Note: Descriptions are shown in the official language in which they were submitted.
CA 02479641 2004-09-16
WO 03/082783 PCT/US02/09310
"PROCESS FOR PRODUCING SELECTED
PHENYL-ALKANES WITH ADSORPTIVE SEPARATION STEP "
BACKGROUND OF THE INVENTION
The invention relates to a process for the selective production of phenyl-
alkane and phenyl-alkane sulfonate compositions and to compositions and to
uses of those compositions.
More than thirty years ago, many household laundry detergents were
io made of branched alkylbenzene sulfonates (BABS). BABS are manufactured
from a type of alkylbenzenes called branched alkylbenzenes (BAB).
Alkylbenzenes (phenyl-alkanes) refers to a general category of compounds
having an aliphatic alkyl group bound to a phenyl group and having the general
formula of (m;-alkyl;);-n-phenyl-alkane. The aliphatic alkyl group may also
consist
of one or more alkyl group branches designated by the corresponding "(m;-
alkyl;);".
The standard process used by the petrochemical industry for producing
BAB consists of oligomerizing light olefins, particularly propylene, to
branched
olefins having 10 to 14 carbon atoms and then alkylating benzene with the
2o branched olefins in the presence of a catalyst such as HF. Although the
product
BAB comprises a large number of alkyl-phenyl-alkanes having the general
formula (m;-alkyl;);-n-phenyl-alkane, two examples of BAB are m-alkyl-m-alkyl-
n-
phenyl-alkanes where m# n, and m-alkyl-m-phenyl-alkanes where m _ 2.
The most prominent common characteristic of BAB is that, for a large
proportion of BAB, there is attached to the aliphatic alkyl chain of BAB
generally
at least one alkyl group branch, and more commonly three or more alkyl group
branches. If any alkyl group branch itself is branched, then the aliphatic
alkyl
group in BAB has even more primary carbon atoms. Thus the aliphatic alkyl
group in BAB usually has three, four, or more primary carbon atoms. Each alkyl
group branch is usually a methyl group branch, although ethyl, propyl, or
higher
alkyl group branches are possible.
CA 02479641 2004-09-16
WO 03/082783 PCT/US02/09310
Another typical characteristic of BAB is that the attachment of the phenyl
group to any non-primary carbon atom of the aliphatic alkyl chain. Except for
1-
phenyl-alkanes whose formation is known to be disfavored due to the relative
instability of the primary carbenium ion and neglecting the relatively minor
effect
of the branches of the branched paraffins, the oligomerization step produces a
carbon-carbon double bond that is randomly distributed along the length of the
aliphatic alkyl chain, and the alkylation step nearly randomly attaches the
phenyl
group to a carbon along the aliphatic alkyl chain. Thus, for example, a BAB
that
has an aliphatic alkyl chain having 10 carbon atoms would be expected to be an
io approximately random distribution of 2-, 3-, 4-, and 5-phenyl-alkanes, and
the
selectivity to 2-phenyl alkane would be 25 if the distribution was perfectly
random, but is typically between 10 and 40.
BAB's commonly have one of the quaternary carbon as one of the carbon
atoms of the aliphatic alkyl group. The quaternary carbon may bind by a carbon-
is carbon bond to a carbon in the phenyl group. When it does the molecule is
referred to as a "quaternary alkyl-phenyl-alkane" or simply hereinafter a
"quat."
and comprise alkyl-phenyl-alkanes having the general formula m-alkyl-m-phenyl-
alkane. "End quats" constitute 2-alkyl-2-phenyl-alkanes where the second
carbon atom from an end of the alkyl side chain is a quat. Quats containing
the
2o quaternary carbon in other locations result in an alkyl-phenyl-alkane
referred to
as an "internal quat." Known processes for producing BAB, create a relatively
high proportion of internal quats, typically greater than 10 mol-%.
Household laundry detergents made of BABS were gradually polluting
rivers and lakes. It was found that BABS were slow to biodegrade. The use
25 linear alkylbenzene sulfonates (LABS), which biodegrade more rapidly than
BABS reduced the problem. LABS are manufactured from linear alkylbenzenes
(LAB). The petrochemical industry produces LAB by dehydrogenating linear
paraffins to linear olefins which are then alkylated with benzene in the
presence
of HF or a solid catalyst. LABs comprise a linear aliphatic alkyl group and a
30 phenyl group and have the general formula n-phenyl-alkane. LAB has no alkyl
group branches and normally two primary carbon atoms (i.e., n _ 2). The
standard LAB process attaches the phenyl group to any secondary carbon atom
-2-
CA 02479641 2004-09-16
WO 03/082783 PCT/US02/09310
of the linear aliphatic alkyl group. HF catalyst alkylation is slightly more
likely to
attach the phenyl group to a secondary carbon near the center of the linear
aliphatic alkyl group. LAB produced by the DetalTM process contains
approximately 25-35 mol-% of n-phenyl-alkanes as 2-phenyl-alkanes.
Recent research has identified modified alkylbenzene sulfonates "MABS",
which differ from all alkylbenzene sulfonates used currently in commerce, and
from all alkylbenzene sulfonates produced by prior alkylbenzene processes,
including those catalyzed by HF, aluminum chloride, silica-alumina, fluorided
silica-alumina, zeolites, and fluorided zeolites. MABS relative to LABS have
io improved laundry cleaning performance, hard surface cleaning performance,
and excellent efficiency in hard and/or cold water, while also having
biodegradability comparable to that of LABS.
MABS can be produced by sulfonating a third type of alkylbenzenes
called modified alkylbenzenes (MAB), and the desired characteristics of MAB
is are determined by the desired solubility, surfactancy, and biodegradability
properties of MABS. MAB comprises a large number of phenyl-alkanes, some
of which may be found in LAB and BAB, but the matching phenyl-alkanes are
not desirable phenyl-alkanes for MAB. The phenyl-alkanes in MAB are phenyl-
alkanes comprising a lightly branched aliphatic alkyl group and a phenyl group
2o and has the general formula (m;-alkyl;);-n-phenyl-alkane. Phenyl-alkanes in
MAB
usually have only one alkyl group branch and where n# 1, the MAB has three
primary carbons. A preferred MAB phenyl-alkane is a monomethyl-phenyl-
alkane. However, the MAB may have two primary carbon atoms if there is only
one alkyl group branch and n = 1, or, if there are two alkyl group branches
and n
25 # 1, four primary carbons. Thus, the first characteristic of MAB is an
average
number of primary carbons in the aliphatic alkyl groups of the phenyl-alkanes
intermediate that in BAB and in LAB. A high proportion of 2-phenyl-alkanes
also
characterizes MAB, namely that from 40 to 100% of phenyl groups selectively
attach to the second carbon atom of the alkyl side chain.
30 As a final characteristic MAB alkylate has a relatively low proportion of
internal quats, typically less than 10 mol-%. Some internal quats such as 5-
-3-
CA 02479641 2004-09-16
WO 03/082783 PCT/US02/09310
methyl-5-phenyl-undecane produce MABS with slower biodegradation. MABS
with end quats such as 2-methyl-2-phenyl-undecane show biodegradation
similar to LABS. See the article entitled "Biodegradation of Coproducts of
Commercial Linear Alkylbenzene Sulfonate," by A. M. Nielsen et al., in
Environmental Science and Technology, Vol. 31, No. 12, 3397-3404 (1997).
PCT International Publication Nos. WO 99/05082, WO 99/05084,
99/05241, and WO 99/05243, all four of which were published on February 4,
1999, disclose alkylation processes for uniquely lightly branched or
delinearized
alkylbenzenes. PCT International Publication No. W099/07656, published on
lo February 18, 1999, discloses processes for such alkylbenzenes using
adsorptive
separation.
Because of the advantages of MABS over other alkylbenzene sulfonates,
catalysts and processes are sought that produce MAB with a selectivity to
2-phenyl-alkanes and selectivity away from internal quaternary phenyl-alkanes.
SUMMARY OF THE INVENTION
In one aspect, this invention is a process for the production of phenyl-
alkanes, in particular modified alkylbenzenes (MAB), by adsorptive separation,
dehydrogenation, and alkylation. The process is characterized by the
composition of the adsorbent and desorbent pair used in the process. The
adsorbent used is silicalite and the desorbent comprises a C5-C8 linear
paraffin,
a C5-C8 cycloparaffin, and/or preferably a branched paraffin such as
isooctane.
In a process embodiment a paraffinic feed stream comprising a first
concentration of a first acyclic paraffin having a carbon number range of C8 -
C28 and 2 or 3 primary carbon atoms and a second acyclic paraffin pass to an
adsorption zone. The adsorption zone comprises a bed of an adsorbent
comprising silicalite at adsorption promoting conditions to selectively adsorb
at
least portion of the acyclic paraffin having 2 or 3 primary carbon atoms. A
desorbent stream comprising at least one of a C5-C8 cycloparaffin, a C5-C8
3o normal paraffin, and a C5-C8 branched paraffin contacts the bed of
adsorbent.
An adsorption extract having a second concentration of the first acyclic
-4-
CA 02479641 2004-09-16
WO 03/082783 PCT/US02/09310
hydrocarbon that is greater than the first concentration is recovered from the
adsorption zone. At least a portion of the adsorption extract passes to a
dehydrogenation zone that operates at dehydrogenation conditions sufficient to
dehydrogenate the first acyclic paraffin. A dehydrogenated product stream
comprising a C8 - C28 acyclic monoolefin having 2 or 3 primary carbon atoms is
recovered from the dehydrogenation zone. An aromatic feedstock comprising a
phenyl compound and at least a portion of the dehydrogenated product stream
pass to an alkylation zone that operates at alkylation conditions sufficient
to
alkylate the phenyl compound with the acyclic monoolefin in the presence of an
io alkylation catalyst. The alkylation zone allows recovery of a phenyl-alkane
comprising a molecule having one phenyl portion and one C8-C28 aliphatic alkyl
portion with 2 or 3 primary carbon atoms and no quaternary carbon atoms
except for quats. The alkylation has a selectivity to 2-phenyl-alkanes of from
40
to 100 and a selectivity to internal quaternary phenyl-alkanes of less than
10. In
a preferred embodiment, the alkylation has a selectivity to non-quats of less
than
10, and more preferably less than 1.
In a preferred process embodiment this invention produces an MAB
composition comprising phenyl-alkanes having one phenyl group and one
aliphatic alkyl group. The the phenyl-alkanes futher have an average weight of
the aliphatic alkyl groups of the phenyl-alkanes of between the weight of a
Clo
aliphatic alkyl group and a C13 aliphatic alkyl group; a content of phenyl-
alkanes
having the phenyl group attached to the 2- and/or 3-position of the aliphatic
alkyl
group of greater than 55 wt-% of the phenyl-alkanes; and an average level of
branching of the aliphatic alkyl groups of the phenyl-alkanes of from 0.25 to
1.3
alkyl group branches per phenyl-alkane molecule when the sum of the contents
of 2-phenyl-alkanes and 3-phenyl-alkanes is more than 55 wt-% and less than or
equal to 85 wt-% of the phenyl-alkanes, or an average level of branching of
the
aliphatic alkyl groups of the phenyl-alkanes of from 0.4 to 1.3 alkyl group
branches per phenyl-alkane molecule when the sum of the concentrations of 2-
phenyl-alkanes and the 3-phenyl-alkanes is greater than 85 wt-% of the phenyl-
alkanes. In addition the aliphatic alkyl groups of the phenyl-alkanes comprise
primarily linear aliphatic alkyl groups and mono-branched aliphatic alkyl
groups,
-5-
CA 02479641 2004-09-16
WO 03/082783 PCT/US02/09310
and wherein the alkyl group branches on the aliphatic alkyl chain of the
aliphatic
alkyl groups comprise primarily small substituents, such as methyl group
branches, ethyl group branches, or propyl group branches, and wherein the
alkyl
group branches attach to any position on the aliphatic alkyl chain of the
aliphatic
alkyl groups provided that phenyl-alkanes having at least one quaternary
carbon
atom on the aliphatic alkyl group comprise less than 20% of the phenyl-
alkanes.
