Note: Descriptions are shown in the official language in which they were submitted.
CA 02484950 2004-10-28
WO 2004/002827 PCT/US2003/020348
DEVICE AND METHOD FOR CONTROLLING THE FLOW OF A POWDER
BACKGROUND
The need for effective therapeutic treatment of patients has resulted
in the development of a variety of techniques for delivering a pharmaceutical
formulation to a patient. One traditional technique involves the oral delivery
of a
pharmaceutical formulation in the form of a pill, capsule, or the like.
Inhaleable
drug delivery, where an aerosolized pharmaceutical formulation is orally or
nasally
inhaled by a patient to deliver the formulation to the patient's respiratory
tract, has
also proven to be an effective manner of delivery. In one inhalation
technique, a
pharmaceutical formulation is delivered deep within a patient's lungs where it
may
be absorbed into the blood stream. In another inhalation technique, a
pharmaceutical formulation is delivered to a targeted region in the
respiratory tract
to provide local treatment to the region. Many types of inhalation devices
exist
including devices that aerosolize a dry powder pharmaceutical formulation.
The pharmaceutical formulation is often packaged so that it may be
made easily available to a user. For example, a dose or a portion of a dose
may be
stored between layers of a multi-layered package, conventionally referred to
as a
blister or blister pack. Typically, a cavity is formed in a lower layer, the
pharmaceutical formulation is deposited within the cavity, and.an upper layer
is
sealed onto the lower layer, such as by heating and/or compressing the layers,
to
secure the pharmaceutical formulation within the cavity. Alternatively, the
dose
may be stored in a capsule that is to be swallowed or from which the
pharmaceutical formulation may be aerosolized. Other packages, such as
bottles,
vials, and the like, may also be used to store the pharmaceutical formulation.
It is often difficult to effectively fill packages with the
pharmaceutical formulation. For example, during a powder filling process, it
is
difficult to sufficiently fluidize the powder and/or to maintain consistent
flow
properties of the powder. Poorly controlled powder flow can result in
inconsistently filled packages. For example, the fill mass may vary from
package
to package thereby affecting the dose to be delivered to a patient for a unit
dose
CA 02484950 2004-10-28
WO 2004/002827 PCT/US2003/020348
package or resulting in too many or too few doses being packaged in a multi-
dose
package. Additionally, the packing characteristics of a powder in a package
may
vary as a result of inconsistent powder flow during the filling process.
Therefore, it is desirable to be able to control the flow of a powder,
particularly a powder pharmaceutical formulation. It is further desirable to
be able
to control the flow of a powder pharmaceutical formulation so that a package
may
be effectively and consistently filled with the pharmaceutical formulation. It
is still
further desirable to control the flow of a pharmaceutical formulation in a
manner
that reduces any adverse effects on the pharmaceutical formulation.
SUMMARY
The present invention satisfies these needs. In one aspect of the
invention the flow of powder from a hopper is controlled in an improved
manner.
In another aspect of the invention, an apparatus for filling a chamber
comprises a hopper adapted to contain a powder pharmaceutical formulation, the
hopper comprising an outlet. The apparatus also comprises a disturbance member
capable of disturbing a medium within the hopper, the disturbance of the
medium
being sufficient to control the flow of powder through the outlet. The chamber
may
be filled by powder flowing through the outlet and into the chamber.
In another aspect of the invention, an apparatus for filling a chamber
comprises a hopper adapted to contain a powder pharmaceutical formulation, the
hopper comprising an outlet. The apparatus also comprises a vibratable member
positioned in, on, or near the hopper so that the vibratable member is spaced
from
powder in the hopper, the vibratable member being capable of fluidizing the
powder in the hopper. The chamber may be filled with powder flowing through
the
outlet and into the chamber.
In another aspect of the invention, a method of filling a chamber
comprises providing a powder pharmaceutical formulation in a hopper;
disturbing a
medium in the hopper to fluidize the powder; and passing the powder through an
outlet and into the chamber.
In another aspect of the invention, a method of filling a chamber
-2-
CA 02484950 2004-10-28
WO 2004/002827 PCT/US2003/020348
comprises providing a powder pharmaceutical formulation; vibrating a member
spaced from the powder to fluidize the powder; and passing the powder through
an
outlet and into the chamber.
In another aspect of the invention, a pharmaceutical package is made
by a process comprising providing a receptacle; filling the receptacle with a
powder
pharmaceutical formulation that has been fluidized by a fluidization member
spaced
from the powder; and sealing the receptacle to secure the powder
pharmaceutical
formulation therein.
DRAWINGS
These features, aspects, and advantages of the present invention will
become better understood with regard to the following description, appended
claims, and accompanying drawings which illustrate exemplary features of the
invention. However, it is to be understood that each of the features can be
used in
the invention in general, not merely in the context of the particular
drawings, and
the invention includes any combination of these features, where:
Figure 1 is a schematic sectional side view of a powder filling
apparatus of the invention;
Figures 2A through 2C are schematic sectional side views of various
receptacles that may be filled using the powder filling apparatus of the
invention;
Figure 3 is a schematic sectional side view of another version of a
powder filling apparatus;
Figures 4A and 4B are schematic sectional side views of the
operation of another version of a powder filling apparatus;
Figure 5 is a schematic sectional side view of another version of a
powder filling apparatus;
Figures 6A and 6B are schematic sectional side views of the powder
filling apparatus of Figure 5 during a powder filling process;
Figure 7 is a schematic sectional front view of a multiple chamber
powder filling apparatus;
-3-
CA 02484950 2004-10-28
WO 2004/002827 PCT/US2003/020348
Figures 8A and 8B are schematic sectional front views of other
versions of multiple chamber powder filling apparatus;
Figure 9 is a schematic cut-away view showing the interior of a
version of a powder filling apparatus;
Figure 10 is a schematic sectional side view of a powder filling
apparatus together with a bulk powder container; and
Figure 11 is a more detailed schematic sectional side view of a
version of a powder filling apparatus with a bulk powder container.
DESCRIPTION
The present invention relates to controlling the flow of a powder,
such as by controlling the flow of powder during a package filling process.
Although the process is illustrated in the context of packaging a powder
pharmaceutical formulation, the present invention can be used in other
processes
and should not be limited to the examples provided herein.
