Note: Descriptions are shown in the official language in which they were submitted.
CA 02484627 2004-11-03
WO 03/101586 PCT/AU03/00661
-1-
CELL RECOVERY
Background of the Invention
The present invention relates to a method and apparatus for recovering
selected cells.
Description of the Prior Art
The reference to any prior art in this specification is not, and should not be
taken as, an
acknowledgement or any form of suggestion that the prior art forms part of the
common general
knowledge in Australia.
It is often desirable to obtain cells having predetermined properties from a
group of cells or tissue
having a range of properties. One way in which this may be achieved is through
the use of flow
cytometry. Flow cytometry is used to quantitatively measure physical or
chemical characteristics
of particles in fluid samples, as they are presented, in single file, into a
focused light beam.
In this case, the cells are labelled with markers, such as fluorescent
markers, such that the markers
couple only to cells having the desired predetermined properties. The labelled
cells are then
injected into a stream of fluid flowing through the cytometer. A light beam is
focused onto the
stream of fluid, such that as the cells pass through the light beam, the
markers fluoresce, allowing
the cells having the desired properties to be detected. The cells may then be
sorted using either
droplet deflection or a mechanical sorter.
In the case of droplet deflection, a piezoelectric transducer is used to
create droplets of sheath fluid.
An electric field is applied to the drops to sort them in accordance with the
cells contained therein.
Alternatively, a mechanical capture tube or the like may be inserted into the
fluid flow to recover
cells contained therein, as described for example, in US Patent Number US-
5,030,002.
Typically however flow cytometers are sophisticated instruments that require
at least daily
alignment by a highly skilled operator. Setting up the apparatus is also
difficult and requires
complex calibration.
Furthermore these techniques can generally only be used to sort large numbers
of cell populations,
however it is somewhat limited when the number of the cells within the
population are low, or
when only small cultures are available. FACS machines can operate (sort) down
to around 1000
cells. The present invention intends to have the ability to operate down to
single cells
CA 02484627 2004-11-03
WO 03/101586 PCT/AU03/00661
However, as there are often only small numbers of cells having desired
properties, it is necessary to
have apparatus to detect, recover and isolate single cells in a fashion that
is non detrimental to the
cell. These cells are then available for further analysis.
Summary of the Present Invention
In a first broad form the present invention provides apparatus for recovering
selected cells from a
number of cells, the selected cells having predetermined properties, the
apparatus including:
a) A stimulation system;
b) A detection system;
c) A retriever; and,
d) A control system, coupled to the stimulation and detection systems and a
pipette, the
control system being adapted to:
i) Attempt to stimulate one or more of the cells using the stimulation system;
ii) Detect at least one stimulated cell using detection system, the stimulated
cell having
the predetermined properties; and,
iii) Recover a stimulated cell using the pipette.
The retriever is typically a pipette, although other devices for retrieving
the cells could be used.
The pipette is preferably coupled to a drive system, and includes an actuator
adapted to actuate the
pipette to thereby expel or draw in cells through a port. In this case, the
control system is typically
adapted to:
a) Operate the drive system to position port adjacent the stimulated cell;
and,
b) Recover the cell by operating the actuator to thereby draw the cell into
the pipette.
In this case, the drive system is typically a micromanipulator.
The detection system may be adapted to:
a) Determine the position of the stimulated cell; and,
b) Transfer an indication of the position of the stimulated cell to the
control system, the
control system being adapted to operate the drive system in accordance with
the indicated
stimulated cell position.
The stimulation system may be coupled to the pipette, thereby allowing the
stimulation system to
stimulate cells near the port.
CA 02484627 2004-11-03
WO 03/101586 PCT/AU03/00661
-3-
The control system is typically operated to:
a) Operate the drive system until a number of cells are detected by the
detection system;
b) Attempt to stimulate the cells; and,
c) Repeat steps (a) and (b) until one or more stimulated cells are detected.
The cells having the predetermined properties may be coupled to respective
markers, in which case
the stimulation system is typically adapted to stimulate the cells by
stimulating the markers.
However, direct stimulation of the cells may be possible in some
circumstances.
The markers may be fluorescent markers, in which case the stimulation system
typically includes a
radiation source for stimulating the fluorescent markers. However, other
markers, such as
magnetic markers, or the like, may be used.
The radiation source is typically a laser, although LED or other radiation
sources may be used.
Alternatively, the markers may be magnetic markers, in which case, the
stimulation system is
formed from a magnetic field generator adapted to generate a magnetic field.
The magnetic field can be adapted to attract stimulated particles towards the
retriever for recovery.
The stimulated particles can be drawn into the pipette under the action of the
magnetic field.
Additionally or alternatively, standard use of the pipette can be used.
The detection system may be formed from the magnetic field generator, with the
stimulated
particles being determined by the attraction of the particles to the magnetic
field.
The detection system typically includes:
a) An imaging system for generating images of one or more of the cells; and,
b) A processing system for detecting cells and/ox stimulated cells in the
images.
The imaging system is preferably being coupled to the drive system to thereby
generate images of a
region near the port. However, a separate respective drive system may be used.
The particles may be any form of particles, although the apparatus is
particularly suitable for use
with cells.
CA 02484627 2004-11-03
WO 03/101586 PCT/AU03/00661
-4-
In a second broad form, the present invention provides a method of recovering
selected cells from a
number of cells, the selected cells having predetermined properties, the
method including:
a) Attempt to stimulate one or more of the cells;
b) Detect at least one stimulated cell having the predetermined properties;
and,
c) Recover a stimulated cell using a retriever.
