Note: Descriptions are shown in the official language in which they were submitted.
CA 02477905 2004-08-31
WO 03/074093 PCT/US03/05946
VTN 582 PCT
IN-LINE STEAM STERILIZER
Related Applications
This application claims priority of a provisional application U.S. Ser. No.
60/360,904, filed on March 1, 2002 and entitled "In-Line Steam Sterilizer."
Field of the Invention
The present invention relates generally to a contact lens manufacturing
arts, and, in particular to a novel in-line sterilizer for an ophthalmic
contact
lens manufacturing system, sterilization tray and method for performing in-
line sterilization of packaged contact lenses more efficiently and with
increased throughput.
Automated ophthalmic contact lens production processes are known
wherein each lens is formed by sandwiching a monomer between back curve
(upper) and front curve (lower) mold structure transported in a mold cavity.
The
monomer is polymerized (cured) to form a lens blank, and is subject to further
processing including, but not limited to: removing the lens blanks from their
mold
structures, i.e., de-molding; subjecting the lenses to a hydration process;
transferring of the lenses to an individual blister package; automatic lens
inspection of the lens, e.g., while contained in their blister pack; lens
sterilization;
and final packaging for consumer use. The reader may refer to U.S. Patent No.
5,555,504 entitled PRODUCTION LINE TRACKING AND QUALITY CONTROL
SYSTEM for a description of an exemplary prior art ophthalmic lens production
and packaging control system.
With respect to lens sterilization, a manufactured ophthalmic lens in an
aqueous solution is sealed in a blister pack and sterilized in a steam
sterilizer.
Example descriptions of packaged contact lenses and techniques for their
sterilization are described in U.S. Pat Nos. 5,488,815 and 5,577,367.
Particularly, in commonly-owned issued U.S. Patent No. 5,488,815, an
apparatus is disclosed that contemplates the utilization of a conveyor system
for the conveyance of a plurality of trays, each adapted to house therein a
specific quantity of interleaved pairs of arrays of blister packages, which
are
sequentially folded into paired interleaved positions, and then conveyed
through the intermediary of a transfer conveyor into a respective metal tray
so
CA 02477905 2004-08-31
WO 03/074093 PCT/US03/05946
as to fill spaces in the latter arranged in specified rows and columns. The
metal firay, which is placed into an upended position in order to be able to
receive the interleaved pairs of arrays of blister packages from a shuttle
conveyor, upon being filled is then tilted back into a normally horizontal
orientation and, if desired depending upon production requirements, up to
three such array-filled metal trays may then be vertically stacked or
superimposed, and also conveyed in a series of such stacked trays. A
conveyor is adapted to convey the metal trays with the arrays of blister
packages contained therein into a sterilizing chamber, such as an autoclave,
in which the arrays of blister packages are collectively sterilized.
Subsequent
to the sterilization cycle having been completed, a secondary packaging
procedure is implemented where the trays together with the sterilized arrays
of blister packages are transported by a further conveyor towards an
unloading arrangement in which the trays are unstacked and individual trays
then sequentially upended. This enables the contents of the trays to be
transferred to an unloading shuttle conveyor which, in turn, facilitates
specified quantities of interleaved pairs of arrays of blister packages to be
advanced in succession into a cartoner having open-ended cartons therein
adapted to receive the arrays of blister packages.
Central to the prior art sterilization apparatuses and techniques is that the
blister package array trays must be transferred from the manufacturing line to
the sterilizer which is in a separate area off-line from the manufacturing
stations. Thus, a major problem with that process is a potential for mixing
sterile and non-sterile product at the off-line area. Other problems include
extra material handling equipment; additional manufacturing floor space, and
added in-process product inventory. Therefore, there has been a need, prior
to the invention, to provide an in -line sterilization apparatus for a contact
lens
manufacturing and packaging facility that obviates the need for off-loading
contact lens product for sterilization.
Additionally, these prior art lens sterilization processes provide for
sterilization relatively late in the manufacturing process, prior to secondary
packaging, i.e., placement of labeled lens blister packages in cartons, for
2
CA 02477905 2004-08-31
WO 03/074093 PCT/US03/05946
example. Given the nature of the equipment involved, i.e., the materials, the
prior art equipment and ophthalmic lens sterilizing processes further do not
adequately dry the packages which reduces the throughput as packages have
to be rejected. This phenomenon currently prevents sterilized packages from
immediately being packaged in a secondary packaging process, e.g., to cartons
for shipping. In order to solve the "dryness" problem, longer sterilization
process cycle times in excess of 1 hour are typically required in order to
achieve the degree of dryness of the packages for secondary packaging.
Therefore, there has been a need, prior to the invention, to provide an in -
line
sterilization apparatus for a contact lens manufacturing and packaging
facility
that ensures packages subject to a sterilization process meet requirements of
dryness so that they may be immediately placed in cartons in a subsequent
packaging step. It would be highly desirable to provide for an automated
contact lens manufacturing facility, an in-line sterilization machine that
sterilizes
ophthalmic lens packages without having to offload product from the line.
