Note: Descriptions are shown in the official language in which they were submitted.
CA 02479048 2004-09-13
WO 03/076690 PCT/AU03/00306
- 1 -
REDUCTION OF METAL OXIDES IN AN ELECTROLYTIC CELL
The present invention relates to reduction of
metal oxides in a solid state in an electrolytic cell.
The present invention was made during the course
of an on-going research project on solid state reduction
of titania (Ti02) carried out by the applicant.
During the course of the research project the
applicant carried out experimental work on the reduction
of titania using an electrolytic cell that included a
graphite crucible that formed an anode of the cell, a pool
of molten CaC12-based electrolyte in the crucible, and a
range of cathodes that included solid titania.
One objective of the experimental work was to
reproduce the results reported in International
application PCT/GB99/01781 (publication no. W099/64638) in
the name of Cambridge University Technical Services
Limited and in technical papers published by the inventors
of that International application.
The Cambridge International application discloses
two potential applications of a "discovery" in the field
of metallurgical electrochemistry.
One application is the direct production of a
metal from a metal oxide.
In the context of this application, the
"discovery" is the realisation that an electrolytic cell
can be used to ionise oxygen contained in a metal oxide so
that the oxygen dissolves in an electrolyte. The
Cambridge International application discloses that when a
suitable potential is applied to an electrolytic cell with
a metal oxide as a cathode, a reaction occurs whereby
CA 02479048 2004-09-13
WO 03/076690 PCT/AU03/00306
2 -
oxygen is ionised and is subsequently able to dissolve in
the electrolyte of the cell.
European patent application 9995507.1 derived
from the Cambridge International application has been
allowed by the European Patent Office.
The allowed claims of the European patent
application inter alia define a method of electrolytically
reducing a metal oxide (such as titania) that includes
operating an electrolytic cell at a potential at an
electrode formed from the metal oxide that is lower than
the deposition potential of cations in the electrolyte at
a surface of the electrode.
The Cambridge European patent application does
not define what is meant by deposition potential and does
not include any specific examples that provide values of
the deposition potential for particular cations.
However, submissions dated 2 October 2001 to the
European Patent Office by the Cambridge patent attorneys,
which pre-dated'the lodgement of the claims that were
ultimately allowed, indicate that they believe that the
decomposition potential of an electrolyte is the
deposition potential of a cation in the electrolyte.
Specifically, page 5 of the submissions state
that:
"The second advantage described above is achieved in part
through carrying out the claimed invention below the
decomposition potential of the electrolyte. If higher
potentials are used then, as noted in DI and D2, the
cation in the electrolyte deposits on the metal or semi-
metal compound. In the example of DI, this leads to
calcium deposition and therefore consumption of this
CA 02479048 2011-07-28
3 -
reactive metal............During operation of the method, the
electrolytic cation is not deposited on the cathode".
Contrary to the findings of Cambridge, the
experimental work carried out by the applicant has
established that it is essential that the electrolytic
cell be operated at a potential that is above the
potential at which Ca" cations in the electrolyte can
deposit as Ca metal on the cathode.
Accordingly, the present invention provides a
method of reducing a metal oxide in a solid state in an
electrolytic cell, which electrolytic cell includes an
anode, a cathode, a molten electrolyte, the electrolyte
includes cations of a metal that is capable of chemically
reducing the metal oxide, and the metal oxide in a solid
state immersed in the electrolyte, and which method
includes a step of operating the cell at a potential that
is above a potential at which cations of the metal that is
capable of chemically reducing the metal oxide can deposit
as the metal on the cathode, whereby the metal chemically
reduces the metal oxide.
More particularly, the present invention provides
a method of reducing a metal oxide in a solid state in
an electrolytic cell, which electrolytic cell includes an anode,
a cathode formed at least in part from the metal oxide, a molten
electrolyte, the electrolyte includes cations of a metal that is
capable of chemically reducing the metal oxide, and the metal
oxide in a solid state is immersed in the electrolyte, and which
method includes a step of operating the cell at a potential that
is above a potential at which cations of the metal that is
capable of chemically reducing the metal oxide can deposit as
the metal on the cathode, whereby the metal chemically reduces
the metal oxide.
CA 02479048 2011-07-28
- 3a -
In another aspect, the invention provides an
electrolytic cell reducing a metal oxide in a solid state,
which electrolytic cell includes an anode, a cathode formed
at least in part from the metal oxide, a molten electrolyte,
which electrolyte includes cations of a metal that is capable
of chemically reducing the metal oxide, and a metal oxide in
a solid state immersed in the electrolyte, and which
electrolytic cell operates at a potential that is above a
potential at which cations of the metal that is capable of
chemically reducing the metal oxide deposit as the metal on
the cathode, whereby the metal chemically reduces the metal
oxide.
The applicant does not have a clear understanding
of the electrolytic cell mechanism at this stage.
