Note: Descriptions are shown in the official language in which they were submitted.
CA 02476034 2004-08-26
' 22935-1180D
PROCESS FOR THE RECOVERY OF A CARBONYLATION PRODUCT
This application is a divisional application of
copendirig application 2,119,609, filed March 22, 1994.
The present invention relates, in one aspect, to a
process for the recovery of a carbonylation product from a
liquid carbonylation reaction composition and in particular to
a process for the recovery of a carbonylation product from a
liquid carbonylation reaction composition comprising free or
combined iridium carbonylation catalyst. In another aspect
the invention relates to a process for preparing a carboxylic
acid.
British Patent GH 1,234,641 describes a process for
the production of an organic acid or an ester by carbonylation
of an alcohol, halide, ester, ether or phenol in the presence
of a noble metal catalyst selected from iridium, platinum,
palladium, osmium and ruthenium and their compounds and a
promoter which is halogen or halogen compound. In the liquid-
phase embodiment illustrated in Figure 1 in GB 1,234,641 the
liquid effluent from the reactor has its pressure let down and
is then introduced into a distillation or flash column 30
where the lower boiling compounds consisting principally of
methyl acetate, methyl iodide and unreacted methanol are
separated from acetic acid and the other less volatile
components such as the catalyst system. The lower boiling
components are recycled to the reactor. The acetic acid and
other higher boiling compounds are removed from column 30 and
enter distillation or flash column 40. In this column acetic
acid which may contain water is separated from the other high
boiling components, principally comprised of the catalyst.
The acetic acid is withdrawn and may be further purified to
- 1 -
CA 02476034 2004-08-26
remove water. The high boiling components are recycled to t he
reactor. If no component higher boiling than acetic acid,
such as a high boiling
- 1a -
CA 02476034 2004-08-26
solvent, is present, then some acetic acid may be recycled to return
the catalyst to the reactor.
A similar reaction scheme is described in related US patent US
3,772,380 which relates to a process for the preparation of
carboxylic acids and esters by carbonylation of alcohols and their
ester, ether and halide derivatives in the presence of an
iridium/halogen catalyst system.
According to British patent GB 1,355,146 conventional processing
schemes involving distillation for the separation of carbonylation
products from the liquid reaction mass pose problems of catalyst
inactivation and precipitation for rhodium and iridium carbonylation
catalyst systems such as those described in GB 1,234,461 because
these tend to decompose and become inactive when they come in to
contact with the hot surfaces in distillation column reboilers. One
solution proposed in GB 1,355,146 is to utilise extremely large
distillation column reboilers. Another solution proposed in GB
1,355,146 is to pass at least a portion of the liquid reaction mass
to a separation zone maintained at a pressure substantially below
that of the reaction zone thus vapourising at least a portion of the
carbonylation products without the addition of heat. In a preferred
process the liquid remaining in the separation zone after
vapourisation of at least a portion of the carbonylation products is
re-cycled to the reaction zone. The examples given only relate to the
use of rhodium catalysts and there are no details given of what
components are present in the unvaporised liquid fraction when
iridium carbonylation catalysts are used.
The technical problem to be solved by the pi:esent invention is to
provide a process for the recovery of a carbonylation product from a
liquid carbonylation reaction composition comprising free or combined
iridium carbonylation catalyst in which the catalyst has reduced
tendancy to lose its stability and/or solubility.
thus, according to the present invention there is provided a
process for the recovery of a carbonylation product from a liquid
reaction composition of an iridium-catalysed carbonylation reaction
of a carbonylatable reactant, which liquid composition comprises
CA 02476034 2004-08-26
carbonylation product and free or combined iridium carbonylation
catalyst, which process comprises subjecting the liquid carbonylation
reaction composition to a vaporisation, with ox' without the addition
of heat, to produce a vapour fraction comprising carbonylation
product and a liquid fraction comprising iridium carbonylation
catalyst and separating the liquid and vapour fractions, and in which
process there is maintained in the liquid fraction a concentration of
water of at least 0.5~ by weight.
The present invention solves the technical problem presented
above by the use of water to stabilise the iridium catalyst during
the recovery process.
