Note: Descriptions are shown in the official language in which they were submitted.
CA 02489676 2004-12-16
WO 2004/002543 PCT/US2002/020436
PROSTHETIC DEVICES HAVING DIFFUSION-HARDENED SURFACES AND
BIOCERAMIC COATINGS
BACKGROUND OF THE INVENTION
[0001] Orthopedic implant materials must combine high strength, corrosion
resistance and tissue compatibility. The longevity of the implant is of prime
importance
especially if the recipient of the implant is relatively young because it is
desirable that the
implant function for the complete lifetime of a patient. Because certain metal
alloys have the
required mechanical strength and biocompatibility, they are ideal candidates
for the
fabrication of prostheses. These alloys include 316L stainless steel, chrome-
cobalt-
molybdenum alloys and, more recently, titanium alloys wluch have proven to be
the most
suitable materials for the fabrication of load-bearing prostheses.
[0002] It has also been found that metal prostheses are not completely inert
in the
body. Body fluids act upon the metals causing them to slowly corrode by an
ionizing process
that thereby releases metal ions into the body. Metal ion release from the
prosthesis is also
related to the rate of wear of load bearing surfaces because the passive oxide
film, which is
formed on the surface, is constantly removed. The repassivation process
constantly releases
metal ions during the ionizing process. Furthermore, the presence of third-
body wear
(cement or bone debris) accelerates this process and microfretted metal
particles increase
friction.
[0003] The excellent corrosion resistance of zirconium has been known for many
years. Zirconium displays excellent corrosion resistance in many aqueous and
non-aqueous
media and for this reason has seen an increased use in the chemical process
industry and in
medical applications. A limitation to the wide application of zirconium in
these areas is its
relatively low resistance to abrasion and its tendency to gall. This
relatively low resistance to
abrasion and the tendency to gall is also demonstrated in zirconium alloys.
[0004] U.S. Patent 2,987,352 to Watson first disclosed a method of producing
zirconium bearings with a specific form of oxidized zirconium as a surface
layer. The
specific form of oxidized zirconium .is a blue-blaclc or blue oxidized
zirconium. The method
of Watson was refined by Haygarth (U.S. Patent 4,671,824) resulting in
improved abrasion
resistance and better dimensional control of the oxidized product. U.S. Patent
No. 5,037,438
to Davidson first demonstrated the many advantages that are realized through
the use of the
specific form of oxidized zirconium on zirconium and zirconium alloy
substrates in prosthetic
CA 02489676 2004-12-16
WO 2004/002543 PCT/US2002/020436
devices. Davidson extended this work to include surfaces subject to
nitridation in U.S. Patent
No. 5,180,394. Other U.S. Patents of Davidson (5,152,794; 5,370,694;
5,372,660; 5,496,359;
and 5,549,667) demonstrate the use of this specific form of zirconium oxide or
zirconium
nitride in other prosthetic application. All of the aforementioned patents of
Davidson are
incorporated by reference as though fully disclosed herein. The advantages of
these surfaces
include increased strength, low friction and high wear resistance. U.S. Patent
Nos 5,037,438
and 5,180,394 to Davidson, respectively, disclose a method of producing
zirconium alloy
prostheses with an oxidized zirconium surface and a nitrified zirconium
surface. The work of
Watson and Davidson teach a specific form of oxidized or ivtrided zirconium
which
possesses all of the advantages of ceramic materials while maintaining the
strength of
metallic surfaces. While the present invention is not intended to be limited
by theory, the
oxide or nitride layer are believed to be characterized by the presence of
free oxygen or
nitrogen which diffuses into the interior of the material, near the metallic
substrate. The
resulting "diffusion hardened" surfaces have oxide or nitride layers that
possess properties
that combine the unique advantages of both ceramic and metal surface, while
simultaneously
minimizing the disadvantages of these materials. All of the U.S. Patents cited
above to
Davidson, Watson, and Haygarth are incorporated by reference as though fully
set forth
herein. While the early work of Davidson focused on pure zirconium and alloys
of zirconium
in which zirconium was the predominant metal, later work has shown that this
is not
necessary in order to form the desired diffusion hardened oxide. For instance,
an alloy of 74
wt% titanium, 13 wt% niobium and 13 wt% zirconium ("Ti-13-13") will form the
diffusion
hardened oxidation layer used herein. Ti-13-13 is taught in U.S. Patent
5,169,597 to
Davidson et al. By effectively taking advantage of the unique properties of
such diffusion-
hardened layers on prosthetic devices, the useful service life of the device
is greatly
improved. The improvement was realized by improving the wear resistance of the
contacting
surfaces of an implant (most notably the articulating surfaces), thereby
lengthening the useful
service life of the implant.
[0005] Apart from the issue of wear, another important performance criterion
for
medical implants as it relates to service life is the degree of fixation
stability. The integrity of
the fixation stability of the implant in the implanted tissue is another major
factor in the
service life of the implant. Fixation stability is typically accomplished
through ingrowth of
surrounding tissue into the implant and its ability to become firmly anchored
to other
components such as bone cement with a large shear strength. A typical hip
joint prosthesis
2
CA 02489676 2004-12-16
WO 2004/002543 PCT/US2002/020436
includes a stem fixated into the femur, a femoral head, and an acetabular cup
against which
the femoral head articulates. A typical knee joint prosthesis has a femoral
and tibial
component, both of which are fixated to their respective bones. This fixation
could be to any
tissue, and it is oftentimes assisted through the use of other materials, such
as bone cement,
etc.
[0006] Because of the improvements in wear resistance realized through the use
of diffusion-hardened surfaces, fixation stability remains as a maj or
limiting factor, among
others, in the overall service life of implants. Fixation stability of the
oxidized and nitrified
zirconium prostheses of Davidson was accomplished through the use of porous
metal beads
or wire mesh coatings that promoted bone in-growth. These methods relied
exclusively on an
increase in the surface area for improving bone in-growth and on-growth into
the implant.
These techniques are taught in U.S. Patent 5,037,438 and U.S. Patent 5,180,394
as well as
other patents of Davidson, and when combined with the advantages of oxidized
or nitrified
zirconium, represented an improvement in performance of medical implants in
numerous
areas. Nevertheless, these fixation methods have not kept up with the
breakthrough
advancement in prosthesis service life realized through the use of diffusion-
hardened oxide
surfaces such as oxidized zirconium. Fixation stability remains a weak link in
the chain in
the goal of long service life prosthetic devices. Accordingly, continued
improvement in the
fixation stability of such implants is desirable.
[0007] Recent efforts at improving fixation stability have been directed
toward the
use of textured surfaces. These techniques typically involve the use of
chemical or
electrochemical etching. Examples in the prior art include the U.S. Patents of
Wagner,
(5,922,029; 5,258,098; 5,507,815: and 6,193,762) in which the etching
methodology is used.