This invention, when used for detergent alkylation, produces detergents
that meet the increasingly stringent requirements of 2-phenyl-alkane
selectivity
and internal quaternary phenyl-alkane selectivity for the production of MAB
that
io can be sulfonated to produce MABS with improved cleaning effectiveness in
hard and/or cold water and biodegradability comparable LAS.
In another aspect the process of this invention produces particular MAB
and MABS product compositions with specially tailored carbon branching that
differs from prior art processes. In another aspects these MAB and MABS
produced may be used as a lubricant or a lubricant additive, respectively.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 shows an embodiment of the invention.
Figure 2 is a concentration profile for a pulse test separation.
DETAILED DESCRIPTION OF THE INVENTION
The process uses a feed mixture comprising a paraffin and a feedstock
comprising a phenyl compound are consumed in the subject process. The feed
mixture comprises acyclic paraffins having 8 to 28 carbon atoms. The acyclic
paraffin is preferably a "lightly branched paraffin," which as used herein,
refers
to a paraffin having three or four primary carbon atoms no quaternary carbon
atoms. Normally, the lightly branched paraffin has a total number of from 9 to
16
carbon atoms, preferably from 10 to 14 carbon atoms, and highly preferably
from 10 to 13 carbon atoms. The lightly branched paraffin generally comprises
3o an aliphatic alkane having the general formula of (p;-alkyl;);-alkane.
-6-
CA 02479641 2004-09-16
WO 03/082783 PCT/US02/09310
The lightly branched paraffins comprise generally more than 30 mol-%
and preferably more than 70 mol-%, of the feed mixture. Branching alkyl groups
generally comprise methyl, ethyl, and propyl groups with shorter branches
being
preferred. Preferably, the lightly branched paraffin has only one alkyl group
branch and comprise preferably more than 85 mol-% of the total lightly
branched
paraffins. Lightly branched paraffins having either two alkyl group branches
or
four primary carbon atoms comprise generally less than 30 mol-%, and
preferably less than 15 mol-%, of the total lightly branched paraffins.
The feed mixture may also contain one or more nonbranched (linear) or
io normal paraffin molecules having a total number of carbon atoms per
paraffin
molecule of generally from 8 to 28 carbon atoms, and highly preferably from 10
to 13 carbon atoms. The concentration of nonbranched paraffins in the feed
mixture is often above 0.3 mol-%.
In addition to lightly branched and nonbranched paraffins, other more
is highly branched acyclic compounds may be in the feed mixture. However, on
dehydrogenation such highly branched paraffins tend to form highly branched
monoolefins which on alkylation tend to form BAB. For example, paraffin
molecules consisting of at least one quaternary carbon atom tend on
dehydrogenation followed by alkylation to form phenyl-alkanes that have in the
2o aliphatic alkyl portion a quaternary carbon atom that is not a quat.
Therefore, the
quantity of these highly branched paraffins charged to the process is
preferably
minimized. Paraffin molecules consisting of at least one quaternary carbon
atom generally comprise less than 10 mol-%, preferably less than 5 mol-%,
more preferably less than 2 mol-%, and most preferably less than 1 mol-% of
the
25 feed mixture.
The production of the feed mixture is not an essential element of this
invention, and any suitable method for producing the feed mixture may be used.
Since the carbon number range of the feed mixture desired for the production
of
MAB is normally between 9 and 16. This range corresponds to paraffins boiling
in
30 the kerosene boiling point range, therefore kerosene fractions form
suitable feed
mixture precursors. Kerosene preparation methods are inherently imprecise and
produce a mixture of compounds. The feed mixtures to the process may contain
-7-
CA 02479641 2005-05-16
quantities of paraffins having multiple branches and paraffins having multiple
carbon atoms in the branches, cycloparaffins, branched cycloparaffins, or
other
compounds having boiling points relatively close to the desired compound
isomer.
Keroserie fractions contain a very large number of different hydrocarbons and
the
feed mixture to the subject process can therefore contain 200 or more
different
compounds including sizable quantities of aromatic hydrocarbons. Fractions
recovered from crude oil by fractionation will typically require hydrotreating
for
removal of sulfur and/or nitrogen prior to being fed to the subject process.
It is expected, however, that separation rather than oligomerization or
io other forms of synthesis will provide a lower cost adequate feed mixture
and will
therefore be the predominate source of the feed mixture. A preferred method
for
the production of the feed mixture is the separation of nonbranched (linear)
hydrocarbons or lightly branched hydrocarbons from a kerosene boiling range
petroleum fraction. Several known processes that accomplish such a separation
is are known. One process, the UOP MolexTM process, is an established,
commercially proven method for the liquid-phase adsorption separation of
normal paraffins from isoparaffins, cycloparaffins, and aromatics using the
UOP
~
Sorbex separation technology. Another suitable, established, and proven
process is the UOP Kerosene IsosivTM Process, which employs vapor-phase
2o adsorption for separating normal paraffins from nonnormal paraffins using
molecular sieves in an adsorber vessel. See Chapters 10.3, 10.6 and 10.7 in
the
book entitled Handbook of Petroleum Refining Process, Second Edition, edited
by Robert A. Meyers, published by McGraw-Hill,. New York, 1997.
The raffinate stream of an adsorptive separation process, such as the UOP
25 MolexTM process which selectively recovers the nonbranched (linear)
paraffins in
an extract stream, is an especially preferred feed mixture for the subject
process.
The raffinate stream from such a process will be free of contaminants such as
sulfur or nitrogen containing compounds, and will also have a suitably low
concentration of nonbranched paraffins.and olefins. The use of such a
raffinate
30 stream as the feed mixture allows integration of the subject process into
an
existing LAB facility, with the two adsorptive separation steps being
performed in
series. The separately recovered normal paraffin stream and feed mixture can
* Trade-mark
-8-
CA 02479641 2004-09-16
WO 03/082783 PCT/US02/09310
then be processed in a variety of ways. For instance, each of the nonbranched
paraffin stream and the feed mixture could be processed independently via
dehydrogenation and aromatic alkylation to produce two separate products.
Alternatively, the nonbranched paraffin stream and the feed mixture could be
used
to form a desired paraffin blend. That is, the stream charged to the
dehydrogenation zone of the subject process can comprise the product of the
separation zone of the subject process plus from 10 to 50 vol-% nonbranched
paraffins.
The composition of a mixture of linear, lightly branched, and branched
io paraffins as well as olefins can be determined by analytical methods that
are
well-known to a person of ordinary skill in the art of gas chromatography. The
article written by H. Schulz, et al. and published starting at page 315 of the
Chromatographia 1, 1968, describes a temperature-programmed gas
chromatograph apparatus and method that is suitable for identifying components
Is in complex mixtures of paraffins or olefins. A person of ordinary skill in
the art
can separate and identify the components in a mixture of paraffins using
essentially the apparatus and method described in the article by Schulz et al.
The aromatic-containing feedstock to the subject process comprises a
phenyl compound, which is benzene when the process is detergent alkylation.
20 In a more general case, the phenyl compound of the aromatic feedstock may
be
alkylated or otherwise substituted derivatives or of a higher molecular weight
than benzene, including toluene, ethylbenzene, xylene, phenol, naphthalene,
etc.,
The adsorptive separation section recovers acyclic, lightly branched
25 paraffins from the feed mixture. This separation can be performed in a
batch or
continuous mode including the use of two or more adsorbent beds in cyclic
operation. In this mode one or more beds are used for the separation while
another bed is being regenerated. Significant operational and economic
advantages accrue to performing the separation on a continuous basis.
Simulated
30 moving bed technology (SMB) is the preferred method of achieving continuous
operation and uniform products. The separation preferably recovers monomethyl
paraffins from the feed mixture.
-9-
CA 02479641 2004-09-16
WO 03/082783 PCT/US02/09310
SMB adsorptive separation units for simulating movement of the adsorbent
relative to the feed stream are well known. This simulation is performed using
established commercial technology wherein the adsorbent is held fixed in place
as
a number of subbeds retained in one or more cylindrical adsorbent chambers.
The positions at which the streams involved in the process enter and leave the
chambers are slowly shifted from subbed to subbed along the length of the
adsorbent chambers so that the streams enter or leave different subbeds as the
operational cycle progresses. Normally there are at least four streams (feed,
desorbent, extract and raffinate) employed in this procedure, and the location
at
io which the feed and desorbent streams enter the chamber and the extract and
raffinate streams leave the chamber are simultaneously shifted in the same
direction at set intervals. Only one line is normally employed for each
subbed, and
each bed line carries one of the four process streams at some point in the
cycle.
Cyclic advancement of the input and output streams of this simulation can be
accomplished by a manifolding system or by rotary disc valves. This simulation
usually includes the use of a variable flow rate pump which pushes liquid
leaving
one end of the adsorbent vessel(s) to the other end in a single continuous
loop.
Simulated moving bed processes typically include at least three or four
separate steps which are performed sequentially in separate zones within a
mass
of adsorbent. Each of these zones normally is formed from a plurality of
subbeds
with the number of beds per zone ranging from 2 or 3 up to 8 to 10. The most
widely practiced commercial process units typically contain 24 beds. All of
the
beds are contained in one or more vertical vessels referred to herein
collectively
as the adsorbent chamber. The general technique employed in the performance
of a simulated moving bed adsorptive separation is well described at page 70
of
the September 1970 edition of Chemical Engineering Progress (Vol. 66, No 9).
as
shown in US-A-3,040,777 and US-A-3,422,848.
During the adsorption step of the process a feed mixture containing a
mixture of compounds is contacted with the adsorbent at adsorption conditions
3o and one or more compound(s) or a class of compounds is selectively adsorbed
and retained by the adsorbent while the other compounds of the feed mixture
are
relatively unabsorbed. Normally the desired compound is adsorbed. The feed
-10-
CA 02479641 2004-09-16
WO 03/082783 PCT/US02/09310
mixture may contain a large variety of compounds including isomers of the
desired
compound. For instance, a mixed xylene feed stream may contain ethylbenzene
and/or C9 aromatics and can be processed to recover a specific isomer by a
suitable adsorbent/desorbent pair operated at suitable conditions. Differing
sieve/desorbent combinations are used for different separations. For instance,
X
zeolites, specifically X zeolites exchanged with barium or barium and
potassium
ions at their exchangeable sites, are the preferred adsorbents for p-xylene
recovery from xylene mixtures.
In the next step of the process, the unabsorbed (raffinate) components of
io the feed mixture are then removed from the void spaces between adsorbent
particles and from the surface of the adsorbent as a raffinate stream. The
adsorbed compound is then recovered from the adsorbent by contact a stream
comprising the desorbent material at desorption conditions in a desorption
step.
The desorbent displaces the desired compound to form an extract stream, which
is normally transferred to a fractionation zone for recovery of the desired
compound from desorbent of the extract stream. In some instances the desired
product of the process can be in the raffinate stream rather than the extract
stream.
For purposes of this description, various terms used herein are defined as
follows. A "feed mixture" is a mixture containing one or more extract
components
and one or more raffinate components to be separated by the adsorption section
of the subject process. The term "feed stream" indicates a stream of a feed
mixture passed into contact with the adsorbent. An "extract component" is a
compound or class of compounds that is more selectively adsorbed by the
adsorbent. A "raffinate component" is a compound or class of compound that is
less selectively adsorbed. The term "desorbent material" means a material
capable of desorbing an extract component. The term "raffinate stream" means a
stream for removed from the adsorbent bed after the adsorption of extract
compounds that can vary from essentially 100% desorbent material to
essentially
100% raffinate components. The term "extract stream" means a stream desorbed
by a desorbent material and removed from the adsorbent bed and varying from
essentially 100% desorbent material to essentially 100% extract components.
-11-
CA 02479641 2005-05-31
Separation means, typically fractional distillation columns, recover all or
portions of the extract stream and the raffinate stream. The stream containing
the
undesired compound may be recycled to isomerization. The extract stream may
be rich in the desired compound or may only contain an increased
concentration.
When used relative to a process stream the term "rich" is intended to indicate
a
concentration of the indicated compound or class of compounds greater than 50
mole %.
