Note: Descriptions are shown in the official language in which they were submitted.
CA 02487079 2004-11-24
WO 03/099179 PCT/AU03/00639
1
GENERATION OF ELECTRICAL STIMULI FOR
APPLICATION TO A COCHLEA
The present invention relates generally to the generation of electrical
stimuli for application to a cochlea via an auditory prosthesis electrode
array.
The multi-channel cochlear implant was first implanted in 1978. Early
signal processing designs extracted the second formant (F2) and pitch (F0) to
control electrode stimulation. The frequency of F2 controlled the location of
electrode stimulation, and FO controlled the rate of stimulation. Improvements
were made by also extracting the first formant (Fl) and adding a second
stimulated electrode for each pitch period. The MULTIPEAK stimulation
strategy added stimulation of a number of fixed electrodes to better represent
high-frequency information. The next stages of development were the Spectral
Maxima Sound Processor (SMSP) strategy, described in Australian Patent No.
657,959, and SPEAK strategy, described in US Patent No. 5,597,380. These
were a departure from the others as they used a fixed stimulation rate and
stimulated electrodes that corresponded to maxima in the sound spectra.
Another fixed-rate strategy, CIS, is described in US Patent No. 4,207,441.
This
strategy stimulated all of a small number of electrodes to represent the sound
spectra. All of the above processing strategies involve fixed-rate sound
processing.
Kitazawa et al. (Kitazawa, S, Muramoto, K. and Ito, J. (1994) "Acoustic
simulation of auditory model based speech processor for cochlear implant
system", Proceedings of the International Conference on Spoken Language
Processing, ICSLP'94, pp. 2043-2046) describes a strategy for the spectra/CI-
22
system using an auditory model for choosing electrodes to stimulate and for
extracting fundamental frequency to control rate of stimulation. This strategy
CA 02487079 2004-11-24
WO 03/099179 PCT/AU03/00639
2
was tested by simulation with normal hearing listeners. Stimulation was
restricted to low rates and required explicit extraction of pitch information.
Performance was reduced for word recognition compared to the Wearable
Speech Processor (WSP) running MULTIPEAK.
The SMSP strategy was further expanded by an invention entitled
"Emphasis of short-duration transient speech features" (PCT/AUOO/01310,
International Publication Number WO 01/31632 Al). Together with SMSP,
this strategy is called the "Transient Emphasis Spectral Maxima Sound
Processor" (TESM). This strategy used the same signal processing and
electrode selection method as SMSP except that the relative amplitude of short-
duration amplitude transitions was increased before electrode selection in
order
to assist in perception of short, low-amplitude speech sounds.
More recently the "Multi-rate cochlear stimulation strategy and
apparatus" (PCT/AUOO/00838, International Publication Number WO 01/03622
Al) has been developed that does not use fixed stimulation rates. The strategy
determines rate of stimulation for each implant electrode by measuring average
intervals between positive zero-crossings of the filtered signals for each
band.
Another recent development is "Sound processor for a cochlear implant"
(PCT/AU01/00723, International Publication Number WO 01/99470 Al) called
the "Travelling Wave Strategy". This device models the spatio-temporal neural
excitation patterns induced by basilar membrane motion in the normally hearing
listener to produce electrical stimulation patterns for the cochlear implant
user.
The device contains a basilar membrane motion model, and inner and outer hair
cell models, to provide 3-dimensional (position, time, amplitude) excitation
patterns that may more closely mimic those of the normally hearing listener.
CA 02487079 2010-07-06
=
3
Meyer-Base et al. (Meyer-Base, U., Meyer-Base, A., and Scheich, H.
(1997) "Auditory Neuron Models for Cochlea Implants", SPIE AeroSense,
April 1997, Orlando, Florida, UAS; Vol 3077, pp. 582-593; Meyer-Base, U.
(1998) "An interspike interval method to compute speech signals from neural
firing", SPIE AeroSense, April 1998, Orlando, Florida, UAS; Vol 3390, pp.