Furthermore, as prior attempts to segregate sterile and non-sterile
product implement manual procedures, it would be highly desirable to provide
for an automated contact lens manufacturing facility, an in-line sterilization
machine that inherently prevents the mixing of sterile and no-sterile product.
It
would be highly desirable to provide for an automated contact lens
manufacturing facility, an in-line sterilization machine that sterilizes
ophthalmic
lens packages without having to offload product from the line, and, that is of
improved design and capable of sterilizing a greater number of package
containing trays that can be achieved in current systems, thus increasing
throughput per unit volume. It would be highly desirable to provide for an
automated contact lens manufacturing process a novel tray for supporting
stacks of contact lens packages for conveyance in an in-line sterilization
machine that employs a temperature and steam pressurization sterilization
process in a manner such that packaged lens are sterilized, in a more
expedient
fashion, with virtually no package damage due to moisture, thereby enabling
acceptable packages to immediately proceed to a subsequent packaging
station. It would be highly desirable to provide for an automated contact lens
3
CA 02477905 2004-08-31
WO 03/074093 PCT/US03/05946
manufacturing process a novel tray and tray stacking structure that carries
nested arrays of blister packages for simultaneous sterilization and durable
to
withstand the steam and temperature conditions in the sterilizer.
Objectives of the Invention
Accordingly, it is an object of the present invention to provide an in-line
sterilizing apparatus for sterilizing contact lens containing packages without
off-loading the packages from the manufacturing line. It is another object of
the present invention to provide a novel tray structure for carrying contact
lens
containing packages in an in-line sterilizing apparatus that enables increased
throughput per unit volume.
It is a further object of the present invention to provide a novel process
for sterilizing contact lens containing packages in a novel in-line
sterilizing
apparatus that requires less sterilization time.. It is still another object
of the
present invention to provide a novel tray structure for carrying contact lens
packages in a novel in-line sterilization apparatus that performs a
sterilization
under optimum timing, temperature and pressure conditions such that, the
sterilization process provides adequately dry packages prior to their
secondary packaging which immediately follows the sterilization.
Another object of the present invention is to provide a control system
and method for maintaining information relating to the tracking of contact
lens
packages conveyed on a novel transport structure for sterilization in a novel
sterilization apparatus. It is yet still another object of the present
invention to
provide an in-line sterilizing apparatus for sterilizing contact lens
containing
packages in an in-line sterilizing apparatus that is designed to inherently
prevent mixing of sterile and non-sterile product. With respect to soft
contact
lens production, according to an aspect of the present invention, there is
provided a sterilization system and method for automatically transporting a
plurality of soft contact lens packages containing soft contact lenses to be
sterilized through an in-line apparatus, without off-loading packages from the
line, but rather, placing them in novel sterilization trays for transport
through a
sterilizer under optimum conditions of temperature and time for enabling
4
CA 02477905 2004-08-31
WO 03/074093 PCT/US03/05946
increased throughput, wherein dryness of said packages is ensured so that
the packages may immediately proceed for further secondary packaging.
Advantageously, the integration of the ophthalmic lens package
sterilizer within the manufacturing line provides physical separation of
sterile
and non-sterile product. The result is that the product cannot move the
sterile
packaging area without going through the sterilizer. The integration also
allows complete tracking of the product through the lens machine by the
machine controller, ensuring that the product must go through a complete
sterilization cycle before unloading to the secondary packaging area.
Brief Description of the Drawings
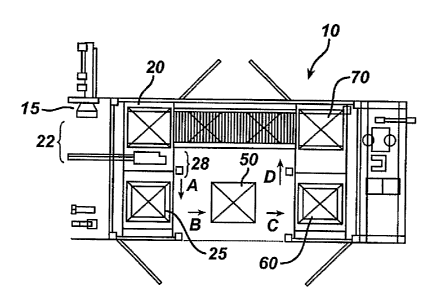
Figure 1 is a conceptual top plan view of a portion of the lens production
facility including the various stations involved with loading of ophthalmic
lens
containing blister packages in novel tray units and their in-line
sterilization
according to the principles of the invention;
Figure 2 is a side elevation view of the in-line sterilizer station 50
including the
tray stacking, novel sterilizer machine, and tray de-stacking stations;
Figure 3(a) is a top plan view of the novel tray unit according to the
principles
of the invention;
Figure 3(b) is a detailed close-up view of the circled portion 100a of the
tray
100 illustrated in Figure 3(a);
Figure 4(a) is a side cross-sectional view of the novel tray 100 taken along
line "E-E" illustrated in Figure 3(a);
Figure 4(b) is a front cross-sectional view of a portion of the novel tray 100
taken along line "F-F" of the tray 100 illustrated in Figure 3(a);
Figure 5 depicts the interleaved pairs of blister arrays 150 deposited in a
respective cavity 120 of the novel tray 100;
5
CA 02477905 2004-08-31
WO 03/074093 PCT/US03/05946
Figure 6 illustrates the front elevation view of the in-line sterilizer
machine
shown receiving a stack of trays from a conveyor;
Figure 7 is a piping and instrumentation diagram illustrating the devices and
operating parameters for performing steam sterilization according to the
invention; and,
Figure 3 is a side elevation view of the latch mechanism 175 that retains the
stack of trays at the tray stacking station 25 of Figure 2.
