Note: Descriptions are shown in the official language in which they were submitted.
CA 02479163 2004-08-25
NOVEL LEPTIN ANTAGONIST
The present invention relates to an antagonist or inhibitor of leptin
signalling via the leptin
receptor. The leptin antagonist binds to the leptin receptor, but is unable to
induce JAK-STAT
signal transduction via the leptin receptor. By binding to the leptin
receptor, the leptin
antagonist impairs binding of leptin to the leptin receptor and blocks leptin
signalling.
After cleavage of its 21 amino acids signal peptide (Cohen et al., 1996),
mature human leptin
is secreted as a 146 amino acid protein; with a typical type II interleukin
structure, consisting of
a bundle of 4 helices (helix 1-4), with an up-up-down-down topology (Zhang et
al., 1997).
Leptin is secreted into the bloodstream primarily by adipocytes, and blood
concentrations of
leptin correlate with white adipose tissue mass. Leptin acts as an energy
homeostasis
hormone, regulating energy expenditure and food intake.
Leptin does so by binding to the leptin receptor in certain areas in the
hypothalamus, which
leads to phosphorylation of STAT molecules that subsequently migrate to the
cell nucleus and
induce transcription of different genes.
In addition to its adipostatic function, leptin has many other functions: it
can induce
proliferation, differentiation and functional activation of haemopoietic cells
(Gainsford et al.,
1996), and induces angiogenesis (Sierra-Honigmann et al., 1998).
Leptin also interacts with the immune and inflammatory responses (Loffreda et
al., 1998).
Leptin levels are acutely increased by inflammatory stimuli and by pro-
inflammatory cytokines
TNF-a, and IL-1 (Grunfeld et al., 1996). Leptin itself regulates the
production of several
cytokines in vitro, regulates the T helper (Th1ITh2) balance, and can up-
regulate inflammatory
responses (Loffreda et al., 1998; Faggioni et al., 1998; Lord et al., 1998).
The human leptin receptor is expressed at the cell surface of many different
tissues. At least
six different splice variants of the human leptin receptor were found at
present. The longest
isoform of the human leptin receptor consists of 1162 amino acids, with an
extracellular region
between residues 1 and 840, a transmembrane region between residues 841 and
863 and an
intracellular region between residues 864 and 1162.
The extracellular part of the human leptin receptor contains at least 7
structural domains (Fong
et al., 1998).
Domain 1 (residue 62-178) and 2 (residue 235-328) have a fibronectin type III
fold and
together form a cytokine receptor module (CRM), named CRM1.
Domain 3 (residue 329 - 427) has a Immunoglobulin type fold.
Domain 4 (residue 428 - 535) and 5 (residue 536 - 635) also have a
fibronectine type III fold
and together form a second cytokine receptor module (CRM), named CRM2.
1
CA 02479163 2004-08-25
Domain 6 and 7 have a fibronectin type III domain structure.
Like all members of the class I cytokine receptor family, the leptin receptor
has no intrinsic
kinase activity, and uses a cytoplasmic associated Janus kinase (JAK2 in case
of the leptin
receptor) for intracellular signalling (Ghilardi et al., 1997). In a generally
accepted model, leptin
binding leads to formation of a receptor complex, allowing activation of JAK2
by cross-
phosphorylation. Activated JAK2 then rapidly phosphorylates several tyrosine
residues in the
cytosolic domain of the leptin receptor. These phosphorylated tyrosine
residues provide
docking sites for SH2 containing signalling molecules. In the mouse leptin
receptor, tyrosine
1138 serves as a binding site for signal transducer and activator of
transcription 3
(STAT3)(Baumann et al., 1996). STAT3 itself is a substrate for JAK2 and
dimerizes upon
phosphorylation, translocates to the nucleus and modulates transcription of
target genes.
The leptin receptor shows the highest sequence similarity with the cytokine
receptors of the IL-
6 family and with the Granulocyte Colony-Stimulating Factor (G-CSF) Receptor.
FSSP (Holm
and Sander, 1997) structural similarity searches reveal that leptin shows the
highest structural
similarity with the cytokines of the IL-6 family and G-CSF, and to a lesser
extent with other long
chain cytokines, such as the growth hormone and placental lactogen. The
crystal structure of
the Kaposi's sarcoma-associated herpesvirus IL-6 (vIL6, viral tL-6) in a 2:2
complex with the
three N-terminal extracellular domains of human gp130 reveals two binding
sites, binding site
II and binding site III, for interaction between vIL6 and gp130 (Chow et al,
2001). Binding site
II, consisting of residues in helices 1 and 3 of vIL6, interacts with the
cytokine receptor module
(CRM) of gp130. Binding site III in vIL6 consists of residues in the N-
terminus of helix 4, in the
loop connecting helix 3 and 4 and in the loop connecting helix 1 and 2, and
interacts with the
Immunoglobulin-like domain of gp130. Corresponding site II and III residues
were identified in
other members of the IL-6 family of cytokines by site directed mutagenesis:
human IL-6,
human IL-11, Leukemia inhibitory factor (LIF), oncostatin M (OSM) and Ciliary
neurotrophic
factor (CNTF) (Kalai et al., 1997; Savino et al., 1993; DiMarco et al., 1996;
Hudson et al.,
1996; Inoue et al., 1995; Barton et al., 1999; Bravo and Heath, 2000).
IL-6 contains a third binding site, binding site 1, for interaction with the
IL-8 a receptor. Human
IL-6 forms a hexameric 2:2:2 complex with its gp130 and IL-6 a receptor
chains: each IL-6
molecule binds two gp130 molecules by its site II and III binding sites, and
one IL-6 receptor a
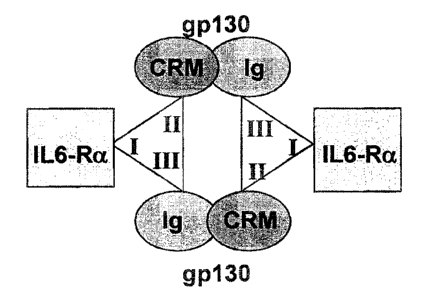
subunit (IL-6Ra) by its binding site I (figure 1). (Boulanger et al., 2003).
Activation of the leptin receptor by binding of leptin plays a role in several
physiological
processes. Several variant and mutant forms of leptin have been described,
that can be used
in different applications. W002082833 describes modified leptin polypeptides
that are
substantially non-immunogenic or less immunogenic than any non-modified
counterpart when
2
CA 02479163 2004-08-25
used in vivo. These polypeptides can be administered to humans of therapeutic
use.
W09700319 discloses chimeric leptin polypeptides comprising leptin or a mutant
or variant
thereof fused to a human immunoglobulin domain. These chimeric derivatives
have prolonged
clearing rates and may be useful in the treatment or prophylaxis of obesity,
or diseases and
conditions associated with obesity such as atherosclerosis, hypertension and
type II diabetes.
W09720933 discloses mutational variants of the mammalian leptin. These
molecules can
serve as agonist or antagonist of the wild type leptin; their capacity to
induce the signaling
pathway upon binding of the receptor varies for the different muteins.
W09812224 describe
the use of fragments, derived from leptin, as leptin antagonist, especially
for treating type II
diabetes.
There is, however, still need for a leptin mutant that is able to bind to the
receptor, with a
similar or higher affinity as the wild type leptin, but without remaining
signaling activity. Such
leptin mutant would be a powerful antagonist and can be used to treat leptin-
mediated
diseases.
