Note: Descriptions are shown in the official language in which they were submitted.
CA 02488588 2004-11-30
GUIDEWIRE FOR CROSSING OCCLUSIONS OR STENOSIS
BACKGROUND OF THE INVENTION
The present invention is generally related to medical devices, kits, and
methods. More specifically, the present invention provides a system for
crossing stenosis,
partial occlusions, or total occlusions in a patient's body.
Cardiovascular disease frequently arises from the accumulation of
atheromatous material on the inner walls of vascular lumens, particularly
arterial lumens of
the coronary and other vasculature, resulting in a condition known as
atherosclerosis.
Atheromatous and other vascular deposits restrict blood flow and can cause
ischemia which,
in acute cases, can result in myocardial infarction or a heart attack.
Atheromatous deposits
can have widely varying properties, with some deposits being relatively soft
and others being
fibrous and/or calcified. In the latter case, the deposits are frequently
referred to as plaque.
Atherosclerosis occurs naturally as a result of aging, but may also be
aggravated by factors
such as diet, hypertension, heredity, vascular injury, and the like.
Atherosclerosis can be treated in a variety of ways, including drugs, bypass
surgery, and a variety of catheter-based approaches which rely on
intravascular widening or
removal of the atheromatous or other material occluding the blood vessel.
Particular
catheter-based interventions include angioplasty, atherectomy, laser ablation,
stenting, and
the like. For the most part, the catheters used for these interventions must
be introduced over
a guidewire, and the guidewire must be placed across the lesion prior to
catheter placement.
Initial guidewire placement, however, can be difficult or impossible in
tortuous regions of the
vasculature. Moreover, it can be equally difficult if the lesion is total or
near total, i.e. the
lesion occludes the blood vessel lumen to such an extent that the guidewire
cannot be
advanced across.
To overcome this difficulty, forward-cutting atherectomy catheters have been
proposed. Such catheters usually can have a forwardly disposed blade (U.S.
4,926,858) or
rotating burr (U.S. 4,445,509). While effective in some cases, these catheter
systems, even
with a separate guidewire, have great difficulty in traversing through the
small and tortuous
body lumens of the patients and reaching the target site.
1
_
CA 02488588 2004-11-30
For these reasons, it is desired to provide devices, kits, and methods which
can
access small, tortuous regions of the vasculature and which can remove
atheromatous,
thrombotic, and other occluding materials from within blood vessels. In
particular, it is
desired to provide atherectomy systems which can pass through partial
occlusions, total
occlusions, stenosis, and be able to macerate blood clots or thrombotic
material. It is further
desirable that the atherectomy system have the ability to infuse and aspirate
fluids before,
during, or after crossing the lesion. At least some of these objectives will
be met by the
devices and methods of the present invention described hereinafter and in the
claims.
SUMMARY OF THE INVENTION
The present invention provides systems and methods for removing occlusive
material and passing through occlusions, stenosis, thrombus, and other
material in a body
lumen. More particularly, the present invention can be used passing through
stenosis or
occlusions in a neuro, cardio, and peripheral body lumens. Generally, the
present invention
includes an elongate member that is positioned adjacent the occlusion or
stenosis. A drive
shaft having a distal tip is rotated and advanced from within the elongate
member to create a
path forward of the elongate member to form a path in the occlusion or
stenosis. To facilitate
passing through the occlusion or stenosis, the distal end of the elongate
member can be
steerable to provide better control the creation of the path through the
occlusion or stenosis.
Optionally, the target site can be infused and/or aspirated before, during,
and after creation of
the path through the occlusion.
In an exemplary embodiment, the elongate member is a hollow guidewire that
has a flexibility, pushability and torqueability to be advanced through the
tortuous blood
vessel without the use of a separate guidewire. Additionally, the hollow
guidewire may be
sized to fit within a conventional support or access catheter system and
inserted into the
blood vessel and delivered to the target site. The catheter system can be
delivered either
concurrently with the advancement of the hollow guidewire or after the
guidewire has
reached the target site. The position of the hollow guidewire and catheter
system can be
maintained and stabilized while the drive shaft is rotated and translated out
of the axial lumen
of the hollow guidewire. The distal tip of the drive shaft can be deflected,
coiled, blunted,
flattened, enlarged, twisted, basket shaped, or the like. In some embodiments,
to increase the
rate of removal of the occlusive material, the distal tip is sharpened or
impregnated with an
abrasive material such as diamond chips, diamond powder, glass, or the like.
2
CA 02488588 2013-11-20
rotate the drive shaft as the distal tip of the drive shaft is advanced
distally to create a path that
is large enough to pass the guidewire through the occlusion or stenosis.
Various embodiments of this invention provide a kit comprising: a flexible
hollow guidewire having an axial passage, an outer diameter between
approximately 0.009
inches and 0.035 inches, and a torqueability and pushability to be advanced
through a body
lumen without the need of a separate guidewire; a rotatable drive shaft having
a shaped distal
tip, the rotatable wire being removably received within the passage of the
hollow guidewire;
instructions for use in passing through occlusions in a body lumen; and a
package adapted to
contain the hollow guidewire, rotatable wire, and the instructions for use.
Various embodiments of this invention provide use of an assembly, system or
kit of this invention for the purposes recited above.
The hollow guidewire may be sized to fit within a conventional support or
access catheter system and inserted into the blood vessel and delivered to the
target site. The
catheter system can be delivered either concurrently with the advancement of
the hollow
guidewire or after the guidewire has reached the target site. The position of
the hollow
guidewire and catheter system can be maintained and stabilized while the drive
shaft is rotated
and translated out of the axial lumen of the hollow guidewire. The distal tip
of the drive shaft
can be deflected, coiled, blunted, flattened, enlarged, twisted, basket
shaped, or the like. In
some embodiments, to increase the rate of removal of the occlusive material,
the distal tip is
sharpened or impregnated with an abrasive material such as diamond chips,
diamond powder,
glass, or the like.
2b
CA 02488588 2004-11-30
The drive shaft can be a counter-wound guidewire construction or be of a
composite structure consisting of a fine wire around which a coil is wrapped.