560-571; Meyer-Base, U., Meyer-Base, A., and Scheich, H. (2000) "An inter-
spike interval method for computing phase locking from neural firing", Biol.
Cybern. 82, 283-290) developed a neural model with the aim of extracting inter-
spike intervals for control of cochlear implant stimulation. However, this
work
was on the development of hardware for implementing auditory models and the
papers give no details of how the cochlear implant strategy would be
implemented.
It would be desirable to provide a method and system for generating
electrical stimuli for application to a cochlea via an auditory prosthesis
electrode
array that more closely replicates cochlea stimulation of a normal hearing
listener.
It would also be desirable to provide a method and system for generating
electrical stimuli for application to a cochlea that ameliorates or overcomes
one
or more disadvantages of known electrical stimuli generation methods and
systems.
One aspect of the present invention provides a method for processing sound
signals to generate electrical stimuli for an auditory prosthesis electrode
array
including a plurality of electrodes, the method including:
deriving one or more filtered representations of an incoming audio signal;
CA 02487079 2010-07-06
4
monitoring the one or more filtered signal representations for a threshold
crossing, to detect when the filtered signal representation crosses a
predetermined
magnitude threshold;
generating a series of spikes from each filtered signal representation to
directly control electrode stimulation, and
determining for each spike within each of the series of spikes, a different
and
sequential electrode stimulation time based upon when the corresponding
filtered
signal representation crossed the predetermined threshold.
The amplitude of each spike may be derived from the amplitude of a
filtered signal representation peak following the threshold crossing. Spike
amplitude may be equal to the difference between the predetermined threshold
and the amplitude of a filtered signal representation peak following the
threshold crossing. Alternatively, spike amplitude may equal to the amplitude
of
a filtered signal representation peak following the threshold crossing.
In one embodiment, each spike may have a temporal position based upon
an instant at which the filtered signal representation crosses a zero axis in
a
positive direction. The amplitude of each spike may be derived from the
amplitude of a filtered signal representation peak following the zero axis
crossing. Spike amplitude may be equal to the difference between a
predetermined threshold and the amplitude of a filtered signal representation
peak following the zero axis crossing. Alternatively, spike amplitude may be
equal to the amplitude of a filtered signal representation peak following the
zero
axis crossing.
The method may further include delaying the temporal position of each
CA 02487079 2010-07-06
4a
spike according to a travelling wave delay for a normally hearing person along
the length of the electrode array.
The method may further include adapting the threshold based on long
term average energy of the filtered signal representation.
The method may further include computing a neural adaptation variable
CA 02487079 2004-11-24
WO 03/099179 PCT/AU03/00639
from an integral of previous spikes in the spike series and from a decay
function; and
applying a gain derived from the neural adaptation variable to the
amplitude of each spike.
5
The method may further include limiting the number of spikes used to
generate the electrical stimuli for each electrode so that the number of times
a
particular electrode is stimulated within a stimulation sequence window does
not exceed a fixed maximum average rate.
The method may further include sorting spikes occurring within a
stimulation sequence window in order of importance; and selecting a number of
the sorted spikes in order of importance to derive an electrode stimulation
sequence for generating electrical stimuli. The spike importance may be
determined by normalised amplitude.
The method may further include configuring normalisation coefficients
for comparison and prioritisation of spikes in different frequency regions.
The method may further include obtaining the electrode stimulation
sequence by progressively placing spikes occurring within the stimulation
sequence window in stimulation time slots corresponding to the temporal
positions of the spikes in decreasing order of importance from a sorted list
of
normalised amplitude spikes.
The method may further include mapping electrical stimuli to a current
level for a particular user using a loudness growth function and stored
threshold
and comfort levels for that particular user, wherein the mapping uses a
special
comfort level for short duration transition events to provide increased
CA 02487079 2010-07-06
6
stimulation levels during neural response adaptation.