Surprisingly, we found that leptin, similar to IL-6 and G-CSF, has a binding
site II and III, which
are both involved in binding to the leptin receptor. As is the case for G-CSF
and for members
of the IL-6 family, binding site II consists of residues in helix 1 (residues
4 - 26) and 3 (residues
71 - 93). These residues are involved in high affinity binding to the CRM2
module of the leptin
receptor, and mutations in binding site II of mouse leptin decrease or even
abolish the affinity
of the mutant leptin to the mouse leptin receptor. Due to the decreased
binding, these mutants
show a decreased induction of JAK~STAT signalling via the leptin receptor.
Binding site III consists of residues in the loop connecting helix 1 and 2
(residues 27 - 50) and
of residues at the N-terminus of helix 4 (residues 105 - 122). Mutations in
this binding site do
not affect the high affinity binding to the leptin receptor or to the CRM2
module, but still show a
decreased induction of JAK-STAT signalling via the leptin receptor. The
S120AT121A
mutation in mouse leptin binding site III is unable to induce any JAK-STAT
reporter activity via
the mouse leptin receptor, although the binding affinity of this mutant for
the leptin receptor or
for CRM2 of the mouse leptin receptor is similar to that of wild type leptin.
Even more surprisingly, we found that the S120AT121A mouse leptin mutant can
inhibit the
binding of wild type leptin to its receptor, and can inhibit the JAK-STAT
signal induced by wild
type leptin. This is because the S120AT121A leptin mutant shows an intact
binding site II, and
avidly binds to CRM2 of the leptin receptor, thus competing for binding of
wild type leptin.
Unlike wild type leptin, however, binding of the S120AT121A mutant does not
induce JAK
STAT signalling.
3
CA 02479163 2004-08-25
A first aspect of the invention is a leptin antagonist of polypeptidic nature,
comprising SEQ ID
N°1. Preferably, said leptin antagonist is comprising SEQ ID N°2
or 3, even more preferably
said leptin antagonist is comprising SEQ ID N° 4. Preferably, said
leptin antagonist is binding
to the leptin receptor, without inducing the signaling pathway. A preferred
embodiment is a
leptin antagonist according to the invention, capable of binding the leptin
receptor without
inducing the signaling pathway, whereby said antagonist is mutated in amino
acid 120 andlor
121 of SEQ ID N°5. Preferably, amino acid 120 or amino acid 121 are
mutated into an alanine.
Even more preferably, both amino acids are changed into alanine.
Another preferred embodiment is a leptin antagonist of polypeptidic nature,
capable of binding
the leptin receptor without inducing the signaling pathway, comprising SEQ ID
N°5 or a
functional fragment or variant thereof. A functional fragment as used here is
a fragment that
still can bind to the leptin receptor. ~4 variant as used here implies that
besides the amino acids
120 and 121, other amino acids may differ from the wild type human leptin
sequence.
Chemical modifications of the antagonist are also considered as variants.
Chemical
modifications of polypeptides are known to the person skilled in the art, and
include but are not
limited to natural occurring modifications such as glycosylation,
phosphorylation,
ubiquitinilation and artificial modifications such as PEGylation. Preferably,
said chemical
modification is increasing the half-life time of the polypeptide. Even more
preferably, said
chemical modification is PEGylation. Preferably said variant is at least 70%
identical to said
wild type sequence, more preferably it is at least 80% identical to said wild
type sequence,
even more preferably it is at least 90% identical to said wild type sequence,
most preferable it
is 95% identical to said wild type sequence. Said functional fragment can be
used as such, or it
may be fused to another polypeptide. In the fatter case, the fusion
polypeptide has preferably
an increased antagonistic capacity. Possible fusion partners are, as a non-
limiting example,
polypeptides that increase the half-life time of the polypeptide in vivo. Such
polypeptides are
disclosed, amongst others, in W09700319. Alternatively, the fusion partner may
capture leptin
itself, thereby increasing the antagonistic activity. Polypeptides that
capture leptin are, as a
non-limiting example, leptin antibodies preferably single chain antibodies, or
a domain of the
leptin receptor that is binding leptin.
A preferred fusion partner for the antagonist it the immunoglobulin type fold
domain of the
leptin receptor. This domain is involved in leptin dependent oligomerisation
of the leptin
receptor, and subsequent signaling: Fusion of this domain to the antagonist
will therefore give
an increased antagonistic affect, by disturbing the leptin induced
oligomerization of the
receptor.
Another aspect of the invention is the use of a leptin antagonist according to
the invention to
treat T-cell mediated immune andlor autoimmune diseases. Indeed, leptin
enhances T cell
mediated immune responses, by signalling through the long form of the leptin
receptor on
4
CA 02479163 2004-08-25
CD4+ T lymphocytes (Lord et al., 1998). Leptin shifts the T-cell responses
towards a Th1 type,
with increased secretion of pro-inflammatory cytokines IL-2 and interferon-y,
and decreased IL-
4 production. A leptin antagonist can therefore be used for
modifyinglattenuating the T-cell
immune responses, with use as a drug for the treatment of T-cell mediated
(auto-) immune
diseases.
Still another aspect of the invention is the use of an antagonist according to
the invention to
treat intestinal inflammation diseases. Preferably, said intestinal
inflammation diseases are
selected from the group consisting of Crohn's disease, ulcerative colitis and
intestinal
infectious diseases. In experimental mouse model systems, where chronic and
acute colitis is
induced by dextran sulfate sodium or trinitrobenzene sulfonic acid, leptin
deficient mice (oblob
mice), show a 72% reduction of colitis severity and a similar decrease of pro-
inflammatory
cytokines in the intestine, compared to wild type mice (Siegmund et al.,
2002). Administration
of leptin in the leptin deficient mice abolishes the resistance against
experimentally induced
colitis (Siegmund et al., 2002). Administration of Clostridium difficile toxin
A induces severe
colitis in mice. Leptin deficient (oblob) mice, as well as leptin receptor
deficient (dbldb) mice
are partially protected against the toxin A- induced intestinal secretion and
inflammation
(Mykoniatis et al., 2003). In oblob; but not in dbldb mice, leptin
administration reverses the
protection against toxin A-induced intestinal secretion and inflammation
(Mykoniatis et al.,
2003). A leptin antagonist can therefore be used as a drug for treatment of
intestinal
inflammation diseases, such as Crohn's disease, ulcerative colitis and
intestinal infectious
diseases.
In case of intestinal inflammation diseases, a preferred delivery method for
the leptin
antagonist according to the invention is an in vivo delivery system, as
described in
W09714806. Therefore, another aspect of the invention is a lactic acid
bacterium, producing a
leptin antagonist according to the invention. Preferably, said lactic acid
bacterium is a
Lactobacillus, even more preferably said lactic acid bacterium is a
Lactococcus.
A further aspect of the invention is the use of a leptin antagonist according
to the invention to
treat rheumathoid arthritis. Administration of methylated BSA in the knees of
mice leads to the
development of Antigen-induced arthritis. As compared to wild type mice,
leptin deficient
(oblob) mice and leptin receptor deficient (db/db) mice develop less severe
arthritis, with
decreased IL-1a and TNF-a in the knee synovial fluid, decreased serum levels
of anti-
methylated BSA antibodies and a decreased antigen-specific T cell
proliferative response
(Busso et al., 2002). A leptin antagonist can therefore be used as a drug for
treatment of
rheumatoid arthritis.
Still another aspect of the invention is the use of a leptin antagonist
according to the invention
to treat multiple sclerosis. The clinical onset of experimental autoimmune
encephalomyelitis
(EAE), a mouse model for multiple sclerosis, in disease-susceptible C57BLI6J(H-
2b) and
5
CA 02479163 2004-08-25
SJLIJ(H-2s) mice is preceded by an increase in serum leptin concentrations
(Sanna et al.,
2003). This increase is correlated with disease susceptibility. Acute
starvation, which reduces
serum leptin levels, delays disease onset and attenuates the EAE symptoms.