The counter-
wound or composite constructions are more flexible than a single wire drive
shaft and can
provide a tighter bending radius while still retaining the torque transmitting
ability so that it
can still operate as a lesion penetration mechanism.
In a specific configuration, the drive shaft has spiral threads or external
riflings extending along the shaft. The spirals typically extend from the
proximal end of the
shaft to a point proximal of the distal tip. As the drive shaft is rotated and
axially advanced
into the occlusive material, the distal tip creates a path and removes the
material from the
body. The rotating spirals act similar to an "Archimedes Screw" and transport
the removed
material proximally up the lumen of the elongate member and prevent the loose
atheromatous
material from escaping into the blood stream.
Systems and kits of the present invention can include a support system or
access system, such as a catheter or guidewire having a body adapted for
intraluminal
introduction to the target blood vessel. The dimensions and other physical
characteristics of
the access system body will vary significantly depending on the body lumen
which is to be
accessed. In the exemplary case, the body of the support or access system is
very flexible
and is suitable for introduction over a conventional guidewire or the hollow
guidewire of the
present invention. The support or access system body can either be for "over-
the-wire"
introduction or for "rapid exchange," where the guidewire lumen extends only
through a
distal portion of the access system body. Optionally, the support or access
system can have at
least one axial channels extending through the lumen to facilitate infusion
and/or aspiration
of material from the target site. Support or access system bodies will
typically be composed
of an organic polymer, such as polyvinylchloride, polyurethanes, polyesters,
polytetrafluoroethylenes (PTFE), silicone rubbers, natural rubbers, or the
like. Suitable
bodies may be formed by extrusion, with one or more lumens that extend axially
through the
body. For example, the support or access system can be a support catheter,
interventional
catheter, balloon dilation catheter, atherectomy catheter, rotational
catheter, extractional
catheter, laser ablation catheter, guiding catheter, stenting catheter,
ultrasound catheter, and
the like.
In other embodiments, a hollow guidewire can be used as the support or access
system. The hollow guidewire can be navigated to and positioned at the target
site, with or
without the use of a separate guidewire. The hollow guidewire support system
provides the
3
_
CA 02488588 2004-11-30
flexibility, maneuverability, torqueability (usually 1:1), and columnar
strength necessary for
accurately advancing through the tortuous vasculature. The hollow guidewire
support system
can act as a working channel inside of which other interventional devices can
be delivered to
the target site. Such devices include, but are not limited to a rotating
guidewire, infusion
guidewire, clot maceration guidewire, normal guidewire, and the like. Because
the hollow
guidewire is not composed of polymer, the hollow guidewire working channel
does not
soften at body temperatures.
The hollow guidewire working channel typically has a thin wall construction
which allows the lumen of the working channel to be maximized when compared
with
polymeric based catheter designs. This allows larger diameter devices to be
inserted into it
than can be inserted through similar sized catheter-based devices. The larger
lumen of the
hollow guidewire working channel allows devices such as clot macerators and
other larger
devices to be delivered to the target lesion. Additionally the larger diameter
lumen allows
infusion or clot dissolving fluid and/or aspiration of the debris created in
the clot maceration
process.
In use, the access system can be delivered to the target site over a
conventional
guidewire. Once the access system has been positioned near the target site,
the conventional
guidewire can be removed and the elongate member can be advanced through the
access
system to the target site. Alternatively, because the elongate member can have
the flexibility,
pushability, and torqueability to be advanced through the tortuous regions of
the vasculature,
it is possible to advance the elongate member through the vasculature to the
target site
without the use of a separate guidewire. The access system can be advanced
over the
elongate member to the target site. Once the elongate member has been
positioned at the
target site, the drive shaft is rotated and advanced into the occlusive
material. The rotation of
the distal tip creates a path forward of the elongate member. In some
embodiments the path
created by the distal tip has a path radius which is larger than the radius of
the distal end of
the elongate member. In other embodiments, the path created by the distal tip
has a path
radius which is the same size or smaller than the radius of the elongate
member.
One exemplary system for crossing an occlusion or stenosis within a body
lumen comprises a drive shaft that is rotatably and translatably received
within an axial
lumen of an elongate member. Means at a distal portion of the drive shaft
creates a path in
front of the elongate member to facilitate crossing of the occlusion or
stenosis. The means is
moveable between an axially retracted configuration and an axially extended
configuration.
4
CA 02488588 2004-11-30
The means in the axially extended configuration creates a profile that is at
least as large as the
diameter of the distal end of the elongate member. In alternative
implementations, the path
creating means can move from the retracted position to an extended
configuration that has a
profile with the same or smaller profile than the distal end of the elongate
member.
In another aspect, the present invention provides a system for crossing an
occlusion or stenosis within a body lumen. The system comprises an elongate
member
having a proximal end, a distal end, and a lumen. A drive shaft is rotatably
and translatably
disposed in the elongate member and is removably attached to a rotating
mechanism. The
rotating mechanism rotates the drive shaft so that a distal tip can be
advanced beyond the
distal end of the elongate member to create a path through the occlusion or
stenosis such that
the elongate member can be advanced past the occlusion or stenosis. In a
specific
implementation, the rotating mechanism can be detached from the drive shaft
and an access
system can be delivered to the target site over the elongate member.
Thereafter, the rotating
mechanism can be reattached and the drive shaft can be rotated.
In yet another aspect, the present invention provides an assembly for crossing
an occlusive or stenotic material in a body lumen. The assembly comprises a
guidewire
having an axial lumen. A drive shaft rotatably and translatably extends
through the axial
lumen of the guidewire. The drive shaft has a distal tip that can be rotated
and advanced to
create a path through the occlusive or stenotic material. In some embodiments,
the guidewire
has an outer diameter or periphery similar to conventional passive guidewires
used for neuro,
cardio, and peripheral interventions. The outer diameter or periphery of the
guidewire having
an axial lumen is typically between approximately .040 inches and .009 inches,
and
preferably between approximately .024 inches and .009 inches, and typically
between 0.013
and 0.014 inches. Depending on the body lumen that is accessed, the outer
diameter of the
guidewire can be larger or smaller. In most embodiments, the guidewire has the
torqueability, pushability, and steerability to be advanced through the body
lumen.
