Note: Descriptions are shown in the official language in which they were submitted.
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
- 1 -
WARMING AND NONIRRTTATING ANHYDROUS LUBRICANT COMPOSITIONS
This application is a continuation-in-part of patent
application United States Serial No. 10/137,509 and of copending
patent application United States Serial No. (Attorney
Docket No. PPC 834 CIP 1) which are hereby incorporated herein by
reference.
FIELD OF THE INVENTION
This invention relates to substantially anhydrous, personal
lubricant gel and jelly compositions that are warming and
nonirritating when applied to the skin or mucous membranes,
especially the vaginal or oral mucosa. In some embodiments, the
compositions of this invention are substantially anhydrous and
contain one or more polyhydric alcohol. This invention also relates
to the method that can be used to test and compare the irritation
of the compositions of this invention and other personal lubricants
known to the art. Unlike previously-known compositions that use
exothermic reactions to generate warmth or use irritants to convey
a perception of warmth, the compositions of this invention use
"heat of solution'° to generate warmth. The feeling of warmth
generated by the compositions of this invention is very pleasant
and mild in comparison with previously-known compositions. The
compositions of this invention are also more lubricating to tissue
than previously-known warming compositions. Furthermore, the
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
- 2 -
lubricity of the compositions of this invention increases upon
dilution with water; the lubricity of previously-known aqueous
compositions tends to decrease upon dilution with water. In actual
use, therefore, the compositions of this invention will be
considerably more lubricating than those previously known to the
art.
BACKGROUND OF THE INVENTION
Humans are warm-blooded animals that maintain a constant body
temperature of 98.6°F (37°C). Human skin and external organs
have
a very efficient circulatory and nervous system with the result
that the human body can very quickly perceive changes in
temperature. Personal lubricants and medicaments are usually
applied to humans' mucous membranes at room temperature, i.e.,
between at 60°F and 80°F. Because there is an appreciable
difference in temperature between room temperature and human body
temperature, users of such lubricants and medicaments perceive them
to be quite cold. This feeling of cold can be quite uncomfortable
for the user. From time to time, attempts have been made to
develop products that overcome this perception of cold.
An appreciable number of personal lubricant compositions are
known to the art. These compositions range from jellies to liquids
to vaginal suppositories and vary from being aqueous to oils to
silicone based. The majority of the compositions actually used
today are aqueous jellies or aqueous liquids. Almost all personal
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
- 3 -
lubricants known and available for use today are cold to touch, a
feeling that can be uncomfortable.
A number of compositions are known to the trade or described
in the literature that claim to impart a warming sensation upon
application to the skin or mucosa. These compositions generally
fall into a few main categories based upon mechanism of action.
Some of these compositions use plant extracts which are
irritating to the skin and mucous membranes and give a feeling or
perception of warmth by virtue of their irritant action. Others
claim to enhance blood flow in order to cause tissue warming. Still
others are alleged to work on the principle of freezing point
depression and are well suited for heating in a microwave or
cooling in a refrigerator. There is one cosmetic composition
rendered self-heating by inclusion of compound containing a boron-
to-boron linkage, which reacts exothermally with water.
One category of warming compositions use plant extracts or
agents, such as methyl salicylate, that are irritating to the skin
or mucous membranes. For example, WO 97/02273A describes phosphate
derivatives useful in oral and topical compositions to provide a
perceived sensation of warmth. The compositions contain warming
components such as vanillyl derivatives. The compositions also
incorporate an additional warming agent, including ethanol, niacin,
jambu, nicotinic acid, zingerone, vanillyl alcohol isopropyl ether,
gingerol, methyl salicylate, shogaol, paradol, zingerone,
capsaicin, dihydrocapsaicin, nordihydrocapsaicin homocapsaicin,
tincture capsicum, eucalyptus oil.
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
- 4 -
Another category of warming products utilize the mechanism of
increased blood circulation. U.S. Patent No. 5,895,658, entitled
"Delivery of L-Arginine to Cause Tissue Warming, Sustained Release
of Nitric Oxide to treat effects of Diabetes, Stimulate Hair Growth
and Heal Wounds," describes a preparation for producing enhanced
blood flow in tissues thus causing beneficial effects, such as
warming cold tissues of hands and feet.
Yet another category of warming products uses inorganic
compounds to create exothermic reactions resulting in the evolution
l0 of heat. WO 200260408 A1, for example, relates to anhydrous
cosmetic hair care compositions containing inorganic salts such as
sodium sulfate, calcium sulfate, aluminum sulfate, calcium
chloride, magnesium chloride or calcium oxide, or magnesium
sulfate. Upon mixing with water, these compositions generate heat.
KR20010227018 describes exothermic cosmetic Containing active
zeolite compositions that promote blood circulation and metabolism
by giving warm-sense to the skin. Active zeolite contains
ethoxylated alcohol. JP63159490A relates to exothermic cosmetic
compositions especially for softening hair containing reducing
agents such as sodium sulphite, sodium thiosulphate, sodium
pyrosulphite or sodium hydrogen sulphite and an oxidizing agent
such as sodium bromate, potassium bromate or sodium perbromate.
EP2001306347 refers to compositions for use in preparing hair
for shaving. The compositions contain at least one substance that
undergoes a discernible chemical change when mixed during shaving.
The substance changes temperature, emits a scent or undergoes
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
- 5 -
oxidation, hydration, an acid-base reaction or an exothermic
reaction.
Another category of heat-producing products uses mechanisms
other than the three aforementioned categories. US 20020142015 A1
and JP 2002179517 A describe warming compositions for cosmetics,
toiletries, bath additives and pharmaceuticals that contain a
cooling agent and a specific p-hydroxybenzaldehyde derivative. The
use of this p-hydroxybenzaldehyde cooling agent is intended to
product a warming effect for a longer duration. FR2810240 A1
describes cosmetic compositions containing a component that can
absorb or release heat thereby providing a cooling and refreshing
effect during exposure to heat or a warming effect during exposure
to cold, such as a combination of long chain hydrocarbon compounds
that can absorb thermal energy and store or exchange heat.
US3632516A describes a self-heating lather that is rapidly heated
by hydrogen peroxide via a thiosulphredox reaction.
Another category of heat-producing products uses mechanisms
other than the three aforementioned categories. US 20020142015 A1
and JP 2002179517 A describe warming compositions for cosmetics,
toiletries, bath additives and pharmaceuticals that contain a
cooling agent and a specific p-hydroxybenzaldehyde derivative. The
use of this p-hydroxybenzaldehyde cooling agent is intended to
product a warming effect for a longer duration. FR2810240 A1
describes cosmetic compositions containing a component that can
absorb or release heat thereby providing a cooling and refreshing
effect during exposure to heat or a warming effect during exposure
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
- 6 -
to cold, such as a combination of long chain hydrocarbon compounds
that can absorb thermal energy and store or exchange heat.