Although the techniques of Wagner et al. represent one potential source of
methods for
surface texture modification it is expected that any other surface .texture
modification
techniques would be similarly useful in aiding fixation. For example,
mechanical etching
would also produce an acceptable textured surface. Notably, in copending U.S.
Application
No. 60/338,420, the use of textured surfaces is combined with diffusion-
hardened oxidation
surfaces to produce a prosthetic device having superior articulating surfaces
and improved
fixation stability.
[0008] Bioceramics in general, calcium phosphates, and hydroxyapatite in
particular, have been used to promote bone growth. These chemical species are
similar in
chemical composition to bone and teeth. Much attention has been given in the
art to the
3
CA 02489676 2004-12-16
WO 2004/002543 PCT/US2002/020436
development of apatite materials to assist in the regeneration of bone defects
and injuries.
Very early on, similarities had been observed between the powder X-ray
diffraction pattern of
the ifa ~ivo mineral and hydroxyapatite. Calcium compomids, or calcium slats,
including
calcium sulfate (Nielson, 1944), calcium hydroxide (Peltier, 1957), and
tricalcium phosphate
(Albee et al., 1920), have been observed to stimulate new bone growth when
implanted or
injected into bone cavities (Hulbert et al., 1983). These materials also
exhibit good
biocompatibility and compositional similarities to human bone and tooth and
can serve as
resorbable or non-resorbable implants depending on their degree of
microporosity. They
have also been used as coatings on conventional implant devices (See e.g.,
U.S. Patent Nos.
6,350,126; 6,261,322; 5,279,831; 5,164,187; and 5,039,546). The chemical
similarity
between the calcium-based bioceramics and the material found in natural bone
is believed to
be the mechanism by which these chemical species promote bone growth.
[0009] While all of these advancements have aided in the principle goal of
lengthening of the useful life of prosthetic devices, further improvements are
needed. A
delay or complete prevention of failure of an implant avoids the need for
revision surgery and
is always desirable. There exists a need for a method to produce medical
implants combining
good fixation and low wear. This invention herein relates to metallic
orthopedic implants
having surfaces of a thin, dense, highly wear-resistant coating of diffusion-
hardened
oxidation or nitridation layer in addition to surfaces coated with one or more
bioceramic or
bone growth promoting materials such as one or more apatite compounds. The
oxidation
layer is formed by an iu-situ process characterized by the diffusion of oxygen
or nitrogen into
the surface toward the unoxidized substrate below. The combination of high-
strength, highly
wear-resistant diffusion-hardened prosthetic surfaces with bioceramic-coated
surfaces
produces a prosthetic device with exceptionally long service life. The
combination
synergistically improves the implants' service life by addressing the two
major failure
mechanisms: wear of the articulating surfaces and implant loosening.
SUMMARY OF THE INVENTION
[0010] In one aspect of the present invention, a prosthesis comprises a
femoral
component having an implant portion for inserting into body tissue and a
bearing surface
comprising at least one condyle. The femoral component is formed of zirconium,
hafnium,
niobium, tantalum or alloys of any of those metals. The prosthesis also
comprises a tibial
component having an articulating surface, the articulating surface being
comprised of an
4
CA 02489676 2004-12-16
WO 2004/002543 PCT/US2002/020436
organic polymer or polymer-based composite and is adapted to cooperate with
said bearing
surface. The prosthesis also has a diffusion-hardened oxide or nitride coating
on at least a
part of said bearing surface for reducing wear of the tibial component and has
at least one
bioceramic compound coating at least a part of said implant portion.
[0011] In a specific embodiment, the diffusion-hardened oxide or nitride
coating
is selected from the group consisting of oxidized zirconium, oxidized hafnium,
oxidized
niobium, oxidized tantalum, nitrided zirconium, nitrided hafnium, nitrided
niobium, nitrided
tantalum and combinations thereof.
[0012] In a preferred embodiment, the femoral component is formed of zirconium
or zirconium alloy and the diffusion-hardened oxide coating comprises blue-
black or black
oxidized zirconium.
[0013] In an alternative embodiment, the tibial component further comprises an
attachment portion formed of zirconium, hafnium, niobium, tantalum, or alloys
thereof: The
attachment portion may have a diffusion-hardened oxide or nitride coating, and
that coating
may be oxidized zirconium, oxidized hafnium, oxidized niobium, oxidized
tantalum, nitrided
zirconium, nitrided hafnium, nitrided niobium, nitrided tantalum or
combinations thereof.
[0014] In a preferred embodiment, the attachment portion is comprised of
zirconium or zirconium alloy and the diffusion-hardened oxide coating is made
of blue-black
or black oxidized zirconium.
[0015] In other embodiments, the bioceramic compound may be one or more of
the compounds hydroxyapatite, fluoroapatite, chloroapatite, bromoapatite,
iodoapatite,
calcium sulfate, calcium phosphate, calcium carbonate, calcium tartarate,
bioactive glass, and
combinations thereof. In a preferred embodiment, the compound comprises
hydroxyapatite.
[0016] In another embodiment, a prosthesis comprises a femoral component
having an implant portion for inserting into body tissue, a head portion
comprising a bearing
surface, the femoral component being formed of zirconium, hafnium, niobium,
tantalum or
alloys thereof. The prosthesis also has an acetabulax cup having an inner
surface comprising
an organic polymer or a polymer-based composite and an outer surface, the
inner surface
being adapted to cooperate with said bearing surface The prosthesis also has a
diffusion-
hardened oxide or nitride coating on at least a part of said bearing surface
for reducing wear
of said inner surface and at least one bioceramic compound coating on at least
a part of the
implant portion or the outer surface or both the implant portion and said
outer surface.
CA 02489676 2004-12-16
WO 2004/002543 PCT/US2002/020436
[0017] In a specific embodiment, the prosthesis has a diffusion-hardened oxide
or
nitride coating which is selected from the group consisting of oxidized
zirconium, oxidized
hafnium, oxidized niobium, oxidized tantalum, nitrified zirconium, nitrified
hafiuum, nitrified
niobium, nitrified tantalum, and combinations thereof.
[0018] In a preferred embodiment, the femoral component is formed of zirconium
or zirconium alloy and the diffusion-hardened oxide coating is made up of blue-
black or
black oxidized zirconium.
(0019] In an alternative embodiment, the outer surface formed of zirconium,
hafnium, niobium, tantalum or alloys thereof. The outer surface of the device
may be at least
partly comprised of a diffusion-hardened oxide or nitride coating which may be
oxidized
zirconium, oxidized hafnium, oxidized niobium, oxidized tantalum, nitrified
zirconium,
nitrified hafnium, nitrified niobium, nitrified tantalum, or combinations
thereof.
[0020] In a preferred embodiment, the outer surface is comprised of zirconium
or
zirconium alloy. In a specific embodiment, the diffusion-hardened oxide
coating is made up
of blue-black or black oxidized zirconium.