In the process of the present invention, the vaporisation may be
performed as a flash vaporisation with or without the addition of
heat. In an adiabatic flash the pressure of the composition at
elevated temperature is reduced without the addition of heat. In an
isothermal flash the pressure of the composition at elevated
temperature is reduced and the temperature of the composition is
maintained by the addition of heat. Either of these types of flash
vaporisations may be used or a combination of both, for example
addition of only some heat and reducing pressure or addition of heat
without a change in pressure.
Thus, for example, in one embodiment using an adiabatic flash,
the liquid carbonylation reaction composition at elevated temperature
and pressure such as, for example, that required for the
carbonylation reaction, is introduced into a flash zone which is at a
substantially lower pressure than the elevated pressure of the
carbonylation reaction composition. This causes at least a portion of
the liquid carbonylation reaction composition to vaporise and produce
the vapour and liquid fractions, which may be removed separately from
the flash zone. A suitable adiabatic flash may be performed by, for
example, introducing liquid carbonylation reaction composition having
a temperature of about 100 to 250°C and a pressure of about 10 to 100
barg into a flash zone maintained at a temperature of about 80 to
200°C and a pressure of about 0 to 20 barg.
The vaporisation may also be performed by the addition of heat to
3
CA 02476034 2004-08-26
the liquid carbonylation reaction composition to vaporise at least a
portion of the composition and produce the vapour and liquid
fractions. This may be an isothermal flash wherein the temperature of
the composition i.s maintained by the addition o:E heat. A suitable
isothermal flash vaporisation may be performed at a temperature of 80
to 200°C and a pressure of 0 to 20 barg.
The vaporisation may be performed in a short: residence vaporiser
wherein heat is supplied to the liquid carbonylation reaction
composition to vaporise a portion thereof whether or not the pressure
is reduced.
The vaporisation may also be performed in a fractional
distillation zone. In this embodiment, liquid carbonylation reaction
composition is introduced into a distillation zone, the liquid
fraction comprising iridium carbonylation catalyst is removed from
the base of the distillation zone. The vapour fraction comprising
carbonylation product passes up the distillation zone and may be
removed either as a liquid or vapour at any point above the base of
the distillation zone.
More than one vaporisation stage may be used in the process of
the present invention provided that in each stage the concentration
of water present in the liquid fraction is sufficient to maintain the
stability and solubility of the iridium carbonylation catalyst.
Thus, two or more flash vaporisations may be usFd in sequence each
independently with or without the addition of heat. Alternatively,
one or more flash vaporisations may precede a fractional distillation
zone.
When heat is added to effect the vaporisation a suitable source
of heat is steam heating.
The residence time of the liquid traction in the vaporisation
zone or distillation zone is preferably relatively short, for example
a liquid fraction residence time of 1 to 60 minutes.
'Whatever the design of the equipment used for the vaporisation,
the water concentration in the liquid fraction comprising the iridium
carbonylation catalyst is at least 0.5~ by weight, preferably about
0.5 to 50o by weight, more preferably 1 to loo by weight. The water
4
CA 02476034 2004-08-26
may be introduced to the vaporisation as a component in the liquid
carbonylation reaction composition and/or may be introduced
separately to the vaporisation.
The free or combined iridium carbonylation catalyst concentration
in the liquid fraction may suitably be in the range from 0.01 by
weight iridium up to the limit of solubility of the catalyst in the
liquid fraction, preferably 0.05 to 2.0~ by weight,
Preferably, the liquid fraction also compri;aes halide
carbonylation promoter, for example an alkyl halide, preferably an
iodide promoter and most preferably methyl iodide. Suitably the
halide promoter is present at a concentration of 0.01 to 20o by
weight.
Preferably, the liquid fraction also comprises ester derivative
of the carbonylatable reactants, for example methyl acetate.
Suitably the ester derivative is present at a concentration of 1 to
50~ by weight.
The preferred and most preferred concentrations of these
components in the liquid fraction are independently set out in Table
1 below.
TABLE 1
CONCENTRATIONS OF COMPONENTS IN LIQUID FRACTION
COMPONENT PREFERRED MOST PREFERRED
~ BY WEIGHT $ BY WEIGHT
Water 0.5 - 50 1.0 - 15
Iridium catalyst 0.05 - 2.0 0.1 - 1.0
Halide promoter 0.01 - 20 Q.1 - 10
Ester derivative 2 - 50 3 - 35
5
CA 02476034 2004-08-26
The liquid carbonylation reaction composition of any suitable
liquid-phase, iridium-catalysed carbonylation process of
carbonylatable reactants may be used in the process of the present
invention.
