Note: Descriptions are shown in the official language in which they were submitted.
CA 02491511 1996-10-10
CA 02234S8S 1998
WO 97/L~O~ PGT/0896/162T7
PROLONGED RELEASE OF GM-(~'
Backgrmmd of the Inve~On
The invention is generally in the area of c~~olled,
pholonged release microsphere formulations for recombinant >mman
granulocyte macrophage colony sW feting factor (GM-CSF).
GM-CSF, granulocyte macrophage color stimulating factor, is a
hematopoietic growth factor which promotes the proliferation and
diffaenfiation of hematopoietic progenitor cells. The cloned gene for
GM-CSF has been e~cprrssod in ba~eria, yeast and mammalian cells. The
endogenous human protein is a moaometic glycopratein with a molecular
weight of about 22,000 daltons. GM-CSF produced in a yeast expression
system is commercially available as Leukine' from Immunex Corporation,
Seattle, RTashington. It is a glycoprotein of 127 amino acids
characterized by three primary molecular species having molecular masses
of 19,500, 16,800, and 15,500 daltons.
Gcneratly, GM-CSF is adnoimstered over a period of at least 6 to
7 days in order to obtain the optimal effect on the white blood cells.
Under some circ~unstaaces, it is desirable to have a formulation which
.provides conti~ous, zero order or first order kinetic release of GM-CSF
over a period of approximately one week. Moreover, sustained release
formulation of GM-CSF may have advantageous therapeutic not
shared by standard liquid formuiations. Sustained-release formulations of
GM-CSF, however, are not currr~ly available.
Controlled release formulations are well known for drug delivery.
ZS Both biodegradable and non-biodegradable polymers have been used to
form microcapsules, microspheres or microparticles of various diameters,
porosities, and drug loadings with the goal of obtaining release of the
. encapsulated drug over a period of time. Msny formulations that have
been developed have been designed far administration by injection,
although the majoriiy of co~oiled release formulations have emtric
.. CA 02491511 1996-10-10
CA 02234585 1998
Z
_2_
coatings or are formulations resistant to passage thmugh the
gastrointestinal tract that have been developed for oral administration.
It is difFcult to achieve linear, controlled release using the
standard formulations. Most formulations are designed either to pmvide
very rapid release by diffusion and/or degradation of the polymer forming
the microparticle or provide for a burst release followed by some kind of
linear release which generally plateaus after a period of time. U.S. Patent
No. 5,192,741 to Orsolini, et al., is representative of the literature
regarding the difficulcies in obtaining controlled release from
microspheres formed of poly(lactide-co-glycolides) (PLGAs). Similarly,
Lu and Park J. Pharm. Sci. Te 'cal 49, I3-19 (1995) describes the use
of microcapsules, noting that one cannot obtain good release
characteristics with microspheres and that protein stability in the -
microspheres is a problem. Since GM-CSF is an extremely potent
compound where the effect may vary widely depending upon the given
' dosage, it may be advantageous in soma circumstances to obtain a more
linear release rather than a burst followed by a plateau of drug being
released.
Representative of the many patents relating to controlled release
are U.S. Patent No. 4,767,628 to Fiutchiason, disclosing multighasic
release of a peptide from a PLGA carrier. Blends of polymers are used is
. a large matrix delivery system to avoid multiphasic release. U.S. Patent
No. 4,897,268 to Tice, et al., discloses the use of different PLGAs in the
same composition, but blends microspheres made of the different PLGAs
to achieve linear release. U.S. Patent No. 4,849,228 to Yamamoto, et
al. , claims PLGA microspheres having a very low monobasic acid content
which allegedly have excellent release characteristics. PCT WO 94/01133
by Schering Corporation discloses microsphcres of GM-CSF prepared
using various polymers such as polyanhydrides, polyphosphazenes, and
collagen. PCT WO 9I/12882 by Medgenix Group S.A. discloses
~1MENGED SHE~~'
CA 02491511 1996-10-10
' , CA 02234585 1991.
1
mierospheres for the controlled release of water-soluble substances
prepared using polymers such as poly(lactic-co-glycolic acid). PCT WO
95/06077 by Sandoz Patent GMBH discloses polymeric matrices that
include poly(eihylene carbonate) and pharmaceutical compositions made
from the polymeric matrices. DE 44 06 I72 A1 by Schwarz Pbarma AG
discloses branched polyesters with molecular weights up to 500,000
prepared from electrolyte-substituted polyols and poly(hydroxy acids).
It is therefore an object of the present invention to provide a
formulation encapsulating GM-CSF which provides for controlled,
prolonged release with either zero order kinetics, first order release
kinetics or multiphasic release kinetics over a period of greater than one
day following administration to a patient by injection.
At,AE~ iCtJ Sl',EET
CA 02491511 1996-10-10
CA 02134585 19 '
WO 97/13302 PGTJOS96/16Z77
-3-
It is a further object of the present invention to provide a
formulation for delivery of GM-CSF far administration orally,
t<ansmucosally, topically or by injection.
Summsiy of the Invention
g Formulations for controlled, prolonged release of GM-CSF have
been developed. These are based on solid microparticles formed of the
combination of biodegradable, synthetic polymers such as poly(lactic acid)
(PLA), poly(glycolic acid) (PGA), and copolymers thereof with excipienLs
and drug loadings that yield a sustained release over a period of one day
IO to at least one week, when administered orally, transmucosally, topically
or by injection. In the preferred embodiment, the microparticles have
different diameters depending on their route of administration.
Microparticles administered by injection have diameters sufficiently small
to pass through a needle, in a size range of between 10 and 100 microns.
15 Orally administered microparticles are less than 10 microns in diameter to
facilitate uptake by the Peyer's patches in the small intestine.
Other embodiments have been developed to alter the release
kinetics or the manner in which the drug is, distributed in vivo. For
example, in some cases a polymer is selected which elicits a mild
20 inflammatory reaction, for example, PLGA and polyanhydrides, which
can act as chemoattractant, either due to the polymer itself or minor
contaminants in the polymer. In another embodiment, the GM-CSF is
administered in a hydrogel which can be injected subcutaneous or at a
specific site for controlled release.
25 The microparticles or hydrogel are administered to the patient in
an amount effective to stimulate proliferation of hematopoietic cells,
especially white cells. These are most preferably microspheres
administered by injection.
Easmples demonstrate the preparation of microparticles releasing
30 GM-CSF over a prolonged period with zero order, first order, or
muitiphasic release kinetics. The type of release kinetics are determined
CA 02491511 1996-10-10 ....__
CA 02234585 1998
..W
WO 97/13302 " PGTlIIS96/16Z77 -
-4-
for the particular clinical application. The data demonstrates that it is
possible not only to achieve the desired release characteristics bnt also to
retain extremely high levels of bioactivity of tha encapsulated GM-CSF.
Examples also demonstrate release from hydrogels.
Brief Description of the Drawlags
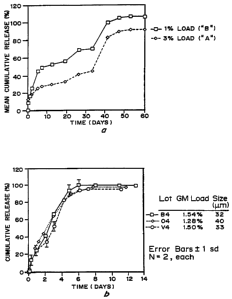
Figure lA is a graph of GM-CSF release, mean percent
cumulative release in vitro over time (days) for a 196 load (squares) and
3 9~ load (diamonds) in microspheres prepared by phase separation with a
single PLGA copolymer. Figure 1B is a graph of GM-CSF release, mean
percent cumulative release in vitro over time (days) for a 1.54 'lh load
(squares, lot B4), 1.28 load (diamonds, lot 04) and 1.5% load (circles,
lot V4) in microspheres prepared by phase separation using a blend of
PLA and PLGA polymers.
Figure 2A is a graph of in vitro release ACS for "Lot O"
microspheres prepared by phase separation of a single molecular weight
PLGA, showing GM-CSF release as cumulative release in vitro
over time (days). Figure 2B is a graph of mouse serum GM-CSF lovels
(ng/ml) over time (days) following microsphere or bolus injections, for 50
mg microspheres, 500 ~cg bolus, and 50 ~cg bolus. Figure 2C is a graph
of the GM-CSF levels following mierosphere injection (diamonds) versus
levels calculated from in vitro release rate and experime~al half life
).
Figure 3A is a graph of GM-CSF release, mean percent
cumulative release in vitro over time (days) far lot V4 microspheres
prepared by phase separation using a blend of two PLGAs of different
molecv3ar weight and a PLPr. Figure 3B is a graph of TF-1 bioactivity of
lot V4 release samples, percent activity at disc~e time points (days).
Figure 3C are graphs of the white blood cell counts (WBC), absolute
acutrophii coin (ANC), and platelet coin in primates injected with
microspheres containing GM-CSF, as a function of time (days).
CA 02491511 1996-10-10
CA 02Z345aS 1998
wo 9'n~oz rcrms9snszr~ -
s-
Figure 4 is a graph of GM-CSF release fiom a PLGA gel, p~
cumulative release over time (days).
Figure 5 is a graph of TF-1 cell activity of GM-CSF extractod
from PLGA microspheles with acetic acid, graphing pert activity
versus microsphere lot.
Figure 6 is a graph of PLGA degradation over time of three types
of microspheres prepared from either PLGA (Cytec. 7 LV.), PLA (R104)
or an 80/20 blend of the two polymers, graphing weight average
molecular weight over time (days).
Detailed Description of the Invention
There are mad advantages for a controlled release formulation of
GM-CSF. Among these are the convenience of a single injection for the
patient and physician, avoidance of peaks and valleys in systemic GM-
CSF concentration which is associated with npeatod injections, the
potential to reduce the overall dosage of GM-CSF, and the potential to
enhance the pharmacological effects of GM-CSF. A controlled release
formulation of GM-CSF also provides an opportunity to use GM-CSF in a
manner not previously exploited, such as a vaccine adjuvani.
