Note: Descriptions are shown in the official language in which they were submitted.
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
BIS-TRANSITION-METAL-CHELATE PROBES
This invention was made with Government support under Grant No. NIH RO1-
GM41376, awarded by the National Institutes of Health. Therefore, the
Government has
certain rights in this invention.
FIELD OF THE INVENTION
This invention relates to compositions and methods for labeling molecules.
More
particularly, the present invention relates to certain transition metal
chelate probes capable of
selectively associating with histidine- containing target sequences on
compounds of interest
and yielding a detectable signal.
BACKGROUND OF THE INVENTION
Characterization of proteins often requires the ability to incorporate
detectable
groups--e.g., fluorochromes, chromophores, spin labels, radioisotopes,
paramagnetic atoms,
heavy atoms, haptens, crosslinking agents, and cleavage agents--at specific,
defined sites.
For proteins that do not contain pre-existing cysteine residues, site-specific
labeling can be
accomplished by use of site-directed mutagenesis to introduce a cysteine
residue at the site of
interest, followed by cysteine-specific chemical modification to incorporate
the labeled
probe. However, for proteins that contain pre-existing cysteine residues, site-
specific
labeling is difficult. Multiple strategies have been reported: (i) intein-
mediated labeling
("expressed protein ligation"), (Muir, et al., Proc. Nat'l. Acad. .Sci. USA,
95:6705-6710
(1998)); (ii) transglutaminase-mediated labeling (Sato et al., Biochem.
35:13072-13080
(1996)); (iii) oxidation-mediated labeling (Geoghegan, et al., Bioconj. Chem.,
3:138-146
(1992)); and (iv) trivalent-arsenic-mediated labeling (Griffin et al., Science
281:269-272,
1998) (U.S. Patent No. 6,008,378). Strategies (i)-(iii) do not permit in situ
labeling (i.e.,
direct labeling of proteins in cuvettes, gels, blots, or biological samples--
without the need for
a subsequent purification step) or in vivo labeling (i.e., direct labeling of
proteins in cells).
Strategy (iv) requires a structural scaffold presenting two trivalent-arsenic
atoms in a
precisely defined spatial relationship and therefore relates only to a limited
number of
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
detectable groups (such as those having a detectable xanthene, xanthanone, or
phenoxazinestructural nucleus).
Transition-metal chelates consisting of a transition-metal ion, such as Ni2+,
Co2+,
Cu2+, or ZnZ+, in complex with a tridentate or tetradentate chelating ligand,
such as
iminodiacetic acid (IDA) or nitrilotriacetic acid (NTA), exhibit high affinity
for oligohistidine
sequences, particularly hexahistidine sequences (Sulkowski, E., Trends
Biotechnol., 3:1-7
(1985); Hochuli, et al., J. Chromat. 411:177-184 (1987); Hochuli, E. et al.
BioTechnol.
6:1321-1325 (1988). Figure 1 shows a proposed model for binding of neighboring
hexahistidine residue to a Ni-NTA resin as disclosed in Crowe, J. et al.,
Methods Mol. Bdol.,
31:371-387 (1994)).
The high affinity of interactions between transition-metal chelates and
oligohistidine
sequences, particularly hexahistidine sequences, has been verified using force
microscopy
experiments, which permit direct measurement of interaction forces on the
single-molecule
level and direct observation of molecular recognition of a single receptor-
ligand pair
(Kienberger, F. et al. Single Mol. 1:59-65 (2000); Schmitt, L. et al. Biophys.
J. 78: 3275-3285
(2000)).
The high affinity of interactions between transition-metal chelates and
oligohistidine
sequences, particularly hexahistidine sequences, has been used advantageously
to purify
biomolecules containing, or modified to contain, "oligohistidine tags,"
particularly
"hexahistidine tags" (Hochuli, E. et al. BioTechnol. 6:1321-1325 (1988);
Crowe, J. et al.,
Methods Mol. Biol., 31:371-387 (1994)). In this application, termed
"immobilized-metal-
chelate affinity chromatography," a transition-metal chelate consisting of a
transition-metal
ion, such as Niz+, Co2+, Cu2+, or Zn2+, in complex with a tridentate or
tetradentate chelating
ligand, such as iminodiacetic acid (1DA) or nitrilotriacetic acid (NTA), is
immobilized on a
solid phase, such as chromatographic resin, and the resulting immobilized
metal chelate is
used to bind, and thereby purify from other components, tagged biomolecules.
The high affinity of interactions between transition-metal chelates and
oligohistidine
tags, particularly hexahistidine tags, also has been used advantageously in
biosensor analysis
2
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
of biomolecules containing, or modified to contain, oligohistidine tags,
particularly
hexahistidine tags (Gershon, et al. J. Immunol. Meths. 183:65-76 (1995);
Nieba, L. et al.
Anal. Biochem. 252:217-228 (1997)). Kienberger et al., Single Mol. 1; S9-65
(2000). In this
application, a transition-metal chelate consisting of a transition-metal ion,
such as Ni2+, Co2+
Cu2+, or Zn2+, in complex with a tridentate or tetradentate chelating ligand,
such as
iminodiacetic acid (mA) or nitrilotriacetic acid (NTA), is immobilized on a
biosensor chip,
such a surface-plasmon-resonance biosensor chip, and the resulting immobilized
metal
chelate is used to detect, quantify, and analyze tagged biomolecules.
It would be advantageous to be able to use the high affinity of interactions
between
transition-metal chelates and oligohistidine tags, particularly hexahistidine
tags, in labeling
and in situ detection of tagged biomolecules.
There is a need for improved methods and compositions for protein labeling. In
particular, there is a need for methods and compositions that permit in situ
labeling, that
permit in vivo labeling, and that encompass a wide range of detectable groups
with different
properties.
SUMMARY OF THE INVENTION
The invention provides a molecule with two pendant metal-chelate moieties
according
to the general structural Formula (I), including tautomers, salts, and acids
thereof:
R2/ X~ R2.
IRS,
N N
~Y \O / .Y,. O
O O O O O O
(I)
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
wherein: (a) Y and Y' are each a transition metal, (b) R' and R'' are each
independently
C(COO-), CH(COOH), or absent; (c) R2 and R2~ are linkers each having a length
of from
about 3.0 to about 20 A; and (d) X is a detectable group. The linkers may be
linear or
branched, may contain aromatic moieties, and optionally may be further
substituted.
Additionally provided herein are methods of synthesis of compounds of the
present
invention involving coupling of:
(a) a synthon which includes a bis-activated-ester derivative of a detectable
group; and
(b) a synthon which includes an amine or hydrazide derivative of a chelator;
and then adding a transition metal.
Additionally provided herein are methods of synthesis of compounds of the
present
invention containing a non-sulfonated cyanine or squaraine detectable group,
involving
coupling of: (a) a synthon selected from mono-chelator-functionalized 2,3,3-
trimethylindole,
mono-chelator-functionalized 2,3,3-trimethylbenzindole, mono-chelator-
functionalized 2-
methyl-pyridine, mono-chelator-functionalized 2-methyl-benzothiazole, mono-
chelator-
functionalized 2-methyl-napthothiazole, mono-chelator-functionalized 2-methyl-
benzoxazole, and mono-chelator-functionalized 2-methyl-napthoxazole; (b) a
synthon,
identical or nonidentical to the synthon in (a), selected from the group in
(a); and
(c) a synthon containing at least one carbon atom;
and then adding a transition metal.
Additionally provided herein are methods of synthesis of compounds of the
present
invention containing a disulfonated cyanine or squaraine detectable group,
involving
coupling of:
(a) a synthon selected from mono-chelator-functionalized 2,3,3-trimethyl-5-
sulfanato-indole,
mono-chelator-functionalized 2,3,3-trimethyl-6-sulfanato-benzindole, mono-
chelator-
functionalized 2-methyl-5-sulfanato-pyridine, mono-chelator-functionalized 2-
methyl-5-
sulfanato-benzothiazole, mono-chelator-functionalized 2-methyl-6-sulfanato-
napthothiazole,
mono-chelator-functionalized 2-methyl-5-sulfanato-benzoxazole, and mono-
chelator-
functionalized 2-methyl-6-sulfanato-napthoxazole;
4
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
(b) a synthon, identical or nonidentical to the synthon in (a), selected from
the group in (a);
and
(c) a synthon containing at least one carbon atom; and then adding a
transition metal.
Additionally provided herein are methods of synthesis of compounds of the
present
invention containing a monosulfonated cyanine or squaraine detectable group,
involving
coupling of: (a) a synthon selected from mono-chelator-functionalized 2,3,3-
trimethylindole,
mono-chelator-functionalized 2,3,3-trimethylbenzindole, mono-chelator-
functionalized 2-
methyl-pyridine, mono-chelator-functionalized 2-methyl-benzothiazole, mono-
chelator-
functionalized 2-methyl-napthothiazole, mono-chelator-functionalized 2-methyl-
benzoxazole, and mono-chelator-functionalized 2-methyl-napthoxazole; (b) a
synthon
selected from mono-chelator-functionalized 2,3,3-trimethyl-5-sulfanato-indole,
mono-
chelator-functionalized 2,3,3-trimethyl-6-sulfanato-benzindole, mono-chelator-
functionalized 2-methyl-5-sulfanato-pyridine, mono-chelator-functionalized 2-
methyl-6-
sulfanato-benzothiazole, mono-chelator-functionalized 2-methyl-6-sulfanato-
napthothiazole,
mono-chelator-functionalized 2-methyl-5-sulfanato-benzoxazole, and mono-
chelator
functionalized 2-methyl-6-sulfanato-napthoxazole; and (c) a synthon containing
at least one
carbon atom; and then adding a transition metal.
Additionally provided herein are methods of synthesis of compounds of the
present
invention containing a xanthene, xanthanone, or phenoxazine detectable group,
involving
reaction of a xanthene, xanthanone, or phenoxazine detectable group, a
secondary-amine
derivative of a chelator, and formaldehyde, according to the Mannich reaction
(Mannich, C.
et al. Arch. Pharm. 250:647, 1912); followed by addition of a transition
metal.
