Note: Descriptions are shown in the official language in which they were submitted.
. .
~i~~s~~
~~yi WO 94/02498
. .. .. t ,; '. PCf/US93106$84
HYBRID OLIGONUCLEOTIDE P8088HOROTHIOATE$
BACROROUND OF TSE INVENTI03d
;;:
Field of Tile ~, nven~~c~n_
The invention relates to synthetic oligonucleotides
that are useful for studies of gene expression and in the
antisense oligonucleotide therapeutic approach. More ~.;;::
particularly, the invention relates to synthetic
oligonucleotides that have improved qualities for such
agplications resulting from modifications in the sugar
phosphate backbone of the oligonucleotides.
Sup ,wary. of The Rglated~~rt
The potential for the development of an antisense
oligonucleotide therapeutic approach was first suggested
in three articles published in 1977 and 1978. Paterson
et al., Proc. Natl. Acad. Sci. USA 7g: 4370-4374 (1987)
discloses that cell-free translation of mRNA can be -.
inhibited by the binding of an oligonucleotide
complementary to the mRNA. Zamecnik and Stephenson,
Proc. Natl. Acad. Sci. USA 75: 280-284 and 285-288 (1978)
discloses that a.13-mer synthetic oligonucleotide that is
complementary to a part of the Rous sarcoma virus (RSV)
genome inhibits RSV replication in infected chicken
tibroblasts and inhibits RSV-mediated transformation of
primary chick fibroblasts into malignant sarcoma cells.
These early indications that synthetic
oiigonucleotides can be used to inhibit virus propagation
and neoplasia have been followed by the use of synthetic
oligonucleotides to inhibit a wide variety of viruses.
Goodchild et al., U.S. Patent No. 4,806,463 (the
teachings of which are hereby incorporated by reference) ; '-.
discloses inhibition of Human immunodeficiency virus ; .
(HIV) by synthetic oligodeoxynucleotides complementary to
various regions of the HIV genome. Leiter et al., Proc.
CA 02490644 1993-07-22
WO 94/02498 '~ ~" i., ~ ~ ~ . .
.'~' ~' .. , ~ ,~ PCT/US93/~b884 :'F
..2 _ , ..<::r .
'
Natl. Acad. Sci.
USA $7: 3430-3434
(1990) discloses
inhibition of
influenza virus
by synthetic
oligonucleotides.
Agris et al.,
Biochemistry
~: 6268-
6275 ~ (1986)
discloses the
use of synthetic
oligonucleotides
to inhibit Vesicular
stomatitis virus
(VSV). Gao et
al., Antimicrob.
Agents Chem.
~4_: 808-812
(1990) discloses
inhibition of
Herpes simplex
virus by
synthetic aligonucleotides.
Birg et al.,
Nucleic Acids
Res. ~$: 2901-2908
(1990) discloses
inhibition of
Simian
virus (SV40) by
synthetic oligonucleotides.
Storey et
al., Nucleic Acids
Res. 19: 4109-4114
(1991} discloses
inhibition of
Human papilloma
virus (HPV) by
synthetic
oligonucleotides.
The use of synthetic_oligonucleotides
'
and their analogs
as antiviral
agents has recently
been
extensively reviewed
by Agrawal, Tibtech
10: 152-158
(1992).
In addition, synthetic
oligonucleotides
have been
used to inhibit
a variety of
non-viral pathogens,
as well
as to selectively
inhibit the expression
of certain
cellular genes.
Thus, the utility
of synthetic
oligonucleotides
as
agents to inhibit
virus propagatioe,
propagation of
non-
viral pathogens
and selective
expression of
cellular
genes has been
well. established,
However, there
is a
need for improved
oligonucleotides
that have greater
efficacy in inhibiting
such viruses,
pathogens and
selective gene
expression. Various
investigators
have
attempted to meet
this need by
preparing and
testing
oligonucleotides
having modifications
in their
~nternucleotide
linkages. Several
investigations
have
w
~
., , , " ;
shown that such
modified oligonucleotides
are more
effective thaw
their unmodified
counterparts.
Sarin et
al., Proc. Natl.
Acad. Sci. USA
85: 7448-7451
(1988) , ~'
teaches that oligodeoxynucleoside
methylphosphonates
are
more. active as
inhibitors of
HIV-1 than conventional
,
i
oligvdeoxynucleotides.
Agrawal et al.,
Proc. Natl. Acad.
;hcJa r.v-..
-: r '
1
7,'.~ v
! :
a. . ' V -
.:
.C - . .
. . . "
v.
.
.. .....
'.:.... ['. .
.- . . . ..,
' . . .~-. ..J
.c: v.. 1. '
- ..
CA 02490644 1993-07-22
,r. ,'~,
. 1 ~ ~ ~
~
.
.
",'~ WO 02498
94/ '
: ~
~
~ ~ PCT/US93/06884
.;
Sci. USA 85: 7079-7083 (1988) teaches that
oligonucleatide phosphorothioates and various .
oligonucleotide phosphoramidates are more effective at
inhibiting HIV-1 than conventional oligodeoxynucleotides.
Agrawal et al., Proc. Natl. Acad. Sci. USA 86: 7790-7794
(1989) discloses the advantage of oligonucleotide ,.
phosphorothioates in inhibiting HIV-1 in early and
chronically infected cells.