Detailed Description of the Invention and Preferred Embodiments
The invention includes an apparatus for sterilizing ophthalmic lens
packages comprising
a sterilizing chamber designed to accommodate a plurality of ophthalmic
lens packages;
a means for transporting said ophthalmic lens packages to said sterilizing
chamber;
a means for subjecting a plurality of ophthalmic lens packages to
appropriate sterilization cycle when said ophthalmic lens packages are
contained within said sterilizing chamber;
wherein, said sterilizing chamber is disposed contiguously with prior
processing
stations and secondary packaging stations of a ophthalmic lens manufacturing
line.
As used herein the terms "ophthalmic lens" includes but is not limited to hard
contact lenses, soft contact lenses, rigid gas permeable contact lenses, intra-
ocular lenses and lenses for eyeglasses. The ophthalmic lenses inspected in
this invention may or may not contain vision correction. The preferred lenses
are soft contact lenses with or without vision correction. Soft lenses may be
made of conventional hydrogels and are generally prepared from monomers
including but not limited to hydroxyethyl methacrylate (HEMA), vinyl
pyrrolidone, glycerol methacrylate, methacrylic acid and acid esters; or
silicone hydrogels. Examples of soft contact lenses include but are not
limited
6
CA 02477905 2004-08-31
WO 03/074093 PCT/US03/05946
to etafilcon A, genfilcon A, lenefilcon A, polymacon, acquafilcon A,
balafilcon
A, lotrafilcon A and silicone hydrogels as prepared in U.S. Pat. No.
5,998,498,
US Pat. App. No. 09/532,943, a continuation-in-part of US Pat App. No.
09/532,943, filed on August 30, 2000, U.S. Pat. Ser. No. 09/957, 299 filed on
September20, 2001, U.S. Patent No. 6,087,415, U.S. Pat. No. 5,760,100,
U.S. Pat. No.5,776, 999, U.S. Pat. No. 5,789,461, U.S. Pat. No. 5,849,811,
U.S. Pat. No. 5,965,631, U.S. Pat. App. No. 60/318,536, entitled Biomedical
Devices Containing Internal wetting Agents," filed on September 10, 2001 and
its non-provisional counterpart of the same title, filed on September 6, 2002.
These patents as well as all other patent disclosed in this application are
hereby incorporated by reference in their entirety.
Numerous processes are known for making ophthalmic lenses,
including various processes to make soft contact lenses. While the present
invention is applicable across the board to all ophthalmic lens processes, a
preferred practice, along with its correlative manufacturing line stations,
will
now be described in the context of a soft contact lens, it being understood
that
the present invention is not limited to such lenses.
As used herein the phrase "ophthalmic lens packages" refers to the
primary packaging for individual ophthalmic lenses, or more commonly known
a blister packages. Examples of said packages include but are not limited to
the packages disclosed in U.S. Patent Nos. 4,691,820; 5,054,610; 5,337,888;
5,375,698; 5,409,104; 5,467,868; 5,515,964; 5,609,246; 5,695,049;
5,697,495; 5,704,468; 5,711,416; 5,722,536; 5,573,108; 5,823,327;
5,704,468; 5,983,608; 6,029,808; 6,044,966; and 6,401,915, U.S. Pat. App.
Ser. No. 60/436,109 filed on December 23, 2002 entitled "Contact Lens
Packages Containing Additives" and, U.S. Pat App. Ser. No. 10/183,133 filed
on June 26, 2002, entitled "Contact Lens Packages". All of the
aforementioned patents and patent applications are hereby incorporated by
reference.
As used herein the term "sterilizing chamber" refers to an enclosure
that is used to sterilize ophthalmic lens packages. Said chamber may be
opened, automatically or manually, to permit entry and exit of said packages.
7
CA 02477905 2004-08-31
WO 03/074093 PCT/US03/05946
Further said chamber must be constructed to allow, either or both, heating or
cooling by of any of a number of methods which include but are not limited to
dry heat, steam heat, jacketed cooling, and the like. Temperature
modification of said chamber sterilizes ophthalmic lens packages contained
therein. As used herein "means for transporting" includes but is not limited
to
conveyor belts, pulleys, and other mechanized system used to move items
along a manufacturing line. In the preferred embodiment means for
transporting includes "sterilizing trays." Said sterilizing trays have
external
walls and internal walls dividing said tray in order to enable to hold a
plurality
of ophthalmic lens packages in the most efficient orientation for sterilizing
said
packages. The preferred orientation for sterilizing said packages is with the
package held vertically, as depicted in Figure 5. As used herein "means for
subjecting" includes but is not limited to pressure, heat and steam sources
and regulators.