Additionally provided herein is a labeled target material including a target
sequence
of the form: (H);, wherein H is histidine, and i is 4 to 12, preferably 4 to
8, and most
preferably 6, and wherein the target sequence is bonded with a molecule
according to
Formula (I).
Also included is a detectable complex including a molecule according to
Formula (I)
and a target sequence, bonded thereto. The target sequence includes an amino
acid sequence
5
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
of the form: (H);, wherein H is histidine, and i is 4 to 12, preferably 4 to
8, and most
preferably 6.
The invention also includes a method for imparting fluorescent properties to a
target
material, including the step of reacting: (a) the target material having a
target sequence of the
form (H);, wherein H is histidine, and i is 4 to 12, preferably 4 to 8, and
most preferably 6,
with (b) a molecule according to Formula (I), under conditions sufficient to
permit metal-
chelate moieties of said molecule according to Formula (I) to bond to the
target sequence.
Furthermore, provided herein is a method for detecting a target material of
interest,
including the steps of: (a) providing a target material of interest having a
target sequence of
the form: (H);, wherein H is histidine, and i is 4 to 12, preferably 4 to 8,
and most preferably
6; (b) incubating the polypeptide with a molecule according to Formula (I),
having a
detectable group, for a time period sufficient to allow labeling of the target
material; and (c)
detecting the detectable group, thereby detecting the target material of
interest.
Additionally, a method for imaging the localization, concentration or
interactions of a
target material of interest on or within cells, tissues, organs or organisms
is provided,
including the steps of: (a) providing a target material of interest having a
target sequence of
the form: (H);, wherein H is histidine, and i is 4 to 12, preferably 4 to 8,
and most preferably
6; (b) incubating the target material with a molecule according to Formula (I)
for a time
period sufficient to allow labeling of the polypeptide; and (c) detecting the
detectable group
of said molecule according to Formula (I), thereby imaging the localization,
concentration or
interactions of the target material of interest.
Furthermore, provided herein is an assay method for monitoring a binding
process
including the steps of: (a) reacting a first component of a specific binding
pair with a second
component of the pair, with the first component being labeled with a molecule
according to
Formula (I) having a detectable group; and (b) monitoring the reaction by
monitoring a
change in a signal of the detectable group.
6
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
Also provided herein is an assay method for monitoring a binding process
including
the steps of: (a) reacting a first component of a specific binding pair with a
second
component of the pair, with the first component being labeled with a molecule
according to
Formula (I) having a detectable group; and (b) monitoring the reaction by
monitoring
fluorescence emission intensity, fluorescence lifetime, fluorescence
polarization, fluorescence
anisotropy, or fluorescence correlation of the detectable group.
Additionally provided herein is an assay method for monitoring a binding
process,
including the steps of: (a) reacting a first component of a specific binding
pair with a second
component of the pair, with the first component being labeled with a molecule
according to
Formula (I) wherein X of Formula (I) is a fluorochrome, and with the second
component
containing Y, wherein Y is selected from the group including a fluorochrome
and
chromophore, Y being capable of participating in fluorescence energy transfer,
fluorescence
quenching, or exciton formation with X; and (b) monitoring the reaction by
monitoring
fluorescence of X.
The invention also provides an assay method for monitoring a binding process,
including the steps of: (a) reacting a first component of a specific binding
pair with a second
component of the pair, with the first component being labeled with a molecule
according to
Formula (I) wherein X of Formula (I) is selected from the group consisting of
a fluorochrome
and a chromophore, and with the second component containing Y, wherein Y is a
fluorochrome able to participate in fluorescence energy transfer, fluorescence
quenching, or
exciton formation with X; and (b) monitoring the reaction by monitoring
fluorescence of Y.
The invention further provides an assay method for monitoring a reaction,
including
the steps of: (a) reacting a first participant in a reaction with a second
participant in
the reaction, the first participant being labeled with a molecule according to
Formula (I); and
(b) monitoring the reaction by monitoring a change in a detectable property of
the detectable
group.
Furthermore, provided herein is a method for isolating a target material of
interest
including the steps of: (a) contacting molecules according to Formula (I)
immobilized on a
7
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
solid support, with a solution containing a polypeptide of interest, the
polypeptide including a
target sequence of the form: (H);, wherein H is histidine, and i is 4 to 12,
preferably 4 to 8,
and most preferably 6, under conditions that allow binding of the target
material to
immobilized molecules of Formula (I); and (b) eluting the target material of
interest with a
low-molecular weight monothiol or low-molecular-weight dithiol.
The invention also includes a method for immobilizing a target material of
interest
including the steps of: (a) contacting molecules according to Formula (I)
immobilized on a
solid support, with a solution containing a target material, the target
material containing a
target sequence of the form (H);, wherein H is histidine, and i is 4 to 12,
preferably 4 to 8, and
most preferably 6, under conditions that allow binding of the target material
to immobilized
molecules according to Formula (I).
Additionally provided herein is a kit including: (a) a molecule according to
Formula
(I); and (b) a molecule containing a target sequence including an amino acid
sequence of the
form: (H);, wherein H is histidine, and i is 4 to 12, preferably 4 to 8, and
most preferably 6.
Further provided herein is a kit including: (a) a molecule according to
Formula (I);
and (b) a reagent that promotes the formation of a complex between the
molecule according
to Formula (I) and a peptide having a target sequence of the form: (H);,
wherein H is
histidine, and i is 4 to 12, preferably 4 to 8, and most preferably 6.
8
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 shows a prior-art model for the binding of neighboring hexahistidine
residues
to a NTA:Ni2+ resin.
FIGS. 2 and 3 show results of fluorescence anisotropy experiments verifying
specific
interactions between bis-transition-metal-chelate probes according to the
invention with a
hexahistidine-tagged protein.
FIG. 4 is a model structure of a DNAF-CAP-His6 complex showing the position of
the
fluorescein of DNAF (circle), the position of the hexahistidine tag of each
CAP-His6 promotor
(diamond), the distance between fluorescein and the hexahistidine tag of the
proximal CAP-
His6 promotor (~55 A), and the distance between fluorescein and the
hexahistidine tag of the
distal CAP-Hiss promotor (~80 A).
FIGS. 5 and 6 show results of FRET experiments verifying high-affinity,
specificinteractions of bis-transition-metal-chelate probes according to the
present invention
with a hexahistidinetagged protein.
FIGS. 7 and 8 show results of FRET experiments verifying stoichiometric
interactions
of nickel containing probes according to the present invention with the
hexahistidine tag.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
The inventors have found, as set forth herein, that a molecule having two
transition-metal
chelates and a detectable group binds with high affinity and high specificity
to oligohistidine
target sequences, particularly hexahistidine target sequences.
Furthermore, the inventors have found that a molecule having two transition-
metal
chelates and a detectable group binds with much higher affinity (more than 10
times higher
affinity) and much higher specificity (more than 10 times higher specificity)
to oligohistidine
9
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
target sequences, particularly hexahistidine target sequences, than does a
molecule having
only a single transition-metal chelate and a detectable group.
Furthermore, the inventors have found that a molecule having two transition-
metal
chelates and a detectable group can be used to label, detect, and analyze
target materials
containing, or derivatized to contain, oligohistidine target sequences,
particularly
hexahistidine target sequences.
Furthermore, the inventors have found that a molecule having two transition-
metal
chelates and a detectable group can be used in in situ labeling, detection,
and analysis of
target materials containing, or derivatized to contain, oligohistidine target
sequences,
particularly hexahistidine target sequences (i.e., direct labeling, detection,
and analysis of said
target materials--without the need for a subsequent purification step).
Compositions of the Invention
The present invention provides a probe for detecting a target material of
interest. The
probe includes two transition-metal chelates and a detectable group, according
to the
following general structural Formula (I), and tautomers, salts, and acids
thereof:
R2/ X~ R2,
Rt R1,
N N
O OYO \O O OY~O O
(I)
wherein: (a) Y and Y' are each a transition metal, (b) R' and R'~ are each
independently CH(COO-), CH(COOH), or absent, (c) RZ and R2~ are linkers each
having a
length of about 3.0 to 20 ~, and preferably about 3.0 to 15 ~, and (d) X is a
detectable group.
The linkers may be linear or branched, may contain aromatic moieties, and may
optionally be
further substituted.
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
"Y" in Formula (I) is a transition metal. Y can be any transition metal
capable of specific
interaction with a oligohistidine tag. Transition metals are those metals
having incompletely
filled d-orbitals and variable oxidation states. Examples of suitable
transition metals include:
nickel, cobalt, copper, and zinc. In a preferred embodiment, Y is a divalent
transition-metal
ion. In a particularly preferred embodiment, Y is selected from the group
consisting of Ni2+,
Co2+, Cu2+, and Zn2+.
When R' in Formula (I) is absent, the chelator is iminodiacetic acid (IDA).
When R'
is CH(COO-) or CH(COOH), the chelator is nitrilotriacteic acid (NTA).
Similarly, when R'~ in Formula (I) is absent, the chelator is iminodiacetic
acid (IDA).
When R'' is CH(COO-) or CH(COOH), the chelator is nitrilotriacetic acid (NTA).
R2 and RZ' in Formula (I) are linkers. The structures of R2 and R2' should
permit the
two pendant transition-metal chelates to be separated by a distance comparable
to the
dimensions of a oligohistidine target sequence, particularly a hexahistidine
target sequence.
Thus, the structures of RZ and RZ' should permit the two pendant transition-
metal chelates to
be separated by about 2.5 to 25 A, and preferably by about 5 to 20 A
(distances measured
metal-to-metal). RZ and R2' may be linear or branched, may optionally contain
cyclic groups,
ZO and may optionally be further substituted. RZ and R2' may be the same or
different.
Preferably, R2 and R2' are the same. RZ and R2' may be connected to different
atoms of X
(preferably two atoms on the same edge or face of X). Alternatively, RZ and
RZ' may be
connected to the same atom of X. Alternatively, RZ and RZ' may be connected to
a single
atom, which in turn is connected, directly or through a linker of maximal
length 4 t~, to X.
