Note: Descriptions are shown in the official language in which they were submitted.
CA 02481836 2005-07-29
. APPARATUS FOR DRYING DIP-COATED MEDTCAMNNTS
This application is a division of PCT International
Application No. PCT/US98/13575 bearing Canadian Application Serial
No. 2,266,445 with the international filing date of July 1, 1998.
FI$LD OF TFIB INVgNTION
The present invention relates to a process and
apparatus f or the gelatin coating of medicaments.
BACKGROUND OF INVENTION
Many products, including prescription drugs, over the
counter drugs (e. g., analgesics) and vitamins, come in a solid
dosage (i.e., "medicament") form. Two common shapes for these
medicaments are referred to as "tablets" and "caplets".
Tablets are generally disc-shaped having a diameter that is
greater than their height. Caplets are elongated shapes having
a longitudinal axis that is greater than the greatest thickness
of the medicament along the longitudinal axis, typically by
approximately 2.5 times. Both usually include rounded ends and
edges and a flat surface corresponding to the walls of the die
in which the mixture of ingredients are pressed into the
particular solid dosage form. The flat area is sometimes
referred to as a sidewall and can form a corner or edge
relative to the two sides on opposite sides of the sidewall.
A common problem with both caplets and tablets
(collectively "medicaments") is the texture or feel of their
surfaces. Without any outer coating, both forms have a
"chalky" texture formed by the compressed mixture. Research
has established that some people believe uncoated medicaments
are difficult to swallow. Research also has found, however,
that people believe that medicaments having a gelatin or
similar coating are significantly easier to swallow.
Accordingly, many such processes have been developed and are
known in the art.
One such process, disclosed in U.S. Patent No.
599,865, utilizes a bar or plate which has been coated with a
1
CA 02481836 2004-10-26
cleanly separable adhesive preparation, such as a combination
of beeswax and rosin. Medicaments to be coated are pressed
onto the adhesive and partially dipped into a coating mixture.
The medicaments may then be dried and pressed onto a second
adhesive coated bar or plate so that the remaining portions of -
the medicaments may be dipped.
U.S. Patent No. 540,538 discloses a machine for
dipping medicaments which utilizes a plate having countersunk
holes to retain tablets by the application of a vacuum through
the holes. The plate is placed over a vacuum box and
medicaments are placed in each hole. A vacuum is then applied
and the box and plate are inverted, thereby allowing the
medicaments to be dipped in a coating bath. Once the
medicaments have been dipped, the box is returned to its
upright position, the vacuum is removed and the plate is
manually removed, with the medicaments in place, to allow
drying of the coating. After drying, the medicaments may be
transferred onto a second plate with their uncoated sides
exposed by manually placing the second plate over the
medicaments contained an the first plate and flipping the two
plates over. The uncoated sides may then be coated as
described above.
Another process, disclosed in, Japanese Patent
Publication No. 41-13997, utilizes rigid tubes to retain
tablets on their ends by the application of a suction force
through the tube center. After dipping the tablets to
approximately their midpoints and drying, the tablets are
inverted and transferred onto opposing tubes. The vacuum is
then switched from the first set of tubes to the second thereby
exposing the uncoated portions of the tablets to be dipped. A
drawback of this system is that no means is provided to easily .
center the tablets on the tubes.
U.S. patent No. 2,373,721 refer:> to a system for
coating medicaments in which the medicaments are held over a
2
CA 02481836 2004-10-26
coating tank in an inverted orientation by suction tubes. Cups
slightly larger than the individual medicaments are then raised
from an initial position, submerged in the coating, to immerse
the individual medicaments in coating material contained within
the cups.
U.S. Patent No. 4,965,089 refers to the use of a
caplet holding plate having sets of caplet gripping collets on
both sides thereof. Caplets, initially disposed on one side of
the plate, are dipped in a gelatin coating on one end~then
pushed through the plate so that the other side of the caplet
may be coated. The disadvantages of such a system are that the
caplet holding plate is not suitable for coating tablets having
a height substantially less than their diameter. In addition,
the coated surface may be damaged when pushed through the
collet to the other side of the caplet holding plate.
U.S. Patent No. 5,228,916, refers to the use of
moveable vacuum tubes, which extend through a carrier plate, to
secure tablets to be dipped. Such a process is not, however,
well suited for coating caplets whose ends may not be readily
secured to a vacuum tube. In addition, the use of multiple
moveable vacuum tubes makes it difficult to maintain the
tablets centered on the vacuum tubes in a level plane parallel
with the surface of the coating in which the tablet is to be
dipped. It is, therefore, difficult to obtain a level
transition line when coating tablets with more than one colox
coating.
Therefore, there exists a need for a medicament
coating system which may be easily adapted to coat medicaments
of different sizes and shapes and which produces a uniform
coating with no damage to the finished product.
St7L~iARY OF THE IN~TE~ION
The present invention provides a method and apparatus
for coating medicaments to
3
CA 02481836 2004-10-26
produce a uniform, undamaged coating. Further, the present
invention provides a method and apparatus for coating
medicaments which may be easily adapted to coat medicaments
of different sizes and shapes. The present invention also
provides an apparatus to secure medicaments to a pallet in a
Level orientation such that the pallet may be inverted and
the medicaments dipped in a coating mixture. The present
invention also provides a method and apparatus to evenly and
quickly dry a coating once it has been applied~to a
medicament.
In accordance with a first aspect of the present
invention there is provided an apparatus comprising a
continuous conveyer system to advance a plurality of pallets
through the sequence of stations at wh~_ch the steps necessary
~5 to dip and coat each side of a medicament with a coating are
performed. The stations of the system include a loading
station, at least one dipping station, at least one dryer
station, a reorientation station and an unloading station.
Medicaments to be coated are, loaded into pallets with
a first portion of the medicaments exposed at the loading
station. The pallets are oriented in an upright position so
that the medicaments extend above the surface.of the pallet.
After a pallet is loaded, it proceeds along the conveyer to the
first dipping station. At the first dipping station, a vacuum
is applied to the pallet to secure the medicaments to the
pallet and the pallet to the end effector tooling of a robot
arm. The pallet is then inverted and the first exposed portion
of each of the medicaments is dipped into a bath to coat the
exposed end. The dipping step has a first dipping profile of
insertion rate and depth, dwell time in the bath, and removal
rate to control the coating on the medicament. Preferably, the
4
CA 02481836 2004-10-26
bath is a gelatin or a gelatinous bath for gel coating the
medicament.
After the dipping step, the pallet is returned to its
upright position on the conveyer, and the vacuum is removed.
The pallet is then advanced to the dryer station, where the
coating is dried. The dryer station includes a series of
conveyors similar to those which transport the pallets between
the different stations. The dryer conveyors transport the
pallets through at least one, and preferably two drying rooms.
The drying rooms have a controlled environment and vertical air
flow plenums over each conveyor section. The air plenums are
disposed horizontally over the path of the pallets and include
rows of air directing apertures, preferably in a sinusoidal
arrangement, running in the direction of product flow for
directing a flow of air onto the medicaments. When the pallets
exit the dryer station, the coating on the first exposed
portion of the medicaments has been fully dried.
The pallets are then passed to the reorientation
station where the partially coated.medicaments are transferred
from the first pallet to a second pallet to expose the uncoated
ends. The second pallet is identical to the first pallet. The
transfer is accomplished in the reorientation station by.
indexing,the first pallet under the second pallet, which is in
a face-down orientation. The two pallets are then urged
against a transfer plate to form a sandw:Lch, which is then
rotated 180° to invert the pallets so that the medicaments
free-fall downward from the first pallet,, through apertures in
the transfer plate, into the medicament :receptacles of the
second pallet, leaving a second portion of the medicaments
r
3D exposed. The first pallet, now empty and in a face down
orientation, remains in the reorientation station to be the
"second" pallet for the next full pallet entering the
reorientation station.
The second pallet, now loaded, is then ejected or
5
CA 02481836 2004-10-26
withdrawn from the reorientation station and advanced to a
second dipping station. The second dipping station operates in
the same manner as the first dipping station except that it may °
have a second dipping profile that is different from the first
dipping profile. Preferably, the dipping profiles are
identical. Thus, the second exposed portion of the medicaments
is dipped in the same manner as the first portion. Preferably,
the second dipping station has a bath of similar material to
the bath of the first dipping station, more preferably a
gelatin or gelatinous bath of the same color or a different
color as that of the first dipping station. It also should be
understood that the first and second dipping stations, in one
embodiment, could use a common bath and in-another embodiment,
could use baths of different types of materials.
After the second coating has been applied, the second
pallet is advanced through the dryer station in the same
direction as occurred after the first coating. At the exit of
the dryer station, the medicaments are fully dried and are sent
to the unloading station where the coated medicaments are
ejected from the pallet. It should be uncterstood that the
conveyor system can be a series or a plurality of conveyers
that transfer the pallets along and between the stations.