As used herein "prior processing stations" include but are not limited to
any stations an ophthalmic lens manufacturing line such as lens formation,
hydration, inspection and the like. Specific examples of such processing
stations are disclosed in U.S. Pat No. 4,958,280, entitled "Apparatus and
Method for Satisfying Disposable Contact Lens Prescriptions," which is
hereby incorporated by reference in its entirety. In the preferred embodiment
of this invention, the prior processing stations include any or any
combination
of the following means for loading said sterilizing trays with ophthalmic lens
packages, means for transferring the loaded sterilizing trays to the
sterilizing
chamber, means for unloading said sterilizing trays, and means for
transferring the sterilized ophthalmic lens packages to said second packaging
station.
As used herein the term "sterilization cycle" refers subjecting said
packages to different temperatures and pressures in order to sterilize said
packages and ophthalmic lenses contained therein. As used herein,
"secondary packaging" includes but is not limited to cartons, shrinking
wrapping or other methods of enclosing the individually packaged ophthalmic
lenses in multiple containers. Examples of such secondary packaging are
8
CA 02477905 2004-08-31
WO 03/074093 PCT/US03/05946
disclosed in U.S. Pat. No. 5,577,367 which is hereby incorporated by
reference in its entirety. An example of an embodiment of the invention is
illusfirated in greater detail in reference to the following figures.
Referring to Figure 1, there is shown a simplified diagrammatic top view
of a portion 10 of a contact lens production facility including a processing
station
designed to enable expedient and consolidated packaging of manufactured
ophthalmic lenses in an aqueous solution contained in an individual blister
package, and a processing station designed to enable their expedited
sterilization with increased throughput. Preferably, as known in the art, an
individual formed ophthalmic contact lens is placed into an individual plastic
"blister" package containing an aqueous solution. In the preferred embodiment,
each individual blister package is removably attachable to one or more other
packages to form a blister pack array comprising three blister packages, for
example (not shown). Thus, a formed ophthalmic lens is loaded in each blister
pack of the array and is sealed in accordance with techniques known in the
art.
Next, the formed and sealed blister pack arrays 22 are transferred to a tray
loading station 20, where they are loaded into a novel sterilizer tray (not
shown),
as will be described in greater detail herein. In the preferred embodiment,
two
formed and sealed blister pack arrays 22 are interleaved (nested) in a manner
prior to transference to a tray loading station 20 by rotating one array and
placing a second array on top of the other. The blister pack array has been
designed to provide the interleaving feature. Coordinated with the activity of
packaging and nesting the blister package array in an interleaved manner, a
novel sterilizing tray unit (not shown) designed to receive and support the
blister
packs for conveyance through the in-line sterilizer unit is indexed from an
empty
tray stack (not shown) proximate the tray loading station 20 to receive the
packages at the tray loading station 20. Preferably, at the tray loading
station
20, the novel in-line sterilizer tray is suitably indexed and oriented for
enabling a
gripper mechanism 28 to automatically pick and transfer each interleaved pair
of
blister arrays into a correspondingly indexed cavity formed in the tray.
Preferably, the tray is situated horizontally while the gripper mechanism
picks
five (5) pairs of blister pack arrays and places them vertically into one (1 )
row
9
CA 02477905 2004-08-31
WO 03/074093 PCT/US03/05946
comprising five tray cavities designed to receive the nested pair. Next, the
tray
filled with the plurality of interleaved pairs of blister arrays is
automatically
conveyed by a servo-controlled belt driven conveyor, for example, in the
direction labeled "A", into a tray stacking station 25 where a stack of trays
are
formed. In the preferred embodiment, nine trays, each filled with the
plurality of
interleaved pairs of blister package arrays, is stacked one on top of each
other
and automatically conveyed, in the direction labeled "B", to an immediately
adjacent in-line sterilizer 50 where they are subject to an efficient
steam/heat
sterilization process as will be explained in greater detail herein. The
design of
the in-line sterilization station 50 coupled with the novel sterilization tray
design
that permits stacking enables increased lens sterilization throughput levels
heretofore unachievable. Moreover, the novel in-line sterilizer design permits
implementation of new sterilization process parameters that ensures the
dryness of every blister pack array, which is essential for subsequent
automated
secondary packaging. Further, as shown in Figure 1, after the sterilization
cycle
is complete, the tray stack comprising the sterilized blister pack arrays is
automatically conveyed, in the direction labeled "C", to an immediately
adjacent
tray de-stacking station 60 where, in an essentially a reversed process, the
trays
are one-by-one destacked. The individual de-stacked tray is then automatically
conveyed, in the direction labeled "D", to an unload station 70 where the
blister
packs of each tray are picked-up for transference to a secondary packaging
station where they are suitably placed in cartons as part of a secondary
packaging procedure.
