Note: Descriptions are shown in the official language in which they were submitted.
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
SYSTEMS AND METHODS FOR INVESTIGATING
INTRACRANIAL PRESSURE
CROSS REFERENCE TO RELATED APPLICATIONS
This application is a continuation-in-part application claiming priority to co-
pending, commonly assigned United States Patent Application No. 09/966,367
filed on
October 1, 2001 and also claims priority to United States Provisional
Application No.
60/381, 789, filed May 21, 2002 and United States Provisional Application No.
60/384,819,
filed June 4, 2002. The above applications are expressly incorporated herein
by reference in
their entirety.
BACKGROUND OF THE INVENTION
Technical Field. The present invention relates generally to systems and
methods for
assessing vascular health and for assessing the effects of treatments, risk
factors and
substances, including therapeutic substances, on blood vessels, especially
cerebral blood
vessels, all achieved by measuring various parameters of blood flow in one or
more vessels
and analyzing the results in a defined matter. In addition, the present
invention further
pertains to collecting, analyzing, and using the measurement of various
parameters of blood
flow in one or more vessels to establish protocols for and to monitor clinical
trials. Further,
the present invention relates to an automated decision support system for
interpreting the
values of various parameters of blood flow in one or more vessels in assessing
the vascular
health of an individual.
Background Information. Proper functioning of the vascular system is essential
for
the health and fitness of living organisms. The vascular system carries
essential nutrients
and blood gases to all living tissues and removes waste products for
excretion. The
vasculature is divided into different regions depending on the organ systems
served. If
vessels feeding a specific organ or group of organs are compromised, the
organs and tissues
supplied by those vessels are deleteriously affected and may even fail
completely.
Vessels, especially various types of arteries, not only transmit fluid to
various
locations, but are also active in responding to pressure changes during the
cardiac cycle.
With each contraction of the left ventricle of the heart during systole, blood
is pumped
through the aorta and then distributed throughout the body. Many arteries
contain elastic
membranes in their walls which assist in expansion of the vessel during
systole. These
elastic membranes also function in smoothing pulsatile blood flow throughout
the vascular
system. The vessel walls of such arteries often rebound following passage of
the systolic
pressure waveform.
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
In auto-regulation, cerebral blood vessels maintain constant cerebral blood
flow by
either constricting or dilating over a certain mean arterial blood pressure
range so that
constant oxygen delivery is maintained to the brain. Vascular failure occurs
when the
pressure drops too low and the velocity starts to fall. If the blood pressure
gets too high
and the vessels can no longer constrict to limit flow, then breakthrough,
hyperemia
breakthrough, and loss of auto-regulation occur. Both of these conditions are
pathologic
states, and have been described in the literature in terms of mean arterial
pressure and
cerebral blood flow velocity. But there are outliers that could not be
explained based on
that model. The failure of the model is that it relies upon systemic blood
pressure; the
pressure of blood in the brain itself is not being measured directly. The
resultant pressure
curve has an S-shaped curve.
The force applied to the blood from each heart beat is what drives it forward.
In
physics, force is equivalent to mass times acceleration. But when blood is
examined on a
beat to beat variation, each heartbeat delivers about the same mass of blood,
unless there
is severe loss of blood or a very irregular heart rhythm. Therefore, as a
first
approximation, the force of flow on the blood at that particular moment is
directly
proportional to its acceleration.
Diseased blood vessels lose the ability to stretch. The elasticity or stretch
of the
blood vessel is very critical to maintaining pulsatile flow. When a muscle is
stretched, it
is not a passive relaxation. There is a chemical reaction that happens within
the muscle
itself that causes a micro-contracture to increase the constriction, so that
when a bolus of
blood comes through with each heartbeat, it stretches the blood vessel wall,
but the blood
vessel then contracts back and gives the kick forward to maintain flow over
such a large
surface area with the relatively small organ of the heart. This generates a
ripple of waves,
starting in the large vessel of the aorta and working its way through the rest
of the vessels.
As vessels become diseased, they lose the ability to maintain this type of
pulsatile flow.
Further, if vessels are compromised due to various factors such as narrowing
or
stenosis of the vessel lumen, blood flow becomes abnormal. If narrowing of a
vessel is
extensive, turbulent flow may occur at the stenosis resulting in damage to the
vessel. In
addition, blood may not flow adequately past the point of stenosis, thereby
injuring tissues
distal to the stenosis. While such vascular injuries may occur anywhere
throughout the
body, the coronary and cerebral vascular beds are of supreme importance for
survival and
well-being of the organism. Narrowing of the coronary vessels supplying the
heart may
2
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
decrease cardiovascular function and decrease blood flow to the myocardium,
leading to a
heart attack. Such episodes may result in significant reduction in cardiac
function and
death.
Abnormalities in the cerebral vessels may prevent adequate blood flow to
neural
tissue, resulting in transient ischemic attacks (TIAs), migraines and stroke.
The blood
vessels which supply the brain are derived from the internal carotid arteries
and the
vertebral arteries. These vessels and their branches anastomose through the
great arterial
circle, also known as the Circle of Willis. From this circle arise the
anterior, middle and
posterior cerebral arteries. Other arteries such as the anterior communicating
artery and
the posterior communicating artery provide routes of collateral flow through
the great
arterial circle. The vertebral arteries join to form the basilar artery, which
itself supplies
arterial branches to the cerebellum, brain stem and other brain regions. A
blockage of
blood flow within the anterior cerebral artery, the posterior cerebral artery,
the middle
cerebral artery, or any of the other arteries distal to the great arterior
circle results in
compromised blood flow to the neural tissue supplied by that artery. Since
neural tissue
cannot survive without normal, constant levels of glucose and oxygen within
the blood
and provided to neurons by glial cells, blockage of blood flow in any of these
vessels
leads to death of the nervous tissue supplied by that vessel.
Strokes result from blockage of blood flow in cerebral vessels due to
constriction
of the vessel resulting from an embolus or stenosis. Strokes may also arise
from tearing
of the vessel wall due to any number of circumstances. Accordingly, a blockage
may
result in ischemic stroke depriving neural tissue distal to the blockage of
oxygen and
glucose. A tearing or rupture of the vessel may result in bleeding into the
brain, also
known as a hemorrhagic stroke. Intracranial bleeding exerts deleterious
effects on
surrounding tissue due to increased intracranial pressure and direct exposure
of neurons to
blood.
Regardless of the cause, stroke is a major cause of illness and death. Stroke
is the
leading cause of death in women and kills more women than breast cancer.
Currently,
more than three quarters of a million people in the United States experience a
stroke each
year, and more than 25 percent of these individuals die. Approximately one-
third of
individuals suffering their first stroke die within the following year.
Furthermore, about
one-third of all survivors of a first stroke experience additional strokes
within the next
three years.
3
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
In addition to its terminal aspect, stroke is a leading cause of disability in
the adult
population. Such disability can lead to permanent impairment and decreased
function in
any part of the body. Paralysis of various muscle groups innervated by neurons
affected
by the stroke can lead to confinement to a wheel chair, and muscular
spasticity and
rigidity. Strokes leave many patients with no ability to communicate either
orally or by
written means. Often, stroke patients are unable to think clearly and have
difficulties
naming objects, interacting with other individuals, and generally operating in
society.
Strokes also result in massive expenditures of resources throughout society,
and
place a tremendous economic burden on affected individuals and their families.
It is
estimated that the annual total costs in the United States economy alone is
over $30 billion
per year, with the average acute care stroke treatment costing approximately
$35,000. As
the population increases in age, the incidence of stroke will rise
dramatically. In fact, the
risk of stroke doubles with ever succeeding decade of life. Since the life
expectancy of
the population has increased dramatically during the last 100 years, the
number of
individuals over 50 years old has risen precipitously. In this population of
individuals
living to ages never before expected, the potential for stroke is very high
indeed.
Accordingly, the financial and emotional impact of cerebral vascular damage is
expected
to dramatically increase during the next several decades.
Despite the tremendous risk of stroke, there are presently no convenient and
accurate methods to access vascular health. Many methods rely on invasive
procedures,
such as arteriograms, to determine whether vascular stenosis is occurring.
These invasive
techniques are often not ordered until the patient becomes symptomatic. For
example,
carotid arteriograms may be ordered following a physical examination pursuant
to the
appearance of a clinical symptom. Performing an arteriogram is not without
risks due to
introducing dye materials into the vascular system that may cause allergic
responses.
Arteriograms also use catheters that can damage the vascular wall and dislodge
intraluminal plaque, which can cause an embolic stroke at a downstream site.
Many methods and devices available for imaging cerebral vessels do not provide
a
dynamic assessment of vascular health. Instead, these imaging procedures and
equipment
merely provide a snapshot or static image of a vessel at a particular point in
time.
Cerebral angiography is conventionally held to be the "gold standard" of
analyzing blood
flow to the brain. But this invasive method of analysis only provides the
shape of the
vessels in an imaging modality. To obtain the same type of flow criteria from
an
4
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
angiogram as one obtains from the present invention would entail extraordinary
efforts
and multiple dangerous procedures.
Instruments have been developed to obtain noninvasive measurements of blood
velocity in anterior arteries and veins using Doppler principles. In
accordance with
known Doppler phenomenon, these instruments provide an observer in motion
relative to
a wave source a wave from the source that has a frequency different from the
frequency of
the wave at the source. If the source is moving toward the observer, a higher
frequency
wave is received by the observer. Conversely, if the wave source is moving
away from
the observer, a lower frequency wave is received. The difference between the
emitted and
received frequencies is known as the Doppler shift. This Doppler technique may
be
accomplished through the use of ultrasound energy.
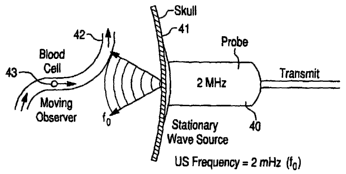
The operation of such instruments in accordance with the Doppler principle may
be illustrated with respect to Figures I to 4. In Figure 1, the ultrasound
probe 40 acts as a
stationary wave source, emitting pulsed ultrasound at a frequency of, e.g., 2
MHz. This
ultrasound is transmitted through the skull 4I and brain parenchyma to a blood
vessel 42.
For purposes of illustration, a blood cell 43 is shown moving toward the probe
and acts as
a moving observer. As illustrated in Figure 2, the blood cell reflects the
pulse of
ultrasound and can be considered a moving wave source. The probe receives this
reflected ultrasound, acting as a stationary observer. The frequency of the
ultrasound
received by the probe, fl is higher than the frequency, fo, originally
emitted. The Doppler
shift of the received wave can then be calculated. Figures 3 and 4 show the
effect on a
pulse of ultrasound when blood flows in a direction away from the probe. In
this case, the
received frequency, f2, reflected from the blood cell, is lower than the
emitted frequency
fo. Again, the Doppler shift can be calculated.
The Doppler effect can be used to determine the velocity of blood flow in the
cerebral arteries. For this purpose, the Doppler equation used is the
following:
2 Ft Y cos O
d- v
0
where
Fd = Doppler frequency shift
F~ = Frequency of the transmitter
V = Velocity of blood flow
O = Angle of incidence between the probe and the artery
5
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
Vo = Velocity of ultrasound in body tissue
Typically, FL is a constant, e.g., 2, 4 or 8 MHz, and Vo is approximately 1540
meters second (m/s) in sob body tissue. Assuming that there is a zero angle of
incidence
between the probe and the artery, the value of cos O is equal to 1. The effect
of the angle
O is only significant for angles of incidence exceeding 30°.
In exemplary instruments, ultrasonic energy is provided in bursts at a pulse
repetition rate or frequency. The probe receives the echoes from each burst
and converts
the sound energy to an electrical signal. To obtain signal data corresponding
to reflections
occurring at a specific depth (range) within the head, an electronic gate
opens to receive
the reflected signal at a selected time after the excitation pulse,
corresponding to the
expected time of arrival of an echo from a position at the selected depth. The
range
resolution is generally limited by the bandwidth of the various components of
the
instrument and the length of the burst. The bandwidth can be reduced by
filtering the
received signal, but at the cost of an increased length of sample volume.
Other body movements, for example, vessel wall contractions, can also scatter
ultrasound, which will be detected as "noise" in the Doppler signal. To reduce
this noise
interference, a high pass filter is used to reduce the low frequency, high
amplitude signals.
The high pass filter typically can be adjusted to have a passband above a
cutoff frequency
selectable between, e.g., about 0 and about 488 Hz.
Many health care providers rarely have such flow diagnostic capabilities at
their
disposal. For example, health care providers may be situated in remote
locations such as
in rural areas, on the ocean or in a battlefield situation. These health care
providers need
access to analytical capabilities for analysis of flow data generated at the
remote location.
Health care providers facing these geographic impediments are limited in
their ability to provide the high quality medical services needed for their
patients,
especially on an emergency basis. Further, both physicians and individuals
concerned for
their own health are often limited in their ability to consult with
specialists in specific
medical disciplines. Accordingly, a system that facilitates access of
physicians in various
locations to sophisticated medical diagnostic and prognostic capabilities
concerning
vascular health is needed. Such access would promote delivery of higher
quality health
care to individuals located throughout the country, especially in remote areas
removed
from major medical centers.
6
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
'There is also a need for a system whereby patient vascular data can be
transmitted
to a central receiving facility, which receives the data, analyzes it,
produces a value
indicative of the state of vascular health, and then transmit this information
to another
location, such as the originating data transmitting station, or perhaps
directly to the health
care provider's office. This system should provide access to sophisticated
computing
capabilities that would enhance the accuracy of health care providers'
diagnostic and
prognostic capabilities concerning vascular health. This system should be able
to receive
high volumes of patient data and rapidly process the data in order to obtain
diagnoses and
prognoses of disease. Such a system could be used for diagnosis and prognosis
of any
disease or condition related to vascular health.
There is a further need for a system that facilitates the ability of a health
care
provider to conveniently and rapidly transmit vascular flow data parameters
obtained from
a patient to a location where consistent, reproducible analysis is performed.
The results of
the analysis can then be transmitted to the health care provider to facilitate
accurate
diagnosis or prognosis of a patient, to recommend treatment options, and to
discuss the
ramifications of those treatment options with the patient.
There is also a need for a system that enables health care providers to
measure the
rate and type of developing vascular disease, and to recommend interventions
that
prevent, minimize, stabilize or reverse the disease.
There is a further need for a system that enables health care providers to
predict
the vascular reaction to a proposed therapeutic intervention, and to modify
the proposed
therapeutic intervention if a deleterious or adverse vascular response is
anticipated.
Physicians often prescribe therapeutic substances for patients with conditions
related to
the cardiovascular system that may affect vascular health. For example,
hypertensive
patients may be prescribed beta-blockers with the intent of lowering blood
pressure,
thereby decreasing the probability of a heart attack. Patients frequently
receive more than
one therapeutic substance for their condition or conditions. The potential
interaction of
therapeutic substances at a variety of biological targets, such as blood
vessels, is often
poorly understood. Therefore, a non-invasive method that can be used to assess
the
vascular effects of a substance, such as a therapeutic substance, or a
combination of
therapeutic substances is needed. A clear understanding of the vascular
effects of one or
more substances on blood vessels may prevent prescriptions of substances with
undesirable and potentially lethal effects, such as stroke, vasospasm and
heart attack.
7
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
Accordingly, what is needed is a system and method that can be used for
repeated
assessment without deleterious effects of potential vascular effects of a
substance, or
combination of substances, in a patient population during a clinical trial.
Such clinical
studies may also reveal dosages of individual substances and combinations of
substances
at specific dosages that provide desirable and unexpected effects on blood
vessels.
Furthermore, a system and method that can provide an assessment of the
vascular
health of an individual is needed. Also needed is a system and method that may
be used
routinely to assess vascular health, such as during periodic physical
examinations. This
system and method preferably is non-invasive and provides information
concerning the
compliance and elasticity of a vessel. Also needed is a system and method that
may be
used to rapidly assess the vascular health of an individual. Such systems and
methods
should be available for use in routine physical examinations, and especially
in the
emergency room, intensive care unit or in neurological clinic. What is also
needed is a
system and method which can be applied in a longitudinal manner for each
individual so
that the vascular health of the individual may be assessed over time. In this
manner, a
problem or a disease process may be detected before the appearance of a major
cerebral
vascular accident or stroke.
In addition, there is a need for a system and method for assessing whether
treatments, risk factors and substances affect blood vessels, particularly
cerebral blood
vessels, so that their potential for causing vascular responses may be
determined. By
determining the vascular effects of treatments, risk factors and substances,
physicians may
recommend that a patient avoid the treatment, risk factor and/or substance.
Alternatively,
desirable vascular effects of a treatment, therapeutic intervention and/or
substance may
result in administration of the treatment, therapeutic intervention and/or
substance to
obtain a desired effect.
In addition, there is also needed a system and method for assessing the
efficacy of
a treatment, including conducting a procedure, carrying out a therapy, and
administering a
pharmaceutical substance, in treating vascular disorders, so that
identification of those
treatments most efficacious in the treatment of vascular disorders can be
determined and
employed to restore vascular health.
As required by federal regulations, treatments, including drugs and other
therapies
intended for treating individuals, have to be tested in people. These tests,
called clinical
trials, provide a variety of information regarding the efficacy of treatment,
such as
8
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
whether it is safe and effective, at what doses it works best, and what side
effects it
causes. This information guides health professionals and, for nonprescription
drugs,
consumers in the proper use of medicines. In controlled clinical trials,
results observed in
patients being administered a treatment are compared to results from similar
patients
receiving a different treatment such as a placebo or no treatment at all.
Controlled clinical
trials are the only legal basis for the United States Food and Drug
Administration
("FDA") in determining that a new treatment provides "substantial evidence of
effectiveness, as well as confirmation of relative safety in terms of the risk-
to-benefit ratio
for the disease that is to be treated."
It is important to test drugs, therapies, and procedures in those individuals
that the
treatments are intended to help. It is also important to design clinical
studies that ask and
answer the right questions about investigational treatment. Before clinical
testing is
initiated, researchers analyze a treatment's main physical and chemical
properties in the
laboratory and study its pharmacological and toxic effects on laboratory
animals. If the
results from the laboratory research and animal studies show promise, the
treatment
sponsor can apply to the FDA to begin testing in people. Once the FDA has
reviewed the
sponsor's plans and a local institutional review board - typically a panel of
scientists,
ethicists, and nonscientists that oversees clinical research at medical
centers - approves
the protocol for clinical trials, clinical investigators give the treatment to
a small number
of healthy volunteers or patients. These Phase 1 studies assess the most
common acute
adverse effects and examine the size of doses that patients can take safely
without a high
incidence of side effects. Initial clinical studies also begin to clarify what
happens to a
drug in the human body, e.g., whether it's changed, how much of it is absorbed
into the
bloodstream and various organs, how long it is retained within the body, how
the body
rids the drug, and the effects) of the drug on the body.
If Phase 1 studies do not reveal serious problems, such as unacceptable
toxicity, a
clinical study is then conducted wherein the treatment is given to patients
who have the
condition that the treatment is intended to treat. Researchers then assess
whether the
treatment has a favorable effect on the condition. The process for the
clinical study
simply requires recruiting one or more groups of patients to participate in a
clinical trial,
administering the treatment to those who agree to participate, and determining
whether the
treatment helps them.
9
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
Treatments usually do not miraculously reverse fatal illnesses. More often,
they
reduce the risk of death but do not entirely eliminate it. This is typically
accomplished by
relieving one or more symptoms of the illness, such as nasal stuffiness, pain,
or anxiety.
A treatment may also alter a clinical measurement in a way that physicians
consider to be
valuable, for example, reduce blood pressure or lower cholesterol. Such
treatment effects
can be difficult to detect and evaluate. This is mainly because diseases do
not follow a
predictable path. For example, many acute illnesses or conditions, such as
viral ailments
like influenza, minor injuries, and insomnia, go away spontaneously without
treatment.
