Note: Descriptions are shown in the official language in which they were submitted.
CA 02485298 2007-08-01
51067-42
SUBSTITUTED PYRAZOLYL COMPOUNDS FOR THE TREATMENT OF
INFLAMMATION
FIELD OF TBE INVENTION
[001) The present invention in general is in the field of anti-inflammatory
pharmaceutical agents and specifically relates to substituted pyrazolyl
derivatives,
compositions comprising such, and methods for treating cancer, inflammation,
and
inflammation-associated disorders, such as arthritis.
BACKGROUND OF THE INVENT]ON
[002] The following description of the bacl:ground of the invention is
provided
to aid in the understanding the invention, but is not admitted to be or
describe prior
art to the invention.
[003J NF-xB is a ubiquitous transcription-factor that plays a prominent role
in
the activation of the immune system and in stress responses by regulating the
transcription of many early, inducible genes including proinflarrvmatory
cytokines,
adhesion molecules, growth factors, enzymes, and receptors (Ghosh S., May, M.
J,
and Kopp.-E (1998) Annu. Rev. Immunol. 16, 115-260; Zandi, E., and Karin, M.
(1999) Mol. Cell. Biol. 19, 4547-455 1; Karin, M. (1999) J. Biot. Chent.
274,.27339-
27342). Specificity of gene expression is deterrnined at a cellular level by a
diverse
array of external stimuli such as bacterial products including LPS, as well as
cytokines, most importantly tumor necrosis factor-a (TNFa) and interleukin-[i
(It.1R). Through the synergistic interaction with other transcription factors,
further
specificity can be achieved while maintaining enormous potential to
coordinately
1
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
induce a large number of functionally related genes. NF-xB is composed of homo
and heterodimers of the Rel protein family and is sequestered in an inactive
form in
the cytoplasm by members of the IxB family of inhibitory proteins (Ghosh S.,
May,
M. J., and Kopp. E (1998) Annu. Rev. Imrnunol. 16, 115-260; Zandi, E., and
Karin,
M. (1999) Mol. Cell. Biol. 19, 4547-455 1; Karin, M. (1999) J. Biol. C12em.
274,
27339-27342). IxBs mask the nuclear localization signal on NF-xB, preventing
nuclear translocation and hence DNA binding to the promoter regions of
responsive
genes. Stimulation of cells with an agonist that activates NF-xB leads to a
series of
biochemical signals, ultimately resulting in the phosphorylation,
ubiquitinylation,
and degradation of IKBs, thereby releasing NF-xB for nuclear translocation
(Ghosh
S., May, M. J., and Kopp. E (1998) Annu. Rev. bnmunol. 16, 115-260; Zandi, E.,
and Karin, M. (1999) Mol. Cell. Biol. 19, 4547-4551; Karin, M. (1999) J. Biol.
Chem. 274, 27339-27342). Recently, two IxB kinases (IKK1 or IKKa and IKK2 or
IKK(3), which phosphorylate IxBs and thereby initiate their degradation, have
been
cloned and characterized by a number of laboratories (Ghosh S., May, M. J.,
and
Kopp. E(1998) Annu. Rev. Immunol. 16, 115-260; Zandi, E., and Karin, M. (1999)
Mol. Cell. Biol. 19, 4547-4551; Karin, M. (1999) J. Biol. Clzem. 274, 27339-
27342). The catalytic subunits, IKK1 and IKK2, are similar structurally as
well as
enzymatically and exist as a heterodimer in a large protein complex referred
to as
the IKK signalsome (Regnier, C., Song, H., Gao, X., Goeddel, D., Cao, Z. and
Rothe, M. (1997) Cell 90, 373-383; DiDonato, J.A., Hayakawa, M., Rothwarf,
D.M., Zandi, E. and Karin, M. (1997) Nature 388, 548-554; Mercurio, F., Zhu,
H.,
Murray, B.W., Shevchenko, A., Bennett, B.L., Li, J.W., Young, D.B., Barbosa,
M.,
Mann, M., Manning, A. and Roa, A. (1997) Science 278, 860-866; Zandi, E.
Rothwarf, D.M., Delhase, M., Hayadawa, M and Karin, M. (1997) Cell 91, 243-
252; Woronicz, J.D., Gao, X., Cao, Z., Rothe, M. And Goeddel, D.V. (1997)
Science 278, 866-869). A third protein, NEMO (IKKy, IKKAP1), is a regtilatory
adapter protein necessary for IKK activation and kinase activity (Yamaoka, S.,
Courtois, G., Bessia, C., Whiteside, S. T., Weil, R., Agou, F., Kirk, H. E.,
Kay, R.
J., and Ireal, A. (1998) Cell 93, 1231-1240; Rothwarf, D. M., Zandi, E.,
Natoli, G.,
Karin, M. (1998) Nature 395, 297; Mercurio, F., Murray, B. W., Shevchenko, A.,
Bennet, B. L., Young, D. B., Li, J. W., Pascual, G., Motiwala, A., Zhu, H.,
Mann,
2
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
M and Manning, A. M. (1999) Mol. Cell. Biol. 2, 1526-1538). IKK1 and IKK2 are
co-expressed in most human adult tissues as well as in different developmental
stages of mouse embryos (Regnier, C., Song, H., Gao, X., Goeddel, D., Cao, Z.
and
Rothe, M. (1997) Cell 90, 373-383; DiDonato, J.A., Hayakawa, M., Rothwarf,
D.M., Zandi, E. and Karin, M. (1997) Nature 388, 548-554; Mercurio, F., Zhu,
H.,
Murray, B.W., Shevchenko, A., Bennett, B.L., Li, J.W., Young, D.B., Barbosa,
M.,
Mann, M., Manning, A. and Roa, A. (1997) Science 278, 860-866; Zandi, E.
Rothwarf, D.M., Delhase, M., Hayadawa, M and Karin, M. (1997) Cell 91, 243-
252; Woronicz, J.D., Gao, X., Cao, Z., Rothe, M. and Goeddel, D.V. (1997)
Science 278, 866-869; Hu, M. C. T., and Wang, Y. (1998) Gene 222, 31-40). This
kinase complex appears to represent a critical, common denominator in the
activation of NF-xB in a number of signal transduction pathways stimulated by
a
variety of agonists including cytokines, such as TNFa and IL1(3, microbial
products
such as LPS and viral proteins such as TAX, as well as phorbol esters,
oxidizing
agents and serine/tyrosine phosphatases (Ghosh S., May, M. J., and Kopp.
E(1998)
Annu. Rev. Immunol. 16, 115-260; Zandi, E., and Karin, M. (1999) Mol. Cell.
Biol.
19, 4547-4551; Karin, M. (1999) J. Biol. Chem. 274, 21339-27342).
[004] IKKl (also termed IKKa, Regnier, C., Song, H., Gao, X., Goeddel, D.,
Cao, Z. and Rothe, M. (1997) Cell 90, 373-383; DiDonato, J.A., Hayakawa, M.,
Rothwarf, D.M., Zandi, E. and Karin, M. (1997) Nature 388, 548-554; Mercurio,
F.,
Zhu, H., Murray, B.W., Shevchenko, A., Bennett, B.L., Li, J.W., Young, D.B.,
Barbosa, M., Mann, M., Manning, A. And Roa, A. (1997) Science 278, 860-866)
was cloned simultaneously by standard biochemical purification of the IxB
kinase
activity from TNFa stimulated HeLa S3 cells and by its interaction with the
MAP3K, NF-xB inducing kinase (NIK), in a yeast two-hybrid screen. IKKI was
identified as the previously cloned serine-threonine kinase, CHUK (Connelly,
M.
and Marcu, K. (1995) Cell. Mol. Biol. Res. 41, 537-549). IKK1 (also termed
IKKa) is an 85 kDa, 745 amino acid protein that contains an N-terminal
serine/threonine kinase catalytic domain, a leucine zipper-like amphipathic
helix,
and a C-terminal helix-loop-helix domain. IKK2 (also termed IKK(3) was also
cloned by standard biochemical purification, copurifying with IKKI from TNFa
3
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
stimulated HeLa S3 cells as well as by being identified in the public database
from
an EST clone with sequence homology to IKK1 (Mercurio, F., Zhu, H., Murray,
B.W., Shevchenko, A., Bennett, B.L., Li, J.W., Young, D.B., Barbosa, M., Mann,
M., Manning, A. and Roa, A. (1997) Science 278, 860-866; Zandi, E. Rothwarf,
D.M., Delhase, M., Hayadawa, M and Karin, M. (1997) Cell 91, 243-252;
Woronicz, J.D., Gao, X., Cao, Z., Rothe, M. And Goeddel, D.V. (1997) Science
278, 866-869). IKK2 is an 87 kDa, 756 amino acid protein with the same over
all
topology as IKK1 except for the addition of an 11 amino acid extension at the
C-terminus. IKK1 and IKK2 are 52% identical overall with 65% identity in the
kinase domain and 44% identity in the protein interaction domains in the C-
terminus. Data obtained using transient mammalian expression analysis, by in
vitro
translation experiments and by coexpression in a baculoviral system reveals
that
IKK1 and IKK2 associate preferentially as a heterodimer through their leucine
zipper motifs. Although homodimers have also been described in these systems,
the
heterodimer is thought to be the physiologic form of the kinase in mammalian
cells
(Zandi, E. Rothwarf, D.M., Delhase, M., Hayadawa, M and Karin, M. (1997) Cell
91, 243-252; Li, J., Peet, G.W., Pullen, S.S., Schembri-King, J., Warren,
T.C.,
Marcu, K.B., Kehry, M.R., Barton, R. and Jakes, S. (1998) J. Biol. Chem. 273,
30736-30741). Finally, NEMO (also termed IKKy) contains three a-helical
regions
including a leucine zipper, interacts preferentially with IKK2 and is required
for
activation of the heterodimeric kinase complex perhaps by bringing other
proteins
into the signalsome complex (Yamaoka, S., Courtois, G., Bessia, C., Whiteside,
S.
T., Weil, R., Agou, F., Kirk, H. E., Kay, R. J., and Ireal, A. (1998) Cell 93,
1231-
1240; Rothwarf, D. M., Zandi, E., Natoli, G., Karin, M. (1998) Nature 395,
297;
Mercurio, F., Murray, B. W., Shevchenko, A., Bennet, B. L., Young, D. B., Li,
J.
W., Pascual, G., Motiwala, A., Zhu, H., Mann, M and Manning, A. M. (1999) Mol.
Cell. Biol. 2, 1526-1538).
[005] The kinase activities of IKK1 and IKK2 are regulated by
phosphorylation and require an intact leucine zipper (LZ) for dimerization as
well as
an intact helix-loop-helix (HLH) domain, which can exert a positive regulatory
effect on kinase activity even when it is expressed in trans with the
remainder of the
4
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
IKK protein (Regnier, C., Song, H., Gao, X., Goeddel, D., Cao, Z. and Rothe,
M.
(1997) Cell 90, 373-383; DiDonato, J.A., Hayakawa, M., Rothwarf, D.M., Zandi,
E.
and Karin, M. (1997) Nature 388, 548-554; Mercurio,F., Zhu, H., Murray, B.W.,
Shevchenko, A., Bennett, B.L., Li, J.W., Young, D.B., Barbosa, M., Mann, M.,
Manning, A. and Roa, A. (1997) Science 278, 860-866; Zandi, E. Rothwarf, D.M.,
Delhase, M., Hayadawa, M and Karin, M. (1997) Cell 91, 243-252; Woronicz,
J.D.,
Gao, X., Cao, Z., Rothe, M. and Goeddel, D.V. (1997) Science 278, 866-869;
Dehase, M., Hayakawa, M., Chen, Y., and Karin, M. (1999) Science 284, 309-
313).
Both IKK subunits contain a canonical MAPKK activation loop motif near the N-
terminus which is the target for phosphorylation and activation of kinase
activity by
MAP3Ks such as NIK and MEKK1, although the physiologic regulation by these
two upstream kinases awaits further characterization (Zandi, E., and Karin, M.
(1999) Mol. Cell. Biol. 19, 4547-4551; Karin, M. (1999) J. Biol. Chem. 274,
27339-
27342; Karin, M., and Delhase, M. (1998) Proc. Natl. Acad. Sci. USA 95, 9067-
9069). Finally, phosphorylation of serines in the C-terminus of IKK2 results
in a
decrease in IKK activity and it is postulated to be responsible for the
transient
kinase activity seen after stimulation of cells with an agonist (Dehase, M.,
Hayakawa, M., Chen, Y., and Karin, M. (1999) Science 284, 309-313).
[006] IKK2 demonstrates a more potent kinase activity compared to IKK1
using IxBa or IicB(3 as a substrate (Mercurio, F., Zhu, H., Murray, B.W.,
Shevchenko, A., Bennett, B.L., Li, J.W., Young, D.B., Barbosa, M., Mann, M.,
Manning, A. and Roa, A. (1997) Science 278, 860-866; Zandi, E. Rothwarf, D.M.,
Delhase, M., Hayadawa, M and Karin, M. (1997) Cell 91, 243-252; Woronicz,
J.D.,
Gao, X., Cao, Z., Rothe, M. and Goeddel, D.V. (1997) Science 278, 866-869;
Dehase, M., Hayakawa, M., Chen, Y., and Karin, M. (1999) Science 284, 309-
313).
Mutations of the phospho-acceptor serine residues within the MAPKK activation
loop alters IKK2 kinase activity; the serine to alanine substitutions result
in
decreased kinase activity whereas the serine to glutamic acid substitutions
result in
a constitutively active kinase. Similar alanine mutations in IKKl do not
result in a
decreased stimulation of total IKK activity in response to TNFa or IL1 J3
(Dehase,
M., Hayakawa, M., Chen, Y., and Karin, M. (1999) Science 284, 309-313). IKK2
5
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
being the dominant kinase activity within the IKK complex is further supported
by
the analysis of fibroblasts from mice deficient in IKK1 or IKK2. Fibroblasts
lacking IKKl retain full IKK activity in response to cytokines and could
activate
NF-xB. In contrast, fibroblasts lacking IKK2 do not exhibit IKK activity when
stimulated with cytokines nor do they activate NF-xB. Furthermore, the
phenotypes
of each IKK knock out is unique with IKK1 deficiency resulting in skin and
skeletal
defects and IKK2 knock out being embryonic lethal due to hepatocyte apoptosis
(Li,
Q., Antwerp, D. V., Mercurio, F., Lee, K., and Verma, I. M. (1999) Science
284,
321-325; Takeda, K., Tekeuchi, 0., Tsujimura, T., Itami, S., Adachi, 0.,
Kawai, T.,
Sanjo, H., Yoshikawa, K., Terada, N, and Akira, S. (1999) Science 284, 313-
316;
Hu, Y., Baud, V., Delhase, M., Zhang, P., Deerinck, T., Ellisman, M., Johnson,
R.,
and Karin, M. (1999) Science 284, 315-320; Li, Q., Lu, Q., Hwang, J. Y.,
Buscher,
D., Lee, K., Izpisua-Belmonte, J. C., and Verma, I. M. (1999) Gene and
Developnaent 13, 1322-1328; Tanaka, M., Fuentes, M. E., Yamaguchi, K., Durnin,
M. H., Dalrymple, S. A., Hardy, K. L., and Goeddel, D. V. (1999) Ifnmunity 10,
421-429).
[0071 It is well-known that NF-KB plays a key role in the regulated expression
of a large number of pro-inflammatory mediators including cytokines such as IL-
6
and IL-8, cell adhesion molecules, such as ICAM and VCAM, and inducible nitric
oxide synthase (iNOS). Such mediators are known to play a role in the
recruitment
of leukocytes at sites of inflammation and in the case of iNOS, may lead to
organ
destruction in some inflammatory and autoimmune diseases. The importance of
NF-xB in inflammatory disorders is further strengthened by studies of airway
inflammation including asthma in which NF-icB has been shown to be activated.
This activation may underlie the increased cytokine production and leukocyte
infiltration characteristic of these disorders. In addition, inhaled steroids
are known
to reduce airway hyperresponsiveness and suppress the inflammatory response in
asthmatic airways. In light of the recent findings with regard to
glucocorticoid
inhibition of NF-xB, one may speculate that these effects are mediated through
an
inhibition of NF-xB. Further evidence for a role of NF-xB in inflammatory
disorders comes from studies of rheumatoid synovium. Although NF-icB is
6
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
normally present as an inactive cytoplasmic complex, recent
immunohistochemical
studies have indicated that NF-xB is present in the nuclei, and hence active,
in the
cells comprising rheumatoid synovium. Furthermore, NF-xB has been shown to be
activated in human synovial cells in response to stimulation with TNF-a. Such
a
distribution may be the underlying mechanism for the increased cytokine and
eicosanoid production characteristic of this tissue. See Roshak, A. K., et
al., J. Biol.
Chem., 271, 31496-31501 (1996).
[008] The NF-xB/Rel and IxB proteins are also likely to play a key role in
neoplastic transformation. Family members are associated with cell
transformation
in vitro and in vivo because of overexpression, gene amplification, gene
rearrangements, or translocations (Gilmore TD, Trends Genet 7:318-322, 1991;
Gillmore TD, Oncogene 18:6925-6937, 1999; Rayet B. et al., Oncogene 18: 6938-
6947, 1991). In addition, rearrangement and/or amplification of the genes
encoding
these proteins are seen in 20-25% of certain human lymphoid tumors. In
addition, a
role for NF-xB in the regulation of apoptosis, cell cycle progression,
invasion, and
metastasis has been reported (Bours V. et al., Biochemical Phaf-macology
60:1085-
1090, 2000) strengthening the role of this transcription factor in the control
of cell
proliferation. The inhibition of NF-xB has been shown to potentiate TNF- and
cancer therapy through increased apoptosis (Wang C-Y et al., Science 274:784-
787,
1996; Wang C-Y et al., Nat Med 5:412-417, 1999). It has also been shown that
human T-cell leukemia virus type 1(HTLV1) infected cells (the etiological
agent of
an aggressive malignancy of activated CD4+ T lymphocytes), IKKa and IKK(3 are
expressed constitutively, which normally function in a transient manner (Chu Z-
L et
al., J of Biological Cl2emistry 273:15891-15894, 1998). The HTLV1 transforming
and transactivating protein (Tax) has been shown to bind MEKK1 and increases
the
activity of IKK(3 to enhance phosphorylation of serine residues in IxBa that
lead to
its degradation.
[009] Pyrazoles have been described for use in the treatment of inflammation.
U.S. Patent No. 5,134,142 to Matsuo et al describes 1,5-diaryl pyrazoles, and
7
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
specifically, 1-(4-fluorophenyl)-5-[4-(methylsulfonyl)phenyl]-3-
trifluoromethyl
pyrazole, as having anti-inflammatory activity.
[0010] U.S. Patent No. 3,940,418 to R. Hamilton describes tricyclic 4,5-
dihydrobenz[g]indazoles as antiinflammatory agents. In addition, R. Hamilton
[J.
Heterocyclic Chem., 13, 545 (1976)] describes tricyclic 4,5- '
dihydrobenz[g]indazoles as antiinflammatory agents. U.S. Patent No. 5,134,155
describes fused tricyclic pyrazoles having a saturated ring bridging the
pyrazole and
a phenyl radical as HMG-CoA reductase inhibitors. European publication EP
477,049, published Mar. 25, 1992, describes [4,5-dihydro-l-phenyl-lH-
benz[g]indazol-3-yl]amides as having antipsychotic activity. European
publication
EP 347,773, published Dec. 27, 1989, describes [4,5-dihydro-1-phenyl-lH-
benz[g]indazol-3-y1]propanamides as immunostimulants. M. Hashem et al [J. Med.
Chem., 19, 229 (1976)] describes fused tricyclic pyrazoles, having a saturated
ring
bridging the pyrazole and a phenyl radical, as antibiotics.
[0011] Certain substituted pyrazolyl-benzenesulfonamides have been described
in the literature as synthetic intermediates. Specifically, 4-[5-(4-
chlorophenyl)-3-
phenyl-lH-pyrazol-l-yl]benzenesulfonamide has been prepared from a pyrazoline
compound as an intermediate for compounds having hypoglycemic activity [R.
Soliman et al, J. Pharm. Sci., 76, 626 (1987)]. 4-[5-[2-(4-Bromophenyl)-2H-
1,2,3-
triazol-4-yl]-3-methyl-IH-pyrazol-1-yl]benzenesulfonamide has been prepared
from
a pyrazoline compound and described as potentially having hypoglycemic
activity
[H. Mokhtar, Pak. J. Sci. Ind. Res., 31, 762 (1988)]. Similarly, 4-[4-bromo-5-
[2-(4-
chlorophenyl)-2H-1,2,3-triazol-4-yl]-3-methyl-lH-pyrazol-1-
y1]benzenesulfonamide has been prepared [H. Mokhtar et al, Pak. J. Sci. Ind.
Res.,
34, 9 (1991)].
[0012] The phytotoxicity of pyrazole derivatives is described [M. Cocco et al,
Il. Farinaco-Ed. Sci., 40, 272 (1985)], specifically for 1-[4-
(aminosulfonyl)phenyl]-
5-phenyl-lH-pyrazole-3,4-dicarboxylic acid.
8
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
[0013] The use of styryl pyrazole esters for antidiabetes drugs is described
[H.
Mokhtar et al, PhaYrnazie, 33, 649-651 (1978)]. The use of styryl pyrazole
carboxylic acids for antidiabetes drugs is described [R. Soliman et al,
Phannazie,
33, 184-5 (1978)]. The use of 4-[3,4,5-trisubstituted-pyrazol-l-
yl]benzenesulfonamides as intermediates for sulfonylurea anti-diabetes agents
is
described, and specifically, 1-[4-(aminosulfonyl)phenyl]-3-methyl-5-phenyl-lH-
pyrazole-4-carboxylic acid [R. Soliman et al, J. Pharm. Sci., 72, 1004
(1983)]. A
series of 4-[3-substituted methyl-5-phenyl-lH-pyrazol-l-yl]benzenesulfonamides
has been prepared as intermediates for anti-diabetes agents, and more
specifically,
4-[3-methyl-5-phenyl-lH-pyrazol-1-yl]benzenesulfonamide [H. Feid-Allah,
PhaYmazie, 36, 754 (1981)]. In addition, 1-(4-[aminosulfonyl]phenyl)-5-
phenylpyrazole-3-carboxylic acid has been prepared from the above described 4-
[3-
methyl-5-phenyl-lH-pyrazol-1-yl]benzenesulfonamide compound [R. Soliman et al,
J. Pharm. Sci., 70, 602 (1981)].
[0014] WO 00/27822 discloses tricyclic pyrazole derivatives, WO 00/59901
discloses dihydroindeno pyrazoles, WO 99/17769 discloses indeno[1,2-c]-,
naphtho[1,2-c]- and benzo[6,7]cyclohepta[1,2-c]pyrazole derivatives, US
5,196,445
discloses heteroaryl-3-oxo-propanenitrile derivatives useful in the treatment
of
rheumatoid arthritis, WO 97/10210 discloses tricyclic pyrrolidine derivatives
as
calcium channel antagonists, WO 95/15315 discloses diphenyl pyrazole
compounds, WO 95/15317 discloses triphenyl pyrazole compounds, WO 95/15318
discloses tri-substituted pyrazole compounds, and WO 96/09293 discloses
benz[g]indazolyl derivatives.
[0015] WO 95/15316 discloses substituted pyrazolyl benzenesulfonamide
derivatives and WO 01/32663 discloses pyrazlecarboxylic acid tricyclic
derivatives
as CB 1 cannabinoid receptor inhibitors.
9
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
DETAILED DESCRIPTION OF THE INVENTION
[0016] A class of compounds, which are useful in treating cancer,
inflammation, and inflammation related disorders, is defined by Formula I:
R2
R12
B
R1 --(
3
X R
Z
R
A
R11
wherein
A is (CH2),,,; wherein each CH2 may be independently substituted
with one or more substitution selected from the group consisting of:
aryl, heteroaryl, alkanoyl, hydroxy, halogen, alkoxy, lower alkyl,
amino, aminoalkyl, alkylamino, alkenyl, and alkynyl;
m is 1 to 4;
B is a 5 or 6 membered heteroaryl, aryl, saturated or unsaturated
heterocyclic wherein said aryl, heteroaryl, or heterocyclic are
optionally substituted with R1, R2, and R12;
X is selected from the group consisting of: N and C;
Y and Z are independently selected from the group consisting of: N,
CH, CR3, S, and 0; ,
R' is selected from the group consisting of: hydrido, halogen, alkyl,
aryl, heteroaryl, alkenyl, alkynyl, haloalkyl, CN, NO2, OR5,
OCOOR5, CO2R~, CON(R6)R7, COR6, SR6, SOR6, SO2R6, NR6R7,
NR6CORI, NR6CONHR7, NR6SO2R7, NR6SO2NHR7, and
SO2N(R6)W wherein R6 and R7 may be taken together to form a 3-7
membered carbocyclic ring having 1 to 3 substituted or unsubstituted
heteroatoms selected from the group consisting of: S, SO, SO2, 0,
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
and NR6; wherein said alkenyl, alkynyl, alkyl, aryl, heteroaryl or OR5
are optional substituted with, hydrido, halogen, alkyl, hydroxyalkyl,
aryl, heteroaryl, haloalkyl, COCF3, CN, NO2, ORS, OCOORS,
COZR7, CON(R6)R7, COR6, SR6, SOR6, S02R6, NR6R7, NR6COR7,
NR6CONHR7, NR6SO2W, NR6SO2NHR7, and SO2N(R)R' wherein
R6 and R7 may be taken together to form a 3-7 membered
carbocyclic ring having 1 to 3 substituted or unsubstituted
heteroatoms selected from the group consisting of: S, SO, SO2, 0,
and NR6;
R2 is selected from the group consisting of: halogen, hydrido,
hydroxyalkyl, alkyl, OR6, CN, NO2, SR6, NHR6, CON(R)R7,
NHCONHR6, CO2H, and haloalkyl;
Rr and R2 may be taken together to form a 5 to 7 membered
saturated or unsaturated carbocyclic ring optionally containing 0 to 3
heteroatoms selected from the group consisting of N, 0, or S, and
wherein said ring is optionally substituted with Rl;
R3 is selected from the group consisting of: substituted or
unsubstituted amidine, alkylamino, aminoalkyl, CONHW, NH2,
NHCOR6, and CH2NHCOR6;
R4 is selected from the group consisting of: halogen, alkylsulfinyl,
alkylsulfonyl, cyano, alkoxycarbonyl, alkyl, haloalkyl, hydrido,
hydroxyalkyl, haloalkoxy, heterocyclic, nitro, acylamino, aryl,
heteroaryl, and alkenyl, OR13, SR$, SO2N(R8)R", NHR9, NHCOR9,
NR9COR9, NHCO(OR9), NR9CO(OR9), NRgS02R10,
NHSOZN(R10)R10', NR6CON(Rl0)Rl0', COR9, C02Rg, CON(R)R8',
wherein R8 and R8' may be taken together to form a 3-7 membered
carbocyclic ring having 1 to 3 substituted or unsubstituted
heteroatoms selected from S, SO, SO2, 0, N, and NR6, and wherein
R10 and R10'may be taken together to form a 3-7 membered
carbocyclic ring having 1 to 3 substituted or unsubstituted
heteroatoms selected from S, SO, SO2, 0, N, and NR6 wherein said
11
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
aryl, heterocyclic, heteroaryl, or alkenyl are optionally substituted
with R9;
R5 is selected from the group consisting of: hydrido, alkyl, aryl,
arylalkyl, heteroaryl, heterocyclicalkyl, and heteroarylalkyl, wherein
aryl, alkyl, arylalkyl, heteroaryl, heterocyclicalkyl, or heteroarylalkyl
are optionally substituted with one or more radicals selected from the
group consisting of OR14, N(R14)R14', and glycols;
R6 is independently selected from the group consisting of: hydrido,
aryl, heteroaryl, lower alkyl, haloalkyl, alkenyl, alkynyl,
hydroxyalkyl, aminoalkyl, alkylaminoalkyl, alkoxy, alkoxyalkyl,
heterocyclicalkyl, and heterocyclic;
R7 is independently selected from the group consisting of: hydrido,
aryl, heteroaryl, lower alkyl, haloalkyl, alkenyl, alkynyl,
hydroxyalkyl, aminoalkyl, alkylaminoalkyl, alkoxy, alkoxyalkyl,
heterocyclicalkyl, and heterocyclic;
R$ is independently selected from the group consisting of: hydrido,
aryl, heteroaryl, arylalkyl, heterocyclic, haloalkyl, arylalkylamino,
alkylaminoalkyl, dialkylaminoalkyl, alkyl, alkenyl, alkynyl,
heteroarylalkyl, and heterocyclicalkyl;
R8'is independently selected from the group consisting of: hydrido,
aryl, heteroaryl, arylalkyl, heterocyclic, haloalkyl, arylalkylamino,
alkylaminoalkyl, dialkylaminoalkyl, alkyl, alkenyl, alkynyl,
heteroarylalkyl, and heterocyclicalkyl;
R9 is independently selected from the group consisting of: hydrido,
lower alkyl, aryl, heteroaryl, arylalkyl, heterocyclic, cycloalkyl,
heterocyclicalkyl, haloalkyl, arylalkylamino, amino, aminoalkyl,
aminoacyl, nitro, azido, and heteroarylalkyl, wherein alkyl, aryl,
heteroaryl, aminoalkyl, or arylalkyl are optionally substituted with
one or more radical selected from the group consisting of:
alkylsulfonamide, sulfamyl, alkyl, alkylthio, alkylsulfinyl,
alkylsulfonyl, alkylamino, aminoalkyl, alkylaminoalkyl, alkoxy,
halogen, acyloxy, oxy, formyl, haloalkyl, cyano, haloalkoxy, acyl,
12
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
carboxyl, hydroxy, hydroxyalkyloxy, phenoxy, nitro, azido,
benzyloxy, dialkylam.inoacyl, thioalkyl, aminoacyloxy, thiocyanate,
isothiocyanate, alkyldioxy, hydroxyalkyl, alkylamino,
alkyloxycarbonyl, alkoxyalkyl, alkenylamino, alkynylamino, alkenyl,
alkynyl, dialkylaminoalkyloxy, and heterocyclic optionally
substituted with alkyl, alkylamino, aminoalkyl, hydroxyalkyl, and
alkylaminoalkyl;
R10 is independently selected from the group consisting of: hydrido,
lower alkyl, heteroaryl, heterocyclic, haloalkyl, arylalkylamino,
heteroarylalkyl, aryl, and arylalkyl, wherein aryl, heteroaryl,
heterocyclic, or arylalkyl are optionally substituted with one or more
radical selected from alkyl, alkoxy, halogen, haloalkyl, cyano,
haloalkoxy, acyl, carboxyl, hydroxy, hydroxyalkyloxy, phenoxy,
benzyloxy, dialkylaminoalkyloxy, and heterocyclic,
RiO' is independently selected from the group consisting of: hydrido,
lower alkyl, heteroaryl, heterocyclic, haloalkyl, arylalkylamino,
heteroarylalkyl, aryl, and arylalkyl, wherein aryl, heteroaryl,
heterocyclic, or arylalkyl are optionally substituted with one or more
radical selected from alkyl, alkoxy, halogen, haloalkyl, cyano,
haloalkoxy, acyl, carboxyl, hydroxy, hydroxyalkyloxy, phenoxy,
benzyloxy, dialkylaminoalkyloxy, and heterocyclic,
R" is selected from the group consisting of: hydrido, halogen,
haloalkyl, CN, C02R5, lower alkyl, lower alkenyl, lower alkynyl,
alkoxy, and CONH2;
R12 is selected from the group consisting of: hydrido, halogen, alkyl,
and alkoxy;
Ri3 is selected from the group consisting of: hydrido, alkyl, aryl,
arylalkyl, heteroaryl, heterocyclicalkyl, and heteroarylalkyl, wherein
aryl, alkyl, arylalkyl, heteroaryl, heterocyclicalkyl, or heteroarylalkyl
are optionally substituted with one or more radicals selected from the
group consisting of OR14 , N(R14)R1, and glycols;
13
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
R14 is independently selected from the group consisting of hydrido,
and lower alkyl; and
R14' is independently selected from the group consisting of hydrido,
and lower alkyl;
or isomers, tautomers, carriers, esters, prodrugs, pharmaceutically
acceptable salts thereof.
[0017] Another class of compounds is defined by Formula .II
R2
R12
R1
B
-N
\\,Rs
R4 ~
A
Rt1
wherein
A is (CH2)m; wherein each CH2 may be independently substituted
with one or more substitution selected from the group consisting of:
aryl, heteroaryl, alkanoyl, hydroxy, halogen, alkoxy, lower alkyl,
amino, aminoalkyl, alkylamino, alkenyl, and alkynyl;
m is 1 to 4;
B is a 5 or 6 membered heteroaryl, aryl, saturated or unsaturated
heterocyclic wherein said aryl, heteroaryl, or heterocyclic are
optionally substituted with R1, R2, and R12;
RI is selected from the group consisting of: hydrido, halogen, alkyl,
aryl, heteroaryl, alkenyl, alkynyl, haloalkyl, CN, NO2, ORS,
14
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
OCOORS, COZR7, CON(R6)R7, COR6, SR6, SOR6, S02R6, NR6R7,
NR6COR', NR6CONHR~, NR6SO2R7, NR6SO2NHR7, and
SO2N(R6)R7 wherein R6 and R7 may be taken together to form a 3-7
membered carbocyclic ring having 1 to 3 substituted or unsubstituted
heteroatoms selected from the group consisting of: S, SO, S02, O,
and NR6; wherein said alkenyl, alkynyl, alkyl, aryl, heteroaryl or OR5
are optional substituted with, hydrido, halogen, alkyl, hydroxyalkyl,
aryl, heteroaryl, haloalkyl, COCF3, CN, NOa, ORS, OCOORS,
C02R7, CON(R6)R7, COR6, SR6, SOR6, S02R6, NR6R7, NRgCOR7,
NR6CONHR7, NR6SO2R7, NR6SO2NHR7, and SO2N(R6)R7 wherein
R6 and R7 may be taken together to form a 3-7 membered
carbocyclic ring having 1 to 3 substituted or unsubstituted
heteroatoms selected from the group consisting of: S, SO, SO2, 0,
and NR6;
R2 is selected from the group consisting of: halogen, hydrido,
hydroxyalkyl, alkyl, OR6, CN, NO2, SR6, NHR6, CON(R6)R7 ,
NHCONHR6, CO2H, and haloalkyl;
Ri' and R2 may be taken together to form a 5 to 7 membered
saturated or unsaturated carbocyclic ring optionally containing 0 to 3
heteroatoms selected from the group consisting of N, 0, or S, and
wherein said ring is optionally substituted with R1;
R3 is selected from the group consisting of: substituted or
unsubstituted amidine, alkylamino, aminoalkyl, CONHR7 , NH2,
NHCOR6, and CH2NHCOR6;
R4 is selected from the group consisting of: halogen, alkylsulfinyl,
alkylsulfonyl, cyano, alkoxycarbonyl, alkyl, haloalkyl, hydrido,
hydroxyalkyl, haloalkoxy, heterocyclic, nitro, acylamino, aryl,
heteroaryl, and alkenyl, OR13, SRB, SO2N(R8)R", NHR9, NHCOR9,
NR9COR9, NHCO(OR9), NR9CO(OR9), NR8SO2R10,
NHSOZN(R10)R"", NR6CON(R10)R"", COR9, CO2R8, CON(R)R",
wherein R8 and R8'may be taken together to form a 3-7 membered
carbocyclic ring having 1 to 3 substituted or unsubstituted
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
heteroatoms selected from S, SO, S02, 0, N, and NR6, and wherein
Rl0 and R10'may be taken together to form a 3-7 membered
carbocyclic ring having 1 to 3 substituted or unsubstituted
heteroatoms selected from S, SO, S02, O, N, and NR6 wherein said
aryl, heterocyclic, heteroaryl, or alkenyl are optionally substituted
with R9;
RS is selected from the group consisting of: hydrido, alkyl, aryl,
arylalkyl, heteroaryl, heterocyclicalkyl, and heteroarylalkyl, wherein
aryl, alkyl, arylalkyl, heteroaryl, heterocyclicalkyl, or heteroarylalkyl
are optionally substituted with one or more radicals selected from the
group consisting of OR14, N(R14)R14', and glycols;
R6 is independently selected from the group consisting of: hydrido,
aryl, heteroaryl, lower alkyl, haloalkyl, alkenyl, alkynyl,
hydroxyalkyl, aminoalkyl, alkylaminoalkyl, alkoxy, alkoxyalkyl,
heterocyclicalkyl, and heterocyclic;
R7 is independently selected from the group consisting of: hydrido,
aryl, heteroaryl, lower alkyl, haloalkyl, alkenyl, alkynyl,
hydroxyalkyl, aminoalkyl, alkylaminoalkyl, alkoxy, alkoxyalkyl,
heterocyclicalkyl, and heterocyclic;
R8 is independently selected from the group consisting of: hydrido,
aryl, heteroaryl, arylalkyl, heterocyclic, haloalkyl, arylalkylamino,
alkylaminoalkyl, dialkylaminoalkyl, alkyl, alkenyl, alkynyl,
heteroarylalkyl, and heterocyclicalkyl;
R ,is independently selected from the group consisting of: hydrido,
aryl, heteroaryl, arylalkyl, heterocyclic, haloalkyl, arylalkylamino,
alkylaminoalkyl, dialkylaminoalkyl, alkyl, alkenyl, alkynyl,
heteroarylalkyl, and heterocyclicalkyl;
R9 is independently selected from the group consisting of: hydrido,
lower alkyl, aryl, heteroaryl, arylalkyl, heterocyclic, cycloalkyl,
heterocyclicalkyl, haloalkyl, arylalkylamino, amino, aminoalkyl,
aminoacyl, nitro, azido, and heteroarylalkyl, wherein alkyl, aryl,
heteroaryl, aminoalkyl, or arylalkyl are optionally substituted with
16
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
one or more radical selected from the group consisting of:
alkylsulfonamide, sulfamyl, alkyl, alkylthio, alkylsulfinyl,
alkylsulfonyl, alkylamino, aminoalkyl, alkylaminoalkyl, alkoxy,
halogen, acyloxy, oxy, formyl, haloalkyl, cyano, haloalkoxy, acyl,
carboxyl, hydroxy, hydroxyalkyloxy, phenoxy, nitro, azido,
benzyloxy, dialkylaminoacyl, thioalkyl, aminoacyloxy, thiocyanate,
isothiocyanate, alkyldioxy, hydroxyalkyl, alkylamino,
alkyloxycarbonyl, alkoxyalkyl, alkenylamino, alkynylamino, alkenyl,
alkynyl, dialkylaminoalkyloxy, and heterocyclic optionally
substituted with alkyl, alkylamino, aminoalkyl, hydroxyalkyl, and
alkylaminoalkyl;
R10 is independently selected from the group consisting of: hydrido,
lower alkyl, heteroaryl, heterocyclic, haloalkyl, arylalkylamino,
heteroarylalkyl, aryl, and arylalkyl, wherein aryl, heteroaryl,
heterocyclic, or arylalkyl are optionally substituted with one or more
radical selected from alkyl, alkoxy, halogen, haloalkyl, cyano,
haloalkoxy, acyl, carboxyl, hydroxy, hydroxyalkyloxy, phenoxy,
benzyloxy, dialkylaminoalkyloxy, and heterocyclic,
R10' is independently selected from the group consisting of: hydrido,
lower alkyl, heteroaryl, heterocyclic, haloalkyl, arylalkylamino,
heteroarylalkyl, aryl, and arylalkyl, wherein aryl, heteroaryl,
heterocyclic, or arylalkyl are optionally substituted with one or more
radical selected from alkyl, alkoxy, halogen, haloalkyl, cyano,
haloalkoxy, acyl, carboxyl, hydroxy, hydroxyalkyloxy, phenoxy,
benzyloxy, dialkylaminoalkyloxy, and heterocyclic,
Rli is selected from the group consisting of: hydrido, halogen,
haloalkyl, CN, C02R5, lower alkyl, lower alkenyl, lower alkynyl,
alkoxy, and CONH2;
R12 is selected from the group consisting of: hydrido, halogen, alkyl,
and alkoxy;
R13 is selected from the group consisting of: hydrido, alkyl, aryl,
arylalkyl, heteroaryl, heterocyclicalkyl, and heteroarylalkyl, wherein
17
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
aryl, alkyl, arylalkyl, heteroaryl, heterocyclicalkyl, or heteroarylalkyl
are optionally substituted with one or more radicals selected from the
group consisting of OR14, N(RI4)R14', and glycols;
R14 is independently selected from the group consisting of hydrido,
and lower alkyl; and
,
R14' is independently selected from the group consisting of hydrido,
and lower alkyl;
or isomers, tautomers, carriers, esters, prodrugs, pharmaceutically
acceptable salts thereof.
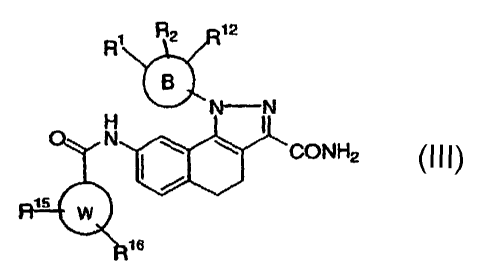
Another class of compounds is defined by Formula III
R1 R2 12
\
NN
N
CONH2
W
16
wherein
B is a 5 or 6 membered heteroaryl, aryl, saturated or unsaturated
heterocyclic wherein said aryl, heteroaryl, or heterocyclic are
optionally substituted with R1, R2, and R12;
W is a 5 or 6 membered heteroaryl, aryl, saturated or unsaturated
heterocyclic;
Rl is selected from the group consisting of: hydrido, halogen, alkyl,
aryl, heteroaryl, alkenyl, alkynyl, haloalkyl, CN, NO2, OR$,
OCOORS, COZR~, CON(R6)R7, COR6, SR6, SOR6, SOZR6, NR6R7,
NR6COR', NR6CONHR~, NR6SO2R7, NR6SO2NHR7, and
SOZN(R6)R7 wherein R6 and R7 may be taken together to form a 3-7
membered carbocyclic ring having 1 to 3 substituted or unsubstituted
18
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
heteroatoms selected from the group consisting of: S, SO, S02, 0,
and NR6; wherein said alkenyl, alkynyl, alkyl, aryl, heteroaryl or OR5
are optional substituted with, hydrido, halogen, alkyl, hydroxyalkyl,
aryl, heteroaryl, haloalkyl, COCF3, CN, NO2, ORS, OCOORS,
C02R7, CON(R6)R7, COR6, SR6, SOR6, S02R6, NR6R7, NR6COW,
NR6CONHR7 , NR6SO2R7, NR6SO2NHR~, and SO2N(R6)R7 wherein
R6 and R7 may be taken together to form a 3-7 membered
carbocyclic ring having 1 to 3 substituted or unsubstituted
heteroatoms selected from the group consisting of: S, SO, SO2, 0,
and NR6;
R2 is selected from the group consisting of: halogen, hydrido,
hydroxyalkyl, alkyl, OR6, CN, NO2, SR6, NHR6, CON(R6)R7,
NHCONHR6, CO2H, and haloalkyl;
Rl and R2 may be taken together to form a 5 to 7 membered
saturated or unsaturated carbocyclic ring optionally containing 0 to 3
heteroatoms selected from the group consisting of N, 0, or S, and
wherein said ring is optionally substituted with R1;
R5 is selected from the group consisting of: hydrido, alkyl, aryl,
arylalkyl, heteroaryl, heterocyclicalkyl, and heteroarylalkyl, wherein
aryl, alkyl, arylalkyl, heteroaryl, heterocyclicalkyl, or heteroarylalkyl
are optionally substituted with one or more radicals selected from the
group consisting of OR14, N(R14)R14~, and glycols;
R6 is independently selected from the group consisting of: hydrido,
aryl, heteroaryl, lower alkyl, haloalkyl, alkenyl, alkynyl,
hydroxyalkyl, aminoalkyl, alkylaminoalkyl, alkoxy, alkoxyalkyl,
heterocyclicalkyl, and heterocyclic;
R7 is independently selected from the group consisting of: hydrido,
aryl, heteroaryl, lower alkyl, haloalkyl, alkenyl, alkynyl,
hydroxyalkyl, aminoalkyl, alkylaminoalkyl, alkoxy, alkoxyalkyl,
heterocyclicalkyl, and heterocyclic;
R 12 is selected from the group consisting of: hydrido, halogen, alkyl,
and alkoxy;
19
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
R15 is selected 'from the group consisting of: alkylsulfonamide,
sulfamyl, alkyl wherein said alkyl is optionally substituted with a
carbocyclic or heterocyclic wherein said carbocyclic or heterocyclic
is optionally substituted with one to six substituents selected from
the group consisting of alkyl, alkylamino, aminoalkyl, hydroxyalkyl,
alkylaminoalkyl, alkylaminoalkylamino, dialkylaminoalkylamino,
alkylamino(alkyl)amino, alkoxy, alkoxyalkyl, oxo, hydroxy, amino,
halogen, cyano, nitro, acyl, heteroaryl wherein said heteroaryl is
optionally substituted with one or more halogen, (CH2)r,C(R')R'
wherein n is 0 to 4 and each R' is independently selected from the
group consisting of hydrido, hydroxy, amino, 0, S, and alkyl,
(CH2)õNHCON(R')R' wherein n is 0 to 4 and each R' is
independently selected from the group consisting of hydrido,
hydroxy, amino, and alkyl, (CH2)õNHC(O)OR' wherein n is 0 to 4
and R' is selected from the group consisting of hydrido, hydroxy,
amino, and alkyl; alkylthio, alkylsulfinyl, alkylsulfonyl, alkylamino,
aminoalkyl, alkylaminoalkyl, dialkylaminoalkyl, alkoxy, halogen,
acyloxy, oxy, formyl, haloalkyl, cyano, haloalkoxy, acyl, carboxyl,
hydroxy, hydroxyalkyloxy, phenoxy, nitro, azido, benzyloxy,
dialkylaminoacyl, thioalkyl, aminoacyloxy, thiocyanate,
isothiocyanate, alkyldioxy, hydroxyalkyl, alkylamino,
alkyloxycarbonyl, alkoxyalkyl, alkenylamino, alkynylamino, alkenyl,
alkynyl, dialkylaminoalkyloxy, and heterocyclic wherein said
heterocyclic is optionally substituted with one to six substituents
selected from the group consisting of alkyl, alkylamino, aminoalkyl,
hydroxyalkyl, alkylaminoalkyl, alkylaminoalkylamino,
dialkylaminoalkylamino, alkylamino(alkyl)amino, alkoxy,
alkoxyalkyl, oxo, hydroxy, amino, halogen, cyano, nitro, acyl,
heteroaryl wherein said heteroaryl is optionally substituted with one
or more halogen, (CH2)nC(R')R' wherein n is 0 to 4 and each R' is
independently selected from the group consisting of hydrido,
hydroxy, amino, 0, S, and alkyl, (CH2)nNHCON(R')R' wherein n is
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
0 to 4, and each R' is independently selected from the group
consisting of hydrido, hydroxy, amino, and alkyl,
(CH2)nNHC(O)OR' wherein n is 0 to 4 and R' is selected from the
group consisting of hydrido, hydroxy, amino, and alkyl,
'~ R17
5'~N / .~S N R17
N
S" =
ON R18
N~
N
\R18 R17 ~R18
R17
N
and R18
R17 is selected from the group consisting of: alkyl, hydroxyalkyl,
alkoxyalkyl, aminoalkyl, haloalkyl, acyl, thioalkyl, dialkylaminoacyl,
alkylsulfonyl, arylsulfonyl, CO(alkyl), CO(aryl), CO(CH2)nOH [n =
0 to 4], C02(alkyl), CON(alkyl)(alkyl'), formyl, cycloalkyl,
heterocyclic, hydroxyalkoxyalkyl, alkenylalkyl, alkynylalkyl,
arylalkyl, and heteroarylalkyl; and
R18 is selected from the group consisting of: alkyl, hydroxyalkyl,
alkoxyalkyl, aminoalkyl, haloalkyl, acyl, thioalkyl, dialkylaminoacyl,
alkylsulfonyl, arylsulfonyl, CO(alkyl), CO(aryl), CO(CH2)nOH [n =
0 to 4], C02(alkyl), CON(alkyl)(alkyl'), formyl, cycloalkyl,
heterocyclic, hydroxyalkoxyalkyl, alkenylalkyl, alkynylalkyl,
arylalkyl, and heteroarylalkyl;
or isomers, tautomers, carriers, esters, prodrugs, pharmaceutically
acceptable salts thereof.
21
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Definitions
[0018] The present invention includes the use of all hydrates, solvates,
complexes and prodrugs of the compounds of this invention. Prodrugs are any
covalently bonded compounds, which releases the active parent drug according
to
Formula I, Formula II, or Formula IH in vivo. If a chiral center or another
form of
an isomeric center is present in a compound of the present invention all forms
of
such isomer or isomers, including enantiomers and diastereomers, are intended
to
be covered herein. Compounds containing a chiral center may be used as a
racemic
mixture, an enantiornerically enriched mixture, or the racemic mixture may be
separated using well-known techniques and an individual enantiomer may be used
alone. In cases in which compounds have unsaturated carbon-carbon double
bonds,
both the cis (Z) and trans (E) isomers are within the scope of this invention.
In cases
wherein compounds may exist in tautomeric forms, such as keto-enol tautomers,
each tautomeric form is contemplated as being included within this invention
whether existing in equilibrium or predominantly in one form.
[0019] The meaning of any substituent at any one occurrence in Formula I,
Formula II or Formula III or any sub-Formula thereof is independent of its
meaning,
or any other substituents meaning, at any other occurrence, unless specified
otherwise.
[0020] The term "alkyl" is used, either alone or within other terms such as
"haloalkyl" and "alkylsulfonyl"; it embraces linear or branched radicals
having one
to about twenty carbon atoms or, preferably, one to about twelve carbon atoms.
More preferred alkyl radicals are "lower alkyl" radicals having one to about
ten
carbon atoms. Most preferred are lower alkyl radicals having one to about five
carbon atoms. Examples of such radicals include methyl, ethyl, n-propyl,
isopropyl,
n-butyl, isobutyl, sec-butyl, tert-butyl, pentyl, isoamyl, hexyl, octyl and
the, like.
The term "hydrido" denotes a single hydrogen atom (H). This hydrido radical
may
be attached, for example, to an oxygen atom to form a hydroxyl radical or two
hydrido radicals may be attached to a carbon atom to form a methylene (-CH2-)
22
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
radical. The term "halo" means halogens such as fluorine, chlorine, and
bromine or
iodine atoms. The term "haloalkyl" embraces radicals wherein any one or more
of
the alkyl carbon atoms is substituted with halo as defined above. Specifically
embraced are monohaloalkyl, dihaloalkyl, and polyhaloalkyl radicals. A
monohaloalkyl radical, for one example, may have a bromo, chloro, or a fluoro
atom within the radical. Dihalo radicals may have two or more of the same halo
atoms or a combination of different halo radicals and polyhaloalkyl radicals
may
have more than two of the same halo atoms or a combination of different halo
radicals. The term "hydroxyalkyl" embraces linear or branched alkyl radicals
having
one to about ten carbon atoms any one of which may be substituted with one or
more hydroxylradicals. The terms "alkoxy" and "alkoxyalkyl" embrace linear or
branched oxy-containing radicals each having alkyl portions of one to about
ten
carbon atoms, such as methoxy radical. The term "alkoxyalkyl" also embraces
alkyl
radicals having two or more alkoxy radicals attached to the alkyl radical,
that is, to
form monoalkoxyalkyl and dialkoxyalkyl radicals. The "alkoxy" or "alkoxyalkyl"
radicals may be further substituted with one or more halo atoms, such as
fluoro,
chloro, or bromo, to provide "haloalkoxy" or "haloalkoxyalkyl" radicals.
Examples
of "alkoxy" radicals include methoxy, butoxy, and trifluoromethoxy. The term
"aryl", alone or in combination, means a carbocyclic aromatic system
containing
one, two, or three rings wherein such rings may be attached together in a
pendent
manner or may be fused. The term "aryl" embraces aromatic radicals such as
phenyl, naphthyl, tetrahydronapthyl, indane, and biphenyl. The term
"heterocyclic"
embraces saturated, partially saturated, and unsaturated heteroatom-containing
ring-
shaped radicals, where the heteroatoms may be selected from nitrogen, sulfur
and
oxygen. Examples of saturated heterocyclic radicals include pyrrolidyl and
morpholinyl. The term "heteroaryl" embraces unsaturated heterocyclic radicals.
Examples of unsaturated heterocyclic radicals, also termed "heteroaryl"
radicals
include thienyl, pyrrolyl, furyl, pyridyl, pyrimidyl, pyrazinyl, pyrazolyl,
oxazolyl,
isoxazolyl, imidazolyl, thiazolyl, and tetrazolyl. The term also embraces
radicals
where heterocyclic radicals are fused with aryl radicals. Examples of such
fused
bicyclic radicals include benzofuran, benzothiophene, and the like. The term
"heterocyclic alkyl" embraces alkyl attached to the heterocyclic. The term
23
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
"sulfonyl", whether used alone or linked to other terms such as alkylsulfonyl,
denotes respectively divalent radicals -SOZ-. "Alkylsulfonyl", embraces alkyl
radicals attached to a sulfonyl radical, where alkyl is defined as above. The
term
"arylsulfonyl" embraces sulfonyl radicals substituted with an aryl radical.
The terms
"sulfamyl" or "sulfonamidyl", whether alone or used with terms such as "N-
alkylsulfamyl", "N-arylsulfamyl", "N,N-dialkylsulfamyl" and "N-alkyl-N-
arylsulfamyl", denotes a sulfonyl radical substituted with an amine radical,
forming
a sulfonamide (-S02-NH2). The terms "N-alkylsulfamyl" and "N,N-
dialkylsulfamyl" denote sulfamyl radicals substituted, respectively, with one
alkyl
radical, a cycloalkyl ring, or two alkyl radicals. The terms "N-arylsulfamyl"
and "N-
alkyl-N-arylsulfamyl" denote sulfamyl radicals substituted, respectively, with
one
aryl radical, and one alkyl and one aryl radical. The terms "carboxy" or
"carboxyl",
whether used alone or with other terms, such as "carboxyalkyl", denotes -CO2H.
The term "carboxyalkyl" embraces radicals having a carboxyradical as defined
above, attached to an alkyl radical. The term "carbonyl", whether used alone
or with
other terms, such as "alkylcarbonyl", denotes -(C=O)-. The term
"alkylcarbonyl"
embraces radicals having a carbonyl radical substituted with an alkyl radical.
An
example of an "alkylcarbonyl" radical is CH3-(C=O)-. The term
"alkylcarbonylalkyl" denotes an alkyl radical substituted with an
"alkylcarbonyl"
radical. The term "alkoxycarbonyl" means a radical containing an alkoxy
radical, as
defined above, attached via an oxygen atom to a carbonyl (C=O) radical.
Examples
of such "alkoxycarbonyl" radicals include (CH3)3CO-C=O)- and -(O=)C-OCH3.
The term "alkoxycarbonylalkyl" embraces radicals having "alkoxycarbonyl", as
defined above substituted to an alkyl radical. Examples of such
"alkoxycarbonylalkyl" radicals include (CH3)3COC(=O) (CHZ)2- and -
(CH2)2(O=)COCH3. The term "amido" when used by itself or with other terms such
as "amidoalkyl", "N-monoalkylamido", "N-monoarylamido", "N,N-dialkylamido",
"N-alkyl-N-arylamido", "N-alkyl-N-hydroxyamido" and "N-alkyl-N-
hydroxyamidoalkyl", embraces a carbonyl radical substituted with an amino
radical.
The terms "N-alkylamido" and "N,N-dialkylamido" denote amido groups which
have been substituted with one alkyl radical and with two alkyl radicals,
respectively. The terms "N-monoarylamido" and "N-alkyl-N-arylamido" denote
,24
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
amido radicals substituted, respectively, with one aryl radical, and one alkyl
and one
aryl radical. The term "N-alkyl-N-hydroxyamido" embraces amido radicals
substituted with a hydroxyl radical and with an alkyl radical. The term "N-
alkyl-N-
hydroxyamidoalkyl" embraces alkyl radicals substituted with an N-alkyl-N-
hydroxyamido radical. The term "amidoalkyl" embraces alkyl radicals
substituted
with amido radicals. The term "aminoalkyl" embraces alkyl radicals substituted
with amino radicals. The term "alkylaminoalkyl" embraces aminoalkyl radicals
having the nitrogen atom substituted with an alkyl radical. The term "amidino"
denotes an -C(=NH)-NH2 radical. The term "cyanoamidino" denotes an -C(=N-
CN)-NH2 radical. The term "heterocycloalkyl" embraces heterocyclic-substituted
alkyl radicals such as pyridylmethyl and thienylmethyl. The term "aralkyl"
embraces
aryl-substituted alkyl radicals such as benzyl, diphenylmethyl,
triphenylmethyl,
phenethyl, and diphenethyl. The terms benzyl and phenylmethyl are
interchangeable. The term "cycloalkyl" embraces radicals having three to ten
carbon
atoms, such as cyclopropyl cyclobutyl, cyclopentyl, cyclohexyl, and
cycloheptyl.
The term "cycloalkenyl" embraces unsaturated radicals having three to ten
carbon
atoms, such as cylopropenyl, cyclobutenyl, cyclopentenyl, cyclohexenyl, and
cycloheptenyl. The term "alkylthio" embraces radicals containing a linear or
branched alkyl radical, of one to ten carbon atoms, attached to a divalent
sulfur
atom. An example of "alkylthio" is methylthio, (CH3-S-). The term
"alkylsulfinyl"
embraces radicals containing a linear or branched alkyl radical, of one to ten
carbon
atoms, attached to a divalent -S(=O)- atom. The terms "N-alkylamino" and "N, N-
dialkylamino" denote amino groups which have been substituted with one alkyl
radical and with two alkyl radicals, respectively. The term "acyl", whether
used
alone, or within a term such as "acylamino", denotes a radical provided by the
residue after removal of hydroxyl from an organic acid. The term "acylamino"
embraces an amino radical substituted with an acyl group. An examples of an
"acylamino" radical is acetylamino (CH3C(=O)-NH-).
[0021] Compounds of Formula I, Formula II or Formula III would be useful for,
but not limited to, the treatment of inflammation in a subject, and for
treatment of
other inflammation-associated disorders, such as, as an analgesic in the
treatment of
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
pain and headaches, or as an antipyretic for the treatment of fever. For
example,
compounds of Formula I, Formula II or Formula III would be useful to treat
arthritis, including but not limited to rheumatoid arthritis, spondylo
arthopathies,
gouty arthritis, osteoarthritis, systemic lupus erythematosus, and juvenile
arthritis.
Such compounds of Formula I, Formula II or Formula III would be useful in the
treatment of asthma, bronchitis, menstrual cramps, tendinitis, bursitis, and
skin
related conditions such as psoriasis, eczema, burns, and dermatitis. Compounds
of
Formula I, Formula II or Formula III also would be useful to treat
gastrointestinal
conditions such as inflammatory bowel disease, Crohn's disease, gastritis,
irritable
bowel syndrome, and ulcerative colitis and for the prevention of colorectal
cancer.
Compounds of Formula I, Formula II or Formula III would be useful in treating
inflammation in such diseases as vascular diseases such as vascularitus,
migraine
headaches, periarteritis nodosa, thyroiditis, aplastic anemia, Hodgkin's
disease,
sclerodoma, rheumatic fever, type I diabetes, myasthenia gravis, sarcoidosis,
nephrotic syndrome, Behcet's syndrome, polymyositis, gingivitis,
hypersensitivity,
conjunctivitis, swelling occurring after injury, myocardial ischemia, and the
like.
The compounds of the present invention may also be used for pain. The
compounds
are useful as antiinflammatory agents, such as for the treatment of arthritis,
with the
additional benefit of having significantly less harmful side effects. The
compounds
of Formula I, II or III are useful as agents for treating cancer or anticancer
agents.
The compounds of Formula 1, 11 or III may be proapoptotic, antiapoptotic,
anticell
cycle progressive, antiinvasive, antiproliferative, antiangiogenic, and
antimetastatic.
The cancer may be colon, ovarian, breast, prostate, gastric, B-cell lymphoma,
and
multiple myeloma. More specifically, the compounds of this invention are
useful in
the treatment of a variety of cancers including, but not limited to: carcinoma
such as
bladder, breast, colon, kidney, liver, lung, including small cell lung cancer,
esophagus, gall-bladder, ovary, pancreas, stomach, cervix, thyroid, prostate,
and
skin, including squamous cell carcinoma; hematopoietic tumors of lymphoid
lineage, including leukemia, acute lymphocytic leukemia, acute lymphoblastic
leukemia, B-cell lymphoma, T-cell-lymphoma, Hodgkin's lymphoma, non-
Hodgkin's lymphoma, hairy cell lymphoma and Burkett's lymphoma; hematopoietic
tumors of myeloid lineage, including acute and chronic myelogenous leukemias,
26
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
myelodysplastic syndrome and promyelocytic leukemia; tumors of mesenchymal
origin, including fibrosarcoma and rhabdomyosarcoma; tumors of the central and
peripheral nervous system, including astrocytoma, neuroblastoma, glioma and
schwannomas; other tumors, including melanoma, seminoma, teratocarcinoma,
osteosarcoma, xeroderma pigmentosum, keratoxanthoma, thyroid follicular cancer
and Kaposi's sarcoma. Due to the key role of PKs in the regulation of cellular
proliferation, these compounds are also useful in the treatment of a variety
of cell
proliferative disorders such as, for instance, benign prostate hyperplasia,
familial
adenomatosis, polyposis, neuro-fibromatosis, psoriasis, vascular smooth cell
proliferation associated with atherosclerosis, pulmonary fibrosis, arthritis
glomerulonephritis and post-surgical stenosis and restenosis. The compounds of
Formula I, II, or III may be used as an anitviral agent. The compounds of this
invention are useful as inhibitors of protein kinases. The compounds of this
invention are useful as inhibitors of IKK1 and/or IKK2, IKKucIIKK(3
heterodimer,
TBK or IKKi. The compounds of the invention may also useful as inhibitors of
other protein kinases such as, for instance, protein kinase C in different
isoforms,
cyclin dependent kinase (cdk), Met, PAK-4, PAK-5, ZC-1, STLK-2, DDR-2,
Aurora 1, Aurora 2, Bub-1, PLK, Chkl, Chk2, HER2, rafl, MEKl, MAPK, EGF-R,
PDGF-R, FGF-R, IGF-R, VEGF-R, P13K, weel kinase, Src, Abl, Akt, ILK, MK-2,
IKK-2, Cdc7, Nek, and thus be effective in the treatment of diseases
associated with
other protein kinases. The present invention preferably includes compounds,
which
selectively inhibit IKK2 over IKK1. Preferably, the compounds have an IKK2
IC50
of less than 1 M, and have a selectivity ratio of IKK2 inhibition over IKK1
inhibition of at least 50, and more preferably of at least 100. Even more
preferably,
the compounds have an IKKl IC50 of greater than 10 M, and more preferably of
greater than 100 M. The compounds of Formula I, II, or III may also be used
to
treat angiogenesis associated cardiovascular, ophthalmology and osteoporosis
disorders. The compounds of the present invention may also be used for
treatment
of knee injury such as sport injuries.
[0022] While it is possible for an active ingredient to be administered alone
as
the raw chemical, it is preferable to present it as a pharmaceutical
formulation.