Figure 2 is a side elevation view of the in-line sterilizer station including
the tray stacking station 25, novel sterilizer machine, and tray de-stacking
stations. As shown in Figure 2, the tray stacking station 25 operates as
follows: a first sterilizer tray 100 filled with the plurality of interleaved
pairs of
blister package arrays is automatically conveyed from the tray loading station
on a tray carrier 160. This tray carrier serves to index the tray one (1 ) row
at
a time through the tray loading process and then indexes to the stacking
position. Under precise program control, the stacker cylinder 170 is actuated
to elevate the tray carrier carrying the tray 100, in the direction labeled
"G",
CA 02477905 2004-08-31
WO 03/074093 PCT/US03/05946
above the level of conveyor 180, e.g. a belt driven roller, or like conveyor
system, where the sterilizer tray is latched and held into place by a latch
mechanism 175. The latch is actuated by an air cylinder which opens with air
and closes with spring force. The spring force allows the trays to push past
the latch and closes on the tray to hold it in place. The cylinder is then
actuated to retract the tray carrier 160 to retrieve the next tray unit
including
the plurality of interleaved pairs of blister package arrays. In the second
iteration, the process repeats and the tray carrier is elevated for placement
underneath the tray stack in a stackable manner. The latch mechanism is
actuated to hold the second tray having the first tray in a stacked
configuration
thereon, as will described herein with respect to Figure 8. After a
predetermined
amount of iterations, e.g., nine (9) in the preferred embodiment, the latch
mechanism is holding a nested stack of nine sterilizer trays 200. The latch is
finally released and the stack 200 of sterilizer trays becomes supported by
conveyor 180 which is driven to convey the stack into the in-line sterilizer
machine 50 in the load direction as indicated in Figure 2. Preferably, the
sterilizer includes a motorized roller conveyor 195 which is synchronized with
the conveyor 180. This allows the stack of trays to load into the sterilizer.
After
sterilization, the stack unloads to the conveyor 181 at the de-stacker station
60
where the top tray 100 of the stack 200 is transferred to another tray carrier
161.
The interleaved arrays of blister packs are unloaded using a similar gripper
mechanism used to load the tray as described with respect to Figure 1.
Figure 8 is a side elevation view of the latch mechanism 175 that retains
the stack of trays at the tray stacking station 25 of Figure 2. As shown in
Figure
8, latching fingers 175a,b under spring control are actuated by the stacking
cylinder 170 when extended (Figure 2) to nest the next successive tray 101 at
the bottom of the tray stack. The elevation of the stack by the tray carrier
and
stacking cylinder causes the latch fingers to disengage from the first tray,
and
catch the next upward moving tray in the stack where the latch fingers again
engage the peripheral ridge portion 107 at each side of the tray 100. Although
not shown in Figure 8, while in upward movement, in the direction labeled "G",
the spring is actuated so that the latch fingers will eventually engage the
11
CA 02477905 2004-08-31
WO 03/074093 PCT/US03/05946
respective ridge portion 107 at each side of the bottommost tray 101 for
supporting the stack thereon.
Figure 6 illustrates the front elevation view of the in-line sterilizer
machine Figure 6 shown receiving the stack of trays 200 on a conveyor.
More particularly, as shown in Figures 5 and 6, a conveyor 195, such as a
roller type conveyor provided within the sterilizer, is communicatively
situated
at an equal height "h" in the sterilizer as the conveyor 180 of the tray
stacking
station so that the tray stack 200 may be directly transported to within the
confines of the sterilizer chamber 53. Prior to transference, under program
control, the doors of the sterilizer are opended, e.g., vertical doors may be
retracted. The stack in conveyed from conveyor 180 to 195 within the
sterilizer in synchrony. After the sterilizer is loaded, the doors are closed
and
the sterilization cycle begins.
As shown in Figures 6 and 7, in the preferred embodiment, the
sterilizer 50 is a jacketed pressure chamber 53 having two doors 56 with the
jacket 307 surrounding the chamber 53 so as to provide extra heat during the
sterilization cycle as shown in Figure 7. In an example embodiment, the
dimensions of the in-line sterilizer chamber is approximately 650 mm in
height, 450 mm wide and 650 mm deep, thus providing less than a cubic
meter (1 m3), which using the novel sterilization trays of the invention, is
sufficient to provide a capacity per unit of chamber volume of approximately
three (3) times greater than prior art versions. Preferably, the sterilizer
machine is a complete system with mechanical/electrical process
components, an internal sterilizer tray conveyor 195, and control system. The
sterilizer 50 is integrated into the manufacturing machine to provide much
higher throughput per unit volume. As will be explained in greater detail,
this
throughput/volume is achieved by implementing the novel sterilization trays
used. Meeting the requisite "dryness" criteria is achieved by the design of
the
sterilizer, use of the novel trays, and the sterilization process parameters
implemented during the sterilization cycle, as will be explained.