Some chronic conditions like arthritis, multiple sclerosis, or asthma often
follow a varying
course, e.g., better for a time, then worse, then better again, usually for no
apparent
reason. Heart attacks and strokes have widely variable death rates depending
on
treatment, age, and other risk factors, making the "expected" mortality for an
individual
patient hard to predict.
A further difficulty in gauging the effectiveness of an investigational
treatment is
that in some cases, measurements of disease are subjective, relying on
interpretation by
the physician or patient. In those circumstances, it's difficult to tell
whether treatment is
having a favorable effect, no effect, or even an adverse effect. The way to
answer critical
questions about an investigational treatment is to subject it to a controlled
clinical trial.
In a controlled trial, patients in one group receive the investigational
treatment.
Those in a comparable group, the control group, receives either no treatment
at all, a
placebo (an inactive substance that looks like the investigational drug), or a
treatment
known to be effective. The test and control groups are typically studied at
the same time.
Usually, the same group of patients is divided into two sub-groups, with each
subgroup
receiving a different treatment.
In some special cases, a study uses a "historical control," in which patients
given
the investigational treatment are compared with similar patients treated with
the control
treatment at a different time and place. Often, patients are examined for a
period of time
after treatment with an investigational treatment, with the investigators
comparing the
patients' status both before and after treatment. Here, too, the comparison is
historical
and based on an estimate of what would have happened without treatment. The
historical
control design is particularly useful when the disease being treated has high
and
predictable death or illness rates. It is important that treatment and control
groups be as
similar as possible in characteristics that can affect treatment outcomes. For
example, all
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
patients in a specific group must have the disease the treatment is meant to
treat or the
same stage of the disease. Treatment and control groups should also be of
similar age,
weight, and general health status, and similar in other characteristics that
could affect the
outcome of the study, such as other treatments) being received at the same
time.
A principal technique used in controlled trials is called "randomization."
Patients
are randomly assigned to either the treatment or control group rather than
deliberately
selected for one group or the other. An important assumption, albeit a
seriously flawed
one, is that when the study population is large enough and the criteria for
participation are
carefully defined, randomization yields treatment and control groups that are
similar in
important characteristics. Because assignment to one group or another is not
under the
control of the investigator, randomization also eliminates the possibility of
"selection
bias," the tendency to pick healthier patients to get the new treatment or a
placebo. In a
double-blind study, neither the patients, the investigators, nor the data
analysts know
which patients got the investigational drug.
Unfortunately, careful definition of selection criteria for matching
participation in
clinical trials has not been conventionally available. Vascular health, and
more
particularly cerebrovascular health, has been a criterion that has been
difficult, if not
impossible, to assess for possible clinical trial participants. Thus, there
remains a need in
the art for the ability to truly randomize clinical trials by choosing trial
participants with
matched vascular and cerebrovascular characteristics.
Moreover, an important aspect of clinical trials is to assess the risk of
adverse
effects of a given treatment. This can be difficult for adverse effects that
manifest
themselves only long after the short run of a clinical trial has run its
course.
Unfortunately, vascular effects, and more particularly cerebrovascular adverse
effects, are
difficult, if not impossible, to assess during the course of a clinical trial.
Thus, there
remains a need in the art for the ability to accurately assess adverse effects
brought about
by a treatment upon vascular and cerebrovascular health characteristics.
There is also needed a system and method for assessing the efficacy of a
treatment,
including conducting a procedure, carrying out a therapy, and administering a
pharmaceutical substance or combinations thereof in treating vascular
disorders, so that
identification of deleterious treatments can be determined and no longer be
prescribed.
Further, there is a need for a system and method for assessing the impact of a
treatment, including conducting a procedure, carrying out a therapy, and
administering a
11
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
pharmaceutical substance, or combinations of pharmaceutical substances, upon
vascular
health, so that the impact of a treatment which have an effect upon vascular
health can be
ascertained.
SUMMARY OF THE INVENTION
The present invention provides a solution to the above described shortcomings
by
providing a system and method for assessing the vascular health of an
individual. This
system and method is inexpensive, rapid, non-invasive, and provides superior
data
concerning the dynamic function of the vasculature. Accordingly, this system
and method
may be used in a wide variety of situations including, but not limited to,
periodic physical
examinations, in an intensive care unit, in an emergency room, in the field
such as in
battlefield situations or at the scene of an emergency on the highway or in
the country,
and in a neurological clinic. The use of this system and method enables
physicians to
evaluate individuals not only for their current state of vascular health, but
also to detect
any deviations from vascular health by evaluating specific parameters of
vascular
function.
In addition to use during routine physical examinations, the present system
and
method may be used to evaluate individuals with the risk factors for cerebral
vascular
malfunction. Such risk factors include, but are not limited to a prior history
of stroke, a
genetic predisposition to stroke, smoking, alcohol consumption, caffeine
consumption,
obesity, hypertension, aneurysms, arteritis, transient ischemic episodes
(TIAs), closed
head injury, history of migraine headaches, prior intracranial trauma,
increased
intracranial pressure, and history of drug abuse.
In addition to providing a system and method for evaluating individuals with
high
risk factors, the present system and method also provides a mechanism for
selecting
patient groups for clinical trials and monitoring patient populations in
specific clinical
groups. For example, a patient population of individuals at high risk of
stroke may be
evaluated systematically over time to determine whether ongoing vascular
changes may
indicate an incipient cerebral vascular event, such as stroke. In this manner,
it may be
possible to predict the occurrence of a first stroke, thereby preventing the
stroke. In
another embodiment, the present invention provides a mechanism for monitoring
individuals who have experienced a stroke.
In yet a further embodiment of the present invention, the vascular reactivity
of an
individual to various substances, including but not limited to drugs,
nutrients, alcohol,
12
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
nicotine, caffeine, hormones, cytokines and other substances, may be
evaluated. Through
the use of this system and method, research studies may be conducted using
animals or
humans to evaluate the effects of various substances on the vascular system.
By
performing the noninvasive, low cost and efficient tests of the present
invention, valuable
information concerning the potential vascular effects of a substance may be
collected and
assessed before the substance is medically prescribed. Furthermore, vascular
effects of
dosages of individual substances and combinations of substances at different
dosages may
be evaluated-in selected clinical populations using the system and method of
the present
invention. Accordingly, the present invention provides a system and method for
performing non-invasive clinical research studies to evaluate potential
vascular effects of
substances, or combinations of substances, at selected dosages and in selected
patient
populations.
In another embodiment, the present invention may be applied to specific
populations of individuals who have had specific illnesses to determine
whether
application of a substance may produce undesirable effects in that population.
For
example, a population of diabetic individuals may react differently to a
specific substance
such as a drug than a non-diabetic population. Further, a population of
hypertensive
individuals may react differently to a specific substance, such as a
catecholaminergic
agonist drug or an ephedrine-containing natural extract, than a non-
hypertensive
population. The use of the present invention permits an assessment of vascular
reactivity
in any individual or any population, whether it be a population of individuals
with specific
diseases, conditions or prior exposures to various therapies.
By means of the present invention, a method of assessing vascular health in a
human or an animal is provided. In one embodiment, this assessment method
comprises
the steps of obtaining information concerning flow velocity within a vessel;
calculating a
mean flow velocity value for the vessel; calculating a systolic acceleration
value for the
vessel; and inserting the mean flow velocity value and the systolic
acceleration value into
a schema for further analysis of the calculated values. Such schema can
consist of
multiple arrangements of such values, including but not limited to diagrams,
graphs,
nomograms, spreadsheets and databases, thereby permitting operations such as
mathematical calculations, comparisons and ordering to be performed that
include the
calculated values.
13
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
In one embodiment, the assessment method may further comprise calculating a
pulsatility index. With the pulsatility index calculated, the assessment
method of is able
to plot the pulsatility index, the systolic acceleration value, and the mean
flow velocity
value for the vessel in a 3-dimensional space, wherein the plot of the
pulsatility index, the
systolic acceleration value, and the mean flow velocity value in 3-dimensional
space
produce a first characteristic value for the vessel. This first characteristic
value for the
vessel may then be compared to other first characteristic values obtained from
measurements of flow velocity collected from similar vessels from other humans
or
animals to determine whether the vessel is in an auto-regulation mode.
The assessment method may further comprise collecting information concerning
an additional variable, transforming the information into a value, and
plotting the value in
n-dimensional space together with the pulsatility index, the systolic
acceleration value,
and the mean flow velocity value to produce a second characteristic value for
the vessel.
The second characteristic value can then be compared to second characteristic
values
obtained from measurements of flow velocities collected from similar vessels
from other
humans or animals to determine whether the vessel is in an auto-regulation
mode.
The vessel of the assessment method as described above can be an intracranical
vessel. Further, the vessel can be an artery. The artery can be one that
supplies the
central nervous system. Further, the artery can be selected from the group
consisting of
the common carotid, internal carotid, external carotid, middle cerebral,
anterior cerebral,
posterior cerebral, anterior communicating, posterior communicating,
vertebral, basilar,
ophthalmic, and branches thereof.
The information collected in the assessment method described above concerning
flow velocity can be gathered using ultrasound energy. This gathering of flow
velocity
information can further be gathered by use of a Doppler probe.
The effects of a substance on a vessel can be determined by applying the
assessment method as described above both before and after administering the
substance.
This substance can be a drug. The drug may be a vasoactive drug. The substance
may be
suspected of having vascular activity.
The assessment method described above may be utilized in the instance wherein
the human or the animal is suspected of having or has a vascular disease or a
condition
that affects vascular function. The human or the animal can be analyzed at a
time of
normal and at a time of abnormal health.
14
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
The present invention further provides for a method of assessing vascular
effects
of a treatment in a human or an animal. This method includes the steps of
collecting a
first set of information concerning flow velocity within a vessel;
administering the drug;
collecting a second set of information concerning flow velocity within the
vessel;
calculating a mean flow velocity value for the vessel; calculating a systolic
acceleration
value for the vessel; and inserting the mean flow velocity value and the
systolic
acceleration value into a schema for analysis of the calculated values.
The step of administering a treatment in the vascular effects assessment
method
can be selected from the group consisting of administering a drug, conducting
a
procedure, and carrying out a therapy. When the administration comprises
administering
a drug, the drug may include a statin. The statin administered can include
Atorvastatin
calcium.
The steps of collecting the first set of information and collecting the second
set of
information in the vascular assessment method described above can be performed
using
ultrasound energy. More specifically, the collection steps can be performed
using a
Doppler probe.
The present invention further provides for a method of assessing vascular
effects
of a treatment in a human or an animal. The treatment can include conducting a
procedure, carrying out a therapy, and administering a drug. This method
includes the
steps of collecting a first set of information concerning flow velocity within
a vessel;
obtaining a first mean flow velocity value before administration of the
treatment;
obtaining a first systolic acceleration value before administration of the
treatment;
administering the treatment; collecting a second set of information concerning
flow
velocity within the vessel; obtaining a second mean flow velocity value
following
administration of the treatment; obtaining a second systolic acceleration
value after
administration of the treatment; comparing the first mean flow velocity value
and the
second mean flow velocity value; and comparing the first systolic acceleration
value and
the second systolic acceleration value to determine if the treatment had a
vascular effect.
The method of assessing the vascular effects of a treatment as described above
may further include the steps of calculating a first pulsatility index from
the first set of
information; calculating a second pulsatility index from the second set of
information;
plotting the first pulsatility index, the first mean flow velocity value, and
the first systolic
acceleration value to produce a first characteristic value for the vessel;
plotting the second
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
pulsatility index, the second mean flow velocity value and the second systolic
acceleration
value to produce a second characteristic value for the vessel; and comparing
the first
characteristic value and the second characteristic value to determine if the
drug had a
vascular effect.
The step of administering a treatment in the method of assessing vascular
effects
of a treatment as described above can be selected from the group consisting of
administering a drug, conducting a procedure, and carrying out a therapy. When
the
administration includes administering a drug, the drug can include a statin.
When a statin
is administered, the statin can include Atorvastatin calcium.
The steps of collecting the first set of information and collecting the second
set of
information in the method of assessing vascular effects of a treatment as
described above
can be performed using ultrasound energy. More specifically, the collection
can be
performed by means of a Doppler probe.
The method of assessing vascular effects of a treatment as described above may
be
used when the human or the animal has a risk factor for a stroke. The human or
the
animal may have received at least one medication before collecting the first
set of
information.
The method of assessing vascular effects of a treatment as described above may
be
used to determine if the drug may cause undesirable vascular effects in the
human or the
animal receiving the medication.
The method of assessing vascular effects of a drug as described above can be
used
when the human or the animal has a vascular disease or a condition that
affects vascular
function.
In another embodiment of the present invention, a method of assessing vascular
effects of a treatment in humans or animals is provided. The method of
accessing the
vascular effects includes assigning individual humans or animals to different
groups for
each human or animal by performing the steps of obtaining a first set of
information
concerning flow velocity within a vessel; obtaining a first mean flow velocity
value before
administration of the drug; obtaining a first systolic acceleration value
before
administration of the treatment; administering the treatment; obtaining a
second set of
information concerning flow velocity within the vessel; obtaining a second
mean flow
velocity value following administration of the treatment; obtaining a second
systolic
acceleration value after administration of the treatment; comparing the first
mean flow
16
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
velocity value and the second mean flow velocity value; comparing the first
systolic
acceleration value and the second systolic acceleration value to determine if
the treatment
had a vascular effect; and statistically analyzing data for each individual
before and after
administration of the treatment.
The administration of the treatment in the method of assessing vascular
effects of a
treatment by assigning individual humans or animals to different groups as
described
above can be selected from the group consisting of administering a drug,
conducting a
procedure, and carrying out a therapy. When the administration of a drug is
selected, the
drug may include a statin. The statin can be Atorvastatin calcium.
The data collection step in the method of assessing vascular effects of a
treatment
by assigning individual humans or animals to different groups as described
above can be
performed using ultrasound energy. Further, the data collection step can be
performed
using a Doppler probe.
The method of assessing vascular effects of a treatment by assigning
individual
humans or animals to different groups as described above can further include
statistically
analyzing data within each group before and after administration of the
treatment.
In one embodiment, the present invention further provides for a method of
screening for adverse effects of a treatment. The screening method includes
the steps of
applying the treatment to a number of individuals; monitoring the
cerebrovascular blood
flow of such individuals after applying the treatment; and identifying adverse
effects to
cerebrovascular blood flow in such individuals arising after applying the
treatment.
The data regarding cerebrovascular health status obtained by the screening
method
of the present invention can include both the mean flow velocity value for
intracranial
blood vessels of the individuals and systolic acceleration value for
intracranial blood
vessels of the individuals. The intracranial vessels can be arteries. The
arteries can be
selected from the group consisting of is the common carotid, internal carotid,
external
carotid, middle cerebral, anterior cerebral, posterior cerebral, anterior
communicating,
posterior communicating, vertebral, basilar, and branches thereof. The data
obtained may
also include a pulsatility index.
The screening method permits quantitative data regarding the cerebrovascular
blood flow of a number of individuals to be obtained. The quantitative data
obtained may
be collected by the use of ultrasound energy. Further, a Doppler probe can be
used to
collect the data regarding cerebrovascular health status.
17
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
The screening method treatment applied can include at least one treatment
selected
from the group consisting of administering a drug, conducting a procedure, and
carrying
out a therapy.
When the treatment selected is administration of a drug, the drug or substance
can
be a vasoactive drug, or a drug suspected of having vascular activity
The screening method for adverse effects of a treatment on a vessel as
described
above may be applied both before and after administration of the treatment.
The screening method for adverse effects of a treatment on a vessel as
described
above may be applied on individuals suspected of having or actually having a
vascular
disease or a condition that affects vascular function.
The present invention comprises measurements of parameters of vascular
function.
Specifically, the present invention uses energy including, but not limited to,
sound energy
and any form of electromagnetic energy, to determine the rate of movement of
cells
through vessels. While not wanting to be bound by the following statement, it
is believed
that red blood cells account for the majority of cells detected with this
technique. In a
preferred embodiment, ultrasound energy is utilized.
According to the present invention, a sample volume of red blood cells is
measured utilizing sound energy. Because not all blood cells in the sample
volume are
moving at the same speed, a range or spectrum of Doppler shifted frequencies
are
reflected back to the probe. Thus, the signal from the probe may be converted
to digital
form by an analog-to-digital converter, with the spectral content of the
sampled Doppler
signal then calculated by computer or digital signal processor using a fast
Fourier
transform method. This processing method produces a velocity profile of the
blood flow,
which varies over the period of a heartbeat. The process is repeated to
produce a beat-to-
beat flow pattern, or sonogram, on a video display. The instrument can be
configured to
analyze multiple separate frequency ranges within the spectrum of Doppler
signals. Color
coding may be used to show the intensity of the signal at different points on
the spectral
line. The intensity of the signal represents the proportion of blood cells
flowing within
that particular velocity range. The information displayed on the video screen
can be used
by a trained observer to determine blood flow characteristics at particular
positions within
the brain of the individual being tested, and can be used to detect anomalies
in that blood
flow such as the presence of a blockage or restriction, or the passage of an
embolus
through the artery, which introduces a transient distortion of the displayed
information.
18
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
The instrument can also include a processing option that provides a maximum
frequency
follower or envelope curve displayed on the video screen as the white outline
of the flow
spectrum.
In another preferred embodiment, coherent light in the form of lasers may be
employed. In yet another embodiment, infrared or ultraviolet radiation may be
employed.
In one preferred embodiment, the system and method of the present invention
permits a determination of vascular health based on an analysis of two blood
flow
parameters, mean flow velocity and systolic acceleration.
Earlier studies have analyzed how blood velocity correlates with blood flow to
the
brain. Flow is a concept different from velocity; flow is the quantity per
unit time
delivered to a certain region of the brain. This is partially dependent on
velocity.
Accordingly, the earlier studies demonstrate a one-to-one relationship between
flow and
velocity. Therefore, mean flow velocity is a very good indicator of cerebral
blood flow.
Thus, conventionally, this theory has been relied upon to determine blood flow
to the
brain. There is a second calculated number called the pulsatility index, which
is the
resistance of blood flow downstream, which others have also measured. Still,
there is a
need to examine any combination of flow parameters to assess vascular health
or auto-
regulation.
In a more preferred embodiment of the present invention, transcranial Doppler
is
used to obtain the velocity measurements described above. Application of a
selected form
of energy to cells within the vessels permits a calculation of the flow rate
of the cells
within the vessels. By measuring specific parameters involved in the flow of
cells
through vessels, a data analysis may be performed.
One parameter of relevance to the present invention is mean blood flow
velocity
(Vm). The value of this parameter is given by the equation
Vm =VS 3Vd +Vd
where
Vs = peak systolic velocity, and
Vd = end diastolic velocity.
A second parameter of relevance to the present invention is the pulsatility
index
(P;). The value of this parameter is given by the equation
P, = V _ Va
Vm
19
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
where
Vm = mean blood flow velocity
Vg = peak systolic velocity, and
Vd = end diastolic velocity.
Another parameter of relevance to the present invention is systolic
acceleration.
This variable is determined by measuring the flow velocity at the end of
diastole,
measuring the flow velocity at peak systole, and then dividing the difference
between
these measures by the length of time between the end of diastole and the time
of peak
systolic velocity. This is an index of systolic acceleration. The value of
this parameter is
given by the equation -
A - ys - ya
rs - to
where
tg = time at VS and td = time at Vd
Vg = peak systolic velocity, and
Vd = end diastolic velocity.
In one preferred embodiment of the present invention, a characteristic
signature for
each vessel is defined by plotting the systolic acceleration against the mean
flow velocity.