Thus, a suitable carbonylation process may z:omprise a liquid
phase, iridium-catalysed carbonylation of an alcohol, ester,
hydrocarbyl halide and/or hydrocarbyl ether reactant to produce a
corresponding carboxylic acid and/or carboxylic acid ester. In such
a process carbon monoxide is contacted with a liquid carbonylation
reaction composition comprising carbonylatable reactant and/or an
ester derivative thereof, iridium carbonylation catalyst, halide
carbonylation promoter and preferably, a finite concentration of
water.
A suitable alcohol carbonylatable reactant is any alcohol having
from 1 to 20 carbon atoms and at least one hydroxyl group.
Preferably, the alcohol is a monofunctional aliphatic alcohol having
from 1 to 8 carbon atoms. Most preferably, the alcohol is methanol,
ethanol and/or propanol. A mixture comprising more than one alcohol
may be used. The carbonylation product of the alcohol will be a
carboxylic acid having one carbon atom more than the alcohol and/or
an ester thereof with the alcohol reactant. A particularly preferred
reactant is methanol, the carboxylic acid produces of which is acetic
acid and/or methyl acetate.
A suitable ester carbonylatable reactant is any ester of an
alcohol and a carboxylic acid. Preferably the ester reactant is an
ester of a carboxylic acid and an alcohol which alcohol has from 1 to
20 carbon atoms. More preferably the ester reactant is an ester of a
carboxylic acid and a monofunctional aliphatic alcohol which alcohol
has from 1 to 8 carbon atoms. Most preferably the ester reactant is
an ester of a carboxylic acid and methanol, ethanol or propanol.
Preferably the ester reactant is an ester of an alcohol and the
carboxylic acid product. Preferably the ester reactant has up to 20
carbon atoms. A mixture of ester reactants may be used. The
carboxylic acid carbonylation product of the ester reactant will be a
carboxylic acid having one carbon atom more than the alcohol
6
CA 02476034 2004-08-26
component of the ester reactant. A particularly preferred ester
reactant is methyl acetate, the carboxylic acid carbonylation product
of which is acetic acid.
A suitable halide carbonylatable reactant is any hydrocarbyl
S halide having up to 20 carbon atoms. Preferably the halide reactant
is an iodide or a bromide. More preferably the halide component of
the hydrocarbyl halide reactant is the same halide as that of the
halide carbonylation promoter. Most preferably the hydrocarbyl
halide is a hydrocarbyl iodide, most preferably methyl iodide, ethyl
iodide or propyl iodide. A mixture of hydrocarbyl halide reactants
may be used. The carboxylic acid product of the hydrocarbyl halide
reactant will be a carboxylic acid having one more carbon atom than
the hydrocarbyl halide reactant. The ester carbonylation product of
the hydrocarbyl halide will be the ester of the hydrocarbyl halide
and a carboxylic acid having one more carbon atom than the
hydrocarbyl halide.
A suitable ether carbonylatable reactant is any hydrocarbyl ether
having up to 20 carbon atoms. Preferably the ether reactant is a
dialkyl ether, most preferably dimethyl ether, diethyl ether or
dipropyl ether. A mixture of ethers may be used. The carbonylation
products of the ether reactant will be carboxylic acids having one
carbon atom more than each of the hydrocarbyl groups of the ether
and/or esters derivatives thereof. A particularly preferred ether
carbonylation reactant is dimethyl ether, the carboxylic acid product
of which is acetic acid.
A mixture of alcohol, ester, halide and ether carbonylatable
reactants may be used in the carbonylation process. More than one
alcohol, ester, halide and/or ether may be used. A particularly
preferred carbonylatable reactant is methanol and/or methyl acetate,
the carboxylic acid earbonylation products of which are acetic acid.
The iridium carbonylation catalyst in the liquid carbonylation
reaction composition may comprise any iridium-containing compound
which is soluble in the liquid reaction composition. It may be added
to the liquid carbonylation reaction composition. for the
carbonylation reaction in any suitable form which dissolves in the
7
CA 02476034 2004-08-26
liquid reaction composition or is convertible to a soluble form.