27
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
The present invention comprises a pharmaceutical composition comprising a
therapeutically effective amount of a compound of the present invention in
association with at least one pharmaceutically acceptable carrier, adjuvant,
or
diluent. The present invention also comprises a method of treating
inflammation or
inflammation associated disorders in a subject, the method comprising
administering to the subject having such inflammation or disorders a
therapeutically
effective amount of a compound of the present invention. Also included in the
family of compounds of the present invention are the pharmaceutically
acceptable
salts thereof. The term "pharmaceutically acceptable salts" embraces salts
coniinonly used to form alkali metal salts and to form addition salts of free
acids or
free bases. The nature of the salt is not critical, provided that it is
pharmaceutically
acceptable. Suitable pharmaceutically acceptable acid addition salts of
compounds
of the present invention may be prepared from an inorganic acid or from an
organic
acid. Examples of such inorganic acids are hydrochloric, hydrobromic,
hydroiodic,
nitric, carbonic, sulfuric, and phosphoric acid. Appropriate organic acids may
be
selected from aliphatic, cycloaliphatic, aromatic, araliphatic, heterocyclic,
carboxylic and sulfonic classes of organic acids, examples of which are
formic,
acetic, propionic, succinic, glycolic, gluconic, lactic, malic, tartaric,
citric, ascorbic,
glucuronic, maleic, fumaric, pyruvic, aspartic, glutamic, benzoic,
anthranilic,
mesylic, salicyclic, salicyclic, phydroxybenzoic, phenylacetic, mandelic,
embonic
(pamoic), methanesulfonic, ethanesulfonic, benzenesulfonic, pantothenic,
toluenesulfonic, 2-hydroxyethanesulfonic, sulfanilic, stearic,
cyclohexylaminosulfonic, algenic, 0-hydroxybutyric, salicyclic, galactaric and
galacturonic acid. Suitable pharmaceutically acceptable base addition salts of
compounds of the present invention include metallic salts made from aluminum,
calcium, lithium, magnesium, potassium, sodium and zinc or organic salts made
from N,N'-dibenzylethylenediamine, chloroprocaine, choline, diethanolamine,
ethylenediamine, meglumine (N-methyl-glucamine) and procaine. All of these
salts
may be prepared by conventional means from the corresponding compound of the
present invention by reacting, for example, the appropriate acid or base with
the
compound of the present invention.
28
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
[0023] Also embraced within this invention are pharmaceutical compositions
comprising one or more compounds of the present invention in association with
one
or more non-toxic, pharmaceutically acceptable carriers and/or diluents and/or
adjuvants and/or excipient (collectively referred to herein as "carrier"
materials)
and, if desired, other active ingredients. Accordingly, the compounds of the
present
invention may be used in the manufacture of a medicament. Pharmaceutical
compositions of the compounds of the present invention prepared as herein
before
described may be formulated as solutions or lyophilized powders for parenteral
administration. Powders may be reconstituted by addition of a suitable diluent
or
other pharmaceutically acceptable carrier prior to use. The liquid formulation
may
be a buffered, isotonic aqueous solution. The compounds of the present
invention
may be administered by any suitable route, preferably in the form of a
pharmaceutical composition adapted to such a route, and in a dose effective
for the
treatment intended. The compounds and composition may, for example, be
administered intravascularly, intraperitoneally, intravenously,
subcutaneously,
intramuscularly, intramedullary, orally, or topically. For oral
administration, the
pharmaceutical composition may be in the form of, for example, a tablet,
capsule,
suspension, or liquid. The active ingredient may also be administered by
injection
as a composition wherein, for example, normal isotonic saline solution,
standard
5% dextrose in water or buffered sodium or ammonium acetate solution may be
used as a suitable carrier. Such formulation is especially suitable for
parenteral
administration, but may also be used for oral administration or contained in a
metered dose inhaler or nebulizer for insufflation. It may be desirable to add
excipients such as polyvinylpyrrolidone, gelatin, hydroxy cellulose, acacia,
polyethylene glycol, mannitol, sodium chloride, or sodium citrate. The
pharmaceutical composition is preferably made in the form of a dosage unit
containing a particular amount of the active ingredient. Examples of such
dosage
units are tablets or capsules. The amount of therapeutically active compound
that is
administered and the dosage regimen for treating a disease condition with the
compounds and/or compositions of this invention depends on a variety of
factors,
including the age, weight, sex and medical condition of the sub-ject, the
severity of
the disease, the route and frequency of administration, and the particular
compound
29
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
employed, and thus may vary widely. The pharmaceutical compositions may
contain active ingredient in the range of about 0.1 to 2000 mg, preferably in
the
range of about 0.5 to 500 mg and most preferably between about 1 and 100 mg. A
daily dose of about 0.01 to 100 mg/kg bodyweight, preferably between about 0.1
and about 50 mg/kg body weight and most preferably between about 1 to 20 mg/kg
bodyweight, may be appropriate. The daily dose can be administered in one to
four
doses per day. For therapeutic purposes, the compounds of this invention are
ordinarily combined with one or more adjuvants appropriate to the indicated
route
of administration. If administered orally, the compounds may be admixed with
lactose, sucrose, starch powder, cellulose esters of alkanoic acids, cellulose
alkyl
esters, talc, stearic acid, magnesium stearate, magnesium oxide, sodium and
calcium salts of phosphoric and sulfuric acids, gelatin, acacia gum, sodium
alginate,
polyvinylpyrrolidone, and/or polyvinyl alcohol, and then tableted or
encapsulated
for convenient administration. Such capsules or tablets may contain a
controlled
release formulation as may be provided in a dispersion of active compound in a
sustained release material such as glyceryl monostearate, glyceryl distearate,
hydroxypropylmethyl cellulose alone or with a wax. Formulations for parenteral
administration may be in the form of aqueous or non-aqueous isotonic sterile
injection solutions or suspensions. These solutions and suspensions may be
prepared from sterile powders or granules having one or more of the carriers
or
diluents mentioned for use in the formulations for oral administration. The
compounds may be dissolved in water, polyethylene glycol, propylene glycol,
ethanol, corn oil, cottonseed oil, peanut oil, sesame oil, benzyl alcohol,
sodium
chloride, and/or various buffers. The pharmaceutical preparations are made
following the conventional techniques of pharmacy involving milling, mixing,
granulating, and compressing, when necessary, for tablet forms; or milling,
mixing
and filling for hard gelatin capsule forms. When a liquid carrier is used, the
preparation will be in the form of a syrup, elixir, emulsion, or an aqueous or
non-
aqueous suspension. Such a liquid formulation may be administered orally or
filled
into a soft gelatin capsule. For rectal administration, the compounds of the
present
invention may also be combined with excipients such as cocoa butter, glycerin,
gelatin, or polyethylene glycols and molded into a suppository. The methods of
the
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
present invention include topical administration of the compounds of the
present
invention. By topical administration is meant non-systemic administration,
including the application of a compound of the invention externally to the
epidermis, to the buccal cavity and instillation of such a compound into the
ear, eye,
and nose, wherein the compound does not significantly enter the blood stream.
By
systemic administration is meant oral, intravenous, intraperitoneal, and
intramuscular administration. The amount of a compound of the present
invention
(hereinafter referred to as the active ingredient) required for therapeutic or
prophylactic effect upon topical administration will, of course, vary with the
compound chosen, the nature and severity of the condition being treated and
the
animal undergoing treatment, and is ultimately at the discretion of the
physician.
[0024] The topical formulations of the present invention, both for veterinary
and for human medical use, comprise an active ingredient together with one or
more
acceptable carriers therefore, and optionally any other therapeutic
ingredients. The
carrier must be "acceptable" in the sense of being compatible with the other
ingredients of the formulation and not deleterious to the recipient thereof.
Formulations suitable for topical administration include liquid or semi-liquid
preparations suitable for penetration through the skin to the site of where
treatment
is required such as: liniments, lotions, creams, ointments or pastes, and
drops
suitable for administration to the eye, ear or nose. The active ingredient may
comprise, for topical administration, from 0.01 to 5.0 wt%. of the
formulation.
[0025] Drops according to the present invention may comprise sterile aqueous
or oily solutions or suspensions and may be prepared by dissolving the active
ingredient in a suitable aqueous solution of a bactericidal and/or fungicidal
agent
and/or any other suitable preservative, and preferably including a surface
active
agent. The resulting solution may then be clarified by filtration, transferred
to a
suitable container, which is then sealed and sterilized by autoclaving, or
maintaining
at 90-100 C for half an hour. Alternatively, the solution may be sterilized
by
filtration and transferred to the container by an aseptic technique. Examples
of
bactericidal and fungicidal agents suitable for inclusion in the drops are
31
CA 02485298 2004-11-08
51067-42
phenylmercuric nitrate or acetate (0.00217c), benzalkonium chloride (0.0196)
and
chlorhexidine acetate (0.0 1%). Suitable solvents for the preparation of an
oily
solution include glycerol, diluted alcohol, and propylene glycol.
[0026] Lotions according to the present invention include those suitable for
application to the skin or eye. An eye lotion may comprise a sterile aqueous
solution
optionally containing a bactericide and may be prepared by methods similar to
those
for the preparation of drops. Lotions or liniments for application to the sldn
may
also include an agent to basten drying and to cool the skin, such as an
alcohol or
acetone, and/or a moisturizer such as glycerol or an oil such as castor oil or
arachis
oil. Creams, ointments, or pastes according to the present invention are semi-
solid
formulations of the active ingredient for external application. They may be
made by
mixing the active ingredient in finely divided or powdered form, alone or in
solution or suspension in an aqueous or non-aqueous fluid, with the aid of
suitable
machinery, with a greasy or non-greasy basis. The basis may comprise
hydrocarbons such as hard, soft or liquid paraffin, glycerol, beeswax, a
metallic
soap; a mucilage; an oil of natural origin such as almond, corn, arachis,
castor or
olive oil; wool fat or its derivatives, or a fatty acid such as stearic or
oleic acid
together with an alcohol such as propylene glycol or macrogols. The
formulation
may incorporate any suitable surface-active agent such as an anionic,
cationic, or
non-ionic surface-active agent such as sorbitan esters or polyoxyethylene
derivatives thereof. Suspending agents such as natural gums, cellulose
derivatives
or inorganic materials such as silicaceous silicas, and other ingredients sucb
as
lanolin may also be included. Other adjuvants and modes of administration are
well
and widely known in the pharmaceutical art. Although this invention has been
described with respect to specific embodiments, the details of these
embodiments
are not to be construed as linzitations.
Pharmaceutical compositions/formulations of the
invention may be contained in a commercial package, which
may optionally further comprise instructions for the
therapeutic use thereof as herein described.
GENERAL SYNTHTIC PROCEDURES
[0027] The starting materials used herein are commercially available or are
prepared by routine methods well known to those of ordinary skill in the art
and
32
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
can be found in standard reference books, such as the COMPENDIUM OF
ORGANIC SYNTHETIC METHODS, Vol. I-VI (published by Wiley-
Interscience).
[0028] The compounds of the invention can be synthesized according to the
following procedures of Schemes I-XI, wherein the R1-R14 substituents are as
defined for Formula I, Formula II or Formula III, above, except where further
noted.
[0029] SCHEME I
R2
HN~NH2 HCI R~~I_
O OH
R3 + I ~ EtOH or MeOH
R4%' ~ A O Ri 1~R2 orHOAc N; R3
R4'~ / A 0
1 2 3
Synthetic Scheme I illustrates the procedure used to prepare the
antiinflammatory
pyrazoles of the present invention. 1,3-Dicarbonyl compounds such as 1, or the
shown enol form which is in equilibrium with the 1,3-diketone, are allowed to
react
with a substituted hydrazine hydrochloride 2 in warm methanol or ethanol or
acetic
acid to provide the pyrazoles 3 via a condensation reaction. When A = -CH2CH2-
,
the central ring may be aromatized to provide A = -CH=CH-, by using an oxidant
such as DDQ, Pd or Pt on carbon with cyclooctadiene or other H2 acceptor, or
sulfur
in an appropriate solvent or without solvent.
[0030] SCHEME II
O O OH
R4 O T,HIVIDS R4 R3
~~ + O O/
~~ A (or) NaOMe / MeOH A 0
O
4 1
Synthetic Scheme II illustrates the procedure for the preparation of
substituted
diketones 1. An appropriately substituted ketone 4, including, but not limited
to; 1-
indanones, 1-tetralones, and 1-benzosuberones, is first treated with base,
such as
sodium methoxide, lithium bistrimethylsilylamide or lithium diisopropylamide
33
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
(LDA), followed by condensation with a suitable acylating agent, such as,
dimethyl
or diethyl oxalate, in an appropriate solvent, such as methanol, diethyl ether
or
tetrahydrofuran, to provide 1,3-dicarbonyl compounds 1 which are suitable for
conversion into antiinflammatory pyrazoles as illustrated in Scheme I.
Alternatively, the dicarbonyl compounds 1 can be directly prepared from
commercially available cyclic ketones 4.
[0031] SCHEME III
O
R4 + O A1C13 R4 I~ 00
I
/ ~ H
O O
5 6
HZ / Pd/C
O
O
~ TFAA / TFA R4
R4 ~ / OH
/
7
8
Synthetic Scheme III illustrates a three-step procedure used for the
preparation of
substituted 1-tetralones. In step one, an appropriate substituted benzene 5 is
condensed with succinic anhydride and a catalyst such as aluminum chloride
into
the corresponding 4-phenyl-4-ketobutanoic acid derivatives 6. In step two, the
keto
group of the 4-phenyl-4-ketobutanoic acids 6 is reduced using catalytic
hydrogenation or Wolff-Kishner type reductions, thus providing 4-
phenylbutanoic
acids 7. In addition, ketone reductions can be carried out using metal
amalgams. In
step three, the 4-phenylbutanoic acids are treated with a mixture of
trifluoroacetic
anhydride, and trifluoroacetic acid to effect intramolecular Friedel-Crafts
acylation
affording selected tetralones 8. Alternatively, the Friedel-Crafts acylation
can be
affected with other strong acids such as polyphosphoric acid, sulfuric acid,
or
aluminum chloride.
34
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
[0032] SCHEME IV
0
0
O BrMg , R4 f11Clg R4 i~
Rd i + / I /
O
9 10 8
Synthetic Scheme IV describes an alternate synthetic route to 1-tetralones S.
In step
one, addition of allylmagnesium bromide in a suitable solvent such as, THF or
diethyl ether, to an appropriately substituted benzoate 9 affords the 1-
phenylbut-3-
ene-l-ones 10. In step two, the 1-phenylbut-3-ene-l-ones 10 can be cyclized
under
Friedel-Crafts alkylation conditions, provided R4 is a ring activating
substituent,
using catalysts such as aluminum chloride to produce 1-tetralones 8.
[0033] SCHEME V
O O
E+
g
Scheme V describes the direct modification of 1-tetralone to substituted
tetralones.
Commercially available 1-tetralone may be treated with a variety of
electrophilic
reagents such as bromine, ammonium nitrite or vinylsilanes, represented by E,
with
or without a catalyst to generate directly a substituted tetralone 8,
containing bromo,
nitro or vinyl groups. Such tetralones 8 can be further embellished to provide
the
desired substitution patterns. Mixtures may be readily separated using
chromatographic techniques.
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
[0034] SCHEME VI
0
E+ Ra I KMnO4 Ra if
11 8
An alternate to Scheme V is Scheme VI wherein an appropriately substituted
decaline is subjected to electrophilic addition to generate substituted
decalins 11.
Substituted decalins may also be prepared by Friedel-Crafts alkylation of
substituted
benzenes. Substituted decalins 11 can then be oxidized to the tetralones 8
using
oxidants such as KMnO4 or Se02.
[0035] SCHEME VII
0
F3C- CF3
\ /%Q \ Pd(OAc)z, R3P, CO O\~ 1
HO j O Tf0 ~
/ ~ MeOH
8a 8b 12
Scheme VII describes the modification of existing tetralones into analogs
containing differing functional groups that can also be further modified. By
example, hydroxy tetralone (8a where R4 = OH) can be converted to the triflate
8b
by treatment with trifluoromethane sulfonic anhydride. Triflate 8b can the be
subjected to Pd(OAc)2 an appropriate phosphine and CO in the presence of
methanol to generate tetralone 12 containing a carboxy methyl group. Triflates
can
be used in a variety of palladium coupling reactions to introduce additional
functional groups.
36
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
[0036] SCHEME VIII
0
0
R4 H + R4 i
o-I
14 O
13
1) H2 / Pd/C
2) NaOH
O
R4 i TFAA/TFA R4
O
16 15
Synthetic Scheme VIII illustrates a three step procedure used for the
preparation of
substituted 1 -indanones 16. In step one, an appropriate substituted
benzaldehyde 13
is condensed with methyl acetate and a catalyst such as triethylamine into the
corresponding methyl cinnamate derivatives 14. Additionally, commercially
available cinnamates may be used in the following steps. In step two the
olefin
group of the cinnamate 14 is reduced using catalytic hydrogenation and the
ester
hydrolyzed with base, such as NaOH, thus providing 3-phenylpropanoic acids 15.
In step three, the 3-phenylprapanoic acids are treated with a mixture of
trifluoroacetic anhydride and trifluoroacetic acid to effect intramolecular
Friedel-
Crafts acylation affording selected 1-indanones 16. Alternatively, the Friedel-
Crafts
acylation can be effected with other strong acids such as sulfuric acid or
aluminum
chloride.
[0037] SCHEME IX
0 0 0
4 i~ A1C13 Rq
R4 + R 1
9 17 16
37
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Synthetic Scheme IX illustrates a two-step route for the preparation of
substituted
1-indanones 16. Commercially available methyl benzoates 9, or other alkyl
esters,
may be treated with a vinyl lithium reagent to afford phenylvinyl ketones 17.
Alternatively, dimethylamides or N-methyl-O-methylhydroxamides may be used in
place of the esters. Also, other vinyl metals, such as; vinylmagnesium bromide
may
be used in place of the vinyl lithium reagent. The resulting phenylvinyl
ketones
may be cyclized using Friedel-Crafts alkylating catalysts, such as aluminum
chloride.
[0038] SCHEME X
0
~ A1C13 a I \
R4 i/ +
C O OH
0 O O
5 18
HZ / Pd/C
TFAA/TFA R4 I
R4 ~ / OH
19 O
15 Synthetic Scheme X illustrates a three step procedure used for the
preparation of
substituted 1-benzosuberones 20. In step one, an appropriate substituted
benzene 5
is condensed with glutaric anhydride and a catalyst such as aluminum chloride
into
the corresponding 5-phenyl-5-ketopentanoic acid derivatives 18. In step two,
the
keto group of the 5-phenyl-5-ketopentanoic acids 18 is reduced using catalytic
20 hydrogenation or Wolff-Kishner type reductions, thus providing 5-
phenylpentanoic
acids 19. In addition, ketone reductions can also be carried out using metal
amalgams. In step three, the 5-phenylpentanoic acids are treated with a
mixture of
trifluoroacetic anhydride, and trifluoroacetic acid to effect intramolecular
Friedel-
38
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Crafts acylation affording selected benzosuberones 20. Alternatively, the
Friedel-
Crafts acylation can be affected with other strong acids such as
polyphosphoric acid,
H2SO4 or A1C13. Alternatively, 5-phenyl-5-ketopentanoic acids 18, can be
prepared
from glutaric acid and a phenyllithium or a phenyl Grignard reagent
appropriately
substituted and compatible with reaction conditions.
[0039] Scheme XI
\
0 0 Nz~ NHNH2'HCI N-N
O2N OEt R;/ 02N OEt
CO O - r ! \ O
AcOH, reflux
ethyl(7-nitro-l-oxo-1,2,3,
4-tetrahydronaphthalen-2-yl)
(oxo)acetate 1
R \
SnC12 N-N OEt NH3, MeOH N-N NH2
EtOH H2N 700 psi H2N / I~ O
~
R~ l
R9CO2H, HATU or \ H O N N-N NH2 TFA
Y RT
R9COCI, Pyridine R9 O
HO' /
\
O N N-N NH2
Y
O
R9
R = 3- or 4-benzyloxyl
Scheme XI describes the synthesis of the pyrazoles with phenols at N-position.
In
step one; 3- or 4-benzyloxylphenylhydrazine was refluxed with ethyl (7-nitro-l-
oxo-1,2,3,4-tetrahydronaphthalen-2-yl)(oxo)acetate 1 in acetic acid to give
pyrazole.
39
CA 02485298 2007-08-01
51067-42
Then the nitro group was reduced to amine by using tin (II) chloride in
ethanol. In
the following step, the conversion of ester to arnide was achieved by reacting
with
liquid ammonia in a pressured tube at high temperature. The resulting compound
can either react with acid and HATU in DMF or acid chloride in pyridine to
give the
desired amide. The benzyl group was deprotected by stirring with TFA at room
temperature.
[0040] Although the foregoing invention has been
described in some detail by way of illustration and example for the purposes
of
clarity of understanding, it will be readily apparent to one skilled in the
art in light
of the teachings of this invention that changes and modifications can be made
without departing from the spirit and scope of the present invention. The
following
examples are provided for exemplification purposes only and are not intended
to
limit the scope of the invention, which has been described in broad terms
above.
EXAMPLES
[0041] Example 1
1-{ 4-[(aminothio)peroxy]phenyl }-8-nitro-4,5-dihydro-lH-benzo[g]indazole-3-
carboxamide
HZNSO2~aN-N
0N ~ ' NHp
[0042] Step 1
0 ou
OpN O\--,
~I O
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
To 7-nitro-l-tetralone (4.6 g, 0.024 mol) and ethyl oxalate (3.5 mL, 0.026
mol) in
ether (100 mL) was added dropwise lithium bis(trimethylsilyl)amide (1M in THF,
26 mL). The slurry was stirred overnight and filtered to give the product as
an olive
green solid, 6.2 g (87% yield). 1H NMR (DMSO-d6l 300 MHz) 8.45 (d, 1H); 8.05
(d of d, 1H); 7.42 (d, 1H); 4.08 (q, 2H); 2.82-2.72 (m, 2H); 2.51-2.43 (m,
2H); 1.21
(t, 3H).
[0043] Step 2
Ethyl 1- { 4-[(aminothio)peroxy]phenyl }-8-nitro-4, 5-dihydro-1 H-benzo [g]
indazole-
3-carboxylate
H2NS021a
N-N
02N \ I O\11-
O
The material of Step 1 (6.2 g, 0.021 mol) and 4-sulfonamidophenylhydrazine
hydrochloride (5.1 g, 0.023 mol) were stirred in methanol (100 mL) overnight.
Conc. HCl (2 mL) was added to the thick slurry and the contents were heated on
a
steam bath for 1 hour. Contents were allowed to cool and filtered to give an
off-
white solid, 6.9 g. NMR and LC/MS analysis show the solid to contain two
components, the desired and the hydrated pyrazole. TFA (60 mL) and TFAA (20
mL) were added to the solid and heated on a steam bath for 1 hour. Contents
were
concentrated in vacuo leaving the product as a solid, 6.4 g (69% yield).
FABHRMS
m/z 443.1020 (M+H, C20H19N406S requires 443.1025). 1H NMR (DMSO-d6/ 300
MHz) 8.10 (d of d, 1H); 8.03 (d, 2H); 7.82 (d, 2H); 7.70 (d, 1H); 7.62 (s,
1H); 7.50
(d, 1H); 4.33 (q, 2H); 3.20-2.95 (m, 4H); 1.33 (t, 3H).
Anal. Calcd for C20H18N406S: C, 54.29; H, 4.10; N, 12.66. Found: C, 54.49; H,
4.00; N, 12.52.
[0044] Step 3
41
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
The material of Step 2 (718 mg, 0.0016 mol), conc. ammonium hydroxide (30 mL),
and methanol (15 mL) were stirred in a stoppered flask for 72 hours. Contents
were
filtered to give a light amber solid (606 mg). The solid was recrystallized
from
acetonitrile to give the product as a light arnber solid, 450 mg (68% yield).
FABHRMS m/z 414.0902 (M+H, Cl$H16N505S requires 414.0872). 'H NMR
(DMSO-d6/ 300 MHz) 8.15 - 7.95 (m, 3H); 7.83 (d, 2H); 7.80-7.40 (m, 6H); 3.20-
2.95 (m, 4H).
Anal. Calcd for Cz8Hi5N505S: C, 52.30; H, 3.66; N, 16.94. Found: C, 52.04; H,
3.64; N, 16.61.
[0045] Example 2
8-amino-l-{ 4-[(aminothio)peroxy]phenyl }-4,5-dihydro-lH-benzo[g]indazole-3-
carboxamide
H2NSO2 <IN-N
HZN / ~ ~ NH2
~ I O
The compound was prepared similarly to Example 1 in 70 % yield. FABHRMS m/z
384.1136 (M+H, C18H18N503S requires 384.1130). 'H NMR (DMSO-d6/ 300
MHz) 7.95 (d, 2H); 7.75 (d, 2H); 7.53 (br s, 1H); 7.43 (br s, 1H); 7.32 (br s,
1H);
7.01 (d, IH); 6.44 (d of d, 1H); 6.03 (s, 1H); 4.81 (s, 2H); 2.93-2.65 (m,
4H).
Anal. Calcd for C18H17N503S: C, 56.38; H, 4.47; N, 18.27. Found: C, 56.31; H,
4.42; N, 18.31.
[0046] Example 3
8-(acetylamino)-1-{ 4-[(aminothio)peroxy]phenyl } -4,5-dihydro-lH-
benzo [g]indazole-3-carboxamide
42
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
H2NS0z
N-N
AcHN / ' NH2
~ O
To the material of Example 2 (1.0 g, 0.0026 mol) in DMF (15 mL) was added
dropwise a mixture of acetic anhydride (0.283 mL, 0.003 mol) and pyridine
(0.243
mL, 0.003 mol) in DMF (5 mL). Contents were stirred overnight, diluted with
water (75 mL), and filtered to give the desired as a white solid, 1.0 g (90%
yield).
FABHRMS m/z 426.1235 (M+H, C20H20N504S requires 426.1236). 1H NMR
(DMSO-d6 / 300 MHz) 9.80 (s, 1H); 8.00 (d, 2H); 7.75 (d, 2H); 7.60 (s, 1H);
7.48
(s, 2H); 7.39 (s, 1H); 7.30 (d, 1H); 7.15 (s, 1H); 2.90 (s, 4H); 1.92 (s, 3H).
Anal. Calcd for C20H19N504S (1H20): C, 54.17; H, 4.77; N, 15.79. Found: C,
54.20; H, 4.97; N, 15.77.
[0047] Example 4
1- { 4-[(aminothio)peroxy]phenyl } -8-{ [(methylthio)peroxy] amino }-4,5-
dihydro-lH-
benzo [g] indazole-3-carboxamide
HZNS02
N-N
MeSO2HN / ~ ~ NH2
\I O
To the material of Example 2 (1.2 g, 0.003 mol) and triethylamine (0.278 mL,
0.0035 mol) in DMF (10 mL) at 0 C, was added dropwise methanesulfonyl chloride
(0.278 mL, 0.0035 mol) in CH2C12 (2 mL). Contents were stirred overnight,
slowly
coming to room temperature. Contents were diluted with water (50 mL) and
filtered to give the product as an off-white solid, 524 mg (37% yield).
FABHRMS
m/z 462.0917 (M+H, C19H2ON505S2 requires 462.0906). 1H NMR (DMSO-d6 /
300 MHz) 9.60 (s, 1H); 7.98 (d, 2H); 7.80 (d, 2H); 7.60 (s, 1H); 7.50 (s, 2H);
7.40
(s, 1H); 7.37 (d, 1H); 7.02 (s, 1H); 6.75 (s, 1H); 2.93 (s, 4H); 2.75 (s, 3H).
43
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Anal. Calcd for C19Hz9N505S2: C, 49.45; H, 4.15; N, 15.17. Found: C, 49.19; H,
3.77; N, 15.53.
[0048] Examples 5-40
Synthesis of the sulfonamide/amide/urea library
Scheme XII
Q,O
H2N- S ~ 0
H2N
N-N 1. RSO2CI or RCOCI, Pyridine
NH2 N_N
H2N 2.MP-trisamine resin NH2
O 3. MP-TsOH resin in DMF R-HN
O
The sulfonamides, amides, and urea were synthesized in a library format by
using a
Bohdan reaction block. The starting materials are the product of Example 2(8-
amino-l- { 4-[(aminothio)peroxy]phenyl }-4,5-dihydro-1H-benzo[g]indazole-3-
carboxamide) and appropriate sulfonyl chlorides, acyl chlorides and
isocyanates.
Thirty-five reactions constituted this library.
The general procedure is as follows: 48 mg of the product of Example 2(8-amino-
1-{ 4-[(aminothio)peroxy]phenyl } -4,5-dihydro- lh-benzo[g]indazole-3-
carboxamide)
in 1 mL pyridine was placed in each reaction vessel, then 1.2 eq. of a
sulfonyl
chloride was added, and the mixture was shaken overnight. Then 3 mL methylene
chloride and 300 mg of resin PS-trisamine were added, and then shaken over
night.
After filtration and washing with 2 mL methanol twice, the filtrates were
combined
and solvents evaporated. The residue was dissolved in 2 mL dimethylformamide,
and MS-TsOH resin (450 mg) was added and shaken for 48 hours. After filtration
and washing with 2 mL DMF, the combined filtrate was analyzed by LC-MS and
LC. Then the filtrate was evaporated on a SpeedVac and the residue were
suspended
in 2 mL of H20/tBuOH, and lyophilized for 2 days. All compounds were obtained
in solid form, and the majority of the compounds have about 90% purity. Table
1
44
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
shows the substitutions, compound identification, and IKK heterodimer assay
values for the compounds from the sulfonamide library. The structures of the
compounds of Examples 5-40 were confirmed Mass Spectroscopy and/or NMR
analysis.
[0049] Synthesis of Compounds of Examples 41-45
Scheme XIII
0
11 H N /\ O HZN 0 X= -H 70%a Example 41
z 0 I X H~ N N O -CH3 65% Example 42
N-N O H_ ~ ~ -OCH3 71% Example 43
HZN NaB(OAc)3H ~ NHZ Cl 74% Example 44
NHZ DMF-HOAc
Example 2 x
HzN
R--e O R=-iBu 61% Example 45
H H N 0
--~
NaB(OAc)3H N
DMF-HOAc R NHz
[0050] Example 41
1-[4-(aminosulfonyl)phenyl]-8-(benzylamino)-4,5-dihydro-1 H-benzo[g]indazole-3-
carboxamide
O, ,O
H2N S~ lal N~N O
H
N I NHz
~
1~,
To a mixture of the product of example 2(8-amino-l-{4-
[(aminothio)peroxy]phenyl }-4,5-dihydro-lh-benzo[g]indazole-3-carboxamide) (76
mg, 0.20 mmol), acetic acid (0.3 mL) and sodium triacetoxyborohydride (213 mg,
1.00 mmol) in DMF (3 mL) was added benzaldehyde (64 mg, 0.60 mmol). The
resulted mixture was stirred at RT for 18 h, added water (10 mL), extracted
with
EtOAc (3x10 mL). The combined organic layers was washed with water (3x10
mL), dried over MgSO4, filtered through a silica gel pad with EtOAc, and
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
concentrated. The crude product was triturated with diethyl ether to give 1-[4-
(aminosulfonyl)phenyl]-8-(benzylamino)-4,5-dihydro-1 H-benzo [g] indazole-3-
carboxamide as a yellow solid (66 mg, 70%). Its structure was confirmed by 1H
NMR and MS (474, M+1). C25H23N503S, Calc.: C: 63.41, H: 4.90, N: 14.79;
Found, C: 63.11, H: 4.70, N: 13.54.
[0051] Example 42
1-[4-(aminosulfonyl)phenyl]-8-[(4-methylbenzyl)amino]-4,5-dihydro-1 H-
benzo [g] indazole-3-carboxamide
0,, 010
H2N"S
N-N
N NH2
O
1-[4-(aminosulfonyl)phenyl]-8-[(4-methylbenzyl)amino]-4,5-dihydro-1 H-
benzo[g]indazole-3-carboxamide (32 mg, 65%) was synthesized by the same
procedure as in Example 41, starting with the product of Example 2(8-amino-l-
{4-
[(aminothio)peroxy]phenyl }-4,5-dihydro-lH-benzo[g]indazole-3-carboxamide)
(38.3 mg, 0.10 mmol) and p-Tolualdehyde (36 mg, 0.30 mmol). Its structure was
confirmed by 1H NMR and MS (488, M+1). C26H25N503S, Calc.: C: 64.05, H:
5.17, N: 14.36; Found, C: 63.78, H: 4.99, N: 14.12.
[0052] Example 43
1-[4-(aminosulfonyl)phenyl]-8-[(4-methoxybenzyl)amino]-4,5-dihydro-1 H-
benzo [g] indazole-3-carboxamide
46
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
O , ,O
H2N/S <:IN-N
N NH2
o
The title compound (36 mg, 71 %) was synthesized by the same procedure as in
Example 41 starting with the product of Example 2(8-amino-1-{4-
[(aminothio)peroxy]phenyl }-4,5-dihydro-lH-benzo[g]indazole-3-carboxamide)
(38.3 mg, 0.10 mmol) and p-anisaldehyde (38 mg, 0.30 mmol). Its structure was
confirmed by IH NMR and MS (504, M+1). C26H25N504S.(Et2O) 0.6, Calc.: C:
62.24, H: 5.70, N: 12.78; Found, C: 61.68, H: 5.43, N: 12.54.
[0053] Example 44
1-[4-(aminosulfonyl)phenyl]-8-[(4-chlorobenzyl)amino]-4,5-dihydro-lH-
benzo[g]indazole-3-carboxamide
O,, O
H2N"S~
N-N
N N H2
O
CI
The title compound (37 mg, 74%) was synthesized by the same procedure as in
Example 41 starting with the product of Example 2(8-amino-1-{4-
[(aminothio)peroxy]phenyl } -4,5-dihydro-1 H-benzo[g]indazole-3-carboxamide)
(38.3 mg, 0.10 mmol) and p-chlorobenzaldehyde (42 mg, 0.30 mmol). Its
structure
was confirmed by'H NMR and MS (508, M+1). C25H22N503SC1, Calc.: C: 59.11,
H: 4.37, N: 13.79; Found, C: 58.78, H: 4.25 N: 13.18.
47
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
[0054] Example 45
1-[4-(aminosulfonyl)phenyl]-8-(isobutylamino)-4,5-dihydro-lH-benzo[g]indazole-
3-carboxamide
o,,o
H2N r ~
\ N,.N O
N
"C I NH2
The title compound (27 mg, 61%) was synthesized by the same procedure as in
Example 41 starting with the product of Example 2(8-amino-1-{4-
[(aminothio)peroxy]phenyl }-4,5-dihydro-lH-benzo[g]indazole-3-carboxamide)
(38.3 mg, 0.10 mmol) and isopropyl aldehyde (22 mg, 0.30 mmol). Its structure
was confirmed by 'H NMR and MS (440, M+1). C22H25N503S.H20.(Et20) 0.2,
Calc.: C: 57.97, H: 6.19, N: 14.83; Found, C: 57.63, H: 5.76 N: 14.04.
[0055] Procedures for the synthesis of Compounds of Example 46 and 47
Scheme XIV
o R o
H2N-S R.N-~S /
0 ~ N_N o 1) 2eq. NaH / DMF ~ ~ N,N 0
~ H N , ~
H2N NHZ 2) 2eq. MeI or 2 ~ ~ NH2
"~~ Br
Example 2
R= CH3 Example 46
R = CH,CH=CH2 Example 47
[0056] Example 46
8-amino-l-{4-[(dimethylamino)sulfonyl]phenyl )-4,5-dihydro-lH-
benzo[g]indazole-3-carboxamide
48
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
O~' "O
-~NI S
~ \ I N-
NH2
H2N
O
To a stirred solution of the product of Example 2(8-amino-1-{4-
[(aminothio)peroxy]phenyl}-4,5-dihydro-lH-benzo[g]indazole-3-carboxamide) (38
mg, 0.10 mmol) in DMF (1 mL) at RT under Ar was added sodium hydride in
mineral oil (60%, 8 mg, 0.20 mmol). After 2 h, iodomethane (28.4 mg, 0.20
mmol)
in DMF (1 mL) was added and the resulted mixture was stirred at RT for 18 h,
added water (10 mL), extracted with EtOAc (3x10 mL). The combined organic
layers was washed with water (3x 10 mL), dried over MgSO4, filtered through a
silica gel pad with EtOAc, and concentrated. The crude product was triturated
with
diethyl ether to give 8-amino-l-{4-[(dimethylamino)sulfonyl]phenyl}-4,5-
dihydro-
1H-benzo[g]indazole-3-carboxamide as a yellow solid (29 mg, 70%). Its
structure
was confirmed,by IH NMR and MS (412, M+1). C20H21N503S.(H20) 0.3.(Et2O)o.3,
Calc.: C: 57.99, H: 5.65, N: 15.95; Found, C: 57.31, H: 5.18, N: 15.26.
[0057] Example 47
8-amino-l- { 4-[(diallylamino)sulfonyl]phenyl } -4,5-dihydro-1 H-benzo
[g]indazole-3-
carboxamide
~N ~ S
n
N NH2
H2N
1 o
The title compound was synthesized by the same procedure used as for Example
46
except iodomethane was replaced by allyl bromide (24.2 mg, 0.20 mmol). The
title
compound is a yellow solid (28 mg, 61%). Its structure was confirmed by 'H NMR
49
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
and MS (464, M+1). C24H25N503S, Calc.: C: 62.18, H: 5.44, N: 15.11; Found, C:
61.76, H: 5.10, N: 14.77.
[0058] Synthesis of Examples 48 and 49
Scheme XV
OH
HN
s ~ Boc 0
~ ~ \ ~
H
H2N \ / EDC, HOB 't, TEA H2N Q HCI 2N
H2O/
NH2 DMF N-N NH2 30 min N~ ~ NH2
\ ct, overnight N
H2N H
\ HNN HCl.H2N
6oo IOI 0 1
Example 2 Example 48
HN,~OCH3
Boc 0 ~OH
OH
~ f HBoc 0 ~ e \ ~
2N
H2 EDC, HOBt, TEA HZN H HCI
H2Oldioxane rt,
-N DMF = -N 30 min = N-N NH
/~ ~N 2
NH2 tt, ovemight : H NH2
H2N I \ \ HN~N + \ \ HCi .H2N I \ \
/ Boc 0 0
Example 2 Example 49
[0059] Example 48
8-(L-alanylamino)-1-[4-(aminosulfonyl)phenyl]-4,5-dihydro-lH-benzo[g]indazole-
3-carboxamide
o~ so
HZN Q N N
H NH2
N
HzN O
O
To a stirring solution of 8-amino-l-{4-[(aminothio)peroxy]phenyl}-4,5-dihydro-
1H-benzo[g]indazole-3-carboxamide (Example 2) (153 mg, 0.40 mmol) in DMF (6
mL) were added N-Boc-L-alanine (90 mg, 0.48 mmol), EDC (88 mg, 0.46 mmol),
HOBt (60 mg, 0.44 mmol), and triethylamine (0.06 mL, 0.44 mmol). The reaction
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
mixture was allowed to stir overnight at room temperature. The DMF was then
removed under reduced pressure and the resulting residue was purified by
reverse
phase preparative HPLC to give a beige powder (83 mg, 38 %). The powder was
then dissolved in dioxane/water (2 mL, 1:1) and 5 M HCl (1 mL) was added at
room temperature. After stirring for 3 hours, the solvent was removed under
reduced pressure to give an oily residue. The residue was dissolved in a
minimum
amount of methanol and ether was added. The resulting precipitate was filtered
to
give the title compound as a pale yellow solid (66 mg, 90 %). 1H NMR (400 MHz,
d6-DMSO): 1.33 (d, 3H, J= 6 Hz), 2.88-2.97 (m, 4H), 3.92 (m, 1H), 7.24-8.15
(7H,
m); M+1=456, Anal. Calcd for C21H23N604SC1 containing MeOH (1) and CH2C12
(1): C, 45.44; H, 4.83; N, 13.82. Found C, 45.37; H, 4.83; N, 13.60.
[0060] Example 49
8-(D-alanylamino)-1-[4-(aminosulfonyl)phenyl]-4,5-dihydro-1 H-benzo[g]indazole-
3-carboxamide
0 o
H2N-S
N-N
H2N ~ 'N / 6~ NH2
~I{ ~ ~ O
I
0
To a stirring solution of 8-amino-1-{4-[(aminothio)peroxy]phenyl}-4,5-dihydro-
1H-benzo[g]indazole-3-carboxamide (Example 2) (156 mg, 0.40 mmol) in DMF (6
mL) were added N-Boc-D-alanine (81 mg, 0.43 mmol), EDC (85 mg, 0.44 mmol),
HOBt (58 mg, 0.43 mmol), and triethylamine (0.06 mL, 0.44 mmol). The reaction
mixture was allowed to stir overnight at room temperature. The DMF was then
removed under reduced pressure and the resulting residue was purified by
reverse
phase preparative HPLC to give a beige powder (122 mg, 56 %). The powder was
then dissolved in dioxane/water (2 mL, 1:1) and 5 M HCl (1 mL) was added at
room temperature. After stirring for 3 hours, the solvent was removed under
51
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
reduced pressure to give the title compound as a light orange solid (92 mg, 87
%).
'H NMR (400 MHz, d6-DMSO): 1.35 (d, 3H, J= 6 Hz), 2.80-2.94 (m, 4H), 3.91 (m,
1H), 7.13-8.14 (7H, m); M+1=456.
[0061] Example 50
8-[(2-chlorobenzoyl)amino]-1-{4-[(dimethylamino)sulfonyl]phenyl } -4,5-dihydro-
1 H-benzo [g] indazole-3 -carboxamide
0
0 CI N-S
p 0 \ ~ N
H
/ ~ \ ( N- p
N-N Q CI O N, ~
H2N Pyridine CI NH2
~ ~ NH2 / I
Example 46 Example 50
To a stirred solution of 8-amino-1-{4-[(dimethylamino)sulfonyl]phenyl}-4,5-
dihydro-lH-benzo[g]indazole-3-carboxamide (Example 46) (1.52 g, 3.70 mmol) in
pyridine (25 mL) at RT was added 2-chlorobenzoic chloride (970 mg, 5.55 mmol).
After 14 h, trisamine (1 g) was added and the mixture was stirred for 2 h. The
mixture was filtered through a silica gel pad with EtOAc and concentrated.
Column
chromatography (silica gel, EtOAc) gave the title compound as a yellow solid
(900
mg, 1.63 mmol, 44%). Its structure was confirmed by 1H NMR and MS (551,
M+1). C27H24C1N504S, Calc.: C: 58.96, H: 4.40, N: 12.73; Found, C: 58.66, H:
4.65, N: 12.58.
[0062] Examples 51-91
Synthesis of the sulfonamide/arnide/urea library
The sulfonamides, amides, and ureas of Examples 51-91 were synthesized in a
library format as described in Examples 5-40. The starting materials are the
product
of Example 4 (8-amino-l-{4-[(aminothio)peroxy]phenyl}-4,5-dihydro-lH-
benzo[g]indazole-3-carboxamide) and appropriate sulfonyl chlorides, acyl
chlorides
and isocyanates. Table I shows the compound identification, compound, IKK
resin
52
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
assay values, formula weight, and mass spectroscopy characterization for the
compounds from the library.
Table 1
COMPOUND STRUCTURE EXAMPLE IKK2 Formula Mass
-resin Weight Spec
1-[4 Example 1 > 100 413.41 414
(aminosulfonyl)phenyl]-8- HZN,S o M
nitro-4,5-dihydro-1H-
benzo[g]indazole-3-
~ N-N
carboxamide o=,N NH2
8-amino-l-[4- Example 2 <1 383.43 384
(aminosulfonyl)phenyl]- HzN-so 0
M
4,5-dihydro-lH- o'' C~N-N
benzo[g]indazole-3-
carboxamide HNNH
z
8-(acetylamino)- 1-[4- Example 3 10 < 425.47 426
(aminosulfonyl)phenyl]- H N-s o 100
4,5-dihydro-lH- 2 0'
benzo[g]indazole-3- M>
carboxamide ILN \ \ N NHZ
/
1-[4- Example 4 <1 461.52 462
(aminosulfonyl)phenyl]-8- HZN_s M
[(methylsulfonyl)amino]- .
4,5-dihydro-lH- N_N
benzo[g]indazole-3- -s N NH2
carboxamide 0 o
1-[4- Example 5 10 < 515.49 516
(aminosulfonyl)phenyl]-8- H2N;s 100
{ [(trifluoromethyl)sulfony o ~
1]amino}-4,5-dihydro-1H- F o N-N NHZ M
benzo[g]indazole 3 F* s_N carboxamide F o o
1-[4- Example 6 <1 475.55 478
(aminosulfonyl)phenyl]-8- H2N.S:o
M
[(ethylsulfonyl)amino]-
4,5-dihydro-1 H-
NHZ
benzo[g]indazole-3- s N-N
carboxamide o
53
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
COMPOUND STRUCTURE EXAMPLE IKK2 Formula Mass
-resin Weight Spec
1-[4- Example 7 1< 529.52 530
(aminosulfonyl)phenyl]-8- HZN,s 10
1[(2,2,2
trifluoroethyl)sulfonyl]am F F
( o H N-N NN2 M
ino}-4,5-dihydro-lH- F~-s-"
11
benzo[g]indazole-3- o
carboxamide
1-[4- Example 8 1< 489.58 490
(aminosulfonyl)phenyl]-8- HZ".S 10
[(propylsulfonyl)amino]- 0'
4,5-dihydro-lH- o N N pM
11
benzo[g]indazole-3- ",."-s
_N NH2
carboxamide 0
1-[4- Example 9 1< 489.58 490
(aminosulfonyl)phenyl]-8- HZN,s 10
[(isopropylsulfonyl)amino o
]-4,5-dihydro-lH- o H N-N NHZ ItM
benzo[g]indazole-3- s'"
carboxamide 1~ ~
1-[4- Example 1< 503.60 531
(aminosulfonyl)phenyl]-8- HZN,s 10 10
[(butylsulfonyl)amino]- o
4,5-dihydro-lH- o u N-N NH M>
benzo[g]indazole-3- s-" carboxamide 0 1-[4- Example 1< 537.62 538
(aminosulfonyl)phenyl]-8- H2N,s 11 10
[(benzylsulfonyl)amino]- o
4,5-dihydro-lH- 0 N-N NHz M>
benzo[g]indazole-3-
carboxamide 60
1-[4- HN.S Example 10 < 573.65 574
(aminosulfonyl)phenyl]-8- ~ o
[(1- O HN-N NH 12 100
naphthylsulfonyl)amino]- / s-" z M
4,5-dihydro-lH-
benzo[g]indazole-3-
carboxamide
1-[4- Example 1< 616.72 617
(aminosulfonyl)phenyl]-8- ~=S 13 10
({[5-(dimethylamino)-1- \ so
aphthyl]sulfonyl}amino) " "-N "~ M
n
-4,5-dihydro-lH- " \ ~
benzo[g]indazole-3-
carboxamide
54
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
COMPOUND STRUCTURE EXAMPLE IKK2 Formula Mass
-resin Weight Spec
1-[4- Example 1< 574.64 575
(aminosulfonyl)phenyl]-8- HzN.sP
14 10
[(isoquinolin-5- N 6' ~
ylsulfonyl)amino]-4,5- - o H N-N NH M
dihydro-1H- o N~ ~ o Z
benzo[g]indazole-3-
carboxamide
1-[4- Example > 100 574.64 575
(aminosulfonyl)phenyl]-8- H2N. S 15 M
[(quinolin-7-
ylsulfonyl)amino]-4,5- o N-N
dihydro-lH- s_N ~ NHZ
benzo[g]indazole-3- 0 C o
carboxamide
1-[4- Example 1< 565.59 575
(aminosulfonyl)phenyl]-8- HzN.s 16 10
[(2,1,3-benzoxadiazol-4- o, 6
' ~
ylsulfonyl)amino]-4,5- N~ /N o H / N-N NH M
dihydro-1 H- o 0 2
benzo[g]indazole-3-
carboxamide
1-[4- Example 1< 599.69 600
(aminosulfonyl)phenyl]-8- ~".S
[(1,1'-biphenyl-4- o 1 17 10
ylsulfonyl)amino]-4,5- S-N N-N "~ M
dihydro-lH- ~o o
benzo[g]indazole-3-
carboxamide
1-[4- Example 10 < 606.71 607
(aminosulfonyl)phenyl]-8- Hz". So
{ [(5-pyridin-2-ylthien-2- o I ~ 18 100
yl)sulfonyl]amino}-4,5- CN S H~\/N-N "F~ pM
dihydro-1 H- o " o
benzo[g]indazole-3-
carboxamide
1-[4- Example 10 < 527.58 528
(aminosulfonyl)phenyl]-8- HZN esP 19 100
{ [(1-methyl-lH-imidazol- o ~
4-yl)sulfonyl]amino}-4,5- ~ 0 H ~ N-N NH2 PM
dihydro-lH- N s'N benzo[g]indazole-3- N O
carboxamide
1-[4- Example 10 < 541.61 542
(aminosulfonyl)phenyl]-8- HZN.s 20 100
{[(1,2-dimethyl-1H- o 1
imidazol-4- \ o H N-N NH M
yl)sulfonyl]amino}-4,5- N ~ S-N ~ Z
01
dihydro-lH-
benzo[g]indazole-3-
carboxamide
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
COMPOUND STRUCTURE EXAMPLE IKK2 Formula Mass
-resin Weight Spec
8-({ [2-(acetylamino)-4- Example 1< 601.69 602
methyl-l,3-thiazol-5- H2N=s 21 10
yl]sulfonyl}amino)-1-[4- o
(aminosulfonyl)phenyl]- N~o H N-N Nl~ M
4,5-dihydro-lH- "-~',s o "
benzo[g]indazole-3-
carboxamide
1-[4- Example 10 < 537.62 538
(aminosulfonyl)phenyl]-8- HzN,s~ 22 100
{[(4- 6 1
methylphenyl)sulfonyl]am 0- H N-N NHZ M 3>
ino}-4,5-dihydro-lH- s"
benzo[g]indazole-3-
carboxamide
1-[4- Example 10 < 553.62 554
(aminosulfonyl)phenyl]-8- HzNs= 23 100
M
methoxyphenyl)sulfonyl]a o H N-N NH,
mino}-4,5-dihydro-lH- o"
benzo[g]indazole-3-
carboxamide
1-[4- Example 1< 541.58 542
(aminosulfonyl)phenyl]-8- HZN.s 24 10
{ [(4- (3 fluorophenyl)sulfonyl]ami o H N-N Ny2 M
no)-4,5-dihydro-lH- F / S-"
benzo[g]indazole-3- 0
carboxamide
1-[4- Example 1< 558.04 559
(aminosulfonyl)phenyl]-8- HZN=sP 25 10
{[(4- o
chlorophenyl)sulfonyl]am o H N-N NHz M
ino}-4,5-dihydro-lH- s'"
benzo[g]indazole-3- o
carboxamide
1-[4- Example 1< 602.49 603
(aminosulfonyl)phenyl]-8- HN.s 26 10
{ [(4- o bromophenyl)sulfonyl]am o H N-N NHZ M
ino}-4,5-dihydro-lH- er / o" o
benzo[g]indazole-3-
carboxamide
1-[4- Example 10 < 591.59 592
(aminosulfonyl)phenyl]-8- HzN.s= 27 100
\{ [3- F F
M
(trifluoromethyl)phenyl]s o H N-N NH,
ulfonyl}amino)-4,5- F s"
dihydro-lH- + o
benzo[g]indazole-3-
carboxamide
56
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
COMPOUND STRUCTURE EXAMPLE IKK2 Formula Mass
-resin Weight Spec
1-[4- Example 1< 592.48 593
(aminosulfonyl)phenyl]-8- HZN.s 28 10
{[(3,4-
C1
dichlorophenyl)sulfonyl]a }~ o H ~ N-N NH2 M
mino}-4,5-dihydro-lH- 01- o N
benzo[g]indazole-3-
carboxamide
1-[4- Example 1< 592.48 593
(aminosulfonyl)phenyl]-8- HZN.s 29 10
([(2,5- o
dichlorophenyl)sulfonyl]a ci o H N-N NHZ M
mino}-4,5-dihydro-1H- S-N
benzo[g]indazole-3- C1 ~
carboxamide
1-[4- Example 1 < '592.48 593
(aminosulfonyl)phenyl]-8- H2N.s 30 10
{ [(2,4- ~ I ~
dichlorophenyl)sulfonyl]a ~~ o H ~ N-N NH2 M
mino}-4,5-dihydro-lH- ci ~ I ~ N~ o
benzo[g]indazole-3-
carboxamide
1-[4- Example 10 < 559.57 560
(aminosulfonyl)phenyl]-8- HZN.sP 31 100
([(2,4- o 1
difluorophenyl)sulfonyl]a ~ o N-N NHz M
mino}-4,5-dihydro-lH- F / sN
benzo[g]indazole-3- \ F o
carboxamide
1-[4- Example 10 < 559.57 560
(aminosulfonyl)phenyl]-8- H2N.s 32 100
{ [(3,4- 6
difluorophenyl)sulfonyl]a }~ o H ~ N-N NHZ M
mino}-4,5-dihydro-lH- F-\/ S-N i ~
benzo[g]indazole-3- ~ I
carboxamide
1-[4- Example 1< 620.48 621
(aminosulfonyl)phenyl]-8- HZN.s 33 10
{ [(4-bromo-2- o
fluorophenyl)sulfonyllami F o H N N NH2 M
no}-4,5-dihydro-lH- BI s
benzo[g]indazole-3- \ /
carboxamide
1-[4- Example 10 < 583.64 584
(aminosulfonyl)phenyl]-8- H2N-
s 34 100
dimethoxyphenyl)sulfonyl -o o N N-N N~ M
91 ]amino}-4,5-dihydro-lH- 0 oN \ ~ o
benzo[g]indazole-3-
carboxamide
57
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
COMPOUND STRUCTURE EXAMPLE IKK2 Formula Mass
-resin Weight Spec
1-[4- Example 1< 659.59 660
(aminosulfonyl)phenyl]-8- r-4N.s 35 10
({[3,5- F F d
[tM
bis(trifluoromethyl)phenyl F o" N-N NH2
]sulfonyl}amino)-4,5- s-N
dihydro-1H- o
benzo[g]indazole-3- F pF
carboxamide
1-[4- Example 1< 626.93 627
(aminosulfonyl)phenyl]-8- HZN.S 36 10
{[(2,4,5- o
trichlorophenyl)sulfonyl]a ci H N-N N"2 M
mino}-4,5-dihydro-1H o
benzo[g]indazole-3- ci o
carboxamide
1-[4- Example 1< 439.49 440
(aminosulfonyl)phenyl]-8- HZN o 37 10
(propionylamino)-4,5-
dihydro-lH- o N-N pM
NH
benzo[g]indazole-3- v
~J1--r" 2
carboxamide I o
1-[4- Example 1< 487.54 489
(aminosulfonyl)phenyl]-8- "ZN,so 38 10
(benzoylamino)-4,5- o
dihydro-lH- o N-N M
benzo[g]indazole-3- "i N"2
carboxamide o
1-[4- Example 1< 454.51 455
(aminosulfonyl)phenyl]-8- HZN, 0 39 10
{ [(ethylamino)carbonyl]a os
mino}-4,5-dihydro-lH- o N-N M
benzo[g]indazole-3- r"i-~-rHi ~ ' N"Z
carboxamide
1-[4- Example 1< 502.55 503
(aminosulfonyl)phenyl]-8- ,%N :o 40 10
[(anilinocarbonyl)amino]- CS
4,5-dihydro-lH- o N-N M
NH2
benzo[g]indazole-3 N_1Lr", ,
carboxamide
1-[4- Example 1 473.56 474
(aminosulfonyl)phenyl]-8- 0; ,0 41 10
(benzylamino)-4,5- HZNS ,
dihydro-lH- " N-N o M
benzo[g]indazole-3- N NHZ
ca
rboxamide 6-6
58
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
COMPOUND STRUCTURE EXAMPLE IKK2 Formula Mass
-resin Weight Spec
1-[4- Example 10 < 487.58 488
(aminosulfonyl)phenyl]-8- 0. .0 42 100
[(4-methylbenzyl)amino]- H2N"S
4,5-dihydro-lH- H N-N NH M
benzo[g]indazole-3- N~ \ o z
carboxamide
1-[4- Example 1 < 503.58
(aminosulfonyl)phenyl]-8- 0, .0 43 10
[(4- HzN"S
methoxybenzyl)amino]- H N-N NH !tM
4,5-dihydro-lH- N~ \ Z
o
benzo[g]indazole-3-
carboxamide
o-
1-[4- Example 10 <- 508.00 509
(aminosulfonyl)phenyl]-8- 0 0 44 100
[(4-chlorobenzyl)amino]- HZN"S
4,5-dihydro-lH- N-N M
benzo[g]indazole-3- NHZ
~ \ o
carboxamide
ci
1-[4- Example 10 < 439.54 440
(aminosulfonyl)phenyl]-8- o, 45 100
(isobutylamino)-4,5- H2N"s /
M
dihydro-lH- H~ I N-N 0
benzo[g]indazole-3- N Nrl2
carboxamide
8-amino-1-{4- Example 1< 411.48 412
[(dimethylamino)sulfonyl] o. ;0 46 10
phenyl}-4,5-dihydro-lH- _N"S abenzo[g]indazole-3- N"N NH2 M
carboxamide HZN &-po
8-amino-l-{4- Example 1< 463.56 464
[(diallylamino)sulfonyl]ph (? , 47 10
enyl)-4,5-dihydro-lH-
benzo[g]indazole-3- M
carboxamide H N ~N NHZ
2
0
59
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
COMPOUND STRUCTURE EXAMPLE IKK2 Formula Mass
-resin Weight Spec
8-(L-alanylamino)-1-[4- Example 1< 490.97 492
(aminosulfonyl)phenyl]- p 48 10
4,5-dihydro-lH- HZNOS
benzo[g]indazole-3- N-N NHZ M
carboxamide HCI
H
hydrochloride ~" v i o
HZN o
8-(D-alanylamino)-1-[4- Example 1< 490.97 492
(aminosulfonyl)phenyl]- o. ..0 49 10
4,5-dihydro-lH- "~"'S ~
benzo[g]indazole-3- /~ M
carboxamide "-"
hydrochloride H01 " \ o "~
8-[(2- Example <1 550.04 551
chlorobenzoyl)amino]-1- 50 M
14- -"= .o
[(dimethylamino)sulfonyl] -s
1
phenyl }-4,5-dihydro-lH-
benzo[g]indazole-3- o N-N
NHZ
carboxamide ci 6 -(o
1-[4- H2N\ Example 1< 467.55 467
(aminosulfonyl)phenyl]-8- I 51 10
(pentanoylamino)-4,5-
dihydro-lH- M
benzo[g]indazole-3- ~ L Acarboxamide ~/
1-[4- Example 1< 493.59 494
(aminosulfonyl)phenyl]-8- 52 10
[(cyclohexylcarbonyl)ami
no]-4,5-dihydro-lH- e; F~M
benzo[g]indazole-3- $ o
carboxamide
1-[4- HZN\f Example 1< 470.56 480
(aminosulfonyl)phenyl]-8- 53 10
[(cyclopentylcarbonyl)ami
no]-4,5-dihydro-lH- x2 M
benzo[g]indazole-3- 8
carboxamide
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
COMPOUND STRUCTURE EXAMPLE IKK2 Formula Mass
-resin Weight Spec
1-[4- H N Example 1< 465.53 466
(aminosulfonyl)phenyl]-8- 2 \~ \ 54 10
[(cyclobutylcarbonyl)ami
no]-4,5-dihydro-lH- M
benzo[g]indazole-3- $
carboxamide
1-[4- õZN\~ Example 1< 451.51 452
(aminosulfonyl)phenyl]-8- 55 10
[(cyclopropylcarbonyl)am
ino]-4,5-dihydro-lH- ~ -\ H2 M
benzo[g]indazole-3- H
carboxamide
1-[4- HZN\~ Example 1< 453.52 454
(aminosulfonyl)phenyl]-8- so 56 10
(butyrylamino)-4,5-
dihydro-lH- M
benzo[g]indazole-3- $ e \
carboxamide
1-[4- Example 1< 501.57 502
(aminosulfonyl)phenyl]-8- 57 10
[(phenylacetyl)amino]-
4,5-dihydro-lH- benzo[g]indazole-3- 0J-11
carboxamide
1-[4- 112N 4 Example 1< 455.49 456
(aminosulfonyl)phenyl]-8- 58 10
[(methoxyacetyl)amino]-
4,5-dihydro-lH- M
benzo[g]indazole-3- J-H I \
carboxamide i
1-[4- H2N\g Example 1< 453.52 454
(aminosulfonyl)phenyl]-8- 59 10
(isobutyrylamino)-4,5-
dihydro-lH- M
benzo[g]indazole-3-
R \
carboxamide
61
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
COMPOUND STRUCTURE EXAMPLE IKK2 Formula Mass
-resin Weight Spec
Example 1< 555.54 556
(aminosulfonyl)phenyl]-8- 60 10
{[4-
(trifluoromethyl)benzoyl]a M
mino}-4,5-dihydro-lH-
benzo[g]indazole-3-
carboxamide
!3
1-[4- g,N~ Example <1 517.56 518
(aminosulfonyl)phenyl]-8- 61 M
[(4-
methoxybenzoyl)amino]- \ pa:
4,5-dihydro-lH-
benzo[g]indazole-3-
carboxamide
~
1-[4- $ZN~~ Example <1 521.98 522
(aminosulfonyl)phenyl]-8- e 62 M
[(4-chlorobenzoyl)amino]-
4,5-dihydro-lH- benzo[g]indazole-3- $ \
carboxamide
1-[4- $ZN~ Example <1 505.53 506
(aminosulfonyl)phenyl]-8-
[(4-fluorobenzoyl)amino]- 63 M
4,5-dihydro-lH-
H:
benzo[g]indazole-3-
carboxamide
1-[4- Example 1< 547.59 548
(aminosulfonyl)phenyl]-8-
[(3,4- 64 10
dimethoxybenzoyl)amino] e \ ~ H: M
-4,5-dihydro-lH-
benzo[g]indazole-3-
carboxamide
1-[4 ~ Example 10 < 477.5 478
(aminosulfonyl)phenyl]-8- Z i ~ 65 100
(2-furoylamino)-4,5-
dihydro-lH- M
benzo[g]indazole-3-
carboxamide
62
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
COMPOLTND STRUCTURE EXAMPLE IKK2 Formula Mass
-resin Weight Spec
1-[4- AzN Example 10 < 493.57 494
(aminosulfonyl)phenyl]-8- \
66 100
[(thien-2-
ylcarbonyl)amino]-4,5- M
dihydro-lH- H
benzo[g]indazole-3-
carboxamide
1-[4- AzN Example <1 488.53 489
(aminosulfonyl)phenyl]-8- 67 M
(isonicotinoylamino)-4,5-
dihydro-lH- benzo[g]indazole-3-
carboxarxride
8-[(1- Example 1< 545.66 546
adamantylcarbonyl)amino
]-1-[4- 68 10
(aminosulfonyl)phenyl]- I N M
4,5-dihydro-lH-
benzo[g]indazole-3-
carboxamide
1-[4- ,,,N Example 1< 523.59 526
(aminosulfonyl)phenyl]-8-
69 10
[(phenylsulfonyl)amino]-
4,5-dihydro-lH- H; M
benzo[g]indazole-3-
carboxamide
1-[4- H2N Example 1< 555.54 556
(aminosulfonyl)phenyl]-8- ~/
{[3- 0 70 10
(trifluoromethyl)benzoyl]a õ, M
mino}-4,5-dihydro-lH-
benzo[g]indazole-3- kt,
carboxamide
1-[4- H2N Example <1 501.57 502
(aminosulfonyl)phenyl]-8- ~J~,
[(3- ~\/I\ 71 M
methylbenzoyl)amino]-
H,
4,5-dihydro-lH-
benzo[g]indazole-3-
carboxamide
63
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
COMPOUND STRUCTURE EXAMPLE IKK2 Formula Mass
--resin Weight Spec
1-[4- xzN,, Example <1 566.43 567
(aminosulfonyl)phenyl]-8- I 72 M
[(3-bromobenzoyl)amino]-
4,5-dihydro-lH-
benzo[g]indazole-3-
carboxamide
1-[4= $2N ~ Example 1< 531.59 532
(aminosulfonyl)phenyl]-8- 73 10
{[(3-
methoxyphenyl)acetyl]ami B , M
no }-4,5-dihydro-lH-
benzo[g]indazole-3-
carboxamide
1-[4- Example <1 488.53 489
(aminosulfonyl)phenyl]-8- a2N- ~ 74 M
[(pyridin-3-
ylcarbonyl)amino]-4,5-
dihydro-lH- g2
benzo[g]indazole-3-
carboxamide
1-[4- Example <1 521.98 522
(aminosulfonyl)phenyl]-8- aZN-~~ 75 M
[(2-chlorobenzoyl)amino]-
4,5-dihydro-1 H- 8
benzo[g]indazole-3- Z
carboxamide
1-[4- Example 1< 602.49 603
(aminosulfonyl)phenyl]-8-
{ [(3- 76 10
bromophenyl)sulfonyl]ami gZ M
no}-4,5-dihydro-lH-
benzo[g]indazole-3-
carboxamide
1-[4- Example 1 558.04 559
(aminosulfonyl)phenyl]-8- ezN-
{ [(3 77 10
chlorophenyl)sulfonyl]ami g, M
no}-4,5-dihydro-lH-
benzo[g]indazole-3-
carboxamide
64
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
COMPOUND STRUCTURE EXAMPLE IKK2 Formula Mass
-resin Weight Spec
1-[4- ~ Example <1 512.55 513
(aminosulfonyl)phenyl]-8- ~ 78 M
[(3-cyanobenzoyl)amino]- $ZN
4,5-dihydro-lH-
gZ
benzo[g]indazole-3-
carboxamide
-N
1-[4- Example 10 < 537.62 538
(aminosulfonyl)phenyl]-8- azNi 79 100
{[(3-
methylphenyl)sulfonyl]am 132 M
ino}-4,5-dihydro-lH-
benzo[g]indazole-3-
carboxamide
1-[4- Example <1 517.56 518
(aminosulfonyl)phenyl]-8- 80 M
[(3-
methoxybenzoyl)amino]- \ Q2
4,5-dihydro-lH- ~ -
benzo
[g]indazole-3-
carboxamide
1-[4- L' Example <1 521.98 522
(anunosulfonyl)phenyl]-8- $'Ni
[(3-chlorobenzoyl)amino]- 81 M
4,5-dihydro-lH-
$ / ~ \ Naa
benzo[g]indazole-3-
carboxamide cx
1-[4- Example 10 < 517.56 518
(aminosulfonyl)phenyl]-8-
N'N~ 82 100
[(2-
methoxybenzoyl)amino]- M
4,5-dihydro-lH- H,
benzo[g]indazole-3-
carboxamide
1-[4- Example <1 555.54 556
(aminosulfonyl)phenyl]-8- H1H~ 83 M
{ [2-
(trifluoromethyl)benzoyl]a
mino}-4,5-dihydro-lH- $ \ N1
benzo[g]indazole-3- C
carboxamide 3
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
COMPOUND STRUCTURE EXAMPLE IKK2 Formula Mass
-resin Weight Spec
1-[4- Example <1 501.57 502
(aminosulfonyl)phenyl]-8- H=N-
84 M
[(2-
methylbenzoyl)amino]-
4,5-dihydro-lH- ~ N \ NN=
benzo[g]indazole-3-
carboxamide
1-[4- Example <1 556.43 557
(aminosulfonyl)phenyl]-8- H=N~ ~110 85 M
[(2,6- r
dichlorobenzoyl)amino]- I ) 1
4,5-dihydro-lH- ~ \ \ NH=
benzo[g]indazole-3-
c7 o
carboxamide 0
1-[4- Example <1 571.53 572
$,N
(aminosulfonyl)phenyl]-8-
- 86 M
{ [2(trifluoromethoxy)benzoyl
]amino}-4,5-dihydro-lH- $ ~ \ $2
benzo[g]indazole-3-
carboxamide
1-[4- Example <1 556.43 557
(aminosulfonyl)phenyl]-8- $=N- ~ 87 M
[(2>3-
dichlorobenzoyl)amino]-
4,5-dihydro-lH- ~ 8 \ ~ Na=
benzo a1 J
[g]indazole-3-
cl o
carboxamide
1-[4- " Example <1 505.53 506
(aminosulfonyl)phenyl]-8- H=N- ~
[(2-fluorobenzoyl)amino]- 88 M
4,5-dihydro-lH-
[g]
indazole-3- carboxamide
benzo ?Y.
