 8 6.32 (d, J = 6Hz, 1H), 8 7.19 -
7.55 (m, I4H), 8 7.76
-138-
CA 02475069 2004-08-20
(Tn~ ~~ {~~37N3~6'S~i
benzyl 3-meth~rl-1 j2-hvdroxy-1-phenethyl_~
4- 3- hen 1 ro Icarbamo I oxazol-2- I eth lcarbamo I bu lcarbamate (Compound
321);
benzyl_I-T2-hydroxy-2-f4-(2-indol-3- ly ethylcarbamo lY)oxazal-2-vli-
I-phenerhyleth~lcarbamo-yl~-3-methylbutvlcarbamate (Compound 322); MS (ESI)
m/z = 666 {M
+ 1); 'H-NMR (300 MHz, CDC13): 8 0.90 (d, J = 6 Hz, 6fI); 81.40 - L80 (m, 6H),
8 2:(10 (m,
1H); 8 2.6? (m, 2H), S 3.09 (m, 2H), & 3.52 - 3.85 (m, ZH), 8 3.99 - 4.20 (m,
ZH), 8 4.26 - 4:44
(m, IH), S 4.81 (s, 1H), S 5.09 (s, 2H), ~ 5.50 (m, IH), 8 6.?2 (d, J = 6 Hz,
IH), 8 6.99 - 7.41
(m, 14H), 8 8.18 {s, IH), 8 8.39 (s, 1H), (C3$H43IVs0~;
benzvl3-methyl-1-f2-hydroxy-2-(4-methylcarbamoyloxazol-2-vl)-'
1-phenethvlethylcarbamoylibuty,~carbamate (Compound 323);, MS (ESn mlz = 53?
(M + I);
'H-NMR (300 MHz, CDC13): 8 0.90 (d, J = 6 Hz, 6H), b L33 - L80 (m, 6H), 8 2.00
(m,
1H), 8 2.b7 (m, 2H), 2.89 (m, 3H), 8 4.10 (m, 1H), 8 4.25 (m, 1H), 8 4.81 (s,
1H), 8 5.09 (m,
3H), 8 6.68 (d, J = 4 Hz, IH), 8 ?.09 - ?.38 (m, l OH), 8 8.18 (s, IH),
(CZgH3bN4~,~}i
b_enzvl 2-f 2-f2-(2-henzyloxvcarbonylamino-4-rnethvlvalerylamino)-I-hydroxy-
4-phenvlbutylioxazol-2-vlcarbonylamino? valerate (Compound 324); MS (ESn inlz
= ?2? (M +
I);'H-NMR (300 MHz, CDC13): 8 0.95 (m, 12H~, fi 1.45 - 1.80 {m, 9H), S 2.00
(m, IH), $ 2.6?
(m, 2H); 8 3.99 - 4.15 {m, 2H), b 4.85 (m, 2H), 8 5.09 (m, 4H), 6 5.50 (m,
IH), b 6.88 (m,1H),
8 ?.12 - ?.45 (m, ISH), S 8.18 (s, lI-~, (C4~H~IV408);
benzyllS-12-f4-(4-benzylpiperidin-1-vlcarbonyl)oxazol-2-yli-2-hydroxv-
1S-phenethvlethvlcarbamovl~-3-methvlbu"t~lcarbamate (Compound 325);
benzvl 1 S-f 2-l4-fur-2-vlmethylcarbam ovl ox azol-2-~rI)-2-h)rdroxv-
IS-phenethvlethvlcarbamovll-3-meth~rlbutylcarbamate (Compound 326);
benzyl 3-methyl-I S-T2-hydroxy_1 S-nhenethyl:. .
