Note: Descriptions are shown in the official language in which they were submitted.
CA 02475248 2007-03-21
61368-1244
1
NANOCARBIDE PRECIPITATION STRENGTHENED ULTRAHIGH-STRENGTH,
CORROSION RESISTANT, STRUCTURAL STEELS
BACKGROUND OF THE INVENTION
In a principal aspect, the present invention relates to cobalt, nickel,
chromium stainless
martensitic steel alloys having ultrahigh strength and corrosion resistance
characterized by
15 nanoscale sized carbide precipitates, in particular, M2C precipitates.
Main structural components in aerospace and other high-performance structures
are
almost exclusively made of ultrahigh-strength steels because the weight, size
and, in some
cases, cost penalties associated with use of other materials is prohibitive.
However, ultrahigh-
strength steels with a tensile strength in the range of at least 240 ksi to
300 ksi have poor
20 general corrosion resistance and are susceptible to hydrogen and
environmental embrittlement.
Thus,' to provide general corrosion, resistance in aerospace and other
structural steel
components, cadmium plating of the components is typically employed, and when
wear
resistance is needed, hard chromium plating is predominantly used. These
coatings have
disadvantages from a cost, manufacturing, environmental and reliability
standpoint.
25 Consequently, a goal in the design or discovery of ultrahigh-strength steel
alloys is elimination
of the need for cadmium and chromium coatings without a mechanical deficit or
diminishment
of strength. One performance objective for alloys of the subject invention is
replacement of
non-stainless structural steels with stainless or corrosion resistant steels
that have tensile
strengths greater than about 240 ksi, that do not require cadmium coating and
which
30 demonstrate wear resistance without chromium plating or other protective
and wear resistant
coatings.
One of the most widely used ultrahigh-strength steels in use for aerospace
structural
applications is 300M steel. This alloy is essentially 4340 steel modified to
provide a slightly
CA 02475248 2007-03-21
61368-1244
2
higher Stage I tempering temperature, thereby allowing the bakeout of
embrittling hydrogen
introduced during processing. Aerospace Material Specification AMS 6257A [SAE
International, Warrendale, PA, 2001] covers a majority of the
use of 300M steel in aerospace applications. Within this specification minimum
tensile
properties are 280 ksi ultimate tensile strength (UTS), 230 ksi yield strength
(YS), 8%
elongation and a reduction of area of 30%. The average plane strain mode I
fracture toughness
is 52 ksi,/in [Philip, T. V. and T. J. McCaffrey, Ultrahigh-Strength Steels,
Properties and
Selection: Irons, Steels, and High-Performance Alloys, Materials Park, OH, ASM
International,
1: 430-448, 1990]. Stress corrosion cracking resistance in a
3.5% by weight aqueous sodium chloride solution is reported as 10 ksiJin .
The high tensile strength of 300M steel allows the design of lightweight
structural
components in aerospace systems such as landing gear. However, the lack of
general corrosion
resistance requires cadmium coating, and the low stress corrosion cracking
resistance results in
significant field failures due to environmental embrittlement.
. Precipitation hardening stainless steels, primarily 15-5PH, [AMS 5659K, SAE
International, Warrendale, PA, 1998] may also be used in
structural aerospace components, but typically only in lightly loaded
applications where the
weight penalties due to its low strength are not large. Corrosion resistance
is sufficient for such
an alloy so that cadmium plating can be eliminated; however minimum tensile
properties of
15-5PH in the maximum strength H900 condition are only 190 ksi UTS and 170 ksi
YS. This
limits the application to components that are not strength limited.
Another precipitation strengthening stainless steel, Carpenter Custom 465TH
steel
[Alloy Digest, SS-716, Materials Park, OH, ASM International, 1998]
uses intermetallic precipitation and reaches a maximum of UTS of slightly
below 270
ksi. At that strength level Custom 465TH steel has a low Charpy V-notch impact
energy of
about 5 ft-lb [Kimmel, W. M., N. S. Kuhn, et al., Cryogenic Model Materials,
39th AIAA
Aerospace Sciences Meeting & Exhibit, Reno, NV, 20011. For
most structural applications Custom 465TM steel must be used in a condition
that limits its UTS
to well below 270 ksi in order to maintain adequate Charpy V-notch impact
resistance.
A number of secondary hardening stainless steels have been developed that
reach
ultimate strength levels of up to 270 ksi. These are disclosed in U.S. Patent
Nos. Re. 26,225,
3,756,808, 3,873,378, and 5,358,577. These stainless steels use higher
chromium levels to
CA 02475248 2007-03-21
61368-1244
3
maintain corrosion resistance and therefore compromise strength. A primary
feature of these
alloys is the large amount of austenite, both retained and formed during
secondary hardening.
The austenite modifies the flow behavior of the alloys and, while they may
achieve an UTS as
high as 270 ksi, their yield strength is no more than 200 ksi. This large gap
between yield and
ultimate limits the applications for which these steels can be used. Thus
there has remained the
need for ultrahigh strength, noncorrosive steel alloys that have a yield
strength of at least about
230 ksi and an ultimate tensile strength of at least about 280 ksi.
SUMMARY OF THE INVENTION
Briefly, the invention comprises stainless steel alloys comprising, by weight,
about: 0.1
to 0.3% carbon (C), 8 to 17% cobalt (Co), less than 10% nickel (Ni), greater
than 6% and less
than 11% chromium (Cr), and less than 3% molybdenum (Mo) along with other
elemental
additives including minor amounts of Si, Cu, Mn, Nb, V, Ta, W, Ti, Zr, rare
earths and B, the
remainder iron (Fe) and incidental elements and impurities, processed so as to
be principally in
the martensitic phase with ultrahigh strength and noncorrosive physical
characteristics as a
result of the choice and amount of constituents and the processing protocol.
The alloys of the subject invention can achieve an ultimate tensile strength
(UTS) of
about 300 ksi with a yield strength (YS) of about 230 ksi and also provide
corrosion resistance
with greater than about 6% and less than about 11%, preferably less than about
10% by weight
chromium. The alloys of the invention provide a combination of the observed
mechanical
properties of structural steels, that are currently cadmium coated and used in
aerospace
applications, and the corrosion properties of stainless steels without special
coating or plating.
Highly efficient nanoscale carbide (M2C) strengthening of the described alloys
provides
ultrahigh strengths with lower carbon and alloy content while improving
corrosion resistance
due to the ability of the nanoscale carbides to oxidize and supply chromium as
a passivating
oxide film. This combination of ultrahigh strength and corrosion resistance
properties in a
single material eliminates the need for cadmium coating without a weight
penalty relative to
current structural steels. Additionally, alloys of the subject invention
reduce environmental
embrittlement driven field failures because they no longer rely on an
unreliable coating for
protection from the environment.
Thus, the invention provides a new class of ultrahigh-strength, corrosion
resistant,
structural steel alloys.
CA 02475248 2007-03-21
61368-1244
4
Further, the invention provides ultrahigh-
strength, corrosion resistant, structural steel alloys that
do not require plating or coating to resist corrosion.
The invention also provides ultrahigh-strength,
corrosion resistant, structural steel alloys having cobalt,
nickel and chromium alloying elements in combination with
other elements whereby the alloys are corrosion resistant.
Further, the invention provides ultrahigh-
strength, corrosion resistant, structural steel alloys
having an ultimate tensile strength (UTS) greater than about
240 ksi and preferably greater than about 280 ksi, and a
yield strength (YS) greater than about 200 ksi and
preferably greater than about 230 ksi.
The invention also provides ultrahigh-strength,
corrosion resistant, structural steel alloys characterized
by a lath martensitic microstructure and by M2C nanoscale
sized precipitates in the grain structure and wherein other
MxC precipitates where x > 2 have generally been solubilized.
The invention also provides ultrahigh-strength,
corrosion resistant, structural steel alloys which may be
easily worked to form component parts and articles while
maintaining its ultrahigh strength and noncorrosive
characteristics.
Further, the invention provides processing
protocols for the disclosed stainless steel alloy
compositions that enable creation of an alloy microstructure
having highly desirable strength and noncorrosive
characteristics.
In one aspect, the invention provides a
structural, stainless steel alloy comprising, in
CA 02475248 2007-03-21
61368-1244
4a
combination, by weight: about 0.15 to 0.30% carbon, about 8
to 17% cobalt, 5.0 to 10.0% nickel, about 8.0 to 11.0%
chromium, about 1.0 to 3.0% molybdenum, less than about 0.8%
vanadium and less than about 3% tungsten, the balance
essentially iron and incidental elements and impurities,
wherein the alloy has a predominantly lath martensite
microstructure essentially without topologically close
packed intermetallic phases and said carbon is in
predominantly a dispersion of nanoscale, M2C carbide
particles having a nominal dimension less than about 10
nanometers in diameter, wherein M is two or more elements
selected from the group consisting of Cr, Mo, W, V, Nb and
Ta.
In a further aspect, the invention provides a
structural, stainless steel alloy comprising, in combination
by weight: about 0.15 to 0.3% carbon, about 8 to 17% cobalt,
5.0 to 10% nickel, about 8 to 11% chromium, molybdenum,
tungsten and vanadium, the molybdenum being present in an
amount by weight greater than about 1.0 and less than about
3%, the tungsten being present in an amount by weight less
than about 3% and the vanadium being present in an amount by
weight less than about 0.8%, the balance essentially iron
and incidental elements and impurities, wherein the steel
alloy comprises a corrosion resistant, lath martensitic
microstructure essentially without topologically close
packed intermetallic phases and said carbon is predominantly
in a dispersion of nanoscale, M2C carbide particles having a
diameter of 10 nm or less, wherein M comprises Mo and one or
more elements selected from the group consisting of Cr, W
and V, and wherein cementite dissolution is effectively
complete.
In a still further aspect, the invention provides
a stainless, structural steel alloy comprising, in
CA 02475248 2007-03-21
61368-1244
4b
combination, by weight: about 0.15 to 0.30% carbon, about 8
to 17% cobalt, 5.0 to less than 10.0% nickel, about 8.0 to
11.0% chromium, about 1.0 to 3.0% molybdenum, less than
about 0.8% vanadium and less than about 3% tungsten, the
balance essentially iron and incidental elements and
impurities, wherein the alloy is a predominantly lath
martensite microstructure essentially without topologically
close packed intermetallic phases and said carbon is
predominantly in a dispersion of nanoscale, M2C carbide
particles having a nominal dimension less than about 10
nanometers in diameter, wherein M is two or more elements
selected from the group consisting of Cr, Mo, W and V.
These and other aspects, advantages and features
will be set forth in the detailed description which follows.