Nevertheless, whilst not wishing to be bound by
the comments in this and the following paragraphs, the
applicant offers the following comments by way of an
outline of a possible cell mechanism.
The experimental work carried out by the
applicant produced evidence of Ca metal dissolved in the
electrolyte. The applicant believes that, at least during
the early stages of operation of the cell, the Ca metal
was the result of electrodeposition of Ca" cations as Ca
metal on electrically conductive sections of the cathode.
CA 02479048 2004-09-13
WO 03/076690 PCT/AU03/00306
4 -
The experimental work was carried out using a
CaCl2-based electrolyte at a cell potential below the
decomposition potential of CaCl2. The applicant believes
that the initial deposition of Ca metal on the cathode was
due to the presence of Ca++ cations and O-- anions derived
from CaO in the electrolyte. The decomposition potential
of CaO is less than the decomposition potential of CaC12.
In this cell mechanism the cell operation is dependent, at
least during the early stages of cell operation, on
decomposition of CaO, with Ca++ cations migrating to the
cathode and depositing as Ca metal and O`- anions migrating
to the anode and forming CO and/or CO2 (in a situation in
which the anode is a graphite anode).
The applicant believes that the Ca metal that
deposited on electrically conductive sections of the
cathode was deposited predominantly as a separate phase in
the early stages of cell operation and thereafter
dissolved in the electrolyte and migrated to the vicinity
of the titania in the cathode and participated in chemical
reduction of titania.
The applicant also believes that at later stages
of the cell operation part of the Ca metal that deposited
on the cathode was deposited directly on partially
deoxidised titanium and thereafter participated in
chemical reduction of titanium.
The applicant also believes that the O--anions,
once extracted from the titania, migrated to the anode and
reacted with anode carbon and produced CO and/or CO2 (and
in some instances CaO) and released electrons that
facilitated electrolytic deposition of Ca metal on the
cathode.
Preferably the cathode is formed at least in part
CA 02479048 2004-09-13
WO 03/076690 PCT/AU03/00306
-
from the metal oxide.
Preferably the method includes operating the cell
at the potential that is above the potential at which
5 cations of the metal that is capable of chemically
reducing the metal oxide deposit as the metal on the
cathode so that the metal deposits on the cathode.
Preferably the metal deposited on the cathode is
soluble in the electrolyte and can dissolve in the
electrolyte and thereby migrate to the vicinity of the
metal oxide.
In a situation in which the metal oxide is a
titanium oxide, such as titania, it is preferred that the
electrolyte be a CaC12-based electrolyte that includes CaO
as one of the constituents of the electrolyte. In this
context, it is noted that the present invention does not
require the addition of substantial amounts of CaO to the
electrolyte.
In such a situation it is preferred that the cell
potential be above a potential at which Ca metal can
deposit on the cathode, i.e. at a potential that is above
the decomposition potential of CaO.
The decomposition potential of CaO can vary over
a considerable range depending on factors such as the
composition of the anode, the electrolyte temperature and
electrolyte composition.
in a cell containing CaO saturated CaC12 at 1373K
(1100 C) and a graphite anode this would require a minimum
cell potential of 1.34V.
It is also preferred that the cell potential be
below the potential at which Cl- anions can deposit on the
CA 02479048 2004-09-13
WO 03/076690 PCT/AU03/00306
6 -
anode and form chlorine gas, i.e. the decomposition
potential of CaC12.
In a cell containing CaO saturated CaC12 at 1373K
(1100 C) and a graphite anode this would require that the
cell potential be less than 3.5V.
The decomposition potential of CaC12 can vary
over a considerable range depending on factors such as the
composition of the anode, the electrolyte temperature and
electrolyte composition.
For example, a salt containing 80% CaC12 and 20%
KC1 at a temperature of 900K (657 C), decomposes to Ca
(metal) and C12 (gas) above 3.4V and a salt containing 100%
CaC12 at 1373K (1100 C) decomposes at 3.OV.
In general terms, in a cell containing CaO-CaC12
salt (not saturated) at a temperature in the range of 600-
1100 C and a graphite anode it is preferred that the cell
potential be between 1.3 and 3.5V.
The CaC12-based electrolyte may be a commercially
available source of CaC12, such as calcium chloride
dehydrate, that partially decomposes on heating and
produces CaO or otherwise includes CaO.
Alternatively, or in addition, the CaC12-based
electrolyte may includeCaC12 and CaO that are added
separately or pre-mixed to form the electrolyte.
it is preferred that the anode be graphite or an
inert anode.
The applicant found in the experimental work that
there were relatively significant amounts of carbon
transferred from the graphite anode to the electrolyte and
CA 02479048 2004-09-13
WO 03/076690 PCT/AU03/00306
7 -
to a lesser extent, to the titanium produced at the
cathode under a wide range of cell operating conditions.