With mean flow velocity plotted on the y-axis and systolic acceleration
plotted on the x-
axis, a vessel may be represented as a point on this graph.
The present invention reveals that vessels are in a state of normal auto-
regulation
when their vascular state values fall within the auto-regulating regions of
the above
described graph. A point on the graph represents a vascular state of a vessel.
It has also
been determined that when the value for an individual vessel falls within
other regions of
the graph outside the zone of auto-regulation, serious problems have either
occurred or
may be ongoing. Accordingly, the present invention permits not only a
determination of
the location of each individual vessel on such a graph, but also provides
insight into the
vascular health of a vessel in view of its deviation in distance and/or
direction from what
may be considered within the normal range of such vessels.
In another preferred embodiment of the present invention, another
characteristic
signature for each vessel is defined by plotting the systolic acceleration
relative to the
mean flow velocity and the pulsatility index. With mean flow velocity plotted
on the y-
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
axis, pulsatility index plotted on the z-axis, and systolic acceleration
plotted on the x-axis,
a vessel may be represented as a point in this 3-dimensional space.
The present invention further reveals that vessels are in a state of normal
auto-
regulation when their values fall in certain regions of this 3-dimensional
space. The 3-
dimensional plot provides a characteristic shape representing a cluster of
points, wherein
each point represents the centroid from an individual's specific vessel. It
has further been
determined that when the value for an individual vessel falls in other regions
of the 3-
dimensional space outside the zone of auto-regulation, serious problems have
either
occurred or may be ongoing. Accordingly, the present invention permits not
only a
determination of the location of each individual vessel on such a graph, but
also provides
insight into the vascular health of a vessel in view of its deviation, either
in distance
and/or direction, from what may be considered within the normal range of such
vessels.
By means of the present invention, it has been determined that each cerebral
vessel
has a characteristic state and signature represented in a 3-dimensional graph.
The
characteristic state and signature for one vessel of an individual can be
represented as a
point in the vascular state diagram, and the characteristic states and
signatures for a
population of the same vessel type can be represented by a set of points
described as a
mathematical centroid. This value for the centroid is obtained through those
analyses
described above. The present invention reveals that individual vessels,
especially
individual cerebral vessels, display a clustering of points in 3-dimensional
space that
defines a shape.
It is to be understood that other variables may be employed in addition to
systolic
acceleration, mean flow velocity, and pulsatility index to provide additional
information
concerning specific vessels. When additional variables are employed, the data
may then
be plotted in a 4-dimensional or more dimensional space. Analysis of a
specific centroid
value for a vessel from an individual, in terms of its distance from the mean
value for
centroids for the same named vessel taken from other individuals, provides a
basis for
assessing the significance of differences between normal and abnormal vessels
and
enables predictions of abnormality. Accordingly, the present invention is not
limited to 3-
dimensional space. Further, individual vessels may be represented in n-
dimensional
space, wherein each dimension may be a relevant clinical parameter. For
example,
additional dimensions or variables may include, but are not limited to, age,
clinical history
or prior stroke, risk factors such as obesity, smoking, alcohol consumption,
caffeine
21
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
consumption, hypertension, closed head injury, history of migraine headaches,
vasculitis,
TIAs, prior intracranial trauma, increased intracranial pressure, history of
drug abuse,
steroid administration including estrogen and/or progesterone, lipid
deposition,
hyperlipidemia, parathyroid disease, abnormal electrolyte levels, adrenal
cortical disease,
atherosclerosis, arteriosclerosis, calcification, diabetes, renal disease,
prior administration
of therapeutic agents with vascular effects, prior administration of
therapeutic agents with
effects on the release or reuptake of norepinephine at postganglionic
sympathetic nerve
endings, prior administration of therapeutic agents with effects on the
release or reuptake
of acetylcholine at postganglionic parasympathetic nerve endings, vascular
denervation,
shock, electrolyte levels, pH, p0z, pCOz, or any combination thereof.
The present invention permits analysis of all the vessels of an individual.
These
analytical methods provide an index of the vascular health of the individuals,
especially
the compliance of individual vessels. In a preferred embodiment, the present
invention
permits analysis of a vessel's ability to auto-regulate. Any such vessel may
be analyzed
provided it can be located with the device used to analyze blood flow. Both
arteries and
veins may be analyzed with the system and method of the present invention.
Regarding
arteries, both cerebral and non-cerebral vessels may be analyzed. For example,
the
common carotid, internal carotid artery, external carotid artery and other
extracranial
arteries may be evaluated. Further, analysis of the cerebral vessels of an
individual can be
performed with the system and method of the present invention, including the
vessels
contributing to the great arterial circle and their primary branches. The
present invention
further permits analysis of individual cerebral vessels from individuals in
different groups,
for example, groups within specific age ranges or at specific ages, groups
considered
healthy, groups which may fall into a clinically defined group, such as
diabetics, groups of
individuals who share common risk factors such as obesity, groups of
individuals exposed
to similar substances, such as nicotine, or pharmaceuticals, such as beta
Mockers.
The present invention includes a system having the capability for a variety of
communication mechanisms such as access to the Internet that provides accurate
prediction of the future occurrence of vascular disease, vascular disease
diagnosis,
determination of the severity of vascular disease, and/or vascular disease
prognosis. The
present invention provides one or more highly sophisticated computer-based
databases
trained to diagnose, prognose, determine the severity of and predict the
future occurrence
of vascular disease, and provide increased accuracy of diagnosis and
prognosis.
22
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
The system of the present invention can operate by receiving patient vascular
data
from another location through a receiver or data receiving means, transmitting
the data
into a computer or through several computers containing vascular data for that
specific
vessel or numerous vessels in normal and/or diseased states, comparing the
patient's
vascular data to the database to produce one or more results, and transmitting
the one or
more results to another location. The other location may be a computer in a
remote
location, or other data receiving means.
In one embodiment of an automated decision support system for interpreting the
values of various parameters of blood flow in one or more vessels in assessing
the
vascular health of an individual according to the present invention, at least
three different
modules are presented, each interactive with the other. These modules include
a module
for accessing data, a module for interfacing with a user, and module for
processing patient
data, or reasoning module.
The data access module provides access and storage methods for transcranial
Doppler and clinical data inputted by a user, and for inferences from the
reasoning engine.
This data may be stored by any method known to those skilled in the art,
including but not
limited to storage on a network server, or storage in a file on a personal
computer. The
data access module is able to respond to a variety of commands, including but
not limited
to a command to initialize the module, one to retrieve patient data, a command
to save
patient data and/or graphs, a command to delete patient data and/or graphs, a
command to
retrieve a list of patients, and a command to query the database.
The user interface module performs various functions, including but not
limited to
processing user input to be sent to the data access module, running commands
for the
reasoning module, querying about patient data for the data access module, and
querying
about inference results from the reasoning module. The user interface module
may further
be designed to display patient data for at least one patient received from the
data access
module and concept instances received from the reasoning module. The user
interface
module can also be designed to display clinical and demographic data for a
patient, raw
transcranial Doppler velocimetry data, and an analysis of a patient's
hemodynamic state.
The analysis of the patient's hemodynamic state includes, but is not limited
to the
condition of each artery, any global conditions detected, and an assessment of
the
patient's risk for stroke. The user interface preferably provides a user the
ability to drill
23
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
down from a patient's assessment of the risk for stroke in order to determine
how
conclusions were reached.
The reasoning interface module performs various functions, including but not
limited to accepting commands to process patient data for inferred concepts,
searching for
instances of particular concepts or evidence of a given concept instance in a
concept
graph, and saving the concept graph or loading an old concept graph. The
reasoning
interface can be further broken down into at least two other modules - an
analysis module
for performing analysis of the data inputted, including but not limited to any
user input,
saved concepts and/or data, clinical data, and transcranial Doppler data; and
an interface
module for hiding the details of the interaction of the analysis module with
the other
modules. The interface module allows other modules to access data and concept
graphs
residing in the analysis module without exposure to the analysis interface.
Preferably,
those files created by the reasoning module are stored by the data access
module.
According to the present invention, patient data includes all data derived
from
transcranial Doppler readings and all clinical data. Preferably, patient data
is accessed
and stored as a single block of data for each patient, referenced by a unique
patient ID.
In one embodiment of the present invention, transcranial Doppler data and
clinical
data is inputted by a user at the user interface. Once the input has been
completed, the
user can either save the data to a file for later access, or can immediately
analyze the data
before saving it. In either instance, patient data is retrieved by the
reasoning module from
the data access module. Both modules retrieve patient data based on patient
ID.
Preferably, a user is able to retrieve a list of all patients saved in a file
in order to be able
to select a particular patient's data to view, edit, or analyze. Preferably,
although not
necessary, the set of parameters sent to the data access module includes a
user ID.
. The analysis module is able to provide one or more classes of service. For
example, the module includes methods for commanding the analysis module,
including
commands for initializing, starting, running and stopping the module. Another
class of
service provided by the module may include methods for setting and/or
retrieving concept
attribute values.
As defined by the above described modules, the present invention is able to
provide the sequences for an automated decision support system for
interpreting the
values of various parameters of blood flow in one or more vessels in assessing
the
vascular health of an individual. These sequences include but are not limited
to saving
24
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
patient data, analyzing patient data, loading an analysis to an analysis page,
and retrieving
evidence from a concept graph.
By means of the above described modules, the present invention is able to
provide
the software design for an automated decision support system for interpreting
the values
of various parameters of blood flow in one or more vessels in assessing the
vascular
health of an individual.
With the use of the above described modules, the present invention is able to
provide the use cases for an operational prototype for an automated decision
support
system for interpreting the values of various parameters of blood flow in one
or more
vessels in assessing the vascular health of an individual. These use cases, or
user
interface commands, include but are not limited to entering new patient data,
loading
existing patient data, viewing clinical data, viewing transcranial Doppler
velocimetry,
analyzing patient data, viewing analyses, and gathering the evidence behind an
analysis.
In a preferred embodiment of the present invention, there is provided a
process by
which the vascular health assessment can be carried out remotely, allowing for
interrogation of a patient's vascular health at one location, while processing
the patient's
data information obtained by ultrasound measurements of the cerebral vascular
health
state from various flow parameters is done at another location. This process
is preferably
managed in a stepwise fashion using a decision matrix developed to obtain the
appropriate
data set given the patient's particular situation at the time. Therefore, the
process can be
remotely managed and the data can be remotely processed.
For example, a technician or physician would assist a patient by applying to
the
patient's head an appropriate device that would obtain the necessary
transcranial Doppler
data, or alternatively, a probe would be placed at appropriate windows on the
skull to
obtain the Doppler data. The vascular health data would then be collected and
transmitted
to another device that would perform the vascular health assessment. The data
would then
be processed and an interpretation generated, as well as potential
recommendations for
additional measurements. The assessment process itself could be done one test
at a time
in batch mode, or it could be done continuously on an online system. The
interpretation
and potential recommendations can then be relayed to another location, this
location can
be any of several choices, including the location of the patient, the location
of the health
care provider, or the location where the diagnosis will be communicated.
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
In executing the analysis, the analyst, e.g., a computer or assessor, would
perform
the analysis and, preferably, do a comparison to a reference population. The
reference
population could be the group of patients evaluated that day or it could be
the population
that is appropriate in some other respect. In any case, it is important to
consider the
reference population and to have a current data set on the reference
population because the
predictive value would be affected by the underlying prevalence of individuals
in that
particular reference group.
It will be appreciated that the transmission of the vascular health
information from
the measurement device to the vascular health assessor and the transmission of
the
interpretation of vascular health to a communication location can be
accomplished
through a variety of communication links, including, modem, cable modem, DSL,
Tl, and
wireless transmission. The transmissions could be batch or continuous.
It will be appreciated that in a client-server informatics embodiment, some
assessment functions might reside on the client side while others would reside
on the
server side, the ratio of what is placed on each being a function of optimal
bandwidth,
computer speed and memory. Other considerations include remote transmission of
the
data, either in stepwise manner or in a batch mode, through a computational
device
attached to the ultrasound probe.
The present invention further includes a system, combined with access to the
Internet and other communication mechanisms, that provides substantially
accurate
prediction of the future occurrence of vascular disease, vascular disease
diagnosis,
determination of the severity of vascular disease, and/or vascular disease
prognosis. The
present invention further provides one or more highly sophisticated computer-
based
databases trained to interrogate, diagnose, prognose, determine the severity
of and predict
the future occurrence of vascular disease, and provide increased accuracy of
diagnosis and
prognosis. The present invention also provides a sensitive tool to assess
subtle differences
in flow characteristics following exposure to substances such as drugs in a
clinical
environment.
The present invention may also be combined with a file system, such as an
electronic file system, so that the individual patient's vascular data file,
the results from
the analysis of vascular flow characteristics, may be stored in the patient
file. In this
manner, the health care provider or patient may have rapid access to
information in the
patient file. Changes in vascular health since previous visits to the health
case provider
26
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
may be determined quickly, thereby indicating whether vascular disease
progression has
changed or, if recommended, interventional strategies or therapeutics are
effective. The
present invention also provides physicians with the ability to rapidly advise
patients
concerning recommended additional diagnostic testing and available treatment
options
following receipt of information from the computer-based database about the
prediction of
the future occurrence of vascular disease, disease diagnosis, determination of
the severity
of vascular disease, and/or vascular disease prognosis.
It is therefore an object of the present invention to provide a new method for
assessing vascular health.
It is further an object of the present invention to provide a method for
routine
evaluation of cerebral vascular health.
Yet another object of the present invention is to evaluate the vascular health
of
individuals at risk for disease.
Still another object of the present invention is to provide a method for
monitoring
patients who have experienced a vascular problem, such as stroke.
Another object of the present invention is to provide a method for evaluating
the
response of vessels to treatment(s), including conducting procedures, carrying
out
therapies, and administering substances.
A specific object of the present invention is to evaluate the vascular
response to
substances in individuals at risk of cerebral vascular pathology.
Yet another object of the present invention is to evaluate the vascular
response to
treatment(s), including conducting procedures, carrying out therapies, and
administering
drugs which may be used in a therapeutic manner.
Another object of the present invention is to provide ongoing evaluation of
the
vascular health of patients following stroke, closed head injury, contra coup
lesions, blunt
force trauma, transient ischemic attacks, migraine, intracranial bleeding,
arteritis,
hydrocephalus, syncope, sympathectomy, postural hypotension, carotid sinus
irritability,
hypovolemia, reduced cardiac output, cardiac arrhythmias, anxiety attacks,
hysterical
fainting, hypoxia, sleep apnea, increased intracranial pressure, anemia,
altered blood gas
levels, hypoglycemia, partial or complete carotid occlusion, atherosclerotic
thrombosis,
embolic infarction, carotid endarterectomy, oral contraceptives, hormone
replacement
therapy, drug therapy, treatment with blood thinners including coumadin,
warfarin, and
antiplatelet drugs, treatment with excitatory amino acid antagonists, brain
edema, arterial
27
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
amyloidosis, aneurysm, ruptured aneurysm, arteriovenous malformations, or any
other
conditions which may affect cerebral vessels. In addition, changes in vascular
flow
following aneurysm rupture can also be monitored.
It is another object of the present invention to evaluate drugs or other
substances
suspected to have vascular activity.
Yet another object of the present invention is to evaluate drugs with
suspected
vascular activity in individuals known to be at risk of vascular disease.
Another object of the present invention is to evaluate substances, such as
drugs,
suspected of having vascular activity in individuals following stroke.
Yet other object of the present invention is to provide a non-invasive method
to
evaluate substances, such as drugs, suspected of have vascular activity in
individuals with
no apparent vascular problems.
Another object of the present invention is to provide a non-invasive method to
evaluate different dosages of substances, such as drugs, suspected of have
vascular
activity in individuals.
Still another object of the present invention is to provide a non-invasive
method to
evaluate different combinations of substances, such as drugs, suspected of
have vascular
activity in individuals.
Yet another object of the present invention is to provide a non-invasive
method to
evaluate different combinations of selected dosages of substances, such as
drugs,
suspected of have vascular activity in individuals.
A further object of the present invention is to evaluate the vascular health
of
specific vessels or vascular beds following vascular insult in another region
of the cerebral
vasculature. In this manner, the capacity of other vessels to properly auto-
regulate and
distribute collateral blood flow may be assessed.
An advantage of the present invention is that it is not invasive.
A further advantage of the present invention is that it is rapid and
inexpensive to
perform.
Another advantage of the present invention is that the characteristics of each
cerebral vessel may be established as a baseline in order to monitor the
vascular health of
the individual over time, especially during routine physical examination,
following a
vascular insult or injury, or exposure to drugs.
28
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
Yet another advantage of the present invention is that analysis of individual
vessels and their deviation from a normal value for a corresponding vessel in
another
individual may indicate specific medical conditions. Treatment of those
medical
conditions may then be evaluated with the present invention to determine
whether the
treatment was effective on the specific vessel being evaluated.
Accordingly, it is an object of the present invention to provide a system for
efficient delivery of information concerning the vascular health of an
individual.
Yet another object of the present invention is to provide a system which
health
care providers can utilize to provide more precise and accurate prediction of
the future
occurrence of vascular disease, diagnosis of vascular disease, determination
of the
severity of vascular disease and prognosis of vascular disease.
An object of the present invention is to provide a system which health care
providers can utilize to provide more precise and accurate prediction,
diagnosis and
prognosis of vascular diseases, and associated treatment options, such
diseases including,
but not limited to, cerebrovascular disease.
It is further an object of the present invention to provide a computer-based
database that may receive vascular flow data from an input device, interpret
the vascular
flow data in view of existing data for the same vessel or vessels in normal or
disease
states, produce a values) that provides useful information concerning vascular
health and
then optionally transmit the information to another location.
It is yet another object of the present invention to provide a system that
delivers to
the health care provider a complete patient report within a short time
interval.
It is another object of the present invention to provide point-of care
analytical
capabilities linked through communication means to local or remote computers
containing
a computer-based database that may receive vascular flow data from an input
device,
interpret the vascular flow data in view of existing data for the same vessel
or vessels in
normal or disease states, produce a value that provides useful information
concerning
vascular health, and then optionally transmit the information to another
location. Such
output values may be transmitted to a variety of locations including the point-
of care in
the health care provider's office that transmitted results from the point-of
care flow
measuring device. The present invention provides accurate, efficient and
complete
information to health care providers using in order to enhance affordable and
quality
health care delivery to patients.
29
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
These and other objects, features and advantages of the present invention will
become apparent after a review of the following detailed description of the
disclosed
embodiments.