It has become customary in the art to group the numerous beds in the
adsorption chambers into a number of zones. Zone I, the adsorption zone, makes
io contact between the feed stream and the adsorbent. Zone II, the
purification zone,
removes undesired isomers as raffinate. In Zone Ili, the desorption zone,
desorbent releases the desired isomers from the adsorbent for recovery in the
extract stream. Zone IV contains quantity of adsorbent located between Zone I
and Zone III that segregates Zones I and III and partially removes desorbent
from
the adsorbent. The liquid flow through Zone IV prevents contamination of Zone
III
by Zone I liquid by flow cocurrent to the simulated motion of the adsorbent
from
Zone III toward Zone I. A more thorough explanation of simulated moving bed
processes is given in the Adsorption, Liquid Separation section of the Kirk-
Othmer
Encyclopedia of Chemical Technology, Fourth Edition, John Wiley and Sons,
U.S.A.. 1991.
The objectives of this invention are achieved by employing a novel
adsorbent-desorbent pair comprising a siticalite adsorbent and a desorbent
containing a branched paraffin; a linear paraffin and/or cycloparaffin; a
linear
paraffin and a branched paraffin; or a linear paraffin, a cycloparaffin, and a
branched paraffin. The preferred desorbent is a C5 to C8 branched paraffin.
The preferred branched paraffin for the desorbent is isooctane.
The preferred adsorbent comprises silicalite. Silicalite is well described in
the article, "Silicalite, A New Hydrophobic Crystalline Silica Molecular
Sieve,"
Nature, Vol. 271, Feb. 9, 1978. Silicate is a hydrophobic crystalline silica
molecular sieve having an MFI type structure of intersecting bent-orthogonal
channels formed with two cross-sectional geometries, 6A circular and 5.1-5.7 A
elliptical on the major axis. This gives silicalite great selectivity as a
size selective
-12-
CA 02479641 2004-09-16
WO 03/082783 PCT/US02/09310
molecular sieve. Due to its aluminum free structure composed of silicon
dioxide
silicalite does not show ion-exchange behavior. Thus silicalite is not a
zeolite.
The practice of the subject invention requires no significant variation in
operating conditions, adsorbent or desorbent composition within the adsorbent
chambers or during different process steps. That is, the adsorbent preferably
remains at the same temperature and pressure throughout the process.
The active component of the adsorbent is normally used in the form of
small agglomerates having high physical strength and attrition resistance. The
agglomerates contain the active adsorptive material dispersed in an amorphous,
io inorganic matrix referred to as the binder and having channels and cavities
therein
which enable fluid access to the adsorptive material. Methods for forming the
crystalline powders into such agglomerates include the addition of an
inorganic
binder, generally a clay comprising a silicon dioxide and aluminum oxide, to a
high
purity adsorbent powder in a wet mixture. Silica is a suitable binder. The
adsorbent particles may be in the form of extrudates, tablets, macrospheres or
granules having a desired particle range, preferably from 16 to 60 mesh
(Standard
U.S. Mesh) (1.9 mm to 250 microns). Clays of the kaolin type, water permeable
organic polymers or silica are generally used as binders.
Those skilled in the art will appreciate that the performance of a particular
2o adsorbent is often greatly influenced by a number of variables not related
to its
composition such as operating conditions, feed stream composition, and the
water
content of the adsorbent. One such variable is the water content of the
adsorbent
which is expressed herein in terms of the recognized Loss on Ignition (LOI)
test.
In the LOI test the volatile matter content of the zeolitic adsorbent is
determined by
the weight difference obtained before and after drying a sample of the
adsorbent
at 500 C under an inert gas purge such as nitrogen for a period of time
sufficient
to achieve a constant weight. For the subject process it is preferred that the
water
content of the adsorbent results in an LOI at 900 C of less than 7.0 wt-% and
preferably within the range of from 0 to 4.0 wt-%.
A silicalite or other microporous active component of the adsorbent will
ordinarily be in the form of small crystals present in the adsorbent particles
in
amounts ranging from 75 to 98 wt-% of the particle based on volatile-free
-13-
CA 02479641 2004-09-16
WO 03/082783 PCT/US02/09310
composition. Volatile-free compositions are generally determined after the
adsorbent has been calcined at 900 C in order to drive off all volatile
matter. The
remainder of the adsorbent will generally be the inorganic matrix of the
binder.
The present invention, passes a feed mixture comprising one or more
monomethyl branched hydrocarbons and at least one nonnormal hydrocarbon of
like carbon number but different structure through one or more beds of
adsorbent
that selectively adsorbing the monomethyl branched hydrocarbon and passes
other components through the adsorption zone. At some point in time the flow
of
the feed stream through the adsorbent bed stops and the adsorption zone is
io flushed to remove nonadsorbed materials surrounding the adsorbent.
Thereafter
passing a desorbent through the adsorbent bed recovers the desired isomer.
The selectivity, (f3), of an adsorbent/desorbent pair is defined as the ratio
of the two components in the adsorbed phase divided by the ratio of the same
two components in the unabsorbed phase at equilibrium conditions. Relative
selectivity is given by the equation:
Selectivity = wt. percent C/wt. percent DA
wt. percent C/wt. percent Du
where C and D are two components of the feed stream represented in weight
percent and the subscripts A and U represent the adsorbed and unabsorbed
phases, respectively. The equilibrium conditions are determined when the feed
stream passing over a bed of adsorbent does not change composition, in other
words, when there is no net transfer of material occurring between the
unabsorbed and adsorbed phases. Relative selectivity can be expressed not only
for one feed stream compound as compared to another but can also be
expressed between any feed mixture component and the desorbent material.
An important characteristic of an adsorbent is the rate of exchange of the
desorbent for the extract component of the feed mixture or, in other words,
the
3o relative rate of desorption of the extract component. Faster rates of
exchange
reduce the amount of desorbent material needed to remove the extract
component and consequently the operating cost of the process. Exchange rates
are often temperature dependent. Ideally, desorbent materials should have a
-14-
CA 02479641 2004-09-16
WO 03/082783 PCT/US02/09310
selectivity for extract components equal to or slightly less than 1 to desorb
extract
components as a class with reasonable flow rates of desorbent material, and so
that extract components can displace desorbent material in subsequent
adsorption steps.
s In continuous liquid phase adsorptive separation processes the desorbent
material must be judiciously selected to satisfy many criteria. Expressed in
terms
of the selectivity, the adsorbent should have more selectivity for all extract
components with respect to a raffinate component than for the desorbent
material
with respect to a raffinate component. Desorbent materials must also be
io compatible with the particular adsorbent and the particular feed mixture
and have
a reasonable cost.
Adsorption conditions in general include a temperature range of from 20 C
to 250 C, with from 40 C to 150 C being more preferred. Temperatures from
80 C to 140 C are highly preferred. Adsorption conditions also preferably
include
15 a pressure sufficient to maintain the process fluids in liquid phase; which
may be
from atmospheric to 600 psi(g). Desorption conditions generally include the
same
temperatures and pressure as used for adsorption conditions.
The preferred desorbent comprises a mixture of normal paraffin, a
cycloparaffin (naphthene) and/or a branched paraffin. Preferred cycloparaffins
are
20 cyclopentane, cyclohexane and methyl cyclohexane. The preferred normal
paraffins are n-pentane and n-hexane. Normal paraffins are strong desorbents
and n-hexane is actually the strongest desorbent of these compounds. A blend
of
normal paraffins and cycloparaffins or of normal paraffins and isooctane, is
often
desirable to adjust the strength of the desorbent stream. These blends may
25 contain from 10 to 90 vol-% cycloparaffin or isooctane, with the remainder
being
the normal paraffin.
The extract stream comprises paraffins having a total number of carbon
atoms per paraffin molecule of generally from 8 to 28, preferably from 8 to
15,
and more preferably from 10 to 15 carbon atoms. The extract stream contains a
3o higher concentration of lightly branched paraffins, based on the total
paraffins in
the extract stream, than the concentration of lightly branched paraffins in
the
-15-
CA 02479641 2004-09-16
WO 03/082783 PCT/US02/09310
feed mixture, based on the total paraffins in the feed mixture. The lightly
branched paraffins having either two alkyl group branches or four primary
carbon atoms comprise generally less than 60 mol-%, preferably less than 30
mol-%, and more preferably less than 15 mol-%, of the total lightly branched
paraffins in the portion of the extract stream that passes to the
dehydrogenation
zone of the process. The lightly branched paraffins having either one alkyl
group branch and more desirably a methyl group comprise preferably more than
85 mol-% of the total lightly branched paraffins in the portion of the extract
stream charged to the dehydrogenation zone. When present in the extract
io stream with the lightly branched paraffins, the linear paraffin content
should be
as no more than, 75 mol-% of the total paraffins in that portion of the
extract
stream that is charged to the dehydrogenation zone. Paraffin molecules
consisting of at least one quaternary carbon atom generally comprise less than
mol-%, preferably less than 5 mol-%, more preferably less than 2 mol-%, and
most preferably less than 1 mol-%, of that portion of the extract stream that
passes to the dehydrogenation zone.
The dehydrogenation section may be configured substantially in the
manner shown in the drawing. Briefly, a stream containing paraffins combines
with recycled hydrogen to form a dehydrogenation reactant stream that is
heated
2o and contacted with a dehydrogenation catalyst in a fixed bed maintained at
dehydrogenation conditions. The effluent of the fixed catalyst bed, which is
referred to herein as the dehydrogenation reactor effluent stream, is cooled,
partially condensed, and passed to a vapor-liquid separator. The vapor-liquid
separator produces a hydrogen-rich vapor phase and a hydrocarbon-rich liquid
phase. The condensed liquid phase recovered from the separator passes to a
stripping column, which removes all compounds which are more volatile than the
lightest hydrocarbon which is desired to be passed to the alkylation section.
The olefin-containing net stream that passes from the dehydrogenation section
to the alkylation section of the process is referred to herein as the
3o dehydrogenated product stream.
This invention is not limited to any one particular flow scheme for the
dehydrogenation section which may include moving or fixed bed
-16-
CA 02479641 2004-09-16
WO 03/082783 PCT/US02/09310
dehydrogenation catalyst, catalyst-containing reaction zones with heat
exchangers there between and introduction of hot hydrogen-rich gas streams.
Hydrocarbons may contact any catalyst bed in an upward-, downward-, or radial-
flow fashion.
Dehydrogenation catalysts are well known in the prior art as exemplified
by US-A-3,274,287; US-A-3,315,007; US-A-3,315,008; US-A-3,745,112; US-A-
US-A-4,430,517; US-A-4,716,143; US-A-4,762,960; US-A-4,786,625; and US-A-
4,827,072. It is believed that the choice of a particular dehydrogenation
catalyst
is not critical to the success of this invention. The preferred catalyst is a
layered
io composition of an inner core bonded to an outer layer comprising a
refractory
inorganic oxide containing at least one uniformly dispersed platinum group
(Group VIII (IUPAC 8-10)) metal and at least one promoter metal, and where at
least one modifier metal is dispersed on the catalyst composition. Preferably,
the outer layer is bonded to the inner core to the extent that the attrition
loss is
less than 10 wt-% based on the weight of the outer layer.
The dehydrogenation conditions are selected to minimize cracking and
polyolefin by-products. Typical dehydrogenation conditions will not result in
any
appreciable isomerization of the hydrocarbons in the dehydrogenation reactor.
The hydrocarbon may contact the catalyst the liquid phase, mixed vapor-liquid
phase, or preferably in the vapor phase. Dehydrogenation conditions include a
temperature of generally from 400 C (752 F) to 900 C (1652 F) and preferably
from 400 C (752 F) to 525 C (977 F), a pressure of generally from 1 kPa(g)
(0.15 psi(g)) to 1013 kPa(g) (147 psi(g)), and a LHSV of from 0.1 to 100 hr'.
As
used herein, the abbreviation "LHSV" means liquid hourly space velocity, which
is defined as the volumetric flow rate of liquid per hour divided by the
catalyst
volume. Generally for normal paraffins, the lower the molecular weight the
higher the temperature required for comparable conversion. The
dehydrogenation zone maintain pressure as low as practicable, usually less
than
345 kPa(g) (50 psi(g)), consistent with equipment limitations, to maximize
chemical equilibrium advantages.
