Note: Descriptions are shown in the official language in which they were submitted.
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
MACROLIDE ANTIBACTERIAL COMPOUNDS
TECHNICAL FIELD
This invention is directed to compounds with
antibacterial activity, processes for making the compounds
and intermediates used in the processes, compositions
containing the compounds, and methods for prophylaxis or
treatment of bacterial infections using the compounds.
BACKGROUND OF THE INVENTION
Because the effectiveness of many drugs currently
available for prophylaxis or treatment of bacterial
infections is being compromised by the emergence of
drug-resistant bacteria, novel antibacterial compounds would
be beneficial for their therapeutic value and their
contribution to the antibacterial arts.
-1-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
SUMMARY OF THE INVENTION
A first embodiment of this invention, therefore, is
directed to compounds, and salts, prodrugs, and salts of
prodrugs thereof, having antibacterial activity, the
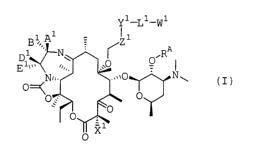
compounds having formula (I)
Y1 ~1-W1
B1,A 1
i M
N\ ~ O~RA
E~~~~~,, ,O
Nn, ~ .,,~0 N~
O
O ;' O
~O
O
_ X1
(I) .
in which
two of A1, B~, D1, and E1 are hydrogen, alkyl, alkenyl,
alkynyl, cycloalkyl, aryl, heteroaryl, heterocyclyl, -CN,
-OH, -SH, -C (O) H, -C (O) R1, -C (0) OH, -C (O) OR1, -C (O) NRZR3, or
alkyl substituted by one, two, or three substituents
independently selected from the group consisting of -CN,
-OH, -SH, halo, aryl, heteroaryl, heterocyclyl, -OR1, -SR1,
-C (O) H, -C (0) R~', -C (O) OH, -C (O) OR1, -CH=N-OR1, -OC (O) R1,
-OC (O) OR1, -C (O) NR2R3, -OC (O) NR2R3, -NR2R3, -N (R4 ) C (O) H,
-N (R4) C (O) Rl, -N (R4) C (O)NR2R3, -N (R4) SO~R1, -ORl, -SR1,
-S (O) R1, -SO~R1, and -SOzNR2R3, and the remainder are
hydrogen; or
A1 and D~, A1 and Ei, B1 and D1, or B1 and D1 together
are one- to five-membered alkylene or two- to five-membered
heteroalkylene, and the remainder are hydrogen; or
A1 and B~ together are one- to seven-membered alkylene
or two- to seven-membered heteroalkylene, and D1 and E1 are
hydrogen; or
-2-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
D1 and E1 together are one- to seven-membered alkylene
or two- to seven-membered heteroalkylene, and A1 and B1 are
hydrogen;
X1 is hydrogen or fluoride;
M1 is (E)-CH=CH, (Z)-CH=CH, or C=C;
Y1 is arylene or heteroarylene;
Z1 is drawn from left to right and is alkylene,
alkenylene, alkynylene, CH=N-0-CHI-(alkenylene), CH~N(R5),
CH2N (R5) (CH2) m, C (0) N (R5) , N (R5) C (0) N (R6) , CH=N-N (R5) ,
CH=N-N ( R5 ) C ( O ) , O, CH=N-0 , CH=N-0- ( CH2 ) m, C ( O ) N ( R5 ) ( CH2
) m, or
CH=N-O ( CH2 ) n-O,
in which m is one, two, three, or four, and n is two,
three, or four;
W1 is hydrogen aryl, heteroaryl, or heterocyclyl;
Rl is alkyl, aryl, heteroaryl, or heterocyclyl;
R2 and R3 are independently hydrogen or alkyl; or
R2 and R3 together are 3- to 7-membered alkylene or 3-
to 7-membered heteroalkylene;
R4 is hydrogen or alkyl;
R5 and R6 are independently hydrogen or alkyl; and
RA is hydrogen or Rp in which RP is a hydroxyl
protecting moiety;
in which, for the foregoing,
each aryl, arylene, heteroaryl, heteroarylene,
heterocyclyl, and heterocycloalkylene is unsubstituted or
substituted by one, two, three, four, or five substituents
independently selected from the group consisting of alkyl,
alkenyl, alkynyl, cycloalkyl, halo, -CN, -OH, -SH, -NH2,
-NO~, =0, -CF3, -CH~CF3, -CF2CF3, -OCF3, -OCH~CF3, -OCF~CF3,
-OR3~, -SR3~, -S (0) R35~ -S02R35~ -C (O) H~ -C (0) R35r -C (0) OHM
-C (0) OR35~ -NH (R35) ~ -N (R35) (R35) ~ -C (0) NH2, -C (O) NH (R35) ,
-3-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
-C (O) N (R35) (R3s) , -OC (O) R35, -OC (O) OR35, -OC (O) NHS,
-OC (0) NH (R35) , -OC (0) N (R35) (R36) , -NHC (O) H, -NHC (O) R35,
-NHC (O) OR35, -NHC (0) NH2, -NHC (O) NH (R35) , -NHC (O) N (R35) (R36) ,
-SO~NH~, -S02NH (R35) , -SON (R35) (R36) , R4o, and alkyl
substituted with one or two substituents independently
selected from the group consisting of halo, -CN, -OH, -SH,
=0, -OR3o, -SR3o, -C (O) OH, -C (0) OR35, -NHS, -NH (R35) ,
-N (R35) (R36) ~ -C (O) NH2, -C (O) NH (R35) , -C (0) N (R35) (R36) i
-OC (O) R35, -OC (0) NHS, -OC (0) NH (R35) , -OC (O) N (R35) (R36) '
-SO~NH2, -SO~NH (R35) , -S02N (R35) (R3s) , and R4o;
R3o is alkyl or alkyl substituted with a substituent
selected from the group consisting of halo and -OR45;
R35 and R36 are independently selected alkyl;
R4o is phenyl, naphthyl, furyl, thienyl, pyrrolyl,
oxazolyl, thiazolyl, imidazolyl, pyrazolyl, isoxazolyl,
isothiazolyl, 1,2,3-oxadiazolyl, 1,2,3-thiadiazolyl,
1,3,4-thiadiazolyl, 1,2,3-triazolyl, tetrazolyl, pyridyl,
pyrazinyl, pyrimidinyl, pyrrolidinyl, inidazolidinyl,
piperidinyl, piperazinyl, morpholinyl, or thiomorpholinyl,
each of which is unsubstituted or substituted with one, two,
or three substituents independently selected from the group
consisting of alkyl, alkenyl, alkynyl, cycloalkyl, halo,
-CN, -OH, -SH, -N02, =0, -CF3, -CH2CF3, -CF~CF3, -OCF3,
-OCH2CF3, -OCF~CF3, -OR45, -SR45, -S (0) RSO~ -SO~RSO, _C (O) Hr
-C (0) RSO, -C (0) OH, -C (O) ORSO. -NH2~ -NH (R5o) , -N (R5o) (R51) ~
-C (O) NHS, -C (0) NH (R5o) , -C (O) N (R5o) (R51) , -OC (0) RSO,
-OC (0) ORSO, -OC (O) NHS, -OC (0) NH (R5o) , -OC (O) N (R5o) (R51) ~
-NHC (0) H, -NHC (0) RSO, -NHC (0) ORSO, -NHC (0) NH2, -NHC (0) NH (R5o) ,
-NHC (0) N (R5o) (R51) , -SO~NH2, S02NH (R5o) , and -S02N (R5o) (R51) ;
R45 is alkyl; and
-4-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
R5~ and R51 are independently selected alkyl.
A second embodiment of this invention is directed to
processes for making the compounds of the first embodiment.
A third embodiment of this invention is directed to
intermediates which are useful in the second embodiment.
A fourth embodiment of this invention is directed to
compositions comprising a therapeutically effective amount
of a compound of the first embodiment.
A fifth embodiment of this invention is directed to
methods for prophylaxis or treatment of bacterial infections
in a fish or a mammal comprising administering thereto a
therapeutically effective amount of a compound of the first
embodiment.
In a preferred fifth embodiment of this invention, the
beneficiary of prophylaxis or treatment of bacterial
infections is a mammal.
In a more preferred fifth embodiment of this invention,
the beneficiary of prophylaxis or treatment of bacterial
infections is a human.