BRIEF DESCRIPTION OF THE FIGURES
Figures 1 to 4 are illustrative views showing the manner in which ultrasonic
pulses
are applied to the head of an individual to obtain information on the velocity
of blood
flowing in an intracranial blood vessel;
Figures Sa to Sd provide schematic representations of transcranial Doppler
ultrasound analyses in which velocity is indicated on the y-axis and time is
provided on
the x-axis;
Figure 6 is a schematic representation of a 2-dimensional nomogram in which
mean flow velocity is indicated on the y-axis and systolic acceleration is
provided on the
x-axis;
Figure 7 shows the nomogram of Figure 6, as well as areas of the nomogram
which indicate deviations from normal, auto-regulatory conditions;
Figure 8 shows a schematic representation of a 3-dimensional nomogram;
Figures 9a to 9d show schematic representations of a 2-dimensional nomogram in
which mean flow velocity is indicated on the y-axis and systolic acceleration
is provided
on the x-axis of a patient who presented with slight feelings of unsteadiness;
Figure 10 is a block diagram of an illustrative system architecture of a
preferred
embodiment of the invention;
Figure 11 is a concept graph of left extracranial frontal artery concepts of a
preferred embodiment of the invention;
Figure 12 is a concept graph of left intracranial frontal artery concepts of a
preferred embodiment of the invention;
Figure 13 is a concept graph of right intracranial frontal artery concepts of
a
preferred embodiment of the invention;
Figure 14 is a concept graph of right extracranial frontal artery concepts of
a
preferred embodiment of the invention;
Figure 15 is a concept graph of posterior artery concepts of a preferred
embodiment of the invention;
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
Figure 16 is a concept graph of collateral flow concepts of a preferred
embodiment
of the invention;
Figure 17 is a concept graph of parameter concepts of a preferred embodiment
of
the invention;
Figure 18 is a concept graph of stroke candidate concepts of a preferred
embodiment of the invention;
Figure 19 is a concept graph of small vessel disease concepts of a preferred
embodiment of the invention;
Figure 20 is a concept graph of data concepts of a preferred embodiment of the
invention;
Figure 21 is a concept graph of arterial condition concepts of a preferred
embodiment of the invention;
Figure 22 is a concept graph of arterial condition concepts of a preferred
embodiment of the invention;
Figure 23 is a block diagram for an application service provider architecture
of a
preferred embodiment of the invention;
Figure 24 is an illustration of a logon page of a preferred embodiment of the
invention;
Figure 25 is an illustration of a user startup window of a preferred
embodiment of
the invention;
Figure 26 is an illustration of a transcranial Doppler data window of a
preferred
embodiment of the invention;
Figure 27 is an illustration of a hemodynamic analysis window of a preferred
embodiment of the invention;
Figure 28A depicts the global vascular status of a subject basedon data from a
number of vessel at the initial onset of symptoms associated with an increase
of intracranial
pressure;
Figure 28B depicts a shift in vascular status in individual vessels as the
subject's
symptoms have progressively worsened;
Figure 28C depicts a dramatic globalized shift in vascular status of
individual
vessels after the subject's symptoms have increase to the point of requiring
hospitalization;
Figure 28D depicts a return of vascular status to a near normal state after
treatment
to decrease intracranial pressure;
31
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
Figure 29 demonstrates that traditional blood flow tests would not detect the
intracranial pressure changes occurring in the subject that were observable
using
transcranial based dynamic vascular assessment;
Figure 30 is a schematic representation of correlated MFV and SA data from the
two
series of subjects presented in Table 8;
Figure 31 is a bar graph of Trendelenberg PI data for the two series of
subjects from
Table 8;
Figure 32 is a schematic representation of correlated PI and SA data from the
two
series of subjects presented in Table 8; and
Figures 33 and 34 depict 19 intracranial vessel segments available for
evaluation by
the invention.
DETAILED DESCRIPTION OF THE INVENTION
This application expressly incorporates herein by reference in their entirety
co-
pending and commonly assigned United States Patent Applications Nos.
09/966,366,
09/966,368, 09/966,360, and 09/966,359, all filed on October 1,2001.
The present invention provides a novel system and method for evaluating
vascular
health. This invention may be used to evaluate individuals for risk of
cerebral vascular
disease. The invention may also be used for evaluating vascular health in
individuals
following a vascular insult or stroke. The present invention may also be used
for
assessing the effects of individual substances and combinations of substances
on cerebral
vessels.
As noted above, the present invention comprises measurements of parameters of
vascular function. Specifically, the present invention uses energy including,
but not
limited to, sound energy or any form of electromagnetic energy, to determine
the rate of
movement of cells through vessels. In a preferred embodiment, ultrasound
energy is
utilized.
Description of Flow Data Acquisition and Analysis
According to the system and method of the present invention, a noninvasive
instrument is utilized to obtain measurements of blood velocity in
intracranial arteries and
veins using Doppler principles. Since body movements such as vessel wall
contractions
are detected as "noise" in the Doppler signal scattering ultrasound, a high
pass filter is
used to reduce these low frequency, high amplitude signals. The high pass
filter typically
32
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
can be adjusted to have a passband above a cutoff frequency selectable between
0 and,
e.g., 488 Hz.
Because not all blood cells in the sample volume are moving at the same speed,
a
range or spectrum of Doppler-shifted frequencies are reflected back to the
probe. Thus,
the signal from the probe may be converted to digital form by an analog-to-
digital
converter, and the spectral content of the sampled Doppler signal calculated
by a
computer or digital signal processor using a fast Fourier transform method.
This
processing method produces a velocity profile of the blood flow, which varies
over the
period of a heartbeat. The process is repeated to produce a beat-to-beat flow
pattern, or
sonogram, on a video display. The instrument can be configured to analyze
multiple
separate frequency ranges within the spectrum of Doppler signals. Color coding
may be
used to show the intensity of the signal at different points on the spectral
line. The
intensity of the signal will represent the proportion of blood cells flowing
within that
particular velocity range. The information displayed on the video screen can
be used by a
trained observer to determine blood flow characteristics at particular
positions within the
brain of the individual being tested, and can detect anomalies in such blood
flow, for
example, the possible presence of a blockage or restriction, or the passage of
an embolus
through the artery which introduces a transient distortion of the displayed
information.
The instrument can also include a processing option which provides a maximum
frequency follower or envelope curve which is displayed on the video screen as
the white
outline of the flow spectrum.
Figures Sa to Sd illustrate Doppler waveform definitions provided by a system
according to the present invention. Figure Sa is a graph, providing the
results of a
transcranial Doppler ultrasound analysis in which velocity is indicated on the
y-axis and
time is provided on the x-axis. The peak systole velocity is indicated in the
Figure.
Figure Sb is a graph providing the results of a transcranial Doppler
ultrasound
analysis in which velocity is indicated on the y-axis and time is provided on
the x-axis.
The end diastole velocity is indicated in the Figure.
Figure Sc is a graph providing the results of a transcranial Doppler
ultrasound
analysis in which velocity is indicated on the y-axis and time is provided on
the x-axis.
The mean flow velocity is indicated in the Figure.
33
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
Figure Sd is a graph providing the results of a transcranial Doppler
ultrasound
analysis in which velocity is indicated on the y-axis and time is provided on
the x-axis.
The systolic upstroke time or acceleration is indicated in the Figure.
The present invention provides a plot on a two-dimensional graph of the
systolic
acceleration and mean flow velocity. Referring back to the auto-regulation
model, one
now finds that the auto-regulation curve more accurately describes the
vascular health of a
system. Addition of a third dimension, the pulsatility index, provides a three-
dimensional
plot, that gives a much more accurate look at how blood is flowing in that
particular
subsection of the vessel. Thus, the present invention combines different blood
flow
parameters to give a nomogram or a graphical representation of how blood is
flowing
within the brain itself.
The present invention permits the interrogation of cerebral vessels to
determine the
state of vascular health or disease by examining the flow parameters for a
vessel and
comparing then with a normal value. This also permits a clinical trial to be
run since an
entire population can be interrogated with this relatively quick and
noninvasive technique,
thereby obtaining readings not only for each individual patient, but also for
the
population. In addition, one can monitor the flow dynamics of the group as a
whole over
time and determine if either the non-treatment group becomes more diseased or
if the
treatment group stabilizes, improves, or has a lower rate of disease, all
determined by
clinical measurements. Thus, the present invention provides a very sensitive
blood flow
interrogation tool for the brain to determine whether a drug is going to be
safe or effective
for use in patients.
Using an ultrasound probe, one can determine the velocity of blood. The
relationship of the velocity of blood at two separate points within the points
will provide
the flow parameters of the present invention. Analyzing the relationship of
the three
parameters in each individual segment in relationship to a normal population
can
determine the state of disease of that particular segment of vessel. Further,
assessing all
the segments of vessels in the brain as a whole, one can determine the
interconnections
and the states of abnormal flow into whole regions of the brain. The more
regions of the
brain at risk, the higher the stroke risk for the patient. Thus, the present
invention permits
one to quantitate stroke risk in patients.
According to the present invention, values for various transcranial Doppler
sonography measurements for a number of patients are collected into a database
of the
34
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
present invention. The database may further provide ranges of transcranial
Doppler
sonography measurements for various cerebral arteries. Figure 6 provides a
nomogram of
the values for mean flow velocity on the y-axis and systolic acceleration on
the x-axis for
transcranial Doppler ultrasound analyses of the ophthalmic artery in a number
of
individuals. It will be appreciated that the majority of the data points are
grouped in the
lower left-hand side of the nomogram. These represent the values corresponding
to
vascular health. The aberrant points found in the upper left-hand portion of
the
nomogram correspond to a state of vascular disorder, specifically,
vasodilation. In
addition, the aberrant points found in the lower right-hand portion of the
nomogram also
correspond to a state of vascular disorder; however, here these points
correspond to
stenosis. These observations are provided in Figure 7.
In another preferred embodiment, the system and method of the present
invention
permits a determination of vascular health based on an analysis of three blood
flow
parameters, mean flow velocity, systolic acceleration, and pulsatility index.
For example,
Figure 8 provides a nomogram of the values for mean flow velocity on the y-
axis, systolic
acceleration on the x-axis, and pulsatility index on the z-axis for
transcranial Doppler
ultrasound analyses of a cerebral artery in a number of individuals. It will
be appreciated
that the majority of the data points are grouped in a centroid located in the
first octant (x >
0, y > 0, z > 0) close to the origin of the nomogram. If plotted as the
logarithm of the
value, these exhibit a normal distribution. The normal range of the log of
these values
represent the values corresponding to the vascular health of the reference
population.
Thus, the present invention permits the construction of any and all reference
populations
based on the data collected from the reference population. The data set is the
ideal
reference set because the reference population can be defined in any manner,
e. g., those
patients who are exhibiting a certain set of symptoms or desired
characteristics.
The aberrant points found distal to the origin and having a large mean flow
velocity (y value) in the nomogram correspond to a state of vascular disorder,
specifically,
vasodilation. In addition, the aberrant points found distal to the origin and
having a large
systolic acceleration (x value) in the nomogram also correspond to a state of
vascular
disorder; however, here these points correspond to stenosis.
The measurements, gathered on a substantial number of individuals to date,
demonstrate that the observed values for a normal population show
statistically normal
distributions of values for the three parameters, mean blood flow, systolic
acceleration,
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
and pulsatility index. Scrutinized by means of standard multivariate
statistical methods,
such as tests of significance, multivariate distances, and cluster analysis,
the observed
values for all three parameters all show a statistically normal distribution.
An aspect of a preferred embodiment of the present invention is the collection
of
data by means of transcranial Doppler sonography. As discussed previously,
instrumentation for conducting transcranial Doppler sonography is commonly a 2
MHz
pulsed Doppler and a spectrum analyzer, in which the examiner interrogates the
intracranial vessels without the aid of an image. Such a technique is referred
to as
freehand, blind, or non-imaging transcranial Doppler sonography. Recently,
duplex
ultrasound systems incorporating B-mode imaging and color and power Doppler
have
been employed to perform transcranial Doppler studies. However, despite
advances in
duplex ultrasound technology, freehand transcranial Doppler sonography is
commonly
performed because the technique can be equally accurate and the
instrumentation less
expensive and more portable when compared to the duplex ultrasound.
Although freehand transcranial Doppler sonography can be characterized as
operator dependent, the technique is objective and reproducible. The operator,
in
conducting transcranial Doppler sonography, considers the relevant anatomy,
natural
cranial windows, and recognized examination techniques. Specifically, an
understanding
of the extracranial arterial circulation contributing to the intracranial
flow, the intracranial
arterial circulation, carotid arteries, vertebral arteries, basilar artery,
and their common
anatomical variations is a prerequisite.
Additionally, in conducting the examination the examiner must also identify
the
vessel. Such identification is often premised upon the acoustical window being
utilized,
the depth of the volume sample, the direction of the blood flow relative to
the transducer,
the relative velocity, and spatial relationships.
The examiner must also recognize that there are three acoustical windows or
regions over the cranium where the bone is either thin enough or through which
there are
natural openings to allow sufficient ultrasound energy to be passed into and
back out of
the skull to permit performance of a transcranial Doppler examination, i.e.,
the signal-to-
noise ratio is adequate at the "window." However, enhanced phase array
detectors may
provide sufficiently improved signal-to-noise ratio that a "window" may not be
necessary.
The three acoustical windows are the transtemporal window located superior to
the
zygomatic arch over the temporal bone; the transorbital window where the
transducer is
36
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
oriented directly over the closed eyelid in a direct anteroposterior direction
with a slight
angulation toward midline; and the transforamenal window located midline over
the back
of the neck approximately 1 inch below the palpable base of the skull. It is
to be
understood that other windows may be used for other approaches using sound or
other
electromotive forces for detection of cell movement within vessels. It will be
recognized
that many texts provide sufficient instruction to examiners so as to enable
them to perform
optimal transcranial Doppler sonography. One such text is L. Nonoshita-Karr
and K.A.
Fujioka, "Transcranial Doppler Sonography Freehand Examination Techniques," J.
Vasc.
Tech., 24, 9 (2000), which is incorporated herein by reference.
In another preferred embodiment of the present invention, ultrasound beam
alignment is controlled rapidly and automatically in two dimensions. Devices
that scan
azimuth angle rapidly while varying elevation angle in small increments have
been used
for 3-dimensional image construction, but lack speed in controlling elevation.
In the
analogous area of laser scanning, it is common to steer a light beam in two
dimensions
using a pair of orthogonally-rotating mirrors driven by galvanometer
movements. The
double mirror approach does not work as well with ultrasound, however. The
size and
cumbersomeness of a pair of galvanometer driven mirrors is a disadvantage in
medical
applications, especially for limited space uses such as transesophageal and
transrectal
probes. Another design constraint is that the wavelengths of diagnostic
ultrasound waves
are much larger than optical wavelengths, of necessity, since attenuation of
ultrasound
waves rises steeply with decreasing wavelength. As a rule of thumb, ultrasound
wavelength cannot be much less than 1% of the maximum depth to be imaged, with
an
even larger wavelength required for imaging through tissues with high
attenuation. With
relatively large wavelengths, diffraction effects make it impossible to
produce very thin
collimated beams that can be steered by small mirrors, as with lasers.
For sharp focusing of ultrasound, a relatively large aperture is needed to
avoid
angular dispersion by diffraction. A well focused near field ultrasound beam
has the
shape of a converging cone connecting to a diverging cone through a short
focal neck,
representing a small depth of near-optimum focus in the target area.
Resolution
approaching a practical minimum spot diameter of a little under two
wavelengths at the
focus demands an included cone angle on the order of 60°. If the
originating end of the
columnar beam is made smaller while maintaining a fixed focus depth, then
diffraction
causes the focal neck to become thicker, sacrificing resolution at optimum
depth for an
37
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
increased depth range of relatively good focus. To achieve fine focus with a
double
minor apparatus, the mirrors must be comparatively large, increasing the
difficulty of
attaining fast angular response.
Typical electromechanical ultrasound image scanners employ multiple
transducers
on a rotating head, or an ultrasound minor rotationally vibrating at an
angular resonance -
approaches that achieve desired azimuth scanning by sacrificing the
possibility of precise
angular servo-control in a non-scanning mode.
In radar, phased arrays permit rapid scanning and abrupt alignment changes in
two
dimensions from a fixed transmit/receive surface. A comparable approach is
applicable to
medical ultrasound. One dimensional ultrasound phased arrays are finding
increasing use,
and limited control of alignment in a second dimension is beginning to appear.
In one
preferred embodiment, a stepper motor is used to rotate the scanning plane of
a one-
dimensional phased array through small incremental steps in order to construct
a 3-
dimensional digital image. This approach requires that the target and the
ultrasound
scanner be mechanically stabilized so that frames of a slow scan are in
precise
registration. A phased array with dual sets of electrodes that permit beam
steering in
either of two selected scanning planes can be used. For example, a system that
employs a
one dimensional ultrasound array can achieve controllable alignment and focus
depth in a
plane, for use in range-gated pulsed Doppler to characterize the flow velocity
profile over
the cross-section of an artery. The device is also useful to quantify angular
relationships,
through comparing Doppler velocities at different axial locations along an
artery, so that
the relationship between Doppler frequency shift and flow velocity can be
determined
accurately.
In many emerging ultrasound applications, visual image scanning takes on a
supporting role of identifying structures and defining their positions, in
preparation for
analytic measurements in a small region, which is concerned with measuring
flow
velocity profiles over the dimensions of an artery and over time, to
characterize
volumetric flow and to detect the flow disturbances caused by stenotic
lesions. Using
fixed alignment defocused beams or beams electromechanically aligned with
respect to
two axes, ultrasound can be used to track the time-varying positions of organ
surfaces
generating specular reflections, for the purposes of vibration tracking and
diameter
pulsation tracking, in a system to determine blood pressure, intraocular
pressure and
mechanical tissue properties. One preferred embodiment would consist a non-
focusing 2-
38
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
axis ultrasound aiming device, consisting of an ultrasound transducer disk
stacked on a
short magnet cylinder and the transducer-magnet pair mounted in a 2-axis
gimbal bearing,
consisting of pins and engaging bearing cups on the ring and the magnet, with
flexible
wires connecting the gimbaled part to fixed housing. Surrounding the gimbal is
a
torroidal ferromagnetic core in four sections, with four windings on the four
90° quadrants
of the core. Opposite windings are interconnected, giving two electrical
circuits that
generate two orthogonal magnetic fields crossing the gimbaled transducer-
magnet pair.
The gimbaled part tilts in response to the two applied fields, aiming the
ultrasound beam.
In this aiming device, the axially-poled center magnet is inherently unstable
in its
center alignment, being attracted to point across the torroid. To stabilize
alignment, the
torsional restoration of the connecting wires must overcome the magnetic
instability.
Alignment direction is determined open-loop by the balance of mechanical and
magnetic
forces, without direct sensing for servo-control. In an uncompensated open-
loop control
situation, if the net alignment restoration is weak, then settling is slow,
and if restoration
is made stronger, then the steady power needed to maintain off center
alignment becomes
excessive. A compensated open-loop controller whose action takes into account
the
known dynamic properties of a particular design, i.e., inertia, angular spring
coefficient,
damping, and electromagnetic coupling strength, can speed response. The term
"pole-
zero compensation" is often applied to this kind of a controller, since
LaPlace pole-zero
analysis is commonly used to design the controller transfer function. To speed
responses,
the controller transfer function cancels electromechanical low frequency
zeroes with poles
and low frequency poles with zeroes, generally replacing the poles removed
with new
poles as far to the left of the origin as is practical within bandwidth
constraints.
Something much needed and unavailable in existing designs is fast mechanical
alignment capability together with alignment sensing and error feedback for
rapid, fast
settling changes in alignment. In areas of alignment tracking and analysis of
echo features
and their movements or velocities, particularly for extended monitoring in
unanesthetized
subjects, there is need for a combined ability to scan rapidly for image
presentation and to
fine-tune 2-dimensional beam alignment under continuous software control, to
maintain
alignment dynamically on a tissue structure subject to extended monitoring.
In the area of combined scanning and fixed beam alignment monitoring, a phased
array device that switches readily between B-Mode image scanning and Doppler
tracking
at a specified alignment within the image plane can be employed. A device like
this, with
39
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
phased array speed, can alternate between scanning sweeps and brief periods of
Doppler
data gathering at a fixed alignment in a time-multiplexed mode, achieving
relative
continuity of both image and Doppler data. Electronic alignment control is
restricted to a
single axis, while manual control is needed for the second axis. One can also
employ a
dual beam ultrasound device, using one beam for tracking data from a fixed
target and the
other beam for ongoing scanning to aid the operator in maintaining alignment
on the
desired target. Again, the other axis of alignment is controlled manually.
For many applications it is advantageous to achieve a device small enough so
that
it can be affixed directly to the subject's body and ride body motions, rather
than
obtaining measurements in a clinical setting. The advantages of the present
invention in
fulfilling these and other needs will be seen in the following specification
and claims.
