Note: Descriptions are shown in the official language in which they were submitted.
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
6,11 Bicyclic Erythromycin Derivatives
TECHNICAL FIELD
The present invention relates to novel semisynthetic macrolides having
antibacterial
activity and useful in the treatment and prevention of bacterial infections.
More particularly,
the invention relates to 6,11-3-carbon bridged erythromcyin derivatives,
compositions
containing such compounds and methods for using the same, as well as processes
for making
such compounds.
BACKGROUND OF THE INVENTION
Erythromycins A through D, represented by formula (E) as illustrated below,
O NMe2
HQ 2
9 OH Erythromycin Ra Rb
,
Ha,,,... 6 A -OH -CH3
Ra 12 O
B -H -CH3
O O
0 C -OH -H
4"
0 D -H -H
>(OH o
(E) Rb
are well-known and potent antibacterial agents, used widely to treat and
prevent bacterial
infection. As with other antibacterials, however, bacterial strains having
resistance or
insufficient susceptibility to erythromycin have been identified. Also,
erythromycin A has
only weak activity against Gram-negative bacteria. Therefore, there is a
continuing need to
identify new erythromycin derivative compounds which possess improved
antibacterial
activity, which have less potential for developing resistance, which possess
the desired Gram-
negative activity, or which possess unexpected selectivity against target
microorganisms.
Consequently, numerous investigators have prepared chemical derivatives of
erythromycin in
an attempt to obtain analogs having modified or improved profiles of
antibiotic activity.
Kashimura et al. have disclosed 6-O-methylerythromycin derivatives having a
tricyclic basic nuclear structure in European Application 559896, published
November 11,
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
1991. Also, Asaka et al. have disclosed 5-O-desosminylerythronolide
derivatives containing
a tricyclic carbamate structure in PCT Application WO 93/21200, published
Apri122, 1992.
Recently erythromycin derivatives containing a variety of substituents at the
6-0
position have been disclosed in U.S. patent Nos. 5,866,549, 6,075,011 and
6,420,555 B 1 as
well as PCT Applications WO 00/78773 and WO 03/024986. Furthermore, Ma et. al.
have
described erythromycin derivatives with aryl groups tethered to the C-6
position in J. Med
Chem., 44, pp 4137-4156 (2001).
SUMMARY OF THE INVENTION
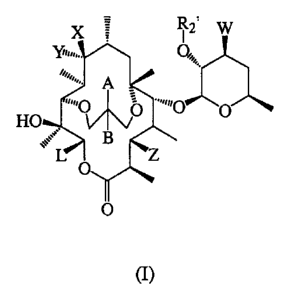
The present invention provides a novel class of C6-C 11 bridged erythromycin
compounds that possess antibacterial activity.
In one aspect of the present invention there are disclosed novel bridged
erythromycin
compounds represented by formula I as illustrated below:
X ; R2= W
O O -,,,0 O
HO
B
L Z
O
O
(I)
as well as its pharmaceutically acceptable salts, esters and prodrugs,
wherein
A is selected from:
a) -OH;
b) -ORp, where RP is a hydroxy protecting group;
c) -RI, where R, is independently selected from:
(1) aryl;
(2) substituted aryl;
(3) heteroaryl; and
(4) substituted heteroaryl;
2
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
d) -ORI, where R1 is as previously defined;
e) -R2, where R2 is selected from:
(1) hydrogen;
(2) halogen;
(3) Cl=C12 alkyl optionally containing 0, 1, 2, or 3 heteroatoms selected from
0, S
or N, optionally substituted- with one or more substituents selected from
halogen, aryl, substituted aryl, heteroaryl, and substituted heteroaryl;
(4) C2-C12 alkenyl optionally containing 0, 1, 2, or 3 heteroatoms selected
from 0,
S and N, optionally substituted with one or more substituents selected from
halogen, aryl, substituted aryl, heteroaryl, and substituted heteroaryl; and
(5) C2-C 12 alkynyl optionally containing 0, 1, 2, or 3 heteroatoms selected
from 0,
S and N, optionally substituted with one or more substituents selected from
halogen, aryl, substituted aryl, heteroaryl, and substituted heteroaryl;
f) -OR2, where R2 is independently previously defined;
g) -S(O)õR11, where n = 0, 1 or 2, and Ri1 is independently hydrogen, Rl or
R2,
where R1 and R2 are as previously defined;
h) -NHC(O)R11, where R>> is as previously defined;
i) -NHC(O)NHR11, where R11 is as previously defined;
j) -NHS(O)2Rl1, where R11 is as previously defined;
k) NR14R15, where R14 and R15 are each independently R11, where R11 is as
previously defined; and
1) -NHR3, where R3 is an amino protecting group;
B is selected from:
a) hydrogen;
b) deuterium;
c) halogen ;
d) -OH;
e) -R1, where R, is as previously defined;
f) -R2, where R2 is as previously defined; and
g) -ORp, where Rp is as previously defined, provided that when B is halogen, -
OH, or
-ORP, A is R, or R2;
or alternatively, A and B taken together with the carbon atom to which they
are attached are
selected from:
3
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
a) C=O;
b) C(OR2)2, where R2 is as previously defined;
c) C(SR2)2, where R2 is as previously defined;
d) C(OR12) (ORt3), where R12 and R13 are independently CI-C6 alkyl or taken
together are -(CH2),n , where m = 2 or 3;
e) C(SR12) (SR13), where R12, R13, and m are as previously defined;
f) C=CHR11, where R, 1 is as previously defined;
g) C=N-O-R>>, where R11 is as previously defined;
h) C=N-O-ArI-M-Ar2, wherein
(1) -Arl- is R31, where R31 is independently selected from:
(a) -RI, where R1 is as previously defined;
(b) -C1-C12 alkyl optionally containing 0, 1, 2, or 3 heteroatoms selected
from
0, S or N, optionally substituted with one or more substituents selected
from halogen, aryl, substituted aryl, heteroaryl, and substituted heteroaryl;
(c) -C2-CI2 alkenyl optionally containing 0, 1, 2, or 3 heteroatoms selected
from 0, S and N, optionally substituted with one or more substituents
selected from halogen, aryl, substituted aryl, heteroaryl, and substituted
heteroaryl; or
(d) -C2-C12 alkynyl optionally containing 0, 1, 2, or 3 heteroatoms selected
from 0, S and N, optionally substituted with one or more substituents
selected from halogen, aryl, substituted aryl, heteroaryl, and substituted
heteroaryl;
(2) -M- is absent or selected from:
(a) -CI -C12 alkyl optionally containing:
1. 0-3 heteroatoms selected from 0, S or N; and
2. 0-3 groups selected from -C=N-, -N=N- or C(O);
(b) -C2-C12 alkenyl optionally containing:
1. 0-3 heteroatoms selected from 0, S or N; and
2. 0-3 groups selected from -C=N-, -N=N-, or C(O);
(c) -C2-C 12 alkynyl optionally containing;
1. 0-3 heteroatoms selected from 0, S or N; and
2. 0-3 groups selected from -C=N-, -N=N-, or C(O);
(d) substituted aryl;
4
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
(e) substituted heteroaryl; or
(f) substituted heterocycloalkyl; and
(3) -Ar2 is selected from:
(a) aryl;
(b) substituted aryl;
(c) heteroaryl; or
(d) substituted heteroaryl;
i) C=NNHR11, where R11 is as previously defined;
j) C=NNHC(O)R11, where R11 is as previously defined;
k) C=NNHC(O)NHR11, where RI I is as previously defined;
1) C=NNHS(O)2R11, where R, 1 is as previously defined;
m) C=NNHR3, where R3 is as previously defined;
n) C=NR11, where Rl1 is as previously defined; or
o) C=N-N=CHR11, where R11 is as previously defined;
one of X and Y is hydrogen and the other is selected from:
a) hydrogen;
b) deuterium;
c) -OH;
d) -ORp, where Rp is as previously defined;
e) -NR4R5, where R4 and R5 are each independently selected from:
(1) hydrogen;
(2) C1-C12 alkyl, optionally substituted with one or more substituents
selected
from halogen, aryl, substituted aryl, heteroaryl and substituted heteroaryl;
or
(3) R4 and R5, taken together with the nitrogen atom to which they are
attached
form a 3-10 membered heteroalkyl ring containing 0-2 additional hetero atoms
selected from 0, S, and N; or
altematively, X and Y taken together with the carbon atom to which they are
attached are
selected from:
a) C=O;
b) C=N-Q, wherein Q is selected from:
(1) R11, where R, 1 is as previously defined;
(2) amino protecting group;
(3) C(O)RI I, where R11 is as previously defined; or
5
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
(4) OR6, where R6 is independently selected from:
(a) hydrogen;
(b) -CH2O(CH2)20CH3 ,
(c) -CH2O(CH2O)õCH3, where n is as previously defined;
(d) -C1-Clz alkyl, optionally substituted with one or more substituents
selected
from aryl, substituted aryl, heteroaryl and substituted heteroaryl;
(e) C3-C12 cycloalkyl;
(f) C(O)- C1-C12 alkyl;
(g) C(O)-C3-C12 cycloalkyl;
(h) C(O)-R, 1, where R11 is as previously defined; or
(i) -Si(Ra)(Rb)(Rj, wherein Ra, Rb and Rc are each independently selected
from C1-CIZ alkyl, aryl, and substituted aryl; or
(5) O-C(R7)(R8)-O-R6, where R6 is as previously defined, provided that R6 is
not
C(O)- C1-C12 alkyl, C(O)-C3-C12 cycloalkyl, or C(O)-R1, and R7 and R8 taken
together with the carbon atom to which they are attached form a C3-C12
cycloalkyl group or each independently is selected from:
1. hydrogen; or
2. CI-C1Z alkyl;
L is selected from:
b) -CH3;
c) -CH2CH3;
d) -CH(OH)CH3;
e) C1-C6 alkyl, optionally substituted with one or more substituents selected
from
aryl, substituted aryl, heteroaryl, and substituted heteroaryl;
f) C2-C6 alkenyl, optionally substituted with one or more substituents
selected from
aryl, substituted aryl, heteroaryl, and substituted heteroaryl; or
g) C2-C6 alkynyl, optionally substituted with one or more substituents
selected from
aryl, substituted aryl, heteroaryl, and substituted heteroaryl;
W is -NR14R15, where R15 and R15 are each independently selected from:
a) hydrogen;
b) Ci-C12 alkyl, optionally substituted with one or more substituents selected
from
halogen, aryl, substituted aryl, heteroaryl and substituted heteroaryl;
6
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
c) CZ-C12 alkenyl, optionally substituted with one or more substituents
selected from
halogen, aryl, substituted aryl, heteroaryl and substituted heteroaryl;
d) C2-C12 alkynyl, optionally substituted with one or more substituents
selected from
halogen, aryl, substituted aryl, heteroaryl and substituted heteroaryl; or
e) R14 and R15, taken together with the nitrogen atom to which they are
attached form
a heterocycloalkyl cyclic moiety;
Z is selected from:
(a) hydrogen;
(b) -OH;
(c) -ORP, where RP is as previously defined;
(d) -OR11, where R11 is as previously defined;
(e) -OC(O)RI l, where RI I is as previously defined;
(f) -OC(O)NHRI I, where R11 is as previously defined;
(g) -S(O)õR11, where n and RlI are as previously defined; or
(h) -
-0,,,, O
G
0
1
R3 , where
i. R3" is hydrogen or methyl; and
ii. where one of J or G is hydrogen, the other is selected from:
1. hydrogen;
2. deuterium;
3. -OH;
4. -ORP, where RP is previously defined;
5. -OR4", where R4" is hydrogen or RP, where RP is as previously
defined; or
6. -NR4R5, where R4 and R5 is as previously defined; or
iii. in the alternative, J and G are taken together with the carbon atom to
which they are attached to form a group selected from:
1. C=O;
2. C=N-Q, wherein Q is as previously defined; and
R2' is hydrogen or RP, where Rp, is as previously defined.
7
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
In another embodiment of the present invention there are disclosed
pharmaceutical
compositions comprising a therapeutically effective amount of any compound of
the present
invention in combination with a pharmaceutically acceptable carrier or
excipient. In yet
another embodiment of the invention are methods of treating antibacterial
infections in a
subject with said pharmaceutical compositions. Suitable carriers and methods
of formulation
are also disclosed.
In a further aspect of the present invention there are provided processes for
the
preparation of 6,11-3C-bridged erythromycin derivatives of formula (I) via any
synthetic
route delineated herein.
DETAILED DESCRIPTION OF THE INVENTION
A first embodiment of the present invention is a compound of formula I as
defined
herein, or its pharmaceutically acceptable salt, ester, or prodrug.
Representative subgenera of the present invention are:
A compound according to claim 1, which is represented by formula II:
Q R2' w
N~
A
HO 0
,~ B
.~
O Z
O
(n)
where A, B, Q, RZ', W, and Z are as previously defined;
A compound according to claim 1, which is represented by formula III:
8
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
Q = R2, \N/
N O
A
0~~
HO
B
O Z
O
(III)
where A, B, Q, R2', and Z are as previously defined;
A compound according to claim 1, which is represented by formula IV:
Q R2, \N
N~
A
O ,,,0 O
HO
,ol" B
O O
0 "',*,0_R4"
O
(
(IV)
where A, B, Q, R2', and R4" are as previously defined;
A compound according to claim 1, which is represented by formula V:
Q _ R2, \N/
N~
A
,,,,,0 O ==.,,,,0 O
HO
B
O OH
O
(V)
where A, B, Q, and R2' are as previously defined;
A compound according to claim 1, which is represented by formula VI:
9
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
Ar2
M
Arl
Q _ ~ R2 w
N ~ 0~,,=
~~~~''''== N
,,,,,0 z ~~,l0 O
HO ~
O Z
O
(VI)
where Arl, Ar2, M, Q, R2', W, and Z are as previously defined;
A compound according to claim 1, which is represented by formula VII:
Ar2
M
I
= ~1
Q R2' N
N O
~~~~''''== N
0
HO
\\\',,.
O Z
O
(VII)
where Arl, Ar2, M, Q, R2', and Z are as previously defined;
A compound according to claim 1, which is represented by formula VIII:
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
Ar2
M
I
Ac ~ 1 R2, \N/
N~ ~
p'~.,
N
,,,,,p
HO J\/
O Z
O
(VIII)
where Arl, Ar2, M, R2', and Z are as previously defined;
A compound according to claim 1, which is represented by formula IX:
Ar2
M
I
Q = ~1 R2' N
N p I
O',1.,
N
,,,,,p I O ==,,,,,p
HO J\/
e,,.
p coyo
0 "O
I
(IX)
where Arl, Ar2, M. Q, R2', and R4" are as previously defined; and
A compound according to claim 1, which is represented by formula X:
11
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
Ar2
M
I
= ~1
Q R2' N
N\ O
~ O''.=
~14' N
0
HO
O OH
O
(X)
where Arl, Ar2, M, Q, and R2' are as previously defined.
Representative compounds according to the invention are those selected from:
1) Compound of formula IV: A and B taken together with the carbon atom to
which they are attached = C=CH2, Q = OH, R2' is H, and R4" = Ac;
2) Compound of formula IV: A and B taken together with the carbon atom to
which are attached = C=CH2, Q = H, R2' = H, and R4" = Ac;
3) Compound of formula V: A and B taken together with the carbon atom to
which they are attached = C=CH2, Q = H, and R2' = H;
4) Compound of formula V: A and B taken together with the carbon atom to
which they are attached = C=CH2, Q = Ac, and R2' = H;
5) Compound of formula IV: A and B taken together with the carbon atom to
which they are attached = C=CH2, Q = O-CH2OCH3, R2' = H, and R4" = Ac;
6) Compound of formula V: A and B taken together with the carbon atom to
which they are attached = C=CH2, Q = O-CH2OCH3, and R2' = H;
7) Compound of formula I: A and B taken together with the carbon atom to
which they are attached = C=CH2, X and Y taken together with the carbon
atom to which they are attached = C=NAc, L = CH2CH3, W is N(CH3)2, Z = H
and R2' = H;
8) Compound of formula I: A and B taken together with the carbon atom to
which they are attached = C=CH2, X and Y taken together with the carbon
atom to which they are attached = C=NAc, L = CH2CH3, Z = OC(O)(p-
nitrophenyl) and R2' = H;
12
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
9) Compound of formula I: A and B taken together with the carbon atom to
which they are attached = C=CH2, X and Y taken together with the carbon
atom to which they are attached = C=NAc, L = CH2CH3, W is N(CH3)2, Z
OC(O)[2-(N02), 4-(CF3)Phenyl] and R2' = H;
10) Compound of formula I: A and B taken together with the carbon atom to
which they are attached = C=CH2, X and Y taken together with the carbon
atom to which they are attached = C=NAc, L = CH2CH3, W is N(CH3)2, Z
OC(O)CH2(p-methoxyphenyl) and R2' = H;
11) Compound of formula IV: A and B taken together with the carbon atom to
which they are attached = C=CH2, Q = Ac, R2' = H, and R4" = Ac;
12) Compound of formula I: Compound of formula IV: A and B taken together
with the carbon atom to which they are attached = C=O, Q = Ac, R2' = H, and
R4" = Ac;
13) Compound of formula IV: A and B taken together with the carbon atom to
which they are attached = C=OBz, Q = Ac, R2' = H, and R4" = Ac;
14) Compound of formula IV: A and B taken together with the carbon atom to
which they are attached = C=(3-quinolyl), Q = Ac, R2' = H, and R4" = Ac;
15) Compound of formula I: A and B taken together with the carbon atom to
which they are attached = C=CH2, X and Y taken together with the carbon
atom to which they are attached = C=O, L = CH2CH3, W is N(CH3)2, Z = 4-
acetoxycladinose and R2' = H;
16) Compound of formula I: A and B taken together with the carbon atom to
which they are attached = C=CH-quinolin-3-yl, X and Y taken together with
the carbon atom to which they are attached = C=O, L = CH2CH3, W is
N(CH3)2, Z = 4-acetoxycladinose and R2' = H;
17) Compound of formula I: A and B taken together with the carbon atom to
which they are attached = C=CH-quinolin-3-yl, X and Y taken together with
the carbon atom to which they are attached = C=O, L = CH2CH3, W is
N(CH3)2, Z = OH, and R2' = H;
18) Compound of formula I: A and B taken together with the carbon atom to
which they are attached = C=CH2, X and Y taken together with the carbon
atom to which they are attached = C=O, L = CH2CH3, W is N(CH3)2, Z = OH
and R2' = H;
13
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
19) Compound of formula IV: A and B taken together with the carbon atom to
which they are attached = C=CH2-phenyl, Q = OH, R2' = H, and R4" = Ac;
20) Compound of formula V: A and B taken together with the carbon atom to
which they are attached are C=CH-phenyl, Q= Ac, and R2' = H;
21) Compound of formula I: A and B taken together with the carbon atom to
which they are attached = C=O, X and Y taken together with the carbon atom
to which they are attached =C=NAc, L = CH2CH3, W is N(CH3)2, Z
OCH2CH=CH(quinolin-3-yl), and R2' = H;
22) Compound of formula I: A and B taken together with the carbon atom to
which they are attached = C=CHCHCH-phenyl, X and Y taken together with
the carbon atom to which they are attached = C=NAc, L = CH2CH3, W is
N(CH3)2, Z= OC(O)-benzyl and R2' = H;
23) Compound of formula I: A and B taken together with the carbon atom to
which they are attached = C=CHCHCH-phenyl, X and Y taken together with
the carbon atom to which they are attached = C=NAc, L = CH2CH3, W is
N(CH3)2, Z = OC(O)CH2(2-pyridyl) and R2' = H;
24) Compound of formula I: A and B taken together with the carbon atom to
which they are attached = C=CH2, X and Y taken together with the carbon
atom to which they are attached = C=NOH, L = CH2CH3, W is N(CH3)2, Z
4-oxocladinose and R2' = H;
25) Compound of formula I: A and B taken together with the carbon atom to
which they are attached = C=CH2, X and Y taken together with the carbon
atom to which they are attached = C=NOH, L = CH2CH3, W is N(CH3)2, Z
4-oximecladinose and R2' = H;
26) Compound of formula IV: A and B taken together with the carbon atom to
which they are attached are C=CH2, Q = OH, and R2 = R4" = H;
27) Compound of formula I: Compound of formula IV: A and B taken together
with the carbon atom to which they are attached = C=CH2, Q = OH, R2' = H,
and R4" = Ac.
In another aspect, the present invention relates to a method for controlling a
bacterial
infection in a subject (e.g., mammal, human, horse, dog, cat, fish) comprising
administering
to the subject a therapeutically effective amount of a pharmaceutical
composition described
herein. The method includes administering to the subject (including a subject
identified as in
14
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
need of such treatment) a therapeutically effective amount of any compound
delineated
herein, or any pharmaceutical composition delineated herein to produce such
effect.
Yet another aspect of this invention relates to a method of treating a subject
(e.g.,
mammal, human, horse, dog, cat, fish) having bacterial infection or disease or
disease
symptom related to having a bacterial infection (including diseases delineated
herein). The
method includes administering to the subject (including a subject identified
as in need of such
treatment) an effective amount of a compound described herein, or a
composition described
herein to produce such effect. Identifying a subject in need of such treatment
can be in the
judgment of a subject or a health care professional and can be subjective
(e.g. opinion) or
objective (e.g. measurable by a test or diagnostic method).
Also within the scope of this invention is a packaged product. The packaged
product
includes a container, one of the aforementioned compounds in the container,
and a legend
(e.g., a label or an insert) associated with the container and indicating
administration of the
compound for treating a disorder associated with bacterial infection,
including the diseases
delineated herein.
In a further aspect of the present invention is a process of making any
compound
delineated herein via any synthetic route delineated herein.
Definitions
Listed below are definitions of various terms used to describe this invention.
These
definitions apply to the terms as they are used throughput this specification
and claims, unless
otherwise limited in specific instances, either individually or as part of a
larger group.
The terms " C1-C3 alkyl," " C1-C6 alkyl," or "C1-C12 alkyl," as used herein,
refer to
saturated, straight- or branched-chain hydrocarbon radicals containing between
one and three,
one and twelve, or one and six carbon atoms, respectively. Examples of C1-C3
alkyl radicals
include methyl, ethyl, propyl and isopropyl radicals; examples of C1-C6 alkyl
radicals
include, but are not limited to, methyl, ethyl, propyl, isopropyl, n-butyl,
tert-butyl, neopentyl
and n-hexyl radicals; and examples of C1-C12 alkyl radicals include, but are
not limited to,
ethyl, propyl, isopropyl, n-hexyl, octyl, decyl, dodecyl radicals.