Examples of suitable iridium-containing compounds which may be used
include IrCl3, IrI3, IrBr3, Ir(CO)2I2, Ir(CO)2C12 Ir(CO)2Br2,
IrC13.4H20, IrBr34H20, Ir2(CO)8, iridium metal, iridium acetate,
Ir203, Ir02, Ir(acac)(CO)2 and Ir(acac)2. Preferably, the iridium
catalyst concentration in the liquid carbonylation reaction
composition is in the range 50 to 10000 ppm by weight of iridium,
more preferably 100 to 6000 ppm by weight of iridium.
The halide carbonylation promoter for the suitable carbonylation
reaction may be an iodide or bromide compound preferably an iodide.
Preferably, the halide promoter is the halide derivative of the
carbonylatable reactant, that is a hydrocarbyl halide. Most
preferably, the halide carbonylation promoter is methyl iodide.
Preferably the concentratian of halide carbonylation promoter in the
liquid carbonylation reaction composition is in the range 1 to 20~ by
weight, more preferably 1 to 10~ by weight.
The carbon monoxide feed to the suitable ca:rbonylation reaction
may be essentially pure or may contain inert impurities such as
carbon dioxide, methane, nitrogen, noble gases, water and C1 to C4
paraffinic hydrocarbons. Hydrogen may be present in the suitable
carbonylation reactor. Hydrogen may be generated in situ or fed to
the carbonylation reactor with the carbon monoxide and/or separately
therefrom. The partial pressure of carbon monoxide in the suitable
carbonylation reaction may suitably be in the range 1 to 70 barg.
The pressure of the suitable carbonylation reaction is suitably
in the range 10 to 100 barg. The temperature of the suitable
carbonylation reaction is suitably in the range 100 to 250°C.
The liquid carbonylation reaction composition may also comprise
ester derivative of the carbonylatable reactants preferably in the
range 0.1 to 75~ by weight, more preferably in 'the range 1.0 to 60%
by weight.
'The liquid carbonylation reaction composition may comprise water.
The water may be formed in situ in the carbonylation reaction, for
example by the esterification reaction between alcohol reactant and
carboxylic acid product. The water may be introduced to the
CA 02476034 2004-08-26
carbonylation reactor together with or separately from the other
liquid reactants such as esters, for example methyl acetate. water
may be separated from reaction composition withdrawn from the reactor
and recycled in controlled amounts to maintain the required
concentration in the carbonylation reaction composition. The
concentration of water in the liquid carbonylation reaction
composition may be at least 0.1~ by weight. Typically, and depending
upon the other components of the liquid reaction composition, the
water concentration in the liquid carbonylation reaction composition
may be at least 0.1~ by weight and up to 30~ by weight preferably up
to 15~ by weight, most preferably the water concentration is about 2
to 8~ by weight.
The components in the liquid carbonylation reaction composition
which are more volatile than carbonylation product may be recovered
from the carbonylation reaction composition in a preliminary recovery
stage before the carbonylation product is recovered from the
remaining carbonylation reaction composition. These more volatile
components may be, for example, carbonylatable reactant and/or ester
derivative thereof and carbonylation halide promoter. These volatile
components may be recycled to the carbonylation reaction. A suitable
preliminary recovery stage may comprise flash vaporisation with or
without the addition of heat.
In particular, it has been found that in the liquid phase,
iridium-catalysed carbonylation of an alcohol, ester, hydrocarbyl
halide and/or hydrocarbyl ether reactant to produce carboxylic acid,
the concentration of ester derivative of the reactant necessary in
the liquid phase reaction composition to achieve a suitable rate of
reaction is relatively high. This ester derivative may be recovered
from the liquid carbonylation reaction composition in a preliminary
vaporisation before the carbonylation product is recovered.