Description ofData Telemetry
The present invention provides an integrated system which combines several
unique technologies to assist physicians in the control, management and
delivery of
improved, efficient and timely medical care for patients. Key components of
this
integrated system include, but are not limited to, (1) a processor which may
include, but is
not limited to, a desktop personal computer, a laptop computer, or a multi-
user server
system; (2) an output device for displaying information from the processor,
such as
monitors, printers, liquid crystal displays, and other output devices known to
one skilled
in the art; and optionally including (3) analyzers for assessing a patient's
clinical profile.
Such analyzers may be used for analyzing flow characteristics of a vessel or
number of
vessels.
All patient data may be placed in a form, such as a digitized form or other
computer readable and communication acceptable form, and transmitted to
another
location. In one embodiment, the computer-based database may be located in the
office of
the health case provider, perhaps in the computer in a physician's office. In
another
embodiment, the computer-based database may be located in a centralized
hospital
facility, in a emergency room/service, in a clinical chemistry laboratory, or
in a facility
dedicated solely to housing and maintaining the computer-based database. In
yet another
embodiment, the computer-based database may be located in a home computer. In
a
further embodiment, the computer-based database may be portable for uses such
as on a
battlefield, in rural areas and at events.
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
Another component in the system of the present invention includes a
transmission
device such as a modem or other communication device known to one of skill in
the art.
Such devices include, but are not limited to satellites, radios, telephones,
cables, infrared
devices, and any other mechanism known to one of skill in the art for
transmitting
information. The transmission device modem transmits information to the
central
computer-based database. In a preferred embodiment, modems are used for
computer
access to the Internet. Such communication means may be essential for
transmission of
patient information from assessment of vascular flow parameters, from the
health care
provider's point of care, such as an office, to another facility housing the
computer-based
database. It is to be understood that the facility housing the computer-based
database may
be located locally, in the same office, the same building, or across town, or
at a remote
location such as in another city, state, country, or on a ship, plane or
satellite.
The computer-based system may be configured to take advantage of data
communications technologies and distributed networks, which makes it possible
to deliver
data to virtually anywhere in the world in an efficient and timely manner.
This system in
accordance with the present invention is capable of transferring clinical
vascular flow data
from a remote source to a central server via one or more networks. The central
server
hosts the computer-based database and related components. Accordingly, the
central
server is operable to analyze the received laboratory and clinical vascular
data using an
expert system, in order to produce information related to diagnoses,
prognoses, decision
supports, clinical data analyses and interpretations. The resulting
information may then
be delivered from the central server to one or more remote client stations via
one or more
networks. The entire process of transferring data from a remote source to a
central server,
analyzing the data at the central server to produce information, and
transferring the
information to a remote client site may thus be performed on-line and in real
time.
In automated decision support system for interpreting the values of various
parameters of blood flow in one or more vessels in assessing the vascular
health of an
individual, the data which are collected on an individual vessel are analyzed
individually
for each patient and then are also analyzed as an ensemble over that patient.
In other
words, all the vessels and their respective parameters, their respective
health states, are
compared to one another and an overall system analysis is made. The points of
data in n-
dimension states describing the health state of a vessel are tracked over time
so as to
determine a starting point and a velocity. The velocity in this case would be
a direction of
41
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
change as well as a rate of change in n-dimensional space. In more
conventional terms, if
noncompliance was detected in a vessel as one of the dimensions in n-
dimensional space,
then after a treatment one might see that number which represents
noncompliance, or a
degree of noncompliance migrate in a certain direction - for example, toward
compliance
- as the vessel becomes more compliant with the treatment intended to make it
more
compliant. The significance of that change will be assessed by looking at the
velocity of
health state movement in dimensional space across all of the individual's
cerebral
vasculature.
The movement from the baseline of any single vessel point may be hard to
assess
for statistical significance. However, there are statistical tools which are
appropriate for
analyzing the movement of the health states of all of the vessel points
simultaneously. An
example of that would be the Wilcox Test, which allows comparison of a group
of non-
parametric values to ascertain whether the variables are statistically
different from one
another or not. Other tests may be appropriate given the data set. However,
fundamentally the process is to quantitate the health state of each vessel of
an individual
in a n-dimensional space and determine the significance of change and the
direction of
change, such that if the directions and the degrees of change are, when
considered
together, significant, it can then be concluded that the treatment is
effective. In an
individual case it is also possible to stop treatment and confirm that the
effect being
observed was in fact due to the drug by observing a reversal of the same.
When comparing a clinical trial treatment group to a control group, the
process can
be similar to what is being done with the individual. There, it is a matter of
assessing
whether or not the numbers quantitating particular characteristics of the
vessel health state
with regard to each of the dimensions in dimensional space can be construed to
be
significant. A discussion of the statistical analysis employed here is found
in Jerrold H.
Zar, Biostatistical Analysis, Prentice Hall, Inc. New Jersey, pp. 153-161,
which is
incorporated herein by reference.
One way in which the system of the present invention is trained is one wherein
the
software quantitates the rationale being used by the expert. In such a system,
during this
process the expert and the system come to mirror each other. In the process
the expert is
very specific, concrete and quantitative regarding the data analysis. In its
turn, the
software maintains a detailed bookkeeping of the analytical process. Thus, the
software
42
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
system and the expert each begin to diversify their respective roles in the
development of
this knowledge. The purpose of the software is to capture the expert's
analysis.
According to the expert system of the present invention, characteristics of
various
functions for an automated decision support system for interpreting the values
of various
parameters of blood flow in one or more vessels in assessing the vascular
health of an
individual are provided. These characteristics can be derived from various
functions, for
example, transcranial Doppler readings at a left anterior carotid artery or
basal artery test
point, flow parameters for various arteries, a summary of patient data, a
summary of
clinical tests) performed on a patient, the presence of vasodilators and/or
vasoconstrictors
in a patient, a stenotic pattern or pattern indicating constriction of an
artery at a particular
test point, a vasodilation pattern or pattern indicating dilation of a blood
vessel, a
noncompliance pattern or pattern indicating loss of compliance in an artery
such as in the
example of hardening of the artery, a normal pattern or pattern indicating a
blood vessel
with normal radius, a global vasoconstriction or reversible stenosis of
vessels in the brain,
global vasodilation or dilation of all cranial blood vessels, a pseudo-
normalized pattern of
constriction or dilation at an arterial test point, a pseudo-normalized
pattern of loss of
compliance in an artery, stenosis of a vessel due to blockage, dilation of an
artery to
compensate for loss of flow elsewhere, permanent dilation of an artery,
noncompliance or
a state in which a vessel's walls have lost flexibility, collateral flow
through an artery or
via reversal of flow, and/or patient risk assessment for any type of stroke.
Parameters for determining the various functions can include, but are not
limited
to, identification of the person taking the Doppler reading, the date of the
reading, patient
identification, a patient's sex, a patient's ethnic group, a patient's date of
birth, a patient's
drug usage including specific drugs, Doppler values, Doppler times,
acceleration, flow
direction, reading depth, the mean and/or standard deviation of the flow
velocity in a
vessel, the mean and/or standard deviation of the systolic acceleration in a
vessel, the
pulsatility index of a vessel. These parameters can be static values, inputted
or retained
within a database, or calculated ones. Other calculated parameters may include
the
calculation of the belief of whether there are vasodilators or
vasoconstrictors present in
the patient, which may be based upon the presence of vasoactive substances
such as
caffeine and/or methylxanthine. An example of another calculated parameter may
include
the belief of the severity of the constriction of an artery at a particular
test point, which
may be characterized as none, minimal, moderate or severe. An example of
another
43
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
calculated parameter of the present invention may include the belief of
dilation of a blood
vessel, which may be characterized as none, hyperemic, normal or pathological.
An
example of another calculated parameter of the present invention may include
the belief of
loss of compliance in an artery, which may be characterized as none, normal or
pathological. An example of another calculated parameter of the present
invention may
include the belief of a blood vessel with normal radius, which may be
characterized as
none, hyperemic, normal or pathological. An example of another calculated
parameter of
the present invention may include the belief of a blood vessel with a high
pulsatility
index, or wherein the pulsatility index of one vessel is higher than another,
which may be
characterized as true or false. As can be seen from the above examples,
various beliefs
may be calculated according to the expert system of the present invention
based upon the
function studied.
An automated decision support system according to the present invention
provides
a domain ontology for interpreting the values of various parameters of blood
flow in one
or more vessels in assessing the vascular health of an individual. These
parameters may
be determined by means of a transcranial Doppler velocimetry technique, which
is a non-
invasive technique for measuring blood flow in the brain. According to this
technique, an
ultrasound beam from a transducer is directed through one of three natural
acoustical
windows in the skull to produce a waveform of blood flow in the arteries using
Doppler
sonography. The data collected to determine the blood flow may include values
such as
the pulse cycle, blood flow velocity, end diastolic velocity, peak systolic
velocity, mean
flow velocity, total volume of cerebral blood flow, flow acceleration, the
mean blood
pressure in an artery, and the pulsatility index, or impedance to flow through
a vessel.
From this data, the condition of an artery may be derived, those conditions
including
stenosis, vasoconstriction, irreversible stenosis, vasodilation, compensatory
vasodilation,
hyperemic vasodilation, vascular failure, compliance, breakthrough, and pseudo-
normalization.
In order to best analyze a patient's risk of stroke, additional patient data
is utilized
by the automated decision support system according to the present invention.
This data
may include personal data, such as date of birth, ethnic group, sex, physical
activity level,
and address. The data may further include clinical data such as a visit
identification,
height, weight, date of visit, age, blood pressure, pulse rate, respiration
rate, and so forth.
The data may further include data collected from blood work, such as the
antinuclear
44
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
antibody panel, B-vitamin deficiency, C-reactive protein value, calcium level,
cholesterol
levels, entidal COz, fibromogin, amount of folic acid, glucose level,
hematocrit
percentage, H-pylori antibodies, hemocysteine level, hypercapnia, magnesium
level,
methyl maloric acid level, platelets count, potassium level, sedrate (ESR),
serum osmolality,
sodium level, zinc level, and so forth. The data may further include the
health history data
of the patient, including alcohol intake, autoimmune diseases, caffeine
intake, carbohydrate
intake, carotid artery disease, coronary disease, diabetes, drug abuse,
fainting, glaucoma,
head injury, hypertension, lupus, medications, smoking, stroke, family history
of stroke,
surgery history, and so forth.
The automated decision support system according to the present invention
further
considers related pathologies in analyzing a patient's risk of stroke,
including but not
limited to gastritis, increased intracranial pressure, sleep disorders, small
vessel disease, and
vasculitis. In a preferred embodiment, the invention includes a decision
support system and
method for screening potential participants in a drug trial. General
references detailing
principles and terms known to those skilled in the art of decision support
systems include
(1) Schank, R.C. and Abelson, R., Scripts, Plans Goals and Understanding,
Hillsdale, NJ:
Lawrence Erlbaum Associates (1977); (2) Schank, R.C. and Riesbeck, C.K.,
Inside
Computer Understanding, Hillsdale, NJ: Lawrence Erlbaum Associates (1981); (3)
Sacerdoti, E.D., A Structure for Plans and Behaviors, New York: Elsevier
(1978); (4)
Rinnooy Kan, A.H.G., Machine Scheduling Problems, The Hague: Martinus Nijhoff
(1976); and (5) Charniak, E., Riesbeck, C.K. and McDermott, D., Artificial
Intelligence
Programming, Hillsdale, NJ: Lawrence Erlbaum Associates (1980).
Several terms used in disclosure of the present invention are described
generally by
the following definitions accepted by those skilled in the art-
Concept Graph: a knowledge representation of the dependencies between
observable data values and higher level computations and assertions made about
the data. A
concept graph can be implemented as a directed acyclic graph of concept nodes
that is a
particular type of augmented transition network (ATN).
Decision Support System: a computer program that uses a knowledge base to
assist
in solving problems. Most expert systems use an inference engine to derive new
facts and
beliefs using a knowledge base.
Inference Engine: a computer program that infers new facts or beliefs from
known
facts or beliefs using a knowledge base and a set of logical operations.
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
Knowledge Base: a collection of knowledge (e.g., objects, concepts,
relationships,
facts, rules, etc.) expressed in a manner such that it can be used by an
inference engine. For
example, a knowledge base may include rules and facts or assertions as in
traditional
expert systems.
One preferred embodiment of a decision support system of the present invention
includes the ability to assess the hemodynamic state of a subject's
cerebrovasculature
through the use of transcranial Doppler measurements. Referring to Figure 10
the
embodiment consists of three software modules: a Data Access 1010 module, a
Reasoning 1020 module, and a Graphical User Interface (GUI) module 1030. The
Reasoning 1020 module consists of two sub-modules: a situation assessment
module
comprising the PreAct DSA 1022 sub-module from Applied System Intelligence,
Inc.,
including the domain knowledge base 2362; and Reasoning Interface 1024 sub-
module.
Cognitive engines, other than DSA, may be used. The Reasoning Interface 1024
sub-
module serves to hide the details of interacting with the DSA 1022 sub-module
from other
objects. In this embodiment, these modules run sequentially as part of the
same process,
with one instance of each module.
The Data Access 1010 module provides access and storage methods for TCD
measurementldata, clinical data, and inferences from the Reasoning 1020
module. In a
preferred laptop personal computer configuration this collection of data is
stored in a file.
The GUI 1030 module processes user input to be sent to the Data Access 1010
module, runs commands for the Reasoning 1020 module, queries about patient
data for
the Data Access 1010 module, and queries about inference results for the
Reasoning 1020
module. The GUI 1030 module also displays patient data received from the Data
Access
1010 module and concept instances, related to the concept graph instances
received from
the Reasoning 1020 module.
The PreAct DSA 1022 sub-module accepts leaf level concepts representing
patient
data and processes them for inferred concepts such as disease. The current
concept graph
may be queried for all instances of a particular concept pattern or for
evidence supporting
a particular instance. The current graph may be saved for future queries and
saved
concept graphs may be reloaded for querying. The DSA 1022 sub-module also has
access
to the underlying knowledge base 2362. The Reasoning Interface 1024 sub-module
accepts commands to process patient data for inferred concepts, to search for
instances of
particular concepts or evidence for a given concept instance in the active
concept graph,
46
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
and to save the current concept graph or load a saved concept graph. The
Reasoning
Interface 1024 sub-module converts these commands into a command language
understood by the DSA 1022 sub-module.
This preferred embodiment makes use of the data structures found in Table 1.
DATA STRUCTURE DEFINITION
Patient ID Uni uel identifies each atient
Grou ID Uni uel identifies each grou of patients
in the system
Patient data blockContains TCD data and clinical data
for a patient. This
includes:
0 Data and measurement times for each
vessel test point;
O Demographic data, e.g., date of birth,
ethnic group;
0 Clinical data, e.g., vital signs,
test results
Filename Name of a conce t ra h file
Concept pattern Unique identifier of a concept pattern
ID
Conce t ke ID Uni ue ke of a conce t in stance
Concept instance Concept instance from a concept graph.
Derived concepts
include belief values.
List ofconcept List of concept instances from a conce
instances t gra h
List of concept List of keys for instances of a certain
keys pattern
Table 1
Patient data consists of data derived from TCD measurements and clinical data.
This data is used to fill in the leaf level concepts in the concept graph.
Patient data is
accessed and stored as a single block of data for each patient, referenced by
a unique
patient ID.
TCD measurements and data may be input in a streaming fashion via a network or
direct connection or as a file. Clinical data may be input as a file or
manually through the
GUI 1030 module. After completing data input, the user may elect to save the
data or file
for later access or to analyze the data. In either case, the Reasoning 1020
module
retrieves patient data via the Data Access 1010 module. For this purpose, the
GUI 1030
module stores data in a file. Both modules retrieve patient data by patient
ID.
Additionally, in order to allow a user to select a patient's data to view,
edit, or analyze,
the interface allows the GUI 1030 module to retrieve a list of all patients
saved in a file.
In preferred embodiments, the set of parameters passed to Data Access 1010
module
functions includes a user ID.
Inference data includes concept instances in the concept graph for a
particular
patient. The DSA 1022 sub-module provides its own accessors for loading a
concept
graph from a text file and saving a concept graph to a text file. The Data
Access 1010
47
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
sub-module is responsible for storing the file created by the Reasoning 1020
module.
Table 2 identifies commands used by the Data Access 1010 module.
COMMAND USED BY PARAMETERS RETURN
Initialize ModuleSystem layerNone Success/failure
Retrieve PatientGUI control,patient ID, Patient data
Data user ID block
Reasoning
Save Patient GUI controlPatient ID, Success/failure
Data user ID
Delete Patient GUI controlPatient ID, Success/failure
Data and user ID
Concept Graph
Retrieve List GUI controlUser ID List of patient
of Patients IDs
Store Patient Reasoning Patient ID, Success/failure
Concept user ID,
Graph filename accessible
b
Data Access
Module
Retrieve PatientReasoning, Patient ID, Filename accessible
Concept GUI user ID
Graph by Reasoning
Module
Query Database GUI SQL Query Query result
Table 2
The GUI 1030 module accepts input from the user, converts the user's input in
to
data and commands for other modules, and displays the values returned on the
screen or in a
printout. The GUI 1030 module provides for display of clinical and demographic
data for a
patient, raw TCD data and measurements, and an analysis of a patient's
hemodynamic state.
The analysis of a patient's hemodynamic state includes the condition of each
artery for
which TCD measurements are available, any global conditions found, and an
assessment of
the patient's risk for stroke. The GUI 1030 also allows a user to drill down
from a patient's
risk for stroke to determine how that conclusion was reached.
The Reasoning Interface 1024 sub-module allows other modules to access the
concept stored in the DSA 1022 sub-module without being exposed to all the
details of the
DSA 1022's interface. Reasoning Interface 1024 sub-module commands include
those in
Table 3.
COMMAND USED BY PARAMETERS RETURN
Initialize System layer None Success/failure
module
48
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
Run module GUI control Patient ID, Success/failure
with a user ID
patient's data
Get concept GUI control Concept patternList of concepts
ID
instances
Get concept GUI control Concept patternConcept
instance ID,
concept key
ID
Get concept GUI control Concept patternList of concepts
ID,
evidence concept key
ID
Load a patient'sGUI control Patient ID, Success/failure
user ID
concept graph
Save a patient'sGUI control Patient ID, Success/failure
user ID
concept graph
Table 3
The DSA 1022 sub-module includes methods for commanding the sub-modules,
including commands for initializing, starting, running, and stopping. The DSA
1022 sub-
module also includes services for setting and retrieving concept attribute
values.
Requests for DSA 1022 sub-module data are responded to with one of three
values: 1 - data found correctly; 0 - data not found but no critical error
occurred; and - 1
- critical error, see exception log file. In addition to requesting the value
of a particular
attribute in a known concept instance, the invention can request both an index
of concepts
and a deep copy of a particular concept instance. The system also responds to:
a user
request for a list of all child concept instances of a particular concept
instance; a user
request to clear all concept instances from the concept graph (patterns will
remain
loaded); a user request to save a concept graph to a specified file name (in
preferred
embodiments, this file will be saved as an XML file); and a user request to
load a saved
concept graph from a specified file name.
In a broad sense, this preferred embodiment allows as user to enter new
patient
data through the GUI 1030 and save the data; load existing patient data from a
database;
view raw data, e.g., clinical data and TCD data; analyze patient data for
inferences about
the patient's hemodynamic state; view results of an analysis; and view the
evidence used
to reach a particular inference.
Upon initialization, a main program instantiates and initializes the modules
and
sub-modules in the following order: Data Access 1010, Reasoning Interface 1024
(which
49
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
will initialize the DSA 1022), and GUI 1030. After initialization is complete,
control is
passed to the GUI 1030. Control remains with the GUI 1030 until the user signs
out, at
which point the main program shuts down the modules in the reverse order of
initialization. The Reasoning Interface 1024 module shuts down the DSA 1022.