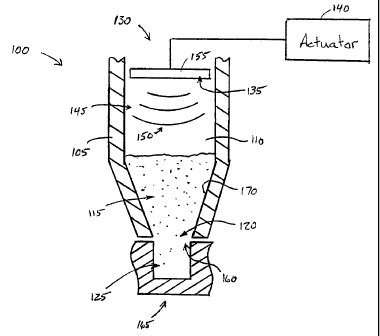
A powder filling apparatus 100 according to the present invention is
shown schematically in Figure 1. The powder filling apparatus 100 comprises a
hopper 105 having a reservoir 110 capable of containing a bed of powder 115,
such
as a powder pharmaceutical formulation. The hopper 105, which may be of any
suitable size and shape, comprises an outlet 120 through which fluidized
powder
may flow. A chamber 125 may be positioned in proximity to the outlet 120 so
that
powder flowing through the outlet 120 will flow into the chamber 125 to fill
the
chamber 125.
A powder fluidizer 130 may be positioned in, on, or near the hopper
105. The powder fluidizer 130 comprises a disturbance member 135 that provides
a
disturbance within the hopper 105. In one version, the disturbance member 135
may be actuated by an actuator 140 to cause a disturbance within the hopper
105 to
control the flow of powder 115 in the hopper 105. For example, the disturbance
member 135 may disturb a medium 145, such as air or other gas, that is in the
hopper 105 in such a manner that the disturbed medium 145 may cause
fluidization
-4-
CA 02484950 2004-10-28
WO 2004/002827 PCT/US2003/020348
of the powder 115. Accordingly, at least a portion of the disturbance member
135
may be positioned so that it is separated from the powder 115 by the medium
145.
The powder fluidizer 130 may be used to control a powder filling
process within the hopper 105. In one version, the powder fluidizer 130 may
operate continuously or periodically in short intervals to maintain the powder
115 in
a constantly fluidized state. In this version, powder 115 may flow through the
outlet 120 until the hopper 105 is empty or until the chamber 125 is filled.
In
another version, the powder fluidizer 130 may control the timing of the flow
of
powder 115 and/or may control the amount of powder 115 that flows through the
outlet 120 of the hopper 105 and into the chamber 125. For example, the outlet
120
in the hopper 105 may be sufficiently small that undisturbed powder 115 does
not
flow through the outlet 120 or does not consistently flow through the outlet
120.
When it is desired for the powder 115 to flow into the chamber 125, the
actuator
140 causes the disturbance member 135 to disturb the medium 145 and thereby
fluidize the powder 115 to allow the powder 115 to flow through the outlet 120
and
into the chamber 125. When the chamber 125 is sufficiently filled, the
actuator 140
may cause the disturbance to stop or be reduced, thereby reducing the amount
of
powder 115 flowing through the outlet 120, for example by terminating the flow
of
powder through the outlet 120.
In one version, the powder fluidizer 130 provides a disturbance
within the hopper 105, and the disturbance comprises vibrations 150. The
disturbance member 135 may comprise a vibratable object, such as a membrane
155, within, on or near the hopper 105, the membrane 155 being capable of
vibrating when excited by the actuator 140 to produce vibrations. The
vibrating
membrane 155 disturbs the medium 145. For example, as the membrane 155
moves in a downward direction, the portion of the medium 145 immediately in
front is compressed causing a slight increase in pressure, it then moves back
past its
rest position and causes a reduction in the pressure. The process may continue
so
that one or more waves of alternating high and low pressure are radiated away
from
the membrane 155. The waves contact the powder 115 and the resulting impact is
sufficient to at least momentarily fluidize the powder 115.
-5-
CA 02484950 2004-10-28
WO 2004/002827 PCT/US2003/020348
The frequency of the vibrations 150 may be selected to fluidize a
particular powder 115 and/or to best suit a particular filling process. In one
particular version, the vibrations 150 may be in the audible range. In yet
another
version, the membrane 155 may vibrate at a frequency in a non-audible range to
lessen operator annoyance. The vibration may be at any frequency, or multiple
frequencies, that desirably fluidizes or otherwise controls the flow of the
powder
115. For example, in the version shown in Figure 1, the membrane 155 may
vibrate
at one or more frequencies comprising a frequency of from about 10 Hz to about
1000 Hz, more preferably from about 90 Hz to about 500 Hz, more preferably
from
about 100 Hz to about 200 Hz, and most preferably at about 120 Hz. In one
version, the frequency may be selectable. For example, through experimentation
or
modeling, a particularly desirable frequency for a particular configuration
and/or
powder may be selected, such as a frequency that is determined through
experimentation or analysis to cause a resonance within the hopper 105.
The chamber 125 comprises an opening 160 positionable in relation
to the outlet 120 in the hopper 105 to receive powder flowing from the hopper
105
through the outlet 120. In one version, such as the version shown in Figure 1,
the
opening 160 into the chamber 125 is substantially the same shape and size and
as
the outlet 120 to prevent excessive amounts of powder 115 from getting trapped
between the hopper 105 and a member 165 that contains or supports the chamber
125. As also shown in the version of Figure 1, the hopper 105 may comprise
converging side walls 170 that provide a convergent flow path towards the
outlet
120 for the powder 115. The convergent flow path allows for increased
reservoir
volume in the hopper 105. In another version, the chamber opening 160 and the
outlet 120 may be differently sized. For example, the outlet 120 may be
smaller
than the opening 160 when it is desirable to fill a relatively large chamber
with a
precisely controlled amount of powder 115 or when it is not desirable to
provide a
mechanism for precisely positioning the chamber 125 beneath the outlet 120.
Alternatively, the opening 160 may be smaller than the outlet 120 when it is
desirable to use the hopper 105 to fill varying sizes of chambers 125 or in
situations
where the loss of powder 115 to spaces between the hopper 105 and the member
165 is not of critical concern.
-6-
CA 02484950 2010-07-26
WO 2004/002827 PCT/US2003/020348
The chamber 125 may be within a receptacle 175 used to store the powder
115. For example, the receptacle 175 may be in the form of primary or
secondary
packaging used to store a powder pharmaceutical formulation. In one version,
the
receptacle 175 comprises a multi-layered package, conventionally referred to
as a
blister or blister pack, and the chamber 125 is within the multi- layered
package.