Further the invention includes a method of sterilizing ophthalmic lens
packages comprising
12
CA 02477905 2004-08-31
WO 03/074093 PCT/US03/05946
transporting a plurality of said ophthalmic lens packages to a sterilizing
chamber;
subjecting said plurality of ophthalmic lens packages at least one
sterilization cycle within said sterilizing chamber;
wherein, said sterilizing chamber is disposed contiguously with prior
processing
stations and secondary packaging stations of a ophthalmic lens manufacturing
line.
The terms ophthalmic lens, packages, sterilizing chamber, prior processing
station, sterilization cycle, and secondary packaging stations all have their
aforementioned meanings and preferred ranges. An embodiment, that
illustrates, but does not limit, this invention is disclosed below.
Figure 7 is a piping and instrumentation diagram illustrating the major
devices and controls utilized for performing steam sterilization according to
the
invention. As shown in Figure 7, and explained in greater detail herein, the
expedited and efficient lens package sterilization process is enabled under
the
programmed control and supervision of an enhanced programmable logic
control system (PLC) or like equivalent control device 99. In a first
sterilization
cycle referred to as the PREHEAT Phase, the function is to pre-heat product
and internal chamber with dry heat only, to prevent steam condensation on
product. The steps involved with this process includes: starting the fan motor
which is indicated at 302 in Figure 7; initiating the start of the Preheat
Sequence Timer with a pre-set value of 2.0 minutes in an example
embodiment described. In a next step, a jacket steam valve that is indicated
at 304 in Figure 7 is opened and a Jacket Pressure Switch 308 setpoint is
controlled, which, in the example embodiment described, is at a switch
setpoint value of about 2.0 bar, for example. Air valves 310 and drain valves
320 are then actuated to control the air pressure of the chamber at air over-
pressure setpoint with a small percentage of the alarm limits. In an example
embodiment, the air overpressure setpoint value is a saturated steam
pressure at + 1.05 bar and is set within the overpressure alarm limits of +/-
0.2
bar, for example. When the Preheat Sequence Timer reaches the pre-set
value, the process advances to the next phase.
13
CA 02477905 2004-08-31
WO 03/074093 PCT/US03/05946
In the second sterilization cycle referred to as the Sterilization Cycle
HEAT LOAD Phase, the function is to heat the product with steam and dry
heat to a sterilization temperature. The steps involve opening a jacket steam
valve 304 and setting the jacket pressure switch to a setpoint value of
approximately 2.0 bar. Then, one or more air valves and drain valves 310,
320 are actuated in order to control the chamber pressure at air over-pressure
setpoint within a +/- percentage of the alarm limits. In an example
embodiment, the air overpressure setpoint value is a saturated steam
pressure at + 1.05 bar and is set within the overpressure alarm limits of +/-
0.2
bar, for example. The Steam Control Valve that is indicated at 305 in Figure 7
is opened to achieve a temperature setpoint value plus overshoot. In an
example embodiment described, the temperature setpoint value is 124.0°
C
with an overshoot value of about 0.6° C. When the minimum chamber
temperature reaches the temperature setpoint value minus the overshoot
value (123.4° C), an Exposure Start delay timer, preset to a value of
1.0
minute begins counting and when the Exposure Start timer reaches 1.0
minute, the process advances to the next phase.
In a third sterilization cycle referred to as the Sterilization Cycle
EXPOSURE Phase, the function is to hold the product at sterilization
temperature for minimum sterilization time. After the 1.0 minute time period
of
the preceding paragraph is reached, an Exposure Sequence Timer
commences which is pre-set to an Exposure Timer Value of 18.0 minutes.
Then, one or more air valves 310 are actuated in order to control the chamber
pressure at air over-pressure setpoint within a +/- percentage of the alarm
limits. In an example embodiment, the air overpressure setpoint value is a
saturated steam pressure at + 1.05 bar and is set within the overpressure
alarm limits of +/- 0.2 bar, for example. Then, the Steam Control Valve 305
opens to the setpoint value, which, in the example embodiment, is
124.0° C.
In the preferred embodiment, all temperatures are maintained within no more
than 1 ° C of the Exposure Temperature setpoint. Then, the jacket steam
valve 304 is then closed. When the Exposure Timer reaches a pre-set value,
the cycle advances to the next phase.