US3632516A describes a self-heating lather that is rapidly heated
by hydrogen peroxide via a thiosulphredox reaction.
JP2001335429 describes gel-like Cosmetics that contain 40-75%
by weight of polyethylene glycol, 20-55% by weight of glycerol and
carboxyvinyl polymer. These compositions are used for generating
heat for promoting blood Circulation and metabolism in fatigue
conditions and to provide warm feeling during shaving. One example
of a composition known to the trade, ProsensualTM, distributed by
Lexie Trading, Inc., Fairlaw, New Jersey, contains plant extracts
such as Cinnamon cassia (Cinnamon), Zingiber officinalis (Ginger),
Mint, Sandalwood, Orange and Clove, which are all known to be skin
irritants. Such a composition has the disadvantage of Causing
irritation to the mucosa, which Can be problematic in relation to
the vaginal or oral mucosa as irritation may promote the growth of
unwanted bacteria and cause infection.
Another current composition, WETT"' Heating Massage Oil,
distributed by International, Valencia, California, uses Retinyl
Palmitate (Vitamin A Palmitate), Prunus amygdalis (Prunes), Amara
(Almond), Persica gratissima (Avacado Oil), Macdamia ternifulia
Seed 0i1, Kakeri Nut Oil, Helianthus annus (hybrid Sunflower),
Cannabis sativa (Hemp) Seed Oil and Aloe vera. Most of these
ingredients are known irritants that are not suitable for use on
mucous membranes.
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
U.S. Patent No. 5,513,629 entitled "Microwavable Heat
Releasing and Absorbing Compositions and Container, Pliable Gel
Comprising Humectant, Freezing Point Depressant, Gel Sealer,
Polyacrylamide Absorbent, Corn Starch Binder, Mineral Oil and
Plasticizers, Durability, Efficacy" describes compositions that
have a high vapor points and are, therefore, suited for heating in
a microwave oven or cooling in a freezer and placement in a
suitable container or vinyl package, such as a hot-and-cold pack,
but not for human consumption or use.
However, none of the foregoing compositions are actually
"warm", or at a relatively higher temperature than the ambient
temperature of the product or the surrounding environment.
U.S. Patent No. 4,110,426, entitled "Method of Treating Skin
and Hair with a Self Heated Cosmetic, Organic Boron-Oxygen-Boron
Compounds" describes non-aqueous compositions such as shaving
creams, that are rendered self-heating by including therein a
compound containing at least one boron-oxygen-boron linkage, such
as triethoxyboroxine. The boron-containing compound reacts
exothermally with water or other protic material to increase
temperature. Such compositions are not suitable for vaginal or oral
use due to the potential toxicity of boron-containing compounds to
the human reproductive system (Fail PA, et al., general,
reproductive, developmental, and endocrine toxicity of boronated
compounds., Reprod toxicol 12: 1, 1-18, Jan-Feb, 1998).
Physical energy forms have been utilized to enhance material
transport across a membrane for therapeutic purposes. Such energy
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
_ g _
forms include electricity, ultrasound and thermal energy (e. g.,
heat-assisted drug delivery), (reviewed by Sun, in "Skin Absorption
Enhancement by Physical Means: Heat, Ultrasound, and Electricity",
Transdermal and Topical Drug Delivery Systems, Interpharm Press,
Inc., 1997, pages 327-355). Local heating of a drug delivery
system or formulation, as well as the skin or mucosal tissues, not
only increases thermodynamic energy of drug molecules and membrane
permeability to facilitate drug movement across a barrier membrane,
it improves blood circulation in the tissue to expedite drug
removal from the local tissue into the systemic circulation. Both
processes leads to an enhanced absorption of the drug. Experimental
evidence demonstrates that low-level heating (i.e., a tissue
temperature of less than about 42°C) significantly enhances
percutaneous drug absorption.
U.S. Patent No. 5,658,583 describes a heat-generating
apparatus for improved dermal permeation of pharmaceuticals. The
apparatus includes a thin drug formulation reservoir and a heat-
generating chamber of oxidation reaction separated by a non-
permeable wall. The drug formulation reservoir houses a
predetermined amount of a formulation containing pharmaceutical
agents. The heat-generating/temperature-regulating chamber includes
a heat-generating medium consisting of carbon, iron, water and/or
salt which is activated upon contact with oxygen in the air.
However, a complicated heating device such as this is not suitable
for use in the vaginal or oral cavity for obvious safety concerns.
Locally applied heat (such as an abdominal heating patch) has
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
_ g _
also been used to treat dysmenorrhea, or menstrual cramps, with
demonstrated efficacy (Akin MD et al., Continuous low-level topical
heat in the treatment of dysmenorrhea., Obstet Gynecol 97: 3, 343-
9, Mar, 2001).
U.S. Patent No. 6,019,782 describes disposable thermal body
pads with heat generation via an oxidation reaction intended for
relieving menstrual pain when applied onto the abdominal skin.
There is currently a commercial product in the U.S. market for
dysmenorrhea treatment based on abdominal heating, ThermaCare~ Air-
Activated Heatwraps, Menstrual Cramp Relief patches manufactured by
Procter & Gamble (Cincinnati, OH). However, there are no products
or description of internal localized heating to treat dysmenorrhea.
SUMMARY OF THE INVENTION
The compositions and methods of this invention relate to
substantially anhydrous non-irritating gel and jelly compositions.
The compositions and methods of this invention contain cellulose
gelling derivatives and polyhydric alcohols. Preferably, the
cellulose gelling derivatives are hydrocolloids. The compositions
of this invention may be used as warming lubricant compositions
that are non-toxic and non-irritating and that can be used as
personal lubricants designed to come into contact with the skin or
mucosa. When mixed with water, the gel and jelly compositions of
this invention increase in temperature or generate warmth. This has
a soothing effect on the tissues to which these compositions are
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
- 10 -
applied. This substantially eliminates the feeling or perception of
cold that conventional personal lubricants convey upon use.
The compositions of this.invention have excellent lubrication
characteristics. The gels and jelly compositions of this invention
are more lubricating than even aqueous lubricant products currently
available on the market. The compositions of this invention, in
particular, the jelly compositions of this invention, are novel in
that their lubricity increases upon dilution with water. Known,
commercially available aqueous compositions decrease in lubricity
upon dilution with water. This is a particular advantage in that
the compositions of this invention may be used in connection with
moist vaginal or oral mucosa and will become increasingly
lubricious upon exposure to the moisture therein.