BRIEF DESCRIPTION OF THE DRAWING
In the detailed description that follows,
reference will be made to the drawing comprised of the
following figures:
FIG. 1 is a flow block logic diagram that
characterizes the design concepts of the alloys of the
invention;
FIG. 2A is an equilibrium phase diagram depicting
the phases and composition of carbides at various
temperatures in an example of an alloy of the invention;
FIG. 2B is a diagram of the typical processing
path for alloys of the invention in relation to the
equilibrium phases present;
FIG. 3 is a graph correlating peak hardness and M2C
driving forces for varying carbon (C) content, with values
in weight percent;
CA 02475248 2004-08-03
WO 03/076676 PCT/US03/03682
FIG. 4 is a graph showing contours of M2C driving force (AG) and scaled rate
constant
for varying molybdenum (Mo) and vanadium (V) contents, where temperature has
been set to
482 C, and amounts of other alloying elements have been set to 0.14% by weight
carbon (C),
9% by weight chromium (Cr), 13% by weight cobalt (Co), and 4.8% by weight
nickel (Ni);
5 FIG. 5 is a phase diagram at 1000 C used to determine final vanadium (V)
content for a
carbon (C) content of 0.14% by weight, where other alloying element amounts
have been set to
9% by weight chromium (Cr), 1.5% by weight molybdenum (Mo), 13% by weight
cobalt (Co),
and 4.8% by weight nickel (Ni);
FIG. 6 is a graph showing contours of Ms temperature and M2C driving force
(AG) for
varying cobalt (Co) and nickel (Ni) contents, where temperature has been set
to 482 C, and
other alloying element amounts have been set to 0.14% by weight carbon (C), 9%
by weight
chromium (Cr), 1.5% by weight molybdenum (Mo), and 0.5% by weight vanadium (V)
in an
embodiment of the invention; and;
FIG. 7 is a 3-dimensional atom-probe image of an M2C carbide in an optimally
heat
treated preferred embodiment and example of the invention.
DETAILED DESCRIPTION OF THE INVENTION
The steel alloys of the invention exhibit various physical characteristics and
processing
capabilities. These characteristics and capabilities were established as
general criteria, and
subsequently the combination of elements and the processing steps appropriate
to create such
steel alloys to meet these criteria were identified. FIG. 1 is a system flow-
block diagram which
illustrates the processing/structure/properties/performance relationships for
alloys of the
invention. The desired performance for the application (e.g. aerospace
structures, landing gear,
sports equipment, tools, etc.) determines a set of alloy properties required.
Alloys of the
invention exhibit the structural characteristics that can achieve the desired
combination of
properties and can be assessed through the sequential processing steps shown
on the left of
FIG. 1. Following are the criteria for the physical properties and the
processing capabilities or
characteristics for the alloys. This is followed by a description of the
analytical and
experimental techniques relating to the discovery and examples of the alloys
that define, in
general, the range and extent of the elements, physical characteristics and
processing features
of the present invention.
CA 02475248 2004-08-03
WO 03/076676 PCT/US03/03682
6
Physical Characteristics
The physical characteristics or properties of the most preferred embodiments
of the
invention are generally as follows:
Corrosion resistance equivalent to 15-5PH (H900 condition) as measured by
linear polarization.
Strength equivalent to or better than 300M alloy, i.e.:
Ultimate Tensile Strength (UTS) X80 ksi.
Yield Strength (YS) X30 ksi.
Elongation (EL) >_8%.
Reduction of Area (RA) ~0%.
Stress Corrosion Cracking Resistance (K1 ) >_ 15 ksi in .
K' >_ 0.21
YS
Surface hardenable to ?67 Rockwell C (HRC) for wear and fatigue resistance.
Optimum microstructural features for maximum fatigue/corrosion fatigue
resistance.
Processability Characteristics
A principal goal of the subject invention is to provide alloys with the
objective physical
properties recited above and with processability that renders the alloys
useful and practical.
With a number of possible processing paths associated with the scale of
manufacture and the
resulting cleanliness and quality for a given application, compatibility of
the alloys of the
subject invention with a wide range of processes is desirable and is thus a
feature of the
invention.
A primary objective for and characteristic of the alloys is compatibility with
melting
practices such as Vacuum Induction Melting (VIM), Vacuum Arc Remelting (VAR),
and
Electro-Slag Remelting (ESR) and other variants such as Vacuum Electro-Slag
Remelting
(VSR). Alloys of the subject invention can also be produced by other processes
such as air
melting and powder metallurgy. Of importance is the behavior of the alloys to
exhibit limited
solidification microsegregation under the solidification conditions of the
above processes. By
selection of appropriate elemental content in the alloys of the'subject
invention, the variation of
composition that results from solidification during processing across a
secondary dendrite can
be minimized. Allowable variation results in an alloy that can be homogenized
at commercially
feasible temperatures, usually at metal temperatures in excess of 1 100 C and
up to the incipient
CA 02475248 2007-03-21
61368-1244
7
melting of the alloy, and for reasonable processing times, typically less than
seventy-two hours
and preferably less than thirty-six hours.
Alloys of the subject invention also possess reasonable hot ductility such
that hot
working after homogenization can be accomplished within temperature and
reduction
constraints typical of current industrial practice. Typical hot working
practice for alloys of the
subject invention should enable cross-sectional reduction ratios in excess of
three to one and
preferably in excess of five to one. In addition, initial hot working of the
ingot should be
possible below 1100 C, and finish hot working to the desired product size
should be possible at
temperatures below 950 C.
Objectives regarding solution heat treatment include the goal to fully
dissolve all
primary alloy carbides (i.e. MXC where X > 2) while maintaining a fine scale
grain refining
dispersion (i.e. MC) and a small grain size, generally equal to or smaller
than ASTM grain size
number 5 in accordance with ASTM El 12 [ASTM, ASTM El 12-96, West
Conshohocken, PA,
19961. Thus with the alloys of the invention, during solution
heat treatment into the austenite phase field, coarse scale alloy carbides
that formed during
prior processing are dissolved, and the resulting carbon in solution is then
available for
precipitation strengthening during tempering. However, during the same process
the austenite
grains can coarsen, thereby reducing strength, toughness and ductility. With
alloys of the
invention, such grain coarsening is slowed by MC precipitates that pin the
grain boundaries
and, as solution heat treatment temperature increases, the amount of this
grain refining
.dispersion needed to avoid or reduce grain coarsening increases. Alloys of
the subject invention
generally thoroughly dissolve all coarse scale carbides, i.e. MxC where x > 2,
while
maintaining an efficient grain refining dispersion at reasonable solution heat
treatment
temperatures in the range of 850 C to 1100 C, preferably 950 C to 1050 C.
After the solution heat treatment, components manufactured from the alloys of
the
Subject invention are typically rapidly cooled or quenched below temperatures
at which
martensite forms. The preferred result of this process is a microstructure
that consists of
essentially all martensite with virtually no retained austenite, other
transformation products
such as bainite or ferrite, or other carbide products that remain or are
formed during the
process. The thickness of the component being cooled and the cooling media
such as oil, water,
or air determine the cooling rate of this type of process. As the cooling rate
increases, the risk
of forming other non-martensitic products is reduced, but the distortion in
the component
CA 02475248 2004-08-03
WO 03/076676 PCT/US03/03682
8
potentially increases, and the section thickness of a part that can be
processed thus decreases.
Alloys of the subject invention are generally, fully martensitic after cooling
or quenching at
moderate rates in section sizes less than three inches and preferably less
than six inches when
cooled to cryogenic temperatures, or preferably to room temperature.
After cooling or quenching, components manufactured using alloys of the
subject
invention maybe tempered in a temperature range and for a period of time in
which the carbon
in the alloy will form coherent nanoscale M2C carbides while avoiding the
formation of other
carbide products, i.e. M2C where x>2. During this aging or secondary hardening
process the
component is heated to the process temperature at a rate determined by the
power of the
furnace and the size of the component section and held for a reasonable time,
then cooled or
quenched to room temperature.
If the prior solution treatment has been ineffective in avoiding retained
austenite, the
tempering process may be divided into multiple steps where each tempering step
is followed by
a cool or quench to room temperature and preferably a subsequent cool to
cryogenic
temperatures to form martensite. The temperature of the temper process would
typically be
between 200 C to 600 C, preferably 450 C to 540 C and be less than twenty-four
hours in
duration, preferably between two to ten hours. The outcome of the desired
process is a
martensitic matrix (generally free of austenite) strengthened by a nanoscale
M2C carbide
dispersion, devoid of transient cementite that forms during the early stages
of the process, and
without other alloy carbides that may precipitate if the process time becomes
too long.
A significant feature of alloys of the invention is related to the high
tempering
temperatures used to achieve its secondary hardening response. Although a
specific goal is to
avoid cadmium plating for corrosion resistance, many components made from an
alloy of the
invention may require an electroplating process such as nickel or chromium
during
manufacture or overhaul. Electroplating processes introduce hydrogen into the
microstructure
that can lead to embrittlement and must be baked out by exposing the part to
elevated
temperatures after plating. Alloys of the invention can be baked at
temperatures nearly as high
as their original tempering temperature without reducing the strength of the
alloy. Since
tempering temperatures are significantly higher in alloys of the invention
compared to
commonly used 4340 and 300M alloys, the bake-out process can be accomplished
more
quickly and reliably.
Certain surface modification techniques for wear resistance, corrosion
resistance, and
decoration, such as physical vapor deposition (PVD), or surface hardening
techniques such as
CA 02475248 2004-08-03
WO 03/076676 PCT/US03/03682
9
gas or plasma nitriding, are optimally performed at temperatures on the order
of 500 C and for
periods on the order of hours. Another feature of alloys of the subject
invention is that the heat-
treating process is compatible with the temperatures and schedules typical of
these surface
coating or hardening processes.
Components made of alloys of the subject invention are typically manufactured
or
machined before solution heat treatment and aging. The manufacturing and
machining
operations require a material that is soft and exhibits favorable chip
formation as material is
removed. Therefore alloys of the subject invention are preferably annealed
after the hot
working process before they are supplied to a manufacturer. The goal of the
annealing process
is to reduce the hardness of an alloy of the subject invention without
promoting excessive
austenite. Typically annealing would be accomplished by heating the alloy in
the range of
600 C to 850 C, preferably in the range 700 C to 750 C for a period less than
twenty-four
hours, preferably between two and eight hours and cooling slowly to room
temperature. In
some cases a multiple-step annealing process may provide more optimal results.
In such a
process an alloy of the invention may be annealed at a series of temperatures
for various times
that may or may not be separated by an intermediate cooling step or steps.
After machining, solution heat treatment and aging, a component made of an
alloy of
the subject invention may require a grinding step to maintain the desired
final dimensions of
the part. Grinding of the surface removes material from the part by abrasive
action against a
high-speed ceramic wheel. Damage to the component by overheating of the
surface of the part
and damage to the grinding wheel by adhesion of material needs to be avoided.
These
complications can be avoided primarily by lowering the retained austenite
content in the alloy.
For this and the other reasons stated above, alloys of the subject invention
exhibit very little
retained austenite after solution heat treatment.