DETAILED DESCRIPTION OF THE INVENTION
The compounds of this invention comprise both fixed and
variable moieties, the latter of which are identified by a
capital letter and accompanying numerical or alphabetical
superscript, in which
the term "alkenyl" means a monovalent, straight or
branched hydrocarbon, having two to eight carbon atoms and
at least one carbon-carbon double bond, attached through a
carbon atom;
the term "alkenylene" means a divalent, straight or
branched hydrocarbon, having two to eight carbon atoms and
one carbon-carbon double bond, attached through carbon
atoms;
-5-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
the term "alkynyl" means a monovalent, straight or
branched hydrocarbon, having two to eight carbon atoms and
at least one carbon-carbon triple bond, attached through a
carbon atom;
the term "alkynylene" means a divalent, straight or
branched hydrocarbon, having two to eight carbon atoms and
one carbon-carbon triple bond, attached through carbon
atoms;
the term "alkyl" means a monovalent, saturated,
straight or branched hydrocarbon, having one to eight carbon
atoms, attached through a carbon atom;
the term "alkylene" means a divalent, saturated,
straight or branched hydrocarbon, having one to eight carbon
atoms, attached through carbon atoms;
the term "aryl" means monovalent phenyl, attached
through a carbon atom, unfused or fused with cycloalkyl,
cycloalkenyl, heteroaryl, another phenyl, naphthyl, or the
saturated part of indan;
the term "arylene" means divalent phenyl, attached
through phenyl carbon atoms, unfused or fused with
cycloalkyl, cycloalkenyl, another phenyl, naphthyl, or the
saturated part of indan;
the term "cycloalkenyl" means a monovalent, cyclic or
bicyclic hydrocarbon, having four to eight carbon atoms and
one or two carbon-carbon double bonds, attached through a
carbon atom;
the term "cycloalkyl" means a monovalent, saturated
cyclic hydrocarbon, having three to eight carbon atoms,
attached through a carbon atom;
the term "cycloalkylene" means a divalent, saturated
cyclic hydrocarbon, having three to eight carbon atoms,
attached through carbon atoms;
the term "halo" means fluoro (-F), chloro (-C1), bromo
(-Br), and iodo (-I);
-6-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
the term "heteroaryl" means a monovalent, aromatic,
five-membered ring having two double bonds and one oxygen or
one sulfur atom, one, two, three, or four nitrogen atoms, or
one or two nitrogen atoms and one oxygen or one sulfur atom
and the remaining atoms are carbon atoms, attached through a
carbon or nitrogen atom, and unfused or fused with phenyl,
cycloalkyl, cycloalkenyl, heterocycle, or another
heteroaryl; and a monovalent aromatic, six-membered ring
having three double bonds and one, two, or three nitrogen
atoms and the remaining atoms are carbon atoms, attached
through a carbon atom and unfused or fused with phenyl,
cycloalkyl, cycloalkenyl, heterocycle, or another
heteroaryl;
the term "heteroarylene" means a divalent, aromatic,
five-membered ring having two double bonds and one oxygen or
one sulfur atom, one, two, three, or four nitrogen atoms, or
one or two nitrogen atoms and one oxygen or one sulfur atom
and the remaining atoms are carbon atoms, attached through
carbon atoms; and a divalent aromatic, six-membered ring
having three double bonds and orie, two, or three nitrogen
atoms and the remaining atoms are carbon atoms, attached
through carbon atoms;
the term "heterocyclyl" means a monovalent, non-
aromatic three- or four-membered ring having one nitrogen,
oxygen, or sulfur atom and the remaining atoms are carbon
atoms, zero double bonds, attached through a carbon or
nitrogen atom and unfused or fused with phenyl or
heteroaryl; a monovalent, non-aromatic five-membered ring
having one or two nitrogen, oxygen, or sulfur atoms, and the
remaining atoms are carbon atoms, and zero or one double
bonds, attached through a carbon or nitrogen atom and
unfused or fused with phenyl or heteroaryl; and a
monovalent, non-aromatic six or seven-membered ring having
one, two, or three nitrogen, oxygen, or sulfur atoms and the
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
remaining atoms are carbon atoms, and zero, one, or two
double bonds, attached through a carbon or nitrogen atom and
unfused or fused with phenyl or heteroaryl; and
the term "heteroalkylene" means an alkylene of three to
eight atoms, connected through a carbon atom, in which one,
two, or three CH2 moieties have been independently replaced
by 0, NH, N(alkyl), S, C(0), S(0), or 502.
Preferred A1, B1, Dl, and E1 moieties are hydrogen.
A preferred X1 moiety is hydrogen.
A preferred M1 moiety is C--__C.
Preferred Y1 moieties are 1,3-phenylene,
1,4-phenylene, and 2,5-thienylene.
Preferred Z1 moieties are alkynylene,
CH=N-0-CH2- ( alkenylene ) , CH2N ( R5 ) , CH2N ( R5 ) ( CHZ ) m, C ( O ) N (
R5 ) ,
N ( R5 ) C ( O ) N ( R6 ) , CH=N-N ( R5 ) , CH=N-N ( R5 ) C ( 0 ) , O, CH=N-0,
CH=N-0 ( CH2 ) m, C ( O ) N ( R5 ) ( CH2 ) m, and CH=N-0 ( CH2 ) n-O,
in which m is one to three, and n is three.
Preferred W1 moieties are phenyl, 3-fluorophenyl,
4-(1,2,3-thiadiazol-4-yl)phenyl, phenyl fused with another
phenyl (naphthyl), pyridyl, and pyridyl fused with phenyl
(quinolinyl) .
Preferred R5 moieties are hydrogen and alkyl.
A preferred R6 moiety is hydrogen.
A preferred RA moiety is hydrogen.
These preferred variable moieties combine with the
parent moiety to form a preferred first embodiment of this
invention, the preferred first embodiment comprising
compounds, and salts, prodrugs, and salts of prodrugs
thereof, having formula (I)
-g_
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
YZ ZZ-Ws
B1 A 1
1 M
N~ ~ p~RAI
E Zy ~~,, ,O
Nn, .,,v0 Nw
O~'
O \;: 0
. w0 .
O
~1
(I),
in which Al, B1, D1, and Ei are hydrogen; X1 is hydrogen; M1
is C---C; Y1 is arylene or heteroarylene, in which the Y1
arylene is 1,3-phenylene or 1,4-phenylene, and in which the
Y1 heteroarylene is 2,5-thienylene; L1 is drawn from left to
right and is alkynylene, CH=N-O-CH2-(alkenylene), CH2N(R5),
CH2N (R5) (CH2) m, C (O) N (R5) , N (R5) C (0) N (R6) , CH=N-N (R5) , CH=N-
N (R5) C (O) , O, CH=N-O, CH=N-O (CH2),n, C (O) N (R5) (CHZ),r" or
CH=N-O(CH2)n-0, in which m is one, two, or three, and n is
three; W1 is hydrogen, aryl, or heteroaryl, in which the
aryl is phenyl or phenyl fused with another phenyl
(naphthyl), each of which is unsubstituted or substituted by
one substituent selected from the group consisting of halo
and R4°, in which R4° is 1,2,3-thiadiazolyl, and in which the
heteroaryl is pyridyl or pyridyl fused with phenyl
(quinolinyl); with the proviso that W1 is hydrogen only when
Z1 is CH=N-O(CH2)m; R5 is hydrogen or alkyl; R6 is hydrogen;
and RA is hydrogen.
These preferred variable moieties also combine to form
another preferred first embodiment of this invention, the
preferred first embodiment comprising compounds, and salts,
prodrugs, and salts of prodrugs thereof, having formula (I)
-9-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
Y1 ~1_W1
Il
I
RA
L
E ~ Nw
0
(Z)
in which A1, B1, D~, and E1 are hydrogen; X1 is hydrogen; M1
is C=C; Y~ is arylene or heteroarylene, in which the arylene
is 1,3-phenylene or 1,4-phenylene, and in which the
heteroarylene is 2,5-thienylene; L1 is drawn from left to
right and is C~-alkynylene, CH=N-0-CH2-(C2-alkenylene),
CHgN (R5) r CH2N (R5) (CHZ) mr C (0) N (R5) r N (RS) C (0) N (R6) r
CH=N-N ( R5 ) , CH=N-N ( R5 ) C ( O ) , O, CH=N-0, CH=N-0 ( CH2 ) m,
C (0)N (R5) (CH2)m, or CH=N-0 (CHI) n-O, in which m is one, two,
or three, and n is three; W1 is hydrogen, aryl, heteroaryl,
or heterocyclyl, in which the aryl is phenyl or phenyl fused
with another phenyl (naphthyl), each of which is
unsubstituted or substituted by one substituent selected
from the group consisting of halo and R4°, in which R4° is
1,2,3-thiadiazolyl, and in which the heteroaryl is pyridyl
or pyridyl fused with phenyl (quinolinyl); with the proviso