X in Formula (I) is a detectable group. "Detectable group" as used herein
refers to
any chemical moiety that can be detected. Examples of detectable groups
include fluorescent
moieties, phosphorescent moieties, luminescent moieties, absorbent moieties,
photosensitizers, spin labels, radioisotopes, isotopes detectable by nuclear
magnetic
resonance, paramagnetic atoms, heavy atoms, haptens, crosslinking agents,
cleavage agents,
and combinations thereof.
11
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
In one embodiment, X is detected by monitoring a signal. Some signals which
may
be monitored due to the presence of a detectable group include, for example,
fluorescence
(fluorescence emission intensity, fluorescence lifetime, fluorescence
polarization,
fluorescence anisotropy, or fluorescence correlation), luminescence,
phosphorescence,
absorbance, singlet-oxygen production, electron spin resonance, radioactivity,
nuclear
magnetic resonance, and X-ray scattering.
In another embodiment, X is detected by receptor-binding, protein-protein or
protein-
nucleic acid crosslinking, or protein or nucleic acid cleavage.
Preferred detectable groups include fluorescent moieties. In one preferred
embodiment, cyanine fluorescent moieties are used. These include, but are not
limited to:
Cy3: 1-R-2-[3-[1-R-1,3-dihydro-3,3-dimethyl-5-sulfo-2H-indol-2-ylidene]-1-
propeny 1]-3,3-
dimethyl-5-sulfo-3H-indolium, CyS: 1-R-2-[5-[1-R-1,3-dihydro-3,3-dimethyl-5-
sulfo-2H-
indol-2-ylidene]-1,3-penta dienyl]-3,3-dimethyl-5-sulfo-3H-indolium, Cy7: 1-R-
2-[7-[1-R-
1,3-dihydro-3,3-dimethyl-5-sulfo-2H-indol-2-ylidene]-1,3,5-heptatrienyl]-3,3-
dimethyl-5-
sulfo-3H-indolium, indocyanine green and IRDye (1-R-2-[2-[2-R'-3-[(1-R-1,3-
dihydro-3,3-
dimethyl-5-sulfo-2H-indol-2-ylidene) ethylidene]-1-cyclohexen-1-yl]ethenyl]-
3,3-dimethyl-
5-sulfo-3H-indolium), and mono- and non-sulfonated derivatives thereof. In
another
?0 preferred embodiment, squaraine fluorescent moieties are used. In another
preferred
embodiment, xanthene, xanthanone,
and phenoxazine fluorescent moieties are used.
Examples of cyanine, squaraine, xanthene, xanthanone, and phenoxazine
detectable
?5 groups fluorescent moieties are described, inter alia, in Southwick et al.,
1990, Cytometry
11:418-430; Mujumdar et al., 1993, Bioconjugate Chemistry 4:105-111; Waggoner
and
Ernst, Fluorescent Regents for Flow Cytometry, Part 1: Principles of Clinical
Flow
Cytometry (1993) and Haugland, Molecular Probes Handbook of Fluorescent Probes
and
Research Chemicals, Molecular Inc. 6'h edition (1996) and Berling and Reiser,
Methoden der
30 Organischer Chemie, p 231-299 (1972), Oswald et al., Analytical
Biochemistry 280: 272-277
(2000), Oswald et al. Photochemistry and Photobiology 74(2): 237-245 (2001),
Oswald et al.
12
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
Bioconjugate Chemistry 10: 925-931 (1999), U.S. Patent No: 6,086,737. The
structures in
these publications are all incorporated herein by reference.
In a preferred embodiment, X may be selected from the following cyanine
detectable
groups:
3
R ~ ~ R3,
U V
N \ \ ~N+
n~
(II)
R3
R3,
/ U\ \ V
n~
(III)
13
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
R3,
R3
- \
(IV)
R3 R3.
U
N \ ~ ~N+
n~
(V)
R3
R3,
~I
N \ ~ ~N+
(VI)
14
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
wherein U and V are each independently C(R4)Z, NH, O, S, or (CH)Z; R3 and R3'
are
each independently H or sulfonate; R4 is H, CH3, CHZCH3, or (CH2)ZCH3; and n
is 0 or an
integer of from 1 to 6.
In another preferred embodiment, X may be selected from the following
squaraine
detectable groups:
R5
R3 ~ I ~ R3,
U O V
N \ \ ~N+
Jn~~ Jn l
(VII)
R3,
R3
R5
I
U O V
N \ \ ~N+
n. n'
(VIII)
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
R3,
R3
Rs
I
\
N N+
(IX)
R3 Rs R3,
\
N+
..
(X)
16
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
R3,
3 '
R
N N+
.. U ..
(XI)
R3 R3,
Rs
I
U O V
N \ \ ~N+
n, O n.
(XII)
17
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
Rs R3'
R3
U O V
I~
....
(XIII)
R3'
R3 Rs
N N+
(XIV)
18
N N
N N
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
R3 R3,
Rs
I
N N+
(XV)
wherein U and V are each independently C(R4)Z, NH, O, S, or (CH)2; R3 and R3~
are
each independently H or sulfonate; R4 is H, CH3, CHZCH3, or (CHZ)2CH3; RS is
absent or is
selected from the group consisting of H, an alkyl group, and an aryl group;
and n' is 0 or an
integer of from 1 to 3.
In another preferred embodiment, X may be selected from the following
xanthene,
xanthanone, and phenoxazine detectable groups:
R6,
OH
(XVI)
19
N N
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
R6,
OH
(XVII)
O
R6,
H OH
(XVIII)
R6,
OH
(XIX)
6 6'
R / ~N / R
O ~ O ~ OH
(XX)
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
R~ R6,
OH
(XXI)
wherein R6, RG', R6", R6"', R6~~", and R6"'~~ are each independently hydrogen,
halogen,
hydroxyl, or alkoxyl; and R7, when present, is hydrogen, carboxyl, carboxylate
or sulfonate.
One preferred molecule of the present invention includes two pendant
transition-metal
chelates and a cyanine detectable group according to the following general
structural formula:
R3
R3,
U V
N \ \ ~N+
R2. n IR2,
R1 R1,
I
N N
O OYO O O Oy0 p
(XXII)
wherein Y, Y', R', R'~, R2, and RZ' are as defined previously; wherein U and V
are each
independently C(R4)2, NH, O, S, or (CH)2; R3 and R3' are each independently H
or sulfonate;
R4 is H, CH3, CHZCH3, or (CH2)ZCH3; and n is 0 or an integer of from 1 to 6.
Particularly preferred embodiments include the aforementioned structure where
n is 1,
2 or 3. In an even more preferred embodiment, n is 1, 2, or 3; and R2 and RZ'
are identical
21
n 6" wT D 6",
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
and are about 3.0 to 15 A in length. In an especially preferred embodiment, n
is l, 2, or 3; R2
and R2' are identical and about 3.0 to 15 ~ in length; and Y and Y' are each
Niz+.
One preferred molecule of the present invention includes two pendant
transition-metal
chelates and a cyanine detectable group according to the following general
structural formula:
R3
R3,
U V
N+
O
NH HN
O O
1~
O~ N ~i
N ~ i
O- \Y~ ~y~ -O.
O O
(XXIII)
wherein Y and Y' are as defined previously; U and V are each independently
C(R4)Z, NH, O,
S, or (CH)Z; R3 and R3' are each independently H or sulfonate; R4 is H, CH3,
CHZCH3, or
(CHZ)zCH3; and n is 0 or an integer of from 1 to 6. In a particularly
preferred embodiment, n
is 1, 2, or 3; and Y and Y' are each Ni2+.
Furthermore, provided herein is a molecule with two pendant transition-metal
chelates
and a detectable group according to the following general structural formula:
22
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
O O
NH HN
O O
~O O
O
N N i
O- \Y~ ~ ~ ~y~ ~O_
O
O O
(XXIV)
wherein Y and Y' are as defined previously; R3 and R3~ are each independently
H or sulfonate;
and n is 1, 2, 3, or 4. In a particularly preferred embodiment, n is 1, 2, or
3; and Y and Y' are
each Ni2+
There are no particular limitations to the detectable groups of the compounds
of the
present invention, so long as the ability of the bis-transition-metal-chelate
moieties to bind to
a target sequence is maintained. The points) of attachment between the bis-
transition-metal-
chelate moieties and the detectable group may vary.
Modifying groups that aid in the use of the bis-transition-metal-chelate
derivative may
also be incorporated. For example, the bis-transition-metal-chelate derivative
may be
substituted at one or more positions to add a solid-phase binding group or a
crosslinking
group.
For applications involving labeling of target materials within cells, the bis-
transition-
metal-chelate derivative preferably is capable of traversing a biological
membrane. Smaller
molecules are generally able to traverse a biological membrane better than
larger derivatives.
23
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
Bis-transition-metal-chelate derivatives of less than 2000 Daltons are
preferable for
membrane traversal.
The polarity of the bis-transition-metal-chelate derivative can also determine
the
ability of the bis-transition-metal-chelate derivative to traverse a
biological membrane.
Generally, a hydrophobic bis-transition-metal-chelate derivative is more
likely to traverse a
biological membrane. The presence of polar groups can reduce the likelihood of
a molecule
to traverse a biological membrane. A bis-transition-metal-chelate derivative
that is unable to
traverse a biological membrane may be further derivatized by addition of
groups that enable
or enhance the ability of the molecule to traverse a biological membrane.
Preferably, such
derivatization does not significantly alter the ability of the bis-transition-
metal-chelate
derivative to react subsequently with a target sequence. The bis-transition-
metal-chelate
derivative may also be derivatized transiently. In such instances, after
traversing the
membrane, the derivatizing group is eliminated to regenerate the original bis-
transition-
metal-chelate derivative. Examples of derivatization methods that increase
membrane
traversability include ether formation with acyloxyalkyl groups. For example,
an
acetoxymethyl ether is readily cleaved by endogenous mammalian intracellular
esterases.
Jansen, A. and Russell, T.J., J. Chem. Soc., 2127-2132 (1965). Also, pivaloyl
ester is useful
in this regard. Madhu et al., J. Occul. Pharmaco. Ther., 14:389-399 (1998).
