Note: Descriptions are shown in the official language in which they were submitted.
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
METHOD FOR PREVENTING DAMAGE TO OR REJUVENATING A
CELLULAR BLOOD COMPONENT USING MITOCHONDRIAL ENHANCER
CROSS-REFERENCE TO RELATED APPLICATIONS
This application is a continuation-in-part of 09/586,147 filed June 2, 2000;
and
09/596,429, filed June 15, 2000, which is a continuation-in-part of
09/357,188, filed
July 20, 1999, now issued U.S. patent number 6,277,337; and claims priority to
U.S.
provisional application number 60/378,374, filed May 6, 2002; the disclosures
of
which are incorporated herein by reference to the extent not inconsistent
herewith.
BACKGROUND OF THE INVENTION
. Whole blood collected from volunteer donors for transfusion recipients is
typically separated into its components, red blood cells, platelets, and
plasma, by
apheresis or other known methods. Each of these fractions are individually
stored and
used to treat a multiplicity of specific conditions and disease states. For
example, the
red blood cell component is used to treat anemia, the concentrated platelet
component
is used to control bleeding, and the plasma component is used frequently as a
source
of Clotting Factor VIII for the treatment of hemophilia.
In the United States, blood storage procedures are subject to regulation by
the
government. The maximum storage periods for the blood components collected in
these systems are specifically prescribed. For example, whole blood components
collected in an "open" (i.e., non-sterile) system must, under governmental
rules, be
transfused within twenty-four hours and in most cases within six to eight
hours. By
contrast, when whole blood components are collected in a "closed" (i.e.,
sterile)
system the red blood cells can be stored up to forty-two days (depending upon
the
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
2
type of anticoagulant and storage medium used) and plasma may be frozen and
stored
for even longer periods. Platelets can be frozen with dimethyl sulfoxide
(DMSO) and
stored for years (Valeri et al. (2000) Chapter 6, Frozen Platelets, pages 105-
130, in
Platelet Therapy: Current Status and Future Trends, Eds. Seghatchian, J. et
al.,
Elsevier, Amsterdam). With 6% DMSO platelets can be stored for about three
years
at -80°C and with 5% DMSO for about two years at -150°C.
While red cells are stored in the cold, Murphy and Gardner, New Eng. J. Med.
280:1094 (1969), demonstrated that platelets stored as platelet-rich plasma
(PRP) at
22 °C. possessed a better in vivo half life than those stored at 4
°C. Thus, more
acceptable platelet concentrates could be transfused after storage at room
temperature.
Until recently, the rules allowed for platelet concentrate storage at room
temperature
for up to seven days (depending upon the type of storage container). However,
it was
recognized that the incidence of bacterial growth and subsequent transfusion
reactions
in the recipient increased to unacceptable levels with a seven-day-old
platelet
concentrate. Platelet concentrates may currently be stored for no more than
five days.
Contamination of blood supplies with infectious microorganisms such as
malaria, West Nile virus, HIV, hepatitis and other viruses and bacteria
presents a
serious health hazard for those who must receive transfusions of whole blood
or
administration of various blood components such as platelets, red cells, blood
plasma,
Factor VIII, plasminogen, fibronectin, anti-thrombin III, cryoprecipitate,
human
plasma protein fraction, albumin, immune serum globulin, prothrombin complex,
plasma growth hormones, and other components isolated from blood. Blood
screening procedures may miss contaminants, and sterilization procedures,
which do
not damage cellular blood components but effectively inactivate all or reduce
infectious viruses and other microorganisms, are needed in the art. Systems
that use
the same chemistry to inactivate or reduce microorganisms in different fluids,
for
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
3
example separate blood components, are desired for many reasons, including
ease of
use in a blood bank setting. It is also desired that the inactivation or
reduction
treatment be easily implemented in a blood bank setting, and produce
inactivation or
reduction in a short period of time.
Bacteria can easily be introduced to blood components by at least two
different
means. First, if the donor is experiencing a mild bacteremia, a condition
comprising
bacteria in the blood, the blood will be contaminated, regardless of the
collection or
storage method. Adequate donor histories and physicals will decrease but not
eliminate this problem. See B. J. Grossman et al., Transfusion 31:500 ( 1991
).
A second, more pervasive source of contamination is the venepuncture
employed when drawing blood. Even when "sterile" methods of skin preparation
are
employed, it is extremely difficult to sterilize the crypts around the sweat
glands and
hair follicles. During venepuncture, this contaminated skin is often cut out
in a small
"core" by a sharp needle. This core can serve to "seed" the blood bag with
bacteria
that may grow and become a risk to the recipient.
Indeed, many patients requiring platelet transfusions lack host-defense
mechanisms for normal clearing and destruction of bacteria because of either
chemotherapy or basic hematologic disease. The growth of even seemingly
innocuous
organisms in stored platelets can, upon transfusion, result in recipient
reaction and
death. See e.g., B. A. Myhre, JAMA 244:1333 (1980) and J. M. Heal et al.,
Transfusion 27:2 ( 1987).
It has been found that platelets which have been treated with a
photosensitizer
and light to inactivate or reduce pathogens which may be present may show re-
activation of pathogens during long-term storage after such a treatment. In
addition to
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
4
platelet aggregation, platelets may show high activation and low extended
shape
change response by day 5 of storage, both of which may be indications of
cytoskeletal
changes in the platelets. Such changes may be indications of platelet damage
due to
the storage conditions. It is therefore necessary to improve the quality of
stored
photoradiated platelets.
There is a need for methods allowing for better pathogen reduction and/or
inactivation while maintaining cell quality above acceptable limits and for
methods
allowing for improved cell quality while maintaining pathogen reduction and/or
inactivation. Large quantities of blood and blood products are discarded by
blood
banks after certain periods of storage due to expiration of the blood and
blood
products. By improving the cell quality of blood components during storage and
after
pathogen reduction and/or inactivation, the shelf life blood components is
increased.
In cells, food is oxidized to produce high-energy electrons that are converted
to
stored energy. This energy is stored in high-energy phosphate bonds in ATP.
Ingested sugars are broken down by enzymes that split them into a six-carbon
molecule called glucose. Glucose may also be provided to cells in media or
storage
solutions. The breakdown of glucose to provide energy to cells is an important
mechanism in cellular metabolism. This mechanism, known as glycolysis,
produces
ATP (adenosine triphosphate) in the presence or absence of oxygen. The
production
of ATP is essential for cellular energy metabolism. Glucose enters the cell by
special
molecules in the membrane called "glucose transporters." Once inside the cell,
glucose is broken down to make ATP in two pathways. The first pathway requires
no
oxygen and is called anaerobic metabolism. Anaerobic metabolism or glycolysis
occurs in the cytoplasm outside the mitochondria. During glycolysis, glucose
is
broken down into pyruvate, a three-carbon molecule. This conversion involves a
sequence of nine enzymatic steps that create phosphate-containing
intermediates.
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
Each reaction is designed to produce hydrogen ions (electrons) that can be
used to
make energy in the form of ATP. Only two ATP molecules can be made by one
molecule of glucose run through this pathway. This pathway is also used to
produce
two lactate molecules from every one glucose molecule.
For most animal cells, glycolysis is merely the first stage in the breakdown
of
sugar into cellular energy, since the pyruvic acid that is formed at the last
step quickly
enters the cell's mitochondria to be completely oxidized to C02 and H20 in the
citric
acid cycle. The citric acid cycle is also known in the art as the Kreb's cycle
or the
tricarboxylic acid (TCA) cycle. The citric acid cycle occurs in the
mitochondria and
is the common pathway to completely oxidize fuel molecules, which are mostly
acetyl
CoA, the product from the oxidative decarboxylation of pyruvate. Acetyl CoA
enters
the cycle and passes through ten steps of reactions that yield energy (ATP)
and C02.
In the case of organisms which are anaerobic (those that do not use molecular
oxygen) and for tissues like skeletal muscle that can function under anaerobic
conditions, glycolysis is a major source of the cell's ATP. This also occurs
in an
aerobic cell if the mitochondria of the cell are damaged in some way, thereby
preventing the cell from entering the citric acid cycle.
Since ATP is essential to continued cell function, when aerobic metabolism is
slowed or prevented by lack of oxygen, anaerobic pathways for producing ATP
are
stimulated and become critical for maintaining cell viability. Here, instead
of being
degraded in the mitochondria, the pyruvate molecules stay in the cytosol and
can be
converted into ethanol and C02 (as in yeast) or into lactate (as in muscle).
Lactate accumulation in cells causes an increased concentration of hydrogen
ions and a decrease in pH. Blood cells in storage that experience a decrease
in pH
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
6
may be only undergoing glycolysis. Such a drop in pH indicates as well as
contributes
to a decrease in cell quality during cell storage.
Factors which cause cells to enter glycolysis and thereby accumulate lactic
acid
or lactate include events which occur internally in a body such as strokes or
infarctions, as well as external events such as treatment of the cells after
removal from
a body. One example of an external treatment which might cause cells to
accumulate
lactate is a procedure to inactivate or reduce pathogens which might be
contained in
cells or fluids containing cells to be transfused into a recipient. Currently
used
methods to sterilize pathogenic contaminants which may be present in blood or
blood
components can cause damage to the mitochondria of the cells being treated. If
this
occurs, the cells can only make ATP through the glycolysis pathway, causing a
buildup of lactic acid in the cell and a subsequent drop in pH during storage.
Mitochondria are critical subcellular organelles of blood components. They are
involved in aerobic energy metabolism and the oxidative reactions therein.
Mitochondria are sensitive to endogenous and exogenous influences and may be
easily damaged or destroyed. Dysfunctional energy metabolism and, more
severely,
damaged mitochondria, lead to a decline in platelet quality and eventual cell
death.
Possible causes of damage to blood components may be storage, pathogen
inactivation, and pathogen reduction processes. A reason for changes in
platelet
viability after pathogen reduction or inactivation may be that irradiation of
the
platelets to kill pathogens may be causing damage to the platelet mitochondria
(Chavez et al. (September 1998) Biochem. Mol. Biol. Int. 46(1):207-214; Masaki
et
al. (March 1997) J. Dermatol. Sci. 14(3):207-216; and Salet et al. (Apr 1995)
Int. J.
Radiat. Biol. 67(4):477-80). It has been observed that a side effect of a
pathogen
reduction process is that when platelets are subjected to UV light, the
mitochondria of
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
7
the platelets have a greater chance of suffering at least some damage than
when they
have been subjected to visible light. Mitochondria are present in all oxygen-
utilizing
organisms in which energy in the form of adenosine triphosphate (ATP) is
generated
and oxygen is reduced to water. Ninety percent of the oxygen taken in by the
organism is consumed by the mitochondria. A substantial byproduct of ATP
generation is the formation of potentially toxic oxygen radicals. For example,
it is
estimated that 1-2% of all reduced oxygen yields superoxide (02~) and hydrogen
peroxide (H202). Other reactive oxygen species (ROS) that form are singlet
oxygen
(02) and hydroxyl radicals (~OH). Under stress conditions in the cell this can
rise to
10% of all consumed oxygen. Mitochondrial membranes are sensitive to lipid
peroxidation and depolarization resulting from these ROS.
Furthermore, photochemical methods for pathogen inactivation and reduction
of blood products which generate singlet oxygen species in the process of
photolysis
of the photosensitizer cause further damage to mitochondrial membranes. It is
therefore necessary to protect platelet mitochondria of platelets and other
blood cells
from ROS generated by both photochemical decontamination and stress conditions
of
storage.
There is a need in the art for methods to prevent damage to mitochondria, to
reduce damage to and degradation of blood components during storage and
before,
during, and after pathogen inactivation and reduction procedures.
All references cited are incorporated herein by reference in their entirety to
the
extent that they are not inconsistent with the disclosure herein. Citation of
the above
documents is not intended as an admission that any of the foregoing is
pertinent prior
art. All statements as to the date or representation as to the contents of
these
documents is based on subjective characterization of the information available
to the
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
8
applicant, and does not constitute any admission as to the accuracy of the
dates or
contents of these documents.
SUMMARY OF THE INVENTION
This invention provides a method for treating a fluid comprising a cellular
blood component to improve a vital quality of said cellular blood component,
said
method comprising adding an effective, substantially non-toxic amount of a
mitochondria) enhancer to said fluid wherein said mitochondria) enhancer is
selected
from the group consisting of alloxazines, endogenous alloxazines, non-
endogenous
alloxazines, endogenously-based derivative alloxazines, endogenous
photosensitizers,
and non-endogenous photosensitizers. The concentration of mitochondria)
enhancer
in the fluid can be from any amount sufficient to provide measurable
enhancement of
a vital quality of a cell in the fluid up to a toxic amount. Preferably the
mitochondria)
enhancer is present at a final concentration from about one to about 200
micromolar.