that W1 is hydrogen only when L1 is CH=N-0(CHZ)m and m is
one; R5 is hydrogen or Cl-alkyl; R6 is hydrogen; and RA is
hydrogen.
These preferred variable moieties also combine to form
still yet another preferred first embodiment of this
invention, the preferred first embodiment comprising
compounds, and salts, prodrugs, and salts of prodrugs
thereof, which are
-10-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
(2R, 4R, 5R, 6R, 8R, 118, 125,198, 208) -11-ethyl-
2,4,6,8,12,19-hexamethyl-7,9,14-trioxo-4-(3-(5-
((phenylamino)methyl)thien-2-yl)prop-2-ynyl)-10,13-dioxa-
15,18-diazatricyclo[10.6.2.015,2o]icos-1(18)-en-5-yl 3,4,6-
trideoxy-3-(dimethylamino)-(3-D-xylo-hexopyranoside,
(2R,4R,5R,6R,8R,11R,12S,19R,20R)-11-ethyl-
2,4,6,8,12,19-hexamethyl-7,9,14-trioxo-4-(3-(5-((E)-
(((phenylmethyl)oxy)imino)methyl)thien-2-yl)prop-2-ynyl)-
10,13-dioxa-15,18-diazatricyclo[10.6.2.015,2o~icos-1(18)-en-
5-yl 3,4,6-trideoxy-3-(dimethylamino)-(3-D-xylo-
hexopyranoside,
(2R,4R,5R,6R,8R,11R,12S,19R,20R)-11-ethyl-
2,4,6,8,12,19-hexamethyl-4-(3-(5-((E)-
((methyloxy)imino)methyl)thien-2-yl)prop-2-ynyl)-7,9,14-
trioxo-10,13-dioxa-15,18-diazatricyclo[10.6.2.015,2o~icos-
1(18)-en-5-yl 3,4,6-trideoxy-3-(dimethylamino)-(3-D-xylo-
hexopyranoside,
(2R,4R,5R,6R,8R,11R,12S,19R,20R)-11-ethyl
2,4,6,8,12,19-hexamethyl-7,9,14-trioxo-4-(3-(5-((E)
((phenyloxy)imino)methyl)thien-2-yl)prop-2-ynyl)-10,13-
dioxa-15,18-diazatricyclo[10.6.2.015,zo~icos-1(18)-en-5-yl
3,4,6-trideoxy-3-(dimethylamino)-[3-D-xylo-hexopyranoside,
(2R, 4R, 5R, 6R, 8R, 118, 125,198, 208) -11-ethyl-
2,4,6,8,12,19-hexamethyl-4-(3-(5-((E)-(((naphthalen-1-
ylmethyl)oxy)imino)methyl)thien-2-yl)prop-2-ynyl)-7,9,14-
trioxo-10,13-dioxa-15,18-diazatricyclo[10.6.2.015,2o]icos-
1(18)-en-5-yl 3,4,6-trideoxy-3-(dimethylamino)-(3-D-xylo-
hexopyranoside,
(2R,4R,5R,6R,8R,11R,12S,19R,20R)-11-ethyl-
2,4,6,8,12,19-hexamethyl-4-(3-(5-((E)-(((3-naphthalen-1-
ylprop-2-enyl)oxy)imino)methyl)thien-2-yl)prop-2-ynyl)-
7,9,14-trioxo-10,13-dioxa-15,18-
-11-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
diazatricyclo[10.6.2,015,~o]icos-1(18)-en-5-yl 3,4,6-
trideoxy-3-(dimethylamino)-(3-D-xylo-hexopyranoside,
(2R,4R,5R,6R,8R,11R,12S,19R,20R)-11-ethyl-
2,4,6,8,12,19-hexamethyl-7,9,14-trioxo-4-(3-(5-((E)-(((2-
(phenyloxy)ethyl)oxy)imino)methyl)thien-2-yl)prop-2-ynyl)-
10,13-dioxa-15,18-diazatricyclo[10.6.2,015,2o~icos-1(18)-en-
5-yl 3,4,6-trideoxy-3-(dimethylamino)-(3-D-xylo-
hexopyranoside,
(2R,4R,5R,6R,8R,11R,12S,19R,20R)-11-ethyl-
2,4,6,8,12,19-hexamethyl-7,9,14-trioxo-4-(3-(5-
(((phenylmethyl)amino)methyl)thien-2-yl)prop-2-ynyl)-10,13-
dioxa-15,18-diazatricyclo[10.6.2.015,2o]icos-1(18)-en-5-yl
3,4,6-trideoxy-3-(dimethylamino)-(3-D-xylo-hexopyranoside,
(2R,4R,5R,6R,8R,11R,12S,19R,20R)-11-ethyl-
2,4,6,8,12,19-hexamethyl-7,9,14-trioxo-4-(3-(5-((E)-
(pyridin-2-ylhydrazono)methyl)thien-2-yl)prop-2-ynyl)-10,13-
dioxa-15,18-diazatricyclo[10.6.2,015,zo]icos-1(18)-en-5-yl
3,4,6-trideoxy-3-(dimethylamino)-(3-D-xylo-hexopyranoside,
N' - ( ( 1E ) - ( 5- ( 3- ( ( 2R, 4R, 5R, 6R, 8R, 11R, 125, 19R, 2 0R) -11-
ethyl-2,4,6,8,12,19-hexame~thyl-7,9,14-trioxo-5-((3,4,6-
trideoxy-3-(dimethylamino)-(3-D-xylo-hexopyranosyl)oxy)-
10,13-dioxa-15,18-diazatricyclo[10.6.2.015,2o~ic~s-1(18)-en-
4-yl)prop-1-ynyl)thien-2-yl)methylidene)pyridine-2-
carbohydrazide,
(2R,4R,5R,6R,8R,11R,12S,19R,20R)-11-ethyl-
2,4,6,8,12,19-hexamethyl-7,9,14-trioxo-4-(3-(5-((E)-
(((quinolin-3-ylmethyl)oxy)imino)methyl)thien-2-yl)prop-2-
ynyl)-10,13-dioxa-15,18-diazatricycl~[10.6.2.015,20]icos-
1(18)-en-5-yl 3,4,6-trideoxy-3-(dimethylamino)-(3-D-xylo-
hexopyranoside,
(2R,4R,5R,6R,8R,11R,12S,19R,20R)-11-ethyl
2,4,6,8,12,19-hexamethyl-7,9,14-trioxo-4-(3-(5-(((2
-12-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
phenylethyl)amino)methyl)thien-2-yl)prop-2-ynyl)-10,13-
dioxa-15,18-diazatricyclo[10.6.2.p15,2o~icos-1(18)-en-5-yl
3,4,6-trideoxy-3-(dimethylamino)-(3-D-xylo-hexopyranoside,
(2R,4R,5R,6R,8R,11R,12S,19R,20R)-11-ethyl-
2,4,6,8,12,19-hexamethyl-7,9,14-trioxo-4-(3-(5-(((3-
phenylpropyl)amino)methyl)thien-2-yl)prop-2-ynyl)-10,13-
dioxa-15,18-diazatricyclo[10.6.2.015,2o]icos-1(18)-en-5-yl
3,4,6-trideoxy-3-(dimethylamino)-(3-D-xylo-hexopyranoside,
(2R,4R,5R,6R,8R,11R,12S,19R,20R)-11-ethyl-
2,4,6,8,12,19-hexamethyl-7,9,14-trioxo-4-(3-(3-(pyridin-2-
yloxy)phenyl)prop-2-ynyl)-10,13-dioxa-15,18-
diazatricyclo[10.6.2.015,zo~icos-1(18)-en-5-yl 3,4,6-
trideoxy-3-,(dimethylamino)-(3-D-xylo-hexopyranoside,
5-(3-((2R,4R,5R,6R,8R,11R,12S,19R,20R)-11-ethyl-
2,4,6,8,12,19-hexamethyl-7,9,14-trioxo-5-((3,4,6-trideoxy-3-
(dimethylamino)-(3-D-xylo-hexopyranosyl)oxy)-10,13-dioxa-
15,18-diazatricyclo[10.6.2.015,2o]icos-1(18)-en-4-yl)prop-1-
ynyl)-N-(3-fluorophenyl)thiophene-2-carboxamide,
5-(3-((2R,4R,5R,6R,8R,11R,12S,19R,20R)-11-ethyl-
2,4,6,8,12,19-hexamethyl-7,9,14-trioxo-5-((3,4,6-trideoxy-3-
(dimethylamino)-(3-D-xylo-hexopyranosyl)oxy)-10,13-dioxa-
15,18-diazatricyclo[10.6.2.015,2o]icos-1(18)-en-4-yl)prop-1-
ynyl)-N-(3-fluorophenyl)-N-methylthiophene-2-carboxamide,
(2R,4R,5R,6R,8R,11R,12S,19R,20R)-11-ethyl-4-(3-(3-((3-
fluorophenyl)oxy)phenyl)prop-2-ynyl)-2,4,6,8,12,19-
hexamethyl-7,9,14-trioxo-10,13-dioxa-15,18-
diazatricyclo[10.6.2.015,2o]icos-1(18)-en-5-yl 3,4,6-
trideoxy-3-(dimethylamino)-(3-D-xylo-hexopyranoside,
(2R,4R,5R,6R,8R,11R,12S,19R,20R)-11-ethyl
2,4,6,8,12,19-hexamethyl-7,9,14-trioxo-4-(3-(5-(pyridin-2
ylethynyl)thien-2-yl)prop-2-ynyl)-10,13-dioxa-15,18-
-13-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
diazatricyclo[10.6.2,015,20]icos-1(18)-en-5-yl 3,4,6-
trideoxy-3-(dimethylamino)-(3-D-xylo-hexopyranoside,
(2R,4R,5R,6R,8R,11R,12S,19R,20R)-11-ethyl-
2,4,6,8,12,19-hexamethyl-7,9,14-trioxo-4-(3-(4-
(phenyloxy)phenyl)prop-2-ynyl)-10,13-dioxa-15,18-
diazatricyclo[10.6.2.015,2o]icos-1(18)-en-5-yl 3,4,6-
trideoxy-3-(dimethylamino)-(3-D-xylo-hexopyranoside,
5- (3- ( (2R, 4R, 5R, 6R, 8R, 11R, 125, 19R, 20R) -11-ethyl-
2,4,6,8,12,19-hexamethyl-7,9,14-trioxo-5-((3,4,6-trideoxy-3-
(dimethylamino)-(3-D-xylo-hexopyranosyl)oxy)-10,13-dioxa-
15,18-diazatricyclo[10.6.2.015,2o~icos-1(18)-en-4-yl)prop-1-
ynyl)-N-pyridin-3-ylthiophene-2-carboxamide,
5- (3- ( (2R, 4R, 5R, 6R, 8R, 11R, 125, 19R, 20R) -11-ethyl-
2,4,6,8,12,19-hexamethyl-7,9,14-trioxo-5-((3,4,6-trideoxy-3-
(dimethylamino)-(3-D-xylo-hexopyranosyl)oxy)-10,13-dioxa-
15,18-diazatricyclo[10.6.2,015,2o~icos-1(18)-en-4-yl)prop-1-
ynyl)-N-(4-(1,2,3-thiadiazol-4-yl)phenyl)thiophene-2-
carboxamide,
5-(3-((2R,4R,5R,6R,8R,11R,12S,19R,20R)-11-ethyl-
2,4,6,8,12,19-hexamethyl-7,9,14-trioxo-5-((3,4,6-trideoxy-3-
(dimethylamino)-(3-D-xylo-hexopyranosyl)oxy)-10,13-dioxa-
15,18-diazatricyclo[10.6.2.015'~°~icos-1(18)-en-4-yl)prop-1-
ynyl)-N-(3-quinolin-3-ylpropyl)thiophene-2-carboxamide,
(2R,4R,5R,6R,$R,11R,12S,19R,20R)-11-ethyl-
2,4,6,8,12,19-hexamethyl-4-(3-(5-
((methyl(phenylmethyl)amino)methyl)thien-2-yl)prop-2-ynyl)-
7,9,14-trioxo-10,13-dioxa-15,18-
diazatricyclo [ 10 . 6 . 2 , 015, 20~ icos-1 ( 18 ) -en-5-yl 3, 4, 6-
trideoxy-3-(dimethylamino)-(3-D-xylo-hexopyranoside, and
N-(5-(3-((2R,4R,5R,6R,8R,11R,12S,19R,20R)-11-ethyl-
2,4,6,8,12,19-hexamethyl-7,9,14-trioxo-5-((3,4,6-trideoxy-3-
(dimethylamino)-[3-D-xylo-hexopyranosyl)oxy)-10,13-dioxa-
-14-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
15,18-diazatricyclo[10.6.2.Oi5'2°]icos-1(18)-en-4-yl)prop-1-
ynyl)thien-2-yl)-N'-pyridin-4-ylurea.
The compounds of this invention comprise asymmetrically
substituted carbon atoms in the R or S configuration.
Asymmetric carbon atoms with equimolar amounts of R and S
configurations are racemic. Atoms with an excess of one
configuration over the other are assigned the configuration
in the higher amount, preferably an excess of about 85%-900,
more preferably an excess of about 95a-99%, and still more
preferably an excess greater than about 990.
The terms "R" and "S" are as defined by the IUPAC 1974
Recommendations for Section E, Fundamental Stereochemistry,
Pure Appl. Chem. (1976) 45, 13-10.