Methods of Synthesis of Compositions of the Invention
The invention provides methods of synthesis of compounds of the present
invention
which include coupling of: (a) a synthon which includes a bis-activated-ester
derivative of a
detectable group; and (b) a synthon which includes an amine or hydrazide
derivative of a
chelator; and then adding a transition metal.
The invention also provides methods of synthesis of non-sulfonated cyanine or
squaraine compounds of the present invention which include coupling of: (a) a
synthon
selected from mono-chelator-functionalized 2,3,3-trimethylindole, mono-
chelator-
functionalized 2,3,3-trimethylbenzindole, mono-chelator-functionalized 2-
methyl-pyridine,
mono-chelator-functionalized 2-methyl-benzothiazole, mono-chelator-
functionalized 2-
methyl-napthothiazole, mono-chelator-functionalized 2-methyl-benzoxazole, and
mono-
24
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
chelator-functionalized 2-methyl-napthoxazole; (b) a synthon, identical or
nonidentical to the
synthon in (a), selected from the group in (a); and (c) a synthon containing
at least one carbon
atom; and then adding a transition metal.
The invention also provides methods of synthesis of disulfonated cyanine or
squaraine
compounds of the present invention which include coupling of: (a) a synthon
selected from
mono-chelator-functionalized 2,3,3-trimethyl-5-sulfanato-indole, mono-chelator-
functionalized 2,3,3-trimethyl-6-sulfanato-benzindole, mono-chelator-
functionalized 2-
methyl-5-sulfanato-pyridine, mono-chelator-functionalized 2-methyl-5-sulfanato-
benzothiazole, mono-chelator-functionalized 2-methyl-6-sulfanato-
napthothiazole, mono-
chelator-functionalized 2-methyl-5-sulfanato-benzoxazole, and mono-chelator-
functionalized 2-methyl-6-sulfanato-napthoxazole; (b) a synthon, identical or
nonidentical to
the synthon in (a), selected from the group in (a); and (c) a synthon
containing at least one
carbon atom; and then adding a transition metal.
The invention also provides methods of synthesis of monosulfonated cyanine or
squaraine compounds of the present invention which include coupling of: (a) a
synthon
selected from mono-chelator-functionalized 2,3,3-trimethylindole, mono-
chelator-
functionalized 2,3,3-trimethylbenzindole, mono-chelator-functionalized 2-
methyl-pyridine,
mono-chelator-functionalized 2-methyl-benzothiazole, mono-chelator-
functionalized 2-
methyl-napthothiazole, mono-chelator-functionalized 2-methyl-benzoxazole, and
mono-
chelator-functionalized 2-methyl-napthoxazole; (b) a synthon selected from
mono-chelator-
functionalized 2,3,3-trimethyl-5-sulfanato-indole, mono-chelator-
functionalized 2,3,3-
trimethyl-6-sulfanato-benzindole, mono-chelator-functionalized 2-methyl-5-
sulfanato-
pyridine, mono-chelator-functionalized 2-methyl-6-sulfanato-benzothiazole,
mono-chelator-
functionalized 2-methyl-6-sulfanato-napthothiazole, mono-chelator-
functionalized 2-methyl-
5-sulfanato-benzoxazole, and mono-chelator-functionalized 2-methyl-6-sulfanato-
napthoxazole; and (c) a synthon containing at least one carbon atom; and then
adding a
transition metal.
Coupling of the synthons referred to herein can be accomplished in a single
step, or in
two steps. For example, for symmetric compounds (i.e., where (a) and (b) are
identical),
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
coupling of the reactants (a), (b), and (c) desirably is carned out in a
single step. For
asymmetric compounds (i.e., where (a) and (b) are non-identical), coupling of
the reactants
(a), (b), and (c) desirably is carned out in two steps: i.e., reaction of (a)
with (c), followed by
reaction of the resultant product with (c); or, alternatively, reaction of (b)
with (c), followed
by reaction of the resultant product with (a).
Coupling of the synthons referred to herein can be performed in solution, or
with one
or more synthons attached to a solid support.
Coupling of the synthons referred to herein can be performed with the chelator
in an
unprotected form, or with the chelator in a protected form initially and
deprotected thereafter.
The invention also provides methods of synthesis of xanthene, xanthanone, or
phenoxazine compounds of the present invention which include reaction of a
xanthene,
xanthanone, or phenoxazine detectable group, a secondary-amine derivative of a
chelator, and
formaldehyde, according to the Mannich reaction (Mannich, C. et al. Arch.
Pharm. 250:647,
1912); followed by addition of a transition metal.
The Mannich reaction referred to herein can be performed with the chelator in
an
unprotected form, or with the chelator in a protected form initially and
deprotected thereafter.
Target Materials and Target Seguences of the Invention
The invention provides detectable complexes of molecules according to Formula
(I)
with target sequences. Detectable complexes as used herein refer to the
association between
target amino acid sequences and bis-transition-metal-chelate derivatives
according to the
invention.
Suitable target materials include, but are not limited to, polypeptides, and
polypeptide
mimetics (such as peptide nucleic acid). Preferably, the target material is a
polypeptide.
As used herein, "polypeptide" refers to both short chains, commonly referred
to as
"peptides, "oligopeptides," or "oligomers," and to longer chains, generally
referred to as
26
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
"proteins." Polypeptides may contain amino acids other than the 20 gene-
encoded amino
acids. Polypeptides may include amino acid sequences modified either by
natural processes,
such as post-translational processing, or by chemical modification techniques
which are well-
known in the art. Such modifications are well described in basic texts and in
more detailed
monographs, as well as in research literature. Thus "polypeptide" includes
peptides,
oligopeptides, polypeptides and proteins, all of which terms are used
interchangeably herein.
The target material contains, or is modified to contain, at least one copy of
an
oligohistidine target sequence, herein referred to interchangeably as the
"target sequence" or
"tag." The target sequence is generally of the form: (H);, wherein H is
histidine and i is an
integer of from 4 to 12 (i.e., SEQ B7 NOS. 1-9), preferably 4 to 8, and most
preferably 6.
The target sequence may be incorporated at any desired site, or set of sites,
within a
target material, but preferably is incorporated at a site that is (a)
accessible and (b) not
essential for structure and function of the target material.
For example, when the target material is a protein, the target sequence
preferably is
incorporated at the N-terminal region, at the C-terminal region, at an
internal loop region, at a
surface-exposed non-essential loop, at an internal linker region, or at
combinations thereof.
The specific site, or set of sites, can be chosen to accommodate the
functional requirements
of a protein. For example, it is known that N-terminal modification of
chemokines can affect
their activity; therefore, in applications with chemokines, either C-terminal
modification or
internal modification would be preferable. Since labeling is performed at
defined, user-
selected sites, effects on the activity of target material can be avoided.
When it is important
to preserve the activity of the tagged target material, specific activity
testing of the tagged vs.
the untagged tareget material may be conducted to verify activity. See, for
example, Mas
et al,. Science, 233:788-790 (1986).
Target-sequence-containing polypeptides may be generated by total synthesis,
partial
synthesis, in vitro translation, or in vivo bacterial, archaeal, or eukaryotic
production.
27
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
In one preferred embodiment, the target sequences and/or target-sequence-
containing
polypeptides used in the invention are prepared using solid-phase synthesis
(see, e.g.,
Merrifield et al. J. Am. Chem. Soc., 85:2149, (1962) Steward and Young, Solid
Phase
Pe tides Synthesis, Freeman, San Francisco, (1969), and Chan and White, Fmoc
Solid Phase
Peptide Synthesis - A Practical Approach, Oxford Press (2000)).
In another preferred embodiment, the target sequences and/or target-sequence-
containing polypeptides used in the invention are prepared using native
chemical ligation
(Dawson et al., Science, 266, 1994).
In an especially preferred embodiment, the target sequences and/or target-
sequence-
containing polypeptides are generated by in vivo bacterial, archaeal, or
eukaryotic expression
of a recombinant nucleic acid sequence encoding the target-sequence-containing
polypeptide.
Methods for the construction of recombinant nucleic acid sequences encoding a
tag-
containing polypeptide are well known in the art (Sambrook and Russel,
Molecular Cloning
A Laboratory Manual, 3rd Ed., Cold Spring Harbor Laboratory, New York (2001),
the
entirety of which is herein incorporated by reference. In addition, techniques
for transient or
stable introduction of recombinant nucleic acid sequences into cells (see, for
example,
Ausubel et al., Current Protocols In Molecular Biolo~y, John Wiley & Sons,
Inc. (1995)), for
replacement of native nucleic acid sequences by recombinant nucleic acid
sequences in cells
(see, for example, Ausubel et al., Current Protocols In Molecular Biolo~y,
John Wiley &
Sons, Inc. (1995)), and for expression of recombinant nucleic acid sequences
in cells (see
e.g., Lee and Arthans, H.J. Biol. Chem., 263:3521, (1988); Rosenberg, et al.,
Gene, 56:125
(1987)), are well known in the art.
The bis-transition-metal-chelate moieties of the molecules according to
Formula (I)
bind to the oligohistidine target sequence. The transition metals of the bis-
transition-metal-
chelate moieties bind to imidazole groups of histidines of the oligohistidine
target sequence.
Although not intending to be limited to such interpretation, it is believed
that the
affinity of the bis-transition-metal-chelate probe for oligohistidine target
sequences relates to
the presence of two tridentate (where R' or R'~ is absent) or tetradentate
(where R' or R'~ is
28
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
CH(COO-) or CH(COOH)) transition-metal chelates, each having a transition
metal with at
least two coordination sites available for interaction with electron-donor
groups.
Oligohistidine target sequences comprising 4 to 12 histidine residues have
appropriate
electron-donor functionality, size, and flexibility to interact with available
coordination sites
of the bis-transition-metal-chelate probe, creating a stable linkage
therewith.