As shown in Figure 2A, powder 115 flows from the hopper 105 to a cavity 180 in
a lower layer 185 of the multi-layered package. An upper layer (not shown) may
then be sealed onto the lower layer 185, such as by heating and/or compressing
the layers, to secure the powder within the cavity 180, as described for
example in
U.S. Patent 5,865,012 and in U.S. Patent Application Publication No.
2005-0051453.
In one version, the multi-layered package may comprise a lower layer
comprising a metal containing layer, such as a layer comprising aluminum,
and/or
an upper layer comprising a metal containing layer. The metal containing
layers
may be sufficiently thick to substantially prevent a significant amount of
moisture
from passing therethrough. For example, the metal containing layers may be
from
about 10 gm to about 100 gm, and more preferably from about 20 gm to about 80
gm. The lower layer and the upper layer may be sealed together by a layer of
sealing material, such as a layer of lacquer that may be from about 1 gm to
about 20
gm. In another version, the receptacle 175 comprises a capsule, such as a
capsule
that is to be swallowed or from which the pharmaceutical formulation may be
aerosolized, and the chamber 125 is within the capsule. As shown in Figure 2B,
a
first portion 190 of a capsule is positioned to receive powder flowing through
the
outlet 120 of the hopper. After filling, a second portion (not shown) may be
placed
over the first portion 190 to form the a capsule shape and to contain the
powder
within the capsule, as described in U.S. Patent 4,247,066, U.S. Patent
4,864,876,
U.S. Patent 6,357,490, and in the PCT application WO 00/07572 published on
February 17, 2000.
In another version, as shown in Figure 2C, the chamber 125 may be
within a container 195, such as a bottle, vial or the like. For example, in
this
version, the container 195 may be use to contain multiple doses of a powder
-7-
CA 02484950 2010-07-26
WO 2004/002827 PCT/US2003/020348
pharmaceutical formulation, such as a container described in U.S. Patent
4,524,769.
In another version, the chamber 125 may be a transfer chamber 200
that transfers powder that flows from the hopper 115 into the transfer chamber
200
to another chamber, such as a chamber within a package 175. For example, as
shown in Figure 3, the transfer chamber 200 may be provided in a movable
member
205. The transfer chamber 200 receives powder from the hopper 115 when in a
filling position as shown in Figure 3. The movable member 205 then transports
the
transfer chamber 200 to a position in proximity to the package 175 where at
least a
portion of the contents of the transfer chamber 200 may be emptied into the
package 175. The transport chamber 200 may be sized so that it contains a
predetermined amount of powder. For example, the transport chamber 200 may be
sized to collect a dose of a powder pharmaceutical formulation, and the
accurate
dose may be delivered to the package 175. A doctor blade 210 may be provided
to
scrape off any excess powder in the transport chamber 200.
In the version shown in Figures 4A and 4B, a powder transfer
assistance mechanism 215 is provided. In one version, the powder transfer
assistance mechanism 215 comprises a channel 220 in communication with the
transfer chamber 200. The channel 200 is connectable to a source of suction
when
the transfer chamber 200 is in the powder collecting position shown in Figure
4A.
In this way, suction 225 can be provided to the transfer chamber 200 to assist
in
collecting powder 115 within the transfer chamber 200. A filter 230 may be
provided in the transfer chamber 200 to prevent powder 115 from being
suctioned
into the channel 200. For example, the filter may comprise apertures having a
diameter of from about 0.10 micrometers to about 0.65 micrometers, most
preferably about 0.65 micrometers. When the transfer chamber 200 is moved to a
powder ejecting position as shown in Figure 4B, the channel 220 may be
connectable to a source of pressurized gas to create pressure 235 within the
channel
220 to cause the powder in the transfer chamber 200 to be ejected into the
receptacle 175. An example of a powder transfer assistance mechanism is
described in U.S. Patents 5,826,633.
-8-
CA 02484950 2010-07-26
WO 2004/002827 PCT/US2003/020348
The powder filling apparatus 100 provides for an advantageous
powder filling process. One such advantage is that there is a reduction in the
amount of physical contact between powder in the hopper 105 and other objects.
This reduced contact can be useful in preventing undesirable conditions in the
powder pharmaceutical formulation. For example, excessive physical contact can
in some situations cause one or more of the following situations: formation of
aggregates, increased electrostatic interactions, denaturation, and reduced
aerosol
performance. Though these undesirable effects have been prevented or
compensated for in costly and encumbering manners, the reduction of the amount
of direct physical contact provides a particularly simplified and useful
alternative.
In another version, the powder filling apparatus 100 comprises a
powder fluidizer 130 of the type discussed above in combination with an
additional
powder fluidizing member. For example, as shown in Figure 5, the powder
filling
apparatus may comprise a second powder fluidizer 240. The second powder
fluidizer 240 comprises a powder fluidizing member 250 and an actuator 255
that
drives the powder fluidizing member 250 to in a manner that fluidizes the
powder
115 in the hopper 105. For example, the powder fluidizing member 250 may be a
member that directly contacts the powder 115, and the movement of the
fluidizing
member 250 causes the powder 115 to fluidize. In the version shown, the powder
fluidizing member 250 comprises a rod 260 that extends downwardly into the bed
of powder 115. A holding arm 265 holds the rod 260 in the hopper 105. The
actuator 255 may be connected to drive the arm 265 to drive the rod 260 or may
be
connected directly to the rod 260, such as by being connected between the rod
260
and the arm 265. The fluidization of powder in this manner is described in
U.S.
Patent 6,182,712. The rod 260 may be caused to vibrate by the actuator 240.
For
example, as shown in Figure 5, the rod may have a distal end that is
positionable
near the outlet 120, and the actuator 240 may drive the rod in an up and down
motion 270 to fluidize the powder to cause it to flow through the outlet 120
and
into the chamber 125. In one particular version, the rod 260 may be attached
to a
motor, such as a piezoelectric motor, and is vibrated at a frequency of from
about
1000 Hz to about 180,000 Hz,
-9-
CA 02484950 2004-10-28
WO 2004/002827 PCT/US2003/020348
more preferably from about 10,000 Hz to about 40,000 Hz, and most preferably
from about 15,000 Hz to about 25,000 Hz. Additionally or alternatively, the
rod
260 may be vibrated or moved in another direction, such as laterally or
rotationally.