Although anhydrous compositions are ordinarily perceived to
be irritating to the skin and mucous membranes, the gel and jelly
compositions of this invention are surprisingly non-irritating.
The compositions of this invention may be applied to the skin
or mucous membranes, preferably the vaginal or oral mucosa. The
compositions of this invention are preferably substantially
anhydrous and preferably contain at least one polyhydric alcohol.
We theorize that, when the polyhydric alcohols contained in
the compositions of this invention come into contact with water or
body moisture in humans, they react with the ambient water
molecules to cause an increase in temperature or generate warmth,
thus having a soothing effect on the tissues to which these
compositions are applied.
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
- 11 -
Surprisingly, and contrary to the general belief that
polyhydric alcohols in compositions are irritating to the mucosa,
compositions of this invention containing such polyhydric alcohols
have been found to be non-irritating. We theorize that the
hydrocolloids useful in the compositions and methods of this
invention swell when they come into contact with water, yielding a
lubrication coating gel. This coating physically blocks the
irritant action of other anhydrous elements of the compositions of
this invention. Furthermore, as the polyhydric alcohols useful in
the compositions of this invention are humectants and moisturizers,
when the hydrocolloids swell and form thin films over mucosal
tissues, the films retain the moisturizers on the surface of the
tissues. Thus, the compositions of this invention overcome dry
conditions such as vaginal dryness and mouth dryness caused by
various factors including menopause and aging, as well as various
disease conditions.
Thus, the compositions of this invention are very mild to the
skin and mucous membranes. The compositions of this invention are
soothing when applied to oral mucous membranes and may function to
relieve minor irritation of the mouth and throat.
The combination of polyhydric alcohols in the compositions of
this invention may also be used as a vehicle to solubilize
otherwise insoluble drugs, including, but not limited to,
antifungals, antibacterials, antivirals, analgesics, anti-
inflammatory steroids, contraceptives, local anaesthetics, hormones
and the like.
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
- 12 -
Preferably, the compositions of this invention are maintained
at an acidic pH. An acidic pH is very helpful for the maintenance
of healthy vaginal and oral flora, particularly for the maintenance
of Lactobacilli in the vaginal area. Conventional acids or buffers
are known to be insoluble in anhydrous compositions. Preferably,
the compositions of this invention contain an organic acid that is
soluble therein to maintain an acidic pH. More preferably, the
organic acid is lactic acid. Lactic acid is not only soluble in the
anhydrous compositions of this invention, it is a natural acid
l0 generated in human tissue and is very safe for use in the
compositions of this invention. Such an organic acid is
particularly useful as an acidifying agent that may assist in
lowering the pH of the tissues where the compositions of this
invention are applied. This will help maintain the natural acidic
environment of the mucosa and encourage the growth of appropriate
f lora .
The compositions of this invention may also preferably
contain an insulating agent which functions to preserve the
temperature increase by maintaining the heat within the composition
after it has been applied to the skin or mucosa. More preferably,
honey may be utilized as an insulating agent.
BRIEF DESCRIPTION OF THE DRAWINGS
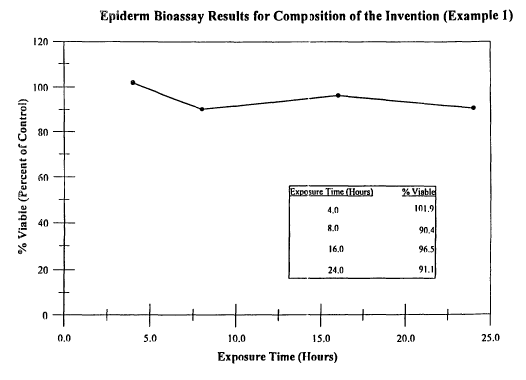
Fig. 1 is a graph depicting the % viable Epiderm cells vs
~5 Exposure Time using the composition of Example 1.
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
- 13 -
Fig. 2 is a graph depicting the % viable Epiderm cells vs
Exposure Time using the composition of Example 2.
Fig. 3 is a graph depicting the % viable Epiderm cells vs
Exposure Time using a State-of-the-Art non-irritating Product (K-Y
Liquid°) .
Fig. 4 is a graph depicting the % viable Epiderm cells vs
Exposure Time using a State-of-the-Art warming Product
(Prosensual°)
Fig. 5 is a graph comparing the Lubricity vs Time (Seconds)
of the composition of Example 1 and three leading Personal
Lubricants on the market.
DETAILED DESCRIPTION OF THE
PREFERRED EMBODIMENTS
The compositions of this invention are substantially
anhydrous, preferably containing less than about 20% water, more
preferably containing less than about 5% water and, most
preferably, containing less than about 3% water.
Preferably, the compositions of this invention contain at
least one polyhydric alcohol, and more preferably, two polyhydric
alcohols. Preferably, at least one of the polyhydric alcohols of
the compositions of this invention is a polyalkylene glycols or
others selected from the following group: glycerine, propylene
glycol, Dutylenes glycol, hexalene glycol or polyethylene glycol of
various molecular weight and the like and/or combination thereof.
More preferably, the compositions of this invention contain a
polyethylene glycol; most preferably, the polyethylene glycol may
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
- 14 -
be selected from the following group: polyethylene glycol 400 or
polyethylene glycol 300. The compositions of this invention should
contain polyhydric alcohols in an amount from about 80% to about
98% by weight of the composition.
The compositions of this invention should also preferably
contain one or more water-soluble cellulose-derived film-forming
polymers, gums, chitosans or the like. Preferably, such cellulose-
derived polymers are hydroxyalkylcellulose polymers. More
preferably, the hydroxyalkylcellulose polymer is selected from the
following group: hydroxyethylcellulose,
carboxyboxymethylcellulose, hydroxypropylcellulose and
hydroxypropylmethylcellulose and the like. Most preferably, the
hydroxyalkylcellulose polymer is hydroxypropylcellulose, such as
Klucel° which is available commercially from Hercules Incorporated
of Wilmington, Delaware. Most of the cellulose-derived polymers
are water soluble and insoluble in anhydrous solvents but for
hydroxypropylcellulose, which is completely soluble in the
anhydrous polyhydric portion of the compositions of this invention.
Preferably, the compositions of this invention contain from about
0.15% to about 0.6% by weight of hydroxypropylcellulose to yield
pourable gels and from about 1% to about 4% of
hydroxpropylcellulose to yield thixotropic jellies.