In additian, chimeric oligonucleotides having more
than one type of internucleotide linkage within the
oligonucleotide have been developed. Chimeric
oligonucleotides contain deoxyribonucleosides only, but
have regions containing different internucleotide
linkages. Pederson et al., U.S. Patent No. S,XXX,XXX
(Ser. No. 07/480,269; allowed on 12/24/91) , the teachings
of which are hereby incorporated by reference, discloses
chimeric oligonucleotides having an oligonucleotide
phosphodiester or oligonucleotide phosphorothioate core
sequence flanked by oligonucleotide phasphoramidates, =.
methylphosphonates or phosphoramidates. Furdon et al.,
Nucleic Acids Res. 17: 9193-9204 (1989) discloses
chimeric oligonucleotides wing regions of ,
oligonucleotide phosphodiesters in addition to either
oligonucleotide phosphorothioate or ,methylphosphonate
regions. Quartin et al., Nucleic Acids Res. 37: 7523-
7562 (1989) discloses chimeric oligonucleotides having
regions of oligonucleotide phosphodiesters and
oligonucleotide methylphosphonates. Each of the above w
compounds uses deoxyribonucleotide '.
phosphorothioates,which have reduced duplex stability. .:
Atabekov et al., FEBS Letters 232: 96-98 (1988} discloses
chimeric oligonucl.eotides in which all internucleotide
linkages are phosphodiester linkages, but in which
regions .of oligoribonucleotides and
oligodeoxyribonucleotides are mixed. Inoune et al., FENS , ,.
'Letters, ?~: 237-250 (1987) discloses chimeric .
CA 02490644 1993-07-22
~~. !,~..~~~,).~;, ....~~. ;..
WO 94/02498 ~ PCT/US93/06884
4
r ~
-4 -
oligonucleotides having only phosphodiester linkages, and
regions of oligodeoxyribonucleotides and 2'-oMe- ,
ribonucleotides. None of these compounds having solely
phosphodiester linkages exhibit either endonuclease or
exonuclease resistance.
Many of these modified oligonucleotides have i
contributed to improving the potential efficacy of the
antisense oligonucleotide therapeutic approach. However,
certain deficiencies remain in the known
oligonucleotides, and these deficiencies can limit the
effectiveness of such oligonucleotides as therapeutic
agents. Wickstrom, J. Biochem. Biophys. Methods 13: 97-
102 (1986) teaches that oligonucleotide phosphodiesters
are susceptible to nuclease-mediated degradation. Such
nuclease susceptibility can limit the bioavailability of
oligonucleotides _in vivo. Agrawal et al., Proc. Natl.
Aced. ~Sai. USA 87: 1401-1405 (1990) teaches that
ol3gonucleotide phosphoramidates or methylphosphonates
when hybridized to RNA do not activate RNase H, the
activation of which can be important to the function of
antisense oligonucleotides. Agrawal et al., Nucleosides
& Nucleotides 8_: 5-6 (189) teaches that
oligodeoxyribonucleatide phosphorothioates have reduced
duplex stability when hybridized to RNA. '
There is, therefore, a need for improved
oliganucleotides that overcome the deficiencies of
oligonucleotides that are known in the art. Ideally,
such oligonuclecitides should be resistant to nucleolytic
degradation, should form stable duplexes with RNA, and
should activate RNase H when hybridized with RNA.
. . . . . :::
~.
,.
~ :.:
j
..:.
CA 02490644 1993-07-22
21: 4 4 ~ ~:~,~ = i
.'..~~ y; WO 94/42498
PGT/US93/06884
,..
-5-
$RIEF BUMM~R'~ OF THE INVENTION
The invention provides hybrid oligonucleotides that
resist nucleolytic degradation, form stable duplexes with
RNA or DNA, and activate RNase H when hybridized with
RNA. Oligonucleotides according to the invention provide w
these features by having phosphorothioate and/or
phosphorodithioate internucleotide linkages and segments
of oligodeoxyribonucleotides as well as segments of
either oligoribonucleotides or 2'-substituted
l0 oligoribonucleotides. For purposes of the invention, the
term "Z'-substituted" means substitution of the 2'-off of
the ribose molecule with, era, 2 ~ -OMe, 2 ~ -allyl, 2 ~ -aryl,
Z'-alkyl, 2'-halo, or 2'-amino, but not with 2'-H,
wherein allyl, aryl, or alkyl groups may be unsubstituted
or substituted, e.a.,, with halo, hyd~oxy,
trifluoromethyl, cyano, nitro, acyl, acyloxy, alkoxy,
carboxyl, carbalkoxyl or amino groups,
An object of the invention is to provide
oligonucleotides that can be used to analyze and explain w
ZO the- importance to the effectiveness of antisense
oligonucleotides of the parameters of nuclease
resistance, duplex stability anQ RNase H activation.
Another object of the invention is to provide
oligonucleotides that are effective for regulating
cellular, pathogen, or viral gene expression at the mRNA
level. Yet another object of the invention is to provide
therapeutic oligonucleotides that have great efficacy in
the antisense oligonucleotide therapeutic approach.
Oligonucleotides according to the invention are useful in
satisfying each of these objects of the invention..
,: , , .
,e..: ..: ~ ' ; .;._ ; ;:; ..;;, , ;y , ,.; ,., ;,: , : ~ : ~' , : ; ; . .;,
y: . : :. ~.,' w ; . .
liE....... :.;~. ~. t ~ :. .,.~-.n.. ~.n ,...: . ~ . ~~~ ~:. :..,... ;,, .
..:. .:~ . '.~'.:,. . --~:;.. -~',;...: . . '.'. ~ . . .:.. ,. ." _ . : . . ~.