14
CA 02477905 2004-08-31
WO 03/074093 PCT/US03/05946
In a fourth sterilization cycle referred to as the Sterilization Cycle AIR
COOL Phase, the function is to cool the package product with air flowing into
the chamber to remove steam vapor. In this phase, an Air Cool Phase
Sequence Timer is commenced which is at a pre-set value of about 4.0
minutes. Then, one or more air valves and drain valves 310, 320 are
actuated in order to control the chamber pressure at air over-pressure
setpoint within a +/- percentage of the alarm limits. In an example
embodiment, the air overpressure type is a ramp control; ramp setpoint value
is set to -0.04 bar/minute; and, an overpressure alarm limit values: +/- 0.2
bar.
Then, the steam valve 305 is closed; and, when Air Cool Timer reaches a pre-
set value, the cycle advances to the next phase.
In a fifth sterilization cycle referred to as the Sterilization Cycle WATER
COOL LOAD Phase, the function is to cool the product with water flowing into
the internal heat exchanger chamber indicated at 315 in Figure 7, in order to
condense remaining steam vapor and cool product to final temperature for
unloading to secondary packaging area. First, one or more air valves and
drain valves 310, 320 are actuated in order to control the chamber pressure at
air over-pressure setpoint within a +/- percentage of the alarm limits. In an
example embodiment, the air overpressure type is a ramp control; a ramp
setpoint value being set to -0.04 bar/minute; and, an overpressure alarm limit
values of +/- 0.2 bar. Then, a Water Valve 325 opens to fill the internal heat
exchanger 315. When maximum chamber temperature reaches a second
cool phase setpoint, the cycle advances to an Exhaust phase. In an example
embodiment, the water cool load phase setpoint value is equal to 65.0°
C.
In a sixth sterilization cycle referred to as the Sterilization Cycle
EXHAUST Phase, the Chamber drain valves 320 are opened and the Air
valves 310 are closed. The Water valve 325 is opened. When the chamber
pressure reaches a setpoint value, the cycle advances to an End of Cycle
phase. In an example embodiment, the Exhaust phase setpoint value is 0.1
bar. The sterilization cycle is now complete.
In the preferred embodiment, given the design of the sterilizer chamber
and the process parameters utilized, the total sterilization cycle time is
CA 02477905 2004-08-31
WO 03/074093 PCT/US03/05946
approximately forty (40) minutes which is a fairly dramatic improvement over
the sterilization cycle times in prior art sterilizer equipment.
Referring now to Figure 3(a), there is illustrated the novel plastic tray
unit 100 for carrying nested stacks of blister pack arrays carrying ophthalmic
lenses for input to the sterilizer machine. A detailed close-up view of a
portion
100a of the tray 100 is illustrated in Figure 3(b). In the top view of the
plastic
tray 100 shown in Figure 3(a), the tray includes an external wall 102 bounding
the perimeter of the tray, and four internal divider walls 104a - 104d, which
form five tray columns 110a-110e. As shown in Figure 4(a) which illustrates a
side cross-sectional view of the novel tray 100 taken along line "E-E"
illustrated in Figure 3(a), the cavities 120a, 120b,...,120v are shown
separated by the divider walls 115 formed there between. When the tray 100
is indexed for loading at the blister pack loading station 20 (Figure 1 ), the
tray
is oriented horizontally with the divider walls 115 extending upward. Thus, as
. shown in Figure 5, the interleaved pairs of blister arrays 150 may easily be
deposited in a respective cavity 120 separated from each other by the divider
walls 115 in the manner illustrated, and supported by the plastic tray bottom
101. It should be understood that the configured interleaving of a nested
array of packages for sterilization according to the invention, is
substantially
similar to the configuration of the interleaved nested array of packages that
will be ultimately packaged in a carton at the subsequent secondary
packaging station (not shown).
Referring back to Figure 4(a), along the perimeter of each plastic tray
100, is the external wall 102 which is shown as extending above height of the
divider walls 115. As shown in Figure 4(b) which depicts a front cross-
sectional view of a portion of the novel tray 100 taken along line "F-F" of
the
tray 100 illustrated in Figure 3(a), a top portion 103 of the external wall
102
includes a recessed or "lip" portion 106 that preferably forms an internal
shelf
about the substantially the whole periphery of the tray and that is designed
to
stackably engage a bottom portion of the next tray that is to be stacked
thereon. Thus, the bottom portion 101 of the tray is designed for easy
stacking placement within the peripheral recess portion 106 of each tray for
16
CA 02477905 2004-08-31
WO 03/074093 PCT/US03/05946
enabling nested stacking of the trays at the tray stacking station. Figure
3(b)
further shows openings 109 formed in the bottom of the tray 100 to provide
circulation of steam and air to the sealed blister pack arrays. Furthermore,
in
view of Figure 4(b), the external wall is provided with a peripheral ridge or
flanged portion 107 that accommodates the placement of the latch fingers for
supporting the trays during at the tray stacking station, as described with
respect to Figure 8. In the preferred embodiment, a single plastic tray 100 is
designed to support the weight of at least a stack of nine trays for input to
the
sterilizer machine. Moreover, the material of the plastic tray is not only
designed for strength to support the weight of the trays but to additionally
endure the temperature and pressure of the steam sterilization cycle. In the
preferred embodiment, the tray is injection molded plastic such as Polyether
Imide Ultem~ brand manufacture by General Electric.