Accordingly, all stereoisomers of the compounds of this
invention, including racemic mixtures, mixtures of
diastereomers, and single diastereomers, are meant to be
embraced by this invention.
The compounds of this invention may also comprise
carbon-carbon double bonds as being in the Z or E
configuration, in which the term "Z" represents the larger
two of the four substituents disposed on same side of a
carbon-carbon double bond and the term "E" represents the
larger two of the four substituents disposed on opposite
sides of a carbon-carbon double bond. The compounds may
also exist as an equilibrium mixture comprising Z or E
configurations.
The compounds of this invention containing hydroxyl,
amino, or carboxylic acids may have attached thereto
prodrug-forming moieties. The prodrug-forming moieties are
removed by metabolic processes and release the compounds
having the freed hydroxyl, amino, or carboxylic acid in
vivo. Prodrugs are useful for adjusting such
pharmacokinetic properties of the compounds as solubility
and/or hydrophobicity, absorption in the gastrointestinal
-15-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
tract, bioavailability, tissue penetration, and rate of
clearance.
The compounds of this invention may be prepared by
synthetic processes or metabolic processes. Metabolic
processes include those processes occurring in vitro in
V1V0.
The compounds of this invention may exist as acid
addition salts, basic addition salts, or zwitterions. Salts
of the compounds are prepared during their isolation or
following their purification. Acid addition salts of the
compounds are those derived from the reaction of the
compounds with an acid. For example, the acetate, adipate,
alginate, citrate, aspartate, benzoate, benzenesulfonate,
bisulfate, butyrate, camphorate, camphorsufonate,
digluconate, glycerophosphate, hemisulfate, heptanoate,
hexanoate, formate, fumarate, hydrochloride, hydrobromide,
hydroiodide, lactate, maleate, mesitylenesulfonate,
methanesulfonate, naphthylenesulfonate, nicotinate, oxalate,
pamoate, pectinate, persulfate, picrate, propionate,
succinate, tartrate, thiocyanate, trichloroacetic,
trifluoroacetic, phosphate, glutamate, bicarbonate, para-
toluenesulfonate, lactobionate, and undecanoate salts of the
compounds and prodrugs thereof are contemplated as being
within the scope of this invention. When the compounds
contain carboxylic acids, basic addition salts may be
prepared therefrom by reaction with a base such as the
hydroxide, carbonate, or bicarbonate of cations such as
lithium, sodium, potassium, calcium, and magnesium.
The compounds of this invention may be administered
with or without an excipient. Excipients include
encapsulating materials or formulation additives such as
absorption accelerators, antioxidants, binders, buffers,
coating agents, coloring agents, diluents, disintegrating
agents, emulsifiers, extenders, fillers, flavoring agents,
-16-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
humectants, lubricants, perfumes, preservatives,
propellants, releasing agents, sterilizing agents,
sweeteners, solubilizers, wetting agents, and mixtures
thereof. Excipients for orally administered compounds in
solid dosage forms include agar, alginic acid, cocoa butter,
gelatin, isotonic saline, malt, powdered tragacanth,
Ringer's solution, talc, water, aluminum hydroxide,
magnesium hydroxide, sodium and potassium phosphate salts,
cellulose, cellulose acetate, ethyl cellulose, sodium
carboxymethyl cellulose, ethyl laureate, ethyl oleate,
magnesium stearate, sodium lauryl sulfate, castor oil, corn
oil, cottonseed oil, germ oil, groundnut oil, olive oil,
peanut oil, safflower oil, sesame oil, soybean oil, benzyl
alcohol, benzyl benzoate, 1,3-butylene glycol, ethanol,
ethyl acetate, ethyl carbonate, glycerol, isopropanol,
propylene glycol, tetrahydrofurfuryl alcohol, corn starch,
potato starch, lactose, glucose sucrose, and mixtures
thereof. Excipients for ophthalmically and orally
administered compounds in liquid dosage forms include water,
ethanol, isopropanol, ethyl carbonate, ethyl acetate, benzyl
alcohol, benzyl benzoate, propylene glycol, 1,3-butylene
glycol, cottonseed oil, groundnut oil, corn oil, germ oil,
olive oil, castor oil, sesame oil, glycerol,
tetrahydrofurfuryl alcohol, polyethylene glycols, fatty acid
esters of sorbitan, and mixtures thereof. Excipients for
osmotically administered compounds include water, ethanol,
isopropanol, chlorofluorohydrocarbons, and mixtures thereof.
Excipients for parenterally administered compounds include
water, 1,3-butanediol, Ringer's solution, U.S.P. or isotonic
sodium chloride solution, oleic acid, castor oil, corn oil,
cottonseed oil, germ oil, groundnut oil, olive oil, peanut
oil, safflower oil, sesame oil, soybean oil, liposomes, and
mixtures thereof. Excipients for rectally and vaginally
-17-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
administered compounds include cocoa butter, polyethylene
glycol, wax, and mixtures thereof.
The compounds of this invention may be administered
parenterally (subcutaneously, intravenously,
intramuscularly, and intrasternally), orally, osmotically,
ophthalmically, rectally, topically, and vaginally. Orally
administered compounds in solid dosage forms may be
administered as capsules, dragees, granules, pills, powders,
and tablets. Ophthalmically and orally administered
compounds in liquid dosage forms may be administered as
elixirs, emulsions, microemulsions, solutions, suspensions,
and syrups. Osmotically and topically administered
compounds may be administered as creams, gels, inhalants,
lotions, ointments, pastes, powders, solutions, and sprays.
Parenterally administered compounds may be administered as
aqueous or oleaginous solutions or aqueous or oleaginous
suspensions, the latter of which contains crystalline,
amorphous, or otherwise insoluble forms of the compounds.
Rectally and vaginally administered compounds may be
administered as creams, gels, lotions, ointments, and
pastes.
Dosage forms for the compounds of this invention depend
on the species being treated, the disorder being treated and
the severity thereof, the composition comprising the
compounds, the time of administration, the route of
administration, the duration of treatment, the potency of
the compounds, and the rate of excretion of the compounds.
The daily therapeutically effective amount of the compounds
administered to a patient in single or divided doses range
from about 0.1 to about 200 mg/kg body weight, preferably
from about 0.25 to about 100 mg/kg body weight. Single dose
compositions contain these amounts of the compounds or
combinations of submultiples thereof.
-18-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
To determine antibacterial activity of the compounds of
this invention, twelve petri dishes, each containing
successive aqueous dilutions of test compounds in sterilized
Brain Heart Infusion agar (Difco 0418-01-5) (10 mL), were
inoculated with 1:100 dilutions of the representative
microorganisms in TABLE 1 using a Steers replicator block
(or 1:10 dilutions for slow-growing Streptococcus strains),
co-incubated at 35-37 °C for 20-24 hours with a plate with
an erythromycin A standard and a control plate with no
compound, and inspected visually to provide the minimum
inhibitory concentration (MIC), in ~g/mL, by which is meant
the lowest concentration of the test compound which yielded
no growth, a slight haze, or sparsely isolated colonies on
the inoculum spot as compared to growth in the control
plate.
TABLE 1
Microorganism Code
Staphylococcusaureus NCTC10649M AA
Staphylococcusaureus A5177 BB
Staphylococcus'aureus PIU 2043 CC
Streptococcus pyrogenes EES&1 DD
Streptococcus pyrogenes 930 EE
Streptococcus pyrogenes PIU 2548 FF
Streptococcus pneumoniae ATCC 6303 GG
Streptococcus pneumoniae 5979 HH
Streptococcus pneumoniae 5649 JJ
All of the compounds of this invention tested displayed
antibacterial activity superior to their respective controls
and are therefore useful as antibacterials.
The following schemes illustrate representative
processes by which the compounds of this invention may be
prepared, with the understanding that the order of the steps
-19-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
in the processes may be varied, other reagents may be
substituted for those specifically mentioned, and vulnerable
substituents may be protected and deprotected during the
process.
Abbreviations used are: DME for 1,2-dimethoxyethane;
DMF for N,N-dimethylformamide; and THF for tetrahydrofuran.
-20-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
SCHEME 1
RP
0 ~ OH I O ~ ~/
O ,,0 N\ ~~, , O ,,0 N\
HOn,. HOn,. O
O
HO :: O
HO,;:~ ~ _
~Orr.,.
Orr,,, 0 Q P
0
O . OOH 0 ~,,~O~R
( 1 ) rOCH3 ( 2 ) ~OCH3
RP ~ O.RP
Nw ~ ~/ I 0 ~ N
El~~~' .,,, ,0 ~,0 N~ ~~0 ~ \
w
N~., O / 0
0~ O
0 \; . 0 ~ N~ 0 ;; ,
Orr,, O
O
r,. RP .,,, ,R
P
0
0 O
OCH
(5) ,~~OCH3 (3) 3
E
3
(6) (7)
Compounds having formula (1) may be converted to
compounds having formula (2), in which RP is acetyl
(CH3C(O)), benzoyl (C6H5C(0)), or trimethylsilyl, by reacting
the former, a hydroxyl protecting reagent, a first base,
and, optionally, N,N-dimethylaminopyridine. Hydroxyl
protecting reagents include benzoic anhydride, acetic
-21-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
anhydride, benzoyl chloride, acetyl chloride, and
trimethylsilyl chloride. First bases include triethylamine,
diisopropylethylamine, pyridine, and lutidine. The reaction
is typically conducted at about 0 °C to 60 °C, over about 4
to 24 hours, in solvents such as dichloromethane,
chloroform, THF, DME, and
tert-butyl methylether.
Compounds having formula (2) may be converted to
compounds having formula (3) by reacting the former,
carbonyldiimidazole, a second base, and, optionally, N,N-
dimethylaminopyridine. Second bases include 1,8-
diazabicyclo-[5.4.0]under-7-ene, lithium
bis(trimethylsilyl)amide, sodium bis(trimethylsilyl)amide,
and potassium bis(trimethylsilyl)amide. The reaction is
typically conducted at about 25 °C, over about 6 to 24
hours, in solvents such as THF, DMF, 1,4-dioxane, and
N-methylpyrrolidine.