The compositions of this invention preferably also contain an
insulating agent. More preferably, the insulating agent should be
honey or esters of isopropyl alcohol and saturated high molecular
weight fatty acids such as myristic or palmitic acid,
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
- 15 -
e.g.,isopropyl myristate and isopropyl palmitate . The insulating
agent should be present in the compositions of this invention in an
amount of from about 1% to about 5% by weight of the composition.
The compositions of this invention are unexpectedly self-
preserving and may not require a preservative. However, a
preservative may be added to impart an additional guarantee against
microbial growth. A preservative may be selected from preservatives
known to those of skill in the art, including, but not limited to,
one or more of the following: methylparaben, benzoic acid, sorbic
acid, gallic acid, propylparaben or the like. The preservative may
be present in the compositions of this invention in an amount from
about 0.01% to about 0.75% by weight of the composition.
The compositions of this invention may also preferably
contain an ester. More preferably, the ester is a fatty acid ester.
Most preferably, the ester may include, but is not limited to:
isopropyl stearate, isopropyl myristate, isopropyl palmitate,
isopropyl laurate and the like. Most preferably, the ester is
isopropyl myristate.
The compositions of this invention may contain one or more
water-soluble cellulose-derived polymers, gums, chitosans or the
like. Such polymers contribute to the viscosity and bioadhesiveness
of the compositions of this invention. Preferably, such cellulose-
derived polymers are hydroxyalkylcellulose polymers. More
preferably, the hydroxyalkylcellulose polymer is
hydroxypropylcellulose or Klucel°, available commercially from
Hercules Incorporated, Wilmington, Delaware.
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
- 16 -
The polyhydric alcohols used in the compositions of this
invention are theorized to be useful as warming and heat-generating
agents. Honey functions as an insulating agent, protecting the
compositions from becoming too cold. The ester, preferably a fatty
acid ester, functions as an emollient and lubricant. The cellulose
polymer is useful as a viscosity building agent. The compositions
of this invention are unique in that they lubricate, warm and
soothe the tissues of the user, especially the oral and vaginal
mucous membranes, without conveying a feeling of cold. Moreover,
they are smooth and lubricating.
The compositions of this invention may be a liquid, a semi-
solid, or a solid depending upon the particular intended use
thereof. The compositions of this invention may be formulated as
syrupy liquid-gels, pourable gel or thick jellies. Preferably,
their viscosities should range from about 1,000 cps to about 7,000
cps for the gels and from about 60,000 cps to about 500,000 cps for
the jellies. The compositions of this invention may also be
formulated into soft or hard gelatin capsules, suppositories and
impregnated into fabrics or polymers.
The compositions of this invention may be used as personal
lubricants which convey a feeling of warmth. The feeling of warmth
generated by the compositions of this invention is soothing to the
skin or mucous membranes where they are applied. The compositions
of the invention also possess a sweet and pleasant taste, which is
of particular benefit when these compositions are used orally.
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
- 17 -
The compositions of this invention may also be used as
personal moisturizers, which convey a feeling of warmth when
applied to vaginal or oral mucosa. They may be used as
moisturizers which convey a feeling of warmth and relieve vaginal
dryness or dry mouth.
The compositions of this invention may also be used as a
vehicle to deliver medication or other treatment agents to
biomembranes including, but not limited to, hormones,
antimicrobials, antibacterials, antibiotics, non-steroidal anti-
inflammatory agents, spermicides, immunodilators, anaesthetics,
plant extracts, vitamins, corticosteroids or antifungal agents and
the like.
Antifungal agents are preferably azoles or imidazoles,
including but not limited to, miconazole, econazole, terconazole,
saperconazole, itraconazole, butaconazole, clotrimazole,
tioconazole, fluconazole and ketoconazole, vericonazole,
fenticonazole, sertaconazole, posaconazole, bifonazole,
oxiconazole, sulconazole, elubiol, vorconazole, isoconazole,
flutrimazole, tioconazole and their pharmaceutically acceptable
salts and the like. Other antifungal agents may include an
allylamine or one from other chemical families, including but not
limited to, ternafine, naftifine, amorolfine, butenafine,
ciclopirox, griseofulvin, undecyclenic acid, haloprogin,
tolnaftate, nystatin, iodine, rilopirox, BAY 108888, purpuromycin
and their pharmaceutically acceptable salts.
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
- 18 -
Another embodiment of the invention are compositions for
vulvovaginal or other mucosal use containing one or more
antibiotics. The antibiotic may be chosen from the group including,
but not limited to, metronidazole, clindamycin, tinidazole,
ornidazole, secnidazole, refaximin, trospectomycin, purpuromycin
and their pharmaceutically acceptable salts and the like.
Another embodiment of the compositions of this invention
include compositions for vulvovaginal or other mucosal use
containing one or more antiviral agents. Antiviral agents may
preferably include, but are not limited to, immunomodulators, more
preferably imiquimod, its derivatives, podofilox, podophyllin,
interferon alpha, reticolos, cidofovir, nonoxynol-9 and their
pharmaceutically acceptable salts and the like.
Still other embodiments of the compositions of this invention
are compositions that include one or more spermicides. The
spermicides may preferably include, but are not limited to,
nonoxynol-9, octoxynol-9, dodecaethyleneglycol monolaurate, Laureth
105, and Methoxypolyoxyethyleneglycol 550 Laurate and the like.
Still other embodiments of the compositions of this invention
are compositions containing antimicrobial agents. The antimicrobial
agents may preferably include, but are not limited to,
chlorohexidine gluconate, sodium polystyrene sulfonate, sodium
cellulose sulfate, silver particles of micro- and sub-micrometer
sizes, silver salts and other antibacterial agents known to the
art.
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
- 19 -
Yet other embodiments of the compositions of this invention
are compositions that may include local anesthetics. The local
anesthetics may preferably include, but are not limited to,
benzocaine, lidocaine, dibucaine, benzyl alcohol, camphor,
resorcinol, menthol and diphenylhydramine hydrochloride and the
like.
Compositions of the invention may also include plant extracts
such as aloe, witch hazel, chamomile, hydrogenated soy oil and
colloidal oatmeal, vitamins such as vitamin A, D or E and
corticosteroids such as hydrocortisone acetate.
Another embodiment of the compositions and methods of this
invention include compositions for vulvovaginal use containing one
or more hormones for treating a decrease in estrogen secretion in
the woman in need of estrogen replacement such as women with
vaginal atrophy. The hormones may preferably include, but are not
limited to, estrogen selected from the group consisting of
estradiol, estradiol benzoate, estradiol cypionate, estradiol
dipropionate, estradiol enanthate, conjugated estrogen, estriol,
estrone, estrone sulfate, ethinyl estradiol, estrofurate,
quinestrol and mestranol.