Leptin-deficient
C57BL/6J-oblob mice are resistant against EAE, while this resistance is
abolished by
administration of leptin (Matarese et al.; 2001 ). These data strongly
indicate that leptin is a
required factor for development of EAE, and thus, probably for multiple
sclerosis. A leptin
antagonist can therefore be used as a drug for treatment of multiple
sclerosis.
A further aspect of the invention is the use of a leptin antagonist according
to the invention to
treat Type 1 diabetes. Type 1 diabetes is an autoimmune disease, in which the
pancreatic (3-
cells are destroyed by inflammatory processes. In the non-obese diabetic (NOD)
mouse, an
animal model for type 1 diabetes, an increased serum level of leptin precedes
the diabetes in
susceptible females, while injection of leptin accelerates the autoimmune
destruction of the
pancreatic a-cells (Matarese et al., 2002). A leptin antagonist can be used as
a drug for
preventingltreating type 1 diabetes.
A further aspect of the invention is the use of a leptin antagonist according
to the invention to
prevent and/or treat diseases characterized by T-cell mediated hepatotoxicity.
Leptin deficient
ob/ob mice are protected from T cell-mediated hepatitis, experimentally
induced with ConA or
Pseudomonas aeruginosa exotoxin A (Faggioni et al., 2000). Injection of leptin
in the leptin
deficient mice restores the hepatotoxicity of these compounds (Faggioni et
al., 2000). A leptin
antagonist can therefore be used for treatment and/ or prevention of diseases
characterized by
T-cell mediated hepatotoxicity.
Still another aspect of the invention is a pharmaceutical composition,
comprising a leptin
antagonist according to the invention, optionally with a pharmaceutical
acceptable excipient.
Suitable excipients are known to the person skilled in the art, and are
inherently non-toxic and
nontherapeutic. Excipients may be, as a non limiting example, Ringer's
solution, dextrose
solution or Hank's solution. Non aqueous solutions such as fixed oils and
ethyl oleate may also
be used. A preferred excipient is 5% dextrose in saline. The excipient may
contain minor
amounts of additives such as substances that enhance isotonicity and chemical
stability,
including buffers and preservatives.
BRIEF DESCRIPTION OF THE FIGURES
Figure 1: composition of the 2 :2 :2 IL-6 : gp130 : IL-6Ra complex.
Figure 2: Activation of luciferase as reporter by WT leptin and the S120AT121A
mutant.
Figure 3: Competition of Leptin mutants for binding of SEAP-Leptin to LepR
CRM2. Inset:
binding of SEAP-Leptin to LepR CRM2 in the presence of undiluted medium
containing WT
leptin or the R20N or S120AT121A mutant leptin.
6
CA 02479163 2004-08-25
Figure 4: Incubation of the transfected HEK293T cells with the wt HA-mouse
leptin induces
luciferase activity. Co-incubation with the HA-tagged S120AT121A mouse leptin,
purified on
the anti-HA affinity column, leads to strong inhibition of the leptin-induced
luciferase activity,
and the luciferase activity drops to the level of non-stimulated cells. 50 pl
of the HEK293T
cells were incubated with WT leptin and 50 NI of different dilutions of
fractions 1 (O),2 (~), and
3(D) eluted from the anti HA column with 1 mglml HA peptide in equilibration
buffer. As a
control (+), 50 NI of the HEK293T cells were incubated with WT leptin and 50
NI of different
dilutions of the elution buffer (1 mg/ml HA peptide in equilibration buffer).
EXAMPLES
Example 1: Detection of binding site II and III in human and mouse leptin by
structural
superposition.
FSSP structural similarity searches reveal that leptin shows the highest
structural similarity
with the cytokines of the IL-6 family and G-CSF, and to a minor extent with
other long chain
cytokines, such as the growth hormone and placental lactogen.
The crystal structures of human leptin (1ax8), human CNTF (1cnt), human IL-6
(1alu), bovine
G-CSF(1bgc), vIL6 (1i1r), ovine placental lactogen (1f6f), murine LIF (11ki)
and human OSM
(1 evs) were superposed, using the FSSP and Prosup algorithms. Human leptin
residues
overlapping with binding site II or III residues in the other cytokines were
considered as
possible binding site II or III residues in human leptin.
A homology model was built for murine leptin, by replacing non-identical
residues in human
leptin structure by the optimal rotamer of the corresponding residue in mouse
leptin, followed
by energy minimization, using moe and the charmm22 forcefield. Residues
aligning with the
possible binding site II or III residues in human leptin were considered as
possible binding site
II or III residues in human leptin. Solvent-exposed residues in the predicted
binding site II and
III were mutated in the pMET7-SIgK-HA-mLep expression vector, and the mutant
leptin was
expressed in COS-1 cells:
Example 2: Generation of the mouse leptin expression vector pMet7-SIgK-HA-
mouse
leptin
The pCDM8 mleptin-AP vector is containing the mouse leptin sequence, followed
by the
human alkaline phosphatase sequence. The wild type (wt) mouse leptin sequence
was
isolated from this vector by PCR, using the 5' forward oligomeric primer 5'-
GCGTCCGGAATCCAGAAAGTCCAGGATG-3', containing a BspEl restriction site, and the
3'
reverse primer 5'-CGCTCTAGATTAGCATTCAGGGCTAACATCC-3' , containing an Xbal
restriction site. The pMET7-SIgK-HA-LRIo plasmid contains the SIgK signal
peptide, followed
by the HA tag sequence and the mouse leptin receptor sequence. The leptin
receptor
7
CA 02479163 2004-08-25
sequence was excised from this vector, using the BspEl and Xbal restriction
enzymes, and the
mouse leptin sequence was ligated into this opened vector. The resulting
vector, pMET7-
SIgK-HA-mLep, allows the expression of a fusion protein, consisting of the
SIgK signal
peptide, followed by the HA-tag sequence, followed by a 4 amino acid GGSG
linker, followed
by amino acids 3 to 146 of mouse leptin. Upon expression in eukaryotic cells,
the SIgK signal
peptide is cleaved off and the HA-tagged protein is secreted in the medium.
Amino acid sequence of SIgK-HA-mouse leptin: the arrow indicates the predicted
cleavage site
of the SIgK signal peptide, the numbers above the sequence indicate the
residue numbers in
mouse leptin:
3 28
i
METDTLLLWVLLLVWPGSTGD YPYDVPDYA GGSG IQKVQDDTKTLIKTIVTRINDISHTQ
SIgK signal peptide ~ HA-tag GGSG mouse leptin
29 88
SVSAKQRVTGLDFIPGLHPILSLSKMDQTLAVYQQVLTSLPSQNVLQIANDLENLRDLLH
89 120121 146
LLAFSKSCSLPQTSGLQKPESLDGVLEASLYSTEWALSRLQGSLQDILQQLDVSPEC
8
CA 02479163 2004-08-25
O-1769 Mutation (-l3fV in mouse ieptin GGATGACACC CC:AACAT~CAAGACAATTGTCACC
GGATC
i i_
O-1770 Mutation L13(V in mouse leptin GATCCTGGTGACAATTGTCTTGATGTTGGTTTTGGTGT
CATGC
! I
lfl AI! mutations were introduced usi~~g the (~uickChange methoc:l
(~tratagene) according to the
manufacturer's specifications.