In a second aspect of the invention, an apparatus to
hold the medicaments to be coated is provided. In one
embodiment, the apparatus comprises pallets that include a
plate-like support member, on which rests a resilient and
deformable mat having a rectangular array of medicament
receptacles. The surface of the support member that abuts the
mat contains a matrix of very fine grooves that provide
channels for communication of a vacuum. These channels are
connected to centrally located holes or apertures, e.g., three,
extending through the support member. These hales can be
coupled to a vacuum source, e.g., in the end effector of a
robot arm capable of engaging the pallets and inverting them
6
CA 02481836 2004-10-26
for dipping in the bath.
One such mat is configured with receptacles for
medicaments that are tablets. This mat ha.s raised hollow
tubular portions that provide holes through which the vacuum
can act to hold tablets seated on the tubular portion. In this
embodiment, each tubular portion holds only one tablet at a
time by resting the tablet upon the top end portion of the
tube. The mat also includes portions that: project upwardly
from the mat, adjacent to and around the upwardly extending
tubular portions. These projections serve as rests and guides
to support and center each of the tablets on a tubular portion
in the absence of any vacuum holding the tablet thereon. These
projections are mounted on compliant diaphragm portions of the
mat. When the vacuum is applied to secure the tablet to the
tubular portion and hence the mat, the diaphragm portions flex
'and the support projections are deflected away from the tablet,
which remains centrally contained within 'the receptacle. When
the vacuum is removed, the diaphragms return to their normal
position and the projections will support the tablet so that it
will not fall off the tubular portion.
Another such mat is configured for medicaments that
are caplets. In this embodiment, the medicament receptacles of
the caplet.mat do not have holes through the mat, but instead,
include small compartments formed by raised portions in the
mat. These raised portions form the walls of the caphet-
holding compartments. These' walls are mounted on compliant
diaphragm portions of the mat such that when the vacuum is
applied, the diaphragms flex and the walls are deflected toward
the caplet contained within the receptacle. This action
results in securing the caplets to the mat. It also results in
straightening the caplets in the pallet, thereby to provide
them with a relatively uniform orientation for dipping.
In another aspect of the present invention, an
improved apparatus for drying the coated medicaments is
7
CA 02481836 2004-10-26
provided. The improved drying means inclue~es air directing
plates under which the medicaments to be dz~ied are passed.
Each air directing plate has at least one grow of air directing
apertures arranged in a sinusoidal layout running in the
direction of product flow. Preferably, the: air directing
apertures are directed normal to the plane of the pallets and
there is approximately one row of apertures corresponding to,
and parallel with, each row of medicaments on the pallets
passing thereunder. An air plenum is used to force air through
the air directing plates as the coated pallets pass thereunder.
This results in a wash of air that swirls around the coated
medicament as the medicament moves through the drying section,
and provides an effective drying of the coated medicaments,
particularly gelatin coated medicaments.
25 These and other features and advantages of the
present invention will be apparent and fully understood from
the following detailed description of they preferred
embodiments, taken in connection with the appended drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
The present invention can be better understood when
considered with the following drawings in 'which like reference
numerals refer to like elements and in which:
FIGURE 1 is a top view of the system for the dip
coating of medicaments in accordance with ~~ preferred
embodiment of the present invention
FIGURE 2 is an exploded perspective view of a pallet
in accordance with a preferred embodiment o f the present
invention;
FIGURE 3 is a top view of the support member of the
pallet of FIGURE 2;
FIGURE 3A is a partial top view of the grooves of the
support member of FIGURE 3;
FIGURE 3B is a partial cross-sectional view of the
8
CA 02481836 2004-10-26
grooves of the support member of FIGURE 3;
FIGURE 4 is a perspective view of a tablet mat and
support member in accordance with a preferred embodiment of the
present invention
FIGURE 5 is a top view of the tablet mat of the
pallet of FIGURE 4;
FIGURE 5 is a partial cross-sectional view of the
tablet mat and support member of FIGURE 4;
FIGURE 6A is a partial cross-sectional view of the
tubular portion of the tablet mat of FIGURE 6;
FIGURE 7 is a partial cross-sectional view of the
tablet mat and support member of FIGURE 4 with a vacuum being
applied by the end effector tooling in accordance with a
preferred embodiment of the present invention;
FIGURE 8 is a perspective view c>f a caplet mat and
support member in accordance with a preferred embodiment of the
present invention;
FIGURE 9 is a top view of the caplet mat of the
pallet of FIGURE 8;
FIGURE 10 is a partial cross-seta Tonal view of the
caplet mat and support member of FIGURE 8;
FIGURE 11 is a partial cross-sectional view of the
caplet mat_.and support member of FIGURE 8 with a vacuum being
applied by the end effector tooling in accordance with a
preferred embodiment of the present invention;
FIGURE 12 is a side elevational view of the loading
station of the present invention;
FIGURE 13A is a front elevational view of the caplet
drop tube of the present invention;
FIGURE 13B is a side elevational view of the caplet
drop tube of FIGURE 13A;
FIGURE 13C is a top view of the caplet drop tube of
FIGURE I3A;
FIGURE 14A is a partial cross-sectional top view of a
9
CA 02481836 2004-10-26
caplet being loaded into a caplet mat by a caplet drop tube of
the present invention;
FIGURE 14B is an elevational view of a caplet being -
loaded into a caplet mat by a caplet drop tube of the present
invention;
FTGURE 15A is a front elevational view of the tablet
drop tube of the present invention:
FIGURE 15B is a side elevational view of the tablet
drop tube of FIGURE 15A;
FIGURE 15C is a top view of the tablet drop tube of
FIGURE 15A;
FIGURE 16 is a top view of the dipping stations and
automatic drawdown system of the present invention;
FIGURE 17 is a top view of one of a dipping station
of the present invention:
FIGURE 28 is a top view of the capture station and
end effector tooling of FIGURE 17;
FIGURE 19 is a cross-sectional view of the end
effector tooling and centering plate used 1.o center caplets at
a dipping station of the present invention;.
FIGURE 20 is an cross-sectional view of caplets being
dipped at a dipping station of the present invention:
FIGURE 21 is an cross-sectional view of tablets being
dipped at a dipping station of the present invention;
FIGURE 22 is a partial top view of a dryer air plenum
plate of the present invention;
FIGURE 23 is a schematic~diagram of the process air
flow handling system of the present invention:
FIGURE 24 is a side elevational view of the
reorientation station of the present invention:
FIGURE 25 is a front cross-sectional view of the
reorientation station of FIGURE 24 configured for caplets;
FIGURE 26 is a front cross-sectional view of the
reorientation station of FIGURE 24 configured for tablets:
CA 02481836 2004-10-26
FIGURE 2? is a top view df the unloading station of
the present invention;
FIGURE 28.is a side elevational v~_ew of the unloading
station of FIGURE 27.; and
Figure 29 is a schematic process diagram of the
automatic drawdown system (ADS) of the present invention.
DETAILED DESCRIPTION OF THE PREFERRED E1~ODIMENT
The system for coating medicaments according to the
present invention comprises two major sections. One section,
the Carrier Handling System (CHS), is shown on the right side
of Figure 1 and the second section, known as the Automatic
Drawdown System (ADS), is shown on the left hand portion of
Figure 1.
In the preferred embodiment, the medicaments are dip
coated with a gelatinous or gelatin material, as is discussed
below. However, it should be understood that this invention is
applicable to dip coating medicaments with materials other than
gelatin and gelatinous materials, as will be appreciated by a
person of ordinary.skill in the art,
Further, in the preferred embodiment, the medicament
is preferably acetylsalicylic acid (commonly known as ASA and
by its trademark ASPIRIN) and preferably has been spray-coated
with a subcoating. It should, nevertheless, be 'understood that
the present invention is applicable to dip coating medicaments
having a composition other than ASA and the particular
disclosed subcoating. For example, other analgesics such as
acetaminophen, ibuprofen, ketoprofin and.combinations thereof,
alone or together with one or more other active ingredients
such as decongestants, antihistamines, expectorants, caffeine,
sleep-aides, as well as inactive ingredients and excipients, as
well as other over the counter and prescription or ethical
pharmaceutical products or nutritionals could be used.
The currently preferred subcoating is applied in a
conventional one-step spraying operation and consists of a
water soluble film former ~hydroxypropyl methylcellulose
11
CA 02481836 2004-10-26
(HPMC)), a slightly soluble plasticizer (triacetin), and a
hydrophobic surfactant (sorbitan trioleate). A suitable
formulation for the subcoating is given in Table 1.