Controlled Release Formulations
As used herein, "sustained" or "extended" release of tine GM-CSF
can be continuous or discontinuous, linear or non-linear. This can be
accomplished using one or more types of polymer compositions, drug
loadings, inclusion of excipierlrs or degradation enhancela, or other
modifiers, administered alone, in combination or sequentially to produce
the desired effect. Zero order or linear release is generally construed to
mean that the amount of GM-CSF released over time remains relatively
constant as a function of amountlunit time during the desired time frame,
for example, six to seven days. Multi phasic is generally constived to
mean that release occurs in more than one "burst" .
As used herein, "microparticles" refers to particles having a
diameter of less thaw one mm, more typically less than 100 microns.
CA 02491511 1996-10-10 -
CA 01234585 1998-
w0 97- PCT/US96/16Z77
-6-
Microparticles can refer to microspher~es, which are solid spherical
micmparticles, and anicrocapsnles, which are sp1>ericat micropardcles
having a core of a different polymer, drug, or composition. Unless
otherwise stated herein, microparticles refers to solid particles, not
microcapsules. '
Polymers for Formation of Mlcroparttdes
Many polymers have been used for controlled drug delivery.
Polymers typically arc thermoplastic synthetic polymers, such as
ethylenevinyl acetate and poly(acrylic acid), which are generally viewed
as non-biodegradable since they remain in relatively the same form over a
period of at least two or three years following implantation in the body,
and biodegradable polymers, such as poly(hydroxy acids) including
polylactic acid, polyglycolic acid, and copolymers thereof,
polyanhydrides, polyorthoestrrs, and certain types of protein and
IS polysaccharide polymers. The te~cm bioerod~le or biodegrable, as used
herein, means a polymer that dissolves or degrades within a period that is
acceptable in the desired application (usually in vivo therapy), less than
about five years and most preferably less than about one year, once
exposed to a physioiogicaI solution of pH 6-8 at a temperature of between
about25°C and 38°C.
A preferred polymer material is one which is biodegradable and
which retains sufficient form to control release for a period following
implantation of at least six to seven days. The poly (hydroxy acids),
Y PoIY~ acid-co-glycolic acid) ("PLfIrA"), is a particularly
preferred polymer since it has been used in the manufacture of degradable
sutures for several decades. The polymer degrades by hydrolysis
following exposure to the aqueous environment of the body. The polymer
is hydrolyzed to yield lactic and glycolic acid monomers, which are '.
normal byproducts of cellular metabolism. The rate of polymer
disintegration can vary from several weeks to periods of greater than one
year, depending on several factors inchiding polymer molecular weight,
ratio of lactide to glycolide monomers in the polymer chain, and
CA 02491511 1996-10-10
' CA 02234585 1998
$ -7-
stereoregularity of the monomer subututs (miztzu~es. of L and D
stereoisomers disrupt the polymer crystallinity enhancing polymer
breakdown). Particularly usefell results are obtained by blending PLGA
having different molecular weights, and/or different ratios of lactide to
glycolide. The molecular weight and monomer ratios can be optimized to
tailor the release kinetics over a defined period of time. The higher
molecular weights, result is polymer matrices which retain their strucuu'al
integrity for longer periods of time; while lower molecular weights, result
in both faster release and shorter matrix lives.
IS In a preferred embodiment described herein, the microspheres
contain blends of at least two and more preferably three or more
biodegradable polymers, preferably hydrolytically unstable polymers,
most preferably poly(hydroxy acids) of different molecular weight and/or
monomer ratio. In a preferred embodiment, three different molecular
weight PLGAs are blended to form a composition that has linear release
over a defined period of time, ranging from at least one day to about sixty
days. In a more preferred embodiment to obtain release from about one
to sweaty-one days, the PLGAs have molecular weights between 1000 and
20,000, more preferably between 5,000 and 10,000, between 20,000 and
35,000, more preferably between 25,000 and 30,000, and between 35,000
and 70,000. In the most preferred embodiment for release over a period
. of about one week, PLGAs having molecular weights of about 6,000,
30,000, and 41,000 are combined.
PLA polymers are usually prepared from the cyclic esters of lactic
acids. Both L(+) and D(-) forms of tactic acid can be used to prepare
the PLA polymers, as well as the optically inactive DL-lactic acid mixture
of D(-) and L(+) lactic acids. Methods of prepazing polylactides are well
documented in the patent literature. The following U.S. Patents describe
in detail suitable polylactides, their properties and their preparation: U.S.
Patent Nos. 1,995,970 to borough; 2,703,316 to Schneider; 2,758,987 to
r1~!ENDED SNE~T
CA 02491511 1996-10-10
'>
CA 02234585 1998-
WO 97/13502 - PGTlU596/16277
_g_
SaLberg; 2,951,828 to Zeile; 2,676,945 to Higgins; and 2,683,136;
3,531,561 to Treliu.
Since it is desirable to target delivery of GM-CSF to white cells,
particularly in the case where the GM-CSF is being used as an adjuvant,
alone or in combination vv~ith antigen, the polymer may be selected based
on properties other than just controlled release. For example, it is known
that certain polymers are inflammatory and therefore attract leukocytes,
macrophages and other "white" cells. Examples of "chemoattractant"
polymers include the polyhydroxy acids (PL, PG, PLGAs),
polyanhydrides. poly(ortho esters), and the polyphosphazenes.
In the case where the microparticles are intended for transmucosal
or oral delivery, it may be desirable to select polymers which are
bioadhesive. F~camples of bioadhesive polymers include hydrophilic
polymers, especially those containing carboxylic groups, such ss
poly(acrylic acid). Rapidly bioerodible polymers such as poly(lactide-co-
glycolide), polyanhydrides, and polyorthoesters having carboxylic groups
exposed on the external surface as their smooth surface erodes, are
particularly useful. Representative natural polymers are proteins, such as
z~eia, albumin, and collagen, and polysaccharides, such as cellulose,
dextrans, and alginic acid. Other representative synthetic polymers
include polyamides, polycarbonates, polyalkylenes, polyalkylene glycols,
poIyalkylene oxides, polyalkylene terephthalates, polyvinyl alcohols,
polyvinyl ethers, polyvinyl esters, polyvinyl halides,
polyvinylpyrrolidone, polyglycolides, polysiloxanes, polyuretbanes,
celluloses including alkyl cellulose, hydrozyalkyl celluloses, cellulose
ethers, cellulose esters, and nitrocelluloses, polymers of acrylic and
methacrylic esters, poiy(lactide-co-glycolide), polyanhydrides,
polyorthoesters blends and copolymers thereof.
Polymers for Formation of Hydrogels
Other polymeric materials that may be useful include hydrogels
such as the naturally occturing polysaccharides like alginate, as well as
synthetic hydrogel materials such as some of the polyacrylic acids,
CA 02491511 1996-10-10 ...
CA 01134585 1998
' T-
WO 97/13502 - PCT/QS96/l6ZTf '
_9-
polyphosphazenes, polyethylene glycol-PLGA copolymers and other
synthetic biodegradable polymers which absorb up to 90% of the final
weight of waoer.
The polymeric material which is mined with GM-CSF for
implantation into the body should form a hydrogel. A hydrogel is defined
as a substance formed when an organic polymer (natural or synthetic) is
cross-linked via covalent, ionic, or hydrogen bonds to create a three-
dimensional open-lattice structure which entraps water molecules to form
a gel. Examples of materials which can be used to form a hydrogel
include polysaccharides such as alginate, polyphosphazenes, and
polyacrylates, which are crosslinked ionically, or block copolymers such
as Pluronics'rM or TetronicsTM, polyethylene oxide polypropylene glycol
block copolymers which are crosslinked by temperature or pH,
respectively. Other materials include proteins such as fibrin, polymers
such as polyvinylpyrrolidone, hyaluronic acid and collagen. U.S. Patent
Nos. 5,286,495 and 5,410,016 to Hubbell, et al., dcscri'tx useful
materials for forming biocompatible hydrogels.
In general, these polymers are at least partially soluble in aqueous
solutions, such as water, buffered salt solutions, or aqueous alcohol
solutions, that have charged side groups, or a monovalent ionic salt
thereof. Examples of polymers with acidic side groups that can be
reacted with rations are poly(phosphazenes), poly(acrylic acids).
poly(methaerylic acids), copolymers of acrylic acid and methacrylic acid,
polyvinyl acetate), and sulfonated polymers, such as sulfonated
polystyrene. Copolymers having acidic side groups formed by reaction of
acrylic or methacrylic acid and vinyl ether monomers or polymers can
also be used. Examples of acidic groups are carboxylic acid groups,
sulfonic acid groups, halogenated (preferably fluorinated) alcohol groups,
phenolic OH groups, and acidic OH gmups.
Examples of polymers with basic side groups that can be reacted
with anions are polyvinyl amines), polyvinyl PYri~). PoIY(~YI
imidazole), and some imino substituted polyphosphaunes. The
CA 02491511 1996-10-10
' CA 01234585 1998-
-10-
ammonium or quaternary salt of the polymers can also be formed from
the backbone nitrogens or pendant imino groups. Examples of basic side
groups are amino and imiao groups.
Calcium alginate and certain other polymers can form ionic
hydrogels which are malleable can be used to encapsulate GM-CSF.
Alginate can be ionically cross-linked with divalent cations, in water, at
room temperature, to form a hydrogel matrix. The hydrogel is produced
by cross-linking the anionic salt of alginic acid, a carbohydrate polymer
isolated from seaweed, with divalent canons, whose strength increases
with either increasing concentrations of calcium ions or alginate.