.. '
CA 02490644 1993-07-22
Image
. . .21'4v064g,.::,,, ~ ~, .
y~=;. WO 94/OZ498 PCT/US93/06884
DETAILED DESCRIPTION OF THE ~R,~FERRED EMBODIMENTS
In a first aspect, the invention provides
oiigonucleotides that are useful for studying the
parameters that are important for effective antisense
oligonucleotide action. For purposes of the invention,
the term oligonucleotide includes polymers of two or more
ribonucleotides, deoxyribonucleotides, or both, with
ribonucleotide and/or deoxyribonucleotide monomers being
connected together via 5' to 3' linkages which may
include any of the linkages that are known in the
antisense oligonucleotide art. In addition, the term
oligonucleotides includes such molecules having modified
nucleic acid/bases and/or sugars, as well as such
molecules having added substituents, such as diamines,
cholesteryl or other lipophilic groups. Certain
preferred combinations of monomers and inter-monomer
linkages are~discussed in greater detail below.
It is generally believed that the activity of an
antisense oligonucleotide depends on the binding of the
oligonucleotide to the target nucleic acid, thus
disrupting the, function of the target, either by
hybridization arrest or by clestryction of target RNA by
RNase H. These mechanisms of action suggest that two
parameters should be important to antisense
oligonucleotide activity: duplex stability and RNase H -.
activation. Duplex .stability is important, since the
oligonucleotide presumably must form a duplex (or triplex
in the Hoogsteen pairing mechanism) with the target
nucleic acid to act either by hybridization arrest or by
RNase H-mediated target destruction. RNase F~ activation
(the ability to activate RNase H when hybridized with
target RNA) is implicated when the target nucleic acid is
RNA, since. such activation can lead to the effective '
destruction of the target RNA molecule. In addition, for
an antisense oligonucleotide to act in ~, it must
survive long enough to interact with the target nucleic
CA 02490644 1993-07-22
Image
214~OG4~:-:.yt:;~ .... y
~z ~'~ WO 94/02498
!<;t~~:, ~ : '~ '. .~ P(.'T/US93/06884
'9_ , 1
s
TABLE I
PROPERTIES OF QI~IGOO~]~TLJCLEOTID~S
Duplex Nuclease RNase H .
Oligonudeotide Stability'Resistance=Activation'
Oligodeoxytibonucleotide
(phosphate) -- __ Yes
Oligodeoxyriboaucieotide
phosphorothioate Lower + Yes
Oligodooxyribonucleodde
phosphorodithioate Lower + + Yes
Oligodeoxyribonucleotide
selenoste Lower + N.K.
Oligodeoxyribonucleotide
phosphoramidate Lower +++ No
Oligoribonucleotide
(phosphate) Higher -- No .
~
Otigoribonucleotida
phosphomthioate Higher + No
2'-OMe-Oligoauclcotide
(phosphate) Higher + No
2 0 Z'-OMe-Oiigoribonucleotide
(phosphorothioate) Higher + + No .
Oligodeoxyribonucleotide
methylphosphonate Lower + + + No
1. Duplex stability of oligonucleotide to complementary oligoribonucleotide
under
2 S physiological conditions, compared to DNA-RNA stability.
2. Compared from DNA (phosphodiesterase digestion).
3. Activation of RNase H by the duplex formed between oiigonucleotide and RNA.
Hybrid oligonucleotides according to the invention
form more stable duplexes with complementary RNA than
30 ' 'oligodeoxyribonucleotide phosphorothioates. In addition,
they are more resistant to endonucleolytic and
i
exonucleolytic degradation than oligodeoxyribonucleotide
phosphorothioates and they normally activate RNase H.
Consequently, oligonucleotides according to the invention
3b complement the oligonucleotides shown in Table I in
i
CA 02490644 1993-07-22
WO 9 4 ~ ~ ~..~~~.'' ' ~ ~ ' '
/ .21
. .
4
02498
PCT/US93/06884
,~:~.~,
-10- . ,
~'involved in the effectiveness
studies of the parameters
,
of antisense oligo'~~tlc'leotides.
With respect to this first aspect of the invention, i
oligonucleotides according to the invention can have any .
oligonucleotide sequence, since complementary
oligonucleotides used in such study can be prepared
having any oligonucleotide sequence. oligonucleotides
according to this aspect of the invention are
characterized only by the following features. First, at :v
least some of the internucleotide linkages present in
oligonucleotides according to the invention are
phosphorathioate and/or phosphorodithioate linkages. In
various embodiments, the number of phosphorothioate
and/or phosphorodithioate internucleotide linkages can
15, range from l,to as many internucleotide linkages as are
present in the oligonucleotide. Thus, for purposes of
the invention, the term oligonucleatide phosphorothioate
and/or phosphorodithioate is intended to encompass every
such embodiment. In a preferred embodiment,
oligonucleotides according to the invention will range
'
s:
y,
from about 2 to about 50 nucleotides in length, and most
preferably from about 6 to abq~at 50 nucleotides in ,
length: Thus, in this preferred embodiment,
oligonucleotides according to the invention will have
from ~1 to about 49 p3iosphorothioate and/or ,
phosphorodithioate internucleotide linkages.
A second feature of oligonucleotides according to
this aspect of the invention is the presence of
deoxyribonucleosides. Oligonucleotides according to the
invention contain at least one deoxyribonucleoside.
~~Prefera~ly ol.igonucleotides.according to the invention
contain fo~ir or more deoxyribonucleotides in a contiguous
' block, so ws to provide an activating segment for RNase .