TA6LF 1: SUBCOATING FORMUIATlON
Raw Material ~~ ~~ ~ of Raw Material
hydroxypropyl methylcelluiose (HPMC) 82.12
triacetin 16.42
sorbitan uioleate 1.4fi
After the subcoating has been applied, the
medicaments are "conditioned" by placing them in a_ bin and
exposing them to compressed air. In the preferred embodiment,
the bin is closed, but not sealed and includes one or more air
distribution wands to which a compressed air source may be
attached. Each distribution wand is essentially a pipe having
a plurality of holes along its length to distribute the
compressed air throughout the bin. The compressed air is at
ambient temperature and supplied to the bin so as to provide a
positive pressure in the bin. At the end of approximately 24
hours of exposure to the compressed air, the medicaments are
sampled arid the moisture content analyzed. Provided the
moisture content is verified to be below a predetermined level
(i.e., 1.5~) the medicaments are sent to the loading station to
be loaded onto the pallets as described below.
The Carrier Handling System (CHS) ,
The major components of the CHS, as illustrated in
Figure 1, are a loading station 10, a first dipping station 20,
a dryer station 30, a reorientation station 50, a second
dipping station 60, and an unloading station '~0. Medicaments
are transported between the above stations by a conveyer system
12
CA 02481836 2004-10-26
100. The conveyer system 100 is comprised of flexible plastic
links and is designed far 24 hour a day operation. Preferably,
the conveyor system 100 is actually a series of separate
conveyor belts that, together with the various operating
stations, form a continuous path. One suitable conveyor system
is to' use the known conveyor systems for transporting cases of
carbonated beverages.
The medicaments are carried on the conveyer system
140 by specially designed pallets 200 which are adapted to hold
the medicaments when inverted through the application of a
vacuum.
The Pallets
Referring to Figures 2-11, the pallets 200 used in
the system of the current invention to transport the
medicaments between stations include a plate-like support
member 210, on which rests a mat 270 (or Z40) having a
rectangular array of receptacles 272 (or 230) designed to
receive the medicaments. Because of the differences in
geometry, the tablet mats 240 (Figures 4-7) differ in design
from the caplet mats 270 (Figures 2 and 8-~11), but in both
cases the support member 2I0 is identical. In the preferred
embodiment,- the mats 240 and 270, are formed of rubber,
however, other resilient, impermeable materials may be used,
e.g., silicone.
Referring to Figures 2-3B, the support member 210 is
preferably a rectangular tray having a rim 212 around its
perimeter and a flat receiving surface 214 within the area
defined by the rim 212 for receiving the mat 240 or 270. The
mat 240 or 270 rests on the receiving surface 214 of the
support member 210. A rib 215 is integrally formed on the
receiving surface 214 of the support member 210 just inside the
rim 212. Rib 216 extends around the perimeter of the support
member 210 and fits within a corresponding sealing channel 232
13
CA 02481836 2004-10-26
formed in the mat to provide a substantially air tight seal
therebetween. (See Figures 6, 7, 10 and 11). It is important
that the mat 240 or 270 be seated squarely in support member '
210 so that the receptacles 230 or 272 are in a substantially
rectangular array of straight lines, and not curved, which
could adversely affect the ability to fill the pallet with
medicaments. This adjustment may be made by manual
manipulation of the mat.
The receiving surface 219 of the support member 210,
which abuts the mat 240 or 270, contains a matrix of very fine
grooves 218, which are formed in the process of molding the
support member 210. fihese grooves 218 provide channels for
comrnunication of a vacuum. As best seen in Figures 3-3B, the
grooves 218 of the preferred embodiment have a triangular
cross-section, are approximately .015'° deep and .030'° wide,
and
are in a cross-hatched arrangement with approximately .090"
between adjacent groves. The grooves 218 are connected to four
centrally located holes 220 which extend through the support
member 210 to which a vacuum may be applied.
In the preferred embodiment, the support members 210
are formed of a rigid plastic, such as polycarbonate, so as to
be dimensionally stable in the operating environment of the CHS
and to not~warp to any significant degree, despite repeated use
and thermal cycling. It should, however, be apparent to one
skilled in the art that other materials, such as steel or
aluminum, could be used. In addition, other groove
configurations and other means of communicating the vacuum
across the receiving surface of the support member may be used
so long as the support member 210 provides. support to the mats
at the required locations. One example of an alternative
embodiment would include raised members strategically placed on
the receiving surface of the support tray to allow deflection
of the diaphragm portions of the mat while, in the case of the
tablet mat, simultaneously supporting the tubular sections and
19
CA 02481836 2004-10-26
T
allowing the vacuum to be communicated through their center
apertures. (See description of mats 240 and 270 below),
The receptacles in the tablet mat 240, illustrated in
Figures 4-?, consist of raised tubular portions 242 that
provide holes 243 through which a vacuum can act. Each tubular
portion 242 holds only one tablet at a time by resting the
tablet upon the top end 244 of the tubular portion 242. (See
Figure 6.) The bottom end 246 of the tubular portion 242 rests
on the receiving surface 214 of the support member 210 and is
aligned with at least one of the grooves 218 therein.
Accordingly, when a vacuum is applied to the support member 210
at holes 220, it is communicated through the grooves 218 to the
tubular portions 242 of the mat 240 to secure the tablets 204
to the mat 240. (See Figure ?). Because the bottom ends 246
of tubular portions 242 are in contact with the receiving
surface 214 of the support member 210, the tubular portions 242
do not move in a direction perpendicular to the receiving
surface 214.
In the preferred embodiment, the tubular portions
have an outer diameter of .200" and an inner diameter of .120".
Thus, each tube wall is approximately .040" thick. At the top
end of each tubular portion, the inner .030" is angled inward
at 45°. (.See Figure 6A).
The tubular portions 242 of the mat 240 are
integrally connected to compliant diaphragm portions 250.of the
mat 240 at a point 248 between the bottom end 246 and the top
end 244 of the tubes 242. Thus, the compliant diaphragm
portions 250 are held at a distance away from the receiving
surface 214 of the support~member 210. (See Figures 6 and ?).
The compliant diaphragm portions 250 of the mat 240 are further
arranged so that each compliant diaphragm portion 250 is
aligned with at least one groove 218 in the receiving surface
214 of the support member 210.
The tablet mat 240 also includes guide portions 260
CA 02481836 2004-10-26
that project upwardly from the mat (i.e., a.way from the support
member 210), adjacent to the upwardly extending tubular
portions 242. These projections 250 serve as guides or
stabilizing elements to center each of the tablets 204 on one
of the tubular portions 242. Preferably, there are tour such
projections spaced at 90° around the tubular portions, although
more or fewer projections could be used. The spacing of the
guide portions 260 is determined by the sine and shape of the
tablets to be coated. In the preferred embodiment each group
of four projections defines a circular tablet receptacle with
an inner diameter of .436°'. In an alternate example, five
projections are used, particularly if the medicament has a
hexagonal shape.
To ensure compete coverage of the tablets 204 by the
coating, the guide portions 260 are mounted on the compliant
diaphragm portions 250 of the mat 240. Thus, as illustrated in
Figure 7, when the vacuum is applied to secure the tablets 204
to the tubular portion 242, the compliant diaphragm 250 is
simultaneously drawn toward the support member 210, thereby
deflecting the projections 260 radially away from the tablet
204 and somewhat downwardly. This action exposes a retained
tablet.
sn the illustrated embodiment, the tablet receptacles
230 are arranged in a plurality of rows 231 best seen in
Figures 4 and 5, each spaced approximately 0.743" center-to-
center from the adjacent row 231 of receptacles 230.
Receptacles 230 within a row 231 are spaced approximately
0.495" center-to-center from the adjacent receptacles. This
close spacing within each row 231 is accom.piished by forming
adjacent guide projections 250 as a single piece. It should,
therefore, be apparent that when deflected., the shared guide
projections 260 are deflected-primarily towards the adjacent
row 231 of receptacles 230.
The pallet used to transport the caplets is similar
16
CA 02481836 2004-10-26
x
to the pallet used for tablets. The caplet pallet employs an
identical plate-like support member 210, on which rests a
somewhat different mat 270 (shown in Figures 2 and 8-11) having
a rectangular array of receptacles 272. In this case, there
. 5 are no holes through the mat 270. Instead, the receptacles
consist of small compartments 272 formed by raised gripping
portions 280 formed on the top surface of the mat 2?0. These
gripping portions 280 form the walls of the caplet-holding
compartments 272.
In the preferred embodiment, the gripping portions
280 are formed by raised gripping ribs 282 which are integrally
formed with and run along the length of the caplet mat 270.
Pairs of adjacent gripping ribs.282 include a plurality of
gripping fingers 284, each gripping finger 289 being integrally
formed with one of the gripping ribs 282 and the mat 270,
extending toward a corresponding gripping finger 284 on the
opposing gripping rib 282. These gripping fingers 284 divide
the regions between adjacent gripping rib pairs 282 alternately
into caplet-holding compartments 272 and drop tube receiving
compartments 274.