The method may further include encoding the electrode stimulation
sequence; and transmitting the encoded electrode stimulation sequence to the
auditory prosthesis electrode array to enable stimulation of an auditory nerve
or
cochlea nucleus.
Another aspect of the invention provides a system for stimulating an auditory
prosthesis electrode array, including:
a stimulator unit for selectively stimulating electrodes in the electrode
array;
and
a processor for processing received sound signals and controlling the
operation of the stimulator unit to generate electrical stimuli for an
auditory
prosthesis electrode array including a plurality of electrodes, said processor
being
configured to:
derive one or more filtered representations of an incoming audio signal;
monitor the one or more filtered signal representations for a threshold
crossing to detect when the filtered signal representation crosses a
predetermined
magnitude threshold;
generate a series of spikes from each filtered signal representation to
directly
control electrode stimulation and to determine for each spike within each of
the
series of spikes, a different and sequential electrode stimulation time based
upon
when the corresponding filtered signal representation crossed the
predetermined
threshold.
Yet another aspect of the invention provides a processor for use in a system
for stimulating an auditory prosthesis electrode array, the system including a
stimulator unit for selectively stimulating electrodes in the electrode array,
the
processor including digital signal processing means for processing received
sound
CA 02487079 2010-07-06
7
signals and controlling the operation of the stimulator unit to generate
electrical
stimuli for an auditory prosthesis electrode array, the processor being
further
configured to:
derive one or more filtered representations of an incoming audio signal;
monitor the one or more filtered signal representations for a threshold
crossing, to detect when the filtered signal representation crosses a
predetermined
magnitude threshold; and
generate a series of spikes from each filtered signal representation to
directly
control electrode stimulation, and to determine for each spike within each of
the
series of spikes, a different and sequential electrode stimulation time based
upon
when the corresponding filtered signal representation crossed the
predetermined
threshold.
The strategy implemented by the present invention simulates the behaviour
of the hair cells of the cochlea and of the auditory nerve to create a spiked-
based
representation modelled on action potentials generated in the auditory
neurons.
The cochlea is electrically stimulated using a sequence of electrical pulses
that
replicates this spike-based representation as closely as possible within
device
limitations.
The Spike-based Temporal Auditory Representation (STAR) strategy of
the present invention improves speech perception ability in the presence of
noise, including both pseudo-stationary random noise and multi-talker babble.
This is achieved by providing an improved representation of fine-grained
temporal information, especially phase locking to formants across multiple
stimulation channels. Adaptation effects improve detection of transient
events,
such as plosive onsets and formant transitions, and help to overcome pseudo-
CA 02487079 2010-07-06
7a
stationary background noise by a neural adaptation process similar to spectral
subtraction.
The incoming acoustic signal may be passed through a filter bank in
which the centre-frequencies are distributed according to an
auditory/psychophysics-based distribution of frequencies. The filters may have
significant overlap based on equivalent rectangular bandwidth. Either linear
or
non-linear filters may be used.
Preferably, a series of "spikes" are extracted from each filtered
representation of the signal, each spike having a temporal position or "spike
time"
based upon the instance at which the signal crosses a threshold in a positive-
going
direction. The amplitude of the spike may be determined by the difference by
the
difference between the threshold and the following maximum of the signal.
Neural adaptation effects may be incorporated to emphasise onsets of
signals relative to the steady-state amplitudes as is observed in
physiological
studies. A time-dependent integral may be computed from the series of spikes
in each channel (the value of this integral decays in time if there are no
subsequent spikes), and a gain applied to the amplitude of each spike by a
measure related to this sum.
CA 02487079 2004-11-24
WO 03/099179 PCT/AU03/00639
8
The value of the threshold may be adjusted based on signal history (i.e.
threshold adaptation), in order to take account of differing listening
conditions
and levels of background noise. The amplitudes of the spikes may also be
adjusted based on the recent signal history, in order to model neural
adaptation
effects and allow increased responses at the onset of acoustical stimuli.