2-(4-pvrid-2-ylmethylcarbamoyloxazol-2=ylleth~)carbamoylibu~t rlcarbamate
(Compound 32?);
benzyl 3-methyl-1S-f2-hydroxY lS~henethyl-
2_-_(4-pyrid-3-ylmethylcarbamoyloxazo~-2-~I ethylcarbamoyllbutylcarbamate
(Compound 328);
benzvl 3-methyl-1S-f2-hydroxy-1S-pheneth_yl-
2-(4-pvrid-4 ylmeth~rlcarbamoyloxazol-2-yl)eth~rlcarbamo-yl~butvJcarbamate
(Compound 329);
benzvl3-methyl-1S-(2-f4-(2-chlorobenzylcarbamoyl)oxazol-2-vll-2-hydroxv-
1S-phenethyleth~bamoyllbu~lcarbamate (Compound 330);
benzvl 3-methyl-1S-f 2-14-(3-chlorobenzylcarbamovI)oxazol-2-~~Il-2-hydroxv-
-139-
CA 02475069 2004-08-20
IS-nhenethylethvlcarbamoyl Ibut~carbamate (Compound 331);
benzvl 3-meth~rl-1S-I2 j4-(4-chlorobenzylcarbamovl)oxazoI-2-y11-2-hvdroxv-
1 S-nheneth,Xlethvlcarbamoyl }but~carbamate (Compound 332);
benzvl 3-methyl-1S-{,2-hvdroxv-1S-ahenethvl-
- 4- 2R- hen lc clo ro -1S- Icarbamo 1 oxa ol-2- 1 eth lcarbarno 1 bu
lcarbamate
(Compound 333);
benzyl 1 S-f 2-(4-adamantan-1-ylmethylcarbamovloxazol-2-vl~
2-hvdroxv-methyl)-IS-nhenethvlethylcarbamovlT-3-methvlbutylcarbamate (Compound
334);
benzvI 3-methy-1 S-f2-~droxy-I S-phenethyl-2-(4-dinhenylmethylcarbamoyloxazgl-
2-vllethylcarbamo ly lbutylcarbaxnate (Compound 33~);
benzyI 3-methyl-1-I2-hydroxv-2-f4-(I-methyIethylcarbamovl~oxazol-2-yll-
I- h~ enet~lethyl_carbamoyl lbutvlcarbamate (Compound 336);
benzvl 3-methvt-1-I2-hydroxy-I-phenethvl-
2- 4-f , (IS:phenvlethylcarbamoyl,~oxazol-2- lY lethylcarbamovl
lbutylcarbamate (Compound 337);
benzyl3-methyl-I-I2-hxdroxy-I-ghenethvl-
2-f4-(IR_phenvlethylcarbamovl)oxazol-2- ly lethylcarbamoyl but rLlcarbamate
(Compound 338);
benzSrl 3-meth~I-1~2-f4-(N benzyl-N meth lcarbamoylOoxazol-2- ly 1-2-hydroxy_
I-nhenethvlethylcarbamoyl Ibut~lcarbamate (Compound 339);
benzyI 3-methyl-I~j2-hydroxv-1-ohenethyl-
2~gyrrolidin-I-vlcarbonyloxazol-2-~rl~eth~rlcarbamoylibutylcarbamate (Compound
340);
benzvl 3-methyl-I-f2-hydroxv-I-nhenethvl-
2 S4-pi~eridin-1-~carbonvloxazol-2-vi~ethylcarbamoyllbutylcarbamate (Compound
34I);
benz~ 3-methyl-I-( 2-~4-(2,3-dihydroindol-1 ylcarbonyl)oxazol-2_yl_]-2-
hydroxy_
1-phenethylethvlcarbamovl Iburylcarbamate (Compound 342);
benzvl 3-methyl-I-I2-f4-X3,4-dihydro-IH isoqninol-2-ylcarbonyl~oxazoI-2- ly 1-
2-hvdroxy
l~henethylethvlcarbamovllbut~rlcarbamate (Compound 343);
bent I 3-meth 1-I- 2- 4- 3 4-dih dro- H- uinot-1- Icarbon- 1 oxazol-2- 1 -2-
dr x -
1-phenethylethylcarbamovl Ibutylcarbamate (Compound 344);
benz~rI3-inethvl-I-f2-hydroxy-2-(4-naphth-1-ylmeth l~bnyloxazol-2-yl)-
I-pheneth~eth~lcarbamovllbuylcarbamate (Compound 345); and
benzyl 1S-I2-f4-(3,4-dihvdro-2H quinol-I-~ibonyl)oxazoi-2_,yll-2-hydroxv-
IS-methylethylcarbamovl I-3-rrZethylbut~carbama~e (Compound 3415).
-144-
CA 02475069 2004-08-20
.. v vv..wa. . .
Proceeding by methods analogous to those described above provided the
following
compounds of Formula I:
N f3-methvl-?S-f1S-thiazol-2-ylcarbon;rlethylcarbamoyllbutyll~
4-moz~holin-4-ylbenzamide (Compound 34?); and
N ?S- 2-benzooxazo -2- 1-1 1-dimeth l-2-oxoeth lcarbamo 1 -3-meth Ibu f - .