Many components manufactured from alloys of the subject invention may require
joining by various welding process such as gas-arc welding, submerged-arc
welding, friction-
stir welding, electron-beam welding and others. These processes require the
material that is
solidified in the fusion zone or in the heat-affected zone of the weld to be
ductile after
processing. Pre-heat and post-heat may be used to control the thermal history
experienced by
the alloy within the weld and in the heat-affected zone to promote weld
ductility. A primary
driver for ductile welds is lower carbon content in the material, however this
also limits
strength. Alloys of the subject invention achieve their strength using very
efficient nanoscale
CA 02475248 2004-08-03
WO 03/076676 PCT/US03/03682
M2C carbides and therefore can achieve a given level of strength with lower
carbon content
than steels such as 300M steel, consequently promoting weldability.
Microstructure and Composition Characteristics
The alloy designs achieve required corrosion resistance with a minimum Cr
content
5 because high Cr content limits other desired properties in several ways. For
example, one result
of higher Cr is the lowering of the martensite Ms temperature which, in turn,
limits the content
of other desired alloying elements such as Ni. High Cr levels also promote
excessive
solidification microsegregation that is difficult to eliminate with high-
temperature
homogenization treatments. High Cr also limits the high-temperature solubility
of C required
10 for carbide precipitation strengthening, causing use of high solution heat
treatment
temperatures for which grain-size control becomes difficult. Thus, a feature
of the alloys of the
invention is utilization of Cr in the range of greater than about 6% and less
than about 11%
(preferably less than about 10%) by weight in combination with other elements
as described to
achieve corrosion resistance with structural strength.
Another feature of the alloys is to achieve the required carbide strengthening
with a
minimum carbon content. Like Cr, C strongly lowers Ms temperatures and raises
solution
temperatures. High C content also limits weldability, and can cause corrosion
problems
associated with Cr carbide precipitation at grain boundaries. High C also
limits the extent of
softening that can be achieved by annealing to enhance machinability.
Both of the primary features just discussed are enhanced by the use of Co. The
thermodynamic interaction of Co and Cr enhances the partitioning of Cr to the
oxide film
formed during corrosion passivation, thus providing corrosion protection
equivalent to a higher
Cr steel. Co also catalyzes carbide precipitation during tempering through
enhancement of the
precipitation thermodynamic driving force, and by retarding dislocation
recovery to promote
heterogeneous nucleation of carbides on dislocations. Thus, C in the range of
about 0.1% to
0.3% by weight combined with Co in the range of about 8% to 17% by weight
along with Cr as
described, and the other minor constituent elements, provides alloys with
corrosion resistance
and ultrahigh strength.
The desired combination of corrosion resistance and ultrahigh strength is also
promoted
by refinement of the carbide strengthening dispersion down to the
nanostructural level, i.e., less
than about ten nanometers in diameter and preferably less than about five
nanometers.
Compared to other strengthening precipitates such as the intermetallic phases
employed in
maraging steels, the relatively high shear modulus of the M2C alloy carbide
decreases the
CA 02475248 2007-03-21
61368-1244
11
optimal particle size for strengthening down to a diameter of only about three
nanometers.
Refining the carbide precipitate size to this level provides a highly
efficient strengthening
dispersion. This is achieved by obtaining a sufficiently high thermodynamic
driving force
through alloying. This refinement provides the additional benefit of bringing
the carbides to the
same length scale as the passive oxide film so that the Cr in the carbides can
participate in film
formation. Thus the carbide formation does not significantly reduce corrosion
resistance. A
further benefit of the nanoscale carbide dispersion is effective hydrogen
trapping at the carbide
interfaces to enhance stress corrosion cracking resistance. The efficient
nanoscale carbide
strengthening also makes the system well suited for surface hardening by
nitriding during
tempering to produce M2(C,N) carbonitrides of the same size scale for
additional efficient
strengthening without significant loss of corrosion resistance. Such nitriding
can achieve
surface hardness as high as 1100 Vickers Hardness (VHN) corresponding to 70
HRC.
Toughness is further enhanced through grain refinement by optimal dispersions
of grain
refining MC carbide dispersions that maintain grain pinning during
normalization and solution
treatments and resist microvoid nucleation during ductile fracture. Melt
deoxidation practice is
controlled to favor formation of Ti-rich MC dispersions for this purpose, as
well as to minimize
the number density of oxide and oxysulfide inclusion particles that form
primary voids during
fracture. Under optimal conditions, the amount of MC, determined by mass
balance from the
available Ti content, accounts for less than 10% of the alloy C content.
Increasing Ni content
within the constraints of the other requirements enhances resistance to
brittle fracture.
Refinement of M2C particle size through precipitation driving force control
allows ultrahigh
strength to be maintained at the completion of M2C precipitation in order to
fully dissolve Fe3C
cementite carbides that precipitate prior to M2C and limit fracture toughness
through microvoid
nucleation. The cementite dissolution is considered effectively complete when
M2C accounts
for 85% of the alloy C content, as assessed by the measured M2C phase fraction
using
techniques described by Montgomery [Montgomery, J. S. and G. B. Olson, M2C
Carbide
Precipitation in AF1410, Gilbert R. Speich Symposium: Fundamentals of Aging
and
Tempering in Bainitic and Martensitic Steel Products, ISS-AIME, Warrendale,
PA, 177-214,
1992], Precipitation of other phases that can limit toughness
such as other carbides (e.g. M23C6, M6C and M7C3) and topologically close
packed (TCP)
intermetallic phases (e.g. U and p. phases) is avoided by constraining the
thermodynamic
driving force for their formation.
CA 02475248 2004-08-03
WO 03/076676 PCT/US03/03682
12
In addition to efficient hydrogen trapping by the nanoscale M2C carbides to
slow
hydrogen transport, resistance to hydrogen stress-corrosion is further
enhanced by controlling
segregation of impurities and alloying elements to prior-austenite grain
boundaries to resist
hydrogen-assisted intergranular fracture. This is promoted by controlling the
content of
undesirable impurities such as P and S to low levels and gettering their
residual amounts in the
alloy into stable compounds such as La2O2S or Ce2O2S. Boundary cohesion is
further enhanced
by deliberate segregation of cohesion enhancing elements such as B, Mo and W
during heat
treatment. These factors promoting stress corrosion cracking resistance will
also enhance
resistance to corrosion fatigue.
All of these conditions are achieved by the class of alloys discovered while
maintaining
solution heat treatment temperatures that are not excessively high. Martensite
Ms temperatures,
measured by quenching dilatometry and 1% transformation fraction, are also
maintained
sufficiently high to establish a lath martensite microstructure and minimize
the content of
retained austenite which can otherwise limit yield strength.
Preferred Processing Techniques
The alloys can be produced via various process paths such as for example
casting,
powder metallurgy or ingot metallurgy. The alloy constituents can be melted
using any
conventional melt process such as air melting but more preferably by vacuum
induction
melting (VIM). The alloy can thereafter be homogenized and hot worked, but a
secondary
melting process such as electro slag remelting (ESR) or vacuum arc remelting
(VAR) is
preferred in order to achieve improved fracture toughness and fatigue
properties. In order to
achieve even higher fracture toughness and fatigue properties additional
remelting operations
can be utilized prior to homogenization and hot working. In any event, the
alloy is initially
formed by combination of the constituents in a melt process.
The alloy may then be homogenized prior to hot working or it may be heated and
directly hot worked. If homogenization is used, it may be carried out by
heating the alloy to a
metal temperature in the range of about 1100 C or 1110 C or 1120 C to 1330 C
or 1340 C or
1350 C or, possibly as much as 1400 C for a period of time of at least four
hours to dissolve
soluble elements and carbides and to also homogenize the structure. One of the
design criteria
for the alloy is low microsegregation, and therefore the time required for
homogenization of the
alloy is typically shorter than other stainless steel alloys. A suitable time
is six hours or more in
the homogenization metal temperature range. Normally, the soak time at the
homogenization
temperature does not have to extend for more than seventy-two hours. Twelve to
eighteen
CA 02475248 2004-08-03
WO 03/076676 PCT/US03/03682
13
hours in the homogenization temperature range has been found to be quite
suitable. A typical
homogenization metal temperature is about 1240 C.
After homogenization the alloy is typically hot worked. The alloy can be hot
worked by,
but not limited to, hot rolling, hot forging or hot extrusion or any
combinations thereof. It is
common to initiate hot working immediately after the homogenization treatment
in order to
take advantage of the heat already in the alloy. It is important that the
finish hot working metal
temperature is substantially below the starting hot working metal temperature
in order to assure
grain refinement of the structure through precipitation of MC carbides. After
the first hot
working step, the alloy is typically reheated for continued hot working to the
final desired size
and shape. The reheating metal temperature range is about 950 C or 960 C or
970 C to 1230 C
or 1240 C or 1250 C or possibly as much as 1300 C with the preferred range
being about
1000 C or 1010 C to 1150 C or 1160 C. The reheating metal temperature is near
or above the
solvus temperature for MC carbides, and the objective is to dissolve or
partially dissolve
soluble constituents that remain from casting or may have precipitated during
the preceding hot
working. This reheating step minimizes or avoids primary and secondary phase
particles and
improves fatigue crack growth resistance and fracture toughness.
As the alloy is continuously hot worked and reheated the cross-sectional size
decreases
and, as a result, the metal cools faster. Eventually it is no longer possible
to use the high
reheating temperatures, and a lower reheating temperature must be used. For
smaller cross-
sections the reheating metal temperature range is about 840 C or 850 C or 860
C to 1080 C or
1090 C or 1100 C or possibly as much as 1200 C with the preferred range being
about 950 C
or 960 C to 1000 C or 1010 C. The lower reheating metal temperature for
smaller cross-
sections is below the solvus temperature for other (non-MC) carbides, and the
objective is to
minimize or prevent their coarsening during reheating so that they can quickly
be dissolved
during the subsequent normalizing or solution heat treatment.
Final mill product forms such as, for example, bar stock and forging stock are
typically
normalized and/or annealed prior to shipment to customers. During normalizing
the alloy is
heated to a metal temperature above the solvus temperature for all carbides
except MC
carbides, and the objective is to dissolve soluble constituents that may have
precipitated during
the previous hot working and to normalize the grain size. The normalizing
metal temperature
range is about 880 C or 890 C or 900 C to 1080 C or 1090 C or 1100 C with the
preferred
range being about 1020 C to 1030 C or 1040 C. A suitable time is one hour or
more and
CA 02475248 2004-08-03
WO 03/076676 PCT/US03/03682
14
typically the soak time at the normalizing temperature does not have to extend
for more than
three hours. The alloy is thereafter cooled to room temperature.
After normalizing the alloy is typically annealed to a suitable hardness or
strength level
for subsequent customer processing such as, for example, machining. During
annealing the
alloy is heated to a metal temperature range of about 600 C or 610 C to 840 C
or 850 C,
preferably between 700 C to 750 C for a period of at least one hour to coarsen
all carbides
except the MC carbide. A suitable time is two hours or more and typically the
soak time at the
annealing temperature does not have to extend for more than twenty-four hours.