Another embodiment of the compositions and methods of this
invention include compositions for vulvovaginal use containing one
or more analgesics and/or nonsteroidal anti-inflammatory agents for
treating dysmenorrhea or mentrual cramping. The analgesics and
nonsteroidal anti-inflammatory agents may preferably include, but
are not limited to, aspirin, ibuprofen, indomethacin,
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
- 20 -
phenylbutazone, bromfenac, fenamate, sulindac, nabumetone,
ketorolac, and naproxen and the like.
In another embodiment of the compositions and methods of this
invention, the compositions may be useful for treating female
sexual dysfunction by themselves as they may serve to increase
blood flow to areas on which they are applied by increasing
temperature thereon. Alternatively, they may contain agents known
to those of skill in the art to treat female sexual dysfunction
(including different aspects of female sexual dysfunction such as
female sexual arousal disorder, hypoactive sexual desire disorder,
orgasmic disorder and the like) as well as those that treat
dyspareunia and/or vaginismus, or vulvodynia and to relieve pain
upon intercourse. Such agents include hormones such as estrogen,
prostaglandin, testosterone; calcium channel blockers, cholinergic
'15 modulators, alpha-adrenergic receptor antagonist, beta-adrenergic
receptor agonists, camp-dependent protein kinase activtors,
superoxide scavengers, potassium channel activators, estrogen-like
compounds, testosterone-like compounds, benzodiazepines, adrenergic
nerve inhibitors, HMG-CoA reductase inhibitors, smooth muscle
relaxants, adenosine receptor modulators and adenylyl cyclase
activators. Such agents include phosphodiesterase-5 inhibitors and
the like.
Yet another embodiment of the compositions and methods of
this invention include compositions for oral and vulvovaginal or
other mucosal use relates to a method of enhancing the absorption
of active agents from the applied compositions into the mucosal
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
- 21 -
membrane by increasing the composition and mucosal tissue
temperature via interaction of the polyhydric alcohols in the
compositions and moisture on the mucosa and subsequently released
heat.
Yet another embodiment of the compositions of this invention
include compositions for vulvovaginal use relates to compositions
and methods for preventing and/or treating dysmenorrhea by
intravaginal warming or heating. Preferably, the composition heats
the intravaginal area to a temperature preferably between about 37°C
and about 42°C, more preferably between about 38°C and about
41°C.
The compositions of invention for use in such a method may
optionally contain active agents such as analgesics and
nonsteroidal anti-inflammatory agents for dysmenorrhea treatment.
The composition of the invention may be administered directly into
the vagina by an applicator, or be impregnated into vaginal devices
such as tampon for intravaginal applications.
The compositions of this invention may be manufactured as a
coating of a tampon, or dispersing throughout the absorbent tampon
material, or enclosed inside as a core of a tampon. The
compositions of this invention for the warming tampon for
preventing and/or treating dysmenorrhea preferably include a
mixture of polyethylene glycols of various molecular weights
,produced by The Dow Chemical Company (Midland, MI) under the trade
names of CARBOWAX SENTRY PEG 300 NF, CARBOWAX SENTRY PEG 400 NF,
CARBOWAX SENTRY PEG 600 NF, CARBOWAX SENTRY PEG 900 NF, CARBOWAX
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
- 22 -
SENTRY PEG 1000 NF, CARBOWAX SENTRY PEG 1450 NF, CARBOWAX SENTRY
PEG 3500 NF, CARBOWAx SENTRY PEG 4000 NF, CARBOWAX SENTRY PEG 4600
NF, and CARBOWAX SENTRY PEG 8000 NF. The compositions of this
invention for dysmenorrhea prophylaxis and treatment may contain
one or more water-soluble cellulose-derived polymers and gums that
form gels around the polyhydric alcohols such as glycerin,
propylene glycol and polyethylene glycols thus reducing the
dissolution of the polyhydric alcohols, prolonging the solvation
heat release, and regulating the elevated temperature in the
preferred temperature range.
This invention also relates to a method of determining and
comparing relative amounts of irritation caused by particular
sources using the EpiDermTM Skin Model Assay as described in Example
1, such as compositions applied to skin or mucosal cells. The
following Example 1 exemplifies the use of the method of this
invention.
Example 1: EpiDerm~ Skin Model Assay to Test Irritation of
Lubricants
The method designated as EpiDermT"' Skin Model assay uses the
epithelial cells derived from human skin as target cells and is
commercially available from the MatTek Corporation. This assay is
described in Berridge, M.V., et al. (1996) The Biochemical and
Cellular Basis of Cell Proliferation Assays That Use Tetrazolium
Salts. Biochemica 4: 14-19. The test materials are applied directly
to the epithelial cell culture surface. This test has not
previously been used for determining toxicity of test materials.
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
- 23 -
The toxicity of the test material is evaluated on the basis of
relative tissue viability vs time. The actual Tissue Viability is
determined by NAD(P)H-dependent microsomal enzyme reduction of MTT
in control and test article treated cultures. The negative control
used in this assay was deionized water and the positive control was
Triton X-100. The exposed cell cultures were incubated for 4, 8, 16
and 24 hours and assayed for reduction of MTT. The data is
presented below in Figures 1 through 4 in the form of Relative
Survival (relative MTT reduction) versus Exposure Time. Relative
irritation in this assay is expressed by the percent survival rate
of epiderm cells over a period of 24 hours. Products with higher
relative survival rates are less toxic or less irritating while the
ones with lower survival rates are more toxic or irritating. The
survival rate of four compositions of this invention ranged between
81.3% and 90.3%, indicating that the compositions of this invention
are essentially non-irritating.
Figures 1 through 4 summarize the results of Epiderm Skin
Model Bioassay. The data is plotted as % Viable Cells vs the
Exposure Time ranging from 4 to 24 hours. Figures 1 and 2
represent the results for two compositions of this invention,
Composition 1 and Composition 2 respectively. Figure 3 represents
the results of K-Y° Liquid that is an established personal
lubricant on the market. K-Y° Liquid is established as safe and
nonirritating in animal and human testing and long-term human use
history. Results for K-Y° Liquid showed 100.3% viable cells after
24 hour of exposure (Figure 3).