1-[4- Exarnple <1 522.97 523
(aminosulfonyl)phenyl]-8-
"=N- 89 M
{[(2-chloropyridin-3-
yl)carbonyl]amino }-4,5-
dihydro-lH- I H g=
benzo[g]indazole-3- ~
carboxamide 1 0
66
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
COMPOUND STRUCTURE EXAMPLE IKK2 Formula Mass
-resin Weight Spec
1[4 Example 10 < 558.04 558
(aminosulfonyl)phenyl]-8- x2N-
90 100
{ c [(2hlorophenyl)sulfonyl]ami C1 M
no}-4,5-dihydro-lH- Hz
benzo[g]indazole-3-
carboxamide
1-[4- Example <1 566.43 567
(aminosulfonyl)phenyl]-8- A2N-
[(2-bromobenzoyl)amino]- 91 M
4,5-dihydro-lH-
benzo[g]indazole-3- er R,
carboxamide
[0063] Examples 92-125
[0064] Examples 92-125 shown in Table 2 were synthesized using the
following synthesis procedure similar to scheme I where R9 is the appropriate
aryl,
substituted aryl, heteroaryl, substituted heteroaryl, substituted arylalkyl,
substituted
heteroarylalkyl, or cycloalkyl.
Scheme XV
67
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
%S o
O O 4-(Methylsulfonyl)-
phenylhydrazine N-N OEt
OzN OEt AcOH, A OZN
~ 0 1 0
ethyl (7-nitro-l-oxo-1,2,3, ethyli-[4-(methylsulfonyl)phenyl)-8-nftro-4,5-
4-tetrahydronaphthalen-2-yl) dihydro-1 H-benzo[glindazole-3-carboxylate
(oxo)acetate 1
~ S'O O~S IO
/
Pd(OH)2 ~ / NH3, MeOH Q-
H2 N-N
HzN \ N OEt 700 psl NH2
H
~ O zN
O
Example 92
OSO
/
N-N
N\~\JJ NHZ
R9CO2H, HATU or Ry~~
O ~~ O
R9COCI, Pyridine
[0065] Example 92
8-amino-l-[4-(methylsulfonyl)phenyl]-4,5-dihydro-1 H-benzo[g]indazole-3-
carboxamide hydrochloride
O\\ S 0
N-N
H2N CONH2
[0066] Step 1
O\\ S O
h
N-N
02N r \ \ COOEt
I
A mixture of (17.2 mmoles) of diketo ester and (17.2 mmoles) 4-
(Methylsulfonyl)-
68
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
phenylhydrazine in 100m1 of acetic acid was refluxed with stirring for 3h, and
then
cooled. The mixture was concentrated, and the residue triturated with ethyl
acetate
'affording a brown solid, which was filtered, washed with ethyl acetate, and
dried to
give the title compound. The structure was supported by 1H NMR.
[0067] Step 2
0 S 0
N-N
H2N COOEt
(
A solution of the title product of Step 1 in acetic acid was treated at room
temperature with 5% palladium on carbon under an atmosphere of hydrogen gas at
5 psi. The reaction was followed by LC-MS. When the conversion was complete,
the mixture was filtered and concentrated to give the title compound as a
brownish
oil that was used directly for the next step.
[0068] Step 3
8-amino-l-[4-(methylsulfonyl)phenyl]-4,5-dihydro-lH-benzo[g]indazole-3-
carboxamide hydrochloride
O\\ S 0
/
N-N
H2N CONHz
I
The title product of Step 2 was dissolved in anhydrous ethanol and then an
approximately equal volume of liquid ammonia was added. The resulting mixture
was sealed in a pressure vessel and then stirred overnight at 100 C. After
cooling,
the mixture was concentrated. The residue was taken up in dichloromethane -
69
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
methanol and chromatographed over silica gel using ethyl acetate as eluent to
give
the title compound, as an oil which crystallized on standing.
Table 2.
Structure Formula Name IKK Example
Weight Resin
IC50 0 418.91 8-amino-l-[4- 1< 92
~S%o
(methylsulfonyl)phen 10
/ yl]-4,5-dihydro-lH-
~ N_N benzo[g]indazole-3- M
H2N NH2 carboxamide
/
\ ~ o hydrochloride
HCI
0 554.55 1-[4- <1 93
-S-o (methylsulfonyl)phen
yl]-8-{ [2- M
_ (trifluoromethyl)benz
~/ N N NH2 oyl]amino}-4,5-
,~ dihydro-lH-
~ F o benzo[g]indazole-3-
F carboxamide
_S o 500.58 8-[(2- <1 94
n/\ methylbenzoyl)amino M
]-1-[4-
\ _ (methylsulfonyl)phen
N N NHZ yl]-4,5-dihydro-IH-
/ benzo[g]indazole-3-
\ o carboxamide
s,o 521.00 8-[(2- <1 95
a,,-- chlorobenzoyl)amino M
]-1-[4- [t
N-N (methylsulfonyl)phen
N NH 2 yl]-4,5-dihydro-lH-
benzo[g]indazole-3-
~
CI 0 carboxamide
_ 555.44 8-[(2,3- <1 96
~Q o
S- dichlorobenzoyl)ami
/ ~ no]-1-[4- M
(methylsulfonyl)phen
c~ N ~\ NH2 yl]-4,5-dihydro-lH-
benzo[g]indazole-3-
ci o \ ~ o carboxamide
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Structure Formula Name IKK Example
Weight Resin
IC50 (
0
%S,O 504.54 8-[(2- <1 97
\ fluorobenzoyl)amino] PM
/ -1-[4-
~ (methylsulfonyl)phen
~' H N_\ NH2 yl]-4,5-dihydro-lH-
F N benzo[g]indazole-3-
o 0 carboxamide
0S,o 521.99 8-{ [(2-chloropyridin- <1 98
3 M
yl)carbonyl]amino}- p
I _ 1-[4-
N-~ N H N\ NH2 (methylsulfonyl)phen
c~ o \ o yl]-4,5-dihydro-lH-
\ benzo[g]indazole-3-
carboxamide
seo 476.52 1-[4- 1< 99
-~ a"-- (methylsulfonyl)phen 10
yl]-8-[(1H-pyrazol-4-
NI H N" N NH2 ylcarbonyl)amino]- M
H~N 0 4,5-dihydro IH-
o benzo[g]indazole-3-
carboxamide
~ 0 501.57 8-{[(2-methylpyridin- <1, 100
-s, 3-
~ yl)carbonyl]amino)
- M
1-[4-
~ N- N
H NH 2 (methylsulfonyl)phen
N N yl]-4,5-dihydro-lH-
0 0 benzo[g]indazole-3-
carboxamide
0 522.53 8-[(2,3- <1 101
~S o difluorobenzoyl)amin
M
~ N-N (methylsulfonyl)phen
~ , N ~ ~ NH2 yl]-4,5-dihydro-lH-
F ( o benzo[g]indazole-3-
F o \ carboxamide
0 531.55 1-[4- <1 102
-S"O (methylsulfonyl)phen
yl]-8-[(2- M
nitrobenzoyl)amino]-
I~ N \ N NH2 4,5-dihydro-lH-
O benzo[g]indazole-3-
No o carboxamide
0
71
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Structure Formula Name IKK Example
Weight Resin
ICso (
0 558.61 3-[({3- 1< 103
(aminocarbonyl)-1-
[4-
(methylsulfonyl)phen M
N- ~ NH2 yl]-4,5-dihydro-lH-
0 ~o o benzo[g]indazol-8-
yl}amino)carbonyl]-
2-methylphenyl
acetate
_ 538.99 8-[(3-chloro-2- <1 104
~S-o fluorobenzoyl)amino]
-1-[4- pM
N-N NH (methylsulfonyl)phen
2 yl]-4,5-dihydro-lH-
N
ei o benzo[g]indazole-3-
F 0 carboxamide
0
11 521.99 8-{ [(4-chloropyridin- 1 < 105
~s=~ 3-
J 10
yl)carbonyl]amino}-
N 1-[4- M
N~ H N- i NHZ (methylsulfonyl)phen
I N yl]-4,5-dihydro-lH-
o benzo[g]indazole-3-
carboxamide
11 0 514.61 8-[(2,3- 1 < 106
0
dimethylbenzoyl)ami
~ 10
no]-1-[4-
~ N \ NH2 (methylsulfonyl)phen M
yl]-4,5-dihydro-lH-
~ o benzo[g]indazole-3-
0 carboxamide
0
s~o 516.58 8-[(3-hydroxy-2- <1 107
methylbenzoyl)amino M
]-1-[4-
H N-N NH (methylsulfonyl)phen
N, ~ 2 yl]-4,5-dihydro-lH-
Ho o \ o benzo[g]indazole-3-
carboxamide
0
"10 555.44 8-[(2,5- <1 108
''S, d
ichlorobenzoyl)ami
no]-1-[4- M
H N- N NH (methylsulfonyl)phen
N 2 yl]-4,5-dihydro-lH-
0- o benzo[g]indazole-3-
carboxamide
72
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Structure Formula Name IKK Example
Weight Resin
(
IC50
0 501.57 8-[(2- 1 < 109
0
aminobenzoyl)amino 10
H N-N NH (methylsulfonyl)phen M
(2 yl]-4,5-dihydro-lH-
o benzo[g]indazole-3-
carboxamide
0 487.54 8- <1 110
is (isonicotinoylamino) M
1-[4-
NH N \ NH2 (methylsulfonyl)phen
~ N, yl]-4,5-dihydro-lH-
0 o benzo[g]indazole-3
carboxamide
s 566.00 8-[(2-chloro-5- <1 111
nitrobenzoyl)amino]- M
No2 1-[4-
H \ NH (methylsulfonyl)phen
N 2 yl]-4,5-dihydro-lH-
0 o benzo[g]indazole-3-
ci
carboxamide
o ~0 572.47 8-[(5-amino-2- <1 112
HCI -~S~ chlorobenzoyl)amino
NH2 ]-1 [4 M
(methylsulfonyl)phen
~ \ NHz yl]-4,5-dihydro-lH-
~ benzo[g]indazole-3-
I o o carboxamide
hydrochloride
,~0 536.01 8-[(3-amino-4- <1 113
Q,-- chlorobenzoyl)amino M
]-1-[4-
cl N- N (methylsulfonyl)phen
NH
~ 2 yl]-4,5-dihydro-lH-
HZN o benzo[g]indazole-3-
carboxamide
S~o 536.01 8-[(4-amino-2- <1 114
~ chlorobenzoyl)amino pM
i
H2N H N-N NH2 (methylsulfonyl)phen
N yl]-4,5-dihydro-lH-
O o benzo[g]indazole-3-
ci
carboxamide
0
",p 536.01 8-[(2-amino-5- 1 < 115
chlorobenzoyl)amino
N-N (methylsulfonyl)phen M
N/ NH2 yl] 4,5 dihydro 1H
benzo[g]indazole-3-
NHZ 0- carboxamide
73
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Structure Formula Name IKK Example
Weight Resin
(
IC50
536.01 8-[(3-amino-2- <1 116
chlorobenzoyl)aniino
M
H NH2 (methylsulfonyl)phen
" \
HZN yl]-4,5-dihydro-lH-
ci o \ ~ benzo[g]indazole-3-
carboxamide
s~o 536.01 8-[(2-amino-4- <1 117
chlorobenzoyl)amino pM
ci
N- N NH (methylsulfonyl)phen
Z ylj-4,5-dihydro-lH-
o benzo[g]indazole-3-
NHZ
carboxamide
0
%\,O 536.01 8-[(2-amino-3- <1 118
~ chlorobenzoyl)amino M
\ N'"N (methylsulfonyl)phen
~ ~ N "HZ yl]-4,5-dihydro-lH-
C, N \ I benzo[g]indazole-3-
H2 carboxamide
0 i~o 657.58 8-({2-chloro-5- <1 119
-NH [(N,N M
dimethylglycyl)amin
'\ ~~ NHZ o]benzoyl}amino}-1-
[4-
Z~l (methylsulfonyl)phen
yl]-4,5-dihydro-1H-
HcI benzo[g]indazole-3-
carboxamide
hydrochloride
o 564.07 8-{ [2-chloro-5- <1 120
's'
(dimethylamino)benz M
~ oyljamino}-1-[4-
ci 0 H N-N NHz (methylsulfonyl)phen
N: o yl]-4,5-dihydro-lH-
~ benzo[g]indazole-3-
,~N carboxamide
O 572.56 8-[(5-azido-2- <1 121
N3 -S--~-ro -N NH2 nitrobenzoyl)amino]- M
/ V1-[4- u
(methylsulfonyl)phen
yl]-4,5-dihydro-lH-
NO2 O benzo[g]indazole-3-
carboxamide
74
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Structure Formula Name IKK Example
Weight Resin
(
IC50
0 527.57 8-[(4- <1 122
/Si azidobenzoyl)amino]
N3 ~ o -1-[4 M
\ ~ N \N NH 2 (methylsulfonyl)phen
yl]-4,5-dihydro-lH-
o benzo[g]indazole-3-
carboxamide
11 ~O 556.43 8-1[(2,5- <1 123
0
~S~ O1N..N dichloropyridin-3-
CI ) yl)carbonyl]amino}- M
1-[4-
~ ~ NH2 (methylsulfonyl)phen
N ~ N y1]-4,5-dihydro-lH-
O O benzo[g]indazole-3-
CI
carboxamide
O, O 583.09 8-{ [2-chloro-5- <1 124
g%O S~ (methylsulfinyl)benz M
oyl]amino}-1-[4-
N-N (methylsulfonyl)phen
N NH2 yl]-4,5-dihydro-lH-
CI o I.~ O benzo[g]indazole-3-
carboxamide
0 565.01 8-{ [(6-chloro-1,3- <1 125
o benzodioxol-5- M
ro QV\' yl)carbonyl] amino}-
o o 1-[4-
N-N NHz (methylsulfonyl)phen
HN
~
c yl]-4,5-dihydro-lH-
~ o benzo[g]indazole-3-
carboxamide
Meo2s a~,- 619.13 8-{[2-chloro-5-(4- <1 126
methylpiperazin-l- M
N N yl)benzoyl]amino}-1-
O CONH2 [4
ci ~ (methylsulfonyl)phen
~ ~ yl]-4,5-dihydro-lH-
N- benzo[g]indazole-3-
~ N~ carboxamide
[0069] Example 126
8- { [2-chloro-5-(4-methylpiperazin-l-yl)benzoyl]amino }-1-[4-
(methylsulfonyl)phenyl]-4,5-dihydro-1 H-benzo[g] indazole-3-carboxamide
Example 126 was synthesized using the following scheme.
Scheme XVI
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
CI ~ CI O~ rNH CI O
C02H -' I\ O
conc. H2SO4 Pd2(dba)3
Br 70% Br BINAP / N
Na'BuO
(' Jl
95%
N
R
R
CI 0
C1'NN
N H TFA OH HN j, ~ CONHZ O N I CONH
a
CH-= N CI
88% N-~)
N ~,N
NI
R 4-F, 4-SO2Me, 3,4-MDO, etc.
Me02S~_aN-N
O N I ~ \ CONHZ
CI /
N~
~N~
To a mixture of 2-chloro-5-(4-methylpiperazin-1-yl)benzoic acid (0.9 g, 0.0035
mol), the title compound of Example 92 (0.0024 mol) and 1 mL of
diisopropylethylamine in 25 mL of DMF was added HATU (1.3 g, 0.0035 mol) in
one portion. The reaction mixture was stirred at room temperature for 16 h.
Solvent was removed and the residue was purified on preparative HPLC to give
the
product as a pale white solid (89% yield); mp: 194-195 C; 'HNMR (DMSO + TFA-
d, 400 MHz) 8: 10.29 (s, 1H), 8.10 (d, J = 8.7 Hz, 2H), 7.86 (d, J = 8.7 Hz,
2H),
7.64 (s, 1H), 7.43 (dd, J = 2.1, 8.2 Hz, 1H), 7.41 (s, 1H), 7.37 (dd, J = 2.0,
8.2 Hz,
1H), 7.35 (d, J 8.2Hz, 1H), 7.08 (dd, J = 3.0, 8.9 Hz, 1H), 7.04 (d, J= 3.0
Hz,
1H), 3.89 (d, J 12.8 Hz, 2H), 3.51 (d, J= 12.8 Hz, 2H), 3.23(s, 3H), 3.11 (m,
2H),
76
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
2.95 (m, 4H), 2.86 (m, 2H). The bioactivity in the IKK 2 Resin assay for the
compound of Example 126 is shown in Table 2.
[0070] Examples 127-158 shown in Table 3 were synthesized by the following
synthesis scheme were R9 is the appropriate aryl, substituted aryl,
heteroaryl,
substituted heteroaryl, substituted arylalkyl, substituted heteroarylalkyl, or
cycloalkyl.
Scheme XVII
OOS CH3 O S CH3 O~S,CH3
~ ~ ~
NH2
N-N OEt hydrolysis N-N Rink resin N-N
02N \ \ \ OH 02N NH
O2N I~ \ O HOBt, DIC, 0
DMF /
Exam le 92 Resin 1
p Example 127
SnC12, DMF
TFA
~ S CH3 O S CH3 O S CH3
O ~\ p ~ p' ~
[\\~~ - \ / \ ~
N TFA
N NH2 E N-N NH N-N NH2
H2N H2N \ ~\ O 02N ~ O
o ~/
Example 129 RiCOOH, Resin 2 Example 128
PyBrop,
DIEA,
NMP
O S CH3
O' S CH3 0
N-N NH
s H TFA Rs N \ ~\ 0 2
N NH
R~N 0 I
O
Examples 130-158.
Resin 3
[0071] Example 127
1-[4-(methylsulfonyl)phenyl]-8-nitro-4,5-dihydro-1 H-benzo [g] indazole-3-
carboxylic acid
77
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
To 5.0 g (11.3 mmol) of Example 92 (ethyl 1-[4-(methylsulfonyl)phenyl]-8-nitro-
4,5-dihydro-lH-benzo[g]indazole-3-carboxylate) in 115 mL of THF was added 115
mL of 1N NaOH and the mixture allowed to stir overnight at RT. The solution
was
acidified with 2N HC1 and extracted three times with ethyl acetate. Combined
extracts were washed with 10% aq. HCI, brine, dried with Na2SO4 and
concentrated
to afford 4.97 g (100%) of a yellow solid: IH NMR (d6-DMSO) 3.00 (m, 2H), 3.11
(m, 2H), 3.31 (s, 3H), 7.39 (d, 1H), 7.67 (d, 1H), 7.90 (d, 2H), 8.07 (dd,
1H), 8.16
(d, 1H); MS (ESI+) 414 (M+1).
[0072] Example 128
Resin 1 and 1-[4-(methylsulfonyl)phenyl]-8-nitro-4,5-dihydro-lH-
benzo [g] indazole-3-carboxamide
Commercially available Rink amide resin (10 g, NovaBiochem #01-64-0013, 100-
200 mesh, 0.61 mmol/g) was washed sequentially with dichloromethane (DCM)
and dimethylformamide (DMF). The resin was filtered, treated twice with 50%
piperidine in DMF for 15 min, and subsequently washed three times each with
DMF, DCM, and anhydrous DMF. To the resin was added 4.65 g of Example 127,
1.52 g of HOBt and 1.75 mL of DIC in 35 mL anhydrous DMF. After 3 h at RT,
the reagents were removed by filtration and the resin washed three times each
with
DMF, methanol, and DCM. The resin was used directly in the next step. A small
portion (approx. 100 mgs) of resin was cleaved by treatment with 20% TFA in
CH2C12 for 30 min. The resin washed twice with CH2C12 and the collected
filtrates
concentrated in vacuo. The product was purified by silica chromatography to
give
the title compound as a light yellow solid: 'H NMR(CDC13) 9.03 (s, 2H),
8.20(d,
J=8.4Hz, 2H), 8.10(dd, J=2Hz, 8.4Hz, 1H), 7.79(d, J=8.4Hz, 2H), 7.53(d,
J=8.4Hz,
1H), 7.51(d, J=2Hz, 1H), 3.25(s, 3H), 3.16(m, 4H); MS(ESI+) 413 (M+1, 100).
[0073] Example 129
Resin 2 and 8-amino-l-[4-(methylsulfonyl)phenyl]-4,5-dihydro-lH-
benzo[g]indazole-3-carboxamide, trifluoroacetic acid salt
78
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
To resin 2 (6.0 mmol) was added 50 mL of 2M SnC12-2H20 in wet DMF. After
agitation of the mixture overnight, the reagents were removed by filtration
and the
resin washed three times each with DMF, THF, and DCM. The resin was filtered
and dried to give 10.96 g of resin 2. A 92.1 mg portion of the resin was
treated
twice with 20% TFA in CH2C12 and washed three times with CHaC12. The
combined filtrates were concentrated in vacuo and resin loading determined by
direct cleavage NMR of the title compound: resin loading of 0.46 meq/g; 1H NMR
(CDC13/TFA) 3.14 (brs, 4H), 3.31 (s, 3H), 6.78 (d, 1H, 2.0 Hz), 7.33 (dd, 1H),
7.54
(d, 1H, 8.0 Hz), 7.83 (d, 2H), 8.16 (d, 2H); MS(ESI+) 383 (M+1, 100).
[0074] Resin 3
Resin 2 (0.45 mmol/g, 0.200g, 90 mol) was washed three times with anhydrous
NMP and subsequently treated with 0.45 mmol of carboxylic acid R9COOH in 0.5
mL of anhydrous NMP, 0.45 mmol of PyBrop in 0.5 niL of anhydrous NMP and 0.9
mmol of DIEA. The resin was shaken at RT under N2 for 2h. Subsequently, the
reagents were removed by filtration, the resins retreated with the appropriate
carboxylic acid, PyBrop, and DIEA in the same manner as described above. After
agitation of the reaction vessels overnight at RT, the reagents were removed
by
filtration and the resins washed three times each with NMP, DMF, methanol, and
DCM. The products were cleaved from the resins by adding 0.5 mL of 20%
TFA/DCM and agitating the mixture for 15 min. The filtrate was collected and
the
resin retreated with additional TFA/DCM for 15 min. The resin was washed twice
with DCM and the filtrates combined and concentrated in vacuo to afford the
final
products.
[0075] Example 130
8-[(cyclobutylcarbonyl)amino]-1-[4-(methylsulfonyl)phenyl]-4,5-dihydro-1 H-
benzo[g]indazole-3-carboxamide
Using the method described from resin 3, the product was obtained in 57% yield
as
a light yellow solid: 1H NMR(CDC13/CD3OD). 8.13 (d, J=8.4 Hz, 2H), 7.76(d,
79
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
J=8.4 Hz, 2H), 7.38(d, J=1.6 Hz, 1H), 7.24(d, J=8 Hz, 1H), 6.97(dd, J=2 Hz, 8
Hz,
1H), 6.88 (s, 1H), 3.23(s, 3H), 3.10(m, 2H), 3.02(m, 1H), 2.95(m, 2H), 2.16(m,
4H), 1.95(m, 1H), 1.82(m, 1H). 13C NMR(CDC13/CD30D): 165.0, 144.2, 142.9,
140.4, 137.1, 133.0, 129.3, 129.2, 126.4, 126.0, 123.3, 119.4, 114.8, 114.7,
60.7,
44.8, 40.6, 31.7, 29.6, 25.3, 22.8, 20.1, 18.1, 14.3. High resolution Mass:
M+H+
=465.1591 (observed), 465.1608 (theoretical).
[0076] Example 131
8-[(2-chloro-4,5-dimethoxybenzoyl)amino]-1-[4-(methylsulfonyl)phenyl]-4,5-
dihydro-lH-benzo[g]indazole-3-carboxamide
Using the method described from resin 3, the product was obtained in 62% yield
as
a light yellow solid: 1H NMR(CDC13). 8.14(d, J=8.8Hz, 2H), 8.03(s, 1H),
7.79(d,
J=8.8Hz, 2H), 7.40(d, 1H), 7.29(m, 1H), 7.21(s, 1H), 7.16(m, 1H), 6.81(s, 1H),
3.89(s, 3H), 3.88(s, 3H), 3.14(m, 2H), 3.13(s, 3H), 2.99(m, 2H). 13C
NMR(CDC13):
171.4, 164.4, 163.7, 151.7, 148.4, 144.3, 143.1, 140.8, 140.2, 136.4, 134.0,
129.6,
129.4, 126.7, 126.4, 126.1, 123.3, 122.4, 119.7, 115.3, 113.2, 113.0, 60.6,
45.0,
29.8, 21.3, 20.2. High resolution Mass: M+H+ = 581.1277 (observed),
5 81.1256(theoretical).
[0077] Examples 132-158
The compounds of Examples 132-158 were prepared as previously described for
Example 130 using the appropriate aryl, substituted aryl, heteroaryl,
substituted
heteroaryl, substituted arylalkyl, substituted heteroarylalkyl, or cycloalkyl
and are
listed in Table 3.
[0078] Example 159
8- { [2-chloro-5-(methylsulfonyl)benzoyl]amino }-1-[4-(methylsulfonyl)phenyl]-
4,5-
dihydro-1 H-benzo[g]indazole-3-carboxamide
Scheme XVIII
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
R CH3 0 0i ,CH3 O;8 CH3
,S
~S \ O
O I OH SCH3 ~/ Q S02CH3 / N
_N ~ / CI N_N 1) MCPBA H \ N
N
NH NH -~ NHz
HzN I\ PyBrop, DIFJ1, N O 2) TFA/CH2CI2 N \ ~
\~\/%O NMP CI O I i CI O I O
Resin 2 Example 159
To 0.30 g of amino resin 2 (pretreated in DCM for two hrs and washed with dry
NMP) was added PyBrop (0.42g, 0.90 mmol), 2-chloro-5-methylthiobenzoic acid
(182mg, 0.9 mmol), DIEA(314 uL, 1.8 mmol) and dry NMP (2 mL). The resin was
shaken for two hrs. The excess reagents were drained, and the resin was washed
with DMF(x3), methanol (x3), and DCM (x3), and treated with 20% TFA/DCM
mixture containing 1% triisopropylsilane (2 x 12 min x 3 mL). The resin was
washed with DMC (2 x 4 mL). The combined filtrate and washings were
evaporated to a solid, which was further dissolved in 10 mL of DCM. To the
resulting solution was added MCPBA (440 mg, 77% pure, 1.96 mmol ). After 3h,
the reaction was quenched with 30 mL ethylacetate. The organic phase was
washed
with sat. sodium bicarbonate (x3) and brine (x2), dried over anhydrous
magnesium
sulfate and concentrated in vacuo. The residue was purified by silica
chromatography with 8:2 EtOAc/hexane. The product was isolated as light yellow
solid, 37 mg (34%): 1H NMR(CDC13/CD3OD) 8.00(d, J=8.8Hz, 2H), 7.83(s, 1H),
7.81(dd, 1H), 7.68(d, J=8.4Hz, 2H), 7.51(d, J=8.4 iHz, 1H), 7.34(s, 1H), 7.25-
7.17(m, 2H), 3.02(s, 3H), 2.98(s, 3H), 2.96(m, 2H), 2.88(m, 2H). LC-MS: 599.0
(M+H+). High resolution Mass: M+NH4+=616.1077 (observed),
616.1086(theoretical).
[0079] Example 160
8-(L-histidylamino)-1-[4-(methylsulfonyl)phenyl]-4,5-dihydro-lH-
benzo[g]indazole-3-carboxamide
SCHEME XIX
81
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
O' ,CH3 R, sCH3 Rl,CH3
O~ HN O~ ,S
HN ~
N-N (? Fmoc-Hls-OH 'N I H N-N 1)p(peridine/DMF ~~ ~ H NHZ
NH NH -'' N N-N
H N PyBrop, DIEA, Fmoc~N N 2) TFA/CHzC12 N ~ ~
z I o NMP H o ~ O HzN ~ ~ O
2 Example 143
Amino resin 3 (0.402 mmols/g, 0.0804 mmols, 0.200g) was pre-treated in DCM for
one hour followed by washing using anhydrous NMP. To this resin added 5.0
equiv. of Fmoc-His-OH (0.402 mmols, 152 mg) followed by addition of PyBroP
(NovaBiochem, 0.402 mmols, 187 mg). To this mixture was added 10.0 equiv. of
DIEA (0.804 mmols, 140 l) followed by addition of anhydrous NMP (1 ml).
Reaction vessel was capped and agitated under nitrogen for two hours. Reagents
were removed by filtration and the resin washed as follows: NMP (x3), DMF
(x3),
DCM (x3), and anhydrous NMP (xl). Retreated resin as described above and let
agitate under nitrogen overnight. Drained vessel and washed as follows: NMP
(x3), DMF (x3), MeOH (x3), DCM (x3), and DMF (xl). Deprotected Fmoc group
using 50:50 piperidine/DMF (x2, 2ml) 40 minutes each. Washed resin as follows:
DMF (x3), MeOH (x3), and DCM (x3). Let resin air dry for approximately one
hour. Resin was then treated with 20:80 TFA/DCM containing 1%
triisopropylsilane (x2, 1 ml, 45 minutes). Collected filtrates and washed
resin with
DCM (x3, lml). Collected all washings and remove volatiles under nitrogen to
afford 17.2 mg of an orange solid. MS+ +1 (C25H25N704S): 520 (measured).
Table 3.
Structure Formula Name IKK Example
Weight Resin
IC50
s~ 464.55 8- 1< 10 130
C [(cyclobutylcarbonyl)a M
N-N o mino]-1-[4- p
N NHZ (methylsulfonyl)pheny
0 11-4,5-dihydro-lH-
~ O benzo[g]indazole-3-
carboxamide
82
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Structure Formula Name IKK Example
Weight Resin
ICso
581.05 8-[(2-chloro-4,5- <1 M 131
dimethoxybenzoyl)ami
N'N o no]-1-[4-
o N NH2 (methylsulfonyl)pheny
oi 1]-4,5-dihydro-lH-
benzo[g]indazole-3-
~ o- carboxamide
~o
0 566.00 8-[(4-chloro-2- <1 M 132
's' ~ nitrobenzoyl)amino]-
~ 1-[4-
cl ~ N_N (methylsulfonyl)pheny
NH2 1]-4,5-dihydro-lH-
~ benzo[g]indazole-3-
~ o No2 0 carboxamide
%S 545.58 8-[(2-methyl-3- 1< 10 133
nitrobenzoyl)amino]- M
1-[4-
N-N (methylsulfonyl)pheny
N NHz 1]-4,5-dihydro-lH-
benzo[g]indazole-3-
0 o
0 carboxamide
"~0 538.99 8-[(2-chloro-6- <1 M 134
0
iS fluorobenzoyl)amino]-
~ ~ 1-[4-
/ N-N (methylsulfonyl)pheny
H NH2 1]-4,5-dihydro-lH-
N benzo[g]indazole-3-
carboxamide
CI 0
o,0 566.00 8-[(2-chloro-3- <1 M 135
--S' ~ nitrobenzoyl)amino]-
~ 1-[4-
'~ N-N (methylsulfonyl)pheny
~ H NH
~ N 2 1] 4,5 dihydro-1H
No2 o benzo[g]indazole-3-
ci o carboxanude
QS,o 599.89 8-[(5-bromo-2- <1 M 136
chlorobenzoyl)amino]-
Br 1-[4-
(methylsulfonyl)pheny
( N N O NHz 1]-4,5-dihydro-lH-
~ benzo[g]indazole-3-
cl 0 carboxamide
83
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Structure Formula Name IKK Example
Weight Resin
IC50
S~o 567.09 8-{ [2-chloro-5- <1 M 137
~ (methylthio)benzoyl]a
s ~ N nnino}-1-[4-
_\ (methylsulfonyl)pheny
H NH2 1]-4,5-dihydro-lH-
4N benzo[g]indazole-3-
cl o o carboxamide
%S~o 536.01 8-{[(2-chloro-6- <1 pM 138
methylpyridin-3-
/ yl)carbonyljamino}-1-
N CI N-N NH [4-
\ 2 (methylsulfonyl)pheny
1]-4,5-dihydro-lH-
o benzo[g]indazole-3-
carboxamide
0 0 566.00 8-[(5-chloro-2- <1 pM 139
nitrobenzoyl)amino]-
ci 1-[4-
N-N (methylsulfonyl)pheny
N \ NHZ 1]-4,5-dihydro-lH-
0 \ ~ o benzo[g]indazole-3-
NO2
carboxamide
o, s ,0 486.55 8-(benzoylamino)-l- <1 M 140
[4-
~ / (methylsulfonyl)pheny
N-N NH2 11-4,5-dihydro-lH-
\ ! N ~ benzo[g]indazole-3-
o o carboxamide
0= 0 532.64 1-[4- 1< 10 142
(methylsulfonyl)pheny M
~ / 11-8-{[2-
N-N NH (methylthio)benzoyl]a
Hi Z
r mino}-4,5-dihydro-
"Is o o 1H-benzo[g]indazole-
3-carboxamide
o. 516.58 8-[(3- 1< 10 142
methoxybenzoyl)amin M
/ o]-1-[4-
/ N-N H2 (methylsulfonyl)pheny
~ ~ N l1-4,5-dihydro-lH-
~ benzo[g]indazole-3-
o I carboxamide
84
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Structure Formula Name IKK Example
Weight Resin
IC50
01,90 516.58 8-[(4- <1 M 143
methoxybenzoyl)amin
o / o]-1-[4-
/ N-N NHz (methylsulfonyl)pheny
N 1]-4,5-dihydro-lH-
o benzo[g]indazole-3-
0
carboxamide
o0 511.56 8-[(4- <1 M 144
~s
cyanobenzoyl)amino]-
N 1-[4-
N_N (methylsulfonyl)pheny
N ~ NHz 1]-4,5-dihydro-lH-
~ benzo[g]indazole-3-
0 o
carboxamide
0 ; 0 554.63 8-1[(3,7- 1 < 10 145
S dimethylimidazo[1,2-
pM
a]pyridin-2-
~ N-N NH yl)carbonyl]amino}-1-
N ~ [4-
o (methylsulfonyl)pheny
1]-4,5-dihydro-lH-
benzo[g]indazole-3-
carboxamide
0 566.00 8-[(2-chloro-4- <1 M 146
%S'o nitrobenzoyl)amino]-
1-[4-
~ \
No2 N-N (methylsulfonyl)pheny
NMZ 1]-4,5-dihydro-lH-
o o benzo[g]indazole-3-
oi carboxaniide
QS ~ 561.58 8-[(5-methoxy-2- <1 M 147
' _ nitrobenzoyl)amino]-
o~ / 1-[4-
(methylsulfonyl)pheny
N \N NH2 1]-4,5-dihydro-lH-
benzo[g]indazole-3-
0-N .0 o carboxamide
QS o 588.63 3-[({3- <1 M 148
N
(aminocarbonyl)-1-[4-
g (methylsulfonyl)pheny
_ 1]-4,5-dihydro-lH-
~ N NH2 benzo[g]indazol-8-
N yl}amino)carbonyl]-4-
o'N o 0 nitrophenyl
thiocyanate
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Structure Fonnula Name IKK Example
Weight Resin
IC50
o, S~ 567.53 8-[(4,5-difluoro-2- <1 M 149
~" _ nitrobenzoyl)amino]-
F ~ ~ 1-[4-
F N-N (methylsulfonyl)pheny
H NH2 1]-4,5-dihydro-lH-
N benzo[g]indazole-3-
-N o carboxamide
o,,,Q 591.60 8-[(4,5-dimethoxy-2- <1 M 150
nitrobenzoyl)amino]-
~ o 1-[4-
o N_N (methylsulfonyl)pheny
N ~ NHZ 1]-4,5-dihydro-lH-
~ benzo[g]indazole-3-
oao o I/ o carboxamide
o; ,~ 549.54 8-[(5-fluoro-2- <1 M 151
~-S nitrobenzoyl)amino]-
F ' ~ 1-[4-
~ (methylsulfonyl)pheny
N \N NH2 1]-4,5-dihydro-lH-
~ benzo[g]indazole-3-
O 0 I ~ carboxamide
o,P, 599.55 1-[4- <1 [tM 152
~S _ (methylsulfonyl)pheny
F F \ ~ I]-8-{[2-nitro-4-
(trifluoromethyl)benzo
F H NNH2 yl]amino}-4,5-
N dihydro-1 H-
oo I)CY o benzo[g]indazole-3-
carboxamide
o,,,,0 545.58 8-[(5-methyl-2- <1 M 153
~ S nitrobenzoyl)amino]-
' ~ 1-[4-
~ (methylsulfonyl)pheny
/ = N-N
H NHz 1]-4,5-dihydro-lH-
N o benzo[g]indazole-3-
o-.N,o o I/ carboxamide
,~ 545.58 8-[(3-methyl-2- 1< 10 154
''S nitrobenzoyl)amino}-
1-[4- M
(methylsulfonyl)pheny
~H,,6-/N~ NH2 1]-4,5-dihydro-lH-
N benzo[g]indazole-3-
'o o o
carboxamide
86
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Structure Formula Name IKK Example
Weight Resin
IC50
00 576.55 8-[(2,4- <1 M 155
's dinitrobenzoyl)amino]
~* / 1 [4
o N_N NH (methylsulfonyl)pheny
N 2 1]-4,5-dihydro-lH-
; o 0 benzo[g]indazole-3
0 o carboxamide
S o,,,0 500.58 8-[(3- 1< 10 156
'"S methylbenzoyl)amino]
-1-[4- M
(methylsulfonyl)pheny
~ ~ M N-N NHZ 1]-4,5-dihydro-lH-
benzo[g]indazole-3-
o carboxamide
o,,~ 530.61 8-{[(3- <1 M 157
S methoxyphenyl)acetyl]
amino}-1-[4-
H N NH2 (methylsulfonyl)pheny
\ o N 1]-4,5-dihydro-lH-
o benzo[g]indazole-3-
I~
carboxamide
0 0 500.58 1-[4- 1< 10 158
's (methylsulfonyl)pheny
A 1]-8- M
H N-N NH [(phenylacetyl)amino]-
Z
N 4,5-dihydro-lH-
I o benzo[g]indazole-3-
o carboxamide
Qs~o 599.09 8-{ [2-chloro-5- <1 M 159
o (methylsulfonyl)benzo
o:s ~ yl]amino}-1-[4-
_ (methylsulfonyl)pheny
H N\ NH2 1]-4,5-dihydro-lH-
N
benzo[g]indazole-3-
clill 0 carboxamide
s 519.59 8-(L-histidylamino)-1- 1< 10 160
[4 M
M
(methylsulfonyl)pheny
O N-N 1]-4,5-dihydro-IH-
HZN,, rHi NHZ benzo[g]indazole-3-
~ o carboxamide
NH
[0080] Examples 161-206
87
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Examples 161-206 shown in Table 4 were synthesized using the following
synthesis
procedure similar to Scheme I where R9 is the appropriate aryl, substituted
aryl,
heteroaryl, substituted heteroaryl, substituted arylalkyl, substituted
heteroarylalkyl,
or cycloalkyl. The detailed synthesis of 1-(1,3-benzodioxol-5-yl)-8-{[(2-
chloropyridin-3-yl)carbonyl]amirio }-4,5-dihydro-1 H-benzo[g]indazole-3-
carboxamide (Example 161) is described below and is illustrative for the
compounds of Table 4.
88
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
SCHEME XX
o
O 0 3,4-(Methylenedioxy)-
~
phenylhydrazine N-N OEt
OzN 1-1 ~OE2 ACOH, 4 I O2N
ethyl (7-nitro-l-oxo-1,2,3,
4-tetrahydronaphthalen-2-yl)
(oxo)acetate i
~O
/'-
_ O
Pd(OH)2 \ I N-N \ ~
NH3, MeOH ~
H2 Et N-
H2N 700 psi N-N
NH2
O H2N O
O/'- ~
\ ~
R9CO2H, HATU or Ry N N-N NH2
0 O
R9COCI, Pyridine
[0081] Example 161
1-(1,3-benzodioxol-5-yl)-8-{ [(2-chloropyridin-3-yl)carbonyl]amino }-4,5-
dihydro-
1 H-benzo [g]indazole-3-carboxamide
/--0
0
CI 0
N-N
N \ HN r ~ \ CONH2
~ / \ I
[0082] Step 1
89
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
/'-0
0
\ ~ ~ HCI
H-.NH2
The title compound of step 1 was prepared by the method disclosed by T.
Komatsu
et al, Azneim.-Forsch. (1972) 22(12), 2099-104.
[0083] Step 2
/-0
0
N-N
02N COOEt
I
A mixture of 5.OOg (17.2 mmoles) of diketo ester and 3.24g (17.2 mmoles) of
the
title product of Step 1 in 100ml of acetic acid was refluxed with stirring for
3h, and
then cooled. The mixture was concentrated, and the residue triturated with
ethyl
acetate affording a brown solid which was filtered, washed with ethyl acetate,
and
dried to give the title compound, 4.79g. The structure was supported by 1H
NMR.
[0084] Step 3
/-0
0
N-N
H2N C00Et
I
A solution 4.79g of the title product of Step 2 in acetic acid was treated at
room
temperature with 5% palladium on carbon under an atmosphere of hydrogen gas at
5 psi. The reaction was followed by LC-MS. When the conversion was complete,
the mixture was filtered and concentrated to give the title compound as a
brownish
oil that was used directly for the next step.
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
[0085] Step 4
8-amino-l-(1,3-benzodioxol-5-yl)-4,5 -dihydro-1 H-benzo [g] indazole-3-
carboxamide
/'-0
0
N-N
H2N CONHz
The title product of Step 3 was dissolved in anhydrous ethanol and then an
approximately equal volume of liquid ammonia was added. The resulting mixture
was sealed in a pressure vessel and then stirred overnight at 100 C. After
cooling,
the mixture was concentrated. The residue was taken up in dichloromethane -
methanol and chromatographed over silica gel using ethyl acetate as eluent to
give
the title compound, 890mg, as an oil which crystallized on standing. Anal. for
C19H16N403=0.75 H20 (MW 361.87): Calc'd.: C, 63.06;, H, 4.46, N, 15.48.
Found: C, 63.24; H, 4.70, N, 14.58.
[0086] Step 5
/-0
0
CI 0
N-N
N \ HN / \ \ CONH2
A mixture of the title product of Example 4 (3.6 g, 0.01 mol) and 2-
chloronicotinyl
chloride (1.8 g, 0.01 mol) in 50 ml of pyridine was stirred at room
temperature
overnight. Solvent was removed and the residue was triturated with a mixture
of
acetonitrile and methanol (20:1) to give 2.6 g of the title compound as a
light brown
solid. The mother liquor was concentrated and purified the same way to give
91
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
another 0.69 g of product (68% yield); mp: 165-166C. Anal. Calcd. for
C25H18C1N504= 0.5 H20: C, 60.43; H, 3.85; 14.09. Found: C, 60.27; H, 3.59; N,
14.14.
Table 4
Structure Mol. Compound Name(s) IKK2 Resin Example
Wt. IC50
o O 487.90 1-(1,3-benzodioxol-5-yl)-8- <1 M 161
{[(2-chloropyridin-3-
yl)carbonyl]amino }-4,5-
ci O N-N NH2 N/ 2 benzo[g]indazole-3-
I o carboxamide
/- 522.35 1-(1,3-benzodioxol-5-yl)-8- <1 M 162
ci 0 {[(2,5-dichloropyridin-3-
~ yl)carbonyl]amino}-4,5-
I H N' ; dihydro-lH-
N - N NHa benzo[g]indazole-3-
ci 0 i o carboxamide
0 o 565.01 1-(1,3-benzodioxol-5-yl)-8- <1 M 163
{[2-chloro-4-
~s ~ (methylsulfonyl)benzoyl]amin
N N-N o}-4,5-dihydro-lH-
NH2
benzo[g]indazole-3-
ci o 0
carboxamide
o 504.91 1-(1,3-benzodioxol-5-yl)-8- <1 M 164
[(2-chloro-4-
F/ c~ o \/ N_N fluorobenzoyl)amino]-4,5-
~ r"~ CONH2 dihydro-lH-
o benzo[g]indazole-3-
carboxamide
0 0 486.92 1-(1,3-benzodioxol-5-yl)-8- <1 M 165
[(2-chlorobenzoyl)amino]-
4,5-dihydro-lH-
ca 0 N-N benzo[g]indazole-3-
H NHZ
carboxamide
Nz~ N o
o_ 522.90 1-(1,3-benzodioxol-5-yl)-8- <1 M 166
F o / [(2-chloro-4,5-
F N-N difluorobenzoyl)amino]-4,5-
~ cONHz dihydro-lH-
ci 0 benzo[g]indazole-3-
carboxamide
92
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Structure Mol. Compound Name(s) IKK2 Resin Example
Wt. IC50
0 0 501.93 1-(1,3-benzodioxol-5-yl)-8- <1 M 167
{ [(2-chloro-6-methylpyridin-
3-yl)carbonyl]amino }-4,5-
cl O N-N NH dihydro-lH-
~ 2 benzo[g]indazole-3-
ry o carboxamide
0 /~ 530.93 1-(1,3-benzodioxol-5-yl)-8- <1 M 168
ro { [(6-chloro-1,3-benzodioxol-
0 5-yl)carbonyl]amino }-4,5-
~ \ O N-N NH dihydro-lH-
~I HN 2 benzo[g]indazole-3-
~ o carboxaniide
0 0 501.93 8-[(2-amino-6- <1 M 169
chlorobenzoyl)amino]-1-(1,3-
NHz ~ benzodioxol-5-yl)-4,5-
~ o N-N dihydro-lH-
~ ~ NH2 benzo[g]indazole-3-
c1 HN + ~ o carboxamide
/--0 487.91 1-(1,3-benzodioxol-5-yl)-8- <1 M 170
o [(3-
~ chloroisonicotinoyl)amino]-
N ~ 4,5-dihydro-lH-
N-N
~ i N coNH2 benzo[g]indazole-3-
carboxannide
CI o 0 0 538.39 8-[(3-amino-2- <1 M 171
chlorobenzoyl)amino]-1-(1,3-
~ ~ benzodioxol-5-yl)-4,5-
~ N-N dihydro-lH-
H2N CI cIa,NH2 benzo[g]indazole-3-
o carboxamide hydrochloride
HCI
0 0 501.93 8-[(5-amino-2- <1 M 172
HZN chlorobenzoyl)amino]-1-(1,3-
~ benzodioxol-5-yl)-4,5-
~ o N_N dihydro-lH-
~ NHZ benzo[g]indazole-3-
CI HN /
o carboxamide
93
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Structure Mol. Compound Name(s) IKK2 Resin Example
Wt. IC50
~--0 531.92 1-(1,3-benzodioxol-5-yl)-8- <1 M 173
[(2-chloro-5-
NOz nitrobenzoyl)amino]-4,5-
H N NH2 dihydro-lH-
~ N/ benzo[g]indazole-3-
carboxamide
cl
o 546.97 1-(1,3-benzodioxol-5-yl)-8- <1 M 174
[(2-chloro-4,5-
~ dimethoxybenzoyl)amino]-
\ p / \ N Nrl~ 4,5-dihydro-lH-
benzo[g]indazole-3-
~ I Y \ carboxamide
1~
0 o 546.97 1-(1,3-benzodioxol-5-yl)-8- <1 M 175
[(2-chloro-3,4-
dimethoxybenzoyl)amino]-
cl o N-N NHZ 4,5-dihydro-lH-
~0~ benzo[g]indazole-3-
1 &100 carboxamide
/-0 549.01 1-(1,3-benzodioxol-5-yl)-8- <1 M 176
0
{[2-chloro-5-
S (methylsulfinyl)benzoyl]amin
H N- \ o}-4,5-dihydro-lH-
N NH2 benzo[g]indazole-3-
ci o o carboxamide
.~
o 531.37 1-(1,3-benzodioxol-5-yl)-8- <1 M 177
[(2-bromobenzoyl)amino]-
/ ~ 4,5-dihydro-lH-
sr benzo[g]indazole-3-N \ N CONH carboxamide
2
0 /~0 516.95 1-(1,3-benzodioxol-5-yl)-8- <1 M 178
[(2-chloro-5-
methoxybenzoyl)amino]-4,5-
cl 0 H N-N NH2 dihydro-lH-
N benzo[g]indazole-3-
~ r \ carboxamide
0
~
494.51 8-[(4-acetylbenzoyl)amino]-1- 1< 10 pM 179
o (1,3-benzodioxol-5-yl)-4,5-
N-N dihydro-lH-
I r"J CONHZ benzo[g]indazole-3-
0 carboxamide
94
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Structure Mol. Compound Name(s) IKK2 Resin Example
Wt. IC50
0 0 501.93 8-[(4-anuno-2- <1 M 180
chlorobenzoyl)amino]-1-(1,3-
~ benzodioxol-5-yl)-4,5-
' N--N NHZ dihydro-lH-
ci HN benzo[g]indazole-3-
~ o carboxanlide
0 555.90 1-(1,3-benzodioxol-5-yl)-8- <1 M 181
({[2-chloro-6-
~ (trifluoromethyl)pyridin-3-
~
o NHZ yl]carbonyl}amino)-4,5-
N dihydro-lH-
cF3 o benzo[g]indazole-3-
carboxamide
516.95 1-(1,3-benzodioxol-5-yl)-8- <1 M 182
[(2-chloro-4-
~o _ methoxybenzoyl)amino]-4,5-
~ I p N~ NHz dihydro-lH-
ci o o benzo[g]indazole-3-
carboxamide
0 0 529.99 1-(1,3-benzodioxol-5-yl)-8- <1 M 183
{[2-chloro-5-
(dimethylamino)benzoyl]amin
cl 0 N- N NHZ o}-4,5-dihydro-lH-
N benzo[g]indazole-3-
~ carboxamide
,N\
0 0 531.92 1-(1,3-benzodioxol-5-yl)-8- <1 M 184
[(2-chloro-4-
~ nitrobenzoyl)amino]-4,5-
Noa '' 0 N_N NH dihydro-lH-
ci HN 2 benzo[g]indazole-3-
~ o carboxamide
/~o 565.01 1-(1,3-benzodioxol-5-yl)-8- <1 M 185
0 Si 0 {[2-chloro-5-
~ (methylsulfonyl)benzoyl]amin
H N-N NH o}-4,5-dihydro-lH-
N Z benzo[g]indazole 3-
a carboxamide
0 469.46 1-(1,3-benzodioxol-5-yl)-8- <1 M 186
[(6-hydroxypyridin-3-
Ho / N-N yl)carbonyl]amino}-4,5
N~ ~ coNH dihydro-1 H-
o [g]indazole-3-
~ 2 benzo
carboxamide
l
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Structure Mol. Compound Name(s), IKK2 Resin Example
Wt. IC50
Co 467.49 1-(1,3-benzodioxol-5-yl)-8- <1 [IM 187
o ~/j { [(4-methylpyridin-3-
yl)carbonyl] amino }-4,5-
Cj~ N -N CONH dihydro-lH-
~ 2 benzo[g]indazole-3-
0 I carboxamide
o O 466.50 1-(1,3-benzodioxol-5-yl)-8- 1< 10 M 188
[(2-methylbenzoyl)amino]-
~ ~ 4,5-dihydro-lH-
~ N-N benzo[g]indazole-3-
H NNHz carboxamide
N O
Co 521.36 1-(1,3-benzodioxol-5-yl)-8- <1 M 189
[(2,5-
/ N_N dichlorobenzoyl)amino]-4,5-
ci CONH dihydro-lH-
2 benzo[g]indazole-3-
0 carboxamide
0 0 533.01 1-(1,3-benzodioxol-5-yl)-8- <1 M 190
~S {[2-chloro-5-
~ (methylthio)benzoyl]amino }-
~ H N-N 4,5-dihydro-lH-
N NHZ benzo[g]indazole-3
c~ o ,4- o carboxamide
o O 574.04 1-(1,3-benzodioxol-5-yl)-8- 1< 10 M 191
({2-chloro-5-[2-
(dimethylamino)ethoxy]benzo
ci 0 N-N NH2 yl}amino)-4,5-dihydro-lH-
N benzo[g]indazole-3-
~ o carboxamide
0
N
0 0 497.47 1-(1,3-benzodioxol-5-yl)-8- <1 M 192
[(2-nitrobenzoyl)amino]-4,5-
/ dihydro-lH-
benzo[g]indazole-3-
H N carboxamide
N CONHZ
NOz O I /
96
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Structure Mol. Compound Name(s) IKK2 Resin Example
Wt. IC50
Co 474.50 1-(1,3-benzodioxol-5-yl)-8- 1< 10 M 193
o { [(4-methyl-1,2,3-thiadiazol-
N 5-yl)carbonyl]amino }-4,5-
N S ~ nHi N CONHZ dihydro-lH-
benzo[g]indazole-3-
o ~ carboxamide
/~0 521.36 1-(1,3-benzodioxol-5-yl)-8- <1 M 194
[(2,3-
dichlorobenzoyl)amino]-4,5-
ci N-N dihydro-lH-
cl I ~ N NHZ benzo[g]indazole-3-
~ ~ carboxamide
Co 521.36 1-(1,3-benzodioxol-5-yl)-8- <1 M 195
[(2,4-
ci N_N dichlorobenzoyl)amino]-4,5-
~ N CONH dihydro-lH-
~ 2 benzo[g]indazole-3-
0 carboxamide
0 0 453.46 1-(1,3-benzodioxol-5-yl)-8- <1 M 196
(isonicotinoylaniino)-4,5-
~ ~ dihydro-lH-
o ~ N_N benzo[g]indazole-3-
H NHz carboxamide
I ~ N /
N / ~ ~ O
o _ 527.50 1-(1,3-benzodioxol-5-yl)-8- 1< 10 M 197
oMe o ~ ~ [(5-methoxy-2-
OMe
N N-N o dihydro-lH-
~ benzo[g]indazole-3-
NOZ 0 ~ / NH2 carboxamide
0 0 482.50 1-(1,3-benzodioxol-5-yl)-8- <1 M 198
[(3-hydroxy-2-
~ methylbenzoyl)amino]-4,5-
H N-N NH dihydro-lH-
o N 2 benzo[g]indazole-3-
~ carboxamide
i I
HO
o_ 469.46 1-(1,3-benzodioxol-5-yl)-8- 1< 10 M 199
o / [(1-
l N'H N-N oxidoisonicotinoyl)alnlno]-
~ N cONH2 4,5-dihydro-lH-
0 benzo[g]indazole-3-
carboxamide
97
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Structure Mol. Compound Name(s) IKK2 Resin Example
Wt. IC50
0 442.44 1-(1,3-benzodioxol-5-yl)-8- 1< 10 M 200
(3-furoylamino)-4,5-dihydro-
1H-benzo[g]indazole-3-
N \ \ ~ CONHZ carboxamide
O
501.93 8-[(3-amino-4- 1< 10 M 201
0
/ chlorobenzoyl)amino]-1-(1,3-
ci benzodioxol-5-yl)-4,5-
N-N NH dihydro-lH-
HZN HN r-I benzo[g]indazole-3-
\ carboxamide
467.49 1-(1,3-benzodioxol-5-yl)-8- 1 < 10 M 202
{ [(2-methylpyridin-3-
I \ H N_N yl)carbonyl]amino}-4,5-
N / N CONH dihydro-lH-
\ \ z benzo[g]indazole-3-
carboxamide
'N o O 587.04 1-(1,3-benzodioxol-5-yl)-8- 1 < 10 M 203
0 ~ ({ 2-chloro-4-[(N,N-
~ ~ \ o ~ ~ dimethylglycyl)amino]benzoy
' N-N NHz 1}amino)-4,5-dihydro-lH-
i H benzo[g]indazole-3-
\ carboxamide
o O 536.46 1-(1,3-benzodioxol-5-yl)-8- 1< 10 pM 204
{[2-
Ft F (trifluoromethoxy)benzoyl]am
0 0 ~ N-N ino}-4,5-dihydro-lH-
H NH2 benzo[g]indazole-3-
~ j \ carboxamide
0 469.46 1-(1,3-benzodioxol-5-yl)-8- 1< 10 M 205
0 ' / {[(6-hydroxypyridin-2-
~ N-N yl)carbonyl] amino } -4,5-
\
~ ~ N CONH2 dihydro-lH-
Ho N \ benzo[g]indazole-3-
O carboxamide
o -N 477.48 1-(1,3-benzodioxol-5-yl)-8- 1< 10 M 206
[(4-cyanobenzoyl)amino]-4,5-
/ ~ dihydro-lH-
Nc benzo[g]indazole 3-
/ I
\ N CONH2 carboxan--ide
O
98
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
[0087] SCHEME XXI
Ar-, N_N RiR2NH (5 equiv.), EtOH, H Ar-, N-N
O N i~ CONH2 100 C O N CONH2
CI R2RiN
N~ I N,
[0088] Example 207
1-(1,3-benzodioxol-5-yl)-8-( { [2-(methylamino)pyridin-3-yl]carbonyl } amino)-
4,5-
dihydro-1 H-benzo [g] indazole-3-carboxamide
[--O
O llz:~
1,
N-N
H
O N I ~ \ N CONH2
HN /
N~
A mixture of the title product of Example 161 (1.4 g, 0.0028 mol) and
methylamine
(0.014 mol) in 6 mL EtOH was heated in a sealed tube to 100 C for 48 h. The
off-
white precipitate that formed in the crude reaction mixture was filtered and
washed
with EtOH and Et20 to afford 1.05 g of title compound (yield: 75%). Mp: 273-
275 C. 1H NMR (300 MHz, d6-DMSO): S 2.88-2.95 (m, 4H + 3H), 6.12 (s, 2H),
6.57-6.61 (dd, 1H, J = 7.6 Hz, 4.7 Hz), 6.95-6.98 (dd, 1H, J =, 8 Hz, 2 Hz),
7.07 (d,
1H, J = 8 Hz), 7.13 (d, 1H, J = 2 Hz), 7.25-7.34 (m, 3H), 7.45-7.50 (m, 2H),
7.82-
7.83 (m, 1H), 7.91-7.95 (dd, 1H, J= 7.6 Hz, 1.7 Hz), 8.18-8.20(dd, 1H, J= 4.8
Hz,
1.8 Hz), 10.02 (s, 1H). M + 1 = 483.
[0089] Example 208
1-(1,3-benzodioxol-5-yl)-8-[( { 2-[(2-hydroxyethyl)amino]pyridin-3-
yl } carbonyl)amino]-4,5-dihydro-1 H-benzo[g]indazole-3-carboxamide
99
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
~O
O 1-1:
OH H ~1!5:~: N-N
' O N I ~ ~ CONH2
H1N /
N~ I
The title compound was synthesized by the same procedure as in Example 207
starting with product of Example 161 (lg, 0.0020 mol) and ethanolamine
(0.626g,
0.010 mol) in 4 mL of EtOH to afford 0.475 g of title compound (yield: 46 %).
Mp:
250-253 C. iH NMR (300 MHz, d6-DMSO): S 2.86-2.93 (m, 4H), 3.43-3.47 (m,
2H), 3.53-3.54 (m, 2H), 4.76 (s, IH), 6.12 (s, 2H), 6.56-6.59 (dd, 1H, J = 7.5
Hz,
4.8 Hz), 6.95-6.97 (dd, 111, J = 8 Hz, 2 Hz), 7.05 (d, 1H, J = 8 Hz), 7.11 (d,
IH, J =
2 Hz), 7.26-7.37 (m, 4H), 7.51 (s, 1H), 7.89-7.91 (dd, 1H, J = 7.6 Hz, 1.5
Hz), 7.97-
7.99 (t, 1H, J= 5 Hz), 8.14-8.15 (dd, 1H, J= 4.7 Hz, 1.6 Hz), 10.02 (s, 1H).
Anal.
Calcd. for C27H24N605: C, 63.27; H, 4.72; N, 16.40. Found: C, 63.38; H, 4.7;
N,
16.34. M+1=513.
[0090] Example 209
1-(1,3-benzodioxol-5-yl)-8-[({ 2-[(4-methoxybenzyl)amino]pyridin-3-
yl}carbonyl)amino]-4,5-dihydro-IH-benzo[g]indazole-3-carboxamide
r-- O
/ 0 0
I /
- H N-N
O N I CONH2
N
N~
The title compound was synthesized by the same procedure as in Example 207
starting with product of Example 161 (2 g, 0.0040 mol) and p-
methoxybenzylamine
(2.8 g, 0.020 mol) in 10 mL EtOH to yield 1.96 g of the title compound (yield:
60%). Mp: 181-182 C. 1H NMR (300 MHz, d6-DMSO): S 2.88-2.94 (m, 4H), 3.71
(s, 3H), 4.56 (d, 2 H, J = 5.6 Hz), 5.98 (s, 2H), 6.60-6.64 (dd, 1H, J = 7.5
Hz, 4.7
Hz), 6.88 (d, 2H, J = 8.4 Hz), 6.96 (s, 2H), 7.11 (s, 1H), 7.23-7.35 (m, 5 H),
7.39 (s,
100
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
1H), 7.49 (s, 1H), 7.95 (d, 1H, J= 7.45 Hz), 8.17 (d, 1H, J= 4.7 Hz), 8.23 (t,
1H, J
= 5.6 Hz), 10.06 (s, 1H). Anal. Calc. for C33H28N605: C, 67.34; H, 4.79; N,
14.28.