The retriever is preferably a pipette, although other retrievers could also be
used.
The pipette is typically being coupled to a drive system, and includes an
actuator adapted to actuate
the pipette to thereby expel or draw in cells through a port. The method
preferably includes:
a) Operate the drive system to position port adjacent the stimulated cell;
and,
b) Recover the cell by operating the actuator to thereby draw the cell into
the pipette.
Generally the method includes using a detection system to:
a) Determine the position of the stimulated cell; and,
b) Operate the drive system in accordance with the indicated stimulated cell
position.
The method typically includes:
a) Using an imaging system for generating images of one or more of the cells;
and,
b) Using a processing system for detecting stimulated cells in the images.
The method generally includes causing the control system to:
a) Operate the drive system until a number of cells are detected by the
imaging system;
b) Attempt to stimulate the cells; and,
c) Repeat steps (a) and (b) until one or more stimulated cells are detected.
The markers may alternatively be magnetic markers, in which case the the
method including
applying a magnetic field to the particles to thereby stimulate the particles.
The magnetic field can be adapted to attract stimulated particles towards the
retriever for recovery.
In this case, the stimulated particles being drawn into the pipette under the
action of the magnetic
field.
The method can include detecting stimulation of the particles by detecting the
attraction of the
particles towards the retriever.
CA 02484627 2004-11-03
WO 03/101586 PCT/AU03/00661
-5-
The method is preferably performed by apparatus according to the first broad
form of the invention.
In a third broad form the present invention provides a processing system for
controlling apparatus
for recovering selected cells from a number of cells, the selected cells
having predetermined
properties, the apparatus including a stimulation system, a detection system
and a retriever, the
processing system being adapted to:
a) Attempt to stimulate one or more of the cells using the stimulation system;
b) Detect at least one stimulated cell using detection system, the stimulated
cell having the
predetermined properties; and,
c) Recover a stimulated cell using the retriever.
The processing system is typically being adapted to perform the method of the
second broad form
of the invention, and operate as the control system of the first broad form of
the invention.
In a fourth broad form the present invention provides a computer program
product for recovering
selected cells from a number of cells, the selected cells having predetermined
properties, the
apparatus including a stimulation system, a detection system and a retriever,
the computer program
product including computer executable code which when executed by a suitable
processing system
causes the processing system to perform the method of the second broad form of
the invention.
In a fifth broad form the present invention provides a pipette system for
manipulating particles, the
pipette system including:
a) A pipette for containing fluid in use, the pipette including a port;
b) An actuator coupled to the pipette, the actuator being adapted to draw in
and/or expel fluid
through the port;
c) A radiation source; and,
d) A waveguide having a first end coupled to the radiation source and a second
end coupled to
the pipette adjacent the port to thereby allow radiation from the radiation
source to impinge
on particles positioned adjacent to the port in use.
The pipette system typically includes a detector adapted to detect radiation
emitted by the particle.
In this case, the detector is preferably coupled to the first end of the
waveguide, to thereby detect
radiation emitted from the particle.
The radiation source may be a laser.
CA 02484627 2004-11-03
WO 03/101586 PCT/AU03/00661
-6-
The waveguide can be a fibre optic cable, or alternatively may be formed from
the pipette, the
pipette including a shaped portion to allow the radiation from the radiation
source to enter the
pipette and pass along at least a portion of the pipette, the radiation being
emitted from the pipette
through the port.
The pipette system typically includes a controller adapted to perform at least
one of:
a) Activating the actuator to thereby cause fluid to be drawn in and/or
expelled through the
port; and,
b) Activating the radiation source, to thereby expose a particle to radiation.
The pipette system typically includes a drive system adapted to move the
pipette system to be with
respect to a fluid filled container to thereby allow particles to be
positioned in or removed from
fluid in the container.
The drive system may be coupled to a controller, the controller being adapted
to recover particles
having predetermined properties from the container by:
a) Positioning the pipette system such that the port is adjacent to a
particle;
b) Activating the radiation source to thereby expose the particle to
radiation;
c) Detect any radiation emitted by the particle;
d) Determine if the particle has the predetermined properties in accordance
with the detected
radiation; and,
e) In accordance with a successful comparison, activate the actuator to
thereby draw fluid into
the pipette through the port, thereby recovering the particle.
The controller is preferably formed from a suitably programmed processing
system.
In a sixth broad form the present invention provides a pipette system for
manipulating particles, the
pipette system including:
a) A pipette for containing fluid in use, the pipette including a port;
b) An actuator coupled to the pipette, the actuator being adapted to draw in
and expel fluid
through the port, the actuator including:
i) A fluid reservoir;
ii) A flexible tube coupling the pipette to the fluid reservoir;
iii) An arm positioned so as to partially compress the tube;
iv) An actuator drive system adapted to move the arm so as to perform at least
one of:
(1) Further compressing the tube to thereby expel fluid from the port; and,
CA 02484627 2004-11-03
WO 03/101586 PCT/AU03/00661
_ '7 _
(2) Decompressing the tube to thereby draw fluid in through the port.
The actuator drive system typically includes:
a) A first actuator drive for moving the arm with respect to the tube; and,
b) A second actuator drive formed from an arm end portion, the arm end portion
being in
contact with the tube in use, the second actuator drive being adapted to cause
the arm end
portion to expand or contract.
The pipette system typically includes a controller coupled to the actuator
drive system, the
controller being adapted to operate the actuator drive system to thereby draw
fluid in or expel fluid
through the port.