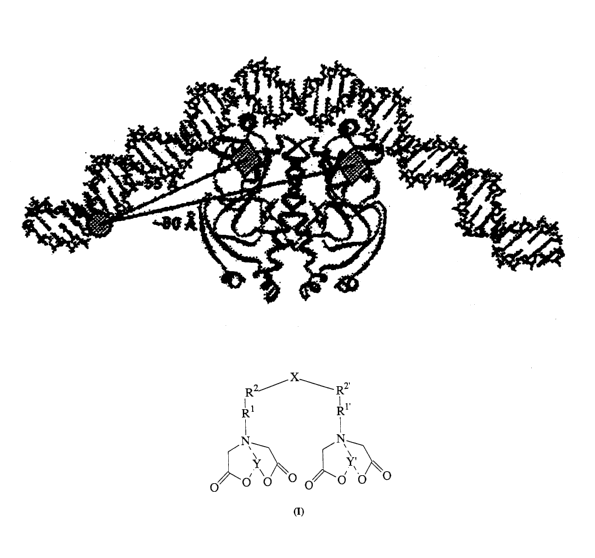
An example of a transition-metal-chelate probe of the invention in association
with a
oligohistidine target sequence, in this case a hexahistidine target sequence,
is depicted as
follows:
~3$ $~7
/ ~ ~ \
N ~ N+
n
O O
NH HN
O O
O O
O O N~/
i
N
w ~ ~O.
~o% v
. o ,
N
N \N N \N N
NI1 ' NH NH \ NH NH
R- N N N N N N R°
H H H H H H
O O O O O O
Labeling is accomplished by contacting a bis-transition-metal-chelate molecule
according to Formula (I) with a target-sequence-containing target material.
The bis-
transition-metal-chelate molecule may be contacted with a target-sequence-
containing target
material located in, for example, a test tube, a microtiter-plate well, a
cuvette, a flow cell, or a
capillary, or immobilized on, for example a surface or other solid support.
Alternatively, the
29
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
bis-transition-metal-chelate molecule may be contacted with a target-sequence-
containing
target material located within a cell, tissue, organ, or organism (in which
embodiment, the
bis-transition-metal-chelate derivative preferably is capable of traversing an
intact biological
membrane).
In one embodiment, the bis-transition-metal-chelate molecules according to
Formula
(I) are used to label target-sequence-containing molecules within cells. The
bis-transition-
metal-chelate molecules of this invention may be introduced into cells by
diffusion (for bis-
transition-metal-chelate derivatives capable of traversing biological
membranes) or by
microinjection, electroporation, or vesicle fusion (for any bis-transition-
metal-chelate
derivative). The target-sequence-containing molecules may be introduced into
cells by
microinjection, electroporation, or vesicle fusion, or by expression of
recombinant genes in
situ.
In one preferred embodiment, a target-sequence-containing protein produced by
expression of a recombinant gene within cells is contacted with a probe of
this invention by
incubating cells in medium containing the probe. Following labeling, and
optionally
following further manipulations, cells are imaged using an epi-illumination,
confocal, or
total-internal-reflection optical microscope with an optical detector, such as
a CCD camera,
an intensified CCD camera, a photodiode, or a photomultiplier tube, and
fluorescence signals
are analyzed.
Uses of the Compositions of the Invention
It is contemplated that bis-transition-metal-chelate molecules of the
invention may be
used in a variety of in vitro and in vivo applications.
The bis-transition-metal-chelate molecules of the invention may be used in
numerous
standard assay formats, as are well known in the art. Some examples of assay
formats
include fluorescence emission intensity, fluorescence polarization (FP),
fluorescence
anisotropy (FA), fluorescence resonance energy transfer (FRET), fluorescence
correlation
spectroscopy (FCS), fluorescence-activated cell--or particle--sorting (FACS),
x/y-fluorescence scanning (FluorImaging), epi-illumination optical microscopy,
confocal
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
optical microscopy, total-internal-reflection optical microscopy, absorbance
spectroscopy,
enzyme-linked immunosorbent assay (ELISA), radioimmunoassay (RIA),
scintillation
proximity assay (SPA), autoradiography, and assays formats that involve use of
biotin or
other hapten incorporation to provide a recognition event for binding or
immobilization of
one or more components.
Some examples, which are intended to be illustrative and not limiting of
possible
assay formats and applications that could use site specific bis-transition-
metal-chelate-labeled
target materials, are set forth below.
For example, the bis-transition-metal-chelate derivatives of the present
invention may
be used to detect and/or quantify a polypeptide of interest containing, or
derivatized to
contain, a target sequence. The target-sequence-containing polypeptide is
incubated with a
molecule according to Formula (I) for a time period sufficient to allow
labeling thereof.
Labeled target-sequence-containing polypeptide optionally may be separated
from unbound
material before the detection step using any method known in the art, and the
detectable
group X is detected, thereby detecting the polypeptide of interest. The target-
sequence-
containing polypeptide may be included in any material, including, but not
limited to,
cuvettes, microtiter plates, capillaries, flow cells, test tubes, gels, blots,
and biological
samples.
The invention also provides an assay method for monitoring a binding process.
In
this method, a first component of a specific reaction pair is labeled with a
molecule according
to Formula (I) and is reacted with a second component of the pair. The
reaction can be
monitored by monitoring a change in a signal of the detectable group X.
Examples of specific reaction pairs include, but are not restricted to,
antibodies/antigens, hormone/receptor, enzyme/substrate, and protein/analyte.
In a fluorescence-emission-intensity assay, the sample is exposed to light of
a first
wavelength (able to be absorbed by a fluorescent moiety), and fluorescence-
emission
intensity is monitored at a second wavelength (emitted by said fluorescent
moiety).
31
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
Fluorescence-emission intensity is dependent on the quantity of the
fluorescent moiety and on
the local environment of the fluorescent moiety.
A fluorescence-emission-intensity assay to detect and quantify binding between
two
molecules, molecule 1 and molecule 2, may be configured as follows: A reaction
mixture is
prepared by combining molecule 1 labeled with fluorescent moiety X according
to the current
invention and molecule 2. Complex formation results, directly or indirectly,
from a change in
the local environment of X, and, correspondingly, in a change in the
fluorescence emission
intensity of X. The progress of the reaction is monitored by observing the
change in
fluorescence emission intensity of X. Equilibrium association and dissociation
constants may
be extracted from the concentration-dependence of the reaction.
In a fluorescence-polarization (FP) or fluorescence-anisotropy (FA) assay, a
sample is
exposed to polarized light of a first wavelength (able to be absorbed by a
fluorescent moiety),
and fluorescence-emission polarization or anisotropy is monitored at a second
wavelength
(emitted by said fluorescent moiety). Fluorescence-emission polarization or
anisotropy is
inversely related to the rotational dynamics, and thus to the size, of said
fluorescent moiety
(or, if said fluorescent moiety is attached to a molecule or complex, to the
rotational
dynamics, and thus to the size, of the molecule or complex). FP or FA assays
permit
detection of reactions that result in changes in size of molecules or
complexes, including
especially, macromolecule-association and macromolecule-dissociation
reactions.
An FP or FA assay to detect and quantify binding between two molecules,
molecule 1
and molecule 2, may be configured as follows: A reaction mixture is prepared
by combining
ZS molecule 1 labeled with fluorochrome X according to the current invention
and molecule 2.
Complex formation results in formation of a higher-molecular-weight, higher-
FP, higher-FA
species. The progress of the reaction is monitored by observing the decrease
in FP or FA.
Equilibrium association and dissociation constants are extracted from the
concentration-
dependence of the reaction.
A further FP or FA assay may be used to detect and quantify proteolytic
activity and
may be configured as follows: A reaction mixture is prepared by combining a
substrate
32
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
molecule labeled with fluorochrome X according to the present invention and a
sample
containing a proteolytic enzyme. Cleavage of the substrate molecule by the
proteolytic
enzyme results in the production of lower-molecular-weight, lower-FP, lower-FA
fragments.
The progress of the reaction is monitored by observing the decrease in FP or
FA.
Fluorescence resonance energy transfer (FRET) is a physical phenomenon that
permits measurement of distance). FRET occurs in a system having a fluorescent
probe
serving as a donor and a second fluorescent probe serving as an acceptor,
where the emission
spectrum of the donor overlaps the excitation spectrum of the acceptor. In
such a system,
upon excitation of the donor with light of the donor excitation wavelength,
energy can be
transferred from the donor to the acceptor, resulting in excitation of the
acceptor and
emission at the acceptor emission wavelength. FRET readily can be detected--
and the
efficiency of FRET readily can be quantified--by exciting with light of the
donor excitation
wavelength and monitoring emission of the donor, emission of the acceptor, or
both. The
efficiency of energy transfer, E, is a function of the Forster parameter, R~,
and of the distance
between the donor and the acceptor, R:
E = [1 + (R/R~)6]n
0
wherein the Forster parameter (in angstroms, A), is:
Ro (in ~) _ (0.211 X lO~s)(n-4QDK2.~1/6
wherein n is the refractive index of the medium, QD is the donor quantum yield
in the
absence of the acceptor, Kz is the orientation factor relating the donor
acceptor transition
dipoles, and J is the spectral overlap integral of the donor emission spectrum
and the acceptor
excitation spectrum.
If one performs FRET experiments under conditions where Ro is constant,
measured
changes in E permit detection of changes in R, and, if one performs
experiments under
conditions where Ro is constant and known, the measured absolute magnitude of
E permits
determination of the absolute magnitude of R.
33
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
With fluorochromes and chromophores known in the art, FRET is useful over
distances of about 1 nm to about 15 nm, which are comparable to the dimensions
of
biological macromolecules and macromolecule complexes. Thus, FRET is a useful
technique
for investigating a variety of biological phenomena~that produce changes in
molecular
proximity. When FRET is used as a detection mechanism, colocalization of
proteins and
other molecules can be imaged with spatial resolution beyond the limits of
conventional
optical microscopy.
A FRET assay to detect and quantify binding between two molecules, molecule 1
and
molecule 2, may be configured as follows: A reaction mixture is prepared by
combining
molecule 1 labeled with a molecule according to Formula (I) where detectable
group X is a
fluorescent moiety and molecule 2 is labeled with a fluorescent moiety Y or a
chrompohore
Y, wherein X and Y are able to participate in FRET. Complex formation results
in increased
proximity between X and Y, and, correspondingly, in increased FRET. The
progress of the
reaction is monitored by observing the increase in FRET. Equilibrium
association and
dissociation constants may be extracted from the concentration-dependence of
the reaction.