Referring to Figures 10 and 11, each gripping portion
280 is integrally formed on a compliant diaphragm section 290
of the mat~2.70 which also forms the bottom section of the
caplet-holding compartment 272. These compliant diaphragm
portions 290 of the mat 270 are further arranged so that each
compliant diaphragm portion 290 is aligned with at least one
groove 218 in the receiving surface 219 of the support member
220.
The compliant diaphragm portion 290 of each caplet-
holding compartment is maintained at a distance from the
support member 210 by spacer ribs 299 integrally formed on the
support member facing surface o-f the mat 270. Thus, as shown
in Figure 11, when suction is applied to the pallet assembly,
the diaphragm 290 is drawn toward the support member 210 and
17
CA 02481836 2004-10-26
the raised gripping portions 280 are deflecaed radially inward
towards the caplet 200 contained within the caplet-holding
compartment 272, thereby frictionally engaging (i.e., '
"gripping°') the caplet 206. As a result, the array of caplets
206 in the pallet are straightened to have their longitudinal
axes substantially all normal to the pallet: receiving surface
214. This gives a uniform orientation and results in a
reasonably consistent dipping from pallet to pallet.
In the preferred embodiment, the spacer ribs 294 run
parallel to the gripping ribs 282 and are disposed between
adjacent gripping rib pairs, although they are located on the
opposite (i.e., support member facing) surface of the mat 270.
In addition, the preferred embodiment includes stop ribs 298
also integrally farmed on the support member facing surface of
the mat 270, parallel to the spacer ribs 299 and centrally
located below the caplet-holding compartments 272. These stop
ribs 298 have a lower profile than the spacer ribs 294 and,
therefore, do not contact the receiving surface 214 of the
support member 210 when the diaphragm 290 is in its undeflected
state as shown in Figure 10. However, when a vacuum is applied
and the diaphragm 290 is drawn toward the support member 210,
as illustrated in Figure 11, the stop rib 298 contacts the
receiving..surface 214 and prevents full deflection of the
diaphragm 290.
It can be observed that the caplet holding
compartments 272 of the illustrated embodiment, like the tablet
receptacles 230, .are arranged in a plurality of rows 271.
Further, as in the case of the tablet receptacle rows 231, the
caplet compartment rows 271 are each spaced approximately
0.743" center-to-center from the adjacent rows 271. This
permits the same dryer~section configuration to be used for
both caplets and tablets without modification of the dryer air
plenum plates 42 (described below). However, because the drop
tube receiving compartments 274 are located between adjacent
18
CA 02481836 2004-10-26
caplet compartments 272, compartments 272 within a row 271 are
spaced further apart than the tablet receptacles 230, for
example, approximately 1.5" apart center-to~-center for 500 mg
caplets .
In the case of both the tablets and the caplets, a
medicament is placed in each of the receptacles by using drop
tubes tdescribed below) which allow the medicaments to free-
fall into the receptacles under the force of gravity. The
dimensions of the receptacles are such that the caplets or
tablets become fully seated in a reasonably centered position.
After loading, a vacuum may be applied to the support member to
secure the mat to the support member and to secure the
medicaments to the mat, thus allowing the pallet to be inverted
without the medicaments falling out of the receptacles.
The Loading Station
Empty pallets are initially located on the conveyer
100 at the area labeled 102 in Figure d with the medicament
receptacle rows (232 or 271) oriented transverse to the
direction of travel, The pallets are then sent to loading
station 10 which is illustrated in more detail in Figure 12.
The loading station 10 preferably includes a. feed assembly 420
of the type designed for filling blister packaging With solid
dosage forms. Such a feed system is available from Aylward
Corp., New Bern, North Carolina. The feed assembly 420 is
modified for loading the particular dimensions of medicaments
in the desired orientation into the pallet mats utilizing an
array of modified drop tubes. Because of differences in the
geometry of the medicaments and their respective mats 240 and
270, the caplet drop tubes 300 differ in construction from the
tablet drop tubes 350, but in both cases each drop tube is long
enough to hold more than one medicament at a time.
The caplet drop tube mechanism 300, shown in Figures
13A-13C, is a tube of appropriate dimensions to hold caplets
19
CA 02481836 2004-10-26
206 in a vertical orientation, i.e.., with the longitudinal
caplet axis in a vertical orientation. At the top of the
caplet drop tube 300 are two guide flanges 302 mounted opposite
each other. These guide flanges 302 help align the caplet 206
properly as it enters the top of the drop tube 300. A pair of
holding pins 306 and 308 are located inside each caplet drop
tube 300 near the bottom, one spaced vertically above the
other, separated by a distance approximately equal to the
length of a single caplet 206. These holding pins 306 and 308
act in tandem to hold the caplets 206 in place, and to release
them one at a time into a pallet receptacle..
In operation, a pallet is first indexed under the
array of drop tubes 300 and a lifting device raises~the pallet
such that it engages the bottom of the drop tubes 300 to
I5 deposit one medicament in each receptacle, one medicament per
drop tube 300 at a time. In a preferred embodiment, the
lifting device is located below the conveyor_ and consists of a
plate with four locating pins in each corner. (Two pins are
located on each side of the conveyer). Bolted to the plate
with the pins are two Parker"'slide mechanisms so as to achieve
motion in two dimensions (Left to right and up and down). As a
pallet to be filled approaches the load station, a pallet stop
located behow the conveyor is triggered. From below the
conveyor, the pins are raised and guided into the holes at each
corner of the pallet. Upward motion continues, lifting the
pallet off of the conveyor until the pallet makes contact with
the bottom of the drop tubes 300.
In a preferred embodiment, the bottom of each caplet
drop tube 300 is shaped with chamfered portions 304. The
34 chamfered portions 304 contact the gripping ribs 282 of the
caplet mat 270 at the drag tube receiving compartments 274.
The chamfered portions 304 cause gripping ribs 282 (and
consequently the caplet-holding compartments 272) to spread
open slightly. (See Figures 14A-14B). This. provides for a
CA 02481836 2004-10-26
larger target for the caplet 206 to enter fully into the empty
caplet-holding compartment 272 in a desirable position and
orientation. When the pallet is lowered, the caplet 206 is
left behind in the receptacle 272.
Referring to Figure 22, caplets 206 initially located
in a supply hopper 400 are controllably released and allowed to
slide down a supply slide 410 onto a commercial sorter assembly
430, which sorts out partial or damaged caplets. One
TM
acceptable sorter 430 is the GMP model commercially available
from the Ackley Corp. From the sorter 430, the whole,
undamaged caplets 206 are sent down a second slide 432 to the
feed assembly 9'20, described above.
In the feed assembly 420, the guide flanges 302
located at the top ends of the drop tubes 300 protrude through
openings in a holding tray. The holding tray includes a number
of hills and valleys and is designed to reciprocate (oscillate)
up and down. This action causes the caplets 206 in the holding
tray to move about the holding tray and eventually fall into
the drop tubes 300 with the correct orientation. Accordingly,
it is important that the caplets 206 in the holding tray be
sufficient in number to fill all of the drop tubes 300, but not
so great in number to impede the motian of the caplets 206 in
the.~tray to permit the caplets 206 to fall into all of the drop
tubes 300. The caplets 206 that fall. into the drop tubes 300
2~ are then held in place by the holding pins 306 and 308.
Each drop tube 300 feeds tree caplets 206 one at a
time by first retracting the upper holding pin 306, thereby
allowing a caplet 206 to fall towards the lower holding pin
308, which is in its extended position. The upper holding pin
306 is then extended to block the next caplet 206 in the drop
tube 300 and the lower holding pin 308 is retracted, thereby
allowing the first caplet 206 to free-fall out of the tube 300
and into a caplet-holding compartment 272. of the pallet below.
As noted, one caplet 206 from each drop tube 300 is
21
CA 02481836 2004-10-26
simultaneously released into the pallet.
The lifting device then lowers the pallet slightly,
indexes to the next adjacent row, and repeats the above
process. In the described embodiment, each pallet is loaded in
two steps because the spacing between the drop tube center
lines is farther apart than the spacing of the receptacles in
the pallet.
Fox the tablets, the modified drop tube 350 shown in
Figures 15A-15C is used. The tablet drop tubes 350 are similar
to the caplet drop tubes 300 but have been modified to include
a J-shaped curved section 360 at the bottom of each tube 350.
The J-shaped curve provides that as the tablets 204 slide down
the tablet drop tubes 350, they rotate 90° from a vertical-
orientation (i.e., sidewall vertically oriented) to a
horizontal orientation (i.e., sidewall horizontally oriented).