The pipette system preferably includes a drive system adapted to move the
pipette system to be
with respect to a fluid filled container to thereby allow particles to be
positioned in or removed
from fluid in the container.
The drive system is typically coupled to the controller, the controller being
adapted to recover
particles from the fluid by:
a) Positioning the pipette system such that the port is adjacent to a
particle; and,
b) Activate the actuator drive system to thereby draw fluid into the pipette
through the port,
thereby recovering the particle.
The tube may be formed from silicon tubing.
Typically the pipette system according to the sixth broad form of the
invention is a pipette
according to the fifth broad form of the invention.
In a sixth broad form the present invention provides a pipette system for
manipulating particles, the
pipette system including:
a) A pipette for containing fluid in use, the pipette including a port;
b) An actuator coupled to the pipette, the actuator being adapted to draw in
and/or expel fluid
through the port; and,
c) A magnetic field generator for exposing particles to a magnetic field.
The magnetic field generator may be formed from a solenoid, or the like.
PCT/AU03/00661
CA 02484627 2004-11-03
Received 17 March 2004
_g_
The pipette can include a nozzle, in which case the solenoid is formed from a
graphite layer and a
number of associated windings arranged on the nozzle.
The pipette system may include a controller adapted to perform at least one of
a) Activating the actuator to thereby cause fluid to be drawn in and/or
expelled through the
port; and,
b) Activating a current supply coupled to the solenoid, to thereby expose the
particles to a
magnetic field.
The pipette system typically includes a drive system adapted to move the
pipette system to be with
respect to a fluid filled container to thereby allow particles to be
positioned in or removed from
fluid in the container. .
Brief Description of the Drawings
An example of the present invention will now be described with reference to
the accompanying
drawings, in which: -
Figure 1 is a block diagram of an example of apparatus for implementing the
present invention;
Figure 2A is a schematic diagram of the pipette of Figure 1;
Figure 2B is a schematic diagram of the operation of the actuator of Figure
2A;
Figure 2C is a schematic diagram of a first example of the pipette of Figure
2A modified for use
with a bladder;
Figure 2D is a schematic diagram of a second example of the pipette of Figure
2A modified for use
with a bladder; '
Figure 3 is a schematic diagram of the stimulation system of Figure 1;
Figure 4 is a schematic diagram of the apparatus of Figure 1;
Figures SA to SC are a flow chart of the process implemented by the apparatus
of Figure l;
Figures 6A to 6E are schematic diagrams of cells in the selection and recovery
wells of Figure 4;
Figures 7A and 7B are schematic diagrams of cells being drawn into and
expelled from the pipette
of Figure 2;
Figure 8 is a schematic diagram of the distribution of cells into a well
plate;
Figure 9 is a schematic diagram of the pipette nozzle holding a number of
cells;
Figure 10 is a schematic diagram of an example of an alternative pipette
actuator; and,
Figure 11 is an example of the pipette of Figure 2 modified to include an
electrode;
Figure 12 is an example of a modified pipette; and
Figure 13 is an example of a suitable cutting tool.
AMEi'~1~."?.E'~ urH~~l'~
lP~'~/AU
CA 02484627 2004-11-03
WO 03/101586 PCT/AU03/00661
_g_
Detailed Description of the Preferred Embodiments
An example for apparatus suitable for implementing the present invention will
now be described
with reference to Figures 1.
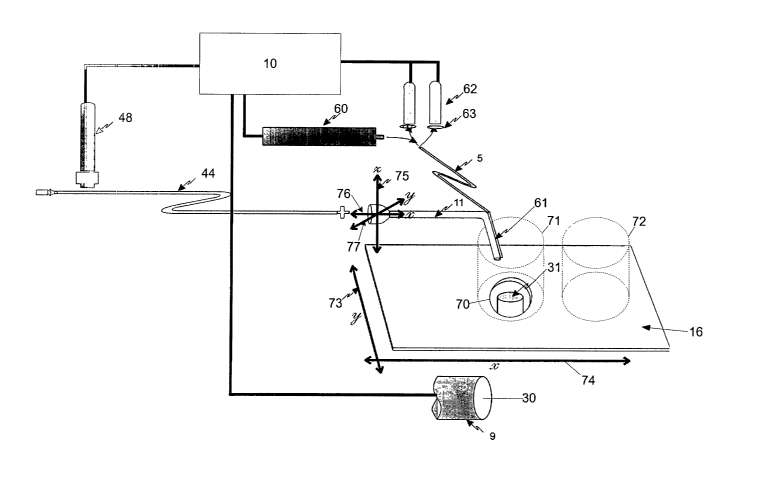
As shown in Figure 1, the apparatus includes a processing system 10 coupled to
an imaging system
11, a first drive system 12, a second drive system 13 and a stimulation system
14. The first drive
system 12 is coupled to a pipette 15, with the second drive system being
coupled to a stage 16, as
shown.
The processing system 10 includes a processor 20, a memory 21, an input/output
(I/O) device 22,
an image interface 23, a drive interface 24, and a stimulation interface 25,
coupled together via a
bus 26. The processing system may therefore be any one of a number of
processing systems, such
as a suitably programmed computer, specialised hardware, or the like.
In any event, the I/O device typically includes a display, such as a computer
monitor or the like, a
keyboard, and one or more other input devices such as a mouse, joystick,
trackball or the like.
The imaging system 11 includes a camera 30 such a CCD camera or the like which
is coupled to a
microscope 31. The imaging system 11 is connected to the processing system via
the image
interface 23.