In yet another aspect the present invention provides a guidewire system for
passing through occlusions or stenosis. The system comprises a hollow
guidewire having a
distal end, a proximal end, and a lumen. A drive shaft is movably disposed
within the hollow
guidewire such that a distal tip portion can extend beyond the distal end of
the hollow
guidewire. A rotating mechanism can rotate the drive shaft and an actuator can
be used to
control the axial movement of the drive shaft. Activation of the actuator
moves the distal end
5
- --
CA 02488588 2004-11-30
of the rotating drive shaft along its longitudinal axis to create a path
through the occlusion or
stenosis.
In yet another aspect, the present invention provides a method of crossing an
occlusion or stenosis within a body lumen. The method comprises positioning an
elongate
member and a drive shaft in the body lumen. The drive shaft is rotated. The
drive shaft is
expanded from a retracted configuration to an expanded configuration. In the
expanded
configuration, the drive shaft creates a path that is at least as large as the
perimeter of the
distal end of the elongate member. The distal portion of the drive shaft is
then advanced into
the occlusion or stenosis to create a path in the occlusion or stenosis.
In another aspect the present invention provides a method of crossing an
occlusion or stenosis within a body lumen. The method comprises advancing a
guidewire
=
through the body lumen. An access or support system is moved over the
guidewire to the
occlusion or stenosis. The guidewire is removed from the body lumen and a
steerable
elongate member having a drive shaft is passed through the lumen of the access
system. The
drive shaft is rotated within a lumen of the elongate member. The drive shaft
is advanced
from a retracted position to an extended position to create a path through the
occlusion or
stenosis.
In yet another aspect, the present invention provides a method of passing
through an occlusive or stenotic material in a body lumen. The method
comprises
positioning a hollow guidewire with a drive shaft adjacent the occlusion. A
drive shaft is
rotated and advanced out of the hollow guidewire and into the occlusive or
stenotic material
to create a path through the occlusive or stenotic material. In some
embodiments, the
guidewire can then be moved through the occlusive or stenotic material and an
access system
can be positioned in the path through the occlusive or stenotic material. The
remaining
occlusive or stenotic material can then removed with the access system.
In another aspect, the present invention provides a kit. The kit has a hollow
guidewire having a lumen. A rotatable drive shaft having a shaped distal tip
is removably
received within the lumen of the hollow guidewire. Instructions for use in
passing occlusions
or stenosis in a body lumen comprise rotating the inner wire within the
steerable hollow
guidewire and advancing the drive shaft into the occlusive or stenotic
material to create a
path through the occlusive or stenotic material. A package is adapted to
contain the hollow
guidewire, rotatable wire, and the instructions for use. In some embodiments,
the instructions
6
CA 02488588 2004-11-30
can be printed directly on the package, while in other embodiments the
instructions can be
separate from the package.
These and other aspects of the invention will be further evident from the
attached drawings and description of the invention.
BRIEF DESCRIPTION OF THE DRAWINGS
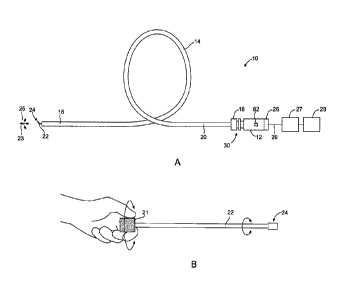
Fig. 1A shows an elevational view of a system of the present invention;
Fig. 1B shows manual manipulation of the drive shaft;
Fig. 2 shows a distal end of the elongate member and a distal tip of a drive
shaft of the present invention;
Fig. 3 is a cross sectional view of the device along A-A of Fig. 2;
Fig. 4 shows a diamond chip embedded distal tip of the drive shaft;
Fig. 5A shows a deflected distal tip in a position forward of the distal end
of
the elongate member;
Fig. 5B shows the flexible deflected distal tip in a fully retracted position
within the axial lumen of the elongate member;
Fig. 5C shows a deflected distal tip in a retracted position with the distal
tip
partially extending out of the elongate member;
Fig. 6A shows a sharpened deflected distal tip extending out of the elongate
member;
Figs. 6B and 6C show the cutting edges on the deflected distal tip of Fig. 6A;
Fig. 6D shows the distal tip deflected off of the longitudinal axis of the
drive
shaft;
Figs. 6E and 6F is a partial cut away section of two counter-wound drive
shafts of the present invention;
Fig. 6G shows the relative flexibility between a conventional drive shaft and
a
counter-wound drive shaft of the present invention;
Figs. 7A to 7C illustrate a method of forming the deflected distal tip using a
fixture;
Figs. 8A-8K show a variety of tip configurations;
Fig. 8L shows a distal tip having a flattened and twisted configuration;
Figs. 8M-8P show an exemplary method of manufacturing the distal tip of Fig.
8L;
7
_
CA 02488588 2004-11-30
Fig. 9 shows a drive shaft having spirals or external riflings which
facilitate
the proximal movement of the removed occlusive or stenotic material;
Fig. 10 shows a linkage assembly between the motor shaft and the drive shaft;
Figs. 11A and 11B show an alternative linkage assembly coupling the motor
shaft and the drive shaft;
Figs. 12-14 show a luer connection assembly which couples the elongate
member to the housing;
Figs. 15 shows a system having an access system, a hollow guidewire with a
deflectable distal end, and a drive shaft;
Figs. 16A to 16E illustrate a method of the present invention;
Figs. 17A to 17E illustrate another method of the present invention;
Figs. 18A to 18B illustrate yet another method of the present invention; and
Fig. 19 shows a kit of the present invention.