[0021] In other embodiments, the bioceramic compound may be one or more of
hydroxyapatite, fluoroapatite, chloroapatite, bromoapatite, iodoapatite,
calcium sulfate,
calcium phosphate, calcium carbonate, calcium tartarate, bioactive glass, and
combinations
thereof. In a preferred embodiment, the compound comprises hydroxyapatite.
[0022] In a general embodiment of the invention, a prosthesis comprises a body
having an implant portion for inserting into body tissue, the body being
formed of zirconium,
hafnium, niobium, tantalum or alloys thereof. The prosthesis also has a
bearing surface on
said body, the bearing surface being sized and shaped to engage or cooperate
with a second
bearing surface, the second bearing surface being a part of another prosthesis
portion. The
prosthesis also has a diffusion-hardened oxide or utride coaxing on said
bearing surface of
said body and at least one bioceramic compound coating at least a part of said
body.
[0023] In a specific embodiment, the diffusion-hardened oxide coating is
selected
from the group consisting of oxidized zirconium, oxidized hafiiium, oxidized
niobium,
oxidized tantalum, nitrified zirconium, nitrified hafiiium, nitrified niobium,
nitrified tantalum,
and combinations thereof.
[0024] In a preferred embodiment, the body is formed of zirconium or zirconium
alloy and the diffusion-hardened oxide or nitride coating comprises blue-black
or black
oxidized zirconium.
6
CA 02489676 2004-12-16
WO 2004/002543 PCT/US2002/020436
[0025] In an alternative embodiment, the other prosthesis portion comprises
zirconium, hafiiium, niobium, tantalum, or alloys thereof. In a specific
embodiment, the
other prosthesis portion comprises a diffusion-hardened ox=de or nitride
coating. In yet
another specific embodiment, the diffusion-hardened oxide or nitride coating
of the other
prosthesis portion comprises oxidized zirconium, oxidized hafnium, oxidized
niobium,
oxidized tantalum, nitrided zirconium, nitrided hafiiium, nitrided niobium,
nitrided tantalum,
or combinations thereof.
[0026] In a preferred embodiment, the other prosthesis portion comprises
zirconium or zirconium alloy. It may have a diffusion-hardened oxide coating
made up of
blue-black or black oxidized zirconium.
[0027] In a specific embodiment, the bioceramic compound on the body may be
one or more of hydroxyapatite, fluoroapatite, chloroapatite, bromoapatite,
iodoapatite,
calcium sulfate, calcium phosphate, calcium tartarate, bioactive glass, and
combinations
thereof. In a preferred embodiment, the compound comprises hydroxyapatite.
[0028] In an alternative embodiment, the other prosthesis portion comprises a
coating of at least one bioceramic compound.
[0029] In a specific embodiment, the coating of at least one bioceramic
compomld
on the other prosthesis portion comprises a compound selected from the group
consisting of
hydroxyapatite, fluoroapatite, chloroapatite, bromoapatite, iodoapatite,
calcium sulfate,
calcium phosphate, calcium tartarate, bioactive glass, and combinations
thereof. Preferably,
the compound comprises hydroxyapatite.
[0030] In a preferred embodiment, the second bearing surface comprises an
organic polymer or polymer composite.
[0031] In another embodiment, a prosthesis comprises a body having an implant
portion for inserting into the body tissue of a patient, the body being formed
of zirconium,
hafiuum, niobium, tantalum or alloys thereof. The prosthesis also has a
bearing surface on
the body, a counter bearing surface adapted to cooperate with the bearing
surface, a diffusion-
hardened oxide or nitride coating at least a part of said bearing surface, and
at least one
bioceramic compound coating at least a part of said implant portion.
[0032] In a specific embodiment, the diffusion-hardened oxide or nitride
coating
is selected from the group consisting of oxidized zirconium, oxidized niobium,
oxidized
hafnium, oxidized tantalum, nitrided zirconium, nitrided hafiuum, nitrided
iuobium, nitrided
tantalum, and combinations thereof.
7
CA 02489676 2004-12-16
WO 2004/002543 PCT/US2002/020436
[0033] In a preferred embodiment, the body is formed of zirconium or zirconium
alloy and the diffusion-hardened oxide coating comprises blue-black or black
oxidized
zirconium.
[0034] In an alternative embodiment, the counter bearing surface is comprised
of
a diffusion-hardened oxide or nitride coating. The diffusion-hardened oxide or
nitride
coating on the cotulter bearing surface may be selected from the group
consisting of oxidized
zirconium, oxidized niobium, oxidized hafiiium, oxidized tantalum, nitrided
zirconium,
nitrided hafnium, nitrided niobium, nitrided tantalum, and combinations
thereof.
[0035] In a preferred embodiment, the counter bearing comprises blue-black or
black oxidized zirconium.
[0036] In one embodiment, the at least one bioceramic compound comprises a
compound selected from the group consisting of hydroxyapatite, fluoroapatite,
chloroapatite,
bromoapatite, iodoapatite, calcium sulfate, calcium phosphate, calcium
tartarate, bioactive
glass, and combinations thereof. Preferably, the compound comprises
hydroxyapatite.
[0037] In a preferred embodiment, the counter bearing surface comprises an
organic polymer or polymer composite.
[0038] In another embodiment, the prosthesis further comprises an irregular
surface formed of beads of zirconium, hafnium, niobium, tantalum, or alloys
thereof. In a
specific embodiment, the prosthesis further comprises a diffusion-hardened
surface on the
beads or a coating of at least one apatite compound on the beads or both a
diffusion-hardened
surface and a coating of at least one bioceramic compound on the beads.
[0039] In another embodiment, the prosthesis further comprises an irregular
surface formed of wire mesh of zirconium, hafnium, niobium, tantalum, or
alloys thereof. In
a specific embodiment, the prosthesis further comprises a diffusion-hardened
surface on the
wire mesh or a coating of at least one bioceramic compound on the wire mesh or
both a
diffusion-hardened surface and a coating of at least one bioceramic compound
on the wire
mesh.
[0040] In another embodiment, the prosthesis further comprises a textured
surface
formed of zirconium, hafnium, niobium, tantalum, or alloys thereof. In a
specific
embodiment, the prosthesis further comprises a diffusion-hardened surface on
said textured
surface or a coating of at least one bioceramic compound on said textured
surface or both a
diffusion-hardened surface and a coating of at least one bioceramic compound
on said
textured surface.