In use, the drive systems 12, 14 are coupled to the processor via the drive
interface 24, thereby
allowing the processor 20 to control motion and operation of the pipette 15
and the stage 16, as will
be described in more detail below. Similarly, the stimulation system 14 is
coupled to the
stimulation interface 25, to allow the stimulation system to be activated, as
will be described in
more detail below.
In use, this allows cells having predetermined properties to be recovered from
a group of cells held
in suspension in a suitable container. In order to achieve selection the cells
are labelled with
markers, which are adapted to adhere and or permeate only the cells having the
required
predetermined properties. The processing system 10 can then activate the
stimulation system 14 to
stimulate the marker cells and thereby identify the cells having the
predetermined properties.
It will be appreciated that this may be achieved in a number of ways depending
on the
characteristics of the markers, and the stimulation system. Thus for example,
the markers could be
magnetic markers, with the stimulation system being adapted to generate a
magnetic field. This
CA 02484627 2004-11-03
WO 03/101586 PCT/AU03/00661
-10-
could be adapted to cause movement of the markers, and hence the cells having
the predetermined
properties, thereby allowing the cells to be identified. Alternatively,
optical markers may be used,
as will be described in more detail below.
In any event, once the cells having the predetermined properties have been
identified, the
processing system 10 can then control the pipette 14 to remove cells from the
well 40. This rnay be
achieved automatically or manually in accordance with input commands received
from the user via
the I/O device 22.
In order to achieve this, the processor 20 executes appropriate application
software, which is stored
in the memory 21, to control the operation of the apparatus.
A detailed example will now be described with reference to Figures 2 to 7.
The pipette 14 is shown in more detail in Figure 2A. As shown, the pipette is
formed from a glass
nozzle 40 having a port 41. The glass nozzle includes a female coupling 42
that is adapted to
cooperate with a male coupling 43 on a flexible tube 44. In use, the tube 44
is connected via a
stopcock 45 and a reservoir 46 to a pump 47. An actuator 48 is positioned
adjacent the flexible
tube 44, to allow the tube to be clamped as shown in Figure 2B.
It will be appreciated from this that any form of actuator, such as a
solenoid, may be used.
However, in this example, the actuator is formed from a threaded screw drive
49, coupled a DC or
stepper motor 50, which forms part of the drive system 12. In use, this allows
the actuator to be
moved in the direction of the arrow 51, an amount of ~Smm.
The actuator tip can have a piezo electric stack 52 coupled thereto, to allow
fine control
(displacement of ~ 20~m) of the end of the actuator. Again, the piezo stack
forms part of the drive
system 12.
In use, the pipette is loaded with a suitable fluid medium by placing the port
41 into a container that
has sufficient fluid to fill the system. The pump or other such means of
drawing fluid through the
system 47 is activated and fluid is drawn through the pipette. When the system
is loaded and there
are no air bubbles present in the tubing, the stopcock 45 is closed to prevent
further fluid flow, and
the pump 47 turned off.
Whilst the port 41 is still immersed in the fluid medium, the actuator 48 is
adjusted such that the
CA 02484627 2004-11-03
WO 03/101586 PCT/AU03/00661
-11-
silicon tubing is compressed to about half its diameter, as shown in Figure
2B. Thus, in use, with
the port 41 positioned in fluid in a well causing the actuator 48 to move in
the direction of the
arrow 51 compresses or releases the tubing 44 which, in turn, either expels or
draws in fluid
through the port 41. This allows cells to be recovered from a well, as will be
explained in more
detail below.
Variations on this design are shown in Figures 2C and 2D. In these examples,
the actuator 48 is
positioned adjacent a bladder 44A provided in the flexible tube 44. In this
case, the bladder has a
larger cross sectional area than the tube and will therefore contain a greater
volume of fluid per unit
length compared to the tubing 44. This has two main benefits. In particular,
the larger cross
sectional area provides for a greater range of movement of the actuator. This
coupled with the
increased fluid volume in the bladder allows for a greater amount of fluid to
be displaced when
compared to the action of the actuator on the tube 44.
As a xesult this provides greater control over the amount of fluid expelled or
drawn in through the
aperture 41, allowing for greater accuracy in retrieving individual cells
using the pipette.
In this instance, it will be appreciated that by providing sufficient liquid
in the bladder, it is not
necessary to provide the stopcock 45, the reservoir 46 or the pump 47, as
shown in Figure 2C. In
particular, the bladder and pipette can be filled, with an amount of fluid
being expelled from the
bladder before the bladder is positioned so as to cooperate with the actuator,
thereby allowing the
actuator position to be adjusted to allow fluid to be drawn in or expelled
through the aperture 41.
Alternatively, the bladder can be connected to a stopcock 45, reservoir 46 and
pump 47, by a tube
44B, as shown in Figure 2D.
For the remainder of the description, the example will focus on the use of the
actuator on the tube
44, although it will be appreciated that the techniques will also apply to the
actuator acting on an
appropriate bladder.
In this example, the stimulation system 14 is coupled to the pipette 15, as
shown in more detail in
Figure 3. As shown, the stimulation system includes a radiation source 60,
such as a UV burner
with suitable filters, a laser, or the like, coupled to an optical fibre 61.
The optical fibre is coupled to the pipette nozzle 40, using appropriate
fixing means, such as a
rubber tube (not shown). The optical fibre is also coupled to detectors 62,
such as photo-diode
CA 02484627 2004-11-03
WO 03/101586 PCT/AU03/00661
-12-
tubes, via suitable filters 63, to detect emissions from cells, as will be
explained in more detail
below.