The water soluble polymer with charged side gmups is crosslinked
by reacting the polymer with an aqueous solution containing multivalent
ions of the opposite charge, either multivalent canons if the polymer has
acidic side groups or multivalent anions if the polymer has basic side
groups. The preferred cations for cross-linking of the polymers with
acidic side groups to form a hydrogel are divalent and trivalent cations
such as copper, calcium, aluminum, magnesium, strontium, barium, and
tin, although di-, tri- or tetra-functional organic canons such as
alkylammonium salts, e.g., R31V* -C6-*NR3 Can also be used. Aqueous
solutions of the salts of these canons are added to the polymers to form
soft, highly swollen hydrogels and membranes. The higher the
- concentration of catioa, or the higher the valence, the greater the degree
of cross-linking of the polymer. Concentrations from as low as 0.005 M
have been demonstrated to cross-link the polymer. Higher concentrations
are limited by the solubility of the salt.
The preferred anions for cross-linking of the polymers to form a
hydrogel are divalent and trivalem anions such as low molecular weight
dicarboxylic acids, for example, terephthalic acid, sulfate ions and
carbonate ions. Aqueous solutions of the salts of these anions are added
to the polymers to form soft, highly swollen hydrogcls and membranes, as
described with respect to canons.
~r~he~~ s~;E~
CA 02491511 1996-10-10
CA 03234585 1998-
1'
-lI-
A variety of polycations can be used to complex and thereby
stabilize the polymer hydrogel into a semi-permeable surface membrane.
Examples of materials that can be used include polymers having basic
reactive groups such as amine or imine groups, having a preferred
molecular weight between 3,000 and 100,000, such as polyethyleneimine
and polylysine. These are commercially available. One polycation is
poly(L-lysine); examples of synthetic polyamines are: polyethyleneimine,
poly(vinylamine), and poly(allyl amine). There are also natural
polycations such as the polysaccharide, chitosan. Polyanions that can be
used io form a semi-permeable membrane by reaction with basic surface
groups on the polymer hydrogcl include polymers and copolymers of
acrylic acid, methacrylic acid, and other derivatives of acrylic acid,
polymers with pendant SO~H groups such as sulfonated polystyrene, and
polystyrene with carboxylic acid groups.
These polymers are either commercially available or can be
synthesized using methods known to those skilled in the art. See, for
example concise Encyclop~,te yg" of Polvrner Science and Polymeric Amines
and Ammonium Salts, E. Goethals, editor (Pergamea Press, Elmsford,
NY 1980).
GM-CSF
GM-CSF, granulocyte macrophage colony stimulating factor, is a
hematopoietic growth factor which promotes the proliferation and
differentiation of hematopoietic progenitor cells. The cloned gene for
GM-CSF has been expressed in bacteria, yeast and mammalian cells. The
endogenous protein is a monomeric glycoproteia with a molecular weight
of about 22,000 daltons. The recombinant preparation expressed in
bacterial cells is unglycosylated. GM-CSF produced in a yeast expression
system is marketed as Leukine~ by Immunex Corporation, Seattle,
Washington. Laukine'~ is sold is lyophilized form. It is a glycoproteia
of 127 amino acids characterized by three primary molecular species
having molecular masses of 19,500, 16,800, and IS,500 daltons.
.,.rit ~~- ._ .
CA 02491511 1996-10-10
CA D1234585 1998-
- i
-12-
GM-CSF is described in U.S, Patent No. 5,078,996 to Coalon, et
al. Analogs of GM-CSF are described in U.S. Patent Nos. 5,229,496,
5,393,870, anti 5,391,485 to Deeley, et al. Ia the preferred embodiment
the GM-CSF is recombinant protein having a molecular weight of
between approximately 14,000 and 20,000, made in yeast which
hyperglycosylates the protein presumably limiting the amount of non
specifc absorption observed with the protein. GM-CSF fusion proteins
can also be used. Examples with GM-CSF fusion proteins include fusion
proteins with I;L,-3 and other lymphokines or growth factors.
Pre~p~ration of Miq~rarticles
Microspheres, or solid micropatticles, can be prepared using any
of a number of techniques known to those skilled in the art. GM-CSF
appears to be unusually stable to processing, especially in the presence of
organic solvents, which facilitates microparticle formation containing GM-
CSF having very high levels of bioactivity, typically greater than 90% as
compared to the GM-CSF prior to incorporation into the microparticles.
Examples of methods for preparation include solvent evaporation, spray
drying, solvent extraction and other methods known to those skilled in the
art. As discussed above, hydrogels are typically formed by ionic
crossiinking, by addition of ions or polyions, or photocrosslinkiag or
other forms of chemical crosslinking.
11?icrosphere Preparation
Bioerodi'ble microspheres can be prepared using nay of the
methods developed for making microspheres for drug delivery, for
example, as described by Mathiowitz and Larger, J. Controlled Release
5,13-22 (1987); Mathiowitz, et al., Reactive Poly~rg 6, 275-283 (I987);
and Mathiowitz, et al., J. Agpt. PQ,~,vmer Sci. 35, 755-774 (I988). The
selection of the method depends on the polymer selection, the size,
external morphology, and crysrauinity that is desired, as described, for
example, by Mathiowitz, et al., Sca_nr,;nQ Microsconv 4,329-340 (I990);
Mathiowitz, et al., J. Appj,, Pollvmer Sci. 45, 125-134 (1992); and Benita,
et al., J. ,pharm. Sc~ 73,
~t~Ei~~J~Q ~i~:E~'
CA 02491511 1996-10-10
CA 02134585 1998-
_ _ _ ...
-13-
1721-1724 (1984). Methods include solvent evaporation, phase
separation, spray drying, and hot melt encapsulation. U.S. Patent Nos.
3,773,919; 3,737,337; and 3,523,906 are representative of methods for
making microspheres.
A preferred method is described in U.S. Patent No. 5,000,886 to
Lawter, et aI. The GM-CSF is dispersed in an aqueous solution which is
then mixed with an organic solution of the polymer. The dispersion is
added to a non-solvent for the polymer and the GM-CSF, then the
microparticles hardened by extraction of the polymer solvent into a
volatile silicone fluid.
In solvent evaporation, described for example, in Mathiowitz, et
al., (1990), Benita, and U.S. Patent No. 4,272,398 to Jaffe, the polymer
is dissolved in a volatile organic solvent. The GM-CSF, either in soluble
form or dispersed as fine particles, is added to the polymer solution, and
the mixture is suspended in an aqueous phase that contains a surface
active agent such as polyvinyl alcohol). The resulting emulsion is stirred
until most of the organic solvent evaporates, leaving solid microspheres.
In general, the polymer can be dissolved in methylene chloride.
Several different polymer concentrations can be used, for example,
between 0.01 and 0.50 g/ml. After loading the solution with GM-CSF,
the solution is suspended in 200 ml of vigorously stirring distilled water
. containing 19'0 (w/v) poly(vinyl alcohol) (Sigma Chemical Co., St. Louis,
MO). After four hours of stirring, the organic solvent will have
evaporated from the polymer, and the resulting microspheres will be
washed with water and dried overnight in a lyophilizer.
Microspheres with different sizes (between 1 and 1000 microns)
and morphologies can be obtained by this method which is useful for
relatively stable polymers such as polyesters and polystyrene. However,
labile polymers such as polyaahydrides may degrade duo to exposure to
water. For these polymers, hot melt encapsulation and solvent removal
may be preferred.
AMENDED SHEc'~'
CA 02491511 1996-10-10
CA 02134585 1998-
-14-
Solvent removal is particularly useful with polyanhydrides. In this
method, the drug is dispersed or dissolved in a solution of a selected
polymer in a volatile organic solvent like methylene chloride. The
mixture is then suspended in oil, such as silicone oil; by stirring, to form
an emulsion. Within 24 hours, the solvent diffuses into the oil phase and
the emulsion droplets harden into solid polymer microspheres. Unlike
solvent evaporation, this method can be used to make microspheres from
polymers with high melting points and a wide range of molecular weights.
Microspheres having a diameter between one and 300 microns can be
obtained with this procedure. The external morphology of the spheres is
highly dependent on the type of polymer used.
In spray drying, the polymer is dissolved in methylene chloride
(0_04 g/mI). A known amount of active drug is suspended (if insoluble)
or co-dissolved (if soluble) in the polymer solution. The solution or the
dispersion_ is then spray-dried. _ _ Microspheres. rang'tag in diameter
between
one and ten microns can be obtained with a morphology which depends
on the selection of polymer.
Hydrogel microparticles made of gel-type polymers such as
alginate or polyphosphazcnes or other dicarboxylic polymers can be
prepared by dissolving the polymer in an aqueous solution, suspending the
material to be incorporated into the mixture, and extruding the polymer
- mixture through a microdroplet forming device, equipped with a nitrogen
gas jet. The resulting microparticles fall into a slowly stirring, ionic
hardening bath, as described, for example, by Salib, et al. ,
PharnZazeutisc~e I~d~ 40-11A, 1230 (1978). The advantage of this
system is the ability to further modify the surface of the microparticies by
coating them with polycationic polymers such as polylysine, after
fabrication, for example, as described by Lim, et al., J. Pharm. Set. 70,
351-354 (1981). As described by Lim, et al., in the case of alginate, a
hydrogel can be formed by ionically crosslinking the alginate with calcium
ions, then crosslinking the outer surface of the microparticle with a
polycation such
A~r~:'~ :-. .