Electrodes may be stimulated at threshold crossing times (delayed across
all electrodes by up to 10 msec to allow peak detection) using stimulus levels
proportional to the adapted amplitudes. Owing to limitations in existing
cochlear implants, a limited number of electrodes may be chosen for
stimulation
by selecting channels with the highest normalised average amplitude. Temporal
contention (i.e. simultaneous spikes) may be accounted for by systematically
shifting spikes to other times based on their importance, which are determined
using normalised amplitudes. Average rate of stimulation may be limited on
each electrode by ensuring that spikes in high frequency channels are not
stimulated above a fixed maximum average rate.
The present invention builds upon a rich history of auditory modelling.
One of the most prominent such models is that developed by Allen & Ghitza
(US Patent 4,905,285) in the context of developing improved strategies for
automatic speech recognition based upon the human auditory system. A similar
system was developed by Kim et al. (Kim, D-S, Lee, S-Y and Rhee, M (1999)
"Auditory processing of speech signals for robust speech recognition in real-
world noisy environments", IEEE T-SAP 7(1), 55-69) that used a single zero-
crossing measure to locate spikes and determined their amplitudes using the
subsequent peak of the filtered signal.
The present invention uses a similar auditory model to Kitazawa et al.
(1994) for the pre-processing of the incoming waveform, but uses a spike-train
CA 02487079 2004-11-24
WO 03/099179 PCT/AU03/00639
9
directly to control the electrode simulation rather than extract FO explicitly
to
control rate of stimulation across the electrode array. Explicit extraction of
pitch is not required to control the overall rate of stimulation but may be
represented by the stimulation sequence of the more apical electrodes and by
the
amplitude modulation over the whole electrode array. Direct extraction of
spike
trains with fine-grained temporal information also distinguishes this
invention
from the Multi-rate strategy.
The Travelling Wave Strategy is similar to the STAR strategy of the
present invention in that it extracts precise timing information that is
useful for
higher levels of processing in the cochlear nucleus and other stages of the
brainstem. However, the Travelling Wave Strategy relies on specific basilar
membrane properties that are not used in STAR, while STAR relies more on
auditory nerve behaviour. The model used by STAR allows various non-linear
properties to be implemented such as neural response adaptation and threshold
adaptation. Also, the stimulus times of STAR are based on threshold crossing
times rather than at times of local maxima as specified for the Travelling
Wave
Sound Processor.
The TESM strategy contains an element similar to the neural adaptation
effect that is part of the present invention. However, the increasing of
amplitude of short-duration amplitude transitions in the TESM strategy is
based
on a direct estimate of the slowly varying signal envelope and then rules are
applied for applying a gain factor. A consequence of the method used by TESM
is that a delay of 30 ms is introduced in the signal. In one embodiment, the
present invention applies the adaptation function continuously to the sequence
of spikes and does not rely on examination of time periods to estimate gain as
TESM does. Thus, not only is the technique different, but no extra time delay
is
introduced.
CA 02487079 2004-11-24
WO 03/099179 PCT/AU03/00639
The following description refers in more detail to the various features of
the method and system for generating electrical stimuli of the present
invention.
To facilitate an understanding of the invention, reference is made in the
5 description to the accompanying drawings where the invention is illustrated
in a
preferred embodiment. It is to be understood however, that the invention is
not
limited to the preferred embodiment as shown in the drawings.
In the drawings:
10 Figure 1 is a schematic diagram of one embodiment of a system for
stimulating an electrode array implanted into a cochlea;
Figure 2 is a schematic diagram showing the function blocks of a
processor forming part of the electrode stimulation system of Figure 1; and
Figure 3 is an exemplary filtered representation of an audio signal
received by the electrode stimulation system of Figure 1.
Referring now to Figure 1, there is shown generally a system for
stimulating an electrode array in accordance with a processed signal. An
electrode array 1, implanted into a cochlea, connects via cable 2 to a
receiver-
stimulator unit (RSU) 3. The implanted system receives control signals and
power from an external speech processor unit, preferably via a tuned coil RF
system 5, 6 as illustrated. However, any alternative connection technique such
as percutaneous connection may be employed.