In use, the system can select and remove individual cells using the pipette 15
from a group of cells
held in suspension.
In order to achieve this, the apparatus is arranged as shown schematically in
Figure 4. As shown,
the stage 16 includes an aperture 70, having the microscope 31 mounted
therein. From this it will
be appreciated that the microscope 31 is typically an inverted microscope.
In use the stage 16 is adapted to receive a selection well 71 containing the
cells to be recovered.
The stage may also receive a recovery well 72 for receiving the recovered
cells. In use, the
selection well 71 is positioned on top of the aperture 70, to allow the camera
30 to obtain an image
of the inside of the selection well 71, via the microscope 31. In use, the
processing system 10 is
adapted to control the drive system 14, to cause the stage to be move in the
directions shown by the
arrows 73, 74.
This allows a representation of the contents of a selected well can be
captured by the processing
system using the image interface, which is typically a frame grabber or the
like. Images may then
be used by the processing system to control the drive system 12 and the
stimulation system 13.
Additionally or alternatively, images may be displayed to a user using the I/O
device 22.
The pipette is positioned adjacent the stage 16 as shown, to allow the nozzle
to be inserted into the
well 71. The pipette is coupled to the drive system 12, to allow the pipette
to moved with respect
to the well, as shown by the arrows 75, 76, 77. Accordingly, the drive system
12 typically includes
a micromanipulator system having three independently controlled axis with
resolution tolerances
and repeatabilities of <S~m. This system is controlled by dedicated software
executed by the
processor 20.
An example of the method of operation of the apparatus of Figure 4 to recover
selected cells will
now be described in detail with reference to Figures SA, SB and SC.
In particular, the process involves obtaining a group of cells at step 200,
with at least some of the
cells having predetermined properties that are of interest to the user of the
apparatus.
At step 210 the user labels the cells with fluorescent markers, such that each
cell having the
CA 02484627 2004-11-03
WO 03/101586 PCT/AU03/00661
-13-
predetermined properties becomes labelled with a respective marker, whilst
cells having different
properties do not. As a result of this, only cells having the predetermined
properties are labelled
the fluorescent markers.
At step 220 the labelled cells are placed in the selection well 71 above the
aperture 70, as shown in
Figure 4. It will be appreciated therefore that the cells may therefore be
labelled with the markers
whilst the cells are held in suspension in the selection cell 71, allowing the
selection well to be
placed above the aperture as required.
In any event, the processing system 10 then uses the camera 30 to obtain an
image of the cells
within the selection well 71, at step 230, using the image to determine the
position of the cells at
step 240. This is achieved by having the imaging system 30 generate a sequence
of images of the
inside of the selection well 71. The image interface then selects an
appropriate one of the images
and transfers this to the processor 20. The processor 20 will then analyse the
image to determine
the position of the cells. In particular, the processor 20 will typically use
edge detection software
to detect edges in the image representing the edge of the cells in the
solution.
At step 250 the processing system 10 activates the drive system 12 causing the
pipette 15 to be
positioned in the selection well 71, with the port 41 adjacent to and above a
selected cell. In
particular, the pipette 15 is positioned such that radiation emitted from the
fibre optic cable 61
impinges on the selected cell.
At step 260, the processing system 10 activates the laser 60, causing
radiation to be emitted from
the fibre optic cable 61. In particular, the laser is activated such that the
radiation from the laser
impinges on the selected cell. It will be appreciated that if the cell has the
predetermined
properties, the marker bound to the cell will fluoresce under the influence of
the radiation. An
example of this is shown, for example in Figure 6A, in which a selected cell
80, amongst other
cells 81, is fluorescing.
At step 270, the processing system uses the detectors to detect any
fluorescent markers, by
examining for fluorescence in the image. If no fluorescence is detected at
step 280, then this
indicates that no marker has been exposed, and hence that the cell does not
have the predetermined
properties.
Accordingly, the processing system moves the position of the pipette 15 and
hence the position of
the fibre optic cable 61, such that different cells are positioned adjacent
the end of the fibre optic
CA 02484627 2004-11-03
WO 03/101586 PCT/AU03/00661
-14-
cable. The processing system then repeats step 260 to 280, so that different
cells are exposed to
radiation.
This process is repeated until a fluorescent marker is detected, allowing the
processing system 10 to
select a cell that is suitable for recovery.
At step 300 the processing system 10 determines the position of the selected
cell. At step 310 the
processing system 10 positions the port 41 adjacent the selected cell. This is
achieved by using
feedback to monitor the position of the port 41 as the drive system 12 is
activated, as will be
appreciated by those skilled in the art.
At step 320 the processing system 10 activates the actuator 48, as described
above, to draw fluid
into the port 41. At this stage, the drawing in of the fluid should cause the
cell to be drawn into the
pipette nozzle 40. At step 330, the processing system operates to determine if
the cell has entered
the nozzle 40.
This could be achieved for example by having the camera 30 image the inside of
the pipette nozzle
40. Alternatively, the processing system 10 may be adapted to track the cells
in the selection well
71, by comparing subsequent images captured by the camera 30. This allows the
processing
system to track movement of the cells and determine when a respective cell has
been removed from
the selection well 71 and hence is contained within the pipette nozzle 40.