This invention also provides methods for increasing the storage life of
cellular
blood components, extending platelet storage life, treating a cell comprising
a
mitochondrion, treating a fluid comprising cells containing mitochondria to
improve a
quality of said fluid, said methods comprising adding an effective,
substantially non-
toxic amount of a mitochondria) enhancer to said fluid wherein said
mitochondria)
enhancer is selected from the group consisting of alloxazines, endogenous
alloxazines,
non-endogenous alloxazines, endogenously-based derivative alloxazines,
endogenous
photosensitizers, and non-endogenous photosensitizers. Fluids treatable by the
methods of this invention include fluids containing living cells with
mitochondria or
fluids that come into contact with living cells such as peritoneal solutions,
blood, and
fluids comprising a blood product. Cells treatable by the methods of this
invention
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
9
include plant cells, animal cells, yeast cells, cellular blood components,
platelets, and
cells in a wound surface.
In the practice of this invention, the fluid is optionally exposed to
photoradiation greater than ambient light. When the fluid is exposed to
photoradiation
greater than ambient light, the photoradiation may be of sufficient energy to
activate a
photosensitizer in the fluid. When the fluid is exposed to photoradiation, the
wavelength of the light can be in the visible or ultraviolet spectrum. When
the fluid is
exposed to photoradiation, it can be performed at a time selected from the
group
consisting of before, after, and simultaneously with treating the fluid with
mitochondrial enhancer. When the fluid is exposed to photoradiation, it can be
of
energy between about 5 J/cm2 and about 150 J/cm2.
When the fluid is exposed to photoradiation of sufficient energy to activate a
photosensitizer which is also in the fluid, the photoradiation can also be of
sufficient
energy to substantially reduce pathogens which may be present in the fluid.
Pathogens which can be reduced by the methods of this invention include
extracellular
and intracellular viruses, bacteria, bacteriophages, fungi, blood-transmitted
parasites,
and protozoa, and mixtures of any two or more of the foregoing.
When photosensitizer is added to the fluid, the photosensitizer can be the
same
as the mitochondrial enhancer that is added to the fluid. The concentration of
photosensitizer can be any amount sufficient to provide a measurable reduction
of
pathogens in the fluid up to an amount which would be toxic to the cells.
Preferably
the photosensitizer is present at a concentration from about 1 to about 200
micromolar. In one embodiment of this invention, the cellular blood component
is not
stored prior to said treating. In another embodiment of this invention, the
cellular
blood component is stored prior to said treating. When the cellular blood
component
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
is stored prior to treating, it is stored for an amount of time between about
1 hour and
about 7 days prior to said treating. Alternatively the cellular blood
component is
stored for more than about one hour prior to said treating.
Mitochondrial enhancers useful in the practice of this invention, which may
simultaneously act as photosensitizers, include 7,8-dimethyl-10-ribityl
isoalloxazine,
7,8-dimethylalloxazine, 7,8,10-trimethylisoalloxazine, alloxazine
mononucleotide,
isoalloxazine-adenosine dinucleotide, vitamin Kl, vitamin Kl oxide, vitamin
K2,
vitamin KS, vitamin K6, vitamin K7, vitamin K-S(II), and vitamin L. When the
mitochondrial enhancer is 7,8-dimethyl-10-ribityl isoalloxazine it is in the
fluid at a
concentration of about one to about 200 micromolar.
Additional mitochondrial enhancers useful in the practice of this invention
include molecules of the formula:
R3 R~
R4 N N
N
R5 N ~ ~ wR2
Rg O
wherein R1, R2, R3, R4, RS and R6 are, independently from one another,
selected
from the group consisting of hydrogen, optionally substituted hydrocarbyl,
alcohol,
amine, polyamine, sulfate, phosphate, halogen selected from the group
consisting of
chlorine, bromine and iodine, salts of the foregoing; and -NRa-(CRbR')"-X
wherein X
is a halogen selected from the group consisting of chlorine, bromine and
iodine, Ra, Rb
and R~ are, independently of each other, selected from the group consisting of
hydrogen, optionally substituted hydrocarbyl, and halogen selected from the
group
consisting of chlorine, bromine and iodine, and n is an integer from 0 to 20;
provided
that Rl is not -OH or a straight chain alkyl group where the second carbon of
the
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
11
chain is substituted with -OH or =O and Rl, R4, RS are not all methyl groups
when
R2, R3 and R6 are hydrogen. R1, R2, R3, R4, RS and R6 can be, independently
from
one another, selected from the group consisting of hydrogen, optionally
substituted
alcohol, straight chain or cyclic saccharide, amino acid, amine, polyamine,
polyether,
polyalcohol, sulfate, phosphate, carbonyl, glycol, halogen selected from the
group
consisting of chlorine, bromine and iodine, aldehyde, ketone, carboxylic acid
and
ascorbate. These compounds may also act as photosensitizers.
An example of a cellular blood component treatable by the methods of this
invention is platelets. In an embodiment of this invention, the cellular blood
component is stored for more than about one hour after said mitochondria)
enhancer is
added.
The methods of this invention can also include adding nitric oxide to the
fluid,
adding quencher to the fluid, adding process enhancer to the fluid, adding
oxygen to
the fluid, and/or adding glycolysis inhibitor to the fluid.
Vital qualities that are improved by the methods of this invention include
oxygen consumption, rate of oxygen consumption, lactate production, rate of
lactate
production, pH, rate of pH change, activation, hypotonic shock response,
glucose
consumption, rate of glucose consumption, platelet swirl, platelet
aggregation, carbon
dioxide production, rate of carbon dioxide production, cell count, and extent
of shape
change.
In an embodiment of this invention, the oxygen consumption is increased by at
least about 5%. In an embodiment of this invention, the rate of lactate
production is
decreased by at least about 25%. In an embodiment of this invention, the pH is
increased by at least about 0.1 units. In an embodiment of this invention, the
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
12
hypotonic shock response is increased by at least about S%. In an embodiment
of this
invention, the glucose consumption is decreased by at least about 10%. In an
embodiment of this invention, the platelet swirl is increased by at least
about S%. In
an embodiment of this invention, the platelet aggregation is decreased by at
least
about 5%. In an embodiment of this invention, the carbon dioxide production is
increased by at least about 5%. In an embodiment of this invention, the cell
count is
increased by at least about 5%. In an embodiment of this invention, the extent
of
shape change is increased by at least about 5%. In an embodiment of this
invention,
the activation is decreased by at least about 5%.
BRIEF DESCRIPTION OF THE DRAWINGS
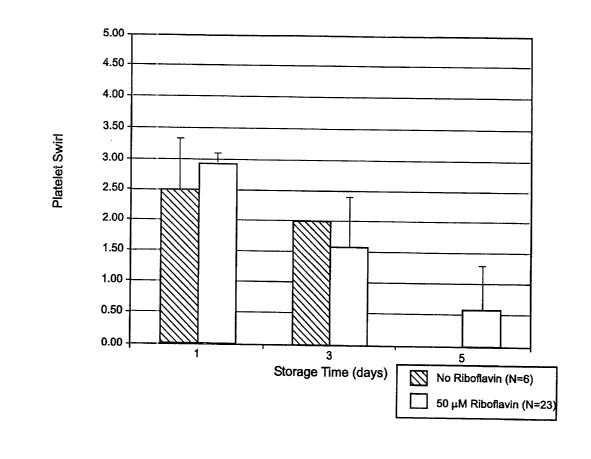
Figure 1 is a graph showing the effect of mitochondria) enhancer on platelet
swirl (0-4 units) of cellular blood components as a function of storage time
(days).
Figure 2 is a graph showing the effect of mitochondria) enhancer on hypotonic
shock response (HSR), % reversal, of platelets as a function of storage time
(days).
Figure 3 is a graph showing the effect of mitochondria) enhancer on pH of the
stored fluid as a function of storage time (days).
Figure 4 is a graph showing the effect of mitochondria) enhancer on % extent
of shape change (ESC) of platelets as a function of storage time (days).
Figure S is a graph showing the effect of mitochondria) enhancer on lactate
production by platelets as a function of storage time (days).
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
13
Figure 6 is a graph showing the effect of mitochondria) enhancer on the rate
of
lactate production by platelets as a function of mitochondria) enhancer
concentration.
Figure 7 is a graph showing the effect of mitochondria) enhancer on lactate
production by platelets as a function of storage time (days).
Figure 8 is a graph showing the effect of mitochondria) enhancer on glucose
consumption by platelets as a function of storage time (days).
Figure 9 is a graph showing the effect of mitochondria) enhancer on p-selectin
expression (% activation), by platelets as a function of storage time (days).
Figure 10 is a graph showing the effect of mitochondria) enhancer on oxygen
consumption by platelets as a function of storage time (days).
Figure 11 is a graph showing the effect of mitochondria) enhancer on reduction
kinetics of vaccinia virus as a function of photoradiation exposure time
(delivered
energy).
Figure 12 is a graph showing the effect of various concentrations of
mitochondria) enhancer on reduction of Herpes Virus 2 (HSV-2) as a function of
photoradiation exposure time (delivered energy).
Figure 13 is a graph showing the effect of various energy doses on reduction
of
S. epidermidis as a function of concentration of mitochondria) enhancer
(micromoles).
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
14
Figure 14 is a graph showing the effect of various concentrations of
mitochondria) enhancer on reduction of X174 as a function of delivered
photoradiation energy.
DETAILED DESCRIPTION OF THE INVENTION
Definitions
As used herein, "mitochondria) enhancer" refers to a composition which
enhances a vital quality of mitochondria or of cells containing mitochondria.
Mitochondria) enhancers useful in the practice of this invention include, but
are not
limited to alloxazines, endogenous alloxazines, non-endogenous alloxazines,
endogenously based derivative alloxazines, endogenous photosensitizers, and
non-
endogenous photosensitizers.
As used herein, "ambient light" refers to natural light such as sunlight,
including sunlight through glass or plastic, or overhead room light, such as
from
incandescent, fluorescent, and/or halogen bulbs. Ambient light is generally
not of
enough energy and/or of the appropriate wavelengths to sufficiently activate a
photosensitizer in a solution to substantially reduce pathogens therein.
As used herein, "substantially inactivate pathogens" refers to reducing the
ability of pathogens to reproduce, preferably by killing them. When the
treated fluid
comprises a cellular blood component, the level of pathogens in the fluid can
be
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
decreased such that the cellular blood component may be safely administered to
a
patient.
As used herein, "substantially reduce pathogens" refers to reducing the
ability
of pathogens to reproduce, preferably by killing them. When the treated fluid
comprises a cellular blood component, the level of pathogens in the fluid can
be
decreased such that the cellular blood component may be safely administered to
a
patient.
As used herein, "storage" refers to the amount of time after formulation or
collection before a fluid is utilized for its intended purpose. As used
herein, storage of
blood or blood product refers to time between the collection of the blood or
blood
product and the utilization of the blood or blood product for its intended
purpose, such
as the administration of that blood product to a patient.
As used herein, "an amount of mitochondrial enhancer sufficient to improve
storage life" refers to an amount that measurably increases a vital cell
quality.
As used herein, "glycolysis inhibitor" refers to compositions that interfere
with
the biochemical pathway of glycolysis. 2-deoxy-D-glucose is an example of a
glycolysis inhibitor.
As used herein, "adding nitric oxide to a fluid" refers to increasing the
amount
of nitric oxide within a fluid. In the practice of this invention nitric oxide
may be
added to a fluid by any method known in the art. Methods for adding nitric
oxide to a
fluid include, but are not limited to, adding liquids, solids, or gases
containing nitric
oxide and adding nitric oxide generators. Nitric oxide generators are
chemicals that
are able to react, directly or indirectly, to produce nitric oxide. Nitric
oxide generators
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
16
may react with components already in a fluid to produce nitric oxide, or they
may
require the addition of one or more different nitric oxide generators to the
fluid, with
which they may react to produce nitric oxide. Nitric oxide generators that do
not
require the addition of one or more different nitric oxide generators are
nitric oxide
donors. Nitric oxide donors are well known in the art (Bauer et al., (1995)
Advances
in Pharmacology 34:361 and U.S. Patent No. 6,232,434) and are available for
purchase from companies such as Cayman Chemical, Ann Arbor, MI. Nitric oxide
donors include, but are not limited to L-arginine, N-acetyl-L-cysteine, DEA-
NO,
DETA-NO, DETA-NONOate, PAPA-NO, sodium nitroprusside, and nitroglycerine.
Liquids containing nitric oxide include, but are not limited to liquids
comprising two
nitric oxide generators combined in a fluid to produce nitric oxide, saline in
which
nitric oxide gas has been bubbled, and nitric oxide-saturated water.
As used herein, "adding oxygen" refers to adding oxygen to a fluid to increase
the dissolved oxygen in the fluid to an amount greater than would be present
in the
fluid when the fluid is under an air atmosphere at ambient conditions without
mixing.