The coil 6 carries a signal modulated by the processor 7 so as to cause the
RSU 3 to stimulate the electrodes in the electrode array in the desired
sequence,
timing and amplitude. The processor 7 in turn receives electrical analog
signals
from a microphone 8 worn by the user. The present invention is concerned with
the operation of the processor and particularly the method of processing the
CA 02487079 2004-11-24
WO 03/099179 PCT/AU03/00639
11
incoming electrical signal.
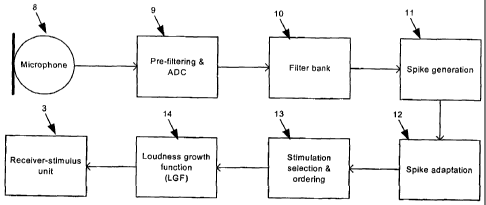
Figure 2 illustrates the various functional blocks of the processor 7,
including a pre-filtering & ADC block 9, a filter bank 10, a spike generation
block 11, a spike adaptation block 12, a stimulation selection & ordering
block
13 and a loudness growth function block 14. The pre-filtering & ADC block 9
may be implemented using known electronic circuitry and analog signal
sampling techniques, whilst the functional blocks 10 to 14 may be implemented
using known digital signal processing techniques.
Pre-filtering and Analog-to-Digital Conversion
Sound is recorded by the microphone 8, which may inherently apply pre-
emphasis to the incoming signal. This signal is low-pass filtered, to prevent
aliasing during sampling, and is then sampled by an analog-to-digital
converter
in the pre-filtering and ADC block 9. In an embodiment of the invention using
the CI-24M cochlear implant, the signal is sampled by the analog-to-digital
converter at 14.4 kHz.
Filter Bank
The digitally sampled audio signal is passed through the filter bank 10
spanning the region of approximately 200-7200 Hz. The number of filters will
depend on the cochlear implant device used and the number of electrodes
available for a particular implant user, which is typically 22 channels in the
Cochlear Ltd NucleusTM electrode array but varies across devices. The centre-
frequencies (CFs) of the filters are distributed using a psychophysical pitch
scale, such as mel-scale or bark scale, in a similar way to standard frequency
distributions for electrodes in commonly used cochlear implant frequency-
electrode maps. The filter bandwidth is proportional to the equivalent
rectangular bandwidth obtained from the psychophysical critical band
CA 02487079 2004-11-24
WO 03/099179 PCT/AU03/00639
12
measurements. The filter bank is designed along the lines of that designed by
Kim et al. (1999, op cit) for automatic speech recognition, except that the
number of filters is different and IIR filters may be used instead of FIR
filters.
Spike Generation
Spikes are generated in the spike generation block 11 from the filtered
signal representation in a manner similar to that used by Kim et al. (1999, op
cit) except that threshold-crossing times rather than zero-crossing times are
used. This allows adaptation of the strategy to ambient long-term noise
levels.
As can be seen in Figure 3, a spike is generated at time tT of a positive-
going
crossing of the threshold 0. An amplitude as is assigned to the spike by
subtracting the peak amplitude a p from the threshold 0. The peak amplitude is
found by searching ahead in the channel for the maximum amplitude of the
filtered signal representation before a following negative-going threshold
crossing. The search is limited to 10 ms, or 1440 samples, to prevent
significant
processing time lag in the strategy.
The spike time, or temporal position of a spike, is the sample time closest
to the threshold-crossing time plus the number of sample periods corresponding
to the travelling-wave delay associated with the distance along the electrode
array of the electrode corresponding to the particular filter. If this delay
time is
a(n), where n is the filter number, then the spike time is is = tT + a(n).