8
CA 02489676 2004-12-16
WO 2004/002543 PCT/US2002/020436
[0041] In another embodiment of the present invention, an endoprosthesis is
described. In this embodiment, the prosthesis comprises a prosthesis body
formed of
zirconium, hafnium, niobium, tantalum or alloys thereof, and the prosthesis
body forming one
component of a two-component joint and having a bearing surface at least a
portion of which
is adapted to cooperate with and slide against body tissue of a second joint
component. The
prosthesis also comprises a diffusion-hardened oxide or nitride coating on at
least a part of a
bearing surface adapted to cooperate and slide against the body tissue, said
coating selected
from the group consisting of oxidized zirconium, oxidized hafnium, oxidized
niobium,
oxidized tantalum, nitrified zirconium, nitrified hafnium, nitrified niobium,
nitrified tantalum,
and combinations thereof. The prosthesis of this embodiment also comprises at
least one
bioceramic compound coating on at least a part of said prosthesis body.
[0042] In a specific embodiment, the bearing surface is a femoral head adapted
to
cooperate with and slide against cartilage tissue of a pelvis.
[0043] In another embodiment, the bearing surface is a head of a hmneral
implant
adapted to cooperate with natural body tissue of a glenoid of a recipient.
[0044] In an alternative embodiment, the bearing surface is a bearing surface
of a
glenoid prosthesis adapted to cooperate with natural tissue of a humerus.
[0045] In another embodiment, the bearing surface is a bearing surface of at
least
one condyle of a femoral component of a knee joint prosthesis adapted to
cooperate against
natural tissue of a tibia.
[0046] In an alternative embodiment, the bearing surface is a bearing surface
of a
tibial component of a knee joint prosthesis adapted to cooperate with natural
tissue of
condyles.
[0047] Various other embodiments include those wherein the at least one
bioceramic compound is selected from the group consisting of hydroxyapatite,
fluoroapatite,
chloroapatite, bromoapatite, iodoapatite, calcium sulfate, calcium phosphate,
calcium
tartarate, bioactive glass, and combinations thereof.
[0048] In a preferred embodiment, the prosthesis body is formed of zirconium
or
alloys thereof and the diffusion-hardened oxide coating comprises blue-black
or black
oxidized zirconium.
[0049] W another embodiment of the present invention, there is a prosthesis
comprising a body formed of alloy having a composition comprising from about
10 to about
20 wt % niobium or from about 35 to about 50 wt % niobium; from about 13 to
about 20 wt
9
CA 02489676 2004-12-16
WO 2004/002543 PCT/US2002/020436
zirconium; and the balance titanium; a diffusion-hardened oxide or nitride
coating on at
least a paxt of the body; and at least one bioceramic compound coating at
least a part of the
body.
[0050] In a specific embodiment, the prosthesis has a composition consisting
essentially of about 74 wt % titanium, about 13 wt % niobium, aald about 13 wt
% zirconium.
DESCRIPTION OF THE DRAWINGS
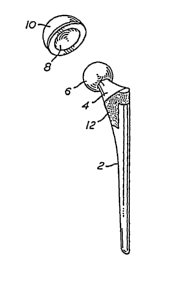
[0051]Figure 1. Typical hip prosthesis
shown ifa vivo.
[0052]Figure 2. Typical hip prosthesis
shown ex vivo.
[0053]Figure 3. Typical knee prosthesis
shown ih vivo.
[0054]Figure 4. Typical knee prosthesis
shown ex vivo.
DETAILED DESCRIPTION OF THE INVENTION
[0055] As used herein, "a" or "an" may mean one or more. As used herein in the
claim(s), when used in conjunction with the word "comprising", the words "a"
or "an" may
mean one or more than one. As used herein, "another" may mean at least a
second or more.
[0056] As used herein, "apatite" means any chemical species of the genus
having
the empirical formula Ca$(PO4)3X where X is any univalent ligand satisfying
the
electroneutrality of the general formula. "Hydroxyapatite" is defined as the
apatite wherein
X = OH, or having the empirical formula Cas(P04)30H.
[0057] [0057] As used herein, "bioceramic" means any ceramic material
including apatites (hydroxyapatite, fluoroapatite, chloroapatite,
bromoapatite, and
iodoapatite), calcium sulfate, calcium phosphate, calcium carbonate, calcium
taxtarate,
bioactive glass, and combinations thereof.
[0058] As used herein, the term, bone growth-promoting material means any
material that promotes growth of bone tissue by any mechanism. These include,
but are not
limited to, the bioceramic materials defined above.
[0059] As used herein, as it refers to interacting surfaces on a prosthetic
device,
the term "cooperate" is defined as any type of interaction, including an
articulating
interaction, a non-articulating interaction, and any and all intermediate
levels of interaction.
[0060] As used herein, "diffusion-hardened surface" is defined as a type of
abrasion resistant surface formed by certain specific in-situ oxidation or
nitridation processes.
The surface is characterized by being oxidized or nitrided relative to the
substrate upon which
CA 02489676 2004-12-16
WO 2004/002543 PCT/US2002/020436
it is situated. It is oxidized or nitrided by an in-situ oxidation or
nitridation process by which
oxygen or nitrogen diffuses from the surface toward the interior substrate
domain. Specific
examples of the oxidation or nitridation processes are provided herein. When
used in
reference to the underlying substrate material, it is synonymous with "surface
hardened".
Also synonymously, the surface oxide or nitride layer is also referred to as
"diffusion-
bonded". An oxidized or nitrided zirconium surface, as those terms are used
herein, are
examples of a diffusion hardened surface; other metals or metal alloys may
also form
diffusion-hardened surfaces by oxidation or nitridation. In all discussions
herein referring to
various applications and embodiments of diffusion-hardened surfaces on
prosthetic devices, it
should be understood that discussions with respect to oxidized surfaces apply
equally to
nitrided surfaces.
[0061] As used herein, "metallic" may be a pure metal or an alloy.
[0062] As used herein, "nitridation" is defined as the chemical process by
which a
substrate material, preferably a metal is combined with nitrogen to form the
corresponding
nitride.
[0063] As used herein, "zirconium alloy" is defined as any metal alloy
containing
zirconium in any amount greater than about 10 % by weight of zirconium. Thus,
an alloy in
which zirconium is a minor constituent at about 10 % by weight or greater is
considered a
"zirconium alloy" herein. Similarly, a "metal alloy" of any other named metal
(e.g., a
hafiiium alloy or a niobium alloy; in these cases, the named metal is hafnium
and niobium,
respectively) is defined as any alloy containing the named metal in any amount
greater than
about 10 % by weight.
[0064] One aspect of the invention is to combine low friction, wear resistant
surfaces with surfaces which promote bone in-growth and on-growth.
Illustrative examples
of such articulating surfaces are shown in the schematic diagrams, FIGS. 1-4.