The tablet drop tubes 350 are further modified in that the
lower holding pin 308 has been moved from a position in the
tube above the J-shaped curve 360, as in the prior art, to a
place that is below the J-shaped curve 360 as illustrated in
Figure 15A.
In operation, the tablet pallets a.re loaded in a
similar manner as the caplet pallets. Indeed, the same loading
station, except for the drop tubes, may be used. With
reference to Figure 12, tablets 204 initially located in a
supply hopper 400 are controllably released down slide 410 to
sorter 430, which sorts out damaged and partial tablets 204.
From sorter 430, the whole, undamaged tablets 204 are fed to
the feed assembly 420 by way of slide 432. ,As with the caplet
drop tubes 300, guide flanges 302 located at the top ends of '
the tablet drop tubes 350 protrude through openings in the
holding tray of the feed assembly as the holding tray
reciprocates (oscillates) up and down. This causes the tablet s
204 in the holding tray to fall into the.drop tubes 3 50 with
the correct orientation. These tablets 204 are then held in
22
CA 02481836 2005-07-29
place in the drop tubes 350 by the~holding pins 306 and 308.
One tablet 204 from each drop tube 350 is then simultaneously
released into a pallet which as been indexed under the drop
tubes 350 and raised by the lifting device to engage the bottom
of the drop tubes 350.
The tablet loading process differs from the caplet
loading process in that there are no chamfered portions on the
tablet drop tubes 350 to act on the tablet mat 240. In
addition, as described above, the tablet drop tubes 350 include
a J-shaped curved section 360 at the bottom of each tube 350 so
that as the tablets 204 slide down the tablet drop tubes 350,
they rotate 90° from.a vertical orientation to a horizontal
orientation. However, as in the case of the caplet loading
procedure, tablets 204 are loaded into a pallet in two steps,
due to fact that the spacing between the drop tube center lines
is-farther apart than the spacing of the receptacles in the
tablet mat 240. It should be understood that the dimensions of
the medicament and the loading station drop tubes will
determine whether more or less than two loading steps are
needed to fill a pallet.
The First Dipping-Station
After a pallet is loaded, it is carried along by the
conveyer 100 through section 104 in Figure 1 to the first
dipping station 20. As illustrated in Figures 16 and 17, each
dipping station includes a pallet capture station 22, a
commercial robot 24 and a dipping vat 26 full of the liquid
coating material.
The robot system of the preferred embodiment is a
FanuC Model S-700 Robot. The robot system includes a System R-
J Controller with TPP and KAREL software, the specifically
designed end effector tooling 23, and the Model S-700' robot 24.
The robot 24 is a series of mechanical links driven by servo
motors, which working from .the robot rotating base up through
23
CA 02481836 2004-10-26
the end effector tooling 23, includes six axes of rotation.
fihe software controls robot motion in all .six axes as well as
the input/output that may be used between the controller and
any other devices. Control of the robot provides for accuracy
of motion to within .00i degrees or .023 millimeters in terms
of increments of motion at higher end motion speeds. Maximum
speed of movement as measured at end effector tooling is
approximately 700 in./min.
Each capture station 22 includes an actuator
mechanism for capturing pallets and loading two pallets at a
time onto the end effectar tooling 23 of the robot 24.
Appropriate pallet stops, pus::ing mechanisms, microswitches,
linear actuators and the like are used to temporarily retain
and precisely and positively load the two pallets into the
correct position on the end effector tooling 23. In a
preferred embodiment, a pneumatically controlled pallet stop
stops pallets from entering into the capture station 22 until
the previous dip cycle has been completed. The pallet stop is
then released allowing two pallets to advance into the station.
At the same time pallets are advancing, a slide mechanism
located below the pallets is re-indexed and sets a slide finger
in an upward position to then push the two pallets onto the end
effector tooling 23. tThis pushing of two pallets on also
pushes two off the end effector tooling 23 at the same time).
Referring to Figures 7, 11, 18 and 19, the end
effector tooling 23, which is specifically designed for the
particular operation, is essentially a platform 150 having a
pair of end rails 170 along its outer edges, parallel to the
direction of pallet travel. Each end rail 170 includes a slot
172 adapted to receive a corresponding edge 224 of the pallet
support member 210 (see Figures 7 and I1). Thus, when the end
effector tooling 23 is positioned between the conveyor sections
104 and 106 in the capture station 22 with the top surface 152
of the platform 154 at approximately the same level as the
24
CA 02481836 2004-10-26
conveyor surfaces, pallets may be indexed onto the tooling
platform 150 where they are engaged by the slots 172 of the end
rails 1?0.
The platform 150 of the preferred embodiment also has
a pair of square air bladders 154. Each air bladder 154
comprises a flat rubber liner 156, approximately '/z inch wide,
forming a continuous square band. As best seen in Figures 7,
11 and 19, the rubber liners 156 are held in place by inner and
outer .frames 158, 160, between which are channels 162 in the
platform 150 for communicating air pressure throughout the
rubber liners 156. Each air bladder 154 is positioned on the
platform 150 to align with the periphery of one of the pallets
when properly indexed on the platform 150. Thus, after two
pallets have been indexed onto the platform 150, the air
bladders 154 are pressurized to inflate slightly and push on
the underside of the corresponding pallets at a smooth region
226 which forms a continuous square ring inboard of the
periphery of the pallets. The pallets are thereby frictionally
secured to the end effector tooling 23 between the pressurized
bladders 154 and the slots 172 in the side rails 170. (See,
Figures 7 and 11). The air bladders 154 also function to form
a positive seal with the pallets for the application of a
vacuum, as_described below.
The end effector tooling 23 also houses a pair of
eductors 1-68 mounted to the underside of the platform 150. The
eductors 168 take compressed air and convert that energy into a
vacuum. One acceptable eductor 168'is commercially available
from PIAB.
In operation, pallets are indexed onto the tooling
platform 150 from conveyor section 109 two at a time by a servo
driven slide tab. The air bladders 154 are 'then pressurized to
secure the pallets to the tooling 23 and the eductors 168 are
activated to apply a vacuum to the bottom of pallets. As best
seen in Figure 18, the vacuum is applied through apertures 164
CA 02481836 2004-10-26
located in the tooling platform 150. One vacuum aperture 164
is centrally located within each of the areas defined by the
air bladders 154. In addition, two vacuum distribution '
channels 166 are formed in the platform 150 perpendicular to
each other and intersect at the vacuum aperture 164. The
vacuum is communicated through four holes in each pallet
support member~210 to act on the rubber mat 240 or 2?0
positioned thereon to secure the medicaments to the pallet, as
previously described. The robot 24 then extracts the pallets
(with vacuum on) from their position in the conveyor system 100
and moves them to the dipping vat 26.
Upon completion of the dip cycle (described below), -
the pallets are returned to the capture station 22 by the robot
arm 24. The vacuum is then deactivated and the air bladders
154 are depressurized. The just-dipped pallets are then urged
off of the tooling platform 150 and onto the next conveyor
section 106. The next two pallets, which have been queued up,
may then be indexed into the tooling platform and the process
repeated. The robot does not control the end effector tooling
23 except for positioning. The actuation of the vacuum on/off,
and setting conditions under which the robot can start and stop
its cycle, is controlled by the main programmable logic
controller- of the carrier handling system (dlescribecl below) ,
When coating caplets, each capture station 22
optionally may include a centering plate 28 having a series of
countersunk holes 29. Trais plate 28 may be used to center and
orient axially the caplets to ~ 5° vertical. Figure 19
illustrates how the centering is obtained by using the robot
arm end effector tooling 23 to engage the two pallets and raise
the pallets vertically against the centering plate 28 so that
the caplets engage the corresponding~holes 29. The pallets are
urged against the centering plate 28 with a vacuum being
applied so ws to hold the caplets in a properly oriented
position. When coating tablets, this step is generally not
26
CA 02481836 2004-10-26
necessary, It also may be omitted~for caplets depending on the
size of the caplets and the dimensions of the receptacles.
Further, the centering plate 28 could alternatively be lowered
onto the pallets and then raised after the centering operation.
The dipping vat 26 contains the first coating which
in the preferred embodiment is a white gelatin coating. The
robot 24, after withdrawing two pallets from the pallet capture
station 22, and, in the case of caplets, optionally centering
them using the centering plate 28, inverts the pallets with the
vacuum engaged and dips the medicaments into the gelatin bath,
using precisely controlled times and rates for lowering,
holding and raising the medicaments. Figures 20 and 21 show
caplets and tablets, respectively, being dipped by the
currently preferred embodiment. As illustrated, the
medicaments are dipped into the gelatinous bath to coat
slightly more than half the medicament.