DESCRIPTION OF THE SPECIFIC EMBODIMENTS
The systems, devices and methods according to the present invention will
generally be adapted for the intraluminal treatment of a target site within a
body lumen of a
patient, usually in a coronary artery or peripheral blood vessel which is
occluded or stenosed
with atherosclerotic, stenotic, thrombotic, or other occlusive material. The
systems, devices
and methods, however, are also suitable for treating stenoses of the body
lumens and other
hyperplastic and neoplastic conditions in other body lumens, such as the
ureter, the biliary
duct, respiratory passages, the pancreatic duct, the lymphatic duct, and the
like. Neoplastic
cell growth will often occur as a result of a tumor surrounding and intruding
into a body
lumen. Removal of such material can thus be beneficial to maintain patency of
the body
lumen. While the remaining discussion is directed at passing through
atheromatous or
thrombotic occlusive material in a coronary artery, it will be appreciated
that the systems and
methods of the present invention can be used to remove and/or pass through a
variety of
occlusive, stenotic, or hyperplastic material in a variety of body lumens.
An apparatus 10 embodying features of the present invention is illustrated in
Fig. 1A. The apparatus 10 generally includes a housing 12 coupled to an
elongate member
14 which has a proximal end 16, a distal end 18, and an axial lumen 20. As
shown by arrows
23, 25, the drive shaft 22 is movably received within the axial lumen 20 of
the elongate
member 14. The distal tip 24 of the drive shaft 22 has a shaped profile such
that movement
8
CA 02488588 2004-11-30
of the drive shaft creates a path forward of the distal end of the elongate
member 14 for
passing through the occlusive or stenotic material. In most configurations,
wires 29 couple
the drive motor 26 to the control system 27 and power supply 28. In some
embodiments, the
power supply 28 is covered with a plastic sheath cover so as to maintain a
sterile environment
(not shown).
The drive motor 26 is attachable to the proximal end of the drive shaft 14 to
move (i.e., rotate, translate, reciprocate, vibrate, or the like) the drive
shaft 22 and shaped
distal tip 24. An input device 82 is attached to the housing 12 to control the
rotation and/or
axial movement of the drive shaft 22. The proximal end 16 of elongate member
14 is
coupled to the housing 12 through a connector assembly 30. The connector
assembly limits
the motion of the elongate member 14 while allowing the drive shaft 22 to
rotate and translate
within the elongate member 14. Optionally, some embodiments of the connector
assembly
30 includes an aspiration or infusion port (not shown) for facilitating fluid
exchange (e.g.,
delivery or removal) at the target site.
As shown in Fig. 1B, in order to macerate clots and to penetrate soft lesions,
some drive shafts of the present invention can be configured to be manually
rotated. In such
embodiments, the proximal end of the drive shaft 22 can be grasped between the
fingers and
manually turned to rotate the distal tip 24. The proximal end can be
optionally fit with a
knurled knob 21 or other mechanism which allows manual manipulation of the
proximal end
of the drive shaft 22.
An exemplary embodiment of the elongate member 14 is best seen in Figs. 2
and 3. The elongate member 14 is preferably a flexible, hollow guidewire that
has the
flexibility, pushability, and torqueability to allow a user to advance the
hollow guidewire
directly through a tortuous blood vessel to the target site. Because of the
high columnar
strength of the hollow guidewire 14 there is typically no need for a separate
guidewire.
In the exemplary embodiment illustrated in Fig. 2, the hollow guidewire has
an helically wound elongated shaft which defines an axial lumen 20 that
receives the drive
shaft 22 and which can be used for infusion or aspiration. The elongated shaft
includes a
proximal outer tube 32, an intermediate coil 34, and a distal coil tip 36. In
some
embodiments the intermediate coil 34 is made of a stainless steel or nitinol
coil, while the
distal tip 36 is composed of a flexible, radiopaque coil, such as platinum-
iridium. As shown,
the intermediate coil 34 is threadedly engaged with the outer tube 32 and
distal tip 36, but it
will be appreciated that the intermediate coil 34 can be connected to the
outer tube 32 and
9
CA 02488588 2004-11-30
distal tip 36 by any conventional means, e.g. solder, adhesive, or the like.
The proximal end
of the elongate member 14 can be coupled to a vacuum source or a fluid source
(not shown)
such that the target site can be aspirated or infused during the procedure.
Hollow guidewire 14 is typically sized to be inserted through coronary, neuro,
or peripheral arteries and can have a variety of diameters. The outer diameter
of the hollow
guidewire is typically between approximately 0.009 inches and 0.040 inches and
preferably
between approximately 0.009 inches and 0.024 inches so as to ensure
compatibility with
existing interventional cardiology catheters and stent systems. The length of
the hollow
guidewire 14 may be varied to correspond to the distance between the
percutaneous access
site and the target site. For example, for a target site within the heart that
is being accessed
through the femoral artery, the hollow guidewire will typically have a length
of
approximately 175 cm. It should be noted however, that other embodiments of
the hollow
guidewire 14 may have dimensions that are larger or smaller than the above
described
embodiments and the present invention is not limited to the above recited
dimensions.
Referring now to Fig. 3, a cross section of one embodiment of the hollow
guidewire 14 is shown. An inner tube 38 and outer tube 40 are positioned
around coils 34, 36
to provide a flexible, structural support which prevents liquids from moving
between the
blood vessel and the axial lumen of the elongate member 14. A reinforcing wire
42 can be
positioned between the inner tube 38 and the coils 34, 36 to provide for
deflection or steering
of the distal end 18. The reinforcing wire 42 can be formed of a material
having sufficient
strength so that a thin profile is possible. For example, the reinforcing wire
can be an at least
partially flattened strip of stainless steel that can retain its shape until
it is re-shaped to a
different configuration. In one configuration, the reinforcing wire 42 is
soldered or otherwise
connected to the distal end of coil 36 and the remainder of the reinforcing
wire 42 extends
proximally to the housing 12. Manipulation of the proximal end of the
reinforcing wire 42
allows the user to deflect or steer the distal tip 18 without permanently
impairing the inner
structure of the hollow guidewire 14. The steerable distal tip provides a user
with greater
intraluminal control of removing the occlusive or stenotic material from the
blood vessel and
also aids in navigating the hollow guidewire to the target site. In another
configuration, the
reinforcing wire is 42 can be soldered or otherwise connected to both the
distal end and to the
junction between coils 34, 36. Therefore, if the coils 34, 36, break, the
attached reinforcing
wire 42 can prevent the coils 34, 36 from detaching from the system 10. A more
complete
description of the hollow guidewire can be found in commonly owned U.S. Patent
CA 02488588 2004-11-30
Application No. 09/030,657, filed February 25, 1998, the complete disclosure
of which was
previously incorporated by reference.