Found C, 67.08; H, 4.78; N, 14.19. M + 1 = 589.
[0091] Example 210
8- { [(2-aminopyridin-3-yl)carbonyl]amino } -1-(1,3-benzodioxol-5-yl)-4,5-
dihydro-
1 H-benzo [g] indazole-3-carboxamide
o
N-N
O N I ~CONH2
H2N 10 N'
The title compound of Example 209 (1.96 g, 0.0033 mol) was dissolved in 6 mL
CH2Cl2 and reacted with 5 mL TFA at room temperature for 36 h. The crude
reaction mixture was diluted with CH2C12 and basified with a saturated aqueous
solution of Na2CO3. The layers were separated and the organic layer was dried
over
MgSO4. The residue obtained after removal of the solvent under vacuum was
triturated with EtOH to afford 0.503 g of title compound (yield: 32%). Mp: 265-
267 C. 1H NMR (300 MHz, d6-DMSO): S 2.88-2.95 (m, 4H), 6.12 (s, 2H), 5.58-
6.62 (dd, 1H, J= 7.6 Hz, 4.7 Hz), 6.97-7.08 (m, 4H), 7.17 (d, 1H, J= 1.9 Hz),
7.28
(s, IH), 7.32 (s, 2H), 7.55 (s, 2H), 7.95-7.98 (dd, 1H, J= 7.7 Hz, 1.7 Hz),
8.11-8.13
(dd, 1H, J= 4.7 Hz, 1.7 Hz), 9.99 (s, 1H). M + 1= 469.
SCHEME XXII
Ar,, RCOOH, HATU, Et3N, Ar~N-N
N-N DMF, rt 0 N
NH2
HzN ~ \ \ CONH2 y CO
R ID 25
101
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
[0092] Example 211
1-(1,3-benzodioxol-5-yl)-8-[(2,5-dichloroisonicotinoyl)amino]-4,5-dihydro-lH-
benzo [g] indazole-3-carboxamide
/~'- O
O
1
N-N
O N CONH2
CI
N CI
2,5-Dichloroisonicotinic acid (1.65g, 0.0086 mol), HATU (3.27g, 0.0086 mol)
and
finally Et3N (2.32 mL, 0.0166 mol) were added to a solution of the title
compound
of step 4 of Example 161 (2g, 0.00574 mol) in 29 mL of DMF. The reaction
mixture was stirred at room temperature for 3 h. The completion of the
reaction
was confirmed by monitoring the disappearance of the title compound of step 4
of
Example 161 in LC/MS. The crude reaction mixture was concentrated to about 10
mL of DMF. Upon addition of water to this DMF residue, a white solid was
formed. This white solid was triturated in water for 20 min and filtered. The
solid
was collected, dissolved in THF, and dried with MgSO4. Removal of the solvent
afforded a brown solid that was triturated in warm CH3CN (80 C) to give 2.2g
of
the title compound (yield 73%). Mp: 292-293 C. 'H NMR (300 MHz, d6-DMSO):
S 2.90-2.92 (m, 4H), 6.09 (s, 2H), 6.94-7.04 (m, 2H), 7.12 (d, 1H, J = 2 Hz),
7.26-
7.38 (m, 4H), 7.51 (s, 1 H), 7.81 (s, 1 H), 8.61 (s, 1 H), 10.54 (s, 1 H). M +
1= 523.
[0093] Example 212
1-(1,3-benzodioxol-5-yl)-8-[(5-chloro-2-morpholin-4-ylisonicotinoyl)amino]-4,5-
dihydro-1 H-benzo [g] indazole-3 -carboxamide
102
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
r--O
O ~
H f~
N-N
O N CONH2
CI
N
~O -
[0094] Step 1
5-chloro-2-morpholin-4-ylisonicotinic acid
0 OH
CI
N
2,5-Dichloroisonicotinic acid, prepared by the method of E. Marzi, A. Bigi, M.
Schlosser, Eur. J. Org. Chem. 2001, 1371 - 1376, (1.6g, 0.0083 mol) and
morpholine (10.9g, 0.125 mol) in 4 mL of N,N-dimethylacetamide were heated at
80 C for 4 days. The volatiles were removed under vacuum and the resulting
yellow
solid partitioned between water and Et20. The aqueous layer was acidified to
pH =
1.5 using an aqueous solution of HC1 and extracted once with Et20 (25 mL) and
three times with CH2C12 (25 mL). The organic extracts were combined and the
solvents removed under vacuum. The resulting yellow solid was crystallized
from
MeOH to afford the title compound 1.07 g (yield: 34 %). 1H NMR (300 MHz, d6-
DMSO): 8 3.45 (t, 4H, J = 4.8 Hz), 3.67 (t, 4H, J= 4.8 Hz), 7.06 (s, 1H), 8.21
(s,
1H), 13.79 (s (broad), 1H). 13C HMR (100 MHz, d6-DMSO): S 45.6, 66.4, 107.3,
116.1, 141.3, 148.3, 158.5, 166.8. M+ 1= 243.
[0095] Step 2
1-(1,3-benzodioxol-5-yl)-8-[(5-chloro-2-morpholin-4-ylisonicotinoyl)amino]-4,5-
dihydro-1 H-benzo[g]indazole-3-carboxamide
103
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
r--O
O
1
H N-N
O N CONH2
CI
II
N
The title compound was synthesized by the same procedure as in Example 211
starting with the title material from step 1 (0.35 g, 0.00144 mol), the title
compound
of step 4 of Example 161 (0.333 g, 0.00096 mol), HATU (0.54 g, 0.00142 mol)
and
Et3N (0.39 mL, 0.00279 mol) in DMF (8 mL) to yield 0.487 g of the title
compound
(yield: 88%). Mp: 269-271 C. 1H NMR (300 MHz, d6-DMSO): 6 2.88-2.93 (m,
4H), 3.46 (t, 4H, J = 4.6 Hz), 3.66 (t, 4H, J= 4.6 Hz), 6.09 (s, 2H), 6.94 (s,
IH),
6.97 (d, IH, J = 2 Hz), 7.02 (d, 1H, J = 8.2 Hz), 7.11 (d, 1H, J = 1.9 Hz),
7.25 (s,
1H), 7.29-7.32 (m, 2 H), 7.39-7.42 (dd, 1H, J= 8.2 Hz, 2 Hz), 7.5 (s, 1H),
8.19 (s,
1H), 10.36 (s, 1H). M + 1 = 574.
[0096] Example 213
1-(1,3-benzodioxol-5-yl)-8-( { [5-chloro-2-(methylthio)pyrimidin-4-
yl]carbonyl } amino)-4,5-dihydro-lH-benzo[g]indazole-3-carboxamide
r--O
O I-lLN-N ~ ,
H 0
O N \ \ \
CI NH2
N
~ I
N S-~
The title compound was synthesized by the same procedure as in Example 211
starting with 5-chloro-2-(methyl-thio)pyrimidine-4 carboxylic acid (1.76 g,
0.00861
mol), the title compound of step 4 of Example 161 (2 g, 0.00574 mol), HATU
(3.27
g, 0.00857mo1), and Et3N (2.32 mL, 0.0166 mol) in DMF (29 mL) to yield 1.3 g
of
104
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
the title compound (yield: 42%). 'H NMR (300 MHz, d6-DMSO): S 2.52 (s, 3H),
2.88-2.93 (m, 4H), 6.08 (s, 2H), 6.94-6.97 (dd, 1H, J= 8.2 Hz, 1.9 Hz), 7.01
(d, 1H,
J= 8.2 Hz), 7.11 (d, 1H, J= 1.9 Hz), 7.25-7.33 (m, 3H), 7.38-7.41 (dd, 1H, J=
8.2
Hz, 1.9 Hz), 7.5 (s, 1H), 8.88 (s, 1H), 10.59 (s, 1H). M + 1= 536.
SCHEME XXIII
H Ar-, N-N H Ar-, N-N
O N I~ CONHZ RiR2NH (20 equiv.), neat O N CONH2
CI or EtOH, 100 C CI
N CI N NRyR2
[0097] Example 214
1-(1,3-benzodioxol-5-yl)-8-{ [5-chloro-2-(4-methylpiperazin-l-
yl)isonicotinoyl] amino } -4,5-dihydro- 1 H-benzo[g]indazole-3-carboxamide
r--O
O ~
N
-N
O N )::Io~
CONH2
CI ID~
IJ
N N-~
A mixture of the title compound of Example 211 (1.2g, 0.0023 mol) and N-
methylpiperazine (4.6 mL, 0.046 mol) was heated at 100 C in a sealed tube for
24 h.
The completion of the reaction was checked by HPLC. After removal of the
volatiles under vacuum, the residue was partitioned between water and CH2Cl2.
The
organic layer was washed an additional time with water and dried over MgSO4.
The crude product mixture was purified by chromatography on silica gel using
CH2C12/MeOH: 12/1 to 10/2 to give 0.62g of the title product, yield: 46%. Mp:
305-307 C. 'H NMR (400 MHz, d6-DMSO): S 2.18 (s, 3H), 2.34-2.35 (d, 2H, J= 5
105
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Hz), 2.89-2.91 (m, 4H), 3.49 (d, 2H, J = 5 Hz), 6.08 (s, 2H), 6.91-7.03 (m,
3H), 7.1
(d, 1H, J = 2 Hz), 7.24-7.3 (m, 3H), 7.38-7.41 (dd, 1H, J = 8.3 Hz, 2 Hz),
7.49 (s,
1H), 8.13 (s, 1H), 10.33 (s, 1H). M + 1 = 587.
[0098] Example 215
1-(1,3-benzodioxol-5-yl)-8-[(5-chloro-2-piperazin-1-ylisonicotinoyl)amino]-4,5-
dihydro-1 H-benzo[g]indazole-3-carboxamide
/--0
O t~N-N
O N CONH2
CI
N QH
The title compound was synthesized by the same procedure as in Example 214
starting with the title compound of Example 211 (1 g, 0.0018 mol) and
piperazine (3
g, 0.036 mol) in EtOH (4 mL). The reaction was run at 95 C for 24h. After
allowing the reaction nlixture to cool down, the volatiles were removed under
vacuum. The residue was triturated with H20 and finally with EtOH to yield
0.572
g of the title compound (yield: 55%). 1H NMR (300 MHz, d6-DMSO): 6 2.71 (s,
broad, 4H), 2.86-3.30 (m, 4H), 3.39 (s, broad, 4H), 6.07 (s, 2H), 6.85 (s,
1H), 6.93-
6.95 (dd, 1 H, J= 8.2 Hz, 1.9 Hz), 7.00 (d, 1 H, J= 8.2 Hz), 7.09 (d, 1 H, J=
1.9 Hz),
7.24-7.30 (m, 3H), 7.38-7.41 (dd, 1H, J = 8.2 Hz, 1.74 Hz), 7.49 (s, 1H), 8.12
(s,
1H), 10.32 (s, 1H). M + 1= 573.
[0099] Example 216
1-(1,3-benzodioxol-5-yl)-8-{ [(3,6-dichloropyridin-2-yl)carbonyl]amino } -4,5-
dihydro-lH-benzo[g]indazole-3-carboxamide
106
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
/--O
O
CI
H N_\ O
CI N N \ \
O I NH2
The title compound was synthesized from 1.653 g of 3,6-dichloro-2-pyridine
carboxylic acid (CP92740, prepared by the method of E. Marzi, A. Bigi, M.
Schlosser, Eur. J. Org. Chem. 2001, 1371 - 1376) and the title compound of
step 4
of Example 161 (2.0 g) by the same procedure used for Example 211. The title
compound is a brown solid (2.4 g, 80%), m.p. 263-265 C. Its structure was
confirmed by 'H NMR and LClMS: 1H NMR (d6-DMSO): S 2.82-3.01 (m, 4H)),
6.11 (s, 2H), 6.97-7.07 (m, 2H), 7.38 d, 1H, J = 1 Hz), 7.81 (s, 1H), 7.33-
7.42 (m,
3H), 7.52 (s, 1H), 7.51 (d, 1H, J= 9 Hz), 8.15 (d, 1H, J= 9 Hz), 10.57 (s,
1H). ESI
mass spectrum for C25H18C12N5O4+: 522 (M + 1).
[00100] Example 217
1-(1,3-benzodioxol-5-yl)-8-( { [3-chloro-6-(4-methylpiperazin-1-yl)pyridin-2-
yl]carbonyl}amino)-4,5-dihydro-lH-benzo[g]indazole-3-carboxamide
/'O
0
cl
I ~ H N-N
~N N N I \ \ CONH2
/NJ O
[00101] Step 1
Potassium 3-chloro-6-(4-methylpiperazin-1-yl)pyridine-2-carboxylate
107
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
0 OK+
cl / N
N--)
~\,N\
Potassium 3-chloro-6-(4-methylpiperazin-1-yl)pyridine-2-carboxylate was
synthesized by the reaction used in Example 250 step 1 starting with 3,6-
dichloro-2-
pyridine carboxylic acid (0.60 g, 3.125 mmol) N-methylpiperazine (7.2 g, 72
mmol). The reaction was carried out at 95 C for 3 days. The volatiles were
removed
under vacuum. The resulting residue was washed with a saturated solution of
K2C03 and with CH2Cla. Three layers were formed. The middle was separated,
dried, and the solvent was removed under reduced pressure giving 0.89 g of
Potassium 3-chloro-6-(4-methylpiperazin-1-yl)pyridine-2-carboxylate (96%). Its
structure was confirmed by 1H NMR and LC/MS: 1H NMR (D20): S 2.18 (s, 3H),
2.43 (s, broad, 4H), 3.37 (s, broad, 411), 6.77 (d, 1H, J = 9 Hz), 7.58 (d,
1H, J = 9
Hz). ESI mass spectrum for C11H15C1N302+: 256 (M + 1) in the presence of TFA.
[00102] Step 2
1-(1,3-benzodioxol-5-yl)-8-({ [3-chloro-6-(4-methylpiperazin-1-yl)pyridin-2-
y1]carbonyl } amino)-4,5-dihydro-1 H-benzo[g]indazole-3-carboxamide
/'o
ci
I ~ H N-N
N N N I \ \ CONH2
NJ O
The title compound was synthesized from 0.30 g of 3-chloro-6-(4-
methylpiperazin-
1-yl)-2-pyridine carboxylic acid, obtained by acidification of its K-salt from
step 1,
and the title compound of step 4 of Example 161 (0.217 g) by the same
procedure
used for Example 260. The title compound is a brown solid (0.23 g, 61%), m.p.
108
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
264-266 C(decomposition). Its structure was confirmed by 'H NMR and LC/MS:
1H NMR (d6-DMSO): S 2.70 (s, 3H), 2.72-4.30 (m, 12H), 6.02 (s, 2H), 6.92-7.12
(m, 4H), 7.22-7.36 (m, 3H), 7.42-7.53 (m, 2H), 7.77 (d, 1H, J = 9 Hz), 10.32
(s,
1H). ESI mass spectrum for C30H29C1N7O4+: 586 (M + 1).
[00103] Example 218
1-(1,3-benzodioxol-5-yl)-8- { [(3-chloro-6-{ [2-(dimethylamino)ethyl]thio }
pyridin-2-
yl)carbonyl] amino }-4, 5-dihydro-1 H-benzo [g] indazole-3-carboxamide
/--o
0
' ci
N-N
\ \ \ CONH2
~N\/"~,g ! N NI/
The title compound was synthesized from 0.36 g of the title compound of step 1
of
Example 262, and the title compound of step 4 of Example 161 (0.35 g) by the
same
procedure used for Example 260. The title compound is a white solid (0.51 g,
86%). Its structure was confirmed by 'H NMR and LC/MS: 1H NMR (d6-DMSO):
S 2.18 (s, 6H), 2.55 (m, 2H), 2.85 (m, 4H), 3.24 (m, 2H), 6.10 (s, 2H), 6.90-
7.08 (m,
2H), 7.14 (s, 1H), 7.22-7.35 (m, 211), 7.36-7.47 (m, 3H), 7.52 (s, 1H), 7.84
(d, IH, J
= 9 Hz), 10.40 (s, IH). ESI mass spectrum for C29H28C1N6O4S+: 591 (M + 1).
[00104] Example 219
1-(1,3-benzodioxol-5-yl)-8-{ [(3-chloro-6-morpholin-4-ylpyridin-2-
yl)carbonyl] amino } -4,5-dihydro-lH-benzo[g]indazole-3-carboxamide
109
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
O
~/
CI
\
I ~ H N-N
O
~N N N \ \
NH2
O
The title compound was synthesized from 0.27 g of morpholine and the title
compound of Example 216 (0.522 g) by the same procedure used for Example 207
except that EtOH was replaced by 0.5 ml of DMA. The reaction was carried out
at
80 C for 36 hours. The title compound, isolated by preparative HPLC, is a
white
solid (0.30 g, 52%), m.p. 260-262 C (decomposition). Its structure was
confirmed
by 1H NMR and LC/MS: 1H NMR (CDC13) S 2.96 (m, 2H), 3.15 (m, 2H)), 3.48 (m,
4H), 3.86 (m, 4H), 5.41 (s, 1H), 6.74-6.86 (m, 2H), 6.90-7.04 (m, 2H), 7.16
(d, 1H,
J = 1 Hz), 7.28-7.35 (m, 2H), 7.54-7.22 (m, 2H), 9.32 (s, 1H). ESI mass
spectrum
for C29H26C1N6O5+: 573 (M + 1).
[00105] Example 220
1-(1,3-benzodioxol-5-yl)-8-({ [3-chloro-6-(methylamino)pyridin-2-
yl]carbonyl } amino)-4,5-dihydro-lH-benzo[g]indazole-3-carboxamide
/--O
O
~~ ~
N-N
H N N ONH2
0 I
The title compound was synthesized from N-methylamine (6 mL of 33 w% solution
in EtOH) and the compound of Example 216 (1.04 g) by the same procedure used
for Example 207 except that 1 mL of DMA was added. The reaction was carried
out at 82 C for 5 days. The title compound, isolated by preparative HPLC, is a
white solid (0.29 g, 52%), M.p. 269-270 C (decomposition). Its structure was
110
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
confirmed by 1H NMR and LC/MS: 'H NMR (d6-DMSO): S 2.76 (d, 3H, J = 5.5
Hz), 2.84-3.99 (m, 4H), 6.16 (s, 2H), 6.57 (d, 1H, J = 9 Hz), 6.91-7.04 (m,
3H),
7.12 (d, 1H, J = 1 Hz), 7.22-7.34 (m, 3H), 7.46-7.54 (m, 3H), 10.19 (s, 1H).
ESI
mass spectrum for C26H22C1N6O4+: 517 (M + 1).
[00106] Example 221
SCHEME XXIV
CI CI O~ rNH CI O~
CO2H ~ ~ I\ O iN O
conc. H2SO4 Pd2(dba)3
Br 70% Br BINAP 'N)
Na BuO
rN
95%
R
R.
ci O
N-N H N-N
11~H HN CONH2 N
TFA I\ CONH2
/
CH2CI2 N
88% ~ J \ I N--)
R= 4-F, 4-SO2Me, 3,4-MDO, etc.
/-O
O
N N
O N
CONH2
CI
N-
N\
iii
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
[00107] Step 1: A solution of 2-chloro-5-bromobenzoic acid (23.6 g, 0.1 mol),
conc. sulfuric acid (5 mL) and condensed isobutene (400 mL) was prepared in a
pressure vessel and stirred at room temperature under 12 psi for 2 days. The
vessel
was opened and the excess isobutene was released. The remaining liquid was
treated with sat. NaHCO3 solution and extracted with methylene chloride. The
organic layer was washed with brine, dried over MgSO4, and filtered. The
filtrate
was concentrated in vacuum to give 20.5 g of the crude product as brown oil,
which
was used without further purification (70%).
[00108] Step 2: A mixture of tert-butyl 2-chloro-5-bromobenzoate (2.95 g, 0.01
mol), N-methylpiperazine (1.5 g, 0.015 mol), NatBuO (1.5 g, 0.015 mol),
Pd2(dba)3
(0.18 g, 0.0002 mol) and BINAP (0.2 g, 0.0003 mol) in toluene was heated at
100 C
under nitrogen for 16 h. The solution was cooled to room temperature and
filtered
through a pad of Celite . The filtrate was concentrated and the residue was
partitioned between methylene chloride and water. The organic layer was washed
with brine, dried over MgSO4, and filtered. The filtrate was concentrated in
vacuum
to give 3.0 g of the crude product as a dark brown oil (97%). The NMR and MS
were consistent with the proposed structure.
[00109] St~: To a solution of tert-butyl 2-chloro-5-(4-methylpiperazin-l-
yl)benzoate (7.3 g, 0.023 mol) in methylene chloride (150 mL) was added
trifluoroacetic acid (62 mL, 0.8 mol) dropwise at 0-5 C. The reaction mixture
was
stirred overnight while allowing to warm up to room temperature. Solvent and
excess TFA was removed and the residue was triturated with ether to give 7.5 g
of
acid as a light brown solid;'H NMR (DMSO, 400 MHz) S: 10.11 (s, 1H), 7.39 (d,
J
= 8.9 Hz, 1H), 7.33 (d, J 3.1 Hz, 1H), 7.16 (dd, J= 3.1, 8.9 Hz, 1H), 3.90 (d,
J=
12.2 Hz, 2H), 3.52 (d, J 11.1 Hz, 2H), 3.15 (m, 2H), 2.98 (m, 2H), 2.87 (s,
3H);
Anal. Calcd. for C12H15C1N202 + 1.0 TFA: C, 45.60; H, 4.37; N, 7.60. Found: C,
45.99; H, 4.62; N, 7.21.
[00110] St~: To a mixture of 2-chloro-5-(4-methylpiperazin-1-yl)benzoic
acid (0.9 g, 0.0035 mol), the title compound of step 4 of Example 161 (0.82 g,
112
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
0.0024 mol) and 1 mL of diisopropylethylamine in 25 mL of DMF was added
HATU (1.3 g, 0.0035 mol) in one portion. The reaction mixture was stirred at
room
temperature for 16 h. Solvent was removed and the residue was purified on
preparative HPLC to give 1.25 g of the product as a pale white solid (89%
yield);
mp: 185-187 C; 'HNMR (DMSO + TFA-d, 400 MHz) S: 10.26 (s, 1H), 9.81 (brs,
1H), 7.52 (brs, 1H), 7.44 (dd, J= 2.0, 8.1 Hz, 1H), 7.37 (d, J= 8.6 Hz, 1H),
7.33 (d,
J = 2.1 Hz, 1H), 7.31 (d, J = 8.4 Hz, 1H), 7.30 (s, 1H), 7.12 (d, J = 2.0 Hz,
1H), 7.09
(m, 2H), 7.03 (d, J = 8.2 Hz, 1H), 6.98 (dd, J = 2.1, 8.2 Hz, 1H), 6.11 (s,
2H), 3.90
(m, 2H), 3.50 (m, 2H), 3.14 (m, 2H), 2.96 (m, 6H), 2.86 (s, 3H).
[00111] The bioactivity in the IKK2 Resin assay for the compounds of Examples
207-221 is shown in Table 5.
Table 5
Structure Mol. Compound Name(s) IKK2
wt. Resin Avg. Example
IC50 (uM)
o,-0 482.50 1-(1,3-benzodioxol-5-yl)-8- <1 M 207
~ ~ [2-(methylamino)pyridin-3-
-H 0 ~ N-N yl]carbonyl}amino)-4,5-
H NHz dihydro-lH-benzo[g]indazole-
" 0 3-carboxamide
o/- 512.53 1-(1,3-benzodioxol-5-yl)-8- 51 M 208
OH tz [({2-[(2-
hydroxyethyl)amino]pyridin-
NH 0 H N-N NHZ 3-yl}carbonyl)amino]-4,5-
N e-, "\ ) o dihydro-1H benzo[g]indazole-
3-carboxamide
- 588.63 1-(1,3-benzodioxol-5-yl)-8- 10 < 100 209
'1 b [(12-[(4- M
NH o H N-N N~ methoxybenzyl)amino]pyridin
N ~ N \ ~ ~ -3-yl}carbonyl)amino]-4,5-
dihydro-1 H-b en zo [ g]indazole-
3-carboxamide
/-o 468.48 8-{ [(2-aminopyridin-3- 1< 10 pM 210
/~ ~ yl)carbonyl]amino}-1-(1,3-
benzodioxol-5-yl)-4,5-
J N-N dihydro-lH-benzo[g]indazole-
o ~
HZN ~ ~ \ NHZ 3-carboxamide
N~
113
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
o~0 522.35 1-(1,3-benzodioxol-5-yl)-8- <1 M 211
[(2,5-
~ dichloroisonicotinoyl)amino]-
ci 0 N N NHZ 4,5-dihydro-lH-
N'' o benzo[g]indazole-3-
" carboxamide
ci
573.01 1-(1,3-benzodioxol-5-yl)-8- 51 M 212
~ [(5-chloro-2-morpholin-4-
o~ ylisonicotinoyl)amino]-4,5-
~
ci 0 \N NHZ dihydro-lH-benzo[g]indazole-
i 0 3-carboxamide
N
Co~
0 0 534.99 1-(1,3-benzodioxol-5-yl)-8- <1 M 213
~ ({[5-chloro-2-
/ ~ (methylthio)pyrimidin-4-
ci 0 N-N NHZ yl]carbonyl}amino)-4,5-
i\ H~ \ o dihydro-lH-benzo[g]indazole-
"Y" \ 3-carboxamide
,S
0 /-0 586.06 1-(1,3-benzodioxol-5-yl)-8- <1 M 214
{[5-chloro-2-(4-
~ methylpiperazin-l-
cl 0 H N N NH2 yl)isonicotinoyl]amino}-4,5-
~ " \ ) o dihydro-lH-benzo[g]indazole
3-carboxamide
C ~
N
o 572.03 1-(1,3-benzodioxol-5-yl)-8- <1 M 215
[(5-chloro-2-piperazin-l-
, ylisonicotinoyl)amino]-4,5-
c~ o H \N NH2 dihydro-lH-benzo[g]indazole-
~ " ) 0 3-carboxamide
N
C ~
N
H
o,.o 522.35 1-(1,3-benzodioxol-5-yl)-8- 1< 10 M 216
{[(3,6-dichloropyridin-2-
ci / yl)carbonyl]amino}-4,5-
, H N-N o dihydro-lH-benzo[g]indazole-
ci N N / NH 3-carboxamide
z
0
,1 586.06 1-(1,3-benzodioxol-5-yl)-8- <1 M 217
0
({[3-chloro-6-(4-
[3-chloro-6-(4-
ci N_N methylpiperazin-1-yl)pyridin-
NN~ 2-yl]carbonyl}amino)-4,5-
i"~J dihydro-lH-benzo[g]indazole-
3-
114
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
õ 591.09 1-(1,3-benzodioxol-5-yl)-8- <1 M 218
{[(3-chloro-6-{[2-
N (dimethylamino)ethyl]thio}py
\ O1 N-N
CONHi ridin-2-yl)carbonyl]amino}-
" ( 4,5-dihydro-lH-
benzo[g]indazole-3-
carboxamide
- 573.01 1-(1,3-benzodioxol-5-yl)-8- <1 M 219
{ [(3-chloro-6-morpholin-4-
I C1 / N-N ylpyridin-2-
O -4,5-
NN ~, \ NH yl)carbonyl]amino}
J = dihydro-1H-benzo[g]indazole-
3-carboxamide
,- 516.95 1-(1,3-benzodioxol-5-yl)-8- <1 M 220
({[3-chloro-6-
~ (methylamino)pyridin-2-
~ I H N N. yl]carbonyl}amino)-4,5-
H N CONHx
dihydro-lH-benzo[g]indazole-
3-carboxamide
o 585.07 1-(1,3-benzodioxol-5-yl)-8- <1 M 221
{[2-chloro-5-(4-
~ N-N methylpiperazin-l-
N CONHZ yl)benzoyl]amino}-4,5-
ci dihydro-lH-benzo[g]indazole-
~ 3-carboxamide
N,_
[00112] Examples 222-243
Examples 222-243 shown in Table 6 were synthesized with the corresponding
starting compounds using the following synthesis procedure similar to Scheme I
where R9 is the appropriate aryl, substituted aryl, heteroaryl, substituted
heteroaryl,
substituted arylalkyl, substituted heteroarylalkyl, or cycloalkyl.
115
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
[00113] SCHEME XXV
F
O O 4-(fluoro)- /
phenylhydrazine N-N OEt
O2N / OEt AcOH, 4 02N
~ 51YO
ethyl (7-nitro-l-oxo-1,2,3,
4-tetrahydronaphthalen-2-yl)
(oxo)acetate 1
F F
Pd(OH)2
)2 NH3, MeOH /
N-N OEt
H2 HZN ~~ 700 psi H N-N NH2
2N
5:~, N~
F
Q
R9CO2H, HATU or R9 N N-N NH2
O
O
R9COCI, Pyridine
Table 6
Structure Mol. Wt. Compound Name(s) IKK2 Example
Resin
IC50 (
\S O F 522.99 8-{[2 chloro-5 <1 M 222
(methylsulfinyl)benzoyl] a
N-N mino } 1 (4-fluorophenyl)-
~ N N NH2 4,5-dihydro-lH-
~ benzo[g]indazole-3-
CI 0 O carboxamide
F 496.33 8-{ [(2,5-dichloropyridin-3- <1 M 223
yl)carbonyl]amino}-1-(4-
fluorophenyl)-4,5-dihydro-
N-N 1H benzo[g]indazole-3-
N ~ ~ ~ carboxamide
CI ~ ~ NHz
N
CI
116
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Structure Mol. Wt. Compound Name(s) IKK2 Example
Resin
IC50 (
F 504.90 8{[(6-chloro 1,3 <1 M 224
benzodioxol-5-
yl)carbonyl]amino }-1-(4-
O H N O fluorophenyl)-4,5-dihydro-
CI N 1H-benzo[g]indazole-3-
' ~ NH2 carboxamide
O,/O
F 461.88 8-{[(2-chloropyridin-3- <1 M 225
yl)carbonyl]amino}-1-(4-
-~ fluorophenyl)-4,5-dihydro-
N-N 1H-benzo[g]indazole-3-
N \ carboxamide
CI NH2
N
F ~ 538.99 8-1[2-chloro-4- <1 M 226
0 -o (methylsulfonyl)benzoyl]a
~
H N-N mino}-1-(4-fluorophenyl)-
N NH 4,5-dihydro-lH-
c- 0 benzo[g]indazole-3-
carboxamide
F 475.91 8-{ [(2-chloro-6- <1 M 227
~ methylpyridin-3-
yl)carbonyl]amino }-1-(4-
CI 0 H N-N NH2 fluorophenyl)-4,5-dihydro-
N 0 1H-benzo[g]indazole-3-
~ carboxamide
F 498.35 8-[(3- <1 M 228
chloroisonicotinoyl)amino]
-1-(4-fluorophenyl)-4,5-
N N-N dihydro-lH-
/ N 2 benzo[g]indazole-3-
cl 0 carboxamide hydrochloride
HCI
F 475.91 8-[(3-amino-2- <1 M 229
chlorobenzoyl)amino]-1-
N-N (4-fluorophenyl)-4,5-
N O dihydro-lH-
CI benzo[g]indazole-3-
NH2 carboxamide
HzN
117
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Structure Mol. Wt. Compound Name(s) IKK2 Example
Resin
IC50 (
F 460.90 8-[(2- <1 M 230
chlorobenzoyl)amino]-l-
(4-fluorophenyl)-4,5-
N- dihydro-lH-
N i ~ o benzo[g]indazole-3-
ci NH2 carboxamide
F 456.48 1-(4-fluorophenyl)-8-[(3- <1 M 231
hydroxy-2-
methylbenzoyl)amino]-4,5-
N-N dihydro-1 H-
O
N o benzo[g]indazole-3-
/ NH2 carboxamide
HO
F 475.91 8-[(4-amino-2- <1 M 232
chlorobenzoyl)amino]-1-
(4-fluorophenyl)-4,5-
0 N-N dihydro-lH-
N o benzo[g]indazole-3-
Cil NH2 carboxamide
H2N
F 490.92 8-[(2-chloro-4- <1 M 233
metho xybenzoyl) amino]-1-
N_N (4-fluorophenyl)-4,5-
o N ~ \ dihydro 1H-
c, I NH benzo[g]indazole-3-
carboxamide
0
\
F 529.88 8-({ [2-chloro-6- <1 M 234
(trifluoromethyl)pyridin-3-
~- yl]carbonyl } amino)-1-(4-
ct 0 N- ~ NHZ fluorophenyl)-4,5-dihydro-
N 1H-benzo[g]indazole-3-
I ,~ carboxamide
CF3
\S F 506.99 8-{ [2-chloro-5- <1 M 235
(methylthio)benzoyl]amino
i H N-N )-1-(4-fluorophenyl)-4,5-
N NH2 dihydro-lH-
benzo[g]indazole 3
CI 0 carboxamide
118
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Structure Mol. Wt. Compound Name(s) IKK2 Example
Resin
IC50 (
F 475.91 8-[(5-amino-2- <1 M 236
chlorobenzoyl)amino]-1-
(4-fluorophenyl)-4,5-
N-N dihydro-lH-
N ~ p benzo[g]indazole-3-
cI NH2 carboxamide
NH2
F 503.97 8-{[2-chloro-5- <1 [tM 237
N~ (dimethylamino)benzoyl]a
mino }-1-(4-fluorophenyl)-
N-N 4,5-dihydro-lH-
~ N CONH2 benzo[g]indazole-3-
CI p ~ carboxamide
F 495.34 8-[(2,3- <1 M 238
dichlorobenzoyl)amino]-1-
(4-fluorophenyl)-4,5-
N-N dihydro-lH-
p N , \ ~NH2 o benzo[g]indazole-3-
CI carboxamide
CI
F 520.95 8-[(2-chloro-4,5- <1 M 239
\N dimethoxybenzoyl)amino]-
1-(4-fluorophenyl)-4,5-
N-N dihydro-lH-
p N benzo[g]indazole-3-
CI NH2 carboxamide
0
-o I
F 440.48 1-(4-fluorophenyl)-8-[(2- <1 M 240
methylbenzoyl)amino]-4,5-
dihydro-lH-
N-N benzo[g]indazole-3-
\ o carboxamide
NH2
I \
119
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Structure Mol. Wt. Compound Name(s) IKK2 Example
Resin
IC50 (
F 505.89 8-[(2-chloro-3- <1 M 241
nitrobenzoyl)amino]-1-(4-
fluorophenyl)-4,5-dihydro-
N-N 1H-benzo[g]indazole-3-
0 N \ a carboxamide
CZ NH
N O
F 400.43 1-(4-fluorophenyl)-8- 1< 10 M 242
[(methylsulfonyl)amino]-
4,5-dihydro-lH-
N-N benzo[g]indazole-3-
carboxamide
O,
O+S~,N
NH2
F 427.44 1-(4-fluorophenyl)-8- 1< 10 M 243
(isonicotinoylamino)-4,5-
dihydro-lH-
N-N benzo[g]indazole-3-
0 ~ O carboxamide
H O NH2
N
120
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
[00114] Example 244
8-amino- i-(4-fluorophenyl)-4,5-dihydro-lH-benzo[g]indazole-3-carboxamide
F
r
N--H2
H2N 106~
This material was prepared from 4-fluorophenyl hydrazine by the method
described for
Example 92.
[00115] Example 245
1-(4-fluorophenyl)-8-{ [(2-piperazin-l-ylpyridin-3-yl)carbonyl]amino }-4,5-
dihydro-
1 H-benzo [g] indazole-3-carboxamide
F
H N-N
HN--'-) O N I\ \\ CONH2
N~
The title compound was synthesized by the same procedure as in Example 207
starting
with the title compound of Example 225 (0.81 g, 0.017 mol) and piperazine (3
g, 0.0348
mol) in 4 mL Et OH to afford 0.523 g of title compound (yield: 58 %). Mp: 156-
157 C.
1H NMR (300 MHz, d6-DMSO): 6 2.63 (t, 4H, J= 4.5 Hz), 2.86-2.93 (m, 4H), 3.08
(t, 4H,
J = 4.5 Hz), 6.86-6.90 (dd, 1H, J = 7.4 Hz, 4.8 Hz), 7.23 (s, 1H), 7.28 (s,
1H), 7.32 (d, 1H,
J = 8.3 Hz), 7.40 (t, 2H, J = 8.7 Hz), 7.54-7.6 (m, 4H), 7.63-7.66 (dd, 1H, J
= 7.4 Hz, 1.7
Hz), 8.22-8.25 (dd, 1H, J= 4.8 Hz, 1.9 Hz), 10.25 (s, 1H). M + 1 = 512.
[00116] Example 246
8- { [(6-chloro-4-methylpyridin-3-yl)carbonyl] amino } -1-(4-fluorophenyl)-4,5-
dihydro-lH-
benzo [g] indazole-3-carboxamide
121
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
F
CI r H N_\
O
N
O N H2
[00117] Step 1
6-Hydroxy-4-methylnicotinic acid
0
I oH
HO N
A modification of the procedure of Weglinski and Talik (Rocz. Chem. 1977, 51,
2401) was
used. Potassium carbonate powder (-325 mesh) was dried under vacuum at 200 C
overnight prior to use. A layer of the dried potassium carbonate (28.56 g,
0.207 mol) was
placed in the bottom of a 300-mL Hastelloy-B autoclave followed by a layer of
2-hydroxy-
4-methylpyridine (28.51 g, 0.2612 mol) mixed with dried potassium carbonate
(28.70 g,
0.208 mol). The vessel was sealed, carefully purged with dry carbon dioxide (5
x 80 psig),
pressurized with dry carbon dioxide to 800 psig, and heated to 130 C for 18
h. After
cooling and careful venting, the product mixture was dissolved in water (560
mL) and
added to 132 mL of 6 N HCl with vigorous stirring over about 20 min. The pH of
the
resulting slurry was 2.43 and potassium carbonate (ca. 0.25 g) was added to
pH=2.53.
After stirring the slurry for 1 h at 25 C, the precipitate was recovered by
vacuum filtration,
washed with water (3 x 35 mL), and dried in vacuo at 100 C to afford 7.11 g
of a tan
powder. 1H NMR analysis of the tan powder was consistent with a mixture of
about 5.6
mol% 2-hydroxy-4-methylpyridine and 94.4 mol% of the desired 2-hydroxy-4-
methyl-5-
pyridinecarboxylic acid. The yield was estimated to be 17.0% based on the NMR
assay of
the recovered product. 1H NMR (400 MHz, d6-DMSO) S 2.36 (d, T= 1 Hz, 3H), 6.19
(apparent s, 1H), 7.99 (s, 1H), 12.2 (broad s, 2H). 13C NMR (100 MHz, d6-DMSO)
8 21.6,
109.1, 119.5, 141.1, 151.8, 161.8, 165.8.
[00118] Step 2
122
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
4-Methyl-6-chloronicotinic acid
O
OH
CI N
6-Hydroxy-4-methylnicotinic acid (10 g, 65.3 mmol) and phosphorus oxychloride
(33 mL)
were combined and refluxed for 3 hours. The reaction solution was poured into
300 mL of
ice and then 600 mL of water was added. The solution was boiled for 30 minutes
before
cooling and extracting the product into ether. The solvent was removed and the
residue
was recrystallized from 900 mL of hot water. Yellow solid, 9.06g (81% yield).
Mp 170-
172 C. 1H NMR (CD3OD): S 2.61 (s, 3H), 7.41 (s, 1H), 8.80 (s, IH). LC-MS, M +
1=
172.
[00119] Step 3
8- { [(6-chloro-4-methylpyridin-3-yl)carbonyl] amino 1- 1 -(4-fluorophenyl)-
4,5-dihydro- I H-
benzo [g] indazole-3-carboxamide
F
~ ~
~ N_ \
CI / H O
N' N
O N H2
The compound of example 244 (3.872 mmoles), 4-methyl-6-chloronicotinic acid
from step
2 (5.828 mmoles), and HATU (5.844 mmoles) were dissolved in 20 mL DMF followed
by
the addition of 1.9 mL triethylamine. The mixture was stirred at room
temperature
overnight. The solvent was then stripped and the residue suspended in water,
filtered, and
washed with water. The solid was recrystallized from acetonitrile, then
redissolved in
acetonitrile, decolorized with decolorizing carbon, and dried over anhydrous
MgSO4. The
solvent was then stripped down to a solid. Mp: 280-284 C. 1H NMR (d6-DMSO, 400
MHz): S 2.28 (s, 3H); 2.84-2.98 (m, 4H); 7.25-7,44 (m, 6H); 7.49 (s, 1H); 7.51-
7.62 (m,
3H); 8.35 (s, 1H); 10.31 (s, 1H). 13C NMR (DMSO, 100 MHz): S 19.13, 20.35,
29.67,
115.01, 117.07, 117.30, 120.08, 121.34, 126.19, 126.60, 128.72, 128.81,
129.58, 132.80,
123
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
133.42, 136.81, 136.84, 137.64, 139.71, 143.19, 148.42, 149.96, 151.59,
161.44, 163.88,
164.47, 164.78. M + 1= 476.
[00120] Example 247
1-(4-fluorophenyl)-8-{ [(4-methyl-6-morpholin-4-ylpyridin-3-yl)carbonyl]amino
}-4,5-
dihydro-1 H-benzo [g] indazole-3-carboxamide
F
C
O N-N
O
NN
0 NH2
The title compound of Example 246 (1.47 mmoles) and morpholine (23.1 mmoles)
were
dissolved in 10 mL of N,N-dimethylacetamide. The reaction mixture was then
placed
under nitrogen and stirred in an oil bath at 100 C for 27 h. The mixture was
partially
stripped of solvent then added to water, filtered, and washed with water. The
solid was
recrystallized from acetonitrile, then dissolved in acetonitrile and dried
over anhydrous
MgSO4. The solvent was then stripped down to a solid. Mp: 301 C (decomp.) 1H
NMR
(d6-DMSO, 400 MHz): S 2.22 (s, 3H); 2.80-2.94 (m, 4H); 3.46 (t, 4H, J = 4.8
Hz); 3.62 (t,
4H, J= 4.8 Hz); 6.67 (s, 1H); 7.22-7.27 (m, 3H); 7.31-7.38 (m, 2H); 7.42 (dd,
1H, J =
1.9Hz, 8.2Hz); 7.49-7.57 (m, 3H); 8.11 (s, 1H); 9.92 (s, 1H). 13C NMR (d6-
DMSO, 100
MHz): S 20.35, 20.38, 29.63, 45.55, 66.54, 108.46, 114.91, 117.02, 117.25,
119.91,
121.23, 122.78, 126.42, 128.64, 128.73, 129.36, 132.60, 136.82, 136.85,
138.38, 139.82,
143.15, 147.70, 148.03, 160.04, 161.39, 163.84, 164.47, 166.18. M+ 1= 527
[00121] Example 248
8-[(2,5-dichloroisonicotinoyl)amino]-1-(4-fluorophenyl)-4,5-dihydro-1 H-
benzo[g]indazole-3-carboxamide
F a_
H N N
O N I CONH2
CI
N CI
124
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
2,5-Dichloroisonicotinic acid (1.65g, 0.0086 mol), HATU (3.27g, 0.0086 mol),
and Et3N
(2.32 mL, 0.0166 mol) were added to a solution of the title compound of
Example 244
(1.85g, 0.00574 mol) in 29 mL of DMF. The reaction mixture was stirred at room
temperature for 3 h. The completion of the reaction was confirmed by
monitoring the
disappearance of the title compound of Example 246 step 2 in LC/MS. The crude
reaction
mixture was concentrated to about 10 mL of DMF. Upon addition of water to this
DMF
residue, a white solid was formed. This white solid was triturated in water
for 20 min and
filtered. The solid was collected, dissolved in THF, and dried with MgSO4.
Removal of
the solvent afforded a brown solid that was crystallized from CH3CN to give
2.1 g of the
title compound as white needles (yield 73%). 1H NMR (300 MHz, d6-DMSO): 2.86-
2.91
(m, 4H), 7.18 (d, 1H, J= 1.2 Hz), 7.25 (s, 1H), 7.32-7.36 (m, 4H), 7.52-7.56
(m, 3H), 7.75
(s, 1H), 8.57 (s, 1H), 10.49 (s, 1H). M + 1= 497.
[00122] Example 249
8-[(5-chloro-2-morpholin-4-ylisonicotinoyl)amino]-1-(4-fluorophenyl)-4,5-
dihydro-
1 H-benzo [g] indazole-3-carboxamide
F
N \ CI N_'
O
N
N
NH2
Oi O
The title compound of Example 244 (0.757 mmoles), 5-chloro-2-morpholin-4-
ylisonicotinic acid, (1.38 mmoles), and HATU (1.57 mmoles) were dissolved in 5
mL
DMF followed by the addition of 0.4 mL of triethylamine. The mixture was
stirred at room
temperature for 3 hrs and partially stripped of DMF. The reaction mixture was
then added
to water, filtered, and washed with water. The solid was dissolved in THF,
decolorized
with decolorizing carbon, and dried over anhydrous MgSO4. The THF was stripped
and
the solid triturated in diethyl ether three times, then triturated in ethanol,
and twice more
with acetonitrile, filtered and dried under vacuum. Mp: 309-313 C. 'H NMR (d6-
DMSO,
400 MHz): 8 2.80-2.95 (m, 4H); 3.42 (t, 4H, J = 5 Hz); 3.62 (t, 4H, J = 5 Hz);
6.88 (s,
125
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
1H); 7.17-7.21 (m, 1H); 7.23-7.41 (m, 5H); 7.49-7.58 (m, 3H); 8.14 (s, 1H);
10.31 (s, 1H).
13C NMR (d6- DMSO, 100 MHz): 5 20.34, 29.70, 45.74, 66.50, 106.55, 114.97,
115.87,
117.09, 117.32, 120.05, 121.41, 126.67, 128.64, 128.73, 129.62, 133.47,
136.80, 136.82,
137.48, 139.64, 143.21, 145.28, 147.54, 158.30, 161.39, 163.85, 164.45. M + 1
= 547.
[00123] Example 250
8- { [5-chloro-2-(4-methylpiperazin- 1 -yl)isonicotinoyl] amino } -1-(4-
fluorophenyl)-
4,5dihydro-1 H-benzo[g]indazole-3-carboxamide
F
H N-N
CONH2
O N ID~
CI N N-
[00124] Step 1
5-chloro-2-(4-methylpiperazin- 1 -yl)isonicotinic acid hydrochloride
0 OH
CI
I = HCI
N N
5-chloro-2-(4-methylpiperazin-1-yl)isonicotinic acid hydrochloride was
synthesized by the
same procedure as for example 212 step 1 starting with 2,5-dichloroisonico-
tinic acid (3 g,
0.0156 mol) N-methylpiperazine (30.7g, 0.30 mol) in 10 mL of N,N-
dimethylacetamide.
The reaction was carried out at 100 C for 8 days. The volatiles were removed
under
vacuum. The resulting residue was washed with a saturated solution of K2C03
and with
CH2C12. The solvents were removed under vacuum and the resulting residue
dissolved in
the minimum amount of water, acidified to pH = 1 with an aqueous solution of
HCl (1N)
and washed with CH2C12. Upon standing at room temperature, the acidic aqueous
layer
126
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
afforded 2g (yield: 44%) of title compound. 1H NMR (300 MHz, D20): S 2.85 (s,
3H),
3.13 (t, 2H, J= 12.28 Hz), 3.34 (t, 2H, J= 14.3 Hz), 3.56 (d, 2H, J= 12.28
Hz), 4.18 (d,
2H, J = 14.3 Hz), 7.08 (s, 1H), 8.00 (s, 1H). 13C NMR (75 MHz, D20): 8 43.1,
43.4,
52.54, 109.5, 117.6, 142.3, 147.9, 154.4, 169.5.
[00125] Step 2
8-{ [5-chloro-2-(4-methylpiperazin- 1 -yl)isonicotinoyl] amino } -1-(4-
fluorophenyl)-
4,5 dihydro-1 H-benzo [g] indazole-3-carboxamide
F O N )aN-N
CONH2
C!
N
N~
The title compound was synthesized by the same procedure as in Example 211
starting
with 5-chloro-2-(N-methyl-piperazinyl)isonicotinic acid hydrochloride from
step 1)) (0.59
g, 0.00202 mol), the title compound of Example 244 (0.432 g, 0.00134 mol),
HATU
(0.755g, 0.00198mo1) and Et3N (1.09 mL, 0.0078 mol) in DMF (8 mL) to yield
0.305 g of
the title compound (yield: 40%). 1H NMR (300 MHz, d6-DMSO): 6 2.17 (s, 3H),
2.33 (t,
4H, J = 4.8 Hz), 2.87-2.93 (m, 4H), 3.47 (t, 4H, J = 4.8 Hz), 6.87 (s, 1H),
7.2 (d, 1H, J =
1.9 Hz), 7.27 (s, 1H), 7.29-7.41 (m, 5H), 7.53-758 (m, 3H), 8.13 (s, 1H),
10.31 (s, 1H). M
+1=561.
[00126] Example 251
1-(4-fluorophenyl)-8-{ [2-(4-methylpiperazin- 1 -yl)isonicotinoyl] amino } -
4,5-dihydro- 1 H-
benzo[g] indazole-3-carboxamide
127
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
= F
):~Y H N N N O
\ \
O I /
/NJ
NH2
[00127] Step 1
8-[ (2-chloroisonicotinoyl) amino] - 1 -(4-fluorophenyl)-4,5-dihydro- 1 H-
benzo[g]indazole-3-carboxamide
F
O
N N
H
CI / N \ \
O NH2
This material was prepared from Example 244 (8-amino-l-(4-fluorophenyl)-4,5-
dihydro-
1H-benzo[g]indazole-3-carboxamide) and 2-chioroisonicotinic acid by the method
described for Example 246 step 3.
[00128] Step 2
The material from step 1 (2.17 mmoles) and N-methyl piperazine (32.9 mmoles)
were
dissolved in 5.0 mL N,N-dimethylacetamide. The reaction mixture was then
placed under
nitrogen and stirred in an oil bath at 100 C for 88 h. The mixture was
partially stripped of
solvent then added to water, filtered, and washed with water. The solid was
then dissolved
in acetonitrile, decolorized with decolorizing carbon, and dried over
anhydrous MgSO4.
The solvent was stripped, then the solid residue was recrystallized from
acetonitrile. Mp:
277-279 C. 1H NMR (400 MHz, d6-DMSO): S 2.17 (s, 3H); 2.35 (t, 4H, J = 5 Hz);
2.82-
2.95 (m, 4H); 3.47 (t, 4H, J = 5 Hz); 6.85 (d, 1H, J = 5 Hz); 7.01 (s, 1H);
7.21-7.44 (m,
128
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
6H); 7.50-7.59 (m, 3H); 8.16 (d, 1H, J = 5 Hz); 10.10 (s, 1H). 13C NMR (DMSO-
d6, 100
MHz): S 20.32, 29.69, 45.20, 46.46, 55.00, 105.43, 111.23, 115.50, 117.07,
117.30,
120.66, 121.37, 126.54, 128.60, 128.69, 129.48, 133.36, 136.82, 136.85,
137.70, 139.71,
143.19, 144.36, 148.89, 159.88, 161.37, 163.81, 164.44, 165.15. M + 1 526.
[00129] Example 252
8- { [5-chloro-2-(4-methyl- 1,4-diazepan- 1 -yl)isonicotinoyl] amino } -1-(4-
fluorophenyl)-4,5-dihydro-1 H-benzo[g]indazole-3-carboxamide
F llz:~
H N_N
CONH2
O N ID~
CI J
N N
C
'N
The title compound was synthesized by the same procedure as in Example 214
starting
with the title compound of Example 248 (1 g, 0.0020 mol) and 1-
methylhomopiperazine
(4.6 g, 0.040 mol). The reaction was run at 95 C for 24 h. After allowing the
reaction
mixture to cool, the volatiles were removed under vacuum. The residue was
triturated with
H2O to yield 0.899 g of the title compound as a tetrahydrate (yield: 71%). 1H
NMR (300
MHz, d6-DMSO): S 1.81-1.83 (m, 2H), 2.21 (s, 3H), 2.41 (t, 2H, J= 5.5 Hz),
2.53 (t, 2H, J
= 4.56 Hz), 2.87-2.93 (m, 4H), 3.53 (t, 2H, J = 5.5 Hz), 3.63-3.67 (m, 2H),
6.62 (s, 1H),
7.19 (d, 1H, J = 1.9 Hz), 7.27-7.38 (m, 4H), 7.42-7.45 (dd, IH, J= 8.12 Hz,
1.88 Hz),
7.53-7.58 (m, 3H), 8.07 (s, 1H), 10.31 (s, 1H). M + 1= 575.
[00130] Example 253
8-[(5-chloro-2-piperazin-1-ylisonicotinoyl)amino]-1-(4-fluorophenyl)-4,5-
dihydro-
1 H-benzo [g] indazole-3-carboxami de
129
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
F a
H N-N
O N I \ \ ~ CONH2
CI
N N'
~~NH
The title compound was synthesized by the same procedure as in Example 214
starting
with the title compound of Example 248 (lg, 0.0020 mol) and piperazine (3.44g,
0.040
mol) in 5 mL of EtOH. The reaction was run at 100 C for 24 h. The off-white
precipitate
that formed in the crude reaction mixture was filtered and washed with EtOH to
afford
0.579 g of title compound (yield: 53%). 1H NMR (300 MHz, d6-DMSO): 2.67 (t,
4H, J=
4.9 Hz), 2.85-2.90 (m, 4H), 3.36 (t, 4H, J= 4.9 Hz), 6.80 (s, 1H), 7.18-7.19
(m, 1H), 7.25
(s, 1H), 7.27-7.35 (m, 3H), 7.37-7.39 (dd, 1H, J = 8 Hz, 2Hz), 7.51-7.55 (m,
3H), 8.09 (s,
1H), 10.28 (s, 1H). M= 1= 547
[00131] Example 254
8-( { 5-chloro-2-[[2-(dimethylamino)ethyl] (methyl)amino]isonicotinoyl }
amino)-1-(4-
fluorophenyl)-4,5-dihydro-1 H-benzo [g] indazole-3-carboxamide
F
N-N
O N CONH2
CI / I /
ZI
N N~
~-N
The title compound was synthesized by the same procedure as in Example 214
starting
with the title compound of Example 248 (0.8 g, 0.0016 mol) and N,N,N'-
trimethylethylene
diamine (3.3 g, 0.032 mol). The reaction was run at 100 C for 24 h. After
removal of the
volatiles under vacuum, the residue was partitioned between water and CH2C12.
The
130
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
organic layer was washed an additional time with water and dried over MgSO4.
The crude
product mixture was purified by preparative HPLC to give 0.424 g of the title
product,
yield: 47%. 1H NMR (400 MHz, d6-DMSO): 2.13 (s, 6H), 2.34 (t, 2H, J = 6.7 Hz),
2.87-
2.95 (m, 4H), 2.96 (s, 3H), 3.59 (t, 2H, J = 6.7 Hz), 6.58 (s, IH), 7.2 (d,
1H, J = 2Hz),
7.27 (s, 1H), 7.29-7.37 (m, 3H), 7.41-7.44 (dd, 1H, J = 8 Hz, 2 Hz), 7.53-7.58
(m, 3H),
8.13 (s, 1H), 10.36 (s, 1H). M + 1= 563.
[00132] Example 255
8- {[(3-chloro-6-morpholin-4-ylpyridin-2-yl)carbonyl] amino } - 1-(4-
fluorophenyl)-4,5-
dihydro-lH-benzo[g]indazole-3-carboxamide
F
CI
N_N
(~N N CONH2
IO~ I
[00133] Step 1
3-chloro-6-morpholinyl-2-pyridine carboxylic acid
ci
~ ~
N N OH
O
3,6 Dichloropyridine-2-carboxylic acid (0.55g, 2.86 mmol), morpholine (1.37g,
15.7
mmol) and N,N dimethylacetamide (1.37 mL) were combined and stirred for 24 h
at 80
C. An additional volume of morpholine (1.39 g, 15.7 mmol) was added and
heating
continued for 40 hours more. After cooling the DMA was removed in the presence
of
toluene. The residue was dissolved in water and extracted with ether to remove
excess
morpholine. The aqueous was acidified to pH = 2 and the product extracted into
ether.
Crystallization from water gave a white solid, 369 mg (53% yield). 1H NMR
(CD3OD): S
3.52 (t, 4H), 3.78 (t, 4H), 6.97 (d, l H), 7.65 (d, l H). LC-MS, M + H: 243.
131
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
[00134] Step 2
8-1 [(3-chloro-6-morpholin-4-ylpyridin-2-yl)carbonyl]amino}-1-(4-fluorophenyl)-
4,5-
dihydro-lH-benzo [g]indazole-3-carboxamide
F
ci
I ~ H N-N
~N N N CONH2
OJ O
The title compound was synthesized from 0.294 g of 3-chloro-6-morpholinyl-2-
pyridine
carboxylic acid (from step 1) and (0.258 g) of the title compound of example
244 by the
same procedure used for Example 211 except that HATU was replaced by HBTU
(BF4).
The title compound is a brown solid (0.37 g, 84%), m.p. 252-254 C. Its
structure was
confirmed by 1H NMR and LC/MS: 1H NMR (CDC13): S 2.95 (m, 2H), 3.12 (m, 2H),
3.48
(m, 4H), 3.88 (m, 414), 5.39 (s, 1H), 6.65-6.82 (m, 3H), 7.16 7.18-7.23 (m,
3H), 7.43-756
(m, 3H), 7.62 (m, 1H), 9.18 (s, IH). ESI mass spectrum for C28H25C1FN6O3+: 547
(M +
1).
[00135] Example 256
8-( { [3-chloro-6-(4-methylpiperazin-1-yl)pyridin-2-yl]carbonyl } amino)-1-(4-
fluorophenyl)-
4,5-dihydro-1 H-benzo[g]indazole-3-carboxamide
F
CI
N-N
rNI N-- N CONH2
O
The title compound was synthesized from 0.501 g of 3-chloro-6-piperazinyl-2-
pyridine
carboxylic acid, obtained by acidification of its K-salt (from step 1 of
Example 217), and
the title compound of Example 244 (0.37 g) by the same procedure used for
Example 217.
132
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
The title compound is a brown solid (0.56 g, 88%), m.p. 218-220 C. Its
structure was
confirmed by 'H NMR and LC/MS: 'H NMR (d6-DMSO): 8 2.19 (s, 3H), 2.38 (m, 4H),
2.85 (m, 4H), 3.45 (m, 4H), 6.95 (d, 1H, J = 9 Hz), 7.20 (d, 1H, J = 2 Hz),
7.26-7.40 (m,
4H), 7.49-7.62 (m, 4H), 7.63 (d, 1H, J = 9 Hz), 10.20 (s, 1H). ESI mass
spectrum for
C29H28C1FN7O2+: 560 (M + 1).
[00136] Example 257
8- { [(3-chloro-6- { [2-(dimethylamino)ethyl]thio }pyridin-2-yl)carbonyl]amino
}-1-(4-
fluorophenyl)-4,5-dihydro-1 H-benzo[g]indazole-3-carboxamide
F
CI
N~ Nr N N-N
CONH2
; S
0
[00137] Step 1
2-[(6-carboxy-5-chloropyridin-2-yl)thio]-N,N-dimethylethanaminium chloride
cl
ci I O
g N
i~H OH
3,6-Dichloropyridine-2-carboxylic acid (1.0g, 5.23 mmol.), sodium hydroxide
(1.64g, 15.7
mmol.), and anhydrous THF (10 mL) were combined before slowly adding the N,N-
dimethylaminoethanethiol (1.9 g, 18.3 mmol). After stirring for several hours
under
nitrogen, two aliquots of DMF (101nL each) were added. Several hours later,
additional
DMF (10 mL) and dimethylaminoethanethiol (1.9 g, 18.3 mmol) were added. The
reaction
was stirred overnight at room temperature. The solution was diluted with water
and
extracted three times with methylene chloride. The aqueous was acidified to pH
5 and
extracted four times with methylene chloride. The aqueous was acidified to pH
1, the
solvent was removed, and the residue was recrystallized from hot water. Yellow
solid,
133
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
0.786 g (51% yield). 1H NMR (300 MHz, CD3OD): S 2.98 (s, 6H), 3.53 (m, 4H),
7.50 (d,
1H), 7.85 (d, 1H). 13C NMR (75 MHz, CD3OD): S 24.4, 42.8, 58.2, 125.7, 128.1,
140.0,
147.3, 158.2, 166Ø
[00138] Step 2
8-{ [(3-chloro-6-{ [2-(dimethylamino)ethyl]thio }pyridin-2-yl)carbonyl]amino}-
1-(4-
fluorophenyl)-4,5-dihydro-1 H-benzo [g] indazole-3-carboxamide
F
CI \
N-N
N )C(Y- CONH2
o 10
The title compound was synthesized from 0.355 g of the title compound of Step
1 and the
title compound of Example 244 (0.32 g) by the same procedure used in Example
255. The
title compound is a white solid (0.49 g, 87%). Its structure was confirmed by
1H NMR and
LC/MS: 1H NMR (CD3CN): S 2.18 (s, 6H), 2.57 (m, 2H), 2.85 (m, 4H), 3.00 (m,
4H), 3.26
(m, 2H), 5.88 (s, 1H), 6.94 (d, 1H), 7.27-7.38 (m, 5H), 7.47-7.62 (m, 3H),
7.69 (d, 1H, J
9 Hz), 9.40 (s, 1H). ESI mass spectrum for C28H27CIFN6O2S+: 565 (M + 1).
[00139] Example 258
N-N
N CONH2
CI
N'
This compound was synthesized by using the same procedure described for
Example 221;
mp: 194-195 C; 1HNMR (DMSO + TFA-d, 400 MHz) S: 10.24 (s, 1H), 9.69 (s, 1H),
7.60
134
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
(m, 2H), 7.46 (d, J = 4.2 Hz, 1H), 7.38 (m, 411), 7.10 (dd, J= 2.6, 8.9 Hz,
1H), 7:06 (s,
1H), 3.90 (d, J = 13.0 Hz, 2H), 3.50 (d, J = 12.0 Hz, 2H), 3.12 (m, 2H), 2.95
(m, 411), 2.91
(s, 3H), 2.86 (s, 2H).
[00140] The IKK2 bioactivity for Examples 245-257 is shown in Table 7.