Specific operation of the GUI 1030 module can include being initialized by one
or
more external commands. Operation of the GUI 1030 can further include
accepting a user
commands to sign in to the system; change the group of patients currently
being processed
(contingent upon authority of that user to have access to the data for the new
group);
create a new group; sign out of the system; create a new patient record;
process a patient's
data for inferences; edit data for a new or existing patient; save a patient's
data; display a
list of subjects in the specified group (including an indication of whether or
not a
hemodynamic analysis has been done on the patient's data; display patient data
for an
existing patient; display patient's overall risk of stroke; display an
explanation of a
patient's stroke risk, including concepts used as evidence and the ability to
drill down in
to evidence for further detailed display; and display the status of arterial
flow in all the
patient/subject's arteries for which data is available, including flow
characteristics at each
test point, global characterizations of blood flow, and the direction of blood
flow.
Specific operation of the Data Access 1010 module can include serving as an
interface to an existing relational database management system; accepting
commands for
initialization, shutdown, creation of a new patient record, retrieval of the
patient data
block for a specified patient, update of a patient's data, deletion of a
record, retrieval of a
concept graph, update of a concept graph, deletion of a concept graph; and
accepting a
query for a list of all patients in the database.
Specific operation of the Reasoning Interface 1024 sub-module can include
initialization by one or more external commands; accepting commands for
processing a
patient's data, saving the analysis of the current patient's data, loading a
saved analysis,
and stop processing; and accepting queries for instances of particular concept
patterns in
the concept graph, a particular concept instance, and further explanation of a
concept
instance.
Specific operation of the DSA 1022 sub-module can include initialization by
one
or more external commands; and use of knowledge bases to store concept
patterns and
knowledge base algorithms used to infer concepts from leaf level data
provided, with the
basis for the inferences being the TCD data and clinical data. The algorithms
infer the
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
concepts in several intermediate steps, each represented in the concept graph,
such that it
is sufficient for one skilled in the art of the problem domain to follow the
chain of
reasoning. The conditions represented in the concept graph include, but are
not limited to,
vasodilation, hyperemic vasodilation, pathological vasodilation, non-
compliance, and
irreversible stenosis. The concept graphs provide a path for following a chain
of
reasoning backwards from a conclusion. The algorithms use a plurality of
reasoning
techniques, e.g., Bayesian reasoning, to look for supporting data in related
concepts.
Further operation of the DSA 1022 sub-module can include loading knowledge
bases;
accepting patient data to be processed through transactions; allowing the user
to save the
concepts resulting from an inference and load saves concepts; and querying for
instances
of particular concept patterns in the current concept graph, particular
concept instances,
and further explanation of a concept instance. This querying can include
accepting a clear
command, and in response, clearing all concept instances from the current
graph; concept
patterns remain loaded; accepting a kill command to release all allocated
memory and
terminate; and writing non-fatal errors to a log file.
In another preferred embodiment, the invention is a networked based system and
method for analyzing the hemodynamic state of a subject based on TCD
measurements.
When using this embodiment, a user submits data to a centralized system for
analysis
similar to that described in the previous embodiment.
Referring to Figure 23 a block diagram illustrating the context and
relationship
between modules for the preferred Application Service Provider (ASP)
embodiment is
shown. The modules run in separate process spaces. The user interface (one or
more
instances of a Web Browser 2310) and System Interface 2320 are connected via a
network, in this case the Internet, using connection protocols known to those
skilled in the
art of computing. The System Interface 2320 Manager provides an adaptive layer
between the web server and the remainder of the system. The Accounts Manager
2340
maintains authorization and accounting data for each user account. The
Reasoning
Manager 2350 manages requests for analysis of data and queries of existing
analyses. It
also maintains connections to one or more instances of the Reasoning Module
2360. The
Reasoning Module 2360 encapsulates a DSA component in a fashion similar to the
earlier
described embodiment. The DSA component uses the invention's knowledge base to
analyze TCD data and provide access to results. The Reasoning Module 2360
provides
translations to and from the interface language use by the DSA component. The
51
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
Watchdog 2370 monitors invention performance for functioning within acceptable
parameters.
The invention is accessed via the Internet through a web site, using a
standard
browser 2310. Figures 24 through 27 illustrate the data available through
typical pages
displayed at the browser in response to appropriate user actions. The system
is entered
through a login page, an example of which is illustrated in Figure 24. In this
embodiment,
the same login page is used by both users and administrators. Based on the
identity of the
account, the invention will present either the administrator startup page or
the user startup
page. The administrator startup page provides an administrator with access to
administration functionality described below. The user startup page,
illustrated in Figure
25, lists those patients that are associated with the user. From this point,
the user may add
new patient data, edit existing patient data or delete patient data.
The patient data page, illustrated in Figure 26, displays clinical data on a
patient and
allows a user to edit this data. The patient data page also provides access to
the TCD data
tab for that patient. The TCD data tab for a patient, provides access to TCD
measurements.
The user may add new TCD measurements, view existing measurements, edit, or
delete
measurements. This page provides further access to the hemodynamic analysis
tab,
illustrated in Figure 27, for the patient. The hemodynamic analysis tab
displays the result of
an analysis of a patient's TCD data. If no analysis has been performed on a
set of TCD
readings, the user may request that such analysis be performed from this page.
T'he Knowledge base 2362 maintains the lrnowledge for TCD analysis. The
inventions analytical techniques may be modified by changing these Knowledge
base 2362
files. The Patient database 2382 stores data about a patient pertinent to
analysis of his TCD
data. Each patient is assigned a unique ID by the user of the system.
Information contained
in the Patient database 2382 includes that shown in Table 4
ITEM DESCRIPTION
User ID Unique identifier for the user of the system
Patient ID Unique identifier for this patient within
this user's patients
Date of birthPatient's date of birth
Sex Patient's generic sex
Ethnic group Patient's ethnic group
For each set
of TCD readings
for this
patient:
Reading date Date of reading
For each reading
within a
set of TCD
readings
52
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
ITEM DESCRIPTION
Segment ID Arterial segment from which the reading
was taken
Depth Depth of the reading (mm)
PSV Peak systolic velocity
PSVTime Timestamp of PSV reading (sec)
EDV End diastolic velocity
EDVTime Timestamp of EDV reading (sec)
Table 4
The Patient Analysis Database 2384 stores the Reasoning 1020 module's analysis
of
a set of TCD data. The analysis is stored as a file in a format that can be
read into the
Reasoning 1020 module, e.g., an extensible Markup Language (XML) file.
Information
contained in an entry in the Patient Analysis Database 2384 includes the
information in
Table 5.
ITEM DESCRIPTION
User ID Unique identifier for the user of the system
Patient Unique identifier for this patient within
ID this user's patients
Reading Patient's date of birth
ID
Analysis Output file from the patient's concept graph
Table 5
The Authorization Database 2342 stores the IDs and passwords of authorized
users
and administrators. Information contained in an entry in the Authorization
Database 2342
includes the information in Table 6.
ITEM DESCRIPTION
User ID Unique identifier for the user of the system
Password Encrypted password for the user
Account User or Administrator
type
Table 6
53
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
The Transaction Log 2344 records activity of users and administrators in the
system,
Information contained in the Transaction Log 2344 includes the types found in
Table 7.
TRANSACTION NAME TRANSACTION FIELDS
Log in User ID
Timestamp
Failed log in User ID
Invalid password
Timestamp
Log out User ID
Timestamp
Add new patient User ID
Patient ID
Timestamp
Edit patient data User ID
Patient ID
Timestamp
Delete patient User ID
Patient ID
Timestamp
Analyze patient User ID
Patient ID
Reading ID
Timestamp
Display patient listUser ID
Timestamp
54
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
Display patient User ID
Patient ID
Timestamp
Create new account Administrator ID
New account ID
Account type (user or administrator)
Timestamp
Delete account Administrator ID
Account ID
Timestamp
Download Transaction Administrator ID
Log
Timestamp
Download AuthorizationAdministrator ID
Database
Timestamp
TimPCtamn
Table 7
System Database 2390 stores data used to provision the application's process.
Examples include parameters for the IPC connections and the location of the
data files
specified in the above description.
Knowledge structures are defined and developed over the lifecycle of the
invention; both for this embodiment and for other preferred embodiments. The
knowledge structures identify broad functionality to envision the invention's
behavior.
Preferred embodiment of the present invention use a concept graph (CNG) for
knowledge
representation. The CNG, see Figures I I through 22, contain input data to the
system and
inferred states form the input data. Arrows in the concept graph represent the
direction of
inference. The inferences culminate in the top-level Stroke Risk concept.
The system provides various functionality to authorized users, including
logging in
using an existing account; setting up a new patient record; editing an
existing patient
record; requesting and obtaining an analysis of a previously entered set of
patient TCD
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
readings; requesting and obtaining a list of all patients for which that user
has entered
data, with the existence of an analysis indicated; requesting and obtaining a
display of
previously entered data and, if available the analysis of that data; deleting
patient data
entered by that user; deleting a TCD reading set; and logging off.
The system provides various functionality to authorized system administrators,
including logging in; creating a new account; listing all existing accounts;
deleting an
existing account; downloading transaction data; changing the e-mail address to
which
notifications are sent by the Watchdog 2370; and logging off.
Upon initialization, a main program instantiates and initializes the modules
in the
following order: Watchdog 2370, System Interface 2320, Accounts Manager 2340,
Data
Manager 2380, Reasoning Manager 2350. These modules run in separate process
spaces
from the main program. Upon shutdown, a main program shuts down the modules in
the
following order: Reasoning 1020 module, Data Manager 2380, Accounts Manager
2340,
System Interface 2320, Watchdog 2370.
The System Interface 2320 is initialized by external command. It converts data
submitted in hypertext markup language (HTML) into commands for other system
modules, and conversely, reformats data from other system modules into
outbound HTML
pages for presentation to a user. The System Interface 2320 module maintains a
list of
users currently logged into the system and automatically logs a user off after
some time of
inactivity. The System Interface 2320 accepts a shutdown command accepts
requests for
system data from other modules.
The Data Manager 2380 can be initialized by an external command, and maintains
data in persistent storage. The Data Manager 2380 is able to accept and
respond to
various commands, such as retrieve the IDs of patients entered by a particular
user; set up
a new patient record; retrieve a patient's data; modify a patient's data;
store the analysis
of a particular TCDV reading; retrieve the analysis of a particular TCDV
reading; delete a
patient's records; and shut down.
The Accounts Manager 2340 can be initialized by external command, and can
accept
transactions to be recorded in a Transaction Log 2344. The Accounts Manager
2340 can
accept and respond to commands such as create a new account; delete an
existing account;
validate an account ID and password (if the account ID and password are valid,
the
Accounts Manager 2340 can indicate in the reply whether this account is a
regular user or
56
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
an administrator); download the Transaction Log 2344; download the
Authorization
Database 2342; and shut down.
'The Reasoning Manager 2350 can be initialized by an external command. Upon
initialization, the Reasoning Manager 2350 initializes one instance of the
Reasoning 1020
module. The Reasoning Manager 2350 maintains connections to all existing
instances of
the Reasoning 1020 module. The Reasoning 1020 modules run in a separate
process space
from the Reasoning Manager 2350. The Reasoning Manager 2350 initialize
additional
instances of the Reasoning 1020 module or delete instances of the Reasoning
1020 module
as necessary to optimize the system load.
The Reasoning Manager 2350 is able to accept and respond to various commands
such as analyze a patient's data. The patient's data is assumed to be
accessible through the
Data Manager. T'he Reasoning Manager 2350 retrieves the data from the Data
Manager,
loads it into a particular Reasoning 1020 module, and issues a command to the
Reasoning
1020 module to analyze the data. The Reasoning Manager 2350 is further able to
accept
and respond to other various commands such as query a patient's analysis for a
particular
concept instance. In this instance, the Reasoning Manager 2350 loads the
analysis into a
Reasoning 1020 module, if necessary, and sends a query to the Reasoning 1020
module.
The Reasoning Manager 2350 is further able to accept and respond to other
various
commands such as query a patient's analysis for all instances of a particular
concept pattern.
In this instance, the Reasoning Manager 2350 loads the analysis into a
Reasoning 1020
module, if necessary, and sends a query to the Reasoning 1020 module. The
Reasoning
Manager 2350 is further able to accept and respond to other various commands
such as
query a patient's analysis for further explanation of a concept instance. If
necessary, the
Reasoning Manager 2350 loads the analysis into a Reasoning 1020 module and
sends a
query to the Reasoning 1020 module. The Reasoning Manager 2350 is further able
to
accept and respond to other various commands such as shut down. When shutting
down,
the Reasoning Manager 2350 preferably shuts down all instances of the
Reasoning 1020
module.
Reasoning 1020 module is initialized by an external command. No other commands
are processed until the module is initialized. The Reasoning 1020 module
Applied System
Intelligence, Inc.'s PreAct DSA 1022 module to store and analyze data using a
concept
graph. The Reasoning 1020 module uses a knowledge bases independent of the
PreAct
library to store the concept patterns and necessary algorithms. These
knowledge base
57
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
2362s are loaded after the module is initialized. The algorithms use various
reasoning
techniques, e.g., Bayesian reasoning, to propagate belief values through the
graph.
Sample concept graphs can be found at Figures 11 through 22. T'he Reasoning
Module
2360 provides accessors to input patient data into the concept graph.
The Reasoning Module 2360 accepts and responds to various commands such as
clear the current concept graph; analyze a patient's data (preferably, the
module sends a
notification when the analysis is complete); save the analysis of the current
patient's data
(preferably, the module sends a notification when the save is complete); load
a saved
patient analysis; and stop.
The Reasoning 1020 module can accept and respond to one or more queries for
all
instances of a particular concept pattern in the concept graph; a particular
concept
instance; and further explanation of a concept instance. The Reasoning 1020
module is
further able to write non-fatal errors to a log file.
The Watchdog 2370 includes an off the-shelf module chosen to be initialized by
an external command which will set all necessary parameters; to send a
notification to a
specified set of e-mail addresses when the available disk space drops below a
preset level;
to send a notification to a specified set of e-mail addresses when the system
load exceeds
a preset level; and to accept and respond to a command to change the set of e-
mail
addresses to which notifications are sent.
An exemplary network architecture of an exemplary system in accordance with
the
present invention is described below. The exemplary system comprises one or
more client
stations, a central server and a communications link. The one or more client
stations
function as remote access points to the central server. A client station may
be located in a
laboratory, a physician office and/or at any other appropriate site. A client
station may be
configured for transmitting and/or receiving information to or from the
central server in
either an interactive mode or a batch mode.
Client stations may comprise any type of computer-like device that is capable
of
sending and/or receiving data. For example, a client station may comprise a
desktop
computer, a laptop computer, a hand-held device, or the like. A client station
may also
comprise a laboratory instrument having functionality for collecting raw data
(such as
patient vascular data), and for transferring that raw data to the central
server via the
communications link. A client station may also comprise a device for receiving
raw data
from a laboratory instrument, such as a flow analytical device, or a device
holding data
58
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
transmitted from a flow analytical device, and then passing that data to the
central server
via the communications link. These and other examples of client station
configurations
will be apparent to those of ordinary skill in the art.
A first client station may be configured to transmit raw data to the central
server
via the communications link and a second client station may be configured to
receive
processed data (results) from the central server via the communications link.
A client
station may implement various user interfaces, printing and/or other data
management
tasks and may have the ability to store data at least temporarily.
The communications link may comprise a dedicated communications link, such as
a dedicated leased line or a modem dial up connection. Alternately, the
communications
link may comprise a network, such as a computer network, a telecommunications
network, a cable network, a satellite network, or the like, or any combination
thereof. The
communications link may thus comprise a distributed network and/or one or more
interconnected networks. In an exemplary embodiment, the communications link
may
comprise the Internet. As should be apparent to those of skill in the art, the
communications link may be land-line based and/or wireless. Communications
over the
communication link between the client station and the central server may be
carried out
using any well-known method for data transmission, such as e-mail, facsimile,
FTP,
HTTP, and any other data transmission protocol.
The central server comprises the computer-based database of vascular
information.
The central server implements analytic and interpretive algorithms. It will be
apparent to
those of skill in the art, however, that the communication station and the
computation
station may be implemented in a single computer. The configuration of an
exemplary
central server will be described in greater detail below.
A system in accordance with an exemplary embodiment of the present invention
may operate in an interactive mode or a batch mode. In the interactive
operating mode,
data samples are processed one by one interactively. For example, in an
interactive
processing mode, a user connects to the central server through a client
station. A data
sample to be processed is then sent from the client station to the central
server. The
processed data (result file) is returned from the central server to the client
station, where it
may be printed and/or archived. After the result file is received at the
client station, a
subsequent data sample may then be transmitted from the client station to the
central
server.
59
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
An exemplary system configured for an interactive processing mode is now
described. A client station may be configured for execution of a communication
browser
program module and one or more printing and/or archiving program modules. As
is
known in the art, a convenient and effective communication link for
facilitating
interactive operations is the Internet. Communication browsers are also known
as World
Wide Web browsers or Internet browsers.
The components of the central server may be distributed among two stations, a
communications station and a computation station. Configured for an
interactive
processing mode, the communications station may comprise a communications
server,
such as a standard http server, for interacting with the communication browser
executed at
the client station. Communications between the communications server and the
communication browser may occur using html pages and computer graphics
interface
(CGI) programs transferred by way of TCP/IP.
Substances
In one preferred embodiment of the present invention, vascular reactivity to
substances may be evaluated. Substances include, but are not limited to,
alcohol, nicotine,
foodstuffs, extracts of plants, nutraceuticals, and drugs. Many drugs are
known to have
effects on the vascular system. A non-limiting list of classes of drugs and
drugs known to
have affects on the vascular system includes the following: beta
adrenoreceptor
antagonists; calcium channel antagonists; angiotensin I converting enzyme
inhibitors;
alpha adrenoreceptor antagonists; cholesterol antagonists; angiotensin II 1
antagonists;
HMGCoA reductase inhibitors; thrombin inhibitors; adrenoreceptor antagonists;
endothelin A receptor antagonists; NMDA antagonists; platelet aggregation
antagonists;
NMDA antagonists; platelet aggregation antagonists; sodium channel
antagonists; 5-
hydroxytrypltamine la agonists; AMPA receptor antagonists; GPIIb IIIa receptor
antagonists; lipase clearing factor stimulants; potassium channel agonists;
potassium
channel antagonists; 5-alpha reductase inhibitors; acetylcholine agonists;
dopaminergic
agonists; endopeptidase inhibitors; estrogen antagonists; GABA receptor
agonists;
glutamate antagonists; peroxisome proliferator-activated receptor agonists;
plasminogen
activator stimulants; platelet-derived growth factor receptor kinase
inhibitors; prostacyclin
agonists; sodium/hydrogen exchange inhibitors; vasopressin 1 antagonists; 15-
lipoxygenase inhibitors; acetyl CoA transferase inhibitors; adenosine A1
receptor agonists;
aldose reductase inhibitors; aldosterone antagonists; angiogenesis stimulants;
apoptosis
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
antagonists; atrial peptide antagonist; beta tubulin antagonists; bone
formation stimulants
caspase inhibitors; CC chemokine receptor 2 antagonists; CD18 antagonists;
cholesterol
ester transfer protein antagonists; complement factor inhibitors;
cyclooxygenase
inhibitors; diuretics; DNA topoisomerase ATP hydrolyzing inhibitors; elastase
inhibitors;
endothelial growth factor agonists; enkephalinase inhibitors; excitatory amino
acid
antagonists; factor Xa inhibitors; fibrinogen antagonists; free radical
scavengers;
glycosylation antagonists; growth factor agonists; guanylate cyclase
stimulants;
imidazoline I1 receptor agonists; immunostimulants; immunosuppressants;
interleukin 1-
beta converting enzyme inhibitors; interleukin 8 antagonists; LDL receptor
function
stimulants; MCP-1 antagonists; melanocortin MC-4 antagonists;
mineralocorticoid
antagonists; nerve growth factor agonists; neuropeptide Y antagonists; oxygen
scavengers; phosphodiesterase inhibitors; potassium sparing diuretics; proline
hydroxylase inhibitors; prostaglandin El agonists; purinoreceptor P2T
antagonists;
reducing agents; thromboxane A2 antagonists; thyroid hormone function
agonists;
transcription factor inhibitors; vasopressin 2 antagonists; and vitronectin
antagonists,
among others.