Compounds having formula (3) may be converted to
compounds having formula (5) by (a) reacting the former and
a compound having formula (4)
D1 B1 A1
E ~~
HEN NHZ
(4)
and (b) reacting the product of step (a) with a dilute first
acid. First acids include hydrochloric acid, triflic acid,
para-toluenesulfonic acid, and trifluoroacetic acid.
Step (a) is typically conducted at about 25 °C, over about
24 hours to 72 hours, in solvents such as acetonitrile, DMF,
water, and mixtures thereof. Step (b) is typically
conducted at about 70 °C to 100 °C, over about 12 hours to
about 24 hours, in solvents such as benzene, toluene,
xylene, and mixtures thereof.
-22-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
Compounds having formula (5) may be converted to
compounds having formula (6) by reacting the former and a
second acid. Second acids include hydrochloric acid,
triflic acid, para-toluenesulfonic acid, and trifluoroacetic
acid. The reaction is typically conducted at about 60 °C,
over about 12 to 24 hours, in solvents such as ethanol,
acetone, THF, water, and mixtures thereof.
Compounds having formula (6) may be converted to
compounds having formula (7) by reacting the former, a first
oxidizing agent, and, optionally, a first additive. First
oxidizing agents include dimethylsulfide/N-
chlorosuccinimide, dimethylsulfoxide/1-(3-
dimethylaminopropyl)-3-ethylcarbodiimide, and
dimethylsulfoxide/oxalyl chloride. First additives include
phosphoric acid, pyridinium trifluoroacetate, silica gel,
triethylamine, and pyridine. The reaction is typically
conducted at about -10 °C to 25 °C, over about 3 to 24 hours,
in solvents such as THF, DMSO, and dichloromethane.
SCHEME 2
Rp
O,
N~ N~
0
(8) (9)
Compounds having formula (8) may be converted to
compounds having formula (9) by reacting the former, a
fluorinating agent, and, optionally, a third base.
Fluorinating agents include 3,5-dichloro-1-fluoropyridinium
tetrafluoroborate, N-fluorobenzenesulfonimide, 3,5-dichloro-
1-fluoropyridinium triflate, N-fluoro-N-methyl-para-
-23-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
toluenesulfonamide, N-fluoropyridinium triflate, or
N-fluoroperfluoropiperidine. Third bases include sodium
hydride, potassium hydride, lithium diisopropylamide,
triethylamine, and N,N-diisopropylethylamine. The reactions
are typically conducted at about -78 °C to 0 °C, over about 2
to 24 hours, in solvents such as DMF, THF, and diethyl
ether.
SCHEME 3
C (0) H
1
~RP ~Rp
O I J
N~ N~
0\ J
(10) (12)
i (0) OH
~,1
B1 A1
Rp
D1 N
O
1~'~ ~~,, '~O
E ~ ,~, ~ ,,~0 N ~
0
0
0 ::
' _ O
X1
(13)
Compounds having formula (10) may be converted to
compounds having formula (12) by reacting the former, a
compound having formula (11)
Qs-Y1-C (O) H
(11) ,
in which Q1 is Cl, Br, or I,
-24-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
a first base, a coupling catalyst, and, optionally, a second
additive. Coupling catalysts include
dichlorobis(triphenylphosphine)palladium(II),
tris(dibenzylideneacetone)dipalladium(0),
tetrakis(triphenylphosphine)palladium(0), and
dichlorobis(triphenylphosphine)nickel(II). Second additives
include triphenylphosphine, triphenylarsine, 1,2-
bis(diphenylphosphino)butane, 1,2-
bis(diphenylphosphino)ethane, copper(I) iodide, and mixtures
thereof. The reactions are typically conducted at about 50
°C to 80 °C, over about 12 to 48 hours, in solvents such as
acetonitrile, DME, THF, and mixtures thereof.
Compounds having formula (12) may be converted to
compounds having formula (13) by reacting the former and a
second oxidizing agent. Second oxidizing agents include
molecular oxygen, potassium permanganate, sodium chlorite,
and silver oxide. The reactions are typically conducted at
about 0 °C to 35 °C, over about 1 to 48 hours, in solvents
such as acetonitrile, DME, THF, tert-butanol, water, and
mixtures thereof.
-25-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
SCHEME 4
R5
~ WI
I (0) H ~~~Q2~Q3i
Yl Yi,
Bse Al / Bye A1
B1~N -.RP D1~N' ' ~RP
E1~. ,,~ ,o o I E1~. ,,~ ,o o
N, , ,,~0 N~ N~ , ~ ,~~0 N~
0~ O ~ 0~ 0
0 O
0
~ X1 ~ o Xi
_ R5
( 12 ) ( I ) a N~Q2~Q3iW1
1 Y
Bi, A
Rp
.O
E ~ ~s, '' ,.v0 N ~
O
O
O ~''
O
(I)-b
Compounds having formula (12) may be converted to
compounds having formula (I)-a by reacting the former and a
compound having formula (14)
HN ( R5 ) -Q2- ( CHZ ) ~-Q3-W1
(14)
in which Q2 is absent, O, or N(R5);
z is zero to four;
Q3 is absent, O, or alkenylene; and
in which Q~, z, and Q3 may combine with the fixed
moieties between Y1 and W1 to form moieties
represented by Z1,
and the dilute first acid. The reaction is typically
conducted at about 25 °C, over about 24 hours to 72 hours,
-2 6-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
in solvents such as acetonitrile, DMF, water, and mixtures
thereof.
Compounds having formula (I)-a may be converted to
compounds having formula (I)-b by reacting the former and a
reducing agent. Reducing agents include sodium
cyanoborohydride and sodium borohydride. The reaction is
typically conducted at about 0 °C to 25 °C, over about 24
hours to 72 hours, in solvents such as methanol, ethanol,
iso-propyl alcohol, acetonitrile, DMF, water, and mixtures
thereof.
SCHEME 5
R5
3
C ( O ) OH O~N~'Q \W 1
Y1
1 /
P
D D 1~ N\ O,R
I E 1~~ ~,,- ~ 0 I
E N~ N,,, ~ ,,.0 N~
( ~ O~ O
O
(1g) (I)-c
R5 0 1
W
i=C=O
1 11 I6.
Y 1 Y R
B1 °, j~~' / B1~, A ~ P
D1 ~RP D1~N', ~R °
'0 ~O O N
E1~,~~ . N\ ~O O I E N~ ,~ w
N~ , - ,~.0 N~ ,,
O
0 0 O
0 0''.I 'n O
O Xl
O
(16) (I)-d
_27_
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
Compounds having formula (13) may be converted to
compounds having formula (I)-c by reacting the former, a
compound having formula (15)
HN ( R5 ) ( CH2 ) z-Q3-W1
(15),
and a dehydrating agent. Dehydrating agents include 1,3-
dicyclohexylcarbodiimide and 1-(3-dimethylaminopropyl)-3-
ethylcarbodiimide. The reaction is typically conducted at
about 0 °C to 25 °C, over about 24 hours to 72 hours, in
solvents such as acetonitrile, DMF, water, and
dichloromethane.
Compounds having formula (13) may be converted to
compounds having formula (I)-d by (a) reacting the former
and diphenylphosphoryl azide to form a compound having
formula (16) and (b) reacting the product of step (a) with a
compound having formula (17)
HN (R6) (W1)
(17) .
Step (a) is typically conducted at about 25 °C to
80 °C, over about 1 to 8 hours, in solvents such as benzene,
toluene, and acetonitrile. Step (b) is typically conducted
at about 25 °C to 100 °C, over about 1 to 24 hours, in
solvents such as benzene, toluene, and acetonitrile, DMF,
and DME.
_28_
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
SCHEME 6
1 Y~ Br
B ;, A
O~RP Dl~Nw O~Rp
N~ E1~~' \N~ ~'''~ v0,~~0 N~
0
0..::
O
~1
O
(10) (19)
Compounds having formula (10) may be converted to
compounds having formula (19) by reacting the former and a
compound having formula (18)
Br-Y1-Br
(18)
under the same conditions described for the conversion of
compounds of this invention having formula (10) to compounds
having formula (12) in Scheme 3.
-29-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
SCHEME 7
Y1 Br / Y1 C=C- ( CH2 ) y-W1
B :, A / B ., A
P P
D1 N ~ ~R D~ , N~ O~R
w 0 E1v~ , , ,0 I
E1~,~~ ,,, ~O ~ ~ ~O N
~N, , ~ ~,v0 N~ ~Ni~, ~, w
O O O
0 O ;;
0 '':
n _ 0
I v~c~ i ~ c
'1
O X
(19) (I)-f
1!/ ( CH2 ) Y W1
Y
1 /
B 1,> A /
P
D ~~Nw O~R
,, , O
~~~0 N~
O
~ ,; O
O
(I)-g
Compounds having formula (19) may be converted to
compounds having formula (I)-f by reacting the former and a
compound having formula (20)
H-C=C- ( CH2 ) y-W1
(20) ,
in which y is zero to four,
or compounds having formula (I)-g by reacting the former and
a compound having formula (21)
H-CH=CH- ( CH2 ) y-W1
(21) ,
under the same conditions described for the conversion of
compounds having formula (10) to compounds having formula
(12) in Scheme 3.
-30-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
SCHEME 8
Y1 L1-wl
1 1
B Zo A ~ B to A
RP RP
DZ~,,... Nw .O O. I Dy,,... Nw .O O
E N, , ~'' ,,.0 N~ E N~ , ''' ° ,~~0 N~
O ~ 0~ 0
O \,,y ~ 0 ';:
rI O O
0 X1 O XZ
(10) (I)-h
Compounds having formula (10) may be converted to
compounds having formula (I)-h by reacting the former and a
compound having formula (22)
~1-~,1-~1-Wl
(22)
under the same conditions described for the conversion of
compounds having formula (10) to compounds having formula
(12) in Scheme 3.
SCHEME 9
W1 Z
BsoA1 ~ BleA~
D2 ,. N~ 0 O~RA D1~ .. N~ 0 H O~RA
E ,' ,,,, , I E ~' ~''-,
O~ n, ~ ,,'vp N~ 0~ n, ,,'v0 N
0\;. O 0 \,: O
0\ - O 0\ 0
(I)-h (I)-i
Compounds having formula (I)-h may be converted to
compounds having formula (I)-i by reacting the former,
hydrogen gas, a hydrogenation catalyst, and, optionally,
quinoline. Hydrogenation catalysts include Lindlar catalyst
and palladium on barium sulfate. The reaction is typically
conducted at 25 °C, over about 1 to 6 hours, in solvents
-31-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
such as methanol, ethanol, propanol, butanol, iso-propanol,
tert-butanol, acetonitrile, THF, ethyl acetate, and mixtures
thereof.