As used herein, "process enhancer" refers to a composition that enhances a
pathogen reduction process. Process enhancers can be included in
photoradiation
processes of this invention. Such enhancers include antioxidants or other
agents to
prevent damage to desired fluid components or to improve the rate of reduction
of
microorganisms and are exemplified by adenine, histidine, cysteine, tyrosine,
tryptophan, ascorbate, N-acetyl-L-cysteine, propyl gallate, glutathione,
mercaptopropionylglycine, dithiothreotol, nicotinamide, BHT, BHA, lysine,
serine,
methionine, glucose, mannitol, trolox, glycerol, vitamin E, alpha tocopherol
acetate,
and mixtures thereof. These process enhancers may be added in dried medium
form
(including powder or pill) or in the form of liquids.
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
17
As used herein, "agitator" refers to an apparatus which can agitate, e.g.
shake or
rotate, the container containing the product to be irradiated, such as the
Helmer
platelet incubator/agitator (Helmer Company, Noblesville, IN).
As used herein with respect to platelet compositions, "100% plasma carryover"
and "100% PCO" refer to plasma to which about 20% by volume of anticoagulant
has
been added. 100% PCO is therefore about 80% plasma. By a similar calculation,
90% PCO is about 72% plasma. The balance of the platelet composition which is
neither plasma nor anticoagulant can contain additional ingredients such as
mitochondrial enhancer and/or photosensitizer. Anticoagulants known to the art
are
useful in the practice of this invention, including ACD-A (anticoagulant
citrate
dextrose formula A) and CPD (citrate phosphate dextrose). .
As used herein, "vital quality" refers to an indicator of cellular blood
component quality, i.e., a parameter of a fluid containing cells or a cellular
blood
component that can be measured to assess its quality. Indicators of cellular
blood
component quality (vital qualities) are described below and include but are
not limited
to activation, hypotonic shock response, amount and rate of lactate
production,
amount and rate of glucose consumption, pH and rate of pH change, platelet
swirl,
platelet aggregation, amount and rate of oxygen consumption, amount and rate
of
carbon dioxide production, cell count (cell survival), and extent of shape
change
(ESC). As used herein, to "improve a vital quality of a cellular blood
component"
refers to improving a parameter of a cellular blood component that can be
measured to
assess quality, including the previously mentioned parameters. An improved
cellular
blood component provides better results when utilized, for example, to treat
patients.
Measurement of these vital qualities provides information on the status of
mitochondria health. Additional qualities which provide information on the
status of
mitochondria health include activation, hypotonic shock response, glucose
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
18
consumption, platelet swirl, platelet aggregation, carbon dioxide production,
cell
count, and ESC.
As used herein, "amount of mitochondria) enhancer effective to improve a vital
quality" refers to enough mitochondria) enhancer to cause a measurable
improvement
in a vital cell quality, but not so much as to be toxic to cells containing
mitochondria.
When the mitochondria) enhancer is naturally present in the cell or its
environment,
the mitochondria) enhancer of this invention is added in an amount sufficient
to cause
a measurable improvement in a vital quality. When the mitochondria) enhancer
riboflavin is added to blood cells, between about 0.1 micromolar and about 1
millimolar, between about 1 micromolar and about 200 micromolar, and between
about 5 micromolar and about 75 micromolar final concentrations are effective.
The term "biologically active" means capable of effecting a change in a living
organism or component thereof.
The terms "blood product" and "blood component" as used herein include
blood, plasma, blood constituents, and therapeutic protein compositions
containing
proteins derived from blood.
As used herein, "cellular blood component" refers to blood components that
contain a substantial amount of or are cellular components of blood. Cellular
blood
components include platelets, erythrocytes (red blood cells), eosinophils,
neutrophils,
leukocytes (white blood cells), monocytes, lymphocytes, basophils, and blood
stem
cells. If a sample of plasma contains a substantial amount a cellular blood
component,
i.e. enough so that the cells therein are useful, such as platelets or white
blood cells, it
is a cellular blood component. As used herein, a "blood component comprising
platelets" includes platelets in plasma and platelets in media.
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
19
As used herein, "cells in a wound surface" refers to cells at or near the
surface
of a wound. Wounds towards the surface of a mammalian body can include white
blood cells, red blood cells, fibroblasts, epidermal cells, and endothelial
cells. Cells in
a wound surface can include cells of ectodermal, mesodermal, and endodermal
origin.
"Substantially non-toxic" amounts of elements of this invention are those
which do not destroy the biological activity of such fluid components other
than
microorganisms.
As used herein, "pathogen" refers to an individual pathogenic organism of one
species, a plurality of such organisms of one species, or a plurality of
pathogenic
organisms of two or more species. As used herein, "increase pathogen
inactivation"
with respect to the effects of a procedure described herein refers to
reduction of a
greater quantity of pathogens after using the procedure than in the absence of
the
procedure. As used herein, "increase pathogen reduction" with respect to the
effects
of a procedure described herein refers to reduction of a greater quantity of
pathogens
after using the procedure than in the absence of the procedure.
The pathogens which may be present in fluid and decontaminated by the
processes of this invention typically include those selected from the group
consisting
of extracellular and intracellular viruses, bacteria, bacteriophages, fungi,
blood-
transmitted parasites, and protozoa, and mixtures of any two or more of the
foregoing.
If one of the pathogens is a virus, it may be selected from the group
consisting of
human immunodeficiency virus (HIV), hepatitis A, B and C viruses, sindbis
virus,
cytomegalovirus, vesicular stomatitis virus, herpes simplex viruses, e.g.
types I and II,
human T-lymphotropic retroviruses, HTLV-III, lymphadenopathy virus LAV/IDAV,
parvovirus, transfusion-transmitted (TT) virus, and Epstein-Barr virus, bovine
viral
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
diarrhea virus, pseudorabies, West Nile virus, and mixtures of any two or more
of the
foregoing. If one of the pathogens is a bacteriophage, it may be selected from
the
group consisting of X174, ~6, ~, R17, T4, and T2, and mixtures of any two or
more
of the foregoing. If one of the pathogens is a bacterium, it may be selected
from the
group consisting of P. aeruginosa, S. aureus, S. epidermidis, E. coli, K.
pneumoniae,
E. faecalis, B. subtilis, S. pneumoniae, S. pyrogenes, S. viridans, B. cereus,
E.
aerogenes, propionabacter, C. perfringes, E. cloacae, P. mirabilis, S.
cholerasuis, S.
liquifaciens, S. mitis, Y. entercolitica, P. fluorescens, S. enteritidis, C.
freundii, and S.
marcescens, and mixtures of any two or more of the foregoing. In an embodiment
of
this invention, if one of the pathogens is a protozoon, it may be P.
falciparum.
As used herein "photosensitizer" refers to any compound which absorbs
radiation of one or more defined wavelengths and subsequently utilizes the
absorbed
energy to carry out a chemical process. The photosensitizers useful in this
invention
include any photosensitizers known to the art to be useful for reducing
microorganisms. Examples of such photosensitizers include porphyrins,
psoralens,
dyes such as neutral red, methylene blue, acridine, toluidines, flavine
(acriflavine
hydrochloride) and phenothiazine derivatives, coumarins, quinolones, quinones,
and
anthroquinones. Photosensitizers of this invention may include compounds which
preferentially adsorb to nucleic acids, thus focusing their photodynamic
effect upon
microorganisms and viruses with little or no effect upon accompanying cells or
proteins. Other photosensitizers of this invention are also useful, such as
those using
singlet oxygen-dependent mechanisms. Photosensitizers useful in the practice
of this
invention include endogenous photosensitizers.
As used herein, "activate a photosensitizer" refers to altering a
photosensitizer
to make it capable of substantially reducing pathogens. An activated
photosensitizer
is capable of reducing microorganisms in a fluid, such as by interfering to
prevent
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
21
their replication. Specificity of action of the photosensitizer can be
conferred by the
close proximity of the photosensitizer to nucleic acid of the microorganism
and this
may result from binding of the photosensitizer to the nucleic acid. "Nucleic
acid"
includes ribonucleic acid (RNA) and deoxyribonucleic acid (DNA).
Photosensitizers
can also act by binding to cell membranes or by other mechanisms.
The term "endogenous" means naturally found in a human or mammalian body,
either as a result of synthesis by the body or because of ingestion as an
essential
foodstuff (e.g. vitamins) or formation of metabolites and/or byproducts in
vivo. The
term "non-endogenous" means not naturally found in a human or mammalian body,
either as a result of synthesis by the body or because of ingestion of an
essential
foodstuff or formation of metabolites and/or byproducts in vivo.
Examples of endogenous photosensitizers include alloxazines. Alloxazines are
molecules comprising an alloxazine backbone. The term "alloxazine" includes
isoalloxazines. Examples of endogenous photosensitizers include 7,8-dimethyl-
10-
ribityl isoalloxazine (riboflavin), 7,8,10-trimethylisoalloxazine
(lumiflavin), 7,8-
dimethylalloxazine (lumichrome), isoalloxazine-adenine dinucleotide (flavine
adenine
dinucleotide [FAD]), alloxazine mononucleotide (also known as flavine
mononucleotide [FMN] and riboflavine-5-phosphate), vitamin Ks, vitamin L,
their
metabolites and precursors, and napththoquinones, naphthalenes, naphthols and
their
derivatives having planar molecular conformations. Endogenously based
derivative
photosensitizers include synthetically derived analogs and homologs of
endogenous
photosensitizers which may have or lack lower (1-S) alkyl or halogen
substituents of
the photosensitizers from which they are derived, and which preserve the
function and
substantial non-toxicity thereof. When endogenous photosensitizers or
endogenously
based derivative photosensitizers are used in the practice of this invention,
particularly
when such photosensitizers are not inherently toxic or do not yield toxic
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
22
photoproducts after photoradiation, no removal or purification step is
required after
decontamination, and treated product can be directly returned to a patient's
body or
administered to a patient in need of its therapeutic effect.
Non-endogenous photosensitizers that are mitochondria) enhancers and are
based on endogenous structures, such as those described in U.S. Patent
Application
09/420,652 are useful in the practice of this invention. These non-endogenous
mitochondria) enhancers and endogenously based derivative mitochondria)
enhancers
are referred to herein as endogenously based derivative mitochondria)
enhancers. The
include molecules of the formula:
R3 R~
R4 / N N
N
R5 N II ~R2
R6 O
wherein Rl, R2, R3, R4, RS and R6 are, independently from one another,
selected
from the group consisting of hydrogen, optionally substituted hydrocarbyl,
alcohol,
amine, polyamine, sulfate, phosphate, halogen selected from the group
consisting of
chlorine, bromine and iodine, salts of the foregoing, and -NRa-(CRbR°)n-
X wherein X
is a halogen selected from the group consisting of chlorine, bromine and
iodine, Ra, Rb
and R~ are, independently of each other, selected from the group consisting of
hydrogen, optionally substituted hydrocarbyl, and halogen selected from the
group
consisting of chlorine, bromine and iodine, and n is an integer from 0 to 20;
provided
that Rl is not -OH or a straight chain alkyl group where the second carbon of
the
chain is substituted with -OH or =O and R1, R4 and RS are not all methyl
groups
when R2, R3 and R6 are all hydrogen.
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
23
In one group of compounds, n is an integer between 0 and 5. In another group
of compounds, n is an integer from 0 to 10. In another group of compounds, n
is an
integer from 0 to 20.
In another group of compounds, R1 is not -OH or a straight chain alkyl group
where the second carbon of the chain is substituted with -OH or =O; and Rl is
not a 2-
3-, 4- or 5- carbon straight chain alkyl that terminates in -OH, -COH, or -H
when R2,
R3 and R6 are H, and R4 and RS are CH3; R1 is not - CHZCH2-(CHOH)2-CH3 or -
CHZCH2-(CHOH)2-CH2S04 or 1'-D-sorbityl or 1'-D-dulcityl or 1'-D-rhamnityl or
1'-
D,L-glyceryl or -CHZ-O-C(O)-CH3 or -CHZ-O-C(O)-CH2CH3 or 2', 3', 4', 5'-di-O-
isopropyridene-riboflavin or 8-aminooctyl when R2, R3 and R6 are H and R4 and
RS
are CH3; R1 is not 1'-D-sorbityl or 1'-D-dulcityl when R4 and RS are both
chlorines
and when R2, R3 and R6 are all hydrogens; RS is not ethyl or chloro when R1
and R4
are methyl and R2, R3 and R6 are all hydrogens; R4 and RS are not both methoxy
or
both tetramethylene when Rl is methyl and R2, R3 and R6 are all hydrogens; R2
is
not -CH2CH2NH when Rl, R4 and RS are CH3 and R3 and R6 are H; R2 is not
\N~
~O
when Rl, R4 and RS are CH3 and R3 and R6 are H; RS is not chloro when R4 is
methoxy and R1 is ethyl-2'N-pyrrolidino and R2, R3, and R6 are hydrogen; Rl is
not
N,N-dimethylaminopropyl or N,N-diethylaminoethyl when RS is chloro or methyl
and R2, R3, R4 and R6 are hydrogen; R3 is not -NH(CHZCH2)Cl when R6 is -NHZ
and R1, R2, R4 and RS are H; Rl, R4, RS are not all methyl groups when all of
R2,
R3 and R6 are hydrogens; Rl, R4, RS and R2 are not all methyl groups when R3
and
R6 are hydrogens; R2 is not carboxymethyl when R1, R4 and RS are methyl and R3
and R6 are hydrogen; R4 is not -NHZ when Rl and RS are methyl and R2, R3 and
R6
are all hydrogen; R1 is not a phenyl group when R4 and RS are methyl and R2,
R3
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
24
and R6 are all H; R1 is not methyl or N,N-dimethylaminoethyl when all of R2,
R3,
R4, RS and R6 are hydrogen; R2, R4, RS are not all methyl when Rl is
acetoxyethyl
and R3 and R are hydrogen; RS is not methyl when Rl is N,N-diethylaminoethyl
and
R2, R3, R4 and R6 are all hydrogen; R4 and RS are not both chlorine when R1 is
methyl and R2, R3 and R6 are all hydrogen; R1 is not ethyl, ~-chloroethyl, n-
butyl,
anilino, benzyl, phenyl, p-tolyl or p-anisyl when RS is NH2 and R2, R3, R4 and
R6 are
all hydrogen; and R4 is not chlorine when Rl is N,N-dimethylaminopropyl and
R2,
R3, RS and R6 are all hydrogen.