Amino acid sequence of the mouse leptin mutants: vertical arrows indicate the
position of the
mutations, the numbers above the; sequence indicate the residue numbers in
mouse ieptin:
20
1 o R20I~
3 20 2~
9
CA 02479163 2004-08-25
METDTLLLWVLLLV1lVPGSTGD YPYD~PDYA GGSG IQKVQDDTKTLIKTIVTNINDiSHTQ
29 gg
SVSAKQRVTGLDFIPGLHPlLSLSKI1~DQTLAVYQQVLTSLPSQNVLQIANDLENLRDLLH
89 120 121 146
~I [
LLAFSKSCSLPQTSGLQKPESLDGVLEASLYSTEWALSRLQGSLQDILQQLDVSPEC
2a L13N
3 13 28
a
l~ NEETDTLLLVWLLLWVPGSTGD YPYDVPDYA GGSG IQKVQDDTKTNIKTIVTRINDISHTQ
29 gg
SVSAKQRVTGLDFIPGLHPlLSLSK;fVIDQTLAVYQQVLTSLPSQNVLQIANDLENLRDLLH
89 12x121 146
~E
LLAFSKSCSLPQTSGLQKPESLDGVLEASLYSTEWALSRLQGSLQDI LQQLDVSPEC
3a S120AT121A
8 28
I
iVIETDTLLLWVLLLIWPGSTGD YPYDV DYA GGSG iQKVQDDTKTLIKTIVTRINDISHTQ
29 gg
SVSAKQRVTGLDFIPGLHPiLSLSK11~DQTLAVYQQVLTSLPSQNVLQlANDLENLRDLLH
8g 120 121 146
LLAFSKSCSLPQTSGLQKPESLDC~VLEASLYAAEWALSRi..t'~GSLC~DILQQLDVSPEC
CA 02479163 2004-08-25
Example 4: Expression of the HA-tagged mouse leptin and HA-tagged mouse leptin
mutants in COS-1 cells
COS-1 cells were seeded at 4 x 105 ceilslwell in 6-well plates, and grown
overnight.
The cells were then transfected using the polyethyleneimine (PEI) transfection
method with the
pMet7-SIgK-HA-mLep or the R20N, L13N or S120AT121A mutants in this vector.
The medium was replaced after 4 hours transfection, and cells were incubated
overnight, after
which the medium was replaced by 2 ml OPTI-MEM medium (Gibco-BRL). After
another 72
hours, the OPTI-MEM medium containing the secreted HA-tagged leptin or HA-
tagged leptin
mutant was collected, and cells were removed by centrifugation.
Example 5: Purification of HA-tagged S120AT121A mouse leptin
COS-1 cells were seeded at 8 x 106 cells per 175 cm2 flask in DMEM medium. Ten
175 cm2
flasks were transfected with the S120AT121A pMET7-SIgK-HA-mLep mutant by
transfection
using polyethyleneimine. After 4 hours, the medium was replaced by fresh DMEM
medium,
and cells were grown overnight. The medium was then replaced by 50 ml OPTI-
MEM, and
cells were incubated in this medium for another 72 hours. The medium with the
secreted
S120AT121A HA-tagged mouse leptin was collected and filtered through a 0.22 pm
filter, and
complete (Roche Diagnostics) protease inhibitor was added.
The S120AT121A HA-tagged mouse leptin was purified on a 1ml anti-HA affinity
column
(Roche diagnostics). The medium was loaded at a flow rate of 0.3 mllmin. The
column was
washed with 25 ml equilibration buffer (20 mM TrisIHCI, pH 7.5, 100 mM NaCI,
0.1 mM EDTA)
+ 0.05% Tween-20, followed by 10 ml equilibration buffer without Tween-20. The
S120AT121A HA-mouse leptin was eluted with HA peptide (1 mglml) in
equilibration buffer.
Example 6: The S120AT121A mouse leptin mutant does not induce JAK-STAT
signalling
of the mouse leptin receptor
HEK293T cells were seeded in 6 well plates at 4 x 105 cellslwell and grown for
20 hours. The
cells were then transfected with the pMET7-mLRlo and pXP2d2-rPAP1 plasmids,
using the
standard calcium phosphate precipitation technique. One day after
transfection, the cells were
washed with phosphate buffered saline, and cultured in DMEM medium
supplemented with
10% foetal calf serum and 50 pg/ml gentamycin. Two days after transfection,
the cells were
dissociated with cell dissociation buffer (Invitrogen) and resuspended in 2ml
of DMEM medium
supplemented with 10% foetal calf serum and 50 Ng/ml gentamycin. 50 pl of the
cell
suspension was seeded in each well of a black 96-well plate (Costar). In each
well, 50 pl of the
11
CA 02479163 2004-08-25
appropriate dilution of medium containing either HA-tagged mouse leptin, R20N
mouse leptin,
L13N mouse leptin or S120AT121A mouse leptin was added.
The pMET7-mLRlo plasmid encodes the long form of the mouse leptin receptor,
with a C-
terminal myc tag. The pXP2d2-rPAP1 plasmid encodes the luciferase gene, under
control of
the STAT3-dependent rat pancreatitis-associated protein-1 promotor.
Incubation of the transfected HEK293T cells with wt HA-tagged mouse leptin
leads to
concentration-dependent luciferase activity. Incubation of the transfected
HEK293T cells with
the R20N mutant HA-tagged mouse leptin, the L13N mutant HA-tagged mouse leptin
or the
S120AT121A mouse leptin does not lead to an appreciable increase of luciferase
activity in the
transfected cells.
Exampte 7: The S120AT121A mouse leptin inhibits binding of wt leptin to the
leptin
1 S receptor
8 x 106 COS-1 cells were seeded in a 175 cm2 flask, and grown overnight in
DMEM medium
supplemented with 10% foetal calf serum and 50 pg/ml gentamycin.
The cells were transfected with the pMET7-mLRCRM2-his6 using the PEI
transfection method.
pMET7-mLRCRM2-his6 encodes amino acids 407- 604, corresponding to the CRM2
module
of the murine leptin receptor, followed by a C-terminal his6 tag. After 4
hours, the medium was
replaced by fresh DMEM medium supplemented with 10% foetal calf serum and 50
Nglml
gentamycin, and cells were grown overnight. The medium was then replaced by 50
ml OPTI-
MEM, and cells were incubated in this medium for another 72 hours. The medium
with the
secreted His-tagged CRM2 leptin was collected and cells were removed by
centrifugation. This
medium is referred to as CRM2-his medium.
8 x 106 COS-1 cells were seeded in a 175 cm2 flask, and grown overnight in
DMEM
supplemented with 10% foetal calf serum and 50 pg/ml gentamycin.
The cells were transfected with the pCDMB AP-mLep plasmid using the PEI
transfection
method. The pCDM8 AP-mLep pfasmid allows expression of a fusion protein,
consisting of
murine leptin linked to the C-terminus of human secreted alkaline phosphatase
(SEAP). After 4
hours, the medium was replaced by fresh DMEM medium, and cells were grown
overnight.
The medium was then replaced by 50 ml OPTI-MEM; and cells were incubated in
this medium
for another 72 hours. The medium with the secreted SEAP-leptin was collected
and cells were
removed by centrifugation. This medium is referred to as SEAP-leptin medium.
Maxisorp plates (Nunc) were coated overnight at 6 °C with 0.25 pglml
anti-His5 antibody
(Qiagen). Plates were washed four times with phosphate buffered saline pH 7.5
containing 0.1
Tween 20. The free protein binding sites on the plates were blocked by
incubation with 1
12
CA 02479163 2004-08-25
casein in phosphate buffered saline pH 7.5 at 37 °C for two hours.