SCHEME 10
B:eA / B1,A \ B (V1) 2
D1 1Nw ~ A D1 1Nw
E1~~..,, ,.0 O'R Ey~,,,,, ,.O ~R
N\ O~ n, ,,,v0 N
Ni~,
0.::~0 O O~ ~ 0..:;~0 O O
(10) (24)
1 A1 Y1 L1_W1
B ,>, \
N~ ~ RA
E 1, ~ ' ~ ~,,, ,.O 0.
r~, ~ .,v0 N
O
O '0
~Z
0
(I)-7
Compounds having formula (10) may be converted to
compounds having formula (24) by reacting the former and
compounds having formula (23)
' B (V1) 3v
(23)
in which each V1 is independently hydrogen, alkyl, OH,
or OR45.
The reaction is typically conducted at about -20 °C to 25
°C,
over about 1 to 6 hours, in solvents such as THF, DME, and
diethyl ether.
-32-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
Compounds having formula (24) may be converted to
compounds having formula (I)-j by reacting the former,
compounds having formula (25)
X1-Y1-L1-Wi
(25) ,
a coupling catalyst, a fourth base, and, optionally, a
second'additive. Coupling catalysts include
dichlorobis(triphenylphosphine)palladium(II),
tris(dibenzylideneacetone)dipalladium(0),
tetrakis(triphenylphosphine)palladium(0), and
dichlorobis(triphenylphosphine)nickel(II). Fourth bases
include sodium carbonate, sodium bicarbonate, potassium
carbonate, cesium carbonate, triethylamine, and
diisopropylethylamine. The reaction is typically conducted
at about 50 °C to 80 °C over about 12 to 48 hours, in
solvents such as acetonitrile, THF, DMF, and DME.
Compounds having formula (I), in which RA is Rp, and RP
is acetyl or benzoyl, may be converted to compounds having
formula (I), in which RA is hydrogen, by reacting the former
and methanol. The reaction is typically conducted at about
°C to 65 °C, over about 2 to 60 hours, in methanol.
Compounds having formula (I), in which RA is RP, and RP
is trimethylsilyl, may be converted to compounds having
formula (I), in which RA is hydrogen, by reacting the former
25 and a fluoride-donating agent. Fluoride-donating agents
include tetrabutylammonium fluoride, polymer-bound ammonium
fluoride, tetrabutylammonium fluoride, pyridine~HF, and
triethylamine~trihydrofluoride. The reaction is typically
conducted at about 0 °C to 50 °C, over about 1 to 24 hours,
in solvents such as THF and 1,4-dioxane.
-33-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
The following examples illustrate methods by which
certain preferred first embodiments of this invention may be
prepared.
EXAMPLE 1
This example was prepared as described in commonly
owned US 6,075,133, EXAMPLE 246, step 246c.
EXAMPLE 2
A solution of EXAMPLE 1 (10 g), 4-dimethyl-
aminopyridine (50 mg), benzoic anhydride (7.02 g), and
triethylamine (3.8 mL) in dichloromethane (70 mL) at 15 °C
was stirred for 20 minutes at 15 °C and at ambient
temperature for 7 hours, diluted with ethyl acetate, washed
with 5% sodium carbonate, water, and brine, and dried
(Na2S04), filtered, and concentrated.
EXAMPLE 3'
A solution of EXAMPLE 2 (9.80 g), carbonyldiimidazole
(4.05 g), and 4-dimethylaminopyridine (122 mg) in THF (45
mL) and DMF (13 mL) was treated with 1,8-diazabicyclo-
[5.4.0]under-7-ene (2.24 mL), stirred at ambient temperature
for 12 hours, diluted with ethyl acetate, washed with water
and brine, and dried (Na2S09), filtered, and concentrated.
EXAMPLE 4
A solution of EXAMPLE 3 (11.09 g) and ethylenediamine
(6.67 mL) in acetonitrile (50 mL) and water (5 mL) was
stirred at ambient temperature for 3 days and concentrated.
The concentrate was dissolved in toluene (140 mL) and acetic
acid (7 mL), stirred at 80 °C for 12 hours and at ambient
temperature for 12 hours, diluted with dichloromethane,
washed with saturated potassium carbonate, and dried
(Na2S04), filtered, and concentrated; and the concentrate was
-34-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
flash chromatographed on silica gel with 95:5:0.5
dichloromethane/methanol/concentrated ammonium hydroxide.
EXAMPLE 5
A solution of EXAMPLE 4 (5.95 g) and 2M HCl (5 mL) in
ethanol (5 mL) was heated to 55 °C for 12 hours and
concentrated. The concentrate was dissolved in water,
washed with diethyl ether, treated with saturated ammonium
hydroxide and extracted with dichloromethane; and the
extract was concentrated.
EXAMPLE 6
A solution of N-chlorosuccinimide (5.46 g) in
dichloromethane (200 mL) at -10 °C was treated with dimethyl
sulfide (3.50 mL), stirred for 10 minutes, treated with a
solution of EXAMPLE 5 (20.9 g) in dichloromethane (90 mL)
over 30 minutes, stirred for 1 hour, treated with
triethylamine (3.79 mL), stirred for 90 minutes, washed with
5o sodium bicarbonate and brine, and dried (Na2S0~),
filtered, and concentrated.
EXAMPLE 7
A solution of EXAMPLE 6 (14 g) in methanol (100 mL) was
heated at 60 °C for 16 hours and concentrated; and the
concentrate was flash chromatographed on silica gel with
97:2:1 dichloromethane/methanol/concentrated ammonium
hydroxide.
EXAMPLE 8
A mixture of EXAMPLE 7 (3.75 g), 5-bromothiophene-2-
carboxaldehyde (1.55 g), dichlorobis(triphenylphosphine)-
palladium(II) (76 mg), and copper(I) iodide (10.2 mg) in
acetonitrile (60 mL) and triethylamine (20 mL) was heated at
75 °C for 14 hours and concentrated; and the concentrate was
-35-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
flash chromatographed on silica gel with 97:2:1
dichloromethane/methanol/concentrated ammonium hydroxide.
EXAMPLE 9
A mixture of EXAMPLE 8 (350 mg), aniline (82.9 ~.L), and
acetic acid (77.8 ~,L) in methanol (8 mL) was stirred at
ambient temperature for 2 hours, treated with sodium
cyanoborohydride (57 mg), heated at 60 °C for 1 hour,
treated with dichloromethane, washed with 5o sodium
bicarbonate and brine, and dried (Na2S04), filtered, and
concentrated; and the concentrate was flash chromatographed
on silica gel with 98':1:1 dichloromethane/methanol/
concentrated ammonium hydroxide.
EXAMPLE 10
A mixture of EXAMPLE 8 (280 mg), and O-benzylhydroxyl-
amine hydrochloride (86.6 mg) in methanol (4 mL) was heated
at 55 °C for 2 hours, poured into 5% sodium bicarbonate, and
extracted with dichloromethane. The extract was washed with
brine, dried (Na2S04), filtered, and concentrated; and the '
concentrate was flash chromatographed on silica gel with
97.5:2:0.5 dichloromethane/methanol/concentrated ammonium
hydroxide.
EXAMPLE 11
This example was prepared by substituting O-
phenylhydroxylamine hydrochloride for 0-benzylhydroxylamine
hydrochloride in EXAMPLE 8.
EXAMPLE 12
This example was prepared by substituting 0-naphthalen-
1-ylmethylhydroxylamine and catalytic para-toluenesulfonic
acid for O-benzylhydroxylamine hydrochloride in EXAMPLE 8.
-36-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
EXAMPLE 13
This example was prepared by substituting O-(3-
naphthalen-1-yl-allyl)hydroxylamine for 0-naphthalen-1-
ylmethylhydroxylamine in EXAMPLE 12.
EXAMPLE 14
This example was prepared by substituting O-(2-phenoxy
ethyl,)hydroxylamine for O-naphthalen-1-ylmethylhydroxylamine
hydrochloride in EXAMPLE 12.
EXAMPLE 15
A mixture of EXAMPLE 7 (350 mg), N-benzyl-N-((5-
bromothien-2-yl)methyl)amine (172 mg), dichlorobis-
(triphenylphosphine)palladium(II) (7.4 mg), triethylamine (2
mL) and copper(I) iodide (2 mg) in acetonitril.e (6 mL) was
heated at 80 °C for 20 hours and concentrated; and the
concentrate was flash chromatographed on silica gel with
98:1.5:0.5 dichloromethane/methanol/concentrated ammonium
hydroxide.
EXAMPLE 16
A mixture of EXAMPLE 8 (200 mg), 2-hydrazinopyridine
(42.3 mg), and magnesium sulfate (50 mg) in THF was heated
at 66 °C for 16 hours, and filtered. The filtrate was
treated with ethyl acetate, washed with water and brine,
dried (Na2S04);, filtered, and concentrated; and the
concentrate was flash chromatographed on silica gel with
97.5:2:0.5 dichloromethane/methanol/concentrated ammonium
hydroxide.
EXAMPLE 17
This example was prepared by substituting pyridine-2-
carbohydrazide for 2-hydrazinopyridine in EXAMPLE 16.
-37-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
EXAMPLE 18
This example was prepared by substituting O-quinol-3-
ylmethylhydroxylamine for O-naphthalen-1-
ylmethylhydroxylamine hydrochloride in EXAMPLE 12.
EXAMPLE 19
This example was prepared by substituting N-((5-
bromothien-2-yl)methyl)-N-(2-phenylethyl)amine for N-benzyl-
N-((5-bromothien-2-yl)methyl)amine in EXAMPLE 15.
EXAMPLE 20
This example was prepared by substituting N-((5-
bromothien-2-yl)methyl)-N-(3-phenylpropyl)amine for N-
benzyl-N-((5-bromothien-2-yl)methyl)amine in EXAMPLE 15.