Figs. 4-9 show various embodiments of the drive shaft 22 of the present
invention. In most embodiments, the drive shaft 22 is a wire, a counter-wound
multiple
strand wire, or a plurality of braided wires having a body 44 and a shaped
distal tip 24. The
proximal end of the drive shaft 22 can be removably coupled to a rotatable
motor shaft 48
(Figs. 10 and 11A) or manually manipulated (Fig. 1B). The body 44 of the drive
shaft 22
extends through the elongate member 14 so that the distal tip 24 of the drive
shaft is
positioned near the distal end of the elongate member 14. The detachable
connection to the
motor shaft 48 allows the drive shaft 22 and elongate member 14 to be detached
from the
motor shaft 48 and connector assembly 30 so that an access or support system
can be placed
over the elongate member and advanced through the body lumen.
As shown in Figs. 4 and 5A-5C, the distal tip can be shaped or deflected from
the longitudinal axis 50 to extend beyond the radius of the elongate member 14
such that
rotation of the drive shaft 22 creates a path radius 52 that is as at least as
large as the radius
54 of the distal end of the elongate member 14. In other embodiments, the
distal tip 24 will
be deflected and shaped so as to create a path radius 52 which is the same or
smaller than the
radius of the distal end of the elongate member 14 (Figs. 8B-8G). For example,
in one
exemplary configuration shown in Fig. 5C, a portion of the distal tip 24
extends beyond the
distal end 18 of the elongate member when in the fully retracted position.
When the drive
shaft 22 is advanced out of the elongate member 14, the flexible distal tip 24
maintains a
deflected shape (Fig. SA). In alternative configurations, it is contemplated
that the deflection
at the distal tip 24 can straighten somewhat under the force from the walls of
the elongate
member 14 when the drive shaft 22 is retracted into the elongate member 14
(Fig. 5B). Thus,
in the axially retracted configuration, the drive shaft 22 will have a profile
that is smaller than
the radius of the distal tip of the elongate member. When the drive shaft is
advanced out of
the distal end of the elongate member, the drive shaft will expand to an
axially extended
configuration in which the distal tip of the drive shaft 22 will have a
profile that is larger than
the axially retracted configuration, and in some embodiments will have a
larger profile than
the distal end of the elongate member 14.
Referring again to Fig. 4, in some configurations a layer of abrasive material
56 can be attached and distributed over at least a portion of the distal tip
24 of the drive shaft
22 so that the abrasive material 56 engages the stenotic or occlusive material
as the drive
11
CA 02488588 2004-11-30
shaft 22 is advanced into the occlusion or stenosis. The abrasive material 56
can be diamond
powder, diamond chips, fused silica, titanium nitride, tungsten carbide,
aluminum oxide,
boron carbide, or other conventional abrasive particles.
Alternatively, as shown in Figs. 6A-6D, the distal tip 24 of the drive shaft
22
can be sharpened to facilitate passing through the occlusion or stenosis. A
distal edge of the
tip 24 can be sharpened so as to define a cutting edge 58 which rotatably
contacts the
occlusive or stenotic material. In an exemplary embodiment illustrated in
Figs. 6B-6C, a tip
60 of the drive shaft can be sharpened to create a plurality of cutting edges
58. Furthermore,
as shown in Fig. 6D and as described above, the distal tip 24 can be deflected
from its
longitudinal axis 50 to create the cutting path radius 52 of the drive shaft
24 that is smaller,
larger, or the same length as the radius of the elongate member 14.
The drive shaft 22 can be composed of a shape retaining material, a rigid
material, a flexible material, or can be composed of a plurality of materials.
For example in
some configurations, the drive shaft 22 can be comprised of nitinol, stainless
steel, platinum-
iridium, or the like. The distal tip 24 of the drive shaft 22 can have an
enlarged tip, a
preformed curve, or a preformed deflection (Fig. 5A). Figs. 6E and 6F show
exemplary
embodiments of a counter-wound and composite drive shafts of the present
invention. The
counter-wound drive shaft 22 shown in Fig. 6E is made of a 0.004 inch OD
center wire 67
having a right-hand wound surrounding wire 69 coiled around the center wire
67. The
surrounding wire 69 can be soldered to the center wire at both ends of the
center wire. In the
embodiment of Fig. 6F, multiple strand wires 51 can be wound around a central
coil 71 to
form the drive shaft 22. The counter-wound drive shafts are significantly more
flexible than
a single wire guidewire and allows for a tighter bending radius over
conventional guidewire.
Fig. 6G illustrates the flexibility of both a 0.007 inch OD single wire
stainless steel wire drive
shaft 22a and a 0.007 inch OD counter-wound stainless steel drive shaft 22b.
As shown by
Fig. 6F, the counter-wound drive shaft has better flexibility, while still
maintaining its
torqueability, maneuverability, and columnar strength.
Additionally, in some embodiments, the distal portion of the drive shaft 22 is
radiopaque so that a physician can track the position of the drive shaft 22
using fluoroscopy.
The drive shaft 24 typically has a diameter between approximately 0.010 inches
and 0.005
inches. It should be appreciated that the dimension of the drive shaft will be
slightly less than
the inner diameter of the hollow guidewire so as to allow rotation without
significant heat
generation. Consequently, the dimensions of the drive shaft will vary
depending on the
12
CA 02488588 2004-11-30
relative inner diameter of the elongate member 14 and the present invention is
not limited to
the above described dimensions of the drive shaft.