Plates were washed four
times with phosphate buffered saline pH 7.5 containing 0.1 % Tween 20. The
plates were
incubated for 2 hours at 37 °C with the undiluted medium containing the
his-tagged CRM2 of
the murine leptin receptor. Plates were washed four times with phosphate
buffered saline pH
7.5 containing 0.1 % Tween 20. The plates were then incubated with SEAP-Ieptin
medium
and different concentrations of either HA-tagged mouse leptin or S120AT121A
ieptin
antagonist. Addition of S120AT121A mouse leptin or WT leptin, but not of
mutant R20N
inhibits the binding of the SEAP-lepfin to the his-tagged CRM2 of the murine
leptin receptor.
The results are shown in Figure 3.
Example 8: The S120AT121A mouse ieptin mutant inhibits the ,IAK-STAT
signalling of
the mouse leptin receptor
HEK 293T cells were seeded in 5 well plates at 4 x 105 cellslwell and grown
for 20 hours. The
cells were then transfected with the pMET7-mLRlo and pXP2d2-rPAP1 plasmids,
using the
standard calcium phosphate precipitation technique. One day after
transfection, the cells were
washed with phosphate buffered saline, and cultured in DMEM medium
supplemented with
10% foetal calf serum and 50 Ng/ml gentamycin. Two days after transfection,
the cells were
dissociated with cel( dissociation buffer (Invitrogen) and resuspended in 2ml
of DMEM
medium, supplemented with 10°/~ foetal calf serum and 50 pglml
gentamycin. 50 pl of the cell
suspension was seeded in each well of a black 96-well plate (Costar). OPTI-MEM
medium,
containing wt HA-tagged leptin (see example 4) was diluted 16 fold in OPT-MEM.
50 pl of this
diluted medium was added to the cells, together with 50 NI of dilution of anti-
HA purified (see
example 5) HA tagged S120AT121A leptin. The cells were then incubated
overnight. The
medium was removed and the cells were incubated with 50 ~I of lysis buffer (25
mM Tris, pH
7.8; 2 mM EDTA; 2 mM DTT; 10% glycerol; 1% Triton X-100) for 10 minutes. 35 pl
of
luciferase substrate buffer (20 rnM Tricine; 1.07 mM (MgC03)4Mg(OH)2.5H20;
2.67 mM
MgS04.7Hz0; 0.1 mM EDTA; 33.3 mM DTT; 270 mM Coenzyme A; 470 mM Luciferin; 530
mM
ATP; final pH 7.8) was then added to the lysate, and the light emission was
measured in a
TopCount Chemiluminescence Counter (Packard).
The results are shown in Figure 4. Incubation of the transfected HEK293T cells
with the wt HA-
tagged mouse leptin induces luciferase activity. Co-incubation with the
S120AT121A mutant
inhibits the leptin-induced luciferase activity. This inhibitory effect is not
seen with the L13N nor
the R20N mutants.
13
CA 02479163 2004-08-25
REFERENCES
Barton, V.A., Hudson, K.R. and Heath, J.K. (1999) Identification of three
distinct receptor
binding sites of murine interieukin-11. J. Biol. Chem., 274, 5755-5761.
Baumann, H., Morella, K.K., White, D.W., Dembski, M., Bailon, P.S., Kim, H.,
Lai, C.-F. and
Tartaglia, L.A. (1996) The full-length leptin receptor has signalling
capabilities of interleukin 6-
type cytokine receptors. Proc. Natl. Acad. Sci., 93, 8374-8378.
Boulanger, M.J., Chow, D.-C., Brevnova, E.E. and Garcia, K.C. (2003) Hexameric
structure
and assembly of the interleukin-6I IL-6 a-receptor I gp130 complex. Science,
300, 2101-2104.
Bravo, J., and Heath, J.K. (2000) Receptor recognition by gp130 cytokines.
EMBO J., 19,
2399-2411.
Busso, N., So, A., Chobaz-Peclat V., Morard, C., Martinet-Soria E., Talabot-
Ayer D. and
Gabay C. (2002) Leptin signalling deficiency impairs humoral and cellular
immune responses
and attenuates experimental arthritis. J. lmmunol., 168, 875-882.
Chow, D.-C., He, X.-L., Snow, A.L., Rose-John, S. and Garcia, K.C. (2001)
Structure of an
extracellular gp130 cytokine receptor signalling complex. Science, 291, 2150-
2155.
Cohen, S.L., Halaas, J.L., Friedman, J.M., Chait, B.T., Bennett, L., Chang,
D., Hecht, R. and
Collins, F. (1996) Human leptin characterization. Nature. 382, 589.
Di Marco, A.; Gloaguen, L, Graziani, R., Paonessa, G., Saggio, L, Hudson,
K.R., and Laufer,
R. (1996) Identification of Ciliary neurotrophic factor (CNTF) residues
essential for leukemia
inhibitory factor receptor binding and generation of CNTF receptor
antagonists. Proc. Natl.
Acad. Sci. USA, 93, 9247-9252.
Faggioni, R., Fantuzzi, G., Fuller, J., Dinarello, C.A., Feingold, K.R.,
Grunfeld, C. (1998) IL-1
beta mediates leptin induction during inflammation. Am J Physiol., 274, 8204-
208
Fong, T.M., Huang,R.-R.C., Tota, M.R., Mao, C., Smith, T., Varnerin,J.,
Karpitskiy, V.V.,
Krause, J.E., Van Der Ploeg, L.H.T. (1998) Localization of Leptin Binding
Domain in the leptin
receptor. Molecular Pharmacology; 63, 234-240.
Gainsford, T., Willson, T.A., Metcalf, D., Handman, E., McFarlane, C., Ng, A.,
Nicola, N.A.,
Alexander, W.S., Hilton, D.J. (1996) Leptin can induce proliferation,
differentiation, and
functional activation of hemopoietic cells. Proc. Natl. Acad. Sci. USA, 93,
14564-14568.
Ghilardi, N., Skoda, R.C. 1997 The leptin receptor activates janus kinase 2
and signals for
proliferation in a factor-dependent cell line. Mol Endocrinol, 11, 393-9.
14
CA 02479163 2004-08-25
Grunfeld, C., Zhao, C., Fuller, J., Pollack, A., Moser, A., Friedman, J.,
Feingold, K.R. (1996)
Endotoxin and cytokines induce expression of leptin, the ob gene product, in
hamsters. J Clin
Invest., 97, 2152-2157.
Holm, L. and Sander, C. (1997). Dali/FSSP classification of three-dimensional
protein folds.
Nucleic Acids Res.,25, 231-234.
Hudson, K.R., Vernallis, A.B., and Heath J.K. (1996) Characterization of the
receptor binding
sites of human leukemia inhibitory factor and creation of antagonists. J.
Biol. Chem. 271,
11971-11978.
Inoue M., Nakayam, C., Kikuchi, K., Kimura, T., Ishige, Y., Ito, A., Kanaoka,
M. and Noguchi,
H. (1995) D1 cap region involved in the receptor recognition and neuronal cell
survival activity
of human Ciliary neurotrophic factor. Proc. Natl. Acad. Sci. USA, 92, 8579-
8583.
Kalai, M.; Montero-Julian., F., Grotzinger, J. ; Fontaine, V., Vandenbussche,
P.,
Deschuyteneer, R., Wollmer, A., Brailly, F., and Content, J. (1997) Analysis
of the human
Interleukin-61 Human Interleukin-8 Receptor binding interface at the amino
acid level: proposed
mechanism of interaction. Blood, 89, 1319-1333.