In addition, other agents are suspected of having vascular activity. These
agents
are include, but are not limited to, danaparoid sodium, nitric acid
scavengers,
clomethiazole, remacemide, TP 10, cerivastatin, nimodipine, nitrendipine, BMS-
204352,
BIII-890, dipyridamole +ASA, fradafiban, irampanel hydrochloride,
lefradafiban,
aptiganel, sipatrigine, NRTs, cromfiban, eptifibatide, nematode anticoagulant
protein
NAPc2, UK-279276, Flocor, DMP-647, ASA, GPI-6150, dermatan sulfate, NOS
inhibitors, ancrod, PARP inhibitors, tinzaparin sodium, NOX-100, LDP-O1,
argatroban,
fosphenytoin, tirilazad mesylate, dexanabinol, CPC-211, CPC-111, bosentan,
clopidogrel
hydrogen sulfate, nadroparin, ticlopidine, NS-1209, ADNF III, vinconate, ONO-
2506,
cilostazol, SUN-N4057, SR-670291, nicardipine, YM-337, and YM-872.
The present invention may be utilized following administration of the drug
through
acceptable methods of administration to evaluate the effects on vessels. It is
to be
understood that the present invention may be practiced with regard to
different vessels,
including but not limited to, vessels in the extremities, in the coronary
circulation, and
extracranial and intracranial cerebral vessels. In a preferred embodiment, the
extracranial
and intracranial cerebral vessels are examined with the present invention.
61
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
Measurements may be taken before administration of the drug, and at specific
times following administration of the drug to determine the effect of the drug
on vascular
reactivity. In this manner, each individual subject and each individual vessel
acts as its
own control to assess the effects of that drug on that specific vessel.
All cerebral vessels may be analyzed to determine whether the drug has
differential effects on different cerebral vessels. By performing such an
analysis over
numerous individuals, valuable data may be obtained concerning the vascular
effects of a
specific drug. Furthermore, by choosing individuals from different groups,
such as (a)
individuals with no known pathology, (b) individuals with no known pathology
in specific
age groups, (c) individuals with known pathology in a specific disease group,
(d)
individuals with known pathology in a specific disease group in a specific age
range or in
a specific stage of the progression of the disease, and (e) individuals in a
specific disease
group currently receiving specific therapeutic mediations.
Through application of the present invention to individuals from the desired
group,
valuable information may be obtained concerning the effects of different
disease
processes, or prior or co-administration of other drugs, on the vascular
effects of the test
drug in different individuals, at different ages, and in different conditions.
It will be appreciated that a preferred embodiment of the present invention
allows
for the assaying of the efficacy of a treatment comprising collecting data
regarding
cerebrovascular health status of a number of individuals serving as patients
in the clinical
trial; grouping the patients into at least two groups of patients such that
patients with a
similar cerebrovascular health status are grouped together; applying the
treatment to the at
least two groups of patients; monitoring outcomes of the treatment for each of
the at least
two groups of patients; and determining the efficacy of the treatment based on
the
outcomes of the treatment for each of the at least two groups of patients. In
a preferred
embodiment of the present invention, the data regarding cerebrovascular health
status
comprises mean flow velocity value for at least three cerebrovascular vessels
of the
individuals and systolic acceleration value for at least three cerebrovascular
vessel s of the
individuals. In another preferred embodiment of the invention, the data
regarding
cerebrovascular health status further comprises calculating a pulsatility
index.
Another preferred embodiment of the present invention provides a method of
screening for adverse effects of a treatment comprising: applying the
treatment to a
number of individuals; monitoring the cerebrovascular blood flow of such
individuals
62
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
after applying the treatment; and identifying adverse effects to
cerebrovascular blood flow
in such individuals arising after applying the treatment. In a preferred
embodiment,
quantitative data regarding the cerebrovascular blood flow of a number of
individuals is
obtained. In a still further preferred embodiment of the present invention,
the data
regarding cerebrovascular health status comprises mean flow velocity value for
at least
three cerebrovascular vessels of the individuals and systolic acceleration
value for at least
three cerebrovascular vessels of the individuals. In still a further preferred
embodiment,
the data regarding cerebrovascular health status further comprises calculating
a pulsatility
index.
It will be appreciated that the present invention allows for the creation of
matched
groups with a suite of blood vessel issues, e.g., plaque and general
vasculitis, among
others. The present invention also provides for the creation of matched groups
with a
particular circulatory problem, e. g., stenosis in a particular vessel,
inadequate profusion of
small blood vessels in posterior of brain, migraines, and apnea, among others.
Under conventional approaches to clinical trials, one cannot identify
participants
with such problems, much less match participants wherein both groups have
essentially
the same severity and incidence of the pathology being examined. Thus, the
conventional
approach to clinical trials (1) address much less specific conditions, e. g.,
overall stroke
risk, rather than the precise severity and incidence of the pathology being
examined, (2)
include individuals who show no disease/deterioration, and (3) include
individuals who
are likely to suffer immediate catastrophic failure. Despite numerous attempts
to conduct
clinical trials related to primary stroke prevention where there is no
previous history of
stroke or acute cardiac event, this problem has remained unsolved until now.
EXAMPLE I
Effects ofPropranolol on YascularReactivity
Propranolol, also known as Inderal, is prescribed routinely for individuals
with
hypertension, one of the major risk factors for stroke. In order to assess the
effects of
propranolol on vascular reactivity, a transcranial Doppler analysis was
performed on the
cerebral vessels of a 46 year old hypertensive man. Propranolol was then
administered at
an oral dosage of about 40 mg. Another transcranial Doppler analysis was
performed
approximately two hours after administration of the propranolol. Changes in
specific
vessels were compared to pre-administration readings. By analyzing pre- and
post-
63
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
administration vessel dynamics, an indication of the effect of the beta
adrenergic blocker,
propranolol, on dynamics of flow in specific cerebral vessels is obtained.
EXAMPLE 2
Analysis of the Effects of Plavix oh Cerebral Vessels
Plavix is a member of a class of drugs known as blood thinners or anti-
platelet
drugs. Plavix is often prescribed following stroke to minimize platelet
aggregation and
clot formation. However, one of the major dangers of Plavix is intracranial
hemorrhage.
Therefore, when using Plavix to prevent or minimize the possibility of a
stroke due to
infarction, one may increase the possibility of a hemorrhagic stroke.
Accordingly,
properly selecting the appropriate patient for Plavix is critical for
maintenance of vascular
health.
A 63 year old male with a history of hypertension experiences a first stroke
in the
left middle cerebral artery resulting in deficits in the right hand, leg, and
some deficits in
motor speech. These are the symptoms upon presentation in the neurological
clinic.
Transcranial Doppler analysis of all cerebral vessels is performed in addition
to analyzing
the common carotid artery and the internal carotid artery. The analysis
reveals alterations
in vascular flow in the internal carotid artery just distal to the bifurcation
of the common
carotid artery. A stenotic area is observed. Further, additional flow
abnormalities are
detected in the left middle cerebral artery, consistent with the patient's
presentation of
right-sided motor paralysis. Transcranial Doppler analysis reveals excellent
collateral
flow to the contralateral hemisphere and no deficits in the left anterior
cerebral and left
posterior cerebral arteries.
The physician considers prescription of Plavix together with a calcium channel
blocker. Transcranial Doppler analysis was performed at monthly intervals. By
analyzing
changes in the individual cerebral vessels as a function of Plavix +/- calcium
channel
blocker administration, the physician observes no effect on the cerebral
vessels. The
physician subsequently administers a higher dose. Again, transcranial Doppler
analysis is
performed on all cerebral vessels. The physician observes marked changes in
the vascular
dynamics of the vessel studied as the pulsatility index decreases and the auto-
regulation
curve left-shifts toward normal. The physician, based on these results,
determined a
proper dosage of the vasoactive medication for the patient.
64
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
The patient is then monitored on a monthly basis after the initial
prescription of
Plavix in order to determine whether vascular changes are occurring which
necessitate
alteration in the therapy.
EXAMPLE 3
Assessment of Cerebral Vascular Status During Battlefield Situations
A 21 year old paratrooper jumps from an airplane to reach the battlefield
below.
While parachuting to the surface, his parachute becomes entangled in the
branches of a
large tree. The serviceman hears gunfire in the vicinity of his location and,
in an attempt
to free himself, cuts one of the lines connecting the parachute to his
harness. He falls to
the earth but his head strikes a major branch of the tree during descent. The
serviceman is
found unconscious by a field medic. After determining that no cervical
fracture is
present, the medic removes the serviceman to a field hospital. Transcranial
Doppler is
performed by the medic trained in such techniques. The data is acquired and
transmitted
by an uplink satellite communication to a battlefield command center hospital.
Prior data
on the serviceman is compiled during routine physical examination at the time
of
induction into the service. The new transcranial Doppler data is compared to
the prior
data. The results indicate dramatic changes in auto-regulation of the left
anterior cerebral
artery. This is caused by vasospasm due to a subarachnoid hemorrhage from
blunt force
trauma at the fronto-parietal suture. There is also a subdural hematoma. The
field
physician suspects this possibility in view of the contusions evident in the
region of this
suture. The results of the comparative analysis of the cerebral vessels are
transmitted to
the field physician who then performs an emergency craniotomy in the region of
the left
fronto-parietal suture. Following release of pressure on the brain and
stabilization of the
patient, a transcranial Doppler analysis is performed immediately post
surgery, and at 12
and 24 hours thereafter. The results indicate that the left anterior cerebral
artery flow
dynamics are changing and the characteristic of this vessel moves from the
lower right
quadrant on the plot of flow velocity versus systolic acceleration toward the
region of
normal auto-regulation.
Another scenario is development of spasm or post-traumatic hyperemia at
24° C
with clinical deterioration. Transcranial Doppler analysis was performed at
the field
hospital. Worsening vasospasm was found and the treatment altered in response.
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
EXAMPLE 4
Application of Transcranial Doppler Analysis in the Emergency Room
A 23 year old is admitted into the emergency room in a state of extreme
agitation
and mania While the medical staff is attempting to obtain a blood workup and
waits for
the results of the analysis, the patient suddenly falls unconscious. Blood
pressure is
observed to drop precipitously. Transcranial Doppler analysis is performed on
the
cerebral vessels of the patient. The results indicate a shifting to the lower
left of the
normal regulation curve for the left middle cerebral artery.
Electrocardiagraphic analysis
reveals atrial fibrillation. Blood chemistry reveals that the patient took a
large dosage of
cocaine together with amphetamine. The results of the transcranial Doppler
analysis are
consistent with induction of cerebral vascular failure which was secondary to
a heart
attack due to extreme vessel constriction of the coronary vasculature.
EXAMPLE 5
Case Study of a Female Who Presented With Unsteady Gait
A 62 year old female presented in the neurological clinic complaining of
slight
feelings of unsteadiness during walking. Transcranial Doppler analysis was
performed
and the different cerebral vessels were analyzed. The initial nomogram
schematic
representation of a 2-dimensional nomogram of the transcranial Doppler
sonography data,
in which mean flow velocity is indicated on the y-axis and systolic
acceleration is
provided on the x-axis, is provided in Figure 9a. Shortly thereafter, the
patient's
symptoms worsened, however, no definitive diagnosis was yet established.
Transcranial
Doppler analysis was performed a second time and the transcranial Doppler
sonography
data was represented in a second nomogram provided in Figure 9b. The results
were
compared to the first test and showed a clear shifting to the right on the
flow velocity
versus systolic acceleration plot.
Next, the patient was hospitalized in critical condition and yet no diagnosis
had
been established. The technician performed another transcranial Doppler test
and the
transcranial Doppler sonography data was represented in a third nomogram
provided in
Figure 9c. A dramatic shifting to the right of many of the vascular points was
observed.
A cisternogram revealed hydrocephalus, so a shunt was inserted. The
neurologist
concluded that an increased intracranial pressure had exerted a deleterious
effect on the
66
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
cerebral vessels displacing them from the normal auto-regulation zone.
Following
surgery, a fourth transcranial Doppler analysis was performed and the
transcranial
Doppler sonography data was represented in a fourth nomogram provided in
Figure 9d.
The results showed a clear return toward baseline, i.e., a left shifting in
the characteristic
data points for the vessels analyzed toward their prior location at the time
of the second
test.
This example demonstrated that the results from the transcranial Doppler
analysis,
a non-invasive and highly accurate test, provided valuable information for the
neurologist
to select an appropriate course of action thereby probably preventing a
massive increase in
intracranial pressure resulting in an occlusive stroke and probable death.
These results
also provided an indication of the onset of the life-threatening changes that
occurred
between tests 2 and 3.
EXAMPLE 6
Use of Transcranial Doppler to Analyze Blunt Force Trauma in an Athlete
During a soccer match, a 17 year old high school student receives a severe
blow to
the forehead when he and an opponent jumped together to head the ball. The
student
becomes unconscious but is then revived with smelling salts. After the game,
he
complains of changes in his vision. He is taken to the emergency room and a
transcranial
Doppler analysis is performed. The results of the analysis are compared to a
transcranial
Doppler analysis performed at the beginning of the soccer season. Transcranial
Doppler
analysis shows a slight change in the flow dynamics of the left posterior
cerebral artery
indicating hyperemia or increased flow often observed in patients with
cerebral
contusions. Twenty-four hours later the patient's mental state deteriorates
and a CT scan
only reveals subarachnoid blood. A repeat transcranial Doppler analysis shows
vasospasm of the same artery. An interventional neuroradiologist is called
into the case
and performs angioplasty. Following the procedure, transcranial Doppler
analysis is
performed periodically over a 6 week period. The results are compared to the
transcranial
Doppler profile at the time of admission to the emergency room and also to the
normal
readings obtained at the beginning of the soccer season. The results show a
gradual return
to the normal flow patterns for the left posterior cerebral vessel.
67
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
.... ..
EXAMPLE 7
Use of Transcranial Doppler to Analyze Blunt Force Trauma in the Vascular
Effects of a
Drug
A pharmaceutical company has developed a new substance which it suspects may
have antihypertensive activity by inducing partial dilation of blood vessels.
The company
selects a patient population of individuals with normal blood pressure, a
population with
mild hypertension, and a population with severe hypertension. Sub-populations
are
constructed based on age (fourth, fifth and six decades of life) and sex.
The cerebral vessels of all patients are analyzed using transcranial Doppler
analysis, as described in the present invention, two hours before and two
hours following
oral administration of 25 mg of the test substance. Blood pressure was
monitored af30
minute intervals for the two hours before and two hours following oral
administration of
the new substance. The results demonstrate no discernable effect in the
normotensive and
mildly hypertensive group, and a significant anti-hypertensive effect in the
severely
hypertensive patients in all age groups tested. Analysis of the data obtained
with
transcranial Doppler revealed a decreased flow velocity in the vessels of the
great arterial
circle.
Significant variation is detected in the data set from the female test groups
in the
fifth and sixth decades of life. Further questioning of these individuals
revealed use of
antimenopausal hormone replacement therapy through combined administration of
estrogen
and progesterone. Removal of data contributed from these individuals
dramatically
decreases variance in these test groups. The pharmaceutical company initiates
a new study
to examine the potential interactions of the test substance with estrogen,
progesterone, or a
combination of estrogen, and progesterone, in normotensive, mildly
hypertensive, and
severely hypertensive females in premenopausal and postmenopausal groups,
further
subdivided by history of hormone replacement therapy or exposure to oral
contraceptives.
The invention as disclosed above is also applicable as both a system and
method for
assessing and treating hydrocephalus. Specifically, the invention provides a
system and
method for identifying critical variables affecting the intracranial space,
including increased
intracranial pressure (ICP), and is capable of being used to distinguish
patients suffering
from one of several forms of hydrocephalus from the normal population.
Hydrocephalus is a condition characterized by increased intracranial pressure
resulting in decreased intracranial blood flow. Raised intracranial pressure
puts additional
68
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
external force on vessels, compressing small vessels such as terminal
capillaries andlor the
capillaries of the vaso-vasorum, which supplies blood to arterial walls.
Diminished flow to
the vaso-vasorum reduces the ability of the smooth muscle of an arterial wall
to relax,
thereby diminishing the compliance of the conductance vessels. The combination
of
diminished compliance and increased impedance limits vascular performance.
Specifically,
this flow limitation affects the deeper brain structures fed by deep
penetrating arteries such
as those in the periventricular space. This decrease in flow
characteristically results in
edema formation at the ventricular horns which is believed to be a watershed
ischemic
event.
Very little is known in most cases about the cause of hydrocephalus. It has
been
observed to affect patients with a variety of conditions including, for
example, meningitis or
intracranial hemorrhage (e.g. subarachnoid hemorrhage) and it has been
speculated that it
can be precipitated by certain metabolic disorders or general inflammatory
states. It may
also affect people, particularly the elderly, who exhibit no preexisting
condition. The
hydrocephalus condition often seen in the elderly is known as Normal Pressure
Hydrocephalus (NPH).
NPH is a neurological disorder. While its exact cause is unknown, there are
several
competing theories as to its cause. The main postulated theory is that NPH
results from
increased intracranial pressure on brain tissue due to improper or inefficient
reabsorption or
clearance of accumulated cerebrospinal fluid. Spinal fluid is generated at a
rate of half a
liter a day and must be reabsorbed. Given that the cranium represents a finite
space, an
equilibrium must exists between fluids entering and leaving that space
otherwise the
pressure within will increase. Modern studies indicate that the generation and
reabsorption
of spinal fluid is an active process, as opposed to a passive one. As such, it
is predisposed
to deterioration and breakdown from various causes that can lead to an
accumulation of
excess fluid and a resulting increase in intracranial pressure. A second
theory asserts that
the increased intracranial pressure associated with NPH is caused by disease
of the small
vessels in the brain leading to cortical atrophy (i.e. diminished flow to the
small vessels
leading to a relative enlargement of the ventricles). It is also possible that
NPH results from
a combination of these theories-- a concurrent vascular change due to
transient spinal fluid
accumulation when a patient is recumbent at night that is associated with
diminished venous
flow outside of the cranium resulting in a blood volume build-up within the
cranial vascular
space causing a relative increase in pressure. Data derived from the invention
speaks
69
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
conclusively to the fact that NPH is the result fluid accumulation that in
turn creates
vascular disorder. The invention has further enabled the specific
characterization (i.e.
monitoring and diagnosis) of that vascular disorder throughout the onset,
treatment and
follow-up care of NPH.
Considerable confusion exists in modem medicine distinguishing these two
suspected root causes of NPH. Conventional imaging studies show nothing more
than an
increase in the space occupied by cerebrospinal fluid. These studies, however
cannot
comment directly on the behavior of the fluid. That is, MRI or CAT scans can
only show
concurrent fluid dilation associated with brain atrophy. These "causes"
standing alone,
however, are commonly interpreted .as nothing more than age-related changes
instead of
treatable causes of another condition (i.e. NPH).