H. In certain preferred embodiments, more than one such
activating segment will be present. Such segments may be
pz'esent .at any location within the oligonucleotide.
CA 02490644 1993-07-22
. ~ ~ 21 ~~0 ~:~4:J ' - .
':, j WO 94/02498 ~ ~ , PCT/U593/06884
t
,. ,
-I1
There may be a majority".of deoxyribonucleosides in
oligonucleotides . -
according to the invention. In fact, such
oligonucleotides may have as many as all but one
nucleoside~~being deoxyribonucleosides. Thus, in a
preferred embodiment, having from about 2 to about 50
nucleosides or most preferably from about 6 to about 50
nucleosides, the number of deoxyribonucleosides present
will range from 1 to about 49 deoxyribonucleosides.
A third feature of oligonucleotides according to
this aspect of the invention is the presence of
ribonucleosides, 2'-substituted ribonucleosides or
combinations thereof. For purposes of the invention, the
term "2'-substituted" means substitution of the 2'-OH of
the ribose molecule with, e-cr, 2'-OMe, 2'-allyl, 2'-aryl,
2'-alkyl, 2'-halo, or 2'-amino, but not with 2'-H,
wherein allyl, aryl, or alkyl groups may be unsubstituted
or substituted, era.., with halo, hydroxy,
trifluoromethyl, cyano, vitro, acyl, acyloxy, alkoxy,
carboxyl, carbalkoxyl or amino groups. oligonucleotides
according to the invention contain at least one
ribonucleoside and/or 2'-substituted ribonucleoside. In
a preferred embodiment, such oligonucleotides have 6 or
more ribonucleosides and/or 2'~-substituted
ribonucleosides to enhance duplex stability. Such -..
ribonucleosides and/or2'-substituted ribonucleosides can
be present singly, in pairs, or in larger contiguous
segments, and may be present at any position within the
oligonucleotide or at multiple positions within the
oligonucleotide. Such ribonucleosides and/or 2'
'substituted ribonucleosides may comprise as many as all
but one nucleoside within the oligonucleotides. Thus, in
a preferred embodiment, having from about 2 to about 50 . .
nucleosides or mast preferably from aboT~t 6 to about 50 ;.
nucleosides, the number of ribonuc~::~osides or 2'- '.
CA 02490644 1993-07-22
~i4os~s . .
WO 94102498 , ' PC'T/US93/46884
-12-
substituted ribonucleosides will range from about 1 to
about 49 deoxyribonucleosides. ,
The ability to vary.,the numbers and positions of ,
phosphorothioate andf'or phosphorodithioate , ,
internucleotide linkages, deoxyribonucleosides, and ,
ribonucleosides or 2:!~substituted ribonucleosides allows
the investigator to examine in detail how each of these
variables affects the parameters of nuclease resistance,
duplex stability and RNase H activation. The ability to
vary the size of the oligonucleotide allows examination
of yet another parameter. Tn addition, smaller oligos
(era., dimers) can be used as building blocks for larger
oligos. Thus, every such possible embodiment described
above is useful in such studies.
Tn a second aspect, the invention provides hybrid
oligonucleotides that are effective in inhibiting
viruses, pathogenic organisms, or the expression of
cellular genes. The ability to inhibit such agents is
clearly important to the treatment of a variety of
disease states. ~ligonucleotides according to this
aspect of the invention share the,~haracteristics of the
above-described oligonucleotides, except that the
oligonucleotide sequence of oligonucleotides according to
this aspect of the invention is complementary to a
nucleic acid sequence that is from a virus, a pathogenic
organism or a cellular gene. Preferably such
oligonucleotides are from about 6 to about 50 nucleotides
in length. For purposes of the invention, the term
"oligonucleotide sequence that is complementary to a
30nucleic 'acid' sequence" is intended to mean an
oligonucleotide sequence (2 to about 50 nucleotides) that i
.;
hybridizes to the nucleic acid sequence under
physiological conditions, e.a, by Watson-Crick base
pairing (interaction between oligonucleotide and single-
35. stranded nucleic acid) or by Hoogsteen base pairing
:,
,. ..
CA 02490644 1993-07-22
2mos.~~~.: .
. .. ...;:..
~~ ~~ WO 94/02d98 . , PGT/US93/0688d
-13-
(interaction between oligonucleotide and double-stranded
nucleic acid) or by any other means. Such hybridization
under physiological conditions is measured ~.s a practical
matter by observing interference with the function of the
nucleic acid sequence.
The nucleic acid sequence to which an
oligonucleotide according to the invention is
complementary will vary, depending upon the agent to be
inhibited. In many cases the nucleic acid sequence will
be a virus nucleic acid sequence. The use of antisense
oligonucleotides to inhibit various viruses is well
known, and has recently been reviewed in Agrawal, Tibtech
x,_0:152-158 (1992) . Viral nucleic acid sequences that are
complementary to effective antisense oligonucleotides
have been described for many viruses, including human .
immunodeficiency virus type 1 (U.S. Patent No. 4,806,463,
the teachings of which are herein incorporated by
reference), Herpes simplex virus (U.S. Patent No. w
4, 689, 320, the teachings of which are hereby incorporated
by reference), Influenza virus (U.S. Patent No.