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
The term "substituted alkyl," as used herein, refers to a"C2-C12 alkyl" or "CI-
C6
alkyl" group as previously defined, substituted by independent replacement or
one, two, or
three of the hydrogen atoms thereon with substituents including, but not
limited to, -F, -Cl,
-Br, -I, -OH, protected hydroxy, -NOz, -CN, -CI-C12-alkyl optionally
substituted with
halogen, C2-C12-alkenyl optionally substituted with halogen, -Cz-C12-alkynyl
optionally
substituted with halogen, -NH2, protected amino, -NH -C1-C12-alkyl, -NH -CZ-
C12-alkenyl,
-NH -C2-C12-alkenyl, -NH -C3-C12-cycloalkyl, -NH -aryl, -NH -heteroaryl, -NH -
heterocycloalkyl, -dialkylamino, -diarylamino, -diheteroarylamino, -O-C1-C1z-
alkyl, -O-
C2-C12-alkenyl, -O-C2-C1z-alkenyl, -O-C3-C12-cycloalkyl, -0-aryl, -0-
heteroaryl, -0-
heterocycloalkyl, -C(O)- C1-CIZ-alkyl, -C(O)- C2-C12-alkenyl, -C(O)- CZ-C12-
alkenyl,
-C(O)-C3-C12-cycloalkyl, -C(O)-aryl, -C(O)-heteroaryl, -C(O)-heterocycloalkyl,
-CONH2,
-CONH- C1-C12-alkyl, -CONH- C2-C12-alkenyl, -CONH- C2-C12-alkenyl, -CONH-C3-
C12-
cycloalkyl, -CONH-aryl, -CONH-heteroaryl, -CONH-heterocycloalkyl, -OCOZ- CI-
CIZ-
alkyl, -OCO2- Cz-C12-alkenyl, -OCOz- C2-C12-alkenyl, -OCOz-C3-C12-cycloalkyl, -
OC02-
aryl, -OC02-heteroaryl, -OCO2-heterocycloalkyl, -OCONH2, -OCONH- C1-C12-alkyl,
-OCONH- C2-CI2-alkenyl, -OCONH- CZ-C12-alkenyl, -OCONH- C3-Clz-cycloalkyl,
-OCONH- aryl, -OCONH- heteroaryl, -OCONH- heterocycloalkyl, -NHC(O)- CI-CIZ-
alkyl, -NHC(O)-C2-C12-alkenyl, -NHC(O)-C2-C12-alkenyl, -NHC(O)-C3-Cj2-
cycloalkyl,
-NHC(O)-aryl, -NHC(O)-heteroaryl, -NHC(O)-heterocycloalkyl, -NHCO2- CI-C12-
alkyl,
-NHCOz- CZ-C12-alkenyl, -NHCOz- C2-C12-alkenyl, -NHCOz- C3-C12-cycloalkyl, -
NHCO2-
aryl, -NHCOz- heteroaryl, -NHCOZ- heterocycloalkyl, -NHC(O)NH2, NHC(O)NH- CI-
C12-
alkyl, -NHC(O)NH-C2-C12-alkenyl, -NHC(O)NH-C2-Q2-alkenyl, -NHC(O)NH-C3-ClZ-
cycloalkyl, -NHC(O)NH-aryl, -NHC(O)NH-heteroaryl, -NHC(O)NH-heterocycloalkyl,
NHC(S)NH2, NHC(S)NH- C1-C12-alkyl, -NHC(S)NH-C2-C12-alkenyl, -NHC(S)NH-C2-ClZ-
alkenyl, -NHC(S)NH-C3-C12-cycloalkyl, -NHC(S)NH-aryl, -NHC(S)NH-heteroaryl,
-NHC(S)NH-heterocycloalkyl, -NHC(NH)NH2, NHC(NH)NH- CI-CIZ-alkyl,
-NHC(NH)NH-C2-C12-alkenyl, -NHC(NH)NH-C2-CI2-alkenyl, -NHC(NH)NH-C3-C12-
cycloalkyl, -NHC(NH)NH-aryl, -NHC(NH)NH-heteroaryl, -NHC(NH)NH-
heterocycloalkyl, NHC(NH)-CI-C12-alkyl, -NHC(NH)-C2-C12-alkenyl, -NHC(NH)-C2-
CI2-
alkenyl, -NHC(NH)-C3-C12-cycloalkyl, -NHC(NH)-aryl, -NHC(NH)-heteroaryl,
-NHC(NH)-heterocycloalkyl, -C(NH)NH-Cj-C12-alkyl, -C(NH)NH-Cz-Cj2-alkenyl,
-C(NH)NH-C2-C12-alkenyl, -C(NH)NH-C3-C12-cycloalkyl, -C(NH)NH-aryl, -C(NH)NH-
16
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
heteroaryl, -C(NH)NH-heterocycloalkyl, -S(O)-CI-C12-alkyl, - S(O)-C2-C1Z-
alkenyl, -
S(O)-CZ-CI2-alkenyl, - S(O)-C3-C1z-cycloalkyl, - S(O)-aryl, - S(O)-heteroaryl,
- S(O)-
heterocycloalkyl -SO2NH2, -SOZNH- C1-ClZ-alkyl, -SO2NH- Cz-C12-alkenyl, -SO2NH-
C2-
C12-alkenyl, -SO2NH- C3-C12-cycloalkyl, -SO2NH- aryl, -SO2NH- heteroaryl, -
SO2NH-
heterocycloalkyl, -NHSO2-C1-C12-alkyl, -NHSO2-C2-C12-alkenyl, - NHSO2-Cz-C12-
alkenyl,
-NHSOZ-C3-ClZ-cycloalkyl, -NHSO2-aryl, -NHSO2-heteroaryl, -NHSO2-
heterocycloalkyl,
-CH2NH2, -CH2SOZCH3, -aryl, -arylalkyl, -heteroaryl, -heteroarylalkyl, -
heterocycloalkyl,
-C3-ClZ-cycloalkyl, -methoxymethoxy, -methoxyethoxy, -SH, -S-C1-C12-alkyl, -S-
C2-C12-
alkenyl, -S-C2-C12-alkenyl, -S-C3-C12-cycloalkyl, -S-aryl, -S-heteroaryl, -S-
heterocycloalkyl, or methylthiomethyl.
The terms "C2-C12 alkenyl" or "C2-C6 alkenyl," as used herein, denote a
monovalent
group derived from a hydrocarbon moiety containing from two to twelve or two
to six carbon
atoms having at least one carbon-carbon double bond by the removal of a single
hydrogen
atom. Alkenyl groups include, but are not limited to, for example, ethenyl,
propenyl, butenyl,
1-methyl-2-buten-1-yl, and the like.
The term "substituted alkenyl," as used herein, refers to a"CZ-ClZ alkenyl" or
"C2-C6
alkenyl" group as previously defined, substituted by independent replacement
or one, two, or
three of the hydrogen atoms thereon with substituents including, but not
limited to, -F, -Cl,
-Br, -I, -OH, protected hydroxy, -NO2, -CN, -Cl-C12-alkyl optionally
substituted with
halogen, C2-C12-alkenyl optionally substituted with halogen, -Cz-C12-alkynyl
optionally
substituted with halogen, -NH2, protected amino, -NH -C1-C12-alkyl, -NH -C2-
C12-alkenyl,
-NH -C2-C12-alkenyl, -NH -C3-C12-cycloalkyl, -NH -aryl, -NH -heteroaryl, -NH -
heterocycloalkyl, -dialkylamino, -diarylamino, -diheteroarylamino, -O-C1-C12-
alkyl, -O-
C2-ClZ-alkenyl, -O-C2-C12-alkenyl, -O-C3-Q2-cycloalkyl, -0-aryl, -0-
heteroaryl, -0-
heterocycloalkyl, -C(O)- CI-C12-alkyl, -C(O)- CZ-C12-alkenyl, -C(O)- C2-C12-
alkenyl,
-C(O)-C3-C12-cycloalkyl, -C(O)-aryl, -C(O)-heteroaryl, -C(O)-heterocycloalkyl,
-CONH2,
-CONH- Cl-C12-alkyl, -CONH- C2-C12-alkenyl, -CONH- C2-CIZ-alkenyl, -CONH-C3-
C12-
cycloalkyl, -CONH-aryl, -CONH-heteroaryl, -CONH-heterocycloalkyl, -OCO2- C1-
C12-
alkyl, -OCO2- C2-C12-alkenyl, -OC02- C2-C12-alkenyl, -OCO2-C3-Q2-cycloalkyl, -
OCO2-
aryl, -OCO2-heteroaryl, -OCO2-heterocycloalkyl, -OCONH2, -OCONH- C1-C12-alkyl,
17
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
-OCONH- C2-C12-alkenyl, -OCONH- C2-C12-alkenyl, -OCONH- C3-C12-cycloalkyl,
-OCONH- aryl, -OCONH- heteroaryl, -OCONH- heterocycloalkyl, -NHC(O)- C1-C12-
alkyl, -NHC(O)-C2-C12-alkenyl, -NHC(O)-C2-ClZ-alkenyl, -NHC(O)-C3-C12-
cycloalkyl,
-NHC(O)-aryl, -NHC(O)-heteroaryl, -NHC(O)-heterocycloalkyl, -NHCOz- C1-C12-
alkyl,
-NHCO2- C2-C12-alkenyl, -NHCO2- C2-C12-alkenyl, -NHCO2- C3-C12-cycloalkyl, -
NHCO2-
aryl, -NHCO2- heteroaryl, -NHCO2- heterocycloalkyl, -NHC(O)NH2, NHC(O)NH- C1-
C12-
alkyl, -NHC(O)NH-C2-CI2-alkenyl, -NHC(O)NH-C2-C12-alkenyl, -NHC(O)NH-C3-CI2-
cycloalkyl, -NHC(O)NH-aryl, -NHC(O)NH-heteroaryl, -NHC(O)NH-heterocycloalkyl,
NHC(S)NH2, NHC(S)NH- CI-C12-alkyl, -NHC(S)NH-C2-C12-alkenyl, -NHC(S)NH-C2-Q2-
alkenyl, -NHC(S)NH-C3-C12-cycloalkyl, -NHC(S)NH-aryl, -NHC(S)NH-heteroaryl,
-NHC(S)NH-heterocycloalkyl, -NHC(NH)NH2, NHC(NH)NH- C1-C12-alkyl,
-NHC(NH)NH-C2-C 12-alkenyl, -NHC(NH)NH-C2-C 12-alkenyl, -NHC(NH)NH-C3-C I Z-
cycloalkyl, -NHC(NH)NH-aryl, -NHC(NH)NH-heteroaryl, -NHC(NH)NH-
heterocycloalkyl, NHC(NH)-C1-C12-alkyl, -NHC(NH)-C2-C12-alkenyl, -NHC(NH)-C2-
C12-
alkenyl, -NHC(NH)-C3-C12-cycloalkyl, -NHC(NH)-aryl, -NHC(NH)-heteroaryl,
-NHC(NH)-heterocycloalkyl, -C(NH)NH-C1-C12- alkyl, -C(NH)NH-Cz-C12-alkenyl,
-C(NH)NH-Cz-C12-alkenyl, -C(NH)NH-C3-C12-cycloalkyl, -C(NH)NH-aryl, -C(NH)NH-
heteroaryl, -C(NH)NH-heterocycloalkyl, -S(O)-CI-C12-alkyl, - S(O)-CZ-C12-
alkenyl, -
S(O)-C2-ClZ-alkenyl, - S(O)-C3-C1z-cycloalkyl, - S(O)-aryl, - S(O)-heteroaryl,
- S(O)-
heterocycloalkyl -SO2NH2, -SO2NH- C1-C12-alkyl, -SO2NH- C2-C12-alkenyl, -SO2NH-
Cz-
ClZ-alkenyl, -SO2NH- C3-C12-cycloalkyl, -SOZNH- aryl, -SO2NH- heteroaryl, -
SO2NH-
heterocycloalkyl, -NHSO2-C1-C12-alkyl, -NHSO2-C2-C12-alkenyl, - NHSO2-C2-C12-
alkenyl,
-NHSOz-C3-C12-cycloalkyl, -NHSO2-aryl, -NHSO2-heteroaryl, -NHSO2-
heterocycloalkyl,
-CH2NH2, -CH2SO2CH3, -aryl, -arylalkyl, -heteroaryl, -heteroarylalkyl, -
heterocycloalkyl,
-C3-C12-cycloalkyl, -methoxymethoxy, -methoxyethoxy, -SH, -S-C1-C12-alkyl, -S-
C2-C12-
alkenyl, -S-C2-C1z-alkenyl, -S-C3-C12-cycloalkyl, -S-aryl, -S-heteroaryl, -S-
heterocycloalkyl, or methylthiomethyl.
The terms "C2-ClZ alkynyl" or "C2-C6 alkynyl," as used herein, denote a
monovalent
group derived from a hydrocarbon moiety containing from two to twelve or two
to six carbon
atoms having at least one carbon-carbon triple bond by the removal of a single
hydrogen
18
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
atom. Representative alkynyl groups include, but are not limited to, for
example, ethynyl, 1-
propynyl, 1-butynyl, and the like.
The term "substituted alkynyl," as used herein, refers to a"C2-C12 alkynyl" or
"C2-C6
alkynyl" group as previously defined, substituted by independent replacement
or one, two, or
three of the hydrogen atoms thereon with substituents including, but not
limited to, -F, -Cl,
-Br, -I, -OH, protected hydroxy, -NO2, -CN, -C1-C12-alkyl optionally
substituted with
halogen, C2-C12-alkenyl optionally substituted with halogen, -C2-C12-alkynyl
optionally
substituted with halogen, -NH2, protected amino, -NH -C1-C12-alkyl, -NH -C2-
C12-alkenyl,
-NH -C2-C12-alkenyl, -NH -C3-C12-cycloalkyl, -NH -aryl, -NH -heteroaryl, -NH -
heterocycloalkyl, -dialkylamino, -diarylamino, -diheteroarylamino, -O-C1-C12-
alkyl, -O-
C2-C1z-alkenyl, -O-C2-C12-alkenyl, -O-C3-C12-cycloalkyl, -0-aryl, -O-
heteroaryl, -0-
heterocycloalkyl, -C(O)- C1-C12-alkyl, -C(O)- Cz-C12-alkenyl, -C(O)- C2-C12-
alkenyl,
-C(O)-C3-C12-cycloalkyl, -C(O)-aryl, -C(O)-heteroaryl, -C(O)-heterocycloalkyl,
-CONH2,
-CONH- CI-C12-alkyl, -CONH- CZ-C12-alkenyl, -CONH- C2-C12-alkenyl, -CONH-C3-
C1Z-
cycloalkyl, -CONH-aryl, -CONH-heteroaryl, -CONH-heterocycloalkyl, -OCO2- CI-
C12-
alkyl, -OCO2- CZ-C12-alkenyl, -OCOZ- C2-C1z-alkenyl, -OCO2-C3-C12-cycloalkyl, -
OCO2-
aryl, -OCO2-heteroaryl, -OCO2-heterocycloalkyl, -OCONH2, -OCONH- CI-C12-alkyl,
-OCONH- C2-C12-alkenyl, -OCONH- C2-CIZ-alkenyl, -OCONH- C3-C12-cycloalkyl,
-OCONH- aryl, -OCONH- heteroaryl, -OCONH- heterocycloalkyl, -NHC(O)- C1-C12-
alkyl, -NHC(O)-C2-ClZ-alkenyl, -NHC(O)-C2-C12-alkenyl, -NHC(O)-C3-ClZ-
cycloalkyl,
-NHC(O)-aryl, -NHC(O)-heteroaryl, -NHC(O)-heterocycloalkyl, -NHCO2- CI-C12-
alkyl,
-NHCOZ- C2-C12-alkenyl, -NHCOz- C2-C12-alkenyl, -NHCO2- C3-C12-cycloalkyl, -
NHCO2-
aryl, -NHCO2- heteroaryl, -NHCOZ- heterocycloalkyl, -NHC(O)NH2, NHC(O)NH- C1-
C12-
alkyl, -NHC(O)NH-C2-C12-alkenyl, -NHC(O)NH-C2-C12-alkenyl, -NHC(O)NH-C3-C12-
cycloalkyl, -NHC(O)NH-aryl, -NHC(O)NH-heteroaryl, -NHC(O)NH-heterocycloalkyl,
NHC(S)NH2, NHC(S)NH- C1-C12-alkyl, -NHC(S)NH-C2-C12-alkenyl, -NHC(S)NH-C2-CI2-
alkenyl, -NHC(S)NH-C3-C12-cycloalkyl, -NHC(S)NH-aryl, -NHC(S)NH-heteroaryl,
-NHC(S)NH-heterocycloalkyl, -NHC(NH)NH2, NHC(NH)NH- CI-C12-alkyl,
-NHC(NH)NH-C2-C12-alkenyl, -NHC(NH)NH-C2-C12-alkenyl, -NHC(NH)NH-C3-CI2-
cycloalkyl, -NHC(NH)NH-aryl, -NHC(NH)NH-heteroaryl, -NHC(NH)NH-
heterocycloalkyl, NHC(NH)-C1-C12-alkyl, -NHC(NH)-C2-C12-alkenyl, -NHC(NH)-CZ-
C12-
19
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
alkenyl, -NHC(NH)-C3-C12-cycloalkyl, -NHC(NH)-aryl, -NHC(NH)-heteroaryl,
-NHC(NH)-heterocycloalkyl, -C(NH)NH-Cj-C12-alkyl, -C(NH)NH-C2-C12-alkenyl,
-C(NH)NH-Cz-C12-alkenyl, -C(NH)NH-C3-Clz-cycloalkyl, -C(NH)NH-aryl, -C(NH)NH-
heteroaryl, -C(NH)NH-heterocycloalkyl, -S(O)-C1-C12-alkyl, - S(O)-C2-C12-
alkenyl, -
S(O)-C2-C12-alkenyl, - S(O)-C3-C12-cycloalkyl, - S(O)-aryl, - S(O)-heteroaryl,
- S(O)-
heterocycloalkyl -SO2NH2, -SO2NH- CI-C12-alkyl, -SO2NH- C2-C12-alkenyl, -SO2NH-
C2-
C12-alkenyl, -SOZNH- C3-CIZ-cycloalkyl, -SO2NH- aryl, -SO2NH- heteroaryl, -
SO2NH-
heterocycloalkyl, -NHSO2-C1-C12-alkyl, -NHSO2-C2-C12-alkenyl, - NHSO2-C2-C12-
alkenyl,
-NHSO2-C3-ClZ-cycloalkyl, -NHSO2-aryl, -NHSO2-heteroaryl, -NHSOz-
heterocycloalkyl,
-CH2NH2, -CH2SO2CH3, -aryl, -arylalkyl, -heteroaryl, -heteroarylalkyl, -
heterocycloalkyl,
-C3-C12-cycloalkyl, -methoxymethoxy, -methoxyethoxy, -SH, -S-C1-C]2-alkyl, -S-
C2-C12-
alkenyl, -S-C2-C12-alkenyl, -S-C3-CIZ-cycloalkyl, -S-aryl, -S-heteroaryl, -S-
heterocycloalkyl, or methylthiomethyl.
The term "C1-C6 alkoxy," as used herein, refers to a C1-C6 alkyl group, as
previously
defined, attached to the parent molecular moiety through an oxygen atom.
Examples of Cl-
C6-alkoxy include, but are not limited to, methoxy, ethoxy, propoxy,
isopropoxy, n-butoxy,
tert-butoxy, neopentoxy and n-hexoxy.
The terms "halo" and "halogen," as used herein, refer to an atom selected from
fluorine, chlorine, bromine and iodine.
The term "aryl," as used herein, refers to a mono- or bicyclic carbocyclic
ring system
having one or two aromatic rings including, but not limited to, phenyl,
naphthyl,
tetrahydronaphthyl, indanyl, idenyl and the like.
The term "substituted aryl," as used herein, refers to an aryl group, as
previously
defined, substituted by independent replacement or one, two, or three of the
hydrogen atoms
thereon with substituents including, but not limited to, -F, -Cl, -Br, -I, -
OH, protected
hydroxy, -NO2, -CN, -Cl-C12-alkyl optionally substituted with halogen, C2-C12-
alkenyl
optionally substituted with halogen, -Cz-C12-alkynyl optionally substituted
with halogen,
-NH2, protected amino, -NH -Cl-C12-alkyl, -NH -C2-C12-alkenyl, -NH -CZ-C12-
alkenyl,
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
-NH -C3-C12-cycloalkyl, -NH -aryl, -NH -heteroaryl, -NH -heterocycloalkyl,
-dialkylamino, -diarylamino, -diheteroarylamino, -O-Cl-C1Z-alkyl, -O-C2-C12-
alkenyl, -O-
C2-C12-alkenyl, -O-C3-C12-cycloalkyl, -0-aryl, -0-heteroaryl, -0-
heterocycloalkyl, -C(O)-
C1-ClZ-alkyl, -C(O)- CZ-Ct2-alkenyl, -C(O)- C2-C12-alkenyl, -C(O)-C3-Cl2-
cycloalkyl,
-C(O)-aryl, -C(O)-heteroaryl, -C(O)-heterocycloalkyl, -CONH2, -CONH- C1-C1Z-
alkyl,
-CONH- CZ-C12-alkenyl, -CONH- C2-C12-alkenyl, -CONH-C3-C12-cycloalkyl, -CONH-
aryl, -CONH-heteroaryl, -CONH-heterocycloalkyl, -OCO2- Cl-C12-alkyl, -OCOZ- C2-
C12-
alkenyl, -OCO2- C2-C12-alkenyl, -OC02-C3-C12-cycloalkyl, -OC02-aryl, -OCO2-
heteroaryl,
-OCOZ-heterocycloalkyl, -OCONH2, -OCONH- C1-ClZ-alkyl, -OCONH- C2-C12-alkenyl,
-OCONH- Cz-C12-alkenyl, -OCONH- C3-C12-cycloalkyl, -OCONH- aryl, -OCONH-
heteroaryl, -OCONH- heterocycloalkyl, -NHC(O)- Cl-C12-alkyl, -NHC(O)-C2-C12-
alkenyl,
-NHC(O)-C2-C12-alkenyl, -NHC(O)-C3-C12-cycloalkyl, -NHC(O)-aryl, -NHC(O)-
heteroaryl, -NHC(O)-heterocycloalkyl, -NHCO2- C1-C12-alkyl, -NHCO2- C2-C12-
alkenyl,
-NHCO2- Cz-C12-alkenyl, -NHCO2- C3-ClZ-cycloalkyl, -NHCOz- aryl, -NHCO2-
heteroaryl, -NHCO2- heterocycloalkyl, -NHC(O)NH2, NHC(O)NH- C1-C12-alkyl,
-NHC(O)NH-C2-C 12-alkenyl, -NHC(O)NH-C2-C 12-alkenyl, -NHC(O)NH-C3-C IZ-
cycloalkyl, -NHC(O)NH-aryl, -NHC(O)NH-heteroaryl, -NHC(O)NH-heterocycloalkyl,
NHC(S)NH2, NHC(S)NH- C1-C12-alkyl, -NHC(S)NH-C2-ClZ-alkenyl, -NHC(S)NH-C2-Clz-
alkenyl, -NHC(S)NH-C3-C1z-cycloalkyl, -NHC(S)NH-aryl, -NHC(S)NH-heteroaryl,
-NHC(S)NH-heterocycloalkyl, -NHC(NH)NH2, NHC(NH)NH- C1-C12-alkyl,
-NHC(NH)NH-C2-C12-alkenyl, -NHC(NH)NH-C2-C12-alkenyl, -NHC(NH)NH-C3-C]2-
cycloalkyl, -NHC(NH)NH-aryl, -NHC(NH)NH-heteroaryl, -NHC(NH)NH-
heterocycloalkyl, NHC(NH)-C1-C12-alkyl, -NHC(NH)-C2-C12-alkenyl, -NHC(NH)-C2-
C12-
alkenyl, -NHC(NH)-C3-C12-cycloalkyl, -NHC(NH)-aryl, -NHC(NH)-heteroaryl,
-NHC(NH)-heterocycloalkyl, -C(NH)NH-C1-C12-alkyl, -C(NH)NH-C2-C1z-alkenyl,
-C(NH)NH-C2-C12-alkenyl, -C(NH)NH-C3-C12-cycloalkyl, -C(NH)NH-aryl, -C(NH)NH-
heteroaryl, -C(NH)NH-heterocycloalkyl, -S(O)-Cl-C12-alkyl, - S(O)-C2-C12-
alkenyl, -
S(O)-C2-C12-alkenyl, - S(O)-C3-C12-cycloalkyl, - S(O)-aryl, - S(O)-heteroaryl,
- S(O)-
heterocycloalkyl -SO2NH2, -SOZNH- C1-C12-alkyl, -SO2NH- C2-ClZ-alkenyl, -SO2NH-
C2-
C12-alkenyl, -SO2NH- C3-C12-cycloalkyl, -SO2NH- aryl, -SO2NH- heteroaryl, -
SO2NH-
heterocycloalkyl, -NHSO2-Cl-C12-alkyl, -NHSO2-C2-C12-alkenyl, - NHSO2-C2-C12-
alkenyl,
21
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
-NHSO2-C3-C12-cycloalkyl, -NHSO2-aryl, -NHSO2-heteroaryl, -NHSO2-
heterocycloalkyl,
-CH2NH2, -CH2SO2CH3, -aryl, -arylalkyl, -heteroaryl, -heteroarylalkyl, -
heterocycloalkyl,
-C3-C12-cycloalkyl, -methoxymethoxy, -methoxyethoxy, -SH, -S-C1-C12-alkyl, -S-
C2-C,2-
alkenyl, -S-CZ-C12-alkenyl, -S-C3-CIZ-cycloalkyl, -S-aryl, -S-heteroaryl, -S-
heterocycloalkyl, or methylthiomethyl.