Referring back to Figures 3(a), 3(b), and 5, as mentioned, each column
110a-110e of the tray 100 formed between internal divider walls 104a - 104d
includes a plurality of internal divider walls 115, which in the example
embodiment shown in Figures 5, 3(a), and 3(b), amounts to twenty-one (21 )
dividers. Between each pair of dividers 115a,b are formed the cavity 120 for
retaining an individual array of blister packs (not shown). In this
embodiment,
the twenty internal dividers form twenty-two (22) cavities, each cavity 120
for
carrying a nested pair of interleaved packages carrying 3-pack arrays in a
proper orientation for sterilization. Thus, in a preferred embodiment, there
are
110 cavities formed in the tray, with each cavity accommodating a total of six
lens packages (interleaved pair of three blister packs each). Thus, the
amount of packages carried per tray amounts to 660. With the single tray
capable of supporting nine tray stacks in the preferred embodiment, the total
number of blister packages that may be conveyed for simultaneous
sterilization in the novel sterilizing chamber, amounts to about 5,940. A
further advantage is that the molded plastic tray provides the additional
benefit of being lightweight relative to a metal tray (for example), which
makes
it easier to convey in and out of the sterilizer. A stack of metal trays weigh
17
CA 02477905 2004-08-31
WO 03/074093 PCT/US03/05946
approximately two (2) times the weight, which would require a larger motor,
rollers and more durable system for conveying the stack.
The integration of the sterilizer in-line with the rest of the ophthalmic
lens manufacturing facility that enables sterilization and packaging without
disrupting the product flow, inherently provides a physical barrier between
sterile and non-sterile packaging areas. That is, the product cannot move the
sterile packaging area without going through the sterilizer. Further, the
design
enables continuous uninterrupted processing whereby as a new stack of trays
containing ophthalmic lenses are input from the stacking station to the
sterilizing chamber, the previous tray stack that had just been sterilized are
output from the chamber. Further, the integration also allows complete
tracking of the product through the lens machine by the machine controller,
insuring.the product must go through a complete sterilization cycle before
unloading to the secondary packaging area. Integration of the sterilizer
control system 99 (Figure 7) with the lens machine control system further
provides complete tracking of the product through the packaging and
sterilization process.
Another advantage of the in-line sterilizer design is a higher output per
unit volume of sterilizer chamber. This capacity reduces floor space required
for sterilization and in-process product inventory. The sterilization process
time and use of the stacking sterilization trays provide this capacity. The
process also provides a dry package following sterilization, required by
immediate packing of the product following sterilization.
Still further the invention includes a sterilizing tray for conveying arrays
of ophthalmic lens packages, each of which includes at least one ophthalmic
lens immersed in a sterile aqueous solution, through a sterilizing chamber in
a
manufacturing line for sterilizing said packages prior to packaging in
secondary packaging, said sterilizing tray comprises an external wall
surrounding a perimeter of said tray and having and a number of internal
divider walls forming columns therebetween, each divider wall comprising a
plurality of cavities for holding a plurality of packages in a nested
configuration, said cavities for holding the plurality of packages, in an
18
CA 02477905 2004-08-31
WO 03/074093 PCT/US03/05946
orientation optimized for sterilization in a sterilizing chamber, wherein said
tray
is designed for strength to support the weight of a plurality of trays and
endure
the temperature, and pressure of the steam sterilization. In the preferred
embodiment of this invention, said sterilizing tray is made of a lightweight
plastic material. In another embodiment of this invention said sterilizing
tray
comprises openings either or both, above and below said the perimeter wall
said tray to provide optimum heating or cooling, of said packages contained
therein. Yet still further the invention includes An ophthalmic lens
manufacturing line comprising:
a first station for successively loading arrays of packaged ophthalmic
lenses into respective cavities formed in a plastic tray receptacle;
a second station for successively stacking a plurality of plastic tray
receptacles each loaded with an array of packaged ophthalmic lenses;
a third station comprising a sterilizing chamber designed to
accommodate simultaneous sterilization of a plurality of ophthalmic
lens packages;
a conveyor means for synchronously conveying said stack of loaded
plastic tray receptacles from said second station to said third station for
sterilizing said ophthalmic lens included in said plastic tray;
wherein, said sterilizing chamber of said third station is disposed
contiguously
with said second station to enable continuous sterilization of said ophthalmic
lens packages without disrupting said manufacturing line.
While the invention has been described in connection with a preferred
embodiment, it is not intended to limit the scope of the invention to the
particular form set forth, but on the contrary, it is intended to cover such
alternatives, modifications, and equivalents as may be included within the
spirit and scope of the invention as defined by the appended claims
19