CA 02491511 1996-10-10
_r
CA 02234585 1998-
WO 97/13502 - PGT/US96/iG277 -
-15-
as polylysine, after fabrication. The microsphere particle size are
controlled using various size extruders, polymer flow rates and gas flow
Chitosan microparticles can be prepared by dissolving the polymer
in acidic solution and crosslinking with tripolyphosphate. For example,
carboxymethylcellulose (CMC) microsphere are prepared by dissolving
the polymer in an acid solution and precipitating the microparticles with
lead ions. Alginate/polyethylene imide (PF.>7 can be prepared to reduce
the amount of carboxyl groups on the alginate microparticles .
_. .. Loading of GM-CSF
The range of loading of the GM-CSF to be delivered is typically
between about 0.001 % and 10~, by weight. GM-CSF can be
incorporated into a polymeric matrix at a ratio of between 0.00196 by
weight up to 109b by weight. In a preferred embodiment, GM-CSF is
incorporated into PLGA blends to 2 96 by weight.
Loading is dependent on the disorder to be treated as well as the
time period over which the GM-CSF is to be released. Lower dosages
are required for use as a vaccine adjuvant, in the range of 0.001 to 0.196.
Microparticles for treatment of a severe infection would typically be
delivered in microparticles with 29b by weight drug loading.
Additives to Microparticles Altering Release
Polymer hydrolysis is accelerated at acidic or basic pH's and thus
the inclusion of acidic or basic excipients can be used to modulate the
polymer erosion rate. The excipients can be added as particulates, can be
mixed with the incorporated GM-CSF or can be dissolved within the
polymer.
Degradation enhancers are based on weight relative to the polymer
weight. They can be added to the protein phase, added as a separate
phase (i.e., as patticulates) or can be codissoived in the polymer phase
depending on the compound. In all cases the amount should be between
0.1 and thirty percent (wlw, polymer). Types of degradation enhancers
include inorganic acids such as ammonium sulfate and ammonium
CA 02491511 1996-10-10
.I
CA 02234585 1998-
W0 97/13502 - PCT/US96/16277
-16-
chloride, organic acids such as citric acid, benzoic acids, heparin, and
ascorbic acid, inorganic bases such as sodium carbonate, potassium
carbonate, calcium carbonate, zinc carbonate, and zinc hydroxide, and .
organic bases such as protamine sulfate, spermine, choline, ethanolamine,
diethanolamine, and triethanolamine and surfactants such as TweeaTM and '
Pluronic'rM.
Pore forming agents are used to add microstructure to the matrices
(i.e., water soluble compounds such as inorganic salts and sugars). They
are added as particulates. The range should be between one and thirty
percent (w/w, polymer).
Excipients can be also added to the GM-CSF to maintain its
potency depending on the duration of release. Stabilizers include
carbohydrates, amino acids, fatty acids, and surfactants and are known to
those skilled in the art. In addition, excipients which modify the
solubility of GM-CSF such as salts, complexing agents (albumin,
protamine) can be used to control the release rate of the protein from the
microparticles.
Stabilizers for the GM-CSF are basod on the ratio by weight of
stabilizer to the GM-CSF on a weight basis. Examples include
carbohydrate such as sucrose, lactose, mannitol, dexttan, and heparin,
proteins such as albumin and protamine, amino acids such as arginine,
glycine, and threonine, surfactants such as Tween''"s and Pluronic'''M, salts
such as calcium chloride and sodium phosphate, and lipids such as fatty
acids, phospholipids, and bile salts.
The ratios are generally 1:10 to 4:1, carbohydrate to protein,
amino acids to protein, protein stabilizer to protein, and salts to protein;
1:1000 to 1:20, surfactant to protein; and 1:20 to 4:1, lipids to protein.
Clinical Indications to be Treated '
Systemic Delivery for Proliferation of Cells
GM-CSF is approved for treatment of patterns requiring increased
proliferation of white blood cells. Data indicates that GM-CSF is also
useful as a vaccine adjuvant Morrissey, et al., J. ImmunoloQV 139, 1113-
CA 02491511 1996-10-10
CA 02234585 1998-
WO 97/13502 - PGT/US96/16Z77 -
-I7-
1119 (1987). GM-CSF microparticles can also be used to treat patie~
prone to infection such as those undergoing high risk bowel surgery,
trauma victims and individuals with HIV. The protocols and clinical
efficacy of GM-CSF is well known to those skilled in the art. As
described herein, the protocols are modified to reflect the changes in
delivery rates and dosages resulting from the release profiles from
microparticIes or hydrogels, as appropriate.
In vitro data regarding release profiles for GM-CSF, as well as
efficacy, appears to be predictive, although not identical, of in vivo data.
As demonstrated by the following examples, Rhesus monkey data show
maximum increases in Ieucocyte numbers within four days following
administration of GM-CSF, while in vitro results demonstrated that six to
seven days are required for complete release of the incorporated GM-
CSF. The advantage of using GM-CSF is that the protein is itself
extremely stable, with at least 60 % , in many cases 90 to 100 9b , of the
bioactivity being retained after incorporation into microparticles using auy
one of several processes.
Local Administration as Adjuvant
Enhanced vaccine response can be obtained through the use of
GM-CSF alone, but is more preferably obtained through a selection of the
polymer in combination with the controlled release of the GM-CSF. It is
known that certain polymers serve as chemoattractants for white cells.
PLGA is mildly inflammatory, as are polyanhydrides and polyorthoesters.
Through the selection of the chemoattractant polymer as the matrix for
GM-CSF, in a form yielding controlled release over a period of
approximately one week, maximum vaccine enhancement can be obtained.
In this embodiment, release can be from polymeric matrices in a variety
~ of forms, not just microparticles or hydrogels. The GM-CSF and
polymer may even act synergistically . to enhance the adjuvant effect of the
' 30 GM-CSF, as well as targeting of the GM-CSF to the white cells.
The GM-CSF can also be injected with a tumor antigen or tumor
cells that express antigens or their surfaces for use as a tumor vaccine.
CA 02491511 1996-10-10
CA 02234585 1998-04-09
WO 97/13502 - PCT/ITS96116Z77
-18-
Topical or Transmuc~al Administration
The hydrogel formulations are particularly useful for topical
applications. For example, GM-CSF has been shown by Brau~stein ei
al., west. Dermatol. I03, 601-604 (1994) to stimulate loeratinocyte
proliferation in human skin and could thus be utilized ss a topical wound
healing agent. Mucosal delivery of GM-CSF microparticles could also be
efficacious in the stimulation of mucosal immunity since the protein has
been shown to play a role in antibody production (Grabstein, et al., ~
Mol. Cell. Immunol. 2, 199 207 (1986)).
A in',.sc_ration of the GM-CSF Micronarticles
In the preferred embodiment for stimulation of proliferation of
hematopoietic progenitor cells, GM-CSF is administered incorporated is
microparticles which dcg~rade over a period of 1 of 2 months. The
microparticles preferably range in size from 10 to 60 microns, with an
average of 35 microns in diameter, and are injected simultaneously with
the aid of a suspension media. In one embodiment, the suspension media
consists of 3 '~ methyl cellulose, 4 9b mannitol, and 0.1 % Tween"' 80,
using a 22 gauge needle. In another embodiment the 3 ~ methylcellulose
is replaced by 1 ~ carboxy methylcellulose. _ One ml of viscous
suspension media is required to suspend 100 milligrams of microparticles
which contain enough GM-CSF to deliver 125 micrograms/mz/day over a
period of 7 days. Larger doses may be achieved by injecting more
microparticles. For example, a 250 microgram/ms/day dose would
require two I ml injections, each containing 100 mg of micropartieles.
The present invention will be further understood by reference to
the following non-limiting examples.
Example 1: Preparation of Microspheres Using Phase Separation
Process.
A. Lot #14223-I33. Sa~mnle "A"
The encapsulating polymer was a poly(glycolide-co-d,l lactide)
having an inherent viscosity of 0.43 dLg (as determined in a 0.5qb wlv
hexafluoroisopropanol sohrtion at 30°C), and a glycolide to lactide
ratio
CA 02491511 1996-10-10
CA 02234585 1998-04-09
w0 97/13302 - PCT/US96/16ZT7 -
-19-
of 45:55. It was prepared with glycolic acid as the initiator and stannous
chloride dihydrate as the catalyst. The distribution of lactoyl and glycoyl
groups within the copolymer was shown to be randa~ by C13 NMR and
solubility measurements. The residual lactide content was reduced by
~ 5 vacuum stripping. The encapsulating polymer solution was prepared by
adding 100 g of the polymer to 900 g of methylene chloride, and stirring
the mixture until the polymer dissolved.
0.978 ml of a GM-CSF solution (at 63.3 mg/ml in 100 mM tris
buffer) was added to a 20 g portion of the encapsulating polymer solution.
The mixture was stirred with a homogenizes using a 10-mm head at
10,000 RPM for 2 minutes to create a water-in-oil (W/O) emulsion.
18.0 g of Dow Corning~ 360 Fluid (polydimethylsiloxane) was
added to the W/O emulsion, and the mixture was homogenized at 10,000
RPM for 2 minutes. The mixture was then added to 2.4 kg of Dow
Corningm 244 Fluid (octamethylcyclotetrasiloxane) under stirring at 750
RPM to harden the microspheres. Stirring was continued for 90 minutes.
Microspheres were collected with a strainer fitted with a 1 ~m stainless
steel screen, then dried under vacuum.
Particle size distribution was analyzed with a Malvern 2600
Particle Sizes. Approximately 50 mg of microspheres were suspended in
about 10 ml of Dow Corningm 244 Fluid, and was sonicated for 2 minutes
to fully disperse the micmspheres. A few drops of this suspension were
then added to the optical cell which coined Dow Corningm 244 Fluid.
The particle size distribution was then measured. The sample had a
volume median diameter of 66.7 um, 10 ~ of the microspheres were
under 24.1 ~cm, 9096 of the microspheres were under 118.6 ~cm.