In one embodiment, the distal tip 24 of the drive shaft is created using a
shaped fixture 64. As shown in Figs. 7A and 78, the distal tip 24 is
positioned on the fixture
64 and bent to a desired angle 66. The distal tip 24 can be bent to almost any
angle 66
between 0 degrees and 90 degrees from the longitudinal axis 50, but is
preferably deflected
between 0 degrees and 50 degrees. As shown in Fig. 7C, a sharpened edge 58
can be
created on the distal tip using a wafer dicing machine used in the production
of silicon
microchips (not shown). The angle of the sharpened edge 58 can be almost any
angle, but the
angle is typically between 0 degrees and 45 degrees, and is preferably
between
approximately 8 degrees and 18 degrees. Naturally, it will be appreciated
that a variety of
methods can be used to manufacture the distal tip of the drive shaft and that
the present
invention is not limited to drive shafts produced by the described method.
As mentioned above, the distal tip 24 can take various shapes. One
embodiment having a deflected distal tip 24 is shown in Fig. 8A. In an
exemplary
configuration, the deflected tip is offset at an angle such that rotation of
the drive wire 22
defines a profile or path that is at least as large as the outer diameter of
the distal end of the
elongate member 14. As shown in Figs. 88 and 8C, in other embodiments, the tip
can be
deflected at other angles and may have a length that creates a path that is
smaller or the same
diameter as the distal end of the elongate member. The deflected distal tip
can extend
radially any feasible length beyond the perimeter or diameter of the elongate
member 14. It
should be understood that the invention is not limited to a single deflected
tip. For example,
the drive shaft can comprise a plurality of deflected tips. Alternatively, the
drive shaft may
have a distal tip 24 that is twizzle shaped, spring shaped, twisted metal
shaped (Fig. 8D), ball
shaped (Fig. 8E), a discontinuous surface (Fig. 8F), or the like.
Alternatively, the drive shaft
may comprise a plurality of filaments (Fig. 8G), rigid or flexible brush
elements, a plurality
of coils, or the like.
The distal tip of the drive shaft can be configured optimally for the type of
occlusion or stenosis to be penetrated. Some lesions are made up substantially
of clot or
thrombotic material that is soft and gelatinous. Figs. 8H and 8K shows distal
tip
embodiments which may be used to macerate a soft clot, thrombotic material, or
stenosis.
Fig. 8H shows a distal tip 24 having a basket like construction which is made
up of a plurality
of strands 59 that are connected at their ends 61, 63. In another embodiment
illustrated in
13
CA 02488588 2004-11-30
Fig. 81, the distal tip 24 can be composed of a plurality of strands 59 that
are unconnected at
their distal ends 63. Additionally, the distal ends 63 of the strands 59 can
be turned inward so
that the distal ends 63 do not penetrate the body lumen when rotated. Fig. 8J
shows a
corkscrew spiral distal tip having a blunt distal end 63. Fig. 8K shows a
distal tip having a
loop configuration.
In use, the distal tip 24 is rotated and advanced distally from a retracted
position to an expanded position into the soft material in the target lesion.
If slow speed
rotation is desired the user can rotate the drive shaft slowly by hand by
grasping a knurled
knob attached to the proximal end of the drive shaft (Fig. 1B). If high speed
rotation is
desired, the proximal end of the drive shaft 22 can be attached to the drive
motor 26. As the
expanded wire basket tip is rotated, the tip macerates the soft clot and
separates the clot from
the wall of the body lumen. If a large diameter hollow guidewire working
channel is used to
deliver the drive shaft to the target area, the macerated clot can be
aspirated through the
guidewire working channel. Alternatively or additionally, a fluid, such as
thrombolytic
agents, can be delivered through the working channel to dissolve the clot to
prevent "distal
trash" and blockage of the vasculature with debris from the macerated clot.
In another exemplary embodiment shown in Fig. 8L, the distal tip 24 of the
drive shaft 22 can be flattened and twisted to create a screw lie tip that can
create a path
through the occlusion. The flattened and twisted distal tip 24 can have a same
width, a
smaller width or a larger width than the drive shaft 24. For example, in one
configuration for
a drive shaft having an outer diameter of 0.007 inches, the distal tip 24 can
be flattened to
have a width between approximately 0.015 inches and 0.016 inches, or more. It
should be
appreciated, however, that the distal tip can be manufactured to a variety of
sizes.
Figs. 8M-8P show one method of manufacturing the flattened and distal tip of
the present invention. The round drive shaft 22 (Fig. 8M) is taken and the
distal end is
flattened (Fig. 8N). The distal end can be sharpened (Fig. 80) and twisted two
or two and a
half turns (Fig. 8P). If a different amount of twists are desired, the distal
tip can be
manufactured to create more (or less) turns.
As shown in Figs. 9 and 15 in some embodiments the drive shaft 22 can
optionally have spiral threads or external riflings 64 which extend along the
body 44. As the
drive shaft 22 is rotated and axially advanced into the atheromatous material,
the distal tip 24
creates a path and removes the atheromatous material from the blood vessel.
The rotating
spirals 64 act similar to an "Archimedes Screw" and transport the removed
material
14
CA 02488588 2004-11-30
proximally up the axial lumen of the elongate member 14 and prevent the loose
atheromatous
material from blocking the axial lumen of the elongate member 14 or from
escaping into the
blood stream.
In use, drive shaft 24 is rotated and advanced to create a path distal of the
elongate member 14 to create a path through the occlusion. The drive shaft 24
can be
advanced and rotated simultaneously, rotated first and then advanced, or
advanced first and
then rotated. The drive shaft 22 is typically ramped up from a static position
(i.e. 0 rpm) to
about 5,000 rpm, 20,000 rpm with a motor. It should be noted, however, that
the speed of
rotation can be varied (higher or lower) depending on the capacity of the
motor, the
dimensions of the drive shaft and the elongate member, the type of occlusion
to be bypassed,
and the like. For example, if desired, the drive shaft can be manually rotated
or reciprocated
at a lower speed to macerate soft clots or to pass through lesions.