The term "arylalkyl," as used herein, refers to a C1-C3 alkyl or C1-C6 alkyl
residue
attached to an aryl ring. Examples include, but are not limited to, benzyl,
phenethyl and the
like.
The term "substituted arylalkyl," as used herein, refers to an arylalkyl
group, as
previously defined, substituted by independent replacement or one, two, or
three of the
hydrogen atoms thereon with substituents including, but not limited to, but
not limited to, -F,
-Cl, -Br, -I, -OH, protected hydroxy, -NOZ, -CN, -Cl-C12-alkyl optionally
substituted with
halogen, C2-C12-alkenyl optionally substituted with halogen, -C2-ClZ-alkynyl
optionally
substituted with halogen, -NH2, protected amino, -NH -C1-C12-alkyl, -NH -C2-
C12-alkenyl,
-NH -C2-Clz-alkenyl, -NH -C3-CIZ-cycloalkyl, -NH -aryl, -NH -heteroaryl, -NH -
heterocycloalkyl, -dialkylamino, -diarylamino, -diheteroarylamino, -O-C1-C12-
alkyl, -O-
C2-C1z-alkenyl, -O-C2-C12-alkenyl, -O-C3-C12-cycloalkyl, -0-aryl, -0-
heteroaryl, -0-
heterocycloalkyl, -C(O)- CI-C12-alkyl, -C(O)- C2-C1z-alkenyl, -C(O)- C2-C12-
alkenyl,
-C(O)-C3-ClZ-cycloalkyl, -C(O)-aryl, -C(O)-heteroaryl, -C(O)-heterocycloalkyl,
-CONH2,
-CONH- CI-C12-alkyl, -CONH- C2-C12-alkenyl, -CONH- C2-Clz-alkenyl, -CONH-C3-
C12-
cycloalkyl, -CONH-aryl, -CONH-heteroaryl, -CONH-heterocycloalkyl, -OCO2- C1-
C12-
alkyl, -OC02- CZ-C12-alkenyl, -OCO2- Cz-CIZ-alkenyl, -OCO2-C3-C12-cycloalkyl, -
OC02-
aryl, -OCO2-heteroaryl, -OCOz-heterocycloalkyl, -OCONH2, -OCONH- C1-C12-alkyl,
-OCONH- C2-C12-alkenyl, -OCONH- CZ-C12-alkenyl, -OCONH- C3-CIZ-cycloalkyl,
-OCONH- aryl, -OCONH- heteroaryl, -OCONH- heterocycloalkyl, -NHC(O)- C1-C12-
alkyl, -NHC(O)-C2-CI2-alkenyl, -NHC(O)-C2-C12-alkenyl, -NHC(O)-C3-C12-
cycloalkyl,
-NHC(O)-aryl, -NHC(O)-heteroaryl, -NHC(O)-heterocycloalkyl, -NHCO2- C1-C12-
alkyl,
-NHCO2- Cz-C1z-alkenyl, -NHCOZ- C2-ClZ-alkenyl, -NHCO2- C3-C12-cycloalkyl, -
NHCO2-
aryl, -NHCO2- heteroaryl, -NHCO2- heterocycloalkyl, -NHC(O)NHZ, NHC(O)NH- CI-
C12-
alkyl, -NHC(O)NH-C2-C12-alkenyl, -NHC(O)NH-CZ-CIZ-alkenyl, -NHC(O)NH-C3-CI2-
22
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
cycloalkyl, -NHC(O)NH-aryl, -NHC(O)NH-heteroaryl, -NHC(O)NH-heterocycloalkyl,
NHC(S)NH2, NHC(S)NH- C1-C1z-alkyl, -NHC(S)NH-C2-C12-alkenyl, -NHC(S)NH-C2-C12-
alkenyl, -NHC(S)NH-C3-C12-cycloalkyl, -NHC(S)NH-aryl, -NHC(S)NH-heteroaryl,
-NHC(S)NH-heterocycloalkyl, -NHC(NH)NH2, NHC(NH)NH- C1-C12-alkyl,
-NHC(NH)NH-C2-C12-alkenyl, -NHC(NH)NH-C2-C12-alkenyl, -NHC(NH)NH-C3-C12-
cycloalkyl, -NHC(NH)NH-aryl, -NHC(NH)NH-heteroaryl, -NHC(NH)NH-
heterocycloalkyl, NHC(NH)-Cj-C12-alkyl, -NHC(NH)-C2-C12-alkenyl, -NHC(NH)-C2-
C12-
alkenyl, -NHC(NH)-C3-C12-cycloalkyl, -NHC(NH)-aryl, -NHC(NH)-heteroaryl,
-NHC(NH)-heterocycloalkyl, -C(NH)NH-Cj-C12-alkyl, -C(NH)NH-C2-C12-alkenyl,
-C(NH)NH-C2-C12-alkenyl, -C(NH)NH-C3-C1z-cycloalkyl, -C(NH)NH-aryl, -C(NH)NH-
heteroaryl, -C(NH)NH-heterocycloalkyl, -S(O)-C1-C12-alkyl, - S(O)-C2-ClZ-
alkenyl, -
S(O)-C2-C12-alkenyl, - S(O)-C3-C12-cycloalkyl, - S(O)-aryl, - S(O)-heteroaryl,
- S(O)-
heterocycloalkyl -SO2NHZ, -SO2NH- C1-C12-alkyl, -SO2NH- C2-C12-alkenyl, -SO2NH-
C2-
C12-alkenyl, -SO2NH- C3-C1z-cycloalkyl, -SO2NH- aryl, -SO2NH- heteroaryl, -
SO2NH-
heterocycloalkyl, -NHSO2-CI-C12-alkyl, -NHSOz-CZ-CIZ-alkenyl, - NHSO2-C2-C12-
alkenyl,
-NHSO2-C3-C12-cycloalkyl, -NHSOZ-aryl, -NHSO2-heteroaryl, -NHSO2-
heterocycloalkyl,
-CH2NH2, -CH2SO2CH3, -aryl, -arylalkyl, -heteroaryl, -heteroarylalkyl, -
heterocycloalkyl,
-C3-C12-cycloalkyl, -methoxymethoxy, -methoxyethoxy, -SH, -S-Cl-C12-alkyl, -S-
C2-C12-
alkenyl, -S-C2-C12-alkenyl, -S-C3-C12-cycloalkyl, -S-aryl, -S-heteroaryl, -S-
heterocycloalkyl, or methylthiomethyl.
The term "heteroaryl," as used herein, refers to a mono-, bi-, or tri-cyclic
aromatic
radical or ring having from five to ten ring atoms of which one ring atom is
selected from S,
O and N; zero, one or two ring atoms are additional heteroatoms independently
selected from
S, 0 and N; and the remaining ring atoms are carbon, wherein any N or S
contained within
the ring may be optionally oxidized. Heteroaryl includes, but is not limited
to, pyridinyl,
pyrazinyl, pyrimidinyl, pyrrolyl, pyrazolyl, imidazolyl, thiazolyl, oxazolyl,
isooxazolyl,
thiadiazolyl, oxadiazolyl, thiophenyl, furanyl, quinolinyl, isoquinolinyl,
benzimidazolyl,
benzooxazolyl, quinoxalinyl, and the like.
The term "substituted heteroaryl," as used herein, refers to a heteroaryl
group as
previously defined, substituted by independent replacement or one, two, or
three of the
23
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
hydrogen atoms thereon with substituents including, but not limited to, -F, -
Cl, -Br, -I,
-OH, protected hydroxy, -NOz, -CN, -Cl-C12-alkyl optionally substituted with
halogen, C2-
C12-alkenyl optionally substituted with halogen, -Cz-C12-alkynyl optionally
substituted with
halogen, -NH2, protected aniino, -NH -Cl-C12-alkyl, -NH -C2-C12-alkenyl, -NH -
C2-C12-
alkenyl, -NH -C3-C12-cycloalkyl, -NH -aryl, -NH -heteroaryl, -NH -
heterocycloalkyl,
-dialkylamino, -diarylamino, -diheteroarylamino, -O-C1-C12-alkyl, -O-C2-C12-
alkenyl, -O-
C2-C12-alkenyl, -O-C3-C12-cycloalkyl, -0-aryl, -0-heteroaryl, -0-
heterocycloalkyl, -C(O)-
CI-C12-alkyl, -C(O)- C2-C12-alkenyl, -C(O)- C2-CI2-alkenyl, -C(O)-C3-C12-
cycloalkyl,
-C(O)-aryl, -C(O)-heteroaryl, -C(O)-heterocycloalkyl, -CONH2, -CONH- C1-C12-
alkyl,
-CONH- C2-C12-alkenyl, -CONH- C2-C12-alkenyl, -CONH-C3-C12-cycloalkyl, -CONH-
aryl, -CONH-heteroaryl, -CONH-heterocycloalkyl, -OCOZ- Cl-C12-alkyl, -OCOz- CZ-
C12-
alkenyl, -OCO2- C2-C12-alkenyl, -OCO2-C3-C12-cycloalkyl, -OC02-aryl, -OCO2-
heteroaryl,
-OC02-heterocycloalkyl, -OCONH2, -OCONH- C1-C12-alkyl, -OCONH- C2-C12-alkenyl,
-OCONH- C2-C1z-alkenyl, -OCONH- C3-CI2-cycloalkyl, -OCONH- aryl, -OCONH-
heteroaryl, -OCONH- heterocycloalkyl, -NHC(O)- C1-C12-alkyl, -NHC(O)-C2-CI2-
alkenyl,
-NHC(O)-C2-C12-alkenyl, -NHC(O)-C3-C1z-cycloalkyl, -NHC(O)-aryl, -NHC(O)-
heteroaryl, -NHC(O)-heterocycloalkyl, -NHCOZ- C1-C12-alkyl, -NHCOZ- C2-C12-
alkenyl,
-NHCO2- Cz-C12-alkenyl, -NHCO2- C3-C12-cycloalkyl, -NHCO2- aryl, -NHCO2-
heteroaryl, -NHCO2- heterocycloalkyl, -NHC(O)NH2, NHC(O)NH- C1-C12-alkyl,
-NHC(O)NH-C2-C12-alkenyl, -NHC(O)NH-C2-C12-alkenyl, -NHC(O)NH-C3-Clz-
cycloalkyl, -NHC(O)NH-aryl, -NHC(O)NH-heteroaryl, -NHC(O)NH-heterocycloalkyl,
NHC(S)NH2, NHC(S)NH- C1-C12-alkyl, -NHC(S)NH-C2-C12-alkenyl, -NHC(S)NH-C2-Clz-
alkenyl, -NHC(S)NH-C3-C12-cycloalkyl, -NHC(S)NH-aryl, -NHC(S)NH-heteroaryl,
-NHC(S)NH-heterocycloalkyl, -NHC(NH)NH2, NHC(NH)NH- C1-C12-alkyl,
-NHC(NH)NH-Cz-CIZ-alkenyl, -NHC(NH)NH-C2-C12-alkenyl, -NHC(NH)NH-C3-C12-
cycloalkyl, -NHC(NH)NH-aryl, -NHC(NH)NH-heteroaryl, -NHC(NH)NH-
heterocycloalkyl, NHC(NH)-C1-C12-alkyl, -NHC(NH)-C2-C12-alkenyl, -NHC(NH)-C2-
CI2-
alkenyl, -NHC(NH)-C3-C1z-cycloalkyl, -NHC(NH)-aryl, -NHC(NH)-heteroaryl,
-NHC(NH)-heterocycloalkyl, -C(NH)NH-C1-C12-alkyl, -C(NH)NH-C2-C12-alkenyl,
-C(NH)NH-Cz-C12-alkenyl, -C(NH)NH-C3-CI2-cycloalkyl, -C(NH)NH-aryl, -C(NH)NH-
heteroaryl, -C(NH)NH-heterocycloalkyl, -S(O)-C1-C12-alkyl, - S(O)-C2-C1z-
alkenyl, -
24
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
S(O)-C2-C12-alkenyl, - S(O)-C3-C12-cycloalkyl, - S(O)-aryl, - S(O)-heteroaryl,
- S(O)-
heterocycloalkyl -SO2NH2, -SO2NH- C1-C12-alkyl, -SO2NH- C2-CI2-alkenyl, -SO2NH-
C2-
C12-alkenyl, -SO2NH- C3-CI2-cycloalkyl, -SO2NH- aryl, -SO2NH- heteroaryl, -
SO2NH-
heterocycloalkyl, -NHSO2-C1-ClZ-alkyl, -NHSO2-C2-C12-alkenyl, - NHSO2-C2-C12-
alkenyl,
-NHSO2-C3-C12-cycloalkyl, -NHSOz-aryl, -NHSO2-heteroaryl, -NHSO2-
heterocycloalkyl,
-CH2NH2, -CH2SO2CH3, -aryl, -arylalkyl, -heteroaryl, -heteroarylalkyl, -
heterocycloalkyl,
-C3-C12-cycloalkyl, -methoxymethoxy, -methoxyethoxy, -SH, -S-Cl-C1z-alkyl, -S-
C2-C12-
alkenyl, -S-C2-ClZ-alkenyl, -S-C3-CIZ-cycloalkyl, -S-aryl, -S-heteroaryl, -S-
heterocycloalkyl, or methylthiomethyl.
The term "C3-C1z-cycloalkyl," as used herein, denotes a monovalent group
derived
from a monocyclic or bicyclic saturated carbocyclic ring compound by the
removal of a
single hydrogen atom. Examples include, but not limited to, cyclopropyl,
cyclobutyl,
cyclopentyl, cyclohexyl, bicyclo [2.2.1] heptyl, and bicyclo [2.2.2] octyl.
The term "substituted C3-C12-cycloalkyl," as used herein, refers to a C3-C12-
cycloalkyl group as previously defined, substituted by independent replacement
or one, two,
or three of the hydrogen atoms thereon with substituents including, but not
limited to, -F,
-Cl, -Br, -I, -OH, protected hydroxy, -NO2, -CN, -C1-C12-alkyl optionally
substituted with
halogen, C2-ClZ-alkenyl optionally substituted with halogen, -C2-C12-alkynyl
optionally
substituted with halogen, -NHz, protected amino, -NH -C1-C12-alkyl, -NH -C2-
C12-alkenyl,
-NH -C2-C12-alkenyl, -NH -C3-C]Z-cycloalkyl, -NH -aryl, -NH -heteroaryl, -NH -
heterocycloalkyl, -dialkylamino, -diarylamino, -diheteroarylamino, -O-C1-C12-
alkyl, -O-
C2-C12-alkenyl, -O-CZ-C12-alkenyl, -O-C3-C12-cycloalkyl, -O-aryl, -O-
heteroaryl, -0-
heterocycloalkyl, -C(O)- C1-C12-alkyl, -C(O)- C2-C12-alkenyl, -C(O)- Cz-C12-
alkenyl,
-C(O)-C3-C12-cycloalkyl, -C(O)-aryl, -C(O)-heteroaryl, -C(O)-heterocycloalkyl,
-CONH2,
-CONH- C1-C12-alkyl, -CONH- C2-C12-alkenyl, -CONH- C2-CIz-alkenyl, -CONH-C3-
C12-
cycloalkyl, -CONH-aryl, -CONH-heteroaryl, -CONH-heterocycloalkyl, -OCOz- C1-
C12-
alkyl, -OCO2- C2-C12-alkenyl, -OC02- CZ-C12-alkenyl, -OCOZ-C3-C12-cycloalkyl, -
OC02-
aryl, -OCO2-heteroaryl, -OCOz-heterocycloalkyl, -OCONHZ, -OCONH- C1-C12-alkyl,
-OCONH- C2-CI2-alkenyl, -OCONH- CZ-C12-alkenyl, -OCONH- C3-C1z-cycloalkyl,
-OCONH- aryl, -OCONH- heteroaryl, -OCONH- heterocycloalkyl, -NHC(O)- CI-C12-
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
alkyl, -NHC(O)-C2-C12-alkenyl, -NHC(O)-C2-C12-alkenyl, -NHC(O)-C3-C12-
cycloalkyl,
-NHC(O)-aryl, -NHC(O)-heteroaryl, -NHC(O)-heterocycloalkyl, -NHCOZ- CI-C12-
alkyl,
-NHCO2- CZ-C1z-alkenyl, -NHCO2- C2-C12-alkenyl, -NHCOZ- C3-C12-cycloalkyl, -
NHCO2-
aryl, -NHCO2- heteroaryl, -NHCO2- heterocycloalkyl, -NHC(O)NH2, NHC(O)NH- CI-
C1Z-
alkyl, -NHC(O)NH-C2-C12-alkenyl, -NHC(O)NH-C2-ClZ-alkenyl, -NHC(O)NH-C3-C12-
cycloalkyl, -NHC(O)NH-aryl, -NHC(O)NH-heteroaryl, -NHC(O)NH-heterocycloalkyl,
NHC(S)NH2, NHC(S)NH- CI-C12-alkyl, -NHC(S)NH-C2-C12-alkenyl, -NHC(S)NH-C2-C12-
alkenyl, -NHC(S)NH-C3-C12-cycloalkyl, -NHC(S)NH-aryl, -NHC(S)NH-heteroaryl,
-NHC(S)NH-heterocycloalkyl, -NHC(NH)NH2, NHC(NH)NH- C1-C12-alkyl,
-NHC(NH)NH-C2-C12-alkenyl, -NHC(NH)NH-C2-C12-alkenyl, -NHC(NH)NH-C3-C12-
cycloalkyl, -NHC(NH)NH-aryl, -NHC(NH)NH-heteroaryl, -NHC(NH)NH-
heterocycloalkyl, NHC(NH)-C1-C12-alkyl, -NHC(NH)-C2-C12-alkenyl, -NHC(NH)-C2-
C12-
alkenyl, -NHC(NH)-C3-C12-cycloalkyl, -NHC(NH)-aryl, -NHC(NH)-heteroaryl,
-NHC(NH)-heterocycloalkyl, -C(NH)NH-C1-C12-alkyl, -C(NH)NH-C2-C12-alkenyl,
-C(NH)NH-C2-C12-alkenyl, -C(NH)NH-C3-C12-cycloalkyl, -C(NH)NH-aryl, -C(NH)NH-
heteroaryl, -C(NH)NH-heterocycloalkyl, -S(O)-Q-C12-alkyl, - S(O)-C2-C12-
alkenyl, -
S(O)-C2-ClZ-alkenyl, - S(O)-C3-C12-cycloalkyl, - S(O)-aryl, - S(O)-heteroaryl,
- S(O)-
heterocycloalkyl -SO2NH2, -SOZNH- C1-C12-alkyl, -SO2NH- C2-ClZ-alkenyl, -SO2NH-
C2-
C12-alkenyl, -SO2NH- C3-C12-cycloalkyl, -SO2NH- aryl, -SO2NH- heteroaryl, -
SO2NH-
heterocycloalkyl, -NHSO2-C1-C12-alkyl, -NHSO2-C2-C1z-alkenyl, - NHSO2-C2-C12-
alkenyl,
-NHSO2-C3-C12-cycloalkyl, -NHSO2-aryl, -NHSOz-heteroaryl, -NHSO2-
heterocycloalkyl,
-CH2NH2, -CH2SO2CH3, -aryl, -arylalkyl, -heteroaryl, -heteroarylalkyl, -
heterocycloalkyl,
-C3-C12-cycloalkyl, -methoxymethoxy, -methoxyethoxy, -SH, -S-C1-C12-alkyl, -S-
C2-C12-
alkenyl, -S-C2-C12-alkenyl, -S-C3-C12-cycloalkyl, -S-aryl, -S-heteroaryl, -S-
heterocycloalkyl, or methylthiomethyl.
The term "heterocycloalkyl," as used herein, refers to a non-aromatic 5-, 6-
or 7-
membered ring or a bi- or tri-cyclic group fused system, where (i) each ring
contains between
one and three heteroatoms independently selected from oxygen, sulfur and
nitrogen, (ii) each
5-membered ring has 0 to 1 double bonds and each 6-membered ring has 0 to 2
double bonds,
(iii) the nitrogen and sulfur heteroatoms may optionally be oxidized, (iv) the
nitrogen
heteroatom may optionally be quaternized, and (iv) any of the above rings may
be fused to a
26
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
benzene ring. Representative heterocycloalkyl groups include, but are not
limited to,
[1,3]dioxolane, pyrrolidinyl, pyrazolinyl, pyrazolidinyl, imidazolinyl,
imidazolidinyl,
piperidinyl, piperazinyl, oxazolidinyl, isoxazolidinyl, morpholinyl,
thiazolidinyl,
isothiazolidinyl, and tetrahydrofuryl.