A FRET assay to detect and quantify proteolytic activity may be configured as
follows: A reaction mixture is prepared by combining a) a substrate molecule
labeled at site
1 with Formula (I) wherein detectable group X is a fluorescent moiety and
labeled at site 2
with fluorochrome Y, wherein sites 1 and 2 are on opposite sides of the
proteolytic-cleavage
site, and wherein X and Y are able to participate in FRET, and b) a sample
containing a
proteolytic enzyme. Cleavage of the substrate molecule by the proteolytic
enzyme results in
decreased proximity between X and Y and, correspondingly, in decreased FRET.
The
progress of the reaction is monitored by observing the decrease in FRET.
A FRET assay to detect conformation change within molecule 1 induced upon
interaction with molecule 2, may be configured as follows: A reaction mixture
is prepared by
combining (a) molecule 1 labeled at one site with fluorochrome X according to
the current
invention and labeled at another site with fluorochrome Y, wherein X and Y are
able to
participate in FRET, and (b) molecule 2. Conformation change within molecule 1
induced
34
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
upon interaction with molecule 2 results in a change in proximity between X
and Y, and,
correspondingly, a change in FRET. The progress of the reaction is monitored
by observing
the change in FRET.
A FRET assay to measure the distance between two sites, 1 and 2, within a
molecule
of interest, may be configured as follows: the molecule of interest is labeled
at site 1 with
fluorochrome X according to the current invention and is labeled at site 2
with fluorochrome
Y, wherein X and Y are able to participate in FRET; fluorescence excitation
and emission
spectra are collected for X and Y; and the distance, R, is calculated as
described supra.
Fluorescence emission intensity, lifetime, polarization, aniosotropy and FRET
are
further described in the following references: Brand, L. and Johnson, M.L.,
Eds.,
Fluorescence Spectroscopy (Methods in Enzymology, Volume 278), Academic Press
(1997),
Cantor, C.R. and Schimmel, P.R., Biophysical Chemistry Part 2, W.H. Freeman
(1980) pp.
433-465. Dewey, T.G., Ed., Biophysical and Biochemical Aspects of Fluorescence
Spectroscopy, Plenum Publishing (1991). Guilbault, G.G., Ed., Practical
Fluorescence,
Second Edition, Marcel Dekker (1990). Lakowicz, J.R., Ed., Topics in
Fluorescence
Spectroscopy: Techniques (Volume l , 1991 ); Principles (Volume 2, 1991 );
Biochemical
Applications (Volume 3, 1992); Probe Design and Chemical Sensing (Volume 4,
1994);
Nonlinear and Two-Photon Induced Fluorescence (Volume 5, 1997); Protein
Fluorescence
(Volume 6, 2000), Plenum Publishing.
Fluorescence imaging using epi-illumination, confocal, or total-internal-
reflection
optical microscopy permits characterization of the quantities, locations, and
interactions of
fluorochrome-labeled target materials within cells. All fluorescence
observables that can be
analyzed in vitro--emission intensity, emission lifetime, fluorescence
correlation, FP/FA, and
FRET--also can be analyzed in cells (See Nakanishi et al. Anal. Chem. 73:2920-
2928 (2001);
Maiti, S. et al. Proc. Natl. Acad. Sci. USA 94: 11753-11757 (1997); Eigen and
Rigler, Proc.
Natl. Acad. Sci. USA 91:5740-5747 (1994) for example of uses of fluorescence
in cells).
The bis-transition-metal-chelate derivatives of this invention may be used to
label
target-sequence-containing molecules within cells. The bis-transition-metal-
chelate
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
derivatives of this invention may be introduced into cells by diffusion (for
bis-transition-
metal-chelate derivatives capable of traversing biological membranes) or by
microinjection,
electroporation, or vesicle fusion (for any bis-transition-metal-chelate
derivative). The target-
sequence-containing molecules may be introduced into cells by microinjection,
electroporation, or vesicle fusion, or by expression of recombinant genes in
situ.
In one embodiment, a target-sequence-containing protein produced by expression
of a
recombinant gene within cells is contacted with a bis-transition-metal-chelate
derivative of
this invention by incubating cells in medium containing the bis-transition-
metal-chelate
derivative. Following labeling, and optionally following further
manipulations, the cells are
imaged using an epi-illumination, confocal, or total-internal-reflection
optical microscope
with an optical detector, such as a CCD camera, an intensified CCD camera, a
photodiode, or
a photomultiplier tube, and fluorescence signals are analyzed.
The fluorescent molecules of the present invention also can be used, in vitro
or in
vivo, in single-molecule fluorescence assays with single-molecule detection,
wherein
fluorescence emission intensity, fluorescence correlation, FPlFA, or FRET is
analyzed from
individual single molecules.
The fluorescent molecules of the present invention also can be used, in vitro
or
in vivo, in fluorescence assays with "multiplex" detection, wherein a
plurality of different
fluorescent molecules are attached to a plurality of different primary
molecules, molecule la,
lb, ...ln, with each primary molecule being specific for a different secondary
component, 2a,
2b, ...2n, in order to monitor a plurality of reactions between primary
molecules and
secondary molecules in a single reaction mixture. According to this method of
use, each of
the primary molecules is separately labeled with a fluorochrome having a
different,
distinguishable excitation and/or emission wavelength. The primary molecules
are then
reacted, as a group, with the secondary molecules, as a group, and
fluorescence is monitored
at each of different, distinguishable excitation and/or emission wavelengths.
The fact that the present invention is compatible with fluorochromes having
different,
distinguishable excitation and emission wavelengths (see, e.g., Table 1 for
excitation maxima
36
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
and emission maxima of derivatives of Cy3, CyS, and Cy7 in Examples), makes
the invention
particularly important for applications involving multiplex detection.
Most or all of the assays above, in vitro or in vivo, can be adapted for high-
throughput
screening, using formats, equipment, and procedures apparent to persons
skilled in the art.
Examples of fluorochromes and chromophores suitable for use in assays above,
in
conjunction with the molecules of the invention, are presented in Haugland R.
P. Handbook
of Fluorescent Probes and Research Chemicals, Molecular Probes, sixth edition
(1996), ISBN
0-9652240-0-7 (Spence, MTZ, ed). Said fluorochromes and chromophores can be
incorporated into polypeptides and other molecules of interest by any suitable
method, many
of which are well known in the art, including, but not limited to, chemical
synthesis,
enzymatic synthesis, ribosomal synthesis, chemical ligation, chemical
modification, and
hapten binding (see Haugland R. P. Handbook of Fluorescent Probes and Research
Chemicals, supra). Alternatively, fusions of autofluorescent proteins, such as
green
fluorescent protein, to a polypeptide of interest can be encoded as nucleic-
acid fusion
constructs, produced in cells, and analyzed in cells or in vitro.
The methods of the invention may be used in many areas of biology and
biological
research including drug screening, diagnostics and academic research.
It further is contemplated that the bis-transition-metal-chelate molecules of
the
invention may be used for immobilization andlor affinity-purification of
target-sequence-
containing molecules.
Immobilization may be accomplished by: (a) covalently attaching a bis-
transition-
metal-chelate derivative to a surface or other solid support (via detectable
group X or via a
linker); (b) contacting the resulting bis-transition-metal-chelate-derivative-
containing surface
or other solid support with a solution containing a target-sequence-containing
target material;
and (c) optionally washing the surface or the solid support to remove unbound
material.
37
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
Affinity purification may be accomplished by: (a) covalently attaching a bis-
transition-metal-chelate derivative to a surface or other solid support, (b)
contacting the
resulting bis-transition-metal-chelate-derivative-containing surface or other
solid support
with a solution containing a target-sequence-containing molecule, (c)
optionally washing the
surface or other solid support to remove unbound material, and (d) eluting the
target-
sequence-containing molecule with a low-molecular-weight monothiol (e.g., (3-
mercaptoethanol) or, preferably, a low-molecular-weight dithiol (e.g.,
dithiothreitol or
ethanedithiol).
The invention also provides a kit including a molecule according to Formula
(I) and a
target material including a target sequence of the form: (H);, wherein H is
histidine and i is
an integer of from 4 to 12 (i.e., SEQ )D NOS. 1-9), preferably 4 to 8, and
most preferably 6.
The invention also provides a kit. The kit includes a molecule according to
Formula
(I) and a reagent the promotes the formation of a complex between the molecule
of Formula
(I) and a target sequence of the invention.
It will be apparent that the present invention has been described herein with
reference
to certain preferred or exemplary embodiments. The preferred or exemplary
embodiments
described herein may be modified, changed, added to, or deviated from without
departing
from the intent, spirit and scope of the present invention, and it is intended
that all such
additions, modifications, amendments and/or deviations be included within the
scope of the
following claims.
38
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
EXAMPLES
FX A MPT .F. 1
Synthesis of (Ni2+-NTA)~-Cy3
A. Swnthesis of (NTA)~-Cy3
N-(5-amino-1-carboxypentyl)iminodiacetic acid (Dojindo; 26 mg, 80 p,mol) was
dissolved in 1.6 ml O.1M sodium carbonate and was added to Cy3 bis-
succinimidyl-ester
("Cy3 Reactive Dye" from Amersham-Pharmacia Biotech). Following reaction for 1
hour
(with vortexing at 15-min intervals) at 25°C in the dark, products were
purified from excess
N-(5-amino-1-carboxypentyl)iminodiacetic acid using a Sep-Pak C18 cartridge
((Millipore;
pre-washed with 10 ml of acetonitrile and 10 ml water; washed with 20 ml
water; eluted with
1 ml 60% methanol), dried, re-dissolved in 200 ~.l methanol, and purified by
preparative TLC
[1000A silica gel (Analtech); NH40H:ethanol:water 55:35:10 v/v/v]. Three bands
were
resolved, corresponding to (NTA)2-Cy3 (rf=0.2), (NTA),-Cy3 mono acid (r,=0.5),
and
(NTA)2-Cy3 bis acid (rt=0.8). (NTA)Z-Cy3 was eluted using 60% methanol, dried,
re-
dissolved in 2 ml water and quantified spectrophotometrically (esso-150,OOOM-
'crri'). The
yield was 64 nmol, 8%. ES-MS: mle 1197.0 (calculated 1197.4).