Table 7
Structure Mol. Wt. Compound Name(s) IKK2 Example
Resin IC50
F 511.56 1-(4-fluorophenyl)-8- <1 M 245
Q {[(2-piperazin-l-
N-N ylpyridin-3-
~-> o N yl)carbonyl]amino}-4,5-
N \ NHZ dihydro-lH-
N benzo[g]indazole-3-
carboxamide
F 475.91 8-{ [(6-chloro-4- <1 M 246
methylpyridin-3-
c' / N-N o yl)carbonyl]amino}-1-(4-
N. fluorophenyl) 4,5-
0 NH2 dihydro-lH-
benzo[g]indazole-3-
carboxamide
F 526.58 1-(4-fluorophenyl)-8- <1 M 247
~ ~ { [(4-methyl-6-morpholin-
N N I N N-N 4-ylpyridin-3-
yl)carbonyl]amino}-4,5-
dihydro-lH-
benzo[g]indazole-3-
carboxamide
F 496.33 8-[(2,5- <1 M 248
dichloroisonicotinoyl)ami
N ci N N-N o no]-1-(4-fluorophenyl)-
ci 4,5-dihydro-lH-
0 NHz benzo[g]indazole-3-
carboxamide
F 546.99 8-[(5-chloro-2-morpholin- <1 M 249
4-ylisonicotinoyl)amino]-
N C1 y N-N 1-(4-fluorophenyl)-4,5-
rN N dihydro-lH-
'J benzo[g]indazole-3-
carboxamide
135
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
F 560.04 8-{[5-chloro-2-(4- <1 M 250
/ methylpiperazin-l-
ci 0 N-N NH yl)isonicotinoyl]amino}-
N Z 1-(4-fluorophenyl)-4,5-
N I ~ dihydro-lH-
CNJ \ benzo[gjindazole-3-
carboxamide
N
F 525.59 1-(4-fluorophenyl)-8-{[2- <1 M 251
(4-methylpiperazin-l-
N N-N o yl)isonicotinoyl]amino}-
~N J o NHz 4,5-dihydro-lH-
benzo[gjindazole-3-
carboxamide
F 574.06 8-{ [5-chloro-2-(4-methyl- <1 M 252
1,4-diazepan-l-
ci 0 N-N yI)isonicotinoyljamino}-H ~ N ~~ NHZ 1-(4-fluorophenyl)-4,5-
N dihydro-lH-
C benzo[g]indazole-3-
carboxamide
N
F 546.01 8-[(5-chloro-2-piperazin- <1 M 253
1 1-ylisonicotinoyl)amino]-
ci o N-N 1-(4-fluorophenyl)-4,5-
N ~ NHz dihydro-lH-
N benzo[g]indazole-3-
CN\ carboxamide
NJ
H
F 562.05 8-({5-chloro-2-[[2- <1 M 254
(dimethylamino)ethyl](m
ci 0 N-N ethyl)amino]isonicotinoyl
} amino)-1-(4-
NHZ
N fluorophenyl)-4,5-
~N\ dihydro-lH-
benzo[g]indazole-3-
N
carboxamide
F_ 546.99 8-{ [(3-chloro-6- 1< 10 M 255
cI ~ / morpholin-4-ylpyridin-2-
~ H N-" yl)carbonyl]amino}-1-(4-
N"N-/~( N coNHz fluorophenyl)-4,5-
01-li O dihydro-lH-
benzo[g]indazole-3-
carboxamide
136
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
F_ 560.04 8-({[3-chloro-6-(4- <1 M 256
\ ~i I methylpiperazin-l-
~N I N N N CON~ yl)pyridin-2-
NJ o I yl]carbonyl}amino)-1-(4-
~ fluorophenyl)-4,5-
dihydro-lH-
benzo[g]indazole-3-
carboxamide
F_ 565.07 8-{[(3-chloro-6-{[2- <1 M 257
c, ~ ~ (dimethylamino)ethyl]thi
ON~~S N q \\N caNHi o}pyridin-2-
o yl)carbonyl]amino}-1-(4-
fluorophenyl)-4,5-
dihydro-lH-
benzo[g]indazole-3-
carboxamide
I 559.05 8-{ [2-chloro-5-(4- <1 M 258
methylpiperazin-l-
O N N-N yl)benzoyl]amino}-1-(4-
~ CONH2 fluorophenyl)-4,5-
c- dihydro-lH-
benzo[g]indazole-3-
carboxamide
~ a______________
[00141] Examples 259-263
Examples 259-263 were synthesized with the corresponding starting compounds
using the following synthesis procedures similar to scheme I where R9 is the
appropriate aryl, substituted aryl, heteroaryl, substituted heteroaryl,
substituted
arylalkyl, substituted heteroarylalkyl, or cycloalkyl.
137
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
14112?PCT
N-N OEt
arYihydrazine
O AcOH, fl 02N O
OEt
OZN O
~...
ethyl(7-nitro-1-o)(O 1,2,3,
4-tetrahydronaPhthalen 2 Yl)
(Oxo)acetate 1 2
RiC-U/
2 NH3, MeOH N-N NH2
Pd(OH)2 OEt 700 H2N
t t2 HaN O
2.
R1 ~d
N-N NHz
HATl, or
R9COaH, O
0 R9COCi PYridine
139
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
SCHEME XXVII
~
0 0 ~\ NHNH2'HCI N-N OEt
O2N OEt R ; / O2N ~
\ I O \ ~ O
ethyl (7-nitro-l-oxo-1,2,3, AcOH, reflux
4-tetrahydronaphth ale n-2-yl)
(oxo)acetate 1
HO \ I HO CQ P
d(OH)2 N-N OEt NH3, MeOH N-N NH2
- - ~ 2
H
2 H2N \ I \ O 700 psi H2N OJ \ O
TBDMSOQF/ TB
DMSCI, imidazole N-N 1) R9CO2H, HATU
NH2
DMF, RT H2N / I \
O 2) TBAF/THF
HO \ I R10 \
H N-N NH2 RiCI or R"OTs H N-N NH2
O\/N / I \ O Base, DMF O~N / I \ O
'R( \ R
OH
1NHCI
H N-N NH2
MeOH, reflux ON
R9 O
R = 3- or 4-benzyloxyl
139
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
[00142] Example 259
1- [4-(benzyloxy)phenyl]-8- [(2-chlorobenzoyl)amino] -4,5-dihydro-1 H-
benzo [g] indazole-3-carboxamide
0,,
~I
N-N
N ~ ~ I CONHZ
Cj I /
[00143] St~: A mixture of 4-benzyloxylphenylhydrazine hydrochloride
(6.42 g, 0.03 mol) and ethyl (7-nitro-l-oxo-1,2,3,4-tetrahydronaphthalen-2-
yl)(oxo)acetate 1 (9.2 g, 0.03 mol) in 200 mL of acetic acid was refluxed for
16 h,
then cooled to room temperature. The precipitate was collected by filtration
and
air-dried to give 8.5 g of product as a light green solid (60% yield); 1HNMR
(DMSO, 400 MHz) S: 8.07 (dd, 1H), 7.66 (d, 1H), 7.35-7.53 (m, 8H), 7.24 (dd,
2H), 5.26 (s, 2H), 4.32 (q, 2H), 3.11 (m, 2H), 3.30 (m, 2H), 1.32 (t, 3H).
[00144] Step 2: A mixture of the product from step 1(15.0 g, 0.032 mol) and
tin chloride (21.6 g, 0.096 mol) in 400 mL of ethanol was heated at reflux
overnight. Another two equivalent of tin chloride was added and stirred for 6
h.
The reaction mixture was cooled to room temperature and the precipitate was
filtered and washed with ether to give 13.5 g of the amine as a light yellow
solid
(96% yield); 1HNMR (DMSO, 400 MHz) 8: 8.07 (dd, 1H), 7.66 (d, 1H), 7.35-7.53
(m, 8H), 7.24 (dd, 2H), 5.26 (s, 2H), 4.32 (q, 2H), 3.11 (m, 2H), 3.30 (m,
2H), 1.32
(t, 3H).
[00145] Step 3: A sealed reaction vessel containing the product from step
(6.2 g, 0.014 mol) and 40.mL of liquid ammonia in 200 mL of absolute alcohol
was
heated at 120 C and 600 psi for 24 h. After cooling, solvent was removed and
the
residue was purified by chromatography on silica gel (ethyl acetate/hexane,
6:4) to
give 4.0 g of product as a pale yellow solid (70% yield); 1HNMR (CDC13, 400
140
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
MHz) b: 7.35-7.47 (m, 7 H), 7.07 (m, 3H), 6.81 (s, 1H), 6.50 (dd, 1H), 6.07
(d, 1H),
5.49 (s, 1H), 5.15 (s, 2H), 3.37 (s, 2H), 3.08 (m, 2H), 2.86 (m, 2H).
[00146] Step 4: To a solution of the product from step 3(7.08 g, 0.017 mol)
in 100 mL of pyridine was added 2-chlorobenzoyl chloride (3.4 g, 0.019 mol) in
one
portion and the reaction mixture was stirred at room temperature overnight.
Solvent
was removed and the residue was stirred with water. The precipitate was
collected
by filtration and air-dried to give 7.5 g of product as a white solid (80%
yield);
1HNMR (DMSO, 400 MHz) 8: 10.29 (s, 1H), 7.15-7.51 (m, 18 H), 5.16 (s, 2H),
2.90 (m, 4H); Anal. Calcd. for C32H25C1N403: C, 70.01; H, 4.59; N, 10.20.
Found:
C, 69.62; H, 4.44; N, 10.24.
[00147] Example 260
1-[4-(benzyloxy)phenyl]-8- { [(2-chloropyridin-3-yl)carbonyl] amino 1-4,5-
dihydro-
1 H-benzo [g] indazole-3-carboxamide
r
~ '
N-N
O N I CONH2
N'~
CI / / r
This compound was synthesized by following the same procedure as Example 259
except using 2-chloronicotinyl chloride in step 4; 1HNMR (DMSO, 400 MHz) S:
10.42 (s, 1H), 8.50 (d, 1H), 7.96 (d, 1H), 7.16-7.54 (m, 15H), 5.16 (s, 2H),
2.93 (m,
4H); Anal. Calcd. for C31H24C1N503: C, 67.70; H, 4.40; N, 12.73. Found: C,
66.75;
H, 4.17; N, 12.41.
[00148] Example 261
8-[(2-chlorobenzoyl)amino]-1-(4-hydroxyphenyl)-4,5-dihydro-lH-
benzo [g] indazole-3-carboxamide
141
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
HO,,a
N-N
N \ \ I CONH2
CI /
The final product from Example 259 (7.5 g, 0.014 mol) was dissolved of TFA
(120
mL) and the dark brown solution was stirred at room temperature for 84 h.
Solvent
was removed and the residue was taken up with 200 mL of water. The solid was
collected and air-dried to give 6.5 g of product as a pale white solid (83%
yield);
'HNMR (DMSO, 400 MHz) S: 10.29 (s, 1H), 9.84 (s, 1H), 7.24-7.51 (m, 11 H),
6.87 (d, 2H), 2.90 (m, 4H); Anal. Calcd. for C25H19C1N4O3 + 1.0 H20: C, 62.96;
H,
4.44; N, 11.75. Found: C, 62.92; H, 4.28; N, 11.76.
[00149] Example 262
8-[(2-chlorobenzoyl)amino]-1-(3-hydroxyphenyl)-4,5-dihydro-1 H-
benzo [g] indazole-3 -carboxamide
OH
N
bN- H
~
O N \ \ CONH2
CI I /
This compound was synthesized by following the same procedure as Example 261
except using 3-benzyloxyphenyhydrazine hydrochloride in step 1; 1HNMR (DMSO,
400 MHz) S: 10.31 (s, 1H), 7.27-7.54 (m, lOH), 6.89 (m, 3H), 2.92 (m, 4H);
Anal.
Calcd. for C25HI9C1N403 + 0.5 H20: C, 64.17; H, 4.31; N, 11.97. Found: C,
64.29;
H, 4.36; N, 11.63.
[00150] Example 263
142
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
8- { [(2-chloropyridin-3-yl)carbonyl] amino }-1-(4-hydroxyphenyl)-4,5-dihydro-
lH-
benzo [g]indazole-3-carboxamide
HO a
I
N-N
O N ~ CONH2
CI I i
N~ I
This compound was synthesized by following the same procedure as Example 261
except using 2-chioronicotinyl chloride in step 4; 1HNMR (DMSO, 400 MHz) b:
10.31 (s, 1H), 7.27-7.54 (m, 10H), 6.89 (m, 3H), 2.92 (m, 411); Anal. Calcd.
for
C24H18C1N5O3: C, 62.68; H, 3.95; N, 15.23. Found: C, 62.03; H, 3.89; N, 14.83.
[00151] The bioactivity in the IKK2 resin assay of Examples 259-263 is
shown Table 8.
Table 8
Compound No., Structure Mol. Compound Name(s) IKK2 MS Example
Wt. Resin (M+H)
IC50
\ 549.03 1-[4-(benzyloxy)phenyl]-8-[(2- > 10 550 259
~ ~ chlorobenzoyl)amino]-4,5-
/ N-~ dihydro-lH-benzo[g]indazole- PM
b / \ N"~ 3-carboxamide
ci~ \ ~ o
550.02 1-[4-(benzyloxy)phenyl]-8- 1< 10 551 260
{[(2-chloropyridin-3- M
\ yl)carbonyl]amino}-4,5-
I / dihydro-lH-benzo[g]indazole-
I / N-N 3-carboxamide
O N NH2
cl \ \ I O
143
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Compound No., Structure Mol. Compound Name(s) IKK2 MS Example
Wt. Resin (M+H)
IC50
458.91 8-[(2-chlorobenzoyl)amino]-1- <1 gM 459 261
(4-hydroxyphenyl)-4,5-
"o \ dihydro-lH-benzo[g]indazole-
N-N 3-carboxamide
O N 1 NHa
CI~ I / O
458.90 8-[(2-chlorobenzoyl)amino]-1- <1 M 459 262
(3-hydroxyphenyl)-4,5-
o" dihydro-lH-benzo[g]indazole-
I 3-carboxamide
N-N
O N ~ NFL
CI \ \ O
459.89 8-{[(2-chloropyridin-3- <1 M 460 263
yl)carbonyl] amino } -1-(4-
"o \ hydroxyphenyl)-4,5-dihydro-
I
" / N-N 1H-benzo[g]indazole-3-
0 N NHz carboxamide
CI \ \ O
I
N
[00152] Example 264
8-[(2-chlorobenzoyl)amino]-1-[4-(2-morpholin-4-ylethoxy)phenyl]-4,5-dihydro-1
H-
benzo[g]indazole-3-carboxamide
N~~O
~ HN'
IN
O N ~ ~ CONH2
To a suspension of the product from Example 261 (0.6 g, 0.001 mol) and cesium
carbonate in 10 mL of DMF was added 4-(2-chloroethyl)morpholine hydrochloride
(0.19 g, 0.001 mol) in one portion. The reaction mixture was stirred at room
temperature overnight. After the removal of solvent, the residue was
partitioned
between water and ethyl acetate. The organic layer was washed with brine,
dried
over magnesium sulfate, and concentrated. This crude was purified by HPLC to
144
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
give 0.15 of product as a light yellow (26% yield);1HNMR (DMSO, 400 MHz) S:
11.49 (brs, 1H), 10.33 (s, 1H), 7.14-7.54 (m, 10H), 4.53 (s, 2H), 3.82 (m,
2H), 3.58
(m, 8H), 3.08 (m, 2H), 2.93 (m, 4H); Anal. Calcd. for C31H30C1N5041.0 H20- 1.0
HC1: C, 59.43; H, 5.31; N, 11.18. Found: C, 59.58; H, 5.26; N, 10.92.
[00153] Example 265
8-[(2-chlorobenzoyl)amino]-1-{ 4-[(2,2-dimethyl-1,3-dioxolan-4-
yl)methoxy]phenyl }-4,5-dihydro-1 H-benzo[g]indazole-3-carboxamide
0
oJ,o
\ I N-N
N I CONH2
CI
This compound was synthesized by following the same procedure as Example 264
except using 2,2-dimethyl-1,3-dioxolan-4-ylmethyl p-toluenesulfonate (78%
yield);
1HNMR (CDC13, 400 MHz) 5: 7.69 (d, 1H), 7.59 (m, 211), 7.44 (d, 2H), 7.30-7.40
(m, 3H), 7.05 (d, 211), 6.83 (d, 2H), 4.52 (m, 1H), 4.13 (m, 2H), 4.02 (m,
2H), 3.93
(m, 2H), 3.14 (m, 2H), 2.98 (m, 2H), 1.46 (s, 3H), 1.26 (s, 3H); Anal. Calcd.
for
C31H29C1N405: C, 64.98; H, 5.10; N, 9.78. Found: C, 64.56; H, 4.97; N, 9.68.
[00154] Example 266
8-[(2-chlorobenzoyl)amino]-1-[4-(2,3-dihydroxypropoxy)phenyl]-4,5-dihydro-lH-
benzo[g]indazole-3-carboxamide
OH
HO,,~,,O~
N-N
O N ~ Z~-, CONH2
CI I i
To a suspension of the product from Example 265(0.36 g, 0.00063 mol) in
145
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
methanol was added 1N HC1 and the mixture was heated at reflux for 16 h.
Solvent
was removed and the crude was recrystallized from water and methanol to give
0.24
g of the desired product as a white solid (72%); 'H NMR (DMSO, 400 MHz) S:
10.30 (s, 1H), 7.36-7.57 (m, 6H), 7.33 (d, 1H), 7.28 (brs, 1H), 7.18 (d, 1H),
7.09 (d,
2H), 4.98 (d, 1H), 4.68 (t, 1H), 4.10 (q, 1H), 4.05 (dd, 1H), 3.92 (dd, 1H),
3.83 (m,
1H), 3.45 (t, 1H), 3.16 (d, 2H), 2.94 (m, 4H); Anal. Calcd. for C28H25C1N405:
C,
63.10; H, 4.73; N, 10.51. Found: C, 62.81; H, 4.45; N, 10.16.
[00155] The compounds of Examples 267-275 were synthesized as described
in Example 264 using the appropriate aryl, substituted aryl, heteroaryl,
substituted
heteroaryl, substituted arylalkyl, substituted heteroarylalkyl, or cycloalkyl.
[00156] The bioactivity in the IKK2 Resin assay for the compounds of
Examples 264-275 is shown in Table 9.
Table 9
Compound No., Structure Mol. Compound Name(s) IKK2 MS Example
Wt. Resin (M+H)
IC50
o N-N 572.07 8-[(2-chlorobenzoyl)amino]-1- <1 M 573 264
~ J 1 [4-(2-morpholin-4-
0v~
o r", I N~ ylethoxy)phenyl]-4,5-dihydro-
cJ 1 0 1H benzo[g]indazole 3
1 carboxamide
573.05 8-[(2-chlorobenzoyl)amino]-1- <1 M 574 265
'xo {4-[(2,2-dimethyl-1,3-
~ 1 N-N dioxolan-4-
o r, NF~ yl)methoxy]phenyl}-4,5-
ci 1 o dihydro-lH-benzo[g]indazole-
~ 1 3-carboxamide
Ho 532.99 8-[(2-chlorobenzoyl)amino]-1- <1 M 533 266
HoD~"c i [4-(2,3-
~ 1 N-N dihydroxypropoxy)phenyl]-4,5-
0 rNi ~ 6 fl NH, dihydro-1H-benzo[g]indazole-
ci e 1 ~ 0 3-carboxamide
146
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Compound No., Structure Mol. Compound Name(s) IKK2 MS Example
Wt. Resin (M+H)
IC50
Ho 533.98 8-[(3- <1 M 534 267
Ho~ chloroisonicotinoyl)amino]-1-
~ [4-(2,3-
\
o r" N-N NH, dihydroxypropoxy)phenyl]-4,5-
c,~ o dihydro-lH-benzo[g]indazole-
l= 3-carboxamide
N
Ho 611.08 8-{[2-chloro-4- <1 M 612 268
Ho~ (methylsulfonyl)benzoyl]amino
N-N }-1-[4-(2,3-
w NH, dihydroxypropoxy)phenyl]-4,5-
ci (.~ o dihydro-lH-benzo[g]indazole-
~ 3-carboxamide
0=5.
0
Ho 548.00 8-[(5-amino-2- <1 M 548 269
Ho~ chlorobenzoyl)amino]-1-[4-
~ N-N (2,3-
o r, , NHz dihydroxypropoxy)phenyl]-4,5-
ci o dihydro-lH-benzo[g]indazole-
~ ~ 3-carboxamide
NHz
Ho 533.98 8-([(2-chloropyridin-3- <1 M 534 270
Ho~ yl)carbonyl]amino}-1-[4-(2,3-
~ I N-N dihydroxypropoxy)phenyl]-4,5-
o rHi NHz dihydro-lH-benzo[g]indazole-
ci~ 0 3-carboxamide
N_\J~
N''1 530.03 8-[(2-chlorobenzoyl)amino]-l- 1< 10 530 271
H I N-N {4-[2-
.~
0 N NHz (dimethylamino)ethoxy]phenyl M
C1 ~ }-4,5-dihydro-lH-
~~
benzo[g]indazole-3-
carboxamide
544.06 8-[(2-chlorobenzoyl)amino]-1- 1< 10 544 272
-N-~, O
- {4-[3 M
o H \ ~ N N N NHz (dimethylamino)propoxy]pheny
C1 ~ ~ 0 1}-4,5-dihydro-lH-
~ benzo[g]indazole-3-
carboxamide
4 651.14 8-{[2-chloro-4- 1 < 10 651 273
s' ~ O (methylsulfonyl)benzoyl]amino
M
N-N }-1-{4-[(2,2-dimethyl-l,3-
~ON):~~NH,
dioxolan-4-
C1 yl)methoxy]phenyl}-4,5-
dihydro-1 H-benzo [g] indazole-
o 3-carboxamide
147
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Compound No., Structure Mol. Compound Name(s) IKK2 MS Example
Wt. Resin (M+H)
IC50
\~ 574.04 8-{[(2-chloropyridin-3- 1< 10 574 274
~' ~ 0 yl)carbonyl]amino}-1-{4-[(2,2- M
H N-N dimethyl-1,3 dioxolan 4
C1" I "",
yl)methoxy]phenyl}-4,5-
N I dihydro-lH-benzo[g]indazole-
3-carboxamide
'1/'0 574.04 8-[(3- 1< 10 574 275
0
6N-N chloroisonicotinoyl)amino]-1-
{3-[(2,2-dimethyl-1,3- M
" JI ""2 dioxolan-4-
C1 ~ yl)methoxy]phenyl}-4,5-
" dihydro-lH-benzo[g]indazole-
3-carboxamide
[001571 Example 276
8-[(2-chlorobenzoyl)amino]-1-(4-cyanophenyl)-4,5-dihydro-lH-benzo[g]indazole-
3-carboxamide
HO~
N-N
H
O N CONH2
CI
0--/
[00158] Stepl: The product (45.0 g, 0.096 mol) from step 1 of Example
261 was hydrogenated in 400 mL of acetic with Pd(OH)2/C as catalyst for 17 h
under 15 psi in a Parr shaker. After the removal of solvent, the residue was
triturated with a mixture of methanol and ether (1:2) to give 23.0 g of the
desired
product as a white solid (68% yield); 1HNMR (DMSO, 400 MHz) 8: 10.06 (s, 1H),
7.27 (d, 2H), 6.98 (d, 1H), 6.92 (d, 2H), 6.43 (dd, 1H), 6.02 (d, 1H), 4.82
(brs, 2H),
4.29 (q, 2H), 2.75 (m, 2H), 1.30 (t, 3H).
[00159] Step 2: A sealed reaction vessel containing the product from step 1
(25.0 g, 0.072 mol) and 40 mL of liquid ammonia in 250 mL of absolute alcohol
148
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
was heated at 120 C and 600 psi for 30 h. After cooling, the precipitate was
collected by filtration to give 16.7 g of product as a pale yellow solid (73%
yield);
1HNMR (CDC13, 400 MHz) S: 7.44 (s, 1H), 7.27 (d, 2H), 7.20 (s, 1H), 6.97 (d,
1H),
6.91 (d, 2 H), 6.40 (d, 1H), 6.03 (s, 1H), 4.77 (brs, 2H), 2.85 (m, 2H), 2.72
(m, 2H).
[00160] Step 3: To a solution of the product form step 1 (5.25 g, 0.016 mol)
and TBDMSCI (3.0 g, 0.02 mol) in 100 mL of DMF was added imidazole (2.72 g,
0.04 mol) in one portion. The reaction mixture was stirred at room temperature
for
36 h. Solvent was removed and the residue was partitioned between water and
ethyl
acetate. The organic layer was washed with brine, dried over magnesium
sulfate,
and concentrated. This crude was purified by chromatography on silica gel
(ethyl
acetate/hexane, 1:1) to give 4.0 g of product as a white solid (57% yield).
The
NMR was consistent with the proposed structure.
[00161] Step 4: To a mixture of the product from step 3 (1.05 g, 0.0024 mol)
and 2-chloro-4,5-methylenedioxanylbenzoic cid (0.73 g, 0.0036 mol) in 25 mL of
DMF was added 1 mL of diisopropylethylamine, followed by the addition of HATU
(1.37 g, 0.0036 mol). The reaction was stirred at room temperature for 16 h
and
concentrated. The residue was partitioned between water and ethyl acetate and
the
organic phase was concentrated. This crude was then dissolved in 20 mL of THF
and treated with 10 eq of TBAF for lh at RT. After the removal of solvent, the
residue was partitioned between water and ethyl acetate. The organic layer was
washed with brine, dried over magnesium sulfate, and filtered. The filtrate
was
concentrated and triturated with a mixture of acetonitrile to give 1.04 g of
desired
product as an off-white solid (86% yield);1HNMR (DMSO, 400 MHz) S: 10.17 (s,
1H), 9.85 (s, 1H), 7.47 (m, 2H), 7.27 (m, 5H), 7.14 (s, 1H), 7.04 (s, 1H),
6.88 (d,
2H), 6.12 (s, 2 H), 2.92 (m, 4H).
[00162] Example 277
8- { [(6-chloro-1,3-benzodioxol-5-yl)carbonyl]amino}-1-(3-hydroxyphenyl)-4,5-
dihydro-1 H-benzo [g] indazole-3-carboxamide
149
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
OH
/ I
\ N-N
O N ~ I CONH2
CI I /
O
0-J
This compound was synthesized by following the same procedure as Example 276
except using 3-benzyloxyphenyhydrazine hydrochloride in step 1; iHNMR (DMSO,
400 MHz) S: 10.19 (s, 1H), 9.86 (s, 1H), 7.51 (m, 2H), 7.31 (m, 3H), 7.24 (d,
1H),
7.14 (s, 1H), 7.04 (s, 1H), 6.88 (m, 3H), 6.12 (s, 2 H), 2.91 (m, 4H); Anal.
Calcd.
for C26H19C1N405: C, 62.10; H, 3.81; N, 11.14. Found: C, 61.52; H, 3.53; N,
11.11.
[00163] Example 278
8-[(2-chloro-5-nitrobenzoyl)amino]-1-(4-hydroxyphenyl)-4,5-dihydro-lH-
benzo [g] indazole-3-carboxamide
HO,~Ci N-N
H ~
0- N ~ ~ CONH2
CI I /
NO2
This compound was synthesized by following the same procedure as Example 276
except using 2-chloro-5-nitrobenzoic acid in step 4; Anal. Calcd. for
C25H18C1N505'1.5 H20: C, 56.56; H, 3.99; N, 13.19. Found: C, 56.89; H, 4.45;
N,
12.81.
[00164] Example 279
8-{ [2-chloro-5-(methylsulfinyl)benzoyl]amino}-1-(4-hydroxyphenyl)-4,5-dihydro-
1H-benzo [g]indazole-3-carboxamide
150
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
CLNN
H I
O N CONHZ
CI SOMe
This compound was synthesized by following the same procedure as Example 276
except using 2-chloro-5-(methylthio)benzoic acid in step 4 and then oxidized
to the
desired product with mCPBA; 'HNMR (DMSO, 400 MHz) 8: 10.43 (s, 1H), 9.85
(s, 1H), 7.26-7.79 (m, 10H), 6.88 (d, J = 8.5 Hz, 2H), 2.92 (m, 4H), 2.80 (s,
3H);
Anal. Calcd. for C26H21C1N404S: C, 59.94; H, 4.06; N, 10.75; S, 6.15. Found:
C,
59.48; H, 4.09; N, 10.54; S, 6.18.
[00165] Example 280
8- [ (5-amino-2-chlorobenzoyl) amino] -1-(4-hydroxyphenyl)-4,5-dihydro-1 H-
benzo [g] indazole-3 -carboxamide
HO 5111
N-N
0 N ~ CONH2
CI
NH2
This compound was synthesized by following the same procedure as Example 276
except using 5-[(tert-butoxycarbonyl)amino]-2-chlorobenzoic acid in step 4 and
then deprotected with 4N HCI in dioxane; 1HNMR (DMSO, 400 MHz) 8: 10.19 (s,
1H), 9.84 (s, 1H), 7.49 (d, 1H), 7.46 (d, 1H), 7.28-7.31 (m, 5H), 7.12 (d,
1H), 6.88
(d, 2H), 6.64 (m, 2H), 2.90 (m, 4H), 2.80; Anal. Calcd. for Ca5H2OC1N5O3'3.0
H20:
C, 56.87; H, 4.96; N, 13.26. Found: C, 56.18; H, 5.10; N, 13.09.
[00166] The compounds of Examples 281-287 listed in the Table 10 were
prepared according to the procedure of Example 276 using the appropriate
acylating
agent.
151
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
[00167] The bioactivity in the IKK2 Resin assay for the compounds of
Examples 276-287 is shown in Tab1e10.
Table 10
Compound No., Structure Mol. Compound Name(s) IKK2 MS Example
Wt. Resin (M+H)
IC50
HO 502.91 8-{ [(6-chloro-1,3- <1 M 503 276
_ benzodioxol-5-
o r", ~ N N,6 yl)carbonyl]amino}-1-(4-
oi o hydroxyphenyl)-4,5-dihydro-
~ 1H-benzo[g]indazole-3-
J carboxamide
0
oH 502.92 8-{[(6-chloro-1,3- <1 M 503 277
6N-N benzodioxol-5-
yl)carbonyl]amino}-1-(3-
o r", NH, hydroxyphenyl)-4,5-dihydro-
oi 0 1H-benzo[g]indazole-3-
~ carboxamide
0
o~
HO 503.91 8-[(2-chloro-5- <1 M 504 278
s _ nitrobenzoyl)amino]-1-(4-
o rHi N~ N NHz hydroxyphenyl) 4,5 dihydro
:rI
1H-benzo[g]indazole 3-
carboxaniide
O
HO 521.00 8-{ [2-chloro-5- <1 M 521 279
N-N (methylsulfinyl)benzoyl]amin
o r", ~ ~ NHo}-1-(4-hydroxyphenyl)-4,5-
~ o dihydro-lH-
~ benzo[g]indazole-3-
s carboxamide
0
Ho 473.92 8-[(5-amino-2- <1 M 474 280
_ chlorobenzoyl)amino]-1-(4-N Z'-'I , N~ N,~ hydroxyphenyl)-4,5-dihydro
1H-benzo[g]indazole-3
ca rboxamide
NHs
OH 459.90 8-[(3- <1 M 460 281
6,N- chl oroisonicotinoyl)amino]-
N 1-(3-hydroxyphenyl)-4,5-
0 r "~ NHz dihydro-lH-
c~~ o benzo[g]indazole-3-
~) carboxamide
N
152
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Compound No., Structure Mol. Compound Name(s) IKK2 MS Example
Wt. Resin (M+H)
TC50
oH 510.38 8-[(5-amino-2- <1 M 511 282
chlorobenzoyl)amino]-1-(3-
H 6 N-N hydroxyphenyl)-4,5-dihydro-
o N N~ 1H-benzo[g]indazole-3-
ol o carboxamide hydrochloride
NHz
HCI
OH 488.94 8-[(2-chloro-4- <1 M 489 283
/ methoxybenzoyl)amino]-1-
H N-N (3-hydroxyphenyl)-4,5-
o I NHs dihydro-lH-
ol o benzo[g]indazole-3-
~ carboxamide
o1~
OH 459.90 8-{ [(2-chloropyridin-3- <1 M 460 284
6,1-- yl)carbonyl]amino}-1-(3-
N-N hydroxyphenyl)-4,5-dihydro-
0 0, / ~ NHz 1H-benzo[g]indazole-3-
ci o carboxamide
oH 398.44 1-(3-hydroxyphenyl)-8- 1< 10 399 285
6N-N [(methylsulfonyl)amino]-4,5-
dihydro-lH- pM
.r"~ NH2 benzo[g]indazole-3-
0 o carboxamide
OH 518.96 8-[(2-chloro-3,4- <1 M 519 286
6~N-N dimethoxybenzoyl)amino]-1-
(3-hydroxyphenyl)-4,5-
o rHi NHz dihydro-lH-
ci benzo[g]indazole-3-
o carboxamide
o~
OH 473.92 8-{ [(2-chloro-4- <1 M 474 287
6,N- met hylpyridin-3-
/ N yl)carbonyl]amino}-1-(3-
No~/r"~ ~ NHz hydroxyphenyl)-4,5-dihydro-
Tci (of 1H-benzo[g]indazole-3-
carboxamide
[00168] Example 288
8- { [(2-chloropyridin-3-yl)carbonyl] amino 1- 1 -(4-morpholin-4-ylphenyl)-4,5-
dihydro-IH-benzo [g]indazole-3 -carboxamide
153
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
O<:~N-N
N ~ ~ I CONH2
ci
I /
N,
This compound was synthesized in an analogous manner to Example 3 by
substituting 4-morpholinylphenylhydrazine hydrochloride and 2-chloronicotinyl
chloride.; 'HNMR (DMSO, 400 MHz) 8: 10.39 (s, 1H), 8.50 (d, 1H), 7.94 (d, 1H),
7.05-7.53 (m, 10H), 3.74 (m, 4H), 3.18 (m, 4H), 2.92 (m, 4H); Anal. Calcd. for
C28H25C1N603: C, 63.57; H, 4.76; N, 15.89. Found: C, 63.19; H, 4.61; N, 15.48.
IKK-2 resin IC50 1<10 M.
[00169] Example 289
8-[(2-chlorobenzoyl)amino]-1- { 4-[( IE)-3-hydroxy-3-methylbut-l-enyl]phenyl }
-4,5-
dihydro- IH-benzo[g]indazole-3-carboxamide
OH
py N N NH2 15
CI O O
A 50 mL round bottomed flask with a magnetic stir bar was charged with 1-(4-
bromophenyl)-8-[(2-chlorobenzoyl)amino]-4,5-dihydro-lH-benzo[g]indazole-3-
carboxamide (987 mg, 1.89 mmol), palladium (II) acetate (44 mg, 0.19 mmol),
1,1'-
biphenyl-2-yl[di(tert-butyl)]phosphine (254 mg, 0.822 mmol), and
dimethylformamide (20 mL). The resulting solution was sparged with argon for
10
minutes. To the solution was added 2-methyl-3-buten-2-ol (823 mg, 6.07 mmol)
and triethylamine (614 mg, 6.07 mmol). The solution was sparged with argon for
an additional 2 minutes. The flask was sealed with a rubber septum and heated
to
154
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
100 C in an oil bath for 90 minutes. The reaction was allowed to cool to room
temperature and water was added. The resulting precipitate was collected and
purified by silica gel chromatography (100% hexane to 100% ethyl acetate). The
pure fractions were combined, concentrated to dryness, triturated with diethyl
ether,
and dried under vacuum to give 215 mg of 8-[(2-chlorobenzoyl)amino]-1-{4-[(lE)-
3-hydroxy-3-methylbut-l-enyl]phenyl }-4,5-dihydro-lH-benzo[g]indazole-3-
carboxamide (0.408 mmol, 21% yield) as a solid. 1H NMR (400 MHz, DMSO-d6)
S 1.29 (s, 6 H), 2.88-2.98 (m, 4 H), 4.76 (s, 1 H), 6.48 (d, 1 H), 6.58 (d, 1
H), 7.23
(d, 1 H), 7.30-7.50 (m, 8 H), 7.53-7.59 (m, 3 H), 10.28 (s, 1 H); MS (ESI+)
for
C30H27C1N403 m/z 527 (M+H)+.
[00170] The compounds of Example 290-308 listed in the Table 11 were
prepared according to the procedure of Example 289 using the appropriate
alkene.
[00171] The bioactivity in the IKK2 Resin assay for the compounds of
Examples 289-308 is shown in Tablel 1.
Table 11
Compound No., Structure Mol. Compound Name IKK2 MS Example
Wt. Resin (M+H)
IC50
o" 527.03 8-[(2-chlorobenzoyl)amino]-1- <1 M 527 289
/ { 4-[(1E)-3-hydroxy-3-
_ methylbut-l-enyl]phenyl}-4,5-
~ / dihydro-lH-benzo[g]indazole-
Q--Ir H N-N NHz 3-carboxamide
N
0
CI O
541.01 ethyl (2E)-3-(4-{3- 1< 10 541 290
o (aminocarbonyl)-8-[(2- M
o / chlorobenzoyl)amino]-4,5-
~ dihydro-lH-benzo[g]indazol-l-
~ / yl}phenyl)prop-2-enoate
\ I CI N \N NHz
\
O /
155
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Compound No., Structure Mol. Compound Name IKK2 MS Example
Wt. Resin (M+H)
IC50
oH 512.96 (2E)-3-(4-{3-(aminocarbonyl)- <1 M 513 291
~ 8-[(2-chlorobenzoyl)amino]-
~ 4,5-dihydro-lH-
~ / benzo[g]indazol-l-
~ c+ N ~" ""2 yl}phenyl)prop-2-enoic acid
0
o
511.97 1-{4-[(lE)-3-amino-3- <1 M 512 292
~ oxoprop-l-enyl]phenyl}-8-[(2-
~ chlorobenzoyl)amino]-4,5-
cl dihydro-lH-benzo[g]indazole-
\ n"~ " N"2 3-carboxamide
O
O /
'N 535.01 8-[(2-chlorobenzoyl)amino]-1- <1 M 535 293
{4-[(E)-2-(1H-imidazol-l-
~ yl)ethenyl]phenyl}-4,5-
~ / dihydro-lH-benzo[g]indazole-
/ ~ O1 H "-" NHz 3-carboxamide
N
I/
o 539.00 8-[(2-chlorobenzoyl)aniino]-1- 1< 10 539 294
{4-[(E)-(2-oxodihydrofuran- M
3(2H)-ylidene)methyl]phenyl}-
C1 ~ / 4,5-dihydro-lH-
~ N ~" "", benzo[g]indazole-3-
o I 1~ o carboxamide
513.00 8-[(2-chlorobenzoyl)amino]-1- <1 M 513 295
{ 4-[( lE)-3-hydroxybut-l-
~ enyl]phenyl}-4,5-dihydro-lH-
benzo[g]indazole-3-
ci
~ I N ~" "H= carboxamide
o
513.00 8-[(2-chlorobenzoyl)amino]-1- <1 M 513 296
[4-(3-oxobutyl)phenyl]-4,5-
~ dihydro-lH-benzo[g]indazole-
c~ ~ / 3-carboxamide
~ \ N-N NHz
N \
\ \
O I /
H,N 0 547.04 8-[(2-chlorobenzoyl)amino]-1- <1 M 547 297
, { 4-[(E)-2-
"'N I I \ (methylsulfonyl)ethenyl]phenyl
}-4,5-dihydro-lH-
~ HN o benzo[g]indazole-3-
~ Ci carboxamide
o:
Oa8\
156
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Compound No., Structure Mol. Compound Name IKK2 MS Example
Wt. Resin (M+H)
IC50
H~N 513.00 8-[(2-chlorobenzoyl)aniino]-1- <1 M 513 298
{4-[(lE)-4-hydroxybut-l-
"'N enyl]phenyl}-4,5-dihydro-lH-
~ benzo[g]indazole-3-
~ HN o carboxamide
~cl
Ho
HzN 529.00 8-[(2-chlorobenzoyl)amino]-1- <1 M 529 299
[4-(4-hydroxy-3-
"'N oxobutyl)phenyl]-4,5-dihydro-
~ 1H-benzo[g]indazole-3-
~ / HN o carboxamide
~I ~ cl
Ho
499.96 8-{[(2-chloropyridin-3- <1 M 500 300
Ho yl)carbonyl]amino}-1-{4-
N-N [(1Z)-3-hydroxyprop-l-
0 N ~ ~ enyl]phenyl}-4,5-dihydro-1H-
cl N' i I NHz benzo[g]indazole- 3-
carboxamide
N 0 526.99 8-{ [(2-chloropyridin-3- <1 M 527 301
H yl)carbonyl]amino}-1-{4-
_ [(1E)-3-(methylamino)-3-
~ / oxoprop-l-enyl]phenyl}-4,5-
N-N dihydro-lH-benzo[g]indazole-
N) N \ \N
3-carboxamide
CI 0
513.99 8-{[(2-chloropyridin-3- <1 M 514 302
yl)carbonyl]amino }-1-[4-(3-
- oxobutyl)phenyl]-4,5-dihydro-
~ 1H-benzo[g]indazole-3-
N N ~" carboxamide
\ 11
/ F~
cl 0 N
F'N 512.96 1-{4-[(1E)-3-amino-3- <1 M 513 303
~ oxoprop-l-enyl]phenyl}-8-
_ { [(2-chloropyridin-3-
~ yl)carbonyl]amino}-4,5-
N N \" dihydro-lH-benzo[g]indazole-
~
cl 0 / NH2 3-carboxaniide
H 528.01 8-{[(2-chloropyridin-3- 1< 10 528 304
yl)carbonyl]amino}-1-{4- M
[(1E)-3-hydroxy-3-methylbut-
1-enyl]phenyl}-4,5-dihydro-
N N ~", 1H-benzo[g]indazole-3-
"H= carboxamide
cl 0
157
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Compound No., Structure Mol. Compound Name IKK2 MS Example
Wt. Resin (M+H)
IC50
541.01 8-{[(2-chloropyridin-3- 1< 10 541 305
i yl)carbonyl]amino}-1-{4
M
, [(1E)-3-(dimethylamino)-3-
~ ~ oxoprop-l-enyl]phenyl}-4,5-
N N N-" NHz dihydro-lH-benzo[g]indazole-
\ ~ 3-carboxamide
ci 0 452.54 8-[(methylsulfonyl)amino]-1- 1< 10 453 306
[4-(3-oxobutyl)phenyl]-4,5- M
~ dihydro-lH-benzo[g]indazole-
3-carboxamide
H N-N NHz
S.N
O'0
465.54 1-{4-[(1E)-3-(methylamino)-3- <1 [LM 566 307
H / oxoprop-l-enyl]phenyl}-8-
_ [(methylsulfonyl)amino]-4,5-
~ / dihydro-lH-benzo[g]indazole-
H N-N NHZ 3-carboxanlide
S.N
O'0
562.50 8-[(2-chlorobenzoyl)amino]-1- 1< 10 526 308
i I ~ {4-[(1E)-3-
qj'~ N N-" NH, (dimethylamino)prop-l- M
c o\ I o enyl]phenyl}-4,5 dihydro 1H
benzo[g]indazole-3-
HO1 carboxamide hydrochloride
[00172] Example 309
8-[(2-chlorobenzoyl)amino]-1-(4-{ ( lE)-3-[(2-methoxyethyl)amino]-3-oxoprop-l-
enyl }phenyl)-4,5-dihydro-lH-benzo[g]indazole-3-carboxamide
~o 0
N
H
N NHZ
p
cl O
To a 50 mL syringe barrel equipped with a fritted disk was added 2.02 g of PS-
MB-
CHO (Argonaut Technologies, 1.46 mmol/g loading). The resin was washed with
N,N-dimethylformamide. To the resin was added a solution of sodium
158
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
triacetoxyborohydride (3.20 g, 15.1 mmol) dissolved in trimethylorthoformate
(1.5
mL), acetic acid (1.5 mL), and N,N-dimethylformamide (12 mL). 2-
Methoxyethylamine (1.11 g, 14.8 mmol) was added to the mixture. The mixture
was allowed to shake on an orbital shaker for 16 hrs. The solution was drained
from the resin and washed with a solution of 8 parts N,N-dimethylfromamide to
1
part trimethylorthoformate to 1 part acetic acid. The resin was then washed
with
N,N-dimethylformamide, 1 part N,N-dimethylformamide to 1 part triethylamine,
N,N-dimethylformamide, dichloromethane, and diethyl ether. The resin was dried
under vacuum.
[00173] To a 4 mL peptide flask was added 100mg of the resin. A solution
of HBTU (127 mg, 0.336 mmol), 1-hydroxybenzotriazole (52 mg, 0.38 mmol),
triethylamine (34 mg, 0.34 mmol), and (2E)-3-(4-{3-(aminocarbonyl)-8-[(2-
chlorobenzoyl)amino]-4,5-dihydro-lH-benzo[g]indazol-l-yl }phenyl)prop-2-enoic
acid (172 mg, 0.335 mmol) in 2mL of N,N-dimethylformamide was added to the
resin. The peptide flask was agitated on an orbital shaker for 16 hrs, after
which the
solution was drained and the resin was washed with DMF, dichloromethane, and
diethyl ether. The resin was dried under vacuum.
[00174] The resin was suspended in 2 mL of 90% aqueous trifluoroacetic
acid and agitated for 30 minutes. The solution was filtered. The resin was
washed
with 2 mL of 90% aqueous trifluoroacetic acid, with the wash being collected.
The
TFA solutions were combined, diluted to 15 mL with water, and concentrated to
dryness. The resulting oil was triturated with methanol to yield the title
compound.
MS (ESI+) for C31H28C1N504 m/z 570.2 (M+H)+.
[00175] The compounds of Examples 310-315 listed in the Table 12 were
prepared according to the procedure of Example 309 using the appropriate
amine.
[00176] The bioactivity in the IKK2 Resin assay for the compounds of
Examples 309-315 is shown in Table 12.
159
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Table 12
Compound No., Structure Mol. Compound Name(s) IKK2 MS Example
Wt. Resin (M+H)
IC50
-O\-\ 0 570.0 8-[(2-chlorobenzoyl)amino]-1-(4- <1 M 570 309
a / {(1E)-3-[(2-methoxyethyl)amino]-
_ 3-oxoprop-1-enyl }phenyl)-4,5-
~ / dihydro-lH-benzo[g]indazole-3-
\ NHz carboxaniide
l\
ci o 0
~ / 0 592.1 8-[(2-chlorobenzoyl)amino]-1-(4- <1 M 592 310
b / {(lE)-3-[(2-furylmethyl)amino]-3-
_ oxoprop-l-enyl}phenyl)-4,5-
~ / dihydro-lH-benzo[g]indazole-3-
\ \ carboxaniide
ot o NH,
0
526.0 8-[(2-chlorobenzoyl)amino]-1-{4- <1 M 526 311
/ [(1E)-3-(methylamino)-3-
_ oxoprop-l-enyl]phenyl}-
~ / 4,5-dihydro-lH-benzo[g]indazole-
\ \ 0 NHz 3-carboxamide
ct o
N 540.0 8-[(2-chlorobenzoyl)amino]-1-{4- <1 M 540 312
H / [(lE)-3-(ethylamino)-3-oxoprop-
_ 1-enyl]phenyl}-4,
5-dihydro-lH-benzo[g]indazole-3-
\ NHz carboxanude e
\
0
ci o
0 602.1 1-{4-[(1E)-3-(benzylamino)-3- 1< 10 602 313
oxoprop-l-enyl]phenyl}-8-[(2-
chlorobenzoyl)amino]- PM
\ / 4,5-dihydro-IH-benzo[g]indazole-
\ \ ~ NHz 3-carboxamide
ci o I\
s
ra- 387.44 8-amino-l-{4-[(lE)-3- 1 < 10 388 314
/ (methylamino)-3-oxoprop-l- M
_ enyl]phenyl}-4,5-dihydro-lH-
~ / benzo[g]indazole-3-
N-N
HzN \ \ ' carboxamide
O
479.56 1-{4-[(lE)-3-(dimethylamino)- 1< 10 480 315
N
. 3-oxoprop-1-enyl]phenyl}-8-
[(methylsulfonyl)amino]-4,5-
H N-N N~ M
i
dihYdro-lH-benzoLg1ndazole-
\S.N
3-carboxamide
0
160
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
[00177] Example 316
8-{ [(2-chloropyridin-3-y1)carbonyl]amino}-1-[4-(3-hydroxypropyl)phenyl]-4,
5-dihydro-1 H-benzo [g] indazole-3-carboxamide
HO
-N
O N 0
CI NHZ
/ ~
N~
Using standard hydrogenation conditions 8-{ [(2-chloropyridin-3-
yl)carbonyl] amino }-1-{ 4-[(1Z)-3-hydroxyprop-l-enyl]phenyl }-4,5-dihydro-1H-
benzo[g]indazole-3-carboxamide was converted to 8-{ [(2-chloropyridin-3-
yl)carbonyl]amino}-1-[4-(3-hydroxypropyl)phenyl]-4,5-dihydro-lH-
benzo[g]indazole-3-carboxamide. MS (ESI+) for ni/z 502 (M+H)+. IKK-2 resin
ICSO<1 M.
[00178] Example 317
8-[(2-chlorobenzoyl)amino]-1-[4-(3-furyl)phenyl]-4,5-dihydro-lH-
benzo [g]indazole-3-carboxamide
0
U
~
CI H ~ / N-N
I N J' NH2
O ) / O
[00179] Step 1: A suspension of ethyl 1-(4-bromophenyl)-8-nitro-4,5-
dihydro-lH-benzo[g]indazole-3-carboxylate (4.4 g) in THF (80 mL) was treated
with 1 N aq. NaOH (80 mL) and stirred vigorously overnight. The reaction
mixture
was diluted with ethyl acetate and acidified to pH = 2 with 1 N aq. HCI. The
organic layer was separated and the aqueous fraction extracted with EtOAc
(3x).
161
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Combined extracts were washed with brine, dried over NazSO4, filtered and
concentrated in vacuo to afford 4.0 g (97%) of 1-(4-bromophenyl)-8-nitro-4,5-
dihydro-IH-benzo[g]indazole-3-carboxylic acid as a solid: MS(ESI+) 414 [M+H]+.
1H NMR (400 MHz, d6 DMSO) S 8.07 (dd, IH), 7.83 (d, 2H), 7.66 (d, 1H), 7.56
(d,
2H), 7.42 (d, 1H), 3.10 (m, 2H), 2.99 (m, 2H).
[00180] Step 2: A suspension of Rink amide resin (5.3g, 2.5 mmol, 0.47
meq/g, NovaBiochem), in 30 % piperidine/DMF was prepared in a solid phase
reactor equipped with an overhead stirrer. The mixture was stirred for 15 min,
filtered, and treated a second time with 30 % piperidine/DMF for 15 min. The
solvent was removed by filtration and the resin washed with DMF ( 3x), MeOH
(3x), and DCM (4x). A solution of 1-(4-bromophenyl)-8-nitro-4,5-dihydro-IH-
benzo[g]indazole-3-carboxylic acid (2.07 g) in 1:1 DCM/DMF (10 mL) was
prepared and added to the resin, followed by 1M HOBt in DMF (5 mL) and 1M
DIC in DMF (5 mL). The resin was stirred at RT. After stirring for 16 h, the
resin
was washed with DMF (3x), MeOH (3x), DCM (4x), and filter to afford loaded
resin. Resin loading was determined by direct cleavage 'H NMR: 0.569 mmol/g.
Evaporated direct cleavage NMR sample from the resin gave an oil: MS(ESI+) 413
[M+H]+. 'H NMR (400 MHz, 10%TFA/CDC13): 6 8.10 (dd, 1H), 7.76 (d, 2H), 7.63
(d, 1H), 7.53 (d, 1H), 7.41 (d, 2H), 3.16 (m, 4H).
[00181] Step 3: In a solid phase reactor equipped with an overhead stirrer to
a suspension of resin from step 2 (7.8 g, 4.43 mmol) in NMP (15 mL) was added
2M SnC12 in NMP (15 mL). The mixture was stirred for 1 h, filtered, and
retreated
with 2M SnC12 in NMP (15 mL). After stirring overnight the resin was filtered,
washed with DMF (3x), MeOH (3x), DCM (4x), filtered, and air dried to afford
the
intermediate amine resin. Determined resin loading by direct cleavage 'H NMR:
0.414 mmol/g. In a solid phase reactor equipped with an overhead stirrer was
prepared a suspension of 0.4 g of the amine resin in NMP.
[00182] Step 4: The resin was allowed to stir for 5 min and subsequently
treated with a solution of 2-chlorobenzoic acid (126 mg) in NMP (1 mL). The
162
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
mixture was treated with HATU (307 mg), DIEA (0.28 mL) and stirred for lh. The
resultant resin was filtered, subjected to a second treatment of 2-
chlorobenzoic acid,
HATU and DIEA in NMP, and allowed to stir. After stirring overnight, the resin
was filtered, washed with DMF (3x), MeOH (3x), and DCM (4x). The resin was
filtered and air dried to afford resin. Determined resin loading by direct
cleavage 'H
NMR: 0.702 mmol/g.
[00183] Step 5: To a reaction vessel was added resin from step 4 (0.20g,
0.09 mmol) in a suspension of toluene/EtOH (2:1). The vessels were purged with
argon for 5 min and subsequently treated with Pd(PPh3)4 (41.6 mg, 0.036mmol),
an
3-furylboronic acid (0.2mmo1), and 2M Na.ZCO3 (200 L, 0.4mmol). The vessels
were heated to 100 C and allowed to agitate for 30 h. Each vessel was quenched
with 25% NH4OH for 30min, filtered and washed three times each with DMF,
MeOH, MeOH:H20 (1:1), 0.2N HCt, MeOH:H20 (1:1), MeOH and DCM. The
resins were allowed to dry and cleaved with 10% TFA/DCM (2 mL) for 30 min.
The resins were washed twice with 0.5 mL DCM and the combined filtrates
concentrated to afford the desired final product.
[00184] The compounds of Examples 318-323 listed in the Table 13 were
prepared according to the procedure of Example 317 using the appropriate
boronic
acid in step 5.
[00185] The bioactivity in the TKK2 Resin assay for the compounds of
Examples 316-323 is shown in Table 13.
163
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Table 13
Compound No., Structure Mol. Compound Name IKK2 MS Example
Wt. Resin (M+H)
IC50
501.96 8-{ [(2-chloropyridin-3- <1 M 502 316
Ho yl)carbonyl]amino}-1-[4-(3-
p \ N hydroxypropyl)phenyl]-4,5-
0 dihydro-lH-benzo[g]indazole-
C1 N \ ~ NHz 3-carboxaniide
0 508.97 8-[(2-chlorobenzoyl)amino]-1- 1< 10 509 317
~ [4-(3-furyl)phenyl]-4,5-
~ / dihydro-lH-benzo[g]indazole- M
\ ~ C1 N N-N N~ 3-carboxamide
o ~ i
,N 520.00 8-[(2-chlorobenzoyl)amino]-1- 1< 10 520 318
~ (4-pyridin-3-ylphenyl)-4,5- M
/ dihydro-lH-benzo[g]indazole-
N
\ ~ C1 N-N NH2 3-carboxamide
o I i
oH 549.03 8-[(2-chlorobenzoyl)amino]-1- 1< 10 549 319
[3'-(hydroxymethyl)-1,1'- M
\ / biphenyl-4-yl]-4,5-dihydro-lH-
benzo[g]indazole-3-
\ ' 01 N-N NH2 carboxamide
\
0
o /
""= 534.02 1-(3'-amino-1,1'-biphenyl-4- 1< 10 534 320
yl)-8-[(2- M
- chlorobenzoyl)amino]-4,5-
, cl N-N dihydro-lH-benzo[g]indazole-
N \ ~ ' ""~ 3-carboxamide
o ~,~ o
oH 563.02 4'-{3-(aminocarbonyl)-8-[(2- 1< 10 563 321
chlorobenzoyl)amino]-4,5- M
dihydro-lH-benzo[g]indazol-l-
yl}-1,1'-biphenyl-3-carboxylic
\ C1 N ~N NHz acid
O
563.02 4'-{3-(aminocarbonyl)-8-[(2- 1< 10 563 322
"o chlorobenzoyl)amino]-4,5- M
dihydro-lH-benzo[g]indazol-l-
N " yl}-1,1'-biphenyl-4-carboxylic
H \ NHacid
o
164
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Compound No., Structure Mol. Compound Name IKK2 MS Example
Wt. Resin (M+H)
IC50
~ o- 579.06 8-[(2-chlorobenzoyl)amino]-1- 1 < 10 579 323
0 (3',4'-dimethoxy-1,1'-biphenyl- M
, 4-yl)-4,5-dihydro-IH-
benzo[g]indazole-3-
a
\ N N-N
NHz carboxamide
p ~ O
[00186] The compounds of Examples 324-366 in Table 14 were prepared in a
manner analogous to Example 3 using the appropriate hydrazine and acylating or
sulfonating agent. The bioactivity in the IKK2 Resin assay for the compounds
of
Examples 324-366 is shown in Table 14.
Table 14
Compound No., Structure Mol. Wt. Compound Name(s) IKK2 MS Example
Resin (M+H)
IC50
No2 487.91 8-[(2-chlorobenzoyl)amino]- <1 gM 488 324
/ 1-(4-nitrophenyl)-4,5-
~ N-N o dihydro-IH-
" - ~ N,~ benzo[g]indazole-3
ci 0 ~ ~ carboxamide
2 487.91 8-[(2-chlorobenzoyl)amino]- 1< 10 488 325
C~ 1-(3-nitrophenyl)-4,5- M
N-N dihydro-lH-
~ ~ N ~\ cONH, benzo[g]indazole-3-
O1 0 ~ s carboxamide
ci 511.80 8-[(2-chlorobenzoyl)amino]- 1< 10 512 326
O1-(3,4-dichlorophenyl)-4,5-
dihydro-lH- M
P-ir " ~ ~ N"~ benzo[g]indazole-3-
cl 0 ~ carboxamide
N~ 443.90 8-[(2-chlorobenzoyl)amino]- 1< 10 444 327
~~ 1 pyridin 3-y1-4,5-dihydro M
N ~" NHz 1H-benzo[g]indazole-3- o ~ I o carboxamide
a
~ 442.91 8-[(2-chlorobenzoyl)amino]- <1 M 443 328
~ / 1-phenyl-4,5-dihydro-lH-
ci
01 ly N ~" N~ benzo[g]indazole-3-
0 0 o carboxamide
165
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Compound No., Structure Mol. Wt. Compound Name(s) IKK2 MS Example
Resin (M+H)
IC50
0 486.92 4-{3-(aminocarbonyl)-8-[(2- 1< 10 487 329
Hp- chlorobenzoyl)amino]-4,5-
~ ~ dihydro-lH-benzo[g]indazol- M
1-yl}benzoic acid
pioyi N NH
CI
\ 382.44 8-[(methylsulfonyl)amino]-1- 1< 10 383 330
~ e phenyl-4,5 dihydro-lH-
N N-N NNZ benzo[g]indazole-3- M
N / \
o'o \ o carboxamide
443.90 8-[(3- <1 M 444 331
chloroisonicotinoyl)amino]-
I hen 14 5 dih dro-1H-
~ N \ "\ " ~ coNt~ P Y-~- Y
ol o benzo[g]indazole-3-
carboxamide
c( ~ 477.35 8-[(2-chlorobenzoyl)amino]- <1 M 478 332
r/~~~ ---\)~" 1-(4-chlorophenyl)-4,5-
N-N dihydro-lH-
o " o benzo[g]indazole-3-
o' N~ carboxamide
F~o 526.91 8-[(2-chlorobenzoyl)amino]- 1< 10 527 333
F 1-[4 M
(trifluoromethoxy)phenyl]-
o N ~ ~" 0 4,5-dihydro-lH-
o~ \ I N~ benzo[g]indazole-3-
I carboxamide
477.35 8-[(2-chlorobenzoyl)amino]- <1 M 478 334
ci 1-(3-chlorophenyl)-4,5-
0 N ~ N dihydro-lH-
o~ ~ I NNO benzo[g]indazole-3-
~ \ carboxamide
456.94 8-[(2-chlorobenzoyl)amino]- <1 M 457 335
1 1-(4-methylphenyl)-4,5-
N-N dihydro-lH-
o N \ ' o benzo[g]indazole-3-
oi\ N~ carboxamide
456.94 8-[(2-chlorobenzoyl)amino]- <1 M 457 3336
1-(3-methylphenyl)-4,5-
dihydro-lH-
o r, ~ " o benzo[g]indazole-3-
CI \ NHz carboxamide
166
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Compound No., Structure Mol. Wt. Compound Name(s) IKK2 MS Example
Resin (M+H)
IC50
484.99 8-[(2-chlorobenzoyl)amino]- 1< 10 485 337
1-(4-isopropylphenyl)-4,5- M
dihydro-lH-
N-N benzo[g]indazole-3-
carboxamide
CI~" k2
4
43.89 8-[(2-chlorobenzoyl)amino]- 1 M 444 338
1-pyridin-4-y1-4,5-dihydro-
Q
N-N 1H-benzo[g]indazole-3-
N / carboxaniide
CI \ I NH
~ \
/
460.89 8-[(2-chlorobenzoyl)amino]- <1 M 461 339
1-(3-fluorophenyl)-4,5-
N-N dihydro-lH-
~ benzo[g]indazole-3-
"
0 N / \
cl NI~ carboxamide
11'"J/,
F F 372.35 8-amino-l-[4- 1< 10 373 340
F (trifluoromethyl)phenyl]-4,5- M
dihydro-lH-
N-" o benzo[g]indazole-3-
H~N N"z carboxamide
I~ F 460.89 8-[(2-chlorobenzoyl)amino]- <1 M 461 341
~ 1-(2-fluorophenyl)-4,5-
N-N dihydro-lH-
"
O N \ benzo[g]indazole-3 CI \ N"2 carboxamide
F 462.43 8-[(2,3- 1< 10 463 342
difluorobenzoyl)amino]-1-(3-
-~ fluorophenyl)-4,5-dihydro- M
N / ~" 1H-benzo[g]indazole-3-
F \ \ ~ Nr carboxamide
F ~
F 498.51 3-({[3-(aminocarbonyl)-1-(3- 1< 10 499 343
cz fluorophenyl)-4,5-dihydro- M
1H-benzo[g]indazol-8-
~ N yl]amino}carbonyl)-2-
H methylphenyl acetate
o~
o--4-
167
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Compound No., Structure Mol. Wt. Compound Name(s) IKK2 MS Example
Resin (M+H)
IC50
F 495.34 8-[(2,3- 1< 10 496 344
dichlorobenzoyl)amino]-1-(3-
fluorophenyl)-4,5-dihydro-
N ~M
o H I~" 0 1H-benzo[g]indazole-3-
/
N~ carboxamide
c~
F 461.88 8-{ [(2-chloropyridin-3- <1 M 462 345
yl)carbonyl]amino}-1-(3-
fluorophenyl)-4,5-dihydro-
O H ~ " 0 1H-benzo[g]indazole-3-
ci " N~ carboxamide
N
F 478.88 8-[(2-chlorobenzoyl)amino]- <1 M 479 346
1-(3,5-difluorophenyl)-4,5-
F 0 dihydro-1 H-
~ H ~ " o benzo[g]indazole-3-
N /
c~~ NHz carboxamide
F 479.87 8-{ [(2-chloropyridin-3- <1 M 480 347
~ \ yl)carbonyl]amino}-1-(3,5-
F difluorophenyl)-4,5-dihydro-
0 H ~ " c 1H-benzo[g]indazole-3-
/
ci "~ NNz carboxamide
i~
F 513.33 8-[(2,3- 1< 10 513 348
~ \ dichlorobenzoyl)amino]-1- M
F (3,5-difluorophenyl)-4,5-
H
ci ~ " dihydro-lH-
c NHz benzo[g]indazole-3-
~ carboxaniide
a /
F F 510.91 8-[(2-chlorobenzoyl)amino]- 1< 10 511 349
1-[4- M
1 (trifluoromethyl)phenyl]-4,5-
N-N
H ~ dihydro-lH-
o N ~ o benzo[g]indazole-3-
ci , ""~ carboxamide
I/
sr 521.81 1-(4-bromophenyl)-8-[(2- <1 M 521 350
chlorobenzoyl)amino]-4,5-
\ O1 N \N N~ dihydro-lH-
o benzo[g]indazole-3-
0 carboxamide
168
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Compound No., Structure Mol. Wt. Compound Name(s) IKK2 MS Example
Resin (M+H)
IC50
er 501.39 1-(4-bromophenyl)-8-[(2- <1 M 502 351
a methylbenzoyl)amino]-4,5-
, ~ I H N-N dihydro-lH-
benzo[g]indazole-3-
~ "
carboxamide
B' 383.25 8-amino-l-(4-bromophenyl)- 1< 10 383 352
0 4,5-dihydro-lH-
~N \ ~", NHz benzo[g]indazole-3- M
~ o carboxamide
F 478.89 8-[(2-chlorobenzoyl)amino]- <1 M 479 353
1-(2,4-difluorophenyl)-4,5-
~
cl 0 H \N NN dihydro-lH-
I " o benzo[g]indazole-3-
carboxamide
F 479.87 8-[(3- <1 gM 480 354
F ~ chloroisonicotinoyl)amino]-
I
N 01 N\NcoN 1-(3,4-difluorophenyl)-4,5-
"~ dihydro-IH-
benzo[g]indazole-3-
carboxamide
F 479.87 8-[(3- <1 M 480 355
loroisonicotinoyl)amino]-
b ch
HF N-N 1-(3,5-difluorophenyl)-4,5-
~ " ~ 0ON1~ dihydro-lH-
benzo[g]indazole-3-
carboxamide
FCl 495.34 8-[(2-chlorobenzoyl)amino]- <1 M 495 356
1-(3-chloro-4-fluorophenyl)-
\ N \N N,~ 4,5-dihydro-lH-
benzo[g]indazole-3-
cl 0 o
carboxamide
o 436.42 8-aniino-1-14- 1< 10 437 357
cFs s [(trifluoromethyl)sulfonyl]ph
N-N enyl}-4,5-dihydro-1H- M
""~ benzo[g]indazole-3-
carboxamide
0 0 612.42 8-[(3- <1 M 576 358
cF~- s chloroisonicotinoyl)amino]-
1-{4-
N N-N NH~ [(trifluoromethyl)sulfonyl]ph
ci H enyl}-4,5-dihydro-lH-
N benzo[g]indazole-3-
carboxamide hydrochloride
o 575.96 8-{ [(2-chloropyridin-3- <1 M 576 359
cFs s yl)carbonyl]amino}-1-{4-
[(trifluoromethyl)sulfonyl]ph
N N-N NHenyl}-4,5-dihydro-lH-
c~ benzo[g]indazole-3-
"J carboxamide
169
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Compound No., Structure Mol. Wt. Compound Name(s) IKK2 MS Example
Resin (M+H)
IC50
1 478.34 1-(4-chlorophenyl)-8-{ [(4- <1 M 478 360
chloropyridin-3-
\ 0
N ~N. N~ yl)carbonyl]amino}-4,5-
N o dihydro-lH-
benzo[g]indazole-3-
carboxamide
0' 472.94 8-[(2-chlorobenzoyl)amino]- 1< 10 473 361
Q 1-(3-methoxyphenyl)-4,5- M
N-N dihydro-lH-
r, benzo[g]indazole-3-
c~ carboxamide
F 478.88 8-[(3-chloro-2- 1 < 10 479 362
/ fluorobenzoyl)amino]-1-(3-
/ N-N fluorophenyl)-4,5-dihydro- pM
ra ' 1H-benzo[g]indazole-3-
F~ N carboxamide
F, ~ 478.88 8-[(2-chlorobenzoyl)amino]- 51 M 479 363
]I"~~ 1-(3,4-difluorophenyl)-4,5-
N-N dihydro-lH-
N benzo[g]indazole-3-
C1H N~ carboxamide
~
p F 479.87 8-{ [(2-chloropyridin-3- <1 M 480 364
0 yl)carbonyl]amino}-1-(3,4-
N-N difluorophenyl)-4,5-dihydro-
0 N 1H-benzo[g]indazole-3-
C1 NH~ carboxanllde
N i
F F 513.33 8-[(2,3- 1< 10 513 365
0 dichlorobenzoyl)amino]-1-
N-N (3,4-difluorophenyl)-4,5- M
N ' dihydro-lH-
01H N"~ benzo[g]indazole-3-
c~ carboxaniide
F F 479.88 8-{ [(2-chloropyridin-3- <1 M 480 366
yl)carbonyl]amino } -1-(2,4-
o H N-N, difluorophenyl)-4,5-dihydro-
C1 NHz 1H-benzo[g]indazole-3-
N carboxamide
[00187] Example 367
8-[(2-chlorobenzoyl)amino]-1-[5-(methylsulfonyl)pyridin-2-yl]-4,5-dihydro- lH-
benzo[g]indazole-3-carboxamide
170
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
O1,
N-N
N
CI CONH2
[00188] Step 1
~ "'0
S I ~N
~ Br
To a suspension of 2,5-dibromopyridine (12.0 g, 0.05 mol) in ether was added
nBuLi (32 mL of 1.6 N in hexane, 0.05 mol) at -78 C dropwise. The purple
suspension was stirred for 1 hour and then treated with dimethyl disulfide.