The term "substituted heterocycloalkyl," as used herein, refers to a
heterocycloalkyl
group, as previously defined, substituted by independent replacement or one,
two, or three of
the hydrogen atoms thereon with substituents including, but not limited to, -
F, -Cl, -Br, -I,
-OH, protected hydroxy, -NOz, -CN, -CI-C12-alkyl optionally substituted with
halogen, C2-
C12-alkenyl optionally substituted with halogen, -C2-C12-alkynyl optionally
substituted with
halogen, -NH2, protected amino, -NH -C1-C12-alky], -NH -C2-C12-alkenyl, -NH -
C2-Clz-
alkenyl, -NH -C3-C12-cycloalkyl, -NH -aryl, -NH -heteroaryl, -NH -
heterocycloalkyl,
-dialkylamino, -diarylamino, -diheteroarylamino, -O-C1-C12-alkyl, -O-Cz-C12-
alkenyl, -O-
C2-C12-alkenyl, -O-C3-C12-cycloalkyl, -0-aryl, -0-heteroaryl, -0-
heterocycloalkyl, -C(O)-
CI-C12-alkyl, -C(O)- Cz-C]2-alkenyl, -C(O)- C2-C12-alkenyl, -C(O)-C3-C12-
cycloalkyl,
-C(O)-aryl, -C(O)-heteroaryl, -C(O)-heterocycloalkyl, =CONH2, -CONH- C1-C12-
alkyl,
-CONH- C2-C12-alkenyl, -CONH- C2-C12-alkenyl, -CONH-C3-C12-cycloalkyl, -CONH-
aryl, -CONH-heteroaryl, -CONH-heterocycloalkyl, -OCO2- C1-C12-alkyl, -OC02- C2-
C12-
alkenyl, -OCO2- C2-C12-alkenyl, -OCO2-C3-C12-cycloalkyl, -OCO2-aryl, -OCO2-
heteroaryl,
-OC02-heterocycloalkyl, -OCONH2, -OCONH- C1-CIZ-alkyl, -OCONH- C2-C12-alkenyl,
-OCONH- C2-C12-alkenyl, -OCONH- C3-C12-cycloalkyl, -OCONH- aryl, -OCONH-
heteroaryl, -OCONH- heterocycloalkyl, -NHC(O)- CI-C12-alkyl, -NHC(O)-C2-C1z-
alkenyl,
-NHC(O)-C2-C1z-alkenyl, -NHC(O)-C3-CI2-cycloalkyl, -NHC(O)-aryl, -NHC(O)-
heteroaryl, -NHC(O)-heterocycloalkyl, -NHCOZ- CI-C12-alkyl, -NHCO2- C2-C12-
alkenyl,
-NHCO2- C2-C12-alkenyl, -NHCO2- C3-C12-cycloalkyl, -NHCO2- aryl, -NHCO2-
heteroaryl, -NHCOZ- heterocycloalkyl, -NHC(O)NH2, NHC(O)NH- C1-C12-alkyl,
-NHC(O)NH-C2-C12-alkenyl, -NHC(O)NH-C2-C12-alkenyl, -NHC(O)NH-C3-C12-
cycloalkyl, -NHC(O)NH-aryl, -NHC(O)NH-heteroaryl, -NHC(O)NH-heterocycloalkyl,
NHC(S)NH2, NHC(S)NH- C1-C1Z-alkyl, -NHC(S)NH-C2-CI2-alkenyl, -NHC(S)NH-C2-C12-
alkenyl, -NHC(S)NH-C3-C1z-cycloalkyl, -NHC(S)NH-aryl, -NHC(S)NH-heteroaryl,
-NHC(S)NH-heterocycloalkyl, -NHC(NH)NH2, NHC(NH)NH- C1-C12-alkyl,
-NHC(NH)NH-C2-C12-alkenyl, -NHC(NH)NH-C2-C12-alkenyl, -NHC(NH)NH-C3-C12-
227
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
cycloalkyl, -NHC(NH)NH-aryl, -NHC(NH)NH-heteroaryl, -NHC(NH)NH-
heterocycloalkyl, NHC(NH)-C1-C12-alkyl, -NHC(NH)-C2-C12-alkenyl, -NHC(NH)-C2-
C1z-
alkenyl, -NHC(NH)-C3-C12-cycloalkyl, -NHC(NH)-aryl, -NHC(NH)-heteroaryl,
-NHC(NH)-heterocycloalkyl, -C(NH)NH-CI-C12-alkyl, -C(NH)NH-C2-CI2-alkenyl,
-C(NH)NH-C2-C12-alkenyl, -C(NH)NH-C3-CIZ-cycloalkyl, -C(NH)NH-aryl, -C(NH)NH-
heteroaryl, -C(NH)NH-heterocycloalkyl, -S(O)-Cl-C1z-alkyl, - S(O)-C2-C12-
alkenyl, -
S(O)-C2-ClZ-alkenyl, - S(O)-C3-ClZ-cycloalkyl, - S(O)-aryl, - S(O)-heteroaryl,
- S(O)-
heterocycloalkyl -SO2NH2, -SO2NH- CI-C12-alkyl, -SO2NH- CZ-C12-alkenyl, -SO2NH-
C2-
C12-alkenyl, -SO2NH- C3-C12-cycloalkyl, -SO2NH- aryl, -SO2NH- heteroaryl, -
SOZNH-
heterocycloalkyl, -NHSO2-C1-C12-alkyl, -NHSO2-C2-C12-alkenyl, - NHSO2-C2-CI2-
alkenyl,
-NHSO2-C3-C12-cycloalkyl, -NHSOZ-aryl, -NHSO2-heteroaryl, -NHSO2-
heterocycloalkyl,
-CH2NH2, -CH2SO2CH3, -aryl, -arylalkyl, -heteroaryl, -heteroarylalkyl, -
heterocycloalkyl,
-C3-C1z-cycloalkyl, -methoxymethoxy, -methoxyethoxy, -SH, -S-CI-C12-alkyl, -S-
C2-C12-
alkenyl, -S-C2-C12-alkenyl, -S-C3-ClZ-cycloalkyl, -S-aryl, -S-heteroaryl, -S-
heterocycloalkyl, or methylthiomethyl.
The term "heteroarylalkyl," as used herein, refers to a C1-C3 alkyl or CI-C6
alkyl
residue residue attached to a heteroaryl ring. Examples include, but are not
limited to,
pyridinylmethyl, pyrimidinylethyl and the like.
The term "substituted heteroarylalkyl," as used herein, refers to a
heteroarylalkyl
group, as previously defined, substituted by independent replacement or one,
two, or three of
the hydrogen atoms thereon with substituents including, but not limited to, -
F, -Cl, -Br, -I,
-OH, protected hydroxy, -NO2, -CN, -C1-C12-alkyl optionally substituted with
halogen, C2-
C12-alkenyl optionally substituted with halogen, -C2-C12-alkynyl optionally
substituted with
halogen, -NH2, protected amino, -NH -C1-C12-alkyl, -NH -C2-C12-alkenyl, -NH -
C2-Clz-
alkenyl, -NH -C3-CIZ-cycloalkyl, -NH -aryl, -NH -heteroaryl, -NH -
heterocycloalkyl,
-dialkylamino, -diarylamino, -diheteroarylamino, -O-C1-C12-alkyl, -O-C2-ClZ-
alkenyl, -O-
C2-C12-alkenyl, -O-C3-C12-cycloalkyl, -0-aryl, -O-heteroaryl, -0-
heterocycloalkyl, -C(O)-
C1-C12-alkyl, -C(O)- C2-C12-alkenyl, -C(O)- C2-ClZ-alkenyl, -C(O)-C3-CIz-
cycloalkyl,
-C(O)-aryl, -C(O)-heteroaryl, -C(O)-heterocycloalkyl, -CONH2, -CONH- C1-C12-
alkyl,
-CONH- C2-C12-alkenyl, -CONH- Cz-C12-alkenyl, -CONH-C3-C12-cycloalkyl, -CONH-
28
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
aryl, -CONH-heteroaryl, -CONH-heterocycloalkyl, -OCO2- C1-C12-alkyl, -OC02- C2-
C12-
alkenyl, -OCOZ- C2-C1z-alkenyl, -OC02-C3-C,Z-cycloalkyl, -OCO2-aryl, -OC02-
heteroaryl,
-OCOz-heterocycloalkyl, -OCONH2, -OCONH- C1-C12-alkyl, -OCONH- C2-C12-alkenyl,
-OCONH- C2-C12-alkenyl, -OCONH- C3-C1z-cycloalkyl, -OCONH- aryl, -OCONH-
heteroaryl, -OCONH- heterocycloalkyl, -NHC(O)- Cl-C12-alkyl, -NHC(O)-C2-C12-
alkenyl,
-NHC(O)-C2-C12-alkenyl, -NHC(O)-C3-C12-cycloalkyl, -NHC(O)-aryl, -NHC(O)-
heteroaryl, -NHC(O)-heterocycloalkyl, -NHCO2- C1-C12-alkyl, -NHCO2- C2-C12-
alkenyl,
-NHCO2- C2-C12-alkenyl, -NHCOZ- C3-C12-cycloalkyl, -NHCO2- aryl, -NHCOz-
heteroaryl, -NHCOZ- heterocycloalkyl, -NHC(O)NHZ, NHC(O)NH- C1-C12-alkyl,
-NHC(O)NH-C2-C12-alkenyl, -NHC(O)NH-C2-ClZ-alkenyl, -NHC(O)NH-C3-C12-
cycloalkyl, -NHC(O)NH-aryl, -NHC(O)NH-heteroaryl, -NHC(O)NH-heterocycloalkyl,
NHC(S)NH2, NHC(S)NH- C1-C1z-alkyl, -NHC(S)NH-C2-C12-alkenyl, -NHC(S)NH-C2-C12-
alkenyl, -NHC(S)NH-C3-QZ-cycloalkyl, -NHC(S)NH-aryl, -NHC(S)NH-heteroaryl,
-NHC(S)NH-heterocycloalkyl, -NHC(NH)NH2, NHC(NH)NH- C1-C12-alkyl,
-NHC(NH)NH-C2-C12-alkenyl, -NHC(NH)NH-C2-ClZ-alkenyl, -NHC(NH)NH-C3-C12-
cycloalkyl, -NHC(NH)NH-aryl, -NHC(NH)NH-heteroaryl, -NHC(NH)NH-
heterocycloalkyl, NHC(NH)-Cl-C12-alkyl, -NHC(NH)-C2-C12-alkenyl, -NHC(NH)-C2-
ClZ-
alkenyl, -NHC(NH)-C3-C12-cycloalkyl, -NHC(NH)-aryl, -NHC(NH)-heteroaryl,
-NHC(NH)-heterocycloalkyl, -C(NH)NH-C1-C12-alkyl, -C(NH)NH-C2-C1z-alkenyl,
-C(NH)NH-C2-C12-alkenyl, -C(NH)NH-C3-C12-cycloalkyl, -C(NH)NH-aryl, -C(NH)NH-
heteroaryl, -C(NH)NH-heterocycloalkyl, -S(O)-C1-C12-alkyl, - S(O)-C2-C12-
alkenyl, -
S(O)-CZ-C12-alkenyl, - S(O)-C3-C12-cycloalkyl, - S(O)-aryl, - S(O)-heteroaryl,
- S(O)-
heterocycloalkyl -SO2NH2, -SO2NH- Cl-C12-alkyl, -SOZNH- C2-C,2-alkenyl, -SO2NH-
C2-
C12-alkenyl, -SOZNH- C3-C12-cycloalkyl, -SOZNH- aryl, -SO2NH- heteroaryl, -
SO2NH-
heterocycloalkyl, -NHSO2-C1-C12-alkyl, -NHSO2-C2-C12-alkenyl, - NHSO2-C2-CI2-
alkenyl,
-NHSO2-C3-C12-cycloalkyl, -NHSO2-aryl, -NHSO2-heteroaryl, -NHSO2-
heterocycloalkyl,
-CH2NH2, -CH2SO2CH3, -aryl, -arylalkyl, -heteroaryl, -heteroarylalkyl, -
heterocycloalkyl,
-C3-C12-cycloalkyl, -methoxymethoxy, -methoxyethoxy, -SH, -S-C1-C12-alkyl, -S-
Cz-C12-
alkenyl, -S-C2-C12-alkenyl, -S-C3-C12-cycloalkyl, -S-aryl, -S-heteroaryl, -S-
heterocycloalkyl, or methylthiomethyl.
29
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
The term "C1-C6 alkoxy," as used herein, refers to a C1-C6 alkyl group, as
previously
defined, attached to the parent molecular moiety through an oxygen atom.
Examples of C1-
C6-alkoxy include, but are not limited to, methoxy, ethoxy, propoxy,
isopropoxy, n-butoxy,
tert-butoxy, neopentoxy and n-hexoxy.
The term "C1-C3-alkyl-amino," as used herein, refers to one or two C1-C3-alkyl
groups, as previously defined, attached to the parent molecular moiety through
a nitrogen
atom. Examples of C1-C3-alkyl-amino include, but are not limited to,
methylamino,
dimethylamino, ethylamino, diethylamino, and propylamino.
The term "alkylamino" refers to a group having the structure -NH(C1-C12 alkyl)
where C1-C12 alkyl is as previously defined.
The term "dialkylamino" refers to a group having the structure -N(C1-C12
alkyl) (C1-
C12 alkyl), where CI-C12 alkyl is as previously defined. Examples of
dialkylamino are, but
not limited to, dimethylamino, diethylamino, methylethylamino, piperidino, and
the like.
The term "alkoxycarbonyl" represents an ester group, i.e., an alkoxy group,
attached
to the parent molecular moiety through a carbonyl group such as
methoxycarbonyl,
ethoxycarbonyl, and the like.
The term "carboxaldehyde," as used herein, refers to a group of formula -CHO.
The term "carboxy," as used herein, refers to a group of formula -COOH.
The term "carboxamide," as used herein, refers to a group of formula -
C(O)NH(C1-
C12 alkyl) or - C(O)N(CI-C12 alkyl) (Ci-CIz alkyl), -C(O)NH2, and the like.
The term "hydroxy protecting group," as used herein, refers to a labile
chemical
moiety which is known in the art to protect a hydroxyl group against undesired
reactions
during synthetic procedures. After said synthetic procedure(s) the hydroxy
protecting group
as described herein may be selectively removed. Hydroxy protecting groups as
known in the
are described generally in T.H. Greene and P.G. M. Wuts, Protective Groups in
Or anic
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
S nty hesis, 3rd edition, John Wiley & Sons, New York (1999). Examples of
hydroxy
protecting groups include, but are not limited to, methylthiomethyl, tert-
butyl-dimethylsilyl,
tert-butyldiphenylsilyl, acyl substituted with an aromatic group and the like.
The term "protected hydroxy," as used herein, refers to a hydroxy group
protected
with a hydroxy protecting group, as defined above, including benzoyl, acetyl,
trimethylsilyl,
triethylsilyl, methoxymethyl groups, for example.
The term "amino protecting group," as used herein, refers to a labile chemical
moiety
which is known in the art to protect an amino group against undesired
reactions during
synthetic procedures. After said synthetic procedure(s) the amino protecting
group as
described herein may be selectively removed. Amino protecting groups as known
in the are
described generally in T.H. Greene and P.G. M. Wuts, Protective Groups in
Organic
Synthesis, 3rd edition, John Wiley & Sons, New York (1999). Examples of amino
protecting
groups include, but are not limited to, t-butoxycarbonyl, 9-
fluorenylmethoxycarbonyl,
benzyloxycarbonyl, and the like.
The term "protected amino," as used herein, refers to an amino group protected
with
an amino protecting group as defined above.
The term "aprotic solvent," as used herein, refers to a solvent that is
relatively inert to
proton activity, i.e., not acting as a proton-donor. Examples include, but are
not limited to,
hydrocarbons, such as hexane and toluene, for example, halogenated
hydrocarbons, such as,
for example, methylene chloride, ethylene chloride, chloroform, and the like,
heterocyclic
compounds, such as, for example, tetrahydrofuran and N-methylpyrrolidinone,
and ethers
such as diethyl ether, bis-methoxymethyl ether. Such compounds are well known
to those
skilled in the art, and it will be obvious to those skilled in the art that
individual solvents or
mixtures thereof may be preferred for specific compounds and reaction
conditions, depending
upon such factors as the solubility of reagents, reactivity of reagents and
preferred
temperature ranges, for example. Further discussions of aprotic solvents may
be found in
organic chemistry textbooks or in specialized monographs, for example: Organic
Solvents
Physical Properties and Methods of Purification, 4th ed., edited by John A.
Riddick et al.,
Vol. II, in the Techniques of Chemistry Series, John Wiley & Sons, NY, 1986.
31
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
The term "protogenic organic solvent," as used herein, refers to a solvent
that tends to
provide protons, such as an alcohol, for example, methanol, ethanol, propanol,
isopropanol,
butanol, t-butanol, and the like. Such solvents are well known to those
skilled in the art, and
it will be obvious to those skilled in the art that individual solvents or
mixtures thereof may
be preferred for specific compounds and reaction conditions, depending upon
such factors as
the solubility of reagents, reactivity of reagents and preferred temperature
ranges, for
example. Further discussions of protogenic solvents may be found in organic
chemistry
textbooks or in specialized monographs, for example: Organic Solvents Physical
Properties
and Methods of Purification, 4th ed., edited by John A. Riddick et al., Vol.
II, in the
Techniques of Chemistry Series, John Wiley & Sons, NY, 1986.
"An effective amount," as used herein, refers to an amount of a compound which
confers a therapeutic effect on the treated subject. The therapeutic effect
may be objective
(i.e., measurable by some test or marker) or subjective (i.e., subject gives
an indication of or
feels an effect). An effective amount of the compound described above may
range from
about 0.1 mg/Kg to about 500 mg/Kg, preferably from about 1 to about 50 mg/Kg.
Effective
doses will also vary depending on route of administration, as well as the
possibility of co-
usage with other agents.
Combinations of substituents and variables envisioned by this invention are
only
those that result in the formation of stable compounds. The term "stable", as
used herein,
refers to compounds which possess stability sufficient to allow manufacture
and which
maintains the integrity of the compound for a sufficient period of time to be
useful for the
purposes detailed herein (e.g., therapeutic or prophylactic administration to
a subject).
The synthesized compounds can be separated from a reaction mixture and further
purified by a method such as column chromatography, high pressure liquid
chromatography,
or recrystallization. As can be appreciated by the skilled artisan, further
methods of
synthesizing the compounds of the formulae herein will be evident to those of
ordinary skill
in the art. Additionally, the various synthetic steps may be performed in an
alternate
sequence or order to give the desired compounds. Synthetic chemistry
transformations and
protecting group methodologies (protection and deprotection) useful in
synthesizing the
compounds described herein are known in the art and include, for example,
those such as
described in R. Larock, Comprehensive Organic Transformations, VCH Publishers
(1989);
32
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
T.W. Greene and P.G.M. Wuts, Protective Groups in Organic Synthesis, 2d. Ed.,
John Wiley
and Sons (1991); L. Fieser and M. Fieser, Fieser and Fieser's Reagents for
Organic Synthesis,
John Wiley and Sons (1994); and L. Paquette, ed., Encyclopedia of Reagents for
Organic
Synthesis, John Wiley and Sons (1995), and subsequent editions thereof.
The term "subject" as used herein refers to an animal. Preferably the animal
is a
mammal. More preferably the mammal is a human. A subject also refers to, for
example,
dogs, cats, horses, cows, pigs, guinea pigs, fish, birds and the like.
The compounds of this invention may be modified by appending appropriate
functionalities to enhance selective biological properties. Such modifications
are known in
the art and may include those which increase biological penetration into a
given biological
system (e.g., blood, lymphatic system, central nervous system), increase oral
availability,
increase solubility to allow administration by injection, alter metabolism and
alter rate of
excretion.
The compounds described herein contain one or more asymmetric centers and thus
give rise to enantiomers, diastereomers, and other stereoisomeric forms that
may be defined,
in terms of absolute stereochemistry, as (R)- or (S)- , or as (D)- or (L)- for
amino acids. The
present invention is meant to include all such possible isomers, as well as
their racemic and
optically pure forms. Optical isomers may be prepared from their respective
optically active
precursors by the procedures described above, or by resolving the racemic
mixtures. The
resolution can be carried out in the presence of a resolving agent, by
chromatography or by
repeated crystallization or by some combination of these techniques which are
known to
those skilled in the art. Further details regarding resolutions can be found
in Jacques, et al.,
Enantiomers, Racemates, and Resolutions (John Wiley & Sons, 1981). When the
compounds
described herein contain olefinic double bonds, other unsaturation, or other
centers of
geometric asymmetry, and unless specified otherwise, it is intended that the
compounds
include both E and Z geometric isomers or cis- and trans- isomers. Likewise,
all tautomeric
forms are also intended to be included. The configuration of any carbon-carbon
double bond
appearing herein is selected for convenience only and is not intended to
designate a particular
configuration unless the text so states; thus a carbon-carbon double bond or
carbon-
heteroatom double bond depicted arbitrarily herein as trans may be cis, trans,
or a mixture of
the two in any proportion.
33
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
As used herein, the term "pharmaceutically acceptable salt" refers to those
salts which
are, within the scope of sound medical judgment, suitable for use in contact
with the tissues
of humans and lower animals without undue toxicity, irritation, allergic
response and the like,
and are commensurate with a reasonable benefit/risk ratio. Pharmaceutically
acceptable salts
are well known in the art. For example, S. M. Berge, et al. describes
pharmaceutically
acceptable salts in detail in J. Pharmaceutical Sciences, 66: 1-19 (1977). The
salts can be
prepared in situ during the final isolation and purification of the compounds
of the invention,
or separately by reacting the free base function with a suitable organic acid.
Examples of
pharmaceutically acceptable include, but are not limited to, nontoxic acid
addition salts are
salts of an amino group formed with inorganic acids such as hydrochloric acid,
hydrobromic
acid, phosphoric acid, sulfuric acid and perchloric acid or with organic acids
such as acetic
acid, maleic acid, tartaric acid, citric acid, succinic acid or malonic acid
or by using other
methods used in the art such as ion exchange. Other pharmaceutically
acceptable salts
include, but are not limited to, adipate, alginate, ascorbate, aspartate,
benzenesulfonate,
benzoate, bisulfate, borate, butyrate, camphorate, camphorsulfonate, citrate,
cyclopentanepropionate, digluconate, dodecylsulfate, ethanesulfonate, formate,
fumarate,
glucoheptonate, glycerophosphate, gluconate, hemisulfate, heptanoate,
hexanoate,
hydroiodide, 2-hydroxy-ethanesulfonate, lactobionate, lactate, laurate, lauryl
sulfate, malate,
maleate, malonate, methanesulfonate, 2-naphthalenesulfonate, nicotinate,
nitrate, oleate,
oxalate, palmitate, pamoate, pectinate, persulfate, 3-phenylpropionate,
phosphate, picrate,
pivalate, propionate, stearate, succinate, sulfate, tartrate, thiocyanate, p-
toluenesulfonate,
undecanoate, valerate salts, and the like. Representative alkali or alkaline
earth metal salts
include sodium, lithium, potassium, calcium, magnesium, and the like. Further
pharmaceutically acceptable salts include, when appropriate, nontoxic
ammonium,
quaternary ammonium, and amine cations formed using counterions such as
halide,
hydroxide, carboxylate, sulfate, phosphate, nitrate, alkyl having from 1 to 6
carbon atoms,
sulfonate and aryl sulfonate.
As used herein, the compounds of this invention, including the compounds of
formulae described herein, are defined to include pharmaceutically acceptable
derivatives or
prodrugs thereof. A"pharmaceutically acceptable derivative or prodrug" means
any
pharmaceutically acceptable salt, ester, salt of an ester, or other derivative
of a compound of
34
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
this invention which, upon administration to a recipient, is capable of
providing (directly or
indirectly) a compound of this invention.
When the compositions of this invention comprise a combination of a compound
of
the formulae described herein and one or more additional therapeutic or
prophylactic agents,
both the compound and the additional agent should be present at dosage levels
of between
about 1 to 100%, and more preferably between about 5 to 95% of the dosage
normally
administered in a monotherapy regimen. The additional agents may be
administered
separately, as part of a multiple dose regimen, from the compounds of this
invention.
Alternatively, those agents may be part of a single dosage form, mixed
together with the
compounds of this invention in a single composition.
As used herein, unless otherwise indicated, the term "bacterial infection(s)"
or
"protozoa infections"; includes, but is not limited to, bacterial infections
and protozoa
infections that occur in mammals, fish and birds as well as disorders related
to bacterial
infections and protozoa infections that may be treated or prevented by
administering
antibiotics such as the compounds of the present invention. Such bacterial
infections and
protozoa infections and disorders related to such infections include, but are
not limited to, the
following: pneumonia, otitis media, sinusitus, bronchitis, tonsillitis, and
mastoiditis related to
infection by Streptococcus pneumoniae, Haemophilus influenzae, Moraxella
catarrhalis,
Staphylococcus aureus, or Peptostreptococcus spp. Pseudomonas spp.;
pharynigitis,
rheumatic fever, and glomerulonephritis related to infection by Streptococcus
pyogenes,
Groups C and G streptococci, Clostridium diptheriae, or Actinobacillus
haemolyticum;
respiratory tract infections related to infection by Mycoplasma pneumoniae,
Legionella
pneumophila, Streptococcus pneumoniae, Haemophilus influenzae, or Chlamydia
pneumoniae; uncomplicated skin and soft tissue infections, abscesses and
osteomyelitis, and
puerperal fever related to infection by Staphylococcus aureus, coagulase-
positive
staphylococci (i.e., S. epidermidis, S. hemolyticus, etc.), S. pyogenes, S.
agalactiae,
Streptococcal groups C-F (minute-colony streptococci), viridans streptococci,
Corynebacterium spp., Clostridium spp., or Bartonella henselae; uncomplicated
acute urinary
tract infections related to infection by S. saprophyticus or Enterococcus
spp.; urethritis and
cervicitis; and sexually transmitted diseases related to infection by
Chlamydia trachomatis,
Haemophilus ducreyi, Treponema pallidum, Ureaplasma urealyticum, or Nesseria
gonorrheae; toxin diseases related to infection by S. aureus (food poisoning
and Toxic shock
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
syndrome), or Groups A, S. and C streptococci; ulcers related to infection by
Helicobacter
pylori; systemic febrile syndromes related to infection by Borrelia
recurrentis; Lyme disease
related to infection by Borrelia burgdorferi; conjunctivitis, keratitis, and
dacrocystitis related
to infection by C. trachomatis, N. gonorrhoeae, S. aureus, S. pneumoniae, S.
pyogenes, H.
influenzae, or Listeria spp.; disseminated Mycobacterium avium complex (MAC)
disease
related to infection by Mycobacterium avium, or Mycobacterium intracellulare;
gastroenteritis related to infection by Campylobacterjejuni; intestinal
protozoa related to
infection by Cryptosporidium spp. odontogenic infection related to infection
by viridans
streptococci; persistent cough related to infection by Bordetella pertussis;
gas gangrene
related to infection by Clostridium perfringens or Bacteroides spp.; Skin
infection by S.
aureus, Propionibacterium acne; atherosclerosis related to infection by
Helicobacter pylori or
Chlamydia pneumoniae; or the like.