Typically after the alloy has been delivered to a customer and processed to,
or near, its
final form and shape it is subjected to solution heat treatment preferably in
the metal
temperature range of about 850 C or 860 C to 1090 C or 1100 C, more preferably
about
950 C to 1040 C or 1050 C for a period of three hours or less. A typical time
for solution heat
treatment is one hour. The solution heat treatment metal temperature is above
the solvus
temperature for all carbides except MC carbides, and the objective is to
dissolve soluble
constituents that may have precipitated during the preceding processing. This
inhibits grain
growth while enhancing strength, fracture toughness and fatigue resistance.
After solution heat treatment it is important to cool the alloy fast enough to
about room
temperature or below in order to transform the microstructure to a
predominantly lath
martensitic structure and to prevent or minimize boundary precipitation of
primary carbides.
Suitable cooling rates can be achieved with the use of water, oil, or various
quench gases
depending on section thickness.
After quenching to room temperature the alloy may be subjected to a cryogenic
treatment or it may be heated directly to the tempering temperature. The
cryogenic treatment
promotes a more complete transformation of the microstructure to a lath
martensitic structure.
If a cryogenic treatment is used, it is carried out preferably below about -70
C. A more
preferred cryogenic treatment would be below about -195 C. A typical cryogenic
treatment is
in the metal temperature range of about -60 C or -70 C to -85 C or -95 C.
Another typical
cryogenic treatment is in the metal temperature range of about -180 C or -190
C to -220 C or
-230 C. Normally, the soak time at the cryogenic temperature does not have to
extend for more
than ten hours. A typical time for cryogenic treatment is one hour.
After the cryogenic treatment, or if the cryogenic treatment is omitted,
immediately
following quenching, the alloy is tempered at intermediate metal temperatures.
The tempering
treatment is preferably in the metal temperature range of about 200 C or 210 C
or 220 C to
CA 02475248 2004-08-03
WO 03/076676 PCT/US03/03682
580 C or 590 C or 600 C, more preferably about 450 C to 530 C or 540 C.
Normally, the
soak time at the tempering temperature does not have to extend for more than
twenty-four
hours. Two to ten hours in the tempering temperature range has been found to
be quite suitable.
During the tempering treatment, precipitation of nanoscale M2C-strengthening
particles
5 increases the thermal stability of the alloy, and various combinations of
strength and fracture
toughness can be achieved by using different combinations of temperature and
time.
For alloys of the invention with lower MS temperatures, it is possible to
further enhance
strength and fracture toughness through multi-step thermal treatments by
minimizing retained
austenite. Multi-step treatments consist of additional cycles of cryogenic
treatments followed
10 by thermal treatments as outlined in the text above. One additional cycle
might be beneficial
but multiple cycles are typically more beneficial.
An example of the relationship between the processing path and the phase
stability in a
particular alloy of the invention is depicted in FIGS. 2A and 2B.
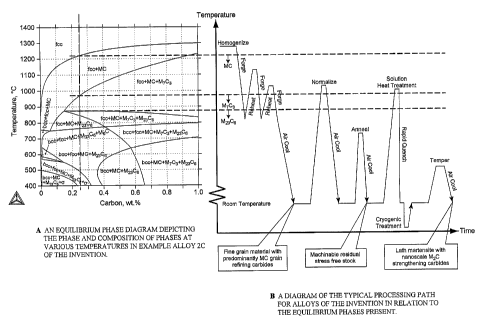
FIG. 2A depicts the equilibrium phases of alloy 2C of the invention wherein
the carbon
15 content is 0.23% by weight as shown in TABLE 1.
FIG. 2B then discloses the processing sequence employed with respect to the
described
alloy 2C. After forming the melt via a melt processing step, the alloy is
homogenized at a metal
temperature exceeding the single phase (fcc) equilibrium temperature of about
1220 C. All
carbides are solubilized at this temperature. Forging to define a desired
billet, rod or other
shape results in cooling into a range where various complex carbides may form.
The forging
step may be repeated by reheating at least to the metal temperature range (980
C to 1220 C)
where only MC carbides are at equilibrium.
Subsequent cooling (air cool) will generally result in retention of primarily
MC
carbides, other primary alloy carbides such as M7C3 and M23C6 and the
formation of generally a
martensitic matrix. Normalization in the same metal temperature range followed
by cooling
dissolves the M7C3 and M23C6 primary carbides while preserving the MC
carbides. Annealing
in the metal temperature range 600 C or 610 C to 840 C or 850 C and cooling
reduces the
hardness level to a reasonable value for machining. The annealing process
softens the
martensite by precipitating carbon into alloy carbides that are too large to
significantly
strengthen the'alloy yet are small enough to be readily dissolved during later
solution treatment.
This process is followed by delivery of the alloy product to a customer for
final manufacture of
a component part and appropriate heat treating and finishing.
CA 02475248 2007-03-21
61368-1244
16
Typically the customer will form the alloy into a desired shape. This will be
followed
by solution heat treatment in the MC carbide temperature range and then
subsequent rapid
quenching to maintain or form the desired martensitic structure. Tempering and
cooling as
previously described may then be employed to obtain strength and fracture
toughness as
desired.
Experimental Results and Examples
A series of prototype alloys were prepared. The melt practice for the refining
process
was selected to be a double vacuum melt with La and Ce impurity Bettering
additions.
Substitutional grain boundary cohesion enhancers such as W and Re were not
considered in the
making of the first prototype, but an addition of twenty parts per million B
was included for
this purpose. For the deoxidation process, Ti was added as a deoxidation
agent, promoting TiC
particles to pin the grain boundaries and reduce grain growth during solution
treatment prior to
tempering.
The major alloying elements in the first prototype are C, Mo, and V (M2C
carbide
formers), Cr (M2C carbide former and oxide passive film former), and Co and Ni
(for various
required matrix properties). The exact alloy composition and material
processing parameters
were determined by an overall design synthesis considering the linkages and a
suite of
computational models described elsewhere [Olson, G. B, "Computational Design
of
Hierarchically Structured Materials.", Science 277, 1237-1242, 1997]. The
following is a
summary of the initial prototype procedure. Selected parameters
are indicated in FIGS. 3-6 by a star (*).
The amount of Cr was determined by the corrosion resistance requirement and a
passivation thermodynamic model developed by Campbell [Campbell, C, Systems
Design of
High. Performance Stainless Steels, Materials Science and Engineering,
Evanston, IL,
Northwestern 243, 1997]. The amount of C was determined by
the strength requirement and an M2C precipitation/strengthening model
according to the
correlation illustrated in FIG. 3. Based on the goal of achieving 53 HRC
hardness, a C content
of 0.14% by weight was selected. The tempering temperature and the amounts of
M2C carbide
formers Mo and V were determined to meet the strength requirement with
adequate M2C
precipitation kinetics, maintain a 1000 C solution treatment temperature, and -
avoid
microsegregation. FIGS. 4 and 5 illustrate how the final V and Mo contents
were determined.
Final contents by weight of 1.5% Mo and 0.5% V were selected. The level of
solidification
microsegregation is assessed by solidification simulation for the
solidification cooling rate and
CA 02475248 2004-08-03
WO 03/076676 PCT/US03/03682
17
associated dendrite arm spacing of anticipated ingot processing. Amounts of Co
and Ni were
determined to (1) maintain a martensite start temperature of at least 200 C,
using a model
calibrated to Ms temperatures measured by quenching dilatometry and 1%
transformation
fraction, so a lath martensite matrix structure can be achieved after
quenching, (2) maintain a
high M2C carbide initial driving force for efficient strengthening, (3)
improve the bcc cleavage
resistance by maximizing the Ni content, and (4) maintain the Co content above
8% by weight
to achieve sufficient dislocation recovery resistance to enhance M2C
nucleation and increase Cr
partitioning to the oxide film by increasing the matrix Cr activity. FIG. 6
shows that, with other
alloy element amounts and the tempering temperature set at their final levels,
optimization of
the above four factors results in the selection of Co and Ni amounts of about
13% and 4.8% by
weight, respectively. The material composition and tempering temperature were
fine-tuned by
inspecting the driving force ratios between M2C and other carbides and
intermetallic phases
with reference to past studies of other precipitation hardened Ni-Co steels.
The composition of the first design prototype designated 1 is given in TABLE 1
along
with later design iterations. The initial design included the following
processing parameters:
= a double vacuum melt with impurity gettering and Ti deoxidation;
= a minimum solution treatment temperature of 1005 C, where this temperature
is limited
by vanadium carbide (VC) formation according to thermodynamic equilibrium; and
= a tempering temperature of 482 C with an estimated tempering time of three
hours to
achieve optimum strength and toughness.
Evaluation of the first prototype (entry 1 in TABLE 1) gave promising results
for all
properties evaluated. The most significant deficiencies were a lower than
desired Ms
temperature by 25 C to 50 C and a strength level 15% below objectives. A
second series of
designs denoted 2A, 2B and 2C in TABLE 1 were then evaluated. All three second-
iteration
prototypes gave satisfactory transformation temperatures, and the best
mechanical properties of
the second iteration were exhibited by alloy 2C. Based on the latter base
composition, a third-
iteration series of alloys designated 3A, 3B and 3C in TABLE 1 explored minor
variations in
grain-refining MC carbides, comparing TiC, (Ti,V)C, and NbC. Principal
parameters were MC
phase fraction and coarsening resistance at solution temperatures, subject to
the constraint of
full MC solubility at homogenization temperatures. Selecting (Ti,V)C as the
optimal grain
refining approach, a fourth-iteration design series designated 4A through 4G
in TABLE 1
examined (a) refinement of martensitic transformation kinetics to minimize
retained austenite
CA 02475248 2010-07-07
61368-1244
18
content, (b) increased stability of competing M2C carbides to promote full
dissolution of
cementite during M2C precipitation strengthening in order to enhance fracture
toughness and
(c) utilized lower temperature iron (Fe) based M2C precipitation strengthening
to completely
avoid the precipitation of cementite and enhance cleavage resistance.
Modification of carbide
thermodynamics and kinetics in the latter two series included additions of W
and Si.
A fifth series of alloys, designated 5B through 5F in TABLE 1, examined the
limits of
Ni that can be added to the alloy to improve fracture toughness by lowering
the ductile to brittle
transition temperature. While the alloy Ms for these compositions falls below
room temperature
as the 'Ni content reaches to about 10 percent by weight, it was found that
tempering the alloy
in multiple steps with cryogenic cooling between each step was able to convert
the majority of
the retained austenite to martensite. This allows good strength properties to
be achieved in
combination with high Ni content to control ductile fracture behavior even in
alloys that are
fully austenitic after quenching. Although multiple tempering has been
commonly used to
minimize retained austenite in steels, it was unexpected that the technique
could be used
effectively in alloys with such high Ni contents and high austenite contents.