B. Synthesis of (Niz+-NTA)z-Cy3
NiCl2 (Aldrich; 350 nmol of NiCl2 in 3 p,l of O.O1N HCl) was added to (NTA)2-
Cy3
(70 nmol in 2 ml water), and the solution was brought to pH 7 by addition of
0.8 ml 50 mM
sodium acetate (pH 7), 200 mM NaCI. Following reaction for 30 min. at
25°C in the dark,
the product was purified using a Sep-Pak C18 cartridge ((Millipore; procedure
as above) and
dried. ES-MS: mle 1316.8 (calculated 1315.7). Niz+ content [determined by
performing
analogous reaction with ~3NiC12 (New England Nuclear) and quantifying
reactivity in product
by scintillation counting in Scintiverse II (Fischer)]: 1.4 mol Ni2+ per mol.
Spectroscopic
properties are reported in Table 1.
39
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
o~
NH HN
O O
-o 0
N N
o l '
/ ' ~~O_
_ O--Ni ~ ~ IYi
O
~O O O
XXV
TABLE 1
S ectrosco
is Pro erties
of (Ni2+-NTA)2-C
3 and (Ni2+-NTA)2-C
3a
fluorochrome ~ max exc ~ max em (nm)uantum yield
(nm) (Q)
(Ni'-NTA)Z- 552 565 0.04
C 3
(Ni'-NTA)2- 650 668 0.05
Cy5
a Ni'+-free
analogues
exhibit identical
~, n,ax, eXC
and ~, max,em
and 3.8-fold
higher Q (with
the higher
Q presumably
reflecting
the unavailability
of
nonradiative
decay involving
Ni2+ unoccu
ied d orbitals).
EXAMPLE 2
Synthesis of (Ni2+-NTA)z-CySA. Synthesis of (NTA)?-Cy5
N-(5-amino-1-carboxypentyl)iminodiacetic acid (Dojindo; 40 mg; 125 ~.mol) was
dissolved in 0.8 ml O.1M sodium carbonate and was added to Cy5 bis-
succinimidyl-ester
("Cy5 Reactive Dye" Amersham-Pharmacia Biotech; 800 nmol). Following reaction
for 1 h
(virtexed at 15 minute intervals) at 25°C in the dark, products were
purified from excess N-
(5-amino-1-carboxypentyl)iminodiacetic acid using a Sep-Pak C18 cartridge
((Millipore;
procedure as above), dried, re-dissolved in 200 ~.1 methanol, and purified in
100 ~,m portions
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
by preparative TLC [silica gel, 1000 A (Analtech); NH~OH:ethanol:water in a
55:35:10 v/v/v.
Three bands were resolved, corresponding to (NTA)2-Cy5 (rt=0.2), (NTA)~-Cy5
mono acid
(rf=0.6), and (NTA)2-Cy5 bis acid (rt=0.8). The (NTA)Z-Cy5 was eluted with 60%
methanol,
dried, re-dissolved in 2 ml water and quantified spectrophotometrically (e5so
= 250,OOOM-
lcm-~). Yield: 60 nmol; 7.5%.
B. Synthesis of (Ni2+-NTA)~-Cy5
NiCl2 (Aldrich; 90 nmol in 1 p,l of 0.01 N HCl) was added to (NTA)Z-Cy5 (30
mmol
in 1 ml water), and the solution was bought to pH 7 by addition of 0.5 ml 50
mM sodium
acetate (pH 7), 70 mM NaCI. Following reaction for 30 min. at 25°C in
the dark, the product
was purified using a Sep-Pak C18 cartridge ((Millipore; procedure as above)
and dried. ES-
MS: mle 1341.0 (calculated 1341.7). Spectroscopic properties are reported in
Table 1.
'nc
O
mn
~ N ~~
N ~ i
o- it ~ ~ ~ ;_o_
'~o~0 0 0
(XXVI)
41
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
EXAMPLE 3
Preparation of a C-terminall~hexahistidine tagged derivative of the
transcriptional activator
CAP (CAP-Hiss)
A. Preparation of CAPHis6
Plasmid pAKCRP-Hiss encodes CAP-His6 under the control of bacteriophage T7
gene
promotor. Plasmid AKCRP-His6 was constructed from plasmid pAKCRP (as described
in
Kapanidis, A. et al., J. Mol. Biol. 312:453-468 (2001) by using site-directed
mutagenesis (as
10 described in Kukel, et al., J. Meths. Enzymol., 204:125-138 (1991)) to
insert six His codons
(CAC-CAC-CAC-CAC-CAC-CAC) after codon 209 of the crp gene.
To prepare CAP-His6, a culture of E. coli strain BL21(DE3) (Novagen)
transformed
with pAKCRP-Hiss was shaken at 37°C in 1 L LB (as described in Miller,
J., Experiments in
Molecular Genetics, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
(1972))
containing 200 mg/ml ampicillin until OD600 = 0.5, induced by addition of
isopropyl-thio-(3-
D-galactoside to 1 mM, and shaken an additional 3 h at 37°C. The
culture was harvested by
centrifugation (4,500 x g; 15 min. at 4°C), the cell pellet was re-
suspended in 15 ml buffer A
[20 mM Tris-HCl (pH 7.9), 500 mM NaCI, 5 mM imidazole], cells were lysed by
sonication,
ZO and the lysate was cleared by centrifugation (30,000 x g; 30 min. at
4°C). The sample was
adjusted to 15 ml with buffer A, adsorbed onto 2 ml Ni2+-NTA agarose (Qiagen)
in buffer A,
washed with 12 ml buffer A containing 20 mM imidazole, and eluted with 6 x 1
ml buffer A
containing 200 mM imidazole.
Fractions containing CAP-Hiss were pooled, desalted twice into buffer B [40 mM
Tris-HCl (pH 8), 100 mM NaCI, 1 mM dithiothreitol, 5% glycerol] by gel-
filtration
chromatography on NAP-10 (Amersham-Pharmacia Biotech), quantified
spectrophotometrically (s27g, protomer = 20,000 M-' cm'), and stored in
aliquots at -80°C. Yield
20 mg/L culture. Purity > 99%.
42
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
EXAMPLE 4
Verification of Affinit a~~ecificity of Association of (Ni2+-NTA)2Cy3 and
(Ni2+-
NTA)~CyS with Target Material
Affinity and specificity of association of the probe with target material were
evaluated
using fluorescence anisotropy assays (methods as in Jameson and Dwyer, Methods
Enzymol.,
246:283-300 (1995)). Formation of a complex of the probe with a tagged protein
was
detected as an increase in fluorescence anisotropy, A, arising from the
increase in molecular
size and corresponding decrease in rotational dynamics.
A. Titration of (Ni2+-NTA)~Cy3 and (Ni2+-NTA)~CyS with CAP-His6
Reaction mixtures [200 p,l, in 100 ~.l quartz micro-cuvettes (Starna)]
contained 50 nM
of (Ni2+-NTA)Z-Cy3 or (Ni2+-NTA)2-Cy5 in buffer C [40 mM Tris-HCl (pH 8), 100
mM
NaCI, 1 mM dithiothreitol, 0.5 mM imidazole, 0.2 mM cAMP, 100 ~.g/ml bovine
serum
albumin, and 5% glycerol]. Reaction mixtures were titrated with 0-3 ~,M CAP-
His6 (or CAP)
by successive addition of 0.5-4 ~.l aliquots of 2-4 ~.M CAP-Hiss (or CAP) in
the same buffer.
Fluorescence anisotropy was determined at the start of the titration and 5 min
after each
successive addition in the titration. All solutions were maintained at
25°C.
B. Detection of Fluorescence Anisotropy
Fluorescence measurements were performed using a commercial steady-state
fluorescence instrument (QM-1, PTI) equipped with T-format Glan-Thompson
polarizers
(PTI). Excitation wavelengths were 530 nm for (Ni2+-NTA)2-Cy3 and 630 nm for
(Ni2+-
NTA)Z-CyS; emission wavelengths were 570 nm for Ni2+-NTA)Z-Cy3 and 670 nm for
Ni2+-
NTA)z-CyS. Slit widths were lOmn. Fluorescence emission intensities were
corrected for
background by subtraction of fluorescence emissions intensities for control
reactions
containing identical concentrations of CAP-His6 or CAP but not containing
probe.
Fluorescence anisotropy, A, was calculated using: A = (Ivy-GIvH)/(Ivv + 2GvH)
where
Ivy and IvH are the fluorescent intensities with the excitation polarizer at a
vertical position
and the emission polarizers at vertical and horizontal positions,
respectively, and G is the
grating correction factor. Data were plotted as: (A-A~/Ao) where A is the
fluorescence
43
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
anisotropy in the presence of the indicated concentration of CAP-His6 or CAP,
and Ao is the
fluorescence anisotropy in the absence of CAP-His6 or CAP. Equilibrium
dissociation
constants were calculated using linear regression.
Referring now to FIG. 2, a graphical representation of results of titration of
(Ni2~-NTA)2-Cy3 with His6-CAP is shown (filled circles). Specific interaction
between
(Ni2+-NTA)2-Cy3 and CAP-His6 is evidenced by a large, saturable increase in
fluorescence
anisotropy. High affinity of interaction is evidenced by a low equilibrium
dissociation
constant (KD = 1.0 p,M). Specificity of interaction is evidenced by the
absence of a
significant increase in fluorescence anisotropy in a control titration with
CAP (open circles;
>95% specificity). ).
Referring now to FIG. 3, a graphical representation is shown of titration of
(NTA)Z-
Cy5 with CAP-His6 is shown (filled circles). Specific interaction between
(Ni2+-NTA)2-Cy5
and Hiss-CAP is evidenced by a large, saturable increase in fluorescence
anisotropy. High
affinity of interaction is evidenced by a low equilibrium dissociation
constant (KD = 0.4 pM).