Accordingly, the temporal position of each spike is delayed by a travelling
wave
delay that would exist for a normally hearing person along the length of the
electrode array,
a(n) = CDeeLxh=>
where CD is the traveling wave delay coefficient, CL is the length coefficient
for the traveling wave delay and x(n) is the propagation distance for
electrode
CA 02487079 2004-11-24
WO 03/099179 PCT/AU03/00639
13
n. Typical values for CD and CL may be obtained from measurements
reported in the literature. For example, for one person described in
Donaldson,
GS and Ruth, RA (1993, Derived band auditory brain-stem response estimates
of traveling wave velocity in humans. I: Normal-hearing subjects, J. Acoust.
Soc. Am. 93(2):940-951) the coefficients are CD = 0.3631, CL = 0.11324 and
x(n) is distance from the stapes in mm. In the preferred implementation, x(n)
is the distance of electrode n from the most basal electrode that is in use.
In
another implementation of the invention, x(n) is computed by determining the
effective distance of electrode n from the round window based on the location
in the normal cochlea of the centre frequency of the filter for that electrode
using a frequency-place map function.
In this embodiment, each spike has a temporal position based upon an
instant at which the filtered signal representation crosses the threshold 0 in
a
positive direction. The amplitude of each spike is derived from the amplitude
ap of the filtered signal representation peak following the threshold
crossing. In
this case, spike amplitude is equal to the difference between the threshold 0
and
the amplitude a. However, spike amplitude may alternatively be equal to the
amplitude ap itself.
In other embodiments though, the threshold may be the zero axis and
each spike may have a temporal position based upon an instant at which the
filtered signal representation crosses the zero axis (referenced 0 in Figure
3) in a
positive direction. The amplitude of each spike may be derived from the
amplitude a p of a filtered signal representation peak following the zero axis
crossing. Spike amplitude may be equal to the difference between the threshold
0 and the amplitude a p . Alternatively, spike amplitude may be equal to the
amplitude a p itself.
CA 02487079 2004-11-24
WO 03/099179 PCT/AU03/00639
14
Spike Adaptation
Adaptation effects modelled on neural adaptation are applied to each
generated spike by the spike adaptation block 12 based on the previous history
of the spike train in each channel. The adaptation is controlled by a variable
obtained by integrating over all previous spikes with an appropriate decay
function. A gain derived from the adaptation variable is applied to the spike
amplitude (as) to obtain an adapted version (as) that may be greater than the
original amplitude when the integral is small. With sustained activity, the
integral will increase thus making as reduce back to as . The equation for
this
complete operation is
as =as + CA as('-FA), FA =YA Y,ase-(t'-ts,)lzA
Cs ts,<ts
where CA is the maximum value permissible for an adapted spike, Cs is the
maximum sustained amplitude permissible for a spike sequence, YA is a
normalising term to control rate of adaptation, zA is a time constant
controlling
rate of decay of previous spike train influence on the current spike, is is
the
time of the current spike, t,s represents the times of the previous spikes and
as
represents the amplitudes of the previous spikes. The summation is
implemented in recursive form in the digital signal processor as a single
value
for each channel that is continually updated with each spike arrival.
The spike adaptation block 12 also acts to adapt the value of the
threshold based on the long-term average energy of the filtered signal
representation.
In one implementation of this procedure, the threshold, 0, is adjusted by the
previous sequence of generated spikes for each filter. An equation of the form
CA 02487079 2004-11-24
WO 03/099179 PCT/AU03/00639
o s B e_(`5_ts_')1 z" + C a
s s-1 ~t s
may be used, where Os is the new threshold computed after the generation of
the current spike s at time ts, based on the previous threshold s_1 that was
computed at the previous spike time ts_1. The time constant r, is set for each
5 filter based on the centre frequency of that filter to control the rate of
decay of
the threshold. The value as is the amplitude of the current spike, which was
computed using the threshold established for the previous spike, and Cõ is a
constant for each filter to control the rate of growth of the threshold.