Carbon in the titanium is an undesirable
contaminant. In addition, carbon transfer was partially
responsible for low energy efficiency of the cell. Both
problems could present significant barriers to
commercialisation of electrolytic reduction technology.
The applicant also found that the dominant
mechanism of carbon transfer is electrochemical rather
than erosion and that one way of minimising carbon
transfer and therefore contamination of titanium produced
at the cathode by electrochemical reduction of titania is
to position a membrane that is permeable to oxygen anions
and is impermeable to carbon in ionic and non-ionic forms
between the cathode and the anode and thereby prevent
migration of carbon to the cathode.
Accordingly, in order to minimise contamination
of titanium produced at the cathode resulting from carbon
transfer, it is preferred that the electrolytic cell
includes a membrane that is permeable to oxygen anions and
is impermeable to carbon in ionic and non-ionic forms
positioned between the cathode and the anode to thereby
prevent migration of carbon to the cathode.
The membrane may be formed from any suitable
material.
Preferably the membrane is formed from a solid
electrolyte.
One solid electrolyte tested by the applicant is
yttria stabilised zirconia.
According to the present invention there is also
CA 02479048 2004-09-13
WO 03/076690 PCT/AU03/00306
8 -
provided an electrolytic cell as described above and
operating in accordance with the above described method.
The present invention is described further with
reference to the following example.
1. Experimental Method and Electrolytic Cell
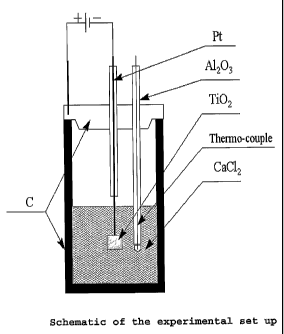
The electrolytic cell is shown in Figure 1.
With reference to Figure 1, the electrochemical
cell included a graphite crucible equipped with a graphite
lid. The crucible was used as the cell anode. A stainless
steel rod was used to secure electrical contact between a
d/c power supply and the crucible. The cell cathode
consisted of Kanthal or platinum wire connected at one end
to the power supply and Ti02 pellets suspended from the
other end of the wire. An alumina tube was used as an
insulator around the cathode. The cell electrolyte was a
commercially available source of CaC12, namely calcium
chloride dihydrate, that partially decomposed on heating
at the operating temperature of the cell and produced CaO.
A thermocouple was immersed in the electrolyte in close
proximity to the pellets.
Two types of pellets were used. One type was
slip-cast and the other type was pressed. Both types of
pellets were made from analytical grade Ti02 powder. Both
types of pellets were sintered in air at 850 C. One
pressed and one slip-cast pellet were used in the
experiment.
The cell was positioned in a furnace and the
experiment was conducted at 950 C. Voltages up to 3V were
applied between the crucible wall and the Kanthal or
platinum wire. The voltage of 3V is below the potential
at which C1- anions can deposit on the anode at that
CA 02479048 2004-09-13
WO 03/076690 PCT/AU03/00306
- 9 -
temperature. In addition, the voltage of 3V is above the
decomposition potential of CaO and below the decomposition
potential of CaC12.
The power-supply maintained a constant voltage
throughout the experiment. The voltage and resulting cell
current were logged using LabVIEW (TM) data acquisition
software.
At the end of the experiment the cell was
removed from the furnace and quenched in water. The solid
CaC12 was dissolved by water and the two pellets were
recovered.
II. Experimental Results
With reference to Figures 2 and 3, the constant
voltage (3V) used in the experiment produced an initial
current of approximately 1.2A. A continuous drop in the
current was observed during the initial 2 hours. After
that a gradual increase in the current up to 1A was
observed.
SEM images of the cross-sections of the two
recovered pellets are shown in Figures 4 and 5. The SEM
images indicate the presence of metallic titanium in both
pellets, thereby establishing that the method successfully
electrochemically reduced titania.
The presence of virtually pure metallic titanium
in both pellets was confirmed by EPMA analysis. The
analysis also showed areas of partially reduced titania.
The EPMA results are shown in Figures 6 and 7.
Carbon was detected at various locations within
the pellets and its content varied up to 18wt%.
CA 02479048 2004-09-13
WO 03/076690 PCT/AU03/00306
- 10 -
Many modifications may be made to the present
invention as described above without departing from the
the spirit and scope of the invention.
By way of example, whilst the above description
of the invention focuses on reduction of titania, the
invention is not so limited and extends to reduction of
other titanium oxides and to oxides of other metals and
alloys. Examples of other potentially important metals
are aluminium, silicon, germanium, zirconium, hafnium,
magnesium and molybdenum.
Furthermore, whilst the above description focuses
on CaC12-based electrolyte, the invention is not so limited
and extends to any other suitable electrolytes (and
mixtures of electrolytes). Generally, suitable
electrolytes will be salts and oxides that are soluble in
salts. One example of a potentially suitable electrolyte
is BaC12.