The sixth series of alloys, designated 6A through 6M in TABLET, was determined
to
incorporate the features represented in the first five series and are
considered preferred
embodiments of the invention. Thus, appropriate processing of the described
alloys provides
an essentially martensitic phase.
Following is a summary of the described experiments and alloys:
CA 02475248 2004-08-03
WO 03/076676 PCT/US03/03682
19
TABLE 1
Note: All values in % by weight
Alloy c Co Ni Cr Mo W Si V Ti Nb
1 0.15 13.0 4.8 9.0 1.5 - - 0.50 0.02 -
2A 0.18 12.5 2.8 9.1 1.3 - - 0.29 0.03 -
2B 0.11 16.7 3.7 9.2 2.0 - - 0.50 0.03 -
2C 0.23 12.5 2.8 9.0 1.3 - - 0.30 0.03 -
3A 0.24 12.4 2.8 9.0 1.3 - - 0.29 0.02 -
3B 0.24 12.4 2.8 9.1 1.3 - - 0.37 0.03
3C 0.24 12.4 2.8 9.0 1.3 - - 0.34 - 0.03
4A 0.24 12.2 2.0 9.1 1.3 - - 0.29 0.02 -
4B 0.25 12.4 2.7 8.2 1.3 - - 0.29 0.02 -
4C 0.20 12.4 2.1 8.2 1.3 - - 0.29 0.02 -
4D 0.19 14.2 2.8 6.8 2.4 1.3 - 0.28 0.02 -
4E 0.19 12.1 2.0 8.2 1.3 2.0 - 0.28 0.02 -
4F 0.21 14.2 2.6 8.2 1.3 - 0.6 0.29 0.02 -
4G 0.26 12.6 1.7 8.5 0.29 - - 0.30 0.02 -
5B 0.24 13.0 5.1 8.9 1.7 - - 0.29 0.03 -
5C 0.25 12.2 6.2 9.0 1.3 - - 0.29 0.03 -
5D 0.22 15.3 4.6 8.5 1.5 0.5 - 0.28 0.03 -
5E 0.24 13.0 7.4 8.9 1.7 - - 0.28 0.03 -
5F 0.24 13.0 8.7 8.9 1.5 - - 0.28 0.02 -
6A 0.24 14.0 6.0 9.0 1.5 0.9 0.25 0.02
6B 0.22 14.0 5.4 9.0 1.0 1.5 0.30 0.02
6C 0.22 14.0 7.2 9.0 1.0 1.0 0.30 0.02
6D 0.23 14.0 6.5 9.0 1.0 1.2 0.30 0.02
6E 0.23 14.1 7.0 9.1 1.0 1.0 0.31 0.02
6F 0.21 14.0 5.5 10.0 2.0 1.0 0.30 0.02
6G 0.24 13.0 7.4 8.5 1.4 2.0 0.30 0.02
6H 0.23 12.5 2.8 9.0 1.3 2.75 0.30 0.02
61 0.22 14.0 5.5 8.5 2.0 1.4 0.7 0.30 0.02
6J 0.21 14.0 7.5 9.0 1.0 1.0 0.30 0.02
6K 0.19 14.0 8.1 9.0 1.0 1.0 0.30 0.02
6L 0.21 8.0 6.0 9.0 2.0 2.0 0.30 0.02
6M 0.20 8.0 6.0 9.0 2.0 2.0 0.7 0.30 0.02
CA 02475248 2004-08-03
WO 03/076676 PCT/US03/03682
Example 1
Alloy 1 in TABLE 1 was vacuum induction melted (VIM) to a six inch diameter
electrode which was subsequently vacuum arc remelted (VAR) to a eight inch
diameter ingot.
The material was homogenized for seventy-two hours at 1200 C, forged and
annealed
5 according to the preferred processing techniques described above and
depicted in FIG 2A and
2B. Dilatometer samples were machined and the MS temperature was measured as
175 C by
quenching dilatometry and 1 % transformation fraction.
Test samples were machined, solution heat treated at 1025 C for one hour, oil
quenched, immersed in liquid nitrogen for one hour, warmed to room temperature
and
10 tempered at 482 C for eight hours. The measured properties are listed in
TABLE 2 below.
TABLE 2
Various measured properties for Alloy 1
Property Value
Yield Strength 205 ksi
Ultimate Tensile Strength 245 ksi
Elongation 10%
Reduction of Area 48%
Hardness 51 HRC
Example 2
Alloy 2A in TABLE 1 was vacuum induction melted (VIM) to a six inch diameter
15 electrode which was subsequently vacuum arc remelted (VAR) to a eight inch
diameter ingot.
The ingot was homogenized for twelve hours at 1190 C, forged and rolled to
1.500 inch square
bar starting at 1120 C, and annealed according to the preferred processing
techniques described
above and depicted in FIG 2A and 2B. Dilatometer samples were machined and the
MS
temperature was measured as 265 C by quenching dilatometry and 1%
transformation fraction.
20 Test samples were machined from the square bar, solution heat treated at
1050 C for
one hour, oil quenched, immersed in liquid nitrogen for one hour, warmed to
room temperature,
tempered at 500 C for five hours, air cooled, immersed in liquid nitrogen for
one hour, warmed
to room temperature and tempered at 500 C for five and one-half hours. The
measured
properties are listed in TABLE 3 below. The reference to the corrosion rate of
15-5PH (H900
CA 02475248 2004-08-03
WO 03/076676 PCT/US03/03682
21
condition) was made using a sample tested under identical conditions. The
average corrosion
rate for 15-5PH (H900 condition) for this test was 0.26 mils per year (mpy).
TABLE 3
Various measured properties for Alloy 2A
Property Value
Yield Strength 197 ksi
Ultimate Tensile Strength 259 ksi
Elongation 14%
Reduction of Area 64%
Hardness 51.5 HRC
KI, Fracture Toughness 41 ksi in
Open Circuit Potential (OCP) -0.33 V
Average Corrosion Rate 0.52 mpy (200% of 15-5PH H900 Condition)
Kiser. 25 ksi in
Nitrided Surface Hardness 1100 HV (70 HRC)
Tensile samples were machined from the square bar, solution heat treated at
1025 C for
seventy-five minutes, oil quenched, immersed in liquid nitrogen for one hour,
warmed to room
temperature, multi-step tempered at 496 C for either four hours or six hours
with liquid
nitrogen (LN2) treatments for one hour in between the temper steps. The
measured tensile
properties are listed in TABLE 4 below.
TABLE 4
Measured tensile properties for Alloy 2A
Ultimate
Yield Tensile
Strength Strength Elongation Reduction of Area
Temper Treatment (ksi) (ksi) (%) (%)
12h 208 264 17 64
6h+LN2+6h 216 261 17 65
4h+LN2+4h+LN2+4h 203 262 15 64
CA 02475248 2004-08-03
WO 03/076676 PCT/US03/03682
22
Example 3
Alloy 2B in TABLE 1 was vacuum induction melted (VIM) to a six inch diameter
electrode which was subsequently vacuum arc remelted (VAR) to a eight inch
diameter ingot.
The ingot was homogenized for twelve hours at 1190 C, forged and rolled to
1.000 inch
diameter round bar starting at 1120 C and annealed according to the preferred
processing
techniques described above and depicted in FIG 2A and 2B. Dilatometer samples
were
machined and the MS temperature was measured as 225 C by quenching dilatometry
and 1%
transformation fraction.
Test samples were machined from the round bar, solution heat treated at 1100 C
for
70 minutes, oil quenched, immersed in liquid nitrogen for one hour, warmed to
room
temperature and tempered at 482 C for twenty-four hours. The measured
properties are listed in
TABLE 5 below.
TABLE 5
Various measured properties for Alloy 2B
Property Value
Yield Strength 211 ksi
Ultimate Tensile Strength 247 ksi
Elongation 17%
Reduction of Area 62 %
Hardness 51 HRC
Example 4
Alloy 2C in TABLE 1 was vacuum induction melted (VIM) to a six inch diameter
electrode which was subsequently vacuum arc remelted (VAR) to a eight inch
diameter ingot.
The ingot was homogenized for twelve hours at 1190 C, forged to 2.250 inch
square bar
starting at 1120 C and annealed according to the preferred processing
techniques described
above and depicted in FIG 2A and 2B. Dilatometer samples were machined and the
MS
temperature was measured as 253 C by quenching dilatometry and 1%
transformation fraction.
Test samples were machined from the square bar, solution heat treated at 1025
C for
75 minutes, oil quenched, immersed in liquid nitrogen for one hour, warmed to
room
CA 02475248 2004-08-03
WO 03/076676 PCT/US03/03682
23
temperature, tempered at 498 C for eight hours. The measured properties are
listed in TABLE
6 below.
TABLE 6
Various measured properties for Alloy 2C
Property Value
Yield Strength 221 ksi
Ultimate Tensile Strength 297 ksi
Elongation 12.5%
Reduction of Area 58%
Hardness 55 HRC
KI Fracture Toughness 42 ksi in
Test samples were machined from the square bar, solution heat treated at 1025
C for
75 minutes, oil quenched, immersed in liquid nitrogen for one hour, warmed to
room
temperature, tempered at 498 C for twelve hours. The measured properties are
listed in TABLE
7 below.
TABLE 7
Various measured properties for Alloy 2C
Property Value
Yield Strength 223 ksi
Ultimate Tensile Strength 290 ksi
Elongation 13%
Reduction of Area 62%
Hardness 54 HRC
KI, Fracture Toughness 43 ksi in
Corrosion test samples were machined from the square bar, solution heat
treated at
1025 C for 75 minutes, oil quenched, immersed in liquid nitrogen for one hour,
warmed to
room temperature, tempered at 498 C for eight hours, air cooled and tempered
at 498 C for
four hours. The measured properties are listed in TABLE 8 below. The reference
to the
corrosion rate of 15-5PH (H900 condition) was made using a sample tested under
identical
CA 02475248 2004-08-03
WO 03/076676 PCT/US03/03682
24
conditions. The average corrosion rate for 15-5PH (H900 condition) for this
test was 0.26 mils
per year (mpy).
TABLE 8
Various measured properties for Alloy 2C
Property Value
Open Circuit Potential (OCP) -0.32 V
Average Corrosion Rate 0.40 mpy (150% of 15-5PH H900 Condition)
Tensile samples were machined from the square bar, solution heat treated at
1025 C for
75 minutes, oil quenched, immersed in liquid nitrogen for one hour, warmed to
room
temperature, multi-step tempered at 496 C for either four hours or six hours
with liquid
nitrogen (LN2) treatments for one hour in between the temper steps. The
measured tensile
properties are listed in TABLE 9 below.