In another version, the additional powder fluidizing member may comprise a
stirrer
or other fluidizing mechanism.
The powder fluidizer 130 and second powder fluidizer 240 may
work in tandem or alone to fluidize powder 115 in the hopper 105. For example,
as
shown in Figure 5, the powder fluidizer 130 may be actuated concurrently with
the
second powder fluidizer 240 to simultaneously generate vibrations 150 in the
medium 145 and to directly vibrate 270 the powder 115. For some powders this
combined action provides superior fluidization capabilities. In another
version, the
powder fluidizer 130 and the second powder fluidizer 240 may be actuated at
different times or actuated in a manner to supplement one another. For
example, as
shown in Figures 6A and 6B, the powder fluidizer 130 may serve to supplement
the
action of the second powder fluidizer 240. As shown in Figure 6A, for some
powders, the vibration of the rod 260 may be sufficient for a chamber 125 to
be
filled but may also result in the formation of one or more voids 275 in the
area in
proximity to the rod 260. After the void 275 has been created, vibration of
the rod
260 would have little fluidization capability. To fill the void 275, the
powder
fluidizer 130 may be actuated, as shown in Figure_,6B. The disturbance to the
medium 145 is sufficient to cause the powder 115 to again contact the rod 260
so
that the rod 260 may again be vibrated to fluidize the powder 115. The powder
fluidizer 130 and/or the second powder fluidizer 240 may operate continuously
to
maintain the powder 115 in a continuously fluidized condition during the
filling of
multiple chambers 125 through the outlet 120. Alternatively, the powder
fluidizer
130 and/or the second powder fluidizer 240 may operate only when it is desired
to
have the powder 115 fluidized, and the outlet 120 may be sized such that the
powder does not substantially flow though the outlet 120 in the absence of
such
operation of the fluidizers.
In one version, as shown in Figure 7, the powder filling apparatus
100 is configured to simultaneously fill a plurality of chambers 125. In this
version
the hopper 105 comprises a plurality of outlets 120, such as two, three, four,
or
-10-
CA 02484950 2010-07-26
WO 2004/002827 PCT/US2003/020348
more. The powder fluidizer 130 is positioned to fluidize the powder 115 in the
hopper 105 across all of the outlets 120. In the version shown in Figure 7,
the
powder flowing through an outlet 120 passes into a transfer chamber 200 in a
moveable member 205, which in this version is a rotatable member. When the
transfer chamber 200 is filled, the moveable member 205 is rotated from the
filling
position shown in the figure to an ejecting position where the transfer
chambers 200
are positioned above respective receptacles 175. The receptacles 175 are
supported
by a platform 300. The platform 300 may be moveable relative to the moveable
member 205 so as to able to bring the receptacles 175 into the position shown
in
Figure 7 and to take the receptacles 175 away after they are filled, at which
time the
transfer chambers 200 are moved back to their filling positions. This process
may
continue until a desired number of receptacles have been filled. In one
version, the
platform 300 may be a moveable and indexable plate having openings for
receiving
receptacles. In another version, the platform 300 may be a belt on a roller
system.
Figures 8A and 8B show versions of a powder filling apparatus 100
capable of simultaneously filling a plurality of chambers 120 and comprising a
powder fluidizer 130 and a second powder fluidizer 240. In the version of
Figure
8A, the second powder fluidizer 240 comprises a rod 260 that may be vibrated
in an
up and down direction 270. In addition, a mechanism is provided that allows
the
rod 260 to translate laterally 310 across each of the openings. An exemplary
translation mechanism is described in aforementioned U. S. Patent 6,182, 712
as
discussed above. In the version of Figure 8B, a plurality of vibrating rods
260 are
provided. For example, a rod 260 may be associated with a respective outlet
120.
A detailed view of an embodiment of a powder filling apparatus in
accordance with the version of Figure 8A is shown in Figure 9. In this
version, a
power fluidizer 130 and a second powder fluidizer 240 are used to control the
flow
of powder in the hopper through a plurality of outlets 120. The rod 260 of the
second powder fluidizer is connected to a piezoelectric actuator or motor 320
to
cause the rod 260 to vibrate up and down. A mechanism, such as a screw drive,
is
provided within the arm 265 that causes the rod 260 to translate 310 across
the
hopper 105. A first enclosure 325 and a second enclosure 330 are provided to
- 11 -
CA 02484950 2004-10-28
WO 2004/002827 PCT/US2003/020348
maintain desirable conditions within the powder filling apparatus 100. For
example, for some powders, such as powder pharmaceutical formulations, it may
be
desirable to maintain a clean or sterile environment for the powder. It may
also be
desirable to maintain a certain relative humidity within enclosures,
particularly
when filling powders that undergo a change when subjected to significant
amounts
of moisture. One or more of the enclosures may comprise, for example, a
medical
grade stainless steel, engineering polymer, PVC, or the like. In one version,
multiple powder fluidizers 130 may be provided within the second enclosure.
This
may be advantageous when very large hoppers 105 are utilized. An inlet 335
through the enclosure 325 allows for the introduction of bulk powder into the
hopper 105.
At least a portion of the powder fluidizer 130 may be housed within
the second enclosure 330. In one version, the membrane 155 may be a portion of
a
speaker cone from a conventional audio speaker. The speaker is connected to a
function generator that can provide power and frequency ranges to the speaker
through an amplifier. As the speaker cone vibrates, fluidizing sound is
created.
The speaker cone may comprise, for example, a 3 inch woofer, a 4 inch woofer,
a
6.5 inch woofer, or the like. In another version, the powder fluidizer may
comprise a membrane that is spaced from the speaker cone so that when the
speaker
cone vibrates, the membrane is caused to vibrate. This configuration may be
useful
in maintaining a controlled environment within the hopper 105 in that the
speaker
may be housed completely within the second enclosure 330 and is not directly
exposed to the hopper 105.
Additionally or alternatively, a bulk powder fluidizer 350 may be
provided to fluidize bulk powder 355 contained in a bulk powder container 360.
The bulk powder container 360 may be used to supply powder to the hopper 105,
as
shown in Figure 10. In this version, the bulk powder container 360 comprises
an
outlet 365 that is in communication with the inlet 335 into the hopper 105.
The
bulk powder fluidizer 350, which may comprise a membrane 370 and actuator 375
similar to those described above, is actuated when it is desired to fluidize
the bulk
powder 355 to cause it to flow through the outlet 365 and into the hopper 105.