Thus, according to one embodiment of the present invention there
is provided a process for the recovery of a carboxylic acid
carbonylation product of an alcohol, ester, hydrocarbyl halide and/or
hydrocarbyl ether carbonylatable reactant, from a liquid
carbonylation reaction composition comprising carboxylic acid
9
CA 02476034 2004-08-26
carbonylation product, free or combined iridium carbonylation
catalyst and ester derivative of the carbonylatable reactant which
process comprises (a) subjecting the liquid carbonylation reaction
composition to a vapourisation in a first vaporisation zone to
S produce, with or without the addition of heat, a first vapour
fraction comprising at least a portion of the ester derivative in the
liquid carbonylation reaction composition and a first liquid fraction
comprising the remainder of the ester derivative in the liquid
carbonylation reaction composition, at least a portion of the
carboxylic acid product and the iridium carbonylation catalyst, and
maintaining a concentration of water of at least 0.5~ by weight in
the first liquid fraction and (b) passing the first liquid fraction
to a second vapourisation zone wherein the first liquid fraction is
subjected to a vapourisation, with or without the addition of neat,
to produce a second vapour .fraction comprising carboxylic acid
carbonylation product and a second liquid fraction comprising iridium
carbonylation catalyst and maintaining in the second liquid fraction
a concentration of water of at least 0.5~ by weight.
In this embodiment, the first vapour fraction and the second
liquid fraction may be recycled to the carbonylation reaction. The
second vapour fraction comprising carboxylic acid carbonylation
product may be further purified by conventional means such as
fractional distillation in one or more fractional distillation zones
to recover carboxylic acid carbonylation product from the other
components which may be recycled to the carbonylation reaction. Thus,
for example, the second vapour fraction may be introduced into a
distillation zone and subjected to fractional distillation in which a
heads process stream comprising halide or halide compound
carbonylation promoter, ester derivative of the carbonylatable
reactant and optionally water is removed from the distillation zone
and may be recycled to the carbonylation reaction; and a base process
strum comprising carboxylic acid carbonylation product and
optionally water is removed as a vapour or liquid from the base of
the distillation zone and may be subjected to further conventional
purification if necessary, :for example to remove water and trace
CA 02476034 2004-08-26
impurities such as iodides and oxidisable impurities by, for
example, passing through a silver loaded ion exchange resin.
In this embodiment of the present invention the
first vapourisat ion is preferably an adiabat is f lash vapour-
isation and the second vapourisation is performed in a
fract Tonal dist illat ion zone or, preferably is a part ial
vaporiser with addit ion of heat .
As stated above, in another aspect the invention
provides a process for reacting carbon monoxide with a
carbonylatable reactant and/or an ester derivative thereof in
a liquid reaction composition comprising an iridium
carbonylation catalyst, a hydrocarbyl halide carbonylation
promoter, water and carbonylation reaction product,
characterised in that the liquid reaction composi.t ion
comprises water at a concentration about 2 to 8~ by weight,
hydrocarbyl halide carbonylation promoter at a concentration
in the range 1 to 20~ by weight and ester derivative of the
carbonylatable reactant at a concentration in the range 1.0 to
60~ by weight. In a preferred embodiment the carbonylatable
reactant is methanol, the hydrocarbyl halide pfomoter is
methyl iodide and the ester derivative is methyl acetate, and
the carbonyl reaction product is acetic acid.
The invention will now be illust rated by way of
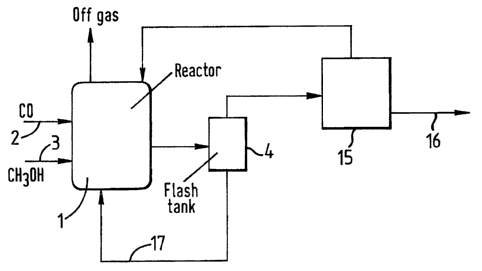
example only by reference to Figures 1 to 3. Figure 1 repre-
sents in schematic form a flow diagram of a process according
to the present invention incorporating a single flash separa-
tion stage. Figures 2 and 3 show schematic flow diagrams of
- 1.1 -
CA 02476034 2004-08-26
two processes according to the present invention each using
two separation stages.
The processes in Figures 1 to 3 may be used for the
production of acetic acid by carbonylation of methanol.