B. Lot #14223-134. Sample "B°
The encapsulating polymer and its solution in methylene chloride
were the same as described in "A°.
0.320 ml of a GM-CSF solution (at 63.3 mg/ml in 100 mM tris
buffer) was addod to a 20 g portion of the encapsulating polymer solution.
CA 02491511 1996-10-10
' CA 02234585 1998-04-09
-20-
The mixture was stirred with a homogenizer using a 10-mm head at
10,000 RPM for 2 minutes to create a water-in-oil (W/O) emulsion.
18.0 g of Dow Corning~ 360 Fluid (polydimethylsiloxane) were
added to the W/O emulsion, and the mixture was homogenized at 10,000
RPM for 2 minutes. The mixture was then added to 2.4 kg of Dow
Corning~ 244 Fluid (octamethylcyclotetrasiloxane) under stirring for 90
minutes at 750 RPM to harden the microspheres. Microspheres were
collected, dried and pazticle size distribution analyzed as described in
"A". The sample had a volume median diameter of 43.8 ~cm, 10 % of the
microspheres were under 7.0 Ecm, 90 % of the microspheres were under
77.9 ~cm.
As shown in Figure lA, samples "A" and "B" demonstrate that
PLGA microspheres can be fabricated to release GM-CSF in a triphasic
manner. In the first phase, the protein is released continuously over
approximately 5 days. This phase is followed by a period of minimal
GM-CSF release until day 35. At this time another pulse of GM-CSF is
released from the system. The duration of each phase can be controlled
by the type of polymers used to prepare the microspheres.
C. Lot #96~'i-96A. Sample "~~4"
The encapsulating polymer was a 60:20:20 mixture of I) a
poly(glycolide-co-d,l lactide) having a glycolide to lactide ratio of 47:53
and an inherent viscosity of 0.72 dLlg as determined in a 0.5 % wlv
- hexafluoroisopropanol solution at 30°C (polymer n, 2) a
poly(glycolide
co-d,l lactide) having a glycolide to lactide ratio of 50:50 and an inherent
viscosity of 0.33 dL/g as determined in a 0.1 % w/v chloroform solution
at 25°C (polymer In, and 3) a poly(d,l lactide) with an average
molecular
weight of 1938 as determined by end group titration (polymer III).
Polymer II and polymer III were reprccipitated before use. Tl~
encapsulating polymer solution was prepared by adding 1.20 g of polymer
I, 0.40 g of polymer II, and 0.40 g of polymer BI to 18.00 g of
methylene chloride and stirring the mixture until the polymers dissolved.
AliritNC~ED SHEFf
CA 02491511 1996-10-10
CA 02234585 1998-04-09
WO 97/13502 - PGT/US9G/16Z77
-21-
0.481 ml of a GM-CSF solution at 84.8 mg/ml in 100 mM tris
buffer) was added to the encapsulating polymer solution, homogenized
with a 20-mm head at 10,000 RPM for 60 seconds to created a water-in-
__________-~0 (~~O) emulsion.
The beaker containing the W/O emulsion was placed under a
mixer equipped with a 3-blade Teflon stirrer and stirred at 1000 RPM.
20 ml of Dow Corning~ 360 Fluid was added to the W/O emulsion while
it was being stirred at 1000 RPM over a 1-minute period of time using a
syringe Pump.
_ _ The mixture was then added to 2.0 kg of Dow Corningm 244 Fluid
under stirring for 90 minutes at 400 RPM to harden the microspheres.
Microspheres were collected, dried and Particle size distribution
was analyzed as described above. The sample had a volume median
diameter of 31. 8 ~cm, 10 % were under 14.1 ~cm and 90 Rb of the
microsphers were under 52.2 /am.
D. Lot #9663-135C Sample "04 "
The encapsulating polymer was a 60:20:20 mixture of: 1) a
poly(glycolide-co-d,l lactide) having a glycolide to lactide redo of 47:53
and an inherent viscosity of 0.72 dL/g as determined in a 0.5 '~ w/v
hexafluoroisopropanol solution at 30°C (polymer I), 2) a poly(glycolide-
co-d,l lactide) having a glycolide to lactide ratio of 50:50 and an inherent
viscosity of 0.33 dLJg as determined in a 0.19b wlv chloroform solution
at 25°C (polymer In, and 3) a poly(d,l lactide) with an average
molecular
weight of 1938 as determined by end group titration (polymer ~.
Polymer II and polymer III were reprecipitated before use. The
encapsulating polymer solution was prepared by adding 1.20 g of polymer
I, 0.40 g of polymer II, and 0.40 g of polymer III to 18.00 g of
methylene chloride and stirring the mixture until the polymers dissolved.
0.462 ml of a GM-CSF solution (at 88.4 mg/ml in 100 mM tris
buffer) was added to the encapsulating polymer solution, homogenized
with a 20-mm head at 10,000 RPM for 60 seconds to create a water-in-oil
(W/O) emulsion. The W/O emulsion was stirred at 1000 RPM. 20 ml of
CA 02491511 1996-10-10
CA OZ234585 1998-04-09
WO 97n350~
Dow Coraingm 360 Fluid was added to the W/O emulsion while it was
being stirred over a 1-minute period of time usiag a syringe gump. The
mixture was then added to 2.0 kg of Dow Corningm 244 Fluid under
stirring at 400 RPM for 90 minutes to harden the microsph~et~es.
~ Microspheres were collected, dried and particle size distn'bution
was analyzed as described above. The sample had a volume median
diameter of 39.5 Ecm, 10% of the microspheres were under 14.9 ~cm, and
909b of the microspheres were under 65.1 Eam.
E. QO-168. Sample "V4." Ascetic Pros
Microspheres were prepared aseptically as follows. Glassware,
mixer shafts and heads, and stainless steel vessels were autoclaved prior
to use.
The encapsulating polymer was a 60:20:20 mW 4ire of 1)
poly(glycolide-co-d,l lactide) having a glycolide to lactide ratio of 47:53
and an inhcnent viscosity of 0.72 dL/g as deternnined in a 0.596 w/v
hexatluoroisopmpanol solution at 30°C (polymer n, 2) a poly(glycolide-
co-d,l lacdde) having a glycolide to lactide ratio of 50:50 and an inherent
viscosity of 0.33 dLJg as determined is a 0.19b w/v chloroform solution
at 25°C (polymer In, and 3) a poly(d,l lactide) with an average
molecular
weight of 1938 as determined by end group titration (polymer 1II).
Polymer II and polymer III were reprecipitated before use. The
encapsulating polymer sohrtion was prepared by adding 3.00 g of polymer
I, 1.00 g of polymer II, and 1.00 g of polymer BI to 45.00 g of
methylene chloride and stirring the mixture uatil the polymers dissolved.
20.00 g of this solution was filtered through a glass fiber prefilter and a
0.45 pm Teflon filter for microencapsulstion.
Approximately 100 g of Dow Corningm 360 Fiuid was heated at
160°C for 1 hour in a glass bea>oer covertd with aluminum foil, then '
cooled to room t~pe~tture.
2.0 kg of Dow Coraingm 244 Fluid was filtered through a
"Millipak'" 20 ~ 0.22 Ecm filter into the hardening vessel.
CA 02491511 1996-10-10
CA 02234585 1998-04-09
WO 97113302 PGT/OS96/16Z77 '
0.485 ml of a GM-CSF solution (at 87.3 mg/ml in 100 mM tris
buffer, filtered through a 0.2 Ecm filter) was added to the 20 grams of
. filtered encapsulating polymer sohnion, stirred with a homogenizes using
a 20-nun head at 10,000 RPM for 60 seconds to create a water-in-oil
t',W10) emulsion.
The beaker containing the W/O emulsion was placed under a
mixer equipped with a 3-blade Teflon stirrer and stirred at 1000 RPM.
20 ml of the heat-treated Dow Corning~ 360 Fluid was added to the Rt/O
emulsion while it was being stirred at 1000 RPM over a 1 minute period
of time using a syringe pump.
The mixture was then added to the 2.0 kg of filtered Dow
Corningm 244 Fluid under stirring at 400 RPM to harden the
anicrospheres. Stirring was continued for 90 minutes.
Microspheres were collected, dried and the particle size
distribution was determined with a Malvern 2600 Particle Sizes. The
particle size distribution was then measured. The sample had a volume
median diameter of 32.5 pm, 10% of the microspheres were under 14.7
lam, and 90 9~ of the microspheres were under 54.8 ~cm.
Example 2: Analysis of GM-CSF Incorporated into Microsphet; es
Usiag Reversed Phase ugh Performance Liquid
Chronnatography.
GM-CSF was first extracted from microspheres using acetic acid,
methylene chloride and phosphate buffered saline. A control consisting of
blank microspheres with freshly added stock GM-CSF was concurnntly
extracted. The eatunets were then evaluated by RP-HPLC for glycoform
distn'bution.
Recombinant human (rhu) GM-CSF glycosytated variaais are
measured using high performance liquid chromatography (HPI~. The
cohimn is a C 18 10 micron, 300 angstrom cohimn (4.6 mm x 25 cm)
from Vydac. The glycofot:ms of GM-CSF can be resolved in this method
using a mobile phase gradient of 25 to 65 % 0.1 ~b trifluoroacetxc acid
(TFA)/acetonitrile in 0.1 ~ TFA/water with constant 200 mM NaCI.
CA 02491511 1996-10-10
CA 02234585 1998-04-09
WO 97/I3302 - PCT/US96/16277 -
_2ø
Results show that the GM-CSF glycofolm distribution of the
control is not altered due to the extraction protocol and the RP-HPLC
profile of GM-CSF incorporatod into microspheres remains unchanged.