4.-(4-methvlniperazin-1-yl~benzamide (Compound 348).
Proceeding by methods analogous to those set forth in this Application
compounds of
Formula I are provided which are comprised by the elements A, B, C and D
listed in the
following Table 1.
, TABLE 1
A B " R,i C ~ ~ ~ D
_ ~ ~~x A Its .
- ~~(
rt'
A1 B1 Ci ' Dl
f l
.o
r
C« /
''o _ N ,..-
* * .,
Nf ~C *N c*
H O ~t o
A2 82 / ~ C2 . D2,
o ~
0
~.-,
~ ,C, * *N C*
H O
8 O
I I i 1 ~ I ~ 6
_~4~-
CA 02475069 2004-08-20
A3 B3 ~ C3 D3
O O
O.vr
*~ C* N
H O
8 0
A4 B4 C4 D4
I N *N C,k 01
F i ~ » o ~N''
*N ~*
. a a
8 O
AS BS / ~ CS DS
H ~ *N c* NJ
N* C*- i~ a~ '
l a
H O
A6 ~ ' ~ B6 F o F C6 ~ , D6
l ~ O1
w
~S . ~, N
~O
*N i~~"'
H O .~ O
A? B7 / C? ~ D7
o O \ 0.:*
NJ
*N r*
N* C*
a 8 O
H O.
-142-
CA 02475069 2004-08-20
~~ V VVI~7Jt~
AS o B8 / ~ C8 D8
F . *N . C*
'J
8 O
Ni '(;*
A9 B9 N i ~ C9 D9
~/
0
I *N/~.O* <D
I II .
8 O
*~
Nj IC
H O
A10 B10 N C10 D10
o ~
N~NH
o,
*N~C.~*
1 II
*~ * 8 O
N~ IC
All B11 N Cll Dil
i
p \~ O.
~p~ ~yS~O (~ NH
N* C*
N~ IC* H O
H. O
i
-143-
CA 02475069 2004-08-20
A12 B12 N~ ~ C12 D12
~ ~ O_S,, o
O~S~ O HN-~C
.O ~ .NH .
~ N* C,
N! n H O
' H
Al3 B13 ~ C13 ~ D13
~ *.O,
O~S~~O NL~C* ~N 1
~ .
*~ * H O
N! C
H O
A14 B14 C14 D14
0
O, S''
O *~ * i N
N I ~C'
* H
*N c O
m
H O
A15 B15 ~ C15 D15
O
w O' S.~O . N I 'C' I : *''N
W
H O
*N C*
s n
A16 B16 ~ C16 i D16 .
O N ! SN
*~ * * *
NH O NH O ~ '
CA 02475069 2004-08-20
A17 B17 CI7 D17
o D ~~
NI C*
e~
*N e* H o
a O
AI8 i B18 ~ C1$ '~ D18
I / ° ~ 1 ~gIN ..
F *yq C~
N* C* r 1~
H ~ ~ $ O
A19 B19 S CI9 D19
~ /
o~ o
>~ ~ N* c~ ;N
o r
y i ,~ " °
N* C'
( n
H
Azo szo , CZO Dza
~ ~
o wI N
°;S " <~ ~~
~ ~ a ~ ~ NH O'k
N' C*
I rr
H O
-145-
CA 02475069 2004-08-20
azi B2i ~ cm Dzi
~N
o . O
o~~r
N
NH O NI ,C*
H O
,~2 B22 O C22 D22
O ~ / N
1 . O,, .O s
O S *~ I
N*~~G,* ,
* ~ 1~
l NI n M O .
H O
A23 ~ B23 / ~ C23 D23
\ Ohl
_ *I
N* C* N* C*
H p H O
A24 B24 ' ~ ~ C2~E ~ D24
O
~ NJ * o
\ ~F
*
N~ C'"
C~ NH ~ H p
I I I J I i I
CA 02475069 2004-08-20
11 V VVIJJt'ri
A25 B25 / C25 D25
N ~/
O . .. <O,
S I F N..
r \ I N* . C* NH OC*
I ii
H O
A26 B26 ~ ~ a6 ~6
OH
F
F
*
N I ~~
H O
N* C*
I n
H O
p27 B2~ ~ C27 D27
r N OH
t . F *~
Q
* *
F
r v N~ ,~
H o
N* .~ C*
n
H O
A28 B28 C28 D28
y
~ ,,,.
c pH
I i \ F F
NI ~C*
H O
N* C* ~ v
I il
H O '
I 1 L l I 1 I I I
-147-
CA 02475069 2004-08-20
WVUUI~'d~1°1 _ avarv~avwvvw..