The
reaction mixture was kept at this temperature for 1 h and 1/a h at 0 C. The
reaction
was quenched with a mixture of concentrated HCI and ether. The organic phase
was washed with brine, dried over MgSO4, and filtered. The filtrate was
concentrated to give 10.3 g of crude as brown oil, which was used without
purification. To a solution of this crude (10.0 g, 0.05 mol) in methanol (200
mL)
was added a solution of OXONE in 300 mL of water. The reaction was stirred at
room temperature for 72 h. Solvent was removed and the residue was basified
with
50% NaOH solution. The precipitate was collected by filtration, air-dried to
give
8.2 g of product as a white crystal (72% yield over two step). NMR spectrum
was
consistent with the proposed structure.
[00189] Step 2
0, 00
S N
~
NHNH2
A mixture of the product from step 1(8.0 g, 0.034 mol) and hydrazine (2.3 g,
0.068
mol) in 100 mL of ethanol was heated at reflux for 2 h. cooled to room
temperature,
and the solid was collected by filtration, washed with sat. NaHCO3, water, air-
dried
to give 4.0 g of crude as a pale white solid (63% yield); NMR spectrum was
consistent with the proposed structure.
171
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
[00190] Step 3
O111,O
N-N
z~
02N C02Et
A mixture of the product from step 2(1.4 g, 0.007 mol) and ethyl (7-nitro-l-
oxo-
1,2,3,4-tetrahydronaphthalen-2-yl)(oxo)acetate (2.03 g, 0.007 mol) in 75 mL of
acetic acid was heated at reflux under nitrogen for 3 h. The solvent was
removed
and the residue was treated with a mixture of methanol/ethyl acetate/ether to
give
1.67 g of the product as a yellow solid (54% yield); 1HNMR (DMSO, 400 MHz) S:
8.98 (dd, 1H), 8.68 (dd, 1H), 8.21 (d, 1H), 8.13 (dd, 1H), 7.95 (d, 1H), 7.68
(d, 1H),
4.37 (q, 2H), 3.42 (s, 3H), 3.11 (m, 2H), 3.00 (m, 2H), 1.35 (t, 3H); Anal.
Calcd. for
C20H18N406S: C, 54.29; H, 4.10; N, 12.66; S, 7.23. Found: C, 53.71; H, 4.41;
N,
12.56; S, 7.14.
[00191] Step 4
Q. ,o
iS
~
N-N
H2N CO2Et
The product from step 3(1.6 g, 0.0036 mol) was hydrogenated in a Parr shaker
with
20% Pd(OH)2/C in acetic acid for 2 h at 5 psi. After the removal of solvent,
the
residue was triturated with a mixture of methanol and ether to give 1.0 g of
the
product as a white solid (67% yield): 1HNMR (DMSO, 400 MHz) S: 9.01 (dd, IH),
8.62 (dd, 1H), 8.07 (dd, 1H), 7.01 (d, IH), 6.46 (dd, 1H), 6.12 (d, 1H), 4.90
(brs,
2H), 4.33 (q, 2H), 3.42 (s, 3H), 2.87 (m, 2H), 2.78 (m, 2H), 1.33 (t, 3H);
Anal.
Calcd. for C20H2ON404S: C, 58.24; H, 4.89; N, 13.58; S, 7.77. Found: C, 57.70;
H,
4.68; N, 13.43; S, 7.60.
172
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
[00192] Step 5
o, o
iS / N
\ I
N-N
HZN I CONH2
To a suspension of the product from step 4(0.95 g, 0.0023 mol) in 25 mL of
methanol was added liquid ammonia through a dry-ice condenser. The solution
was
sealed with a septum and stirred at room temperature for 48 h. Solvent was
removed and the solid was triturated with methanol to give 0.68 g of product
as a
yellow solid (77% yield): 1HNMR (CDC13, 400 MHz) 8: 8.99 (d, 1H), 8.63 (ddd,
1H), 8.10 (d, 1H), 7.66 (s, 1H), 7.43 (s, 1H), 7.00 (d, IH), 6.45 (d, 1H),
6.22 (s,
1H), 4.89 (s, 2H), 3.41 (s, 3H), 2.86 (m, 2H), 2.74 (m, 2H); Anal. Calcd. for
C18H17N503S: C, 56.39; H, 4.47; N, 18.27; S, 8.36. Found: C, 55.48; H, 4.29;
N,
17.84; S, 8.19.
[00193] Step 6
o, ,so
N-N
N \ \ I CONH2
CI
To a suspension of the product from step (0.62 g, 0.0016 mol) in 10 mL of
pyridine was added 2-chlorobenzoyl chloride (0.29 g, 0.0016 mol) in one
portion
and the reaction mixture was stirred at room temperature overnight. Solvent
was
removed and the residue was partitioned between ethyl acetate and water. The
organic phase was washed with brine, dried over MgSO4, and filtered. The
filtrate
was concentrated and purified by chromatography on silica gel (ethyl acetate)
to
give 0.6 g of the product as a yellow solid (72% yield); iHNMR (DMSO, 400 MHz)
S: 10.36 (s, 1H), 9.00 (d, 1H), 8.62 (dd, 1H), 8.15 (d, 1H), 7.74 (s, 1H),
7.39-7.54
(m, 7 H), 7.34 (d, 1H), 3.32 (s, 3H), 2.93 (m, 4H); Anal. Calcd. for
C25H20C1N504S:
173
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
C, 57.53; H, 3.86; N, 13.42; S, 6.14. Found: C, 56.69; H, 4.37; N, 12.82; S,
5.86.
IKK-2 resin IC50 <1 M.
[00194] Example 368
8-[(2-chlorobenzoyl)amino]-1-[6-(methylsulfonyl)pyridin-3-yl]-4,5-dihydro-lH-
benzo [g] indazole-3-carboxamide
O1, SO N
I
N-N
N \ ~ I CONH2
CI / I /
[00195] Step 1
o\SO Na,-_ N
O2
A mixture of 2-chloro-5-nitropyridine (20.5 g, 0.13 mol) and sodium
thiomethoxide
(10.9 g, 0.16 mol) in DMS was heated at 100 C under nitrogen for 3 h. Cooled
to
room temperature and water was added. The precipitate was collected, air-dried
to
give 14.5 g of product as a brown solid. To a solution of this solid (16.5 g,
0.097
mol) in 100 mL of acetone was added 170 mL of 2N sulfuric acid solution. Then
a
solution of K1VInO4 (20.0 g, 0.126 mol) in 375 mL of water was added dropwise
to
the above suspension. The reaction mixture was stirred at RT overnight and
then it
was filtered. The solid was stirred with 400 mL of hot ethanol, then cooled
and
filtered. The filtrate was concentrated to half volume and the precipitate was
collected and air-dried to give 12.5 g of the desired product as a pale yellow
solid,
which was used without further purification. The NMR and MS were consistent
with the proposed structure.
174
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
[00196] Step 2
OSO N\
I /
NHNH2
A mixture of the product from step 1(12.3 g, 0.061 mol), iron (6.5 g, 0.11
mol) and
1 mL of acetic acid in 250 mL of water was heated at reflux for 4 h. Cooled to
room temperature, 400 mL of sat. NaHCO3 solution was added and extracted with
ethyl acetate. The organic layer was washed with brine, dried over MgSO4, and
concentrated to give 3.5 g of crude as a dark brown solid. To a solution of
this
crude in conc. HCI at -10 C was added a solution of NaNO2 dropwise. The
mixture
was stirred at this temperature for 2 h and then a solution tin chloride in
conc. HCI
was added slowly to keep the temperature under -5 C. The reaction was stirred
overnight while allowing to warm up to RT. NaOH solution was added to adjust
pH to 9 and filtered through a pad of Celite . The aqueous phase was extracted
with THF and the organic layer was washed with brine, dried over MgSO4, and
concentrated. The crude was triturated with methanol to give the hydrazine as
a
yellow solid. The NMR and MS were consistent with the proposed structure.
[00197] Steps 3-6
0S~ N
\ I
H N-N
O N \ \ CONH2
CI I /
The title compound was synthesized by using the same procedure from step 3 to
step 6 for Example 367 except using the above hydrazine; 1H NMR (DMSO, 400
MHz) 10.38 (s,1H), 9.08 (s, 1H), , IH), 8.41 (d, 1H), 8.25 (d, 1H), 7.71 (s,
1H),
7.35-7.53 (m, 8H), 3.28 (s, 3H), 2.96 (m, 4H); Anal. Calcd. for C25H2OCIN5O4S:
C,
57.53; H, 3.86; N, 13.42; S, 6.14. Found: C, 56.62; H 4.09; N, 13.09; S, 5.99.
IKK-2 resin IC50 < 1 M.
175
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
[00198] Example 369
8-[(2-chlorobenzoyl)amino]-1-(4-cyanophenyl)-4,5-dihydro-lH-benzo[g]indazole-
3-carboxamide
NC~n
T'~~i N-N
0 \ NH2
. HN
CI O
~
N~ ~
1-(4-Brornophenyl)-8-[(2-chlorobenzoyl)amino]-4,5-dihydro-lH-benzo[g]indazole-
3-carboxamide (5.2g) and Zn(CN)2 (0.9g) were dissolved in 100m1 DMF under NZ.
Then Pd(Ph3P)4 (1.38g) was added. The reaction mixture was heated up to 100 C
under NZ for 12 hours. After the reaction is completed by HPLC, the solvent
was
evaporated, and the residue was suspended in ethyl acetate and water. After
filtration, and washing with water and ethyl acetate, the filtrate of the
organic layer
was separated and dried with NazSO4. After filtration and evaporation of
solvent, the
residue was triturated with ether. Solid obtained was filtered and washed with
ether,
then dried under vacuum. The desired compound (3.4g) was obtained and
characterized by 'H NMR, LC-MS (468, M+1), and CHN analysis. IKK-2 resin
IC50 < 1 M.
[00199] The compounds of Examples 370-380 in the Table 15 were prepared
by the reduction and/or acylation or sulfonation from either Example 324 or
Example 325 using standard conditions. The bioactivity in the IKK2 Resin assay
for the compounds of Examples 370-380 is shown in Table 15.
176
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Table 15
Compound No., Structure Mol. Compound Name(s) IKK2 MS Example
Wt. Resin (M+H)
IC50
H2N 457.92 1-(4-aminophenyl)-8-[(2- <1 M 458 370
/ chlorobenzoyl)amino]-4,5-
I H N-N dihydro-lH-benzo[g]indazole-
i N \ ~ CONHz
ol o ~ 3-carboxamide
499.96 1-[4-(acetylamino)phenyl]-8- <1 pM 500 371
[(2-chlorobenzoyl)amino]-4,5-
N-N dihydro-lH-benzo[g]indazole-
( N ~ CONH6 3-carboxaniide
ci 0
CH,S y 536.01 8-[(2-chlorobenzoyl)amino]-1- <1 M 536 372
o N {4-
/ [(methylsulfonyl)amino]phenyl
H N-N }-4,5-dihydro-lH-
f, N ooN", benzo[g]indazole-3-
ci o carboxamide
~ 562.03 1-[4-(benzoylamino)phenyl]-8- 1< 10 562 373
~ [(2-chlorobenzoyl)amino]-4,5- M
o dihydro-lH-benzo[g]indazole-
N N 3 carboxamide
P-r N CONHx
CI 0 /
o~N 529.99 8- (2-chlorobenzoyl)amino]-1- <1 M 530 374
{
N_N [(methoxyacetyl)amino]phenyl
~N ooN~ }-4,5-dihydro-lH-
Cl 0 ~ benzo[g]indazole-3-
carboxamide
NHz 457.92 1-(3-aminophenyl)-8-[(2- <1 M 458 375
chlorobenzoyl)an-uno]-4,5-
~ N-N dihydro-lH-benzo[g]indazole-
~ r, oONHs 3-carboxaniide
oi c
Q-Y 596.48 8-[(2-chlorobenzoyl)amino]-1- 1< 10 596 376
r, ~ {4-[(2-
ci 0 chlorobenzoyl)amino]phenylQ }- uM
H N-N 4,5-dihydro-lH-
N ~ CONHi
ol 0 ~ benzo[g]indazole-3-
carboxamide
507.98 8-[(2-chlorobenzoyl)amino]-1- 1< 10 508 377
N [4-(1 H-pyrrol-1-yl)phenyl]-
~ / M
4,5-dihydro-1 H-
I " N " benzo[g]indazole-3-
/ N t CONHz
a 0 1 carboxaniide
177
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Compound No., Structure Mol. Compound Name(s) IKK2 MS Example
Wt. Resin (M+H)
IC50
606.09 1-(4- 1< 10 606 378
o~o {[(benzyloxy)acetyl]amino}ph
HN M
enyl)-8-[(2-
I ~ H \ / N-N chlorobenzoyl)amino]-4,5-
~N ~ ~ ooN~ dihydro-lH-benzo[g]indazole-
01 o I ~ 3-carboxamide
657.18 8-[(2-chlorobenzoyl)amino]-1- 1< 10 657 379
{4-[(5-{[(2,2-
H-r;o dimethylpropanoyl)oxy]amino} M
pentanoyl)amino]phenyl}-4,5-
dihydro-1 H-benzo [ g] indazole-
HN o 3-carboxamide
\ ~l
N-N
N CONHz
OI 0
0 593.52 8-[(2-chlorobenzoyl)amino]-1- 1< 10 557 380
H [4-({ [2-
~ N-N (dimethylamino)ethyl]amino}c M
0 arbonyl)phenyl]-4,5-dihydro-
oi 1H-benzo[g]indazole-3-
carboxamide hydrochloride
HCI
[00200] Example 381
1-(6-aminopyridin-3-yl)-8-[(3-chloroisonicotinoyl)amino]-4,5-dihydro-lH-
benzo[g]indazole-3-carboxamide
H2N N
N-N
O N I \ \
CONH2
CI / /
~ I
N
[00201] Step 1: A 100 mL 3-neck flask was charged with (in order) Cul (300
mg, 1.6 mmol), 1,10-phenanthroline (350 mg, 1.94 mmol) and 25 mL DMF. A
dark cherry red solution resulted. To this solution was added (in order) 2-
Chloro-5-
iodopyridine (2.0 g, 8.4 mmol), t-butyl carbamate (1.33 g, 10.1 mmol), 25 mI,
DMF
178
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
and Cs2CO3 (4.75 g, 14.6 mmol). To the flask was attached a reflux condenser
with
a nitrogen inlet, a thermometer and a glass stopper. With stirring the slurry
was
heated to 70 C for 3 h. The reaction was allowed to cool to room temperature.
The
crude reaction mixture was poured into 200 mL water giving a rust colored
solid.
The product was extracted from this aqueous slurry using 2 x 200 mL diethyl
ether.
The ether layers were extracted with 200 mL water, dried over MgSO4, filtered,
and
then concentrated giving a dark oil. The oil was chromatographed on silica gel
(25g) using 20% EtOAc/80% hexane giving 0.9 g (3.7 mmol, 44%) of product
(light yellow oil which slowly solidified). NMR and MS were consistent with
the
proposed structure.
[00202] Step 2: To a mixture of the product from step 1(6.35 g, 0.023mo1)
and ethyl (7-nitro-l-oxo-1,2,3,4-tetrahydronaphthalen-2-yl)(oxo)acetate (6.6
g,
0.023 mol) in 200 mL of ethanol was added 10 mL of 1N HCI and the reaction
mixture was heated at reflux under nitrogen for 6 h. After the solution was
cooled,
the precipitate was collected by filtration and air-dried to give 7.5 g of the
product
as a yellow crystal (83% yield); IHNMR (DMSO, 400 MHz) S: 8.76 (dd, 1H), 8.22
(dd, 1H), 8.12 (dd, 1H), 7.85 (dd, 1H), 7.71 (d, 1H), 7.44 (d, 1H), 4.35 (q,
2H), 3.14
(m, 2H), 3.03 (m, 2H), 1.33 (t, 3H); Anal. Calcd. for C19H15CIN404: C, 57.22;
H,
3.79; N, 14.05; Cl, 8.89. Found: C, 57.03; H, 3.95; N, 13.71; Cl, 9.04.
[00203] Step 3: The product from step 2(7.5 g, 0.019 mol) was
hydrogenated in a Parr shaker with 5% Pt/C in acetic acid for 2 h at 5 psi.
After the
removal of solvent, the residue was triturated with a mixture of methanol and
ether
to give 6.5 g of the product as a pale yellow solid (94% yield): 1HNMR (DMSO,
400 MHz) S: 8.64(dd, 1H), 8.09 (dd, 1H), 7.75 (dd, 1H), 7.02 (d, 1H), 6.45
(dd,
1H), 6.03 (d, 1H), 4.99 (brs, 2H), 4.31 (q, 2H), 2.88 (m, 2H), 2.78 (m, 2H),
1.31 (t,
3H); Anal. Calcd. for C19H17C1N402: C, 61.88; H, 4.65; N, 15.19; Cl, 9.61.
Found:
C, 60.97; H, 5.06; N, 14.65; Cl, 9.50.
[00204] Step 4: To a mixture of the product from step 3(0.96 g, 0.0026 mol)
and 3-chloroisonicotinic cid (0.65 g, 0.004 mol) in 25 mL of DMF was added 1
1mI,
179
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
of diisopropylethylamine, followed by the addition of HATU (1.50 g, 0.004
mol).
The reaction was stirred at room temperature for 16 h and concentrated. The
residue was triturated with methanol and acetonitrile to give 1.08 g of
product as a
pale yellow solid (82% yield); IHNMR (DMSO, 400 MHz) S: 10.56 (s, IH), 8.76
(s, 1H), 8.69 (d, 1H), 8.63 (d, 1H), 8.14 (dd, 1H), 7.76 (d, 1H), 7.56 (d,
1H), 7.53
(dd, 1H), 7.40 (d, 1H), 7.26 (d, 1H), 4.32 (q, 2H), 2.97 (s, 4H), 1.31 (t,
3H).
[00205] Step 5: A sealed reaction vessel containing the product from step 4
(0.8 g, 0.0016 mol) and 10 mL of liquid ammonia in 50 mL of absolute alcohol
was
heated at 120 C and 600 psi for 24 h. After cooling, solvent was removed and
the
residue was triturated with a niixture of methanol and acetonitrile to give
0.38 g of
product as a pale yellow solid (52% yield);1HNMR (DMSO, 400 MHz) 8: 10.57 (s,
1H), 8.75 (s, 1H), 8.63 (d, 1H), 8.02 (d, 1H), 7.47-7.56 (m, 3H), 7.35 (d,
1H), 7.32
(d, 1H), 7.27 (s, 1H), 6.54 (d, 1H), 6.36 (s, 2H), 2.92 (m, 4H); Anal. Calcd.
for
C23H18C1N702: C, 60.07; H, 3.95; N, 21.32. Found: C, 59.26; H, 3.99; N, 20.85.
[00206] Example 382
8- [(3-chloroisonicotinoyl)amino]-1-thien-2-yl-4,5-dihydro-1 H-
benzo[g]indazole-3-
carboxamide
S N-N
O N CONH2
CI
N
~ooxo~~ Ste_p 1: To a mixture of tert-butyl 1-thien-2-ylhydrazinecarboxylate
(3.9 g, 0.016 mol, synthesized by using the same method as the previous
example)
and ethyl (7-nitro-l-oxo-1,2,3,4-tetrahydronaphthalen-2-yl)(oxo)acetate (4.6
g,
0.016 mol) in 100 mL of ethanol was added 5 mL of 1N HCl and the reaction
mixture was heated at reflux under nitrogen for 6 h. After the solution was
cooled,
the precipitate was collected by filtration and air-dried to give 2.6 g of the
product
180
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
as a brown solid (44% yield). This solid was refluxed with 3eq of tin chloride
in
ethanol under nitrogen for 3 h. Solvent was removed and the residue was
partitioned between THF and sat. NaHCO3 solution. Organic layer was washed
with brine, dried over MgSO4, and concentrated to give 1.4 g of crude as a
yellow
solid, used without further purification. The MS and NMR were consistent with
the
proposed structure.
[00208] Step 2: A sealed reaction vessel containing the crude product from
step 1(1.3 g, 0.004 mol) and 10 mL of liquid ammonia in 50 mL of absolute
alcohol was heated at 120 C and 600 psi for 24 h. After cooling, solvent was
removed and the residue was triturated with a mixture of methanol and
acetonitrile
to give 1.0 g of product as a pale yellow solid. To a mixture of this solid
(0.56 g,
0.0018 mol) and 3-chloroisonicotinic cid (0.39 g, 0.0027 mol) in 20 mL of DMF
was added 1 mL of diisopropylethylamine, followed by the addition of HATU
(1.03
g, 0.0027 mol). The reaction was stirred at room temperature for 16 h and
concentrated. The residue was triturated with methanol and water to give 0.31
g of
product as pale yellow solid (38% yield); 1HNMR (DMSO, 400 MHz) S: 10.55 (s,
IH), 8.75 (s, IH), 8.62 (d, 1H), 7.67 (d, 1H), 7.62 (s, 1H), 7.55 (d, 1H),
7.44 (s,
3H), 7.42 (s, 1H), 7.35 (m, 3H), 7.12 (m, 1H), 2.93 (m, 4H); Anal. Calcd. for
C22H16C1N502S: C, 58.73; H, 3.58; N, 15.57. Found: C, 58.26; H, 3.62; N,
15.48.
[00209] Example 383
8-[(3-chloroisonicotinoyl)amino]-1-thien-3-yl-4,5-dihydro-lH-benzo[g]indazole-
3-
carboxamide
sa__ N-N
O N
CONH2
cl
N
181
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
This compound was synthesized by using the same method as previously described
in Example 381. 1HNMR (DMSO, 400 MHz) 8: 10.54 (s, 1H), 8.75 (s, 1H), 8.62 (d,
1H), 7.85 (dd, 1H), 7.75 (dd, 1H), 7.58 (s, 1H), 7.54 (d, 1H), 7.38 (m, 3H),
7.31 (s,
1H), 7.28 (dd, 1H), 2.92 (m, 4H); Anal. Calcd. for C22HI6C1N502S: C, 58.73; H,
3.58; N, 15.57. Found: C, 58.65; H, 3.77; N, 15.59.
[00210] Example 384
1-(4-amino-3,5-difluorophenyl)-8-[(3-chloroisonicotinoyl)amino]-4,5-dihydro-1
H-
benzo [g] indazole-3-carboxamide
H2N F
F O
N-N
N CONH2
CI
N
[00211] Step 1: 1-Bromo- 3,4,5 trifluorobenzene (5 g, 24 mmol) was added
to a slurry of Pd(OAc)2 (0.269 g , 5 mol %), bisdiphenylphosphino-ferrocene
(1.0 g,
7 mol %) in anhydrous toluene (50 mL) at rt. Benzophenone hydrazone (4.9 g)
was
added, stirred for 5 min following by addition of dried cesium acetate (9.33
g) and
toluene (40 mL). The flask was removed from a glove box and heated to 86 C
for
72 hours. The reaction was monitored by disappearance of
bromotrifluourobenzene
by LC (210 nm) or 19 F NMR. The reaction mixture was cooled down to room
temperature and filtered through a sintered glass funnel. The solvent was
removed
under the vacuum. The orange solid residue was re-dispersed in ether (15 mL)
and
hexane (150 mL) and heated up to 58 C, stirred for 20 min. The hot solution
was
quickly filtered, solid discarded and the solution was allowed to cool to room
temperature, stirred for 30 min, then 1 hour at 4 C. The formed slurry was
filtered,
washed with cold hexane (2x25 mL). Crystals were dried in air then in the
vacuum
182
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
at 80 C for 1 hour to give 5 g of diphenylmethanone (3,4,5-
trifluorophenyl)hydrazone (64 % yield) as a yellowish solid.
[00212] Step 2: The product from step 1(1.9 g) and ethyl (7-nitro-l-oxo-
1,2,3,4-tetrahydronaphthalen-2-yl)(oxo)acetate (1.66 g) were dispersed in 1M
HC1
in ethanol (125 mL), heated to reflux, and stirred until the starting material
disappeared (overnight). The solution was cooled down to 4 C and stirred for 2
hrs.
The cold slurry was filtered, solid washed with anhydrous ethanol (2x25 mL),
dried
on air and in the vacuum oven at 70 C for 1 hour to give 1.4 g of 8-nitro-1-
(3,4,5-
trifluorophenyl)-4,5-dihydro-lH-benzo[g]indazole-3-carboxamide as a brown
powder (58 % yield).
[00213] Step 3: The product from step 2(1.17 g, 0.0028mo1) was
hydrogenated in a Parr shaker with 5% Pt/C in acetic acid for 4 h at 5 psi.
After the
removal of solvent, the residue was triturated with a mixture of methanol and
ether
to give 1.0 g of the product as a pale yellow solid. A sealed reaction vessel
containing this solid and 10 mL of liquid ammonia in 50 mL of absolute alcohol
was heated at 120 C and 600 psi for 24 h. After cooling, solvent was removed
and
the residue was triturated with a mixture of methanol and acetonitrile to give
0.8 g
of 8-amino-l-(4-amino-3,5-difluorophenyl)-4,5-dihydro-lH-benzo[g]indazole-3-
carboxamide as a pale yellow solid (80% yield over two steps); NMR spectrum
was
consistent with the proposed structure.
[00214] Step 4: To a mixture of the product from step 3(0.75 g, 0.0021 mol)
and 3-chloroisonicotinic cid (0.33 g, 0.0023 mol) in 20 mL of DMF was added 1
mL of diisopropylethylamine, followed by the addition of HATU (0.9 g, 0.0023
mol). The reaction was stirred at room temperature for 16 h and concentrated.
The
crude was purified by reverse phase HPLC to give 0.15 g of 1-(4-amino-3,5-
difluorophenyl)-8-[(3-chloroisonicotinoyl)amino]-4,5-dihydro-lH-
benzo[g]indazole-3-carboxamide as a pale white solid (15% yield); 1HNMR
(DMSO, 400 MHz) S: 10.56 (s, 1H), 8.76 (s, 1H), 8.63 (d, 1H), 8.56 (s, 1H),
7.54
(d, 1H), 7.49 (dd, 1H), 7.35 (m, 2H), 7.31 (s, 1H), 6.71 (m, 2H), 5.88 (s,
2H), 2.92
183
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
(m, 4H); Anal. Calcd. for Ca4H17FC1N602 + 0.5 H20: C, 57.21; H, 3.60; N,
16.68.
Found: C, 56.91; H, 3.70; N, 16.64.
[00215] Example 385
1-(4-amino-2,5-difluorophenyl)-8-[(3-chloroisonicotinoyl)amino]-4,5-dihydro-1
H-
benzo [g] indazole-3-carboxamide
H2N
F F
N N-N
O I \ \
CONH2
CI
N
[00216] This compound was synthesized by using the same method as
Example 384; mp: 289-290 C; 1HNMR (DMSO, 400 MHz) S: 10.55 (s, 1H), 8.75
(s, 1H), 8.63 (d, 1H), 7.57 (d, 1H), 7.55 (s, 1H), 7.39 (m, 4H), 7.29 (s, 1H),
6.70 (m,
1H), 5.90 (brs, 211), 2.89(s, 4H); Anal. Calcd. for CZ4H17FC1N602: C, 58.25;
H,
3.46; N, 16.98. Found: C, 57.65; H, 3.73; N, 16.82.
[00217] Additional analytical data is presented in Table 16 for the
compounds of Examples 381-385.
Table 16
Compound No., Structure Mol. Compound Name(s) IKK2 MS Example
Wt. Resin (M+H)
IC50
HzN N~ 459.90 1-(6-aminopyridin-3-yl)-8- <l M 460 381
I [(3-
o r", /~ N chloroisonicotinoyl)amino]-
~ 1 H2 4,5-dihydro-lH-
ci
benzo[g]indazole-3-
N carboxamide
184
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Compound No., Structure Mol. Compound Name(s) IKK2 MS Example
Wt. Resin (M+H)
IC50 449.92 8-[(3- <1 M 450 382
H s N-N chloroisonicotinoyl)amino]-
o N r, ~ NH6 1-thien-2-yl-4,5-dihydro-lH-
ci benzo[g]indazole-3-
N carboxamide
s 449.92 8-[(3- <1 M 450 383
~ N-N chloroisonicotinoyl)amino]-
o rv " NHZ 1-thien-3-yl-4,5-dihydro-1H-
cl o benzo[g]indazole-3-
~ N carboxamide
F 494.89 1-(4-amino-3,5- <1 M 495 384
H" )J~ difluorophenyl)-8-[(3-
F ~ N.-N chloroisonicotinoyl)amino]-
o r", NHz 4,5-dihydro-lH-
cl~ benzo[g]indazole-3-
~ carboxamide
N
H." 494.89 1-(4-amino-2,5- <1 M 495 385
F F difluorophenyl)-8-[(3-
chIoroisonicotinoyl)amino]-
N-N
NHz 4,5-dihydro-lH-
' benzo[g]indazole-3-
carboxamide
[00218] Example 386
8-{ [(2-chloropyridin-3-yl)carbonyl]amino}-1-[4-(3-hydroxyprop-1-ynyl)phenyl]-
4,5-dihydro-lH-benzo[g]indazole-3-carboxamide
HO
N-N
O N O
CI ~ I NHZ
~
N ~
The following scheme was used for the synthesis of the title compound of
Example
386.
185
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
SCHEME XXVIII
Br
0 TBSO
N-N Pd(PPh3)4
H2N CONH2 CuI-DMF O CI
TBSO HzN N- CI
CO
NH2
8-amino-l-(4-bromop henyl)
-4,5-dihydro-1 H-benzo[g]indazole-
<X~
3-carboxamide Pyr
TBSO \\\ NO N
kll / TBAF ~ /
H N-N H N-N
N~. CONH2 40% N/ ~ CONH2
CI 3 steps cl I
N'~ N, I
Example 386
A stirred solution of 8-amino-l-(4-bromophenyl)-4,5-dihydro-lH-
benzo[g]indazole-3-carboxamide (2.30 g, 6 mmol) and tert-butyldimethyl-(2-
propynl-oxy)silane (1.12 g, 6.6 mmol) in DMF-triethylamine (6 mL-6 mL) was
added CuI (114 mg, 0.6 mmol) and tetrakis(triphenylphosphine) palladium (346
mg,
0.3 mmol) and the resulted mixture was heated at 100 C for 14 h before cooled
to
rt. The mixture was filtered through silica gel pad, washed with EtOAc, and
concentrated. The crude material was taken into pyridine (20 mL), treated 2-
chloronicotinyl chloride (1.23g, 7 mmol) at RT for 14 h. Tetrabutylammonium
fluoride (25 mL of 1M THF solution, 25 mmol) was added at RT and stirred
overnight. Aqueous ammonium chloride was added, the mixture extracted with
EtOAc (5x30 mL). The organic portions were combined, dried over MgSO4,
filtered, and separated by silica gel column (EtOAc). This gave 8-{ [(2-
chloropyridin-3-yl)carbonyl]amino }-1-[4-(3-hydroxyprop-1-ynyl)phenyl]-4,5-
dihydro-lH-benzo[g]indazole-3-carboxamide as a pale yellow solid (1.19g, 40%
over 3 steps). 1H NMR was consistent with its structure.
[00219] Example 387
8- { [(2-chloropyridin-3-yl)carbonyl]amino 1-1-[4-(5-hydroxypent-l-
ynyl)phenyl]-
4,5-dihydro- l H-benzo [g] indazole-3-carboxamide
186
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
HO /~
N-N
N
CI NHz
N,
/
The compound was prepared in a similar manner as Example 386. 1H NMR was
consistent with its structure.
[00220] Example 388
8-{ [(2-chloropyridin-3-yl)carbonyl] amino } -1-(4-ethynylphenyl)-4,5-dihydro-
lH-
benzo [g] indazole-3 -c arboxamide
N-N
o N \ t O
/
CI NH2
A stirred solution of 8-amino-l-(4-bromophenyl)-4,5-dihydro-lH-
benzo[g]indazole-3-carboxamide (5.74 g, 15 mmol) and tert-
butyldimethylsilylacetylene (2.31 g, 16.5 mmol) in DMF-triethylamine (20 mL-10
mL) was added CuI (285 mg, 1.5 mmol) and tetrakis(triphenylphosphine)
palladium
(870 mg, 0.75 mmol) and the resulted mixture was heated at 100 C for 14 h
before
cooled to rt. The mixture was filtered through silica gel pad, washed with
EtOAc,
and concentrated. The crude material was taken into pyridine (30 mL), treated
with
2-chloronicotinyl chloride (2.90 g, 16.5 mmol) at RT for 14 h.
Tetrabutylammonium fluoride (25 mL of 1M THF solution, 25 mmol) was added at
RT and stirred overnight. Aqueous ammonium chloride was added, the mixture
extracted with EtOAc (5x30 mL). The organic portions were combined, dried over
MgSO4, filtered, and separated by silica gel column (EtOAc). This gave product
as
187
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
a pale yellow solid (3.5 g, 49% over 3 steps). 1H NMR was consistent with its
structure. CNH calculated for C26H18N502C1(H20)1.3: C(63.5%), H(4.2%),
N(14.3%); found: C(63.6%), H(4.0%), N(14.3%).
[00221] Example 389
8- { [(2-chloropyridin-3-yl)carbonyl]amino }-1-(4-vinylphenyl)-4,5-dihydro-1 H-
benzo[g]indazole-3-carboxamide
N-N
O N / ~ I
O
CI NHZ
N ~
A solution of 8-{ [(2-chloropyridin-3-yl)carbonyl]amino}-1-(4-ethynylphenyl)-
4,5-
dihydro-lH-benzo[g]indazole-3-carboxamide (557 mg, 1.19 mmol) in DMF-DMSO
(20 mL - 1 mL) was treated at RT with H2 (5 psi) and Pd-CaSO4 (5%, 100 mg) for
12 min. The mixture was filtered through celite pad, concentrated and added
water.
The solid product was collected via filtration, washed with water and ether,
and
dried to give product (262 mg, 47%). 1H NMR was consistent with its structure.
[00222] Example 390
1-(4-acetylphenyl)-8-{ [(2-chloropyridin-3-yl)carbonyl] amino } -4,5-dihydro-1
H-
benzo[g]indazole-3-carboxamide
O
/ ~
~
N-N
O O
Cl NHZ
/ ~
N ~
188
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
A mixture of 8-{[(2-chloropyridin-3-yl)carbonyl]amino}-1-(4-ethynylphenyl)-4,5-
dihydro-lH-benzo[g]indazole-3-carboxamide (442 mg, 1.2 mmol), water (65 mg,
3.6 mmol), and triflic acid (270 mg, 1.8 mmol) in dioxane (10 mL) was heated
to
100 C for 18 h. The mixture was cooled to RT, aqueous NaHCO3 was added, and
filtered. The product was washed with water and ether, and dried. This gave
product (293 mg, 67%). 1H NMR was consistent with its structure.
[00223] Example 391
8- { [(2-chloropyridin-3-yl)carbonyl]amino }-1-[4-(1-hydroxyethyl)phenyl]-4,5-
dihydro-lH-benzo[g]indazole-3-carboxamide
OH
N-N
O N 0[~ N
0
CI NHZ
1 ~
N
A mixture of 1-(4-acetylphenyl)-8-{ [(2-chloropyridin-3-yl)carbonyl]amino}-4,5-
dihydro-lH-benzo[g]indazole-3-carboxamide (200 mg, 0.41 mmol) in MeOH
(3 mL) and water (0.3 mL) was added NaBH4 (10 mg, 0.25 mmol) at RT and stirred
for 14 h. The mixture was separated on silica gel column (EtOAc) to give
product
(100 mg, 50%). 'H NMR was consistent with its structure.
[00224] Additional analytical data for the compounds of Examples 386-391
is presented in Table 17
189
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Table 17.
Compound No., Structure Mol. Compound Name(s) IKK2 MS Example
Wt. Resin (M+H)
IC50
Ho ~ 497.94 8-{ [(2-chloropyridin-3- 51 M 498 386
yl)carbonyl]amino}-1-[4-(3-
hydroxyprop-1-ynyl)phenyl]-
"-" 4,5-dihydro-lH-
o, o o benzo[g]indazole-3-
~ ~ N~4
N ~ carboxamide
525.99 8-{ [(2-chloropyridin-3- <1 pM 526 387
-
Ho yl)carbonyl] amino }- 1-[4-(5
~ hydroxypent-1-ynyl)phenyl]-
H 4,5-dihydro-lH-
"-"
o " N~ benzo[g]indazole-3-
01 -- carboxamide
N~
~ 467.91 8-{[(2-chloropyridin-3- 1< 10 468 388
y1)carbonyl]amino}-1-(4-
/ N-N ethynylphenyl)-4,5-dihydro- M
o 0 1H-benzo[g]indazole-3-
cl NHz carboxamide
~
N/~
469.93 8-{[(2-chloropyridin-3- <1 gM 470 389
yl)carbonyl]amino}-1-(4-
vinylphenyl)-4,5-dihydro-lH-
"-" benzo[g]indazole-3-
H CI O N~ ~ \ N~ carboxamide
N ~
0 485.93 1-(4-acetylphenyl)-8-{ [(2- 1< 10 486 390
chloropyridin-3-
yl)carbonyl]amino}-4,5- pM
N-N
o o dihydro-lH-benzo[g]indazole-
cl NHZ 3-carboxanude
w ~
OH 487.95 8-{ [(2-chloropyridin-3- <1 M 488 391
yl)carbonyl]amino}-1-[4-(1-
hydroxyethyl)phenyl]-4,5-
N ~" o dihydro-lH-benzo[g]indazole-
ol NHz 3-carboxamide
N~
[00225] Example 392
8-{ [5-(acetylamino)-2-chlorobenzoyl] amino }-1-[4-(ethylsulfonyl)phenyl]-4,5-
dihydro-1 H-benzo[g]indazole-3-carboxamide
190
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
n 00
NH
N /\N NH2
O
CI O \
[00226] Step 1
F
S
To a stirring solution of NaOH (11.26 g, 281.6 mmol) in water (500 mL) was
added
dropwise a solution of 4-fluorothiophenol (25 mL, 234.6 mmol) in methanol (50
mL). After 15 minutes ethyl bromide (14.4 mL, 281.6 mmol) was added. After 6
hours more NaOH (1 g) was added and the reaction mixture was extracted with
ether (3 X 300 mL). The combined organic extracts were treated with brine
followed by MgSO4 then concentrated down to give 1-(ethylthio)-4-fluorobenzene
(29 g, 80%) as a slightly yellow colored liquid.
[00227] Step 2
~ F
O~ (/
0= ~
To a solution of the crude product of step 1 in CH2Cl2 (500 mL) was added
m-CPBA (82 g of 77% max powder, 368 mmol) portion-wise with vigorous
stirring. After 5 hours the reaction mixture was concentrated down and ethyl
acetate (750 mL) was added. The organic phase was then washed with 4% aqueous
NaOH (2 X 100 mL), water (100 mL), then brine (75 mL), and finally dried over
191
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
MgSO4. The solution was concentrated down to yield a white solid (17.9 g) that
was carried onto the next step without purification.
[00228] Step 3
NHNH2
0 '
0=S
\
The crude product of step 2 was dissolved in ethanol (200 mL) and hydrazine
(24
mL, 758 mmol) was added. The reaction mixture was heated to reflux for 6 hours
then left at room temperature overnight. The ethanol was concentrated down to
a
smaller volume then water was added. A white precipitate formed and was
collected (12.87 g, 68%). The desired compound (1-[4-
(ethylsulfonyl)phenyl]hydrazine) was used in the next step without
purification.
[00229] Step 4
~ ~ ,o
NH
~N NH2 CI O
o-Ir N
O
The title compound was prepared in a manner analogous to Example 3 using 1-[4-
(ethylsulfonyl)phenyl]hydrazine and the appropriate acylating agent. The
desired
product crystallizes out of the reaction media in 81% yield. Anal. Calcd for
C29H26C1NSO5S (MW = 591.13): C, 58.83; H, 4.43; N, 11.83. Found: C, 58.69;
H, 4.46; N, 12.16.
[00230] Example 393
8-[(2-chlorobenzoyl)amino]-1-[4-(isopropylsulfonyl)phenyl]-4,5-dihydro-lH-
benzo [g] indazole-3-carboxamide
192
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
?O
N ~N NHz
O
cl O
[00231] Step 1
O ~ F
~ /
0=S
To a 250 mL round-bottomed flask was placed KH (35 wt % in mineral oil) (1.64
g,
14.35 mmol). The solid was washed with hexane (2 X 10 mL) under nitrogen.
THF (50 mL) was added and the suspension was cooled to 0 C. lodomethane (0.89
mL, 14.35 mmol) followed by a solution of 4-fluorophenylmethylsulfone (1.25 g,
7.17 mmol) in THF (10 mL) was added. The reaction mixture was stirred at 0 C
for 1 hour then allowed to warm to room temperature overnight. A mixture of
ethyl
and isopropyl sulfone was observed, so LiHMDS (7.2 mL, 14.35 mmol) and
iodomethane (0.45 mL, 14.35 mmol) were added. After 2.5 hours water was added
to the reaction mixture and the aqueous phase was extracted with ether (3 X
150
mL). The combined organic extracts were treated with brine and dried over
MgSO4. The ether solution was concentrated down to give the desired compound
as a yellow solid. The crude material was used in the nest step without
further
purification.
[00232] Step 2
N, NHNH2
0=S
193
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
The product of step 1 (640 mg, 3.15 mmol) was dissolved in absolute ethanol
(12
mL), the system was flushed with N2 and hydrazine ( 404 mg, 12.6 mmol) was
added and the reaction refluxed overnight. HPLC showed 78% product and 21%
starting material. To drive the reaction to completion 2 equivalents of
additional
hydrazine was added and reaction was refluxed for additional 5 hours, at this
time
HPLC indicated 95% product. The reaction mixture was concentrated and the
residue was stirred with water. A white solid, 390 mg (58%) was isolated. HPLC
indicated 86% product and 14% starting material. The aqueous phase was
extracted
with ethyl acetate (3 x 50 mL). The organic phase was dried over MgSO4, and
concentrated to yield 258 mg (38%) of 1-[4-(isopropylsulfonyl)phenyl]hydrazine
with 99% purity. MH+ = 215. The 390 mg was re-dissolved in 10 mL ethanol and
treated once more with additional hydrazine to obtain additional product with
the
desired purity.
[00233] Step 3
00
\/
I N ~N NH2
CI O ~ I O
~ I O
The title compound was prepared in a manner analogous to Example 3 using 1-[4-
(isopropylsulfonyl)phenyl]hydrazine and the appropriate acylating agent. The
desired compound was recovered in 86% yield. HPLC indicated the compound had
94% purity. Anal. Calcd for C28H25C1N404S +.1 H20 (MW = 550.05): C, 61.05; H,
4.61; N, 10.17. Found: C, 60.67; H, 4.44; N, 10.14.
[00234] Example 394
1-{ 4-[(3-aminopropyl)sulfonyl]phenyl } -8-{ [(2-chloropyridin-3-
yl)carbonyl]amino } -
4,5-dihydro-lH-benzo[g]indazole-3-carboxamide hydrochloride
194
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
H2N
O!O
H-CI
0/, N H2
/ I
N~ N ~
C! O ~ I O
[00235] Stgp 1
Br"v~NHBoc
Commercially available 3-bromopropylamino hydrogen bromide (10 g, 45.7 mmol)
was suspended in CH2C12 (125 mL). Triethylamine (10 mL, 98 mmol) was added
followed by (Boc)20 (11g, 50 mmol) as a solid. After stirring overnight at
room
temperature the reaction mixture was diluted with CH2Cl2 (100 mL). The organic
phase was washed with 1M HCl (100 mL), sat. aq. NaHCO3 (50 mL), and brine (50
mL) then dried over MgS04. Evaporation under reduced pressure yielded the
desired compound as a slightly yellow liquid (10.55g, 97%) and no purification
was
necessary.
[00236] Ste p 2
F
S
BocHN
To a stirring solution of NaOH (624 mg, 15.6 mmol) in water (20 mL) was added
drop-wise a solution of 4-fluorobenzenethiol (2 g, 15.6 mmol) in methanol (5
mL)
at room temperature. After 30 minutes a solution of the product of step 2
(3.71 g,
15.6 mmol) in methanol (5 mL) was added drop-wise and the reaction mixture was
allowed to stir at room temperature overnight. Ether (450 mL) was added and
the
aqueous layer separated. The organic phase was then washed successively with
1N
195
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
NaOH (75 mL), conc. aq. NH4C1 (75 mL), and brine (50 mL). The solution was
dried over MgSO4 and concentrated down to give the desired compound as a
colorless liquid (3.2 g, 72%). The compound was used in the next step without
further purification.
[00237] Step 3
~ F
O ~ ,
0=
BocHN
To a stirring solution of the product of step 2 (3.2 g, 11.2 mmol) in CH2C12
(150
mL) was added m-CPBA (77% max powder) (9.56 g, mmol) portion-wise at room
temperature. The reaction was left overnight then it was concentrated down and
ethyl acetate (750 mL) was added. The organic phase was then washed with 4%
aqueous NaOH (2 X 100 mL), water (100 mL), then brine (75 mL), and finally
dried over MgSO4. The solution was concentrated down to yield a white solid
that
was carried onto the next step without purification.
[00238] Step 4
~ NHNH2
~ /
0=S
BocHN
[00239] All of the crude material from step 3 was dissolved in ethanol (30
mL) and hydrazine (2.4 mL, 75 mmol) was added. The reaction mixture was
refluxed overnight then concentrated down to a volume of 5 mL and added to
water.
The resulting precipitate was collected to yield tert-butyl 3-[(4-
196
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
hydrazinophenyl)sulfonyl]propylcarbamate as a white solid (1.88 g, 51%). The
compound was used in the next step without further purification.
[00240] Step 5
H2N
0
IWO
H-CI
/ I
N-N
N y N NH2
CI 0 ~ I O
The title compound was prepared in a manner analogous to Example 3 using tert-
butyl 3-[(4-hydrazinophenyl)sulfonyl]propylcarbamate and the appropriate
acylating
agent. The title compound was isolated as a tan colored HCl salt following
standard
Boc deprotection using 4N HCI in dioxane.
[00241] The compounds of Examples 395-409 in Table 18 were prepared in a
manner analogous to Examples 392, 393, and 394 using the appropriate hydrazine
and acylating agent.
[00242] The bioactivity in the IKK2 Resin assay for the compounds of
Examples 392-409 is shown in Table 18.
Table 18
Compound No., Structure Mol. Compound Name IKK2 MS Example
Wt. Resin (M+H)
IC50
o 0 592.08 8-{[5-(acetylamino)-2- <1 pM 592 392
/- NH I~S ~ ~ cblorobenzoyl]amino}-1-[4-
~ ~ H ~ N-N (ethylsulfonyl)phenyl]-4,5-
~ N N'~ dihydro-lH-benzo[g]indazole-
ci 0 3-carboxamide
0 0 550.05 8-[(2-chlorobenzoyl)amino]-1- <1 M 550 393
[4-(isopropylsulfonyl)phenyl]-
N-N 4,5-dihydro-lH-
"
~ r, ~ \ ~ NHz benzo[g]indazele-3-
ci o ~ ~ carboxaniide
197
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Compound No., Structure Mol. Compound Name IKK2 MS Example
Wt. Resin (M+H)
IC50
601.52 1-{4-[(3- 1< 10 565 394
H-Cl aminopropyl)sulfonyl]phenyl }-
o. ,0 8-1 [(2-chloropyridin-3- M
yl)carbonyl]amino}-4,5-
N N """ NHz dihydro-lH-benzo[g]indazole-
ol 0 I~ 0 3-carboxamide hydrochloride
0 0 535.03 8-[(2-chlorobenzoyl)amino]-1- <1 M 535 395
/ [4-(ethylsulfonyl)phenyl]-4,5-
/ H N-N dihydro-lH-benzo[g]indazole-
" NHz 3-carboxamide
ci 0
0 0 536.01 8-{ [(2-chloropyridin-3- <1 M 536 396
-,_'s / yl)carbonyl]amino}-1-[4-
H \ N-N (ethylsulfonyl)phenyl]-4,5-
") " ~ ~ "H, dihydro-lH-benzo[g]indazole-
ci 0 / 0 3-carboxamide
o, 0 650.16 tert-butyl 3-[({3- <1 M 650 397
ci S \ ~ (aminocarbonyl)-1-[4-
N "-N NF~ (ethylsulfonyl)phenyl]-4,5-
HN o o ~ o dihydro-lH-benzo[g]indazol-8-
yl } amino)carbonyl]-4-
chlorophenylcarbamate
o. 0 550.04 8-[(5-amino-2- <1 M 550 398
0 chlorobenzoyl)amino]-1-[4-
~ H N-N (ethylsulfonyl)phenyl]-4,5-
HzN" ~ 6 "'~ dihydro-lH-benzo[g]indazole-
o 0 3-carboxamide
0 0 474.56 1-[4-(ethylsulfonyl)phenyl]-8- 1< 10 475 399
~'s [(methylsulfonyl)amino]-4,5
N_N dihydro-lH-benzo[g]indazole- M
H
~ "'~ 3-carboxamide
o S-" ~/ o
o
0 0 549.05 8-[(2-chlorobenzoyl)amino]-1- <1 M 549 400
[4-(propylsulfonyl)phenyl]-4,5-
\ N-N dihydro-lH-benzo[g]indazole-
~ " N
"H, 3-carboxamide
ci 0 0 0 550.04 8-{ [(2-chloropyridin-3- 1< 10 550 401
-'~s yl)carbonyl]aniino}-1-[4
~ N-N (propylsulfonyl)phenyl]-4,5- M
N) N NHz dihydro-lH-benzo[g]indazole-
ol 0 3-carboxamide
198
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Compound No., Structure Mol. Compound Name IKK2 MS Example
Wt. Resin (M+H)
IC50
o. ~ 678.09 8-[(5-amino-2- <1 pM 678 402
S
0 ci chlorobenzoyl)amino]-1-[4-
F'O,k.H=N 1 p ~" NH, (propylsulfonyl)phenyl]-4,5-
~ r 0 dihydro-lH-benzo[g]indazole-
3-carboxamide trifluoroacetate
0 0 488.59 8-[(methylsulfonyl)amino]-1- 1< 10 489 403
[4-(propylsulfonyl)phenyl]-4,5
dih dro-lH-benzo M
H y [g]indazole-
s "'~ 3-carboxamide
o r o
H-oi q. 0 600.53 1-{4-[(3- <1 gM 564 404
H2N'-'~9 ~ aminopropyl)sulfonyl]phenyl}-
~ ~ I N N 8-[(2-chlorobenzoyl)amino]-
~ r", NF6 4,5-dihydr6-1H-
oi o benzo[g]indazole-3-
carboxamide hydrochloride
=lo 563.08 1-[4-(butylsulfonyl)phenyl]-8- 1< 10 563 405
wS
[(2-chlorobenzoyl)amino]-4,5-
~ N "-" N,~ dihydro-lH-benzo[g]indazole- M
c~ o . \ 0 3-carboxamide
o, 0 396.47 8-amino-l-[4- 1< 10 397 406
"'S ~I
~ (ethylsulfonyl)phenyl]-4,5-
" " dihydro-lH-benzo[g]indazole- M
HzN I/ o NHz 3-carboxamide
o, 0 410.50 8-amino-l-[4- 1<10 411 407
(propylsulfonyl)phenyl]-4,5-
1 j~N,~ dihydro-lH-benzo[g]indazole- uM
~~ \ 0 3-carboxamide
=-9 664.19 tert-butyl 3 [({3- 1< 10 664 408
~ ~S ~ N N (aminocarbonyl)-1-[4- M
HN ~ " N"6 (propylsulfonyl)phenyl]-4,5-
( o dihydro-lH-benzo[g]indazol-8
-I yl } amino)carbonyl]-4-
chlorophenylcarbamate
0 0 561.06 8-[(2-chlorobenzoyl)amino]-1- 1< 10 561 409
{4-
~ N \ N-N NN [(cyclopropylmethyl)sulfonyl]p M
~~ o ~ o henyl}-4,5-dihydro-lH-
benzo[g]indazole-3-
carboxamide
[00243] Example 410
8-[(2-chlorobenzoyl)amino]-1-[4-(methylthio)phenyl]-4,5-dihydro-1 H-
benzo [g] indazole-3-carboxamide
199
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
N N-N NHZ
CI O O
[00244] Step 1
IaNHNH2
4-Methylthiol-aniline (10.0 g; 7.2 mmoles) was suspended in 6N HCI (50 mL).
The
solution was cooled to 0 C and sodium nitrite (5.26 g; 7.6 mmoles) dissolved
in
water (20 mL) was added dropwise keeping the temperature at 0 C. When the
addition is complete, the reaction mixture is homogeneous and has changed from
a
dark brown to an orange color. After letting this stir for an hour, SnC12.2H20
(42.5 g; 18.8 mmoles) dissolved in conc. HCl (35 mL) was added to the cold
solution over a period of 15 minutes. The reaction was allowed to stir for 2
hours
allowing the temperature to reach room temperature. The white solid was
filtered
of and suspended in ice water (300 mL). 50% NaOH solution was added till
reaction mixture becomes basic (pH - 12). Any undissolved solid was filtered
off
and the aqueous phase was extracted with ethyl ether (3 x 300 mL). The organic
phase was dried over MgSO4 and concentrated to a yellow solid (7.8 g; 71 %
yield)
HPLC indicated that product is 64% pure and has M+1=155 (other impurity was
starting material); 1H NMR (CDC13) b 2.44 (s, 3H), 6.78 (m, 2 H), 7.23 (m,
2H).
[00245] Step 2
\ N N-N NHZ
CI O I / O
200
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
The title compound was prepared in a manner analogous to Example 3 using 1-[4-
methylsulfonyl)phenyl]hydrazine and the appropriate acylating agent.
[00246] The compounds of Examples 411-414 in Table 19 were prepared in a
manner analogous to Examples 410 using the appropriate acylating agent.
[00247] The bioactivity in the IKK2 Resin assay for the compounds of
Examples 410-414 is shown in Table 19.
Table 19
Compound No., Structure Mol. Compound Name IKK2 MS Example
Wt. Resin (M+H)
IC50
s ~ 489.00 8-[(2-chlorobenzoyl)amino]-1- 1< 10 489 410
~ [4-(methylthio)phenyl]-4,5- M
r", \\" N"2 dihydro-lH-benzo[g]indazole-
o~ o I~ 0 3-carboxaniide
428.54 8-[(methylsulfonyi)amino]-1- <1 M 429 411
N-N [4-(methylthio)phenyl]-4,5-
,N \ \ ~ NHZ dihydro-lH-benzo[g]indazole-
~ 3-carboxamide
,s 526.45 8-[(3- <1 pM 490 412
HCI I chloroisonicotinoyl)amino]-1-
N~ N "-N NHz [4-(methylthio)phenyl]-4,5-
ci o I \ dihydro-lH-benzo[g]indazole-
3-carboxamide hydrochloride
~s 533.01 8-{[(6-chloro-l,3-benzodioxol- <1 M 533 413
~ N-N 5-yl)carbonyl]amino}-I-[4-
~ ~ ,"~ \ \ N,6 (methylthio)phenyl]-4,5-
oi o dihydro 1H-benzo[g]indazole-
3-carboxamide
s 540.48 8-[(5-amino-2- <1 M 504 414
i N-N chlorobenzoyl)amino]-1-[4-
~ I N \ \ NN (methylthio)phenyl]-4,5-
c~ o dihydro-IH-benzo[g]indazole-
HoI 3-carboxamide hydrochloride
[00248] The compounds of Examples 415-420 in Table 20 were prepared
by oxidation with m-CPBA with the appropriate sulfide.
201
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
[00249] The bioactivity in the IKK2 Resin assay for the compounds of
Examples 415-420 is shown in Table 20.
Table 20
Compound No., Structure Mol. Compound Name(s) IKK2 MS Example
Wt. Resin (M+H)
IC50
0 444.54 1-[4-(methylsulfinyl)phenyl]-8- 1< 10 445 415
[(methylsulfonyl)amino]-4,5- pM
H N_N dihydro-lH-benzo[g]indazole-
-~ N,~\NHz 3 carboxamide
S
o o
0
HCI 542.45 8-[(3- <1 M 543 416
"5 ~ ~ chloroisonicotinoyl)amino]-1-
~H ~ N_N [4-(methylsulfinyl)phenyl]-4,5-
N NHi dihydro-lH-benzo[g]indazole-
cl o ( 0 3-carboxamide hydrochloride
0 549.01 8-{[(6-chloro-1,3-benzodioxol- <l M 550 417
ro 5 5-yl)carbonyl]amino}-1-[4-
N_N (methylsulfinyl)phenyl]-4,5-
N NF~ dihydro-lH-benzo[g]indazole-
cl o 3-carboxamide
0 556.48 8-[(5-amino-2- <1 M 557 418
HCI NHa s ~ chlorobenzoyl)amino]-1-[4-
N-N (metbylsulfinyl)phenyl]-4,5-
~ NH4 dihydro-lH-benzo[g]indazole-
ei 0 3-carboxan-ude hydrochloride
0 505.00 8-[(2-chlorobenzoyl)amino]-1- <1 M 505 419
~s ~ [4-(methylsulfinyl)phenyl]-4,5-
\ N N-N N~ dihydro-lH-benzo[g]indazole-
~ 3-carboxarmde
I ~ o
cl 0
0 505.99 8-{ [(2-chloropyridin-3- <1 M 506 420
~s yl)carbonyl]amino}-1-[4-
\ N N_N N~ (methylsulfinyl)phenyl]-4,5-
~ dihydro 1H-benzo[g]indazole-
i 0 3-carboxamide
[00250] Example 421
202
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
1-{4-[(allylamino)sulfonyl]phenyl } -8-[(2-chlorobenzoyl)amino]-4,5-dihydro-lH-
benzo [g] indazole-3-carboxamide
\ oSo
~\H .N 0
N
H NH2
N
C! O
[00251] Step 1
0 0
AH.S ( \
/
N'NH2
H
\ N a~c
ct O 10 To a stirring solution of 1-[4-(aminosulfonyl)phenyl]-8-[(2-
chlorobenzoyl)amino]-
4,5-dihydro-lH-benzo[g]indazole-3-carboxamide (2967 mg, 5.7 mmol) in THF
(120 mL) was added DMAP (348 mg, 2.8 mmol), then tri-ethyl amine (0.95 mL, 6.8
mmol), followed by acetic anhydride (1.62 mL, 17.1 mmol). The reaction mixture
was stirred at room temperature for overnight and then concentrated. To the
residue
was added 5 % aq. NaHCO3 (100 mL). All compounds were dissolved. The aq.
Layer was washed with EA (100 mL x 2), CH2C12 (100 mL). The aq. layer was
separated. To the aq. Layer was added 1N HCl to pH=6. A white precipitate was
formed. It was filtered and washed with ether to give a white solid. The solid
was
dried under reduced pressure at 45 C to give 1-{4-
[(acetylamino)sulfonyl]phenyl}-
8-[(2-chlorobenzoyl)amino]-4,5-dihydro-lH-benzo[g]indazole-3-carboxamide
(2255.4 mg, 70%).
203
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
[00252] Step 2
O Cs+
~ S/o
N'~
N- N NHO
9U2
CI O
To a stirring solution of 1-{4-[(acetylamino)sulfonyl]phenyl}-8-[(2-
chlorobenzoyl)amino]-4,5-dihydro-lH-benzo[g]indazole-3-carboxamide (563 mg, 1
mmol) in H20 (5 mL) was added cesium carbonate (163 mg, 0.5 mmol). The
suspension was stirred at room temperature for overnight. All compounds were
dissolved. It was dried to give the Cs+ salt (650 mg, 93 %).
[00253] Step 3
oso
~N I \
O
/O / N.N
- NH2
N
CI 0
To a stirring solution of the Cs+ salt of 1-{4-[(acetylamino)sulfonyl]phenyl}-
8-[(2-
chlorobenzoyl)amino]-4,5-dihydro-lH-benzo[g]indazole-3-carboxamide (637 mg,
0.92 mmol ) in DMF (8 mL) was added a solution of allyl bromide (111 mg,
0.92 mmol). The solution was stirred at room temperature for over the weekend
and
concentrated. To the mixture was added MeOH- H20 (2:1). A precipitate was
formed. It was filtered to give a yellow solid of 1-(4-
[acetyl(allyl)amino] sulfonyl }phenyl)-8-[(2-chlorobenzoyl)amino]-4,5-dihydro-
1H-
benzo[g]indazole-3-carboxamide (360 mg, 65%.)
[00254] Step 4
~S~
0
H I / N.N
NH2
N
CI O
204
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
A solid of 1-(4-{[acetyl(allyl)amino]sulfonyl}phenyl)-8-[(2-
chlorobenzoyl)amino]-
4,5-dihydro-lH-benzo[g]indazole-3-carboxamide (360 mg, 0.6 mmol) was
dissolved in 0.5 N solution of NaOH in EtOH (10 mL) 'and stirred at room
temperature for overnight. The mixture was concentrated. It was purified by
HPLC
to give the desired compound (210 mg, 62 %.) IKK-2 resin IC50 < 1 M.