The speed with which the medicaments are lowered and
raised from the gelatin, the amount of time the medicaments are
held in the gelatin, and the gelatin temperature and viscosity,
are particularly important to ensure that the medicament is
covered by the gelatin to the desired degree and with the
desired coating thickness, and migration is kept to a minimum
when the pallet is returned to an upright orientation. The
currently preferred gelatin (described below) is maintained at
a temperature range of 120-130°F and has a viscosity of from
400 to 625 centipoise. It has been found that the parameters
listed in Table 2 yield the most desirable results for a gel
temperature of i24°F for yellow and white and 127nF for red,
and viscosity of 400-600 centipoise. Table 3 contains the
presently preferred parameters for the first dipping station
20, as well as the second dipping station 60 (described below).
27
CA 02481836 2004-10-26
TABLE 2: DIPPING PROFILE RANGES
MOVEMENT VARIABLE RANGE OF VALUES
Approach Rate tin/min)550-650
Approach Dwelf (sec) 0.0-2.0
Dip Rate (inlmin) 30-45
Dip Dwell (sec) 0.0-4.0
Withdrawal Rate (in/min)4.5-7.5
Withdrawal Dwell (sec)0.0-1.5
Departure Rate (in/min)550-650
Depart Dwelt (sec) 0.0-3.0
28
CA 02481836 2004-10-26
O O O O is O O O O O ~ N
_ ~ O ~ .- tCicV ~ CO t-
d
Q- ~O
O O
Z J
.O Q Q
V
O O O O ~ ch O O O O tt~ N
Cp ~ ~- tC ~ ~ O r-
Z O H.
Z
d ~.
D Z
O
~_ Q 4
t~!J U
O O O O O ~ O O O O et~ N
J C~ Cn 'l> CO Q ~ P' W O ~ O r- O Q
O
a M t,JtJ
O
Z
D Z O
H
~ t'd'
4
U
O O O O O tc~ O O O O ua N
CO ,O ~ co p ~ p ~ o0
' N
M a M
t/~ O J m
~'i' t~!~
U
J .... "
O C
Q c 'E ~ c
'E V N ...N ~E ,. _ V
Q ~., _ G ~ w. ~ ~ G C
,_" V
C_ N ~",~ ~ .Q5
E ~ D ~ o .-.~ ..
W as a
o a as ~ 3 3 .. ~ v ~
~ y ar ~ ~ O ~ C
a a ~ O .~c~ ' ra ~ a~ m d
a a c. a = " ~ ~ > :z? ;~ ;o
Q 4 G D 'S tS' D O ~ cn in in
29
CA 02481836 2004-10-26
Referring to Tables 2 and 3, once the medicaments
have been positioned over the dipping vat in an inverted
orientation, they are lowered towards the gelatin coating by
the robot arm 24 at the indicated "Approach Rate" until they '
are just above (i.e., about '/e inch above) the fluid level in
the dipping vat 26 where they remain for the indicated
"Approach Dwell" time. The medicaments are then lowered at the
prescribed "Dipping Rate" into the gelatin coating until they
reach the desired dip depth and allowed to remain partially
submerged for the indicated "Dip Dweli" time. The medicaments
are then raised from the gelatin coating at the listed
"Withdrawal Rate" to a point where the end~of the medicament
has just passed the fluid surface, i.e., ,005"-.010" above the
fluid level, and held in place above the gelatin bath for the
''Withdrawal Dwell" time. The medicaments are next raised
further from just above the gelatin bath, at the indicated
''Departure Rate", to the initial position, where they remain at
rest for the ''Departure Dwell" time before being inverted to
their upright orientation. After being returned to their
upright orientation, the medicaments remain in position above
the dipping vat 26 for ''Invert Dwell" time and are then
returned to the capture station 22 by the robot arm 24.
The "Slide accel/decel°' rates listed in Table 3 are
the acceleration and deceleration rates of the slide which
pushes the pallets onto the end effector tooling 23 of the
robot arm 24. Similarly, "Slide Speed'' is the speed of the
pallet indexing slide at the end of the acceleration cycle.
If either of these values is improperly set, the medicaments
could be dislodged from the pallets when being indexed onto or
removed from the end effector tooling 23. The "Slide
Initiation" is the time required before the slide motion can b~e
initiated. This time is required, for example, to clear the
pallets on the end effector tooling 23 before a subsequent pair
CA 02481836 2004-10-26
of pallets can be loaded onto the tooling 23.
These parameters provide for a rapid chill set of the
gelatin coating with minimal runback as the medicaments are
extracted from the bath and inverted 180° to the coated side up
position. They advantageously avoid the need for dabbing the
medicaments to remove excess gelatin at the bottom of the
medicaments, and for additional angular movement, e.g., 360°
rotation about the longitudinal axis or the plane of the
pallet, thereby reducing the processing steps required.
At the conclusion of the first dipping sequence, the
robot 24 restores the two pallets to the upright orientation
and returns them to the capture station 22, where the vacuum is
turned off and the air bladders 154 are depressurized. The
capture station 22 then operates a microswitch and uses a
pushing mechanism (e. g., a pusher tab mounted on a servo driven
slide) to release and push controllably the next two pallets,
which may have been backed up on the conveyor, into the capture
station 22, thereby ejecting the two just-dipped pallets out of
capture station 22 and onto the following conveyor section 106.
Thus, the next two pallets are loaded into the capture station
22 of the dipping station 20.
The Dryer-'Station
The just-dipped pallets advance along the conveyor
100 through section 106 in figure 1 into the dryer loading area
designated at 32. The pallets are then transferred in grougs
of four onto a dryer conveyor that advances the pallets four
abreast through the dryer station 30. The dryer station 30
includes two drying rooms 34 and 36 separated by a wall, with
each room having two parallel 40 foot long dryex conveyors
running through it, one conveyor for first pass drying arid the
second for second pass drying (described below). Thus, on each
pass the medicaments travel through 80 feet; in total.
As the pallets are advanced through the dryer station
31
CA 02481836 2004-10-26
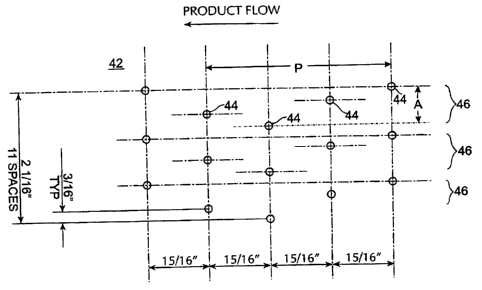
30, they pass under air plenum plates 42, each of which has a
plurality of apertures 44 through which a downward air flow is
directed. The plates are '/a inch thick and are disposed
approximately 1 to 1'/a inches above the medicaments. Each
aperture 44 is 3/32 inch in diameter with a 1/16 inch
countersink on the air supply side. Using the process air
handling system described below, this results in a jet velocity
through each aperture 44 of approximately 8,000 feet per
minute.
In the currently preferred embodiment, the air flow
apertures 44 in the plates 42 are arranged in a plurality of
sinusoidal rows 46, each running in the direction of product
flow. Figure 22 is a partial top view of an air plenum plate
42 illustrating the arrangement of air flow apertures 44. The
apertures are longitudinally spaced at intervals approximately
15/16 of an inch from each other in the direction of product
flow. Apertures in adjacent rows are laterally spaced
approximately 3/a of an inch apart. To forrct a sinusoidal-like
arrangement, the holes in each row are offset laterally from a
common line by varying distances. Looking at Figure 22 from
left to right, the holes in each row are offset by 0, 6/16,
9/16 and 3/I6 inches, which pattern repeats itself every 33/a
inches. -
In the currently preferred embodiment, it is
desirable to provide approximately one sinusoidal row 46 of
apertures 44 for each row (231 or 271) of medicaments on the
pallets as they pass below. Since the spacing between adjacent
caplet compartment rows 271 and between adjacent tablet
receptacle rows 231 is approximately equal to each other and to
the spacing of the sinusoidal air aperture rows 46, this is
accomplished by passing the pallets through the dryer sections
with the medicament rows {231 or 271) oriented in the same
direction as the product flow. As a result of the high air
velocity and sinusoidal aperture arrangement, each medicament
32
CA 02481836 2004-10-26
passes through numerous high speed air swirls as it travels
linearly through the dryer tunnel. This results in~quick
uniform drying of the entire coated medicament surface.
The first and second dryer rooms operate at
essentially the same conditions which are 34°C dry bulb 18°C
dewpoint, nominal, with the total airflow from the air plenums
being sufficient to generate a five inch static pressure
(approximately 22,500 cubic feet per minute in each room). By
contrast, the ambient temperature and humidity of the room in
which the CHS is located is typically maintained at 72~5° F and
50~5o relative humidity. The moisture driven off of the
medicaments is exhausted by a process air exhaust system.
Figure 23 is a schematic diagram of the presently preferred
process air flow handling system.