Bacterial infections and protozoa infections and disorders related to such
infections
that may be treated or prevented in animals include, but are not limited to,
the following:
bovine respiratory disease related to infection by P. haemolytica., P.
multocida, Mycoplasma
bovis, or Bordetella spp.; cow enteric disease related to infection by E. coli
or protozoa (i.e.,
coccidia, cryptosporidia, etc.), dairy cow mastitis related to infection by S.
aureus, S. uberis,
S. agalactiae, S. dysgalactiae, Klebsiella spp., Corynebacterium, or
Enterococcus spp.; swine
respiratory disease related to infection by A. pleuropneumoniae., P.
multocida, or
Mycoplasma spp.; swine enteric disease related to infection by E. coli,
Lawsonia
intracellularis, Salmonella spp., or Serpulina hyodyisinteriae; cow footrot
related to infection
by Fusobacterium spp.; cow metritis related to infection by E. coli; cow hairy
warts related to
Infection by Fusobacterium necrophorum or Bacteroides nodosus; cow pink-eye
related to
infection by Moraxella bovis, cow premature abortion related to infection by
protozoa (i.e.
neosporium); urinary tract infection in dogs and cats related to infection by
E. coli; skin and
soft tissue infections in dogs and cats related to infection by S.
epidermidis, S. intermedius,
coagulase neg. Staphylococcus or P. multocida; and dental or mouth infections
in dogs and
oats related to infection by Alcaligenes spp., Bacteroides spp., Clostridium
spp., Enterobacter
spp., Eubacterium spp., Peptostreptococcus spp., Porphfyromonas spp.,
Campylobacter spp.,
Actinomyces spp., Erysipelothrix spp., Rhodococcus spp., Trypanosoma spp.,
Plas,odium
spp., Babesia spp., Toxoplasma spp., Pneumocystis spp., Leishmania spp., and
Trichomonas
spp. or Prevotella spp. Other bacterial infections and protozoa infections and
disorders related
to such infections that may be treated or prevented in accord with the method
of the present
36
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
invention are referred to in J. P. Sanford at al.,"The Sanford Guide To
Antimicrobial
Therapy," 26th Edition, (Antimicrobial Therapy, Inc., 1996).
Antibacterial Activity
Susceptibility tests can be used to quantitatively measure the in vitro
activity of an
antimicrobial agent against a given bacterial isolate. Compounds were tested
for in vitro
antibacterial activity by a micro-dilution method. Minimal Inhibitory
Concentration (MIC)
was determined in 96 well microtiter plates utilizing the appropriate Mueller
Hinton Broth
medium (CAMHB) for the observed bacterial isolates. Antimicrobial agents were
serially
diluted (2-fold) in DMSO to produce a concentration range from about 64 g/ml
to about
0.03 g/ml. The diluted compounds (2 l/well) were then transferred into
sterile,
uninoculated CAMHB (0.2 mL) by use of a 96 fixed tip-pipetting station. The
inoculum for
each bacterial strain was standardized to 5 x 105 CFU/mL by optical comparison
to a 0.5
McFarland turbidity standard. The plates were inoculated with 10 l/well of
adjusted
,
bacterial inoculum. The 96 well plates were covered and incubated at 35 +/- 2
C for 24 hours
in ambient air environment. Following incubation, plate wells were visually
examined by
Optical Density measurement for the presence of growth (turbidity). The lowest
concentration of an antimicrobial agent at which no visible growth occurs was
defined as the
MIC. The compounds of the invention generally demonstrated an MIC in the range
from
about 64 g/ml to about 0.03 g/ml.
All in vitro testing follows the guidelines described in the Approved
Standards M7-
A4 protocol, published by the National Committee for Clinical Laboratory
Standards
(NCCLS).
Pharmaceutical Compositions.
The pharmaceutical compositions of the present invention comprise a
therapeutically
effective amount of a compound of the present invention formulated together
with one or
more pharmaceutically acceptable carriers or excipients.
As used herein, the term "pharmaceutically acceptable carrier or excipient"
means a
non-toxic, inert solid, semi-solid or liquid filler, diluent, encapsulating
material or
formulation auxiliary of any type. Some examples of materials which can serve
as
37
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
pharmaceutically acceptable carriers are sugars such as lactose, glucose and
sucrose; starches
such as corn starch and potato starch; cellulose and its derivatives such as
sodium
carboxymethyl cellulose, ethyl cellulose and cellulose acetate; powdered
tragacanth; malt;
gelatin; talc; excipients such as cocoa butter and suppository waxes; oils
such as peanut oil,
cottonseed oil, safflower oil, sesame oil, olive oil, corn oil and soybean
oil; glycols such as
propylene glycol; esters such as ethyl oleate and ethyl laurate; agar;
buffering agents such as
magnesium hydroxide and aluminun hydroxide; alginic acid; pyrogen-free water;
isotonic
saline; Ringer's solution; ethyl alcohol, and phosphate buffer solutions, as
well as other non-
toxic compatible lubricants such as sodium lauryl sulfate and magnesium
stearate, as well as
coloring agents, releasing agents, coating agents, sweetening, flavoring and
perfuming
agents, preservatives and antioxidants can also be present in the composition,
according to
the judgment of the formulator.
The pharmaceutical compositions of this invention may be administered orally,
parenterally, by inhalation spray, topically, rectally, nasally, buccally,
vaginally or via an
implanted reservoir, preferably by oral administration or administration by
injection. The
pharmaceutical compositions of this invention may contain any conventional non-
toxic
pharmaceutically-acceptable carriers, adjuvants or vehicles. In some cases,
the pH of the
formulation may be adjusted with pharmaceutically acceptable acids, bases or
buffers to
enhance the stability of the formulated compound or its delivery form. The
term parenteral as
used herein includes subcutaneous, intracutaneous, intravenous, intramuscular,
intraarticular,
intraarterial, intrasynovial, intrasternal, intrathecal, intralesional and
intracranial injection or
infusion techniques.
Liquid dosage forms for oral administration include pharmaceutically
acceptable
emulsions, microemulsions, solutions, suspensions, syrups and elixirs. In
addition to the
active compounds, the liquid dosage forms may contain inert diluents commonly
used in the
art such as, for example, water or other solvents, solubilizing agents and
emulsifiers such as
ethyl alcohol, isopropyl alcohol, ethyl carbonate, ethyl acetate, benzyl
alcohol, benzyl
benzoate, propylene glycol, 1,3-butylene glycol, dimethylformamide, oils (in
particular,
cottonseed, groundnut, corn, germ, olive, castor, and sesame oils), glycerol,
tetrahydrofurfuryl alcohol, polyethylene glycols and fatty acid esters of
sorbitan, and
mixtures thereof. Besides inert diluents, the oral compositions can also
include adjuvants
38
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
such as wetting agents, emulsifying and suspending agents, sweetening,
flavoring, and
perfuming agents.
Injectable preparations, for example, sterile injectable aqueous or oleaginous
suspensions, may be formulated according to the known art using suitable
dispersing or
wetting agents and suspending agents. The sterile injectable preparation may
also be a sterile
injectable solution, suspension or emulsion in a nontoxic parenterally
acceptable diluent or
solvent, for example, as a solution in 1,3-butanediol. Among the acceptable
vehicles and
solvents that may be employed are water, Ringer's solution, U.S.P. and
isotonic sodium
chloride solution. In addition, sterile, fixed oils are conventionally
employed as a solvent or
suspending medium. For this purpose any bland fixed oil can be employed
including
synthetic mono- or diglycerides. In addition, fatty acids such as oleic acid
are used in the
preparation of injectables.
The injectable formulations can be sterilized, for example, by filtration
through a
bacterial-retaining filter, or by incorporating sterilizing agents in the form
of sterile solid
compositions which can be dissolved or dispersed in sterile water or other
sterile injectable
medium prior to use.
In order to prolong the effect of a drug, it is often desirable to slow the
absorption of
the drug from subcutaneous or intramuscular injection. This may be
accomplished by the use
of a liquid suspension of crystalline or amorphous material with poor water
solubility. The
rate of absorption of the drug then depends upon its rate of dissolution,
which, in turn, may
depend upon crystal size and crystalline form. Alternatively, delayed
absorption of a
parenterally administered drug form is accomplished by dissolving or
suspending the drug in
an oil vehicle. Injectable depot forms are made by forming microencapsule
matrices of the
drug in biodegradable polymers such as polylactide-polyglycolide. Depending
upon the ratio
of drug to polymer and the nature of the particular polymer employed, the rate
of drug release
can be controlled. Examples of other biodegradable polymers include
poly(orthoesters) and
poly(anhydrides). Depot injectable formulations are also prepared by
entrapping the drug in
liposomes or microemulsions that are compatible with body tissues.
Compositions for rectal or vaginal administration are preferably suppositories
which
can be prepared by mixing the compounds of this invention with suitable non-
irritating
39
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
excipients or carriers such as cocoa butter, polyethylene glycol or a
suppository wax which
are solid at ambient temperature but liquid at body temperature and therefore
melt in the
rectum or vaginal cavity and release the active compound.
Solid dosage forms for oral administration include capsules, tablets, pills,
powders,
and granules. In such solid dosage forms, the active compound is mixed with at
least one
inert, pharmaceutically acceptable excipient or carrier such as sodium citrate
or dicalcium
phosphate and/or: a) fillers or extenders such as starches, lactose, sucrose,
glucose, mannitol,
and silicic acid, b) binders such as, for example, carboxymethylcellulose,
alginates, gelatin,
polyvinylpyrrolidinone, sucrose, and acacia, c) humectants such as glycerol,
d) disintegrating
agents such as agar-agar, calcium carbonate, potato or tapioca starch, alginic
acid, certain
silicates, and sodium carbonate, e) solution retarding agents such as
paraffin, f) absorption
accelerators such as quaternary ammonium compounds, g) wetting agents such as,
for
example, cetyl alcohol and glycerol monostearate, h) absorbents such as kaolin
and bentonite
clay, and i) lubricants such as talc, calcium stearate, magnesium stearate,
solid polyethylene
glycols, sodium lauryl sulfate, and mixtures thereof. In the case of capsules,
tablets and pills,
the dosage form may also comprise buffering agents.
Solid compositions of a similar type may also be employed as fillers in soft
and hard-
filled gelatin capsules using such excipients as lactose or milk sugar as well
as high
molecular weight polyethylene glycols and the like.
The solid dosage forms of tablets, dragees, capsules, pills, and granules can
be
prepared with coatings and shells such as enteric coatings and other coatings
well known in
the pharmaceutical formulating art. They may optionally contain opacifying
agents and can
also be of a composition that they release the active ingredient(s) only, or
preferentially, in a
certain part of the intestinal tract, optionally, in a delayed manner.
Examples of embedding
compositions that can be used include polymeric substances and waxes.
Dosage forms for topical or transdermal administration of a compound of this
invention include ointments, pastes, creams, lotions, gels, powders,
solutions, sprays,
inhalants or patches. The active component is admixed under sterile conditions
with a
pharmaceutically acceptable carrier and any needed preservatives or buffers as
may be
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
required. Ophthalmic formulation, ear drops, eye ointments, powders and
solutions are also
contemplated as being within the scope of this invention.
The ointments, pastes, creams and gels may contain, in addition to an active
compound of this invention, excipients such as animal and vegetable fats,
oils, waxes,
paraffins, starch, tragacanth, cellulose derivatives, polyethylene glycols,
silicones, bentonites,
silicic acid, talc and zinc oxide, or mixtures thereof.
Powders and sprays can contain, in addition to the compounds of this
invention,
excipients such as lactose, talc, silicic acid, aluminum hydroxide, calcium
silicates and
polyamide powder, or mixtures of these substances. Sprays can additionally
contain
customary propellants such as chlorofluorohydrocarbons.
Transdermal patches have the added advantage of providing controlled delivery
of a
compound to the body. Such dosage forms can be made by dissolving or
dispensing the
compound in the proper medium. Absorption enhancers can also be used to
increase the flux
of the compound across the skin. The rate can be controlled by either
providing a rate
controlling membrane or by dispersing the compound in a polymer matrix or gel.
According to the methods of treatment of the present invention, bacterial
infections
are treated or prevented in a patient such as a human or other animals by
administering to the
patient a therapeutically effective amount of a compound of the invention, in
such amounts
and for such time as is necessary to achieve the desired result.
By a "therapeutically effective amount" of a compound of the invention is
meant a
sufficient amount of the compound to treat bacterial infections, at a
reasonable benefit/risk
ratio applicable to any medical treatment. It will be understood, however,
that the total daily
usage of the compounds and compositions of the present invention will be
decided by the
attending physician within the scope of sound medical judgment. The specific
therapeutically
effective dose level for any particular patient will depend upon a variety of
factors including
the disorder being treated and the severity of the disorder; the activity of
the specific
compound employed; the specific composition employed; the age, body weight,
general
health, sex and diet of the patient; the time of administration, route of
administration, and rate
of excretion of the specific compound employed; the duration of the treatment;
drugs used in
41
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
combination or contemporaneously with the specific compound employed; and like
factors
well known in the medical arts.
The total daily dose of the compounds of this invention administered to a
human or
other animal in single or in divided doses can be in amounts, for example,
from 0.01 to 50
mg/kg body weight or more usually from 0.1 to 25 mg/kg body weight. Single
dose
compositions may contain such amounts or submultiples thereof to make up the
daily dose.
In general, treatment regimens according to the present invention comprise
administration to
a patient in need of such treatment from about 10 mg to about 1000 mg of the
compound(s)
of this invention per day in single or multiple doses.
The compounds of the formulae described herein can, for example, be
administered
by injection, intravenously, intraarterially, subdermally, intraperitoneally,
intramuscularly, or
subcutaneously; or orally, buccally, nasally, transmucosally, topically, in an
ophthalmic
preparation, or by inhalation, with a dosage ranging from about 0.5 to about
100 mg/kg of
body weight, alternatively dosages between 1 mg and 1000 mg/dose, every 4 to
120 hours,
or according to the requirements of the particular drug. The methods herein
contemplate
administration of an effective amount of compound or compound composition to
achieve the
desired or stated effect. Typically, the pharmaceutical compositions of this
invention will be
administered from about 1 to about 6 times per day or alternatively, as a
continuous infusion.
Such administration can be used as a chronic or acute therapy. The amount of
active
ingredient that may be combined with the carrier materials to produce a single
dosage form
will vary depending upon the host treated and the particular mode of
administration. A
typical preparation will contain from about 5% to about 95% active compound
(w/w).
Alternatively, such preparations may contain from about 20% to about 80%
active
compound.
Lower or higher doses than those recited above may be required. Specific
dosage and
treatment regimens for any particular patient will depend upon a variety of
factors, including
the activity of the specific compound employed, the age, body weight, general
health status,
sex, diet, time of administration, rate of excretion, drug combination, the
severity and course
of the disease, condition or symptoms, the patient's disposition to the
disease, condition or
symptoms, and the judgment of the treating physician.
42
CA 02483875 2007-07-24
Upon improvement of a patient's condition, a maintenance dose of a compound,
composition or combination of this invention may be administered, if
necessary.
Subsequently, the dosage or frequency of administration, or both, may be
reduced, as a
function of the symptoms, to a level at which the improved condition is
retained when the
symptoms have been alleviated to the desired level. Patients may, however,
require
intermittent treatment on a long-term basis upon any recurrence of disease
symptoms.
The pharmaceutical compositions of this invention can be administered orally
to fish
by blending said pharmaceutical compositions into fish feed or said
pharmaceutical
compositions may be dissolved in water in which infected fish are placed, a
method
commonly referred to as a medicated bath. The dosage for the treatment of fish
differs
depending upon the purpose of administration (prevention or cure of disease)
and type of
administration, size and extent of infection of the fish to be treated.
Generally, a dosage of 5
- 1000 mg, preferably 20 - 100 mg, per kg of body weight of fish may be
administered per
day, either at one time or divided into several times. It will be recognized
that the above-
specified dosage is only a general range which may be reduced or increased
depending upon
the age, body weight, condition of disease, etc. of the fish.
Unless otherwise defined, all technical and scientific terms used herein are
accorded
the meaning commonly known to one with ordinary sldll in the art.
Abbreviations
Abbreviations which have been used in the descriptions of the scheme and the
examples that follow are:
Ac for acetyl;
AIBN for azobisisobutyronitrile;
Bu3SnH for tributyltin hydride;
CDI for carbonyldiimidazole;
dba for dibenzylidene acetone;
dppb for diphenylphosphino butane;
DBU for 1,8-diazabicyclo[5.4.0]undec-7-ene;
DEAD for diethylazodicarboxylate;
43
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
DMAP for dimethylaminopyridine;
DMF for dimethyl formamide;
DPPA for diphenylphosphoryl azide;
EtOAc for ethyl acetate;
MeOH for methanol;
NaN(TMS)2 for sodium bis(trimethylsilyl)amide;
NMMO for N-methylmorpholine N-oxide;
TEA for triethylamine;
THF for tetrahydrofuran;
TPP or PPh3 for triphenylphosphine;
MOM for methoxymethyl;
Boc for t - butoxycarbonyl;
Bz for benzyl;
Ph for phenyl;
POPd for dihydrogen dichlorobis(di-tert-butylphosphinito-xP)palladate(II);
TBS for tert-butyl dimethylsilyl; or
TMS for trimethylsilyl.
Synthetic Methods
The compounds and processes of the present invention will be better understood
in
connection with the following synthetic schemes that illustrate the methods by
which the
compounds of the invention may be prepared.
A preferred intermediate for the preparation of compounds represented by
formula I is
a compound represented by the formula
R6'~,0
R2' NMe2
N~
HO~,,,,,.. =.. Hd ..,,,180
HO
\,,.
O a,,,, O
0 =-,,~0 a
(XI)
44
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
where R6, R2' and R4" are as previously defined.
A second preferred intermediate for the preparation of compounds represented
by
formula I is a compound represented by the formula
R6,, 0
R2' NMe2
N-- 0'~..
HOm... HO.~' ,nO
HO
0 OH
0
(XII)
where R6 and R2' are as previously defined.
Scheme 1
R6'-0
OH = ~~ R2' NMe2
N OHHO, N CZ
OH "
HO O HO O
Ery A
a,,,,. 0 0 a,,,. O
O
O :,:0H O "O R4õ
O Q
(1-2)
R6\O =
( = R2' NMe2
N 0
(1_3) oo
HO
0 a-. 0
0 ,.O_R4õ
O
(1-4) 1
A process of the invention, as illustrated in Scheme 1, involves preparing a
compound
of formula I(1-4) by reacting a compound of formula (1-2) with a suitable
alkylating agent.
In accordance with Scheme 1, the 9-keto group of erythromycins can be
initially
converted into an oxime by methods described in U.S. Patent 4,990,602,
followed by the
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
protection of the 2'- and 4"-hydroxyl and, if desired, the oxime groups of the
erythromycin
derivatives to obtain the compounds of formula (1-2).
The preparation of protected erythromycins is also described in U.S. Patents
4,990,602; 4,331,803; 4,680,386; 4,670,549; or European Patent Application EP
260,938.
The 2'- and 4"-hydroxyl groups are protected by reaction with suitable
hydroxyl
protecting agents in an aprotic solvent. Typical hydroxyl protecting reagents
include, but are
not limited to, acetylating agents, silylating agents, acid anhydrides, and
the like. Examples
of hydroxyl protecting reagents include, but ar not limited to, acetyl
chloride, acetic
anhydride, benzoyl chloride, benzoic anhydride, benzyl chloroformate,
hexamethyldisilazane,
and trialkylsilyl chlorides.
Examples of aprotic solvents include, but are not limited to, dichloromethane,
chloroform, tetrahydrofuran, N-methylpyrrolidinone, dimethylsulfoxide, N,N-
dimethylformamide, N,N -dimethylacetamide, hexamethylphosphoric triamide, a
mixture
thereof or a mixture of one of these solvents with ether, tetrahydrofuran, 1,2-
dimethoxyethane, 1,2-dichloroethane, acetonitrile, ethyl acetate, acetone and
the like. Aprotic
solvents do not adversely affect the reaction. Preferably, the solvent is
selected from
dichloromethane, chloroform, N,N -dimethylformamide, tetrahydrofuran, N-
methylpyrrolidinone or mixtures thereof. A more thorough discussion of
solvents and
conditions for protecting the hydroxyl group can be found in T.W. Greene and
P.G.M. Wuts,
"Protective Groups in Organic Synthesis" 3'd ed., John Wiley & Son, Inc, 1999.
Protection of 2'- and 4"-hydroxyl groups may be accomplished sequentially or
simultaneously to provide compound (1-2) where R2' and/or R4" can be, for
example, acetyl,
benzoyl, trimethylsilyl, and the like. Preferred protecting groups include
acetyl, benzoyl, and
trimethylsilyl. A particularly preferred group for protecting the hydroxyl and
oxime groups is
the acetyl protecting group, wherein R2' = R4" = R6 = Ac.
Acetylation of the hydroxyl group is typically accomplished by treating the
compound
(1-1) with an acetylating reagent, for example, acetic anhydride or acetyl
chloride.
The erythromycin derivative of formula (1-1) is then reacted with an
alkylating agent
of the formula:
R12-OC(O)O-CH2[C=CHR11]CH2-OC(O)-OR12 (1-3)
where R12 is Cl -C12-alkyl and R1I is as previously defined.
Most palladium (0) catalysts are expected to work in this process. Some
palladium (II)
catalysts, such as palladium (II) acetate, which is converted into a palladium
(0) species in-
situ by the actions of a phosphine, will work as well. See, for example,
Beller et al. Angew.
46
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
Chem. Int. Ed. Engl., 1995, 34 (17), 1848. The palladium catalyst can be
selected from, but
not limited to, the group consisting of palladium (II) acetate,
tetrakis(triphenylphospine)palladium (0),
tris(dibenzylideneacetone)dipalladium,
tetradibenzylideneacetone)dipalladium and the like. Palladium on carbon and
palladium (II)
halide catalysts are less preferred than other palladium catalysts for this
process.
Suitable phosphines include, but are not limited to, triphenylphosphine,
bis(diphenylphosphino)methane, bis(diphenylphosphino)ethane,
bis(diphenylphosphino)propane, 1,4-bis(diphenylphosphino)butane,
bis(diphenylphosphino)pentane, and tri-o-tolyl-phosphine, and the like.
The reaction is carried out in an aprotic solvent, preferably at elevated
temperature,
preferably at or above 50 C. Suitable aprotic solvents include, but are not
limited to,
tetrahydrofuran, N, N-dimethylformamide, dimethyl sulfoxide, N-methyl-2-
pyrrolidone,
hexamethylphosphoric triamide, 1,2-dimethoxyethane, methyl-tert-butyl ether,
heptane,
acetonitrile, isopropyl acetate and ethyl acetate. The most preferred solvents
are
tetrahydrofuran or toluene.
Generally, the alkylating agents have the formula (1-3), previously described.
The
preferred alkylating agents are those wherein R12 is tert-butyl, isopropyl or
isobutyl. The
alkylating reagents are prepared by reaction of a diol with a wide variety of
compounds for
incorporating the di-carbonate moiety. The compounds include, but are not
limited to, tert-
butyl chloroformate, di-tert-butyl dicarbonate, and 1-(tert-
butoxycarbonyl)imidazole and the
reaction is carried out in the presence of an organic or an inorganic base.
The temperature of
the reaction varies from about -30 C to about 30 C. Preferably the
alkylating reagent is di-
tert-butyl dicarbonate.
An alternative method of converting the alcohol into the carbonate involves
treating
the alcohol with phosgene or triphosgene to prepare the chloroformate
derivative of the diol.