[00255] Example 422
8-[(2-chlorobenzoyl)amino]-1- { 4-[(methylamino)sulfonyl]phenyl }-4,5-dihydro-
lH-
benzo [g] indazole-3-carboxamide
O~ o0
N \
H ~ / N~N O
~ NHp
N
cl O
[00256] St~
oo
N
,~,
'O I ~ N' N p
NH2
H N
ci o
To a stirring solution of 1-{4-[(acetylamino)sulfonyl]phenyl}-8-[(2-
chlorobenzoyl)amino]-4,5-dihydro-lH-benzo[g]indazole-3-carboxamide (563 mg,
1 mmol ) in DMF (20 mL) was added NaH2 (40 mg, 1 mmol). The mixture was
stirred at room temperature for 1 h. To the mixture was added a solution of
lodomethane (170.3 mg, 1.2 mmol) in DMF (1 mL). The solution was stirred at
room temperature for 4 h and concentrated. It was purified by HPLC to give a
solid
of the desired compound 1-(4-{ [acetyl(methyl)amino]sulfonyl}phenyl)-8-[(2-
chlorobenzoyl)amino]-4,5-dihydro-lH-benzo[g]indazole-3-carboxamide (155 mg,
27 %.)
205
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
[00257] Step 2
o, vo
~ is
0
H N
/ Y H NH2
N
cl O
[00258] A solid of 1-(4-{[acetyl(methyl)amino]sulfonyl}phenyl)-8-[(2-
chlorobenzoyl)amino]-4,5-dihydro-lH-benzo[g]indazole-3-carboxamide was
dissolved in 0.5 N solution of NaOH in EtOH (3 mL) and stirred at room
temperature for 2 h. A suspension was formed. It was filtered and washed to
give
the desired compound (29 mg, 53 %.) IKK-2 resin IC50 < 1 M.
[00259] The compounds of Examples 423-433 in Table 21 were prepared in a
manner analogous to Example 46 using the appropriate alkylating agent and when
appropriate acylating or sulfonating agent.
Table 21
Compound No., Structure Mol. Compound Name IKK2 Resin MS Example
Wt. IC50 (M+H)
o,0 435.51 8-amino-l-[4-(2,5- 1 < 10 M 436 423
G""S dihydro-lH-pyrrol-l-
ylsulfonyl)phenyl]-4,5-
~" N~ dihydro-IH-
~ I o benzo[g]indazole-3-
carboxamide
0 0 453.52 8-amino-1-[4- 1< 10 M 454 424
~"" S ~/ \) (morpholin-4-
ylsulfonyl)phenyl]-4,5-
N-" NF~
dihydro-IH-
\ I o benzo[g]indazole-3-
carboxamide
o,0 459.53 8-amino-l-{4-[(diprop- <1 pM 460 425
N.s' 2-
~ N- " NH2 ynylamino)sulfonyl]phe
fizN L ~-< o nyl}-4,5-dihydro-1H-
/ '
benzo[g]indazole-3-
carboxamide
206
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Compound No., Structure Mol. Compound Name IKK2 Resin MS Example
Wt. IC50 (M+H)
0 0 489.58 1-{4- <1 M 490 426
[(dimethylamino)sulfon
yl]phenyl}-8-
N-S~
H "'", [(methylsulfonyl)amino
-3-N \ o ]-4,5-dihydro-lH-
benzo[g]indazole-3-
carboxamide
550.04 8-[(2- <1 M 550 427
-" o chlorobenzo 1 amino
oS/~ 1{4- y) l
/ N-N [(dimethylamino)sulfon
N ~ \ ""~ yl]phenyl}-4,5-dihydro-
t / \ \ ~ 1H-benzo[g]indazole-
3-carboxamide
0; ;0 593.11 8-[(2- <1 M 594 428
'",,,'H=S chlorobenzoyl)amino]-N 1-[4-({[2-
o N ~" o (dimethylamino)ethyl]a
ol I NH, mino } sulfonyl)phenyl]-
~ ~ 4,5-dihydro-IH-
~ benzo[g]indazole-3-
carboxamide
0 550.04 8-[(3- 15 10 M 550 429
N-S~
' ~ ~ chlorobenzoyl)amino]-
H N-N 1-{4-
o N / \ ' NHZ [(dimethylamino)sulfon
~ yl]phenyl}-4,5-dihydro-
oI 1H-benzo[g]indazole-
3-carboxamide
s..o 453.52 8-(acetylamino)-1-{4- 15 10 M 454 430
/t ) [(dimethylamino)sulfon
H N-N yl]phenyl}-4,5-dihydro-
~" 1H-benzo[g]indazole-
~ 3-carboxamide
0 541.65 1-{4- 1< 10 M 542 431
N.s
J [(diallylamino)sulfonyl]
o= N N-N phenyl}-8-
0 ''" o "~ [(methylsulfonyl)amino
]-4,5-dihydro-lH-
benzo[g]indazole-3-
carboxamide
~N 8<o 598.08 8-[(2- 1 10 M 598 432
a chlorobenzoyl)amino]-
N N-N 1-{4-[(diprop-2-
0 " ynylamino)sulfonyl]phe
"., nyl}-4,5-dihydro-lH-
benzo[g]indazole-3-
carboxamide
207
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Compound No., Structure Mol. Compound Name IKK2 Resin MS Example -
Wt. IC50 (M+H)
H 9..0 454.56 8-amino-l-[4-({ [2- 1< 10 M 455 433
N~/ N-S' (dimethylamino)ethyl]a
N-N mino}sulfonyl)phenyl]-
",N 4,5-dihydro-+H-
benzo[g]indazole-3-
carboxamide
[00260] The following scheme was used for the synthesis of Examples 434
SCHEME XXIX
0 0 O o
~ I H2gO4 02N LiHMDS 02N OEt
\ KNO3 iCO O
~O
~ O O~
AcOH ~ ~ ~
-_ y ~~
O NHNH2 N-N OEt SnCI2 H N N-N OEt
O 02N \ I \ O EtOH \ I \ O
O O
/\p
-- cl
~ ~ oN-/ O
NH3, EtOH O N-N NH CI H N-N NH2
H2N N( II N/
O
O Pyridine ci O
[00261] Example 434
1 -(1,3-benzodioxol-5-yl)-8-{ [(2-chloropyridin-3-yl)carbonyl]amino}-5,5-
dimethyl-
4,5-dihydro- IH-benzo[g]indazole-3-carboxamide
208
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
0 /'-O
N-N
O NH2
cl 0
/ I
N~
[00262] Step 1
4,4-dimethyl-tetralone (6g) was suspended in 120m1 conc. HZSOQ at 0 C, then
KNO3(3.8g)/H2SO4 (15m1) solution was added drop wise at 0 C. The reaction
mixture was stirred at 0 C for three hours until the starting material was
gone, then
poured into about 100g of ice. After cooling down, the mixture was filtered,
and the
solid obtained was washed with water, hexane, and then dried under vacuum.
5.6g
of desired product was obtained. It was used as it was without further
purification.
They structure was proved by LC-MS(220, M+1), HPLC, 'HNMR.
[00263] Step 2
The title compound of Step 1(5.6g) was dissolved in 100m1 THF, then (COOEt)Z
(5.6g) was added. The mixture was cooled down to -40 C, and then, LiHMDS/1M
THF solution (40m1) was added slowly. The reaction mixture was warmed up to r.
t.
slowly, then stirred overnight, and then neutralized with 2N aq. HCI. The
mixture
was extracted with EA (3x200m1). The EA solution was dried over Na.ZSO4. After
filtration and evaporation of solvent, the residue was purified by HPLC
(50%CH3CN to 90%CH3CN in 30 minutes). 1.3g of desired product was obtained.
The structure was proved by LC-MS(320, M+1) and HPLC.
[00264] Step 3
209
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
The title compound of step 2(1.3g) and 3,4-methylenedioxyphenylhydrazine
hydrochloride ( 0.85g ) were suspended in 100 ml of HOAc. The mixture was
heated up to reflux for 3 hours, and then the solvent was evaporated, and the
residue
was purified by HPLC (50% CH3CN/H2O to 90%CH3CN/HZO in 30 minutes). 0.65g
of desired product was obtained and characterized by LC-MS(436, M+1), 'HNMR,
HPLC analysis.
[00265] Step 4
The title compound of step 3 (0.65g) was suspended in 100m1 of EtOH, and then
SnClZ (1.2g) were added. The reaction mixture was refluxed overnight. Then the
solvent was evaporated, and residue was dissolved in 15m1 CH3CN and filtered.
The solution was purified by HPLC (40% CH3CN/HZO to 90% CH3CN/H2O in 30
minutes). 0.25g of desired product was obtained and characterized by HPLC and
LC-MS(406, M+1).
[00266] Step 5
The title compound of step 4 (0.25g) was dissolved in EtOH and liquid ammonia,
and heated up to 100C under 600 PSI for 36 hours. After releasing pressure and
evaporating solvent, the residue is purified by HPLC (5% CH3CN/H2O to 60%
CH3CN/H20 in 30 minutes). 200mg of desired product was obtained, and
characterized by LC-MS(376, M+1) and HPLC analysis.
[00267] Step 6
The title compound of step 5 ( 90mg) was dissolved in pyridine(5m1), then 2-
chloro-nictinoyl chloride (52mg) were added. The reaction mixture was stirred
overnight, and then solvent was evaporated. The residue was purified by
HPLC(30% CH3CN/H2O to 90%CH3CN/Hz0 in 30 minutes) 49 mg of desired
compound 1-(1,3-benzodioxol-5-yl)-8-{ [(2-chloropyridin-3-yl)carbonyl]amino}-
5,5-dimethyl-4,5-dihydro-lH-benzo[g]indazole-3-carboxamide
210
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
O/'-O
N-N
0 NH2
cl O
N
The resulting compound was obtained and analyzed by 'HNMR, LC-MS(516,
M+l), HPLC and CHN analysis.
[00268] Example 435
1-(1,3-benzodioxol-5-yl)-8-[(3-chloroisonicotinoyl)amino]-5,5-dimethyl-4,5-
dihydro-lH-benzo[g]indazole-3-carboxamide
O~O
\ ~
H N-N NH2
\ I N
cl O ~ \ O
The tile compound of Example 332 (90mg) was dissolved in 5ml DMF, then 2-
chloroisonicotinic acid(100mg), HATU(150mg) and diiospropylethylamine(0.5m1)
were added. The reaction mixture was stirred at r. t. for two days. After the
reaction
was complete, solvent was evaporated, and the residue was purified by HPLC(30%
CH3CN/HO to 90% CH3CN/HZO in 30 minutes). 100mg of product was obtained
and characterized by'HNMR, LC-MS (516, M+1), HPLC, CHN analysis.
[00269] The compounds of Examples 436-443 in Table 22 were prepared in a
manner analogous to Examples 434 and 435
[00270] The bioactivity ilr the IKK2 Resin assay for the compounds of
Examples 434-443 is shown in Table 22.
211
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Table 22
Compound No., Structure Mol. Compound Name IKK2 MS Example
Wt. Resin (M+H)
IC50
0 515.96 1-(1,3-benzodioxol-5-yl)-8- <1 p.M 516 434
J { [(2-chloropyridin-3-
yl)carbonyl] amino }-5,5-
N ~N NHZ dimethyl-4,5-dihydro-lH-
~ benzo[g]indazole-3-
oi I ~ o carboxamide
N.
0 >'0 515.96 1-(1,3-benzodioxol-5-yl)-8- <1 M 552 435
[(3-
chloroisonicotinoyl)amino]-
N ~N NHz 5,5-dimethyl-4,5-dihydro-lH-
benzo[g]indazole-3-
cl o carboxamide
N
F 475.91 8-[(3- <1 M 476 436
J chloroisonicotinoyl)amino]-1-
~ o ~ N_N NH (4-fluorophenyl)-5-methyl-4,5-
Ci HN ~ ~ ~ z dihydro-lH-benzo[g]indazole-
~ 0 3-carboxamide
F 475.91 8-{[(2-chloropyridin-3- <1 M 476 437
Q yl)carbonyI]amino}-1-(4-
N y llo N-N fluorophenyi)-5-methyl-4,5-
Ci HN / ~ ~ NHZ dihydro-lH-benzo[g]indazole-
~ 0 3-carboxamide
F F 354.36 8-amino-l-(2,4- nd 355 438
di#luorophenyl)-5-methyl-4,5-
N-N NH dihydro-lH-benzo[g]indazole-
HzN , r Z
3-carboxamide
~ HzN 351.39 8-amino-l-(4-amino-2- nd 352 439
fluorophenyl)-5-methyl-4,5-
Q_F fluorophenyl)-5-methyl-4,5-
N-N NHz dihydro-lH-benzo[g]indazole-
H2N 3-carboxamide
0
212
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Compound No., Structure Mol. Compound Name IKK2 MS Example
Wt. Resin (M+H)
IC50
F 493.90 8-[(3- <1 M 494 440
/ chloroisonicotinoyl)amino]-1-
~ o
N-N NH2 (2,4-difluorophenyl)-5-methyl-
Ci HN 4,5-dihydro-lH-
~ o benzo[g]indazole-3-
carboxamide
I 573.08 8-{[2-chloro-5-(4- <1 M 574 441
N methY1P erazin-l-
1 iP
NJ F yl)benzoyl]amino}-1-(4-
n\ fluorophenyl)-5-methyl-4,5-
~ o ~ dihydro-lH-benzo[g]indazole-
CI HN N-N NHZ 3-carboxamide
O
HzN F 490.93 1-(4-amino-2-fluorophenyl)-8- <1 M 491 442
N~ ' \
[(3-
N-N NH chloroisonicotinoyl)amino]-5-
a HN / ' Z methyl-4,5-dihydro-IH-
~ o benzo[g]indazole-3-
carboxamide
F 493.90 8-{[(2-chloropyridin-3- nd 494 443
~ / \ F yl)carbonyl]amino}-1-(2,4-
Ny I C ~
N-N NHZ difluorophenyl)-5-methyl-4,5-
ci HN dihydro-lH-benzo[g]indazole-
~ 0 3-carboxamide
nd = not determined
[00271] Example 444
1-(1,3-benzodioxol-5-yl)-8-[(N-isopropylglycyl)amino]-4,5-dihydro-1 H-
benzo [g] indazole-3-carboxamide
0 /,--o
N_N
NHN CONHZ
0 10
213
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
[00272] Step 1
To a solution of 8-amino-l-(1,3-benzodioxol-5-yl)-4,5-dihydro-lH-
benzo[g]indazole-3-carboxamide (1.00 g, 2.87mmol) in DMA (5.0 mL) was added
chloroacetyl chloride (0.27 mL, 3.44 mmol) and triethylamine (0.48 mL, 3.44
mmol) and the mixture was stirred at RT overnight. The reaction mixture was
triturated with distilled water (3x40 mL) and the solid product precipitated
out once
the water was added. The mixture was filtered and the solid was dried under
the
vacuum oven overnight to give 8-[(chloroacetyl)amino]-1-(4-fluorophenyl)-4,5-
dihydro-lH-benzo[g]indazole-3-carboxamide. MS (ESI+) for C21H17C1N404 m/z
425 (M+H)+.
[00273] Step 2
To a solution of product from step 1(0.10 g, 0.24 mmol) dissolved in DMA (2.0
mL) was added isopropyl amine (3.0 eq) followed by the PS-DIEA resin (2.0 eq).
The mixture was stirred and heated at 100 C overnight. The reaction mixture
was
cooled to RT and the solution was filtered. The filtrate was then evaporated
under a
stream of nitrogen overnight. The sample was purified on the SPE silica
column.
The clean fractions were combined and concentrated to give the title material.
1H
NMR (CD3OD) 8 7.38 (dd, 1H), 7.28 (d, 1H), 7.18 (d, 1H), 7.0 (t, 1H), 6.96 (d,
2H), 6.12 (s, 2H), 3.29 (s, 2H), 3.04 (m, 2H), 2.95 (m, 2H), 2.79 (m, 1H),
1.08 (d,
6H); MS (ESI+) for C24H25N504 m/z 448 (M+H)+.
[00274] The compounds of Examples 445-4521isted in the table below
where prepared according to the procedure of Example 444 using the
appropriately
substituted aniline and appropriate amine.
[00275] The bioactivity in the IKK2 Resin assay for the compounds of
Examples 444-452 is shown in Table 23
214
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Table 23
Compound No., Structure Mol. Compound Name(s) IKK2 LCMS Example
Wt. Resin (M+H)
TC50
0 0 447.50 1-(1,3-benzodioxol-5- <1 M 448 444
/ yl)-8-[(N-
~ isopropylglycyl)amino]-
I N-N 4,5-dihydro-lH-
~N~N ~ ~ CONH2 benzo[g]indazole-3-
" 0 carboxamide
os1o 495.97 1-(1,3-benzodioxol-5- <1 M 460 445
yl)-8-[(N-
H-Cl cyclobutylglycyl)amino]
N-N -4,5-dihydro-lH-
~H~rHi CONHz benzo[g]indazole-3-
0 carboxamide
hydrochloride
0 o 495.97 1-(1,3-benzodioxol-5- 1< 10 460 446
HCI yl)-8-[(pyrrolidin-l- pM
/ ~ ylacetyl)amino]-4,5-
~ N_N dihydro-lH-
r~ CONH2 benzo[g]indazole-3-
CN0 carboxamide
hydrochloride
0 524.02 1-(1,3-benzodioxol-5- 1< 10 488 447
yl)-8-[(N-
HCI cyclohexylglycyl)amino M
~H.~rHi ~N CONHa ]-4,5-dihydro-lH-
benzo[g]indazole-3-
0 carboxamide
hydrochloride
os.o 455.91 1-(1,3-benzodioxol-5- 1< 10 420 448
yl)-8-[(N- M
HCI ~ methylglycyl)amino]-
N_N 4,5-dihydro-lH-
H~r"~ \ CONH2 benzo[g]indazole-3-
o I carboxamide
hydrochloride
0 /.0 495.97 1-(1,3-benzodioxol-5- 1< 10 460 449
/ \' yl)-8-{ [N- M
HCI (cyclopropylmethyl)glyc
N- N yl] amino } -4,5-dihydro-
N ~
coNH2 1H-benzo[g]indazole-3-
~ -
o carboxamide
hydrochloride
o~o 461.53 1-(1,3-benzodioxol-5- <1 M 462 450
\' yl)-8- { [N-(tert-
butyl)glycyl]amino } -
N- N 4,5-dihydro-1 H-
xN~ r"i ~ ~ CoNHz benzo[g]indazole-3-
o carboxamide
215
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Compound No., Structure Mol. Compound Name(s) IKK2 LCMS Example
Wt. Resin (M+H)
IC50
,-0 461.53 1-(1,3-benzodioxol-5- 51 M 462 451
/ ~ ~ 1)8-[(N-
Y
isobutylglycyl)amino]-
H N-N 4,5-dihydro-lH-
\~HN CONHZ benzo[g]indazole-3-
IT o~ i carboxamide
F 433.49 8-[(N- <1 M 434 452
OY\' cyclobutylglycyl)amino]
N-N -1-(4-fluorophenyl)-4,5-
N \ CONN dihydro-lH-
H~ 2 benzo[g]indazole-3-
carboxamide
Example 453
8- { [5-Chloro-2-(4-methylpiperazin-1-yl)isonicotinoyl]amino}-1-[4-
(methylsulfonyl)phenyl]-4,5-dihydro-lH-benzo[g]indazole-3-carboxamide
Step 1
8- [(2, 5-Dichloroisonicotinoyl)amino]-1- [4-(methylsulfonyl)phenyl]-4, 5-
dihydro-
1 H-benzo [g] indazole-3-carboxamide
Q. O
N' I C~ H N-N
CI ~ N
~ I NH2
[00276] The product of Example 92 (8-amino-l-[4-(methylsulfonyl)phenyl]-
4,5-dihydro-lH-benzo[g]indazole-3-carboxamide hydrochloride) (1.20 mmol), 2,5-
dichloroisonicotinic acid (1.83 mmol), HATU (1.83 mmol), and 0.7 mL
triethylamine were dissolved in 10 mL DMF. The mixture was stirred at r.t.
overnight before more triethylamine (0.4 rnL), HATU (1.29 mmol), and 2,5-
dichloroisonicotinic (2.24 mmol) were added to drive the reaction. After
another 2.5
h, the mixture was added to water, causing a precipitate, which was filtered
and
washed with water. After trituration in water, the product was dissolved in
216
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
tetrahydrofuran, decolorized with activated carbon, dried over MgSO4, and the
solvent stripped. The solid was dissolved in anhydrous acetonitrile, filtered,
and the
acetonitrile stripped. The residue was purified by recrystallizations from
absolute
ethanol followed by triturations with anhydrous acetonitrile. Mp: 215-222 C.
Mass
spectrum: M + 1 = 556. 1H NMR (S, d6-DMSO, 400 MHz): 2.87-2.98 (m, 4H); 3.18
(s, 3H); 7.29-7.38 (m, 4H); 7.60 (s, 1H); 7.76 (s, 1H); 7.80-7.85 (m, 2H);
8.06-8.11
(m, 2H); 8.58 (s, 1H); 10.56 (s, 1H).
Step 2
8- { [5-Chloro-2-(4-methylpiperazin-l-yl)isonicotinoyl]amino}-1-[4-
(methylsulfonyl)phenyl]-4,5-dihydro-lH-benzo[g]indazole-3-carboxamide
0 0
N~ Ci H N-N
N
N O NH2
[00277] The product of step 1 (0.92 mmol) and N-methylpiperazine (9.0
mmol) were combined with 2.6 mL DMA. The reaction mixture was placed under
nitrogen and stirred in an oil bath at 100 C for 15 h. The mixture was then
added to
water, causing a precipitate, which was filtered and washed with water. The
solid
was dissolved in acetonitrile, dried over MgSO4, and the solvent stripped.
Mass
spectrum: M + 1= 620.
Example 454
1-(1,3-Benzodioxol-5-yl)-8-{ [(6-chloro-4-methylpyridin-3-yl)carbonyl] amino }-
4,5-
dihydro-1 H-benzo [g] indazole-3-carboxamide
217
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
O~ ~O
/ ~
CI I N_N O
\
O ~ i NH2
[00278] The product of Example 161, Step 4(5.93 mmol), 6-chloro-4-
methylnicotinic acid (Example 246, Step 1) (8.91 mmol), HATU (8.73 mmol), and
3.0 mL triethylamine were dissolved in 30 mL DMF. The mixture was stirred at
r.t.
for 5.4 h. The solvent was then partially stripped and the residue suspended
in
water, filtered, and washed with water. The solid was dissolved in
tetrahydrofuran,
decolorized with activated carbon, dried over MgSO4, and-the solvent stripped.
The
product was purified by recrystallizations in tetrahydrofuran and a
trituration in
ethanol/water. Mp: 303 C (decomp.). Mass spectrum: M + 1 = 502. 1H NMR (S, d6-
DMSO, 400 MHz): 2.28 (s, 3H); 2.81-2.96 (m, 4H); 6.06 (s, 2H) ; 6.90-6.94 (m,
1H); 6.97-7.01 (m, 1H); 7.08 (d, 1H, J = 2.0 Hz); 7.20-7.39 (m, 4H); 7.45-7.49
(m,
2H); 8.34 (s, 1H); 10.30 (s, 1H)
[00279] Examples 455, 456, and 457 were synthesized by the following
general synthesis procedure, illustrated for Example 455:
Zn2
/ CI $N-N Pd2(a)a)s> dPPf / CI N H2, 5%Pd/C
~ H
CI N N/ CONH2 DMF/BN, 90 C~ N N D
N/ CONH2 HCI, rt
O
\
/ CI H N-N
N I N CONH2
H2 O
218
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Example 455
[00280] 8-({ [6-(Aminomethyl)-3-chloropyridin-2-yl]carbonyl}amino)-1-(1,3-
benzodioxol-5-yl)-4,5-dihydro-1 H-benzo[g]indazole-3-carboxamide
Step 1
1-(1,3-Benzodioxol-5-y1)-8-{ [(3-chloro-6-cyanopyridin-2-yl)carbonyl]amino }-
4,5-
dihydro-1 H-benzo [g] indazole-3-carboxamide
/ H N-N
Cl
I
NC N N/ ONH2
O
[00281] The product of Example 216 (1-(1,3-benzodioxol-5-yl)-8-{ [(3,6-
dichloropyridin-2-yl)carbonyl]amino } -4,5-dihydro- 1 H-benzo[g]indazole-3-
carboxamide) (1.045 g, 2 mmol), Zn(CN)2 (0.138 g, 1.2 mmol), Pd2(dba)3 (0.0904
g, 0.1 mmol) (dba = dibenzylideneacetone), and dppf (dppf =
bis[diphenylphosphino]ferrocene) (0.121 g, 0.22 mmol) were mixed in a flask
containing stir bar. The mixture was placed under vacuum then nitrogen three
times
to remove oxygen. Degassed anhydrous solvents (9 mL of DMF and 3 mL of
benzonitrile) were added under N2, and the flask was placed in an oil bath at
90 C
for 6 h. After 6 h solvent was removed under vacuum, 50 n1L of ether was added
and stirred 3 h. Ether was removed by filtration, and the solid was triturated
with
50 mL of water 3 h, dried and purified by HPLC. The title compound is a yellow
solid (0.234 g, 23%). 1H NMR (d6-DMSO): 8 2.82-3.01 (m, 4H), 6.09 (s, 2H),
6.97-7.07 (m, 2H), 7.14 (d, 1H, J= 3 Hz), 7.27 (s, 1H), 7.33-7.40 (m, 3H),
7.52 (s,
1H), 8.21 (d, 1H, J= 8 Hz), 8.39 (d, 1H, J = 8 Hz), 10.60 (s, 1H). ESI mass
spectrum for C26H18C1N6O4+: 513 (M + 1).
Step 2
219
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
8-( { [6-(Aminomethyl)-3-chloropyridin-2-yl]carbonyl } amino)-1-(1,3-
benzodioxol-
5-yl)-4,5-dihydro-1 H-benzo[g]indazole-3-carboxatnide
/lo
~
CI ~ 1N-H
/ ~
N N ONH2
NH2 O
[00282] The product of step 1 (0.24 g, 0.47 mmol) was dissolved in a mixture
of anhydrous solvents, containing glyme (3 mL), methanol (9 mL), and 1.2 M
solution of HCl in methanol (3 mL). The mixture was placed under N2, in Fisher-
Potter bottle, containing magnetic stir bar and 0.02g of 5% Pd/C catalyst
(Degussa -
Frankfurt, Germany). Previously, the catalyst was dried and preactivated (H2,
50
psi, 30 minutes). The hydrogenation was carried out under 3 psi pressure for 3
hours at room temperature. The catalyst was removed by filtration, and the
filtrate
was purified by HPLC to give 0.152 g of TFA salt of 8-({ [6-(Aminomethyl)-3-
chloropyridin-2-yl] carbonyl } amino)-1-(1,3-benzodioxol-5-yl)-4,5-dihydro-1 H-
benzo[g]indazole-3-carboxamide, which was triturated in water-triethyl amine
mixture (2:1) for 30 minutes. The title compound (0.12 g, 49%) was obtained as
the free base (a white solid) after filtration and drying. 1H NMR (db-DMSO): S
2.03
(s, 2H), 2.82-3.00 (m, 4H)), 3.80 (s, 2H), 6.09 (s, 2H), 6.92-7.03 (m, 2H),
7.15 (d,
1H, J = 2 Hz), 7.25 (s, 1H), 7.28-7.44 (m, 3H), 7.49 (s, 1H), 7.58 (d, 1H, J =
8.5
Hz), 7.96 (d, 1H, J= 8.5 Hz), 10.40 (s, 1H). ESI mass spectrum for
C26H22C1N6O4+: 517 (M + 1).
Example 456
8- { [2-(Aminomethyl)-5-chloroisonicotinoyl] amino }-1-(4-fluorophenyl)-4,5-
dihydro-IH-benzo[g]indazole-3-carboxamide
Step 1
220
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
8-[(5-Chloro-2-cyanoisonicotinoyl) amino] -1-(4-fluorophenyl)-4,5-dihydro-1 H-
benzo [g]indazole-3-carboxamide
F
N~ CI N-N
N \ N / I \ CONH2
O
[00283] The compound of step 1 was synthesized by the same procedure as
in step 1 of Example 455 for l-(1,3-Benzodioxol-5-yl)-8-{ [(3-chloro-6-
cyanopyridin-2-yl)carbonyl] amino } -4,5-dihydro-lH-benzo[g]indazole-3-
carboxamide starting with the compound of Example 248 (8-[(2,5-
dichloroisonicotinoyl)amino]-1-(4-fluorophenyl)-4,5-dihydro-lH-
benzo[g]indazole-
3-car'boxamide) (1.67 g, 3.2 mmol), Zn(CN)2 (0.230 g, 2 mmol), Pd2(DBA)3
(0.203
g, 0.22 mmol), and DPPF (0.121 g, 0.52 mmol), in a mixture of DMF (6 mL) and
benzonitrile (2 mL). The mixture was heated at 90 C for 6 h. Solvents were
removed under reduced pressure. The residue was triturated with 50 mL of ether
overnight and filtered to give 2.2 g of a brown solid. The title compound,
isolated
from 0.8 g of the crude reaction mixture by preparative HPLC, is a white solid
(0.30
g), M.p. 275-276 C. 1H NMR (CD3OD): b 2.94 - 3.17 (m, 4H)), 7.20-7.48 (m, 9H),
8.01 (s, 1H), 8.82 (s, 1H). ESI mass spectrum for Ca5H17C1FN6O2+: 487 (M + 1).
Step 2
8- { [2-(Aminomethyl)-5-chloroisonicotinoyl] amino } -1-(4-fluorophenyl)-4,5-
dihydro-1 H-benzo [g] indazole-3-carboxamide
N~ I CI N-N
N CONH2
HCI
H2 O
221
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
[00284] The title compound was synthesized by the same procedure as in step
2 of Example 455 for 8-( {[6-(Aminomethyl)-3-chloropyridin-2-yl]carbonyl }
amino)-
1-(1,3-benzodioxol-5-yl)-4,5-dihydro-lH-benzo[g]indazole-3-carboxamide
starting
with 1.2 g of the crude reaction mixture containing the compound of step 1 and
0.4
g of 10% Pd/C (Aldrich) in a mixture of glyme (3 mL), methanol (14 mL), and
1.2
M solution of HCI in methanol (6 mL). The hydrogenation was carried out under
5
psi pressure for 8 h at room temperature. The catalyst was removed by
filtration,
and the filtrate was purified by HPLC to give 0.43 g of the TFA salt of 8-{[2-
(Aminomethyl)-5-chloroisonicotinoyl] amino } -1-(4-fluorophenyl)-4,5-dihydro-1
H-
benzo[g]indazole-3-carboxamide, which was triturated in 2N HCl for 3 hours.
The
title compound (0.35 g) as the HCI salt (white solid) was obtained after
filtration
and drying. M.p., 312-314 C. 1H NMR (CD3OD): 6 2.91 - 3.15 (m, 4H), 4.33 (s,
2H), 6.09 (s, 2H), 7.22 - 7.42 (m, 5H), 7.38 - 7.52 (m, 3H), 8.78 (s, 1H). ESI
mass
spectrum for C25H21C1FN6O2+: 491 (M + 1).
Example 457
8- { [2-(Aminomethyl)-5-chloroisonicotinoyl]amino 1- l-(l,3-benzodioxoI-5-yI)-
4,5-
dihydro-lH-benzo[g]indazole-3-carboxamide
Step 1
1-(1,3-Benzodioxol-5-yl)-8-[(5-chloro-2-cyanoisonicotinoyl)amino]-4,5-dihydro-
1 H-benzo [g] indazole-3-carboxamide
/'-O
J Ci N
N
N N CONH2
O
[00285] The compound of step 1 was synthesized by the same procedure as
in step 1 of Example 455 for 1-(1,3-Benzodioxol-5-yl)-8-{ [(3-chloro-6-
cyanopyridin-2-yl) c arb onyl] amino }-4, 5-dihydro-1 H-benzo [g] indaz ole-3 -
222
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
carboxamide starting with the compound of Example 211 (1-(1,3-benzodioxol-5-
yl)-8- [(2, 5-dichloroisonicotinoyl)amino] -4, 5-dihydro-1 H-
benzo[g]indazole-3-carboxamide) (2.16 g, 4.1 mmol), Zn(CN)2 (0.287 g, 2.5
mmol), Pd2(DBA)3 (0.203 g, 0.24 mmol), and DPPF (0.121 g, 0.55 mmol), in a
mixture of DMF (9 mL) and benzonitrile (3 mL). The mixture was heated at 90 C
for 6 hours. Solvents were removed under reduced pressure. The residue was
triturated with 50 mL of ether overnight and filtered to give 2.8 g a brown
solid.
The product, isolated from 0.9 g of the crude reaction mixture by preparative
HPLC,
is a white solid (0.35 g), M.p. 321-322 C. 1H NMR (d6-DMSO): S 2.82-3.01 (m,
4H)), 6.10 (s, 2H), 6.94-7.06 (m, 2H), 7.11 (d, 1H, J = 2 Hz), 7.24-7.38 (m,
4H),
7.52 (s, 1H), 8.38 (s, 1H), 8.97 (s, 1H). ESI mass spectrum for C26H18C1N6O4+:
513
(M+1).
Step 2
8-{[2-(Aminomethyl)-5-chloroisonicotinoyl]amino}-1-(1,3-benzodioxol-5-yl)-4,5-
dihydro-1 H-benzo [g] indazole-3-carboxamide
/--O
N~ I Ci H N-N
~ N / ~ ONH2
H2 O ~ I
[00286] The title compound was synthesized by the same procedure as in step
2 of Example 455 for 8-({[6-(Aminomethyl)-3-chloropyridin-2-yl]carbonyl}amino)-
1-(1,3-benzodioxol-5-yl)-4,5-dihydro-lH-benzo[g]indazole-3-carboxamide
starting
with 1.4 g of the crude reaction mixture containing the compound of step 1 and
0.6
g of 10% Pd/C (Aldrich) in a mixture of glyme (20 mL), methanol (30 mL), and
1.2
M solution of HC1 in methanol (10 mL). The hydrogenation was carried out under
5 psi pressure for 8 h at room temperature. The catalyst was removed by
filtration,
and the filtrate was purified by HPLC to give 0.52 g of TFA salt of the title
compound, which was triturated in 1N HC1(2:1) for 3 h. The title compound
(0.40
223
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
g) as the HCl salt (a white solid) was obtained after filtration and drying.
M.p.,
125-130 C. 1H NMR (CD3OD): S 2.90 -3.16 (m, 4H), 4.34 (s, 2H), 6.09 (s, 2H),
6.92 - 7.03 (m, 3H), 7.38(s, 2H), 7.40 (s, 1H), 7.57 (s, 1H), 8.78 (s, 1H).
ESI mass
spectrum for C26H23C1N6O4+: 518 (M + 1).
[00287] Examples 458 and 459 were synthesized by the following synthesis
procedure, illustrated for Example 458.
SCHEME XXX
CO2H CO2Et 1. Zn(CN)2, Pd2(dba)3 C02Et
CI SOCI2 CI dppf, DMF, PhCN CI
N I CI CZH50H~ N I CI 2. H2, 10% Pd/C C2H5OH N NH2
CO2 Et CO2H
(Br(CH2)z)20 CI O HCI O
(i-Pr)~EtN, MeCN N N reflux
NJ
F
F
N
H2N \~ CONH N CI N-N
/ 2
~ I O N N/ \ t CONH2
HBTU (BF~ ), DMSO, NEt3 O \ I
Example 458
8-( { [3-Chloro-6-(morpholin-4-ylmethyl)pyridin-2-yl] carbonyl } amino)- 1 -(4-
fluorophenyl)-4,5-dihydro-lH-benzo[g]indazole-3-carboxamide
Step 1
Ethy12,5-dichloroisonicotinate
C02Et
CI
I
N CI
224
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
[00288] 2,5-Dichloroisonicotinic acid (7.2 g, 37.5 mmol) and 180 mL of
anhydrous C2H5OH were placed in a flask containing magnetic stir bar under N2.
The mixture was cooled to 0 C and 5.6 mL of SOC12 (38 mmol) was added
dropwise. The solution was heated under reflux overnight. Volatiles were
partially
evaporated to decrease the volume to 30 mL. The residue was partitioned
between
a water solution of Na2CO3 with pH = 8.5 (75 mL) and CH2C12 (75 mL). The
organic layer was separated. The aqueous layer was extracted with CH2Cl2 (2 x
25
mL). The organic layers were combined, dried over MgSO4, and concentrated in
vacuo to give 7.8 g of product (97.5% yield). 'H NMR (DCC13): S 1.42 (t, 3H, J
= 7
Hz), 4.44 (q, 2H, J = 7 Hz), 7.68 (s, 1H), 8.50 (s, 1H). ESI mass spectrum for
C8H8C12NO2+: 220 (M + 1).
Step 2
Ethy12-(aminomethyl)-5-chloroisonicotinate hydrochloride
CO2Et
CI HCI
N NH2
[00289] All dried reagents (7.8 g (35.45 mmol) of ethyl 2,5-
dichloroisonicotinate, 4.5 g (3.5 mmol) of Zn(CN)2, 0.226 g (0.41 mmol) of
dppf
(dppf = bis[diphenylphosphino]ferrocene), and 0.170 g(0.19 mmol) of Pd2(dba)3)
(dba = dibenzylideneacetone) were mixed in a flask containing a stir bar.
Degassed
anhydrous solvents (10 mL of DMF and 3.3 mL of benzonitrile) were added under
N2, and the flask was placed in an oil bath at 90 C for 6 h. After 6 h
solvents were
removed under vacuum, then 100 mL of water was added and the residue was
triturated for 3 h. After filtration, the solid was dried under reduced
pressure to give
about 7 g of product mixture, which was used for reduction without further
purification. Both starting material and ethyl dicyanoisonicotinate were
detected.
[00290] This product mixture (7 g) was placed in a Fisher-Porter bottle with
1.8 g of 10% Pd/C (Aldrich) in 120 mL of EtOH and 30 mL of Et,)O-HC1(2M
225
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
solution) under a hydrogen atmosphere (2-3 psi) and stirred at RT for 3 hours.
After removing solvent, the oil was triturated with ether to give a brownish
solid,
which, after filtration, was triturated with 10 mL of CH3CN to give 4.2 g of
the title
compound as the HCl salt (47% yield). 1H NMR (CD3OD): 8 1.41 (t, 3H, J = 7
Hz), 4.38 (s, 2H), 4.46 (q, 2H, J = 7 Hz), 7.80 (s, 1H), 8.78 (s, 1H). ESI
mass
spectrum for C9H12C1aN2O2+: 215 (M + 1).
Step 3
Ethy15-chloro-2-(morpholin-4-ylmethyl)isonicotinate trifluoroacetate
CO2Et CF3CO2H
CI / ~O
N
[00291] The product from step 2 (1.77 g, 7 mmol), 2-bromoethyl ether (2.18
g, 9.5 mmol), and ethyldiisopropylamine (2.5 g, 19 mmol) in 10 mL of
acetonitrile
were placed in a flask containing magnetic stir bar. The reaction mixture was
stirred for 8 days at room temperature. Volatiles were removed under reduced
pressure, and the desired product (solid, 1.3 g, 65% yield) was isolated with
preparative HPLC as the TFA salt. 1H NMR (CD3OD): S 1.42 (t, 3H, J = 7 Hz),
3.41 (s, broad, 4H), 4.46 (q, 2H, J = 7 Hz), 4.58 (s, 2H), 4.88 (s, broad,
4H), 7.90 (s,
1H), 8.83 (s, 1H). ESI mass spectrum for C13H18ClN2O3+: 285 (M + 1).
Step 4
5-Chloro-2-(morpholin-4-ylmethyl)isonicotinic acid hydrochloride
CO2H
CI / ,/~O -
I
\N NJHCI
[00292] The compound of step 3 (2.69 g, 10.3 mmol) as a suspension in 3N
HCl (15 mL) was heated under reflux for 3 hours. Volatiles were removed under
226
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
reduced pressure to give 1.88 g (95% yield) of the title compound. 1H NMR
(CD3OD): S 3.30 (s, broad, 2H), 3.41 (s, broad, 2H), 3.83 (s, broad, 2H), 4.05
(s,
broad, 2H), 4.58 (s, 2H), 7.92 (s, 1H), 8.83 (s, 1H). ESI mass spectrum for
C1iH14C1N2O3+: 257 (M + 1).
Step 5
8-( { [3-Chloro-6-(morpholin-4-ylmethyl)pyridin-2-yl]carbonyl } amino)-1-(4-
fluorophenyl)-4,5-dihydro-1 H-benzo [g]indazole-3-carboxamid.
F
CI
N H N-N
O N N/ CONH2
lo ~ o \ I
[00293] The title compound was synthesized from 0.94 g (3.2 mmol) of 5-
chloro-2-(morpholin-4-ylmethyl)isonicotinic acid hydrochloride (step 4) and
the
compound of Example 244 (8-amino-l-(4-fluorophenyl)-4,5-dihydro-lH-
benzo[g]indazole-3-carboxamide) (0.69 g, 2.1 mmol) by the same procedure used
for Example 255 except that DMF was replaced with DMSO (4 mL). The title
compound is a brownish solid (0.31 g, 26%), M.p., 310-315 C(decomp.). 1H
NMR (CD3OD): S 2.78 - 2.82 (m, 4H), 2.92 - 3.12 (m, 4H), 3.76 - 3.80 (m, 4H),
3.98 (s, 2H), 7.21 - 7.42 (m, 5H), 7.50 - 7.63 (m, 3H), 8.63 (s, 1H). ESI mass
spectrum for C?9HZ7CIFN6O3+: 561 (M + 1).
Example 459
1-(1,3-Benzodioxol-5-yl)-8-({ [3-chloro-6-(morpholin-4-ylmethyl)pyridin-2-
yl]carbonyl } amino)-4,5-dihydro-lH-benzo[g]indazole-3-carboxamide
227
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
O O
2 HC1 \ /
CI N
N x5H
[00294] The title compound was synthesized from 1.0 g (3.4 mmol) of 5-
chloro-2-(morpholin-4-ylmethyl)isomicotinic acid hydrochloride (step 4 of
Example
458) and the product of Example 161, Step 3 (0.78 g, 2.2 mmol) by the same
procedure used for Example 255 except that DMF was replaced with DMSO (5
mL). The title compound was initially isolated as the TFA salt by preparative
HPLC. After trituration with 2N HCI (2 x 20 mL) it was filtered and dried
under
reduced pressure to give the product as the bis(hydrochloride) tetrahydrate
(0.35 g,
22% yield), M.p., 231-235 C(decomp.). 1H NMR (CD3OD): S 2.50 - 2.54 (m,
4H), 2.92 - 3.12 (m, 4H), 3.76 - 3.80 (m, 6H), 6.09 (s, 2H), 6.90 - 7.10 (m,
3H),
7.38 - 7.42 (m, 3H), 7.58 (s, IH), 8.60 (s, 1H). ESI mass spectrum for
C30H28C1N605+: 587 (M + 1).
Examples 460 and 461 were synthesized by the following synthesis procedure,
illustrated for the synthesis of Example 461.
SCHEME XXXI
CO2Me CO2Me tributylvinyl tin, CO2Me
CI , SOCI2, MeOH CI / Pd(Ph3)4, dioxane CI
CI ~N I CI N
228
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
O O
~
O O
CO2H ~ / ~ N-N CI IN~ ~
HN ~ CONH2 O N N-N
1. morpholine, ~ CONH2
AcOH, EtOH HCI
2. NaOH, EtOH
3. HCI OJ HATU, DMF, NEt3
N N
O
Example 460
1-(1,3-Benzodioxol-5-y1)-8- { [5-chloro-2-(2-morpholin-4-
ylethyl)isonicotinoyl]amino}-4,5-dihydro-1H-benzo[g]indazole-3-carboxamide
Step 1
Methyl 2,5-dichloroisonicotinate
CO2Me
CI ,
I
N CI
[00295] Thionyl chloride (9.13 g, 5.6 mL, 76 mmol) was added at 0 C to a
suspension of 2,5-dichloroisonicotinic acid (7.3 g, 38 mmol) in 170 mL of
MeOH.
The reaction mixture was refluxed overnight. The volatiles were evaporated and
the
resulting residue was basified (pH = 9) with a saturated aqueous solution of
Na2CO3
and extracted with CH2CL.2. The organic extract was dried over MgSO4.
Purification
by chromatography on silica gel (hexanes / EtOAc : 4/1) afforded the title
compound as a white solid, yield 89%. 1H NMR (300 MHz, CDC13): 8 3.98 (s, 3H),
7.7 (s, 1H), 8.49 (s, 1H), 13C NMR (300 MHz, CDC13): 8 53.4, 125.2, 129.2,
139.3,
150.1, 151.1, 163.4. Mass spectrum, M+ 1= 207.
Step 2
Methyl 2-vinyl-5-chloroisonicotinate
229
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
CO2Me
CI
. ~ /
N
[00296] Pd(PPh3)4 (263 mg, 0.6 %) was added to a degassed mixture of
methy12,5-dichloroisonicotinate (step 1) (8.39 g, 40.7 mmol) and
tributyl(vinyl)tin
(15.4 g, 14.24 mL, 48.5 mmol) in 100 mL THF. The reaction mixture was stirred
at
60 C for 4 days. The solvent was evaporated and the resulting residue
partitioned
between CH2C12 and a saturated aqueous solution of KF. The CH2C12 extract was
washed with H20 and dried over MgSO4. Purification by chromatography on silica
gel (hexanes / EtOAc : 20/1 to 10/1) afforded the title compound as a
colorless oil,
yield 74 %. 1H NMR (300 MHz, CDC13): S 3.96 (s, 3H), 5.55 (d, 1H, Jc,s = 10.8
Hz), 6.24 (d, 1H, JTrans = 17.5 Hz), 6.78 (dd, 1H, JT,-QõS = 17.5 Hz, Jcis =
10.8 Hz),
7.65 (s, 1H), 8.62 (s, 1H), 13C NMR (300 MHz, CDC13): 8 53.2, 120.2, 121.9,
128.6, 135.3, 137.2, 151.1, 154.7, 164.8.1H-13C and 1H-15N correlation
spectroscopy (ghmbc) confirmed the structure.
Step 3
2-(Morpholin-4-ylethyl)-5-chloroisonicotinic acid hydrochloride
CO2H
CI , = HCI
~ ~
N Oo
[00297] A mixture of the material from step 2 (1.5 g, 7.5 mmol), morpholine
(0.97 g, 0.97 mL, 11 mmol) and acetic acid (0.66 g, 0.63 mL, 11 mniol) in 7 mL
EtOH was refluxed for 2 days. The reaction mixture was concentrated under
vacuum and the resulting residue basified to pH = 8 to 9 with a saturated
aqueous
solution of Na2CO3 and extracted with CHaC12. The organic extracts were dried
over MgSO4. The crude product, consisting of a mixture of methyl and ethyl5-
chloro-2-(morpholinoethyl)-isonicotinic esters, and was stirred with NaOH (1
mol/L, 7.5 mL, 7.5 mmol) in 20 mL of EtOH, at room temperature for 2 h. The
230
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
volatiles were removed and the basic aqueous residue washed once with CH2C12.
The aqueous phase was acidified to pH = 3 with an aqueous solution of HC1(1N)
and washed with CH2C12 before concentration under vacuum affording a white
solid, which was washed with MeOH to give the title product, yield 49%. 1H NMR
(300 MHz, d6-DMSO): S 2.47-2.48 (m, 2H), 2.82-2.87 (m, 4H), 2.96-3.015 (m,
2H), 3.6-3.3-63 (m, 2H + H20), 7.49 (s, 1H), 8.53 (s, 1H),13C NMR (300 MHz,
D20 ): S 30.3, 52.0, 56.1, 63.9, 121.4, 125.6, 147.9, 149.0, 154.9, 172.6.
Mass
spectrum, M + 1 = 271.
Step 4
1-(1,3-Benzodioxol-5-yl)-8-{ [5-chloro-2-(2-morpholin-4-
ylethyl)isonicotinoyl] amino }-4,5-dihydro-1 H-benzo[g]indazole-3-carboxamide
O~O
\ ~
H N-N
O N CONH2
C! 1)~
N co
[00298] The product of step 4 was synthesized by the same procedure as in
Example 211, starting with 8-amino-1 -(4-fluorophenyl)-4,5-dihydro-1H-
benzo[g]indazole-3-carboxamide (0.3 g, 0.86 mmol), 2-(Morpholin-4-ylethyl)-5-
chloroisonicotinic acid hydrochloride (step 2) (0.399 g, 0.86 mmol), HATU
(0.48 g,
1.28 mmol) and Et3N (0.511 g, 0.70 mL, 5.05 mmol) in 6 mL of DMF. Purification
by chromatography on silica gel (CH2C12 / MeOH: 11/1) afforded the title
compound as a yellow solid, yield 41%. 1H NMR (300 MHz, d6-DMSO): 6 2.38 (s
(broad), 4H), 2.62 (t, 2H, J= 7.12 Hz), 2.87-2.92 (m, 4H + 2H), 3.52 (t, 4H,
J=
3.23), 6.07 (s, 2H), 6.94 (dd, 1H, J= 8.2 Hz, 1.88 Hz), 6.99-7.01 (m, 1H),
7.09 (d,
1H, J= 1.88 Hz), 7.24 (s, IH), 7.27-7.31 (m, 2H), 7.38 (dd, 1H, J= 8.2 Hz,
1.88
Hz), 7.43 (s, 1H), 7.49 (s,1H), 8.58 (s, 1H), 10.44 (s, 1H). Mass spectrum, M
+ 1
262.
231
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Example 461
8- { [5-Chloro-2-(2-morpholin-4-ylethyl)isonicotinoyl]amino }-1-(4-
fluorophenyl)-
4,5-dihydro-1 H-benzo [g]indazole-3-carboxamide
\ ~
~
H F\ N-N
O N CO
NH2
CI lxr
N N~
~O
[00299] The title compound was synthesized by the same procedure as in
Example 211, starting with 8-amino-l-(4-fluorophenyl)-4,5-dihydro-lH-
benzo[g]indazole-3-carboxamide (0.489 g, 1.52 mmol), 2-(Morpholin-4-ylethyl)-5-
chloroisonicotinic acid hydrochloride (step 3 of Example 460) (0.7 g, 2.27
mmol),
HATU (0.852 g, 2.24 mmol) and Et3N (0.885 g, 1.22 mL, 8.75 mmol) in 10 mL of
DMF. Purification by preparative reverse phase HPLC afforded the title
compound
as a white solid, yield 25 %. 'H NMR (300 MHz, d6-DMSO): S 2.35 (s (broad),
4H), 2.58 (t, 2H, J = 7.58 Hz), 2.84-2.90 (m, 4H + 2H), 3.54 (t, 4H, J= 4.36
Hz),
7.18 (s, 1H), 7.25 (s, 1H), 7.28-7.37 (m, 4H), 7.39 (s, 1H), 7.53-7.56 (rn,
3H), 8.55
(s, 1H), 10.41 (s, 1H). Mass spectrum, M + 1= 576.
Example 462 was prepared using a synthesis method similar to that of Example
460
and 461, differing only in the amine added to the methyl 2-vinyl-5-
chloropyridine.
SCHEME XXXII
CO2Me CO2Me CO2H
CI CI , Cf , = HCI
N NMe2H, AcOH, N N 1. NaOH, EtOH ~N N
MeOH, 37% ~ 2. HCI
40%
232
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Example 462
8-({ 5-Chloro-2-[2-(dimethylamino)ethyl]isonicotinoyl } amino)-1-(4-
fluorophenyl)-
4,5-dihydro-lH-benzo [g]indazole-3-carboxamide
Step 1
Methyl 5-chloro-2-[2-(dimethylamino)ethyl]isonicotinate
CO2Me
CI
N N
[00300] A mixture of inethyl2-vinyl-5-chloroisonicotinate (step 2 of
Example 460) (2.48 g, 12.5 mmol), methylamine (2 M solution in MeOH) (11.5
mL, 23 mmol) and acetic acid (1.38 g, 1.32 mL, 23 mmol) in 3 mL MeOH was
heated at 78 C in a sealed tube for 3 days. The reaction mixture was
concentrated
under vacuum and the resulting residue basified to pH = 8 to 9 with a
saturated
aqueous solution of Na2CO3 and extracted with CH2C12. The organic extracts
were
dried over MgSO4. Purification by chromatography on silica ge1(CH2C12)
afforded
the title compound in 37% yield. 1H NMR (400 MHz, CDC13 ): S 2.29 (s, 6H),
2.71
(t, 2H, J = 7.36 Hz), 2.97 (t, 2H, J = 7.36 Hz), 3.9 (s, 3H), 7.5 (s, 1H),
8.56 (s, 1H),
13C NMR (300 MHz, CDC13): S 35.5, 45.4, 53.1, 59.0, 123.8, 127.8, 137.1,
150.8,
159.3, 164.9. Mass spectrum, M + 1 = 243.
Step 2
5-Chloro-2-[2-(dimethylamino)ethyl]isonicotinic acid hydrochloride
CO2H
CI / = HCI
~N N"
233
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
[00301] An aqueous solution of NaOH (1 mol/L) (5.8 mL, 5.8 mmol) was
added to a solution of inethyl5-chloro-2-[2-(dimethylamino)ethyl]isonicotinate
(step 1) (1.41 g, 5.8 mmol) in 15 mL MeOH. The reaction mixture was stirred at
room temperature for 3 h and concentrated under vacuum. The basic aqueous
residue was washed once with CHaC12. The aqueous phase was acidified to pH = 3
with an aqueous solution of HCl (1N) and washed with CH2Cl2 before
concentration under vacuum affording a white solid, which was washed with MeOH
to give the title product, yield 40 %. iH NMR (300 MHz, D20): S 2.76 (s, 6H),
3.12
(t, 2H, J= 7.24 Hz), 3.38 (t, 2H, J= 7.24 Hz), 7.30 (s, 1H), 8.40 (s, 1H).
Mass
spectrum, M + 1= 229.
Step 3
8-( { 5-Chloro-2-[2-(dimethylamino)ethyl]isonicotinoyl } amino)-1-(4-
fluorophenyl)-
4,5-dihydro-1 H-benzo [g] indazole-3 -carboxamide
F
H N-N
O N ' CONH2
CI
I
N Ni
1
[00302] The title compound was synthesized by the same procedure as in
Example 211, starting with 8-amino-l-(4-fluorophenyl)-4,5-dihydro-lH-
benzo[g]indazole-3-carboxamide (0.3 g, 0.86 mmol) (0.478 g, 1.48 mmol), 2-
(Morpholin-4-ylethyl)-5-chloroisonicotinic acid hydrochloride (step 3 of
Example
460) (0.59 g, 2.22 mmol), HATU (0.833 g, 2.19 mmol) and Et3N (0.871 g, 1.2 mL,
8.6 mmol) in DMF (8 mL). Purification by preparative reverse phase HPLC
afforded the title compound as a white solid in 33% yield. 1H NMR (300 MHz, d6-
DMSO): S 2.10 (s, 6H), 2.54 (t, 2H, T= 7 Hz), 2.81-2.90 (m, 6H), 7.18 (s, 1H),
7.26-737 (m, 6H), 7.52-7.56 (m, 3H), 8.54 (s, 1H), 10.42 (s, 1H). Mass
spectrum,
M+1=534
234
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Example 463
8-{ [(5-Chloro-2,4'-bipyridin-4-yl)carbonyl] amino }-1-(4-fluorophenyl)-4,5-
dihydro-
1 H-benzo [g]indazole-3-carboxamide
Step 1
Methyl 5-chloro-2,4'-bipyridine-4-carboxylate
C02Me
CI
N
N
[00303] The product of step 1 was synthesized by the same procedure as for
methyl 2-vinyl-5-chloroisonicotinate (step 2 of Example 461) starting with
methyl
2,5-dichloroisonicotinate (step 1 of Example 460) (1.4 g, 6.8 mmol), 4-
tributylstanylpyridine (2.5 g, 6.8 mmol) and Pd(PPh3)4 (45 mg) in 20 mL of
dioxane. The reaction mixture was refluxed for 7 days. Purification by
chromatography on silica gel (CH2CI2/MeOH: 18/1) afforded the title compound
as
a colorless oil, yield 44 %.1H NMR (300 MHz, CDC13): S 4.02 (s, 3H), 7.89-7.91
(m, 2H), 8.17 (s, 1H), 8.76 (d, broad, 2H, J = 6.0 Hz), 8.81 (s, 1H). Mass
spectrum,
M+1 =249.
Step 2
5-Chloro-2,4'-bipyridine-4-carboxylic acid hydrochloride
CO2H
CI HCI
N
N
[00304] The compound of step 2 was synthesized by the same procedure as
for 5-chloro-2-[2-(dimethylamino)ethyl]isonicotinic acid hydrochloride (step2
of
235
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Example 462) starting with the product of step 1 (0.755 g, 3 mmol) and NaOH (1
mol/L) (4.5 mL, 4.5 mxnol) in 5 mL MeOH. The reaction mixture was stirred at
room temperature for lh before acidification to pH = 2 with an aqueous HCl
(1N)
solution. The acidic mixture was washed once with CH2C12. White needles
crystallized from the acidic aqueous layer. These needles were filtered and
washed
with water to give the title compound in 36% yield. 1H NMR (300 MHz, CDC13): S
8.1 (dd, 2H, J = 4.6 Hz, 1.6 Hz), 8.4 (s, 1H), 8.72 (dd, 2H, J = 4.6 Hz, 1.6
Hz), 8.9
(s, 1H). Mass spectrum, M + 1(-HCl) = 235.
Step 3
8- { [(5-Chloro-2,4'-bipyridin-4-yl)carbonyl] amino } - 1 -(4-fluorophenyl)-
4,5-dihydro-
1H-benzo [g] indazole-3-carboxamide
F
-
H N N
O N CONH2
CI
N NII
[00305] The title compound was synthesized by the same procedure as in
Example 211 starting with 8-amino-l-(4-fluorophenyl)-4,5-dihydro-lH-
benzo[g]indazole-3-carboxamide (0.3 g, 0.86 mmol) (0.227 g, 0.7 mmol), the
product of step 2 (0.287 g, 1.0 mmol), HATU (0.395 g, 1 mmol) and Et3N (0.413
g,
1.2 mL, 4 mmol) in DMF (6 mL). Crystallization from CH3CN afforded the title
compound as a white solid, yield 45%. 1H NMR (400 MHz, d6-DMSO): S 2.9-2.95
(m, 4H), 7.26 (d, 1H, J = 2 Hz), 7.28 (s, 1H), 7.34-7.42 (m, 4H), 7.55 (s,
1H), 7.57-
7.6 (m, 2H), 8.07 (dd, 2H, J= 4.6 Hz, 1.5 Hz), 8.3 (s, 1H), 8.7 (dd, 2H, J=
4.6 Hz,
1.5 Hz), 8.86 (s, 1H), 10.5 (s, 1H). Mass spectrum, M + 1 = 539.
[00306] Example 464 was synthesized by the following synthesis method.
The first two bicyclic compounds were not isolated pure, but subjected to
236
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
successive reactions as described in step 3 to give 8-{ [(5-chloro-1'-methyl-
1',2',3',6'-
tetrahydro-2,4'-bipyridin-4-yl)carbonyl] amino } -1-(4-fluorophenyl)-4,5-
dihydro-1 H-
benzo [g]indazole-3-carboxamide.
0 OTf SnMe3
Me6Sn2, Pd(PPh3)4,
LHMDS, PhN(OTf)2 ~ LiCI, dioxane, 98 C 65 1 ~ N
CO2Me
cl C02Me C02H
N I CI CI i I 1. NaOH, MeOH CI
Pd(PPh3)4, N 2. HCI N
dioxane 98 C N HCI N
F _ F
\
N-N H N-N
H2N CONH2 O N CONH2
cl ~
HATU, Et3N, DMF -N
Step 1
1-Methyl-1,2,3,6-tetrahydropyridin-4-yl trifluoromethanesulfonate
OTf
\
N
1
[00307] LiHMDS (1M, THF) (48.6 mL, 48.6 mmol) was added to a solution
of 1-methyl-4-piperidone (5 g, 5.43 mL, 44.2 mmol) in 80 mL THF at -75 C. The
mixture was stirred at this temperature for 2 h and warmed up to -50 C. A
solution
of N-phenyltrifluoro-methanesulfonimide (17.36 g, 48.6 mmol) in 55 mL THF was
added to the reaction mixture, which was then allowed to warm up to room
237
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
temperature over a period of 3 h. The volatiles were removed under vacuum.
Purification of the resulting residue by chromatography on silica gel
(EtOAc/hexanes: 7/3) afforded the title compound in 73% yield. 1H NMR (300
MHz, CDC13): S 2.36 (s, 3H), 2.43-2.45 (m, 2H), 2.65 (t, 2H, J= 5.8 Hz), 3.05-
3.07
(m, 2H), 5.68 - 5.70 (m, 1H).
Step 2
1 -Methyl-4-(trimethylstannyl)- 1,2,3,6-tetrahydropyridine
SnMe3
N
)
1
[00308] Pd(PPh3)4 (0.31 g, 0.26 mmol) was added to a degassed mixture of
1-methyl-1,2,3,6-tetrahydropyridin-4-yl trifluoromethanesulfonate (step 1)
(3.28 g,
13.3 mmol), hexamethylditin (4.60 g, 14 mmol), LiCI (1.73 g, 40 mmol) and a
few
crystals of 2,6-di-tert-butyl-4-methylphenol in 60 mL dioxane. The reaction
mixture
was heated at 98 C for 4 h. After cooling the reaction mixture was poured into
an
aqueous solution of KF and extracted with Et20. The organic extracts were
washed
once with H20 and once with brine before being dried over MgSO4. The residue
obtained after removal was purified by chromatography on silica gel
(CH2C12/MeOH : 10/1) to afford the title compound in a 41% yield. 1H NMR (300
MHz, CDC13): S 0.0 (t, 9H, Jsn-x = 26.4 Hz), 2.21 (s, 3H), 2.23-2.27 (m, 2H),
2.39
(t, 2H, J = 5.6 Hz), 2.82-2.85 (m, 2H), 5.70 - 5.72 (m, 1H).
Step 3
8-{ [(5-chloro-1'-methyl-1',2',3',6'-tetrahydro-2,4'-bipyridin-4-
yl)carbonyl]amino }-1-
(4-fluorophenyl)-4, 5-dihydro-1 H-benzo [g] indazole-3-carboxamide
238
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
F
H N-N
O N ~ CONH2
CI ~ /
N
N
[00309] Pd(PPh3)4 (30 mg, 0.025 mmol) was added to a degassed mixture of
the product of step 2 (1.82 g, 7 mmol) and methy12,5-dicloroisonicotinate
(step 1 of
Example 460) (0.96 g, 4.66 mmol) in 10 mL of dioxane. The reaction mixture was
kept at 98 C overnight. After cooling the reaction mixture was poured into an
aqueous solution of KF and extracted with EtOAc. The organic extracts were
washed once with H20 and once with brine before being dried over MgSO4. The
residue, obtained after removal of the solvents, was purified by
chromatography on
silica gel (CH2C12/MeOH: 15/1) to afford 0.606 g of a 2/1 mixture of the
methyl 5-
chloro-1'-methyl-1',2',3',6'-tetrahydro-[2,4']bipyridinyl-4-carboxylate and
the
product of step 2 which was dissolved in 5 mL of MeOH and reacted at room
temperature with an aqueous solution of NaOH (1M) (2.5 mL, 2.5 mmol) for 1.5
h.