S,XXX,XXX; Ser. No. 07/516,275, allowed June 30, 1992;
the teachings of which are hereby incorporated by
reference), and Human papilloma virus (Storey et al.,
Nucleic Acids Res. 19:4109-4114 (1991)). Sequences
complementary to any of these nucleic acid sequences can
be used for oligonucleotides according to the invention,
as can be oligonucleotide sequences complementary to
nucleic acid sequences from any other virus. Additional
viruses that have known nucleic acid sequences against
which antisense oligonucleotides can be prepared include
Foot and'Mvuth Disease Virus (S~e_ Robertson et ah., J.
Virology ~,: 651 (1985)' Harris et al., J. Virology 36: .
659 (1980)), Yellow Fever Virus (~ge_ Rice et al., Science
2,~,Q: 726 (1985) ) , Varicella-Zoster Virus (See Davison and
Scott, J. Gen. Virology 67: 2279 (1986), and Cucumber
CA 02490644 1993-07-22
X140649 ~.::::~~.~~:4~;..~ .; ,
WO 94/02498 PCT/US93/06884 .:~
_14_ ,
Mosaic Virus (See Richards et al., Virology 89: 395
(197$) )..
Alternatively, oligonucleotides according to the
invention can have an oligonucleotide sequence
complementary to a nucleic acid sequence of a pathogenic . -.
organism. The nucleic acid sequences of many pathogenic
organisms have been described, including the malaria
organism, Plasmndi,um falciparq~m, and many pathogenic
bacteria. Oligonucleotide sequences complementary to
nucleic acid sequences from any such pathogenic organism
can be used in oligonucleotides according to the
invention. Examples of pathogenic eukaryotes having
known nucleic acid sequences against which antisense
oligonucleotides can be prepared include Try,~~a~posoma
~~ cei qambiense and ~eishmania (See Campbell et al.,
Nature ~,1: 350 (1984) ) , ,~asciola hegatiga ( ee Zurita et
al., Proc. Natl. Acad. Sci. USA 8~: 2340 (1987).
Antifungal .oligonucleotides can be prepared using a
target hybridizing region having an oligonucleotide
sequence that is complementary to a nucleic acid sequence
from, era. , the chitin synthetase gene, and antibacterial
oligonucleotides can be prepared using, e.a., the alanine ,_
racemase gene.
Tn yet another embodiment, the oligonucleotides
according to the invention can have an oligonucleotide
sequence complementary to a cellular gene or gene
transcript, the abnormal expression or product of which
results in a disease state. The nucleic acid sequences
:;.:
of several such cellular genes have been described, .
~ ' including prior protein (Stahl and Prusiner, FASEH 'J. 5_: ~ ,
2799-2807 (1991)), the amyloid-like protein associated
with Alzheimer's disease (U.S..Patent No. 5,015,570, the
teachings of which are hereby incorporated by reference) , .
and various well-known oncogenes and proto-oncogenes,
such as c-mob,, c-mvc, c-~, and n-xas. In addition, ..
.'
.:::. :.. . .,. , :.: ., .. . ::.:... . ~. :. : . ~ ~:~. .: _ .;:, :_ _ ...
::. .... .~. .. . :, .:,.: e. .. :' , :..:: :. .: .: r:. .~:. : .:::. _ ::. .
CA 02490644 1993-07-22
'~ ;. WO 94/02498 ~ 214' 0 ~ 4 9 _: ; , ~ ; : ~; ; ;
. ._ .~ PGT/US93/06884
-15-
oligonucleotides that inhibit the synthesis of structural
proteins or enzymes involved largely or exclusively in .
spermatogenesis, sperm motility, the binding of the sperm
to the egg or-any other step affecting sperm viability
may be used as contraceptives for men. Similarly,
contraceptives for women may be oligonucleotides that
inhibit proteins or enzymes involved in ovulation,
fertilization, implantation or in the biosynthesis of
hormones involved in those processes.
ZO Hypertension can be controlled by
oligodeoxynucleotides that suppress the synthesis of
angiotensin converting enzyme or related enzymes in the
renin/angiotensin system; platelet aggregation can be
controlled by suppression of the synthesis of enzymes
necessary for the synthesis of thromboxane A2 for use in
myocardial and cerebral circulatory disorders, infarcts,
arteriosclerosis, embolism and thrombosis; deposition of
cholesterol in arterial wall can be inhibited by
suppression of the synthesis of fattyacryl co-enzyme A:
cholesterol aryl transferase in arteriosclerosis;
inhibition of the synthesis of cholinephosphotransferase
may be useful in hypolipidemia. ~
There are numerous neural disorders in which
hybridization arrest can be used to reduce or eliminate
adverse effects of the disorder. For example,
suppression of the synthesis of monoamine oxidase can be
used in Parkinson°s disease; suppression of catechol
o-methyl transferase can be used to treat depression; and .
suppression of indole N-methyl transferase can be used in
treating schizophrenia.
Suppression of selected enzymes. in the arachidonic
acid cascade which leads to prostaglandins and
leukotrienes ~ may be useful in the control of platelet
aggregation, allergy, inflammation, pain and asthma.
Suppression of the protein expressed by the
multidrug resistance (mdr) gene, which is responsible for
CA 02490644 1993-07-22
WO 94!02498 ~ 1 ~ ~ ~ ~ , ~, . ~ . PCT/US93/06884 .
..
-16-
development of resistance to a variety of anti-cancer
drugs and is a major impediment in chemotherapy may prove
to be beneficial in the treatment of cancer.
Oligonucleotide sequences complementary to nucleic acid .
sequences from any of these genes can be used for
oligonucleotides according~to the invention, as can be ,
oligonucleotide sequences complementary to any other .
cellular gene or gene transcript, the abnormal expression
or product of which results in a disease state.