[0065] A typical hip joint assembly is shown in situ in FIGS. 1 and 2. The hip
joint stem 2 fits into the femur wlule the femoral head 6 of the prosthesis
fits into and
aa~ticulates against the inner lining 8 of an acetabular cup 10 which in turn
is affixed to the
pelvis as shown in FIG. 1. In prior art devices, a porous metal bead or wire
mesh coating 12
is incorporated to allow stabilization of the implant by ingrowth of
surrounding tissue into the
porous coating. More recently, textured surfaces have been employed in various
surfaces
which directly contact bone (such as area 12), in order to increase surface
area and allow the
implant to dig in to the bone. Similarly, such a coating can also be applied
to the outer
11
CA 02489676 2004-12-16
WO 2004/002543 PCT/US2002/020436
surface (contacting the pelvis) of the acetabular component. The femoral head
6 may be an
integral part of the hip joint stem 2 or may be a separate component mounted
upon a conical
taper at the end of the neck 4 of the hip joint prosthesis. This allows the
fabrication of a
prosthesis having a metallic stem and neclc but a femoral head of some other
material, such as
ceramic. This method of construction is often desirable because ceramics have
been found to
generate less frictional torque and wear when articulating against the lining
of an acetabular
cup. The lining is typically formed of ultra-high molecular weight
polyethylene (UHMWPE)
or cross-linked polyethylene (XLPE); however, other suitable materials may be
used.
Regardless of the materials, however, the femoral head articulates against the
inner surface of
the acetabular cup thereby causing wear and, in the long term, this may
necessitate prosthesis
replacement. This is especially the case where the femoral head is of metal
and the
acetabular cup is lined with an organic polymer or composite thereof. While
these polymeric
surfaces provide good, relatively low friction surfaces and are biocompatible,
they are subject
to wear and accelerated creep due to the frictional heat and torque to which
they are subj ected
during ordinary use. The use of a diffusion-hardened oxide layer surface such
as oxidized
zirconium significantly extends the useful service life of the articulating
couple.
[0066] A typical knee joint prosthesis is shown in situ in FIGS. 3 and 4. The
knee
joint includes a femoral component 20 and a tibial component 30. The femoral
component
includes condyles 22 which provide the articulating surface of the femoral
component and
pegs 24 for affixing the femoral component to the femur. The pegs (24) and the
surfaces
adjacent to the pegs directly contact the femur. The pegs (24) and the
adjacent surfaces have
been subjected to the same stabilization techniques as were discussed for hip
prostheses; i.e.,
porous metal beads, wire mesh coatings, and more recently, textured surfaces.
The tibial
component 30 includes a tibial base 32 with a peg 34 for mounting the tibial
base onto the
tibia. A tibial platform 36 is mounted atop the tibial base 32 and is supplied
with grooves 38
similar to the shape of the condyles 22. The bottom surfaces of the condyles
26 contact the
tibial platform's grooves 38 so that the condyles articulate within these
grooves against the
tibial platform. While condyles are typically fabricated of metals, the tibial
platform may be
made from an organic polymer or a polymer-based composite. Thus, the hard
metallic
condyle surfaces 26 would articulate against a relatively softer organic
composition. As
previously explained, this may result in wear of the organic material, i.e.
the tibial platform
necessitating the replacement of the prosthesis. As in the case of the hip
joint, porous bead or
12
CA 02489676 2004-12-16
WO 2004/002543 PCT/US2002/020436
wire mesh coatings can also be applied to either the tibial or femoral
components of the knee
or both.
[0067] The invention provides orthopedic implants having diffusion-hardened
oxide or nitride surfaces such as oxidized zirconium or nitrified zirconium.
More generally,
metals or metal alloys of titanium, vanadium, niobium, hafnium and/or tantalum
may be used
as substrate materials to form suitable diffusion-hardened oxide surface
layers. Most of the
examples herein deal with zirconium or zirconium alloy substrates and surface
layers of
oxidized zirconium or nitrified zirconium; however, it should be understood
that other metals
such as hafnium, vanadium, titanium, niobium, tantalum, and their alloys, are
amenable to the
present invention. In order to form continuous and useful oxide or nitride
coatings over the
desired surface of the metal alloy prosthesis substrate, the metal alloy
should preferably
contain from about ~0 to about 100 wt. % of the desired metal, and more
preferably from
about 95 to about 100 wt. %. It should be noted that in some cases, lower
amount of the
desired metal are possible. In some cases, alloys where the desired metal is
at about 10 % by
weight or greater may yield acceptable results. For example, an alloy of about
74 wt
titanium, about 13 wt % niobium and about 13 wt % zirconium ("Ti-13-13") can
be
successfully used herein. Ti-13-13 is taught in U.S. Patent 5,169,597 to
Davidson et al.
Thus, while levels of the desired metal of about 10 % by weight or greater are
known to
produce acceptable results, increasing this level continuously gives
progressively better
results, with at least 80 % by weight, and at least 95 % by weight, being the
preferred and
most preferred levels, respectively.
[0068] In the case of either oxidized or nitrified zirconium, oxygen, niobium,
and
titanium, among others, may be included as common alloying elements in the
alloy with often
times the presence of hafnium. Yttrium may also be alloyed with the zirconium
to enhance
the formation of a tougher, yttria-stabilized zirconium oxide coating during
the oxidation of
the alloy. While oxidized or nitrified zirconium is used for illustrative
purposes herein, it
should be understood that the teachings apply analogously to the other
possible metal
candidates as well. While such zirconium containing alloys may be custom
formulated by
conventional methods known in the art of metallurgy, a number of suitable
alloys are
commercially available. In the case of oxidized zirconium. some commercial
alloys include,
among others Zircadyne 705, Zircadyne 702, and Zircalloy.
[0069] The base metal and metal alloys are cast or machined by conventional
methods to the shape and size desired to obtain a suitable prosthesis
substrate. The substrate
13
CA 02489676 2004-12-16
WO 2004/002543 PCT/US2002/020436
is then subjected to process conditions which cause the ira situ formation of
a tightly adhered,
diffusion-bonded coating of zirconium oxide or zirconiwn nitride on its
surface. The term
"diffusion-hardened" and "diffusion-bonded" are used in reference to the
desired oxides or
nitrides because the formation of these particular surfaces is characterized
by the diffusion of
oxygen or nitrogen from the surface towards the interior (i.e., approaching
the unoxidized
substrate, native metal or metal alloy). It is believed that this diffusion of
oxygen or nitrogen
is what imparts the high strength and high wear resistance to these surfaces.
The process
conditions for formation include, for instance, air, steam, or water oxidation
or oxidation in a
salt bath. These processes ideally provide a thin, hard, dense, low friction,
wear-resistant
zirconium nitride or blue-black or black wear-resistant zirconium oxide film
or coating of
thicknesses typically on the order of several microns (10-~ meter) on the
surface of the
prosthesis substrate. Below this coating, diffused oxygen or nitrogen from the
oxidation or
nitridation process increases the hardness and strength of the underlying
substrate metal.