10 Stimulation Selection and Ordering
There are a number of issues that are important to consider when
selecting an electrode stimulation sequence. These are:
1. The maximum pulse rate delivered to a single cochlear implant electrode
may be limited to a fixed maximum average rate. This may be usually
15 controlled by limiting the maximum average stimulation rate per electrode,
for
example, to 2000 pulses per second (pps).
2. The cochlear implant has a maximum possible total rate of stimulation.
For example, in the Cochlear CI-24M device, this is 14,400 pps.
3. Stimulation of multiple electrodes should be non-simultaneous to avoid
current interactions within the cochlea. Therefore, there should be a means of
arbitrating contention between spikes with simultaneous arrival times.
4. Each cochlear implant user has a different mapping between current level
on each electrode and perceived loudness. Thus threshold (T) and comfort (C)
levels should be established for each user and for each electrode. Overall
adjustments of T and C levels may often be made, usually downward, after
fitting the map to account for loudness summation across multiple electrodes
when the device is actually being used with sound input. A loudness growth
function (LGF) should be applied to transform spike height to current level.
CA 02487079 2004-11-24
WO 03/099179 PCT/AU03/00639
16
The creation of a stimulation sequence by the stimulation selection &
ordering block 13 commences in each cycle by establishing a stimulation
selection window of duration Tw = mXTE where TE is the number of stimulation
intervals permitted within the bound of the maximum average stimulation rate.
For a 2000 Hz maximum average rate of stimulation per electrode, the
minimum time between successive stimuli on a single electrode should be, on
average, greater than or equal to 0.5 ms. A maximum total stimulation rate for
an implant of 14,400 Hz provides about 0.07 ms per stimulus. In this example,
TE = 7.2 stimulation intervals. An integer value of mx can be used to select a
longer window for a cycle of electrode sequence selection and also the limit
on
the number of times each electrode may be stimulated within that window such
that the maximum stimulation rate is not exceeded. In the preferred
implementation of the invention, mx =1. It will be appreciated that in other
embodiments of the invention higher maximum total stimulation rates and
higher maximum average stimulation rates may be used.
The spikes from each channel that occur with the window Tw are then
considered for selection as electrode stimuli. The spikes may be combined
across channels and are ordered by normalised amplitude. The normalisation is
determined from configuration of the entire prosthesis, particularly the
characteristics of the microphone 8 and the filter bank 10, as well as the
properties of speech, so that the priority of spikes in different frequency
regions
can be incorporated into the sorting procedure.
After sorting, the list of sorted normalised amplitude spikes is
progressively examined to obtain a stimulation sequence for the window Tw.
The top candidate is selected (i.e. the candidate with the largest normalised
amplitude) and a stimulation pulse is placed in a stimulation time slot
CA 02487079 2004-11-24
WO 03/099179 PCT/AU03/00639
17
corresponding to the closest time to the spike time is . The process continues
until all time slots within Tyy have been filled. If a stimulation pulse is to
be
placed in a slot that is already filled, then neighbouring slots are examined
in the
following order: [-1 +1 -2 +2 -3 +3 ...] where -1 indicates the previous slot,
+1
is the next slot, -2 is the slot before the previous one, etc. Only slots
within
window Tw may be examined in this way.
Loudness Growth Function
After each cycle of electrode selection, the stimuli are mapped to current
levels using the standard loudness growth function (LGF) and the stored map (T
and C levels) for the user by the loudness growth function block 14. The LGF
is a logarithmic function relating stimulus level to loudness to obtain an
appropriate increase in subjective loudness. The stored map specifies the
minimum and maximum current levels permitted for a user. A special C level
may be specified for short duration transient events to allow increased levels
during an adaptation step.
Implant Stimulation
The stimulus sequence is then transmitted to the receiver-stimulus unit 3
that interfaces with the cochlear implant and encodes the electrode selection
and
current level information to the device.
Finally, it is to be understood that various modifications and/or additions
may be made to the method and system for generating electrical stimuli
described herein without departing from the spirit or ambit of the present
invention.