This
actuation may be continuous so that a small amount of powder is continuously
-12-
CA 02484950 2004-10-28
WO 2004/002827 PCT/US2003/020348
being supplied to the hopper 105 at about the rate that powder is flowing
through
the one or more outlets 120 in the hopper 105. Alternatively, the actuation
may be
periodic. In one version, the bulk powder fluidizer 350 may be actuated when
the
level of the powder in the hopper 105 falls below a predetermined level. This
may
involve manual actuation or a level sensor, such as a capacitive sensor, may
be
provided to allow for automatic refilling. A gate or valve may also be
provided
near the inlet 335.
A powder filling apparatus 100 incorporating the features of the
version of Figures 9 and 10 is shown in Figure 11. In this version, a valve
380, is
provided to selectively introduce powder for the bulk powder container 360
into the
hopper 115. In this version, the valve 380 is opened when the level of the
powder
bed 115 in the hopper 105 falls below a predetermined level. The bed level is
detected by a capacitive sensor 385 operatively positioned to generate a
signal when
the bed level falls below the predetermined height. The signal is provided to
a
controller which controls the opening and closing of the valve 380.
Alternatively or
additionally, a laser sensor may be utilized. In the version shown, a second
enclosure 390 is also provided for at least a portion of the bulk powder
fluidizer
350.
The powder filling apparatus 100 has been found to fill powder into
receptacles in an improved manner. The powder filling apparatus 100 is
particularly effective in filling fine dry powders into unit dose receptacles.
For
example, Table 1 shows a comparison of filling a fine dry, powder
pharmaceutical
formulation, Powder A, using a prior art powder filler and using a powder
filling
apparatus 100 according to the present invention. The prior art powder filler
is
described in U.S. Patent 6,182,712. The powder filling apparatus 100 shown in
present Figure 11 with a transfer chamber as shown in Figures 4A and 4B was
used
for the comparison. In the Table, N represents the number of receptacles
filled; SD
represents the standard deviation; and RSD represents the relative standard
deviation. As can be seen, in each of five separate runs, the prior art system
was
unable to match the filling consistency of the powder filling apparatus 100.
In fact,
in even the best run using the prior art system, the filling range was more
than twice
the range using the powder filling apparatus 100 of the present invention.
-13-
CA 02484950 2004-10-28
WO 2004/002827 PCT/US2003/020348
Mean Fill
SD RSD Range
Filler Used N Mass
(mg) (%) (mg) (mg)
Prior Art Powder Filler,
404 0.11 1.5 7.52 1.2
Run 1
Prior Art Powder Filler,
264 0.18 2.5 7.55 1.94
Run 2
Prior Art Powder Filler,
491 0.16 2.1 7.52 1.11
Run 3
Prior Art Powder Filler,
488 0.15 2.0 7.51 1.39
Run 4
Prior Art Powder Filler,
356 0.32 4.3 7.50 1.99
Run 5
Present Powder Filler
288 0.08 1.1 7.50 0.55
100
Table 1
The powder filling apparatus 100 of the present invention has also
shown universal adaptability for filling various powders. The powder filling
apparatus 100 shown in present Figure 11 with a transfer chamber as shown in
Figures 4A and 4B was used for a comparison of different powders, and the
results
are shown in Table 2. Six different powders were filled into unit dose
receptacles.
The powders were of varying size, compositions, active agents, excipients, and
properties. However, as can be seen from the data, very consistent filling was
achieved with each of the powders. Very low RSD's were achieved for each of
the
powders. In addition, the powder filling apparatus 100 demonstrated the
ability to
consistently fill both small and large doses into a receptacle.
-14-
CA 02484950 2004-10-28
WO 2004/002827 PCT/US2003/020348
Powder Filled Using Mean Fill
SD RSD Range
Present Powder Filler N Mass
100 (mg) (%) (mg) (mg)
Powder A 288 0.08 1.1 7.50 0.59
Powder B 120 0.03 0.9 4.06 0.16
Powder C 60 0.06 1.2 4.90 0.28
Powder D 270 0.91 1.8 50.09 4.90
Powder E 89 0.55 1.1 51.32 3.04
Powder F 30 0.18 1.8 10.09 0.82
Table 2
A computer controller may be provided to control the actuation of
the bulk powder fluidizer 350 and/or to control the actuation of the powder
fluidizer
130 and/or the second powder fluidizer 240. The controller may control the
operation of the entire powder filling apparatus 100. The controller may be a
single
controller device or may be a plurality of controller devices that may be
connected
to one another or a plurality of controller devices that may be connected to
different
components of the packaging apparatus 100.
In one embodiment, the controller comprises electronic hardware
including electrical circuitry comprising integrated circuits that is suitable
for
operating or controlling the powder filling apparatus 100. Generally, the
controller
is adapted to accept data input, run algorithms, produce useful output
signals, and
may also be used to detect data signals from one or more sensors and other
device
components, and to monitor or control the process in the powder filling
apparatus
100. However, the controller may merely perform one of these tasks. In one
version, the controller may comprise one or more of (i) a computer comprising
a
central processor unit (CPU) which is interconnected to a memory system with
peripheral control components, (ii) application specific integrated circuits
(ASICs)
that operate particular components of the powder filling apparatus 100 or
operate a
particular process, and (iii) one or more controller interface boards along
with
suitable support circuitry. Typical CPUs include the PowerPCTM, PentiumTM, and
-15-
CA 02484950 2004-10-28
WO 2004/002827 PCT/US2003/020348
other such processors. The ASICs are designed and preprogrammed for particular
tasks, such as retrieval of data and other information from the powder filling
apparatus 100 and/or operation of particular device components. Typical
support
circuitry includes for example, coprocessors, clock circuits, cache, power
supplies
and other well known components that are in communication with the CPU. For
example, the CPU often operates in conjunction with a random access memory
(RAM), a read-only memory (ROM) and other storage devices well known in the
art. The RAM can be used to store the software implementation of the present
invention during process implementation. The programs and subroutines of the
present invention are typically stored in mass storage devices and are
recalled for
temporary storage in RAM when being executed by the CPU.