Compounds containing any combination of substituents or members of the
Markush groups specified above are useful in the practice of this invention.
All
compounds useful in the practice of this invention have the ability to enhance
mitochondria) function. All substituents of the compounds of the invention may
be
the same, all substituents may be different, or any combination of
substituents may be
the same or different. Substituents with a specified function, for example
those that
impart water solubility to the compound, may be included at any of R1-26.
Compounds useful in the practice of this invention include all those compounds
with the isoalloxazine backbone (shown below):
R3 R~
Ra N N
N
R5 N ~ ~ wR2
R6 O
where R1-R6 are substituted with various substituents, as described elsewhere,
except
those previously known to the art. The substituents included in the compounds
and
used in the methods of the invention may be any substituent not having
structures or
reactivity which would substantially interfere with the desired microorganism
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
neutralization of the microorganism neutralizer, as may readily be determined
without
undue experimentation by those skilled in the art. The foregoing isoalloxazine-
related
photosensitizers also function as enhancers of mitochondria) function.
This invention provides methods for treating cell-containing fluids to improve
a
vital quality of the fluid by enhancing the vital quality and/or preventing
damage to
mitochondria within the cells. Compositions useful for treating fluids to
enhance
mitochondria) function and/or prevent damage to mitochondria comprise
mitochondria) enhancers. Mitochondria) enhancers provided by this invention
include
alloxazines such as endogenous alloxazines, non-endogenous alloxazines, and
endogenously based derivative alloxazines, and photosensitizers such as
endogenous
photosensitizers, non-endogenous photosensitizers, and endogenously based
derivative photosensitizers. Molecules with alloxazine backbones are
alloxazines.
The methods provided by this invention comprise adding a substantially non-
toxic
amount of mitochondria) enhancer to a fluid, whereby the function of a
mitochondrion
within a cell in the fluid is enhanced. Mitochondria are enhanced when they
are
prevented from being damaged, when they are rejuvenated, and/or when a vital
cell
quality of a cell containing mitochondria is improved. When a method step is
performed on the fluid which damages mitochondria within cells in the fluid,
such as
storing or photoradiating to reduce pathogens which may be present within the
fluid,
the mitochondria) enhancer prevents damage to or rejuvenates mitochondria
within the
cells. Mitochondria) enhancer may be added before, during, or after the
damaging
method step. The methods of this invention include the use of mitochondria)
enhancer
to prevent damage to and/or rejuvenate non-lymphocytic blood components
before,
during, and after a lymphocytic population reduction process.
Any fluid comprising a cell containing a mitochondrion is treatable by the
methods of this invention. Mitochondria) enhancer is used in an amount which
is
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
26
effective at preventing damage to and/or rejuvenating mitochondria and which
is also
non-toxic to the mitochondria, to the cells containing the mitochondria, and
to the
recipient of the treated fluid. Fluids treatable by the methods of this
invention include
blood, fluids comprising a cellular blood product or a cellular blood
component, and
bodily fluids. Cellular blood components treatable by the methods of this
invention
include platelets, blood, and plasma containing platelets or other cellular
blood
components. When the fluid to be treated by the methods of this invention is
to be
consumed by a human or an animal, the mitochondria) enhancer must also be non-
toxic to the human or animal, or be removed from the fluid before consumption.
This
invention also provides methods for using mitochondria) enhancer to treat
cells having
mitochondria. Cells treatable by the methods of this invention include cells
known in
the art to contain mitochondria, including plant cells, animal cells, and
yeast cells.
This invention also provides methods for using mitochondria) enhancer to treat
a
wound surface. Cells at or near a wound surface can include white blood cells,
red
blood cells, fibroblasts, epidermal cells, and endothelial cells. Cells in a
wound
surface can include cells of ectodermal, mesodermal, and endodermal origin.
Treating
mitochondria-containing cells in a wound surface with mitochondria) enhancer
improves the health of the cells, prevents infection, and speeds healing.
Any fluid that will be in contact with a cell containing a mitochondrion (e.g.
peritoneal fluid and saliva) is treatable by the methods of this invention.
Peritoneal
fluid is the fluid within the peritoneal space that houses the
gastrointestinal organs of
the mammalian body. Peritoneal fluid can contain cells and can be treated by
the
methods of this invention. Additionally, peritoneal fluid can be removed from
a body,
mitochondria) enhancer added, and the treated fluid administered back to a
body.
Alternatively mitochondria) enhancer can be directly administered to
peritoneal fluid
inside a body, without removing peritoneal fluid. The mitochondria) enhancer
in the
peritoneal fluid inside the body enhances the mitochondria) function of cells
within
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
27
the fluid and/or lining the peritoneal space. Adding mitochondrial enhancer to
saliva
enhances the mitochondria) function of cells lining the digestive tract.
When blood or blood components are collected from an individual and the
blood or blood components are to be transfused into the same or another
individual,
the processes of collection and transfusion, the passage of time between
collection and
transfusion, and processes performed on blood and blood components between
collection and transfusion may decrease the quality of the blood or the blood
components. Change in the quality of blood or blood component are detectable
as a
change in a vital quality of the blood or blood component and can be assayed
by any
method known to the art. Indicators of cellular blood component quality (vital
qualities) include but are not limited to activation, hypotonic shock
response, amount
and rate of lactate production, amount and rate of glucose consumption, pH and
rate of
pH change, platelet swirl, platelet aggregation, amount and rate of oxygen
consumption, amount and rate of carbon dioxide production, cell count, and
extent of
shape change (ESC).
The processes of collection and transfusion, the passage of time between
collection and transfusion, and processes performed on blood and blood
components
between collection and transfusion may decrease the quality of blood or
cellular blood
components by damaging mitochondria or mitochondria) processes that occur
within
the blood or cellular blood components. As previously described, when
mitochondria
or mitochondria) processes are damaged, the energy-producing metabolism of a
cell
switches to favor anaerobic glycolysis over oxidative phosphorylation in
combination
with the citric acid cycle and electron transport chain process, which occurs
within the
mitochondria, optionally in combination with glycolysis in the cytoplasm. As a
result,
less oxygen is consumed by the fluid comprising the blood or blood product,
lactate
production is increased (lactate is a product of glycolysis), and the
resulting increase
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
28
in lactic acid causes a decrease in the pH of the fluid. Therefore, indicators
of cell
quality that are also indicators of mitochondria) health include amount and
rate of
oxygen consumption, amount and rate of lactate production, pH and rate of pH
change. Other indicators of cell quality, including, but not limited to,
activation,
hypotonic shock response, amount, and rate of glucose consumption, platelet
swirl,
platelet aggregation, amount and rate of carbon dioxide production, cell
count, and
ESC, also provide information on mitochondria) health.
Processes that can decrease the quality of blood or cellular blood components
include, but are not limited to, the passage of time between collection and
transfusion
and photoradiation to reduce pathogens. The passage of time between collection
and
transfusion, or other use for collected blood or blood product, may be as
short as a few
minutes to a few hours, to days, or as long as several weeks. As described
above,
whole blood components collected in an "open" (i.e., non-sterile) system must,
under
governmental rules, be transfused within twenty-four hours and in most cases
within
six to eight hours. When whole blood components are collected in a "closed"
(i.e.,
sterile) system, the red blood cells can be stored up to forty-two days
(depending upon
the type of anticoagulant and storage medium used), and plasma may be frozen
and
stored for even longer periods. Currently, platelet concentrate may be stored
at room
temperature for up to no more than five days.
This invention provides a method for treating a fluid comprising a cellular
blood component to improve a vital quality of said blood component, said
method
comprising adding a substantially non-toxic amount of a mitochondria) enhancer
to
said fluid wherein said mitochondria) enhancer is selected from the group
consisting
of endogenous alloxazines, non-endogenous alloxazines, endogenously-based
derivative alloxazines, photosensitizers, endogenous photosensitizers, non-
endogenous photosensitizers, and endogenously-based derivative
photosensitizers. In
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
29
one embodiment of this invention, the fluid is not exposed, before, after, or
during
addition of a mitochondria) enhancer, to photoradiation greater than ambient
light.
Adding mitochondria) enhancer to a fluid comprising blood or a cellular blood
component improves the quality of the blood or cellular blood component
thereby
increasing the allowed storage life of the blood or cellular blood component,
allowing
the blood or cellular blood component to be stored for a longer amount of time
before
it is administered to a patient. The FDA provides Guidance for Industry: An
Acceptable Circular of Information for the Use of Human Blood and Blood
Components (http://www.fda.gov/cber/gdlns/circbld.pdfJ and allowed storage
times of
blood and blood components are known in the art. In one embodiment of this
invention involving platelets, the vital cell qualities of platelets treated
with
mitochondria) enhancer are improved such that the treated platelets can be
administered to a patient after seven days of storage.
Mitochondria) enhancer may be added to a fluid before, after, and/or during
storage. In the practice of this invention, if a fluid is utilized for its
intended purpose
immediately after creation or acquisition, it is considered to have not been
stored. If
time passes between creation or acquisition, including processing time, this
is storage
time. In one embodiment of this invention, mitochondria) enhancer is added at
the
beginning of storage. The beginning of storage is about the first 10% of total
storage
time. In another embodiment mitochondria) enhancer is added during the middle
of
storage. The middle of storage is about the central 80% of total storage time.
In
another embodiment, mitochondria) enhancer is added towards the end of
storage.
The end of storage is about the last 10% of total storage time. In an
embodiment of
this invention, a fluid comprising blood or a cellular blood component has
been stored
for an amount of time between about one minute and about forty-five days
before
adding mitochondria) enhancer. In an embodiment of this invention, a fluid
comprising blood or a cellular blood component has been stored for an amount
of time
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
between about one hour and about seven days before adding mitochondria)
enhancer.
In an embodiment of this invention, the fluid comprises platelets that have
been stored
for six days before mitochondria) enhancer is added to the fluid. In an
embodiment of
this invention, a fluid comprising blood or a cellular blood component is
stored for an
amount of time between about one hour and about five days after adding
mitochondria) enhancer. In another embodiment of this invention, a fluid
comprising
blood or a cellular blood component is stored for an amount of time between
about
one minute and about forty-five days before adding mitochondria) enhancer, and
the
fluid to which mitochondria) enhancer had been added is subsequently stored
for an
amount of time between about one minute and about forty-five days. In another
embodiment of this invention, a fluid comprising platelets is stored for an
amount of
time up to about three years after adding mitochondria) enhancer. In yet
another
embodiment of this invention, a fluid comprising a cellular blood component is
stored
for an amount of time up to about three years before adding mitochondria)
enhancer.
Additional methods steps may be performed on a fluid in the practice of this
invention. In one embodiment of this invention, the method also comprises
exposing
the fluid to photoradiation of energy greater than ambient light. In one
embodiment,
the method also comprises performing a pathogen reduction process on the
fluid. In
one embodiment of this invention, the pathogen reduction process comprises
exposing
the fluid to photoradiation of sufficient energy to substantially reduce
pathogens
which may be present in the fluid. In one embodiment, a pathogen reduction
process
is performed before adding mitochondria) enhancer to the fluid. In another
embodiment, mitochondria) enhancer is added during a pathogen reduction
process.
In another a pathogen reduction process is performed after adding
mitochondria)
enhancer. In one embodiment, mitochondria) enhancer is added during a pathogen
reduction process, resulting in a pathogen reduction process being performed
before,
during, and after adding mitochondria) enhancer. As known in the art, pathogen
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
31
reduction processes utilizing photoradiation also typically utilize a
photosensitizer. In
one embodiment of this invention, the method also comprises adding a
photosensitizer
to the fluid. A mitochondria) enhancer may also be a photosensitizer and vice
versa.