The distal tip of the drive shaft 22 can extend almost any length beyond the
distal portion of the hollow guidewire. In most embodiments, however, the
distal tip
typically extends about 5 centimeters, more preferably from 0.05 centimeters
to 5
centimeters, and most preferably between 0.05 centimeter and 2 centimeters
beyond the distal
portion of the hollow guidewire.
Referring now to Figs. 10, 11A, and 11B, the motor shaft 48 and the proximal
end 46 of the drive shaft 22 are coupled together with a detachable linkage
assembly 70. In
one embodiment shown in Fig. 10, linkage assembly 70 has a first flange 72
attached to the
motor shaft 48. The first flange can be snap fit, snug fit, or permanently
attached to the drive
shaft 48. A second flange 74 can be permanently or removably coupled to the
proximal end
46 of the drive shaft 22 so that the first flange 72 of the motor shaft 48 can
threadedly engage
the second flange 74. In some embodiments, the proximal end of the drive shaft
46 can be
enlarged so as to improve the engagement with the second flange 74. An o-ring
76 is
preferably disposed within a cavity in the first flange 72 to hold the first
flange 72 and second
flange 74 in fixed position relative to each other.
As shown generally in Figs. 1 and 11B, the motor 26 can be removably
coupled to the housing 12. To detach the motor 26 and power supply 28 from the
drive shaft
22, the user can unlock the luer assembly 30 so as to release the elongate
member 14 from the
housing 12. The drive shaft 22 and elongate member 14 are then both free to
move axially.
The motor 26 can be moved proximally out of the housing 12 and the proximal
end 46 of the
drive shaft 22 can be detached from the motor shaft 48. After the motor 26,
housing 12, and
_
CA 02488588 2004-11-30
luer assembly 30 have been uncoupled from the elongate member 14 and drive
shaft 22, a
support or access system (not shown) can be advanced over the free proximal
end of the
elongate member 14. Thereafter, the luer assembly and motor shaft 48 can be
recoupled to
the elongate member 14.
In the embodiment shown in Figs. 11A and 11B, the linkage assembly 70
includes a connecting shaft 78 that can be snugly fit over the motor shaft 48.
The connecting
shaft 78 preferably tapers from a diameter slightly larger than the motor
shaft 48 to a
diameter of that of the approximately the proximal end 46 of the drive shaft
22. In the
embodiment shown, the connecting shaft 78 is coupled to the drive shaft
through shrinkable
tubing 80. Because the connecting shaft 78 is snug fit over the motor shaft,
(and is not
threadedly attached to the drive shaft) the size of the connecting shaft 78
can be smaller than
the linkage assembly 70. While the exemplary embodiments of the connection
assembly
between the drive shaft and motor shaft have been described, it will be
appreciated that drive
shaft and motor shaft can be attached through any other conventional means.
For example,
the motor shaft 48 can be coupled to the drive shaft 22 through adhesive,
welding, a snap fit
assembly, or the like.
As shown in Fig. 11B, the drive shaft 22 extends proximally through the
housing 12 and is coupled to the motor shaft 48. An actuator 82 can be
activated to advance
and retract the drive shaft 22. In some embodiments, the motor is press fit
into the actuator
housing 12. The drive shaft 22 is attached to the motor shaft 26 via o-rings
such that the
drive shaft 22 can be moved axially through axial movement of the actuator 82.
In most embodiments, actuation of the drive motor 26 and power supply 28
(e.g. rotation of the drive shaft) will be controlled independent from
advancement of the drive
shaft 22. However, while the actuator 82 is shown separate from the control
system 27 and
power supply 28 (Fig. 1), it will be appreciated that actuator 82 and control
system 27 can be
part of a single, consolidated console attached to the housing 12 or separate
from the housing
12. For example, it is contemplated that that the drive shaft 22 can be
rotated and advanced
simultaneously by activation of a single actuator (not shown).
A connection assembly 30 is positioned on a proximal end of the housing to
couple the hollow guidewire 14 and the drive shaft 22 to the housing 12. In a
preferred
embodiment shown in Figs. 12-14, the connection assembly 30 is a detachable
luer which
allows the drive shaft 22 to be moved (e.g. rotated, reciprocated, translated)
while the
elongate member is maintained in a substantially static position. Fig. 12 best
illustrates an
16
CA 02488588 2004-11-30
exemplary luer connection assembly 30 which couples the elongate member 14 and
the
housing 12. The luer has a gland 86 which is rotatably connected to a fitting
88 and a tubular
portion 90. Rotation of the gland 86 rotates and torques the elongate member
14 while the
elongate member 14 is advanced through the blood vessel. Fitting 88 is
threaded into the
gland 86 such that a distal end of the fitting engages an o-ring 92 and a
surface wall 94 of the
gland. The longitudinal axis 96 of the fitting 88 and gland 86 are aligned so
as to be able to
receive the axial lumen of the elongate member 14. As the fitting 88 engages
the o-ring 92,
the o-ring is compressed radially inward to squeeze and maintain the position
of the elongate
member 14. Accordingly, as illustrated in Fig. 13, when the drive shaft 22 is
rotated within
the elongate member 14, the o-ring 92 is able to substantially maintain the
position and
orientation of the elongate member 14. Tubular portion 90 attached to the
proximal end of
the fitting 88 threadedly engages the housing 12 and enables the luer
connection assembly 30
to be removed from the housing 12 (Fig. 14). A more complete description of
the connection
assembly 30 can be found in commonly owned U.S. Patent Application No.
09/030,657, filed
February 25, 1998, the complete disclosure of which was previously
incorporated by
reference. It should be appreciated that the present invention is not limited
to the specific
luer assembly described. Any luer assembly can be used to connect the elongate
member 14
to the housing 12. For example, a Y-luer assembly (not shown) can be used with
the system
of the present invention to infuse or aspirate of fluids through the lumen of
the hollow
guidewire 14.