[0070] The air, steam and water oxidation processes are described for
zirconium
and zirconium alloys in now-expired U.S. Pat. No. 2,987,352 to Watson, the
teachings of
which are incorporated by reference as though fully set forth. These methods
may also be
applied to metals and alloys of hafnium, titanium, vanadium, niobium, and
tantalum. In the
case of zirconium or zirconium alloy, the air oxidation process provides a
firmly adherent
black or blue-black layer of zirconium oxide of highly oriented monoclinic
crystalline form.
If the oxidation process is continued to excess, the coating will whiten and
separate from the
metal substrate. The oxidation step may be conducted in either air, steam or
hot water. For
convenience, the metal prosthesis substrate may be placed in a furnace having
an oxygen-
containing atmosphere (such as air) and typically heated at 700 °F -
1100 °F up to about 6
hours. However, other combinations of temperature and time are possible. When
higher
temperatures are employed, the oxidation time should be reduced to avoid the
formation of
the white oxide.
[0071] The oxide layer should range in thickness from about 1 to about 20
microns; however, a range of from about 1 to about 5 microns is preferred. The
overall
average thickness can be controlled by the parameters of time and temperature.
For example,
furnace air oxidation at 1000 °F for 3 hours will form an oxide coating
on Zircadyne 705
about 2 - 3 microns thick, oxidation at 1175 °F for 1 hour results in
an overall average oxide
coating of about 4 - 5 microns thick, and oxidation at 1175 °F for 3
hours results in an overall
average oxide coating of about 10 - 11 microns thick. As additional examples,
one hour at
14
CA 02489676 2004-12-16
WO 2004/002543 PCT/US2002/020436
1300 °F will form an oxide coating about 14 microns in thickness, while
21 hours at 1000 °F
will form an oxide coating thickness of about 9 microns. Using different
combinations of
oxidation times and higher oxidation temperatures will increase or decrease
this thickness,
but higher temperatures and longer oxidation times may compromise coating
integrity,
depending upon the nature of the substrate and other factors. For thicker
coatings of oxide,
some trial and error may be necessary. Of course, because in the usual case
only a thin oxide
is necessary on the surface, only very small dimensional changes, typically
less than 10
microns over the thickness of the prosthesis, will result. In general, thinner
coatings (1 - 4
microns) have better attachment strength.
[0072] One of the salt-bath methods that may be used to apply the oxide
coatings
to the metal alloy prosthesis, is the method of U.S. Pat. No. 4,671,824 to
Haygarth, the
teachings of which are incorporated by reference as though fully set forth. In
the case of
oxidized zirconium, the salt-bath method provides a similar, slightly more
abrasion resistant
blue-black or black zirconium oxide coating. The method requires the presence
of an
oxidation compound capable of oxidizing zirconium in a molten salt bath. The
molten salts
include chlorides, nitrates, cyanides, and the like. The oxidation compound,
sodium
carbonate, is present in small quantities, up to about 5 wt %. The addition of
sodium
carbonate lowers the melting point of the salt. As in air oxidation, the rate
of oxidation is
proportional to the temperature of the molten salt bath and the '824 patent
prefers the range
550 °C -800 °C (1022 °F -1470 °F). However, the
lower oxygen levels in the bath produce
thinner coatings than for furnace air oxidation at the same time and
temperature. A salt bath
treatment at 1290 °F for four hours produces an oxide coating thickness
of roughly 7 microns.
[0073] Whether air oxidation in a furnace or salt bath oxidation is used, the
oxide
coatings are quite similar in hardness. For example, if the surface of a
wrought Zircadyne 705
(Zr, 2-3 wt. % Nb) prosthesis substrate is oxidized, the hardness of the
surface shows a
dramatic increase over the 200 Knoop hardness of the original metal surface.
The surface
hardness of the resulting blue-black zirconium oxide surface following
oxidation of
Zircadyne 705 by either the salt bath or air oxidation process is
approximately 1700-2000
Knoop hardness.
[0074] In the case of nitridation of zirconium. and zirconium alloys, an
analogous
procedure is used. As in the oxide case, the nitride layer should range in
thickness from
about 1 to about 20 microns; however, a range of from about 1 to about 5
microns is
preferred. Even though air contains about four times as much nitrogen as
oxygen, when
CA 02489676 2004-12-16
WO 2004/002543 PCT/US2002/020436
zirconium or a zirconium alloy is heated in air as described above, the oxide
coating is
formed in preference to the nitride coating. This is because the thermodynamic
equilibrium
favors oxidation over nitridation under these conditions. Thus, to form a
nitride coating the
equilibrium must be forced into favoring the nitride reaction. This is readily
achieved by
elimination of oxygen and using a nitrogen or ammonia atmosphere instead of
air or oxygen
when a gaseous environment (analogous to "air oxidation") is used. In order to
form a
zirconium nitride coating of about 5 microns in thickness, the zirconium or
zirconium alloy
prosthesis should be heated to about 800 °C for about one hour in a
nitrogen atmosphere.
Thus, apart from the removal of oxygen (or the reduction in oxygen partial
pressure), or
increasing the temperature, conditions for forming the zirconium nitride
coating do not differ
significantly from those needed to form the blue-black or blaclc zirconium
oxide coating. Any
needed adjustment would be readily apparent to one of ordinary slcill in the
art.
[0075] When a salt bath method is used to produce a nitride coating, then the
oxygen-donor salts should be replaced with nitrogen-donor salts, such as, for
instance
cyanide salts. Upon such substitution, a nitride coating may be obtained under
similar
conditions to those needed for obtaining an oxide coating. Such modifications
as are
necessary, may be readily determined by those of ordinary skill in the art.
Alternatively, the
zirconium nitride may be deposited onto the zirconium or zirconium alloy
surface via
standard physical or chemical vapor deposition methods, including those using
an ion-
assisted deposition method. It is preferred that the physical or chemical
vapor deposition
methods be carned out in an oxygen-free enviromnent. Techniques for producing
such an
environment are known in the art, for instance the bulk of the oxygen may be
removed by
evacuation of the chamber and the residual oxygen may be removed with an
oxygen
scavenger.
[0076] When the zirconium or zirconium alloy is provided with a zirconium
porous bead, zirconium wire mesh surface, or textured surface, then this
surface layer can
also be coated with zirconium oxide or nitride, as the case may be, to provide
protection
against metal ionization in the body.
[0077] These diffusion-bonded, low friction, highly wear resistant oxidized or
nitrided zirconium coatings are grown in-situ and used on the surfaces of
orthopedic implants
subject to conditions of wear. Such surfaces include, but are not limited to,
the articulating
surfaces of knee joints, elbows and hip joints. As mentioned before, in the
case of hip joints,
the femoral head and stem are typically fabricated of metal alloys while the
acetabular cup
16
CA 02489676 2004-12-16
WO 2004/002543 PCT/US2002/020436
may be fabricated from ceramics, metals or organic polymer-lined metals or
ceramics.
However, the acetabular cup may be fabricated of a metal or metal alloy that
forms a
diffusion-hardened surface.