The extract stream may be admixed with a diluent material before, while,
or after flowing to the dehydrogenation zone. The diluent material may be
-17-
CA 02479641 2004-09-16
WO 03/082783 PCT/US02/09310
hydrogen, steam, methane, ethane, carbon dioxide, nitrogen, argon, and the
like, or a mixture thereof. Hydrogen is the preferred diluent. Ordinarily,
when
hydrogen is utilized as the diluent it is utilized in amounts sufficient to
ensure a
hydrogen to hydrocarbon mole ratio of 0.1:1 to 40:1.
Water or a material decomposable to water at dehydrogenation
conditions such as an alcohol, aidehyde, ether, or ketone may be added to the
dehydrogenation zone continuously or intermittently in an amount calculated on
the basis of equivalent water of 1 to 20,000 weight ppm of the extract stream.
1
to 10,000 weight ppm of water addition gives best results when dehydrogenating
io paraffins having from 2 to 30 or more carbon atoms.
The dehydrogenated product stream is typically a mixture of unreacted
paraffins, linear (unbranched) olefins, and branched monoolefins including
lightly
branched monoolefins. Typically from 0 to 75 mol-%, and preferably from 0 to
50 mol-%, of the olefins in the monoolefin-containing stream from the paraffin
dehydrogenation process are linear (unbranched) olefins. The dehydrogenated
product may also contain monoolefins having a total number of carbon atoms of
from 8 to 28 with preferably less than 10 mol-%, and preferably less than 1
mol-
%, of the monoolefins having quaternary carbon atoms.
The dehydrogenated product stream may comprise a highly branched
monoolefin or a linear (unbranched) olefin, but is preferably a lightly
branched
monoolefin. The term "lightly branched monoolefin," as used herein, refers to
a
monoolefin having a total number of carbon atoms and of from 8 to 28, of which
three or four of the carbon atoms are primary carbon atoms and none of the
remaining carbon atoms are quaternary carbon atoms. Preferably, the lightly
branched monoolefin has a total number of from 8 to 15 carbon atoms, and
more preferably from 10 to 15 carbon atoms.
The lightly branched monoolefin generally comprises an aliphatic alkene
having the general formula of (p;-alkyl;);-q-alkene. The lightly branched
monoolefin may be an alpha monoolefin or a vinylidene monoolefin, but is
3o normally an internal monoolefin. As used herein, the term "alpha olefins"
refers
to olefins having the chemical formula, R-CH=CH2. The term "internal olefins,"
-18-
CA 02479641 2004-09-16
WO 03/082783 PCT/US02/09310
as used herein, includes di-substituted internal olefins having the chemical
formula R-CH=CH-R; tri-substituted internal olefins having the chemical
formula
R-C(R)=CH-R; and tetra-substituted olefins having the chemical formula R-
C(R)=C(R)-R. The di-substituted internal olefins include beta internal olefins
having the chemical formula R-CH=CH-CH3. As used herein, the term
"vinylidene olefins" refers to olefins having the chemical formula R-C(R)=CH2.
Suitable lightly branched monoolefins include octenes, nonenes, decenes,
undecenes, dodecenes, tridecenes, tetradecenes, pentadecenes, hexadecenes,
heptadecenes, octadecenes, nonadecenes, eicosenes, heneicosenes,
io docosenes, tricosenes, tetracosenes, pentacosenes, hexacosenes,
heptacosenes, and octacosenes.
For lightly branched monoolefins the alkyl group branch or branches of
the lightly branched monoolefin are generally selected from methyl, ethyl, and
propyl groups, with shorter and normal branches being preferred. For all
lightly
branched monoolefins passed to the alkylation section, preferably the lightly
branched monoolefin has only one alkyl group branch, but two alkyl group
branches are also possible. Lightly branched monoolefins having either two
alkyl group branches or four primary carbon atoms comprise generally less than
30 mol-%, and preferably less than 15 mol-%, of the total lightly branched
monoolefins passed to the alkylation section, with the remainder of the
lightly
branched monoolefins passed to the alkylation section having one alkyl group
branch. Monoolefins having either two alkyl group branches or four primary
carbon atoms and a quaternary carbon atom comprise generally less than 10
mol-%, and preferably less than 1 mol-%, of the total lightly branched
monoolefins passed to the alkylation section. Lightly branched monoolefins
having either one alkyl group branch or three primary carbon atoms comprise
preferably more than 85 mol-% of the total lightly branched monoolefins passed
to the alkylation section. Lightly branched monoolefins having only one alkyl
group branch and where the sole alkyl group branch is a methyl group are
3o referred to herein as monomethyl-alkenes and are a preferred component of
the
dehydrogenated product stream.
-19-
CA 02479641 2004-09-16
WO 03/082783 PCT/US02/09310
Although vinylidene monoolefins may be present in the dehydrogenated
product stream, they are normally a minor component and have a concentration
of usually less than 0.5 mol-%, and more commonly less than 0.1 mol-%, of the
olefins in the dehydrogenated product stream.
The skeletal structures of the monoolefins in a mixture comprising lightly
branched monoolefins can be determined by analytical methods that are well-
known to a person of ordinary skill in the art of gas chromatography and need
not be described here in detail. A person of ordinary skill in the art can
modify
the apparatus and method in the previously mentioned article by Schulz et al.
to
io equip the injector with a hydrogenator insert tube in order to hydrogenate
the
lightly branched monoolefins to lightly branched paraffins in the injector.
The
lightly branched paraffins are then separated and identified using essentially
the
apparatus and method described in the article by Schulz et al. This apparatus
and method, however, will not determine the location of the carbon-carbon
double bond in any of the monoolefins in the mixture.
In addition to the lightly branched monoolefin, other acyclic compounds
may be charged to the alkylation section via the dehydrogenated product
stream. One of the advantages of this invention is that the stream containing
the lightly branched monoolefins can be passed directly to the alkylation
reaction
section despite the fact that that stream also contains acyclic paraffins
having
the same number of carbon atoms as the lightly branched monoolefins. Thus,
this invention avoids the need to separate the paraffins from the monoolefins
prior to passing to the alkylation section. Other acyclic compounds include
nonbranched (linear) olefins and monoolefins. Nonbranched (linear) olefins
which may be charged have a total number of carbon atoms per paraffin
molecule of generally from 8 to 28, preferably from 8 to 15, and more
preferably
from 10 to 13 carbon atoms. The nonbranched olefin may be an alpha
monoolefin but is preferably an internal monoolefin. When present in the
dehydrogenated product stream with the lightly branched monoolefins, the
linear
olefin content should be no more than, 75 mol-% of the total monoolefins in
the
dehydrogenated product stream, but is generally less than 60 mol-% of the
total
monoolefins in the dehydrogenated product stream.
-20-
CA 02479641 2004-09-16
WO 03/082783 PCT/US02/09310
Because of the possible presence in the dehydrogenated product stream
of linear monoolefins, in addition to the lightly branched monoolefins, the
bulk
dehydrogenated product stream may contain, on average, fewer than 3, or
between 3 and 3.4, primary carbon atoms per monoolefin molecule in the
dehydrogenated product stream. Depending on the relative proportions of linear
and lightly branched monoolefins, the dehydrogenated product stream, or the
sum of all the monoolefins that pass to the alkylation zone, may have from
2.25
to 3.4 primary carbon atoms per monoolefin molecule.
Linear and/or nonlinear paraffins which pass to the alkylation section, via
io the dehydrogenated product stream, have a total number of carbon atoms per
paraffin molecule of generally from 8 to 28, preferably from 8 to 15, and more
preferably from 10 to 13 carbon atoms. The nonlinear paraffins in the
dehydrogenated product stream may include lightly branched paraffins and may
also include paraffins having at least one quaternary carbon atom. Such linear
is and nonlinear paraffins are expected to act as a diluent in the alkylation
step and
not to materially interfere with the alkylation step. Monoolefin molecules
consisting of at least one quaternary carbon atom generally comprise less than
mol-%, preferably less than 5 mol-%, more preferably less than 2 mol-%, and
most preferably less than 1 mol-% of the dehydrogenated product stream or of
the sum of all the monoolefins that pass to the alkylation zone.
The alkylation section reacts the monoolefins in the dehydrogenated
product stream with a phenyl compound. In the general case, the monoolefins
could be reacted with benzene or substituted derivatives of benzene including
toluene and ethylbenzene. For detergent alkylation, the preferred phenyl
compound is benzene. A solid alkylation catalyst typically reacts the phenyl
compound and the monoolefins.
Only minimal skeletal isomerization of the olefins is believed to occur in
the alkylation section. Minimal skeletal isomerization of the monoolefins
means
that generally less than 25 mol-%, and preferably less than 10 mol-%, of the
olefin, the aliphatic alkyl chain, and any reaction intermediate undergoes
skeletal
isomerization. Thus, the extent of light branching of the lightly branched
-21-
CA 02479641 2005-05-16
monoolefin is identical to the extent of light branching in the aliphatic
alkyl chain
in the phenyl-alkane product molecule and the number of primary carbon atoms
also remains essentially the same. Finally, although the formation of 1-phenyl-
alkane product is not significant at alkylation conditions the number of
primary
carbon atoms in the phenyl-alkane product will be slightly less than the
number
of primary carbon atoms in the lightly branched monoolef in.
The alkylation of the phenyl compound with the lightly branched
monoolefins produces (m;-alkyl;); n-phenyl-alkanes, where the aliphatic alkyl
group has two, three, or four primary carbon atoms per phenyl-alkane molecule.
io Preferably, the aliphatic alkyl group has three primary carbon atoms per
phenyl-
alkane molecule, and more preferably one of the three primary carbon atoms is
in a methyl group at one end of the aliphatic alkyl chain, the second primary
carbon atom is in a methyl group at the other end of the chain, and the third
primary carbon atom is in a single methyl group branch attached to the chain.
Generally from 0 mol-% to 75 mol-%, and preferably from 0 mol-% to 50 mol-%,
of the (m;-alkyl;); n-phenyl-alkanes produced may have 2 primary carbon atoms
per phenyl-alkane molecule. Typically from 25 mol-% to 100 mol-%, of the (m;-
alkyl;)i-n-phenyl-alkanes produced may have 3 primary carbon atoms per phenyl-
alkane molecule. Generally from 0 mol-% to 40 mol-% of the (m; alkyl;);-n-
2o phenyl-alkanes produced may have 4 primary-carbon atoms. Thus, (m-methyl)-
n-phenyl-alkanes having only one methyl group branch are preferred and are
referred to herein as monomethyl-phenyl-alkanes. It is expected that the
number of primary, secondary, and tertiary carbon atoms per product phenyl-
alkane molecule can be determined by high resolution multipulse nuclear
magnetic resonance (NMR) spectrum editing and distortionless enhancement by
polarization transfer (DEPT), which is described in the brochure entitled
"High
Resolution Multipulse NMR Spectrum Editing and DEPT," which is distributed by
Bruker Instruments, Inc., Manning Park, Billerica, Massachusetts, USA.
The alkylation of the phenyl compound with the monoolefins has a
selectivity of 2-phenyl-alkanes of generally from 40 to 100 and preferably
from
-22-
CA 02479641 2004-09-16
WO 03/082783 PCT/US02/09310
60 to 100, and an internal quaternary phenyl-alkane selectivity of generally
less
than 10 and preferably less than 5.
The alkylation of the phenyl compound with the monoolefins has a
selectivity to phenyl-alkanes containing non-quat quaternary carbons of less
than 10, and preferably less than 1. A suitable approximation of the
selectivity
to such quaternary phenyl-alkanes can be arrived at by using the following
formula:
T=100 CQO
Co
where
T = selectivity to non-quats quarternary carbons
CQO = moles of monoolefins having a quaternary carbon atom entering
the selective alkylation zone
Co = moles of monoolefins entering the selective alkylation zone
The values of CQO and Co can be determined using the molar flow rate of
monoolefins entering the selective alkylation zone and the previously
mentioned
modified apparatus and method of Schulz et al. The selectivity, T, can be
estimated using this formula if each monoolefin entering the selective
alkylation
zone has an equal probability of alkylating the phenyl compound, regardless of
whether the monoolefin has a quaternary carbon atom. As a first approximation,
this condition is met when more than 40 wt-% of the monoolefins entering the
selective alkylation zone are lightly branched monoolefins or normal
monoolefins.