In one embodiment of this invention, the photosensitizer is the same as the
mitochondria) enhancer utilized. In one embodiment, the step of adding
photosensitizer to the fluid is performed by adding mitochondria) enhancer to
the
fluid. Pathogens that are substantially reduced by the methods of this
invention
include extracellular and intracellular viruses, bacteria, bacteriophages,
fungi, blood-
transmitted parasites, protozoa, and mixtures of any two or more of the
foregoing.
When performing a pathogen reduction process utilizing photoradiation on a
fluid, an amount of photoradiation is chosen that substantially reduces
pathogens
without destroying desired biological activities within the fluid. Preferably
an amount
of photoradiation is chosen that minimally damages or decreases desired
biological
activities within the fluid. In an embodiment of this invention, visible
photoradiation
is of about 419nM. In an embodiment of this invention, ultraviolet radiation
is of
about 320nM. This invention provides methods for adding mitochondria) enhancer
to
a fluid, wherein addition of mitochondria) enhancer protects or rejuvenates
fluid
components from damage caused by photoradiation steps of a pathogen reduction
process performed on the fluid, enabling the use of more photoradiation energy
which
in turn enables better pathogen reduction. In an embodiment of this invention,
mitochondria) enhancer is added to a fluid comprising platelets before a
pathogen
reduction process utilizing between about 5 J/cm2 and about 360 J/cm2
ultraviolet
photoradiation is performed on the fluid. In another embodiment of this
invention, the
pathogen reduction process utilizes more than about 5 J/cm2, more than about
30
J/cm2, more than about 50 J/cm2, more than about 80 J/cm2, more than about 100
J/cm2, more than about 120 J/cm2, more than 120 J/cm2, more than about 180
J/cm2,
more than 180 J/cm2, more than 200 J/cm2, between about 5 J/cm2 and about 360
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
32
J/cm2, between about 25 J/cmz and about 180 J/cm2, between about 75 J/cm2 and
about 120 J/cm2, or between about 120 J/cmZ and about 180 J/cm2 ultraviolet
radiation.
In one embodiment of this invention, more than one mitochondria) enhancer is
added. In one embodiment, mitochondria) enhancer is added more than once.
In one embodiment of this invention, the mitochondria) enhancer is riboflavin,
also known as 7,8-dimethyl-10-ribityl isoalloxazine. In one embodiment,
riboflavin is
added to a final concentration in the fluid of about 1 micromolar to about 200
micromolar. In one embodiment about ten micromolar is added. In another
embodiment about 50 micromolar is added. Riboflavin can be a photosensitizer
and a
mitochondria) enhancer. In one embodiment, riboflavin is added in an amount
between about five micromolar and about 100 micromolar.
In the practice of this invention, at least one vital quality of the fluid
being
treated, e.g. a fluid comprising blood or cellular blood component, is
improved after
adding mitochondria) enhancer to the fluid. Any indicator of cell quality
known in the
art may be measured. During cell metabolism, cells consume glucose and make
two
lactate molecules, which lowers the pH. In the U.S. the specified lower limit
for pH
of the surrounding fluid is about 6.2 as measured at 22°C. Limits can
be different in
different countries. A fixed amount of glucose is provided to cells in
storage. If the
cells use up the glucose too quickly, they will die. A slower consumption of
glucose
is better, resulting in less lactose production and maintenance of a pH above
6.2.
Glucose consumption, lactose production, and pH indicators of cell quality are
measured in rate as well as absolute change. Three activity parameters that
can be
measured to determine whether platelets and other cellular blood components
have
retained their functional ability after storage are: cell count, hypotonic
stress response,
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
33
and aggregation, as induced by collagen in combination with adenosine
diphosphate
(ADP). In an embodiment of this invention, indicators of cell quality measured
in the
practice of this invention include activation, hypotonic shock response,
amount and
rate of lactate production, amount and rate of glucose consumption, pH, rate
of pH
change, platelet swirl, platelet .aggregation, amount and rate of oxygen
consumption,
amount and rate of carbon dioxide production, cell count, and/or ESC.
Indicators of
cell quality are typically measured periodically, e.g., cell quality for
platelets is
typically measured each hour or on Days 1, 3, 5, and/or 7 after adding
mitochondria)
enhancer. All methods known in the art for measuring indicators of cell
quality are
useful in the practice of this invention. Oxygen concentration, carbon dioxide
concentration, and pH may be measured using a blood gas analyzer.
P-selectin, also known as GMP-140, measures activation. When cells are
activated, p-selectin is expressed and appears on the surface of the cells.
Platelet cells
must retain the ability to activate when they are taken out of long-term
storage to
function normally for transfusion purposes. Cells need to be activated in
vivo, so
premature activation in vitro needs to be prevented. There is improved
recovery and
survival of platelets in vivo when p-selectin is kept low (Transfusion
2002;42:847-854
and Transfusion 2002;42:1333-1339). Limits for values of certain vital
qualities of
blood components for use in treating patients are set by the Food and Drug
Administration (FDA) of the United States (See Circular of Information for the
Use of
Human Blood and Blood Components or
http://www.fda.gov/cber/gdlns/circbld.pdf).
Expression of p-selectin is optionally measured before adding mitochondria)
enhancer
and then at one or more time points after adding mitochondria) enhancer. At a
selected time point, the percentage of cells expressing p-selectin is measured
for both
treated and untreated samples. The percentage of cells expressing p-selectin
is the
percent of cells activated (percent activation). The amount of change in
activation due
to the addition of mitochondria) enhancer is calculated by ((percent
activation of
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
34
treated - percent activation of untreated) / percent activation of untreated))
* 100 =
percent change in activation. If the percent change in activation is negative:
absolute
value of the percent change in activation = amount of decrease in activation,
in
percent, at that time point. In the practice of this invention, cellular
activation, is
decreased as compared to cellular activation in a fluid to which mitochondria)
enhancer has not been added. Preferably activation is measured by detecting
alpha
granule release, such as by the presence of p-selectin expression, but any
method
known in the art may be used. Activation can be decreased by at least about
3%, by at
least about 10%, and up to at least about 15%. In an embodiment of this
invention,
activation can be decreased by an amount between about 5% and about 50%.
Hypotonic stress response (HSR) is an assay used to determine if platelets
have
retained metabolic viability. It measures the ability of the cells to respond
to osmotic
shock after about a ten-minute recovery period. Percent HSR measures the
percentage
of cells that are able to recover in about ten minutes. The percentage of
cells that are
able to recover is the percent of reversal. The specified lower limit for HSR
is about
36%. This assay is a photometric measurement of the platelets' ability to
overcome
the addition of a hypotonic solution. This activity reflects cell function
(i.e., ability to
maintain a functional membrane water pump) and is indicative of platelet
recovery
following storage. Hypotonic stress response has been demonstrated to be an
important indicator of platelets' ability to survive in circulation following
transfusion.
Consequently, hypotonic stress response represents an important parameter for
evaluating platelet biochemistry following storage. HSR is optionally measured
before adding mitochondria) enhancer and then at one or more time points after
adding mitochondria) enhancer. At a selected time point, HSR is measured for
both
treated and untreated samples. The amount of change in HSR due to the addition
of
mitochondria) enhancer is calculated by ((HSR of treated - HSR of untreated) /
HSR
of untreated)) * 100 = percent change in HSR. If the percent change in HSR is
not
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
negative, the percent change in HSR is the amount of increase in HSR, in
percent, at
that time point. In the practice of this invention, hypotonic shock response
(HSR)
is increased, as compared to hypotonic shock response in a fluid to which
mitochondria) enhancer has not been added. HSR can be increased by at least
about
5%, by at least about 20%, and up to at least about SO%. In an embodiment of
this
invention, HSR can be increased by an amount between about S% and about 25% as
measured on Day 5 after adding mitochondria) enhancer.
Platelet swirl is a subjective, qualitative indicator of cell quality. When a
blood bag is squeezed, healthy cells will swirl, creating a pattern which can
be
observed by the light reflecting off and through the cells. Platelet swirl is
scored on a
scale of from zero to three or four, with three or four being the healthiest.
The
quantity of cells swirling and the strength of the swirl are two
characteristics that are
considered. Platelet swirl is optionally measured before adding mitochondria)
enhancer and then at one or more time points after adding mitochondria)
enhancer. At
a selected time point, platelet swirl is measured for both treated and
untreated
samples. The amount of change in platelet swirl due to the addition of
mitochondria)
enhancer is calculated by ((platelet swirl of treated - platelet swirl of
untreated) /
platelet swirl of untreated)) * 100 = percent change in platelet swirl. If the
percent
change in platelet swirl is not negative, the percent change in platelet swirl
is the
amount of increase in platelet swirl, in percent, at that time point. In the
practice of
this invention, platelet swirl is increased, as compared to platelet swirl in
a fluid to
which mitochondria) enhancer has not been added. Platelet swirl can be
increased by
at least about 5% and by at least about 20%. In an embodiment of this
invention,
platelet swirl can be increased by an amount between about 5% and about 1000%
as
measured on Day 5 after adding mitochondria) enhancer.
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
36
' In the practice of this invention, the pH of the fluid is increased and the
rate of
pH decrease can be decreased, as compared to pH and rate of pH decrease in a
fluid to
which mitochondrial enhancer has not been added. The increased rate of pH
increase
can also be measured, if pH increases. pH is optionally measured before adding
mitochondrial enhancer and then at one or more time points after adding
mitochondrial enhancer. At a selected time point, pH is measured for both
treated and
untreated samples. The amount of change in pH in pH units is the pH of treated
samples - pH of untreated samples. To calculate the change in the rate of pH,
pH is
measured at two or more selected time points, wherein at least one of the time
points
occurs after adding mitochondrial enhancer. Time point 2 is measured after
time point
1. The rate of pH change is calculated by (pH at time point 2 - pH at time
point 1 ) /
(time point 2 - time point 1) = rate of pH change in pH units per unit of time
(such as
hours or days). If the rate of pH change is negative, the pH is decreasing
with time.
pH of a fluid comprising a cellular blood component can decrease after
photoradiation. The rate of pH change is calculated for both treated and
untreated
samples. The percent change in rate of pH change is ((rate of change of pH of
treated
- rate of change of pH of untreated) / rate of change of pH of untreated) *
100. If the
rate of pH change of treated, the rate of change of untreated, and the percent
change in
rate of pH change are negative, the rate of pH change is decreased by adding
mitochondrial enhancer. The rate of pH decrease is the absolute value of the
percent
change in rate of pH change. The rate of pH decrease can be decreased by at
least
about 2%, by at least about 20%, and up to at least about 40%. In an
embodiment of
this invention, the rate of pH decrease can be decreased by an amount between
about
2% and about SO%. The pH can be increased by at least about 0.1 units, by at
least
about 0.2, by at least about 0.35, and up to at least about 0.5. In an
embodiment of
this invention, the pH can be increased by an amount between about 0.1 and
about
0.75. In an embodiment of this invention, the rate of pH decrease can be
decreased by
an amount between about 15% and about 50% on Day 5. In an embodiment of this
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
37
invention, the pH is increased by an amount between about 0.1 and about 0.5 pH
units, about twenty-four hours after mitochondria) enhancer is added to
platelets that
were stored for six days after apheresis.
In the practice of this invention, the rate of lactate production by cells in
the
fluid being treated and the amount of lactate produced are decreased, as
compared to
rate of lactate production and amount of lactate produced in a fluid to which
mitochondria) enhancer has not been added. Amount of lactate in a fluid can be
measured by any method known in the art. Amount of lactate is optionally
measured
before adding mitochondria) enhancer and then at one or more time points after
adding mitochondria) enhancer. Amount of lactate can be measured as the
concentration of lactate in the fluid. At a selected time point, amount of
lactate is
measured for both treated and untreated samples. The percent change of lactate
production is the ((amount of lactate in treated samples - amount of lactate
in
untreated samples) / amount of lactate in untreated samples) * 100. If the
percent
change of lactate production is negative, the percent decrease in lactate
production due
to adding mitochondria) enhancer is the absolute value of the percent change
of lactate
production. To calculate the rate of lactate production, amount of lactate is
measured
at two or more selected time points, wherein at least one of the time points
occurs
after adding mitochondria) enhancer. Time point 2 is measured after time point
1.
The rate of lactate production is calculated by (amount of lactate at time
point 2 -
amount of lactate at time point 1 ) / (time point 2 - time point 1 ) = rate of
lactate
production in units in which lactate was measured (e.g. concentration) per
unit of time
(e.g. hours or days). The rate of change of lactate production is calculated
for both
treated and untreated samples. The percent change in the rate of lactate
production is
((rate of change of lactate production of treated - rate of change of lactate
production
of untreated) / rate of change of lactate production of untreated) * 100. If
the rate of
lactate production of treated and the rate of lactate production of untreated
are
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
38
positive, and the percent change in rate of lactate production is negative,
the rate of
lactate production is decreased by adding mitochondria) enhancer. The decrease
in
the rate of lactate production is the absolute value of the percent change in
rate of
lactate production. The rate of lactate production (micromoles per hour) can
be
decreased by at least about 5%, by at least about 50%, by at least about 80%
and up to
at least about 125%. In an embodiment of this invention, the rate of lactate
production
can be decreased by an amount between about 25% and about 100%. In an
embodiment of this invention, the amount of lactate produced can be decreased
by an
amount between about 15% and about 100%. In an embodiment of this invention,
the
amount of lactate produced can be decreased by about 75%. In an embodiment of
this
invention, the rate of lactate production can be decreased by an amount
between about
75% and about 100% about twenty-four hours after mitochondria) enhancer is
added
to platelets.