[0078) When the diffusion-hardened oxide or nitride coated femoral head is
used
in conjunction with any of these acetabular cups, the coefficient of friction
between the
femoral head and the inner surface of the cup is reduced so that less heat and
torque is
generated and less wear of the mating bearing surface results. Tlvs reduction
in heat
generation, frictional torque, and wear is particularly important in the case
of acetabular cups
lined with organic polymers or composites of such polymers. Organic polymers,
such as
UHMWPE, exhibit rapidly increased rates of creep when subjected to heat with
consequent
deleterious effect on the life span of the liner. Wear debris of the polymer
leads to adverse
tissue response and loosening of the device. The diffusion-hardened coating
serves to protect
the prosthesis substrate and increase its mechanical strength properties but,
as a result of its
low friction surface, it also protects those surfaces against which it is in
operable contact and
consequently enhances the performance and life of the prosthesis.
[0079] The usefulness of prostheses employing diffusion-hardened surfaces is
not
limited to load-bearing surfaces of load-bearing prostheses, but are also
applicable to non-
load bearing prostheses, especially joints, where a high rate of wear may be
encountered.
Because the diffusion-hardened surface is firmly bonded to the metal or metal
alloy
prosthesis substrate, it provides a barrier between the body fluids and the
metal or metal
alloy, thereby preventing the corrosion of the alloy by the process of
ionization and its
associated metal ion release. Because these diffusion-hardened surfaces offer
advantages in
both mechanical wear and for the prevention of metal ion release, they are
applicable to any
and all surfaces of a prosthetic device.
[0080] The substrate metal or metal alloy has been used to provide a porous
bead,
wire mesh, or textured surface to which surrounding bone or other tissue may
integrate to
stabilize the prosthesis. The porous metal beads, wire mesh or textured
surface can have
diffusion-hardened surfaces as well. As a result, these special surfaces can
be rendered non-
ion releasing in a way similar to the oxidation or nitridation of the base
prosthesis for the
elimination or reduction of metal ion release. These roughened surfaces
improve bone
ingTOwth and on-growth by providing an increased surface area for adhesion,
and by
providing an increased surface area onto which bone in-growth and on-growth
may occur.
17
CA 02489676 2004-12-16
WO 2004/002543 PCT/US2002/020436
[0081] The inventors have discovered that extending the useful service life of
prosthetic devices can be realized by combining the advantages of diffusion-
hardened
surfaces with the fixation stability imparted by the use of bioceramic
compomlds. These
material surfaces enhance fixation by both a physical/mechanical mechanism
(increasing
surface area for better adhesion) and a chemical mechanism (e.g., the
promotion of bone
growth by apatites in general and hydroxyapatites in particular). The porous
metal
beads/wire mesh/textured surface techniques operate only by the
physicallmechanical
mechanism of increasing surface area for better adhesion. In this way,
bioceramics have
better fixation-enhancing abilities by virtue of their multiple modes of
action. In the case of
apatite compounds, the apatite surfaces interact with bone or provide for bone
ingrowth
enhancing the fixation stability of the device. Because of the chemical
similarities between
the apatites and the natural material in bones, it is believed that there is a
chemical driving
force which promotes bone growth in the presence of apatites. Coating with
apatite can also
increase the attachment area on the implant which is available for bone in-
growth and on-
growth area while simultaneously chemically promoting bone growth. This dual
mechanism
of promoting bone in-growth and on-growth results in improved fixation
stability of implants
employing apatite coatings. Bioceramics in general exhibit similar beneficial
properties. The
inventors have applied these synergies for the fabrication of exceptionally
long-life prosthetic
devices.
[0082] The biocermaic or apatite coatings may be produced by any conventional
or non-conventional means. One of ordinary skill in the art is familiar with
these methods,
particularly those employing apatite compounds, especially hydroxyapatite. For
the purposes
of discussion, much of the remainder of the discussion focuses on the apatite
compounds with
the understanding that the invention is not so limited. Use of other
bioceramic and bone
growth-promoting materials is largely analogous, with only minor modifications
perhaps
necessary and otherwise known to one of ordinary skill in the art. These other
materials
include, but are not limited to, calcium sulfate, calcium phosphate, calcium
carbonate,
calcium tartarate, bioactive glass, and combinations thereof.
[0083] In the preferred embodiment, an apatite coating is used and the apatite
coatings will be hydroxyapatite, Ca5(P04)3(OH), and possess a large surface
area owing to
the fibrous nature of the hydroxyapatite crystals. The surface area will
generally range from
about 1-25 m2/cm2 of area. The coatings may be as thin as about 2 ~.m,
preferably being at
least about 5 pm, and more preferably at least about 10 ~,m, and may range to
40 ~.m thick or
18
CA 02489676 2004-12-16
WO 2004/002543 PCT/US2002/020436
greater, depending upon need. Usually, a relatively thin coating will be
employed to avoid
thick brittle ceramic interfaces between the substrate and the ductile bone.
The high surface
of this coating presents orders of magnitude more binding surface than the
uncoated implant
or the conventional calcium phosphate coatings.
[0084] The apatite composition may be modified in a variety of ways by the
introduction of other ions, as required. Other ions include fluoride,
carbonate, sodium,
chloride, hydrogen ions, HP04, HC03, etc., and the like. Usually fewer than
about 50%,
more usually fewer than about 20% of the total number of phosphate and
hydroxide anions
and up to 50% of calcium cation will be substituted with other ions. These
substitutions will
influence the ih vivo dissolution behavior of the coating which may be
resorbable or non-
resorbable.
[0085] Hydroxyapatite possesses a net positive charge at physiological pH
which i
attracts negatively charged proteins, such as collagen or other exogenous or
endogenous
proteins, which may serve as growth factors and result in other interfacial
chemistry. Thus,
the coating may provide for the presence of such products on the surface of
the
hydroxyapatite or analogs or as part of the structure of the hydroxyapatite.
[0086] The coatings may be applied by any conventional or non-conventional
methods of applying bioceramic, and n particular, hydoxyapatite or apatite
compounds. All
patent references describing such techniques are incorporated by reference as
though fully
described herein. For example, the bioceramic may be applied to solid
surfaces, porous
surfaces, etched surfaces, or any other type of surface. Because the coating
may be applied in
a liquid medium which is able to penetrate channels, pores, indentations and
other structural
features, a uniform coating may be obtained which can coat substantially the
entire surface,
without leaving exposed areas. In one solution-based deposition of
hydroxyapatite, small,
sticky hydroxyapatite colloidal particles in suspension are formed in
proximity to the
substrate to be coated by the addition of calcium and phosphate reactants in
solution. (See
U.S. Patents 5,188,670; 5,279,831; and 5,164,187). Alternatively, the
bioceramic may be
applied as dry particulates as taught in U.S. Patent 4,693,986, which is
incorporated by
reference herein as though fully described. Vapor deposition techniques,
plasma spray
deposition (see U.S. Patent 6,280,789), electrodeposition (U.S. Patent
5,759,376) are
additional illustrative examples of known methods for applying surface
coatings of
bioceramic compounds. The precise mode of deposition is unimportant and any
and all
means yielding a coating of apatite having good structural integrity are
acceptable. In the
19
CA 02489676 2004-12-16
WO 2004/002543 PCT/US2002/020436
preferred embodiment, the bioceramic compound or compounds are deposited via
plasma
spray deposition or chemical vapor deposition.