Alkylation of the phenyl compound by the monoolefins may be conducted
as a batch method but a continuous method is preferred. The alkylation
catalyst
may be used as a packed bed or a fluidized bed in upflow, downflow, or
horizontal flow mode. The benzene and the dehydrogenated product stream
containing the lightly branched monoolefins that enters the alkylation zone
typically has a total phenyl compound:monoolefin molar ratio of between 2.5:1
and 50:1, and more typically between 8:1 and 35:1. Portions of the
-23-
CA 02479641 2004-09-16
WO 03/082783 PCT/US02/09310
dehydrogenation product stream may feed into several discrete points within
the
alkylation reaction zone, and at each zone the phenyl compound:monoolefin
molar ratio exceed 50:1. However, the total benzene:olefin ratio used in the
foregoing variant of this invention will remain within the stated range. The
total
combined feed passes through the packed bed at a liquid hourly space velocity
(LHSV) between 0.3 and 6 hr'. The alkylation conditions include a temperature
in the range between 80 C (176 F) and 225 C (437 F). Preferably the alkylation
occurs in at least partial liquid phase, and preferably in either an all-
liquid phase
or at supercritical conditions. The requisite pressure necessarily depends
upon
lo the olefin, the phenyl compound, and temperature, but normally ranges from
1379-6895 kPa(g) (200-1000 psi(g)), and most usually 2069-3448 kPa(g) (300-
500 psi(g)). The alkylation reaction usually leaves little unreacted olefin
and
typically goes to at least 98% conversion based on the monoolefin.
Any alkylation catalyst that meets the requirements for conversion,
selectivity, and activity may be used. Preferred alkylation catalysts comprise
zeolites having a zeolite structures BEA, MOR, MTW, or NES. Such zeolites
include mordenite, ZSM-4, ZSM-12, ZSM-20, offretite, gmelinite, beta, NU-87,
and gottardiite. These zeolite structure types, the term "zeolite structure
type,"
and the term "isotypic framework structure" are used herein as they are
defined
2o and used in the Atlas of Zeolite Structure Types, by W. M. Meier, et al.,
published on behalf of the Structure Commission of the International Zeolite
Association by Elsevier, Boston, Massachusetts, USA, Fourth Revised Edition,
1996. Alkylations using NU-87 and NU-85, which is an intergrowth of zeolites
EU-1 and NU-87, are described in US-A-5,041,402 and US-A-5,446,234,
respectively. Gottardiite, which has an isotypic framework structure of the
NES
zeolite structure type, is described in the articles by A. Alberti et al., in
Eur. J.
Mineral., 8, 69-75 (1996), and by E. Galli et al., in Eur. J. Mineral., 8, 687-
693
(1996). Most preferably, the alkylation catalyst comprises mordenite.
Useful zeolites for the alkylation catalyst in the present invention generally
3o have at least 10 percent of the cationic sites thereof occupied by ions
other than
alkali or alkaline-earth metals. Such other ions include aluminum, zinc,
copper,
aluminum, and preferably ammonium, hydrogen, rare earth, or combinations
-24-
CA 02479641 2004-09-16
WO 03/082783 PCT/US02/09310
thereof. In a preferred embodiment, the zeolites are converted to the
predominantly hydrogen form, generally by replacement of the originally
present
ions with hydrogen ion precursors, e.g., ammonium ions, which upon calcination
yield the hydrogen form. This exchange is conveniently carried out by contact
of
the zeolite with an ammonium salt solution, e.g., ammonium chloride, utilizing
well known ion exchange techniques. In certain embodiments the replacement
produces a zeolite material in which at least 50 percent of the cationic sites
are
occupied by hydrogen ions. Although the hydrogen form of the zeolite catalyzes
the reaction successfully, the zeolite may also be partly in the alkali metal
form.
io The zeolites may be subjected to various chemical treatments, including
alumina extraction (dealumination) and combination with one or more metal
components, such as the metals of Groups IIIB (IUPAC 3), IVB (IUPAC 4), VIB
(IUPAC 6), VIIB (IUPAC 7), VIII (IUPAC 8-10), and IIB (IUPAC 12). The zeolites
may, in some instances, be subjected to thermal treatment. including steaming
or calcination in air, hydrogen, or an inert gas, e.g. nitrogen or helium. A
suitable steaming treatment comprises contacting the zeolite with an
atmosphere containing from 5 to 100% steam at a temperature of from 250 C
(482 F) to 1000 C (1832 F) for 0.25 and 100 hours at pressures ranging from
sub-atmospheric to several hundred atmospheres.
A matrix material or binder that is resistant to the temperature and other
conditions used in the process may contain the zeolite. Suitable matrix
materials include synthetic substances, naturally occurring substances, and
inorganic materials such as clay, silica, and/or metal oxides. Naturally
occurring
clays for compositing with the zeolite include those of the montmorillonite
and
kaolin families. In addition to the foregoing materials, the zeolite used in
this
invention may be compounded with a porous matrix material, such as alumina,
silica-alumina, silica-magnesia, silica-zirconia, silica-thoria, silica-
beryllia, silica-
titania, and aluminum phosphate as well as ternary combinations, such as
silica-
alumina-thoria, silica-alumina-zirconia, silica-alumina-magnesia, and silica-
magnesia-zirconia. The relative proportions of and matrix material may vary
widely, with the zeolite weight content ranging from between 1% to 99%,
-25-
CA 02479641 2004-09-16
WO 03/082783 PCT/US02/09310
typically from 5% to 80% by weight, and preferably from 30% to 80%, of the
combined weight of zeolite and matrix material.
The zeolites in the alkylation catalyst generally have a framework
silica:alumina molar ratio of from 5:1 to 100:1. A mordenite for use in
alkylation
has a framework silica:alumina molar ratio generally of from 12:1 to 90:1, and
preferably of from 12:1 to 25:1. The term "framework silica:alumina molar
ratio"
means the molar ratio of Si02 per A1203, in the zeolite framework.
Zeolites prepared in the presence of organic cations may not be
sufficiently catalytically active for alkylation. Insufficient catalytic
activity is
io believed to result from the organic cations of the forming solution
occupying the
intracrystalline free space. Heating in an inert atmosphere at 540 C (1004 F)
for
one hour, ion exchanging with ammonium salts, and calcining at 540 C (1004 F)
in air may activate such catalysts. Calcination temperatures higher than 540 C
(1004 F) may ensure decomposition of any ammonia on the catalyst.
is The alkylation reaction zone produces an alkylation reaction effluent that
enters separation facilities for the recovery of products and recyclable feed
compounds. The alkylation reaction effluent passes into a benzene column to
produce an overhead stream containing benzene for recycled to the alkylation
reaction zone and a bottoms stream containing the phenyl-alkane product. This
2o bottoms stream passes into a paraffin column to produce an overhead stream
containing unreacted paraffins and a bottoms stream containing the product
phenyl-alkanes and any higher molecular weight by-product hydrocarbons
formed in the alkylation reaction zone. The paraffin column bottoms stream may
pass to a rerun column to produce an overhead phenyl-alkane product stream
25 containing the MAB and a rerun column bottoms stream containing polymerized
olefins and polyalkylated benzenes (heavy alkylate). Alternatively, a
sufficiently
low heavy alkylate content of the paraffin column bottoms stream renders the
rerun column unnecessary and the paraffin column bottoms stream may be
recovered as the net MAB stream, which may be subsequently sulfonated to
30 produce MABS.
-26-
CA 02479641 2004-09-16
WO 03/082783 PCT/US02/09310
Several variants of the subject process are possible. Selective
hydrogenation may saturate diolefins in the dehydrogenated product stream to
desired monoolefins. The process may selectively remove deleterious aromatic
by-products contained in the dehydrogenated product stream which may be
formed during the catalytic dehydrogenation of paraffins and that may cause
deactivation of the catalyst in the alkylation section, decreased selectivity
to the
desired phenyl-alkanes, and accumulation to unacceptable concentrations. A
selective removal zone may take the aromatic by-products from the extract
stream, the dehydrogenated product steam, the overhead liquid stream of the
io paraffin column, the dehydrogenation zone or, where present, the selective
diolefin hydrogenation product stream.
This process can produce a preferred MAB composition by adsorptively
separating paraffins having an average weight between the weight of a C1o
paraffin and a C13 paraffin to produce extract paraffins having an average
level
of branching of from 0.25 to 1.3, or of from 0.4 to 1.3, alkyl group branches
per
paraffin molecule. These extract paraffins primarily comprise linear paraffins
and mono-branched paraffins, and the alkyl group branches on the aliphatic
alkyl chain of the extract paraffins primarily comprise small substituents,
such as
methyl group branches, ethyl group branches, or propyl group branches. The
2o extract paraffins are dehydrogenated to produce the corresponding mono-
olefins, which alkylate a phenyl compound to produce phenyl-alkanes. The
resultant phenyl-alkanes have the characteristics that the phenyl-alkanes
having
the phenyl group attached to the 2- and/or 3-position of the aliphatic alkyl
group
comprise greater than 55 wt-% of the phenyl-alkanes, and the phenyl-alkanes
having at least one quaternary carbon atom on the aliphatic alkyl group
comprise less than 20% of the phenyl-alkanes.
Sulfonation of the phenyl-alkanes produced by the processes of this
invention can be accomplished by contacting the phenyl-alkane compounds with
any of the well-known sulfonation systems, including those described in
3o Detergent Manufacture Including Zeolite Builders and Other New Materials,
by
Marshall Sittig, Noyes Data Corporation, Park Ridge, New Jersey, 1979, and in
Volume 56 of "Surfactant Science" series, Marcel Dekker, Inc., New York, NY,
-27-
CA 02479641 2004-09-16
WO 03/082783 PCT/US02/09310
1996. Sulfonation of the phenyl-alkane compounds produces a sulfonated
product comprising phenyl-alkane sulfonic acids. After sulfonation,
neutralization
of the sulfonated product with any suitable alkali, such as sodium, potassium,
ammonium, magnesium, calcium, and substituted ammonium alkalis, and
mixtures thereof produces a neutralized product comprising phenyl-alkane
sulfonates.
In yet another aspect of the present invention, this invention is the use of
the MAB compositions produced by the processes disclosed herein as
lubricants. These phenyl-alkanes are believed to have properties of viscosity,
io temperature-dependence of viscosity, and density that make them
advantageous for use as petroleum lubricants. The use of phenyl-alkanes as
lubricants is described, for example, in the article by E. R. Booser in Kirk-
Othmer
Encyclopedia of Chemical Technology, Fourth Edition, Volume 15, John Wiley
and Sons, New York, New York, USA, 1995, pp. 463-517, to which reference is
is made for a description of such lubricants and their use.
In still another aspect, this invention is the use of the MABS compositions
produced by the processes disclosed herein as lubricant additives. It is
believed
that phenyl-alkane sulfonates, either in the form of normal salts or basic
salts of
phenyl-alkane sulfonic acids, produced as disclosed herein, have the ability
to
2o reduce or prevent deposits in engines operating at high temperatures. The
term
"normal salt" of an acid means a salt which contains the stoichiometric amount
of metal required for the neutralization of the acidic group or groups
present, and
the term "basic salt" means a salt which contains more metal than is required
for
the neutralization reaction. The excess metal in the form of basic salts is
25 believed to be capable of neutralizing oil oxidation combustion products
and
"blow-by" fuel combustion products. Phenyl-alkane sulfonates and their use as
lubricant additives, in particular as detergents, is described, for example,
in the
above-mentioned Booser article; in Lubricant Additives, by C. V. Smalheer and
R. K. Smith, The Lezius-Hiles Co., Cleveland, Ohio, USA, 1967, pp. 2-3; and in
30 the article by R. W. Watson and T. F. McDonnell, Jr., entitled "Additives-
The
Right Stuff for Automotive Engine Oils," in Fuels and Lubricants Technology:
An
-28-
CA 02479641 2008-01-15
Overview SP-603, Society of Automotive Engineers, Warrendale, Pennsylvania,
USA, October 1984, pp. 17-28.