The di-chloroformate derivative is then converted into the di-carbonate by the
methods
described in Cotarca, L., Delogu, P., Nardelli, A., Sunijic, V, Synthesis,
1996, 553. The
reaction can be carried out in a variety of organic solvents such as
dichloromethane, toluene,
diethyl ether, ethyl acetate and chloroform in the presence of a base.
Examples of suitable
bases include, but are not limited to, sodium hydroxide, potassium hydroxide,
ammonium
hydroxide, sodium carbonate, potassium carbonate, ammonium carbonate,(4-
dimethylamino)pyridine, pyridine, triethylamine and the like. The temperature
can vary from
0 C to about 60 C. The reaction typically takes about 3 to 5 hours to run to
completion.
47
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
Scheme 2
R6,,
R6\ R2 NMe o Rz' NMe2
N\ z N~
,,,,,p alcohol O
p~~ p HO
HO 1 M HC
0 p
(1-4) (2-1)
Another process of the invention involves the removal of the cladinose moiety
of the
compounds of formula I. The cladinose moiety of the macrolide compound (1-4)
is removed
either by mild acid hydrolysis or by enzymatic hydrolysis to give compounds of
formula (2-
1) in Scheme 2. Representative acids include dilute hydrochloric acid,
sulfuric acid,
perchloric acid, chloroacetic acid, dichloroacetic acid or trifluoroacetic
acid. Suitable solvents
for the reaction include methanol, ethanol, isopropanol, butanol, water and
mixtures there of.
Reaction times are typically 0.5 to 24 hours. The reaction temperature is
preferably 0 to 80
OC.
Scheme 3
R6,, ~
Y Rz NMez R '
Q HN 2 NMeI
noii p~O ....nq p z
HO
Q nnu~~ _=,,yl p O
HO
0
o ..='
~~p-R4" O ""O-R4õ
,I
(1~,) (3-1)
Compounds of formula (1-4) where R6 is Ac can be converted into the
corresponding
imine as outlined in Scheme 3. Selective deprotection of the oxime is
typically accomplished
via alkaline hydrolysis in protic solvents. Representative alkali include
lithium hydroxide,
sodium hydroxide, potassium hydroxide, and the like. Solvents which are
applicable include,
but are not limited to, tetrahydrofuran, 1,4-dioxane, 1,2-dimethoxyethane,
isopropanol,
48
CA 02483875 2007-07-24
ethanol, butanol , water and mixtures thereof. The reaction temperature is
preferably 0 to 35
C and reaction time is preferably 0.5 to 24 hours.
In a like fashion, simultaneous deprotection of both the oxime and the 2'
hydroxyl can
be accomplished under a variey of conditions. Conditions for deprotection
include, but are
not limited to, treating with an alcoholic solvent at from room temperature to
reflux, or
treatment with a primary amine, such as butylamine. Alcoholic solvents
preferred for the
deprotection are methanol and ethanol. A more thorough discussion of the
procedures,
reagents and conditions for removing protecting groups is described in the
literature, for
example, by T.W. Greene and P.G.M. Wuts in "Protective Groups in Organic
Synthesis" 3rd
ed., John Wiley & Son, Inc, 1999.
Deoxygenation of compounds of formula (1-4) where R6 is H under reducing
conditions gives the macrolide imine of formula (3-1). Many reducing agents
can be used to
effect this transformation including, but not limited to: lithium aluminum
hydride, titanium
thrichloride, sodium cyanoborohydride, borane, and various sulfides such as
sodium
hydrogen sulfide, sodium ethoxide. For a more detailed account of oxime
reduction see J.
March in "Advanced Organic Chemistry" 4ded., Wiley & Son, Inc, 1992.
A particularly useful method for the reduction of oximes to the corresponding
imine
uses a sulfite reducing agent, such as sodium hydrogensulfite or titanium
trichloride under
acidic conditions, typically in protic solvents. Representative acids include,
but are not
limited to, acetic acid, formic acid, dilute hydrochloric acid, dilute
phosphoric acid, dilute
sulfuric acid, and the like. Protic solvents include, but are not limited to,
mixtures of water
and methanol, ethanol, isopropanol, or butanol. The reaction is typically
carried out at 25 to
110 C, preferably for between 1 and 10 hours.
49
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
Scheme 4
O R,
= RZ NMe2 R2' NMe2
HN IN
HO ~ H p
O
p 0~,,,,=. O p O
0 ~ ~''O R4õ 0 ~
(3-3) ~ (4-1) I
0 R2
R2 NMe2 Rz' NMe2
HN IN
HO HO p
.' ,
p p p 0
O 0 ~p
(3-3) ~ (4-2)
Another process of the invention, as illustrated in Scheme 4, involves a
procedure for
the acylation of imines of the formula (3-3). The imine is acylated under
basic conditions
using a suitable acylating agent in an aprotic solvent. Typical acylating
agents include, but
are not limited to, acid chlorides, acid anhydrides, and chloroformates.
Typical bases include, but are not limited to, pyridine, triethylamine,
diisopropyl
ethylamine, N-methyl morpholine, N-methyl pyrrolidine, 2,6-lutidine, 1,8-
diazabicyclo[5.4.0]undec-7-ene. For a more extensive discourse on acylating
conditions see
for example, T.W. Greene and P.G.M. Wuts in "Protective Groups in Organic
Synthesis" 3'a
ed., John Wiley & Son, Inc, 1999, referred to above herein.
Conversion of alkene (1-4) into ketone (5-1) can be accomplished by exposure
of the
alkene to ozone followed by decomposition of the ozonide with the appropriate
reducing
agent, as outlined in Scheme 5. The reaction is typically carried out in a
solvent such as, but
not limited to methanol, ethanol, ethyl acetate, glacial acetic acid,
chloroform, methylene
chloride or hexanes, preferably methanol, preferably at -20 to -78 C.
Representative
reducing agents are, for example, triphenylphosphine, trimethyl phosphite,
thiourea, and
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
dimethyl sulfide, preferably triphenylphosphine. A more thorough discussion of
ozonolysis
and the conditions there for can be found in J. March "Advanced Organic
Chemistry" 4th ed.,
Wiley & Son, Inc, 1992.
Scheme 5
R6~0 =
Rz NMe2 R6~0 =
R
N 2 NMe2
N~
~~ O
HO 03, HO 0 ...,,,,0
3'
O 11-10
,,. O
O
,O 0 I 'O
(1-4)
~
'5-1)
R6'-0
ysO4 =
N\ R2 NMe2 NaIO4
O
O
HO
OH
O O
0 ,O
'-2)
An alternative method for the preparation of ketone (5-1) involves
dihydroxylation of
the alkene followed by diol cleavage. The glycol (5-2) is first prepared by
reacting the alkene
(1-4) with osmium tetroxide. This reaction can be carried out either with
stochiometric
amounts of osmium tetraoxide, or with catalytic amounts of osmium tetraoxide,
if an oxidant
such as hydrogen peroxide, tert-butyl hydroperoxide, or N-methylmorpholine-N-
oxide is
present. These reactions can be run in a variety of solvents including: 1,4-
dioxane,
0
tetrahydrofuran, tert-butanol and diethyl ether, preferably at 0 to 50 C.
The glycol can be cleaved by a variety of reagents including, but not limited
to,
periodic acid, lead tetraacetate, manganese dioxide, potassium permanganate,
sodium
metaperiodate, and N-iodosuccinamide. Depending on the cleavage reagent, a
variety of
solvents can be used. Preferably the cleavage reagent is sodium metaperiodate,
the solvent is
51
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
preferably a mixture of ethanol, methanol or 1,4-dioxane and water and the
reaction
temperature is 0 C to 25 C.
Scheme 6
~~ R6-,0 R6
I = p Rj 2 NMe2
0 R2NMe2
N~ N~
O N
,,w0 O,,,,,0 O R 0
0
+ ~6 HO
HO H2N'
O 0,,,,,, O O O'~== O
0 0
O "O R4.,
,O
(5-1) (6-1) I
Compounds of formula (5-1) represent useful intermediates which can be further
functionalized in a variety of ways. Scheme 6 details a procedure for the
conversion of the
ketone (5-1) into an oxime of formula (6-1). Oxime formation can be
accomplished under
either acidic or basic conditions in a variety of solvents. Representative
acids include, but are
not limited to, hydrochloric, phosphoric, sulfuric, para-toluenesulfonic, and
pyridinium p-
toluene sulfonate. Likewise bases which are useful are, for example,
triethylamine, pyridine,
diisopropylethyl amine, 1,5-lutidine, imidazole, and the like. Appropriate
solvents include,
but are not limited to, methanol, ethanol, water, tetrahydrofuran, 1,2-
dimethoxyethane, and
ethyl acetate. Preferably the reaction is run in ethanol using triethylamine
as the base. The
reaction temperature is generally 25 C to 50 C and reaction time is 1 to 12
hours.
25
52
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
Scheme 7
R~I
P-6-p R SORp
N~ z' ~e2 RI 1 6? _
' Rz NMe2
=.
OH (7-1) N
p ak
Rp0 OBoc HO//,,,,
HO p p
Pd2(dba)3, dppb HO
p TIIF, reflux
0 O//,,,.. O
="/p_R4" p
(1 2) (7-2) p R4
Rl l R1 t
ORp ORp
OH = R2 NMe2 = RR 2 NMez
H
HO õ p O =,,,,,p p
HO
.~'' \'',=,
p p
O " 'O R4 p
~-P
14
7
~ 3) RIi (?-4) I
OBoc
R2 NMe2 R~ 1 R2' NMez
O py' p p'q
--õ~~p - ,,,,, = I ~ =., O
HO Pdz(dba)3, dppb HO 'p
TI4F, reflux
p ., O p
p p ~
O ="",0 R4., 0 _
(? 5) (7-6) 1
Scheme 7 details a procedure for the stepwise formation of the 6-11 bridged
macrolide of formula (7-6). In a similar manner as before, the procedure
involves reacting a
compound of formula (1-2) with a suitable alkylating agent. As before, the
erythromycin
derivative of formula (1-2) is reacted with an alkylating agent of the
formula:
R12-OC(O)O-CH2[C=CHR11]CH2-O-Rp (7-1)
where R12 is C1-C12-alkyl and Rp and R1, are as previously defined.
Most palladium (0) catalysts are expected to work in this process. Some
palladium (II)
catalysts, such as palladium (II) acetate, which is converted into a palladium
(0) species in-
53
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
situ by the actions of a phosphine, will work as well. See, for example,
Beller et al. Angew.
Chem. Int. Ed. Engl., 1995, 34 (17), 1848. The palladium catalyst can be
selected from, but
not limited to, the group consisting of palladium (lI) acetate,
tetrakis(triphenylphospine)palladium (0),
tris(dibenzylideneacetone)dipalladium,
tetradibenzylideneacetone)dipalladium and the like. Palladium on carbon and
palladium (II)
halide catalysts are less preferred than other palladium catalysts for this
process.
Suitable phosphines include, but are not limited to, triphenylphosphine,
bis(diphenylphosphino)methane, 1,2-bis(diphenylphosphino)ethane, 1,3-
bis(diphenylphosphino)propane, 1,4-bis(diphenylphosphino)butane, 1,5-
bis(diphenylphosphino)pentane, and tri(o-tolyl)phosphine, and the like.
The reaction is carried out in an aprotic solvent, preferably at elevated
temperature,
preferably at or above 50 C. The aprotic solvents include, but are not limited
to,
tetrahydrofuran, N,N-dimethylformamide, dimethyl sulfoxide, N-methyl-2-
pyrrolidone,
hexamethylphosphoric triamide, 1,2-dimethoxyethane, methyl-tert-butyl ether,
heptane,
acetonitrile, isopropyl acetate and ethyl acetate. The most preferred solvents
are
tetrahydrofuran and toluene.
The alkylating agents useful in the process of the invention are the mixed
silyl ether
carbonates (7-1). Generally, the alkylating agents have the formula (7-1),
previously
described. The preferred alkylating agents are those wherein R12 is tert-
butyl, isopropyl or
isobutyl and Rp is tert-butyl dimethyl silyl, triisopropyl silyl, tert-butyl
diphenyl silyl or the
like.
The alkylating reagents of formula (7-1) are prepared by reaction of a diol
sequentially with a wide variety of compounds for incorporating the carbonate
moiety,
followed by a wide variety of compounds for incorporating the silyl moiety.
Alkylating
reagents include, but are not limited to, tert-butyl chloroformate, di-tert-
butyl dicarbonate,
and 1-(tert-butoxycarbonyl)imidazole; whereas silylating reagents include, but
are not limited
to, tert-butyl dimethyl silyl chloride, tert-butyl dimethyl silyl triflate,
tert-butyl dimethyl silyl
cyanide, and tert-butyl dimethyl silyl imidazole. Both reactions are carried
out in the
presence of an organic or an inorganic base. The temperature of the reactions
varies from
about -30 C to about 30 C. Preferably, the alkylating reagent is di-tert-butyl
dicarbonate and
the silylating reagent is tert-butyl dimethyl silyl chloride.
The free oxime (7-3) is prepared using essentially the same procedure as for
the
deprotection of oxime (1-4) where R6 is Ac.
54
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
Compounds of formula (7-4) can be formed directly from compounds of formula (7-
3) by the application of the previously described procedure for the reduction
of oximes of
formula (1-4), where R6 is hydrogen, to the corresponding.imine of formula (3-
1) in Scheme
3.
The protecting group (Rp) is then removed from the hydroxyl of the compound of
formula (7-4) using the appropriate conditions as outlined in T.W. Greene and
P.G.M. Wuts
in "Protective Groups in Organic Synthesis" 3'd ed., John Wiley & Son, Inc,
1999. For
example, when the protecting group is TBS, tetra-n-butyl ammonium fluoride,
hydrofluoric
acid or trifluoroacetic acid may be used. Using standard conditions, the
primary hydroxyl is
converted to the tert-butyl carbonate, and subsequently the 1 1-hydroxyl group
is alkylated by
means of a palladium (0) catalyst as described previously. In this way
compounds of formula
(7-5) can be prepared readily.
Scheme 8
R2' NMe2 v = 1 R2 NMe2
v o,
,
,,,,,,
o~o =,,,,,o
0~o O o
HO HO
.\ .
.''O OH 0
-1 Y--Ar
0 0 0
V: 0 or N-OR6 or NC(O)F
(8-2) L-3) or NC(O)R2
Y: 0 or (CH2)n or N, or S
n : 0-4
Scheme 8 illustrates a procedure for the acylation of the C-3 hydroxyl of
compounds
of formula (8-2). The hydroxyl group is acylated under basic conditions using
a suitable
acylating agent in an aprotic solvent. Typical acylating agents include, but
are not limited to,
acid chlorides, acid anhydrides, and chloroformates.
Typical bases include, but are not limited to, pyridine, triethylamine,
diisopropyl
ethylamine, N-methyl morpholine, N-methyl pyrrolidine, 2,6-lutidine, 1,8-
diazabicyclo[5.4.0]undec-7-ene. For a more extensive discourse on acylating
conditions see
for example, T.W. Greene and P.G.M. Wuts in "Protective Groups in Organic
Synthesis" 3a
ed., John Wiley & Son, Inc, 1999.
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
Scheme 9
R2' NMe2
U p= R2 NMe2
V
o~o p
HO p~ p O
HO
O O pH
p Oy U
O S
(8-2)
(9-1)
= R2' NMe2
V
0
~0
HO V: 0 or N-OR6 or NC(O)R1
or NC(O)R2
O
U: SR or OR
0
(9-2)
Another process of the invention, as illustrated in Scheme 9, involves the C-3
deoxygenation of the macrolide (8-2) which can be accomplished via the two
step procedure
shown therein. In the first step the xanthate or thiocarbonate is formed by
the reaction of
alkoxide of alcohol (8-2) with the appropriate thiocarbonyl. For instance,
formation of the
xanthate can be accomplished by reaction of the alkoxide with either
carbondisulfide
followed by methyliodide, or a dithiocarbonyl imidazole; whereas the
thiocarbonate can be
prepared by the reaction of the alkoxide with either thiocarbonyldimidazole
followed by
methanol, ethanol or the like, or a thiochloroformate. One skilled in the art
will appreciate
that other reagents and conditions exist to perform these transformations and
that the
examples above are for illustrative purposes only and do not limit the scope
of this invention.
These reactions are typically run in a polar aprotic solvent, preferably THF,
acetonitrile, or
DMF.
In the second step of Scheme 9, the thiocarbonate or xanthate is decomposed to
give
the alkane. Most typically this is done under radical conditions using, for
example, a silyl
hydride such as SiH(TMS)3, SiH2Ph2 or the like, a tin hydride such as Bu3SnH,
Ph3SnH or
the like, and a radical initiator such as AIBN or t-butyl peroxide. The
preferred solvent is
toluene.
56
CA 02483875 2007-07-24
Scheme 10
Q RZ, \ N/
,=
N, Q R2' W
.,,. A
' ~.
.,0~~ =.,O O = p
HO
'.= ~'00 '0 O
B HO
O Z --~ ,=
~ ' B
O Z
O
O
iai i0-2
It will be appreciated that compounds of the present invention include
modification of
the 3'-N of compounds of the formula (10-1). Compounds of formula (10-2) can
be made via
the methods delineated in U.S. Patents 6,034,069 and 6,387,885.
Examples
The compounds and processes of the present invention will be better understood
in
connection with the following examples, which are intended as an illustration
only and not
limiting of the scope of the invention. Various changes and modifications to
the disclosed
embodiments will be apparent tothose skilled in the art and such changes and
modifications
including, without limitation, those relating to the chemical structures,
substituents,
derivatives, formulations and/or methods of the invention may be made without
departing
from the spirit of the invention and the scope of the appended claims.
ExamQle 1
Compound of formula IV: A and B taken together with the carbon atom to which
they are
attached = C=CH2. Q = OH. RZis H. and Rg" = Ac.
Step la: Compound of formula 1-2: = Ac. Rg' = Ac and RZ Ac:
Acetic anhydride (35.9 ml, 0.38 mol), triethylamine (55.7 ml, 0.4 mol) and
DMAP
(3.7 g, 0.03 mol) were added to a solution of the compound of formula (1-2)
where R6 = R2'
= R4" = H (74.9 g, 0.1 mol) in 400 ml of TIF at room temperature and the
resulting mixture
57
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
was stirred at room temperature for about 16 hours. The reaction mixture was
concentrated to
about 200 ml under reduced pressure, diluted with 300 ml of ethyl acetate,
washed with
saturated NaHCO3 (4X500 ml) and brine (500 ml). The organic phase was dried
over sodium
sulfate and the solvent was removed in vacuo. The solid residue was
recrystallized from ethyl
acetate to give the title compound (78g).
MS (ESI) m/z: 875.46 (M+H)+.
13C-NMR(100 MHz, CDC13): S 178.5, 175.4, 170.6, 170.2, 168.2,100.2, 96.1,
83.3, 79.3,
78.7, 75.2, 74.5, 72.9, 70.0, 67.6, 63.4, 63.2, 60.6, 49.5, 44.7, 40.9, 35.4,
31.8, 28.5, 22.8,
21.7,21.6, 21.5, 21.3, 21.2, 21.1, 19.9, 18.6, 18.4, 16.7, 14.9, 14.4, 14.3,
10.8, 9.2
Step lb: Compound of formula 1-4: R6 = Ac, R2' = Ac and R4" = Ac.
A mixture of the compound from step la (21.9 g, 25 mmol), 2-methylene-1,3-
propane-[bis-(tert-butyl)carbonate] (18.02 g, 62.25 mmol) and 1,4-
bis(diphenylphosphino)-
butane (640 mg, 1.5 mmol) was dissolved in freshly distilled THF (250 ml). To
the solution
was added Pd2(dba)3 (687 mg, 0.75 mmol). The reaction mixture was heated to
reflux slowly.
After refluxing for 14 hours, the reaction was cooled to room temperaure,
diluted with 400 ml
ethyl acetate, and washed with saturated NaHCO3 (400 ml) and brine (400 ml).
The organic
phase was dried over Na2SO4, the solvent was removed in vacuo and the solid
residue was
purified by silica gel chromatography (acetone : hexane/1:2) to give the title
compound (22g).
MS (ESI) m/z: 927.64 (M+H)+.
13C-NMR(100 MHz, CDC13): S 176.5, 175.9, 170.7, 170.1, 169.9, 141.6, 124.7,
100.4, 96.0,
79.1, 78.7, 78.2, 78.0, 77.4, 76.5, 73.5, 73.0, 72.4, 72.1, 67.8, 66.1, 63.4,
63.3, 49.6, 44.1,
41.2, 40.9, 37.3, 35.4, 35.1, 31.3, 29.5, 28.5, 27.1, 23.4, 21.7, 21.3, 21.1,
20.9, 20.3, 18.8,
18.3, 17.4, 15.7, 13.4, 12.7, 8.6.
Step lc: Compound of formula IV: A and B taken together with the carbon atom
to
which they are attached = C=CH2, O= OH, RZ' = H, and R4" = Ac.
A solution of the compound from step lb (22g) in 400 ml methanol was refluxed
for
48 hours. The solvent was removed in vacuo and the compound was purified by
column
chromatography (CH2C12: 2M ammonia in MeOH195:5) to give the title compound
(18.5 g).
MS (ESI) m/z: 843.67 (M+H)+.
13C-NMR(100 MHz, CDC13): S 176.2, 170.8, 168.8, 142.0, 124.2, 102.5, 95.9,
79.4, 78.7,
78.1, 78.0, 76.6, 73.0, 71.8, 71.1, 68.2, 65.6, 63.2, 49.7, 44.2, 41.7, 40.5,
37.7, 35.0, 34.4,
29.3, 25.8, 23.5, 21.9, 21.3, 21.1, 19.0, 18.1, 17.5, 15.3, 13.2, 12.7, 8.7.
58
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
Example 2
Compound of formula IV: A and B taken together with the carbon atom to which
are attached
= CCH2, Q= H, R2' = H, and R4" = Ac.
Formic acid (2.33 ml, 61.7 mmol), water (115 ml) and sodium thiosulfate (9.7g,
55.5
mol) were added sequentially into a solution of the title compound of example
1 (15.6 g, 18.5
mmol) in isopropanol (10 ml) at room temperature. The reaction mixture was
refluxed for 1.5
hour, cooled to room temperature, diluted with ethyl acetate (300 ml), and
washed with
saturated sodium bicarbonate (2x200 ml) and brine (200 ml). The organic phase
was dried
over sodium sulfate and the solvent was removed in vacuo. The residue was
purified by silica
gel chromatography to give the title compound (8.0g).
MS (ESI) m/z: 827.59 (M+H)+.
13C-NMR(100 MHz, CDC13): S 190.8, 176.6, 170.7, 142.5, 122.2, 102.4, 96.0,
95.9, 79.9,
78.9, 78.7, 77.9, 76.3, 72.9, 71.1, 68.2, 65.5, 63.2, 50.3, 49.7, 44.5, 41.5,
40.5, 37.5, 35.4,
35.0, 29.1, 23.2, 21.9, 21.4, 21.3, 21.1, 19.8, 18.1, 17.2, 14.6, 13.7, 12.4,
8.8.
Example 3
Compound of formula V: A and B taken together with the carbon atom to which
they are
attached = C=CH2, O= H, and R,' = H.
Method 1
Hydrochloric acid (0.5N, 60 ml) was added to a solution of the compound from
Example 2 (5.8 g, 7 mmol) in ethanol (30 ml) at room temperature. The mixture
was heated
to 65 C for 2 hours, cooled to room temperature and the pH was adjusted to
pH=10 by slow
addition of 3N aqueous sodium hydroxide. The aqueous solution was extracted
with ethyl
acetate (200m1) and the organic phase was washed once with saturated sodium
bicarbonate
(200 ml), dried over sodium sulfate and sovent was removed in vacuo. The
residue was
purified by silica gel chromatography (CH2C12: 2M ammonia in methanol/95:5) to
give the
title compound (2.8 g).
MS (ESI) m/z: 627.56(M+H)+.