Approximately l00 (9,500 cubic feet per minute) of
the total process air requirement is drawn from outside (or
inside) of the building by a make-up air handling unit 120.
This air passes through a commercially available air handler
that features a pre-filter and filter section, and heating and
cooling coils. Air is then drawn into a commercially available
fan and the fan's discharge is equally split to deliver the
pre-conditioned air (constant drybulb and dewpoint
temperatures) to two process,air handling units 122. This is a
once through system.
Supply air from the make-up air handler 120 and
return air from the drying room is mixed and drawn into the
suction side of a commercially available unitary air handling
units (process air handling units) 122. In the process air
handler 122, air passes through a pre-filter and filter section
for removal of gross particulate. Air then passes through
heating and cooling coils to temper the air to the desired dry
bulb temperature for drying the product. Air is then drawn
into a commercially available fan that has a discharge capacity
of 22,500 cubic feet per minute.
33
CA 02481836 2004-10-26
After the fang moisture is introduced into the air
stream to provide the desired dewpoint delivered to the
product. The dry bulb and dewpoint conditioned air then
travels through the delivery ductwork to then feed the air
delivery plenums directly over the exposed product in the dryer
rooms 34 and 36. Inside the plenums, air passes through final
high efficiency particulate air (HEPA) filters for particulate
removal then passes through the air plenum plates 42 which
provide the impingement drying of the product. The static
pressure of the system is measuxed between the HEPA filters and
the plenum plates 42. After the drying, the air is then drawn
back to the suction side of the process ai;r handler to repeat
the process.
Each of the two drying rooms are connected to an
exhaust fan 124 that extracts air and driven off moisture from
the space. The amount of air exhausted is dependent an the
amount of air delivered by the make-up air handling unit 120.
(Typically, the volume of air exhausted is greater than that
supplied by the make-up air handling unit so as to keep the
drying room under a negative pressure relative to the CHS
area).
After passing through the two dryer rooms 34, 36 the
pallets ar-a advanced to the exit area, designated 38 in Figure
1. At area 38, the four pallets are held .against a stop, and
then transferred onto the next conveyor section 108 in groups
of four. The four pallets then advance to the reorientation
station 50 where they are stopped, held and flipped one pallet
at a time.
The pallets are advanced through the dryers at
approximately 2.5 feet per minute. Thus, it takes
approximately 32 minutes to pass through both 90 foot rooms.
Typically, each group of four pallets is spaced between 12 and.
13 inches from the preceding group, and contains 1008
medicaments (252 tablets per pallet). This provides a
34
CA 02481836 2004-10-26
throughput of from 60,000 to 90,000 medicaments per hour,
assuming no waste.
The Reorientation Station
° The reorientation station 50, which is illustrated in
Figures 24-26, includes a reorientation mechanism 59 which is
essentially a drum mounted to rotate about a horizontal axis.
The rotation of the drum is driven by a pneumatic motor capable
of clock wise and counter-clock wise rotation, which is
connected by toothed belt to the drum assembly and controlled
by the computer system described below.
The reorientation mechanism 59 houses two lift plates
51 for receiving like pallets in opposition, and a transfer
plate 53 interposed between the lift plates 51. Each lift
plate is supported by four pilot rods 58 along which guide
bushings 51a slide to allow vertical movement of the lift
plates 51. Two pneumatic lift mechanisms 56 are secured to
opposite sides of the body of the reorientation mechanism 59
with one.lift mechanism 56 connected to each lift plate 51 so
as to urge the lift plate vertically along the pilot rods 58.
Each lift plate 51 also includes end rails 52, similar to the
platform end rails 170 of the dipping station. The end rails
52 are mounted along the edges of the lift plates 51, parallel
to the direction of pallet travel. Like end rails 170, end
rails 52 include slots adapted to receive the edge 224 of the
pallet support member 210.
The transfer plate 53 includes an array of apertures
54 which correspond to the medicament receptacles of the
pallets for transferring the medicament from one pallet to the
other. (See Figures 25-26). In additian, when coating
° caplets, the transfer plate 53 of the preferred embodiment,
illustrated in Figure 25, includes chamfered protrusions 55
similar to the chamfered ends 304 of the caplet drop tubes
previously described. The chamfered protrusions 55 are located
CA 02481836 2004-10-26
on both surfaces of the transfer plate adjacent to the.
apertures 54 in positions corresponding to the drop~tube
receiving compartments 274 in the caplet mats 270. ,
In operation, the pallet to be unloaded is indexed
onto the reorientation mechanism 59 and secured to the lower
lift plate 51 by end rails 52 by a stop and slide mechanism
similar to that in the capture station 22 of: the dipping
station 20. An empty pallet is already located on the upper
lift plate 51 in an inverted (i.e., medicament receptacles
down) orientation. The loaded pallet is then urged against the
transfer plate 53 by lift mechanism 56 and aligned with the
empty pallet and the transfer plate 53 by way of alignment pins
57 which engage the corners of each pallet support member 210
at alignment holes 222 (see Figure 3). When coating caplets
according to the preferred embodiment, the chamfered
protrusions 55 on either side of the transfer plate 53 contact
the gripping ribs.28.2 of the caplet mats 270 at the drop tube
receiving compartments 274 thereby causing the gripping ribs
282 (and consequently the caplet-holding compartments 272) in
both pallets to spread open slightly. This allows for a larger
target for the caplet to enter the empty pallet and eases the
exit of the half coated caplet from the loaded pallet. It also
can act to-break a gelatin-mat seal, if one has formed.
The reorientation mechanism 59 is then rotated 180°
in a first direction. As a result, the half-coated medicaments
fall through the transfer plate apertures 54 into the empty
pallet, which is now in the lower position, under the force of
gravity. The now loaded pallet (located on the lower lift
plate 51) is then moved away from the transfer plate 53 and
pushed onto the conveyer by, e.g., a following loaded pallet to
be unloaded. For the next pallet, the same sequence occurs .
except that the reorientation mechanism 59 rotates 180° in the
other direction. The back and forth rotation is used for
simplicity of hydraulic (pneumatic) and electronic control and
36
CA 02481836 2004-10-26
wiring.
In coating different sized products, different
transfer plates 53 may be required (e. g., 500 milligram caplets
may require a transfer plate 53 with larger apertures 54 than
' 325 milligram caplets). For tablets, the 'transfer plate 53 may
be omitted as shown in Figure 26, in which case the two pallets
are urged against each other in the reorientation mechanism
prior to being inverted. Preferably, however, a relatively
thin transfer plate (not shown) is used for tablets to ensure a
centered transfer.
In the preferred embodiment, only the transfer plate
53 need be changed for different medicament praducts, rather
than having to use multiple reorientation mechanisms 59. This
saves time and makes it significantly easier to reconfigure the
system in preparation for coating different products.
The Second Dipoina Station
After reorientation, the pallet with the array of
medicaments having their uncoated ends facing upwards is then
transported by conveyer system 100 to a second dipping station
60. The second dipping station 60 operates in the same manner
as the first dipping station 20, except that a different.
coating material may be applied. In the currently preferred
embodiment, the second coating is either a. white, red or yellow
gelatin coating, depending on the size of the medication and
whether one color or two color medicaments. are desired.
Optionally, a single dipping station may be used, for example,
so as to coat the medicaments with a single color.
Alternatively, two dipping vats 26 could be supplied from a
single gelatin supply (tanks 80 and 90).
In the preferred embodiment, the medicaments are
dipped into the gelatinous bath according to the profile listed
in Tables 2 and 3 (above). At the first dipping station,
caplets are dipped to a depth of approximately '/2 the caplet
37
CA 02481836 2004-10-26
length plus .015"-.020" and at the second dipping station, they
are dipped to a depth of approximately ~/2 the caplet length.
The extent of dipping of each "half" can be controlled to
achieve coverage which extends from no over3.ap between the
first and second coating, to some overlap which is not visible
to the eye and may not be felt, to a substantial overlap which
provides a surface discontinuity which can be felt and/or seen.
Tt should be understood that the first and second
dipping profiles can be selected to obtain the desired coating,
ranging from no overlap, abutting coatings, and overlapped
coatings. When combined with the viscosity of the gelatinous
baths, the configuration and thickness profile of the gelatin
coating on the medicaments can be selected as desired. For
example, both coatings can be of the same v=~scosity and produce
the same thickness.
The Second Pass through the Dryer Sections
After the second coating has been applied, the
pallets are again advanced through both rooms 34 and 36 of the
dryer station 30, this time passing through the second tunnel.
The pallets travel in the same direction as occurred after the
first coating and under the same conditions, again four pallets
abreast at-~a.time.. At the exit 38 of the second dryer section
36, the medicaments are fully dried and ready for unloading.
Before unloading, however, it may be desirable to subject the
fully coated and dried medicaments to a curing step, the curing
step being substantially identical to the conditioning that was
employed after the subcoating was applied.