Example 3: GM-CSF Release ginetics from NRcrosphere
Preparations. ,
Release kinetics of GM-CSF microspheres were analyzed using the
following methods. Microsphere lots were stored in their original
containers at 2 - 8°C. The in vitro release studies were initiated
within a
few days of preparation. Studies were set up in duplicate for screening
and in triplicate for increased confidence in accuracy and precision. The
release buffer was phosphate buffered saline (PBS, I2 mM sodium
phosphate, 3.7 mM potassium phosphate, 150 mM sodium chloride, pH
7.0, 0.22 ~m filtered) containing 0.02 9b sodium azide as preservative,
although it is recommended to leave out the azide and handle the release
study as aseptically as possible when bioactivity analysis of the released
GM-CSF is planned. Collection timc intervals in a study may include
times at 2, 6, 24, 48, 72 hrs, 5, 7, 10, and 14 days.
To set up an in vitro release assay, approximately 50 to 75 mg of
microspheres were placed in the 9 mm 1D x 13 mm OD x 5 mm teflon
spacer which is within the teflon jacket of the device. The spacer was
anchored at both ends with I3 mm diameter, 10 fcm mesh stainless steel
screens which allow circulation of release buffer through the
microspheres.
The loaded device was placed into a wide, 30 ml polypropylene
test tube with 6 ml release buffer. The open tubes containing the loaded
device and release buffer were covered with loose caps or Kimwipesm
secured with rubber bands and placed in a vacuum desiccator. Exposure
to vacuum for approximately 2 hours helps remove any trapped air
bubbles and promotes initial wetting of the microspherrs. The tubes were
then capped and incubated at 37°C on an orbital shaker set at 100 rpm.
At appropriate time intervals the release buffer was retrieved by
decanting into preweighed, labelled 15 ml polypropylene test tubes. The
CA 02491511 1996-10-10
CA 02234585 1998-04-09
WO 97/13502 - PCT/US96/16Z77
-
volume obtained was determined by weight (ml = g, approximately).
The device, with an addition of firsh release buffer (6 mn, was they
returned to the Orbital shalaer at 37°C.
. _- _._. _she microspherereleasesamples were evaluated b~ the Bio-Rad ___
Total Protein Assay to quantify the release of GM-CSF. A total protein
assay can be used since there is no other protein present.
The results of the release kinetics analysis are shown in Figures 1B
and 3A. Samples "B4", "04" and "V4", prepared in Example 1, show
that PLGA microspheres can be fabricated to contimwusly release GM-
CSF over a period of approximately 6 days. The release is linear over
this time and approaches zero order. Furthermore, the GM-CSF is
released with essentially complete retention of bioactivity as shown in
Figure 3B (given the standard deviation of the bioassay, the released GM-
CSF can be considered completely active.) These examples also show
. that the microsphere fabrication process is highly reproducible as
evidenced by the very similar GM-CSF release profiles generated from
the three preparations.
Example 4: In ~vo Release Profile of huGM-CSF is a Marine
Model
A. Lot #9402-94. Samnie "O"
Microspheres were prepared aseptically as follows:
Glassware, mixer shafts and heads, and stainless steel vessels were
autoclaved prior to use. 4.0 kg of Dow Corningm 244 Fhtid
(octamethylcyclotetrasiloxane) was filtered through a "Millipak'°' 40"
0.22
~.m filter into the hardening vessel. Approximately 100 g of Dow
Corningm 360 Fluid (polydimethylsiloxane, 350 centistrokes) was heated
at 160°C for 80 minutes in a glass beaker covered with aluminum foil,
then cooled to room temperature.
A 40-gram portion of a lOq6 poly(glycolide-co-d,l lactide) solution
in methylene chloride was filtered through a polyvi~lidene fluoride 0.22
Ecm filter for microencapsulation. The polymer had an inherent viscosity
of 0.43 dLlg as determined in a 0.5 Rlo w/v hexafluoroisopropanol solution
CA 02491511 1996-10-10
CA 02134585 1998-04-09
WO 97/13302 - PCT1ITS96/i6IT7 -
at 30°C, and a glycolide to lactide ratio of 45:55. It was prepared
with
glycolic acid as the initiator and stannous chloride dehydrate as the
catalyst. The distribution of lactoyl and glycoyl groups withim the .
copolymer was shown to be random by CI3 NMR sad sohibility
measurements. The residual lactide contest was reduced by vacuum '
stripping.
0.664 ml of a GM-CSF solution (about 63.1 mg/ml is 100 mM
tris buffer, filtered through a 0.2 hum filter) was added to the 40 grams of
filtered polymer solution, stirnd with a homogenizer usinig a 20-mm head
at 10.000 RPM for 60 seconds to create a water-in-oil (W/O) emulsion.
36 ml of the heat-treated Dow Corningm 360 Fluid was added to the W/O
emulsion, and the mixture was homogenized at 5000 RPM for 90
seconds. The mixture was then added to the 4.0 kg of filtered Dow
Coraing~ 244 Fluid under stiaing at 500 RPM to harden the
microspheres. Stirring was continued for I hour. Microspheres were
collected, dried and particle size distribution analyzed as described above.
The sample had a volume median diameter of 56.0 ~ma, l O gb of the
mierospheres were under 26.3 Ecm, 909b of the microspheres were under
91.6 Ecm.
The microspheres were first analyzed for in vitm release
characteristics to ensure continuous release of huGM-CSF over a period
of greater than 7 days (Figure 2A). The microspheres were then weighed
and loaded into 3 cc syringes with 50 mg of microspheres/syringe. The
injection vehicle for the microspheres was an aqueous solution of low
viscosity grade methyl cellulose, confining 3 9b (w/w) methyl cellulose,
0.1 °6 Tween 80, and 49b mannitol (final osmolality = 292 mOsmlkg).
Vials were loaded with 1 g each of sterile filtered injection vehicle
solution. For injection, an 18-gauge needle was attached to an empty 3
cc syringe and used to withdraw 0.5 - 0.6 ml of injection vehicle. The
needle was then removed from the syringe and the syringe containing the
vehicle was attached to a syringe containing microspheres through a
"syringe connector°. Mixing was achievod by pushing the syringes back-
CA 02491511 1996-10-10
CA 02234585 1998-04-09
wo ~rn~soi - rcrms96nsir
-27-
and-forth 25 times in each direction. The empty syringe and the syringe
connector were then removed. A 22 gauge needle was then attached to
the syringe with suspended micxospha~a ready for injection.
Release_S'tudies in Mine
Release studies wcra conducted on mice as follows. Male B6 mice
(6 weeks old) were obtained from Jackson Laboratories (Bar Harbor, ME)
and were housed in the Ianmuaex animal laboratory facility for an
additional IO weeks prior to initiating the study. Seventeen groups of
mice were used in the study, with three mice used per group. For the test
group 50 mg of microspheres containing 500 ~,g of huGM-CSF (196 by
wt) were injected subcutaneously in 0.5 ml of the methyl cellulose
injection vehicle. Groups of mice receiving these injections were
sacrificxd at intervals of 1, 2, 6, 24 hr, 3, 5, 7, and 9 days. As a
negative control, one group of mice was sacrificed without receiving
huGM-CSF in any form. As a final control a bolus of huGM-CSF was
injected subcutaneously at a dose of either 500 or 50 ~cg of huGM-CSF.
The 500 ~cg dose represented the entire amount of huGM-CSF contained
in a 50 mg injection of microspheres, and the 50 ~cg dose represented an
approximation of the amount of huGM-CSF released by 50 mg of
micmspheres over a period of 1 day in vitro. Groups of mice receiving
bolus injections were sacrificed at intervals of 1, 2, 6, and 24 hr post-
injection.
Following sacrifice of the mice, blood samples were obtained and
allowed to clot at 4°C. The sera was then harvested and the clot was
discarded. Remaining cxlh~lar debris was removed by centrifugation, and
the serum samples were then frozen at -70°C until further analysis.
CA 02491511 1996-10-10
CA OI234585 1998-04-09
WO 97/13302 PGTlIJS96/16Z77 '
-28-
Table I. In yvo Micros~~her~ R~t_~4e S~dy_ Outiine
G~,~, Post-Infection
Sa-~ficr Tim
1 no injection, negative cool 0 hr .
2 500 ~,g huGM-CSF bolus injection
1 hr
3 50 ~,g huGM-CSF bolus injection 1 hr
4 50 mg microspheres injected 1 hr
500 ~.g huGM-CSF bolus injection
2 hr
6 50 ~cg huGM-CSF bolus injection 2 hr
7 50 mg microspheres injected 2 hr
8 500 ~,g huGM-CSF boh~s injection
6 hr
9 50 ~,g huGM-CSF bolus injection 6 hr
50 mg microsphens injected 6 hr
11 500 ug huGM-CSF bolus injection 24 hr
'12 50 ~cg huGM-CSF bolus injection 24 hr
13 50 mg microspheres injected 24 hr
14 50 mg microspheres injected 72 hr (3 day)
50 mg microspheres injected I20 hr (5 day)
16 50 mg microspheres injected 168 hr (7 day)
17 50 mg microspheres injected 216 hr (9 day)
Serum samples we=e thawed and analyzed by ELiSA for
determination of huGM-CSF concentrations. The GM-CSF enzyme
linked immunoassay (EIA) is an assay designed to quantitate levels of
recombinant human (rhu) GM-CSF in an unknown sample. An anti-GM-
5 CSF marine monoclonal antibody is adsorbed onto a 96-well polystynen~
plate overnight. After washing, a standard curve and samples are added
to the plate and incubatal. The plate is washed to remove any excess
unabsorbed rhu-GM-CSF. A polyclonal antibody to rhu-GM-CSF is then
added to each well and incubated. The plate is washed to remove any
10 unbound polyclonal antibody and a solution containing donkey anti-sheep
IgG antibody conjugated to horseradish peroxidase (HRP) enzyme is -
addcd to each well. Following incubation the plate is washed to remove
any excess HRP-linkod a~'body which did not bind to the sheep '
antibodies present. A developing solution containing the chromogenic
15 substrate for the HRP conjugate is added to the plate. Color development
CA 02491511 1996-10-10
_ .._
CA 02234585 1998-04-09
W0 99/13302 - PCT/~18961162T1
29-
is directly Proportional to the amount of HRP-conjugate present. The
optical density readiags at the correct wavelength give numerical values
for each well. These wells can be compared with the staadard curve
values, permitting quantitation of the levels of rhu-GM-CSF. A
monoclonal anti GM-CSF antibody can be obtai~d from Immunea. A
donkey anti-sheep IgG antibody HRP conjugate is obtained from Jackson
Immunoresearch Laboratories.