If it is determined that the cell is not in the nozzle at step 340, the
processing system 10 returns to
step 310 to reposition the pipette port adjacent the selected cell. The
processing system 10 then
repeats steps 310 to 340 until it is determined that the cell is in the nozzle
40.
At step 350 the processing system determines if any other cells are also
contained within the nozzle
40. This may occur for example if two cells are positioned adjacent each other
in the selection well
71. In particular, when the fluid is drawn into the pipette nozzle 40, this
can cause multiple cells to
be drawn in through the port.
It will be appreciated that this may be desirable if all the cells in the
nozzle have the predetermined
properties, in which case the processing system can simply move onto step 380.
However, if cells
that don't have the predetermined properties are recovered, this could
contaminate the group of
cells that are eventually recovered.
CA 02484627 2004-11-03
WO 03/101586 PCT/AU03/00661
-15-
Accordingly if the processor determines that more than one cell is included in
the pipette at step
360 the processing system 10 will attempt to remove one of the cells at step
370. This can be
achieved by repeatedly operating the pipette to cause the pipette to
repeatedly draw in and expel
fluid via the pipette aperture 41. Agitation of the fluid medium and repeated
movement of the cells
through the pipette aperture 41 will usually allow a cell to be separated from
surrounding cells.
An example of this is shown in Figure 7A, which shows the hydrodynamic stream-
lines 83 as fluid
is expelled from the pipette aperture 41. As shown, the hydrodynamic stream-
lines, which
represent lines of constant force, spread out away from the pipette aperture
41. Similarly, as the
selected cell and unwanted cell 80, 81, are entrained in the fluid flow, this
will tend to cause the
cells 80, 81 to separate as they are expelled away from the pipette aperture
41.
Accordingly, repeated activation of the pipette allows the cells 80, 81, to be
separated, allowing the
selected marked cell to be collected alone.
At step 380 the processing system 10 re-positions the pipette in a recovery
well 72 and activates the
pipette to expel the cell into the recovery well 72 at step 390.
It will be appreciated from this that this provides a system for automatically
recovering cells having
predetermined properties. In particular, the apparatus can be provided with
cells in the selection
well 71 and then left to operate to automatically remove cells 80 having the
desired properties, to
the recovery well 72, as shown in Figure 6B.
By repeating this procedure, this allows a large number of cells 80 having
predetermined properties
to be recovered to the recovery well 72. During this procedure, the processing
system 10 can be
adapted to distribute the recovered cells 80 in a predetermined pattern
throughout the recovery
well, as shown for example in Figure 6C, or Figure 6D.
Alternatively, individual cells may be positioned in different recovery wells,
as will be appreciated
by persons skilled in the art. An example of this is shown in Figure 8, in
which the cells 80 are
distributed into a well plate 84, including a number of recovery wells 85
arranged in a grid like
fashion. This allows the cells 80 to be positioned in the recovery wells 85,
either individually, or
with multiple cells per recovery well, as shown.
In general, the recovery well will include a growth medium to encourage growth
of the recovered
cell. However, the recovery well may instead include a localised section of
the culture/tissue, as
CA 02484627 2004-11-03
WO 03/101586 PCT/AU03/00661
-16-
shown for example at 84 in Figure 6E. Micro-injecting the recovered cell
directly into the culture,
as shown in Figure 6E, can further aid cell recovery.
A particular benefit of this process is that cells are recovered on an
individual basis. This allows
cells having very strict criteria to be collected. As cells are collected on
an individual basis, this
prevents the opportunity of contamination of a sample cell in the recovery
well by cells not having
the required properties. Furthermore, this allows a larger number of cells to
be recovered
automatically.
Variations
A number of variations on the above are possible.
Stimulation
It will be appreciated that it may not be necessary to use markers, if the
cells having the
predetermined properties can be distinguished from other cells in the group
using some other
techniques. Accordingly, if the cells have different properties, this would
allow direct stimulation
of the cells to distinguish those having the required predetermined
properties.
These properties rnay include optical and magnetic properties. However, in
addition to this, other
properties, such as dimensional properties may also be used to distinguish the
cells. In this
instance, the stimulation will involve using laser measurements to allow the
dimensions of the cells
to be determined.
Pipette
It will be appreciated as well that the pipette can be used to provide
additional functionality. Thus,
for example, the pipette could be used to remove fluid from a well and replace
it with fresh /
different media.
A further variation is for the processing system to collect a number of cells
80 having the
predetermined properties from the selection well 71, with the cells being
stored in the nozzle 40, as
shown for example in Figure 9. The cells can then be placed into one or more
recovery wells 72,
individually as required.
A number of other pipette modifications are also shown in our copending patent
application
entitled "Cell Fusion" and persons skilled in the art will appreciate that
these modifications may be
incorporated with each other.
CA 02484627 2004-11-03
WO 03/101586 PCT/AU03/00661
-17-
Thus for example, the pipette system may use an alternate actuator to the
actuator 48 shown in
Figure 2. This may include a piezo-electric actuator, shown for example in
Figure 10.
As shown, the actuator is formed from a housing 90 defining a chamber that is
divided into two
portions 91A, 91B by a piezo-electric element 92, as shown. The chamber 91B is
coupled by a
port 93 to the flexible tube 44, of the pipette shown in Figure 2.
In use, the chamber 91B, the port 93, and the flexible tube 44, and the nozzle
40, are filled with
fluid, with the chamber 91A being filled with air and sealed. Applying a
current to the piezo-
electric element 92, via leads 94, causes the element to move, with the
direction of movement
depending on the polarity of the applied current.