A29 B29 ~ C29 D29
i N a
y /.,
... O . NI c*
er
* H
NI oC;
H O
A30 B30 C30 D30
O NJ ~ ".N\
° ~N~
i ~ ,.,,
~*
8~ N* Cr* ee
n H O
H O
A31 B31 F F C31 D31
F ~'
\ N O-- H
r
H s I O y. N N
O
a.. *...J
*~ * H
N~ ~C
H O
A32 B32 C32 D32
o . N~ o-H
O ~ /: ,N
N. c. Nf C* ~l~ ~~
H O e~
H O
A33 B33 C33 D33
*-N'
NH ; r. ~ ~
NH O
*
N) ~~
H O
I 1 1 I I I 1 I 1
~~48-
CA 02475069 2004-08-20
B34 C34 D34
NrH
H
0
* ~*
NI ~
~* ~* H
H O
A35 B35 ~ C35 ~ D35
N~H *N C*
a
N* wCa.
H O
A36 B36 C36 ~ D3b
0 0.,~. S..O
1
I w N,H
cF, ~
* *
NH p ~ *N C*
I II
H
A37 B37~ D37
_ ~
w. N~H
N*~
I C
H O
A38 B38 ~ D38
o N.H
i
~~O
I NH O
> > > ~ i ~
-149-
CA 02475069 2004-08-20
A39 B39 ~ F D39
~ /
o ~ ~ . ~ y\.\
~ / ~ /
NI e~
H O '
A40 B40 ~ F'~ ~ ' D40
~~
S O
\ \ .\
I / ~ /
N* C*
i n
H O
A41 . B41 F F D41
p /
1 O~~S ~ ! w \ \
I / ~ /
N* C*
I a
H O
A42 B42 D42
P-1. .~
NO
,~~ \ \ \
N *~ *
NI 'C
H O
I I I I I I
-15U-
CA 02475069 2004-08-20
A43 B43 D43
~ ..O
s..o ~ ~ s>
ao
a~ a
NH O
Aaa B44 ~. ~ na4
o N
~.~J t
o
' aN a*
s
A45 ~,g,w~ B45 ~ D45
'' N
~* O
aN Its
H ~
A46 B45 L~46
o =s .o ,
s
N C ' ~ \
O
H O~ *r~~
A47 i B47 ' ' ~7
W \
aN '~°C*
1 11
ti O
-151-
CA 02475069 2004-08-20
A48 O B48 ~ ~ D48
f ~ \
N ~ /
cs ci
i t i~
H O
A49 ~VB49 N D49
~N
O S O
*N C*
! ii
H O
ASO i B50 ~ D50
~ ~ /
'N / sN
H
*N C*
! !I
Ei O
-152-
CA 02475069 2004-08-20
nsa ~ B5a F nsa
N
0
. ,i W N
...~~
~540
*N C*
i il
H
A32 i B52 F D52
S
~ I
O=S=O
*N C*
1 it
H O
A53 ~ B53 F D53
* / ~N
J F / ~
. _
.F
1~. i
*N C*
1 tt
H O
-153-
CA 02475069 2004-08-20
A54 ~ B54 N DS4
~ I ~ *.N
<° , _ > ,
N
o~s'o
*N C*
1 11
H O
A55 ' ~ DSS' I w y
o i s
A56 j DS6 ~ '*
~ i i
A57 ~ D57
-. \
A58 ~ D58 ~ \
w \ - _ ~*
N
A59 j D59 ~ \
N
On
O
-1S4-
CA 02475069 2004-08-20
A60 i . D~ /
N ' \
/, N
Ab! . ~ D61
- N
\ N~
A6~ ~ D62 ~ \
\ N- v
HO N
Ab3 ~ D63 * / \
a \
~N
N
A64 ~ D64
1~"~ r w
LN
A65 ~ ~ D65 / \
N
*-N
A66 ~ D66 , ~ N ~
~ N ~~~
Asp ~o~~~ ~s7
__ l i ! I I ~ I I I
CA 02475069 2004-08-20
A68 ~ D68 ~ . \
~*
A69 ~~ D69 \
~.~" (i
A70 °S° D70 ~ \
. . las!*.