The Unloading Station
Unloading preferably occurs one pallet at a time at
station 70. The unloading station 70, illustrated in Figures
27 and 28, captures a pallet at capture station 71 and indexes
it under an unloading plate 73 and unloading cone 76.
38
CA 02481836 2004-10-26
Unloading plate 73 retains the mat 240 or 270 in its place
against support member 2I0 as the pallet is inverted to
' discharge the coated medicaments into unloading cone 76.
In a preferred embodiment, the unloading cone 76 is
connected to the unloading plate 73, such that they pivot about
a common axis as the pallet of medicaments is raised from the
capture station 71 and inverted. This minimizes the risk of
ejecting the medicaments or having the medicaments fall out of
the pallet so as to become waste during the unloading
operation. The unloading cone 76 and unloading plate ?3 are
driven about the pivot axis by a pneumatically actuated motor
capable of rotating clock wise and counter-clockwise.
In operation, a pneumatically actuated pallet stop
allows the loaded pallets to enter the capture .station 71 one
at a time. The pallet entering the station slides in between
two spring loaded carrier guides 62 which include slots 64 to
engage the perimeter of the pallet in a similar manner to that
in the reorientation station 50. Once the pallet is in the
correct position, the motor is actuated to rotate the unload
mechanism. Starting the rotation of the unload mechanism
causes the two spring loaded carrier guides 62 to "pull up'° the
pallet so as to compress the pallet against the unloading plate
73. The pallet, along with the plate 73 and cone 76 are then
flipped over to strike stop member 78. This. angular momentum
is thus rapidly decelerated, which ejects the coated
medicaments into the unloading cone 76.
In one embodiment, the unloading plate 73 is similar
to the transfer plate 53 previously described. Like the
. transfer plate 53, the unloading plate 73 may include a
plurality of apertures 74 which correspond t:o the medicament
' receptacles of the pallets. In addition, when unloading
caplets, the unloading plate 73 may include chamfered
protrusions 75 (see, Figure 28) in a similar arrangement to
that found on the transfer plate 53. Unlike the transfer plate
39
CA 02481836 2004-10-26
53, however, the, chamfered protrusions 75 of the unloading
plate 73 are only required on one side, the side facing the
pallet to be unloaded. ~ In operation, the chamfered protrusions
75 on the unloading plate 73 contact the gripping ribs 282 of
the caplet mat 270 at the drop tube receiving compartments 274,
thereby causing the caplet-holding compartments 272 to spread
open slightly. This breaks any adhesion formed between the
gelatin and mat, and also allows the fully coated caplet to
free-fall under the influence of gravity out of the pallet,
minimizing the force used to rotate the pallet against the stop
member 78.
In another embodiment, the unloading plate 73 has a
mesh screen or grid which contacts a certain number of
receptacles, but not every receptacle. For example, a grid
that contacts every row and every third column of the pallet is.
suitable.
Once the fully coated medicaments are ejected from
the inverted pallet into unloading cone 76, they fall through
the opening 77 and onto a diverter 79 which directs the
unloaded medicaments into either a collecting container or a
refuse container. The diverter 79 is e:>sentially a short chute
which is rotatable between two positions, the first (shown in
Figures 27 and 28) to direct the medicaments into the
collecting container, and the other (shown in phantom in Figure
28) to direct the medicaments into the refuse container. The
diverter ?9 is controlled by the computer system described
below to selectively reject defective batches. Medicaments
which are not rejected are collected in the collecting
container and taken to be packaged as the finished product.
The. now empty pallet is then :returned to an upright
orientation; the unloading plate 73 is raised and the pallet is
pushed onto the next conveyor section 102, which was the
initial starting position for the pallet. For the next pallet,
the same sequence occurs. The unloaded pallets are transported
CA 02481836 2005-07-29
to the loading station by the conveyer system 100 and the
entire process is repeated.
The Automatic Drawdown System (ADS)
The ADS provides the two supplies of coating material,
one for each dipping station. Referring to Figures 1 and 29,
a batch of coating material is first made in each of the large
tanks 80, which is then transferred into the respective smaller
holding tanks 90. Holding tanks 90 are continuous flow tanks
that continuously feed the dipping vats 26. A holding tank 90
is periodically refilled from a large tank 80 whenever a new
batch is provided or whenever another volume of coating
material is needed to continue production. A clean-in-place
system is used to clean out tanks 80 (including the associated
piping, pumps and hoses) after a batch is made, and the whole
dipping system (tanks 80 and 90, and vats 261 at the end of a
production run.
In the preferred embodiment, the medicaments are
coated with a gelatinous material. The gelatinous material
includes water, gelatin, glycerin, a plasticizes. a surfactant
and preservatives. The currently preferred gelatin
formulations, along with the tested ranges, are given below in
Table 4, with the balance of the mixture in each case being
made up of purified water.
It should, of course, be understood that one of
ordinary skill in the art of gelatin use in the pharmaceutical
industry would be able to determine equivalents without undue
experimentation, given the disclosure provided. For example,
other known plasticizers, such as polyethylene, glycols,
triacetin, mineral oil or caster oil, may be used in place of
titanium dioxide. Likewise, other known~surfactants, such as
Spans or Tween~, may be used instead of sodium lauryl
sulfate. Further, as indicated in Table 4, various dyes
may be added to impart color to the gelatin.
91
CA 02481836 2004-10-26
TABLE 4: GEL FORMULATIONS
Raw Material % of RM
Tested PreferredRed White Yellow
Range Range Gelatin Gelatin Gelatin
Gelatin 25-40 28-30 29.16 29.16 29.17
l
Glycerin 2-10 4 3.65 3.65 3.65
I FD&C Red 1.82
FD&C Yetfow .83
Titanium Dioxide 1-3.5 1.5 1.37 1.37 1.37
Sodium Lauryl Sulfate.2-.6 .3 .27 .27 .27
Methytparaben 0-.44 .44 .44 .44
Propylparaben 0-.04 .04 .04 .04
Butyfp_ araben 0-.02 .02 .02 .02
_ _. _ I , p II
The process for making the gelatin is computer
controlled with operator inputs required. Grater is added to
tank 80, along with the gelatin, coloring, preservatives, and
other materials, as indicated. The gelatin is hydrated and
mixed under a vacuum of about 29 inches of mercury for thirty
minutes. The heat is then turned on to melt. the hydrated
gelatin at a temperature of 104° to 108° F for approximately
forty-five minutes, being careful not to boil the solution.
After the melting, the vacuum is broken and tank 80
is heated to a temperature below 140° F, preferably between
120° and 130° F. Once the temperature matches that of the
smaller holding tank 90, the batch is transferred into the
holding tank 90. The holding tank 90 continues to circulate
t he warm gelatin into the dipping vat 26 through feed valve 2?
42
CA 02481836 2004-10-26
located near the bottom of dipping vat 26. Overflow is
captured by overf low vat 25 and returned to tank 90 via gravity
and vacuum assist to be mixed with the rest of the batch. This
arrangement maintains a constant gelatin level in dipping vat
26 and provides for uniform gelatin consistency. Tank 90 is
maintained at a low vacuum, about 30 inches of water, in order
to degas the circulating gelatin. Heat exchangers are used to
maintain temperature and a peristaltic pump (not shown) is used
to inject water to compensate for water loss due to
evaporation.
Computer Control
A network of Man Machine Interface (MML) industrial
computers are used to monitor and control t:he operation of the
entire system. Each computer is allowed to directly control
the apparatus within a local area, i.e., within the operator's
field of view, and otherwise is capable of monitoring all other
systems operating conditions. This allows an operator to view
what is going on in a different room, but not to control it.
Each pallet is provided with a bar code or other
identifying indicia, which is scanned as the pallet enters each
station. This provides the system with the ability to monitor
the process and track and identify any problem that. may occur
at the various processing stations. Thus, if a given step in
the process is defective, when the pallet gets to the unloading
station, the diverter 79 is actuated to divert the unloaded
medicaments into a separate waste container. Similarly, if
there was a detected problem with some other part of the
process that is out of tolerances far the process, then certain
subsequent process steps will be skipped. For example, if
there was a problem with the first dipping station 20, then the
pallet will not be reoriented or dipped a second time, but it
will be unloaded and the contents diverted as waste. Indeed,
the pallet may pass directly through the reorientation station
43
CA 02481836 2004-10-26
50 if so desired. Further, appropriate programming and
scanning of bar codes cari allow one robot 2~ to be used to dip
the pallets in the appropriate one of two or more dipping vats
26.
'The present invention has been described in terms of
preferred embodiments thereof. Other ~mbod:iments, features and
variations within the scope of the invention will,. given the
benefit of this disclosure, occur to those having ordinary
skill in the art.
44