Serum samples were also analyzed for bioactivity by the cell
proliferation assay TF-1. A TFl bioassay is used to detect the presence
and amount of human GM-CSF. The TFl bioassay utilizes a human
erythroleukemia cell Iine, TFl, to detect the presence of huGM-CSF, hu
IIr3, or rhu PIXY321 in test samples. These cells are dependent upon
huGM-CSF for growth and are maintained is medium supplemented with
huGM-CSF. The addition of huGM-CSF, hu II~3, or rhu PIXY321 to
these cells stimulates a dose-dependent proliferation, allowing for
qua~itation of hnGM-CSF, hu IL-3, or rhu PIXY321 in test samples as
compared to a standard of known huGM-CSF, hu ITr3, or rhu PIXY321
concentration.
The amount of proliferation is measured by "pulsing" each
microwell with tritisted thymidine (~H-TdR) for 4 hours at 37°C.
Proliferating TF-1 cells will incorporaOe (~H-TdR) which is added to the
medium into their DNA as they divide. The cells from each well are then
harvested onto a glass fiber filter paper which traps the labeled GM-CSF.
The amount of'H-Tit t=apped on each filter paper is then counted on a
beta co~mter. The rnimber of counts per minute (cpm) for each well is
directly proportional to the amount of proliferation by the activated TF-1
cells in response to huGM-CSF, hu IL-3, or ru PIXY321. The resulting
counts per minute are directly proportional to the amount of GM-CSF that
was stimulating the cell colony.
To determine bioa~ivit3r of GM-CSF in the release samples all
samples are dilutod to 0.2 ng GM-CSF/ml and submiued for analysis.
The resulting activities expressed as unitslml are compared to the activity
CA 02491511 1996-10-10
CA 02234585 1998-04-09
WO 97113302 - PCT/US96/i6177 '
-30-
of as untreated stock sample of GM-CSF analyzed simultaz>eously. In
theory, all samples should have approximatiely 10096 activity relative to
the stock sample. Hetvveen assays control values can range atrnmd 20 to
2596, a precision level not unusual with assays based on cell growth.
The percentage of specific activity retained at each time point was
determined by dividing the specific activity measured at each time point
by the specific activity of stock huGM-CSF of the same lot which had not
been incorporated into microsphei~es.
Results of the release study arc shown in Figure 2A, which is a
graph of the in vitro release kinetics showing release over a period of
about ten days. Figure 2B is a graph of the circulating mouse serum
huGM-CSF levels (determined by ELISA) as a function of time. Both the
500 and 50 ~cg bolus injections were rapidly cleared from mouse serum.
Due to the rapid decline of detectable huGM-CSF in mouse serum only a
rough estimate of the ~ elimination half life could be made (t~,~B = 1.57
hr); however, this estimate agrees closely with previously reported half-
lives for huGM-CSF circulating in a mouse model. Levels of serum
huGM-CSF in the mice which received microspheres dropped rapidly
over the first 6 hours post-injection (from 218 to 35 nglml), and then
remained relatively constant over the remaining 9 days of the study.
Presumably, given the in vitro release profile for this lot of microspheres
(approximately 309b release after 9 days) huGM-CSF would have released
from the microsphrres beyond the 9 day period whore the in vivo study
was terminated.
As shown in Figure 2C, the in vivo release data was compared to
the in vitro release data as follows: (1) the in vitro release data was first
mathematically modeled to fit a power series; (2) a theoretical in vivo
serum huGM-CSF concentration profile was then calf by takiztg a
mass balance in a single compartment model (i.e. the huGM-CSF
conce~ration in the manse at a~ time equals the concentration of huGM-
CSF already in the mouse at a previous time pout plus the amount of
huGM-CSF released from the microspheres over that time period minus
CA 02491511 1996-10-10
CA OZ234585 1998-04-09
WO 97/l3sOZ ~- PCT/US96/16277
-31-
the amount of huGM-CSF cleared by normal physiological clearance
mechanisms over that same time period). The resulting comparison of
serum concentration based on in vitro release and actual in view serum
huGM-CSF levels is shown in Figure 2C. As demonsti~aLed in this figtn~e
the actual in viva serum huGM-CSF levels were lower than those
predicted by the in vitro release, however, the profiles were similar is
shape and remarkably close in values at later time points.
The bioactivity of huGM-CSF released from microspheres in viva
was estimated by TF-1 bioassay. The percent of specific bioactivity
varied from a high of 67% at 1 hour and gradually declined to a low of
33 % after 9 days.
Example 5: Release of Human GM-CSF from Microspheres in a
Primate Model.
Microspheres containing huGM-CSF were prepared for in viva
injection in a rhesus monkey model (Lot #9490-168, sample "V4°).
Microspheres were first characterized in vitro for protein loading (1.4896
wt/wt by amino acid analysis), release kinetics (see Figure 3A) and
bioactivity of released material by TF-1 bioassay (see Figures 1B and
3B). Based on the in vitro release profile, microspheres were weighed
out such that primates would receive approximately 25 ~,cg/kg/day for 7
days. Syringes were loaded with microspheres which included an extra
5 % for the hold-up volume encountered on injection (50 ~cl holdup
volume for 1 ec tuberculin syringe). Three primates received injections
with microspheres containing GM-CSF. One primate received placebo
nucrospheres which did not contain microspheres. The quantity of
microsphe~rs injected into each of the primates was 39.4 mg, 35.5 mg,
42.1 mg, and 36.8 mg, for 3.2 kg, 2.9 kg, 3.4 kg, and 3.0 kg animals,
respectively.
The injection vehicle for the microspheres was an aqueous solution
- 30 of low viscosity grade methyl cellulose, containing 3 96 (wt/wt) methyl
cellulose, 0.196 Tween 80, and 4 96 maanitol.
CA 02491511 1996-10-10
CA 02234585 1998-04-09
-32-
Serum samples went collected and analyzed for white blood cell
count (WBC), absolute neutrophil count (ANC), and platelet count on
days 3 and 1 prior to injection, on the day of injection, and daily
following the injection for 10 days. Daily blood cells counts are shown in
Figure 3C.
The WBCs and ANCs were clearly elevated on days 1 through 4
in each of the animals receiving GM-CSF containing microspheres. No
changes in blood cell counts were measured for the primate receiving the
placebo injection.
This example shows that recombinant human GM-CSF released
from PLGA microspheres in vivo, is capable of eliciting a biological
response in a non-human primate model.
Highly localized inflammatory response seen is the monkeys was
characterized by a significant localized swelling (1 - 2 cm disiaeter lump)
at the site of injection as a result of recruitment of neutrophils,
macrophages, dendritic cells and monocytes.
Example 6: Release of GM-CSF From a PLGA Gel.
A 2096 solution of PLGA (50:50 lactide glycolide ratio, 0.38 dL/g
intrinsic viscosity (LV.)) was prepared by heating 2 g of PLGA is 8 g of
glycerol triacetate (triacetin) at 70°C for 30 minutes. Lyophilized GM-
CSF was added to the PLGA solution at 10 mg/mI and sonicated to
- complete mixing. Screw top vials (5 ml) were filled with 3 ml PBS and
approximately 250 ~cg of the PLGA/GM-CSF solution (containing
approximately 2.5 mg of GM-CSF) was added to each vial by pipetting.
The vials were shaken gently at 37°C for 6 days. The injected
solution
formed a gel on injection into the PBS. At intervals of 4 hr, 8 hr, I day,
3 days, and 6 days, the solutions were removed from the vials and
analyzed for GM-CSF content by a BioRad total protein assay.
The protein release kinetics are shown in Figure 4 and show fwst
order kinetics over a period of about six days.
Af ;ut~~t7 S'rs'E"t3'
_CA 02491511 1996-10-10
CA 02234585 1998-04-09
WO 97/13302 - - PGT/US96J16Z77
-33-
Example 7: Preparation of Microspheres Uslag a W/O/W-Methanol
Fatraction Process. (i.ot #14254-13~
The encapsulating polymer was a 70:20:10 mixture of 1) a
poly(glycolide-co-d,l lactide) having a glycolide to lactide ratio of 50:50
and an inherent viscosity of 0.33 dL/g as determined in a 0.196 w/v
chloroform solution at 25 °C (polymer n, 2) a poly(L-lactide) with an
average molecular weight of 1786 as determined by end group titration
(polymer In, and 3) a poly(d,l lactide) with an average molecular weight
of 1938 as determined by end group titration (polymer Iin. The polymers
were reprecipitated before use. The encapsulating polymer solution was
prepared by adding 1.40 g of polymer I, 0.40 g of polymer II, and 0.20 g
of polymer III to 8.00 g of methylene chloride and stirring the mixture
until the polymers dissolved.