0
A7I ~~~ D71 * .
~N
N \ s . ~ . ' i ~N
N
A72 ~ °~ D72.
I w I w
a .i i N
A73 °ya D73 * \ ..'~N
F) ~ S ' / ~N
F
A74 ~ ~p D74 \ N'
". S' . *~~~~ .
A75 pro D'75 ~.~ ~ N
LN~N
~ ~ ~ t
-i~~-
CA 02475069 2004-08-20
~~V VVIJJJ<'~~
A76 u D76 N
\ '~
F 'N N
A7 . o~s~o . D~ \ N1
7 ~
v
L~N~
F
A78 S D78
\ Nr ~.
Cl~
0 o D79 ~ N
A79 ~ ~
y f I - ~ i
N
A$0 ,so D80 * ~ ~ .:.
h y
l
A8! D8! ~ wN
~
*
r ~
N
A82 ~ ~ D82 ~ ~ N
~~ J
N
A83 , ono ' D83 ~ N;
i , i
'~ ''
-I57-
CA 02475069 2004-08-20
A~ p~ , \ N1
~"'
Dss
Ass
*.N ~
A86 OSO D86 *,O a
~N ~
N
.
A87 ' S~ D87 ;o , ~
N
A88 D88 \O ~ w N
N r
0 0
0
S
A89 s s~,o ~ D89
N r
A90 OHO _ ~ \ N''*
I r ..J
N
A91 O~O D91 1
S O
Het
O
-158-
CA 02475069 2004-08-20
WO OOISSI44 _ d'i:l/UJUU1UOD~ _"_
A9a s~p ~ *,N
W
A93 p JQ D93
~O~
F
OH
A94 S ~ ~ D94 ' O
NR
/ ~
A95 D O D95 a
~w
S ~
A96 p ~ D96
R
_
A97 °S D97
I ~ ~
N
A98 OSO D98 *. R
l
,...
A99 O O
R
-IJr9-
CA 02475069 2004-08-20
A100 D100 p
...
AIOI . ~ O D101 *.~ ~
O S vN I /
A102 w,° ~ D102
s
~i ~ . 1 w
o°
A103 O O D103 O
vS w
1~ ~
'* .
r
N
A104 s° D104 O
w
/
'O
A105 O Diu$ O
~O~S ~
r of
A106 O'~O D106 =,W~.
S ' S \\S ~ /
-iso-
CA 02475069 2004-08-20
A107 O S D10~
1
S . ~ a ~.N
vt
A108 . ~ J~DI08 \ w i
NsN
A109 ~ O O ~ D109 a
g ~S
\l
e1
A110 S OS DI10 ' O
m
DII1
A111 OS
(/
CI
A112 °g° D1I2 '
n . N
A113 ~.p~ DI13 ~*
i N
A114 ~~° D114
~ N
I I I 1 ~ ~ ~ ~ J
-161-
CA 02475069 2004-08-20
AI O S~ DI o
IS l5.