TABLE 9
Measured tensile properties for Alloy 2C
Ultimate
Yield Tensile Reduction
Strength Strength Elongation of Area Hardness
Temper Treatment [ksi] [ksi] [%] [%] [HRC]
12h 213 293 17 63 55.5
6h+LN2+6h 227 295 15 51 56
4h+LN2+4h+LN2+4h 223 294 18 64 55.5
Example 5
Alloy 3A in TABLE 1 was vacuum induction melted (VIM) to a six inch diameter
electrode which was subsequently vacuum are remelted (VAR) to a eight inch
diameter ingot.
The ingot was homogenized for twelve hours at 1260 C, forged to 2.250 inch
square bar
starting at 1090 C and annealed according to the preferred processing
techniques described
above and depicted in FIG 2A and 2B. Dilatometer samples were machined and the
MS
temperature was measured as 250 C by quenching dilatometry and 1%
transformation fraction.
CA 02475248 2004-08-03
WO 03/076676 PCT/US03/03682
Test samples were machined from the square bar, solution heat treated at 1025
C for
75 minutes, oil quenched, immersed in liquid nitrogen for one hour, warmed to
room
temperature, tempered at 510 C for five hours. The measured properties are
listed in TABLE
10 below.
5 TABLE 10
Various measured properties for Alloy 3A
Property Value
Yield Strength 228 ksi
Ultimate Tensile Strength 284 ksi
Elongation 16%
Reduction of Area 60%
Hardness 54 HRC
KI, Fracture Toughness 37 ksi in
Test samples were machined from the square bar, solution heat treated at 1025
C for
75 minutes, oil quenched, immersed in liquid nitrogen for one hour, warmed to
room
temperature, multi-step tempered at 510 C for four hours followed by liquid
nitrogen (LN2)
10 treatment for one hour and finally tempered at 510 C for an additional four
hours. The
measured properties are listed in TABLE 11 below.
TABLE 11
Various measured properties for Alloy 3A
Property Value
Yield Strength 226 ksi
Ultimate Tensile Strength 279 ksi
Elongation 16%
Reduction of Area 61%
Hardness 54 HRC
KI, Fracture Toughness 38 ksi in
Corrosion test samples were machined from the square bar, solution heat
treated at
15 1025 C for 75 minutes, oil quenched, immersed in liquid nitrogen for one
hour, warmed to
CA 02475248 2004-08-03
WO 03/076676 PCT/US03/03682
26
room temperature, and tempered at 200 C for one hour. The measured properties
are listed in
TABLE 12 below. The reference to the corrosion rate of 15-5PH (H900 condition)
was made
using a sample tested under identical conditions. The average corrosion rate
for 15-5PH (H900
condition) for this test was 0.20 mils per year (mpy).
TABLE 12
Various measured properties for Alloy 3A
Property Value
Open Circuit Potential (OCP) -0.29 V
Average Corrosion Rate 0.51 mpy (255% of 15-5PH H900 Condition)
Corrosion test samples were machined from the square bar, solution heat
treated at
1025 C for 75 minutes, oil quenched, immersed in liquid nitrogen for one hour,
warmed to
room temperature, and tempered at 510 C for eight hours. The measured
properties are listed in
TABLE 13 below.
TABLE 13
Various measured properties for Alloy 3A
Property Value
Open Circuit Potential (OCP) -0.26 V
Average Corrosion Rate 0.38 mpy (190% of 15-5PH H900 Condition)
Example 6
Alloy 3B in TABLE 1 was vacuum induction melted (VIM) to a six inch diameter
electrode which was subsequently vacuum arc remelted (VAR) to a eight inch
diameter ingot.
The ingot was homogenized for twelve hours at 1260 C, forged to 2.250 inch
square bar
starting at 1090 C and annealed according to the preferred processing
techniques described
above and depicted in FIG 2A and 2B. Dilatometer samples were machined and the
MS
temperature was measured as 240 C by quenching dilatometry and 1%
transformation fraction.
Test samples were machined from the square bar, solution heat treated at 1025
C for
75 minutes, oil quenched, immersed in liquid nitrogen for one hour, warmed to
room
CA 02475248 2004-08-03
WO 03/076676 PCT/US03/03682
27
temperature, and finally tempered at 510 C for five hours. The measured
properties are listed in
TABLE 14 below.
TABLE 14
Various measured properties for Alloy 3B
Property Value
Yield Strength 235 ksi
Ultimate Tensile Strength 288 ksi
Elongation 16%
Reduction of Area 60%
Hardness 54 HRC
KI, Fracture Toughness 38 ksi in
Test samples were machined from the square bar, solution heat treated at 1025
C for
75 minutes, oil quenched, immersed in liquid nitrogen for one hour, warmed to
room
temperature, multi-step tempered at 510 C for four hours followed by liquid
nitrogen (LN2)
treatment for one hour and finally tempered at 510 C for an additional four
hours. The
measured properties are listed in TABLE 15 below.
TABLE 15
Various measured properties for Alloy 3B
Property Value
Yield Strength 234 ksi
Ultimate Tensile Strength 281 ksi
Elongation 15%
Reduction of Area 62%
Hardness 54 HRC
KI, Fracture Toughness 35 ksi in
Example 7
Alloy 4A in TABLE 1 was vacuum induction melted (VIM) to a four inch diameter
electrode which was subsequently vacuum arc remelted (VAR) to a five inch
diameter ingot.
CA 02475248 2004-08-03
WO 03/076676 PCT/US03/03682
28
The ingot was homogenized for twelve hours at 1250 C, hot rolled to two inch
round corner
square using frequent reheats at 1015 C, hot rolled to 0.750 inch thick by
2.250 inch wide
rectangular bar, normalized and annealed according to the preferred processing
techniques
described above and depicted in FIG 2A and 2B. Dilatometer samples were
machined and the
MS temperature was measured as 275 C by quenching dilatometry and 1%
transformation
fraction.
Corrosion test samples were machined from the rectangular bar, solution heat
treated at
1025 C for 75 minutes, oil quenched, immersed in liquid nitrogen for one hour,
warmed to
room temperature, and tempered at 510 C for twelve hours. The measured
properties are listed
in TABLE 16 below. The reference to the corrosion rate of 15-5PH (11900
condition) was made
using a sample tested under identical conditions. The average corrosion rate
for 15-5PH (11900
condition) for this test was 0.20 mils per year (mpy).
TABLE 16
Various measured properties for Alloy 4A
Property Value
Open Circuit Potential (OCP) -0.28 V
Average Corrosion Rate 0.45 mpy (225% of 15-5PH H900 Condition)
Corrosion test samples were machined from the rectangular bar, solution heat
treated at
1025 C for 75 minutes, oil quenched, immersed in liquid nitrogen for one hour,
warmed to
room temperature, and tempered at 510 C for twenty-four hours. The measured
properties are
listed in TABLE 17 below.
TABLE 17
Various measured properties for Alloy 4A
Property Value
Hardness 53 HRC
Open Circuit Potential (OCP) -0.38 V
Average Corrosion Rate 0.88 mpy
CA 02475248 2004-08-03
WO 03/076676 PCT/US03/03682
29
Example 8
Alloy 4B in TABLE 1 was vacuum induction melted (VIM) to a four inch diameter
electrode which was subsequently vacuum arc remelted (VAR) to a five inch
diameter ingot.
The ingot was homogenized for twelve hours at 1250 C, hot rolled to two inch
round corner
square using frequent reheats at 1015 C, hot rolled to 0.750 inch thick by
2.250 inch wide
rectangular bar, normalized and annealed according to the preferred processing
techniques
described above and depicted in FIG 2A and 2B. Dilatometer samples were
machined and the
MS temperature was measured as 285 C by quenching dilatometry and 1%
transformation
fraction.
Corrosion test samples were machined from the rectangular bar, solution heat
treated at
1025 C for 75 minutes, oil quenched, immersed in liquid nitrogen for one hour,
warmed to
room temperature, and tempered at 510 C for twelve hours. The measured
properties are listed
in TABLE 18 below. The reference to the corrosion rate of 15-5PH (H900
condition) was made
using a sample tested under identical conditions. The average corrosion rate
for 15-5PH (H900
condition) for this test was 0.20 mils per year (mpy).
TABLE 18
Various measured properties for Alloy 4B
Property Value
Hardness 54 HRC
Open Circuit Potential (OCP) -0.33 V
Average Corrosion Rate 1.05 mpy (525% of 15-5PH H900 Condition)
Example 9
Alloy 4C in TABLE 1 was vacuum induction melted (VIM) to a four inch diameter
electrode which was subsequently vacuum arc remelted (VAR) to a five inch
diameter ingot.
The ingot was homogenized for twelve hours at 1250 C, hot rolled to two inch
round corner
square using frequent reheats at 1015 C, hot rolled to 0.750 inch thick by
2.250 inch wide
rectangular bar, normalized and annealed according to the preferred processing
techniques
described above and depicted in FIG 2A and 2B. Dilatometer samples were
machined and the
MS temperature was measured as 310 C by quenching dilatometry and 1%
transformation
fraction.
CA 02475248 2004-08-03
WO 03/076676 PCT/US03/03682
Test samples were machined from the rectangular bar, solution heat treated at
1025 C
for 75 minutes, oil quenched, immersed in liquid nitrogen for one hour, warmed
to room
temperature, multi-step tempered at 200 C for two hours followed by liquid
nitrogen (LN2)
treatment for one hour and finally tempered at 200 C for an additional two
hours. The
5 measured properties are listed in TABLE 19 below.
TABLE 19
Various measured properties for Alloy 4C
Property Value
Yield Strength 197 ksi
Ultimate Tensile Strength 258 ksi
Elongation 11%
Reduction of Area 37%
Hardness 51 HRC
Example 10
Alloy 4D in TABLE 1 was vacuum induction melted (VIM) to a four inch diameter
10 electrode which was subsequently vacuum arc remelted (VAR) to a five inch
diameter ingot.
The ingot was homogenized for twelve hours at 1250 C, hot rolled to two inch
round corner
square using frequent reheats at 1015 C, hot rolled to 0.750 inch thick by
2.250 inch wide
rectangular bar, normalized and annealed according to the preferred processing
techniques
described above and depicted in FIG 2A and 2B. Dilatometer samples were
machined and the
15 MS temperature was measured as 300 C by quenching dilatometry and 1%
transformation
fraction.
Test samples were machined from the rectangular bar, solution heat treated at
1025 C
for 75 minutes, oil quenched, immersed in liquid nitrogen for one hour, warmed
to room
temperature, multi-step tempered at 200 C for two hours followed by liquid
nitrogen (LN2)
20 treatment for one hour and finally tempered at 200 C for an additional two
hours. The
measured properties are listed in TABLE 20 below.