13C-NMR(100 MHz, CDC13): S 188.5, 176.0, 143.9, 118.9, 106.9, 90.8, 79.8,
79.6, 79.2, 77.4,
75.9, 75.3, 70.8, 70.4, 65.8, 65.3, 44.6, 42.1, 40.4, 38.6, 36.4, 35.3, 28.2,
22.9, 21.5, 20.0,
19.7, 16.8, 15.1, 14.9, 11.5, 8.3.
Method 2
59
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
Titanium trichloride (40 ml, 20% in 3% hydrochloric acid) was added dropwise
during 10 minutes into a stirred solution of the compound from step lc (9.5 g,
11.3 mmol)
and ammonia acetate (17.4 g, 226 mmol) in 120 ml of methanol at 0 C. The
reaction mixture
was allowed to warm up to room temperature and stirred overnight. The pH of
the reaction
mixture was adjusted to pH=10 by slow addition of 3N aqueous sodium hydroxide.
The
aqueous solution was extracted with ethyl acetate (200 ml) and the organic
phase was washed
once with saturated sodium bicarbonate (200m1), dried over sodium sulfate and
solvent was
removed in vacuo. The residue was purified by silica gel chromatography
(CH2CI2: 2M
ammonia in methanol/95:5) to give the title compound (3.0 g).
MS (ESI) m/z: 627.56 (M+H)+.
Example 4
Compound of formula V: A and B taken together with the carbon atom to which
they_are
attached = C=CH2, Q= Ac, and R2' = H.
Step 4a: Compound of formula V: A and B taken together with the carbon atom to
which they are attached = C=CH2, Q= Ac, and R,' = Ac.
Acetic anhydride (1.36 ml, 14.4 mmol) was added to a solution of the title
compound
of Example 3(3 g, 4.8 mmol) and triethylamine (2.8 ml, 20 mmol) in
dichloromethane (40
ml). The reaction mixture was stirred at room temperature for 4 hours, diluted
with 100 m1 of
dichloromethane and washed with saturated sodium bicarbonate (3x100 ml) and
brine (100
ml). The organic phase was dried over sodium sulfate and the solvent was
removed in vacuo.
The residue was purified by silica gel chromatography (hexanes:acetone/1:1) to
give title
compound (2.9 g).
MS (ESI) m/z: 711.50 (M+H)+.
13C-NMR(100 MHz, CDC13): S 184.7, 176.9, 174.9, 170.1, 141.9, 122.2, 99.4,
81.2, 79.0,
77.8, 77.7, 76.1, 73.5, 71.7, 68.8, 65.7, 63.2, 43.7, 40.8, 39.9, 38.2, 36.2,
35.6, 31.0, 25.5,
23.2, 21.6, 21.2, 19.9, 19.5, 17.1, 15.8, 14.7, 11.8, 7.9.
Step 4b: Step 4a: Compound of formula V: A and B taken together with the
carbon
atom to which they are attached = C=CH2, Q= Ac, and R,' = H.
The title compound is prepared by refluxing the compound from step 4a in
methanol
according to the procedure described in Example 1(Step lc).
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
Example 5
Compound of formula IV: A and B taken together with the carbon atom to which
they are
attached = C=CH9, Q= O-CH2OCH3 Rz,' = H, and R4" = Ac.
Step 5a: Compound of formula IV:A and B taken together with the carbon atom to
which they are attached = C=CH2. 0 = OH, R,' = Ac, and R4" = Ac.
1M LiOH (10 ml)was added to a solution of the compound from step lb (1.85g, 2
mmol) in 10 ml THF and 10 ml isopropanol at room temperature. After stirring
at room
temperature for 30 minutes, the reaction mixture was diluted with saturated
sodium
bicarbonate (40 ml) and extracted with ethyl acetate (2x40 ml). The organic
phase was
washed with brine (2x 40 ml), dried over sodium sulfate and the solvent was
removed in
vacuo. The residue was purified by silica gel chromatography (hexanes:acetone,
1:1) to give
the title compound (1.65g).
MS (ESI) m/z: 885.45 (M+H)+.
13C-NMR(100 MHz, CDC13): 8 175.9, 170.7, 170.1, 168.7, 142.0, 142.2, 100.5,
95.9, 79.4,
78.6, 78.1, 77.9, 76.6, 73.0, 72.8, 72.0, 71.7, 67.7, 65.5, 63.5, 63.2, 49.6,
44.1, 41.4, 40.9,
37.4, 35.0, 34.4, 31.7, 31.3, 25.7, 23.5, 21.7, 21.3, 19.0, 18.1, 17.5, 15.3,
13.2, 12.7, 8.6
Step 5b: Compound of formula IV: A and B taken together with the carbon atom
to
which they are attached = C=CH9, O= O-CH2OCH3 Rz= Ac, and R4" = Ac.
Sodium hydride (33 mg, 1.3 mmol) was added into a solution of the title
compound of
example 5a (885 mg, 1 mmol) in 10 ml DMF at 0 C. MOM-Cl (90 l, 1.2 mmol) was
added
and stirred at 0 C overnight. The reaction mixture was diluted with ethyl
acetate (20 ml) and
quenched with saturated NaHCO3, washed with brine (20 ml) and dried over
anhydrous
sodium sulfate. The residue was purified by silica gel chromatography to give
the title
compound (0.4 g).
MS (ESI) m/z: 929.49 (M+H)+.
13C-NMR(100 MHz, CDC13): 8 175.9, 170.7, 170.1, 169.5, 142.1, 124.5, 100.5,
98.4, 96.0,
79.3, 78.7, 78.2, 78.1, 77.2, 76.5, 72.9, 72.0, 71.8, 67.7, 65.9, 63.4, 63.2,
56.6, 49.6, 44.1,
41.4, 40.9, 37.2, 35.0, 34.8, 34.6, 31.3, 28.0, 27.0, 25.4, 23.4, 21.7, 21.3,
21.1, 20.8, 19.0,
18.2, 17.4, 15.5, 13.3, 12.7, 8.6.
Step 5c. Compound of formula IV: A and B taken together with the carbon atom
to
which they are attached = C=CH2, O= O-CHZOCH3 R,' =H, and R4" = Ac.
The title compound was prepared by refluxing the compound from Step 5b in
methanol according to the procedure described in Example 1(Step lc).
61
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
MS (ESI) m/z: 887 (M+H)+.
Example 6
Compound of formula V: A and B taken together with the carbon atom to which
they are
attached = C=CH2, Q= O-CH2OCH3, and R2' = H.
Step 6a: Compound of formula v: A and B taken together with the carbon atom to
which they are attached = C=CH2,Q= OH, and RZ' = Ac.
To a solution of the title comound of example 1 (4.2 g, 4.5 mmol) in 50 ml
methanol
was added 2M HCl (10 ml). The reaction mixture was refluxed for 1.5 hours and
then
condensed to 30 ml. Saturated sodium bicarbonate (30 ml) was added and the
mixture was
extracted with ethyl acetate (50 ml). The organic phase was dried over sodium
sulfate and
solvent was removed in vacuo. The residue was purified by silica gel
chromatography
(hexane:acetone/1:1) to give the title compound (2.5g).
MS (ESI) m/z: 685.45 (M+H)+.
13C-NMR(100 MHz, CDC13): S 175.2, 170.2, 166.3, 143.6, 119.3, 99.6, 82.2,
79.5, 78.1,
77.5, 76.0, 73.7, 71.7, 68.9, 65.5, 63.3, 43.8, 40.8, 37.4, 35.9, 34.3, 31.1,
25.6, 23.3, 21.7,
21.3, 19.9, 19.6, 17.1, 15.7, 14.7, 11.9, 7.9
Step 6b: Compound of formula I: A and B taken together with the carbon atom to
which they are attached = C=CH2, Q= O-CH2CH3, and Rz' = Ac.
To a solution of the compound from step 6a (6.85g, 10 mmol) in 40 ml DMF was
added NaH (303 mg, 1.3 mmol) at 0 C portion wise. After 10 minutes, MOM-Cl
(900 l,
1.15 mmol) was added at 0 C during 15 minutes. The reaction mixture was
stirred at 0 C for
16 hours, diluted with ethyl acetate (100 ml) and quenched with saturated
sodium bicarbonate
(60 ml). The organic layer was separated, washed with brine (60 ml) and dried
over sodium
sulfate. The solvent was removed on vacuo and the residue was purified by
silica gel
chromatography (hexane:acetone/1:1) to give the title compound (4.5 g).
MS (ESI) m/z: 729 (M+H)+.
Step 6c. Compound of formula I: A and B taken together with the carbon atom to
which they are attached = C=CH,, O= O-CH2OCH3, and R2' = H.
The title compound of Example 6 was prepared by refluxing the compound from
Step
6b in methanol according to the procedure described in Example 1 (Step lc).
MS (ESI) m/z: 687 (M+H)+.
62
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
Example 7
Compound of formula I: A and B taken together with the carbon atom to which
they are
attached = C=CH?, X and Y taken together with the carbon atom to which they
are attached =
C=NAc, L = CH2CH3, W is N(CH32, Z= H and R_~' = H
Step 7a: Compound of formula I: A and B taken together with the carbon atom to
which they are attached = C=CH2, X and Y taken together with the carbon atom
to
which they are attached = C=NAc, L = CH2CH3, W is N(CHZ = H and RZ' = Ac.
The title compound is prepared from the compound of Step 4a , sodium hydride,
carbon disulfide and tri-n-butyl tin hydride according to the procedures
described in the
literature. For further details, see Elliott, Richard L.; Pireh, Daisy;
Griesgraber, George;
Nilius, Angela M.; Ewing, Patty J.; Bui, Mai Ha; Raney, Patti M.; Flamm,
Robert K.; Kim,
Ki; Henry, Rodger F.; Chu, Daniel T. W.; Plattner, Jacob J.; Or, Yat Sun. J.
Med. Chem.
(1998), 41(10), 1651-1659.
Step 7b: Compound of formula I: A and B taken together with the carbon atom to
which they are attached = C=CH2, X and Y taken together with the carbon atom
to
which they are attached = C=NAc, L = CH,CH3, W is N(CH7, Z = H and R2' = H.
The title compound is prepared by refluxing the compound from step 7a in
methanol
according to the procedure described in Example 1 (Step lc).
Example 8
Compound of formula I: A and B taken together with the carbon atom to which
they are
attached = C=CH2, X and Y taken together with the carbon atom to whichthey are
attached =
C=NAc, L = CHZCH3, Z = OC(O)(p-nitrophenyl) and R2' = H.
Step 8a: Compound of formula I: A and B taken together with the carbon atom to
which they are attached = C=CH2, X and Y taken toizether with the carbon atom
to
which they are attached = C=NAc, L = CH?CH3, W is N(CH7, Z = OC(O)(p-
nitrophenyl) and R,' = Ac.
The title compound is prepared from the compound of Step 4a of Example 4,
sodium
hydride, and para-nitrobenzoyl fluoride according to the procedures described
in the
literature. For further details, see Misawa et al, 6-O-
Desosaminylerythronolide derivatives,
U.S. Patent No. 5,602,239.
Step 8b. Compound of formula I: A and B taken together with the carbon atom to
which they are attached = C=CH2, X and Y taken together with the carbon atom
to
63
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
which they are attached = C=NAc, L = CH2CH3, W is N(CHZ = OC(O)(n-
nitrophenyl) and RZ' = H.
The title compound is prepared by refluxing the compound from step 8a in
methanol
according to the procedure described in Example 1 (Step lc).
Example 9
Compound of formula I: A and B taken together with the carbon atom to which
they are
attached = C=CH2, X and Y taken together with the carbon atom to which they
are attached =
C=NAc, L= CH2CH3, W is N(CH3),, Z = OC(O)f2-(NO2), 4-(CF3)Phenyll and R2' = H.
The title compound is prepared from the compound of Step 4a of Example 4, 2-
nitro-
4-trifluoromethylbenzoyl fluoride and sodium hydride followed by reaction in
methanol
according to the procedures described in Example 8.
Example 10
Compound of formula I: A and B taken together with the carbon atom to which
they are
attached = C=CH2, X and Y taken together with the carbon atom to which they
are attached =
C=NAc, L= CH2CH3, W is N(CH2, Z = OC(O)CH,(p-methoxyphen,yl) and R2' = H.
Step 10a: Compound of formula I: A and B taken together with the carbon atom
to
which they are attached = C=CH2, X and Y taken together with the carbon atom
to
which they are attached = C=NAc, L = CH2CH3, W is N(CH3),, Z = OC(O)CHApz
methoxyphenyl) and R?' = Ac.
The title compound is prepared from the compound of Step 4a of Example 4, p-
methoxyphenyl acetic acid, pivaloyl chloride, and triethylamine in a solution
of pyridine and
dichloromethane according to the procedure described in the literature. For
further details, see
Morimoto et al, 5-O-Desosaminylerythronolide derivatives, EP 0619320, and WO
99/21868.
Step lOb: Compound of formula I: A and B taken together with the carbon atom
to
which they attached = C=CH2, X and Y taken together together with the carbon
atom
to which they are attached = C=NAc, L = CH2C3, W is N(CH2, Z = OC(O)CH2-(P-
methoxyphenyl) and RZ' = H.
The title compound is prepared by refluxing the compound from Step l0a in
methanol according to the procedure described in Example 1 (Step lc).
64
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
Example 11
Compound of formula IV: A and B taken together with the carbon atom to which
they are
attached = C=CH2, Q = Ac, Rz' = H, and R4" = Ac.
Step 11a. Compound of formula IV: A and B taken together with the carbon atom
to
which they are attached = C=CH2, Q= Ac, RZ' = Ac, and R4" = Ac.
The title compound was prepared from the title compound of Example 6, acetic
anhydride, triethylamine and di methylami nopyri dine according to the
procedures described in
Example 4 (Step 4a).
MS (ESI) m/z: 911 (M+H)+.
Step 1 lb. Compound of formula IV: A and B taken together with the carbon atom
to
which they are attached = C=CH2, Q= Ac, R2' = H, and R4" = Ac.
The title compound of Example 11 is prepared by refluxing the compound from
Step
l la in methanol according to the procedure described in Example 1 (Step lc).
Example 12
Compound of formula I: Compound of formula IV: A and B taken together with the
carbon
atom to which they are attached = C=O, O= Ac, R2' = H, and R4" = Ac.
Ozone is bubbled into a solution of the title compound of Example 11 in
methanol
and dichloromethane at -78 C until the solution turns light blue. Excess
ozone is removed by
bubbling with nitrogen. Triphenylphosphine is added and the solution is
allowed to warm up
to room temperature. The solvent is removed in vacuo and the solid residue is
re-dissolved in
tetrahydrofuran. The resulting solution is refluxed overnight. The title
compound of Example
12 is purified by silica gel chromatography.
Example 13
Compound of formula IV: A and B taken together with the carbon atom to which
they are
attached = C=OBz, O= Ac, R2' = H, and R4" = Ac.
O-benzyl hydroxylamine and the title compound of Example 12 are dissolved in
ethanol. The reaction mixture is stirred at room temperature for 1 hour. The
solvent is
removed and the title compound is purified by silica gel chromatography.
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
Example 14
Compound of formula IV: A and B taken together with the carbon atom to which
they are
attached = C=(3-quinolyl), Q= Ac, R,' = H. and R4" = Ac.
The title compound is prepared from the title compound of Example 13, 18, 3-
bromoquinoline, and palladium (II) catalyst according to the procedures
described in the
literature. For further details, see Or, Yat Sun; Clark, Richard F.; Wang,
Sanyi; Chu, Daniel
T. W.; Nilius, Angela M.; Flamm, Robert K.; Mitten, Michael; Ewing, Patty;
Alder, Jeff; Ma,
Zhenkun. J. Med. Chem. (2000), 43(6), 1045-1049.
Example 15
Compound of formula I: A and B taken together with the carbon atom to which
they are
attached = C=CH2, X and Y taken together with the carbon atom to which they
are attached =
C=O, L CHzCH3, W is N(CH3 2, Z = 4-acetoxycladinose and R2' = H
Step 15a: Compound of formula 7-2: R6 = Ac, R11= H, RP = tert-
butyldimethylsilyl,
Rz' = Ac, and R4" = Ac.
tert-Butyl-OC(O)-OCHZ(C=CH2)CHZ-O-tert-butyldimethylsilyl (0.9 g, 3 mmol) and
1,4-bis(diphenylphosphino)butane (170 mg, 0.4 mmol) and Pd2(dba)3 (183 mg, 0.2
mmol)
were added into a solution of the compound of formula (1-2), R6 = R2' = R4" =
Ac (1.75 g, 2
mmol) in tetrahydrofuran (10 ml) at room temperature. The reaction mixture was
refluxed
under nitrogen overnight, cooled to room temperature and the solvent was
removed in vacuo.
The residue was purified by silica gel chromatography (acetone:hexane/1:3) to
give the title
compound (1.5 g).
MS (ESI) m/z: 1059.65 (M+H)+.
13C-NMR(100 MHz, CDC13): S 181.2, 179.3, 175.9, 175.5, 173.5, 148.5, 116.5,
104.8, 102.0,
85.2, 84.3, 83.9, 83.6, 82.8, 82.2,79.7, 78.1, 77.6, 75.6, 72.4, 70.4, 69.0,
68.6, 54.6, 49.9,
46.2, 43.2, 40.8, 36.5, 33.6, 31.4, 27.1, 27.0, 26.6, 26.3, 25.2, 25.1, 24.0,
23.7, 22.0, 20.4,
16.0, 15.2, 0.5, 0.0
Step 15b: Compound of formula 7-2: R6 = H, Rõ = H, RI-2 = tert-
butyldimethylsilyl,
R,' = H, and R4" = Ac.
A solution of the compound from Step 15a (3.18 g, 3 mmol) in methanol (80 ml)
was
refluxed for 8 hours. The reaction was cooled to room temperature, the solvent
was removed
in vcauo and the residue was purified by silica gel chromatography (2M ammonia
in
methanol: dichloromethane/3:97) to give the title compound (2.6 g).
66
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
MS (ESI) m/z: 975.47 (M+H)+.
13C-NMR(100 MHz, CDC13): S 179.5, 178.9, 175.7, 150.6, 121.5, 106.8, 101.4,
85.3, 83.9,
83.7, 82.4, 82.0, 79.3, 77.8, 76.7, 76.5, 72.7, 70.4, 70.1, 69.3, 68.3, 54.6,
49.8, 45.5, 43.0,
42.9, 40.6, 38.1, 34.0, 31.1, 30.5, 27.1, 26.3, 26.1, 26.0, 24.3, 23.7, 23.5,
21.5, 19.9, 15.7,
14.8, 0.5, 0Ø
Step 15c: Compound of formula 7-4: RI 1= H, Rp = H, R,' = H, and R4" = Ac.
Forniic acid (0.38 ml, 10 mmol) and Na2S2O4 (1.39, 8 mmol) was added into an
emulsion of the compound from Step15 b(2.44g, 2.5 mmol) in isopropanol (25 ml)
and water
(30 ml). The mixture was heated to 90 C and stirred at that temperature for 8
hours. The
reaction mixture was cooled to room temperature, diluted with ethyl acetate
(60 ml), washed
with saturated sodium bicarbonate (3x60 ml), and dried over sodium sulfate.
The solvent was
removed in vacuo and the residue was purified by silica gel chromatography (2M
ammonia in
methanol:dichloromethane/3: 97) to give the title compound (1.7 g).
MS (ESI) m/z: 846.54 (M+H)+.
13C-NMR(100 MHz, CDC13): S 221.3, 175.3, 170.6, 147.0, 114.1, 101.8, 96.6,
79.9, 79.2,
78.8, 78.7, 77.4, 75.0, 72.8, 71.4, 68.8, 67.8, 65.4, 65.3, 63.7, 63.4, 60.6,
49.6, 45.5, 44.8,
40.4, 38.2, 38.0, 35.6, 22.0, 21.2, 21.1, 19.6, 18.6, 16.5, 14.4, 12.2, 10.6,
9.8.
Step 15d: Compound of formula 7-4: R11 = H. R- = H, RZ' = Ac, and R4" = Ac.
Acetic anhydride (94 1, 1 mmol) was added to a solution of the compound from
Step
15c (338.4mg, 0.4 mmol) in dichloromethane (5 ml). The mixture was stirred at
room
temperature for 16 hours. The solvent was removed in vacuo and the residue was
purified by
silica gel chromatography (acetone:hexane/4 : 6) to give the title compound
(330 mg).
MS (ESI) m/z: 888.58 (M+H)+.
13C-NMR(100 MHz, CDC13): 8 221.3, 175.1, 170.6, 170.3, 146.8, 114.2, 99.6,
96.5, 79.9,
79.1, 78.5, 78.4, 77.1, 74.9, 72.8, 72.1, 68.9, 67.1, 65.1, 63.7, 63.5, 63.1,
49.3, 45.5, 44.8,
40.6, 38.0, 37.7, 37.6, 35.5, 29.4, 21.8, 21.3, 21.1, 21.0, 19.4, 18.6, 16.6,
12.2, 10.6, 9.6.
Step 15e: Compound of formula 7-4: R = H, R_= tert-butox cy arbonyl, R?' = Ac,
and R4" = Ac.
Di-tert-butyl-dicarbonate (69 l, 0.3 mmol) was added to a solution of the
compound
of Step 15d (178 mg, 0.2 mmol) and triethylamine (56 l, 0.4 mmol) in
dichloromethane (8
ml) at room temperature. After 10 minutes, N,N-dimethylamino pyridine (12.2
mg, 0.1
mmol) was added. The resulting solution was stirred at room temperature for 2
hours. The
67
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
solvent was removed in vacuo and the residue was purified by silica gel
chromatography
(acetone:hexane/1:3) to give the title compound (180 mg).
MS (ESI) m/z: 988.41 (M+H)+.
13C-NMR(100 MHz, CDC13): S 219.6, 174.6, 170.6, 170.3, 153.8, 141.3, 116.8,
99.6, 96.5,
82.0, 80.2, 79.4, 78.7, 78.6, 76.8, 74.9, 72.9, 72.4, 69.1, 67.9, 67.2, 64.8,
63.6, 63.4, 49.4,
45.2, 44.8, 41.0, 37.9, 37.7, 37.6, 35.6, 31.8, 31.3, 31.2, 28.2, 28.1, 22.9,
21.8, 21.5, 21.4,
21.1, 19.4, 18.7, 16.7, 16.6, 14.4, 12.5, 10.7, 9.7.
Step 15f= Compound of formula I: A and B taken together with the carbon atom
to
which they are attached = C=CHZ. X and Y taken together with the carbon atom
to
which they are attached = C=O, L= CH2CH3, W is N(CH2, Z = 4-acetoxycladinose
and R2' = Ac.
1,4-bis(diphenylphosphino)butane (8.5 mg, 0.02 mmol) and Pd2(dba)3 (9.2 mg,
0.01
mmol) were added to a solution of the compound of Step 15e (98.8 mg, 0.1 mmol)
in 2 ml
anhydrous THF at room temperature. The resulting mixture was refluxed for 30
minutes. The
solvent was removed in vacuo and the residue was used for next step reaction
without
purification.
MS (ESI) m/z: 870.49 (M+H)+.
Step 15g Compound of formula I: A and B taken together with the carbon atom to
which they are attached = C=CH2, X and Y taken together with the carbon atom
to
which they are attached = C=O, L = CH3CH3, W is N(CH9, Z = 4-acetoxycladinose
and R,' = H.
A solution of the compound of Step 15f (87 mg, 0.01 mmol) in 5 ml methanol was
refluxed for 8 hours. The solvent was removed in vacuo and the residue was
purified by silica
gel chromatography (CH2C12 : 2M ammonia in CH30H/97:3) to give the title
compound (70
mg).
MS (ESI) m/z: 828.50 (M+H)+.
13C-NMR(100 MHz, CDC13): S 219.1, 17601, 170.8, 14103, 125.7, 102.7, 96.0,
79.2, 78.8,
77.9, 77.2, 76.9. 73.0, 72.3, 71.1, 70.2, 68.4, 65.8, 65.6, 63.3, 49.9, 46.6,
44.5, 41.9, 40.6,
39.4, 38.9, 35.2, 29.1, 23.3, 21.9, 21.4, 21.2, 20.7, 18.3, 17.9, 13.5, 12.6,
8.7.