S
AI16 . . o~~ D116 O
S ~ /
A117 OSO , DI17
Br
A118 p'"o DII$ ~
v J
S / N
~ ' H
r
AI19 . DI19 w
A
i
/
N
N
A120 .. DI20
r N
i
H
A121 , o~S - D121
o
<
~
0
A1Z2 0~,,,0 DI22 w
~ I
~rs
0 N
N
-162-
CA 02475069 2004-08-20
A123, O D123
O.C *N
. C\ N
AI24 O
ii
C
A125 O
a
y.C
Ales O
ii
C
Ai27 F O
U
C
A128 O
n
of
A129 O
n
C
N
-1 G3-
CA 02475069 2004-08-20
AI30 O~ O
O~C
A131 N
~~ o
n
\ C
A 132 i
CN,
i
A133 0
F $*
F~o
F
While any combination of the elements A, B snd C may comprise the compounds of
the
Invention, certain combinations are preferred For example; the following
combinations
All-BS-C4-Dl A17-BS-C4-D1 A66-BS-C4-D1 A75-BS-C4-Dl
A128-BS-C4-Dl All-Bb-C4-Dl A17-Bb-C4-Dl A66-B6-C4-Dl
f0 A75-Bb-C4-Dl A128-Bb-C4-Dl All-B8-C4-Dl A17-B8-C4-Dl
A66-B8-C4-Dl A75-B8-C4-D1 AI28-B8-C4-DI Al I-B12-C4-DI
A17-B12-C4-Dl Ab6-B12-C4-Dl A75-B12-C4 Dl , A128-B12-C4-Dl
All-B11-C4-Dl A17-B11-C4-Dl A66-B1I-C4-Dl A75-B11-C4-Dl
A128-BIi-C4-Dl All-B14-C4-Dl A17-BI4-C4-Dl A66-BI4-C4-DI
i5 A75-B14-C4-Dl A128-B14-C4-DI All-B3-C4-D2 A17-BS-C4-D2
A66-BS-C4-D2 A75-BS-C4-D2 A128-BS-C4-D2 All-B6-C4-D2
AI7-Bb-C4-D2 A66-B6-C4-D2 A75-Bb-C4-D2 AI28-Bb-C4-D2
All-B8-C4-D2 A17-B8-C4-D2 A66-B8-C4 D2 A75-B8-C4-D2
-164-
CA 02475069 2004-08-20
AI28-B8-C4-D2 All-B12-C4-D2 ~A17-B12-C4-D2A66-BI2-C4-DZ
A75-B12-C4-D2 Ai28-B12-C4-D2 All-B11-C4-D2 A17-B1I-C4-D2
A66-B i I-C4-D2A75-B 11-C4-D2 A128-B I 1-C4-D2Al l-B 14-C4-D2
~
A17-B i 4-C4-D2A66-B 14-C4-D2 A75-B 14-C4-D2_AI28-B 14-C4-D2
_
A61-B5-C4-Dl. A64-B5-C4-Dl A37-B5-C4-D1 A38-B5-C4-Dl
A90-B5-C4-Dl A92-B5-C4-Dl Ai33-B5-C4-Dl A61-B6-C4-Dl
A64-B6-C4-Dl A37-B6-C4-D1 A38-B6-C4-Dl A90-B6-C~4-Di
A92-B6-C4-Di A133-B6-C4-DI A61-B12-C4-DI A64-B12-C4-Dl
A37-B12-C4-DI A38-B12-C4-DI A90-B12-C4-D1 A92-B12-C4-Dl
10A133-B12-C4-Dl
All-B31-C4-D1 A75-B31-C4-Dl. AI28-B31-C4-DlAli-BI3-C4-Dl
A75-Bi3-C4-Dl A128-B13-C4-Di All-B21-C4-D1 A75-B21-C4-D1
A128-B21-C4-DlAlI-B46-C4-D1 A75-B46-C4-Dl A128-B46-C4-Dl
Aii-B49-C4-D1 A75-B49-C4-D1 ~ A128-B49-C4-DlAI1-B50-C4-D1
15A75-B50-C4-Dl A128-B50-C4-Dl All-B51-C4-Dl A75-B51-C4-Dl
Ai28-B51-C4-DIAli-B52-C4-Dl A75-B52-C4-D1 AI28-B52-C4-Dl
All-B53-C4-D1 A75-B53-C4-Dl AI28-B53-C4-Dl
Al i-BS-C36-DlA75-B5-C36-Di A128-BS-C36-DlAl l-B6-C36 Dl
B6-C36-DI AI28-B6-C36-Di Ail-B12-C36-D1A75-B12-C36-Dl
A75 =
20A128-BI2-C36-DlAI1-B5-CI1-Dl A75-BS-Cll-DB Ai28-B5-C11-Dl
Ali-B6-C11-D1 A75-B6-C11-Dl AI28-B6-C11-DlAil-B12~~C11-DI
A75-BI2-Cli-D1A128-B12-Cll-DIAll-BS-C10-Dl A75-BS-~10-Dl
A128-BS-C10-DIAll-B6-C10-D1 A75-B6-C10-Dl A128-Bfr-CIO-DI
All-B12-C10-DlA75-B12-C10-Dl A128-B12-C10-DlAll-B5-C35-DI
25A75-B5-C35-Dl Ai28-BS-C35-D1 All-B6-C35-Dl A75-B6-C35-DI
A128-B6-C35-DlAli-B12-C35-D1 A75-B12-C35-DlAi28-B12-C35-Di
All-B5-C4-D33 A75-B5-C4-D33 A128-B5-C4-D33AI1-B6-C4-D33
A75-B6-C4-D33.Ai28-B6-C4-D33 AIi-B12-C4-D33A75-B12-C4-D33
AI28-B 12-C4-D33Al l-B5-C4-D83 A75-B5-C4-D83 A128-B5-C4-D83
30Ail-86-C4-D83 A75-B6-C4-D83 A128-B6-C4-D83Ai1-B12-C4-D83
A75-B12-C4-D83A128-B12-C4-D83AII-B5-C4-D86 A75-BS-C4-D86