Thus, in use, with the pipette port 41 positioned in a fluid filled well,
causing the piezo-electric
element 92 to move in the direction of the arrow 95 will increase the volume
of the chamber 91B,
thereby causing fluid to be drawn through the port 41. Similarly, causing the
piezo-electric
element 92 to move in the direction of arrow 96 will decrease the volume of
the chamber 91B,
thereby causing fluid to be expelled through the port 41.
Accordingly, the pipette can be activated to draw in or expel fluid through
the port 41 depending on
the polarity of the current applied to the leads 94. Accordingly, in use, the
leads 94 are coupled to
the processing system 10, to allow a suitable signal to activate the pipette
as required.
Similarly, the pipette may be adapted to incorporate an electrode, for use in
apply an electric field
to the cell, as used for example in cell dielectropherisis (DEP). And cell
fusion techniques. An
example of this shown in Figure 11.
As shown, an electrode 100 formed from a cylindrical tube 101 is coupled to
the nozzle 40 of the
pipette 40, such that the port 41 is contained in the tube 101. In use, the
pipette may be used
substantially as described with respect to the pipette of Figure 2.
Additionally however, the electrode 100 can be coupled to a field generator
102, which is also
coupled to a second electrode 103, as shown by the leads 104. In use, the
electrodes 100, 103
cooperate to allow electric fields to be applied to one or more cells 105,
positioned therebetween.
It will be appreciated that the electrode 103 may be formed from an electrode
coupled to another
pipette.
PCT/AU2003/00661
CA 02484627 2004-11-03 Received OS April 2004
-18-
~yia~netic Labeliin~
As mentioned above techniques exist for labelling cells that allows them to be
magnetically sorted.
1n this example, small metal beads are used, as markers to identify cells of
interest. This is achieved
by ensuring that cells having desired properties can be fused to the beads and
thereby extracted
from a mixture of cells.
This can be achieved for example by coating tl~e beads with an arifiibody of
interest and then mixing
the beads into a culture of cells. Cells that axe expressing the appropriate
receptor on the surface
X 0 bind to the beads. The culture is then filtered tl~xough a tube, placed in
an external magnetic held
containing thousands of small beads that attract and hold tire labelled cells,
whilst allowing the
unlabelled cells to be washed through and discarded. Once the external
magnetic field is removed
the bound cells can then be washed through the tube and isolated as desired.
It. will be appreciated that this may be achieved on a smaller scale using a
pipette modified to
incorporate an electromagnet.
An example of a suitably modified pipette will now be dCS~ibed with reference
to Figure 12. ltt
this example, the pipette shown generally at l I0 includes a graphite layer
111 positioned around
the pipette nozzle 1I2. A number of caper windings 113 are provided around a
graphite core to
form an electromagnet. In use the copper windings are coupled to a DC power
'supply shown
generally at 114, so that the windings act as a solenoid to generate a
magnetic ~xeld represented by
the field lines 1 ! 5.
The copper windings may be provided in a number of layers depending on the
implementation, and
may be embedded in a layer of epoxy in order to prevent electrolysis from
occurring. '.fE~e graphite
layer 111 acts as a paratuagtletiC substance increasing the strength of the
magnetic field within the
pipette.
1'he ends of the wire arc coimected to a variable TaC power supply and a
resister (R). Fussing a
current through the wire (taking account of Lenz's Law) will induce a magnetic
field, flit strength
of which is proportional to the applied laC Voltage (V), as given by the
equation:
~ = n~ - nuY
where: n = the nuntbcr of turns per unit Itngth
AMENDED SHEET. ~ -
____ _ ._ fPEA/AU
PCT/AU2003/00661
CA 02484627 2004-11-03 Received OS April 2004
_19_
tt = the permeability of free space.
Tn use, the pipette is positioned near a number of cells which may suspended
in a fluid medium or
resting on a substrate 116 as shown at 117. In this case, at least some of the
cells are attached to
appropriate magnetic marlaers, such as the beads outlined above.
In use, the metal particles, and hence the cells they are attached to, will be
attracted into the
magnetic field and can therefore be dxawn into the pipette in the normal way.
This allows ceps
coupled to the magnetic markers, and hence colts having ctrtain properties to
be selected.
It will be appreaiat~ed that cells with a higher dtnsity of receptors (a
higher number of magnetic
marl<crs), should have a larger force exerted on them than Lolls with less
receptors for the same
magnetic field strength. Therefore as the )=?C voltage is ini;reased, a larger
number of cells should
' be drawn into the magnetic fselds influence. This field gradient can allow
for a further sorting
I S aritGrxa.
In order to ensure no wanted cells have been collected, it is possible to
flush out the pipette by
Cxpclling fluid from the nozzle. In this case, airy cells not bound magnetic
markers will be
expelled from the pipette together with the fluid, whilst the cells bound to
markers will be held in
plane by the action of the magnetic field. In this case, when the selected
cells are to be expelled,
the magnetic field can be deactivated,.allawing the cells and attached markers
to be expelled in the
normal way.
A further development that earl be utilised within the examples described
above is for a cuttit~ final
to bG provided to allow cells to be cut, as well as to allow cells that hav'c
adhered to the well surface
or electrodes to be released. An example of a suitable cutting tool is shawr'r
in figure 13. As
shown, the.cutting toot includes a support post 120 having a blade 121
pivotally mounted thereto
by a hinge 122 or other appropriate conneotion.