In Figures 1 to 3 a carbonylation reactor (1) is
provided with a supply of carbon monoxide (2) and a supply of
methanol (3). In use, the carbonylation reactor contains a
liquid reaction composition comprising acetic acid
carbonylation product, iridium carbonylation catalyst, methyl
acetate derivative of methanol carbonylation reactant; methyl
iodide carbonylation promoter and a finite concentration of
water of at least 0.1~ by weight. In use, the reactor is
maintained at a pressure of 10 to 100 berg and a temperature
of 100 to 250°C. In use, liquid carbonylation reaction
composition is withdrawn from the reactor (1) and passed to
flash zone (4) operated at a pressure below that of the
reactor (for example 0 to 20 berg). This is preferably an
adiabative flash zone.
In the process shown in Figure 1 a vapour fraction
comprising methyl acetate, methyl iodide, acetic acid and
water is passed from the flash zone (4) to separation zone
(15). This is shown schematically as a single block and may
comprise one or more separation stages, for example fractional
distillation zones. In this separation zone (15;1 acetic acid
product is separated from the methyl iodide, methyl acetate
and water which are recycled separately or together in one or
more process streams back to the carbonylation reactor (1).
The acetic acid product taken from the separation zone
- lla -
CA 02476034 2004-08-26
along line (16) may be further purified by conventional processes.
In the process shown in Figure 1 a liquid fraction comprising acetic
acid, at least 0.5$ by weight water and involatile iridium
carbonylation catalyst is passed from the flash zone (4) and recycled
along line (17) to the reactor (1).
In the embodiments shown in Figures 2 and 3 the flash zone (4) is
operated as a preliminary separation zone to separate some of the
methyl acetate and methyl iodide from the removed liquid
carbonylation reaction composition. Thus, in Figures 2 and 3 in
flash zone (4) a first vapour fraction comprising a significant
amount of the methyl acetate and methyl iodide from the carbonylation
reaction composition is recycled from the flash zone (4) to the
reactor along line (5). A first liquid fraction comprising the
remainder of the methyl acetate and methyl iodide, the ir:ldium
carbonylation catalyst and at least 0.5~ by weight water :LS passed
from the flash zone (4) along line (6).
In the embodiment shown in Figure 2, the first liquid fraction is
passed to a stripper distillation zone (7). From the top of the
distillation zone (7) a process stream comprising methyl acetate and
methyl iodide is taken along line (8) and recycled direct:Ly or
indirectly back to the carbonylation reactor. A crude acetic acid
carbonylation product is taken from the distillation zone (7) as a
vapour or liquid at a point above the base of the distill<ition zone
and passed along line (10) to a distillation zone(11). In
distillation zone (11) water is removed as a head product and
recycled to the reactor along line (12) and acetic acid product is
taken as a base product. A second liquid fraction compri:aing iridium
carbonylation catalyst and at least 0.5~ by weight water :LS taken
from the base of the distillation zone (7) and recycled to the
reactor along line (9).
In the embodiment shown in Figure 3, the first liquid fraction is
passed to a partial vaporiser (20) in which part of the fraction is
vaporised by the addition of heat to form the second vapour and
liquid fractions. The second liquid fraction comprising iridium
carbonylation catalyst and at least 0.5~ by weight water is recycled
12
CA 02476034 2004-08-26
to the reactor along line (21). The second vapour fractic>n
comprising methyl acetate, methyl iodide, water. and acetic acid is
passed along line (22) to distillation zone (23). The methyl iodide
and methyl acetate are taken from the distillation zone (23) as a
heads product and are recycled to the reactor along line (24). A
base product from the distillation zone (23) comprising acetic acid
and water is taken along line (25) and passed to a distillation zone
(26) from which acetic acid is recovered as a base product and water
is taken as a head product and recycled along line (27) to the
reactor. In the embodiment shown in Figures 2 and 3 the recovered
acetic acid may be further purified by conventional means (not shown)
to remove for example iodide and oxidisable impurities.
The invention will now be further illustrated by reference to the
following examples.
A stock solution of iridium carbonylation catalyst was prepared
by charging the following components to a 100m1 Hastelloy B2(trade
mark) batch autoclave fitted with a Dispersimax (trade mark)
stirrer:-
IrC13.4H20 1.5g
methyl iodide 2.5g
water 0.758
acetic acid balance to 50g
The autoclave was pressurised with carbon monoxide to 45 barg
and then heated to 195°C with stirring for 2 hours. After cooling to
room temperature and depressurising, the stock solution was analysed
by Inductively Coupled Plasma spectroscopy (ICP) for iridium content
(typically about 7500 ppm). High pressure infrared analysis of
similarly prepared solutions had previously indicated that they
contain the species [Ir(CO)2I4]-. This stock carbonylation catalyst
solution was used in subsequent experiments.