EXAMPLE 21
A mixture of EXAMPLE 7 (350 mg), 2-(3-
bromophenoxy)pyridine (251 mg), dichlorobis(triphenyl-
phosphine)palladium(II) (11 mg), and copper(I) iodide (1
mg), and triethylamine (2 mL) in acetonitrile (6 mL) was
heated at 70 °C for;20 hours and concentrated; and the
concentrate was flash chromatographed on silica gel with
98:1.5:0.5 dichloromethane/methanol/concentrated ammonium
hydroxide.
EXAMPLE 22
This example was prepared by substituting 5-bromo-N-(3-
fluorophenyl)thiophene-2-carboxamide for 2-(3-
bromophenoxy)pyridine in EXAMPLE 21.
EXAMPLE 23
-38-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
This example was prepared by substituting 5-bromo-N-(3-
fluorophenyl)-N-methylthiophene-2-carboxamide for 2-(3-
bromophenoxy)pyridine in EXAMPLE 21.
EXAMPLE 24
This example was prepared by substituting 1-bromo-3-(3-
fluorophenoxy)benzene for 2-(3-bromophenoxy)pyridine in
EXAMPLE 21.
EXAMPLE 25
A solution of EXAMPLE 6 (5 g), 2,5-dibromothiophene
(4.74 g), dichlorobis(triphenylphosphine)palladium(II) (184
mg), and copper(I) iodide (17 mg), and triethylamine (9 mL)
in acetonitrile (27 mL) was heated at 80 °C for 24 hours,
concentrated, treated with dichloromethane, washed with 50
sodium bicarbonate and brine, dried (Na2SQ4), filtered, and
concentrated; and the concentrate was flash chromatographed
on silica gel with 98.5:1:0.5 dichloromethane/methanol/
concentrated ammonium hydroxide.
EXAMPLE 26
A solution of EXAMPLE 25, (300 mg), 2-ethynylpyridine
(41.7 mg), dichlorobis(triphenylphosphine)palladium(II) (4.5
mg), copper(I) iodide (0.6 mg) and triethylamine (1 mL) in
acetonitrile (3 mL) was heated at 75 °C for 14 hours and
concentrated.
EXAMPLE 26A
A solution of EXAMPLE 26 in methanol (10 mL) was heated
at 60 °C for 14 hours and concentrated; and the concentrate
was flash chromatographed on silica gel with 98:1:1
dichloromethane/methanol/concentrated ammonium hydroxide.
EXAMPLE 27
-39-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
This example was prepared by substituting 1-bromo-4-
phenoxybenzene for 2-(3-bromophenoxy)pyridine in EXAMPLE 21.
EXAMPLE 28
This example was prepared by substituting 5-bromo-N-
pyridin-3-ylthiophene-2-carboxamide for 2-(3-
bromophenoxy)pyridine in EXAMPLE 21.
EXAMPLE 29
This example was prepared by substituting 5-bromo-N-(4-
(1,2,3-thiadiazol-4-yl)benzyl)thiophene-2-carboxamide for 2-
(3-bromophenoxy)pyridine in EXAMPLE 21.
EXAMPLE 30
This example was prepared by substituting 5-bromo-N-(3-
(quinolin-3-yl)propyl)thiophene-2-carboxamide for 2-(3-
bromophenoxy)pyridine in EXAMPLE 21.
EXAMPLE 31
This example was prepared by substituting N-benzyl-N-
((5-bromothien-2-yl)methyl)-N-methylamine for 2-(3-
bromophenoxy)pyridine in EXAMPLE 21.
EXAMPLE 32
A mixture of EXAMPLE 7, (300 mg), N-(3-bromophenyl)-N'-
pyridin-4-ylurea (123 mg), tris(dibenzylideneacetone)-
dipalladium(0) (16.6 mg), 1,2-bis(diphenylphosphino)ethane
(14.4 mg), triethylamine (1.5 mL), and copper(I) iodide
(0.86 mg) in acetonitrile (5 mL) was heated at 75 °C for l8
hours and concentrated; and the concentrate was flash
chromatographed on silica gel with 98:1:1 dichloromethane/
methanol/concentrated ammonium hydroxide.
EXAMPLE 33
-40-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
A solution of EXAMPLE 6 (672 mg) in DMF (5 mL) at 0 °C
was treated with 600 oily sodium hydride (70 mg), stirred
for 40 minutes, treated with N-fluorobenzenesulphonimide
(314 mg), stirred for 3 hours, diluted with ethyl acetate,
washed with water and brine, dried (Na2S04), filtered, and
concentrated; and the concentrate was flash chromatographed
on silica gel with 95:5:0.5 dichloromethane/methanol/
concentrated ammonium hydroxide.
' 10 SPECTRAL DATA FOR REPRESENTATIVE COMPOUNDS
EXAMPLE 7
i3C NMR (CDC13) 8 204.7, 169.5, 156.0, 103.4, 81.59,
81.53, 80.36, 77.2 (C-5), 77.2 (C-13), 74.3, 70.26, 69.6,
65.9, 60.1, 51.1, 50.6, 49.3, 47.1, 42.6, 42.2, 40.2, 40.2,
37.9, 36.3, 28.3, 22.3, 21.2, 20.3, 19.6, 15.0, 14.5, 12.9,
11.0, 10.6.
EXAMPLE 10
13C NMR (CDC13) 8 204.88, 204.85, 179.86, 179.80;
169.56,169.53, 156.0, 143.16, 140.37, 132.18, 131.31,
131.28, 128.95, 128.45, 128.42, 128.02, 127.93, 103.60,
103.56, 93.97, 93.34, 81.48, 80.43, 78.94, 77.45, 77.34,
77.29, 76.87, 76.71, 70.32, 69.64, 65.89, 60.25, 51.41,
51.20, 49.47, 47.40, 47.32, 42.71, 42.28, 40.24, 37.99,
36.29, 28.20, 22.29, 21.25, 20.26, 19.65, 15.39, 15.28,
14.57, 13.03, 11.09, 10.53.
EXAMPLE 11
13C NMR (CDC13) 8 204.86, 180.02, 179.94, 169.62,
169.59, 156.00, 145.61, 142.32, 132.54, 132.29, 131.39,
131.36, 130.40, 129.33, 129.29, 122.86, 122.4'1, 114.91,
114.42, 103.60, 103.54, 94.59, 93.88, 81.51, 80.49, 78.74,
77.43, 77.40, 77.28, 70.30, 69.66, 65.87, 51.45, 51.39,
-41-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
51.20, 49.50, 49.47, 47.40, 47.29, 42.73, 42.28, 40.24,
38.02, 37.99, 36.31, 28.20, 22.27, 21.25, 20.26, 20.22,
19.65, 15.36, 15.23, 14.60, 13.03, 11.08, 10.53.
EXAMPLE 12
13C NMR (CDC13) 8 204.87, 204.83, 179.76, 179.73,
169.52, 156.0, 143.19, 140.19, 136.79, 133.72, 132.18,
131.86, 131.31, 131.28, 129.11, 129.01, 128.53, 128.49,
127.62, 127.12, 126.35, 125.78, 125.26, 124.11, 123.99,
103.60, 103.54, 93.92, 93.37, 81.48, 81.43, 80.45, 80.41,
78.94, 78.89, 77.45, 77.32, 77.307, 75.41, 75.08, 70.30,
69.64, 65.87, 60.23, 51.39, 51.20, 51.17, 49.47, 49.39,
47.40, 47.29, 42.69, 42.28, 42.23, 40.22, 37.96, 36.29,
28.19, 22.27, 21.24, 20.26, 19.64, 15.39, 15.23, 14.55,
13.023, 11.06, 10.51.
EXAMPLE 13
13C NMR (CDC13) 8 204.89, 204.85, 179.79, 169.45, 156.0,
155.97, 143.19, 143.15, 140.29, 132.19, 131.33, 131.28,
130.90, 130.45, 128.98, 12$.43, 128.16, 128.06, 128.12,
128.09, 126.05, 125.73, 125.69, 125.57, 124.08, 123.89,
123.85, 103.60, 103.54, 93.88, 93.37, 81.48, 80.46, 80.39,
79.0, 77.45, 77.34, 77.27, 77.29, 75.71, 75.30, 70.30,
69.64, 65.89, 60.25, 51.41, 51.22, 51.17, 49.49, 49.41,
47.42, 47.32, 42.69, 42.28, 40.22, 38.00, 36.29, 28.20,
22.32, 22.27, 21.24, 20.28, 19.63, 15.39, 15.25, 14.57,
13.06, 13.04, 11.08, 10.56.
EXAMPLE 14
13C NMR (CDC13) b 204.89, 204.85, 179.86, 179.80,169.56,
169.45, 156.0, 155.97, 143.57, 140.48, 132.21, 131.87,
131.37, 131.33, 129.40, 129.16, 120.89, 114.81, 114.70,
103.62, 103.57, 93.92, 93.42, 81.48, 80.46, 80.39, 78.89,
-42-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
77.43, 77.36, 77.31, 77.28, 73.12, 72.84, 70.30, 69.66,
66.23, 65.91, 60.25, 51.41, 51.22, 51.19, 49.49, 49.43,
47.44, 47.37, 42.69, 42.27, 40.24, 37.99, 36.29, 28.20,
22.32, 22.27, 21.25, 20.226, 19.63, 15.43, 15.29, 14.56,
13.06, 13.04, 11.08, 10.56.
EXAMPLE 15
13C NMR (CDC13) ~ 204.9, 179.8, 169.6, 156.0, 146.4,
139.8, 132.1, 128.4, 128.4, 128.1, 128.1, 126.9, 124.6,
121.5, 103.5, 90.9, 81.5, 80.2, 79.4, 77.4, 77.3 70.3, 69.6,
65.9, 60.2, 52.6, 51.4, 51.2, 49.4, 47.6, 47.4, 42.7, 42.2,
40.2, 38.0, 36.2, 28.2, 22.3.