68
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
Example 16
Compound of formula I= A and B taken together with the carbon atom to which
they are
attached = C=CH-quinolin-3-yl X and Y taken together with the carbon atom to
which they
are attached = C=O, L = CHZCH3, W is N(CH~?, Z = 4-acetoxycladinose and R2' =
H.
To a solution of the title compound from Example 15 (992 mg, 1.2 mmol), 3-
bromoquinoline (340 l, 2.5 mmol) and triethyl amine (836 l, 6 mmol) in
acetoitrile (12 ml)
was added P(o-Tol)3 (146 mg, 0.48 mmol) and palladium acetate (54 mg, 0.24
mmol) at room
temperature. The mixture was degassed and heated to 80 C and stirred for 16
hours. The
mixture was diluted with ethyl acetate (50 ml) and washed with NaHCO3 (60 ml x
2) and
brine (60 ml). The solvent was removed in vacuo and the residue was purified
by silica gel
chromatography (CH2C12 : 2M ammonia in CH30H/97:3) to give the title compound.
MS (ESI) m/z: 955 (M+H)+.
Example 17
Compound of formula I= A and B taken together with the carbon atom to which
they are
attached = C=CH-quinolin-3-yl X and Y taken together with the carbon atom to
which they
are attached = C=O, L= CH2CH3, W is N(CH,iZ = OH, and Rz' = H.
To a solution of the title compound from Example 16 (150 mg, 0.16 mmol) in
ethanol(3 ml) was added 5 ml of 5N HCI. The mixture was heated to 65 C and
stirred for 2
hours. The reaction was quenched with saturated NaHCO3 (25 ml) and extracted
with ethyl
acetate (25 ml). The extract was washed with brine and dried ove anhydrous
Na2SO4. The
solvent was removed in vacuo and the residue was purified by silica gel
chromatography
(CHZC12 : 2M ammonia in CH30H/97:3) to give the title compound.
MS (ESI) m/z: 755 (M+H)+.
Example 18
Compound of formula I: A and B taken together with the carbon atom to which
they are
attached = C=CH2, X and Y taken together with the carbon atom to which they
are attached =
C=O, L= CH,CH3, W is N(CH2, Z= OH and R2' = H.
Step 18a: Compound of formula I: A and B taken together with the carbon atom
to
which they are attached = C=CH2. X and Y taken together with the carbon atom
to
which they are attached = C=O, L = CH)CH3, W is N(CH2. Z = OH and R?' = Ac.
69
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
To a solution of the compound of Stepl5f of Example 15 (700 mg, 0.8 mmol) in
10
ml of ethanol was added 25 ml of 1M HCI. The mixture was refluxed for 2 hours
and then
cooled to room temperature. The pH of the mixture was adjusted to pH=10 by
addition of 2M
NaOH and the mixture was extracted with ethyl acetate (25 ml x 3). The organic
phases were
dried over Na2SO4 and the solvent was removed in vacuo. The residue was
purified by silica
gel chromatography (hexanes:acetone / 1:1) to give the title compound (480
mg).
MS (ESI) m/z: 670.23 (M+H)+.
I3C-NMR(100 MHz, CDC13): S 216.3, 175.0, 170.1, 141.8, 122.1, 99.4, 81.1,
79.0, 77.7,
77.5, 76.2, 75.6, 72.1, 71.7, 68.8, 65.6, 63.2, 60.5, 46.5, 43.7, 40.8, 39.1,
38.6, 35.9, 31.1,
23.0, 21.6, 21.3, 21.2, 19.8, 18.5, 17.3, 14.8, 14.3, 13.0, 11.7, 7.9.
Step 18b. Compound of formula I: A and B taken together with the carbon atom
to
which they are attached = C=CH2, X and Y taken together with the carbon atom
to
which they are attached = C=O, L = CH2CH3, W is N(CH3)?, Z = OH and R,.' = H.
The title compound is prepared by refluxing the compound from Step 18a in
methanol
according to the procedure described in Example 1 (Step lc).
Example 19
Compound of formula IV: A and B taken together with the carbon atom to which
they are
attached = C=CH2-phenyl, Q= OH, R2' = H, and R4" = Ac.
Step 19a: t-BuOC(O)OCHC, (O)CH,OC(O)OtBu
To a solution of 1,3-dihydroxyacetone dimer (36.03 g, 0.20 mol) and DMAP (1.22
g,
10.0 mmol) in dichloromethane (80 mL) and pyridine (97.0 mL, 1.20 mol) was
added a
solution of di-tert-butyl dicarbonate (200.0 g, 0.92 mol) in dichloromethane
(40 mL) via a
dropping funnel over 3 hours at room temperature. After stirring at room
temperature for 15
hours, the reaction mixture was condensed in vacuo. The residue was diluted
with
hexane:diethyl ether/1:1 and washed with saturated aqueous CuSO4, water and
brine. The
organic phase was dried over Na2SO4 and the solvent was removed in vacuo. The
residue was
purified by silica gel chromatography (hexane:ethyl acetate/95:5-85:15) to
give the title
compound (45.0 g, 39% yield).
13C-NMR (125 MHz, CDC13): S 198.5, 152.6, 83.5, 68.5, 27.6.
Step 19b: Compound of formula 1-3 of Scheme 1: R = Phenyl and R11= t-Butyl.
A suspension of benzyltriphenylphosphonium bromide (520 mg, 1.20 mmol) in THF
(5.0 mL) was treated with n-butyl lithium (1.6 M in hexane, 0.81 mL, 1.30
mmol) at -78 C
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
under nitrogen with stirring. The mixture was warmed to -15 C over 1 hour
before a solution
of the compound from Step 19a (290 mg, 1.0 mmol) in THF (2.5 mL) was charged
at -70 C.
The reaction mixture was warmed to room temperature over 1 hour and left
stirring for
another 14 hours before partition (ethyl acetate and water). The organic phase
was washed
with water, brine and dried over Na2SO4. The solvent was evaporated and the
residue was
purified by silica gel chromatography (hexane:CH2C12/1:1) to give the title
compound (253
mg, 70% yield).
13C-NMR (125 MHz, CDC13): S 153.1, 153.0, 135.1, 134.6, 130.4, 128.6, 128.2,
127.6, 82.0,
81.9, 68.4, 62.7, 27.6, 27.5.
Step 19c: Cogipound of formula IV: A and B taken together with the carbon atom
to
which they are attached = C=CH2_phenyl, O= OAc, R2' = Ac, and R4" = Ac.
A mixture of Erythromycin A oxime triacetate (525 mg, 0.60 mmol), the compound
from Step 19b (250 mg, 0.69 mmol), 1,4-bis(diphenylphosphino)butane (51.2 mg,
0.12
mmol), and tris(dibenzylideneacetone)dipalladium (54.9 mg, 0.06 mmol) in THF
(5.0 mL)
was degassed and heated to and kept at 75 C for 15 hours before evaporation.
The residue
was purified by silica gel chromatography (hexane:acetone/4:1-1.5:1) to give
the title
compound as a 2.6:1 isomeric mixture (330 mg, 55% yield).
MS (ESI) m/z: 1003 (M+H)+.
Step 19d: Compound of formula IV: A and B taken together with the carbon atom
to
which they are attached = C=CH,=phenyl, Q= OH, R2' = H, and R4" = Ac.
The title compound was prepared by refluxing the compound from Step 19c in
methanol according to the procedure described in Example 1 (Step lc).
MS (ESI) mlz: 919 (M+H)+.
Example 20
Compound of formula V: A and B taken together with the carbon atom to which
they are
attached are C=CH-phenyl, Q = Ac, and R2' = H.
Step 20a: Compound of formula V: A and B taken together with the carbon atom
to
which they are attached are C=CH-phenyl, Q= H, and R2' = Ac.
A solution of the compound of Example 19 (0.30 mmol) in methanol (5.0 mL) was
treated with titanium(III) chloride (20% in 3% HCI, 0.77 mL) for 2 hours at
room
temperature then for 1 hour at 50 C before partition (CH2C12 and aqueous
saturated
NaHCO3). The aqueous solution was extracted with CH2C12. The combined extracts
were
71
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
washed with brine and dried over Na2SO4. After evaporation, the residue was
purified by
silica gel chromatography (CH2C12:2 M NH3 in MeOH/98:2--93:7) to give the
title compound
as a 4:1 isomeric mixture (105 mg, 50% yield).
MS (ESI) m/z: 703 (M+H)+.
Step 20b: Compound of formula V: A and B taken together with the carbon atom
to
which they are attached are C=CH-phenyl, Q= Ac, and R2' = Ac.
A solution of the compound from Step 20a (105 mg, 0.15 mmol) in CH2C12 (3.0
mL)
was treated with triethylamine (104 L, 0.74 mmol) and acetic anhydride (42
L, 0.45 mmol)
at room temperature for 19 hours before evaporation and drying in vacuo to
give the title
compound.
MS (ESI) m/z: 787 (M+H)+.
Step 20c. Compound of formula V: A and B taken together with the carbon atom
to
which they are attached are C=CH-phenyl, = Ac, and R,' = H.
The title compound is prepared by refluxing the compound from Step 20b in
methanol
according to the procedure described in Example 1(Step lc).
Example 21
Compound of formula I: A and B taken together with the carbon atom to which
they are
attached = C=0 X and Y taken together with the carbon atom to which they are
attached
=C=NAc, L= CH,CH3, W is N(CH), Z = OCH7CH=CH(duinolin-3-yl), and RZ' = H.
Step 21a: Compound of formula I: A and B taken together with the carbon atom
to
which they are attached = C=O, X and Y taken together with the carbon atom to
which they are attached = C=NH, L = CH)CH~, W is N(CH31LZ=
OCH2CH=CH(duinolin-3-yl) and R2' = H.
A solution of the title compound from Example 3 (0.5g, 0.8 mmol) in methanol
(5 ml)
and dichloromethane (5 ml) was cooled to -78 C and ozone was bubbled through
the reaction
until the solution became light blue. Then nitrogen was bubbled through the
reaction mixture
to remove excess ozone and triphenyl phosphine (1.0g, 3.8 mmol) was added. The
solution
was allowed to warm to room temperature over 1 hour. The solvent was
evaporated and the
residue was dissolved in 20 ml of THF and refluxed overnight. The solvent was
removed
under vacuum and the residue was purified by flash chromatography (Si02,
CH2C12:2M
ammonia in methanol = 95 : 5) to give the title compound (0.33g, 66%)
MS (ESI) m/z 629.2 (M+H)+
72
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
Step 21b. Compound of formula I: A and B taken together with the carbon atom
to
which they are attached = C=0 X and Y taken together with the carbon atom to
which they are attached = C=NAc, L= CH2CH3, W is N(CH,, Z= OH and Rz' _
Ac.
Acetic anhydride (0.17 ml, 1.5 mmol) was added to a solution of the compound
from
Step 21a (0.3 g, 0.5 mmol) and triethylamine (0.4 ml, 3.0 mmol) in
dichloromethane (10 ml).
The reaction mixture was stirred at room temperature for 4 hours, diluted with
100 ml of
dichloromethane and washed with saturated sodium bicarbonate (3x100 ml) and
brine (100
ml). The organic phase was dried over sodium sulfate and the solvent was
removed in vacuo.
The residue was purified by silica gel chromatography (hexanes/acetone:1:1) to
give the title
compound ( 0.2 g).
MS (ESI) m/z: 713.1 (M+H)+.
Step 21c. Compound of formula I: A and B taken together with the carbon atom
to
which they are attached = C=O, X and Y taken together with the carbon atom to
which they are attached = C=NAc, L = CH7CH3, Z = OCH2CH=CH(cuinolin-3-yl)
and Rz' = Ac.
A mixture of the compound from Step 21b (55 mg, 0.08 mmol), 3-(t-
butoxycarboxy)-
3-(3-quinolinyl)-1-propene (60 mg, 0.21 mmol), and 1,4-bis(diphenylphosphino)-
butane (10
mg, 0.02 mmol) was dissolved in freshly distilled THF (5.0 ml). To the
solution was added
Pd2(dba)3 (12 mg, 0.01 mmol). The reaction mixture was heated to reflux
slowly. After
refluxing for 14 hours, the reaction was worked up as described in Step lb of
Example 1 and
the solid residue was purified by silica gel chromatography (acetone :
hexane/1:1) to give the
title compound (27 mg).
MS (ESI) m/z 880.3 (M+H)+.
Step 21d: Compound of formula I= A and B taken together with the carbon atom
to
which they are attached = C=O, X and Y taken together with the carbon atom to
which they are attached = C=NAc, L = CH2CH3, Z = OCH,CH=CH(quinolin-3-yl)
and R2' = H.
The title compound was prepared by refluxing the compound from Step 21c in
methanol according to the procedure described in Step lc of Example 1.
MS (ESI) m/z 838.2 (M+H)+.
Selected 13C NMR: S 206.0, 184.7, 176.2, 176.1, 149.1, 147.8, 133.0, 129.7,
129.6, 129.5,
128.2, 128.1, 127.8, 127.4, 101.1.
73
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
Example 22
Compound of formula I: A and B taken together with the carbon atom to which
they are
attached = C=CHCHCH-phenyl, X and Y taken together with the carbon atom to
which they
are attached = C=NAc, L = CH2CH3, W is N(CH9. Z = OC(O)-benzyl and R2' = H.
Step 22a. Compound of formula I: A and B taken together with the carbon atom
to
which they are attached are C=CHCH=CH-phenyl, X and Y taken together with the
carbon atom to which they are attached are C=NAc, L = CH2CH3, W is N(CH3j2, Z=
OH, and RZ' = Ac.
To a solution of the compound of formula 1: A and B taken together with the
carbon
atom to which they are attached are C=CH2, X and Y taken together with the
carbon atom to
which they are attached are C=NAc, L = CH2CH3, W is N(CH3)2, Z= OH, and R2 =
Ac (0.5
g, 0.7 mmol) in 8 ml anhydrous DMF, (3-bromostyrene (0.15 ml, 1.2 mmol) and
K2CO3 (200
mg, 1.5 mmol) were added at room temperature. The mixture was degassed briefly
and a
catalytic amount of dihydrogen dichlorobis(di-tert-butylphosphinito-
xP)palladate(II) (POPd
from Combiphos catalysts, Inc.) was added. The reaction mixture was heated to
100 C in a
sealed tube for 48 hours. Ethyl acetate (50 mL) was added and the solution was
washed 3
times with aqueous NaHCO3. The organic layer was dried over anhydrous Na2SO4.
The
solvent was evaporated under vacuum and the residue was purified by flash
chromatography
(Si02, acetone: hexanes/ 1: 1) to provide the title compound.
MS (ESI) m/z 813 (M+H)+
Step 22b. Compound of formula I: A and B taken together with the carbon atom
to
which they are attached are C=CHCH=CH-phenyl, X and Y taken together with the
carbon atom to which they are attached are C=NAc, L = CH2CH3, W is N(CH3j2, Z=
OC(O)CH;(2-pyridyl) and RZ' = Ac.
Into a solution of 2-pyridylacetic acid (85.4 mg, 0.48mmol) in CH2C12(1 ml)
was
added Et3N (140 l) at room temperature. Then trimethyl acetic chloride (60
1, 0.48mmol)
was added to the suspension at 0 C. After stirring at 0 C for 30 mins, a
solution of the
compound from Step 22b (100 mg, 0.12 mmol) in CH2C12(1 ml) in pyridine (100
1) was
added at 0 C. The reaction mixture was warmed up to room temperature and
stirred for
overnight. The mixture was treated with saturated NaHCO3, extracted with ethyl
acetate and
washed with brine. The organic phase was dried over sodium sulfate and the
solvent was
74
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
removed in vacuo. The crude residue was purified by column chromatography
(Aceton/Hexane 1:1) to give the title compound (83 mg).
MS (ESI) m/z: 932 (M+H)+.
Step 22c. Compound of formula I: A and B taken together with the carbon atom
to
which they are attached = C=CHCH=CH-phenyl, X and Y taken together with the
carbon atom to which they are attached = C=NAc, L = CHZCH3, W is N(CHz, Z
OC(O)CH,(2-p,yridyl) and RZ' = H.
The title compound (71 mg) was prepared by stirring the compound from Step 22b
in
methanol overnight.
MS (ESI) m/z: 890 (M+H)+.
Example 23
Compound of formula I: Compound of formula I: A and B taken together with the
carbon
atom to which they are attached = C=CHCHCH-phenyl, X and Y taken together with
the
carbon atom to which they are attached = C=NAc, L = CH2CH, W is N(CH3)2, Z=
OC(O)CH,(3-pyridyl) and R,' = H.
Step 23a. Compound of formula I: Compound of formula I: A and B taken to eg
ther
with the carbon atom to which they are attached = C=CHCH=CH-phenyl, X and Y
taken together with the carbon atom to which they are attached = C=NAc, L
CHzCH, W is N(CH,, Z = OC(O)CH7(3-Qyridyl) and R2' = Ac.
The title compound (59 mg) was prepared with the title compound from step 22a
and
3-pyridylacetic acid according to the procedure described in Step22 Step lc).
MS (ESI) m/z: 932 (M+H)+.
Step 23c. Compound of formula I: A and B taken together with the carbon atom
to
which they are attached = C=CHCH=CH-phenyl, X and Y taken together with the
carbon atom to which they are attached = C=NAc, L = CHZCH3, W is N(CH2, Z
OC(O)CH,(3-p,Yridyl) and RZ' = H
The title compound (52 mg) was prepared by stirring the compound from Step 23b
in
methanol for overnight.
MS (ESI) m/z: 890 (M+H)+.
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
Example 24
Compound of formula I: A and B taken together with the carbon atom to which
they are
attached = C=CH2 X and Y taken together with the carbon atom to which they are
attached =
C=NOH, L = CH,CH3, W is N(CH3.7. Z = 4-oxocladinose and R2= H.
Step 24a. Compound of formula IV: A and B taken together with the carbon atom
to
which they are attached = C=CHZ, O= OH, and Rz= R4" = H.
Into a solution of the compound from Step 22b of example 22 (lg, 1.07 mmol) in
5 ml
methanol was added LiOH(150 mg, 6.25 mmol) at rt. After stirring for overnight
the mixture
was diluted with ethyl acetate, and washed with saturated NH4C1 and brine. The
organic
phase was dried over NazSO4, the solvent was removed in vacuo to give the
title compound
(0.9g).
MS (ESI) m/z: 800 (M+H)+.
Step 24b. Compound of formula IV: A and B taken together with the carbon atom
to
which they are attached = C=CH7, Q= OAc, and R,= Ac, and R4" = H.
Into a solution of the compound from step 24a (lg, 1.25 mmol) in 2.5 ml CH2C12
was
added Ac20 (240 ul, 2.5 mmol) and DIEA(870 ul, 5 mmol) at room temperature.
After
stirring for 1 hour the mixture was diluted with ethyl acetate, and washed
with saturated
NaHCO3 and brine. The organic phase was dried over Na2SO4, and the solvent
removed in
vacuo to give the crude title compound (1.2 g).
MS (ESI) m/z: 885 (M+H)+.
Step 24c. Compound of formula I= A and B taken together with the carbon atom
to
which they are attached = C=CH2, X and Y taken together with the carbon atom
to
which they are attached = C=NOAc, L = CH2CH3, W is N(CH2, Z = 4-oxocladinose
and R2' = Ac.
Into a solution of the crude compound from step 24b in 7 ml of CH2ClZ was
added
DMSO (1.3 ml, 18.75 mmol) and EDC-HCl (1.2 g, 6.25 mmol) at 0 C. Then Py-TFA
(1.2 g,
6.25 mmol) was added at 0 C. The mixture was warmed to room temperature and
stirred
overnight. The mixture was diluted with ethyl acetate, and washed with
saturated NaHCO3
and brine. The organic phase was dried over Na2SO4, the solvent removed in
vacuo and the
solid residue was purified by silica gel chromatography (acetone : hexane/1:1)
to give the title
compound (890 mg).
MS (ESI) m/z: 883 (M+H)+.
Step 24d. Compound of formula I: A and B taken together with the carbon atom
to
which they are attached = C=CH,, X and Y taken together with the carbon atom
to
76
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
which they are attached = C=NOH, L = CH2CH3, W is N(CH2, Z = 4-oxocladinose
and R2 = H.
A solution of the compound from Step 24c (50 mg) in 2 ml methanol was heated
at
55 C for 48 hours. The solvent was removed in vacuo and the compound was
purified by
column chromatography (CH2C12: 2M ammonia in MeOH/97:3) to give the title
compound
(20 mg).
MS (ESI) m/z: 799 (M+H)+.
Example 25
Compound of formula I: A and B taken together with the carbon atom to which
they are
attached = C=CH2, X and Y taken together with the carbon atom to which they
are attached =
C=NOH, L = CHZCH3, W is N(CH2, Z = 4-oximecladinose and R2' = H.
Into a solution of the compound from Step 24c of Example 24 (840 mg, 0.95
mmol)
in 4 ml of 2-PrOH was added H2NOH-HC1(140 mg, 1.9 mmol) at room temperature
followed by the addition of Et3N (260 ul, 1.9 mmol). The mixture was stirred
at room
temperature for 2 hours and heated at 45 C for 20 minutes. Then acetic acid
(108 ul) was
added and the mixture heated at 40 C overnight. Another 70 mg of H2NOH-HCl was
added
and the mixture heated at 45 C for 6 hourrs. The mixture was diluted with
ethyl acetate, and
washed with saturated NaHCO3 and brine. The organic phase was dried over
Na2SO4, the
solvent removed in vacuo and the solid residue was purified by silica gel
chromatography
(CH2C12: 2M ammonia in MeOH/97:3) to give the title compound (400 mg).
MS (ESI) m/z: 814 (M+H)+.
Example 26
Compound of formula IV: A and B taken together with the carbon atom to which
they are
attached are C=CH2, O= OH, and RZ' = R4" = H.
A solution of the compound from Step 24b of example 24 (50 mg) in 2 ml
methanol
was heated at room temperature for 48 hours. The solvent was removed in vacuo
and the
compound was purified by column chromatography (CH2CI2: 2M ammonia in
MeOH/97:4)
to give the title compound (23 mg).
MS (ESI) m/z: 801 (M+H)+.
77
CA 02483875 2004-10-29
WO 03/095466 PCT/US03/14914
Example 27
Compound of formula I: Compound of formula IV: A and B taken together with the
carbon
atom to which they are attached = C=CH2, Q= OH, R,' = H, and R4" = Ac.
Step 27a. Compound of formula IV: A and B taken together with the carbon atom
to
which they are attached = C=CH2, Q= OCH3 R,' = Ac, and R4" = Ac.
A mixture of the compound from Step 5a of Example 5 (100 mg, 0.11 mmol) and
NaH (60% in mineral oil, 7 mg, 0.17 mmol) in 0.5 ml DMF was stirred at room
temperature
for one minute before methyl iodide (0.007 mL, 0.11 mmol) was added. After 15
minutes the
mixture was quenched with water. The mixture was extracted with hexanes-ether
(1:1). The
extracts were dried over Na2SO4 and the solvent was removed in vacuo to give
the crude title
compound (80 mg).
MS (ESI) m/z: 899 (M+H)+.
Step 27b. Compound of formula IV: A and B taken together with the carbon atom
to
which they are attached = C=CH2, Q= OCH3 R?' = H, and R4" = H.
The title compound (40 mg) was prepared by stirring the compound from Step 27a
(80 mg) in methanol at room temperature for 16 hours and refluxing for 2 hours
according to
the procedure described in Example 1(Step lc).
MS (ESI) m/z: 857 (M+H)+.
Although the invention has been described in detail with respect to various
preferred
embodiments it is not intended to be limited thereto, but rather those skilled
in the art will
recognize that variations and modifications may be made therein which are
within the spirit
of the invention and the scope of the appended claims.
78