The software implementation and computer program code product of
the present invention may be stored in a memory device, such as an EPROM, and
called into RAM during execution by the controller. The computer program code
may be written in conventional computer readable programming languages, such
as
for example, assembly language, C, C", Pascal, or native assembly. Suitable
program code is entered into a single file, or multiple files, using a
conventional
text editor and stored or embodied in a computer-usable medium, such as a
memory
of the computer system. If the entered code text is in a high level language,
the code
is compiled to a compiler code which is linked with an object code of
precompiled
windows library routines. To execute the linked and compiled object code, the
system user invokes the object code, causing the computer system to load the
code
in memory to perform the tasks identified in the computer program.
In one version, the controller may comprise a microprocessor or
ASIC of sufficiently small size and power consumption to be housed on or in
the
powder filling apparatus 100. For example, suitable microprocessors for use as
a
local microprocessor include the MC68HC711E9 by Motorola, the PIC16C74 by
Microchip, and the 82930AX by Intel Corporation. The microprocessor can
include
one microprocessor chip, multiple processors and/or co-processor chips, and/or
digital signal processor (DSP) capability.
In one particularly useful implementation, the powder filling
apparatus 100 may be used to fill a pharmaceutical receptacle, such as a
blister,
-16-
CA 02484950 2010-07-26
WO 2004/002827 PCT/US2003/020348
capsule, vial, bottle, or the like, with a powder pharmaceutical formulation.
For
example, the powder filling apparatus 100 has proven to be particularly
advantageous in filling dry powder inhaleable pharmaceutical formulations into
receptacles from which the pharmaceutical formulation may be aerosolized for
inhalation by a user. For example, when in a powdered form, the powder may be
initially stored in the sealed package, which is opened prior to
aerosolization of
the powder, as described in U.S. Patent 5,785,049, U.S. Patent 5,415,162, and
U.S. Patent No. 6,606,992. Alternatively the powder may be contained in a
capsule, as described in U.S. Patent 4,995,385, U.S. Patent 3,991,761, U.S.
Patent
6,230,707, and PCT Publication WO 97/27892, the capsule being openable
before, during, or after insertion of the capsule into an aerosolization
device. In
either the bulk, blister, capsule, or the like form, the powder may be
aerosolized
by an active element, such as compressed air, as described in US Patent
5,458,135, U.S. Patent 5,785,049, and U.S. Patent 6,257,233, or propellant, as
described in PCT Publication WO 00/72904. Alternatively the powder may be
aerosolized in response to a user's inhalation, as described for example in
the
aforementioned US Patent No. 6,606,992 and U. S. Patent 4,995, 385.
The pharmaceutical formulation may comprise an active agent. The
active agent described herein includes an agent, drug, compound, composition
of
matter or mixture thereof which provides some pharmacologic, often beneficial,
effect. This includes foods, food supplements, nutrients, drugs, vaccines,
vitamins,
and other beneficial agents. As used herein, the terms further include any
physiologically or pharmacologically active substance that produces a
localized or
systemic effect in a patient. An active agent for incorporation in the
pharmaceutical
formulation described herein may be an inorganic or an organic compound,
including, without limitation, drugs which act on: the peripheral nerves,
adrenergic
receptors, cholinergic receptors, the skeletal muscles, the cardiovascular
system,
smooth muscles, the blood circulatory system, synoptic sites, neuroeffector
junctional sites, endocrine and hormone systems, the immunological system, the
-17-
CA 02484950 2010-07-26
WO 2004/002827 PCT/US2003/020348
reproductive system, the skeletal system, pulmonary system, autacoid systems,
the
alimentary and excretory systems, the histamine system, and the central
nervous
system. Suitable active agents may be selected from, for example, hypnotics
and
sedatives, psychic energizers, tranquilizers, respiratory drugs,
anticonvulsants,
muscle relaxants, antiparkinson agents (dopamine antagnonists), analgesics,
anti-
inflammatories, antianxiety drugs (anxiolytics), appetite suppressants,
antimigraine
agents, muscle contractants, anti-infectives (antibiotics, antivirals,
antifungals,
vaccines) antiarthritics, antimalarials, antiemetics, anepileptics,
bronchodilators,
cytokines, growth factors, anti-cancer agents, antithrombotic agents,
antihypertensives, cardiovascular drugs, antiarrhythmics, antioxicants, anti-
asthma
agents, hormonal agents including contraceptives, sympathomimetics, diuretics,
lipid regulating agents, antiandrogenic agents, antiparasitics,
anticoagulants,
neoplastics, antineoplastics, hypoglycemics, nutritional agents and
supplements,
growth supplements, antienteritis agents, vaccines, antibodies, diagnostic
agents,
and contrasting agents. The active agent, when administered by inhalation, may
act
locally or systemically.
The active agent may fall into one of a number of structural classes,
including but not limited to small molecules, peptides, polypeptides,
proteins,
polysaccharides, steroids, proteins capable of eliciting physiological
effects,
nucleotides, oligonucleotides, polynucleotides, fats, electrolytes, and the
like.
Examples of active agents suitable for use in this invention include
but are not limited to one or more of calcitonin, amphotericin B,
erythropoietin
(EPO), Factor VIII, Factor IX, ceredase, cerezyme, cyclosporin, granulocyte
colony
stimulating factor (GCSF), thrombopoietin (TPO), alpha-1 proteinase inhibitor,
elcatonin, granulocyte macrophage colony stimulating factor (GMCSF), growth
hormone, human growth hormone (HGH), growth hormone releasing hormone
(GHRH), heparin, low molecular weight heparin (LM)W, interferon alpha,
interferon beta, interferon gamma, interleukin-1 receptor, interleukin-2,
interleukin-
1 receptor antagonist, interleukin-3, interleukin-4, interleukin-6,
luteinizing
hormone releasing hormone (LHRH), factor IX, insulin, pro-insulin, insulin
analogues (e.g., mono-acylated insulin as described in U.S. Patent No.