In the practice of this invention, the rate of glucose consumption by cells in
the
fluid, such as cellular blood components, and the amount of glucose consumed
are
decreased, as compared to rate of glucose consumption and amount of glucose
consumed in a fluid to which mitochondria) enhancer has not been added.
Glucose
consumption is decreased by adding mitochondria) enhancer because adding
mitochondria) enhancer enables a cell to better utilize mitochondria)
biochemical
pathways (citric acid cycle and electron transport chain) to generate energy,
which
generate more energy per glucose molecule compared to cytoplasmic biochemical
pathways (glycolysis). Additionally, in platelets, mitochondria) biochemical
pathways
for generating energy don't require glucose. Glucose consumption can be
measured
by measuring the amount of glucose remaining in the fluid at a selected time
point.
The decrease in glucose consumption can be measured as the increase in the
amount
of glucose remaining in the fluid. The amount of glucose remaining in a fluid
can be
measured by any method known in the art. The amount of glucose remaining is
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
39
optionally measured before adding mitochondria) enhancer and then at one or
more
time points after adding mitochondria) enhancer. The amount of glucose can be
measured as the concentration of glucose (e.g. molecules/volume or
molecules/cells)
in the fluid. At a selected time point, amount of glucose is measured for both
treated
and untreated samples. The percent change of glucose consumption is the
((amount of
glucose in treated samples - amount of glucose in untreated samples) / amount
of
glucose in untreated samples) * 100. By this calculation, the percent change
of
glucose remaining in the fluid is the percent change of glucose consumption
due to
adding mitochondria) enhancer. To calculate the rate of glucose consumption,
the
amount of glucose remaining in the fluid is measured at two or more selected
time
points, wherein at least one of the time points occurs after adding
mitochondria)
enhancer. Time point 2 is measured after time point 1. The rate of glucose
consumption is calculated by (amount of glucose remaining at time point 2 -
amount
of glucose remaining at time point 1 ) / (time point 2 - time point 1 ) = rate
of glucose
consumption in units in which glucose was measured (e.g. concentration) per
unit of
time (e.g. hours or days). The rate of glucose consumption, as calculated
using
amount of glucose remaining (FIG. 8) can be negative. The change in the rate
of
glucose consumption is calculated for both treated and untreated samples. The
percent change in the rate of glucose consumption is ((rate of glucose
consumption of
treated - rate of glucose consumption of untreated) / rate glucose consumption
of
untreated) * 100. If the rate of glucose consumption of treated and the rate
of glucose
consumption of untreated are negative, and the percent change in rate of
glucose
consumption is positive, the rate of glucose consumption is decreased by
adding
mitochondria) enhancer. The decrease in the rate of glucose consumption is the
percent change in rate of glucose consumption. When the rate of glucose
consumption is decreased, more glucose is left in the fluid at a selected time
point.
The rate of glucose consumption can be preferably decreased by at least about
5%, by
at least about 25%, at least about 75%, and up to at least about 100%. In an
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
embodiment of this invention, the rate of glucose consumption can be decreased
by an
amount between about 25% and about 150%. The amount of glucose consumed, as
measured by the amount of glucose remaining, can be decreased by at least
about
10%, by at least about 25%, and up to at least about 80%. In an embodiment of
this
invention, the amount of glucose consumed, as measured by the amount of
glucose
remaining, can be decreased by an amount between about 10% and about 150%, or
between about 25% and about 100% on Day 3.
The extent of shape change (ESC) is the extent to which cellular blood
component cells are able to change shape when contacted with an agonist.
Healthy
cells are able to change shape. Percent ESC measures the percent of cells able
to
change shape. The specified lower limit for ESC is 10%. ESC is optionally
measured
before adding mitochondrial enhancer and then at one or more time points after
adding mitochondrial enhancer. At a selected time point, ESC is measured for
both
treated and untreated samples. The amount of change in ESC due to the addition
of
mitochondrial enhancer is calculated by ((ESC of treated - ESC of untreated) /
ESC of
untreated)) * 100 = percent change in ESC. If the percent change in ESC is not
negative, the percent change in ESC is the amount of increase in ESC, in
percent, at
that time point. In the practice of this invention, when the cellular blood
component
comprises platelets, the extent of cell shape change (ESC) is increased, as
compared to
the ESC of a fluid containing platelets to which mitochondrial enhancer has
not been
added. In an embodiment of this invention, the ESC can be increased by an
amount
between about 5% and about 125%. In an embodiment of this invention, the ESC
can
be increased by at least about 5%, by at least about 25%, by at least about
75%, and
up to at least about 100%. In an embodiment of this invention, the ESC can be
increased by at least about 75% on Day 5.
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
41
In the practice of this invention, the amount and/or the rate of oxygen
consumption of the cellular blood component are/is increased, as compared to
the
amount and/or rate of oxygen consumption of a cellular blood component in a
fluid to
which mitochondria) enhancer has not been added. Amount of oxygen present in a
gas above a fluid can be measured by any method known in the art. Glycolysis
does
not require oxygen, but the citric acid cycle does, therefore, when cells are
utilizing
mitochondria) biochemistry to generate energy (citric acid cycle), more oxygen
is
consumed and consequently less is left in the gas above the fluid containing
the cells.
Amount of oxygen is optionally measured before adding mitochondria) enhancer
and
then at one or more time points after adding mitochondria) enhancer. At a
selected
time point, amount of oxygen is measured for both treated and untreated
samples. The
percent change of oxygen consumption is also the percent change in oxygen
remaining in the gas above the fluid. The percent change of oxygen consumption
is
the ((amount of oxygen in treated samples - amount of oxygen in untreated
samples) /
amount of oxygen in untreated samples) * 100. If the percent change of oxygen
consumption is negative, the percent increase in oxygen consumption due to
adding
mitochondria) enhancer is the absolute value of the percent change of oxygen
consumption. To calculate the rate of oxygen consumption, amount of oxygen is
measured at two or more selected time points, wherein at least one of the time
points
occurs after adding mitochondria) enhancer. Time point 2 is measured after
time point
1. The rate of oxygen consumption is calculated by (amount of oxygen at time
point 2
- amount of oxygen at time point 1 ) / (time point 2 - time point 1 ) = rate
of oxygen
consumption in units in which oxygen was measured (e.g. partial pressure) per
unit of
time (e.g. hours or days). The rate of change of oxygen consumption is
calculated for
both treated and untreated samples. The percent change in the rate of oxygen
consumption is ((rate of change of oxygen consumption of treated - rate of
change of
oxygen consumption of untreated) / rate of change of oxygen consumption of
untreated) * 100. If the rate of oxygen consumption of treated and the rate of
oxygen
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
42
consumption of untreated are positive, and the percent change in rate of
lactate
production is negative, the rate of oxygen consumption is increased by adding
mitochondria) enhancer. The increase in the rate of oxygen consumption is the
absolute value of the percent change in rate of oxygen consumption. In an
embodiment of this invention, the oxygen consumption can be increased by an
amount
between about 5% and about 125%. In an embodiment of this invention, the
oxygen
consumption can be increased by at least about 5%, by at least about 15%, by
at least
about 75%, by at least about 100%, and up to at least about 175%. In an
embodiment
of this invention, the oxygen consumption can be increased by at least about
50% at
twenty-four hours after adding mitochondria) enhancer. In an embodiment of
this
invention, the rate of oxygen consumption can be increased by at least about
10% or
between about 10% and about 50%.
In the practice of this invention, the rate of carbon dioxide production by
cells
in the gas above a fluid being treated and the amount of carbon dioxide
produced are
increased, as compared to the rate of carbon dioxide production and amount of
carbon
dioxide produced by a cell-containing fluid to which mitochondria) enhancer
has not
been added. Carbon dioxide is produced during mitochondria) biochemical
pathways
(citric acid cycle) for producing energy. Amount of carbon dioxide in the gas
above a
fluid can be measured by any method known in the art. Amount of carbon dioxide
is
optionally measured before adding mitochondria) enhancer and then at one or
more
time points after adding mitochondria) enhancer. Amount of carbon dioxide can
be
measured as the concentration (e.g. partial pressure) of carbon dioxide in the
fluid.
At a selected time point, amount of carbon dioxide is measured for both
treated and
untreated samples. The percent change of carbon dioxide production is percent
change of carbon dioxide in the gas above a treated sample compared to an
untreated
sample. The percent change of carbon dioxide production is the ((amount of
carbon
dioxide in treated samples - amount of carbon dioxide in untreated samples) /
amount
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
43
of carbon dioxide in untreated samples) * 100. If the percent change of carbon
dioxide production is positive, it is the percent increase in carbon dioxide
production
due to adding mitochondria) enhancer. To calculate the rate of carbon dioxide
production, amount of carbon dioxide is measured at two or more selected time
points,
wherein at least one of the time points occurs after adding mitochondria)
enhancer.
Time point 2 is measured after time point 1. The rate of carbon dioxide
production is
calculated by (amount of carbon dioxide at time point 2 - amount of carbon
dioxide at
time point 1 ) / (time point 2 - time point 1 ) = rate of carbon dioxide
production in
units in which carbon dioxide was measured (e.g. concentration) per unit of
time (e.g.
hours or days). The rate of change of carbon dioxide production is calculated
for both
treated and untreated samples. The percent change in the rate of carbon
dioxide
production is ((rate of change of carbon dioxide production of treated - rate
of change
of carbon dioxide production of untreated) / rate of change of carbon dioxide
production of untreated) * 100. If the rate of carbon dioxide production of
treated and
the rate of carbon dioxide production of untreated are positive, and the
percent change
in rate of carbon dioxide production is positive, the rate of carbon dioxide
production
is increased by adding mitochondria) enhancer.
Potential for aggregation is another vital quality that indicates whether
blood
platelets have maintained their functional integrity during storage. This
potential is
measured by using ADP and collagen to induce aggregation. An agonist is an
agent
that binds to a receptor and initiates a certain response. In an agonist-
induced
aggregation, aggregation or clumping is the response to the agonist. The
agonists
ADP and collagen are used to induce aggregation to determine if platelets have
retained their ability to aggregate. In addition, when performing aggregation
response
tests, one can detect the presence of spontaneous aggregation, that is the
platelets
adhering to each other without the addition of an agonist. The occurrence of
spontaneous aggregation has been correlated with removal of platelets from
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
44
circulation, indicating the platelets have short survival times. Aggregation
can be
measured by any method known in the art. Aggregation is optionally measured
before
adding mitochondria) enhancer and then at one or more time points after adding
mitochondria) enhancer. At a selected time point, aggregation is measured for
both
treated and untreated samples. The amount of change in aggregation due to the
addition of mitochondria) enhancer is calculated by ((aggregation of treated -
aggregation of untreated) / aggregation of untreated)) * 100 = percent change
in
aggregation. If the percent change in aggregation is negative, the absolute
value of the
percent change in aggregation is the amount of decrease in aggregation, in
percent, at
that time point.
Vital cell qualities that determine allowed storage life of blood components
are
determined by the U.S. FDA (See Circular of Information for the Use of Human
Blood and Blood Components or http://www.fda.gov/cber/gdlns/circbld.pdf). To
increase storage life of a blood component, the vital cell quality that is
limiting the
storage life of that blood component must be improved. Additional vital cell
qualities
can be improved as well. In the practice of this invention, when 10 to 50
micromolar
riboflavin is added to platelets, the vital quality of cellular blood
component activation
can be decreased by at least about 3%, and/or the vital quality of HSR can be
increased by at least about S%, and/or the vital quality of platelet swirl can
be
increased by at least about 5%, and/or the vital quality of pH of the fluid
containing
the cellular blood component can be decreased by at least about 0.1 pH units,
and/or
the vital quality of rate of lactate production can be decreased by at least
about 5%,
and/or the vital quality of rate of glucose consumption can be decreased by at
least
about 5%, and/or the vital quality of rate of oxygen consumption can be
decreased by
at least about 5%, and/or the vital quality of carbon dioxide production can
be
decreased by about 5%. In an embodiment of this invention, the cellular blood
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
component is platelets and they can be stored, e.g. for a period greater than
five days,
for between about five days and about seven days.
Collected platelets can be concentrated before treating using the methods of
this invention. Platelets can be concentrated by any method known in the art,
such as
using apheresis devices while collecting blood or from previously collected
samples
of whole blood. In an embodiment of this invention, platelets are
hyperconcentrated
to form hyperconcentrated platelets (HCP) by centrifugation at 3000 times
gravity
(3000xG) for fifteen minutes and allowed to rest for one hour. In an
embodiment of
this invention, HCP are resuspended in autologous plasma, resulting in
approximately
five trillion platelets per milliliter. Fluid can consist essentially of
platelets in plasma
or cell culture media, e.g. comprising platelets and between about 5% and
about 95%
plasma or media.