Antisense regulation of gene expression in plant
cells has been described in U.S. Patent No. 5,107,065,
the teachings of which are hereby incorporated by .
reference.
Tn a third aspect, the invention provides
therapeutic pharmaceutical formulations of
oligonucleotides that are effective for treating virus .,~~
infection, infections by pathogenic organisms, or disease
resulting from abnormal gene expression or from the
expression of an abnormal gene product. Such therapeutic
pharmaceutical formulations comprise the oligonucleotides
according to the second aspect~af the invention in a
pharmaceutically acceptable carrier.
In a fourth aspect, the invention provides a method
for inhibiting the gene expression of a virus, a
pathogenic organism or a cellular gene, the method
comprising the step of providing oligonucleotides
according to the invention to cells infected with the
virus or pathogenic organism in the former two cases or
to cells generally in the latter case. Such methods are
useful in studying gene expression and the function of
specific genes.
In a fifth aspect, the invention provides a method
of treating a diseased human or animal in which the
CA 02490644 1993-07-22
214064-9:..5~- . -
'' ~: WO 94/02498 - .. ... p~/US93106884
~'.~ J
17 --
disease results from infection with a virus or pathogenic
organism, or from the abnormal expression or product of
a cellular gene. ~ The method comprises administering
therapeutic pharmaceutical formulations of
oligonucleotides according to the invention to the _
diseased human or animal. Preferably, the routes of such
administration will include oral, intranasal, rectal and
togical administration. In such methods of treatment
according to the invention the oligonucleotides may be
1a administered in conjunction with other therapeutic
agents, e,~g_, AZT in the case of AIDS.
A variety of viral diseases may be treated by the
method of treatment according to the invention, including
AIDS, ARC, oral or genital.herpes, papilloma warts, flu,
foot and mouth disease, yellow fever, chicken pox,
shingles, HTLV-leukemia, and hepatitis. ~ Among fungal
diseases treatable by the method of treatment according
to the invention are candidiasis, histoplasmosis,
cryptococcocis, blastomycosis, aspergillosis,
2o sporotrichosis, chromomycosis, dermatophytosis and
coccidioidomycosis. The method can also be used to treat
rickettsial diseases (ea., txphus, Rocky Mountain
spotted fever), as well as sexually transmitted diseases
caused by C~,ar~,yd'~,a tr~,chomatis or ~ymphoara~uloma
veDe3~ua~. A variety of parasitic diseases can be treated
by the method according to the invention, including
amebiasis, Chegas~ disease, toxoplasmosis,
pneumocystosis, giardiasis; cryptosporidiosis,
trichomoniasis, and Pneumocvstis carini pneumonia; also
worm (helminthic diseases) such as ascariasis,
'.filariasis, trichinosis, schistosomiasis and nematode or
cestode infections. Malaria can be treated by the method
of treatment.of the invention regardless of whether it is
caused by ,~ . f~;,, ci aR rum, P. yivax, P. o ale, or P.
malarial.
CA 02490644 1993-07-22
WO 94/02498 214 0 6 ~ ~ ~~ ~ ' ° . ~ pCT/US93/OG884 k~,~ . .
_18_ , % r
The infectious diseases identified above can all be
treated by the method of treatment according to the
invention because the in,Fectious agents for these
diseases are known and th~s,':oligonucleotides according to .
the invention can be prepared, having oligonucleotide
sequence that is complementary to a nucleic acid sequence ~ ,
that is an essential nucleic acid sequence for the
propagation of the infectious agent, such as an essential ' -~
gene.
other disease states or conditions that are
treatable by the method according to the invention result
from an abnormal expression or product of a cellular
_ gene. These conditions can be treated by administration
of oligonucleotides according to the invention., and have
been discussed earlier in this disclosure.
Oligonucleotides according to the invention can be
synthesized by procedures that are well known in the art.
Alternatively, and preferably such oligonucleotides can
be synthesized by the H-phosphonate approach described in .
U.S. Patent No, 5,XXX,XXX (Ser. No. 07/334,679; allowed
an March 10, 1992), the teachings of which are hereby
incorporated by reference, and in Agrawal and Tang, ,
Tetrahedron Lett. _3~: 7541-7544 (1990). Oligonucleotides
according to the invention can be made even more
resistant to nucleolytic degradation through the addition
of cap structures at the 5~ and/or 3r end.
The following examples are intended to further
illustrate certain preferred embodiments of the invention .
'v'' ~ and are'not intended to be limiting,in nature. '
i
r.
CA 02490644 1993-07-22
~~.; W
E,~~fi,~ O 94/02498 , ~~ ~ ~ ~ s 4 9 = . : : ., .; ,, :~:
PGT/US93/06884
-19-
Exan~gle Z
8y~t"ha~~s o~ Rybrid Oligonueleotide Phosp,~orothioates .
Hybrid oligonucleptide phosphorothioates were
Synthesized .on CPG on a 5-6 ,umole scale on an automated
synthesizer (model 8700, Millipore, Milford, MA) using
the H-phosphonate approach described in U.S. Patent No.