5,922,675), amylin, C-peptide,
-18-
CA 02484950 2004-10-28
WO 2004/002827 PCT/US2003/020348
somatostatin, somatostatin analogs including octreotide, vasopressin, follicle
stimulating hormone (FSH), insulin-like growth factor (IGF), insulintropin,
macrophage colony stimulating factor (M-CSF), nerve growth factor (NGF),
tissue
growth factors, keratinocyte growth factor (KGF), glial growth factor (GGF),
tumor
necrosis factor (TNF), endothelial growth factors, parathyroid hormone (PTH),
glucagon-like peptide thymosin alpha 1, IIb/IIIa inhibitor, alpha-1
antitrypsin,
phosphodiesterase (PDE) compounds, VLA-4 inhibitors, bisphosponates,
respiratory syncytial virus antibody, cystic fibrosis transmembrane regulator
(CFTR) gene, deoxyreibonuclease (Dnase), bactericidal/permeability increasing
protein (BPI), anti-CMV antibody, 13-cis retinoic acid, macrolides such as
erythromycin, oleandomycin, troleandomycin, roxithromycin, clarithromycin,
davercin, azithromycin, flurithromycin, dirithromycin, josamycin, spiromycin,
midecamycin, leucomycin, miocamycin, rokitamycin, andazithromycin, and
swinolide A; fluoroquinolones such as ciprofloxacin, ofloxacin, levofloxacin,
trovafloxacin, alatrofloxacin, moxifloxicin, norfloxacin, enoxacin,
grepafloxacin,
gatifloxacin, lomefloxacin, sparfloxacin, temafloxacin, pefloxacin,
amifloxacin,
fleroxacin, tosufloxacin, prulifloxacin, irloxacin, pazufloxacin,
clinafloxacin, and
sitafloxacin, aminoglycosides such as gentamicin, netilmicin, paramecin,
tobramycin, amikacin, kanamycin, neomycin, and streptomycin, vancomycin,
teicoplanin, rampolanin, mideplanin, colistin, daptomycin, gramicidin,
colistimethate, polymixins such as polymixin B, capreomycin, bacitracin,
penems;
penicillins including penicllinase-sensitive agents like penicillin G,
penicillin V,
penicillinase-resistant agents like methicillin, oxacillin, cloxacillin,
dicloxacillin,
floxacillin, nafcillin; gram negative microorganism active agents like
ampicillin,
amoxicillin, and hetacillin, cillin, and galampicillin; antipseudomonal
penicillins
like carbenicillin, ticarcillin, azlocillin, mezlocillin, and piperacillin;
cephalosporins
like cefpodoxime, cefprozil, ceftbuten, ceftizoxime, ceftriaxone, cephalothin,
cephapirin, cephalexin, cephradrine, cefoxitin, cefamandole, cefazolin,
cephaloridine, cefaclor, cefadroxil, cephaloglycin, cefuroxime, ceforanide,
cefotaxime, cefatrizine, cephacetrile, cefepime, cefixime, cefonicid,
cefoperazone,
cefotetan, cefmetazole, ceftazidime, loracarbef, and moxalactam, monobactams
like
aztreonam; and carbapenems such as imipenem, meropenem, pentamidine
-19-
CA 02484950 2004-10-28
WO 2004/002827 PCT/US2003/020348
isethiouate, albuterol sulfate, lidocaine, metaproterenol sulfate,
beclomethasone
diprepionate, triamcinolone acetamide, budesonide acetonide, fluticasone,
ipratropium bromide, flunisolide, cromolyn sodium, ergotamine tartrate and
where
applicable, analogues, agonists, antagonists, inhibitors, and pharmaceutically
acceptable salt forms of the above. In reference to peptides and proteins, the
invention is intended to encompass synthetic, native, glycosylated,
unglycosylated,
pegylated forms, and biologically active fragments and analogs thereof.
Active agents for use in the invention further include nucleic acids,
as bare nucleic acid molecules, vectors, associated viral particles, plasmid
DNA or
RNA or other nucleic acid constructions of a type suitable for transfection or
transformation of cells, i.e., suitable for gene therapy including antisense.
Further,
an active agent may comprise live attenuated or killed viruses suitable for
use as
vaccines. Other useful drugs include those listed within the Physician's Desk
Reference (most recent edition).
The amount of active agent in the pharmaceutical formulation will
be that amount necessary to deliver a therapeutically effective amount of the
active
agent per unit dose to achieve the desired result. In practice, this will vary
widely
depending upon the particular agent, its activity, the severity of the
condition to be
treated, the patient population, dosing requirements, and the desired
therapeutic
effect. The composition will generally contain anywhere from about 1% by
weight
to about 99% by weight active agent, typically from about 2% to about 95% by
weight active agent, and more typically from about 5% to 85% by weight active
agent, and will also depend upon the relative amounts of additives contained
in the
composition. The compositions of the invention are particularly useful for
active
agents that are delivered in doses of from 0.001 mg/day to 100 mg/day,
preferably
in doses from 0.01 mg/day to 75 mg/day, and more preferably in doses from 0.10
mg/day to 50 mg/day. It is to be understood that more than one active agent
may
be incorporated into the formulations described herein and that the use of the
term
"agent" in no way excludes the use of two or more such agents.
The pharmaceutical formulation may comprise a pharmaceutically
acceptable excipient or carrier which may be taken into the lungs with no
significant adverse toxicological effects to the subject, and particularly to
the lungs
-20-
CA 02484950 2004-10-28
WO 2004/002827 PCT/US2003/020348
of the subject. In addition to the active agent, a pharmaceutical formulation
may
optionally include one or more pharmaceutical excipients which are suitable
for
pulmonary administration. These excipients, if present, are generally present
in the
composition in amounts ranging from about 0.01 % to about 95% percent by
weight, preferably from about 0.5 to about 80%, and more preferably from about
1
to about 60% by weight. Preferably, such excipients will, in part, serve to
further
improve the features of the active agent composition, for example by providing
more efficient and reproducible delivery of the active agent, improving the
handling
characteristics of powders, such as flowability and consistency, and/or
facilitating
manufacturing and filling of unit dosage forms. In particular, excipient
materials
can often function to further improve the physical and chemical stability of
the
active agent, minimize the residual moisture content and hinder moisture
uptake,
and to enhance particle size, degree of aggregation, particle surface
properties, such
as rugosity, ease of inhalation, and the targeting of particles to the lung.
One or
more excipients may also be provided to serve as bulking agents when it is
desired
to reduce the concentration of active agent in the formulation.