The drawing shows a preferred arrangement for an integrated separation-
dehydrogenation-alkylation scheme of this invention. The following description
of the drawing is not meant to preclude other arrangements for the process
flow
of this invention and is not intended to limit this invention as set forth in
the
claims.
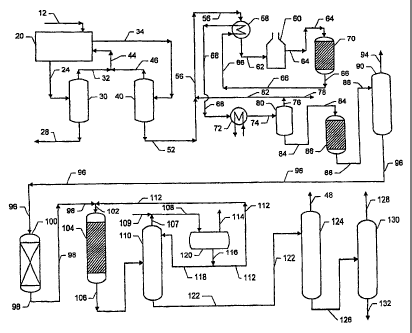
Referring now to the drawing, a line 12 charges a feed mixture comprising
an admixture of CIa-C13, including lightly branched paraffins, more highly
io branched paraffins, and normal (nonbranched) paraffins to an adsorptive
separation zone 20 which employs normal paraffin and/or cycloparaffin and/or
isooctane as the desorbent. A line 24 passes a raffinate stream comprising
more highly branched paraffins and a cycloparaffin or isooctane from the
adsorptive separation zone 20 to a raffinate column 30. The conditions of the
is raffinate column 30 produce an overhead stream 32 comprising desorbent
material and a bottom stream comprising more highly branched paraffins in a
line 28. Line 34 passes an extract stream comprising lightly branched
paraffins,
normal paraffins, and desorbent materials from adsorptive separation zone 20
to
an extract column 40. The extract column 40 recovers adsorbent materials in
20 line 46. The overhead streams in lines 32 and 46 combined recycle desorbent
via a line 44. The extract column 40 also produces a bottom stream comprising
lightly branched paraffins and normal paraffins in a line 52.
Line 52 admixes the bottom stream from the extract column 40 with
recycled hydrogen from a line 82 to form a mixture of paraffins and hydrogen
25 that flows through a line 56. An indirect heat exchanger 58 heats the
contents of
line 56 that pass via a line 62 to a fired heater 60. A line 64 carries the
heated
stream to a dehydrogenation reactor 70. Dehydrogenation reactor 70 contacts
the paraffins with a dehydrogenation catalyst at conditions that effect
significant
conversion of the paraffins to the corresponding olefins. Lines 66 and 68 cool
a
30 dehydrogenation reactor effiuent stream comprising a mixture of hydrogen,
paraffins, monoolefins including lightly branched monoolefins, diolefins, C9-
-29-
CA 02479641 2004-09-16
WO 03/082783 PCT/US02/09310
minus hydrocarbons, and aromatic hydrocarbons by passage serially through
heat exchangers 58 and 72. This cooling condenses substantially all of the C4-
plus hydrocarbons into a liquid phase stream and separates the liquid phase
stream from the remaining hydrogen-rich vapor. Line 74 passes the
dehydrogenation reactor effluent to vapor-liquid separation vessel 80 that
divides it into a hydrogen-rich vapor phase stream removed through a line 76
and a dehydrogenation product stream removed through a line 84. Line 76
supplies recycle hydrogen to line 82 and line 78 recovers a net hydrogen
product stream.
io Line 84 passes the bottom of the separation vessel 80, containing normal
paraffins, lightly branched paraffins, normal monoolefins, lightly branched
monoolefins, Cg-minus hydrocarbons, diolefins, aromatic by-products, and some
dissolved hydrogen, to a selective hydrogenation reactor 86. Selective
hydrogenation reactor 86 contacts the dehydrogenated product stream with a
is selective hydrogenation catalyst at conditions that convert a significant
amount
of the diolefins to the corresponding monoolefins and uses dissolved hydrogen
in the dehydrogenated product stream and/or additional make-up hydrogen (not
shown) for the conversion. Line 88 carries a selective hydrogenation reactor
effluent stream comprising a mixture of hydrogen, normal paraffins, lightly
20 paraffins, normal monoolefins, lightly branched monoolefins, Cg-minus
hydrocarbons, and aromatic by-product hydrocarbons to a stripping column 90.
Stripping column 90 recovers a net overhead stream in line 94 that comprises
the Cg-minus hydrocarbons produced in the dehydrogenation reactor as by-
products and any remaining dissolved hydrogen. are separated from the and
25 concentrated into a net overhead stream removed from the process through a
line 94.
A line 96 passes the remaining Clo-plus hydrocarbons from stripping
column 90 to an aromatics removal zone 100 that contacts this effluent with an
adsorbent to remove the aromatic by-products. Line 98 transfers from the
3o aromatics removal zone 100 an effluent comprising an admixture of the
normal
paraffins, lightly branched paraffins, normal monoolefins, and lightly
branched
monoolefins, and which has a greatly reduced concentration of aromatic by-
-30-
CA 02479641 2004-09-16
WO 03/082783 PCT/US02/09310
products compared to the stripping effluent stream. After addition of benzene
via a line 112 a line 102 passes the admixture into an alkylation reactor 104
where the admixture contacts an alkylation catalyst at alkylation-promoting
conditions to produce phenyl-alkanes.
A line 106 carries the alkylation reactor effluent stream into a benzene
fractionation column 110 by a line 106. This stream comprises an admixture of
benzene, normal paraffins, lightly branched paraffins, and MAB. The phenyl-
alkanes characterize the MAB which in addition to the phenyl portion have one
aliphatic alkyl portion with either 1 or 2 primary carbon atoms, or with 2, 3,
or 4
io primary carbon atoms and no quaternary carbon atoms except for quats.
Column 110 separates the effluent into a bottom stream and an overhead
stream comprising benzene and possibly light gases. A line 107 combines the
overhead stream of line 107 with make-up benzene from a line 109 into a fine
108 that passes the combined stream to a separator drum 120. A line 114
removes any noncondensed light gases from drum 120 while a line 116 supplies
condensed liquid as reflux to column 110 via a line 118 and supplies benzene
for recycle by a line 112. A line 122 carries the remainder of the alkylation
effluent stream from column 110 to a paraffin column 124 from which a line 48
withdraws an overhead stream containing a mixture of paraffins and generally
less then 0.3 wt-% monoolefins. A line 126 takes the paraffin column bottom
stream containing the phenyl-alkanes and heavy alkylate by-products to a rerun
column 130 that separates paraffin column bottom stream into a bottom stream
132 comprising heavy alkylate and an overhead alkylate product stream 128
containing the phenyl-alkane compounds. Sulfonation of the phenyl-alkane
compounds in the overhead alkylate product stream 128 will produce phenyl-
alkane sulfonic acids, which can be neutralized.
EXAMPLE 1
A "pulse test" procedure tests adsorbents with a particular feed mixture and
desorbent material to measure adsorptive capacity, selectivity, resolution and
exchange rate of the adsorbent. The basic pulse test apparatus consists of a
tubular adsorbent chamber of approximately 70 cc volume having an inlet and
-31-
CA 02479641 2004-09-16
WO 03/082783 PCT/US02/09310
outlet at opposite ends of the chamber. The chamber is contained within a
temperature control means and pressure control equipment maintains the
chamber at a constant predetermined pressure. Attachment of quantitative and
qualitative analytical equipment such as refractometers, polarimeters and
chromatographs to an outlet line of the chamber and will detect quantitatively
and/or determine qualitatively one or more components in the effluent stream
leaving the adsorbent chamber. A pulse test begins by passing the desorbent
material through the adsorbent chamber to fill the adsorbent to equilibrium
with a
particular desorbent material, then injecting a pulse of the feed mixture,
lo sometimes diluted in desorbent, for a duration of one or more minutes and
then
resuming desorbent flow elute the feed mixture components as in a liquid-solid
chromatographic operation. The effluent can be analyzed on-stream and/or
effluent fractions can be collected and later analyzed separately to permit
plotting
the traces of the envelopes of corresponding component peaks in terms of
component concentration versus quantity of effluent.
From information derived from the pulse test the adsorbent/desorbent
system performance can normally be rated in terms of retention volume for an
extract or a raffinate component, selectivity for one component with respect
to the
other, stage time, the resolution between the components and the rate of
2o desorption of an extract component by the desorbent. The distance between
the
center of the peak envelope of an extract or a raffinate component and the
peak
envelope of a tracer component or some other known reference point will
determine retention volume of an extract or a raffinate in terms of the volume
in
cubic centimeters of desorbent pumped during the time interval corresponding
to
the distance between the peak envelopes.
Table 1 lists variables and results of small scale "pulse tests" performed
to evaluate various desorbents and conditions on several feed mixtures. The
materials labeled Raffinate A and B are the raffinate streams of commercial
adsorptive separation units which recover normal paraffins from a C,o - C14
3o hydrocarbon fraction. The desorbent column in Table 1 indicates the volume
percent of each component of the desorbent as specified by the footnotes.
-32-
CA 02479641 2004-09-16
WO 03/082783 PCT/US02/09310
Table 1
Run No. Feed Mixture Temp, C Desorbent *
9577-77 Raffinate A 150 50 C5/50 N6
9577-85 Raffinate A 100 50 C5/50 N6
9937-01 Raffinate A 150 100 C6
9937-06 Raffinate A 150 70 C6/30 N6
9937-17 Raffinate A 150 50 C6/50 N6
9937-25 Raffinate B 150 50 C6/50 N6
9953-06 Kerosene 150 50 C6/50 N6
*C5 indicates cyclopentane
C6 indicates cyclohexane
N6 indicates n-hexane
All tests used the adsorbent comprised 80% silicalite and 20% silica binder
and
the chromatographic column having an adsorbent volume of 70 ml. The flow
rate through the column was 1.21 cc/min.
io In view of the very large number of different compounds in the feed
mixture pulse and the limitations inherent of the simple pulse test procedure
the
effectiveness of the separation was determined by collecting fractions of the
effluent every two minutes and analyzing each fraction. The initial fractions
had
high concentrations of desorbent and were followed by fractions having high
concentrations of the more highly branched nonnormal hydrocarbons. The
desired acyclic hydrocarbons having only 3 primary carbon atoms (i.e.,
monomethyl hydrocarbons) tended to concentrate in the fractions collected at
the end of the pulse. Table 2 gives the concentration (wt percent) of acyclic
paraffins having only 3 primary carbon atoms (i.e., monomethyl paraffins)
present in several different fractions of Run No. 9937-06.
-33-
CA 02479641 2004-09-16
WO 03/082783 PCT/US02/09310
Table 2
Acyclic Paraffins Having Only 3
Fraction No. Primary Carbon Atoms, %
18 34
19 48
20 60
22 55
24 60
26 77
28 77
32 79
38 96
Run No. 9937-06 combined liquid collected as fractions No. 19 to 100.
Analysis found the combined liquid to contain the weight percentages of
different
structural classes of compounds on a desorbent free basis as shown in Table 3.
Table 3
acyclic paraffins having only 3 primary carbon 64%
atoms (monomethyl branched)
acyclic paraffins having only 4 primary carbon 2.7%
atoms (dimethyl branched)
acyclic paraffins having only 2 primary carbon 4.6%
atoms (normal paraffins)
Aromatics 4.1%
Naphthenes 13.9%
Unknowns 10.7%
io
Run No. 9937-17 combined liquid collected as fractions No. 23 to 50.
Analysis found the liquid to contain the weight percentages of different
structural
classes of compounds shown in Table 4:
-34-
CA 02479641 2008-01-15
Table 4
acyclic paraffins having only 3 primary carbon 77%
atoms (monomethyl branched)
acyclic paraffins having oniy 4 primary carbon 0.1%
atoms (dimethyl branched)
acyclic paraffins having only 2 primary carbon 9.6%
atoms (normal)
Aromatics 4.1%
Naphthenes 3.8%
Unknowns 4.8%
Analysis of a sample formed by combining liquid from fractions 23 to
48 of Run 9953-6 found 67% acyclic paraffins having only 3 primary carbon
atoms (i.e., monomethyl branched compounds) and 9.3% acyclic paraffins having
only 2 primary carbon atoms (i.e., normal paraffins).