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
- 24 -
Example 1 of the invention (Figure 1) and Example 2 of the
invention (Figure 2) showed 91.1% and 96.9% viable cells
respectively. Figure 4 shows the results of a warming composition
known to the trade. This product uses plant materials like
cinnamon, clove, ginger cloves and orange and others for a warming
sensation. The results show only 37.6% viable cells after 24 hours
of exposure to this product. This indicates that such compositions
will be irritating to the skin and mucous membranes. Compositions 1
and 2 of this invention, with 91.1% and 96.9% viable cells
respectively, will be practically nonirritating. Positive control
(Triton X-100) has only 22.4% viable cells at the 8-hour interval.
Example 2: Generation Of Warmth
The compositions of this invention are anhydrous and contain
one or more polyhydric alcohol. When combined with water, the
polyhydric alcohols used in the compositions of this invention
generate an increase in temperature that has a soothing effect on
the tissues these compositions are applied. In actual use the
compositions of the invention interact with the moisture of the
vaginal or oral mucosa, thereby increasing the temperature or
generating feeling of warmth.
The "Generation of warmth" data summarized in Table 1 below,
was generated by mixing 20 ml of each of the ingredients in
Composition 1 and Composition 1 of this invention with 20 ml of
water. The temperature of the product and that of water were
recorded before water was added to the product. After the addition
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
- 25 -
of water the mixture was mixed for two minutes and the actual
temperature was recorded. Glycerin, Propylene Glycol and Honey are
the ingredients in Composition 1. It is clear from Table 1. that
when mixed with water the temperature of the mixture rises by 9.0°
F for Glycerin, 13.5° F for Propylene Glycol, 17.0° F for
Polyethylene Glycol 400 and 12.5° F for composition Examplel of
this invention. The calculated rise in temperature for Composition
1, based on the rise in temperature and the % w/w quantity of each
individual ingredient in the Composition was 10.875° F. The actual
recorded temperature rise for Composition 1. was 12.5° F which is
1.625° F higher than expected which indicates that there is an
unexpected increase in temperature resulting from the Combination
of ingredients.
GENERATION OF WARMTH (RISE IN TEMPERATURE °F) DATA BY MIXING EQUAL
QUANTITY
OF EACH PRODUCT WITH WATER
Product TemperatureTemperatureAverage Actual Rise in
Name
of the of Water Expected TemperatureTemperature
Product (F)
(F) Temperature(F) (F)
(F) (Expected
Minus Actual)
Glycerin
Assay 69.0 71.0 70.0 79.0 9.0
Propylene
Glycol 72.4 71.0 71.7 85.2 13.5
Assay
Honey
74.0 71.0 72.5 74.0 1.5
K - Y Warm~
74.0 71.0 72.5 85.0 12.5
Isopropyl
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
- 26 -
Myristate 75.0 74.1 74.5 75.2 0.7
Polysoxbate
60
70.9 74.1 72.5 83.1 10.6
Polyethylene
Glycol
400
72.0 71.0 71.5 88.5 17.0
Calculated Rise in Temperature: In order to determine the expected
rise in temperature from each composition, the percentage of each
component in such composition was multiplied by the temperature
increase generated by such component alone to obtain its expected
contribution to the temperature increase. These values were added
together to calculate the total expected temperature rise. These
values were then compared with the actual temperature rise
generated by each composition. For example, the calculated rise in
temperature generated by the "K-Y Warm~" composition in the table
above was found as follows and compared with the actual temperature
rise to determine the unexpectedly higher generation of warmth of
the composition:
Propylene Glycol (50% of 13.5) - 6.75
Glycerin (45% Of 9.0) - 4.05
Honey (5% of 1.5) - 0.075
Total 10.875
Difference: 12.5 - 10.875 = 1.625
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
- 27 -
Example 3: Effect of Water Content on Generation of Warmth
On contact with moisture or water the heat of solution is
responsible for the warming action of the compositions of this
invention. There is a concern that accidental contamination with
water or prolonged exposure to excessive moisture, the warming
capacity of the product may be adversely effected. According to
this example, water was added to compositions of this invention
varying from about 1% to about 10% as outlined in Table 2 below.
The contents were thoroughly mixed and the samples were allowed to
stay at room temperature for 24 hour following which the generation
of warmth was determined as outlined in the following paragraph.
The results show that rise in temperature is proportionately
decreased depending on the quantity of water added but there is
still an 8.5°F increase in temperature at about 10% water addition.
The results of this example are set forth in Table 2 below.
Table 2: Effect Of water Content On Generation Of Warmth For K-Y
Warm~.
Product TemperatureTemperatureAverage Actual Rise in
Name of the of Water Expected TemperatureTemperature
Sample (F) Temperature(F) (F)
(F) (F) (Expected
Minus
Ac tual
)
No Water 73.80 70.00 71.90 83.50 11.60
1% Water 73.90 70.00 71.95 82.20 10.25
2% Water 72.30 70.00 71.95 81.70 9.85
3% Water 72.30 70.00 71.15 80.40 9.25
4% water 72.20 70.00 71.10 80.70 9.60
5% Water 71.60 70.00 70.80 80.40 9.60
6% Water 71.60 70.00 70.80 80.40 9.60
7% Water 71.50 70.00 70.75 80.20 9.45
8% Water 71.60 70.00 70.80 80.20 9.40
9% Water 70.90 70.00 70.45 79.50 9.05
10% Water 70 50 70.00 70.25 179.00 X8.50
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
- 28 -
Example 4: Perception Of Warmth In Human Use
A Human Use Study was conducted with 246 subjects. The data
generated by this study are summarized below in Table 2. The
subjects were asked to use compositions of this invention. They
were asked three questions regarding the perception of warmth while
using the product, as follows:
1. Does it warm on contact?
2. Does it feel warm?
3. Does it not feel cold?
The subjects were asked to register their response as
Excellent, Very Good, Good, Fair and Poor. The positive responses
are summarized in Table 2.
Table 3: PERCEPTION OF WARMTH IN HUMAN USE STUDY WITH 246
HUMAN SUBJECTS USING COMPOSITION EXAMPLE 1 OF THE INVENTION
QUESTION ASKED POSITIVE RESPONSE (%)
Warms on Contact I
Excellent 25.12
Very Good 31.88
Good 24.64
Total 81.64
Feels Warm
Excellent 30.88
Very Good 28.92
Good 25.98
Total 85.78
Does Not Feel Cold
Excellent 54.37
Very Good 29.61
Good 10.19
Total I 94.53 I
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
- 29 -
As set forth in Table 3 above, 81.64% of the subjects
registered a positive response that the product "warms on contact",
85.78% subjects felt that the product "feels warm" while 94.53%
subjects registered that the product "does not feel cold".