The methods provided by this invention optionally further comprise
photoradiating, adding photoactivator, adding nitric oxide, adding quencher,
adding
glycolysis inhibitor, adding oxygen, and/or adding process enhancers to the
fluid
being treated.
Pathogen reduction using photoradiation requires mixing a photosensitizer with
the material to be decontaminated. Mixing may be done by simply adding the
photosensitizer or a solution containing the photosensitizer to the fluid to
be
decontaminated. In one system, the material to be decontaminated to which the
photosensitizer has been added is flowed past a photoradiation source, and the
flow of
the material provides sufficient turbulence to distribute the photosensitizer
throughout
the fluid to be decontaminated. In another system, the fluid and
photosensitizer are
placed in a photopermeable container and irradiated in batch mode, preferably
while
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
46
agitating the container to fully distribute the photosensitizer and expose all
the fluid to
the radiation.
The amount of photosensitizer to be mixed with the fluid will be an amount
sufficient to adequately reduce microorganisms therein, but less than a toxic
(to
humans or other mammals) or insoluble amount. Optimal concentrations of
desired
photosensitizers may be readily determined by those skilled in the art without
undue
experimentation.
The fluid containing the photosensitizer is exposed to photoradiation of the
appropriate wavelength to activate the photosensitizer, using an amount of
photoradiation of sufficient energy to activate the photosensitizer as
described above,
but less than that which would cause non-specific damage to the biological
components or substantially interfere with biological activity of other
proteins present
in the fluid. The wavelength used will depend on the photosensitizer selected,
as is
known in the art or readily determinable without undue experimentation
following the
teachings hereof. After exposure to the light energy, the pathogen reduced
cellular
blood component may be kept in the pathogen reduction solution, or may be
transferred to a storage solution.
Photoradiation to reduce pathogens is performed by methods known in the art
or by methods described in references included herein. An amount of energy is
supplied to the fluid using photoradiation. Preferably the amount of energy
supplied
is sufficient to reduce pathogens which may exist in the fluid, but also does
not
substantially interfere with the biological activity of the blood components)
contained
in the fluid. The biological activity of blood components) in the fluid at
least meets
minimum standards for medical and veterinary use for standard storage times
for the
specific blood component, e.g., five or preferably seven days for platelets.
Pathogen
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
47
reduction methods of this invention are described as using flux (energy) in
units of
joules (J) per unit area (cm2) per unit time (min). A time length of
photoradiation is
selected to accomplish delivering a total amount of energy selected to
substantially
reduce pathogens in the fluid being treated. Some lamps useful for providing
the
required energy are VHO lights with a mercron ballast, T8 lights with an
icecap
ballast, or T8 lights with an icecap ballast and quartz attenuator.
Photoradiation can
be delivered continuously or in a segmented (interrupted) fashion. The
photoradiation
is preferably within the ultraviolet range or the visible range. A
photoradiation light
source can be selected that is capable of providing light of about 300 nm to
about 700
nm, or between about 340 nm to about 650 nm of radiation. The energy delivered
to
the fluid is an amount sufficient to activate a photosensitizes, e.g., between
about 5
J/cm2 and about 360 J/cm2. In an embodiment of this invention, the total time
of
photoradiation is sufficient to substantially reduce pathogens, e.g., between
about
three and about thirty minutes. To "substantially inactive pathogens" means to
reduce
their ability to reproduce, preferably by killing them, to levels in a blood
component
such that the blood component may be safely administered to a patient.
Ultraviolet
wavelength of 320 nm is useful in the practice of this invention.
This invention provides methods for improved pathogen reduction. Pathogen
reduction processes using photoradiation are improved by the addition of
mitochondrial enhances. Addition of mitochondrial enhances to a fluid
containing a
blood product containing mitochondria prevents damage to the blood product
from
photoradiation, which allows the use of high photoradiation energies, which
are more
effective at reducing pathogens. Prevention of damage to the blood product is
indicated by improvement to vital cell qualities in fluids treated with
mitochondrial
enhances compared to fluids treated at equivalent energies without addition of
mitochondrial enhances, before, after, or during photoradiation. High energies
useful
in the practice of this invention include more than about 30 J/cm2, more than
about 50
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
48
J/cmz, more than about 80 J/cm2, more than about 100 J/cmz, more than about
120
J/cm2, more than 120 J/cm2, more than about 180 J/cm2, more than 180 J/cm2,
between about 5 J/cmz and about 360 J/cm2, between about 25 J/cm2 and about
180
J/cmz, between about 75 J/cm2 and about 120 J/cm2, and between about 120 J/cmz
and
about 180 J/cm2. ultraviolet photoradiation. This invention provides an
improved
method for treating a fluid comprising a cellular blood component to reduce
pathogens
which may be present therein, comprising the steps of:
(a) adding an reduction-effective, substantially non-toxic amount of a
photosensitizer to said fluid;
(b) adding an effective amount of mitochondria) enhancer to said fluid and
in an amount sufficient to improve a vital quality of said cellular blood
component; and
(c) exposing said fluid to photoradiation of sufficient energy to activate
said
photosensitizer, for a sufficient time to substantially reduce said
pathogens.
In the practice of this invention the photoradiation is preferably applied at
an energy
more than about 25 J/cm2, wherein the photoradiation is substantially non-
toxic to the
cellular blood component. Amounts of photoradiation energy and mitochondria)
enhancer are selected such that extent of pathogen reduction and vital cell
quality
immediately after photoradiation and at about 24 hours after photoradiation
are about
the same or better than an equivalent process without mitochondria) enhancer
at a
lower energy. Examples of lower energies are about five to about 15 J/cm2. In
the
practice of this invention, photoradiation is preferably delivered at more
than about 25
J/cm2 or more than about 40 J/cm2. In the practice of this invention,
photoradiation is
delivered at an energy between about 25 J/cmZ and about 120 J/cmz, between
about 25
J/cm2 and about 180 J/cm2, or between about 25 J/cm2 and about 360 J/cm2.
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
49
Fluids may optionally be mixed before or during photoradiation. Mixing may
enhance dissolution of components. Before or during photoradiation, the fluid
may be
mixed by mixing and/or shaking at a speed between about 70cpm and about
150cpm,
or between about 120cpm and about 135cpm. When performed before
photoradiation,
mixing can be performed for about one to about ten minutes. The mixing and
shaking
may be performed in any motion known to the art, including mixing and shaking
using a to-and-fro motion. One or more of the light sources may move in a
coordinated manner with the movement of the mixing. Mixing enables the
majority of
the photosensitizer and fluid contained within the container to be exposed to
the light
emitted from each of the discrete radiation sources by continually replacing
the
exposed fluid at the light-fluid interface with fluid from other parts of the
bag not yet
exposed to the light. Such mixing continually brings to the surface new fluid
to be
exposed to light. Photoradiation can be performed at a temperature that allows
for
reduction of pathogens and does not interfere with the biological activity of
cellular
blood components. Photoradiated fluids containing cellular blood components
can be
stored at temperatures known in the art for storing blood products.
Materials which may be treated by methods of this invention that involve
photoradiation greater than ambient light include any materials which are
adequately
permeable to photoradiation to provide sufficient light to achieve pathogen
reduction,
,or which can be suspended or dissolved in fluids which have such permeability
to
photoradiation. Plasma and cell culture media containing cellular blood
components
are permeable to photoradiation.
Decontamination methods of this invention do not destroy the biological
activity of fluid components other than microorganisms. As much biological
activity
of these components as possible is retained, although in certain instances,
when the
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
methods are optimized, some loss of biological activity, e.g., denaturization
of protein
components, must be balanced against effective decontamination of the fluid.
In addition to treating whole blood, fluids containing cellular blood products
and cell-containing bodily fluids, this method is useful for treating other
cell-
containing fluids including fluids which are meant for nourishment of humans
or
animals such as fruit and vegetable juices.
EXAMPLES
Example 1 Treatment of Platelets with Riboflavin
Platelets were collected using standard collection methods using a COBE~
SpectraTM apheresis machine (manufactured by Gambro BCT, Lakewood, CO, USA).
Fresh platelets were less than 24 hours old after collection via apheresis.
Other
apheresis machines useful for collecting transfusion-quality platelets are
useful in the
practice of this invention. Collected platelets were diluted in a solution
containing
0.9% sodium chloride with either 0 or 10 ~.M riboflavin. Samples were
saturated with
air via vigorous mixing. Platelets were exposed to no photoradiation greater
than
ambient light. Results are shown in Table 1.
Table 1
Riboflavin Photoradiation Lactate Glucose 02 pH
Concentration Production Consumption Consum tion
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
51
Rate Rate (pM/hr/1012
(pM/hr) (pM/hr) plts)
Euv Evis Day 24 Day
J/cm2 J/cm2 0 hrs 0
0 0 0 31 18 92 114
0 0 29 19 136 124
0 0 0 50 87 6.92
10 0 0 68 186 6.91
Cell quality indicators were improved or unaffected by the addition of
riboflavin.
Example 2 Treatment of Platelets with Riboflavin and Visible Light
Platelets were collected using standard collection methods using a COBE~
SpectraTM apheresis system (manufactured by Gambro BCT, Lakewood, CO, USA)
and TRIMA~ apheresis system (available from Gambro BCT, Lakewood, CO, USA) .
Fresh platelets were less than 24 hours old after collection via apheresis.
Other
apheresis machines useful for collecting transfusion-quality platelets are
useful in the
practice of this invention. Collected platelets were diluted in a solution
containing
0.9% sodium chloride with either 0 or 10 ~M riboflavin. Samples were saturated
with
air via vigorous mixing. The samples were irradiated with a Dymax light source
using
one bulb at 15.5 mW/cm2 for varying times. The UV component of the light was
filtered out using a polycarbonate filter. Exposure time was 31 minutes.
Results are
shown in Table 2.
Table 2
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
52
Riboflavin Photoradiation Lactate Glucose OZ pH
Concentration Production Consumption Consumption
(~M) Rate Rate (pM/hr/10'Z
(pM/hr) (p,M/hr) pits)
Euv Eves Day 24 hrs Day
J/cmz J/cm2 0 0
0 0 40 83 42 80 63
0 40 30 13 117 94
0 0 40 237 91 6.89
10 0 40 239 140 6.84
Cell quality indicators were improved or unaffected by addition of riboflavin.
Example 3 Treatment of Platelets with Riboflavin and Ultraviolet and Visible
Light
Platelets were collected using standard collection methods using a COBE~
SpectraTM apheresis machine (manufactured by Gambro BCT, Lakewood, CO, USA).
Fresh platelets were less than 24 hours old after collection via apheresis.
Other
apheresis machines useful for collecting transfusion-quality platelets are
useful in the
practice of this invention. Collected platelets were diluted in a solution
containing
0.9% sodium chloride with either 0 or 10 pM riboflavin. Samples were saturated
with
air via vigorous mixing. The samples were irradiated with a Dymax light source
using
one bulb at 15.5 mW/cm2 for varying times. Results are shown in Tables 3 and
4.
Table 3
Riboflavin Photoradiation Lactate Glucose 02 pH
Concentration Production Consumption Consumption
(~M) Rate Rate (pM/hr/10'2
(pM/hr) (pM/hr) pits)
Euv Ems Day 24 Day
hrs
J/cmz J/cmZ 0 0
0 29 40 327 115 20 0
10 29 40 60 39 55 111
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
53
When riboflavin was added, lactate production rate was decreased by about 82%,
glucose consumption rate was decreased by about 66%, and oxygen consumption
rate
was increased by about 175%. Other cell quality indicators were improved as
well.
The energy of both ultraviolet and visible light combined was 40 J/cm2.
Table 4
Riboflavin WavelengthExposure Oxygen pH
Concentration Time Consumption
(pM) (min) (pM/hr/10~2
lts)
Day 24 Day 24
0
hrs 0 hrs
0 UV+VIS 16 22 6.87
- -~ -UV+VIS 16 ~ ~ 86 6.86
~
When riboflavin was added, oxygen consumption was increased by about 13%.
Other
cell quality indicators were improved as well.
In a related experiment, 30m1 aliquots of concentrated platelets, with varying
amounts of riboflavin from 0 to 10 micromolar, were photoradiated with 365 nm
UV
to 29 J/cmZ and 419 nm visible light to 40 J/cm2. Cell quality indicators were
measured for about 25 hours following photoradiation. Figure 5 is a graph
showing
the effect of mitochondrial enhancer on lactate production (lactate
concentration
mM/1000 cells) by platelets as a function of storage time (days). The amount
of
lactate produced decreased about 70% with riboflavin. Figure 6 is a graph
showing
the effect of mitochondrial enhancer on the rate of lactate production by
platelets as a
function of mitochondrial enhancer concentration. The rate of lactate
production
decreased about 80% when 10 micromolar riboflavin was added compared to no
riboflavin. The rate of lactate production decreased with increasing amounts
of
riboflavin.