CA 02475248 2004-08-03
WO 03/076676 PCT/US03/03682
31
TABLE 20
Various measured properties for Alloy 4D
Property Value
Yield Strength 199 ksi
Ultimate Tensile Strength 263 ksi
Elongation 13%
Reduction of Area 17%
Hardness 53 HRC
Corrosion test samples were machined from the rectangular bar, solution heat
treated at
1000 C for 75 minutes, oil quenched, immersed in liquid nitrogen for one hour,
warmed to
room temperature, and tempered at 510 C for twelve hours. The measured
properties are listed
in TABLE 21 below. The reference to the corrosion rate of 15-5PH (H900
condition) was made
using a sample tested under identical conditions. The average corrosion rate
for 15-5PH (H900
condition) for this test was 0.20 mils per year (mpy).
TABLE 21
Various measured properties for Alloy 4D
Property Value
Open Circuit Potential (OCP) -0.35 V
Average Corrosion Rate 1.12 mpy (5 60% of 15-5PH H900 Condition)
Example 11
Alloy 4E in TABLE 1 was vacuum induction melted (VIM) to a four inch diameter
electrode which was subsequently vacuum arc remelted (VAR) to a five inch
diameter ingot.
The ingot was homogenized for twelve hours at 1250 C, hot rolled to two inch
round corner
square using frequent reheats at 1015 C, hot rolled to 0.750 inch thick by
2.250 inch wide
rectangular bar, normalized and annealed according to the preferred processing
techniques
described above and depicted in FIG 2A and 2B. Dilatometer samples were
machined and the
MS temperature was measured as 300 C by quenching dilatometry and 1%
transformation
fraction.
CA 02475248 2004-08-03
WO 03/076676 PCT/US03/03682
32
Example 12
Alloy 4F in TABLE 1 was vacuum induction melted (VIM) to a four inch diameter
electrode which was subsequently vacuum arc remelted (VAR) to a five inch
diameter ingot.
The ingot was homogenized for twelve hours at 1250 C, hot rolled to two inch
round corner
square using frequent reheats at 1015 C, hot rolled to 0.750 inch thick by
2.250 inch wide
rectangular bar, normalized and annealed according to the preferred processing
techniques
described above,and depicted in FIG 2A and 2B. Dilatometer samples were
machined and the
MS temperature was measured as 300 C by quenching dilatometry and 1%
transformation
fraction.
Test samples were machined from the rectangular bar, solution heat treated at
1025 C
for 75 minutes, oil quenched, immersed in liquid nitrogen for one hour, warmed
to room
temperature, multi-step tempered at 200 C for two hours followed by liquid
nitrogen (LN2)
treatment for one hour and finally tempered at 200 C for an additional two
hours. The
measured properties are listed in TABLE 22 below.
TABLE 22
Various measured properties for Alloy 4F
Property Value
Yield Strength 202 ksi
Ultimate Tensile Strength 267 ksi
Elongation 11%
Reduction of Area 15%
Hardness 51 HRC
Corrosion test samples were machined from the rectangular bar, solution heat
treated at
1000 C for 75 minutes, oil quenched, immersed in liquid nitrogen for one hour,
warmed to
room temperature, and tempered at 510 C for twelve hours. The measured
properties are listed
in TABLE 23 below. The reference to the corrosion rate of 15-5PH (H900
condition) was made
using a sample tested under identical conditions. The average corrosion rate
for 15-5PH (H900
condition) for this test was 0.20 mils per year (mpy).
CA 02475248 2004-08-03
WO 03/076676 PCT/US03/03682
33
Table 23
Various measured properties for Alloy 4F
Property Value
Open Circuit Potential (OCP) -0.33 V
Average Corrosion Rate 0.62 ropy (3 10% of 15-5PH H900 Condition)
Example 13
Alloy 4G in TABLE 1 was vacuum induction melted (VIM) to a four inch diameter
electrode which was subsequently vacuum arc remelted (VAR) to a five inch
diameter ingot.
The ingot was homogenized for twelve hours at 1250 C, hot rolled to two inch
round corner
square using frequent reheats at 1015 C, hot rolled to 0.750 inch thick by
2.250 inch wide
rectangular bar, normalized and annealed according to the preferred processing
techniques
described above and depicted in FIG 2A and 2B. Dilatometer samples were
machined and the
MS temperature was measured as 320 C by quenching dilatometry and 1%
transformation
fraction.
Example 14
Alloy 5B in TABLE 1 was vacuum induction melted (VIM) to a four inch diameter
electrode which was subsequently vacuum arc remelted (VAR) to a five inch
diameter ingot.
The ingot was homogenized for twelve hours at 1250 C, hot rolled to two inch
round corner
square using frequent reheats at 1015 C, hot rolled to 0.750 inch thick by
2.250 inch wide
rectangular bar, normalized and annealed according to the preferred processing
techniques
described above and depicted in FIG 2A and 2B. Dilatometer samples were
machined and the
MS temperature was measured as 200 C by quenching dilatometry and 1%
transformation
fraction.
Test samples were machined from the rectangular bar, solution heat treated at
1025 C
for 75 minutes, oil quenched, immersed in liquid nitrogen for one hour, warmed
to room
temperature, multi-step tempered at 468 C for twenty-four hours followed by
liquid nitrogen
(LN2) treatment for one hour and finally tempered at 468 C for an additional
twenty-
four hours. The measured properties are listed in TABLE 24 below.
CA 02475248 2004-08-03
WO 03/076676 PCT/US03/03682
34
TABLE 24
Various measured properties for Alloy 5B
Property Value
Yield Strength 204 ksi
Ultimate Tensile Strength 265 ksi
Elongation 16%
Reduction of Area 63%
Hardness 52 HRC
Test samples were machined from the rectangular bar, solution heat treated at
1025 C
for 75 minutes, oil quenched, immersed in liquid nitrogen for one hour, warmed
to room
temperature, multi-step tempered at 468 C for thirty-six hours followed by
liquid nitrogen
(LN2) treatment for one hour and finally tempered at 468 C for an additional
thirty-six hours.
The measured properties are listed in TABLE 25 below.
TABLE 25
Various measured properties for Alloy 5B
Property Value
Yield Strength 211 ksi
Ultimate Tensile Strength 294 ksi
Elongation 15%
Reduction of Area 55%
Hardness 55 HRC
Example 15
Alloy 5C in TABLE 1 was vacuum induction melted (VIM) to a four inch diameter
electrode which was subsequently vacuum arc remelted (VAR) to a five inch
diameter ingot.
The ingot was homogenized for twelve hours at 1250 C, hot rolled to two inch
round corner
square using frequent reheats at 1015 C, hot rolled to 0.750 inch thick by
2.250 inch wide
rectangular bar, normalized and annealed according to the preferred processing
techniques
described above and depicted in FIG 2A and 2B. Dilatometer samples were
machined and the
CA 02475248 2004-08-03
WO 03/076676 PCT/US03/03682
MS temperature was measured as 180 C by quenching dilatometry and 1%
transformation
fraction.
Test samples were machined from the rectangular bar, solution heat treated at
1025 C
for 75 minutes, oil quenched, immersed in liquid nitrogen for one hour, warmed
to room
5 temperature, multi-step tempered at 468 C for sixteen hours followed by
liquid nitrogen (LN2)
treatment for one hour and finally tempered at 468 C for an additional sixteen
hours. The
measured properties are listed in TABLE 26 below.
TABLE 26
Various measured properties for Alloy 5C
Property Value
Yield Strength 204 ksi
Ultimate Tensile Strength 261 ksi
Elongation 16%
Reduction of Area 63%
Hardness 49 HRC
10 Example 16
Alloy 5D in TABLE 1 was vacuum induction melted (VIM) to a four inch diameter
electrode which was subsequently vacuum are remelted (VAR) to a five inch
diameter ingot.
The ingot was homogenized for twelve hours at 1250 C, hot rolled to two inch
round corner
square using frequent reheats at 1015 C, hot rolled to 0.750 inch thick by
2.250 inch wide
15 rectangular bar, normalized and annealed according to the preferred
processing techniques
described above and depicted in FIG 2A and 2B. Dilatometer samples were
machined and the
MS temperature was measured as 240 C by quenching dilatometry and 1%
transformation
fraction.
Test samples were machined from the rectangular bar, solution heat treated at
1025 C
20 for 75 minutes, oil quenched, immersed in liquid nitrogen for one hour,
warmed to room
temperature, multi-step tempered at 468 C for twenty-four hours followed by
liquid nitrogen
(LN2) treatment for one hour and finally tempered at 468 C for an additional
twenty-
four hours. The measured properties are listed in TABLE 27 below.
CA 02475248 2004-08-03
WO 03/076676 PCT/US03/03682
36
TABLE 27
Various measured properties for Alloy 5D
Property Value
Yield Strength 228 ksi
Ultimate Tensile Strength 276 ksi
Elongation 16%
Reduction of Area 61%
Hardness 53 HRC
Test samples were machined from the rectangular bar, solution heat treated at
1025 C
for 75 minutes, oil quenched, immersed in liquid nitrogen for one hour, warmed
to room
temperature, and finally tempered at 468 C for twenty-eight hours. The
measured properties are
listed in TABLE 28 below.
TABLE 28
Various measured properties for Alloy 5D
Property Value
Yield Strength 225 ksi
Ultimate Tensile Strength 300 ksi
Elongation 14%
Reduction of Area 46%
Hardness 55 HRC
Test samples were machined from the rectangular bar, solution heat treated at
1025 C
for 75 minutes, oil quenched, immersed in liquid nitrogen for one hour, warmed
to room
temperature, and finally tempered at 468 C for seventy-two hours. The measured
properties are
listed in TABLE 29 below.
CA 02475248 2004-08-03
WO 03/076676 PCT/US03/03682
37
TABLE 29
Various measured properties for Alloy 5D
Property Value
Yield Strength 233 ksi
Ultimate Tensile Strength 294 ksi
Elongation 14%
Reduction of Area 11%
Hardness 54 HRC
Example 17
Alloy 5E in TABLE 1 was vacuum induction melted (VIM) to a four inch diameter
electrode which was subsequently vacuum are remelted (VAR) to a five inch
diameter ingot.
The ingot was homogenized for twelve hours at 1250 C, hot rolled to two inch
round corner
square using frequent reheats at 1015 C, hot rolled to 0.750 inch thick by
2.250 inch wide
rectangular bar, normalized and annealed according to the preferred processing
techniques
described above and depicted in FIG 2A and 2B. Dilatometer samples were
machined and the
MS temperature was measured as 165 C by quenching dilatometry and 1%
transformation
fraction.
Test samples were machined from the rectangular bar, solution heat treated at
1025 C
for 75 minutes, oil quenched, immersed in liquid nitrogen for one hour, warmed
to room
temperature, multi-step tempered at 468 C for sixteen hours followed by liquid
nitrogen (LN2)
treatment for one hour and finally tempered at 468 C for an additional sixteen
hours. The
measured properties are listed in TABLE 30 below.