The reaction mixture was acidified to pH = 2 with an aqueous solution of HCI
(1N)
and concentrated to give a white solid residue. This residue was reacted with
8-
amino-l-(4-fluorophenyl)-4,5-dihydro-lH-benzo[g]indazole-3-carboxamide (0.52
g, 1.6 mmol) in 10 niL DMF in the presence of Et3N (0.94 g, 1.3 mL, 9.3 mmol)
and HATU (0.906 g, 2.38 mmol). After stirring 1.5 h, the mixture was
concentrated
and a white solid precipitated upon addition of water. The solid was filtered,
dissolved in THF and dried over MgSO4. Preparative HPLC afforded the title
compound in 36% yield. 'H NMR (400 MHz, d6-DMSO): S 2.25 (s, 3H), 2.51-2.53
(m, 4H), 2.86-2.91 (m, 4H), 3.04 (s, broad, 2H), 6.73 (s, broad, 1H), 7.19 (t,
1H, J
2.5 Hz), 7.25 (s, 1H), 7.29-7.38 (m, 4H), 7.52-7.56 (m, 3H), 7.59 (s, IH),
8.59 (s,
1H), 10.4 (s, 1H). Mass spectrum, M + 1= 558
239
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Example 465
1-(4-Fluorophenyl)-8- [(2-morpholin-4-ylisonicotinoyl)amino] -4, 5-dihydro-1 H-
benzo[g]indazole-3-carboxamide
Step l
8-[(2-Chloroisonicotinoyl)amino]-1-(4-fluorophenyl)-4,5-dihydro-1 H-
benzo[g]indazole-3-carboxamide
N-N
~ N
C~ O NH2
[00310] The product of Example 244 (8-amino-l-(4-fluorophenyl)-4,5-
dihydro-lH-benzo[g]indazole-3-carboxamide) (8.46 nunol), 2-chloroisonicotinic
acid (12.70 mmol), HATU (12.63 mmol), and 4.2 mL triethylamine were dissolved
in 42 mL of DMF. The mixture was stirred at r.t. for 4 h. The solvent was then
partially stripped and the residue suspended in water, filtered, and washed
with
water. The solid was triturated in water, dissolved in acetonitrile,
decolorized with
activated carbon, dried over MgSO4, and recrystallized from acetonitrile. Mp:
258-
262 C. Mass spectrum: M + 1= 462. 'H NMR (S, d6-DMSO, 400 MHz): 2.83-2.95
(m, 4H); 7.26 (s, 1H); 7.30-7.42 (m, 5H); 7.50-7.58 (m, 3H); 7.65-7.68 (m,
IH);
7.78 (s, 1H); 8.53 (d, 1H, J = 5.1 Hz); 10.36 (s, 1H).
Step 2
1-(4-Fluorophenyl)-8-[(2-morpholin-4-ylisonicotinoyl)amino]-4,5-dihydro-1 H-
benzo [g] indazole-3-carboxamide
240
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
F
NH N-N 0~N N
, \ NH
O~ 0
[00311] The product of step 1 (2.17 mmol) and morpholine (32.7 mmol)
were dissolved in 10 mL N,N-dimethylacetamide. The reaction mixture was placed
under nitrogen and stirred in an oil bath at 80 C for 160 h then 100 C for 172
h.
The mixture was partially stripped of solvent then added to water causing a
precipitate, filtered, and washed with water. The solid was recrystallized
from
acetonitrile, and triturated in diethyl ether, ethyl acetate, acetonitrile,
and ethanol.
The product was dissolved in tetrahydrofuran, dried over MgSO4, and the
solvent
stripped. Finally, the product was dissolved in ethanol followed by stripping
to
remove trapped solvents. Mp: 269-274 C (decomp.). Mass spectrum: M + 1= 513.
1H NMR (S, d6-DMSO, 400 MHz): 2.56-2.59 (m, 4H); 3.44 (t, 4H, J = 4.8 Hz);
3.66
(t, 4H, J = 4.8 Hz); 6.88-6.92 (m, 1H); 7.02 (s, 1H); 7.24-7.43 (m, 6H); 7.50-
7.58
(m, 3H); 8.19 (d, 1H, J = 5.1 Hz); 10.12 (s, 1H).
Example 466
8- { [5-chloro-2-(1,4-diazepan-1-yl)isonicotinoyl]amino }-1-(4-fluorophenyl)-
4,5-
dihydro-1 H-benzo [g] indazole-3-carboxamide
F
CI O H N-N NH2
N
N O
N
"
NH
C
[00312] The title compound was synthesized by the same procedure as in
Example 214 starting with 8-[(2,5-dichloroisonicotinoyl)amino]-1-(4-
fluorophenyl)-
241
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
4,5-dihydro-lH-benzo[g]indazole-3-carboxamide (Example 248) (1 g, 2 mmol) and
homopiperazine (4 g, 40 mmol) in 5 mL EtOH. The reaction was run at 100 C for
24 h. The white precipitate that formed in the crude reaction mixture was
filtered
and washed with EtOH to afford the title product, yield: 82%. 1H NMR (400 MHz,
d6-DMSO): 6 1.68-1.72 (m, 2H), 2.63 (t, 2H, J= 5.84 Hz), 2.79 (t, 2H, J= 5
Hz),
2.91-2.93 (m, 4H), 3.59 (t, 2H, J= 5 Hz), 3.64 (t, 2H, J= 5.84 Hz), 6.62 (s,
1H), 7.2
(d, 1H, J = 2 Hz), 7.28-7.4 (m, 4H), 7.44 (dd, 1H, J = 8.2 Hz, 2 Hz), 7.55-
7.60 (m,
3H), 8.08 (s, 1H), 10.32 (s, 1H). Mass spectrum, M + 1= 561.
Example 467
8-({ 5-Chloro-2-[4-(2-hydroxyethyl)piperazin-1-yl]isonicotinoyl } amino)-1-(4-
fluorophenyl)-4, 5-dihydro-1 H-benzo [g] indazole-3 -carboxamide
F
H N-N
CONH2
O N [/
CI
I
N N~
''NNO~OH
[00313] The title compound was synthesized by the same procedure as in
Example 214 starting with 8-[(2,5-dichloroisonicotinoyl)amino]-1-(4-
fluorophenyl)-
4,5-dihydro-lH-benzo[g]indazole-3-carboxamide (Example 248) (0.8 g, 1.6 mmol)
and 1-(2-hydroxyethyl)piperazine (4.19 g, 3.94 mL, 32 mmol). The reaction was
run
at 100 C for 24 h. The white precipitate that formed in the crude reaction
mixture
was filtered and washed with EtOH and finally triturated with CHC13 to afford
the
title product, yield: 66%. 1H NMR (400 MHz, d6-DMSO): S 2.38 (t, 2H, J = 6.2
Hz), 2.44 (t, 4H, J= 4.7Hz), 2.87-2.93 (m, 4H), 3.45-3.52(m, 4H + 2H), 4.39
(t, 1H,
J= 5.3 Hz), 6.87 (s, 1H), 7.2 (d, 1H, J= 2 Hz), 7.27 (s, 1H), 7.29-7.38 (m,
3H), 7.4
(dd, 1H, J= 8.2 Hz, 2 Hz), 7.54-7.58 (m, 3H), 8.12 (s, 1H), 10.31 (s, 1H).
Mass
spectrum, M + 1 = 591.
242
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Example 468
8-( { 5-Chloro-2- [4-(2-methoxyethyl)piperazin-1-yl]isonicotinoyl } amino)- 1 -
(4-
fluorophenyl)-4,5-dihydro-1 H-benzo [g] indazole-3-carboxamide
F
N~ CIH N-N
rN i N
~J O I i \ NH2
OS N
1
[00314] The title compound of Example 248 (8-[(2,5-
dichloroisonicotinoyl)amino]-1-(4-fluorophenyl)-4,5-dihydro-IH-
benzo[g]indazole-
3-carboxamide) (1.61 mmol) and 1-(2-methoxyethyl)-piperazine (16.25 mmol) were
combined with 4.0 mL of DMA. . The reaction mixture was placed under nitrogen
and stirred in an oil bath at 100 C for 25 h. The mixture was added to water
causing
a precipitate, filtered, and washed with water. The product was dissolved in ,
tetrahydrofuran, dried over MgSO4, and the solvent stripped. The product was
purified by nearly dissolving in ethanol, adding acetonitrile, the solid
triturated in
the solvent mix, the solvent partially stripped, and slurry filtered.
Elemental
analysis: 61.65% C, 5.22% H, 16.04% N (theory: 61.64% C, 5.17% H, 16.23% N).
Mp: 238-240 C. Mass spectrum: M + 1 = 604.
Example 469
8-[(5-Chloro-2-{ [2-(dimethylamino)ethyl]amino }isonicotinoyl)amino]-1-(4-
fluorophenyl)-4,5-dihydro-1 H-benzo[g]indazole-3-carboxamide
243
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
F
H N-N
O N I ~ CONH2
CI /
N I H~
/1 \
[00315] The title compound was synthesized by the same procedure as in
Example 214 starting with 8-[(2,5-dichloroisonicotinoyl)amino]-1-(4-
fluorophenyl)-
4,5-dihydro-lH-benzo[g]indazole-3-carboxamide (Example 248) (1 g, 2 mmol) and
N,N-dimethylethylenediamine (3.55 g, 4.42 mL, 40 mmol). The reaction was run
at
100 C for 24 h. The white precipitate that formed in the crude reaction
mixture was
filtered and washed with EtOH and finally purified by reverse phase
preparative
HPLC to afford the title product, yield: 14%. 1H NMR (400 MHz, d6-DMSO): S
2.13 (s, 6H), 2.34 (t, 2H, J= 6.5 Hz), 2.87 - 2.92 (m, 4H), 3.29 - 3.30 (m,
2H), 6.49
(s, 1H), 6.78 (t, 1H, J= 5.3 Hz), 7.2 (d, 1H, J= 2 Hz), 7.27 (s, 1H), 7.29 -
7.40 (m,
4H), 7.53 - 7.58 (m, 3H), 7.99 (s, 1H), 10.3 (s, 1H). Mass spectrum, M + 1=
549.
Example 470
8-({5-Chloro-2-[(3R)-3-methylpiperazin-1-yl]isonicotinoyl}amino)-1-(4-
fluorophenyl)-4,5-dihydro-1H-benzo [g]indazole-3-caxboxamide
F
Q
CI H N-~ ON 1)6--c2
~
[00316] The title compound of Example 248 (8-[(2,5-
dichloroisonicotinoyl)amino]-1-(4-fluorophenyl)-4,5-dihydro-lH-
benzo[g]indazole-
3-carboxamide) (2.62 mmol) and (R)-2-methylpiperazine (25.6 mmol) were
244
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
combined with 6.5 mL of DMA. The reaction mixture was placed under nitrogen
and stirred in an oil bath at 100 C for 15.5 h. The mixture was then added to
water,
causing a precipitate, which was filtered and washed with water. The solid was
dissolved in acetonitrile, dried over MgSO4, and the solvent stripped. Mp.,
275-
278 C (decomp.). Mass spectrum: M + 1 = 560.
Example 471
8-( { 5-Chloro-2-[(3S)-3-methylpiperazin-1-yl]isonicotinoyl } amino)-1-(4-
fluorophenyl)-4,5-dihydro-1 H-benzo[g]indazole-3-carboxamide
F
/ ~
N~ CI H ~ N-N
~N N
~ ~
HNJ O J i NH2
[00317] The title compound of Example 248 (8-[(2,5-
dichloroisonicotinoyl)amino]-1-(4-fluorophenyl)-4,5-dihydro-1 H-benzo
[g]indazole-
3-carboxamide) (2.63 mmol) and (S)-2-methylpiperazine (25.1 mmol) were
combined with 6.5 mL N,N-dimethylacetamide. The reaction mixture was placed
under nitrogen and stirred in an oil bath at 100 C for 15.5 h. The mixture was
then
added to water causing a precipitate, which was filtered, and washed with
water.
The solid was dissolved in acetonitrile, dried over MgSO4, and the solvent
stripped.
Mp: 275-278 C (decomp.). Mass spectrum: M + 1 = 560.
Example 472
8-( { 5-Chloro-2-[(3R,5S)-3,5-dimethylpiperazin-1-yl]isonicotinoyl } amino)-1-
(4-
fluorophenyl)-4,5-dihydro-1 H-benzo[g]indazole-3-carboxamide
245
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
F
CIH -N
N O NH2
H
[00318] The title compound of Example 248 (8-[(2,5-
dichloroisonicotinoyl)amino]-1-(4-fluorophenyl)-4,5-dihydro-1 H-benzo [g]
indazole-
3-carboxamide) (2.62 mmol) and cis-2,6-dimethyl piperazine (26.2 mmol), were
combined with 6.5 mL DMA. The reaction mixture was placed under nitrogen and
stirred in an oil bath at 100 C for 24 h. The mixture was then added to water,
causing a precipitate, which was filtered and washed with water. The solid was
dissolved in acetonitrile, dried over MgSO4, and the solvent stripped. Mp: 250
C
(decomp.). Mass spectrum: M+' 1 = 574.
[00319] Examples 473, 474, 475 were synthesized by the following synthesis
method, illustrated for 473.
SCHEME XXXIII
r-~ CbzCI, Et3N /-\ CH2O, NaBH(OAc)3 Pd/C, H2(15 psi), ~
HN NH CHCI3 Cbz-N NH DCE, rt, 2h Cbz-N/ N- rt HN N-
~{'
F \ H F
I ~ N
I ~ N-N
H
O N \ \ N CONH2 ;N' O N \ ~\ CONH2
ci ! / C1
EtOH, 100 C
N CI N
N'
Example 473
8-( { 5-Chloro-2-[(3R,5S)-3,4,5-trimethylpiperazin-l-yl]isonicotinoyl } amino)-
1-(4-
fluorophenyl)-4,5-dihydro-1 H-benzo[g]indazole-3-carboxamide
246
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Step 1
Benzyl (3R,5S)-3,5-dimethylpiperazine-l-carboxylate
Cbz-N NH
'
[00320] Benzyl chloroformate (9.7g, 8.12 mL, 54.1 mmol) was slowly added
to a mixture of 2,6-dimethylpiperazine (6.25g, 54.7 mmol) and Et3N (5.53g, 7.6
mL, 54.7 mmol) in 100 mL CHC13. The reaction mixture was stirred at room
temperature for 4h. Water was added and the organic phase separated and dried
over
MgSO4. Purification by chromatography on silica gel (CH2C12/MeOH: 12/1)
afforded the title compound in 60% yield. 1H NMR (300 MHz, CDC13): S 1.04 (d,
6H, J = 6.2 Hz), 1.46 (s, broad, 1H), 2.38 (m, broad, 2H), 2.78 (s, broad,
2H), 3.99 -
4.03 (m, broad, 2H), 5.12 (s, 2H), 7.34 - 7.36 (m, 5H), 13C NMR (300 MHz,
CDC13): S 19.5, 50.9, 67.3, 128.1, 128.2, 128.7, 137.0, 155.3.
Step 2
Benzyl (3R,5S)-3,4,5-trimethylpiperazine-l-carboxylate
Cbz-N N-
[00321] NaBH(OAc)3 (2.96 g, 14 mmol) was added to a mixture of the
product of step 1 (2.34 g, 10 mmol) and formaldehyde (0.86 g, 0.79 mL, 10
mmol)
in 35 mL dichloroethane. The reaction mixture was stirred at room temperature
for
1 h 40 min and quenched by adding an aqueous saturated solution of NaHCO3. The
product was extracted with EtOAc and dried over MgSO4. Evaporation of the
volatiles afforded the titled compound in 76% yield. 1H NMR (300 MHz, CDC13):
S
1.09 (d, 6H, J= 6.1 Hz), 2.16-2.17 (m, 2H), 2.25 (s, 3H), 2.65 (m, broad, 2H),
3.95
(m, broad, 2H), 5.13 (s, 2H), 7.33 - 7.36 (m, 5H). Mass spectrum, M + 1 = 263.
247
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Step 3
(2R,6S)-1,2,6-Trimethylpiperazine
HN N-
[00322] A mixture of the product of step 2(2.75 g, 10 mmol) and Pd/C (5%,
1.1 g) in 20 mL MeOH was reacted for 2 h at room temperature under H2 (15psi).
The mixture was filtered through Celite and the filtrate evaporated to afford
the title
compound in 80% yield. 1H NMR (400 MHz, CDC13): S 1.0 (d, 6H, J = 6.1 Hz),
1.78 (m, broad, 1H), 2.0-2.05 (m, 2H), 2.21 (s, 3H), 2.5 (t, 2H, J = 10.7),
2.82 (dd,
2H, J = 12.5 Hz, 1.8 Hz). Mass spectrum, M + 1 = 129.
Step 4
8-({5-Chloro-2-[(3R,5S)-3,4,5-trimethylpiperazin-1-yl]isonicotinoyl}amino)-1-
(4-
fluorophenyl)-4,5-dihydro-1 H-benzo [g] indazole-3-carboxamide
F
_
H N N
O N jEI5CONH2
CI
N N
NI-I
1-f I
[00323] The title compound was synthesized by the same procedure as in
Example 214 starting with 8-[(2,5-dichloroisonicotinoyl)amino]-1-(4-
fluorophenyl)-
4,5-dihydro-lH-benzo[g]indazole-3-carboxamide) (0.49 g, 1.0 mmol) and (2R,6S)-
1,2,6-trimethylpiperazine (step 3) (1.03 g, 8.0 mmol) in 5 mL of EtOH. The
reaction was run at 100 C for 5 days. The off-white precipitate that formed in
the
248
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
crude reaction mixture was filtered and washed with EtOH to afford the title
compound in 77% yield. 1H NMR (400 MHz, d6-DMSO): 81.01 (d, 6H, J= 12.3
Hz), 2.05 (m, broad, 2H), 2.13 (s, 3H), 2.47-2.53 (2H, m, overlaps with DMSO),
2.88-2.93 (m, 4H), 4.11 (d, 2H, J= 12.3 Hz), 6.92 (s, 1 H), 7.21 (d, 1 H, J=
2.0Hz),
7.27 (s, 1H), 7.3-7.38 (m, 3H), 7.41 (dd, 1H, J= 8.2 Hz, 2.0 Hz), 7.54-7.58
(m, 3H),
8.11 (s, 1H), 10.32 (s, 1H). HRMS calc. for C31H32C1FN702 588.2285, found
588.2212.
Example 474
8-({5-Chloro-2-[(3R)-3,4-dimethylpiperazin-1-yl]isonicotinoyl}amino)-1-(4-
fluorophenyl)-4,5-dihydro-1 H-benzo [g]indazole-3-carboxamide
Step 1
Benzyl (3R)-3-methylpiperazine-l-carboxylate
Cbz-N NH
[00324] The product of step 1 was synthesized by the same procedure as for
compound of step 1 of Example 473 starting with 2-(R)-methylpiperazine (6 g,
59.9
mmol), triethylamine (6.04 g, 8.32 mL, 59.7 mmol) and benzyl chloroformate
(10.62 g, 59.2 mmol) in 100 mL of CHC13. Purification by chromatography on
silica
gel (CH2C12/MeOH: 12/1) afforded the title compound in 30% yield.1H NMR (300
MHz, CDC13): 8 1.05 (d, 3H, J= 6.2 Hz), 1.59 (s, 1H), 2.47 (s, broad, 1H),
2.71 -
2.9 (m, 4H), 4.02 (s, broad, 1H), 5.13 (s, 2H), 7.30 - 7.36 (m, 5H).
Step 2
Benzyl (3R)-3,4-dimethylpiperazine-l-carboxylate
Cbz-N N-
249
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
[00325] The title compound of,step 2 was synthesized by the same procedure
as for the compound of step 2 of Example 473 starting with compound of step 1
(3.77 g, 16 mmol), formaldehyde (1.46 g, 1.34 mL, 16 mmol), and NaHB(OAc)3
(4.98 g, 23 mmol) in 60 mL of dichioroethane to afford the title compound in
95 %
yield. 1H NMR (300 MHz, CDC13): S 1.05 (d, 3H, J= 6.24 Hz), 2.02 - 2.08 (m,
1H), 2.13-2.21 (m, 1H), 2.28 (s, 3H), 2.72-2.76 (m, 2H), 3.08 (t, 1H, J= 1.2
Hz),
3.95 - 3.99 (m, 2H), 5.12 (s, 2H), 7.32 - 7.36 (m, 5H).
Step 3
(2R)-1,2-Dimethylpiperazine
HN N-
[00326] The title compound of step 3 was synthesized by the same procedure
as fo'r step 3 of Example 473 starting with the compound of step 2 (3.78 g,
15.2
mmol), (Pd/C (5%, 1.6g) in 30 mL of MeOH under H2 (15 psi). The reaction
mixture was stirred 4 h to afford the title compound in 53% yield. 1H NMR (300
MHz, CDC13): S 1.03 (d, 3H, J= 6.24 Hz), 1.96-2.03 (m, 1H), 2.12 - 2.21 (m,
1H),
2.27 (s, 1H), 2.34 (s, broad, 1H), 2.45 - 2.52 (m, 1H), 2.73 - 2.92 (m, 4H).
Mass
spectrum, M + 1= 115. HRMS calc. for C6H15N2115.1230, found 115.1199.
Step 4
8-( { 5-Chloro-2-[(3R)-3,4-dimethylpiperazin-l-yl]isonicotinoyl } amino)-1-(4-
fluorophenyl)-4,5-dihydro-1 H-benzo[g]indazole-3-carboxamide
250
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
F
_
H N N
O N CONH2
CI
J
N
("'N
[00327] The title compound was synthesized by the same procedure as in
Example 214 starting with 8-[(2,5-dichloroisonicotinoyl)amino]-1-(4-
fluorophenyl)-
4,5-dihydro-lH-benzo[g]indazole-3-carboxamide (Example 248) (0.434 g, 0.87
mmol) and (2R)-1,2-dimethylpiperazine (step 3) (0.9 g, 7.8 mmol) in 5 mL of
EtOH. The reaction was run at 100 C for 4 days. The off-white precipitate that
formed in the crude reaction mixture was filtered and washed with EtOH to
afford
the title compound in 55% yield. 1H NMR (400 MHz, d6-DMSO): S 1.0 (d, 3H, J
6.17 Hz), 1.96 - 2.0 (m, 1 H), 2.05 - 2.06 (m, IH), 2.15 (s, 3H), 2.48 - 2.54
(m, 1 H),
2.74 (d, broad, 1H, J= 11.5 Hz), 2.85 - 2.93 (m, 4H), 4.01-4.09 (m, 2H), 6.89
(s,
1 H), 7.21 (d, 1 H, J= 2.0 Hz), 7.27 (s, 1 H), 7.29 - 7.38 (m, 2H), 7.41 (dd,
1 H, J=
8.2 Hz, 2.0 Hz), 7.53 - 7.58 (m, 2H), 8.11 (s, 1H), 10.33 (s, 1H). HRMS calc.
for
C30H30C1FN702 574.2128, found 574.2094.
Example 475
8-( { 5-Chloro-2-[(3S)-3,4-dimethylpiperazin-l-yl]isonicotinoyl } amino)-1-(4-
fluorophenyl)-4,5-dihydro-1 H-benzo[g]indazole-3-carboxamide
Step 1
Benzyl (3S)-3-methylpiperazine-l-carboxylate
/-\
Cbz-N NH
251
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
[00328] The compound of step 1 was synthesized by the same procedure as
for step 1 of Example 473 starting with 2-(S)-methylpiperazine (6 g, 59.9
mmol),
triethylamine (6.04 g, 8.32 mL, 59.7 mmol) and benzyl chloroformate (10.62 g,
59.2
mmol) in 100 mL CHC13. Purification by chromatography on silica gel
(CH2C12/MeOH: 12/1) afforded the title compound in 22% yield. 1H NMR (300
MHz, CDC13): 8 1.05 (d, 3H, J= 6.2 Hz), 1.89 (s, 1H), 2.48 (s, broad, 1H), 2.7-
2.97
(m, 4H), 4.02 (s, broad, 1H), 5.12 (s, 2H), 7.32 - 7.35 (m, 5H).
Step 2
Benzyl (3S)-3,4-dimethylpiperazine-l-carboxylate
/-1
Cbz-N N-
[00329] The compound of step 2 was synthesized by the same procedure as
for step 2 for Example 473 starting with the compound of step 1 (3.1 g, 13
mmol),
formaldehyde (1.2 g, 1.1 mL, 13 mmol), and NaHB(OAc)3 (4.09 g, 19 mmol) in 50
mL dichloroethane to afford the title compound in 96 % yield. 1H NMR (300 MHz,
CDC13): S 1.05 (d, 3H, J= 6 Hz), 2.02-2.05 (m, 1H), 2.13-2.21 (m, 1H), 2.28
(s,
3H), 2.71-2.75 (m, 2H), 3.08 (t, 1H, J= 1.5 Hz), 3.95 - 3.99 (m, 2H), 5.12 (s,
2H),
7.32 - 7.36 (m, 5H).
Step 3
(2S)-2-Methylpiperazine
H N-
[00330] (2S)-2-Methylpiperazine was synthesized by the same procedure as
for step 3 of Example 473 starting with the material from step 2 (3.1 g, 12.4
mmol),
(Pd/C (5%, 1.37g) in 25 mL MeOH under H2 (15 psi). The reaction mixture was
252
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
stirred 4 h to afford the title compound in 55% yield. 1H NMR (300 MHz,
CDC13):
S 1.03 (d, 3H, J= 6.24 Hz), 1.97 - 2.03 (m, 1H), 2.12 - 2.21 (m, 1H), 2.27 (s,
1H),
2.42 (s, broad, 1H), 2.45 - 2.52 (m, 1H), 2.73 - 2.92 (m, 4H). Mass spectrum,
M +
1=115.
Step 4
8-( { 5-Chloro-2-[(3S)-3,4-dimethylpiperazin-l-yl]isonicotinoyl } amino)-1-(4-
fluorophenyl)-4,5-dihydro-1 H-benzo[g]indazole-3-carboxamide
F
_
H N N
O N CONH2
CI
N N
[00331] The title compound was synthesized by the same procedure as in
Example 214 starting with 8-[(2,5-dichloroisonicotinoyl)amino]-1-(4-
fluorophenyl)-
4,5-dihydro-lH-benzo[g]indazole-3-carboxamide (Example 248) (0.380 g, 0.76
mmol) and (2S)-2-methylpiperazine (step 3) (0.79 g, 6.8 mmol) in 3 mL of EtOH.
The reaction was run at 100 C for 3 days. The off-white precipitate that
formed in
the crude reaction mixture was filtered and washed with EtOH to afford the
title
compound in 55% yield. 1H NMR (400 MHz, d6-DMSO): 81.0 (d, 3H, J= 6.17
Hz), 1.96-1.98 (m, 1H), 2.02 - 2.09 (m, 1H), 2.16 (s, 3H), 2.48 - 2.54 (m,
1H), 2.74
(d, broad, IH, J= 11.7 Hz), 2.85-2.93 (m, 4H), 4.02-4.09 (m, 2H), 6.89 (s,
1H),
7.21 (d, 1H, J= 2.0 Hz), 7.27 (s, 1H), 7.29 - 7.38 (m, 2H), 7.41 (dd, 1H, J=
8.2 Hz,
2.0 Hz), 7.54 - 7.58 (m, 2H), 8.11 (s, 1H), 10.33 (s, 1H). HRMS calc. for
C30H30C1FN702 574.2128, found 574.2098.
253
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Example 476
8-{ [5-Chloro-2-(4-ethylpiperazin-1-yl)isonicotinoyl]amino }-1-(4-
fluorophenyl)-4,5-
dihydro-1 H-benzo [g] indazole-3-carboxamide
F
/ ~
N~ I CIH N_N N N
I ~ \ 10"
~N"o
[00332] The title compound of Example 248 (8-[(2,5-
dichloroisonicotinoyl)amino]-1-(4-fluorophenyl)-4,5-dihydro-lH-
benzo[g]indazole-
3-carboxamide) (1.81 mmol) and 1-ethyl piperazine (18.1 mmol) were combined
with 4.5 mL of DMA. The reaction mixture was placed under nitrogen and stirred
in
an oil bath at 100 C for 21 h. The mixture was added to water, causing a
precipitate,
which was filtered and washed with water. The solid was dissolved in
acetonitrile,
dried over MgSO4, filtered, and washed with acetonitrile. Mp: 251-254 C. Mass
spectrum: M + 1 = 574.
Example 477
8- { [5-Chloro-2-(4-isopropylpiperazin-1-yl)isonicotinoyl]amino}-1-(4-
fluorophenyl)-4,5-dihydro-1 H-benzo [g] indazole-3 -carboxamide
F
N~ ~ CIH / N-N O
N ~ N
NH2
-y
[00333] The title compound of Example 248 (8-[(2,5-
dichloroisonicotinoyl) amino]-1-(4-fluorophenyl)-4, 5-dihydro-1 H-benzo [g]
indazole-
3-carboxamide) (1.81 mmol) and (1-isopropyl) piperazine (18.1 mmol) were
254
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
combined with 4.5 mL of DMA. The reaction mixture was placed under nitrogen
and stirred in an oil bath at 100 C for 21 h. The mixture was added to water,
causing a precipitate, which was filtered and washed with water. The solid was
dissolved in acetonitrile, dried over MgSO4, and the solvent stripped. The
product
was then filtered and washed with acetonitrile. Mp: 220-226 C. Mass spectrum:
M
+1=588.
Example 478
8-1 [5-Chloro-2-(4-methylpiperazin- 1 -yl)isonicotinoyl] amino } -1-pyridin-4-
yl-4, 5-
dihydro-1H-benzo[g]indazole-3-carboxamide
Step 1
8-[(2,5-Dichloroisonicotinoyl)amino]-1-pyridin-4-yl-4,5-dihydro-1 H-
benzo [g]indazole-3-carboxamide
H N-N
O N ~ CONH2
ci I
N CI
[00334] The compound of step 1 was synthesized by the same procedure as
in Example 211, starting with 8-amino-1-pyridin-4-yl-4,5-dihydro-lH-
benzo[g]indazole-3-carboxamide (1.56 g, 5.1 mmol), 2,5-dichloroisonicotinic
acid
(1.47 g, 7.6 mmol), HATU (2.91 g, 7.3 mmol) and Et3N (2.85 g, 2.07 mL, 28
mmol) in DMF (25 mL). The crude reaction mixture was concentrated and the
product precipitated upon addition of water and filtered. Trituration in 250
mL hot
CH3CN afforded the title compound as a yellow solid, yield 40%. 1H NMR (400
MHz, d6-DMSO): S 2.92 (s, 4H), 7.33-7.4 (m, 3H), 7.52 (dd, 1H, J= 8.15 Hz, 2
Hz), 7.62-7.64 (m, 3H), 7.8 (s, 1H), 8.61 (s, 1H), 8.74 (dd, 2H, J= 4.6 Hz,
1.5 Hz),
10.61 (s, 1H). Mass spectrum, M + 1 480.
255
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Step 2
8-{ [5-Chloro-2-(4-methylpiperazin-1-yl)isonicotinoyl]amino}-l-pyridin-4-yl-
4,5-
dihydro-1 H-benzo jg] indazole-3-carboxamide
H N-N
O N I CONH2
CI
I
N N ~
[00335] The title compound was synthesized by the same procedure as in
Example 214 starting with the compound of step 1 (0.8 g, 1.66 mmol) and N-
methylpiperazine (3 g, 3.34 mL, 30mmol) in 1.5 mL EtOH. The reaction was run
at
100 C for 2 days. The white precipitate that formed in the crude reaction
mixture
was filtered and washed with EtOH to afford the title product, yield: 73%. 1H
NMR
(400 MHz, d6-DMSO): S 2.16 (s, 3H), 2.32 (t, 4H, J= 4.8 Hz), 2.9 (s, 4H), 3.47
(t,
4H, J= 4.8 Hz), 6.89 (s, 1H), 7.34-7.38 (m, 3H), 7.54 (dd, 1H, J = 8.3 Hz, 2
Hz),
7.60-7.61 (m, 3H), 8.12 (s, 1H), 8.72 (dd, 2H, J= 4.5 Hz, 1.6 Hz), 10.4 (s,
1H).
Mass spectrum, M + 1= 544.
Example 479
1-(1,3-Benzodioxol-5-yl)-8-( { [5-chloro-2-(4-methylpiperazin-1-yl)pyrimidin-4-
yl]carbonyl } amino)-4,5-dihydro-1 H-benzo [g]indazole-3-carboxamide
Step 1
5-Chloro-2-(methylsulfonyl)-4-pyrimidinecarboxylic acid
O OH
CI N
~ i
N O,S~
256
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
[00336] 5-Chloro-2-(methylsulfonyl)-4-pyrimidinecarboxylic acid was
prepared by the method of Liang Yong-min, Luo Sheng-jun, Zhang Zhao-xin and
Ma Yong-xiang, Synthetic Commun., 32 (1), 153-157, 2002.
Step 2
1-(1,3-Benzodioxol-5-yl)-8-({ [5-chloro-2-(methylsulfonyl)pyrimidin-4-
yl]carbonyl } amino)-4,5-dihydro-1 H-benzo[g]indazole-3-carboxamide
/-O
O
1
H N-N
O N CONH2
CI N
N OSO
[00337] The title compound of step 2 was synthesized by the same procedure
as in Example 211 starting with 5-chloro-2-(methylsulfonyl)-4-
pyrimidinecarboxylic acid (step 1) (0.987 g, 4.17 mmol), Example 161, step 3(8-
amino-1 -(1,3-benzodioxol-5-yl)-4,5-dihydro-1H-benzo[g]indazole-3-carboxamide)
(0.968 g, 2.78 mmol), HATU (1.58 g, 4.15 mmol) and Et3N (1.54 g, 1.12 mL, 15
mmol) in DMF (8 mL). Trituration with CH2C12 afforded the title compound in
38% yield. 1H NMR (400 MHz, d6-DMSO): 6 2.86-2.91 (m, 4H), 3.42 (s, 1H), 6.05
(s, 2H), 6.94 (dd, 1H, J = 8.2 Hz, 2 Hz), 7 (d, 111, J = 2.2 Hz), 7.1 (d, 1H,
J = 2 Hz),
7.23-7.26 (m, 2H), 7.31-7.37 (m, 2H), 7.48 (s, 1H), 9.35 (s, 1H), 10.73 (s,
1H).
Mass spectrum, M + 1 = 567.
Step 3
1-(1,3-Benzodioxol-5-yl)-8-( { [5-chloro-2-(4-methylpiperazin-1-yl)pyrimidin-4-
yl]carbonyl } amino)-4,5-dihydro-lH-benzo[g]indazole-3-carboxamide
257
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
/-O
O
H N-N
O N I \ CONH2
CI N /
N N
~N~
[00338] The title compound was synthesized by the same procedure as in
Example 214 starting with the compound of step 2 (0.625 g, 1.1 mmol) and N-
methylpiperazine (1.98 g, 2.2 mL, 20 mmol) in 3 mL EtOH. The reaction was run
at
95 C for 2.5 h. The volatiles were removed and the residue partitioned between
H20 and CHZCI2. The organics were dried over MgSO4. Purification by reverse
phase preparative HPLC afforded the title product in 16% yield. 1H NMR (400
MHz, d6-DMSO): S 2.18 (s, 3H), 2.33 (t, 4H, J = 4.7 Hz), 2.86-2.92 (m, 4H),
3.67
(t, 4H, J = 4.7 Hz), 6.08 (s, 2H), 6.94 (dd, 1H, J = 8.2 Hz, 2.1 Hz), 7 (d,
1H, J = 8.2
Hz), 7.1 (d, 1H, J = 2 Hz), 7.27-7.31 (m, 2H), 7.4 (dd, 1H, J = 8.2 Hz, 2 Hz),
7.49
(s, 1H), 8.5 (s, 1H), 10.34 (s, 1H). Mass spectrum, M + 1 = 567.
Example 480
8-({ [5-Chloro-2-(4-methylpiperazin-1-yl)pyrimidin-4-yl]carbonyl}amino)-1-(4-
fluorophenyl)-4,5-dihydro-lH-benzo[g]indazole-3-carboxamide
Step 1
8-({ [5-Chloro-2-(4-methylpiperazin-1-yl)pyrimidin-4-yl]carbonyl } amino)-1-(4-
fluorophenyl)-4,5-dihydro-lH-benzo[g]indazole-3-carboxamide
F a-
H N N
O N N~ CONH2
CI N /
N OAS~O
258
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
[00339] The compound of step 1 was synthesized by the same procedure as
in Example 211 starting with the compound of step 1 of Example 479 (2.7 g,
11.4
mmol), 8-amino-l-(1,3-benzodioxol-5-yl)-4,5-dihydro-lH-benzo[g]indazole-3-
carboxamide (2.45 g, 7.6 mmol), HATU (4.32 g, 11.3 mmol) and Et3N (2.17 g, 3
mL, 21 mmol) in DMF (8 mL). Purification by preparative reverse phase HPLC
afforded the title compound in 62% yield. 1H NMR (400 MHz, d6-DMSO): 8 2.88-
2.92 (m, 4H), 3.41 (s, 3H), 7.22 (s, broad, 1H), 7.25 (s, broad, 1H), 7.34-
7.37 (m,
4H), 7.52-7.58 (m, 3H), 9.34 (s, IH), 10.72 (s, 1H). Mass spectrum, M + 1=
541.
Step 2
8-( { [5-Chloro-2-(4-methylpiperazin-1-yl)pyrimidin-4-yl]carbonyl } amino)-1-
(4-
fluorophenyl)-4,5-dihydro-1 H-benzo [g]indazole-3 -carboxamide
F
_
H N N
O N ~ \ ~ CONH2
CI / N
N N "')
~N'~'
[00340] The title compound was synthesized by the same procedure as in
Example 214 starting with the material from step 1 (0.9 g, 1.66 mmol) and N-
methylpiperazine (1.66 g, 1.84 mL, 16.5 mmol) in 3 mL EtOH. The reaction was
run at 45 C for 7 h. The yellow precipitate that formed in the crude reaction
mixture
was filtered and purified by reverse phase preparative HPLC to afford the
title
product in 15% yield. 1H NMR (400 MHz, d6-DMSO): S 2.18 (s, 3H), 2.32 (t, 4H,
J = 4.9 Hz), 2.88-2.93 (m, 4H), 3.68 (t, 4H, J= 4.9 Hz), 7.18 (t, 1H, J = 2.4
Hz),
7.27 (s, 1H), 7.31-7.39 (m, 3H), 7.42 (dd, 1H, J = 8.2 Hz, 2 Hz), 7.53-7.59
(m, 3H),
8.49 (s, 1H), 10.412 (s, 1H). Mass spectrum, M + 1 = 562.
259
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
[00341] The bioactivity in the IKK2 Resin assay for the compounds of
Examples 452-480 is shown in Table 24
Table 24
Compound No., Structure Compound Name IKK2 Resin Example
IC50
0 $-[(2,$- <1 M 453 step I
Dichloroisonicotinoyl)
amino]-1-[4-
N' H N-N O (methylsulfonyl)pheny
N 1]-4,5-dihydro-lH-
0 NH2 benzo[g]indazole-3-
carboxamide
0 8-{ [5-Chloro-2-(4- <i M 453
OY\' methylpiperazin-l-
ci N-N yl)isonicotinoyl]amin
N ~ 0}-1-[4-
~N NH2 (methylsulfonyl)pheny
1]-4,5-dihydro-lH-
benzo[g]indazole-3-
carboxamide
O~O 1-(1,3-Benzodioxol-5- <i M 454
yl)-8-{[(6-chloro-4-
CI ~ methylpyridin-3-
Nol N N-N O yl)carbonyl]amino}-
O ~ NH2 4,5-dihydro-lH-
benzo[g]indazole-3-
carboxamide
1-(1,3-Benzodioxol-5- 1< 10 M 455 step 1
yl)-8-{[(3-chloro-6-
r c~ N-N cyanopyridin-2-
~ n~i yl)carbonyl]amino}-
N N o \~ ONH2 4,5-dihydro-lH-
benzo[g]indazole-3-
carboxamide
O 8-( {[6- 1< 10 M 455
(Aminomethyl)-3-
~ a ~ N-N chloropyridin-2-
, N yl]carbonyl } amino)-1-
N ~ CONH2 (1,3-benzodioxol-5-
NH2 0 ~ yl)-4,5-dihydro-lH-
benzo[g]indazole-3-
carboxamide
260
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Compound No., Structure Compound Name IKK2 Resin Example
TC50
F 8-[(5-Chloro-2- <1 M 456 step 1
ci cyanoisonicotinoyl)am
H ino]-1-(4-
N N CONH2 fluorophenyl)-4,5-
o Z~'
dihydro-1 H-
benzo[g]indazole-3-
carboxamide
8-{ [2-(Aminomethyl)- <1 M 456
ANr'II 5-
r"~ CONH2 chloroisonicotinoyl]a
"c~
NHZ 0 mino }-1-(4-
~ I
fluorophenyl)-4,5-
dihydro-lH-
benzo[g]indazole-3-
carboxamide
1-(1,3-Benzodioxol-5- <1 M 457 step 1
yl)-8-[(5-chloro-2-
Nr ci ~ N cyanoisonicotinoyl)am
N N/ CONHZ ino]-4,5-dihydro-1H-
0 benzo[g]indazole-3-
carboxamide
8-{ [2-(Aminomethyl)- 51 M 457
H 5-
N~ ci N chloroisonicotinoyl]a
$N-
~ ~ N mino}-1-(1,3-
CONHZ
benzodioxol-5-yl)-
N"2 0 4,5-dihydro-lH-
benzo[g]indazole-3-
carboxamide
~ 8-({ [3-Chloro-6- <1 M 458
N~ ~ cl H~ N-N (morpholin-4-
vN_/~N .CONH ylmethYl)pyridin-2-
~ Y1]carbonY1 } amino)-1-
(4-fluorophenyl)-4, 5-
dihydro-lH-
benzo[g]indazole-3-
carboxamid
o 1-(1,3-Benzodioxol-5- <1 M 459
ZxC, yl)-8-({[3-chloro-6-
~ cl " N-N NH2 (morpholin-4-
0 ylmethyl)pyridin-2-
0 y1]carbony1} amino)-
4,5-dihydro-lH-
benzo[g]indazole-3-
carboxamide
261
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Compound No., Structure Compound Name IKK2 Resin Example
IC50
0 O 1-(1,3-Benzodioxol-5- <1 M 460
y1)-8-{[5-chloro-2-(2-
~ morpholin-4-
H N-N ylethyl)isonicotinoyl]a
O N CONH2 mino}-4,5-dihydro-
CI 1H-benzo[g]indazole-
3-carboxamide
N co
F 8-{[5-Chloro-2-(2- <1 M 461
morpholin-4-
H N-N ylethyl)isonicotinoyl]a
O N CONHZ mino}-1-(4-
CI fluorophenyl)-4,5-
~ dihydro-lH-
N N) benzo[g]indazole-3-
~'O carboxamide
8-( { 5-Chloro-2-[2- <1 M 462
/ (dimethylamino)ethyl]
H N-N isonicotinoyl } amino)-
O N CONH2 1-(4-fluorophenyl)-
J 4,5-dihydro-lH-
CI benzo
[g]indazole-3-
N carboxamide
8-{[(5-Chloro-2,4'- <i M 463
N-N bipyridin-4-
o N yl)carbonyl]amino}-1-
I CONH2 (4-fluorophenyl)-4,5-
CI dihydro-
~ 1H-benzo[g]indazole-
N 3-carboxamide
N
F 8-{[(5-chloro-1'- <1 M 464
methyl-1',2',3',6'-
N-N tetrahydro-2,4'-
N bipyridin-4-
CI O J CONH2 yl)carbonyl]amino}-1-
i (4-fluorophenyl)-4,5-
N dihydro-1 H-
N", benzo[g]indazole-3-
carboxamide
262
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Compound No., Structure Compound Name IKK2 Resin Example
IC50
F 1-(4-Fluorophenyl)-8- 51 M 465
~\
~ [(2-morpholin-4-
N H N-N o ylisonicotinoyl)amino
~J o N O13'iH2 ]-4,5-dihydro-lH-
benzo[g]indazole-3-
carboxamide
F 8-{[5-chloro-2-(1,4- <1 M 466
diazepan-l-
ci 0 N-N NH yl)isonicotinoyt]amin
H ~ 2 0 } -1-(4-fluorophenyl)-
N N 0 4,5-
dihydro-lH-
N~ benzo[g]indazole-3-
\\\~~~~ carboxamide
NH
F.
8-( { 5-Chloro-2-[4-(2- 51 pM 467
hydroxyethyl)piperazi
H N-N n-1-
O N CONH2 yl]isonicotinoyl}amin
Ci o)-1-(4-
~ fluorophenyl)-4,5-
N N dihydro-1 H-
~N,~oH benzo[g]indazole-3-
carboxamide
F 8-({5-Chloro-2-[4-(2- <_1 M 468
ci N-N methoxyethyl)piperazi
N o n-1-
N JN 0 1) NH2 yl]isonicotinoyl}amin
o f o)-1-(4-fluorophenyl)-
4,5-dihydro-1 H-
benzo[g]indazole-3-
carboxamide
F 8-[(5-Chloro-2-{[2- <1 M 469
Q (dimethylamino)ethyl]
H N-N amino } isonicotinoyl)a
O N CONH2 mino]-1-(4-
Cl fluorophenyl)-4,5-
~ dihydro-lH-
N N) benzo[g]indazole-3-
N*~' carboxamide
263
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Compound No., Structure Compound Name IKK2 Resin Example
IC50
F~ 8-({5-Chloro-2-[(3R)- 51 M 470
3-methylpiperazin-1-
N% N N-N o yl]isonicotinoyl}amin
NH o)-1-(4-fluorophenyl)-
~ o 2 4,5-dihydro-lH-
benzo[g]indazole-3-
carboxamide
F ~ 8-({5-Chloro-2-[(3S)- <1 M 471
3-methylpiperazin-l-
ci
H N-N o yl]isonicotinoyl}amin
HN,) o N I~ \ NH2 o)-1-(4-fluorophenyl)-
4,5-dihydro-lH-
benzo [g]indazole-3-
carboxamide
F ~ 8-({5-Chloro-2- <1 gM 472
, [(3R,5S)-3,5-
N~ H N-N o dimethylpiperazin-l-
N I ~ \ NH yl]isonicotinoyl}amin
HN~ o ~ 2 o)-1-(4-fluorophenyl)-
4,5-dihydro-lH-
benzo[g]indazole-3-
carboxamide
F ~ 8-({5-Chloro-2- <1 M 473
~ ~ _ [(3R,5S)-3,4,5-
O N ~ N trimethylpiperazin-l-
CONH2 yl]isonicotinoyl}amin
CI ~ o)-1-(4-
I fluorophenyl)-4,5-
N N~ dihydro-lH-
N", benzo[g]indazole-3-
carboxamide
F I~ 8-( { 5-Chloro-2-[(3R)- <1 M 474
_ 3,4-
O N N dimethylpiperazin-l-
CONH2 yl]isonicotinoyl}amin
cl o)-1-(4-
~ ~ fluorophenyl)-4,5-
N ~ dihydro-lH-
N benzo[g]indazole-3-
~ carboxamide
264
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Compound No., Structure Compound Name IKK2 Resin Example
IC50
8-({5-Chloro-2-[(3S)- <1 [tM 475
3,4-
H N_N dimethylpiperazin-l-
O CONH2 y1]isonicotinoyl}amin
cl o)-1-(4-
~ ~ fluorophenyl)-4,5-
N N dihydro-1 H-
benzo[g]indazole-3-
N carboxamide
F 8-{ [5-Chloro-2-(4- <1 M 476
ci ethylpiperazin-1-
N H N-N O yl)isonicotinoyl]amin
~NJ \ ~ N \ NH2 o}-1-(4-fluorophenyl)-
4,5-dihydro-lH-
benzo[g]indazole-3-
carboxamide
F ~ 8-{[5-Chloro-2-(4- <1 M 477
c ~, isopropylpiperazin-l-
N H N-N
o yl)isonicotinoyl]amin
NJ o N NH2 01-1-(4-fluorophenyl)-
4,5-dihydro-lH-
benzo[g]indazole-3-
carboxamide
8-[(2,5- <1 M 478 step 1
Dichloroisonicotinoyl)
H N-N amino]-1-pyridin-4-yl-
O N CONH2 4,5-dihydro-lH-
CI benzo[g]indazole-3-
~ ~ carboxamide
N Cl
N 8-{ [5-Chloro-2-(4- <1 M 478
methylpiperazin-l-
H N-N yl)isonicotinoyl]amin
O N CONH2 o}_1-pyridin-4-yl-4,5-
CI dihydro-lH-
benzo[g]indazole-3-
carboxamide
N ON,
265
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Compound No., Structure Compound Name IKK2 Resin Example
IC50
/_O 1-(1,3-Benzodioxol-5- <1 M 479 step 2
0 yl)-8-({[5-chloro-2-
I (methylsulfonyl)pyrim
H O N ~ N idin-4-
0 yl]carbonyl}amino)-
CI N 4,5-dihydro-lH-
~ benzo[g]indazole-3-
~N o S o carboxamide
/-O 1-(1,3-Benzodioxol-5- <1 M 479
0 yl)-8-({[5-chloro-2-(4-
I methylpiperazin-l-
H N N CONH yl)pyrimidin-4-
2 yl]carbonyl } amino)-
cl N I 4,5-dihydro-lH-
~ benzo[g]indazole-3-
N carboxamide
NNI
8-({ [5-Chloro-2-(4- <1 M 480
~ , methylpiperazin-l-
H N-N yl)pyrimidin-4-
0 N CONH2 yl]carbonyl}amino)-1-
CI N (4-
~ ~ fluorophenyl)-4,5-
N N~ dihydro-lH-
~ N ~ benzo[g]indazole-3-
carboxamide
BIOLOGICAL EVALUATION
Materials
[00342] SAM2 TM 96 Biotin capture plates were from Promega. Anti-FLAG
affinity resin, FLAG-peptide, NP-40 (Nonidet P-40), BSA, ATP, ADP, AMP, LPS
(E. coli serotype 0111:B4), and dithiothreitol were obtained from Sigma
Chemicals.
Antibodies specific for NEMO (IKKy) (FL-419), IKK1(H-744), IKK2(H-470) and
ItcBoc(C-21) were purchased from Santa Cruz Biotechnology. Ni-NTA resin was
purchased from Qiagen. Peptides were purchased from American Peptide
Company. Protease inhibitor cocktail tablets were from Boehringer Mannheim.
266
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Sephacryl S-300 column was from Pharmacia LKB Biotechnology. Centriprep-10
concentrators with a molecular weight cutoff of 10 kDa and membranes with
molecular weight cut-off of 30 kDa were obtained from Amicon. [Y'-33P] ATP
(2500 Ci/mmol) and [Y' 32P] ATP (6000 Ci/mmol) were purchased from Amersham.
The other reagents used were of the highest grade commercially available.
Cloning and Expression
[00343] cDNAs of human IKK1 and IKK2 were amplified by reverse
transcriptase-polymerase chain reaction from human placental RNA (Clonetech).
hIKK1 was subcloned into pFastBac HTa (Life Technologies) and expressed as N-
terminal His6-tagged fusion protein. The hIKK2 cDNA was amplified using a
reverse oligonucleotide primer which incorporated the peptide sequence for a
FLAG-epitope tag at the C-terminus of the IKK2 coding region (DYKDDDDKD).
The hIKK2:FLAG cDNA was subcloned into the baculovirus vector pFastBac. The
rhIKK2 (S177S, E177E) mutant was constructed in the same vector used for wild
type rhIKK2 using a QuikChangeTm mutagenesis kit (Stratagene). Viral stocks of
each construct were used to infect insect cells grown in 40L suspension
culture. The
cells were lysed at a time that maximal expression and rhIKK activity were
demonstrated. Cell lysates were stored at -80 C until purification of the
recombinant proteins was undertaken as described below.
Enzyme Isolation
[00344] All purification procedures were carried out at 4 C unless otherwise
noted. Buffers used are: buffer A: 20 mM Tris-HC1, pH 7.6, containing 50 mM
NaC1, 20 mM NaF, 20 mM (3-Glycerophosphate, 500 uM sodiumortho-vanadate,
2.5 mM metabisulfite, 5 mM benzamidine, 1 mM EDTA, 0.5 mM EGTA, 10%
glycerol, 1 mM DTT, 1X CompleteTm protease inhibitors; buffer B: same as
buffer
A, except 150 mM NaCl, and buffer C: same as buffer A, except 500 mM NaC1.
Isolation of rhIKKl hofnodimer
[00345] Cells from an 8-liter fermentation of baculovirus-expressed IKK1
tagged with His peptide were centrifuged and the cell pellet (MOI 0.1, I=72
hr) was
267
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
re-suspended in 100 ml of buffer C. The cells were microfluidized and
centrifuged
at 100,000 X g for 45 min. The supernatant was collected, imidazole added to
the
final concentration of 10 mM and incubated with 25 ml of Ni-NTA resin for 2
hrs.
The suspension was poured into a 25 ml column and washed with 250 ml of buffer
C and then with 125 inl of 50 mM imidazole in buffer C. rhIKKl homodimer was
eluted using 300 mM invidazole in buffer C. BSA and NP-40 were added to the
enzyme fractions to the final concentration of 0.1 %. The enzyme was dialyzed
against buffer B, aliquoted and stored at -80 C.
Isolation of rhIKK2 homodim.er
[00346] A 10-liter culture of baculovirus-expressing IKK2 tagged with FLAG
peptide was centrifuged and the cell pellet (MOI=0.1 and 1=72 hrs) was re-
suspended in buffer A. These cells were microfluidized, and centrifuged at
100,000
X g for 45 min. Supernatant was passed over a G-25 column equilibrated with
Buffer A. Protein peak was collected and incubated with anti-FLAG affinity
resin
on a rotator overnight in buffer B. The resin was washed in batch with 10-15
bed
volumes of buffer C. Washed resin was poured into a column and rhIKK2
homodimer was eluted using 5 bed volumes of buffer B containing FLAG peptide.
5 mM DTT, 0.1% NP-40 and BSA (concentrated to 0.1% in final amount) was
added to the eluted enzyme before concentrating in using an Amicon membrane
with a molecular weight cut-off of 30 kDa. Enzyme was aliquoted and stored at -
80
C.
Isolation of rhIKK1/IKK2 heteroditner
[00347] The heterodimer enzyme was produced by coinfection in a
baculovirus system (FLAG IKK2/IKK1 His; MOI=0.1 and 1=72 hrs). Infected cells
were centrifuged and the cell pellet (10.0 g) was suspended in 50 ml of buffer
A.
The protein suspension was microfluidized and centrifuged at 100,000 X g for
45
min. Imidazole was added to the supernatant to a final concentration of 10 mM.
The protein was allowed to bind 25 ml of Ni-NTA resin by mixing for 2 hrs. The
protein-resin slurry was poured into a 25 ml column and washed with 250 ml of
buffer A containing 10 mM imidazole followed by 125 ml of buffer A containing
268
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
50 mM imidazole. Buffer A, containing 300 mM imidazole, was then used to elute
the protein. A 75 ml pool was collected and NP-40 was added to a final
concentration of 0.1%. The protein solution was then dialyzed against buffer
B.
The dialyzed heterodimer enzyme was then allowed to bind to 25 ml of anti-FLAG
M2 agarose affinity gel overnight with constant mixing. The protein-resin
slurry
was then centrifuged for 5 min at 2,000 rpm. The supernatant was collected and
the
resin re-suspended in 100 ml of buffer C containing 0.1% NP-40. The resin was
washed with 375 ml of buffer C containing 0.1 % NP-40. The protein-resin was
poured into a 25 ml column and the enzyme eluted using buffer B containing
FLAG
peptide. Enzyme fractions (100 ml) were collected and concentrated to 20 ml
using
an Amicon membrane with molecular weight cut-off of 30 kDa. Bovine serum
albumin was added to the concentrated enzyme to final concentration of 0.1 %.
The
enzyme was then aliquoted and stored at -80 C.
Cell Culture
[00348] The wild type (wt) human pre-B cell line, 70Z/3, and its mutant,
1.3E2, were generously provided by Dr. Carol Sibley. Wt 70Z/3 and 1.3E2 cells
were grown in RPMI 1640 (Gibco) supplemented with 7 % defined bovine serum
(Hyclone) and 50 M 2-mercaptoethanol. Human monocytic leukemia THP-1 cells,
obtained from ATCC, were cultured in RPMI 1640 supplemented with 10% defined
bovine serum, 10 mM HEPES, 1.0 mM sodium pyruvate and 50 M 2-
mercaptoethanol. For experiments, cells were plated in 6 well plates at 1x106
cells/ml in fresh media. Pre-B cells were stimulated by the addition of 10
g/ml
LPS for varying lengths of time ranging from 0-4 hr. THP-1 cells were
stimulated
by the addition of 1 g/ml LPS for 45 minutes. Cells were pelleted, washed
with
cold 50 mM sodium phosphate buffer, pH 7.4 containing 0.15 M NaC1 and lysed at
4 C in 20 mM Hepes buffer, pH 7.6 containing 50 mM NaCl, 1 mM EDTA, 1 mM
EGTA, 1 mM sodium orthovanadate, 10 mM 0-glycerophosphate, 1 mM NaF, 1
mM PMSF, 1 mM DTT and 0.5 % NP40 (lysis buffer). The cytosolic fractions
obtained following centrifugation at 10,000 X g were stored at -80 C until
used.
269
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Irnrnunoprecipitation and Western Blotting
[00349] SF9 cells paste containing rhIKKs were centrifuged (100,000 X g, 10
min) to remove debris. rhIKKs were immunoprecipitated (100 g of cell paste)
from the cell supematant using 3 g of anti-NEMO antibody ( FL-419), followed
by
coupling to protein A sepharose beads. rhIKKs were also immunoprecipitated
from
affinity chromatography purified protein preparations (1 g) using anti-FLAG,
anti-
His or anti-NEMO antibodies (1-4 g) followed by protein A sepharose coupling.
The native, human IKK complex was immunoprecipitated from THP-1 cell
homogenates (300 g/condition) using the anti-NEMO antibody. Immune
complexes were pelleted and washed 3 times with 1 ml cold lysis buffer.
Immunoprecipitated rhIKKs were chromatographed by SDS-PAGE (8% Tris-
glycine) and transferred to nitrocellulose membranes (Novex) and detected by
chemiluminescense (SuperSignal) using specific anti-IKK antibodies (IKK2 H-
470,
IKK1 H-744). Native IKK2, IxBa, and NEMO proteins from cytosolic lysates (20-
80 g) were separated by SDS-PAGE and visualized by chemiluminescense using
specific antibodies.
Phosphatase Treatment
[00350] Immunoprecipitated rhIKKs were washed 2 times in 50 mM Tris-
HCI, pH 8.2 containing 0.1 mM EDTA, 1 mM DTT, 1 mM PMSF and 2 mM
MnC12 and resuspended in 50 l. Phosphatase (XPPase, 1000 U) was pre-diluted
in
the same buffer and added to the IKK samples. Following an incubation at room
temperature for 30 minutes with intermittent mixing, cold lysis buffer was
added to
the tubes to stop the reaction. After several washes, 10 % of the beads were
removed for Western analysis, and the remaining material was pelleted and
resuspended in 100 l of the buffer used for the in vitro kinase assay.
IKKa SAM Enzynie Assay
[00351] IKKa kinase activity was measured using a biotinylated IxBa
peptide (Gly-Leu-Lys-Lys-Glu-Arg-Leu-Leu-Asp-Asp-Arg-His-Asp-Ser32-Gly-Leu-
270
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
Asp-Ser36-Met-Lys-Asp-Glu-Glu), a SAM2 Tm 96 Biotin capture plate and a vacuum
system. The standard reaction mixture contained 5 M biotinylated IxBa
peptide, 1
M [7-33P] ATP (about 1 X 105 cpm), 1 mM DTT, 50 mM KCI, 2 mM MgC12, 2
mM MnC12, 10 mM NaF, 25 mM Hepes buffer, pH. 7.6 and enzyme solution (1-10
1) in a final volume of 50 l. After incubation at 25 C for 30 min, 25 l of
the
reaction mixture was withdrawn and added to a SAM2 'rm 96 Biotin capture 96-
well
plate. Each well was then washed successively with 800 12 M NaCl, 1.2 ml of
NaC1 containing 1 Io H3PO4, 400 l H20, and 200 195 Io ethanol. The plate was
allowed to dry in a hood at 25 C for 1 hr and then 25 l of scintillation
fluid
(Microscint 20) was added to each well. Incorporation of [7-33P] ATP was
measured using a Top-Count NXT (Packard). Under each assay condition, the
degree of phosphorylation of IxBa peptide substrate was linear with time and
concentration for all purified enzymes. Results from the biotinylated peptide
assay
were confirmed by SDS-PAGE analysis of kinase reaction utilizing a GST-
IKBa1_54
and [y-32P] ATP. The resulting radiolabeled substrate was quantitated by
Phosphoimager (Molecular Dynamics). An ion exchange resin assay was also
employed using [y-33P] ATP and GST-IxBal_54 fusion protein as the substrates.
Each assay system yielded consistent results in regard to Km and specific
activities
for each of the purified kinase isoforms. One unit of enzyme activity was
defined as
the amount required to catalyze the transfer of 1 nmole of phosphate from ATP
to
IxBa peptide per min. Specific activity was expressed as units per mg of
protein.
For experiments related to K. determination of purified enzymes, various
concentrations of ATP or IxBa peptide were used in the assay at either a fixed
IxBa
or ATP concentration. For IxBa peptide Km, assays were carried out with 0.1 g
of
enzyme, 5 M ATP and IxBa peptide from 0.5 to 20 M. For ATP Km, assays
were carried out with 0.1 g of enzyme, 10 M ItxBa peptide and ATP from 0.1
to
10 M. For Km determination of rhIKKl homodimer, due to its low activity and
higher Km for IKBa peptide, rhIKKl homodimer (0.3 g) was assayed with 125 M
IKBa peptide and a 5-fold higher specific activity of ATP (from 0.1 to 10 M)
for
ATP Km experiments and a 5-fold higher specific activity of 5 gM ATP and IxBa
peptide (from 5 to 200 M) for IxBa peptide Km experiments.
271 '
CA 02485298 2004-11-08
WO 03/095430 PCT/US03/08917
IKKfl Resin Enzyme Assay
[00352] IKK(3 kinase activity was measured using a biotinylated IxBa
peptide (Gly-Leu-Lys-Lys-Glu-Arg-Leu-Leu-Asp-Asp-Arg-His-Asp-Ser32-Gly-Leu-
Asp-Ser36-Met-Lys-Asp-Glu-Glu) (American Peptide Co.). 20 ul of the standard
reaction mixture contained 5 M biotinylated IxBa peptide, 0.1 Ci/reaction
W3P]
ATP (Amersham) (about 1 X 105 cpm), 1 M ATP (Sigma), 1 mM DTT (Sigma), 2
mM MgC12 (Sigma), 2 mM MnC12 (Sigma), 10 mM NaF (Sigma), 25 mM Hepes
(Sigma) buffer, pH 7.6 and 20 1 enzyme solution and 10 ul inhibitor in a
final
volume of 50 1. After incubation at 25 C for 30 min, 150 l resin (Dowex
anion-
exchange resin AG1X8 200-400 mesh) in 900 mM formate, pH 3.0 was added to
each well to stop the reaction. Resin was allowed to settle for one hour and
50 ul of
supernatant was removed to a Micolite-2 flat bottom plate (Dynex). 150 l of
scintillation fluid (Microscint 40) (Packard) was added to each well.
Incorporation
of [y-33P] ATP was measured using a Top-Count NXT (Packard).
IKK heterodimer Resin Enzyme Assa.y
[00353] IKK heterodimer kinase activity was measured using a biotinylated
IxBa peptide (Gly-Leu-Lys-Lys-Glu-Arg-Leu-Leu-Asp-Asp-Arg-His-Asp-Ser32-
Gly-Leu-Asp-Ser36-Met-Lys-Asp-Glu-Glu) (American Peptide Co.). 20 ul of the
standard reaction mixture contained 5 M biotinylated IrxBa peptide, 0.1
Ci/reaction [y-33P] ATP (Amersham) (about 1 X 105 cpm), 1 M ATP (Sigma), 1
mM DTT (Sigma), 2 mM MgC12 (Sigma), 2 mM MnC12 (Sigma), 10 mM NaF
(Sigma), 25 mM Hepes (Sigma) buffer, pH 7.6 and 20 1 enzyme solution and 10
l
inhibitor in a final volume of 50 l. After incubation at 25 C for 30 min,
150 t
resin (Dowex anion-exchange resin AG1X8 200-400 mesh) in 900 mM formate, pH
3.0 was added to each well to stop the reaction. Resin was allowed to settle
for one
hour and 50 ul of supernatant was removed to a Micolite-2 flat bottom plate
(Dynex). 150 l of scintillation fluid (Microscint 40) (Packard) was added to
each
well. Incorporation of [y-33P] ATP was measured using a Top-Count NXT
(Packard).
272