Example 5: Comparison Of Lubricity
Ahmad et al. in U.S. Patent No. 6,139,848, which is hereby
incorporated herein by reference, describe a method to test
lubricity of various personal lubricants known to the trade. In the
described test method, the lubricity of various marketed personal
lubricants was determined over a period of 300 seconds (5 minutes).
The lubricity data disclosed in this patent indicates that K-Y
Liquid° lubricant had a higher lubrioity and was longer lasting
during the 300 seconds test period than the competitive products.
The lubricity data set forth in U.S. Patent No. 6,139,848 has a
negative (-) sign during the "push" and positive (+) sign during
the "pull" phase of the experiment. Compositions of this invention
were tested using the lubricity test set forth in U.S. Patent No.
6,129,848. However, the test duration was suCCessfully extended to
16 minutes (960 seconds) and the data was treated to "curve-fit" to
eliminate the negative (-) sign. The lubricity data for the
composition 1 of this invention is compared with the data for K-Y
Liquid in Figure 5. The data indicate that Composition 1 of this
invention has a higher lubricity as compare to K-Y Liquid and that
Composition 1 maintains the high lubricity for an extended period
of 16 minutes (960 minutes) and is therefore longer lasting.
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
- 30 -
Example 6: Heat of Solution
The warming effect of the compositions of this invention is
believed to be caused by generating heat of solution, as opposed to
creating the conditions for exothermic reactions. Exothermic
reactions result in evolution of heat due to a chemical reaction
between two chemicals and are uncontrolled. Such an exothermic
chemical reaction may generate new products or chemical entities,
some of which may not be suitable for human tissues. In contrast,
when a solution is formed there is an energy change because of the
difference between the forces of attraction of unlike and like
molecules. Specifically, bonds are broken between molecules of the
each component being mixed and new bonds are formed between
neighboring molecules of the product mixture or solution. This
mechanism is different from a Heat of Reaction because there is no
chemical rearrangement of the constituent atoms to form products
from reactants. As can be seen from the following experiment,
maximum heat generated or the maximum rise in temperature is no
more than 18.8 °F, which makes these compositions very mild and
safe.
The solution process for the compositions of this invention
(COMPOSITION 15 of Example 9 below) in, for example, vaginal fluids
("X H20") can be represented by the following physical equation:
COMPOSITION 15 (l) + X HZO (1) ~ COMPOSITION 15 (X H20)
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
- 31 -
The designation "COMPOSITION 15 (X HZO)" represents that the product
is a solution of 1 (mol) of COMPOSITION 15 in X (mol) of H20.
Thus, using COMPOSITION 15, a composition according to this
invention, as a personal lubricant does not change the existing
amount of naturally occurring vaginal fluids. It simply forms a
solution with them.
The maximum temperature increase possible from the generation
of heat by use of the compositions of this invention may be
measured using thermodynamic principles. For example, Differential
Scanning Calorimetry (DSC) was employed to characterize the heat
released by the compositions of this invention when they come into
contact with water to form a solution. In this testing, the energy
released when a thin film of a particular composition was applied
to a thin film of water was measured. The results of a typical
test are presented in Figure 6. The area of the exothermic (i.e.,
negative) peak represents the total energy released during the
formation of a solution of the composition of this invention and
water. Table 1 summarizes the energy released for this series of
experiments.
Table 1: Summary of DSC Measurements of Heat Released By
COMPOSITION 15 / Water
Experiment # Composition of the Energy Released
Invention (mJ)
(mg)
1 17.85 398.878
2 22.5 355.108
3 28.32 267.229
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
- 32 -
Average 22.89 340.405
Standard Deviation 5.25 67.045
The energy release measured by the DSC is representative of the
maximum energy which would be seen on the surface of the vaginal
tissue. This is because the heat flux (energy flow) into the thin
film of water during the formation of the solution measured by the
DSC is equivalent to the heat flux (energy flow) which would be in
the fluid on the surface of the vaginal tissue. Therefore,
thermodynamics can be used to calculate the maximum possible
temperature rise as follows:
Qmax = Cpm OTmax (Equation 1)
where, Qmax represents the Maximum Energy Released during contact
(formation of solution) of Composition 15 and water; Cpm represents
Heat Capacity of Solution of Composition 15 and Water; and 4T",ax
represents Maximum Temperature Rise. Thus, rearranging Equation 1,
we can calculate 4Tmax, the Maximum Temperature Rise, based upon the
known or measured values of the Maximum Energy Released and the
Heat Capacity of Solution of Composition 15, as follows:
OTm~ = Qm~ / Cpm (Equat7.on 2 )
By assuming a normal distribution, the experimental results in
Table 1 can be used to arrive at a worst case estimate for the
maximum value of Qmax as follows
Qmax = Average Experimental Energy Release +
3 x (Standard Deviation of Experimental Energy Release)
/ (Average Quantity of Composition 15)
- ~ (340.405 mJ) + (3) ( 67.045 mJ) ~ / (22.89 mg)
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
- 33 -
- (541.539 mJ / 22.89mg)
(Equation 3)
(Using this as the upper limit represents the 99.73% upper
confidence limit for the normal distribution.)
In the case of Cpm, the smaller of the Cp for Composition 15 and the
Cp for Water can be used to arrive at a worst case estimate for its
minimum value. Since,
Cp (Composition 15) - 0.54 cal / (g - °C)
Cp (Composition 15) - 1.00 cal / (g - °C)
then,
Cpm (worst case minimum) = 0.54 cal / (g - °C)
(Equation 4)
Therefore, a worst case estimate of the maximum temperature
increase possible from the generation of heat by for Composition 15
can be arrived at by using the combining Equations 2, 3, and 4 as
follows:
2 0 OZ'max - Qmax / L'pm
((541.539 mJ)/(22.89 mg))/( 0.54 cal / (g - °C) x 0.23901
cal / J
- 10.5 °C or = 18.8 °F
Thus, the maximum heat released upon use of Composition 15 is, at
the most, about 10.5°C or 18.8 °F, a relatively small increase
in
heat, indicating that the temperature increase effected by the
compositions of this invention are safe and comfortable to the
user.
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
- 34 -
Example 7: Generation of Warmth
Compositions 10, 11 and 12 were tested in accordance with the
following procedure to determine the extent to which said
compositions generate warmth upon mixture with water. Data was
generated by mixing 20 ml of each composition with 20 ml of water.
The temperature of the composition and that of water were recorded
before water was added to the composition After the addition of
water the contents were mixed for two minutes and the actual
temperature was recorded. The results are set forth in the
following Table:
GENERATION OF WARMTH (RISE IN TEMPERATURE °F) DATA BY MIXING EQUAL
QUANTITY OF EACH COMPOSITION WITH WATER.