Loffreda, S., Yang, S.Q., Lin, H.Z., Karp, C.l_., Brengman, M.L., Wang, D.J.,
Klein, A.S.,
Bulkley, G.B., Bao, C., Noble, P.W., Lane, M.D., Diehl, A.M. (1998) Leptin
regulates
proinflammatory immune responses. Faseb J., 12, 57-65.
Lord, G.M., Matarese, G., Howard, J.K., Baker, R.J., Bloom, S.R., Lechler,
R.I. (1998) Leptin
modulates the T-cell immune response and reverses starvation-induced
immunosuppression.
Nature, 394, 897-901.
Matarese, G., Di Giacomo, A., Sanna, V., Lord, G.M., Howard, J.K., DiTuoro,
A., Bloom, S.R.,
Lechler, R.L, Zappacosta, S. and Fontana, S. (2001) requirement for leptin in
the induction
and progression of autoimmune encephalomyelitis. J. Immunology, 166, 5909-
5916.
Matarese, G., Sanna, V., Lechler, R.I., Sarvetnick, N., Fontana, S.,
Zappacosta, S., and La
Cava, A. (2002) Leptin accelerates autoimmune diabetes in female NOD mice.
Diabetes, 51,
1356-1361.
Mykoniatis A., Anton, P.M., WLK, M., Wang, C.C., Ungsunan, L., Bliaher, S.,
Venihaki, M.,
simeonidis, S., Zacks, J., Zhao, D., Sougioultzis, S., Karalis, K., Mantzoros,
C., and
Pothoulakis, C. (2003) Leptin mediates Clostridium Difficile Toxin A- induced
enteritis in mice.
Gastroenterology, 124, 683-691.
Sanna, V., Di Giacomo, A., La Cava, A., Lechler, R.I., Fontana, S.,
Zappacosta, S., and
Matarese, G. (2003) Leptin surge precedes onset of autoimmune
encephalomyelitis and
correlates with development of pathogenic T cell responses. J. Clin, Invest.,
111, 241-250.
CA 02479163 2004-08-25
Savino, R., Lahm, A., Giogio, M., Cabibbo, A., Tramontano, A, and Ciliberto,
G. (1993)
Saturation mutagenesis of the human interleukin 6 receptor-binding site:
Implications for its
three-dimensional structure. Proc. Natl. Acad. Sci. USA, 90, 4067-4071.
Siegmund, B., Lehr, H.A., and Fantuzzi, G. (2002) Leptin: A pivotal mediator
of intestinal
S inflammation in mice. Gastroenterology, 122, 2011-2025.
Sierra-Honigmann, M.R., Nath, A.K., Murakami, C., Garcia-Cardetia, G.,
Papapetropoulos, A.,
Sessa, W.C., Madge, L.A., Schechner, J.S., Scwabb, M.B., Polverini, P.J.,
Flores-Riveros, J.R.
(1998) Biological action of leptin as an angiogenic factor. Science, 281, 1683-
1686.
Zhang, F., Basinski, M.B., Beals, J.M., Briggs, S.L., Churgay, L.M., Clawson,
D.K., DiMarchi,
R.D., Furman, T.C., Hale, J.E., Hsiung, H.M., Schoner, B.E., Smith, D.P.,
Zhang, X.Y., Wery,
J.P., Schevitz, R.W. (1997) Crystal structure of the obese protein leptin-
E100. Nature, 387,
206-209.
16
CA 02479163 2004-12-21
SEQUENCE LISTING
(1) GENERAL INFORMATION:
(i) APPLICANT: VIB VZW; UNIVERSITEIT GENT
(ii) TITLE OF INVENTION: NOVEL LEPTIN ANTAGONIST
(iii) NUMBER OF SEQUENCES: 17
(iv) CORRESPONDENCE ADDRESS:
(A) ADDRESSEE: FETHERSTONHAUGH & CO.
(B) STREET: P.O. BOX 2999, STATION D
(C) CITY: OTTAWA
(D) STATE: ONT
(E) COUNTRY: CANADA
(F) ZIP: K1P 5Y6
(v) COMPUTER READABLE FORM:
(A) MEDIUM TYPE: Floppy disk
(B) COMPUTER: IBM PC compatible
(C) OPERATING SYSTEM: PC-DOS/MS-DOS
(D) SOFTWARE: ASCII (text)
(vi) CURRENT APPLICATION DATA:
(A) APPLICATION NUMBER: 2,479,163 CA
(B) FILING DATE: 25-AUG-2004
(C) CLASSIFICATION:
(vii) PRIOR APPLICATION DATA:
(A) APPLICATION NUMBER:
(B) FILING DATE:
(viii) ATTORNEY/AGENT INFORMATION:
(A) NAME: FETHERSTONHAUGH & CO.
(B) REGISTRATION NUMBER:
(C) REFERENCE/DOCKET NUMBER: 29775-46
(ix) TELECOMMUNICATION INFORMATION:
(A) TELEPHONE: (613)-235-4373
(B) TELEFAX: (613)-232-8440
(2) INFORMATION FOR SEQ ID NO.: 1:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 16
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY:
(ii) MOLECULE TYPE: polypeptide
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE
(C) OTHER INFORMATION: a leptin antagonist
(ix) FEATURE
(A) NAME/KEY: MISC_FEATURE
(B) LOCATION: (1). (1)
(C) OTHER INFORMATION: X can be any amino acid, but not S
(ix) FEATURE
(A) NAME/KEY: MISC_FEATURE
(B) LOCATION: (2) . (2)
(C) OTHER INFORMATION: X can be any amino acid, but not T
(ix) FEATURE
(A) NAME/KEY: MISC_FEATURE
(B) LOCATION: (9). (9)
(C) OTHER INFORMATION: X can be an R or W
(ix) FEATURE
(A) NAME/KEY: MISC_FEATURE
(B) LOCATION: (12) .(12)
(C) OTHER INFORMATION: X can be an G, A or R
(ix) FEATURE
(A) NAME/KEY: MISC FEATURE
17
CA 02479163 2004-12-21
(B) LOCATION: (13)..(13)
(C) OTHER INFORMATION: X can be an S or A
(xi) SEQUENCE DESCRIPTION: SEQ ID NO.: 1:
Xaa Xaa Glu Val Val Ala Leu Ser Xaa Leu Gln Xaa Xaa Leu Gln Asp
1 5 10 15
(2) INFORMATION FOR SEQ ID NO.: 2:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 16
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY:
(ii) MOLECULE TYPE: polypeptide
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE
(C) OTHER INFORMATION: a leptin antagonist
(ix) FEATURE
(A) NAME/KEY: MISC_FEATURE
(B) LOCATION: (2) . (2)
(C) OTHER INFORMATION: X can be any amino acid, but not T
(ix) FEATURE
(A) NAME/KEY: MISC_FEATURE
(B) LOCATION: (9). (9)
(C) OTHER INFORMATION: X can be an R or W
(ix) FEATURE
(A) NAME/KEY: MISC_FEATURE
(B) LOCATION: (12) .(12)
(C) OTHER INFORMATION: X can be an G, A or R
(ix) FEATURE
(A) NAME/KEY: MISC_FEATURE
(B) LOCATION: (13) .(13)
(C) OTHER INFORMATION: X can be an S or A
(xi) SEQUENCE DESCRIPTION: SEQ ID NO.: 2:
Ala Xaa Glu Val Val Ala Leu Ser Xaa Leu Gln Xaa Xaa Leu Gln Asp
1 5 10 15
(2) INFORMATION FOR SEQ ID NO.: 3:
(1) SEQUENCE CHARACTERISTICS
(A) LENGTH: 16
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY:
(ii) MOLECULE TYPE: polypeptide
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE
(C) OTHER INFORMATION: a leptin antagonist
(ix) FEATURE
(A) NAME/KEY: MISC_FEATURE
(B) LOCATION: (1). (1)
(C) OTHER INFORMATION: X can be any amino acid, but not S
(ix) FEATURE
(A) NAME/KEY: MISC_FEATURE
(B) LOCATION: (9). (9)
(C) OTHER INFORMATION: X can be an R or W
(ix) FEATURE
(A) NAME/KEY: MISC_FEATURE
(B) LOCATION: (12) .(12)
(C) OTHER INFORMATION: X can be an G, A or R
(ix) FEATURE
(A) NAME/KEY: MISC FEATURE
18
CA 02479163 2004-12-21
(B) LOCATION: (13)..(13)
(C) OTHER INFORMATION: X can be an S or A
(xi) SEQUENCE DESCRIPTION: SEQ ID NO.: 3:
Xaa Ala Glu Val Val Ala Leu Ser Xaa Leu Gln Xaa Xaa Leu Gln Asp
1 5 10 15
(2) INFORMATION FOR SEQ ID NO.: 4:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 16
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY:
(ii) MOLECULE TYPE: polypeptide
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE
(C) OTHER INFORMATION: a leptin antagonist
(ix) FEATURE
(A) NAME/KEY: MISC_FEATURE
(B) LOCATION: (9). (9)
(C) OTHER INFORMATION: X can be an R or W
(ix) FEATURE
(A) NAME/KEY: MISC_FEATURE
(B) LOCATION: (12) .(12)
(C) OTHER INFORMATION: X can be an G, A or R
(ix) FEATURE
(A) NAME/KEY: MISC_FEATURE
(B) LOCATION: (13) . (13)
(C) OTHER INFORMATION: X can be an S or A
(xi) SEQUENCE DESCRIPTION: SEQ ID NO.: 4:
Ala Ala Glu Val Val Ala Leu Ser Xaa Leu Gln Xaa Xaa Leu Gln Asp
1 5 10 15
(2) INFORMATION FOR SEQ ID NO.: 5:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 146
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY:
(ii) MOLECULE TYPE: polypeptide
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE
(C) OTHER INFORMATION: a leptin antagonist
(ix) FEATURE
(A) NAME/KEY: MISC_FEATURE
(B) LOCATION: (120)..(120)
(C) OTHER INFORMATION: X can be any amino acid, but not S
(ix) FEATURE
(A) NAME/KEY: MISC_FEATURE
(B) LOCATION: (121)..(121)
(C) OTHER INFORMATION: X can be any amino acid, but not T
(xi) SEQUENCE DESCRIPTION: SEQ ID NO.: 5:
Val Pro Ile Gln Lys Val Gln Asp Asp Thr Lys Thr Leu Ile Lys Thr
1 5 10 15
Ile Val Thr Arg Ile Asn Asp Ile Ser His Thr Gln Ser Val Ser Ser
20 25 30
Lys Gln Lys Val Thr Gly Leu Asp Phe Ile Pro Gly Leu His Pro Ile
35 40 45
19
CA 02479163 2004-12-21
Leu.Thr Leu Ser Lys Met Asp Gln Thr Leu Ala Val Tyr Gln Gln Ile
50 55 60
Leu Thr Ser Met Pro Ser Arg Asn Val Ile Gln Ile Ser Asn Asp Leu
65 70 75 80
Glu Asn Leu Arg Asp Leu Leu His Val Leu Ala Phe Ser Lys Ser Cys
85 90 95
His Leu Pro Trp Ala Ser Gly Leu Glu Thr Leu Asp Ser Leu Gly Gly
100 105 110
Val Leu Glu Ala Ser Gly Tyr Xaa Xaa Glu Val Val Ala Leu Ser Arg
115 120 125
Leu Gln Gly Ser Leu Gln Asp Met Leu Trp Gln Leu Asp Leu Ser Pro
130 135 140
Gly Cys
145
(2) INFORMATION FOR SEQ ID NO.: 6:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 28
(B) TYPE: nucleic acid
(C) STRANDEDNESS:
(D) TOPOLOGY:
(ii) MOLECULE TYPE: DNA
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE
(C) OTHER INFORMATION: forward oligomeric primer used in example 2
(xi) SEQUENCE DESCRIPTION: SEQ ID NO.: 6:
GCGTCCGGAA TCCAGAAAGT CCAGGATG 28
(2) INFORMATION FOR SEQ ID NO.: 7:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 31
(B) TYPE: nucleic acid
(C) STRANDEDNESS:
(D) TOPOLOGY:
(ii) MOLECULE TYPE: DNA
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE
(C) OTHER INFORMATION: reverse primer used in example 2
(xi) SEQUENCE DESCRIPTION: SEQ ID NO.: 7:
CGCTCTAGAT TAGCATTCAG GGCTAACATC C 31
(2) INFORMATION FOR SEQ ID NO.: 8:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 178
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY:
(ii) MOLECULE TYPE: polypeptide
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Mus musculus
(ix) FEATURE
(A) NAME/KEY: MISC_FEATURE
(C) OTHER INFORMATION: SlgK-HA-mouse leptin
CA 02479163 2004-12-21
(xi) SEQUENCE DESCRIPTION: SEQ ID NO.: 8:
Met Glu Thr Asp Thr Leu Leu Leu Trp Val Leu Leu Leu Trp Val Pro
1 5 10 15
Gly Ser Thr Gly Asp Tyr Pro Tyr Asp Val Pro Asp Tyr Ala Gly Gly
25 30
Ser Gly Ile Gln Lys Val Gln Asp Asp Thr Lys Thr Leu Ile Lys Thr
35 40 45
Ile Val Thr Arg Ile Asn Asp Ile Ser His Thr Gln Ser Val Ser Ala
50 55 60
Lys Gln Arg Val Thr Gly Leu Asp Phe Ile Pro Gly Leu His Pro Ile
65 70 75 80
Leu Ser Leu Ser Lys Met Asp Gln Thr Leu Ala Val Tyr Gln Gln Val
85 90 95
20 Leu Thr Ser Leu Pro Ser Gln Asn Val Leu Gln Ile Ala Asn Asp Leu
100 105 110
Glu Asn Leu Arg Asp Leu Leu His Leu Leu Ala Phe Ser Lys Ser Cys
115 120 125
Ser Leu Pro Gln Thr Ser Gly Leu Gln Lys Pro Glu Ser Leu Asp Gly
130 135 140
Val Leu Glu Ala Ser Leu Tyr Ser Thr Glu Val Val Ala Leu Ser Arg
145 150 155 160
Leu Gln Gly Ser Leu Gln Asp Ile Leu Gln Gln Leu Asp Val Ser Pro
165 170 175
Glu Cys
(2) INFORMATION FOR SEQ ID NO.: 9:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 40
(B) TYPE: nucleic acid
(C) STRANDEDNESS:
(D) TOPOLOGY:
(ii) MOLECULE TYPE: DNA
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE
(C) OTHER INFORMATION: primer 0-1701
(xi) SEQUENCE DESCRIPTION: SEQ ID NO.: 9:
CAAGACCATT GTCACCAACA TTAATGACAT TTCACACACG 40
(2) INFORMATION FOR SEQ ID NO.: 10:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 40
(B) TYPE: nucleic acid
(C) STRANDEDNESS:
(D) TOPOLOGY:
(ii) MOLECULE TYPE: DNA
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE
(C) OTHER INFORMATION: primer O-1702
21
CA 02479163 2004-12-21
~(xi) SEQUENCE DESCRIPTION: SEQ ID NO.: 10:
CGTGTGTGAA ATGTCATTAA TGTTGGTGAC AATGGTCTTG 40
(2) INFORMATION FOR SEQ ID NO.: 11:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 37
(B) TYPE: nucleic acid
(C) STRANDEDNESS:
(D) TOPOLOGY:
(ii) MOLECULE TYPE: DNA
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE
(C) OTHER INFORMATION: primer O-1821
(xi) SEQUENCE DESCRIPTION: SEQ ID NO.: 11:
GGAAGCCTCA CTCTACGCCG CGGAGGTGGT GGCTTTG 37
(2) INFORMATION FOR SEQ ID NO.: 12:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 37
(B) TYPE: nucleic acid
(C) STRANDEDNESS:
(D) TOPOLOGY:
(ii) MOLECULE TYPE: DNA
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE
(C) OTHER INFORMATION: primer 0-1822
(xi) SEQUENCE DESCRIPTION: SEQ ID NO.: 12:
CAAAGCCACC ACCTCCGCGG CGTAGAGTGA GGCTTCC 37
(2) INFORMATION FOR SEQ ID NO.: 13:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 43
(B) TYPE: nucleic acid
(C) STRANDEDNESS:
(D) TOPOLOGY:
(ii) MOLECULE TYPE: DNA
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE
(C) OTHER INFORMATION: primer O-1769
(xi) SEQUENCE DESCRIPTION: SEQ ID NO.: 13:
GGATGACACC AAAACCAACA TCAAGACAAT TGTCACCAGG ATC 43
(2) INFORMATION FOR SEQ ID NO.: 14:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 43
(B) TYPE: nucleic acid
(C) STRANDEDNESS:
(D) TOPOLOGY:
(ii) MOLECULE TYPE: DNA
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE
(C) OTHER INFORMATION: primer O-1770
(xi) SEQUENCE DESCRIPTION: SEQ ID NO.: 14:
GATCCTGGTG ACAATTGTCT TGATGTTGGT TTTGGTGTCA TCC 43
22
CA 02479163 2004-12-21
(2)~INFORMATION FOR SEQ ID NO.: 15:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 178
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY:
(ii) MOLECULE TYPE: polypeptide
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE
(C) OTHER INFORMATION: mouse leptin mutant R20N
(xi) SEQUENCE DESCRIPTION: SEQ ID NO.: 15:
Met Glu Thr Asp Thr Leu Leu Leu Trp Val Leu Leu Leu Trp Val Pro
1 5 10 15
Gly Ser Thr Gly Asp Tyr Pro Tyr Asp Val Pro Asp Tyr Ala Gly Gly
25 30
Ser Gly Ile Gln Lys Val Gln Asp Asp Thr Lys Thr Leu Ile Lys Thr
20 35 40 45
Ile Val Thr Asn Ile Asn Asp Ile Ser His Thr Gln Ser Val Ser Ala
50 55 60
Lys Gln Arg Val Thr Gly Leu Asp Phe Ile Pro Gly Leu His Pro Ile
65 70 75 80
Leu Ser Leu Ser Lys Met Asp Gln Thr Leu Ala Val Tyr Gln Gln Val
85 90 95
Leu Thr Ser Leu Pro Ser Gln Asn Val Leu Gln Ile Ala Asn Asp Leu
100 105 110
Glu Asn Leu Arg Asp Leu Leu His Leu Leu Ala Phe Ser Lys Ser Cys
115 120 125
Ser Leu Pro Gln Thr Ser Gly Leu Gln Lys Pro Glu Ser Leu Asp Gly
130 135 140
Val Leu Glu Ala Ser Leu Tyr Ser Thr Glu Val Val Ala Leu Ser Arg
145 150 155 160
Leu Gln Gly Ser Leu Gln Asp Ile Leu Gln Gln Leu Asp Val Ser Pro
165 170 175
Glu Cys
(2) INFORMATION FOR SEQ ID NO.: 16:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 178
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY:
(ii) MOLECULE TYPE: polypeptide
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE
(C) OTHER INFORMATION: mouse leptin mutant L13N
(xi) SEQUENCE DESCRIPTION: SEQ ID NO.: 16:
Met Glu Thr Asp Thr Leu Leu Leu Trp Val Leu Leu Leu Trp Val Pro
1 5 10 15
23
CA 02479163 2004-12-21
Gly~Ser Thr Gly Asp Tyr Pro Tyr Asp Val Pro Asp Tyr Ala Gly Gly'
20 25 30
Ser Gly Ile Gln Lys Val Gln Asp Asp Thr Lys Thr Asn Ile Lys Thr
35 40 45
Ile Val Thr Arg Ile Asn Asp Ile Ser His Thr Gln Ser Val Ser Ala
50 55 60
Lys Gln Arg Val Thr Gly Leu Asp Phe Ile Pro Gly Leu His Pro Ile
65 70 75 80
Leu Ser Leu Ser Lys Met Asp Gln Thr Leu Ala Val Tyr Gln Gln Val
85 90 95
Leu Thr Ser Leu Pro Ser Gln Asn Val Leu Gln Ile Ala Asn Asp Leu
100 105 110
Glu Asn Leu Arg Asp Leu Leu His Leu Leu Ala Phe Ser Lys Ser Cys
115 120 125
Ser Leu Pro Gln Thr Ser Gly Leu Gln Lys Pro Glu Ser Leu Asp Gly
130 135 140
Val Leu Glu Ala Ser Leu Tyr Ser Thr Glu Val Val Ala Leu Ser Arg
145 150 155 160
Leu Gln Gly Ser Leu Gln Asp Ile Leu Gln Gln Leu Asp Val Ser Pro
165 170 175
Glu Cys
(2) INFORMATION FOR SEQ ID NO.: 17:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 178
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY:
(ii) MOLECULE TYPE: polypeptide
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(1x) FEATURE
(C) OTHER INFORMATION: mouse leptin mutant S120AT121A
(xi) SEQUENCE DESCRIPTION: SEQ ID NO.: 17:
Met Glu Thr Asp Thr Leu Leu Leu Trp Val Leu Leu Leu Trp Val Pro
1 5 10 15
Gly Ser Thr Gly Asp Tyr Pro Tyr Asp Val Pro Asp Tyr Ala Gly Gly
20 25 30
Ser Gly Ile Gln Lys Val Gln Asp Asp Thr Lys Thr Leu Ile Lys Thr
35 40 45
Ile Val Thr Arg Ile Asn Asp Ile Ser His Thr Gln Ser Val Ser Ala
50 55 60
Lys Gln Arg Val Thr Gly Leu Asp Phe Ile Pro Gly Leu His Pro Ile
70 75 80
Leu Ser Leu Ser Lys Met Asp Gln Thr Leu Ala Val Tyr Gln Gln Val
85 90 95
24
CA 02479163 2004-12-21
Leu~Thr Ser Leu Pro Ser Gln Asn Val Leu Gln Ile Ala Asn Asp Leu~
100 105 110
Glu Asn Leu Arg Asp Leu Leu His Leu Leu Ala Phe Ser Lys Ser Cys
115 120 125
Ser Leu Pro Gln Thr Ser Gly Leu Gln Lys Pro Glu Ser Leu Asp Gly
130 135 140
Val Leu Glu Ala Ser Leu Tyr Ala Ala Glu Val Val Ala Leu Ser Arg
145 150 155 160
Leu Gln Gly Ser Leu Gln Asp Ile Leu Gln Gln Leu Asp Val Ser Pro
165 170 175
Glu Cys