In comparing this data to the performance normally desired in
io commercial separations requires recognition that better selectivity will
result from
optimization in terms of adsorbent composition, desorbent composition and
operating conditions. Further the use of simulated moving bed (SMB)
technology or even better batch separation technology will improve the
performance of the process.
EXAMPLE 2
This example subjects a representative mixture of C,o pure components to a
pulse test procedure that uses of a pre-pulse of C. isoparaffin. The test used
a feed
mixture containing equal volumes of 3,3,5-trimethylheptane, 2,6-
dimethyloctane, 2-
methyinonane, normal decane, and 1,3,5-trimethylbenzene (1,3,5 TMB) in pulse
test
column having a volume of 70 cc and temperature maintained at
-35-
CA 02479641 2004-09-16
WO 03/082783 PCT/US02/09310
120 C (248 F). The flow rate through the column was 1.1 cc/min. The test used
a silicalite adsorbent and a 70/30 volume % mixture of normal heptane and
isooctane as desorbent. The test injected a pre-pulse of 40 ml isooctane into
the test loop immediately before the feed mixture injection.
Figure 2 graphically represents the results in a plot of the relative
concentrations of the components versus time, as measured by the volume of
collected effluent. Figure 2 shows a useful separation between the monomethyl
paraffin and the normal paraffin on the one hand and the di- and tri-methyl
paraffins on the other hand. Although the use of the pre-pulse is believed to
io improve the separation of the monomethyl paraffin band in the effluent, a
useful
separate band of the monomethyl paraffin is produced as a result of the
presence of isooctane in the desorbent, even in the absence of a pre-pulse.
EXAMPLE 3
An olefinic stream was used.
Table 5: Composition of Olefinic Stream
Olefin Component Content wt-%
Li hts 0.64
Linear olefins 30.11
6-methyl undecene 7.66
5-methyl undecene 15.33
4-methyl undecene 11.82
3-methyl undecene 12.95
2-methyl undecene 8.87
Other alkyl olefins 9.05
Heavies 3.53
Total 100
1 Lights include olefins having fewer than 12 carbon atoms.
2 Linear olefins include C12 linear olefins.
3 Other alkyl olefins include dimethyl, trimethyl, and other C12 olefins
4 Heavies include C12 olefin dimers and trimers.
Mixture of an olefinic stream comprising a blend of monomethyl C12
olefins and having the composition shown in Table 5 with benzene produced a
combined stream consisting of 93.3 wt-% benzene and 6.7 wt-% olefinic stream,
-36-
CA 02479641 2005-05-16
which corresponds to a molar ratio of benzene per olefin of 30:1. A
cylindrical
reactor with an inside diameter of 0.875 in (22.2 mm), was loaded with 75 cc
(53.0 g) of a mordenite-alumina extruded catalyst prepared from the hydrogen
form of a mordenite having a SiO2/AI203 of 18.
The combined stream passed to the reactor and contacted the extrudate
at a LHSV of 2.0 hr'', a total pressure of 500 psi(g) (3447 kPa(g)), and a
reactor
inlet temperature of 125 C (257 F). At these conditions, the reactor lined out
over a period of 24 hours and then a selective liquid product was collected
over
the period of the next 6 hours.
io The selective liquid product was analyzed by 13 C nuclear magnetic
resonance (NMR) in order-to determine the selectivity to 2-phenyl-alkanes and
end quaternary phenyl-alkanes. The NMR analytical method typically consists
of diluiting a 0.5 g sample of phenyl-alkane mixture to 1.5 g with anhydrous
deuterated chloroform, mixing a 0.3 milliliter aliquot of the diluted phenyl-
alkane
mixture with 0.3 milliliter of 0.1 M chromium (111) acetylacetonate in
deuterated
chloroform in a 5 mm NMR tube, adding A small amount of tetramethylsilane
(TMS) to the mixture as a 0.0 ppm chemical shift reference and running the
carbon spectrum on a Bruker ACP-300 FT-NMR spectrometer, available from
Bruker Instruments, Inc., Billerica, Massachusetts, USA at a field strength of
2o 7.05 Tesla or 75.469 MHz in a 5 mm QNP probe with a sweep width of 22727
Hz (301.1 ppm) to collect 65000 data points.. The quantitative carbon spectrum
is obtained using gated on-acquisition 'H decoupling (inverse gated
decoupling).
The quantitative 13C spectrum is run with 7.99 microsecond (90 ) pulses, 1.442
second acquisition time, a 5 second delay between pulses, a decoupler power,
using composite pulse decoupling (CPD), of 18H with a pulse width of 105
microseconds (90 ) and at least 2880 scans. The number of scans depends on
whether benzene is stripped from the liquid product prior to taking the above-
mentioned 0.5 g sample. The data processing is done with The Bruker PC
software WINNMR-1 D* Version 6.0, does data scanning. During data
processing a line broadening of 1 Hz is applied to the data. Specific peaks
are
integrated in the region between 152 ppm and 142 ppm. Table 6 shows the 13C
NMR peak identifications of the chemical shifts of the benzylic carbon of the
'` Trade-mark .37-
CA 02479641 2004-09-16
WO 03/082783 PCT/US02/09310
phenyl-alkane isomers. The term "benzylic carbon" means the carbon in the ring
of the phenyl group bound to the aliphatic alkyl group.
Table 6: 13C NMR Peak Identifications
Chemical Shift of the Phenyl-alkane Isomer Type of Quat'
Benzylic Carbon (ppm)
149.6 2-methyl-2-phenyl End
148.3 4-methyl-2-phenyl NQ
m-methyl-m-phenyl, m>3 Internal
148.0 5-methyl-2-phenyl NO
m-methyl-2-phenyl, m>5 NQ
147.8 5-methyl-2-phenyl NQ
2-phenyl (linear) NQ
3-methyl-3-phenyl Internal
147.6 4-methyl-2-phenyl NQ
147.2 3-methyl-2-phenyl NO
146.6 3-methyl-2-phenyl NQ
146.2 - 146.3 m-methyl-4-phenyl, m#4 NQ
145.9 - 146.2 m-methyl-3-phenyl, m>5 NQ
145.9 3-phenyl (linear) NQ
' NQ = Nonquat
The peak at 148.3 ppm is identified both with 4-methyl-2-phenyl-alkanes and
with m-methyl-m-phenyl-alkanes (m>3). However, the presence of m-methyl-m-
phenyl-alkanes (m>3) at more than 1%, reveals them as a distinct peak at 0.03
io ppm upfield of the peak for the 4-methyl-2-phenyl-alkanes. The peak at
147.8
ppm is identified with the 2-phenyl-alkanes as shown in Table 6, with possible
interference from 3-methyl-3-phenyl-alkanes.
The end quaternary phenyl-alkane selectivity is computed by dividing the
integral of the peak at 149.6 ppm by the sum of the integrals of all of the
peaks
listed in Table 6, and multiplying by 100. The 2-phenyl-alkane selectivity can
be
-38-
CA 02479641 2005-05-16
estimated if the amount of internal quaternary phenyf-alkanes contributing to
the
peaks at 148.3 ppm and 147.8 ppm is less than 2%, as determined by the
hereinafter-described gas chromatography/mass spectrometry method. As a
first approximation, this condition is met when the sum of the integrals of
the
s 4-phenyl-alkane and 3-phenyl-alkane peaks at 146.2 - 146.3 ppm and 145.9 -
146.2 ppm (respectively) is small relative to the sum of the integrals of all
the
peaks from 145.9 ppm to 149.6 ppm and the end quaternary phenyl-alkane
selectivity is less than 10. In such case, the 2-phenyl-alkane selectivity is
computed by dividing the sum of integrals of the peaks from 149.6 to 146.6 ppm
to by the sum of the integrals of all of the peaks listed in Table 6, and -
multiplying
by 100.
Analysis by gas chromatography/mass spectrometry of the selective liquid
product also determines the selectivity to internal quaternary phenyl-alkanes.
The gas chromatography/mass spectrometry analytical method typically consists
15 of analyzing the liquid product by an HP 5890 Series Il*gas chromatograph
(GC)
equipped with an HP 7673*autosampler and an HP 597emass spectrometer
(MS) detector used with an HP Chemstationlo control the data acquisition and
analysis, both available from Hewlett Packard Company, Palo Alto, California,
USA. The GC has a 30 meter x 0.25 mm DB1 HT(df = 0.1 m) column or
2o equivalent obtained from J&W Scientific Incorporated, 91 Blue Ravine Road,
Folsom, Califomia, USA. Helium carrier gas at 15 psi(g) (103 kPa(g)) and 70 C
(158 F) passes in constant pressure mode as the injector temperature holds at
275 C (527 F). The transfer line and MS source temperatures are held at
250 C (482 F). An oven temperature program of 70 C (158 F) for 1 minute,
25 then to 180 C (356 F) at 1 C per minute (1.8 F per minute), then to 275 C
(527 F) at 10 C per minute (18 F per minute), then hold at 275 C (527 F) for 5
minutes is used. The HP Chemstation software tunes the MS with the software
set to standard spectra autotune. The MS detector is scanned from 50-550 Da
with a threshold = 50.
30 The concentrations of internal quaternary phenyl-alkanes in the selective
liquid product are determined (i.e., the selective liquid product is
quantitated)
' Trade-mark
-39-
CA 02479641 2004-09-16
WO 03/082783 PCT/US02/09310
using the method of standard addition. Chapter 7 of the book entitled, Samples
and Standards, by B. W. Woodget et al., published on behalf of ACOL, London
by John Wiley and Sons, New York, in 1987 provides background on this
method.
First, a stock solution of internal quaternary phenyl-alkanes is prepared
and quantitated by alkylating benzene with a monomethyl alkene using a
nonselective catalyst such as aluminum chloride. The stock solution comprising
this nonselective liquid product of this alkylation contains a blend of
internal
quaternary phenyl-alkanes. A standard GC methodology identifies the largest
io peaks corresponding to the stock solution and the concentrations of the
internal
quaternary phenyl-alkanes in the stock solution are determined (i.e., the
stock
solution is quantitated) using a flame ionization detector (FID). The
retention
times of the peaks for the internal quaternary phenyl-alkanes decrease as the
index m in the formula m-methyl-m-phenyl-alkane increases and as the number
is of carbon atoms in the aliphatic alkyl group of the internal quaternary
phenyl-
alkane decreases. The concentration of each internal quaternary phenyl-alkane
is computed by dividing the area of the peak of that internal quaternary
phenyl-
alkane by the sum of the areas of all of the peaks.
Next, a spiking solution of internal quaternary phenyl-alkanes is prepared
2o by diluting an aliquot portion of the stock solution with dichloromethane
(methylene chloride) to attain a nominal concentration of 100 wppm of one
particular internal quaternary phenyl-alkane of interest (e.g., 3-methyl-3-
phenyl
decane). The concentration of any other particular internal quaternary phenyl-
alkane in the spiking solution may be greater or less than 100 wppm, depending
25 on the concentration of that internal quaternary phenyl-alkane in the stock
solution.
Third, a sample solution is prepared by adding 0.05 g of an aliquot portion
of the selective liquid product to a 10 milliliter volumetric flask and then
diluting
its contents with dichloromethane up to the 10 milliliter mark.
30 Fourth, a resultant solution is prepared by adding 0.05 g of an aliquot
portion of the selective liquid product to a 10 milliliter volumetric flask
and
-40-
CA 02479641 2005-03-14
diluting its contents with spiking solution up to the 10 milliliter mark to
dilute the
contents.
Both the sample solution and the resultant solution are analyzed by
GC/MS under the above-described conditions. Table 7 lists the extracted ions
from the full MS scan, plotted, and integrated using the HP Chemstation
software. The HP Chemstation software determines the individual extracted
ion peak areas that correspond to the internal quats listed in Table 7.