A 5 ~ aqueous solution of polyvinyl alcohol (PVA) was prepared
by adding 20.00 g of low molecular weight (M.W. = 31,000 - 50,000,
87-89 °6 hydrolyzed) PVA to 380 g of deioniud water, and stirring with
heating (to approximately 70°C) until the PVA is dissolved. The
solution
was filtered through a 0.2 Esm filter after cooling to room temperature.
0.389 ml of a GM-CSF solution (about 87.3 mg/ml in 100 mM
tris buffer) was added to 8.50 g of the encapsulating polymer solution in a
30-ml glass beaker, and was homogenized with a 20-mm head at 6000
RPM for 60 seconds to create a water-in-oil (W/O) emulsion.
The above emulsion was added to 400 g of the 5 ~Yo PVA solution
in a stainless steel vessel while it was being stirred with a homogenizer at
6000 RPM using a 20-mm head to create a water-in-oil-in-water (W/O/V~
emulsion. Total elapsod time was 1 minute.
The vessel containing the W/O/W emulsion was placed under a
mixer equipped with a "high shear disperser" and stirred at 400 RPM.
400 g of methanol was added to the W/0/W emulsion over a 45-minute
- 30 period w extract the methylene chloride from the microspheres. Stirring
was continued for another 45 minutes after the addition of methanol.
CA 02491511 1996-10-10
CA 02234585 1998-04-09
WO 97/13302 - PCT/I1S96/16277
_3ø
Microspheres were collected, dried and particle size distribution
was determined as described above.
The sample had a volume median diameter of 24.1 /,ctn, 1096 of
the microspheres were under 10.8 ~cna, sad 9096 of the microspheres were
under 42.3 fcm. '
Example 8: Microsphere Preparation using a W/OIW Methanol
Extraction Process. (Lot #14254-160)
The encapsulating polymer was a 80:10:10 mixture of 1) a
poly(glycoIide-co-d,l lactide) having a glycolide to Iactide ratio of 50:50
and an inherent viscosity of 0.33 dL/g as determined in a 0.19b w/v
chloroform solution at 25°C (polymer n, 2) a poly(Lrlactide) with an
average molecular weight of 1786 as determined by end group titration
(polymer In, and 3) a poly(d,l lactide) with an average molecular weight
of 1938 as determined by end group titration (polymer IIn. The polymers
were reprecipitated before use. The encapsulating polymer solution was
prepared by adding 1.60 g of polymer I, 0.20 g of polymer II, and 0.20 g
of polymer BI to 8.00 g of methylene chloride and stirring the mixture
until the polymers dissolved.
A 5 9b aqueous solution of polyvinyl alcohol (PVA) was prepared
by adding 20.00 g of low molecular weight (M.W. = 31,000 - 50,000,
87-89 ~ hydrolyzed) PVA to 380 g of deionized water, and stirring with
heating (to approximately 70°C) unfit the PVA is dissolved. The
solution
was filtered through a 0.2 ~cm filter after cooling to room temperature.
0.389 mI of a GM-CSF solution (about 87.3 mg/ml in 100 mM
Tris buffer) were added to 8.50 g of the encapsulating polymer solution in
a 30-ml glass beaker, and was homogenized with a 20-mm head at 6000
RPM for 60 seconds to create a water-in-oil (W/O) emulsion.
The above emulsion was added to 400 g of the 5 96 PVA solution '
in a stainless steel vessel while it was being stirred with a homogenizes at
6000 RPM using a 20-mm head to create a water-in-oil-is water (W/Ol~
emulsion. Total elapsed time was 1 minaue.
CA 02491511 1996-10-10
CA 02234585 1998-04-09
WO 97/13502 - PCT/US96/16277
-35-
The vessel containing the W/O!W emulsion was placed under a
mixer equipped with a "high shear dispenser" and stirnd at 400 RPM.
400g of methanol was then pumped into the W/O/W emulsion at a
constant rate over a 5-minute period to extract the methylene chloride
~ 5 firom the microspheres. Stirring was continued for another 85 minutes
after the addition of methanol.
Microspheres were collected, dried, and particle size distribution
was determined as described above.
The sample had a volume median diameter of 26.1 fcm, 109b of
the microspheres were under 11.6 ~cm, 90'~ of the micmspheres were
under 46.7 E.cm.
Example 9: Preparation of mierospheres Lot #14259-100 (Hydrogel)"
by a W/O/W methanol e~ctraction process.
The encapsulating polymer was a 67:23:10 block tripolymer of
caprolactone, trimethylene carbonate, and polyethylene oxide 8000. 3.27
g of the polymer was mixed with 18.53 g of methylene chloride and
stirred until the polymer dissolved.
A 1 °~b aqueous solution of polyvinyl alcohol (PVA) was prepared
by adding 11.0 g of low molecular weight (M.W. = 31,000 - 50,000, 87-
8996 hydrolyzed) PVA to 1089.00 g of deionized water, and stirring with
heating (to approximately 70°C) until the PVA dissolved. The solution
was filtered through a 0.2 hum filter after cooling to room temperature.
0.174 ml of a GM-CSF solution (si 87.1 mg/ml in 100 mM Tris
buffer) was added to a 10.00 g portion of the encapsnlati~g polymer
solution in a 30-ml glass beaker, and was homogenized with a 20-mm
head at 6000 RPM for 60 seconds to create a water-in-oil (W/O)
emulsion.
The above emulsion was added to a 500 g portion of the 19b PVA
solution in a stainless steel vessel while it was being stirred with a
homogenizer at 6000 RPM using a 20-mm head to create a water-in-oil-
in-water (W/O/~ emulsion. Total elapsod time was 1 minute.
CA 02491511 1996-10-10
CA 02234585 1998-04-09
WO 97/13502 - PCT/~596/162T1
-36-
The vessel coning the W/O/W emulsion was placed under a
mixer equipped with a "high shear disperser" and stirred at 400 RPM.
500 g of methanol was pumped i~o the W/O/W emulsion over a 5-minaue
period to extract the methylene chloride from the microsphetrs. Stirring
was continued for another 55 minutes after the addition of methanol. '
Microspheres were collected, dried and particle size distribution
was determined with a Malvern 2600 Particle Sizer. Approximately 50
mg of microspheres was suspended in about 10 ml of methanol, and was
sonicated for 2 minutes to fully disperse the microspheres. A few drops
of this suspension were then added to the optical cell which contained
methanol. The particle size distribution was then measured. The sample
had a volume median diameter of 68.3 ~cm, 109b of the microspheres
were under 14.3 E.cm and 909b of the microspheres were under 177.3 Erm.
Example 10: Extraction of GM-CSF from Microspheres for In V'~v
Determination of Bioactivity.
This method of extracting the protein from the microspheres is
both quantitative and nondestructive and therefore can be used to
determine the integrity of the incorporated protein. Approximately 20 mg
microspheres (Lots L3, M3, N3, K3, J3, and E3) prepared by the phase
separation method described in Example 1 were weighed into a 2 ml
Eppendorf tube. 500 ~,1 of glacial acetic acid was then added and the
tubes were capped and periodically vortexed to completely dissolve the
microspheres. 500 ~cl methylene chloride was then added and the tubes
were capped and vortexed periodically for about 5 minutes. Finally 500
Wl PBS were added and, again, the tubes were capped and vortexed
continually for about 2 minutes. The capped tubes were next centrifuged
in a microfuge at high speed for 2 minutes to facilitate clean separation of
the aqueous and organic solvent phases. The absorbance of 280 nm of
the upper aqueous layer (1 ml) containing the GM-CSF was determined
and the concentration of GM-CSF in mg/ml was calculated by dividing
the absorbance by the extinction coefficient of 1.08. The samples wen
CA 02491511 1996-10-10
CA 02134585 1998-04-09
wo ~rn3so~ - rc~rszrr -
-37-
then submitted for TFl bioassay to determine the bioactivity of the GM-
CSF.
Figeu~e 5 shows the petoart bid of the GM-CSF eat
from the micraspheres. The control is an aqueous GM-CSF sample that
has gone through the same extraction process. These results indicate that
the GM-CSF extiscted from the microspheres has retained complete
bioactivity.
Example I1: Preparation of PLGA microspheres and PLGA
degradation profiles.
Microspheres containing 100 Cytec, 0.7 dIJg intrinsic viscosity
(LV.) PLGA, 100Yb 8104 PLA, and an 80:20 mixture of the PLGA:PLA
were prepared by the silicone oil hardening proxss. Samples of each of
these microspheres were incubated at 37°C in PBS for periods of 1, 2,
4,
6, 8, 10, 14, 28, 42, and 56 days. Following incubation the microsphens
1S were dissolved in terrahydroti~ran (TZ~ making 1 to 29b solutions
(microsphere wrlTT~ volume). The samples were then analyzed by gel
permeation chromatography (GPC) using Waters HPLC system with a
styragel HR-4E column (Waters) which was maid at 30°C
throughout the GPC nm. Polystyrene narrow molecular weight range
standards were used to calibrate the column. 'Weight and number average
molecxtlar weights of the degraded PLGA polymers were determia~od with
Millenium GPC software (Waters). Figure 6 below illustrates the weight
average molecular weights of each micxosphere formulation as a function
of incubation time. Note that the degradation of microspheres prepared
from the 1009b Cytec 0.7 LV PLGA was incomplete over a 14 day
incubation period whereas the 80:20 mixture of Cytec 0.7 LV./R104 was
essentially degraded. In this example the 8104 PLA acted as a
degradation enhancer for the microsphetts.
Modifications and variations of the compositions and methods for
maaoufacture and use descnbod herein will be obvious to those skilled in
the art from the foregoing description. Such modifications and variations
ane intended to come within the scope of the appended claims.