[0087] In the hip joint assembly shown in situ in FIGS. 1 and 2, areas 12 and
2
are, among others, examples of areas wherein the apatite coating of the
present invention
could be applied. In many conventional prosthetic devices, these areas,
particularly area 12,
comprise porous metal beads, wire mesh, and/or textured surfaces to enhance
fixation
stability. As per the present invention, these areas as well as other areas,
could have an
bioceramic coating (most preferably, a hydroxyapatite coating) in lieu of, or
alternatively in
addition to, the conventional porous metal beads, wire mesh coatings, andJor
textured
surfaces used to promote bone in-growth or on-growth. Positioning the coating
on the
femoral stem affords a good deal of surface .area contact with the femor and
allows
stabilization of the implant by ingrowth of surrounding tissue into the porous
coating.
Similarly, such a coating can also be applied to the outer surface of the
acetabular
component. The femoral head 6 may be an integral part of the hip joint stem 2
or may be a
separate component mounted upon a conical taper at the end of the neck 4 of
the hip joint
prosthesis. This allows the fabrication of a prosthesis having a metallic stem
and neck but a
femoral head of some other material, such as ceramic. Regardless of the
materials, however,
the use of bioceramic coatings in any area where the prosthetic device
contacts bone will
result in enhanced fixation stability and allow prosthetic devices employing
diffusion-
hardened surfaces to realize the full service-enhancing attributes of those
surfaces. In this
way, fixation stability is no longer a major limiting factor in the effort to
fabricate a truly life-
long prosthetic devices employing diffusion-hardened oxide surfaces. The use
of diffusion-
hardened surfaces coupled with bioceramic-promoted fixations markedly extends
the life of
such prostheses.
[0088] As in the case of the hip joint, in a knee prosthesis, porous metal
beads,
wire mesh coatings, or textured surfaces can also be applied to either the
tibial or femoral
components of the knee or both. In the typical knee joint prosthesis shown in
situ in FIG. 4,
selected area within the femoral component 20, particularly :hose areas around
the pegs 24
may be coated with bioceramics such as, for example, the haloapatites such as
fluoroapatite
and chloroapatite, but preferably hydroxyapatite. The tibial component 30
includes a tibial
base 32 with a peg 24 for mounting the tibial base onto the tibia. The
underside of the tibial
base directly contacts the tibia and is an example of an ideal location for a
coating of
bioceramic. It should be noted that the aforementioned areas are non-limiting
examples of
CA 02489676 2004-12-16
WO 2004/002543 PCT/US2002/020436
areas wherein the bioceramic coating could be applied to a prosthetic having a
diffusion-
hardened oxidized or nitrided surface.
[0089] The invention described herein is also useful in endoprostheses or
unipolar
prostheses. These include, but are not limited to shoulder, lrnee, and hip
endoprostheses. In
these devices, the bearing surface cooperates with body tissue, most commonly
cartilage. At
least a part of the bearing surface of the endoprosthesis will have a
diffusion-hardened oxide
or nitride coating and at least part of the body of the endoprosthesis will
have an coating of at
least one bioceramic compound. The methods for applying the diffusion-hardened
oxide or
nitride coating are the same as those for other prosthetic devices. Similarly,
the bioceramic
coating may be applied in any manner, conventional or otherwise.
[0090] It is important to note that the areas where the bioceramics could be
applied may vary, but it is preferred that the application occur in areas of
maximum contact
with bone, as such would promote maximum bone in-growth and on-growth.
Bioceranic may
or may not be applied on at least part of the diffusion-hardened surface. This
would also be
true in the case of other prosthetic devices such as shoulders, fingers, jaws,
elbows, and
others. The invention is broadly described to encompass any prosthetic device
having at least
part of its surface comprising a diffusion-hardened surface and at least part
of its surface
comprising one or more bioceramic materials.
[0091] The use of bioceramic coatings and diffusion-hardened surfaces on
prosthetic devices can be performed in conjunction with conventional
techniques for
effecting fixation stability of such devices. These include, but are not
limited to, the use of
irregular surfaces of beads and/or wire mesh or the use of textured surfaces
such as those
known in the art and formed by techniques such as chemical, electrochemical,
and/or
mechanical etching. These conventional fixation surfaces may themselves
comprise a
diffusion-hardened surface or an bioceramic coating or both.
[0092] One skilled in the art readily appreciates that the present invention
is well
adapted to carry out the objectives and obtain the ends and advantages
mentioned as well as
those inherent therein. Systems, methods, procedures and techniques described
herein are
presently representative of the preferred embodiments and are intended to be
exemplary and
are not intended as limitations of the scope. Changes therein and other uses
will occur to
those skilled in the art which axe encompassed within the spirit of the
invention or defined by
the scope of the claims.
21
CA 02489676 2004-12-16
WO 2004/002543 PCT/US2002/020436
U.S. Patent Documents
2,987,352 6/1961 Watson
4,671,824 6/1987 Haygarth
4,673,409 6/1987 Van Kampen
4,644,942 2/1987 Sump
4,272,855 6/1981 Frey
4,865,603 9/1989 Noiles
5,922,029 711999 Wagner et al.
5,507,815 4/1996 Wagner et al.
5,258,098 1111993 Wagner et al.
6,193,762 2/2001 Wagner et a1.
5,037,438 8/1991 Davidson
5,152,794 10/1992 Davidson
5,169,597 12/1992 Davidson et al.
5,180,394 1/1993 Davidson
5,370,694 12/1994 Davidson
5,372,660 12/1994 Davidson et al.
5,496,359 3/1996 Davidson
5,549,667 8/1996 Davidson
5,188,670 2/1993 Constantz
5,279,831 1/1994 Constantz
5,164,187 11 / 1992 Constantz -
Other References
Albee, et al., "Studies in Bone Growth," Ann. Surg., 71:32-39, 1920.
Hulbert et al., "History of Bioceramics," Ceramics in Surgery, 3-27, 1983.
Nielson, "Filling of Sterile and Infected Bone Cavities by Means of Plaster of
Paris,"
Acta Chir. Scandanav., 91:17-27, 1944.
22
CA 02489676 2004-12-16
WO 2004/002543 PCT/US2002/020436
Peltier, et al., "The Using of Plaster of Paris to Fill Defects in Bone," Ann.
Suxg.,
146:61-69, 1957.
23