TABLE 7:
io Ratio of Mass to Charge of Ion for Peaks of Extracted"Ions
Internal Number of Carbon Ratio of Mass to Charge
Quaternary Atoms in Aliphatic (m/z) of Two Extracted
Phenyl-Alkane Alkyl Group of the Ions Corresponding to
Internal Quaternary Internal Quaternary
Phenyl-Alkane Phenyl-Alkane
11 133 and 203
3-methyl-3-phenyl 12 133 and 217
13 133 and 231
11 147 and 189
4-methyl-4-phenyl 12 147 and 203
13 147and217
11 161 and 175
5-methyl-5-phenyl 12 161 and 189
13 161 and 203
The concentration of each internal quaternary phenyl-alkane in Table 7 is
computed using the following formula:
-41-
CA 02479641 2004-09-16
WO 03/082783 PCT/US02/09310
Ai
C=S
A2 - Ai
where
C = concentration of internal quaternary phenyl-alkane in sample solution,
wt-%
S = concentration of internal quaternary phenyl-alkane in spiking solution,
wt-%
A, = peak area of internal quaternary phenyl-alkane in sample solution,
area units
A2 = peak area of internal quaternary phenyl-alkane in resultant solution,
area units
Using consistent units the concentration of each internal quaternary phenyl-
alkane in the selective liquid product is computed from the concentration of
that
internal quaternary phenyl-alkane in the sample solution by accounting for the
dilution effect of the dichloromethane in the sample solution. In this manner,
the
is concentration in the selective liquid product of each of the internal
quaternary
phenyl-alkanes in Table 7 is computed. The total concentration of internal
quaternary phenyl-alkanes in the selective liquid product, C,QPA, is computed
by
summing the individual concentrations of each of the internal quaternary
phenyl-
alkanes in Table 7.
The selective liquid product may contain internal quaternary phenyl-
alkanes other than those listed in Table 7, such as m-methyl-m-phenyl-alkanes
where m > 5, depending on the number of carbon atoms in the aliphatic alkyl
groups of the phenyl-alkanes. It is believed that, with the C12 olefinic
stream and
the conditions of this example, the concentrations of such other internal
quaternary phenyl-alkanes are relatively low compared to those of the internal
quaternary phenyl-alkanes listed in Table 7. For this example, the total
concentration of internal quaternary phenyl-alkanes in the selective liquid
-42-
CA 02479641 2004-09-16
WO 03/082783 PCT/US02/09310
product, CIQPA, is computed by summing only the individual concentrations of
each of the internal quaternary phenyl-alkanes in Table 7. However, if the
olefinic stream had comprised olefins having, say, up to 28 carbon atoms, then
the total concentration of internal quaternary phenyl-alkanes in the selective
liquid product, CIQPA, would be computed by summing individual concentrations
of m-methyl-m-phenyl-alkanes, where m is from 3 to 13. In more general terms,
if the olefinic stream contains olefins having x carbon atoms, then the total
concentration of internal quaternary phenyl-alkanes in the selective liquid
product, CIPQA, is computed by summing individual concentrations of m-methyl-
io m-phenyl-alkanes where m is from 3 to x/2. Without undue experimentation at
least one peak with a ratio of mass to charge (m/z) of an extracted ion
corresponding to each internal quaternary phenyl-alkane is identifiable, so
that
the concentration of all internal quaternary phenyl-alkanes may be determined
and then summed to arrive at C,QPA.
is The selectivity to internal quaternary phenyl-alkanes in the selective
liquid
product is computed using the following formula:
Q=100 CIQPA
C MAB
where
Q= selectivity to internal quaternary phenyl-alkanes
20 CIQPA = concentration of internal quaternary phenyl-alkanes in selective
liquid product, wt-%
CMAB = concentration of modified alkylbenzenes in selective liquid product,
wt-%
The concentration of modified alkylbenzenes, CMAB, in the selective liquid
25 product is determined using a gas chromatography method to find the
concentration of impurities in the selective liquid product. In the
determinination
of CMAB, "impurities" means components of the selective liquid product that
lie
outside a specific retention time range used in the gas chromatography method.
-43-
CA 02479641 2005-05-16
"Impurities" generally includes benzene, some dialkylbenzenes, olefins,
paraffins, etc.
A gas chromatography method determines the amount of impurities from
the selective liquid product. Equivalent equipment, equivalent sample
preparation, and equivalent GC parameters that are different but that produce
equivalent results to those described below may also be used to determine the
amount of impurities in the selective liquid product.
Equipment:
io = Hewlett Packard Gas Chromatograph HP 5890 Series II equipped with
a split/splitless injector and flame-ionization detector (FID)
= J&W Scientific capillary column DB-1 HT, 30 meter length, 0.25 mm
inside diameter, 0.1 micro-meter film thickness, catalog no. 1221131.
= Restek Red lite*Septa 11 mm, catalog no. 22306. (Available from
Restek Corporation, 110 Benner Circle, Bellefonte, Pennsylvania,
USA).
= Restek 4 mm Gooseneck inlet sleeve with a carbofrit, catalog no.
20799-209.5.
= 0-ring for inlet liner Hewlett Packard, catalog no. 5180-4182.
= J. T. Baker HPLC grade methylene chloride, catalog no. 9315-33, or
equivalent. (Available from J. T. Baker Co., 222 Red School Lane,
Phillipsburg, New Jersey, USA).
= 2 ml gas chromatograph autosampler vials with crimp tops, or
equivalent.
Sample Preparation:
= Weigh 4-5 mg of sample into a 2 ml GC autosampler vial.
= Add 1 ml methylene chloride to the GC vial; seal with 11 mm crimp
vial Teflor*iined closures (caps), HP part no. 5181-1210 (available
from Hewlett Packard Company), using crimper tool, HP part no.
8710-0979 (available from Hewlett Packard Company); and mix well.
= The sample is now ready for injection into the GC.
* Trade-mark -44-
CA 02479641 2004-09-16
WO 03/082783 PCT/US02/09310
GC Parameters:
= Carrier gas: hydrogen.
= Column head pressure: 9 psi.
= Flows: column flow, 1 mI/min; split vent, 3 mI/min; septum purge, 1
mI/min.
= Injection: HP 7673 Autosampler, 10 microliter syringe, 1 microliter
injection.
= Injector temperature: 350 C (662 F)
= Detector temperature: 400 C (752 F)
= Oven temperature program: initial hold at 70 C (158 F) for 1 minute;
heating rate of 1 C per minute (1.8 F per minute); final hold at 180 C
(356 F) for 10 minutes.
This gas chromatography method requires two standards freshly distilled
to a purity of more than 98 mol-% and generally comprising a 2-phenyl-alkane.
The "light standard" of the 2-phenyl-alkane has at least one fewer carbon atom
in its aliphatic alkyl group than that of the olefin in the olefinic stream
charged to
the alkylation zone that has the fewest number of carbon atoms. The "heavy
standard" of the 2-phenyl-alkane standard has at least one more carbon atom in
its aliphatic alkyl group than that of the olefin in the olefinic stream
charged to
the alkylation zone that has the most number of carbon atoms. For example, if
the olefins in the olefinic stream charged to the alkylation zone have from 10
to
14 carbon atoms, then the suitable standards include 2-phenyl-octane as the
light standard and 2-phenyl-pentadecane as the heavy standard.
Gas chromatography methods using the conditions specified above
determine retention time of each standard, and the two standard retention
times
in turn define a retention time range. Then, an aliquot sample of the
selective
liquid product is analyzed by the gas chromatography method using the above
conditions. If more than 90% of the total GC area is within the retention time
3o range, then the impurities in the selective liquid product are deemed to be
not
more than 10 wt-% of the selective liquid product, and, for the sole purpose
of
-45-
CA 02479641 2004-09-16
WO 03/082783 PCT/US02/09310
computing the selectivity to internal quaternary phenyl-alkanes, CMAB is
assumed
to be 100 wt-%.
If the percent of the total GC area within the retention time range is not
more than 90%, then the impurities in the selective liquid product are deemed
to
be more than 10 wt-% of the selective liquid product. In this case, to
determine
CMqg, impurities are distilled from the selective liquid product by adding
2200 to
2300 g of the selective liquid product to 5-liter, 3-necked round bottom flask
with
24/40 joints and containing a magnetic stir bar and a few boiling chips. A 9-
1/2
inch (24.1 cm) long Vigreux condenser with a 24/40 joint is placed in the
center
io neck of the flask and a water cooled condenser fitted with a calibrated
thermometer attaches to the top of the Vigreux. A vacuum receiving flask
attaches to the end of the condenser. A stopper occupies one side arm of the
flask and a calibrated thermometer extends from the other side arm. Aluminum
foil wrapping surrounds the flask and the Vigreux. A vacuum line applies
vacuum
to the receiving flask as the selective liquid product is stirred in the flask
and
upon reaching maximum vacuum (at least 25.4 mm Hg by gauge or less), an
electric heating mantle heats the selective liquid product.
After the heating begins "fraction A" is first collected from 25 C (77 F) to
the light standard boiling point temperature at the pressure at the top of the
Vigreux, as measured by the calibrated thermometer at the top of the Vigreux.
"Fraction B" is then collected from the temperature of the light standard
boiling
point to the heavy standard boiling point temperature both measured by the
calibrated thermometer at the top of the Vigreux for the pressure at the top
of
the Vigreux. Low-boiling fraction A and high-boiling pot residues are
discarded.
Fraction B contains the modified alkylbenzenes of interest, and is weighed.
Appropriate temperatures for collecting fractions A and B can be determined
from the article written by Samuel B. Lippincott and Margaret M. Lyman,
published in Industrial and Engineering Chemistry, Vol. 38, in 1946, and
starting
at page 320. Using the Lippincott et al. Article.
Next, an aliquot sample of fraction B is analyzed by the gas
chromatography method using the above conditions. If more than 90% of the
-46-
CA 02479641 2004-09-16
WO 03/082783 PCT/US02/09310
total GC area for fraction B is within the retention time range, then the
impurities
in fraction B are deemed to be not more than 10 wt-% of the selective liquid
product, and, for the sole purpose of computing the selectivity to internal
quaternary phenyl-alkanes, CMAB is computed by dividing the weight of fraction
B
collected by the weight of the aliquot portion of the selective liquid product
charged to the 5-liter flask in the above distillation method. If the percent
of the
total GC area for fraction B within the retention time range is less than 90%,
then
the impurities in fraction B are deemed greater than 10 wt-% of fraction B and
impurities removal again follows using the above distillation method to
separate
io a low-boiling fraction C and pot residues from a fraction D containing the
modified alkylbenzenes of interest. Fraction D is recovered, weighed, and
analyzed by the gas chromatography method to determine if it meets the same
90% criteria for the total GC area for fraction within the retention time
range. If
so, CMAB is computed by previously described procedure. If not, the
distillation
and gas chromatography methods are repeated for fraction D.
The above-described distillation and gas chromatography methods can
be repeated until a fraction containing the modified alkylbenzenes of interest
and
having less than 10 wt-% impurities is collected, provided that sufficient
quantity
of material remains after each distillation for further testing by these
methods.
2o Then, once CMAB is determined, the selectivity to internal quaternary
phenyl-
alkanes, 0, is computed using the above formula.
The results of these analyses are shown in the Table 8:
Table 8: Liquid Product Analysis
2-Phenyl-Alkane End Quaternary Phenyl- Internal Quaternary
Selectivity Alkane Selectivity Phenyl-Alkane
Selectivity
82.0% 6.98% 1.9%
In the absence of shape selectivity, most of the 2-methyl undecene would
be expected to form 2-methyl-2-phenyl undecane (that is, an end quat).
-47-
CA 02479641 2004-09-16
WO 03/082783 PCT/US02/09310
Likewise, most of the 6-methyl undecene, 5-methyl undecene, 4-methyl
undecene, and 3-methyl undecene would be expected to form internal quats.
The linear olefins would be expected to produce a statistical distribution of
2-
phenyl-dodecane, 3-phenyl-dodecane, 4-phenyl-dodecane, 5-phenyl-dodecane,
and 6-phenyl-dodecane. Thus, if the lights, the heavies, and the other alkyl
olefins listed in Table 5 are excluded from the computations, the 2-phenyl-
alkane selectivity would be no greater than 17 and the internal quaternary
phenyl-alkane selectivity would approach 55. Table 8 shows that the 2-phenyl-
alkane selectivity is significantly higher than expected in the absence of
shape
io selectivity and that the internal quaternary alkylbenzene selectivity
obtained
using the mordenite catalyst is much less than the internal quaternary
alkylbenzene selectivity that would be expected in the absence of shape
selectivity.
-48-