A128-BS-C4-D86All-B6-C4-D86 A75-B6-C4-D86 A128-B6-C4-D86
-165-
CA 02475069 2004-08-20
All-B12-C4-D86 A75-B12-C4-D85 AI28-B12-C4-D85 All-BS-C4-D123
A75-BS-C4-DI23 AI28-BS-C4-DI23 AII-Bb-C4-DI23 A75-Bb-CøDI23
A128-Bb-C4-DI23 All-Bi2-C4-DI23 A75-BI2-C4-D123 A128-B12-C4-D123
EXAMPLE 28
Cathepsin B Assay
Solutions of test compounds in varying concentrations were prepared in I0 ~.L
of
dimethyl sulfoxide (DMSO) and then diluted into assay buffer (40 p,L,
comprising:
N,N bis(2-hydroxyethyl)-2-aminoethanesulfonic acid (BES), 50 mM (pI~ 6);
polyoxyethylenesorbitan monolaurate, 0.05%; and dithiothreitol (DTT), 2.5 mM).
Human
cathepsin B (0.025 pMoles in 25 p.L of assay buffer) was added to the
dilutions. The assay
solutions were 'mixed for 5-10 seconds on a shaker plate, covered and
incubated for 30 minutes -
at room temperature. Z-FR-AMC (20 nMoles in 25 ~L of assay buffer) was added
to the assay
solutions and hydrolysis was followed spectrophotometrically at ( 7~. 460 nm)
for 5 minutes.
Apparent inhibition constants (K.~ were calculated from the enzyme progress
curves using
standard mathematical models.
Compounds of the invention were tested by the above-described assay and
observed to
exhibit cathepsin B inhibitory activity with a K; of less than or equal to 10
~M.
EXAMPLE 29
Cathepsin K Assay
Solutions of test compounds in varying concentrations were prepared in 10 JCL
of
dimethyl sulfoxide (DMSO) and then diluted into assay buffer (40 ~L,
comprising: MES, 50 mM
(pH 5.5); EDTA, 2.5 mM; and DTT, 2.5 mM]. Human cathepsin K (0.0906 pIVdoles
in 25 p,L of
assay buffer) was added to the dilutions. The assay solutions were mixed for 5-
10 seconds on a
shaker plate, covered and incubated for 30 minutes at room temperature. Z-Phe-
Arg-AMC (4
nMoles in 25 ~tL of assay buffer) was added to the assay solutions and
hydrolysis was followed
spectrophotometrically at ( ~, 4b0 nm) for 5 minutes. Apparent inhibition
constants (K;) were
calculated from the enzyme progress curves using standard mathematical models.
Compounds of the invention were tested by the above-described assay and
observed to
-166-
CA 02475069 2004-08-20
~~ V Vweira--~
exhibit cathepsin K inhibitory activity with a K; of less than or equal to 10
ACM.
EXAMPLE 30
Cathepsin L Assay
Solutions of test compounds in varying concentrations were prepared in 10 fCL
of
dimethyl sulfoxide (DMSO) and then diluted into assay buffer (40 fCL,
comprising: MES, 50 mM
{pH 5.5); EDTA, 2.5 mM; and DTT, 2.5 uiM). Human cathepsin L (0.05 pMoles in
25 pL of
assay buffer) was added to the dilutions. The assay solutions were mixed for 5-
.10 seconds on a
shaker plate, covered and incubated for 30 minutes at room temperature. ~ Z-
Phe-Arg-AMC (I
nMoles in 25 fCL of assay buffer) was added to the assay solutions and
hydrolysis was followed
spectrophotometrically at ( ~, 460 nm) for 5 minutes. Apparent inhibition
constants (Ka were
calculated from the enzyme progress curves using standard mathematical models.