. S,XXX,XXX (Ser. No. 07/344,679; allowed on March 19,
1992). Deoxynucleoside H-phosphonates were obtained from
Millipore. 2'-OMe ribonucleotide H-phosphonates were
l0 synthesized by standard procedures. Segments of
oligonucleotides containing 2'-OMe nucleoside were
assembled by using 2~-OMe ribonucleoside H-phosphonates
for the desired cycles. Similarly, segments of
oligonucleotides containing deoxyribonucleosides were
~ assembled by using deoxynucleoside H-phosphonates for the
desired cycles. After assembly, CPG bound
oligonucleotide~ H-phasphcinate was oxidized with sulfur to
generate the phosphorothioate linkage. Oligonucleotides
were then deprotected in concentrated NH40H at 4 0C f or 4 8
hours.
Crude oligonucleotide (about,~l2~ units) was analyzed
an reverse low pressure chromatography on a Ci8 reversed
phase medium. The DMT group was removed by treatment '
with 80% aqueous acetic acid, then the oligonucleotides
were dialyzed against distilled water and lyophilized.
The oligonucleotides synthesized are shown in Table
Ix, below.
,. r ,
. . ..
~..
~.
. i -:
i :.
CA 02490644 1993-07-22
WO 94/OZ498 ~ ~ ~ ~ ~ ~ ~ . , . . 1 PCT/US93/06884
w
-2 0-
TABLE II I
I E T TESL' YNTHE ED
s--
Oligo ~.~.. ,Structure
A 'A C A C C C A A cvT' T C T G A A A A
T G G',
B A C A C C C A A T T C U G A A A A U G '
G
C A C A C C C A A T T C T G A A A A U G
G
D ~ A C C ~__A_ A T T C t~ G A A A A ~ G
E A ~ A t'~C C A A U T C T G A A A A T G ,
G
F A~A.('~~"s;AA UCUGAA.~,~,UGG
Underlined sequences~coataln 2'-OMe ribonucleoside.
* All intemudeotide linkages are phosphorothioate linkages for oligos A-G.
F,,~aal,Pls 2 ..
Relative Nuclease Resistance of
Bybrid Ol~gQ,nualeot~.de plhosvphorothioates
To test the relative nuclease resistance of various
hybrid oligonucleotide phosphorothioates, the
aligonucleotides were treated with snake venom
phosphodiesterase (SVPD). About 0.2 Az~ units of oligos
A, C and F were dissolved in 500u1 buffer (40mM NHaC03, pH
0.4 + 20mM MgClz) and mixed with units SvPD. The
mixture was incubated at 37°C for 420 minutes. After o, ..
200 and 420 minutes, 165~c1 aliquots were removed and
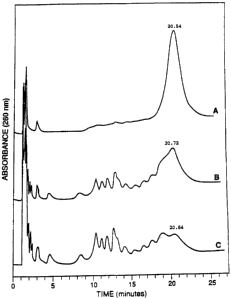
' ' analyzed- using ion exchange HPLC. The: results are shown .
in Figure 1. Oligonucleotide F was very resistant to
phosphodiesterase, whereas oligonucleotide A was digested , ,
almost to completion and oligonucleotide C was digested
,.
to 50% (panel A). An oligonucleotide phosphodiester was , ;.~.
.:.
. _ ,..:n.~ .:.:::-4,~...v ::.: :~..~~ ~':._ 'Y..~~ ' "~.... .::~.. .: . .
:..;. , . ;..... , ..;.. ..~.,. ~~.. : .. ...
CA 02490644 1993-07-22
. . _. ._ s
~~;~~ WO 94/02498 ~ ~ v ~ ~ ~ ~ PGT/US93/06884
i
-21-
digested to about 80% in one minute using one tenth of
the concentration of SVPD.
These results~indicate that the presence of 2'-OMe
ribonucleosides in an oligonucleotide phosphorothioate
enhances resistance to exonucleolytic digestion and that
this enhanced resistance. increased when a larger
proportion of 2'-OMe ribonucleotides are used. Due to
the similar character and behavior of ribonucleotides, w
other 2'-substituted ribonucleotides and 2'-OMe ..
ribonucleotides, these results also suggest that similar ..
enhancement of nuclease resistance would be obtained for
hybrid oligonucleotide phosphorothioates and/or
phosphorodithioates having ribonucleotides, 2'-
substituted ribonucleotides, or a mixture of
ribonucleotides and 2'-substituted ribonucleotides.
~samyle 3
Relative Duplex 8tsbility of "
Hybr~.d Oliqonualeatide Phosnhorothioates
Oligonucleotides A-F were tasted for their relative
stability of duplexes formed' with complementary
oligodeoxyribonucleatides, and with complementary
oligoribonucleotides. In separate reactions, each
oligonucleotide A-F was mixed with an equivalent quantity
(0..2 A~'units) of Sts complementary oligonucleotide in
150 mM NaCl, lOmM Na2P04, lmM EDTA, pH 7. The mixture was
heated to 85C for 5 minutes, then cooled o 30C. The
temperature was then increased from 30C to 80C at a rate
., .
.
. ..:
_.,,.
,;,, , r iof ' 1C per minute and Az~ was recorded :as a function
~ of
temperature. The results are shown in Table III, below. i':
:3 .;:..,,
iv
.,
T
.. ru- rrr-r-... ., ~. , s, . d
.. . ..-_
.-.e..-- _. .~ . , ~. _ ., ";1.__:: ., .:,.. .:,"":... , . ~ ':: :._;..
..-.f ., ..;. ~. r- , ~., ,. .:_v .~., ~ ,.-- . : :...,. ..-:: . .
. . r.. . ... - !_.. _.~.:, , -,'::
CA 02490644 1993-07-22
214064 :,: - ~:..:. .
WO 94102498
,. .