Further complicating accurate diagnosis of NPH is that it is characterized by
the
"classical symptom triad" of incontinence, dementia and unsteadiness of gait,
though other
symptoms are often present or more prevalent. These symptoms can often be
mistakenly
attributed to other causes. As a result, NPH is frequently misdiagnosed
because it
historically requires a high index of suspicion on the part of the treating
physician. Once
suspected, NPH is difficult to definitively assess and diagnose accurately.
Conventionally,
confirming a diagnosis of NPH entails performing an invasive procedure, known
as a
cisternogram, comprising injection of a radioactive tracer substance into the
subdural space
(i. e. the cerebrospinal fluid space) and monitoring the uptake of the tracer
at particular
points in the cranium using a nuclear detector at 24, 48 and 72 hour intervals
after the initial
injection in an effort to semi-quantitate the clearance of that radionuclide
tracer. Other
methods of diagnosing hydrocephalus and NPH include repeated lumbar puncture
testing,
which is the withdrawal of anywhere from 20 to 40 cc's of spinal fluid to see
if a patient
gains clinical improvement. The most marked improvements being in gait and
mentation.
Continuous pressure monitoring of the spinal fluid pressure can also be
performed via an
indwelling catheter. However, this methodology is performed only at those
institutions
having specialized critical care units dedicated to this task. Furthermore,
this method
entails a very high risk of infection (i.e. a meningitis).
While a cisternogram or other clinical study can be indicative of NPH
condition,
alone they typically cannot definitively diagnose a patient with NPH because
they do not
sufficiently exclude other causes of the observed symptoms. The only
definitive diagnostic
procedure entails a major invasive neurosurgical procedure. The presence of
the symptoms
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
alone, however, usually does not warrant performing such a procedure.
Accordingly, it has
been notoriously difficult to both accurately and quickly assess and diagnose
NPH.
Finally, by the time the classic triad of symptoms appears in a patient
sufficient to
arouse the suspicions of the treating physician, considerable injury to the
central nervous
system has already occurred. Given that the central nervous system has very
little capacity
for damage repair, especially in the elderly, it is highly desirable to have a
system capable
of being used to both preventively monitor patients before symptoms become
evident and to
quickly and accurately diagnosis a patient once the symptoms have been
expressed.
The use of the dynamic vascular analysis (DVA) (formerly described as DCA or
Dynamic Cerebrovascular Analysis) methodology described above has been
uniquely
applied for the diagnosis and evaluation of hydrocephalus, including NPH, both
before and
after surgical correction. It has been used to track the natural history and
progression of the
onset of NPH. It has also been used to generate a reference database useful
for future
diagnoses that includes a variety of intracranial pressure data such as
natural history NPH
data, supine data, Trendelenberg (head down tilt of approximately 15 degrees).
Finally, the
invention provides a reliable, non-invasive, portable, inexpensive method for
diagnosing
and monitoring hydrocephalus and, in particular, NPH.
In accordance with an embodiment of the invention, a representative
DVA/hydrocephalus protocol involves interrogation with a fixed TCD
probe/device, as
depicted in Figures 1-4, such that the artery being studied is continuously
monitored.
Alternatively, other forms of emissive and reflective wave technology, such as
laser
technology, can be utilized. Monitoring occurs with the patient placed in a
Trendelenberg
position of varying degrees (optimally between ~15 and ~20°). followed
by data collection
at 30, 60, 90 and 120 seconds intervals. Following analysis in the
Trendelenberg position,
the patient is brought to the supine position. Again, data is collected at 30,
60, 90 and 120
second intervals. In a normative patient state there will be no statistically
significant change
in flow dynamics of the vessel being interrogated. Patients experiencing
global intracranial
change (i.e. experiencing increasing intracranial pressure) will demonstrate
dramatically
changing and shifting flow dynamics between hyperdynamic states characterized,
in part, by
stiffening of the vessel, increasing acceleration and slight impedance
increases but with very
little change of the velocity.
While in the Trendelenberg position the relationship between the middle
cerebral
and the ophthalmic artery is observed for the patient. There will be a
reversal of the
71
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
impedance index relative to a normal baseline state in a patient experiencing
increased
intracranial pressure associated with hydrocephalus. It is also helpful to
similarly diagnose
increased intracranial pressure prior to evaluating the subject in the
Trendelenberg position.
The protocol is also applicable after a patient has undergone an intracranial
shunting
procedure.
One common shortcoming of most diagnostic systems relates to the lack of
sensitivity and specificity associated with the differential diagnosis of
various conditions
(i.e. increased intracranial pressure and/or flow variations) that may
explained by any
number of physiological phenomenon. The invention has enabled observation of
the
abnormal flow characteristics in patients suffering from hydrocephalus which
are especially
apparent during a tilt table (Trendelenberg) test. The fundamental feature of
the test is the
ability to detect and observe a homogenous global increase in both the
pulsatility index and
flow acceleration, thus enabling discrimination between homogenous and
heterogeneous
effects from global intracranial events. For example, a global event could be
global
inflammation which would typically cause a patchy distribution when the TCD
data was
correlated (i.e. a heterogeneous event) or it could be a metabolic disorder
affecting all
vessels homogeneously without necessarily excluding any particular region.
These
metabolic disorders may include, for example, Fabry Disease or Diabetes.
One example of an application of the invention involved an elderly patient who
represents the first documented natural history study of the development of
increasing
intracranial pressure. In other words, it represented the first progressive
study of the onset
of NPH. Figures 28A-28D illustrate this progressive study. It was observed
that the onset
of NPH over time was characterized by global blood flow accelerations in the
cerebral
vasculature, as well as an increase in the pulsatility index. There was also
an observed
reversal in the impedance index of the middle cerebral artery to ophthalmic
artery
relationship. Typically in a normal state, the ophthalmic artery is considered
an end artery
and has higher impedance values (or index of pulsatility) than the middle
cerebral artery
which is considered a conductance artery. If an impedance reversal occurs, the
impedance
is greater in the conductance vessel than the end artery. Furthermore, when an
impedance
reversal occurs, it exists bilaterally in the cranium. As such, it is probable
that the reversal
is a result of increased intracranial pressure. Figure 29 demonstrates that
traditional blood
flow tests would not have detected the intracranial pressure changes occurring
in the subject
that were observable using transcranial-based dynamic vascular assessment.
72
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
As an extension of the above study, Table 8 contains mean flow velocity,
systolic
acceleration and pulsatility index data for two series of subjects suffering
from increased
intracranial pressure obtained by TCD when the subjects were moved from a
supine to a
head-down tilt position. Figures 30-32 illustrate this same data after being
subjected to
DVA analysis.
Grou Mean Flow VelocityS stolic AccelerationPulsatili Index
41 693 1.72
59 1537 1.78
Series 64 1138 1.64
1
61 1372 1.91
55 1327 2.01
59 1932 1.94
52 437 0.76
54 458 0.90
52 473 0.81
54 451 0.83
58 656 0.84
Series 56 467 0.76
2
55 390 0.70
55 428 0.76
46 539 0.95
54 614 0.74
47 478 0.75
43 593 0.79
Table 8.
Once calculated, the TCD data was analyzed by Dynamic Vascular Analysis (DVA),
as described above. The DVA for each subject comprised a) a simultaneous
consideration
of the TCD values (peak systolic velocity(PSV), end diastolic velocity (EDV),
peak systolic
time (PST), end diastolic time (EDT), mean flow velocity (MFV), systolic
acceleration
(SA), pulsatility index (PI), the natural logarithm of the SA (LnSA)) for each
of the
established I9 vessel segments within the cerebral vasculature; b) a
comparison of the TCD
values against a reference database to quantify the degree of variance from
mean values;
and c) a series of indices (blood flow velocity rations) derived from the TCD
values that are
representative of the vascular status/performance/health of each the 19 vessel
segments.
The derived indices include:
1 ) Acceleration/Mean Flow Velocity Index (VAI) (Systolic Acceleration value
divided by the Mean Flow Velocity value and/or reciprocals thereof);
73
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
2) Velocity/Impedance index (VPI) (Mean Flow Velocity value divided by the
Pulsatility Index value and/or reciprocals thereof); and
3) Acceleration Impedance Index (API) (Systolic Acceleration value divided by
the Pulsatility Index value and/or reciprocals thereof).
The 19 intracranial vessel segments considered are depicted in Figures 33 and
34.
The vessel segments depicted in Figures 33 and 34 represent the left and right
vertebral
artery (VA), basilar artery (BA), posterior cerebral artery/PCA t (towards)
(P1), posterior
cerebral artery/PCA a (away) (P2), internal carotid artery/ICA t (towards)
(Cl), middle
cerebral artery (M1 ), anterior cerebral artery (Al ), anterior communicating
artery (ACOM),
carotid siphon (towards) (C4), carotid siphon (away) (C2), and the ophthalmic
artery (OA).
The data revealed that patients suffering from hydrocehalus had higher than
normal
PSV values for the M1 and C1 segments. These patients also exhibited a PI
increase in the
M1, A1, C1 and C2 segments as well as an increase in the SA in the M1, A1 and
C4
segments. The LnSA was also increased in the M1, A1 and C4 segments.
Conversely, the
acceleration-impedance ratios were diminished in the M1, A1 and C1 segments.
The
velocity-impedance ratio was also decreased in the A1 segment. The invention
further
disclosed that increased PI is predictive of hydrocephalus in the A1 and C1
segments.
Increased SA in the C4 segment is also an indicator of hydrocephalus. Finally,
a collective
increase in SA, PI and LnSA in the M1 segment was also predictive. It has been
concluded
based on this data that observation in blood flow changes in the C1 segment
provides the
most effective indicators and predictors of hydrocephalus. Blood flow data
derived from
the M1 and C1 segments is also well suited for predicting and monitoring
hydrocephalus.
The invention has been particularly adapted for use in evaluating and
assessing
hydrocephalus and NPH. The methodology for doing so involves measuring one or
more
points in the cerebrovasculature by TCD and performing a DCA analysis in
either or both
the supine and Trendelenberg positions on patients suspected of having or at
risk of
experiencing increased intracranial pressures associated with hydrocephalus
and NPH.
The invention has further application than the direct detection and monitoring
of
patients with hydrocephalus. For example, there currently exists a
programmable shunt
system. A shunt is a tube placed in the fluid space in the brain that drains
into the belly
cavity and which usually passes through a pressure control valve. The valve
activates the
shunt to drain after a preset intracranial pressure level is reached.
Continuous drainage is
undesirable because creates the risk of over-drainage and the formation of a
causing a
74
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
subdural hematoma The programmable shunt system was developed whereby the
shunt is
initially set at a high opening pressure and progressively adjusted according
to clinical
effect. The difficulty with such a process is that it usually takes two to
three weeks to
observe an adequate clinical effect in order to change the pressure setting of
the shunt
system. The invention enables observation of any dynamic shift in vessel
performance long
before there is a clinical change in the patient. In fact, the invention
enables almost
instantaneous changes in vessel performance. It is thus possible to make
adjustments to
these types of shunting systems much more quickly and accurately. For example,
a
monitoring physician can utilize the invention as an indicator of when to
reduce the valve
opening pressure level without go so low as to risk patient development of a
subdural
hematoma. It also enables the physician to optimize the normalization of
cerebral perfusion
over a two or three day period rather than a several month period because it
eliminates the
need to follow the traditional process of adjusting the pressure level
followed by a several
week wait to observe a clinical effect.
The device is also of practical value to makers and distributors of shunts and
related
devices. The invention enables makers and sellers of such devices because in
enables better
product development and marketing practices and in turn facilitates expansion
of product
markets. For example, the invention could be given to a care facility as part
of a contract to
exclusively purchase shunts from a particular manufacturer or distributor.
It is also envisioned that the invention will be used a screening device at
hospitals,
nursing homes and other care facilities. Specifically, it will help facilitate
resource
management by enabling administrators and treating physicians to forecast
demand for,
among other things, intracranial shunts, as well as the staff needed for
implanting the same.
The invention further facilitates more effective monitoring and tracking of
patients with
known intracranial conditions that predispose them to suffering intracranial
pressure
increases. These patients would include, for example, those having experienced
or disposed
to experiencing a hemorrhagic stroke or patients with altered mental status
suspected to be
related to increased intracranial pressure. Further, because the invention is
disposed to
being operated both as a monitor and/or remotely, it can be operated from a
central location
within a care facility (e.g. a nurses station), thus enabling one person to
simultaneously
monitor a number of patients.
The invention is well suited to the development and optimization of drugs,
treatments and therapies of NPH. That is, the invention can be readily
utilized to evaluate
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
the effects of various hydrocephalus treatment methodologies by monitoring
patients both
pre- and post-treatment. Furthermore, the treatment data can be further
combined with
longitudinal patient data to particularly tailor patient treatment regimens.
Finally, as will be appreciated by those skilled in the art, the invention as
a
methodology for diagnosing and treating hydrocephalus can be further applied
in an
automated fashion, locally or remotely, via telecommunications line or simple
local bedside
test. As with any diagnostic test, the present invention is intended in at
least one
embodiment to be a fully-automated, remotely-controlled diagnostic system for
the
detection and monitoring of increased intracranial pressure.
In a controlled study, it has been discovered that the invention is also
applicable as
both a system and method for assessing and treating dementia. Specifically, in
a study of 56
patients with a diagnosis of dementia, Alzheimer's type, and 39 age-matched
controls, it has
been observed that the invention can identify critical variables that affect
intracranial blood
flow that in turn cause dementia.
Participants were categorized into either the patient or control group based
on
several factors. Members of the patient group had a pre-existing diagnosis of
dementia and
had below average performance on the Mini Mental Status Exam (MMSE). The
control
group was selected from friends and family of the dementia patients based on
the absence of
a dementia diagnosis, no reported history of cognitive impairment, and an
above average
score on the MMSE.
Study subjects were evaluated using TCD, though other forms of emissive and
reflective wave technology, such as laser technology, can alternatively be
utilized. TCD
measurements were conducted in a small 10'X10' dimly lit room and asked to sit
in a
recliner-style chair using traditional TCD methodologies. TCD measurements
were
obtained non-invasively and provided blood flow velocity data of the major
arteries
supplying blood to the brain. Waveforms were obtained from several cranial
windows. The
transtemporal windows were used bilaterally to view segments of the middle
cerebral
arteries, anterior cerebral arteries, internal carotid artery, and the
posterior cerebral arteries.
The transophthalmic windows were used bilaterally to view segments of the
ophthalmic
arteries as well as the internal carotid arteries. The transoccipital window
was used to view
the right and left vertebral arteries as well as several depths of the basilar
artery. A sweep
speed of 4 seconds per screen was used yielding 3-7 quality waveforms per page
based on
the participant's heart rate. The display screen was saved when the
technologist identified
76
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
at least one waveform on which a clear diastolic trough and a systolic peak
could be
measured on one waveform that was among several contiguous waves. The vessels
were
insonated at well-established depths corresponding to the 19 established
vessel segments.
Analysis of the TCD data comprised software-assisted determination of time and
velocity. Specifically, the TCD technologist placed the computer cursor on the
end
diastolic trough immediately prior to the up-sloping and second cursor at the
ensuing peak
systole. The x- and y-axis values for each cursor position yielded,
respectively, the time and
velocity. From this data, the peak systolic velocity, peak systolic time, end
diastolic
velocity, and end diastolic time values were determined. Using traditional TCD
formulae,
this data was used to calculate the Mean Flow Velocity, Systolic Acceleration,
and
Pulsatility Index values for each subject.
Once calculated, the TCD data was analyzed by Dynamic Vascular Analysis (DVA),
as described above. The DVA for each subject comprised a) a simultaneous
consideration
of the TCD values (MFV, SA, and PI) from a single wave form for each of the
established
19 vessel segments within the cerebral vasculature; b) a comparison of the TCD
values
collected from a single wave against a reference database to quantify the
degree of variance
from mean values; and c) a series of indices (blood flow velocity rations)
derived from the
TCD values that are representative of the vascular status/performance/health
of each the 19
vessel segments depicted in Figures 33 and 34. The derived indices include:
3) Acceleration/Mean Flow Velocity Index (Systolic Acceleration value divided
by the Mean Flow Velocity value and/or reciprocals thereof);
4) Velocity/Impedance index (Mean Flow Velocity value divided by the
Pulsatility Index value and/or reciprocals thereof); and
3) Acceleration Impedance Index (Systolic Acceleration value divided by the
Pulsatility Index value and/or reciprocals thereof).
The data revealed that the patients suffering from dementia had a decrease in
mean
flow velocity and a corresponding increase in the pulsitility index within the
M1, Al, C1,
C2, C4, VA, BA, P1 and the P2 vessel segments. Except for a decrease in the
basilar artery,
it was observed that the systolic upstroke acceleration was unchanged in the
patient group
relative to the control groups.
The blood flow velocity ratios were also determined to be important to the
evaluation of the patients suffering from dementia. First, the
acceleration/velocity ratio, an
indicator of the kinetic energy transfer into forward blood flow, was
increased in the M1,
77
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
A1, C1, C2, C4, VA, BA, P1 and the P2 vessel segments. Conversely, the
acceleration
impedance ratios, indicating the result of downstream impedance force on the
forward force
of blood flow, and the velocity impedance ratio, indicating the effect of
downstream
impedance force on the forward mean flow velocity and a surrogate marker for
relative
blood flow, were diminished in the M1, Al, C1, C2, C4, VA, BA, P1 and P2
vessel
segments of the dementia patients.
The holocephalic diminution of mean cerebral blood flow velocities in a number
of
vessel segments in the dementia subjects (relative to the control group) is
consistent with
previous cerebral blood flow studies demonstrating diminished cerebral
perfusion in
dementia (i.e. changes in mean cerebral blood flow velocities have been
associated with
diminished cerebral blood flow). The discovery that systolic upstroke
acceleration remains
unchanged in patients suffering from dementia is significant when related to
the global
diminishing blood flow velocities otherwise associated with this condition. If
diminished
blood flow to the cerebrum is secondary erect of global low blood flow, then
the cerebral
vessels should dilate to compensate for the diminishing force of flow up to
the point of
autoregulation failures. Under this "traditional" scenario, systolic
acceleration should
exhibit a continual to decline. The present invention, however, has
demonstrated the
opposite effect in patients suffering from dementia (i.e. declining mean flow
velocities did
not correspond to a change in systolic upstroke acceleration). In other words,
the invention
has been used to specifically quantify and demonstrate that in patients
affected by dementia,
a static forward force on blood flow has, over time, less direct effect on the
forward
movement of the blood. The invention expresses this effect on blood flow as
the
acceleration-velocity ratio which is reflective of the amount of kinetic
energy required for
forward blood movement. The invention has demonstrated that the acceleration-
velocity
ratio is increased in all vessels, except the ophthalmic arteries, in patients
suffering from
dementia. This discovery is buttressed by the observed increases in the
pulsatility index in
the M1, A1, Cl, C2, C4, VA, BA, P1 and the P2 vessel segments.
In sum, the assumption that dementia is an apoptotic process secondary to
toxic
substance deposition, is inconsistent with the data developed by the
invention; if dementia is
the result of atrophy or the loss of brain tissue, the amount of work (i.e.
kinetic energy)
needed to move blood forward should be decreased. The invention has
demonstrated
conclusively, therefore, that dementia is at least in large part a direct
function of blood flow
dynamics as opposed to the result of the deterioration brain matter.
Accordingly, the
78
CA 02491044 2004-12-23
WO 03/099131 PCT/US03/15820
invention provides a reliable and efficient means for diagnosing and assessing
patients
suffering from dementia as well as monitoring and optimizing treatments and
regimens
designed to combat the onset and progression of the condition.
Various preferred embodiments of the invention have been described in
fulfillment
of the various objects of the invention. It should be recognized that these
embodiments
are merely illustrative of the principles of the invention. Numerous
modifications and
adaptations thereof will be readily apparent to those skilled in the art
without departing
from the spirit and scope of the present invention.
79