Product TemperatureTemperatureAverage Actual Rise in
Name
of the of:'Water Expected TemperatureTemperature
Product (F) Temperature(F) (F)
(F)
F) (Expected
Minus Actual)
Rise In
Temperature
For Compositions
For Compositions
Of The
Invention
Composition73.00 70.3 71.6 87.3 15.7
10
Composition73.00 70.3 71.6 83.2 11.6
11
Composition73.00 70.3 71.6 87.1 15.5
12
Rise In
Temperature.For
The Individual
Components
Of The
Compositions
Polyethylene72.0 71.0 71.5 88.5 17.0
Glycol 400
Propylene 72.4 71.0 71.7 85.2 13.5
Glycol
Glycerin 69.0 71.0 70.0 79.0 9.0
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
- 35 -
We calculated the rise in temperature For Compositions 10, 11 and
12
Composition 10
Propylene Glycol (38 % of 13.5) = 5.13
Polyethylene Glycol 400 (61.5% Of 17.0) = 10.45
Total: 15.58 °F
Composition 11
For Composition 11 the calculated Rise in Temperature is 15.58 °F
Composition 12
For Composition 12 the calculated Rise in Temperature is 15.15 °F
Calculated temperature for all three compositions is very close to
the Actual Rise in Temperature
Example 8: Comparison of Lubricity
Using the method to test and compare lubricity of various
personal lubricants set forth in Example 3 above, the lubricity of
the compositions of this invention was determined. The following is
the summary of the results:
The Gel compositions of this invention are as lubricating as
the aqueous gel compositions described in the US Patent No.
6,139,848 by Ahmad et al. In Figure 7, the lubricity of
commercially available KY° Jelly was measured both in its
commercially-available form and in a 1:1 dilution with water. Upon
dilution, the lubricity did not increase substantially.
The Jelly compositions of this invention are more lubricating
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
- 36 -
as compared to the state of the art aqueous jellies known to the
trade. Composition 14 of this invention was measured with respect
to lubricity as initially made and in a 1:1 dilution with water.
Surprisingly, its lubricity increases substantially (about four-
s fold) upon dilution. These data are represented in Figure 8.
Thus, the jelly compositions of this invention become more
lubricious or their lubricity is increased when these compositions
are diluted with water in a 1:1 ratio. Figure 9 demonstrates a
comparison of the lubricities of KY° Jelly and Composition l4 in
their initial forms. Figure 10 illustrates the lubricities of two
warming gel compositions of this invention, Compositions 13 and 14,
showing their high lubricities. Figure 11 shows KY* Ultragel and
diluted Composition 14.
Example 9: Compositions Of The Invention
The following compositions of this invention were made as
follows: first, propylene glycol and glycerin were mixed. A
preservative and the insulating agent were then added to the
mixture in the same container. The mixture was then heated to from
about 35° C to about 45 ° C to completely dissolve the
preservative.
The mixture was then cooled. The gel and jelly compositions were
made by mixing propylene glycol, polyethylene glycol and
hydroxypropylcellulose in a high-speed mixer at a temperature of
between about 60° C to about 70 ° C until a smooth gel or jelly
was
obtained. The resulting gel or jelly was cooled to a temperature
of between about 45° C and about 55 ° C and lactic acid was
added.
The mass was continuously mixed for about 15 minutes or until the
lactic acid was dissolved. The mass was then cooled to room
temperature.
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
- 37 -
Composition 1:
Propylene Glycol 50.00%
Glycerin 45.00%
Honey 5.00%
Composition 2:
Propylene Glycol 50.00%
Glycerin 20.00%
Isopropyl Myristate 27.00%
Polysorbate 60 3.00%
Composition 3:
Propylene Glycol 95.00%
Honey 5.00%
Composition 4:
Propylene Glycol 50.00%
Glycerin 20.00%
Isopropyl Myristate 29.50%
Klucel HF 0.50%
Composition 5:
Propylene Glycol 99.50%
Klucel HF 0.50%
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
- 38 -
Composition 6:
Propylene Glycol 49.80%
Glycerin 45.00%
Honey 5.00%
Preservative 0.20%
Composition 7:
Miconazole Nitrate 2.00%
Propylene Glycol 49.80%
Glycerin 43.00%
Honey 5.00%
Preservative 0.20%
Composition 8:
Fluconazole 2.00%
Propylene Glycol 49.80%
Glycerin 43.00%
Honey 5.00%
Preservative 0.20%
Composition 9:
Metronidazole 3.00%
Propylene Glycol 49.80%
Glycerin 42.00%
Honey 5.00%
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
- 39 -
Preservative 0.20°s
Composition 10 (Gel):
Propylene Glycol 38.00
Polyethylene Glycol 400 61.05
Lactic Acid 00.20
Hydroxypropylcellulose 0.75
Composition 11 (Jelly):
Propylene Glycol 37.00
Polyethylene Glycol 400 61.05
Lactic Acid 00.20
Hydroxypropylcellulose 1.75
Composition 12 (Gel):
Propylene Glycol 48.00
Polyethylene Glycol 400 51.30
Lactic Acid 0,20
Hydroxypropylcellulose 0.50
Composition 13 (Jelly):
Propylene Glycol 48.55
Polyethylene Glycol 4007 50.00
Lactic Acid 0.20
Hydroxypropylcellulose 1.25
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
- 40 -
Composition 14 (Jelly:
Propylene Glycol 98.55
Lactic Acid 0.20
Hydroxypropylcellulose 1.25
Composition 15 (Jelly):
Polyethylene Glycol 400 98.55
Lactic Acid 0.20
Hydroxypropylcellulose 1.25
Composition 16 (Gel):
Polyethylene Glycol 400 99.50
Lactic Acid 0.20
Hydroxypropylcellulose 0.30
Composition 17 (Gel):
Propylene Glycol 74.50
Glycerin 25.00
Lactic Acid 0.20
Hydroxypropylcellulose 0.30
Composition 18 (Gel):
Propylene Glycol 74.50
Polyethylene Glycol 400 25.00
Lactic Acid 0.20
Hydroxypropylcellulose 0.30
CA 02484329 2004-10-29
WO 03/092652 PCT/US03/13554
- 41 -
Composition 19 (Gel):
Propylene Glycol 69.50
Polyethylene Glycol 400 15.00
Glycerin 15.00
Lactic Acid 2.00
Hydroxypropylcellulose 0.30
Composition 20 (Jelly):
Propylene Glycol 73.55
Polyethylene Glycol 400 25.00
Lactic Acid 0.20
Hydroxypropylcellulose 1.25