Pharmaceutical excipients and additives useful in the present
pharmaceutical formulation include but are not limited to amino acids,
peptides,
proteins, non-biological polymers, biological polymers, carbohydrates, such as
sugars, derivatized sugars such as alditols, aldonic acids, esterified sugars,
and
sugar polymers, which may be present singly or in combination. Suitable
excipients are those provided in WO 96/32096, which is incorporated herein by
reference in its entirety. The excipient may have a glass transition
temperature
(Tg) above about 35 C, preferably above about 40 C, more preferably above 45
C, most preferably above about 55 C.
Exemplary protein excipients include albumins such as human serum
albumin (HSA), recombinant human albumin (rHA), gelatin, casein, hemoglobin,
and the like. Suitable amino acids (outside of the dileucyl-peptides of the
invention), which may also function in a buffering capacity, include alanine,
glycine, arginine, betaine, histidine, glutamic acid, aspartic acid, cysteine,
lysine,
leucine, isoleucine, valine, methionine, phenylalanine, aspartame, tyrosine,
tryptophan, and the like. Preferred are amino acids and polypeptides that
function
-21-
CA 02484950 2004-10-28
WO 2004/002827 PCT/US2003/020348
as dispersing agents. Amino acids falling into this category include
hydrophobic
amino acids such as leucine, valine, isoleucine, tryptophan, alanine,
methionine,
phenylalanine, tyrosine, histidine, and proline. Dispersibility- enhancing
peptide
excipients include dimers, trimers, tetramers, and pentamers comprising one or
more hydrophobic amino acid components such as those described above.
Carbohydrate excipients suitable for use in the invention include, for
example, monosaccharides such as fructose, maltose, galactose, glucose, D-
mannose, sorbose, and the like; disaccharides, such as lactose, sucrose,
trehalose,
cellobiose, and the like; polysaccharides, such as raffinose, melezitose,
maltodextrins, dextrans, starches, and the like; and alditols, such as
mannitol,
xylitol, maltitol, lactitol, xylitol sorbitol (glucitol), pyranosyl sorbitol,
myoinositol
and the like.
The pharmaceutical formulation may also include a buffer or a pH
adjusting agent, typically a salt prepared from an organic acid or base.
Representative buffers include organic acid salts of citric acid, ascorbic
acid,
gluconic acid, carbonic acid, tartaric acid, succinic acid, acetic acid, or
phthalic
acid, Tris, tromethamine hydrochloride, or phosphate buffers.
The pharmaceutical formulation may also include polymeric
excipients/additives, e.g., polyvinylpyrrolidones, derivatized celluloses such
as
hydroxymethylcellulose, hydroxyethylcellulose, and
hydroxypropylmethylcellulose, Ficolls (a polymeric sugar), hydroxyethylstarch,
dextrates (e.g.,cyclodextrins, such as 2-hydroxypropyl-(3-cyclodextrin and
sulfobutylether-(3-cyclodextrin), polyethylene glycols, and pectin.
The pharmaceutical formulation may further include flavoring
agents, taste-masking agents, inorganic salts (for example sodium chloride),
antimicrobial agents (for example benzalkonium chloride), sweeteners,
antioxidants, antistatic agents, surfactants (for example polysorbates such as
"TWEEN 20" and "TWEEN 80"), sorbitan esters, lipids (for example
phospholipids such as lecithin and other phosphatidylcholines,
phosphatidylethanolamines), fatty acids and fatty esters, steroids (for
example
cholesterol), and chelating agents (for example EDTA, zinc and other such
suitable
cations). Other pharmaceutical excipients and/or additives suitable for use in
the
-22-
CA 02484950 2010-07-26
WO 2004/002827 PCT/US2003/020348
compositions according to the invention are listed in "Remington: The Science
&
Practice of Pharmacy", 19`" ed., Williams & Williams, (1995), and in the
"Physician's Desk Reference", 52 d ed., Medical Economics, Montvale, NJ
(1998),
both of which are incorporated herein by reference in their entireties.
"Mass median diameter" or "MMD" is a measure of mean particle
size, since the powders of the invention are generally polydisperse (i.e.,
consist of a
range of particle sizes). MMD values as reported herein are determined by
centrifugal sedimentation, although any number of commonly employed techniques
can be used for measuring mean particle size. "Mass median aerodynamic
diameter" or "NIlv AD" is a measure of the aerodynamic size of a dispersed
particle.
The aerodynamic diameter is used to describe an aerosolized powder in terms of
its
settling behavior, and is the diameter of a unit density sphere having the
same
settling velocity, generally in air, as the particle. The aerodynamic diameter
encompasses particle shape, density and physical size of a particle. As used
herein,
MMAD refers to the midpoint or median of the aerodynamic particle size
distribution of an aerosolized powder determined by cascade impaction.
In one version, the powdered formulation for use in the present
invention includes a dry powder having a particle size selected to permit
penetration
into the alveoli of the lungs, that is, preferably 10 m mass median diameter
(MMD), preferably less than 7.5 m, and most preferably less than 5 m, and
usually being in the range of 0.1 m to 5 m in diameter. The delivered dose
efficiency (DDE) of these powders may be greater than 30%, more preferably
greater than 40%, more preferably greater than 50% and most preferably greater
than 60% and the aerosol particle size distribution is about 1.0 - 5.0 m mass
median aerodynamic diameter (MMAD), usually 1.5 - 4.5 m MMAD and
preferably 1.5 - 4.0 m MMAD. These dry powders have a moisture content below
about 10% by weight, usually below about 5% by weight, and preferably below
about 3% by weight. Such powders are described in WO 95/24183, WO 96/32149,
WO 99/16419, and WO 99/16422.
-23-
CA 02484950 2004-10-28
WO 2004/002827 PCT/US2003/020348
Although the present invention has been described in considerable
detail with regard to certain preferred versions thereof, other versions are
possible,
and alterations, permutations and equivalents of the version shown will become
apparent to those skilled in the art upon a reading of the specification and
study of
the drawings. For example, the relative positions of the elements in the
expedients
for carrying out the relative movements may be changed. Also, the various
features
of the versions herein can be combined in various ways to provide additional
versions of the present invention. Furthermore, certain terminology has been
used
for the purposes of descriptive clarity, and not to limit the present
invention. For
example, the use of the terms such as "up" and "down" and "first" and "second"
may be reversed in the specification. Therefore, the appended claims should
not be
limited to the description of the preferred versions contained herein and
should
include all such alterations, permutations, and equivalents as fall within the
true
spirit and scope of the present invention.
-24-