PCT/US93/06884
8 $ 's v~~~
j' ~'
. ~1 .. o,.r ~
~ ., ~o 0oeo ,;,
H s ~ ~ .
3
~' e, V ~ h h N ~
v . , .. . ..
l
od .
.
.
I yp O COA V! .
h ~' V1M M
H
a
a
,
. .
p '9 ~l 'R~'.~ ..
_ 3 ~ o < n ~n n e. ~"
.
t~ $ ~ t~ 8
~ a
t
v, o; v. a ..
v~ v v~v~ w '
H V
s
U ~ o~
H ~ 1!!N1
p ~ ..
F ..
x
A
~ r~ o~ ..~y ~o
Q a oo a '~~ s
M 1 V
s, ,.
f0 V 'DW 4.
z
. ,
.
~
t
i
3
i
CA 02490644 1993-07-22
j )
~~n~ WO
94/02498
~ ~ .
~ ~ .PCT/US93/06884
-23- ,
These results reveal that when the complementary ,
oligonucleotide is an oligoribonucleotide, the presence of
2'-OMe ribonucleotides enhances duplex stability, and that
this enhancement increases with increased proportions of
2'-OMe ribonucleosides. These results should be similarly
applicable to hybrid oligonucleotide phosphorothioates
and/or phosphorodithioates containing ribanucleotides, 2~-
substituted ribonucleotides, or~mixtures of ribonucleotides
and 2'-substituted ribonucleotides. Thus; the hybrid
oligonucleotide phosphorothioates and/or
phosphorodithioates according to the invention should bind
viral RNA or virus, pathogenic organism or cellular mRNA
with greater affinity than ordinary oligodeoxynucleotide
phosphorothioates.
F~ampie 4
Activation of RNase H by
Hybrid ol3~,qg~,cieotide T~hosphorothioates
Oligonucleotide phosphorothioates and various hybrid
oligonucleotide phosphorothioates were studied fox their
RNase H activation properties. Oligonucleotide A. (Table
II), an oligonucleotide phosphorothi"bate which is known to
activate RNase H, was used as a control. Oligonucleotide
F (a.2'-OMe analog of oligonualeotide phosphorothioate) and r
:::.;
oligonucleotides C, B, and E, hybrid oligonucleotides, were
studied for their ability to activate RNase H.
To carry out the experiment, a complementary 32-mer
oligoribonuclevtide was synthesized (Figure 2) and kinased
32P-labeled 32-mer RNA ( 0 . 003 Az~ units; 0 , 01
at the 5' -end
,
,;, , .; ,~cg~) :and oli~gonucleotides (0.4635 A2~ units; ~1.9, fig) ~ were,
.
mixed in the 24 ~1 of buffer (0.15 M NaCl, O.OW MgCl2, o.ol
M Tris chloride, pH 7.9, containing 0.001 M DTT. The
mixture was incubated with 6 units of RNase H (E. Coli) at
37C. Aliquots of 4.f ul were removed at 0, 15, 30, and 60
minutes and analyzed on polyacrylamide gel electrophoresis. -
,v,~,,w r r . ~ r r. ' ~r .. r: .-
' w~~u.. At..nw, n aT~,., ~--'a l . ~ .. , r ~.:-
no . 1., ..... ....,.:mr:..,.:-,~ ~..e..::.~ ~~ .:;'::n ~. ." ,~ r
'~N. .ICY.:..:V'. . ., . V.,r,.'- ._.5..' S
, .. ~.n.i.o'' ... ... . . '. ,._... , ..:. p .
21054.0
CA 02490644 1993-07-22
2140G4~~ - ~ v
WO 94/02498 . . ~PGT/US93/06884 ~.
-24-
Oligonucleotide A (Duplex A) showed site specific
cleavage of RNA by RNase H. ~Oligonucleotide F (2'-oMe
analog; Duplex B) showed no.cheavage of RNA in presence of ;
RNase H. Hybrid oli.gonuclei?~tide B, C, and E (Duplexes C, . '
;,
D, E, resp.) showed site specific cleavage of RNA by RNase
H. Duplex F, in which a mismatched oligonucleotide .
phosphorothioate was studied showed no cleavage of RNA.
Lane G shows that in presence of RNase H, RNA was not
cleaved.
1o FLxamole 5 w:
Inhibition of HIV by
Hybrid oligpnuclevtide Phostahorothioates .
Hybrid oligonucleotide phosphorothioates were tested
for their ability to :inhibit HIV-1 in tissue culture. H9
lymphocytes were infected with HZV-1 virions 00.01 - 0.1
TCID~/cell) for one hour at 37°C. After one hour,
unadsorbed virions were washed and the infected cells were
divided among wells of 24 well plates. To the infected
cells, an appropriate concentration (from stock solution)
of oligonucleotide was added to obtain the required
concentration in 2 ml medium. ~n a positive control
experiment ddC or AZT was added. The cells were then
cultured for three days. At the end of three days,
supernatant from the infected culture was collected and
measured fox p24 expression by ELISA. The level of
expression of -24 was compared between oligonucleotide
treated and untreated (no drug) infected cells.
r. ,- ~'~ All of the hybrid oligonucleotide phosphorothioates
tested showed significant inhibition of p24 expression at
~tg/ml concentrations, without significant cytotoxicity ,
(data not shown). ~ These results indicate that hybrid
oligonucleotide phosphorothioates containing 2'-oMe , ,
ribonucleotides are effective as inhibitors of gene
CA 02490644 1993-07-22
Image