CA 02475248 2004-08-03
WO 03/076676 PCT/US03/03682
38
TABLE 30
Various measured properties for Alloy 5E
Property Value
Yield Strength 224 ksi
Ultimate Tensile Strength 260 ksi
Elongation 16%
Reduction of Area 59%
Hardness 50 HRC
Test samples were machined from the rectangular bar, solution heat treated at
1025 C
for 75 minutes, oil quenched, immersed in liquid nitrogen for one hour, warmed
to room
temperature, multi-step tempered at 468 C for twenty-four hours followed by
liquid nitrogen
(LN2) treatment for one hour and finally tempered at 468 C for an additional
twenty-
four hours. The measured properties are listed in TABLE 31 below.
TABLE 31
Various measured properties for Alloy 5E
Property Value
Yield Strength 233 ksi
Ultimate Tensile Strength 291
Elongation 13%
Reduction of Area 51%
Hardness 55 HRC
Test samples were machined from the rectangular bar, solution heat treated at
1025 C
for 75 minutes, oil quenched, immersed in liquid nitrogen for one hour, warmed
to room
temperature, multi-step tempered at 468 C for fourteen hours followed by
liquid nitrogen (LN2)
treatment for one hour and finally tempered at 468 C for fourteen hours. The
measured
properties are listed in TABLE 32 below.
CA 02475248 2004-08-03
WO 03/076676 PCT/US03/03682
39
TABLE 32
Various measured properties for Alloy 5E
Property ' Value
Yield Strength 218 ksi
Ultimate Tensile Strength 294 ksi
Elongation 14%
Reduction of Area 47%
Hardness 55 HRC
Example 18
Alloy 5F in TABLE 1 was vacuum induction melted (VIM) to a four inch diameter
electrode which was subsequently vacuum arc remelted (VAR) to a five inch
diameter ingot.
The ingot was homogenized for twelve hours at 1250 C, hot rolled to two inch
round corner
square using frequent reheats at 1015 C, hot rolled to 0.750 inch thick by
2.250 inch wide
rectangular bar, normalized and annealed according to the preferred processing
techniques
described above and depicted in FIG 2A and 2B. Dilatometer samples were
machined and the
MS temperature was measured to be lower than 25 C by quenching dilatometry and
1%
transformation fraction.
Test samples were machined from the rectangular bar, solution heat treated at
1025 C
for 75 minutes, oil quenched, immersed in liquid nitrogen for one hour, warmed
to room
temperature, multi-step tempered at 468 C for sixteen hours followed by liquid
nitrogen (LN2)
treatment for one hour and finally tempered at 468 C for an additional sixteen
hours. The
measured properties are listed in TABLE 33 below.
CA 02475248 2004-08-03
WO 03/076676 PCT/US03/03682
TABLE 33
Various measured properties for Alloy 5F
Property Value
Yield Strength 234 ksi
Ultimate Tensile Strength 254 ksi
Elongation 14%
Reduction of Area 62%
Hardness 49 HRC
Test samples were machined from the rectangular bar, solution heat treated at
1025 C
for 75 minutes, oil quenched, immersed in liquid nitrogen for one hour, warmed
to room
5 temperature, and finally tempered at 468 C for twenty-eight hours. The
measured properties are
listed in TABLE 34 below.
TABLE 34
Various measured properties for Alloy 5F
Property Value
Yield Strength 168 ksi
Ultimate Tensile Strength 265 ksi
Elongation 14%
Reduction of Area 52%
Hardness 50 HRC
Test samples were machined from the rectangular bar, solution heat treated at
1025 C
10 for 75 minutes, oil quenched, immersed in liquid nitrogen for one hour,
warmed to room
temperature, and finally tempered at 468 C for forty-eight hours. The measured
properties are
listed in TABLE 35 below.
CA 02475248 2007-03-21
61368-1244
41
TABLE 35
Various measured properties for Alloy 5F
Property Value
Yield Strength 168 ksi
Ultimate Tensile Strength 246 ksi
Elongation 15%
Reduction of Area 57%
Hardness 49 HRC
The examples of Tables 34 and 35 illustrate the benefits of multi-step
tempering of the
alloys to provide higher strength.
Important to the alloy design is the achievement of efficient strengthening
while
maintaining corrosion resistance and effective hydrogen trapping for stress-
corrosion
resistance. All of these attributes are promoted by refinement of the
strengthening M2C carbide
particle size to an optimal size of about three nanometers at the completion
of precipitation.
FIG. 7 shows the atomic-scale imaging of a three nanometer M2C carbide in the
optimally heat
treated alloy 2C using three-dimensional Atom-Probe microanalysis [M. K.
Miller, Atom Probe
Tomography, Kluwer Academic/Plenum Publishers, New York, NY, 2000)
verifying that the designed size and particle composition have in fact
been achieved. This image is an atomic reconstruction of a slab of the alloy
where each atom is
represented by a dot on the figure with a color and size corresponding to its
element. The drawn
circle in FIG. 7 represents the congregation of alloy carbide formers and
carbon which define
the M2C nanoscale carbide in the image.
As a consequence, the alloys discovered have a range of combinations of
elements as
set forth in TABLE 36.
TABLE 36
All values in % by weight
C Co Ni Cr Si Mn Cu
0.1 to 0.3 8 to 17 0 to 10 6 to 11 <1 <0.5 <0.15
CA 02475248 2004-08-03
WO 03/076676 PCT/US03/03682
42
With one or more of:
Mo Nb V Ta W
<3 <0.3 <0.8 <0.2 <3
And one or more of:
Ti La or other Zr B
rare earths
<0.2 <0.2 <0.15 <0.005
And the balance Fe
Preferably, impurities are avoided; however, some impurities and incidental
elements
are tolerated and within the scope of the invention. Thus, by weight, most
preferably, S is less
than 0.02%, P less than 0.012%, 0 less than 0.015% and N less than 0.015%. The
microstructure is primarily martensitic when processed as described and
desirably is
maintained as lath martensitic with less than 2.5% and preferably less than 1%
by volume,
retained or precipitated austenite. The microstructure is primarily inclusive
of M2C nanoscale
carbides where M is one or more element selected from the group including Mo,
Nb, V, Ta, W
and Cr. The formula, size and presence of the carbides are important.
Preferably, the carbides
are present only in the form of M2C and to some extent, MC carbides,without
the presence of
other carbides and the size (average diameter) is less than about ten
nanometers and preferably
in the range of about three nanometers to five nanometers. Specifically
avoided are other larger
scale incoherent carbides such as cementite, M23C6, M6C and M7C3. Other
embrittling phases,
such as topologically close packed (TCP) intermetallic phases, are also
avoided.
The martensitic matrix in which the strengthening nanocarbides are embedded
contains
an optimum balance of Co and Ni to maintain a sufficiently high Ms temperature
with
sufficient Co to enhance Cr partitioning to the passivating oxide film,
enhance M2C driving
force and maintain dislocation nucleation of nanocarbides. Resistance to
cleavage is enhanced
by maintaining sufficient Ni and promoting grain refinement through stable MC
carbide
dispersions which resist coarsening at the normalizing or solution treatment
temperature. Alloy
composition and thermal processing are optimized to minimize or eliminate all
other dispersed
particles that limit toughness and fatigue resistance. Resistance to hydrogen
stress corrosion is
enhanced by grain boundary segregation of cohesion enhancing elements such as
B, Mo and W,
and through the hydrogen trapping effect of the nanoscale M2C carbide
dispersion. Alloy
CA 02475248 2004-08-03
WO 03/076676 PCT/US03/03682
43
composition is constrained to limit microsegregation under production-scale
ingot solidification
conditions.
The specific alloy compositions of TABLE 1 represent the presently known
preferred
and optimal formulations in this class of alloys, it being understood that
variations of
formulations consistent with the physical properties described, the processing
steps and within
the ranges disclosed as well as equivalents are within the scope of the
invention.
These preferred embodiments can be summarized as seven subclasses of alloy
compositions
presented in TABLE 37. Subclass 1 is similar in composition to alloys 2C, 3A
and 3B of
TABLE 1 and is optimal for a secondary hardening temper at about 400 C to 600
C to
precipitate Cr-Mo base M2C carbides providing a UTS in the range of about 270
ksi to 300 ksi.
Subclass 2 is similar in composition to alloys 4D and 4E of TABLE 1 and
includes additions of
W and/or Si to destabilize cementite and provide greater thermal stability
with a secondary
hardening temper at about 400 C to 600 C to precipitate Cr-Mo-W base M2C
carbides. For
applications requiring higher fracture toughness, subclass 3 is similar in
composition to alloys
1, 2A and 2B in TABLE 1 and provides an intermediate UTS range of about 240
ksi to 270 ksi.
Subclass 4 is similar in composition to alloys 4F and 4G of TABLE 1 and is
optimal for low-
temperature tempering at about 200 C to 300 C to precipitate Fe-base M2C
carbides without the
precipitation of cementite. Alloy subclass 5 is a most preferred embodiment of
subclass 1.
Subclass 6 is similar in composition to alloys 5B through 5F and 6A through
6K. Subclass 6
provides optimal toughness due to the higher Ni content but may require
multiple tempering
treatments with cryogenic treatments between steps in order to avoid
significant amounts of
retained austenite in the final microstructure. Subclass 7 is a further
optimization of fracture
toughness and is similar to alloys 6L and 6M where the lower Co content lowers
the ductile to
brittle transition temperature of the alloy.
CA 02475248 2004-08-03
WO 03/076676 PCT/US03/03682
44
TABLE 37
All values in % by weight
Alloy
C Co Ni Cr Mo W Si V Ti
subclass
1 0.20 to llto 2.0 to 7.5 to 1.0 to < 0.1 < 0.25 0.1 to 0.01 to
0.26 15 3.0 9.5 2.0 0.5 0.05
2 0.20 to 12 to 2.0 to 7.0 to 1.0 to < 2.5 < 0.75 0.1 to 0.01 to
0.25 15 3.0 9.0 3.0 0.5 0.05
3 0.10 to 12 to 2.5 to 8.5 to 1.0 to < 0.1 < 0.25 0.1 to 0.01 to
0.20 17 5.0 9.5 2.0 0.5 0.05
4 0.25 to 11 to 1.0 to 7.0 to < 1.0 < 0.1 < 1.0 0.1to 0.01 to
0.28 15 3.0 9.0 0.5 0.05
0.22 to 12 to 2.5 to 8.5 to 1.0 to < 0.1 < 0.25 0.1 to 0.01 to
0.25 13 3.0 9.5 1.5 0.5 0.05
6 0.18 to 10 to 4.0 to 8.0 to 1.0 to < 3.0 < 1.0 0.1 to 0.01 to
0.25 15 8.0 10.0 3.0 0.5 0.05
0.18to 4.0 to 8.0 to 1.0 to 0.1 to 0.01 to
7 6to10 <3.0 <1.0
0.25 8.0 10.0 3.0 0.5 0.05
Therefore, the invention including the class of ultrahigh-strength, corrosion
resistant,
5 structural steel alloys and the processes for making and using such alloys
is to be limited only
by the following claims and equivalents thereof.