Specificity of interaction is evidenced by the absence of a significant
increase in fluorescence
anisotropy in a control titration with CAP (open circles; (>95% specificity).
ZO
EXAMPLE 5
Verification of Affinit ~,~Specificity, and Stoichiometry of Association of
(Ni2+-NTA)?
and (Ni2+-NTA)aCyS with Target Material Using_FRET
ZS The affinity, specificity, and stoichiometry of interactions between probes
according
to the invention and the His6 tag also were verified using FRET assays. A His6-
tagged
protein-DNA complex, (CAP-His6)-DNAF, was prepared. FRET assays using the
probes
according to the invention then were performed to verify interactions, to
detect a target
material, and to measure an intermolecular distance.
A. Preparation of DNAF
44
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
DNAF, 53 base pair fluorescein-labelled DNA fragment containing the consensus
DNA site for CAP (fluorescein incorporated at position -9 relative to the
consensus DNA site
for CAP) was prepared as described in Ebright, R. et al., J. Mol. Biol.
312:453-468 (2001).
B. FRET Assays--Standard TitrationsReaction mixtures [200 p.l, in 50 p.l
quartz
micro-cuvettes (Starna)] contained 5 nM DNAF and 50 nM CAP-His6 (or CAP) in
buffer C.
Reaction mixtures were titrated with 0-3.2 p.M 2a or 2b by successive addition
of 0.3-1.2 gl
aliquots of 30-300 pM of (Niz+-NTA)z-Cy3 or (Niz+-NTA)z-Cy5 in the same
buffer.
Fluorescence anisotropy was determined at the start of the titration and 5 min
after each
successive addition in the titration. All solutions were maintained at
25°C.
Fluorescent emission intensities, F, were measured using a commercial steady-
state
fluorescence instrument (QM-1, PTI) equipped with T-format Glan-Thompson
polarizers
(PTI) set at 54.7° ("magic angle"). Excitation wavelength was 480 nm;
emission wavelength
range were 500-600 nm (titrations with (Niz+-NTA)z-Cy3) or 500-700 (titrations
with
(Niz+:NTA)z-CyS; excitation slit width was 10 nm; emission slit width was 15
nm.
Fluorescence emission intensities were corrected for background (by
subtraction of
fluorescence emission intensities for control reaction mixtures containing
identical
concentrations of (Niz+-NTA)z-Cy3 or (Niz+-NTA)z-CyS, but not containing CAP-
His6 or
CAP) and for dilution.
Efficiencies of FRET, E, were calculated as: E = 1 -
(Fszo,aso~szoiago°) where Fszo,4ao is
the fluorescence emission intensity of the fluorescein label at the indicated
concentration of
(Niz+-NTA)z-Cy3 or (Niz+-NTA)z-Cy5 and Fszoiaso° is the fluorescence
emission intensity of
the fluorescein label at 0 ~.M of (Niz+-NTA)z-Cy3 or (Niz+-NTA)z-CyS. Data
were plotted as
E vs. titrant concentration, and binding curves and equilibrium dissociation
constants were
calculated using non-linear regression (as described in Gunasekera, A. et al.,
J. Biol. Chem.,
267:14,713-14,720 (1992)).
Referring now to FIG. 5, a graphical representation of results of titration of
the (CAP-
His6)-DNAF complex with (Niz~-NTA)z-Cy3 is shown (filled circles). Specific
interaction
between the (CAP-His6)-DNAFCOmplex and N( iz+-NTA)z-Cy3 is evidenced by a
large,
saturable increase in FRET. High affinity of interaction is evidenced by a low
equilibrium
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
dissociation constant (Kp = 0.9 pM). Specificity of interaction is evidenced
by the absence
of a significant increase in fluorescence anisotropy in a control titration
with the CAP-DNAF
complex (open circles; (>95% specificity).
Refernng now to FIG. 6, a graphical representation of results of titration of
the (CAP-
His6)-DNAF complex with (Ni2~-NTA)Z-Cy5 is shown (filled circles). Specific
interaction
between the (CAP-His6)-DNAF complex and (Ni2+-NTA)2-Cy5 is evidenced by a
large,
saturable increase in FRET. High affinity of interaction is evidenced by a low
equilibrium
dissociation constant (Kp = 0.3 pM). Specificity of interaction is evidenced
by the absence
of a significant increase in fluorescence anisotropy in a control titration
with the CAP-DNAF
complex (open circles; (>95% specificity).
C. FRET Assays--Stoichiometric Titrations
Stoichiometric titrations were performed analogously to standard titrations
(as
described in Example 5B), using reaction mixtures containing 0.6-2.6 p.M (CAP-
His6)-DNAF
[prepared by equilibration of DNAF with excess CAP-His6 for 20 min. at
25°C, followed by
removal of unbound CAP-His6 by filtration through Bio-Rex 70 (Bio-Rad)
(according to
methods described in Kapanidis, A.N., et al., J. Mol. Biol. 312:453-468
(2001)], and titrating
with 0-12 ~.M of (Ni2+-NTA)z-Cy3 or (Ni2+-NTA)2-Cy5 by successive addition of
0.3-1.2 ~.l
aliquots of p,M (Ni2+-NTA)2-Cy3 or (Ni2+-NTA)z-CyS. Fluorescence emission
intensities
were corrected for dilution and background, and values of E were corrected for
non-specific
interactions (by subtraction of values of E for control reaction mixtures
omitting CAP-His6).
Corrected values of E were plotted as E/Esac vs. titrant concentration where
Esat is the E at
saturating titrant concentrations).
Referring now to FIG. 7, a graphical representation of results of
stoichiometric
titration of the (CAP-Hiss)-DNAF complex with (Niz~-NTA)2-Cy3 is shown (filled
circles).
The interaction between with (Ni2~-NTA)2-Cy3 and His6 has a stoichiometry is
1:1, as
evidenced inflection of the titration curve at a ratio of 1 mole (Ni2~-NTA)2-
Cy3 to 1 mole
CAP-His6 protomer.
46
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
Referring now to FIG. 8, a graphical representation of results of
stoichiometric
titration of the (CAP-His6)-DNAF complex with (Ni2~-NTA)2-Cy5 is shown (filled
circles).
The interaction between with (Ni2~-NTA)Z-Cy5 and His6 has a stoichiometry is
1:1, as
evidenced inflection of the titration curve at a ratio of 1 mole (Ni2~-NTA)2-
Cy5 to 1 mole
CAP-His6 protomer.
D. FRET Assays--Distance Determinations
Donor-acceptor distances, R, were determined using the measured efficiencies
of
FRET at saturation, Esa~ (0.45 for titration with (Ni2~-NTA)2-CyS; 0.25 for
titration
(Ni2~-NTA)2-CyS; see FIGS. 5, 6), and the measured Forster parameters, Ro:
E = Ro6/(Ro6 + R~)
Ro (in A) _ (0.2 11 X lO-5)(n-4QDKZJ)1~6
wherein n is the refractive index of the medium (1.4 for dilute protein
solutionsg), QD is the
donor quantum yield in the absence of acceptor [0.4; measured using quinine
sulfate in 0.1 N
N2S04 as standard (QQs = 0.51)], KZ 1S the orientation factor relating the
donor emission
dipole and acceptor dipole [approximated as 2/3 due to the low fluorescent
anisotropy of the
donor], and J is the spectral overlap integral of the donor emission spectrum
and the acceptor
excitation spectrum:
J = [JFD(~)~a,(~)~4d~]/[JFu(~)d~]
wherein FD(7~) is the normalized corrected emission spectrum of donor, EA(~,)
is the molar
extinction coefficient of acceptor, and ~, is the wavelength.
The analysis above yields a donor-acceptor distance of 56(~4) ~. This distance
is in
excellent agreement with the distance of about 55 A expected based on
structural information
as illustrated in FIG. 3 (corresponding to the distance between the
fluorescein on DNA and
the Hisb of the proximal CAP-His6 protomer).
47
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
It will be apparent that the present invention has been described herein with
reference
to certain preferred or exemplary embodiments. The preferred or exemplary
embodiments
described herein may be modified, changed, added to, or deviated from without
departing
from the intent, spirit and scope of the present invention, and it is intended
that all such
additions, modifications, amendments and/or deviations be included within the
scope of the
following claims.
48
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
SEQUENCE LISTING
<110> Ebright, Richard H.
Ebright, Yon W.
<120> Transition Metal Containing Chelete Probes
<130> 744-37P
<150> 60/410,267
<151> 2002-09-13
<150> 60/367,775
<151> 2002-03-28
<160> 9
<170> PatentIn version 3.1
<210> 1
<211> 4
<212> PRT
<213> Artificial Sequence
<220>
<223> Target Sequence
<400> 1
His His His His
1
<210> 2
<211> 5
<212> PRT
<213> Artificial Sequence
<220>
<223> Target Sequence
<400> 2
His His His His His
1 5
<210> 3
<211> 6
<212> PRT
<213> Artificial Sequence
<220>
<223> Target sequence
<400> 3
1
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
His His His His His His
1 5
<210> 4
<211> 7
<212> PRT
<213> Artificial Sequence
<220>
<223> Target sequence
<400> 4
His His His His His His His
1 5
<210> 5
<211> 8
<212> PRT
<213> Artificial Sequence
<220>
<223> Target sequence
<400> 5
His His His His His His His His
1 5
<210> 6
<211> 9
<212> PRT
<213> Artificial Sequence
<220>
<223> Target sequence
<400> 6
His His His His His His His His His
1 5
<210> 7
<211> 10
<212> PRT
<213> Artificial Sequence
<220>
<223> Target Sequence
<400> 7
His His His His His His His His His His
2
CA 02488819 2004-12-07
WO 03/091689 PCT/US02/36180
1 5 10
<210> 8
<211> 11
<212> PRT
<213> Artificial Sequence
<220>
<223> Target sequence
<400> 8
His His His His His His His His His His His
1 5 10
<210> 9
<211> 12
<212> PRT
<213> Artificial Sequence
<220>
<223> Target sequence
<400> 9
His His His His His His His His His His His His
1 5 10
3