In use, the post is coupled to a micro manipulator (not shown), to allow the
post to be positioned
within the rcspcative well. The post can be rotated as shown by the axraw 123,
allowing the blade
to be positioned above a cell to be cut. If the cell is a free cell 124, the
cell will generally be held in
place using a pipette, or other suitable manipulator, as shown at 125.
tance positioned, the post is lowered such that the tip of the blade 'bites'
into the soft plastic of the
AMENDED SHEET
lPF,.AIAU
CA 02484627 2004-11-03
WO 03/101586 PCT/AU03/00661
-20-
bottom of the plastic plate. Further lowering of the post will cause the blade
to pivot around the
hinge 122 and 'guillotine' through object, such as the cell, placed in its
path. Motion is stopped
when the blade has cut through the object of interest and is completely
parallel with bottom of
plate.
It will be appreciated that the functionality of the different examples
described above may be
combined in any one of a number of arrangements. This allows for example cells
to be selected
automatically in accordance with magnetic or radiation sensitive markers. The
cells can then be
arranged in a fusion well, and fused, with the~fusate being automatically
retrieved and positioned in
a recovery well.
Manual Operation
Whilst the operation of the above described apparatus is being described in an
automatic process, it
will be appreciated that the apparatus may be controlled manually. In order to
achieve this, the
processing system 10 is adapted to respond to input command provided by the
user. The
processing system 10 is adapted to respond to input commands to perform any
one or more of:
.Activate the laser;
.Select marked cells from examination of images presented on the I/O device
22;
.Control the positioning of the pipette; and,
.Activating the pipette to recover one or more cells.
In this instance, the images of the inside of the selection well 71 can be
displayed to the user on a
suitable display, or the like. The user can then control the apparatus using
suitable input commands
to allow cells to be detected and recovered as described above, using the
displayed images to
determine the cell positions.
Cloning
The above described apparatus and method are particularly useful in the field
of cloning, which
refers to the isolation of a single cell into a vessel, plate, well etc.
containing suitable growth
media. In particular, the apparatus could be used as follows:
l.Single cell cloning based on visual identification by an operator. Using
input commands and
manual operation to isolate and recover single cells from within a culture,
targeting cells by
using the pipette system mounted on an inverted microscope.
2.Single cell cloning based on image analysis carried out by a computer. Using
a camera mounted
on an inverted microscope, live images can be captured and analysed by
software, allowing the
apparatus to target cells based on pre-set criteria such as shape, size and
texture.
CA 02484627 2004-11-03
WO 03/101586 PCT/AU03/00661
-21-
3.Single cell cloning based on fluorescent markers. Using either of the above
methods but
employing a fluorescent dye conjugated to a ligand, receptor, antibody or
other molecule of
interest. The dye could also be unconjugated. The dye could be stimulated by
either a UV
burner with suitable filters or a laser. Detection could be by either image
capture or
photodiode, either signal being interpreted by computer software and the
pipette targeted to the
cell of interest.
4.Multiple cell cloning. As above but with any number of cells as pre-set by
the user.
In this instance, cells could be cloned from the following sources:
l.Non adherent cells contained in, and resting on the bottom of, a standard
biological cell culture
vessel.
2.As above but for adherent cells whereby a small dose (micro-injection) of
suitable enzyme,
typically a protease, could be administered using a multi-pipette to effect
the release of cells
from the surface of the vessel. Alternatively, suction alone might provide
sufficient force in
some instances to remove the adherent cells.
3.As above but for cells in a slice of tissue immersed in suitable media,
whereby a small dose
(micro-injection) of suitable enzyme could be administered using a mufti-
pipette to effect the
release of cells from the tissue sample.
Accordingly, the system described above allows individual cells to be easily
selected. As the cells
are selected using the pipette as shown in Figure 3, this makes individual
cell selection easier than
in the prior art. This therefore helps increase the speed and ease with which
individual cells can be
selected, recovered and used is subsequent procedures.
In addition to this, the apparatus as a whole is generally less complicated,
thereby helping reduce
the cost, as well as easing use of the apparatus to perform cell recovery. As
a result, recovery using
the system described above can generally be achieved more rapidly and cheaper
than in the prior
art.
Accordingly, it will be appreciated that the apparatus is ideal for use in the
following applications:
l.Rare Cell Recovery: Whereby there is a large number of cells are in culture
and a small sub-
population need to be recovered.
2.Diagnostics: Cell recovery from tissue obtained from a needle biopsy, along
with the ability to
clone single cell cultures of these cells and monitor subsequent growth and
other characteristics
such as surface marker expression.
CA 02484627 2004-11-03
WO 03/101586 PCT/AU03/00661
-22-
Persons skilled in the art will appreciate that numerous variations and
modifications will become
apparent. All such variations and modifications which become apparent to
persons skilled in the
art, should be considered to fall within the spirit and scope that the
invention broadly appearing
before described.
Accordingly, while the above description has focused on cell selection, it
will be appreciated that
the techniques may generally be applied to any cells, vectors, particles,
molecules, liposomes, and
other such vesicles. Cells are defined as, but not limited to as being cells
from vertebrate
(including all mammalian species), invertebrate, plant, fungus and bacterial
organisms, including
all cells of eukaryotic and prokaryotic origin.
Furthermore, the techniques may be advantageously used in conjunction with the
techniques
described in the copending applications entitled "A Method of Cell Therapy"
and "Cell Fusion".
In particular, in this latter case, the automated selection and retrieval of
cells advantageously allows
single cell fusion to be performed at a high rate, allowing a large number of
high quality fusates to
be obtained.