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
54
Example 4 Treatments of Platelets with NaCN to Simulate Electron Transport
Chain
Damage
Sodium cyanide was used as a control to simulate damage to the electron
transport chain. Results are shown in Table 5.
Table 5
Sodium Riboflavin WavelengthExposureOxygen Lactate pH
Cyanide Concentration Time Consumption Production
(wM) (min) (pM/hr/1012 (pM/hr)
Its)
Day 24 24 hrs Day 24
0
hrs 0 hrs
absent 0 0 0 87 50 6.92
present 0 0 0 14 ~ 6.92
Example 5 Treatment of Stored Platelets with Riboflavin
The platelets were 6-day-old apheresis platelets which had been stored under
standard conditions at a local blood bank. Products were placed into 30 mL
bags for
irradiation and 24 hour storage. Results are shown in Table 6.
Table 6
PlateletRiboflavin WavelengthExposure Oxygen Lactate pH
StorageConcentration Time Consumption Production
(days) (p,M) (min) (pM/hr/10~2 (pM/hr)
Its
Day 24 24 hrs Day 24
0
hrs 0 hrs
6 0 0 0 118 63 444 7.10 6.97
6 10 0 0 116 168 40 7.08 6.71
When riboflavin was added to stored platelets, lactate production decreased by
about
91 %. Other cell quality indicators were improved as well.
Example 6 Treatment of Stored Platelets with Riboflavin and Visible
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
The platelets were 6-day-old apheresis platelets which had been stored under
standard conditions at a local blood bank. Products were placed into 30 mL
bags for
irradiation and 24 hour storage. The light source used was a Dymax light
source with
a single bulb. LJV light was filtered out using a polycarbonate sheet.
Exposure time
was adjusted to deliver 40 J/cm2. Results are shown in Table 7.
Table 7
PlateletConc. WavelengthExposureLactate pH
StorageBZ Time Production
da s ( M) min M/hr
24 hrs Day 24
0 hrs
6 0 VIS 31 404 7.096.48
6 10 VIS 31 ~ 7.07~6.84~
Example 7 Treatment of Stored Platelets with Riboflavin and Visible and
Ultraviolet
Light
The platelets were 6-day-old apheresis platelets which had been stored under
standard conditions at a local blood bank. Products were placed into 30 mL
bags for
irradiation and 24 hour storage. The light source used was a Dymax light
source with
a single bulb. UV light was filtered out using a polycarbonate sheet. Exposure
time
was adjusted to deliver 40 J/cmz combined visible light and ultraviolet light.
Results
are shown in Table 8.
Table 8
PlateletConc. Wavelength ExposureLactate pH
StorageBz Time Production
(da ( M (min ( M/hr
s
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
56
24 hrs Day 24
0 hrs
6 0 UV+VIS 16 545 7.086
6 10 UV+VIS 16 177 7.076.12
Example 8 Treatment of Platelets with Riboflavin and Ultraviolet Light
278m1 of 90% PCO in Sengewald bags, with 50 micromolar riboflavin (23
samples) and without riboflavin (6 samples), while stirring at 120 cpm (adding
oxygen), were irradiated with 320 nm UV photoradiation to a total of 7 J/cm2.
Vital
cell quality indicators were measured at various storage times after
photoradiation.
Figure 8 is a graph showing the effect of mitochondria) enhancer on glucose
consumption (glucose concentration mM/10~2 cells) by platelets as a function
of
storage time (days). The amount and rate of glucose consumption decreased with
the
addition of riboflavin. Figure 1 is a graph showing the effect of
mitochondria)
enhancer on platelet swirl (0-4 units) of cellular blood components as a
function of
storage time (days). Platelet swirl increased on Day 1 with the addition of
riboflavin.
Figure 2 is a graph showing the effect of mitochondria) enhancer on hypotonic
shock
response (HSR), % reversal, of platelets as a function of storage time (days).
HSR
increased with the addition of riboflavin. Figure 3 is a graph showing the
effect of
mitochondria) enhancer on pH of the stored fluid as a function of storage time
(days).
pH was higher with riboflavin. pH decreased after photoradiation at a slower
rate
with riboflavin, compared to without riboflavin. Figure 9 is a graph showing
the
effect of mitochondria) enhancer on p-selectin expression (GMP-140 (granule
membrane protein-140) expression, also called % activation, by platelets as a
function
of storage time (days). Percent activation is the percentage of cells
expressing p-
selectin. P-selectin expression was decreased with riboflavin. Figure 4 is a
graph
showing the effect of mitochondria) enhancer on percent extent of shape change
(ESC) of platelets as a function of storage time (days). ESC% was increased
with
riboflavin.
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
57
Example 9 Treatment of Platelets with Riboflavin and Ultraviolet Light
278m1 of 90% PCO in Sengewald bags, with 50 micromolar riboflavin (23
samples) and without riboflavin (6 samples), while stirring at 120 cpm (adding
oxygen), were irradiated with 320 nm UV photoradiation to a total of 7 J/cm2.
Vital
cell quality indicators were measured at various storage times after
photoradiation.
Figure 7 is a graph showing the effect of mitochondria) enhancer on lactate
production
by platelets as a function of storage time (days). Lactate production was
decreased
with riboflavin by between about 20% and about 30%. The rate of lactate
production
was decreased by about 25%. Figure 8 is a graph showing the effect of
mitochondria)
enhancer on glucose consumption by platelets as a function of storage time
(days).
Glucose consumption was decreased by riboflavin by between about 15% and about
85%. Rate of glucose consumption was decreased by about 36%. Figure 9 is a
graph
showing the effect of mitochondria) enhancer on p-selectin expression (%
activation),
by platelets as a function of storage time (days). Activation was reduced by
riboflavin
by about 10%. Figure 10 is a graph showing the effect of mitochondria)
enhancer on
oxygen consumption by platelets as a function of storage time (days). Figure
10 is
alternatively labeled as a graph of oxygen concentration which is indicative
of
consumption because the lower the oxygen concentration, the higher the oxygen
consumption. Oxygen consumption was increased by about 30%. The rate of oxygen
consumption was increased by about 37.5%.
Example 10 Effect of Riboflavin on Reduction of Vaccinia Virus at Various
Ener~y
Levels
Vaccinia virus was used to innoculate Isolyte S media (Halpern, et al. (1997)
Crit Care Med. 25(12):2031-8). Samples also contained 10 micromolar
riboflavin.
Vaccinia virus was titered before and after exposure and measured as TCIDso
(tissue
culture infection dose for 50% of the tissue culture cells). UV photoradiation
was
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
58
continued for a total of 30 minutes (40 J/cm2), with vaccinia virus titered at
ten minute
intervals. More energy was delivered with longer exposure times. Figure 11 is
a
graph showing the effect of mitochondria) enhancer on reduction kinetics of
vaccinia
virus as a function of photoradiation exposure time (delivered energy).
Vaccinia virus
reduction increased with increasing exposure time (energy). There was no
reduction
without riboflavin. Results are shown in Table 9.
Table 9
Log Reduction
in the presence
of 10 ~,M
TIME Riboflavine
0 6.56
3.22
1.5
1.5
Example 11 Effect of Riboflavin on Reduction of HSV-2 at Various Energy Levels
and Riboflavin Concentrations
HSV-2 was used to innoculate 90% PCO, wherein the balance was Isolyte S
media. HSV-2 was titered before and after exposure to photoradiation from a
DYMAX 2000 irradiator. Figure 12 is a graph showing the effect of various
concentrations of mitochondria) enhancer on reduction of Herpes Virus 2 as a
function
of photoradiation exposure time (delivered energy). Photoradiation included
both
visible and ultraviolet wavelengths. The four arrows on figure 12 indicate
that the
actual log inactivation data points might be higher than those shown because
these
samples were at the detection limit of the assay. Log reduction observed, in
cfu/ml, is
calculated by measuring an initial titer before treatment, measuring a titer
after
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
59
treatment, and subtracting the latter titer from the initial titer. Reduction
was greatest
with 10 micromolar riboflavin. Results are shown in Table 10.
Table 10
Riboflavin
Concentration60 J/cmz120 J/cm2 150 J/cm2
micromolar
0 0 0 0
0.5 3.25 2.75
1.75 3.75 3.1
50 1.5 3.5 ~ 2
Example 12 Effect of Riboflavin on Reduction of S. epidermidis at Various
Ener~y
Levels and Riboflavin Concentration
S. epidermidis was used to innoculate 90% PCO, wherein the balance was
Isolyte S media. S. epidermidis was titered before and after exposure to
photoradiation from a DYMAX 2000 irradiator. Photoradiation was delivered at
40
J/cm2, 80 J/cm2, and 120 J/cm2. Photoradiation included both visible and
ultraviolet
wavelengths. Riboflavin concentration was 0, 10, 25, 50, and 100 micromolar.
Figure 13 is a graph showing the effect of various energy doses on reduction
of S.
epidermidis as a function of concentration of mitochondria) enhancer.
Reduction was
greatest with higher energy delivery and with 10 micromolar riboflavin.
Results are
shown in Table 11.
Table 11
Riboflavin Energy Energy Energy
ConcentrationDose Dose Dose
micromolar 40 J/cm280 J/cm2 120 J/cmz
0 0 0 0
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
10 0 2.4 4.8
25 2.4 3.4 4.6
50 1.8 3.2 4
100 1.7 3 3.6
Example 13 Effect of Riboflavin on Reduction of ~X 174 at Various Energy
Levels
and Riboflavin Concentration
X174 was used to innoculate 90% PCO, wherein the balance was Isolyte S
media. Photoradiation was delivered at 40 J/cm2, 80 J/cm2, and 120 J/cm2.
Photoradiation included both visible and ultraviolet wavelengths. Riboflavin
was at 0,
50, or 100 micromolar. Figure 14 is a graph showing the effect of various
concentrations of mitochondria) enhancer on reduction of X174 as a function of
delivered photoradiation energy. Reduction was greatest at higher riboflavin
concentrations ( 100 micromolar) and higher energies ( 120 J/cm2). Results are
shown
in Table 12.
Table 12
Ener 0 Micromolar 50 Micromolar 100 Micromolar
0 0 0 0
40 0.3 0.3 0.7
80 0.3 1 1.3
120 0.4 1.2 1.9
Example 14 Treatment of Platelets with Non-Endogenous Alloxazine and
Ultraviolet
Light
Non-endogenous alloxazine is added to a fluid containing platelets and
photosensitizer. The fluid is exposed to about 30 J/cm2 ultraviolet light.
After five
days of storage, cell quality indicators are improved compared to an
equivalent
process not using non-endogenous alloxazine.
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
61
Example 15 Treatment of Platelets with Endogenously Based Derivative
Alloxazine
and Ultraviolet Light
Endogenously based derivative alloxazine is added to a fluid containing
platelets and photosensitizer. The fluid is exposed to about 30 J/cm2
ultraviolet light.
After five days of storage, cell quality indicators are improved compared to
an
equivalent process not using endogenously based derivative alloxazine.
Example 16 Treatment of Platelets with Vitamin K and Ultraviolet Light
Vitamin K is added to a fluid containing platelets and photosensitizer. The
fluid is exposed to about 30 J/cm2 ultraviolet light. After five days of
storage, cell
quality indicators are improved compared to an equivalent process not using
vitamin
K.
Example 17 Treatment of Platelets with Vitamin L and Ultraviolet Light
Vitamin L is added to a fluid containing platelets and photosensitizer. The
fluid is exposed to about 30 J/cm2 ultraviolet light. After five days of
storage, cell
quality indicators are improved compared to an equivalent process not using
vitamin
L.
Example 18 Treatment of a Peritoneal Solution with Mitochondria) Enhancer
Peritoneal solution is removed from a body, mitochondria) enhancer is added,
and the peritoneal solution with mitochondria) enhancer is administered to the
peritoneal space of a body. The body may be the same body from which the
peritoneal solution was removed. Alternatively, mitochondria) enhancer is
administered directly to the peritoneal space of a body. Cells within the
peritoneal
solution and/or cells that are in contact with the mitochondria) enhancer
containing
peritoneal space are mitochondrially enhanced.
CA 02483046 2004-10-21
WO 03/094979 PCT/US03/14070
62
Example 19 Treatment of a Wound Surface with Mitochondria) Enhancer
A wound is treated by administration of mitochondria) enhancer. Cells on and
near the surface of the wound, including but not limited to white blood cells,
red blood
cells, and fibroblasts, are mitochondrially enhanced.
It will be appreciated by those of ordinary skill in the art that blood
collection
apheresis systems, cellular blood components, mitochondria) enhancers,
alloxazines,
pathogen reduction processes, photoradiation methods, storage times, cell
quality
indicators, and pathogens other than those specifically disclosed herein are
available
in the art and can be employed in the practice of this invention. All art-
known
functional equivalents are intended to be encompassed within the scope of this
invention.