Catalyst Stability Test 1
In a first stability test, stock carbonylation catalyst
solution prepared as hereinbeforedescribed and containing about 9300
ppm iridium (8.578), methyl iodide (O.Olg), methyl acetate (0.73g)
and water (0.61g) were charged to a Fischer-Porter tube, purged with
13
CA 02476034 2004-08-26
carbon monoxide sealed and then heated to 100°C with stirring for 15
minutes under autogenous pressure. This simulated the conditions
which would be expected to prevail during the recovery of
carbonylation product from a carbonylation composition in the second
of a two-stage vaporisation according to the process of the present
invention. At the end of the heating period the contents of the
Fischer-Porter tube were cooled and analysed for iridium content by
ICP. The Fisher-Porter tube was then reassembled and the test
continued using the same solution maintained at 100°C for 2 hours,
before, repeating the analysis. The results are shown in Table 2
below.
TABLE 2
Test Test DurationInitial iridiumFinal iridium iridium
No. (minutes) concentration concentration a__~emaining
in
(ppm) (ppm) solution
1 15 9327 8986 96
120 9120 8854 97
Catalyst Stability Test 2
In a second stability test, stock carbonylation catalyst
solution prepared as hereinbeforedescribed and containing about 9300
ppm iridium (8.51g), methyl iodide (0:02g), methyl acetate (0.84g)
and water (0.48g) were charged to a Fischer-Porter tube, purged with
carbon monoxide and then pressurised to 1 barg with carbon monoxide
before being heated to 130°C with stirring for 15 minutes. The
pressure in the Fischer-Porter tube when at temperature wa.s about 2.4
barg. This simulated the conditions which would be expected to
prevail during the recovery of carbonylation product from a
carbonylation composition in the second of a two stage vaporisation
according to the process of the present invention. At the end of the
heating period, the contents of the Fischer-Porter tube were cooled
14
CA 02476034 2004-08-26
and analysed for iridium content by ICP. The Fischer-Porter tube was
then reassembled and the test continued using the same soP_ution
maintained at 130°C for 2 hours, before, repeating the analysis. The
results are set out in the Table 3 below:
Catalyst Stability Test 3
Test 2 was repeated using a fresh charge of reagents in which
the iridium catalyst stock solution contained about 17000 ppm iridium
and had been prepared by removing about 50% of the acetic acid under
vacuum from stock solution prepared as hereinbeforedescribed. The
results are set out in Table 3 below:
TABLE 3
Test Test DurationInitial IridiumFinal Iridium Iridium
No. (minutes) concentration concentration remaining
(ppm) (ppmj in
solution
(%)
2 15 9286 9538 100
120 9126 8976 94
3 15 17283 18097 96
120 I 18316
18035 ~ 100
The results of tests 1 to 3 show that the iridium catalyst. is stable
in the presence of at least 0.5% water.
Catalyst Stability Test 4
Test 2 was repeated but without addition of methyl iodide to
the Fischer Porter tube. The results are shown in Table 4 below.
Catalyst Stability Test 5
Test 2 was repeated but without addition of methyl acetate to
the Fischer Porter tube. The results are shown in Table 4 below.
CA 02476034 2004-08-26
Comparative Example A
Test 2 was repeated using the following initial charge:
catalyst stock solution 9.0948
methyl acetate 0.8738
methyl iodide 0.0248.
The water content of the mixture charged was measured by i~he Karl
Fischer method to be only 0.33% by weight. The analysis of iridium
concentration after 120 minutes shown in Table 5 below shows that
when the water content is less than 0.5% by weight the iridium
catalyst does not remain in solution.
TABLE 4
Test Test DurationInitial IridiumFinal Iridium Iridium
No. (minutes) concentration concentration remaining
in
(ppm) (ppm) solution
(%)
4 120 9839 9572 9?
5 120 9851 9895 100
TABLE 5
Test Test DurationInitial IridiumFinal Iridium Iridium
No. (minutes) concentration concentration remaining
in
(ppm) (ppm) solution
(%)
A 120 9342 8263 88
20
16