EXAMPLE 16
~3C NMR (CDC13) 8 204.88, 204.85, 180.04, 179.83,
169.51, 169.48, 156.0, 147.61, 147.63, 138.15, 138.09,
137.97, 133.00, 132.54, 130.40, 129.30, 126.78, 116.67,
115.23, 107.94, 107.96, 103.57, 94.34, 93.03, 81.53, 81.49,
80.48, 80.38, 79.28, 78.28, 77.40, 77.31, 70.30, 69.64,
65.88, 60.23, 51.39, 51.20, 49.47, 47.40, 42.68, 42.26,
40.22, 37.99, 36.28, 28.20, 25.23, 22.29, 21.25, 20.28,
20.25, 19.62, 15.39, 15.35, 14.55, 13.04, 11.08, 10.54.
EXAMPLE 17
13C NMR (CDC13) 8 204.91, 180.07, 169.39, 156.10,
132.67, 103.59, 81.66, 80.43, 77.36, 77.28, 70.32, 69.66,
65.87, 60.26, 51.39, 51.19, 49.39, 47.42, 42.65, 42.24,
40:24, 38.00, 36.23, 28.20, 22.35, 21.25, 20.26, 19.60,
15.38, 14.54, 13.08, 11.09, 10.59.
EXAMPLE 18
isC NMR (CDC13) 5204.91, 204.85, 179.99, 179.83, 169.54,
169.37, 156.0, 155.97, 151.07, 150.89, 147.88, 143.78,
-43-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
140.85, 136.39, 135.66, 135.42, 132.22, 131.65, 131.44,
130.21, 130.00, 129.53, 129.47, 129.32, 129.29, 128.02,
127.95, 126.77, 126.72, 103.61, 103.54, 94.03, 93.51, 81.51,
80.46, 80.39, 78.86, 77.39, 77.28, 77.19, 77.21, 74.46,
74.15, 70.30, 69.64, 65.89, 60.23, 51.39, 51.20, 51.16,
49.47, 49.42, 47.42, 47.34, 42.69, 42.26, 42.23, 40.24,
38.00, 37.97, 36.28, 28.20, 22.36, 22.29, 21.24, 20.28,
20.23, 19.63, 15.41, 15.25, 14.54, 13.10, 13.04, 11.09,
10.58.
EXAMPLE 19
13C NMR (CDC13) 8 204.9, 179.8, 169.6, 156.0, 146.4,
139.8, 132.1, 128.4, 128.4, 128.1, 128.1, 126.9, 124.6,
121.5, 103.5, 90.9, 81.5, 80.2, 79.4, 77.4, 77.3 70.3, 69.6,
65.9, 60.2, 52.6, 51:4, 51.2, 49.4, 47.6, 47.4, 42.7, 42.2,
40.2, 38.0, 36.2, 28.2, 22.3.
EXAMPLE 20
i3C NMR (CDC13) 8 204.9, 179.8, 169.3, 156.0, 146.6,
142.1, 132.1, 128.4 128.3, 125.7, 124.5, 121.5, 103.6, 91.0,
81.5, 80.2, 79.4, 77.4, 77.3, 70.3, 69.7, 69.6, 65.9, 60.2,
51.4, 51.2, 49.4, 48.5, 48.5, 47.4, 42.7, 42.2, 40.2, 38.0,
36.2, 33.5, 31.6, 28.2, 22.3, 21.2, 20.3, 19.6, 15.4, 14.5,
13.1, 11.1.
EXAMPLE 21
13C NMR (CDC13) 8 204.8, 180.1, 169.6, 163.4, 155.9,
153.9, 147.7, 139.4, 129.5, 127.8, 124.4, 124.0, 121.3,
118.6, 111.5, 103.5, 88.1, 84.9, 81.4, 80.3, 77.5, 77.1,
70.2, 69.5, 65.9, 60.2, 51.3, 51.2, 49.3, 47.2, 42.6, 41.2,
40.2, 38.0, 36.2, 28.3, 22.2, 21.2, 20.2, 19.6, 15.2, 14.6,
13.0, 11.0, 10.4.
-44-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
EXAMPLE 22
13C NMR (CDC13) 8 205.0, 180.6, 169.2, 164.5, 159.2,
156.3, 139.4, 139.3, 138.8, 132.7, 130.1, 129.9, 129.4,
127.6, 115.5, 115.5, 111.3, 111.0, 107.8, 107.5, 103.6,
93.7, 81.8, 80.4, 78.5, 77.7, 77.1, 72.2, 70.2, 69.6, 65.9,
60.3, 51.3, 51.2, 49.3, 47.6, 42.6, 42.4, 40.2 38.1, 36.1,
28.2, 22.5, 21.2, 20.3, 19.6, 15.6, 13.2, 11.2, 10.7.
EXAMPLE 23
13C NMR (CDC13) S 204.8, 179.7, 169.6, 164.7, 161.8,
155.9, 145.3, 145.2, 137.7, 132.3, 131.5, 131.1, 127.9,
123.9, 115.7,115.5, 115.4, 115.3, 103.6, 93.7, 81.4, 80.4,
78.3, 77.4, 77.2, 70.3, 69.6, 65.9, 60.1, 51.3, 51.2, 49.4,
47.3, 42.6, 42.2, 40.2, 38.9, 37.9, 36.2, 28.1, 22.2, 21.2,
20.2, 19.6, 15.3, 14.5, 12.9, 11.0, 10.5.
EXAMPLE 24
13C NMR (CDC13) 8 204.9, 179.8, 169.6, 160.5, 157.5,
156.0, 129.6, 126.3, 124.5, 121.0, 120.8, 120.7, 118.8,
118.4, 116.5, 116.2, 103.5, 88.0, 84.9, 81.5, 80.3, 77.4,
77.2, 70.3, 69.6, 65.9, 60.2, 51.3, 51.2, 49.4, 47.2, 42.7,
42.2, 40.2, 38.0, 36.3, 28.2, 22.3, 21.2, 20.2, 19.6, 15.2,
14.6, 13.0, 11.1, 10.5.
EXAMPLE 25
13C NMR (100 MHz, CDC13) 8 204.9, 179.9, 169.6, 156.0,
150.1, 142.9, 136.1, 133.0, 132.1, 127.1, 125.3, 123.4,
122.9, 103.6, 92.9, 92.8, 82.2, 81.5, 80.5, 78.6, 77.4,
77.3, 70.3, 69.7, 65.9, 60.2, 51.4, 51.2, 49.5, 47.3, 42.7,
42.3, 40.2, 38.0, 36.3, 28.2, 22.3, 21.2, 20.2, 19.7, 15.3,
14.6, 13.0, 11.0, 10.5.
EXAMPLE 27
-45-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
13C NMR (CDC13) b 204.9, 179.9, 169.5, 157.4, 156.0,
133.1, 129.8, 123.7, 119.3, 118.8, 118.3, 117.6, 103.5,
86.7, 85.1, 81.5, 80.2, 77.4, 77.3, 70.3, 69.6, 65.9, 60.2,
i 51.4, 51.2, 49.4, 47.3, 42.7, 42.2, 40.2, 38.1, 36.3, 28.2,
22.3, 21.2, 20.2, 19.6, 15.3, 14.6, 13.1, 11.1, 10.6.
EXAMPLE 28
13C NMR (CDC13) 8 204.9, 180.3, 169.1, 159.7, 156.3,
145.2, 141.7, 138.8, 135.0, 132.6, 129.4, 127.9, 127.7,
123.6, 103.6, 93.8, 81.8, 80.4, 78.4, 77.6, 77.1, 70.3,
69:6, 65.8, 60.3, 57.3, 51.2, 49.3, 47.5, 42.6, 42.3, 40.2,
38.0, 36.1, 28.2, 22.4, 21.2, 20.3, 19.5, 15.5, 14.5, 13.1,
11.2, 10.6.
EXAMPLE 29
13C NMR (CDC13) b 204.8, 180.0, 169.4, 162.4, 161.1,
156.1, 139.4, 138.8, 132.5, 130.0, 128.5, 128.5, 127.7,
127.1, 103.6, 93.5, 81.6, 80.4, 78.4, 77.4, 77.3, 70.3,
69.6, 65.8, 60.2, 51.3, 51.2, 49.4, 47.4, 43.6, 42.6, 42.2,
40.2, 38.0, 36.2, 28.2, 22.3, 21.2, 20.2, 19.6, 15.4, 14.5,
11.1, 10.6.
EXAMPLE 30
13C NMR (CDC13) 8 204.9, 180.2, 169.3, 161.2, 156.1,
151.7, 146.9, 138.9, 134.3, 134.0, 132.5, 129.1, 128.7,
128.4, 128.1, 127.4, 126.6, 103.6, 93.3, 81.7, 80.4, 78.5,
77.x, 77.2, 70.3, 69.6, 65.9, 60.3, 51.3, 51.2, 49.4, 47.5,
42.6, 42.3, 40.2, 39.6, 38.0, 36.2, 30.9, 30.5, 28.2, 22.4,
21.2, 20.3, 19.6, 15.5, 14.5, 13.1, 11.1, 10.6.
EXAMPLE 31
13C NMR (CDC13) 8 204.8, 179.7, 169.4, 156.0, 145.1,
138.8, 131.9, 128.8, 128.2, 127.0, 125.2, 122.0, 103.5,
-46-
CA 02476223 2004-08-12
WO 03/068790 PCT/US03/04117
91.0, 81.5, 80.2, 79.5, 77.3, 77.3, 70.3, 69.6, 65.9, 61.1,
60.2, 56.0, 51.4, 51.2, 49.4, 47.3, 42.7, 42.3, 42.0, 40.2,
38.0, 36.2, 28.2, 22.3, 21.2, 20.2, 19.6, 15.3, 14.5, 13.0,
11.1, 10.5.
EXAMPLE 32
13C NMR (CDC13) 8 205.3, 180.7, 169.1, 156.7, 152.1,
150.1, 146.8, 138.6, 129.2, 126.3, 123.3, 121.8, 119.5,
112.9, 103.9, 87.0, 85.5, 82.3, 80.3, 77.7, 77.6, 70.4,
69.7, 65.8, 60.5, 51.3, 51.3, 49.1, 47.5, 42.5, 42.3, 40.2,
38.2, 36.1, 28.1, 22.6, 21.2, 20.1, 19.5, 15.5, 14.7, 13.2,
11.2, 10.7.
The foregoing is merely illustrative of this invention
and is not intended to limit the same to the disclosed
compounds. Variations and changes which are obvious to one
skilled in the art are intended to be within the scope and
nature of this invention which is defined in the appended
claims.
-47-