As shown in Figs. 3 and 15, systems of the present invention can further
include an access or support system 98. The access or support system 98 can be
an
intravascular catheter such as a hollow guidewire support device, support
catheter, balloon
dilation catheter, atherectomy catheters, rotational catheters, extractional
catheters,
conventional guiding catheters, an ultrasound catheter, a stenting catheter,
or the like. In an
exemplary configuration shown in Fig. 15, the system includes an infusion or
aspiration
catheter which has at least one axial channel 100, and preferably a plurality
of axial channels
100 which extends through the catheter lumen 102 to the distal end of the
catheter. The
elongate member 14 and drive shaft 22 can be positioned and advanced through
the lumen
102 of the catheter. The axial channel 20 of the elongate member 14 and/or the
axial
channels 100 of the catheter 98 can also be used to aspirate the target site
or infuse
therapeutic, diagnostic material, rinsing materials, dyes, or the like.
17
CA 02488588 2004-11-30
The access or support system can be guided by the elongate member to the
target site in a variety of ways. For example, as illustrated in Figs. 16A to
16E, a
conventional guidewire 104 can be advanced through the blood vessel BV from
the access
site (Fig. 16A). Once the guidewire 104 has reached the target site, the
support or access
system 98 can be advanced over the guidewire 104 (Fig. 16B). Alternatively,
the guidewire
104 and support or access system 98 can be simultaneously advanced through the
body lumen
(not shown). Once the support or access system 98 has reached the target site,
the
conventional guidewire 104 can be removed and the hollow guidewire 14 having
the drive
shaft 22 can be introduced through the lumen 102 of the access system 98 (Fig.
16C). Even if
the distal tip 24 of the drive shaft 22 is not fully retracted into the axial
lumen 20, the lumen
102 of the support or access system protects the blood vessel BV from damage
from the
exposed distal tip 22. In most methods, the support or access system is
positioned or
stabilized with balloons, wires, or other stabilization devices 106 to provide
a more controlled
removal of the occlusive or stenotic material OM. Once the drive shaft 22 has
reached the
target site, the drive shaft can be rotated and advanced into the occlusive or
stenotic material
OM to create a path (Figs. 16D and 16E).
In another method of the present invention, the hollow guidewire 14 can be
used to guide the support or access system to the target site without the use
of a separate
guide wire. The hollow guidewire 14 provides the flexibility, maneuverability,
torqueability
(usually 1:1), and columnar strength necessary for accurately advancing
through the tortuous
vasculature and positioning the distal end of the support or access system at
the target site.
The steerable distal portion can be deflected and steered through the tortuous
regions of the
vasculature to get to the target site. As shown in Fig. 17A, the hollow
guidewire is advanced
through the tortuous blood vessel to the target site. Due to the small size of
the guidewire 14
relative to the blood vessel, even if the distal tip 24 of the drive shaft 22
extends partially out
of the hollow guidewire 14, any potential damage to the blood vessel BY will
be minimal.
Once the hollow guidewire reaches the target site within the blood vessel, the
motor shaft 48, luer assembly 30, and housing 12 can be detached from the
proximal end 46
of the drive shaft 22 so that the support or access system can be placed over
the hollow
guidewire. After the motor has been detached, the support or access system can
be advanced
over the guidewire and through the body lumen to the target site (Fig. 17B).
To reattach the
drive motor 26 to the drive shaft 22, the hollow guidewire 14 and drive shaft
22 are inserted
through the luer assembly 30. The luer assembly 30 is tightened to lock the
position of the
18
CA 02488588 2004-11-30
hollow guidewire 14. The drive shaft 22 will extend proximally through the
housing 12
where it can be recoupled to the motor shaft using the above described linkage
assemblies 70
or other conventional linkage assemblies. Once at the target site, the
position of the support
or access system 98 can be stabilized by a balloon, wires, or other
stabilizing devices 106,
and the drive shaft 22 can be rotated and advanced into the occlusive or
stenotic material OM
(Figs. 17C and 17D). The rotation of the drive shaft creates a path forward of
the distal end
18 of the hollow guidewire 14. As noted above, the path can have the same
diameter, smaller
diameter, or larger diameter than the distal end of the hollow guidewire.
Before, during, or
after the rotation of the drive shaft, the user can steer or deflect the
distal end 18 of the hollow
guidewire 14 to guide the hollow guidewire to the desired location within the
blood vessel.
For example, as shown in Fig. 17E, once a portion of the occlusion or stenosis
has been
removed, the distal end 18 of the hollow guidewire 14 can be guided to angle
the distal end
so that the drive shaft is extended into a different portion of the occlusive
or stenotic material
OM.
While the apparatus of the present invention is sufficient to create a path
through the occlusion OM without the use of a support or access system, the
apparatus 10 of
the present invention can be used in conjunction with other atherectomy
devices to facilitate
improved removal or enlargement of the path through the occlusion. For example
as shown
in the above figures, the hollow guidewire 14 and the atherectomy device 108
can be
advanced through the body lumen and positioned adjacent the occlusion OM. The
drive shaft
22 is rotated and advanced to make an initial path through the occlusion (Fig.
18A). The
hollow guidewire 14 is then moved through the path in the occlusion and the
atherectomy
device 108 can then be advanced over the hollow guidewire 14 into the path in
the occlusion
OM to remove the remaining occlusion with cutting blades 110, or the like
(Fig. 18B). While
Fig. 18B shows cutting blades 110 to remove the occlusive material OM, it will
be
appreciated that other removal devices and techniques can be used. Some
examples include
balloon dilation catheters, other atherectomy catheters, rotational catheters,
extractional
catheters, laser ablation catheters, stenting catheters, and the like.
In another aspect, the invention provides medical kits. As shown in Fig. 19,
the medical kit generally includes a system 10, instructions for use (IFU) 120
which describe
any of the above described methods, and a package 130. The IFU can be separate
from the
package or they can be printed on the package. The kits can also optionally
include any
19
_
CA 02488588 2013-11-20
combination of a second guidewire, a motor, a power supply, a plastic sheath
cover, connection
assemblies, support or access systems, or the like.
While the above is a complete description of the preferred embodiments of the
invention, various alternatives, modifications, and equivalents may be used.
The scope of the
following claims should not be limited by the embodiments set forth in the
examples, but
should be given the broadest interpretation consistent with the specification
as a whole.