Compounds of the invention were tested by the above-described assay and
observed to
exhibit cathepsin L inhibitory activity with a K; of less than or equal to 10
~M.
EXAMPLE 31
Cathepsin S Assay
Solutions of test compounds in varying concentrations were prepared in 10 ~tI.
of
dimethyl sulfoxide (DMS~) and then diluted into assay buffer (40 p,L,
comprising: MF.S, SO mM
(pH 6.5); EDTA, 2.5 mM; and NaCI, 100 mM). Human cathepsin S (0.158 pMoles in
25 p.L of
assay buffer) was added to the dilutions. The assay solutions were mixed for 5-
10 seconds on a
shaker plate, covered and incubated for 30 minutes at room temperature. Z-Val-
Val-Arg-AMC
(9 nMoIes in 251CL of assay buffer) was added to the assay solutions and
hydrolysis was
followed spectrophotometricaIly at ( ~. 460 nm) for 5 minutes. Apparent
inhibition constants (I~
were calculated from the enzyme progress curves using standard mathematical
models.
Compounds of the invention were tested by the above-described: assay and
observed to
exhibit cathepsin S inhibitory activity with a I~ of less than or equal to 10
p,M.
EXAMPLE 32
Ovalbunun Challenge Mouse .
-167-
CA 02475069 2004-08-20
C57 mice (female) were sensitised with ovalbumin (10~g, i.p.) administered
together
with aluminium hydroxide adjuvant (20 mg; i.p.) on days 0 and 12. Mice are
challenged on
either day 22,'23 or 24 by exposure for 60 minutes to an aerosol of ovalbumin
(10.g / 1) twice, 4
hours apart. Mice are dosed p.o. with either vehicle 5 ml/kg (0.5%MCI0..2
°!o Tween 80 in
Hi0) or test compound at 0, 8, 23.5 29, 33, 48 and 56 hours.
Mice were euthanized with pentobarbitone i.p. after 86 hours (72 haurs after
the first
challenge). The lungs were insufflated for histological examination as soon as
possible after
euthanization. Lungs were insufflated with IO°~o neutral buffered
formalin (NBF), at 30 cm
water pressure. The Lungs were removed and placed in pots of 10% NBF. After
fixation in
109b NBF for a minimum of 24 hours the lungs were processed through graded
alcohols to wax.
The lungs were blocked longitudinally and one 2 Icm section for each animal
was cut at the level
of the main bronchi. Sections then were stained with haematoxylin and eosin.
Pathological
assessment of sections is performed and a grading is assigned.
Histopathological evaluation of the lung tissue demonstrate a dose dependant
anti-
inflammatory effect on vascular and mucosal beds after treatment with
compounds of the
invention between 0.03 and 30 mg/kg.
-168-
CA 02475069 2004-08-20
~/ V VW VV1~~
EXAMPLE 32
Representative Pharmaceutical Formulations Containing a Compound of Formula I
ORAL FORMULATION
Compound of Formula I 10-100 mg
Citric Acid Monohydrate 105 mg
Sodinrn Hydroxide 18 mg
Flavoring
Water qa. to 100
~~ .
INTRAVENOUS FORMULATION
Compound of Formula I 0.1-10 mg
Dextrose Monohydrate q.s. to make
isotonic
Citric Acid Monohydrate ~ 1.05 mg
Sodium Hydroxide 0.18 mg
Water for Injection q.s. to 1.0
mL
l5
TABLET FORMULATION
Compound of Formula I 1R6
Microcrystalline Cellulose ?3%
Stearic Acid 2596
Colloidal Silica ~ 1°k.
The resulting tablets are useful for administration in accordance with the
methods of this
invention for treating or preventing a cathepsin mediated disease state, such
as osteoporosis,
juvenile onset diabetes, multiple sclerosis, pemphigus vulgaris, Craves'
disease, myasthenia
gravis; systemic lupus erythemotasus, rheumatoid arthritis,~Hashimoto's
thyroiditis, asthma,
organ transplant or tissue graft rejections, chronic obstructive pulmonary
disease, bronchioIitis,
excessive airway eIastolysis in asthma and bronchitis, pneumonities, plaque
rupture, atheroma
and systemic amyloidosis.
-169-